Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Kiniksa Pharmaceuticals, Ltd. | a18-30113_1ex99d1.htm |
| 8-K - 8-K - Kiniksa Pharmaceuticals, Ltd. | a18-30113_18k.htm |
First-In-Human Study of KPL-716, Anti-Oncostatin M Receptor Beta Monoclonal Antibody, in Healthy Volunteers and Subjects With Atopic Dermatitis Zamaneh Mikhak1, Joel M. Neutel2, Robert Bissonnette3, Dareen Siri4, Thomas Wade5, Stephen K. Tyring6, Eben Tessari1, Rohan Gandhi1, Fang Fang1, John F. Paolini1 1Kiniksa Pharmaceuticals Corp., Lexington, Massachusetts, USA; 2Orange County Research Center, Tustin, California, USA; 3Innovaderm Research, Inc., Montreal, Quebec, Canada; 4Sneeze, Wheeze and Itch Associates, Normal, Illinois, USA; 5QPS Miami Research Associates, Miami, Florida, USA; 6Houston Skin Associates, Houston, Texas, USA Presented at: The 27th Congress of the European Academy of Dermatology and Venereology; September 12–16, 2018; Paris, France 1
2 Acknowledgments: Study Sponsor: Kiniksa Pharmaceuticals, Ltd. Disclosures: Zamaneh Mikhak, Eben Tessari, Rohan Gandhi, Fang Fang, John F. Paolini: Employees at Kiniksa Pharmaceuticals Corp. Robert Bissonnette: Investigator, Consultant, Advisory Board Member, Speaker for and/or receives honoraria from Aquinox Pharma, Antiobix, Asana, Astellas, Brickell Biotech, Dermavant, Dermira, Dignity Sciences, Eli Lilly, Galderma, Glenmark, GSK-Stiefel, Hoffman-LaRoche Ltd, Leo Pharma, Neokera, Pfizer, Regeneron, Sienna, and Vitae; Shareholder of Innovaderm Research; Investigator for Kiniksa Pharmaceuticals, Ltd. Joel M. Neutel: Investigator for Kiniksa Pharmaceuticals, Ltd. Dareen Siri: Investigator for Regeneron, Pfizer, AnaptysBio, Vanda, Kiniksa Pharmaceuticals, Ltd., Speaker, Advisory Board, and Steering Committee for Regeneron /Sanofi. Stephen K. Tyring: Investigator for Abbvie, Aclaris, BMS, BI, Celgene, Dermik, Galderma, GSK, Janssen, Leo, Merck, Novartis, Ortho, Pfizer, Regeneron, Roche, Kiniksa Pharmaceuticals, Ltd. Thomas Wade: Investigator for Kiniksa Pharmaceuticals, Ltd. 2 Acknowledgements and Disclosures
