Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - Cardiovascular Systems Inc | ex322-63018.htm |
| EX-32.1 - EXHIBIT 32.1 - Cardiovascular Systems Inc | ex321-63018.htm |
| EX-31.2 - EXHIBIT 31.2 - Cardiovascular Systems Inc | ex312-63018.htm |
| EX-31.1 - EXHIBIT 31.1 - Cardiovascular Systems Inc | ex311-63018.htm |
| EX-23.1 - EXHIBIT 23.1 - Cardiovascular Systems Inc | ex231-63018.htm |
| EX-10.57 - EXHIBIT 10.57 - Cardiovascular Systems Inc | ex1057-secondleaseamendmen.htm |
| EX-10.26 - EXHIBIT 10.26 - Cardiovascular Systems Inc | ex1026-executiveseverancep.htm |
| EX-10.6 - EXHIBIT 10.6 - Cardiovascular Systems Inc | ex106fy19directorcompensatio.htm |
| EX-10.5 - EXHIBIT 10.5 - Cardiovascular Systems Inc | ex105-fy19execincentiveplan.htm |
| EX-10.4 - EXHIBIT 10.4 - Cardiovascular Systems Inc | ex104-fy19basesalaries.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2018
OR
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 000-52082
CARDIOVASCULAR SYSTEMS, INC.
(Exact name of registrant as specified in its charter)
Delaware | 41-1698056 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
1225 Old Highway 8 Northwest St. Paul, Minnesota | 55112-6416 | |
(Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code:
(651) 259-1600
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of each exchange on which registered | |
Common Stock, One-tenth of One Cent ($0.001) Par Value Per Share | The Nasdaq Stock Market LLC | |
Securities registered pursuant to Section 12(g) of the Act:
None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes þ No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer x | Accelerated filer o | Non-accelerated filer o | Smaller reporting company o | |||
Emerging growth company o | (Do not check if a smaller reporting company) | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No þ
As of December 31, 2017, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was approximately $761.7 million based on the closing sale price as reported on the Nasdaq Global Market.
The number of shares of the registrant’s common stock outstanding as of August 17, 2018 was 33,505,560.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the proxy statement for the registrant’s 2018 Annual Meeting of Stockholders are incorporated by reference into Items 10, 11, 12, 13 and 14 of Part III of this report.
Table of Contents
Page No. | ||
Item 1. | ||
Item 1A. | ||
Item 1B. | ||
Item 2. | ||
Item 3. | ||
Item 4. | ||
Item 5. | ||
Item 6. | ||
Item 7. | ||
Item 7A. | ||
Item 8. | ||
Item 9. | ||
Item 9A. | ||
Item 9B. | ||
Item 10. | ||
Item 11. | ||
Item 12. | ||
Item 13. | ||
Item 14. | ||
Item 15. | ||
We make available, free of charge, copies of our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act on our website, http://www.csi360.com, as soon as reasonably practicable after filing such material electronically or otherwise furnishing it to the Securities and Exchange Commission (“SEC”). We are not including the information on our website as a part of, or incorporating it by reference into, this Form 10-K.
The SEC maintains a website that contains reports, proxy and information statements, and other information regarding issuers, including the Company, that file electronically with the SEC. The public can obtain any documents that we file with the SEC at http://www.sec.gov. We file annual reports, quarterly reports, proxy statements, and other documents with the SEC under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The public may read and copy any materials that we file with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
PART I
Item 1. Business.
Special Note Regarding Forward Looking Statements
This Form 10-K contains plans, intentions, objectives, estimates and expectations that constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Exchange Act, which are subject to the “safe harbor” created by those sections. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “intend,” “should,” “could,” “would,” “expect,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” and similar expressions intended to identify forward-looking statements. Examples of these statements include, but are not limited to, any statements regarding our future financial performance, results of operations or sufficiency of capital resources to fund our operating requirements, and other statements that are other than statements of historical fact. Our actual results could differ materially from those discussed in these forward-looking statements due to a number of factors, including the risks and uncertainties that are described more fully by us in Part I, Item 1A and Part II, Item 7 of this Form 10-K and in our other filings with the Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which apply only as of the date of this Form 10-K. You should read this Form 10-K completely and with the understanding that our actual future results may be materially different from what we expect. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
Corporate Information
Cardiovascular Systems, Inc. (“CSI”) was incorporated in Delaware in 2000. Our principal executive office is located at 1225 Old Highway 8 Northwest, St. Paul, Minnesota 55112. Our telephone number is (651) 259-1600, and our website is www.csi360.com. The information contained in or accessible through our website is not incorporated by reference into, and should not be considered part of, this Form 10-K.
We have received 22 federal registrations in the U.S. Patent and Trademark Office (“USPTO”) of certain marks, including “CSI®” (a first and second), “CSI® (Stylized)” (a first and second), “CSIQ®”, “CSIQ (Stylized)”, “DIAMONDBACK®”, “DIAMONDBACK 360®” (a first and second), “DIAMONDBACK 360® (Stylized)”, “GLIDEASSIST®”, “STAY A STEP AHEAD OF PAD®”, “STEALTH 360®”, “TAKE A STAND AGAINST AMPUTATION®”, “TAKE A STAND AGAINST AMPUTATION® (Stylized), “VIPERWIRE®”, “VIPERWIRE ADVANCE®”, “VIPERWIRE ADVANCE® (Stylized)”, “VIPERSLIDE®”, VIPERSLIDE® (Stylized)”, “VIPERTRACK®” and “VIPERTRACK® (Stylized)”. We have applied for federal trademark registration with the USPTO of certain marks, including “VIPERCATH”, “ZILIENT”, and “ZILIENT (Stylized)”. All other trademarks, trade names and service marks appearing in this Form 10-K are the property of their respective owners.
Business Overview
We are a medical technology company leading the way in the effort to successfully treat patients suffering from peripheral and coronary artery diseases, including those with arterial calcium, the most difficult form of arterial disease to treat. We are committed to clinical rigor, constant innovation and a defining drive to set the industry standard to deliver safe and effective medical devices that improve the lives of patients facing this difficult disease state. We have developed a patented orbital atherectomy systems (“OAS”) for both peripheral and coronary commercial applications. The primary base of our business is catheter-based platforms capable of treating a broad range of vessel sizes and plaque types, including calcified plaque, and address many of the limitations associated with other treatment alternatives. To date, over 392,000 of our OAS devices have been sold to leading institutions across the United States and Japan.
Peripheral
Our peripheral arterial disease (“PAD”) products are catheter-based platforms capable of treating a broad range of plaque types in leg arteries both above and below the knee, including calcified plaque, and address many of the limitations associated with other existing surgical, catheter and pharmacological treatment alternatives. The micro-invasive devices use small access sheaths that can provide procedural benefits and allow physicians to treat PAD patients in even the small and tortuous vessels located below the knee and facilitate access through alternative sites in the ankle, foot and wrist, as well as in the groin.
1
The United States Food and Drug Administration (“FDA”) granted 510(k) clearance for the following PAD products as a therapy in patients with PAD. We refer to these products in this Form 10-K as the “Peripheral OAS.”
FDA 510(k) Clearance Granted | Product | Commercial Introduction | ||
August 2007 | Diamondback 360 Peripheral(1) | September 2007 | ||
March 2009 | Predator 360(1) | April 2009 | ||
March 2011 | Stealth 360® Peripheral OAS (“Stealth 360”) | March 2011 | ||
February 2014 | Diamondback 360 60cm Peripheral | April 2014 | ||
April 2015 | Diamondback 360 Low Profile Peripheral | July 2015 | ||
October 2015 | Diamondback 360 1.50 Peripheral | January 2016 | ||
October 2015 | Diamondback 360 2.00 Peripheral | January 2016 | ||
June 2017 | Diamondback 360 200cm Peripheral | February 2018 | ||
June 2017 | Diamondback 360 180cm Peripheral | February 2018 | ||
(1) We are not currently marketing this product. | ||||
Sales of Peripheral OAS during the fiscal year ended June 30, 2018 represented 68% of revenue.
In January 2018, we announced that we entered into an original equipment manufacturer agreement with Integer Holdings Corporation to manufacture our ZILIENT™ guidewires. The full U.S. market launch of the ZILIENT peripheral guidewires began in early fiscal 2019. We anticipate that additional ZILIENT guidewires for coronary interventions and radial peripheral interventions will become available in the future.
In February 2018, we announced that the first patients were treated using our FDA-cleared extended length Diamondback 360 Peripheral OAS to treat PAD. The extended length makes it easier for physicians to reach and treat lower limb PAD lesions through the radial artery in the wrist, providing an alternative access point and more options to treat complicated and at-risk patients. We are currently in a limited market release with an anticipated full commercial launch in fiscal 2019.
Coronary
Our coronary arterial disease (“CAD”) product, the Diamondback 360 Coronary OAS (“Coronary OAS”), is a catheter-based platform designed to facilitate stent delivery in patients with CAD who are acceptable candidates for percutaneous transluminal coronary angioplasty or stenting due to de novo, severely calcified coronary artery lesions. The Coronary OAS design is similar to technology used in our Peripheral OAS, customized specifically for the coronary application.
In October 2013, we received premarket approval (“PMA”) from the FDA to market the Coronary OAS as a treatment for severely calcified coronary arteries and we commenced a commercial launch that same month. Sales of Coronary OAS during the fiscal year ended June 30, 2018 represented approximately 23% of revenue.
In January 2018, we announced our relationship with OrbusNeich® to be the exclusive U.S. distributor of OrbusNeich balloon products. In March 2018, the FDA granted 510(k) clearance for the OrbusNeich 1.0mm Sapphire® II Pro coronary balloon (“1.0mm balloon”). The 1.0mm balloon, the first and only 1.0mm coronary balloon available in the United States, offers industry-leading entry and crossing profiles and is precision engineered for crossing and treating extremely tight and complex lesions. We anticipate OrbusNeich’s full coronary balloon product portfolio will become available in the United States during fiscal 2019 and fiscal 2020.
In addition to the Peripheral and Coronary OAS, we offer multiple accessory products required for use with the Peripheral and Coronary OAS. Sales of accessory products, primarily guide wires, represented 9% of revenue during the fiscal year ended June 30, 2018.
International
In November 2016, we signed an exclusive distribution agreement with Medikit Co., Ltd. to sell our Coronary and Peripheral OAS in Japan. In March 2017, we received approval from Japan’s Ministry of Health, Labor and Welfare (“MHLW”) for our Coronary OAS Micro Crown. In February 2018, the Coronary OAS Micro Crown received reimbursement approval in Japan, followed by the first commercial sales in Japan. This represents the first international market for any of our products, and most
2
importantly, an opportunity to provide physicians in Japan a cost-effective treatment option for the difficult-to-treat patient population with severely calcified coronary lesions.
In October 2014, we received CE Mark for our Stealth 360 device. In July 2018, we entered into an exclusive Distribution Agreement with OrbusNeich to sell our Peripheral and Coronary OAS outside of the United States and Japan. We expect that OrbusNeich will commence sales of our products in certain countries in Southeast Asia, Europe and the Middle East during fiscal year 2019.
Market Overview
Peripheral Artery Disease (“PAD”)
Peripheral artery disease is widespread and can be life threatening. The disease is characterized by narrowed, hardened arteries in the legs, limiting blood flow to the legs and feet. If left untreated, PAD may continue to progress to Critical Limb Ischemia (“CLI”), a condition in which the amount of oxygenated blood being delivered to the limb is insufficient to keep the tissue viable. CLI may lead to non-healing ulcers, infections, gangrene, limb amputation or death. According to estimates by the American Heart Association (“AHA”), as many as 18 million Americans, mostly over the age of 65, have PAD. An aging population, coupled with high rates of diabetes and obesity, is likely to continue to increase the prevalence of PAD. In many older PAD patients, particularly those with diabetes, PAD is characterized by fibrotic (moderately hard) or calcified (extremely hard) plaque deposits that can be very challenging to treat. Although we believe the rate of PAD diagnoses is increasing, we also believe that under-diagnosis continues, due to patient and physician awareness. Emphasis on PAD education from industry, medical associations, insurance companies and other groups, coupled with publications in medical journals and public news channels, is increasing physician and patient awareness of PAD risk factors, symptoms, and treatment options. Physicians manage a significant portion of the PAD diagnosed population by recommending lifestyle changes, such as diet and exercise, and by prescribing prescription drugs, such as statins. While medications, diet and exercise may improve blood flow, they do not treat the underlying vascular occlusions, and many patients have difficulty maintaining lifestyle changes. As a result of these challenges, many medically managed patients develop more severe symptoms that require procedural intervention.
Coronary Artery Disease (“CAD”)
Heart disease is the leading cause of death in both men and women in the United States. Coronary artery disease is the most common type of heart disease in the United States and is a life-threatening condition. CAD occurs when a fatty material called plaque builds up on the walls of arteries that supply blood to the heart. The plaque buildup causes the arteries to harden and narrow (atherosclerosis), reducing blood flow. The risk of CAD increases if a person has one or more of the following: high blood pressure, abnormal cholesterol levels, diabetes, or family history of early heart disease. According to the AHA, 16.3 million people in the United States (or 6.3% of the adult population) have CAD, the most common form of heart disease. According to the U.S. Centers for Disease Control and Prevention, over 370,000 lives are claimed in the United States each year from CAD. According to estimates, significant arterial calcium is present in nearly 40% of patients (Genereux et al., 2014; Bourantas et al., 2014), and severe calcium affects up to 20% of patients (Bourantas et al., 2014), undergoing a percutaneous coronary intervention (“PCI”). Significant calcium contributes to poor outcomes and higher treatment costs in coronary interventions when traditional therapies are used, including a significantly higher occurrence of death and major adverse cardiac events.
Our Peripheral OAS and Coronary OAS
Our orbital atherectomy systems represent a unique and innovative approach to the treatment of PAD and CAD that provide physicians and patients with a procedure that addresses many of the limitations of other treatment alternatives. The Peripheral OAS and Coronary OAS devices are single-use catheters that incorporate a control handle and flexible drive shaft with an eccentrically mounted diamond-coated crown. The peripheral device is often used for vessel preparation to enable low pressure percutaneous transluminal angioplasty, including the use of drug-coated balloons (“DCB”), and results in lower use of bailout stents. The coronary device is similarly used to prepare a vessel by treating severe calcium prior to stent delivery to help facilitate vessel access and stent expansion and prevent malapposition of stent struts for optimal stent performance.
The OAS treats atherosclerotic plaque, which is harder than a normal vessel wall. The OAS is designed to differentiate between hard, diseased plaque and healthy, compliant arterial tissue, a concept that we refer to as “differential sanding.” The diamond-coated crown preferentially engages and sands away harder material, but is designed not to damage more compliant parts of the artery, which flex away from the crown. Physicians position the crown at the site of a lesion containing arterial plaque and orbit the crown against it to sand away the superficial, or surface, plaque and create a smooth lumen, or channel, in the vessel. In addition, the crown’s rotating eccentric mass and orbital motion deliver pulsatile mechanical energy into the vessel wall. These
3
pulsatile forces may break up deeper plaque and contribute to improving the compliance change of the diseased segment of the artery.
Components of the OAS
Our OAS uses a single-use, low-profile catheter that travels over our proprietary ViperWire guide wires and is electrically powered by a small, portable saline infusion pump that also helps cool the system and remove debris. The Peripheral OAS reduces plaque on peripheral vessel walls by using an orbiting, diamond-coated crown within peripheral arteries. Similarly, the Coronary OAS uses the same method to reduce severely calcified plaque on coronary vessel walls within coronary arteries in order to facilitate stent performance.
Catheter. The catheter for our OAS consists of:
• | an electrically-powered control handle, which allows movement of the crown and predictable crown location; |
• | a flexible drive shaft with an eccentrically mounted diamond-coated crown, which tracks and orbits over the guide wire; and |
• | a sheath, which covers the drive shaft and permits delivery of saline or medications to the treatment area. |
ViperWire Advance Peripheral Guide Wire, ViperWire Advance Peripheral Guide Wire with Flex Tip and ViperWire Advance Coronary Guide Wire. The ViperWire guide wires are required for using the OAS and were designed to offer the ability to maneuver through tortuous, twisting blood vessels and cross challenging lesions. The OAS travels over this wire to the lesion and operates on this wire.
ViperSlide Lubricant. ViperSlide is an exclusive lubricant designed to optimize the smooth operation of the OAS.
Saline Infusion Pump. The saline infusion pump is a small, portable device that bathes the OAS shaft and crown and provides an electric power supply for the operation of the catheter. The constant flow of saline during orbit reduces the risk of heat generation and improves the flush of particulates.
Mechanism of Action
The mechanism of action is a function of the centrifugal force generated by the eccentrically mounted crown as it rotates and orbits inside the vessel. As the speed of the crown’s rotation increases, centrifugal force increases the crown’s radius of orbit and presses the diamond-coated crown against the lesion or plaque, removing a small amount of plaque with each orbit. The centrifugal force exerted onto the vessel wall decreases as the orbital radius increases, reducing the likelihood of adverse events during treatment. The characteristics of the orbit and the resulting lumen size can be adjusted by modifying the following two variables:
• | Speed. An increase in speed creates a larger orbital radius, thus accommodating larger diameter vessels. Our Peripheral OAS allows the user to choose between three rotational speeds. Our Coronary OAS Classic Crown allows the user to choose between two rotational speeds, in addition to engaging a recently added Glide Assist® feature, which is an innovative solution that facilitates device tracking, provides easier device repositioning, and provides enhanced performance in challenging anatomies. |
• | Crown Characteristics. The crowns for the OAS are designed with various weights (as determined by crown geometry and material density) and are coated with diamond particles. The Peripheral OAS crowns are available in three configurations: classic, micro and solid. Physicians select crown sizes and configurations based on several case criteria, including reference vessel size, lesion length and degree of stenosis, stenosis morphology, and anatomy tortuosity. The crown for the Coronary OAS is available in two configurations: 1.25 millimeter Classic Crown (currently sold in the United States), and the 1.25 millimeter Micro Crown (currently sold in Japan). |
Centrifugal force propels the crown outward against the arterial wall as the crown rotates. This force is offset by the counterforce exerted by the arterial wall and the guidewire. Normal arteries are compliant and have the ability to expand and contract as needed to supply blood flow. If the tissue is compliant, it flexes away, minimizing the engagement of the diamond-grit and protecting the integrity of the healthy tissue. Diseased tissue is less flexible or non-compliant and provides resistance to the centrifugal force, which generates an opposing force that enables the diamond-coated crown to engage and sand the plaque. The sanded plaque and calcium are broken down into particles generally smaller than circulating red blood cells that are washed away downstream with the patient’s natural blood flow.
4
The small particle size and short treatment time minimizes the risk of vascular bed overload, or a saturation of the peripheral or coronary vessels with large particles, which may cause slow or reduced blood flow. The small size of the particles allows them to be naturally cleared from the blood via various types of white blood cells and macrophages.
We believe the OAS offers the following key benefits:
Strong Safety Profile
• | Differential Sanding Reduces Risk of Adverse Events. The OAS is designed to differentiate between hard, non-compliant plaque and soft, compliant arterial tissue. Arteries are composed of three tissue layers (from inside to out): the intima, media, and adventitia. The eccentrically mounted diamond-coated crown at the working end of the device engages and removes plaque from the artery wall with minimal likelihood of penetrating or damaging the fragile intima, or inner layer of the arterial wall because soft, compliant tissue flexes away from the crown. |
• | Eliminates Need for Distal Protection. The small size of the particles produced during sanding avoids the need for ancillary distal protection devices, commonly used with directional cutting atherectomy devices. The small particulate size also significantly reduces the risk of macroembolization, or larger pieces of removed plaque capable of blocking blood flow downstream. |
• | Allows Continuous Blood Flow During Procedure. The OAS allows for continuous blood flow while orbiting, as well as constant flushing of particulates during treatment. Other devices may restrict blood flow due to the size of the catheter required or the use of distal protection devices, which could result in complications such as excessive heat and tissue damage. |
• | Benefits of Smaller Sheaths. The Diamondback 360 Peripheral OAS portfolio is uniquely compatible with 4 French (“Fr”) to 6Fr sheaths. Centrifugal force enables the OAS to treat large vessels through small sheaths; for example, it can treat up to 5mm vessel through a 4Fr sheath. Smaller sheaths may be associated with fewer bleeding complications, shortened post-procedure ambulation time and reduced radiation exposure. |
Proven Efficacy
• | Efficacy Demonstrated for Both Peripheral OAS and Coronary OAS. |
◦ | Peripheral OAS - Our pivotal OASIS clinical trial was designed to evaluate the safety and effectiveness of the OAS in treating patients with symptomatic PAD. Performance targets were established cooperatively with the FDA before the trial began. Despite 55% of the lesions consisting of calcified plaque, the Diamondback 360 Peripheral OAS successfully met the study endpoints. |
◦ | Coronary OAS - Our pivotal ORBIT II Coronary OAS trial was designed to evaluate the safety and efficacy of the OAS in treating de novo severely calcified coronary lesions. The trial met both the primary safety and efficacy endpoints by significant margins. Preparation of severely calcified plaque with the Coronary OAS not only helped facilitate stent delivery, but also improved both peri-procedural and 30-day clinical outcomes compared with the outcomes of historic control subjects in this difficult-to-treat patient population. |
• | Treats Difficult, Fibrotic and Calcified Lesions. The Peripheral OAS enables physicians to remove plaque from long, fibrotic, or calcified lesions, as well as bifurcated lesions or lesions with softer plaque, in peripheral arteries both above and below the knee. In the coronary arteries, the Coronary OAS enables physicians to treat complex, severely calcified lesions, enabling stent placement in these difficult to treat lesions. To date, the Coronary OAS is the only FDA-approved device approved specifically for treatment of severely calcified coronary lesions. |
• | Orbital Motion Improves Lesion Compliance. The orbiting action of the OAS removes the hard plaque in the artery by sanding, while the centrifugal motion of the eccentrically mounted crown creates pulsatile forces. Compliance change is achieved as the OAS differentiates between hard, diseased plaque and healthy, compliant arterial tissue, a concept that we refer to as “differential sanding.” The diamond-coated crown preferentially engages and sands away harder material, but is designed to not damage more compliant healthy parts of the artery, which flex away from the crown. Physicians position the crown at the site of a lesion containing arterial plaque and orbit the crown against it to sand away the superficial, or surface, plaque and create a smooth lumen, or channel, in the vessel. In addition, the crown’s rotating eccentric mass and orbital motion deliver pulsatile mechanical energy into the vessel wall. These pulsatile forces may break up deeper plaque and contribute to compliance change of the diseased segment of the artery. |
5
Together, these mechanistic components sufficiently remove or modify hard plaque, allowing for low pressure balloon inflation. The orbital motion and speed of the eccentrically mounted crown increases, thus allowing for continuous reduction of plaque with differential sanding and pulsatile forces, as the opening of the lumen increases during the operation of the devices.
• | Differential Sanding Creates Smooth Lumens. The differential sanding of the OAS creates a smooth lumen surface, or channel, inside the vessel. We believe that the smooth lumens created by the device increase the velocity of blood flow and decrease the resistance to blood flow, which may decrease the potential for restenosis, or re-narrowing of the arteries. |
Ease of Use
• | Set Up Time. Given the relative simplicity of the OAS, physicians and lab staff can usually set up and begin using the device in under two minutes. |
• | Utilizes Familiar Techniques. Physicians using the OAS employ techniques similar to those used in angioplasty, which are familiar to interventional cardiologists, vascular surgeons and interventional radiologists who are trained in endovascular techniques. The devices’ simple user interfaces require minimal additional training. |
• | Single Crown Treats Multiple Lesions in Various Sized Vessels. Centrifugal force unique to the OAS allows for a single access site to treat multiple lesions, in most cases. In the coronary arteries, Coronary OAS is the only atherectomy device able to treat 2.5 to 4mm vessels with one device through a 6Fr radial approach. In the peripheral vasculature, the OAS is capable of treating multiple lesions in multiple arteries through a single access site, thus reducing the need for multiple devices or the need for multiple access sites. |
• | No Need for Collection Reservoir. Because the particles of plaque sanded away are of such small sizes, the OAS does not require a collection reservoir that needs to be repeatedly emptied or cleaned during the procedure, which would potentially add time and cost to the procedure. |
Multiple Applications
The unique OAS mechanism of action used in both the Peripheral OAS and Coronary OAS can be used to treat multiple anatomic locations.
• | Below-the-Knee and Behind-the-Knee Peripheral Artery Disease. Arteries below and behind the knee are small in diameter and may be diffusely stenosed, calcified or both. Reaching and treating these small vessels requires a low profile, which most competitive devices do not offer. Behind-the-knee, or popliteal, lesions also present challenges if a stent is used because stents frequently fracture in this area due to the forces exerted on the vessels when the knee bends or flexes. The Diamondback 360 Peripheral OAS is effective in treating those vessels. The Peripheral OAS offers a shorter shaft length (60cm), a smaller profile and a more flexible shaft than the predecessors for improved ease of use, and includes a 4-Fr catheter that enables physicians to access lesions below-the-knee using retrograde access through arteries in the ankle or foot. |
• | Above-the-Knee Peripheral Artery Disease. Arteries above the knee are typically longer, straighter and wider than below-the-knee vessels. Plaque in these arteries may also be diffuse, fibrotic and calcific. Physicians often use higher speeds or larger crown sizes of our products to treat lesions above the knee. Our newest Peripheral OAS innovation includes the addition of extended length OAS that can treat above-the-knee disease through trans-radial access (access through the radial artery in the wrist). The ability to treat the larger above-the-knee arteries with OAS via the small trans-radial access sites is made possible by the unique features of the OAS including its small crossing profile and ability to orbit at higher speeds for treatment of larger vessels. |
• | Coronary Artery Disease. The individuals more at risk for being diagnosed with CAD are those that are suffering from high blood pressure, abnormal cholesterol levels, diabetes, renal insufficiency, or have a family history of heart disease. The pathogenesis of CAD is marked by the accumulation of a fatty material called plaque on the walls of arteries that supply blood to the heart. The plaque buildup causes the arteries to harden and narrow (atherosclerosis), reducing blood flow. The Coronary OAS is the only atherectomy device specifically indicated for severe coronary calcium. |
6
Cost and Time Efficient Procedure
• | Short Procedure Time. The OAS has a short treatment time, typically less than two minutes. |
• | Single Crown Can Treat Various Lumen Sizes Helping Limit Hospital Costs. The OAS orbital mechanism of action allows one device to treat various diameter lumens inside the artery. Adjusting the rotational speed of the crown changes the orbit to create the desired lumen diameter, thereby potentially avoiding the need to use multiple catheters of different sizes to treat multiple lesions. |
• | Trans-Radial Access Provides Multiple Benefits. The low profile of the OAS allows for trans-radial access with benefits to both physicians and patients. Radial access can enable treatment of complex, calcified coronary and, often, peripheral arteries that are challenging to access. In addition, the radial access site is associated with lower vascular and bleeding complication rates, faster patient recovery time, and the ability to treat bilateral disease in one setting, address obese patients and work around previous, compromised access sites. For patients, this contributes to comfort during- and post-operation, earlier ambulation, reduced risk of infection, and faster healing. |
• | Retrograde Access Treatment Option Benefits. Many of the patients treated with the Peripheral OAS have advanced PAD and suffer from CLI. These patients often have complex, calcified lesions in their lower leg, which are challenging to access and treat using the traditional femoral artery access site. If left untreated, these cases may result in lower limb amputation. Our family of 1.25mm Peripheral OASs with 4Fr compatibility allows for more options to treat those lesions by providing a low-profile system that is fully compatible with alternative access sites in the foot or ankle. Smaller sheaths have been shown to reduce procedure times and decrease complications. |
Our Strategy
Our goal is to be the leading provider of solutions for the treatment of PAD and CAD. We intend to broaden our product offering and expand to new international markets. The key elements of our strategy include:
• | Drive Adoption through Our Direct U.S. Sales Organization, Medical Education and Key Opinion Leaders. We expect to continue to drive adoption of the OAS in both hospital and office-based lab settings through the strong support of a clinically knowledgeable direct U.S. sales force focused on the needs of interventional cardiologists, vascular surgeons, interventional radiologists and their cath lab teams. A key element of our strategy is a focus on educating and training physicians about disease states, our clinical data, and proper use and application of OAS technology through programs delivered via physician faculty, our direct sales force and seminars where physician industry leaders discuss case studies and treatment techniques using the devices. |
• | Build a Strong Portfolio of Clinical Evidence on Safety, Effectiveness and Economic Benefits of the OAS. Physicians and payors are increasingly interested in clinical and economic evidence to support decisions regarding optimal treatment of patients. We are focused on conducting robust clinical studies that provide insight into and demonstrate the effectiveness of the OAS in treating complex peripheral and coronary artery disease. We believe that demonstrating the clinical advantages and cost-effectiveness of our OAS technology is critical to support physician adoption of the OAS, drive best clinical practice, and sustain ongoing reimbursement coverage for our devices. |
• | Enhance OAS and Expand Product Portfolio within the Market for Treatment of Peripheral and Coronary Arteries. In addition to continued innovation and product development on our peripheral and coronary OAS platforms, we are growing our product portfolio to offer new accessories and devices that improve outcomes and expand the patient population we can treat. See “Pursue Strategic Acquisitions and Partnerships” and “Research and Development Activities - Development Activities” for descriptions of new products in development. |
• | Expand Internationally. In November 2016, we signed an exclusive distribution agreement with Medikit Co., Ltd. to sell the Diamondback 360 Coronary OAS Micro Crown in Japan. In March 2017, we received approval of that device from Japan’s MHLW. In February 2018, we announced reimbursement approval and our first commercially treated patient in Japan. This represented our first entry into the international market, and most importantly, an opportunity to provide physicians in Japan with a cost-effective treatment option for the difficult-to-treat patient population with severely calcified coronary lesions. |
In October 2014, we received CE Mark for our Stealth 360 Peripheral OAS device and we are planning to seek approval of other products in Europe.
