Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - Zero Gravity Solutions, Inc. | ex_116571.htm |
| EX-31.1 - EXHIBIT 31.1 - Zero Gravity Solutions, Inc. | ex_116570.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
☑ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) |
|
|
|
OF THE SECURITIES ACT OF 1934 |
|
For the fiscal year ended December 31, 2017
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) |
|
|
|
OF THE SECURITIES ACT OF 1934 |
|
Commission File Number: 000-55345
ZERO GRAVITY SOLUTIONS, INC.
(Exact name of registrant as specified in its charter)
|
Nevada |
|
46-1779352 |
|
(State or other jurisdiction of Incorporation or organization) |
|
(IRS Employer Identification No.) |
190 NW Spanish River Boulevard, Boca Raton, Florida 33431
(Address, including zip code, of principal executive offices)
(561) 416-0400
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: None.
Securities registered pursuant to Section 12(g) of the Act:
|
Title of Each Class |
|
Name of Each Exchange on Which Registered |
|
Common Stock, $0.001 par value per share |
|
N/A |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐ No ☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act.
Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☐ No ☑
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registration statement was required to submit and post such files). Yes ☑ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
Non-accelerated filer |
☐ |
(Do not check if a smaller reporting company) |
|
|
|
|
Smaller reporting company |
☒ |
|
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐ No ☑
As of June 30, 2017, the aggregate market value of the registrant's common stock held by non-affiliates of the registrant was $17,565,231 in a private placement transaction of approximately $1.40 per share based on management's estimate of fair value (as there have been nominal to no trades that have occurred in the past twelve months on OTC Pink).
As of August 13, 2018, the registrant had 40,954,112 shares of common stock outstanding.
Documents incorporated by reference: None.
FORM 10-K ANNUAL REPORT
DECEMBER 31, 2017
TABLE OF CONTENTS
|
|
|
Page |
|
|
|
|
|
Item 1. |
1 |
|
|
Item 1A. |
18 |
|
|
Item 1B. |
19 |
|
|
Item 2. |
19 |
|
|
Item 3. |
19 |
|
|
Item 4. |
19 |
|
|
|
|
|
|
|
|
|
|
Item 5. |
Market for the Registrant’s Common Stock and Related Stockholder Matters |
19 |
|
Item 6. |
21 |
|
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
21 |
|
Item 7A. |
28 |
|
|
Item 8. |
28 |
|
|
Item 9. |
Changes in and/or Disagreements with Accountants on Accounting and Financial Disclosure |
28 |
|
Item 9A. |
29 |
|
|
Item 9B. |
30 |
|
|
|
|
|
|
|
|
|
|
Item 10. |
30 |
|
|
Item 11. |
34 |
|
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
37 |
|
Item 13. |
Certain Relationships and Related Transactions and Director Independence |
40 |
|
Item 14. |
43 |
|
|
|
|
|
|
|
|
|
|
Item 15. |
44 |
FORWARD-LOOKING STATEMENTS
Statements in this report may be "forward-looking statements." Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates and projections about our business based, in part, on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Therefore, actual outcomes and results may, and are likely to, differ materially from what is expressed or forecasted in the forward-looking statements due to numerous factors, including those described above and those risks discussed from time to time in this report, including the risks described under "Risk Factors" and any risks described in any other filings we make with the SEC. Any forward-looking statements speak only as of the date on which they are made, and we do not undertake any obligation to update any forward-looking statement to reflect events or circumstances after the date of this report.
Management’s discussion and analysis of financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. On an on-going basis, we evaluate these estimates, including those related to useful lives of property and equipment, the allowance for doubtful accounts and stock based compensation. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. There can be no assurance that actual results will not differ from those estimates.
|
BUSINESS |
Company Overview
Organizational structure
Zero Gravity Solutions, Inc. (“we”, “us”, “our”, the "Company", “ZGSI” or the "Registrant"), a Nevada corporation, is an agricultural based biotechnology company focused on commercializing technology derived from, and designed for long term spaceflight and planetary colonization with significant applications to agriculture on Earth. These technologies are focused on improving world agriculture by providing valuable solutions to challenges facing humanity, including climate change stress and soil degradation threats to agricultural production and the increasing inability to feed the world’s rapidly growing population. The Company’s business model focuses on two primary business segments: 1) BAM-FX™ which is a cost effective, ionic nutrient delivery platform for plants that delivers minerals and micronutrients systemically at the cellular level of a plant, and 2) Directed Selection™ which relates to the production and alteration of new varieties of novel stem cells with unique and beneficial characteristics in the prolonged zero/micro gravity environment of the International Space Station. These novel stem cells, if developed, could be patented for commercial sale to third parties in the agricultural and human regenerative medical markets. ZGSI is headquartered in Boca Raton, Florida.
Our business activities are separated between two wholly owned subsidiaries BAM Agricultural Solutions, Inc. (“BAM Inc.”) oversees BAM-FX™ commercial introduction and business development through product trials and validation with crop growers and distributors that have established business networks in agricultural markets, manufacturing, sales and agronomy support. Zero Gravity Life Sciences Inc. (“ZGLS”) is responsible for any space research projects, life science applications of our technology and conducting research on future BAM Inc. product lines. We believe that the separation of these functions and the corresponding allocation of management by expertise will enable us to improve our performance and provide focus on our different business activities.
Operating Activity
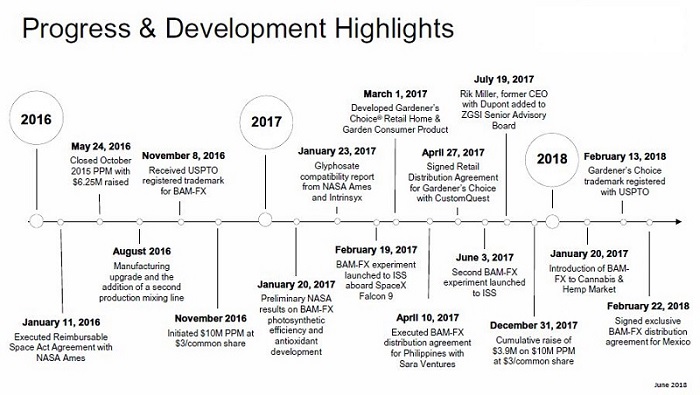
The Company is focused on near-term revenue generation through the introduction of the Company’s first commercial product, BAM-FX™, to domestic and international agricultural markets. BAM-FX™ is an enabling technology platform, registered as a fertilizer application with thirty-three (33) domestic states and approved for commercial import and sale in Chile, Colombia, China, Paraguay and Brazil.
During 2017, the Company devoted significant resources to domestic and international trials with both growers and independent third parties. Commercial agricultural markets generally adopt new products after a number of years of successful trials showing consistent results. The Company believes that BAM-FX™ has begun to gain market recognition and acceptance. With continuing product technical support for application timing and dosage rates, validation of the technology attributes and proper product pricing providing growers an appropriate return on their investment, the Company expects to see growers purchase commercial quantities of BAM-FX™ either directly from the company or through the Company’s distributors during 2018, domestically and internationally.
In excess of one hundred and fifty trials were conducted during 2017. The trials included both grower experience and third-party validation on crops including, but not limited to, tomato, grape, berry, onion, soybean, wheat, cotton, melon, stone fruit, corn, tobacco and cannabis. We believe successful grower experience trials positively affect marketing effort by word of mouth. For commercial markets to develop rapidly, third party validation trials are necessary. A number of third-party validation trials planned for 2017 were not funded due to lack of financial resources. As a result, our team of six agronomists and certified crop advisors confined their 2017 efforts to identifying appropriate product application timing in crop growth cycles and dosage rates to maximize yield, quality and plant health.
For 2018, the Company plans to focus on a limited number of crops that have high market value for the grower and have shown significant yield and quality improvement from prior tests. Final trial results for tobacco crops from 2017 were completed during the first quarter of 2018 and showed an increase in crop yield of 25% using BAM-FX™ over the control crop in certain trials. Although we believe that these yields are replicable in growing conditions conducted, we continue to refine our application protocols for yield improvement in both domestic and international growing conditions. The Company expects to target tobacco crop growers in 2018 in addition to growers in other high market value crops, with which successful trials have been conducted including grape, soy bean, avocado and berries. Also, based upon significant improvements in yield and quality on medicinal cannabis from testing with growers in Mendocino and Humboldt Counties in California, the Company will add cannabis to its list of target crops for 2018.
BAM Agricultural Solutions, Inc., has entered into a License and Distribution Agreement with a group of Mexican businessmen with longstanding relationships with markets in Mexico and a successful history in building companies in Mexico and in the US. The initial crops they will be working with are avocados and strawberries.
Domestic commercial activity:
The Company began conducting domestic product trials on a variety of crops in laboratory and academic settings as well as in field applications on grower/end-user crops during 2014 and expanded field applications with those and additional trial participants in subsequent years. Results from trials during 2016 on a variety of field crops, vegetables and fruits, a number of which were validated by independent accredited third-party laboratories or academic institutions, showed significant yield, nutrient and biomass improvements for crops treated.
In 2016, the Company shifted its sales focus from direct sales to growers toward developing relationships with potential channel partners. In general, a channel partner is an established agricultural product distributor and/or service provider with which the Company has executed a distribution agreement. Three distribution agreements with agricultural product distributors in Ohio and California were signed during 2016, two of which are active, including an agreement with Pinnacle Agriculture Distribution, Inc., ("Pinnacle"). Pinnacle is a multifaceted agricultural retail distribution business with operations including agricultural chemicals, fertilizer bulk handling and precision agricultural services. During 2017, the Company executed distribution agreements with two additional regional domestic channel partners, one in Ohio and one in Pennsylvania. We expect our channel partner strategy will begin generating product revenue during the 2018 growing season, given sustained technical support for the product introduction process with their customers and appropriate product pricing for the channel partner’s financial incentive and growers’ investment return.
While continuing to focus on a channel partner strategy and our technical product development fieldwork, the Company began discussions with three large prospective domestic agricultural industry leaders. The goal is to explore technological synergies and product integration strategies adding BAM-FX™ into their existing product lines to enhance the effectiveness of their products. We believe the Company’s technology is a delivery platform or product enhancement that, when incorporated into other agricultural products, for example fertilizer, the resulting product's effectiveness is improved. The Company plans to pursue these opportunities as part of its 2018 business strategy.
International commercial activity:
International business development efforts that begun in 2015 continued throughout fiscal 2017. The Company’s international business strategy, in general, is to engage existing agricultural entities, which have strong distribution channels within a country, that are financially able to introduce new products into local agricultural markets. We have initiated business development activities through existing management relationships or trusted referrals. The Company undertakes the cost of developing the relationship, provides technical product support including research, trial data and product application protocols. The local entity contracts directly with the Company and incurs the cost of business development efforts within the country. By the end of 2017, we completed two years of product efficacy and business development work in India and one year in China, Colombia, Paraguay and Brazil. Product efficacy and registration for import in Haiti began in 2017.
The Company executed an exclusive distribution agreement with a distributor in Paraguay during 2016, with a minimum purchase requirement of seventy-four thousand (74,000) gallons during the first year of the agreement to maintain exclusivity. We expected to deliver an initial order of BAM-FX™ to our Paraguay distributor during the fourth quarter of 2016. The order was delayed until the second quarter 2017, due to the distributor’s need to finalize Paraguay’s government product testing requirements for commercial import and obtain import approval from Servicio Nacional de Calidad y Sanidad Vegetal y de Semillas ("SENAVE"), the government agency overseeing agricultural imports. BAM-FX™ tests were successfully completed by the Paraguay Agricultural and Technological Center (CETAPAR) in January 2017 and submitted to SENAVE. BAM-FX™ was registered and approved for commercial sale in Paraguay during the second quarter of 2017.
The distributor in Paraguay completed commercial soybean trials and compiled results from trials begun in 2017 during the first quarter of 2018, which showed grower benefits resulting in an increased return on their investment. We are currently evaluating and exploring mutually beneficial business models to exploit this market, which will include re-negotiating the terms of our existing distribution agreement. The distributor in Paraguay, with the Company's financial support, began to target sales to sub-distributors and commercial growers of row crops, primarily wheat, corn and soybeans during 2017. If the Company reaches acceptable terms with our distributor, we believe these ongoing efforts will result in a pragmatic strategy to begin realizing revenue during the third quarter of 2018.
BAM-FX™ was introduced to the South American countries of Colombia and Brazil during 2016. The Company contracted with Fundacao de Apoio A Pesquisa Agricola (FUNDAG), a private nonprofit entity established under the laws and registered with the Brazilian government, which provided positive product test results for the Brazilian government’s product registration and import approval process. A Brazilian channel partner was identified during 2017, introduced to the Company by one of the Company’s agronomy and science consultants affiliated with the University of Florida, familiar with agricultural markets and distributors in Brazil. The channel partner is finalizing the registration of the product for import and sale.
The Company contracted with an independent Colombian laboratory during 2016 to test and provide evidence of effectiveness of BAM-FX™ for the Colombian government’s registration and approval to commercially import product, which we received during 2017. The Company engaged an import-export company with existing business affiliations and operations in Colombia during 2016 to introduce BAM-FX™ to commercial agricultural markets in Colombia, pursuant to a foreign business development consulting agreement. The Company expects to negotiate and conclude product distribution agreements with Colombian entities during 2018.
Pursuant to a non-exclusive distribution agreement executed in 2015 for certain countries in the Asia Pacific markets, a distributor engaged a potential sub-distributor in India during 2016, Nurture Earth R&D Pvt. Ltd, Nurture Earth. Nurture Earth conducted a number of trials during 2016 and 2017 primarily on pigeon pea, cotton, vegetables and tomato. Some vegetable results showed yield improvements of approximately fifty percent (50%). Tomato trials showed a yield increase of approximately forty eight percent (48%) of harvested fruit weight and approximately eighty three percent (83%) in seeds. Furthermore, seed treat results on pigeon pea showed positive germination and survival results using specific product dilution rates. The Company expects to complete registration to import BAM-FX™ in India for commercial sale during the third quarter of 2018.
The Company contracted a business development entity with offices in the US and China, Asia 21 Communications, LLC, "Asia 21", as its project manager for marketing and product testing in China during 2017. The results of the trails during 2017 in China were positive in certain crops and have encouraged growers to replicate the trials in 2018, the second year of tests in grapes, rice and tobacco. Trials in wheat, soybean, berries, tomato and stone fruit showed either marginally improved crop yield or yield less than ten percent (10%), which growers consider statistically insignificant. We believe these marginal results are due to application protocols which were not optimized. A number of potential distributors have been identified in China, however, the Company has no formal distribution agreements with distributors in China, at this time. Although we expect to generate nominal revenue from sales to growers through Asia 21, we do not expect to generate significant revenue through distributors in China until 2019. On February 12, 2018, the Company was notified that BAM-FX™ was approved for import and commercial sale within China.
During the fourth quarter of 2017, the Company entered into a distribution agreement with a group for product sale and distribution to the government of Haiti. The distributor’s contract for sale and distribution is currently being finalized with the government of Haiti with an expected initial order of 50,000 gallons of BAM-FX™ during 2018. Orders pursuant to the distribution agreement are currently pending Haitian government approval for import and sale. The approval, depends upon results of product efficacy testing with expected completion during the third or fourth quarter of 2018.
After a review of ongoing costs associated with the Company’s operations in Chile and potential for near term commercial sales and collection of accounts receivable, the Company determined during the fourth quarter of 2017 that the cost associated with penetrating and maintaining the Chilean market would require significant additional resource commitments during 2018, which we determined would be better utilized in markets with shorter term sales prospects. The Company began supporting the Chilean operations financially during 2015. Initially the Company expected the Chilean operation would obtain and provide its own financing to support operating as a distributor in the country. The expected funding was not secured and the probability of obtaining funding during 2018 appeared unlikely. We believe it is necessary to engage an established and financially stable distributor to continue developing the market for BAM-FX™ in Chile. The Company expects to resume business development efforts when financial resources are available to identify, engage and support a distributor.
We employ the technical talents of certified crop advisors and agronomists, both domestically and internationally, to support our commercialization and revenue generation efforts. During 2016, the Company added qualified sales management and agronomy personnel to develop domestic channel partner relationships and provide technical product support to growers and channel partners. During the second half of 2017, the Company began to focus on crops with high market value for growers enabling the grower to realize a return on the cost of purchasing and applying BAM-FX™. As a result of the change in focus, the Company began to reduce the number of technical consultants and advisors employed domestically. Internationally, in India, China and Paraguay, the Company has maintained or increased the use of contracted technical and business development talent to introduce BAM-FX™ into those markets.
During the third quarter of 2016, the Company formed an agronomy and science team to integrate agronomy and crop advisor personnel and centralize technical product know-how in Asia and North and South America. The agronomy and science team provides technical support to growers and channel partners in those markets. In addition, the team maintains the Company’s relationships with science advisors at the NASA-Ames Research Center, NASA ARC, which were developed pursuant to a Reimbursable Space Act Agreement ("RSAA") executed in January 2016. The Company expects to maintain its domestic agronomy trained personnel during 2018 while adding technical consultants, as needed, for business development in the Asia Pacific and South American markets.
Domestic Retail Market Activity
During 2016, the Company began developing a business strategy to enter the home and garden retail market with a specially formulated ready to use offering of BAM-FX™. During 2017, the Company applied for the trademark, Gardner’s Choice, the name of the planned retail product. On January 10, 2018 the Company received notice of allowance for the Gardner's Choice trademark from USPTO and expects to receive official registration during 2018.
Although we expected to test market acceptance using pilot programs in certain big box stores during 2017, the Company determined that additional product development work was needed. During 2017, product efficacy, stability and safety validation work was expanded beyond the work initiated in 2016 which, we expect to complete during the third quarter of 2018. We engaged a seasoned retail market and brand consultant to assist us with our retail product business strategy and roll out during 2017 and designated personnel within the Company to execute the strategy.
The Company executed a non-exclusive distribution agreement in 2017 with CustomQuest, Inc., a subsidiary of Victory Wholesale Group, to lead the introduction into the retail market through their existing infrastructure and relationships. Sales and presentation materials for retail consumers and buyers, product packaging and retail point-of-sale displays have been developed along with a strong electronic and social media presence. The Company has contracts in place with a product fulfillment vendor to mix, bottle, label and ship the product. The Company also works with a service group to provide state registration and regulatory and compliance assistance.
Our response from attending industry trade shows, including the Ace Hardware 2018 Spring Convention and the GIE Expo 2017, and initial marketing efforts to several co-op and big box buyers at both regional and national levels has been positive with strong interest in third quarter 2018 product placement. We expect additional follow on activities to encourage sales which, include increased marketing through traditional, electronic and social media.
Specialty Crop Activity
In 2016, a licensed grower in Mendocino Country provided the Company anecdotal information on significant yield improvement the grower realized by applying BAM-FX™ to cannabis plants. The grower conducted the work without the Company’s participation. As a result of yield improvement reported, the Company participated in two independent trials during 2017 on medical cannabis in California with growers licensed with the State of California. A trial in Mendocino County resulted in an increase in cannabinoid levels from 15% in the control crop to approximately 23% for the crop treated with BAM-FX™. Complete yield results were not available for this trial as wildfires caused partial crop damage. A second trial in Humboldt County, California, with a licensed grower, showed an approximate forty four percent (44%) yield increase using BAM-FX™ over the control group.
With the positive trial results obtained and the number of inquiries the Company received about the use of BAM-FX™ on cannabis crops during the fourth quarter of 2017, the Company has added cannabis and hemp as target crops for 2018. The Company will restrict its business activities to sale and distribution of product, providing the picks and shovels to growers. During 2018, the Company expects to refine and optimize product formulations for this market, which is expected to create premium pricing for the product.
Manufacturing Operations
We operate a manufacturing facility in Okeechobee, Florida. Manufacturing is conducted on an as needed batch process. During 2016, the Company made process improvements and modifications to existing equipment and facilities to enhance efficiency, comply with regulatory requirements, control the quality of raw materials used in production and consistently provide customers a quality product. The Company added a second product mixing line to increase production capability for expected product demand and a reverse osmosis water treatment system for consistent water quality. These improvements addressed product variability resulting from raw material inputs. Manufacturing operations during the second half of 2017 concentrated on process and procedure documentation, quality control and production precision.These efforts addressed variances that could affect product performance. The benefits expected from these efforts include consistent product chemistry and process replication, providing the ability to scale and replicate the production process in other locations.
Directed Selection™
The Company’s long-term objectives include the commercialization of ZGSI’s space derived Directed Selection™ technology, which predicts that plant and animal stem cells exposed to prolonged microgravity in space can be endowed with new characteristics beneficial to society. In previous years, with our collaborators, the National Aeronautics and Space Administration (“NASA”), the United States Department of Agriculture (“USDA”), and the University of Florida, we conducted scientific studies on six NASA sponsored flights to the International Space Station (“ISS”). These experiments demonstrated that the gene expression of plant cells in zero/microgravity changes substantially, and furthermore, provided us with strong evidence that these changes can be directed toward beneficial attributes.
Future Directed Selection™ research will aim to produce new varieties of proprietary, patentable stem cells for plants with desirable traits, such as the ability to better survive environmental challenges (i.e. temperature or climate change) or to resist disease. Adaptive changes in the plant will be accomplished without the need for additive or subtractive genetic engineering, thus eliminating public concerns about genetically modified organisms (“GMO”). The plant still uses the capabilities that are a natural part of its genetic potential through altered gene expression that enables adaptation toward valuable traits. Another important aspect of our space derived intellectual property capitalizes on space flight experiments showing the unique effects of zero/microgravity on mammalian cells. Space-based technology imparts the likely ability to reproduce undifferentiated multi-potent human stem cells in substantially larger quantities and more rapidly than is possible on Earth. Long-term goals include revolutionary advancements in stem cell differentiation, cellular macrostructure assembly, vascularization and wound healing. Because both humans and animals use very similar metabolic pathways, we also expect to produce patented stem cells that can provide beneficial treatments to commercial livestock. We believe that this area may lead to significant future business opportunities for the Company. We expect that research funding will be deferred beyond 2018 unless cash flows from our operations provide sufficient funds to support those efforts or funding is designated by equity investors or grants.
Research and Development Activity
The Company developed scientific relationships with NASA ARC, Ohio State University. UC Davis, Utah state, University of Iowa and Penn State University during 2016 to understand and validate the science incorporated in BAM-FX™ and identify additional commercial attributes. The Company did not have the financial resources to fully exploit these relationships during 2017 due to financial constraints.
Pursuant to the Company's Reimbursable Space Act Agreement, with NASA ARC, the Company has access to NASA scientists and laboratories. Because the RSAA is reimbursable agreement, whereby the Company reimburses NASA for technical services, the Company retains ownership of intellectual property resulting from the research work performed.
NASA ARC used its unique, ground-based, controlled environment facilities and research personnel to address a series of questions relevant to both ZGSI and NASA during 2016 through the first half of 2017. The scientific data is jointly evaluated by ZGSI and NASA ARC to determine utility of the ZGSI products to commercial agriculture and NASA applications. First year research pursuant to the RSAA was completed during the first half of 2017. The studies were conducted in both soil pots and hydroponic systems with near optimal nutrient supply. The results, which showed a significant improvement in plant productivity, suggest that the effects of BAM-FX™ include improvement in nutrient use efficiency and enhancement of photosynthetic efficiency which causes an increase in the amount of biomass produced per unit of light energy absorbed by the plant.
As jointly agreed and based on evaluation of the scientific data, space flight opportunities have been identified to test our potential role in achieving NASA goals in plant-based space biology and life support pursuits. On February 21, 2017, the Company’s research experiment using BAM-FX™ was successfully delivered to the ISS, launched from the Kennedy Space Center on February 18, 2017. In collaboration with NASA and Inrinsyx Technologies Corporation (“Intrinsyx”), the experiments studied the growth and nutritional effects of BAM-FX™ in broccoli seedlings in microgravity, advancing scientific knowledge to promote the growth of fresh, nutrient-dense food for astronauts on long duration space missions. In a report issued by Intrinsyx, the space flight experiment test was successful as the results obtained during space flight supported the initial hypothesis that using BAM-FX™ in plant growth media enhanced broccoli growth for both roots and shoots over untreated controls with an approximate doubling of growth size and fivefold greater dry weights obtained on orbit. The Company had an additional launched research equipment on June 3, 2017 in collaboration with Intrinsyx and will have third launched research experiment during the third quarter of 2018.
From the scientific work of NASA ARC, ZGSI expects to understand BAM-FX’s mode of action and physiological effects leading to increased yields currently observed on earth in commercial agricultural systems and address the science questions of interest to ZGSI and queries from channel partners. Additionally, ZGSI expects to discover if BAM-FX™ truly has applications for growing plants in the controlled conditions of spaceflight and for human colonization of other worlds. ZGSI plans to optimize BAM-FX™ application methods in both controlled environment agricultures on earth and in space flight applications.
Pending adequate financial resources, we plan to fund additional research and continue working with NASA ARC in 2018 to finalize: the preliminary results of our 2016 and 2017 studies; identify the scientific evidence of the action of BAM-FX™ within the plant structure; and refine predictable crop and environmental dosage application rates to support precision agricultural applications for growers.
During January 2017, Intrinsyx and NASA ARC jointly issued results of their research showing BAM-FX™ is compatible with glyphosate resistant crops, primarily Roundup Ready crops. Roundup Ready crops are crops genetically modified to be resistant to the herbicide Roundup. Roundup is the brand name of an herbicide produced by Monsanto Corporation with the active ingredient, glyphosate. Glyphosate is a broad-spectrum systemic herbicide and plant desiccant or drying agent. The 2014 Agricultural Chemical Use Survey published by the United States Department of Agriculture’s National Agricultural Statistics Service reported that glyphosate was applied to 38% of domestic planted acres. The result of this joint scientific research allowed the Company to remove labeling and instructions warning against use with glyphosate, thereby eliminating a significant marketing impediment for our product.
We believe the Company’s proprietary technologies enable the development of a diverse product line with unique solutions to big problems, representing significant potential revenue and profit opportunities. Due to the market scope and the associated regulatory requirements to reach full profit potential, ZGSI’s strategy is to enter into channel partnerships by executing distribution agreements, domestically and internationally, with industry-leading agriculture product distributors which could leverage our collective resources and expedite revenue generation. In addition, we believe our proprietary technologies represent a delivery platform or enabling system that could enhance the performance of agricultural inputs for other agricultural companies’ products. We initiated discussions related to product integration strategies with three companies during 2017. These companies expressed interest in the following collaborative efforts: integration of our technology in retail fertilizer products; integration of our technology in wholesale fertilizer products; and seed treat applications to improve germination and add new competitive product claims. We believe collaborative product integration and associated research will be a significant component of our business activity in 2018.
