Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Protagonist Therapeutics, Inc | a18-18323_48k.htm |
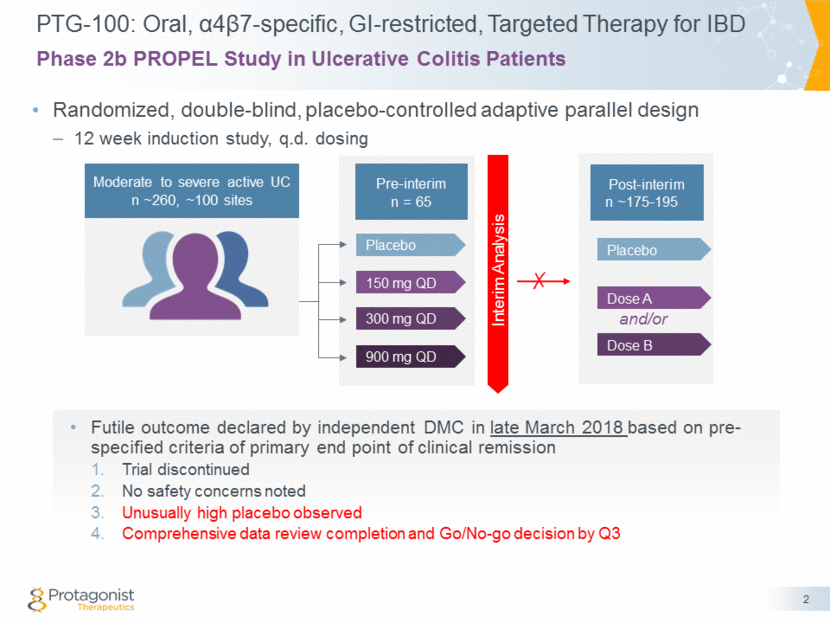
PTG-100: Oral, α4β7-specific, GI-restricted, Targeted Therapy for IBD Randomized, double-blind, placebo-controlled adaptive parallel design 12 week induction study, q.d. dosing 2 Phase 2b PROPEL Study in Ulcerative Colitis Patients 300 mg QD Placebo 900 mg QD 150 mg QD Dose B Placebo Dose A Moderate to severe active UC n ~260, ~100 sites Pre-interim n = 65 Interim Analysis and/or Futile outcome declared by independent DMC in late March 2018 based on pre-specified criteria of primary end point of clinical remission Trial discontinued No safety concerns noted Unusually high placebo observed Comprehensive data review completion and Go/No-go decision by Q3 Post-interim n ~175-195 ✗
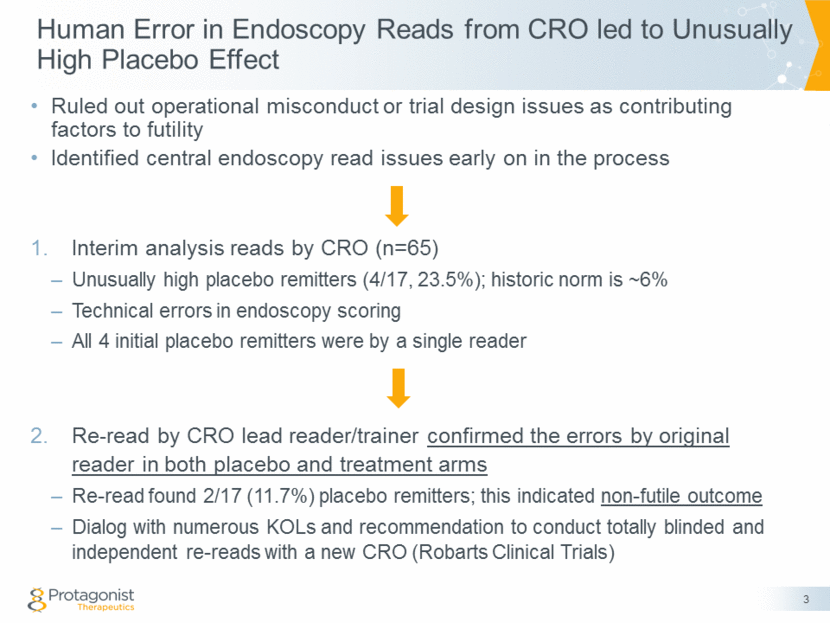
Human Error in Endoscopy Reads from CRO led to Unusually High Placebo Effect 3 Ruled out operational misconduct or trial design issues as contributing factors to futility Identified central endoscopy read issues early on in the process Interim analysis reads by CRO (n=65) Unusually high placebo remitters (4/17, 23.5%); historic