Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Sienna Biopharmaceuticals, Inc. | d554785dex991.htm |
| 8-K - 8-K - Sienna Biopharmaceuticals, Inc. | d554785d8k.htm |

SNA-001 Acne Pivotal Trials: Results with 1064 nm and 810 nm Lasers July 30, 2018 March 2017 Exhibit 99.2
Forward Looking Statements This presentation contains forward-looking statements. All statements other than descriptions of historical facts contained in this presentation, including statements regarding future operational and financial results and positions, business strategy, prospective products, potential market, commercial opportunity and market share, availability and potential sources of funding, clinical trial results, product approvals and regulatory pathways, research and development costs, timing (including but not limited to clinical development and regulatory timelines), strategies for completion and likelihood of success for our business activities, and plans for future operations, are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Such risks and uncertainties include, among others, those inherent in the preclinical and clinical development process and the regulatory approval process; the risks and uncertainties in commercialization and gaining market acceptance; the risks associated with protecting and defending our patents or other proprietary rights; the risk that our proprietary rights may be insufficient to protect our product candidates; the risk that we will be unable to obtain necessary capital when needed on acceptable terms or at all; competition from other products or procedures; our reliance on third-parties to conduct our clinical and non-clinical trials; and our reliance on single-source third-party suppliers to manufacture clinical, non-clinical and any future commercial supplies of our product candidates. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to our business in general, see our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission and our future current and periodic reports. Except as required by applicable law, we assume no obligation to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Topical Photoparticle TherapyTM Platform (SNA-001)
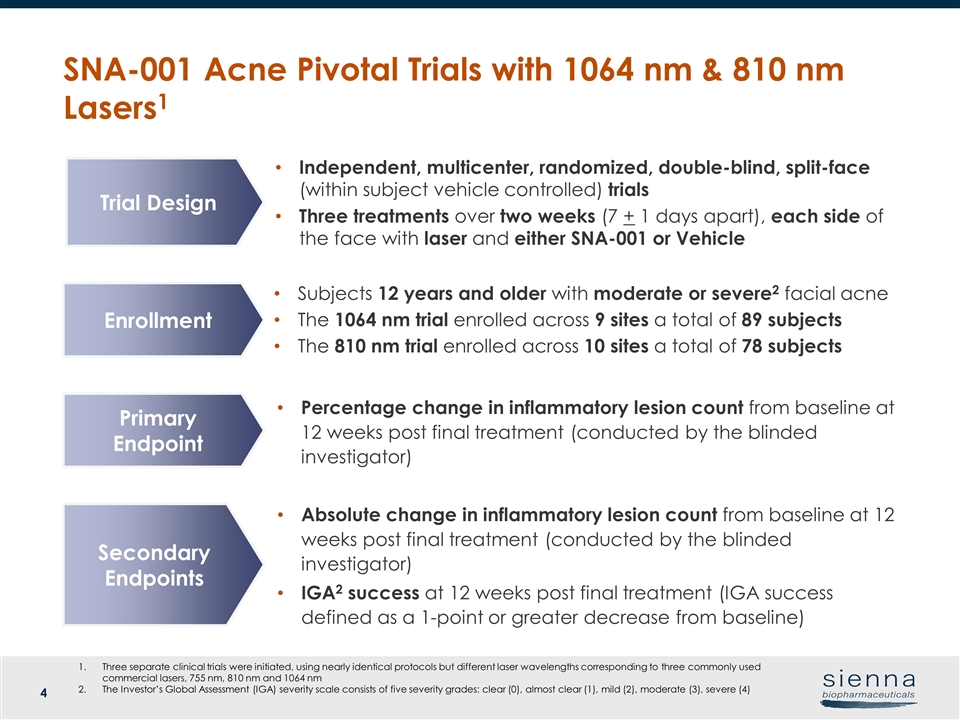
Enrollment Primary Endpoint Secondary Endpoints Independent, multicenter, randomized, double-blind, split-face (within subject vehicle controlled) trials Three treatments over two weeks (7 + 1 days apart), each side of the face with laser and either SNA-001 or Vehicle Three separate clinical trials were initiated, using nearly identical protocols but different laser wavelengths corresponding to three commonly used commercial lasers, 755 nm, 810 nm and 1064 nm The Investor’s Global Assessment (IGA) severity scale consists of five severity