Corporate History and Structure
Zero Gravity Solutions, Inc. was initially incorporated in the State of Idaho as Hazelwood-Gable, Inc. on August 19, 1983 to engage in the business of developing mineral resources. The Company engaged in limited operations until 2003. On June 19, 2002, the Company changed its corporate domicile to the State of Nevada and, on November 25, 2003, changed its name to American Thorium, Inc. On December 10, 2003, the Company entered into an agreement to acquire certain mining claims and changed its name to Thorium Nuclear Energy, Inc. on March 5, 2004. On March 8, 2006, the Company changed its name to Hazelwood Ventures, Inc. and began looking for new business ventures. On June 14, 2006, the Company changed its name to Monarch Molybdenum & Resources, Inc. and on November 27, 2007 entered into a merger agreement, whereby it was to acquire certain mining claims from Thorium Energy, Inc. and changed the Company's name to Thorium Energy, Inc. The merger agreement was rescinded on March 19, 2008 and the Company changed its name to Monolith Ventures Inc. on April 2, 2008 and with the intent to acquire or merge with one or more businesses. On December 30, 2011, the Company acquired ElectroHealing Holdings, Inc., which held certain patents, patent applications and other technologies and/or licenses pertaining to medical device technology and changed its name to ElectroHealing Technologies, Inc. on January 12, 2012. Subsequent to the transaction, management re-evaluated the merits and benefits of the ElectroHealing Holdings, Inc. acquisition and began to explore possible alternatives. Accordingly, we executed a rescission agreement on December 20, 2012 that returned the previously acquired patents and technologies in exchange for the cancellation of the shares of our common stock previously issued in the acquisition.
Formation of ZGSI and the Company’s Current Structure
On December 3, 2012, we entered into a Patent Acquisition Agreement with John W. Kennedy whereby we acquired certain patents applications and technologies related to the Company’s current Directed Selection™ business segment. In connection with the acquisition of this technology, we changed our name to Zero Gravity Solutions, Inc. on January 11, 2013 to better reflect our new business endeavors, and issued 11,500,000 shares of our authorized, but previously unissued common stock. In connection with this acquisition, we moved our corporate headquarters from Salt Lake City, Utah to its current location at 190 NW Spanish River Boulevard, Suite 101, Boca Raton, FL 33431.
On July 30, 2013, John W. Kennedy assigned to the Company the entire right, title and interest in, to and under the invention and provisional patent application for Bioavailable Minerals for Plant Health which became the basis for the Company’s BAM-FX™ business segment.
The Company currently has two wholly owned subsidiaries: BAM Agricultural Solutions, Inc. (“BAM Inc.”) and Zero Gravity Life Sciences, Inc. (“ZGLS”). BAM Inc. is a Florida Corporation that holds and controls the manufacturing, sales and revenue generation of the Company's agricultural product, BAM-FX™. ZGLS is a Florida Corporation that holds and controls the Company’s Directed Selection™ business segment and the related research and development work. The Company had previously wholly owned Zero Gravity Solutions, LTD (ZGS Ltd.), and BAM Agricultural Solutions, LTD. (both private United Kingdom limited companies based in the United Kingdom) which had been created to service the Company's expected operations and interests in the European Union (EU). During the fourth quarter 2015, both UK companies were liquidated, and the financial resources redeployed to markets in the United States and Chile.
The Company currently has 29 employees, of which 22 are full-time employees. The Company has selected December 31st as its fiscal year end.
Principal Product Lines
BAM-FX™
BAM-FX™ is a mineral delivery platform for food crops. BAM-FX™ was originally developed by John W. Kennedy, our Chief Science Officer and former director, to grow food crops in space vehicles designed for deep space human missions, but has been found to have potentially far reaching applications for agriculture on Earth. BAM-FX™ is a proprietary formulation, currently including micronutrients zinc and copper, used to improve plant performance by providing a delivery platform that allows plants to more efficiently uptake nutrients. BAM-FX™ is not classified as a genetic modification (“GMO”). The BAM-FX™ formula, works as an enhancement of other agricultural plant treatment regiments. BAM-FX™ enhances the uptake of nutrients and it is capable of improving crop yields and potentially reducing input costs.
BAM-FX™ can be applied by seed soak/treat, soil application using drip irrigation systems or as a foliar treatment of the plant at later stages of the plant’s development.
Academic and grower product trials, validated by independent laboratories and academic institutions, showed BAM-FX™ treated plants exhibit faster germination, higher yields, increased plant resistance to harmful environmental factors and higher quality fruit and vegetables. Trials have shown that applications of BAM-FX™ on various crops reduces the need for multiple applications of other products, which we believe will save growers time and money while creating faster growing, healthier, and more robust plants. Additionally, we believe BAM-FX™ may provide significant value for farmers, growers, and world agriculture through:
|
● |
Observed increased plant stress tolerance; |
|
● |
Faster time to maturity and harvest with higher farm yields for food crops; |
|
● |
Improved delivery of targeted nutrients and minerals to crops requiring lower energy to uptake, creating a more robust plant and nutritious crop; |
|
● |
Increased nutritional quality of grains and produce including the potential to engineer nutrition into our food; and |
|
● |
Reduced ecological concerns associated with agricultural runoff pollution into water sources as phosphate or nitrate usage is improved. |
Food crops offer the best source of nutrition for humans since many supporting nutrients require organic carriers and are not normally absorbed by using vitamin and mineral supplements alone. As BAM-FX™ can be applied to any plant, we anticipate a substantial demand for BAM-FX™ to support improved crop health and nutrition.
BAM-FX™ Trials
In 2014, BAM-FX™ product development and commercialization activities spanned a wide range of studies in terms of crop plants, test parameters, geographic region, and potential customer base. In 2015, we conducted second season tests with a number of growers to replicate the first year’s positive results. The trials were primarily foliar applications during the growth cycle of the crops, including but not limited to wine grapes, table grapes, avocados, beans, tomatoes, rice, berries, stone fruit, corn, onions, barley, wheat, and leafy greens. In addition to showing product efficacy, the trials enabled us to begin branding BAM-FX™ and provided word of mouth support within the local farming community to attract potential channel partner interest in BAM-FX™ and its performance.
During 2016, we shifted some of our resources from field trials for performance data to scientific efforts with universities and NASA ARC to obtain third party data scientifically validating the results and understanding BAM-FX™ mode of action. Scientific efforts were conducted through NASA ARC under the RSAA and in connection with studies at the University of Florida, Tropical Research and Education Center ("TREC") which is a division of the University of Florida, Ohio State University, Penn State University, University of Iowa and Florida International University. BAM Inc. began a product marketing approach on grower’s field validations, whereby specific expected crop results were identified at the inception of the validation process and upon achieving the expected results over multi-year growing cycles, a grower would agree to purchase the product. In addition to being part of our business development effort, the Company expected to develop brand name recognition and provide pull through business for prospective channel partners.
The Company initiated trials on the effects of BAM-FX™ on medicinal cannabis with certified licensed growers on outdoor crops as well as crops in a highly controlled internal environment during 2017. The same positive results we have seen with commercial agriculture crops were also evidenced with cannabis.
The Company conducted in excess of one hundred and fifty (150) domestic trials in addition to trials conducted in India, China, Chile, Paraguay, Brazil and Columbia during 2017. The majority of our domestic trials were intended to either replicate prior year results or introduce BAM-FX™ to the grower and the grower’s agricultural input supplier, either an existing or potential channel partner. In addition, the Company’s agronomy team worked to identify protocols for application rates and timing for a number of crops to maximize BAM-FX™ performance. Based upon the result of those trials the Company identified a target list of crops for 2018 efforts which include berry, grape, avocado, soy bean, tobacco, cotton and cannabis. We expect to continue work on certain row and vegetable crops, however the emphasis for those crops is expected to include seed treat applications, which have shown significant potential economic benefit to growers.
Revenue Cycle
The commercialization cycle for new agricultural products is minimally two to three growing seasons. Our 2015 trials included a mix of independent laboratory tests, first year small-scale trials with growers and second season multiple acre trials with growers. Demonstrating consistent product performance is one key to obtain product orders from growers in the United States. During 2016, in connection with NASA ARC, we began developing product application dose rates and response curves scientifically to enable us to predict outcomes and support consistent performance.
Although United States growers have not historically been innovators and have required multiple growing season trials before adopting new products and technologies, our 2014 and 2015 domestic trial results were instrumental in opening commercial markets in Asia, Mexico and South American countries.
During the fourth quarter of 2016, the Company entered into an agreement with Global Biotech, S.A.S. a Colombia agricultural testing and analytic laboratory, to evaluate BAM-FX™ through a series of product application tests to obtain approval for commercial import. We received registration approval through the Colombia Institute of Agriculture (ICA) during 2017 for commercial import. We are working with a business development consultant to identify a Colombian channel partner to sell, market and distribute BAM-FX™ within Colombia.
The Company conducted initial market analysis in China with the assistance of a contractor, Asia 21, focusing on the sale and distribution of products and solutions into China and providing local operational support, management and services. Asia 21 has offices in the United States and China. Registration with the China Ministry of Agriculture for approval to import and sell BAM-FX™, which began during the fourth quarter of 2016, was received in February 2018. In connection with BAM-FX™ registration testing requirements, the Company contracted with the Shanghai Agricultural Institute to develop BAM-FX™ test data and technical reports for government import approval.
The Company began introducing BAM-FX™ to Asia Pacific markets in 2016, targeting potential distributors through existing business relationships. High-value crop and row crop product evaluation is currently in process in a number of Asia Pacific countries including India and Philippines.
During February 2017, the Company announced that it had signed an exclusive distribution agreement with Bison Africa Capital (Pty) Ltd. (“Bison”), an emerging investment holding company based in South Africa and a leader in building competitive and representative South Africa companies. The parties did not actively pursue South African markets in 2017 and have mutually agreed to continue business development efforts with the expectation of beginning the registration process for commercial sale during 2018.
DIRECTED SELECTION™
Directed Selection™ is a proprietary technological method designed to use the unique conditions of near-zero gravity in low earth orbit to create plants and animal cells that have beneficial traits that we believe would have value to society. This technology predicts that plant and animal stem cells exposed to prolonged microgravity in space can be endowed with new characteristics.
Life on Earth has always developed within the confines of gravity. About 50% of the energy expended by terrestrial-bound plants is dedicated to structural support in order to overcome gravity. By removing gravity from the equation, plant cells in a weightless environment have an excess of energy. This relatively benign environmental change causes the plant to engage its survival mechanisms, thereby enabling differential gene expression. The plant is able to adapt quickly to changing environments or disease-causing organisms, stressors that we introduce artificially while the plant is in microgravity thereby directing gene expression. This indicates that we can produce new varieties of plants, with required new attributes, faster than traditional methods.
All aspects of our Directed Selection™ technology must be conducted in a long-term microgravity environment, currently only available in space. We partnered with the University of Florida to conduct the initial experiments in order to validate the efficacy of this technology in space. Preliminary results showed that the Directed Selection™ technology was able to identify frost resistance capabilities in the Jatropha Curcas plant. This capability could allow cultivation in areas previously impossible.
The Company may use the Directed Selection™ platform technology to create more robust plant varieties adapted toward desirable characteristics. Directed Selection™ research may be aimed to produce new varieties of proprietary, patentable stem cells for plants with desirable traits like the ability to better survive environmental challenges (i.e. temperature or climate change) or to resist disease. Adaptive changes in the plant could be accomplished without the need for additive or subtractive genetic engineering, thus eliminating public concerns about GMOs. The plant still uses the capabilities that are a natural part of its genetic potential through altered gene expression that enables adaptation toward valuable traits.
A key part of our future operations is the expansion of these patents to cover additional crops, animals and humans, and the specific methods and tools that are developed from our research and development. The Company possesses patent applications that contain claims covering biological processes in microgravity, including the growth of cellular plant and animal tissues in orbit, stem cell replication and related processes.
The second part of the Directed Selection™ technology pertains to the mass replication or propagation of stem cells in space, something that can be done on Earth but at much slower rates. Although stem cells can be produced on Earth, current methods are inadequate to create large quantities of healthy cells in short periods of time. Our technology would allow for the increased production of healthy stem cells. Tests, which occurred over six space missions, have provided initial proof-of-concept that the Directed Selection™ technology allows stem cells to replicate en masse. We believe it may eventually allow us to produce large quantities of undifferentiated pluripotent stem cells in the same environment for commercial sale to third parties.
Directed Selection™ Research
A series of space microgravity experiments were executed by means of six space shuttle flights to the ISS spanning from 2007 through 2011, linked to and supported by the NASA Space Act Agreement entered into by ZGSI’s founder, Mr. John W. Kennedy. A new five-year RSAA, signed during January 2016, with NASA ARC, provides us access to NASA scientists and laboratories to continue research on earth. In addition, we expect that there are opportunities through a collaboration with Space Florida to continue research on the ISS. Space Florida is an economic development organization devoted to space research and drives Florida economic development across the global space enterprise. In prior years, tests of chemical compounds, plant cells, and mammalian stem cells in microgravity were conducted in conjunction with the USDA, Agricultural Research Service Labs (Beltsville, MD) and TREC.
Proof of Concept Plant Research to Develop Jatropha Biofuel Strains.
The ZGSI space research program focused, in part, on Directed Selection™ of traits in plants, using microgravity. Initial research focused on in vitro cell culture methods for plants of interest for biofuels or valuable ornamentals. The tropical and sub-tropical plant, Jatropha Curcas (physic nut), produces an easily purified biodiesel or jet fuel, but Jatropha is intolerant of frost and subject to diseases like root rot, leaf spot, and rust. A Jatropha cultivar with a broader climate range, improved frost tolerance, and greater disease resistance, would be a valuable improvement to biofuel production. A parallel study of ornamentals tested an endangered orchid species, two types of tropical flowering tree, and a common plant model organism. Research encompassed a series of six flights, from 2007 through 2011, and was completed by Dr. Wagner Vendrame at TREC. Research support was provided by NASA, BioServe Space Technologies, the Vecellio Group, and Vecenergy Corporation.
Plant cells from test species were grown in microgravity aboard the ISS and returned to ground for follow-up testing. The series of experiments produced several important results. The research team discovered the means to produce and propagate plant stem cells using microgravity. Further, methods were developed to out-grow plants from microgravity stem cells. Plant cells in microgravity were able to form pro-embryonic masses, which would enable quick regeneration of clonal plant material in order to grow new, whole plants. Plant cells also had increased growth, and cell cultures acquired a high stress tolerance in microgravity based upon the ability to recover viable cells after a long-term (290 day) culture under very limited nutrient conditions. As predicted, plant cells grown in microgravity acquired different growth characteristics and differential gene expression, compared to growth in normal gravity conditions. Currently, proprietary Jatropha strains are in keeping at TREC. The Company expects to continue research efforts at the time sufficient financial resources are available.
Mammalian Stem Cell Research in Space
Mammalian stem cell research offers an immeasurable promise for human health through potential means of disease treatment or cure and for tissue or organ regeneration or repair. ZGSI scientists and collaborators recognized this valuable application of space research early on, in 2008, and used the STS-126 flight mission to attempt to grow porcine liver stem cells (PICM-19) in microgravity. The experiment verified that mammalian stem cells would propagate, differentiate, and even form biliary liver structures in microgravity. Cell viability was maintained and cell activity assays indicated that cells maintained hepatocyte detoxification function over the test period. An early stem cell experiment, the PICM-19 was an essential first step toward using microgravity to mass-propagate human differentiated or undifferentiated stem cells to accomplish a myriad of research goals. The Company has no current plan to finance additional research efforts
Timeline and Plan of Operations
While we believe we can realize sustainable revenue executing our plan to leverage our technology and resources by developing strong business relationships with our channel partners, integrate our technology into other agricultural companies’ products and introduce our lawn and garden retail product, Gardner’s Choice, we cannot guarantee we will be successful. Our business is subject to risks inherent in the establishment of a new business enterprise including the financial risks associated with the limited capital resources currently available to us for the implementation of our business strategies. There can be no guarantee that we will be able to obtain the necessary levels of profitability or fundraising needed to remain operational on a long-term basis. Adequate additional financing may not be available to us on acceptable terms, or at all. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our research and product development programs or any future commercialization efforts. We will need to generate significant revenues to achieve profitability, and we may never do so.
Zero Gravity Solutions, Inc.
The Company is a fully reporting company and as such, provides general and financial administration, SEC reporting compliance, marketing support and strategic planning to BAM Inc. and ZGLS. The Company also develops product marketing material, investor oriented materials and provides systems support and market reach on our websites and through social media programs.
During May 2016, the Company announced that it had closed the final tranche of its previous offering of the Company’s Units in an exempt private placement transaction (“Private Placement”). Each Unit in the Private Placement consisted of one share of the Company’s common stock and a five-year warrant to purchase one share of the Company’s common stock at $2.00 for $1.25 per Unit. The Company received gross proceeds of $6,260,000 million pursuant to the Private Placement.
During August 2016, the Company identified a need to raise additional funds to execute its business plan for the remainder of 2016 and future periods. In October 2016, the Company began addressing those requirements through a new private placement of common stock of up to $10,000,000 at $3.00 per share of common stock issued (the "2016 Private Placement").
For the fiscal year ended December 31, 2017, the Company issued 787,133 shares of common stock and received gross proceeds of $2,361,399 pursuant to its 2016 Private Placement.
Commencing in 2016, the Company in cooperation with Space Florida, provided management assistance and strategic planning in the organization of the Planetary Sustainability Institute ("PSI"), a non-profit entity chartered to engage in research and commercialization of new sustainable technologies at the Kennedy Space Center. We expect to continue to play an integral part in the development of PSI’s sustainable agricultural research programs in 2018.
The Company expects to continue providing strategic directives, management and financial support primarily to BAM Inc., including work with Intrinsyx on future BAM-FX™ related research missions to the ISS, as BAM Inc. executes its business strategy during 2018.
BAM Agricultural Solutions, Inc.
Execution of BAM Inc.’s 2018 business strategy is subject to obtaining sufficient funding. BAM Inc. expects to build product demand and brand recognition for BAM-FX™ by generating sales and revenue in domestic and international markets through channel partners, technology integration in other agricultural companies’ retail products and launching a lawn and garden retail product, Gardner’s Choice during 2018. This strategy entails identifying, engaging and cooperating with multiple channel partners to include BAM-FX™ as a component of their agricultural product offerings; engaging major agricultural retail or wholesale chemical companies to identify and implement technology integration strategies; and successfully introducing our retail product to big box stores.
The Company’s core product, BAM-FX™, is an aqueous solution containing ionic zinc and copper micronutrients, in addition to sulfur. Currently, the product is sold in a concentrated form with pre-application dilution in rates ranging from 1:100 to 1:2,000. BAM-FX™ can be used in irrigation or hydroponic, seed treat and foliar applications. Zinc and sulfur promote plant growth and yield. Copper promotes enzyme activities in plants and is essential for chlorophyll and seed production. We believe the combination of micronutrients with other product attributes enables the product to improve crop yields, promote growth and withstand environmental stress by improving the plant’s ability to more efficiently uptake nutrients either available or latent in soils. The composition of micronutrient contained in BAM-FX™ is a contributor to its performance as a growth stimulator. We plan to focus our 2018 sales, agronomy and science efforts on foliar, seed treat and irrigation applications. In 2018, we expect to show scientifically, for specifc crops, how and when, in terms of application timing, these benefits are best expressed during the plant’s life cycle.
BAM Inc. expects to narrow sales and marketing efforts geographically and to certain crops. The Company expects to support existing and expand domestic channel partner activity through 2018. Domestic crop focus for 2018 includes grape, berry, soybean, cotton, tobacco, cannabis and avocado.
Business development efforts, including additional product performance trails, are expected to continue in India and China. The Company received approval to import and sell BAM-FX™ in China during February 2018 and expects to receive approval for import and sale in India during the forth quarter of 2018.
The Company expects to introduce product into Brazil through ongoing efforts, which commenced in 2017, of a potential business partner currently applying for approval to import and sell within the country. Sale of product into Haiti is currently subject to Haitian government approval and is managed by a group of business professionals having high-level relationships within the country.
On February 13, 2018, the Company signed an exclusive business development and distribution agreement for introduction of BAM-FX™ into agricultural markets in Mexico. The initial collaboration in the business development process is expected to be avocado with secondary attention to berries. We expect our agronomy team will work with an agronomy expert in Mexico to develop protocols through numerous tests of BAM-FX™ in diverse geographies and soils. Assuming the success of the trials, we expect product introduction efforts to avocado growers could begin in 2019.
The Company started investigating the retail markets for ready-to-use home and garden applications in 2016 and developed a ready to use product, Gardner’s Choice, during 2017 with an expected market introduction in the third quarter of 2018. BAM Inc. signed a distribution agreement with CustomQuest, Inc., (“CustomQuest”), a subsidiary of Victory Wholesale Group, a respected national distributor of grocery and health and beauty care products to a diversified segment of retail/consumer markets. CustomQuest currently services over 25,000 retail locations and will utilize its infrastructure and resources to begin the rollout of Gardner’s Choice, a ready-to-use formula of BAM-FX™. The Company has identified and is currently working with a third-party vendor for product packaging and labeling fulfillment services and a second third-party vendor to assist with state registration requirements.
The Company believes that current field agricultural technical support and sales personnel are adequate for 2018. The Company’s personnel resources include five agronomists or certified crop advisors in California, the Midwest and Texas. In addition, the Company expects to maintain its current number of sales and sales support personnel.
We believe the Company’s manufacturing facility in Okeechobee, Florida, should have sufficient capacity to fulfill domestic and international orders for product demand in 2018. We have undertaken a number of changes in the manufacturing process to simplify production and more efficiently and consistently produce high quality product. The changes also enable the Company to provide standard operating processes and procedures to third party fulfillment entities for additional production capacity as demand requires.
Zero Gravity Life Sciences, Inc.
We expect ZGLS will assist with agricultural research and identify complimentary technologies for agricultural and any space related initiatives and assist in developing and testing new BAM-FX™-based formulations for specific crops and varying geographic soils and weather conditions. In addition, ZGLS will manage our intellectual property portfolio and assist BAM Inc. with patent strategies related to commercialization of new products. The Company also expects ZGLS to provide focus on the Company’s Planetary Sustainability Institute efforts in 2018 in cooperation with Space Florida.
Strategy for Growth
BAM Agricultural Solutions, Inc.
BAM Inc. is executing the commercial roll out of BAM-FX™ following three years of initial product testing with several universities, end product users, and laboratory work supplementing the science of BAM-FX™ during 2017 through work performed under the RSAA with NASA ARC. BAM-FX™ is licensed in a fertilizer product category and approved for sale in thirty-three states in the United States, China, Colombia, Paraguay and Chile. BAM Inc. is pursuing registration and approval from appropriate government authorities for commercial sale and distribution in Brazil, South Africa, Mexico, India, Haiti and the Philippines.
The Company expects to focus 2018 sales and marketing effort on certain crops, including grape, berry, tobacco, soybean, cotton, avocado and cannabis. Our 2017 trials also showed positive performance in vegetable and fruit crops. We plan to sell our product at wholesale to our distributors or channel partners domestically and internationally and to end-users in the absence of a distributor.
We expect to continue to develop our domestic channel partner network during 2018. International distributor agreements cover Colombia, Paraguay, Mexico and Haiti. We expect to conclude distribution agreements for China during 2018 and are currently working with distributors or potential distributors in business development efforts and for government registration for import and sale in Brazil, India, Philippines and Mexico. The Company curtailed operations in Chile during the fourth quarter of 2017 until adequate resources are available to address the Chilean markets or a channel partner is identified.
On February 20, 2018, BAM Inc. and the Lichtinger Group entered into a License and Business Development Agreement Mexico, effective as of February 13, 2018 (the “Agreement”). Following consummation of the Agreement, the Lichtinger Group formed a company in Mexico, Agro Space Tech SA, “Agro Space”, in which BAM will receive a twenty percent (20%) equity ownership interest.
The Agreement provides Agro Space with the exclusive right to import, package, sell and distribute BAM’s product lines and promote its brand, as well as other “white label” brands as they are introduced, in the Mexican markets, and a limited manufacturing right to modify the presentation of BAM’s products and dilute such products, all during the term of the Agreement. The exclusivity of the rights licensed to Agro Space are conditioned upon (i) meeting specified revenue targets beginning on the third anniversary following the successful completion of specified local trials through the tenth anniversary thereof; and (ii) an expansion of the business to target and add a specified number of crops during each of the five (5) years following the successful completion of specified local trials.
The second phase of the Company’s 2018 sales and marketing strategy is to pursue alliances with large agricultural and chemical manufacturers to incorporate our core technology within their product lines to improve the performance and profitability of existing product lines. We are currently discussing these opportunities with three large-scale integrated agricultural input distributors and one fertilizer manufacturer. We expect each opportunity will involve significant technical analysis including product efficacy testing, which if successful, will represent potentially sizable product sales or licensing revenue.
Gardner’s Choice, a ready to use home and garden product, is expected to be introduced to big box stores and lawn and garden retailers in 2018. The Company has received positive response from purchasers during Gardner’s Choice presentation during the Ace Hardware 2018 Spring Convention and GIE Expo 2017 in the fourth quarter of 2017. We believe we will receive stocking orders from purchasers during the third quarter of 2018.
Recent changes in laws now make the possession of cannabis for medicinal or recreational uses legal in many countries throughout the world. The global legal market for such products in 2016 was $14.3 billion and is expected to reach $140 billion by 2027. These numbers anticipate approval of pending legislation in many countries that would once again allow for the large-scale farming of hemp, a variety of cannabis, which contains very low levels of the psychoactive agent known as THC. Twenty-five of the largest producers of cannabis in North America are reported to have under operation and construction more than seven million square feet of indoor capacity. Preliminary data has shown that BAM-FX™, added to a growers protocol, has produced up to a 44.79% increase in vegetative growth, in weight of processed buds per plant, as well as a significant improvement in quality, as measured by THC, terpene and cannabinoid levels. We will continue to pursue additional field studies to demonstrate the value of BAM-FX™ to the cannabis industry, including hemp. Currently, we are in discussions with groups representing the larger growing operations in the U.S., Israel, and Uruguay, who we expect will be conducting larger-scaled trials with cannabis plants grown under various conditions, and with several of the common varieties of plants under cultivation. We intend to work with these groups to demonstrate the value of BAM-FX™, to make it a preferred, or required, element in the growing process, to improve their competitive advantage. At the same time, we expect to continue discussions with regional distributors of products that target individual cannabis growers.
Currently, our sales and agronomy personnel are sufficient to support our domestic commercial and retail business strategies. As sale and distribution expands internationally, additional technical personnel will be required and added, on an as needed basis.
The Company’s growth strategy, in part, is to engage and support channel partners in domestic and international agricultural markets. We believe our sales approach for 2018 will focus primarily on target crops and select geographies with a combination of foliar and seed treat applications. Working with agricultural input distributors and chemical companies to incorporate our core technology into an existing product line to enhance performance is an integral component of our 2018 revenue strategy. In addition, our revenue strategy is expected to include the introduction of Gardner’s Choice into retail and lawn and garden venues.
Zero Gravity Life Sciences, Inc.
ZGLS’s short to mid-term plans includes the utilization of NASA and PSI relationships to research BAM-FX™ related to space program applications, as well as engaging in research based upon the Company’s zero/micro gravity intellectual property leading toward the development and patenting of unique stem cells developed on the ISS. These potentially patentable plant stem cell lines could lead to new global food cash crops resistant to climate change and other adverse environmental factors.
