Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PIERIS PHARMACEUTICALS, INC. | form8-k.htm |

Cantor Fitzgerald Global Healthcare Conference October 2018 (Nasdaq: PIRS)

Forward Looking Statements This presentation contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, references to novel technologies and methods and our business and product development plans, including the advancement of our proprietary and co-development programs into and through the clinic. Actual results could differ from those projected in any forward- looking statements due to numerous factors. Such factors include, among others, our ability to raise the additional funding we will need to continue to pursue our business and product development plans; the inherent uncertainties associated with developing new products or technologies and operating as a development stage company; our ability to develop, complete clinical trials for, obtain approvals for and commercialize any of our product candidates, including our ability to recruit and enroll patients in our studies; our ability to address the requests of the FDA; competition in the industry in which we operate and market conditions. These forward-looking statements are made as of the date of this press release, and we assume no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law. Investors should consult all of the information set forth herein and should also refer to the risk factor disclosure set forth in the reports and other documents we file with the SEC available at www.sec.gov, including without limitation the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2017 and the Company's Quarterly Reports on Form 10-Q. 2
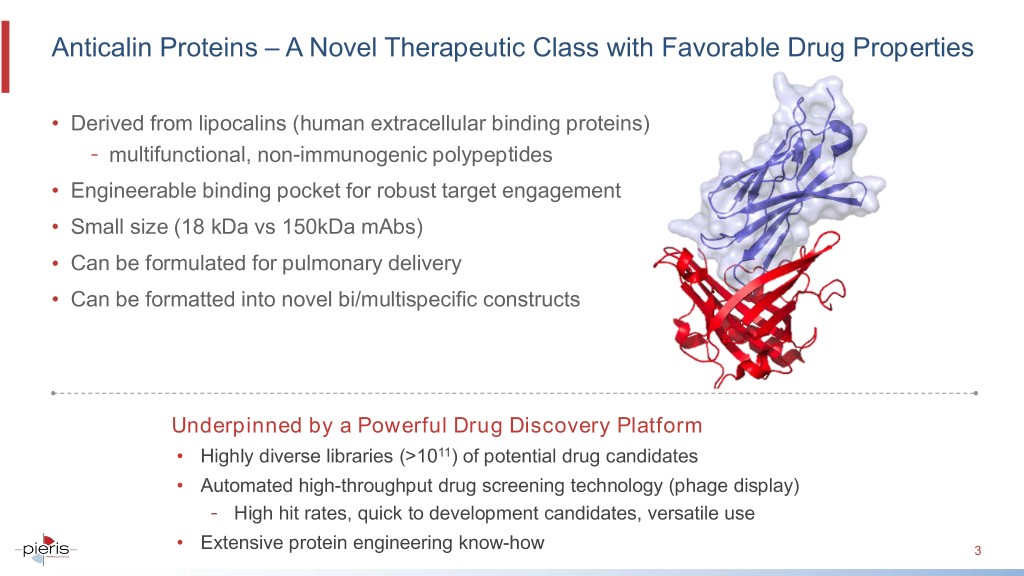