7
In July 2018, we entered into an exclusive Distribution Agreement with OrbusNeich to sell our coronary and peripheral OAS outside of the United States and Japan. We expect that OrbusNeich will commence sales of our products in certain countries in Southeast Asia, Europe and the Middle East during our fiscal year ending June 30, 2019, and we will closely collaborate with OrbusNeich on the timing of product introductions in each country under this Distribution Agreement. OrbusNeich and its authorized sub-distributors will be responsible for selling our products, and we will focus our international efforts on physician training and education on our products.
• | Pursue Strategic Acquisitions and Partnerships. In addition to adding to our product portfolio through internal development efforts, we are opportunistically seeking ways to expand our portfolio through acquisitions, distribution agreements, licensing transactions, manufacturing agreements and other strategic partnerships to add new product lines and technologies that leverage our sales expertise and footprint or complement our strategic objectives. In January 2018, we announced an exclusive U.S. distribution agreement with OrbusNeich to offer their full line of semi-compliant, non-compliant and specialty balloons for both coronary and peripheral vascular procedures. Currently we are focused on offering their coronary balloons, including the Sapphire® II Pro, which obtained FDA clearance in March 2018 as the first and only 1.0mm coronary balloon available in the United States. In addition, we entered into an agreement with Integer Holdings Corporation to manufacture our ZILIENT™ peripheral guidewires, which are designed to provide tip resilience and crossability in challenging peripheral arterial lesions. Finally, we entered into an agreement with Aerolase Corp. for the co-development of a new laser atherectomy device for physicians to use in more effectively treating multiple forms of arterial disease. |
Research and Development Activities
Clinical Studies Summary
We study the most challenging patient populations and are committed to providing relevant clinical evidence that enables physicians to select and utilize the best treatment options for their patients. A total of 6,121 subjects (4,788 PAD and 1,333 CAD) have been enrolled in our clinical studies as of June 30, 2018. Our clinical studies incorporate rigorous long-term clinical and healthcare economic data that are critical to improving patient care and ongoing healthcare changes.
We have completed numerous clinical studies to demonstrate the safety and efficacy of the Peripheral OAS. Its unique mechanism of action differentiates the Peripheral OAS from other endovascular PAD treatment options and in our clinical studies of patients with the most challenging lesions, the Peripheral OAS consistently demonstrates successful lesion modification and durable results.
PAD Studies
The following PAD clinical studies were completed or in process during fiscal 2018:
• | LIBERTY 360°. This prospective, observational, multi-center clinical study is evaluating the procedural and long-term clinical, quality of life and economic outcomes of endovascular device interventions, including orbital atherectomy, for the treatment of PAD. We expect the results from this study to increase our understanding of the clinical and economic outcomes of endovascular treatment for PAD patients, including those with the most advanced form of the disease, Rutherford Class 6. Enrollment of 1,204 subjects at 51 sites in the United States was completed in February 2016. |
LIBERTY 360° two-year data was presented in August 2018. The majority of devices used in the study were balloons and/or atherectomy, and the Peripheral OAS was the most frequently used atherectomy device. The LIBERTY 360° data through two years demonstrated that peripheral interventions can be used successfully across all Rutherford classes, including the most challenging Rutherford Class 6 subjects. Quality of life improved significantly from baseline to two years in all Rutherford groups. An orbital atherectomy subanalysis of the LIBERTY 360° data indicated high freedom from major amputation at two years in all Rutherford Classes (RC2-3, 99.1%; RC4-5, 94.5%; and RC6, 79.8%). Overall, the results of this novel all-comers PAD study suggest that peripheral vascular intervention (“PVI”) is an alternative to “primary amputation” in Rutherford Class 6 patients. Additionally, data from the LIBERTY 360° study provide further evidence to support PVI treatment in Rutherford 2-5 patients.
8
• | OPTIMIZE BTK. This pilot, hypothesis generating, non-powered, multi-center, randomized clinical study conducted in Europe is designed to gather preliminary exploratory data regarding the acute and long-term clinical outcomes of orbital atherectomy with adjunctive DCB angioplasty versus DCB angioplasty alone in PAD patients with calcified, below-the-knee lesions. We completed enrollment of fifty evaluable patients in May 2018, and these patients will be followed for up to two years. |
CAD Studies
We have conducted two clinical studies to evaluate the safety and efficacy of the Coronary OAS Classic Crown device: the ORBIT I feasibility study and the ORBIT II pivotal study. The safety and efficacy of the Coronary OAS Micro Crown device were evaluated in the COAST study.
The following CAD clinical study was in process during fiscal 2018:
• | ECLIPSE. This post-market, randomized one-to-one, multi-center trial is designed to evaluate vessel preparation with Coronary OAS Classic Crown compared to conventional angioplasty technique prior to drug-eluting stent implantation for the treatment of severely calcified lesions. Approximately 2,000 subjects will be enrolled at approximately 150 sites in the United States and subjects will be followed for up to two years. The co-primary endpoints of acute minimum stent area (assessed by optical coherence tomography in a subset of equally randomized 500 subjects) and one-year target vessel failure are powered to demonstrate superiority of OAS vessel preparation vs. conventional angioplasty. |
Our clinical portfolio is expanding as we develop future studies to answer difficult questions about PAD and CAD treatment. Our clinical research continues to highlight the safety and efficacy of the OAS and current and new research illustrates our versatility in the emerging vascular market.
Development Activities
Our product research and development activities are dedicated to the development and commercialization of products that serve the peripheral and coronary vascular disease space, with emphasis towards high margin products and complex arterial disease states treated by our primary customers. The focus and value proposition of our products is to enable positive acute and long-term clinical outcomes, with efficiency and predictability, in challenging patient subsets.
Research and development resources have been strategically allocated between opportunities that maximize the clinical effectiveness and user satisfaction of our OAS product line and the development of additional products that offer portfolio diversification and incremental revenue opportunities.
Specific to the peripheral vascular disease market, we will continue our commitment to patients suffering from CLI through a breadth of above-the-knee and below-the-knee differentiated products that treat or uniquely expand the ability of our devices to treat obstructive lesions throughout the leg and foot. Most recently, we launched a line of extended length Diamondback 360 products (180 cm and 200 cm) that are intended to accommodate patient anatomy and physician preferred access methodology, for example radial access. Specific to the coronary vascular disease market, we are building a portfolio of differentiated products that are used to treat complex CAD. We launched a new coronary OAS feature called GlideAssist. This feature is an innovative solution that facilitates device tracking, provides easier device repositioning, and provides enhanced performance in challenging anatomies. We also recently launched our next generation saline infusion pump. The pump is the reusable component of the system, and the next generation product has been updated to comply with new medical device standards and address numerous user satisfaction opportunities. In addition, we entered into an agreement with Aerolase Corp. for the co-development of a new laser atherectomy device for physicians to use to more effectively treat multiple forms of arterial disease, and we are developing a new temporary hemodynamic support pump. Emphasis in both franchises is placed on novel and differentiated devices that address unmet or under-met clinical or technical needs.
Research and development expenses for the years ended June 30, 2018, 2017, and 2016 were $26.8 million, $22.9 million and $25.9 million, respectively.
9
Sales and Marketing
We market and sell the majority of our products through a direct sales force in the United States. Revenues for the years ended June 30, 2018, 2017, and 2016 were $217.0 million, $204.9 million and $178.2 million, respectively. We have targeted sales and marketing efforts to interventional cardiologists, vascular surgeons and interventional radiologists with experience using similar catheter-based procedures, such as angioplasty, stenting, and directional or laser atherectomy. Professional education is also a key element of our sales strategy.
We target our marketing efforts to practitioners through medical conferences, seminars, peer-reviewed journals and marketing materials. Our sales and marketing program focuses on:
• | clinical results showing safety and efficacy of our products; |
• | educating physicians on the prevalence and complications of calcium in PAD and CAD; and |
• | developing relationships with key opinion leaders. |
We sell the majority of our products through direct shipment to hospitals or clinics.
We are party to a purchasing agreement with HealthTrust Purchasing Group, L.P. (“HPG”), which was renewed effective May 1, 2018 and expires on July 31, 2021. HPG acts as a group purchasing organization for the healthcare providers belonging to HPG as participants. Under the purchasing agreement, all of HPG’s participants located in the United States or its territories are eligible to purchase our orbital atherectomy systems and related products at prices set forth in the purchasing agreement. The purchasing agreement may be terminated at any time, without cause, by HPG upon at least 60 days’ prior written notice to us. Either party may terminate the purchasing agreement upon the occurrence of a material breach by the other party that goes uncured within 30 days following receipt of written notice of such breach. If the purchasing agreement with HPG were to be terminated, our financial results will be materially adversely affected.
Outside of the United States, sales of our products in Japan are through our exclusive distributor, Medikit. In July 2018, we entered into an exclusive distribution agreement with OrbusNeich to sell our Peripheral OAS and Coronary OAS outside of the United States and Japan through OrbusNeich’s current sales and distribution network. For the year ended June 30, 2018, $1.8 million of our revenue was from sales in Japan, while the remaining revenues were from sales in the United States. For the years ended June 30, 2017 and 2016, all of our revenues were from sales in the United States.
We have observed some degree of seasonality in our business, as there tends to be a lower number of procedures that use our products during the three months ending September 30. Interventional procedure volume usually grows throughout the course of the fiscal year, with the quarter ending June 30 usually representing the highest volume of cases and, therefore, the highest amount of revenue generated by us during the course of the fiscal year.
Manufacturing
We use internally-manufactured and externally-sourced components to manufacture the OAS. Most of the externally-sourced components are available from multiple suppliers; however, certain key components, including the diamond-grit-coated crown and our ViperSlide Lubricant, are single sourced. We have strategies and arrangements in place for procuring our key components from alternative suppliers in the event that one or more of our single source suppliers were to discontinue supplying us with a key component. We assemble the shaft, crown and handle components on-site, and test, pack, seal and label the finished assembly before sending the packaged product to a contract sterilization facility. Upon return from the sterilizer, the product is held in inventory prior to shipping to our customers.
We are located in a leased 125,000-square-foot corporate headquarters in Minnesota. This custom-designed building has space for more than 500 employees and contains dedicated research and development, training and education, and manufacturing facilities. Depending on staffing, the facility has the annual capacity to produce in excess of 75,000 devices per shift. The finished goods storage has capacity for approximately 20,000 devices and more than 500 saline infusion pumps, as well as other accessory products.
Our leased Pearland, Texas facility is 46,000 square feet and includes a custom-built clean room and production space for future expansion of value-add processes, including machining and electronics assembly. The facility, when fully staffed and equipped, also has the annual capacity to produce approximately 75,000 devices per shift. This facility has finished goods storage capacity for greater than 15,000 devices and other accessory products and over 500 saline infusion pumps.
10
We believe that our facilities in Minnesota and Texas will provide adequate production, assembly, and warehousing capacity for the foreseeable future.
We are registered with the FDA as a medical device manufacturer and comply with the FDA’s Quality System Regulation (“QSR”). We have opted to maintain a Quality Management System to enable us to market our products in the member states of the European Union, the European Free Trade Association and countries that have entered into Mutual Recognition Agreements with the European Union. We are ISO 13485:2003 certified, and our renewal is due in December 2018. Under these registrations, our plants are audited by the FDA and our Notified Body. The Stealth 360 Peripheral OAS has received CE Mark. We are registered as a Foreign Medical Device Manufacturer in Japan and our registration certificate renewal is due in June 2021.
Third-Party Reimbursement and Pricing
Third-party payors, including private insurers and government insurance programs, such as Medicare and Medicaid, pay for a significant portion of patient care provided in the United States. The single largest payor in the United States is the Medicare program, a federal governmental health insurance program administered by the Centers for Medicare and Medicaid Services (“CMS”). Medicare covers certain medical care expenses for eligible elderly and disabled individuals, including a large percentage of the population with PAD and CAD who could be treated with the OAS. In addition, private insurers often follow the coverage and reimbursement policies of Medicare. Consequently, Medicare’s coverage and reimbursement policies are important to our operations.
CMS establishes Medicare reimbursement coverage policy and payment rates for physician and facility healthcare services, including procedures using atherectomy products. Obtaining and maintaining coding, coverage and payment for our products is critical for commercial success. We believe that physicians and hospitals that treat PAD and CAD with the respective OAS will generally be eligible to receive reimbursement from Medicare, as well as private insurers, that is adequate to cover the costs of OAS, associated materials, and physician’s services.
With respect to reimbursement outside of the United States, in February 2018, the Coronary OAS Micro Crown received reimbursement approval in Japan, followed by the first commercial sales in Japan. In connection with our Distribution Agreement with OrbusNeich, we and OrbusNeich will seek reimbursement approvals in other countries in connection with the commercial introductions of our products, to the extent that reimbursement is available and subject to local rules and regulations.
Competition
The medical device industry is highly competitive, subject to rapid change and significantly affected by new product introductions and other activities of industry participants. Our OAS compete with a variety of other products or devices for the treatment of vascular disease, including stents, balloon angioplasty catheters and atherectomy catheters, as well as products used in vascular surgery. Competitors in the stent and balloon angioplasty market segments include Abbott Laboratories, Boston Scientific, Cook Medical, Johnson & Johnson, Becton Dickinson, and Medtronic. We also compete against manufacturers of atherectomy catheters and other products designed to treat vascular disease, including Medtronic, Philips/Spectranetics, Boston Scientific, Ra Medical, Shockwave and Avinger, as well as manufacturers that may enter the market due to the increasing demand for treatment of vascular disease. Other competitors include pharmaceutical companies that manufacture drugs for the treatment of PAD and CAD and companies that provide products used by surgeons in peripheral and coronary bypass procedures.
Because of the size of the peripheral opportunities, competitors and potential competitors have historically dedicated significant resources to aggressively promote their products. We believe that our Peripheral OAS and Coronary OAS compete primarily on the basis of:
• | safety and efficacy, even in calcified plaque (or severely calcified plaque in the coronaries); |
• | low profile and alternative access site capabilities; |
• | predictable clinical performance; |
• | availability of clinical data; |
• | ease of use; |
• | economic benefit; |
• | key opinion leader support and customer base; |
• | customer service and support. |
11
We are aware of a company, Cardio Flow, Inc., developing an atherectomy system that could potentially compete with our products. On August 27, 2012, we entered into a Settlement Agreement with Lela Nadirashvili, the widow of Dr. Leonid Shturman, our founder, relating to the ownership of certain patents invented by Dr. Shturman. We believe that Ms. Nadirashvili assigned her rights under the Settlement Agreement, including the right to utilize certain patents, to Cardio Flow. On April 6, 2018, we filed a breach of contract action against Cardio Flow in Minnesota District Court in Ramsey County, Minnesota, alleging that Cardio Flow has developed or is in the process of developing an atherectomy device that incorporates elements belonging exclusively to us, in violation of the Settlement Agreement. We are seeking damages and a permanent injunction preventing Cardio Flow from taking further steps to develop or attempt to develop an atherectomy device that incorporates the elements that belong exclusively to us. We are pursuing our claims against Cardio Flow vigorously.
Patents and Intellectual Property
We rely on a combination of patent, copyright and other intellectual property laws, trade secrets, nondisclosure agreements and other measures to protect our proprietary rights. As of June 2018, we held 52 issued U.S. patents and have 38 U.S. patent applications pending, as well as 175 granted foreign patents and 152 foreign patent applications pending, each of which corresponds to aspects of our U.S. patents and applications. Our issued U.S. patents expire between 2019 and 2035, and our patents covering the core technology begin to expire in 2023 while providing core technology coverage through 2035. We have many additional patents relating to our core technology currently pending in the USPTO, which will extend our key covered subject matter and coverage dates significantly. Our issued patents and patent applications relate primarily to the design and operation of interventional atherectomy devices, including the Peripheral OAS and Coronary OAS. These patents and applications include claims covering key aspects of orbital atherectomy devices, including the design, manufacture and therapeutic use of certain atherectomy abrasive heads, drive shafts, control systems, handles and couplings. As we continue to research and develop our atherectomy technology, we intend to file additional U.S. and foreign patent applications related to the design, manufacture and therapeutic uses of atherectomy devices.
In addition, we hold 22 registered U.S. trademarks, 17 registered marks in the Madrid Protocol with protection granted within at least one of Australia, the European Union, China, India, Japan, the United Kingdom, and Mexico, six registered marks in the European Union, 12 registered marks in Canada, eight registered marks in India, four registered marks in Mexico, and nine registered marks in Hong Kong. We have multiple pending trademark applications worldwide.
We also rely on trade secrets, technical know-how and continuing innovation to develop and maintain our competitive position. We seek to protect our proprietary information and other intellectual property by requiring our employees, consultants, contractors, outside scientific collaborators and other advisors to execute non-disclosure and assignment of invention agreements on commencement of their employment or engagement. Agreements with our employees also forbid them from bringing the proprietary rights of third parties to us. We also require confidentiality or material transfer agreements from third parties that receive our confidential data or materials.
Government Regulation of Medical Devices
Governmental authorities in the United States at the federal, state and local levels and in other countries extensively regulate, among other things, the development, testing, manufacture, labeling, promotion, advertising, distribution, marketing and export and import of medical devices such as the Peripheral OAS and Coronary OAS.
Failure to obtain approval to market our products under development and to meet the ongoing requirements of these regulatory authorities could prevent us from marketing and continuing to market our products.
United States
The Federal Food, Drug, and Cosmetic Act (“FDCA”) and the FDA’s implementing regulations govern medical device design and development, preclinical and clinical testing, premarket clearance or approval, registration and listing, manufacturing, labeling, storage, advertising and promotion, sales and distribution, export and import, and post market surveillance. Medical devices and their manufacturers are also subject to inspection by the FDA. The FDCA, supplemented by other federal and state laws, also provides civil and criminal penalties for violations of its provisions.
Unless an exemption applies, each medical device we wish to commercially distribute in the United States will require marketing authorization from the FDA prior to distribution. The two primary types of FDA marketing authorization are premarket notification (also called 510(k) clearance) and PMA.
12
510(k) Clearance. To obtain 510(k) clearance for a medical device, an applicant must submit a premarket notification to the FDA demonstrating that the device is “substantially equivalent” to a predicate device legally marketed in the United States. A device is substantially equivalent if, with respect to the predicate device, it has the same intended use and has either (i) the same technological characteristics or (ii) different technological characteristics and the information submitted demonstrates that the device is as safe and effective as a legally marketed device and does not raise different questions of safety or effectiveness. A showing of substantial equivalence sometimes, but not always, requires clinical data. Generally, the 510(k) clearance process can exceed 90 days and may extend to a year or more.
After a device has received 510(k) clearance for a specific intended use, any modification that could significantly affect its safety or effectiveness, such as a significant change in the design, materials, method of manufacture or intended use, will require a new 510(k) clearance or PMA (if the device as modified is not substantially equivalent to a legally marketed predicate device). The determination as to whether new authorization is needed is initially left to the manufacturer; however, the FDA may review this determination to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing the modified device until 510(k) clearance or PMA is obtained. The manufacturer may also be subject to significant regulatory fines or penalties.
We received 510(k) clearances for the Peripheral OAS as set forth under “Business Overview.”
Premarket Approval. A PMA application requires the payment of significant user fees and must be supported by valid scientific evidence, which typically requires extensive data, including technical, preclinical, clinical and manufacturing data, to demonstrate to the FDA’s satisfaction the safety and efficacy of the device. A PMA application must also include a complete description of the device and its components, a detailed description of the methods, facilities and controls used to manufacture the device, and proposed labeling. After a PMA application is submitted and found to be sufficiently complete, the FDA begins an in-depth review of the submitted information. During this review period, the FDA may request additional information or clarification of information already provided. Also during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a pre-approval inspection of the manufacturing facilities to ensure compliance with the FDA’s QSR, which requires manufacturers to follow design, testing, control, documentation and other quality assurance procedures.
FDA review of a PMA application is required by statute to take no longer than 180 days, although the process typically takes significantly longer, and may require several years to complete. The FDA can delay, limit, or deny approval of a PMA application for many reasons, including:
• | the systems may not be safe or effective to the FDA’s satisfaction; |
• | the data from preclinical studies and clinical trials may be insufficient to support approval; |
• | the manufacturing process or facilities used may not meet applicable requirements; and |
• | changes in FDA approval policies or adoption of new regulations may require additional data. |
If the FDA evaluations of both the PMA application and the manufacturing facilities are favorable, the FDA will either issue an approval letter or an approvable letter, which usually contains a number of conditions that must be met in order to secure final approval of the PMA. When and if those conditions have been fulfilled to the satisfaction of the FDA, the agency will issue a PMA letter authorizing commercial marketing of the device for certain indications. If the FDA’s evaluation of the PMA application or manufacturing facilities is not favorable, the FDA will deny PMA or issue a not approvable letter. The FDA may also determine that additional clinical trials are necessary, in which case the PMA may be delayed for several months or years while the trials are conducted and the data submitted in an amendment to the PMA application. Even if a PMA application is approved, the FDA may approve the device with an indication that is narrower or more limited than originally sought. The agency can also impose restrictions on the sale, distribution or use of the device as a condition of approval, or impose post approval requirements such as continuing evaluation and periodic reporting on the safety, efficacy, and reliability of the device for its intended use.
New PMA applications or PMA supplements may be required for modifications to the manufacturing process, labeling, device specifications, materials or design of a device that is approved through the PMA process. PMA supplements often require submission of the same type of information as an initial PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA application and may not require as extensive clinical data or the convening of an advisory panel.
13
Clinical Trials. Clinical trials are almost always required to support a PMA application and are sometimes required for a 510(k) clearance. These trials generally require submission of an application for an Investigational Device Exemption (“IDE”) to the FDA. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE application must be approved in advance by the FDA for a specified number of patients, unless the product is deemed a non-significant risk device and eligible for more abbreviated IDE requirements. Generally, clinical trials for a significant risk device may begin once the IDE application is approved by the FDA and the study protocol and informed consent are approved by appropriate institutional review boards at the clinical trial sites.
FDA approval of an IDE allows clinical testing to go forward but does not bind the FDA to accept the results of the trial as sufficient to prove the product’s safety and efficacy, even if the trial meets its intended success criteria. With certain exceptions, changes made to an investigational plan after an IDE is approved must be submitted in an IDE supplement and approved by FDA (and by governing institutional review boards when appropriate) prior to implementation.
All clinical trials must be conducted in accordance with regulations and requirements collectively known as good clinical practice. Good clinical practices include the FDA’s IDE regulations, which describe the conduct of clinical trials with medical devices, including the recordkeeping, reporting and monitoring responsibilities of sponsors and investigators, and labeling of investigational devices. They also prohibit promotion, test marketing or commercialization of an investigational device and any representation that such a device is safe or effective for the purposes being investigated. Good clinical practices also include the FDA’s regulations for institutional review board approval and for protection of human subjects (such as informed consent), as well as disclosure of financial interests by clinical investigators.
Required records and reports are subject to inspection by the FDA. The results of clinical testing may be unfavorable or, even if the intended safety and efficacy success criteria are achieved, may not be considered sufficient for the FDA to grant approval or clearance of a product. The commencement or completion of any clinical trials may be delayed or halted, or be inadequate to support approval of a PMA application or clearance of a premarket notification for numerous reasons.
Continuing Regulation. After a device is cleared or approved for use and placed in commercial distribution, numerous regulatory requirements continue to apply. These include:
• | establishment registration and device listing upon the commencement of manufacturing; |
• | the QSR, which requires manufacturers, including third-party manufacturers, to follow design, testing, control, documentation and other quality assurance procedures during medical device design and manufacturing processes; |
• | labeling regulations, which prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling and promotional activities; |
• | medical device reporting regulations, which require that manufacturers report to the FDA if a device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if malfunctions were to recur; |
• | corrections and removal reporting regulations, which require that manufacturers report to the FDA field corrections; and |
• | product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA caused by the device that may present a risk to health. |
In addition, the FDA may require a company to conduct post market surveillance studies or order it to establish and maintain a system for tracking its products through the chain of distribution to the patient level.
Failure to comply with applicable regulatory requirements, including those applicable to the conduct of clinical trials, can result in enforcement action by the FDA, which may lead to any of the following sanctions:
• | warning letters or untitled letters; |
• | fines, injunctions and civil penalties; |
• | product recall or seizure; |
• | unanticipated expenditures; |
• | delays in clearing or approving or refusal to clear or approve products; |
• | withdrawal or suspension of FDA approval; |
• | orders for physician notification or device repair, replacement or refund; |
• | operating restrictions, partial suspension or total shutdown of production or clinical trials; or |
• | criminal prosecution. |
14
We and our contract manufacturers, specification developers and suppliers are also required to manufacture our products in compliance with current good manufacturing practice requirements set forth in the QSR.
The QSR requires a quality system for the design, manufacture, packaging, labeling, storage, installation and servicing of marketed devices, and includes extensive requirements with respect to quality management and organization, device design, buildings, equipment, purchase and handling of components, production and process controls, packaging and labeling controls, device evaluation, distribution, installation, complaint handling, servicing and record keeping. The FDA enforces the QSR through periodic announced and unannounced inspections that may include the manufacturing facilities of subcontractors. If the FDA believes that we or any of our contract manufacturers or regulated suppliers is not in compliance with these requirements, it can shut down our manufacturing operations, require recall of our products, refuse to clear or approve new marketing applications, institute legal proceedings to detain or seize products, enjoin future violations or assess civil and criminal penalties against us or our officers or other employees. Any such action by the FDA would have a material adverse effect on our business.
Fraud and Abuse
Our operations are directly, or indirectly through our customers, subject to various state and federal fraud and abuse laws, including, without limitation, the FDCA, the federal Anti-Kickback Statute and the False Claims Act. These laws may impact, among other things, our sales, marketing, education and clinical programs.
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program, such as the Medicare and Medicaid programs. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Many states have also adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
The federal False Claims Act prohibits persons from knowingly filing or causing to be filed a false claim to, or the knowing use of false statements to obtain payment from, the federal government. Various states have also enacted laws modeled after the federal False Claims Act.
In addition to the laws described above, the Health Insurance Portability and Accountability Act of 1996 created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services.
On June 28, 2016, we entered into a Settlement Agreement with the U.S. government, acting through the U.S. Attorney’s Office for the Western District of North Carolina (the “DOJ”) and on behalf of the Office of Inspector General of the Department of Health and Human Services (the “OIG”) and Travis Thams to resolve the DOJ investigation of whether we violated the False Claims Act. In connection with the resolution of this matter, we entered into a five-year corporate integrity agreement (the “Corporate Integrity Agreement”) with the OIG. The Corporate Integrity Agreement requires that we maintain our existing compliance programs and imposes certain expanded compliance-related requirements during the term of the Corporate Integrity Agreement, including establishment of specific procedures and requirements regarding consulting activities, co-marketing activities and other interactions with healthcare professionals and healthcare institutions and the sale and marketing of our products; ongoing monitoring, reporting, certification and training obligations; and the engagement of an independent review organization to perform certain auditing and reviews and prepare certain reports regarding our compliance with federal health care programs.
The federal Physician Payments Sunshine Act, or the Sunshine Act, and certain state laws require persons to collect and report certain data on payments and other transfers of value to physicians and teaching hospitals. It is widely anticipated that public reporting under the Sunshine Act and implementing Open Payment regulations will result in increased scrutiny of the financial relationships between industry, physicians and teaching hospitals.
