Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Assertio Therapeutics, Inc | a18-28095_18k.htm |
About this Presentation The statements that are not historical facts contained in this presentation are forward-looking statements including, but not limited to, statements relating to the commercialization of Gralise, CAMBIA, and Zipsor, royalties associated with Collegium’s commercialization of NUCYNTA and NUCYNTA ER, regulatory approval and clinical development of cosyntropin depot, and expectations regarding financial results and potential business opportunities. These forward-looking statements involve significant risks and uncertainties, including risks detailed in the Company’s Securities and Exchange Commission filings, including the Company’s most recent Annual Report on Form 10-K and most recent Quarterly Report on Form 10-Q. The inclusion of forward-looking statements should not be regarded as a representation that any of the Company’s plans or objectives will be achieved. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Assertio undertakes no duty or obligation to update any forward-looking statements contained in this presentation as a result of new information, future events or changes in its expectations except as may be required by law. This presentation contains non-GAAP financial measures, including adjusted EBITDA and non-GAAP SG&A. Please refer to the appendix to this presentation for an explanation of these non-GAAP financial measures and for tables that reconcile the non-GAAP figures to their GAAP equivalent. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. 2

New Name, Renewed Mission: Advance Patient Care in the Company’s Core Areas of Neurology, Orphan & Specialty Medicines 3 Assertio Therapeutics, Inc. (Assertio) is a new name that reflects a new business strategy, mission, vision and values Established new headquarters in Lake Forest, IL Transformed into a leaner, more entrepreneurial and faster moving company Location allows the Company to attract and retain new talent Relocation reduces headquarters’ staff by ~40 percent and office space requirement by ~50 percent The Company’s common stock trades under a new NASDAQ ticker symbol “ASRT”