Anticalin Proteins – A Novel Therapeutic Class with Favorable Drug Properties • Derived from lipocalins (human extracellular binding proteins) - multifunctional, non-immunogenic polypeptides • Engineerable binding pocket for robust target engagement • Small size (18 kDa vs 150kDa mAbs) • Can be formulated for pulmonary delivery • Can be formatted into novel bi/multispecific constructs Underpinned by a Powerful Drug Discovery Platform • Highly diverse libraries (>1011) of potential drug candidates • Automated high-throughput drug screening technology (phage display) - High hit rates, quick to development candidates, versatile use • Extensive protein engineering know-how 3
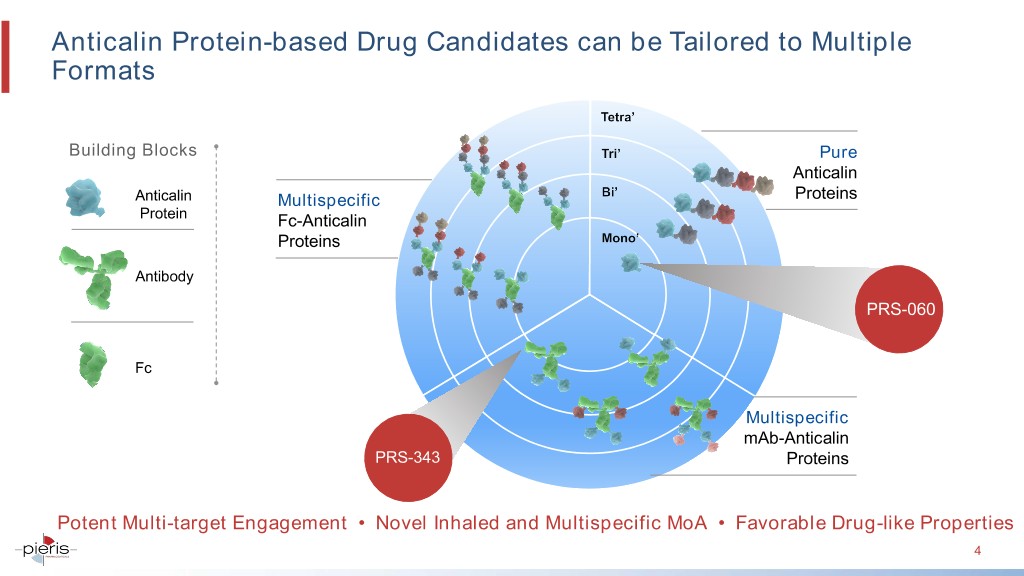
Anticalin Protein-based Drug Candidates can be Tailored to Multiple Formats Building Blocks Pure Anticalin Anticalin Multispecific Proteins Protein Fc-Anticalin Proteins Antibody PRS-060 Fc Multispecific mAb-Anticalin PRS-343 Proteins Potent Multi-target Engagement • Novel Inhaled and Multispecific MoA • Favorable Drug-like Properties 4
Pieris Investment Opportunity • Validation through three anchor partnerships - $120+M in upfront payments and milestones since January 2017 - Each partnership includes co-development & US-focused commercialization rights Immuno-oncology • Near-term, clinical-based inflection points - IO: wholly owned bispecific 4-1BB agonist (PRS-343) - Respiratory: co-developed (AstraZeneca) inhaled IL4Ra antagonist (PRS-060) - Anemia: non-core asset targeting hepcidin (partnered in JP) with additional drug class validation and licensing revenue potential • Significant capital to bridge through near-term clinical datasets • Partnerships and pipeline supported by IND engine yielding several Respiratory drug candidates with excellent drug-like properties ANCHOR PARTNERSHIPS 5
