Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Ovid Therapeutics Inc. | ovid-8k_20180806.htm |

Phase 2 STARS Trial Achieves Primary Endpoint and Shows Statistically Significant Improvements in CGI-I August 6, 2018 Exhibit 99.1

Agenda SPEAKERS AGENDA ITEM Lora Pike Senior Director, IR & PR Welcome Jeremy Levin, DPhil, MB BChir Chairman & CEO Initial Remarks and OV101 Program Strategy Amit Rakhit, MD, MBA Chief Medical & Portfolio Management Officer Overview of topline STARS data Jeremy Levin, DPhil, MB BChir Chairman & CEO Pipeline, timelines and closing remarks Yaron Werber, MD, MBA Chief Business & Financial Officer Claude Nicaise, MD Head, Strategic Orphan Regulatory Affairs Join for Q&A

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “expects,” “anticipates,” “projects,” “estimates,” “intends,” “plans,” “believes,” variations of such words and similar expressions are intended to identify such forward-looking statements. Forward-looking statements contained in this presentation include statements about: timing, scope and design of any future clinical trials for OV101; the timing and results of any discussions with regulatory authorities regarding the registrational path for OV101; the potential clinical benefit of OV101 to treat patients with Angelman syndrome; and the future results of ongoing data analysis of the STARS trial of OV101, and the timing and trial design for the ELARA open label extension study, and the progress, timing and results of clinical trials regarding Ovid’s other product candidates. Each of these forward-looking statements involves risks and uncertainties. These statements are based on the Company’s current expectations and projections made by management and are not guarantees of future performance. Therefore, actual events, outcomes and results may differ materially from what is expressed or forecast in such forward-looking statements. Factors that may cause actual results to differ materially from these forward-looking statements are discussed in the Company’s filings with the U.S. Securities and Exchange Commission, including the "Risk Factors" sections contained therein. Except as otherwise required under federal securities laws, we do not have any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events, changes in assumptions or otherwise.

