Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ALPINE IMMUNE SCIENCES, INC. | d627257d8k.htm |

Creating New Immunotherapies to Fight Disease September, 2018 Exhibit 99.1

Forward Looking Statements This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. These forward-looking statements are not based on historical fact and include statements regarding Alpine’s platform technology, potential therapies, potential milestone and royalty payments, future development plans, clinical and regulatory objectives and the timing thereof, expectations regarding the sufficiency of cash to fund operations into 2020, expectations regarding the plans of its collaborator, expectations of future collaborations and expectations regarding the potential efficacy and commercial potential of Alpine’s and its collaborator’s product candidates. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may”, “will”, “should”, “would”, “expect”, “plan”, “intend”, and other similar expressions among others. These forward-looking statements are based on current assumptions involving risks, uncertainties, and other factors that may cause actual results, events, or developments to be materially different from those expressed or implied by such forward-looking statements. These risks and uncertainties, many of which are beyond our control, include, but are not limited to: Alpine’s discovery-stage and pre-clinical programs may not advance into the clinic or result in approved products on a timely or cost-effective basis or at all; Alpine may not achieve additional milestone payments pursuant to its collaborations; the impact of competition; adverse conditions in the general domestic and global economic markets; as well as the other risks identified in our filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof, Alpine undertakes no obligation to update forward-looking statements, and readers are cautioned not to place undue reliance on such forward-looking statements. “Variant Immunoglobulin Domain”, “vIgD”, “Transmembrane Immunomodulatory Protein”, “TIP”, “Secreted Immunomodulatory Protein”, and “SIP” are registered trademarks or trademarks of Alpine Immune Sciences, Inc. in various jurisdictions. All rights reserved.
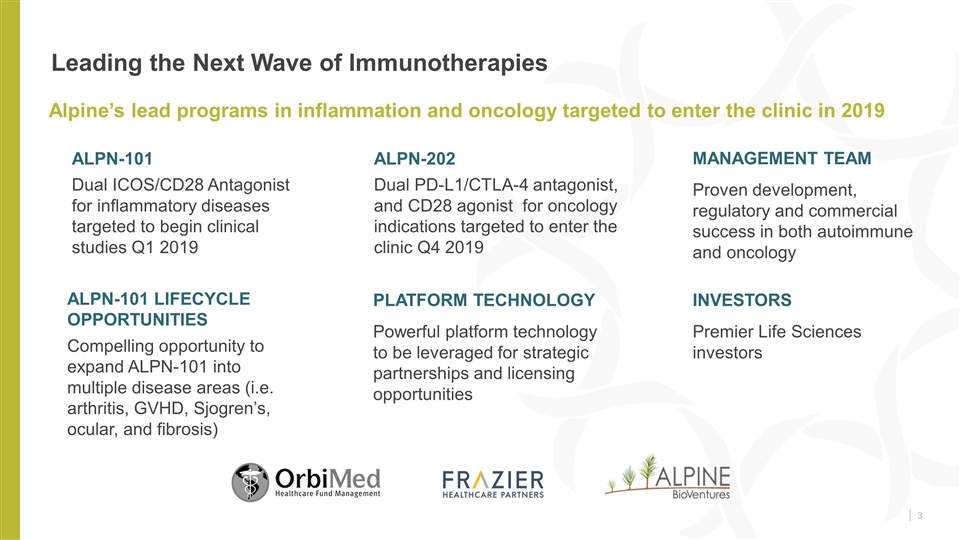
Leading the Next Wave of Immunotherapies PLATFORM TECHNOLOGY Powerful platform technology to be leveraged for strategic partnerships and licensing opportunities Alpine’s lead programs in inflammation and oncology targeted to enter the clinic in 2019 MANAGEMENT TEAM Proven development, regulatory and commercial success in both autoimmune and oncology INVESTORS Premier Life Sciences investors ALPN-101 Dual ICOS/CD28 Antagonist for inflammatory diseases targeted to begin clinical studies Q1 2019 ALPN-202 Dual PD-L1/CTLA-4 antagonist, and CD28 agonist for oncology indications targeted to enter the clinic Q4 2019 ALPN-101 Lifecycle Opportunities Compelling opportunity to expand ALPN-101 into multiple disease areas (i.e. arthritis, GVHD, Sjogren’s, ocular, and fibrosis)
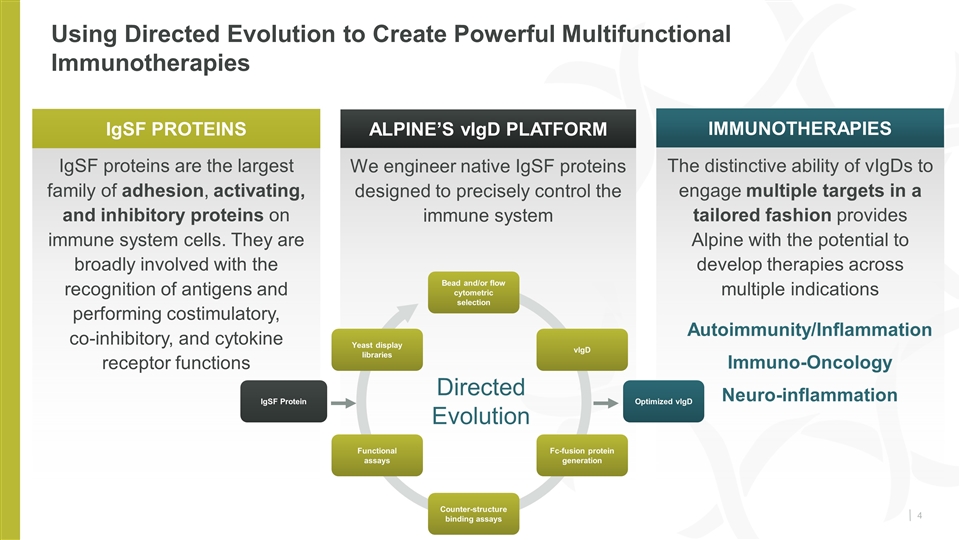
