Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - REGENXBIO Inc. | rgnx-ex991_15.htm |
| 8-K - 8-K - REGENXBIO Inc. | rgnx-8k_20180808.htm |

August 8, 2018 REGENXBIO Reports Second Quarter 2018 Financial and Operating Results and Interim Data from Ongoing Clinical Trials for Wet AMD and HoFH EXHIBIT 99.2

Forward-looking statements This presentation includes “forward-looking statements,” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements express a belief, expectation or intention and are generally accompanied by words that convey projected future events or outcomes such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “design,” “intend,” “expect,” “could,” “plan,” “potential,” “predict,” “seek,” “should,” “would” or by variations of such words or by similar expressions. The forward-looking statements include statements relating to, among other things, REGENXBIO’s future operations, clinical trials, costs and cash flow. REGENXBIO has based these forward-looking statements on its current expectations and assumptions and analyses made by REGENXBIO in light of its experience and its perception of historical trends, current conditions and expected future developments, as well as other factors REGENXBIO believes are appropriate under the circumstances. However, whether actual results and developments will conform with REGENXBIO’s expectations and predictions is subject to a number of risks and uncertainties, including the timing of enrollment, commencement and completion and the success of clinical trials conducted by REGENXBIO, its licensees and its partners, the timing of commencement and completion and the success of preclinical studies conducted by REGENXBIO and its development partners, the timely development and launch of new products, the ability to obtain and maintain regulatory approval of product candidates, the ability to obtain and maintain intellectual property protection for product candidates and technology, trends and challenges in the