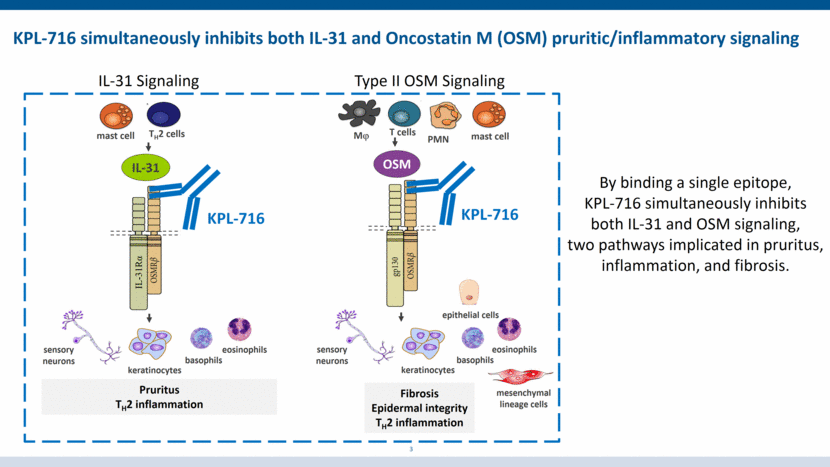
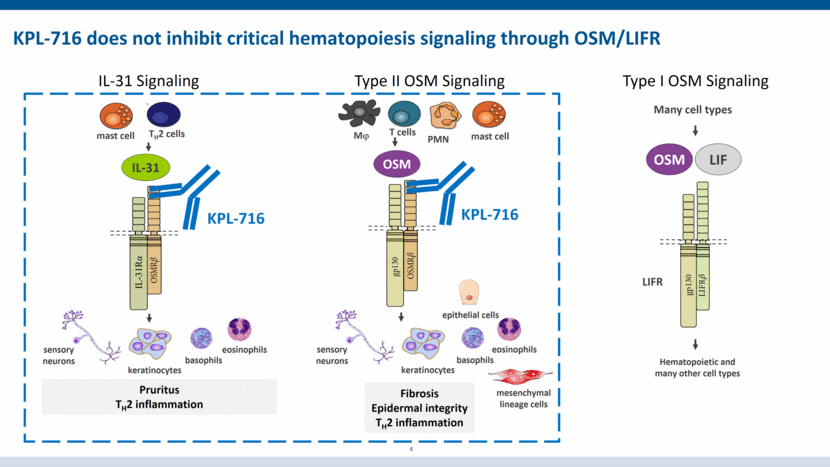
Pruritus TH2 inflammation Fibrosis Epidermal integrity TH2 inflammation IL-31 TH2 cells sensory neurons keratinocytes KPL-716 OSM Mj T cells PMN mast cell epithelial cells KPL-716 basophils eosinophils mast cell sensory neurons keratinocytes basophils eosinophils mesenchymal lineage cells 3 KPL-716 simultaneously inhibits both IL-31 and Oncostatin M (OSM) pruritic/inflammatory signaling By binding a single epitope, KPL-716 simultaneously inhibits both IL-31 and OSM signaling, two pathways implicated in pruritus, inflammation, and fibrosis. IL-31 Signaling Type II OSM Signaling IL-31Rα
KPL-716 does not inhibit critical hematopoiesis signaling through OSM/LIFR Type II OSM Signaling IL-31 Signaling Pruritus TH2 inflammation Fibrosis Epidermal integrity TH2 inflammation IL-31 TH2 cells sensory neurons keratinocytes KPL-716 OSM Mj T cells PMN mast cell epithelial cells KPL-716 basophils eosinophils mast cell sensory neurons keratinocytes basophils eosinophils mesenchymal lineage cells 4 Many cell types LIF LIFR Hematopoietic and many other cell types OSM Type I OSM Signaling IL-31Rα
Atopic Dermatitis (AD) is a proxy for IL-31-driven pruritic diseases 5 1) Raap et al. JACI, 2008; 2) Kim et al. Ann Derm, 2011; 3) Nobbe et al. Acta Derm Venerol, 2012; 4) Bilsborough et al. JACI, 2006; 5) Raap et al. Clin Exp Allergy, 2017; 6) Nemoto et al. BJD, 2016; 7) Ruzicka et al. JEM, 2017; 8) Gazel et al. JID, 2006; 9) Boniface et al. JI, 2007; 10) Fritz et al. JI, 2011; 11) Mozaffarian et al. JI, 2008; 12) Fritz et al. Exp Cell Rsch, 2009; 13) Kwofie et al. Resp Rsch, 2015. 