Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Allena Pharmaceuticals, Inc. | d607528d8k.htm |

Bringing First-in-Class Oral Enzyme Therapeutics to Patients with Rare and Severe Metabolic and Kidney Disorders August 2018 Exhibit 99.1

Allena Pharmaceuticals, Inc. These slides contain forward-looking statements and information. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward-looking statements. All forward-looking statements are based on estimates and assumptions by our management that, although we believe them to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These statements are also subject to a number of material risks and uncertainties that are described under the heading “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2017 filed with the Securities and Exchange Commission on March 27, 2018 and in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2018 filed with the Securities and Exchange Commission on August 7, 2018, as well as discussions of potential risks, uncertainties and other important factors in our subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.
Investment Highlights Significant Unmet Need in Oxalate and Urate Disorders Late-Stage Development Candidate: ALLN-177 Pioneering Expertise in Oral Enzyme Therapeutics Focused on rare and severe metabolic disorders that can cause kidney stones, damage the kidney, and potentially lead to CKD and ESRD No approved oxalate therapies; potential untapped multi-billion dollar market First-in-class, oral therapy for severe hyperoxaluria Enrolling Phase 3 study, URIROX-1, in enteric hyperoxaluria, topline data expected 2H 2019 Enrolling Phase 2 basket study in other severe and orphan indications Approach enables treatment of metabolic diseases with oral, non-absorbed enzyme therapeutics GI MOA reduces subsequent metabolic burden on the kidney Raised $75M in successful initial public offering in 4Q 2017 Founded in 2011 with Frazier Healthcare Partners, Third Rock Ventures, and Bessemer Venture Partners. Additional private financing from HBM, Pharmstandard International, Partner Fund, Fidelity Management & Research Company Strong Support from Leading Biotechnology Investors
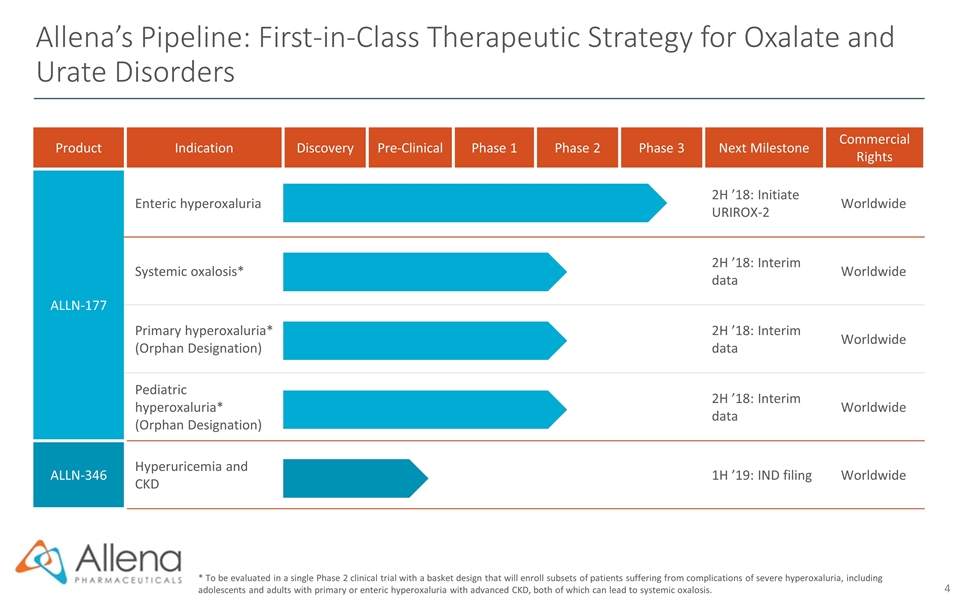
Product Indication Discovery Pre-Clinical Phase 1 Phase 2 Phase 3 Next Milestone Commercial Rights ALLN-177 Enteric hyperoxaluria 2H ’18: Initiate URIROX-2 Worldwide Systemic oxalosis* 2H ’18: Interim data Worldwide Primary hyperoxaluria* (Orphan Designation) 2H ’18: Interim data Worldwide Pediatric hyperoxaluria* (Orphan Designation) 2H ’18: Interim data Worldwide ALLN-346 Hyperuricemia and CKD 1H ’19: IND filing Worldwide Allena’s Pipeline: First-in-Class Therapeutic Strategy for Oxalate and Urate Disorders * To be evaluated in a single Phase 2 clinical trial with a basket design that will enroll subsets of patients suffering from complications of severe hyperoxaluria, including adolescents and adults with primary or enteric hyperoxaluria with advanced CKD, both of which can lead to systemic oxalosis.
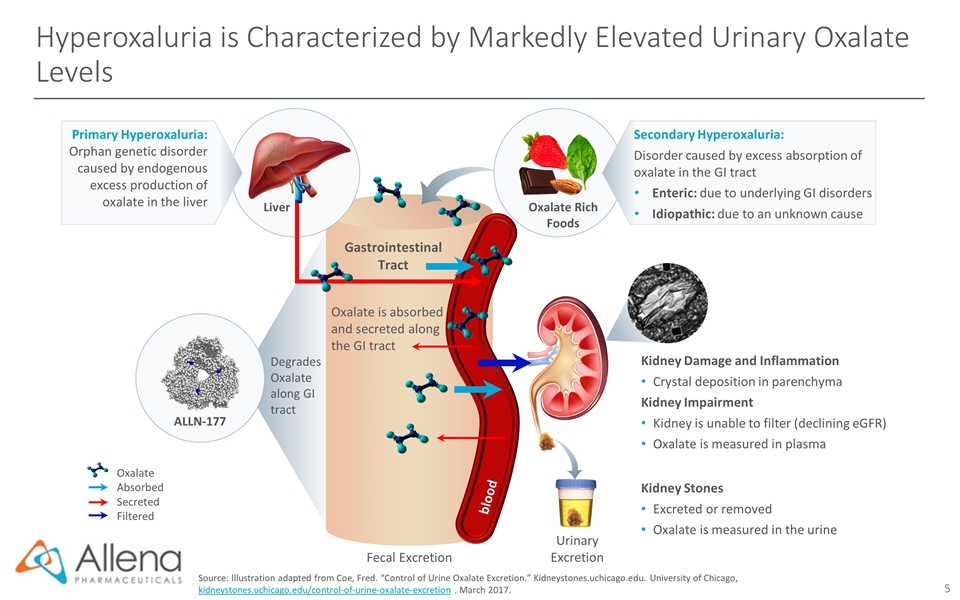