15
Voluntary industry codes, federal guidance documents and a variety of state laws address the tracking and reporting of marketing practices relative to gifts given and other expenditures made to doctors and other healthcare professionals. In addition to impacting our marketing and educational programs, our internal business processes are and will continue to be affected by the numerous legal requirements and regulatory guidance at the state, federal and industry levels.
International Regulation
International sales of medical devices are subject to foreign government regulations, which may vary substantially from country to country. While harmonization of global regulations has been pursued, requirements continue to differ significantly among countries. We expect this global regulatory environment will continue to evolve, which could impact the cost, the time needed to approve, and, ultimately, our ability to maintain existing approvals or obtain future approvals for our products. Regulations of the FDA and, as we continue our international expansions, regulatory agencies outside the United States, may impose extensive compliance and monitoring obligations on us and our operations. Additionally, the time required to obtain approval in a foreign country may be longer or shorter than that required for FDA approval and the requirements may differ. For example, the primary regulatory environment in Europe with respect to medical devices is that of the European Union, which includes most of the major countries in Europe. Other countries, such as Switzerland, have voluntarily adopted laws and regulations that mirror those of the European Union with respect to medical devices. The European Union has adopted numerous directives and standards regulating the design, manufacture, clinical trials, labeling and adverse event reporting for medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear the CE conformity marking, indicating that the device conforms to the essential requirements of the applicable directives and, accordingly, can be commercially distributed throughout the European Union, although actual implementation of these directives may vary on a country-by-country basis. The method of assessing conformity varies depending on the class of the product, but normally involves a combination of submission of a design dossier, self-assessment by the manufacturer, a third-party assessment, and review of the design dossier by a “Notified Body.” This third-party assessment generally consists of an audit of the manufacturer’s quality system and manufacturing site, as well as review of the technical documentation used to support application of the CE Mark to one’s product and possibly specific testing of the manufacturer’s product. An assessment by a Notified Body of one country within the European Union is required in order for a manufacturer to commercially distribute the product throughout the European Union.
In July 2016, we submitted an application to Japan’s Pharmaceuticals and Medical Devices Agency (“PMDA”) for approval of our Coronary OAS Micro Crown and received approval in March 2017. In February 2018, Japan became the first international market for any of our products. As part of our Japan commercialization process we are subject to the requirements of the Japanese Act on Securing Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics (the “PMD Act”). Our quality management system and products are subject to review and examination by PMDA and subject to approval and enforcement by Japan’s MHLW. The critical suppliers named in our application are also subject to this review and examination for the activities they perform for us. Non-compliance with the PMD Act could result in revocation or suspension of our license, revocation of approvals, and criminal sanctions such as fines and/or imprisonment.
In July 2018, we entered into an exclusive distribution agreement with OrbusNeich to sell our Peripheral OAS and Coronary OAS outside of the United States and Japan. In connection with the introduction of our products in each country under this distribution agreement, we will need to seek regulatory approvals of our products under the rules and regulations applicable in each such country and we will be required to comply with ongoing requirements, which may be varied and require us to expend substantial resources.
In addition, our international expansion, operations, distribution and sales will require us to comply with the U.S. Foreign Corrupt Practices Act and similar anti-bribery laws in other jurisdictions and with U.S. and foreign export control, trade embargo and custom laws.
Environmental Regulation
Our operations are subject to regulatory requirements relating to the environment, waste management and health and safety matters, including measures relating to the release, use, storage, treatment, transportation, discharge, disposal and remediation of hazardous substances. We are currently classified and licensed as a Very Small Quantity Hazardous Waste Generator within Ramsey County, Minnesota. There are no regulated wastes requiring licensing in our Texas facility.
16
Employees
As of June 30, 2018, we had 652 full-time employees. None of our employees are represented by a labor union or are parties to a collective bargaining agreement, and we believe that our employee relations are good.
Executive Officers of the Registrant
The names, ages and positions of our current executive officers are as follows:
Name | Age | Position | |
Scott R. Ward | 58 | Chairman, President and Chief Executive Officer | |
Jeffrey S. Points | 41 | Chief Financial Officer | |
Rhonda J. Robb | 50 | Chief Operating Officer | |
Laura J. Gillund | 57 | Chief Talent Officer | |
Alexander Rosenstein | 46 | General Counsel and Corporate Secretary | |
Sandra M. Sedo | 54 | Chief Compliance Officer | |
Scott R. Ward, Chairman President and Chief Executive Officer. Mr. Ward has been a member of our Board of Directors since November 2013 and has served as its Chairman since November 2014. Mr. Ward served as our Interim President and Chief Executive Officer beginning November 30, 2015, and on August 15, 2016, Mr. Ward was appointed as our President and Chief Executive Officer. Since 2013, Mr. Ward has been one of the Managing Directors at SightLine Partners. Following his appointment as our President and Chief Executive Officer, Mr. Ward continues to be a Managing Director of Sightline Opportunity Management Fund II, LLC and may provide limited advisory and consulting services to Sightline Partners in this capacity. From 1981 to 2010, Mr. Ward was employed by Medtronic, Inc. and held a number of senior leadership positions. Mr. Ward was Senior Vice President and President of Medtronic’s CardioVascular business from May 2007 to November 2010. Prior to that he was Senior Vice President and President of Medtronic’s Vascular business from May 2004 to May 2007, Senior Vice President and President of Medtronic’s Neurological and Diabetes business, from February 2002 to May 2004, and President of Medtronic’s Neurological business from January 2000 to January 2002. He was Vice President and General Manager of Medtronic’s Drug Delivery business from 1995 to 2000. Prior to that, Mr. Ward led Medtronic’s Neurological Ventures in the successful development of new therapies. Mr. Ward serves on the boards of several private companies. Until April 2016, Mr. Ward was the Chairman of the Board of Creganna Medical. Mr. Ward served as a member of the Board of Surmodics, Inc. from September 2010 to March 2015.
Jeffrey S. Points, Chief Financial Officer. Mr. Points joined us in September 2007 as Corporate Controller, became Senior Director and Controller in July 2013, Corporate Controller and Treasurer in January 2015, Vice President, Corporate Controller and Treasurer in May 2017 and was promoted to Chief Financial Officer in February 2018. From July 2005 to September 2007, Mr. Points was Assistant Controller at Empi, a manufacturer and provider of non-invasive medical products for pain management and physical rehabilitation. From January 1998 to July 2005, Mr. Points held various leadership positions at CliftonLarsonAllen, a national public accounting firm. Mr. Points also serves as a member of the Board of Directors for The Phoenix Residence, Inc.
Rhonda J. Robb, Chief Operating Officer. Ms. Robb joined us as Chief Operating Officer in January 2018. Prior to joining us, she held several positions at Medtronic, most recently as Vice President and General Manager of the Heart Valve Therapies Business from 2014 to 2018. From 2009 to 2014, Ms. Robb was Vice President and General Manager for Medtronic’s Catheter Based Therapies business. Ms. Robb was employed by Medtronic since 1990 and has served in several other leadership roles, including Vice President of Global Marketing, Coronary and Peripheral; Director Global Marketing, Heart Failure/High Power Therapies; and Director US Marketing, Cardiac Rhythm & Disease Management.
Laura J. Gillund, Chief Talent Officer. Ms. Gillund joined us in September 2013 as Vice President of Human Resources and Professional Development and was promoted to Chief Talent Officer in April 2016. Previously, Ms. Gillund was Vice President of Human Resources for C.H. Robinson Worldwide, Inc. from August 2002 to May 2012. Ms. Gillund serves as a member of the Board of Allina Health System and as member of the Board of Directors of College Possible.
Alexander Rosenstein, General Counsel and Corporate Secretary. Mr. Rosenstein joined us in September 2014 as Corporate Legal and Compliance Counsel, became Corporate Secretary in November 2014, and was promoted to General Counsel in
17
March 2015. From October 2005 to September 2014, Mr. Rosenstein was an attorney at Fredrikson & Byron, P.A., which provides legal services to us from time to time, and from September 1998 to September 2005, he was an attorney practicing in New York City.
Sandra M. Sedo, Chief Compliance Officer. Ms. Sedo joined us in June 2016 as Corporate Compliance Officer and was promoted to Chief Compliance Officer in July 2017. Prior to joining us, Ms. Sedo consulted for medical device companies in the legal and compliance areas. From 2005 to 2015, Ms. Sedo was employed by Medtronic, Inc. in various legal and compliance roles, and prior to that was a partner at Dorsey & Whitney LLP, which provides legal services to us from time to time.
Item 1A. Risk Factors.
Risks Relating to Our Business and Operations
We have a history of net losses and a short commercialization experience, and we may continue to incur losses.
We were profitable in fiscal 2018 but have incurred net losses in each prior fiscal year since our formation in 1989. For the years ended June 30, 2018, 2017, and 2016, we had net income (losses) of $1.7 million, $(1.8) million, and $(56.0) million, respectively. As of June 30, 2018, we had an accumulated deficit of approximately $327.6 million. We commenced commercial sales of the Peripheral OAS in September 2007 and the Coronary OAS in October 2013, and our short commercialization experience makes it difficult for us to predict future performance. We expect to continue to incur significant expenses for sales and marketing, research and development, and manufacturing as we expand our product offering, launch our business in international markets and continue to commercialize the Peripheral OAS and the Coronary OAS and develop and commercialize future versions of the Peripheral OAS, the Coronary OAS, and any future products. Additionally, we expect that our general and administrative expenses will increase to support business growth. If we are unable to balance revenue growth and cost management, our operating losses may continue.
We may be unable to sustain our historical revenue growth.
Other than a 4.9% decline in revenue from sales of our Peripheral OAS during fiscal 2016, our revenue from sales of our OAS devices has grown in each of the fiscal years since we began commercialization in September 2007. Our ability to increase our revenues in future periods will depend on our ability to increase sales of the OAS and other products we introduce, which will, in turn, depend in part on our success in growing our customer base and reorders from those customers. We may not be able to generate, sustain or increase revenues on a quarterly or annual basis. If we cannot achieve or sustain revenue growth for an extended period, our financial results will be adversely affected and our stock price may decline.
The Peripheral OAS, the Coronary OAS and other products may never achieve broad market acceptance.
The Peripheral OAS, Coronary OAS, and other products we develop or market now or in the future may never gain broad market acceptance among physicians, patients and the medical community. The degree of market acceptance of any of our products will depend on a number of factors, including:
• | the actual and perceived effectiveness and reliability of our products; |
• | the prevalence and severity of any adverse patient events involving our products; |
• | the results of any clinical trials relating to use of our products; |
• | the availability, relative cost and perceived advantages and disadvantages of alternative technologies or treatment methods for conditions treated by our products; |
• | the degree to which treatments using our products are approved for reimbursement by public and private insurers; |
• | the degree to which physicians adopt our products; |
• | the extent to which we are successful in educating physicians about PAD and CAD in general and the existence and benefits of our products in particular; |
• | the strength of our marketing and distribution infrastructure; |
• | the level of education and awareness among physicians and hospitals concerning our products; and |
• | our reputation among physicians and hospitals. |
Failure of our products to significantly penetrate current or new markets would negatively impact our business, financial condition and results of operations.
18
Our customers may not be able to achieve adequate reimbursement for using the Peripheral OAS and the Coronary OAS or other products, which could affect the acceptance of our products and cause our business to suffer.
The availability of insurance coverage and reimbursement for newly approved medical devices and procedures is uncertain. The commercial success of our products is substantially dependent on whether third-party insurance coverage and reimbursement for the use of such products and related services are available. We expect our products to continue to be purchased by hospitals and other providers who will then seek reimbursement from various public and private third-party payors, such as Medicare, Medicaid and private insurers, for the services provided to patients. While third-party payors are currently providing reimbursement for our products, we can give no assurance that these third-party payors will continue to provide adequate reimbursement for use of the Peripheral OAS and the Coronary OAS and our other products to permit hospitals and doctors to consider the products cost-effective for patients requiring treatment, or that current reimbursement levels for our products will continue. In addition, the overall amount of reimbursement available for PAD and CAD treatment could decrease in the future. Failure by hospitals and other users of our products to obtain sufficient reimbursement could cause our business to suffer.
Medicare, Medicaid, health maintenance organizations and other third-party payors are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement, and, as a result, they may not cover or provide adequate payment for use of our products. In order to position our products for acceptance by third-party payors, we may have to agree to lower prices than we might otherwise charge.
Governmental and private sector payors have instituted initiatives to limit the growth of healthcare costs using, for example, price regulation or controls and competitive pricing programs. Some third-party payors also require demonstrated superiority, on the basis of randomized clinical trials, or pre-approval of coverage, for new or innovative devices or procedures before they will reimburse healthcare providers who use such devices or procedures. It is uncertain whether our current products or any future products we may develop will be viewed as sufficiently cost-effective to warrant adequate coverage and reimbursement levels.
In addition, in June 2016, we entered into a Settlement Agreement with the U.S. government, acting through the U.S. Attorney for the Western District of North Carolina (the “DOJ”) and on behalf of the Office of Inspector General of the Department of Health and Human Services (the “OIG”), and Travis Thams, and a five-year Corporate Integrity Agreement with the OIG. In the event of a breach of the Settlement Agreement or the Corporate Integrity Agreement, we could be excluded from participation in federal health care programs. If third-party coverage and reimbursement for our products is limited or not available, the acceptance of our products and, consequently, our business will be substantially harmed.
Healthcare reform legislation could adversely affect our operating results and financial condition.
There have been and continue to be proposals by the federal government, state governments, regulators and third-party payors to control healthcare costs and, more generally, to reform the U.S. healthcare system, some of which have been enacted into law, such as the Patient Protection and Affordable Care Act, or the Patient Act. The Patient Act and any additional healthcare proposals and laws that may be enacted in the future could also limit the prices we are able to charge for our products or the amounts of reimbursement available for our products and could limit the acceptance and availability of our products. The Patient Act and future healthcare legislation could adversely affect our revenue and financial condition. The U.S. Congress has in the past considered legislation to repeal, modify or replace the Patient Act. We cannot predict the outcome of these efforts and, as a result, we cannot predict the effect that any such repeal, modification or replacement will have on our business and results of operations.
Our financial performance may be adversely affected by medical device tax provisions in the health care reform legislation.
The imposition of the 2.3% medical device excise tax enacted as part of the Patient Act has adversely affected our financial results and has required, and will continue to require, us to identify ways to reduce spending in other areas or raise additional capital to offset the increased expense. Although the excise tax has been suspended by Congress until the end of 2019, its status is unclear for subsequent years. We have not been able to pass along the cost of the tax to our customers or offset the cost of the tax through higher sales volumes resulting from the expansion of health insurance coverage and do not expect to be able to do so in the future. Ongoing implementation of this legislation could have a material adverse effect on our results of operations and cash flows.
19
We have limited data and experience regarding the safety and efficacy of the Peripheral OAS and Coronary OAS. Any long-term data that is generated may not be positive or consistent with our limited short-term data, which would affect market acceptance of these products.
Because our technology is relatively new in the treatment of PAD and CAD, we have performed clinical trials only with limited patient populations. The long-term effects of using the Peripheral OAS and the Coronary OAS in a large number of patients have not been studied and the results of short-term clinical use of the Peripheral OAS or the Coronary OAS do not necessarily predict long-term clinical benefits or reveal long-term adverse effects. We are conducting and developing several clinical trials, and there are substantial risks and uncertainties involved in these trials. We must devote substantial resources to our clinical trials, clinical trials often take several years to develop and conduct, there are difficulties involved in locating sites and patients to participate in our clinical trials, and the results of every trial are uncertain until the trial is completed. These uncertainties could adversely impact our financial results, our reputation and the reputation of our products.
Clinical trials conducted with the Peripheral OAS and the Coronary OAS have involved procedures performed by physicians who are very technically proficient. Consequently, both short and long-term results reported in these studies may be significantly more favorable than typical results achieved by physicians, which could negatively impact market acceptance of the Peripheral OAS and the Coronary OAS and materially harm our business.
We face significant competition, must innovate to stay competitive, and may be unable to sell the Peripheral OAS, the Coronary OAS or any other products at profitable levels.
The market for medical devices is highly competitive, dynamic and marked by rapid and substantial technological development and product innovation. Our ability to compete depends on our ability to innovate successfully, and, while certain barriers exist to entry into our market, we cannot assure that new entrants or existing competitors will not be able to develop products that compete directly with our products. We compete against very large and well-known stent and balloon angioplasty device manufacturers, atherectomy catheter manufacturers, pharmaceutical companies, and companies that provide products used by surgeons in peripheral and coronary bypass procedures. We may have difficulty competing effectively with these competitors because of their well-established positions in the marketplace, significant financial and human capital resources, established reputations and worldwide distribution channels.
Our competitors may:
• | develop and patent processes or products earlier than we will; |
• | obtain regulatory clearances or approvals for competing medical device products more rapidly than we will; |
• | market their products more effectively than we will; |
• | sell their products at lower prices than we do; or |
• | develop more effective or less expensive products or technologies that render our technology or products obsolete or non-competitive. |
We have encountered and expect to continue to encounter potential customers who, due to existing relationships with our competitors, are committed to or prefer the products offered by these competitors. In addition, increased consolidation in the healthcare industry has resulted in companies with greater market power, which increases competition for goods and services.
We experience significant competition on the pricing of our products and expect to continue to experience pressure from our customers to lower our prices. Our customers may require lower pricing in connection with contract renewals or otherwise for us to continue to sell our products to them. In addition, if our Purchasing Agreement with HealthTrust Purchasing Group, L.P. is terminated, our financial results will be materially adversely affected.
If we are unable to compete successfully, our revenue will suffer. Increased competition might lead to price reductions and other concessions that might adversely affect our operating results. Competitive pressures may decrease the demand for our products and could adversely affect our financial results.
Our efforts to develop new products may not be successful or the new products may not provide the revenue we expect.
We plan to add to our product portfolio through both internal development efforts and through acquisitions, distribution agreements, licensing transactions, manufacturing agreements and other strategic partnerships. For example, we recently entered into an agreement with Aerolase Corp. for the co-development of a new vascular laser device for physicians to use in more effectively treating multiple forms of arterial disease and we are developing a new temporary hemodynamic support
20
pump.
These new products and technologies may fail to reach the market or may only have limited commercial success because of efficacy or safety concerns, failure to achieve positive clinical outcomes, inability to obtain necessary regulatory approvals, limited scope of approved uses, excessive costs to manufacture, failure to establish or maintain intellectual property rights, or infringement of the intellectual property rights of others. Even if we successfully develop new products or enhancements or new generations of our existing products, they may be quickly rendered obsolete by changing customer preferences, changing industry standards, or competitors’ innovations. Innovations may not be accepted quickly in the marketplace because of, among other things, entrenched patterns of clinical practice or uncertainty over third-party reimbursement. We cannot provide certainty as to when or whether any of our products under development will be launched, whether we will be able to develop, license, or otherwise acquire compounds or products, or whether any products will be commercially successful. Failure to launch successful new products or technologies, or new indications or uses for existing products, may cause our products or technologies to become obsolete, causing our revenues and operating results to suffer.
We have limited commercial manufacturing experience and could experience difficulty in producing the Peripheral OAS and the Coronary OAS or may need to depend on third parties to manufacture the products.
We have limited experience in commercially manufacturing the Peripheral OAS, even less experience in commercially manufacturing the Coronary OAS and no experience manufacturing these products in the quantities that we anticipate will be required if we achieve planned levels of commercial sales. As a result, we may not be able to develop and implement efficient, low-cost manufacturing capabilities and processes that will enable us to manufacture the Peripheral OAS and the Coronary OAS or future products in significant volumes, while meeting the legal, regulatory, quality, price, durability, engineering, design and production standards required to market our products successfully.
The forecasts of demand we use to determine order quantities and lead times for components purchased from outside suppliers may be incorrect. Our failure to obtain required components or subassemblies when needed and at a reasonable cost would adversely affect our business.
In addition, we may in the future need to depend upon third parties to manufacture the Peripheral OAS and Coronary OAS and future products. Any difficulties in locating and hiring third-party manufacturers, or in the ability of third-party manufacturers to supply quantities of our products at the times and in the quantities we need, could have a material adverse effect on our business.
We depend upon third-party suppliers, including single source suppliers to us and our customers, making us vulnerable to supply problems and price fluctuations.
We rely on third-party suppliers to provide us with certain components of our products and to provide key components or supplies to our customers for use with our products. We rely on single source suppliers for certain components of the Peripheral OAS and the Coronary OAS, including the diamond-grit-coated crown and our ViperSlide Lubricant. In some cases, we do not have long-term supply agreements with, or guaranteed commitments from, our suppliers, including single source suppliers. We depend on our suppliers to provide us and our customers with materials in a timely manner that meet our and their quality, quantity and cost requirements. These suppliers may encounter problems during manufacturing for a variety of reasons, any of which could delay or impede their ability to meet our demand and our customers’ demands. These suppliers may cease producing the components we purchase from them or otherwise decide to cease doing business with us.
Any supply interruption from our suppliers or failure to obtain additional suppliers for any of the components used in our products would limit our ability to manufacture our products and could have a material adverse effect on our business, financial condition and results of operations.
We are dependent on our senior management team and highly skilled personnel, and our business could be harmed if we are unable to attract and retain personnel necessary for our success.
We are highly dependent on our senior management and other key personnel. Our success will depend on our ability to retain senior management and to attract and retain qualified personnel in the future, including sales and marketing professionals, scientists, clinical specialists, engineers and other highly skilled personnel and to integrate current and additional personnel in all departments. The loss of members of our senior management, sales and marketing professionals, scientists, clinical and regulatory specialists and engineers could prevent us from achieving our objectives of continuing to grow our company. We do not carry key person life insurance on any of our employees.
21
We have increased the size of our organization and may need to do so in the future, and we may experience difficulties managing growth. If we are unable to manage the anticipated growth of our business, our future revenue and operating results may be adversely affected.
We have significantly expanded the size of our organization over the past three years, particularly in the number of sales and marketing personnel, and may need to do so in the future. The growth we may experience in the future may provide challenges to our organization, requiring us to also rapidly expand other aspects of our business, including our manufacturing operations. Rapid expansion in personnel may result in less experienced people producing and selling our products, which could result in unanticipated costs and disruptions to our operations. If we cannot scale and manage our business appropriately, our anticipated growth may be impaired and our financial results will suffer.
We intend to sell our products internationally in the future, but we may experience difficulties in obtaining approval to do so or in successfully marketing our products internationally even if approved.
Currently, substantially all of our revenues are in the United States, and in fiscal 2018, commercial sales of certain of our products commenced in Japan, which became the first international market for our products. We intend to further expand our international sales in the future, both in Japan with Medikit and in the rest of the world under our Distribution Agreement with OrbusNeich. There can be no guarantee that we will receive approval to sell our products in any additional countries or that any of our approvals will be maintained, nor can there be any guarantee that any sales would result even if such approval is received. We will be substantially reliant upon Medikit and OrbusNeich for our international sales, and any failure of such distributors to effectively sell our products could have a material adverse effect on our international efforts and harm our financial position. In addition, we will incur substantial expenses in connection with international expansion. Our inability to successfully enter international markets and manage business on a global scale could negatively affect our financial results.
We may require additional financing, and our failure to obtain additional financing when needed could force us to delay, reduce or eliminate our product development programs or commercialization efforts.
We may be dependent on additional financing to execute our business plan. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. In the event we need or desire additional financing, we may be unable to obtain it by borrowing money in the credit markets or raising money in the capital markets. If adequate funds are not available on a timely basis, we may terminate or delay the development of one or more of our products, or delay establishment of sales and marketing capabilities or other activities necessary to commercialize our products.
We face a risk of non-compliance with the financial covenants in our loan and security agreement with Silicon Valley Bank.
We are party to a loan and security agreement with Silicon Valley Bank. This agreement requires us to maintain, among other things, either (i) minimum unrestricted cash at Silicon Valley Bank and unused availability on our line of credit of at least $10.0 million or (ii) minimum trailing three-month Adjusted EBITDA of $1.0 million and contains customary events of default, including, among others, the failure to comply with certain covenants or other agreements. Upon the occurrence and during the continuation of an event of default, amounts due under the agreements may be accelerated by Silicon Valley Bank. If we are unable to meet the financial or other covenants under the current loan and security agreement or negotiate future waivers or amendments of such covenants, events of default could occur under the agreement. Upon the occurrence and during the continuance of an event of default under the agreement, Silicon Valley Bank has available a range of remedies customary in these circumstances, including declaring all outstanding debt, together with accrued and unpaid interest thereon, to be due and payable, foreclosing on the assets securing the agreement and/or ceasing to provide additional loans under our line of credit, which could have a material adverse effect on us.
The restrictive covenants under this agreement could limit our ability to obtain future financing, withstand a future downturn in our business or the economy in general or otherwise conduct necessary corporate activities. The financial and restrictive covenants contained in this agreement could also adversely affect our ability to respond to changing economic and business conditions and place us at a competitive disadvantage relative to other companies that may be subject to fewer restrictions. Transactions that we may view as important opportunities, such as acquisitions, may be subject to the consent of Silicon Valley Bank, which consent may be withheld or granted subject to conditions specified at the time that may affect the attractiveness or viability of the transaction.
We lease our corporate headquarters, which subjects us to ongoing payment obligations and compliance with certain covenants.
22
On March 30, 2017, we completed the sale of our corporate headquarters. In connection with such sale, we entered into a lease agreement for our corporate headquarters, which has an initial term of fifteen years, with four consecutive renewal options of five years each. Under this lease, we are obligated to pay a base annual rent in the first year of $1,637,500 with annual escalations of 3%. If we are unable to make such rent payments or comply with the other covenants contained in the lease, the landlord could take certain actions against us, up to and including termination of the lease, which could have an adverse impact on our business, results of operations or financial conditions.
Our stock price is volatile and subject to significant fluctuations.
The market price of our common stock could be subject to significant fluctuations. Market prices for securities of early-stage pharmaceutical, medical device, biotechnology and other life sciences companies have historically been particularly volatile. Our common stock traded as low as $20.58 and as high as $34.51 per share during the 12-month period ended June 30, 2018. Factors that may cause the market price of our common stock to fluctuate include, but are not limited to:
• | announcements of technological or medical innovations for the treatment of vascular disease; |
• | quarterly variations in our or our competitors’ results of operations; |
• | failure to meet estimates or recommendations by securities analysts who cover our stock; |
• | failure to meet our own financial estimates; |
• | accusations that we have violated a law or regulation; |
• | recalls of our products; |
• | significant litigation; |
• | sales of large blocks of our common stock, including sales by our executive officers, directors and significant stockholders; |
• | changes in accounting principles; |
• | actual or anticipated changes in healthcare policy and reimbursement levels; |
• | developments relating to our competitors and markets; and |
• | general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors. |
Moreover, the stock markets in general have experienced substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations may also adversely affect the trading price of our common stock.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
Under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes, such as research tax credits, to offset its post-change income or taxes may be limited. In general, an “ownership change” will occur if there is a cumulative change in our ownership by “5-percent shareholders” that exceeds 50 percentage points over a rolling three-year period. Similar rules may apply under state tax laws. We may have experienced an ownership change in the past and we may also experience ownership changes in the future as a result of future transactions in our stock, some of which may be outside our control. As a result, if we earn net taxable income, our ability to use our pre-change net operating loss carryforwards or other pre-change tax attributes to offset U.S. federal and state taxable income or taxes may be subject to limitations.
An interruption in or breach of security of our information or manufacturing systems could cause a loss of business or damage to our reputation.
We rely on information and communication systems in our manufacturing and in the conduct our business. If there is any failure or interruption of these systems, such an incident could cause failures or disruptions in our customer relationship systems or product manufacturing. In addition, we could be subject to a cyber incident, such as an intentional attack or an unintentional event that involves a third party gaining unauthorized access to our systems, which could disrupt our operations, corrupt our data, or result in release of our confidential information. The occurrence of any failures, interruptions or cyber incidents could cause a loss of business or damage to our reputation and have a material effect on our business, financial condition, results of operations and cash flows.
23
The Tax Cuts and Jobs Act of 2017 may have a significant impact on our financial condition and results of operations.
The Tax Cuts and Jobs Act of 2017 (the “Tax Act”) was signed into law on December 22, 2017. The Tax Act made numerous changes to U.S. federal corporate tax law and is expected to reduce our effective tax rate for the year ended June 30, 2018 and future periods. Effective January 1, 2018, the Tax Act lowers the U.S. corporate tax rate from 35% to 21% and prompts various other changes to U.S. federal corporate tax law. We have assessed the impact the Tax Act with our professional advisors which resulted in the remeasurement of our deferred tax assets and valuation allowance. The impact the Tax Act will have on us in future periods is uncertain and may adversely affect our financial condition and results of operations.
The effects of hurricanes, flooding and other natural disasters may impact our sales, inventories and supply availability, which could adversely affect our financial condition and results of operations.