4 Principles Guiding the Way We Do Business Maintain a Strong NUCYNTA Franchise, Grow Our Neurology Business and Build a New Orphan/Specialty Business Leverage Opportunities In a Changing Market Place, Must be Able to Respond Quickly to Drive Superior Performance Committed to Putting the Patient First Opportunistic Clear Strategy Patient Focused
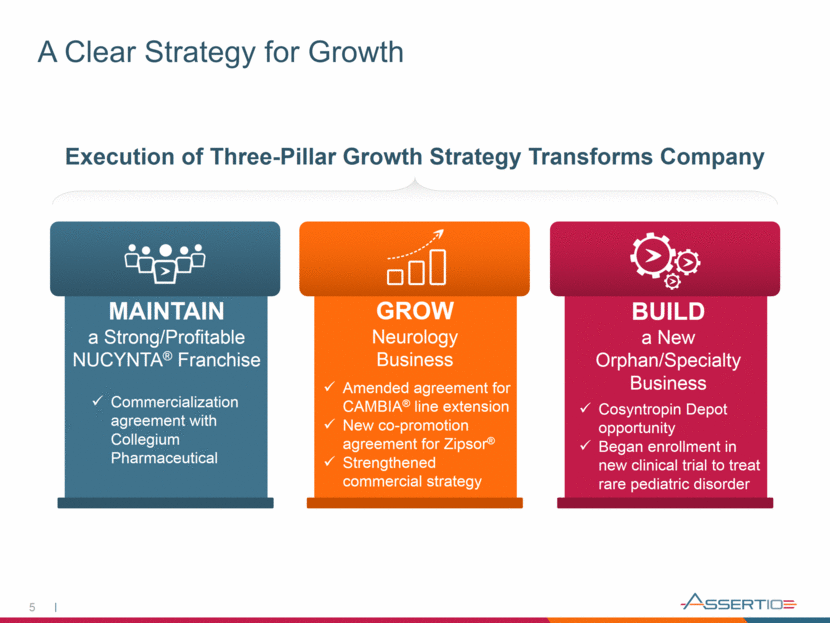
A Clear Strategy for Growth 5 Execution of Three-Pillar Growth Strategy Transforms Company MAINTAIN a Strong/Profitable NUCYNTA® Franchise GROW Neurology Business BUILD a New Orphan/Specialty Business Commercialization agreement with Collegium Pharmaceutical Amended agreement for CAMBIA® line extension New co-promotion agreement for Zipsor® Strengthened commercial strategy Cosyntropin Depot opportunity Began enrollment in new clinical trial to treat rare pediatric disorder
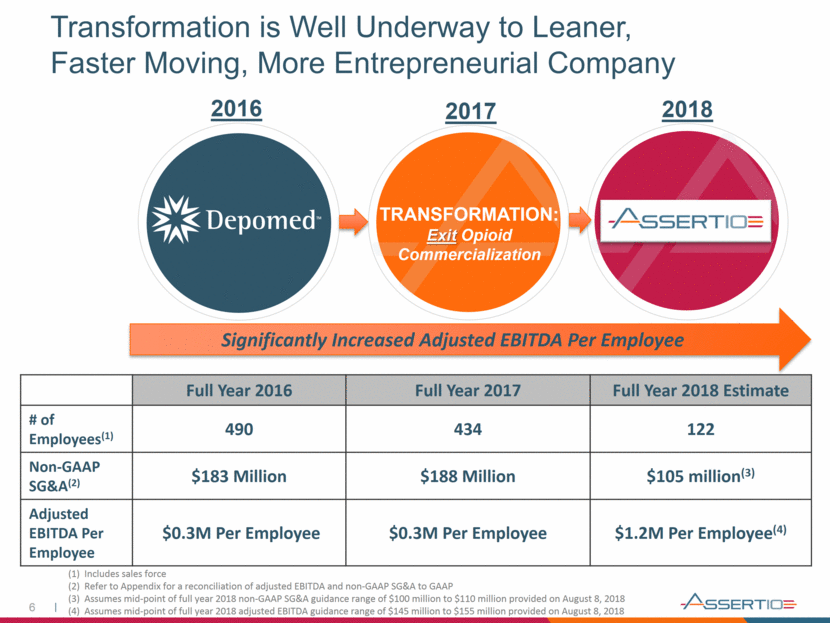
6 Transformation is Well Underway to Leaner, Faster Moving, More Entrepreneurial Company TRANSFORMATION: Exit Opioid Commercialization Full Year 2016 Full Year 2017 Full Year 2018 Estimate # of Employees(1) 490 434 122 Non-GAAP SG&A(2) $183 Million $188 Million $105 million(3) Adjusted EBITDA Per Employee $0.3M Per Employee $0.3M Per Employee $1.2M Per Employee(4) 2016 2017 2018 Significantly Increased Adjusted EBITDA Per Employee (1) Includes sales force (2) Refer to Appendix for a reconciliation of adjusted EBITDA and non-GAAP SG&A to GAAP (3) Assumes mid-point of full year 2018 non-GAAP SG&A guidance range of $100 million to $110 million provided on August 8, 2018 (4) Assumes mid-point of full year 2018 adjusted EBITDA guidance range of $145 million to $155 million provided on August 8, 2018
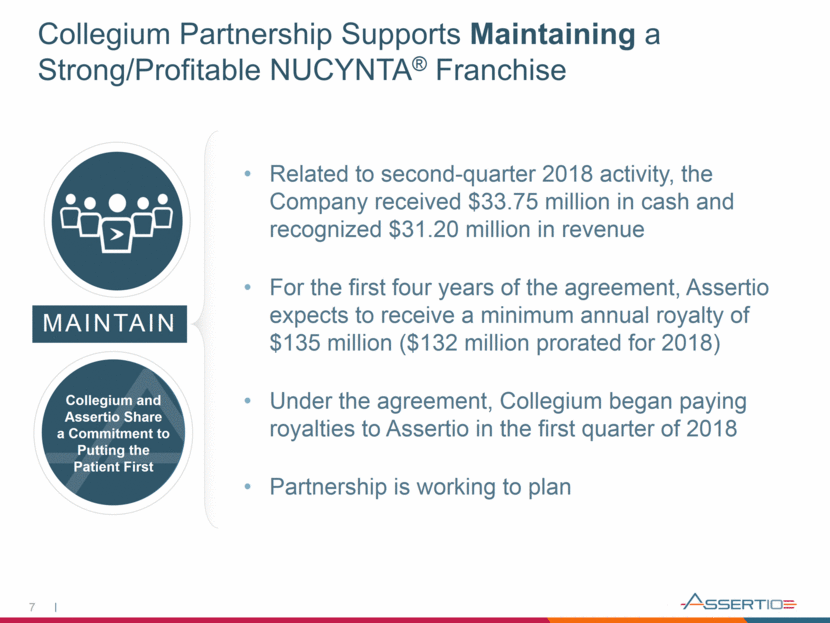
Collegium Partnership Supports Maintaining a Strong/Profitable NUCYNTA® Franchise 7 MAINTAIN Collegium and Assertio Share a Commitment to Putting the Patient First Related to second-quarter 2018 activity, the Company received $33.75 million in cash and recognized $31.20 million in revenue For the first four years of the agreement, Assertio expects to receive a minimum annual royalty of $135 million ($132 million prorated for 2018) Under the agreement, Collegium began paying royalties to Assertio in the first quarter of 2018 Partnership is working to plan