IgSF proteins are the largest family of adhesion, activating, and inhibitory proteins on immune system cells. They are broadly involved with the recognition of antigens and performing costimulatory, co‑inhibitory, and cytokine receptor functions The distinctive ability of vIgDs to engage multiple targets in a tailored fashion provides Alpine with the potential to develop therapies across multiple indications Autoimmunity/Inflammation Immuno-Oncology Neuro-inflammation We engineer native IgSF proteins designed to precisely control the immune system Using Directed Evolution to Create Powerful Multifunctional Immunotherapies IgSF PROTEINS immunotherapies Alpine’s vIgD Platform Directed Evolution IgSF Protein Optimized vlgD Bead and/or flow cytometric selection Counter-structure binding assays Functional assays Fc-fusion protein generation vlgD Yeast display libraries

TWO LEAD PROGRAMS ALPN-101 & ALPN-202 POWERFUL SCIENTIFIC PLATFORM FOCUS ON DELIVERING VALUE

ALPN-101 Autoimmune/Inflammatory Diseases
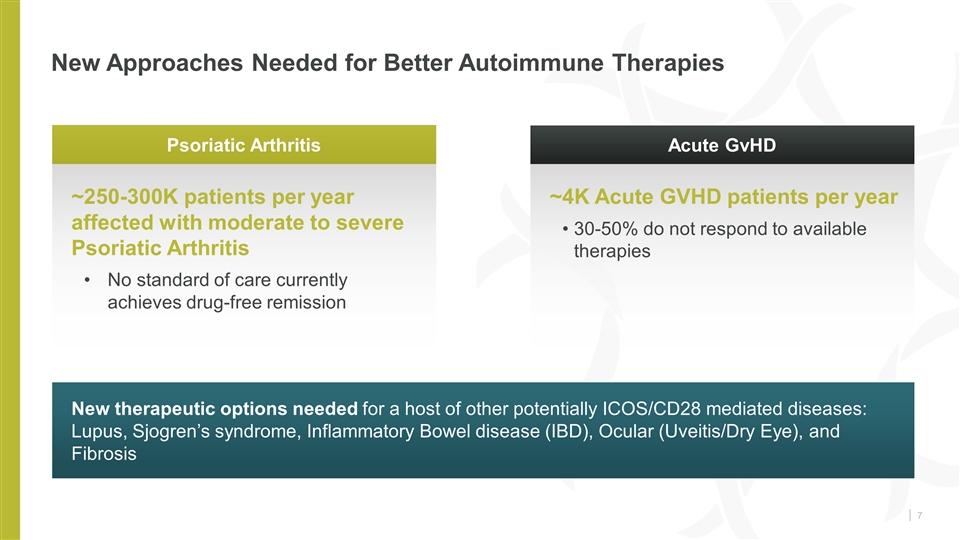
New Approaches Needed for Better Autoimmune Therapies New therapeutic options needed for a host of other potentially ICOS/CD28 mediated diseases: Lupus, Sjogren’s syndrome, Inflammatory Bowel disease (IBD), Ocular (Uveitis/Dry Eye), and Fibrosis Psoriatic Arthritis Acute GvHD ~250-300K patients per year affected with moderate to severe Psoriatic Arthritis No standard of care currently achieves drug-free remission ~4K Acute GVHD patients per year 30-50% do not respond to available therapies
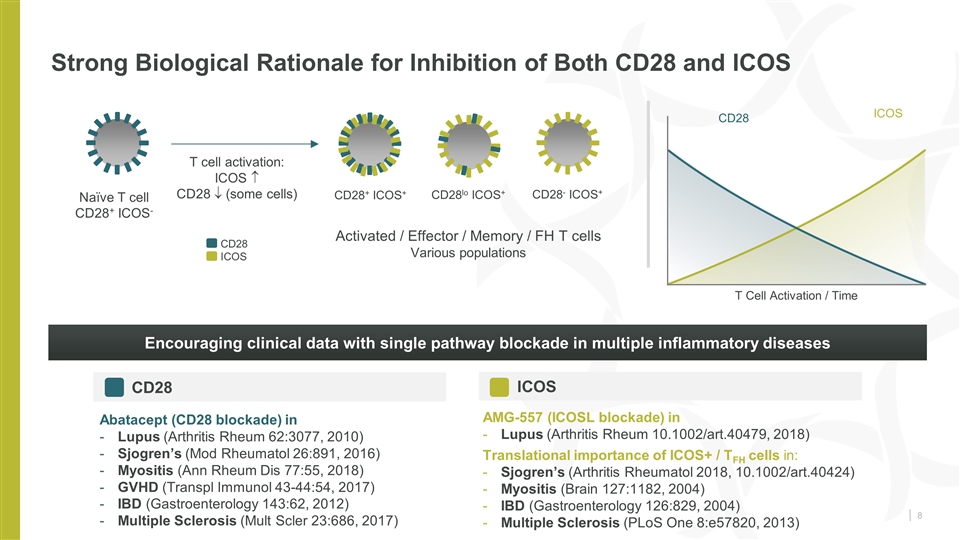