In August and September 2017, Hurricanes Harvey and Irma made landfall along the Texas Gulf Coast and in the State of Florida, respectively, bringing high winds, unprecedented rain and extreme flooding to those areas. A significant portion of our sales is generated from these areas. Procedure volumes in the Houston area and in Florida decreased during the pendency and immediate aftermath of the hurricanes and flooding, which decreased the number of our products used during this time. Any sustained decrease in procedure volumes from hurricanes and other natural disasters that affect any areas in which our customers are located will result in decreased sales in these areas and could have a material adverse effect on our financial condition and results of operations.
In addition, we maintain a 46,000-square foot production facility in Pearland, Texas, which is just outside of Houston in southeast Texas. The storm and its aftermath did not cause damage to our Pearland facility. However, any future loss of operations at the Pearland facility as a result of natural disasters eliminates an alternate production source in the event that our manufacturing capacity at the Minnesota facility is disrupted for any reason.
Any disruptions in our ability to timely manufacture and supply our products to our customers could cause us to experience delays in recognizing revenue or even to lose sales altogether, and any additional hurricanes, flooding or other natural disasters affecting areas in which our products are sold could result in decreased numbers of cases using our products. Any of these events could have a material adverse effect on our financial condition and results of operations.
Risks Related to Government Regulation
Our ability to market the Peripheral OAS in the United States is limited to use as a therapy in patients with PAD and our ability to market the Coronary OAS in the United States is limited to use as a therapy in patients with severely calcified CAD, and if we want to expand our marketing claims, we will need to file for additional FDA clearances or approvals and conduct further clinical trials, which would be expensive and time consuming and may not be successful.
We received FDA 510(k) clearances in the United States for use of the Peripheral OAS as a therapy in patients with PAD, and we received PMA to use the Coronary OAS as a therapy in patients with severely calcified CAD. These general clearances and approvals restrict our ability to market or advertise the Peripheral OAS and the Coronary OAS beyond these uses and could affect our growth.
If we determine to market our orbital technology in the United States for other uses, we would need to conduct further clinical trials and obtain premarket approval from the FDA. Clinical trials are complex, expensive, time consuming, uncertain and subject to substantial and unanticipated delays. There is no assurance that we will be able to obtain FDA approval to use our orbital atherectomy technology for applications other than the treatment of PAD and CAD.
We are or will be subject to an extensive set of post-market controls that apply to us as we commercialize our products, including annual PMA reports, Medical Device Reports on serious adverse events, complaint handling and analysis under the FDA’s QSR, export controls, advertising and promotion requirements, and potential post-market studies required by the FDA.
We and our suppliers are also subject to regulation by various state authorities, which may inspect our or our suppliers’ facilities and manufacturing processes and enforce state regulations. Failure to comply with applicable state regulations may result in seizures, injunctions or other types of enforcement actions.
Our promotion of the Peripheral OAS and the Coronary OAS is closely controlled by the FDA and enforcement activities could limit our ability to inform potential customers of the features of the products.
24
Our products may in the future be subject to product recalls that could harm our reputation and product liability claims that could exceed the limits of available insurance coverage.
The FDA and similar governmental authorities in other countries have the authority to require the recall of commercialized products in the event of material regulatory deficiencies or defects in design or manufacture. For example, since commercialization of the Peripheral OAS, we have had instances of recalls, including, in the year ended June 30, 2017 minor recalls involving guidewires and the OAS saline infusion pump recall discussed below. Any recalls of our products or products that we distribute would divert managerial and financial resources, harm our reputation with customers and have an adverse effect on our financial condition and results of operations.
Also, if any of our products is defectively designed, manufactured or labeled, contain defective components or are misused, we may become subject to costly litigation by our customers or their patients. The use, misuse or off-label use of our products may result in injuries that lead to product liability suits, which could be costly to our business. We cannot prevent a physician from using any of our products for off-label applications. While we have product liability insurance coverage for our products and intend to maintain such insurance coverage in the future, there can be no assurance that we will be adequately protected from claims that are brought against us.
The recall of our saline infusion pumps could adversely affect our business and financial results, harm our reputation and result in legal claims against us.
In April 2017, we initiated a voluntary recall of one type of our saline infusion pumps. While we have made design changes to this pump and have launched a next generation pump to address the issues that led to the recall, it is possible that we did not adequately assess the cause and effect of these issues and we may not have adequately modified the pump design in order to prevent these issues from happening in the future. Any additional problems with our pumps could cause delays in the ability of our customers to perform procedures using our devices and prevent us from adding new customers who may not have access to other pumps that can be used in procedures, which could harm our reputation with customers, adversely affect our ability to generate revenue, and have an adverse effect on our financial condition and results of operations. Any future pump recall would harm our reputation and divert managerial and sales force attention and financial resources from other aspects of our business and would require us to incur substantial expense.
We are subject to many laws and governmental regulations and any adverse regulatory action may materially adversely affect our financial condition and business operations.
Our products and related manufacturing processes, clinical data, adverse events, recalls and corrections and promotional activities are subject to extensive regulation by the FDA and other regulatory bodies. In particular, we are required to comply with the QSR and other regulations, which cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of any product for which we obtain marketing clearance or approval. We are also responsible for the quality of components received by our suppliers. Failure to comply with the QSR requirements or other statutes and regulations administered by the FDA and other regulatory bodies, or failure to adequately respond to any observations, could result in, among other things:
• | warning or other letters from the FDA; |
• | fines, injunctions and civil penalties; |
• | product recall or seizure; |
• | unanticipated expenditures; |
• | delays in clearing or approving or refusal to clear or approve products; |
• | withdrawal or suspension of approval or clearance by the FDA or other regulatory bodies; |
• | orders for physician notification or device repair, replacement or refund; |
• | operating restrictions, partial suspension or total shutdown of production or clinical trials; and |
• | criminal prosecution. |
If any of these actions were to occur, it would harm our reputation and cause our product sales to suffer.
Our operations are also subject to regulatory requirements relating to the environment, waste management and health and safety matters, including measures relating to the release, use, storage, treatment, transportation, discharge, disposal and remediation of hazardous substances. Environmental laws and regulations could become more stringent over time, imposing greater compliance costs and increasing risks and penalties associated with violations.
25
In addition, our relationships with physicians, hospitals and the marketers of our products are subject to scrutiny under various federal anti-kickback, self-referral, false claims and similar laws, often referred to collectively as healthcare fraud and abuse laws, as further described below.
If our operations are found to be in violation of these laws, we, as well as our employees, may be subject to penalties, including monetary fines, civil and criminal penalties, exclusion from federal and state healthcare programs, including Medicare, Medicaid, Veterans Administration health programs, workers’ compensation programs and TRICARE (the healthcare system administered by or on behalf of the U.S. Department of Defense for uniformed services beneficiaries, including active duty and their dependents, retirees and their dependents), and forfeiture of amounts collected in violation of such prohibitions, which could materially adversely affect our financial condition and business operations.
In addition, we have agreements with federal, state and local government agencies, such as the Veterans Administration, and third-party healthcare providers that receive government funding to sell our products. We are subject to extensive regulatory compliance obligations in the award, performance and administration of our government contracts, including regulations relating to procurement integrity, pricing protection, export control, government security, employment practices, accuracy of records and the recording of costs. The other parties to these agreements have the right to audit us to determine whether we are in compliance with these agreements. Failure to comply with these regulations and requirements could result in reductions of the value of contracts, contract modifications or termination, repayment of amounts, the assessment of penalties and fines, and/or suspension or debarment from government contracting or subcontracting in the future, any of which could negatively affect our financial condition and results of operations.
We are subject to federal and state laws prohibiting “kickbacks” and false and fraudulent claims which, if violated, could subject us to substantial penalties. Additionally, any challenges to or investigations into our practices under these laws could cause adverse publicity and be costly to respond to, and thus could harm our business.
The federal healthcare program Anti-Kickback Statute, and similar state laws, prohibit payments that are intended to induce health care professionals or others either to refer patients or to purchase, lease, order or arrange for or recommend the purchase, lease or order of healthcare products or services. A number of states have enacted laws that require pharmaceutical and medical device companies to monitor and report payments, gifts and other remuneration made to physicians and other health care professionals and health care organizations. In addition, some state statutes, most notably laws in Massachusetts and Vermont, impose outright bans on certain gifts to physicians as well as requiring reporting of payments to physicians. Some of these laws, referred to as “aggregate spend” or “gift” laws, carry substantial fines if they are violated. The federal Physician Payments Sunshine Act, or the Sunshine Act, requires us to collect and report certain data on payments and other transfers of value to physicians and teaching hospitals.
It is widely anticipated that public reporting under the Sunshine Act and implementing Open Payments regulations will result in increased scrutiny of the financial relationships between industry, physicians and teaching hospitals. These anti-kickback, public reporting and aggregate spend laws affect our sales, marketing, promotional and clinical activities by limiting the kinds of financial arrangements, including sales programs, we may have with hospitals, physicians or other potential purchasers or users of medical devices. They also impose additional administrative and compliance burdens on us. In particular, these laws influence, among other things, how we structure our sales offerings, including discount practices, customer support, education and training programs, physician consulting and other service arrangements, and clinical trials. If we were to offer or pay inappropriate inducements to purchase our products, we could be subject to a claim under the federal healthcare program Anti-Kickback Statute or similar state laws. If we fail to comply with particular reporting requirements, we could be subject to penalties under applicable federal or state laws. Other federal and state laws generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payments to Medicare, Medicaid or other third-party payors that are false or fraudulent, or for items or services that were not provided as claimed. Although we do not submit claims directly to government healthcare programs or other payors, manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by providing inaccurate billing or coding information to customers, by providing improper financial inducements, or through certain other activities.
26
In providing billing and coding information to customers, we make every effort to ensure that the billing and coding information furnished is accurate and that treating physicians understand that they are responsible for all treatment decisions. Nevertheless, we cannot provide assurance that the government will regard any billing errors that may be made as inadvertent or that the government will not examine our role in providing information to our customers and physicians concerning the benefits of therapy with our devices. Likewise, our financial relationships with customers, physicians, or others in a position to influence the purchase or use of our products may be subject to government scrutiny or be alleged or found to violate applicable fraud and abuse laws. False claims laws prescribe civil, criminal and administrative penalties for noncompliance, which can be substantial. Moreover, an unsuccessful challenge or investigation into our practices could cause adverse publicity, and be costly to respond to, and thus could harm our business and results of operations.
On May 8, 2014, we received a letter from the DOJ stating that it was investigating us to determine whether we had violated the False Claims Act, and on June 28, 2016, we entered into a Settlement Agreement with the United States of America, acting through the DOJ and on behalf of the OIG, and Travis Thams, who filed the qui tam complaint underlying the DOJ’s investigation (the “Civil Action”), to resolve the investigation by the DOJ and the Civil Action. The existence of the investigation and subsequent settlement could negatively affect our reputation and harm our business and results of operations. In addition, the release we received from the government in the Settlement Agreement related to particular conduct alleged in the complaint underlying the investigation. If the government determines that other conduct alleged in the complaint for which the government did not grant us a release merits additional investigation or if the government pursues any action against us relating to this other alleged conduct, then we may need to expend additional amounts to defend ourselves, our management would undergo the distraction of additional investigation and potential litigation, our reputation could be harmed, and our business and results of operations could be materially adversely affected.
Compliance with the terms and conditions of our Corporate Integrity Agreement requires significant resources and management time and, if we fail to comply, we could be subject to penalties or, under certain circumstances, excluded from government healthcare programs, which would materially adversely affect our business.
On June 28, 2016, we entered into a five-year Corporate Integrity Agreement with the OIG. The Corporate Integrity Agreement requires that we maintain our existing compliance programs and imposes certain expanded compliance-related requirements during the term of the Corporate Integrity Agreement, including establishment of specific procedures and requirements regarding consulting activities, co-marketing activities and other interactions with healthcare professionals and healthcare institutions and the sale and marketing of our products; ongoing monitoring, reporting, certification and training obligations; and the engagement of an independent review organization to perform certain auditing and reviews and prepare certain reports regarding our compliance with federal health care programs. Maintaining the broad array of processes, policies and procedures necessary to comply with the Corporate Integrity Agreement will require a significant portion of management’s attention and the application of significant resources. The costs associated with implementation of and compliance with the Corporate Integrity Agreement could be substantial and may be greater than we currently anticipate. In addition, while we have developed and instituted a corporate compliance program, we cannot guarantee that we, our employees, our consultants or our contractors are or will be in compliance with all potentially applicable U.S. federal and state regulations and/or laws, all potentially applicable foreign regulations and/or laws and/or all requirements of the Corporate Integrity Agreement. In the event of a breach of the Corporate Integrity Agreement, we could become liable for payment of certain stipulated penalties or could be excluded from participation in federal health care programs. The costs associated with compliance with the Corporate Integrity Agreement, or any liability or consequences associated with its breach, could have an adverse effect on our business, revenues, earnings and cash flows.
Regulations related to “conflict minerals” may force us to incur additional expenses, may result in damage to our business reputation and may adversely impact our ability to conduct our business.
Pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act, the SEC promulgated final rules regarding disclosure of the use of certain minerals, known as conflict minerals, that are mined from the Democratic Republic of the Congo and adjoining countries, as well as procedures regarding a manufacturer’s efforts to prevent the sourcing of such minerals and metals produced from those minerals. These disclosure requirements require ongoing due diligence efforts and disclosure obligations. There are costs associated with complying with these disclosure requirements, including for diligence in regards to the sources of any conflict minerals used in our products, in addition to the cost of remediation and other changes to products, processes, or sources of supply as a consequence of such verification activities. In addition, our ongoing implementation of these rules could adversely affect the sourcing, supply, and pricing of materials used in our products.
27
Our anticipated international expansion will subject us to increased legal and regulatory requirements, which could have a material effect on our business.
In February 2018, Medikit, our exclusive distributor in Japan, commenced sales of our Coronary OAS Micro Crown, and, in July 2018, we entered into an exclusive Distribution Agreement with OrbusNeich to sell our Peripheral and Coronary OAS outside of the United States and Japan. We expect sales by OrbusNeich to commence in Southeast Asia, Europe and the Middle East in the year ending June 30, 2019. Movement into international markets will subject us and our products to different and increased laws and regulations, including foreign medical device regulations; tax laws; increased financial accounting and reporting burdens and complexities; export laws; and the Foreign Corrupt Practices Act and similar anti-corruption laws. Although we have and will continue to implement policies and procedures designed to ensure compliance with these laws, there can be no assurance that all of our employees, contractors, distributors and agents, as well as those companies to which we will outsource certain aspects of our business operations, including those based in foreign countries where practices that violate such U.S. laws may be customary, will comply with our internal policies. Medikit and OrbusNeich may appoint sub-distributors of our products and we will have limited ability to control the actions of these sub-distributors, but we may be held responsible by governmental authorities for the actions of these sub-distributors. We will incur additional compliance costs associated with global operations, and any alleged or actual violations of these laws and regulations could subject us to government scrutiny, severe criminal or civil fines, sanctions and other liabilities, and prohibitions on business conduct, and could negatively affect our business, reputation, operating results, and financial condition.
New regulatory requirements will impose additional burdens on us, and our business could be adversely affected if we are unable to satisfy all applicable new requirements in a timely fashion.
New regulations impacting our products are periodically adopted. These regulations may require us to change our existing product designs in order to continue marketing our products, which could result in increased expenditures and in risks that we may be unable to successfully change our designs to satisfy the new requirements. For example, IEC 60601-1-2 (4th Edition) was published in July 2014 and updates the performance requirements with respect to electromagnetic interference for medical devices. In the United States, the 4th Edition requirements go into effect on December 31, 2018 for new devices and devices that have undergone substantial changes. We have taken steps to ensure that our products sold in the United States will be compliant with the 4th Edition requirements, but we could experience technical and regulatory delays. If our products do not meet the 4th Edition standards, we may be delayed in launching new products or selling existing products that require material changes, including, for example, as a result of a change of supplier or quality issues. Any delays in selling our products resulting from non-compliance with 4th Edition and other new regulatory requirements could have a material adverse effect on our business.
The impact of restrictive trade policies in the United States and the potential corresponding actions by other countries could adversely affect our financial performance.
The U.S. federal government has recently implemented tariffs on certain products imported into the United States from China, and the Chinese government has responded with retaliatory tariffs on certain products, including medical devices, exported from the United States to China. We cannot predict whether the United States will implement additional trade restrictions with respect to China or other countries and how such countries would respond to such trade restrictions. If these tariffs continue or are expanded, they would make it more difficult to sell our products in China or other markets outside of the United States, if we seek to expand into the Chinese or other markets in the future, and they may increase the costs of procuring component parts for our products from China or other countries. Restrictive trade policies may also harm the United States and global economies generally, which would adversely affect our business in a variety of ways, including reducing the market for our products, causing a downturn in the trading price of our common stock, and restricting access to credit if we seek it for future growth.
Risks Relating to Our Intellectual Property
Our inability to adequately protect our intellectual property could allow our competitors and others to produce products based on our technology, which could substantially impair our ability to compete.
Our success and ability to compete depends, in part, upon our ability to maintain the proprietary nature of our technologies. We rely on a combination of patents, copyrights and trademarks, as well as trade secrets and nondisclosure agreements, to protect our intellectual property. Our issued patents and related intellectual property may not be adequate to protect us or permit us to gain or maintain a competitive advantage. Also, we cannot be assured that any of our pending patent applications will result in the issuance of patents to us. Further, if any patents we obtain or license are deemed invalid and unenforceable, or have their scope narrowed, it could impact our ability to commercialize or license our technology and achieve competitive advantages.
28
Changes in either the patent laws or in interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property. In addition, the laws of some foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States, if at all.
We may, in the future, need to assert claims of infringement against third parties to protect our intellectual property. The outcome of litigation to enforce our intellectual property rights in patents, copyrights, trade secrets or trademarks is highly unpredictable, could result in substantial costs and diversion of resources, and could have a material adverse effect on our financial condition, reputation and results of operations regardless of the final outcome of such litigation.
Despite our efforts to safeguard our unpatented and unregistered intellectual property rights, we may not be successful in doing so or the steps taken by us in this regard may not be adequate to detect or deter misappropriation of our technology or to prevent an unauthorized third party from copying or otherwise obtaining and using our products, technology or other information that we regard as proprietary. In addition, we may not have sufficient resources to litigate, enforce or defend our intellectual property rights. Additionally, third parties may be able to design around our patents.
We also rely on trade secrets, technical know-how and continuing innovation to develop and maintain our competitive position. In this regard, we seek to protect our proprietary information and other intellectual property by having a policy that our employees, consultants, contractors, outside scientific collaborators and other advisors execute non-disclosure and assignment of invention agreements on commencement of their employment or engagement. We cannot provide any assurance that employees and third parties will abide by the confidentiality or assignment terms of these agreements, or that we will be effective in securing necessary assignments from these third parties.
Claims of infringement or misappropriation of the intellectual property rights of others could prohibit us from commercializing products, require us to obtain licenses from third parties or require us to develop non-infringing alternatives, and subject us to substantial monetary damages and injunctive relief.
The medical technology industry is characterized by extensive litigation and administrative proceedings over patent and other intellectual property rights. The likelihood that patent infringement or misappropriation claims may be brought against us increases as we achieve more visibility in the marketplace and introduce products to market. We are aware of numerous patents issued to third parties that relate to the manufacture and use of medical devices for the treatment of vascular disease. The owners of each of these patents could assert that the manufacture, use or sale of our products infringes one or more claims of their patents. There could also be existing patents of which we are unaware that one or more aspects of our technology may inadvertently infringe. In some cases, litigation may be threatened or brought by a patent-holding company or other adverse patent owner who has no relevant product revenues and against whom our patents may provide little or no deterrence.
Any infringement or misappropriation claim could cause us to incur significant costs, place significant strain on our financial resources, divert management’s attention from our business and harm our reputation. If the relevant patents were upheld in litigation as valid and enforceable and we were found to infringe, we could be prohibited from commercializing any infringing products unless we could obtain licenses to use the technology covered by the patent or are able to design around the patent. We may be unable to obtain a license on terms acceptable to us, if at all, and we may not be able to redesign any infringing products to avoid infringement.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
Our principal executive offices are located in our headquarters, a 125,000 square foot facility in St. Paul, Minnesota, which contains dedicated research and development, training and education, and manufacturing facilities, and our central administrative offices. In March 2017, we sold the Minnesota facility and entered into an agreement to lease the facility through March 2032.
In September 2009, we entered into an agreement to lease a 46,000 square foot production facility in Pearland, Texas beginning in April 2010 and continuing through April 2020. This facility primarily accommodates additional manufacturing activities.
We believe that our current facilities are substantially adequate for our current and anticipated future needs for the foreseeable future.
Item 3. Legal Proceedings.
None.
Item 4. Mine Safety Disclosures.
None.
29
PART II
Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Price Range of Common Stock and Dividend Policy
We trade on the Nasdaq Global Market under the symbol “CSII.” The following table sets forth the high and low sales prices for our common stock (based upon intra-day trading) as reported by the Nasdaq Global Market:
Common Stock | |||||||
High | Low | ||||||
Fiscal Year Ended June 30, 2018 | |||||||
First quarter | $ | 33.11 | $ | 26.62 | |||
Second quarter | 29.47 | 23.00 | |||||
Third quarter | 27.80 | 20.58 | |||||
Fourth quarter | 34.51 | 20.89 | |||||
Fiscal Year Ended June 30, 2017 | |||||||
First quarter | $ | 25.22 | $ | 18.00 | |||
Second quarter | 27.38 | 21.29 | |||||
Third quarter | 29.70 | 23.28 | |||||
Fourth quarter | 33.11 | 27.73 | |||||
The number of record holders of our common stock on August 17, 2018 was approximately 139. No cash dividends have been previously paid on our common stock and none are anticipated during the year ending June 30, 2019.
Recent Sales of Unregistered Securities
None.
Issuer Purchases of Equity Securities
None.
Securities Authorized For Issuance Under Equity Compensation Plans
For information on our equity compensation plans, refer to Item 12, “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
30
Performance Graph
The following graph compares the cumulative total stockholder return of our common stock (“CSII”) with the return of the Standard & Poor’s 500 Stock Index (“S&P”) and the S&P Health Care Index (“S&P HC”) from June 30, 2013, through June 30, 2018. The comparisons assume $100 was invested on June 30, 2013 in our common stock, the S&P 500 Stock Index and the S&P Health Care Index and also assumes that any dividends are reinvested. The returns set forth on the following graph are based on historical results and are not intended to suggest future performance.
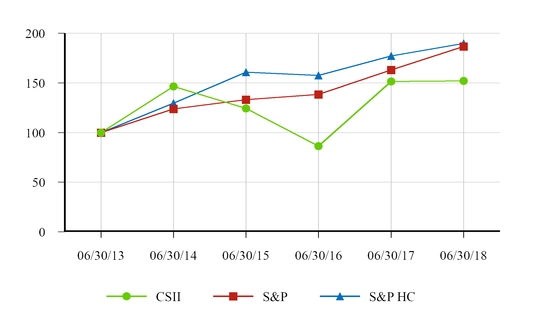
Item 6. Selected Financial Data.
Five-Year Selected Financial Data
(in thousands, except per share amounts)
2018 | 2017 | 2016 | 2015 | 2014 | |||||||||||||||
SUMMARY OF OPERATIONS FOR THE FISCAL YEAR: | |||||||||||||||||||
Net revenues | $ | 217,043 | $ | 204,906 | $ | 178,184 | $ | 181,544 | $ | 136,612 | |||||||||
Income (loss) from operations | $ | 2,234 | $ | (1,542 | ) | $ | (56,077 | ) | $ | (32,637 | ) | $ | (33,489 | ) | |||||
Net income (loss) | $ | 1,712 | $ | (1,792 | ) | $ | (56,024 | ) | $ | (32,822 | ) | $ | (35,290 | ) | |||||
Basic and diluted earnings per share | $ | 0.05 | $ | (0.06 | ) | $ | (1.72 | ) | $ | (1.04 | ) | $ | (1.25 | ) | |||||
Cash dividends declared per share | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||||
FINANCIAL POSITION AT YEAR END: | |||||||||||||||||||
Total assets | $ | 203,352 | $ | 193,940 | $ | 142,406 | $ | 171,328 | $ | 181,901 | |||||||||
Total long-term liabilities | $ | 31,422 | $ | 34,579 | $ | 6,010 | $ | 2,005 | $ | 117 | |||||||||
Stockholders’ equity | $ | 134,470 | $ | 118,389 | $ | 100,897 | $ | 139,435 | $ | 152,055 | |||||||||
31
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of financial condition and results of operations together with our consolidated financial statements and the related notes included elsewhere in this Form 10-K. This discussion and analysis contains forward-looking statements about our business and operations, based on current expectations and related to future events and our future financial performance, that involve risks and uncertainties. Our actual results may differ materially from those we currently anticipate as a result of many important factors, including the factors we describe under “Risk Factors” and elsewhere in this Form 10-K.
OVERVIEW
We are a medical technology company leading the way in the effort to successfully treat patients suffering from peripheral and coronary artery diseases, including those with arterial calcium, the most difficult arterial disease to treat. We are committed to clinical rigor, constant innovation and a defining drive to set the industry standard to deliver safe and effective medical devices that improve lives of patients facing these difficult disease states.
Peripheral
Our Peripheral OAS products are catheter-based platforms capable of treating a broad range of plaque types in leg arteries both above and below the knee, including calcified plaque, and address many of the limitations associated with other existing surgical, catheter and pharmacological treatment alternatives. The micro-invasive devices use small access sheaths that can provide procedural benefits and allow physicians to treat PAD patients in even the small and tortuous vessels located below the knee and facilitate access through alternative sites in the ankle, foot and wrist, as well as in the groin.
The United States Food and Drug Administration (“FDA”) has granted us 510(k) clearances for our Peripheral OAS as a therapy in patients with PAD, as discussed in Item 1 of Part I of this Form 10-K.
Coronary
Our coronary arterial disease (“CAD”) product, the Diamondback 360 Coronary OAS (“Coronary OAS”), is marketed as a treatment for severely calcified coronary arteries. The Coronary OAS is a catheter-based platform designed to facilitate stent delivery in patients with CAD who are acceptable candidates for percutaneous transluminal coronary angioplasty or stenting due to de novo, severely calcified coronary artery lesions. The Coronary OAS design is similar to technology used in our Peripheral OAS, customized specifically for the coronary application.
In January 2018, we announced our relationship with OrbusNeich® to be the exclusive U.S. distributor of OrbusNeich balloon products. In March 2018, the FDA granted 510(k) clearance for the OrbusNeich 1.0mm Sapphire® 11 Pro coronary balloon (“1.0mm balloon”). The 1.0mm balloon, the first and only 1.0mm coronary balloon available in the U.S., offers industry-leading entry and crossing profiles and is precision engineered for crossing and treating extremely tight and complex lesions. We anticipate OrbusNeich’s full balloon product portfolio will become available in the United States in fiscal 2019 and fiscal 2020.
International
In November 2016, we signed an exclusive distribution agreement with Medikit Co., Ltd. (“Medikit”) to sell our Coronary and Peripheral OAS in Japan. In March 2017, we received approval from Japan’s Ministry of Health, Labor and Welfare for our Coronary OAS Micro Crown. On February 1, 2018, the Coronary OAS Micro Crown received reimbursement approval in Japan, followed by the first commercial sales, making Japan the first international market for any of our products. The Coronary OAS Micro Crown is the only atherectomy device designed to both pilot tight, calcific lesions and treat 2.5 to 4mm vessels with a single device.
In October 2014, we received CE Mark for our Stealth 360 Peripheral OAS device. In July 2018, we entered into an exclusive Distribution Agreement with OrbusNeich to sell our Peripheral and Coronary OAS outside of the United States and Japan. We expect that OrbusNeich will commence sales of our products in certain countries in Southeast Asia, Europe and the Middle East during our fiscal year ending June 30, 2019.
FINANCIAL OVERVIEW
32
Net Revenues. We derive substantially all of our revenues from the sale of Peripheral OAS, the Coronary OAS and ancillary products in the United States. The Peripheral OAS and Coronary OAS each use a disposable, single-use, low-profile catheter that travels over our proprietary ViperWire guide wire. The systems use a saline infusion pump as a power supply for the operation of the catheter. Additional ancillary products include the ViperSlide Lubricant, ViperTrack Radiopaque Tape, OrbusNeich balloons, and ZILIENT guidewires.
We have observed some degree of seasonality in our business, as there tends to be a lower number of procedures that use our products during the three months ending September 30. Interventional procedure volume usually grows throughout the course of the fiscal year, with the quarter ending June 30 usually representing the highest volume of cases and, therefore, the highest amount of revenue generated by us during the course of the fiscal year.