business and markets in which REGENXBIO operates, the size and growth of potential markets for product candidates and the ability to serve those markets, the rate and degree of acceptance of product candidates, and other factors, many of which are beyond the control of REGENXBIO. Refer to the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of REGENXBIO’s Annual Report on Form 10-K for the year ended December 31, 2017 and comparable “risk factors” sections of REGENXBIO’s Quarterly Reports on Form 10-Q and other filings, which have been filed with the U.S. Securities and Exchange Commission (SEC) and are available on the SEC’s website at www.sec.gov. All of the forward-looking statements made in this presentation are expressly qualified by the cautionary statements contained or referred to herein. The actual results or developments anticipated may not be realized or, even if substantially realized, they may not have the expected consequences to or effects on REGENXBIO or its businesses or operations. Such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements. Readers are cautioned not to rely too heavily on the forward-looking statements contained in this presentation. These forward-looking statements speak only as of the date of this presentation. REGENXBIO does not undertake any obligation, and specifically declines any obligation, to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.

REGENXBIO second quarter 2018 earnings call agenda Agenda Opening RGX–314 for the treatment of wet AMD – Phase I interim results RGX–501 for treatment of HoFH – Phase I/II interim results Other recent operational highlights Financial results and guidance Closing
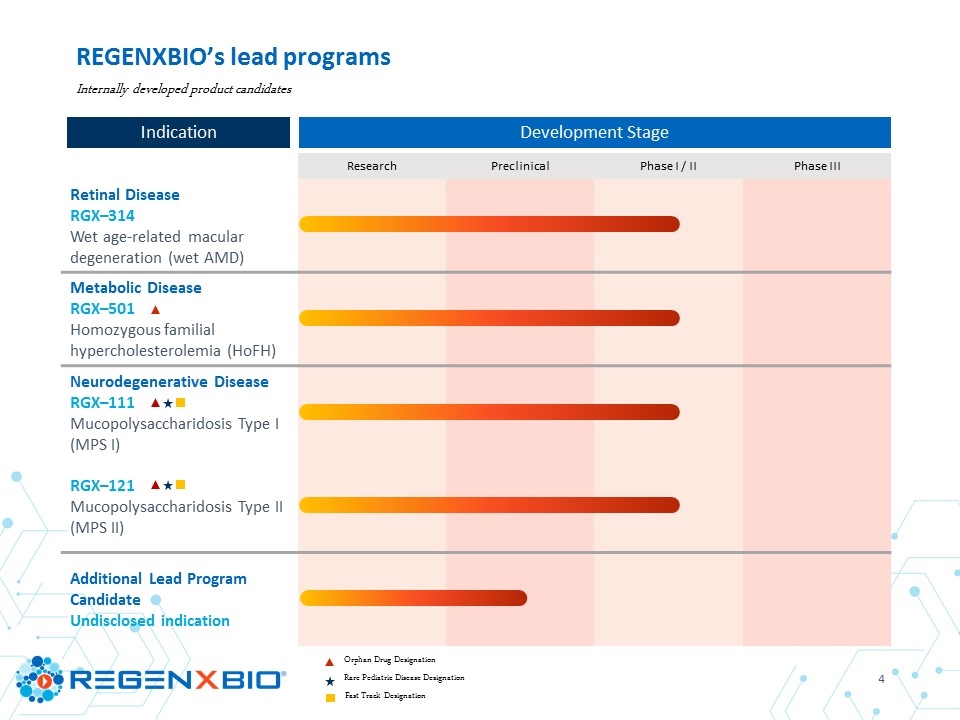
Research Preclinical Phase I / II Phase III Retinal Disease RGX–314 Wet age-related macular degeneration (wet AMD) Metabolic Disease RGX–501 Homozygous familial hypercholesterolemia (HoFH) Neurodegenerative Disease RGX–111 Mucopolysaccharidosis Type I (MPS I) RGX–121 Mucopolysaccharidosis Type II (MPS II) Additional Lead Program Candidate Undisclosed indication REGENXBIO’s lead programs Orphan Drug Designation Rare Pediatric Disease Designation Fast Track Designation Internally developed product candidates Development Stage Indication

