Attached files
As filed with the Securities and Exchange Commission on August 28, 2018
Registration No. 333-226536
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1 To
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Elanco Animal Health Incorporated
(Exact name of registrant as specified in its charter)
| Indiana (State or Other Jurisdiction of Incorporation or Organization) |
2834 (Primary Standard Industrial Classification Code Number) |
82-5497352 (I.R.S. Employer Identification Number) |
2500 Innovation Way
Greenfield, Indiana
46140
(877) 352-6261
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant's Principal Executive Offices)
Michael-Bryant Hicks, Esq.
2500 Innovation Way
Greenfield, Indiana
46140
(877) 352-6261
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent For Service)
| Corey R. Chivers, Esq. Weil, Gotshal & Manges LLP 767 Fifth Avenue New York, New York 10153 (212) 310-8000 (Phone) (212) 310-8007 (Fax) |
Patrick O'Brien Paul Kinsella Tara Fisher Ropes & Gray LLP Prudential Tower 800 Boylston Street Boston, Massachusetts 02199 (617) 951-7000 (Phone) |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company" and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer ý |
Smaller reporting company o Emerging growth company o |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. o
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to Completion, Dated August 28, 2018
Shares

Elanco Animal Health Incorporated
Common Stock
This is an initial public offering of shares of common stock of Elanco Animal Health Incorporated. We are offering shares of our common stock in this offering.
Prior to this offering, there has been no public market for our common stock. It is currently estimated that the initial public offering price per share will be between $ and $ . We intend to apply to have our common stock listed on the New York Stock Exchange ("NYSE") under the symbol "ELAN."
Following this offering, we will be a "controlled company" within the meaning of the corporate governance rules of the NYSE. See "Management — Director Independence and Controlled Company Exemption."
See "Risk Factors" beginning on page 22 to read about factors you should consider before buying shares of our common stock.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
|
Per Share | Total |
|||
| | | | | | |
Initial public offering price |
$ | $ | |||
Underwriting discount(1) |
$ | $ | |||
Proceeds, before expenses, to Elanco |
$ | $ |
- (1)
- We have agreed to reimburse the underwriters for certain expenses in connection with this offering. We refer you to "Underwriting," beginning on page 193 of this prospectus, for additional information regarding total underwriter compensation.
To the extent that the underwriters sell more than shares of common stock, the underwriters have the option to purchase up to an additional shares from us at the initial price to the public less the underwriting discount.
The underwriters expect to deliver the shares to investors against payment in New York, New York on , 2018.
| Goldman Sachs & Co. LLC | J.P. Morgan | Morgan Stanley |
| Barclays | BNP PARIBAS | Citigroup | Credit Suisse | Deutsche Bank Securities |
| Evercore ISI | Cowen |
Prospectus dated ,
TABLE OF CONTENTS
Prospectus
Through and including , 2018 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer's obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
You should rely only on the information contained in this prospectus or in any free writing prospectus we may specifically authorize to be delivered or made available to you. Neither we nor the underwriters (nor any of our or their respective affiliates) have authorized anyone to provide any information other than that contained in this prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. Neither we nor the underwriters (nor any of our or their respective affiliates) take any responsibility for, and neither we nor they provide any assurance as to the reliability of, any other information that others may give you. Neither we nor the underwriters (nor any of our or their respective affiliates) are making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus or any free writing prospectus is only accurate as of its date, regardless of its time of delivery or the time of any sale of shares of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
i
Unless the context requires otherwise: (a) references to "Elanco," our "company," "we," "us" or "our" refer to Elanco Animal Health Incorporated, an Indiana corporation, and its subsidiaries after giving effect to the transactions described under "The Separation and Distribution Transactions — The Separation" or for periods prior to such transactions, the combined businesses operating within Lilly's Elanco animal health division that have been or will be contributed to Elanco as part of such transactions, and (b) references to "Lilly" refer to Eli Lilly and Company, an Indiana corporation, and its subsidiaries other than Elanco.
Market and Industry Information
Unless otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate, including our general expectations and market position, market opportunity and market share, is based on information from third-party sources and management estimates. Certain statements, where indicated, are based on information published by Vetnosis Limited ("Vetnosis"), a research and consulting firm specializing in global animal health and veterinary medicine, and management estimates. Our management estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. Our management estimates have not been verified by any independent source. In addition, assumptions and estimates of our and our industry's future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in "Risk Factors." These and other factors could cause future performance to differ materially from our assumptions and estimates. See "Cautionary Note Regarding Forward-Looking Statements."
Trademarks and Trade Names
The name and mark, Elanco, and other trademarks, trade names and service marks of Elanco appearing in this prospectus are the property of Elanco or, as applicable, licensed to Elanco, or, as applicable, prior to the completion of this offering, are the property of Lilly. The name and mark, Eli Lilly and Company, and other trademarks, trade names and service marks of Lilly appearing in this prospectus are the property of Lilly. This prospectus also contains additional trade names, trademarks and service marks belonging to other companies. We do not intend our use or display of other parties' trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties.
ii
This summary highlights information included elsewhere in this prospectus and does not contain all of the information you should consider in making an investment decision. You should read this entire prospectus carefully, including the sections entitled "Risk Factors," "Cautionary Note Regarding Forward-Looking Statements," "Selected Historical Combined Financial Data," "Unaudited Pro Forma Condensed Combined Financial Statements" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our combined financial statements and the notes thereto before making an investment decision regarding our common stock.
Overview
Founded in 1954 as part of Eli Lilly and Company, Elanco is a premier animal health company that innovates, develops, manufactures and markets products for companion and food animals. Headquartered in Greenfield, Indiana, we are the fourth largest animal health company in the world, with revenue of $2.9 billion for the year ended December 31, 2017. Globally, we are #1 in medicinal feed additives, #2 in poultry and #3 in cattle, measured by 2017 revenue, according to Vetnosis. We also have one of the broadest portfolios of pet parasiticides in the companion animal sector. We offer a diverse portfolio of more than 125 brands that make us a trusted partner to veterinarians and food animal producers in more than 90 countries.
Our vision is to enrich the lives of people through food — making protein more accessible and affordable — and through pet companionship — helping pets live longer, healthier lives. We advance our vision by offering products in four primary categories:
- •
- Companion Animal Disease Prevention ("CA Disease Prevention"): We have one
of the broadest parasiticide portfolios in the companion animal sector based on indications, species and formulations, with products that protect pets from worms, fleas and ticks. Combining our
parasiticide portfolio with our vaccines presence, we are a leader in the U.S. in the disease prevention category based on share of revenue.
- •
- Companion Animal Therapeutics ("CA Therapeutics"): We have a broad pain and
osteoarthritis portfolio across species, modes of action, indications and disease stages. Pet owners are increasingly treating osteoarthritis in their pets, and our Galliprant product is one of the
fastest growing osteoarthritis treatments in the U.S. We also have treatments for otitis (ear infections), as well as
cardiovascular and dermatology indications.
- •
- Food Animal Future Protein & Health ("FA Future Protein &
Health"): Our portfolio in this category, which includes vaccines, nutritional enzymes and animal-only antibiotics, serves the growing demand for
protein and includes innovative products in poultry and aquaculture production, where demand for animal health products is outpacing overall industry growth. We are focused on developing functional
nutritional health products that promote food animal health, including enzymes, probiotics and prebiotics. We are a leader in providing vaccines as alternatives to antibiotics to promote animal health
based on share of revenue.
- •
- Food Animal Ruminants & Swine ("FA Ruminants & Swine"): We have developed a range of food animal products used extensively in ruminant (e.g., cattle, sheep and goats) and swine production. We also deliver value to producers beyond our products through our technical expertise and support.
1
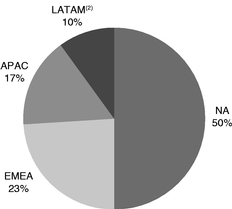
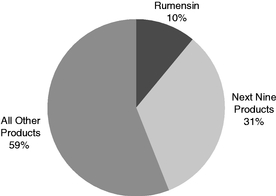
We have a top four presence in all four key industry geographic regions: North America ("NA"); Europe, the Middle East and Africa ("EMEA"); Latin America ("LATAM"); and Asia-Pacific ("APAC"), as measured by 2017 revenue, according to Vetnosis. The following graphs demonstrate our revenue for the year ended December 31, 2017 by product category, geography and our highest revenue products:
Percentage of 2017 Revenue
By Product Category(1)
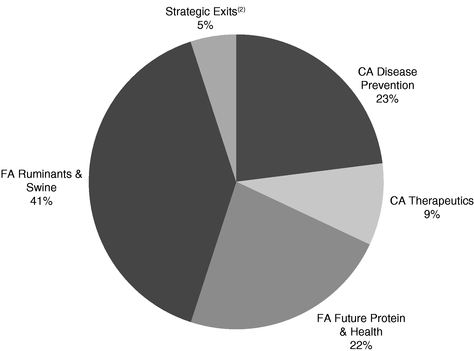
- (1)
- Certain percentages may reflect rounding adjustments.
- (2)
- Strategic Exits includes revenue from third-party manufacturing, distribution and other contractual arrangements, as well as an equine product not core to our business, which we have either exited or made the decision to exit.
| |
|
|
|---|---|---|
| Percentage of 2017 Revenue By Region(1) |
Percentage of 2017 Revenue By Highest Revenue Products(1) |
|
|
|
- (1)
- Certain
percentages may reflect rounding adjustments.
- (2)
- LATAM includes aquaculture in all regions.
2
Through our global sales force of approximately 1,530 sales representatives, our veterinary consultants and our key distributors, we seek to build strong customer relationships and fulfill demand for our food animal products primarily with food animal producers, veterinarians and nutritionists, and for our companion animal products primarily with veterinarians and, in some markets, pet owners. We are also expanding into retail channels in order to meet pet owners where they want to purchase.
Our inclusive approach to sourcing innovation helps us identify, attract, fund and develop new ideas that enhance our pipeline and reduce risk as compared to an in-house only approach. Through this process, we launched nine products from 2015 to 2017 that delivered $143.8 million of revenue in 2017 and $136.6 million of revenue in the first half of 2018.
We believe we have an experienced leadership team that fosters an adaptive, purpose-driven culture among approximately 5,880 employees worldwide as of June 30, 2018 and that our employees share a deep conviction for achieving our vision of food and companionship, enriching life.
For the six months ended June 30, 2018 and 2017, our revenue was $1.5 billion and $1.4 billion, respectively, and for each of the years ended December 31, 2017, 2016 and 2015, our revenue was $2.9 billion. For the six months ended June 30, 2018 and 2017, our net income (loss) was $9.9 million and $(128.5) million, respectively, our adjusted EBITDA was $306.2 million and $278.4 million, respectively, and our adjusted net income was $219.0 million and $156.4 million, respectively. For the years ended December 31, 2017, 2016 and 2015, our net income (loss) was $(310.7) million, $(47.9) million and $(210.8) million, respectively, our adjusted EBITDA was $498.9 million, $540.4 million and $393.7 million, respectively, and our adjusted net income was $250.5 million, $332.7 million and $208.7 million, respectively. For a reconciliation of adjusted EBITDA and adjusted net income to net income (loss), see "— Summary Historical Combined Financial Data and Unaudited Pro Forma Condensed Combined Financial Data."
Industry
Animal Health Industry Overview
Global animal health industry revenue is projected to grow nominally at a compound annual growth rate ("CAGR") of 5% from 2017 to 2023, according to Vetnosis. Importantly, this growing industry, which includes both food and companion animals, benefits billions of people worldwide. The food animal sector focuses on species raised to provide animal protein, such as cattle, other ruminants (e.g., sheep and goats), swine, poultry and aqua. The companion animal — or pet — sector focuses primarily on dogs and cats.
Animal health medicines, vaccines and functional nutritionals represent an estimated global market of $34.3 billion, based on 2017 revenue, according to industry sources. Medicines and vaccines represent a global market of $32.0 billion, based on 2017 revenue, and grew at a CAGR of 4% from 2007 to 2017, according to Vetnosis. Management expects this trend to continue through at least 2023 based on industry projections. Functional nutritionals (specifically enzymes, probiotics and prebiotics) used in food animal production represent a global market of $2.3 billion, according to industry sources. Based on industry projections, management expects functional nutritionals to grow faster than the medicines and vaccines market.
Food Animal. Food animal medicines and vaccines, including aquaculture, represented $21.2 billion of revenue in 2017 and grew at a CAGR of 4% from 2007 to 2017, according to Vetnosis.
3
Factors influencing growth in demand for food animal medicines and vaccines include:
- •
- one in three people needs improved nutrition;
- •
- increased global demand for protein, particularly poultry and aquaculture;
- •
- natural resource constraints, such as scarcity of arable land, fresh water and increased competition for cultivated land, driving the need for
more efficient food production;
- •
- loss of productivity due to food animal disease and death;
- •
- increased focus on food safety and food security; and
- •
- human population growth, increased standards of living, particularly in many emerging markets, and increased urbanization.
Functional nutritionals used in food animal production represent an additional market estimated at $2.3 billion. Growth in functional nutritionals is influenced, among other factors, by demand for antibiotic alternatives that can promote animal health and increase productivity.
Companion Animal. Companion animal medicines and vaccines represented $10.8 billion of revenue in 2017 and grew at a CAGR of 4% from 2007 to 2017, according to Vetnosis.
Factors influencing growth in demand for companion animal medicines and vaccines include:
- •
- increased pet ownership globally;
- •
- pets living longer; and
- •
- increased pet spending as pets are viewed as members of the family by owners.
Key Structural Characteristics of the Animal Health Industry
- •
- Brands often have long, sustainable
value. Branded animal health products often retain significant, and occasionally increased, market share after many years on the market, even
after the loss of patent protection. As an example, five of our top 10 products, based on 2017 revenue, have been on the market for over 25 years. In the food animal sector, the level of
competition is influenced by macro-economic factors, brand loyalty, distribution models and the absence of governmental or third-party payer systems. In the companion animal sector, competition is
influenced by brand loyalty, new innovation, relationships with veterinarians, channel expansion and the overall growth in pet ownership.
- •
- Diversified product
portfolios. Animal health companies often derive their revenue from dozens, if not hundreds, of products and are frequently not dependent on a
select few flagship products. For example, our top 10 products accounted for only 41% of revenue in 2017. We believe companies with diversified global companion and food animal product portfolios can
be more resilient to changing market dynamics and are structured to better balance potential geographic, product and species volatility.
- •
- Deep customer
relationships. Direct customer models allow animal health sales representatives and veterinary consultants to develop a deep understanding of
customer needs, which often facilitate strong and impactful relationships. Representatives and consultants frequently partner with customers through product support and analytics, driving additional
value for the customer.
- •
- Fast and efficient R&D model. Product approvals typically require a limited number of targeted studies in animals, which moderates research expenses. The approval process is generally predictable given the number of studies required, leading to average timelines
4
- •
- Self-pay market. Food animal producers, pet owners and veterinarians typically pay for products out of pocket, making them the primary decision makers. This results in manufacturers being able to price products based primarily on the end customer's realized value.
from initiation of development to approval of three to seven years at a cost of $50 million to $100 million.
Our Competitive Strengths
We believe the following strengths create sustainable competitive advantages that will enable us to continue to grow as a leader in the animal health industry.
- •
- Established leader with a global presence and diversified product
portfolio. We are the fourth largest animal health company in the world, with revenue of $2.9 billion for the year ended
December 31, 2017. Globally, we are #1 in medicinal feed additives, #2 in poultry and #3 in cattle, as measured by 2017 revenue, according to Vetnosis. We also have one of the broadest
portfolios of pet parasiticides in the companion animal sector, based on indications, species and formulations. We have a top four presence in all four key geographic regions (NA, EMEA, LATAM and
APAC), as measured by 2017 revenue, according to Vetnosis, including a strong presence in the emerging markets of Brazil, Thailand, China and Mexico. We have a comprehensive and diversified product
portfolio, with more than 125 brands sold in more than 90 countries. In 2017, our top 10 products accounted for 41% of our revenue, with our top selling product accounting for approximately 10% of our
revenue. Our global footprint includes a direct commercial presence in 62 countries, which we have plans to reduce to fewer than 50 countries, and third-party distribution relationships
serving other relevant markets. Of our approximately 1,530 sales representatives as of June 30, 2018, two-thirds were based outside of North America.
- •
- Strategically positioned to drive innovation and growth in our three targeted growth
categories. Over the past 10 years, we have intentionally transformed Elanco from a food animal focused company into a diversified global company. In addition to our FA
Ruminants & Swine category, we now have established positions in our three targeted growth categories: CA Disease Prevention, CA Therapeutics and FA Future Protein & Health. To achieve
this, among other steps, we have made strategic acquisitions to expand our product portfolio, increase our sales presence globally and obtain R&D and manufacturing capabilities in these categories.
Recent acquisitions include the animal health business of Janssen Pharmaceutica NV, a subsidiary of Johnson and Johnson Company ("Janssen Animal Health"), ChemGen Corp. ("ChemGen"), Lohmann SE
("Lohmann Animal Health"), the animal health business of Novartis AG ("Novartis Animal Health") and the U.S. feline and canine vaccines portfolio of Boehringer Ingelheim Vetmedica, Inc. (the
"BI Vetmedica U.S. vaccines portfolio"). See "Business — Company History." As a result of these acquisitions as well as organic growth, we have grown our companion animal
categories, from a minimal presence in 2007 to more than $900 million in revenue in 2017. We believe that as a result of establishing a strong presence in our targeted growth categories, which
feature favorable industry dynamics, we are strategically positioned to grow our revenue and increase profitability.
- •
- Strength of brands and relationships in our FA Ruminants & Swine category. We provide a range of products for use in ruminant and swine production that we believe have created strong, long-standing customer relationships and provide an important revenue source for our business and for investment capital to support future growth. We have well-established Elanco brands in this category such as Rumensin, a leading cattle feed additive that has
5
- •
- Proven track record of innovation and product launches. We have developed in-house R&D capabilities in the chemical sciences and life sciences, which enable us to discover and develop vaccines and small and large molecules in our targeted areas. We also have an R&D platform that enables us to discover, develop and evaluate future nutritional health opportunities in enzymes, probiotics and prebiotics. Beyond our strong in-house R&D, we also access ideas and innovation from a broad array of sources. This inclusive approach to innovation allows us to identify, attract, fund and develop new ideas in a manner that enhances our pipeline while, we believe, reducing the risk associated with an in-house only innovation model. As a result, we launched nine products from 2015 to 2017 that delivered revenue of $24.7 million in 2015, $97.9 million in 2016, $143.8 million in 2017 and $136.6 million in the first half of 2018. We believe our new products will be an important source of future revenue.
been used for more than 40 years to improve feed efficiency and control coccidiosis. In addition, our technical expertise and analytics help us deliver value to our customers beyond our products. Our analytics help producers analyze large amounts of health and production data, turning that data into actionable information that helps them improve the health of their animals and, as a result, their productivity and profitability. We believe our brands and additional customer support have helped us create broad name recognition and a high level of trust among target customers, which is important to the success of our food animal products. We expect to continue to be a leader in FA Ruminants & Swine.
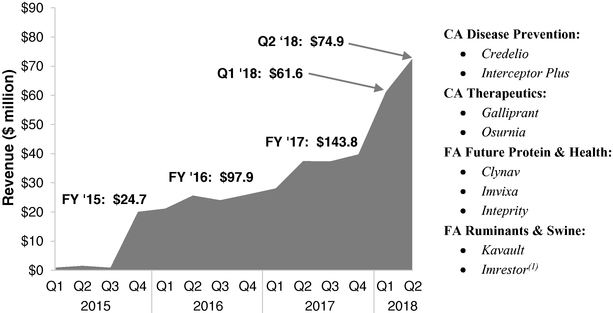
- (1)
- We
suspended commercialization of Imrestor in the second quarter of 2018 and plan to pursue additional indications.
Revenues from Imrestor were $6.5 million for the year ended December 31, 2017 and $1.0 million for the six months ended
June 30, 2018.
Three of these products were developed following the traditional in-house model, while the other products were obtained through an acquisition or venture capital investment. These launches are evidence of our ability to identify innovation from diverse sources and develop them into distinctive products in our targeted categories. They include: Credelio, for the treatment and elimination of fleas and ticks in dogs and puppies; Interceptor Plus, for the prevention of heartworm disease and treatment and control of other endoparasite infections
6
- •
- Expertise in driving cost efficiencies and
productivity. In the last 10 years, we have successfully integrated 10 businesses, including businesses acquired within the last four
years with an aggregate of 4,500 full-time employees, 12 manufacturing sites and eight R&D sites. These acquisitions had a negative impact on operating margins and over the last three years, we have
identified and executed a number of initiatives which improved our operational efficiency and positively impacted our operating margins. Through the reduction of manufacturing and R&D sites, headcount
rationalization, focused procurement initiatives, sales force organizational design and the establishment of an integration center of excellence, we estimate that we delivered more than
$500 million in annualized cost savings from the beginning of 2015 through the end of 2017. Since 2015, in manufacturing we have closed three sites, reduced headcount from approximately 3,500
to approximately 2,330 employees and eliminated over 2,600 stock keeping units, or SKUs (we currently supply approximately 4,400 SKUs). Drawing on these experiences, we are currently executing
additional productivity initiatives throughout the organization that we believe will materially strengthen the margin profile of our business over time.
- •
- Experienced management team and dedicated employees. Our executive management team is comprised of a group of leaders with diverse backgrounds and extensive experience across global animal health and related industries. We believe their experience has provided organizational capabilities to support our targeted growth strategies and helped us create a legacy of growth and transformation in a dynamic industry. Our executives have taken an active role in important initiatives shaping the animal health industry. We also believe we have a loyal, highly engaged, customer-focused and cause-oriented professional workforce. We have recently strengthened our management team by adding executive officers with extensive public company experience.
in dogs and puppies; Galliprant, for the treatment of canine osteoarthritis pain and inflammation; Osurnia, for the treatment of otitis externa in dogs; Clynav, for the immunization of Atlantic salmon against pancreas disease; Imvixa, for the prevention and control of sea lice; Inteprity, for the prevention of mortality caused by necrotic enteritis in broiler chickens; Kavault, for the reduction of diarrhea in weaned pigs; and Imrestor, which we suspended commercialization of in the second quarter of 2018, for the reduction of incidence of clinical mastitis in periparturient dairy cows. In 2017, Clynav and Galliprant were named best food animal and companion animal products, respectively, by Animal Pharm. We currently have R&D projects relating to 36 potential new product innovations (which we define as new chemical entities, new combinations or significant line extensions), which we are investigating as candidates for potential new product launches through 2023. We believe our approach to innovation will enable us to create and maintain an attractive pipeline of novel products.
Our Targeted Value-Generating Strategies
We intend to continue to grow our business and create value for our shareholders through a targeted value-generating strategy with three key pillars: a Portfolio Strategy for our marketed
7
products, an Innovation Strategy for our R&D pipeline and a Productivity Strategy for our margin expansion initiatives.
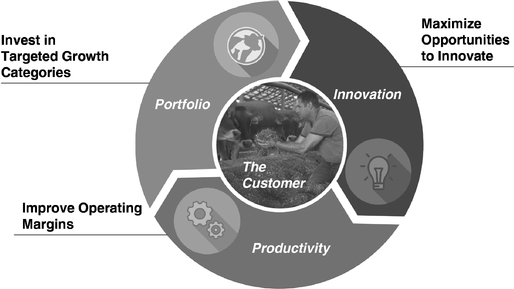
Portfolio Strategy
- •
- Invest in categories with the greatest potential for
growth. We are focusing the majority of our resources, including more than 75% of our R&D funding, on our three targeted growth categories: CA
Disease Prevention, CA Therapeutics and FA Future Protein & Health, where we believe we are well positioned to grow faster than the market. These categories represented 54% of our revenue in
2017.
- •
- CA Disease Prevention — Parasiticides and vaccines are
fundamental to preventing disease in companion animals. We have a strong vaccines portfolio as well as products that protect pets from a broad spectrum of parasites, such as fleas, ticks, heartworms,
roundworms, hookworms, whipworms and tapeworms. We believe we are well positioned to drive additional growth through continued product innovation and sales channel expansion.
- •
- CA Therapeutics — Pets are living longer and owners
increasingly seek treatments for chronic diseases in their pets. To capitalize on these trends, we are focused on driving growth in our CA Therapeutics category by building on our broad base of pain
and osteoarthritis products.
- •
- FA Future Protein & Health — We expect to drive revenue
growth through our poultry and aquaculture portfolios. Poultry and aquaculture are expected to be among the fastest growing animal health protein sources over the next 10 years. We also are
focused on nutritional health products and antibiotic stewardship that address market trends in this category.
- •
- Reinforce our strong presence in our FA Ruminants & Swine category. We plan to continue fortifying our long-standing FA Ruminants & Swine category to meet our customers' needs through targeted product investment and by continuing to strengthen our deep business-to-business relationships through sales force excellence and leadership in industry coalitions. We also plan to continue to utilize analytics, social media and other support to provide value to our customers beyond our products.
8
Innovation Strategy
- •
- Maximize opportunities to innovate within targeted
platforms. Our R&D efforts focus on six areas across our companion and food animal categories where science and our capabilities best match
market opportunities and meet customer needs.
- •
- Companion Animal — We are targeting therapeutics, vaccines and
parasiticides.
- •
- Therapeutics — We are focused on continuing to discover and develop products in areas where we currently
compete such as dermatology, otitis and pain. We are also pursuing novel targets to address unmet needs for chronic conditions in dogs and cats.
- •
- Vaccines — We have a competitive line of core canine, feline and rabies vaccines that we are developing for
expansion into geographies outside the U.S. We are also developing novel delivery technologies for companion animal vaccines, building on the success of the formulation innovation of our current
product line.
- •
- Parasiticides — We leverage proprietary active ingredients to develop and commercialize novel products with
endoparasite and ectoparasite efficacy through combinations and novel formulations. We are also actively pursuing products with novel mechanisms of action to introduce innovation in this category.
- •
- Food Animal — We are targeting pharmaceuticals, vaccines and
the emerging nutritional health space.
- •
- Pharmaceuticals — We focus efforts in discovery and development of novel pharmaceutical and biopharmaceutical
products that could be effective alternatives to antibiotics or address other health challenges encountered in livestock production.
- •
- Vaccines — We have active vaccine R&D programs to discover and develop products to address bacterial and viral
threats in poultry, swine, cattle and fish.
- •
- Nutritional Health — Building on our enzyme product platform and the success of Hemicell, we are targeting R&D
efforts in nutritional health to deliver new products that improve gut health and performance in livestock. We focus on
the role and composition of the microbiome on the health and digestive performance of the animal and look to introduce new products that are enzymes, probiotics or prebiotics.
- •
- Inclusive approach to sourcing innovation. We have a build, buy or ally strategy to identify, attract and develop new ideas in our six R&D focus areas in a manner intended to reduce risk and sustain our pipeline. In addition to traditional corporate R&D, we pursue in-licensing and partnering activities, actively and selectively engaging in funding models that include venture capital, project financing and crowdsourced innovation. This strategy gives us access to a wider range of novel ideas and increases our ability to bring innovative products to market compared to an in-house only model.
Productivity Strategy
- •
- Leverage our productivity capabilities to improve operating margins. We estimate that from the beginning of 2015 through the end of 2017, we generated more than $500 million in annualized cost savings through our productivity initiatives, including the integration of three major acquisitions. Leveraging this track record of productivity improvements and cost
9
- •
- Manufacturing efficiency and cost savings. We plan to continue to execute on
initiatives we have identified to improve manufacturing processes, reduce our manufacturing footprint, advance lean initiatives, consolidate our contract manufacturing organization ("CMO") network,
strategically insource projects and pursue cost savings opportunities for raw materials through a new procurement process. We also plan to leverage our extensive integration experience to continue
identifying cost-savings and delivering on our margin expansion objectives.
- •
- SG&A excellence. Our sales strategy is focused on achieving growth in our targeted product categories while increasing productivity within our sales force. We plan to utilize both our sales force's strong customer relationships and our strategic distributor partnerships to efficiently grow demand for our products. We also have a targeted procurement initiative and are in the process of implementing a G&A steady state organizational design to reduce overhead costs and simplify infrastructure following the termination of our transitional service agreement with Lilly.
savings, we aim to significantly increase our operating margins over time through our initiatives in manufacturing and SG&A. Our productivity strategies include:
Risks Associated with Investing in Our Common Stock
Investing in our common stock involves a number of risks. These risks include, but are not limited to, challenges related to the Separation (as defined below in "—The Separation"), the successful implementation of our strategy and the ability to grow our business. Some of these risks are:
Risks Related to the Separation
- •
- We will incur significant charges in connection with this offering and the Separation and incremental costs as a standalone public company,
including due to replicating or replacing certain functions, systems and infrastructure to which we will no longer have the same access after this offering. When we begin to operate these functions
separately, if we do not have our own adequate systems and business functions in place, or are unable to obtain them from other providers, we may not be able to operate our business effectively or at
comparable costs to the costs of services received under our transitional services agreement with Lilly. See "Certain Relationships and Related Party Transactions — Transitional
Services Agreement."
- •
- Our historical combined financial data is not necessarily representative of the results we would have achieved as a standalone company and may
not be a reliable indicator of our future results. For example, our historical combined financial data reflects expense allocations for certain support functions that are provided on a centralized
basis within Lilly, such as expenses for executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations that
may be higher or lower than the comparable expenses we would have actually incurred, or will incur in the future, as a standalone company.
- •
- We may not be able to replace the services provided by Lilly under the transitional services agreement or enter into appropriate third-party agreements on terms and conditions, including cost, comparable to those that we will receive from Lilly under our transitional services agreement. Additionally, after the transitional services agreement terminates, we may be unable to sustain the services at the same levels or obtain the same benefits as when we were receiving such services and benefits from Lilly. Due to the scope and complexity of the underlying projects relative to these efforts, the amount of total costs
10
- •
- As a result of the Separation, we will lose Lilly's brand, reputation, capital base and other resources, and may experience difficulty
operating as a standalone company. The loss of Lilly's scale, capital base and financial strength may prompt suppliers to reprice, modify or terminate their relationships with us, and Lilly's
reduction of its ownership of our company could potentially cause some of our existing agreements and licenses to be terminated.
- •
- We may not be able to achieve the full strategic and financial benefits expected to result from the Separation.
could be materially higher than our estimate, and the timing of the incurrence of these costs is subject to change.
Risks Related to Our Relationship with Lilly
- •
- Following the completion of the offering, Lilly will continue to have significant control over us for a period of time, which could continue
indefinitely, preventing you and other shareholders from influencing significant decisions. For so long as Lilly controls the majority of the voting power of our outstanding common stock, it will
determine the outcome of all corporate actions requiring shareholder approval.
- •
- Lilly's interests may differ from our interests and the interests of our public shareholders, and therefore actions Lilly takes with respect to
us, as a controlling or significant shareholder, including under the master separation agreement, may not be favorable to us or our public shareholders.
- •
- For so long as Lilly controls a majority of the voting power of our outstanding common stock, we will qualify for, and intend to rely on, exemptions from certain corporate governance requirements. You will not have the same protections afforded to shareholders of companies that are subject to such requirements. For example, we may not have a majority of independent directors or corporate governance and compensation committees consisting entirely of independent directors.
Risks Related to Our Business and Industry
- •
- The animal health industry is highly competitive. Our competitors include standalone animal health businesses, the animal health businesses of
large pharmaceutical companies, specialty animal health businesses and companies that mainly produce generic products. These competitors may have access to greater financial, marketing, technical and
other resources. As a result, they may be able to devote more resources to developing, manufacturing, marketing and selling their products, initiating or withstanding substantial price competition or
more readily taking advantage of acquisitions or other opportunities.
- •
- Disruptive innovations and advances in veterinary medical practices, animal health technologies and alternatives to animal-derived protein,
could negatively affect the market for our products. For example, the market for our companion animal therapeutics has been particularly affected by innovation in new molecules and delivery
formulations in recent years.
- •
- Regulatory restrictions and bans on the use of antibiotics and productivity products in food animals, as well as changing market demand, may continue to negatively affect demand for certain of our food animal products. For example, in certain markets, including the U.S., sales of certain of our food animal products have been negatively affected by an increase in consumer sentiment for "clean" proteins and dairy products (i.e., proteins and dairy products produced without the use of antibiotics or other products intended to increase animal production).
11
- •
- Generic products may be viewed as more cost-effective than our products. Generic competitors are becoming more aggressive in terms of launching
products before patent rights expire, and, because of attractive pricing, sales of generic products are an increasing percentage of overall animal health sales in certain regions.
- •
- We may not successfully implement our business strategies or achieve targeted cost efficiencies and gross margin improvements. Realizing the
anticipated benefits from our strategic initiatives, if any benefits are achieved at all, may take several years. Additionally, we may have insufficient access to capital to fund investments in
strategic initiatives, or our business strategy may change from time to time, which could delay our ability to implement initiatives that we believe are important to our business.
- •
- Consolidation of our customers and distributors could negatively affect the pricing of our products. In recent years, there has been a trend
towards the concentration of veterinarians in large clinics and hospitals. In addition, food animal producers, particularly swine and poultry producers, and our distributors have seen recent
consolidation in their industries. Furthermore, we have seen the expansion of larger cross border corporate customers and an increase in the consolidation of buying groups (cooperatives of veterinary
practices that leverage volume to pursue discounts from manufacturers). If these trends towards consolidation continue, our customers could attempt to improve their profitability by leveraging their
buying power to obtain favorable pricing.
- •
- An outbreak of infectious disease carried by food animals could negatively affect the demand for, and sale and production of, our food animal
products. In recent years, outbreaks of various diseases, including avian influenza, foot and mouth disease, bovine spongiform encephalopathy (otherwise known as BSE or "mad cow" disease) and porcine
epidemic diarrhea virus (otherwise known as PEDV), have negatively impacted sales of our animal health products.
- •
- Our R&D, acquisition and licensing efforts may fail to generate new products or expand the use of our existing products. We may be unable to
determine with accuracy when or whether any of our products now under development will be approved or launched, or we may be unable to develop, license or otherwise acquire product candidates or
products. In addition, we cannot predict whether any products, once launched, will be commercially successful or will achieve sales and revenue that are consistent with our expectations.
- •
- We had losses on an as-reported basis for the last three years, and we expect to continue to incur substantial expenditures to develop, manufacture and market our products and implement our business strategies.
The foregoing is only a summary of some of our risks. For a more detailed discussion of these and other risks you should consider before making an investment in our common stock, see "Risk Factors."
The Separation
Prior to the completion of this offering, we are a wholly-owned subsidiary of Lilly, and all of our outstanding shares of common stock are owned by Lilly.
In connection with the completion of this offering, through a series of equity and other transactions, Lilly will transfer to us substantially all of its animal health businesses that will form our business going forward. In exchange, we will pay to Lilly as consideration (i) all of the net proceeds we will receive from the sale of our common stock in this offering, including any net proceeds we receive as a result of any exercise of the underwriters' overallotment option, (ii) all of the net proceeds (approximately $2.0 billion) we received in the Senior Notes Offering (as defined below)
12
and (iii) all of the net proceeds ($498.6 million) we received from the entry into the Term Facility (as defined below); provided, to the extent the unrestricted cash held by us following the completion of this offering is less than (or more than) $300 million, we will retain a portion of the net proceeds (or pay additional amounts to Lilly) so that the unrestricted cash held by us for working capital and other general corporate purposes following the completion of this offering is $300 million. In addition, a portion of the consideration to be paid to Lilly will be temporarily retained by us as restricted cash in connection with the anticipated transfer to us from Lilly of certain animal health assets in certain jurisdictions that are anticipated to occur following the completion of the offering. We refer to these separation transactions, as described in "The Separation and Distribution Transactions — The Separation," as the "Separation."
In addition, immediately prior to the completion of this offering, we and Lilly intend to enter into certain agreements that will provide a framework for our ongoing relationship with Lilly. For a description of these agreements, see "Certain Relationships and Related Party Transactions — Relationship with Lilly."
Debt Transactions
On August 28, 2018, we issued $500 million aggregate principal amount of 3.912% Senior Notes due 2021, $750 million aggregate principal amount of 4.272% Senior Notes due 2023 and $750 million aggregate principal amount of 4.900% Senior Notes due 2028 (collectively, the "Senior Notes") in a private placement (the "Senior Notes Offering").
On September , 2018, we entered into (i) a revolving credit agreement for a five-year $750 million senior unsecured revolving credit facility (subject to certain conditions) with the ability to incur additional incremental commitments of up to $250 million (the "Revolving Facility"), and (ii) a term credit agreement with a syndicate of banks providing for a three-year senior unsecured term credit facility in an amount of $500 million (the "Term Facility" and, together with the Revolving Facility, the "Credit Facilities"). We refer to the entry into the Credit Facilities and the Senior Notes Offering together as the "Debt Transactions." We refer to the Separation and the Debt Transactions together as the "Transactions."
See "Description of Material Indebtedness" for more information on the Debt Transactions.
The Distribution
Lilly has informed us that, as of the date of this prospectus, it intends, following this offering, to make a distribution to its shareholders of all or a portion of its equity interest in us, which may include one or more distributions effected as a dividend to all Lilly shareholders, one or more offers to Lilly shareholders to exchange their Lilly shares for shares of our common stock, or any combination thereof. We refer to any such potential distribution as the "Distribution."
While, as of the date of this prospectus, Lilly intends to effect the Distribution, Lilly has no obligation to pursue or consummate any further dispositions of its ownership interest in us, including through the Distribution, by any specified date or at all. If pursued, the Distribution may be subject to various conditions, including receipt of any necessary regulatory or other approvals, the existence of satisfactory market conditions and the receipt of an opinion of counsel to the effect that the Separation, together with such Distribution, would be tax-free to Lilly and its shareholders for U.S. federal income tax purposes. The conditions to the Distribution may not be satisfied, Lilly may decide not to consummate the Distribution even if the conditions are satisfied or Lilly may decide to waive one or more of these conditions and consummate the Distribution even if all of the conditions are not satisfied.
13
The Distribution is not being effected pursuant to this prospectus, and the underwriters of this offering are not acting as underwriters for the Distribution.
Corporate Information
Elanco Animal Health Incorporated was incorporated in Indiana on May 3, 2018. Our principal executive offices are located at 2500 Innovation Way, Greenfield, Indiana 46140, and our telephone number is (877) 352-6261. Our corporate website address is www.elanco.com. Our website and the information contained on, or that can be accessed through, our website is not deemed to be incorporated by reference in, and is not considered part of, this prospectus. You should not rely on any such information in making your decision whether to purchase our common stock.
14
Issuer |
Elanco Animal Health Incorporated | |
Common stock offered by us |
shares of common stock (or shares of common stock if the underwriters exercise their option to purchase additional shares in full). |
|
Common stock to be held by Lilly immediately after this offering |
shares of common stock. |
|
Common stock to be outstanding immediately after this offering |
shares of common stock (or shares of common stock if the underwriters exercise their option to purchase additional shares in full). |
|
Option to purchase additional shares of common stock |
The underwriters have an option to purchase an additional shares of common stock from us. The underwriters can exercise this option at any time within 30 days from the date of this prospectus. |
|
Use of proceeds |
We estimate that the net proceeds from the sale of our common stock in this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, will be approximately $ million ($ million if the underwriters exercise their option to purchase additional shares in full) based on an assumed initial public offering price of $ per share (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus). |
|
|
We intend to pay to Lilly as consideration for the portion of its animal health businesses Lilly is contributing to us in connection with the Separation all of the net proceeds we will receive from the sale of our common stock in this offering, including any net proceeds we receive as a result of any exercise of the underwriters' overallotment option, together with the net proceeds we received from the Senior Notes Offering and the entry into the Term Facility; provided, to the extent the unrestricted cash held by us following the completion of this offering is less than (or more than) $300 million, we will retain a portion of the net proceeds (or pay additional amounts to Lilly) so that the unrestricted cash held by us for working capital and other general corporate purposes following the completion of this offering is $300 million. In addition, a portion of the consideration to be paid to Lilly will be temporarily retained by us as restricted cash in connection with the anticipated transfer to us from Lilly of certain animal health assets in certain jurisdictions that are anticipated to occur following the completion of the offering. See "Use of Proceeds." |
15
Dividend policy |
We initially expect to pay quarterly cash dividends to holders of our common stock of $ per share commencing following the fourth quarter of 2018, subject to the discretion of our board of directors. Our ability to pay dividends is subject to certain limitations and we may change our dividend policy at any time. See "Risk Factors — Risks Related to Our Indebtedness," "Risk Factors — Risks Related to Our Initial Public Offering and Ownership of Our Common Stock — While we currently intend to pay a quarterly cash dividend to our common shareholders, we may change our dividend policy at any time" and "Dividend Policy." |
|
Risk factors |
Investing in our common stock involves a high degree of risk. See "Risk Factors" beginning on page 22 and the other information included in this prospectus for a discussion of factors you should carefully consider before investing in our common stock. |
|
Directed share program |
At our request, the underwriters have reserved up to % of the shares of our common stock offered by this prospectus for sale, at the initial public offering price, to our directors, officers and certain employees and other parties with a connection to us, to the extent permitted by local securities laws and regulations. The number of shares available for sale to the general public in this offering will be reduced to the extent these persons purchase reserved shares. Any reserved shares not purchased by these persons will be offered by the underwriters to the general public on the same terms as the other shares. See "Underwriting" for more information. |
|
Principal shareholders |
Upon completion of this offering, Lilly will continue to own a controlling interest in us. Accordingly, we intend to avail ourselves of the "controlled company" exemption under the corporate governance rules of the NYSE. See "Management — Director Independence and Controlled Company Exemption" and "Principal Shareholder." |
|
Listing |
We intend to apply to have our common stock listed on the NYSE under the symbol "ELAN." |
Except as otherwise indicated, the number of shares of our common stock outstanding after this offering and the other information presented in this prospectus:
- •
- give effect to the transactions described under "The Separation and Distribution Transactions — The Separation;"
- •
- assume no exercise of the underwriters' option to purchase additional shares;
- •
- give effect to a -for- stock split of our common stock that will occur prior to the completion of this offering;
- •
- assume an initial public offering price of $ per share (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus);
16
- •
- exclude an aggregate of shares of our common stock that will be available for future equity awards under our equity
incentive
plan, including shares of our common stock issuable upon vesting of the awards we intend to issue following the completion of this offering, as further described in "Executive and Director
Compensation — Anticipated Compensation Program Following this Offering — Awards upon the Completion of this Offering;" and
- •
- give effect to our amended and restated certificate of incorporation and our amended and restated bylaws, which will be in effect prior to the completion of this offering.
17
Summary Historical Combined Financial Data and Unaudited Pro Forma Condensed Combined Financial Data
We report our financial results in accordance with U.S. generally accepted accounting principles ("U.S. GAAP"). The summary historical combined statement of operations data for the six months ended June 30, 2018 and 2017 and the summary historical combined balance sheet data as of June 30, 2018 presented below have been derived from our unaudited combined financial statements included elsewhere in this prospectus. The summary historical combined statement of operations data for the years ended December 31, 2017, 2016 and 2015 and the combined balance sheet data as of December 31, 2017 and 2016 presented below have been derived from our audited combined financial statements included elsewhere in this prospectus.
Our combined financial statements include the attribution of certain assets and liabilities that have historically been held at the Lilly corporate level but which are specifically identifiable or attributable to us. Our combined financial statements also include expense allocations related to certain Lilly corporate functions, including executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations. These expenses have been allocated to us based on direct usage or benefit where specifically identifiable, with the remainder allocated primarily on a pro rata basis of revenue, headcount or other measures. We believe that this expense methodology, and the results thereof, is reasonable for all periods presented. However, the allocations may not be indicative of the actual expense that would have been incurred if we would have operated as an independent, publicly traded company for the periods presented. It is impractical to estimate what our standalone costs would have been for the historical periods presented.
The summary unaudited pro forma condensed combined statement of operations data for the six months ended June 30, 2018 and the year ended December 31, 2017 and summary unaudited pro forma condensed combined balance sheet data as of June 30, 2018 presented below have been derived from our unaudited pro forma condensed combined financial statements included elsewhere in this prospectus. The unaudited pro forma information set forth below reflects our historical combined financial information, as adjusted to give effect to the Transactions as if they had occurred as of January 1, 2017, in the case of statement of operations data, and June 30, 2018, in the case of balance sheet data. The unaudited pro forma information is illustrative and not intended to represent what our results of operations or financial position would have been had the Transactions occurred on the dates indicated or to project our results of operations or financial position for any future period. For an understanding of the pro forma financial statements that give pro forma effect to the Transactions, see "Unaudited Pro Forma Condensed Combined Financial Statements" included elsewhere in this prospectus.
The financial statements included in this prospectus may not be indicative of our future performance and do not necessarily reflect what our financial position and results of operations would have been had we operated as an independent, publicly traded company for the periods presented, including changes that will occur in our operations and capital structure as a result of this offering and the Separation.
18
You should read the information set forth below together with "Selected Historical Combined Financial Data," "Unaudited Pro Forma Condensed Combined Financial Statements," "Management's Discussion and Analysis of Financial Condition and Results of Operations," "Capitalization," and our combined financial statements and the related notes thereto included elsewhere in this prospectus.
|
Six Months Ended June 30, |
Year Ended December 31, |
||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
|
Historical | Historical |
||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
|
Pro Forma 2018 |
2018 | 2017 | Pro Forma 2017 |
2017 | 2016 | 2015 |
|||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
|
(Dollars in millions) | |||||||||||||||||||||
Statement of Operations Data: |
||||||||||||||||||||||
Revenue |
$ | 1,506.4 | $ | 1,506.4 | $ | 1,437.6 | $ | 2,889.0 | $ | 2,889.0 | $ | 2,913.5 | $ | 2,909.1 | ||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Costs, expenses and other: |
||||||||||||||||||||||
Cost of sales |
791.5 | 791.5 | 712.7 | 1,493.9 | 1,493.9 | 1,409.0 | 1,533.7 | |||||||||||||||
Research and development |
126.6 | 126.6 | 127.9 | 251.7 | 251.7 | 265.8 | 291.0 | |||||||||||||||
Marketing, selling and administrative |
371.1 | 371.1 | 388.4 | 779.8 | 779.8 | 784.8 | 916.0 | |||||||||||||||
Amortization of intangible assets |
98.6 | 98.6 | 109.4 | 221.2 | 221.2 | 170.7 | 163.0 | |||||||||||||||
Asset impairment, restructuring and other special charges |
70.4 | 70.4 | 165.6 | 375.1 | 375.1 | 308.4 | 263.3 | |||||||||||||||
Other — net, (income) expense |
65.7 | 10.7 | 1.6 | 109.9 | (0.1 | ) | (2.8 | ) | 1.6 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Income (loss) before income taxes |
$ | (17.5 | ) | $ | 37.5 | $ | (68.0 | ) | $ | (342.6 | ) | $ | (232.6 | ) | $ | (22.4 | ) | $ | (259.5 | ) | ||
Income tax expense (benefit) |
14.4 | 27.6 | 60.5 | 36.3 | 78.1 | 25.5 | (48.7 | ) | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) |
$ | (31.9 | ) | $ | 9.9 | $ | (128.5 | ) | $ | (378.9 | ) | $ | (310.7 | ) | $ | (47.9 | ) | $ | (210.8 | ) | ||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) as a percent of revenue |
(2 | )% | 1 | % | (9 | )% | (13 | )% | (11 | )% | (2 | )% | (7 | )% | ||||||||
Pro forma net income (loss) per share: |
||||||||||||||||||||||
Basic |
$ | $ | ||||||||||||||||||||
Diluted |
$ | $ | ||||||||||||||||||||
Pro forma weighted average shares outstanding: |
||||||||||||||||||||||
Basic |
||||||||||||||||||||||
Diluted |
||||||||||||||||||||||
Statement of Cash Flow Data: |
||||||||||||||||||||||
Net cash provided by (used in): |
||||||||||||||||||||||
Operating activities |
$ | 183.9 | $ | 90.6 | $ | 173.8 | $ | 155.9 | $ | 6.6 | ||||||||||||
Investing activities |
(57.5 | ) | (903.8 | ) | (964.6 | ) | (182.1 | ) | (4,995.4 | ) | ||||||||||||
Financing activities |
(123.7 | ) | 811.9 | 847.5 | (149.6 | ) | 5,353.2 | |||||||||||||||
Other Data (non-GAAP): |
||||||||||||||||||||||
Adjusted EBITDA(1) |
$ | 306.2 | $ | 306.2 | $ | 278.4 | $ | 498.9 | $ | 498.9 | $ | 540.4 | $ | 393.7 | ||||||||
Adjusted net income(1) |
$ | 177.2 | $ | 219.0 | $ | 156.4 | $ | 182.3 | $ | 250.5 | $ | 332.7 | $ | 208.7 | ||||||||
|
As of December 31, |
||||||||||||
| | | | | | | | | | | | | | |
|
As of June 30, 2018 | Historical |
|||||||||||
| | | | | | | | | | | | | | |
|
Pro Forma | Historical | 2017 | 2016 |
|||||||||
| | | | | | | | | | | | | | |
|
(Dollars in millions) | ||||||||||||
Balance Sheet Data: |
|||||||||||||
Current assets |
$ | 2,035.9 | $ | 2,056.9 | $ | 2,123.7 | $ | 1,947.4 | |||||
Current liabilities |
592.5 | 558.4 | 632.6 | 618.9 | |||||||||
Property and equipment, net |
877.7 | 877.7 | 920.3 | 741.8 | |||||||||
Total assets |
8,547.6 | 8,577.4 | 8,940.3 | 8,099.7 | |||||||||
Total liabilities |
3,485.2 | 990.8 | 1,149.5 | 1,071.8 | |||||||||
Long-term debt |
2,475.5 | — | — | — | |||||||||
Total equity |
5,062.4 | 7,586.6 | 7,790.8 | 7,027.9 | |||||||||
19
- (1)
- Non-GAAP Financial Measures
Adjusted EBITDA
We define adjusted EBITDA as net income (loss) adjusted for interest expense, income tax expense (benefit) and depreciation and amortization, further adjusted to exclude purchase accounting adjustments to inventory, integration costs of acquisitions, severance, asset impairment, gain on sale of assets, facility exit costs and other specified significant items, such as unusual or non-recurring items that are unrelated to our long-term operations. For the periods presented, we have not made adjustments for all items that may be considered unrelated to our long-term operations. We believe adjusted EBITDA, when used in conjunction with our results presented in accordance with U.S. GAAP and its reconciliation to net income (loss), enhances investors' understanding of our performance, valuation and prospects for the future. We also believe adjusted EBITDA is a measure used in the animal health industry by analysts as a valuable performance metric for investors.
The following is a reconciliation of adjusted EBITDA to net income (loss), as reported under U.S. GAAP for the six months ended June 30, 2018 and 2017 and the years ended December 31, 2017, 2016 and 2015:
|
| Six Months Ended June 30, |
| Year Ended December 31, | | ||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | |||||||||||
|
| Pro Forma | | Historical | | Pro Forma | | Historical | | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | |||||||
|
| 2018 | | 2018 | | 2017 | | 2017 | | 2017 | | 2016 | | 2015 | | ||||||||||||
| | | | | | | | | | | | | | | | | | |||||||||||
|
| (Dollars in millions) | | ||||||||||||||||||||||||
Reported Net Income (Loss) |
| | $ | (31.9 | ) | | $ | 9.9 | | $ | (128.5 | ) | | $ | (378.9 | ) | | $ | (310.7 | ) | $ | (47.9 | ) | $ | (210.8 | ) | |
Interest expense |
| | 55.0 | | | — | | — | | | 110.0 | | | — | | — | | — | | ||||||||
Income tax expense (benefit) |
| | 14.4 | | | 27.6 | | 60.5 | | | 36.3 | | | 78.1 | | 25.5 | | (48.7 | ) | ||||||||
Depreciation and amortization |
| | 149.6 | | | 149.6 | | 156.1 | | | 318.4 | | | 318.4 | | 254.4 | | 236.9 | | ||||||||
| | | | | | | | | | | | | | | | | | |||||||||||
EBITDA |
| | 187.1 | | | 187.1 | | 88.1 | | | 85.8 | | | 85.8 | | 232.0 | | (22.6 | ) | ||||||||
Purchase accounting adjustments to inventory(a) |
| | — | | | — | | 26.5 | | | 42.7 | | | 42.7 | | — | | 153.0 | | ||||||||
Integration costs of acquisitions(b) |
| | 5.6 | | | 5.6 | | 68.7 | | | 90.3 | | | 90.3 | | 154.8 | | 140.8 | | ||||||||
Severance(b) |
| | (2.6 | ) | | (2.6 | ) | 56.3 | | | 162.0 | | | 162.0 | | 42.1 | | 59.5 | | ||||||||
Asset impairment(b) |
| | 57.7 | | | 57.7 | | 43.8 | | | 110.6 | | | 110.6 | | 98.3 | | 57.5 | | ||||||||
Gain on sale of assets(b) |
| | — | | | — | | (16.0 | ) | | (19.6 | ) | | (19.6 | ) | — | | — | | ||||||||
Facility exit costs(b) |
| | 9.7 | | | 9.7 | | 12.8 | | | 31.8 | | | 31.8 | | 13.2 | | 5.5 | | ||||||||
Contingent consideration(c) |
| | 8.5 | | | 8.5 | | (1.8 | ) | | (4.7 | ) | | (4.7 | ) | — | | — | | ||||||||
Inventory write-off(d) |
| | 40.2 | | | 40.2 | | — | | | — | | | — | | — | | — | | ||||||||
| | | | | | | | | | | | | | | | | | |||||||||||
Adjusted EBITDA |
| | $ | 306.2 | | | $ | 306.2 | | $ | 278.4 | | | $ | 498.9 | | | $ | 498.9 | | $ | 540.4 | | $ | 393.7 | | |
| | | | | | | | | | | | | | | | | | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | |||||||
| | | | | | | | | | | | | | | | | | |||||||||||
- (a)
- See
Note 4: Acquisitions to our audited combined financial statements.
- (b)
- See
Note 4: Asset Impairment, Restructuring and Other Special Charges to our unaudited interim combined financial statements and Note 5:
Asset Impairment, Restructuring and Other Special Charges to our audited combined financial statements.
- (c)
- See
Note 6: Financial Instruments to our unaudited interim combined financial statements.
- (d)
- See Note 5: Inventories to our unaudited interim combined financial statements.
Adjusted Net Income
We define adjusted net income as net income (loss) excluding amortization of intangible assets, purchase accounting adjustments to inventory, integration costs of acquisitions, severance, asset impairment, gain on sale of assets, facility exit costs and other specified significant items, such as unusual or non-recurring items that are unrelated to our long-term operations. For the periods presented, the only other specified significant item included is the exclusion in 2017 of the benefit related to the recently enacted U.S. tax reform legislation. Adjusted net income is an alternative view of performance used by management to evaluate the results of our operations and the discovery, development, manufacture and commercialization of our products, prior to considering certain income statement elements. Specifically, management intends to use adjusted net income for the purpose of analyzing performance results and setting compensation targets. We believe adjusted net income, when used in conjunction with our results presented in accordance with U.S. GAAP and its reconciliation to net income (loss), enhances investors' understanding of our performance, valuation and prospects for the future. We also believe adjusted net income is a measure used in the animal health industry by analysts as a valuable performance metric for investors.
20
The following is a reconciliation of adjusted net income to net income (loss), as reported under U.S. GAAP for the six months ended June 30, 2018 and 2017 and the years ended December 31, 2017, 2016 and 2015:
|
| Six Months Ended June 30, |
| Year Ended December 31, | | ||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | |||||||||||
|
| Pro Forma | | Historical | | Pro Forma | | Historical | | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | |||||||
|
| 2018 | | 2018 | | 2017 | | 2017 | | 2017 | | 2016 | | 2015 | | ||||||||||||
| | | | | | | | | | | | | | | | | | |||||||||||
|
| (Dollars in millions) | | ||||||||||||||||||||||||
Reported Net Income (Loss) |
| | $ | (31.9 | ) | | $ | 9.9 | | $ | (128.5 | ) | | $ | (378.9 | ) | | $ | (310.7 | ) | $ | (47.9 | ) | $ | (210.8 | ) | |
Purchase Accounting Adjustments: |
| | | | | | | | | | | | |||||||||||||||
Amortization of intangible assets |
| | 98.6 | | | 98.6 | | 109.4 | | | 221.2 | | | 221.2 | | 170.7 | | 163.0 | | ||||||||
Purchase accounting adjustments to inventory(a) |
| | — | | | — | | 26.5 | | | 42.7 | | | 42.7 | | — | | 153.0 | | ||||||||
Integration costs of acquisitions(b) |
| | 5.6 | | | 5.6 | | 68.7 | | | 90.3 | | | 90.3 | | 154.8 | | 140.8 | | ||||||||
Severance(b) |
| | (2.6 | ) | | (2.6 | ) | 56.3 | | | 162.0 | | | 162.0 | | 42.1 | | 59.5 | | ||||||||
Asset impairment(b) |
| | 57.7 | | | 57.7 | | 43.8 | | | 110.6 | | | 110.6 | | 98.3 | | 57.5 | | ||||||||
Gain on sale of assets(b) |
| | — | | | — | | (16.0 | ) | | (19.6 | ) | | (19.6 | ) | — | | — | | ||||||||
Facility exit costs(b) |
| | 9.7 | | | 9.7 | | 12.8 | | | 31.8 | | | 31.8 | | 13.2 | | 5.5 | | ||||||||
Contingent consideration(c) |
| | 8.5 | | | 8.5 | | (1.8 | ) | | (4.7 | ) | | (4.7 | ) | — | | — | | ||||||||
Inventory write-off(d) |
| | 40.2 | | | 40.2 | | — | | | — | | | — | | — | | — | | ||||||||
Other: |
| | | | | | | | | | | | |||||||||||||||
U.S tax reform(e) |
| | — | | | — | | — | | | (33.1 | ) | | (33.1 | ) | — | | — | | ||||||||
Tax effect of adjustments(f) |
| | (8.6 | ) | | (8.6 | ) | (14.8 | ) | | (40.0 | ) | | (40.0 | ) | (98.5 | ) | (159.8 | ) | ||||||||
| | | | | | | | | | | | | | | | | | |||||||||||
Adjusted Net Income |
| | $ | 177.2 | | | $ | 219.0 | | $ | 156.4 | | | $ | 182.3 | | | $ | 250.5 | | $ | 332.7 | | $ | 208.7 | | |
| | | | | | | | | | | | | | | | | | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | |||||||
| | | | | | | | | | | | | | | | | | |||||||||||
- (a)
- See
Note 4: Acquisitions to our audited combined financial statements.
- (b)
- See
Note 4: Asset Impairment, Restructuring and Other Special Charges to our unaudited interim combined financial statements and Note 5:
Asset Impairment, Restructuring and Other Special Charges to our audited combined financial statements.
- (c)
- See
Note 6: Financial Instruments to our unaudited interim combined financial statements.
- (d)
- See
Note 5: Inventories to our unaudited interim combined financial statements.
- (e)
- See
Note 11: Income Taxes to our audited combined financial statements.
- (f)
- The tax effect of the adjustments is calculated by applying the applicable tax rate to each adjustment in each relevant jurisdiction. In jurisdictions where we had recorded deferred tax assets related to net operating losses that were offset with valuation allowances, we applied the applicable tax rate to each adjustment and further adjusted for the tax effect of the beneficial reversal of the valuation allowances.
Limitations of Adjusted EBITDA and Adjusted Net Income
The primary material limitations associated with the use of adjusted EBITDA and adjusted net income as compared to U.S. GAAP results include the following: (i) they may not be comparable to similarly titled measures used by other companies, including those in our industry, (ii) they exclude financial information and events, such as the effects of an acquisition or amortization of intangible assets, that some may consider important in evaluating our performance, value or prospects for the future, (iii) they exclude items or types of items that may continue to occur from period to period in the future and (iv) they may not exclude all unusual or non-recurring items, which could increase or decrease these measures, which investors may consider to be unrelated to our long-term operations, such as Strategic Exits. These non-GAAP measures are not, and should not be viewed as, substitutes for U.S. GAAP reported net income (loss). We encourage investors to review our combined financial statements in their entirety and caution investors to use U.S. GAAP measures as the primary means of evaluating our performance, value and prospects for the future, and adjusted EBITDA and adjusted net income as supplemental measures.
21
Investing in our common stock involves a high degree of risk. You should consider carefully the following risks, together with all the other information in this prospectus, including our combined financial statements and notes thereto, before you invest in our common stock. If any of the following risks actually materializes, our business, financial condition and results of operations could be materially adversely affected. As a result, the trading price of our common stock could decline and you could lose part or all of your investment.
Risks Related to Our Business and Industry
The animal health industry is highly competitive.
The animal health industry is highly competitive. Our competitors include standalone animal health businesses, the animal health businesses of large pharmaceutical companies, specialty animal health businesses and companies that mainly produce generic products. We believe many of our competitors are conducting R&D activities in areas served by our products and in areas in which we are developing products. There are also several new start-up companies competing in the animal health industry. We also face competition from manufacturers of drugs globally, as well as producers of nutritional health products. These competitors may have access to greater financial, marketing, technical and other resources. As a result, they may be able to devote more resources to developing, manufacturing, marketing and selling their products, initiating or withstanding substantial price competition or more readily taking advantage of acquisitions or other opportunities. Further, consolidation in the animal health industry could result in existing competitors realizing additional efficiencies or improving portfolio bundling opportunities, thereby potentially increasing their market share and pricing power, which could lead to a decrease in our revenue and profitability and an increase in competition. For example, many of our competitors have relationships with key distributors and, because of their size, the ability to offer attractive pricing incentives, which may negatively impact or hinder our relationships with these distributors. In addition to competition from established market participants, new entrants to the animal health medicines and vaccines industry could substantially reduce our market share, render our products obsolete or disrupt our business model.
To the extent that any of our competitors are more successful with respect to any key competitive factor, or we are forced to reduce, or are unable to raise, the price of any of our products in order to remain competitive, our business, financial condition and results of operations could be materially adversely affected. Competitive pressure could arise from, among other things, more favorable safety and efficacy product profiles, limited demand growth or a significant number of additional competitive products being introduced into a particular market, price reductions by competitors, the ability of competitors to capitalize on their economies of scale, the ability of competitors to produce or otherwise procure animal health products at lower costs than us and the ability of competitors to access more or newer technology than us.
Disruptive innovations and advances in veterinary medical practices, animal health technologies and alternatives to animal-derived protein, could negatively affect the market for our products.
The markets for our products are regularly impacted by the introduction and/or broad market acceptance of newly-developed or alternative products that address the diseases and conditions for which we sell products, including "green" or "holistic" health products, specially bred disease-resistant animals or replacements for meat, milk, eggs or fish from alternative natural or synthetic sources. For example, the market for our companion animal therapeutics has been particularly affected by innovation in new molecules and delivery formulations in recent years. Technological breakthroughs by others may render obsolete our products and reduce or eliminate the market for
22
our products. Introduction or acceptance of competing animal health products and innovation or disruptive protein alternatives could materially adversely affect our business, financial condition and results of operations.
Regulatory restrictions and bans on the use of antibiotics and productivity products in food animals, as well as changing market demand, may continue to negatively affect demand for certain of our food animal products.
Over the past few years, our operational results have been, and will continue to be, affected by regulations and changing market demand. In certain markets, including the U.S., sales of certain of our food animal products have been negatively affected by an increase in consumer sentiment for "clean" proteins and dairy products (i.e., proteins and dairy products produced without the use of antibiotics or other products intended to increase animal production).
There are two classes of antibiotics used in animal health: shared-class, or medically important, antibiotics, which are used to treat infectious disease caused by pathogens that occur in both humans and animals; and animal-only antibiotics, which are used to treat infectious disease caused by pathogens that occur in animals only. See "Business — Products — Antibiotics." Concerns that the use of antibiotics in food animal production may lead to increased antibiotic resistance of human pathogens have resulted in increased regulation and changing market demand. In December 2013, the U.S. Food & Drug Administration (the "FDA") announced final guidance establishing procedures for the voluntary phase-out in the U.S. over a three-year period of the use of shared-class antibiotics in animal feed for growth promotion in food animal production. The guidance allows for continued use of shared-class antibiotics in food-producing animals under the supervision of a veterinarian for treatment, control and, under certain circumstances, for prevention of disease. The FDA indicated that it took this action to help preserve the efficacy of shared-class antibiotics to treat infections in humans. As part of those efforts, stricter guidelines governing the administration of shared-class antibiotics have recently come into effect. As of January 1, 2017, under the FDA guidance and the related rule known as the Veterinary Feed Directive, the use of shared-class antibiotics in the water or feed of food-producing animals requires written authorization by a licensed veterinarian. In addition, other countries in which we sell or plan to sell our products, such as France and Vietnam, have passed restrictions or bans on antibiotic use. Other countries have placed restrictions or bans on the use of specific antibiotics in certain food-producing animals, regardless of the route of administration (in feed or injectable).
From 2015 to 2017, our revenue from shared-class antibiotics declined at a CAGR of 7%, excluding the impact of foreign exchange, driven primarily by changing regulations in many markets, including the Veterinary Feed Directive, as well as changing market demand. Globally, during the first half of 2018, our revenue from shared-class antibiotics was flat, excluding the impact of foreign exchange, and represented 12% (4% from sales in North America and 8% from sales outside of North America) of our total revenue, down from 16% in the first half of 2015. From 2015 to 2017, our revenue from animal-only antibiotics grew at a CAGR of 4%, excluding the impact of foreign exchange, driven by sales outside North America, which offset a slight decline in North America. Globally, during the first half of 2018, our revenue from animal-only antibiotics grew 9%, excluding the impact of foreign exchange, and represented 24% of our total revenue, up from 21% in the first half of 2015. During 2017, as well as the first half of 2018, 86% of our revenue from animal-only antibiotics resulted from the sale of ionophores. Ionophores are a special class of animal-only antimicrobials, and because of their animal-only designation, mode of action and spectrum of activity, their use has not to date been impacted by regulations or changing market demand in many markets outside of North America.
The impact of changes in regulations and market preferences regarding the use of antibiotics in food animals could have a material adverse effect on our business, financial condition and results
23
of operations. If there is an increased public perception that consumption of food derived from animals that utilize our products poses a risk to human health, there may be a further decline in the production of those food products and, in turn, demand for our products. In addition, antibiotic resistance concerns will likely result in additional restrictions or bans, expanded regulations or public pressure to further reduce the use of antibiotics in food animals, increased demand for antibiotic-free protein, or changes in the market acceptance or regulatory treatment of ionophores, any of which could materially adversely affect our business, financial condition and results of operations.
In addition, our revenue has also been impacted by regulatory changes in China and other markets restricting the use of productivity products, such as those containing ractopamine, in food animals. This has resulted in many U.S. food producers who access such markets to eliminate their use of ractopamine. Our FA Ruminants & Swine products Optaflexx and Paylean contain ractopamine. If more producers decide to access such markets or additional markets restrict the use of ractopamine or other productivity products, our business, financial condition and results of operations could be materially adversely affected.
Generic products may be viewed as more cost-effective than our products.
We face competition from products produced by other companies, including generic alternatives to our products. We depend on patents and regulatory data exclusivity periods to provide us with exclusive marketing rights for some of our products. Patents for individual products expire at different times based on the date of the patent filing (or sometimes the date of patent grant) and the legal term of patents in the jurisdictions where such patents are obtained. The extent of protection afforded by our patents varies from jurisdiction to jurisdiction and is limited by the scope of the claimed subject matter of our patents, the term of the patent and the availability and enforcement of legal remedies in the applicable jurisdiction. In 2017, approximately 75% of our revenue was from products that did not have patent protection, including revenue from some of our top products such as Rumensin, Maxiban, Denagard and Tylan Premix. Other products are protected by patents that expire over the next several years. For example, certain patents related to Trifexis expire as early as 2020 in the U.S., 2021 in Japan and 2025 in European territories. As the patents for a brand name product expire, competitors may begin to introduce generic or other alternatives, and as a result, we may face competition from lower-priced alternatives to many of our products. For example, we have experienced significant competitive headwinds from generic ractopamine in the U.S. In the third quarter of 2013, a large established animal health company received U.S. approval for generic ractopamine. U.S. revenue from Optaflexx, our ractopamine beef product, has declined at a CAGR of 28% from 2015 to 2017 as a result of generic competition and international regulatory restrictions. We may face similar competition in the future for existing products that do not benefit from exclusivity, including Rumensin, which has not benefitted from patent protection in the U.S. for over 20 years, or for existing products with material patents expiring in the future. See "Business — Intellectual Property."
Generic competitors are becoming more aggressive in terms of launching products before patent rights expire, and, because of attractive pricing, sales of generic products are an increasing percentage of overall animal health sales in certain regions. Although the impact of generic competition in the animal health industry to date has not typically mirrored that seen in human health, product pricing and the impact of generic competition in the future may more closely mirror human health as a result of changes in industry dynamics, such as channel expansion, consolidation, an increase in the availability and use of pet insurance and the potential for generic competition by established animal health businesses. If animal health customers increase their use of new or existing generic products, our business, financial condition and results of operations could be materially adversely affected.
24
We may not successfully implement our business strategies or achieve targeted cost efficiencies and gross margin improvements.
We are pursuing strategic initiatives that management considers critical to our long-term success, including, but not limited to: improving manufacturing processes, reducing our manufacturing footprint, achieving lean initiatives, consolidating our CMO network, strategically insourcing projects, pursuing cost savings opportunities with respect to raw materials through a new procurement process and improving the productivity of our sales force. We may pursue additional strategic initiatives in the future to improve gross margins and achieve our targeted cost efficiencies. We also have acquired or partnered with a number of smaller animal health businesses, and we intend to continue to do so in the future. There are significant risks involved with the execution of these initiatives, including significant business, economic and competitive uncertainties, many of which are outside of our control. Accordingly, we may not succeed in implementing these strategic initiatives. Realizing the anticipated benefits from these initiatives, if any benefits are achieved at all, may take several years. We may be unable to achieve our targeted cost efficiencies and gross margin improvements. Additionally, we may have insufficient access to capital to fund investments in strategic initiatives, or our business strategy may change from time to time, which could delay our ability to implement initiatives that we believe are important to our business.
Consolidation of our customers and distributors could negatively affect the pricing of our products.
Third-party distributors, veterinarians and food animal producers are our primary customers. In recent years, there has been a trend towards the concentration of veterinarians in large clinics and hospitals. In addition, food animal producers, particularly swine and poultry producers, and our distributors have seen recent consolidation in their industries. Furthermore, we have seen the expansion of larger cross-border corporate customers and an increase in the consolidation of buying groups (cooperatives of veterinary practices that leverage volume to pursue discounts from manufacturers). The pace of consolidation and structure of markets varies greatly across geographies. If these trends towards consolidation continue, our customers could attempt to improve their profitability by leveraging their buying power to obtain favorable pricing. The resulting decrease in our prices could have a material adverse effect on our business, financial condition and results of operations.
An outbreak of infectious disease carried by food animals could negatively affect the demand for, and sale and production of, our food animal products.
Sales of our food animal products could be materially adversely affected by the outbreak of disease carried by food animals, which could lead to the widespread death or precautionary destruction of food animals as well as the reduced consumption and demand for animal protein. In addition, outbreaks of disease carried by food animals may reduce regional or global sales of particular animal-derived food products or result in reduced exports of such products, either due to heightened export restrictions or import prohibitions, which may reduce demand for our food animal products due to reduced herd or flock sizes.
In recent years, outbreaks of various diseases, including avian influenza, foot-and-mouth disease, bovine spongiform encephalopathy (otherwise known as BSE or "mad cow" disease) and porcine epidemic diarrhea virus (otherwise known as PEDV), have negatively impacted sales of our animal health products. The discovery of additional cases of any of these, or new, diseases may result in additional restrictions on animal protein, reduced herd or flock sizes, or reduced demand for animal protein, any of which may have a material adverse effect on our business, financial condition and results of operations. In addition, the outbreak of any highly contagious disease near our main production sites could require us to immediately halt production of our products at such sites or force us to incur substantial expenses in procuring raw materials or products elsewhere.
25
Our R&D, acquisition and licensing efforts may fail to generate new products or expand the use of our existing products.
Our future success depends on both our existing product portfolio and our pipeline of new products, including new products that we may develop through joint ventures and products that we are able to obtain through license or acquisition. We commit substantial effort, funds and other resources to R&D, both through our own dedicated resources and through collaborations with third parties.
We may be unable to determine with accuracy when or whether any of our products now under development will be approved or launched, or we may be unable to develop, license or otherwise acquire product candidates or products. In addition, we cannot predict whether any products, once launched, will be commercially successful or will achieve sales and revenue that are consistent with our expectations. The animal health industry is subject to regional and local trends and regulations and, as a result, products that are successful in some markets may not achieve similar success when introduced into other markets. Furthermore, the timing and cost of our R&D may increase, and our R&D may become less predictable as, among other things, regulations applicable to our industry may make it more time-consuming and/or costly to research, develop and register products. If we are unable to generate new products or expand the use of our existing products, our business, financial condition and results of operations will be materially adversely affected. For example, between 2015 and 2017, prior to our February 2018 launch of Credelio in the U.S., we experienced an innovation lag in the companion animal parasiticide space. In the absence of a competitive combined oral flea and tick product, our U.S. companion animal parasiticide portfolio revenue declined 15% in 2017, excluding the impact on revenue resulting from a reduction in inventory levels within our distribution channel.
In addition, some of our growth has occurred through Lilly's acquisitions, including Novartis Animal Health, Lohmann Animal Health, Janssen Animal Health and the BI Vetmedica U.S. vaccines portfolio. However, following the Separation, we will no longer benefit from Lilly's scale, capital base and financial strength.
We had losses on an as-reported basis for the last three years.
Historically, we have incurred net losses, as reported on a combined basis, including a net income (loss) for each of the years ended December 31, 2017, 2016 and 2015 of $(310.7) million, $(47.9) million and $(210.8) million, respectively. See "Management's Discussion and Analysis of Financial Condition and Results of Operations." We could continue to incur asset impairment, restructuring and other special charges and could report losses in the future. We also expect to continue to incur substantial expenditures to develop, manufacture and market our products and implement our business strategies. We may encounter unforeseen expenses, difficulties, complications, delays, adverse events and other unknown factors that may materially adversely affect our business.
The misuse or off-label use of our products may harm our reputation or result in financial or other damages.
Our products have been approved for use under specific circumstances for the treatment of certain diseases and conditions in specific species. There may be increased risk of product liability claims if veterinarians, food animal producers, pet owners or others attempt to use our products off-label, including the use of our products in species (including humans) for which they have not been approved. Furthermore, the use of our products for indications other than those for which our products have been approved may not be effective, which could harm our reputation and lead to an increased risk of litigation. If we are deemed by a governmental or regulatory agency to have engaged in the promotion of any of our products for off-label use, such agency could request that we modify our training or promotional materials and practices, and we could be subject to
26
significant fines and penalties, and the imposition of these sanctions could also affect our reputation and position within the industry. Any of these events could materially adversely affect our business, financial condition and results of operations.
Animal health products are subject to unanticipated safety, quality or efficacy concerns, which may harm our reputation.
Unanticipated safety, quality or efficacy concerns arise from time to time with respect to animal health products, whether or not scientifically or clinically supported, leading to product recalls, withdrawals or suspended or declining sales, as well as product liability and other claims.
Regulatory actions based on these types of safety, quality or efficacy concerns could impact all, or a significant portion, of a product's sales and could, depending on the circumstances, materially adversely affect our results of operations.
In addition, since we depend on positive perceptions of the safety, quality and efficacy of our products, and animal health products generally, by food producers, veterinarians and pet owners, any concern as to the safety, quality or efficacy of our products, whether actual or perceived, may harm our reputation. These concerns and the related harm to our reputation could materially adversely affect our business, financial condition and results of operations, regardless of whether such reports are accurate.
Our business may be negatively affected by weather conditions and the availability of natural resources.
The animal health industry and demand for many of our products in a particular region are affected by weather conditions, varying weather patterns and weather-related pressures from pests, such as ticks. As a result, we may experience regional and seasonal fluctuations in our results of operations.
Food animal producers depend on the availability of natural resources, including large supplies of fresh water. Their animals' health and their ability to operate could be adversely affected if they experience a shortage of fresh water due to human population growth or floods, droughts or other weather conditions. In the event of adverse weather conditions or a shortage of fresh water, veterinarians or food animal producers may purchase less of our products.
Further, heat waves may cause stress in animals and lead to increased vulnerability to disease, reduced fertility rates and reduced milk production. Droughts may threaten pasture and feed supplies by reducing the quality and amount of forage available to grazing livestock, while climate change may increase the prevalence of parasites and diseases that affect food animals. Adverse weather conditions may also have a material impact on the aquaculture business. Changes in water temperatures could affect the timing of reproduction and growth of various fish species, as well as trigger the outbreak of certain water borne diseases.
In addition, veterinary hospitals and practitioners depend on visits from, and access to, the animals under their care. Veterinarians' patient volume and ability to operate could be adversely affected if they experience prolonged snow, ice or other severe weather conditions, particularly in regions not accustomed to sustained inclement weather.
We may not be able to realize the expected benefits of our investments in emerging markets and are subject to certain risks due to our presence in emerging markets, including political or economic instability and failure to adequately comply with legal and regulatory requirements.
We have taken steps to increase our presence in select emerging markets, including by expanding our sales organization and product offerings in these markets. Failure to continue to
27
maintain and expand our business in emerging markets could materially adversely affect our business, financial condition and results of operations.
In addition, certain emerging markets have legal systems that are less developed. Other jurisdictions in which we conduct business may have legal and regulatory regimes that differ materially from U.S. laws and regulations, are continuously evolving or do not include sufficient judicial or administrative guidance to interpret such laws and regulations. Compliance with diverse legal requirements is costly and time-consuming and requires significant resources. Violations or possible violations of applicable laws or regulations by our employees may result in investigation costs, potential penalties and other related costs which in turn could negatively affect our reputation and our results of operations.
Some countries within emerging markets may be especially vulnerable to periods of local, regional or global economic, political or social instability or crisis. For example, our sales in certain emerging markets have suffered from extended periods of disruption due to natural disasters. Furthermore, we have also experienced lower than expected sales in certain emerging markets due to local, regional and global restrictions on banking and commercial activities in those countries. In addition, certain emerging markets have currencies that fluctuate substantially, which may impact our financial performance. For these reasons, among others, doing business within emerging markets carries significant risks.
Modification of foreign trade policy may harm our food animal product customers.
Changes in laws, agreements and policies governing foreign trade in the territories and countries where our customers do business could negatively impact such customers' businesses and adversely affect our results of operations. A number of our customers, particularly U.S.-based food animal producers, benefit from free trade agreements, such as the North American Free Trade Agreement ("NAFTA"). The U.S. has initiated negotiations with Canada and Mexico aimed at re-negotiating terms of NAFTA. Efforts by the U.S. to withdraw from or materially modify NAFTA or other international trade agreements to which it is a party, as well as trade disputes or the imposition of tariffs, could harm our customers, and as a result, materially adversely affect our business, financial condition and results of operations.
Our business is subject to risk based on global economic conditions.
Macroeconomic business and financial disruptions could have a material adverse effect on our business, financial condition and results of operations. Certain of our customers and suppliers could be affected directly by an economic downturn and could face constraints on the availability of credit or decreased cash flow that could give rise to payment delays, increased credit risk, bankruptcies and other financial hardships that could decrease the demand for our products or hinder our ability to collect amounts due from our customers. If one or more of our large customers, including distributors, discontinues or modifies their relationship with us as a result of economic conditions or otherwise, our business, financial condition and results of operations may be materially adversely affected. In addition, economic concerns may cause some pet owners to forgo or defer visits to veterinary practices or could reduce their willingness to treat pet health conditions or to continue to own a pet. Furthermore, our exposure to credit and collectability risk is higher in certain international markets and our ability to mitigate such risks may be limited. Our procedures intended to monitor and limit our exposure to credit and collectability risk may not effectively limit such risk and avoid losses.