Key Takeaways STARS is the first study to show a positive clinical impact on people with Angelman syndrome since the disorder was characterized > 50 years ago. STARS was an international, randomized, double-blind, placebo-controlled study that utilized prespecified endpoints. The trial met its primary endpoint of safety which demonstrated that OV101 showed a favorable safety profile and was well tolerated in adults and adolescents with Angelman syndrome. Statistically significant improvement (p=0.0006) in the first prespecified efficacy endpoint (CGI-I) observed at 12 weeks of treatment in once-daily dose group compared to placebo. Based on these data, Ovid has identified once-daily dose as the optimal dose to bring forward in future Angelman syndrome studies. The STARS data also provide validation of Ovid’s strategy of developing medicines for rare neurological disorders based on novel scientific pathways and deep commitment to patients, their families and caregivers.
Genetic disorder with core disruptions in behavior, motor, sleep and cognitive function No FDA-approved therapies; treatment limited to supportive care Lifelong disorder that presents early in childhood 1/15,000 prevalence in the general population ~16,000-27,000 patients in the US Monogenetic disorder that results in loss of UBE3A gene expression Angelman Syndrome: Significant Unmet Need
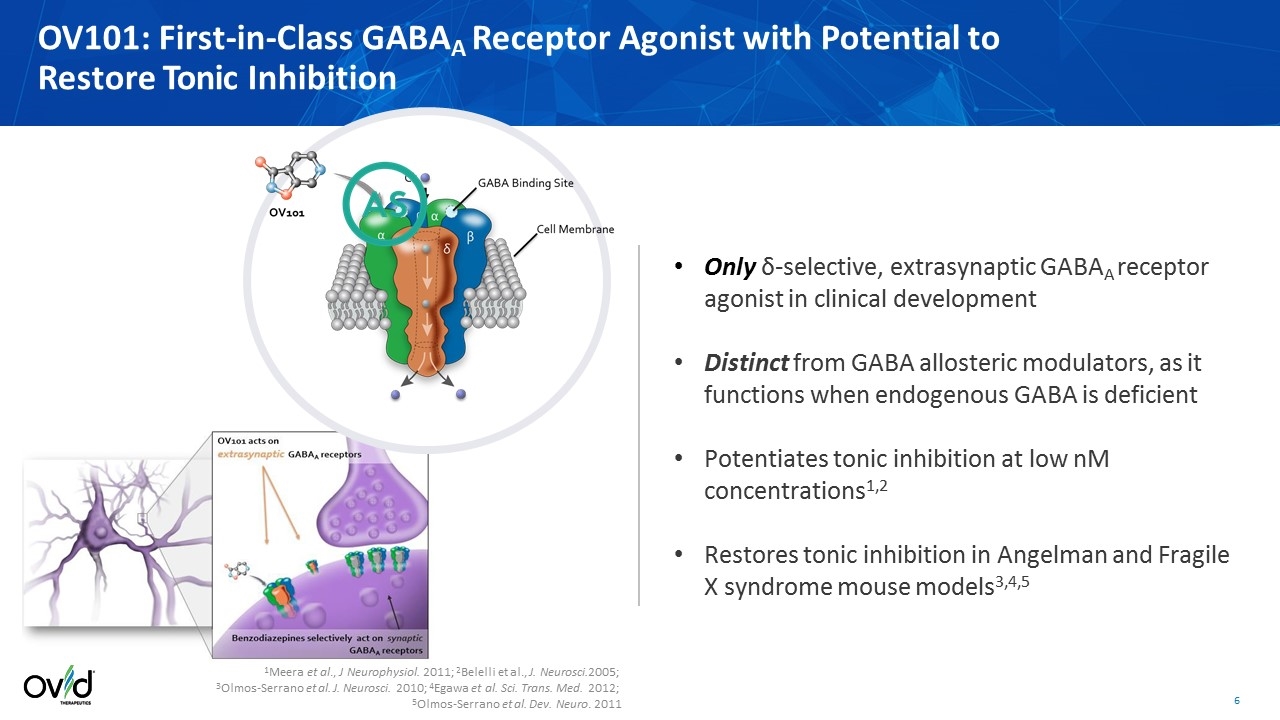
1Meera et al., J Neurophysiol. 2011; 2Belelli et al., J. Neurosci.2005; 3Olmos-Serrano et al. J. Neurosci. 2010; 4Egawa et al. Sci. Trans. Med. 2012; 5Olmos-Serrano et al. Dev. Neuro. 2011 Only δ-selective, extrasynaptic GABAA receptor agonist in clinical development Distinct from GABA allosteric modulators, as it functions when endogenous GABA is deficient Potentiates tonic inhibition at low nM concentrations1,2 Restores tonic inhibition in Angelman and Fragile X syndrome mouse models3,4,5 OV101: First-in-Class GABAA Receptor Agonist with Potential to Restore Tonic Inhibition