Cost of Goods Sold. We assemble the single-use catheter with components purchased from third-party suppliers, as well as with components manufactured in-house. Balloons and guide wires are purchased from third-party suppliers. Our cost of goods sold consists primarily of raw materials, direct labor, manufacturing overhead, and purchased finished goods.
Selling, General and Administrative Expenses. Selling, general and administrative expenses include compensation for executive, sales, marketing, finance, information technology, human resources and administrative personnel, including stock-based compensation and facilities overhead. Other significant expenses include bad debt expense, travel, marketing costs, professional fees and professional education.
Research and Development Expenses. Research and development expenses include costs associated with the design, development, testing, enhancement and regulatory approval of our products. Research and development expenses include employee compensation including stock-based compensation, supplies and materials, patent expenses, consulting expenses, travel and facilities overhead. We also incur significant expenses to operate clinical trials, including trial design, third-party fees, clinical site reimbursement, data management and travel expenses. All research and development expenses are expensed as incurred. Approved patent applications are capitalized and amortized using the straight-line method over their remaining estimated lives. Patent amortization begins at the time of patent application approval, and does not exceed 20 years.
Other (Income) and Expense, Net. Other (income) and expense, net primarily includes interest expense from amounts owed under the lease of our headquarters facility and DOJ settlement, and interest income from money market funds.
Net Operating Loss Carryforwards. We have established valuation allowances to fully offset our deferred tax assets due to the uncertainty about our ability to generate the future taxable income necessary to realize these deferred assets, particularly in light of our historical losses. The future use of net operating loss carryforwards is dependent on us attaining profitable operations and will be limited in any one year under Internal Revenue Code Section 382 due to significant ownership changes (as defined in Section 382) resulting from our equity financings. At June 30, 2018, we had net operating loss carryforwards for federal and state income tax reporting purposes of approximately $275.0 million, which will expire at various dates through fiscal 2037.
CRITICAL ACCOUNTING POLICIES AND SIGNIFICANT JUDGMENTS AND ESTIMATES
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of our consolidated financial statements requires us to make estimates, assumptions and judgments that affect amounts reported in those statements. Our estimates, assumptions and judgments, including those related to revenue recognition, deferred revenue and stock-based compensation, are updated as appropriate at least quarterly. We use authoritative pronouncements, our technical accounting knowledge, cumulative business experience, valuation specialists, judgment and other factors in the selection and application of our accounting policies. While we believe that the estimates, assumptions and judgments that we use in preparing our consolidated financial statements are appropriate, these estimates, assumptions and judgments are subject to factors and uncertainties regarding their outcome. Therefore, actual results may materially differ from these estimates.
Some of our significant accounting policies require us to make subjective or complex judgments or estimates. An accounting estimate is considered to be critical if it meets both of the following criteria: (1) the estimate requires assumptions about matters that are highly uncertain at the time the accounting estimate is made, and (2) different estimates that reasonably could have been used, or changes in the estimate that are reasonably likely to occur from period to period, would have a material impact on the presentation of our financial condition, results of operations, or cash flows.
Revenue Recognition. We sell the majority of our products via direct shipment to hospitals or clinics, and in fiscal 2018 we began shipping to our distributor in Japan. We recognize revenue when all of the following criteria are met: persuasive evidence of an arrangement exists; delivery has occurred; the sales price is fixed or determinable; and collectability is reasonably assured. Revenue recognition may occur upon shipment or upon delivery to the customer site, based on the contract terms. We record estimated sales returns, discounts and rebates as a reduction of net sales.
Deferred revenue associated with the upfront payment received under our distribution agreement with Medikit is recognized in relation to the estimated future sales under the agreement. The short term portion represents the expected amount of deferred revenue that will be recognized over the next year. The estimate of future sales under contract will continue to be assessed and adjusted accordingly.
33
Costs related to products delivered are recognized in the period the revenue is recognized. Cost of goods sold consists primarily of raw materials, direct labor, and manufacturing overhead.
Stock-Based Compensation. We have stock-based compensation plans that include nonvested share awards and an employee stock purchase plan. We determine the fair value of nonvested share awards with market conditions using the Monte Carlo simulation. Fair value of nonvested share awards that vest based upon performance or time conditions is determined by the closing market price of our stock on the date of grant. Stock-based compensation expense is recognized ratably over the requisite service period for the awards expected to vest. Fair value of shares purchased under the employee stock purchase plan are estimated on the grant date, which is the first date in the six-month purchase period. Stock-compensation expense is recognized over the purchase period based on the anticipated amount of shares to be purchased. Management’s key assumptions are developed with input from independent third-party valuation advisors. During the years ended June 30, 2018, 2017 and 2016, we recorded stock-based compensation expense of $10.3 million, $10.4 million, and $13.0 million, respectively.
Legal Proceedings. In accordance with FASB guidance, we record a liability in our consolidated financial statements related to legal proceedings when a loss is known or considered probable and the amount can be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible, but not known or probable, and can be reasonably estimated, the estimated loss or range of loss is disclosed in the notes to the consolidated financial statements. In most cases, significant judgment is required to estimate the amount and timing of a loss to be recorded.
RESULTS OF OPERATIONS
The following table sets forth, for the periods indicated, our results of operations expressed as dollar amounts (in thousands), and, for certain line items, the changes between the specified periods:
Comparison of Fiscal Year Ended June 30, 2018 with Fiscal Year Ended June 30, 2017
Year Ended June 30, | |||||||||||||
2018 | 2017 | Change | Percent Change | ||||||||||
Net revenues | $ | 217,043 | $ | 204,906 | $ | 12,137 | 5.9 | % | |||||
Cost of goods sold | 39,484 | 39,441 | 43 | 0.1 | |||||||||
Gross profit | 177,559 | 165,465 | 12,094 | 7.3 | |||||||||
Gross margin | 81.8 | % | 80.8 | % | 1.0 | % | 1.2 | ||||||
Expenses: | |||||||||||||
Selling, general and administrative | 148,569 | 144,096 | 4,473 | 3.1 | |||||||||
Research and development | 26,756 | 22,911 | 3,845 | 16.8 | |||||||||
Total expenses | 175,325 | 167,007 | 8,318 | 5.0 | |||||||||
Income (loss) from operations | 2,234 | (1,542 | ) | 3,776 | (244.9 | ) | |||||||
Other (income) and expense, net | 390 | 164 | 226 | 137.8 | |||||||||
Income (loss) before income taxes | 1,844 | (1,706 | ) | 3,550 | (208.1 | ) | |||||||
Provision for income taxes | 132 | 86 | 46 | 53.5 | |||||||||
Net income (loss) | $ | 1,712 | $ | (1,792 | ) | $ | 3,504 | (195.5 | ) | ||||
Net Revenues. Net revenues increased by $12.1 million, or 5.9%, from $204.9 million for the year ended June 30, 2017, to $217.0 million for the year ended June 30, 2018. Revenues from our Peripheral OAS increased $6.9 million, or 4.9%, due to an increase in the number of devices sold, partially offset by decreases to average selling price, the adverse effect of Hurricanes Harvey and Irma, and a recall of a version of our saline infusion pump during fiscal 2018. Sales of our Coronary OAS increased by approximately $2.2 million, or 4.7%, reflecting an increase in devices sold. The increase in Peripheral and Coronary OAS sales is primarily due to the expansion of our customer base. We also had $1.8 million of revenue from sales in Japan as commercialization commenced in February 2018, which includes $383,000 related to the deferred up-front payment from Medikit. Other product revenue increased $2.1 million, or 7.4%, during the year ended June 30, 2018, driven by increased sales of our Peripheral and Coronary OAS, which the other products support.
34
Prior to February 2018, all of our revenues have been in the United States; however, sales in Japan commenced in February 2018. In November 2016, we signed an exclusive distribution agreement with Medikit to sell our Peripheral and Coronary OAS in Japan, and in March 2017, we received approval from Japan’s Ministry of Health, Labor and Welfare for our Coronary OAS Micro Crown. On February 1, 2018, the Coronary OAS Micro Crown received reimbursement approval in Japan, followed by the first commercial sales, making Japan the first international market for any of our products. In July 2018, we entered into an exclusive Distribution Agreement with OrbusNeich to sell our Peripheral and Coronary OAS outside of the United States and Japan. We expect that sales under this agreement will commence in fiscal 2019. We expect our revenue to increase as we continue to increase the number of physicians using the devices, increase the usage per physician, introduce new and improved products such as the OrbusNeich balloons and ZILIENT guidewires, generate additional clinical data, and expand into new geographies, partially offset by potential decreases in average selling prices.
Cost of Goods Sold. Cost of goods sold increased 0.1%, from $39.4 million for the year ended June 30, 2017 to $39.5 million for the year ended June 30, 2018. These amounts represent the cost of materials, labor and overhead for single-use catheters, guide wires, pumps, and other ancillary products. The small increase in cost of goods sold was due to greater unit volumes offset by the $1.5 million one-time charge in the year ended June 30, 2017 related to the voluntary recall of one type of our saline infusion pumps, as well as lower costs per unit driven by manufacturing efficiencies and cost reductions in the current year ended June 30, 2018. Gross margin increased to 81.8% for the year ended June 30, 2018 from 80.8% for the year ended June 30, 2017 due to lower costs per unit, as discussed above. Cost of goods sold for the years ended June 30, 2018 and 2017 includes $275,000 and $689,000, respectively, for stock-based compensation. We expect that gross margin for the year ending June 30, 2019 will decrease slightly compared to the year ended June 30, 2018, as an increasing amount of revenue will be derived from lower margin products and international markets. Quarterly margin fluctuations could occur based on production volumes, timing of new product introductions, sales mix, pricing changes, or other unanticipated circumstances.
Selling, General and Administrative Expenses. Selling, general, and administrative expenses increased by $4.5 million, or 3.1%, from $144.1 million for the year ended June 30, 2017 to $148.6 million for the year ended June 30, 2018. The increase was primarily due to increased payroll related expenses due to the expansion of clinical specialists in our sales organization, severance benefits, as well as litigation and other legal expenses. These amounts were partially offset by lower incentive compensation expense. Selling, general, and administrative expenses for the years ended June 30, 2018 and 2017 include $9.1 million and $8.7 million, respectively, for stock-based compensation. We expect our selling, general and administrative expenses to increase as revenue grows in fiscal 2019, but at a rate slightly less than the rate of revenue growth.
Research and Development Expenses. Research and development expenses increased by $3.8 million, or 16.8%, from $22.9 million for the year ended June 30, 2017 to $26.8 million for the year ended June 30, 2018. Research and development expenses relate to the specific projects to develop new products or expand into new markets, such as the development of new versions of our Peripheral OAS and Coronary OAS, and ancillary products, and PAD and CAD clinical studies. The increase was primarily due to the ramp-up of our ECLIPSE clinical study and new development projects. Research and development expenses for the years ended June 30, 2018 and 2017 include $1.0 million for stock-based compensation. We generally expect to incur higher research and development expenses in fiscal 2019 than amounts incurred for the year ended June 30, 2018 as we continue enrollment in the ECLIPSE clinical study and invest in expanding our product portfolio. Fluctuations could occur based on the number of projects and studies and the timing of expenditures.
35
Comparison of Fiscal Year Ended June 30, 2017 with Fiscal Year Ended June 30, 2016
Year Ended June 30, | |||||||||||||
2017 | 2016 | Change | Percent Change | ||||||||||
Net revenues | $ | 204,906 | $ | 178,184 | $ | 26,722 | 15.0 | % | |||||
Cost of goods sold | 39,441 | 35,421 | 4,020 | 11.3 | |||||||||
Gross profit | 165,465 | 142,763 | 22,702 | 15.9 | |||||||||
Gross margin | 80.8 | % | 80.1 | % | 0.7 | % | 0.9 | ||||||
Expenses: | |||||||||||||
Selling, general and administrative | 144,096 | 162,542 | (18,446 | ) | (11.3 | ) | |||||||
Research and development | 22,911 | 25,934 | (3,023 | ) | (11.7 | ) | |||||||
Restructuring | — | 2,364 | (2,364 | ) | 100.0 | ||||||||
Legal Settlement | — | 8,000 | (8,000 | ) | 100.0 | ||||||||
Total expenses | 167,007 | 198,840 | (31,833 | ) | (16.0 | ) | |||||||
Loss from operations | (1,542 | ) | (56,077 | ) | 54,535 | (97.3 | ) | ||||||
Other (income) and expense, net | 164 | (145 | ) | 309 | (213.1 | ) | |||||||
Loss before income taxes | (1,706 | ) | (55,932 | ) | 54,226 | (96.9 | ) | ||||||
Provision for income taxes | 86 | 92 | (6 | ) | (6.5 | ) | |||||||
Net loss | $ | (1,792 | ) | $ | (56,024 | ) | $ | 54,232 | (96.8 | ) | |||
Net Revenues. Net revenues increased by $26.7 million, or 15.0%, from $178.2 million for the year ended June 30, 2016, to $204.9 million for the year ended June 30, 2017. Revenues from our Peripheral OAS increased $13.5 million, or 10.5%, primarily reflecting an 11.5% increase in the number of devices sold. Sales of our Coronary OAS increased by approximately $11.2 million, or 31.2%, reflecting 32.2% more devices sold. The increase in Peripheral and Coronary OAS sales are primarily due to the expansion of our customer base. Other product revenue increased $2.0 million, or 14.1%, during the year ended June 30, 2017, driven by increased sales of our Peripheral and Coronary OAS, which the other products support.
Cost of Goods Sold. Cost of goods sold increased by $4.0 million, or 11.3%, from $35.4 million for the year ended June 30, 2016 to $39.4 million for the year ended June 30, 2017. These amounts represent the cost of materials, labor and overhead for single-use catheters, guide wires, pumps, and other ancillary products. The increase was primarily due to increased sales levels and a one-time charge of $1.5 million related to the initiation of a voluntary recall of one type of our saline infusion pumps, partially offset by lower costs per unit driven by manufacturing efficiencies and cost reductions. Gross margin increased to 80.8% for the year ended June 30, 2017 from 80.1% for the year ended June 30, 2016 due to lower costs per unit, as discussed above. Cost of goods sold for the years ended June 30, 2017 and 2016 includes $689,000 and $794,000, respectively, for stock-based compensation.
Selling, General and Administrative Expenses. Selling, general, and administrative expenses decreased by $18.4 million, or 11.3%, from $162.5 million for the year ended June 30, 2016 to $144.1 million for the year ended June 30, 2017 primarily due to lower payroll-related and travel expenses from a decrease in headcount from the year ended June 30, 2016, commission plan changes, $1.5 million of fiscal 2016 costs associated with the departure of our former CEO, and a reduction in medical device excise tax expense due to the suspension of the tax effective January 1, 2016. Partially offsetting the decreases was a charge of $1.3 million for employment litigation costs and an increase in incentive compensation expense due to performance. Selling, general, and administrative expenses for the years ended June 30, 2017 and 2016 include $8.7 million and $10.4 million, respectively, for stock-based compensation, which decreased due to the reduction in headcount and a change in vesting terms for our performance-based restricted stock awards granted in fiscal 2017 from those granted in fiscal 2016.
Research and Development Expenses. Research and development expenses decreased by $3.0 million, or 11.7%, from $25.9 million for the year ended June 30, 2016 to $22.9 million for the year ended June 30, 2017. Research and development expenses relate to the specific projects to develop new products or expand into new markets, such as the development of new versions of our Peripheral and Coronary OAS, and ancillary products, and PAD and CAD clinical studies. The decrease primarily related to the completion of enrollment in several of our clinical studies and lower payroll-related expenses from a decrease in headcount from the year ended June 30, 2016. Partially offsetting these were higher patent expense and incentive compensation expense due to performance. Research and development expenses for the years ended June 30,
36
2017 and 2016 include $1.0 million and $1.8 million, respectively, for stock-based compensation, which decreased due to the reduction in headcount.
Restructuring Charges. In March 2016, we announced a broad-based restructuring to reduce costs as a key part of our plan to balance revenue growth with a pathway to profitability and positive cash flow. As a result, we recorded a restructuring expense of $2.4 million during the year ended June 30, 2016, which was comprised of severance and other employee related costs. There were no additional charges related to restructuring activities.
Legal Settlement. On June 28, 2016, we entered into a Settlement Agreement with the United States of America, acting through the U.S. Attorney for the Western District of North Carolina (the “DOJ”) and on behalf of the Office of Inspector General of the Department of Health and Human Services, and Travis Thams (the “Relator”), to resolve the previously disclosed investigation by the DOJ and the qui tam complaint filed by the Relator pursuant to the False Claims Act in the United States District Court for the Western District of North Carolina, Charlotte Division. We recorded an $8.0 million legal settlement expense during the year ended June 30, 2016.
NON-GAAP FINANCIAL INFORMATION
To supplement our consolidated financial statements prepared in accordance with GAAP, our management uses a non-GAAP financial measure referred to as “Adjusted EBITDA.” The following table sets forth, for the periods indicated, a reconciliation of Adjusted EBITDA to the most comparable GAAP measure expressed as dollar amounts (in thousands):
Year Ended June 30, | |||||||
2018 | 2017 | ||||||
Net income (loss) | $ | 1,712 | $ | (1,792 | ) | ||
Less: Other (income) and expense, net | 390 | 164 | |||||
Less: Provision for income taxes | 132 | 86 | |||||
Income (loss) from operations | 2,234 | (1,542 | ) | ||||
Add: Stock-based compensation | 10,302 | 10,354 | |||||
Add: Depreciation and amortization | 3,934 | 4,135 | |||||
Adjusted EBITDA | $ | 16,470 | $ | 12,947 | |||
Adjusted EBITDA improved as compared to the prior year due to the increased earnings from operations.
Use and Economic Substance of Non-GAAP Financial Measures Used and Usefulness of Such Non-GAAP Financial Measures to Investors
We use Adjusted EBITDA as a supplemental measure of performance and believe this measure facilitates operating performance comparisons from period to period and company to company by factoring out potential differences caused by depreciation and amortization expense and non-cash charges such as stock-based compensation. Our management uses Adjusted EBITDA to analyze the underlying trends in our business, assess the performance of our core operations, establish operational goals and forecasts that are used to allocate resources and evaluate our performance period over period and in relation to our competitors’ operating results. Additionally, our management is partially evaluated on the basis of Adjusted EBITDA when determining achievement of their incentive compensation performance targets. Management does not use this Adjusted EBITDA measure as a liquidity measure or in the calculation of our financial covenants under the revolving credit facility with Silicon Valley Bank.
We believe that presenting Adjusted EBITDA provides investors greater transparency to the information used by our management for its financial and operational decision-making and allows investors to see our results “through the eyes” of management. We also believe that providing this information better enables our investors to understand our operating performance and evaluate the methodology used by our management to evaluate and measure such performance.
The following is an explanation of each of the items that management excluded from Adjusted EBITDA and the reasons for excluding each of these individual items:
• | Stock-based compensation. Our management believes that excluding this item from our non-GAAP results is useful to investors to understand the application of stock-based compensation guidance and its impact on our operational performance and ability to make additional investments in our company, and it allows for greater transparency to certain line items in our financial statements. |
37
• | Depreciation and amortization expense. Our management believes that excluding these items from our non-GAAP results is useful to investors to understand our operational performance and ability to make additional investments in our company. |
Material Limitations Associated with the Use of Non-GAAP Financial Measures and Manner in which We Compensate for these Limitations
Non-GAAP financial measures have limitations as analytical tools and should not be considered in isolation or as a substitute for our financial results prepared in accordance with GAAP. Some of the limitations associated with our use of these non-GAAP financial measures are:
• | Items such as stock-based compensation do not directly affect our cash flow position; however, such items reflect economic costs to us and are not reflected in our Adjusted EBITDA, and therefore these non-GAAP measures do not reflect the full economic effect of these items. |
• | Non-GAAP financial measures are not based on any comprehensive set of accounting rules or principles and therefore other companies may calculate similarly titled non-GAAP financial measures differently than we do, limiting the usefulness of those measures for comparative purposes. |
• | Our management exercises judgment in determining which types of charges or other items should be excluded from the non-GAAP financial measures we use. |
We compensate for these limitations by relying primarily upon our GAAP results and using non-GAAP financial measures only supplementally.
LIQUIDITY AND CAPITAL RESOURCES
We had cash and cash equivalents of $116.3 million and $107.9 million at June 30, 2018 and 2017, respectively. As of June 30, 2018, we had an accumulated deficit of $327.6 million. The increase in cash and cash equivalents was primarily attributable to net cash provided by our operating and financing activities during the year ended June 30, 2018.
A summary of our cash flow activities is as follows:
Year Ended June 30, | |||||||||||
2018 | 2017 | 2016 | |||||||||
Net cash provided by (used in) operating activities | $ | 9,674 | $ | 19,588 | $ | (23,583 | ) | ||||
Net cash used in investing activities | (5,095 | ) | (1,779 | ) | (3,769 | ) | |||||
Net cash provided by financing activities | 3,769 | 29,465 | 4,148 | ||||||||
Net change in cash and cash equivalents | $ | 8,348 | $ | 47,274 | $ | (23,204 | ) | ||||
Changes in Liquidity
Operating Activities
Net cash provided by (used in) operating activities was $9.7 million, $19.6 million, and $(23.6) million for the years ended June 30, 2018, 2017, and 2016, respectively. For the years ended June 30, 2018, 2017, and 2016, we had a net income (loss) of $1.7 million, $(1.8) million, and $(56.0) million, respectively, and stock based compensation expense of $10.3 million, $10.4 million, and $13.0 million, respectively. Significant changes in working capital during these periods included:
• | Cash (used in) provided by accounts receivable of $(2.9) million, $(5.8) million, and $7.3 million during the years ended June 30, 2018, 2017, and 2016, respectively, was primarily due to the amount and timing of revenue during the periods. |
38
• | Cash provided by (used in) inventories was $292,000, $543,000, and $(3.5) million during the years ended June 30, 2018, 2017, and 2016, respectively. Cash provided by inventory during the years ended June 30, 2018 and 2017 was primarily due to lower inventory levels from improved inventory management. Cash used by inventory during fiscal 2016 was due to higher levels of inventory for future sales growth and new product launches, as well as timing of inventory purchases and sales. |
• | Cash provided by (used in) prepaid expenses and other current assets was $2.3 million, $(1.8) million, and $728,000 during the years ended June 30, 2018, 2017, and 2016, respectively, primarily due to payment timing of vendor deposits and other expenditures. During the year ended June 30, 2018, we also received proceeds from an insurance receivable related to a litigation settlement payment. |
• | Cash provided by (used in) accounts payable of $104,000, $1.8 million, and $(970,000) during the years ended June 30, 2018, 2017, and 2016, respectively, was primarily due to the amount and timing of purchases and vendor payments. |
• | Cash (used in) provided by accrued expenses and other liabilities was $(6.6) million, $725,000, and $10.9 million during the years ended June 30, 2018, 2017, and 2016, respectively. Cash used during the year ended June 30, 2018 was primarily due to the amount and timing of compensation payments, as well as a litigation settlement payment. Cash provided during the year ended June 30, 2017, was primarily due to the amount and timing of compensation payments. Cash provided during the year ended June 30, 2016 was primarily due to the restructuring accrual, benefits related to our former CEO’s departure, and the DOJ legal settlement expense. |
• | Cash provided by deferred revenue was $189,000 and $10.0 million during the years ended June 30, 2018 and 2017. During the year ended June 30, 2017, Medikit made an upfront payment of $10.0 million to us in connection with the exclusive distribution agreement with Medikit to sell our Coronary and Peripheral OAS in Japan. Medikit also provides advance payments for orders under the terms of the agreement, and, therefore, deferred revenue is recorded until products are accepted by Medikit. |
Investing Activities
Net cash used in investing activities was $5.1 million for the year ended June 30, 2018, compared with $1.8 million for the year ended June 30, 2017. The increase was due to a $2.5 million cost method investment made in June 2018, partially offset by the collection of a note receivable.
Net cash used in investing activities was $1.8 million for the year ended June 30, 2017, compared with $3.8 million for the year ended June 30, 2016. The decrease was primarily due to property and equipment purchases and sales of available-for-sale marketable securities under the deferred compensation plan.
Financing Activities
Net cash provided by financing activities was $3.8 million for the year ended June 30, 2018, compared with $29.5 million for the year ended June 30, 2017. The decrease was primarily driven by $20.9 million of cash proceeds received from the sale of the Facility in March 2017, described below and a lesser amount of cash proceeds from stock options received in the year ended June 30, 2018, as there were fewer stock options outstanding.
Net cash provided by financing activities was $29.5 million for the year ended June 30, 2017, compared with $4.1 million for the year ended June 30, 2016. The increase was primarily driven by $20.9 million of cash proceeds received from the sale of the Facility in March 2017, as described below, and a greater amount of cash proceeds from stock option exercises during the year ended June 30, 2017.
Our future liquidity and capital requirements will be influenced by numerous factors, including the extent and duration of future operating losses, the level and timing of future sales and expenditures, the results and scope of ongoing research and product development programs, working capital required to support our business operations, the receipt of and time required to obtain regulatory clearances and approvals, our sales and marketing programs, the continuing acceptance of our products in the marketplace, competing technologies, market and regulatory developments, ongoing facility requirements, potential strategic transactions (including the potential acquisition of, or investments in, businesses, technologies and products), international expansion, and the existence, defense and resolution of legal proceedings. As of June 30, 2018, we believe our current cash and cash equivalents will be sufficient to fund working capital requirements, capital expenditures and operations for the foreseeable future, including at least the next twelve months, as well as to fund payments related to the DOJ settlement, expenses relating
39
to compliance with our Corporate Integrity Agreement (as defined below), payments under our lease agreements, payments under severance agreements, and anticipated costs relating to litigation. We intend to retain any future earnings to support operations and to finance the growth and development of our business. We do not anticipate paying any dividends in the foreseeable future.
Legal Settlement
On June 28, 2016, we entered into a Settlement Agreement with the United States of America, acting through the U.S. Attorney’s Office for the Western District of North Carolina (the “DOJ”) and on behalf of the Office of Inspector General of the Department of Health and Human Services (the “OIG”), and Travis Thams, pursuant to which we will pay $8.0 million as follows: an initial payment of $3.0 million, which we paid on July 1, 2016, with the remaining $5.0 million, which bears interest at 1.8% per annum, payable in 11 equal quarterly installments, beginning January 1, 2017. Under the settlement agreement, if we make a single payment in excess of $2.0 million, which payment is not covered by an insurance policy, in settlement of any claims before paying the full settlement amount, the remaining unpaid balance of the settlement amount will become immediately due and payable, with interest accruing on the unpaid principal portion at an interest rate of 1.8% per annum.
In connection with the resolution of this matter, we entered into a five-year corporate integrity agreement (the “Corporate Integrity Agreement”) with the OIG. The Corporate Integrity Agreement requires that we maintain our existing compliance programs and imposes certain expanded compliance-related requirements during the term of the Corporate Integrity Agreement, including establishment of specific procedures and requirements regarding consulting activities, co-marketing activities and other interactions with healthcare professionals and healthcare institutions and the sale and marketing of our products; ongoing monitoring, reporting, certification and training obligations; and the engagement of an independent review organization to perform certain auditing and reviews and prepare certain reports regarding our compliance with federal health care programs. In the event of a breach of the Corporate Integrity Agreement, we could become liable for payment of certain stipulated penalties or could be excluded from participation in federal health care programs. The Corporate Integrity Agreement requires us to invest additional amounts in our compliance program and pay fees and expenses of the independent review organization.
Facility Sale and Lease
On December 29, 2016, we entered into a Purchase and Sale Agreement, as subsequently amended (collectively, the “Sale Agreement”), with Krishna Holdings, LLC (the “Buyer”), providing for the sale to Buyer of our headquarters facility in St. Paul, Minnesota (the “Facility”), for a cash purchase price of $21.5 million. On March 30, 2017, the sale of the Facility under the Sale Agreement closed. We received proceeds of approximately $20.9 million ($21.5 million less $556,000 of transaction expenses).
We intend to use the net proceeds from the sale for working capital and general corporate purposes, which may include, but are not limited to:
• | the funding of clinical trials and studies; |
• | sales and marketing programs; |
• | expansion into international markets; and |
• | development of new products. |
We may also use a portion of the net proceeds for the potential acquisition of, or investments in, businesses, technologies and products, although we have no current understandings, commitments or arrangements to do so. We cannot specify with certainty all of the particular uses for the net proceeds. Accordingly, we will retain broad discretion over the use of these net proceeds.