Our results of operations are dependent upon the success of our top products.
If any of our top products experience issues, such as disruptive innovations or the introduction of more effective competitive products, negative publicity, changes to veterinarian or customer preferences, loss of patent protection, material product liability litigation, new or unexpected side
28
effects, manufacturing disruptions and/or regulatory proceedings, our revenue could be negatively impacted, perhaps significantly. Our top five products, Rumensin, Trifexis, Maxiban, Denagard and Tylan Premix, contributed approximately 29% of our revenue in 2017. Any issues with these top products, particularly Rumensin, which contributed approximately 10% of our revenue in 2017, could have a material adverse effect on our business, financial condition and results of operations.
Our business is subject to risk based on customer exposure to rising costs and reduced customer income.
Feed, fuel, transportation and other key costs for food animal producers may increase or animal protein prices or sales may decrease. Either of these trends could cause deterioration in the financial condition of our food animal product customers, potentially inhibiting their ability to purchase our products or pay us for products delivered. Our food animal product customers may offset rising costs by reducing spending on our food animal products, including by switching to lower-cost alternatives to our products. In addition, concerns about the financial resources of pet owners could cause veterinarians to alter their treatment recommendations in favor of lower-cost alternatives to our products, which could result in a decrease in sales of our companion animal products, especially in developed countries where there is a higher rate of pet ownership. Rising costs or reduced income for our customers could have a material adverse effect on our business, financial condition and results of operations.
For our companion animal products, increased use of alternative distribution channels, or changes within existing distribution channels, could negatively impact our market share, margins and distribution of our products.
In most markets, pet owners typically purchase their animal health products directly from veterinarians. However, pet owners increasingly have the option to purchase animal health products from sources other than veterinarians, such as online retailers, "big-box" retail stores or other over-the-counter distribution channels. This trend has been demonstrated by the significant shift away from the veterinarian distribution channel in the sale of flea and tick products in recent years. Pet owners also could decrease their reliance on, and visits to, veterinarians as they rely more on internet-based animal health information. Because we market our companion animal prescription products primarily through the veterinarian distribution channel, any decrease in visits to veterinarians by pet owners could reduce our market share for such products and materially adversely affect our business, financial condition and results of operations. In addition, pet owners may substitute human health products for animal health products if human health products are deemed to be lower-cost alternatives.
Legislation has also been proposed in the U.S., and may be proposed in the U.S. or abroad in the future, that could impact the distribution channels for our companion animal products. For example, such legislation may require veterinarians to provide pet owners with written prescriptions and disclosure that the pet owner may fill prescriptions through a third party, which may further reduce the number of pet owners who purchase their animal health products directly from veterinarians. Such requirements may lead to increased use of generic alternatives to our products or the increased substitution of our companion animal products with other animal health products or human health products if such other products are deemed to be lower-cost alternatives. Many states already have regulations requiring veterinarians to provide prescriptions to pet owners upon request and the American Veterinary Medical Association has long-standing policies in place to encourage this practice.
Over time, these and other competitive conditions may increase our use of online retailers, "big-box" retail stores or other over-the-counter distribution channels to sell our companion animal products. We may not be adequately prepared or able to distribute our companion animal products if an increased portion of our sales occurs through these channels. Also, we may realize lower
29
margins on sales through these distribution channels than we do on sales through veterinarians. Any of these events could materially adversely affect our business, financial condition and results of operations.
In addition, if one or more of our companion animal distributors discontinues or modifies their relationship with us, our business, financial condition and results of operations may be materially adversely affected. For example, in 2017, a change in our U.S. inventory management practices resulted in a revenue lag as existing inventory was sold down, which management estimates decreased our revenue by approximately $35 million.
Loss of our executive officers or other key personnel could disrupt our operations.
We depend on the efforts of our executive officers and other key personnel. Our executive officers and other key personnel are not currently, and are not expected to be, subject to non-compete provisions. In addition, we have not entered into employment agreements with our executive officers or other key personnel. Any unplanned turnover or our failure to develop an adequate succession plan for one or more of our executive officer or other key personnel positions could deplete our institutional knowledge base and erode our competitive advantage. The loss or limited availability of the services of one or more of our executive officers or other key personnel, or our inability to recruit and retain qualified executive officers or other key personnel in the future, could, at least temporarily, have a material adverse effect on our business, financial condition and results of operations.
We may be required to write down goodwill or identifiable intangible assets.
Under U.S. GAAP, if we determine goodwill or identifiable intangible assets are impaired, we will be required to write down these assets and record a non-cash impairment charge. As of June 30, 2018, we had goodwill of $2.9 billion and identifiable intangible assets, less accumulated amortization, of $2.5 billion. Identifiable intangible assets consist primarily of marketed products acquired or licensed from third parties, licensed platform technologies that have alternative future uses in R&D, manufacturing technologies, and customer relationships from business combinations. We also have indefinite-lived intangible assets, which consist of acquired in-process R&D projects from business combinations that are subject to impairment and non-cash impairment charges.
Determining whether an impairment exists and the amount of the potential impairment involves quantitative data and qualitative criteria that are based on estimates and assumptions requiring significant management judgment. Future events or new information may change management's valuation of an intangible asset in a short amount of time. The timing and amount of impairment charges recorded in our combined statements of operations and write-downs recorded in our combined balance sheets could vary if management's conclusions change. Any impairment of goodwill or identifiable intangible assets could have a material adverse effect on our business, financial condition and results of operations.
Our R&D relies on evaluations of animals, which may become subject to bans, additional restrictive regulations or increased attention from activism movements.
As an animal health medicines and vaccines business, the evaluation of our existing and new products in animals is required to register our products. Animal testing in certain industries has been the subject of controversy and adverse publicity. Some organizations and individuals have attempted to ban animal testing or encourage the adoption of new regulations applicable to animal testing. To the extent that the activities of such organizations and individuals are successful, our R&D, and by extension our business, financial condition and results of operations, could be materially adversely affected. In addition, negative publicity about us or our industry could harm our reputation.
30
Manufacturing problems and capacity imbalances may cause product launch delays, inventory shortages, recalls or unanticipated costs.
In order to sell our products, we must be able to produce and ship sufficient quantities to our customers. Following the Separation, we will own and operate 13 internal manufacturing sites located in nine countries. We also employ a network of approximately 120 third-party CMOs. Many of our products involve complex manufacturing processes and are sole-sourced from certain manufacturing sites.
Minor deviations in our manufacturing or logistical processes, such as temperature excursions or improper package sealing, could result, and have in the past resulted in, delays, inventory shortages, unanticipated costs, product recalls, product liability and/or regulatory action. In addition, a number of factors could cause production interruptions, including:
- •
- the failure of us or any of our vendors or suppliers, including logistical service providers, to comply with applicable regulations and quality
assurance guidelines;
- •
- mislabeling;
- •
- construction delays;
- •
- equipment malfunctions;
- •
- shortages of materials;
- •
- labor problems;
- •
- natural disasters;
- •
- power outages;
- •
- criminal and terrorist activities;
- •
- changes in manufacturing production sites and limits to manufacturing capacity due to regulatory requirements, changes in types of products
produced, shipping distributions or physical limitations; and
- •
- the outbreak of any highly contagious diseases near our production sites.
These interruptions could result in launch delays, inventory shortages, recalls, unanticipated costs or issues with our agreements under which we supply third parties, which may materially adversely affect our business, financial condition and results of operations.
Our manufacturing network may be unable to meet the demand for our products or we may have excess capacity if demand for our products changes. The unpredictability of a product's regulatory or commercial success or failure, the lead time necessary to construct highly technical and complex manufacturing sites and shifting customer demand (including as a result of market conditions or entry of branded or generic competition) increase the potential for capacity imbalances. In addition, construction of sites is expensive, and our ability to recover costs will depend on the market acceptance and success of the products produced at the new sites, which is uncertain.
We rely on third parties to provide us with materials and services and are subject to increased labor and material costs and potential disruptions in supply.
The materials used to manufacture our products may be subject to availability constraints and price volatility caused by changes in demand, weather conditions, supply conditions, government regulations, economic climate and other factors. In addition, labor costs may be subject to volatility caused by the supply of labor, governmental regulations, economic climate and other factors. Increases in the demand for, availability or the price of, materials used to manufacture our products and increases in labor costs could increase the costs to manufacture our products, result in product delivery delays or shortages, and impact our ability to launch new products on a timely basis or at all. We may not be able to pass all or a material portion of any higher material or labor costs on to our customers, which could materially adversely affect our business, financial condition and results of operations.
31
We may be unable to meet demand for certain of our products if any of our third-party suppliers cease or interrupt operations, fail to renew contracts with us or otherwise fail to meet their obligations to us.
We may incur substantial costs and receive adverse outcomes in litigation and other legal matters.
Our business, financial condition and results of operations could be materially adversely affected by unfavorable results in pending or future litigation matters. These matters may include, among other things, allegations of violation of U.S. and foreign competition law, labor laws, consumer protection laws and environmental laws and regulations, as well as claims or litigations relating to product liability, intellectual property, securities, breach of contract and tort. In addition, changes in the interpretations of laws and regulations to which we are subject, or in legal standards in one or more of the jurisdictions in which we operate, could increase our exposure to liability. For example, in the U.S., attempts have been made to allow damages for emotional distress and pain and suffering in connection with the loss of, or injury to, a companion animal. If such attempts were successful, our exposure with respect to product liability claims could increase materially.
Litigation matters, regardless of their merits or their ultimate outcomes, are costly, divert management's attention and may materially adversely affect our reputation and demand for our products. We cannot predict with certainty the eventual outcome of pending or future litigation matters. An adverse outcome of litigation or legal matters could result in our being responsible for significant damages. Any of these negative effects resulting from litigation matters could materially adversely affect our business, financial condition and results of operations.
Our business is subject to substantial regulation.
As a global company, we are subject to various state, federal and international laws and regulations, including regulations relating to the development, quality assurance, manufacturing, importation, distribution, marketing and sale of our products. Changes in applicable federal, state, local and foreign laws and regulations could have a material adverse effect on our business, financial condition and results of operations. In addition, our manufacturing facilities, including the manufacturing facilities operated by our CMOs, are subject to periodic inspections by regulatory agencies. An inspection may report conditions or practices that indicate possible violations of regulatory requirements. Our failure, or the failure of third parties we rely on, including CMOs, to comply with these regulatory requirements, allegations of such non-compliance or the discovery of previously unknown problems with a product or manufacturer could result in, among other things, inspection observation notices, warning letters or similar regulatory correspondence, fines, a partial or total shutdown of production in one or more of our facilities while an alleged violation is remediated, withdrawals or suspensions of current products from the market, and civil or criminal prosecution, as well as decreased sales as a result of negative publicity and product liability claims. Any one of these consequences could materially adversely affect our business, financial condition and results of operations.
In addition, we will not be able to market new products unless and until we have obtained all required regulatory approvals in each jurisdiction where we propose to market those products. Even after a product reaches market, it may be subject to re-review and may lose its approvals. Our failure to obtain approvals, delays in the approval process, or our failure to maintain approvals in any jurisdiction, may prevent us from selling products in that jurisdiction until approval or re-approval is obtained, if ever. In regard to Brexit, the European Union ("EU") and the United Kingdom ("UK") negotiators have agreed to a transition period, which is scheduled to last until December 2020. It is unclear if the parties will be able to reach an agreement post-separation.
32
The illegal distribution and sale by third parties of counterfeit or illegally compounded versions of our products or of stolen, diverted or relabeled products could have a negative impact on our reputation and business.
Third parties may illegally distribute and sell counterfeit or illegally compounded versions of our products that do not meet the exacting standards of our development, manufacturing and distribution processes. Counterfeit or illegally compounded medicines pose a significant risk to animal health and safety because of the conditions under which they are manufactured and the lack of regulation of their contents. Counterfeit or illegally compounded products are frequently unsafe or ineffective and can be potentially life-threatening to animals. Our reputation and business could suffer harm as a result of counterfeit or illegally compounded products which are alleged to be equivalent and/or which are sold under our brand name. In addition, products stolen or unlawfully diverted from inventory, warehouses, plants or while in transit, which are not properly stored or which have an expired shelf life and which have been repackaged or relabeled and which are sold through unauthorized channels, could adversely impact animal health and safety, our reputation and our business. Public loss of confidence in the integrity of vaccines and/or pharmaceutical products as a result of counterfeiting, illegal compounding or theft could have a material adverse effect on our business, financial condition and results of operations.
We are subject to complex environmental, health and safety laws and regulations.
We are subject to various federal, state, local and foreign environmental, health and safety laws and regulations. These laws and regulations govern matters such as the emission and discharge of hazardous materials into the ground, air or water; the generation, use, storage, handling, treatment, packaging, transportation, exposure to and disposal of hazardous and biological materials, including recordkeeping, reporting and registration requirements; and the health and safety of our employees. Due to our operations, these laws and regulations also require us to obtain, and comply with, permits, registrations or other authorizations issued by governmental authorities. These authorities can modify or revoke our permits, registrations or other authorizations and can enforce compliance through fines and injunctions.
Given the nature of our business, we have incurred, are currently incurring and may in the future incur liabilities for the investigation and remediation of contaminated land under the U.S. Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended, or under other federal, state, local and foreign environmental cleanup laws, with respect to our current or former sites, adjacent or nearby third-party sites, or offsite disposal locations. We could be subject to liability for the investigation and remediation of legacy environmental contamination caused by historical industrial activity as sites that we own or on which we operate. The costs associated with future cleanup activities that we may be required to conduct or finance could be material. Additionally, we may become liable to third parties for damages, including personal injury, property damage and natural resource damages, resulting from the disposal or release of hazardous materials into the environment. Such liability could materially adversely affect our business, financial condition and results of operations.
Furthermore, regulatory agencies are showing increasing concern over the impact of animal health products and food animal operations on the environment. This increased regulatory scrutiny may necessitate that additional time and resources be spent to address these concerns in both new and existing products.
Our failure to comply with the environmental, health and safety laws and regulations to which we are subject, including any permits issued thereunder, may result in environmental remediation costs, loss of permits, fines, penalties or other adverse governmental or private actions, including regulatory or judicial orders enjoining or curtailing operations or requiring corrective measures,
33
installation of pollution control equipment or remedial measures. We could also be held liable for any and all consequences arising out of human exposure to hazardous materials, environmental damage or significant environmental, health and safety issues that might arise at a manufacturing or R&D facility. Environmental laws and regulations are complex, change frequently, have tended to become more stringent and stringently enforced over time and may be subject to new interpretation. It is possible that our costs of complying with current and future environmental, health and safety laws, and our liabilities arising from past or future releases of, or exposure to, hazardous materials could materially adversely affect our business, financial condition and results of operations.
The actual or purported intellectual property rights of third parties may negatively affect our business.
A third party may sue us, or our distributors or licensors, including Lilly, or otherwise make a claim, alleging infringement or other violation of such third-party's patents, trademarks, trade dress, copyrights, trade secrets, domain names or other intellectual property rights. If we, our distributors or licensors do not prevail in this type of litigation, we may be required to:
- •
- pay monetary damages;
- •
- obtain a license in order to continue manufacturing or marketing the affected products, which may not be available on commercially reasonable
terms, or at all; or
- •
- stop activities, including any commercial activities, relating to the affected products, which could include a recall of the affected products and/or a cessation of sales in the future.
The costs of defending an intellectual property claim could be substantial and could materially adversely affect our business, financial condition and results of operations, even if we successfully defend such claim. Moreover, even if we believe that we do not infringe a validly existing third-party patent, we may choose to license such patent, which would result in associated costs and obligations. We may also incur costs in connection with an obligation to indemnify a distributor, licensor or other third party.
The intellectual property positions of animal health medicines and vaccines businesses frequently involve complex legal and factual questions, and an issued patent does not guarantee us the right to practice the patented technology or develop, manufacture or commercialize the patented product. For example, while we generally enter into proprietary information agreements with our employees and third parties which assign intellectual property rights to us, these agreements may not be honored or may not effectively assign intellectual property rights to us under the local laws of some countries or jurisdictions. We cannot be certain that a competitor or other third party does not have or will not obtain rights to intellectual property that may prevent us from manufacturing, developing or marketing certain of our products, regardless of whether we believe such intellectual property rights are valid and enforceable or we believe we would otherwise be able to develop a more commercially successful product, which may materially adversely affect our business, financial condition and results of operations.
If our intellectual property rights are challenged or circumvented, competitors may be able to take advantage of our research and development efforts or harm the value of our brands.
Our long-term success depends on our ability to market innovative, competitive products. We rely and expect to continue to rely on a combination of intellectual property, including patent, trademark, trade dress, copyright, trade secret and domain name protection, as well as confidentiality and license agreements with our employees and others, to protect our intellectual property and proprietary rights. If we fail to obtain and maintain adequate intellectual property
34
protection, we may not be able to prevent third parties from using our proprietary technologies or from marketing products that are very similar or identical to ours.
Our currently pending or future patent applications may not result in issued patents, or be approved on a timely basis, if at all. Similarly, any term extensions that we seek may not be approved on a timely basis, if at all. In addition, our issued patents, or any patents that may issue in the future, may not contain claims sufficiently broad to protect us against third parties with similar technologies or products or provide us with any competitive advantage, including exclusivity in a particular product area.
The validity and scope of our patent claims also may vary between countries, as individual countries have their own patent laws. For example, some countries only permit the issuance of patents covering a novel chemical compound itself, and its first use, and thus further methods of use for the same compound may not be patentable. The validity, enforceability, scope and effective term of patents can be highly uncertain and often involve complex legal and factual questions and proceedings that vary based on the local law of the relevant jurisdiction. Our ability to enforce our patents also depends on the laws of individual countries and each country's practice with respect to enforcement of intellectual property rights. Patent protection must be obtained on a jurisdiction-by-jurisdiction basis, and we only pursue patent protection in countries where we think it makes commercial sense for the given product. In addition, if we are unable to maintain our existing license agreements or other agreements pursuant to which third parties grant us rights to intellectual property, including because such agreements terminate, our financial condition and results of operations could be materially adversely affected.
Patent law reform in the U.S. and other countries may also weaken our ability to enforce our patent rights, or make such enforcement financially unattractive. For instance, in September 2011, the U.S. enacted the America Invents Act, which permits enhanced third-party actions for challenging patents and implements a first-to-invent system. These reforms could result in increased costs to protect our intellectual property or limit our ability to obtain and maintain patent protection for our products in these jurisdictions. Additionally, certain foreign governments have indicated that compulsory licenses to patents may be granted in the case of national emergencies, which could diminish or eliminate sales and profits from those regions and materially adversely affect our financial condition and results of operations.
Our trademarks and brands may provide us with a competitive advantage in the market as they may be known or trusted by consumers. In order to maintain the value of such brands, we must be able to enforce and defend our trademarks. We have pursued and will pursue the registration of trademarks and service marks in the U.S. and internationally; however, enforcing rights against those who knowingly or unknowingly dilute or infringe our brands can be difficult. Effective trademark, service mark, trade dress or related protections may not be available in every country in which our products and services are available. Enforcement is especially difficult in first-to-file countries where "trademark squatters" can prevent us from obtaining adequate protections for our brands. There can be no assurance that the steps we have taken and will take to protect our proprietary rights in our brands and trademarks will be adequate or that third parties will not infringe, dilute or misappropriate our brands, trademarks, trade dress or other similar proprietary rights.
Many of our products are based on or incorporate proprietary information. We actively seek to protect our proprietary information, including our trade secrets and proprietary know-how, by generally requiring our employees, consultants, other advisors and other third parties to execute proprietary information and confidentiality agreements upon the commencement of their employment, engagement or other relationship. Despite these efforts and precautions, we may be unable to prevent a third party from copying or otherwise obtaining and using our trade secrets or
35
our other intellectual property without authorization and legal remedies may not adequately compensate us for the damages caused by such unauthorized use. Further, others may independently and lawfully develop substantially similar or identical products that circumvent our intellectual property by means of alternative designs or processes or otherwise.
We could be subject to changes in our tax rates, the adoption of new U.S. or foreign tax legislation or exposure to additional tax liabilities.
We are subject to income taxes in the U.S. and numerous foreign jurisdictions. Changes in the relevant tax laws, regulations, administrative practices, principles and interpretations could adversely affect our future effective tax rates. The U.S. recently enacted tax reform legislation significantly revising U.S. tax law, and a number of other countries are actively considering or enacting tax changes. Other organizations, such as the Organisation for Economic Cooperation and Development and the European Commission, are active regarding tax-related matters which could influence international tax policy in countries in which we operate. While outcomes of these initiatives continue to develop and remain uncertain, modifications to key elements of the U.S. or international tax framework could have a material adverse effect on our consolidated results of operations and cash flows.
In December 2017, the President of the United States signed into law the Tax Cuts and Jobs Act (the "2017 Tax Act"). The 2017 Tax Act includes significant changes to the U.S. corporate income tax system, such as the reduction in the corporate income tax rate, transition to a modified territorial tax system, changes to business related exclusions, deductions and credits, and modifications to international tax provisions. U.S. GAAP requires that the income tax accounting effects from a change in tax laws or tax rates be recognized in continuing operations in the reporting period that includes the enactment date of the change. These effects include, among other things, re-measuring deferred tax assets and liabilities, evaluating deferred tax assets for valuation allowances and assessing the impact of certain provisions of the 2017 Tax Act. Pursuant to the Staff Accounting Bulletin No. 118 published by the SEC on December 22, 2017 addressing the challenges in accounting for the effects of the Tax Act in the period of enactment, companies must report provisional amounts for those specific income tax effects of the Tax Act for which the accounting is incomplete but a reasonable estimate can be determined. Those provisional amounts will be subject to adjustment during a measurement period of up to one year from the enactment date.
In addition, our effective tax rate is subject to potential risks that various taxing authorities may challenge the pricing of our cross-border arrangements and subject us to additional tax, adversely impacting our effective tax rate and our tax liability. We are also subject to the examination of our tax returns and other tax matters by the Internal Revenue Service (the "IRS") and other tax authorities and governmental bodies. We regularly assess the likelihood of an adverse outcome resulting from these examinations to determine the adequacy of our provision for taxes. There can be no assurance as to the outcome of these examinations. If our effective tax rates were to increase, particularly in the U.S. or other material foreign jurisdictions, or if the ultimate determination of our taxes owed is for an amount in excess of amounts previously accrued, our business, financial condition and results of operations could be materially adversely affected.
Significant portions of our operations are conducted in foreign jurisdictions, including jurisdictions presenting a high risk of bribery and corruption, and are subject to the economic, political, legal and business environments of the countries in which we do business.
Our international operations could be limited or disrupted by any of the following:
- •
- volatility in the international financial markets;
36
- •
- compliance with governmental controls;
- •
- difficulties enforcing contractual and intellectual property rights;
- •
- parallel trade in our products (importation of our products from EU countries where our products are sold at lower prices into EU countries
where the products are sold at higher prices);
- •
- compliance with a wide variety of laws and regulations, such as the U.S. Foreign Corrupt Practices Act (the "FCPA") and similar non-U.S. laws
and regulations;
- •
- compliance with foreign labor laws;
- •
- burdens to comply with multiple and potentially conflicting foreign laws and regulations, including those relating to environmental, health and
safety requirements;
- •
- changes in laws, regulations, government controls or enforcement practices with respect to our business and the businesses of our customers,
including the imposition of limits on our profitability;
- •
- political and social instability, including crime, civil disturbance, terrorist activities and armed conflicts;
- •
- trade restrictions and restrictions on direct investments by foreign entities, including restrictions administered by the Office of Foreign
Assets Control of the U.S. Department of the Treasury and the EU, in relation to our products or the products of farmers and other customers;
- •
- government limitations on foreign ownership;
- •
- government takeover or nationalization of business;
- •
- changes in tax laws and tariffs;
- •
- imposition of anti-dumping and countervailing duties or other trade-related sanctions;
- •
- costs and difficulties and compliance risks in staffing, managing and monitoring international operations, including in the use of overseas
third-party goods and service providers;
- •
- corruption risk inherent in business arrangements and regulatory contacts with foreign government entities;
- •
- longer payment cycles and increased exposure to counterparty risk; and
- •
- additional limitations on transferring personal information between countries or other restrictions on the processing of personal information.
In addition, international transactions may involve increased financial and legal risks due to differing legal systems and customs. Compliance with these requirements may prohibit the import or export of certain products and technologies or may require us to obtain a license before importing or exporting certain products or technologies. A failure to comply with any of these laws, regulations or requirements could result in civil or criminal legal proceedings, monetary or non-monetary penalties, or both, disruptions to our business, limitations on our ability to import and export products, and damage to our reputation. In addition, variations in the pricing of our products between jurisdictions may result in the unauthorized importation or unauthorized re-importation of our products between jurisdictions and may also result in the imposition of anti-dumping and countervailing duties or other trade-related sanctions. While the impact of these factors is difficult to predict, any of them could materially adversely affect our business, financial condition and results of operations.
37
Further, changes in any of these laws, regulations or requirements, or the political environment in a particular country, may affect our ability to engage in business transactions in certain markets, including investment, procurement and repatriation of earnings. For example, Brexit has created political and economic uncertainty, particularly in the UK and the EU. A withdrawal could significantly disrupt the free movement of goods, services, and people between the UK and the EU, and result in increased legal and regulatory complexities, as well as potential higher costs of conducting business in Europe. The UK's vote to exit the EU could also result in similar referendums or votes in other European countries in which we do business. The uncertainty surrounding the terms of the UK's withdrawal and its consequences could adversely impact consumer and investor confidence, and could affect sales or regulation of our products. Any of these effects, among others, could materially adversely affect our business, financial condition and results of operations.
Foreign exchange rate fluctuations and potential currency controls affect our results of operations, as reported in our financial statements.
We conduct operations in many areas of the world, involving transactions denominated in a variety of currencies. In 2017, we generated approximately 50% of our revenue in currencies other than the U.S. dollar, principally the euro, British pound, Brazilian real, Australian dollar, Japanese yen, Canadian dollar and Chinese yuan. We are subject to currency exchange rate risk to the extent that our costs are denominated in currencies other than those in which we earn revenue. In addition, because our financial statements are reported in U.S. dollars, changes in currency exchange rates between the U.S. dollar and other currencies have had, and will continue to have, an impact on our results of operations.
We also face risks arising from currency devaluations and the imposition of cash repatriation restrictions and exchange controls. Currency devaluations result in a diminished value of funds denominated in the currency of the country instituting the devaluation. Cash repatriation restrictions and exchange controls may limit our ability to convert foreign currencies into U.S. dollars or to remit dividends and other payments by our foreign subsidiaries or businesses located in or conducted within a country imposing restrictions or controls. While we currently have no need, and do not intend, to repatriate or convert cash held in countries that have significant restrictions or controls in place, should we need to do so to fund our operations, we may be unable to repatriate or convert such cash, or may be unable to do so without incurring substantial costs.
We depend on sophisticated information technology and infrastructure.
We rely on various information systems to manage our operations, and we increasingly depend on third parties to operate and support our information technology systems, including by way of virtual and cloud-based operations. These third parties include large established vendors as well as small, privately owned companies. Failure by any provider to adequately service our operations, or a change in control or insolvency of one or more providers, may materially adversely affect our business, financial condition and results of operations. Prior to the Separation, we relied on Lilly to negotiate and manage many of our relationships and contracts with these third parties.
Prior to the completion of this offering and in connection with the Separation, we will substantially change a number of our business processes, including changes in our financial reporting and supply chain processes and with respect to where and from whom we obtain information technology systems. In order to support the new business processes under the terms of our transitional services agreement with Lilly, we will make significant configuration, process and data changes within many of the information technology systems we use. If our information technology systems and processes are not sufficient to support our business and financial reporting functions, or if we fail to properly implement our new business processes, our financial reporting
38
may be delayed or inaccurate and, as a result, our business, financial condition and results of operations may be materially adversely affected. Even if we are able to successfully configure and change our systems, all technology systems, even with implementation of security measures, are vulnerable to disability, failures or unauthorized access. If our information technology systems were to fail or be breached, this could materially adversely affect our reputation and our ability to perform critical business functions, and sensitive and confidential data could be compromised.
Breaches of our information technology systems or improper disclosure of confidential company or personal data could have a material adverse effect on our reputation and operations, or we may fail to comply with privacy laws, regulations and our contractual obligations.
We rely on information technology systems to process, transmit and store electronic information in our day-to-day operations, including customer, employee and company data. The secure processing, maintenance and transmission of this information is critical to our operations and the legal environment surrounding information security, storage, use, processing, disclosure and privacy is demanding, with the frequent imposition of new and changing requirements. We also store certain information with third parties. Our information systems and those of our third-party vendors are subjected to computer viruses or other malicious codes, unauthorized access attempts, and cyber- or phishing-attacks and also are vulnerable to an increasing threat of continually evolving cybersecurity risks and external hazards, as well as improper or inadvertent staff behavior, all of which could expose confidential company and personal data systems and information to security breaches. Any such breach could compromise our networks, and the information stored therein could be accessed, publicly disclosed, lost or stolen. Such attacks could result in our intellectual property and other confidential information being lost or stolen, disruption of our operations, and other negative consequences, such as increased costs for security measures or remediation costs, and diversion of management attention. Any actual or perceived access, disclosure or other loss of information or any significant breakdown, intrusion, interruption, cyber-attack or corruption of customer, employee or company data or our failure to comply with federal, state, local and foreign privacy laws or contractual obligations with customers, vendors, payment processors and other third parties, could result in legal claims or proceedings, liability under laws or contracts that protect the privacy of personal information, regulatory penalties, disruption of our operations, and damage to our reputation, all of which could materially adversely affect our business, revenue and competitive position. While we will continue to implement additional protective measures to reduce the risk of and detect cyber-incidents, cyber-attacks are becoming more sophisticated and frequent, and the techniques used in such attacks change rapidly. Our protective measures may not protect us against attacks and such attacks could have a significant impact on our business and reputation. In addition, prior to the Separation we relied on Lilly for certain privacy and compliance functions and personnel and may experience difficulties maintaining and implementing all policies and practices following completion of the Separation.
Increased regulation or decreased governmental financial support relating to the raising, processing or consumption of food animals could reduce demand for our food animal products.
Companies in the food animal sector are subject to extensive and increasingly stringent regulations. See "Business — Regulatory." If food animal producers are adversely affected by new regulations or changes to existing regulations, they may reduce herd or flock sizes or become less profitable and, as a result, they may reduce their use of our products, which may materially adversely affect our business, financial condition and results of operations. Also, many food animal producers benefit from governmental subsidies, and if such subsidies were to be reduced or eliminated, these companies may become less profitable and, as a result, may reduce their use of
39
our food animal products. More stringent regulation of the food animal sector, including regarding the use of food animal products, could have a material adverse effect on our business, financial condition and results of operations.
Our business could be materially adversely affected by labor disputes, strikes or work stoppages.
Some of our employees are members of unions, works councils, trade associations or are otherwise subject to collective bargaining agreements in certain jurisdictions, including the U.S. As a result, we are subject to the risk of labor disputes, strikes, work stoppages and other labor-relations matters. We may be unable to negotiate new collective bargaining agreements on similar or more favorable terms and may experience work stoppages, higher ongoing labor costs or other labor problems in the future at our sites. We may also experience difficulty or delays in implementing changes to our workforce in certain markets.
These risks may be increased by the Separation because we will no longer be able to benefit from Lilly's prior relationships and negotiations relating to such agreements. In addition, in France, we anticipate that we will be required to consult with our works council about the Separation prior to entering into a definitive agreement with respect to the French operations. Due to possible delays in the consultation process or resistance from the works council, there is a risk that the separation of the French operations will not be complete by the closing of the offering.
Further, labor-related issues, including at our suppliers or CMOs, could cause a disruption of our operations, which could have a material adverse effect on our business, financial condition and results of operations, potentially resulting in cancelled orders by customers, unanticipated inventory accumulation or shortages and reduced revenue and net income.
The anticipated benefits of the Separation may not be achieved.
We may not be able to achieve the full strategic and financial benefits expected to result from the Separation. Further, such benefits, if ultimately achieved, may be delayed. These benefits include the following:
- •
- improving strategic and operational flexibility and streamlining decision-making by providing the flexibility to implement our strategic plan
and to respond more effectively to different customer needs and the changing economic and industry environment;
- •
- allowing us to adopt the investment policy and dividend policy best suited to our financial profile and business needs, and allowing us to
raise capital as an independent business;
- •
- creating an independent equity structure that makes possible future acquisitions utilizing our common stock as well as compensation
arrangements; and
- •
- facilitating incentive compensation arrangements for employees more directly tied to the performance of our business, and enhancing employee hiring and retention by, among other things, improving the alignment of management and employee incentives with performance and growth objectives of our business.
We may not achieve the anticipated benefits of the Separation for a variety of reasons. In addition, the Separation could materially adversely affect our business, financial condition and results of operations.
40
We have underfunded pension plan liabilities. We will require current and future operating cash flow to fund these shortfalls reducing the cash available for other uses.
We have certain defined benefit pension plans, predominantly outside of the U.S., that our employees participate in that are either dedicated to our employees or where the plan assets and liabilities that relate to our employees are legally required to transfer to us at the time of the Separation. The funded status and net periodic pension cost for these plans is materially affected by the discount rate used to measure pension obligations, the longevity and actuarial profile of our workforce, the level of plan assets available to fund those obligations and the actual and expected long-term rate of return on plan assets. Significant changes in investment performance or a change in the portfolio mix of invested assets can result in corresponding increases and decreases in the valuation of plan assets or in a change in the expected rate of return on plan assets. As of December 31, 2017, for pension plans with projected benefit obligations in excess of plan assets, the projected benefit obligation was $251.6 million with plan assets of $121.8 million. Any changes in the discount rate could result in a significant increase or decrease in the valuation of pension obligations, affecting the reported funded status of our pension plans as well as the net periodic pension cost in the following years. Similarly, changes in the expected return on plan assets can result in significant changes in the net periodic pension cost in the following years. The need to make additional cash contributions will divert resources from our operations and may have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Our Indebtedness
We have substantial indebtedness.
We have a significant amount of indebtedness, which could materially adversely affect our business, financial condition and results of operations. As of June 30, 2018, pro forma for the Transactions, we had approximately $2.5 billion of outstanding indebtedness, consisting of the Senior Notes and the Credit Facilities. See "Description of Material Indebtedness."
We may incur substantial additional debt from time to time to finance working capital, capital expenditures, investments or acquisitions, or for other purposes. If we do so, the risks related to our high level of debt could intensify. Specifically, our high level of debt could have important consequences, including:
- •
- making it more difficult for us to satisfy our obligations with respect to our debt;
- •
- limiting our ability to obtain additional financing to fund future working capital, capital expenditures, business development or other general
corporate requirements, including dividends;
- •
- increasing our vulnerability to general adverse economic and industry conditions;
- •
- exposing us to the risk of increased interest rates as certain of our borrowings are and may in the future be at variable rates of interest;
- •
- limiting our flexibility in planning for and reacting to changes in the animal health industry;
- •
- impacting our effective tax rate; and
- •
- increasing our cost of borrowing.
41
We may not be able to generate sufficient cash to service all of our indebtedness and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments on or refinance our debt obligations depends on our financial condition and operating performance, which are subject to prevailing economic and competitive conditions and to certain financial, business, legislative, regulatory and other factors beyond our control. We may be unable to maintain a level of cash flows from operating activities sufficient to permit us to pay the principal and interest on our indebtedness.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we could face substantial liquidity problems and could be forced to reduce or delay investments and capital expenditures, or to dispose of material assets or operations, alter our dividend policy, seek additional debt or equity capital or restructure or refinance our indebtedness. We may not be able to effect any such alternative measures on commercially reasonable terms or at all and, even if successful, those alternative actions may not allow us to meet our scheduled debt service obligations. The instruments that will govern our indebtedness may restrict our ability to dispose of assets and may restrict the use of proceeds from those dispositions and may also restrict our ability to raise debt or equity capital to be used to repay other indebtedness when it becomes due. We may not be able to consummate those dispositions or to obtain proceeds in an amount sufficient to meet any debt service obligations when due.
In addition, we conduct our operations through our subsidiaries. Accordingly, repayment of our indebtedness will depend on the generation of cash flow by our subsidiaries, including certain international subsidiaries, and their ability to make such cash available to us, by dividend, debt repayment or otherwise. Our subsidiaries may not have any obligation to pay amounts due on our indebtedness or to make funds available for that purpose. Our subsidiaries may not be able to, or may not be permitted to, make adequate distributions to enable us to make payments in respect of our indebtedness. Each subsidiary is a distinct legal entity and, under certain circumstances, legal, tax and contractual restrictions may limit our ability to obtain cash from our subsidiaries. In the event that we do not receive distributions from our subsidiaries, we may be unable to make required principal and interest payments on our indebtedness.
Our inability to generate sufficient cash flows to satisfy our debt obligations, or to refinance our indebtedness on commercially reasonable terms or at all, may materially adversely affect our business, financial condition and results of operations and our ability to satisfy our obligations under our indebtedness or pay dividends on our common stock.
Risks Related to Our Relationship with Lilly
Following the completion of the offering, Lilly will continue to have significant control over us for a period of time, which could continue indefinitely, preventing you and other shareholders from influencing significant decisions.
Immediately following the completion of this offering, Lilly will own % of our outstanding common stock.
Lilly has indicated that, following completion of the offering, it intends to divest its interest in us. However, Lilly is under no obligation to do so or to dispose of any of its shares of our common stock, whether pursuant to the Distribution or otherwise. A determination whether to effect the Distribution or other disposal of any our shares of common stock, and the timing thereof, is within Lilly's sole discretion.
If the Distribution does not occur, or if Lilly does not otherwise dispose of its shares of our common stock, the risks relating to Lilly's control of us will continue to be relevant to our
42
shareholders. The liquidity of shares of our common stock in the market may be constrained for as long as Lilly continues to hold a significant position in our common stock. A lack of liquidity in our common stock could depress the price of our common stock.
For so long as Lilly controls the majority of the voting power of our outstanding common stock, it will determine the outcome of all corporate actions requiring shareholder approval. Even if Lilly were to dispose of certain of its shares of our common stock such that it would control less than a majority of the voting power of our outstanding common stock, it may be able to influence the outcome of corporate actions so long as it retains a significant portion of our common stock. During the period of Lilly's ownership, investors in this offering may not be able to affect the outcome of such corporate actions. For such time as Lilly owns a controlling interest in or a significant portion of our common stock, it generally will be able to control or significantly influence, directly or indirectly and subject to applicable law, all matters affecting us, including:
- •
- the election of directors;
- •
- determinations with respect to our business direction and policies, including the appointment and removal of officers;
- •
- determinations with respect to corporate transactions, such as mergers, business combinations or the acquisition or the disposition of assets;
- •
- our financing and dividend policy;
- •
- determinations with respect to our tax returns; and
- •
- compensation and benefits programs and other human resources policy decisions.
Lilly's interests may differ from our interests and the interests of our public shareholders.
Lilly's interests may differ from our interests and the interests of our shareholders, and therefore actions Lilly takes with respect to us, as a controlling or significant shareholder, including under the master separation agreement, may not be favorable to us or our public shareholders.
Lilly will have significant rights under the master separation agreement with us.
Until such time, if any, as Lilly divests all or a significant portion of its shares of our common stock, the master separation agreement will give Lilly certain significant rights. The master separation agreement will provide that, for so long as Lilly and its affiliates beneficially own at least 10% of our voting shares, Lilly will be entitled to designate for nomination the number of representatives on the board of directors that is proportionate to its ownership of our voting shares (rounding up to the nearest whole number of directors). For the avoidance of doubt, so long as Lilly and its affiliates beneficially own at least 80% of our voting shares, Lilly will designate for nomination at least 80% of the members of the board of directors (rounding up to the nearest whole number of directors). In addition, so long as Lilly and its affiliates beneficially own at least a majority of our voting shares, Lilly will be entitled to designate the chairman of the board of directors and a majority of the members of each committee of the board of directors.
In addition, subject to certain exceptions, so long as Lilly beneficially owns at least a majority of our voting shares, we will be required to obtain Lilly's prior written approval before undertaking (or permitting or authorizing any of our subsidiaries to undertake) various significant corporate actions, including:
- •
- consolidation or merger transactions;
- •
- dissolution, liquidation or winding up;
43
- •
- incurrence of any indebtedness (as defined in the master separation agreement), other than pursuant to existing debt obligations or unsecured
lines of credit as of the date of completion of the offering;
- •
- altering, amending, terminating, repealing or adopting any provisions inconsistent with our amended and restated articles of incorporation or
our amended and restated bylaws (unless required to comply with applicable law); or
- •
- the issuance, purchase, redemption or other acquisition or retirement for value of any of our equity securities (other than deemed repurchases resulting from the exercise of stock options or tax withholdings).
If Lilly sells a controlling interest in our company to a third party in a private transaction, you may not realize any change-of-control premium on shares of our common stock and we may become subject to the control of a presently unknown third party.
Following the completion of this offering, Lilly will continue to own % of our outstanding common stock. Subject to the provisions of the lock-up agreement entered into in connection with this offering, Lilly will not be restricted from selling some or all of its shares of our common stock in a privately negotiated transaction or otherwise, and a sale of its shares, if sufficient in size, could result in a change of control of our company.
The ability of Lilly to privately sell its shares of our common stock, with no requirement for a concurrent offer to be made to acquire all of the shares of our common stock held by our other shareholders, could prevent you from realizing any change-of-control premium on your shares of our common stock that may otherwise accrue to Lilly on its private sale of our common stock. Additionally, if Lilly privately sells its controlling equity interest in our company, we may become subject to the control of a presently unknown third party. Such third party may have conflicts of interest with those of other shareholders. In addition, if Lilly sells a controlling interest in our company to a third party, our indebtedness may be subject to acceleration, and our other commercial agreements and relationships, including any remaining agreements with Lilly, could be impacted, all of which may adversely affect our ability to run our business as described herein and may have a material adverse effect on our business, financial condition and results of operations.
For so long as Lilly controls a majority of the voting power of our outstanding common stock, we will qualify for, and intend to rely on, exemptions from certain corporate governance requirements. You will not have the same protections afforded to shareholders of companies that are subject to such requirements.
Upon completion of this offering, we will qualify as a "controlled company" within the meaning of the corporate governance standards of the NYSE because Lilly will control a majority of the voting power of our outstanding common stock entitled to vote in the election of directors. A "controlled company" may elect not to comply with certain corporate governance requirements of the NYSE. Consistent with this, the master separation agreement will provide that, for so long as we are a "controlled company," we will use our reasonable best efforts to take advantage of available "controlled company" exemptions from compliance with certain corporate governance requirements under NYSE rules, including:
- •
- the requirement that a majority of the board of directors consist of independent directors;
- •
- the requirement that our corporate governance committee be composed entirely of independent directors with a written charter addressing the committee's purpose and responsibilities;
44
- •
- the requirement that our compensation committee be composed entirely of independent directors; and
- •
- the requirement for an annual performance evaluation of our corporate governance and compensation committees.
While Lilly controls a majority of the voting power of our outstanding common stock, we may not have a majority of independent directors or corporate governance and compensation committees consisting entirely of independent directors and we will have annual performance evaluations of these committees from time to time.
Accordingly, you will not have the same protections afforded to shareholders of companies that are subject to all of the corporate governance requirements of the NYSE.
As a result of the Separation, we will lose Lilly's brand, reputation, capital base and other resources, and may experience difficulty operating as a standalone company.
We believe our association with Lilly has contributed to our building relationships with our customers due to Lilly's globally recognized brand and perceived high-quality products. This offering and the Separation could adversely affect our ability to attract and retain customers, which could result in reduced sales of our products.
The loss of Lilly's scale, capital base and financial strength may also prompt suppliers to reprice, modify or terminate their relationships with us. In addition, Lilly's reduction of its ownership of our company could potentially cause some of our existing agreements and licenses to be terminated. We cannot predict with certainty the effect that this offering, the Separation or the Distribution will have on our business, our clients, vendors or other persons, or whether our Elanco brand will be accepted in the marketplace.
Further, because we have not operated as a standalone company in the past, we may have difficulty doing so. We may need to acquire assets and resources in addition to those provided by Lilly to our company, and in connection with the Separation, may also face difficulty in separating our assets from Lilly's assets and integrating newly acquired assets into our business. Our business, financial condition and results of operations could be materially adversely affected if we have difficulty operating as a standalone company, fail to acquire assets that prove to be important to our operations or incur unexpected costs in separating our assets from Lilly's assets or integrating newly-acquired assets.
Lilly may compete with us.
Lilly will not be restricted from competing with us in the animal health business. Although Lilly has informed us it has no current intention to compete with us in the animal health business, if Lilly in the future decides to engage in the type of business we conduct, it may have a competitive advantage over us, which may cause our business, financial condition and results of operations to be materially adversely affected.
Certain of our directors may have actual or potential conflicts of interest because of their positions with Lilly.
Following this offering, a majority of our directors will retain their positions as employees with Lilly. In addition, such directors may own Lilly common stock or equity awards. For certain of these individuals, their holdings of Lilly common stock or equity awards may be significant compared to their total assets. Their position at Lilly and the ownership of any Lilly equity or equity awards creates, or may create the appearance of, conflicts of interest when these directors are faced with decisions that could have different implications for Lilly than for us. These potential conflicts could
45
arise, for example, over matters such as the desirability of changes in our business and operations, funding and capital matters, regulatory matters, matters arising with respect to the master separation agreement and other agreements with Lilly relating to the Separation or otherwise, employee retention or recruiting, or our dividend policy.
Provisions relating to certain relationships and transactions in our amended and restated articles of incorporation address certain potential conflicts of interest between us, on the one hand, and Lilly and its officers who are directors of our Company, on the other hand. By becoming our shareholder, you will be deemed to have notice of and have consented to these provisions of our amended and restated articles of incorporation. Although these provisions are designed to resolve certain conflicts between us and Lilly fairly, we cannot assure you that any conflicts will be so resolved. The principles for resolving these potential conflicts of interest are described under "Description of Capital Stock — Conflicts of Interest; Corporate Opportunities."
Lilly and its directors and officers will have limited liability to us or you for breach of fiduciary duty.
Our amended and restated articles of incorporation provide that, subject to any contractual provision to the contrary, Lilly will have no obligation to refrain from:
- •
- engaging in the same or similar business activities or lines of business as we do; or
- •
- doing business with any of our clients, customers or vendors.
Under our amended and restated articles of incorporation, neither Lilly nor any officer or director of Lilly, including our directors who are also Lilly employees, except as provided therein, is liable to us or to our shareholders for breach of any fiduciary duty by reason of any of these activities.
To preserve the tax-free treatment to Lilly and its stockholders of the Separation and the potential Distribution, we may not be able to engage in certain transactions.
To preserve the tax-free treatment to Lilly and its stockholders of the Separation and the potential Distribution, under the tax matters agreement, we will be restricted from taking any action that prevents the Separation and the potential Distribution, taken together, from being tax-free for U.S. federal income tax purposes. These restrictions may limit our ability to pursue certain strategic transactions or engage in other transactions, including use of our common stock to make acquisitions and equity capital market transactions that might increase the value of our business.
We will incur significant charges in connection with this offering and the Separation and incremental costs as a standalone public company.
We will need to replicate or replace certain functions, systems and infrastructure to which we will no longer have the same access after this offering. We may also need to make investments or hire additional employees to operate without the same access to Lilly's existing operational and administrative infrastructure. These initiatives may be costly to implement. Due to the scope and complexity of the underlying projects relative to these efforts, the amount of total costs could be materially higher than our estimate, and the timing of the incurrence of these costs is subject to change.
Lilly currently performs or supports many important corporate functions for our company. Our combined financial statements reflect charges for these services on an allocated basis. Following this offering, many of these services will be governed by our transitional services agreement with Lilly. Under the transitional services agreement we will be able to use these Lilly services for a fixed term established on a service-by-service basis. Partial reduction in the provision of any service or
46
termination of a service prior to the expiration of the applicable fixed term requires Lilly's consent. In addition, either party will be able to terminate the agreement due to a material breach of the other party, upon prior written notice, subject to limited cure periods or if the other party undergoes a change of control.
We will pay Lilly mutually agreed-upon fees for these services, which will be based on Lilly's costs (including third-party costs) of providing the services through March 31, 2021 and subject to a mark-up of 7% thereafter, with additional inflation-based escalation beginning January 1, 2022. However, since our transitional services agreement was negotiated in the context of a parent-subsidiary relationship, the terms of the agreement, including the fees charged for the services, may be higher or lower than those that would be agreed to by parties bargaining at arm's length for similar services and may be higher or lower than the costs reflected in the allocations in our historical financial statements. In addition, while these services are being provided to us by Lilly, our operational flexibility to modify or implement changes with respect to such services or the amounts we pay for them will be limited. Prior to the Distribution, if effected, Lilly will have the unilateral right to resolve disputes under the transitional services agreement.
We may not be able to replace these services or enter into appropriate third-party agreements on terms and conditions, including cost, comparable to those that we will receive from Lilly under our transitional services agreement. Additionally, after the transitional services agreement terminates, we may be unable to sustain the services at the same levels or obtain the same benefits as when we were receiving such services and benefits from Lilly. When we begin to operate these functions separately, if we do not have our own adequate systems and business functions in place, or are unable to obtain them from other providers, we may not be able to operate our business effectively or at comparable costs, and our profitability may decline. In addition, we have historically received informal support from Lilly, which may not be addressed in our transitional services agreement. The level of this informal support will diminish or be eliminated following this offering.
In addition, our historical combined financial statements include the attribution of certain assets and liabilities that historically have been held at the Lilly corporate level but which are specifically identifiable or attributable to the businesses being transferred to us in connection with the Separation. The value of the assets and liabilities we assume in connection with the Separation could ultimately be materially different than such attributions, which could have a material adverse effect on our financial condition.
Lilly's rights as licensor under the intellectual property and technology license agreement could limit our ability to develop and commercialize certain products.
Prior to the Separation, we had the ability to leverage certain of Lilly's intellectual property. As part of the Separation, we intend to enter into an intellectual property and technology license agreement. Pursuant to the intellectual property and technology license agreement, Lilly will license to us certain of its intellectual property (excluding trademarks) related to the animal health business and a license for us to use Lilly's proprietary compound library for a period of two years plus up to three additional one-year periods, each such period to be granted under Lilly's sole discretion. If we fail to comply with our obligations under this agreement and Lilly exercises its right to terminate it, our ability to continue to research, develop and commercialize products incorporating that intellectual property will be limited. In addition, this agreement includes limitations that affect our ability to develop and commercialize certain products, including in circumstances where Lilly has an interest in the licensed intellectual property in connection with its human health development programs. These limitations and termination rights may make it more difficult, time consuming or expensive for us to develop and commercialize certain new products, or may result in our products being later to market than those of our competitors. For a summary description of the terms of the
47
intellectual property and technology license agreement, see "Certain Relationships and Related Party Transactions — Relationship with Lilly — Intellectual Property and Technology License Agreement."
Risks Related to Our Initial Public Offering and Ownership of Our Common Stock
An active trading market for our common stock may not develop, and you may not be able to sell your common stock at or above the initial public offering price.
Prior to the completion of this offering, there has been no public market for our common stock. An active trading market for shares of our common stock may never develop or be sustained following this offering. If an active trading market does not develop, you may have difficulty selling your shares of common stock at an attractive price, or at all. The price for our common stock in this offering will be determined by negotiations among Lilly, us and representatives of the underwriters, and it may not be indicative of prices that will prevail in the open market following this offering. Further, certain of our directors, officers, employees and other parties with a connection to us have the opportunity to purchase up to % of the shares of our common stock offered in this offering at the initial public offering price in a directed share program. To the extent these individuals purchase shares in this offering, fewer shares may be actively traded in the public market because these stockholders will be restricted from selling the shares by a 180-day lock-up restriction in the case of our officers and directors, and a day lock-up restriction in the case of other individuals, which would reduce the liquidity of the market for our common stock. Consequently, you may not be able to sell your common stock at or above the initial public offering price or at any other price or at the time that you would like to sell. An inactive market may also impair our ability to raise capital by selling our common stock, and it may impair our ability to attract and motivate our employees through equity incentive awards and our ability to acquire other companies, products or technologies by using our common stock as consideration.
The price of our common stock may fluctuate substantially.
You should consider an investment in our common stock to be risky, and you should invest in our common stock only if you can withstand a significant loss and wide fluctuations in the market value of your investment. Some factors that may cause the market price of our common stock to fluctuate, in addition to the other risks mentioned in this section of the prospectus, are:
- •
- our announcements or our competitors' announcements regarding new products, enhancements, significant contracts, acquisitions or strategic
investments;
- •
- changes in earnings estimates or recommendations by securities analysts, if any, who cover our common stock;
- •
- failures to meet external expectations or management guidance;
- •
- fluctuations in our quarterly financial results or the quarterly financial results of companies perceived to be similar to us;
- •
- changes in our capital structure or dividend policy, including as a result of the Distribution, future issuances of securities, sales of large
blocks of common stock by our shareholders, including Lilly, or our incurrence of additional debt;
- •
- reputational issues;
- •
- changes in general economic and market conditions in or any of the regions in which we conduct our business;
- •
- changes in industry conditions or perceptions;
48
- •
- changes in applicable laws, rules or regulations and other dynamics; and
- •
- announcements or actions taken by Lilly as our principal shareholder.
In addition, if the market for stocks in our industry or related industries, or the stock market in general, experiences a loss of investor confidence, the trading price of our common stock could decline for reasons unrelated to our business, financial condition and results of operations. If any of the foregoing occurs, it could cause our stock price to fall and may expose us to lawsuits that, even if unsuccessful, could be costly to defend and a distraction to management.
You will incur immediate dilution as a result of this offering.
If you purchase common stock in this offering, you will pay more for your shares than the pro forma net tangible book value of your shares. As a result, you will incur immediate dilution of $ per share, representing the difference between the assumed initial public offering price of $ per share (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus) and our pro forma net tangible book deficit per share as of June 30, 2018 after giving effect to the Transactions, of $ . Accordingly, should we be liquidated at our book value, you would not receive the full amount of your investment.
Our historical combined financial data is not necessarily representative of the results we would have achieved as a standalone company and may not be a reliable indicator of our future results.
Our historical combined financial data included in this prospectus does not reflect the financial condition, results of operations or cash flows we would have achieved as a standalone company during the periods presented or those we will achieve in the future. This is primarily the result of the following factors:
- •
- our historical combined financial data does not reflect the Separation;
- •
- our historical combined financial data reflects expense allocations for certain support functions that are provided on a centralized basis
within Lilly, such as expenses for executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations that may be
higher or lower than the comparable expenses we would have actually incurred, or will incur in the future, as a standalone company;
- •
- our cost of debt and our capital structure will be different from that reflected in our historical combined financial statements;
- •
- significant increases may occur in our cost structure as a result of this offering, including costs related to public company reporting,
investor relations and compliance with the Sarbanes-Oxley Act of 2002 (the "Sarbanes-Oxley Act"); and
- •
- this offering may have a material effect on our customers and other business relationships, including supplier relationships, and may result in the loss of preferred pricing available by virtue of our reduced relationship with Lilly.
Our financial condition and future results of operations, after giving effect to the Separation, will be materially different from amounts reflected in our historical combined financial statements included elsewhere in this prospectus. As a result of the Separation, it may be difficult for investors to compare our future results to historical results or to evaluate our relative performance or trends in our business.
49
The pro forma and non-GAAP financial measures included in this prospectus are presented for informational purposes only and may not be an indication of our financial condition or results of operations in the future.
The unaudited pro forma condensed combined financial statements included in this prospectus are presented for information purposes only and are not necessarily indicative of what our actual financial condition or results of operations would have been had the Transactions been completed on the date indicated. The assumptions used in preparing the pro forma financial information may not prove to be accurate and other factors may affect our financial condition or results of operations. Accordingly, our financial condition and results of operations in the future may not be consistent with, or evident from, such pro forma financial information. The non-GAAP financial measures included in this prospectus, adjusted EBITDA and adjusted net income, include information that we use to evaluate our past performance, but you should not consider such information in isolation or as an alternative to measures of our performance determined under U.S. GAAP. For further information regarding such limitations, see "Prospectus Summary — Summary Historical Combined Financial Data and Unaudited Pro Forma Condensed Combined Financial Data."
As a standalone public company, we may expend additional time and resources to comply with rules and regulations that do not currently apply to us, and failure to comply with such rules may lead investors to lose confidence in our financial data.
As a standalone public company, we will be subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act and regulations of the NYSE. We have established all of the procedures and practices required as a subsidiary of Lilly, but we must implement others as a separate, standalone public company. Establishing such procedures and practices will increase our legal, accounting and financial compliance costs, will make some activities more difficult, time-consuming and costly and could be burdensome on our personnel, systems and resources. We will devote significant resources to address these public company requirements, including compliance programs and investor relations, as well as our financial reporting obligations. As a result, we have and will continue to incur significant legal, accounting and other expenses that we did not previously incur to comply with these rules and regulations. Furthermore, the need to establish the corporate infrastructure necessary for a standalone public company may divert some of management's attention from operating our business and implementing our strategy. However, the measures we take may not be sufficient to satisfy our obligations as a public company. In addition, we cannot predict or estimate the amount of additional costs we may incur in order to comply with these requirements.
We have made, and will continue to make, changes to our internal controls and procedures for financial reporting and accounting systems to meet our reporting obligations. In particular, as a public company, our management will be required to conduct an annual evaluation of our internal controls over financial reporting and include a report of management on our internal controls in our annual reports on Form 10-K. Under current rules, we will be subject to these requirements beginning with our annual report on Form 10-K for the year ended December 31, 2019. In addition, we will be required to have our independent registered public accounting firm attest to the effectiveness of our internal controls over financial reporting pursuant to Auditing Standard No. 5 beginning with our annual report on Form 10-K for the year ended December 31, 2019. If we are unable to conclude that we have effective internal controls over financial reporting, or if our registered public accounting firm is unable to provide us with an attestation and an unqualified report as to the effectiveness of our internal controls over financial reporting, investors could lose
50
confidence in the reliability of our financial statements, which could result in a decrease in the value of our common stock.
While we currently intend to pay a quarterly cash dividend to our common shareholders, we may change our dividend policy at any time.
Although we currently intend to pay a quarterly cash dividend to our common shareholders, we have no obligation to do so, and our dividend policy may change at any time without notice to our shareholders. We currently intend to pay a quarterly cash dividend on our common stock of $ per share commencing following the fourth quarter of 2018, subject to the discretion of our board of directors. Returns on your investment will primarily depend on the appreciation, if any, in the price of our common stock. We anticipate that we will retain most of our future earnings, if any, for use in the development and expansion of our business, repayment of indebtedness and for general corporate purposes. The declaration and payment of dividends to holders of our common stock will be at the discretion of our board of directors in accordance with applicable law after taking into account various factors, including our financial condition, results of operations, current and anticipated cash needs, cash flows available in the U.S., impact on our effective tax rate, indebtedness, legal requirements and other factors that our board of directors deems relevant.
The distributions we pay on our common stock may not qualify as dividends for U.S. federal income tax purposes, which could adversely affect the U.S. federal income tax consequences to you of owning our common stock.
Generally, any distributions that we make to a stockholder with respect to its shares of our common stock will constitute a dividend for U.S. federal income tax purposes to the extent of our current or accumulated earnings and profits as determined for U.S. federal income tax purposes. We had no accumulated earnings and profits, as determined for U.S. federal income tax purposes, as of June 30, 2018. Furthermore, our ability to generate earnings and profits, as determined for U.S. federal income tax purposes, in any future year is subject to a number of variables that are uncertain and difficult to predict.
Generally, any distribution not constituting a dividend under the rules described above will be treated as first reducing your adjusted basis in your shares of our common stock and, to the extent that the distribution exceeds your adjusted basis in your shares of our common stock, as gain from the sale or exchange of such shares, and if you are a domestic corporation, you will not be entitled to claim, with respect to such non-dividend distribution, a "dividends-received" deduction, which generally applies to dividends received from other domestic corporations.
Prospective foreign investors should see "Material U.S. Federal Income and Estate Tax Considerations for Non-U.S. Holders" for a more detailed description of the material U.S. federal income tax consequences of the ownership and disposition of shares of our common stock to such investors.
Applicable laws and regulations, provisions of our Amended and Restated Articles of Incorporation and our Amended and Restated Bylaws and certain contractual rights granted to Lilly may discourage takeover attempts and business combinations that shareholders might consider in their best interests.
Applicable laws, provisions of our amended and restated articles of incorporation and our amended and restated bylaws and certain contractual rights that will be granted to Lilly under the master separation agreement may delay, deter, prevent or render more difficult a takeover attempt that our shareholders might consider in their best interests. For example, they may prevent our shareholders from receiving the benefit from any premium to the market price of our common stock
51
offered by a bidder in a takeover context. Even in the absence of a takeover attempt, the existence of these provisions may adversely affect the prevailing market price of our common stock if they are viewed as discouraging takeover attempts in the future.
Our amended and restated articles of incorporation and our amended and restated bylaws contain provisions that are intended to encourage prospective acquirers to negotiate with our board of directors rather than to attempt a hostile takeover, which could deter coercive takeover practices and inadequate takeover bids. These provisions provide for:
- •
- a board of directors divided into three classes with staggered terms;
- •
- advance notice requirements regarding how our shareholders may present proposals or nominate directors for election at shareholder meetings
(except for Lilly's designation of persons for nomination by the board of directors);
- •
- the right of our board of directors to issue one or more series of preferred stock with such powers, rights and preferences as the board of
directors shall determine;
- •
- only the board of directors to fill newly-created directorships or vacancies on our board of directors;
- •
- limitations on the ability of shareholders to call special meetings of shareholders and require that all shareholder action be taken at a
meeting rather than by written consent;
- •
- a two-thirds shareholder vote requirement to amend our amended and restated articles of
incorporation;
- •
- the exclusive right of our board of directors to amend our amended and restated bylaws; and
- •
- the requirement that a 662/3% vote is necessary to remove directors.
These limitations may adversely affect the prevailing market price and market for our common stock if they are viewed as limiting the liquidity of our stock or discouraging takeover attempts in the future.
The Distribution or future sales of our common stock or securities convertible into or exchangeable for common stock, or the perception that the Distribution or such sales may occur, could depress the market price of our common stock.
We are unable to predict with certainty whether or when Lilly will sell a substantial number of shares of our common stock to the extent it retains shares following the Distribution or in the event the Distribution does not occur. The Distribution or sale by Lilly of a substantial number of shares after this offering, or a perception that the Distribution or such sales could occur, could significantly reduce the market price of our common stock.
We may also issue additional shares of common stock or convertible debt securities to finance future acquisitions or for other corporate purposes. Sales of substantial amounts of our common stock in the public market, or the perception that such sales could occur, could adversely affect the market price of our common stock. Further, we cannot predict the size of future issuances of our common stock or the effect, if any, that future issuances and sales of our common stock will have on the market price of our common stock. Any such issuance could result in substantial dilution to our existing shareholders.
52
After the expiration of the lock-up period, there may be sales of a substantial amount of our common stock by our current shareholders, and these sales could cause the price of our common stock to decline.
Lilly and our executive officers and directors have entered into lock-up agreements with the underwriters under which they have agreed, subject to specific exceptions, not to sell, directly or indirectly, any shares of common stock without the permission of each of Goldman Sachs & Co. LLC, J.P. Morgan Securities LLC and Morgan Stanley and Co. LLC for a period of 180 days following the date of this prospectus. We refer to such period as the lock-up period. When the lock-up period expires, we and our shareholders subject to a lock-up agreement will be able to sell shares of our common stock in the public market. In addition, Goldman Sachs & Co. LLC, J.P. Morgan Securities LLC and Morgan Stanley and Co. LLC may, in their sole discretion, release all or some portion of the shares subject to lock-up agreements at any time and for any reason. See "Shares Eligible for Future Sale." Sales of a substantial number of such shares upon expiration of the lock-up agreements, the perception that such sales may occur, or early release of these agreements, could cause our market price to decline or make it more difficult for you to sell your common stock at a time and price that you deem appropriate.
If Lilly makes the Distribution, and there is later a determination that the Separation and the Distribution, taken together, are not tax-free for U.S. federal income tax purposes because the facts, assumptions, representations or undertakings underlying the tax opinion are incorrect or for any other reason, then Lilly and its shareholders could incur significant U.S. federal income tax liabilities, and we could incur significant liabilities.
Completion by Lilly of the Distribution is expected to be conditioned on, among other things, the receipt of an opinion of tax counsel to the effect that, among other things, the Separation, together with the Distribution, will qualify as a transaction that is tax-free for U.S. federal income tax purposes under Sections 355 and 368(a)(1)(D) of the Internal Revenue Code of 1986, as amended (the "Code"). The opinion will rely on certain facts, assumptions, representations and undertakings from Lilly and us regarding the past and future conduct of the companies' respective businesses and other matters. If any of these facts, assumptions, representations or undertakings are incorrect or not otherwise satisfied, the conclusions reached in the opinion could be adversely affected and the Separation and/or the Distribution may not qualify for tax-free treatment. Furthermore, an opinion of counsel is not binding on the IRS or the courts, and the IRS could determine on audit that the Separation, together with the Distribution, is taxable if it disagrees with the conclusions in the opinion or for other reasons, including as a result of certain significant changes in the stock ownership of Lilly or us after the Distribution. Accordingly, no assurance can be given that the IRS will not challenge the conclusions set forth in the opinion or that a court would not sustain such a challenge. If the Separation and/or the Distribution is determined to not be tax-free for U.S. federal income tax purposes, Lilly and its shareholders could incur significant U.S. federal income tax liabilities, and we could incur significant liabilities under applicable law or as a result of our indemnification obligations to Lilly under the tax matters agreement.
53
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements, including, without limitation, statements concerning our industry and our operations, performance and financial condition, including in particular, statements relating to our business, growth strategies, product development efforts and future expenses. Forward-looking statements can be identified by words such as "anticipates," "intends," "plans," "seeks," "believes," "estimates," "expects" and similar references to future periods, or by the inclusion of forecasts or projections. Examples of forward-looking statements include, but are not limited to, statements we make regarding the outlook for our future business and financial performance, such as those contained in "Management's Discussion and Analysis of Financial Condition and Results of Operations."
Forward-looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions. Because forward-looking statements relate to the future, by their nature, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. As a result, our actual results may differ materially from those contemplated by the forward-looking statements. Important factors that could cause actual results to differ materially from those in the forward-looking statements include regional, national, or global political, economic, business, competitive, market, and regulatory conditions and the following:
- •
- heightened competition, including from new innovation or generics;
- •
- the impact of disruptive innovations and advances in veterinary medical practices, animal health technologies and alternatives to
animal-derived protein;
- •
- changes in regulatory restrictions on the use of antibiotics in food animals, as well as changing market demand regarding the use of
antibiotics and productivity products;
- •
- our ability to implement our business strategies or achieve targeted cost efficiencies and gross margin improvements;
- •
- consolidation of our customers and distributors;
- •
- the success of our R&D, acquisition and licensing efforts;
- •
- unanticipated safety, quality or efficacy concerns associated with our products;
- •
- the impact of weather conditions and the availability of natural resources;
- •
- changes in U.S. foreign trade policy, imposition of tariffs or trade disputes;
- •
- the impact of global macroeconomic conditions; and
- •
- the effect of the Separation or the Distribution, if consummated, on our business.
See "Risk Factors" for a further description of these and other factors. Although we have attempted to identify important risk factors, there may be other risk factors not presently known to us or that we presently believe are not material that could cause actual results and developments to differ materially from those made in or suggested by the forward-looking statements contained in this prospectus. If any of these risks materialize, or if any of the above assumptions underlying forward-looking statements prove incorrect, actual results and developments may differ materially from those made in or suggested by the forward-looking statements contained in this prospectus. For the reasons described above, we caution you against relying on any forward-looking statements, which should also be read in conjunction with the other cautionary statements that are included elsewhere in this prospectus. Any forward-looking statement made by us in this prospectus speaks only as of the date thereof. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to publicly update or to revise any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by law. Comparisons of results for current and any prior periods are not intended to express any future trends or indications of future performance, unless specifically expressed as such, and should be viewed as historical data.
54
We estimate that the net proceeds to us from this offering will be approximately $ million, or approximately $ million if the underwriters exercise in full their option to purchase additional shares, assuming an initial public offering price of $ per share (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus), after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to pay to Lilly as consideration for the portion of its animal health businesses Lilly is contributing to us in connection with the Separation all of the net proceeds we will receive from the sale of our common stock in this offering, including any net proceeds we receive as a result of any exercise of the underwriters' overallotment option, together with the net proceeds we received from the Senior Notes Offering and the entry into the Term Facility; provided, to the extent the unrestricted cash held by us following the completion of this offering is less than (or more than) $300 million, we will retain a portion of the net proceeds (or pay additional amounts to Lilly) so that the unrestricted cash held by us for working capital and other general corporate purposes following the completion of the offering is $300 million. In addition, a portion of the consideration to be paid to Lilly will be temporarily retained by us as restricted cash in connection with the anticipated transfer to us from Lilly of certain animal health assets in certain jurisdictions that are anticipated to occur following the completion of the offering (which consideration shall be paid to Lilly if, despite our and Lilly's cooperation and commercially reasonable efforts, such transfers have not occurred prior to a date mutually agreed by us and Lilly).
Assuming no exercise of the underwriters' option to purchase additional shares, each $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus) would increase (decrease) the net proceeds to us from this offering by $ million, assuming the number of shares offered by us, as set forth on the cover of this prospectus, remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, an increase (decrease) of one million shares of common stock sold in this offering by us would increase (decrease) our net proceeds by $ million, assuming the initial public offering price of $ per share (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus), remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. However, any such changes will not impact the cash retained by us following our payment to Lilly of consideration in connection with Separation.
The foregoing represents our current intentions with respect to the use and allocation of the net proceeds of this offering based upon our present plans and business conditions, but our management will have significant flexibility and discretion in applying the net proceeds. The occurrence of unforeseen events or changed business conditions could result in application of the net proceeds of this offering in a manner other than as described in this prospectus.
55
We initially expect to pay quarterly cash dividends to holders of our common stock of $ per share commencing following the fourth quarter of 2018, subject to the discretion of our board of directors.
Our ability to pay dividends may be restricted by any future indebtedness we incur.
We are a holding company that does not conduct any business operations of our own. As a result, our ability to pay cash dividends on our common stock is dependent upon cash dividends and distributions and other transfers from our subsidiaries.
The declaration and payment of dividends to holders of our common stock will be at the discretion of our board of directors and will take into account:
- •
- general economic business conditions;
- •
- our earnings, financial condition and results of operations;
- •
- cash flows available;
- •
- our capital requirements;
- •
- our prospects;
- •
- restrictions in our debt instruments, including the indenture governing our Senior Notes and the credit agreement governing our Credit
Facilities;
- •
- legal restrictions; and
- •
- such other factors as our board of directors may deem relevant.
See "Risk Factors — Risks Related to Our Indebtedness," "Risk Factors — Risks Related to Our Initial Public Offering and Ownership of Our Common Stock — While we currently intend to pay a quarterly cash dividend to our common shareholders, we may change our dividend policy at any time," "Management's Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources," "Description of Material Indebtedness" and "Description of Capital Stock."
56
The following table sets forth our cash and cash equivalents and our capitalization as of June 30, 2018:
- •
- on a historical basis; and
- •
- on a pro forma basis to give effect to (i) the Transactions and (ii) the sale of shares of our common stock in this offering at an assumed public offering price of $ per share, (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus), and the application of the net proceeds received by us from this offering as described under "Use of Proceeds."
The information below is not necessarily indicative of what our cash and cash equivalents and capitalization would have been had the Transactions been completed as of June 30, 2018. In addition, it is not indicative of our future cash and cash equivalents and capitalization. This table should be read in conjunction with "Use of Proceeds," "Selected Historical Combined Financial Data," "Unaudited Pro Forma Condensed Combined Financial Statements," "Management's Discussion and Analysis of Financial Condition and Results of Operations," "Description of Capital Stock" and the combined financial statements and notes thereto appearing elsewhere in this prospectus.
|
June 30, 2018 |
||||||
| | | | | | | | |
|
Historical | Pro Forma For Transactions and Offering(1) |
|||||
| | | | | | | | |
|
(Dollars in millions) | ||||||
Cash and cash equivalents |
$ | 321.0 | $ | 300.0 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Debt(2): |
|||||||
Revolving Credit Facility(3) |
$ | — | $ | — | |||
Term Facility(4) |
— | 500.0 | |||||
3.912% Senior Notes due 2021 |
— | 500.0 | |||||
4.272% Senior Notes due 2023 |
— | 750.0 | |||||
4.900% Senior Notes due 2028 |
— | 750.0 | |||||
Debt issuance costs |
— | (24.5 | ) | ||||
| | | | | | | | |
Total debt |
— | 2,475.5 | |||||
| | | | | | | | |
Equity(5): |
|||||||
Net parent company investment |
7,947.5 | 5,423.3 | |||||
Common stock, no par value, shares authorized, shares issued and outstanding actual; shares authorized, shares issued and outstanding on a pro forma basis |
— | — | |||||
Additional paid in capital |
— | — | |||||
Accumulated other comprehensive income (loss) |
(360.9 | ) | (360.9 | ) | |||
| | | | | | | | |
Total equity |
7,586.6 | 5,062.4 | |||||
| | | | | | | | |
Total capitalization |
$ | 7,586.6 | $ | 7,537.9 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
- (1)
- Each $1.00 increase or decrease in the public offering price per share would increase or decrease, as applicable, our net proceeds, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by $ million (assuming no exercise of the underwriters' option to purchase additional shares), assuming the number of shares offered by us, as set forth on the cover of this prospectus, remains the same. Similarly, an
57
increase or decrease of one million shares of common stock sold in this offering by us would increase or decrease, as applicable, our net proceeds, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by $ million, based on an assumed initial public offering price of $ per share, (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus). However, any such changes will not impact the cash retained by us following our payment to Lilly of consideration in connection with the Separation. See "Use of Proceeds."
- (2)
- For
a description of our indebtedness, see "Description of Material Indebtedness."
- (3)
- On
September , 2018, we entered into a five-year $750 million senior unsecured revolving credit facility. The Revolving Facility
will not be available for borrowings until the date on which certain conditions, including the completion of this offering, are satisfied. We expect that these conditions will be met concurrently with
the completion of this offering. Subject to certain conditions, we expect to have the right to increase the Revolving Facility by up to $250 million. For the pro forma basis presentation, we
have not assumed any borrowings under the Revolving Facility.
- (4)
- On
September , 2018, we entered into a Term Facility in an amount of $500 million.
- (5)
- We have authorized preferred stock, but no preferred shares are assumed to be issued and outstanding on the date of the completion of this offering.
58
If you invest in our common stock in this offering, your ownership interest will be diluted to the extent of the difference between the initial public offering price per share of our common stock and the net tangible book value per share of our common stock upon the completion of this offering. Dilution results from the fact that the per share offering price of our common stock is in excess of the book value per share attributable to new investors.
Our pro forma net tangible book value as of June 30, 2018 was $ million, or $ per share of common stock. Pro forma net tangible book value represents the amount of total tangible assets less total liabilities after giving pro forma effect to the Transactions, and pro forma net tangible book value per share represents pro forma net tangible book value divided by the number of shares of common stock outstanding.
After giving further effect to (i) the sale of shares of common stock in this offering at the assumed initial public offering price of $ per share (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus) and (ii) the application of the net proceeds from this offering, our pro forma as adjusted net tangible book value as of June 30, 2018 would have been $ million, or $ per share. This represents an immediate increase in pro forma as adjusted net tangible book value of $ per share to our existing investors and an immediate dilution in pro forma as adjusted net tangible book value of $ per share to new investors.
The following table illustrates this dilution on a per share of common stock basis:
Assumed initial public offering price per share |
$ | ||||||
Pro forma net tangible book value per share as of June 30, 2018 |
|||||||
Increase in pro forma net tangible book value per share attributable to new investors |
$ | ||||||
| | | | | | | | |
Pro forma as adjusted net tangible book value per share after this offering and the application of the use of proceeds |
|||||||
| | | | | | | | |
Dilution in net tangible book value per share to new investors in this offering |
$ | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
If the underwriters were to fully exercise their option to purchase additional shares of our common stock, our pro forma as adjusted net tangible book value would be $ per share. This represents an increase in pro forma as adjusted net tangible book value of $ per share to our existing investors and an immediate dilution in pro forma as adjusted net tangible book value of $ per share to new investors.
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus), would increase (decrease) our pro forma as adjusted net tangible book value by $ million, or $ per share and the dilution per share to new investors by $ , in each case assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
A one million increase (decrease) in the number of shares offered by us would increase (decrease) our pro forma as adjusted net tangible book value by $ million, or $ per share, and the dilution per share to new investors by $ , in each case assuming the initial public offering price of $ per share (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus) remains the same, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
59
The following table summarizes, on a pro forma as adjusted basis as of June 30, 2018, after giving effect to this offering, the difference between our existing shareholder and new investors with respect to the number of shares of common stock purchased from us, the total consideration paid to us, or to be paid, and the average price per share paid by our existing shareholder or to be paid by new investors purchasing shares in this offering, at an assumed initial public offering price of $ per share, (the midpoint of the estimated public offering price range set forth on the cover page of this prospectus), before deducting the estimated underwriting discounts and commissions:
|
Shares Purchased |
Total Consideration |
Average Price |
|||||||||||||
| | | | | | | | | | | | | | | | | |
|
Number | Percent | Amount | Percent | Per Share |
|||||||||||
| | | | | | | | | | | | | | | | | |
Existing shareholder |
% | $ | % | $ | ||||||||||||
New investors |
||||||||||||||||
| | | | | | | | | | | | | | | | | |
Total |
100.0 | % | $ | 100.0 | % | $ | ||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
If the underwriters were to fully exercise their option to purchase additional shares of our common stock, the percentage of shares of our common stock held by our existing shareholder would be %, and the percentage of shares of our common stock held by new investors would be %.
The foregoing tables and calculations exclude shares of our common stock reserved for future issuance under our equity incentive plan as of the date hereof, which will be effective upon the completion of this offering. To the extent the options are exercised, there will be further dilution to new investors.
The above discussion and tables are based on the number of shares outstanding at June 30, 2018. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of such securities could result in further dilution to our shareholders.
60
SELECTED HISTORICAL COMBINED FINANCIAL DATA
The following tables set forth our selected historical combined financial data for the periods indicated below.
The selected historical combined statement of operations data for the six months ended June 30, 2018 and 2017 and the selected historical combined balance sheet data as of June 30, 2018 and 2017 presented below have been derived from our unaudited combined financial statements included elsewhere in this prospectus. The selected historical combined statement of operations data for the years ended December 31, 2017, 2016 and 2015 and the selected historical combined balance sheet data as of December 31, 2017 and 2016 presented below have been derived from our audited combined financial statements included elsewhere in this prospectus. The selected historical combined statement of operations data for the years ended December 31, 2014 and 2013 and the selected historical combined balance sheet data as of December 31, 2015, 2014 and 2013 presented below have been derived from unaudited combined financial information not included elsewhere in this prospectus.
Our combined financial statements include the attribution of certain assets and liabilities that have historically been held at the Lilly corporate level but which are specifically identifiable or attributable to us. Our combined financial statements also include expense allocations related to certain Lilly corporate functions, including executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations. These expenses have been allocated to us based on direct usage or benefit where specifically identifiable, with the remainder allocated primarily on a pro rata basis of revenue, headcount or other measures. We believe that this expense methodology, and the results thereof, is reasonable for all periods presented. However, the allocations may not be indicative of the actual expense that would have been incurred if we would have operated as an independent, publicly traded company for the periods presented. It is impractical to estimate what our standalone costs would have been for the historical periods presented.
The financial statements included in this prospectus may not be indicative of our future performance and do not necessarily reflect what our financial position and results of operations would have been had we operated as an independent, publicly traded company for the periods presented, including changes that will occur in our operations and capital structure as a result of this offering and the Separation.