Intellectual Property
We have made protection of our intellectual property a strategic priority. We rely on a combination of patent applications, trademarks, trade secrets and other intellectual property laws to protect our proprietary rights.
Patent Applications
We are the owners or licensees of three U.S. patents and many U.S. and foreign pending patent applications that form an important part of our immediate and future business plans. We have been assigned or licensed the rights to these patent applications through agreements with our chief science officer and former director, John W. Kennedy.
We are the assignee of a portfolio of patent applications that pertain to the second-generation bioavailable mineral compositions BAM-FX™. The BAM-FX™ composition is highly useful as a nutrient additive that may fortify against disease or environmental stresses and promotes growth. The basis of which is the ability of the ionized micro nutrient platform to deliver selected minerals into a plant or other complex organism systemically. The U.S. Patent and Trademark Office issued U.S. Patent No. 9,266,785, titled Bioavailable Minerals for Plant Health, on February 23, 2016. The patent will expire on December 27, 2026. Related applications are pending in Chile, Thailand, and Malaysia as well as a child U.S. application.
We are also the licensee of a patent application that pertains to the use of artificial Superoxide Dismutase ("SOD") compositions for the treatment of several plant and animal diseases, including amyotrophic lateral sclerosis (also known as ALS disease). Superoxide is naturally produced by plants and animals, including humans, and its production is accelerated in times of trauma or stress. SOD is produced by the cells to counteract the effects of an over-production of superoxide. Adequate amounts of SOD may increase life-span in humans and may be capable of remediating (and saving) severely stressed plants. This patent application discusses an artificial SOD that can be easily applied and used for treating over-production of superoxide, particularly ALS and other neural disorders. Animal studies of the SOD are being discussed on an ongoing basis with NASA and academic entities. The plant and space radiation applications of the patent applications are being licensed to ZGSI exclusively from Mr. John W. Kennedy for specialized testing and research.
ZGSI is the exclusive assignee of a patent application and an issued U.S. Patent No. 9,816,071, both directed to the replication of stems cells in a weightless environment. These patent applications contain claims covering biological processes in microgravity, including the growth of cellular plant and animal tissues on-orbit, stem cell replication and related processes. The technology accelerates the evolution of organisms, particularly plants, to adapt the organism to thrive in a hostile environment including cold and/or arid climates. Plants adapted using the technology show increased tolerance for the selected hostile environment relative to traditional plants. Specific applications include the development of food crops that are tolerant of cold climates (e.g. frost resistant crops) and arid environments (drought resistant crops). A key part of our ongoing operations is the expansion of this technology to cover additional crops, animals and potentially humans, and the specific methodologies and tools that are developed from our R&D. It is therefore a part of our technology that holds much promise and, from a commercial standpoint, falls into the longer-term plans of the Company.
Trade Secrets
Parts of our portfolio of intellectual property includes trade secrets pertaining to the manufacturing of several of the materials previously mentioned. In particular, BAM-FX™ and its derivatives are made in a complex and very specific process utilizing purpose-built equipment. These manufacturing processes are a separate technology, distinct from the patent applications, and remain highly secure and confidential. Reverse engineering of a product is always possible with sufficient resources. We believe that the experience we have honed over several years of manufacturing these products gives us a substantial competitive edge over any potential newcomers.
Trademarks
In 2015, we filed a trademark application to protect BAM-FX™ which was registered with the USPTO on November 8, 2016. Our trademark "UNLOCKING NUTRITION FOR THE WORLD™" was registered with the USPTO on September 8, 2015. A U.S. trademark application for our ready-to-use consumer product, named Gardener’s Choice™, was filed on May 15, 2017. We anticipate the registration of this trademark to occur shortly. We also hold a number of other common law trademarks that we may register in the future.
As of January 19, 2018, the Company has the following trademark inventory:
|
Mark
|
Country |
|
BAM AG SOLUTIONS |
EUTM |
|
BAM AGRICULTURAL SOLUTIONS |
Chile |
|
BAM AGRICULTURAL SOLUTIONS |
EUTM |
|
BAM AGRICULTURAL SOLUTIONS |
US |
|
BAM FX & Design |
Chile |
|
BAM-FX |
US |

|
EUTM |

|
US |
|
DIRECTED SELECTION |
EUTM |
|
DIRECTED SELECTION |
US |
|
GARDENER'S CHOICE |
US |
|
UNLOCKING NUTRITION FOR THE WORLD |
US |
Strategic Relationships
|
1. |
NASA – During January 2016, the Company executed a new five-year Reimbursable Space Act Agreement, (“RSAA”), with NASA ARC and an existing investor and contractor, Intrinsyx Technologies Corporation (“Intrinsyx”). The RSAA gives the Company access to NASA scientists and laboratories, which are assisting in identifying and documenting attributes of the Company’s product and potential applications in other segments of agricultural markets. The primary objectives of the RSAA are: |
|
a. |
To establish the scientific basis for action of ZGSI products: |
|
b. |
To quantify the impact of ZGSI products on plant growth and productivity: |
|
c. |
To evaluate and test the impact of ZGSI products on yield physiology of selected crops important to commercial agriculture and NASA applications: and |
|
d. |
To evaluate and test the potential utility of ZGSI products to NASA space biology, and life support applications. |
|
2. |
Intrinsyx Technologies Corporation (“Intrinsyx”) is an engineering and information technology services company that has delivered innovative, high-performance IT solutions for space systems and payloads to NASA for over 11 years. Intrinsyx is an important collaborator in our third-party funded field trials of our BAM-FX™ formulation. Intrinsyx also liaises with NASA to support our continued access to NASA-funded research and development on the ISS. |
|
3. |
The Tropical Research and Education Center of the University of Florida Institute of Food and Agricultural Sciences located in Homestead (“IFAS/TREC”) has conducted field trials on BAM-FX™ on multiple crops. TREC was the primary investigator during five trails of our Directed Selection™ technology on flights to the ISS. |
|
4. |
The Center for the Advancement of Science in Space (“CASIS”) is the new non-governmental organization responsible for the management of the United States’ portion of the ISS which has been designated as a national laboratory. We are continuing discussions with CASIS to use the facility at the Kennedy Space Center for lab support of our plant research. |
|
5. |
We continue our discussions with Space Florida to create a PSI, which in part, is expecting to be an agricultural innovation center for the purpose of combining knowledge gathered on the ISS with the need for improved ground focused applications. |
Government Regulations
Although BAM-FX™ is different than common fertilizers available, state regulatory agencies require us to register the product as a fertilizer. The BAM-FX™ product has been registered and issued a license as a liquid fertilizer in thirty-three states. The Company does not claim that BAM-FX™ is a fungicide, bactericide or pesticide, therefore alleviating regulatory oversight associated with those claims. All ingredients in the formulation are categorized as Generally Regarded as Safe (“GRAS”) by the United States Food and Drug Administration (“USFDA”).
With our Directed Selection™ technology utilized in the creation of new varieties of an existing plant’s stem cell with unique characteristics, the resulting plant is developed exclusively through differential gene expression by exposure of the indigenous genome of the original plant to microgravity. No foreign DNA or genetic material is added or introduced to the genome, thus it is not considered to be a GMO and the Company believes that it would likely not fall under regulations that GMOs face. We believe that plants developed with Directed Selection™ technology would be regulated by the USFDA as are other non-genetically modified plants and we do not expect regulation to significantly impact our business plan.
Application of the Directed Selection™ technology to develop human stem cells represents a long-term goal of the Company and therefore we have not fully investigated the level of regulation the resulting products may face. The Company will complete a full review of the applicable regulations before beginning any product development efforts.
We expect that any products utilizing our Superoxide Dismutase (SOD) patent license will be subject to regulatory control. At this time, we have not completed research or development efforts in this area determine how a product may be regulated, if developed, as it relates to our Directed Selection technology.
Competition
Global Market Insights estimates the market size for micronutrients exceeded $4.5 billion in 2014 and is estimated to surpass $8.5 billion by 2023 growing with a cumulative average growth rate of more than 8% from 2016 to 2023. Asia Pacific agricultural micronutrients markets account for over 50% of the total market. The North America market was estimated to exceed $950 million. Foliar applications accounted for more than 22% of the total 2014 volume. There are more than 1,000 major fertilizer manufactures and more than 2,000 suppliers currently servicing both the existing macronutrient and micronutrient markets. We compete with these suppliers and manufacturers who offer a broad product line, as a standalone zinc and copper based product.
A subset of manufacturers and suppliers market zinc and copper foliar products perceived to be similar to BAM-FX™. The products make beneficial use claims similar to those we make, which in most cases are not substantiated, and are generally sold for less than our BAM-FX™ wholesale price. Buyers often have a difficult task distinguishing BAM-FX™ efficacy from other suppliers of zinc and copper foliar micronutrients. We believe our channel partner marketing approach focused on third party field test results and scientific proof of our product claims, allows us to differentiate BAM-FX™ from other competitive products. We believe that our product improves a grower’s return on investment through improved yield and reduction in agricultural inputs when properly applied to crops under appropriate agricultural conditions.
During 2017, the Company increased its understanding of mode of action of the core technology in BAM-FX™. Initial research supports our theory that our core technology acts as a bio-stimulant. A plant bio-stimulant is any substance or microorganism applied to plants to enhance nutrition efficiency, abiotic stress tolerance (drought, extreme temperature, mineral toxicity) and/or crop quality traits, without regard of the bio-stimulant’s nutrients content. Plant bio-stimulants are also contained in numerous commercial agricultural products.
Government agencies and various organizations across the globe have encouraged farmers to adopt bio-stimulants according to Global Marker Insights. In June 2011, the European Bio-stimulants Industry Consortium was formed to foster bio-stimulants to improve yield with less resources with major goals of sustainable agricultural production, economic growth and green innovation. The acid based ingredient bio-stimulant worldwide market is expected to surpass $2.0 billion by 2024.
The bio-stimulant market is at a development stage with the most notable players outside the United States. These players have established markets and adequate financial and technical resources, with which, we may not be able to compete. We expect to develop a bio-stimulant product only in collaboration with a large agricultural or chemical products manufacturer or on behalf of a distributor of agricultural inputs.
Specialty crop markets, specifically for the United States cannabis industry, are estimated to be $6.5 billion in 2016, growing to $30 billion by 2021. Global markets are estimated to be $14.3 billion in 2016, projected to increase to $140 billion by 2027. We believe the potential market for our core technology in agricultural specialty crops, considering only North American markets at the end of 2017, could range from $35 to $75 million at a ten percent penetration rate. Although we believe that this agricultural market represents a significant revenue and growth opportunity for the Company, numerous competitive products currently exist and others are in development by large, well-established companies entering this market such as Scott’s Miracle-Gro Company. Although we believe that we will successfully develop products specifically addressing the specialty markets using our core technology, our ability to enter these markets and generate revenue will depend solely on our product’s performance.
Our core technology can be combined with existing fertilizer and herbicide formulations and conform to existing application protocols. Lab and field research indicates that, by blending BAM-FX™ with existing, widely distributed, “branded” products, we could enhance the performance of those products sold by other agricultural input companies, which we consider as industry collaborators, not competitors.
Competitive Advantage/Barriers to Entry:
The Company believes that ZGSI has adequately protected its intellectual property and has developed a strong, professional technical team that provide it a positive competitive position. We believe our competitive advantages include:
|
● |
Manufacturing methods for our core technology are trade secrets, which we believe would be cost prohibitive and time consuming to reverse engineer. This proprietary and confidential method of manufacturing has been developed and refined over two decades; |
|
● |
Access to the ISS to continue utilizing that resource to advance our core technology; |
|
● |
Innovation of our core technology for agricultural applications, which we expect will generate additional intellectual property and potentially patentable composition and process technology; |
|
● |
Maintaining a working relationship with NASA ARC, USDA Agriculture Research Services Labs, and a number of educational institutions recognized as agricultural industry leaders; |
|
● |
An experienced management team with many years in industry, business operations, corporate finance, intellectual property development and protection as well as a world-class scientific and senior advisory boards. |
|
|
● |
Growing brand recognition. |
|
| ● | Our innovative technology helps provide nutritious food to the worlds rapidly expending population. |
Reports to Security Holders
We are required to file reports and other information with the U.S. Securities and Exchange Commission (“SEC”). You may read and copy any document that we file at the SEC's public reference facilities at 100 F. Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-732-0330 for more information about its public reference facilities. Our SEC filings are available to you free of charge at the SEC's web site at www.sec.gov. We file electronically with the SEC and, as a result, our information is available through the Internet site maintained by the SEC that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. This information may be found at www.sec.gov.
|
RISK FACTORS |
As a “smaller reporting company”, as defined by Item 10 of Regulation S-K, the Company is not required to provide the information required by this item.
|
UNRESOLVED STAFF COMMENTS |
As a “smaller reporting company”, as defined by Item 10 of Regulation S-K, the Company is not required to provide the information required by this item.
|
PROPERTIES |
The Company has an annual lease for its office space located at 190 N.W. Spanish River Blvd, Suites 101/102, Boca Raton, Florida 33431 for a monthly rate of $5,173. BAM Inc. holds a two-year lease for its manufacturing facility located at 1461 NW 25th Drive, Okeechobee, FL 34972 for $1,229 per month. BAM Inc. also leases full service office space in Western Growers’ Association’s facility at 150 Main Street, Salinas, California 93901 for $500 per month. This lease was cancelled in May 2018. BAM Inc. leases a storage facility at 1501 N.W. 25th Drive, Okeechobee, FL 34972 pursuant to an 18-month lease for $856 per month.
We believe our facilities are adequate for our current needs and that we will be able to locate suitable new office space and additional manufacturing capacity if needed.
|
LEGAL PROCEEDINGS |
There are presently no material pending legal proceedings to which the Company, or any of its subsidiaries, is a party or as to which any of its property is subject, and no such proceedings are known to the Registrant to be threatened or contemplated against it.
|
MINE SAFETY DISCLOSURES |
Not applicable.
|
MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED SHAREHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information.
Our common stock is presently quoted on the OTC Pink Market under the symbol “ZGSI”, although there has not been an active trading market for the shares. Accordingly, we are not including a history of reported trades in the public market because of the limited and sporadic trading. There have been 25,500 shares traded in the 12 months ending March 9, 2018.
Holders
As of July 6, 2018, there were 322 record holders of an aggregate of 40,672,395 shares of the common stock issued and outstanding.
Dividends
The Registrant has not paid any cash dividends to date and does not anticipate or contemplate paying dividends in the foreseeable future. It is the present intention of management to utilize all available funds for the development of the Registrant's business.
Securities Authorized for Issuance under Equity Compensation Plan
On November 30, 2015, the Board of Directors authorized and approved the adoption of the 2015 Equity Incentive Plan, (“2015 Plan”) which reserves for issuance 4,000,000 shares of the Company’s common stock, which may be granted from time to time to Directors, employees and certain independent contractors. The 2015 Plan was approved by 51.184% of the stockholders on December 2, 2015. No securities had been approved for issuance as of December 31, 2015. Subsequent to December 31, 2015, a total of 4,000,000 shares have been issued or reserved for issuance under the 2015 Plan.
Recent Sales of Unregistered Securities; Use of Proceeds from Registered Securities
Set forth below is information regarding shares of common stock issued and warrants granted by us since December 31, 2011 that were not registered under the Securities Act. Also included is the consideration, if any received by us, for securities and information relating to the Securities Act, or rules of the SEC, under which exemption from registration was claimed.
|
(1) |
From March 2013 until February 27, 2015, the Company participated in a continuous offering of up to 8,000,000 of its shares of common stock to accredited and non-accredited investors, under which investors purchased shares for a purchase price equal to $0.50 per share. Under this offering, which was oversubscribed, the Company has issued 8,896,594 shares and received a total of $4,448,297. |
|
(2) |
In February-March 2015, the Company issued 200,000 fully vested, non-forfeitable warrants to a Director for his service as a director having a fair value of $92,479 and, in connection to his service as the Company’s Chief Financial Officer, 100,000 shares of the Company’s common stock, along with cashless warrants to purchase 200,000 shares of the Company’s common stock at an exercise price of $0.50 with a term of five years. |
|
(3) |
On July 16, 2015, a member of the Company’s Board of Directors, made an unsecured loan to the Company in the principal face amount of $500,000 in exchange for a promissory note bearing interest at the rate of 8.5% per annum, such interest being payable by the Company quarterly. As additional consideration for the loan, the Company issued a five-year cashless warrant to purchase up to 350,000 shares of the Company’s common stock at an exercise price of $3.00 per share. On July 19, 2016, the promissory note was amended to extend the maturity date to July 16, 2017 and. provided that the principal and accrued but unpaid interest under the Note may be converted, in whole or in part, into the Company’s common stock at a conversion price equal to $1.25 per share. In July 2017, the maturity date of the note was extended to July 2018, the other terms of this note including the conversion feature remain the same. Prepayment of all unpaid principal and interest may be made by the Company prior to the Maturity Date, without penalty or premium. |
|
(4) |
During October 2015, we commenced a private offering of up to $10,000,000 of our securities. The $10,000,000 offering consists of 8,000,000 units at $1.25 per unit; each unit consisting of one share of the Company’s common stock and a five-year warrant to purchase an additional share of the Company’s common stock at $2.00. The Company executed and delivered a side letter with certain investors to retroactively adjust the terms of the Company’s May 2015 offering with the terms of the new offering. Through December 31, 2015, the Company issued 3,634,000 shares of common stock and 3,634,000 warrants to purchase an additional share of the Company’s common stock pursuant to this offering (which includes the retroactive adjustment for the May 2015 offering). |
|
(5) |
During the year ended December 31, 2015, the Company issued 372,000 shares of common stock and 1,613,500 fully vested, non-forfeitable warrants to employees, directors and consultants for services. |
|
(6) |
During the year ended December 31, 2016, pursuant to its October 2015 private offering, the Company issued 1,374,000 units at a price of $1.25 per unit to purchase 1,374,000 shares of common stock. Each unit consisted of one share of the Company’s common stock and a five-year non-forfeitable and fully vested warrant to purchase additional shares of the Company’s common stock at $2.00 per share. In connection with the offering, the Company issued fully vested, non-forfeitable warrants to purchase 152,575 shares of common stock with an exercise price of $2.00 per share to the Company’s placement agent. |
|
(7) |
On January 21, 2016, the Company issued 20,000 shares of common stock to a consultant for services. |
|
(8) |
During the year ended December 31, 2016, the Company issued 110,000 fully vested, non-forfeitable warrants to employees and consultants for services. |
|
| (9) | During 2016, the Company commenced a private offering of up to $10,000,000 of the Company’s securities. The offering consists of 3,333,333 shares of the Company’s common stock at $3.00. Through December 31, 2016, the Company issued 221,667 shares of common stock. In connection with the offering, the Company issued fully vested, non-forfeitable warrants to purchase 11,083 common shares with an exercise price of $3.00 per common share to the Company’s placement agent. | |
| (10) | On March 1, 2017, the Company entered into an agreement with a member of its Board of Directors whereby 350,000 previously issued warrants were exercised in exchange for the issuance of 350,000 shares of the Company's common stock and a cash payment to the Company of $525,000. In connection with the transaction, the Company issued a new five-year warrant to purchase up to 350,000 shares of the Company's common stock at an exercise price of $4.50 per share. |
|
(11) |
On March 16, 2017, the Company also entered into an agreement with a second member of its Board of Directors whereby 400,000 previously issued warrants were exercised in exchange for the issuance of 400,000 shares of the Company's common stock and a cash payment to the Company of $600,000. In connection with the transaction, the Company issued a new five-year warrant to purchase up to 400,000 shares of the Company's common stock at an exercise price of $4.50 per share. |
|
| (12) | During 2017, pursuant to the Company’s 2016 Private Placement, the Company issued 787,133 shares of common stock. In connection with the offering, the Company issued fully vested, non-forfeitable warrants to purchase 53,862 common shares with an exercise price of $3.00 per common share to the Company’s placement agent. | |
| (13) | During the year ended December 31, 2017, the Company issued 100,000 shares of common stock to consultants for services. | |
| (14) | During the year ended December 31, 2017, the Company issued 90,000 fully vested, non-forfeitable warrants to employees and consultants for services. |
In connection with the private offerings noted above, the Company has engaged a broker/dealer, Livingston Securities, LLC, to assist as placement agent for the securities. No underwriters were involved in the foregoing issuances of securities. Each of the above transactions was exempt from the registration requirements of the Securities Act in reliance upon Section 4(a)(2) of the Securities Act or Regulation D promulgated under the Securities Act. The recipient of the securities in each of these transactions represented his, her or its intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed upon the stock certificates or book-entry positions representing the shares issued in each of these transactions. In each case, the recipient had adequate access, through his, her or its relationship with the Company, to information about the Company.
|
SELECTED FINANCIAL DATA |
As a “smaller reporting company”, as defined by Item 10 of Regulation S-K, the Company is not required to provide the information required by this item.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
Overview
Zero Gravity Solutions, Inc., a Nevada corporation, is a biotechnology company focused on commercializing technology derived from and designed for spaceflight with significant applications on Earth. These technologies are focused on improving world agriculture by providing valuable solutions to challenges facing humanity including threats to world agriculture and the ability to feed the world’s rapidly growing population. The Company’s business model is currently focused on its two primary business segments: 1) BAM-FX™, which is a cost effective, nutrient delivery platform for plants that efficiently delivers minerals and micronutrients to plants; and 2) Directed Selection™, which relates to the production and alteration of new varieties of novel stem cells with unique and beneficial characteristics in the prolonged zero/micro gravity environment of the ISS. These novel stem cells, if developed, could be patented for commercial sale to third parties in the agricultural and human regenerative medical markets. ZGSI is headquartered in Boca Raton, Florida.
Our business activities are separated between two primary wholly owned subsidiaries. BAM Agricultural Solutions, Inc., (“BAM Inc.”) oversees product development, introduction and business development through in-field trials and validation tests with crop growers and channel partners, product analysis through academic institutions and NASA, manufacturing, and sale of and agronomy support for our BAM-FX™ product. Zero Gravity Life Sciences Inc. (“ZGLS”) is responsible for any space research projects, life science applications of our technology and conducting research on future BAM Inc. product lines.
A separate wholly owned subsidiary, Zero Gravity Solutions, LTD ("ZGS Ltd."), domiciled in England, was engaged in European market and business development. During 2015, the Company made reductions in personnel and curtailed operations of ZGS Ltd. which was liquidated during the fourth quarter of 2015.
The Company is focused on near-term revenue generation through the introduction of the Company’s first commercial product, BAM-FX™, to domestic and international agricultural markets. BAM-FX™, a micro-nutrient delivery platform, is licensed in and registered for sale as a fertilizer application with thirty-three (33) domestic states and has been approved for import and commercial sales in Chile, Paraguay, Colombia, Brazil and China. The Company is also pursuing import approval through proper governmental officials and agencies to begin commercial sales in South Africa, Indonesia, Haiti, Philippines and India. International operations are conducted through in-country established businesses with which a distribution agreement has been executed.
The Company began domestic product trials on multiple crops in laboratory and academic settings as well as in field applications on grower/end-user crops during 2014 and expanded field applications with those and additional trial participants in subsequent years. The initial and subsequent year trials showed significant yield, nutritional value and biomass improvements for crops treated. We continue to use validation trials with growers to increase our understanding of application rates and protocols for our product and show efficacy. These trials are critical in both market and product development. During the past two years, the Company increasingly focused on obtaining technical, third-party validated, marketing material, through studies with academic institutions and NASA, in accordance with the Company’s RSAA.
During 2016, the Company shifted domestic business development efforts from primarily a direct to grower approach and began developing business relationships with channel partners, in general, large agricultural product distributors with which the Company executed a distribution agreement. Three distribution agreements with agricultural product distributors in Ohio and California were signed during 2016 with three additional in 2017, expanding into Pennsylvania. These efforts have had limited success domestically, due to limited third party crop data from USDA agricultural extension or affiliated universities to support crop yield and performance predictability, combined economic factors including product pricing and risk aversion which, in some instances, did not result in sufficient return on investment for channel partners or growers, and slow adoption practices found within the agricultural industry. We believe part of our slow adoption by channel partners is due to their perceived commitment risk based upon the Company’s lack of adequate capital and capital reserves to address long term operations and sustainability.
The Company began to see limited success penetrating domestic and international markets during 2017. Domestically, the agronomy team conducted in excess of one hundred and fifty grower trials to replicate positive results from prior years using established product protocols and to refine product protocols on a variety of crops in addition to a number of third party validated trials. We believe a number of growers and channel partners will place product orders during the third and fourth quarters of 2018 as BAM-FX™ gains a market foothold in California, Texas, Iowa, Kentucky and Pennsylvania. International trials were conducted in China, India, Paraguay, Colombia, Brazil and Chile during 2017. We believe positive results in soybean in Paraguay could generate revenue in 2018 through a channel partner in Paraguay. We expect to begin generating revenue in other countries during 2018 as a result of our 2017 trial results which will be dependent upon obtaining government approval for commercial import and sale in country and developing and maintaining strong working relationships with distributors in those countries.
We developed a ready to use, home and garden product, Gardner’s Choice, during 2017 for retail markets. Independent third parties successfully tested Gardner’s Choice for plant performance and consumer safety during the last half of 2017 into the first quarter of 2018. During the fourth quarter of 2017, the Company showcased the product at a home and garden trade show and generated significant interest from prospective purchasers. During the first quarter of 2018, the Company introduced Gardner’s Choice directly to Ace Hardware retailers at their annual trade show. We have received positive feedback from retailers and expect to place product in a number of stores during the third quarter of 2018.
The Company engaged a well-established distributor in the mid-west to provide wholesale distribution and assist in marketing Gardner’s Choice to a number of big box retail outlets. We expect that we will complete state registration for sale, labeling and promotional material for Gardner’s Choice during the third quarter of 2018.
To support commercialization and revenue generation efforts, the Company employs certified crop advisors and agronomists for the technical requirements of product introduction domestically and internationally. During 2016, the Company increased the number of its global agronomy and technically trained agricultural personnel and consultants to develop channel partner relationships, improve our technical support to growers and channel partners, and drive scientific academic and NASA related product studies. Reductions in our consulting agronomy staff occurred during the fourth quarter of 2017 due to curtailed operations in Chile and domestically to focus on specific geographies and crops.
The Company continues to develop technical relationships to validate the science incorporated in BAM-FX™ and identify additional commercial markets and licensing opportunities for the product. During the first quarter of 2016, the Company executed a new five-year RSAA with NASA ARC. The RSAA gives the Company access to NASA scientists and laboratories, which assist in identifying and documenting additional attributes of the Company’s science and potential applications in other segments of agricultural markets. We continued working with NASA ARC through the second quarter of 2017 and have not renewed the RSAA due to lack of adequate resources. We maintain our relationship with researchers and specialists and expect to continue research when resources are available.