Hyperoxaluria is Characterized by Markedly Elevated Urinary Oxalate Levels Source: Illustration adapted from Coe, Fred. “Control of Urine Oxalate Excretion.” Kidneystones.uchicago.edu. University of Chicago, kidneystones.uchicago.edu/control-of-urine-oxalate-excretion . March 2017. Fecal Excretion Gastrointestinal Tract Oxalate is absorbed and secreted along the GI tract Urinary Excretion Kidney Damage and Inflammation Crystal deposition in parenchyma Kidney Impairment Kidney is unable to filter (declining eGFR) Oxalate is measured in plasma Kidney Stones Excreted or removed Oxalate is measured in the urine Oxalate Rich Foods Oxalate Absorbed Secreted Filtered Primary Hyperoxaluria: Orphan genetic disorder caused by endogenous excess production of oxalate in the liver Liver blood Secondary Hyperoxaluria: Disorder caused by excess absorption of oxalate in the GI tract Enteric: due to underlying GI disorders Idiopathic: due to an unknown cause Degrades Oxalate along GI tract ALLN-177
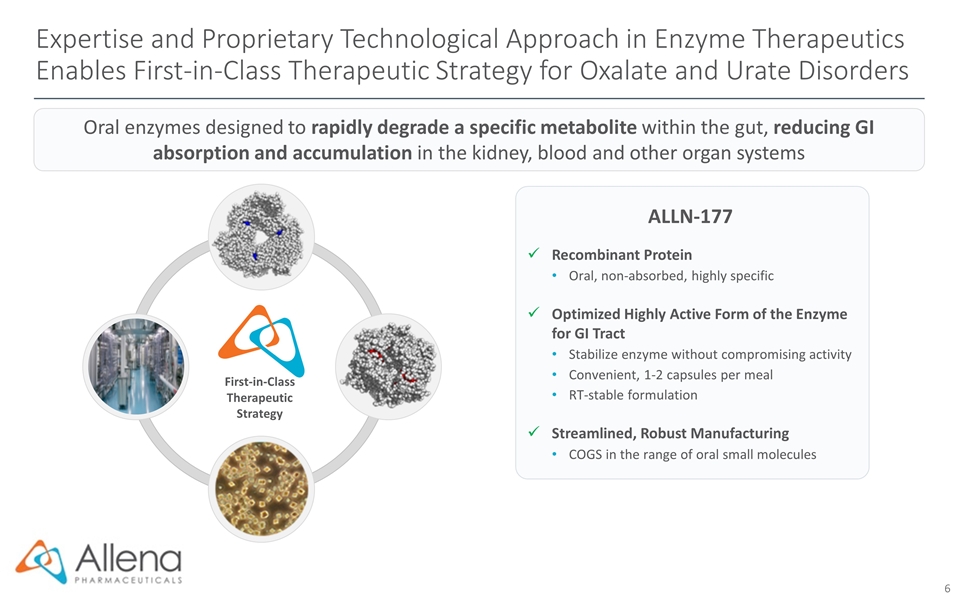
First-in-Class Therapeutic Strategy Expertise and Proprietary Technological Approach in Enzyme Therapeutics Enables First-in-Class Therapeutic Strategy for Oxalate and Urate Disorders ALLN-177 Recombinant Protein Oral, non-absorbed, highly specific Optimized Highly Active Form of the Enzyme for GI Tract Stabilize enzyme without compromising activity Convenient, 1-2 capsules per meal RT-stable formulation Streamlined, Robust Manufacturing COGS in the range of oral small molecules Oral enzymes designed to rapidly degrade a specific metabolite within the gut, reducing GI absorption and accumulation in the kidney, blood and other organ systems
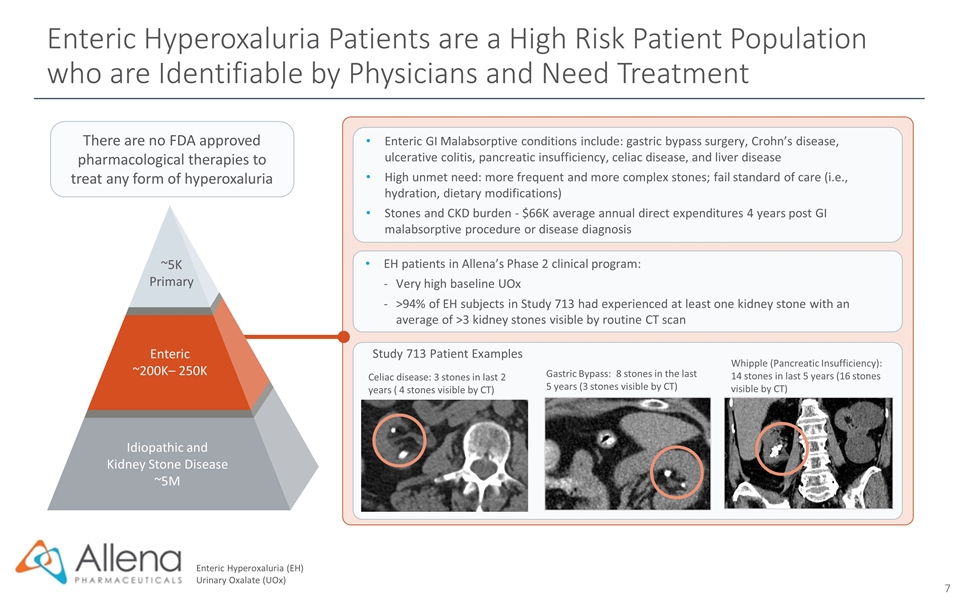
There are no FDA approved pharmacological therapies to treat any form of hyperoxaluria Enteric Hyperoxaluria (EH) Urinary Oxalate (UOx) Study 713 Patient Examples Whipple (Pancreatic Insufficiency): 14 stones in last 5 years (16 stones visible by CT) Celiac disease: 3 stones in last 2 years ( 4 stones visible by CT) Gastric Bypass: 8 stones in the last 5 years (3 stones visible by CT) EH patients in Allena’s Phase 2 clinical program: Very high baseline UOx >94% of EH subjects in Study 713 had experienced at least one kidney stone with an average of >3 kidney stones visible by routine CT scan Enteric GI Malabsorptive conditions include: gastric bypass surgery, Crohn’s disease, ulcerative colitis, pancreatic insufficiency, celiac disease, and liver disease High unmet need: more frequent and more complex stones; fail standard of care (i.e., hydration, dietary modifications) Stones and CKD burden - $66K average annual direct expenditures 4 years post GI malabsorptive procedure or disease diagnosis Idiopathic and Kidney Stone Disease ~5M Enteric ~200K– 250K ~5K Primary Enteric Hyperoxaluria Patients are a High Risk Patient Population who are Identifiable by Physicians and Need Treatment
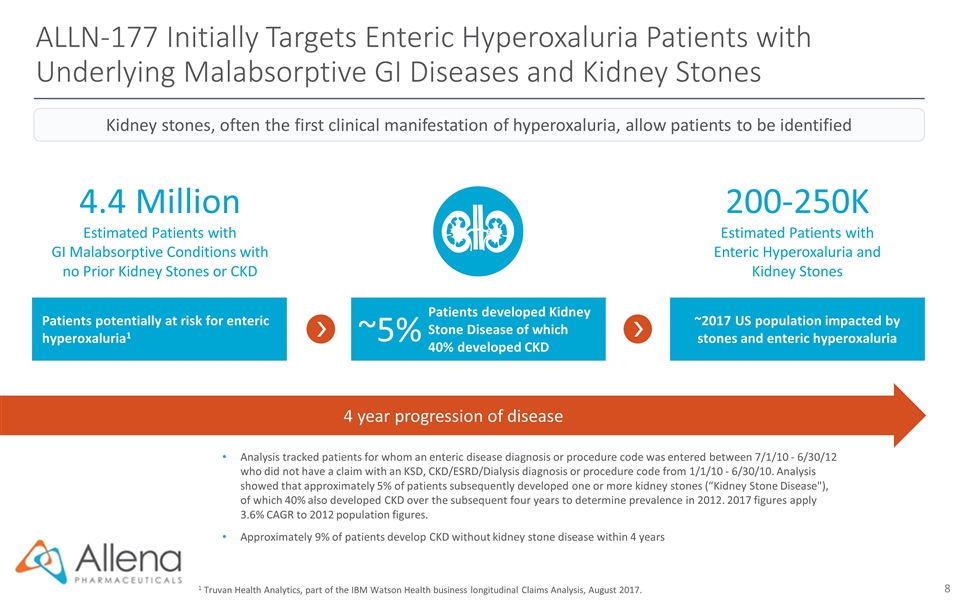