61
You should read the information set forth below together with "Prospectus Summary — Summary Historical Combined Financial Data and Unaudited Pro Forma Condensed Combined Financial Data," "Management's Discussion and Analysis of Financial Condition and Results of Operations," "Capitalization" and our combined financial statements and the related notes thereto included elsewhere in this prospectus.
|
Six Months Ended June 30, |
Year Ended December 31, |
||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
|
2018 | 2017 | 2017 | 2016 | 2015 | 2014 | 2013 |
|||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
|
(Dollars in millions) | |||||||||||||||||||||
Statement of Operations Data: |
||||||||||||||||||||||
Revenue |
$ | 1,506.4 | $ | 1,437.6 | $ | 2,889.0 | $ | 2,913.5 | $ | 2,909.1 | $ | 2,066.0 | $ | 1,928.4 | ||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Costs, expenses and other: |
||||||||||||||||||||||
Cost of sales |
791.5 | 712.7 | 1,493.9 | 1,409.0 | 1,533.7 | 932.6 | 842.1 | |||||||||||||||
Research and development |
126.6 | 127.9 | 251.7 | 265.8 | 291.0 | 208.5 | 216.4 | |||||||||||||||
Marketing, selling and administrative |
371.1 | 388.4 | 779.8 | 784.8 | 916.0 | 561.2 | 511.0 | |||||||||||||||
Amortization of intangible assets |
98.6 | 109.4 | 221.2 | 170.7 | 163.0 | 57.6 | 49.0 | |||||||||||||||
Asset impairment, restructuring and other special charges |
70.4 | 165.6 | 375.1 | 308.4 | 263.3 | 38.8 | — | |||||||||||||||
Other — net, (income) expense |
10.7 | 1.6 | (0.1 | ) | (2.8 | ) | 1.6 | 1.4 | 1.9 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Income (loss) before income tax expense |
37.5 | (68.0 | ) | (232.6 | ) | (22.4 | ) | (259.5 | ) | 265.9 | 308.0 | |||||||||||
Income tax expense (benefit) |
27.6 | 60.5 | 78.1 | 25.5 | (48.7 | ) | 101.0 | 117.0 | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) |
$ | 9.9 | $ | (128.5 | ) | $ | (310.7 | ) | $ | (47.9 | ) | $ | (210.8 | ) | $ | 164.9 | $ | 191.0 | ||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) as a percent of revenue |
1 | % | (9 | )% | (11 | )% | (2 | )% | (7 | )% | 8 | % | 10 | % | ||||||||
|
As of June 30, | As of December 31, |
||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
|
2018 | 2017 | 2017 | 2016 | 2015 | 2014 | 2013 |
|||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
|
(Dollars in millions) | |||||||||||||||||||||
Balance Sheet Data: |
||||||||||||||||||||||
Total assets |
$ | 8,577.4 | $ | 8,996.1 | $ | 8,940.3 | $ | 8,099.7 | $ | 8,433.6 | $ | 2,980.6 | $ | 2,163.7 | ||||||||
Total liabilities |
$ | 990.8 | $ | 1,036.4 | $ | 1,149.5 | $ | 1,071.8 | $ | 993.6 | $ | 541.0 | $ | 595.3 | ||||||||
Total equity |
$ | 7,586.6 | $ | 7,959.7 | $ | 7,790.8 | $ | 7,027.9 | $ | 7,440.0 | $ | 2,439.6 | $ | 1,568.4 | ||||||||
62
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL STATEMENTS
The following unaudited pro forma condensed combined financial statements should be read in conjunction with the section entitled "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our audited and unaudited combined financial statements and accompanying notes included elsewhere in this prospectus.
Our unaudited pro forma condensed combined financial statements consist of an unaudited pro forma condensed combined balance sheet as of June 30, 2018 and unaudited pro forma condensed combined statements of operations for the six months ended June 30, 2018 and 2017 and the year ended December 31, 2017. The unaudited pro forma condensed combined financial statements are based on and have been derived from our historical combined financial statements included elsewhere in this prospectus.
In management's opinion, the unaudited pro forma condensed combined financial statements reflect certain adjustments that are necessary to present fairly our unaudited pro forma condensed combined results of operations and our unaudited pro forma condensed combined balance sheet as of and for the periods indicated. The pro forma adjustments give effect to events that are (i) directly attributable to the transactions described below, (ii) factually supportable, and, with respect to the statement of operations, (iii) expected to have a continuing impact on us. The pro forma adjustments are based on assumptions that management believes are reasonable given the best information currently available.
The unaudited pro forma condensed combined financial statements are for illustrative and informational purposes only and are not intended to represent what our results of operations or financial position would have been had we operated as an independent, publicly traded company during the periods presented or if the transactions described below had actually occurred as of the dates indicated. The unaudited pro forma condensed combined financial statements also should not be considered indicative of our future results of operations or financial position as an independent, publicly traded company.
The unaudited pro forma condensed combined financial statements give effect to the following transactions, which we refer to as the "Transactions," as if they each had occurred on June 30, 2018 for the unaudited pro forma condensed combined balance sheet and on January 1, 2017 for the pro forma condensed combined statements of operations:
- •
- Lilly's transfer to us, through a series of equity transactions, of substantially all of its animal health businesses;
- •
- the impact of the Debt Transactions and the use of the proceeds therefrom; and
- •
- a -for- stock split of our common stock that will occur prior to the completion of this offering.
Due to local regulatory and operational requirements, in certain non-U.S. jurisdictions, the transfer of certain assets and liabilities of Lilly's animal health businesses may not legally occur prior to this offering. We have not adjusted the accompanying unaudited pro forma condensed combined balance sheet for the potential impact of the delayed transfers because these assets and liabilities are not material to our unaudited pro forma condensed combined financial statements, individually or in the aggregate.
Our combined financial statements include expense allocations related to certain Lilly corporate functions, including, but not limited to, executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations. These expenses have been allocated to us based on direct usage or benefit where specifically identifiable, with the remainder allocated primarily on a pro rata basis of revenue,
63
headcount or other measures. We believe that this expense methodology, and the results thereof, is reasonable for all periods presented. However, the allocations may not be indicative of the actual expense that would have been incurred if we would have operated as an independent, publicly traded company for the period presented. Following this offering, we expect Lilly to continue to provide us with some of the services related to these functions on a transitional basis in exchange for agreed-upon fees and we expect to incur other costs to replace the services and resources that will not be provided by Lilly. We will also incur new costs relating to our public reporting and compliance obligations as an independent, publicly traded company. We have not adjusted the accompanying unaudited pro forma condensed combined statements of operations for these estimated costs as they are projected amounts based on estimates and are not factually supportable.
The unaudited pro forma condensed combined statements of operations exclude certain non-recurring costs that we have incurred or expect to incur related to the Separation, including, among other things, the creation of a standalone infrastructure in areas such as information technology, facilities management, distribution, human resources, manufacturing, finance and other functions. We currently estimate these costs in the aggregate to be in a range from $240 million to $290 million, of which a portion will be capitalized and the remainder will be expensed.
64
UNAUDITED PRO FORMA CONDENSED COMBINED BALANCE SHEET
AS OF JUNE 30, 2018
|
Historical | Pro Forma Adjustments |
Notes | Pro Forma |
||||||||
| | | | | | | | | | | | | |
|
(Dollars in millions) | |||||||||||
Assets |
||||||||||||
Current Assets |
||||||||||||
Cash and cash equivalents |
$ | 321.0 | $ | (21.0 | ) | (a) | $ | 300.0 | ||||
Accounts receivable, net |
587.8 | — | 587.8 | |||||||||
Inventories |
1,005.6 | — | 1,005.6 | |||||||||
Other |
142.5 | — | 142.5 | |||||||||
| | | | | | | | | | | | | |
Total current assets |
2,056.9 | (21.0 | ) | 2,035.9 | ||||||||
| | | | | | | | | | | | | |
Long Term Assets |
||||||||||||
Goodwill |
2,932.3 | — | 2,932.3 | |||||||||
Other intangibles, net |
2,534.9 | — | 2,534.9 | |||||||||
Property and equipment, net |
877.7 | — | 877.7 | |||||||||
Other |
175.6 | (8.8 | ) | (b) | 166.8 | |||||||
| | | | | | | | | | | | | |
Total assets |
$ | 8,577.4 | $ | (29.8 | ) | $ | 8,547.6 | |||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Liabilities and Equity |
||||||||||||
Current liabilities |
||||||||||||
Accounts payable |
$ | 193.9 | $ | — | $ | 193.9 | ||||||
Employee compensation |
65.4 | — | 65.4 | |||||||||
Sales rebates and discounts |
127.6 | — | 127.6 | |||||||||
Other |
171.5 | 34.1 | (b) | 205.6 | ||||||||
| | | | | | | | | | | | | |
Total current liabilities |
558.4 | 34.1 | 592.5 | |||||||||
| | | | | | | | | | | | | |
Long Term liabilities |
||||||||||||
Long-term debt |
— | 2,475.5 | (c) | 2,475.5 | ||||||||
Accrued retirement benefits |
142.6 | — | 142.6 | |||||||||
Deferred taxes |
175.5 | — | 175.5 | |||||||||
Other noncurrent liabilities |
114.3 | (15.2 | ) | (b) | 99.1 | |||||||
| | | | | | | | | | | | | |
Total long term liabilities |
432.4 | 2,460.3 | 2,892.7 | |||||||||
| | | | | | | | | | | | | |
Commitments and contingencies |
||||||||||||
Equity |
||||||||||||
Net parent company investment |
7,947.5 | (2,524.2 | ) | (d) | 5,423.3 | |||||||
Common stock, no par value, shares authorized; shares issued and outstanding on a pro forma basis |
— | — | (d) | — | ||||||||
Additional paid-in capital |
— | — | (d) | — | ||||||||
Accumulated other comprehensive income (loss) |
(360.9 | ) | — | (360.9 | ) | |||||||
| | | | | | | | | | | | | |
Total equity |
7,586.6 | (2,524.2 | ) | 5,062.4 | ||||||||
| | | | | | | | | | | | | |
Total liabilities and equity |
$ | 8,577.4 | (29.8 | ) | 8,547.6 | |||||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
See accompanying Notes to Unaudited Pro Forma Condensed Combined Financial Statements.
65
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENTS OF OPERATIONS
SIX MONTHS ENDED JUNE 30, 2018
|
Historical | Pro Forma Adjustments |
Notes | Pro Forma |
||||||||
| | | | | | | | | | | | | |
|
(Dollars in millions) | |||||||||||
Revenue |
$ | 1,506.4 | $ | — | $ | 1,506.4 | ||||||
| | | | | | | | | | | | | |
Costs, expenses and other: |
||||||||||||
Cost of sales |
791.5 | — | 791.5 | |||||||||
Research and development |
126.6 | — | 126.6 | |||||||||
Marketing, selling and administrative |
371.1 | — | 371.1 | |||||||||
Amortization of intangible assets |
98.6 | — | 98.6 | |||||||||
Asset impairment, restructuring and other special charges |
70.4 | — | 70.4 | |||||||||
Other — net expense |
10.7 | 55.0 | (e) | 65.7 | ||||||||
| | | | | | | | | | | | | |
Income (loss) before income tax expense |
37.5 | (55.0 | ) | (17.5 | ) | |||||||
Income tax expense (benefit) |
27.6 | (13.2 | ) | (f) | 14.4 | |||||||
| | | | | | | | | | | | | |
Net income (loss) |
$ | 9.9 | $ | (41.8 | ) | $ | (31.9 | ) | ||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Net income (loss) per share: |
||||||||||||
Basic |
(g) | |||||||||||
Diluted |
(g) | |||||||||||
Weighted average shares outstanding: |
||||||||||||
Basic |
(g) | |||||||||||
Diluted |
(g) | |||||||||||
See accompanying Notes to Unaudited Pro Forma Condensed Combined Financial Statements.
66
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENTS OF OPERATIONS
SIX MONTHS ENDED JUNE 30, 2017
|
Historical | Pro Forma Adjustments |
Notes | Pro Forma |
||||||||
| | | | | | | | | | | | | |
|
(Dollars in millions) | |||||||||||
Revenue |
$ | 1,437.6 | $ | — | $ | 1,437.6 | ||||||
| | | | | | | | | | | | | |
Costs, expenses and other: |
||||||||||||
Cost of sales |
712.7 | — | 712.7 | |||||||||
Research and development |
127.9 | — | 127.9 | |||||||||
Marketing, selling and administrative |
388.4 | — | 388.4 | |||||||||
Amortization of intangible assets |
109.4 | — | 109.4 | |||||||||
Asset impairment, restructuring and other special charges |
165.6 | — | 165.6 | |||||||||
Other — net expense |
1.6 | 55.0 | (e) | 56.6 | ||||||||
| | | | | | | | | | | | | |
Loss before income tax expense |
(68.0 | ) | (55.0 | ) | (123.0 | ) | ||||||
Income tax expense (benefit) |
60.5 | (20.9 | ) | (f) | 39.6 | |||||||
| | | | | | | | | | | | | |
Net loss |
$ | (128.5 | ) | $ | (34.1 | ) | $ | (162.6 | ) | |||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Net loss per share: |
||||||||||||
Basic |
(g) | |||||||||||
Diluted |
(g) | |||||||||||
Weighted average shares outstanding: |
||||||||||||
Basic |
(g) | |||||||||||
Diluted |
(g) | |||||||||||
See accompanying Notes to Unaudited Pro Forma Condensed Combined Financial Statements.
67
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENTS OF OPERATIONS
YEAR ENDED DECEMBER 31, 2017
|
Historical | Pro Forma Adjustments |
Notes | Pro Forma |
||||||||
| | | | | | | | | | | | | |
|
(Dollars in millions) | |||||||||||
Revenue |
$ | 2,889.0 | $ | — | $ | 2,889.0 | ||||||
| | | | | | | | | | | | | |
Costs, expenses and other: |
||||||||||||
Cost of sales |
1,493.9 | — | 1,493.9 | |||||||||
Research and development |
251.7 | — | 251.7 | |||||||||
Marketing, selling and administrative |
779.8 | — | 779.8 | |||||||||
Amortization of intangible assets |
221.2 | — | 221.2 | |||||||||
Asset impairment, restructuring and other special charges |
375.1 | — | 375.1 | |||||||||
Other — net expense (income) |
(0.1 | ) | 110.0 | (e) | 109.9 | |||||||
| | | | | | | | | | | | | |
Loss before income tax expense |
(232.6 | ) | (110.0 | ) | (342.6 | ) | ||||||
Income tax expense (benefit) |
78.1 | (41.8 | ) | (f) | 36.3 | |||||||
| | | | | | | | | | | | | |
Net loss |
$ | (310.7 | ) | $ | (68.2 | ) | $ | (378.9 | ) | |||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Net loss per share: |
||||||||||||
Basic |
(g) | |||||||||||
Diluted |
(g) | |||||||||||
Weighted average shares outstanding: |
||||||||||||
Basic |
(g) | |||||||||||
Diluted |
(g) | |||||||||||
See accompanying Notes to Unaudited Pro Forma Condensed Combined Financial Statements.
68
Notes to Unaudited Pro Forma Condensed Combined Financial Statements
- (a)
- Reflects
an adjustment to present $300 million of cash at the balance sheet date, which is the amount of cash we will have following the completion of the
Separation.
In addition, we will hold restricted cash and an offsetting payable to Lilly at Separation, which if reflected in the pro forma balance sheet would increase total current assets and total current liabilities by the same amount. This restricted cash will be used to pay Lilly further consideration toward the purchase of our animal health businesses from Lilly.
- (b)
- Reflects
the elimination of income tax balances that will remain with Lilly upon the Separation including deferred tax assets, liability for uncertain tax positions
and income tax receivables, which were recorded net against income taxes payable on the historical balance sheet.
- (c)
- Reflects
$2.0 billion of proceeds from the Senior Notes Offering and $500 million of borrowings under the Term Facility, which are offset by estimated
debt issuance costs of $24.5 million.
- (d)
- Represents
the distribution of the net proceeds from the Senior Notes Offering to Lilly and the net impact of the removal of net assets that will not transfer to
Elanco upon Separation. The amount of proceeds distributed to Lilly may vary, based on our cash at the balance sheet date, to result in a cash balance of $300 million following the completion
of the Separation.
- (e)
- Reflects
interest expense related to the Senior Notes Offering, the Credit Facilities and amortization of the associated deferred debt issuance costs. The interest
expense has been calculated based on the fixed interest rates for the notes issued in the Senior Notes Offering and the estimated rate for borrowings under the Term Facility, which in aggregate result
in a blended interest rate of 4.2%, along with an estimated $24.5 million of debt issuance costs amortized over the term of the debt.
- (f)
- Reflects
the impact of the pro forma adjustments on income tax calculated using our U.S. statutory tax rate of 38% for the year ended December 31, 2017 and
the six months ended June 30, 2017 and 24% for the six months June 30, 2018. This represents our U.S. statutory rate during these periods, which differs from our effective rate and does
not include the tax impact of valuation allowances. The pro forma taxes have not been adjusted to reflect any change in our effective tax rate subsequent to the Separation.
- (g)
- Calculated using the number of shares of our common stock expected to be outstanding prior to the completion of the Separation and the completion of this offering.
69
THE SEPARATION AND DISTRIBUTION TRANSACTIONS
The Separation
Prior to the completion of this offering, we are a wholly-owned subsidiary of Lilly, and all of our outstanding shares of common stock are owned by Lilly.
The following are the principal steps of the Separation:
- •
- Lilly formed Elanco Animal Health Incorporated on May 3, 2018.
- •
- Prior to the completion of this offering, Lilly will transfer to us, through a series of equity transactions, substantially all of its animal
health businesses that will form our business going forward.
- •
- In exchange for substantially all of Lilly's animal health businesses, we will pay to Lilly (i) all of the net proceeds we will receive from the sale of our common stock in this offering, including any net proceeds we receive as a result of any exercise of the underwriters' overallotment option, (ii) all of the net proceeds (approximately $2.0 billion) we received in the Senior Notes Offering and (iii) all of the net proceeds ($498.6 million) we received from the entry into the Term Facility; provided, to the extent the unrestricted cash held by us following the completion of this offering is less than (or more than) $300 million, we will retain a portion of the net proceeds (or pay additional amounts to Lilly) so that the unrestricted cash held by us for working capital and other general corporate purposes following the completion of this offering is $300 million. In addition, a portion of the consideration to be paid to Lilly will be temporarily retained by us as restricted cash in connection with the anticipated transfer to us from Lilly of certain animal health assets in certain jurisdictions that are anticipated to occur following the completion of the offering (which consideration shall be paid to Lilly if, despite our and Lilly's cooperation and commercially reasonable efforts, such transfers have not occurred prior to a date mutually agreed by us and Lilly).
In addition, immediately prior to the completion of this offering, we and Lilly intend to enter into certain agreements that will provide a framework for our ongoing relationship with Lilly. For a description of these agreements, see "Certain Relationships and Related Party Transactions — Relationship with Lilly."
Following this offering, Lilly intends to transfer to us certain assets and liabilities of the historic Elanco animal health businesses that, due to business, regulatory or other legal constraints, could not be transferred prior to this offering.
Historically, Lilly has provided significant corporate and shared functions and resources to our business. Our historical financial statements in this prospectus reflect an allocation of these costs within the following statement of operations line items: cost of sales; R&D; marketing, selling and administrative; and other. These expense allocations for certain support functions that are provided on a centralized basis within Lilly include expenses for executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations. Following the Separation, we expect Lilly to continue to provide us with some of the services related to these functions on a transitional basis in exchange for agreed-upon fees, and we expect to incur other costs to replace the services and resources that will not be provided by Lilly. These fees and costs may be greater than or less than levels allocated in our historical financial statements. We will also incur new costs relating to our public reporting and compliance obligations as an independent, publicly traded company.
70
The Distribution
Lilly has informed us that, as of the date of this prospectus, it intends, following this offering, to make a distribution to its shareholders of all or a portion of its equity interest in us, which may include one or more distributions effected as a dividend to all Lilly shareholders, one or more offers to Lilly shareholders to exchange their Lilly shares for shares of our common stock, or any combination thereof. We refer to any such potential distribution as the "Distribution."
While, as of the date of this prospectus, Lilly intends to effect the Distribution, Lilly has no obligation to pursue or consummate any further dispositions of its ownership interest in us, including through the Distribution, by any specified date or at all. If pursued, the Distribution may be subject to various conditions, including receipt of any necessary regulatory or other approvals, the existence of satisfactory market conditions and the receipt of an opinion of counsel to the effect that such Distribution would be tax-free to Lilly and its shareholders for U.S. federal income tax purposes. The conditions to the Distribution may not be satisfied, Lilly may decide not to consummate the Distribution even if the conditions are satisfied or Lilly may decide to waive one or more of these conditions and consummate the Distribution even if all of the conditions are not satisfied.
The Distribution is not being effected pursuant to this prospectus, and the underwriters of this offering are not acting as underwriters for the Distribution.
71
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
The following is a discussion and analysis of our financial condition and results of operations as of, and for, the periods presented. You should read the following discussion and analysis of our financial condition and results of operations together with the sections entitled "Summary — Summary Historical Combined Financial Data and Unaudited Pro Forma Condensed Combined Financial Data," "Risk Factors," "Cautionary Note Regarding Forward-Looking Statements," "Selected Historical Combined Financial Data," "Unaudited Pro Forma Condensed Combined Financial Statements" and our combined financial statements and related notes thereto included elsewhere in this prospectus.
Overview
Founded in 1954 as part of Eli Lilly and Company, Elanco is a premier animal health company that innovates, develops, manufactures and markets products for companion and food animals. Headquartered in Greenfield, Indiana, we are the fourth largest animal health company in the world, with revenue of $2.9 billion for the year ended December 31, 2017. Globally, we are #1 in medicinal feed additives, #2 in poultry and #3 in cattle, measured by 2017 revenue, according to Vetnosis. We also have one of the broadest portfolios of pet parasiticides in the companion animal sector. We offer a diverse portfolio of more than 125 brands that make us a trusted partner to veterinarians and food animal producers in more than 90 countries.
We operate our business in a single segment directed at fulfilling our vision of enriching the lives of people through food — making protein more accessible and affordable — and through pet companionship — helping pets live longer, healthier lives. We advance our vision by offering products in four primary categories:
- •
- Companion Animal Disease Prevention ("CA Disease Prevention"): We have one
of the broadest parasiticide portfolios in the companion animal sector based on indications, species and formulations, with products that protect pets from worms, fleas and ticks. Combining our
parasiticide portfolio with our vaccines presence, we are a leader in the U.S. in the disease prevention category based on share of revenue.
- •
- Companion Animal Therapeutics ("CA Therapeutics"): We have a broad pain and
osteoarthritis portfolio across species, modes of action, indications and disease stages. Pet owners are increasingly treating osteoarthritis in their pets, and our Galliprant product is one of the
fastest growing osteoarthritis treatments in the U.S. We also have treatments for otitis (ear infections), as well as
cardiovascular and dermatology indications.
- •
- Food Animal Future Protein & Health ("FA Future Protein &
Health"): Our portfolio in this category, which includes vaccines, nutritional enzymes and animal-only antibiotics, serves the growing demand for
protein and includes innovative products in poultry and aquaculture production, where demand for animal health products is outpacing overall industry growth. We are focused on developing functional
nutritional health products that promote food animal health, including enzymes, probiotics and prebiotics. We are a leader in providing vaccines as alternatives to antibiotics to promote animal health
based on share of revenue.
- •
- Food Animal Ruminants & Swine ("FA Ruminants & Swine"): We have developed a range of food animal products used extensively in ruminant (e.g., cattle, sheep and goats) and swine production.
For the six months ended June 30, 2018 and 2017, our revenue was $1.5 billion and $1.4 billion, respectively, and for each of the years ended December 31, 2017, 2016 and 2015, our revenue was $2.9 billion. For the six months ended June 30, 2018 and 2017, our net income (loss)
72
was $9.9 million and $(128.5) million, respectively, our adjusted EBITDA was $306.2 million and $278.4 million, respectively, and our adjusted net income was $219.0 million and $156.4 million, respectively. For the years ended December 31, 2017, 2016 and 2015, our net income (loss) was $(310.7) million, $(47.9) million and $(210.8) million, respectively, our adjusted EBITDA was $498.9 million, $540.4 million and $393.7 million, respectively, and our adjusted net income was $250.5 million, $332.7 million and $208.7 million, respectively. For a reconciliation of adjusted EBITDA and adjusted net income to net income (loss), see "Prospectus Summary — Summary Historical Combined Financial Data and Unaudited Pro Forma Condensed Combined Financial Data."
Key Trends and Conditions Affecting Our Results of Operations
Industry Trends
The animal health industry, which focuses on both food animals and companion animals, is a growing industry that benefits billions of people worldwide.
As demand for animal protein grows, food animal health is becoming increasingly important. Factors influencing growth in demand for food animal medicines and vaccines include:
- •
- one in three people needs improved nutrition;
- •
- increased global demand for protein, particularly poultry and aquaculture;
- •
- natural resource constraints, such as scarcity of arable land, fresh water and increased competition for cultivated land, driving the need for
more efficient food production;
- •
- loss of productivity due to food animal disease and death;
- •
- increased focus on food safety and food security; and
- •
- human population growth, increased standards of living, particularly in many emerging markets, and increased urbanization.
Growth in food animal nutritional health products (enzymes, probiotics and prebiotics) is influenced, among other factors, by demand for antibiotic alternatives that can promote animal health and increase productivity.
Factors influencing growth in demand for companion animal medicines and vaccines include:
- •
- increased pet ownership globally;
- •
- pets living longer; and
- •
- increased pet spending as pets are viewed as members of the family by owners.
Product Development and New Product Launches
A key element of our targeted value creation strategy is to drive growth through portfolio development and product innovation, primarily in our three targeted growth categories. Our nine product launches between 2015 and 2017 have had a significant positive impact on our revenue over those periods, and we expect new products and innovation will continue to have a positive impact on our revenue in the future. Revenue from these product launches contributed $143.8 million to revenue for the year ended December 31, 2017 and $136.6 million to revenue for the six months ended June 30, 2018. We continue to pursue the development of new chemical and biological molecules through our approach to innovation. Our future growth and success depends on both our pipeline of new products, including new products that we may develop through joint ventures and products that we are able to obtain through license or acquisition, and the expansion
73
of the use of our existing products. We believe we are an industry leader in animal health R&D, with a track record of product innovation, business development and commercialization.
Impact of Changing Market Demand for Antibiotics
In recent years, our operational results have been, and will continue to be, affected by regulations and changing market demand relating to the use of antibiotics and other products intended to increase food animal production.
There are two classes of antibiotics used in animal health, shared-class, or medically important, antibiotics and animal-only antibiotics. Shared-class antibiotics are used to treat infectious disease caused by pathogens that occur in both humans and animals. As part of our antibiotic stewardship plan and in compliance with FDA guidance, shared-class antibiotics are labeled only for the treatment of an established need in animals and only with veterinarian oversight. However, not all pathogens that cause disease in animals are infectious in humans, and accordingly animal-only antibiotics are not used in human medicine (i.e., not medically important). From 2015 to 2017, our revenue from shared-class antibiotics declined at a CAGR of 7%, excluding the impact of foreign exchange, driven primarily by changing regulations in many markets, including the Veterinary Feed Directive, as well as changing market demand. Globally, during the first half of 2018, our revenue from shared-class antibiotics was flat, excluding the impact of foreign exchange, and represented 12% (4% from sales in North America and 8% from sales outside of North America) of our total revenue, down from 16% in the first half of 2015. From 2015 to 2017, our revenue from animal-only antibiotics grew at a CAGR of 4%, excluding the impact of foreign exchange, driven by sales outside North America, which offset a slight decline in North America. Globally, during the first half of 2018, our revenue from animal-only antibiotics grew 9%, excluding the impact of foreign exchange, and represented 24% of our total revenue, up from 21% in the first half of 2015. During 2017, as well as the first half of 2018, 86% of our revenue from animal-only antibiotics resulted from the sale of ionophores. Ionophores are a special class of animal-only antimicrobials, and because of their animal-only designation, mode of action and spectrum of activity, their use has not to date been impacted by regulations or changing market demand in many markets outside of North America.
Over the past two years, we have intentionally shifted away from shared-class antibiotics, and are focusing on animal-only antibiotics, as well as antibiotic-free solutions. When an animal-only antibiotic exists, we believe it should be the first, preferred antibiotic treatment. Antibiotic resistance concerns, or other health concerns regarding food animal products, may result in additional restrictions, expanded regulations or changes in market demand to further reduce the use of antibiotics in food animals. We believe it is important to protect the benefits of antibiotics in human medicine, while responsibly protecting the health of food animals and the safety of our food supply.
Impact of Competition
The animal health industry is competitive. Established animal health companies who consistently deliver high quality products enjoy brand loyalty from their customers, which often continues after the loss of patent-based or regulatory exclusivity. In 2017, approximately 75% of our revenue was from products that did not have patent protection. In animal health, while potentially significant, erosion from generic competition is often not as steep as in human health, with the originator often retaining a significant market share. While our largest product, Rumensin, has been subject to generic competition for monensin outside the U.S. for more than 10 years, our revenue from Rumensin sales outside the U.S. grew 10% from 2015 to 2017. However, generic competition can nevertheless significantly affect our results. We have experienced significant competitive headwinds from generic ractopamine in the U.S. In the third quarter of 2013, a large, established animal health company received U.S. approval for generic ractopamine. U.S. revenue for Optaflexx,
74
our ractopamine beef product, has declined at a CAGR of 28% from 2015 to 2017 as a result of generic competition and the impact of international regulatory restrictions. In 2017, we had an estimated 64% market share of all U.S. ractopamine-treated beef cattle based on management estimates.
Although we believe brand loyalty is an important contributor to a product's ongoing success, the animal health industry is also impacted by innovation. We experienced an innovation lag in the companion animal parasiticide space from 2015 to 2017. In the absence of a competitive combined oral flea and tick product, our U.S. companion animal parasiticide portfolio revenue declined 15% in 2017, excluding the impact on revenue resulting from a reduction in inventory levels within our distribution channel. In February 2018, we launched Credelio in the U.S. for the treatment of fleas and ticks. Since the launch of Credelio, our U.S. parasiticide portfolio has returned to growth.
Productivity
Our results during the periods presented have benefitted from operational and productivity initiatives that we have implemented following our recent acquisitions and in response to changing market demand for antibiotics and other headwinds. We estimate that these initiatives have generated more than $500 million in annualized cost savings from the beginning of 2015 through the end of 2017.
Our acquisitions of Lohmann Animal Health in 2014, Novartis Animal Health in 2015 and the BI Vetmedica U.S. vaccines portfolio in 2017 added in aggregate $1.4 billion in revenue, 4,500 full-time employees, 12 manufacturing and eight R&D sites. In addition, from 2015 to 2017, changing market demand for antibiotics and other headwinds, such as competition with generics and innovation, affected some of our highest gross margin products, resulting in a change to our product mix and driving operating margin lower. In response, we implemented a number of initiatives across manufacturing, R&D and SG&A. Our manufacturing cost savings strategies included improving manufacturing processes and headcount through lean manufacturing (minimizing waste while maintaining productivity), closing three manufacturing sites, consolidating our CMO network, strategically insourcing certain projects, and pursuing cost savings opportunities with respect to raw materials via a new procurement process. Additional cost savings resulted from reducing the number of R&D sites from 16 to nine, SG&A savings from sales force consolidation, and reducing discretionary and other G&A operating expense.
Foreign Exchange Rates
Significant portions of our revenue and costs are exposed to changes in foreign exchange rates. Our products are sold in more than 90 countries and, as a result, our revenue is influenced by changes in foreign exchange rates. For the year ended December 31, 2017, approximately 50% of our revenue was denominated in foreign currencies. We seek to manage our foreign exchange risk, in part, through operational means, including managing same-currency revenue in relation to same-currency costs and same-currency assets in relation to same-currency liabilities. As we operate in multiple foreign currencies, including the euro, British pound, Brazilian real, Australian dollar, Japanese yen, Canadian dollar, Chinese yuan, and other currencies, changes in those currencies relative to the U.S. dollar will impact our revenue, cost of goods and expenses, and consequently, net income. Exchange rate fluctuations in emerging markets may also have an impact beyond our reported financial results and directly impact operations. These fluctuations may affect the ability to buy and sell our products between markets impacted by significant exchange rate variances. Foreign exchange rates had a negligible effect on revenue in 2017 as compared to 2016 and had a negative impact of 2% in 2016 as compared to 2015.
75
General Economic Conditions
In addition to industry-specific factors, we, like other businesses, face challenges related to global economic conditions. Growth in both the food animal and companion animal sectors is driven in part by overall economic development and related growth, particularly in many emerging markets. In recent years, certain of our customers and suppliers have been affected directly by economic downturns, which decreased the demand for our products.
The cost of our products to food animal producers is small relative to their other production costs, including feed, and the use of our products is intended to improve economic outcomes for food animal producers. Similarly, industry sources have reported that pet owners indicated a preference for reducing spending on other aspects of their lifestyle, including entertainment, clothing and household goods, before reducing spending on pet care. While these factors have mitigated the impact of recent downturns in the global economy, further economic challenges could increase cost sensitivity among our customers, which may result in reduced demand for our products and could have a material adverse effect on our financial condition and results of operations.
Weather Conditions and the Availability of Natural Resources
The animal health industry and demand for many of our animal health products in a particular region are affected by weather conditions, varying weather patterns and weather-related pressures from pests, such as fleas and ticks. As a result, we may experience regional and seasonal fluctuations in our results of operations.
Food animal producers depend on the availability of natural resources, including large supplies of fresh water. Their animals' health and their ability to operate could be adversely affected if they experience a shortage of fresh water due to human population growth or floods, droughts or other weather conditions.
Drought conditions could negatively impact, among other things, the supply of corn and the availability of grazing pastures. A decrease in harvested corn results in higher corn prices, which could negatively impact the profitability of food animal producers of ruminants, pork and poultry. Higher corn prices and reduced availability of grazing pastures contribute to reductions in herd or flock sizes that in turn result in less spending on animal health products. As such, a prolonged drought could have a material adverse effect on our financial condition and results of operations. Factors influencing the magnitude and timing of effects of a drought on our performance include, but may not be limited to, weather patterns and herd management decisions.
In addition, veterinary hospitals and practitioners depend on visits from and access to the animals under their care. Veterinarians' patient volume and ability to operate could be adversely affected if they experience prolonged snow, ice or other severe weather conditions, particularly in regions not accustomed to sustained inclement weather. Adverse weather conditions or a shortage of fresh water may cause veterinarians and food animal producers to purchase less of our products.
Disease Outbreaks
Sales of our food animal products could be adversely affected by the outbreak of disease carried by animals. Outbreaks of disease may reduce regional or global sales of particular animal-derived food products or result in reduced exports of such products, either due to heightened export restrictions or import prohibitions, which may reduce demand for our products. Also, the outbreak of any highly contagious disease near our main production sites could require us to immediately halt production of our products at such sites or force us to incur substantial expenses
76
in procuring raw materials or products elsewhere. Alternatively, sales of products that treat specific disease outbreaks may increase.
Manufacturing and Supply
In order to sell our products, we must be able to reliably produce and ship our products in sufficient quantities. Many of our products involve complex manufacturing processes and are sole-sourced from certain manufacturing sites.
Minor deviations in our manufacturing or logistical processes, unpredictability of a product's regulatory or commercial success or failure, the lead time necessary to construct highly technical and complex manufacturing sites, and shifting customer demand increase the potential for capacity imbalances.
Components of Revenue and Costs and Expenses
Revenue
Our revenue is primarily derived from sales of our products to third-party distributors, and directly to food producers and veterinarians. For additional information regarding our products, including descriptions of our products, see "Business — Products."
We aggregate our products into five categories to understand revenue growth:
- •
- CA Disease Prevention includes parasiticides and vaccine products for dogs and cats;
- •
- CA Therapeutics includes products for the treatment of pain, osteoarthritis, otitis,
cardiovascular and dermatology indications in dogs and cats;
- •
- FA Future Protein & Health includes vaccines, antibiotics, parasiticides and other
products used in poultry and aquaculture production, as well as functional nutritional health products, including enzymes, probiotics and prebiotics;
- •
- FA Ruminants & Swine includes vaccines, antibiotics, implants, parasiticides, and other
products used in ruminants and swine production, as well as certain other food animal products; and
- •
- Strategic Exits includes business activities that we have either exited or made the strategic decision to exit, including the transitional contract manufacturing activity that we acquired in connection with our acquisition of the BI Vetmedica U.S. vaccines portfolio, two terminated legacy U.S. distribution agreements, a terminated distribution agreement outside the U.S. and an equine product not core to our business.
Costs and Expenses and Other
Cost of sales consists primarily of cost of materials, facilities and other infrastructure used to manufacture our products, shipping and handling, inventory losses and expired products.
Marketing, selling and administrative expenses consist of, among other things, the costs of marketing, promotion and advertising and the costs of administration (business technology, facilities, legal, finance, human resources, business development, external affairs and procurement).
Amortization of intangible assets consist of the amortization expense for intangible assets that have been acquired through business combinations.
R&D expenses consist of project costs specific to new product R&D and product lifecycle management, overhead costs associated with R&D operations, regulatory, product registrations and investments that support local market clinical trials for approved indications. We manage overall
77
R&D based on our strategic opportunities and do not disaggregate our R&D expenses incurred by nature or by product as we do not use or maintain such information in managing our business.
Asset impairment, restructuring and other special charges consists primarily of impairment of long-term assets, restructuring charges, costs associated with acquiring and integrating businesses, and certain non-recurring expenses.
Other — (income) deductions consists of net interest (income)/expense, realized or unrealized foreign exchange losses and loss or impairment on other investments.
Comparability of Historical Results
Our historical results of operations for the periods presented may not be comparable with prior periods or with our results of operations in the future. In addition to the factors identified in "— Key trends and Conditions Affecting Our Results of Operations," the following factors, among others, have impacted or may impact the comparability of our results of operations.
Our Relationship with Lilly and Additional Standalone Costs
Our business is currently operated as part of a division of Lilly. Our combined financial statements have been derived from Lilly's consolidated financial statements and accounting records. Our combined financial statements reflect our financial position, results of operations and cash flows of the business that will be transferred to us at the time of the Separation and do not purport to reflect what the results of operations, comprehensive income/(loss), financial position, equity or cash flows would have been had we operated as an independent, publicly traded company during the periods presented.
Our historical results reflect an allocation of costs for certain Lilly corporate costs, including, among others, executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations. These allocations are not necessarily indicative of the expenses we may incur in the future as a standalone public company. Although we intend to enter into certain agreements with Lilly in connection with this offering and the Separation, the amount and composition of our expenses may vary from historical levels since the fees charged for the services under the agreement may be higher or lower than the costs reflected in the historical allocations. In addition, we intend to replace these services over time with ones supplied either internally by our employees or by third parties, the cost of which may be higher or lower than the historical allocations. During the six months ended June 30, 2018 and 2017, corporate overhead and other allocations were $71.1 million and $71.7 million, respectively. During the three years ended December 31, 2017, 2016 and 2015, corporate overhead and other allocations were $151.7 million, $145.3 million and $156.0 million, respectively. See Note 10: Related Party Transactions to our unaudited interim combined financial statements and Note 16: Related Party Transactions to our audited combined financial statements.
We are currently investing in expanding our own administrative functions, including, but not limited to, information technology, facilities management, distribution, human resources and manufacturing, to replace services currently provided by Lilly. Because of initial stand-up costs and overlaps with services currently provided by Lilly, we expect to incur certain temporary, duplicative expenses in connection with the Separation as we replace the services provided by Lilly. We also expect to incur costs related to the build out of processes and systems to support finance and global supply and logistics, among others. We currently estimate these costs in the aggregate to be in a range from $240 million to $290 million, of which a portion will be capitalized and the remainder will be expensed. See "Certain Relationships and Related Party Transactions — Relationship with Lilly."
78
Lilly utilizes a centralized treasury management system. Our combined financial statements reflect cash held only in bank accounts in our legal name and no allocation of combined cash positions. Our combined financial statements also do not reflect an allocation of Lilly's debt and do not reflect the $2.0 billion aggregate principal amount of Senior Notes we issued in a private placement on August 28, 2018 or the $1.25 billion aggregate principal amount of the Credit Facilities that we entered into on September , 2018, and our historical expenses do not reflect the interest expense related thereto that we expect to pay going forward.
For the purposes of our combined financial statements, income tax expense (benefit) is computed on a separate company basis, as if operated as a standalone entity or a separate consolidated group in each material jurisdiction in which we operate. Our financial statements reflect certain deferred tax assets and liabilities and income taxes payable based on this approach that may not transfer to us upon the Separation, as the underlying tax attributes may have been used by Lilly or may be retained by Lilly. As a result of potential changes to our business model and the fact that these deferred tax assets and liabilities and income taxes payable may not transfer to us, income tax expense (benefit) included in the combined financial statements may not be indicative of our future expected tax rate.
See "Unaudited Pro Forma Condensed Combined Financial Statements."
Our historical results also do not reflect the impact of costs we expect to incur as a consequence of becoming a standalone company, including a change in our compensation policies and programs and incremental costs associated with being a publicly traded company.
We expect to institute competitive compensation policies and programs as a standalone public company, the expense for which may differ from the compensation expense allocated by Lilly in our combined financial statements.
As a result of this offering, we will become subject to the reporting requirements of the Exchange Act and the Sarbanes-Oxley Act. We will have additional procedures and practices to establish as a standalone public company. As a result, we will incur additional costs as a standalone public company, including internal audit, external audit, investor relations, stock administration, stock exchange fees and regulatory compliance costs.
Recent Significant Acquisitions
Our financial results have been impacted by acquisitions and integrations. For the periods presented, these include primarily the acquisitions and integrations of Novartis Animal Health, which closed on January 1, 2015, certain rights to develop, manufacture, market and commercialize Galliprant outside the U.S. and co-promote it in the U.S. from Aratana Therapeutics, Inc., which closed on April 22, 2016, and BI Vetmedica U.S. vaccines portfolio, which closed on January 3, 2017. For more information, see Note 4: Acquisitions to our audited combined financial statements.
Asset Impairment, Restructuring and Other Special Charges
During 2015 to 2018, including in connection with the productivity initiatives described above, we incurred charges related to asset impairment, restructuring and other special charges, including integration of acquired businesses. These charges include severance costs resulting from actions taken to reduce our cost structure, asset impairment charges related to competitive pressures for certain companion animal products, product exits and site closures, and integration costs related to acquired businesses, primarily Novartis Animal Health.
For more information on these charges, see Note 4: Asset Impairment, Restructuring and Other Special Charges to our unaudited interim combined financial statements and Note 5: Asset Impairment, Restructuring and Other Special Charges to our audited combined financial statements.
79
Results of Operations
The following discussion and analysis of our combined statements of operations should be read along with our combined financial statements and the notes thereto included elsewhere in this prospectus, which reflect the results of operations of the business to be transferred to us from Lilly. For more information on the combined basis of preparation, see Note 1: Nature of Business and Basis of Presentation to our unaudited interim combined financial statements and Note 1: Nature of Business and Basis of Preparation to our audited combined financial statements.
|
Six Months Ended June 30, |
% Change | Year Ended December 31, | % Change |
|||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
(Dollars in millions) |
2018 | 2017 | 18/17 | 2017 | 2016 | 2015 | 17/16 | 16/15 |
|||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Revenue |
$ | 1,506.4 | $ | 1,437.6 | 5 | % | $ | 2,889.0 | $ | 2,913.5 | $ | 2,909.1 | (1 | )% | 0 | % | |||||||||
Costs, expenses and other: |
|||||||||||||||||||||||||
Cost of sales |
791.5 | 712.7 | 11 | % | 1,493.9 | 1,409.0 | 1,533.7 | 6 | % | (8 | )% | ||||||||||||||
% of revenue |
53 | % | 50 | % | 3 | % | 52 | % | 48 | % | 53 | % | |||||||||||||
Research and development |
126.6 | 127.9 | (1 | )% | 251.7 | 265.8 | 291.0 | (5 | )% | (9 | )% | ||||||||||||||
% of revenue |
8 | % | 9 | % | (1 | )% | 9 | % | 9 | % | 10 | % | |||||||||||||
Marketing, selling and administrative |
371.1 | 388.4 | (4 | )% | 779.8 | 784.8 | 916.0 | (1 | )% | (14 | )% | ||||||||||||||
% of revenue |
25 | % | 27 | % | (2 | )% | 27 | % | 27 | % | 31 | % | |||||||||||||
Amortization of intangible assets |
98.6 | 109.4 | (10 | )% | 221.2 | 170.7 | 163.0 | 30 | % | 5 | % | ||||||||||||||
% of revenue |
7 | % | 8 | % | (1 | )% | 8 | % | 6 | % | 6 | % | |||||||||||||
Asset impairment, restructuring and other special charges |
70.4 | 165.6 | (57 | )% | 375.1 | 308.4 | 263.3 | 22 | % | 17 | % | ||||||||||||||
Other — (income) expense |
10.7 | 1.6 | NM | (0.1 | ) | (2.8 | ) | 1.6 | NM | NM | |||||||||||||||
Income (loss) before income taxes |
$ | 37.5 | $ | (68.0 | ) | NM | $ | (232.6 | ) | $ | (22.4 | ) | $ | (259.5 | ) | NM | NM | ||||||||
% of revenue |
2 | % | (5 | )% | NM | (8 | )% | (1 | )% | (9 | )% | NM | NM | ||||||||||||
Income tax expense (benefit) |
27.6 | 60.5 | NM | 78.1 | 25.5 | (48.7 | ) | NM | NM | ||||||||||||||||
Net income (loss) |
$ | 9.9 | $ | (128.5 | ) | NM | $ | (310.7 | ) | $ | (47.9 | ) | $ | (210.8 | ) | NM | NM | ||||||||
Certain amounts and percentages may reflect rounding adjustments.
80
Revenue
On a global basis, our revenue within our product categories was as follows:
|
Six Months Ended June 30, |
% Change | Year Ended December 31, | % Change |
|||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
(Dollars in millions) |
2018 | 2017 | 18/17 | 2017 | 2016 | 2015 | 17/16 | 16/15 |
|||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
CA Disease Prevention |
$ | 415.3 | $ | 379.3 | 10 | % | $ | 660.2 | $ | 628.4 | $ | 591.2 | 5 | % | 6 | % | |||||||||
CA Therapeutics(a) |
130.6 | 118.3 | 10 | % | 260.8 | 255.6 | 245.2 | 2 | % | 4 | % | ||||||||||||||
FA Future Protein & Health |
339.3 | 291.5 | 16 | % | 649.2 | 630.8 | 633.2 | 3 | % | (0 | )% | ||||||||||||||
FA Ruminants & Swine |
579.6 | 576.9 | 0 | % | 1,175.0 | 1,309.2 | 1,356.6 | (10 | )% | (3 | )% | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Subtotal |
$ | 1,464.8 | $ | 1,366.0 | 7 | % | $ | 2,745.2 | $ | 2,824.0 | $ | 2,826.2 | (3 | )% | (0 | )% | |||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Strategic Exits(a) |
41.6 | 71.6 | (42 | )% | 143.8 | 89.5 | 82.9 | 61 | % | 8 | % | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total |
$ | 1,506.4 | $ | 1,437.6 | 5 | % | $ | 2,889.0 | $ | 2,913.5 | $ | 2,909.1 | (1 | )% | 0 | % | |||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Certain amounts and percentages may reflect rounding adjustments.
- (a)
- On June 30, 2018, we made the decision to exit an equine product not core to our business. Revenue from this product is reflected in Strategic Exits for the six months ended June 30, 2018 and 2017 and in CA Therapeutics for the years ended December 31, 2017, 2016 and 2015. Revenue from this product was $1.6 million and $1.5 million for the six months ended June 30, 2018 and 2017, respectively, and $3.4 million, $3.7 million and $3.4 million, for the years ended December 31, 2017, 2016 and 2015, respectively.
In order to represent the underlying growth trend of our portfolio during the six months ended June 30, 2018 and 2017 and the years ended December 31, 2017, 2016 and 2015, we believe that it is important to also understand revenue growth excluding the impact of incremental revenue of recent significant acquisitions, Strategic Exits and foreign exchange rates.
% Change in Revenue: |
Reported | Resulting from Revenue Growth excluding Acquisition, Strategic Exits and FX(1) |
Resulting from Acquisition(1) |
Resulting from Strategic Exits |
Resulting from FX |
|||||||||||||
| | | | | | | | | | | | | | | | | | | |
First six months of 2018 vs. first six months of 2017 |
||||||||||||||||||
Total revenue |
5 | % | 4 | % | (0 | )% | (2 | )% | 3 | % | ||||||||
CA Disease Prevention |
10 | % | 8 | % | 0 | % | 0 | % | 2 | % | ||||||||
CA Therapeutics |
10 | % | 5 | % | 0 | % | 0 | % | 5 | % | ||||||||
FA Future Protein & Health |
16 | % | 12 | % | 0 | % | 0 | % | 5 | % | ||||||||
FA Ruminants & Swine |
0 | % | (2 | )% | 0 | % | 0 | % | 2 | % | ||||||||
Subtotal |
7 | % | 4 | % | 0 | % | 0 | % | 3 | % | ||||||||
Strategic Exits |
(42 | )% | 0 | % | (9 | )% | (33 | )% | 1 | % | ||||||||
2017 vs. 2016 |
||||||||||||||||||
Total revenue |
(1 | )% | (8 | )% | 7 | % | (1 | )% | 0 | % | ||||||||
CA Disease Prevention |
5 | % | (18 | )% | 22 | % | 0 | % | 1 | % | ||||||||
CA Therapeutics |
2 | % | 2 | % | 0 | % | 0 | % | (0 | )% | ||||||||
FA Future Protein & Health |
3 | % | 3 | % | 0 | % | 0 | % | 0 | % | ||||||||
FA Ruminants & Swine |
(10 | )% | (10 | )% | 0 | % | 0 | % | (0 | )% | ||||||||
Subtotal |
(3 | )% | (8 | )% | 5 | % | 0 | % | 0 | % | ||||||||
Strategic Exits |
61 | % | (0 | )% | 83 | % | (22 | )% | 0 | % | ||||||||
2016 vs. 2015 |
||||||||||||||||||
Total revenue |
0 | % | 2 | % | 0 | % | 0 | % | (2 | )% | ||||||||
CA Disease Prevention |
6 | % | 7 | % | 0 | % | 0 | % | (1 | )% | ||||||||
CA Therapeutics |
4 | % | 5 | % | 0 | % | 0 | % | (1 | )% | ||||||||
FA Future Protein & Health |
(0 | )% | 4 | % | 0 | % | 0 | % | (5 | )% | ||||||||
FA Ruminants & Swine |
(3 | )% | (1 | )% | 0 | % | 0 | % | (2 | )% | ||||||||
Subtotal |
(0 | )% | 2 | % | 0 | % | 0 | % | (3 | )% | ||||||||
Strategic Exits |
8 | % | 0 | % | 0 | % | 8 | % | (0 | )% |
- (1)
- Refers to the acquisition of the BI Vetmedica U.S. vaccines portfolio in January 2017.
81
For geographical information regarding our revenue, see Note 9: Geographic Information to our unaudited interim combined financial statements and Note 15: Geographic Information to our audited combined financial statements.
Total revenue
Six months ended June 30, 2018 vs. six months ended June 30, 2017
Total revenue increased $68.8 million or 5% for the six months ended June 30, 2018 as compared to the six months ended June 30, 2017, reflecting a 3% favorable foreign exchange rate impact, a 3% increase due to higher realized prices and a 1% decrease due to lower volumes.
In summary, the total revenue increase was due primarily to:
- •
- an increase in revenue of $37.3 million due to the positive impact of foreign exchange rates;
- •
- an increase in revenue of $35.8 million or 12% from FA Future Protein & Health products, excluding the impact foreign exchange
rates;
- •
- an increase in revenue of $29.3 million or 8% from CA Disease Prevention products, excluding the impact of foreign exchange rates; and
- •
- an increase in revenue of $6.4 million or 5% from CA Therapeutics products, excluding the impact of foreign exchange rates;
partially offset by:
- •
- a decrease in revenue of $30.6 million from Strategic Exits, excluding the impact of foreign exchange rates; and
- •
- a decrease in revenue of $9.3 million or 2% from FA Ruminants & Swine products, excluding the impact of foreign exchange rates.
The detailed change in revenue by product category was as follows:
- •
- CA Disease Prevention revenue increased by $36.0 million or 10% due primarily to the launches of Credelio and Interceptor
Plus, partially offset by competition in certain parasiticides, primarily
impacting Trifexis and Comfortis and favorable U.S. purchasing patterns for Trifexis in 2017.
- •
- CA Therapeutics revenue increased by $12.3 million or 10% due primarily to the growth of Galliprant, partially offset by a
temporary supply shortage of Percorten V, used for the treatment of
canine Addison's Disease.
- •
- FA Future Protein & Health revenue increased by $47.8 million or 16% due primarily to growth in animal-only antibiotics, Imvixa and AviPro.
- •
- FA Ruminants & Swine revenue increased by $2.7 million or 0% due primarily to growth in animal-only and shared-class antibiotics,
offset by competition from generic ractopamine-based products and a change in purchase patterns in the first half of 2017.
- •
- Strategic Exits revenue decreased by $30.0 million or 42% due primarily to the termination in the third quarter of 2017 of a legacy U.S. distribution agreement acquired as part of our Novartis Animal Health acquisition.
82
2017 vs. 2016
Total revenue decreased $24.5 million or 1% in 2017 as compared to 2016, reflecting a 0% foreign exchange rate impact, a 0% change due to realized prices and a 1% decrease due to lower volumes.
In summary, the total revenue decrease was due primarily to:
- •
- a decline in revenue of $133.6 million or 10% from FA Ruminants & Swine products, excluding the impact of foreign exchanges
rates; and
- •
- a decline in revenue of $113.6 million or 18% from CA Disease Prevention products, excluding the impact of acquisition and foreign exchange rates;
partially offset by:
- •
- the acquisition of the BI Vetmedica U.S. vaccines portfolio which contributed $216.7 million in 2017; and
- •
- an increase in revenue of $18.7 million or 3% from FA Future Protein & Health products, excluding the impact of foreign exchange rates.
The detailed change in revenue by product category was as follows:
- •
- CA Disease Prevention revenue increased by $31.8 million or 5%. Excluding product revenue from the acquisition of the BI Vetmedica U.S.
vaccines portfolio and the impact of foreign exchange rates, revenue declined $113.6 million or 18% due primarily to competition in certain parasiticides, primarily impacting Trifexis and
Comfortis, and a reduction in inventory levels within our U.S. companion animal
distribution channel partially offset by the growth of Interceptor Plus.
- •
- CA Therapeutics revenue increased by $5.2 million or 2% due primarily to the launch of Galliprant, partially offset by
volume declines from competition in our dermatology portfolio.
- •
- FA Future Protein & Health revenue increased by $18.4 million or 3% due primarily to growth in poultry products, including
animal-only antibiotics, enzymes and vaccines, and to a lesser extent aquaculture products.
- •
- FA Ruminants & Swine revenue decreased by $134.2 million or 10% due primarily to competition from generic ractopamine-based
products, as well as declines in shared-class antibiotics and a reduction in inventory levels within our China distribution channel, partially offset by growth in animal-only antibiotics.
- •
- Strategic Exits revenue increased by $54.3 million or 61% due primarily to the acquisition of a transitional contract manufacturing arrangement at Fort Dodge as part of the BI Vetmedica U.S. vaccines portfolio acquisition, partially offset by the termination in the third quarter of 2017 of a legacy U.S. distribution agreement acquired as part of our Novartis Animal Health acquisition.
2016 vs. 2015
Total revenue increased by $4.4 million or 0% in 2016 as compared to 2015, reflecting a 2% unfavorable foreign exchange rate impact, a 1% increase due to higher realized prices and a 1% increase due to higher volume.
In summary, the total revenue increase was due primarily to:
- •
- an increase in revenue of $44.2 million or 7% from CA Disease Prevention products, excluding the impact of foreign exchange rates;
83
- •
- an increase in revenue of $26.9 million or 4% from FA Future Protein & Health products, excluding the impact of foreign exchange
rates; and
- •
- an increase in revenue of $12.2 million or 5% from CA Therapeutics products, excluding the impact of foreign exchange rates;
partially offset by:
- •
- a decline in revenue of $70.9 million due to the negative impact of foreign exchange rates; and
- •
- a decline in revenue of $14.6 million from FA Ruminants & Swine products, excluding the impact of foreign exchange rates.
The detailed change in revenue by product category was as follows:
- •
- CA Disease Prevention revenue increased by $37.2 million or 6% due primarily to the growth of Interceptor
Plus, partially offset by competition in certain parasiticides, primarily impacting Trifexis and Comfortis, and the impact of
foreign exchange rates.
- •
- CA Therapeutics revenue increased by $10.4 million or 4% due primarily to the growth of Osurnia, partially offset by the
impact of foreign exchange rates.
- •
- FA Future Protein & Health revenue decreased by $2.4 million or 0% due primarily to the negative impact of foreign exchange
rates, partially offset by growth in poultry including animal-only antibiotics, enzymes and vaccines.
- •
- FA Ruminants & Swine revenue decreased by $47.4 million or 3% due primarily to the negative impact of foreign exchange rates,
declines in shared-class antibiotics and competition from generic ractopamine-based products, partially offset by growth in vaccines and animal-only antibiotics.
- •
- Strategic Exits revenue increased by $6.6 million or 8% due primarily to revenue growth under two legacy U.S. distribution agreements acquired as part of our Novartis Animal Health acquisition, which have subsequently been terminated.
Costs and Expenses and Other
Cost of sales
Six months ended June 30, 2018 vs. six months ended June 30, 2017
Cost of sales increased $78.8 million for the six months ended June 30, 2018 as compared to the six months ended June 30, 2017 due primarily to:
- •
- an unfavorable product mix as a result of decreasing revenue from higher margin products primarily resulting from generic competition and
favorable U.S. purchasing patterns for Trifexis in 2017;
- •
- higher expenses due to the impact during the first half of 2018 of the implementation of productivity initiatives, including inventory
write-offs of $40.2 million for Imrestor and other products; and
- •
- expensing of capitalized costs related to inventory produced in the prior year and contractual increases in third-party manufacturing agreements;
84
partially offset by:
- •
- approximately $42.7 million in non-recurring costs in 2017 associated with the incremental purchase accounting charges from the acquisition of the BI Vetmedica U.S. vaccines portfolio related to the fair value adjustments to inventory that was subsequently sold.
2017 vs. 2016
Cost of sales increased $84.9 million in 2017 as compared to 2016 due primarily to:
- •
- the addition of approximately $134.1 million of costs in 2017 related to the acquisition of the BI Vetmedica U.S. vaccines portfolio,
including $54.0 million associated with Strategic Exits contract manufacturing obligations and approximately $42.7 million in non-recurring costs associated with the incremental purchase
accounting charges related to the fair value adjustments to inventory acquired that was subsequently sold;
- •
- an unfavorable product mix as a result of disproportional revenue decreases of higher margin products primarily resulting from changing market
demand for antibiotics and competition headwinds; and
- •
- contractual increases in third-party manufacturing agreements;
partially offset by:
- •
- operational efficiencies and cost savings associated with manufacturing footprint consolidation and overall cost reductions.
2016 vs. 2015
Cost of sales decreased $124.7 million in 2016 as compared to 2015 due primarily to:
- •
- approximately $153.0 million in non-recurring costs in 2015 associated with the incremental purchase accounting charges of the Novartis
Animal Health acquisition related to the fair value adjustments to inventory acquired that was subsequently sold; and
- •
- operational efficiencies and cost savings associated with manufacturing footprint consolidation and overall cost reductions;
partially offset by:
- •
- expensing of capitalized costs related to inventory produced in the prior year; and
- •
- an unfavorable product mix as a result of disproportional revenue decreases of higher margin products primarily resulting from changing market demand for antibiotics and competition headwinds.
Research and development
Six months ended June 30, 2018 vs. six months ended June 30, 2017
R&D expenses decreased $1.3 million for the six months ended June 30, 2018 as compared to the six months ended June 30, 2017 due primarily to savings realized from the consolidation of acquired R&D sites and operations, as well as the termination of certain R&D projects.
2017 vs. 2016
R&D expenses decreased $14.1 million in 2017 as compared to 2016 due primarily to savings realized from the consolidation of acquired R&D sites and operations, as well as the termination of
85
certain R&D projects. This decrease was partially offset by expenses incurred in connection with the acquisition of the BI Vetmedica U.S. vaccines portfolio in 2017.
2016 vs. 2015
R&D expenses decreased $25.2 million in 2016 as compared to 2015 due primarily to savings realized from the consolidation of acquired R&D sites and operations, as well as the rationalization of R&D projects.
Marketing, selling and administrative
Six months ended June 30, 2018 vs. six months ended June 30, 2017
Marketing, selling and administrative expenses decreased $17.3 million for the six months ended June 30, 2018 as compared to the six months ended June 30, 2017 due primarily to savings from productivity initiatives related to marketing functions.
2017 vs. 2016
Marketing, selling and administrative expenses decreased $5.0 million in 2017 as compared to 2016 due primarily to savings from productivity initiatives related to salesforce, marketing and administrative functions, more than offsetting the increase from the acquisition of the BI Vetmedica U.S. vaccines portfolio.
2016 vs. 2015
Marketing, selling and administrative expenses decreased $131.2 million in 2016 as compared to 2015 due primarily to cost savings realized from the acquisition and integration of Novartis Animal Health, as well as additional productivity initiatives.
Amortization of intangible assets
Six months ended June 30, 2018 vs. six months ended June 30, 2017
Amortization of intangible assets decreased $10.8 million in the six months ended June 30, 2018 as compared to the six months ended June 30, 2017 due primarily to the acceleration of amortization related to certain product exits that occurred in 2017.
2017 vs. 2016
Amortization of intangible assets increased $50.5 million in 2017 as compared to 2016 due primarily to the impact of the acquisition of the BI Vetmedica U.S. vaccines portfolio and, to a lesser extent, the acceleration of amortization related to certain product exits.
2016 vs. 2015
Amortization of intangible assets increased $7.7 million in 2016 as compared to 2015 due primarily to the impact of the completion of acquired in-process R&D assets, which triggered amortization, as well as the acquisition of Galliprant.
Asset impairment, restructuring and other special charges
For additional information regarding our asset impairment, restructuring and other special charges, see Note 4: Asset Impairment, Restructuring and Other Special Charges to our unaudited interim combined financial statements and Note 5: Asset Impairment, Restructuring and Other Special Charges to our audited combined financial statements.
86
Six months ended June 30, 2018 vs. six months ended June 30, 2017
Asset impairment, restructuring and other special charges decreased $95.2 million in the six months ended June 30, 2018 as compared to the six months ended June 30, 2017 due primarily to:
- •
- higher severance costs recognized in 2017 due to the U.S. voluntary exit program offered to our employees and higher integration costs recognized in 2017 relating to our acquired businesses;
partially offset by:
- •
- higher asset impairments and other charges due to the decision to close a manufacturing facility in the U.S. as well as the decision to suspend commercialization of Imrestor.
2017 vs. 2016
Asset impairment, restructuring and other special charges increased $66.7 million in 2017 as compared to 2016 due primarily to:
- •
- higher severance costs recognized in 2017 due to the U.S. voluntary early retirement program offered to our employees;
partially offset by:
- •
- lower integration costs relating to our acquired businesses.
2016 vs. 2015
Asset impairment, restructuring and other special charges increased $45.1 million in 2016 as compared to 2015 due primarily to:
- •
- higher asset impairment charges due to the closure of a manufacturing facility in Ireland in 2016.
Income Tax Expense (benefit)
Our historical income tax expense (benefit) may not be indicative of our future expected tax rate. See "— Comparability of Historical Results — Our Relationship with Lilly and Additional Standalone Costs."
Six months ended June 30, 2018 vs. six months ended June 30, 2017
Income tax expense, as reported, decreased $32.9 million due primarily to a decrease in the U.S. valuation allowance related to the utilization of prior years' net operating losses.
2017 vs. 2016
Income tax expense, as reported, increased $52.6 million due primarily to an increase in unrecognized deferred tax assets in 2017 due to a valuation allowance and the tax effect of asset impairment, restructuring and other special charges, partially offset by an income tax benefit related to U.S. tax reform.
2016 vs. 2015
Income tax expense (benefit), as reported, increased $74.2 million due primarily to an increase in unrecognized deferred tax assets in 2016 due to a valuation allowance and the tax effect of asset impairment, restructuring and other special charges.
87
Liquidity and Capital Resources
We have historically participated in Lilly's centralized treasury management system, including centralized cash pooling and overall financing arrangements. We have generated and expect to continue to generate positive cash flows from operations. Following the Separation, we expect our primary sources of liquidity will be our cash on hand, cash flows from operations and funds available under our Credit Facilities. As a significant portion of our business is conducted outside the U.S., we expect to hold a significant portion of our cash outside of the U.S. after our Separation. We will monitor and adjust the amount of foreign cash based on projected cash flow requirements. Our ability to use foreign cash to fund cash flow requirements in the U.S. may be impacted by local regulations and, to a lesser extent, following U.S. tax reforms, the income taxes associated with transferring cash to the U.S. See "Business." We currently intend to indefinitely reinvest foreign earnings for continued use in our foreign operations. As our structure evolves as a standalone company, we may change that strategy, particularly to the extent we identify tax efficient reinvestment alternatives for our foreign earnings or we change our cash management strategy.
Our cash used for or provided from financing activities in the historical periods primarily reflect Lilly's funding of animal health acquisitions.
Our principal liquidity needs include funding existing marketed and pipeline products, capital expenditures, business development in our targeted areas and our anticipated dividend.
We believe our cash and cash equivalents on hand, our operating cash flows and our existing financing arrangements will be sufficient to support our cash needs for the foreseeable future, including for at least the next 12 months.
Our ability to meet future funding requirements may be impacted by macroeconomic, business and financial volatility. As markets change, we will continue to monitor our liquidity position. However, a challenging economic environment or an economic downturn may impact our liquidity or our ability to obtain future financing. See "Cautionary Note Regarding Forward-Looking Statements."
Cash Flows
The following table provides a summary of cash flows from operating, investing and financing activities for the periods presented:
|
Six Months Ended June 30, |
% Change | Year Ended December 31, |
% Change |
|||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
(Dollars in millions) |
2018 | 2017 | 18/17 | 2017 | 2016 | 2015 | 17/16 | 16/15 |
|||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net Cash provided by/(used in): |
|||||||||||||||||||||||||
Operating activities |
$ | 183.9 | $ | 90.6 | 103 | % | $ | 173.8 | $ | 155.9 | $ | 6.6 | 12 | % | 2,262 | % | |||||||||
Investing activities |
(57.5 | ) | (903.8 | ) | (94 | )% | (964.6 | ) | (182.1 | ) | (4,995.4 | ) | 430 | % | (96 | )% | |||||||||
Financing activities |
(123.7 | ) | 811.9 | (115 | )% | 847.5 | (149.6 | ) | 5,353.2 | (667 | )% | (103 | )% | ||||||||||||
Effect of exchange-rate changes on cash and cash equivalents |
(5.1 | ) | 6.9 | (174 | )% | 7.9 | (26.0 | ) | (19.8 | ) | (130 | )% | 31 | % | |||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Net increase/(decrease) in cash and cash equivalents |
$ | (2.4 | ) | $ | 5.6 | (143 | )% | $ | 64.6 | $ | (201.8 | ) | $ | 344.6 | (132 | )% | (159 | )% | |||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Certain amounts and percentages may reflect rounding adjustments.
Operating Activities
Six months ended June 30, 2018 vs. six months ended June 30, 2017
88
Our net cash provided by operating activities was $183.9 million for the six months ended June 30, 2018 as compared to cash provided by operating activities of $90.6 million for the six months ended June 30, 2017. This increase in operating cash flows was primarily attributable to:
- •
- net income for the six months ended June 30, 2018 as compared to a net loss for the six months ended June 30, 2017;
partially offset by:
- •
- an increase in receivables for the six months ended June 30, 2018 as compared to a decrease for the six months ended June 30,
2017 due to an increase in sales in the first six months of 2018 as well as the elimination of cash discounts for early customer payments;
- •
- a larger decrease in accounts payable for the six months ended June 30, 2018 as compared to the six months ended June 30, 2017
due to the timing of invoices and payments in the ordinary course of business; and
- •
- a decrease in accruals related to severance cost in the fourth quarter of 2017 due to the U.S. voluntary early retirement program offered to our employees.
2017 vs. 2016
Our net cash provided by operating activities was $173.8 million in 2017 as compared to cash provided by operating activities of $155.9 million in 2016. This increase in operating cash flows was primarily attributable to:
- •
- a decrease in receivables in 2017 as compared to an increase in 2016 due to a one-time impact of standardizing payment terms across our
acquired businesses as well as payment receipt timing due to integration of acquired assets;
- •
- a decrease in other assets in 2017 as compared to an increase in 2016 primarily due to the timing of tax payments;
and
- •
- a smaller increase in inventory levels in 2017 as compared to 2016;
partially offset by:
- •
- increased net losses.
2016 vs. 2015
Our net cash provided by operating activities was $155.9 million in 2016 as compared to cash provided by operating activities of $6.6 million in 2015. This increase in operating cash flows was primarily attributable to:
- •
- decreased net losses;
partially offset by:
- •
- higher inventory levels; and
- •
- a smaller increase in accounts payable and other liabilities in 2016 as compared to 2015 resulting from payments in the ordinary course of business, including income taxes, as well as from an increase in accounts payable following our acquisition of Novartis Animal Health in 2015.
89
Investing Activities
Six months ended June 30, 2018 vs. six months ended June 30, 2017
Our net cash used in investing activities was $57.5 million for the six months ended June 30, 2018 as compared to cash used in investing activities of $903.8 million for the six months ended June 30, 2017. This decrease in net cash flows used in investing activities was primarily attributable to the acquisition of the BI Vetmedica U.S. vaccines portfolio in 2017.
2017 vs. 2016
Our net cash used in investing activities was $964.6 million in 2017 as compared to cash used in investing activities of $182.1 million in 2016. This increase in net cash flows used in investing activities was primarily attributable to the acquisition of the BI Vetmedica U.S. vaccines portfolio in 2017.
2016 vs. 2015
Our net cash used in investing activities was $182.1 million in 2016 as compared to cash used in investing activities of $5.0 billion in 2015. This decrease in net cash flows used in investing activities was primarily attributable to the acquisition of Novartis Animal Health in 2015.
Financing Activities
Six months ended June 30, 2018 vs. six months ended June 30, 2017
Our net cash used in financing activities was $123.7 million for the six months ended June 30, 2018 as compared to cash provided by financing activities of $811.9 million for the six months ended June 30, 2017. This decrease in net cash provided was primarily attributable to financing provided by Lilly for the acquisition of the BI Vetmedica U.S. vaccines portfolio in 2017.
2017 vs. 2016
Our net cash provided by financing activities was $847.5 million in 2017 as compared to cash used in financing activities of $149.6 million in 2016. This increase in net cash provided was primarily attributable to financing provided by Lilly for the acquisition of the BI Vetmedica U.S. vaccines portfolio in 2017.
2016 vs. 2015
Our net cash used in financing activities was $149.6 million in 2016 as compared to cash provided by financing activities of $5.4 billion in 2015. This decrease in net cash provided was primarily attributable to our financing provided by Lilly for the acquisition of Novartis Animal Health in 2015.
Description of Indebtedness
See "Description of Material Indebtedness."
90
Contractual Obligations
Payments due under contractual obligations as of December 31, 2017, are set forth below:
|
Years |
|||||||||||||||
| | | | | | | | | | | | | | | | | |
(Dollars in millions) |
Total(3) | 2018 | 2019 - 2020 |
2021 - 2022 |
Thereafter |
|||||||||||
| | | | | | | | | | | | | | | | | |
Long-term debt obligations |
— | — | — | — | — | |||||||||||
Operating leases |
$ | 109.7 | $ | 20.0 | $ | 34.8 | $ | 27.6 | $ | 27.3 | ||||||
Purchase obligations(1) |
1,114.1 | 1,091.5 | 22.6 | — | — | |||||||||||
Other long-term liabilities(2) |
176.0 | 1.0 | 30.0 | 1.8 | 143.2 | |||||||||||
Certain amounts may reflect rounding adjustments.
- (1)
- Represents
open purchase orders as of December 31, 2017 and contractual payment obligations with each of our significant vendors which are
noncancelable and are not contingent.
- (2)
- Primarily
represents our long-term liabilities associated with our underfunded pension plans. The timing of these payments may vary based on
individual retirement dates and other activities, and the amount may change as it is based on actuarial estimates.
- (3)
- We excluded deferred taxes because we cannot reasonably estimate the timing of future cash outflows associated with those liabilities.
The contractual obligation table is current as of December 31, 2017 and does not give effect to the Debt Transactions.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have a material current effect or that are reasonably likely to have a material future effect on our financial condition, changes in financial condition, revenue or expenses, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Policies
The preparation of financial statements in accordance with U.S. GAAP requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. Certain of our accounting policies are considered critical because these policies are the most important to the depiction of our financial statements and require significant, difficult or complex judgments by us, often requiring the use of estimates about the effects of matters that are inherently uncertain. Actual results that differ from our estimates could have an unfavorable effect on our financial position and results of operations. We apply estimation methodologies consistently from year to year. The following is a summary of accounting policies that we consider critical to the combined financial statements.
Revenue Recognition
Our gross product revenue is subject to deductions that are generally estimated and recorded in the same period that the revenue is recognized and primarily represents revenue incentives (rebates and discounts) and sales returns. For example:
- •
- for revenue incentives, we use our historical experience with similar incentives programs and current sales data to estimate the impact of such programs on revenue and continually monitor the impact of this experience and adjust as necessary; and
91
- •
- for sales returns, we consider items such as: local returns policies and practices; returns as a percentage of revenue; an understanding of the reasons for past returns; estimated shelf life by product; and estimate of the amount of time between shipment and return to estimate the impact of sales returns.
If any of our ratios, factors, assessments, experiences or judgments are not indicative or accurate predictors of our future experience, our results could be materially affected.
Although the amounts recorded for these revenue deductions are dependent on estimates and assumptions, historically our adjustments to actual results have not been material. The sensitivity of our estimates can vary by program, type of customer and geographic location. Amounts recorded for revenue deductions can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions.
Acquisitions and Fair Value
We account for the assets acquired and liabilities assumed in an acquisition based on the fair values as of the acquisition date. The excess of the purchase price over the fair value of the acquired net assets, where applicable, is recorded as goodwill.
The judgments made in determining estimated fair values assigned to assets acquired and liabilities assumed in a business combination, as well as estimated asset lives, can materially affect our consolidated results of operations. The fair values of intangible assets are re-determined using information available near the acquisition date based on expectations and assumptions that are deemed reasonable by management. Depending on the facts and circumstances, we may deem it necessary to engage an independent valuation expert to assist in valuing significant assets and liabilities.
The fair value of any contingent consideration liability that results from a business combination is determined using a market approach based on quoted market values, significant other observable inputs for identical or comparable assets or liabilities, or a discounted cash flow analysis. Estimating the fair value of contingent consideration requires the use of significant estimates and judgments, including, but not limited to, revenue and the discount rate.
Impairment of Indefinite-Lived and Long-Lived Assets
We review the carrying value of long-lived assets (both intangible and tangible) for potential impairment on a periodic basis and whenever events or changes in circumstances indicate the carrying value of an asset (or asset group) may not be recoverable. We identify impairment by comparing the projected undiscounted cash flows to be generated by the asset (or asset group) to its carrying value. If an impairment is identified, a loss is recorded equal to the excess of the asset's net book value over its fair value, and the cost basis is adjusted.
Goodwill and indefinite-lived intangible assets are reviewed for impairment at least annually and when certain impairment indicators are present. When required, a comparison of fair value to the carrying amount of assets is performed to determine the amount of any impairment.
The estimated cash flows and fair values used in our impairment reviews require significant judgment with respect to future volume; use of working capital; foreign currency exchange rates; the selection of appropriate discount rates; product mix; income tax rates and other assumptions and estimates. Such estimates and assumptions are determined based upon our business plans and when applicable, market participants' views of us and other similar companies. We make these judgements based on our historical experience, relevant market size, historical pricing of similar products and expected industry trends. These assumptions are subject to change in future periods because of, among other things, additional information, financial information based on further
92
historical experience, changes in competition, our investment decisions, volatility in foreign currency exchange rates, and results of research and development. A change in these assumptions or the use of alternative estimates and assumptions could have a significant impact on the estimated fair values of the assets, and may result in an impairment of the existing assets in a future period.
During the six months ended June 30, 2018 and 2017, we recorded asset impairments of $57.7 million and $43.8 million, respectively. During the years ended December 31, 2017, 2016 and 2015, we recorded asset impairments of $110.6 million, $98.3 million and $57.5 million due to changes in estimates or judgments related to the use of the assets. For more information related to our impairment charges, see Note 4: Asset Impairment, Restructuring and Other Special Charges to our unaudited interim combined financial statements and Note 5: Asset Impairment, Restructuring and Other Special Charges to our audited combined financial statements.
Deferred Tax Asset Valuation Allowances
We maintain valuation allowances unless it is more likely than not that all or a portion of the deferred tax asset will be realized. Changes in valuation allowances are included in our tax provision in the period of change. In determining whether a valuation allowance is warranted, we evaluate factors such as prior earnings history, expected future earnings, carryback and carryforward periods, and tax strategies that could potentially enhance the likelihood of realization of a deferred tax asset. The realizability assessments made at a given balance sheet date are subject to change in the future, particularly if earnings of a subsidiary are significantly higher or lower than expected, or if we take operational or tax planning actions that could impact the future taxable earnings of a subsidiary. A change in these assumptions may result in an increase or decrease in the realizability of our existing deferred tax assets, and therefore a change in the valuation allowance, in future periods. As of June 30, 2018, we had a valuation allowance of $105.2 million, and as of December 31, 2017 and 2016, we had valuation allowances of $127.7 million and $39.1 million, respectively.
Qualitative and Quantitative Disclosures About Market Risk
Foreign Exchange Risk
We operate on a global basis and are exposed to the risk that our earnings, cash flows and equity could be adversely impacted by fluctuations in foreign exchange rates. We are primarily exposed to foreign exchange risk with respect to net assets denominated in the Euro, British pound, Canadian dollar, Australian dollar and Brazilian real. Lilly maintains a foreign currency risk management program through a central shared entity, which enters into derivative contracts to hedge foreign currency risk associated with forecasted transactions for the entire company, including for our operations. Gains and losses on derivative contracts entered into by Lilly have been allocated to our results to the extent they were to cover exposure related to our business and offset gains and losses on underlying foreign currency exposures. Following the Separation, we intend to implement a foreign currency risk management program on our own behalf.
We also face currency exposure that arises from translating the results of our global operations to the U.S. dollar at exchange rates that have fluctuated from the beginning of the period. We may enter into foreign currency forward or option derivative contracts to reduce the effect of fluctuating currency exchange rates in future periods, but our historical results do not reflect the impact of any such derivatives related to our exposure to foreign currency impacts on translation.
We estimate that a hypothetical 10% adverse movement in all foreign currency exchange rates related to the translation of the results of our foreign operations would increase our net loss by approximately $12 million.
93
Interest Risk
Our combined balance sheets and statements of operations do not include an allocation of third-party debt or interest expense from Lilly because we are not the legal obligor of the debt and because Lilly's borrowings were not directly attributable to our business. We expect to incur indebtedness prior to or in connection with the Separation, at which time our exposure to interest rate risk will increase to the extent we incur or hedge into floating rate obligations.
Recently Issued Accounting Pronouncements
For discussion of our new accounting standards, see Note 3: Implementation of New Financial Accounting Pronouncements to our unaudited interim combined financial statements and Note 3: Implementation of New Financial Accounting Pronouncements to our audited combined financial statements.
