Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Dermira, Inc. | d615533d8k.htm |

QBREXZA™ (glycopyrronium) Cloth Launch Day Our Path to Launch October 1, 2018 Exhibit 99.1

Forward-Looking Statements This presentation contains "forward-looking" statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our business strategy, objectives and opportunities; our projected QBREXZA market opportunity and estimated peak sales potential; our projections relating to quality access and contracted coverage for U.S. commercial lives; future business and product development, clinical, regulatory and commercialization plans; product goals, attributes and performance; the successful completion of, and timing expectations for the receipt and announcement of topline efficacy and safety data from, our Phase 2b lebrikizumab clinical trial; and our 2018 financial guidance, estimated gross-to-net sales range and estimated cash runway. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors that may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements, including, but not limited to, those related to our dependence on third-party clinical research organizations, manufacturers, suppliers and distributors; our ability to obtain necessary additional capital; market acceptance of our product; the impact of competitive products and therapies; our ability to attract and retain key employees; the costs of our commercialization plans and development programs; the design, implementation and outcomes of our clinical trials; our ability to manage the growth and complexity of our organization; our ability to maintain, protect and enhance our intellectual property; and our ability to continue to stay in compliance with applicable laws and regulations. You should refer to the section entitled “Risk Factors” set forth in our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and other filings we make with the Securities and Exchange Commission (SEC) from time to time for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update any forward-looking statements after the date of this presentation except as may be required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. We use our website (www.dermira.com) and LinkedIn page (www.linkedin.com/company/dermira-inc-), Instagram account and corporate Twitter account (@DermiraInc) as channels of distribution of information about our company, product candidates, planned announcements, attendance at upcoming conferences and other matters. Such information may be deemed material information and we may use these channels to comply with our disclosure obligations under Regulation FD. Therefore, investors should monitor our website, Twitter account, Instagram page and our LinkedIn page in addition to following our SEC filings, press releases, public conference calls and webcasts.

QBREXZA™ Cloth – Launched Today! Approved for the treatment of primary axillary hyperhidrosis, or excessive underarm sweating www.qbrexza.com
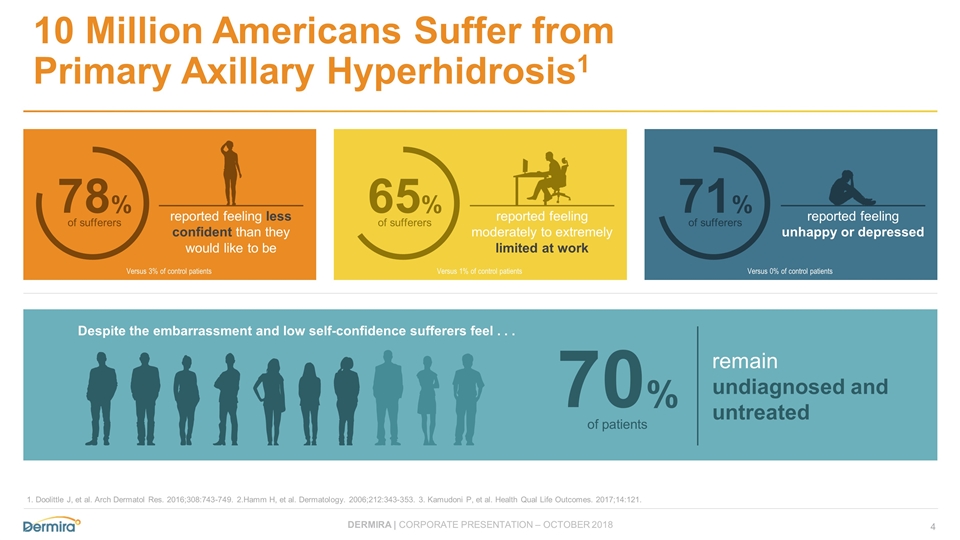
10 Million Americans Suffer from Primary Axillary Hyperhidrosis1 Versus 3% of control patients Versus 1% of control patients Versus 0% of control patients 65% of sufferers 71% of sufferers reported feeling less confident than they would like to be 78% of sufferers reported feeling moderately to extremely limited at work reported feeling unhappy or depressed remain undiagnosed and untreated 70% of patients Despite the embarrassment and low self-confidence sufferers feel . . . 1. Doolittle J, et al. Arch Dermatol Res. 2016;308:743-749. 2.Hamm H, et al. Dermatology. 2006;212:343-353. 3. Kamudoni P, et al. Health Qual Life Outcomes. 2017;14:121.