In connection with the closing of the Facility sale, we entered into a Lease Agreement (the “Lease Agreement”) with Krishna Holdings, LLC, Apex Holdings, LLC, Kashi Associates, LLC, Keva Holdings, LLC, S&V Ventures, LLC, Polo Group LLC, SPAV Holdings LLC, Star Associates LLC, and The Global Villa, LLC. The Lease Agreement has an initial term of fifteen years, with four consecutive renewal options of five years each, with a base annual rent in the first year of $1.6 million and annual escalations of 3%. See Notes 2 and 3 to our Consolidated Financial Statements included in Item 8 of this Annual Report on Form 10-K for additional discussion.
40
Revolving Credit Facility
On March 31, 2017, we entered into a Loan and Security Agreement (the “Loan Agreement”) with Silicon Valley Bank (“SVB”). The Loan Agreement provides for a senior, secured revolving credit facility (the “Revolver”) of $40.0 million (the “Maximum Dollar Amount”).
Advances under the Revolver may be made from time to time up to the Maximum Dollar Amount, subject to certain borrowing limitations. The Revolver has a maturity date of March 31, 2020 and bears interest at a floating per annum rate equal to the Wall Street Journal prime rate, less 0.25%. Interest on borrowings is due monthly and the principal balance is due at maturity. Borrowings up to $10.0 million are available on a non-formula basis. Borrowings above $10.0 million are based on (i) 85% of eligible domestic accounts receivable, and (ii) the lesser of 50% of eligible inventory or $5.0 million, subject to adjustment as defined in Loan Agreement. Upon the Revolver’s maturity, any outstanding principal balance, unpaid accrued interest, and all other obligations under the Revolver will be due and payable. We will incur a fee equal to 1% of the Maximum Dollar Amount upon termination of the Loan Agreement or the Revolver for any reason prior to the maturity date, unless refinanced with SVB.
Our obligations under the Loan Agreement are secured by certain of our assets, including, among other things, accounts receivable, deposit accounts, inventory, equipment, general intangibles and records pertaining to the foregoing. The collateral does not include our intellectual property, but we agreed not to encumber our intellectual property without the consent of SVB. The Loan Agreement contains customary covenants limiting our ability to, among other things, incur debt or liens, make certain investments and loans, enter into transactions with affiliates, undergo certain fundamental changes, dispose of assets, or change the nature of its business. In addition, the Loan Agreement contains financial covenants requiring us to maintain, at all times when any amounts are outstanding under the Revolver, either (i) minimum unrestricted cash at SVB and unused availability on the Revolver of at least $10.0 million or (ii) minimum trailing three-month Adjusted EBITDA of $1.0 million. If we do not comply with the various covenants under the Loan Agreement, the interest rate on outstanding amounts will increase by 5% and SVB may, subject to various customary cure rights, decline to provide additional advances under the Revolver, require the immediate payment of all amounts outstanding under the Revolver, and foreclose on all collateral.
Under the Loan Agreement, we paid SVB a non-refundable commitment fee of $80,000, which will be amortized to interest expense over the term of the Loan Agreement. We are required to pay a fee equal to 0.35% per annum on the unused portion of the Revolver, payable quarterly in arrears. SVB’s obligations to advance funds under the Revolver are subject to an initial collateral examination to be completed within 90 days of the effective date of the Loan Agreement. We are not obligated to draw any funds under the Revolver and no amounts are outstanding as of June 30, 2018. We currently do not have plans of borrowing under the Loan Agreement.
Contractual Cash Obligations. Our contractual obligations and commercial commitments as of June 30, 2018 are summarized below:
Payments Due by Period (in thousands) | |||||||||||||||||||
Contractual Obligations | Total | Less Than 1 Year | 1-3 Years | 3-5 Years | More Than 5 Years | ||||||||||||||
Operating leases(1) | $ | 906 | $ | 510 | $ | 396 | $ | — | $ | — | |||||||||
Financing obligation(2) | 28,397 | 1,699 | 3,553 | 3,770 | 19,375 | ||||||||||||||
Purchase commitments(3) | 18,323 | 18,323 | — | — | — | ||||||||||||||
Legal settlement(4) | 2,314 | 1,847 | 467 | — | — | ||||||||||||||
Severance payments(5) | 975 | 934 | 41 | — | — | ||||||||||||||
Other(6) | 66 | 66 | — | — | — | ||||||||||||||
Total | $ | 50,981 | $ | 23,379 | $ | 4,457 | $ | 3,770 | $ | 19,375 | |||||||||
(1) | The amounts represent future minimum payments under a non-cancellable operating lease for our Texas production facility along with equipment leases. |
(2) | The amounts represent future minimum payments due under the capital lease related to the sale leaseback of our Facility. |
(3) | The amount represents open purchase orders as of June 30, 2018. |
(4) | Consists of payments and related interest associated with the DOJ settlement discussed above. |
(5) | Includes severance related to various severance agreements. |
(6) | Other includes service agreements. |
Due to the uncertainty with respect to the timing of future cash flows associated with our unrecognized tax benefits at June 30, 2018, we are unable to make reasonably reliable estimates of the period of cash settlement with the respective taxing authority. Therefore, $597,000 of unrecognized tax benefits have been excluded from the contractual obligations table above.
41
INFLATION
We do not believe that inflation has had a material impact on our business and operating results during the periods presented.
OFF-BALANCE SHEET ARRANGEMENTS
Since inception, we have not engaged in any off-balance sheet arrangements as defined in Item 303(a)(4) of Regulation S-K.
RECENT ACCOUNTING PRONOUNCEMENTS
In May 2014, the Financial Accounting Standards Board (“FASB”) issued amended revenue recognition guidance to clarify the principles for recognizing revenue from contracts with customers. The guidance requires an entity to recognize revenue in an amount that reflects the consideration to which an entity expects to be entitled in exchange for the transfer of goods or services. The guidance also requires expanded disclosures relating to the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers. Additionally, qualitative and quantitative disclosures are required about customer contracts, significant judgments and changes in judgments, and assets recognized from the costs to obtain or fulfill a contract.
We have completed an adoption plan, which included a review of customer contracts, applying the five-step model of the new standard to the customer contracts and comparing the results to our current accounting. We have elected the modified retrospective method of adoption, which we anticipate will not result in a material adjustment to retained earnings. Our revenue recognition will remain largely unchanged given the majority of our revenue arrangements consist of a single performance obligation that will be recognized at a point in time when control passes to the customer. As a result, we do not anticipate a material impact on the amount and timing of revenue recognized in our consolidated financial statements.
We are also implementing new internal controls around the judgments needed to address risks associated with applying the five-step model, which includes identifying performance obligations and judgments made related to variable consideration. Effective July 1, 2018, we will be revising our revenue recognition accounting policy and disclosures to reflect the requirements of the amended revenue recognition guidance. We will continue to monitor any modifications or interpretations communicated by the FASB that may impact any of its final assessments related to the guidance.
In January 2016, the FASB issued ASU 2016-01, “Recognition and Measurement of Financial Assets and Financial Liabilities,” which revises the accounting requirements related to the classification and measurement of investments in equity securities and the presentation of certain fair value changes for financial liabilities measured at fair value. The update also changes certain disclosure requirements associated with the fair value of financial instruments. These changes will require an entity to measure, at fair value, investments in equity securities and recognize the changes in fair value within net income. ASU 2016-01 will be applied on a modified retrospective basis to all outstanding instruments, with an adjustment recorded to opening retained earnings as of the beginning of the first period in which the guidance becomes effective. The guidance is effective for annual periods beginning after December 15, 2017, including interim periods within those fiscal years, with early adoption permitted. The guidance is effective for us on July 1, 2018. We do not anticipate a material impact on our financial statements upon adoption.
In February 2016, the FASB issued ASU 2016-02, “Leases.” The guidance requires lessees to recognize the assets and liabilities that arise from leases on the balance sheet. ASU 2016-02 is effective for annual periods beginning after December 15, 2018, including interim periods within those annual periods, and should be applied using a modified retrospective approach. Early adoption is permitted. The guidance is effective for us on July 1, 2019. We are currently evaluating the timing, method of adoption and impact of the new lease guidance on our financial statements.
In June 2016, the FASB issued ASU 2016-13, “Measurement of Credit Losses on Financial Instruments,” which revises guidance for the accounting for credit losses on financial instruments within its scope. The new standard introduces an approach, based on expected losses, to estimate credit losses on certain types of financial instruments and modifies the impairment model for available-for-sale debt securities. The new approach to estimating credit losses (referred to as the current expected credit losses model) applies to most financial assets measured at amortized cost and certain other instruments, including trade and other receivables, loans, held-to-maturity debt securities, net investments in leases and off-balance-sheet credit exposures. ASU 2016-13 is effective for annual periods beginning after December 15, 2019, including interim periods within those fiscal years. Early adoption is permitted and should be applied as a cumulative-effect adjustment to retained earnings as of the beginning of the first reporting period in which the guidance is adopted. The guidance is effective for us on July 1, 2020. We do not anticipate a material impact on our financial statements upon adoption.
42
In May 2017, the FASB issued ASU 2017-09, “Scope of Modification Accounting” which clarifies when changes to the terms or conditions of a share-based payment award must be accounted for as modifications. The new guidance will reduce diversity in practice and result in fewer changes to the terms of an award being accounted for as modifications. ASU 2017-09 will be applied prospectively to awards modified on or after the adoption date. The guidance is effective for annual periods, and interim periods within those annual periods beginning after December 15, 2017, with early adoption permitted. We do not anticipate a material impact on our financial statements upon adoption.
PRIVATE SECURITIES LITIGATION REFORM ACT
The Private Securities Litigation Reform Act of 1995 provides a “safe harbor” for forward-looking statements. Such “forward-looking” information is included in this Form 10-K and in other materials filed or to be filed by us with the Securities and Exchange Commission (as well as information included in oral statements or other written statements made or to be made by the Company). Forward-looking statements include all statements based on future expectations. This Form 10-K contains forward-looking statements that involve risks and uncertainties, including, but not limited to, (i) the expectation of selling our products, including recently approved products, future products and products we distribute, domestically and internationally in the future, the timing and structure of our plans to do so, and the specific countries and products to be sold, either by us or through distributors; (ii) our strategy; (iii) the competitive benefits of our products; (iv) potential strategic acquisitions and partnerships; (v) our products in development; (vi) seasonality in our business; (vii) reimbursement of our products; (viii) our intention to expand our product portfolio through internal development and external relationships; (ix) our plan to balance revenue growth with a pathway to profitability and positive cash flow; (x) our current and anticipated clinical studies, including the results and timing of such studies; (xi) our expectation that our revenue will increase; (xii) our expectation of increased selling, general and administrative expenses and the rate of such growth; (xiii) our expectation that gross margin in fiscal 2019 will decrease slightly compared to fiscal 2018; (xiv) our expectation that our current facilities will be adequate for the foreseeable future; (xv) our plans to continue to expand our sales and marketing efforts as well as our product portfolio and clinical studies; (xvi) our intention to file additional patents and our efforts to protect our intellectual property; (xvii) our expectation that we will incur research and development expenses in fiscal 2019 higher than the amounts incurred for fiscal 2018; (xviii) our belief that our current cash and cash equivalents will be sufficient to fund working capital requirements, capital expenditures and operations for the foreseeable future, as well as to fund certain other anticipated expenses; (xix) our intention to retain any future earnings to support operations and to finance the growth and development of our business; (xx) our dividend expectations; (xxi) our plans to use the net proceeds from the sale of our headquarters facility; (xxii) our ability to obtain regulatory approvals to market our products; (xxiii) our plan not to borrow under our loan and security agreement; and (xxiv) the anticipated impact of adoption of recent accounting pronouncements on our financial statements.
In some cases, you can identify forward-looking statements by the following words: “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. Forward-looking statements are only predictions and are not guarantees of performance. These statements are based on our management’s beliefs and assumptions, which in turn are based on their interpretation of currently available information.
These statements involve known and unknown risks, uncertainties and other factors that may cause our results or our industry’s actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements.
These factors include regulatory developments, clearances and approvals; approval of our products for distribution in foreign countries; approval of products for reimbursement and the level of reimbursement in the U.S., Japan and other foreign countries; dependence on market growth; agreements with third parties to sell their products; the ability of OrbusNeich to successfully launch CSI products outside of the United States and Japan; our ability to maintain third-party supplier relationships and renew existing purchase agreements; our ability to maintain our relationships with Medikit and OrbusNeich; the experience of physicians regarding the effectiveness and reliability of our products; the reluctance of physicians, hospitals and other organizations to accept new products; the potential for unanticipated delays in enrolling medical centers and patients for clinical trials; actual clinical trial and study results; the impact of competitive products and pricing; our ability to comply with the financial covenants in our loan and security agreement and to make payments under and comply with the lease agreement for our corporate headquarters; unanticipated developments affecting our estimates regarding expenses, future revenues and capital requirements; the difficulty of successfully managing operating costs; our ability to manage our sales force strategy; actual research and development efforts and needs; our ability to obtain and maintain intellectual property protection for product candidates; fluctuations in results and expenses based on new product introductions, sales mix, unanticipated warranty claims, and the timing of project expenditures; our ability to manage costs; our actual financial resources and our ability to obtain additional financing; investigations or litigation threatened or initiated against us; court rulings and future actions by the FDA and other regulatory bodies; international trade developments; and general economic conditions.
These and additional risks and uncertainties are described more fully in Item 1A of this Form 10-K under “Risk Factors.”
You should read these risk factors and the other cautionary statements made in this Form 10-K as being applicable to all related forward-looking statements wherever they appear in this Form 10-K. We cannot assure you that the forward looking statements in this Form 10-K will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the
43
inaccuracy may be material. You should read this Form 10-K completely. Other than as required by law, we undertake no obligation to update these forward-looking statements, even though our situation may change in the future.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
The primary objective of our investment activities is to preserve our capital for the purpose of funding operations while at the same time maximizing the income we receive from our investments without significantly increasing risk or availability. To achieve these objectives, our investment policy allows us to maintain a portfolio of cash equivalents and investments in a variety of marketable securities, including money market funds, United States government securities, and certain bank obligations. Our cash and cash equivalents as of June 30, 2018 include liquid money market accounts. Due to the short-term nature of these investments, we believe that there is no material exposure to interest rate risk.
Additionally, we have certain available-for-sale marketable securities under our deferred compensation plan. See Note 1 to our Consolidated Financial Statements included in Item 8 of Part II of this Annual Report on Form 10-K for additional information on these available-for-sale marketable securities.
Item 8. Financial Statements and Supplementary Data.
Index to Financial Statements
Page | |
F-1 | |
F-2 | |
F-3 | |
F-4 | |
F-5 | |
F-6 | |
F-7 | |
44
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Cardiovascular Systems, Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Cardiovascular Systems, Inc. and its subsidiaries as of June 30, 2018 and 2017 and the related consolidated statements of operations, comprehensive income (loss), changes in stockholders’ equity and cash flows for each of the three years in the period ended June 30, 2018, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of June 30, 2018, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of June 30, 2018 and 2017 and the results of their operations and their cash flows for each of the three years in the period ended June 30, 2018 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of June 30, 2018, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting under item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
F-1
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Minneapolis, Minnesota
August 23, 2018
We have served as the Company’s auditor since at least 2003, which includes periods before the Company became subject to SEC reporting requirements.
F-2
Cardiovascular Systems, Inc.
Consolidated Balance Sheets
(Dollars in thousands, except per share and share amounts)
June 30, 2018 | June 30, 2017 | ||||||
ASSETS | |||||||
Current assets | |||||||
Cash and cash equivalents | $ | 116,260 | $ | 107,912 | |||
Accounts receivable, net | 31,225 | 28,472 | |||||
Inventories | 16,605 | 16,897 | |||||
Marketable securities | 544 | 704 | |||||
Prepaid expenses and other current assets | 2,977 | 5,074 | |||||
Total current assets | 167,611 | 159,059 | |||||
Property and equipment, net | 27,744 | 29,696 | |||||
Patents, net | 5,231 | 5,056 | |||||
Other assets | 2,766 | 129 | |||||
Total assets | $ | 203,352 | $ | 193,940 | |||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||
Current liabilities | |||||||
Accounts payable | 10,441 | 10,736 | |||||
Accrued expenses | 25,776 | 30,236 | |||||
Deferred revenue | 1,243 | — | |||||
Total current liabilities | 37,460 | 40,972 | |||||
Long-term liabilities | |||||||
Financing obligation | 21,064 | 21,100 | |||||
Deferred revenue | 8,946 | 10,000 | |||||
Other liabilities | 1,412 | 3,479 | |||||
Total liabilities | 68,882 | 75,551 | |||||
Commitments and contingencies | |||||||
Common stock, $0.001 par value; authorized 100,000,000 common shares; issued and outstanding 33,360,032 at June 30, 2018 and 32,849,563 at June 30, 2017 | 33 | 33 | |||||
Additional paid in capital | 461,927 | 447,559 | |||||
Accumulated other comprehensive income | 101 | 100 | |||||
Accumulated deficit | (327,591 | ) | (329,303 | ) | |||
Total stockholders’ equity | 134,470 | 118,389 | |||||
Total liabilities and stockholders’ equity | $ | 203,352 | $ | 193,940 | |||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Cardiovascular Systems, Inc.
Consolidated Statements of Operations
(Dollars in thousands, except per share and share amounts)
Year Ended June 30, | |||||||||||
2018 | 2017 | 2016 | |||||||||
Net revenues | $ | 217,043 | $ | 204,906 | $ | 178,184 | |||||
Cost of goods sold | 39,484 | 39,441 | 35,421 | ||||||||
Gross profit | 177,559 | 165,465 | 142,763 | ||||||||
Expenses: | |||||||||||
Selling, general and administrative | 148,569 | 144,096 | 162,542 | ||||||||
Research and development | 26,756 | 22,911 | 25,934 | ||||||||
Restructuring | — | — | 2,364 | ||||||||
Legal settlement | — | — | 8,000 | ||||||||
Total expenses | 175,325 | 167,007 | 198,840 | ||||||||
Income (loss) from operations | 2,234 | (1,542 | ) | (56,077 | ) | ||||||
Other (income) expense, net: | |||||||||||
Interest expense | 1,717 | 500 | — | ||||||||
Interest income and other, net | (1,327 | ) | (336 | ) | (145 | ) | |||||
Total other (income) expense, net | 390 | 164 | (145 | ) | |||||||
Income (loss) before income taxes | 1,844 | (1,706 | ) | (55,932 | ) | ||||||
Provision for income taxes | 132 | 86 | 92 | ||||||||
Net income (loss) | $ | 1,712 | $ | (1,792 | ) | $ | (56,024 | ) | |||
Basic earnings per share | $ | 0.05 | $ | (0.06 | ) | $ | (1.72 | ) | |||
Diluted earnings per share | $ | 0.05 | $ | (0.06 | ) | $ | (1.72 | ) | |||
Basic weighted average shares outstanding | 33,145,140 | 32,373,709 | 32,537,621 | ||||||||
Diluted weighted average shares outstanding | 33,614,260 | 32,373,709 | 32,537,621 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Cardiovascular Systems, Inc.
Consolidated Statements of Comprehensive Income (Loss)
(Dollars in thousands, except per share and share amounts)
Year Ended June 30, | |||||||||||
2018 | 2017 | 2016 | |||||||||
Net income (loss) | $ | 1,712 | $ | (1,792 | ) | $ | (56,024 | ) | |||
Other comprehensive income (loss): | |||||||||||
Unrealized gain on available for sale securities | 35 | 66 | 20 | ||||||||
Adjustment for net gain realized and included in interest income and other, net | (34 | ) | (6 | ) | (70 | ) | |||||
Total change in unrealized gain (loss) on available for sale securities | 1 | 60 | (50 | ) | |||||||
Comprehensive income (loss) | $ | 1,713 | $ | (1,732 | ) | $ | (56,074 | ) | |||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Cardiovascular Systems, Inc.
Consolidated Statements of Changes in Stockholders’ Equity
(Dollars in thousands, except per share and share amounts)
Common Stock | Additional Paid In Capital | Accumulated Other Comprehensive Income | Accumulated Deficit | Total | ||||||||||||||||||
Shares | Amount | |||||||||||||||||||||
Balances at June 30, 2015 | 31,898,124 | $ | 32 | $ | 410,700 | $ | 90 | $ | (271,387 | ) | $ | 139,435 | ||||||||||
Stock-based compensation related to restricted stock awards, net | 557,756 | 1 | 11,985 | — | — | 11,986 | ||||||||||||||||
Exercise of stock options at $7.90-$12.37 per share | 87,817 | — | 1,006 | — | — | 1,006 | ||||||||||||||||
Employee stock purchase plan activity | 248,800 | — | 4,544 | — | — | 4,544 | ||||||||||||||||
Unrealized gain on marketable securities | — | — | — | 20 | — | 20 | ||||||||||||||||
Net gain reclassified from accumulated other comprehensive income | — | — | — | (70 | ) | — | (70 | ) | ||||||||||||||
Net loss | — | — | — | — | (56,024 | ) | (56,024 | ) | ||||||||||||||
Balances at June 30, 2016 | 32,792,497 | $ | 33 | $ | 428,235 | $ | 40 | $ | (327,411 | ) | $ | 100,897 | ||||||||||
Stock-based compensation related to restricted stock awards, net | (635,018 | ) | — | 9,412 | — | — | 9,412 | |||||||||||||||
Exercise of stock options at $7.90-$12.15 per share | 515,164 | — | 5,362 | — | (100 | ) | 5,262 | |||||||||||||||
Employee stock purchase plan activity | 176,920 | — | 4,550 | — | — | 4,550 | ||||||||||||||||
Unrealized gain on marketable securities | — | — | — | 66 | — | 66 | ||||||||||||||||
Net gain reclassified from accumulated other comprehensive income | — | — | — | (6 | ) | — | (6 | ) | ||||||||||||||
Net loss | — | — | — | — | (1,792 | ) | (1,792 | ) | ||||||||||||||
Balances at June 30, 2017 | 32,849,563 | $ | 33 | $ | 447,559 | $ | 100 | $ | (329,303 | ) | $ | 118,389 | ||||||||||
Stock-based compensation related to restricted stock awards, net | 295,650 | — | 9,546 | — | — | 9,546 | ||||||||||||||||
Exercise of stock options at $7.90-$12.15 per share | 55,880 | — | 514 | — | — | 514 | ||||||||||||||||
Employee stock purchase plan activity | 158,939 | — | 4,308 | — | — | 4,308 | ||||||||||||||||
Unrealized gain on marketable securities | — | — | — | 35 | — | 35 | ||||||||||||||||
Net gain reclassified from accumulated other comprehensive income | — | — | — | (34 | ) | — | (34 | ) | ||||||||||||||
Net income | — | — | — | — | 1,712 | 1,712 | ||||||||||||||||
Balances at June 30, 2018 | 33,360,032 | $ | 33 | $ | 461,927 | $ | 101 | $ | (327,591 | ) | $ | 134,470 | ||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Cardiovascular Systems, Inc.
Consolidated Statements of Cash Flows
(Dollars in thousands)
Year Ended June 30, | |||||||||||
2018 | 2017 | 2016 | |||||||||
Cash flows from operating activities | |||||||||||
Net income (loss) | $ | 1,712 | $ | (1,792 | ) | $ | (56,024 | ) | |||
Adjustments to reconcile net income (loss) to net cash used in operations | |||||||||||
Depreciation of property and equipment | 3,730 | 3,917 | 3,686 | ||||||||
Provision for (recovery of) doubtful accounts (including note receivable) | (225 | ) | 465 | 725 | |||||||
Amortization of patents | 204 | 218 | 231 | ||||||||
Write-off of patent costs | 497 | 733 | 168 | ||||||||
Loss on disposal of property and equipment | 16 | 158 | 170 | ||||||||
Stock-based compensation | 10,302 | 10,354 | 12,977 | ||||||||
Other | — | 138 | — | ||||||||
Changes in assets and liabilities | |||||||||||
Accounts receivable | (2,878 | ) | (5,809 | ) | 7,327 | ||||||
Inventories | 292 | 543 | (3,474 | ) | |||||||
Prepaid expenses and other assets | 2,308 | (1,823 | ) | 728 | |||||||
Accounts payable | 104 | 1,761 | (970 | ) | |||||||
Accrued expenses and other liabilities | (6,577 | ) | 725 | 10,873 | |||||||
Deferred revenue | 189 | 10,000 | — | ||||||||
Net cash provided by (used in) operating activities | 9,674 | 19,588 | (23,583 | ) | |||||||
Cash flows from investing activities | |||||||||||
Expenditures for property and equipment | (1,956 | ) | (981 | ) | (3,818 | ) | |||||
Purchase of investment | (2,538 | ) | — | — | |||||||
Proceeds from (issuance of) convertible note receivable | 318 | — | (350 | ) | |||||||
Purchases of marketable securities | — | — | (37 | ) | |||||||
Sales of marketable securities | 194 | 46 | 1,249 | ||||||||
Costs incurred in connection with patents | (1,113 | ) | (844 | ) | (813 | ) | |||||
Net cash used in investing activities | (5,095 | ) | (1,779 | ) | (3,769 | ) | |||||
Cash flows from financing activities | |||||||||||
Proceeds from the employee stock purchase plan | 3,242 | 3,254 | 3,142 | ||||||||
Exercise of stock options | 513 | 5,263 | 1,006 | ||||||||
Proceeds from financing | 20,944 | — | |||||||||
Other | 14 | 4 | — | ||||||||
Net cash provided by financing activities | 3,769 | 29,465 | 4,148 | ||||||||
Net change in cash and cash equivalents | 8,348 | 47,274 | (23,204 | ) | |||||||
Cash and cash equivalents | |||||||||||
Beginning of period | 107,912 | 60,638 | 83,842 | ||||||||
End of period | $ | 116,260 | $ | 107,912 | $ | 60,638 | |||||
Noncash investing and financing activities | |||||||||||
Change in equipment included in accounts payable | $ | 162 | $ | (319 | ) | $ | (374 | ) | |||
Change in patent costs included in accounts payable | $ | 237 | $ | (150 | ) | $ | 87 | ||||
Supplemental cash flow information | |||||||||||
Interest paid | $ | 1,717 | $ | 500 | $ | — | |||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share and share amounts)
1. Summary of Significant Accounting Policies
Company Description
Cardiovascular Systems, Inc. (the “Company”), based in St. Paul, Minn., is a medical device company focused on developing and commercializing innovative solutions for treating vascular and coronary disease. The Company’s Orbital Atherectomy Systems (“OAS”) treat calcified and fibrotic plaque in arterial vessels throughout the leg and heart in a few minutes of treatment time, and address many of the limitations associated with existing surgical, catheter and pharmacological treatment alternatives.
Principles of Consolidation
The consolidated balance sheets and statements of operations, comprehensive loss, changes in stockholders’ equity, and cash flows include the accounts of the Company and its wholly-owned subsidiary, after elimination of all intercompany transactions and accounts.
Cash and Cash Equivalents
The Company considers all money market funds and other investments purchased with an original maturity of three months or less to be cash and cash equivalents.
Accounts Receivable and Allowance for Doubtful Accounts
Trade accounts receivable are recorded at the invoiced amount and do not bear interest. Customer credit terms are established prior to shipment with the general standard being net 30 days. Collateral or any other security to support payment of these receivables generally is not required. The Company maintains an allowance for doubtful accounts, which is an estimate regularly evaluated by the Company for adequacy by taking into consideration factors such as past experience, credit quality of the customer base, age of the receivable balances, both individually and in the aggregate, and current economic conditions that may affect a customer’s ability to pay. Provisions for the allowance for doubtful accounts attributed to bad debt are recorded in general and administrative expenses.
The following table shows the allowance for doubtful accounts activity:
Amount | |||
Balances at June 30, 2015 | $ | 1,437 | |
Provision for doubtful accounts | 375 | ||
Write-offs | (1,100 | ) | |
Balance at June 30, 2016 | 712 | ||
Provision for doubtful accounts | 465 | ||
Write-offs | (313 | ) | |
Balance at June 30, 2017 | 864 | ||
Provision for doubtful accounts | 125 | ||
Write-offs | (189 | ) | |
Balance at June 30, 2018 | $ | 800 | |
Inventories
Inventories are stated at the lower of cost or net realizable value, with cost determined on a first-in, first-out method of valuation. The establishment of inventory allowances for excess and obsolete inventories is based on estimated exposure on specific inventory items. The Company writes down its inventories as it becomes aware of any situation where the carrying amount exceeds the estimated realizable value based on assumptions about future demands and market conditions.
F-8
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Property and Equipment
Property and equipment is carried at cost, less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over estimated useful lives of 40 years for the building; five to seven years for production equipment and furniture and fixtures; three years for computer equipment and software; and the shorter of their estimated useful lives or the lease term for leasehold improvements. Expenditures for maintenance and repairs and minor renewals and betterments that do not extend or improve the life of the respective assets are expensed as incurred. All other expenditures for renewals and betterments are capitalized. The assets and related depreciation accounts are adjusted for property retirements and disposals with the resulting gains or losses included in the consolidated statement of operations.