94
Overview
Global animal health industry revenue is projected to grow nominally at a CAGR of 5% from 2017 to 2023, according to Vetnosis. Importantly, this growing industry, which includes both food and companion animals, benefits billions of people worldwide. The food animal sector focuses on species raised to provide animal protein, such as cattle, other ruminants (e.g., sheep and goats), swine, poultry and aqua. The companion animal — or pet — sector focuses primarily on dogs and cats.
Animal health medicines, vaccines and functional nutritionals represent an estimated global market of $34.3 billion, based on 2017 revenue, according to industry sources. Medicines and vaccines represent a global market of $32.0 billion, based on 2017 revenue, and grew at a CAGR of 4% from 2007 to 2017, according to Vetnosis. Management expects this trend to continue through at least 2023 based on industry projections. Functional nutritionals (specifically enzymes, probiotics and prebiotics) used in food animal production represent a global market of $2.3 billion, according to industry sources. Based on industry projections, management expects functional nutritionals to grow faster than the medicines and vaccines market.
Food Animal. Food animal medicines and vaccines, including aquaculture, represented $21.2 billion of revenue in 2017 and grew at a CAGR of 4% from 2007 to 2017, according to Vetnosis.
Factors influencing growth in demand for food animal medicines and vaccines include:
- •
- one in three people needs improved nutrition;
- •
- increased global demand for protein, particularly poultry and aquaculture;
- •
- natural resource constraints, such as scarcity of arable land, fresh water and increased competition for cultivated land, driving the need for
more efficient food production;
- •
- loss of productivity due to food animal disease and death;
- •
- increased focus on food safety and food security; and
- •
- human population growth, increased standards of living, particularly in many emerging markets, and increased urbanization.
Functional nutritionals used in food animal production represent an additional market estimated at $2.3 billion. Growth in functional nutritionals is influenced, among other factors, by demand for antibiotic alternatives that can promote animal health and increase productivity.
Companion Animal. Companion animal medicines and vaccines represented $10.8 billion of revenue in 2017 and grew at a CAGR of 4% from 2007 to 2017, according to Vetnosis.
Factors influencing growth in demand for companion animal medicines and vaccines include:
- •
- increased pet ownership globally;
- •
- pets living longer; and
- •
- increased pet spending as pets are viewed as members of the family by owners.
95
Food Animal Sector
Food Animal Growth Drivers
The global food animal sector is primarily focused on the production of cattle (both dairy and beef), other ruminants, swine, poultry and fish. These animals are the basis for most of the worldwide consumption of animal-based protein.
Animal protein now constitutes a larger percentage of the average human diet than ever before. Annual meat production would need to rise by 74%, or over 200 million tons, to reach 470 million tons by 2050 to meet expected global demand. The growth in animal protein consumption is being influenced in part by a growing middle class in developing countries, the global industrialization of food animal production and easier access to safe and affordable meat products. With billions of people currently receiving insufficient daily nutrition, we expect the demand for food animal protein to continue to rise to address this unmet need.
To meet the growing demand for animal protein, additional output is necessary. Simply adding livestock strains the environment and results in the overuse of natural resources. In order to meet the increased demand for animal protein, producers are increasingly looking for ways to drive efficiency and promote animal health through the use of medicines and vaccines.
Industry sources estimate that 20% of production animals are lost to disease and death. By improving health and lowering mortality rates of food animals — predominantly by actively preventing common parasites, diseases and viruses — producers are able to increase production yields and promote more efficient feeding. We believe most food producers find that the positive impact of these therapies outweighs their price, especially as they represent a small portion of the total cost of production. Consequently, we expect that use of these treatments will continue.
Food Animal Product Categories
Food animal medicines, vaccines and functional nutritionals are divided into two main categories: FA Ruminants & Swine and FA Future Protein & Health.
FA Ruminants & Swine
Ruminants and swine, which is comprised of beef and dairy cattle, sheep, goats and pigs, constituted approximately three quarters of the food animal sector revenue by species in 2017, according to Vetnosis. Ruminants and swine are important sources of animal protein throughout the world, and we believe it will continue to be a material category going forward. Management believes this category will continue to grow, albeit at a slower pace than FA Future Protein & Health, and that medicines and vaccines will continue to play a prominent role in the health and productivity of food animals.
FA Future Protein & Health (Poultry, Aquaculture and Functional Nutritionals)
Poultry and fish are among the fastest growing proteins in the food animal sector. The rapid growth of these proteins is expected to continue.
Fish is the fastest growing animal protein globally. Aquaculture — the farming of aquatic organisms such as fish and crustaceans — remains an immature market where low production yields and high costs due to mortality and biological challenges have limited market growth. These factors have led to increased expenditures on aquaculture-specific animal health products.
The use of functional nutritionals to promote animal health and immunity is another path to help producers maximize animal production efficiency and limit use of antibiotics. Enzymes, prebiotics and probiotics are being studied and used across food animal species today. Enzymes in
96
animal feed act to improve feed digestibility and reduce gut inflammation, improving nutrient absorption and reducing cost for producers. Prebiotics are non-digestible functional ingredients fermented in the large colon that feed the beneficial bacteria in the animal's gut and support microbiome health. Probiotics are live bacteria added to feed in order to manage the microbiome and prevent infections. This category is expected to grow faster than the food animal medicines and vaccines market.
Companion Animal Sector
Companion Animal Growth Drivers
Pets are becoming a larger part of the average family dynamic and are increasingly viewed as "members of the family." The number of pets owned in the U.S. has increased in recent years, and in 2017, 68% of all U.S. households owned a pet. With pets living longer, consumers are spending more disposable income to give their "family member" a healthier, more comfortable life. Most veterinary expenditures are paid out of pocket. Even in times of recession, pet owners are less sensitive to the overall price of pet care than to other aspects of their lifestyle. As new innovations emerge, pet owners now have a greater ability to extend the life of their pet by treating chronic diseases and ailments associated with old age.
The U.S. pet ownership trend is being echoed in other parts of the world. Outside the U.S., the number of dogs and cats receiving healthcare is growing with the increasing middle class. In emerging markets, from 2003 to 2016, cat and dog pet ownership grew by approximately 50%.
Companion Animal Product Categories
Companion animal medicines and vaccines are divided into two main categories: CA Disease Prevention and CA Therapeutics.
CA Disease Prevention
CA Disease Prevention consists primarily of parasiticides, which predominantly target fleas, ticks, heartworms, roundworms, hookworms, whipworms and tapeworms; and vaccines, which target rabies, rhinotracheitis, feline leukemia, hepatitis, parainfluenza and other conditions. As pet owners become increasingly willing to spend money on their pets, they are extending the lives and quality of life of their pets through preventative care, mirroring human health trends. Prevention of fleas, ticks, worms and other parasites, as well as vaccination against infection, have become widely adopted by consumers.
CA Therapeutics
CA Therapeutics consists of products used to treat or manage chronic disease in pets. Examples include products for pain, inflammation, arthritis, cardiovascular issues, otitis (ear infections), dermatology conditions, diabetes and many others. These therapies, which offer a higher quality of life for pets, are growing, driven by innovation in new molecules and improved delivery formulations. As pets live longer and owners' willingness to provide them with medical treatment strengthens, innovation has further expanded with therapies influenced by human health, offering the potential for development of new animal health medicines and capabilities.
Key Structural Characteristics of the Animal Health Industry
- •
- Brands often have long, sustainable value. Branded animal health products often retain significant, and occasionally increased, market share after many years on the market, even after the loss of patent protection. As an example, five of our top 10 products, based on
97
- •
- Diversified product
portfolios. Animal health companies often derive their revenue from dozens, if not hundreds, of products and are frequently not dependent on a
select few flagship products. For example, our top 10 products accounted for only 41% of revenue in 2017. We believe companies with diversified global companion and food animal product portfolios can
be more resilient to changing market dynamics and are structured to better balance potential geographic, product and species volatility.
- •
- Deep customer
relationships. Direct customer models allow animal health sales representatives and veterinary consultants to develop a deep understanding of
customer needs, which often facilitate strong and impactful relationships. Representatives and consultants frequently partner with customers through product support and analytics, driving additional
value for the customer.
- •
- Fast and efficient R&D
model. Product approvals typically require a limited number of targeted studies in animals, which moderates research expenses. The approval
process is generally predictable given the number of studies required, leading to average timelines from initiation of development to approval of three to seven years at a cost of $50 million
to $100 million.
- •
- Self-pay market. Food animal producers, pet owners and veterinarians typically pay for products out of pocket, making them the primary decision makers. This results in manufacturers being able to price products based primarily on the end customer's realized value.
2017 revenue, have been on the market for over 25 years. In the food animal sector, the level of competition is influenced by macro-economic factors, brand loyalty, distribution models and the absence of governmental or third-party payer systems. In the companion animal sector, competition is influenced by brand loyalty, new innovation, relationships with veterinarians, channel expansion and the overall growth in pet ownership.
98
Overview
Founded in 1954 as part of Eli Lilly and Company, Elanco is a premier animal health company that innovates, develops, manufactures and markets products for companion and food animals. Headquartered in Greenfield, Indiana, we are the fourth largest animal health company in the world, with revenue of $2.9 billion for the year ended December 31, 2017. Globally, we are #1 in medicinal feed additives, #2 in poultry and #3 in cattle, measured by 2017 revenue, according to Vetnosis. We also have one of the broadest portfolios of pet parasiticides in the companion animal sector. We offer a diverse portfolio of more than 125 brands that make us a trusted partner to veterinarians and food animal producers in more than 90 countries.
Our vision is to enrich the lives of people through food — making protein more accessible and affordable — and through pet companionship — helping pets live longer, healthier lives. We advance our vision by offering products in four primary categories:
- •
- Companion Animal Disease Prevention ("CA Disease Prevention"): We have one
of the broadest parasiticide portfolios in the companion animal sector based on indications, species and formulations, with products that protect pets from worms, fleas and ticks. Combining our
parasiticide portfolio with our vaccines presence, we are a leader in the U.S. in the disease prevention category based on share of revenue.
- •
- Companion Animal Therapeutics ("CA Therapeutics"): We have a broad pain and
osteoarthritis portfolio across species, modes of action, indications and disease stages. Pet owners are increasingly treating osteoarthritis in their pets, and our Galliprant product is one of the
fastest growing osteoarthritis treatments in the U.S. We also have treatments for otitis (ear infections), as well as
cardiovascular and dermatology indications.
- •
- Food Animal Future Protein & Health ("FA Future Protein &
Health"): Our portfolio in this category, which includes vaccines, nutritional enzymes and animal-only antibiotics, serves the growing demand for
protein and includes innovative products in poultry and aquaculture production, where demand for animal health products is outpacing overall industry growth. We are focused on developing functional
nutritional health products that promote food animal health, including enzymes, probiotics and prebiotics. We are a leader in providing vaccines as alternatives to antibiotics to promote animal health
based on share of revenue.
- •
- Food Animal Ruminants & Swine ("FA Ruminants & Swine"): We have developed a range of food animal products used extensively in ruminant (e.g., cattle, sheep and goats) and swine production. We also deliver value to producers beyond our products through our technical expertise and support.
99
We have a top four presence in all four key industry geographic regions: NA; EMEA; LATAM; and APAC, as measured by 2017 revenue, according to Vetnosis. The following graphs demonstrate our revenue for the year ended December 31, 2017 by product category, geography and our highest revenue products:
Percentage of 2017 Revenue
By Product Category(1)

- (1)
- Certain percentages may reflect rounding adjustments.
- (2)
- Strategic Exits includes revenue from third-party manufacturing, distribution and other contractual arrangements, as well as an equine product not core to our business, which we have either exited or made the decision to exit.
| Percentage of 2017 Revenue By Region(1) |
Percentage of 2017 Revenue By Highest Revenue Products(1) |
|

|

|
- (1)
- Certain
percentages may reflect rounding adjustments.
- (2)
- LATAM includes aquaculture in all regions.
100
Through our global sales force of approximately 1,530 sales representatives, our veterinary consultants and our key distributors, we seek to build strong customer relationships and fulfill demand for our food animal products primarily with food animal producers, veterinarians and nutritionists, and for our companion animal products primarily with veterinarians and, in some markets, pet owners. We are also expanding into retail channels in order to meet pet owners where they want to purchase.
Our inclusive approach to sourcing innovation helps us identify, attract, fund and develop new ideas that enhance our pipeline and reduce risk as compared to an in-house only approach. Through this process, we launched nine products from 2015 to 2017 that delivered $143.8 million of revenue in 2017 and $136.6 million of revenue in the first half of 2018.
We believe we have an experienced leadership team that fosters an adaptive, purpose-driven culture among approximately 5,880 employees worldwide as of June 30, 2018 and that our employees share a deep conviction for achieving our vision of food and companionship, enriching life.
For the six months ended June 30, 2018 and 2017, our revenue was $1.5 billion and $1.4 billion, respectively, and for each of the years ended December 31, 2017, 2016 and 2015, our revenue was $2.9 billion. For the six months ended June 30, 2018 and 2017, our net income (loss) was $9.9 million and $(128.5) million, respectively, our adjusted EBITDA was $306.2 million and $278.4 million, respectively, and our adjusted net income was $219.0 million and $156.4 million, respectively. For the years ended December 31, 2017, 2016 and 2015, our net income (loss) was $(310.7) million, $(47.9) million and $(210.8) million, respectively, our adjusted EBITDA was $498.9 million, $540.4 million and $393.7 million, respectively, and our adjusted net income was $250.5 million, $332.7 million and $208.7 million, respectively. For a reconciliation of adjusted EBITDA and adjusted net income to net income (loss), see "Prospectus Summary — Summary Historical Combined Financial Data and Unaudited Pro Forma Condensed Combined Financial Data."
101
Company History
As a unit of Lilly, our business has been built over the course of more than 60 years, including the following key developments:
| Year | Events |
|
| | | |
| 1953 | Introduced one of the first antibiotics exclusively for veterinary use | |
| 1954 | Combined all plant and animal science activities within a single division | |
| 1960 | Launched Elanco Products Company | |
| 1990 | Elanco Products Company renamed Elanco Animal Health and focuses solely on animal health products | |
| 2007 | Acquired Ivy Animal Health, providing access to Ivy's product portfolio and the Benchmark Performance Program data and platform | |
| Launched companion animal business | ||
| 2009 | Formed Elanco Knowledge Solutions (analytics and consultation for customers) | |
| 2011 | Acquired Janssen Animal Health, increasing our portfolio of companion animal products, diversifying our food animal portfolio with poultry products and significantly expanding our European footprint | |
| 2012 | Acquired ChemGen, expanding our business into the enzyme/feed efficiency space | |
| 2014 | Acquired Lohmann Animal Health, strengthening our position in poultry solutions by acquiring a global leader in the supply of poultry vaccines | |
| 2015 | Acquired Novartis Animal Health, increasing our product portfolio, including companion animal and aquaculture, expanding our global commercial presence and enhancing our manufacturing and R&D capabilities | |
| 2016 | Opened a state-of-the-art vaccine R&D facility and entered into a license agreement with Aratana Therapeutics, Inc. to develop, manufacture, market and commercialize Galliprant outside the U.S. and co-promote it in the U.S., expanding our companion animal portfolio | |
| 2017 | Acquired the BI Vetmedica U.S. vaccines portfolio, diversifying our companion animal portfolio and acquiring a fully integrated manufacturing and R&D site |
Our Competitive Strengths
We believe the following strengths create sustainable competitive advantages that will enable us to continue to grow as a leader in the animal health industry.
- •
- Established leader with a global presence and diversified product portfolio. We are the fourth largest animal health company in the world, with revenue of $2.9 billion for the year ended December 31, 2017. Globally, we are #1 in medicinal feed additives, #2 in poultry and #3 in cattle, as measured by 2017 revenue, according to Vetnosis. We also have one of the broadest portfolios of pet parasiticides in the companion animal sector, based on indications, species and formulations. We have a top four presence in all four key geographic regions (NA, EMEA, LATAM and APAC), as measured by 2017 revenue, according to Vetnosis, including a strong presence in the emerging markets of Brazil, Thailand, China and Mexico. We have a comprehensive and diversified product portfolio, with more than 125 brands sold in more than 90 countries. In 2017, our top 10 products accounted for 41% of our revenue, with our top selling product accounting for approximately 10% of our revenue. Our global footprint includes a direct commercial presence in
102
- •
- Strategically positioned to drive innovation and growth in our three targeted growth
categories. Over the past 10 years, we have intentionally transformed Elanco from a food animal focused company into a diversified global company. In addition to our FA
Ruminants & Swine category, we now have established positions in our three targeted growth categories: CA Disease Prevention, CA Therapeutics and FA Future Protein & Health. To achieve
this, among other steps, we have made strategic acquisitions to expand our product portfolio, increase our sales presence globally and obtain R&D and manufacturing capabilities in these categories.
Recent acquisitions include Janssen Animal Health, ChemGen, Lohmann Animal Health, Novartis Animal Health and the BI Vetmedica U.S. vaccines portfolio. See "— Company History." As a
result of these acquisitions as well as organic growth, we have grown our companion animal categories, from a minimal presence in 2007 to more than $900 million in revenue in 2017. We believe
that as a result of establishing a strong presence in our targeted growth categories, which feature favorable industry dynamics, we are strategically positioned to grow our revenue and increase
profitability.
- •
- Strength of brands and relationships in our FA Ruminants & Swine
category. We provide a range of products for use in ruminant and swine production that we believe have created strong, long-standing customer
relationships and provide an important revenue source for our business and for investment capital to support future growth. We have well-established Elanco brands in this category such as Rumensin, a
leading cattle feed additive that has been used for more than 40 years to improve feed efficiency and control coccidiosis. In
addition, our technical expertise and analytics help us deliver value to our customers beyond our products. Our analytics help producers analyze large amounts of health and production data, turning
that data into actionable information that helps them improve the health of their animals and, as a result, their productivity and profitability. We believe our brands and additional customer support
have helped us create broad name recognition and a high level of trust among target customers, which is important to the success of our food animal products. We expect to continue to be a leader in FA
Ruminants & Swine.
- •
- Proven track record of innovation and product launches. We have developed in-house R&D capabilities in the chemical sciences and life sciences, which enable us to discover and develop vaccines and small and large molecules in our targeted areas. We also have an R&D platform that enables us to discover, develop and evaluate future nutritional health opportunities in enzymes, probiotics and prebiotics. Beyond our strong in-house R&D, we also access ideas and innovation from a broad array of sources. This inclusive approach to innovation allows us to identify, attract, fund and develop new ideas in a manner that enhances our pipeline while, we believe, reducing the risk associated with an in-house only innovation model. As a result we have launched nine products from 2015 to 2017 that delivered revenue of $24.7 million in 2015, $97.9 million in 2016, $143.8 million in 2017 and $136.6 million in the first half of 2018. We believe our new products will be an important source of future revenue.
62 countries, which we have plans to reduce to fewer than 50 countries, and third-party distribution relationships serving other relevant markets. Of our approximately 1,530 sales representatives as of June 30, 2018, two-thirds were based outside of North America.
103
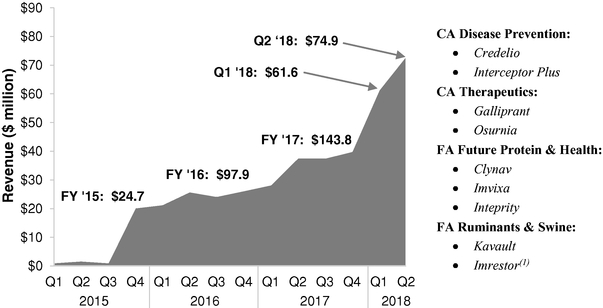
- (1)
- We suspended commercialization of Imrestor in the second quarter of 2018 and plan to pursue additional indications. Revenues from Imrestor were $6.5 million for the year ended December 31, 2017 and $1.0 million for the six months ended June 30, 2018.
- •
- Expertise in driving cost efficiencies and productivity. In the last 10 years, we have successfully integrated 10 businesses, including businesses acquired within the last four years with an aggregate of 4,500 full-time employees, 12 manufacturing sites and eight R&D sites. These acquisitions had a negative impact on operating margins and over the last three years, we have identified and executed a number of initiatives which improved our operational efficiency and positively impacted our operating margins. Through the reduction of manufacturing and R&D sites, headcount rationalization, focused procurement initiatives, sales force organizational design and the establishment of an integration center of excellence, we estimate that we delivered more than $500 million in annualized cost savings
Three of these products were developed following the traditional in-house model, while the other products were obtained through an acquisition or venture capital investment. These launches are evidence of our ability to identify innovation from diverse sources and develop them into distinctive products in our targeted categories. They include: Credelio, for the treatment and elimination of fleas and ticks in dogs and puppies; Interceptor Plus, for the prevention of heartworm disease and treatment and control of other endoparasite infections in dogs and puppies; Galliprant, for the treatment of canine osteoarthritis pain and inflammation; Osurnia, for the treatment of otitis externa in dogs; Clynav, for the immunization of Atlantic salmon against pancreas disease; Imvixa, for the prevention and control of sea lice; Inteprity, for the prevention of mortality caused by necrotic enteritis in broiler chickens; Kavault, for the reduction of diarrhea in weaned pigs; and Imrestor, which we suspended commercialization of in the second quarter of 2018, for the reduction of incidence of clinical mastitis in periparturient dairy cows. In 2017, Clynav and Galliprant were named best food animal and companion animal products, respectively, by Animal Pharm. We currently have R&D projects relating to 36 potential new product innovations (which we define as new chemical entities, new combinations or significant line extensions), which we are investigating as candidates for potential new product launches through 2023. We believe our approach to innovation will enable us to create and maintain an attractive pipeline of novel products.
104
- •
- Experienced management team and dedicated employees. Our executive management team is comprised of a group of leaders with diverse backgrounds and extensive experience across global animal health and related industries. We believe their experience has provided organizational capabilities to support our targeted growth strategies and helped us create a legacy of growth and transformation in a dynamic industry. Our executives have taken an active role in important initiatives shaping the animal health industry. We also believe we have a loyal, highly engaged, customer-focused and cause-oriented professional workforce. We have recently strengthened our management team by adding executive officers with extensive public company experience.
from the beginning of 2015 through the end of 2017. Since 2015, in manufacturing we have closed three sites, reduced headcount from approximately 3,500 to approximately 2,330 employees and eliminated over 2,600 SKUs (we currently supply approximately 4,400 SKUs). Drawing on these experiences, we are currently executing additional productivity initiatives throughout the organization that we believe will materially strengthen the margin profile of our business over time.
Our Targeted Value-Generating Strategies
We intend to continue to grow our business and create value for our shareholders through a targeted value-generating strategy with three key pillars: a Portfolio Strategy for our marketed products, an Innovation Strategy for our R&D pipeline and a Productivity Strategy for our margin expansion initiatives.

Portfolio Strategy
- •
- Invest in categories with the greatest potential for
growth. We are focusing the majority of our resources, including more than 75% of our R&D funding, on our three targeted growth categories: CA
Disease Prevention, CA Therapeutics and FA Future Protein & Health, where we believe we are well positioned to grow faster than the market. These categories represented 54% of our revenue in
2017.
- •
- CA Disease Prevention — Parasiticides and vaccines are fundamental to preventing disease in companion animals. We have a strong vaccines portfolio as well as products that protect pets from a broad spectrum of parasites, such as fleas, ticks, heartworms, roundworms, hookworms, whipworms and tapeworms. We believe we are
105
- •
- CA Therapeutics — Pets are living longer and owners
increasingly seek treatments for chronic diseases in their pets. To capitalize on these trends, we are focused on driving growth in our CA Therapeutics category by building on our broad base of pain
and osteoarthritis products.
- •
- FA Future Protein & Health — We expect to drive revenue
growth through our poultry and aquaculture portfolios. Poultry and aquaculture are expected to be among the fastest growing animal health protein sources over the next 10 years. We also are
focused on nutritional health products and antibiotic stewardship that address market trends in this category.
- •
- Reinforce our strong presence in our FA Ruminants & Swine category. We plan to continue fortifying our long-standing FA Ruminants & Swine category to meet our customers' needs through targeted product investment and by continuing to strengthen our deep business-to-business relationships through sales force excellence and leadership in industry coalitions. We also plan to continue to utilize analytics, social media and other support to provide value to our customers beyond our products.
well positioned to drive additional growth through continued product innovation and sales channel expansion.
Innovation Strategy
- •
- Maximize opportunities to innovate within targeted
platforms. Our R&D efforts focus on six areas across our companion and food animal categories where science and our capabilities best match
market opportunities and meet customer needs.
- •
- Companion Animal — We are targeting therapeutics, vaccines and
parasiticides.
- •
- Therapeutics — We are focused on continuing to discover and develop products in areas where we currently
compete such as dermatology, otitis and pain. We are also pursuing novel targets to address unmet needs for chronic conditions in dogs and cats.
- •
- Vaccines — We have a competitive line of core canine, feline and rabies vaccines that we are developing for
expansion into geographies outside the U.S. We are also developing novel delivery technologies for companion animal vaccines, building on the success of the formulation innovation of our current
product line.
- •
- Parasiticides — We leverage proprietary active ingredients to develop and commercialize novel products with
endoparasite and ectoparasite efficacy through combinations and novel formulations. We are also actively pursuing products with novel mechanisms of action to introduce innovation in this category.
- •
- Food Animal — We are targeting pharmaceuticals, vaccines and
the emerging nutritional health space.
- •
- Pharmaceuticals — We focus efforts in discovery and development of novel pharmaceutical and biopharmaceutical
products that could be effective alternatives to antibiotics or address other health challenges encountered in livestock production.
- •
- Vaccines — We have active vaccine R&D programs to discover and develop products to address bacterial and viral
threats in poultry, swine, cattle and fish.
- •
- Nutritional Health — Building on our enzyme product platform and the success of Hemicell, we are targeting R&D efforts in nutritional health to deliver new products that improve gut health and performance in livestock. We focus on the role and composition of the microbiome on the health and digestive performance of the
106
- •
- Inclusive approach to sourcing innovation. We have a build, buy or ally strategy to identify, attract and develop new ideas in our six R&D focus areas in a manner intended to reduce risk and sustain our pipeline. In addition to traditional corporate R&D, we pursue in-licensing and partnering activities, actively and selectively engaging in funding models that include venture capital, project financing and crowdsourced innovation. This strategy gives us access to a wider range of novel ideas and increases our ability to bring innovative products to market compared to an in-house only model.
animal and look to introduce new products that are enzymes, probiotics or prebiotics.
Productivity Strategy
- •
- Leverage our productivity capabilities to improve operating
margins. We estimate that from the beginning of 2015 through the end of 2017, we generated more than $500 million in annualized cost
savings through our productivity initiatives, including the integration of three major acquisitions. Leveraging this track record of productivity improvements and cost savings, we aim to significantly
increase our operating margins over time through our initiatives in manufacturing and SG&A. Our productivity strategies include:
- •
- Manufacturing efficiency and cost savings. We plan to continue to execute on
initiatives we have identified to improve manufacturing processes, reduce our manufacturing footprint, advance lean initiatives, consolidate our CMO network, strategically insource projects and pursue
cost savings opportunities for raw materials through a new procurement process. We also plan to leverage our extensive integration experience to continue identifying cost-savings and delivering on our
margin expansion objectives.
- •
- SG&A excellence. Our sales strategy is focused on achieving growth in our targeted product categories while increasing productivity within our sales force. We plan to utilize both our sales force's strong customer relationships and our strategic distributor partnerships to efficiently grow demand for our products. We also have a targeted procurement initiative and are in the process of implementing a G&A steady state organizational design to reduce overhead costs and simplify infrastructure following the termination of our transitional service agreement with Lilly.
Products
We have a diverse portfolio of products marketed under more than 125 brands, including products for both food animals and companion animals.
Our food animal products are designed to enable producers to keep animals healthy and deliver more food while using fewer resources. Our antibacterials, anticoccidials, vaccines and parasiticides aim to make food safer by preventing and controlling disease. We offer products and support to enhance the integrity of the food supply, while our productivity enhancers help make food more affordable and abundant by increasing the amount of meat, milk or eggs an animal can supply. Furthermore, our expertise and data analytics help our customers improve production efficiency and business performance. Food animal products represented approximately 63% of our revenue for the year ended December 31, 2017.
Our companion animal products help veterinarians better care for pets. We partner with pet owners and veterinarians for the purpose of providing a consistent flow of innovative and effective products and support. Our R&D focuses on products that prevent and treat disease, improve and extend quality of life and improve the type of care received by pets. We also partner closely with veterinarians to provide technical support and case management for our products. Companion animal products represented approximately 37% of our revenue for the year ended December 31, 2017.
107
We group our products into four principal categories:
- •
- CA Disease Prevention: includes parasiticides and vaccine products for
canines and felines.
- •
- CA Therapeutics: includes products for the treatment of pain,
osteoarthritis, otitis, cardiovascular and dermatology indications in canines and felines.
- •
- FA Future Protein & Health: includes vaccines, antibiotics,
parasiticides and other products used in poultry and aquaculture production, as well as functional nutritional health products, including enzymes, probiotics and prebiotics.
- •
- FA Ruminants & Swine: includes vaccines, antibiotics, implants, parasiticides and other products used in ruminants and swine production, as well as certain other food animal products.
We pursue the development of new chemical and biological molecules through our innovation strategy. Since 2015, we have launched the following nine products:
- •
- In CA Disease Prevention, Credelio and Interceptor
Plus.
- •
- In CA Therapeutics, Galliprant and Osurnia.
- •
- In FA Future Protein & Health, Inteprity, Imvixa and Clynav.
- •
- In FA Ruminants & Swine, Imrestor and Kavault.
In the second quarter of 2018, we suspended commercialization of Imrestor and plan to pursue additional indications.
In 2016, we announced the creation of our Nutritional Health organization, which focuses on functional nutrition products, including enzymes, probiotics and prebiotics, which impact animal microbiomes and other dietary factors to reduce disease incidence, improve gut health and enhance feed digestibility. We first focused on nutritional health in 2012, with the acquisition of ChemGen and the Hemicell brand. In 2016, we entered into an agreement with Agro Biosciences, Inc. to commercialize Correlink — a novel direct-fed microbial (probiotic) product outside the U.S. In early 2018, we announced a new global, exclusive in-licensing agreement with Ab E Discovery to further develop and bring to the market an in feed antibody product focused on reducing and controlling coccidiosis.
Rumensin, our top selling product, contributed approximately 10% of our revenue in 2017, 2016 and 2015. No other product contributed 10% or more of our revenue. Our top five selling products, Rumensin, Trifexis, Maxiban, Denagard and Tylan Premix, collectively contributed approximately 29% of our 2017 revenue. Our top 10 products collectively contributed 41% of our 2017 revenue.
Set forth below is information regarding our principal products.
CA Disease Prevention Products
| Primary | ||||
| Product | Description | Species |
||
| | | | | |
|
Bronchi Shield III and Bronchi Shield Oral (vaccines) |
Bronchi Shield III — To protect against adenovirus, parainfluenza and Bordetella bronchiseptica (Bb) in dogs. Bronchi Shield Oral — To protect against Bb in dogs. |
Dogs |
108
| Primary | ||||
| Product | Description | Species | ||
| | | | | |
|
Comfortis (spinosad) |
To kill fleas and prevent and treat flea infestations (Ctenocephalides felis) in cats 14 weeks of age or older and weighing at least 4.1 lbs. and dogs 14 weeks of age or older and weighing at least 5.0 lbs. | Cats, Dogs | ||
Credelio (lotilaner) |
To kill adult fleas and to treat flea infestations (Ctenocephalides felis) and treat and control tick infestations (Amblyomma americanum (lone star tick), Dermacentor variabilis (American dog tick), Ixodes scapularis (black-legged tick) and Rhipicephalus sanguineus (brown dog tick)) for one month in dogs and puppies 8 weeks of age or older and weighing at least 4.4 lbs. |
Dogs |
||
Duramune (vaccines) |
Includes multiple products that collectively protect against distemper, adenovirus, parvovirus, corona, parainfluenza, leptospira canicola, and other diseases in dogs. |
Dogs |
||
Rabvac (vaccines) |
To protect against rabies, includes a 1-year and 3-year shot. |
Cats, Dogs |
||
Fel-O-Vax (vaccines) |
Includes multiple products that collectively protect against Leukemia, rhinovirus, calicivirus, panleukopenia, and chlamydia in cats. |
Cats |
||
Fel-O-Guard (vaccines) |
Includes multiple products that collectively protect against Leukemia, rhinovirus, calicivirus, panleukopenia, and chlamydia in cats. |
Cats |
||
Interceptor Plus (milbemycin oxime/praziquantel) |
To prevent heartworm disease caused by Dirofilaria immitis and for the treatment and control of adult roundworm (Toxocara canis and Toxascaris leonina), adult hookworm (Ancylostoma caninum), adult whipworm (Trichuris vulpis), and adult tapeworm (Taenia pisiformis, Echinococcus multilocularis, and Echinococcus granulosus) infections in dogs and puppies weighing at least 2 lbs. and 6 weeks of age or older. Interceptor Plus is a relaunch of a previously approved formula. |
Dogs |
||
Milbemax (milbemycin |
To treat and control parasitic infections due to adult hookworm, adult roundworm and adult tapeworm and to prevent heartworm disease caused by Dirofilaria immitis in cats and dogs. |
Cats, Dogs |
||
Trifexis (spinosad + |
To prevent heartworm disease (Dirofilaria immitis) and to kill fleas. Trifexis is indicated for the prevention and treatment of flea infestations (Ctenocephalides felis), and the treatment and control of adult hookworm (Ancylostoma caninum), adult roundworm (Toxocara canis and Toxascaris leonina) and adult whipworm (Trichuris vulpis) infections in dogs and puppies 8 weeks of age or older and weighing at least 5 lbs. |
Dogs |
109
CA Therapeutics Products
| Primary | ||||
| Product | Description | Species |
||
| | | | | |
|
Atopica (cyclosporine A) |
To control atopic dermatitis in dogs weighing at least 4 lbs. | Dogs | ||
Fortekor Plus (benazepril + |
To treat congestive heart failure due to atrioventricular valve insufficiency or dilated cardiomyopathy in dogs. |
Dogs |
||
Galliprant (grapiprant) |
To control pain and inflammation associated with osteoarthritis in dogs. |
Dogs |
||
Onsior (robenacoxib) |
To control postoperative pain and inflammation associated with soft tissue surgery in dogs weighing at least 5.5 lbs. and 4 months of age or older and control postoperative pain and inflammation associated with orthopedic surgery, ovariohysterectomy and castration in cats weighing at least 5.5 lbs. and 6 months of age or older; for up to a maximum of 3 days. |
Cats, Dogs |
||
Osurnia (terbinafine + |
To treat otitis externa in dogs associated with susceptible strains of bacteria (Staphylococcus pseudintermedius) and yeast (Malassezia pachydermatis). |
Dogs |
FA Future Protein & Health
| Primary | ||||
| Product | Description | Species |
||
| | | | | |
|
AviPro (vaccines) |
Includes multiple products that collectively protect against Newcastle disease, infectious bronchitis, fowl cholera, paramyxovirus Type 3, Bursal Disease, other diseases and foodborne pathogens like Salmonella in poultry. | Poultry | ||
Clynav (plasmid deoxyribonucleic acid vaccine) |
To immunize Atlantic salmon to reduce impaired daily weight gain, and reduce mortality, and cardiac, pancreatic and skeletal muscle lesions caused by pancreas disease following infection with salmonid alphavirus subtype 3 (SAV3). |
Fish (Salmon) |
||
Coban / Elancoban (monensin) |
To aid in the prevention of coccidiosis in broiler and replacement chickens (caused by Eimeria necatrix, E. tenella, E. acervulina, E. brunetti, E. mivati, and E. maxima), in turkeys (caused by Eimeria adenoeides, E. meleagrimitis and E. gallopavonis) and in growing Bobwhite quail (caused by Eimeria dispersa and E. lettyae). Coban/Elancoban is an animal-only antibiotic and an ionophore. |
Poultry |
||
Hemicell (endo-1, 4-b-mannanase) |
Enzyme supplement for poultry and swine feeds that contain a source of b-mannanase, which hydrolyses the b-mannans present in soybean and corn meal. |
Poultry, Swine |
110
| Primary | ||||
| Product | Description | Species | ||
| | | | | |
|
Imvixa (lufenuron) |
To prevent and control infestation caused by sea lice, Caligus reogercresseyi, in farmed salmon. | Fish (Salmon) | ||
Maxiban (narasin + |
To prevent coccidiosis in broiler chickens caused by Eimeria necatrix, E. tenella, E. acervulina, E. brunetti, E. mivati and E. maxima. Maxiban is an animal-only antibiotic and an ionophore. |
Poultry |
||
Monteban (narasin) |
To prevent coccidiosis in broiler chickens caused by Eimeria necatrix, E. tenella, E. acervulina, E. brunetti, E. mivati and E. maxima. Monteban is an animal-only antibiotic and an ionophore. |
Poultry |
||
Surmax / Maxus / Inteprity (avilamycin) |
To prevent mortality caused by necrotic enteritis associated with Clostridium perfringens in broiler chickens. Surmax, Maxis and Inteprity are animal-only antibiotics. |
Poultry |
FA Ruminants & Swine
| Primary | ||||
| Product | Description | Species |
||
| | | | | |
|
Denagard (tiamulin) |
To treat Swine Dysentery associated with Serpulinahyodysenteriae susceptible to tiamulin and for treatment of swine bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis sensitive to chlortetracycline and treatment of bacterial pneumonia caused by Pasteurella multocida sensitive to chlortetracycline. Denagard is a shared-class antibiotic. | Swine | ||
Optaflexx / Paylean (ractopamine hydrochloride) |
To increase rate of weight gain, improve feed efficiency and increase carcass leanness, and used as a top dress feed to increase rate of weight gain and improve feed efficiency in cattle fed in confinement for slaughter during the last 28 to 42 days on feed. Ractopamine, the active ingredient in Paylean and Optaflexx, is a beta adrenoreceptor agonist. |
Cattle, Swine |
||
Pulmotil (tilmicosin) |
For swine: To control swine respiratory disease associated with Actinobacillus pleuropneumoniae and Pasteurella multocida. For cattle: To control bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni in groups of beef and non-lactating dairy cattle, where active BRD has been diagnosed in at least 10% of the animals in the group. Pulmotil is a shared-class antibiotic. |
Cattle, Swine |
||
Rumensin (monensin) |
For cattle fed in confinement for slaughter: To improve feed efficiency and prevent and control coccidiosis due to Eimeria bovis and Eimeria zuernii. For dairy cows: To increase milk production efficiency (production of marketable solids-corrected milk per unit of feed intake). |
Cattle |
111
| Primary | ||||
| Product | Description | Species | ||
| | | | | |
For growing cattle on pasture or in dry lot (stocker and feeder and dairy and beef replacement heifers): To increase rate of weight gain and to prevent and control coccidiosis due to Eimeria bovis and Eimeria zuernii. For mature reproducing beef cows: To improve feed efficiency when receiving supplemental feed and to prevent and control coccidiosis due to Eimeria bovis and Eimeria zuernii. For goats: To prevent coccidiosis due to Eimeria crandallis, Eimeria christenseni and Eimeria ninakohlyakimovae in goats maintained in confinement. For calves (excluding veal calves): To prevent and control coccidiosis due to Eimeria bovis and Eimeria zuernii. Rumensin is an animal-only antibiotic and an ionophore. |
||||
Tylan Premix (tylosin phosphate) |
To control porcine proliferative enteropathies associated with Lawsonia intracellularis and to control porcine proliferative enteropathies associated with Lawsonia intracellularis immediately after medicating with Tylan Soluble (tylosin tartrate) in drinking water. Tylan Premix is a shared-class antibiotic. |
Swine, Cattle, Poultry |
||
Vira Shield (vaccines) |
Includes multiple products that protect against infection, bovine rhinotracheitis, bovine viral diarrhea, bovine respiratory syncytial virus, bovine respiratory disease, leptospira canicola and other diseases in cattle. |
Cattle |
Antibiotics
Antimicrobial resistance in humans, or the risk that human pathogens evolve or otherwise emerge that are resistant to antibiotics or other antimicrobials, is a significant health concern, and animal agriculture can play a role in mitigating this risk. As a company dedicated to the health and well-being of animals, we seek to help veterinarians and farmers responsibly use antibiotics when treating animals. In our efforts to address antibiotic resistance while protecting animal health, we introduced a global antibiotic stewardship plan focusing on increasing responsible antibiotic use; reducing the need for shared-class antibiotics; and replacing antibiotics with alternatives to help livestock producers treat and prevent animal disease. Antibiotics, used responsibly, along with good animal care practices, help enhance food safety and animal well-being.
There are two classes of antibiotics used in animal health:
- •
- Animal-only antibiotics and ionophores: Not all pathogens that cause disease in animals are infectious in humans, and accordingly animal-only antibiotics are not used in human medicine (i.e., not medically important). Ionophores are a special class of animal-only antimicrobials uniquely developed only for use in animals. In Europe and certain other jurisdictions, ionophores are not currently classified as antibiotics. Because of their animal-only designation, mode of action, and spectrum of activity, their use is not considered to create the same risk of resistance in human pathogens.
112
- •
- Shared-class antibiotics: These are used in both humans and animals. Some antibiotics are used to treat infectious disease caused by pathogens that occur in both humans and animals. Of the 18 major antibiotic resistance threats that the Centers for Disease Control and Prevention tracks, two are associated with infectious disease in animals. As part of our global antibiotic stewardship plan and in compliance with FDA guidance, shared-class antibiotics are labeled only for the treatment of an established need in animals and only with veterinarian oversight.
We have intentionally shifted away from shared-class antibiotics, and are focusing on animal-only antibiotics, as well as antibiotic-free solutions. In 2017, 13% of our revenue was from products classified as shared-class antibiotics, of which 7% of our revenue was in NA and EMEA and 6% was in APAC and LATAM, whereas 24% of our revenue was from animal-only antibiotics and ionophores, of which ionophores constituted 21% of our revenue. Through our policies and efforts in this area, we seek to protect the benefits of antibiotics in human medicine, while responsibly protecting the health of food animals and the safety of our food supply.
Sales and Marketing
Our sales organization includes sales representatives, veterinary consultants and other value added specialists. In markets where we do not have a direct commercial presence, we generally contract with distributors that provide logistics and sales and marketing support for our products.
Our sales representatives visit our customers, including consultants, veterinarians, food animal producers, and resellers, to inform, promote and sell our products and to support customers. Our veterinary consultants provide scientific consulting focused on disease management and herd management, training and education on diverse topics, including responsible product use, and generally have advanced degrees in veterinary medicine, veterinary nutrition or other agriculture-related fields. These direct relationships with customers allow us to understand their needs. Additionally, our sales representatives and veterinary consultants focus on collaborating with our customers to educate and support them on topics such as local disease awareness and to help them adopt new and more sophisticated animal health solutions, including through the use of our products. As a result of these relationships, our sales and consulting visits provide us with access to customer decision makers. In addition, our sales and marketing organization provides enhanced value by providing support to food animal producers to help maximize their yields and reduce costs. Our analytics help customers analyze large amounts of health and production data. As of June 30, 2018, we had approximately 1,530 sales representatives.
Customers
We primarily sell our food animal products to third-party distributors and directly to a diverse set of food animal producers, including beef and dairy farmers as well as pork, poultry and aquaculture operations. We primarily sell our companion animal products to third-party distributors, as well as directly to veterinarians that typically then sell our products to pet owners. We are also expanding into retail channels in order to meet pet owners where they want to purchase. Our largest customer, an affiliate of AmerisourceBergen Corp., is a third-party veterinary distributor and represented approximately 13% of our revenue for the year ended December 31, 2017. Our next largest customer represented approximately 7% of our revenue for the year ended December 31, 2017 and no other customer represented more than 5% of our revenue for the same period.
Research and Development
Our R&D organization is comprised of internal research, global development, global regulatory and external innovation collaborations and venture investing. As of June 30, 2018, we employed
113
approximately 700 employees in our global R&D and Regulatory Affairs organizations. Our R&D headquarters is located in Greenfield, Indiana. We also have R&D facilities in Basel, Switzerland; Yarrandoo, Australia; Sao Paulo, Brazil; and Shanghai, China and a regulatory facility in Bangalore, India. Additionally, we have R&D operations co-located with manufacturing sites in Fort Dodge, Iowa; Prince Edward Island, Canada; and Cuxhaven, Germany. We incurred R&D expenses of $251.7 million in 2017, $265.8 million in 2016 and $291.0 million in 2015.
New product innovation is a core part of our business strategy. Our R&D investment is focused on projects that target novel product introductions, as well as new indications, presentations, combinations and species expansion. Our approach is a build, buy, or ally strategy to develop compelling targets and concepts that originate from our scientists and innovators, academia, agribusiness, or human pharmaceutical and biotechnology at all stages of R&D. The ability to source our concepts from different areas allows us to create a pipeline that can be competitive in the categories in which we have chosen to compete, while reducing our risk by not owning and funding all aspects of our R&D projects.
We seek to concentrate our resources in areas where we believe the science and our capabilities best match the opportunities in the animal health market. Specifically, our R&D focuses on six areas across companion animals and food animals. For companion animals, we have R&D activities in therapeutics, vaccines and parasiticides, while in food animals we are pursuing pharmaceuticals, vaccines and nutritional health.
Our R&D efforts consist of more than 100 active programs balanced across species and technology platforms. For both food animals and companion animals, we apply both large and small molecule approaches. In vaccines, our efforts encompass a full range of modified live, inactivated and nucleic acid strategies. In nutritional health, we focus on products based on enzymes, probiotics, prebiotics and other approaches that modulate biological activity in the animal digestive tract. Additionally, we employ various delivery strategies for products including in-feed, injectable, oral and topical formulations developed in conjunction with our manufacturing team to assure production that maximizes the capabilities within our internal and external manufacturing network.
We engage in licensing and business development to acquire assets for our pipeline and new R&D platforms and to establish strategic R&D collaborations. We make and maintain capital investments in venture capital vehicles that focus on agribusiness and animal health, and we engage in risk sharing collaborations to expand our external capital sources to augment internal investments. To support collaborations with innovation sources focused on human health we have developed capabilities to conduct translational comparative medical research trials in animals with naturally occurring conditions that mimic a human disease or disorder. This type of collaboration de-risks unproven or less well-validated human hypotheses while potentially defining a clinically validated new approach in veterinary medicine.
Our R&D and commercial leadership allocate R&D investment annually with the goal of aligning near- and long-term strategic opportunities and objectives. Portfolio investment decisions are made based on the probability of technical success and regulatory approval, timing of approval/launch and earlier milestones, feasibility and cost of development and manufacture, intellectual property protection and market attractiveness/commercial forecast. R&D projects are supported by pharmaceutical project management approaches and we aim for all of our supporting R&D functional capabilities and capacities to be managed and matched to the evolving demands of the pipeline. We believe this overall R&D management system has enabled us to consistently gain product approvals while maintaining clear visibility to pipeline breadth and depth to support sustained launches into the future.
114
Manufacturing and Supply Chain
Prior to the Separation, our products have been manufactured at both sites operated by us and sites operated by third-party CMOs.
Following the Separation, we will own and operate 13 internal manufacturing sites, four of which focus on vaccines, six of which focus on other animal health products and three of which are regional sites that focus on packaging:
| Site | Location | Site | Location |
|||
| | | | | | | |
| Clinton | Indiana, U.S. | Winslow | Maine, U.S. | |||
Speke |
Liverpool, U.K. |
Fort Dodge |
Iowa, U.S. |
|||
Kansas City |
Kansas, U.S. |
Cuxhaven |
Germany |
|||
Huningue |
France |
Chungli |
Taiwan |
|||
Wusi |
China |
Cali(a) |
Colombia |
|||
Terre Haute |
Indiana, U.S. |
Barueri |
Brazil |
|||
Prince Edward Island |
Canada |
- (a)
- We have announced our intention to exit ownership of this site
Following the Separation, we will continue to manufacture one product for Lilly at one of these sites for a period of two years. Lilly will have the option to extend for three additional years.
Our global manufacturing and supply chain is also supported by a network of CMOs. As of December 31, 2017, this network was comprised of approximately 120 CMOs. Our External Manufacturing Network centrally governs our global CMO relationships and provides oversight to these CMOs through four hubs.
We select CMOs based on several factors: (i) their ability to reliably supply products or materials that meet our quality standards at an optimized cost; (ii) their access to specialty products and technologies; (iii) capacity; and (iv) financial analyses. Our External Manufacturing Network seeks to ensure that all of the CMOs we use adhere to our standards of manufacturing quality.
We purchase certain raw materials necessary for the commercial production of our products from a variety of third-party suppliers. We utilize logistics service providers as a part of our global supply chain, primarily for shipping and logistics support.
We intend to continue our efficiency improvement programs in our manufacturing and supply chain organization. We have strong globally managed and coordinated quality control and quality assurance programs in place at all internal manufacturing sites and external manufacturing hubs, and we regularly inspect and audit our internal sites and CMO locations. We recently conducted a review of our global manufacturing and supply network to improve efficiency. As a result of this review and our operational efficiency program, we exited ownership of our manufacturing sites in Vacaville, California; Dundee, Scotland; Sligo, Ireland and Larchwood, lowa during the last three years, and we are in the process of exiting our manufacturing site in Cali, Colombia.
Our manufacturing sites experienced approximately 140 external regulatory inspections globally from 2015 to 2017, for which such regulators made no critical findings.
Competition
We face intense competition in the sectors and regions on which we focus. Principal methods of competition vary depending on the particular region, species, product category, or individual
115
product. Some of these methods include new product development, quality, price, service and promotion.
Our primary competitors include animal health medicines and vaccines companies such as Zoetis Inc.; Boehringer Ingelheim Vetmedica, Inc., the animal health division of Boehringer Ingelheim GmbH; Merck Animal Health, the animal health division of Merck & Co., Inc.; and Bayer Animal Health, the animal health division of Bayer AG. We also face competition globally from manufacturers of generic drugs, as well as from producers of nutritional health products, such as DSM Nutritional Products AG and Danisco Animal Nutrition, the animal health division of E. I. du Pont de Nemours and Company. There are also several new start-up companies working in the animal health area. In addition, we compete with numerous other producers of animal health products throughout the world.
Intellectual Property
Our technology, brands and other intellectual property are important elements of our business. We rely on patent, trademark, copyright and trade secret laws, as well as regulatory exclusivity periods and non-disclosure agreements to protect our intellectual property rights. Our policy is to vigorously protect, enforce and defend our rights to our intellectual property, as appropriate.
Our product portfolio and certain product candidates enjoy the protection of approximately 3,000 patents and applications, filed in over 50 countries, with concentration in our major market countries as well as other countries with strong patent systems, such as Australia, Brazil, Canada, Europe, Japan and the U.S. Many of the patents and patent applications in our portfolio are the result of our own work, while other patents and patent applications in our portfolio were at least partially developed, and licensed to us, by third parties. A subset of our current products or product candidates are covered by patents and patent applications in our portfolio.
Patents for individual products expire at different times based on the date of the patent filing (or sometimes the date of patent grant) and the legal term of patents in the countries where such patents are obtained. For example, Galliprant's active ingredient, grapiprant, is encompassed by both compound and physical form patents in the U.S., Europe, Canada and other key markets, with terms that expire between at least October 2021 and March 2026. Various formulation and method of use patents encompass the spinosad pesticide products, Comfortis and Trifexis. The Comfortis formulation patent extends through August 2020 in the U.S., Canada and Australia, and, upon grant of applicable supplementing protection certificate ("SPC"), through August 2025 in Europe. The Trifexis formulation and method of use patents extends through September 2021 in the U.S., Canada and Australia, and, upon grant of applicable SPC, through September 2026 in Europe. We typically maintain all of our patents and assert against third parties as appropriate.
Additionally, many of our vaccine products, including the Duramune family of vaccines, are based on proprietary or patented master seeds and formulations. We actively seek to protect our proprietary information, including our trade secrets and proprietary know-how, through a variety of means including by seeking to require our employees, consultants, advisors and partners to enter into confidentiality agreements and other arrangements upon the commencement of their employment or engagement.
In order to facilitate the Separation and allow Lilly and our operations to continue with minimal interruption, Lilly will license to us the right to use certain intellectual property rights in the animal health field. In addition, Lilly will grant us a transitional license to use certain of Lilly's trademarks for a period of time following the completion of this offering. See "Certain Relationships and Related Party Transactions — Relationship with Lilly — Transitional Trademark License Agreement."
116
We seek to file and maintain trademarks around the world based on commercial activities in most regions where we have, or desire to have, a business presence for a particular product. We currently maintain more than 9,000 trademark applications and registrations in major regions, primarily identifying products dedicated to the care of livestock and companion animals.
Regulatory
The sale of animal health products is governed by the laws and regulations specific to each country in which we sell our products. To maintain compliance with these regulatory requirements, we have established processes, systems and dedicated resources with end-to-end involvement from product concept to launch and maintenance in the market. Our regulatory function actively seeks to engage in dialogue with various global agencies regarding their policies that relate to animal health products. In the majority of our markets, the relevant health authority is separate from those governing human medicinal products.
United States
U.S. Food and Drug Administration. The regulatory body that is responsible for the regulation of animal health pharmaceuticals in the U.S. is the Center for Veterinary Medicine, a division of the FDA. All manufacturers of animal health pharmaceuticals must demonstrate their products to be safe, effective and produced by a consistent method of manufacture as defined under the Federal Food, Drug and Cosmetic Act (the "FFDCA"). The FDA's basis for approving a new animal drug application is documented in a Freedom of Information Summary. Post-approval monitoring of products is required by law, with reports being provided to the CVM's Office of Surveillance and Compliance. Reports of product quality defects, adverse events or unexpected results are maintained and submitted in accordance with the law. Additionally, as part of the drug experience report, we are required to submit all new information pertaining to the safety or effectiveness of a product, regardless of the source.
U.S. Department of Agriculture. The regulatory body in the U.S. for veterinary biologicals is the U.S. Department of Agriculture (the "USDA"). The Center for Veterinary Biologics within the Animal and Plant Health Inspection Service in the USDA is responsible for the regulation of animal health biologicals, which includes but is not limited to vaccines, bacterins, allergens, antibodies, antitoxins, toxoids, immunostimulants, certain cytokines, antigenic or immunizing components of live microorganisms, and diagnostic components of natural or synthetic origin, or that are derived from synthesizing or altering various substances or components of substances such as microorganisms, genes or genetic sequences, carbohydrates, proteins, antigens, allergens or antibodies. All manufacturers of animal health biologicals must show their products to be pure, safe, effective and produced by a consistent method of manufacture as defined under the Virus Serum Toxin Act. Post-approval monitoring of products is required. Reports of product quality defects, adverse events or unexpected results are maintained and submitted in accordance with the agency requirements.
Environmental Protection Agency. The main regulatory body in the U.S. for veterinary pesticides is the Environmental Protection Agency (the "EPA"). The EPA's Office of Pesticide Programs is responsible for the regulation of most pesticide products applied to animals in accordance with a memorandum of understanding (####-##-####) between the FDA and EPA for products that are subject to regulation under both the FFDCA and the Federal Insecticide, Fungicide and Rodenticide Act. All manufacturers of animal health pesticides must show their products will not cause unreasonable adverse effects to man or the environment as stated in the act. Within the U.S., individual state pesticide authorities must, before distribution in that state, also approve pesticide products that are approved by the EPA. Post-approval monitoring of products is required, with reports provided to the EPA and some state regulatory agencies.
117
Food Safety Inspection Service. The FDA is authorized to determine the safety of substances (including "generally recognized as safe" substances, food additives and color additives), as well as prescribe their safe conditions of use. However, although the FDA has the responsibility for determining the safety of substances, the Food Safety and Inspection Service, the public health agency in the USDA, still retains, under the tenets of the Federal Meat Inspection Act and the Poultry Products Inspection Act and their implementing regulations, the authority to determine that new substances and new uses of previously approved substances are suitable for use in meat and poultry products.
In addition, the FCPA prohibits U.S. corporations and their representatives from offering, promising, authorizing or making payments to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business abroad. The scope of the FCPA includes interactions with certain healthcare professionals in many countries. Other countries have enacted similar anti-corruption laws and/or regulations. In some countries in which we operate, the pharmaceutical and life sciences industries are exposed to a high risk of corruption associated with sales to healthcare professionals and institutions. Notwithstanding our reasonable efforts to conduct our operations in material compliance with the FCPA, our international business could expose us to potential liability under the FCPA, which may result in us incurring significant criminal and civil penalties, and to potential liability under the anti-corruption laws and regulations of other jurisdictions in which we operate. In addition, the costs we may incur in defending against an FCPA investigation could be significant.
Outside of the United States
- •
- The European Medicines Agency ("EMA") is a centralized agency of the EU, currently located in London, England. Due to the decision of the UK to
leave the EU, the agency will relocate to Amsterdam. The agency is responsible for the scientific evaluation of Veterinary Medicinal Products ("VMP") developed by pharmaceutical companies for use in
the EU. The agency has a veterinary review section distinct from the medical review section for human products. The Committee for Veterinary Medicinal Products ("CVMP") is responsible for scientific
review of the submissions for VMP and Immunological Veterinary Medicinal Products. If the CVMP concludes that all requirements for quality, safety and efficacy are met, they issue a positive opinion
that is forwarded to the European Commission, who takes the final decision following the European comitology procedure. The centralized marketing authorization (commission decision) of the European
Commission is valid in all of the EU. All countries that are not part of the EU but belong to the European Economic Area ("EEA"), i.e., Norway, Iceland and Liechtenstein, have been part of the
scientific assessment done by the CVMP. These countries issue a national marketing approval in accordance with the Commission Decision. A series of regulations, directives, guidelines, EU Pharmacopeia
Monographs and other legislation provide the requirements for approval in the EU. In general, these requirements are similar to those in the U.S., requiring demonstrated evidence of purity, safety,
efficacy and consistency of manufacturing processes.
If approval is sought for products that either cannot or do not need to follow the centralized procedure, approval can also be achieved by national approval in an EEA country agency. This national authorization can be mutually recognized by other EEA countries/EU member states (Mutual Recognition Procedure). In addition, national and mutual recognition can be done in a combined procedure (Decentralized Procedure).
- •
- The European Food Safety Authority ("EFSA") is the agency of the EU that provides scientific advice and communicates with respect to existing and emerging risks associated
European Union (EU). We are governed by the following EU regulatory bodies:
118
- •
- The European Chemical Agency ("ECHA") is the agency of the EU for the safe use of chemicals. ECHA was founded in 2007 and is based in Helsinki, Finland. Based on ECHA's mandate, the agency conducts the evaluation of biocides for the EU.
with the food chain. EFSA was established in February 2002, is based in Parma, Italy. Based on EFSA's mandate, the agency evaluates applications for feed additives, including enzymes and several nutritionals for animals.
In regard to Brexit, the EU and the UK negotiators have agreed to a transition period which is scheduled to last until December 2020, whereby regulatory processes would operate as they currently do. Post-separation, the UK has indicated they will look to continue working closely with the EMA, and that existing agreements between the EMA and other countries such as Switzerland, the U.S. and Canada provide a precedent which the UK could build on.
Brazil. The Ministry of Agriculture, Livestock Production and Supply ("MAPA") is the regulatory body in Brazil that is responsible for the regulation and control of pharmaceuticals, biologicals and medicinal feed additives for animal use. MAPA's regulatory activities are conducted through the Secretary of Agricultural Defense and its Livestock Products Inspection Department. In addition, regulatory activities are conducted at a local level through the Federal Agriculture Superintendence. These activities include the inspection and licensing of both manufacturing and commercial establishments for veterinary products, as well as the submission, review and approval of pharmaceuticals, biologicals and medicinal feed additives. MAPA is one of the most active regulatory agencies in Latin America, having permanent seats at several international animal health forums, such as Codex Alimentarius, World Organization for Animal Health and Committee of Veterinary Medicines for the Americas. MAPA was also recently invited to be a Latin American representative at International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products ("VICH") meetings. Several normative instructions issued by MAPA have set regulatory trends in Latin America.
Japan. The Ministry of Agriculture, Forestry and Fishery ("MAFF") is the regulatory body in Japan that is responsible for the regulation and control of pharmaceuticals (including biologicals and pesticide/disinfectant) and feed additive/feed for animal use. MAFF's regulatory activities are conducted through the Livestock & Aquaculture Product Safety Control Division under Consumer safety bureau. The animal drug reviews and approvals, reexamination reviews, GxP compliance checks, GxP site inspections and product assay checks (including vaccine national assays) are done by National Veterinary Assay Laboratory ("NVAL"). MAFF coordinates with other agencies such as Ministry of Health, Labor and Welfare ("MHLW") and Food safety commission ("FSC") to perform various license compliance checks (e.g., MA holder, manufacturer and oversea site accreditation) and ensure good promotional activities. Routine inspections, antimicrobial feed additive national assays and manufacturing inspections are done by the Food & Agriculture Material Inspection Center. For food animal products, animal drug review is done by NVAL but the human food safety review is done by FSC (ADI establishment and antimicrobial risk assessment) and MHLW (MRL establishment). These three agencies (NVAL, FSC and MHLW) work together to approve food animal products. In addition to those central government agencies, various licenses are delegated to the local municipal government, such as animal drug wholesaler and retailer licenses and feed additive distributor licenses.
China. The Ministry of Agriculture ("MOA") is the regulatory body that is responsible for the regulation and control of pharmaceuticals, biologicals, disinfectants, medicinal feed additives,
119
pesticide and feed/feed additives for animal use. There are three organizations under the MOA that regulate animal health:
- •
- The Institute of Veterinary Drug Control is responsible for the evaluation of new applications, renewals, variations, manufacturers, quality
methods and tissue residue methods for pharmaceuticals, biologicals, disinfectants and medicinal feed additives.
- •
- The feed/feed additive office is responsible for the registration and renewal of feed and feed additives.
- •
- The pesticide bureau is responsible for the registration and renewal of pesticide products.
Australia. The Australian Pesticides and Veterinary Medicines Authority ("APVMA") is an Australian government statutory authority established in 1993 to centralize the registration of all agricultural and veterinary products into the Australian marketplace. Previously, each state and territory government had its own system of registration. The APVMA assesses applications from companies and individuals seeking registration so they can supply their product to the marketplace. Applications undergo rigorous assessment using the expertise of the APVMA's scientific staff and drawing on the technical knowledge of other relevant scientific organizations, Commonwealth government departments and state agriculture departments. If the product works as intended and the scientific data confirms that when used as directed on the product label it will have no harmful or unintended effects on people, animals, the environment or international trade, the APVMA will register the product. As well as registering new agricultural and veterinary products, the APVMA reviews older products that have been on the market for a substantial period of time to ensure they still do the job users expect and are safe to use. The APVMA also reviews registered products when particular concerns are raised about their safety and effectiveness. The review of a product may result in confirmation of its registration or it may see registration continue with some changes to the way the product can be used. In some cases the review may result in the registration of a product being cancelled and the product taken off the market.
Rest of world. Country-specific regulatory laws typically have provisions that include requirements for certain labeling, safety, efficacy and manufacturers' quality control procedures (to assure the consistency of the products), as well as company records and reports. Other countries' regulatory agencies typically either refer to the FDA, USDA, EU and other international animal health entities, including the World Organization for Animal Health, Codex Alimentarius or VICH (see below), in establishing standards and regulations for veterinary pharmaceuticals and vaccines, or review the quality, safety and effectiveness of the products themselves according to their own national requirements.
Global policy and guidance
Joint FAO/WHO Expert Committee on Food Additives. The Joint FAO/WHO Expert Committee on Food Additives is an international expert scientific committee that is administered jointly by the Food and Agriculture Organization of the United Nations ("FAO") and the World Health Organization ("WHO"). They provide a risk assessment/safety evaluation of residues of veterinary drugs in animal products, exposure and residue definition and maximum residue limit proposals for veterinary drugs. Similarly, the Joint FAO/WHO Meeting on Pesticide Residues ("JMPR") is an international expert scientific group administered jointly by the FAO and WHO. JMPR reviews residues and analytical aspects of the pesticides, estimate the maximum residue levels, review toxicological data and estimate acceptable daily intakes for humans of the pesticides under consideration. We work with these committees to establish acceptable safe levels of residual product in food-producing animals after treatment with veterinary drugs or pesticides. This in turn enables the calculation of appropriate withdrawal times for our products prior to an animal entering the food chain.
120
Advertising and promotion review. Promotion of ethical animal health products is controlled by regulations in many countries. These rules generally restrict advertising and promotion to those claims and uses that have been reviewed and endorsed by the applicable agency. We conduct a review of promotion material for compliance with the local and regional requirements in the markets where we sell animal health products.
Import and Export of Products. The importation and exportation of animal health products is controlled by regulations in many countries. In some jurisdictions this may include obtaining separate permits or licenses by product or by company or filing notices with applicable regulatory agencies prior to import or export of product. We ensure compliance with local and global regulations in the markets where we import/export our animal health products.
International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products. VICH is a trilateral (EU-Japan-USA) program launched in 1996 aimed at harmonizing technical requirements for veterinary product registration. Several other countries have obtained observer status, e.g., Canada, New Zealand, Australia and South Africa, or are linked to VICH on basis of the VICH Outreach Forum, a VICH initiative with the main objective of providing a basis for wider international harmonization of technical requirements. In addition, the World Organization for Animal Health is an associate member of VICH.
The objectives of the VICH are as follows:
- •
- Establish and implement harmonized technical requirements for the registration of veterinary medicinal products in the VICH regions, which meet
high quality, safety and efficacy standards and minimize the use of test animals and costs of product development.
- •
- Provide a basis for wider international harmonization of registration requirements through the VICH Outreach Forum.
- •
- Monitor and maintain existing VICH guidelines, taking particular note of the ICH work program and, where necessary, update these VICH
guidelines.
- •
- Ensure efficient processes for maintaining and monitoring consistent interpretation of data requirements following the implementation of VICH
guidelines.
- •
- By means of a constructive dialogue between regulatory authorities and industry, provide technical guidance enabling response to significant emerging global issues and science that impact regulatory requirements within the VICH regions.
Employees
As of June 30, 2018, we employed approximately 5,640 full time employees. In addition, we employed approximately 240 fixed-duration employees, which are individuals hired for a pre-defined length of time (one to four years). Together, they total approximately 5,880 worldwide. Of the 5,880 employees globally, approximately 2,450 are U.S.-based and approximately 3,430 are employed in other jurisdictions. Some of these employees are members of unions, works councils, trade associations or are otherwise subject to collective bargaining agreements, including approximately 150 union employees in the U.S. located at our Fort Dodge, Iowa manufacturing/R&D facility. Approximately 40% of our global population is in customer-facing roles, including but not limited to, traditional sales roles, technical consultants, account managers and commercial and general managers.
121
Property
We have R&D operations co-located with certain of our manufacturing sites in the U.S. to facilitate the efficient transfer of production processes from our laboratories to manufacturing sites. In addition, we maintain R&D operations at non-manufacturing locations in the U.S., Switzerland, Australia, Brazil and China. As part of the Separation, Lilly will transfer to us its interest in each of these R&D facilities. Our largest R&D facility is our U.S. R&D site located in Fort Dodge, Iowa, which has approximately 0.3 million square feet.
The address of our principal executive offices is currently c/o Elanco, 2500 Innovation Way, Greenfield IN, 46140, and we expect that our principal executive offices will remain at this address following the completion of this offering.
Following the Separation, our global manufacturing network will be comprised of 13 manufacturing sites. The largest manufacturing site in our global manufacturing network is our manufacturing site located in Clinton, Indiana, which has approximately 0.7 million square feet. In addition, our global manufacturing network will continue to be supplemented by approximately 120 CMOs. See "— Manufacturing and Supply Chain."
We own or lease various additional properties for other business purposes including office space, warehouses and logistics centers. In addition, under the transitional services agreement, Lilly will provide us with continued access to certain of its premises currently occupied by our employees for up to two years.
We believe that our existing properties, as supplemented by CMOs and access to Lilly facilities that will be provided under the transitional services agreement, are adequate for our current requirements and for our operations in the near future.
Environmental, Health and Safety
We are subject to various federal, state, local and foreign environmental, health and safety ("EHS") laws and regulations. These laws and regulations govern matters such as the emission and discharge of hazardous materials into the ground, air or water; the generation, use, storage, handling, treatment, packaging, transportation, exposure to, and disposal of hazardous and biological materials, including recordkeeping, reporting and registration requirements; and the health and safety of our employees. Due to our operations, these laws and regulations also require us to obtain, and comply with, permits, registrations or other authorizations issued by governmental authorities. These authorities can modify or revoke our permits, registrations or other authorizations and can enforce compliance through fines and injunctions.
Certain environmental laws impose joint and several liability, without regard to fault, for cleanup costs on persons who have disposed of or released hazardous substances into the environment, including at third-party sites or offsite disposal locations, or that currently own or operate (or formerly owned or operated) sites where such a release occurred. We could be subject to liability for the investigation and remediation of legacy environmental contamination caused by historical industrial activity at sites that we own or on which we operate. In addition to clean-up actions brought by federal, state, local and foreign governmental entities, private parties could raise personal injury or other claims against us due to the presence of, or exposure to, hazardous materials on, from or otherwise relating to such a property.
We have made, and intend to continue to make, necessary expenditures for compliance with applicable EHS laws and regulations. We are also monitoring and investigating environmental contamination from past industrial activity at certain sites. While we cannot predict with certainty our future capital expenditures or operating costs for environmental compliance or the investigation and remediation of contaminated sites, we anticipate capital and operational expenditures for the remainder of the year ending December 31, 2018 and the year ending December 31, 2019 for
122
environmental compliance purposes and for the monitoring, investigation or clean-up of certain past industrial activities as follows:
- •
- environmental-related capital expenditures — $2.9 million; and
- •
- other environmental-related expenditures — $2.2 million.
In connection with past acquisitions and divestitures, we have undertaken certain indemnification obligations that may require us in the future, to conduct or finance environmental cleanups at sites that we no longer own or operate. We have also entered into indemnification agreements in which we are or may be indemnified for various environmental cleanups; however, such indemnities are limited in both time and scope and may be further limited in the presence of new information, or may not be available at all.
Legal Proceedings
We are from time to time subject to claims and litigation arising in the ordinary course of business. These claims and litigation may include, among other things, allegations of violation of U.S. and foreign competition law, labor laws, consumer protection laws and environmental laws and regulations, as well as claims or litigation relating to product liability, intellectual property, securities, breach of contract and tort. We operate in multiple jurisdictions and, as a result, a claim in one jurisdiction may lead to claims or regulatory penalties in other jurisdictions. We intend to vigorously defend against any pending or future claims and litigation, as appropriate.
At this time, in the opinion of management, the likelihood is remote that the impact of such proceedings, either individually or in the aggregate, would have a material adverse effect on our combined results of operations, financial condition or cash flows. However, one or more unfavorable outcomes in any claim or litigation against us could have a material adverse effect for the period in which they are resolved. In addition, regardless of their merits or their ultimate outcomes, such matters are costly, divert management's attention and may materially adversely affect our reputation, even if resolved in our favor.
123
Directors and Executive Officers
The following table sets forth the names and ages, as of June 30, 2018, and titles of the individuals we currently expect to serve as our executive officers and members of our board of directors at the time of the offering. Certain biographical information with respect to those executive officers and directors follows the table.
Name |
Age | Position |
|||
| | | | | | |
Jeffrey N. Simmons |
51 | President, Chief Executive Officer and Director | |||
Lucas E. Montarce |
41 | Acting Chief Financial Officer | |||
Ramiro M. Cabral |
47 | Executive Vice President, Elanco International and Global Customer Value | |||
Michael-Bryant Hicks |
43 | Executive Vice President, General Counsel and Corporate Secretary | |||
David S. Kinard |
51 | Executive Vice President, Human Resources and Corporate Affairs | |||
Sarena S. Lin |
47 | Executive Vice President, Elanco USA and Global Strategy | |||
Aaron L. Schacht |
51 | Executive Vice President, Innovation, Regulatory and Business Development | |||
David A. Urbanek |
52 | Executive Vice President, Manufacturing and Quality | |||
R. David Hoover |
73 | Director (Chairman) | |||
Kapila K. Anand |
64 | Director | |||
Michael J. Harrington |
55 | Director | |||
Lawrence E. Kurzius |
60 | Director | |||
Carl L. McMillian |
52 | Director | |||
David A. Ricks |
51 | Director | |||
Aarti S. Shah |
53 | Director | |||
Joshua L. Smiley |
48 | Director |
Jeffrey N. Simmons serves as our President and Chief Executive Officer and as a director on our board. Mr. Simmons has served as the President of the Elanco Animal Health division of Lilly and Senior Vice President of Lilly since 2008. Prior to 2008, Mr. Simmons held various leadership roles for Elanco, including District Sales Manager, International Marketing Manager, Country Director for Brazil, Area Director for Western Europe and Executive Director for U.S. and Global Research & Development.
Mr. Simmons' experience described above, including his knowledge of our company and the animal health industry and his business and management experience, provides him with the qualifications and skills to serve as a director on our board.
Lucas E. Montarce serves as our Acting Chief Financial Officer. Mr. Montarce was appointed as Acting Chief Financial Officer in August 2018, following the medical leave of absence of our Chief Financial Officer. Mr. Montarce has served as Vice President, Finance, Strategy and Operations of the Elanco Animal Health division of Lilly since June 2017. Since joining Lilly in 2001, he has held a variety of positions across Latin America, Europe and the U.S., including local and regional Chief Finance Officer and Director of Global Treasury.
Ramiro M. Cabral serves as our Executive Vice President, Elanco International and Global Customer Value. Mr. Cabral has served as Vice President and Chief Marketing Officer of the Elanco Animal Health division of Lilly since 2017. Mr. Cabral joined Lilly in 2005 and has held various leadership positions for Elanco, including Vice President and Head of Operations for Elanco EMEA from 2013 to 2017 and Senior Director, U.S. Beef Business Unit from 2011 to 2013.
124
Michael-Bryant Hicks serves as our Executive Vice President, General Counsel and Corporate Secretary. Mr. Hicks has served as General Counsel of the Elanco Animal Health division of Lilly since May 2018. Prior to joining Elanco, Mr. Hicks served in various legal roles, including General Counsel at Mallinckrodt Public Liability Company from 2016 to 2018, Senior Vice President, General Counsel and Corporate Strategy at The Providence Service Corporation from 2014 to 2016 and as Assistant General Counsel at DaVita Inc. from 2011 to 2013.
David S. Kinard serves as our Executive Vice President, Human Resources and Corporate Affairs. Mr. Kinard has served as Vice President of Human Resources and Global Learning and Development for the Elanco Animal Health division of Lilly since May 2018. Prior to May 2018, Mr. Kinard served in various leadership roles for Lilly, including Vice President of Human Resources for a variety of Lilly businesses, including Lilly International in 2017, Bio-Medicines and Emerging Markets from 2015 to 2017 and Lilly Diabetes and Global Employee Relations/HR Operations from 2011 to 2015.
Sarena S. Lin serves as our Executive Vice President, Elanco USA and Global Strategy. Ms. Lin has served as Senior Vice President of North American Operations and Strategy at the Elanco Animal Health division of Lilly since January 2018. Prior to joining Lilly, Ms. Lin served as President of Cargill Feed & Nutrition from 2014 to 2017. Prior to 2014, Ms. Lin served as Global Head of Strategy and Business Development for Cargill from 2011 to 2014. Ms. Lin served on the board of directors for the animal health and dental distributor, Patterson Companies, from 2014 to 2018.
Aaron L. Schacht serves as our Executive Vice President, Innovation, Regulatory and Business Development. Mr. Schacht has served as the Vice President of global research and development of the Elanco Animal Health division of Lilly since 2015. Prior to 2015, Mr. Schacht served in various leadership roles for Lilly, including Global Brand Development Leader of Pain in Lilly BioMedicines in 2014, Senior Advisor of Strategy & Business Development for Lilly BioMedicines from 2012 to 2014 and Executive Director of Global External R&D at Lilly from 2008 to 2012.
David A. Urbanek serves as our Executive Vice President, Manufacturing and Quality. Mr. Urbanek has served as Vice President of Manufacturing at the Elanco Animal Health division of Lilly since November 2017. Prior to that, Mr. Urbanek served in various leadership roles for Lilly's Manufacturing division, including Senior Director of Emerging Markets Manufacturing from 2013 to 2017, Senior Director of Global Diabetes Manufacturing from 2011 to 2013 and Senior Director of External Drug Products Operations from 2009 to 2011.
R. David Hoover serves as the chairman of our board. Mr. Hoover has been retired since 2013. Prior to that, Mr. Hoover served in various roles at Ball Corporation, including Chairman from 2002 to 2013, Chief Executive Officer from 2010 to 2011, President and Chief Executive Officer from 2001 to 2010, Chief Operating Officer from 2000 to 2001 and Chief Financial Officer from 1998 to 2000. Mr. Hoover currently serves on the boards of directors of Ball Corporation and Edgewell Personal Care Company.
Mr. Hoover's experience described above, including his extensive management experience as Chief Executive Officer and Chief Financial Officer at Ball Corporation and corporate governance experience through his service on other public boards, including nine years he previously served as a director for Lilly, provides him with the qualifications and skills to serve as a director on our board.
Kapila K. Anand will serve as a director on our board upon the completion of this offering. Ms. Anand has served as a Senior Advisor to KPMG LLP since 2016. Prior to that, Ms. Anand served in various leadership roles as a partner at KPMG LLP, including Industry Segment Leader — Travel, Leisure and Hospitality from 2011 to 2017, Partner in Charge — Public Policy Business
125
Initiatives from 2009 to 2013, KPMG LLP Board member from 2005 to 2010, Advisory Leader — Private Equity, Real Estate and Hospitality from 2002 to 2009 and Audit Partner — Real Estate and Hospitality from 1989 to 2002. Ms. Anand currently serves on the boards of directors of Extended Stay America, Inc. and Omega Healthcare Investors, Inc.
Ms. Anand's experience described above, including her extensive financial, managerial and corporate governance experience, provides her with the qualifications and skills to serve as a director on our board.
Michael J. Harrington will serve as a director on our board upon the completion of this offering. Mr. Harrington has served as the Senior Vice President and General Counsel for Lilly since January 2013. Prior to 2013, Mr. Harrington served in various legal roles for Lilly, including Vice President and Deputy General Counsel of Global Pharmaceutical Operations from 2010 to 2012 and Vice President and General Counsel, Corporate from 2004 to 2010.
Mr. Harrington's experience described above, including his knowledge of our company and the animal health industry and his business and management experience, provides him with the qualifications and skills to serve as a director on our board.
Lawrence E. Kurzius will serve as a director on our board upon the completion of this offering. Mr. Kurzius has served in various leadership roles at McCormick & Company, including director since 2015 and Chairman of the Board of Directors since 2017, Chief Executive Officer since 2016, President since 2015, Chief Operating Officer from 2015 to 2016, Chief Administrative Officer from 2013 to 2015, President, International Businesses from 2008 to 2013, President, Europe, Middle East and Africa from 2007 to 2008 and President, U.S. Consumer Foods from 2005 to 2006.
Mr. Kurzius' experience described above, including his extensive management experience and corporate governance experience, provides him with the qualifications and skills to serve as a director on our board.
Carl L. McMillian will serve as a director on our board upon the completion of this offering. Mr. McMillian has served as Vice President of Toxicology, Drug Disposition, PKPD, Veterinary Resources & Experimental Medicine of Lilly since 2012. Prior to that, Mr. McMillian served in various leadership roles for Lilly, including Senior Director, Drug Disposition from 2008 to 2012 and Director, Drug Disposition from 2002 to 2008.
Mr. McMillian's experience described above, including his knowledge of our company and the animal health industry and his business and management experience, provides him with the qualifications and skills to serve as a director on our board.
David A. Ricks will serve as a director on our board upon the completion of this offering. Mr. Ricks has served as the Chief Executive Officer and President of Lilly since January 2017; Mr. Ricks joined the board of Lilly in January 2017 and became Chairman in June 2017. Prior to that, Mr. Ricks served in various leadership roles for Lilly, including President of Lilly Bio-Medicines from 2012 to 2016 and President of Lilly USA from 2009 to 2012.