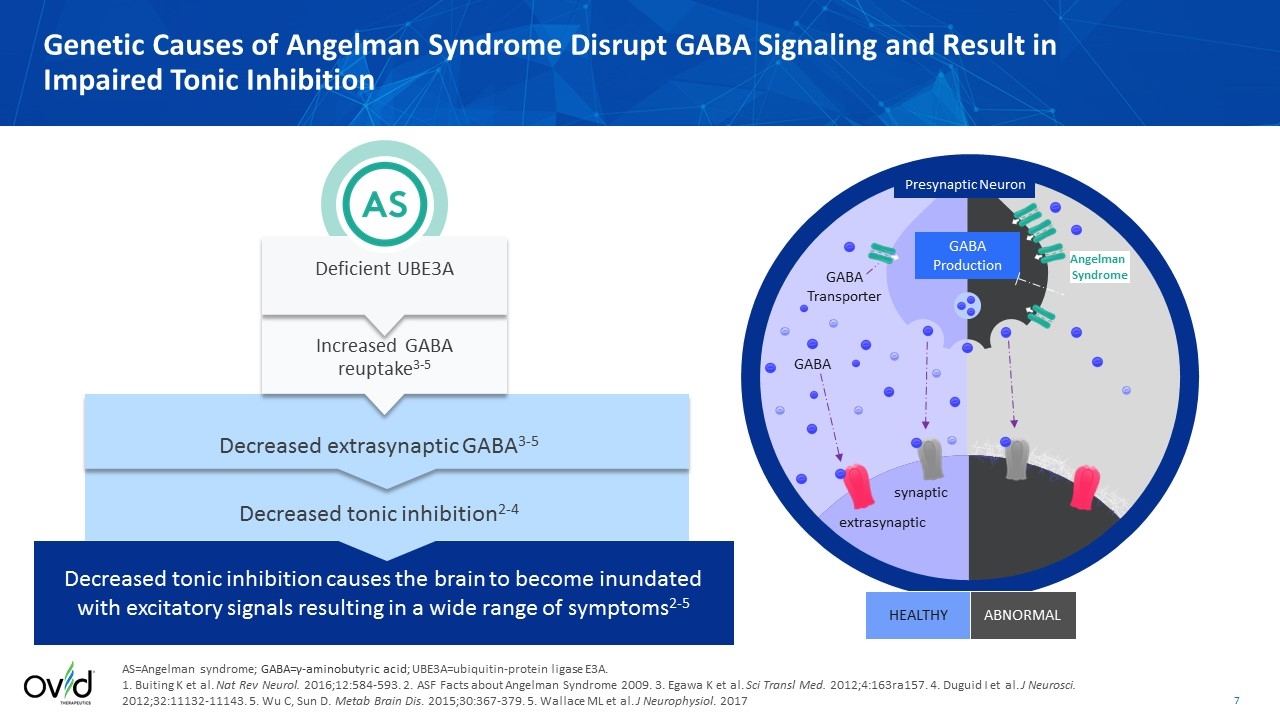
Genetic Causes of Angelman Syndrome Disrupt GABA Signaling and Result in Impaired Tonic Inhibition AS=Angelman syndrome; GABA=γ-aminobutyric acid; UBE3A=ubiquitin-protein ligase E3A. 1. Buiting K et al. Nat Rev Neurol. 2016;12:584-593. 2. ASF Facts about Angelman Syndrome 2009. 3. Egawa K et al. Sci Transl Med. 2012;4:163ra157. 4. Duguid I et al. J Neurosci. 2012;32:11132-11143. 5. Wu C, Sun D. Metab Brain Dis. 2015;30:367-379. 5. Wallace ML et al. J Neurophysiol. 2017 Angelman Syndrome Presynaptic Neuron GABA Production GABA Transporter GABA synaptic extrasynaptic HEALTHY ABNORMAL Decreased tonic inhibition causes the brain to become inundated with excitatory signals resulting in a wide range of symptoms2-5 Deficient UBE3A Decreased extrasynaptic GABA3-5 Decreased tonic inhibition2-4 Increased GABA reuptake3-5

STARS Objectives & Data Main objective of the Phase 2 STARS trial was to provide clear insights into the safety and tolerability of OV101 in patients with Angelman syndrome. We achieved this objective. The second goal of the trial was to provide us with learnings on OV101’s ability to restore tonic inhibition. At the prespecified efficacy analysis at 12 weeks of treatment, OV101 showed a statistically significant improvement compared to placebo in the physician-rated clinical global impressions of improvement (CGI-I) – a measure commonly used in clinical trials that allows the physician to capture a constellation of clinical symptoms. We believe CGI-I is an informative measure of Angelman syndrome activity and plan to incorporate this endpoint in future clinical studies.
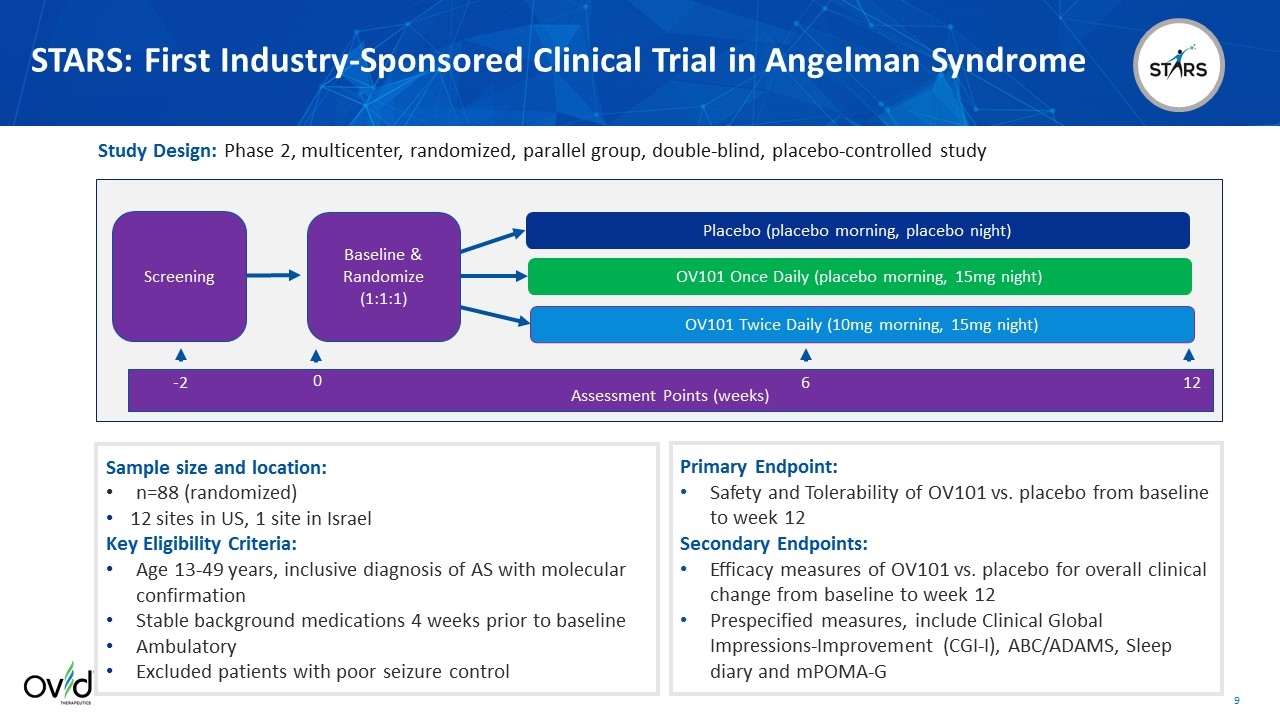