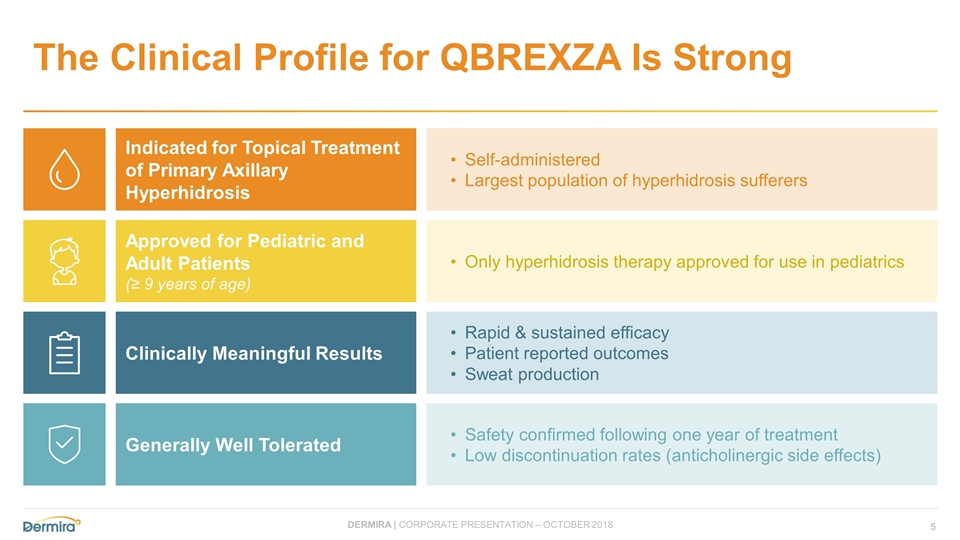
The Clinical Profile for QBREXZA Is Strong Indicated for Topical Treatment of Primary Axillary Hyperhidrosis Approved for Pediatric and Adult Patients (≥ 9 years of age) Clinically Meaningful Results Generally Well Tolerated Self-administered Largest population of hyperhidrosis sufferers Only hyperhidrosis therapy approved for use in pediatrics Rapid & sustained efficacy Patient reported outcomes Sweat production Safety confirmed following one year of treatment Low discontinuation rates (anticholinergic side effects)
Exceeding Expectations on Leading Indicators Educating Physicians Gaining Broad Quality Coverage Activating Patients
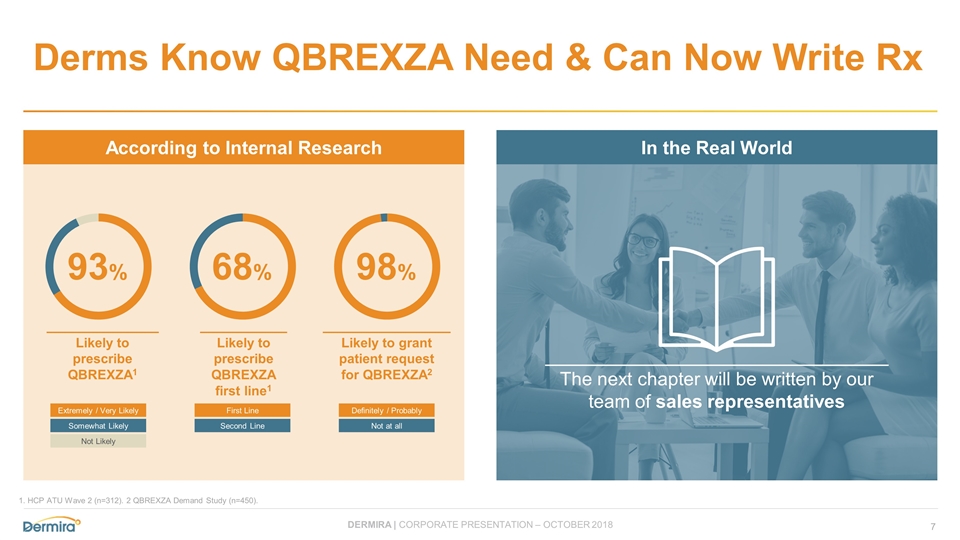
According to Internal Research In the Real World Derms Know QBREXZA Need & Can Now Write Rx 1. HCP ATU Wave 2 (n=312). 