Growing Our Neurology Business 8 GROW Q2 ’18: stabilized core neurology brands with positive sequential total prescription growth for the franchise; but more work to do with Gralise® Co-promotion with Allegis Pharmaceuticals and Zipsor® began in June Added approximately 30 new sales reps that focus exclusively on primary care physicians in targeted geographic regions Q1 ’18: amended existing license agreement for CAMBIA® line extension; potential to expand exclusivity through 2026
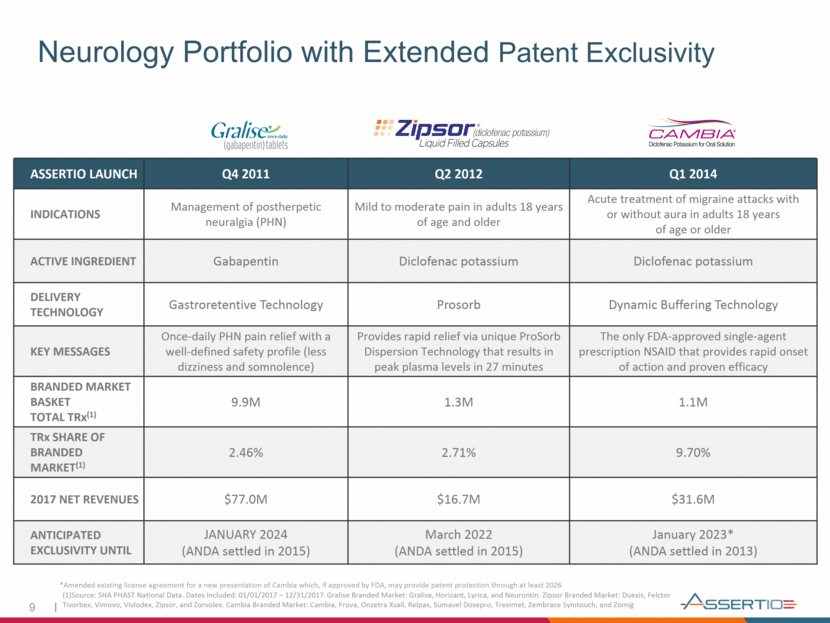
Neurology Portfolio with Extended Patent Exclusivity *Amended existing license agreement for a new presentation of Cambia which, if approved by FDA, may provide patent protection through at least 2026 ASSERTIO LAUNCH Q4 2011 Q2 2012 Q1 2014 INDICATIONS Management of postherpetic neuralgia (PHN) Mild to moderate pain in adults 18 years of age and older Acute treatment of migraine attacks with or without aura in adults 18 years of age or older ACTIVE INGREDIENT Gabapentin Diclofenac potassium Diclofenac potassium DELIVERY TECHNOLOGY Gastroretentive Technology Prosorb Dynamic Buffering Technology KEY MESSAGES Once-daily PHN pain relief with a well-defined safety profile (less dizziness and somnolence) Provides rapid relief via unique ProSorb Dispersion Technology that results in peak plasma levels in 27 minutes The only FDA-approved single-agent prescription NSAID that provides rapid onset of action and proven efficacy BRANDED MARKET BASKET TOTAL TRx(1) 9.9M 1.3M 1.1M TRx SHARE OF BRANDED MARKET(1) 2.46% 2.71% 9.70% 2017 NET REVENUES $77.0M $16.7M $31.6M ANTICIPATED EXCLUSIVITY UNTIL JANUARY 2024 (ANDA settled in 2015) March 2022 (ANDA settled in 2015) January 2023* (ANDA settled in 2013) (1)Source: SHA PHAST National Data. Dates Included: 01/01/2017 – 12/31/2017. Gralise Branded Market: Gralise, Horizant, Lyrica, and Neurontin. Zipsor Branded Market: Duexis, Felctor Tivorbex, Vimovo, Vivlodex, Zipsor, and Zorvolex. Cambia Branded Market: Cambia, Frova, Onzetra Xsail, Relpax, Sumavel Dosepro, Treximet, Zembrace Symtouch, and Zomig 9
Key Focus Next Six Months Grow Gralise® 10 Reduced total call targets from 7,000 to 6,000 Increased call frequency to key Gralise prescribers; ~4,000 high-quality prescribers Gralise has strong managed-care coverage, >75 percent of commercial lives covered Better leverage covered lives Along with the Simple Script™ program, intend to ensure that more of the scripts physicians write get into patients’ hands Gralise is a differentiated product in its combination of true once-daily dosing and favorable side-effect profile Communicate more concise, powerful messaging