The Company manufactures its product in a manufacturing facility in Okeechobee, Florida. Manufacturing is conducted on an as-needed batch process. The Company continually performs regulatory and process efficiency reviews of manufacturing operations. In February 2017, the Company hired a manufacturing plant manager, an experienced biochemical engineer. We have made process improvements and equipment modifications to existing equipment to enhance efficiencies and improve quality control and assurance during 2017. Our process improvements more closely align production with a continuous flow production process. The Company expects to engineer, design and build a scale model of a continuous flow process manufacturing facility during 2019 as financial resources are available.
We began developing new formulations of BAM-FX™ to address nutritional needs of plants that are additive to our product’s current zinc and copper formulation in 2015. New formulations can include boron, manganese, magnesium and iron, which address known plant deficiencies. We believe formulations can be manufactured to include all or numerous combinations of these elements. Certain new formulations have been tested to verify the ability to combine those elements during 2017.
We successfully tested a dry formulation, which maintains the attributes of our product, during 2017. If the dry formulation produces the same efficacy as BAM-FX™, we expect that transportation and handling costs would be reduced significantly and markets to be addressed by that product would expand. We expect to continue developing these new formulations and dry formulations in a laboratory setting, begin to perform efficacy trials and design a scale production process during 2018, with the intent of adding a number of new specialized products to our current product offering.
Although we have started and realized nominal product sales, we anticipate that in the near term, ongoing expenses, including product development, manufacturing process improvement, corporate administration and technical product support, will be funded primarily by proceeds from sales of our securities. On February 27, 2015, we closed a private offering that commenced in March 2013 and raised gross proceeds of $4,448,297 of which $1,253,800 was received during 2015. During May 2015, we began a second offering from which, as of December 31, 2015, we received $4,542,500 in gross proceeds. Direct offering costs relating to these offerings were $536,250 as of December 31, 2015. During 2016, we received an additional $1,717,500 in gross proceeds from this offering.
During 2016, the Company commenced a new private offering of up to $10,000,000 of the Company’s securities consisting of 3,333,333 common shares for $3.00 per common share. Proceeds from the offering were $665,001 with offering costs of $53,200 as of December 31, 2016. As of December 31, 2017, we received an additional $2,370,000 in gross proceeds from this offering with offering costs of $169,472.
We have generated nominal revenues from our operations thus far and expect that product sales will increase significantly in 2018 primarily from direct sales to end users, domestic and international channel partners’ distribution efforts, initial revenues from our retail product, Gardner’s Choice and introduction of product into specialty crop markets.
We cannot, however, guarantee we will be successful in generating significant revenue in 2018 or in the execution of our business strategy. Our business is subject to risks inherent in the establishment of a new business enterprise, including the financial risks associated with the limited capital resources currently available to us for the implementation of our business strategies. To become profitable and competitive, we must raise additional capital to execute our 2018 business plan and realize revenues expected.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations are based on our Consolidated Financial Statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and assumptions that affect the amounts reported in our Consolidated Financial Statements and accompanying notes. Note 1: Summary of Significant Accounting Policies in our Consolidated Financial Statements contains a description of the accounting policies used in the preparation of our financial statements as well as the consideration of recently issued accounting standards and the estimated impact these standards will have on our financial statements. We evaluate our estimates on an ongoing basis, including those related to revenue recognition and stock-based compensation. We base our estimates on historical experience and on various other assumptions that we believe are reasonable under the circumstances. Actual amounts could differ significantly from these estimates under different assumptions and conditions.
We define a critical accounting policy or estimate as one that is both important to our financial condition and results of operations and requires us to make difficult, subjective or complex judgments or estimates about matters that are uncertain. We believe that the following are the critical accounting policies and estimates used in the preparation of our Consolidated Financial Statements. In addition, there are other items within our Consolidated Financial Statements that require estimates but are not deemed critical as defined in this paragraph.
Revenue Recognition
In general, we recognize revenue when (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred, (iii) the price is fixed or determinable and (iv) collectability is reasonably assured. We consider persuasive evidence of an arrangement to be a signed agreement, a binding contract with the customer or other similar documentation reflecting the terms and conditions under which products are sold.
Going Concern
We adopted FASB ASU No. 2014-15, Presentation of Financial Statements- Going Concern, during the first quarter of 2016. This standard defines management’s responsibility to evaluate conditions or events as related to uncertainties that raise substantial doubt about our ability to continue as a going concern and to provide related footnote disclosures, as applicable. Management’s estimates and assumptions, used in the evaluation of our ability to meet our obligations as they become due within one year after the date our financial statements are issued, are based on the facts and circumstances at such date and are subject to a material and high level of subjectivity and uncertainty due to the matters themselves being uncertain and subject to modification. The effect of any individual or aggregate changes in the estimates and assumptions, or the facts and circumstances, could be material to the financial statements. We have concluded that there is substantial doubt about our ability to continue as a going concern. The accompanying financial statements have been prepared assuming we will continue as a going concern, but do not include any adjustments that might result if we were unable to do so.
Employee Stock-Based Compensation and Share-Based payment to non-employees
We measure employee compensation expense for all stock-based awards at fair value on the date of grant and recognize compensation expense over the service period for awards expected to vest.
We use the Black-Scholes option-pricing model to determine the fair value for stock option awards and recognize compensation expense on a straight-line basis over the awards' vesting periods. Determining the fair value of stock-based awards at the grant date requires judgment. The determination of the grant date fair value of options using an option-pricing model is affected by our estimated common stock fair value as well as assumptions regarding a number of other complex and subjective variables. If any of the assumptions used in the Black-Scholes model changes significantly, stock-based compensation for future awards may differ materially compared with the awards granted previously. In valuing our options, we make assumptions about risk-free interest rates, dividend yields, volatility and weighted-average expected lives, including estimated forfeiture rates, of the options.
As the Company's common stock is not traded in an active market, the Company estimates the fair value of its common stock (used in its Black Scholes option pricing model) pursuant to ASC 820. This estimation process maximizes the use of observable inputs, including the quoted price of the Company's common stock in an inactive market, the price of the Company's common stock determined in connection with transactions in the Company's common stock, and an income approach to valuation (discounted cash flow) (Note 2).
The Black-Scholes option-pricing model requires the use of highly subjective and complex assumptions, including the expected term and the price volatility of the underlying stock, which determine the fair value of stock-based awards. These assumptions include:
|
● |
Risk-free rate. Risk-free interest rates are derived from U.S. Treasury securities as of the option grant date. |
|
● |
Expected dividend yields. Expected dividend yields are based on our historical dividend payments, which have been zero to date. |
|
● |
Volatility. Because we have a limited trading history as a public company, we estimate volatility of our share price based on a combination of the published historical volatilities of comparable publicly-traded companies in our vertical markets and the historical volatility of our common stock. |
|
● |
Expected term. We estimate the weighted-average expected life of the options as 5 years. |
|
● |
Forfeiture rate. Forfeiture rates are estimated using historical actual forfeiture trends as well as our judgment of future forfeitures. These rates are evaluated at least annually and any change in compensation expense is recognized in the period of the change. The estimation of stock awards that will ultimately vest requires judgment and, to the extent actual results or updated estimates differ from our current estimates, such amounts will be recorded as a cumulative adjustment in the period in which the estimates are revised. We consider many factors when estimating expected forfeitures, including the types of awards and employee class. Actual results, and future changes in estimates, may differ substantially from management's current estimates. |
The Company accounts for stock-based compensation awards to non-employees in accordance with ASC 505-50, Equity-Based Payments to Non-Employees. Under ASC 505-50, the Company determines the fair value of the warrants or stock-based compensation awards granted as either the fair value of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable. Any stock options or warrants issued to non-employees are recorded in expense and additional paid-in capital in shareholders' deficit over the applicable service periods using variable accounting through the vesting dates based on the fair value of the options or warrants at the end of each period.
Results of Operations
|
For the year ended |
||||||||||||||||
|
2017 |
2016 |
$ Change |
% Change |
|||||||||||||
|
Revenue |
$ | 66,000 | $ | 218,000 | $ | (152,000 |
) |
(69.7 |
)% |
|||||||
|
Cost of Revenue |
12,000 | 51,000 | 39,000 | 76.5 |
% |
|||||||||||
|
Gross Profit |
54,000 | 167,000 | (113,000 |
) |
(67.7 |
)% |
||||||||||
|
Operating Expenses |
7,582,000 | 6,960,000 | (622,000 |
) |
(8.9 |
)% |
||||||||||
|
Loss from Operations |
(7,528,000 |
) |
(6,793,000 |
) |
(735,000 |
) |
(10.8 |
)% |
||||||||
|
Other Income / (Expense) |
(98,000 |
) |
(169,000 |
) |
71,000 | 42.0 |
% |
|||||||||
|
Net Loss |
$ | (7,626,000 |
) |
$ | (6,962,000 |
) |
$ | (664,000 |
) |
(9.5 |
)% |
|||||
|
Net loss per share - basic and diluted |
$ | (0.19 |
) |
$ | (0.18 |
) |
$ | (0.01 |
) |
(5.5 |
)% |
|||||
Revenue for the year ended December 31, 2017 was $66,000, a decrease of $152,000 or 69.7% over $218,000 for the year ended December 31, 2016. Revenue is generated from sales to distributors of agricultural products and to growers who have completed trials in multiple crop-growing seasons with positive crop attributes from applying BAM-FX™ to their crops. During the years ended December 31, 2014, 2015 and 2016, the Company initiated trials of BAM-FX™ with a number of growers. The majority of our revenue in 2016 was generated by purchases of stocking inventory for sales by distributors in the third and fourth quarter. During 2017, the distributors reduced their inventory levels selling to high value crop growers and did not replenish their inventory, resulting in a decrease in revenue from distributors for the year. Most of the distributors, because of their location, were restricted to marketing to lower value crop growers, who in a number of cases had nominal or negative farm operating results. These growers could not, in a large scale application, afford the incremental cost to purchase a newly introduced, albeit more effective product, BAM-FX™, and continued using traditional lower cost and less effective chelated zinc and copper products. The Company addressed its wholesale cost in 2018 and reduced prices for price competitiveness.
For the year ended December 31, 2017, cost of revenue was $12,000, a decrease of $39,000 over $51,000 reported during the same period in 2016. The decrease in cost of revenue is directly related to our decrease in revenue for the year ended December 31, 2017 over 2016.
Operating Expenses increased by $622,000 to $7,582,000 for the year ended December 31, 2017 compared to $6,960,000 for the year ended December 31, 2017, or 8.9%. The increase in operating expense is primarily due to the recording of $927,000 of warrants issued as an inducement to convert previously issue warrants into common shares, an increase in audit fees of $218,000 primarily related to additional expenses insurred due to an accounting change to non-cash employee stock option expense recognition, and an adjustment of the accrual of prior year costs. Additionally due to an increase in corporate insurance expense of $149,000 offset by a decrease in sales, marketing, technical employee and consultant related expenses of $75,000, a decrease in research expense of $285,000 primarily due to the reduction of expenses related to the RSAA; a decrease in automobile, travel and meals expense of $56,000; a decrease in legal expense of $252,000 for services related to the Company’s intellectual property and general corporate matters. Non-cash equity compensation paid to consultants, board members and employees included in general and administrative expense was $1,174,000 for the year ended December 31, 2017, a decrease of $719,000 compared to $1,893,000 for the year ended December 31, 2016. Research and development expense included in operating expenses decreased by $285,000 to $180,000 from $465,000 during the year ended December 31, 2017 from the same period in 2016. The decrease is primarily due to expenses in 2016 related to our RSAA.
Other Expense for the year ended December 31, 2017 decreased by $71,000 to $98,000 from $169,000 for the year ended December 31, 2015. The decrease in other expense is primarily due to an increase in interest expense offset by a decrease in accretion of debt discount in connection with the Promissory Notes.
Net Loss for the year ended December 31, 2017 increased by $664,000 to $7,626,000 from net loss of $6,962,000 for the year ended December 31, 2016. The increase in net loss is primarily due to a decrease in gross profit realized during the period of $113,000, an increase in operating expense for the year ended December 31, 2017 of $622,000, offset by a decrease in other expense of $71,000.
Use of Cash
Net Cash Used in Operating Activities
Net cash used in operating activities for the year ended December 31, 2017 increased by $95,000 to $4,853,000 from $4,758,000 for year ended December 31, 2016. The increase in net cash used in operating activities is primarily due to an increase in net loss of $664,000, a decrease in accounts payable of $146,000 an increase in inventory of $24,000 and a decrease in prepaid expenses of $97,000 offset by a net increase of non-cash warrants and stock options issued for compensation, loan and acquisition costs of $444,000 and a decrease in accounts receivable of $197,000, a decrease in advance on future royalty payments of $41,000 and a decrease in other current assets of $150,000, included in changes in operating assets and liabilities.
Net Cash Used in Investing Activities
Net cash used in investing activities for the year ended December 31, 2017 of $16,000 decreased $75,000 from the year ended December 31, 2016 of $91,000 due to a decrease in cash used to purchase equipment and acquire intellectual property.
Net Cash Provided by Financing Activities
Net cash provided by financing activities increased by $2,422,000 to $4,652,000 for the year ended December 31, 2017 from $2,230,000 for the year ended December 31, 2016. The increase in net cash provided by financing activities is due primarily to an increase in the issuance of promissory notes of $2,285,000, and an increase of $209,000 in notes payable offset by an increase in payments on notes payable of $52,000 and a decrease in proceeds from common stock of $21,000.
Liquidity and Capital Resources
The Company expects to incur significant expenses and operating losses for the foreseeable future. Specifically, we estimate that the costs associated with the execution of our 2018 business plan may exceed $350,000 per month. This expense rate is primarily due to: an increase in costs of additional personnel, personnel-related costs and promotional expenses to develop markets for domestic and international sales of our product, BAM-FX™, our retail product, Gardner’s Choice, and our cannabis market product introduction; improvements to our manufacturing facility and processes; and, research and product development related expense for expansion of our product line, product improvement and dry formulation. The Company has evaluated its ability to continue as a going concern for the next twelve months from the issue date of the December 31, 2017 consolidated financial statements. There is substantial doubt about the Company's ability to continue as a going concern as we do not currently have the funding necessary to support the projected operating costs we expect to be needed to operate the business through mid-2018. The Company is active in its fundraising, and subsequent to December 31, 2017, the Company has raised $2,044,500.
We filed a registration statement on Form 10 with the SEC that became effective in February 2015 and requires us to operate as a fully reporting public company. We expect to continue to incur additional personnel and professional costs associated with operating as a fully reporting public company. Accordingly, we have acknowledged the need to obtain additional funding to operate the Company and have continued to raise funds through a private offering.
On July 16, 2015, Michael T. Smith, a member of the Company’s Board of Directors, made an unsecured loan to the Company in the principal face amount of $500,000. The Company issued Mr. Smith a promissory note (the “Note”) bearing interest at the rate of 8.5% per annum, such interest being payable by the Company to Mr. Smith quarterly. The Note may be repaid in full by the Company, plus all unpaid interest, by July 16, 2016 (“Maturity Date”). Prepayment of all unpaid principal and interest may be made by the Company prior to the Maturity Date, without penalty or premium. As additional consideration for the Note, Mr. Smith also received a five-year cashless warrant to purchase up to 350,000 shares of the Company’s common stock at an exercise price of $3.00 per share. On July 19, 2016, upon approval of the Board of Directors, the Company and Mr. Smith agreed to an amendment of the Note (the “Amendment”) to extend the maturity date of the Note to July 16, 2017 and provide that the principal and accrued but unpaid interest under the Note may be converted, in whole or in part, into the Company’s common stock at a conversion price equal to $1.25 per common share. The Amendment did not amend any other provisions of the Note and became effective as of July 16, 2016. In July 2017, the maturity date of the note was extended to July 2018, the other terms of this note including the conversion feature remain the same.
Adequate additional financing may not be realized from our private offering or otherwise may not be available to us on acceptable terms, or at all. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts. We will need to generate significant revenues to achieve profitability, and we may never do so.
Cash on Hand
As of December 31, 2017, the Company had a cash balance of $14,000 compared to a cash balance of $232,000 as of December 31, 2016.
Total assets were $740,000 and $1,075,000 at December 31, 2017 and December 31, 2016, respectively. Working capital (deficit) was $(1,225,000) and $(594,000) at December 31, 2017 and 2016, respectively. The decrease in working capital of $631,000 during the period was primarily due to cash used in operating activities of $4,853,000 and financing activities of $17,000 offset by cash provided by the sale of common stock, net of offering expenses of $2,192,000 and cash provided by promissory notes, net of offering costs of $2,251,000. Total stockholders’ equity decreased by $1,781,000 to $(1,969,000) at December 31, 2017 from $(188,000) at December 31, 2016.
Outlook
Required Capital Over the Next Year
Due to the fact that our product, BAM-FX™, is new in the agricultural markets, it is difficult to accurately predict revenues and cash flow at this time. We will need additional funding to cover 2018 expenses. Subsequent to December 31, 2018 through June 30, 2019, we issued 1,086,500 shares of common stock pursuant to our private offering for $3,259,500 in gross proceeds.
On or about January 17, 2018, the Company received $500,000 from an accredited investor, and in exchange the Company issued to the investor (a) an unsecured promissory note dated January 16, 2018, maturing on October 26, 2019, in the principal face amount of $500,000 (the “First Note”), and (b) a warrant dated January 18, 2018 to purchase up to 50,000 shares of the Company’s common stock of (the “First Warrant”). A member of the Company’s board of directors is a beneficiary of certain trusts that own Rio Vista Investments, LLC, the holder of the First Note and First Warrant.
On or about January 18, 2018, the Company received $100,000 from an accredited investor, and in exchange the Company issued to the investor (a) an unsecured promissory note dated January 19, 2018, maturing on January 18, 2020, in the principal face amount of $100,000 (the “Second Note”), and (b) a warrant dated January 19, 2018 to purchase up to 10,000 shares of the Company’s common stock of (the “Second Warrant”).
The First Note and Second Note shall collectively be referred to as the “Notes”, and the First Warrant and Second Warrant shall collectively be referred to as the “Warrants”. The Notes and Warrants were made on substantially the same terms, except as noted.
The Notes bear interest at the rate of ten percent (10%) per annum, such interest being payable by the Company to the holders thereof quarterly in cash. The Notes are payable in full by the Company, plus all unpaid interest thereon, by their respective maturity dates (the “Maturity Date”). Prepayment of all unpaid principal and interest on each Note may be made by the Company prior to the Maturity Date, provided that the holder thereof shall receive a minimum amount of interest equal to one year of interest under such Note, based on the full principal amount. In addition to the foregoing, the Company must repay the First Note to Rio Vista Investments, LLC within 30 days of the date the Company possesses an amount of cash equal to the outstanding balance of principal and interest due under the Second Note plus an amount reasonably anticipated to be necessary to operate the Company over the succeeding 6 months. The Warrants are exercisable within 5 years of issuance into shares of the Company’s common stock, at $3.00 per share.
On or about March 8, 2018, the Company received $200,000 from an accredited investor and member of our Board of Directors, and in exchange the Company issued (a) an unsecured promissory note dated March 8, 2018 (the “Third Note”), and (b) a warrant dated March 9, 2018 to purchase up to 20,000 shares of the Company’s common stock (the “Third Warrant”).
On or about March 12, 2018, the Company received $200,000 from Boies Partners, Inc. (“BPI”), an accredited investor, and in exchange the Company issued to BPI (a) an unsecured promissory note dated March 12, 2018 (the “Fourth Note”), and (b) a warrant dated March 12, 2018 to purchase up to 20,000 shares of the Company’s common stock (the “Fourth Warrant”). Alex Boies, a member of our Board of Directors, has no financial interest in or control over BPI and does not otherwise have any beneficial ownership in any securities owned by BPI.
The Third Note and Fourth Note shall collectively be referred to as the “Notes”, and the Third Warrant and Fourth Warrant shall collectively be referred to as the “Warrants”. The Notes were made on substantially the same terms, except as noted. The Warrants were made on the same terms.
The Notes bear interest at the rate of ten percent (10%) per annum, such interest being payable by the Company to the holders thereof quarterly in cash. The Notes are payable in full by the Company, plus all unpaid interest thereon, by March 7, 2020 and March 11, 2020, respectively (as per each, the “Maturity Date”). Prepayment of all unpaid principal due on each Note may be made by the Company prior to each such Note’s Maturity Date, provided that the holder thereof shall receive a minimum amount of interest equal to one year of interest due under such Note, based on such Note’s principal balance as of the origination date. The Notes contains customary provisions for events of default and acceleration of sums due.
The Fourth Note further provides that within 30 days of receipt of payment of any amount principal outstanding under the Fourth Note (the “Conversion Window”), the note holder shall have the right to convert any portion of such payment into the Company’s common stock at the lesser of $3.00 per share or the lowest price per share of any sale by the Company of its common stock occurring between the date of the Fourth Note and the end of the Conversion Window.
The Warrants are exercisable within 5 years of issuance into shares of the Company’s common stock, at $3.00 per share.
On February 20, 2018, BAM Inc. and Pedro Lichtinger Waisman, Isaac Lichtinger Waisman and Victor Lichtinger Waisman (the “Lichtinger Group”) entered into a License and Business Development Agreement Mexico, effective as of February 13, 2018 (the “Agreement”). Following consummation of the Agreement, the Lichtinger Group formed a company in Mexico, Agro Space Tech SA de CV de RV (“Agro Space”), to which BAM will receive a twenty percent (20%) equity ownership interest. BAM received $100,000 following execution of the Agreement, to purchase shares of the Company in connection with the Company’s ongoing private placement of shares of its common stock for USD $3.00 per share.
Without consideration of any revenue or additional fundraising, at the Company’s current rate of expenditure, we expect that our current capital will not be sufficient to cover our future operating costs for twelve months.
The sales cycle for our product BAM-FX™ in agriculture markets is generally two to three growing seasons. We have completed our second season and with certain growers, our third season, of validation and testing as a commercial product and believe that positive trial results on growers’ crops have contributed to product awareness. However, as important as the positive trial results are, third-party validation of the product’s performance and scientific proof of the product’s mode of action are equally important to our channel partners in their purchasing decisions. The scientific studies, which were undertaken in 2016, are expected to continue during 2018. We believe that the combination of validation, testing and technical studies carried out over the past years will lead to revenue generation and growth in 2018.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
As a “smaller reporting company”, as defined by Item 10 of Regulation S-K, the Company is not required to provide the information required by this item.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
See “Index to Consolidated Financial Statements and Financial Statement Schedule” at the end of this Annual Report on page [F-1] for information required by this Item 8.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
During the period from through December 30, 2016, neither the Company nor anyone on its behalf consulted with Crowe with respect to (1) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s financial statements, and neither a written report was provided to the Company nor oral advice was provided that Crowe concluded was an important factor considered by the Company in reaching a decision as to the accounting, auditing, or financial reporting issue or (2) any matter that was either the subject of a “disagreement” (as defined in Item 304(a)(1)(iv) of Regulation S-K and the related instructions) or a “reportable event” (as described in Item 304(a)(1)(v) of Regulation S-K).
|
CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
As of the end of the period covered by this report (the “Evaluation Date”), the Company carried out an evaluation, under the supervision and with the participation of our management, including our Principal Executive Officer and Principal Accounting Officer (our Chief Executive Officer and Chief Financial Officer, respectively), of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Rule 13a-15 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Based upon this evaluation, our Chief Executive Officer concluded that, as of the Evaluation Date, our disclosure controls and procedures were not effective to provide reasonable assurance that (i) information required to be disclosed in the reports that are filed or submitted under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified by the SEC’s rules and forms and (ii) our disclosure controls and procedures are designed to ensure that information required to be disclosed in the reports that the Company files or submits under the Exchange Act is accumulated and communicated to our management including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that the Company’s disclosure controls and procedures will detect or uncover every situation involving the failure of persons within the Company to disclose material information otherwise required to be set forth in the Company’s periodic reports.
Management’s Annual Report on Internal Controls Over Financial Reporting
The Company’s management is also responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. Internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and dispositions of our assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of the financial statements in accordance with U.S. generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In connection with the preparation of our annual financial statements, management has undertaken an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2017 based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Management’s assessment included an evaluation of the design of our internal control over financial reporting and testing of the operational effectiveness of those controls. Based on this evaluation management has concluded that our internal control over financial reporting was not effective as of December 31, 2017
Management has noted the following material weaknesses in internal controls as of December 31, 2017:
|
● |
Management noted that the Company does not have effectively designed and implemented entity level controls. These include not having properly designed and implemented controls to identify risks relevant to financial reporting, communicating financial reporting roles and responsibilities, insuring communications with the audit committee and external parties are complete and accurate, and monitoring the effectiveness of internal control over financial reporting. |
|
● |
Management noted that the Company does not have effectively designed and implemented segregation of duties and period end financial reporting controls. This is mostly due to a limited number of employees among whom duties can be allocated. |
| ● | Management noted that the Company does not have effectively designed and implemented review controls to ensure that the accounting for significant and complex transactions are recorded in accordance with generally accepted accounting principles. Specifically the Company does not have properly designed controls over the review of memoranda to support the accounting for significant transactions, accounts and/or assertions, the accuracy of and conclusions reached in reports prepared by third-party valuation specialists, the completeness and accuracy of the Company's tax provision; and the overall presentation of and disclosures in the consolidated financial statements. |
| ● | Management noted that the Company does not have effectively designed and implemented controls related to information systems user access or change management. The Company has not designed or implemented appropriate user access or change management review controls that would decrease the susceptibility of financially significant systems to unauthorized changes and inappropriate user access. |
Management is in the process of reviewing and revising its internal control framework, including the precision of management review controls, that gave rise to these material weaknesses disclosed above.
This annual report does not include an attestation report of the Company’s registered public accounting firm regarding internal controls over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to rules of the SEC that permit the Company to provide only management’s report in this annual report.
Changes in Internal Controls
There were no changes in our internal control over financial reporting during the fiscal quarter ended December 31, 2017, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
|
OTHER INFORMATION |
Not applicable.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The following table sets forth certain information about our executive officers, key employees and directors:
|
Name |
|
Age |
|
Position |
|
Harvey “Kaye” Klebanoff |
|
77 |
|
Chairman of the Board of Directors of the Company |
|
Alexander M. Boies |
|
32 |
|
Director of the Company |
|
Edward F. Cowle |
|
61 |
|
Director of the Company |
|
Patrick Kennedy |
|
76 |
|
Director and Vice President of Agricultural Business Development of the Company |
|
Timothy A. Peach* |
|
66 |
|
Director and Chief Executive Officer of the Company |
|
Soumyo Sarkar |
|
60 |
|
Director of the Company |
|
Michael T. Smith |
|
74 |
|
Director of the Company |
|
John W. Kennedy |
|
78 |
|
Chief Science Officer of the Company |
| Raveendran Pottahil** | 69 | Chief Operating Officer and Technology Officer of the Company |
* Mr. Peach was appointed as a member of the Board of Directors and Chief Executive Officer on June 15, 2017
** Mr. Pottahil was appointed Chief Operating Officer on June 15, 2017.
Biographies of Directors and Executive Officers
Harvey “Kaye” Klebanoff, Chairman of the Board of Directors of the Company
Mr. Kaye has served as our Chairman since the Company’s inception as its current form in December of 2012. From March 2009 to January 2012, Mr. Kaye was founder, Chairman, Chief Executive Officer and President of Latitude Solutions, Inc. Latitude Solutions, Inc., a publicly traded holding company for several subsidiaries, provided products, processes and services for contaminated water applications. Prior to founding Latitude Solutions, Mr. Kaye was Chief Executive Officer and President of Gulfstream Capital, L.C., a merchant banking, consulting and financial advisory organization, which provided advisory and corporate finance, services to both public and private companies. Gulfstream has acted in a merchant banking, financial advisory and strategic planning capacity for numerous corporations, both public and private. Mr. Kaye has a BS in business from Temple University. Mr. Kaye also serves as a director of Angstrom Technologies, Inc.