norm is ~6% Technical errors in endoscopy scoring All 4 initial placebo remitters were by a single reader Re-read by CRO lead reader/trainer confirmed the errors by original reader in both placebo and treatment arms Re-read found 2/17 (11.7%) placebo remitters; this indicated non-futile outcome Dialog with numerous KOLs and recommendation to conduct totally blinded and independent re-reads with a new CRO (Robarts Clinical Trials)
PTG-100 has Signals of Clinical Efficacy and Merits further Development 4 Blinded re-read of full dataset (n=83) by independent CRO (Robarts) Placebo in sync with historic norms (1/21, 4.8%) Clinical efficacy comparable to other competitive IBD drugs (9-16%) Histopathology and PD biomarker analysis Histopath correlations with endoscopy responses and clinical remissions PD biomarkers illustrate dose dependent target engagement PTG-100 merits further clinical development in UC
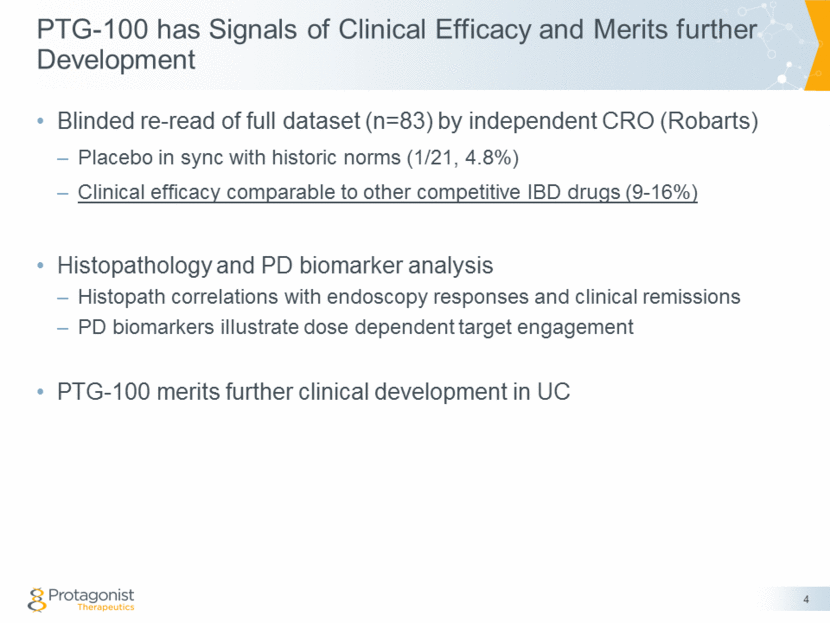
Re-analysis based on Robarts Endoscopy Re-Reads (n = 83) 5 Dataset Summary Placebo rates decrease to ‘historical norms’ 900 mg q.d. shows signals of clinical efficacy Similar to historical norms of efficacy for IBD drugs (next slide) Blinded