Our Heroes

RGX–314 Phase I clinical trial interim results Summary Well–tolerated at all doses Dose–dependent protein expression levels + Reduction in anti–VEGF injections + maintained retinal thickness + maintained to improved vision Cohort 3 (6x1010 GC/eye dose) 50% free of anti–VEGF injections through six months Dose supports Company's plans to proceed to Phase II clinical trial as soon as possible Current clinical trial expanded to include new Cohort 4 dose and the first subject has been dosed Additional data expected to be presented at AAO in October 2018

THE DISEASE Blurring of central vision and progressive vision loss due to formation of leaky blood vessels in the eye VEGF inhibitors are standard of care to treat fluid and associated vision loss Frequency / uncomfortable administration of current anti-VEGF therapies affects compliance and ultimately efficacy >2 million patients estimated in U.S., Europe and Japan Vector: AAV8 Gene: anti-VEGF fab Mechanism of action Reducing leaky blood vessel formation by giving ocular cells the ability to produce an anti-VEGF fab RGX–314 for treatment of wet age-related macular degeneration (wet AMD) RGX–314 PRODUCT CANDIDATE Route of administration Subretinal
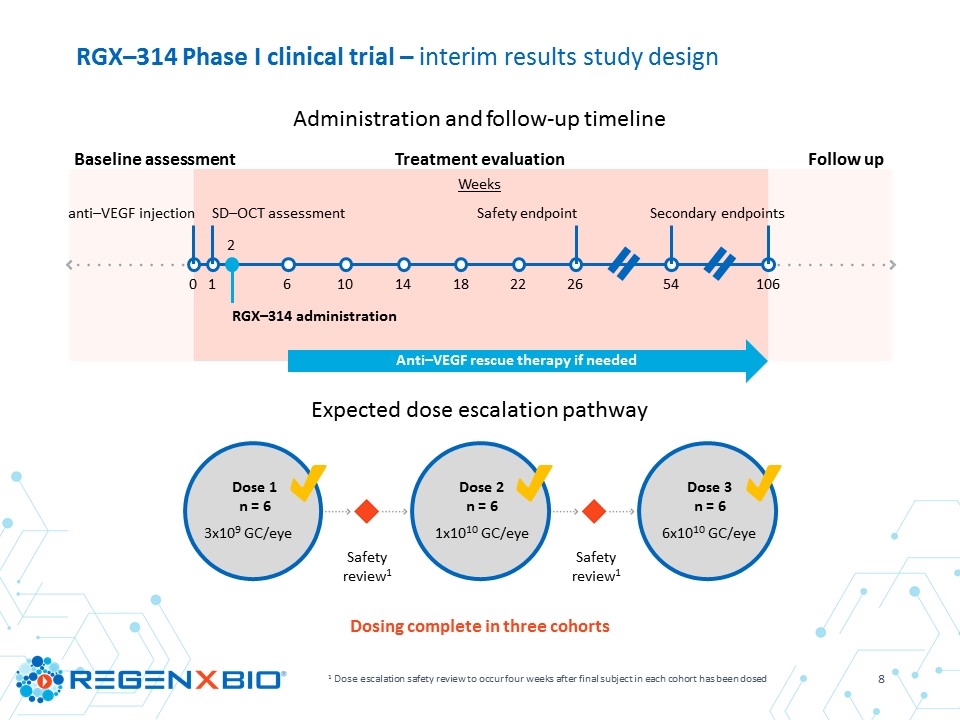
RGX–314 Phase I clinical trial – interim results study design 1 Dose escalation safety review to occur four weeks after final subject in each cohort has been dosed Dosing complete in three cohorts Administration and follow-up timeline Expected dose escalation pathway Dose 1 n = 6 3x109 GC/eye Dose 2 n = 6 1x1010 GC/eye Dose 3 n = 6 6x1010 GC/eye Safety review1 Safety review1 Baseline assessment Treatment evaluation Follow up Weeks 0 1 2 6 10 18 14 22 26 54 106 anti–VEGF injection SD–OCT assessment RGX–314 administration Safety endpoint Secondary endpoints Anti–VEGF rescue therapy if needed