Cosyntropin (Synthetic ACTH Depot) First in a Portfolio of High-Value, High-Touch Orphan/Specialty Medicines Positioned to Address the Needs of Patients, Physicians and Payors 11 BUILD Plan to file for FDA approval of cosyntropin depot by year end 1st Indication (diagnostic - for suspected adrenocortical insufficiency) NDA filing in late 2018 Endocrinologists commonly use exogenous ACTH to trigger the body’s cortisol response Helps determine if a patient’s adrenal glands are functioning properly Goal is to demonstrate that the diagnostic performance of cosyntropin depot is comparable to the reference product (Cortrosyn) 2nd Indication (Infantile Spasms) Investigational New Drug Trial ongoing
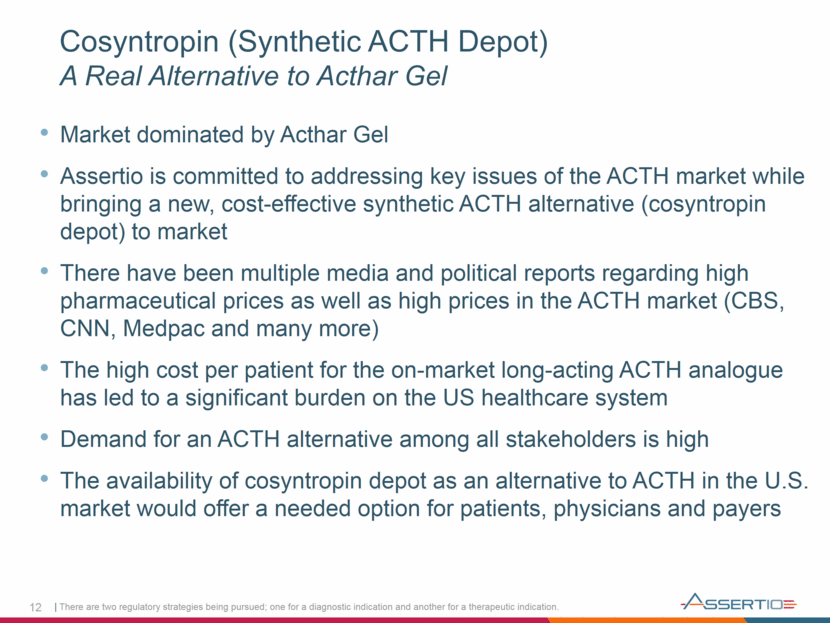
12 Cosyntropin (Synthetic ACTH Depot) A Real Alternative to Acthar Gel Market dominated by Acthar Gel Assertio is committed to addressing key issues of the ACTH market while bringing a new, cost-effective synthetic ACTH alternative (cosyntropin depot) to market There have been multiple media and political reports regarding high pharmaceutical prices as well as high prices in the ACTH market (CBS, CNN, Medpac and many more) The high cost per patient for the on-market long-acting ACTH analogue has led to a significant burden on the US healthcare system Demand for an ACTH alternative among all stakeholders is high The availability of cosyntropin depot as an alternative to ACTH in the U.S. market would offer a needed option for patients, physicians and payers There are two regulatory strategies being pursued; one for a diagnostic indication and another for a therapeutic indication.
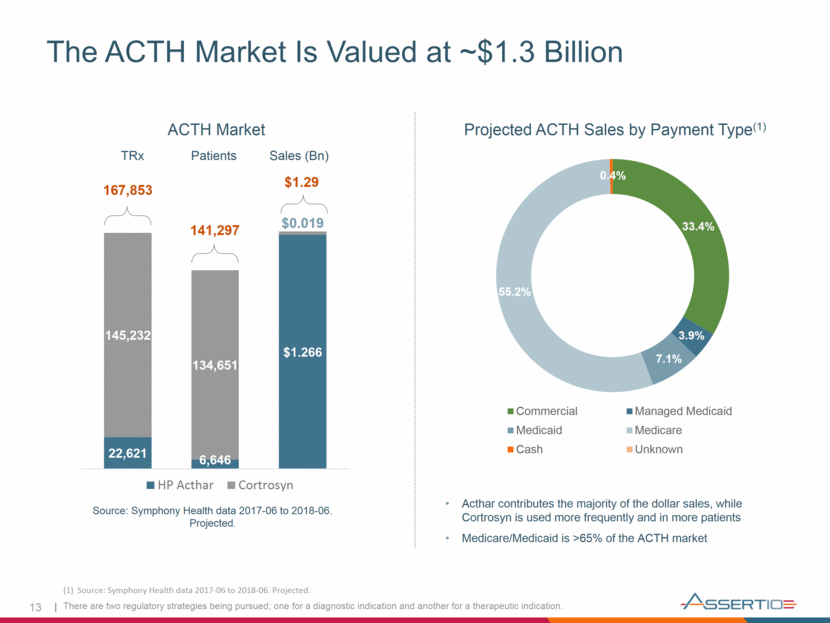
13 The ACTH Market Is Valued at ~$1.3 Billion Acthar contributes the majority of the dollar sales, while Cortrosyn is used more frequently and in more patients Medicare/Medicaid is >65% of the ACTH market Projected ACTH Sales by Payment Type(1) ACTH Market TRx Patients Sales (Bn) Source: Symphony Health data 2017-06 to 2018-06. Projected. 167,853 141,297 $1.29 There are two regulatory strategies being pursued; one for a diagnostic indication and another for a therapeutic indication. (1) Source: Symphony Health data 2017-06 to 2018-06. Projected. $1.266 $0.019 HP Acthar Cortrosyn 22,621 145,232 6,646 134,651 33.4% 3.9% 7.1% 55.2% 0.4% Commercial Managed Medicaid Medicaid Medicare Cash Unknown