STARS: First Industry-Sponsored Clinical Trial in Angelman Syndrome Placebo (placebo morning, placebo night) OV101 Twice Daily (10mg morning, 15mg night) OV101 Once Daily (placebo morning, 15mg night) Assessment Points (weeks) -2 0 6 12 Screening Baseline & Randomize (1:1:1) Study Design: Phase 2, multicenter, randomized, parallel group, double-blind, placebo-controlled study Sample size and location: n=88 (randomized) 12 sites in US, 1 site in Israel Key Eligibility Criteria: Age 13-49 years, inclusive diagnosis of AS with molecular confirmation Stable background medications 4 weeks prior to baseline Ambulatory Excluded patients with poor seizure control Primary Endpoint: Safety and Tolerability of OV101 vs. placebo from baseline to week 12 Secondary Endpoints: Efficacy measures of OV101 vs. placebo for overall clinical change from baseline to week 12 Prespecified measures, include Clinical Global Impressions-Improvement (CGI-I), ABC/ADAMS, Sleep diary and mPOMA-G
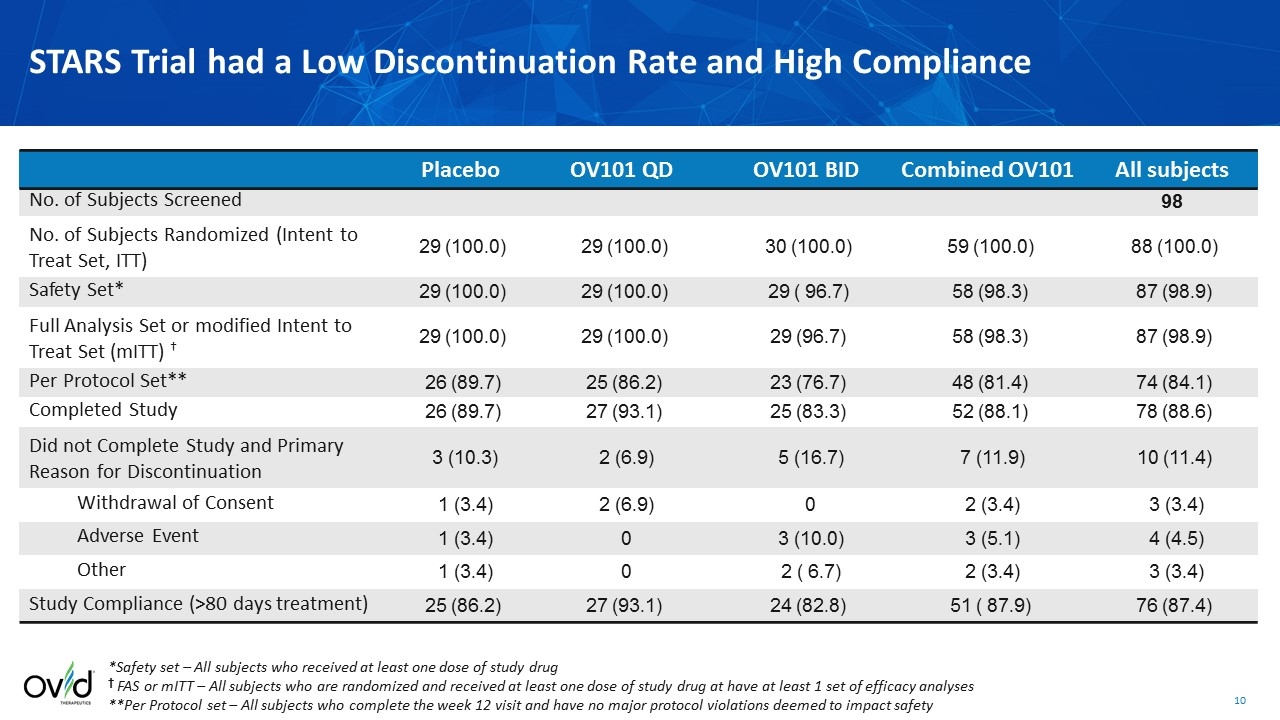
Placebo OV101 QD OV101 BID Combined OV101 All subjects No. of Subjects Screened 98 No. of Subjects Randomized (Intent to Treat Set, ITT) 29 (100.0) 29 (100.0) 30 (100.0) 59 (100.0) 88 (100.0) Safety Set* 29 (100.0) 29 (100.0) 29 ( 96.7) 58 (98.3) 87 (98.9) Full Analysis Set or modified Intent to Treat Set (mITT) † 29 (100.0) 29 (100.0) 29 (96.7) 58 (98.3) 87 (98.9) Per Protocol Set** 26 (89.7) 25 (86.2) 23 (76.7) 48 (81.4) 74 (84.1) Completed Study 26 (89.7) 27 (93.1) 25 (83.3) 52 (88.1) 78 (88.6) Did not Complete Study and Primary Reason for Discontinuation 3 (10.3) 2 (6.9) 5 (16.7) 7 (11.9) 10 (11.4) Withdrawal of Consent 1 (3.4) 2 (6.9) 0 2 (3.4) 3 (3.4) Adverse Event 1 (3.4) 0 3 (10.0) 3 (5.1) 4 (4.5) Other 1 (3.4) 0 2 ( 6.7) 2 (3.4) 3 (3.4) Study Compliance (>80 days treatment) 25 (86.2) 27 (93.1) 24 (82.8) 51 ( 87.9) 76 (87.4) *Safety set – All subjects who received at least one dose of study drug † FAS or mITT – All subjects who are randomized and received at least one dose of study drug at have at least 1 set of efficacy analyses **Per Protocol set – All subjects who complete the week 12 visit and have no major protocol violations deemed to impact safety STARS Trial had a Low Discontinuation Rate and High Compliance
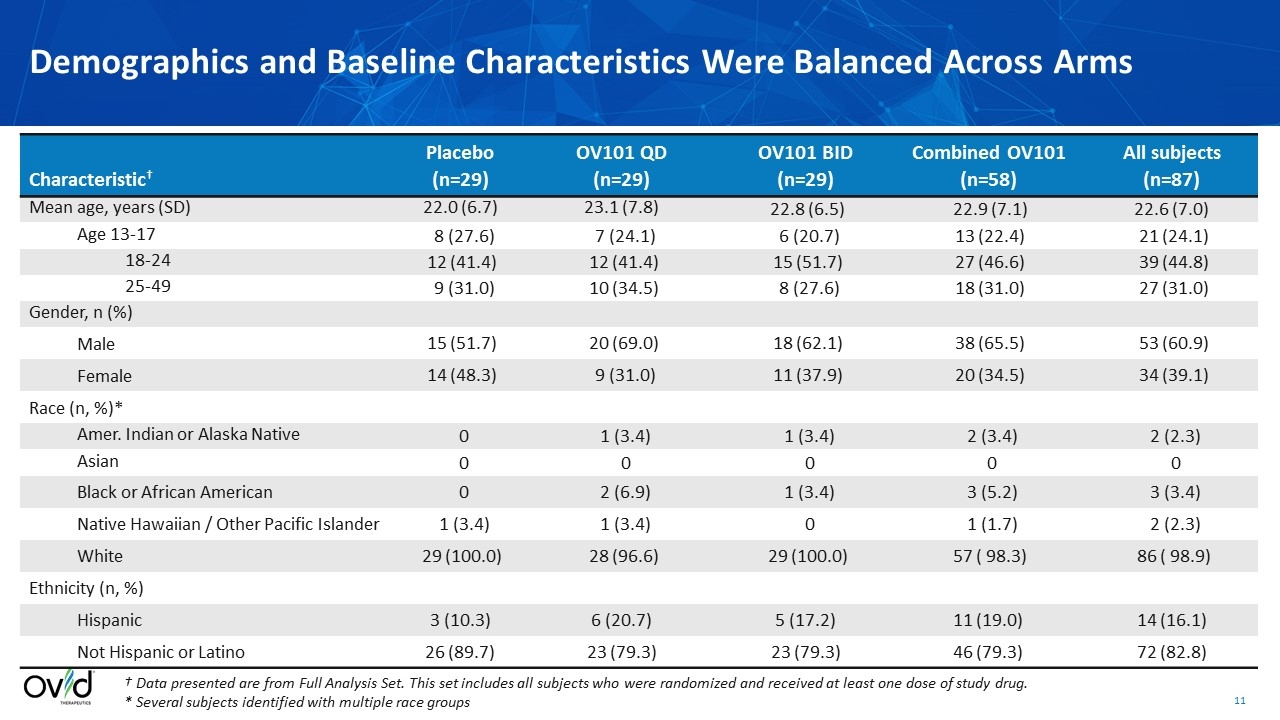