grades: clear (0), almost clear (1), mild (2), moderate (3), severe (4) SNA-001 Acne Pivotal Trials with 1064 nm & 810 nm Lasers1 Trial Design Percentage change in inflammatory lesion count from baseline at 12 weeks post final treatment (conducted by the blinded investigator) Absolute change in inflammatory lesion count from baseline at 12 weeks post final treatment (conducted by the blinded investigator) IGA2 success at 12 weeks post final treatment (IGA success defined as a 1-point or greater decrease from baseline) Subjects 12 years and older with moderate or severe2 facial acne The 1064 nm trial enrolled across 9 sites a total of 89 subjects The 810 nm trial enrolled across 10 sites a total of 78 subjects
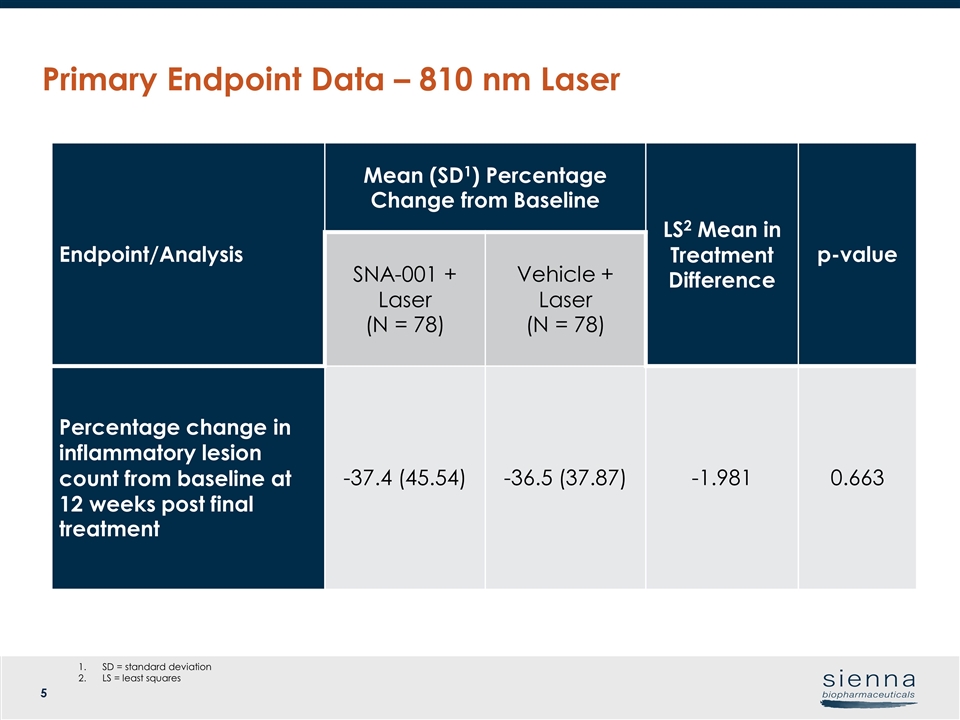
Primary Endpoint Data – 810 nm Laser Endpoint/Analysis Mean (SD1) Percentage Change from Baseline LS2 Mean in Treatment Difference p-value SNA-001 + Laser (N = 78) Vehicle + Laser (N = 78) Percentage change in inflammatory lesion count from baseline at 12 weeks post final treatment -37.4 (45.54) -36.5 (37.87) -1.981 0.663 SD = standard deviation LS = least squares
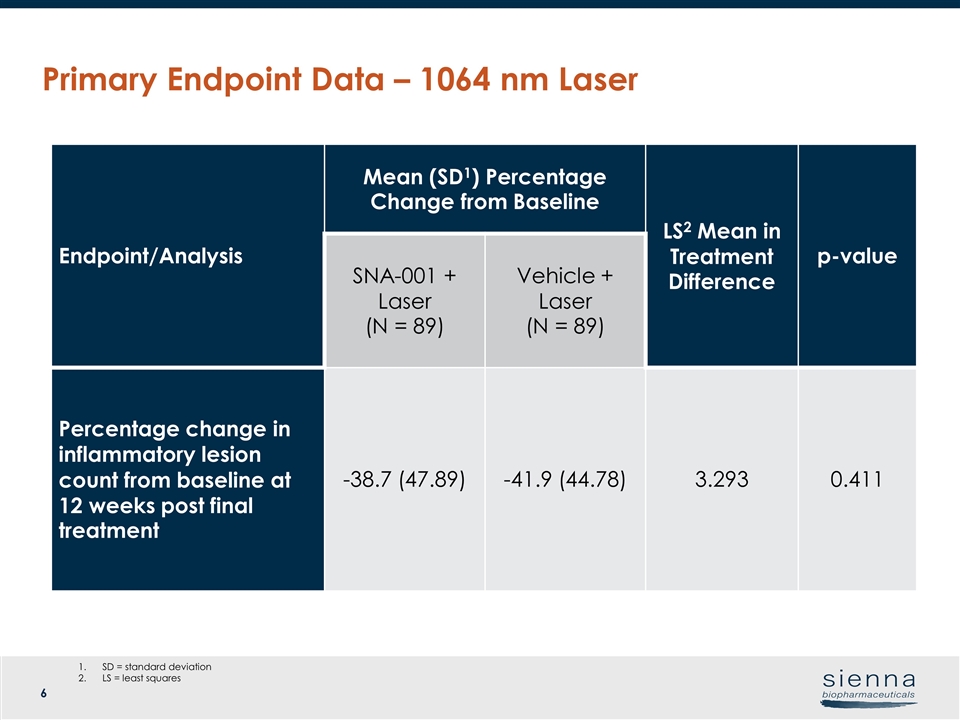
Primary Endpoint Data – 1064 nm Laser Endpoint/Analysis Mean (SD1) Percentage Change from Baseline LS2 Mean in Treatment Difference p-value SNA-001 + Laser (N = 89) Vehicle + Laser (N = 89) Percentage change in inflammatory lesion count from baseline at 12 weeks post final treatment -38.7 (47.89) -41.9 (44.78) 3.293 0.411 SD = standard deviation LS = least squares