Objectives Primary To determine the safety and tolerability of RGX-314 in subjects with wet AMD though six months Secondary Expression of RGX–314 protein in the eye Effect of RGX–314 on best corrected visual acuity (BCVA) and central retinal thickness (CRT) as measured by Spectral Domain Optical Coherence Tomography (SD–OCT) Additional anti–VEGF injections post–RGX–314 RGX–314 Phase I clinical trial – interim results study overview Key inclusion criteria Male or female ≥ 50 to 89 years of age Wet AMD subjects requiring frequent anti–VEGF therapy, with a documented history of response Documented response to anti–VEGF at trial entry (assessed by SD–OCT at week 1) Vision of 20/63 to 20/400 for the initial subject, then 20/40 to 20/400 for the rest of each cohort Pseudophakic (status post cataract surgery) Subjects: 18 subjects dosed Sites: Seven leading retinal surgery centers across the United States

RGX–314 Phase I clinical trial – safety summary RGX–314 was well–tolerated No drug-related AEs or drug-related SAEs Most AEs were assessed as mild (Grade 1 – 83%) No observed immune responses, drug-related ocular inflammation, or any post-surgical inflammation beyond what is expected following routine vitrectomy Five SAEs that were not drug-related were reported in three subjects
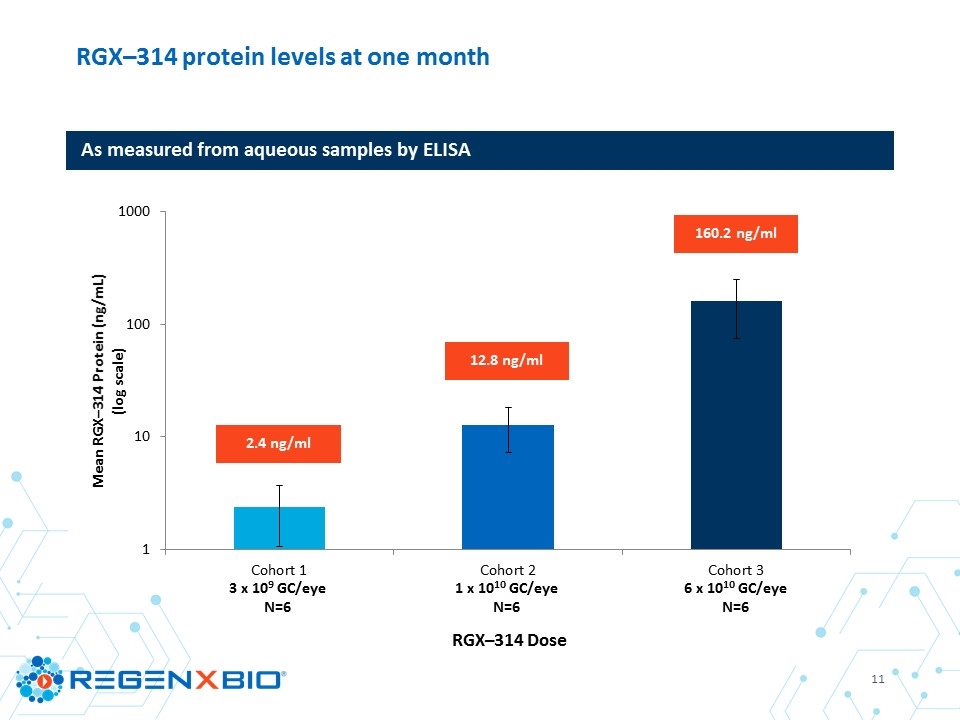
RGX–314 protein levels at one month As measured from aqueous samples by ELISA 12.8 ng/ml 160.2 ng/ml 2.4 ng/ml 3 x 109 GC/eye N=6 1 x 1010 GC/eye N=6 6 x 1010 GC/eye N=6
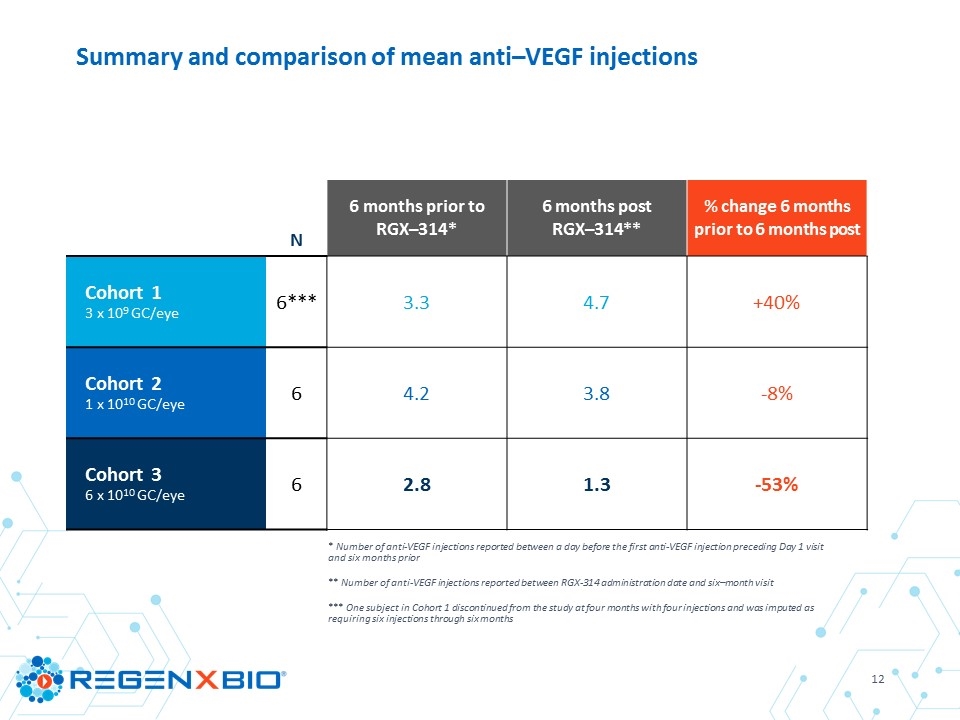
* Number of anti-VEGF injections reported between a day before the first anti-VEGF injection preceding Day 1 visit and six months prior ** Number of anti-VEGF injections reported between RGX-314 administration date and six–month visit *** One subject in Cohort 1 discontinued from the study at four months with four injections