ALLN-177 Initially Targets Enteric Hyperoxaluria Patients with Underlying Malabsorptive GI Diseases and Kidney Stones 1 Truvan Health Analytics, part of the IBM Watson Health business longitudinal Claims Analysis, August 2017. Analysis tracked patients for whom an enteric disease diagnosis or procedure code was entered between 7/1/10 - 6/30/12 who did not have a claim with an KSD, CKD/ESRD/Dialysis diagnosis or procedure code from 1/1/10 - 6/30/10. Analysis showed that approximately 5% of patients subsequently developed one or more kidney stones (“Kidney Stone Disease"), of which 40% also developed CKD over the subsequent four years to determine prevalence in 2012. 2017 figures apply 3.6% CAGR to 2012 population figures. Approximately 9% of patients develop CKD without kidney stone disease within 4 years 4.4 Million Estimated Patients with GI Malabsorptive Conditions with no Prior Kidney Stones or CKD 200-250K Estimated Patients with Enteric Hyperoxaluria and Kidney Stones 4 year progression of disease Kidney stones, often the first clinical manifestation of hyperoxaluria, allow patients to be identified Patients potentially at risk for enteric hyperoxaluria1 Patients developed Kidney Stone Disease of which 40% developed CKD ~2017 US population impacted by stones and enteric hyperoxaluria ~5%
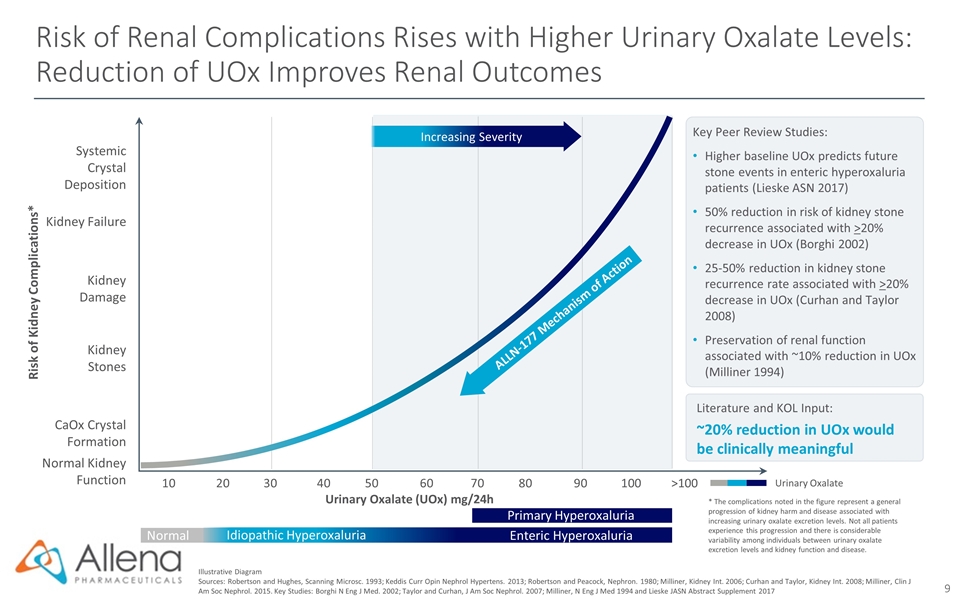
Risk of Renal Complications Rises with Higher Urinary Oxalate Levels: Reduction of UOx Improves Renal Outcomes Illustrative Diagram Sources: Robertson and Hughes, Scanning Microsc. 1993; Keddis Curr Opin Nephrol Hypertens. 2013; Robertson and Peacock, Nephron. 1980; Milliner, Kidney Int. 2006; Curhan and Taylor, Kidney Int. 2008; Milliner, Clin J Am Soc Nephrol. 2015. Key Studies: Borghi N Eng J Med. 2002; Taylor and Curhan, J Am Soc Nephrol. 2007; Milliner, N Eng J Med 1994 and Lieske JASN Abstract Supplement 2017 Urinary Oxalate Literature and KOL Input: ~20% reduction in UOx would be clinically meaningful Key Peer Review Studies: Higher baseline UOx predicts future stone events in enteric hyperoxaluria patients (Lieske ASN 2017) 50% reduction in risk of kidney stone recurrence associated with >20% decrease in UOx (Borghi 2002) 25-50% reduction in kidney stone recurrence rate associated with >20% decrease in UOx (Curhan and Taylor 2008) Preservation of renal function associated with ~10% reduction in UOx (Milliner 1994) Urinary Oxalate (UOx) mg/24h Kidney Damage 102030405060708090100>100 Kidney Failure Normal Kidney Function Kidney Stones CaOx Crystal Formation Systemic Crystal Deposition Risk of Kidney Complications* Increasing Severity ALLN-177 Mechanism of Action * The complications noted in the figure represent a general progression of kidney harm and disease associated with increasing urinary oxalate excretion levels. Not all patients experience this progression and there is considerable variability among individuals between urinary oxalate excretion levels and kidney function and disease. Idiopathic Hyperoxaluria Enteric Hyperoxaluria Primary Hyperoxaluria Normal
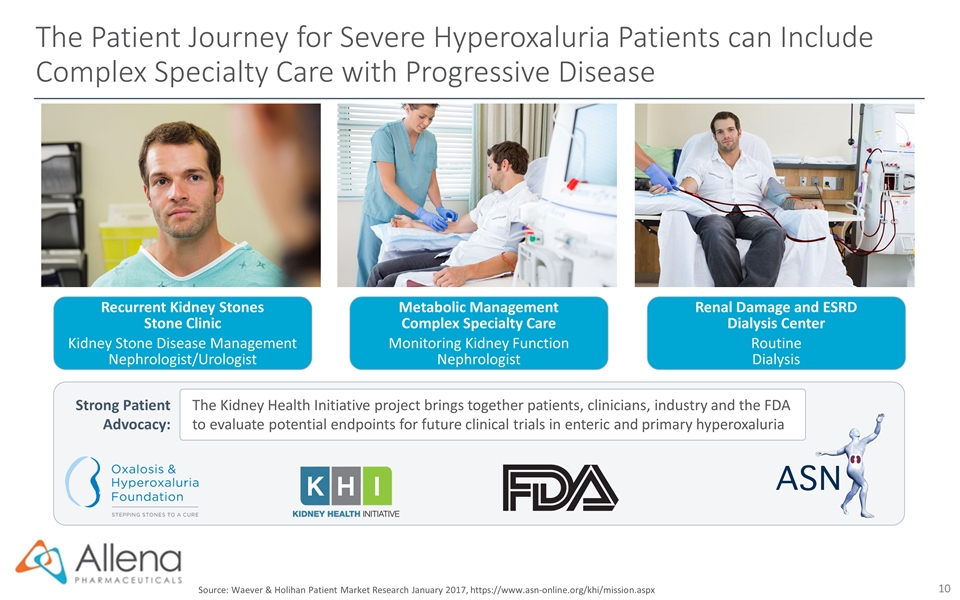