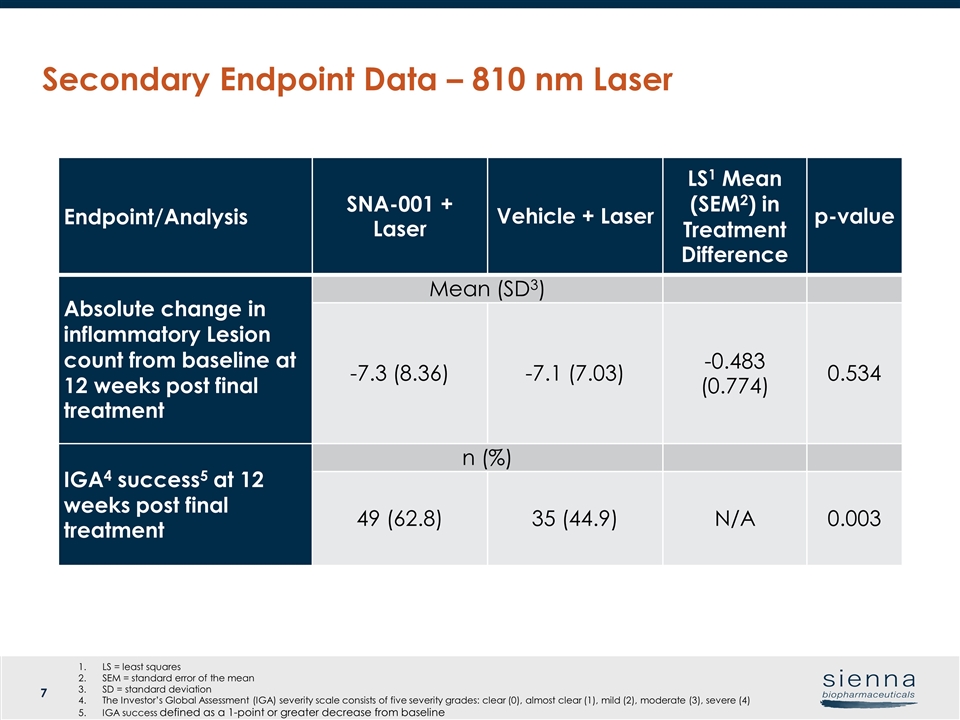
Secondary Endpoint Data – 810 nm Laser Endpoint/Analysis SNA-001 + Laser Vehicle + Laser LS1 Mean (SEM2) in Treatment Difference p-value Absolute change in inflammatory Lesion count from baseline at 12 weeks post final treatment Mean (SD3) -7.3 (8.36) -7.1 (7.03) -0.483 (0.774) 0.534 IGA4 success5 at 12 weeks post final treatment n (%) 49 (62.8) 35 (44.9) N/A 0.003 LS = least squares SEM = standard error of the mean SD = standard deviation The Investor’s Global Assessment (IGA) severity scale consists of five severity grades: clear (0), almost clear (1), mild (2), moderate (3), severe (4) IGA success defined as a 1-point or greater decrease from baseline
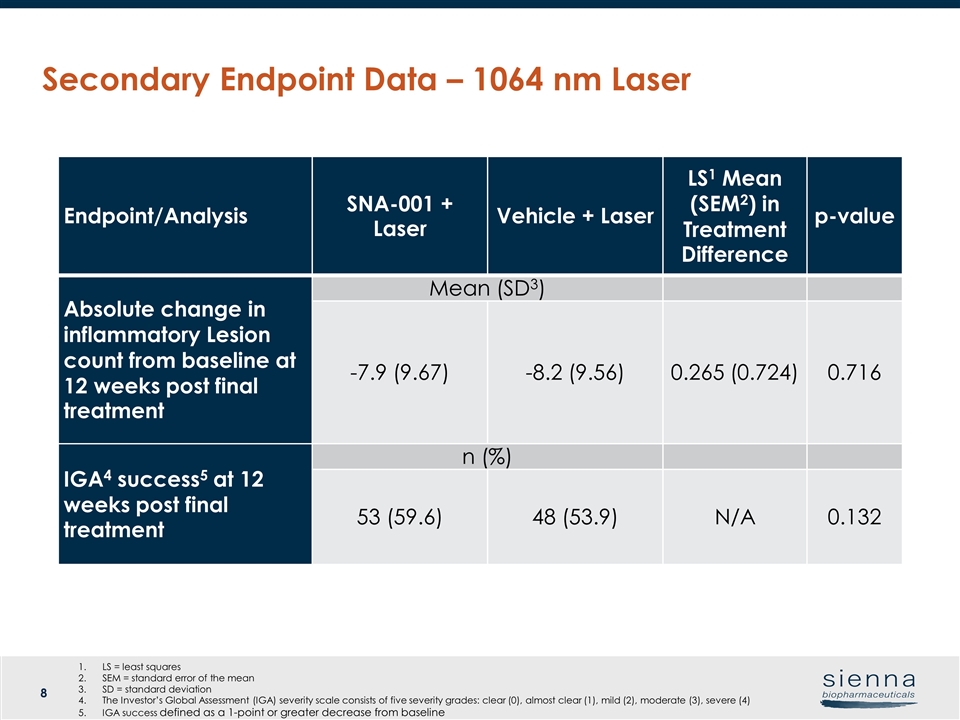
Secondary Endpoint Data – 1064 nm Laser Endpoint/Analysis SNA-001 + Laser Vehicle + Laser LS1 Mean (SEM2) in Treatment Difference p-value Absolute change in inflammatory Lesion count from baseline at 12 weeks post final treatment Mean (SD3) -7.9 (9.67) -8.2 (9.56) 0.265 (0.724) 0.716 IGA4 success5 at 12 weeks post final treatment n (%) 53 (59.6) 48 (53.9) N/A 0.132 LS = least squares SEM = standard error of the mean SD = standard deviation The Investor’s Global Assessment (IGA) severity scale consists of five severity grades: clear (0), almost clear (1), mild (2), moderate (3), severe (4) IGA success defined as a 1-point or greater decrease from baseline