and was imputed as requiring six injections through six months Summary and comparison of mean anti–VEGF injections N 6 months prior to RGX–314* 6 months post RGX–314** % change 6 months prior to 6 months post Cohort 1 3 x 109 GC/eye 6*** 3.3 4.7 +40% Cohort 2 1 x 1010 GC/eye 6 4.2 3.8 -8% Cohort 3 6 x 1010 GC/eye 6 2.8 1.3 -53%
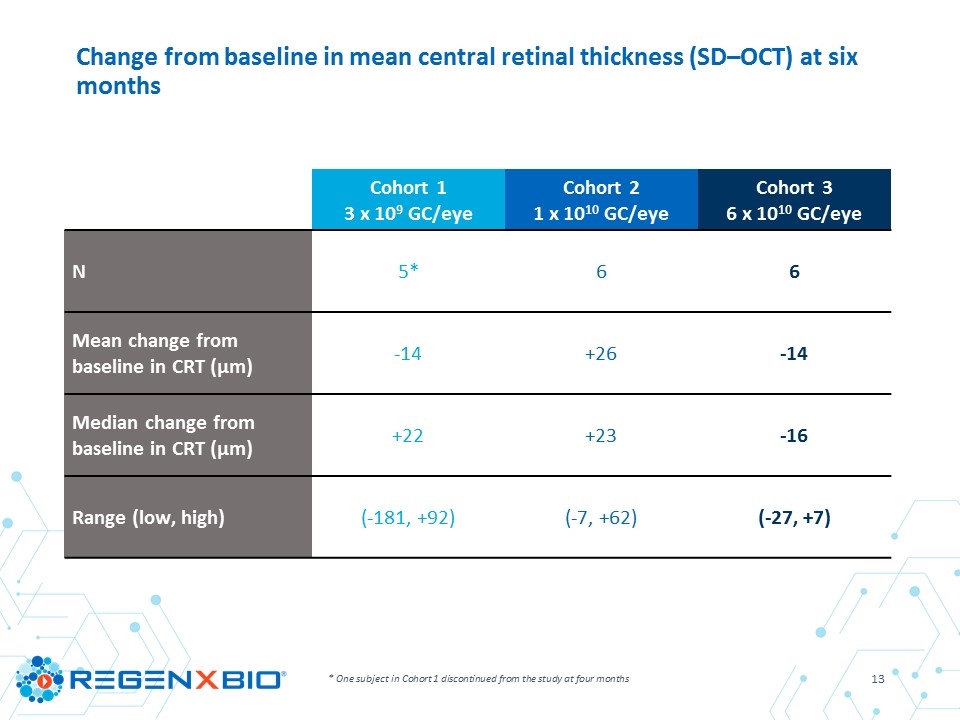
Cohort 1 3 x 109 GC/eye Cohort 2 1 x 1010 GC/eye Cohort 3 6 x 1010 GC/eye N 5* 6 6 Mean change from baseline in CRT (µm) -14 +26 -14 Median change from baseline in CRT (µm) +22 +23 -16 Range (low, high) (-181, +92) (-7, +62) (-27, +7) * One subject in Cohort 1 discontinued from the study at four months Change from baseline in mean central retinal thickness (SD–OCT) at six months
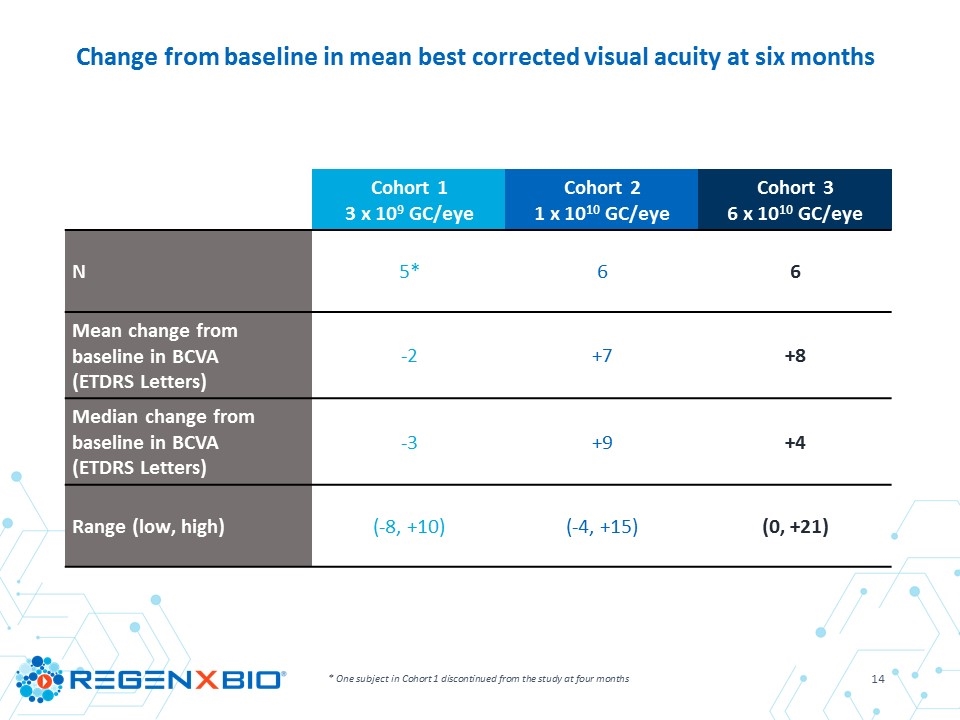
* One subject in Cohort 1 discontinued from the study at four months Change from baseline in mean best corrected visual acuity at six months Cohort 1 3 x 109 GC/eye Cohort 2 1 x 1010 GC/eye Cohort 3 6 x 1010 GC/eye N 5* 6 6 Mean change from baseline in BCVA (ETDRS Letters) -2 +7 +8 Median change from baseline in BCVA (ETDRS Letters) -3 +9 +4 Range (low, high) (-8, +10) (-4, +15) (0, +21)
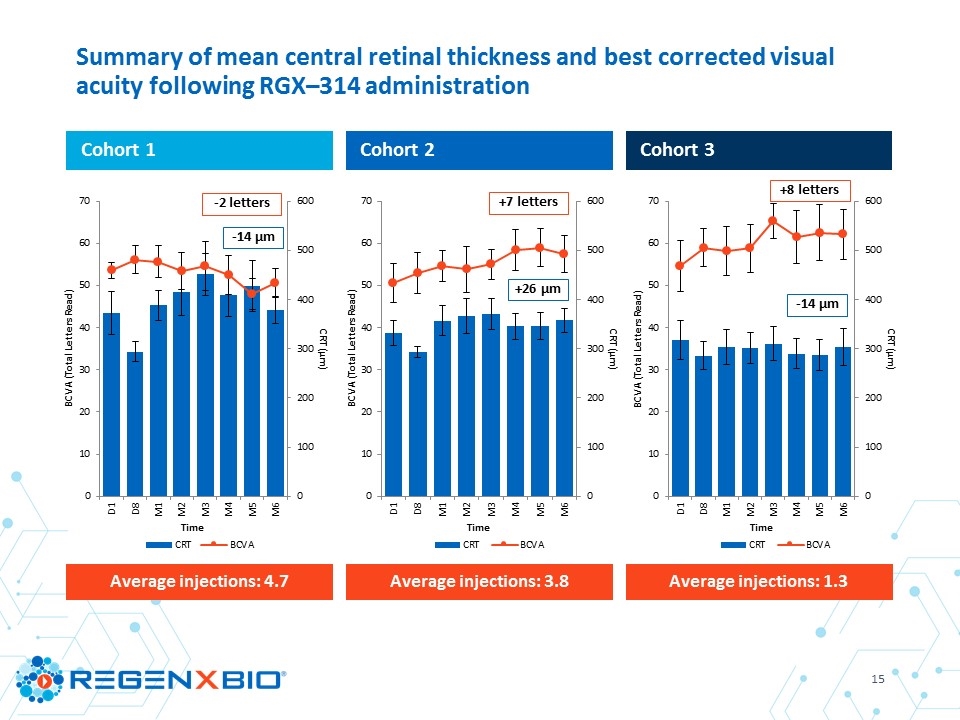
Cohort 1 Cohort 2 Cohort 3 Summary of mean central retinal thickness and best corrected visual acuity following RGX–314 administration Average injections: 4.7 Average injections: 3.8 Average injections: 1.3 +8 letters -14 μm -14 μm -2 letters +7 letters +26 μm
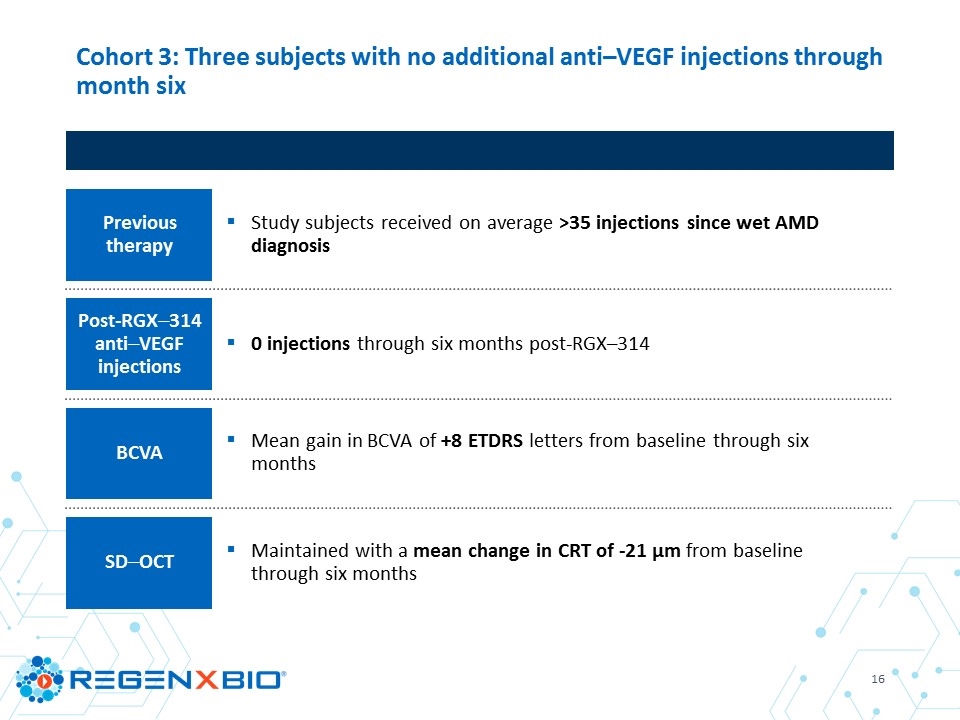
Cohort 3: Three subjects with no additional anti–VEGF injections through month six Previous therapy Study subjects received on average >35 injections since wet AMD diagnosis Post-RGX–314 anti–VEGF injections 0 injections through six months post-RGX–314 BCVA Mean gain in BCVA of +8 ETDRS letters from baseline through six months SD–OCT Maintained with a mean change in CRT of -21 µm from baseline through six months
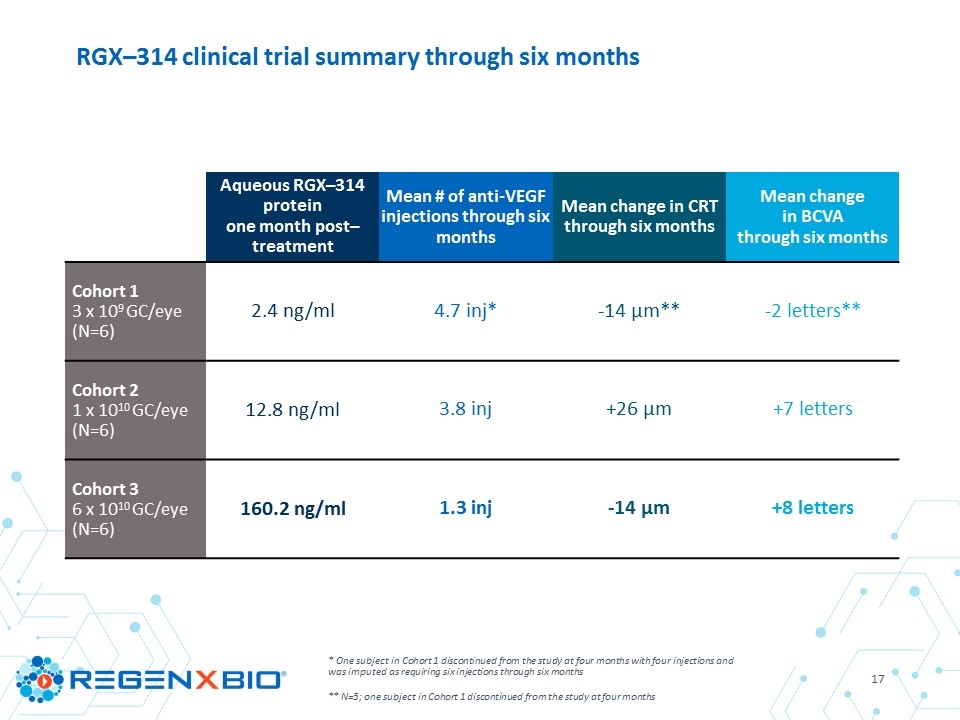