2 QBREXZA Demand Study (n=450). The next chapter will be written by our team of sales representatives Likely to prescribe QBREXZA1 Likely to prescribe QBREXZA first line1 Likely to grant patient request for QBREXZA2 93% 68% 98% Definitely / Probably Not at all First Line Second Line Extremely / Very Likely Somewhat Likely Not Likely
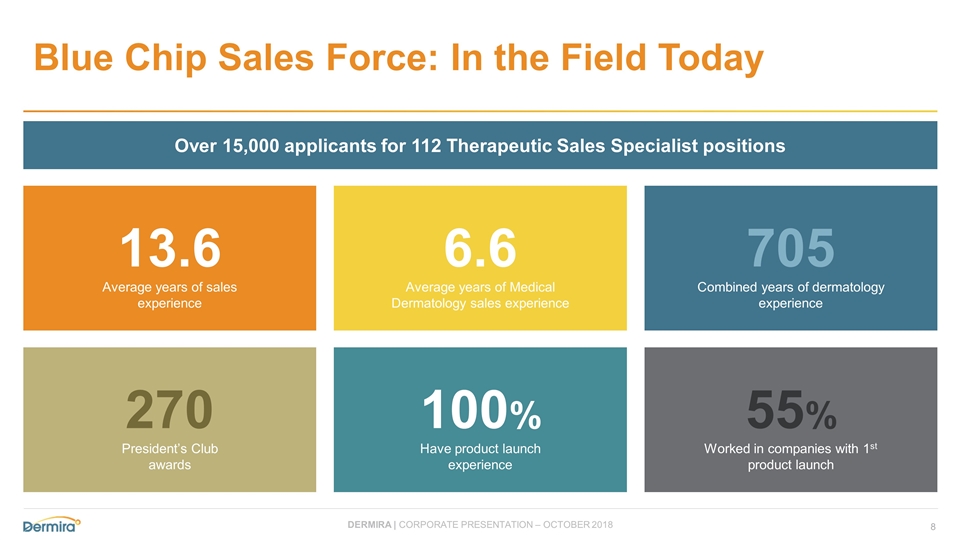
Blue Chip Sales Force: In the Field Today 13.6 Average years of sales experience 6.6 Average years of Medical Dermatology sales experience 705 Combined years of dermatology experience 270 President’s Club awards 100% Have product launch experience 55% Worked in companies with 1st product launch Over 15,000 applicants for 112 Therapeutic Sales Specialist positions
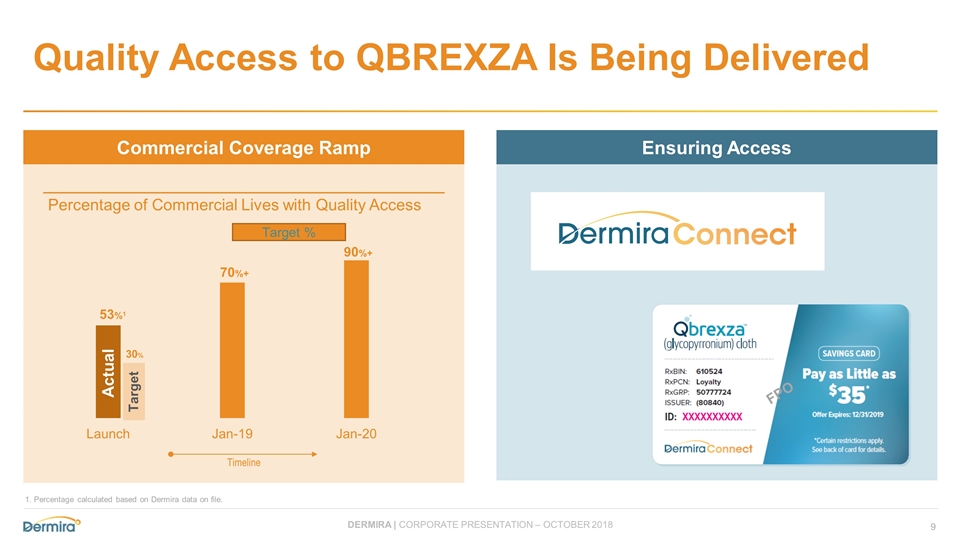
Quality Access to QBREXZA Is Being Delivered FPO Commercial Coverage Ramp Ensuring Access Percentage of Commercial Lives with Quality Access 53%1 70%+ 90%+ 30% Timeline Target % Actual Target 1. Percentage calculated based on Dermira data on file.