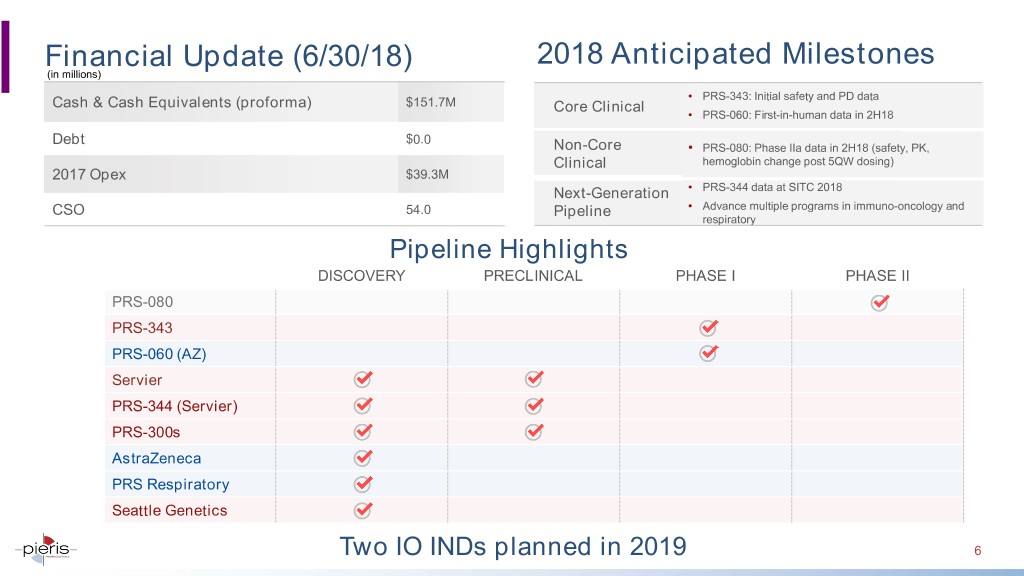
2018 Anticipated Milestones Financial(in millions) Update (6/30/18) • PRS-343: Initial safety and PD data Cash & Cash Equivalents (proforma) $151.7M Core Clinical • PRS-060: First-in-human data in 2H18 Debt $0.0 Non-Core • PRS-080: Phase IIa data in 2H18 (safety, PK, Clinical hemoglobin change post 5QW dosing) 2017 Opex $39.3M Next-Generation • PRS-344 data at SITC 2018 CSO 54.0 • Advance multiple programs in immuno-oncology and Pipeline respiratory Pipeline Highlights DISCOVERY PRECLINICAL PHASE I PHASE II PRS-080 PRS-343 PRS-060 (AZ) Servier PRS-344 (Servier) PRS-300s AstraZeneca PRS Respiratory Seattle Genetics Two IO INDs planned in 2019 6

Immuno-oncology Franchise Prioritizing PRS-343, “fast-followers” and diversified costim agonism beyond 4-1BB Proprietary Clinical (worldwide rights) • PRS-343: First-in-class bispecific to preferentially activate T cells in the tumor microenvironment (TME) • Committed to advancing several additional tumor-localized costimulatory bispecific fusion proteins Servier Collaboration • PRS-344: PD-L1/4-1BB antibody/anticalin bispecific • 5-program deal (all bispecific fusion proteins) • Pieris retains full U.S. rights for 3 out of 5 programs • $31M upfront payment, $1.8B milestone potential • Up to low double-digit royalties on non-codev products Seattle Genetics Collaboration • 3-program partnership based on tumor-localized costimulatory bispecific fusion proteins • Pieris retains opt-in rights for 50/50 global profit split and U.S. commercialization rights on one of the programs • $30 upfront payment, $1.2B milestone potential • Up to double-digit royalties on non-codev products 7