14) Botelho et al. JI, 2013; 15) Wong et al. Lab Investigation; 2014. Role of IL-31 is well-established in pruritus and AD IL-31 levels are elevated in AD and correlate with disease severity1-3 Keratinocytes and macrophages express IL-31Rα, and circulating CLA+ T cells express IL-31 in AD4 Basophils release IL-31, and IL-31 increases IL-4 and IL-13 production in basophils; upregulation inhibited by anti-IL-31Rα and anti-OSMRβ5 Anti-IL-31Rα treatment reduced pruritus in AD6 OSM plays an important role in TH2 inflammation, epidermal integrity, and fibrosis Increases IL-4Rα and IL-13Rα production8-13 Increases IL-4 production; synergizes with IL-4 and IL-13 to increase eotaxin production in fibroblasts and airway smooth muscle cells 8, 10-14 Modulates genes important in keratinocyte activation and differentiation8, 9 Levels are elevated in fibrotic diseases, and OSM over-expression in animal models results in fibrotic changes11, 15
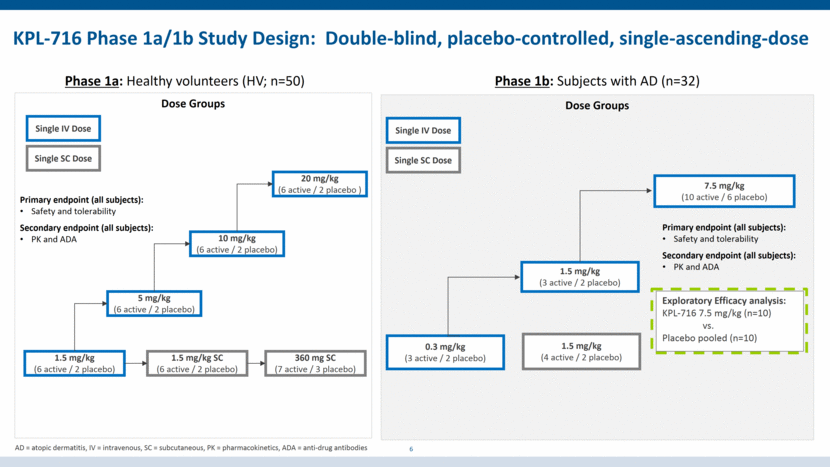
KPL-716 Phase 1a/1b Study Design: Double-blind, placebo-controlled, single-ascending-dose 6 Phase 1b: Subjects with AD (n=32) Phase 1a: Healthy volunteers (HV; n=50) Dose Groups 1.5 mg/kg (6 active / 2 placebo) 5 mg/kg (6 active / 2 placebo) 10 mg/kg (6 active / 2 placebo) 20 mg/kg (6 active / 2 placebo ) 1.5 mg/kg SC (6 active / 2 placebo) 360 mg SC (7 active / 3 placebo) Single IV Dose Single SC Dose Dose Groups 0.3 mg/kg (3 active / 2 placebo) 1.5 mg/kg (3 active / 2 placebo) 7.5 mg/kg (10 active / 6 placebo) 1.5 mg/kg (4 active / 2 placebo) Exploratory Efficacy analysis: KPL-716 7.5 mg/kg (n=10) vs. Placebo pooled (n=10) Single IV Dose Single SC Dose Primary endpoint (all subjects): Safety and tolerability Secondary endpoint (all subjects): PK and ADA Primary endpoint (all subjects): Safety and tolerability Secondary endpoint (all subjects): PK and ADA AD = atopic dermatitis, IV = intravenous, SC = subcutaneous, PK = pharmacokinetics, ADA = anti-drug antibodies