Mr. Kaye is well qualified to serve as the Chairman of Board due to his extensive experience in the management and operation of public and private companies, both large and small, and his previous leadership experience as an entrepreneur, investment banker, chairman, chief executive officer and director.
Alexander M. Boies, Director of the Company
Mr. Boies has been a Director of the Company since December 2015. Mr. Boies is currently employed as an associate at the Boies, Schiller & Flexner LLP law firm in New York, NY, where he works with a wide range of clients on complex litigation matters. After graduating from the University of Michigan, Ann Arbor, with a B.S. in statistics, Mr. Boies was employed by Livingston Securities, LLC from February 2010 until May 2012 wherein he assisted with a number of private placements and IPOs until Mr. Boies resigned in order to pursue his legal degree. From August 2012 until May 2015, Mr. Boies was enrolled as a full-time student at New York University School of Law. During the summer of 2013, Mr. Boies interned at Legal Aid Society in the Bronx Housing Courthouse office. Mr. Boies began working at Boies, Schiller & Flexner LLP as a summer associate in 2014 and commenced full-time employment in October 2015. He was part of the legal team that won a $663M judgment against a guardrail maker in a Qui Tam trial. Additionally, Mr. Boies participated in the management of Hawk and Horse Vineyards, a California company owned and operated by his family since 2001. Mr. Boies also was the Executive Producer on the film, “Escape Plan.” Mr. Boies brings a wide range of legal and financial skills and a diverse set of experiences to the Board.
Edward F. Cowle, Director of the Company
Mr. Cowle has been a Director of the Company since December 2012. Mr. Cowle is currently Chief Executive Officer of Sports Engineering, Inc.(SEI). SEI is the holder of an exclusive license from Worcester Polytechnic Institute (WPI) to commercialize a new type of athletic shoe technology. Mr. Cowle has been a director and former CEO of US Rare Earths, Inc. Mr. Cowle was a founder, and remains a Director, and principal of Laser Technology, Inc. which produces laser based law enforcement speed guns. Mr. Cowle has also worked closely with the Office of Industrial Liaison at New York University Medical School,and the tech transfer office of Johns Hopkins Medical school, investing in and incubating several technologies. Mr. Cowle structured a licensing deal with C. R. Bard for a start-up company that he co-founded with Temple University Office of Technology Development and Commercialization. He was a founding shareholder and investor in Biophan Technology and worked closely with management to develop business, financing, and investor awareness. The company subsequently licensed and sold its technology to Boston Scientific and Medtronic and the core products are currently being sold to the medical community. Mr. Cowle was a founder of Golf Technologies, Inc., the owner and manufacturer of the “Snake Eyes” brand of golf equipment and apparel. The company was bought by Golfsmith who currently sells the Snake Eyes line of products. Mr. Cowle formerly served as Senior Vice President Investments–Paine Webber and Co. and Vice President-Bear Stearns and Co. He graduated from Fairleigh Dickinson University in 1978 with a BA in English and American studies.
We believe Mr. Cowle is well qualified to serve as a member of the Board due to his extensive experience starting, financing and advising successful small businesses and the financial acumen he gained from his experience serving in the financial industry.
Patrick Kennedy, Director and Vice President of Agricultural Business Development of the Company
Mr. Kennedy has been with ZGSI in his current positions since its inception in its current form in December 2012. From May 2000 to June 2007 Mr. Kennedy was the President of American Natural Technology Sciences, Inc. Mr. Kennedy has forty-five years of business experience involving a wide range of skills, including business development, trade, finance, marketing and sales. He has negotiated, marketed and consummated over one billion dollars of sales volume. Mr. Kennedy possesses valuable industry contracts and has been directly responsible for negotiations and structuring of marketing and product distribution. From 1977 to 2000 Mr. Kennedy successfully owned and operated oil companies. Mr. Kennedy also serves as director of Axtel Scientific, Inc. and Mitigation of Disease, Inc. He previously served as director of Digital Stream, Inc.
We believe that Mr. Kennedy is well qualified to serve as a member of the Board due to his extensive understanding of business development and marketing, as well as his significant experience working in and with businesses, large and small.
Timothy A. Peach, Chief Financial Officer of the Company
Mr. Peach hes been a Director and Chief Executive Officer since June 15, 2017 and has served as the Company’s Chief Financial Officer since February 2015. From 2008 until 2014, Mr. Peach served as Chief Financial Officer, Executive Vice President, and Vice President of Finance of Oncure Medical Corp., a radiation oncology treatment center management company located in Englewood, CO. From 2004 until 2008, Mr. Peach served as Chief Financial Officer and Vice President of Finance for VISTA International Technologies, Inc., a waste to energy technology company. He served as an SEC and compliance consultant to growing companies from 2003-2004 and was the Vice President Finance and Chief Accounting Officer/Controller at Convergent Group Corp. from 1998 until 2002. Prior to 2002, he earned his CPA at PricewaterhouseCoopers and held a variety of senior financial and advisory positions at Executive Telecard, Ltd., Kaire International and Telectronics Pacing Systems, Inc. Mr. Peach received his MBA from the University of Pittsburgh. Our Board believes Mr. Peach’s qualifications to serve as our Chief Financial Officer include his extensive financial and operations experience earned in both early stage and established companies.
Soumyo Sarkar, Director of the Company
Mr. Sarkar joined the Company’s Board of Directors in December 2015. Mr. Sarkar has served as Chief Executive Officer and Portfolio Manager of Sumit Capital LLC, an independent institutionally-orientated investment advisory firm since its founding in 2010. Founded in 2010, Sumit Capital is a New York based registered investment advisor. Prior to founding Sumit Capital, Mr. Sarkar was a Managing Director with Deutsche Bank and the Founder and Head of the Deutsche Bank Matched Book Arbitrage Group. He ran the Deutsche Bank Matched Book Arbitrage Group from 1995 to 2009. Prior to joining Deutsche Bank, Soumyo ran a similar strategy within Credit Suisse proprietary trading group from 1991 to 1995. Prior to Credit Suisse, Mr. Sarkar held various research and programming roles for over five years with Solomon Brothers, Merrill Lynch and Lehman Brothers. He has an MBA from the University of Iowa’s Tippie School of Management and a BTech from the BHU Institute of Technology in Varanasi, India. We believe Mr. Sarkar brings an extensive understanding of finance and a wide range of experience to the Board.
Michael T. Smith, Director of the Company
Mr. Smith joined the Company’s Board of Directors in February 2015. He is the former Chairman of the Board and Chief Executive Officer of Hughes Electronics Corporation, having served from October 1997 to May 2001. From 1985 until 1997 he served in a variety of capacities for Hughes, including Vice Chairman of Hughes Electronics, Chairman of Hughes Missile Systems and Chairman of Hughes Aircraft Company. Prior to joining Hughes, he spent nearly 20 years with General Motors Corporation in a variety of financial management positions. Mr. Smith is currently a director, Chair of the Nominating and Governance Committee and Audit Committee member of Teledyne Technologies Incorporated which provides enabling technologies for industrial growth markets. He also serves as a director for WABCO Holdings, Inc. which provides electronic and electromechanical products for the automotive industry and FLIR Systems, Inc., which produces infrared cameras, thermal imaging software and temperature measurement devices. He previously served as a director of ATK Alliant Techsystems, Inc., an advanced weapon and space systems company, from 1997 to 2009, Anteon International Corporation, an information technology and systems engineering solutions company, from 2005 to 2006, and Ingram Micro Corporation, a technology sales, marketing and logistics company from 2001 to 2014. Mr. Smith holds a BA in Political Science from Providence College and an MBA from Babson College and served as an officer in the United States Army.
We believe Mr. Smith brings strong financial skills that are important in the understanding and oversight of our financial reporting and corporate governance matters along with expertise in corporate governance, enterprise risk management and strategic planning, which we believe qualify him to provide guidance as a Director to the Company
Biographies of Key Employees
John W. Kennedy, Chief Science Officer of the Company
John W. Kennedy has been with ZGSI since its inception as the current company in 2012 and was instrumental in its creation. He has 40 years’ experience in applied research, botany, biology, physics, nutrition, biochemistry and discoveries associated with health, disease, plant and biological sciences and technologies. Since November 1979, Mr. Kennedy has been operating John W. Kennedy Consultants, Inc. which represents companies and associations in clearance and registration of pesticides at federal and state agencies. Mr. Kennedy is also president and chairman of Axtel Scientific, Inc., a Nevada corporation, established October 18, 2012 which was licensed by Mr., Kennedy’s IP to commercialize a unique modality for mitigation of several cellular devastating diseases. Zero Gravity Incorporated was a former company owned by Mr. Kennedy that collaborated through a Space Act Agreement with NASA on six space shuttle launches carrying experiments to the International Space Station for plant and animal studies. This corporation was discontinued shortly after the incorporation of ZGSI. Mr. Kennedy holds a BS in Botany and Natural Science from University of Wisconsin. He has received additional educational credits at the United States Department of Agriculture Graduate School in New York, NY with advanced studies in the Biological Sciences. He was also awarded a Space Act Agreement with NASA and is considered a National Lab Pathfinder for his work on plant and human stem cells.
Andrew Koopman, Chief Executive Officer and President of Zero Gravity Life Sciences, Inc.
Since May 2016, Mr. Koopman has served as the Chief Executive Officer and President of the Company's wholly owned subsidiary, ZGLS. From January 2014 to May 2016, Mr. Koopman served on the Company's Scientific Advisory Board as a consultant. He previously served as Director of New Ventures at New York University in the Office of Industrial Liaison from October 2001 to May 2016, where he worked with investors and entrepreneurs to facilitate the formation of new life science companies that focused on the development and commercialization of NYU developed technologies. From August 1997 to October 2001, Mr. Koopman was the Managing Director of Biomedical and Biotechnology Industries at Beroff Associates, a merchant investment banking and strategic advisory group. Prior to joining Beroff Associates, Mr. Koopman served as a laboratory supervisor and project manager with Hoffman-LaRoche from January 1989 to August 1997. Mr. Koopman also held associate scientist positions at Ortho Diagnostic Systems and Unigene Laboratories, Inc. between January 1984 and December 1988. Mr. Koopman holds a BS in Biology from Binghamton University and an MS in molecular biology from Seton Hall University with a minor in business administration from the Stillman School of Business.
Raveendran Pottahil, Chief Operating Officer and Chief Technology Officer of Zero Gravity Solutions, Inc.
Dr. Pottathil is President of Accudx Inc., a strategic technology and consulting company. Dr. Pottathil is an entrepreneur with a 40-year history spanning academia, industry, invention, and company creation.
In 1996, Dr. Pottathil created Accudx as a diagnostic assay manufacturer. This company has progressed into consulting, technology and product development, and intellectual property services in biotech, medtech, and ecotech. His experience in academia and industry has covered various aspects of biochemistry, molecular biology, mammalian genetics, medical virology, tumor biology, and diagnostics, in both academic and industrial settings. As Chief Process Scientist at PetroAlgae, he was responsible for the development and scale up processes for growth, harvesting and processing of micro-algae. As a founding member and Director at Specialty Laboratories International, he successfully set up a large number of esoteric Clinical Reference Laboratories in Mexico, Singapore, Malaysia and India.
Glenn A. Stinebaugh
As reported on Form 8-K, dated June 20, 2017, Mr. Stinebaugh tendered his resignation as a member of the Board of Directors and as the Chief Executive Officer and President of the Company and BAM Inc. On June 15, 2017, Mr. Stinebaugh commenced his position of the Senior Vice President of BAM, Inc.
Term of Office
Our directors are elected to hold office until the next annual general meeting of our stockholders or until removed from office in accordance with our bylaws. Our officers are appointed by our board of directors annually at its annual meeting or at any regular or special meeting of the Board.
Involvement in Certain Legal Proceedings.
Except as noted below, none of our officers, directors, promoters or control persons has had any of the following events occur:
|
● |
any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer, either at the time of the bankruptcy or within two years prior to that time, except that (i) Mr. Kaye held an officer position (until January 2012) and a director position (until April 2012) in Latitude Solutions, Inc. which in December 2012 filed for bankruptcy protection, and (ii) Mr. Peach held an officer position in Oncure Medical Corp. (until June 2014), which filed for reorganization in June 2013 in connection with an acquisition of the company by new ownership: or |
|
● |
any conviction in a criminal proceeding or being subject to a pending criminal proceeding excluding traffic violations and other minor offenses; or |
|
● |
being subject to any order, judgment or decree, not substantially reversed, suspended or vacated, of any court of competent jurisdiction, permanently enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking business; or |
|
● |
being found by a court of competent jurisdiction in a civil action, the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended or vacated; or |
|
● |
been the subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of: |
|
○ |
any Federal or State securities or commodities law or regulation; or |
|
○ |
any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or |
|
○ |
any judicial or administrative proceeding resulting from involvement in mail or wire fraud or fraud in connection with any business entity. |
Corporate Governance
Audit Committee & Compensation Committee
On January 20, 2016, our Board voted to create separate audit and compensation committees of our Board. Before that time, we did not have any standing audit or compensation committees of the Board or committees performing similar functions. On February 25, 2016, our Board adopted both the Audit Committee’s charter, and the Compensation Committee’s charter.
Audit Committee. The Audit Committee is comprised of two of our independent directors, Michael Smith and Soumyo Sarkar. The functions of the Audit Committee include the retention of our independent registered public accounting firm, reviewing and approving the planned scope, proposed fee arrangements and results of our Company’s annual audit, reviewing the adequacy of our Company’s accounting and financial controls and reviewing the independence of our Company’s independent registered public accounting firm. While the Company is not listed on the NASDAQ Stock Market, our Board has determined that each member of the Audit Committee is an “independent director” under the applicable rules and regulations of the SEC. The Board has determined that a member of the Audit Committee does qualify as an “audit committee financial expert” within the applicable requirements of the SEC.
Compensation Committee. The Compensation Committee is comprised of two of our independent directors, Edward F. Cowle and Alexander M. Boies. The functions of the Compensation Committee include the approval of the compensation offered to our executive officers and recommendation to the full Board of the compensation to be offered to our non-employee directors. While the Company is not listed on the NASDAQ Stock Market, our Board has determined that each member of the Compensation Committee is independent in accordance with the applicable rules of regulations of the SEC. In addition, the Compensation Committee evaluates the independence of each compensation consultant, outside counsel and advisor retained by or providing advice to the Compensation Committee.
Code of Ethics. - Our Board has not yet adopted a code of ethics that applies to the Company’s officers and directors. However, our Board is currently evaluating ethics standards and hopes to adopt a code of ethics in 2018 as the Company completes its compliance initiatives.
Section 16 Reporting Compliance - Section 16(a) of the Securities Exchange Act of 1934 requires our directors and executive officers and persons who own more than 10% of our outstanding shares of common stock to file with the SEC initial reports of ownership and reports of changes in ownership in our common stock and other equity securities. Specific due dates for these records have been established, and we are required to report in this report any failure in 2017 to file by these dates. We believe that during 2017, each of the following filed a late Form 3 in relation to the Company becoming subject to Section 16 or the addition of a director to the Board: Harvey Kaye, Patrick Kennedy, Glenn A. Stinebaugh, John Wayne Kennedy, Deworth Williams, Michael Smith, Soumyo Sarkar, Alexander M. Boies, Timothy A. Peach, Richard Godwin and Edward F. Cowle. Each of the aforementioned had a Form 3 that was filed late. The Company has revised its administrative procedures to enhance the ability of the Company’s executive officers and directors to comply with such requirements. All persons subject to Section 16 requirements are current with their filings.
|
EXECUTIVE COMPENSATION |
The following table sets forth the cash and other compensation paid by the Company to our named executive officers for the year ended December 31, 2017.
|
Name and Principal Position |
Year |
Salary |
Stock |
Option |
All other |
Total |
||||||||||||||||
|
Timothy A. Peach, |
2015 |
$ | 96,000 | $ | 50,000 | (4) | $ | 92,473 | (5) | $ | — | $ | 238,473 | |||||||||
|
Chief Financial Officer of the Company (1) |
2016 |
$ | 141,000 | $ | — | $ | 246,384 | (6) | $ | — | $ | 387,384 | ||||||||||
|
Chief Executive Officer of the Company (2) |
2017 |
$ | 150,000 | $ | — | $ | — | $ | — | $ | 150,000 | |||||||||||
|
Harvey “Kaye” Klebanoff, |
2015 | $ | 96,000 | $ | — | $ | — | $ | 15,000 | (7) | $ | 111,000 | ||||||||||
| Chairman and Director of the Company (3) |
2016 |
$ | 141,000 | $ | — | $ | — | $ | — | $ | 141,000 | |||||||||||
|
2017 |
$ | 150,000 | $ | — | $ | — | $ | — | $ | 150,000 | ||||||||||||
|
Andrew Koopman, President of Zero Gravity Life Sciences, Inc. |
2016 |
$ | 93,760 | $ | — | $ | 339,613 | (8) | $ | — | $ | 433,373 | ||||||||||
|
2017 |
$ | 150,000 | $ | — | $ | — | $ | — | $ | 150,000 | ||||||||||||
|
Raveendran Pottahil, Chief Operating Officer and Chief Technology Officer of the Company |
2016 |
$ | 109,375 | $ | — | $ | 169,772 | (9) | $ | — | $ | 279,147 | ||||||||||
|
2017 |
$ | 150,000 | $ | — | $ | — | $ | — | $ | 150,000 | ||||||||||||
(1) On February 20, 2015, Timothy A. Peach was appointed the Chief Financial Officer of the Company.
(2) On June 15, 2017, Timothy A. Peach was appointed the Chief Executive Officer of the Company.
(3) Mr. Kaye served as President and Interim Chief Executive Officer of the Company from October 3, 2014 to November 21, 2014. Mr. Kaye currently serves as Chairman of the Board.
(4) Mr. Peach received 100,000 shares of the Company’s common stock in connection with his execution of an at-will employment agreement with the Company on February 20, 2015.
(5) Mr. Peach received a five-year warrant to purchase 200,000 shares of the Company’s common stock at $0.50 per share in connection with his execution of an at-will employment agreement with the Company on February 20, 2015. After 6 months of satisfactory services, Mr. Peach received an additional five-year warrant to purchase 300,000 shares of the Company’s stock at $0.50 per share.
(6) Mr. Peach received an option to purchase 400,000 shares of the Company’s common stock upon the initialization of the company's 2015 Stock incentive plan. The options vest immediately.
(7) Mr. Kaye’s entire salary of $55,000 earned in 2013 was deferred, of which $40,000 was paid in 2014 and the remainder was paid in fiscal 2015.
(8) Mr. Koopman received an option to purchase 600,000 shares of the Company’s common stock upon his execution of an offer letter with the Company on April 12, 2016, 300.000 are to vest immediately and 300.000 vest after 6 months.
(9) Mr. Pottahil was appointed as Cheif Operating Officer of the Company June 15, 2017. Mr. Potahil received an option to purchase 300,000 shares of the Company’s common stock upon his execution of an offer letter with the Company on April 11, 2016, 150,000 are to vest immediately and 150,000 vest after 6 months.
(10) For valuation considerations, please see Note 7 of the December 31, 2017 Notes to the Financial Statements, included in this report.
Outstanding Equity Awards at December 31, 2017 Fiscal Year End
|
Name |
Number of |
Number of |
Option |
Option |
|||||||||
|
Timothy A. Peach |
200,000 | — | $ | 0.50 |
February 22, 2020 |
||||||||
|
Timothy A. Peach |
300,000 | — | $ | 0.50 |
November 2, 2020 |
||||||||
|
Timothy A. Peach |
400,000 | — | $ | 1.25 |
January 16, 2026 |
||||||||
|
Andrew Koopman |
600,000 | — | $ | 1.25 |
April 12, 2026 |
||||||||
|
Raveendran Pottahil |
300,000 | — | $ | 1.25 |
April 11, 2026 |
||||||||
Narrative Discussion of Compensation Tables
Employee Agreements and Current Compensation Rates for Named Executive Officers
The named executive officers above each serve at the will of our Board of Directors, under the terms discussed below.
As dictated by the Board, Mr. Harvey Kaye’s salary for fiscal year 2015 was $96,000 per annum and $96,000 per annum for 2014. The Compensation Committee and the Board approved an increase in Mr. Kaye’s salary to $150,000 per annum effective March 1, 2016. In addition, Mr. Kaye’s entire salary earned in 2013 of $55,000 was deferred, of which $40,000 was paid in 2014 and the remainder was paid in fiscal 2015.
On February 20, 2015, Mr. Peach entered into an at-will employment agreement with the Company under which he serves as the Chief Financial Officer of the Company. He receives $8,000 per month for his services to the Company, along with 100,000 shares of the Company’s common stock and a five-year warrant to purchase 200,000 shares of the Company’s common stock at $0.50 per share. After six months of satisfactory service Mr. Peach received an additional five-year warrant to purchase 300,000 shares of the Company’s common stock at $0.50 per share. The Compensation Committee and the board approved an increase in Mr. Peach’s compensation to $12,500 per month effective March 1, 2016. On June 15, 2017, Mr. Peach accepted the position of Chief Executive officer.
In March 2016, the Board of Directors, upon the recommendation of the Company’s Compensation Committee, approved increases in the salary of Mr. Kaye, and Mr. Peach, such that each would receive $12,500 per month for their services in their respective positions, to be effective retroactively beginning March 1, 2016. In addition, Mr. Kaye, and Mr. Peach would be eligible for cash bonuses of $12,500 on July 31, 2016 and October 31, 2016, subject to the Company’s achievement of certain revenue targets by such dates, which were not achieved. Accordingly, no cash bonuses were paid in 2016.
Employee Benefit and Incentive Plans
The Company adopted a Stock Incentive Plan for its employees in December 2015, and health care plan for its employees in October 2015, which does not discriminate in favor of our executive officers or directors in scope, terms or operation and is available generally to all salaried employees of the Company.
Director Compensation
|
Name |
Fees earned |
Stock |
(7) |
Option |
(7) |
All other |
Total |
|||||||||||||
|
Harvey Kaye |
$ | - | $ | - | $ | - | $ | 150,000 | (1) | $ | 150,000 | |||||||||
|
Alexander M. Boies (2) |
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
Edward F. Cowle |
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
Soumyo Sarkar (3) |
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
Timothy A. Peach (4) |
$ | - | $ | - | $ | - | $ | 150,000 | $ | 150,000 | ||||||||||
|
Patrick Kennedy |
$ | - | $ | - | $ | - | $ | 126,000 | (5) | $ | 126,000 | |||||||||
|
Michael Smith (6) |
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
(1) Mr. Kaye’s entire salary was earned in connection with his service as Chairman of the Board (including deferred amounts noted in the section above.).
(2) Alexander M. Boies joined the Board of Directors in November 2015.
(3) Soumyo Sarkar joined the Board of Directors in November 2015.
(4) Mr. Peach joined the Board in June 2017.
(5) Mr. P. Kennedy’s entire salary of $96,000 was earned in connection with his service as Vice President of Agricultural Business Development of the Company. He also received $30,000 pursuant to royalties earned or minimum payments under the SOD Royalty Agreement and Royalty Agreement, as discussed in Item 13.
(6) Michael Smith joined the Board of Directors in February 2015.
(7) For valuation considerations, please see Note 7 of the December 31, 2017 Notes to the Financial Statements, included in this report.
There are no written compensation agreements in place regarding the payment to any director for their service as a director. In connection with Mr. Smith’s appointment to the Board, on February 10, 2015 he received warrants to purchase 200,000 shares of the Company's common stock with an exercise price of $0.50 having a fair value of $92,479. In connection with Mr. Boies and Mr. Sarkar joining the Board, on December 2, 2015 each received warrants to purchase 200,000 of the Company’s shares of common stock with an exercise price of $2.00 having a fair value of $238,059.
Indemnification of Officers and Directors
Our bylaws provide that we will indemnify our officers and directors to the fullest extent permitted by applicable law against all liability and loss suffered and expenses (including attorney’s fees) incurred in connection with actions or proceedings brought against them by reason of their serving or having served as officers, directors or in other capacities, provided that the director or officer acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the Company, unless such director or officer will have been adjudged to be liable for negligence or misconduct in the performance of his or her duty to the Company. We shall be required to indemnify a director or officer in connection with an action or proceeding commenced by such director or officer only if the commencement of such action or proceeding by the director or officer was authorized in advance by the Board of Directors.