Strong Biological Rationale for Inhibition of Both CD28 and ICOS Naïve T cell CD28+ ICOS- CD28+ ICOS+ T cell activation: ICOS CD28 ¯ (some cells) CD28- ICOS+ CD28lo ICOS+ Activated / Effector / Memory / FH T cells Various populations Encouraging clinical data with single pathway blockade in multiple inflammatory diseases Abatacept (CD28 blockade) in Lupus (Arthritis Rheum 62:3077, 2010) Sjogren’s (Mod Rheumatol 26:891, 2016) Myositis (Ann Rheum Dis 77:55, 2018) GVHD (Transpl Immunol 43-44:54, 2017) IBD (Gastroenterology 143:62, 2012) Multiple Sclerosis (Mult Scler 23:686, 2017) AMG-557 (ICOSL blockade) in Lupus (Arthritis Rheum 10.1002/art.40479, 2018) Translational importance of ICOS+ / TFH cells in: Sjogren’s (Arthritis Rheumatol 2018, 10.1002/art.40424) Myositis (Brain 127:1182, 2004) IBD (Gastroenterology 126:829, 2004) Multiple Sclerosis (PLoS One 8:e57820, 2013) CD28 ICOS ICOS CD28 T Cell Activation / Time CD28 ICOS
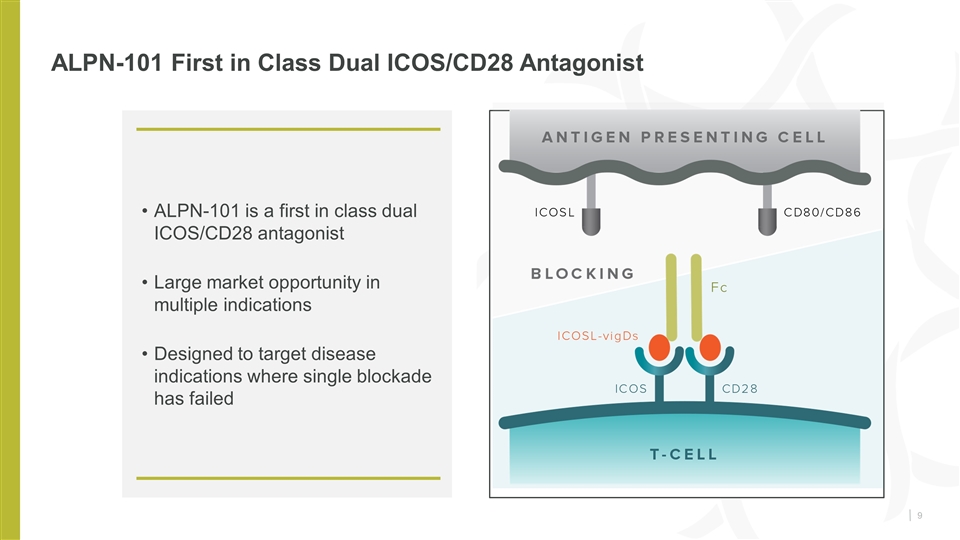
ALPN-101 First in Class Dual ICOS/CD28 Antagonist ALPN-101 is a first in class dual ICOS/CD28 antagonist Large market opportunity in multiple indications Designed to target disease indications where single blockade has failed Company data
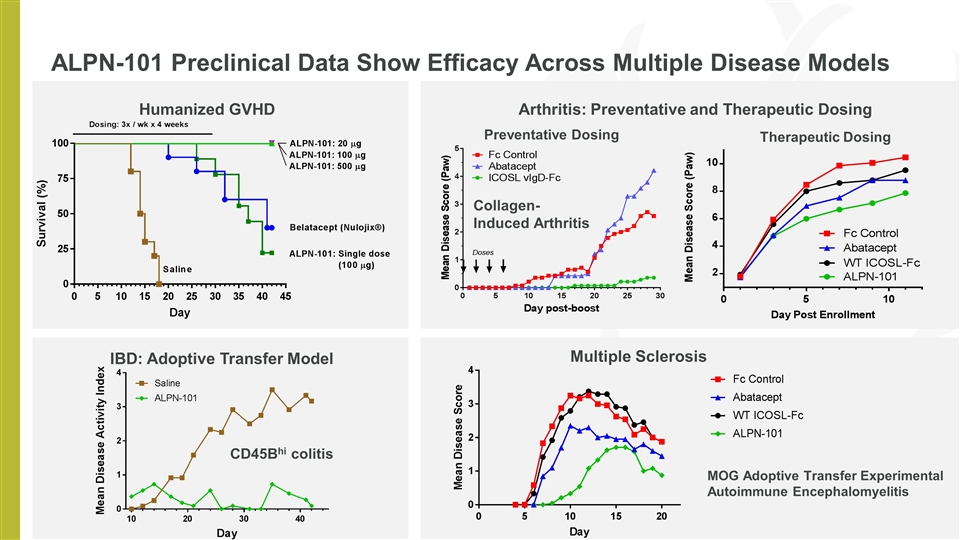
ALPN-101 Preclinical Data Show Efficacy Across Multiple Disease Models IBD: Adoptive Transfer Model Humanized GVHD Arthritis: Preventative and Therapeutic Dosing Preventative Dosing Therapeutic Dosing Multiple Sclerosis Collagen-Induced Arthritis MOG Adoptive Transfer Experimental Autoimmune Encephalomyelitis CD45Bhi colitis
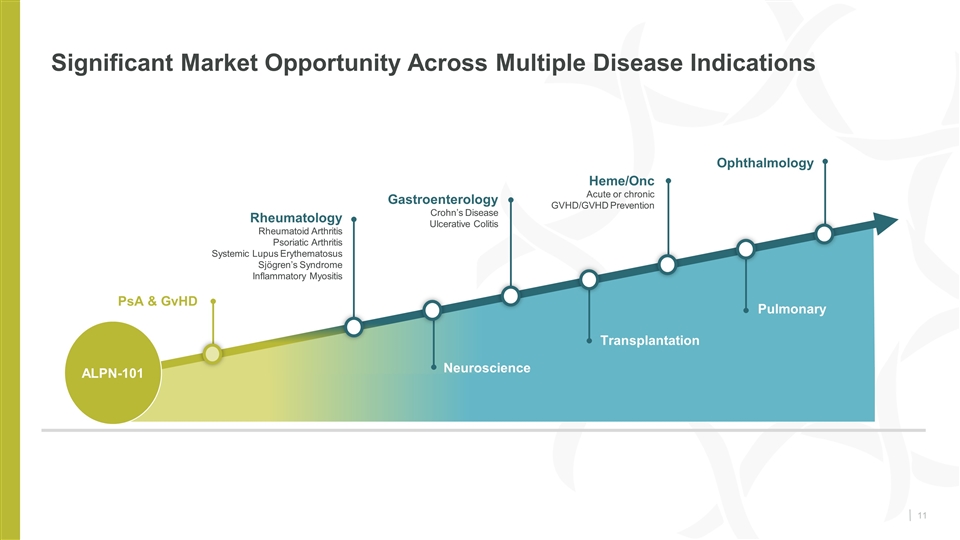
Significant Market Opportunity Across Multiple Disease Indications Rheumatology Rheumatoid Arthritis Psoriatic Arthritis Systemic Lupus Erythematosus Sjögren’s Syndrome Inflammatory Myositis Gastroenterology Crohn’s Disease Ulcerative Colitis Neuroscience Heme/Onc Acute or chronic GVHD/GVHD Prevention Transplantation Ophthalmology Pulmonary ALPN-101 PsA & GvHD