Aqueous RGX–314 protein one month post–treatment Mean # of anti-VEGF injections through six months Mean change in CRT through six months Mean change in BCVA through six months Cohort 1 3 x 109 GC/eye (N=6) 2.4 ng/ml 4.7 inj* -14 µm** ‑2 letters** Cohort 2 1 x 1010 GC/eye (N=6) 12.8 ng/ml 3.8 inj +26 µm +7 letters Cohort 3 6 x 1010 GC/eye (N=6) 160.2 ng/ml 1.3 inj -14 µm +8 letters * One subject in Cohort 1 discontinued from the study at four months with four injections and was imputed as requiring six injections through six months ** N=5; one subject in Cohort 1 discontinued from the study at four months RGX–314 clinical trial summary through six months
Summary of Cohort 3 and Cohort 4 expansion Cohort 3 Positive signals from clinical measures at six months indicate that this may be a clinically meaningful dose which we expect to take into a Phase II trial for wet AMD Mean RGX–314 protein levels >100 ng/ml were observed post–treatment Subjects exhibited signs of biologic activity: maintenance of CRT by SD-OCT, maintenance to improvement in BCVA and majority with minimal to no additional anti–VEGF therapy Cohort 4 To evaluate the potential benefits of a higher dose, we have initiated Cohort 4 (1.6 x 1011 GC/eye) Based on an amendment filed with the FDA, we were cleared to proceed with initiation of dosing The first subject was dosed this week
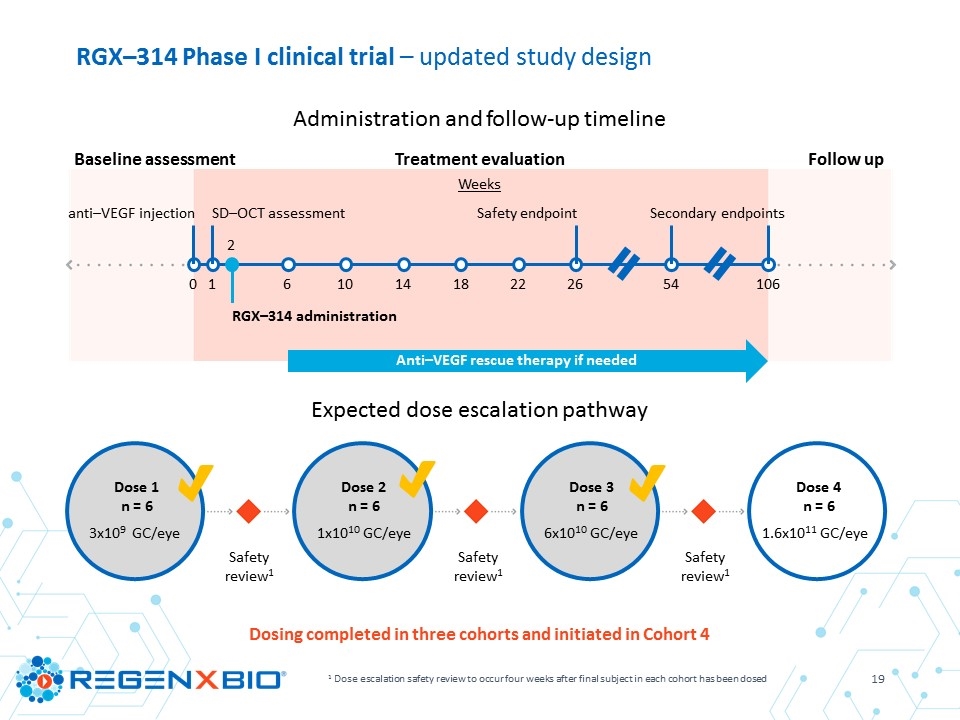
RGX–314 Phase I clinical trial – updated study design 1 Dose escalation safety review to occur four weeks after final subject in each cohort has been dosed Dosing completed in three cohorts and initiated in Cohort 4 Administration and follow-up timeline Expected dose escalation pathway Dose 1 n = 6 3x109 GC/eye Dose 2 n = 6 1x1010 GC/eye Dose 3 n = 6 6x1010 GC/eye Safety review1 Dose 4 n = 6 1.6x1011 GC/eye Safety review1 Safety review1 Baseline assessment Treatment evaluation Follow up Weeks 0 1 2 6 10 18 14 22 26 54 106 anti–VEGF injection SD–OCT assessment RGX–314 administration Safety endpoint Secondary endpoints Anti–VEGF rescue therapy if needed