Mr. Ricks's experience described above, including his knowledge of our company and the animal health industry and his business and management experience, provides him with the qualifications and skills to serve as a director on our board.
Aarti S. Shah will serve as a director on our board upon the completion of this offering. Ms. Shah has served as Senior Vice President and Chief Information Officer of Lilly since July 2016. Prior to that, Ms. Shah served in various leadership roles for Lilly, including Global Brand Leader of the Autoimmune Division of Lilly Bio-Medicines from 2013 to 2016 and Vice President of Biometrics & Advanced Analysis from 2009 to 2013.
126
Ms. Shah's experience described above, including her knowledge of our company and the animal health industry and her business and management experience, provides her with the qualifications and skills to serve as a director on our board.
Joshua L. Smiley will serve as a director on our board upon the completion of this offering. Mr. Smiley has served as Senior Vice President and Chief Financial Officer of Lilly since January 2018. Prior to January 2018, Mr. Smiley held a variety of leadership positions at Lilly, including Senior Vice President and Treasurer in 2017 and Senior Vice President of Finance, Corporate Controller and Chief Financial Officer of Lilly Research from 2011 to 2015.
Mr. Smiley's experience described above, including his knowledge of our company and the animal health industry and his business and management experience, provides him with the qualifications and skills to serve as a director on our board.
Board of Directors
Our business and affairs are managed under the direction of our board of directors. Our amended and restated articles of incorporation and amended and restated bylaws will provide that our board of directors consist of not less than five directors. Contemporaneous with this offering, our board of directors will be composed of nine directors.
Our amended and restated articles of incorporation will provide that our board of directors will be divided into three classes, with one class being elected at each annual meeting of shareholders. Each director will serve a three-year term, with expiration staggered according to class. Each class will initially consist of three directors. The Class I directors, whose terms will expire at the first annual meeting of our shareholders following the filing of our amended and restated articles of incorporation, will be . The Class II directors, whose terms will expire at the second annual meeting of our shareholders following the filing of our amended and restated articles of incorporation, will be . The Class III directors, whose terms will expire at the third annual meeting of our shareholders following the filing of our amended and restated articles of incorporation, will be . See "Description of Capital Stock — Anti-Takeover Effects of Our Amended and Restated Articles of Incorporation and our Amended and Restated Bylaws."
Director Independence and Controlled Company Exemption
We intend to avail ourselves of the "controlled company" exemption under the corporate governance rules of the NYSE. Accordingly, we will not be required to have a majority of "independent directors" on our board of directors as defined under the rules of the NYSE; nor will we have a compensation committee and a corporate governance and nominating committee composed entirely of independent directors. The "controlled company" exemption does not modify the independence requirements for the audit committee, and we intend to comply with the requirements of the Sarbanes-Oxley Act and the NYSE, which require that our audit committee be composed of at least three members, one of whom will be independent upon the listing of our common stock, a majority of whom will be independent within 90 days of listing, and each of whom will be independent within one year of listing.
At such time that we cease to be a "controlled company" under the rules of the NYSE, our board of directors will take all action necessary to comply with the NYSE corporate governance rules, including appointing a majority of independent directors to the board of directors and establishing certain committees composed entirely of independent directors, subject to a permitted "phase-in" period.
Our board of directors has determined that , and are independent directors under the applicable rules of the NYSE.
127
Board Committees
Our board of directors will establish an audit committee, a compensation committee and a nominating and corporate governance committee. Each committee will operate under a charter that will be approved by our board of directors and will have the composition and responsibilities described below. Members will serve on these committees until their resignations or until otherwise determined by our board of directors. The charter of each committee will be available on our website.
Audit Committee. The primary purposes of our audit committee will be to assist our board of directors' oversight of:
- •
- the integrity of our financial statements and any other financial information which will be provided to the shareholders and others;
- •
- the independent auditor's qualifications and independence;
- •
- the systems of internal controls and disclosure controls which management has established;
- •
- the performance of internal and independent audit functions; and
- •
- our compliance with legal and regulatory requirements.
Upon the completion of this offering, and prior to the listing of our common stock, our audit committee will be composed of , and . will serve as chair of the audit committee. qualifies as an "audit committee financial expert" as such term has been defined by the Securities and Exchange Commission in Item 407(d) of Regulation S-K. Our board of directors has affirmatively determined that and meet the definition of an "independent director" for the purposes of serving on the audit committee under applicable NYSE rules and Rule 10A-3 under the Exchange Act. We intend to comply with these independence requirements for all members of the audit committee within the time periods specified under such rules. The audit committee will be governed by a charter that complies with the rules of the NYSE.
Compensation Committee. The primary purposes of our compensation committee will be to assist our board of directors in overseeing our management compensation policies and practices, including:
- •
- determining and approving the compensation of our executive officers; and
- •
- oversight of our compensation plans, including by reviewing and approving incentive compensation and equity compensation policies and programs.
Upon the completion of this offering, and prior to the listing of our common stock, our compensation committee will be composed of , and . will serve as chair of the compensation committee. and , each of whom qualifies as a "non-employee director" under Rule 16b-3 of the Exchange Act, will serve as a subcommittee of our compensation committee for the purpose of reviewing and approving equity awards to our directors and executive officers made pursuant to the 2018 Elanco Stock Plan. We intend to avail ourselves of the "controlled company" exemption under the rules of the NYSE, which exempts us from the requirement that we have a compensation committee composed entirely of independent directors. The compensation committee will be governed by a charter that complies with the rules of the NYSE.
Nominating and Corporate Governance Committee. The primary purposes of our nominating and corporate governance committee will be to:
- •
- recommend to the board of directors the qualifications required for membership on our board of directors and its committees;
128
- •
- identify and recommend to our board of directors candidates for membership on our board of directors and its committees;
- •
- develop and recommend criteria and policies relating to the service of directors; and
- •
- oversee matters of corporate governance.
Upon the completion of this offering, and prior to the listing of our common stock, our nominating and corporate governance committee will be composed of , and . will serve as chair of the nominating and corporate governance committee. We intend to avail ourselves of the "controlled company" exemption under the rules of the NYSE, which exempts us from the requirement that we have a nominating and corporate governance committee composed entirely of independent directors. The nominating and corporate governance committee will be governed by a charter that complies with the rules of the NYSE.
Indemnification of Directors and Officers
See "Description of Capital Stock — Certain Provisions of the Indiana Business Corporation Law."
Code of Business Conduct and Ethics
Prior to the completion of this offering, we will adopt a code of conduct and code of ethical conduct for financial management that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. A copy of the codes will be available on our website located at www.elanco.com. Any amendments to or waivers from our code of ethical conduct for financial management will be disclosed on our Internet website promptly following the date of such amendment or waiver.
Corporate Governance Guidelines
Our board of directors will adopt corporate governance guidelines in accordance with the corporate governance rules of the NYSE, which will serve as a flexible framework within which our board of directors and its committees will operate. These guidelines will cover a number of areas, including the role of the board of directors, board composition, director independence, director selection, qualification and election, director compensation, executive sessions, key board responsibilities, CEO evaluation, succession planning, risk management, board leadership and operations, conflicts of interest, annual board assessments, board committees, director orientation and continuing education, board agenda, materials, information and presentations, director access to management and independent advisers, and board communication with shareholders and others. A copy of our corporate governance guidelines will be posted on our website.
Compensation Committee Interlocks and Insider Participation
We do not have any interlocking relationships between any member of our compensation committee and any of our executive officers that would require disclosure under the applicable rules promulgated under the federal securities laws.
129
EXECUTIVE AND DIRECTOR COMPENSATION
Compensation Discussion and Analysis
Introduction
This compensation, discussion and analysis ("CD&A") provides detailed information regarding the individuals who we currently expect to serve as our Chief Executive Officer and Chief Financial Officer and to be our three most highly-compensated executive officers following this offering (the "Named Executive Officers"):
| Name | Title |
|
| | | |
| Jeffrey N. Simmons | President and Chief Executive Officer | |
| Lucas E. Montarce | Acting Chief Financial Officer | |
| Aaron L. Schacht | Executive Vice President, Innovation, Regulatory and Business Development | |
| David S. Kinard | Executive Vice President, Human Resources and Corporate Affairs | |
| David A. Urbanek | Executive Vice President, Manufacturing and Quality |
We currently operate as part of Lilly. As a result, the 2017 compensation for our Named Executive Officers was determined by Lilly, as described below. We anticipate that Lilly will continue to establish and manage the compensation for all of our Named Executive Officers until the completion of this offering. Accordingly, the discussion in this CD&A primarily relates to Lilly's compensation and compensation philosophy. Our board of directors, through our compensation committee, will establish and oversee our compensation programs following the completion of this offering. The compensation programs that we adopt, and our compensation philosophy, may differ materially from the current Lilly programs summarized in this discussion.
Because Messrs. Montarce, Schacht, Kinard and Urbanek were not executive officers of Lilly, their cash and equity compensation was determined by Lilly's senior management consistent with Lilly's compensation philosophy, but was not specifically determined or reviewed by the Lilly compensation committee. As a Lilly executive officer, Mr. Simmons' compensation was reviewed and determined by Lilly's compensation committee, with the advice of the compensation consultant engaged by Lilly's compensation committee.
This CD&A describes Lilly's compensation philosophy, the elements of each compensation program, the factors that Lilly considered when setting its 2017 executive compensation, and how results affected incentive payouts for 2017 performance for each of our Named Executive Officers, as well as certain elements of the compensation program we currently expect to be in effect following completion of the offering.
Lilly's Philosophy on Compensation
Lilly's compensation programs are designed to help achieve the goals of attracting, engaging and retaining highly talented individuals who are committed to its core values of integrity, excellence and respect for people while balancing the long-term interests of its shareholders and customers.
Lilly's compensation and benefits programs are based on the following objectives:
- •
- Reflect individual and company performance. Lilly reinforces a high-performance culture by linking pay with individual performance and company performance. As Lilly employees assume greater levels of responsibility, the proportion of total compensation based on company performance and shareholder returns increases. Lilly performs an annual review to
130
- •
- Attract and retain talented
employees. Lilly aims for compensation opportunities at Lilly to be competitive with its peer group and reflect the level of job impact and
responsibility. Retention of talent is an important factor in the design of its compensation and benefit programs.
- •
- Implement broad-based
programs. While the amount of compensation paid to employees varies, the overall structure of Lilly's compensation and benefit programs is
broadly similar across the organization to encourage and reward all employees who contribute to its success.
- •
- Consider shareholder input. Lilly management and the Lilly compensation committee consider the results of its annual say-on-pay vote and other sources of shareholder feedback when designing Lilly's compensation and benefit programs.
ensure its programs provide incentives to deliver long-term, sustainable business results while discouraging excessive risk-taking or other adverse behaviors.
Compensation Processes and Analysis
Process for Setting Compensation
The Lilly compensation committee considers individual performance assessments, compensation recommendations from senior leadership, Lilly's company performance, Elanco performance (as applicable), Lilly's peer group data, input from its compensation consultant and its own judgment when determining compensation for its executive officers. When determining the compensation for our other Named Executive Officers, who are not also executive officers of Lilly, Lilly's senior management considers similar factors consistent with Lilly's philosophy, focusing on individual performance assessment, compensation recommendations from senior leadership, Elanco's performance and their own judgment.
- •
- Assessment of individual
performance. Each executive's, including our Named Executive Officers', as applicable, performance assessment is based on achievement of
objectives established at the start of the year, as well as other factors, including the demonstration of Lilly values and leadership behaviors.
- •
- Assessment of company
performance. Lilly company performance and Elanco performance, as applicable, is considered in two
ways:
- •
- Overall performance of the prior year based on a variety of metrics, which is a factor in establishing target compensation for the coming
year.
- •
- Specific performance goals are established at the beginning of each performance year to determine payouts under cash and equity incentive
programs.
- •
- Peer group analysis. Lilly uses
data from its peer group as a market check for compensation decisions, but does not use this data as the sole basis for its compensation targets.
- •
- Input from an independent compensation consultant concerning executive pay. Lilly's compensation committee receives the advice of its independent compensation consultant, Frederic W. Cook & Co., Inc., when setting Lilly executive officer compensation.
Competitive Pay Assessment
Lilly's peer group is comprised of companies that directly compete with Lilly, operate in a similar business model and employ people with the unique skills required to operate an established biopharmaceutical company. Lilly's compensation committee selects a peer group whose median
131
market cap and revenues are broadly similar to Lilly's. Lilly's compensation committee reviews the peer group at least every three years. Lilly's compensation committee reviewed the peer group for purposes of assessing competitive pay in June 2015 and decided to include Abbvie, Amgen, AstraZeneca, Baxter, Biogen, Bristol-Myers Squibb, Celgene, Gilead, GlaxoSmithKline, Hoffman-La Roche, Johnson & Johnson, Medtronic, Merck, Novartis, Pfizer, Sanofi-Aventis and Shire Plc. With the exception of Johnson & Johnson, Novartis and Pfizer, peer companies were no greater than three times Lilly's size on both measures. Lilly's compensation committee included these three companies despite their size because they compete directly with Lilly, have similar business models and seek to hire from the same pool of management and scientific talent.
Components of Executive Compensation
Lilly's executive compensation program, including for our Named Executive Officers, is primarily comprised of three components:
- •
- base salary;
- •
- annual cash bonus programs, including:
- •
- Elanco Corporate Bonus Plan ("Elanco Bonus Plan"), under which bonuses are calculated based on Elanco's performance as compared to Elanco's
internal targets for revenue and income before taxes ("IBT"), Elanco's innovation progress and Lilly's performance under The Eli Lilly and Company Bonus Plan ("Lilly Bonus Plan"), and for
Mr. Simmons bonus payouts under the Elanco Bonus Plan are also subject to the terms of the Lilly Executive Officer incentive Plan (EIOP), as described below;
and
- •
- Lilly Bonus Plan, under which bonuses are calculated based on Lilly's performance as compared to Lilly's internal targets for revenue, earnings
per share ("EPS"), and Lilly innovation progress.
- •
- Lilly equity incentives:
- •
- performance awards ("Lilly PAs"), which are Lilly equity awards with a performance component measuring Lilly's two-year growth in EPS relative
to the expected peer group growth. For performance awards granted to Lilly executive officers, including Mr. Simmons ("Lilly Executive Officer PAs"), the two-year performance period is
immediately followed by a 13-month service-vesting period; and
- •
- shareholder value awards ("Lilly SVAs"), which are performance-based Lilly equity awards that pay out based on absolute Lilly stock price growth over a three-year performance period. For shareholder value awards granted to Lilly executive officers, including Mr. Simmons ("Lilly Executive Officer SVAs"), Lilly also applies a measure of total shareholder return ("TSR") relative to peers and a one-year holding period.
Lilly employees, including our Named Executive Officers, also receive a company benefits package, described below under "Other Compensation Practices and Information — Employee Benefits."
1. Base Salary
Base salaries, including for our Named Executive Officers, are reviewed and established annually by Lilly and may be adjusted upon promotion, following a change in job responsibilities or to maintain market competitiveness. Salaries are based on each person's level of contribution, responsibility, expertise and competitiveness with Lilly peer group data.
132
Base salary increases for 2017 were established based upon a Lilly corporate budget for salary increases, which were set considering Lilly performance over the prior year, expected Lilly performance for the following year and general external trends. In setting salaries, Lilly seeks to retain, motivate and reward successful performers, while maintaining affordability within the company's business plan.
2. Annual Cash Bonus
Our Named Executive Officers, except for Mr. Kinard, participated in the Elanco Bonus Plan during 2017. The Elanco Bonus Plan is designed to reward the achievement of Elanco's financial goals, innovation objectives and contributions to Lilly's overall financial success for the year. The bonus is based on four areas that are measured relative to internal targets: Elanco revenue, Elanco IBT, Elanco innovation progress and Lilly corporate objectives as measured under the Lilly Bonus Plan (referred to as the Lilly Bonus Plan Multiple (LBPM)).
Mr. Kinard participated in the Lilly Bonus Plan for all of 2017. Messrs. Montarce and Urbanek participated in the Lilly Bonus Plan during January 2017 after which they participated in the Elanco Bonus Plan. The Lilly Bonus Plan is designed to reward the achievement of Lilly's financial goals and innovation objectives. The bonus is based on three areas that are measured relative to internal targets: Lilly revenue, Lilly EPS and Lilly innovation progress.
Elanco and Lilly performance goals and individual bonus targets are set at the beginning of each year. Actual payout can range from 0% to 200% of an individual's bonus target. Performance targets and the assessment of the relative weighting for each objective is based upon annual operating plans with a threshold, target and maximum set for each objective (with straight line interpolation for achievement between relevant levels). The 2017 weightings were as follows:
Elanco Bonus Plan
Elanco Goals |
Weighting |
|||
| | | | | |
Elanco revenue performance |
25 | % | ||
Elanco IBT performance |
25 | % | ||
Elanco innovation progress |
25 | % | ||
Lilly Bonus Plan Multiple (LBPM) |
25 | % |
Based on this weighting, the Elanco Bonus Plan multiple is calculated as follows:
(0.25 × revenue multiple) + (0.25 × IBT
multiple) + (0.25 × innovation multiple) + (0.25 × LBPM)
= Elanco Bonus Plan multiple
The annual Elanco Bonus Plan payout is calculated as follows:
Elanco Bonus Plan multiple × individual bonus target × base salary earnings = payout
Lilly Bonus Plan
Lilly Goals |
Weighting |
|||
| | | | | |
Lilly revenue performance |
25 | % | ||
Lilly EPS performance |
50 | % | ||
Lilly innovation progress |
25 | % |
133
Based on this weighting, the Lilly Bonus Plan multiple is calculated as follows:
(0.25 × revenue multiple) + (0.50 × EPS
multiple) + (0.25 × innovation multiple)
= Lilly Bonus Plan multiple
The annual Lilly Bonus Plan payout is calculated as follows:
Lilly Bonus Plan multiple × individual bonus target × base salary earnings = payout
For Mr. Simmons, who is also a Lilly executive officer, annual bonuses are subject to the terms of the Lilly Executive Officer Incentive Plan (EOIP). Under the EOIP, the maximum annual cash bonus allowable is calculated based on Lilly's non-GAAP net income for the year. For Mr. Simmons, the maximum amount for 2017 was 0.15% of non-GAAP net income. None of the Lilly executive officers, including Mr. Simmons, receive an annual cash bonus payment unless Lilly has positive non-GAAP net income for the year.
Under the EOIP, once the maximum payout for Mr. Simmons is determined, the Lilly compensation committee has the discretion to reduce (but not increase) the amount to be paid. In exercising this discretion, the committee's intent is to award the lesser of (i) the bonus Mr. Simmons would have received under the Elanco Bonus Plan or (ii) the EOIP maximum payout. For 2018, Mr. Simmons' bonus payout under the EOIP will be based entirely on Elanco performance.
3. Equity Incentives
Lilly primarily grants two types of equity incentives to executives and certain other employees, including our Named Executive Officers — Lilly PAs and Lilly SVAs. Lilly PAs are designed to focus its leaders on multi-year operational performance relative to peer companies. Lilly SVAs align earned compensation with long-term growth in Lilly shareholder value. Messrs. Schacht and Urbanek received special retention awards during 2017, as described in "Special Retention Awards" below.
Lilly Executive Officer PAs and Lilly Executive Officer SVAs are awarded to Lilly executive officers, including Mr. Simmons. The Lilly compensation committee has the discretion to adjust downward (but not upward) any Lilly executive officer's equity award payout from the amount yielded by the applicable formula.
Performance Awards (PAs and Executive Officer PAs)
Our Named Executive Officers (other than Mr. Simmons) received Lilly PAs which vest over two years. Potential Lilly shares are based on achieving Lilly EPS growth targets over a two-year performance period. The growth-rate targets are set relative to the median expected EPS growth for Lilly's peer group. These awards do not accumulate dividends.
Lilly executive officers, including Mr. Simmons, received the Lilly Executive Officer PAs which use the same two-year EPS growth-rate targets as the Lilly PAs for determining the number of Lilly shares; however, the performance period is followed by an additional 13-month service-vesting period during which the award is held in the form of Lilly restricted stock units.
The Lilly compensation committee believes EPS growth is an effective measure of operational performance because it is closely linked to shareholder value, is broadly communicated to the public, is easily understood by its employees and allows for objective comparisons to its peer group performance. Consistent with its compensation objectives, Lilly company performance exceeding the expected peer group median results in above-target payouts, while Lilly company performance lagging the expected peer group median results in below-target payouts. Possible payouts range from 0% to 150% of the target, depending on Lilly EPS growth over the performance period.
134
Shareholder Value Awards (SVAs and Executive Officer SVAs)
Our Named Executive Officers (other than Mr. Simmons) received Lilly SVAs. These awards are based on Lilly's share price appreciation over a three-year performance period. Lilly SVAs pay above target if Lilly's stock outperforms an expected rate of return and below target if Lilly's stock underperforms that expected rate of return. The expected rate of return is based on the three-year TSR that a reasonable investor would consider appropriate when investing in a basket of large-cap U.S. companies, as determined by the Lilly compensation committee. The minimum price to achieve target is calculated by multiplying the starting share price of Lilly's stock by the three-year compounded expected rate of return less Lilly's dividend yield.
Lilly executive officers, including Mr. Simmons, received Lilly Executive Officer SVAs. These awards are the same as Lilly SVAs except executive officers receive no payout if Lilly's TSR for the three-year period is zero or negative, and a modifier based on Lilly's three-year cumulative TSR relative to its peer companies' median TSR performance will be applied to payouts for SVAs granted in 2016 or later. If Lilly's TSR is above the median of Lilly's peers, the payout is increased by 1% for every percentage point that Lilly's TSR exceeds the median (up to a maximum of 20%). Likewise, if Lilly's TSR is below the median, the payout will be reduced by up to a maximum of 20%. Adding the relative TSR modifier to the Lilly Executive Officer SVAs helps ensure Lilly executive officers' rewards align with shareholder experience while also rewarding strong performance relative to our peer group.
Pay for Performance
The mix of compensation for our Named Executive Officers reflects Lilly's desire to link executive compensation with performance. The graphic below depicts the mix of pay for our CEO and the average for our other Named Executive Officers based on 2017 compensation for all of our Named Executive Officers.
2017 Target Total Compensation
Setting Target Compensation
As described above in "Process for Setting Compensation," in setting its executive officer compensation for 2017, including for Mr. Simmons, Lilly considered individual, Lilly and Elanco (as applicable) performance during 2016, internal pay equity and peer group data. For our Named Executive Officers other than Mr. Simmons, target compensation for 2017 was determined by Lilly senior management, consistent with Lilly's compensation philosophy.
135
The information below reflects total compensation at target for our Named Executive Officers for 2017. The actual compensation received in 2017 is summarized below in "2017 Compensation Payouts."
Base Salary
The following table outlines the annual salary for our Named Executive Officers that was approved by Lilly with respect to 2016 and 2017. Our Named Executive Officers' actual base salary earned during 2017 is reflected in the Summary Compensation Table in the "Executive Compensation" section of this prospectus.
Name |
2016 Annual Base Salary |
2017 Annual Base Salary |
|||||
| | | | | | | | |
Mr. Simmons |
$ | 688,118 | $ | 688,118 | |||
Mr. Montarce |
$ | 209,083 | $ | 280,673 | |||
Mr. Kinard |
$ | 390,028 | $ | 405,632 | |||
Mr. Schacht |
$ | 272,358 | $ | 285,315 | |||
Mr. Urbanek |
$ | 255,979 | $ | 297,174 |
Annual Cash Bonus Targets
Bonus targets for 2016 and 2017 are shown in the table below as a percentage of our Named Executive Officer's base salary earnings:
Name |
2016 Bonus Target |
2017 Bonus Target |
|||||
| | | | | | | | |
Mr. Simmons |
80% | 80% | |||||
Mr. Montarce |
26% | 34% | * | ||||
Mr. Kinard |
45% | 45% | |||||
Mr. Schacht |
34% | 34% | |||||
Mr. Urbanek |
26% | 35% | * |
- *
- The bonus targets for Messrs. Montarce and Urbanek are a weighted average across the positions held during the year.
Total Equity Program — Target Grant Values
For 2017 equity grants, Lilly set the total target value for our Named Executive Officers based on internal pay equity, Lilly and Elanco (as applicable) performance, individual performance and Lilly peer group data (as applicable). Mr. Simmons had 60% of his equity target allocated to Lilly Executive Officer SVAs and 40% to Lilly Executive Officer PAs. Our remaining Named Executive Officers had 50% of their equity allocated to Lilly SVAs and 50% to Lilly PAs. Total target values for the 2016 and 2017 equity grants to our Named Executive Officers were as follows:
Name |
2016 Annual Equity Grant |
2017 Annual Equity Grant |
|||||
| | | | | | | | |
Mr. Simmons |
$ | 2,000,000 | $ | 2,000,000 | |||
Mr. Montarce |
$ | 105,000 | $ | 90,000 | |||
Mr. Kinard |
$ | 400,000 | $ | 415,000 | |||
Mr. Schacht |
$ | 175,000 | $ | 225,000 | |||
Mr. Urbanek |
$ | 85,000 | $ | 80,000 |
136
Messrs. Schacht and Urbanek also received special retention award during 2017, as described in "Special Retention Awards" below, which are not reflected in the table above.
Performance Goals for 2017 Lilly Incentive Programs
Annual Bonus Goals
Performance targets for the Elanco Bonus Plan and the Lilly Bonus Plan were based on the 2017 operating plan for each of Elanco and Lilly, respectively. These targets are described below under "2017 Compensation Payouts."
Performance Award (PAs and Executive Officer PAs)
In February 2017, the Lilly compensation committee established a cumulative, compounded two-year Lilly EPS growth target of 5.3% per year for the 2017-2018 performance period, based on investment analysts' growth estimates for Lilly's peer group companies at that time. Payouts for the 2017-2018 Lilly PAs and 2017-2019 Lilly Executive Officer PAs range from 0% to 150% of the target, as illustrated below:
Shareholder Value Award (SVAs and Executive Officer SVAs)
For purposes of establishing the Lilly stock price target for the 2017-19 Lilly SVAs, the starting price was $72.15 per share, the average Lilly closing stock price for all trading days in November and December 2016. The Lilly target share price was established using the expected annual rate of return for large-cap companies (8%), less an assumed Lilly dividend yield of 2.88%. To determine payouts, the ending price will be the average of the closing prices of Lilly stock for all trading days in November and December 2019.
The Lilly Executive Officer SVAs are designed to deliver no payout if the shareholder return (including projected dividends) is zero or negative.
Mr. Simmons received Lilly Executive Officer SVAs while the remaining Named Executive Officers received Lilly SVAs. Possible payouts based on share price ranges are illustrated in the grids below.
137
Lilly Executive Officer SVA
Ending Stock Price |
Less than $65.80 |
$65.80 to $74.79 |
$74.80 to $83.79 |
$83.80 to $92.79 |
$92.80 to $101.79 |
Greater than $101.79 |
||||||
| | | | | | | | | | | | | |
Compounded Annual Share Price Growth Rate (excluding dividends) |
Less than (3.0%) |
(3.0%) to 1.2% |
1.2% to 5.1% |
5.1% to 8.8% |
8.8% to 12.2% |
Greater than 12.2% |
||||||
Percent of Target |
0% |
50% |
75% |
100% |
125% |
150% |
Lilly SVA
Ending Stock Price |
Less than $36.08 |
$36.08 to $74.79 |
$74.80 to $83.79 |
$83.80 to $92.79 |
$92.80 to $101.79 |
Greater than $101.79 |
||||||
| | | | | | | | | | | | | |
Compounded Annual Share Price Growth Rate (excluding dividends) |
Less than (20.6%) |
(20.6%) to 1.2% |
1.2% to 5.1% |
5.1% to 8.8% |
8.8% to 12.2% |
Greater than 12.2% |
||||||
Percent of Target |
0% |
50% |
75% |
100% |
125% |
150% |
Mr. Simmons' Lilly Executive Officer SVAs are subject to a relative TSR modifier, as outlined in the grid below. The number of Lilly shares to be paid will increase or decrease by 1% for every percentage point Lilly's three-year TSR deviates from Lilly's peer group's median three-year TSR, capped at 20%.
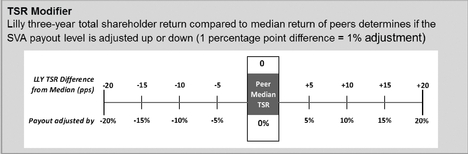
Special Retention Awards
Messrs. Schacht and Urbanek each received a special retention grant of restricted stock units with a grant date fair value of $300,060 and $200,040, respectively, on September 1, 2017. The awards were determined and granted by Lilly management in recognition of their contributions and their importance to Elanco's future success and will vest on the third anniversary of the awards, September 1, 2020, subject to their continued employment with Elanco other than the event of certain qualifying terminations as described below in "Lilly Payments Upon Termination or Change in Control."
2017 Compensation Payouts
The information in this section reflects the amounts paid to our Named Executive Officers under the Elanco Bonus Plan, Lilly Bonus Plan and in respect of Lilly equity awards granted in prior years for which the relevant performance period ended in 2017.
Elanco Performance
In 2017, Elanco did not achieve its annual revenue and IBT targets; however, Elanco made significant progress on its innovation goals. Key innovation highlights include the launches of
138
Galliprant and Clynav, and the first regulatory approval of Credelio. While business performance was below expectations, Elanco made significant progress on its long-term strategic agenda, improving its cost structure, reducing global headcount, rationalizing key assets and products and accelerating important pipeline projects. The 2017 results described below reflect Elanco's 2017 performance with respect to the Elanco Bonus Plan targets and are not presented on the same basis as, and are not directly comparable to, our combined financial results presented in this prospectus.
Elanco Bonus Plan
Elanco's performance compared to the 2017 targets for revenue, IBT, innovation progress and the Lilly Company Bonus Multiple, as well as the resulting bonus multiple, is set forth below.
|
2017 Elanco Target |
2017 Elanco Results |
Multiple |
|||||||
| | | | | | | | | | | |
Revenue |
$ | 3,566M | $ | 3,086M | 0.36 | |||||
IBT |
$ | 943M | $ | 552M | 0.00 | |||||
Innovation |
3.00 | 3.10 | 1.05 | |||||||
Lilly Company Bonus Multiple (LCBM) |
1.34 | |||||||||
Resulting Elanco Bonus Multiple |
0.69 |
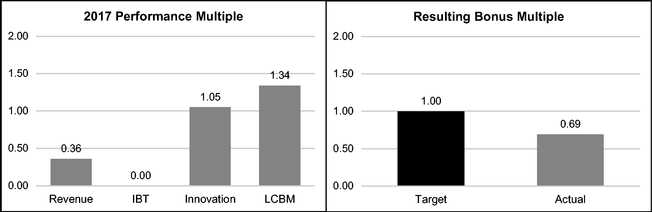
Elanco's 2017 innovation target was 3.0 on a scale of 1.0 to 5.0. Elanco's innovation multiple is comprised of the following factors: (i) achievement of product approvals, (ii) entrants into early and late stage development, (iii) adherence to approval timelines and (iv) a qualitative assessment by Elanco's head of R&D of overall performance. Based on the weighted outcomes of these factors, Elanco achieved a 3.10 score, which correlates to a 1.05 innovation multiple for use in the Elanco bonus calculation.
When combined, the Elanco revenue, IBT, innovation and Lilly Corporate Bonus multiples yielded a 2017 Elanco Bonus Plan multiple of 0.69.
(0.25 × 0.36) + (0.25 × 0.00) + (0.25 × 1.05) + (0.25 × 1.34) = 0.69 bonus multiple
139
The 2017 bonuses paid to our Named Executive Officers under the Elanco Bonus Plan were as follows:
Name |
2017 Bonus ($) |
|||
| | | | | |
Mr. Simmons |
379,841 | |||
Mr. Montarce |
52,647 | |||
Mr. Schacht |
66,935 | |||
Mr. Urbanek |
69,401 |
Lilly Performance
In 2017, Lilly exceeded both its annual revenue and EPS targets. Lilly also made significant progress on its pipeline, meeting or exceeding all of its pipeline targets. Key pipeline highlights include first regulatory approval for Verzenio and Olumiant, along with nine other new approvals, indications or line extensions.
Lilly Bonus Plan
Lilly's performance compared to its 2017 targets for revenue, EPS and pipeline progress, as well as the resulting bonus multiple, is illustrated below.
|
2017 Lilly Target |
2017 Adjusted Results |
Multiple |
|||||||
| | | | | | | | | | | |
Revenue |
$ | 22.3B | $ | 22.9B | 1.30 | |||||
EPS |
$ | 4.15 | $ | 4.28 | 1.37 | |||||
Pipeline Score |
3.00 | 3.65 | 1.33 | |||||||
Resulting Lilly Bonus Multiple |
1.34 |
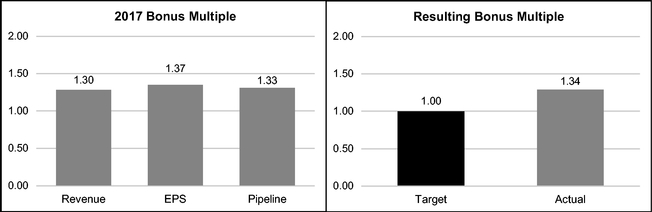
Lilly's Science and Technology Committee assessed Lilly's progress toward achieving product pipeline goals based on the following factors: (i) achievement of product approvals, (ii) new chemical entity entrants into Phase 1 and Phase 3 clinical trials, (iii) new indication or line extension entrants into Phase 3 clinical trials, (iv) speed of development, (v) adherence to timelines and (iv) a qualitative assessment of overall performance. Based on the recommendation of the Science and Technology Committee, Lilly's compensation committee certified a pipeline score of 3.65, resulting in a pipeline multiple of 1.33.
When combined, the Lilly revenue, EPS, and pipeline multiples yielded a 2017 Lilly Bonus Plan multiple of 1.34.
(0.25 × 1.30) + (0.50 × 1.37) + (0.25 × 1.33) = 1.34 bonus multiple
140
The 2017 bonuses paid to Messrs. Montarce, Kinard and Urbanek under the Lilly Bonus Plan were as follows:
| Name | 2017 Bonus ($) |
|
| | | |
| Mr. Montarce | 31,329 | |
| Mr. Kinard | 244,596 | |
| Mr. Urbanek | 7,486 |
Performance Awards (PAs and Executive Officer PAs)
The target cumulative Lilly EPS for the 2016-2017 Lilly PAs and the 2016-2018 Lilly Executive Officer PAs was set in the first quarter of 2016, reflecting expected industry growth of 7.0% each year over the two-year performance period of 2016-2017. Lilly's actual annual EPS growth for the two-year period was 7.0%. This outcome was largely driven by volume growth from newer Lilly products.
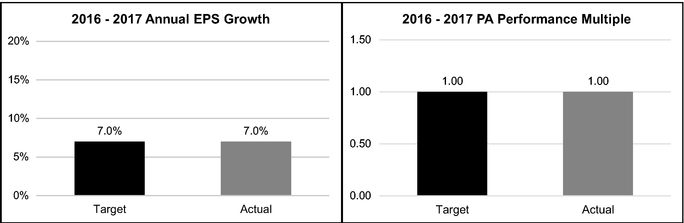
For our Named Executive Officers, the number of Lilly shares earned under the 2016-2017 Lilly PAs or, for Mr. Simmons, the 2016-2018 Lilly Executive Officer PAs is set forth in the table below. Mr. Simmons' shares earned under the 2016-2018 Lilly Executive Officer PA are subject to an additional 13-month service-vesting period.
Name |
Target Shares | Shares Earned |
||
| | | | | |
Mr. Simmons |
11,111 | 11,111 | ||
Mr. Montarce |
729 | 729 | ||
Mr. Kinard |
2,778 | 2,778 | ||
Mr. Schacht |
1,215 | 1,215 | ||
Mr. Urbanek |
590 | 590 |
Shareholder Value Award (SVAs)
The target Lilly stock price range of $80.30 to $86.17 (16.2% to 24.6% stock price growth) for the 2015-2017 Lilly SVAs was set in 2015 based on a beginning Lilly stock price of $69.13, which was the average closing price for Lilly stock for all trading days in November and December 2014. The ending Lilly stock price of $84.70 represents a stock price growth of approximately 22.5% over
141
the relevant three-year period. Lilly's performance compared to target for 2015-2017 Lilly SVAs is shown below.
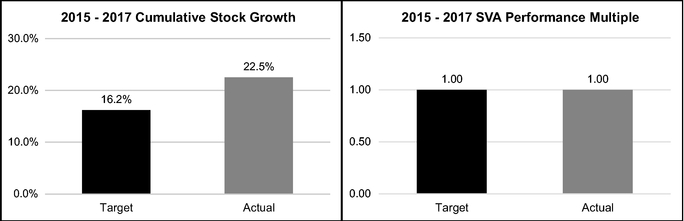
The shares earned by our Named Executive Officers during 2018 under the 2015-2017 Lilly SVAs were as follows
Name |
Target Shares | Shares Paid Out | ||
| | | | | |
Mr. Simmons |
22,507 | 22,507 | ||
Mr. Montarce |
385 | 385 | ||
Mr. Kinard |
3,418 | 3,418 | ||
Mr. Schacht |
684 | 684 | ||
Mr. Urbanek |
598 | 598 |
Other Lilly Compensation Practices and Information
Lilly Employee Benefits
Lilly offers core employee benefits coverage to:
- •
- provide the Lilly workforce with a reasonable level of financial support in the event of illness or injury;
- •
- provide post-retirement income; and
- •
- enhance productivity and job satisfaction through benefit programs that focus on overall well-being.
The benefits available to our Named Executive Officers are generally the same as are available to all U.S. Lilly employees and include medical and dental insurance, disability insurance and life insurance. In addition, The Lilly Employee 401(k) plan ("Lilly 401(k) Plan") and The Lilly Retirement Plan provide U.S. Lilly employees a reasonable level of retirement income reflecting employees' careers with Lilly.
To the extent that any Lilly employee's retirement benefit exceeds Internal Revenue Service (IRS) limits for amounts that can be paid through a qualified plan, Lilly also offers a nonqualified pension plan and a nonqualified savings plan. Our Named Executive Officers participated in these nonqualified plans in 2017. These plans provide only the difference between the calculated benefits and the IRS limits, and the formula is the same for all U.S. Lilly employees. The cost of Lilly employee benefits is partially borne by the employee, including our Named Executive Officers.
142
The Lilly Deferred Compensation Plan
Lilly executive officers, including Mr. Simmons, but not our other Named Executive Officers may defer receipt of all or part of their cash compensation and other U.S. Lilly executives may defer receipt of all or part of their cash bonus under The Lilly Deferred Compensation Plan, which allows participants to save for retirement in a tax-effective way at minimal cost to Lilly. Under this unfunded plan, amounts deferred by the participant are credited at an interest rate of 120% of the applicable federal long-term rate, as described in more detail following the "Nonqualified Deferred Compensation in 2017" table.
Lilly Severance Benefits
Except in the case of a change in control of Lilly, Lilly is not obligated to pay severance to Mr. Simmons upon termination of employment, but severance may be paid at the discretion of the Lilly compensation committee. Lilly has a severance plan in which our other Named Executive Officers participate. The plan generally provides severance benefits to eligible employees of Lilly and certain of its subsidiaries and affiliates in the event of involuntary retirement or termination without cause, which includes separation from Lilly due to their discharge or resignation in lieu of discharge (with certain exceptions), resignation because of disability, the closing of an office or facility in which the employee is employed, or any other reason determined by Lilly to warrant the payment of a severance benefit pursuant to the plan. Such employees will receive a benefit that is a function of their weekly pay and the number of years of service completed at the time of the employee's separation.
Change-in-Control Severance Agreements
Lilly has adopted change-in-control severance pay plans for nearly all Lilly employees, including a plan for select employees, which applies to our Named Executive Officers. The Lilly plans are intended to preserve Lilly employee morale and productivity and encourage retention in the face of the disruptive impact of an actual or rumored change in control. In addition, the Lilly plans are intended to align participating Lilly employee and Lilly shareholder interests by enabling executives to evaluate corporate transactions that may be in the best interests of the Lilly shareholders and other constituents of Lilly without undue concern over whether the transactions may jeopardize the participating employee's own employment.
Highlights of the Lilly change-in-control severance arrangements
- •
- all regular Lilly employees are covered
- •
- double trigger required
- •
- no tax gross-ups
- •
- up to two-year pay protection
- •
- 18-month benefit continuation
The basic elements of the plan applicable to our Named Executive Officers include:
- •
- Double trigger. Unlike "single
trigger" plans that pay out immediately upon a change in control, the select plan requires a "double trigger" — a change in control followed by an involuntary loss of employment
within two years. This is consistent with Lilly's intent to provide employees with financial protection upon loss of employment.
In addition, with respect to unvested equity, pursuant to the terms of the award agreements, performance through the change in control will be used to determine the number of Lilly
143
- •
- Covered terminations. Our
participating Named Executive Officers are eligible for payments under our select plan if, within two years of the change in control, their employment is terminated (i) without cause by Lilly
or (ii) for good reason by the employee, each as is defined in the plan. See "— Lilly Payments Upon Termination or Change in Control (as of December 31, 2017)" for a
more detailed discussion, including a discussion of what constitutes a change in control.
- •
- Up to two years of pay and 18 months of benefits protection upon a covered
termination.
- •
- Severance payment. Our participating Named Executive Officers are eligible for up
to two years' base salary plus two times their target bonus for the then-current year.
- •
- Benefit continuation. Basic employee benefits such as health and life insurance
would continue for 18 months following a participating Named Executive Officer's termination of employment, unless they become eligible for coverage with a new employer before then. Our
participating Named Executive Officers would receive an additional two years of both age and years-of-service credit for purposes of determining eligibility for retiree medical and dental benefits.
- •
- Accelerated vesting of Lilly equity
awards. As described above, unvested equity awards would vest at the time of a covered termination of employment, which includes an involuntary
termination following a change in control.
- •
- Excise tax. In some circumstances, the payments or other benefits received by a participating employee in connection with a change in control could exceed limits established under Section 280G of the Code resulting in an excise tax payment. Lilly does not reimburse or gross-up employees for these taxes. However, the amount of change in control-related benefits will be reduced to the maximum amount that would not result in an excise tax if the effect would be to deliver a greater after-tax benefit than the employee would receive if his or her benefits were not so reduced.
shares earned under an award, but, if awards are assumed or substituted, vesting would only occur upon an involuntary loss of employment following a change in control. Rather, the performance awards will convert to Lilly restricted stock units that continue to vest following the change in control. After a change in control, Lilly shares will vest upon the earlier of the completion of the original award period or upon a covered termination of employment. If the successor entity does not assume, substitute or otherwise replace the awards in connection with a change in control, awards will vest on the change in control.
See "— Lilly Payments Upon Termination or Change in Control (as of December 31, 2017)" below.
Lilly Share Ownership and Retention Guidelines; Prohibition on Hedging and Pledging Shares
Lilly share ownership and retention guidelines help to foster a focus on long-term growth. During 2017, the holding requirement for our Named Executive Officers ranged from 3,500 shares to three times annual base salary depending on the position. The following table shows the share requirements for the applicable Named Executive Officers:
Name |
Share Requirement 2017 |
Owns Required 2017 Shares |
||
| | | | | |
| Mr. Simmons | Three times base salary | Yes | ||
| Mr. Montarce | 5,000 shares | No* | ||
| Mr. Kinard | 7,000 shares | Yes | ||
| Mr. Schacht | 3,500 shares | No* | ||
| Mr. Urbanek | 7,000 shares | Yes |
- *
- Messrs. Montarce and Schacht were compliant with the annual award share retention guideline as they each build toward their respective ownership requirements.
144
Lilly executive officers, including Mr. Simmons, are also required to hold all Lilly shares received from Lilly equity program payouts, net of acquisition costs and taxes, for at least one year, even once Lilly share ownership requirements have been met. For Lilly Executive Officer PAs, this holding requirement is met by the 13-month service-vesting period that applies after the end of the performance period.
Lilly employees, including our Named Executive Officers, are not permitted to hedge their economic exposures to Lilly stock through short sales or derivative transactions. All members of Lilly senior management, including our Named Executive Officers, are prohibited from pledging any Lilly stock (i.e., using Lilly stock as collateral for a loan or trading shares on margin).
Lilly Executive Compensation Recovery Policy
All Lilly incentive awards generally are subject to forfeiture upon termination of employment prior to the end of the performance or vesting period or for disciplinary reasons. In addition, the Lilly compensation committee has adopted a Lilly executive compensation recovery policy that gives the Lilly compensation committee broad discretion to claw back Lilly incentive payouts from any member of Lilly senior management, including our Named Executive Officers, whose misconduct results in a material violation of law or company policy that causes significant harm to Lilly or who fails in his or her supervisory responsibility to prevent such misconduct by others.
The Lilly recovery policy covers any Lilly incentive compensation awarded or paid to an employee at a time when he or she is a member of Lilly senior management. Subsequent changes in status, including retirement or termination of employment, do not affect Lilly's rights to recover compensation under the policy. Recoveries under the Lilly plan can extend back as far as three years.
Treatment of Outstanding Lilly Awards Following this Offering
Following the offering and prior to the Distribution, the Lilly equity awards previously granted to our Named Executive Officers will continue in accordance with their terms, provided that service to Elanco will be counted as service with Lilly for all purposes. Upon the Distribution, it is currently anticipated that the Lilly equity awards will terminate in accordance with their terms for no consideration paid to our Named Executive Officers, and that, in connection with the Separation, the Elanco compensation committee will determine to issue Elanco equity awards of similar value to our Named Executive Officers subject to the requirements of applicable law and the terms of the 2018 Elanco Stock Plan and applicable award agreements. In addition, it is currently anticipated that Elanco will maintain the Elanco Bonus Plan with the same metrics and eligibility for the remainder of 2018 and that for 2019 the Elanco compensation committee will make determinations with respect to the Elanco Bonus Plan as it deems appropriate. See "—Anticipated Compensation Program Following this Offering — Awards upon the Completion of this Offering" below and "Certain Relationships and Related Party Transactions — Relationship with Lilly — Employee Matters Agreement" for additional information with respect to our anticipated compensation programs following this offering.
Anticipated Compensation Program Following this Offering
The following section describes the compensation programs that we intend to implement following this offering, which continues to be subject to development by the Elanco compensation committee.
Elanco Compensation Committee
Following this offering, the Elanco compensation committee, which will be appointed by the Elanco board of directors, will determine the design of our executive and director compensation
145
programs and our compensation philosophy. The Elanco compensation committee is expected to retain its own compensation consultant to advise on its compensation decisions. The Elanco compensation committee will annually review and evaluate our executive compensation plans and programs to ensure they are aligned with our compensation philosophy.
Peer Group
Based on the advice of Willis Towers Watson, the compensation consultant engaged by Lilly management to provide advice on the Elanco peer group in connection with this offering, the following group of 18 companies were identified as our "core" peers.
| Agilent Technologies, Inc. | Endo International plc | PerkinElmer, Inc. | ||
| Alexion Pharmaceuticals, Inc. | Hologic, Inc. | Perrigo Company plc | ||
| Boston Scientific Corporation | IDEXX Laboratories, Inc. | Steris plc | ||
| Catalent, Inc. | Jazz Pharmaceuticals plc | Varian Medical Systems, Inc. | ||
| DENTSPLY SIRONA Inc. | Mallinckrodt Public Limited Company | West Pharmaceutical Services, Inc. | ||
| Edwards Lifesciences Corporation | OPKO Health, Inc. | Zoetis, Inc. |
To determine the elements of our compensation programs for our Named Executive Officers, we expect that the Elanco compensation committee will use the following benchmarks, among others:
- •
- Proxy statement data for the "core" peer group as disclosed in each company's prior year compensation discussion and analysis and executive
compensation tables; and
- •
- Survey data from similarly-sized companies within the life sciences industry, in particular, from the Willis Towers Watson CDB survey that encompassed life-science companies with annual revenues of less than or equal to $20 billion.
We expect the Elanco compensation committee to periodically review our peer group and to make adjustments to its size and composition within its discretion.
Awards upon the Completion of this Offering
We anticipate that certain of our executive officers, including the Named Executive Officers listed below, will receive a Founders' Award that will be granted approximately 30 days after the completion of this offering. It is not expected at this time that Mr. Montarce will receive a Founders' Award. The Founders' Awards are anticipated to be allocated evenly between Elanco RSUs and Elanco stock options. The number of shares subject to such RSUs and stock options will be calculated based upon the executive officer's allocation from a pool to be established for these awards that is expected to equal 0.1% of Elanco's market capitalization measured at the closing of this offering. The anticipated allocation for each of our Named Executive Officers from that pool is as follows:
Name
|
Pool Allocation | |||
|---|---|---|---|---|
Mr. Simmons |
26 | % | ||
Mr. Kinard |
5 | % | ||
Mr. Schacht |
5 | % | ||
Mr. Urbanek |
5 | % | ||
In addition to the Founders' Awards, we anticipate making additional awards to our employees who are not our Named Executive Officers in connection with this offering under the 2018 Elanco Stock Plan, within the limits thereunder.
146
Compensation Arrangements
Executive Compensation
Upon the completion of this offering, the following pay packages are expected to go into effect for our Named Executive Officers. The pay packages for our Named Executive Officers described below are based on the experience profile of the candidate and competitive positioning against peer group benchmarking as described above. All of our Named Executive Officers' compensation packages considered benchmarking data from the Willis Towers Watson life sciences CDB survey regressed for relative company size, and the compensation package for Mr. Simmons was also benchmarked against the compensation of our list of core peer companies.
| Mr. Simmons: | As the President and Chief Executive Officer, Mr. Simmons is expected to receive $1,000,000 in base salary, an annual incentive target equal to 120% of base salary, and an annual equity award with a grant date value targeted at $4,150,000. | |
| Mr. Montarce: | As Acting Chief Financial Officer, Mr. Montarce is expected to receive $336,810 in base salary, an annual incentive target equal of 40% of base salary and an annual equity award with a grant date value targeted at $300,000. | |
| Mr. Kinard: | As the Executive Vice President, Human Resources and Corporate Affairs, Mr. Kinard is expected to receive $430,000 in base salary, an annual incentive target equal to 60% of base salary, and an annual equity award with a grant date value targeted at $625,000. | |
| Mr. Schacht: | As the Executive Vice President, Innovation, Regulatory and Business Development, Mr. Schacht is expected to receive $355,000 in base salary, an annual incentive target equal to 60% of base salary, and an annual equity award with a grant date value targeted at $650,000. | |
| Mr. Urbanek: | As the Executive Vice President, Manufacturing and Quality, Mr. Urbanek is expected to receive $385,000 in base salary, an annual incentive target equal to 60% of base salary, and an annual equity award with a grant date value targeted at $550,000. |
2018 Elanco Stock Plan
Prior to the completion of this offering, we expect our board of directors and our stockholder to approve the 2018 Elanco Stock Plan (the "2018 Stock Plan"), which we expect will become effective on the day immediately prior to the date that the offering of our shares pursuant to this prospectus is declared effective by the SEC and is not expected to be utilized until after the completion of this offering. The following is what we expect to be the material terms of the 2018 Stock Plan.
Awards. Under the 2018 Stock Plan, the following awards may be granted: stock options (including "incentive stock options" within the meaning of Section 422 of the Internal Revenue Code), restricted stock, stock appreciation rights, restricted stock units, other share-based awards, and performance-based awards (all such grants are collectively referred to in this summary as "awards"). Restricted stock units may also be granted as "replacement awards" to employees of the Company (or any affiliate) in substitution of a restricted stock unit covering Lilly common stock that was granted by Lilly to such employees under Lilly's stock plan prior to the effective date that Lilly owns less than fifty percent (50%) of the outstanding Elanco shares.
Shares Reserved. Subject to adjustment in the event of specified capitalization events, the total number of shares of our common stock that will be authorized and available for issuance pursuant to awards granted under the 2018 Stock Plan will be shares as of the date the
147
2018 Stock Plan becomes effective, provided that the number of shares available for issuance will be increased immediately following the date on which the Distribution is completed, by the lesser of (a) shares or (b) such other number of shares as may be determined by our board of directors. Subject to adjustment in the event of specified capitalization events, no more than shares may be issued pursuant to the exercise of incentive stock options.
Shares Reissuable Under the 2018 Stock Plan. The following shares will be available for reissuance pursuant to the Plan: (i) shares that are not issued as a result of the termination, expiration or lapsing of any award for any reason; (ii) shares subject to a "full value" award that are not issued because the award is settled in cash; (iii) shares covered by an option which are surrendered in payment of the option exercise or purchase price or in satisfaction of obligations for tax-related items incident to the exercise of an option; (iv) shares covered by an award which are surrendered in satisfaction of obligations for tax-related items incident to the vesting or settlement of a full value award.
Shares Not Reissuable Under the 2018 Stock Plan. Shares that are repurchased on the open market with the proceeds of the exercise of an option will be counted against the maximum number of shares available for issuance and will not be returned to the 2018 Stock Plan.
Shares Not Counted Against Share Reserve Pool Under the 2018 Stock Plan. To the extent permitted by applicable law or any stock exchange rule, shares issued in assumption of, or in substitution for, any outstanding awards of any entity acquired in any form of combination by the Company or an affiliate will not be counted against shares available for grant pursuant to the 2018 Stock Plan. The payment of a dividend equivalent right in cash in conjunction with any outstanding awards will not be counted against the shares available for issuance under the 2018 Stock Plan.
Eligibility. Incentive stock options may be granted only to our employees and to employees of any of our subsidiaries meeting the requirements of the Internal Revenue Code. Awards other than incentive stock options may be granted to our non-employee directors and to employees of the Company and any of its affiliates.
Administration. The 2018 Stock Plan will be administered by our board of directors or a duly authorized committee of our board of directors (the board of directors or the committee to which administration of the 2018 Stock Plan has been delegated will be referred to in this summary as the "Committee"). The Committee has the sole authority to grant awards and sole and exclusive discretion to interpret and administer the 2018 Stock Plan. The Committee determines the eligible individuals who will receive grants and the precise terms of the grants (including accelerations or waivers of any restrictions, and the conditions under which such accelerated vesting or waivers occur). The Committee has the authority to amend or modify the terms of an outstanding award. To the extent permitted by applicable law, our board of directors also may delegate to a committee of one or more members of our board of directors or one or more officers of the Company the authority to grant or amend awards to participants other than employees who are subject to Section 16 of the Securities Exchange Act of 1934, as amended, or officers or directors of the Company to whom authority to grant or amend awards has been delegated.
Stock Options. The 2018 Stock Plan authorizes the grant of incentive stock options, which are intended to satisfy the requirements of Section 422 of the Internal Revenue Code, and non-qualified stock options, which do not satisfy the requirements of Section 422 of the Code. The exercise price of stock options granted under the 2018 Stock Plan may not be less than 100% (or higher in the case of certain incentive stock options) of the fair market value of a share of our common stock on the date of grant. While the shares are traded on an established stock exchange, "fair market value" means, as of any given date, the closing price of a share as quoted on the principal exchange on which the shares are listed for such date, or if no sale occurred on such date, the first trading date immediately prior to such date during which a sale occurred. Subject to the one-year minimum vesting requirement described below, options granted under the 2018 Stock
148
Plan will vest at the rate specified by the Committee. No stock option will be exercisable for more than ten years after the date it is granted.
Until the shares are issued, no right to vote or receive dividends or dividend equivalents or any other rights as a shareholder will exist with respect to the shares subject to an option, notwithstanding the exercise of an option. If a participant ceases to provide services to the Company or any affiliate, the participant may exercise his or her option within such period of time as is specified in the award agreement to the extent that the option is vested on the date of termination (but in no event later than the expiration of the term of such option as set forth in the award agreement).
Restricted Stock Awards. An award of restricted stock is a direct grant of common stock, subject to such restrictions on transferability and other restrictions as the Committee may impose (including, without limitation, limitations on the right to vote the underlying shares or the right to receive dividends with respect to the underlying shares). The restrictions, if any, may be based on, among other conditions, continued service, the attainment of performance conditions, or a combination of both. Subject to the one-year minimum vesting requirement describe below, these restrictions may lapse separately or in combination at such times, pursuant to such circumstances, in such installments, or otherwise, as the Committee determines at the time of the grant of the award or thereafter. Generally, any shares subject to restrictions are forfeited upon termination of employment. The price, if any, that participants are required to pay for each share of restricted stock will be set by the Committee and will be paid in a form approved by the Committee, which may be cash, services rendered or to be rendered to the Company or an affiliate of the Company, or in another form of payment. To the extent that any dividends are payable with respect to a restricted stock award, the dividends will be accumulated and subject to any restrictions and risk of forfeiture to which the underlying restricted stock is subject.
Stock Appreciation Rights. Stock appreciation rights, or "SARs," typically provide for payments to the holder based upon increases in the price of our shares from the date the SAR was granted to the date that the right is exercised. Subject to the one-year minimum vesting requirements, the Committee will generally determine when the SAR will vest and become exercisable. The vesting conditions, if any, may be based on, among other conditions, continued service, the attainment of performance conditions, or a combination of both. The grant price of a SAR may not be less than the fair market value of a share on the date of grant of the SAR. The Committee determines the term of a SAR, but no SAR will be exercisable more than ten years after the date it is granted.
Until the shares are issued, no right to vote or receive dividends or any other rights as a shareholder will exist with respect to the shares subject to a SAR, notwithstanding the exercise of the SAR. The Committee may elect to settle exercised SARs in cash, in shares, or in a combination of cash and shares. Unless otherwise provided in the 2018 Stock Plan or an award agreement, upon termination of a participant's employment, a SAR will generally be subject to the same conditions as apply to stock options. A SAR may be granted as a standalone right or in connection with an option.
Restricted Stock Units. Restricted stock units are denominated in unit equivalent of shares and are typically awarded to participants without payment of consideration. Subject to the one-year minimum vesting requirements described below, restricted stock units may be subject to vesting conditions based upon continued service, the attainment of performance-based conditions, or both. Except as otherwise determined by the Committee at the time of the grant of the award or thereafter, any restricted stock units that are not vested as of the date of the participant's termination of service will be forfeited.
Unlike restricted stock, the stock underlying restricted stock units will not be issued until the restricted stock units have vested. In addition, recipients of restricted stock units generally have no
149
voting or dividend rights until the vesting conditions are satisfied and the underlying shares are issued. Restricted stock units may be settled in shares, cash or a combination of both.
On the vesting date (or such later date as determined by the Committee and set forth in the agreement evidencing the award), the participant will be issued one unrestricted, fully transferable share for each restricted stock unit scheduled to be paid out on such date and not previously forfeited. Alternatively, settlement of a restricted stock unit may be made in cash (in an amount reflecting the fair market value of shares that would have been issued) or any combination of cash and shares, as determined by the Committee, in its sole discretion. The Committee may authorize dividend equivalents to be paid on outstanding restricted stock units. If dividend equivalents are authorized to be paid, they may be payable in cash or shares, as determined in the discretion of the Committee, only to the extent the underlying award vests.
Other Share-Based Awards. The Committee is authorized under the 2018 Stock Plan to make any other award that is not inconsistent with the provisions of the 2018 Stock Plan and that by its terms involves or might involve the issuance of shares. Subject to the one-year minimum vesting requirements described below, awards may be subject to vesting based on continued service, the attainment of performance conditions, or a combination of both. The Committee may elect to settle these awards in cash, in shares, or in a combination of cash and shares. The Committee may establish the exercise price, if any, of any other share-based awards granted under the Plan, except that the exercise price may not be less than the fair market value of a share on the date of grant for an award that is intended to be exempt from Section 409A of the Internal Revenue Code. The Committee may authorize dividend equivalents to be paid with respect to a share-based award that is a full value award that are payable only to the extent the underlying award vests.
Performance-Based Awards. The Committee may grant to eligible individuals the right to receive performance-based awards. Performance-based awards vest upon the attainment of performance goals based on business criteria specified in the 2018 Stock Plan over a specified performance period. In determining the amount earned by an eligible individual, the Committee has the right to adjust or eliminate the amount payable at a given level of performance to take into account additional factors that the Committee may deem relevant to the assessment of individual or corporate performance for the performance period. The maximum number of shares with respect to which one or more performance-based awards may be granted to any one participant during any calendar year may not exceed 1,500,000 shares.
Minimum Vesting Requirements. No award may vest before the first anniversary of the date of grant, subject to certain accelerated vesting contemplated under the 2018 Stock Plan, with the exception of (i) up to five percent (5%) of the number of shares reserved for issuance under the 2018 Stock Plan, (ii) replacement awards granted under the 2018 Stock Plan, (iii) awards granted in connection with the assumption or substitution of awards as part of a transaction, and (iv) awards that may be settled only in cash.
Non-Employee Directors. Non-employee directors will be eligible to receive all types of awards (except for incentive stock options) under the 2018 Stock Plan. No non-employee director may be granted awards, the grant date fair value of which, when aggregated with cash compensation payable to the director in any calendar year, exceeds $800,000 in any calendar year.
Limits on Transferability of Awards. Except as otherwise provided by the Committee, no award granted under the 2018 Stock Plan may be assigned, transferred, or otherwise disposed of by a participant other than by will or the laws of descent and distribution.
150
Change in Control. Unless precluded by any applicable award agreement, if a Change in Control of the Company occurs, each award outstanding under the 2018 Stock Plan that vests solely on continued service that is not converted, assumed, substituted or replaced by the successor corporation, will vest immediately prior to the Change in Control, and following the Change in Control, the awards will immediately terminate. Awards that vest based on the attainment of performance-based conditions will be subject to the governing impact of a Change in Control in the award agreement, provided the award agreement may not permit vesting of awards at a rate greater than the actual level of attainment and/or will provide for pro-rated vesting based on any reduction to the performance period resulting from the Change in Control. Where awards are assumed or continued after a Change in Control, the Committee may provide that the vesting of one or more awards will automatically accelerate upon an involuntary termination of the participant's employment or service within a designated period following the effective date of a change of control. "Change in Control" has a specified meaning that is defined in the 2018 Stock Plan.
Adjustments Upon Changes in Capitalization. In the event of any stock dividend, stock split, combination or exchange of shares, merger, consolidation, or other distribution (other than normal cash dividends) of the Company's assets to our shareholders, or any other similar event or other change related to a corporate event affecting our shares or the price of our shares other than certain equity restructurings identified in the 2018 Stock Plan, the Committee has discretion to make appropriate adjustments in the number and type of shares subject to the 2018 Stock Plan, the terms and conditions of any award outstanding under the 2018 Stock Plan, and the grant or exercise price of any such award. In the case of certain equity restructurings as specified in the 2018 Stock Plan, the number and type of securities subject to each outstanding award and the grant or exercise price will be equitably adjusted.
Amendment and Termination of Plan. With the approval of our board of directors, at any time and from time to time, the Committee may terminate, amend or modify the 2018 Stock Plan, except that our board may not, without prior shareholder approval, amend the 2018 Stock Plan in any manner that would require shareholder approval to comply with any applicable laws.
Furthermore, absent approval of our shareholders, no option or SAR may be amended to reduce the exercise price or grant price of the shares subject to such option or SAR and (except as permitted under the provisions of the 2018 Stock Plan dealing with certain capitalization adjustments and change in control) no option or SAR may be cancelled in exchange for the grant of an option or SAR having a lower per share exercise price or for a cash payment or another award at a time when the option or SAR has a per share exercise price that is higher than the fair market value of the shares.
Clawback/Recovery. Awards are subject to recoupment under any "clawback" policy adopted by the Company providing for the recovery of awards, shares, proceeds, or payments to participants in the event of fraud or as required by applicable laws or governance considerations or in other similar circumstances.
Plan Term. The 2018 Stock Plan will continue in effect until terminated by our board of directors, but no incentive stock options may be granted under the 2018 Stock Plan after the tenth anniversary of the date the 2018 Stock Plan was approved by our board of directors. Any awards that are outstanding at the time the 2018 Stock Plan terminates will remain in force according to the terms of the 2018 Stock Plan and the applicable agreement evidencing the award.
Elanco Change-in-Control Severance Pay Plan for Select Employees
Prior to the completion of this offering, we expect our board of directors to adopt Elanco change-in-control severance pay plans for nearly all Elanco employees, including a plan which will apply to our Named Executive Officers. The Elanco plans are intended to preserve Elanco employee morale and productivity and encourage retention in the face of the disruptive impact of
151
an actual or rumored change in control. In addition, the Elanco plans are intended to align participating Elanco employee and Elanco shareholder interests by enabling executives to evaluate corporate transactions that may be in the best interests of the Elanco shareholders and other constituents of Elanco without undue concern over whether the transactions would jeopardize the participating employee's own employment.
Highlights of the expected Elanco change-in-control severance arrangements:
- •
- all regular Elanco employees will be covered;
- •
- double trigger required;
- •
- no tax gross-ups;
- •
- up to two-year pay protection; and
- •
- 18-month benefit continuation.
The basic elements of the select plan applicable to our Named Executive Officers are expected to include:
- •
- Double trigger. Unlike "single trigger" plans that pay out immediately upon a change in control, the select plan requires a "double
trigger"—a change in control followed by an involuntary loss of employment within two years. This is consistent with Elanco's intent to provide employees with financial protection upon
loss of employment.
- •
- Covered terminations. Our participating Named Executive Officers would be eligible for payments under our select plan if, within two years of
the change in control, their employment is terminated (i) without cause by Elanco or (ii) for good reason by the employee, each as is defined in the plan.
- •
- The plan would provide for up to two years of pay and 18 months of benefits protection upon a covered termination.
- •
- Severance payment. Named Executive Officers would be eligible for up to two years' base salary plus two times their target bonus for the
then-current year.
- •
- Benefit continuation. Basic employee benefits such as health and life insurance would continue for 18 months following a participating
Named Executive Officer's termination of employment, unless they become eligible for coverage with a new employer before then.
- •
- Excise tax. In some circumstances, the payments or other benefits received by a participating employee in connection with a change in control could exceed limits established under Section 280G of the Code resulting in an excise tax payment. Elanco would not reimburse or gross-up employees for these taxes. However, the amount of change in control-related benefits would be reduced to the maximum amount that would not result in an excise tax if the effect would be to deliver a greater after-tax benefit than the employee would receive if his or her benefits were not so reduced.
Stock Ownership and Holding Requirements
Our board of directors may consider from time to time equity ownership requirements for non-employee directors and executive officers.
Hedging/Pledging Policy
We anticipate adopting a hedging and pledging policy under which our directors and executive officers will be prohibited from hedging their Elanco stock and from pledging, or using as collateral, their Elanco stock.
Executive Compensation
All amounts included in the tables below represent compensation paid by Lilly to our Named Executive Officers for 2017 or the year indicated in the applicable table.
152
Summary Compensation Table
Name and Principal Position |
Year | Salary ($) |
Bonus ($) |
Stock Awards ($)(1) |
Option Awards ($) |
Non-Equity Incentive Plan Compensation ($)(2) |
Change in Pension Value and Nonqualified Deferred Compensation Earnings ($)(3) |
All Other Compensation ($)(4) |
Total Compensation ($) |
|||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Jeffrey Simmons, President and Chief Executive Officer |
2017 | $ | 688,118 | $ | 0 | $ | 2,400,000 | $ | 0 | $ | 379,841 | $ | 1,261,845 | $ | 41,287 | $ | 4,771,091 | |||||||||||
Lucas Montarce, Acting Chief Financial Officer |
2017 | $ | 280,673 | $ | 0 | $ | 112,500 | $ | 0 | $ | 83,976 | $ | 24,896 | $ | 16,200 | $ | 518,245 | |||||||||||
David Kinard, Executive Vice President, Human Resources and Corporate Affairs |
2017 | $ | 405,632 | $ | 0 | $ | 518,750 | $ | 0 | $ | 244,596 | $ | 379,379 | $ | 24,338 | $ | 1,572,696 | |||||||||||
Aaron Schacht, Executive Vice President, Innovation, Regulatory and Business Development |
2017 | $ | 285,315 | $ | 0 | $ | 581,310 | $ | 0 | $ | 66,935 | $ | 310,093 | $ | 16,200 | $ | 1,260,772 | |||||||||||
David Urbanek, Executive Vice President, Manufacturing and Quality |
2017 | $ | 297,174 | $ | 0 | $ | 300,040 | $ | 0 | $ | 76,887 | $ | 333,402 | $ | 17,830 | $ | 1,025,333 |
- (1)
- This
column shows the grant date fair value of the Lilly PAs, Lilly Executive Officer PAs, Lilly SVAs, Lilly Executive Officer SVAs, and Restricted
Stock Unit Awards, as applicable, awarded to our Named Executive Officers in 2017 computed in accordance with FASB ASC Topic 718, based upon the probable outcome of the performance conditions
as of the grant date and the assumptions identified in Note 11 — Stock-based Compensation to Lilly's Annual Report on Form 10-K filed with the SEC on
February 20, 2018. The grant date fair value for the Lilly PAs, Lilly Executive Officer PAs, Lilly SVAs and Lilly Executive Officer SVAs included in the "Stock Awards" column are based on the
probable payout outcome anticipated at the time of grant, which for the Lilly PAs and Lilly Executive Officer PAs was the maximum value and for the Lilly SVAs and Lilly Executive Officer SVAs was
target value.
- For
Mr. Schacht and Mr. Urbanek, the "Stock Awards" column also includes a special retention award of Restricted Stock Units granted on
September 1, 2017 with a grant date fair value of $300,060 for Mr. Schacht and $200,040 for Mr. Urbanek, which they received in recognition of their contributions to Elanco and
their importance to Elanco's future success; both of these grants will vest on September 1, 2020. These grants will be forfeited if Mr. Schacht or Mr. Urbanek terminates
employment with Elanco prior to that date, other than the event of certain qualifying terminations. See " — Lilly Payments Upon Termination or Change in Control (as of
December 31, 2017) — Voluntary Termination" below.
- The supplemental table below shows the total target grant date values approved by the Lilly compensation committee for Mr. Simmons and approved by Lilly management for the remaining Named Executive Officers:
| Name | 2017 Total Equity |
|||
| | | | | |
| Mr. Simmons | $ | 2,000,000 | ||
| Mr. Montarce | $ | 90,000 | ||
| Mr. Kinard | $ | 415,000 | ||
| Mr. Schacht | $ | 225,000 | ||
| Mr. Urbanek | $ | 80,000 |
The table below shows the minimum, target and maximum payouts (using the grant date fair value) for the 2017-2018 Lilly PAs (2017-2019 Lilly Executive Officer PA for Mr. Simmons) included in this column of the "Summary Compensation Table."
| Name | Payout Date |
Minimum Payout |
Target Payout |
Maximum Payout |
||||||||
| | | | | | | | | | | | | |
| Mr. Simmons | January 2020 | $ | 0 | $ | 800,000 | $ | 1,200,000 | |||||
| Mr. Montarce | January 2019 | $ | 0 | $ | 45,000 | $ | 67,500 | |||||
| Mr. Kinard | January 2019 | $ | 0 | $ | 207,500 | $ | 311,250 | |||||
| Mr. Schacht | January 2019 | $ | 0 | $ | 112,500 | $ | 168,750 | |||||
| Mr. Urbanek | January 2019 | $ | 0 | $ | 40,000 | $ | 60,000 |
The table below shows the minimum, target and maximum payouts (using the grant date fair value) for the 2017-2019 Lilly SVAs (2017-2019 Lilly Executive Officer SVAs for Mr. Simmons) included in this column of the "Summary Compensation Table."
| Name | Payout Date |
Minimum Payout |
Target Payout |
Maximum Payout |
||||||||
| | | | | | | | | | | | | |
| Mr. Simmons | January 2020 | $ | 0 | $ | 1,200,000 | $ | 2,160,000 | |||||
| Mr. Montarce | January 2020 | $ | 0 | $ | 45,000 | $ | 67,500 | |||||
| Mr. Kinard | January 2020 | $ | 0 | $ | 207,500 | $ | 311,250 | |||||
| Mr. Schacht | January 2020 | $ | 0 | $ | 112,500 | $ | 168,750 | |||||
| Mr. Urbanek | January 2020 | $ | 0 | $ | 40,000 | $ | 60,000 |
- (2)
- This column shows payments under the Elanco Bonus Plan and/or the Lilly Bonus Plan for performance in 2017. See "— 2017 Compensation Payouts" above for details on 2017 payouts for our Named Executive Officers under the applicable cash bonus plan.
153
- (3)
- The
amounts in this column reflect the change in Lilly pension value, calculated by Lilly's actuary, and are affected by additional service accruals
and pay earned, as well as actuarial assumption changes. The 2017 change in pension values was driven to a large extent by a lower discount rate which increased the net present value of our Named
Executive Officers' pension. The design of the pension benefit did not change. See "— Pension Benefits" below for information about the actuarial assumptions used. Our Named
Executive Officers did not receive any preferential or above-market earnings on deferred compensation.
- (4)
- The amounts in this column consist solely of Lilly matching contributions for each individual's 401(k) plan and nonqualified savings plan contributions. There were no reportable perquisites, personal benefits or tax reimbursements or gross-ups paid to our Named Executive Officers for 2017.
Grants of Plan-Based Awards During 2017
The Lilly compensation plans under which the grants in the following table were made are described in the CD&A and consist of the Elanco Bonus Plan and Lilly Bonus Plan (each plan is a non-equity incentive plan) and the 2002 Lilly Stock Plan (which provides for the grant of performance awards (PAs), shareholder value awards (SVAs) and restricted stock units).
To receive a payout under the Lilly PAs, Lilly Executive Officer PAs, Lilly SVAs or Lilly Executive Officer SVAs, a participant must remain employed with Lilly through the end of the relevant award period (except in the case of death, disability, retirement or redundancy). No dividends accrue on either performance awards or shareholder value awards during the performance period. For the Lilly Executive Officer PAs, non-preferential dividend equivalents accrue during the 13-month service-vesting period (following the two-year performance period) and are paid upon vesting.
|
Lilly compensation committee |
Estimated Future Payouts Under Non-Equity Incentive Plan Awards(1) |
Estimated Future Payouts Under Equity Incentive Plan Awards |
All Other Stock or Option of Stock, Options |
Grant Date Fair Value of Stock and |
|||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name |
Award | Grant Date(2) |
Action Date |
Threshold ($) |
Target ($) |
Maximum ($) |
Threshold (#) |
Target (#) |
Maximum (#) |
or Units (#) |
Option Awards(6) |
|||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Mr. Simmons |
Elanco Bonus Plan | $ | 137,624 | $ | 550,494 | $ | 1,100,989 | |||||||||||||||||||||||||
|
2017 - 2019 Lilly Executive Officer PAs(3) | 2/9/2017 | 12/12/2016 | 5,439 | 10,878 | 16,317 | $ | 1,200,000 | ||||||||||||||||||||||||
|
2017 - 2019 Lilly Executive Officer SVAs(4) | 2/9/2017 | 12/12/2016 | 9,187 | 18,374 | 33,074 | $ | 1,200,000 | ||||||||||||||||||||||||
Mr. Montarce |
Lilly Bonus Plan |
$ |
5,845 |
$ |
23,380 |
$ |
46,760 |
|||||||||||||||||||||||||
|
Elanco Bonus Plan | $ | 19,075 | $ | 76,300 | $ | 152,600 | |||||||||||||||||||||||||
|
2017 - 2018 Lilly PAs(3) | 2/9/2017 | N/A | 306 | 612 | 918 | $ | 67,500 | ||||||||||||||||||||||||
|
2017 - 2019 Lilly SVAs(4) | 2/9/2017 | N/A | 338 | 676 | 1,014 | $ | 45,000 | ||||||||||||||||||||||||
Mr. Kinard |
Lilly Bonus Plan |
$ |
45,634 |
$ |
182,535 |
$ |
365,069 |
|||||||||||||||||||||||||
|
2017 - 2018 Lilly PAs(3) | 2/9/2017 | N/A | 1,411 | 2,882 | 4,233 | $ | 311,250 | ||||||||||||||||||||||||
|
2017 - 2019 Lilly SVAs(4) | 2/9/2017 | N/A | 1,559 | 3,117 | 4,676 | $ | 207,500 | ||||||||||||||||||||||||
Mr. Schacht |
Elanco Bonus Plan |
$ |
24,253 |
$ |
97,007 |
$ |
194,014 |
|||||||||||||||||||||||||
|
2017 - 2018 Lilly PAs(3) | 2/9/2017 | N/A | 765 | 1,530 | 2,295 | $ | 168,750 | ||||||||||||||||||||||||
|
2017 - 2019 Lilly SVAs(4) | 2/9/2017 | N/A | 845 | 1,690 | 2,535 | $ | 112,500 | ||||||||||||||||||||||||
|
RSU Award(5) | 9/1/2017 | N/A | 3,747 | $ | 300,060 | ||||||||||||||||||||||||||
Mr. Urbanek |
Lilly Bonus Plan |
$ |
1,397 |
$ |
5,586 |
$ |
11,173 |
|||||||||||||||||||||||||
|
Elanco Bonus Plan | $ | 25,145 | $ | 100,581 | $ | 201,163 | |||||||||||||||||||||||||
|
2017 - 2018 Lilly PAs(3) | 2/9/2017 | N/A | 272 | 544 | 816 | $ | 60,000 | ||||||||||||||||||||||||
|
2017 - 2019 Lilly SVAs(4) | 2/9/2017 | N/A | 301 | 601 | 902 | $ | 40,000 | ||||||||||||||||||||||||
|
RSU Award(5) | 9/1/2017 | N/A | 2,498 | $ | 200,040 | ||||||||||||||||||||||||||
- (1)
- These
columns show the threshold, target, and maximum payouts for performance under the Elanco Bonus Plan or Lilly Bonus Plan. Bonus payouts range
from 0% to 200% of target.
- (2)
- To
assure grant timing is not manipulated for employee gain, the annual grant date is established in advance by the Lilly compensation committee.
Lilly equity awards to new hires and other off-cycle grants are generally effective on the first trading day of the following month.(1)
- (3)
- This
row shows the possible payouts for 2017-2018 Lilly PAs and the 2017-2019 Lilly Executive Officer PAs, ranging from 0% to 150% of target. The
Lilly PAs, to the extent earned and vested, will pay out in January 2019, and the Lilly Executive Officer PAs, to the extent earned and vested, will pay out in January 2020. The grant date fair
value of the Lilly PAs and Lilly Executive Officer PAs is based on the probable payout outcome at the time of grant, which assumes payout at the maximum value.
- (4)
- This
row shows the range of payouts for 2017-2019 Lilly SVAs and the 2017-2019 Lilly Executive Officer SVAs. These shareholder value awards will pay
out in January 2020, with payouts for the 2017-2019 Lilly SVAs ranging from 0% to 150% and the payouts for the 2017-2019 Lilly Executive Officer SVAs ranging from 0% to 180% of target. Lilly
measures the fair value of Lilly SVAs and the Lilly Executive Officer SVA awards on the grant date using a Monte Carlo simulation model. The grant date fair value of these shareholder value awards is
based on the probable payout outcome at the time of grant, which assumes payout at the target value. The maximum value is listed for these awards Note 1 to the Summary Compensation Table,
above.
- (5)
- The
RSU awards represent the special retention awards granted on September 1, 2017, which they received in recognition of their contributions
to Elanco and their importance to Elanco's future success; both of these grants will vest on September 1, 2020.
- (6)
- This column shows the grant date fair value of the Lilly PAs, Lilly Executive Officer PAs, Lilly SVAs and Lilly Executive Officer SVAs computed in accordance with FASB ASC Topic 718, based upon the probable outcome of the performance conditions as of the grant date and the assumptions identified in Note 11 — Stock-based Compensation to Lilly's Annual Report on Form 10-K filed with the SEC on February 20, 2018 as well as the grant date fair value of the Lilly RSU awards granted to Messrs. Schacht and Urbanek. See notes 3, 4 and 5 to this table. The grant date fair value of the SVAs assuming the maximum level of performance is included in the footnotes to the Summary Compensation Table above.