The Patient Journey for Severe Hyperoxaluria Patients can Include Complex Specialty Care with Progressive Disease Recurrent Kidney Stones Stone Clinic Kidney Stone Disease Management Nephrologist/Urologist Metabolic Management Complex Specialty Care Monitoring Kidney Function Nephrologist Renal Damage and ESRD Dialysis Center Routine Dialysis Strong Patient Advocacy: The Kidney Health Initiative project brings together patients, clinicians, industry and the FDA to evaluate potential endpoints for future clinical trials in enteric and primary hyperoxaluria Source: Waever & Holihan Patient Market Research January 2017, https://www.asn-online.org/khi/mission.aspx

ALLN-177 for the Treatment of Hyperoxaluria
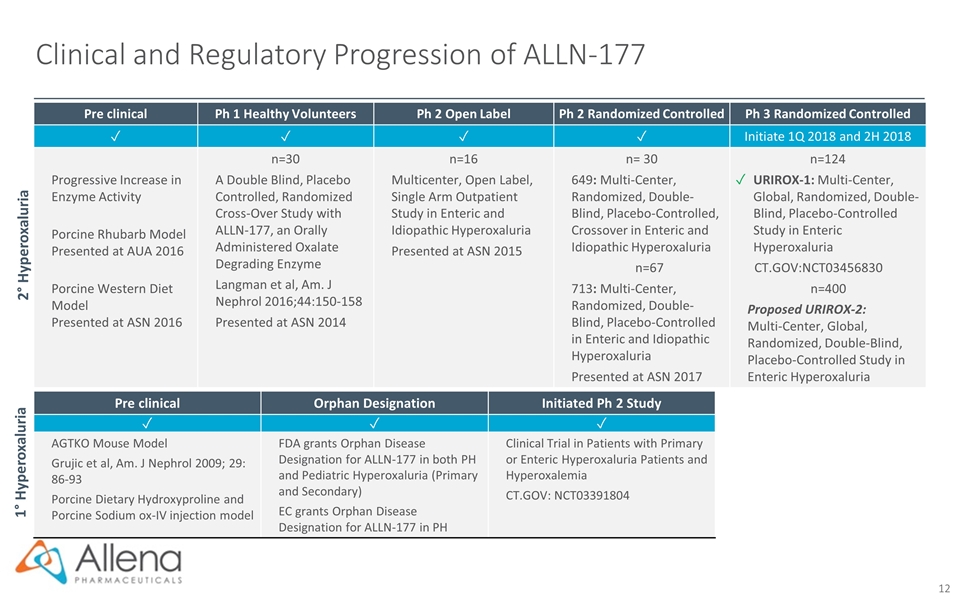
Clinical and Regulatory Progression of ALLN-177 Pre clinical Ph 1 Healthy Volunteers Ph 2 Open Label Ph 2 Randomized Controlled Ph 3 Randomized Controlled ✓ ✓ ✓ ✓ Initiate 1Q 2018 and 2H 2018 Progressive Increase in Enzyme Activity Porcine Rhubarb Model Presented at AUA 2016 Porcine Western Diet Model Presented at ASN 2016 n=30 A Double Blind, Placebo Controlled, Randomized Cross-Over Study with ALLN-177, an Orally Administered Oxalate Degrading Enzyme Langman et al, Am. J Nephrol 2016;44:150-158 Presented at ASN 2014 n=16 Multicenter, Open Label, Single Arm Outpatient Study in Enteric and Idiopathic Hyperoxaluria Presented at ASN 2015 n= 30 649: Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Crossover in Enteric and Idiopathic Hyperoxaluria n=67 713: Multi-Center, Randomized, Double-Blind, Placebo-Controlled in Enteric and Idiopathic Hyperoxaluria Presented at ASN 2017 n=124 ✓ URIROX-1: Multi-Center, Global, Randomized, Double-Blind, Placebo-Controlled Study in Enteric Hyperoxaluria CT.GOV:NCT03456830 n=400 Proposed URIROX-2: Multi-Center, Global, Randomized, Double-Blind, Placebo-Controlled Study in Enteric Hyperoxaluria 2° Hyperoxaluria 1° Hyperoxaluria Pre clinical Orphan Designation Initiated Ph 2 Study ✓ ✓ ✓ AGTKO Mouse Model Grujic et al, Am. J Nephrol 2009; 29: 86-93 Porcine Dietary Hydroxyproline and Porcine Sodium ox-IV injection model FDA grants Orphan Disease Designation for ALLN-177 in both PH and Pediatric Hyperoxaluria (Primary and Secondary) EC grants Orphan Disease Designation for ALLN-177 in PH Clinical Trial in Patients with Primary or Enteric Hyperoxaluria Patients and Hyperoxalemia CT.GOV: NCT03391804
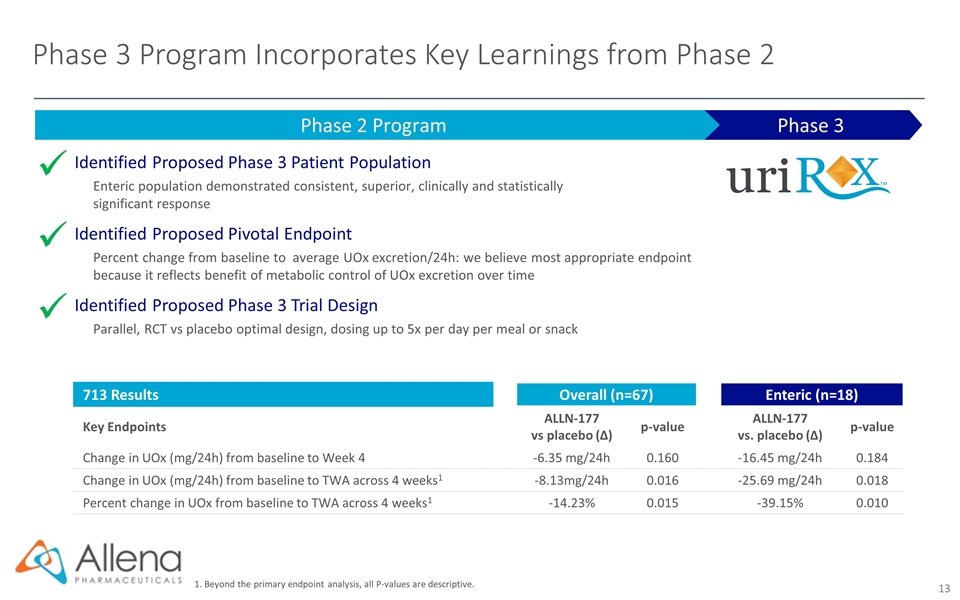
Phase 3 Program Incorporates Key Learnings from Phase 2 Phase 3 Phase 2 Program Identified Proposed Phase 3 Patient Population Enteric population demonstrated consistent, superior, clinically and statistically significant response Identified Proposed Pivotal Endpoint Percent change from baseline to average UOx excretion/24h: we believe most appropriate endpoint because it reflects benefit of metabolic control of UOx excretion over time Identified Proposed Phase 3 Trial Design Parallel, RCT vs placebo optimal design, dosing up to 5x per day per meal or snack ü ü ü 713 Results Overall (n=67) Enteric (n=18) Key Endpoints ALLN-177 vs placebo (∆) p-value ALLN-177 vs. placebo (∆) p-value Change in UOx (mg/24h) from baseline to Week 4 -6.35 mg/24h 0.160 -16.45 mg/24h 0.184 Change in UOx (mg/24h) from baseline to TWA across 4 weeks1 -8.13mg/24h 0.016 -25.69 mg/24h 0.018 Percent change in UOx from baseline to TWA across 4 weeks1 -14.23% 0.015 -39.15% 0.010 1. Beyond the primary endpoint analysis, all P-values are descriptive.