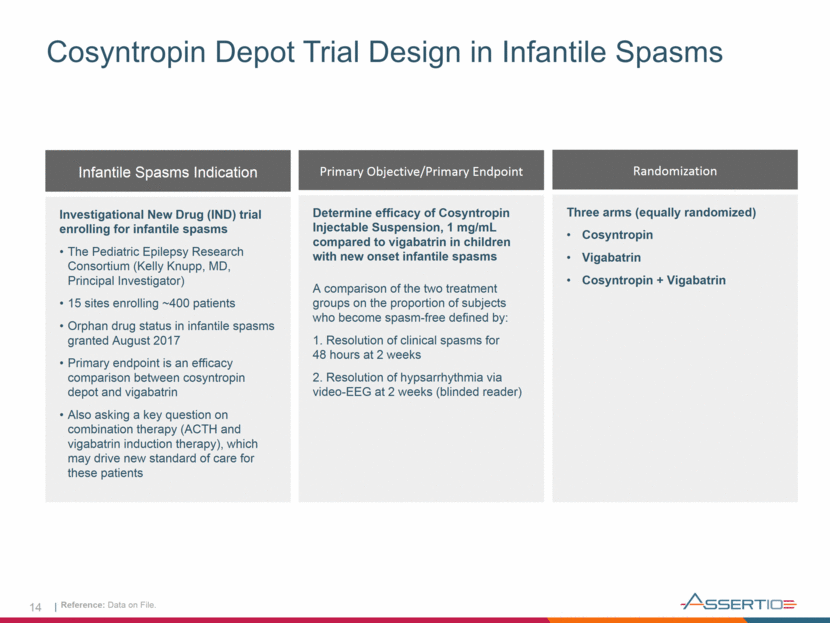
14 Cosyntropin Depot Trial Design in Infantile Spasms Infantile Spasms Indication Investigational New Drug (IND) trial enrolling for infantile spasms The Pediatric Epilepsy Research Consortium (Kelly Knupp, MD, Principal Investigator) 15 sites enrolling ~400 patients Orphan drug status in infantile spasms granted August 2017 Primary endpoint is an efficacy comparison between cosyntropin depot and vigabatrin Also asking a key question on combination therapy (ACTH and vigabatrin induction therapy), which may drive new standard of care for these patients Reference: Data on File. Primary Objective/Primary Endpoint Determine efficacy of Cosyntropin Injectable Suspension, 1 mg/mL compared to vigabatrin in children with new onset infantile spasms A comparison of the two treatment groups on the proportion of subjects who become spasm-free defined by: 1. Resolution of clinical spasms for 48 hours at 2 weeks 2. Resolution of hypsarrhythmia via video-EEG at 2 weeks (blinded reader) Randomization Three arms (equally randomized) Cosyntropin Vigabatrin Cosyntropin + Vigabatrin
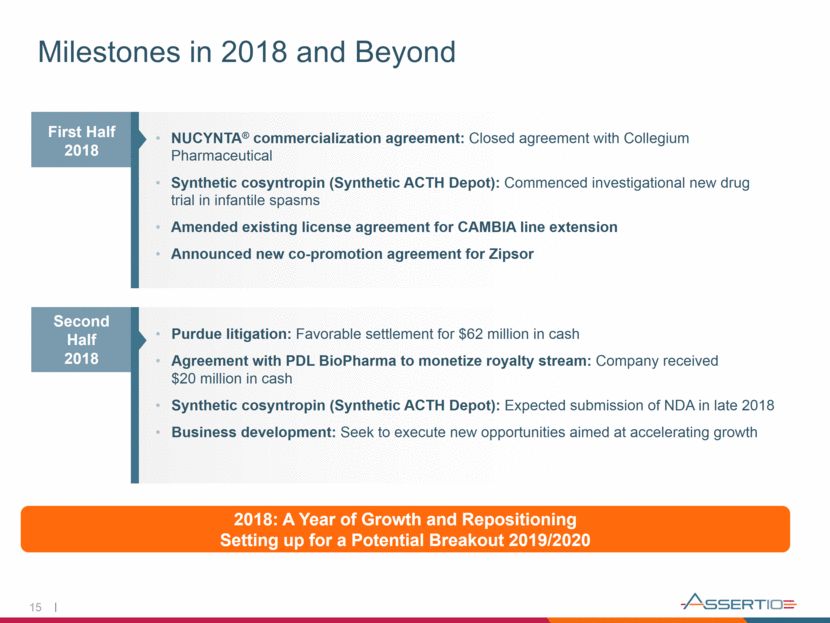
NUCYNTA® commercialization agreement: Closed agreement with Collegium Pharmaceutical Synthetic cosyntropin (Synthetic ACTH Depot): Commenced investigational new drug trial in infantile spasms Amended existing license agreement for CAMBIA line extension Announced new co-promotion agreement for Zipsor Milestones in 2018 and Beyond 15 Purdue litigation: Favorable settlement for $62 million in cash Agreement with PDL BioPharma to monetize royalty stream: Company received $20 million in cash Synthetic cosyntropin (Synthetic ACTH Depot): Expected submission of NDA in late 2018 Business development: Seek to execute new opportunities aimed at accelerating growth Second Half 2018 2018: A Year of Growth and Repositioning Setting up for a Potential Breakout 2019/2020 First Half 2018
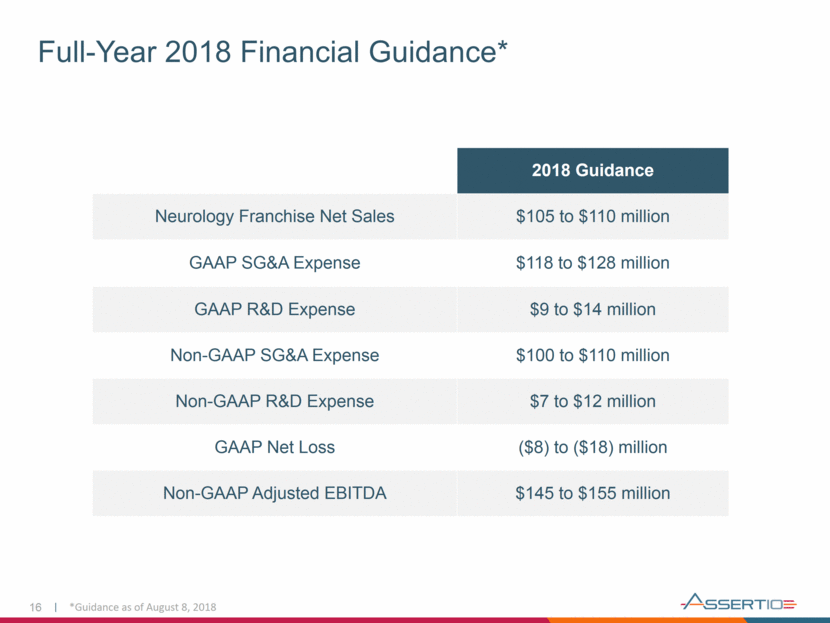