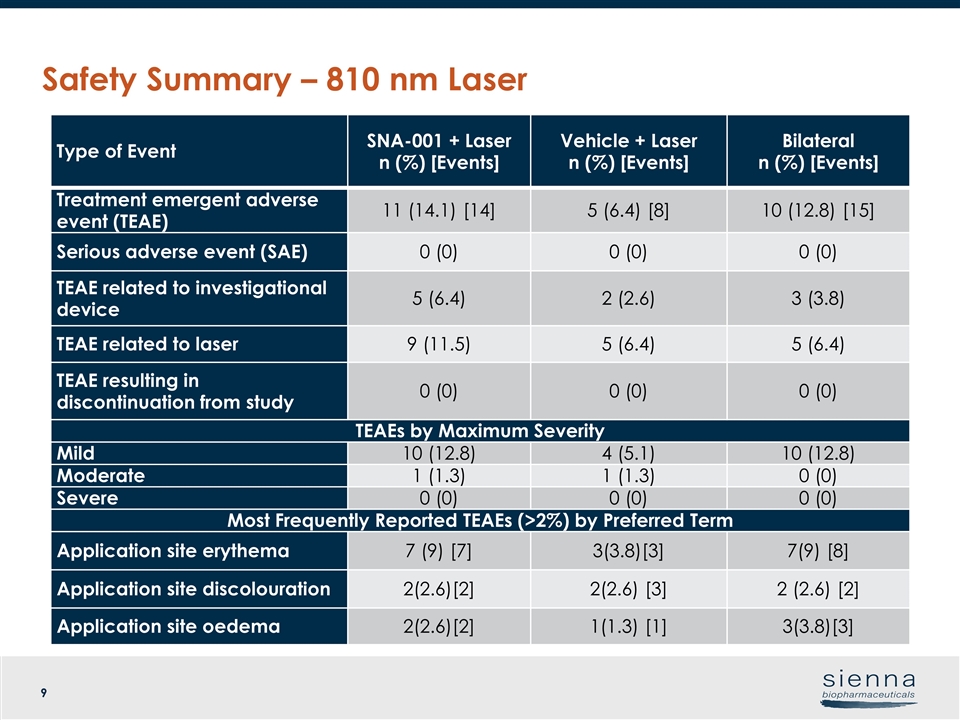
Safety Summary – 810 nm Laser Type of Event SNA-001 + Laser n (%) [Events] Vehicle + Laser n (%) [Events] Bilateral n (%) [Events] Treatment emergent adverse event (TEAE) 11 (14.1) [14] 5 (6.4) [8] 10 (12.8) [15] Serious adverse event (SAE) 0 (0) 0 (0) 0 (0) TEAE related to investigational device 5 (6.4) 2 (2.6) 3 (3.8) TEAE related to laser 9 (11.5) 5 (6.4) 5 (6.4) TEAE resulting in discontinuation from study 0 (0) 0 (0) 0 (0) TEAEs by Maximum Severity Mild 10 (12.8) 4 (5.1) 10 (12.8) Moderate 1 (1.3) 1 (1.3) 0 (0) Severe 0 (0) 0 (0) 0 (0) Most Frequently Reported TEAEs (>2%) by Preferred Term Application site erythema 7 (9) [7] 3(3.8)[3] 7(9) [8] Application site discolouration 2(2.6)[2] 2(2.6) [3] 2 (2.6) [2] Application site oedema 2(2.6)[2] 1(1.3) [1] 3(3.8)[3]
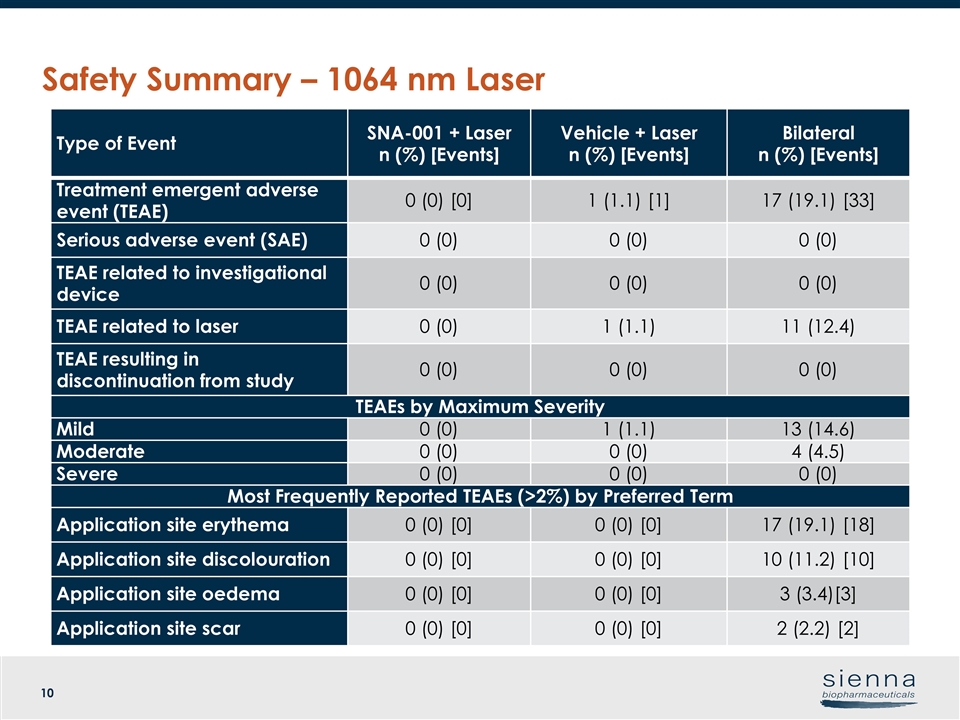
Safety Summary – 1064 nm Laser Type of Event SNA-001 + Laser n (%) [Events] Vehicle + Laser n (%) [Events] Bilateral n (%) [Events] Treatment emergent adverse event (TEAE) 0 (0) [0] 1 (1.1) [1] 17 (19.1) [33] Serious adverse event (SAE) 0 (0) 0 (0) 0 (0) TEAE related to investigational device 0 (0) 0 (0) 0 (0) TEAE related to laser 0 (0) 1 (1.1) 11 (12.4) TEAE resulting in discontinuation