Patents
The capitalized costs incurred to obtain patents are amortized using the straight-line method over their remaining estimated lives. Patent amortization begins at the time of patent application approval, and does not exceed 20 years. The recoverability of capitalized patent costs is dependent upon the Company’s ability to derive revenue-producing products from such patents or the ultimate sale or licensing of such patent rights. Patent recoverability is regularly reviewed and any patents that are abandoned are written off at the time of abandonment.
Long-Lived Assets
The Company regularly evaluates the carrying value of long-lived assets for events or changes in circumstances that indicate that the carrying amount may not be recoverable or that the remaining estimated useful life should be changed. An impairment loss is recognized when the carrying amount of an asset exceeds the anticipated future undiscounted cash flows expected to result from the use of the asset and its eventual disposition. The amount of the impairment loss to be recorded, if any, is calculated by the excess of the asset’s carrying value over its fair value.
Operating Leases
The Company leases its Texas manufacturing facilities under an operating lease agreement. The lease contains rent escalation clauses for which the lease expense is recognized on a straight-line basis over the lease term. Rent expense that is recognized but not yet paid is included in other liabilities on the consolidated balance sheets.
Financing Obligation
In March 2017, the Company entered into an agreement to lease its Minnesota facility. The lease agreement has an initial term of fifteen years, with four consecutive renewal options of five years each at the Company's option. As the lease terms resulted in a capital lease classification, the Company accounted for the sale and leaseback of its Minnesota facility as a financing transaction where the assets remain on the Company's balance sheet and a financing obligation was recorded for $20,944. As lease payments are made, they will be allocated between interest expense and a reduction of the financing obligation, resulting in a value of the financing obligation that is equivalent to the net book value of the assets at the end of the lease term. At the end of the lease (including any renewal option terms), the Company will remove the assets and financing obligation from its balance sheet.
Revenue Recognition
The Company sells the majority of its products via direct shipment to hospitals or clinics. The Company recognizes revenue when all of the following criteria are met: persuasive evidence of an arrangement exists; delivery has occurred; the sales price is fixed or determinable; and collectability is reasonably assured. Revenue recognition may occur upon shipment or upon delivery to the customer site, based on the contract terms. The Company records estimated sales returns, discounts and rebates as a reduction of net sales.
Deferred revenue associated with the upfront payment received under the Company’s distribution agreement with Medikit is recognized in relation to the estimated future sales under the agreement. The short term portion represents the expected amount of deferred revenue that will be recognized over the next year. The estimate of future sales under contract will continue to be assessed and adjusted accordingly.
Costs related to products delivered are recognized in the period the revenue is recognized. Cost of goods sold consists primarily of raw materials, direct labor, and manufacturing overhead.
F-9
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Deferred Revenue
In November 2016, the Company signed an exclusive distribution agreement with Medikit Co., Ltd. (“Medikit”) to sell its Coronary and Peripheral OAS in Japan. To secure exclusive distribution rights, Medikit made an upfront payment of $10,000 to the Company, which is refundable based on the occurrence of certain events during the term of the agreement. The Company has classified the payment as current or long-term based on its expectation of when revenue will be recognized and is re-evaluated on a quarterly basis. Medikit also provides advance payments for orders under the terms of the agreement, and, therefore, deferred revenue is recorded until products are accepted by Medikit.
Warranty Costs
The Company provides its customers with the right to receive a replacement if a product is determined to be defective at the time of shipment. Warranty reserve provisions are estimated based on Company experience, volume, and expected warranty claims. Warranty reserve, provisions and claims were as follows:
Amount | |||
Balances at June 30, 2015 | $ | 126 | |
Provision | 490 | ||
Claims | (471 | ) | |
Balance at June 30, 2016 | 145 | ||
Provision | 1,733 | ||
Claims | (1,361 | ) | |
Balance at June 30, 2017 | 517 | ||
Provision | 328 | ||
Claims | (713 | ) | |
Balance at June 30, 2018 | $ | 132 | |
Pump Recall
In April 2017, the Company initiated a voluntary recall of one type of its saline infusion pumps that resulted in a reserve of approximately $1,535 incurred in fiscal 2017, relating to estimated costs of the recall.
Litigation and Contingent Liabilities
The Company and its operations from time to time are, and in the future may be, parties to or targets of lawsuits, claims, investigations, and proceedings, which are handled and defended in the ordinary course of business. The Company accrues a liability for such matters when it is probable that a liability has been incurred and the amount can be reasonably estimated. When a single amount cannot be reasonably estimated but the cost can be estimated within a range, the Company accrues an amount based on management’s best estimate considering all facts and circumstances. The Company expenses legal costs, including those expected to be incurred in connection with a loss contingency, as incurred.
Medical Device Excise Tax
The Patient Protection and Affordable Care Act of 2010 imposed a medical device excise tax on medical device manufacturers on their sales in the United States of certain devices, which was effective January 1, 2013. The excise tax is 2.3% of the taxable base and applied to a substantial majority of the Company’s sales. Effective January 1, 2016, the excise tax was suspended until the end of 2017, and in January 2018, another temporary two year suspension of the tax was passed, extending the suspension to December 31, 2019. The Company incurred $1,273 of expense related to the tax during the year ended June 30, 2016.
F-10
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Income Taxes
Deferred income taxes are recorded to reflect the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts based on enacted tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
Developing a provision for income taxes, including the effective tax rate and the analysis of potential tax exposure items, if any, requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets. The Company’s judgment and tax strategies are subject to audit by various taxing authorities.
Accounting guidance requires that accounting for uncertainty in income taxes is recognized in the financial statements. The guidance provides that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits of the position. Income tax positions must meet a more-likely-than-not recognition threshold to be recognized. The guidance also provides rules on measurement, derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
Research and Development Expenses
Research and development expenses include costs associated with the design, development, testing, enhancement and regulatory approval of the Company’s products. Research and development expenses include employee compensation (including stock-based compensation), supplies and materials, consulting expenses, patent amortization, travel and facilities overhead. The Company also incurs significant expenses to operate clinical trials, including trial design, third-party fees, clinical site reimbursement, data management and travel expenses. Research and development expenses are expensed as incurred. Expenses associated with patent applications are capitalized and amortized using the straight-line method over their remaining estimated lives. Patent amortization begins at the time of patent application approval, and does not exceed 20 years.
Concentration of Credit Risk
Financial instruments that potentially expose the Company to concentration of credit risk consist primarily of cash and cash equivalents, marketable securities and accounts receivable.
The Company maintains its cash balances primarily with one financial institution. These balances exceed federally insured limits. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant credit risk in cash and cash equivalents.
The Company believes that the credit risk related to marketable securities is limited due to the adherence to an investment policy and that credit risk related to accounts receivable is limited due to a large customer base.
Marketable Securities
The Company’s marketable securities consist solely of available-for-sale securities and were valued in accordance with the fair value measurement guidance discussed below. Available-for-sale securities are carried at fair value with unrealized gains and losses reported as a component of stockholders’ equity as accumulated other comprehensive income (loss), net of tax. Realized gains and losses, if any, are calculated on the specific identification method and are included in interest and other, net in the consolidated statements of operations.
Available-for-sale securities are reviewed for possible impairment at least quarterly, or more frequently if circumstances arise that may indicate impairment. When the fair value of the securities declines below the amortized cost basis, impairment is indicated and it must be determined whether it is other than temporary. Impairment is considered to be other than temporary if the Company: (i) intends to sell the security, (ii) will more likely than not be forced to sell the security before recovering its cost, or (iii) does not expect to recover the security’s amortized cost basis. If the decline in fair value is considered other than temporary, the cost basis of the security is adjusted to its fair market value and the realized loss is reported in earnings. Subsequent increases or decreases in fair value are reported in equity as accumulated other comprehensive income (loss).
F-11
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Cost Method Investments
When the Company controls less than 20% of the decision-making ability over the operations of the investee, the investment is accounted for at cost. Cost method investments are reviewed quarterly for any events or changes in circumstances that may have a significant adverse effect on the fair value of the investment. When the fair value declines below the cost, an impairment loss is recognized unless it is considered temporary. Impairment is considered to be other than temporary if the length of time it will take for the investment’s value to exceed its cost is greater than the Company’s intent and ability to hold the investment.
Fair Value Measurements
Under the authoritative guidance for fair value measurements, fair value is defined as the exit price, or the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants as of the measurement date. The authoritative guidance also establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability developed based upon the best information available in the circumstances. The categorization of financial assets and financial liabilities within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
The hierarchy is broken down into three levels defined as follows:
Level 1 Inputs — quoted prices in active markets for identical assets and liabilities
Level 2 Inputs — observable inputs other than quoted prices in active markets for identical assets and liabilities
Level 3 Inputs — unobservable inputs
As of June 30, 2018, the Company believes that the carrying amounts of its other financial instruments, including accounts receivable, accounts payable and accrued liabilities, approximate their fair value due to the short-term maturities of these instruments. See Note 4 for additional information.
Use of Estimates
The preparation of the Company’s consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates and these differences could be material.
Stock-Based Compensation
The Company has stock-based compensation plans, which include stock options, nonvested share awards, and an employee stock purchase plan. Fair value of option awards is determined using option-pricing models, fair value of nonvested share awards with market conditions is determined using the Monte Carlo simulation, and fair value of nonvested share awards that vest based upon performance or service conditions is determined by the closing market price of the Company’s stock on the date of grant. Stock-based compensation expense is recognized ratably over the requisite service period for the awards expected to vest.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued amended revenue recognition guidance to clarify the principles for recognizing revenue from contracts with customers. The guidance requires an entity to recognize revenue in an amount that reflects the consideration to which an entity expects to be entitled in exchange for the transfer of goods or services. The guidance also requires expanded disclosures relating to the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers. Additionally, qualitative and quantitative disclosures are required about customer contracts, significant judgments and changes in judgments, and assets recognized from the costs to obtain or fulfill a contract.
The Company has completed an adoption plan, which included a review of customer contracts, applying the five-step model of the new standard to the customer contracts and comparing the results to the Company’s current accounting. The Company has elected the modified retrospective method of adoption, which the Company anticipates will not result in a material adjustment
F-12
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
to retained earnings. The Company’s revenue recognition will remain largely unchanged given the majority of the Company’s revenue arrangements consist of a single performance obligation that will be recognized at a point in time when control passes to the customer. As a result, the Company does not anticipate a material impact on the amount and timing of revenue recognized in the Company’s consolidated financial statements.
The Company is also implementing new internal controls around the judgments needed to address risks associated with applying the five-step model, which includes identifying performance obligations and judgments made related to variable consideration. Effective July 1, 2018, the Company will be revising its revenue recognition accounting policy and disclosures to reflect the requirements of the amended revenue recognition guidance. The Company will continue to monitor any modifications or interpretations communicated by the FASB that may impact any of its final assessments related to the guidance.
In January 2016, the FASB issued ASU 2016-01, “Recognition and Measurement of Financial Assets and Financial Liabilities,” which revises the accounting requirements related to the classification and measurement of investments in equity securities and the presentation of certain fair value changes for financial liabilities measured at fair value. The update also changes certain disclosure requirements associated with the fair value of financial instruments. These changes will require an entity to measure, at fair value, investments in equity securities and recognize the changes in fair value within net income. ASU 2016-01 will be applied on a modified retrospective basis to all outstanding instruments, with an adjustment recorded to opening retained earnings as of the beginning of the first period in which the guidance becomes effective. The guidance is effective for annual periods beginning after December 15, 2017, including interim periods within those fiscal years, with early adoption permitted. The guidance is effective for the Company on July 1, 2018. The Company does not anticipate a material impact on its financial statements upon adoption.
In February 2016, the FASB issued ASU 2016-02, “Leases.” The guidance requires lessees to recognize the assets and liabilities that arise from leases on the balance sheet. ASU 2016-02 is effective for annual periods beginning after December 15, 2018, including interim periods within those annual periods, and should be applied using a modified retrospective approach. Early adoption is permitted. The guidance is effective for the Company on July 1, 2019. The Company is currently evaluating the timing, method of adoption and impact of the new lease guidance on its financial statements.
In June 2016, the FASB issued ASU 2016-13, “Measurement of Credit Losses on Financial Instruments,” which revises guidance for the accounting for credit losses on financial instruments within its scope. The new standard introduces an approach, based on expected losses, to estimate credit losses on certain types of financial instruments and modifies the impairment model for available-for-sale debt securities. The new approach to estimating credit losses (referred to as the current expected credit losses model) applies to most financial assets measured at amortized cost and certain other instruments, including trade and other receivables, loans, held-to-maturity debt securities, net investments in leases and off-balance-sheet credit exposures. ASU 2016-13 is effective for annual periods beginning after December 15, 2019, including interim periods within those fiscal years. Early adoption is permitted and should be applied as a cumulative-effect adjustment to retained earnings as of the beginning of the first reporting period in which the guidance is adopted. The guidance is effective for the Company on July 1, 2020. The Company does not anticipate a material impact on its financial statements upon adoption.
In May 2017, the FASB issued ASU 2017-09, “Scope of Modification Accounting” which clarifies when changes to the terms or conditions of a share-based payment award must be accounted for as modifications. The new guidance will reduce diversity in practice and result in fewer changes to the terms of an award being accounted for as modifications. ASU 2017-09 will be applied prospectively to awards modified on or after the adoption date. The guidance is effective for annual periods, and interim periods within those annual periods beginning after December 15, 2017, with early adoption permitted. The Company does not anticipate a material impact on its financial statements upon adoption.
F-13
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
2. Selected Consolidated Financial Statement Information
Accounts Receivable, Net
Accounts receivable consists of the following:
June 30, | |||||||
2018 | 2017 | ||||||
Accounts receivable | $ | 32,025 | $ | 29,336 | |||
Less: Allowance for doubtful accounts | (800 | ) | (864 | ) | |||
Accounts receivable, net | $ | 31,225 | $ | 28,472 | |||
Inventories
Inventories consist of the following:
June 30, | |||||||
2018 | 2017 | ||||||
Raw materials | $ | 6,820 | $ | 7,898 | |||
Work in process | 1,315 | 1,221 | |||||
Finished goods | 8,470 | 7,778 | |||||
Inventories | $ | 16,605 | $ | 16,897 | |||
Property and Equipment, Net
Property and equipment consists of the following:
June 30, | |||||||
2018 | 2017 | ||||||
Land | $ | 500 | $ | 500 | |||
Building | 22,420 | 22,420 | |||||
Equipment | 16,510 | 16,502 | |||||
Furniture | 2,709 | 2,709 | |||||
Leasehold improvements | 438 | 86 | |||||
Construction in progress | 1,110 | 421 | |||||
43,687 | 42,638 | ||||||
Less: Accumulated depreciation | (15,943 | ) | (12,942 | ) | |||
Total Property and equipment, net | $ | 27,744 | $ | 29,696 | |||
On December 29, 2016, the Company entered into a Purchase and Sale Agreement, as subsequently amended (collectively, the “Sale Agreement”), with Krishna Holdings, LLC (the “Buyer”), providing for the sale to Buyer of the Company’s headquarters facility in St. Paul, Minnesota (the “Facility”), for a cash purchase price of $21,500. On March 30, 2017, the sale of the Facility under the Sale Agreement closed. The Company received proceeds of approximately $20,944 ($21,500, less $556 of transaction expenses). The net proceeds are to be used for working capital and general corporate purposes. In connection with the sale, the Company recorded an impairment charge of $158.
Under the Sale Agreement, the Company entered into a Lease Agreement (the “Lease Agreement”) with Krishna Holdings, LLC, Apex Holdings, LLC, Kashi Associates, LLC, Keva Holdings, LLC, S&V Ventures, LLC, Polo Group LLC, SPAV Holdings LLC, Star Associates LLC, and The Global Villa, LLC. As the lease terms resulted in a capital lease classification, the Company accounted for the sale and leaseback of the Facility as a financing transaction where the assets remain on the Company’s balance sheet. See Note 3 for further discussion on future payment obligations under the Lease Agreement.
F-14
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Patents, net
Patents, net consist of the following:
June 30, | |||||||
2018 | 2017 | ||||||
Patents | $ | 6,435 | $ | 6,056 | |||
Less: Accumulated amortization | (1,204 | ) | (1,000 | ) | |||
Total Patents, net | $ | 5,231 | $ | 5,056 | |||
As of June 30, 2018, future estimated amortization of patents is as follows:
2019 | $ | 199 | |
2020 | 193 | ||
2021 | 191 | ||
2022 | 181 | ||
2023 | 174 | ||
Thereafter | 4,293 | ||
$ | 5,231 | ||
This future amortization expense is an estimate. Actual amounts may vary from these estimated amounts due to additional intangible asset acquisitions, approval of patents-in-process, potential impairment, change in useful life or other events.
Accrued Expenses
Accrued expenses consist of the following:
June 30, | |||||||
2018 | 2017 | ||||||
Commissions | $ | 7,234 | $ | 8,217 | |||
Salaries and bonus | 6,624 | 8,247 | |||||
Accrued vacation | 3,557 | 3,436 | |||||
Accrued excise, sales and other taxes | 3,522 | 3,497 | |||||
Legal settlement | 1,847 | 1,814 | |||||
Clinical studies | 1,422 | 657 | |||||
Accrued litigation | — | 2,600 | |||||
Other accrued expenses | 1,570 | 1,768 | |||||
Total Accrued expenses | $ | 25,776 | $ | 30,236 | |||
Legal Settlement
On June 28, 2016, the Company entered into a Settlement Agreement (the “Settlement Agreement”) with the United States of America, acting through the Department of Justice (the “DOJ”) and on behalf of the Office of Inspector General of the Department of Health and Human Services, and Travis Thams, to resolve the investigation by the DOJ and the civil action underlying such investigation. Under the Settlement Agreement, the Company agreed to pay $8,000 (the “Settlement Amount”), as follows: an initial payment of $3,000, paid on July 1, 2016, with the remaining $5,000, which bears interest at 1.8% per annum, payable in 11 equal quarterly installments, beginning January 1, 2017. The amount payable within the next twelve months is included in accrued expenses (as noted in the table above) with the long-term portion included in other liabilities (as noted in the table below). Under the Settlement Agreement, if the Company makes a single payment in excess of $2,000, which payment is not covered by an insurance policy, in settlement of any claims before paying the full Settlement Amount, the remaining unpaid balance of the Settlement Amount will become immediately due and payable, with interest accruing on the unpaid principal portion at an interest rate of 1.8% per annum.
F-15
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Restructuring
On March 31, 2016, the Company announced a restructuring to reduce costs as part of its plan to progress towards profitability and positive cash flow. As a result, the Company recorded a restructuring expense of $2,364 during the year ended June 30, 2016, which was comprised of severance and other employee related costs. There was $22 and $169 of accrued restructuring costs recorded as accrued expenses on the consolidated balance sheet as of June 30, 2018 and June 30, 2017, respectively.
Other Liabilities
The Company’s non-current other liabilities consist of the following:
June 30, | |||||||
2018 | 2017 | ||||||
Legal settlement | $ | 467 | $ | 2,314 | |||
Deferred compensation | 395 | 519 | |||||
Deferred grant incentive | 460 | 473 | |||||
Other liabilities | 90 | 173 | |||||
Total Other liabilities | $ | 1,412 | $ | 3,479 | |||
3. Debt
Revolving Credit Facility
On March 31, 2017, the Company entered into a Loan and Security Agreement (the “Loan Agreement”) with Silicon Valley Bank (“SVB”). The Loan Agreement provides for a senior, secured revolving credit facility (the “Revolver”) of $40,000 (the “Maximum Dollar Amount”).
Advances under the Revolver may be made from time to time up to the Maximum Dollar Amount, subject to certain borrowing limitations. The Revolver has a maturity date of March 31, 2020 and bears interest at a floating per annum rate equal to the Wall Street Journal prime rate, less 0.25%. Interest on borrowings is due monthly and the principal balance is due at maturity. Borrowings up to $10,000 are available on a non-formula basis. Borrowings above $10,000 are based on (i) 85% of eligible domestic accounts receivable, and (ii) the lesser of 50% of eligible inventory or $5,000, subject to adjustment as defined in Loan Agreement. Upon the Revolver’s maturity, any outstanding principal balance, unpaid accrued interest, and all other obligations under the Revolver will be due and payable. The Company will incur a fee equal to 1% of the Maximum Dollar Amount upon termination of the Loan Agreement or the Revolver for any reason prior to the maturity date, unless refinanced with SVB.
The Company’s obligations under the Loan Agreement are secured by certain of the Company’s assets, including, among other things, accounts receivable, deposit accounts, inventory, equipment, general intangibles and records pertaining to the foregoing. The collateral does not include the Company’s intellectual property, but the Company has agreed not to encumber its intellectual property without the consent of SVB. The Loan Agreement contains customary covenants limiting the Company’s ability to, among other things, incur debt or liens, make certain investments and loans, enter into transactions with affiliates, undergo certain fundamental changes, dispose of assets, or change the nature of its business. In addition, the Loan Agreement contains financial covenants requiring the Company to maintain, at all times when any amounts are outstanding under the Revolver, either (i) minimum unrestricted cash at SVB and unused availability on the Revolver of at least $10,000 or (ii) minimum trailing three-month Adjusted EBITDA of $1,000. If the Company does not comply with the various covenants under the Loan Agreement, the interest rate on outstanding amounts will increase by 5% and SVB may, subject to various customary cure rights, decline to provide additional advances under the Revolver, require the immediate payment of all amounts outstanding under the Revolver, and foreclose on all collateral.
Under the Loan Agreement, the Company paid SVB a non-refundable commitment fee of $80, which is being amortized to interest expense over the term of the Loan Agreement. The Company is required to pay a fee equal to 0.35% per annum on the unused portion of the Revolver, payable quarterly in arrears. SVB’s obligations to advance funds under the Revolver are subject to an initial collateral examination to be completed within 90 days of the effective date of the Loan Agreement. The Company is not obligated to draw any funds under the Revolver and no amounts were outstanding as of June 30, 2018 and 2017.
F-16
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Financing Obligation
In connection with the sale of the Facility, the Company entered into a Lease Agreement to lease the Facility. The Lease Agreement has an initial term of 15 years, with four consecutive renewal options of 5 years each at the Company’s option, with a base annual rent in the first year of $1,638 and annual escalations of 3% thereafter. Rent during subsequent renewal terms will be at the then fair market rental rate. The effective interest rate is 7.89%.
Future payments under the initial term of the Lease Agreement as of June 30, 2018 are as follows:
2019 | $ | 1,699 | |
2020 | 1,750 | ||
2021 | 1,803 | ||
2022 | 1,857 | ||
2023 | 1,913 | ||
Thereafter | 19,375 | ||
$ | 28,397 | ||
4. Fair Value
The available-for-sale marketable securities are primarily comprised of investments with a fixed income and equity investments and consist of the following, measured at fair value on a recurring basis:
Fair Value Measurements as of June 30, 2018 Using Inputs Considered as | |||||||||||||
Fair Value | Level 1 | Level 2 | Level 3 | ||||||||||
Mutual funds | $ | 544 | 199 | 345 | $ | — | |||||||
Total marketable securities | $ | 544 | 199 | 345 | $ | — | |||||||
Fair Value Measurements as of June 30, 2017 Using Inputs Considered as | |||||||||||||
Fair Value | Level 1 | Level 2 | Level 3 | ||||||||||
Mutual funds | $ | 704 | 281 | 423 | $ | — | |||||||
Total marketable securities | $ | 704 | 281 | 423 | $ | — | |||||||
During the years ended June 30, 2018 and 2017, there were no purchases of available-for-sale securities and $194 and $40, respectively, of available-for-sale securities that were sold. There were no other-than-temporary impairments during the years ended June 30, 2018 and 2017. During the years ended June 30, 2018 and 2017, there was a realized gain of $34 and $6 that was recorded within interest income and other, net on the consolidated statement of operations.
There were no transfers of assets between Level 1 and Level 2 of the fair value measurement hierarchy during the year ended June 30, 2018. Any transfers between levels are recognized on the date of the event or when a change in circumstances causes a transfer.
Cost Method Investment
The Company holds a cost method investment measured at fair value on a nonrecurring basis and is classified as a Level 3 investment. As of June 30, 2018, the cost of the investment was $2,538. There were no identified events or changes that had a significant adverse effect on the fair value of the investment. The investment is recorded within other long term assets on the consolidated balance sheet.
F-17
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
5. Stock Options and Restricted Stock Awards
On November 15, 2017, the Company’s stockholders approved the 2017 Equity Incentive Plan (the “2017 Plan”) for the purpose of granting equity awards to employees, directors, and consultants. The 2017 Plan replaced the 2014 Equity Incentive Plan (the “2014 Plan”), and no further equity awards may be granted under the 2014 Plan or the 2007 Equity Incentive Plan (the “2007 Plan”) (the 2017 Plan, 2014 Plan and the 2007 Plan are collectively referred to as the “Plans”).
The 2017 Plan allows for the granting of up to 3,607,523 shares of common stock as approved by the Board of Directors or committees thereof in the form of nonqualified or incentive stock options, restricted stock awards, restricted stock unit awards, performance share awards, performance unit awards or stock appreciation rights to officers, directors, consultants and employees of the Company.
Stock Options
All options granted under the Plans become exercisable over periods established at the date of grant. The option exercise price is generally not less than the estimated fair market value of the Company’s common stock at the date of grant, as determined by the Company’s management and Board of Directors. In addition, the Company has granted nonqualified stock options to a director outside of the Plans.
Stock option activity is as follows:
Number of Options | Weighted Average Exercise Price | |||||
Options outstanding at June 30, 2015 | 699,872 | $ | 10.32 | |||
Exercised | (87,817 | ) | $ | 11.46 | ||
Forfeited or expired | (5,176 | ) | $ | 12.37 | ||
Options outstanding at June 30, 2016 | 606,879 | $ | 10.14 | |||
Exercised | (519,297 | ) | $ | 10.33 | ||
Expired | (9,381 | ) | $ | 8.83 | ||
Options outstanding at June 30, 2017 | 78,201 | $ | 9.07 | |||
Exercised | (55,880 | ) | $ | 9.20 | ||
Options outstanding at June 30, 2018 | 22,321 | $ | 8.75 | |||
As of June 30, 2018, all options were fully vested. An employee’s vested options must be exercised at or within 90 days of termination to avoid forfeiture. The Company determined the fair value of options using the Black-Scholes option pricing model. The estimated fair value of options, including the effect of estimated forfeitures, was recognized as expense on a straight-line basis over the options’ vesting periods. There were no options granted during the years ended June 30, 2018, 2017 or 2016.
The aggregate intrinsic value of a stock option award is the amount by which the market value of the underlying stock exceeds the exercise price of the award. The aggregate intrinsic value for vested and outstanding options at June 30, 2018, 2017 and 2016, was $527, $1,811 and $4,025, respectively. The total aggregate intrinsic value of options exercised during the years ended June 30, 2018, 2017 and 2016, was $1,095, $7,955, and $417, respectively. Cash received from option exercises was $514, $5,363 and $1,006 for the years ended June 30, 2018, 2017 and 2016, respectively. The weighted average remaining contractual life of options outstanding at June 30, 2018 was 0.67 years. Shares supporting option exercises are sourced from new share issuances.
Restricted Stock
The fair value of each restricted stock award was equal to the fair market value of the Company’s common stock at the date of grant. Vesting of time based restricted stock awards ranges from one to three years. The estimated fair value of restricted stock awards, including the effect of estimated forfeitures, is recognized on a straight-line basis over the restricted stock’s vesting period.
F-18
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Restricted stock award activity is as follows:
Number of Shares | Weighted Average Grant Date Fair Value | |||||
Outstanding at June 30, 2015 | 843,094 | $ | 25.16 | |||
Granted | 522,415 | $ | 19.30 | |||
Forfeited | (230,710 | ) | $ | 24.83 | ||
Vested | (487,226 | ) | $ | 22.27 | ||
Outstanding at June 30, 2016 | 647,573 | $ | 23.24 | |||
Granted | 258,346 | $ | 21.80 | |||
Forfeited | (103,140 | ) | $ | 22.11 | ||
Vested | (316,195 | ) | $ | 24.21 | ||
Outstanding at June 30, 2017 | 486,584 | $ | 21.26 | |||
Granted | 290,856 | $ | 27.93 | |||
Forfeited | (68,499 | ) | $ | 22.76 | ||
Vested | (253,725 | ) | $ | 22.87 | ||
Outstanding at June 30, 2018 | 455,216 | $ | 24.77 | |||
Total fair value of time-based restricted stock that vested during fiscal 2018, 2017 and 2016 was $5,803, $7,655, and $10,851, respectively. Estimated pre-vesting forfeitures are considered in determining stock-based compensation expense. As of June 30, 2018, 2017 and 2016, the Company estimated its weighted average forfeiture rate at 15.2%, 17.1% and 17.0%, respectively. As of June 30, 2018, there was approximately $8,171 of total unrecognized compensation expense, net of the effect of estimated forfeitures, related to nonvested restricted stock awards, which is expected to be recognized over a weighted-average period of 1.6 years.