Full-Year 2018 Financial Guidance* 16 2018 Guidance Neurology Franchise Net Sales $105 to $110 million GAAP SG&A Expense $118 to $128 million GAAP R&D Expense $9 to $14 million Non-GAAP SG&A Expense $100 to $110 million Non-GAAP R&D Expense $7 to $12 million GAAP Net Loss ($8) to ($18) million Non-GAAP Adjusted EBITDA $145 to $155 million *Guidance as of August 8, 2018
Appendix

18 Note Regarding Use of GAAP and Non-GAAP Financial Measures To supplement our financial results presented on a U.S. generally accepted accounting principles (GAAP) basis, we have included information about non-GAAP adjusted EBITDA and other non-GAAP financial measures as useful operating metrics. We believe that the presentation of these non-GAAP financial measures, when viewed with our results under GAAP and the accompanying reconciliation, provides supplementary information to analysts, investors, lenders, and our management in assessing the Company’s performance and results from period to period. We use these non-GAAP measures internally to understand, manage and evaluate the Company’s performance, and in part, in the determination of bonuses for executive officers and employees. These non-GAAP financial measures should be considered in addition to, and not a substitute for, or superior to, net income or other financial measures calculated in accordance with GAAP. Non-GAAP financial measures used by us may be calculated differently from, and therefore may not be comparable to, non-GAAP measures used by other companies. Non-GAAP measures presented exclude specified items. We consider specified items to be significant income/expense items not indicative of our current operations, including the related tax effect. Specified items include non-cash adjustment to Collegium agreement revenue and cost of sales, release of NUCYNTA and Lazanda sales reserves for products we are no longer selling, interest income, interest expense, amortization, acquired in-process research and development and non-cash adjustments related to product acquisitions, stock-based compensation expense, non-cash interest expense related to debt, depreciation, taxes, transaction costs, CEO transition, restructuring costs, certain types of legal settlements, disputes, fees and costs, and to adjust for the tax effect related to each of the non-GAAP adjustments.
