Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - EYEGATE PHARMACEUTICALS INC | tv502313_8k.htm |
Exhibit 99.1

NASDAQ: EYEG Investor Presentation Two Versatile Platforms Moving Towards Commercialization September 2018

Some of the matters discussed in this presentation contain forward - looking statements that involve significant risks and uncertainties, including statements relating to the prospects for the Company’s OBG and EGP - 437 product candidates , for the timing and outcome of the Company’s clinical trials, the potential approval to market OBG and EGP - 437 , and the Company’s capital needs . Actual events could differ materially from those projected in this presentation and the Company cautions investors not to rely on the forward - looking statements contained in, or made in connection with, the presentation . Among other things, the Company’s clinical trials may be delayed or may eventually be unsuccessful . The Company may consume more cash than it currently anticipates and faster than projected . Competitive products may reduce or eliminate the commercial opportunities of the Company’s product candidates . If the U . S . Food and Drug Administration or foreign regulatory agencies determine that the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them . Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rate due to changes in corporate priorities, the timing and outcomes of clinical trials, regulatory and developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities . If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly alter, delay, scale back or discontinue operations . Additional risks and uncertainties relating to the Company and its business can be found in the “Risk Factors” section of the Company’s Annual Report on Form 10 - K filed with the SEC on March 02 , 2018 . The Company undertakes no duty or obligation to update any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations, except as required by applicable law . The Company uses its website ( www . EyeGatePharma . com ), Facebook page ( https : //www . facebook . com/ EyeGatePharma / ), corporate Twitter account ( https : //twitter . com/EyeGatePharma ), and LinkedIn page ( https : //www . linkedin . com/company/ 135892 / ) as channels of distribution of information about the Company and its product candidates . Such information may be deemed material information, and the Company may use these channels to comply with its disclosure obligations under Regulation FD . Therefore, investors should monitor the Company’s website and its social media accounts in addition to following its press releases, SEC filings, public conference calls, and webcasts . The social media channels that the Company intends to use as a means of disclosing the information described above may be updated from time to time as listed on the Company’s investor relations website . Forward Looking Statements 2