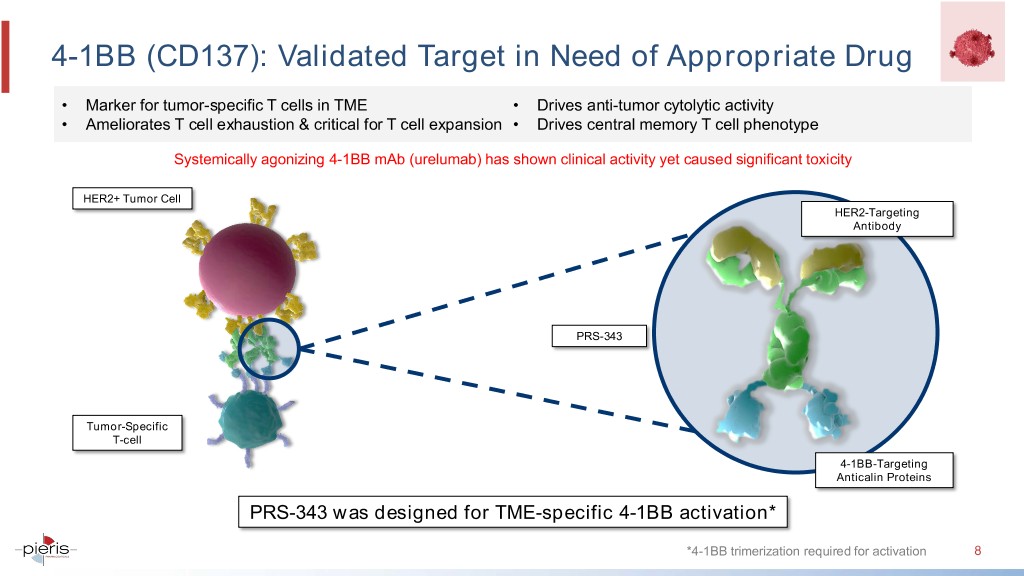
4-1BB (CD137): Validated Target in Need of Appropriate Drug • Marker for tumor-specific T cells in TME • Drives anti-tumor cytolytic activity • Ameliorates T cell exhaustion & critical for T cell expansion • Drives central memory T cell phenotype Systemically agonizing 4-1BB mAb (urelumab) has shown clinical activity yet caused significant toxicity HER2+ Tumor Cell HER2-Targeting Antibody PRS-343 Tumor-Specific T-cell 4-1BB-Targeting Anticalin Proteins PRS-343 was designed for TME-specific 4-1BB activation* *4-1BB trimerization required for activation 8
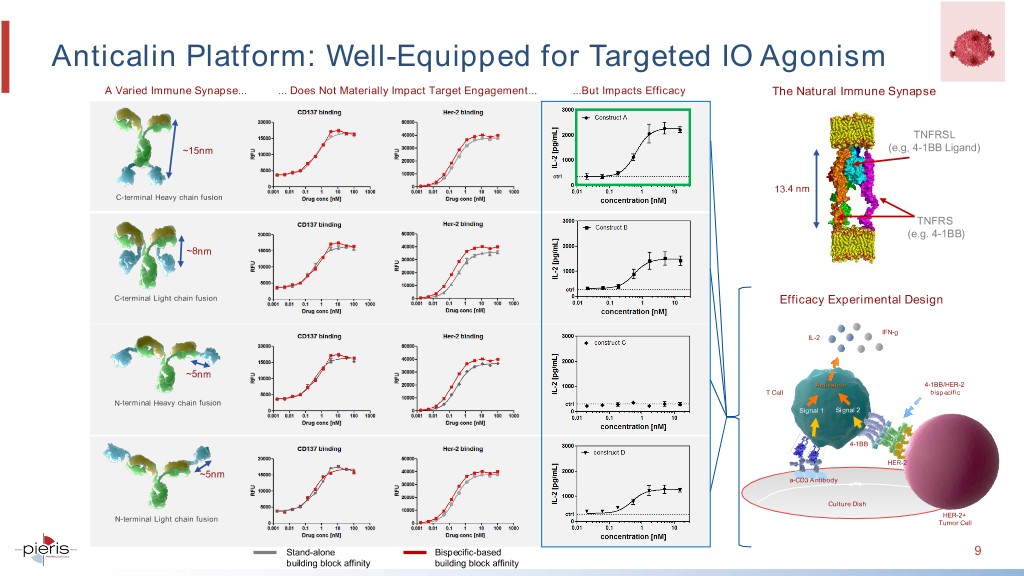
Anticalin Platform: Well-Equipped for Targeted IO Agonism A Varied Immune Synapse... ... Does Not Materially Impact Target Engagement... ...But Impacts Efficacy The Natural Immune Synapse TNFRSL ~15nm (e.g. 4-1BB Ligand) 13.4 nm C-terminal Heavy chain fusion TNFRS (e.g. 4-1BB) ~8nm C-terminal Light chain fusion Efficacy Experimental Design IFN-g IL-2 ~5nm Activation 4-1BB/HER-2 T Cell bispecific N-terminal Heavy chain fusion Signal 1 Signal 2 4-1BB HER-2 ~5nm a-CD3 a-CD3 Antibody antibody Culture Dish HER-2+ N-terminal Light chain fusion Tumor Cell Stand-alone Bispecific-based 9 building block affinity building block affinity
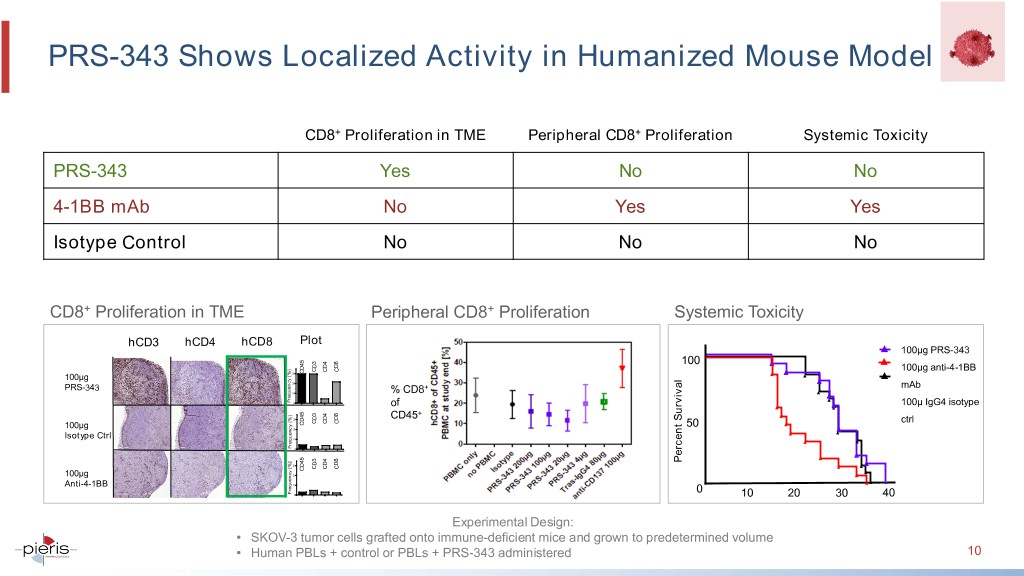
PRS-343 Shows Localized Activity in Humanized Mouse Model CD8+ Proliferation in TME Peripheral CD8+ Proliferation Systemic Toxicity PRS-343 Yes No No 4-1BB mAb No Yes Yes Isotype Control No No No CD8+ Proliferation in TME Peripheral CD8+ Proliferation Systemic Toxicity hCD3 hCD4 hCD8 Plot 100μg PRS-343 100 CD3 CD4 CD8 100μg anti-4-1BB 30 CD45 100µg 20 PRS-343 + mAb 10 % CD8 Frequency [%] 0 of 100μ IgG4 isotype + 30 CD45 CD3 CD4 CD8 ctrl CD45 50 100µg 20 Isotype Ctrl 10 Frequency [%] 0 Percent Survival 30 CD3 CD4 CD8 CD45 100µg 20 Anti-4-1BB 10 Frequency [%] 0 0 10 20 30 40 Experimental Design: • SKOV-3 tumor cells grafted onto immune-deficient mice and grown to predetermined volume • Human PBLs + control or PBLs + PRS-343 administered 10
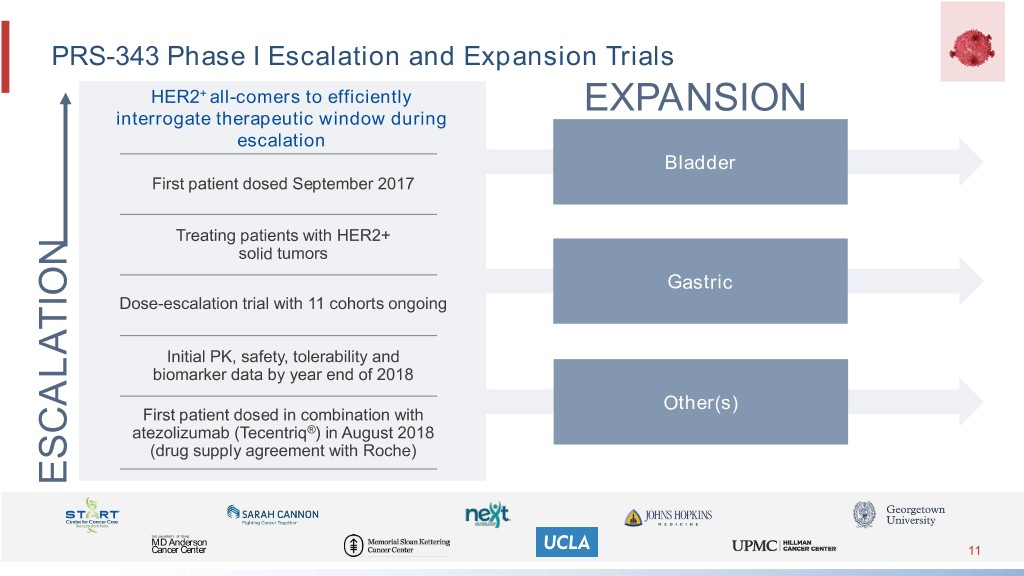
PRS-343 Phase I Escalation and Expansion Trials HER2+ all-comers to efficiently interrogate therapeutic window during EXPANSION escalation Bladder First patient dosed September 2017 Treating patients with HER2+ solid tumors Gastric Dose-escalation trial with 11 cohorts ongoing Initial PK, safety, tolerability and biomarker data by year end of 2018 Other(s) First patient dosed in combination with atezolizumab (Tecentriq®) in August 2018 (drug supply agreement with Roche) ESCALATION THE UNIVERSITY OF TEXAS MD Anderson Cancer Center 11
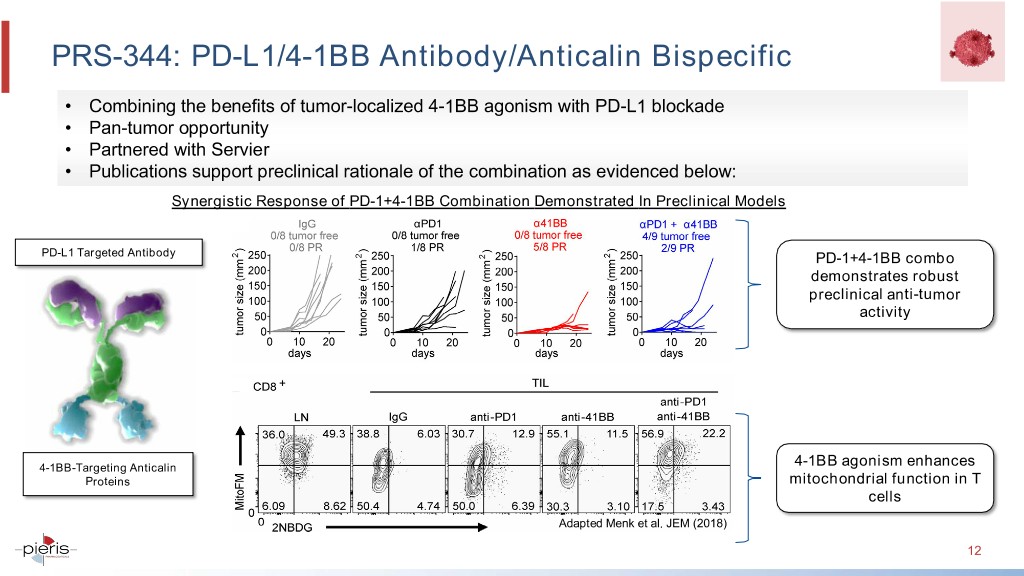
PRS-344: PD-L1/4-1BB Antibody/Anticalin Bispecific • Combining the benefits of tumor-localized 4-1BB agonism with PD-L1 blockade • Pan-tumor opportunity • Partnered with Servier • Publications support preclinical rationale of the combination as evidenced below: Synergistic Response of PD-1+4-1BB Combination Demonstrated In Preclinical Models PD-L1 Targeted Antibody PD-1+4-1BB combo demonstrates robust preclinical anti-tumor activity 4-1BB-Targeting Anticalin 4-1BB agonism enhances Proteins mitochondrial function in T cells Adapted Menk et al. JEM (2018) 12