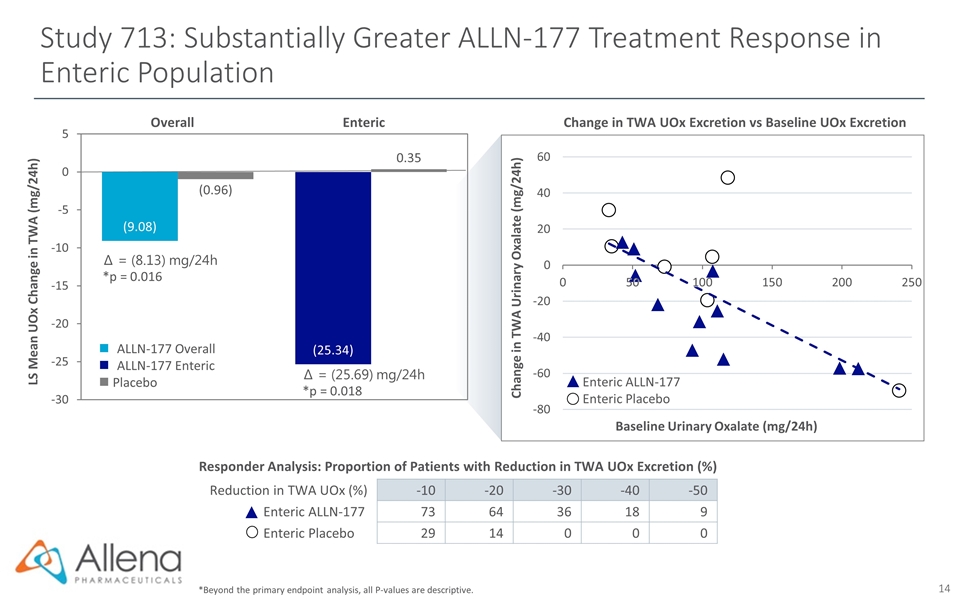
Study 713: Substantially Greater ALLN-177 Treatment Response in Enteric Population *Beyond the primary endpoint analysis, all P-values are descriptive. Enteric ALLN-177 Enteric Placebo Change in TWA Urinary Oxalate (mg/24h) Baseline Urinary Oxalate (mg/24h) Change in TWA UOx Excretion vs Baseline UOx Excretion Responder Analysis: Proportion of Patients with Reduction in TWA UOx Excretion (%) Create table Reduction in TWA UOx (%) -10 -20 -30 -40 -50 Enteric ALLN-177 73 64 36 18 9 Enteric Placebo 29 14 0 0 0 ∆ = (8.13) mg/24h *p = 0.016 ∆ = (25.69) mg/24h *p = 0.018 LS Mean UOx Change in TWA (mg/24h) Overall Enteric ALLN-177 Overall ALLN-177 Enteric n Placebo
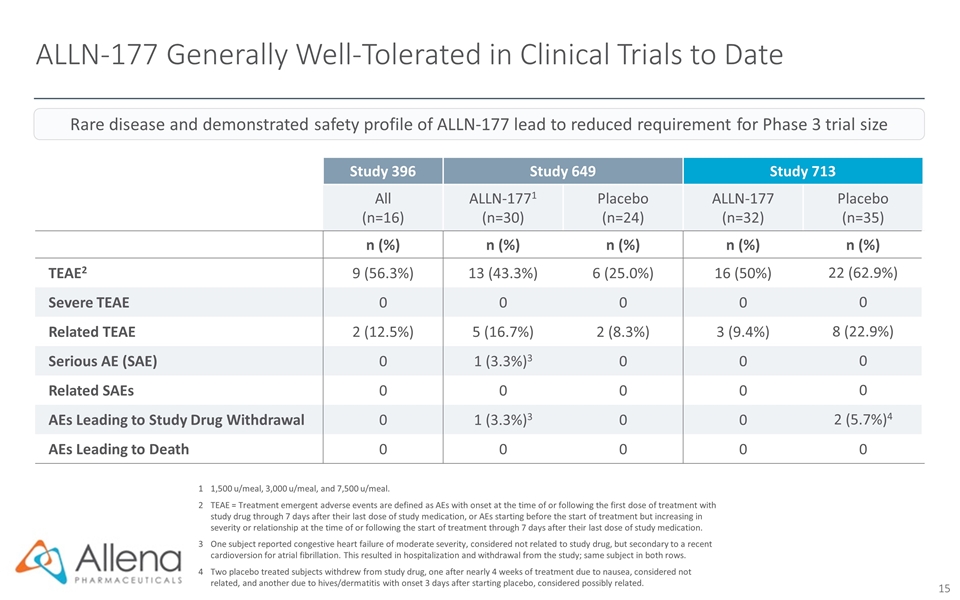
ALLN-177 Generally Well-Tolerated in Clinical Trials to Date 11,500 u/meal, 3,000 u/meal, and 7,500 u/meal. 2TEAE = Treatment emergent adverse events are defined as AEs with onset at the time of or following the first dose of treatment with study drug through 7 days after their last dose of study medication, or AEs starting before the start of treatment but increasing in severity or relationship at the time of or following the start of treatment through 7 days after their last dose of study medication. 3One subject reported congestive heart failure of moderate severity, considered not related to study drug, but secondary to a recent cardioversion for atrial fibrillation. This resulted in hospitalization and withdrawal from the study; same subject in both rows. 4Two placebo treated subjects withdrew from study drug, one after nearly 4 weeks of treatment due to nausea, considered not related, and another due to hives/dermatitis with onset 3 days after starting placebo, considered possibly related. Study 396 Study 649 Study 713 All (n=16) ALLN-1771 (n=30) Placebo (n=24) ALLN-177 (n=32) Placebo (n=35) n (%) n (%) n (%) n (%) n (%) TEAE2 9 (56.3%) 13 (43.3%) 6 (25.0%) 16 (50%) 22 (62.9%) Severe TEAE 0 0 0 0 0 Related TEAE 2 (12.5%) 5 (16.7%) 2 (8.3%) 3 (9.4%) 8 (22.9%) Serious AE (SAE) 0 1 (3.3%)3 0 0 0 Related SAEs 0 0 0 0 0 AEs Leading to Study Drug Withdrawal 0 1 (3.3%)3 0 0 2 (5.7%)4 AEs Leading to Death 0 0 0 0 0 Rare disease and demonstrated safety profile of ALLN-177 lead to reduced requirement for Phase 3 trial size
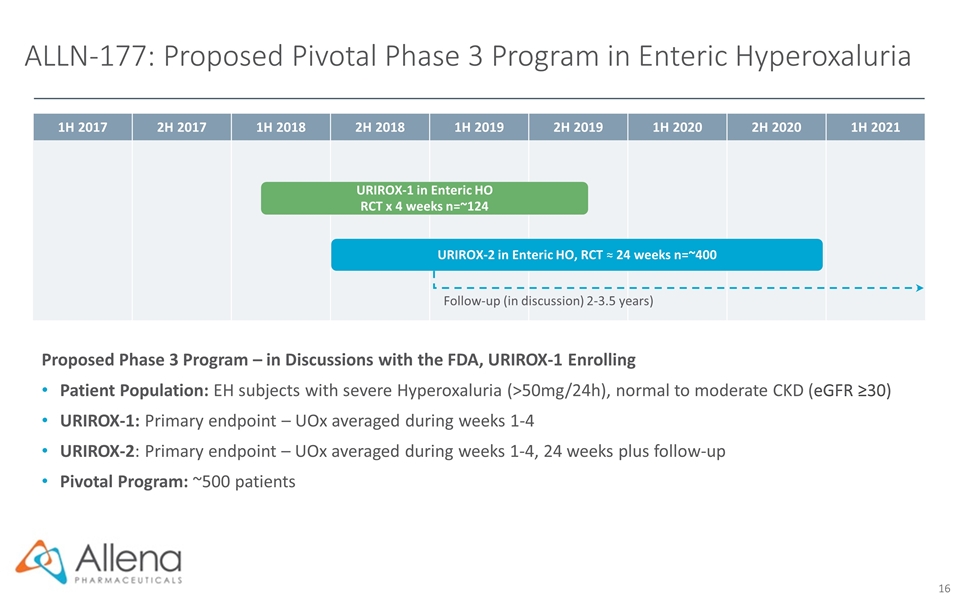
1H 2017 2H 2017 1H 2018 2H 2018 1H 2019 2H 2019 1H 2020 2H 2020 1H 2021 ALLN-177: Proposed Pivotal Phase 3 Program in Enteric Hyperoxaluria Proposed Phase 3 Program – in Discussions with the FDA, URIROX-1 Enrolling Patient Population: EH subjects with severe Hyperoxaluria (>50mg/24h), normal to moderate CKD (eGFR ≥30) URIROX-1: Primary endpoint – UOx averaged during weeks 1-4 URIROX-2: Primary endpoint – UOx averaged during weeks 1-4, 24 weeks plus follow-up Pivotal Program: ~500 patients URIROX-2 in Enteric HO, RCT ≈ 24 weeks n=~400 URIROX-1 in Enteric HO RCT x 4 weeks n=~124 Follow-up (in discussion) 2-3.5 years)
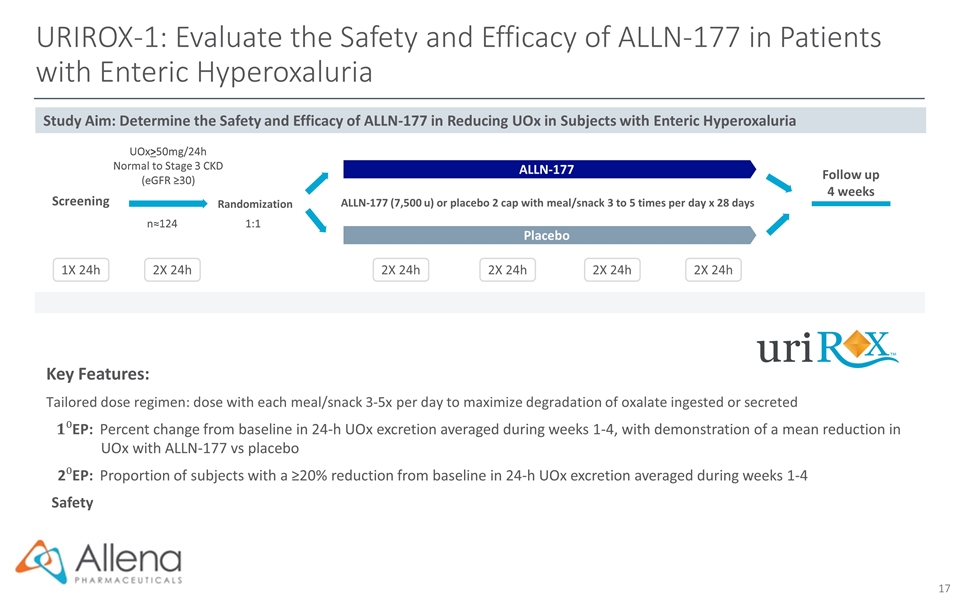