154
Outstanding Lilly Equity Awards at December 31, 2017
The 2017 Lilly closing stock price used to calculate the values in the table below was $84.46.
|
Stock Awards(1) | ||||||||||||||
| | | | | | | | | | | | | | | | |
Name |
Award | Number of Shares or Units of Stock That Have Not Vested (#) |
Market Value of Shares or Units of Stock That Have Not Vested ($) |
Equity Incentive Plan Awards: Number of Unearned Shares, Units, or Other Rights That Have Not Vested (#) |
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units, or Other Rights That Have Not Vested ($) |
||||||||||
| | | | | | | | | | | | | | | | |
Mr. Simmons |
2017 - 2019 Lilly Executive Officer SVA | 33,074 | (2) | $ | 2,793,430 | ||||||||||
|
2016 - 2018 Lilly Executive Officer SVA | 52,542 | (3) | $ | 4,437,697 | ||||||||||
|
2017 - 2019 Lilly Executive Officer PA | 16,317 | (4) | $ | 1,378,134 | ||||||||||
|
2016 - 2018 Lilly Executive Officer PA | 11,111 | (5) | $ | 938,435 | ||||||||||
|
2015 - 2017 Lilly Executive Officer PA | 21,326 | (6) | $ | 1,801,194 | ||||||||||
|
2008 RSU Award | 20,000 | (7) | $ | 1,689,200 | ||||||||||
Mr. Montarce |
2017 - 2019 Lilly SVA |
1,014 |
(2) |
$ |
85,642 |
||||||||||
|
2016 - 2018 Lilly SVA | 1,229 | (3) | $ | 103,801 | ||||||||||
|
2017 - 2018 Lilly PA | 918 | (4) | $ | 77,534 | ||||||||||
Mr. Kinard |
2017 - 2019 Lilly SVA |
4,676 |
(2) |
$ |
394,935 |
||||||||||
|
2016 - 2018 Lilly SVA | 5,781 | (3) | $ | 488,263 | ||||||||||
|
2017 - 2018 Lilly PA | 4,233 | (4) | $ | 357,519 | ||||||||||
|
2008 RSU Award | 6,726 | (8) | $ | 568,078 | ||||||||||
Mr. Schacht |
2017 - 2019 Lilly SVA |
2,535 |
(2) |
$ |
214,106 |
||||||||||
|
2016 - 2018 Lilly SVA | 2,529 | (3) | $ | 213,599 | ||||||||||
|
2017 - 2018 Lilly PA | 2,295 | (4) | $ | 193,836 | ||||||||||
|
2017 RSU Award | 3,747 | (9) | $ | 316,472 | ||||||||||
Mr. Urbanek |
2017 - 2019 Lilly SVA |
902 |
(2) |
$ |
76,183 |
||||||||||
|
2016 - 2018 Lilly SVA | 1,229 | (3) | $ | 103,801 | ||||||||||
|
2017 - 2018 Lilly PA | 816 | (4) | $ | 68,919 | ||||||||||
|
2017 RSU Award | 2,498 | (10) | $ | 210,981 | ||||||||||
- (1)
- The
table does not include stock option awards because Lilly has not awarded stock options to employees since 2006 and there are no outstanding Lilly
stock option awards.
- (2)
- Lilly
SVAs and Lilly Executive Officer SVAs granted for the 2017-2019 performance period, to the extent earned, are scheduled to vest on
December 31, 2019. The number of Lilly shares reported reflects the maximum payout, which will be made if the average closing Lilly stock price in November and December 2019 is over
$101.79. Actual payouts may vary from 0% to 180% of target for the Lilly Executive Officer SVAs and 0% to 150% for the Lilly SVAs. Net Lilly shares received in respect of Lilly Executive Officer SVA
payouts must be held by Mr. Simmons for a minimum of one year.
- (3)
- Lilly
SVAs and Lilly Executive Officer SVAs granted for the 2016-2018 performance period are scheduled to vest on December 31, 2018. The number
of Lilly shares reported reflects the maximum payout, which will be made if the average closing Lilly stock price in November and December 2018 is over $119.58. Actual payouts may vary from 0%
to 180% of target for the Lilly Executive Officer SVA and 0% to 150% for the Lilly SVA. Net Lilly shares from any payout must be held by our Named Executive Officer for a minimum of one year.
- (4)
- For
Mr. Simmons, this number represents the maximum value of Lilly Executive Officer PA shares that could pay out for 2017-2018 performance
period provided performance goals are met. Once the combined cumulative Lilly EPS result and associated payout level is determined at the end of the performance period, the resultant number of shares
owed to Mr. Simmons will be issued as Lilly RSUs that vest in February 2020. For Mr. Kinard, Mr. Schacht and Mr. Urbanek, this number represents the maximum value of
Lilly PA shares that could pay out for 2017-2018 performance period provided performance goals are met. Actual payouts for both Lilly PAs and Lilly Executive Officer PAs may vary from 0% to 150% of
target.
- (5)
- The
performance period ending December 31, 2017 for the 2016-2018 Lilly Executive Officer PA resulted in Mr. Simmons being issued Lilly
RSUs for 150% of target shares. The RSUs are scheduled to vest in February 2019.
- (6)
- RSUs
vested in February 2018 from the 2015-2017 Lilly Executive Officer PA.
- (7)
- This
grant was made in 2008 outside of the normal annual cycle and vested on May 1, 2018.
- (8)
- This grant was made in 2008 outside of the normal annual cycle and will vest on November 3, 2018.
155
- (9)
- This
grant was made in 2017 outside of the normal annual cycle. This grant will vest on September 1, 2020.
- (10)
- This grant was made in 2017 outside of the normal annual cycle. This grant will vest on September 1, 2020.
Lilly Stock Vested in 2017
|
Lilly Stock Awards |
||||||
| | | | | | | | |
Name |
Number of Shares Acquired on Vesting (#) |
Value Realized on Vesting ($)(1) |
|||||
| | | | | | | | |
Mr. Simmons |
10,244 | (2) | $ | 789,095 | |||
|
22,507 | (3) | $ | 1,971,613 | |||
Mr. Montarce |
729 |
(4) |
$ |
243,353 |
|||
|
385 | (3) | $ | 33,726 | |||
|
237 | (6) | $ | 18,304 | |||
Mr. Kinard |
2,778 |
(4) |
$ |
243,353 |
|||
|
3,418 | (3) | $ | 299,417 | |||
Mr. Schacht |
1,215 |
(4) |
$ |
106,434 |
|||
|
684 | (3) | $ | 59,918 | |||
Mr. Urbanek |
590 |
(4) |
$ |
51,684 |
|||
|
598 | (3) | $ | 52,385 | |||
|
4,815 | (5) | $ | 410,816 | |||
- (1)
- Amounts
reflect the market value of the Lilly stock on the day the Lilly stock vested.
- (2)
- Lilly
restricted stock units resulting from the 2014-2016 Lilly Executive Officer PA that vested in February 2017.
- (3)
- Payout
of the 2015-2017 Lilly Executive Officer SVA and 2015-2017 Lilly SVA at 100% of target.
- (4)
- Payout
of the 2016-2017 Lilly PA at 100% of target.
- (5)
- The
last installment of a one-time RSU awarded to Mr. Urbanek in 2007 outside of the normal grant cycle.
- (6)
- Payout of a 2014 RSU grant that vested in February 2017.
Retirement Benefits
Lilly provides retirement income to eligible U.S. Lilly employees, including our Named Executive Officers, through the following plans:
- •
- The Lilly 401(k) Plan, a defined contribution plan qualified under Sections 401(a) and 401(k) of the Internal Revenue Code. Participants
may elect to contribute a portion of their base salary to the plan, and Lilly provides matching contributions on employees' contributions up to 6% of base salary up to IRS limits. The employee
contributions, Lilly contributions and earnings thereon are paid out in accordance with elections made by the participant. See the "All Other Compensation" column in the Summary Compensation Table for
information about Lilly contributions under the 401(k) Plan for our Named Executive Officers.
- •
- The Lilly Retirement Plan, a tax-qualified defined benefit plan that provides monthly benefits to retirees. See the Pension Benefits in 2017 table below for additional information about the value of these pension benefits.
Sections 401 and 415 of the Code generally limit the amount of annual pension that can be paid from a tax-qualified plan ($270,000 in 2017 and $275,000 in 2018) as well as the amount of annual earnings that can be used to calculate a pension benefit. However, since 1975 Lilly has maintained a nonqualified pension plan that pays retirees the difference between the amount payable under the Retirement Plan and the amount they would have received without the Code limits. The Lilly nonqualified pension plan is unfunded and subject to forfeiture in the event of bankruptcy. Likewise, Lilly maintains a nonqualified savings plan that allows participants to contribute up to 6% of base salary exceeding the IRS limit. Lilly matches these contributions as
156
described in the 401(k) Plan. For more information, see the disclosure immediately following the footnotes to the Nonqualified Deferred Compensation in 2017 table.
The following table shows benefits that our Named Executive Officers have accrued under the Lilly Retirement Plan and the Lilly nonqualified pension plan.
Pension Benefits
Name |
Plan | Number of Years of Credited Service |
Present Value of Accumulated Benefit ($)(1) |
Payments During Last Fiscal Year ($) |
||||||||
| | | | | | | | | | | | | |
Mr. Simmons |
Lilly retirement plan (pre-2010) | 21 | $ | 1,080,447 | ||||||||
|
Lilly retirement plan (post-2009) | 8 | $ | 203,352 | ||||||||
|
Lilly nonqualified plan (pre-2010) | 21 | $ | 4,624,126 | ||||||||
|
Lilly nonqualified plan (post-2009) | 8 | $ | 835,649 | ||||||||
| | | | | | | | | | | | | |
|
$ | 6,743,574 | $ | 0 | ||||||||
Mr. Montarce |
Lilly retirement plan (post-2009) | 1 | $ | 21,417 | ||||||||
|
Lilly nonqualified plan (post-2009) | 1 | $ | 6,184 | ||||||||
| | | | | | | | | | | | | |
|
$ | 27,601 | $ | 0 | ||||||||
Mr. Kinard |
Lilly retirement plan (pre-2010) | 13 | $ | 527,840 | ||||||||
|
Lilly retirement plan (post-2009) | 8 | $ | 203,352 | ||||||||
|
Lilly nonqualified plan (pre-2010) | 13 | $ | 758,126 | ||||||||
|
Lilly nonqualified plan (post-2009) | 8 | $ | 280,422 | ||||||||
| | | | | | | | | | | | | |
|
$ | 1,769,740 | $ | 0 | ||||||||
Mr. Schacht |
Lilly retirement plan (pre-2010) | 19 | $ | 933,480 | ||||||||
|
Lilly retirement plan (post-2009) | 8 | $ | 203,352 | ||||||||
|
Lilly nonqualified plan (pre-2010) | 19 | $ | 499,320 | ||||||||
|
Lilly nonqualified plan (post-2009) | 8 | $ | 103,746 | ||||||||
| | | | | | | | | | | | | |
|
$ | 1,739,898 | $ | 0 | ||||||||
Mr. Urbanek |
Lilly retirement plan (pre-2010) | 22 | $ | 1,172,534 | ||||||||
|
Lilly retirement plan (post-2009) | 8 | $ | 211,141 | ||||||||
|
Lilly nonqualified plan (pre-2010) | 22 | $ | 394,285 | ||||||||
|
Lilly nonqualified plan (post-2009) | 8 | $ | 68,559 | ||||||||
| | | | | | | | | | | | | |
|
$ | 1,846,519 | $ | 0 |
- (1)
- The following actuarial assumptions were used to calculate the present value of our Named Executive Officer's accumulated pension benefit:
| Discount rate: | 3.83% for the qualified plan and 3.70% for non-qualified plan | |
| Mortality (post-retirement decrement only): | RP2006 with generational projection using Scale MP2017 | |
| Pre-2010 joint and survivor benefit (% of pension): | 50% until age 62; 25% thereafter | |
| Post-2009 benefit payment form: | life annuity |
157
The Lilly Retirement Plan benefits shown in the table are net present values. The benefits are not payable as a lump sum; they are generally paid as a monthly annuity for the life of the retiree and, if elected, any qualifying survivor. The annual benefit under the Lilly retirement plan is calculated using years of service and the average of the annual earnings (salary plus bonus) for the highest five out of the last 10 calendar years of service (final average earnings).
Post-2009 Lilly Plan Information: Following amendment of the Lilly Retirement Plan formulas, employees hired by Lilly on or after February 1, 2008 have accrued retirement benefits only under the new Lilly plan formula. Employees hired before that date have accrued benefits under both the old Lilly and new Lilly plan formulas. All eligible employees, including those hired on or after February 1, 2008, can retire at age 65 with at least five years of service and receive an unreduced benefit. The annual benefit under the new Lilly plan formula is equal to 1.2% of final average earnings multiplied by years of service. Early retirement benefits under this Lilly plan formula are reduced 6% for each year under age 65. Transition benefits were afforded to employees with 50 points (age plus service) or more as of December 31, 2009. These benefits were intended to ease the transition to the new Lilly retirement formula for those employees who were closer to retirement or had been with Lilly longer at the time the Lilly plan was changed. For the transition group, early retirement benefits are reduced 3% for each year from age 65 to age 60 and 6% for each year under age 60. Mr. Simmons is in this transition group.
Pre-2010 Lilly Plan Information: Employees hired by Lilly prior to February 1, 2008, accrued benefits under both Lilly plan formulas. For these employees, benefits that accrued before January 1, 2010 were calculated under the old Lilly plan formula. The amount of the benefit is calculated using actual years of service through December 31, 2009, while total years of service is used to determine eligibility and early retirement reductions. The benefit amount is increased (but not decreased) proportionately, based on final average earnings at termination compared to final average earnings at December 31, 2009. Full retirement benefits are earned by employees with 90 or more points (the sum of his or her age plus years of service). Employees electing early retirement receive reduced benefits as described below:
- •
- The benefit for Lilly employees with between 80 and 90 points is reduced by 3% for each year under 90 points or age 62.
- •
- The benefit for Lilly employees who have fewer than 80 points, but who reached age 55 and have at least 10 years of service, is reduced as described above and is further reduced by 6% for each year under 80 points or age 65.
158
Nonqualified Deferred Compensation
Name |
Plan | Executive Contributions in Last Fiscal Year ($)(1) |
Registrant (Lilly) Contributions in Last Fiscal Year ($)(2) |
Aggregate Earnings in Last Fiscal Year ($) |
Aggregate Withdrawals/ Distributions in Last Fiscal Year ($) |
Aggregate Balance at Last Fiscal Year End ($) |
||||||||||||
| | | | | | | | | | | | | | | | | | | |
Mr. Simmons |
Lilly nonqualified savings | $ | 25,087 | $ | 25,087 | $ | 41,287 | $ | 0 | $ | 722,166 | |||||||
|
Lilly deferred compensation | $ | 0 | $ | 0 | $ | 54,795 | $ | 1,735,607 | |||||||||
| | | | | | | | | | | | | | | | | | | |
|
Total | $ | 25,087 | $ | 25,087 | $ | 96,082 | $ | 0 | $ | 2,457,773 | |||||||
Mr. Montarce |
Lilly nonqualified savings | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | |||||||
|
Lilly deferred compensation | $ | 0 | $ | 0 | $ | 0 | $ | 0 | |||||||||
| | | | | | | | | | | | | | | | | | | |
|
Total | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | |||||||
Mr. Kinard |
Lilly nonqualified savings | $ | 8,138 | $ | 8,138 | $ | 25,845 | $ | 0 | $ | 171,027 | |||||||
|
Lilly deferred compensation | $ | 62,587 | $ | 0 | $ | 19,261 | $ | 621,727 | |||||||||
| | | | | | | | | | | | | | | | | | | |
|
Total | $ | 70,725 | $ | 8,138 | $ | 45,106 | $ | 0 | $ | 792,754 | |||||||
Mr. Schacht |
Lilly nonqualified savings | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | |||||||
|
Lilly deferred compensation | $ | 0 | $ | 0 | $ | 0 | $ | 0 | |||||||||
| | | | | | | | | | | | | | | | | | | |
|
Total | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | |||||||
Mr. Urbanek |
Lilly nonqualified savings | $ | 1,630 | $ | 1,630 | $ | (2 | ) | $ | 0 | $ | 3,259 | ||||||
|
Lilly deferred compensation | $ | 0 | $ | 0 | $ | 0 | $ | 0 | |||||||||
| | | | | | | | | | | | | | | | | | | |
|
Total | $ | 1,630 | $ | 1,630 | $ | (2 | ) | $ | 0 | $ | 3,259 |
- (1)
- The
amounts in this column are also included in the Summary Compensation Table, in the "Salary" column (nonqualified savings) or the "Lilly Non-Equity
Incentive Plan Compensation" column (deferred compensation).
- (2)
- The amounts in this column are also included in the Summary Compensation Table, in the "All Other Compensation" column as a portion of the savings plan match.
The Nonqualified Deferred Compensation table above shows information about two Lilly programs: the Lilly nonqualified savings plan and the Lilly Deferred Compensation Plan. The Lilly nonqualified savings plan is designed to allow each employee to contribute up to 6% of his or her base salary and receive a Lilly company match beyond the contribution limits prescribed by the IRS with regard to 401(k) plans. The Lilly plan is administered in the same manner as the 401(k) Plan, with the same participation and investment elections. Lilly executive officers may defer receipt of all or part of their cash compensation and other U.S. Lilly executives may defer receipt of all or part of their cash bonus under the Lilly Deferred Compensation Plan. Amounts deferred by executives under the Lilly plan are credited with interest at 120% of the applicable federal long-term rate as established the preceding December by the U.S. Treasury Department under Section 1274(d) of the Code with monthly compounding, which was 2.7% for 2017 and is 3.1% for 2018. Participants may elect to receive the funds in a lump sum or in up to 10 annual installments following termination of employment, but may not make withdrawals while employed by Lilly, except in the event of hardship as approved by the Lilly compensation committee. All deferral elections and associated distribution schedules are irrevocable. Both Lilly plans are unfunded and subject to forfeiture in the event of bankruptcy.
Lilly Payments Upon Termination or Change in Control (as of December 31, 2017)
The following table describes the potential payments and benefits under Lilly's compensation and benefit plans and arrangements to which our Named Executive Officers would be entitled upon a hypothetical termination of employment on December 31, 2017 in the circumstances described in the table. Except for certain terminations following a change in control of Lilly, certain qualifying terminations with respect to his Executive Officer SVAs and Executive Officer PAs as described in this CD&A, there are no agreements, arrangements or plans that entitle Mr. Simmons to severance, perquisites or other enhanced benefits upon termination of his employment. The narrative following
159
the tabular disclosure below contains more details on the treatment of certain equity awards upon a qualifying termination of employment. Other than the payments and benefits described below, any agreement to provide severance payments or benefits (other than following a change in control) would be at the discretion of the Lilly compensation committee or, following this offering, our compensation committee.
|
Cash Severance Payment(1) |
Continuation of Medical / Welfare Benefits (present value) |
Value of Acceleration of Equity Awards |
Total Termination Benefits |
|||||||||
| | | | | | | | | | | | | | |
Mr. Simmons |
|||||||||||||
• Involuntary retirement or termination without cause |
$ | 0 | $ | 0 | $ | 3,490,394 | (4) | $ | 3,490,394 | ||||
• Involuntary or good reason termination after change in control |
$ | 2,477,225 | $ | 315,496 | (2) | $ | 8,591,525 | (5) | $ | 11,384,245 | |||
Mr. Montarce |
|||||||||||||
• Involuntary retirement or termination without cause |
$ | 457,800 | $ | 5,000 | (3) | $ | 0 | (4) | $ | 462,800 | |||
• Involuntary or good reason termination after change in control |
$ | 915,600 | $ | 39,903 | (2) | $ | 177,366 | (5) | $ | 1,132,869 | |||
Mr. Kinard |
|||||||||||||
• Involuntary retirement or termination without cause |
$ | 591,692 | $ | 5,000 | (3) | $ | 568,078 | (4) | $ | 1,164,770 | |||
• Involuntary or good reason termination after change in control |
$ | 1,183,925 | $ | 39,903 | (2) | $ | 1,351,613 | (5) | $ | 2,575,442 | |||
Mr. Schacht |
|||||||||||||
• Involuntary retirement or termination without cause |
$ | 385,381 | $ | 5,000 | (3) | $ | 316,472 | (4) | $ | 706,853 | |||
• Involuntary or good reason termination after change in control |
$ | 770,763 | $ | 315,496 | (2) | $ | 724,245 | (5) | $ | 1,810,503 | |||
Mr. Urbanek |
|||||||||||||
• Involuntary retirement or termination without cause |
$ | 687,383 | $ | 5,000 | (3) | $ | 210,981 | (4) | $ | 903,364 | |||
• Involuntary or good reason termination after change in control |
$ | 1,083,150 | $ | 45,916 | (2) | $ | 365,247 | (5) | $ | 1,494,313 |
- (1)
- Our
Named Executive Officers, other than Mr. Simmons, are entitled to severance under The Lilly Severance Pay Plan upon an involuntary
retirement or termination without cause. Also see "— Lilly Payments Upon Termination or Change in Control (as of December 31, 2017) — Lilly Change - in -
Control Severance Pay Plan" below.
- (2)
- See
"Lilly Accrued Pay and Regular Retirement Benefits" and "Change-in-Control Severance Pay Plan — Continuation of medical and
welfare benefits" below for a discussion of payments following a change in control.
- (3)
- A
one-time payment for any purpose including payment of medical premiums, job placement services or miscellaneous expenses.
- (4)
- Includes
amounts that would be paid under RSU awards. Does not include amounts that would be paid under the SVAs, Executive Officer SVAs, PAs and
Executive Officer PAs, which would result in the following estimated amounts if calculated at target as of December 31, 2018: Mr. Simmons: $3,558,694; Mr. Montarce: $101,859;
Mr. Kinard: $423,933; Mr. Schacht: $207,124; and Mr. Urbanek: $86,008. See "Outstanding Lilly Equity Awards at December 31, 2017" table above.
- (5)
- Includes the acceleration of RSUs (including the Special Retention Awards), SVAs, Executive Officer SVAs, PAs and Executive Officer PAs upon the event of certain qualifying terminations, as applicable. See narrative below.
Lilly Accrued Pay and Regular Retirement Benefits. The amounts shown in the table above do not include certain payments and benefits to the extent they are provided on a
160
non-discriminatory basis to salaried employees generally upon termination of employment or are disclosed above in the Pension Benefits Table or the Nonqualified Deferred Compensation Table. These include:
- •
- accrued salary and vacation pay;
- •
- regular pension benefits under the Lilly Retirement Plan and the Lilly nonqualified pension plan. See "— Retirement
Benefits" above;
- •
- welfare benefits provided to all U.S. retirees, including retiree medical and dental insurance. The amounts shown in the table above as "Lilly
Continuation of Medical / Welfare Benefits" are explained below; and
- •
- distributions of plan balances under the Lilly 401(k) Plan, the Lilly nonqualified savings plan and the Lilly Deferred Compensation Plan. See "— Nonqualified Deferred Compensation" for information about these plans.
Lilly Deferred Compensation. The amounts shown in the table do not include distributions of plan balances under the Lilly deferred compensation plan. See "— Nonqualified Deferred Compensation."
Death and Disability. A termination of employment due to death or disability does not entitle our Named Executive Officers to any cash payments or benefits that are not available to U.S. Lilly salaried employees generally. See "Involuntary Retirement or Termination Without Cause" below for the effect of a termination of employment due to death or disability on Lilly equity awards.
Termination for Cause. Executives terminated for cause, including our Named Executive Officer, receive no severance or enhanced Lilly benefits and forfeit any unvested Lilly equity grants.
Voluntary Termination. Executives, including our Named Executive Officers, receive no severance or enhanced Lilly benefits and forfeit any unvested Lilly equity grants if they leave voluntarily.
Involuntary Retirement or Termination Without Cause. In the event of involuntary retirement (i.e. a retirement predicated on some other involuntary event), death, disability or redundancy, SVAs, Executive Officer SVAs, PAs and Executive Officer PAs are paid on a pro-rated basis based upon the number of days worked and the relevant company performance at the end of the performance period. In addition, upon death, disability or redundancy, RSUs accelerate in full.
Lilly Change in Control Severance Pay Plan. As described in "— Other Lilly Compensation Practices and Information — Lilly Severance Benefits," Lilly maintains a change-in-control severance pay plan for nearly all employees, including our Named Executive Officers and a Lilly severance plan in which our Named Executive Officers other than Mr. Simmons participate. The Lilly change-in-control severance pay plan defines a change in control very specifically, but generally the terms include the occurrence of one of the following: (i) acquisition of 20% or more of Lilly's stock; (ii) replacement by the Lilly shareholders of one half or more of the Lilly board of directors; (iii) consummation of a merger, share exchange or consolidation of Lilly (other than a transaction that results in the Lilly shareholders prior to the transaction continuing to hold more than 60% of the voting stock of the combined entity); or (iv) liquidation of Lilly or sale or disposition of all or substantially all of its assets. The amounts shown in the table for "involuntary or good-reason termination after change in control" are based on the following assumptions and plan provisions:
- •
- Lilly covered terminations. The table assumes a termination of Lilly employment that is eligible for severance under the terms of the Lilly change-in-control severance pay plan, based on our Named Executive Officer's compensation, benefits, age and service credit on December 31, 2017. Eligible terminations include an involuntary termination for reasons
161
- •
- A termination by Lilly is for cause if it is for any of the following reasons: (i) the employee's willful and continued
refusal to perform, without legal cause, his or her material duties, resulting in demonstrable economic harm to Lilly; (ii) any act of fraud, dishonesty or gross misconduct resulting in
significant economic harm or other significant harm to the business reputation of Lilly; or (iii) conviction of or the entering of a plea of guilty or nolo
contendere to a felony.
- •
- A termination is for good reason if it results from: (i) a material diminution in the nature or status of the executive's
position, title, reporting relationship, duties, responsibilities or authority, or the assignment to him or her of additional responsibilities that materially increase his or her workload;
(ii) any reduction in the executive's then-current base salary; (iii) a material reduction in the executive's opportunities to earn incentive bonuses below those in effect for the year
prior to the change in control; (iv) a material reduction in the executive's employee benefits from the benefit levels in effect immediately prior to the change in control; (v) the
failure to grant to the executive stock options, stock units, performance shares or similar incentive rights during each 12-month period following the change in control on the basis of a number of
Lilly shares or Lilly units and all other material terms at least as favorable to the executive as those rights granted to him or her on an annualized average basis for the three-year period
immediately prior to the change in control; or (vi) relocation of the executive by more than 50 miles.
- •
- Lilly cash severance payment. The Lilly cash severance payment amounts to
two times the affected employee's annual base salary plus two times the affected employee's bonus target for that year under the bonus plan.
- •
- Lilly continuation of medical and welfare benefits. This amount represents
the present value of the Lilly change-in-control severance pay plan's provision, following a Lilly covered termination, of 18 months of continued coverage equivalent to the company's current
active employee medical, dental, life and long-term disability insurance together with the value associated with additional credit for purposes of determining eligibility for retiree medical and
dental benefits. Similar actuarial assumptions to those used to calculate incremental pension benefits apply to the calculation for continuation of medical and welfare benefits, with the addition of
actual COBRA rates based on current benefit elections.
- •
- Acceleration of Lilly equity awards. Upon a Lilly change in control, any
unvested Lilly equity awards would convert into Lilly restricted stock units of the new company, with the number of Lilly shares earned under the awards based on accrued performance at the time of the
transaction. The Lilly restricted stock units will continue to vest and pay out upon the earlier of the completion of the original award period, upon a Lilly covered termination, or if the successor
entity does not assume, substitute or otherwise replace the award. The amount in this column represents the value of the acceleration of unvested Lilly equity grants based on target performance, had a
qualifying termination occurred on December 31, 2017.
- •
- Excise taxes. Upon a Lilly change in control, employees may be subject to certain excise taxes under Section 280G of the Code. Lilly does not reimburse the affected employees for those excise taxes or any income taxes payable by the employee. To reduce the employee's exposure to excise taxes, the employee's change in control benefit may be decreased to maximize the after-tax benefit to the individual.
other than for cause or a voluntary termination by the executive for good reason, within two years following the change in control.
Lilly Payments Upon Change in Control Alone. In general, the Lilly change in control severance pay plan is a "double trigger" plan, meaning payments are made only if the employee
162
suffers a covered termination of employment within two years following the change in control, or in the case of Lilly equity awards, if the successor entity does not assume, substitute or otherwise replace the awards.
Lilly Severance Pay Plan. Our Named Executive Officers, other than Mr. Simmons, participate in the Lilly Severance Pay Plan. The plan generally provides severance benefits to eligible employees of Lilly and certain of its subsidiaries and affiliates in the event of involuntary retirement or termination without cause, which includes separation from Lilly due to discharge or resignation in lieu of discharge, other than for cause, resignation because of disability, the closing of an office or facility in which the employee is employed, or any other reason determined by Lilly to warrant the payment of a severance benefit pursuant to the plan. Such employees will receive a benefit that is a function of their weekly pay and the number of years of service completed at the time of their separation.
Director Compensation
Elanco Director Compensation Program
Directors who are employed by us or Lilly (or any of their respective affiliates) are not expected to be eligible to receive compensation for their service on our board of directors. We anticipate that all other members of our board of directors will receive an annual retention fee of $70,000 in cash and an annual equity award in the number of our shares having a grant date value equal to $180,000, that the chairman of our board of directors also will receive an annual retention fee of $100,000 in cash, and the chairman of each of the Audit Committee, Compensation Committee, and Nominating and Corporate Governance Committee each also will receive an annual retention fee of $18,000, $16,000 and $16,000, respectively, in cash. We anticipate that the annual equity awards granted to directors who are not employed by us or Lilly will be subject to mandatory deferral under our Directors' Deferral Plan and that cash compensation will be subject to elective deferral under such plan, as described below.
All of our directors will be reimbursed for reasonable out-of-pocket travel expenses incurred in connection with attendance at board and committee meetings and other board-related activities. Our compensation committee may determine to review and make changes to our director compensation following this offering.
Director Letter Agreement with Chairman
In anticipation of the offering, R. David Hoover was appointed to serve as a director and chairman of our board of directors. In connection therewith, we entered into a letter agreement with Mr. Hoover, which provided, in part, that Mr. Hoover would assist in the identification and recruitment of potential candidates to serve on our board of directors. Mr. Hoover is entitled to a monthly fee of $10,000 for his service prior to the completion of the offering. It is anticipated that following the offering Mr. Hoover's compensation would be consistent with the director and chairman compensation described under "Elanco Director Compensation Program" above as determined by our board of directors in connection with the offering.
Elanco Directors' Deferral Plan
Prior to the completion of this offering we expect our board of directors and our stockholder to approve the Elanco Directors' Deferral Plan (the "Directors' Deferral Plan"), which we expect will become effective on the day immediately prior to the date that the offering of our shares pursuant to this prospectus is declared effective by the SEC. Under the Elanco Directors' Deferral Plan, non-employee directors' equity compensation (but no more than the lesser of 30,000 shares or the number of shares equal in value to $800,000 (as of the applicable valuation date) less the directors' cash compensation for the applicable plan year) are credited annually in a deferred stock account
163
(as described below). The Elanco Directors' Deferral Plan also allows non-employee directors to defer receipt of all or part of their cash compensation until after their service on our board of directors has ended. Each director can choose to invest their deferred cash compensation in one or both of the following two accounts:
Deferred Stock Account. This account allows the director, in effect, to invest his or her deferred cash compensation in company stock. Funds in this account are credited as hypothetical shares of company stock based on the closing stock price on pre-set dates. The number of shares credited in respect of deferred cash compensation is calculated by the amount deferred divided by the closing stock price on pre-set dates. In addition, the annual stock compensation awards described above is also credited to this account. Deferred stock accounts are also credited for dividends as if the credited shares were actual shares, with such credited dividends credited in additional shares.
Deferred Compensation Account. Funds in this account earn interest each year at a rate of 120 percent of the applicable federal long-term rate, compounded monthly, as established the preceding December by the U.S. Treasury Department under Section 1274(d) of the Internal Revenue Code of 1986 (the Internal Revenue Code).
Both accounts may generally only be paid in a lump sum in January of the second plan year following the plan year in which the director separates from service or in annual installments over between two and 10 years, beginning at the same time the lump sum payment would be made. Amounts credited to the director's deferred stock account would generally be paid in shares of company stock and amounts credited to the director's deferred compensation account would be paid in cash.
164
The following table shows information regarding the beneficial ownership of our common stock (i) immediately prior to the completion of this offering and (ii) as adjusted to give effect to this offering by:
- •
- each person or group known by us to own beneficially more than 5% of our common stock;
- •
- each member of our board of directors following the completion of this offering and each of our named executive officers;
and
- •
- all members of our board of directors and our executive officers as a group.
Beneficial ownership of shares is determined under rules of the SEC and generally includes any shares over which a person exercises sole or shared voting or investment power. Except as noted by footnote, and subject to community property laws where applicable, we believe based on the information provided to us that the persons and entities named in the table below have sole voting and investment power with respect to all shares of our common stock shown as beneficially owned by them. Percentage of beneficial ownership is based on shares of common stock outstanding immediately prior to the completion of this offering and shares of common stock outstanding after giving effect to this offering, assuming no exercise of the underwriters' option to purchase additional shares, or shares of common stock, assuming the underwriters exercise their option to purchase additional shares in full. Shares of common stock subject to options currently exercisable or exercisable within 60 days of the date of this prospectus are deemed to be outstanding and beneficially owned by the person holding the options for the purposes of computing the percentage of beneficial ownership of that person and any group of which that person is a member, but are not deemed outstanding for the purpose of computing the percentage of beneficial ownership for any other person. The table does not give effect to any shares that may be acquired by our directors or executive officers pursuant to the directed share
165
program. Unless otherwise indicated, the address for each holder listed below is 2500 Innovation Way, Greenfield, Indiana 46140.
|
Shares of common stock beneficially owned before this offering |
Shares of common stock beneficially owned after this offering (assuming no exercise of the option to purchase additional shares) |
Shares of common stock beneficially owned after this offering (assuming full exercise of the option to purchase additional shares) |
||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Name and address of beneficial owner |
Number of shares |
Percentage of shares |
Number of shares |
Percentage of shares |
Number of shares |
Percentage of shares |
|||||||||||||
| | | | | | | | | | | | | | | | | | | | |
5% shareholder: |
|||||||||||||||||||
Lilly(1) |
100 | % | |||||||||||||||||
Named executive officers and directors: |
|||||||||||||||||||
Jeffrey N. Simmons |
— | — | |||||||||||||||||
David S. Kinard |
— | — | |||||||||||||||||
Lucas E. Montarce |
— | — | |||||||||||||||||
Aaron L. Schacht |
— | — | |||||||||||||||||
David A. Urbanek |
— | — | |||||||||||||||||
R. David Hoover |
— | — | |||||||||||||||||
Kapila K. Anand |
— | — | |||||||||||||||||
Michael J. Harrington |
— | — | |||||||||||||||||
Lawrence E. Kurzius |
— | — | |||||||||||||||||
Carl L. McMillian |
— | — | |||||||||||||||||
David A. Ricks |
— | — | |||||||||||||||||
Aarti S. Shah |
— | — | |||||||||||||||||
Joshua L. Smiley |
— | — | |||||||||||||||||
All board members and executive officers as a group (16 persons) |
— | 0 | % | ||||||||||||||||
- (1)
- Lilly's address is Lilly Corporate Center, Indianapolis, IN 46285.
166
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Relationship with Lilly
Prior to the completion of this offering, all of our outstanding shares of common stock are owned by Lilly. Immediately following the completion of this offering, Lilly will own % of our common stock. See "Risk factors — Risks Related to Our Relationship with Lilly" and "The Separation and Distribution Transactions — The Separation."
In connection with this offering and the Separation, we and Lilly will enter into certain agreements that will effect the Separation of our business from Lilly and provide a framework for our relationship with Lilly after this offering and the Separation. The following is a summary of the terms of the material agreements that we intend to enter into with Lilly prior to the completion of this offering, which will be filed as exhibits to the registration statement of which this prospectus is a part. These summaries set forth the terms of the agreements that we believe are material and are qualified in their entirety by reference to the full text of such agreements.
For further information regarding historical related party transactions, see Note 10: Related Party Transactions to our unaudited interim combined financial statements and Note 16: Related Party Transactions to our audited combined financial statements.
Master Separation Agreement
We intend to enter into a separation agreement with Lilly prior to or concurrently with the completion of this offering. The master separation agreement will govern certain pre-offering transactions, as well as the relationship between Lilly and us following this offering and the Separation.
The separation of our business; contribution of entities. The master separation agreement generally allocates certain assets and liabilities between us and Lilly according to the business to which such assets or liabilities relate. Prior to the closing of the sale of shares pursuant to this offering, Lilly or its affiliates, as applicable, will have conveyed, contributed, assigned, distributed, delivered or otherwise transferred ownership of substantially all of the assets that are used exclusively in, relate exclusively to, or arise exclusively out of, the operation or conduct of Lilly's animal health businesses, to certain direct and indirect subsidiaries of Lilly.
Effective as of the closing of the sale of shares pursuant to this offering, Lilly will contribute to us, pursuant to the master separation agreement, the equity interests of certain entities that will, as of the time of such contribution, hold, either directly or indirectly through the equity ownership of additional entities, substantially all of the assets of Lilly's animal health businesses, which will form our business going forward. The master separation agreement also generally provides for the assumption by us or the entities that will become our subsidiaries pursuant to the master separation agreement, as applicable, of all historical and future liabilities to the extent relating to, arising out of or resulting from the ownership or operation of such animal health businesses. In exchange for the transfer to us of the entities holding substantially all of the assets and liabilities of its animal health businesses, Lilly will receive (i) all of the net proceeds we will receive from the sale of our common stock in this offering, including any net proceeds we receive as a result of any exercise of the underwriters' overallotment option, (ii) all of the net proceeds (approximately $2.0 billion) we received in the Senior Notes Offering and (iii) all of the net proceeds ($498.6 million) we received from the entry into the Term Facility; provided, to the extent the unrestricted cash held by us following the completion of this offering is less than (or more than) $300 million, we will retain a portion of the net proceeds (or pay additional amounts to Lilly) so that the unrestricted cash held by us for working capital and other general corporate purposes following the completion of this offering is $300 million. The determination date that will be used to measure
167
the unrestricted cash held by us following this offering will be the day that is the last day of the month immediately prior to the completion of this offering, if the closing date of this offering is prior to the fifteenth day of the month, or the day that is the last day of the month in which this offering is completed, if the closing date of this offering is on or after the fifteenth day of the month. In addition, a portion of the consideration to be paid to Lilly will be temporarily retained by us as restricted cash in connection with the anticipated transfer to us from Lilly of certain animal health assets in certain jurisdictions that are anticipated to occur following the completion of the offering (which consideration shall be paid to Lilly if, despite our and Lilly's cooperation and commercially reasonable efforts, such transfers have not occurred prior to a date mutually agreed by us and Lilly).
Except as expressly set forth in any of the transaction documents, or as required by law, the assets that have been or will be conveyed, contributed or assigned, transferred, distributed or delivered to us or our subsidiaries (including entities the equity interests of which are being transferred to us by Lilly) are being so transferred on an "as is," "where is" basis, without any representations or warranties, and we have agreed to bear the economic and legal risks that any conveyance was insufficient to vest in us good title, free and clear of any security interest, that any necessary consents or approvals were not obtained or that any conveyance was not done in compliance with any requirements of law or judgments.
Delayed transfers and further assurances. To the extent that the transfers of the assets and the assumptions of the liabilities allocated to us under the master separation agreement have not been completed on or prior to the completion of the offering, we and Lilly will cooperate with each other and use commercially reasonable efforts to effect such transfers and assumptions as promptly as practicable thereafter or at such other time as we and Lilly have agreed. Under the master separation agreement, until the transfer of such assets and the assumption of such liabilities have occurred, the benefits and burdens relating to any such assets and liabilities generally will inure, after the offering, to the entity who would have received such asset or liability, had it been transferred prior to completion of the offering, including, in the case of the jurisdictions in which Lilly and we have agreed to defer the applicable transfers and assumptions, by calculating the net economic benefit and detriment attributable to such assets and liabilities and making payments in connection therewith in the manner agreed upon by us and Lilly. If, despite Lilly and our cooperating with one another and using our respective commercially reasonable efforts, the transfers and assumptions of the applicable assets and liabilities in one or more of such jurisdictions has not occurred on or prior to the date previously agreed upon in writing by us and Lilly, then Lilly shall be paid any remaining consideration retained by us as restricted cash, and shall be entitled to retain, sell, transfer or otherwise dispose of any such remaining asset or liability, in its sole discretion.
We and Lilly have agreed to cooperate with each other and use our respective commercially reasonable efforts to take or cause to be taken all actions, and to do, or cause to be done, all things reasonably necessary, proper or advisable under applicable law, regulations and agreements to consummate and make effective the transactions contemplated by the master separation agreement and the other transaction documents.
Distribution. Lilly has indicated that after this offering it currently intends to effect the Distribution. The master separation agreement provides that we will cooperate with Lilly in all respects to accomplish the Distribution.
Insurance. We will continue to enjoy coverage under Lilly's existing insurance policies until such time as Lilly holds 50% or less of our then outstanding common stock, subject to certain exceptions. After that time, we will arrange for our own insurance policies and will no longer seek benefit from any of Lilly's or its affiliates' insurance policies that may provide coverage for claims
168
relating to the animal health businesses prior to the date on which we obtain our own insurance coverage. The master separation agreement contains procedures for the administration of insured claims and allocates the right to claim coverage and control over the prosecution and defense of claims between us and Lilly.
Mutual releases and Indemnification. Except for each party's obligations under the master separation agreement, the other transaction documents and certain other specified liabilities, under the master separation agreement, we and Lilly will release and discharge the other from any and all liabilities existing or arising from acts or events that occur (or fail to occur) prior to the completion of this offering.
We will indemnify, defend and hold harmless Lilly, each of its affiliates and each of its and their respective directors, officers, managers, members, employees and agents from and against any and all losses relating to, arising out of or resulting from, among others:
- •
- the liabilities of the animal health businesses that are allocated to us;
- •
- any breach by us or our subsidiaries of the master separation agreement or any other transaction document;
- •
- any untrue statement or omission of a material fact in Lilly's governmental or public filings, to the extent caused by information furnished by
us or incorporated by reference from our public filings; or
- •
- any untrue statement or omission of a material fact in our governmental or public filings, to the extent not caused by information furnished by Lilly.
Lilly will indemnify, defend and hold harmless the Company, each of our affiliates and each of our and their respective directors, officers, managers, members, employees and agents from and against any and all losses relating to, arising out of or resulting from, among others:
- •
- the liabilities allocated to Lilly under the master separation agreement;
- •
- any breach by Lilly or its subsidiaries of the master separation agreement or any other transaction document;
- •
- any untrue statement or omission of a material fact in our governmental or public filings, to the extent caused by information furnished by
Lilly or incorporated by reference from Lilly's public filings; or
- •
- any untrue statement or omission of a material fact in Lilly's governmental or public filings, to the extent not caused by information furnished by us.
Exchange of Information. The master separation agreement provides for the mutual sharing of information between Lilly and us in order to comply with applicable law, reporting, filing, audit or tax requirements or other applicable obligations, or for use in judicial or other proceedings after the completion of this offering.
Financial Reporting Covenants. Under the master separation agreement, we have agreed to comply with certain covenants relating to our financial reporting for so long as Lilly is required to consolidate our results of operations and financial position or to account for its investment in us under the equity method of accounting. These include covenants regarding:
- •
- delivery or supply of monthly, quarterly and annual financial information and annual budgets and financial projections to Lilly;
169
- •
- disclosure of information about our financial controls to Lilly; and
- •
- restrictions on modifications to our existing accounting policies and procedures.
We have also agreed that, for so long as Lilly provides us services under the transitional services agreement, we will not change our auditor, nor will we engage our auditor for any non-audit services, in each case, without Lilly's prior consent, and we will generally implement and maintain Lilly's business practices and standards in accordance with certain policies and procedures specified by Lilly, subject to appropriate adjustments to materiality thresholds.
Other covenants. The master separation agreement also contains certain other covenants that place restrictions on our actions, including:
- •
- if Lilly effects a Distribution, then until Lilly and its affiliates beneficially own none of our voting shares, and if Lilly does not effect a
Distribution, then for as long as Lilly and its affiliates beneficially own at least 30% of our voting shares, we will be precluded from taking any action that has the effect, directly or indirectly,
of restricting or limiting Lilly or its affiliates from freely selling, transferring, assigning, pledging or disposing of our common stock;
- •
- for so long as we and Lilly are affiliates of each other, we and Lilly also agree not to, and will cause our respective affiliates not to, take
or fail to take any action that would cause the other to be in breach of or in default under certain material contracts; and
- •
- if Lilly effects a Distribution, then until Lilly and its affiliates beneficially own none of our voting shares, and if Lilly does not effect a Distribution, then for as long as Lilly and its affiliates beneficially own a majority of our then outstanding common stock, and except with respect to certain specified exceptions or to the extent that a designated policy or procedure conflicts with our amended and restated articles of incorporation or bylaws or any transaction document, we and Lilly otherwise agree or as set forth in any other transaction document, we will consistently implement and maintain Lilly's business practices and standards in accordance with certain policies and procedures specified by Lilly, subject to appropriate adjustments to materiality thresholds.
Board representation. The master separation agreement will provide that, for so long as Lilly and its affiliates beneficially own at least 10% of our voting shares, Lilly will be entitled to designate for nomination the number of representatives on the board of directors that is proportionate to its ownership of our voting shares (rounding up to the nearest whole number of directors) and to designate at least one director to each committee of the board of directors other than the Audit Committee. For the avoidance of doubt, so long as Lilly and its affiliates beneficially own at least 80% of our voting shares, Lilly will designate for nomination at least 80% of the members of the board of directors (rounding up to the nearest whole number of directors). In addition, so long as Lilly and its affiliates beneficially own a majority of our voting shares, Lilly will be entitled to designate the chairman of the board of directors and to designate a majority of the members on each committee of the board of directors.
Approval rights. If Lilly effects a Distribution, then until Lilly and its affiliates beneficially own none of our voting shares, and if Lilly does not effect a Distribution, then for so long as Lilly and its affiliates beneficially own a majority of our then outstanding common stock, in each case subject to certain exceptions, we will be required to obtain Lilly's prior written approval before undertaking (or permitting or authorizing any of our subsidiaries to undertake) various significant corporate actions, including:
- •
- consolidation or merger transactions;
170
- •
- dissolution, liquidation or winding up;
- •
- incurrence of any indebtedness (as defined in the master separation agreement), other than pursuant to existing debt obligations or unsecured
lines of credit as of the date of completion of the offering;
- •
- altering, amending, terminating, repealing or adopting any provisions inconsistent with our amended and restated articles of incorporation or
our amended and restated bylaws (unless required to comply with applicable law); or
- •
- the issuance, purchase, redemption or other acquisition or retirement for value of any of our equity securities (other than deemed repurchases resulting from the exercise of stock options or tax withholdings).
No solicitation of employees. Subject to certain customary exceptions, for a period of 12 months following the date on which Lilly and its affiliates no longer own a majority of our outstanding shares of common stock, neither we or our affiliates, nor Lilly or its affiliates, will directly or indirectly solicit or encourage any employee of the other party at the level of senior director and above to leave his or her employment without the prior written consent of the other party.
Dispute resolution. The master separation agreement provides that we and Lilly will use our respective commercially reasonable efforts to resolve disputes expeditiously and on a mutually acceptable negotiated basis by our senior level representatives. Any disputes unable to be resolved through such process will be referred to mediation, for non-binding resolution. Subject to compliance with the terms of the master separation agreement, either we or Lilly, following the escalation and mediation procedures in the master separation agreement, may submit a dispute to a court of competent jurisdiction in Indiana.
Term. Following completion of the offering, the master separation agreement will continue unless terminated by the mutual consent of us and Lilly, although certain rights and obligations may terminate upon a reduction in Lilly's ownership of our outstanding common stock.
Transitional Services Agreement
Historically, Lilly has provided us significant shared services and resources related to corporate functions such as executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations, which we refer to collectively as the "Lilly Services." The transitional services agreement will become operative as of the completion of this offering and the agreement will continue until the expiration or termination of the last Lilly Service to expire or be terminated, unless the agreement is earlier terminated according to the terms of the transitional services agreement.
Under the transitional services agreement, we will be able to use Lilly Services for a fixed term established on a service-by-service basis. Partial reduction in the provision of any Lilly Service or termination of a Lilly Service prior to the expiration of the applicable fixed term requires Lilly's consent. In addition, either party will be able to terminate the agreement due to a material breach of the other party, upon prior written notice, subject to limited cure periods or if the other party undergoes a change of control.
We will pay Lilly mutually agreed-upon fees for the Lilly Services provided under the transitional services agreement, which will be based on Lilly's cost (including third-party costs) of providing the Lilly Services through March 31, 2021 and subject to a mark-up of 7% thereafter, with additional inflation-based escalation beginning January 1, 2020.
171
Tax Matters Agreement
Allocation of taxes. We intend to enter into a tax matters agreement with Lilly immediately prior to the completion of this offering that will govern the parties' respective rights, responsibilities and obligations with respect to tax liabilities and benefits, tax attributes, the preparation and filing of tax returns, the control of audits and other tax proceedings and other matters regarding taxes. In general, under the agreement:
- •
- Lilly will be responsible for any U.S. federal, state, local or foreign taxes (and any related interest, penalties or audit adjustments and
including those taxes attributable to our business) reportable on a consolidated, combined or unitary return that includes Lilly or any of its subsidiaries (and us and/or any of our subsidiaries) for
any periods or portions thereof ending on or prior to the date of the closing of this offering. We will be responsible for the portion of any such taxes for periods or portions thereof beginning after
such date, as would be applicable to us if we filed the relevant tax returns on a standalone basis.
- •
- We will be responsible for any U.S. federal, state, local or foreign taxes (and any related interest, penalties or audit adjustments) that are
reportable on returns that include only us and/or any of our subsidiaries, for all tax periods whether before or after the completion of this offering.
- •
- Lilly will be responsible for certain taxes imposed on Lilly and/or any of its subsidiaries and us and/or any of our subsidiaries arising from, or attributable to, certain transfers of assets or liabilities in the Separation.
We will not generally be entitled to receive payment from Lilly in respect of any of our tax attributes or tax benefits or any reduction of taxes of Lilly. Neither party's obligations under the agreement will be limited in amount or subject to any cap. The agreement will also assign responsibilities for administrative matters, such as the filing of returns, payment of taxes due, retention of records and conduct of audits, examinations or similar proceedings. In addition, the agreement provides for cooperation and information sharing with respect to tax matters.
Lilly will be primarily responsible for preparing and filing any tax return with respect to the Lilly affiliated group for U.S. federal income tax purposes and with respect to any consolidated, combined, unitary or similar group for U.S. state or local or foreign tax purposes that includes Lilly or any of its subsidiaries (including those that also include us and/or any of our subsidiaries), as well as any tax return that includes only Lilly and/or any of its subsidiaries (including such tax returns that reflect taxes attributable to our business). We will generally be responsible for preparing and filing any tax returns that include only us and/or any of our subsidiaries.
The party responsible for preparing and filing a given tax return will generally have exclusive authority to control tax contests related to any such tax return. We will generally have exclusive authority to control tax contests with respect to tax returns that include only us and/or any of our subsidiaries.
Preservation of the tax-free status of certain aspects of the Separation. We and Lilly intend the Separation, the transfer of net cash proceeds from this offering and the Debt Transactions to Lilly and the potential Distribution to qualify as a reorganization pursuant to which no gain or loss is recognized by Lilly or its shareholders for federal income tax purposes under Sections 355, 368(a)(1)(D) and related provisions of the Code. In addition, we and Lilly intend for the Separation, the potential Distribution and certain related transactions to qualify for tax-free treatment under U.S. federal, state and local tax law and/or foreign tax law.
Lilly expects to receive opinions from its outside tax advisors to the effect that, among other things, the Separation, the transfer of net cash proceeds from this offering and the Debt
172
Transactions to Lilly and the potential Distribution will qualify as a transaction that is tax-free for U.S. federal income tax purposes under Sections 355 and 368(a)(1)(D) of the Code. In addition, Lilly expects to receive opinions from its outside tax advisors regarding the tax-free status of certain related transactions. In connection with the opinions, we and Lilly will make certain representations regarding the past and future conduct of our respective businesses and certain other matters.
We will also agree to certain covenants that contain restrictions intended to preserve the tax-free status of the Separation, the transfer of net cash proceeds from this offering and the Debt Transactions to Lilly, the potential Distribution and certain related transactions. We may take certain actions prohibited by these covenants only if Lilly receives a private letter ruling from the IRS or we obtain and provide to Lilly an opinion from a U.S. tax counsel or accountant of recognized national standing, in either case acceptable to Lilly in its sole and absolute discretion, to the effect that such action would not jeopardize the tax-free status of these transactions. We will be barred from taking any action, or failing to take any action, where such action or failure to act adversely affects or could reasonably be expected to adversely affect the tax-free status of these transactions, for all time periods. In addition, during the time period ending two years after the date of the potential Distribution these covenants will include specific restrictions on our:
- •
- issuance or sale of stock or other securities (including securities convertible into our stock but excluding certain compensatory
arrangements);
- •
- sales of assets outside the ordinary course of business; and
- •
- entering into any other corporate transaction which would cause us to undergo a 40% or greater change in our stock ownership.
We will generally agree to indemnify Lilly and its affiliates against any and all tax-related liabilities incurred by them relating to the Separation, the transfer of net cash proceeds from this offering and the Debt Transactions to Lilly, the potential Distribution and/or certain related transactions to the extent caused by an acquisition of our stock or assets or by any other action undertaken by us. This indemnification provision will apply even if Lilly has permitted us to take an action that would otherwise have been prohibited under the tax-related covenants described above.
Employee Matters Agreement
We intend to enter into an employee matters agreement with Lilly immediately prior to the completion of this offering. The employee matters agreement will govern Lilly's, our and the parties' respective subsidiaries' and affiliates' rights, responsibilities and obligations after this offering with respect to employees, compensation, employment, employee benefit plans and related matters. Below is a summary or what we anticipate to be the terms of the employee matters agreement.
Benefit plans generally. Prior to the completion of this offering, except with respect to any cash bonus or equity compensation plans, we will be a participating employer in the Lilly benefit plans in which our employees currently participate to the extent permitted under the plans. We will cease to be a participating employer in the Lilly plans and will adopt our own benefit plans on a date following the completion of this offering, which we refer to as the "Plan Transition Date." Except as otherwise agreed to by the parties, the Plan Transition Date will be the earlier of January 1, 2019 or, with respect to Lilly plans sponsored or maintained primarily for our employees in the United States, the date we are no longer a member of a controlled group with Lilly. An appropriate allocation of our costs incurred under the Lilly benefit plans prior to the Plan Transition Date shall be charged back to us. Lilly will retain the right to amend or terminate its plans. As of the completion of this offering, we intend to adopt or retain, as applicable, cash bonus and equity compensation plans for our employees. Under the cash bonus plans, we will pay our employees on generally the same basis as in effect prior to the offering for the performance period which includes
173
the offering and will assume any liability for the payment of bonuses under Lilly's bonus plans, to the extent applicable. As of the Plan Transition Date, we will establish benefit plans that are comparable to the Lilly plans in which our employees participated prior to the Plan Transition Date. The employee matters agreement does not obligate us to establish any defined benefit pension plan, retiree medical plan or nonqualified plan for our U.S. employees, unless required by law or an applicable collective bargaining agreement, and all liabilities relating to any such plan maintained by Lilly shall remain with Lilly (other than as provided for under "Non-U.S. retirement benefit arrangements").
Employment. We generally intend to offer employment, prior to the completion of this offering, to certain employees who are providing services to our business and who are not otherwise transferring to our entities by operation of law. We refer to the date on which any such transferring employee will be considered to be employed by us (either by operation of law or acceptance of an offer) for purposes of the employee matters agreement as the "Employee Transfer Date." To the extent that severance or termination obligations are triggered by or result from such transfers, or our failure to make offers or continue employment as required by the employee matters agreement, or are required to be paid under applicable law or a Lilly plan Lilly will administer the severance pay or termination pay obligations in accordance with the terms and conditions of the applicable Lilly severance pay or termination pay plan or policy, or as otherwise required by applicable law, and we will indemnify Lilly for such liability. If any of our U.S. employees begins long-term disability leave before the applicable Plan Transition Date, his or her employment will be transferred to Lilly immediately prior the Plan Transition Date. For the period starting as of the completion of this offering and ending on December 31, 2019, our employees will be entitled to receive: (A) (i) at least the same salary or wages, and cash bonus opportunities at target, (ii) equity incentive commitments equal to the equity budget value and (iii) other material terms and conditions of employment as such employees were provided immediately before January 1, 2019; and (B) employee benefits and perquisites (other than cash bonus opportunities, equity incentive commitments, defined benefit pension, retiree medical and nonqualified benefits) that are substantially comparable in the aggregate to the employee benefits and perquisites that such employees were provided under applicable plans of Lilly before January 1, 2019. We will use reasonable efforts to assume, as of the Employee Transfer Date, any applicable employment agreements or other individual benefit or compensation agreement entered into between Lilly and a transferring employee and will indemnify Lilly for all liabilities under such agreements. In addition, we expect to provide to any of our employees whose employment is terminated during the period ending on December 31, 2019, a level of severance benefits that is equal to the greater of (i) the severance benefits the employee would have received under the applicable Lilly plans in effect immediately before January 1, 2019, or (ii) the severance benefits provided under our severance arrangements applicable to similarly-situated employees, in each case, calculated based on the employee's compensation and service.
Unions and collective bargaining agreements. The parties will cooperate to inform and consult with any applicable representative of a labor union, or similar organization, covering any transferring employee, to the extent required by law or the applicable collective bargaining agreement or similar arrangement. As of the Employee Transfer Date, we will assume any collective bargaining, or similar, agreements or arrangements covering any such employees and indemnify Lilly for all related liabilities.
Credited service. We will cause our employee benefit plans to credit our employees, without duplication of benefits, for service with Lilly on or prior to the Employee Transfer Date, and for service with us on or following the Employee Transfer Date, for purposes of eligibility and vesting under all of our employee benefit plans and arrangements and computation of vacation, sick days or severance benefits, or as may otherwise be required by applicable law.
174
U.S. defined benefit and retiree medical plans. Following the Plan Transition Date, U.S. employees will generally be eligible to receive credit for service with us for vesting and eligibility service (but not benefit service) under the Eli Lilly Retirement Plan and the Eli Lilly and Company Retiree Health Plan through a date not later than December 31, 2023, to the extent permitted by and subject to the terms of such plans.
U.S. defined contribution plans. We will establish a 401(k) plan for our U.S. employees effective as of the Plan Transition Date with terms that are substantially similar to Lilly's 401(k) plan, except that it will provide for a 6% Company match and 3% non-elective contribution. Any transferring employee whose Employee Transfer Date is on or before January 1, 2019 will be 100% vested in our 401(k) plan. We will accept the transfer from the U.S. Lilly qualified defined contribution plan to our qualified defined contribution plan of any assets and liabilities allocable to the participants transferring to us. Our employees will be 100% vested in their account balances under the Lilly qualified defined contribution benefit plan as of the Plan Transition Date, and will cease being eligible to receive any employer contributions from Lilly effective December 31, 2018.
U.S. Lilly nonqualified plans. As of the Plan Transition Date, the Eli Lilly Excess Savings Plan and Deferred Compensation Plan will be amended so that any transferring employee will cease being eligible for contributions from Lilly for services rendered after December 31, 2018. Following the Plan Transition Date, U.S. employees will generally be eligible to receive credit for service with us for vesting and eligibility service (but not benefit service) under the Eli Lilly Excess Benefit Retirement Plan through a date not later than December 31, 2023, to the extent permitted by and subject to the terms of the plan.
Non-U.S. retirement benefit arrangements. The employee matters agreement provides for the transfer from Lilly to us of any retirement benefit arrangement covering our employees located outside of the U.S. and of any related obligations or liabilities, unless otherwise agreed by the parties.
Lilly equity compensation. Prior to the completion of the offering, the board of directors of Lilly will determine how any Lilly equity, equity-related and long-term performance awards granted to our transferring employees will be treated under the applicable Lilly plans.
Toll Manufacturing and Supply Agreement
We intend to enter into a toll manufacturing and supply agreement with Lilly immediately prior to the completion of this offering. Lilly has historically manufactured Humatrope drug substance for use in the human health field at its Speke manufacturing site, which site is being transferred to us in connection with this offering. Under the toll manufacturing and supply agreement, we will continue to exclusively manufacture Humatrope for Lilly at the Speke site until December 31, 2020; provided, however, that such obligation may continue through December 31, 2023 (through the exercise of three one-year extensions) if Lilly's replacement third party supplier of Humatrope has not received all necessary governmental approvals or cannot meet Lilly's volume requirements.
The tolling fee that we charge Lilly for the provision of such manufacturing and supply services is based on local value added plus a reasonable arm's length mark-up. By October 1 of each calendar year during the term of the toll manufacturing and supply agreement, we will mutually agree upon a new tolling fee to be effective for the following calendar year.
Under the toll manufacturing and supply agreement, we agree not to manufacture or sell any product that is competitive to Humatrope for a period of five years after the expiration or termination of the agreement. In addition, during the term of the agreement, we agree not to manufacture any product other than Humatrope at certain buildings of the Speke manufacturing site without Lilly's consent.
175
Intellectual Property and Technology License Agreement
We intend to enter into an intellectual property and technology license agreement with Lilly immediately prior to the completion of this offering. Under the intellectual property and technology license agreement, Lilly will grant us an exclusive, perpetual license to exploit products in the animal health field that utilize or use certain of Lilly's intellectual property (excluding trademarks). In addition, Lilly will grant us a non-exclusive, non-sublicenseable license to screen certain compounds in Lilly's compound libraries to exploit products in the animal health field that utilize or use certain of Lilly's intellectual property. This screening license will have an initial term of two years, subject to three one-year extensions, each of which requires Lilly's consent.
If Elanco makes any improvements to the licensed intellectual property, Elanco shall retain ownership of such improvements and provide Lilly with a non-exclusive, perpetual license to use the intellectual property in fields outside animal health (including human health).
For a period of two years following the effective date of the intellectual property and technology license agreement, each party will have a right of first offer with respect to third-party offers that the other party receives to license such other party's intellectual property in the first party's field (animal health versus human health). In connection with such right, we will negotiate exclusively as to such offer for the use of the other party's intellectual property in the first party's field.
Under the intellectual property and technology license agreement, we will provide quarterly reports to Lilly describing any know-how generated under the agreement, including inventions, patentable subject matter, discoveries, and technical data. Elanco will retain ownership of such generated know-how and will provide Lilly with a non-exclusive, perpetual license to use the know-how in fields outside animal health (including human health).
Transitional Trademark License Agreement
We intend to enter into a transitional trademark license agreement with Lilly immediately prior to the completion of this offering. Under the transitional trademark license agreement, Lilly will grant us a transitional license to use certain of Lilly's trademarks for a period of time following the completion of this offering. Such license will be non-exclusive and royalty-free, and will allow us to use certain of Lilly's trademarks on our product packaging, any advertising materials used in connection with the sale and distribution of our products, and generally in connection with the sale and distribution of our products and in the day-to-day operation of our business (including in our books and records).
Such license will terminate on a product-by-product and country-by-country basis. The term of the license will not extend beyond four years; provided, however, that the license can extend for one additional year (beyond such four years) if the parties mutually agree upon such extension. Lilly can terminate the transitional trademark license agreement due to our breach of such agreement, upon prior written notice, subject to a limited cure period.
Registration Rights Agreement
We intend to enter into a registration rights agreement with Lilly immediately prior to the completion of this offering, pursuant to which we will agree that, upon the request of Lilly, we will use our reasonable best efforts to effect the registration under applicable federal and state securities laws of any shares of our common stock retained by Lilly following this offering.
Demand registration. Lilly will be able to request registration under the Securities Act of all or any portion of our shares covered by the agreement and we will be obligated, subject to limited exceptions, to register such shares as requested by Lilly. Lilly will be able to request that we
176
complete two demand registrations, in the aggregate, and four underwritten offerings, in the aggregate, in a twelve-month period, in each case subject to limitations on minimum offering size. Lilly will be able to designate the terms of each offering effected pursuant to a demand registration.
Piggyback registration. If we at any time intend to file on our behalf or on behalf of any of our other security holders a registration statement in connection with a public offering of any of our securities on a form and in a manner that would permit the registration for offer and sale of our common stock held by Lilly, Lilly will have the right to include its shares of our common stock in that offering.
Registration expenses. We will be generally responsible for all registration expenses in connection with the performance of our obligations under the registration rights provisions in the registration rights agreement. Lilly is responsible for its own internal fees and expenses, any applicable underwriting discounts or commissions and any stock transfer taxes.
Indemnification. Generally, the agreement will contain indemnification and contribution provisions by us for the benefit of Lilly and, in limited situations, by Lilly for the benefit of us with respect to the information provided by or failed to be provided by Lilly included or omitted, as applicable, in any registration statement, prospectus or related document.
Transfer. If Lilly transfers shares covered by the agreement, it will be able to transfer the benefits of the registration rights agreement to transferees of at least 5% of the shares of our common stock outstanding immediately following the completion of this offering, provided that each transferee agrees to be bound by the terms of the registration rights agreement.
Term. The registration rights will remain in effect with respect to any shares covered by the agreement until:
- •
- such shares have been sold pursuant to an effective registration statement under the Securities Act;
- •
- such shares have been sold to the public pursuant to Rule 144 under the Securities Act;
- •
- such shares may be sold to the public pursuant to Rule 144 under the Securities Act without being subject to the volume restrictions in
such rule; or
- •
- such shares have been sold in a transaction in which the transferee is not entitled to the benefits of the registration rights agreement.
Directed Share Program
At our request, the underwriters have reserved up to % of the shares of our common stock offered by this prospectus for sale, at the initial public offering price, to our directors, officers and certain employees and other parties with a connection to us, to the extent permitted by local securities laws. If purchased by our directors or officers, these shares will be subject to a 180-day lock-up restriction and if purchased by other individuals, these shares will be subject to a -day lock-up restriction. See "Underwriting" for more information.
Policy Concerning Related Person Transactions
Prior to the completion of this offering, our board of directors will adopt a written policy, which we refer to as the related person transaction approval policy, for the review of any transaction, arrangement or relationship in which we are a participant, if the amount involved exceeds $120,000 and one of our executive officers, directors, director nominees or beneficial holders of more than 5% of our total equity (or their immediate family members), each of whom we refer to as a related
177
person, has a direct or indirect material interest. This policy was not in effect when we entered into the transactions described above.
Each of the agreements between us and Lilly and its subsidiaries that have been entered into prior to the completion of this offering, and any transactions contemplated thereby, will be deemed to be approved and not subject to the terms of such policy. If a related person, other than Lilly and its affiliates, proposes to enter into such a transaction, arrangement or relationship, which we refer to as a related person transaction, the related person must report the proposed related person transaction to our Audit Committee. The policy calls for the proposed related person transaction to be reviewed and, if deemed appropriate, approved by the Audit Committee. In approving or rejecting such proposed transactions, the Audit Committee will be required to consider relevant facts and circumstances. The Audit Committee will approve only those transactions that, in light of known circumstances, are deemed to be in our best interests. In the event that any member of the Audit Committee is not a disinterested person with respect to the related person transaction under review, that member will be excluded from the review and approval or rejection of such related person transaction; provided, however, that such Audit Committee member may be counted in determining the presence of a quorum at the meeting of the Audit Committee at which such transaction is considered. If we become aware of an existing related person transaction which has not been approved under the policy, the matter will be referred to the Audit Committee. The Audit Committee will evaluate all options available, including ratification, revision or termination of such transaction. In the event that management determines that it is impractical or undesirable to wait until a meeting of the Audit Committee to consummate a related person transaction, the chairman of the Audit Committee may approve such transaction in accordance with the related person transaction approval policy. Any such approval must be reported to the Audit Committee at its next regularly scheduled meeting.
A copy of our related person transaction approval policy will be available on our website upon completion of this offering.
178
DESCRIPTION OF MATERIAL INDEBTEDNESS
Senior Notes Offering
On August 28, 2018, we issued $2,000,000,000 aggregate principal amount of our Senior Notes in a private placement, which we refer to as the "Senior Notes Offering." The Senior Notes are comprised of $500,000,000 aggregate principal amount of our 3.912% Senior Notes due 2021, $750,000,000 aggregate principal amount of our 4.272% Senior Notes due 2023 and $750,000,000 aggregate principal amount of our 4.900% Senior Notes due 2028. The interest rate payable on each series of Senior Notes is subject to adjustment if Moody's Investor Services, Inc. or Standard & Poor's Financial Services LLC downgrades, or subsequently upgrades, its ratings on the respective series of Senior Notes. We will pay the net proceeds that we received in the Senior Notes Offering to Lilly in connection with the Separation, subject to certain limitations. See "The Separation and Distribution Transactions—The Separation" and "Use of Proceeds."
The Senior Notes are governed by an indenture and supplemental indenture between us and Deutsche Bank Trust Company Americas, as trustee, which we refer to collectively as the "indenture." The indenture contains certain covenants, including limitations on our and certain of our subsidiaries' ability to incur liens or engage in sale leaseback transactions. The indenture also contains restrictions on our ability to consolidate, merge or sell substantially all of our assets. In addition, the indenture contains other customary terms, including certain events of default, upon the occurrence of which, the Senior Notes may be declared immediately due and payable.
Pursuant to the indenture, we are able to redeem the Senior Notes of any series, in whole or in part, at any time by paying a "make whole" premium, plus accrued and unpaid interest to, but excluding, the date of redemption. Upon the occurrence of a change of control of us and a downgrade of the Senior Notes below an investment grade rating by two or more ratings agencies (or one rating agency, if only one rating agency rates the Senior Notes), we are, in certain circumstances, required to make an offer to purchase each of the Senior Notes at a price equal to 101% of the aggregate principal amount of the Senior Notes together with accrued and unpaid interest to, but excluding, the date of repurchase.
Credit Facilities
Prior to the completion of this offering, we intend to enter into a revolving credit agreement with a syndicate of banks providing for a five-year $750 million senior unsecured revolving credit facility, with the ability (subject to certain conditions) to incur additional incremental commitments of up to $250 million (the "Revolving Facility"), and a term credit agreement with a syndicate of banks providing for a three-year senior unsecured term credit facility in an amount of $500 million (the "Term Facility" and, together with the Revolving Facility, the "Credit Facilities"). The Credit Facilities will not be available for borrowings until the date on which certain conditions, including the completion of this offering, are satisfied. We expect that these conditions will be met concurrently with the completion of this offering. The commitments with respect to the Credit Facilities will terminate if this offering is not completed by June 30, 2019. The Credit Facilities are not guaranteed by our subsidiaries.
Additionally, we will pay a ticking fee on the commitments of each lender with respect to the Term Facility during the period from and including the date of execution of the specific documentation relating to the Term Facility but excluding the date on which such commitments with respect to the Term Facility terminate. With respect to the Revolving Facility, we will pay a facility fee on the aggregate amount of the Revolving Facility (whether drawn or undrawn) during the period from and including the date of execution of the specific documentation relating to the Revolving Facility but excluding the date on which commitments under the Revolving Facility terminate. The applicable margins, the ticking fee and the facility fee are determined based on public ratings of our
179
senior unsecured non-credit enhanced credit rating. Interest on base rate borrowings, the ticking fee and the facility fee are generally payable quarterly in arrears; however, for loans bearing interest based on a Eurocurrency rate, interest is payable on the last day of the applicable interest period and, in the case of any interest period of more than three months' duration, each day prior to the last day of such interest period that occurs at intervals of three months' duration after the first day of such interest period.
We may voluntarily prepay loans and/or reduce the commitment under the Credit Facilities, in whole or in part, without penalty or premium, subject to certain minimum amounts and increments and the payment of customary breakage costs. No mandatory prepayment is required under the Credit Facilities. The Term Facility is subject to amortization payments, payable on the last day of each quarter.
The Credit Facilities contain a financial covenant requiring us to not exceed a maximum consolidated leverage ratio and a financial covenant to maintain a minimum interest coverage ratio. In addition, the Credit Facilities contain customary affirmative and negative covenants that, among other things, limit or restrict our and/or our subsidiaries' ability, subject to certain exceptions, to incur liens, merge, consolidate or sell or otherwise transfer assets, to enter into transactions with our affiliates and incur subsidiary indebtedness. The Credit Facilities also contain customary events of default.
180
In connection with this offering, we will amend and restate our articles of incorporation and bylaws. Copies of the forms of our amended and restated articles of incorporation and bylaws are filed as exhibits to the registration statement of which this prospectus forms a part. The provisions of our articles of incorporation and bylaws and relevant sections of the Indiana Business Corporation Law (the "IBCL"), are summarized below. The following summary is qualified in its entirety by the provisions of our amended and restated articles of incorporation and bylaws and is subject to the applicable provisions of the IBCL.
Authorized Capitalization
Our authorized capital stock shall consist of shares of common stock, no par value, and shares of preferred stock, no par value. Following the completion of this offering, shares of common stock and no shares of preferred stock shall be issued and outstanding.
Common Stock
Holders of our common stock are entitled to the rights set forth below.
Voting Rights
Each outstanding share of our common stock will be entitled to one vote on all matters submitted to a vote of our shareholders. Directors will be elected by a plurality of the votes entitled to be cast. Our shareholders will not have cumulative voting rights. Except as otherwise provided in our amended and restated articles of incorporation or as required by law, all matters to be voted on by our shareholders other than matters relating to the election and removal of directors shall be approved if votes cast in favor of the matter exceed the votes cast opposing the matter at a meeting at which a majority of the outstanding shares entitled to vote on such matter is represented in person or by proxy.
Dividend Rights
Holders of our common stock will share equally in any dividend declared by our board of directors, subject to the rights of the holders of any outstanding preferred stock.
Liquidation Rights
In the event of any voluntary or involuntary liquidation, dissolution or winding up of our affairs, holders of our common stock would be entitled to share ratably in our assets that are legally available for distribution to shareholders. If we have any preferred stock outstanding at such time, holders of the preferred stock may be entitled to distribution and/or liquidation preferences. In either such case, we must pay the applicable distribution to the holders of our preferred stock before we may pay distributions to the holders of our common stock.
Registration Rights
Lilly will be entitled to certain rights relating to the registration of our shares of common stock pursuant to a registration rights agreement. See "Certain Relationships and Related Party Transactions — Relationship with Lilly — Registration Rights Agreement."
181
Other Rights
Our shareholders have no preemptive or other rights to subscribe for additional shares. All outstanding shares are, and all shares offered by this prospectus will be, when sold, validly issued, fully paid and nonassessable.
Preferred Stock
Our board of directors is authorized to provide for one or more series of preferred stock and to fix the terms of such preferred stock, including the preferences, powers and relative, participating, optional or other special rights and qualifications, limitations or restrictions thereof, including the dividend rate, conversion rights, voting rights, redemption rights and liquidation preferences and to fix the number of shares to be included in any such series without any further vote or action by our shareholders. Any preferred stock so issued may rank senior to our common stock with respect to the payment of dividends or amounts upon liquidation, dissolution or winding up, or both. In addition, any such shares of preferred stock may have class or series voting rights. The issuance of preferred stock may have the effect of delaying, deferring or preventing a change in control of our company without further action by the shareholders and may adversely affect the voting and other rights of the holders of our common stock. Our board of directors has not authorized the issuance of any shares of preferred stock, and we have no agreements or plans for the issuance of any shares of preferred stock.
Anti-Takeover Effects of Our Amended and Restated Articles of Incorporation and our Amended and Restated Bylaws
Our amended and restated articles of incorporation and our amended and restated bylaws will contain certain provisions that may be deemed to have an anti-takeover effect and may delay, deter or prevent a tender offer or takeover attempt that a shareholder might consider in its best interest, including those attempts that might result in a premium over the market price for the shares held by shareholders.
Special Meetings
Our amended and restated bylaws will provide that special meetings of holders of common stock may be called only by our board of directors or the Chairman of the board of directors. Holders of our common stock will not be permitted to call a special meeting or to require that our board of directors call a special meeting of shareholders.
Advance Notice Procedures
Our amended and restated bylaws will establish an advance notice procedure for the nomination, other than by or at the direction of our board of directors, of candidates for election as directors as well as for other shareholder proposals to be considered at annual meetings of shareholders. In general, our amended and restated bylaws will provide that notice of intent to nominate a director or raise business at such meetings must be received by us not less than 120 days nor more than 150 days prior to the date on which our proxy statement is released to shareholders in connection with the previous year's annual meeting, or in the event that no annual meeting was held in the previous year, or the date of the annual meeting has been changed by more than 30 days from the date contemplated at the time of the previous year's proxy statement, notice by the proposing shareholder to be timely must be received not later than the close of business on the later of 120 days in advance of such meeting or 10 days following the date on which public disclosure of the date of the meeting is first made and, in each case, must contain
182
certain specified information concerning the person to be nominated or the matters to be brought before the meeting and concerning the shareholder submitting the proposal.
Classified Board
Our amended and restated articles of incorporation and our amended and restated bylaws will provide for our board of directors to be divided into three classes of directors, as nearly equal in number as possible, serving staggered terms of office. Approximately one-third of our board of directors will be elected each year to three-year terms of office, and our directors (other than directors appointed by holders of preferred stock) may be removed only for cause and only upon the affirmative vote of holders of at least 662/3% of our outstanding voting stock.
Under Section 23-1-39-1 of the IBCL, only our board of directors can amend, and shareholders do not have the right to amend, our amended and restated bylaws.
Conflicts of Interest; Corporate Opportunities
In order to address potential conflicts of interest between us and Lilly, our amended and restated articles of incorporation will contain certain provisions regulating and defining the conduct of our affairs to the extent that they may involve Lilly and its officers and directors and our rights, powers, duties and liabilities and those of our officers, directors and shareholders in connection with our relationship with Lilly. In general, these provisions recognize that we and Lilly may engage in the same or similar business activities and lines of business or have an interest in the same areas of corporate opportunities and that we and Lilly will continue to have contractual and business relations with each other, including officers of Lilly serving as our directors.
Our amended and restated articles of incorporation will provide that Lilly will have no duty to communicate information regarding a corporate opportunity to us or to refrain from engaging in the same or similar lines of business or doing business with any of our clients, customers or vendors. Moreover, our amended and restated articles of incorporation will provide that for so long as Lilly owns a majority of all our outstanding voting shares, in the event that any of our directors or officers who is also a director, officer and/or employee of Lilly acquires knowledge of a potential transaction or matter that may be a corporate opportunity for us and Lilly, such director and/or officer shall to the fullest extent permitted by law have fully satisfied and fulfilled his or her fiduciary duty, if any, with respect to such corporate opportunity, and we, to the fullest extent permitted by law, renounce any interest or expectancy in such business opportunity, and waive any claim that such business opportunity constituted a corporate opportunity that should have been presented to us or any of our affiliates, if he or she acts in a manner consistent with the following policy:
- •
- such corporate opportunity offered to any person who is on our board of directors (but is not an officer) and who is also a director, officer
and/or employee of Lilly shall belong to us only if such opportunity is expressly offered to such person solely in his or her capacity as our director and otherwise shall belong to Lilly; and
- •
- such corporate opportunity offered to any person who is our officer and also is a director, officer and/or employee of Lilly shall belong to us unless such opportunity is expressly offered to such person solely in his or her capacity as a director, officer and/or employee of Lilly, in which case such opportunity shall belong to Lilly.
183
Retirement Benefits
Lilly provides retirement income to eligible U.S. Lilly employees, including our Named Executive Officers, through the following plans:
- •
- The Lilly 401(k) Plan, a defined contribution plan qualified under Sections 401(a) and 401(k) of the Internal Revenue Code. Participants
may elect to contribute a portion of their base salary to the plan, and Lilly provides matching contributions on employees' contributions up to 6% of base salary up to IRS limits. The employee
contributions, Lilly contributions and earnings thereon are paid out in accordance with elections made by the participant. See the "All Other Compensation" column in the Summary Compensation Table for
information about Lilly contributions under the 401(k) Plan for our Named Executive Officers.