† Data presented are from Full Analysis Set. This set includes all subjects who were randomized and received at least one dose of study drug. * Several subjects identified with multiple race groups Characteristic† Placebo (n=29) OV101 QD (n=29) OV101 BID (n=29) Combined OV101 (n=58) All subjects (n=87) Mean age, years (SD) 22.0 (6.7) 23.1 (7.8) 22.8 (6.5) 22.9 (7.1) 22.6 (7.0) Age 13-17 8 (27.6) 7 (24.1) 6 (20.7) 13 (22.4) 21 (24.1) 18-24 12 (41.4) 12 (41.4) 15 (51.7) 27 (46.6) 39 (44.8) 25-49 9 (31.0) 10 (34.5) 8 (27.6) 18 (31.0) 27 (31.0) Gender, n (%) Male 15 (51.7) 20 (69.0) 18 (62.1) 38 (65.5) 53 (60.9) Female 14 (48.3) 9 (31.0) 11 (37.9) 20 (34.5) 34 (39.1) Race (n, %)* Amer. Indian or Alaska Native 0 1 (3.4) 1 (3.4) 2 (3.4) 2 (2.3) Asian 0 0 0 0 0 Black or African American 0 2 (6.9) 1 (3.4) 3 (5.2) 3 (3.4) Native Hawaiian / Other Pacific Islander 1 (3.4) 1 (3.4) 0 1 (1.7) 2 (2.3) White 29 (100.0) 28 (96.6) 29 (100.0) 57 ( 98.3) 86 ( 98.9) Ethnicity (n, %) Hispanic 3 (10.3) 6 (20.7) 5 (17.2) 11 (19.0) 14 (16.1) Not Hispanic or Latino 26 (89.7) 23 (79.3) 23 (79.3) 46 (79.3) 72 (82.8) Demographics and Baseline Characteristics Were Balanced Across Arms

OV101 achieved primary endpoint of safety and tolerability as measured by incidence of AEs Overall favorable risk profile and well tolerated Similar incidence of AEs across all treatment arms with majority of AEs being mild Treatment discontinuations due to AEs were low (n=1 placebo, n=0 OV101 QD, n=3 OV101 BID) Most frequent adverse events across all treatment groups Vomiting, somnolence, irritability, aggression, and pyrexia Events occurring in OV101 treatment arms more than placebo Pyrexia, rash, seizure, enuresis, and myoclonic epilepsy 2 subjects with SAEs of seizure – 1 subject in each OV101 treatment group 1 deemed “Possibly Related” and the other “Not Related” No deaths in any treatment groups Summary of Safety and Tolerability Findings
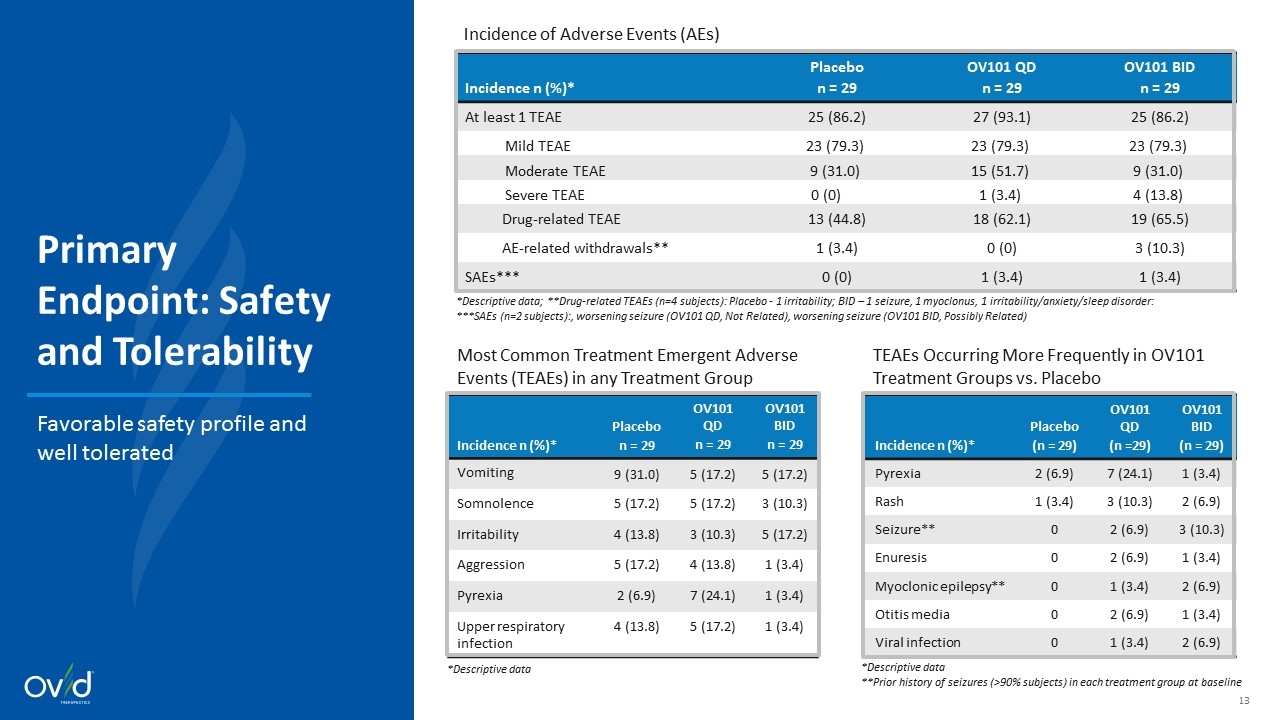