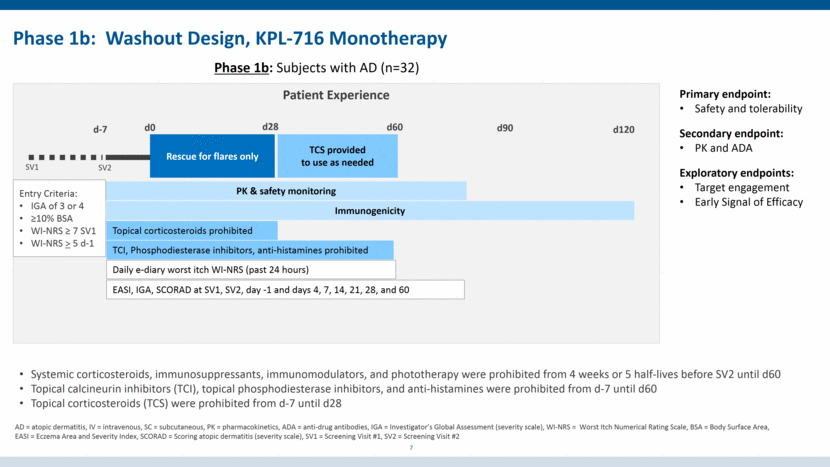
Phase 1b: Washout Design, KPL-716 Monotherapy 7 Phase 1b: Subjects with AD (n=32) Patient Experience Rescue for flares only SV1 SV2 TCI, Phosphodiesterase inhibitors, anti-histamines prohibited TCS provided to use as needed d0 PK & safety monitoring d28 d60 Entry Criteria: IGA of 3 or 4 >10% BSA WI-NRS > 7 SV1 WI-NRS > 5 d-1 EASI, IGA, SCORAD at SV1, SV2, day -1 and days 4, 7, 14, 21, 28, and 60 d90 Daily e-diary worst itch WI-NRS (past 24 hours) Topical corticosteroids prohibited d-7 d120 Immunogenicity Systemic corticosteroids, immunosuppressants, immunomodulators, and phototherapy were prohibited from 4 weeks or 5 half-lives before SV2 until d60 Topical calcineurin inhibitors (TCI), topical phosphodiesterase inhibitors, and anti-histamines were prohibited from d-7 until d60 Topical corticosteroids (TCS) were prohibited from d-7 until d28 Primary endpoint: Safety and tolerability Secondary endpoint: PK and ADA Exploratory endpoints: Target engagement Early Signal of Efficacy AD = atopic dermatitis, IV = intravenous, SC = subcutaneous, PK = pharmacokinetics, ADA = anti-drug antibodies, IGA = Investigator’s Global Assessment (severity scale), WI-NRS = Worst Itch Numerical Rating Scale, BSA = Body Surface Area, EASI = Eczema Area and Severity Index, SCORAD = Scoring atopic dermatitis (severity scale), SV1 = Screening Visit #1, SV2 = Screening Visit #2
Baseline Parameters were Balanced 8 Baseline demographics/disease characteristics: AD KPL-716 7.5 mg/kg IV Placebo Pooled IV Age, mean (SD), years 29.7 (11.2) 41.7 (10.9) Male, % 50 70 White, % 70 70 Elevated IgE, % 60 60 History of any allergic disease, % 40 60 #AD flares in past year, mean (SD) 28.1 (41.6) 3.7 (3.5) Body surface area