- •
- The Lilly Retirement Plan, a tax-qualified defined benefit plan that provides monthly benefits to retirees. See the Pension Benefits in 2017 table below for additional information about the value of these pension benefits.
Our amended and restated articles of incorporation also provide special approval procedures that may be utilized if it is deemed desirable by Lilly, us, our affiliates or any other party, that we take action with specific regard to transactions or opportunities presenting potential conflicts of interest, out of an abundance of caution, to ensure that such transactions are not voidable, or that such an opportunity or opportunities are effectively disclaimed. These special procedures include the following:
- •
- the material facts of the transaction and the director's or officer's interest are disclosed or known to the board of directors or duly
appointed committee of the board of directors and the board of directors or such committee authorizes, approves or ratifies the transaction by the affirmative vote or consent of a majority of the
directors (or committee members) who have no direct or indirect interest in the transaction and, in any event, of at least two directors (or committee members); and
- •
- the material facts of the transaction and the director's interest are disclosed or known to the shareholders entitled to vote and they authorize, approve or ratify such transaction by vote.
Any person purchasing or otherwise acquiring any interest in any shares of our common stock will be deemed to have consented to these provisions of the amended and restated articles of incorporation.
Certain Provisions of the Indiana Business Corporation Law
Shareholder Action by Unanimous Written Consent
Under Chapter 29 of the IBCL, any action required or permitted to be taken by the holders of common stock may be effected only at an annual meeting or special meeting of such holders, and shareholders may act in lieu of such meetings only by unanimous written consent.
Control Share Acquisitions
Our amended and restated articles of incorporation provide that Chapter 42 of the IBCL does not apply to us. However, we could elect to be subject to Chapter 42 in the IBCL in the future. Chapter 42 of the IBCL is designed to protect minority shareholders in the event that a shareholder acquires shares of a corporation's voting stock (referred to as control shares) within one of several specified ranges (one-fifth or more but less than one-third, one-third or more but less than a majority, or a majority or more). Upon the acquisition of control shares, the approval of the rights of the acquirer to vote the shares in excess of each level of ownership must be obtained from a majority of the disinterested shareholders before the acquiring shareholder may vote such shares.
184
Under certain circumstances, including in the event that shareholder approval is not obtained, the shares held by the acquirer may be redeemed by the corporation at the fair value of the shares as determined by the control share acquisition provision.
Certain Business Combinations
Under the business combinations provision of the IBCL, or Chapter 43, any shareholder who acquires a 10%-or-greater ownership position in an Indiana corporation with a class of voting shares registered under Section 12 of the Exchange Act (and that, like us, has not opted-out of this provision) is prohibited for a period of five years from completing a business combination (generally a merger, significant asset sale or disposition or significant issuance of additional shares) with the corporation unless, prior to the acquisition of such 10% interest, the board of directors of the corporation approved either the acquisition of such interest or the proposed business combination. If such board approval is not obtained, then five years after a 10% shareholder has become such, a business combination with the 10% shareholder is permitted if all provisions of the articles of the corporation are complied with and either a majority of disinterested shareholders approves the transaction or all shareholders receive a price per share determined in accordance with the fair price criteria of the business combinations provision of the IBCL. An Indiana corporation may elect to remove itself from the protection provided by the Indiana business combinations provision, but such an election remains ineffective for 18 months and does not apply to a combination with a shareholder who acquired a 10% ownership position prior to the election.
Limitations on Liability and Indemnification of Officers and Directors
Chapter 37 of the IBCL authorizes every Indiana corporation to indemnify its officers and directors under certain circumstances against liability incurred in connection with any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative and whether formal or informal, to which the officers or directors are made a party by reason of their relationship to the corporation. Officers and directors may be indemnified where they have acted in good faith; in the case of official action, the individual reasonably believed that the conduct was in the corporation's best interests and in all other cases, the individual reasonably believed that the conduct was not against the best interests of the corporation; and in the case of criminal proceedings, the individual either had reasonable cause to believe his or her conduct was lawful or no reasonable cause to believe his or her conduct was unlawful. Chapter 37 also requires every Indiana corporation to indemnify any of its officers or directors (unless limited by the articles of incorporation of the corporation) who were wholly successful, on the merits or otherwise, in the defense of any such proceeding against reasonable expenses incurred in connection with the proceeding. A corporation may also, under certain circumstances, pay for or reimburse the reasonable expenses incurred by an officer or director who is a party to a proceeding in advance of final disposition of the proceeding. Chapter 37 states that the indemnification provided for therein is not exclusive of any other rights to which a person may be entitled under the articles of incorporation, bylaws or resolutions of the board of directors or shareholders.
Our amended and restated articles of incorporation and amended and restated bylaws provide for indemnification, to the fullest extent permitted by the IBCL, of our directors, officers and employees against liability and reasonable expenses that may be incurred by them, arising out of any threatened, pending or completed investigation, claim, suit or proceeding, whether civil, administrative, investigative or criminal, in which they may become involved by reason of being or having been a director, officer or employee. To be entitled to indemnification, (a) those persons must have been wholly successful in the claim or action, or (b) the board of directors, independent legal counsel or the shareholders must have determined that such persons acted in good faith in what they reasonably believed to be in our best interest, or in the case of conduct not in the
185
individual's official capacity with us, did not act in opposition to our best interest. In addition, in any criminal action, such persons must have had no reasonable cause to believe that their conduct was unlawful. Our amended and restated bylaws provide for mandatory advancement of expenses to such persons provided certain conditions are met, including provision of a written undertaking to repay such advancements, should it be determined that the person is not entitled to indemnification.
The IBCL permits us to purchase insurance on behalf of our directors, officers, employees and agents against liabilities arising out of their positions with us, whether or not such liabilities would be within the above indemnification provisions. Pursuant to this authority, we will maintain such insurance for our directors, officers and employees and those of our subsidiaries, subject to certain exclusions and deductible and maximum amounts, against loss from claims arising in connection with their acting in their respective capacities, including claims under the Securities Act.
Listing
We intend to apply to have our common stock listed on the NYSE under the symbol "ELAN."
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A.
186
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock. Future sales of our common stock in the public market, or the perception that sales may occur, could materially adversely affect the prevailing market price of our common stock at such time and our ability to raise equity capital in the future.
Sale of Restricted Securities
Upon completion of this offering, we will have shares of our common stock outstanding (or shares, if the underwriters exercise their option to purchase additional shares in full). Of these shares, all shares sold in this offering will be freely tradable without further restriction or registration under the Securities Act, except that any shares purchased by our affiliates, as that term is defined in Rule 144 under the Securities Act, and shares purchased in this offering by participants in our directed share program who are subject to lock-up restrictions or are otherwise restricted from reselling such shares by Rule 144 of the Securities Act. Shares purchased by our affiliates would be subject to the Rule 144 resale restrictions described below. Of the remaining outstanding shares, shares will be deemed "restricted securities" under the Securities Act. Restricted securities may be sold in the public market only if registered or if they qualify for an exemption from registration under Rules 144 or 701 under the Securities Act, which are described below.
Lock-Up Arrangements and Registration Rights
In connection with this offering, we, each of our directors and executive officers and Lilly have entered into lock-up agreements that restrict the sale of our securities for up to 180 days after the date of this prospectus, subject to certain exceptions or an extension in certain circumstances.
In addition, following the expiration of the lock-up period, Lilly will have the right, subject to certain conditions, to require us to register the sale of its shares of our common stock under federal securities laws. See "Certain Relationships and Related Party Transactions — Registration Rights Agreement."
Following the lock-up period described above, all of the shares of our common stock that are restricted securities or are held by Lilly as of the date of this prospectus will be eligible for sale in the public market in compliance with Rules 144 or 701 under the Securities Act.
Rule 144
The shares of our common stock sold in this offering will generally be freely transferable without restriction or further registration under the Securities Act, except that any shares of our common stock held by an "affiliate" of ours may not be resold publicly except in compliance with the registration requirements of the Securities Act or under an exemption under Rule 144 or otherwise. Rule 144 permits our common stock that has been acquired by a person who is an affiliate of ours, or has been an affiliate of ours within the past three months, to be sold into the market in an amount that does not exceed, during any three-month period, the greater of:
- •
- one percent of the total number of shares of our common stock outstanding; or
- •
- the average weekly reported trading volume of our common stock for the four calendar weeks prior to the sale.
Such sales are also subject to specific manner of sale provisions, a six-month holding period requirement (or a one-year holding period if the sale occurs within 90 days of the date of this prospectus), notice requirements and the availability of current public information about us.
187
Approximately shares of our common stock that are not subject to lock-up arrangements described above will be eligible for sale under Rule 144 immediately upon the closing of this offering.
Rule 144 also provides that a person who is not deemed to have been an affiliate of ours at any time during the three months preceding a sale, and who has for at least six months (or one year if the sale occurs within 90 days of the date of this prospectus) beneficially owned shares of our common stock that are restricted securities, will be entitled to freely sell such shares of our common stock subject only to the availability of current public information regarding us. A person who is not deemed to have been an affiliate of ours at any time during the three months preceding a sale, and who has beneficially owned for at least one year shares of our common stock that are restricted securities, will be entitled to freely sell such shares of our common stock under Rule 144 without regard to the current public information requirements of Rule 144.
Rule 701
Rule 701 generally allows a shareholder who purchased shares of our capital stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of our company during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. Rule 701 also permits affiliates of our company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required to wait until 90 days after the date of this prospectus before selling those shares pursuant to Rule 701.
Additional Registration Statements
We intend to file a registration statement on Form S-8 under the Securities Act to register shares of our common stock to be issued or reserved for issuance under our equity incentive plans. Such registration statement is expected to be filed soon after the date of this prospectus and will automatically become effective upon filing with the SEC. Accordingly, shares registered under such registration statement will be available for sale in the open market, unless such shares are subject to vesting restrictions with us or the lock-up restrictions described above.
188
MATERIAL U.S. FEDERAL INCOME AND ESTATE TAX CONSIDERATIONS
FOR NON-U.S. HOLDERS
The following is a general discussion of the material U.S. federal income and estate tax consequences to non-U.S. holders (as defined below) of the purchase, ownership and disposition of our common stock. This discussion does not provide a complete analysis of all potential U.S. federal income and estate tax considerations relating thereto, and does not address any tax considerations related to the Distribution. This description is based on the Code and existing and proposed U.S. Treasury regulations promulgated thereunder, administrative pronouncements, judicial decisions, and interpretations of the foregoing, all as of the date hereof and all of which are subject to change, possibly with retroactive effect. This discussion is limited to non-U.S. holders who hold shares of our common stock as capital assets within the meaning of Section 1221 of the Code (generally for investment). Moreover, this discussion is for general information only and does not address all of the tax consequences that may be relevant to you in light of your particular circumstances, nor does it discuss special tax provisions, which may apply to you if you are subject to special treatment under U.S. federal income tax laws, such as for certain financial institutions or financial services entities, insurance companies, tax-exempt entities, tax-qualified retirement plans, "qualified foreign pension funds" (and entities all of the interests of which are held by qualified foreign pension funds), dealers in securities or currencies, entities that are treated as partnerships or other pass-through entities for U.S. federal income tax purposes (and partners or beneficial owners therein), "controlled foreign corporations," "passive foreign investment companies," former U.S. citizens or long-term residents, persons that have a "functional currency" other than the U.S. dollar, corporations that accumulate earnings to avoid U.S. federal income tax, accrual method taxpayers who are required to recognize income for U.S. federal income tax purposes no later than when such income is taken into account in applicable financial statements, persons deemed to sell common stock under the constructive sale provisions of the Code, and persons that hold common stock as part of a straddle, hedge, conversion transaction, or other integrated investment. In addition, this summary does not address the alternative minimum tax or any state, local or foreign taxes or any U.S. federal tax laws other than U.S. federal income and estate tax laws.
You are urged to consult your own tax advisor concerning the U.S. federal income tax consequences of purchasing, owning and disposing of our common stock, as well as the application of any state, local, foreign income and other tax laws and tax treaties.
As used in this section, a "non-U.S. holder" is a beneficial owner of our common stock (other than a partnership or any other entity treated as a pass-through entity for U.S. federal income tax purposes) that is not, for U.S. federal income tax purposes:
- •
- an individual who is a citizen or resident of the U.S.;
- •
- a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) that is created or organized in or under the laws
of the U.S., any state thereof or the District of Columbia;
- •
- an estate the income of which is subject to U.S. federal income taxation regardless of its source; or
- •
- a trust if (i) a court within the U.S. is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have the authority to control all substantial decisions of the trust or (ii) it has a valid election in effect under applicable U.S. Treasury regulations to be treated as a domestic trust.
A modified definition of "non-U.S. holder" applies for U.S. federal estate tax purposes (as discussed below).
189
If you are an individual, you are a resident alien if you are a lawful permanent resident of the U.S. (e.g., a green card holder) and you may, in many cases, be deemed to be a resident alien, as opposed to a nonresident alien, by virtue of being present in the U.S. for at least 31 days in the calendar year and for an aggregate of at least 183 days during a three-year period ending in and including the current calendar year. For these purposes, all the days present in the U.S. in the current year, one-third of the days present in the immediately preceding year, and one-sixth of the days present in the second preceding year are counted. Resident aliens are subject to U.S. federal income tax as if they are U.S. citizens. Such an individual is urged to consult his or her own tax advisor regarding the U.S. federal income tax consequences of the purchase, ownership or disposition of our common stock.
If a partnership or other entity treated as a pass-through entity for U.S. federal income tax purposes is a beneficial owner of our common stock, the tax treatment of a partner in the partnership or an owner of the other pass-through entity will depend upon the status of the partner or owner and the activities of the partnership or other pass-through entity. Any partnership, partner in such a partnership or owner of another pass-through entity holding shares of our common stock should consult its own tax advisor as to the particular U.S. federal income tax consequences applicable to it.
INVESTORS CONSIDERING THE PURCHASE OF OUR COMMON STOCK ARE URGED TO CONSULT THEIR OWN TAX ADVISORS REGARDING THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AND THE CONSEQUENCES OF OTHER FEDERAL, STATE, LOCAL AND FOREIGN TAX LAWS, AND APPLICABLE TAX TREATIES.
Distributions on Common Stock
We initially expect to pay quarterly distributions on shares of our common stock (as discussed in the section entitled "Dividend Policy"). If we pay distributions on shares of our common stock, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Distributions in excess of our current and accumulated earnings and profits will constitute a return of capital that is applied against and reduces, but not below zero, a non-U.S. holder's adjusted tax basis in shares of our common stock. Any remaining excess will be treated as gain realized on the sale or other disposition of our common stock. See "— Dispositions of Common Stock."
Subject to the discussion below regarding effectively connected income, any dividend paid to a non-U.S. holder on our common stock will generally be subject to U.S. federal withholding tax at a 30% rate. The withholding tax might not apply, however, or might apply at a reduced rate, under the terms of an applicable income tax treaty. You are urged to consult your own tax advisors regarding your entitlement to benefits under a relevant income tax treaty. Generally, in order for us or our paying agent to withhold tax at a lower treaty rate, a non-U.S. holder must certify its entitlement to treaty benefits. A non-U.S. holder generally can meet this certification requirement by providing a valid IRS Form W-8BEN or IRS Form W-8BEN-E (or other applicable form or documentation), as applicable, to us or our paying agent. If the non-U.S. holder holds the stock through a financial institution or other agent acting on the holder's behalf, the holder will be required to provide appropriate documentation to the agent. Even if our current or accumulated earnings or profits are less than the amount of the distribution, the applicable withholding agent may elect to treat the entire distribution as a dividend for U.S. federal withholding tax purposes. A non-U.S. holder that does not timely furnish the required documentation, but that qualifies for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
190
Dividends received by a non-U.S. holder that are effectively connected with a U.S. trade or business conducted by the non-U.S. holder and, if required by an applicable income tax treaty, are attributable to a permanent establishment (or, in certain cases involving individual holders, a fixed base) maintained by the non-U.S. holder in the U.S., are generally not subject to such withholding tax. To obtain this exemption, a non-U.S. holder must provide us with a valid IRS Form W-8ECI properly certifying such exemption. Such effectively connected dividends, although not subject to withholding tax (provided certain certification and disclosure requirements are satisfied), are taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. In addition to the graduated tax described above, such effectively connected dividends received by corporate non-U.S. holders may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty.
The foregoing discussion is subject to the discussion below under "— Backup Withholding and Information Reporting and "— Other Withholding Taxes."
Dispositions of Common Stock
Subject to the discussion below on backup withholding and other withholding requirements, gain realized by a non-U.S. holder on a sale, exchange or other disposition of our common stock generally will not be subject to U.S. federal income or withholding tax, unless:
- •
- the gain (i) is effectively connected with the conduct by the non-U.S. holder of a U.S. trade or business and (ii) if required by
an applicable income tax treaty, is attributable to a permanent establishment (or, in certain cases involving individual holders, a fixed base) maintained by the non-U.S. holder in the U.S. (in which
case the special rules described below apply);
- •
- the non-U.S. holder is an individual who is present in the U.S. for 183 or more days in the taxable year of such disposition and certain other
conditions are met (in which case the gain would be subject to a flat 30% tax, or such reduced rate as may be specified by an applicable income tax treaty, which may be offset by certain U.S. source
capital losses, provided the non-U.S holder has timely filed U.S. federal income tax returns with respect to such losses); or
- •
- we are, or become, a "United States real property holding corporation" (a "USRPHC"), for U.S. federal income tax purposes at any time during the shorter of the five-year period ending on the date of disposition of our common stock and the non-U.S. holder's holding period for our common stock.
Generally, a corporation is a USRPHC if the fair market value of its "United States real property interests" equals 50% or more of the sum of the fair market value of (a) its worldwide real property interests and (b) its other assets used or held for use in a trade or business. The tax relating to stock in a USRPHC does not apply to a non-U.S. holder whose holdings, actual and constructive, amount to 5% or less of our common stock at all times during the applicable period, provided that our common stock is regularly traded on an established securities market. No assurance can be provided that our common stock will be regularly traded on an established securities market at all times for purposes of the rules described above. Although there can be no assurances in this regard, we believe we have not been and are not currently a USRPHC, and do not anticipate being a USRPHC in the future. You are urged to consult your own tax advisor about the consequences that could result if we are, or become, a USRPHC.
If any gain from the sale, exchange or other disposition of our common stock, (1) is effectively connected with a U.S. trade or business conducted by a non-U.S. holder and (2) if required by an applicable income tax treaty, is attributable to a permanent establishment (or, in certain cases
191
involving individuals, a fixed base) maintained by such non-U.S. holder in the U.S., then the gain generally will be subject to U.S. federal income tax at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. If the non-U.S. holder is a corporation, under certain circumstances, that portion of its earnings and profits that is effectively connected with its U.S. trade or business, subject to certain adjustments, generally would also be subject to a "branch profits tax." The branch profits tax rate is generally 30%, although an applicable income tax treaty might provide for a lower rate.
Backup Withholding and Information Reporting
Any dividends that are paid to a non-U.S. holder must be reported annually to the IRS and to the non-U.S. holder. Copies of these information returns also may be made available to the tax authorities of the country in which the non-U.S. holder resides under the provisions of various treaties or agreements for the exchange of information. Dividends paid on our common stock and the gross proceeds from a taxable disposition of our common stock may be subject to additional information reporting and may also be subject to U.S. federal backup withholding if such non-U.S. holder fails to comply with applicable U.S. information reporting and certification requirements. Provision of an IRS Form W-8 appropriate to the non-U.S. holder's circumstances will generally satisfy the certification requirements necessary to avoid the additional information reporting and backup withholding.
Backup withholding is not an additional tax. Any amounts so withheld under the backup withholding rules will be refunded by the IRS or credited against the non-U.S. holder's U.S. federal income tax liability, provided that the required information is timely furnished to the IRS.
Other Withholding Taxes
Provisions commonly referred to as "FATCA" impose withholding (separate and apart from, but without duplication of, the withholding tax described above) at a rate of 30% on payments of U.S.-source dividends (including our dividends) and, beginning January 1, 2019, on gross proceeds from the sale or other disposition of domestic corporate stock (including our stock), paid to "foreign financial institutions" (which is broadly defined for this purpose and in general includes investment vehicles) and certain other non-U.S. entities unless various U.S. information reporting and due diligence requirements (generally relating to ownership by U.S. persons of interests in or accounts with those entities) have been satisfied, or an exemption applies. An intergovernmental agreement between the U.S. and an applicable foreign country may modify these requirements. Accordingly, the entity through which our common stock is held will affect the determination of whether such withholding is required. If FATCA withholding is imposed, a beneficial owner that is not a foreign financial institution generally will be entitled to a refund of any amounts withheld by filing a U.S. federal income tax return containing the required information (which may entail significant administrative burden). Non-U.S. holders are urged to consult their own tax advisors regarding the effects of FATCA on their investment in our common stock.
U.S. Federal Estate Tax
Shares of our common stock owned or treated as owned by an individual who is not a U.S. citizen or resident (as specifically determined for U.S. federal estate tax purposes) at the time of such individual's death will be included in such individual's gross estate for U.S. federal estate tax purposes and may be subject to U.S. federal estate tax unless an applicable estate tax treaty provides otherwise.
THE PRECEDING DISCUSSION OF U.S. FEDERAL INCOME AND ESTATE TAX CONSIDERATIONS IS FOR GENERAL INFORMATION ONLY. IT IS NOT TAX ADVICE. EACH PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR REGARDING THE PARTICULAR U.S. FEDERAL, STATE, LOCAL AND FOREIGN TAX CONSEQUENCES OF PURCHASING, OWNING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS AND TREATIES.
192
We have entered into an underwriting agreement with the underwriters named below with respect to the shares being offered. Subject to certain conditions, each underwriter has severally agreed to purchase the number of shares indicated in the following table. Goldman Sachs & Co. LLC, J.P. Morgan Securities LLC and Morgan Stanley & Co. LLC are the representatives of the underwriters.
Underwriters |
Number of Shares |
|||
| | | | | |
Goldman Sachs & Co. LLC |
||||
J.P. Morgan Securities LLC |
||||
Morgan Stanley & Co. LLC |
||||
Barclays Capital Inc. |
||||
BNP Paribas Securities Corp. |
||||
Citigroup Global Markets Inc. |
||||
Credit Suisse Securities (USA) LLC |
||||
Deutsche Bank Securities Inc. |
||||
Evercore Group L.L.C. |
||||
Cowen and Company, LLC |
||||
| | | | | |
Total |
||||
| | | | | |
| | | | | |
| | | | | |
The underwriters are committed to take and pay for all of the shares being offered, if any are taken, other than the shares covered by the option described below unless and until this option is exercised.
The underwriters have an option to buy up to an additional shares from us to cover sales by the underwriters of a greater number of shares than the total number set forth in the table above. They may exercise that option for 30 days. If any shares are purchased pursuant to this option, the underwriters will severally purchase shares in approximately the same proportion as set forth in the table above.
The following table shows the per share and total underwriting discounts and commissions to be paid by us to the underwriters. Such amounts are shown assuming both no exercise and full exercise of the underwriters' option to purchase additional shares.
Paid by us |
No Exercise | Full Exercise |
|||||
| | | | | | | | |
Per Share |
$ | $ | |||||
Total |
$ | $ |
Shares sold by the underwriters to the public will initially be offered at the initial public offering price set forth on the cover of this prospectus. Any shares sold by the underwriters to securities dealers may be sold at a discount of up to $ per share from the initial public offering price. After the initial offering of the shares, the representatives may change the offering price and the other selling terms. The offering of the shares by the underwriters is subject to receipt and acceptance and subject to the underwriters' right to reject any order in whole or in part.
We, our executive officers and directors and Lilly have agreed with the underwriters, subject to certain exceptions, not to dispose of or hedge any shares of our common stock or securities convertible into or exchangeable for shares of our common stock during the period from the date of this prospectus continuing through the date that is 180 days after the date of this prospectus, except with the prior written consent of each of the representatives. See "Shares Eligible for Future Sale" for a discussion of certain transfer restrictions.
193
At our request, the underwriters have reserved up to % of the shares of our common stock offered by this prospectus for sale, at the initial public offering price, to our directors, officers and certain employees and other parties with a connection to us, to the extent permitted under applicable regulations in the United States and in various countries. Pursuant to the underwriting agreement, the sales will be made by Morgan Stanley & Co. LLC through a directed share program. The number of shares of common stock available for sale to the general public in this offering will be reduced to the extent these persons purchase reserved shares. Any reserved shares not purchased by these persons will be offered by the underwriters to the general public on the same terms as the other shares. We have agreed to indemnify Morgan Stanley & Co. LLC in connection with the directed share program, including for the failure of any participant to pay for its shares. Other than the underwriting discount described on the front cover of this prospectus, the underwriters will not be entitled to any commission with respect to shares of common stock sold pursuant to the directed share program. Shares sold to our directors and officers pursuant to the directed share program will be subject to a 180-day lock-up restriction, and shares sold to other individuals will be subject to a day lock-up restriction.
Prior to the offering, there has been no public market for the shares. The initial public offering price will be negotiated among us and the representatives. Among the factors to be considered in determining the initial public offering price of the shares, in addition to prevailing market conditions, will be our historical performance, estimates of our business potential and earnings prospects, an assessment of management and the consideration of the above factors in relation to market valuation of companies in related businesses.
An application has been made to list our common stock on the NYSE under the symbol "ELAN."
In connection with the offering, the underwriters may purchase and sell shares of common stock in the open market. These transactions may include short sales, stabilizing transactions and purchases to cover positions created by short sales. Short sales involve the sale by the underwriters of a greater number of shares than they are required to purchase in the offering, and a short position represents the amount of such sales that have not been covered by subsequent purchases. A "covered short position" is a short position that is not greater than the amount of additional shares for which the underwriters' option described above may be exercised. The underwriters may cover any covered short position by either exercising their option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to cover the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase additional shares pursuant to the option described above. "Naked" short sales are any short sales that create a short position greater than the amount of additional shares for which the option described above may be exercised. The underwriters must cover any such naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of common stock made by the underwriters in the open market prior to the completion of the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of such underwriter in stabilizing or short covering transactions.
Purchases to cover a short position and stabilizing transactions, as well as other purchases by the underwriters for their own accounts, may have the effect of preventing or retarding a decline in
194
the market price of our common stock, and together with the imposition of the penalty bid, may stabilize, maintain or otherwise affect the market price of our common stock. As a result, the price of our common stock may be higher than the price that otherwise might exist in the open market. The underwriters are not required to engage in these activities and may end any of these activities at any time. These transactions may be effected on the NYSE, in the over-the-counter market or otherwise.
We estimate that our share of the total expenses of the offering, excluding underwriting discounts and commissions, will be approximately $ million. We have agreed to reimburse the underwriters for expenses related to the clearance of the offering with the Financial Industry Regulatory Authority ("FINRA") up to $40,000 and the qualification of the common stock under state securities laws up to $15,000.
We have agreed to indemnify the several underwriters against certain liabilities, including liabilities under the Securities Act.
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include sales and trading, commercial and investment banking, advisory, investment management, investment research, principal investment, hedging, market making, brokerage and other financial and non-financial activities and services. Certain of the underwriters and their respective affiliates have provided, and may in the future provide, a variety of these services to Lilly and its subsidiaries, including us, and to persons and entities with relationships with Lilly and its subsidiaries, including us, for which they received or will receive customary fees and expenses. In particular, certain of the underwriters acted as initial purchasers in the Senior Notes Offering and are lenders under the Credit Facilities. Furthermore, certain underwriters (not in their capacity as such) or their affiliates have separately been engaged to advise Lilly in connection with a strategic review of its animal health businesses, including the potential Distribution. In connection with such engagement, Goldman Sachs & Co. LLC has been granted a right of first refusal, subject to certain limitations, to provide services with respect to certain of our future transactions.
In the ordinary course of their various business activities, the underwriters and their respective affiliates, officers, directors and employees may purchase, sell or hold a broad array of investments and actively trade securities, derivatives, loans, commodities, currencies, credit default swaps and other financial instruments for their own account and for the accounts of their customers, and such investment and trading activities may involve or relate to our assets, securities and/or instruments (directly, as collateral securing other obligations or otherwise) and/or persons and entities with relationships with us. The underwriters and their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such assets, securities or instruments and may at any time hold, or recommend to clients that they should acquire, long and/or short positions in such assets, securities and instruments.
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a "Relevant Member State"), an offer to the public of the common stock may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of the common stock may be made at any time under the following exemptions under the Prospectus Directive:
- (a)
- to any legal entity which is a qualified investor as defined in the Prospectus Directive;
195
- (b)
- to
fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), subject to obtaining the prior consent of the
representatives for any such offer; or
- (c)
- in any other circumstances falling within Article 3(2) of the Prospectus Directive,
provided that no such offer of shares of the common stock shall result in a requirement for the publication by us or any underwriter of a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an "offer to the public" in relation to the common stock in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the common stock to be offered so as to enable an investor to decide to purchase the common stock, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression "Prospectus Directive" means Directive 2003/71/EC (as amended), including by Directive 2010/73/EU, and includes any relevant implementing measure in the Relevant Member State.
This European Economic Area selling restriction is in addition to any other selling restrictions set out below.
United Kingdom
In the UK, this prospectus is only addressed to and directed to qualified investors who are (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the "Order"); or (ii) high net worth entities and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as "relevant persons"). Any investment or investment activity to which this prospectus relates is available only to relevant persons and will only be engaged with relevant persons. Any person who is not a relevant person should not act or rely on this prospectus or any of its contents.
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principals that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions, and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser's province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser's province or territory of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
196
Hong Kong
The shares may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32 of the Laws of Hong Kong) ("Companies (Winding Up and Miscellaneous Provisions) Ordinance") or which do not constitute an invitation to the public within the meaning of the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) ("Securities and Futures Ordinance"), or (ii) to "professional investors" as defined in the Securities and Futures Ordinance and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a "prospectus" as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance, and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to "professional investors" in Hong Kong as defined in the Securities and Futures Ordinance and any rules made thereunder.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor (as defined under Section 4A of the Securities and Futures Act, Chapter 289 of Singapore (the "SFA")) under Section 274 of the SFA, (ii) to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to conditions set forth in the SFA.
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor, the securities (as defined in Section 239(1) of the SFA) of that corporation shall not be transferable for six months after that corporation has acquired the shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer in that corporation's securities pursuant to Section 275(1A) of the SFA, (3) where no consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore ("Regulation 32").
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a trust (where the trustee is not an accredited investor (as defined in Section 4A of the SFA)) whose sole purpose is to hold investments and each beneficiary of the trust is an accredited investor, the beneficiaries' rights and interest (howsoever described) in that trust shall not be transferable for six months after that trust has acquired the shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer that is made on
197
terms that such rights or interest are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction (whether such amount is to be paid for in cash or by exchange of securities or other assets), (3) where no consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32.
Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Act of Japan (Act No. 25 of 1948, as amended) (the "FIEA"). The securities may not be offered or sold, directly or indirectly, in Japan or to or for the benefit of any resident of Japan (including any person resident in Japan or any corporation or other entity organized under the laws of Japan) or to others for reoffering or resale, directly or indirectly, in Japan or to or for the benefit of any resident of Japan, except pursuant to an exemption from the registration requirements of the FIEA and otherwise in compliance with any relevant laws and regulations of Japan.
198
Barnes & Thornburg LLP, Indianapolis, Indiana, has passed upon the validity of the common stock offered hereby on behalf of us. Certain legal matters will be passed upon on behalf of us by Weil, Gotshal & Manges LLP, New York, New York. Certain legal matters will be passed upon on behalf of the underwriters by Ropes & Gray LLP.
The combined financial statements of Elanco as of December 31, 2017 and 2016, and for each of the years in the three-year period ended December 31, 2017 have been included herein in reliance upon the reports of Ernst & Young LLP, independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of our common stock offered by this prospectus. For purposes of this section, the term registration statement means the original registration statement and any and all amendments including the schedules and exhibits to the original registration statement or any amendment. This prospectus, filed as part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules thereto as permitted by the rules and regulations of the SEC. For further information about us and our common stock, you should refer to the registration statement, including the exhibits. This prospectus summarizes provisions that we consider material of certain contracts and other documents to which we refer you. Because the summaries may not contain all of the information that you may find important, you should review the full text of those documents.
The registration statement, including its exhibits and schedules, may be inspected and copied at the public reference facilities maintained by the SEC at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the public reference room by calling 1-800-SEC-0330. Copies of such materials are also available by mail from the Public Reference Branch of the SEC at 100 F Street, N.E., Room 1580, Washington, D.C. 20549 at prescribed rates. In addition, the SEC maintains a website at (http://www.sec.gov) from which interested persons can electronically access the registration statement, including the exhibits and schedules to the registration statement.
We have not authorized anyone to give you any information or to make any representations about us or the transactions we discuss in this prospectus other than those contained in this prospectus or in any free writing prospectus we have prepared. If you are given any information or representations about these matters that is not discussed in this prospectus or in any free writing prospectus we have prepared, you must not rely on that information. This prospectus is not an offer to sell or a solicitation of an offer to buy securities anywhere or to anyone where or to whom we are not permitted to offer or sell securities under applicable law.
199
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Eli Lilly and Company:
Opinion on the Financial Statements
We have audited the accompanying combined balance sheets of the Animal Health Businesses of Eli Lilly and Company to be divested (the Company) as of December 31, 2017 and 2016, the related combined statements of operations, comprehensive loss, equity and cash flows for each of the three years in the period ended December 31, 2017, and the related notes (collectively referred to as the "combined financial statements"). In our opinion, the combined financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2017 and 2016, and the results of its operations and cash flows for each of the three years in the period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company's auditor since 2017.
Indianapolis,
Indiana
May 25, 2018
F-2
Animal Health Businesses of Eli Lilly to be Divested
Combined Statements of Operations
|
Year Ended December 31, |
|||||||||
| | | | | | | | | | | |
(Dollars in millions) |
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Revenue |
$ | 2,889.0 | $ | 2,913.5 | $ | 2,909.1 | ||||
Costs, expenses and other: |
||||||||||
Cost of sales |
1,493.9 | 1,409.0 | 1,533.7 | |||||||
Research and development |
251.7 | 265.8 | 291.0 | |||||||
Marketing, selling and administrative |
779.8 | 784.8 | 916.0 | |||||||
Amortization of intangible assets (Note 8) |
221.2 | 170.7 | 163.0 | |||||||
Asset impairment, restructuring and other special charges (Note 5) |
375.1 | 308.4 | 263.3 | |||||||
Other — net, (income) expense |
(0.1 | ) | (2.8 | ) | 1.6 | |||||
| | | | | | | | | | | |
|
3,121.6 | 2,935.9 | 3,168.6 | |||||||
| | | | | | | | | | | |
Loss before income taxes |
(232.6 | ) | (22.4 | ) | (259.5 | ) | ||||
Income tax expense (benefit) (Note 11) |
78.1 | 25.5 | (48.7 | ) | ||||||
| | | | | | | | | | | |
Net loss |
$ | (310.7 | ) | $ | (47.9 | ) | $ | (210.8 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See notes to combined financial statements.
F-3
Animal Health Businesses of Eli Lilly to be Divested
Combined Statements of Comprehensive Loss
|
Year Ended December 31, |
|||||||||
| | | | | | | | | | | |
(Dollars in millions) |
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Net loss |
$ | (310.7 | ) | $ | (47.9 | ) | $ | (210.8 | ) | |
Other comprehensive income (loss): |
||||||||||
Change in foreign currency translation gains (losses) |
210.1 | (230.7 | ) | (143.6 | ) | |||||
Change in defined benefit pension and retiree health benefit plans, net of taxes |
(9.8 | ) | (4.3 | ) | (11.8 | ) | ||||
| | | | | | | | | | | |
Other comprehensive income (loss), net of taxes |
200.3 | (235.0 | ) | (155.4 | ) | |||||
| | | | | | | | | | | |
Comprehensive loss |
$ | (110.4 | ) | $ | (282.9 | ) | $ | (366.2 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See notes to combined financial statements.
F-4
Animal Health Businesses of Eli Lilly to be Divested
Combined Balance Sheets
|
December 31, |
||||||
| | | | | | | | |
(Dollars in millions) |
2017 | 2016 |
|||||
| | | | | | | | |
Assets |
|||||||
Current Assets |
|||||||
Cash and cash equivalents |
$ | 323.4 | $ | 258.8 | |||
Accounts receivable, net of allowances of $9.8 (2017) and $11.0 (2016) |
567.4 | 585.0 | |||||
Other receivables |
34.5 | 45.7 | |||||
Inventories (Note 6) |
1,062.3 | 875.6 | |||||
Prepaid expenses and other |
136.1 | 182.3 | |||||
| | | | | | | | |
Total current assets |
2,123.7 | 1,947.4 | |||||
| | | | | | | | |
Noncurrent Assets |
|||||||
Investments (Note 7) |
12.3 | 9.0 | |||||
Goodwill (Note 8) |
2,969.2 | 2,576.5 | |||||
Other intangibles, net (Note 8) |
2,672.8 | 2,621.0 | |||||
Other noncurrent assets |
242.0 | 204.0 | |||||
Property and equipment, net (Note 9) |
920.3 | 741.8 | |||||
| | | | | | | | |
Total assets |
$ | 8,940.3 | $ | 8,099.7 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Liabilities and Equity |
|||||||
Current Liabilities |
|||||||
Accounts payable |
$ | 203.8 | $ | 228.2 | |||
Employee compensation |
89.3 | 83.2 | |||||
Sales rebates and discounts |
155.0 | 148.6 | |||||
Income taxes payable (Note 11) |
4.8 | 7.5 | |||||
Other current liabilities |
179.7 | 151.4 | |||||
| | | | | | | | |
Total current liabilities |
632.6 | 618.9 | |||||
| | | | | | | | |
Noncurrent Liabilities |
|||||||
Accrued retirement benefits (Note 12) |
139.0 | 113.8 | |||||
Deferred taxes (Note 11) |
251.9 | 227.5 | |||||
Other noncurrent liabilities |
126.0 | 111.6 | |||||
| | | | | | | | |
Total liabilities |
1,149.5 | 1,071.8 | |||||
| | | | | | | | |
Commitments and Contingencies (Note 13) |
|||||||
Equity |
|||||||
Net parent company investment |
8,047.4 | 7,484.8 | |||||
Accumulated other comprehensive loss (Note 14) |
(256.6 | ) | (456.9 | ) | |||
| | | | | | | | |
Total equity |
7,790.8 | 7,027.9 | |||||
| | | | | | | | |
Total liabilities and equity |
$ | 8,940.3 | $ | 8,099.7 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
See notes to combined financial statements.
F-5
Animal Health Businesses of Eli Lilly to be Divested
Combined Statements of Equity
(Dollars in millions) |
Net Parent Company Investment |
Accumulated Other Comprehensive Loss |
Total Equity |
|||||||
| | | | | | | | | | | |
Balance at January 1, 2015 |
$ | 2,506.1 | $ | (66.5 | ) | $ | 2,439.6 | |||
Net loss |
(210.8 | ) | — | (210.8 | ) | |||||
Other comprehensive loss, net of tax |
— | (155.4 | ) | (155.4 | ) | |||||
Transfers to/from Lilly, net |
5,366.6 | — | 5,366.6 | |||||||
| | | | | | | | | | | |
Balance at December 31, 2015 |
7,661.9 | (221.9 | ) | 7,440.0 | ||||||
Net loss |
(47.9 | ) | — | (47.9 | ) | |||||
Other comprehensive loss, net of tax |
— | (235.0 | ) | (235.0 | ) | |||||
Transfers to/from Lilly, net |
(129.2 | ) | — | (129.2 | ) | |||||
| | | | | | | | | | | |
Balance at December 31, 2016 |
7,484.8 | (456.9 | ) | 7,027.9 | ||||||
Net loss |
(310.7 | ) | — | (310.7 | ) | |||||
Other comprehensive income, net of tax |
— | 200.3 | 200.3 | |||||||
Transfers to/from Lilly, net |
873.3 | — | 873.3 | |||||||
| | | | | | | | | | | |
Balance at December 31, 2017 |
$ | 8,047.4 | $ | (256.6 | ) | $ | 7,790.8 | |||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See notes to combined financial statements.
F-6
Animal Health Businesses of Eli Lilly to be Divested
Combined Statements of Cash Flows
|
Year Ended December 31, |
|||||||||
| | | | | | | | | | | |
(Dollars in millions) |
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Cash Flows from Operating Activities |
||||||||||
Net loss |
$ | (310.7 | ) | $ | (47.9 | ) | $ | (210.8 | ) | |
Adjustments to Reconcile Net Loss to Cash Flows from Operating Activities: |
||||||||||
Depreciation and amortization |
318.4 | 254.4 | 236.9 | |||||||
Change in deferred income taxes |
(13.4 | ) | (5.9 | ) | (76.2 | ) | ||||
Stock-based compensation expense |
25.0 | 20.4 | 13.4 | |||||||
Asset impairment charges |
110.6 | 98.3 | 57.5 | |||||||
Gain on sale of assets |
(19.6 | ) | — | — | ||||||
Other non-cash operating activities, net |
10.0 | 6.0 | 1.5 | |||||||
Other changes in operating assets and liabilities, net of acquisitions and divestitures: |
||||||||||
Receivables — (increase) decrease |
48.4 | (80.7 | ) | (94.6 | ) | |||||
Inventories — (increase) decrease |
(39.0 | ) | (89.1 | ) | 14.9 | |||||
Other assets — (increase) decrease |
52.5 | (36.7 | ) | (52.4 | ) | |||||
Accounts payable and other liabilities — increase (decrease) |
(8.4 | ) | 37.1 | 116.4 | ||||||
| | | | | | | | | | | |
Net Cash Provided by Operating Activities |
173.8 | 155.9 | 6.6 | |||||||
Cash Flows from Investing Activities |
||||||||||
Purchases of property and equipment |
(98.6 | ) | (110.3 | ) | (100.1 | ) | ||||
Disposals of property and equipment |
37.6 | 7.4 | 20.3 | |||||||
Proceeds from sale of product rights (Note 4) |
— | — | 410.0 | |||||||
Cash paid for acquisitions, net of cash acquired (Note 4) |
(882.1 | ) | (45.0 | ) | (5,283.1 | ) | ||||
Other investing activities, net |
(21.5 | ) | (34.2 | ) | (42.5 | ) | ||||
| | | | | | | | | | | |
Net Cash Used for Investing Activities |
(964.6 | ) | (182.1 | ) | (4,995.4 | ) | ||||
Cash Flows from Financing Activities |
||||||||||
Net transactions with Lilly |
848.3 | (149.6 | ) | 5,353.2 | ||||||
Other financing activities, net |
(0.8 | ) | — | — | ||||||
| | | | | | | | | | | |
Net Cash Provided by (Used for) Financing Activities |
847.5 | (149.6 | ) | 5,353.2 | ||||||
Effect of exchange rate changes on cash and cash equivalents |
7.9 | (26.0 | ) | (19.8 | ) | |||||
| | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents |
64.6 | (201.8 | ) | 344.6 | ||||||
Cash and cash equivalents at beginning of year |
258.8 | 460.6 | 116.0 | |||||||
| | | | | | | | | | | |
Cash and Cash Equivalents at End of Year |
$ | 323.4 | $ | 258.8 | $ | 460.6 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See notes to combined financial statements.
F-7
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements
(Tables present dollars in millions)
Note 1: Nature of Business and Basis of Preparation
Nature of Business
Eli Lilly and Company (Lilly) intends to divest substantially all of its animal health businesses through a series of equity transactions. The businesses to be divested are currently held in a combination of dedicated legal entities and commingled entities, which include activities of both Lilly and the divested businesses. Lilly will complete a corporate reorganization prior to the divestiture through which it will transfer the assets, liabilities and businesses to be divested to a single holding company (Elanco Parent). Elanco Parent will ultimately serve as parent company for the businesses to be divested by Lilly.
The accompanying combined financial statements represents the assets, liabilities and results of operations related to the animal health businesses to be transferred to Elanco Parent, which includes the animal health businesses that share people, manufacturing locations and activities. The combined animal health businesses to be transferred from Lilly to Elanco Parent are referred to throughout these combined financial statements as Elanco, the Company, we, us or our.
We are an animal health company that innovates, develops, manufactures and markets products for companion and food animals. We have operations throughout the world with a significant portion of our business in the United States.
Basis of Preparation
The accompanying combined financial statements have been prepared on a standalone basis and are derived from Lilly's consolidated financial statements and accounting records. The combined financial statements reflect the financial position, results of operations and cash flows related to the animal health businesses that will be transferred to Elanco Parent and are prepared in conformity with accounting principles generally accepted in the United States (GAAP). Lilly will transfer to Elanco Parent only the assets, liabilities and operations for business activities that will constitute the ongoing animal health businesses. These businesses operate on an integrated basis with shared people, manufacturing facilities, distribution centers, product types and the associated facilities that are being transferred to Elanco Parent.
These combined financial statements include the attribution of certain assets and liabilities that historically have been held at the Lilly corporate level but which are specifically identifiable or attributable to the businesses being transferred to Elanco Parent. All intercompany transactions and accounts within Elanco have been eliminated. All transactions between us and Lilly are considered to be effectively settled in the combined financial statements at the time the transaction is recorded. The total net effect of the settlement of these intercompany transactions is reflected in the combined statements of cash flows as a financing activity and in the combined balance sheets as net parent company investment.
These combined financial statements include an allocation of expenses related to certain Lilly corporate functions, including executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations. These expenses have been allocated to us based on direct usage or benefit where specifically identifiable, with the remainder allocated primarily on a pro rata basis of revenue, headcount and other measures. We consider the expense methodology and results to be reasonable for all periods presented. However,
F-8
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 1: Nature of Business and Basis of Preparation (Continued)
the allocations may not be indicative of the actual expense that would have been incurred had we operated as an independent, publicly traded company for the periods presented. It is impractical to estimate what the standalone costs of Elanco would have been in the historical periods.
The income tax amounts in these combined financial statements have been calculated based on a separate return methodology and presented as if our operations were separate taxpayers in the respective jurisdictions. We file income tax returns in the U.S. federal jurisdiction and various state, local and non-U.S. jurisdictions. Certain of these income tax returns are filed on a consolidated or combined basis with Eli Lilly and Company and/or its subsidiaries.
Lilly maintains various benefit and combined stock-based compensation plans at a corporate level and other benefit plans at a country level. Our employees participate in such programs and the portion of the cost of those plans related to our employees is included in our financial statements. However, the combined balance sheets do not include any equity issued related to stock-based compensation plans or any net benefit plan obligations unless the benefit plan covers only our dedicated employees or where the legal obligation associated with the benefit plan will transfer to Elanco.
The equity balance in these combined financial statements represents the excess of total assets over total liabilities, including intercompany balances between us and Lilly (net parent company investment) and accumulated other comprehensive loss. Net parent company investment is primarily impacted by contributions from Lilly which are the result of treasury activities and net funding provided by or distributed to Lilly. See Note 16 for further information.
Note 2: Summary of Significant Accounting Policies
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures at the date of the financial statements and during the reporting period. Actual results could differ from those estimates.
Revenue recognition
We recognize revenue from sales of products at the time title of goods passes to the buyer and the buyer assumes the risks and rewards of ownership. Provisions for returns, discounts and rebates are established in the same period the related sales are recognized.
Research and development expenses and acquired in-process research and development
Research and development expenses include the following:
- •
- Research and development costs, which are expensed as incurred.
- •
- Milestone payment obligations incurred prior to regulatory approval of the product, which are accrued when the event requiring payment of the milestone occurs.
F-9
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 2: Summary of Significant Accounting Policies (Continued)
- •
- Acquired in-process research and development (IPR&D) expense, which includes the initial costs of IPR&D projects, acquired directly in a transaction other than a business combination, that do not have an alternative future use.
Foreign Currency Translation
Operations in our subsidiaries outside the United States (U.S.) are recorded in the functional currency of each subsidiary which is determined by a review of the environment where each subsidiary primarily generates and expends cash. The results of operations for our subsidiaries outside the U.S. are translated from functional currencies into U.S. dollars using the weighted average currency rate for the period. Assets and liabilities are translated using the period end exchange rates. The U.S. dollar effects that arise from translating the net assets of these subsidiaries are recorded in other comprehensive loss.
Other significant accounting policies
Our other significant accounting policies are described in the remaining appropriate notes to the combined financial statements.
Note 3: Implementation of New Financial Accounting Pronouncements
The following table provides a brief description of accounting standards that had not yet been adopted as of December 31, 2017 and could have a material effect on our financial statements:
| Standard |
Description |
Effective Date |
Effect on the financial statements or other significant matters |
|||
|---|---|---|---|---|---|---|
| | | | | | | |
| Accounting Standards Update 2014-09 and various other related updates, Revenue from Contracts with Customers | This standard replaced existing revenue recognition standards and requires entities to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. An entity can apply the new revenue standard retrospectively to each prior reporting period presented or with the cumulative effect of initially applying the standard recognized at the date of initial application in retained earnings. We applied the latter approach. | This standard became effective January 1, 2018, and we adopted on that date. | Our evaluation of our contracts subject to this standard is complete and we do not expect the application of the new standard to these contracts to have a material impact to our combined statements of operations or balance sheets at initial implementation. We are also evaluating the new disclosures required by the standard to determine what additional information will need to be disclosed. |
F-10
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 3: Implementation of New Financial Accounting Pronouncements (Continued)
| Standard |
Description |
Effective Date |
Effect on the financial statements or other significant matters |
|||
|---|---|---|---|---|---|---|
| | | | | | | |
| Accounting Standards Update 2016-02, Leases | This standard was issued to increase transparency and comparability among organizations by recognizing lease assets and lease liabilities, including leases classified as operating leases under current GAAP, on the balance sheet and requiring additional disclosures about leasing arrangements. This standard requires a modified retrospective approach to adoption. | This standard is effective January 1, 2019, with early adoption permitted. We intend to adopt this standard on January 1, 2019. | We are in the process of determining the impact on our combined financial statements. We have selected a software solution to be compatible with our enterprise software system. Development of our selected solution is ongoing, as it is not yet fully compliant with the requirements of the standard. The timely readiness of the lease software system is critical to implement an efficient and effective adoption of the standard. | |||
Accounting Standards Update 2016-16, Income Taxes: Intra-Entity Transfers of Assets Other Than Inventory |
This standard requires entities to recognize the income tax consequences of intra-entity transfers of assets other than inventory at the time of transfer. This standard requires a modified retrospective approach to adoption. |
This standard became effective January 1, 2018, and we adopted on that date. |
We currently estimate that the cumulative effect of initially applying the standard will result in a decrease to net parent company investment of $1.8 million. |
F-11
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 3: Implementation of New Financial Accounting Pronouncements (Continued)
| Standard |
Description |
Effective Date |
Effect on the financial statements or other significant matters |
|||
|---|---|---|---|---|---|---|
| | | | | | | |
| Accounting Standards Update 2017-07, Compensation-Retirement Benefits: Improving the Presentation of Net Periodic Pension Cost and Net Periodic Postretirement Benefit Cost | This standard was issued to improve the transparency and comparability among organizations by requiring entities to separate their net periodic pension cost and net periodic postretirement benefit cost into a service cost component and other components. Currently, the costs of the other components along with the service cost component are classified based upon the function of the employee. This standard requires entities to classify the service cost component in the same financial statement line item or items as other compensation costs arising from services rendered by pertinent employees. The other components of net benefit cost will be presented separately from the line items that include the service cost component. When applicable, the service cost component is the only component eligible for capitalization. An entity should apply the new standard retrospectively for the classification of the service cost and other components and prospectively for the capitalization of the service cost component. | This standard became effective January 1, 2018, and we adopted on that date. | Upon adoption of this standard, pension and postretirement benefit cost components other than service costs will be presented in other — net, (income) expense. The application of the new standard did not change combined net income at initial implementation and we do not expect it to have a material impact on an ongoing basis. |
Note 4: Acquisitions
During 2017, 2016, and 2015, we completed the acquisitions of Boehringer Ingelheim Vetmedica, Inc.'s U.S. feline, canine and rabies vaccine portfolio and other related assets (BIVIVP), certain rights to Aratana Therapeutics, Inc.'s (Aratana) Galliprant® and Novartis Animal Health (Novartis AH), respectively. These transactions were accounted for as business combinations under the acquisition method of accounting. Under this method, the assets acquired and liabilities assumed were recorded at their respective fair values as of the acquisition date in our combined financial statements. The determination of estimated fair value required management to make significant estimates and assumptions. The excess of the purchase price over the fair value of the acquired net assets, where applicable, has been recorded as goodwill. The results of operations of these acquisitions are included in our combined financial statements from the dates of acquisition.
F-12
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 4: Acquisitions (Continued)
Boehringer Ingelheim Vetmedica, Inc. Vaccine Portfolio Acquisition
On January 3, 2017, we acquired BIVIVP in a cash transaction for $882.1 million. Under the terms of the agreement, we acquired a manufacturing and research and development site, a U.S. vaccine portfolio including vaccines used for the treatment of bordetella, Lyme disease, rabies and parvovirus, among others.
The following table summarizes the amounts recognized for assets acquired and liabilities assumed as of the acquisition date:
Estimated Fair Value at January 3, 2017 |
||||
| | | | | |
Inventories(1) |
$ | 108.6 | ||
Marketed products(2) |
297.0 | |||
Property and equipment |
148.2 | |||
Other assets and liabilities — net |
8.2 | |||
| | | | | |
Total identifiable net assets |
562.0 | |||
Goodwill(3) |
320.1 | |||
| | | | | |
Total consideration transferred — net of cash acquired |
$ | 882.1 | ||
| | | | | |
| | | | | |
| | | | | |
- (1)
- The
fair value for inventories include a purchase accounting adjustment to write up the inventory value, which resulted in incremental cost of sales
of $42.7 million in 2017. The fair value was determined by estimating the expected sales price of the inventories, reduced for all costs expected to the incurred and a profit on those costs.
- (2)
- These
intangible assets, which are being amortized on a straight-line basis over their estimated useful lives, were expected to have a weighted
average useful life of 10 years.
- (3)
- The goodwill recognized from this acquisition is attributable primarily to expected synergies from combining the operations of BIVIVP with our legacy business, future unidentified projects and products, and the assembled workforce of BIVIVP. The goodwill associated with this acquisition is deductible for tax purposes.
Our combined statement of operations for the year ended December 31, 2017 included BIVIVP revenues of $216.7 million. We are unable to provide the results of operations attributable to BIVIVP as those operations were substantially integrated into our legacy business.
Had BIVIVP been acquired on January 1, 2016, the unaudited pro forma combined revenues of Elanco and BIVIVP would have been $2.89 billion and $3.14 billion for the years ended December 31, 2017 and 2016, respectively. It is impractical to determine the pro forma impact on loss before tax attributable to BIVIVP for 2017 and 2016.
Galliprant Acquisition
On April 22, 2016, we acquired from Aratana, certain rights to Galliprant, a canine pain treatment for osteoarthritis for a total purchase price of $88.6 million, which consisted of an upfront payment of $45.0 million and contingent consideration of $43.6 million. The contingent consideration represented the fair value of potential future payments to Aratana based on the probability of achieving contingent milestones and royalties. At the time of the acquisition, Galliprant was approved in the U.S. and was still under development outside the U.S.
F-13
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 4: Acquisitions (Continued)
Under the terms of the agreement, we were granted co-promotion rights in the U.S. through December 31, 2018, at which time we will control commercialization in the U.S. We received full commercialization rights outside the U.S. The agreement requires payments by us to Aratana associated with certain development, success-based regulatory and sales-based milestones and royalties. As of December 31, 2017, Aratana is eligible to receive up to $8.0 million of potential development and success-based regulatory milestones. Aratana is also eligible to receive up to $75.0 million of potential sales-based milestones. Aratana is eligible to receive royalties based on a percentage of net sales of Galliprant, dependent on the timing and geography of the net sales. There is no cap on the amount of royalties that may be paid pursuant to this arrangement.
The following table summarizes the amounts recognized for assets acquired and liabilities assumed as of the acquisition date:
Estimated Fair Value at April 22, 2016 |
||||
| | | | | |
Deferred tax assets |
$ | 15.3 | ||
Acquired in-process research and development |
31.6 | |||
Marketed products(1) |
57.0 | |||
Deferred tax liabilities |
(15.3 | ) | ||
| | | | | |
Total consideration |
88.6 | |||
Less: Contingent consideration |
(43.6 | ) | ||
| | | | | |
Total cash paid |
$ | 45.0 | ||
| | | | | |
| | | | | |
| | | | | |
- (1)
- These intangible assets, which are being amortized on a straight-line basis over their estimated useful lives, were expected to have a weighted average useful life of 20 years.
Pro forma information has not been included because this acquisition did not have a material impact on the Company's results of operation for the years ended December 31, 2016 and 2015.
Novartis AH Acquisition
On January 1, 2015, we acquired from Novartis AG all of the shares of certain Novartis subsidiaries and the assets and liabilities of other Novartis subsidiaries that were exclusively related to the Novartis AH business in an all-cash transaction for a total purchase price of $5.28 billion.
As a condition to the clearance of the transaction under the Hart-Scott-Rodino Antitrust Improvements Act, following the closing of the acquisition of Novartis AH, we divested certain animal health assets in the U.S. related to the Sentinel® canine parasiticide franchise to Virbac Corporation for approximately $410 million.
The acquired Novartis AH business consisted of the research and development, manufacture, marketing, sale and distribution of veterinary products to prevent and treat diseases in pets, farm animals and farmed fish. Under the terms of the agreement, we acquired manufacturing sites, research and development facilities, a global commercial infrastructure and portfolio of products, a pipeline of projects in development and employees.
F-14
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 4: Acquisitions (Continued)
The following table summarizes the amounts recognized for assets acquired and liabilities assumed as of the acquisition date:
Estimated Fair Value at January 1, 2015 |
||||
| | | | | |
Inventories(1) |
$ | 380.2 | ||
Acquired in-process research and development |
298.0 | |||
Marketed products(2) |
1,953.0 | |||
Property and equipment |
199.9 | |||
Assets held for sale (primarily the U.S. Sentinel rights) |
422.7 | |||
Accrued retirement benefits |
(108.7 | ) | ||
Deferred income taxes |
(60.1 | ) | ||
Other assets and liabilities — net |
(73.0 | ) | ||
| | | | | |
Total identifiable net assets |
3,012.0 | |||
Goodwill(3) |
2,271.1 | |||
| | | | | |
Total consideration transferred — net of cash acquired |
$ | 5,283.1 | ||
| | | | | |
| | | | | |
| | | | | |
- (1)
- The
fair value for inventories include a purchase accounting adjustment to write up the inventory value, which resulted in incremental cost of sales
of $153.0 million in 2015. The fair value was determined by estimating the expected sales price of the inventories, reduced for all costs expected to the incurred and a profit on those costs.
- (2)
- These
intangible assets, which are being amortized on a straight-line basis over their estimated useful lives, were expected to have a weighted
average useful life of 19 years.
- (3)
- The goodwill recognized from this acquisition is attributable primarily to expected synergies from combining the operations of Novartis AH with our legacy business, future unidentified projects and products, and the assembled workforce of Novartis AH. Approximately $1.0 billion of the goodwill associated with this acquisition is deductible for tax purposes.
F-15
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 5: Asset Impairment, Restructuring and Other Special Charges
The Company has historically participated in Lilly's cost-reduction initiatives. The Company's total charges related to asset impairment, restructuring and other special charges, including integration of acquired businesses, in our combined statements of operations consisted of the following:
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Cash expense: |
||||||||||
Severance and other |
$ | 162.0 | $ | 42.1 | $ | 59.5 | ||||
Integration |
90.3 | 154.8 | 140.8 | |||||||
Facility exit costs |
31.8 | 13.2 | 5.5 | |||||||
| | | | | | | | | | | |
Total cash expense |
284.1 | 210.1 | 205.8 | |||||||
| | | | | | | | | | | |
Non-cash expense: |
||||||||||
Asset impairment |
110.6 | 98.3 | 57.5 | |||||||
| | | | | | | | | | | |
Total non-cash expense |
110.6 | 98.3 | 57.5 | |||||||
| | | | | | | | | | | |
Gain on sale of fixed assets |
(19.6 | ) | — | — | ||||||
| | | | | | | | | | | |
Total |
$ | 375.1 | $ | 308.4 | $ | 263.3 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Severance and other costs recognized during the years ended December 31, 2017, 2016 and 2015 were incurred as a result of actions taken to reduce our cost structure, including severance, curtailment loss and special termination benefits costs recognized in 2017 associated with the U.S. voluntary early retirement program offered by Lilly, related to our employees.
Integration costs recognized during the years ended December 31, 2017, 2016 and 2015 were related to our integration efforts as a result of our acquired businesses.
Asset impairment recognized during the year ended December 31, 2017 were primarily related to intangible asset impairments for a certain marketed product and for acquired IPR&D assets. Asset impairment recognized during the years ended December 31, 2016 and 2015 resulted primarily from intangible asset impairments due to product rationalization and to charges related to site closures resulting from our acquisition and integration of Novartis AH, including the closure of a manufacturing facility in Ireland in 2016. See Note 8 for further detail relating to intangible asset impairments.
Gain on sale of fixed assets for the year ended December 31, 2017 represent gain on disposal of two sites that we previously closed as part of our acquisition and integration of Novartis AH.
F-16
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 5: Asset Impairment, Restructuring and Other Special Charges (Continued)
The following table summarizes the activity in our reserves established in connection with these restructuring activities:
|
Facility exit costs |
Severance and other |
Total |
|||||||
| | | | | | | | | | | |
Beginning balance at January 1, 2015 |
$ | — | $ | 2.8 | $ | 2.8 | ||||
Charges |
5.5 | 59.5 | 65.0 | |||||||
Reserve adjustments |
(1.7 | ) | (1.4 | ) | (3.1 | ) | ||||
Cash paid |
— | (50.8 | ) | (50.8 | ) | |||||
| | | | | | | | | | | |
Balance at December 31, 2015 |
3.8 | 10.1 | 13.9 | |||||||
Charges |
13.2 | 42.1 | 55.3 | |||||||
Reserve adjustments |
(0.6 | ) | (3.2 | ) | (3.8 | ) | ||||
Cash paid |
(4.9 | ) | (22.4 | ) | (27.3 | ) | ||||
| | | | | | | | | | | |
Balance at December 31, 2016 |
11.5 | 26.6 | 38.1 | |||||||
Charges |
31.8 | 162.0 | 193.8 | |||||||
Reserve adjustments |
1.4 | (3.9 | ) | (2.5 | ) | |||||
Cash paid |
(9.8 | ) | (141.6 | ) | (151.4 | ) | ||||
| | | | | | | | | | | |
Ending balance at December 31, 2017 |
$ | 34.9 | $ | 43.1 | $ | 78.0 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Substantially all of the reserves are expected to be paid in the next 12 months. The Company believes that the reserves are adequate.
Note 6: Inventories
We state all inventories at the lower of cost or market. We use the last-in, first-out (LIFO) method for the majority of our inventories located in the continental U.S. Other inventories are valued by the first-in, first-out (FIFO) method. FIFO cost approximates current replacement cost.
Inventories at December 31 consisted of the following:
|
2017 | 2016 |
|||||
| | | | | | | | |
Finished products |
$ | 452.0 | $ | 409.5 | |||
Work in process |
580.0 | 452.9 | |||||
Raw materials and supplies |
70.4 | 59.6 | |||||
| | | | | | | | |
Total (approximates replacement cost) |
1,102.4 | 922.0 | |||||
Decrease to LIFO cost |
(40.1 | ) | (46.4 | ) | |||
| | | | | | | | |
Inventories |
$ | 1,062.3 | $ | 875.6 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Inventories valued under the LIFO method comprised $231.4 million and $274.0 million of total inventories at December 31, 2017 and 2016, respectively.
F-17
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 7: Financial Instruments
Financial instruments that potentially subject us to credit risk consist principally of trade receivables. Collateral is generally not required. The risk associated with this concentration is mitigated by our ongoing credit-review procedures and insurance.
A large portion of our cash, which is legally owned by us and is recognized on the combined balance sheets, is held by a few major financial institutions. Lilly monitors the exposure with these institutions and does not expect any of these institutions to fail to meet their obligations. We consider all highly liquid investments with a maturity of three months or less from the date of purchase to be cash equivalents. The cost of these investments approximates fair value.
As of December 31, 2017 and 2016, we had $12.3 million and $9.0 million, respectively, of cost and equity method investments.
The following table summarizes the fair value information at December 31 for contingent consideration liabilities measured at fair value on a recurring basis:
|
Fair Value Measurements Using | |||||||||||||||
| | | | | | | | | | | | | | | | | |
Financial statement line item |
Carrying Amount |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Fair Value |
|||||||||||
| | | | | | | | | | | | | | | | | |
December 31, 2017 |
||||||||||||||||
Other current liabilities-contingent consideration |
$ | 1.3 | — | — | $ | 1.3 | $ | 1.3 | ||||||||
Other noncurrent liabilities-contingent consideration |
45.2 | — | — | 45.2 | 45.2 | |||||||||||
December 31, 2016 |
||||||||||||||||
Other current liabilities-contingent consideration |
4.7 | — | — | 4.7 | 4.7 | |||||||||||
Other noncurrent liabilities-contingent consideration |
41.5 | — | — | 41.5 | 41.5 | |||||||||||
Contingent consideration liabilities relate to Galliprant for which the fair value was estimated using a discounted cash flow analysis and Level 3 inputs, including projections representative of a market participant view for the probability of achieving potential future payments to Aratana and an estimated discount rate. As discussed in Note 4, the amount to be paid is dependent upon certain development, success-based regulatory, and sales-based milestones. In addition, the amount of royalties to be paid is calculated as a percentage of net sales dependent upon the timing and geography and will, therefore, vary directly with increases and decreases in net sales of Galliprant.
Note 8: Goodwill and Other Intangibles
Goodwill
Goodwill was $3.0 billion and $2.6 billion as of December 31, 2017 and 2016, respectively. Goodwill results from excess consideration in a business combination over the fair value of
F-18
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 8: Goodwill and Other Intangibles (Continued)
identifiable net assets acquired. Goodwill is not amortized but is reviewed for impairment at least annually and when impairment indicators are present. Goodwill may be impaired if the carrying amount of a reporting unit exceeds the fair value of that reporting unit, calculated as based on discounted cash flows. The implied fair value of goodwill is then determined by subtracting the fair value of all identifiable net assets other than goodwill from the fair value of the reporting unit. An impairment charge would be recorded for the excess, if any, of carrying amount of goodwill over the implied fair value. The estimated fair value is based on a number of assumptions, including the projected future cash flows and associated growth rate, the discount rate and the terminal value. In assessing the reasonableness of the estimated fair value, we also consider the reasonableness of the implied EBITDA multiple derived based on the estimated fair value. See Note 4 for further discussion of goodwill resulting from recent business combinations. The remaining change in goodwill is primarily the result of foreign exchange translation adjustments.
No impairments occurred with respect to the carrying value of goodwill for the years ended December 31, 2017, 2016 and 2015.
Other Intangibles
The components of intangible assets other than goodwill at December 31 were as follows:
|
2017 | 2016 |
|||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Description |
Carrying Amount, Gross |
Accumulated Amortization |
Carrying Amount, Net |
Carrying Amount, Gross |
Accumulated Amortization |
Carrying Amount, Net |
|||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Finite-lived intangible assets: |
|||||||||||||||||||
Marketed products |
$ | 3,151.2 | $ | (599.8 | ) | $ | 2,551.4 | $ | 2,815.5 | $ | (404.4 | ) | $ | 2,411.1 | |||||
Other |
54.1 | (29.9 | ) | 24.2 | 70.8 | (41.1 | ) | 29.7 | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total finite-lived intangible assets |
3,205.3 | (629.7 | ) | 2,575.6 | 2,886.3 | (445.5 | ) | 2,440.8 | |||||||||||
Indefinite-lived intangible assets: |
|||||||||||||||||||
Acquired in-process research and development |
97.2 | — | 97.2 | 180.2 | — | 180.2 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Other intangibles |
$ | 3,302.5 | $ | (629.7 | ) | $ | 2,672.8 | $ | 3,066.5 | $ | (445.5 | ) | $ | 2,621.0 | |||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Marketed products consist of the amortized cost of the rights to assets acquired in business combinations and approved for marketing in a significant global jurisdiction. For transactions other than a business combination, we capitalize milestone payments incurred at or after the product has obtained regulatory approval for marketing.
Other finite-lived intangibles consist primarily of the amortized cost of licensed platform technologies that have alternative future uses in research and development, manufacturing technologies and customer relationships from business combinations.
F-19
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 8: Goodwill and Other Intangibles (Continued)
Acquired IPR&D consists of the related costs capitalized, adjusted for subsequent impairments, if any. The costs of acquired IPR&D projects acquired directly in a transaction other than a business combination are capitalized if the projects have an alternative future use; otherwise, they are expensed immediately. The fair values of acquired IPR&D projects acquired in business combinations are capitalized as other intangible assets.
Several methods may be used to determine the estimated fair value of other intangibles acquired in a business combination. We utilize the "income method" for other intangibles. This method is a Level 3 fair value measurement and applies a probability weighting that considers the risk of development and commercialization to the estimated future net cash flows that are derived from projected revenues and estimated costs. These projections are based on factors such as relevant market size, patent protection, historical pricing of similar products and expected industry trends. The estimated future net cash flows are then discounted to the present value using an appropriate discount rate. This analysis is performed for each asset independently. The acquired IPR&D assets are treated as indefinite-lived intangible assets until completion or abandonment of the projects, at which time the assets are tested for impairment and amortized over the remaining useful life or written off, as appropriate.
See Note 4 for further discussion of intangible assets acquired in recent business combinations.
Other indefinite-lived intangible assets are reviewed for impairment at least annually and when impairment indicators are present. The fair value of the indefinite lived intangible assets (acquired IPR&D) is estimated using the same assumptions as used for goodwill and by applying a probability weighting that reflects the risk of development and commercialization to the estimated future net cash flows that are derived from projected revenues and estimated costs. Finite-lived intangible assets are reviewed for impairment when an indicator of impairment is present. We compare the carrying amounts of the assets with the estimated undiscounted future cash flows. In the event the carrying amount exceeds the undiscounted cash flows, an impairment charge is recorded for the amount by which the carrying amount of the asset exceeds the estimated fair value, which is determined based on discounted future cash flows.
During 2017, we had impairment charges of $94.5 million (comprised of $56.5 million impairment of finite-lived intangible assets and $38.0 million impairment of indefinite-lived intangible assets) charged to asset impairment, restructuring and other special charges on the combined statements of operations. The impairment of finite-lived intangible assets primarily related to competitive pressures for a certain marketed product resulting in a reduction of projected cash flows. The impairment of indefinite-lived intangible assets primarily related to revised projections of fair value due to competitive pressures and to a lesser extent product rationalization. During 2016, we had impairment charges of $14.0 million primarily related to indefinite-lived intangible assets charged to asset impairment, restructuring and other special charges on the combined statements of operations. During 2015, we had impairment charges of $32.2 million (comprised of $22.5 million impairment of finite-lived intangible assets and $9.7 million impairment of indefinite-lived intangible assets) charged to asset impairment, restructuring and other special charges on the combined statements of operations. The impairments in 2016 and 2015 were related to product rationalization.
F-20
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 8: Goodwill and Other Intangibles (Continued)
Intangible assets with finite lives are capitalized and are amortized over their estimated useful lives, ranging from 3 to 20 years. As of December 31, 2017, the remaining weighted-average amortization period for finite-lived intangible assets is approximately 15 years.
The estimated amortization expense for each of the next five years associated with our finite-lived intangible assets as of December 31, 2017 is as follows:
|
2018 | 2019 | 2020 | 2021 | 2022 |
|||||||||||
| | | | | | | | | | | | | | | | | |
Estimated amortization expense |
$ | 196.5 | $ | 196.2 | $ | 196.2 | $ | 195.4 | $ | 193.6 |
Note 9: Property and Equipment
Property and equipment is stated on the basis of cost. Provisions for depreciation of buildings and equipment are computed generally by the straight-line method at rates based on their estimated useful lives (12 to 50 years for buildings and 3 to 25 years for equipment). We review the carrying value of long-lived assets for potential impairment on a periodic basis and whenever events or changes in circumstances indicate the carrying value of an asset may not be recoverable. Impairment is determined by comparing projected undiscounted cash flows to be generated by the asset to its carrying value. If an impairment is identified, a loss is recorded equal to the excess of the asset's net book value over its fair value, and the cost basis is adjusted.
At December 31, property and equipment consisted of the following:
|
2017 | 2016 |
|||||
| | | | | | | | |
Land |
$ | 25.1 | $ | 17.7 | |||
Buildings |
557.7 | 457.8 | |||||
Equipment |
994.5 | 880.8 | |||||
Construction in progress |
177.1 | 146.6 | |||||
| | | | | | | | |
|
1,754.4 | 1,502.9 | |||||
Less accumulated depreciation |
(834.1 | ) | (761.1 | ) | |||
| | | | | | | | |
Property and equipment, net |
$ | 920.3 | $ | 741.8 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Depreciation expense related to property and equipment and rental expense for all leases was as follows:
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Depreciation expense |
$ | 79.8 | $ | 75.7 | $ | 71.6 | ||||
Rental expense |
47.1 | 41.8 | 37.7 |
The future minimum rental commitments under non-cancelable operating leases that are expected to transfer to Elanco are as follows:
|
2018 | 2019 | 2020 | 2021 | 2022 | After 2022 |
|||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Lease commitments |
$ | 20.0 | $ | 18.1 | $ | 16.7 | $ | 15.1 | $ | 12.5 | $ | 27.3 |
F-21
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 10: Stock-Based Compensation
Lilly maintains various stock-based compensation programs for the benefit of its officers, directors and certain employees including employees of the Company. As we receive the employee services in consideration for the participation of the Company's employees in these plans, stock-based compensation expense for the awards granted to our employees has been reflected in the combined statements of operations.
Lilly's stock-based compensation granted to our employees consists of performance awards (PAs), shareholder value awards (SVAs) and restricted stock units (RSUs). The stock-based compensation expense has been derived from the equity awards granted by Lilly to our employees. The compensation expense is based on the fair value of stock-based awards which is recognized as compensation expense over the requisite service period of the individual grantees, which generally equals the vesting period. The awards are settled by Lilly.
As the stock-based compensation plans are Lilly's plans and the awards are settled by Lilly, the offset to the expense has been recognized through net parent company investment on the combined balance sheets.
Stock-based compensation expense related to our employees for years ended December 31, 2017, 2016 and 2015 was $25.0 million, $20.4 million and $13.4 million, respectively.
Performance Award Program
PAs have been granted to certain of our officers and management and are settled in shares of Lilly's common stock. The number of PA shares actually issued, if any, varies depending on the achievement of certain pre-established earnings-per-share targets over a two-year period. PA shares are accounted for at fair value based upon the closing stock price on the date of grant and fully vest at the end of the measurement period. The fair values of PAs granted for the years ended December 31, 2017, 2016 and 2015 were $73.54, $72.00 and $70.34, respectively. The number of PA shares that will vest for the PA program is dependent upon Lilly's earnings achieved during the vesting period. Pursuant to this program, approximately 69,144 shares, 20,329 shares and 25,197 shares were issued by Lilly to our employees during the years ended December 31, 2017, 2016 and 2015, respectively. As of December 31, 2017, the total remaining unrecognized compensation cost related to nonvested PAs was $6.2 million, which will be amortized over the weighted-average remaining requisite service period of 12 months.
Shareholder Value Award Program
SVAs have been granted to certain of our officers and management and are settled in shares of Lilly's common stock. The number of shares actually issued, if any, varies depending on Lilly's stock price at the end of the three-year vesting period compared to pre-established target stock prices. We measure the fair value of the SVA unit on the grant date using a Monte Carlo simulation model. The model utilizes multiple input variables that determine the probability of satisfying the market condition stipulated in the award grant and calculates the fair value of the award. Expected volatilities utilized in the model are based on implied volatilities from traded options on Lilly's stock, historical volatility of Lilly's stock price and other factors. Similarly, the dividend yield is based on historical experience and Lilly's estimate of future dividend yields. The risk-free interest rate is
F-22
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 10: Stock-Based Compensation (Continued)
derived from the U.S. Treasury yield curve in effect at the time of grant. The weighted-average fair values of the SVA units granted during the years ended December 31, 2017, 2016 and 2015 were $66.25, $48.68 and $54.81, respectively, determined using the following assumptions:
(Percents) |
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Expected dividend yield |
2.50 | % | 2.00 | % | 2.50 | % | ||||
Risk-free interest rate |
1.38 | 0.92 | 0.79 | |||||||
Volatility |
22.91 | 21.68 | 20.37 |
Pursuant to this program, Lilly issued approximately 35,063 shares, 36,071 shares and 21,956 shares to our employees during the years ended December 31, 2017, 2016 and 2015, respectively. As of December 31, 2017, the total remaining unrecognized compensation cost related to nonvested SVAs was $3.7 million, which will be amortized over the weighted-average remaining requisite service period of 20 months.
Restricted Stock Units
RSUs have been granted to certain of our employees and are payable in shares of Lilly's common stock. RSU shares are accounted for at fair value based upon Lilly's closing stock price on the date of grant. The corresponding expense is amortized over the vesting period, typically three years. The fair values of RSU awards granted during the years ended December 31, 2017, 2016 and 2015 were $72.47, $71.46 and $71.69, respectively. The number of shares ultimately issued by Lilly for the RSU program remains constant with the exception of forfeitures. Pursuant to this program, 57,224, 26,468 and 32,695 RSUs were settled by Lilly with its shares to our employees during the years ended December 31, 2017, 2016 and 2015, respectively. As of December 31, 2017, the total remaining unrecognized compensation cost related to nonvested RSUs was $12.9 million, which will be amortized over the weighted-average remaining requisite service period of 21 months.
Note 11: Income Taxes
During the periods presented in the combined financial statements, Elanco was generally included in the tax grouping of other Lilly entities within the respective entity's tax jurisdiction; however, in certain jurisdictions, Elanco filed separate tax returns. The income tax (benefit)/expense included in these combined financial statements has been calculated using the separate return basis, as if Elanco filed separate tax returns.
2017 Tax Act
In December 2017, the President of the U.S. signed into law the Tax Cuts and Jobs Act (2017 Tax Act). The 2017 Tax Act includes significant changes to the U.S. corporate income tax system, such as the reduction in the corporate income tax rate from 35 percent to 21 percent, transition to a territorial tax system, changes to business related exclusions, deductions and credits, and modifications to international tax provisions, including a one-time repatriation transition tax (also known as the 'Toll Tax') on unremitted foreign earnings.
F-23
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 11: Income Taxes (Continued)
GAAP requires that the income tax accounting effects from a change in tax laws or tax rates be recognized in continuing operations in the reporting period that includes the enactment date of the change. These effects include, among other things, re-measuring deferred tax assets and liabilities, evaluating deferred tax assets for valuation allowances and assessing the impact of the Toll Tax and certain other provisions of the 2017 Tax Act. Our accounting for the tax effects of the enactment of the 2017 Tax Act was not complete as of December 31, 2017; however, in certain cases, as described below, we have made a reasonable estimate. In other cases, we have not been able to make a reasonable estimate and continued to account for those items based on our existing accounting model under ASC 740, Income Taxes and the provisions of the tax laws that were in effect immediately prior to enactment. For the items for which we were able to determine a reasonable estimate, we recognized a provisional benefit of $33.1 million, which is included as a component of income tax expense (benefit) from continuing operations. This amount represents $33.1 million attributable to the re-measurement of deferred taxes and no amount for the Toll Tax.