Two ophthalmic platform technologies - FDA market authorization filings expected in 2019 Two separate clinical studies initiated for the OBG eye drop: PRK and PE (e.g. dry eye) EGP - 437’s Phase 3 Uveitis trial misses primary endpoint, data under review to assess strategic options OBG is the first and only Hyaluronic Acid eye drop in the U.S. targeting corneal wound healing OBG eye drop regulated as a device, accelerating the product development plan and time to market Bausch Health Companies Inc. licensed EGP - 437 and EyeGate® II Iontophoresis Delivery System combination product to commercialize for uveitis and post - operative ocular inflammation and pain Company Highlights (NASDAQ: EYEG) 3
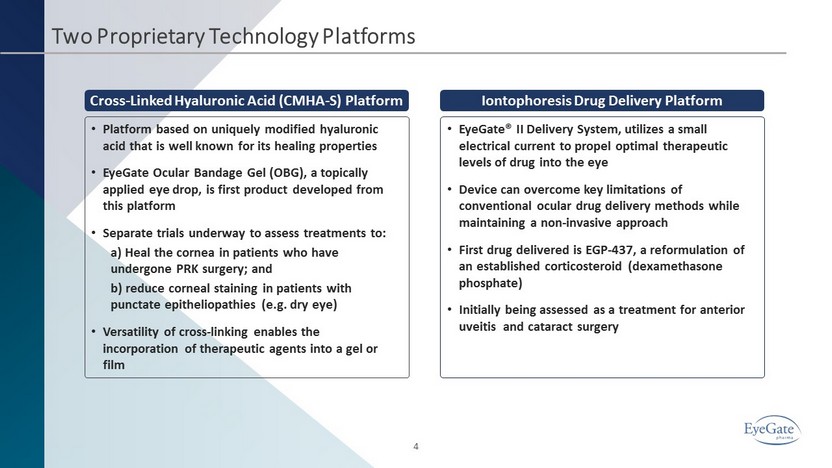
Two Proprietary Technology Platforms • Platform based on uniquely modified hyaluronic acid that is well known for its healing properties • EyeGate Ocular Bandage Gel (OBG), a topically applied eye drop, is first product developed from this platform • Separate trials underway to assess treatments to: a) Heal the cornea in patients who have undergone PRK surgery; and b) reduce corneal staining in patients with punctate epitheliopathies (e.g. dry eye) • Versatility of cross - linking enables the incorporation of therapeutic agents into a gel or film • EyeGate® II Delivery System, utilizes a small electrical current to propel optimal therapeutic levels of drug into the eye • Device can overcome key limitations of conventional ocular drug delivery methods while maintaining a non - invasive approach • First drug delivered is EGP - 437, a reformulation of an established corticosteroid (dexamethasone phosphate) • Initially being assessed as a treatment for anterior uveitis and cataract surgery 4 Cross - Linked Hyaluronic Acid (CMHA - S) Platform Iontophoresis Drug Delivery Platform
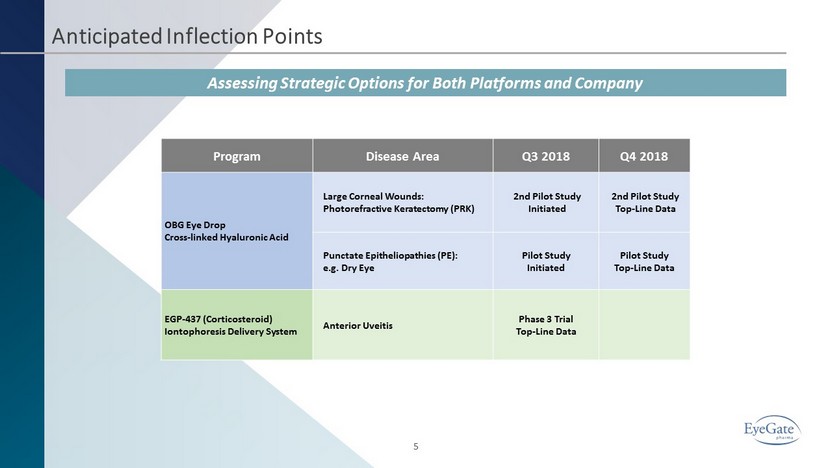
Anticipated Inflection Points 5 Program Disease Area Q3 2018 Q4 2018 OBG Eye Drop Cross - linked Hyaluronic Acid Large Corneal Wounds: Photorefractive Keratectomy (PRK) 2 nd Pilot Study Initiated 2 nd Pilot Study Top - Line Data Punctate Epitheliopathies (PE): e.g. Dry Eye Pilot Study Initiated Pilot Study Top - Line Data EGP - 437 (Corticosteroid) Iontophoresis Delivery System Anterior Uveitis Phase 3 Trial Top - Line Data Assessing Strategic Options for Both Platforms and Company

CMHA - S Platform Ocular Bandage Gel (OBG) Eye Drop

7 OBG: A Differentiated Product on a Rapid Path to Approval Proprietary Formulation of a Known and Trusted Substance Creates a Unique and Differentiated Product » Following a medical device pathway, filing expected in 2019 » Unique crosslinked HA produces a preservative - free, high concentration eye drop that resists degradation and adheres to the ocular surface without blurring vision » Hydrating, protectant and lubricant that facilitates management of corneal re - epithelialization » Addresses continued unmet medical need and research supports favorable economics » Positive results in human trial with two ongoing clinical studies for PRK and PE (dry eye)