We have procured a Directors and Officers Insurance Policy with National Union Fire Insurance Company of Pittsburgh, PA with an $8,000,000 limit of liability.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The following table sets forth, as of April 28, 2018, the number of shares of Common Stock owned of record and beneficially by the named executive officers, directors and persons who beneficially own more than 5% of the outstanding shares of common stock of the Company. Unless otherwise noted below, the address of each director and executive officer of the Company is 190 NW Spanish River Boulevard, Suite 101, Boca Raton, Florida 33431. The addresses for the greater than 5% stockholders are set forth in the footnotes to this table:
|
Common Stock |
||||||||
|
Number of Shares Beneficially Owned (1) |
Percentage Outstanding |
|||||||
|
Directors and Executive Officers |
||||||||
|
Edward F. Cowle |
2,650,000 | 6.58 |
% |
|||||
|
Harvey “Kaye” Klebanoff |
2,483,000 | (3) | 6.17 |
% |
||||
|
Patrick Kennedy |
1,986,666 | 4.93 |
% |
|||||
|
Michael Smith |
1,700,000 | (4) | 4.17 |
% |
||||
|
Timothy A. Peach |
1,000,000 | (6) | 2.50 |
% |
||||
|
Soumyo Sarkar |
940,000 | (5)(7) | 2.48 |
% |
||||
|
Alexander M. Boies |
200,000 | (5) | .50 |
% |
||||
|
All directors and named executive officers as a group (7 persons) |
10,959,666 | 26.87 |
% |
|||||
|
5% Shareholders |
||||||||
|
John W. Kennedy (8) |
7,000,000 | 17.38 |
% |
|||||
|
Deworth Williams (9) |
2,615,000 | 6.49 |
% |
|||||
|
(1) |
The Company believes that each stockholder has sole voting and investment power with respect to the shares of common stock listed, except as otherwise noted. The number of shares beneficially owned by each stockholder is determined under rules of the SEC, and the information is not necessarily indicative of ownership for any other purpose. Under these rules, beneficial ownership includes (i) any shares as to which the person has sole or shared voting power or investment power and (ii) any shares which the individual has the right to acquire within 60 days after April 13, 2018 through the exercise of any stock option, warrant, conversion of preferred stock or other right, but such shares are deemed to be outstanding only for the purposes of computing the percentage ownership of the person that beneficially owns such shares and not for any other person shown in the table. The inclusion herein of any shares of common stock deemed beneficially owned does not constitute an admission by such stockholder of beneficial ownership of those shares of common stock. |
|
(2) |
The percentage outstanding calculation is based on 40,787,448 shares of common stock issued and outstanding as of July 6, 2018. |
|
(3) |
The 2,483,000 shares of our common stock include 983,000 shares of our common stock owned of record by Mr. Kaye and 1,500,000 shares of our common stock owned of record by Ms. Helen Klebanoff, Mr. Kaye’s wife. |
|
(4) |
The 1,700,000 shares of our common stock are made up of (i) 1,050,000 shares of common stock held of record by Mr. Smith, (ii) warrants exercisable into 200,000 shares of our common stock at $0.50 per share, and (iii) warrants exercisable into 350,000 shares of our common stock at $4.50 per share, and (iv) warrants exercisable into 100,000 shares of our common stock at $3.00 per share. |
|
(5) |
Alexander M. Boies and Soumyo Sarkar joined our Board on November 30, 2015. In exchange for their service on the Board, each of Mr. Boies and Mr. Sarkar received warrants to purchase 200,000 shares of the Company’s common stock at an exercise price of $2.00 per share. |
|
(6) |
The 1,000,000 shares of our common stock include 100,000 shares of our common stock owned of record by Mr. Peach and warrants exercisable into 500,000 shares of our common stock at $0.50 per share and options exercisable into 400,000 shares of our common stock at $1.25 per share. |
|
(7) |
The 940,000 shares of our common stock include 620,000 shares of our common stock owned of record by Mr. Sarkar and warrants exercisable into 320,000 shares of our common stock at $2.00 per share. |
|
(8) |
John W. Kennedy is our Chief Science Officer and previously served as a member of our Board of Directors until December 2015. His address is as follows: c/o Zero Gravity Solutions, Inc., 190 NW Spanish River Boulevard, Suite 101, Boca Raton, Florida 33431. |
|
(9) |
Deworth Williams previously served as members of our Board of Directors until December 2015. Mr. William’s address is as follows: 2681 E. Parleys Waye, Suite 204, Salt Lake City, UT 84109. |
Changes in Control
We are unaware of any contract, or other arrangement or provision, the operation of which may at any subsequent date result in a change in control of our Company.
Equity Compensation Plan Information
The following table sets forth equity compensation plan information as of December 31, 2017:
|
Plan category |
Number of securities upon outstanding warrants and |
Weighted- average options, rights |
Number of securities remaining future issuance under compensation plans securities reflected in |
|||||||||
|
(a) |
(b) |
(c) |
||||||||||
|
Equity compensation plans approved by security holders: |
||||||||||||
|
2015 Equity Incentive Plan |
2,750,000 | 1.29 | 1,250,000 | |||||||||
|
Equity compensation plans not approved by security holders |
— | — | — | |||||||||
|
Total |
2,750,000 | 1.29 | 1,250,000 | |||||||||
2015 Equity Incentive Plan. In December 2015, our stockholders approved our 2015 Equity Incentive Plan (“2015 Plan”). Our 2015 Plan provides for the grant of incentive stock options to our employees and our parent and subsidiary corporations' employees, and for the grant of nonstatutory stock options, stock bonus awards, restricted stock awards, performance stock awards and other forms of stock compensation to our employees, including officers, consultants and directors. Our 2015 Plan also provides that the grant of performance stock awards may be paid out in cash as determined by the Committee (as defined herein).
Authorized Shares. A total of 4,000,000 shares of our common stock are reserved for issuance pursuant to the 2015 Plan. Shares issued under our 2015 Plan may be authorized but unissued or reacquired shares of our common stock. Shares subject to stock awards granted under our 2015 Plan that expire or terminate without being exercised in full, or that are paid out in cash rather than in shares, will not reduce the number of shares available for issuance under our 2015 Plan. Additionally, shares issued pursuant to stock awards under our 2015 Plan that we repurchase or that are forfeited, as well as shares reacquired by us as consideration for the exercise or purchase price of a stock award, will become available for future grant under our 2015 Plan.
Administration. Our Board of Directors, or a duly authorized committee thereof (collectively, the “Committee”), has the authority to administer our 2015 Plan. Our Board may also delegate to one or more of our officers the authority to designate employees other than Directors and officers to receive specified stock, which, in respect to those awards, said officer or officers shall then have all that the Committee would have.
Subject to the terms of our 2015 Plan, the Committee has the authority to determine the terms of awards, including recipients; the exercise price or strike price of stock awards, if any; the number of shares subject to each stock award; the fair market value of a share of our common stock; the vesting schedule applicable to the awards, together with any vesting acceleration; the form of consideration, if any, payable upon exercise or settlement of the stock award and the terms and conditions of the award agreements for use under our 2015 Plan. The Committee has the power to modify outstanding awards under our 2015 Plan subject to the terms of the 2015 Plan and applicable law. Subject to the terms of our 2015 Plan, the Committee has the authority to reprice any outstanding option or stock appreciation right, cancel and re-grant any outstanding option or stock appreciation right in exchange for new stock awards, cash or other consideration, or take any other action that is treated as a repricing under generally accepted accounting principles, with the consent of any adversely affected participant.
Stock Options. Stock options may be granted under the 2015 Plan. The exercise price of options granted under our 2015 Plan must at least be equal to the fair market value of our common stock on the date of grant. The term of an incentive stock option may not exceed 10 years, except that with respect to any participant who owns more than 10% of the voting power of all classes of our outstanding stock, the term must not exceed 5 years and the exercise price must equal at least 110% of the fair market value on the grant date. The Committee will determine the methods of payment of the exercise price of an option, which may include cash, shares or other property acceptable to the Committee, as well as other types of consideration permitted by applicable law. No single participant may receive more than 25% of the total options awarded in any single year. Subject to the provisions of our 2015 Plan, the Committee determines the other terms of options.
Performance Shares. Performance shares may be granted under our 2015 Plan. Performance shares are awards that will result in a payment to a participant only if performance goals established by the administrator are achieved or the awards otherwise vest. The Committee will establish organizational or individual performance goals or other vesting criteria in its discretion which, depending on the extent to which they are met, will determine the number and/or the value of performance shares to be paid out to participants. After the grant of a performance share, the Committee, in its sole discretion, may reduce or waive any performance criteria or other vesting provisions for such performance shares. The Committee, in its sole discretion, may pay earned performance units or performance shares in the form of cash, shares or some combination thereof per the terms of the agreement approved by the Committee and delivered to the participant. This agreement will state all terms and condition of the agreements.
Restricted Stock. The terms and conditions of any restricted stock awards granted to a participant will be set forth in an award agreement and, subject to the provisions in the 2015 Plan, will be determined by the Committee. Under a restricted stock award, we issue shares of our common stock to the recipient of the award, subject to vesting conditions and transfer restrictions that lapse over time or upon achievement of performance conditions. The Committee will determine the vesting schedule and performance objectives, if any, applicable to each restricted stock award. Unless the Committee determines otherwise, the recipient may vote and receive dividends on shares of restricted stock issued under our 2015 Plan.
Other Share-Based Awards and Cash Awards. The Committee may make other forms of equity-based awards under our 2015 Plan, including, for example, deferred shares, stock bonus awards and dividend equivalent awards. In addition, our 2015 Plan authorizes us to make annual and other cash incentive awards based on achieving performance goals that are pre-established by our compensation committee.
Change in Control. If the Company is merged or consolidated with another entity or sells or otherwise disposes of substantially all of its assets to another company while awards or options remain outstanding under the 2015 Plan, unless provisions are made in connection with such transaction for the continuance of the 2015 Plan and/or the assumption or substitution of such awards or options with new options or stock awards covering the stock of the successor company, or parent or subsidiary thereof, with appropriate adjustments as to the number and kind of shares and prices, then all outstanding options and stock awards which have not been continued, assumed or for which a substituted award has not been granted shall, whether or not vested or then exercisable, unless otherwise specified in the relevant agreements, terminate immediately as of the effective date of any such merger, consolidation or sale.
Change in Capitalization. If the Company shall affect a subdivision or consolidation of shares or other capital readjustment, the payment of a stock dividend, or other increase or reduction of the number of shares of the common stock outstanding, without receiving consideration therefore in money, services or property, then awards amounts, type, limitations, and other relevant consideration shall be appropriately and proportionately adjusted. The Committee shall make such adjustments, and its determinations shall be final, binding and conclusive.
Plan Amendment or Termination. Our Board has the authority to amend, suspend, or terminate our 2015 Plan, provided that such action does not materially impair the existing rights of any participant without such participant's written consent. The 2015 Plan will terminate ten (10) years after the earlier of (i) the date the 2015 Plan is adopted by the Board, or (ii) the date the 2015 Plan is approved by the stockholders, except that awards that are granted under the 2015 Plan prior to its termination will continue to be administered under the terms of the 2015 Plan until the awards terminate, expire or are exercised.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Certain Relationships and Related Transactions
Except as otherwise indicated herein or in item 11 above, there have been no other related party transactions, or any other transactions or relationships required to be disclosed pursuant to Item 404 of Regulation S-K.
|
● |
On December 12, 2013, our former director and Chief Science Officer, Mr. John W. Kennedy and current director, Mr. Patrick Kennedy, and the Company entered into a royalty agreement (the “Royalty Agreement”) having a term of 25 years, wherein the Company is required to pay royalty fees to Messrs. John W. Kennedy and Patrick Kennedy, in the amount of (1) 5% of gross sales of the BAM-FX™ product (and related products) of which 3% will be paid to John W. Kennedy and 2% to Patrick Kennedy, and/or (2) 10% of any license or sub-license of the product or related products of which 6% will be paid to John W. Kennedy and 4% to P. Kennedy. The Royalty Agreement allowed the Company to pay Messrs. John W. Kennedy and Patrick Kennedy advance royalties as determined by the CEO of the Company, to be deducted from any future royalties due. In March 2015, the Royalty Agreement was superseded by the SOD Royalty Agreement, described below. |
|
● |
On March 13, 2015, the Company entered into a new License and Royalty Agreement with Messrs. John W. Kennedy and Patrick Kennedy in connection with the patent application relating to the Copper/Zinc Superoxide Dismutase (SOD) Formulation for the Treatment of Traumas Including Amyotrophic Lateral Sclerosis for manufacturing, commercial opportunities and/or supply and/or research for products which may be developed by the Company for fertilizers and for humans and animals such as remediation of radiation of astronauts and other oxidative stress conditions discovered in a micro/zero gravity environment (“SOD Royalty Agreement”). The SOD Royalty Agreement superseded the Royalty Agreement. The SOD Royalty Agreement has a term of 25 years and the Company has an obligation to pay royalty fees to Messrs. John W. Kennedy and Patrick Kennedy, in the amount of (1) 5% of gross sales of commercial product developed and sold (and related products) of which 3% will be paid to John W. Kennedy and 2% to Patrick Kennedy, and/or (2) 10% of any license or sub-license of the product or related products, of which 6% will be paid to John W. Kennedy and 4% to Patrick Kennedy. The SOD Royalty Agreement also requires a minimum payment of $2,500 to each individual per month, unearned portions of which may be applied against future royalties earned. John W. Kennedy has waived his minimum payment for the year. In addition, certain other costs, the Company made that were necessary for the maintenance and protection of the Company’s rights in the underlying patents were applied against future royalty obligations of the Company.
For the year ended December 31, 2015, John W. Kennedy and Patrick Kennedy received minimum payments under the SOD Royalty Agreement (or prepayments under the Royalty Agreement) of $15,000 and $30,000, respectively, of which John W. Kennedy and Patrick Kennedy earned royalties of $4,976 and $3,317, respectively. For the year ended December 31, 2016, John W. Kennedy and Patrick Kennedy received minimum payments under the SOD Royalty Agreement (or prepayments under the Royalty Agreement) of $0 and $30,000, respectively, of which John W. Kennedy and Patrick Kennedy earned royalties of $1,261 and $841, respectively. For the year ended December 31, 2017, John W. Kennedy and Patrick Kennedy received minimum payments under the SOD Royalty Agreement of $0 and $30,000, respectively, of which John W. Kennedy and Patrick Kennedy earned royalties of $1,972 and $1.315, respectively, under the SOD Royalty Agreement. |
|
● |
As of December 31, 2015, the Company has paid a total of $124,262 of legal fees related to the approval and issuance of the patent for “Bioavailable Minerals for Plant Health” and legal fees to maintain, protect and litigate the inventor’s rights in the patent for “Mitigation of Plant and Animal Diseases using Bioavailable Minerals”. This patent is held by former director and current Chief Science Officer, Mr. John W. Kennedy and current director, Mr. Patrick Kennedy. The patent is a related patent to be held or licensed by the Company. The Company may utilize this patent in future and reserves the right to negotiate for this patent at a later date. |
|
● |
On March 10, 2015, the Company entered into a Consulting Agreement with Williams Investment Company and Deworth Williams, a former director of the Company. Under the Consulting Agreement, Williams provided advice and recommendations to the Company with respect to investor relations, public relations and general corporate business development advice for a period of six months beginning on the date of the Agreement. The Company paid $50,000 upon execution of the Agreements with additional payments of $150,000 due in equal installments on June 6, 2015 and August 6, 2015. As of December 31, 2017, and to date $75,000 is still payable. |
|
● |
In July 2015, Mr. Michael Smith, a Board member, advanced the Company $500,000 under a note payable for working capital purposes. The unsecured note payable bears interest at 8.5% per annum, payable quarterly, and is due in July 2016. In connection with the note payable, the Company issued warrants to purchase 350,000 shares of the Company’s common stock at an exercise price of $2.00 per share. The Company calculated the fair value of the warrants at $416,618 utilizing the Black-Scholes Model with the following assumptions: expected dividends of 0%, volatility of 184.2%, risk free interest rate of 1.66% and expected life of 5 years. The relative fair value of the debt and warrants was recorded resulting in a debt discount of $227,258 upon execution of the agreement. As of December 31, 2015, accretion of the debt discount of $47,345 was recorded in other expenses on the statement of operations. The total amount of principal and interest paid during the year ended December 31, 2015 was $0 and $18,333, respectively. On July 19, 2016, upon approval of the Board, the Company and Mr. Smith agreed to an amendment of this note to extend the maturity date to July 16, 2017, and to provide the principal & accrued interest may be converted, in whole or part, into the Company's common stock at a conversion equal to $1.25 per share. On July 17, 2017, upon approval of the Board, the Company and Mr. Smith agreed to an amendment of this note to extend the maturity date to July 16, 2018. |
|
● |
On July 16, 2015, Mr. Smith, received a five-year cashless warrant to purchase up to 350,000 shares of common stock (at $3.00 per share) as additional consideration for a loan he made to the Company. On March 1, 2017, the Company and Mr. Smith entered into an amendment of their agreement where Mr. Smith fully exercised his warrant and received 350,000 shares for $525,000 cash payment. As additional consideration for Mr. Smith's entry into the amendment, the Company issued a new Smith warrant for up to 350,000 shares of common stock at $4.50 a share. |
|
● |
On February 19, 2016, Diamond B. Capital LLC ("Diamond") received a five-year warrant to purchase up to 400,000 shares of common stock at $2.00 per share. Alexander M. Boies, a Board member is a 12% ownership of Diamond. On March 16, 2017, the Company entered into an amendment with Diamond B. Capital LLC on their warrant. Diamond B. Capital LLC fully exercised its warrant and received 400,000 shares of common stock for $600,000 payment. |
|
● |
In August 2017, the Company issued an unsecured promissory note to its in-house corporate counsel in the principal face amount of $100,000. The promissory note bears interest at a rate of 10.0%, per annum, payable quarterly. The promissory note shall be repaid in full plus all unpaid interest by August 2019. In connection with the note, the Company issued five-year warrants to purchase up to 10,000 shares of the Company’s common stock at an exercise price of $3.00 per share. |
|
● |
In September 2017, the Company issued an unsecured promissory note to a member of its Board of Directors in the principal face amount of $500,000. The promissory note bears interest at a rate of 10.0%, per annum, payable quarterly. The promissory note shall be repaid in full plus all unpaid interest by September 2019. In connection with the note, the Company issued five-year warrants to purchase up to 50,000 shares of the Company’s common stock at an exercise price of $3.00 per share. |
|
● |
In October 2017, the Company issued an unsecured promissory note to a member of its Board of Directors in the principal face amount of $500,000. The promissory note bears interest at a rate of 10.0% per annum, payable quarterly. The promissory note shall be repaid in full plus all unpaid interest by October 2019. In connection with the note, the Company issued five-year warrants to purchase up to 50,000 shares of the Company’s common stock at an exercise price of $3.00 per share. |
|
● |
On or about January 17, 2018, the Company received $500,000 from an accredited investor, and in exchange the Company issued to the investor (a) an unsecured promissory note dated January 16, 2018, maturing on October 26, 2019, in the principal face amount of $500,000 (the “Second Note”), and (b) a warrant dated January 18, 2018 to purchase up to 50,000 shares of the Company’s common stock of (the “Second Warrant”). A member of the Company’s Board of Directors is a beneficiary of certain trusts that own Rio Vista Investments, LLC, the holder of the note and warrant. The notes bears interest at the rate of ten percent (10%) per annum, such interest being payable by the Company to the holders thereof quarterly in cash and shall be repaid in full by the Company, plus all unpaid interest thereon, by their respective maturity dates (the “Maturity Date”). Prepayment of all unpaid principal and interest on the note may be made by the Company prior to the Maturity Date, provided that the holder thereof shall receive a minimum amount of interest equal to one year of interest under such note, based on the full principal amount. In addition to the foregoing, the Company must repay the Second Note to Rio Vista Investments, LLC within 30 days of the date the Company possesses an amount of cash equal to the outstanding balance of principal and interest due under the Second Note plus an amount reasonably anticipated to be necessary to operate the Company over the succeeding 6 months. The Warrants are exercisable within 5 years of issuance into shares of the Company’s common stock, at $3.00 per share. |
|
● |
On or about March 8, 2018, the Company received $200,000 from Mr. Smith, a member of our Board of Directors, and in exchange the Company issued to Mr. Smith (a) an unsecured promissory note dated March 8, 2018, and (b) a warrant dated March 9, 2018 to purchase up to 20,000 shares of the Company’s common stock. The note bears interest at the rate of ten percent (10%) per annum, such interest being payable by the Company to the holders thereof quarterly in cash. The note shall be repaid in full by the Company, plus all unpaid interest thereon, by March 7, 2020. Prepayment of all unpaid principal due on the note may be made by the Company prior to note’s maturity date, provided that the holder thereof shall receive a minimum amount of interest equal to one year of interest due under such note, based on the note’s principal balance as of the origination date. The warrants are exercisable within 5 years of issuance into shares of the Company’s common stock, at $3.00 per share. |
Director Independence
Our common stock is not quoted or listed on any national exchange or interdealer quotation system with a requirement that a majority of our board of directors be independent and therefore, the Company is not subject to any director independence requirements. Under NASDAQ Rule 5605(a)(2)(A), a director is not considered to be independent if he or she also is an executive officer or employee of the corporation. Under such definition, Harvey Kaye, Patrick Kennedy, and Timothy A. Peach would not be considered independent as they serve currently or have served as the officers of the Company within the past three years. Alexander M. Boies, Soumyo Sarkar, Edward F. Cowle and Michael Smith may be considered independent under this standard.
Policies Regarding Conflicts of Interests and Related Party Transactions
We have not adopted formal policies or procedures for the review or approval of related party transactions or the management of conflicts of interest. However, our Board is in the process of establishing such policies with the intent to have them in place subsequent to the effectiveness of this report.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The Company's Board of Directors approved formation of an Audit Committee on January 20, 2016 which is comprised of two members of the Board of Directors.
As disclosed in our Form 8-K filed on November 5, 2015, we dismissed Sadler, Gibb & Associates, LLC (“Sadler Gibb”) as our independent accountant on October 26, 2015, and engaged GHP Horwath, P.C. (“GHP”) to serve as our new independent registered public accounting firm. In our Form 8-K filed on January 4, 2017, we advised that we had received notice from our independent registered public accounting firm, GHP, that GHP had chosen not to stand for re-appointment as the Company’s auditor, and effective as of December 30, 2016, the client-auditor relationship between the Company and GHP ceased. The resignation of GHP was not recommended by our Audit Committee nor was the Audit Committee’s approval required. We disclosed in our Form 8-K filed on February 10, 2017, that we engaged Crowe Horwath LLP (Crowe) as our new independent registered public accounting firm effective February 8, 2017. Crowe audited our financial statements for the fiscal year ended December 31, 2016, and conducted reviews of the Company’s unaudited quarterly financial statements on an ongoing basis beginning with the financial statements for the quarter ended March 31, 2017. The engagement of Crowe Horwath LLP was approved by our Audit Committee.
The following table sets forth the aggregate fees billed to us by GHP for a portion of the fiscal year ended December 31, 2015, as well as GHP for a portion of the fiscal year ended December 31, 2016 and Crowe fees to be billed in connection with the audit of our financial statements for the year ended December 31, 2016.
|
Crowe |
Crowe |
GHP |
||||||||||
|
2017 |
2016 |
2016 |
||||||||||
|
Audit Fees |
$ | 256,000 | $ | 75,000 | $ | 43,000 | ||||||
|
Audit Related Fees |
- | 8,000 | ||||||||||
|
Tax Fees |
6,000 | - | - | |||||||||
|
Other Fees |
- | - | ||||||||||
|
Totals |
$ | 262,000 | $ | 75,000 | $ | 51,000 | ||||||
Audit fees represent amounts billed for professional services rendered or expected to be rendered for the audit of our annual financial statements.
Audit-related fees represent professional services rendered or expected to be rendered for assurance and related services by the accounting firm that are reasonably related to the performance of the audit or review of our financial statements that are not reported under audit fees. Our Audit Committee is of the opinion that the Audit-Related Fees charged by Sadler Gibb, GHP and Crowe were consistent with each independent accountant maintaining its independence from us.
Tax fees represent professional services rendered or expected to be rendered by the accounting firm for tax compliance.
All other fees represent fees billed for products and services provided by the accounting firm and subcontractors, other than the services reported for the other three categories.
The Audit Committee of the Company approves all auditing services and the terms thereof and non-audit services (other than non-audit services published under Section 10A(g) of the Exchange Act or the applicable rules of the SEC or the Pubic Company Accounting Oversight Board) to be provided to us by the independent auditor; provided, however, the pre-approval requirement is waived with respect to the provisions of non-audit services for us if the "de minimus" provisions of Section 10A(i)(1)(B) of the Exchange Act are satisfied.
|
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
|
(a) |
Financial Statements. |
|
|
Page Number |
|
Reports of Independent Registered Public Accounting Firms |
F-2 |
|
Consolidated Balance Sheets |
F-4 |
|
Consolidated Statements of Operations and Other Comprehensive Loss |
F-5 |
|
Consolidated Statements of Stockholders’ Deficit |
F-6 |
|
Consolidated Statements of Cash Flows |
F-7 |
|
Notes to Consolidated Financial Statements |
F-8 |
(b) Exhibits.
+Previously filed with the Company’s Registration Statement on Form 10, filed with the SEC on December 29, 2014.
++ Previously filed with the Company’s Annual Report on Form 10-K, filed with the SEC on April 1, 2015.
* Filed herewith.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized:
|
Date: August 13, 2018 |
ZERO GRAVITY SOLUTIONS, INC. |
||
|
|
|
||
|
|
By: |
/s/ Timothy A. Peach |
|
|
|
|
Timothy A. Peach Chief Executive Officer and Chief Financial Officer |
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities indicated on August 13, 2018.
|
Signatures: |
Title: |
|
/s/ Harvey Kaye |
Director, Chairman of the Board |
|
Harvey Kaye |
|
|
|
|
|
/s/ Alexander M. Boies |
Director |
|
Alexander M. Boies |
|
|
|
|
|
/s/ Patrick Kennedy |
Director |
|
Patrick Kennedy |
|
|
|
|
|
/s/ Soumyo Sarkar |
Director |
|
Soumyo Sarkar |
|
|
|
|
|
/s/ Michael Smith |
Director |
|
Michael Smith |
|
|
|
|
|
/s/ Edward F. Cowle |
Director |
|
Edward F. Cowle |
|
|
|
|
|
/s/ Timothy A. Peach |
Director, Chief Executive Officer, Chief Financial Officer (Principal Financial Officer) |
|
Timothy A. Peach |
|
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
CONTENTS
Report of Independent Registered Public Accounting Firm
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Zero Gravity Solutions, Inc. (the “Company”) as of December 31, 2017 and 2016, the related consolidated statements of operations, stockholders' deficit, and cash flows for the years then ended, and the related notes (collectively referred to as the "financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has incurred recurring losses from operations, negative cash flows from operating activities, and has a working capital deficit. These factors raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting in accordance with the standards of the PCAOB. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion in accordance with the standards of the PCAOB.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Crowe LLP
We have served as the Company’s auditor since February 8, 2017.