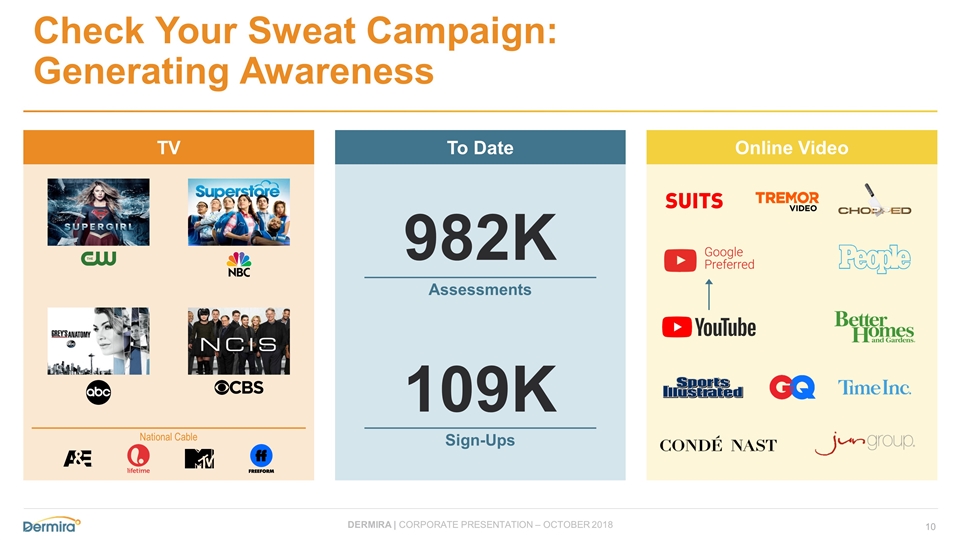
Check Your Sweat Campaign: Generating Awareness TV To Date Online Video Assessments Sign-Ups 982K 109K National Cable
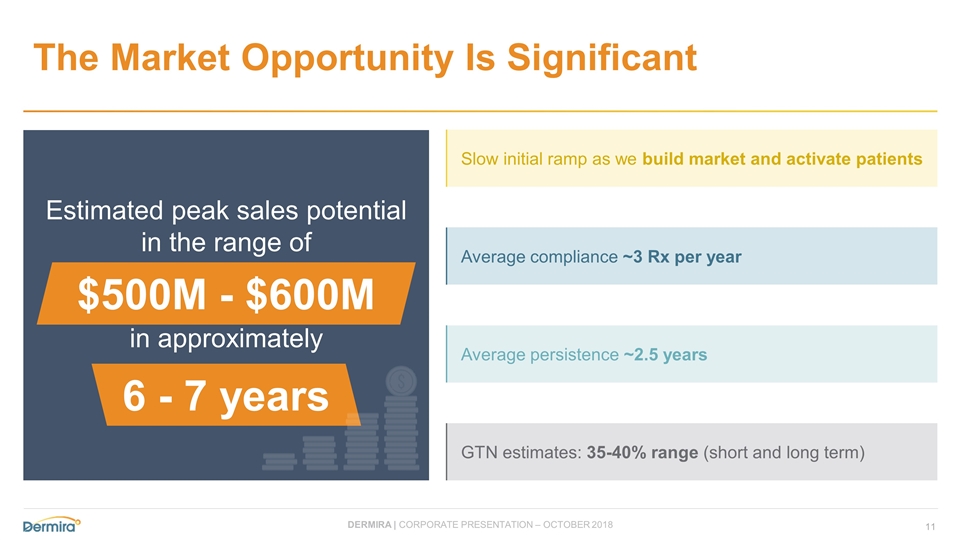
The Market Opportunity Is Significant Estimated peak sales potential in the range of $500M - $600M in approximately 6-7 years Slow initial ramp as we build market and activate patients Average compliance ~3 Rx per year Average persistence ~2.5 years GTN estimates: 35-40% range (short and long term) $500M - $600M 6 - 7 years

What’s Next…
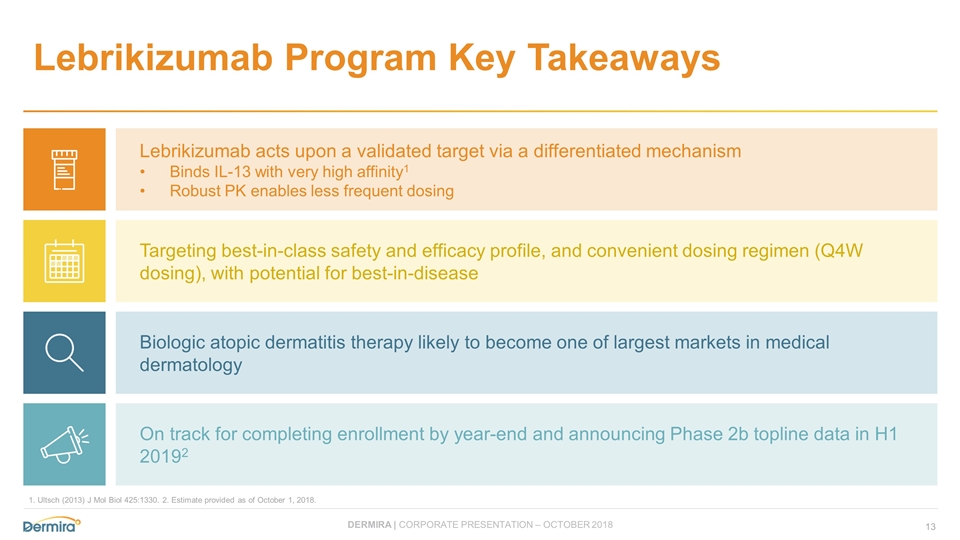
Lebrikizumab Program Key Takeaways Lebrikizumab acts upon a validated target via a differentiated mechanism Binds IL-13 with very high affinity1 Robust PK enables less frequent dosing Targeting best-in-class safety and efficacy profile, and convenient dosing regimen (Q4W dosing), with potential for best-in-disease Biologic atopic dermatitis therapy likely to become one of largest markets in medical dermatology On track for completing enrollment by year-end and announcing Phase 2b topline data in H1 20192 1. Ultsch (2013) J Mol Biol 425:1330. 2. Estimate provided as of October 1, 2018.
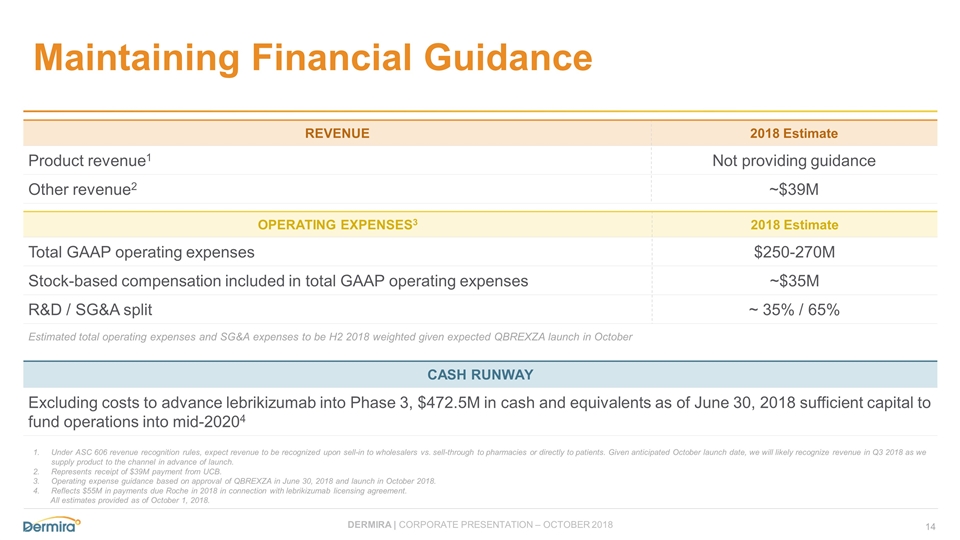
Maintaining Financial Guidance Under ASC 606 revenue recognition rules, expect revenue to be recognized upon sell-in to wholesalers vs. sell-through to pharmacies or directly to patients. Given anticipated October launch date, we will likely recognize revenue in Q3 2018 as we supply product to the channel in advance of launch. Represents receipt of $39M payment from UCB. Operating expense guidance based on approval of QBREXZA in June 