8 Hyaluronic Acid (HA) HA is a Naturally Occurring Molecule that Possesses Beneficial Properties Hyaluronic Acid » Non - immunogenic: does not illicit an immune response » Binds up to 1,000 times its volume in water weight providing hydration and lubrication ideal for the ocular surface » Contributes to cellular proliferation and migration during the wound healing process » Approved in the U.S. for wound and burn management as well as osteoarthritis » A low concentration formulation (0.1% to 0.4%) is the Standard of Care in Europe and Asia for dry eye » Well known and trusted substance in US ophthalmic care

Ocular Bandage Gel (OBG) Eye Drop OBG is Differentiated by its Unique Cross - Linked, High Concentration Formulation of HA The unique cross - linked HA creates a 3D structure that stabilizes the molecule providing: Hyaluronic Acid Hyaluronic Acid Hyaluronic Acid Cross - Link 9 » A scaffolding matrix which protects the ocular surface » Higher viscosity and shear rate that thins during blinking for clear vision » A safe, well tolerated product » Resistance to degradation and prolonged retention on the ocular surface

10 » A clear, viscous, preservative - free eyedrop containing a high concentration (0.75%) of crosslinked hyaluronic acid (CMHA - S) » A long - acting lubricant that protects the corneal surface to promote healing and reduce staining associated with Punctate Epitheliopathies in dry eye patients » Demonstrated ability to accelerate re - epithelialization in PRK patients Ocular Bandage Gel (OBG) Eye Drop Efficacy of CMHA - S has Been Demonstrated in Various Animal Pathologic Conditions A B Molly: 12 year old cat with a non - healing corneal defect A. Non - healing at 42 days B. Ulcer healing after 12 days of using 0.75% CMHA - S » Post traumatic corneal stromal ulcers (real world dogs and cats) » Dry eye (veterinary dogs who failed topical cyclosporine) » Corneal abrasion and alkali burn injuries (rabbit models)
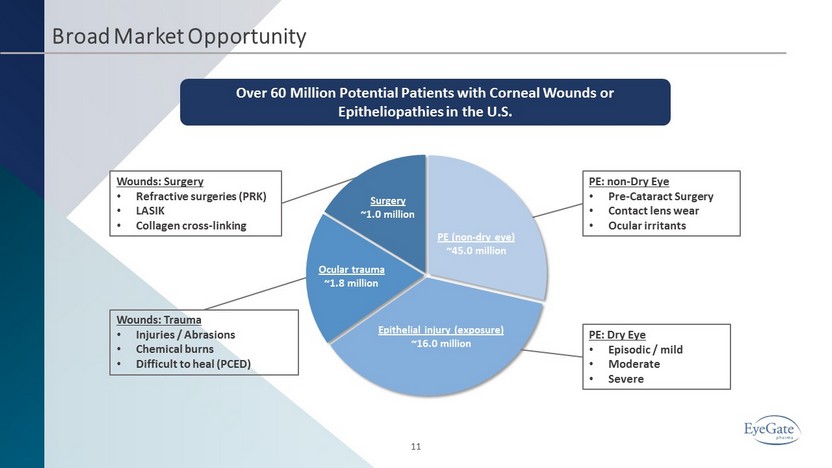
PE (non - dry eye) ~45.0 million Epithelial injury (exposure) ~16.0 million Ocular trauma ~1.8 million Surgery ~1.0 million Broad Market Opportunity 11 Wounds: Surgery • Refractive surgeries (PRK) • LASIK • Collagen cross - linking Wounds: Trauma • Injuries / Abrasions • Chemical burns • Difficult to heal (PCED) PE: Dry Eye • Episodic / mild • Moderate • Severe PE: non - Dry Eye • Pre - Cataract Surgery • Contact lens wear • Ocular irritants Over 60 Million Potential Patients with Corneal Wounds or Epitheliopathies in the U.S.