from study 0 (0) 0 (0) 0 (0) TEAEs by Maximum Severity Mild 0 (0) 1 (1.1) 13 (14.6) Moderate 0 (0) 0 (0) 4 (4.5) Severe 0 (0) 0 (0) 0 (0) Most Frequently Reported TEAEs (>2%) by Preferred Term Application site erythema 0 (0) [0] 0 (0) [0] 17 (19.1) [18] Application site discolouration 0 (0) [0] 0 (0) [0] 10 (11.2) [10] Application site oedema 0 (0) [0] 0 (0) [0] 3 (3.4)[3] Application site scar 0 (0) [0] 0 (0) [0] 2 (2.2) [2]
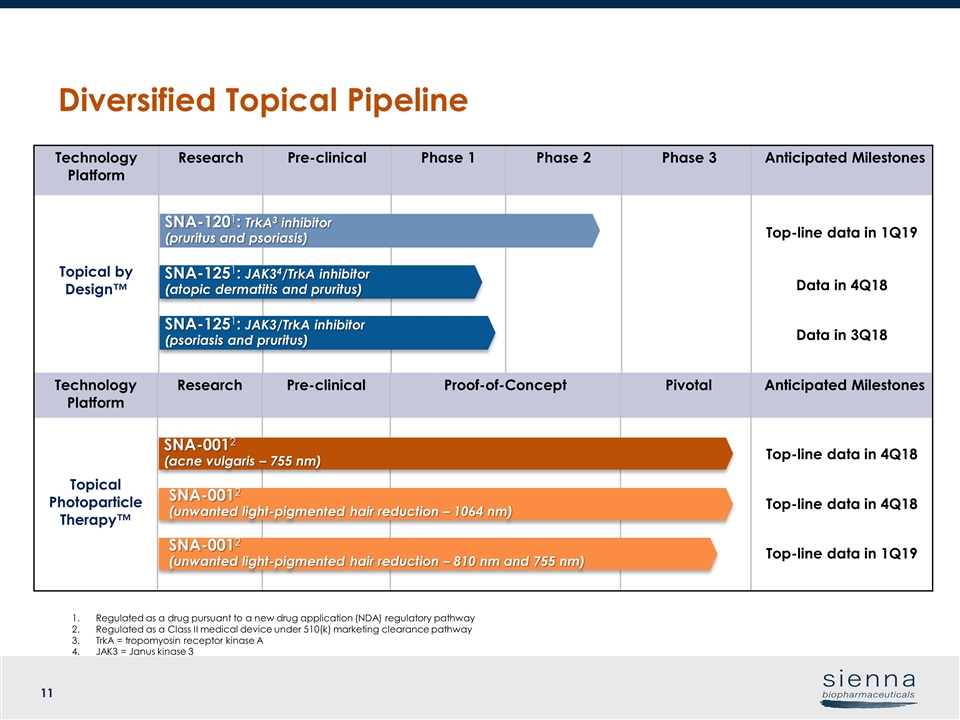
Regulated as a drug pursuant to a new drug application (NDA) regulatory pathway Regulated as a Class II medical device under 510(k) marketing clearance pathway TrkA = tropomyosin receptor kinase A JAK3 = Janus kinase 3 Technology Platform Research Pre-clinical Phase 1 Phase 2 Phase 3 Anticipated Milestones Topical by Design™ Top-line data in 1Q19 Data in 4Q18 Data in 3Q18 Technology Platform Research Pre-clinical Proof-of-Concept Pivotal Anticipated Milestones Topical Photoparticle Therapy™ Top-line data in 4Q18 Top-line data in 4Q18 Top-line data in 1Q19 SNA-1201: TrkA3 inhibitor (pruritus and psoriasis) SNA-1251: JAK3/TrkA inhibitor (psoriasis and pruritus) SNA-0012 (acne vulgaris – 755 nm) SNA-0012 (unwanted light-pigmented hair reduction – 1064 nm) SNA-1251: JAK34/TrkA inhibitor (atopic dermatitis and pruritus) SNA-0012 (unwanted light-pigmented hair reduction – 810 nm and 755 nm) Diversified Topical Pipeline