URIROX-1: Evaluate the Safety and Efficacy of ALLN-177 in Patients with Enteric Hyperoxaluria Key Features: Tailored dose regimen: dose with each meal/snack 3-5x per day to maximize degradation of oxalate ingested or secreted EP:Percent change from baseline in 24-h UOx excretion averaged during weeks 1-4, with demonstration of a mean reduction in UOx with ALLN-177 vs placebo 2EP:Proportion of subjects with a ≥20% reduction from baseline in 24-h UOx excretion averaged during weeks 1-4 Safety Randomization ALLN-177 (7,500 u) or placebo 2 cap with meal/snack 3 to 5 times per day x 28 days UOx>50mg/24h Normal to Stage 3 CKD (eGFR ≥30) 1:1 n≈124 2X 24h 1X 24h 2X 24h 2X 24h 2X 24h 2X 24h Placebo ALLN-177 Screening Follow up 4 weeks Study Aim: Determine the Safety and Efficacy of ALLN-177 in Reducing UOx in Subjects with Enteric Hyperoxaluria
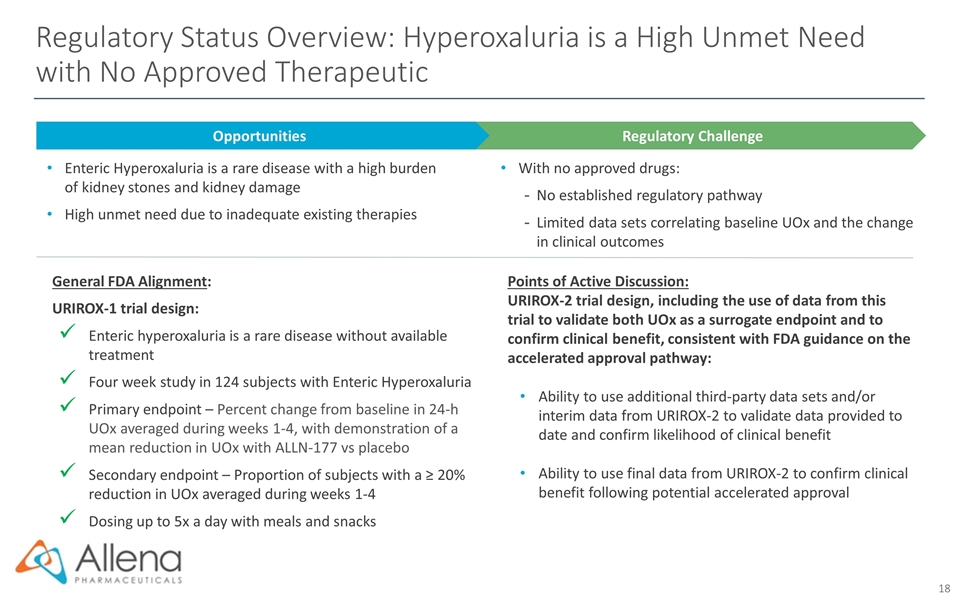
Regulatory Status Overview: Hyperoxaluria is a High Unmet Need with No Approved Therapeutic Regulatory Challenge Opportunities Enteric Hyperoxaluria is a rare disease with a high burden of kidney stones and kidney damage High unmet need due to inadequate existing therapies With no approved drugs: No established regulatory pathway Limited data sets correlating baseline UOx and the change in clinical outcomes General FDA Alignment: URIROX-1 trial design: Enteric hyperoxaluria is a rare disease without available treatment Four week study in 124 subjects with Enteric Hyperoxaluria Primary endpoint – Percent change from baseline in 24-h UOx averaged during weeks 1-4, with demonstration of a mean reduction in UOx with ALLN-177 vs placebo Secondary endpoint – Proportion of subjects with a ≥ 20% reduction in UOx averaged during weeks 1-4 Dosing up to 5x a day with meals and snacks Points of Active Discussion: URIROX-2 trial design, including the use of data from this trial to validate both UOx as a surrogate endpoint and to confirm clinical benefit, consistent with FDA guidance on the accelerated approval pathway: Ability to use additional third-party data sets and/or interim data from URIROX-2 to validate data provided to date and confirm likelihood of clinical benefit Ability to use final data from URIROX-2 to confirm clinical benefit following potential accelerated approval

ALLN-177 Additional Indications
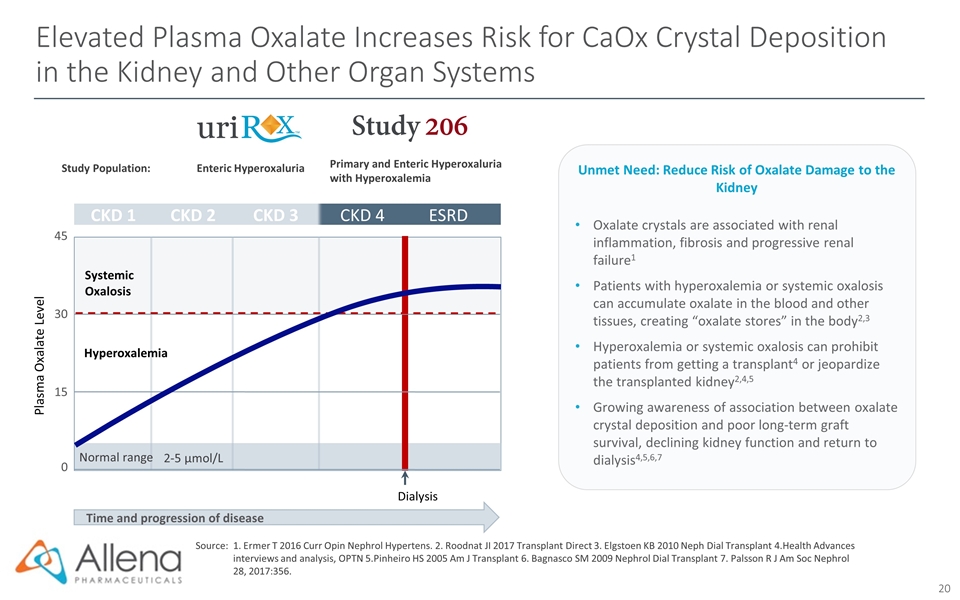
Elevated Plasma Oxalate Increases Risk for CaOx Crystal Deposition in the Kidney and Other Organ Systems Time and progression of disease Plasma Oxalate Level CKD 1 CKD 2 CKD 3 CKD 4 ESRD 0 15 30 45 2-5 µmol/L Normal range Systemic Oxalosis Dialysis Hyperoxalemia Study Population: Enteric Hyperoxaluria Primary and Enteric Hyperoxaluria with Hyperoxalemia Source:1. Ermer T 2016 Curr Opin Nephrol Hypertens. 