Our estimate of the impact of the 2017 Tax Act is based upon our analysis and interpretations of currently available information. Uncertainties remain regarding the impact of the 2017 Tax Act due to future regulatory and rulemaking processes, prospects of additional corrective or supplemental legislation and potential trade or other litigation. These uncertainties, along with our completion of the calculations and potential changes in our initial assumptions as new information becomes available, could cause the actual charge to ultimately differ materially from the provisional amount recorded in 2017 related to the enactment of the 2017 Tax Act.
We have included provisional amounts based upon reasonable estimates for the following:
- •
- Toll Tax
The 2017 Tax Act imposes a one-time Toll Tax on unremitted foreign earnings and profits (E&P) at two different tax rates, with a higher tax rate applied to amounts held in cash and liquid assets. We have not yet completed our calculations of the items composing the Toll Tax, including the total post-1986 E&P of our foreign subsidiaries and amounts held as cash and liquid assets; therefore, we have not recorded a provisional amount for federal and state income taxes. The amount is also subject to change as we assimilate the new laws and subsequent regulations, interpretations and guidance as they are issued. The impact to state income tax expense is also subject to change based upon revisions ultimately made to the Toll Tax calculation, changes in our assumptions related to state taxation of the income used to calculate the Toll Tax and future guidance that may be issued.
- •
- Re-measurement of deferred tax assets and liabilities
The 2017 Tax Act reduced the U.S. corporate income tax rate from 35 percent to 21 percent effective January 1, 2018. GAAP requires deferred tax assets and liabilities to be measured at the enacted tax rate expected to apply when these temporary differences are to be realized or settled. As a result, we determined the amount recorded to income tax expense in continuing operations by using temporary differences that approximated our deferred tax balances at the date of enactment considering any material transactions that occurred between the enactment date and December 31, 2017. We assessed the need for valuation allowances as a result of re-measuring existing temporary differences and considering tax attribute balances; changes recorded to valuation
F-24
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 11: Income Taxes (Continued)
allowances are also reflected in the 2017 Tax Act — provisional adjustment. Re-measurement of the deferred tax assets and liabilities in addition to assessment of valuation allowances is subject to uncertainties given that approximated balances were utilized for the enactment date and tax accounting method changes may be considered.
Under GAAP, the effect of a change in tax law is recorded as a component of the income tax expense (benefit) related to continuing operations in the period of enactment.
- •
- Executive compensation
The 2017 Tax Act includes changes to the taxation of executive compensation. We have recorded a provisional amount based upon our estimates, interpretations of the new law and external guidance.
We could not make a reasonable estimate; therefore, we did not record a provisional amount for the following items:
- •
- The 2017 Tax Act includes an international tax provision for the taxation of Global Intangible Low-Taxed Income (GILTI) effective
January 1, 2018. Questions have surfaced as to whether the income taxes related to GILTI should be recorded in the period the tax arises or whether deferred taxes should be established for
basis differences that upon reversal might be subject to GILTI. ASC 740 does not provide clear guidance on this topic and companies are allowed to make an accounting policy election. We have recorded
no provisional amount for GILTI deferred taxes as more time is needed to analyze the data in order to make an accounting policy election.
- •
- The 2017 Tax Act includes significant changes to the U.S. international tax provisions, including GILTI, Base Erosion Anti-abuse Tax and Foreign Derived Intangible Income. For purposes of analyzing valuation allowances for net operating loss and tax credit carryforwards, we recorded no provisional amount for release of valuation allowances as more time is needed to analyze the data.
We will continue to assess the impact of the 2017 Tax Act on our combined financial statements during the measurement period, which should be no longer than one year from the 2017 Tax Act enactment date. As discussed above, the 2017 Tax Act included numerous changes to the U.S. tax system. We have made a good faith effort to identify items for which no reasonable estimate was made; however, additional items requiring accounting may be identified as we complete our analysis and new information becomes available. Therefore, no reasonable estimate has been made for items in the new tax law that have not been identified.
Deferred taxes are recognized for the future tax effects of temporary differences between financial and income tax reporting based on enacted tax laws and rates.
We recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate resolution.
F-25
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 11: Income Taxes (Continued)
Following is the composition of income (loss) before income tax expense (benefit):
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Federal |
$ | (133.2 | ) | $ | (12.5 | ) | $ | (51.1 | ) | |
Foreign |
(99.4 | ) | (9.9 | ) | (208.4 | ) | ||||
| | | | | | | | | | | |
Loss before income taxes |
$ | (232.6 | ) | $ | (22.4 | ) | $ | (259.5 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Following is the composition of income tax expense (benefit):
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Current: |
||||||||||
Foreign |
$ | 91.6 | $ | 31.1 | $ | 28.0 | ||||
State |
(0.1 | ) | 0.3 | (0.5 | ) | |||||
| | | | | | | | | | | |
Total current tax expense |
91.5 | 31.4 | 27.5 | |||||||
Deferred: |
||||||||||
Federal |
42.6 | 18.4 | (12.6 | ) | ||||||
Foreign |
(16.6 | ) | (26.8 | ) | (66.9 | ) | ||||
State |
(6.3 | ) | 2.5 | 3.3 | ||||||
2017 Tax Act — provisional |
(33.1 | ) | — | — | ||||||
| | | | | | | | | | | |
Total deferred tax benefit |
(13.4 | ) | (5.9 | ) | (76.2 | ) | ||||
| | | | | | | | | | | |
Income taxes |
$ | 78.1 | $ | 25.5 | $ | (48.7 | ) | |||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
F-26
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 11: Income Taxes (Continued)
Significant components of our deferred tax assets and liabilities as of December 31 are as follows:
|
2017 | 2016 |
|||||
| | | | | | | | |
Deferred tax assets: |
|||||||
Compensation and benefits |
$ | 34.8 | $ | 46.0 | |||
Accruals and reserves |
12.0 | 16.9 | |||||
Tax credit carryovers |
19.2 | 15.8 | |||||
Tax loss carryovers |
144.9 | 58.7 | |||||
Other |
26.6 | 36.4 | |||||
| | | | | | | | |
Total gross deferred tax assets |
237.5 | 173.8 | |||||
Valuation allowances |
(127.7 | ) | (39.1 | ) | |||
| | | | | | | | |
Total deferred tax assets |
109.8 | 134.7 | |||||
Deferred tax liabilities: |
|||||||
Intangibles |
(165.2 | ) | (192.9 | ) | |||
Property and equipment |
(43.1 | ) | (55.1 | ) | |||
Other |
(7.4 | ) | (12.6 | ) | |||
| | | | | | | | |
Total deferred tax liabilities |
(215.7 | ) | (260.6 | ) | |||
| | | | | | | | |
Deferred tax liabilities — net |
$ | (105.9 | ) | $ | (125.9 | ) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Deferred tax assets and liabilities reflect the provisional impact of re-measurement resulting from the 2017 Tax Act.
The deferred tax assets and related valuation allowance amounts for U.S. federal and state net operating losses and tax credits shown above have been reduced for differences between financial reporting and tax return filings.
At December 31, 2017, we have tax credit carryovers of $19.2 million available to reduce future income taxes; $1.8 million, if unused, will expire by 2037. The remaining portion of the tax credit carryovers is related to federal tax credits of $4.5 million and state tax credits of $12.9 million, all of which are fully reserved.
At December 31, 2017, we had net operating losses and other carryovers for international and U.S. federal income tax purposes of $628.1 million: $0.7 million will expire by 2022; $540.1 million will expire between 2023 and 2037; and $87.3 million of the carryovers will never expire. Net operating losses and other carryovers for international and U.S. federal income tax purposes are partially reserved. Deferred tax assets related to state net operating losses of $23.2 million are fully reserved.
As described in our significant accounting policies, we have prepared our income taxes on a stand-alone tax basis, and as a result, tax credit and net operating loss carryovers may not be available for our use in future periods as they may have already been used in Lilly consolidated or combined tax return filings or they may be retained by Lilly upon our separation.
F-27
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 11: Income Taxes (Continued)
The movements in the valuation allowance are as follows:
|
2017 | 2016 |
|||||
| | | | | | | | |
January 1 |
$ | (39.1 | ) | $ | (20.5 | ) | |
Increase |
(97.4 | ) | (21.1 | ) | |||
Release |
8.8 | 2.5 | |||||
| | | | | | | | |
December 31 |
$ | (127.7 | ) | $ | (39.1 | ) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The 2017 Tax Act introduces international tax provisions that significantly change the U.S. taxation of foreign earnings. At December 31, 2017, no U.S. taxes or foreign withholding taxes have been accrued on unremitted earnings of our foreign subsidiaries as they are considered indefinitely reinvested for continued use in our foreign operations. This provisional amount is subject to change based upon final calculations of the Toll Tax.
Cash payments of income taxes were as follows:
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Cash payments of income taxes |
$ | 35.7 | $ | 53.6 | $ | 17.5 |
The 2017 Tax Act provides an election to taxpayers subject to the Toll Tax to make payments over an eight year period with the first payment due on the original filing due date of the 2017 federal income tax return. While the Toll Tax calculation is provisional, we believe we will not owe a Toll Tax liability. However, we intend to make this election in the event that our final calculation indicates an amount due; therefore, future cash payments of income taxes may include Toll Tax installments.
The following is a reconciliation of the income tax expense (benefit) applying the U.S. federal statutory rate to income before income taxes to reported income tax expense:
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Income tax at the U.S. federal statutory tax rate |
$ | (81.4 | ) | $ | (7.8 | ) | $ | (90.8 | ) | |
Add (deduct): |
||||||||||
International operations and change in foreign tax rates |
55.6 | 8.4 | 38.4 | |||||||
State taxes |
5.4 | 2.8 | 2.4 | |||||||
Income tax credits |
(1.8 | ) | (1.7 | ) | (1.5 | ) | ||||
Foreign inclusion items |
4.2 | 2.4 | 0.1 | |||||||
Change in uncertain tax positions |
6.2 | 5.2 | 1.5 | |||||||
Change in valuation allowance |
122.2 | 18.1 | 1.7 | |||||||
2017 Tax Act — provisional |
(33.1 | ) | — | — | ||||||
Other |
0.8 | (1.9 | ) | (0.5 | ) | |||||
| | | | | | | | | | | |
Income taxes |
$ | 78.1 | $ | 25.5 | $ | (48.7 | ) | |||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
F-28
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 11: Income Taxes (Continued)
A reconciliation of the beginning and ending amount of gross unrecognized tax benefits is as follows:
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Beginning balance at January 1 |
$ | 25.7 | $ | 25.5 | $ | 18.5 | ||||
Additions based on tax positions related to the current year |
7.9 | 7.4 | 3.7 | |||||||
Additions for tax positions of prior years |
— | — | 6.4 | |||||||
Settlements |
(4.0 | ) | (7.1 | ) | (2.4 | ) | ||||
Changes related to the impact of foreign currency translation |
— | (0.1 | ) | (0.7 | ) | |||||
| | | | | | | | | | | |
Ending balance at December 31 |
$ | 29.6 | $ | 25.7 | $ | 25.5 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The total amount of unrecognized tax benefits that, if recognized, would affect our effective tax rate was $29.6 million and $25.7 million at December 31, 2017 and 2016, respectively. Upon our separation, these tax benefits may not be available to us as the tax benefits are attributed to the legal entity which may remain with Lilly.
We file income tax returns in the U.S. federal jurisdiction and various state, local and non-U.S. jurisdictions. Certain of these income tax returns are filed on a consolidated or combined basis with Eli Lilly and Company and/or its subsidiaries. We are no longer subject to U.S. federal, state and local, or non-U.S. income tax examinations in most major taxing jurisdictions for years before 2013.
As part of Lilly, we are included in its U.S. tax examinations by the Internal Revenue Service. The U.S. examination of tax years 2010-2012 commenced during the fourth quarter of 2013 and was effectively settled in 2016 with an immaterial impact to cash tax payments, gross uncertain tax positions and combined returns of operations. The U.S. examination of 2013-2015 began in 2016. While we believe it is reasonably possible that this audit could reach resolution within the next 12 months, the IRS examination of tax years 2013 - 2015 remains ongoing. Therefore, it is not possible to reasonably estimate the change to unrecognized tax benefits and the related future cash flows.
We recognize both accrued interest and penalties related to unrecognized tax benefits in income tax expense (benefit). We recognized income tax (benefit) expense related to interest and penalties as follows:
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Income tax expense (benefit) |
$ | 2.5 | $ | 5.5 | $ | 2.6 |
At December 31, 2017 and 2016, our accruals for the payment of interest and penalties totaled $17.0 million and $14.5 million, respectively.
Note 12: Retirement Benefits
Shared Lilly Plans
Our employees participated in defined benefit pension and other postretirement plans sponsored by Lilly, which include participants of Lilly's other business. Such plans are accounted
F-29
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 12: Retirement Benefits (Continued)
for as multiemployer plans in these combined financial statements and as a result, no asset or liability was recorded by the Company to recognize the funded status of these plans.
We recorded expense of $73.7 million, $11.3 million and $26.2 million for the years ended December 31, 2017, 2016 and 2015, respectively, relating to our employees' participation in Lilly sponsored plans. Included in the 2017 amount was $67.0 million related to a curtailment loss and special termination benefits for early retirement incentives offered by Lilly to our employees as part of a voluntary early retirement program for the U.S. plan and which has been recorded in asset impairment, restructuring and other special charges. No contributions have been recognized in the combined financial statements as we are not required to make contributions to these plans.
F-30
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 12: Retirement Benefits (Continued)
Pension Plans
There are also certain defined benefit pension plans that our employees participate in that are either dedicated to our employees or where the plan assets and liabilities that relate to our employees are legally required to transfer to Elanco at the time of our separation from Lilly. The plans in Switzerland represent approximately 88 percent of our global benefit obligation. We use a measurement date of December 31 to develop the change in benefit obligation, change in plan assets, funded status and amounts recognized in the combined balance sheets at December 31 for our defined benefit pension plans, which were as follows:
|
2017 | 2016 |
|||||
| | | | | | | | |
Change in benefit obligation: |
|||||||
Benefit obligation at beginning of year |
$ | 225.0 | $ | 234.7 | |||
Service cost |
10.5 | 9.3 | |||||
Interest cost |
1.8 | 1.8 | |||||
Actuarial (gain) loss |
24.4 | (1.6 | ) | ||||
Benefits paid |
(18.5 | ) | (19.8 | ) | |||
Foreign currency exchange rate changes and other adjustments |
15.4 | 0.6 | |||||
| | | | | | | | |
Benefit obligation at end of year |
258.6 | 225.0 | |||||
Change in plan assets: |
|||||||
Fair value of plan assets at beginning of year |
123.7 | 127.4 | |||||
Actual return on plan assets |
13.3 | (1.4 | ) | ||||
Employer contribution |
3.9 | 15.3 | |||||
Benefits paid |
(18.5 | ) | (19.8 | ) | |||
Foreign currency exchange rate changes and other adjustments |
9.1 | 2.2 | |||||
| | | | | | | | |
Fair value of plan assets at end of year |
131.5 | 123.7 | |||||
| | | | | | | | |
Funded status |
(127.1 | ) | (101.3 | ) | |||
Unrecognized net actuarial loss |
29.1 | 17.2 | |||||
Unrecognized prior service cost |
0.7 | 0.6 | |||||
| | | | | | | | |
Net amount recognized |
$ | (97.3 | ) | $ | (83.5 | ) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Amounts recognized in the combined balance sheet consisted of: |
|||||||
Noncurrent assets |
$ | 2.4 | $ | 3.0 | |||
Other current liabilities |
(0.3 | ) | (0.3 | ) | |||
Accrued retirement benefits |
(129.2 | ) | (104.0 | ) | |||
Accumulated other comprehensive loss before income taxes |
29.8 | 17.8 | |||||
| | | | | | | | |
Net amount recognized |
$ | (97.3 | ) | $ | (83.5 | ) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The unrecognized net actuarial loss and unrecognized prior service cost for these pension plans have not yet been recognized in net periodic pension costs and are included in accumulated other comprehensive loss at December 31, 2017.
F-31
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 12: Retirement Benefits (Continued)
During 2018, we expect the following components of accumulated other comprehensive loss to be recognized as components of net periodic benefit cost:
Unrecognized net actuarial loss |
$ | 1.7 | ||
Unrecognized prior service cost |
0.1 | |||
| | | | | |
Total |
$ | 1.8 | ||
| | | | | |
| | | | | |
| | | | | |
We do not expect any plan assets to be returned to us in 2018.
The following represents our weighted-average assumptions related to these pension plans as of December 31:
(Percents) |
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Discount rate for benefit obligation |
1.1 | % | 1.0 | % | 1.0 | % | ||||
Discount rate for net benefit costs |
1.0 | 1.0 | 1.2 | |||||||
Rate of compensation increase for benefit obligation |
2.1 | 3.1 | 3.0 | |||||||
Rate of compensation increase for net benefit costs |
3.1 | 3.0 | 2.0 | |||||||
Expected return on plan assets for net benefit costs |
4.4 | 4.9 | 4.4 |
We annually evaluate the expected return on the plan assets in these pension plans. In evaluating the expected rate of return, we consider many factors, with a primary analysis of current and projected market conditions; asset returns and asset allocations; and the views of leading financial advisers and economists. We may also review our historical assumptions compared with actual results, as well as the assumptions and trend rates utilized by similar plans, where applicable.
The following benefit payments, which reflect expected future service, as appropriate, are expected to be paid as follows:
|
2018 | 2019 | 2020 | 2021 | 2022 | 2022-2026 |
|||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Benefit payments |
$ | 5.5 | $ | 6.0 | $ | 6.0 | $ | 6.1 | $ | 6.3 | $ | 33.8 |
Amounts relating to these pension plans with projected benefit obligations in excess of plan assets were as follows at December 31:
|
2017 | 2016 |
|||||
| | | | | | | | |
Projected benefit obligation |
$ | 251.6 | $ | 218.7 | |||
Fair value of plan assets |
121.8 | 114.4 |
Amounts relating to these defined benefit pension plans with accumulated benefit obligations in excess of plan assets were as follows at December 31:
|
2017 | 2016 |
|||||
| | | | | | | | |
Accumulated benefit obligation |
$ | 223.1 | $ | 180.6 | |||
Fair value of plan assets |
121.8 | 114.4 |
F-32
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 12: Retirement Benefits (Continued)
The total accumulated benefit obligation for these defined benefit pension plans was $230.3 million and $186.9 million at December 31, 2017 and 2016, respectively.
Net pension expense related to these plans included the following components:
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Service cost |
$ | 10.5 | $ | 9.3 | $ | 8.4 | ||||
Interest cost |
1.8 | 1.8 | 2.9 | |||||||
Expected return on plan assets |
(2.4 | ) | (3.4 | ) | (4.5 | ) | ||||
Amortization of prior service cost |
0.1 | 0.1 | — | |||||||
Amortization of net actuarial loss |
1.4 | 1.0 | 0.3 | |||||||
| | | | | | | | | | | |
Net pension expense |
$ | 11.4 | $ | 8.8 | $ | 7.1 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The following represents the amounts recognized for these plans in other comprehensive loss for the years ended December 31, 2017, 2016 and 2015:
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Actuarial loss arising during period |
$ | (17.0 | ) | $ | (6.1 | ) | $ | (13.9 | ) | |
Amortization of prior service cost included in net loss |
0.1 | 0.1 | — | |||||||
Amortization of net actuarial loss included in net loss |
1.4 | 1.0 | 0.3 | |||||||
Foreign currency exchange rate changes and other |
3.5 | 3.0 | 0.3 | |||||||
| | | | | | | | | | | |
Total other comprehensive loss during period |
$ | (12.0 | ) | $ | (2.0 | ) | $ | (13.3 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Benefit Plan Investments
Our benefit plan investment policies are set with specific consideration of return and risk requirements in relationship to the respective liabilities. Our plan assets in our Switzerland pension plans represent approximately 92 percent of our plan assets for these pension plans. Given the long-term nature of our liabilities, these plans have the flexibility to manage an above-average degree of risk in the asset portfolios. At the investment-policy level, there are no specifically prohibited investments. However, within individual investment manager mandates, restrictions and limitations are contractually set to align with our investment objectives, ensure risk control and limit concentrations.
We manage our portfolio to minimize concentration of risk by allocating funds within asset categories. In addition, within a category we use different managers with various management objectives to eliminate any significant concentration of risk.
The investment strategy is to diversify in four major categories with a designated percentage invested in each including 25% fixed income securities, 48% equity securities, a share of 10% in Real Estate Switzerland and 17% in other alternative investments (senior loans, hedge funds and insurance-linked securities). Each category is diversified and comprised of the following:
- •
- Fixed-income securities — Swiss Bonds, Global Aggregates, Global Aggregate Corporates and Emerging Markets Local Currencies.
F-33
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 12: Retirement Benefits (Continued)
- •
- Equity investments — Swiss Equities, World Equities MSCI, Low Volatility Equities (to reduce risk), Emerging Markets
Equities and real estate investment trusts.
- •
- Real Estate in Switzerland — investment foundations and funds
- •
- Other investments — represents primarily private equity like investments, hedge funds, insurance-linked securities, cash and mark-to-market derivatives.
We determine the fair value of the investments based on a market approach using quoted market values, significant other observable inputs for identical or comparable assets or liabilities, or discounted cash flow analyses for all investments except hedge funds, private equity-like investments and real estate.
We determine the fair value of investments using the value reported by the partnership, adjusted for known cash flows and significant events through our reporting date. Values provided by the partnerships are primarily based on analysis of and judgments about the underlying investments. Inputs to these valuations include underlying NAVs, discounted cash flow valuations, comparable market valuations, and may also include adjustments for currency, credit, liquidity and other risks as applicable. The vast majority of these private partnerships provide us with annual audited financial statements including their compliance with fair valuation procedures consistent with applicable accounting standards.
We determine the fair value of real estate investments based on the NAV provided by the fund manager, These NAVs are developed with inputs including discounted cash flow, independent appraisal and market comparable analyses.
F-34
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 12: Retirement Benefits (Continued)
The fair values of these pension plan assets as of December 31, 2017 by asset category are as follows:
|
Fair Value Measurements Using | |||||||||||||||
| | | | | | | | | | | | | | | | | |
Asset Class |
Total | Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Investments Valued at Net Asset Value(1) |
|||||||||||
| | | | | | | | | | | | | | | | | |
Public equity securities |
$ | 0.8 | $ | 0.6 | $ | — | $ | — | $ | 0.2 | ||||||
Fixed income: |
||||||||||||||||
Developed markets |
29.9 | 8.2 | 0.1 | — | 21.6 | |||||||||||
Emerging markets |
7.2 | 0.6 | 0.3 | — | 6.3 | |||||||||||
Private alternative investments: |
||||||||||||||||
Hedge funds |
6.8 | — | — | — | 6.8 | |||||||||||
Equity-like funds |
52.7 | — | — | — | 52.7 | |||||||||||
Real estate |
20.2 | — | — | — | 20.2 | |||||||||||
Other |
13.9 | 0.1 | 0.1 | 13.7 | ||||||||||||
| | | | | | | | | | | | | | | | | |
Total |
$ | 131.5 | $ | 9.5 | $ | 0.5 | $ | — | $ | 121.5 | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- Certain investments that are measured at fair value using the NAV per share (or its equivalent) as a practical expedient have not been classified in the fair value hierarchy.
No material transfers between Level 1, Level 2, or Level 3 occurred during the year ended December 31, 2017. The activity in the Level 3 investments during the year ended December 31, 2017 was not material.
F-35
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 12: Retirement Benefits (Continued)
The fair values of these pension plan assets as of December 31, 2016 by asset category are as follows:
|
Fair Value Measurements Using | |||||||||||||||
| | | | | | | | | | | | | | | | | |
Asset Class |
Total | Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Investments Valued at Net Asset Value(1) |
|||||||||||
| | | | | | | | | | | | | | | | | |
Public equity securities |
$ | 46.2 | $ | 0.1 | $ | — | $ | — | $ | 46.1 | ||||||
Fixed income: |
||||||||||||||||
Developed markets |
31.0 | 9.1 | — | — | 21.9 | |||||||||||
Emerging markets |
5.6 | — | — | — | 5.6 | |||||||||||
Private alternative investments: |
||||||||||||||||
Hedge funds |
6.4 | — | — | — | 6.4 | |||||||||||
Other |
34.5 | 21.7 | — | — | 12.8 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total |
$ | 123.7 | $ | 30.9 | $ | — | $ | — | $ | 92.8 | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- Certain investments that are measured at fair value using the NAV per share (or its equivalent) as a practical expedient have not been classified in the fair value hierarchy.
No material transfers between Level 1, Level 2, or Level 3 occurred during the year ended December 31, 2016. The activity in the Level 3 investments during the year ended December 31, 2016 was not material.
In 2018, we expect to contribute approximately $6 million to these pension plans to satisfy minimum funding requirements for the year. Additional discretionary contributions are not expected to be significant.
Retiree Health Benefit Plan
There is a retiree health benefit plan where the plan liabilities that relate to our employees are legally required to transfer to Elanco at the time of separation from Lilly. The accrued retirement benefits for this plan were $9.8 million and $9.8 million as of December 31, 2017 and 2016, respectively.
Defined Contribution Plans
Lilly has defined contribution savings plans that include certain of our employees worldwide. The purpose of these plans is generally to provide additional financial security during retirement by providing employees with an incentive to save. Our contributions to the plans are based on our employee contributions and the level of our match. Expenses related to our employees under the plans totaled $22.1 million, $19.6 million and $16.2 million for the years ended December 31, 2017, 2016, and 2015, respectively.
F-36
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 13: Contingencies
We are a party to various legal actions in the normal course of business. We record a liability if there is a claim for which it is probable we will make a payment and the amount is estimable. At December 31, 2017 and 2016 we had no liabilities established related to litigation as there are no claims which were probable and estimable. We have not historically had any significant litigation expense and are not currently subject to any claim.
Note 14: Other Comprehensive Loss
The following table summarizes the activity related to each component of other comprehensive loss:
(Amounts presented net of taxes) |
Foreign Currency Translation Gains (Losses) |
Defined Benefit Pension and Retiree Health Benefit Plans |
Accumulated Other Comprehensive Loss |
|||||||
| | | | | | | | | | | |
Beginning balance at January 1, 2015 |
$ | (63.0 | ) | $ | (3.5 | ) | $ | (66.5 | ) | |
Net other comprehensive income (loss) |
(143.6 | ) | (11.8 | ) | (155.4 | ) | ||||
| | | | | | | | | | | |
Balance at December 31, 2015 |
(206.6 | ) | (15.3 | ) | (221.9 | ) | ||||
Net other comprehensive income (loss) |
(230.7 | ) | (4.3 | ) | (235.0 | ) | ||||
| | | | | | | | | | | |
Balance at December 31, 2016 |
(437.3 | ) | (19.6 | ) | (456.9 | ) | ||||
Net other comprehensive income (loss) |
210.1 | (9.8 | ) | 200.3 | ||||||
| | | | | | | | | | | |
Ending balance at December 31, 2017 |
$ | (227.2 | ) | $ | (29.4 | ) | $ | (256.6 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Note 15: Geographic Information
We operate as a single operating segment engaged in the development, manufacturing, marketing and sales of animal health products worldwide for both food animals ("FA") and companion animals ("CA"). Consistent with our operational structure, our President and Chief Executive Officer ("CEO"), as the chief operating decision maker, makes resource allocation and business process decisions globally across our consolidated business. Strategic decisions are managed globally with global functional leaders responsible for determining significant costs/investments and with regional leaders responsible for overseeing the execution of the global strategy. Our global research and development organization is responsible for development of new products. Our manufacturing organization is responsible for the manufacturing and supply of products and for the optimization of our supply chain. Regional leaders are responsible for the distribution and sale of our products and for local direct costs. The business is also supported by global corporate staff functions. Managing and allocating resources at the global corporate level enables our CEO to assess the overall level of resources available and how to best deploy these resources across functions, product types, regional commercial organizations and research and development projects in line with our overarching long-term corporate-wide strategic goals, rather than on a product or geographic basis. Consistent with this decision-making process, our CEO uses consolidated, single-segment financial information for purposes of evaluating performance,
F-37
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 15: Geographic Information (Continued)
allocating resources, setting incentive compensation targets, as well as forecasting future period financial results.
Our products include Rumensin®, Optaflexx®, Denagard®, Tylan®, Maxiban® and other products for livestock and poultry, as well as Trifexis®, Interceptor®, Comfortis® and other products for companion animals. Our results for the year ended December 31, 2017 includes the results of operations from BIVIVP, which was acquired on January 3, 2017 (Note 4).
We have a single customer that accounted for 12.9%, 11.7% and 9.1% of revenue for the years ended December 31, 2017, 2016 and 2015, respectively, and that represented accounts receivable of $88.0 million and $52.8 million as of December 31, 2017 and 2016, respectively.
We are exposed to the risk of changes in social, political and economic conditions inherent in foreign operations and our results of operations and the value of our foreign assets are affected by fluctuations in foreign currency exchange rates.
The following table summarizes our revenue activity:
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
CA Disease Prevention |
$ | 660.2 | $ | 628.4 | $ | 591.2 | ||||
CA Therapeutics |
260.8 | 255.6 | 245.2 | |||||||
CA Other |
143.8 | 89.5 | 82.9 | |||||||
FA Future Protein & Health |
649.2 | 630.8 | 633.2 | |||||||
FA Ruminants Swine |
1,175.0 | 1,309.2 | 1,356.6 | |||||||
| | | | | | | | | | | |
Total Revenue |
$ | 2,889.0 | $ | 2,913.5 | $ | 2,909.1 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Selected geographic area information was as follows:
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Geographic Information |
||||||||||
Revenue — to unaffiliated customers(1): |
||||||||||
United States |
$ | 1,373.0 | $ | 1,361.6 | $ | 1,318.9 | ||||
International |
1,516.0 | 1,551.9 | 1,590.2 | |||||||
| | | | | | | | | | | |
Revenue |
$ | 2,889.0 | $ | 2,913.5 | $ | 2,909.1 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Long-lived assets(2): |
||||||||||
United States |
$ | 604.7 | $ | 463.8 | $ | 406.8 | ||||
United Kingdom |
204.4 | 190.6 | 240.3 | |||||||
Other foreign countries |
190.2 | 173.0 | 223.1 | |||||||
| | | | | | | | | | | |
Long-lived assets |
$ | 999.3 | $ | 827.4 | $ | 870.2 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- Revenue
is attributed to the countries based on the location of the customer.
- (2)
- Long-lived assets consist of property and equipment, net, and certain noncurrent assets.
F-38
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 16: Related Party Transactions
The Company has not historically operated as a standalone business and has various relationships with Lilly whereby Lilly provides services to the Company.
Transfers to/from Lilly, net
As discussed in the basis of preparation, net parent company investment is primarily impacted by contributions from Lilly which are the result of treasury activity and net funding provided by or distributed to Lilly. For the years ended December 31, 2017, 2016, and 2015, the net transfers (to)/from Lilly were $873.3 million, ($129.2) million and $5,366.6 million, respectively. The most significant activity impacting these transfers was the financing by Lilly of Elanco's acquisitions in the amount of $882.1 million for BIVIVP in 2017, $45.0 million for Galliprant in 2016, and $5,283.1 million for Novartis AH business in 2015. Other activities that impacted the net transfers to/from Lilly, but to a lesser extent, include corporate overhead and other allocations, income taxes, retirement benefits and centralized cash management.
Corporate Overhead and Other Allocations
The financial information in these combined financial statements does not necessarily include all the expenses that would have been incurred had the Company been a separate, standalone entity. Lilly provides the Company certain services, including executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations. The Company provides Lilly certain services related to manufacturing support. Our combined financial statements reflect an allocation of these costs. When specific identification is not practicable, a proportional cost method is used, primarily based on sales, and headcount.
The allocations of services from Lilly to the Company were reflected as follows in the combined statements of operations:
|
2017 | 2016 | 2015 |
|||||||
| | | | | | | | | | | |
Cost of sales |
$ | 31.8 | $ | 32.5 | $ | 25.9 | ||||
Research and development |
2.8 | 2.3 | 6.3 | |||||||
Marketing, selling and administrative |
117.1 | 110.5 | 123.8 | |||||||
| | | | | | | | | | | |
Total |
$ | 151.7 | $ | 145.3 | $ | 156.0 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The Company provides Lilly certain services related to manufacturing support. Allocations of manufacturing support from the Company to Lilly of $6.2 million, $5.5 million and $6.3 million for the years ended December 31, 2017, 2016 and 2015, respectively, reduced cost of sales in the combined statements of operations.
The financial information herein may not necessarily reflect the combined financial position, results of operations and cash flows of the Company in the future or what they would have been had the Company been a separate, standalone entity during the periods presented. Management believes that the methods used to allocate expenses to the Company are reasonable.
F-39
Animal Health Businesses of Eli Lilly to be Divested
Notes to Combined Financial Statements (Continued)
(Tables present dollars in millions)
Note 16: Related Party Transactions (Continued)
Stock-based Compensation
As discussed in Note 10, the Company's employees participate in Lilly stock-based compensation plans, the costs of which have been allocated to the Company and recorded in cost of sales, research and development, and marketing, selling and administrative expenses in the combined statements of operations. Stock-based compensation costs related to the Company's employees were $25.0 million, $20.4 million and $13.4 million for the years ended December 31, 2017, 2016 and 2015, respectively.
Retirement Benefits
As discussed in Note 12, the Company's employees participate in defined benefit pension and other postretirement plans sponsored by Lilly, the costs of which have been recorded in the combined statement of operations in cost of sales, research and development, and marketing, selling and administrative expenses and for 2017 in asset impairment, restructuring, and other special charges for the portion related to curtailment and special termination benefits. The costs of such plans related to the Company's employees were $73.7 million, $11.3 million and $26.2 million for the years ended December 31, 2017, 2016 and 2015, respectively.
Centralized Cash Management
Lilly uses a centralized approach to cash management and financing of operations. The majority of the Company's business is party to Lilly's cash pooling arrangements to maximize Lilly's availability of cash for general operating and investing purposes. Under these cash pooling arrangements, cash balances are swept regularly from the Company's accounts. Cash transfers to and from Lilly's cash concentration accounts and the resulting balances at the end of each reporting period are reflected in net parent company investment in the combined balance sheets.
Debt
Lilly's third-party debt and the related interest expense have not been allocated to the Company for any of the periods presented as the Company was not the legal obligor of the debt and Lilly borrowings were not directly attributable to the Company's businesses.
Commercial Operations
The Company sells certain products to and receives certain goods and services from a customer/vendor, whose chairman and Chief Executive Officer is a member of Lilly's Board of Directors. These product sales resulted in revenue of $24.8 million, $14.3 million and $16.5 million for the years ended December 31, 2017, 2016 and 2015, respectively. These product sales resulted in accounts receivable of $2.0 million and $0.8 million at December 31, 2017 and 2016, respectively. The purchase of goods and services resulted in cost of sales and operating expenses of $5.9 million, $7.1 million and $3.6 million for the years ended December 31, 2017, 2016 and 2015, respectively. The purchase of goods and services resulted in accounts payable of $0.3 million and $0.4 million at December 31, 2017 and 2016, respectively.
F-40
Condensed Combined Statements of Operations
(Unaudited)
Animal Health Businesses of Eli Lilly to be Divested
(Dollars in millions)
|
Six Months Ended June 30, |
||||||
| | | | | | | | |
|
2018 | 2017 |
|||||
| | | | | | | | |
Revenue |
$ | 1,506.4 | $ | 1,437.6 | |||
Costs, expenses and other: |
|||||||
Cost of sales |
791.5 | 712.7 | |||||
Research and development |
126.6 | 127.9 | |||||
Marketing, selling and administrative |
371.1 | 388.4 | |||||
Amortization of intangible assets |
98.6 | 109.4 | |||||
Asset impairments, restructuring and other special charges (Note 4) |
70.4 | 165.6 | |||||
Other — net, (income) expense |
10.7 | 1.6 | |||||
| | | | | | | | |
|
1,468.9 | 1,505.6 | |||||
| | | | | | | | |
Income (loss) before income taxes |
37.5 | (68.0 | ) | ||||
Income tax expense |
27.6 | 60.5 | |||||
| | | | | | | | |
Net income (loss) |
$ | 9.9 | $ | (128.5 | ) | ||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
See notes to condensed combined financial statements.
F-41
Condensed Combined Statements of Comprehensive Income (Loss)
(Unaudited)
Animal Health Businesses of Eli Lilly to be Divested
(Dollars in millions)
|
Six Months Ended June 30, |
||||||
| | | | | | | | |
|
2018 | 2017 | |||||
| | | | | | | | |
Net income (loss) |
$ | 9.9 | $ | (128.5 | ) | ||
Other comprehensive income (loss), net of tax |
(104.3 | ) | 235.8 | ||||
| | | | | | | | |
Comprehensive income (loss) |
$ | (94.4 | ) | $ | 107.3 | ||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
See notes to condensed combined financial statements.
F-42
Condensed Combined Balance Sheets
Animal Health Businesses of Eli Lilly to be Divested
(Dollars in millions)
|
June 30, 2018 |
December 31, 2017 |
|||||
| | | | | | | | |
|
(Unaudited) | ||||||
Assets |
|||||||
Current Assets |
|||||||
Cash and cash equivalents |
$ | 321.0 | $ | 323.4 | |||
Accounts receivable, net of allowances of $9.1 (2018) and $9.8 (2017) |
587.8 | 567.4 | |||||
Other receivables |
35.9 | 34.5 | |||||
Inventories (Note 5) |
1,005.6 | 1,062.3 | |||||
Prepaid expenses and other |
106.6 | 136.1 | |||||
| | | | | | | | |
Total current assets |
2,056.9 | 2,123.7 | |||||
| | | | | | | | |
Noncurrent Assets |
|||||||
Investments (Note 6) |
12.8 | 12.3 | |||||
Goodwill |
2,932.3 | 2,969.2 | |||||
Other intangibles, net |
2,534.9 | 2,672.8 | |||||
Other noncurrent assets |
162.8 | 242.0 | |||||
Property and equipment, net of accumulated depreciation of $835.8 (2018) and $834.1 (2017) |
877.7 | 920.3 | |||||
| | | | | | | | |
Total assets |
$ | 8,577.4 | $ | 8,940.3 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Liabilities and Equity |
|||||||
Current Liabilities |
|||||||
Accounts payable |
$ | 193.9 | $ | 203.8 | |||
Employee compensation |
65.4 | 89.3 | |||||
Sales rebates and discounts |
127.6 | 155.0 | |||||
Other current liabilities |
171.5 | 184.5 | |||||
| | | | | | | | |
Total current liabilities |
558.4 | 632.6 | |||||
Noncurrent Liabilities |
|||||||
Accrued retirement benefits |
142.6 | 139.0 | |||||
Deferred taxes |
175.5 | 251.9 | |||||
Other noncurrent liabilities |
114.3 | 126.0 | |||||
| | | | | | | | |
Total liabilities |
990.8 | 1,149.5 | |||||
| | | | | | | | |
Commitments and Contingencies (Note 8) |
|||||||
Equity |
|||||||
Net parent company investment |
7,947.5 | 8,047.4 | |||||
Accumulated other comprehensive loss |
(360.9 | ) | (256.6 | ) | |||
| | | | | | | | |
Total equity |
7,586.6 | 7,790.8 | |||||
| | | | | | | | |
Total liabilities and equity |
$ | 8,577.4 | $ | 8,940.3 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
See notes to condensed combined financial statements.
F-43
Condensed Combined Statements of Equity
(Unaudited)
Animal Health Businesses of Eli Lilly to be Divested
(Dollars in millions)
|
Accumulated Other Comprehensive Income (Loss) |
|||||||||||||||
| | | | | | | | | | | | | | | | | |
|
Net Parent Company Investment |
Foreign Currency Translation |
Defined Benefit Pension and Retiree Health Benefit Plans |
Total | Total Equity |
|||||||||||
| | | | | | | | | | | | | | | | | |
Balance at December 31, 2016 |
$ | 7,484.8 | $ | (437.3 | ) | $ | (19.6 | ) | $ | (456.9 | ) | $ | 7,027.9 | |||
Net loss |
(128.5 | ) | — | — | — | (128.5 | ) | |||||||||
Other comprehensive income, net of tax |
— | 233.3 | 2.5 | 235.8 | 235.8 | |||||||||||
Transfers to/from Lilly, net |
824.6 | — | — | — | 824.6 | |||||||||||
| | | | | | | | | | | | | | | | | |
Balance at June 30, 2017 |
$ | 8,180.9 | $ | (204.0 | ) | $ | (17.1 | ) | $ | (221.1 | ) | $ | 7,959.8 | |||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Balance at December 31, 2017 |
$ |
8,047.4 |
$ |
(227.2 |
) |
$ |
(29.4 |
) |
$ |
(256.6 |
) |
$ |
7,790.8 |
|||
Adoption of Accounting Standards Update 2016-16 |
(0.3 | ) | (0.3 | ) | ||||||||||||
Net income |
9.9 | — | — | — | 9.9 | |||||||||||
Other comprehensive income (loss), net of tax |
— | (105.7 | ) | 1.4 | (104.3 | ) | (104.3 | ) | ||||||||
Transfers to/from Lilly, net |
(109.5 | ) | — | — | — | (109.5 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
Balance at June 30, 2018 |
$ | 7,947.5 | $ | (332.9 | ) | $ | (28.0 | ) | $ | (360.9 | ) | $ | 7,586.6 | |||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
F-44
Condensed Combined Statements of Cash Flows
(Unaudited)
Animal Health Businesses of Eli Lilly to be Divested
(Dollars in millions)
|
Six Months Ended June 30, |
||||||
| | | | | | | | |
|
2018 | 2017 |
|||||
| | | | | | | | |
Cash Flows from Operating Activities |
|||||||
Net income (loss) |
$ | 9.9 | $ | (128.5 | ) | ||
Adjustments to Reconcile Net Income (Loss) to Cash Flows from Operating Activities: |
|||||||
Depreciation and amortization |
149.6 | 156.1 | |||||
Change in deferred income taxes |
10.8 | (0.6 | ) | ||||
Stock-based compensation expense |
13.3 | 12.5 | |||||
Asset impairment charges |
97.9 | 43.8 | |||||
Gain on sale of assets |
— | (16.0 | ) | ||||
Other changes in operating assets and liabilities, net of acquisitions and divestitures |
(98.3 | ) | 20.2 | ||||
Other non-cash operating activities, net |
0.7 | 3.1 | |||||
| | | | | | | | |
Net Cash Provided by Operating Activities |
183.9 | 90.6 | |||||
| | | | | | | | |
Cash Flows from Investing Activities |
|||||||
Net purchases of property and equipment |
(56.5 | ) | (10.0 | ) | |||
Cash paid for acquisitions, net of cash acquired |
— | (882.1 | ) | ||||
Other investing activities, net |
(1.0 | ) | (11.7 | ) | |||
| | | | | | | | |
Net Cash Used for Investing Activities |
(57.5 | ) | (903.8 | ) | |||
| | | | | | | | |
Cash Flows from Financing Activities |
|||||||
Net transactions with Lilly |
(122.8 | ) | 812.1 | ||||
Other financing activities, net |
(0.9 | ) | (0.2 | ) | |||
| | | | | | | | |
Net Cash Provided by (Used for) Financing Activities |
(123.7 | ) | 811.9 | ||||
| | | | | | | | |
Effect of exchange rate changes on cash and cash equivalents |
(5.1 | ) | 6.9 | ||||
| | | | | | | | |
Net increase (decrease) in cash and cash equivalents |
(2.4 | ) | 5.6 | ||||
Cash and cash equivalents at January 1 |
323.4 | 258.8 | |||||
| | | | | | | | |
Cash and Cash Equivalents at June 30 |
$ | 321.0 | $ | 264.4 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
See notes to condensed combined financial statements.
F-45
Notes to Condensed Combined Financial Statements
(Unaudited)
(Tables present dollars in millions)
Note 1: Nature of Business and Basis of Presentation
Nature of Business
Eli Lilly and Company (Lilly) intends to divest substantially all of its animal health businesses through a series of equity transactions. The businesses to be divested are currently held in a combination of dedicated legal entities and commingled entities, which include activities of both Lilly and the divested businesses. Lilly will complete a corporate reorganization prior to the divestiture through which it will transfer the assets, liabilities and businesses to be divested to a single holding company (Elanco Parent). Elanco Parent will ultimately serve as parent company for the businesses to be divested by Lilly.
The accompanying unaudited condensed combined financial statements represent the assets, liabilities and results of operations related to the animal health businesses to be transferred to Elanco Parent, which includes the animal health businesses that share people, manufacturing locations and activities. The combined animal health businesses to be transferred from Lilly to Elanco Parent are referred to throughout these unaudited condensed combined financial statements as Elanco, the Company, we, us or our.
Basis of Presentation
We have prepared the accompanying unaudited condensed combined financial statements in accordance with the requirements for interim reporting and, therefore, they do not include all information and footnotes necessary for a fair presentation of financial position, results of operations, and cash flows in conformity with accounting principles generally accepted in the United States (GAAP). In our opinion, the financial statements reflect all adjustments (including those that are normal and recurring) that are necessary for a fair presentation of the results of operations for the periods shown. In preparing financial statements in conformity with GAAP, we must make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, expenses, and related disclosures at the date of the financial statements and during the reporting period. Actual results could differ from those estimates.
The information included in this interim report should be read in conjunction with our combined financial statements and accompanying notes included elsewhere in this prospectus.
The accompanying unaudited condensed combined financial statements have been prepared on a standalone basis and are derived from Lilly's consolidated financial statements and accounting records. The unaudited condensed combined financial statements reflect the financial position, results of operations and cash flows related to the animal health businesses that will be transferred to Elanco Parent and are prepared in conformity with accounting principles generally accepted in the United States (GAAP). Lilly will transfer to Elanco Parent only the assets, liabilities and operations for business activities that will constitute the ongoing animal health businesses. These businesses operate on an integrated basis with shared people, manufacturing facilities, distribution centers, product types and the associated facilities that are being transferred to Elanco Parent.
These unaudited condensed combined financial statements include the attribution of certain assets and liabilities that historically have been held at the Lilly corporate level but which are specifically identifiable or attributable to the businesses being transferred to Elanco Parent. All intercompany transactions and accounts within Elanco have been eliminated. All transactions
F-46
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 1: Nature of Business and Basis of Presentation (Continued)
between us and Lilly are considered to be effectively settled in the unaudited condensed combined financial statements at the time the transaction is recorded. The total net effect of the settlement of these intercompany transactions is reflected in the condensed combined statements of cash flows as a financing activity and in the condensed combined balance sheets as net parent company investment.
These unaudited condensed combined financial statements include an allocation of expenses related to certain Lilly corporate functions, including executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations. These expenses have been allocated to us based on direct usage or benefit where specifically identifiable, with the remainder allocated primarily on a pro rata basis of revenue, headcount and other measures. We consider the expenses methodology and results to be reasonable for all periods presented. However, the allocations may not be indicative of the actual expense that would have been incurred had we operated as an independent, publicly traded company for the periods presented. It is impractical to estimate what the standalone costs of Elanco would have been in the historical periods.
The income tax amounts in these unaudited condensed combined financial statements have been calculated based on a separate return methodology and presented as if our operations were separate taxpayers in the respective jurisdictions. We file income tax returns in the United States (U.S.) federal jurisdiction and various state, local and non-U.S. jurisdictions. Certain of these income tax returns are filed on a consolidated or combined basis with Eli Lilly and Company and/or its subsidiaries.
Lilly maintains various benefit and combined stock-based compensation plans at a corporate level and other benefit plans at a country level. Our employees participate in such programs and the portion of the cost of those plans related to our employees is included in our financial statements. However, the condensed combined balance sheets do not include any equity issued related to stock-based compensation plans or any net benefit plan obligations unless the benefit plan covers only our dedicated employees or where the legal obligation associated with the benefit plan will transfer to Elanco.
The equity balance in these unaudited condensed combined financial statements represents the excess of total assets over liabilities, including intercompany balances between us and Lilly (net parent company investment) and accumulated other comprehensive loss. Net parent company investment is primarily impacted by contributions from Lilly which are the result of treasury activities and net funding provided by or distributed to Lilly. See Note 10 for further information.
Note 2: Revenue
Effective January 1, 2018, we adopted Accounting Standards Update 2014-09, Revenue from Contracts with Customers (ASU 2014-09) and other related updates. The new standard has been applied to contracts for which performance had not been completed as of the date of adoption. Revenue presented for periods prior to 2018 were accounted for under previous standards and has not been adjusted. Revenue and net income for the six months ended June 30, 2018 do not differ materially from amounts that would have resulted from application of the previous standards.
F-47
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 2: Revenue (Continued)
Product Sales
We recognize revenue primarily from product sales to customers. Revenue from sales of products is recognized at the point where the customer obtains control of the goods and we satisfy our performance obligation, which generally is at the time we ship the product to the customer. Payment terms differ by jurisdiction and customer, but payment terms in most of our major jurisdictions typically range from 30 to 100 days from date of shipment. Revenue for our product sales has not been adjusted for the effects of a financing component as we expect, at contract inception, that the period between when we transfer control of the product and when we receive payment will be one year or less. Any exceptions are either not material or we collect interest for payments made after the due date. Provisions for rebates and discounts, and returns are established in the same period the related sales are recognized. We generally ship product shortly after orders are received; therefore, we generally only have a few days of orders received but not yet shipped at the end of any reporting period. Shipping and handling activities are considered to be fulfillment activities and are not considered to be a separate performance obligation. We exclude from the measurement of the transaction price all taxes assessed by a governmental authority that are imposed on our sales of product and collected from a customer.
Significant judgments must be made in determining the transaction price for our sales of products related to anticipated rebates and discounts, and returns. The following describe the most significant of these judgments:
Sales Rebates and Discounts — Background and Uncertainties
- •
- Most of our animal health products are sold to wholesale distributors. We initially invoice our customers at contractual list prices. Contracts
with direct and indirect customers may provide for various rebates and discounts that may differ in each contract. As a consequence, to determine the appropriate transaction price for our product
sales at the time we recognize a sale to a direct customer, we must estimate any rebates or discounts that ultimately will be due to the direct customer and other customers in the distribution chain
under the terms of our contracts. Significant judgments are required in making these estimates.
- •
- The rebate and discount amounts are recorded as a deduction to arrive at our net product sales. We estimate these accruals using an expected
value approach.
- •
- In determining the appropriate accrual amount, we consider our historical experience with similar incentives programs and current sales data to estimate the impact of such programs on revenue and continually monitor the impact of this experience and adjust as necessary. Although we accrue a liability for rebates related to these programs at the time we record the sale, the rebate related to that sale is typically paid up to six months after rebate or incentive period expires. Because of this time lag, in any particular period our rebate adjustments may incorporate revisions of accruals for several periods.
Our sales rebates and discounts are based on specific agreements and the majority relate to sales in the U.S. The liability for sales rebates and discounts in the U.S. represents approximately
F-48
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 2: Revenue (Continued)
70% of our total liability with the next largest country representing approximately 6% of our total liability.
The following table summarizes the activity in the sales rebates and discounts liability in the United States:
|
Six Months Ended June 30, |
||||||
| | | | | | | | |
|
2018 | 2017 | |||||
| | | | | | | | |
Beginning balance at January 1 |
$ | 104.3 | $ | 105.6 | |||
Reduction of Revenue |
100.7 | 136.3 | |||||
Payments |
(116.4 | ) | (133.7 | ) | |||
| | | | | | | | |
Ending balance at June 30 |
$ | 88.6 | $ | 108.2 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Adjustments to revenue recognized as a result of changes in estimates for the judgments described above during the six months ended June 30, 2018, for product shipped in previous periods were not material.
Sales Returns — Background and Uncertainties
- •
- We estimate a reserve for future product returns related to product sales using an expected value approach. This estimate is based on several
factors, including: local returns policies and practices; returns as a percentage of revenue; an understanding of the reasons for past returns; estimated shelf life by product; and estimate of the
amount of time between shipment and return to estimate the impact of sales returns. Adjustments to the returns reserve have been and may in the future be required based on revised estimates to our
assumptions, which would have an impact on our combined results of operations. We record the return amounts as a deduction to arrive at our net product sales.
- •
- Actual product returns have been less than 2 percent of our net revenue for the six months ended June 30, 2018 and 2017 and have not fluctuated significantly as a percentage of revenue.
F-49
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 2: Revenue (Continued)
Disaggregation of Revenue
The following table summarizes our revenue disaggregated by product category:
|
Six Months Ended June 30, |
||||||
| | | | | | | | |
|
2018 | 2017 | |||||
| | | | | | | | |
Companion Animal Disease Prevention |
$ | 415.3 | $ | 379.3 | |||
Companion Animal Therapeutics |
130.6 | 118.3 | |||||
Companion Animal Other(a) |
41.6 | 71.6 | |||||
Food Animal Future Protein & Health |
339.3 | 291.5 | |||||
Food Animal Ruminants Swine |
579.6 | 576.9 | |||||
| | | | | | | | |
Revenue |
$ | 1,506.4 | $ | 1,437.6 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
- (a)
- The Companion Animal Other for the six months ended June 30, 2018 and 2017 reflects an equine product not core to our business exited in 2018 to facilitate comparability. Revenue from this product was $1.6 million and $1.5 million for the six months ended June 30, 2018 and 2017, respectively.
Note 3: Implementation of New Financial Accounting Pronouncements
The following table provides a brief description of accounting standards that were effective January 1, 2018 and were adopted on that date:
| Standard | Description | Effect on the financial statements or other significant matters |
||
| | | | | |
| Accounting Standards Update 2014-09 and various other related updates, Revenue from Contracts with Customers | This standard replaced existing revenue recognition standards and requires entities to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. An entity can apply the new revenue standard retrospectively to each prior reporting period presented or with the cumulative effect of initially applying the standard recognized at the date of initial application in retained earnings. We applied the latter approach. | Application of the new standard to applicable contracts had no impact to net parent company investment as of January 1, 2018. Disclosures required by the new standard are included in Note 2. |
F-50
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 3: Implementation of New Financial Accounting Pronouncements (Continued)
| Standard | Description | Effect on the financial statements or other significant matters |
||
| | | | | |
| Accounting Standards Update 2016-16, Income Taxes: Intra-Entity Transfers of Assets Other Than Inventory | This standard requires entities to recognize the income tax consequences of intra-entity transfers of assets other than inventory at the time of transfer. This standard requires a modified retrospective approach to adoption. | The cumulative effect of initially applying the standard resulted in a decrease to net parent company investment of approximately $0.3 million. Adoption of this standard did not result in a material change in net income for the six months ended June 30, 2018. | ||
Accounting Standards Update 2017-07, Compensation-Retirement Benefits: Improving the Presentation of Net Periodic Pension Cost and Net Periodic Postretirement Benefit Cost |
This standard was issued to improve the transparency and comparability among organizations by requiring entities to separate their net periodic pension cost and net periodic postretirement benefit cost into a service cost component and other components. Previously, the costs of the other components along with the service cost component were classified based upon the function of the employee. This standard requires entities to classify the service cost component in the same financial statement line item or items as other compensation costs arising from services rendered by pertinent employees. The other components of net benefit cost are now presented separately from the line items that include the service cost component. When applicable, the service cost component is now the only component eligible for capitalization. An entity should apply the new standard retrospectively for the classification of the service cost and other components and prospectively for the capitalization of the service cost component. |
Upon adoption of this standard, pension and postretirement benefit cost components other than service costs are presented in other — net, (income) expense. Retrospective application was not material to the combined statement of operations for the six months ended June 30, 2017. We do not expect application of the new standard to have a material impact on an ongoing basis. |
F-51
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 3: Implementation of New Financial Accounting Pronouncements (Continued)
The following table provides a brief description of the accounting standard that has not yet been adopted and could have a material effect on our financial statements:
| Standard | Description | Effective Date | Effect on the financial statements or other significant matters |
|||
| | | | | | | |
| Accounting Standards Update 2016-02, Leases | This standard was issued to increase transparency and comparability among organizations by recognizing lease assets and lease liabilities, including leases classified as operating leases under current GAAP, on the balance sheet and requiring additional disclosures about leasing arrangements. This standard requires a modified retrospective approach to adoption. | This standard is effective January 1, 2019, with early adoption permitted. We intend to adopt this standard on January 1, 2019. | We are in the process of determining the impact on our combined financial statements. We have selected a software solution to be compatible with our enterprise software system. Development of our selected solution is ongoing, as it is not yet fully compliant with the requirements of the standard. The timely readiness of the lease software system is critical to ensure an efficient and effective adoption of the standard. |
Note 4: Asset Impairment, Restructuring, and Other Special Charges
The Company has historically participated in Lilly's cost-reduction initiatives. The Company's total charges related to asset impairment, restructuring and other special charges, including integration of acquired businesses, in our condensed combined statements of operations consisted of the following:
|
Six Months Ended June 30, |
||||||
| | | | | | | | |
|
2018 | 2017 |
|||||
| | | | | | | | |
Cash expense: |
|||||||
Severance |
$ | (2.6 | ) | $ | 56.3 | ||
Integration |
5.6 | 68.7 | |||||
Exit costs |
9.7 | 12.8 | |||||
| | | | | | | | |
Total cash expense |
12.7 | 137.8 | |||||
| | | | | | | | |
Non-cash expense |
|||||||
Asset impairment |
57.7 | 43.8 | |||||
| | | | | | | | |
Total non-cash expense |
57.7 | 43.8 | |||||
| | | | | | | | |
Gain on sale of fixed assets |
— | (16.0 | ) | ||||
| | | | | | | | |
Total |
$ | 70.4 | $ | 165.6 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Severance costs recognized during the six months ended June 30, 2017 were incurred as a result of actions taken to reduce our cost structure.
F-52
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 4: Asset Impairment, Restructuring, and Other Special Charges (Continued)
Integration costs recognized during the six months ended June 30, 2018 and 2017 were related to our integration efforts as a result of our acquired businesses.
Exit costs primarily represent contract termination costs and reserves for costs related to facilities which we have exited.
Asset impairment and other special charges recognized during the six months ended June 30, 2018 resulted primarily from $19.9 million of intangible asset impairments and $37.8 million of fixed asset impairments. The intangible asset impairments primarily related to revised projections of fair value due to product rationalization. The fixed asset impairments were primarily due to our decision to dispose of a manufacturing facility in the United States and to the suspension of commercial activities for Imrestor®.
Asset impairment recognized during the six months ended June 30, 2017 resulted primarily from intangible asset impairments related to revised projections of fair value due to product rationalization and to a lessor extent competitive pressures. The fair value measurements utilized to determine the intangible asset impairments in 2018 and 2017 represent level three fair value measurements.
Gain on sale of fixed assets for the six months ended June 30, 2017 represent gain on disposal of a site that we previously closed as part of our acquisition and integration of Novartis AH.
The following table summarizes the activity in our reserves established in connection with these restructuring activities:
|
Exit costs | Severance | Total |
|||||||
| | | | | | | | | | | |
Balance at December 31, 2016 |
$ | 11.5 | $ | 26.6 | $ | 38.1 | ||||
Charges |
12.8 | 56.3 | 69.1 | |||||||
Cash paid |
(7.1 | ) | (42.5 | ) | (49.6 | ) | ||||
| | | | | | | | | | | |
Balance at June 30, 2017 |
$ | 17.2 | $ | 40.4 | $ | 57.6 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Balance at December 31, 2017 |
$ | 34.9 | $ | 43.1 | $ | 78.0 | ||||
Charges |
9.7 | (2.6 | ) | 7.1 | ||||||
Cash paid |
(9.9 | ) | (28.4 | ) | (38.3 | ) | ||||
| | | | | | | | | | | |
Balance at June 30, 2018 |
$ | 34.7 | $ | 12.1 | $ | 46.8 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Substantially all of the reserves are expected to be paid in the next 12 months. The Company believes that the reserves are adequate.
Note 5: Inventories
We state all inventories at the lower of cost or market. We use the last-in, first-out (LIFO) method for the majority of our inventories located in the continental U.S. Other inventories are valued by the first-in, first-out (FIFO) method. FIFO cost approximates current replacement cost.
F-53
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 5: Inventories (Continued)
Inventories consisted of the following:
|
June 30, 2018 | December 31, 2017 |
|||||
| | | | | | | | |
Finished products |
$ | 400.0 | $ | 452.0 | |||
Work in process |
578.6 | 580.0 | |||||
Raw materials and supplies |
69.0 | 70.4 | |||||
| | | | | | | | |
Total (approximates replacement cost) |
1,047.6 | 1,102.4 | |||||
Decrease to LIFO cost |
(42.0 | ) | (40.1 | ) | |||
| | | | | | | | |
Inventories |
$ | 1,005.6 | $ | 1,062.3 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
During the six months ended June 30, 2018, we recognized $40.2 million of inventory write-offs in cost of sales primarily related to the suspension of commercial activities for Imrestor.
Note 6: Financial Instruments
Financial instruments that potentially subject us to credit risk consist principally of trade receivables. Collateral is generally not required. The risk associated with this concentration is mitigated by our ongoing credit-review procedures and insurance.
A large portion of our cash, which is legally owned by us and is recognized on the condensed combined balance sheets, is held by a few major financial institutions. Lilly monitors the exposure with these institutions and does not expect any of these institutions to fail to meet their obligations. We consider all highly liquid investments with a maturity of three months or less from the date of purchase to be cash equivalents. The cost of these investments approximates fair value.
As of June 30, 2018 and December 31, 2017, we had $12.8 million and $12.3 million, respectively, of cost and equity method investments.
F-54
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 6: Financial Instruments (Continued)
The following table summarizes the fair value information at June 30, 2018 and December 31, 2017 for contingent consideration liabilities measured at fair value on a recurring basis:
|
Fair Value Measurements Using | |||||||||||||||
| | | | | | | | | | | | | | | | | |
Financial statement line item |
Carrying Amount |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Fair Value |
|||||||||||
| | | | | | | | | | | | | | | | | |
June 30, 2018 |
||||||||||||||||
Other current liabilities- contingent consideration |
$ | 15.9 | — | — | $ | 15.9 | $ | 15.9 | ||||||||
Other noncurrent liabilities- contingent consideration |
41.1 | — | — | 41.1 | 41.1 | |||||||||||
December 31, 2017 |
||||||||||||||||
Other current liabilities- contingent consideration |
1.3 | — | — | 1.3 | 1.3 | |||||||||||
Other noncurrent liabilities- contingent consideration |
45.2 | — | — | 45.2 | 45.2 | |||||||||||
Contingent consideration liabilities relate to Galliprant for which the fair value was estimated using a discounted cash flow analysis and Level 3 inputs, including projections representative of a market participant view for the probability of achieving potential future payments to Aratana Therapeutics, Inc. and an estimated discount rate. The amount to be paid is dependent upon certain development, success-based regulatory, and sales-based milestones. In addition, the amount of royalties to be paid is calculated as a percentage of net sales dependent upon the timing and geography and will, therefore, vary directly with increases and decreases in net sales of Galliprant. There is no cap on the amount that may be paid pursuant to this arrangement. During the six months ended June 30, 2018 as a result of an increase in the projected cash flows related to Galliprant, we increased the fair value of the contingent consideration liabilities by $8.5 million. The additional expense was recognized in other-net, (income) expense.
Note 7: Income Taxes
During the periods presented in the combined financial statements, Elanco was generally included in the tax grouping of other Lilly entities within the respective entity's tax jurisdiction; however, in certain jurisdictions, Elanco filed separate tax returns. The income tax expense included in these combined financial statements has been calculated using the separate return basis as if Elanco filed separate tax returns. As a result, tax credit and net operating loss carryovers may not be available for our use in future periods as they may have already been used in Lilly consolidated or combined tax return filings or they may be retained by Lilly upon separation.
During the six months ended June 30, 2018 we incurred $27.6 million of income tax expense despite earning $37.5 million of income before taxes. Our effective tax rate was 73.6% and the income tax expense recorded relates primarily to our foreign jurisdictions as a valuation allowance was recorded on net operating loss assets generated in the U.S. due to certain asset impairment,
F-55
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 7: Income Taxes (Continued)
restructuring, and other special charges. During the six months ended June 30, 2017 despite reporting a $68.0 million loss before income taxes, we incurred $60.5 million of income tax expense. The tax expense recorded relates primarily to income generated in certain foreign jurisdictions as a valuation allowance was recorded on net operating loss assets generated in the U.S.
In December 2017, the President of the United States signed into law the Tax Cuts and Jobs Act (2017 Tax Act), which includes significant changes to the U.S. corporate income tax system, including a reduction in the corporate income tax rate, transition to a territorial tax system, and modifications to the international tax provisions. At June 30, 2018, our accounting for the 2017 Tax Act is incomplete; however, we expect to complete our accounting by December 2018. As discussed in our 2017 combined financial statements, we recorded provisional adjustments for effects that we were able to reasonably estimate. Those effects included the one-time repatriation transition tax (also known as the 'Toll Tax'), re-measurement of deferred tax assets and liabilities, unremitted earnings, executive compensation, and uncertain tax positions. At December 31, 2017, we were not able to make reasonable estimates for Global Intangible Low-Taxed Income (GILTI) deferred taxes or valuation allowances; therefore, we did not record provisional amounts. We are still evaluating the effects of the GILTI provisions and assessing our valuation allowances, and we have not yet determined our accounting policy election with respect to GILTI deferred taxes or the application of intra-entity transfers of inventory; therefore, the estimated annual effective tax rate reflects GILTI as a period expense. For the six months ended June 30, 2018, we have not made any additional measurement-period adjustments related to these provisional items as we are continuing to collect and analyze additional information as well as evaluate the interpretations and assumptions made. Updates to our calculations may result in material changes to the provisional adjustments recorded at December 31, 2017 and the estimated annual effective tax rate.
As part of Lilly, we are included in its U.S. tax examinations by the Internal Revenue Service ("IRS"). The U.S. examination of tax years 2013-2015 began in 2016. While we believe it is reasonably possible that this audit could reach resolution within the next 12 months, the IRS examination of tax years 2013-2015 remains ongoing. Therefore, it is not possible to reasonably estimate the change to unrecognized tax benefits and the related future cash flows.
Note 8: Contingencies
We are a party to various legal actions in the normal course of business. We record a liability if there is a claim for which it is probable we will make a payment and the amount is estimable. At June 30, 2018 and December 31, 2017 we had no liabilities established related to litigation as there are no claims which were probable and estimable. We have not historically had any significant litigation expense and are not currently subject to any claim.
Note 9: Geographic Information
We operate as a single operating segment engaged in the development, manufacturing, marketing and sales of animal health products worldwide for both food animals and companion animals. Consistent with our operational structure, our President and Chief Executive Officer ("CEO"), as the chief operating decision maker, makes resource allocation and business process
F-56
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 9: Geographic Information (Continued)
decisions globally across our consolidated business. Strategic decisions are managed globally with global functional leaders responsible for determining significant cost/investments and with regional leaders responsible for overseeing the execution of the global strategy. Our global research and development organization is responsible for development of new products. Our manufacturing organization is responsible for the manufacturing and supply of products and for the optimization of our supply chain. Regional leaders are responsible for the distribution and sale of our products and for local direct costs. The business is also supported by global corporate staff functions. Managing and allocating resources at the global corporate level enables our CEO to assess the overall level of resources available and how to best deploy these resources across functions, product types, regional commercial organizations and research and development projects in line with our overarching long-term corporate-wide strategic goals, rather than on a product or geographic basis. Consistent with this decision-making process, our CEO uses consolidated, single-segment financial information for purposes of evaluating performance, allocating resources, setting incentive compensation targets, as well as forecasting future period financial results.
Our products include Rumensin®, Optaflexx®, Denagard®, Tylan®, Maxiban® and other products for livestock and poultry, as well as Trifexis®, Interceptor®, Comfortis® and other products for companion animals.
We have a single customer that accounted for 11.7% and 13.1% of revenue for the six months ended June 30, 2018 and 2017, respectively. The product sales resulted in accounts receivable of $82.3 million and $88.0 million as of June 30, 2018 and December 31, 2017, respectively.
We are exposed to the risk of changes in social, political and economic conditions inherent in foreign operations and our results of operations and the value of our foreign assets are affected by fluctuations in foreign currency exchange rates.
F-57
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 9: Geographic Information (Continued)
Selected geographic area information was as follows:
|
Six Months Ended June 30, |
||||||
| | | | | | | | |
|
2018 | 2017 |
|||||
| | | | | | | | |
Revenue — to unaffiliated customers(1) |
|||||||
United States |
$ | 726.4 | $ | 733.2 | |||
International |
780.0 | 704.4 | |||||
| | | | | | | | |
Revenue |
$ | 1,506.4 | $ | 1,437.6 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
|
June 30, 2018 |
December 31, 2017 |
|||||
| | | | | | | | |
Long-lived assets(2) |
|||||||
United States |
$ | 561.1 | $ | 604.7 | |||
United Kingdom |
194.7 | 204.4 | |||||
Other foreign countries |
189.3 | 190.2 | |||||
| | | | | | | | |
Long-lived assets |
$ | 945.1 | $ | 999.3 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
- (1)
- Revenue
is attributed to the countries based on the location of the customer.
- (2)
- Long-lived assets consist of property and equipment, net, and certain noncurrent assets.
Note 10: Related Party Transactions
The Company has not historically operated as a standalone business and has various relationships with Lilly whereby Lilly provides services to the Company.
Transfers to/from Lilly, net
As discussed in the basis of preparation, net parent company investment is primarily impacted by contributions from Lilly which are the result of treasury activity and net funding provided by or distributed to Lilly. For the six months ended June 30, 2018 and 2017, the net transfers (to)/from Lilly were $(109.5) million and $824.6 million, respectively. The most significant activity impacting the 2017 transfer was the financing by Lilly of Elanco's acquisitions in the amount of $882.1 million for Boehringer Ingelheim Vetmedica, Inc.'s United States feline, canine, and rabies vaccine portfolio and other related assets in 2017. Other activities that impacted the net transfers to/from Lilly include corporate overhead and other allocations, income taxes, retirement benefits, and centralized cash management.
Corporate Overhead and Other Allocations
Lilly provides the Company certain services, including executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations. The Company provides Lilly certain services related to manufacturing support.
F-58
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 10: Related Party Transactions (Continued)
Our combined financial statements reflect an allocation of these costs. When specific identification is not practicable, a proportional cost method is used, primarily based on sales, and headcount.
The allocations of services from Lilly to the Company were reflected as follows in the condensed combined statements of operations:
|
Six Months Ended June 30, |
||||||
| | | | | | | | |
|
2018 | 2017 |
|||||
| | | | | | | | |
Cost of sales |
$ | 14.8 | $ | 15.3 | |||
Research and development |
1.5 | 1.4 | |||||
Marketing, selling and administrative |
54.8 | 55.0 | |||||
| | | | | | | | |
Total |
$ | 71.1 | $ | 71.7 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The Company provides Lilly certain services related to manufacturing support. Allocations of manufacturing support from the Company to Lilly of $2.4 million and $3.0 million for the six months ended June 30, 2018 and 2017, respectively, reduced cost of sales in the condensed combined statements of operations.
The financial information herein may not necessarily reflect the combined financial position, results of operations and cash flows of the Company in the future or what they would have been had the Company been a separate, standalone entity during the periods presented. Management believes that the methods used to allocate expenses to the Company are reasonable.
Stock-based Compensation
The Company's employees participate in Lilly stock-based compensation plans, the costs of which have been allocated to the Company and recorded in cost of sales, research and development, and marketing, selling and administrative expenses in the condensed combined statements of operations. Stock-based compensation costs related to the Company's employees were $13.3 million and $12.5 million for the six months ended June 30, 2018 and 2017, respectively.
Retirement Benefits
The Company's employees participate in defined benefit pension and other postretirement plans sponsored by Lilly, the costs of which have been recorded in the condensed combined statement of operations in cost of sales, research and development, and marketing, selling and administrative expenses. The costs of such plans related to the Company's employees were $1.3 million and $3.4 million for the six months ended June 30, 2018 and 2017, respectively.
Centralized Cash Management
Lilly uses a centralized approach to cash management and financing of operations. The majority of the Company's business is party to Lilly's cash pooling arrangements to maximize Lilly's
F-59
Notes to Condensed Combined Financial Statements (Continued)
(Unaudited)
(Tables present dollars in millions)
Note 10: Related Party Transactions (Continued)
availability of cash for general operating and investing purposes. Under these cash pooling arrangements, cash balances are swept regularly from the Company's accounts. Cash transfers to and from Lilly's cash concentration accounts and the resulting balances at the end of each reporting period are reflected in net parent company investment in the condensed combined balance sheets.
Debt
Lilly's third-party debt and the related interest expense have not been allocated to the Company for any of the periods presented as the Company was not the legal obligor of the debt and Lilly borrowings were not directly attributable to the Company's business.
Commercial Operations
The Company sells certain products to and receives certain goods and services from a customer/vendor, whose chairman and Chief Executive Officer is a member of Lilly's Board of Directors. These product sales resulted in revenue of $12.2 million and $11.2 million for the six months ended June 30, 2018 and 2017, respectively. The product sales resulted in accounts receivable of $1.5 million and $2.0 million at June 30, 2018 and December 31, 2017, respectively. The purchase of goods and services resulted in cost of sales and operating expenses of $1.9 million and $4.2 million for the six months ended June 30, 2018 and 2017, respectively. The purchase of goods and services resulted in accounts payable of $0.6 million and $0.3 million at June 30, 2018 and December 31, 2017, respectively.
F-60
Shares
Elanco Animal Health Incorporated
Common Stock

Goldman Sachs & Co. LLC
J.P. Morgan
Morgan Stanley
Barclays
BNP PARIBAS
Citigroup
Credit Suisse
Deutsche Bank Securities
Evercore ISI
Cowen
Through and including , 2018 (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to an unsold allotment or subscription.
PART II — INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth all costs and expenses, other than the underwriting discount, paid or payable by us in connection with the sale of our common stock being registered. All amounts shown are estimates except for the SEC registration fee, the FINRA filing fee and the listing fee for the NYSE.
|
Amount Paid or to be Paid |
|||
| | | | | |
SEC registration fee |
$ | * | ||
FINRA filing fee |
* | |||
NYSE listing fee |
* | |||
Blue sky qualification fees and expenses |
* | |||
Printing and engraving expenses |
* | |||
Legal fees and expenses |
* | |||
Accounting fees and expenses |
* | |||
Transfer agent and registrar fees and expenses |
* | |||
Miscellaneous expenses |
* | |||
| | | | | |
Total |
$ | * | ||
| | | | | |
| | | | | |
| | | | | |
- *
- To be provided by amendment
Item 14. Indemnification of Officers and Directors.
The Registrant is an Indiana corporation. The Registrant's officers and directors are and will be indemnified under Indiana law and the Amended and Restated Articles of Incorporation and Amended and Restated Bylaws of the Registrant against certain liabilities. Chapter 37 of the Indiana Business Corporation Law (the "IBCL") requires every Indiana corporation to indemnify any of its officers or directors (unless limited by the articles of incorporation of the corporation) who were wholly successful, on the merits or otherwise, in the defense of any proceeding to which the officer or director was a party because the officer or director is or was an officer or director of the Registrant against reasonable expenses incurred in connection with the proceeding. A corporation may also, under certain circumstances, pay for or reimburse the reasonable expenses incurred by an officer or director who is a party to a proceeding in advance of final disposition of the proceeding. The Registrant's Amended and Restated Articles of Incorporation do not contain any provision limiting such indemnification.
Chapter 37 of the IBCL also authorizes every Indiana corporation to indemnify its officers and directors under certain circumstances against liability incurred in connection with any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative and whether formal or informal, to which the officers or directors are made a party by reason of their relationship to the corporation. Officers and directors may be indemnified where they have acted in good faith; in the case of official action, the individual reasonably believed that the conduct was in the corporation's best interests and in all other cases, the individual reasonably believed that the conduct was not against the best interests of the corporation; and in the case of criminal proceedings, the individual either had reasonable cause to believe his or her conduct was lawful or no reasonable cause to believe his or her conduct was unlawful. Chapter 37 states that the indemnification provided for therein is not exclusive of any other rights to which a person may be entitled under the articles of incorporation, bylaws or resolutions of the board of directors or shareholders.
II-1
The Amended and Restated Articles of Incorporation and Amended and Restated Bylaws provide for indemnification, to the fullest extent permitted by the IBCL, of directors, officers and employees of the corporation against liability and reasonable expense that may be incurred by them, arising out of any threatened, pending or completed investigation, claim, suit or proceeding, whether civil, administrative, investigative or criminal, in which they may become involved by reason of being or having been a director, officer or employee. To be entitled to indemnification, (a) those persons must have been wholly successful in the claim or action, or (b) the board of directors, independent legal counsel or the shareholders must have determined that such persons acted in good faith in what they reasonably believed to be in the corporation's best interest, or in the case of conduct not in the individual's official capacity with the corporation, did not act in opposition to the corporation's best interest. In addition, in any criminal action, such persons must have had no reasonable cause to believe that their conduct was unlawful. The Amended and Restated Bylaws provide for mandatory advancement of expenses to such persons provided certain conditions are met, including provision of a written undertaking to repay such advancements, should it be determined that the person is not entitled to indemnification.
The IBCL permits the Registrant to purchase insurance on behalf of directors, officers, employees and agents against liabilities arising out of their positions with the corporation, whether or not such liabilities would be within the above indemnification provisions. Pursuant to this authority, the corporation will maintain such insurance for directors, officers and employees, subject to certain exclusions and deductible and maximum amounts, against loss from claims arising in connection with their acting in their respective capacities, including claims under the Securities Act of 1933, as amended (the "Securities Act").
Reference is made to the form of underwriting agreement to be filed as Exhibit 1.1 hereto for provisions providing that the underwriters are obligated under certain circumstances to indemnify our directors, officers and controlling persons against certain liabilities.
Item 15. Recent Sales of Unregistered Securities
Senior Notes
On August 28, 2018, the Company sold $500,000,000 aggregate principal amount of its 3.912% Senior Notes due 2021 (the "2021 Notes"), $750,000,000 aggregate principal amount of its 4.272% Senior Notes due 2023 (the "2023 Notes") and $750,000,000 aggregate principal amount of its 4.900% Senior Notes due 2028 (the "2028 Notes" and, together with the 2021 Notes and the 2023 Notes, the "Notes") to Goldman Sachs & Co. LLC, J.P. Morgan Securities LLC, Citigroup Global Markets Inc., Barclays Capital Inc., BNP Paribas Securities Corp., Credit Suisse Securities (USA) LLC, Deutsche Bank Securities Inc., Merrill Lynch, Pierce, Fenner & Smith Incorporated, Morgan Stanley & Co. LLC, Academy Securities, Inc., Drexel Hamilton, LLC, Mischler Financial Group, Inc., Samuel A. Ramirez & Company, Inc., and The Williams Capital Group, L.P., as initial purchasers (the "Initial Purchasers"), in reliance on Section 4(a)(2) under the Securities Act. The Notes were resold by the Initial Purchasers to qualified institutional buyers in reliance on Rule 144A and/or non-U.S. persons in offshore transactions in reliance on Regulation S. The Initial Purchasers received customary discounts in connection with the transaction. The net proceeds from the sale of the Notes will be paid to Lilly as consideration for the portion of its animal health businesses Lilly is contributing to us.
II-2
Item 16. Exhibits and Financial Statement Schedules
- (a)
- Exhibits:
II-3
- *
- To be filed by amendment.
- **
- Previously filed.
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreements, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-4
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-5
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Indianapolis, State of Indiana, on August 28, 2018.
| ELANCO ANIMAL HEALTH INCORPORATED | ||||||
By: |
/s/ JEFFREY N. SIMMONS |
|||||
| Name: | Jeffrey N. Simmons | |||||
| Title: | President and Chief Executive Officer | |||||
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities indicated on August 28, 2018.
Signature
|
Title |
|||
| /s/ JEFFREY N. SIMMONS Jeffrey N. Simmons |
President and Chief Executive Officer (Principal Executive Officer) and Director | |||
/s/ LUCAS E. MONTARCE Lucas E. Montarce |
Acting Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
|||
* R. David Hoover |
Chairman |
|||
*By: |
/s/ MICHAEL-BRYANT HICKS Michael-Bryant Hicks Attorney-in-fact |
|||
II-6