affected by AD, mean (SD) 24.2 (8.0) 34.1 (28.0) Weekly average WI-NRS, mean (SD) 8.0 (1.3) 8.2 (0.7) Total EASI, mean (SD) 19.9 (7.6) 25.3 (14.1) Total SCORAD, mean (SD) 66.7 (10.7) 60.7 (13.7) IGA=3, % 80 80 IGA=4, % 20 20 Baseline is defined as the last measurement prior to dosing. AD = atopic dermatitis, IV = intravenous, IGA = Investigator’s Global Assessment (severity scale), WI-NRS = Worst Itch Numerical Rating Scale, EASI = Eczema Area and Severity Index, SCORAD = Scoring atopic dermatitis (severity scale)
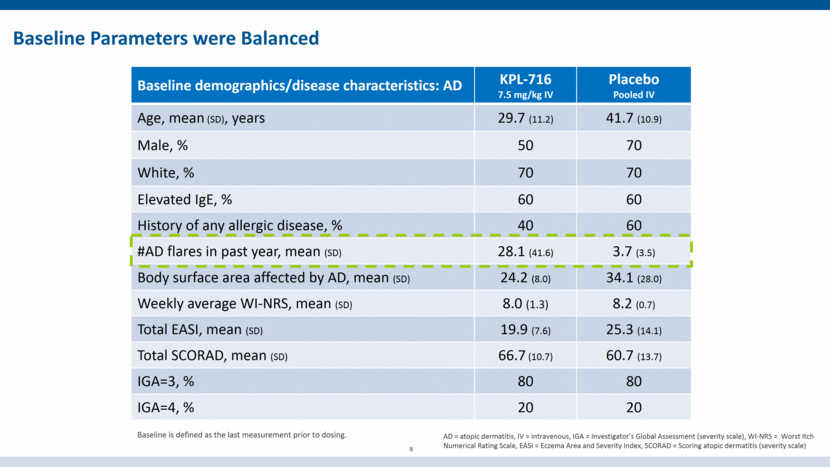
KPL-716 was Well-Tolerated 9 AE KPL-716 (IV) Placebo (IV) 0.3 mg/kg n=3 1.5 mg/kg n=3 7.5 mg/kg n=10 Pooled n=10 DR-TEAE 0 Mild headache (1), Decreased appetite (1) Moderate dizziness (1) Mild somnolence (1) AD flare 1 0 2 3 Study day of AD flare 7 N/A 14, 20 1, 5, 45 AE KPL-716 (IV) Placebo (IV) 1.5 mg/kg n=6 5 mg/kg n=6 10 mg/kg n=6 20 mg/kg n=6 Pooled n=8 DR-TEAE 0 Mild headache (1) 0 0 0 †The only moderate DR-TEAE occurred after a protocol violation. No Deaths No SAEs No Discontinuations due to AEs No Infusion Reactions No Injection Site Reactions No Thrombocytopenia No Peripheral Edema No Conjunctivitis Drug-Related Treatment Emergent Adverse Events (DR-TEAEs) infrequent and not related to dose All resolved without sequalae Healthy volunteers Subjects with atopic dermatitis KPL-716 (SC) Placebo (SC) 1.5 mg/kg n=6 360 mg n=7 Pooled n=5 Mild flushing (1) Mild anemia (1) 0 KPL-716 (SC) Placebo (SC) 1.5 mg/kg n=4 Pooled n=2 Mild dizziness (1) 0 0 0 N/A N/A
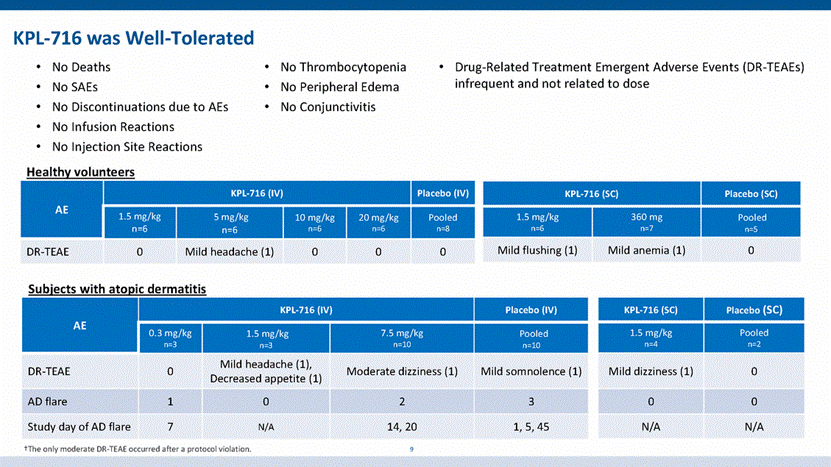
KPL-716 demonstrated dose-dependent elimination (TMDD) 10 TMDD: Target-Mediated Drug Disposition 0.3 1.0 3.0 10.0 30.0 100.0 300.0 1000.0 0 4 8 12 16 Time After Dose (w eeks) C o n c e n t r a t i o n ( m g m L ) 0.3 mg/kg IV 1.5 mg/kg IV 1.5 mg/kg SC 7.5 mg/kg IV