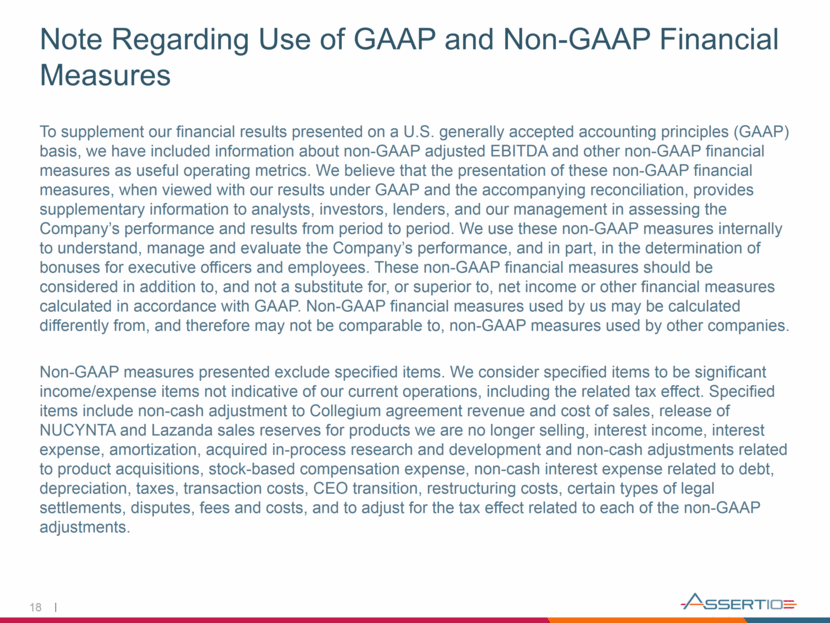
19 Non-GAAP Reconciliation (in millions) (unaudited) (5) Twelve Months Ended December 31, 2017 2016 2018 (unaudited) GAAP net earnings / (loss) (102,496) $ (88,720) $ 12,776 $ Pharmacy benefit manager dispute settlement 4,742 - - Commercialization agreement revenue (1) - - (49,288) Commercialization agreement cost of sales (1) - - 6,200 Nucynta sales reserve (2) - - (10,711) Nucynta and Lazanda revenue reserves (3) - - (1,166) Expenses for opioid-related litigation, investigations and regulations (4) - - 3,047 Intangible amortization related to product acquisitions 102,745 106,845 50,888 Inventory step-up related to product acquisitions - 15 - Contingent consideration related to product acquisitions (6,629) 2,287 (462) Stock based compensation 12,965 17,172 4,946 Interest income (410) (447) (164) Interest expense 78,190 87,088 35,078 Depreciation 2,757 2,530 2,929 Benefit from income taxes (1,429) 24,218 (445) Restructuring and other costs (5) 16,834 5,352 15,477 Acquired in process research and development 24,900 - - Gain on divestiture of Lazanda (17,064) - - Transaction costs 1,435 45 - Non-GAAP adjusted EBITDA 116,540 $ 156,385 $ 69,105 $ # of Employees 434 490 122 Adjusted EBITDA Per Employee 269 319 566 (1) Adjustment for inventory step-up to fair value in revenue recognized and COGS (2) Represents a $12.5 million benefit related to the release of sales reserves for which the Company is no longer financially responsible for, net of $1.8 million in royalties payable to Grunenthal (3) Removal of the impact of revenue reserve adjustment estimates consistent with opioid-related litigation and investigation expense treatment. (4) Legal costs/expenses related to opioid-related litigation, investigations and regulations pertaining to