endoscopy re-reads on complete data set by Robarts Criteria Placebo 150 mg 300 mg 900 mg # Patients 21 22 21 19 Clinical Remission 1 (4.8%) 2 (9.1%) 2 (9.5%) 3 (15.8%) Endoscopy Response 1 (4.8%) 2 (9.1%) 3 (14.3%) 3 (15.8%)
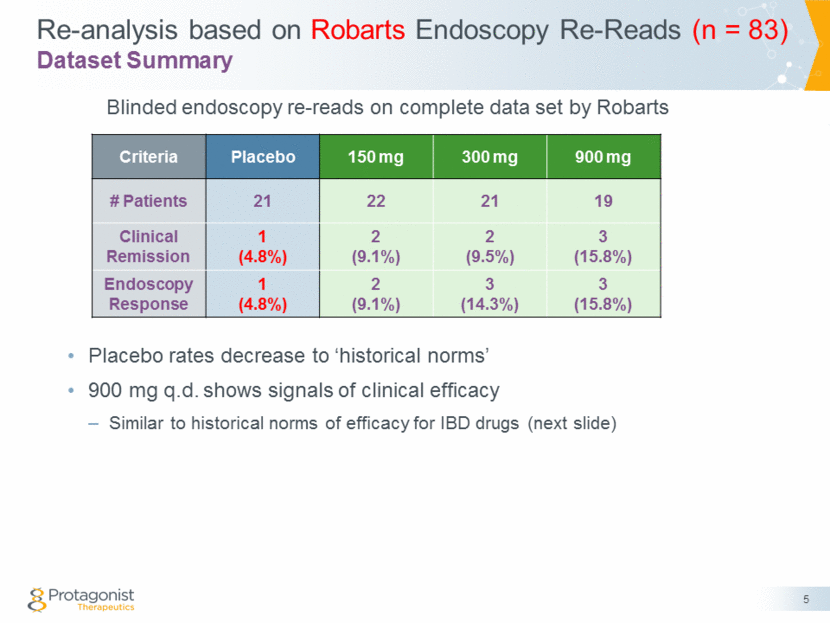
PTG-100 Efficacy Similar to Other IBD Targeted Therapy Drugs 6 *Anti-MadCam mAb No central read endoscopy (Entyvio) a4b7 integrin Oral/S1P1 Oral/JAK N= 905 234 132 65 81 43 284 73 225 149 19 21 Injectable Oral Injectable a4b7/aEb7 Injectable MAdCAM 900 mg qd
PTG-100 Phase 2 PROPEL Study A human error in endoscopy reads led to a futile outcome PTG-100 shows signals of clinical efficacy in UC patients Robarts endoscopy re-reads Histopath and PD data Current data warrants further clinical development of PTG-100 7 Final Interim Analysis Conclusions
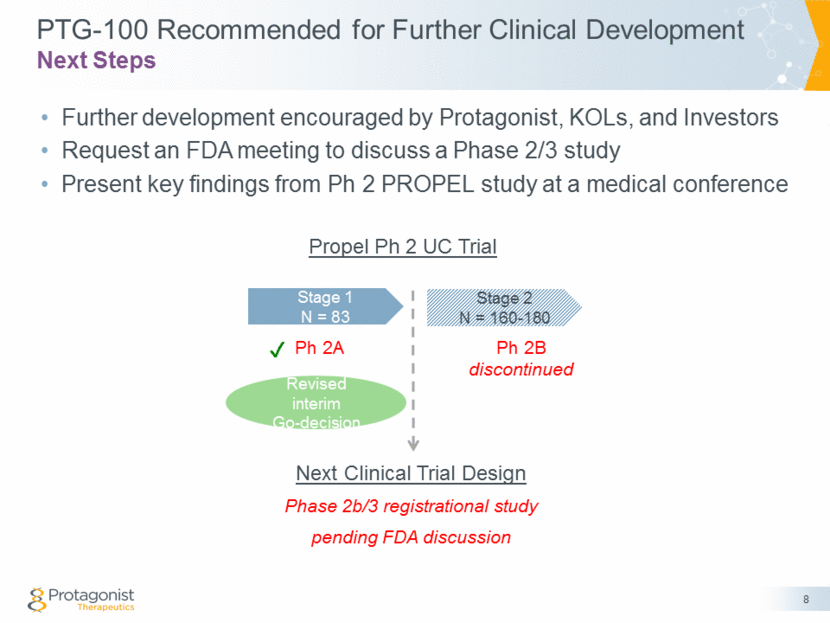
PTG-100 Recommended for Further Clinical Development Further development encouraged by Protagonist, KOLs, and Investors Request an FDA meeting to discuss a Phase 2/3 study Present key findings from Ph 2 PROPEL study at a medical conference 8 Next Steps Stage 1 N = 83 Stage 2 N = 160-180 Ph 2A Ph 2B discontinued Revised interim Go-decision ✔ Next Clinical Trial Design Phase 2b/3 registrational study pending FDA discussion Propel Ph 2 UC Trial