Efficacy Endpoints: Pruritus: Weekly average of daily WI-NRS (worst itch in past 24 hours) collected by daily eDiary Pruritus Visual Analog Scale, a component of SCORAD (average itch in past 3 days) collected at study visits Sleep loss VAS: A component of SCORAD (average sleep loss in past 3 nights) Eczema Area Severity Index (EASI) 11 Efficacy Analysis: 10 KPL-716 subjects (7.5 mg/kg IV) versus 10 placebo subjects (pooled IV) from baseline to Day 28 “Last Observation Carried Forward” approach used for data values after rescue medication administered. Subject was considered non-responder after rescue (responder analysis). Two KPL-716: 2 AD flares (d15 and d21) Three placebo: 2 AD flares (d3, d14), 1 anti-histamine use for upper respiratory infection (d26) Similar results obtained if data values after rescue medication administration were included or excluded Exploratory Efficacy Endpoints and Analysis Plan AD = atopic dermatitis, IV = intravenous, WI-NRS = Worst Itch Numerical Rating Scale, EASI = Eczema Area and Severity Index, SCORAD = Scoring atopic dermatitis (severity scale)
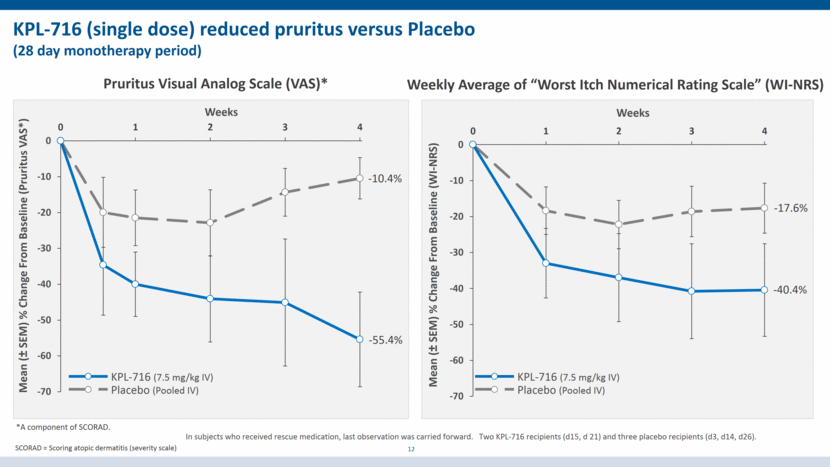
KPL-716 (single dose) reduced pruritus versus Placebo (28 day monotherapy period) 12 Weekly Average of “Worst Itch Numerical Rating Scale” (WI-NRS) Mean (± SEM) % Change From Baseline (WI-NRS) Weeks Pruritus Visual Analog Scale (VAS)* Weeks Mean (± SEM) % Change From Baseline (Pruritus VAS*) *A component of SCORAD. Placebo (Pooled IV) KPL-716 (7.5 mg/kg IV) Placebo (Pooled IV) KPL-716 (7.5 mg/kg IV) In subjects who received rescue medication, last observation was carried forward. Two KPL-716 recipients (d15, d 21) and three placebo recipients (d3, d14, d26). -55.4% -10.4% -17.6% -40.4% SCORAD = Scoring atopic dermatitis (severity scale) -70 -60 -50 -40 -30 -20 -10 0 0 1 2 3 4 -70 -60 -50 -40 -30 -20 -10 0 0 1 2 3 4
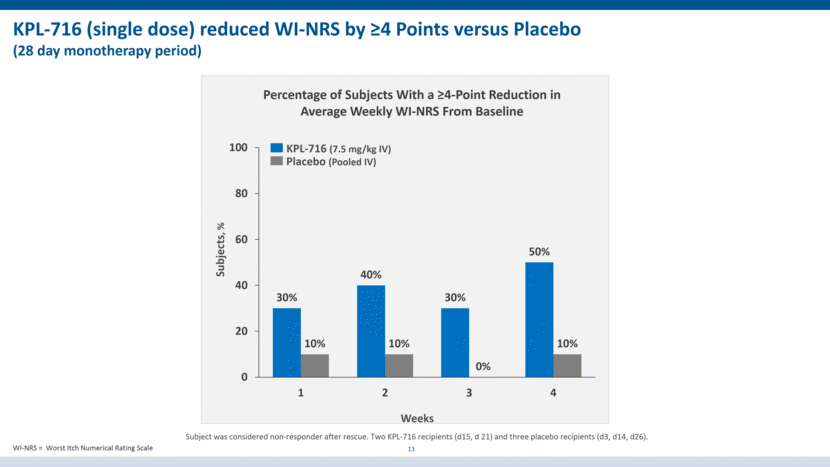