Respiratory Franchise Addressing validated targets through inhalation AstraZeneca Collaboration • PRS-060: IL-4 receptor alpha antagonist in clinical development for the treatment of moderate-to-severe uncontrolled asthma • 4 additional committed novel inhaled Anticalin protein programs • Retained co-development and co-commercialization (US) options on PRS-060 and up to 2 additional programs • Attractive economics • $57.5M upfront & Phase I MS in 2017 • ~$2.1B in milestone potential, plus double-digit royalties • AZ funds all PRS-060 development costs through post-Ph 2a co- development opt-in decision • Access to complementary formulation and device know-how for inhaled delivery Proprietary Clinical (worldwide rights) • Initiated two proprietary respiratory programs for undisclosed targets in 2H18 13
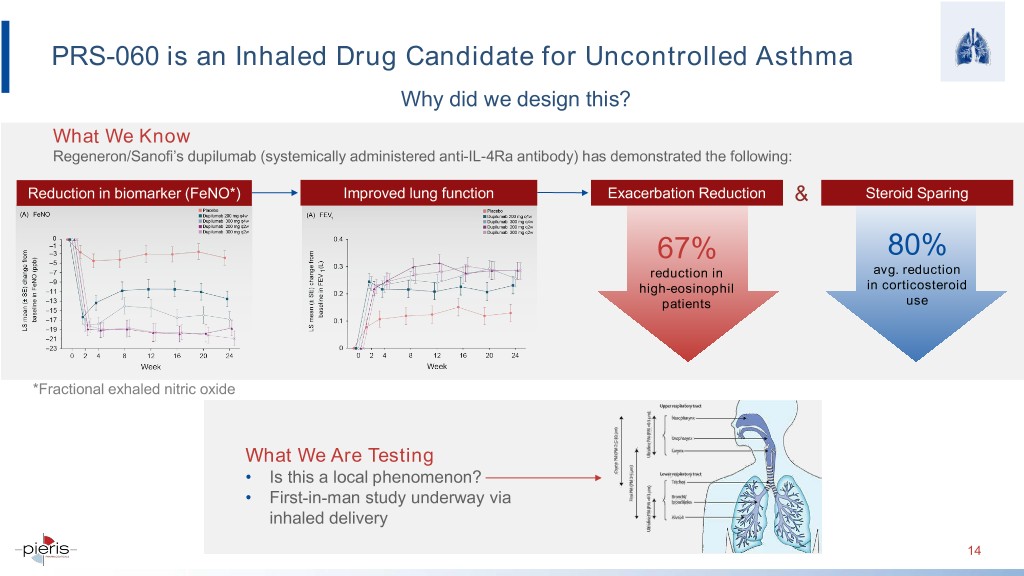
PRS-060 is an Inhaled Drug Candidate for Uncontrolled Asthma Why did we design this? What We Know Regeneron/Sanofi’s dupilumab (systemically administered anti-IL-4Ra antibody) has demonstrated the following: Reduction in biomarker (FeNO*) Improved lung function Exacerbation Reduction & Steroid Sparing 67% 80% reduction in avg. reduction high-eosinophil in corticosteroid patients use *Fractional exhaled nitric oxide What We Are Testing • Is this a local phenomenon? • First-in-man study underway via inhaled delivery 14
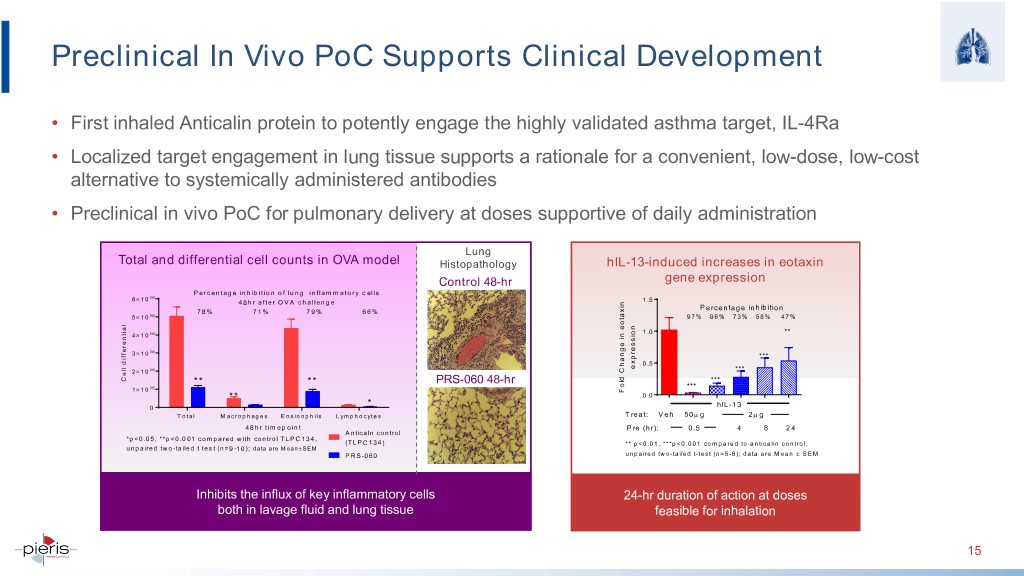
Preclinical In Vivo PoC Supports Clinical Development • First inhaled Anticalin protein to potently engage the highly validated asthma target, IL-4Ra • Localized target engagement in lung tissue supports a rationale for a convenient, low-dose, low-cost alternative to systemically administered antibodies • Preclinical in vivo PoC for pulmonary delivery at doses supportive of daily administration Lung Total and differential cell counts in OVA model Histopathology