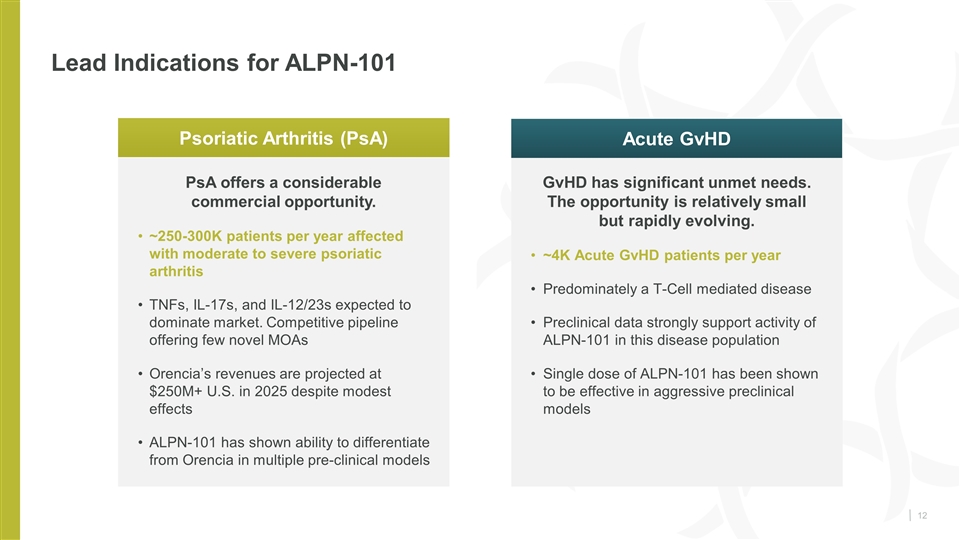
Lead Indications for ALPN-101 PsA offers a considerable commercial opportunity. ~250-300K patients per year affected with moderate to severe psoriatic arthritis TNFs, IL-17s, and IL-12/23s expected to dominate market. Competitive pipeline offering few novel MOAs Orencia’s revenues are projected at $250M+ U.S. in 2025 despite modest effects ALPN-101 has shown ability to differentiate from Orencia in multiple pre-clinical models GvHD has significant unmet needs. The opportunity is relatively small but rapidly evolving. ~4K Acute GvHD patients per year Predominately a T-Cell mediated disease Preclinical data strongly support activity of ALPN-101 in this disease population Single dose of ALPN-101 has been shown to be effective in aggressive preclinical models Psoriatic Arthritis (PsA) Acute GvHD

Executing Initial Clinical Strategy for Lead Program ALPN-101 Phase 1 Healthy Volunteer Study (AUS) Phase 2a Psoriatic Arthritis Phase 1/2 Acute GvHD Preclinical development GMP manufacturing with sufficient drug substance to complete initial Phase 1 Study Phase 2b Proof-of-concept expansion into other indications Key Biomarker and Safety Data

ALPN-202 Oncology
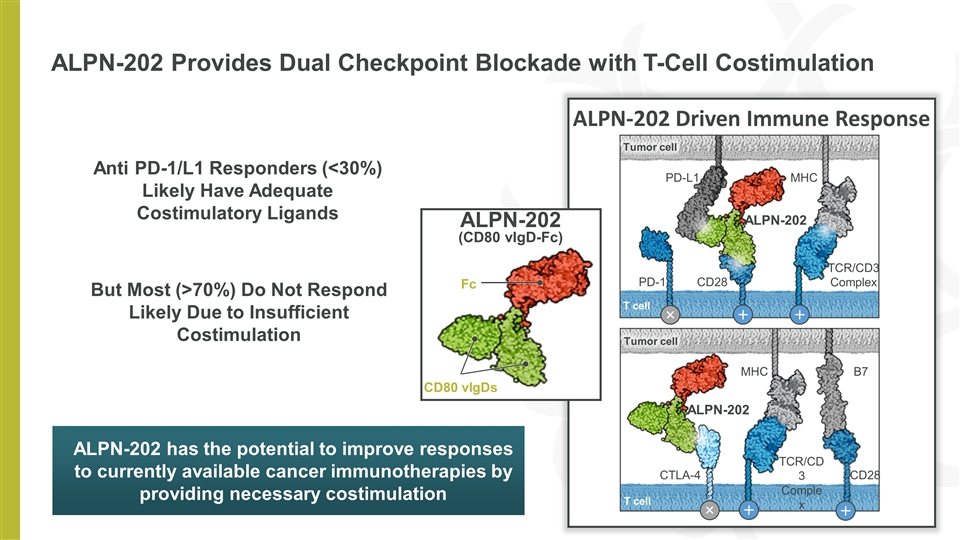