12 Increasing Prevalence of Dry Eye Disease with Favorable Economics 1. Gayton JL. Clin Ophthalmol . 2009;3:405 - 12.; 2. Report of the international dry eye workshop (DEWS). Ocul Surf. 2007;5(2):65 - 204.; 3. Allergan Dry Eye Survey, American Optometric Association, November 6, 2011. Dry Eye Disease is One of the Most Common Chronic Ophthalmologic Diseases 1,2,3 Payer Research, which Anticipates Generic Restasis, Supports Pricing in the Range of $125 - $225 Nearly half of all U.S. adults (48%) experience one or more dry eye symptoms regularly Half of all women (52%) experience one or more dry eye symptoms regularly 2 in 5 women age 45−54 who suffer from dry eye symptoms (42%) experience blurred vision 30% of men 55 and older have experienced dry eye symptoms for more than 10 years Primary focus is on the punctate epitheliopathy (dry eye) market » Patients are currently not adequately managed on artificial tears and/or adjunctive to Restasis and Xiidra » Physician research supports the need for additional treatment options and strong support for OBG profile in dry eye and wound management

Positive Clinical Trial Results 13 Completed First Human Clinical Trial in PRK Patients Healed Wound on Day 3 Day 1 Day 3 Number of Subjects Per Arm Number Percent Horizontal* Vertical* Horizontal Vertical Arm 1 Ocular Bandage Gel 12 10 83.3% 4.1 4.5 0.1 0.2 Arm 2 Ocular Bandage Gel + Bandage Contact Lens 14 9 64.3% 6.3 6.5 0.3 0.3 Arm 3 (Standard of Care) Bandage Contact Lens + Artificial Tears 13 7 53.8% 6.4 6.2 0.6 0.6 54.8% 35.9% 27.4% 83.3% 66.7% Ocular Bandage Gel: % Better than Bandage Contact Lens *Length in mm Re - epithelialization Wound Healing Study 1 : OBG vs. Standard of Care (Bandage Contact Lens + Artificial Tears) x Approximately 55% more patients treated with OBG healed by Day 3 x As early as Day 1 (24 hr. post - op) average wound size was around 36% smaller and 83.3% smaller by Day 3 with OBG alone 1. Open - label, unmasked study. Durrie et al. J Cataract Refract Surg. 2018 Mar;44(3):369 - 375.

Ongoing Clinical Trials Currently Enrolling for Two Studies for Management of Corneal Injury 14 Additional study for patients that have undergone bilateral Photorefractive Keratectomy (PRK) • Objective is to demonstrate re - epithelialization of large corneal epithelial defects • 45 subjects (3 arms): OBG (2 dosing regimens) vs standard of care (bandage contact lens + artificial tears) • Evaluated by a masked reading center (Tufts) using digital photography of fluorescein stained slit lamp photos • Top - line data expected in Q4 2018 New study for patients with Punctate Epitheliopathies (PE) • Objective is to demonstrate a decrease in Fluorescein staining of the cornea: targeting dry eye patients • 30 subjects (2 arms): OBG vs saline • 2 week screening period with wash - out / run - in: all patients stop taking Rx eye drops and receive saline only • Randomized after 2 week screening period if patient meets criteria • Study lasts 4 weeks post randomization: staining completed at Day 7, 14, 21 and 28 • Primary performance is change in corneal staining from baseline to each visit between both arms • Top - line data expected in Q4 2018

Iontophoresis Platform EyeGate® II Delivery System and EGP - 437

EyeGate® II Delivery System and EGP - 437 16 A Non - Invasive Method of Propelling Charged Active Compounds into Ocular Tissues EGP - 437 , a reformulated corticosteroid, Dexamethasone Sodium Phosphate is delivered into the ocular tissues through EyeGate’s proprietary innovative drug delivery system, the EyeGate® II Delivery System A. Applicator B. Small electrical current at electrode C. Charged drug product (in applicator) D. Active product propelled into the eye E. Eye receiving drug product noninvasively Dose is controlled by current strength and application time Drug Product A. C. B. E. D.