KPL-716 (single dose) reduced WI-NRS by >4 Points versus Placebo (28 day monotherapy period) 13 Placebo (Pooled IV) Percentage of Subjects With a >4-Point Reduction in Average Weekly WI-NRS From Baseline Weeks KPL-716 (7.5 mg/kg IV) Subjects, % Subject was considered non-responder after rescue. Two KPL-716 recipients (d15, d 21) and three placebo recipients (d3, d14, d26). WI-NRS = Worst Itch Numerical Rating Scale 30% 40% 30% 50% 10% 10% 0% 10% 0 20 40 60 80 100 1 2 3 4
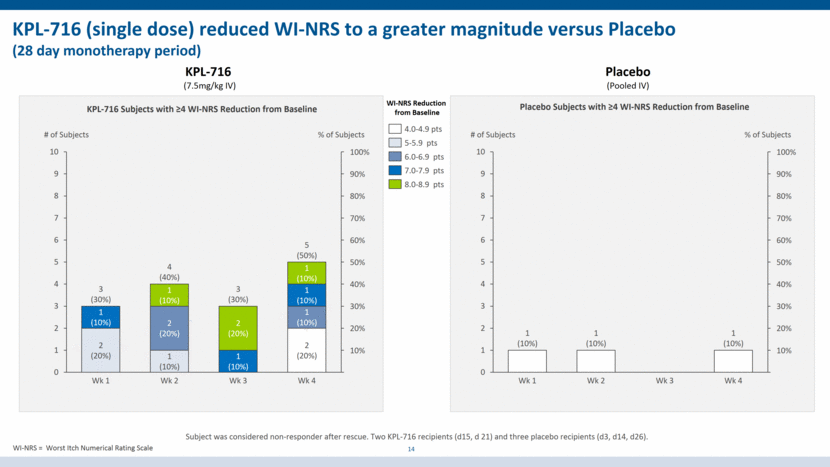
KPL-716 (single dose) reduced WI-NRS to a greater magnitude versus Placebo (28 day monotherapy period) 14 KPL-716 (7.5mg/kg IV) Placebo (Pooled IV) WI-NRS Reduction from Baseline KPL-716 Subjects with >4 WI-NRS Reduction from Baseline Placebo Subjects with >4 WI-NRS Reduction from Baseline 10% 20% 40% 30% 50% 90% 60% 70% 80% 100% # of Subjects 1 (10%) % of Subjects 3 (30%) 1 (10%) Wk 1 2 (20%) 1 (10%) 1 (10%) 2 (20%) 1 (10%) Wk 2 2 (20%) 1 (10%) 2 (20%) 4 (40%) Wk 3 Wk 4 1 (10%) 3 (30%) 5 (50%) 4.0-4.9 pts 5-5.9 pts 6.0-6.9 pts 7.0-7.9 pts 8.0-8.9 pts 20% 10% 30% 70% 60% 40% 50% 80% 90% 100% # of Subjects Wk 2 % of Subjects Wk 1 Wk 3 Wk 4 1 (10%) 1 (10%) 1 (10%) WI-NRS = Worst Itch Numerical Rating Scale Subject was considered non-responder after rescue. Two KPL-716 recipients (d15, d 21) and three placebo recipients (d3, d14, d26). 0 1 2 3 4 5 6 7 8 9 10 0 1 2 3 4 5 6 7 8 9 10
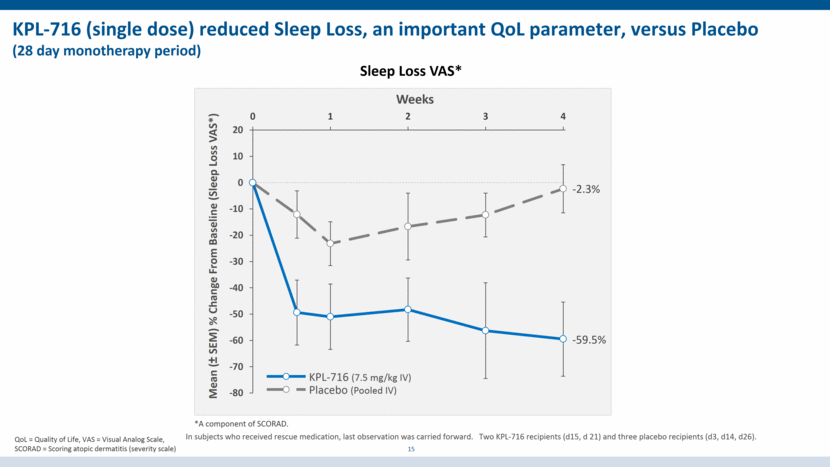
KPL-716 (single dose) reduced Sleep Loss, an important QoL parameter, versus Placebo (28 day monotherapy period) Sleep Loss VAS* *A component of SCORAD. 15 In subjects who received rescue medication, last observation was carried forward. Two KPL-716 recipients (d15, d 21) and three placebo recipients (d3, d14, d26). QoL = Quality of Life, VAS = Visual Analog Scale, SCORAD = Scoring atopic dermatitis (severity scale) Mean (± SEM) % Change From Baseline (Sleep Loss VAS*) Weeks -59.5% -2.3% Placebo (Pooled IV) KPL-716 (7.5 mg/kg IV) -80 -70 -60 -50 -40 -30 -20 -10 0 10 20 0 1 2 3 4