THE DISEASE Defective LDLR gene leads to limited / no LDL cholesterol receptors, allowing LDL to build up in bloodstream Total cholesterol levels >500 mg/dL Coronary artery disease at young age Even with existing standard of care therapies, the mean age of death is 32 years Approx. 11,000 patients worldwide RGX–501 for treatment of homozygous familial hypercholesterolemia (HoFH) RGX–501 PRODUCT CANDIDATE Vector: AAV8 Gene: LDLR Mechanism of action Correction of defective LDLR, reducing circulating LDL cholesterol Route of administration Intravenous Special Regulatory Status Orphan Drug Designation
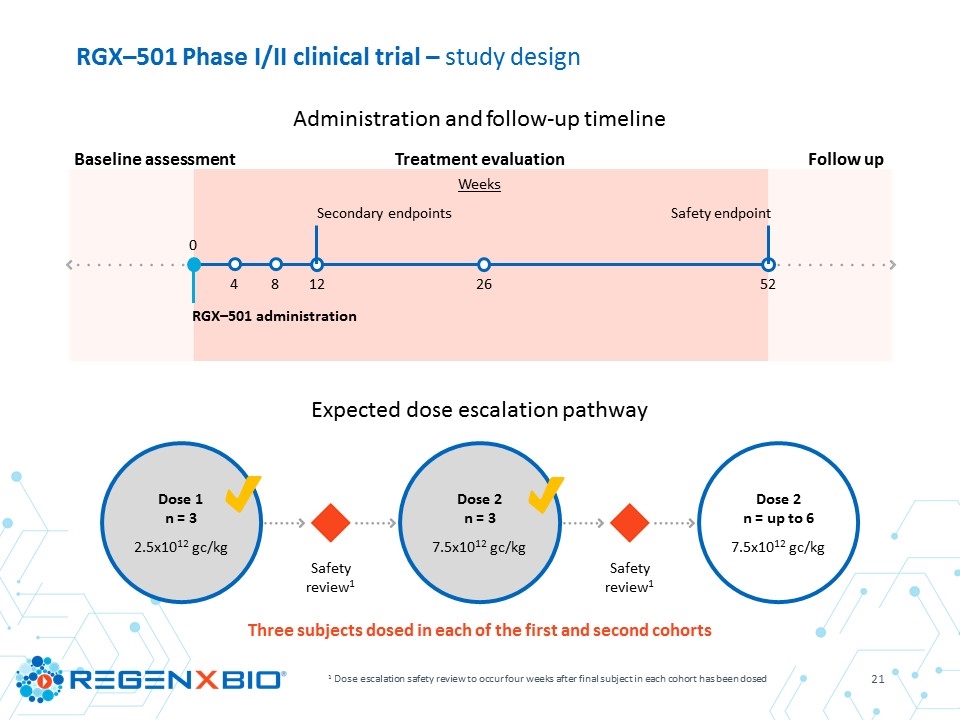
RGX–501 Phase I/II clinical trial – study design 1 Dose escalation safety review to occur four weeks after final subject in each cohort has been dosed Three subjects dosed in each of the first and second cohorts Dose 1 n = 3 Dose 2 n = 3 Dose 2 n = up to 6 7.5x1012 gc/kg 7.5x1012 gc/kg 2.5x1012 gc/kg Safety review1 Safety review1 Administration and follow-up timeline Expected dose escalation pathway Baseline assessment Treatment evaluation Follow up Weeks 0 4 8 26 52 RGX–501 administration Safety endpoint 12 Secondary endpoints

RGX–501 Phase I/II clinical trial interim results Summary Transaminase elevations observed in Cohort 2 Administration of steroid appears to mitigate transaminase elevations and related effects Clinical trial protocol expected to be amended to enroll additional subjects using steroid prophylaxis Additional data expected to be presented in oral presentation in the fourth quarter of 2018
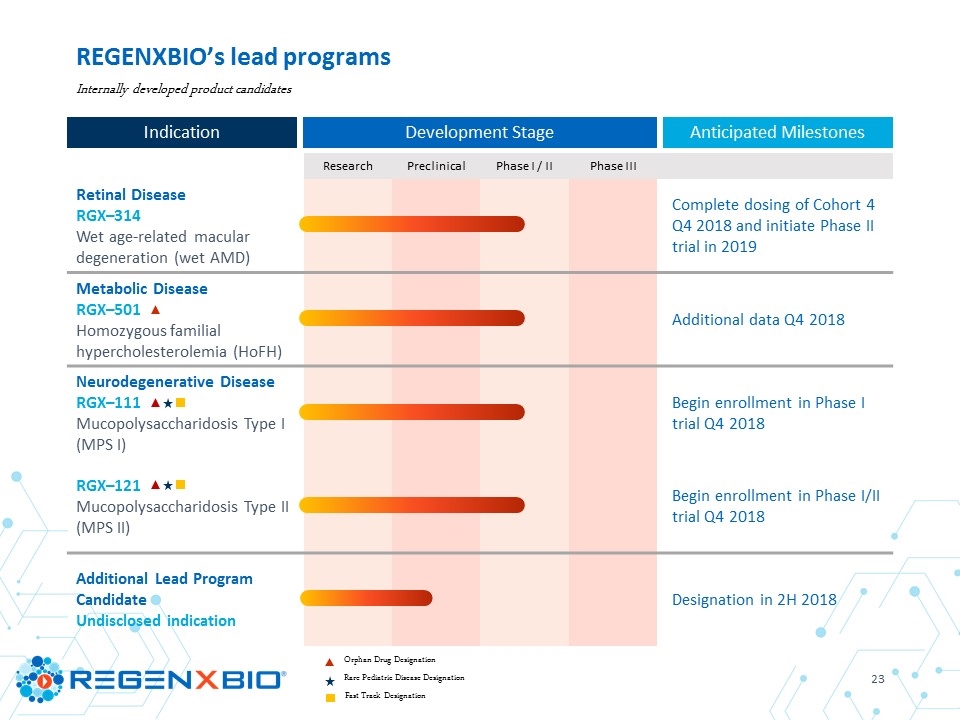
Research Preclinical Phase I / II Phase III Retinal Disease RGX–314 Wet age-related macular degeneration (wet AMD) Complete dosing of Cohort 4 Q4 2018 and initiate Phase II trial in 2019 Metabolic Disease RGX–501 Homozygous familial hypercholesterolemia (HoFH) Additional data Q4 2018 Neurodegenerative Disease RGX–111 Mucopolysaccharidosis