hIL-13-induced increases in eotaxin Control 48-hr gene expression P ercentage inhibition of lung inflam m atory cells 6 × 10 06 48hr after OVA challenge 1 .5 Percentage inhibition 78% 71% 79% 66% 5 × 10 06 97% 86% 73% 58% 47% 1 .0 ** 4 × 10 06 × 06 3 10 *** 0 .5 expression 2 × 10 06 *** Cell differential ** ** PRS-060 48-hr *** 06 *** 1 × 10 eotaxin Change in Fold ** 0 .0 * 0 hIL-13 T otal M acrophages Eosinophils Lymphocytes Treat: Veh 50µ g 2µ g 48hr timepoint Pre (hr): 0.5 4 8 24 Anticaln control *p<0.05, **p<0.001 compared with control TLPC134, (TLPC134) ** p<0.01, ***p<0.001 compared to anticalin control, unpaired two-tailed t test (n=9-10); data are Mean± SEM PRS-060 unpaired two-tailed t-test (n=5-6); data are Mean ± SEM Inhibits the influx of key inflammatory cells 24-hr duration of action at doses both in lavage fluid and lung tissue feasible for inhalation 15
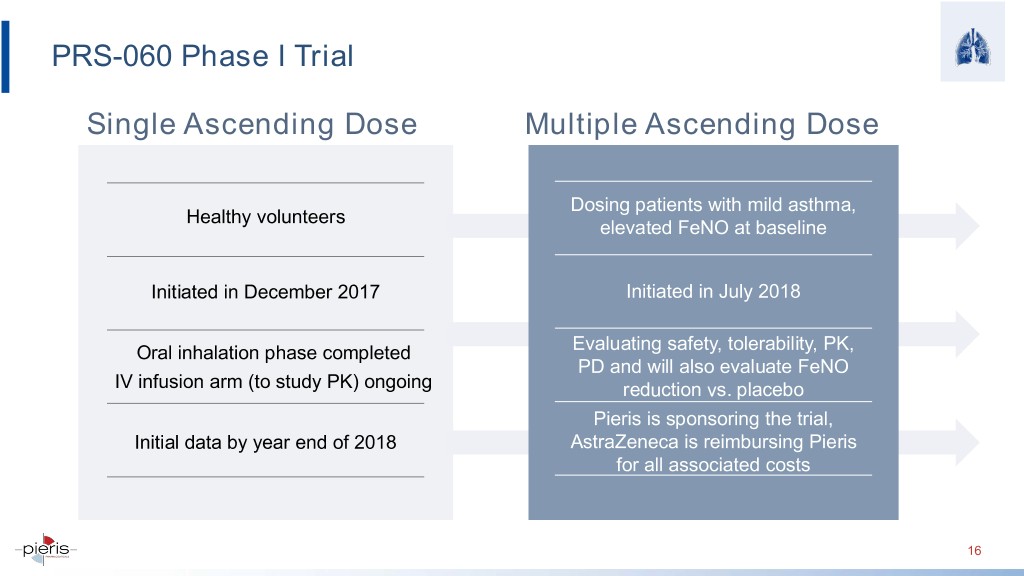
PRS-060 Phase I Trial Single Ascending Dose Multiple Ascending Dose Dosing patients with mild asthma, Healthy volunteers elevated FeNO at baseline Initiated in December 2017 Initiated in July 2018 Oral inhalation phase completed Evaluating safety, tolerability, PK, PD and will also evaluate FeNO IV infusion arm (to study PK) ongoing reduction vs. placebo Pieris is sponsoring the trial, Initial data by year end of 2018 AstraZeneca is reimbursing Pieris for all associated costs 16
Pieris Investment Opportunity • Validation through three anchor partnerships - $120+M in upfront payments and milestones since January 2017 - Each partnership includes co-development & US-focused commercialization rights Immuno-oncology • Near-term, clinical-based inflection points - IO: wholly owned bispecific 4-1BB agonist (PRS-343) - Respiratory: co-developed (AstraZeneca) inhaled IL4Ra antagonist (PRS-060) - Anemia: non-core asset targeting hepcidin (partnered in JP) with additional drug class validation and licensing revenue potential • Significant capital to bridge through near-term clinical datasets • Partnerships and pipeline supported by IND engine yielding several Respiratory drug candidates with excellent drug-like properties ANCHOR PARTNERSHIPS 17

Pieris Pharmaceuticals, Inc. Corporate HQ: 255 State Street, 9th Floor, Boston, MA 02109, USA R&D Hub: Freising, Germany (Munich) info@pieris.com www.pieris.com

APPENDIX

Scientific and Clinical Advisory Boards SCIENTIFIC ADVISORY BOARD: SCIENTIFIC ADVISORY BOARD: CLINICAL ADVISORY BOARD: ONCOLOGY RESPIRATORY ONCOLOGY • E. John Werry, PhD • Gary Anderson, PhD • Sandra Swain, MD University of Pennsylvania University of Melbourne Georgetown University Cancer Center • Vijay Kuchroo DVM, PhD • Peter Barnes, FRS • Noah Hahnm, MD Harvard Medical School Imperial College Johns Hopkins University School of • Michael Curran, PhD • Bruce Levy, MD Medicine MD Anderson Cancer Center Harvard University, Brigham and • David Ilson, MD, PhD • Dario Vignali, PhD Women’s Hospital Memorial Sloan-Kettering Cancer University of Pittsburgh • David Schwartz, MD Center, Weill Cornell Medical College • Padmanee Sharma, PhD University of Colorado, Denver • Funda Eric-Bernstam, MD, PhD MD Anderson Cancer Center • Fan Chung, MD, DSc Institute for Personalized Cancer Imperial College Therapy, MD Anderson Cancer Center • Ian Adcock, PhD Imperial College • Mario Sznol, MD Yale University • Oliver Eickelberg,, MD University of Denver • Sally Wenzel, MD University of Pittsburgh Medical Center 20