ALPN-202 Provides Dual Checkpoint Blockade with T-Cell Costimulation Anti PD-1/L1 Responders (<30%) Likely Have Adequate Costimulatory Ligands But Most (>70%) Do Not Respond Likely Due to Insufficient Costimulation ALPN-202 has the potential to improve responses to currently available cancer immunotherapies by providing necessary costimulation ALPN-202 Driven Immune Response ALPN-202 (CD80 vIgD-Fc) Fc CD80 vIgDs PD-1 CD28 PD-L1 TCR/CD3 Complex MHC Tumor cell T cell ALPN-202 TCR/CD3 Complex MHC B7 CTLA-4 Tumor cell T cell CD28 ALPN-202
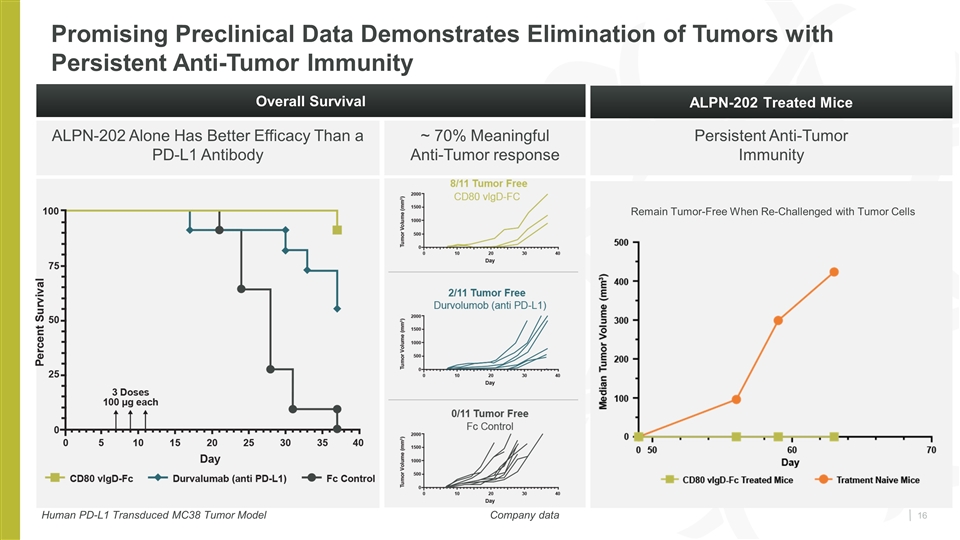
Promising Preclinical Data Demonstrates Elimination of Tumors with Persistent Anti-Tumor Immunity ALPN-202 Alone Has Better Efficacy Than a PD-L1 Antibody Company data Overall Survival ALPN-202 Treated Mice Remain Tumor-Free When Re-Challenged with Tumor Cells Human PD-L1 Transduced MC38 Tumor Model Company data ~ 70% Meaningful Anti-Tumor response Persistent Anti-Tumor Immunity
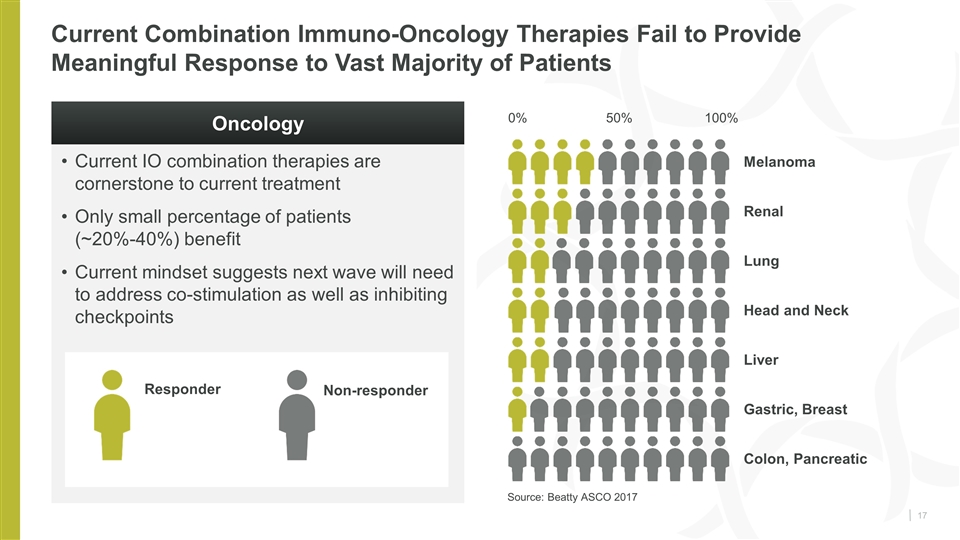
Current IO combination therapies are cornerstone to current treatment Only small percentage of patients (~20%-40%) benefit Current mindset suggests next wave will need to address co-stimulation as well as inhibiting checkpoints Current Combination Immuno-Oncology Therapies Fail to Provide Meaningful Response to Vast Majority of Patients Melanoma Responder Non-responder 0% 100% Renal Lung Head and Neck Liver Gastric, Breast Colon, Pancreatic Oncology 50% Source: Beatty ASCO 2017

ALPN-202 Clinical Strategy Objective is to quickly show treatment effect in tumor “basket” trial at therapeutic doses Limited competition compared to checkpoint inhibition space supports differentiation with early human data Favorable manufacturing and commercial profile PHASE 1 ALPN-202 Planning to Enter Clinic in Q4 2019