30, 2018 and launch in October 2018. Reflects $55M in payments due Roche in 2018 in connection with lebrikizumab licensing agreement. All estimates provided as of October 1, 2018. REVENUE 2018 Estimate Product revenue1 Not providing guidance Other revenue2 ~$39M OPERATING EXPENSES3 2018 Estimate Total GAAP operating expenses $250-270M Stock-based compensation included in total GAAP operating expenses ~$35M R&D / SG&A split ~ 35% / 65% Estimated total operating expenses and SG&A expenses to be H2 2018 weighted given expected QBREXZA launch in October CASH RUNWAY Excluding costs to advance lebrikizumab into Phase 3, $472.5M in cash and equivalents as of June 30, 2018 sufficient capital to fund operations into mid-20204

QBREXZA™ Cloth – Launched Today! Approved for the treatment of primary axillary hyperhidrosis, or excessive underarm sweating www.qbrexza.com

Thank You Company Contact: Ian Clements, PhD ian.clements@dermira.com ©2018 Dermira, Inc. All rights reserved. “Dermira” is a registered trademark in the United States and other countries. A trademark application for “Dermira” and logo is pending in the United States. All other service marks, trademarks and tradenames appearing in this presentation are the property of their respective owners. Solely for convenience, the trademarks and tradenames referred to in this presentation appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable licensor to these trademarks and tradenames. The information herein is for informational purposes only and represents the current view of Dermira, Inc. as of the date of this presentation (or as of an earlier date if specifically noted).