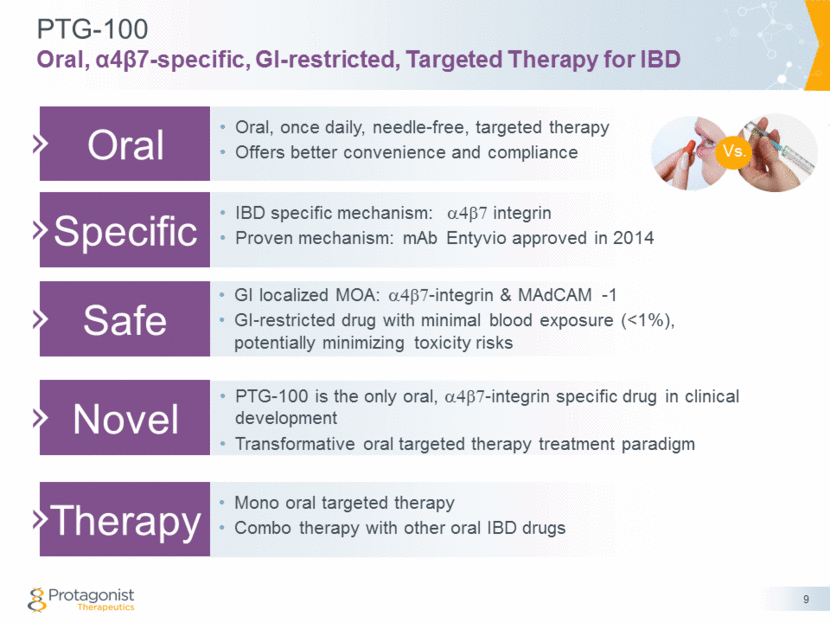
PTG-100 Oral, α4β7-specific, GI-restricted, Targeted Therapy for IBD 9 Oral Oral, once daily, needle-free, targeted therapy Offers better convenience and compliance Specific Safe Novel Therapy IBD specific mechanism: a4b7 integrin Proven mechanism: mAb Entyvio approved in 2014 GI localized MOA: a4b7-integrin & MAdCAM -1 GI-restricted drug with minimal blood exposure (<1%), potentially minimizing toxicity risks PTG-100 is the only oral, a4b7-integrin specific drug in clinical development Transformative oral targeted therapy treatment paradigm Mono oral targeted therapy Combo therapy with other oral IBD drugs Vs.
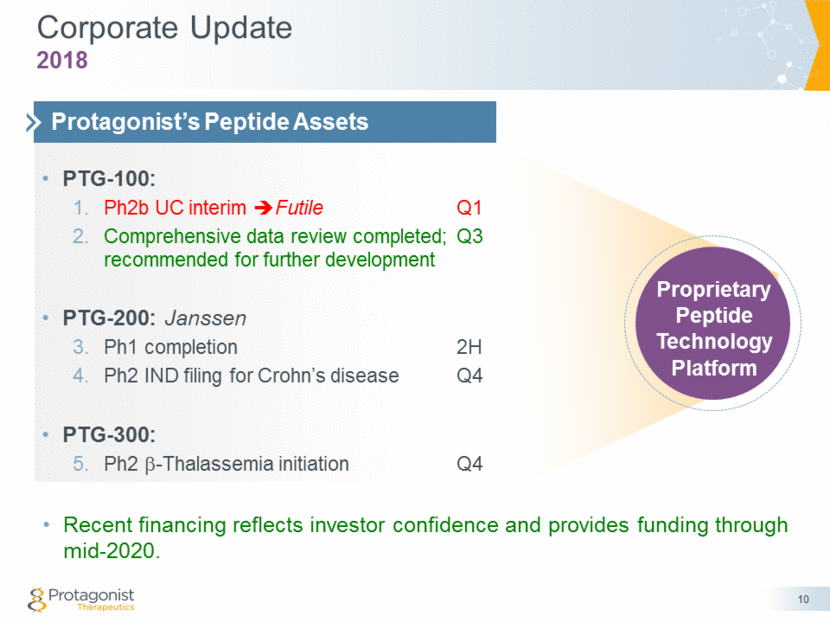
Corporate Update Recent financing reflects investor confidence and provides funding through mid-2020. 10 2018 PTG-100: Ph2b UC interim Futile Q1 Comprehensive data review completed; Q3 recommended for further development PTG-200: Janssen Ph1 completion 2H Ph2 IND filing for Crohn’s disease Q4 PTG-300: Ph2 b-Thalassemia initiation Q4 Protagonist’s Peptide Assets Proprietary Peptide Technology Platform