TWO LEAD PROGRAMS ALPN-101 & ALPN 202 POWERFUL SCIENTIFIC PLATFORM FOCUS ON DELIVERING VALUE
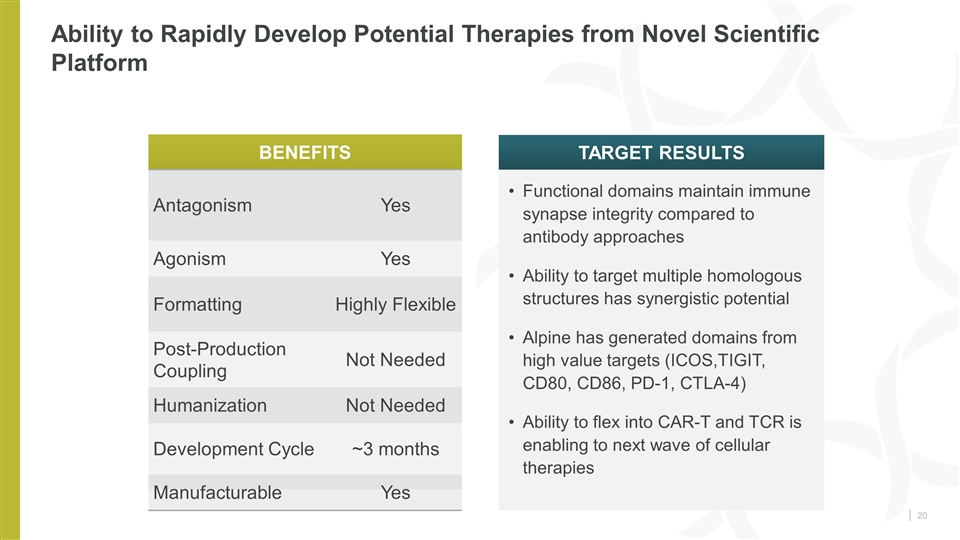
Ability to Rapidly Develop Potential Therapies from Novel Scientific Platform Functional domains maintain immune synapse integrity compared to antibody approaches Ability to target multiple homologous structures has synergistic potential Alpine has generated domains from high value targets (ICOS,TIGIT, CD80, CD86, PD-1, CTLA-4) Ability to flex into CAR-T and TCR is enabling to next wave of cellular therapies Benefits Target Results Antagonism Yes Agonism Yes Formatting Highly Flexible Post-Production Coupling Not Needed Humanization Not Needed Development Cycle ~3 months Manufacturable Yes
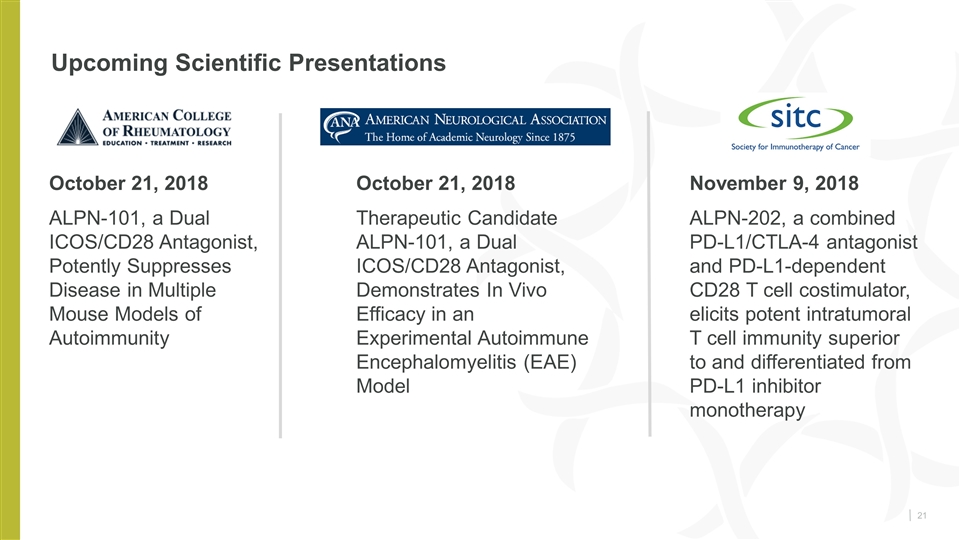
Upcoming Scientific Presentations October 21, 2018 ALPN-101, a Dual ICOS/CD28 Antagonist, Potently Suppresses Disease in Multiple Mouse Models of Autoimmunity October 21, 2018 Therapeutic Candidate ALPN-101, a Dual ICOS/CD28 Antagonist, Demonstrates In Vivo Efficacy in an Experimental Autoimmune Encephalomyelitis (EAE) Model November 9, 2018 ALPN-202, a combined PD-L1/CTLA-4 antagonist and PD-L1-dependent CD28 T cell costimulator, elicits potent intratumoral T cell immunity superior to and differentiated from PD-L1 inhibitor monotherapy

TWO LEAD PROGRAMS ALPN-101 & ALPN 202 POWERFUL SCIENTIFIC PLATFORM FOCUS ON DELIVERING VALUE
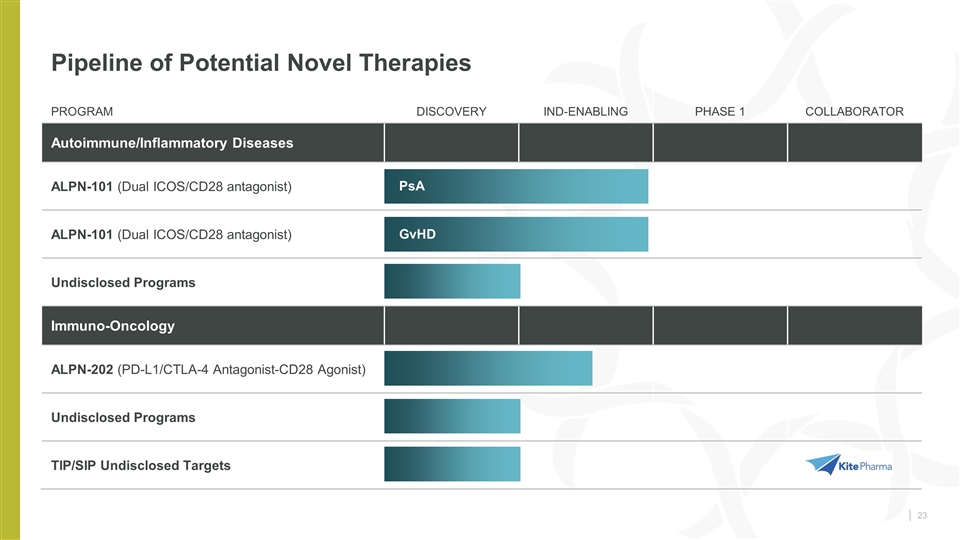
Pipeline of Potential Novel Therapies PROGRAM DISCOVERY IND-ENABLING PHASE 1 COLLABORATOR Autoimmune/Inflammatory Diseases ALPN-101 (Dual ICOS/CD28 antagonist) ALPN-101 (Dual ICOS/CD28 antagonist) Undisclosed Programs Immuno-Oncology ALPN-202 (PD-L1/CTLA-4 Antagonist-CD28 Agonist) Undisclosed Programs TIP/SIP Undisclosed Targets PsA GvHD