2. Roodnat JI 2017 Transplant Direct 3. Elgstoen KB 2010 Neph Dial Transplant 4.Health Advances interviews and analysis, OPTN 5.Pinheiro HS 2005 Am J Transplant 6. Bagnasco SM 2009 Nephrol Dial Transplant 7. Palsson R J Am Soc Nephrol 28, 2017:356. Oxalate crystals are associated with renal inflammation, fibrosis and progressive renal failure1 Patients with hyperoxalemia or systemic oxalosis can accumulate oxalate in the blood and other tissues, creating “oxalate stores” in the body2,3 Hyperoxalemia or systemic oxalosis can prohibit patients from getting a transplant4 or jeopardize the transplanted kidney2,4,5 Growing awareness of association between oxalate crystal deposition and poor long-term graft survival, declining kidney function and return to dialysis4,5,6,7 Unmet Need: Reduce Risk of Oxalate Damage to the Kidney
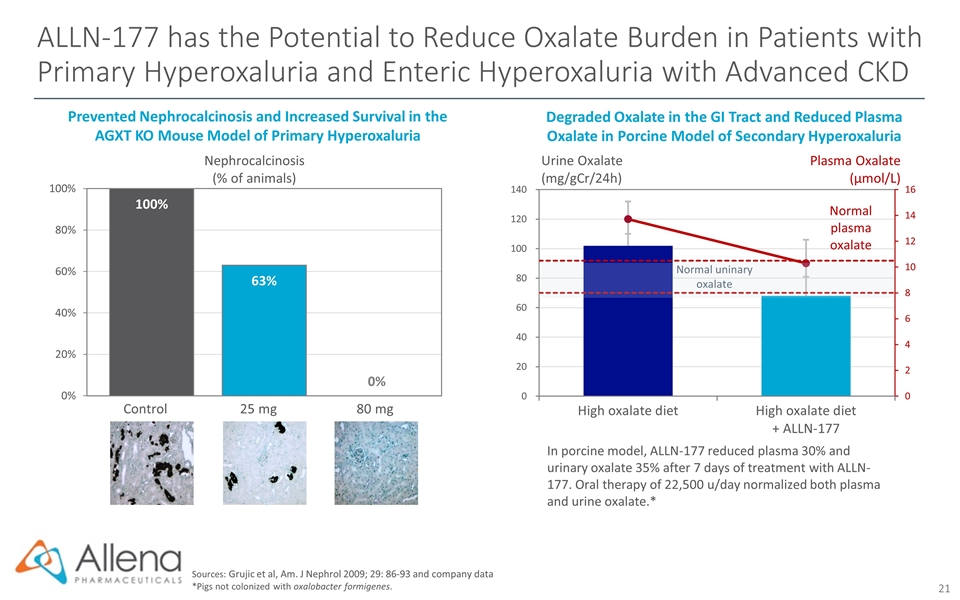
ALLN-177 has the Potential to Reduce Oxalate Burden in Patients with Primary Hyperoxaluria and Enteric Hyperoxaluria with Advanced CKD Sources: Grujic et al, Am. J Nephrol 2009; 29: 86-93 and company data *Pigs not colonized with oxalobacter formigenes. Degraded Oxalate in the GI Tract and Reduced Plasma Oxalate in Porcine Model of Secondary Hyperoxaluria In porcine model, ALLN-177 reduced plasma 30% and urinary oxalate 35% after 7 days of treatment with ALLN-177. Oral therapy of 22,500 u/day normalized both plasma and urine oxalate.* Urine Oxalate (mg/gCr/24h) Plasma Oxalate (µmol/L) Nephrocalcinosis (% of animals) Prevented Nephrocalcinosis and Increased Survival in the AGXT KO Mouse Model of Primary Hyperoxaluria Control 25 mg 80 mg
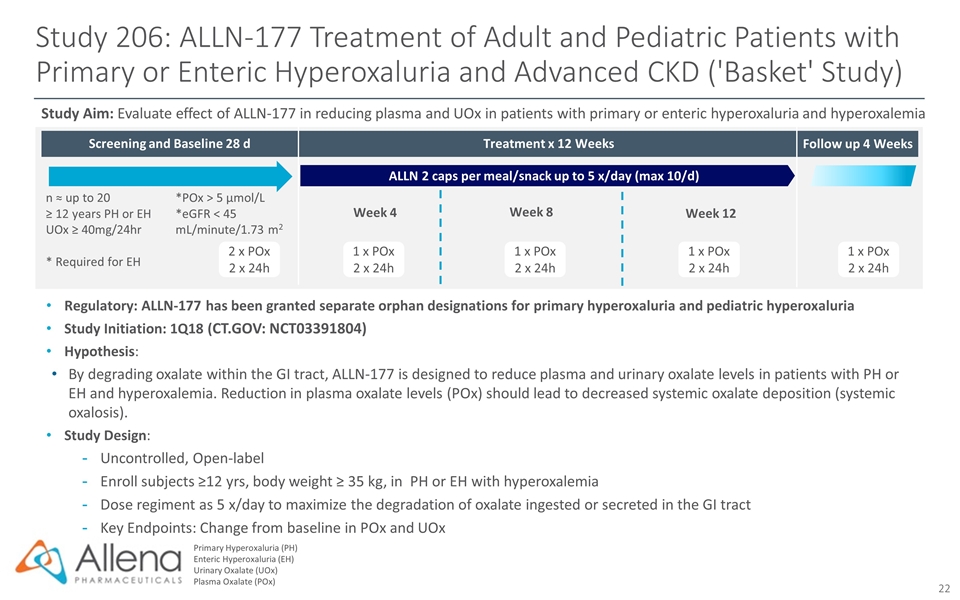
Screening and Baseline 28 d Follow up 4 Weeks Study 206: ALLN-177 Treatment of Adult and Pediatric Patients with Primary or Enteric Hyperoxaluria and Advanced CKD ('Basket' Study) Primary Hyperoxaluria (PH) Enteric Hyperoxaluria (EH) Urinary Oxalate (UOx) Plasma Oxalate (POx) Regulatory: ALLN-177 has been granted separate orphan designations for primary hyperoxaluria and pediatric hyperoxaluria Study Initiation: 1Q18 (CT.GOV: NCT03391804) Hypothesis: By degrading oxalate within the GI tract, ALLN-177 is designed to reduce plasma and urinary oxalate levels in patients with PH or EH and hyperoxalemia. Reduction in plasma oxalate levels (POx) should lead to decreased systemic oxalate deposition (systemic oxalosis). Study Design: Uncontrolled, Open-label Enroll subjects ≥12 yrs, body weight ≥ 35 kg, in PH or EH with hyperoxalemia Dose regiment as 5 x/day to maximize the degradation of oxalate ingested or secreted in the GI tract Key Endpoints: Change from baseline in POx and UOx Study Aim: Evaluate effect of ALLN-177 in reducing plasma and UOx in patients with primary or enteric hyperoxaluria and hyperoxalemia 1 x POx 2 x 24h n ≈ up to 20 ≥ 12 years PH or EH UOx ≥ 40mg/24hr * Required for EH *POx > 5 µmol/L *eGFR < 45 mL/minute/1.73 m2 2 x POx 2 x 24h 1 x POx 2 x 24h 1 x POx 2 x 24h 1 x POx 2 x 24h ALLN 2 caps per meal/snack up to 5 x/day (max 10/d) Treatment x 12 Weeks Week 8 Week 12 Week 4
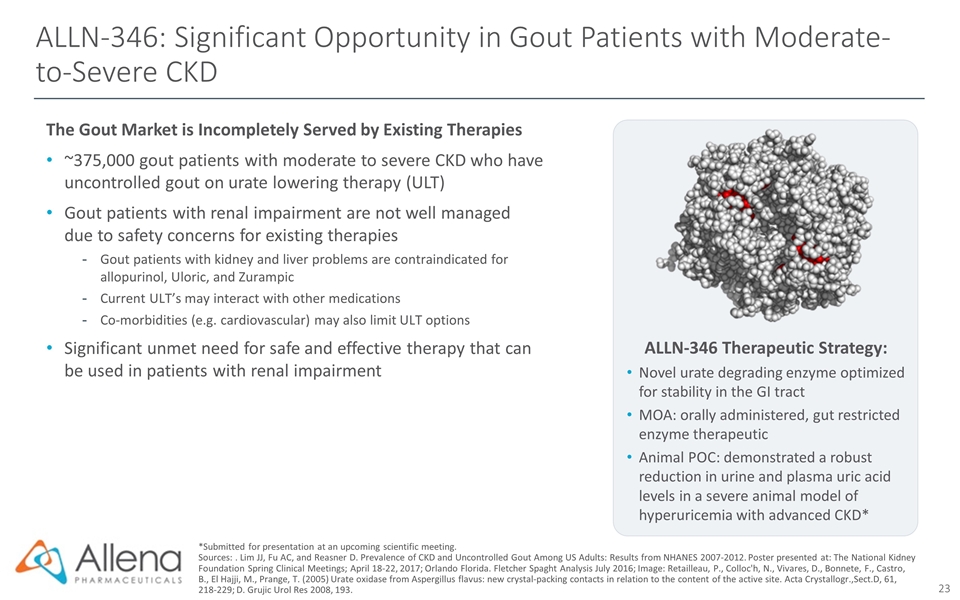