A Highly Differentiated Product 17 The current Standard of Care for both Anterior Uveitis and Cataract Surgery are corticosteroid eye drops The EyeGate®II Delivery System Combined with EGP - 437 Significantly Decreases the Patient Burden by Reducing Treatment Applications by up to 98% The EyeGate® II Delivery System takes less than 5 minutes to administer and over 2,400 treatments performed to date VS 2 to 3 EyeGate treatments Up to 154 eye drop treatments

Exclusive Licensing Agreement with Bausch Health (Formerly Valeant Pharmaceuticals) 18 Worldwide License to Manufacture, Sell, Distribute, and Commercialize EGP - 437 with the EyeGate® II Delivery System for Uveitis and Post - Operative Ocular Inflammation and Pain $136.5 million in potential payments, including up - front, development and commercial milestones Anterior Uveitis • $1 million up - front payment • Up to $32.5 million development and commercials milestones Cataract Surgery • $4 million up - front payment • Up to $99 million development and commercials milestones High Single Digit Royalties based on net sales with upward adjustment to double - digit for cataract surgery EyeGate is responsible for the completion of clinical development and FDA filing for both indications. Bausch holds no rights to “use of system” with other drugs.

19 EGP - 437 Clinical Data Results Inflammation Post Cataract Surgery Phase 2 Clinical Trial Double - masked, placebo - controlled, two - arms, 101 subjects 5.9% 13.7% 27.5% 43.1% 52.9% 4% 10% 24% 32% 36% Day 1 Day 4 Day 7 Day 14 Day 28 EGP-437 (N=51) Vehicle (N=50) Proportion with Total Cell Clearing = 0 x EGP - 437 demonstrated better clinical performance than the vehicle control trending towards statistical significance (p = 0.08) x Secondary endpoints: change in mean cell count and change in mean pain score • Total Cell Clearing count = 0 on Day 7 (p=0.0096) • Pain Score = 0 on Day 1 (p=0.0149) x EGP - 437 arm demonstrated a favorable safety profile with no serious adverse events reported » Non - inferiority was not demonstrated between EGP - 437 and Control (the lower limit of the two - sided 95% confidence interval (CI) for the difference is less than - 10%) » Control group had higher rate of success (ACC count=zero) than the EGP - 437 • The Chi - square test shows significant difference between Control and EGP - 437, preferring the Control group Anterior Uveitis Phase 3 Non - Inferiority Trial Missed Primary Endpoint on Day 15 N Test EGP - 437 Zero Control Zero EGP - Control, Two - Sided 95% CI p - value 1 / p - value 2 251 patients ACC Count 53 (42.4%) 75 (60.3%) ( - 30.25%, - 5.55%) 0.8951/ 0.0052

The Future of Ocular Drug Delivery: Drug - Embedded Contact Lens 20 Potential to Revolutionize the Treatment of Chronic Retinal Conditions at Home by Significantly Reducing or Eliminating Intravitreal Injections and Frequent Office Visits Two Layer Lens Layer 1 : Sits on eye surface with loaded drug Layer 2 : Covers Layer 1 and incorporates iontophoresis electronics First Indication Dexamethasone for macular edema

Two ophthalmic platform technologies - FDA market authorization filings expected in 2019 Two separate clinical studies initiated for the OBG eye drop: PRK and PE (e.g. dry eye) EGP - 437’s Phase 3 Uveitis trial misses primary endpoint, data under review to assess strategic options OBG is the first and only Hyaluronic Acid eye drop in the U.S. targeting corneal wound healing OBG eye drop regulated as a device, accelerating the product development plan and time to market Bausch Health Companies Inc. licensed EGP - 437 and EyeGate® II Iontophoresis Delivery System combination product to commercialize for uveitis and post - operative ocular inflammation and pain Company Highlights (NASDAQ: EYEG) 21

Thank You NASDAQ: EYEG Contact Us Joseph Green Edison Advisors Investor Relations Tel: (646) 653 - 7030 E - mail: jgreen@edisongroup.com