*Descriptive data; **Drug-related TEAEs (n=4 subjects): Placebo - 1 irritability; BID – 1 seizure, 1 myoclonus, 1 irritability/anxiety/sleep disorder: ***SAEs (n=2 subjects):, worsening seizure (OV101 QD, Not Related), worsening seizure (OV101 BID, Possibly Related) Incidence n (%)* Placebo n = 29 OV101 QD n = 29 OV101 BID n = 29 At least 1 TEAE 25 (86.2) 27 (93.1) 25 (86.2) Mild TEAE 23 (79.3) 23 (79.3) 23 (79.3) Moderate TEAE 9 (31.0) 15 (51.7) 9 (31.0) Severe TEAE 0 (0) 1 (3.4) 4 (13.8) Drug-related TEAE 13 (44.8) 18 (62.1) 19 (65.5) AE-related withdrawals** 1 (3.4) 0 (0) 3 (10.3) SAEs*** 0 (0) 1 (3.4) 1 (3.4) Incidence n (%)* Placebo n = 29 OV101 QD n = 29 OV101 BID n = 29 Vomiting 9 (31.0) 5 (17.2) 5 (17.2) Somnolence 5 (17.2) 5 (17.2) 3 (10.3) Irritability 4 (13.8) 3 (10.3) 5 (17.2) Aggression 5 (17.2) 4 (13.8) 1 (3.4) Pyrexia 2 (6.9) 7 (24.1) 1 (3.4) Upper respiratory infection 4 (13.8) 5 (17.2) 1 (3.4) Most Common Treatment Emergent Adverse Events (TEAEs) in any Treatment Group *Descriptive data Incidence n (%)* Placebo (n = 29) OV101 QD (n =29) OV101 BID (n = 29) Pyrexia 2 (6.9) 7 (24.1) 1 (3.4) Rash 1 (3.4) 3 (10.3) 2 (6.9) Seizure** 0 2 (6.9) 3 (10.3) Enuresis 0 2 (6.9) 1 (3.4) Myoclonic epilepsy** 0 1 (3.4) 2 (6.9) Otitis media 0 2 (6.9) 1 (3.4) Viral infection 0 1 (3.4) 2 (6.9) TEAEs Occurring More Frequently in OV101 Treatment Groups vs. Placebo *Descriptive data **Prior history of seizures (>90% subjects) in each treatment group at baseline Incidence of Adverse Events (AEs) Primary Endpoint: Safety and Tolerability Favorable safety profile and well tolerated
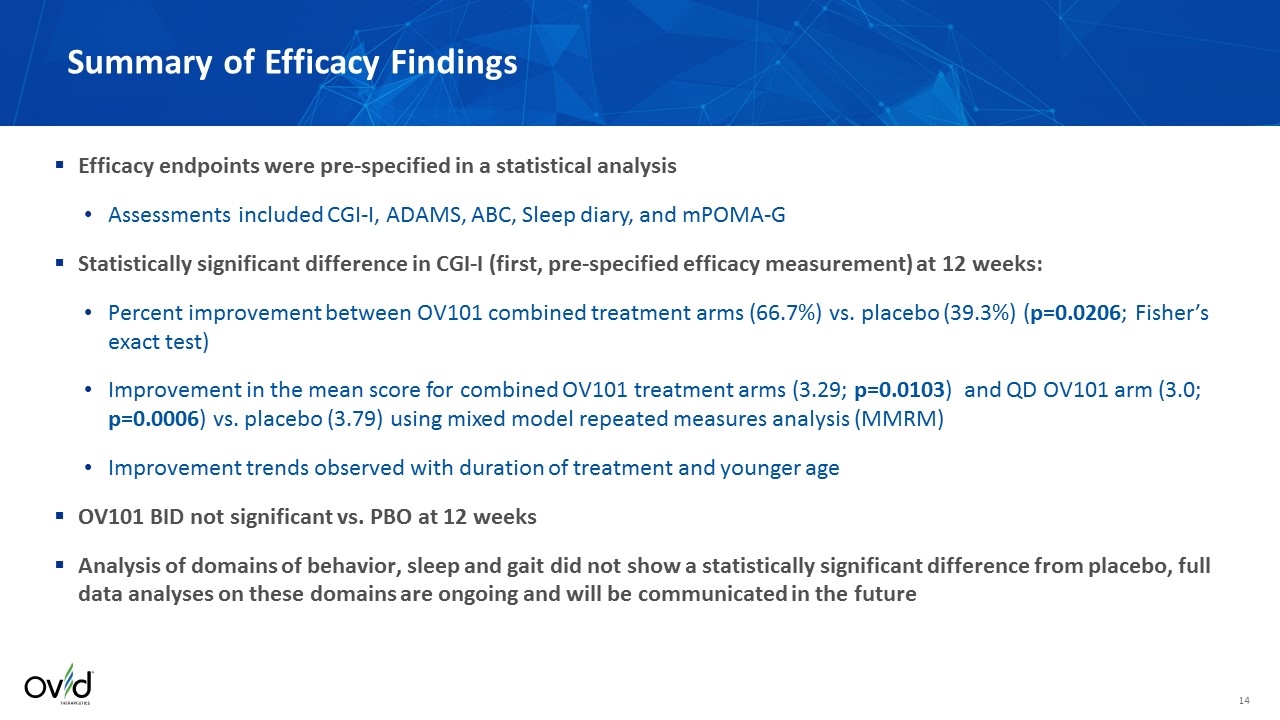
Efficacy endpoints were pre-specified in a statistical analysis Assessments included CGI-I, ADAMS, ABC, Sleep diary, and mPOMA-G Statistically significant difference in CGI-I (first, pre-specified efficacy measurement) at 12 weeks: Percent improvement between OV101 combined treatment arms (66.7%) vs. placebo (39.3%) (p=0.0206; Fisher’s exact test) Improvement in the mean score for combined OV101 treatment arms (3.29; p=0.0103) and QD OV101 arm (3.0; p=0.0006) vs. placebo (3.79) using mixed model repeated measures analysis (MMRM) Improvement trends observed with duration of treatment and younger age OV101 BID not significant vs. PBO at 12 weeks Analysis of domains of behavior, sleep and gait did not show a statistically significant difference from placebo, full data analyses on these domains are ongoing and will be communicated in the future Summary of Efficacy Findings