ALLN-346: Significant Opportunity in Gout Patients with Moderate-to-Severe CKD The Gout Market is Incompletely Served by Existing Therapies ~375,000 gout patients with moderate to severe CKD who have uncontrolled gout on urate lowering therapy (ULT) Gout patients with renal impairment are not well managed due to safety concerns for existing therapies Gout patients with kidney and liver problems are contraindicated for allopurinol, Uloric, and Zurampic Current ULT’s may interact with other medications Co-morbidities (e.g. cardiovascular) may also limit ULT options Significant unmet need for safe and effective therapy that can be used in patients with renal impairment *Submitted for presentation at an upcoming scientific meeting. Sources: . Lim JJ, Fu AC, and Reasner D. Prevalence of CKD and Uncontrolled Gout Among US Adults: Results from NHANES 2007-2012. Poster presented at: The National Kidney Foundation Spring Clinical Meetings; April 18-22, 2017; Orlando Florida. Fletcher Spaght Analysis July 2016; Image: Retailleau, P., Colloc'h, N., Vivares, D., Bonnete, F., Castro, B., El Hajji, M., Prange, T. (2005) Urate oxidase from Aspergillus flavus: new crystal-packing contacts in relation to the content of the active site. Acta Crystallogr.,Sect.D, 61, 218-229; D. Grujic Urol Res 2008, 193. ALLN-346 Therapeutic Strategy: Novel urate degrading enzyme optimized for stability in the GI tract MOA: orally administered, gut restricted enzyme therapeutic Animal POC: demonstrated a robust reduction in urine and plasma uric acid levels in a severe animal model of hyperuricemia with advanced CKD*
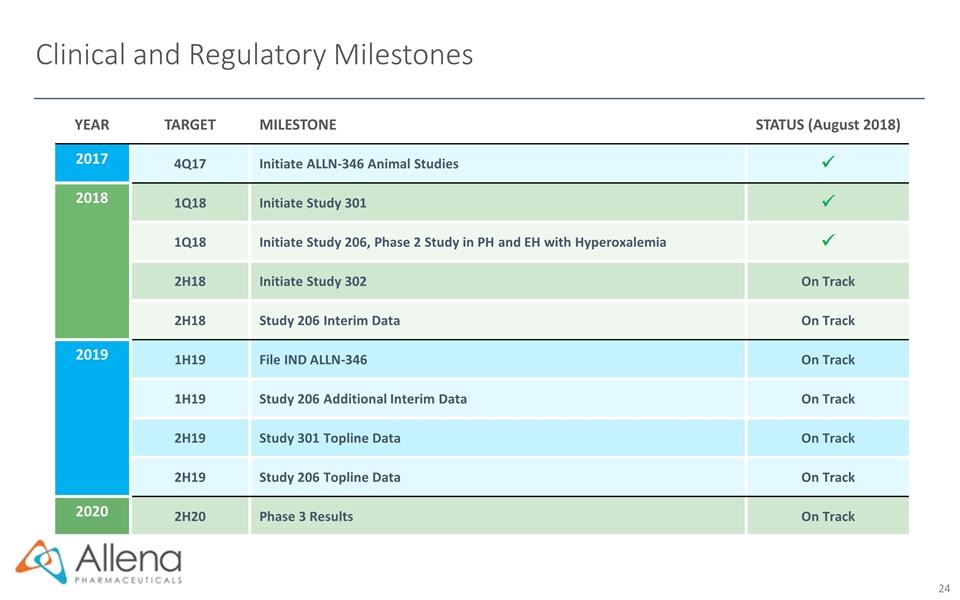
Clinical and Regulatory Milestones YEAR TARGET MILESTONE STATUS (August 2018) 2017 4Q17 Initiate ALLN-346 Animal Studies ü 2018 1Q18 Initiate Study 301 ü 1Q18 Initiate Study 206, Phase 2 Study in PH and EH with Hyperoxalemia ü 2H18 Initiate Study 302 On Track 2H18 Study 206 Interim Data On Track 2019 1H19 File IND ALLN-346 On Track 1H19 Study 206 Additional Interim Data On Track 2H19 Study 301 Topline Data On Track 2H19 Study 206 Topline Data On Track 2020 2H20 Phase 3 Results On Track
Investment Highlights Significant Unmet Need in Oxalate and Urate Disorders Late-Stage Development Candidate: ALLN-177 Pioneering Expertise in Oral Enzyme Therapeutics Focused on rare and severe metabolic disorders that can cause kidney stones, damage the kidney, and potentially lead to CKD and ESRD No approved oxalate therapies; potential untapped multi-billion dollar market First-in-class, oral therapy for severe hyperoxaluria Enrolling Phase 3 study, URIROX-1, in enteric hyperoxaluria, topline data expected 2H 2019 Enrolling Phase 2 basket study in other severe and orphan indications Approach enables treatment of metabolic diseases with oral, non-absorbed enzyme therapeutics GI MOA reduces subsequent metabolic burden on the kidney Raised $75M in successful initial public offering in 4Q 2017 Founded in 2011 with Frazier Healthcare Partners, Third Rock Ventures, and Bessemer Venture Partners. Additional private financing from HBM, Pharmstandard International, Partner Fund, Fidelity Management & Research Company Strong Support from Leading Biotechnology Investors