Fort Lauderdale, Florida
August 13, 2018
Item 1. Consolidated Financial Statements
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Consolidated Balance Sheets
|
December 31, |
December 31, |
|||||||
|
ASSETS |
||||||||
|
CURRENT ASSETS |
||||||||
|
Cash |
$ | 13,862 | $ | 232,394 | ||||
|
Accounts receivable |
1,393 | 121,747 | ||||||
|
Prepaid expenses |
216,139 | 254,269 | ||||||
|
Other current assets |
- | 28,140 | ||||||
|
Inventory |
68,643 | 33,401 | ||||||
|
Total current assets |
300,037 | 669,951 | ||||||
|
Property and equipment - net |
104,590 | 119,057 | ||||||
|
OTHER ASSETS |
||||||||
|
Deposit |
4,617 | 3,617 | ||||||
|
Intellectual property |
11,458 | 5,500 | ||||||
|
Advance on future royalties - related parties |
319,441 | 277,038 | ||||||
|
Total other assets |
335,516 | 286,155 | ||||||
|
TOTAL ASSETS |
$ | 740,143 | $ | 1,075,163 | ||||
|
LIABILITIES AND STOCKHOLDERS' DEFICIT |
||||||||
|
CURRENT LIABILITIES |
||||||||
|
Accounts payable and other payables |
$ | 696,195 | $ | 480,853 | ||||
|
Accounts payable, related party |
112,347 | 75,000 | ||||||
|
Deferred compensation, related party |
12,500 | 12,500 | ||||||
|
Note payable - related party |
500,000 | 500,000 | ||||||
|
Note payable |
204,419 | 195,355 | ||||||
|
Total liabilities (all current) |
1,525,461 | 1,263,708 | ||||||
|
LONG TERM LIABILITIES |
||||||||
|
Note payable, net of discount of $18,218 and $0 |
181,782 | - | ||||||
|
Notes payable, related parties, net of discount of $97,796 and $0 |
1,002,204 | - | ||||||
|
Total long-term liabilities |
1,183,986 | - | ||||||
|
Commitments (Note 4) |
||||||||
|
STOCKHOLDERS' DEFICIT |
||||||||
|
Common stock; 100,000,000 shares authorized, at $0.001 par value, 40,650,397 and 38,973,264 shares issued and outstanding, respectively |
40,650 | 38,973 | ||||||
|
Additional paid-in capital |
21,970,266 | 16,126,129 | ||||||
|
Accumulated deficit |
(23,980,220 |
) |
(16,353,647 |
) |
||||
|
Total stockholders' deficit |
(1,969,304 |
) |
(188,545 |
) |
||||
|
TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT |
$ | 740,143 | $ | 1,075,163 | ||||
See accompanying notes to consolidated financial statements.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Consolidated Statements of Operations
|
Year Ended |
||||||||
|
2017 |
2016 |
|||||||
|
REVENUE |
||||||||
|
Sale of goods |
$ | 65,733 | $ | 218,166 | ||||
|
Total revenue |
65,733 | 218,166 | ||||||
|
COST OF REVENUE |
||||||||
|
Cost of goods sold |
10,276 | 40,882 | ||||||
|
Royalty expense |
2,102 | 10,685 | ||||||
|
Total cost of revenue |
12,378 | 51,567 | ||||||
|
GROSS PROFIT |
53,355 | 166,599 | ||||||
|
OPERATING EXPENSES |
||||||||
|
General and administrative |
7,402,271 | 6,495,350 | ||||||
|
Research and development |
179,351 | 464,570 | ||||||
|
Total operating expenses |
7,581,622 | 6,959,920 | ||||||
|
LOSS FROM OPERATIONS |
(7,528,267 |
) |
(6,793,321 |
) |
||||
|
OTHER INCOME (EXPENSE) |
||||||||
|
Other income (expense) |
(387 | ) | 2,576 | |||||
|
Interest expense |
(82,451 |
) |
(49,068 |
) |
||||
|
Accretion of debt discount |
(15,468 |
) |
(122,631 |
) |
||||
|
Total other income (expense) |
(98,306 |
) |
(169,123 |
) |
||||
|
NET LOSS |
$ | (7,626,573 |
) |
$ | (6,962,444 |
) |
||
|
NET LOSS PER SHARE - BASIC AND DILUTED |
$ | (0.19 |
) |
$ | (0.18 |
) |
||
|
WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING - BASIC AND DILUTED |
40,110,298 | 38,573,651 | ||||||
See accompanying notes to consolidated financial statements.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Consolidated Statements of Stockholders' Deficit
For the years ended December 31, 2017 and 2016
|
Additional |
Total |
|||||||||||||||||||
|
Common Stock |
Paid-In |
Accumulated |
Stockholders' |
|||||||||||||||||
|
Shares |
Amount |
Capital |
Deficit |
Equity(Deficit) |
||||||||||||||||
|
Balance, December 31, 2015 |
37,357,597 | $ | 37,358 | $ | 12,129,502 | $ | (9,391,203 |
) |
$ | 2,775,657 | ||||||||||
|
Common stock and warrants issued for cash, net of cash offering costs of $204,700 |
1,595,667 | 1,595 | 2,176,206 | - | 2,177,801 | |||||||||||||||
|
Common stock issued for services |
20,000 | 20 | 24,980 | - | 25,000 | |||||||||||||||
|
Warrants issued for services |
- | - | 11,899 | - | 11,899 | |||||||||||||||
|
Stock options issued for services |
- | - | 1,783,542 | - | 1,783,542 | |||||||||||||||
|
Net loss for the year ended December 31, 2016 |
- | - | - | (6,962,444 |
) |
(6,962,444 |
) |
|||||||||||||
|
Balance, December 31, 2016 |
38,973,264 | $ | 38,973 | $ | 16,126,129 | $ | (16,353,647 |
) |
(188,545 |
) |
||||||||||
|
Common stock issued for cash, net of offering costs of $169,472 |
787,133 | 787 | 2,191,140 | - | 2,191,927 | |||||||||||||||
|
Exercise of warrants, net of offering costs of $33,750 |
790,000 | 790 | 1,150,460 | - | 1,151,250 | |||||||||||||||
|
Common stock issued for services |
100,000 | 100 | 299,900 | - | 300,000 | |||||||||||||||
|
Warrants issued for services |
- | - | 1,019,855 | - | 1,019,855 | |||||||||||||||
|
Costs of exercise of warrants |
- | - | 926,885 | - | 926,885 | |||||||||||||||
|
Warrants issued for loan costs |
- | - | 131,482 | - | 131,482 | |||||||||||||||
|
Stock options issued for services |
- | - | 124,415 | - | 124,415 | |||||||||||||||
|
Net loss for the year ended December 31, 2017 |
- | - | - | (7,626,573 |
) |
(7,626,573 |
) |
|||||||||||||
|
Balance December 31, 2017 |
40,650,397 | $ | 40,650 | $ | 21,970,266 | $ | (23,980,220 |
) |
$ | (1,969,304 |
) |
|||||||||
See accompanying notes to consolidated financial statements.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
|
Year Ended |
||||||||
|
December 31, |
||||||||
|
2017 |
2016 |
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES |
||||||||
|
Net loss |
$ | (7,626,573 |
) |
$ | (6,962,444 |
) |
||
|
Adjustments to reconcile net loss to net cash used by operating activities: |
||||||||
|
Depreciation expense |
24,890 | 18,095 | ||||||
|
Amortization expense |
1,018 | - | ||||||
|
Common stock issued for services |
300,000 | 25,000 | ||||||
|
Warrants issued for services |
1,019,855 | 11,899 | ||||||
|
Stock options issued for services |
124,415 | 1,783,542 | ||||||
|
Amortization of debt issuance costs |
15,468 | 122,631 | ||||||
|
Cost of exercise of warrants |
926,885 | - | ||||||
|
Changes in operating assets and liabilities: |
||||||||
|
Accounts receivable |
120,354 | (76,871 |
) |
|||||
|
Other current assets |
66,270 | (83,992 |
) |
|||||
|
Prepaid expenses |
- | 97,330 | ||||||
|
Advance on future royalties - related parties |
(42,403 |
) |
(83,756 |
) |
||||
|
Inventory |
(35,242 |
) |
(11,467 |
) |
||||
|
Deposit |
(1,000 |
) |
2,917 | |||||
|
Accounts payable and other payables |
215,342 | 413,908 | ||||||
|
Accounts payable, related party |
37,347 | (15,000 |
) |
|||||
|
Net cash used in operating activities |
(4,853,374 |
) |
(4,758,208 |
) |
||||
|
CASH FLOWS FROM INVESTING ACTIVITIES |
||||||||
|
Cash paid to acquire intellectual property |
(6,976 |
) |
(5,500 |
) |
||||
|
Cash paid to purchase equipment |
(10,423 |
) |
(85,416 |
) |
||||
|
Net cash used in investing activities |
(17,399 |
) |
(90,916 |
) |
||||
|
CASH FLOWS FROM FINANCING ACTIVITIES |
||||||||
|
Proceeds from notes payable |
404,419 | 195,355 | ||||||
|
Payments of notes payable |
(195,355 |
) |
(142,756 |
) |
||||
|
Proceeds from exercise of warrants - related party |
1,185,000 | - | ||||||
|
Payment of offering costs – related party |
(33,750 |
) |
- | |||||
|
Proceeds from notes payable - related party |
1,100,000 | - | ||||||
|
Proceeds from sale of common stock |
2,361,399 | 2,382,501 | ||||||
|
Payment of offering costs |
(169,472 |
) |
(204,700 |
) |
||||
|
Net cash provided by financing activities |
4,652,241 | 2,230,400 | ||||||
|
NET DECREASE IN CASH |
(218,532 |
) |
(2,618,724 |
) |
||||
|
CASH AT BEGINNING OF PERIOD |
232,394 | 2,851,118 | ||||||
|
CASH AT END OF PERIOD |
$ | 13,862 | $ | 232,394 | ||||
|
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION |
||||||||
|
CASH PAID FOR: |
||||||||
|
Interest |
$ | 82,451 | $ | 3,420 | ||||
|
SUPPLEMENTAL DISCLOSURES OF NON-CASH INVESTING AND FINANCING ACTIVITIES: |
||||||||
|
Warrants issued with debt – related party |
$ | 131,482 | $ | 227,258 | ||||
|
Warrants issued as direct offering costs |
$ | 178,217 | $ | 110,794 | ||||
See accompanying notes to consolidated financial statements.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
NOTE 1 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Nature of operations
Zero Gravity Solutions, Inc. (the “Company”) is a biotechnology company focused on commercializing technology derived from and designed for spaceflight with significant applications on Earth. These technologies are focused on improving world agriculture by providing valuable solutions to challenges facing humanity including threats to world agriculture and the ability to feed the world’s rapidly growing population.
The Company owns proprietary technology for its initial commercial product, BAM-FX™ that can boost the nutritional value and enhance the immune system of food crops without the use of Genetic Modification. The Company’s focus is the commercialization of BAM-FX™ in both domestic and international markets. The Company’s headquarters are located in Boca Raton Florida.
The Company operates through two wholly owned subsidiaries: BAM Agricultural Solutions, Inc. and Zero Gravity Life Sciences, Inc., both Florida corporations formed by the Company in 2014.
Going Concern and Management’s Plans
The Company has a working capital deficiency as of December 31, 2017, and a loss from operations and negative cash flows from operating activities for the year then ended. At December 31, 2017, the Company had an approximate cash balance of $14,000, a working capital deficiency of approximately $(1,225,000), a loss from operations of approximately $(7,528,000), and negative cash flows from operating activities of approximately $(4,853,000) for the year then ended. To date, the Company has relied on equity financing and has entered into related party promissory notes to fund its operations. The Company has also issued stock-based compensation in exchange for services. Although the Company intends to raise additional capital, the Company expects to continue to incur losses from operations and have negative cash flows from operating activities for the near-term and these losses could be significant as product development, regulatory, contract research, and technical marketing personnel related expenses are incurred. Management has evaluated its ability to continue as a going concern for the next twelve months from the issuance of these December 31, 2017 consolidated financial statements, and has determined that there is substantial doubt as to its ability to continue as a going concern. The Company has satisfied its obligations using cash from successful capital raising efforts through December 31, 2017, however there are no assurances that such successful efforts will continue for up to a year after these financial statements are issued. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classifications of liabilities that may result should the Company be unable to continue as a going concern. The Company has executed product distribution agreements with domestic and international commercial agricultural distributors and generated initial product orders. Additional technical and marketing effort must be devoted to those distributors to insure the product is properly utilized and validated by end users. To fund these capital needs, the Company has and continues to raise capital through a private placement offering in connection with its investment banker and through its internal efforts, as well as raising funds through issuances of debt. Subsequent to December 31, 2017, the Company has raised approximately $5,311,000 (Note 8). If the Company does not obtain additional capital, the Company would potentially be required to reduce the scope of its business development activities or cease operations. The Company continues to explore obtaining additional capital financing and the Company is closely monitoring its cash balances, cash needs and expense levels.
Management’s strategic plans include the following:
● Continuing to advance commercialization of the Company’s principal product, BAM-FX™ in both domestic and international markets;
● Pursuing additional capital raising opportunities;
● Continuing to explore and execute prospective partnering or distribution opportunities; and
● Identifying unique market opportunities that represent potential positive cash flow.
Basis of Presentation
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Significant estimates include the valuation of equity based compensation. Actual results could differ from those estimates.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
Cash and Cash Equivalents
For the purposes of the statement of cash flows, the Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. The Company had no cash equivalents at December 31, 2017 and 2016.
Segment Reporting
The Company views its operations and manages its business as one reportable segment. As a result the financial statements presented within, relate to our principal operating segment. Customers in the United States account for 100% of our revenues. We do not have any property or equipment outside of the United States.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Zero Gravity Solutions, Inc. and its wholly-owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
Inventory
Inventory is valued on a lower of first in, first out (FIFO) cost or net realizable value. At December 31, inventory consisted of:
|
2017 |
2016 |
|||||||
|
Raw materials |
$ | 23,562 | $ | 9,081 | ||||
|
Consignment |
- | 19,238 | ||||||
|
Finished product |
45,081 | 5,082 | ||||||
|
Total Inventory |
$ | 68,643 | $ | 33,401 | ||||
Property and Equipment
Property and equipment is stated at cost, less accumulated depreciation. Expenditures for maintenance and repairs are charged to expense as incurred.
Depreciation is computed on a straight-line basis over estimated useful lives. Property and equipment is reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. There were no impairment charges taken during the years ended December 31, 2017 and 2016.
Concentration of Credit Risk
In January 2016, the Company transferred a portion of the cash balance to another major national financial institution and as a result, the Company believes that its credit risk exposure is limited. The Company has never suffered a loss due to such excess balances.
Fair Value of Financial Instruments
The Company accounts for financial instruments under Financial Accounting Standards Board (FASB) Accounting Standards Codification Topic (ASC) 820, Fair Value Measurements. This statement defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. To increase consistency and comparability in fair value measurements, ASC 820 establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three levels as follows:
|
Level 1 — quoted prices (unadjusted) in active markets for identical assets or liabilities; |
|
|
|
|
|
Level 2 — observable inputs other than Level 1, quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, and model-derived prices whose inputs are observable or whose significant value drivers are observable; and |
|
|
|
|
|
Level 3 — assets and liabilities whose significant value drivers are unobservable. |
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
Observable inputs are based on market data obtained from independent sources, while unobservable inputs are based on the Company’s market assumptions. Unobservable inputs require significant management judgment or estimation. In some cases, the inputs used to measure an asset or liability may fall into different levels of the fair value hierarchy. In those instances, the fair value measurement is required to be classified using the lowest level of input that is significant to the fair value measurement. Such determination requires significant management judgment.
As the Company's common stock is not traded in an active market, the Company estimates the fair value of its common stock (used in its Black Scholes option pricing model) pursuant to ASC 820. This estimation process maximizes the use of observable inputs, including the quoted price of the Company's common stock in an inactive market, the price of the Company's common stock determined in connection with transactions in the Company's common stock, and an income approach to valuation (a discounted cash flow technique that considers the future cash flows) (Note 2).
The carrying amounts of the Company’s accounts receivable and accounts payable approximate fair value due to the relatively short period to maturity for these instruments. The carrying value of the Company’s notes payable approximates fair value due to their short period to maturity and their stated interest rates, combined with historic interest rate levels.
Revenue recognition and accounts receivable:
We recognize revenues from the sale of agricultural biotechnology products to distributors pursuant to the provisions of ASC 605, “Revenue Recognition”.
Revenues for agricultural chemical products are recognized when title to the products is transferred. We recognize revenue on products we sell to distributors when, according to the terms of the distribution agreements, delivery has occurred, performance is complete, no right of return exists unless required by law, pricing is fixed or determinable, and collection of sales proceeds are reasonably assured.
Amounts billed to customers for shipping and handling fees are included in net sales, and costs incurred by the company for the delivery of goods are classified as cost of goods sold in our Statements of Operations.
The Company determined that no reserve for estimated product returns and allowances was necessary during fiscal 2017 and 2016. Determination of the reserve for estimated product returns and allowances is based on management's analyses and judgments regarding certain conditions. Should future changes in conditions prove management's conclusions and judgments on previous analyses to be incorrect, revenue recognized for any reporting period could be materially affected.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
At December 31, 2017, three customers accounted for 100% of total accounts receivable. Each of these three customers represented 46.5%, 34.9% and 18.6% respectively. During the year ended December 31, 2017, one customers accounted for 22.8% of net sales.
At December 31, 2016, five customers accounted for 99.4% of accounts receivable. Each of these five customers represented 40.3%, 24.1%, 13.0%, 11.7%, and 10.3%, respectively. During the year ended December 31, 2016, three customers accounted for 65% of net sales, representing 28%, 23.7%, and 13.3%, respectively.
The Company extends credit to customers generally without requiring collateral. The Company monitors its exposure for credit losses and maintains allowances for anticipated losses. The Company records an allowance for doubtful accounts when it is probable that the accounts receivable balance will not be collected. When estimating the allowance, the Company takes into consideration such factors as its day-to-day knowledge of the financial position of specific clients, and the industry and size of its clients. The allowance for doubtful accounts as of December 31, 2017 and 2016 was $32,663 and $83,697, respectively.
Warrants
The Company recognizes the cost of warrants issued with debt as debt issuance costs in the financial statements and is measured based on the grant date fair value of the award. The Company estimates the fair value of each warrant at the grant date by using the Black-Scholes option pricing model.
Stock based compensation
The Company recognizes the cost of employee services received in exchange for an award of equity instruments in the financial statements and is measured based on the grant date fair value of the award. Stock based compensation expense is recognized over the period during which an employee is required to provide service in exchange for the award (generally the vesting period). The Company estimates the fair value of each stock award at the grant date by using the Black-Scholes option pricing model.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
Costs equal to these fair values are recognized ratably over the requisite service period based on the number of awards that are expected to vest, or in the period of grant for awards that vest immediately and have no future service condition. The expense resulting from employee and share-based payments is recorded in general and administrative expense in 2017 and 2016.
The Company also grants share-based compensation awards to non-employees for service provided to the Company. The Company measures and recognizes the fair value of such transactions based on the fair value of consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable.
Loss per Share
Loss per share is calculated by dividing the Company’s net loss by the weighted average number of common shares outstanding during the period. Diluted earnings loss per share is calculated by dividing the Company’s net income (loss) by the diluted weighted average number of shares outstanding during the year. The diluted weighted average number of shares outstanding is the basic weighted number of shares adjusted for any potentially dilutive debt or equity. The effect of the inclusion of the dilutive shares would have resulted in a decrease in loss per share. Accordingly, the weighted average shares outstanding have not been adjusted for dilutive shares. Outstanding warrants are not considered in the calculation as the impact of the potential common shares (totaling 10,713,175, shares and 9,764,733 shares for the years ended December 31, 2017 and 2016, respectively), would be to decrease net loss per share.
Research and Development
Research and development costs are charged to expenses as incurred.
Warranty Expense
The Company's distribution agreements provide for a warranty on products sold. As sales under such distribution agreements have been nominal through 2017 and 2016, there has been no warranty expense in 2017 or 2016. A provision for estimated future warranty costs is to be recorded as cost of sales when products are shipped, and warranty costs are to be based on historical trends in warranty charges as a percentage of gross product shipments. A resulting accrual is to be reviewed regularly, and periodically adjusted to reflect changes in warranty cost estimates.
Income Taxes
The Company accounts for income taxes under the asset and liability method, in which deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis and operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date. A valuation allowance is required to the extent any deferred tax assets may not be realizable.
The Company does not have an accrual for uncertain tax positions as of December 31, 2017 and 2016. The Company files corporate income tax returns with the Internal Revenue Service and the States where the Company determines it is required to do so. The Company’s policy is to recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. At December 31, 2017, the Company did not have any accrued interest or penalties associated with any unrecognized tax benefits, nor was any interest expense recognized during the years ended December 31, 2017 or 2016.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
Recently Issued Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued ASC 606, “Revenue from Contracts with Customers” which has been further clarified and amended. The core principle of the new standard is for companies to recognize revenue to depict the transfer of goods or services to customers in amounts that reflect the consideration (that is, payment) to which the company expects to be entitled in exchange for those goods or services. The new standard also will result in enhanced disclosures about revenue, provide guidance for transactions that were not previously addressed comprehensively (for example, service revenue and contract modifications) and clarify guidance for multiple-element arrangements. Entities have the option to apply the new guidance under a retrospective approach to each prior reporting period presented or a modified retrospective approach with the cumulative effect of initially applying the new guidance recognized at the date of initial application within the Statement of Financial Position. The standard is effective for fiscal years, and interim periods within those years, beginning after Dec. 15, 2017. The Company has finalized its assessment of ASC 606 and determined there will be no material effect on our financial position and results of operations. The timing and amount of revenue recognized based on the new standard is consistent with the revenue recognition policy under previous guidance, however, certain additional financial statement disclosures will be required beginning with 2018 reporting, including additional disaggregated view of revenue. We have adopted the new standard effective January 1, 2018, using the modified retrospective transition method
In July 2015, the FASB issued ASU No. 2015-11, Simplifying the Measurement of Inventory. ASU 2015-11 requires that inventory within the scope of the guidance be measured at the lower of cost and net realizable value. Inventory measured using last-in, first-out (LIFO) and retail inventory method (RIM) are excluded from this new guidance. This ASU replaces the concept of market with the single measurement of net realizable value and is intended to create efficiencies for preparers and more closely align U.S. GAAP with IFRS. This ASU is effective for public business entities in fiscal years and interim periods within those years, beginning after December 15, 2016. The implementation of this standard did not have a material impact on the Company’s consolidated financial statements. The Company's disclosures reflect the adoption of this new standard.
In November 2015, the FASB issued ASU. No. 2015-17, Balance Sheet Classification of Deferred Taxes. ASU No. 2015-17 simplifies the presentation of deferred taxes on the balance sheet by requiring classification of all deferred tax items as noncurrent including valuation allowances by jurisdiction. The ASU is effective for public entities for annual and interim periods beginning after December 15, 2016, and interim periods within those annual reporting periods. The Company adopted this standard in the first quarter of 2017. The implementation of this standard did not have a material impact on the Company’s consolidated financial statements.
In March 2016, the FASB issued ASU No. 2016-02, Leases. The main difference between the provisions of ASU No. 2016-02 and previous U.S. GAAP is the recognition of right-of-use assets and lease liabilities by lessees for those leases classified as operating leases under previous U.S. GAAP. ASU No. 2016- 02 retains a distinction between finance leases and operating leases, and the recognition, measurement, and presentation of expenses and cash flows arising from a lease by a lessee have not significantly changed from previous U.S. GAAP. For leases with a term of 12 months or less, a lessee is permitted to make an accounting policy election by class of underlying asset not to recognize right-of-use assets and lease liabilities. The accounting applied by a lessor is largely unchanged from that applied under previous U.S. GAAP. In transition, lessees and lessors are required to recognize and measure leases at the beginning of the earliest period presented using a modified retrospective approach. This ASU is effective for public business entities in fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. Early adoption is permitted as of the beginning of any interim or annual reporting period. The Company has not finalized the analysis to determined the effect of the standard, but believes that it will not have a material impact on the statement of operations.
In March 2016, the FASB issued ASU 2016-09, Compensation - Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting, which simplifies several aspects of the accounting for share-based payment transactions. Under ASU 2016-09, excess tax benefits and tax deficiencies are recognized as income tax expense or benefit in the income statement. ASU 2016-09 also provides entities with the option to elect an accounting policy to continue to estimate forfeitures of stock-based awards over the service period (current GAAP) or account for forfeitures when they occur. Under ASU 2016-09, previously unrecognized excess tax benefits should be recognized using a modified retrospective transition. In addition, amendments requiring recognition of excess tax benefits and tax deficiencies in the income statement, as well as changes in the computation of weighted-average diluted shares outstanding, should be applied prospectively. ASU 2016-09 is effective beginning in the first quarter of 2017. The Company adopted ASU 2016-09, and elected to account for the forfeiture of awards as they occur, during the first quarter of 2017, with no material impact.
In May 2017, the FASB issued ASU 2017-09 Compensation - Stock Compensation (Topic 718): Scope of Modification Accounting, which provides guidance on determining which changes to the terms and conditions of share-based payment awards require an entity to apply modifications. ASU 2017-09 is effective for all entities for annual periods, including interim periods within those annual periods, beginning after December 15, 2017. Early adoption is permitted, including adoption in any interim period, for:
- Public business entities for reporting periods for which financial statements have not yet been issued.
- All other entities for reporting periods for which financial statements have not yet been made available for issuance.
In June 2018, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2018-07, “Compensation - Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting.” ASU No 2018-07 expands the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. The guidance also specifies that Topic 718 applies to all share-based payment transactions in which a grantor acquires goods or services to be used or consumed in a grantor’s own operations by issuing share-based payment awards. This guidance is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. Early adoption is permitted, but no earlier than an entity’s adoption date of Topic 606. This guidance is applicable to the Company’s fiscal year beginning January 1, 2019. The Company is currently evaluating the potential effects of this guidance on its consolidated financial statements.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
NOTE 2 – PROPERTY AND EQUIPMENT
|
December 31, 2017 |
December 31, 2016 |
|||||||
|
Computer equipment |
$ | 15,332 | $ | 15,332 | ||||
|
Equipment and furniture |
133,940 | 123,517 | ||||||
|
Leasehold improvements |
7,593 | 7,593 | ||||||
| 156,865 | 146,442 | |||||||
|
Accumulated Depreciation |
(52,275 |
) |
(27,385 |
) |
||||
|
Property and Equipment - Net |
$ | 104,590 | $ | 119,057 | ||||
Depreciation expense for the years ended December 31, 2017 and 2016 was $24,890 and $18,095, respectively
NOTE 3 – RELATED PARTY TRANSACTIONS
Notes Payable
In July 2015, a Director advanced the Company $500,000 under a note payable for working capital purposes. The unsecured note payable bears interest at 8.5% per annum, is payable quarterly, and was originally due in July 2016. In connection with the note, the Company issued warrants to purchase 350,000 shares of the Company’s common stock at an exercise price of $3 per share. The Company calculated the fair value of the warrants at $416,618 utilizing the Black-Scholes Model with the following assumptions: expected dividends of 0%, volatility of 184.2%, risk free interest rate of 1.66% and expected life of 5 years. The relative fair value of the debt and warrants was recorded resulting in a debt discount of $227,258 upon execution of the agreement. For the year ended December 31, 2016, accretion of the debt discount was $122,631. The accretion of debt discount is presented in other expenses on the statements of operations. In July 2016, the maturity date of the note was extended to July 2017, and a conversion feature was added. The conversion feature allows the holder to convert the debt into common shares at his option at a conversion price of $1.25 per share (400,000 shares). The addition of the conversion feature represented a substantial modification to the debt instrument but the modification was determined to not be material. During 2016, $43,882 was recorded as interest expense and $10,625 was included in payables at December 31, 2016. During 2017, the maturity date of the note was extended to July 2018. During 2017, $42,736 was recorded as interest expense and $10,861 was included in payables at December 31, 2017. This note is reflected in the current liabilities section of the consolidated balance sheets.
In August 2017, the Company issued an unsecured promissory note to its in-house corporate council in the principal face amount of $100,000. The promissory note bears interest at a rate of 10.0%, per annum, payable quarterly. The promissory note is payable in full plus all unpaid interest by August 2019. In connection with the note, the Company issued five-year warrants to purchase up to 10,000 shares of the Company’s common stock at an exercise price of $3.00 per share. The relative fair value of the debt and warrants was recorded resulting in debt discount of $10,435 upon execution of the promissory note. For the year ended December 31, 2017, accretion of the debt discount was $1,844. The accretion of debt discount is presented in other expenses on the statements of operations.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
In September 2017, the Company issued an unsecured promissory note to a member of its Board of Directors in the principal face amount of $500,000. The promissory note bears interest at a rate of 10.0%, per annum, payable quarterly. The promissory note is payable in full plus all unpaid interest by September 2019. In connection with the note, the Company issued five-year warrants to purchase up to 50,000 shares of the Company’s common stock at an exercise price of $3.00 per share. The relative fair value of the debt and warrants was recorded resulting in a debt discount of $52,166 upon execution of the promissory note. For the year ended December 31, 2017, accretion of the debt discount was $8,718. The accretion of debt discount is presented in other expenses on the statements of operations.