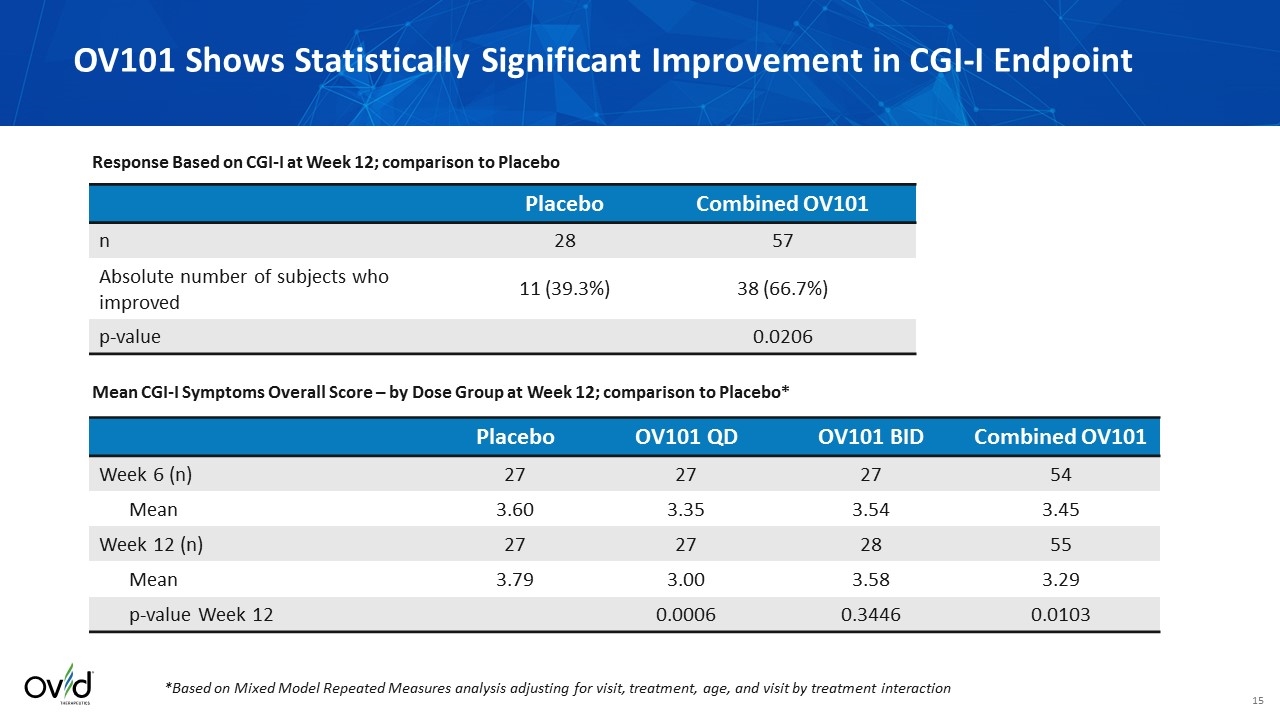
Placebo OV101 QD OV101 BID Combined OV101 Week 6 (n) 27 27 27 54 Mean 3.60 3.35 3.54 3.45 Week 12 (n) 27 27 28 55 Mean 3.79 3.00 3.58 3.29 p-value Week 12 0.0006 0.3446 0.0103 OV101 Shows Statistically Significant Improvement in CGI-I Endpoint *Based on Mixed Model Repeated Measures analysis adjusting for visit, treatment, age, and visit by treatment interaction Placebo Combined OV101 n 28 57 Absolute number of subjects who improved 11 (39.3%) 38 (66.7%) p-value 0.0206 Response Based on CGI-I at Week 12; comparison to Placebo Mean CGI-I Symptoms Overall Score – by Dose Group at Week 12; comparison to Placebo*
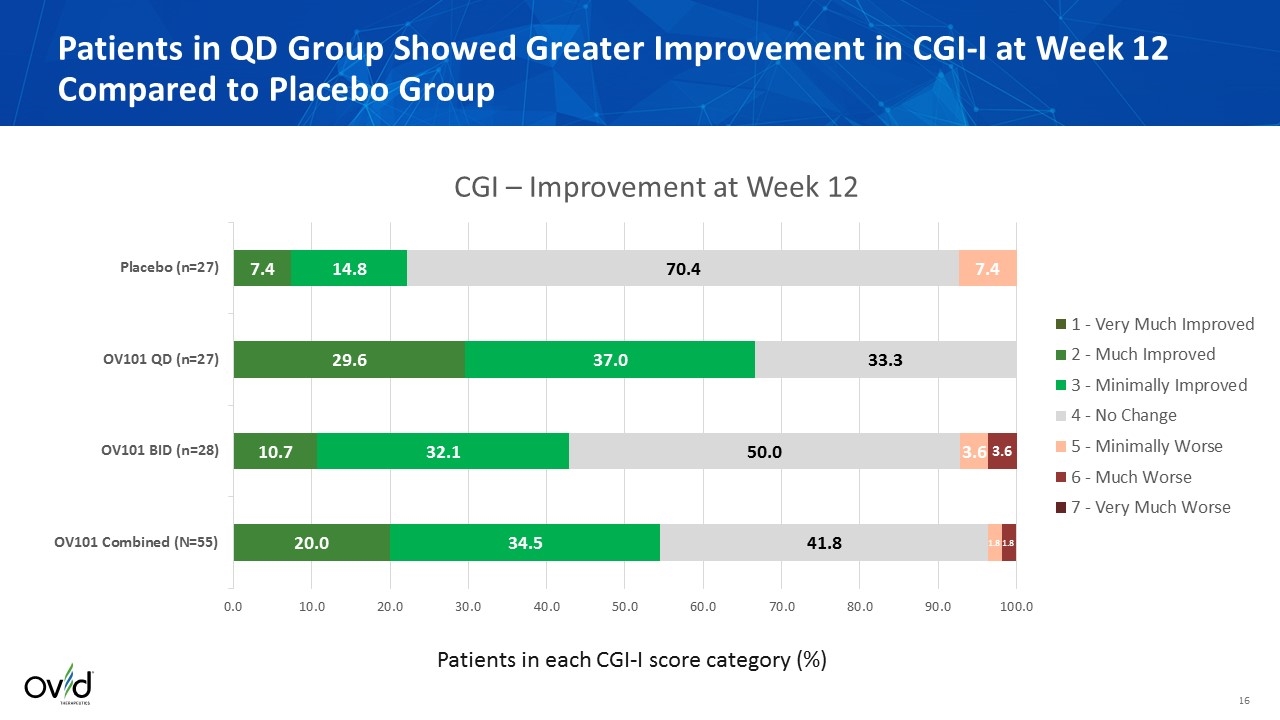
Patients in QD Group Showed Greater Improvement in CGI-I at Week 12 Compared to Placebo Group Patients in each CGI-I score category (%)
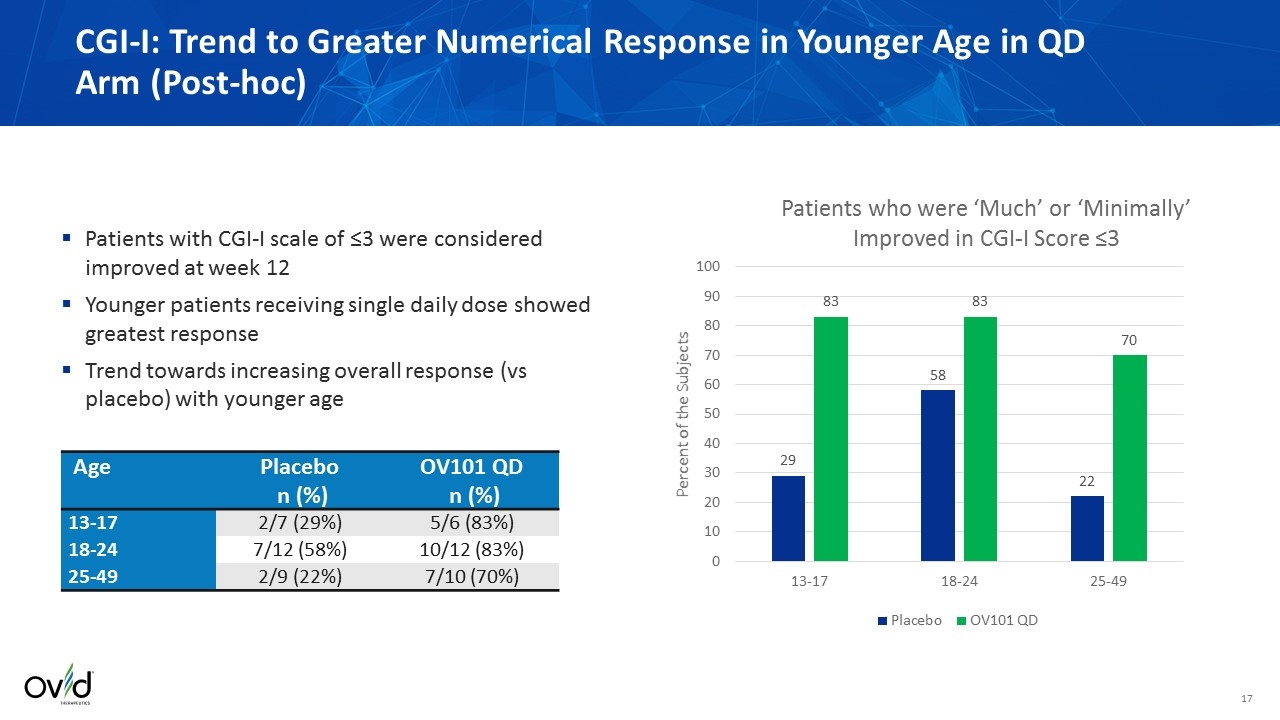
CGI-I: Trend to Greater Numerical Response in Younger Age in QD Arm (Post-hoc) Patients with CGI-I scale of ≤3 were considered improved at week 12 Younger patients receiving single daily dose showed greatest response Trend towards increasing overall response (vs placebo) with younger age Age Placebo n (%) OV101 QD n (%) 13-17 2/7 (29%) 5/6 (83%) 18-24 7/12 (58%) 10/12 (83%) 25-49 2/9 (22%) 7/10 (70%)
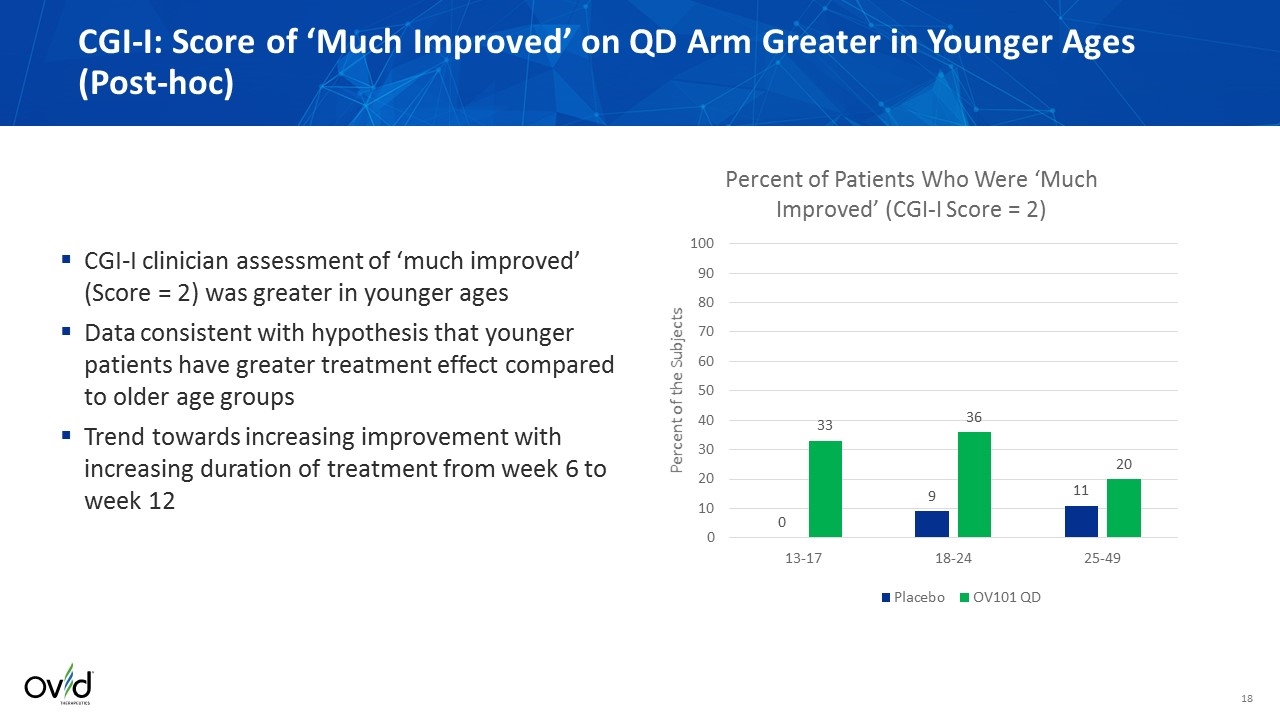
CGI-I: Score of ‘Much Improved’ on QD Arm Greater in Younger Ages (Post-hoc) CGI-I clinician assessment of ‘much improved’ (Score = 2) was greater in younger ages Data consistent with hypothesis that younger patients have greater treatment effect compared to older age groups Trend towards increasing improvement with increasing duration of treatment from week 6 to week 12
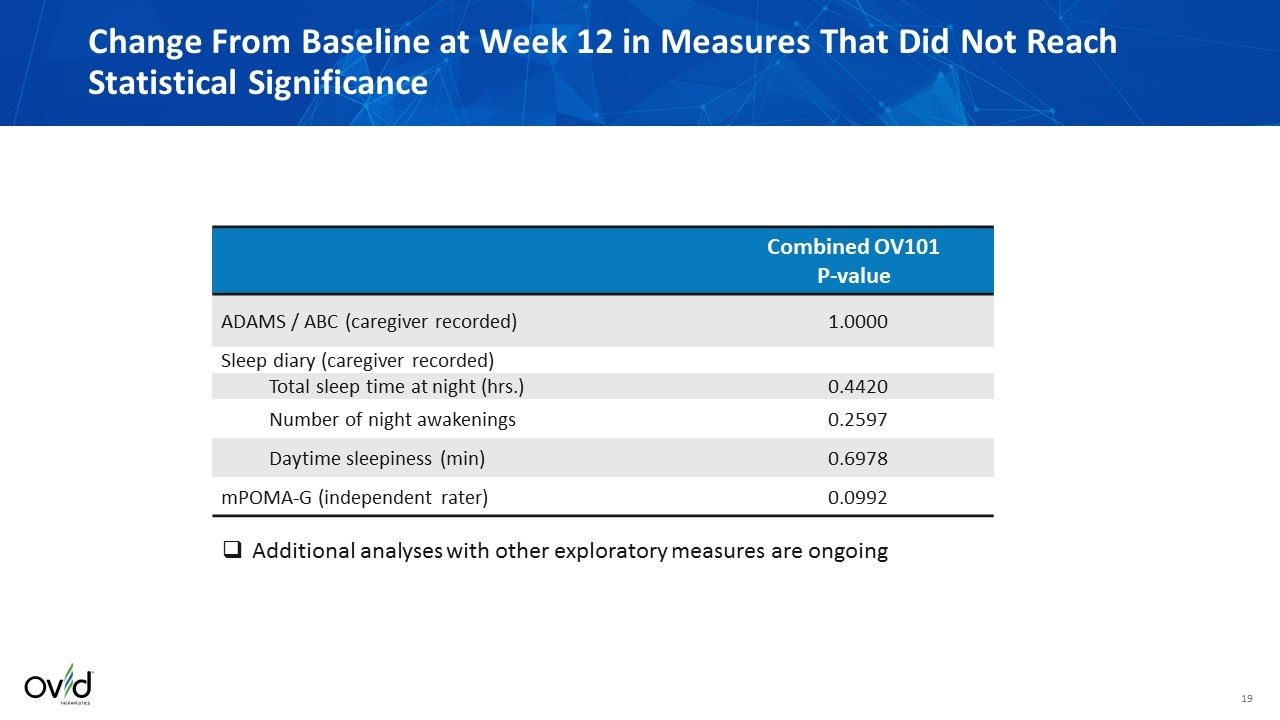