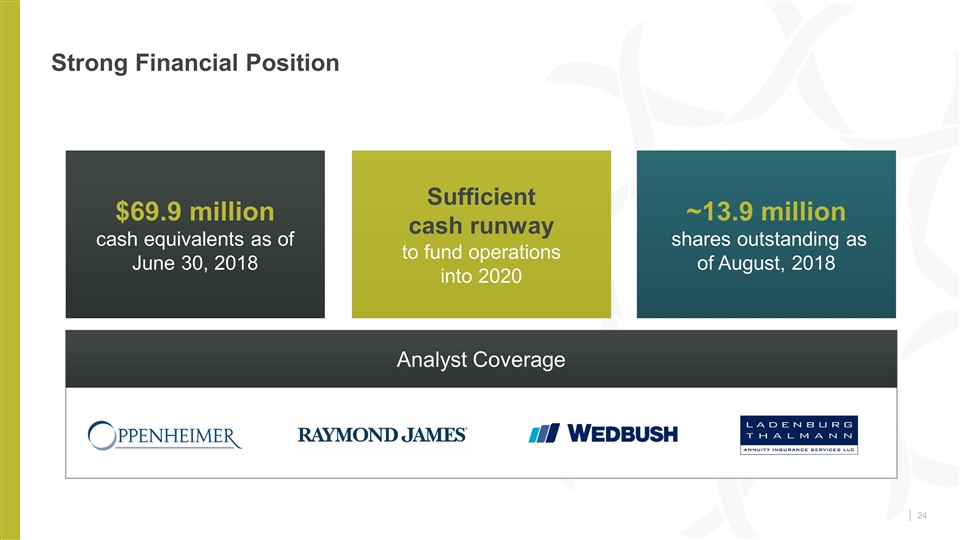
Strong Financial Position $69.9 million cash equivalents as of June 30, 2018 Sufficient cash runway to fund operations into 2020 ~13.9 million shares outstanding as of August, 2018 Analyst Coverage

Strong Leadership Team with Deep Clinical, Regulatory and Commercial Expertise LEADERSHIP TEAM PRINCIPAL INVESTORS Mitchell H. Gold, M.D. Executive Chairman & CEO Stanford Peng, M.D., Ph.D. EVP of R&D, Chief Medical Officer Ryan Swanson VP of Immunology & Co-Founder Paul Rickey Chief Financial Officer Kristine Swiderek, Ph.D. Senior VP of Research Mark Litton, Ph.D. President & Chief Operating Officer
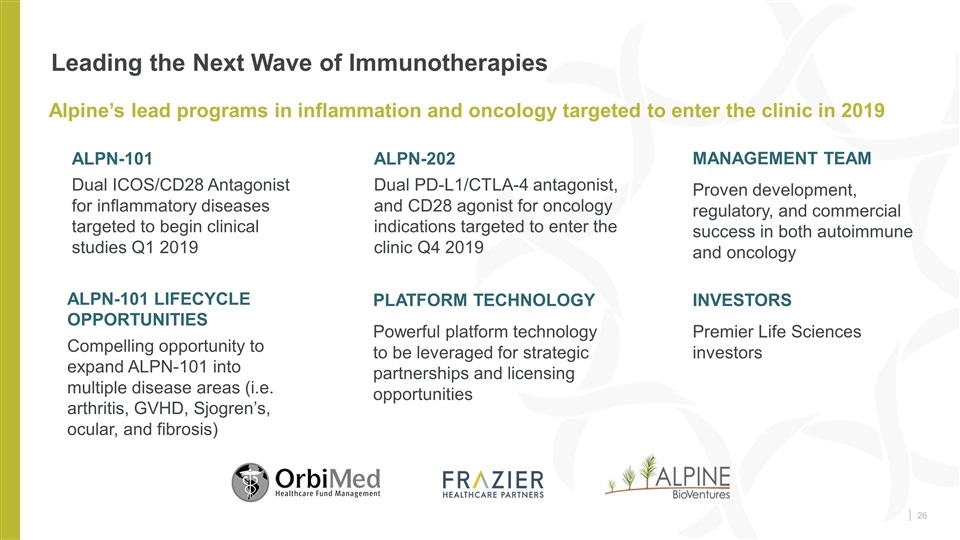
Leading the Next Wave of Immunotherapies PLATFORM TECHNOLOGY Powerful platform technology to be leveraged for strategic partnerships and licensing opportunities Alpine’s lead programs in inflammation and oncology targeted to enter the clinic in 2019 MANAGEMENT TEAM Proven development, regulatory, and commercial success in both autoimmune and oncology INVESTORS Premier Life Sciences investors ALPN-101 Dual ICOS/CD28 Antagonist for inflammatory diseases targeted to begin clinical studies Q1 2019 ALPN-202 Dual PD-L1/CTLA-4 antagonist, and CD28 agonist for oncology indications targeted to enter the clinic Q4 2019 ALPN-101 Lifecycle Opportunities Compelling opportunity to expand ALPN-101 into multiple disease areas (i.e. arthritis, GVHD, Sjogren’s, ocular, and fibrosis)

Thank You