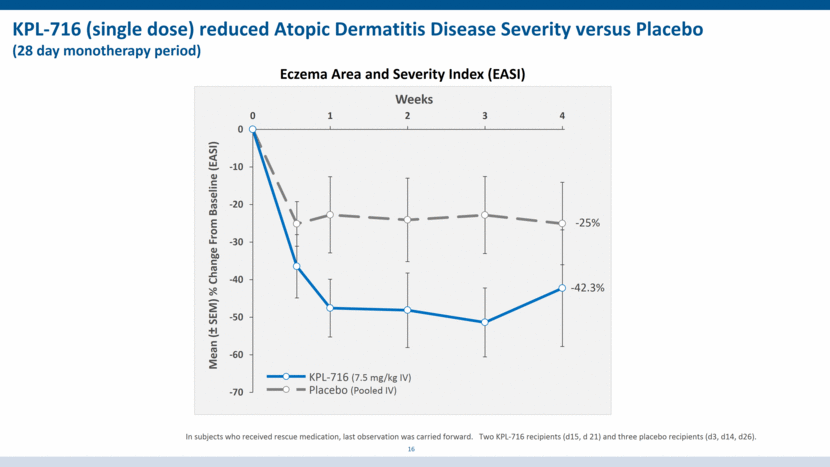
KPL-716 (single dose) reduced Atopic Dermatitis Disease Severity versus Placebo (28 day monotherapy period) Mean (± SEM) % Change From Baseline (EASI) Weeks Eczema Area and Severity Index (EASI) 16 Placebo (Pooled IV) KPL-716 (7.5 mg/kg IV) -42.3% -25% In subjects who received rescue medication, last observation was carried forward. Two KPL-716 recipients (d15, d 21) and three placebo recipients (d3, d14, d26). -70 -60 -50 -40 -30 -20 -10 0 0 1 2 3 4
First-in-Human, double-blind, placebo-controlled study of KPL-716 met the primary endpoint: KPL-716 was well-tolerated in both healthy volunteers and subjects with AD KPL-716 engaged its target and demonstrated an Early Signal of Efficacy with pruritus reduction Reduction in disease severity (EASI) and sleep loss were also demonstrated Repeated-Single-Dose study in subjects with AD is ongoing; longer duration will provide additional efficacy data Data support further development of KPL-716 in chronic pruritic diseases Summary 17
Thank You to the Patients, Investigators and Study Staff 18 Joel M. Neutel, MD Orange Country Research Center Tustin, California, USA Robert Bissonnette, MD Innovaderm Montreal, Quebec, Canada Stephen K. Tyring, MD Houston Skin Associates Houston, Texas, USA Thomas Wade, MD QPS-MRA, LLC Miami, Florida, USA Dareen Siri, MD Sneeze, Wheeze and Itch Associates Normal, Illinois, USA Iftikhar Hussain, MD Vital Prospects Tulsa, Oklahoma, USA Jonathan S. Dosik, MD TKL Research Fair Lawn, New Jersey, USA Jamshid Lofti, MD Clinical Trial Network Houston, Texas, USA Douglas Denham, DO, CPI Clinical Trials of Texas, Inc. San Antonio, Texas, USA