Performance-Based Restricted Stock
The Company also grants performance-based restricted stock awards to certain executives and other management. Fiscal 2018 awards vest based on the Company’s total shareholder return relative to total shareholder return of the peer group (a market condition), as measured by the closing prices of the stock of the Company and its peer group for the 90 trading days preceding July 1, 2017 compared to the closing prices for the 90 trading days preceding July 1, 2020. Fiscal 2017 awards vest based on the Company’s total shareholder return relative to total shareholder return of the peer group (a market condition), as measured by the closing prices of the stock of the Company and its peer group for the 90 trading days preceding July 1, 2016 compared to the closing prices for the 90 trading days preceding July 1, 2019. Fiscal 2016 awards included grants that vested based upon the achievement of certain thresholds measuring total shareholder return during periods within the fiscal year as compared to a pre-determined peer group of companies, and grants that vested based upon achievement of certain thresholds measuring annual revenue growth during the fiscal year as compared to a pre-determined peer group of companies. The aggregate maximum shares granted were as follows:
Performance Measurement | 2018 | 2017 | 2016 | ||||||
Total shareholder return | 278,889 | 336,826 | 156,509 | ||||||
Annual revenue growth | N/A | N/A | 156,509 | ||||||
The results of the Company’s performance based restricted stock awards for fiscal 2016 was a 0% achievement and zero shares vesting for both performance measurements.
F-19
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Performance-based restricted stock award activity is as follows:
Number of Shares | Weighted Average Grant Date Fair Value | |||||
Outstanding at June 30, 2015 | 152,224 | $ | 22.03 | |||
Granted | 313,018 | $ | 16.67 | |||
Forfeited | (52,680 | ) | $ | 28.64 | ||
Vested | (102,451 | ) | $ | 25.58 | ||
Outstanding at June 30, 2016 | 310,111 | $ | 16.67 | |||
Granted | 336,826 | $ | 11.97 | |||
Forfeited | (328,353 | ) | $ | 16.41 | ||
Outstanding at June 30, 2017 | 318,584 | $ | 11.97 | |||
Granted | 278,889 | $ | 13.63 | |||
Forfeited | (66,295 | ) | $ | 13.17 | ||
Outstanding at June 30, 2018 | 531,178 | $ | 12.69 | |||
Total fair value of performance-based restricted stock that vested during fiscal 2018, 2017 and 2016 was $0, $0, and $2,621, respectively. Estimated pre-vesting forfeitures are considered in determining stock-based compensation expense. As of June 30, 2018, there was approximately $2,791 of total unrecognized compensation expense related to nonvested performance-based restricted stock awards, which is expected to be recognized over a weighted-average period of 1.6 years.
Restricted Stock Units
The Company grants restricted stock units to members of its Board of Directors. Restricted stock units represent the right to receive payment in the form of shares of the Company’s common stock or in cash at the Company’s option. Restricted stock unit payments would occur within 30 days following the six month anniversary of the date that the director ceases to serve on the Board of Directors or, if the restricted stock units are granted in lieu of an annual cash retainer, on the payment date selected by the director that is at least two years after the grant date. The estimated fair value of restricted stock awards is recognized on a straight-line basis over the vesting period.
Restricted stock unit activity is as follows:
Number of Shares | Weighted Average Grant Date Fair Value | |||||
Restricted stock units outstanding at June 30, 2015 | 262,943 | $ | 12.62 | |||
Granted | 47,586 | $ | 22.27 | |||
Converted to common stock | (5,713 | ) | $ | 22.18 | ||
Restricted stock units outstanding at June 30, 2016 | 304,816 | $ | 13.95 | |||
Granted | 54,064 | $ | 21.21 | |||
Forfeited | (2,974 | ) | $ | 21.01 | ||
Converted to common stock | (6,476 | ) | $ | 29.34 | ||
Restricted stock units outstanding at June 30, 2017 | 349,430 | $ | 14.73 | |||
Granted | 28,364 | $ | 31.02 | |||
Converted to common stock | (41,925 | ) | $ | 16.07 | ||
Restricted stock units outstanding at June 30, 2018 | 335,869 | $ | 15.94 | |||
F-20
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Stock-Based Compensation Expense
The following amounts were recognized as stock-based compensation expense in the consolidated statements of operations:
Year Ended June 30, 2018 | Restricted Stock Awards | Employee Stock Purchase Plan | Restricted Stock Units | Total | ||||||||||||
Cost of goods sold | $ | 207 | $ | 68 | $ | — | $ | 275 | ||||||||
Selling, general and administrative | 7,462 | 848 | 750 | 9,060 | ||||||||||||
Research and development | 817 | 150 | — | 967 | ||||||||||||
Total stock-based compensation expense | $ | 8,486 | $ | 1,066 | $ | 750 | $ | 10,302 | ||||||||
Year Ended June 30, 2017 | Restricted Stock Awards | Employee Stock Purchase Plan | Restricted Stock Units | Total | ||||||||||||
Cost of goods sold | $ | 588 | $ | 101 | $ | — | $ | 689 | ||||||||
Selling, general and administrative | 6,568 | 1,065 | 1,024 | 8,657 | ||||||||||||
Research and development | 879 | 129 | — | 1,008 | ||||||||||||
Total stock-based compensation expense | $ | 8,035 | $ | 1,295 | $ | 1,024 | $ | 10,354 | ||||||||
Year Ended June 30, 2016 | Restricted Stock Awards | Employee Stock Purchase Plan | Restricted Stock Units | Total | ||||||||||||
Cost of goods sold | $ | 679 | $ | 115 | $ | — | $ | 794 | ||||||||
Selling, general and administrative | 8,215 | 1,167 | 1,000 | 10,382 | ||||||||||||
Research and development | 1,681 | 120 | — | 1,801 | ||||||||||||
Total stock-based compensation expense | $ | 10,575 | $ | 1,402 | $ | 1,000 | $ | 12,977 | ||||||||
Shares Available for Grant
The following summarizes shares available for grant under the 2017 Plan:
Reserved | 3,607,523 | |
Transferred from 2014 Plan | (1,057,523 | ) |
Granted | (81,060 | ) |
Forfeited or cancelled | 95,525 | |
Shares available for grant at June 30, 2018 | 2,564,465 | |
6. Employee Stock Purchase Plan
The Company maintains an employee stock purchase plan that was approved by the Company’s stockholders in November 2015 (“2015 ESPP”) and replaced the previous employee stock purchase plan that expired on May 31, 2016. The 2015 ESPP provides eligible employees the opportunity to acquire common stock in accordance with Section 423 of the Internal Revenue Code of 1986. Stock can be purchased each six-month period per year (twice per year). The purchase price is equal to 85% of the lower of the price at the beginning or the end of the respective period. Employees purchased 158,939 shares at an average price of $20.40 per share during the year ended June 30, 2018. Shares reserved under the 2015 ESPP at June 30, 2018 totaled 1,685,431.
F-21
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
7. Income Taxes
The components of the Company’s overall deferred tax assets and liabilities are as follows:
June 30, | |||||||
2018 | 2017 | ||||||
Deferred tax assets | |||||||
Stock-based compensation | $ | 2,505 | $ | 5,107 | |||
Deferred revenue | 2,351 | — | |||||
Accrued expenses | 970 | 1,650 | |||||
Inventories | 305 | 433 | |||||
Compensation accruals | 133 | 261 | |||||
Depreciation and amortization | 320 | 409 | |||||
Other | 397 | 1,019 | |||||
Research and development credit carryforwards | 5,048 | 4,650 | |||||
Net operating loss carryforwards | 64,101 | 87,502 | |||||
Total deferred tax assets | 76,130 | 101,031 | |||||
Valuation allowance | (76,130 | ) | (101,031 | ) | |||
Net deferred tax assets | $ | — | $ | — | |||
On December 22, 2017, the United States enacted tax reform legislation commonly known as the Tax Cuts and Jobs Act (the “Act”), resulting in significant modifications to existing law. Accounting for the income tax effects of the Act which impact our tax provision has been completed as of the current year and included in the Company’s financial statements as of June 30, 2018. As a result of the Act, the Company remeasured deferred tax assets and liabilities from 34% to 21%, but given the Company is in a full valuation allowance position, there was no tax expense impact.
The Company has established valuation allowances to fully offset its deferred tax assets due to the uncertainty about the Company’s ability to generate the future taxable income necessary to realize these deferred assets, particularly in light of the Company’s historical losses. The future use of net operating loss carryforwards is dependent on the Company attaining profitable operations, and may be limited in any one year under Internal Revenue Code Section 382 due to significant ownership changes, as defined under such Section, as a result of the Company’s equity financings. A summary of the valuation allowances are as follows:
Balances at June 30, 2015 | $ | 84,319 | |
Additions | 16,898 | ||
Balance at June 30, 2016 | 101,217 | ||
Reductions | (186 | ) | |
Balance at June 30, 2017 | 101,031 | ||
Reductions | (24,901 | ) | |
Balance at June 30, 2018 | $ | 76,130 | |
As of June 30, 2018 and 2017, the Company had federal tax NOL carryforwards of approximately $275,030 and $287,598, respectively. These NOL carryforwards are available to offset taxable income through 2037. The Company also had various state NOL carryforwards available to offset future state taxable income. These state NOL carryforwards typically have the same expirations as the Company’s federal tax NOL carryforwards.
As of June 30, 2018 and 2017, the Company had approximately $4,244 and $4,137 of federal research and development credit carryforwards, respectively. As of June 30, 2018 and 2017, the Company had approximately $1,560 and $1,729 of state research and development credit carryforwards. The federal and state research and development credit carryforwards will expire through fiscal 2037 and 2032, respectively.
As required by FASB ASC Topic 740, “Income Taxes,” the Company recognizes the financial statement benefit of a tax position only after determining that the relevant tax authority would more likely than not sustain the position following an
F-22
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
audit. For tax positions meeting the more likely than not threshold, the amount recognized in the financial statements is the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate settlement with the relevant tax authority. The Company recorded a liability relating to unrecognized tax benefits of $597 and $570 at June 30, 2018 and 2017, respectively. Due to the Company having a full valuation allowance, this liability has been netted against the deferred tax asset. The Company recognizes interest and penalties related to uncertain tax provisions as part of the provision for income taxes. The Company has not currently reserved for any interest or penalties for such reserves due to the Company being in an NOL position. The Company does not expect to recognize any benefits from the unrecognized tax benefits within the next twelve months. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
Balances at June 30, 2015 | $ | 494 | |
Increases related to prior year tax positions | 10 | ||
Increases related to current year tax positions | 41 | ||
Balances at June 30, 2016 | 545 | ||
Decreases related to prior year tax positions | (8 | ) | |
Increases related to current year tax positions | 33 | ||
Balance at June 30, 2017 | 570 | ||
Decreases related to prior year tax positions | (3 | ) | |
Increases related to current year tax positions | 30 | ||
Balance at June 30, 2018 | $ | 597 | |
The Company is subject to income taxes in the U.S. federal jurisdiction and various state jurisdictions. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment to apply. The Company is potentially subject to income tax examinations by tax authorities for the tax years ended June 30, 2018, 2017, 2016, and 2015. The Company is not currently under examination by any taxing jurisdiction.
8. Commitments and Contingencies
Operating Leases
The Company leases manufacturing space, equipment and apartments under lease agreements that expire at various dates through March 2020. Rental expenses were $652, $656, and $1,049, for the years ended June 30, 2018, 2017, and 2016, respectively. In March 2017, the Company sold the Facility and began leasing the Facility.
Future minimum lease payments under the agreements as of June 30, 2018 are as follows:
2019 | $ | 510 | |
2020 | 376 | ||
2021 | 20 | ||
2022 | — | ||
2023 | — | ||
Thereafter | — | ||
$ | 906 | ||
Other Matters
In the ordinary conduct of business, the Company is subject to various lawsuits and claims covering a wide range of matters including, but not limited to, employment claims and commercial disputes. While the outcome of these matters is uncertain, the Company does not believe there are any significant matters as of June 30, 2018 that are probable or estimable, for which the outcome is reasonably possible of having a material adverse impact on its consolidated balance sheets or statements of operations.
F-23
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
9. Employee Benefits
The Company offers a 401(k) plan to its employees. Eligible employees may authorize up to $19 of their annual compensation as a contribution to the plan, subject to Internal Revenue Service limitations. The plan also allows eligible employees over 50 years old to contribute an additional $6 subject to Internal Revenue Service limitations. All employees must be at least 21 years of age to participate in the plan. The Company did not provide any employer matching contributions for the years ended June 30, 2018, 2017, and 2016.
The Company offers certain members of management and highly compensated employees the opportunity to defer up to 100% of their base salary (after 401(k), payroll tax and other deductions), performance bonus and discretionary bonus and elect to receive the deferred compensation at a fixed future date of participant’s choosing. Each participant may, at the time of his or her deferral election, choose to allocate the deferred compensation into investment alternatives set by the Human Resources and Compensation Committee. The amount payable to each participant under the plan will change in value based upon the investment selected by that participant and is classified as current or long-term on the Company’s balance sheet based on the disbursement elections made by the participants. As of June 30, 2018, $149 of the amount is included in accrued expenses and $395 is included in other liabilities on the consolidated balance sheet.
10. Earnings Per Share
The following table presents a reconciliation of the numerators and denominators used in the basic and diluted earnings per common share computations (in thousands except share and per share amounts):
Year Ended June 30, | |||||||||||
2018 | 2017 | 2016 | |||||||||
Numerator | |||||||||||
Net income (loss) | $ | 1,712 | $ | (1,792 | ) | $ | (56,024 | ) | |||
Income allocated to participating securities | (19 | ) | — | — | |||||||
Net income (loss) available to common stockholders | $ | 1,693 | $ | (1,792 | ) | $ | (56,024 | ) | |||
Denominator | |||||||||||
Weighted average common shares outstanding — basic | 33,145,140 | 32,373,709 | 32,537,621 | ||||||||
Effect of dilutive stock options(1) | 15,039 | — | — | ||||||||
Effect of dilutive restricted stock units(2) | 335,869 | — | — | ||||||||
Effect of performance-based restricted stock awards (3) | 118,212 | — | — | ||||||||
Weighted average common shares outstanding — diluted | 33,614,260 | 32,373,709 | 32,537,621 | ||||||||
Earnings per common share — basic | $ | 0.05 | $ | (0.06 | ) | $ | (1.72 | ) | |||
Earnings per common share — diluted | $ | 0.05 | $ | (0.06 | ) | $ | (1.72 | ) | |||
(1) | At June 30, 2018, 2017, and 2016; 22,321, 78,201, and 606,879 stock options, respectively, were outstanding. The effect of the shares that would be issued upon exercise of these options has been excluded from the calculation of diluted loss per share because those shares are anti-dilutive. |
(2) | At June 30, 2018, 2017, and 2016; 335,869, 349,430 and 304,816 additional shares of common stock, respectively, were issuable upon the settlement of outstanding restricted stock units. The effect of the shares that would be issued upon settlement of these restricted stock units has been excluded from the calculation of diluted loss per share because those shares are anti-dilutive. |
(3) | At June 30, 2018 and 2017, 531,178 and 318,584, respectively, of performance-based restricted stock awards were outstanding. The effect of the shares that would be issued upon vesting of these awards has been excluded from the calculation of diluted loss per share because those shares are anti-dilutive. |
Unvested time-based restricted stock awards that contain nonforfeitable rights to dividends are participating securities and included in the computation of earnings per share pursuant to the two-class method. Under this method, earnings attributable to the Company are allocated between common stockholders and the participating awards, as if the awards were a second class of stock. During periods of net income, the calculation of earnings per share excludes the income attributable to participating securities in the numerator and the dilutive impact of these securities from the denominator. In the event of a net loss, undistributed earnings are not allocated to participating securities and the denominator excludes the dilutive impact of these
F-24
CARDIOVASCULAR SYSTEMS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
securities as they do not share in the losses of the Company. During the year ended June 30, 2018, undistributed earnings allocated to participating securities were based on 382,476 unvested time-based restricted stock awards.
11. Quarterly Data (Unaudited)
The following table sets forth the Company’s unaudited quarterly summary consolidated statements of operations in each of the quarters for the years ended June 30, 2018 and 2017. The information for each of these quarters is unaudited and has been prepared on the same basis as the consolidated financial statements. This data should be read in conjunction with the consolidated financial statements and related notes. These operating results may not be indicative of results to be expected for any future period (amounts in thousands, except per share data).
2018 | |||||||||||||||||||
Q1 | Q2 | Q3 | Q4 | Total | |||||||||||||||
Net revenue | $ | 49,676 | $ | 52,628 | $ | 55,587 | $ | 59,152 | $ | 217,043 | |||||||||
Gross profit | $ | 40,474 | $ | 43,129 | $ | 45,618 | $ | 48,338 | $ | 177,559 | |||||||||
Net income (loss) | $ | (1,977 | ) | $ | (413 | ) | $ | 365 | $ | 3,737 | $ | 1,712 | |||||||
Earnings per common share - basic(1) | $ | (0.06 | ) | $ | (0.01 | ) | $ | 0.01 | $ | 0.11 | $ | 0.05 | |||||||
Earnings per common share - diluted(1) | $ | (0.06 | ) | $ | (0.01 | ) | $ | 0.01 | $ | 0.11 | $ | 0.05 | |||||||
2017 | |||||||||||||||||||
Q1 | Q2 | Q3 | Q4 | Total | |||||||||||||||
Net revenue | $ | 49,800 | $ | 50,043 | $ | 52,144 | $ | 52,919 | $ | 204,906 | |||||||||
Gross profit | $ | 40,334 | $ | 40,880 | $ | 41,005 | $ | 43,246 | $ | 165,465 | |||||||||
Net income (loss) | $ | (1,858 | ) | $ | 1,043 | $ | (1,749 | ) | $ | 772 | $ | (1,792 | ) | ||||||
Earnings per common share - basic(1) | $ | (0.06 | ) | $ | 0.03 | $ | (0.05 | ) | $ | 0.02 | $ | (0.06 | ) | ||||||
Earnings per common share - diluted(1) | $ | (0.06 | ) | $ | 0.03 | $ | (0.05 | ) | $ | 0.02 | $ | (0.06 | ) | ||||||
(1) The summation of quarterly per share data may not equate to the calculation for the full fiscal year as quarterly calculations are performed on a discrete basis.
F-25
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our Chief Executive Officer and our Chief Financial Officer, referred to collectively herein as the Certifying Officers, are responsible for establishing and maintaining our disclosure controls and procedures. The Certifying Officers have reviewed and evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 240.13a-15(e) and 15d-15(e) promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) as of June 30, 2018. Based on that review and evaluation, which included inquiries made to certain other employees of the Company, the Certifying Officers have concluded that, as of the end of the period covered by this Annual Report on Form 10-K, our disclosure controls and procedures, as designed and implemented, are effective.
Management’s Annual Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) for us. Management conducted an evaluation of the effectiveness of internal control over financial reporting based on the framework in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this evaluation, management concluded that our internal control over financial reporting was effective as of June 30, 2018.
PricewaterhouseCoopers LLP, the independent registered public accounting firm that audited the consolidated financial statements included in this Annual Report on Form 10-K, has also audited the effectiveness of our internal control over financial reporting as of June 30, 2018, as stated in their attestation report included in Part IV, Item 15 of this Annual Report on Form 10-K.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the three months ended June 30, 2018 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
Base Salaries
On August 21, 2018, the Human Resources and Compensation Committee (the “Committee”) of the Board of Directors (the “Board”) approved base salaries for Jeffrey S. Points, our Chief Financial Officer, and Laura J. Gillund, our Chief Talent Officer, of $302,500 and $315,655, respectively, for the year ending June 30, 2019.
On August 22, 2018, the Board approved a base salary for Scott R. Ward, our Chairman, President and Chief Executive Officer, of $670,000 for the year ending June 30, 2019.
Fiscal 2019 Incentive Plan
On August 21, 2018 and August 22, 2018, the Board and the Committee approved the incentive compensation plans for our executive officers for the year ending June 30, 2019, as described below.
Cash Bonus Plan
For the 12-month period ending June 30, 2019, each executive officer is eligible to receive cash incentive compensation pursuant to the Fiscal 2019 Executive Officer Bonus Plan (the “Bonus Plan”), based on our achievement of revenue and Adjusted EBITDA financial goals for such period. Adjusted EBITDA is defined as EBITDA with stock compensation added back into the calculation. Target bonus amounts are weighted 75% for the revenue goal and 25% for the Adjusted EBITDA goal. Target bonus levels as a percentage of base salary are 115% for the Chief Executive Officer, 90% for the Chief Operating
47
Officer, 75% for the Chief Financial Officer, 65% for the Chief Talent Officer and General Counsel, and 50% for the other executive officers. Depending upon our performance against the goals, participants are eligible to earn up to 200% of each of the Adjusted EBITDA and revenue portions of their target bonus amount. The Bonus Plan criteria are the same for all of the executive officers.
Long-term Incentive Plan
Each executive officer will receive grants of restricted stock under the fiscal 2019 long-term incentive plan, effective three business days following the filing of this Form 10-K. The restricted stock grants will be based on a target equity percentage of each executive officer’s base salary, with 40% of such target amount allocated to time-vesting restricted stock and 60% of such target amount allocated to performance-vesting restricted stock; provided, that the performance-vesting restricted stock will be granted to each executive officer at 200% of the target number of shares allocated to performance-vesting restricted stock, and any shares not earned will be forfeited upon confirmation of performance achievement. Target equity grants as a percentage of base salary are 400% for the Chief Executive Officer, 200% for the Chief Operating Officer, 150% for the Chief Financial Officer, and 125% for the other executive officers.
The time-vesting restricted stock grants will vest in equal installments of 1/3 in August 2019, 2020 and 2021. The performance-vesting restricted stock grants will vest based on our total shareholder return relative to total shareholder return of our peer group (as determined by the Committee), as measured by the closing prices of our stock and the peer group members for the 90 trading days preceding July 1, 2018 compared to the closing prices of our stock and the stock of the peer group members for the 90 trading days preceding July 1, 2021. Vesting of the performance-vesting shares will be determined on the date that our Form 10-K for the fiscal year ending June 30, 2021 is filed.
48
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Other than the information included in this Form 10-K under the heading “Employees—Executive Officers of the Registrant,” which is set forth at the end of Item 1 and incorporated herein by reference, the information required by Item 10 is incorporated by reference to the sections labeled “Election of Directors,” “Information Regarding the Board of Directors and Corporate Governance” and “Section 16(a) Beneficial Ownership Reporting Compliance,” all of which will appear in our definitive proxy statement for our 2018 Annual Meeting.
Item 11. Executive Compensation.
The information required by Item 11 is incorporated herein by reference to the sections entitled “Executive Compensation,” “Director Compensation,” “Human Resources and Compensation Committee” and “Compensation Committee Interlocks and Insider Participation,” all of which will appear in our definitive proxy statement for our 2018 Annual Meeting.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by Item 12 is incorporated herein by reference to the sections entitled “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information,” which will appear in our definitive proxy statement for our 2018 Annual Meeting.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by Item 13 is incorporated herein by reference to the sections entitled “Independence of the Board of Directors” and “Transactions With Related Persons,” which will appear in our definitive proxy statement for our 2018 Annual Meeting.
Item 14. Principal Accounting Fees and Services.
The information required by Item 14 is incorporated herein by reference to the section entitled “Principal Accountant Fees and Services,” which will appear in our definitive proxy statement for our 2018 Annual Meeting.
49
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(a) Documents filed as part of this report.
(1) | Financial Statements. The following financial statements are included in Part II, Item 8 of this Annual Report on Form 10-K: |
• | Report of Independent Registered Public Accounting Firm |
• | Consolidated Balance Sheets as of June 30, 2018 and 2017 |
• | Consolidated Statements of Operations for the years ended June 30, 2018, 2017 and 2016 |
• | Consolidated Statements of Comprehensive Loss for the years ended June 30, 2018, 2017 and 2016 |
• | Consolidated Statements of Stockholders’ Equity for the years ended June 30, 2018, 2017 and 2016 |
• | Consolidated Statements of Cash Flows for the years ended June 30, 2018, 2017 and 2016 |
• | Notes to Consolidated Financial Statements |
(2) Financial Statement Schedules.
• | All financial statement schedules have been omitted, because they are not applicable, are not required, or the information is included in the Financial Statements or Notes thereto |
(3) Exhibits.
Exhibit No. | Description | |
3.1 | ||
3.2 | ||
4.1 | ||
4.2 | ||
10.1† | ||
10.2† | ||
10.3† | ||
10.4†* | ||
10.5†* | ||
10.6†* | ||
10.7† | ||
10.8† | ||
10.9† | ||
50
Exhibit No. | Description | |
10.10† | ||
10.11† | ||
10.12† | ||
10.13† | ||
10.14† | ||
10.15† | ||
10.16 | ||
10.17 | ||
10.18+ | ||
10.19† | ||
10.20† | ||
10.21† | ||
10.22† | ||
10.23† | ||
10.24† | ||
10.25† | ||
10.26†* | ||
10.27† | ||
10.28† | ||
51
Exhibit No. | Description | |
10.29† | ||
10.30 | ||
10.31+ | ||
10.32 | ||
10.33 | ||
10.34† | ||
10.35† | ||
10.36† | ||
10.37 | ||
10.38 | ||
10.39 | ||
10.40 | ||
10.41 | ||
10.42 | ||
10.43 | ||
10.44† | ||
10.45† | ||
10.46† | ||
52
Exhibit No. | Description | |
10.47† | ||
10.48† | ||
10.49† | ||
10.50† | ||
10.51† | ||
10.52† | ||
10.53† | ||
10.54† | ||
10.55† | ||
10.56+ | ||
10.57* | ||
23.1* | ||
24.1* | ||
31.1* | ||
31.2* | ||
32.1** | ||
32.2** | ||
101** | Financial statements from the Annual Report on Form 10-K of the Company for the year ended June 30, 2018, formatted, in Extensible Business Reporting Language (XBRL): (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Operations, (iii) the Consolidated Statements of Comprehensive Loss, (iv) the Consolidated Statements of Changes in Stockholders’ Equity, (v) the Consolidated Statements of Cash Flows, and (vi) the Notes to Consolidated Financial Statements. | |
* Filed herewith.
** Furnished herewith.
† Compensatory plan or agreement.
+ | Confidential treatment has been granted for certain portions omitted from this exhibit pursuant to Rule 24b-2 under the Securities Exchange Act of 1934, as amended. |
53
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
CARDIOVASCULAR SYSTEMS, INC. | |||
Date: August 23, 2018 | By: | /s/ Scott R. Ward | |
Scott R. Ward | |||
Chairman, President and Chief Executive Officer | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Each person whose signature appears below constitutes and appoints Scott R. Ward and Jeffrey S. Points as the undersigned’s true and lawful attorneys-in fact and agents, each acting alone, with full power of substitution and resubstitution, for the undersigned and in the undersigned’s name, place and stead, in any and all amendments to this Annual Report on Form 10-K and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granted unto said attorneys-in-fact and agents, each acting alone, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all said attorneys-in-fact and agents, each acting alone, or his substitute or substitutes, may lawfully do or cause to be done by virtue thereof.
Signature | Title | Date | ||
/s/ Scott R. Ward | Chairman, President and Chief Executive Officer (principal executive officer) | August 23, 2018 | ||
Scott R. Ward | ||||
/s/ Jeffrey S. Points | Chief Financial Officer (principal financial and accounting officer) | August 23, 2018 | ||
Jeffrey S. Points | ||||
/s/ Martha Goldberg Aronson | Director | August 23, 2018 | ||
Martha Goldberg Aronson | ||||
/s/ Scott Bartos | Director | August 23, 2018 | ||
Scott Bartos | ||||
/s/ Brent G. Blackey | Director | August 23, 2018 | ||
Brent G. Blackey | ||||
/s/ Edward Brown | Director | August 23, 2018 | ||
Edward Brown | ||||
/s/ William E. Cohn | Director | August 23, 2018 | ||
William E. Cohn | ||||
/s/ Augustine Lawlor | Director | August 23, 2018 | ||
Augustine Lawlor | ||||
54