Important Safety Information about QBREXZA Contraindications: QBREXZA is contraindicated in patients with medical conditions that can be exacerbated by the anticholinergic effect of QBREXZA (e.g., glaucoma, paralytic ileus, unstable cardiovascular status in acute hemorrhage, severe ulcerative colitis, toxic megacolon complicating ulcerative colitis, myasthenia gravis, Sjogren’s syndrome). WARNINGS AND PRECAUTIONS Worsening of Urinary Retention: QBREXZA should be used with caution in patients with a history or presence of documented urinary retention. Prescribers and patients should be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, distended bladder), especially in patients with prostatic hypertrophy or bladder-neck obstruction. Instruct patients to discontinue use immediately and consult a physician should any of these signs or symptoms develop. Patients with a history of urinary retention were not included in the clinical studies. Control of Body Temperature: In the presence of high ambient temperature, heat illness (hyperpyrexia and heat stroke due to decreased sweating) can occur with the use of anticholinergic drugs such as QBREXZA. Advise patients using QBREXZA to watch for generalized lack of sweating when in hot or very warm environmental temperatures and to avoid use if not sweating under these conditions. Operating Machinery or an Automobile: Transient blurred vision may occur with use of QBREXZA. If blurred vision occurs, the patient should discontinue use until symptoms resolve. Patients should be warned not to engage in activities that require clear vision such as operating a motor vehicle or other machinery, or performing hazardous work until the symptoms have resolved. ADVERSE REACTIONS The most common adverse reactions seen in ≥2% of subjects treated with QBREXZA were dry mouth (24.2%), mydriasis (6.8%), oropharyngeal pain (5.7%), headache (5.0%), urinary hesitation (3.5%), vision blurred (3.5%), nasal dryness (2.6%), dry throat (2.6%), dry eye (2.4%), dry skin (2.2%) and constipation (2.0%). Local skin reactions, including erythema (17.0%), burning/stinging (14.1%) and pruritus (8.1%) were also common. DRUG INTERACTIONS Anticholinergics: Coadministration of QBREXZA with anticholinergic medications may result in additive interaction leading to an increase in anticholinergic adverse effects. Avoid coadministration of QBREXZA with other anticholinergic-containing drugs. INSTRUCTIONS FOR ADMINISTERING QBREXZA Instruct patients to use one cloth to apply QBREXZA to both axillae by wiping the cloth across one underarm, ONE TIME. Using the same cloth, apply the medication to the other underarm, ONE TIME. Inform patients that QBREXZA can cause temporary dilation of the pupils and blurred vision if it comes in contact with the eyes. Instruct patients to wash their hands with soap and water immediately after discarding the used cloth. USE IN SPECIFIC POPULATIONS Pregnancy: There are no available data on QBREXZA use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. Lactation: There are no data on the presence of glycopyrrolate or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for QBREXZA and any potential adverse effects on the breastfed infant from QBREXZA or from the underlying maternal condition. Renal Impairment: The elimination of glycopyrronium is severely impaired in patients with renal failure. Healthcare providers: Please see QBREXZA Full Prescribing Information. Patients: Please see QBREXZA Patient Product Information