In October 2017, the Company issued to Rio Vista Investments, LLC, a Nevada limited liability company (“Rio Vista”), (i) an unsecured promissory note in the principal face amount of $500,000 (the “Note”) and (ii) a warrant to purchase up to 50,000 shares of the Company’s common stock (the “Warrant”). The Note issued to Rio Vista bears interest at the rate of ten percent (10%) per annum, such interest being payable by the Company to Rio Vista quarterly in cash. The Note is payable in full by the Company, plus all unpaid interest, by October 26, 2019 (“Maturity Date”). Prepayment of all unpaid principal and interest may be made by the Company prior to the Maturity Date without penalty or premium. Additionally, the Company issued to Rio Vista a five-year Warrant to purchase up to 50,000 shares of the Company’s common stock at an exercise price of $3.00 per share. The relative fair value of the debt and warrants was recorded resulting in a debt discount of $50,229 upon execution of the promissory note. For the year ended December 31, 2017, accretion of the debt discount was $4,472. The accretion of debt discount is presented in other expenses on the statements of operations. A member of the Company’s board of directors is a beneficiary of certain trusts that own Rio Vista.
Royalty Agreement
In 2013, the Company entered into a royalty agreement, which was amended in 2015, with a key employee and principal stockholder of the Company and a current Director of the Company. The agreement has a term of 25 years, requires payments of royalties equal to 5% of gross sales of products derived from certain patents held or licensed by the Company, including the BAM-FX™ product, and also a minimum monthly payment of $2,500 to be offset against future royalty obligations of the Company. In addition certain other costs the Company made that were necessary for the maintenance and protection of the Company’s rights in the underlying patents were applied against future royalty obligations of the Company.
Sales subject to the royalty agreement were $65,733 and $213,367 for the years ended December 31, 2017 and 2016, respectively. As of December 31, 2017, and 2016, $319,441 and $277,038 of prepaid royalties, respectively, are available to be offset against future royalty obligations.
Consulting Agreement
In March 2015, the Company entered into a consulting agreement with a former Director. The agreement had a term of six months and required payments of $200,000 of which $200,000 was recorded as a component of general and administrative expense in the 2015 statement of operations. An obligation of $75,000 was payable to the former Director and is included in accounts payable at December 31, 2017 and 2016.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
NOTE 4 – COMMITMENTS
Lease Commitments
The Company leases its offices and building space under short term leases. These leases are renewable either monthly or annually. The Company also has a two-year lease for its warehouse in Okeechobee, which began September 1, 2016 and will expire August 31, 2018. The future minimum lease payments are $14,748 for 2017 and $9,832 for 2018. Payments included in 2016 for this lease were $4,916. Lease expense was $79,255 and $84,903 for the years ended December 31, 2017 and 2016, respectively.
Research Commitment
In January 2016, the Company entered into a Reimbursable Space Act Agreement (the “SAA”) with the National Aeronautics and Space Administration Ames Research Center (“NASA ARC”). Pursuant to the SAA, NASA ARC will evaluate the Company’s nutrient delivery system for commercial agriculture and NASA applications and the potential development of new agricultural technologies and products. The Company shall provide funding and reimbursement for the costs incurred by NASA ARC under the SAA, but shall own any resulting intellectual property created pursuant to the SAA. The Company paid NASA ARC a total of $373,750, which will serve as reimbursement for NASA ARC’s estimated expenses to carry out its first-year responsibilities pursuant to the SAA. The SAA remains in effect until the earlier of completion of all obligations contemplated in the SAA or five years from the date of agreement, For the years ended December 31, 2017 and 2016, $29,610 and $344,140, respectively, was expensed to research and development expense pursuant to the SAA.
NOTE 5 – NOTE PAYABLE
The Company has an outstanding note payable for financing corporate insurance premiums. The original principle value was $223,707, the note carries a rate of interest of 7.5% and is due in November 2018. The note calls for eleven payments of $19,353. The balance at December 31, 2017 was $204,419.
In December 2017, the Company received $200,000 from an investor, and in exchange the Company issued the investor (a) an unsecured promissory note dated December 14, 2017, maturing on December 13, 2019, in the principal face amount of $200,000, and (b) a warrant dated December 18, 2017 to purchase up to 50,000 shares of the Company’s common stock. The note bears interest at the rate of ten percent (10%) per annum, payable quarterly. The note is repayable in full by the Company, plus all unpaid interest thereon, by the maturity date (the “Maturity Date”). Prepayment of all unpaid principal and interest on the note may be made by the Company prior to the Maturity Date, provided that the holder thereof shall receive a minimum amount of interest equal to one year of interest under the note, based on the full principal amount. The warrants are exercisable within 5 years of issuance into shares of the Company’s common stock, at $3.00 per share. The relative fair value of the debt and warrants was recorded resulting in a debt discount of $18,652 upon execution of the promissory note. For the year ended December 31, 2017, accretion of the debt discount was $434. The accretion of debt discount is presented in other expenses on the statements of operations.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
NOTE 6 – EQUITY
2017 transactions:
Common Stock
Private placement offerings
During 2016, the Company commenced a private offering of up to $10,000,000 of the Company’s securities for $3.00 per share of common stock. Pursuant to the 2016 offering, the Company issued 787,133 shares of common stock during the year ended December 31, 2017. Gross proceeds from the offering were $2,361,399, with offering costs of $169,472. In connection with the offering, the Company issued fully vested, non-forfeitable warrants to purchase 50,409 shares of common stock with an exercise price of $3.00 per share to the placement agent. These warrants are netted in additional paid in capital as non-cash offering costs of the placement (approximately $46,000).
Common stock issued for services
During the year ended December 31, 2017, the Company issued for services, 100,000 shares of common stock at $3.00 per share with a value of $300,000, which was recorded as general and administrative expense.
Warrants
In March 2017, the Company and a Director entered into an amendment to a prior warrant, pursuant to which (a) the exercise price was decreased to $1.50 per share for that portion of the prior warrant to be exercised on such date and (b) the exercise of the prior warrant was to be on a cash basis. Subsequent to the execution of the amendment, the Director fully exercised the prior warrant. As a result of the prior warrant exercise, the Director received 350,000 shares of the Company's common stock in exchange for a cash payment of $525,000. As additional consideration for execution of the amendment and exercise of the prior warrant, the Company issued to the Director a warrant to purchase up to 350,000 shares of the Company's common stock at an exercise price of $4.50 per share. The estimated fair value of this warrant of approximately $402,000 was based on the following assumptions: expected dividends of 0, volatility of 148.4%, risk-free interest rate of 1.89%, and expected life of the warrants of 5 years.
In February 2016, Diamond B Capital, LLC (“Diamond B”) received from the Company a five-year warrant to purchase up to 400,000 shares of the Company’s common stock at an exercise price of $2.00 per share. A member of the Company’s Board of Directors holds membership interests representing a 12% ownership interest in Diamond B. In March 2017, the Company also entered into an amendment to the warrant, pursuant to which the exercise price was decreased to $1.50 per share for that portion of the prior warrant to be exercised by on such date. Subsequent to the execution of the prior warrant, in 2017, Diamond B fully exercised the prior warrant. As a result of the exercise, Diamond B received 400,000 shares of the Company’s common stock in exchange for a cash payment of $600,000. As additional consideration for Diamond B's entry into an amendment and exercise of the prior warrant, in March 2017, the Company issued to Diamond B a warrant to purchase up to 400,000 shares of the Company's common stock at an exercise price of $4.50 per share. The estimated fair value of this warrant of approximately $463,000 was based on the following assumptions: expected dividends of 0, volatility of 148.4%, risk-free interest rate of 1.89%, and expected life of the warrants of 5 years.
In February 2017, an employee exercised a five-year warrant to purchase 40,000 shares of common stock, which had an original exercise price of $2.00 per share, at an exercise price of $1.50 per share for a cash payment of $60,000. In addition, the employee was given a five-year warrant to purchase up to 40,000 shares of common stock with an exercise price of $4.50 per share. The estimated fair value of this warrant of approximately $46,000 was based on the following assumptions: expected dividends of 0, volatility of 148.4%, risk-free interest rate of 2.05%, and expected life of the warrants of 5 years.
The modifications to the exercise price of the warrants are considered a conversion incentive, resulting in an expense (including the expense of the new warrants) of $926,885, recorded as general and administrative expense and additional paid in capital for the year ended December 31, 2017.
Warrants issued for services
During the year ended December 31, 2017, the Company issued fully vested, non-forfeitable five-year warrants to purchase 890,000 common shares at an exercise price of $3.00 per common share to consultants for services. The value of those services was $1,019,855, which was recorded as general and administrative expense.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
Warrants issued with debt
In December 2017, the Company received $200,000 from an investor, and in exchange the Company issued the investor (a) an unsecured promissory note dated December 14, 2017, maturing on December 13, 2019, in the principal face amount of $200,000, and (b) a warrant dated December 18, 2017 to purchase up to 50,000 shares of the Company’s common stock. The warrants are exercisable within 5 years of issuance into shares of the Company’s common stock, at $3.00 per share. The relative fair value of the debt and warrants was recorded resulting in a debt discount of $18,652 upon execution of the promissory note. For the year ended December 31, 2017, accretion of the debt discount was $434. The accretion of debt discount is presented in other expenses on the statements of operations.
Warrants issued with debt – related party
In August 2017, the Company issued an unsecured promissory note to its in-house corporate council in the principal face amount of $100,000. In connection with the note, the Company issued five-year warrants to purchase up to 10,000 shares of the Company’s common stock at an exercise price of $3.00 per share. The relative fair value of the debt and warrants was recorded resulting in debt discount of $10,435 upon execution of the promissory note. For the year ended December 31, 2017, accretion of the debt discount was $1,844. The accretion of debt discount is presented in other expenses on the statements of operations.
In September 2017, the Company issued an unsecured promissory note to a member of its Board of Directors in the principal face amount of $500,000. In connection with the note, the Company issued five-year warrants to purchase up to 50,000 shares of the Company’s common stock at an exercise price of $3.00 per share. The relative fair value of the debt and warrants was recorded resulting in a debt discount of $52,166 upon execution of the promissory note. For the year ended December 31, 2017, accretion of the debt discount was $8,718. The accretion of debt discount is presented in other expenses on the statements of operations.
In October 2017, the Company issued to Rio Vista Investments, LLC, a Nevada limited liability company (“Rio Vista”), (i) an unsecured promissory note in the principal face amount of $500,000 (the “Note”) and (ii) a warrant to purchase up to 50,000 shares of the Company’s common stock (the “Warrant”). The Company issued to Rio Vista a five-year Warrant to purchase up to 50,000 shares of the Company’s common stock at an exercise price of $3.00 per share. The relative fair value of the debt and warrants was recorded resulting in a debt discount of $50,229 upon execution of the promissory note. For the year ended December 31, 2017, accretion of the debt discount was $4,472. The accretion of debt discount is presented in other expenses on the statements of operations. A member of the Company’s board of directors is a beneficiary of certain trusts that own Rio Vista.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
The following is a summary of the Company’s warrant activity for the year ended December 31, 2017:
|
Number of Warrants |
Weighted Average Exercise Price |
Weighted Average Remaining ContractualLife (in Years) |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding - January 1, 2016 | 8,117,075 | $ | 1.44 | |||||||||||||
| Granted | 1,547,658 | 2.01 | ||||||||||||||
|
Exercised |
- | - | ||||||||||||||
|
Cancelled/Forfeited |
- | - | ||||||||||||||
|
Outstanding and exercisable - December 31, 2016 |
9,664,733 | $ | 1.53 | 3.5 | $ | 14,383,083 | ||||||||||
|
Outstanding - January 1, 2017 |
9,664,733 | $ | 1.53 | |||||||||||||
|
Granted |
1,838,442 | 2.01 | ||||||||||||||
|
Exercised |
(790,000 |
) |
2.00 | |||||||||||||
|
Cancelled/Forfeited |
- | - | ||||||||||||||
|
Outstanding and exercisable - December 31, 2017 |
10,713,175 | $ | 1.86 | 2.8 | $ | 2,606,698 | ||||||||||
The aggregate intrinsic value in the table above represents the total intrinsic value (the difference between the estimated fair value of the Company’s stock price on December 31, 2017 and the exercise price, multiplied by the number of in-the-money warrants) that would have been received by the warrant holders, had all warrant holders been able to, and in fact had, exercised their warrants on December 31, 2017.
Stock incentive plan options
During November 2015, the Company adopted the Company’s 2015 Incentive Plan. ("The Plan") provides stock based compensation to employees, directors and consultants. The Company has reserved 4,000,000 shares under the Plan. The Company issued 440,000 options to employees and consultants in 2017 and 2,915,000 options to employees and consultants in 2016. The estimated fair value of options issued was $455,046 and $1,842,233 for 2017 and 2016 respectively and was based upon the following management assumptions: expected dividends of 0, volatility of 146.00-148.39%, risk free interest rates of 1.10% - 1.30%, and expected life of the options of 5 years. During the years ended December 31, 2017 and 2016, 30,000 and 160,000 of options were forfeited, respectively. The expense of options vested, recorded in general and administrative expense for the years ended December 31, 2017 and 2016, was $124,415 and $1,783,542, respectively.
The Company recognizes compensation expense for stock option grants based on the fair value at the date of grant using the Black-Scholes option pricing model. As the Company does not currently have reliably determined historic stock prices, the Company uses management estimates of stock value. The Company uses historical data, among other factors, to estimate the expected option life and the expected forfeiture rate. The Company estimates expected term considering factors such as historical exercise patterns and the recipients of the options granted. The risk-free rate is based on the United States Treasury Department yield curve in effect at the time of grant for the expected life of the option. The Company assumes an expected dividend yield of zero for all periods. For employee, consultant and director stock based compensation, the Company used management's fair value estimates of $0.63 and $1.25 per share of common stock during 2016.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
The Company has elected to account for forfeitures as they occur.
|
Number of Options |
Weighted Average Exercise Price |
Weighted (in Years) |
Aggregate Intrinsic Value |
|||||||||||||
|
Outstanding - January 1, 2016 |
- | $ | - | |||||||||||||
|
Granted |
2,915,000 | 1.25 | ||||||||||||||
|
Exercised |
- | - | ||||||||||||||
|
Cancelled/Forfeited |
160,000 | 1.25 | ||||||||||||||
|
Outstanding - December 31, 2016 |
2,755,000 | $ | 1.25 | 8.7 | $ | 192,850 | ||||||||||
|
Exercisable - December 31, 2016 |
2,500,000 | $ | 1.25 | 8.7 | $ | 175,350 | ||||||||||
|
Outstanding - January 1, 2017 |
2,755,000 | $ | 1.25 | |||||||||||||
|
Granted |
440,000 | 3.00 | ||||||||||||||
|
Exercised |
- | - | ||||||||||||||
|
Cancelled/Forfeited |
30,000 | 1.25 | ||||||||||||||
|
Outstanding - December 31, 2017 |
3,165,000 | $ | 1.49 | 8.5 | $ | 192,850 | ||||||||||
|
Exercisable - December 31, 2017 |
2,750,000 | $ | 1.27 | 8.2 | $ | 192,850 | ||||||||||
The aggregate intrinsic value in the table above represents the total intrinsic value (the difference between the estimated fair value of the Company’s stock price on December 31, 2017 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders, had all option holders been able to, and in fact had, exercised their options on December 31, 2017.
2016 transactions:
Common Stock
Private placement offerings
During the year ended December 31, 2016, pursuant to its October 2015 private offering, the Company issued 1,374,000 units at a price of $1.25 per unit. Each unit consisted of one share of the Company’s common stock and a five-year non-forfeitable and fully vested warrant to purchase an additional share of the Company’s common stock at $2.00 per share. Gross proceeds from the offering were $1,717,500 with offering costs of $151,500. In connection with the offering, the Company issued fully vested, non-forfeitable warrants to purchase 152,575 shares of common stock with an exercise price of $2.00 per share to the Company’s placement agent, this is netted in additional paid in capital as non-cash offering costs of the placement (approximately $153,000).
During 2016, the Company commenced a private offering of up to $10,000,000 of the Company’s securities for $3.00 per share of common stock. Pursuant to the 2016 offering, the Company issued 221,667 shares of common stock during the year ended December 31, 2016. Gross proceeds from the offering were $665,001, with offering costs of $53,200. In connection with the offering, the Company issued fully vested, non-forfeitable warrants to purchase 11,083 shares of common stock with an exercise price of $3.00 per share to the placement agent. These warrants are netted in additional paid in capital as non-cash offering costs of the placement (approximately $11,000).
Common stock issued for services
During the year ended December 31, 2016, the Company issued for services, 20,000 shares of common stock at $1.25 per share with a value of $25,000, which was recorded as general and administrative expense.
Warrants
Warrants issued for services
During the year ended December 31, 2016, the Company issued fully vested, non-forfeitable five-year warrants to purchase 10,000 common shares at an exercise price of $1.25 per common share to a consultant for services. The value of those services was $11,899, which was recorded as general and administrative expense.
ZERO GRAVITY SOLUTIONS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
Note 7 - Income taxes:
Income taxes at the federal statutory rate are reconciled to the Company’s actual income taxes as follows:
|
2017 |
2016 |
|||||||
|
Federal income tax benefit at 34% |
$ | (2,593,000 |
) |
$ | (2,367,000 |
) |
||
|
State income tax net of federal tax effect |
(381,000 |
) |
(348,000 |
) |
||||
|
Permanent items |
11,000 | 13,000 | ||||||
| Other | 7,000 | 4,000 | ||||||
| Tax reform | 2,790,000 | - | ||||||
|
Valuation allowance |
(166,000 | ) | 2,698,000 | |||||
| $ | — | $ | — | |||||
As of December 31, 2017, the Company has net operating loss carry forwards of approximately $17.0 million for federal and state tax purposes, which are available to offset future taxable income, if any, expiring through December 2037. A valuation allowance was recorded at December 31, 2017 due to the uncertainty of realization of deferred tax assets in the future.
The tax effects of temporary differences that give rise to significant portions of deferred tax assets and liabilities at December 31, 2017 and 2016 are as follows:
|
2017 |
2016 |
|||||||
|
Deferred tax assets (liabilities): |
||||||||
|
Net operating loss carry forwards |
$ | 4,601,000 | $ | 4,306,000 | ||||
|
Property and equipment |
(21,000 |
) |
(19,000 |
) |
||||
|
Debt discount related to warrants |
66,000 | 89,000 | ||||||
|
Patent and trademarks net of amortization |
16,000 | 21,000 | ||||||
| Bad Debt | 42,000 | - | ||||||
|
Accruals and other |
43,000 | 33,000 | ||||||
|
Stock-based compensation |
1,530,000 | 1,681,000 | ||||||
|
Deferred tax asset |
6,277,000 | 6,111,000 | ||||||
|
Valuation allowance |
(6,277,000 |
) |
(6,111,000 |
) |
||||
| $ | — | $ | — | |||||
New Tax Reform Legislation
In December 2017, the Tax Cuts and Jobs Act (the “Tax Act”) was enacted. The Tax Act makes significant change in U.S. tax law including a reduction in the corporate tax rates, changes to net operating loss carryforwards and carrybacks, and a repeal of the corporate alternative minimum tax. The Tax Act reduced the U.S. corporate tax rate from the current rate of 34% to 21%. As a result of the Tax Act, the Company was required to revalue deferred tax assets and liabilities at the enacted rate.
NOTE 8 – SUBSEQUENT EVENTS
On or about January 17, 2018, the Company received $500,000 from an accredited investor, and in exchange the Company issued to the investor (a) an unsecured promissory note dated January 16, 2018, maturing on October 26, 2019, in the principal face amount of $500,000 (the “First Note”), and (b) a warrant dated January 18, 2018 to purchase up to 50,000 shares of the Company’s common stock of (the “First Warrant”). A member of the Company’s board of directors is a beneficiary of certain trusts that own Rio Vista Investments, LLC, the holder of the First Note and First Warrant.
On or about January 18, 2018, the Company received $100,000 from an accredited investor, and in exchange the Company issued to the investor (a) an unsecured promissory note dated January 19, 2018, maturing on January 18, 2020, in the principal face amount of $100,000 (the “Second Note”), and (b) a warrant dated January 19, 2018 to purchase up to 10,000 shares of the Company’s common stock of (the “Second Warrant”).
The First Note and Second Note shall collectively be referred to as the “Notes”, and the First Warrant and Second Warrant shall collectively be referred to as the “Warrants”. The Notes and Warrants were made on substantially the same terms, except as noted.
The Notes bear interest at the rate of ten percent (10%) per annum, such interest being payable by the Company to the holders thereof quarterly in cash. The Notes are payable in full by the Company, plus all unpaid interest thereon, by their respective maturity dates (the “Maturity Date”). Prepayment of all unpaid principal and interest on each Note may be made by the Company prior to the Maturity Date, provided that the holder thereof shall receive a minimum amount of interest equal to one year of interest under such Note, based on the full principal amount. In addition to the foregoing, the Company must repay the First Note to Rio Vista Investments, LLC within 30 days of the date the Company possesses an amount of cash equal to the outstanding balance of principal and interest due under the Second Note plus an amount reasonably anticipated to be necessary to operate the Company over the succeeding 6 months. The Warrants are exercisable within 5 years of issuance into shares of the Company’s common stock, at $3.00 per share.
Notes to Consolidated Financial Statements
YEARS ENDED DECEMBER 31, 2017 AND 2016
On or about March 8, 2018, the Company received $200,000 from an accredited investor and member of our Board of Directors, and in exchange the Company issued (a) an unsecured promissory note dated March 8, 2018 (the “Third Note”), and (b) a warrant dated March 9, 2018 to purchase up to 20,000 shares of the Company’s common stock (the “Third Warrant”).
On or about March 12, 2018, the Company received $200,000 from Boies Partners, Inc. (“BPI”), an accredited investor, and in exchange the Company issued to BPI (a) an unsecured promissory note dated March 12, 2018 (the “Fourth Note”), and (b) a warrant dated March 12, 2018 to purchase up to 20,000 shares of the Company’s common stock (the “Fourth Warrant”). Alex Boies, a member of our Board of Directors, has no financial interest in or control over BPI and does not otherwise have any beneficial ownership in any securities owned by BPI.
The Third Note and Fourth Note shall collectively be referred to as the “Notes”, and the Third Warrant and Fourth Warrant shall collectively be referred to as the “Warrants”. The Notes were made on substantially the same terms, except as noted. The Warrants were made on the same terms.
The Notes bear interest at the rate of ten percent (10%) per annum, such interest being payable by the Company to the holders thereof quarterly in cash. The Notes are payable in full by the Company, plus all unpaid interest thereon, by March 7, 2020 and March 11, 2020, respectively (as per each, the “Maturity Date”). Prepayment of all unpaid principal due on each Note may be made by the Company prior to each such Note’s Maturity Date, provided that the holder thereof shall receive a minimum amount of interest equal to one year of interest due under such Note, based on such Note’s principal balance as of the origination date. The Notes contains customary provisions for events of default and acceleration of sums due.
The Fourth Note further provides that within 30 days of receipt of payment of any amount principal outstanding under the Fourth Note (the “Conversion Window”), the note holder shall have the right to convert any portion of such payment into the Company’s common stock at the lesser of $3.00 per share or the lowest price per share of any sale by the Company of its common stock occurring between the date of the Fourth Note and the end of the Conversion Window. This note was subsequently converted at $3.00 per share.
The Warrants are exercisable within 5 years of issuance into shares of the Company’s common stock, at $3.00 per share.
On May 2, 2018, the Company received $300,001 from an accredited investor and member of our Board of Directors, and in exchange the Company issued (a) an unsecured promissory note dated May 2, 2018 (the “Fifth Note”), and (b) a warrant dated May 2, 2018 to purchase up to 30,000 shares of the Company’s common stock (the “Fifth Warrant”). This Note and Warrant was made on the same terms as the "Notes".
On February 20, 2018, BAM Agricultural Solutions, Inc. (“BAM”), a wholly-owned subsidiary of the Company, and Pedro Lichtinger Waisman, Isaac Lichtinger Waisman and Victor Lichtinger Waisman (the “Lichtinger Group”) entered into a License and Business Development Agreement Mexico, effective as of February 13, 2018 (the “Agreement”). Following consummation of the Agreement, the Lichtinger Group formed a company in Mexico, Agro Space Tech SA de CV de RV (“Agro Space”), to which BAM will receive a twenty percent (20%) equity ownership interest. The Lichtinger Group will contribute up to USD $500,000 in capital into Agro Space, with BAM having no initial capital requirement. If Agro Space requires more than the initial USD $500,000 capital contribution, BAM will have a right to contribute capital pro rata and maintain its ownership percentage.
The Agreement provides Agro Space with the exclusive right to import, package, sell and distribute BAM’s product lines and promote its brand, as well as other “white label” brands as they are introduced, in the Mexican markets, and a limited manufacturing right to modify the presentation of BAM’s products and dilute such products, all during the term of the Agreement. BAM will retain the right to all of its intellectual property, including any materials produced in connection with the Agreement and BAM’s products, and Agro Space will have a royalty-free right to reproduce, translate, summarize or otherwise use such materials for the sole purpose of performing pursuant to the Agreement. The exclusivity of the rights licensed to Agro Space are conditioned upon (i) meeting specified revenue targets beginning on the third anniversary following the successful completion of specified local trials through the tenth anniversary thereof; and (ii) an expansion of the business to target and add a specified number of crops during each of the five (5) years following the successful completion of specified local trials.
In addition to BAM receiving an ownership interest in Agro Space, the Company received $100,000 following execution of the Agreement, to purchase shares of the Company in connection with the Company’s ongoing private placement of shares of its common stock for USD $3.00 per share. The Agreement further provides that Agro Space will pay to BAM up to USD $900,000, in the aggregate, upon reaching specified milestones based on completing government/academic trials and revenue hurdles.
On June 29, 2018, the Company received $1M from an affiliate of a board member pursuant to a secured note containing 10% interest and warrant coverage provisions. A second member of the board provided additional funding of $250,000 on July 2, 2018 with an additional $250,000 on July 17. In addition, the Company received $550,000 for a secured note from seven other current shareholders. These notes include warrants to purchase 2,050,000 warrants exercisable within 5 years of issuance into shares of the Company's stock at $1 per share.
Subsequent to December 31, 2017, the Company issued fully vested, non-forfeitable warrants to purchase 165,000 common shares at an exercise price of $3.00 per common share to consultants for services.
Subsequent to December 31, 2017, the Company issued 237,051 shares of common stock pursuant to the August 15, 2016 offering. Proceeds from the offering were $711,154 with offering costs of $25,680, and warrants issued to purchase 7,000 shares of common stock.
F-21