Change From Baseline at Week 12 in Measures That Did Not Reach Statistical Significance Combined OV101 P-value ADAMS / ABC (caregiver recorded) 1.0000 Sleep diary (caregiver recorded) Total sleep time at night (hrs.) 0.4420 Number of night awakenings 0.2597 Daytime sleepiness (min) 0.6978 mPOMA-G (independent rater) 0.0992 Additional analyses with other exploratory measures are ongoing

STARS Data Executive Summary STARS is the first industry-sponsored interventional clinical trial in Angelman syndrome Safety: Overall favorable risk profile and well tolerated Efficacy and Dosing: Consistent, statistically significant improvement in the key, pre-specified endpoint of CGI-I in QD and combined OV101 treatment groups Optimal dose identified as QD Next steps: Complete all tertiary and post-hoc analyses Plan to engage with regulatory authorities to discuss registrational path Plan to initiate open-label extension study (ELARA) in Q4’ 18

Acknowledgements Ovid Therapeutics would like to thank the patients, families, caregivers, and clinicians for their support and active involvement in the STARS clinical trial program
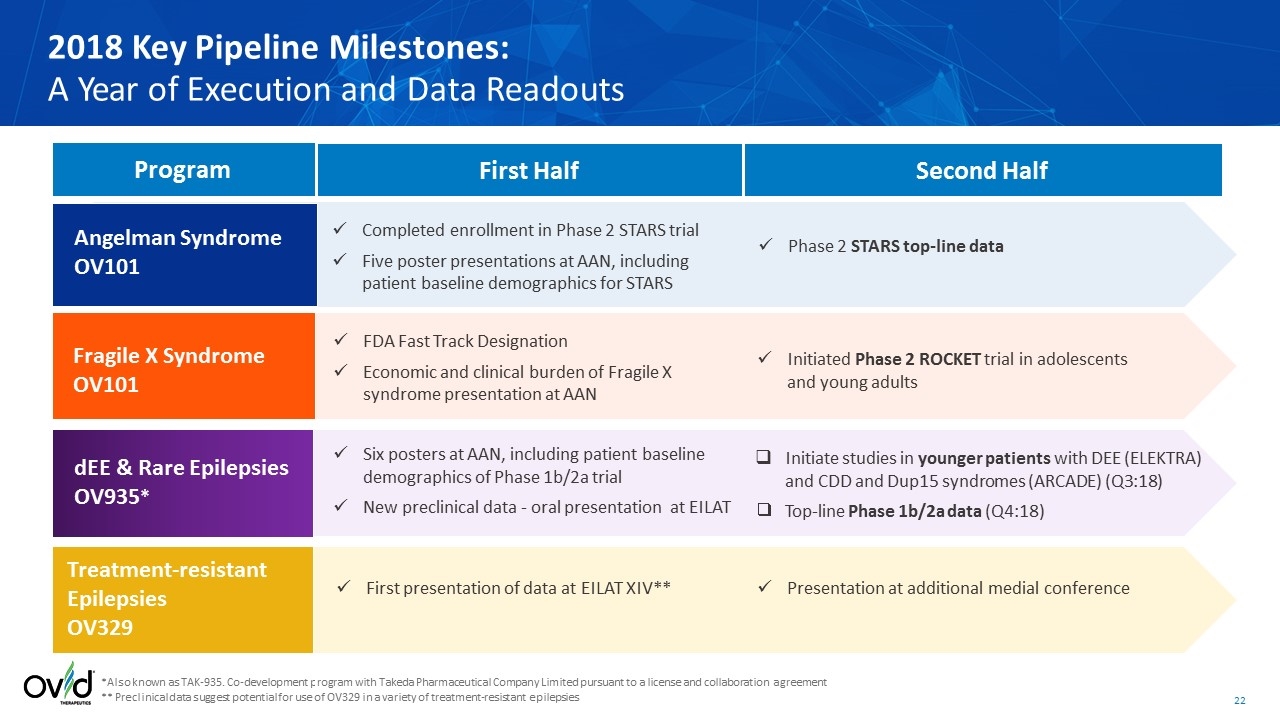
*Also known as TAK-935. Co-development program with Takeda Pharmaceutical Company Limited pursuant to a license and collaboration agreement ** Preclinical data suggest potential for use of OV329 in a variety of treatment-resistant epilepsies 2018 Key Pipeline Milestones: A Year of Execution and Data Readouts Angelman Syndrome OV101 First Half Second Half Fragile X Syndrome OV101 dEE & Rare Epilepsies OV935* Treatment-resistant Epilepsies OV329 Program Completed enrollment in Phase 2 STARS trial Five poster presentations at AAN, including patient baseline demographics for STARS FDA Fast Track Designation Economic and clinical burden of Fragile X syndrome presentation at AAN Six posters at AAN, including patient baseline demographics of Phase 1b/2a trial New preclinical data - oral presentation at EILAT First presentation of data at EILAT XIV** Phase 2 STARS top-line data Initiated Phase 2 ROCKET trial in adolescents and young adults Initiate studies in younger patients with DEE (ELEKTRA) and CDD and Dup15 syndromes (ARCADE) (Q3:18) Top-line Phase 1b/2a data (Q4:18) Presentation at additional medial conference