the Company's historical commercialization of opioid products (5) Restructuring and other costs represents non-recurring costs associated with the Company's restructuring, special meeting requests of an activist investor, CEO transition and an attempted debt refinancing. RECONCILIATION OF GAAP NET EARNING (LOSS) TO NON-GAAP ADJUSTED EBITDA Six Months Ended June 30, (unaudited) (unaudited) (in thousands)
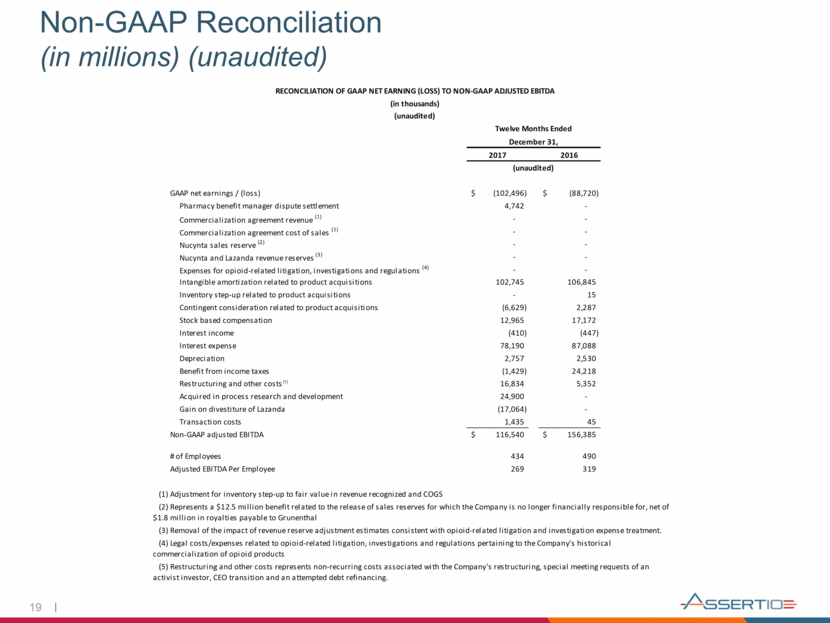
20 Non-GAAP Reconciliation (in millions) (unaudited) For the six months ended 2016 2017 June 30, 2018 GAAP as reported 204,498 $ 195,695 $ 60,341 $ Contingent consideration related to product acquisitions 120 7,708 541 Stock based compensation (16,633) (12,157) (4,849) Expenses for opioid-related litigation, investigations and regulations - - (3,047) Restructuring and Other costs (5,352) (3,587) (652) Non-GAAP adjusted (prior methodology) 182,633 $ 187,659 $ 52,334 $ For the twelve months ended December 31, RECONCILATIONS OF GAAP REPORTED TO NON-GAAP ADJUSTED INFORMATION (unaudited) (in thousands) Selling, general and administrative expense
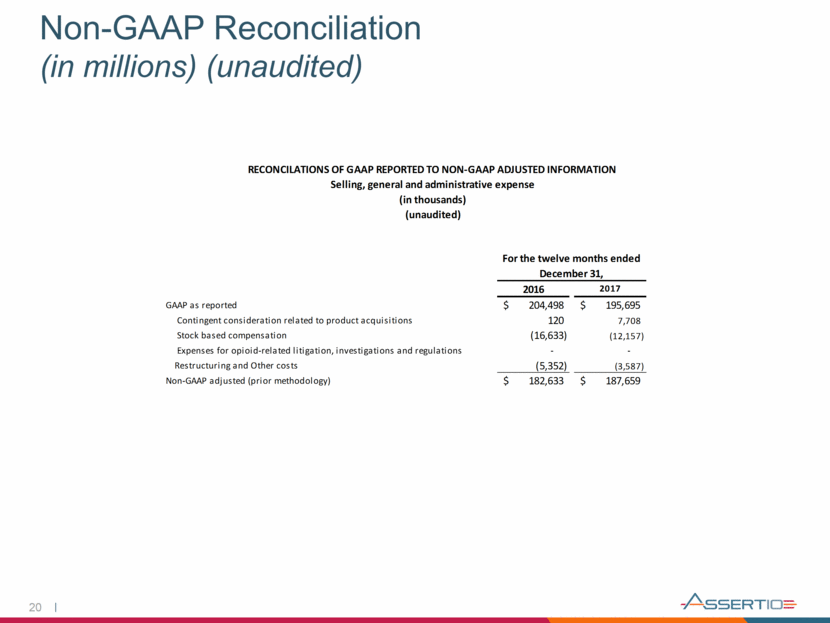
21 Full-Year 2018 Non-GAAP Guidance Reconciliation (in millions) (unaudited) Low End High End Low End High End Low End High End GAAP ($8) ($18) $9 $14 $118 $128 Specified Items (2) $153 $173 ($2) ($2) ($18) ($18) Non-GAAP $145 $155 $7 $12 $100 $110 (2) For purposes of this forward-looking reconciliation, a description of the categories of specified items included in this reconciliation are detailed in the slide titled "Note Regarding Use of GAAP and Non-GAAP Financial Measures". Full Year 2018 Guidance Earnings (1) R&D SG&A (1) GAAP Earnings guidance refers to GAAP Net Loss and Non-GAAP Earnings Guidance refers to Non-GAAP Adjusted EBITDA.
September 2018