Type I (MPS I) Begin enrollment in Phase I trial Q4 2018 RGX–121 Mucopolysaccharidosis Type II (MPS II) Begin enrollment in Phase I/II trial Q4 2018 Additional Lead Program Candidate Undisclosed indication Designation in 2H 2018 REGENXBIO’s lead programs Orphan Drug Designation Rare Pediatric Disease Designation Fast Track Designation Internally developed product candidates Development Stage Indication Anticipated Milestones
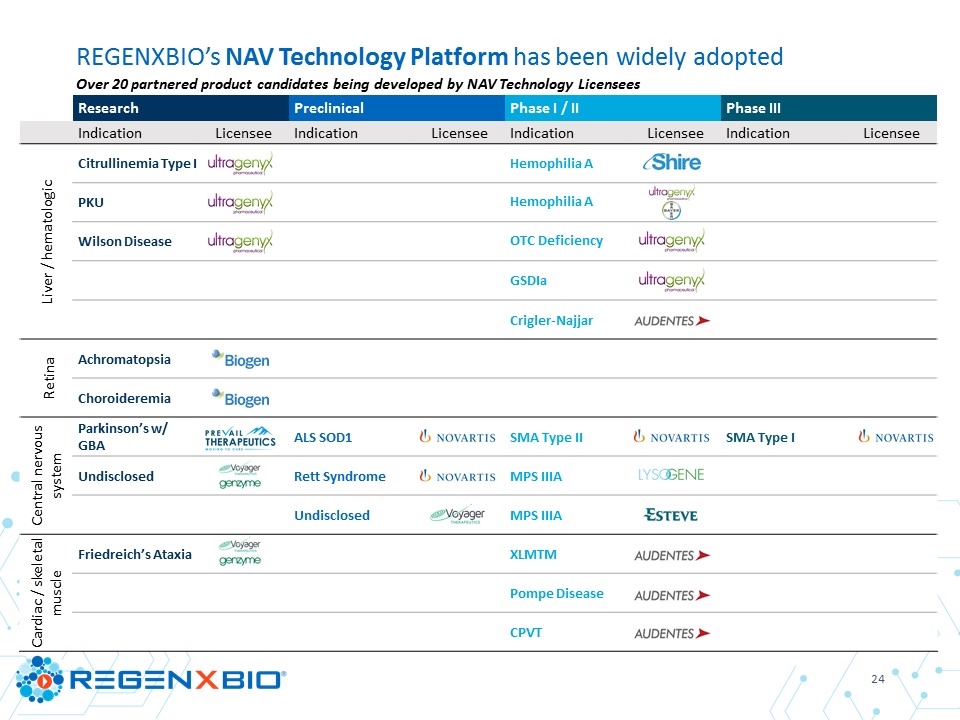
REGENXBIO’s NAV Technology Platform has been widely adopted Over 20 partnered product candidates being developed by NAV Technology Licensees Research Preclinical Phase I / II Phase III Indication Licensee Indication Licensee Indication Licensee Indication Licensee Liver / hematologic Citrullinemia Type I Hemophilia A PKU Hemophilia A Wilson Disease OTC Deficiency GSDIa Crigler-Najjar Retina Achromatopsia Choroideremia Central nervous system Parkinson’s w/ GBA ALS SOD1 SMA Type II SMA Type I Undisclosed Rett Syndrome MPS IIIA Undisclosed MPS IIIA Cardiac / skeletal muscle Friedreich’s Ataxia XLMTM Pompe Disease CPVT
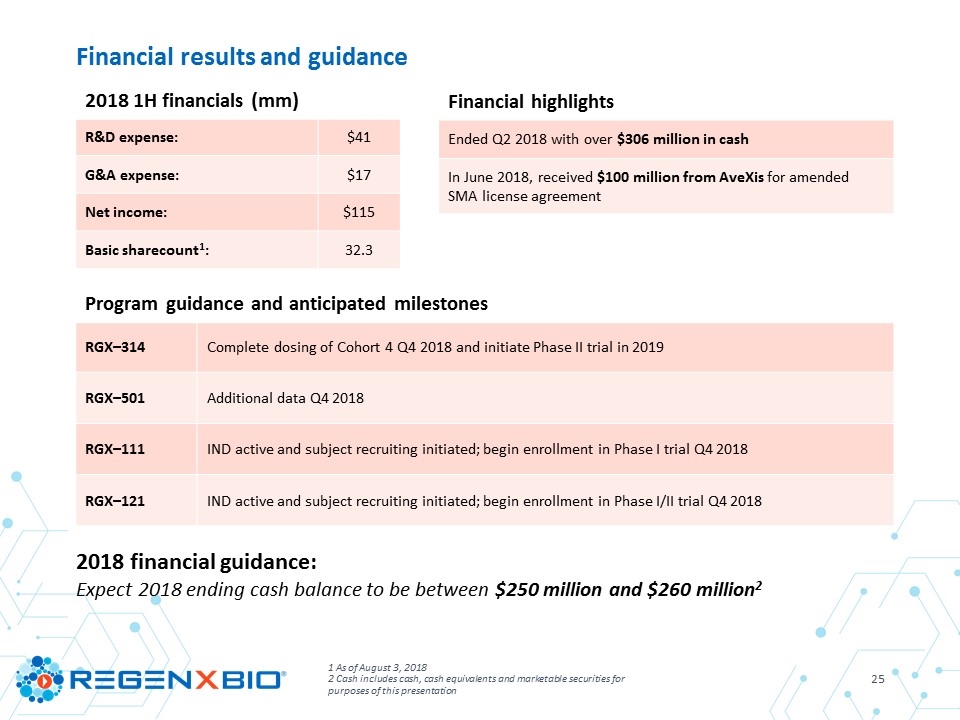
1 As of August 3, 2018 2 Cash includes cash, cash equivalents and marketable securities for purposes of this presentation Financial results and guidance 2018 financial guidance: Expect 2018 ending cash balance to be between $250 million and $260 million2 2018 1H financials (mm) R&D expense: $41 G&A expense: $17 Net income: $115 Basic sharecount1: 32.3 Financial highlights Ended Q2 2018 with over $306 million in cash In June 2018, received $100 million from AveXis for amended SMA license agreement Program guidance and anticipated milestones RGX–314 Complete dosing of Cohort 4 Q4 2018 and initiate Phase II trial in 2019 RGX–501 Additional data Q4 2018 RGX–111 IND active and subject recruiting initiated; begin enrollment in Phase I trial Q4 2018 RGX–121 IND active and subject recruiting initiated; begin enrollment in Phase I/II trial Q4 2018

Closing and Q&A
