Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - aTYR PHARMA INC | life-ex991_6.htm |
| 8-K - 8-K - aTYR PHARMA INC | life-8k_20180727.htm |
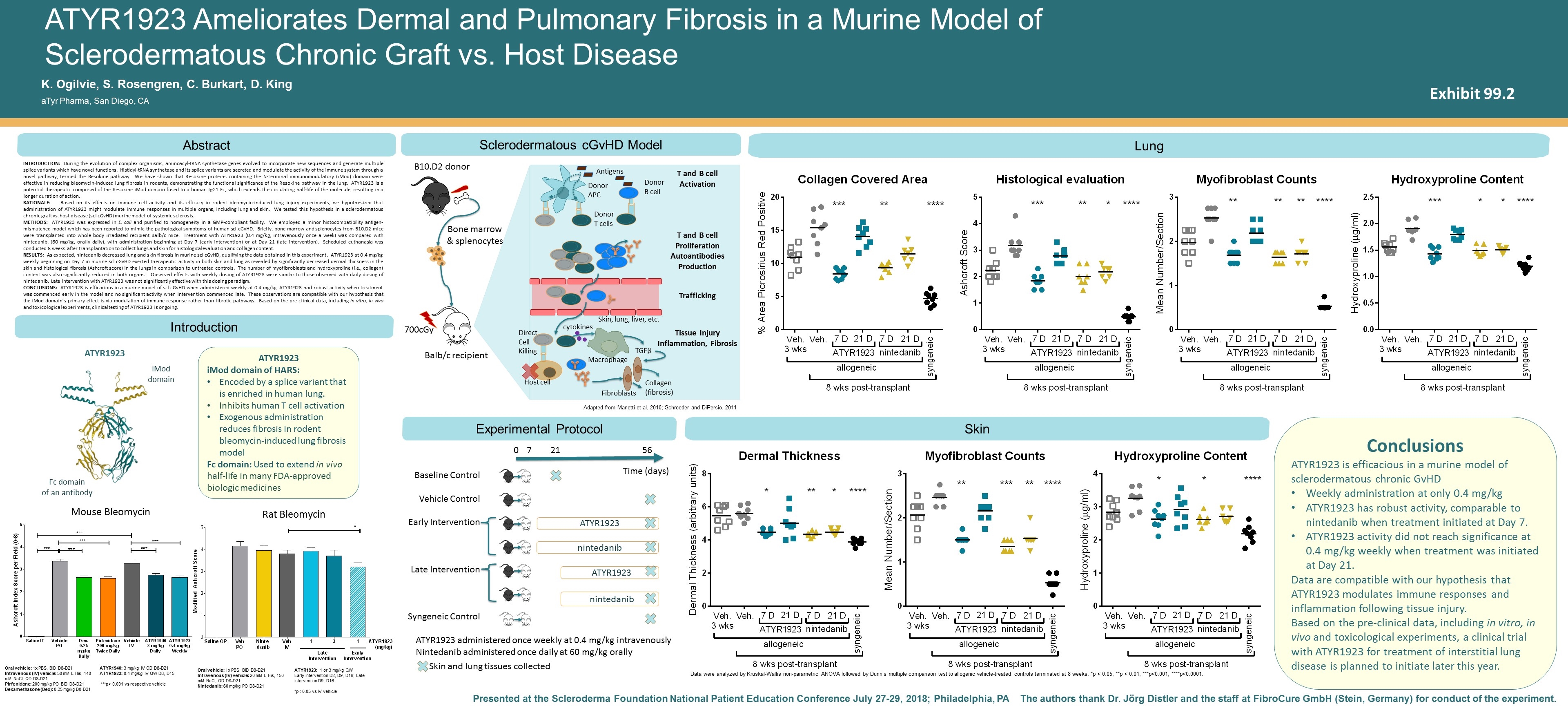
ATYR1923 iMod domain of HARS: Encoded by a splice variant that is enriched in human lung. Inhibits human T cell activation Exogenous administration reduces fibrosis in rodent bleomycin-induced lung fibrosis model Fc domain: Used to extend in vivo half-life in many FDA-approved biologic medicines Abstract The authors thank Dr. Jörg Distler and the staff at FibroCure GmbH (Stein, Germany) for conduct of the experiment. Presented at the Scleroderma Foundation National Patient Education Conference July 27-29, 2018; Philadelphia, PA ATYR1923 Ameliorates Dermal and Pulmonary Fibrosis in a Murine Model of Sclerodermatous Chronic Graft vs. Host Disease Introduction K. Ogilvie, S. Rosengren, C. Burkart, D. King aTyr Pharma, San Diego, CA INTRODUCTion: During the evolution of complex organisms, aminoacyl-tRNA synthetase genes evolved to incorporate new sequences and generate multiple splice variants which have novel functions. Histidyl-tRNA synthetase and its splice variants are secreted and modulate the activity of the immune system through a novel pathway, termed the Resokine pathway. We have shown that Resokine proteins containing the N-terminal immunomodulatory (iMod) domain were effective in reducing bleomycin-induced lung fibrosis in rodents, demonstrating the functional significance of the Resokine pathway in the lung. ATYR1923 is a potential therapeutic comprised of the Resokine iMod domain fused to a human IgG1 Fc, which extends the circulating half-life of the molecule, resulting in a longer duration of action. Rationale: Based on its effects on immune cell activity and its efficacy in rodent bleomycin-induced lung injury experiments, we hypothesized that administration of ATYR1923 might modulate immune responses in multiple organs, including lung and skin. We tested this hypothesis in a sclerodermatous chronic graft vs. host disease (scl cGvHD) murine model of systemic sclerosis. Methods: ATYR1923 was expressed in E. coli and purified to homogeneity in a GMP-compliant facility. We employed a minor histocompatibility antigen-mismatched model which has been reported to mimic the pathological symptoms of human scl cGvHD. Briefly, bone marrow and splenocytes from B10.D2 mice were transplanted into whole body irradiated recipient Balb/c mice. Treatment with ATYR1923 (0.4 mg/kg, intravenously once a week) was compared with nintedanib, (60 mg/kg, orally daily), with administration beginning at Day 7 (early intervention) or at Day 21 (late intervention). Scheduled euthanasia was conducted 8 weeks after transplantation to collect lungs and skin for histological evaluation and collagen content. Results: As expected, nintedanib decreased lung and skin fibrosis in murine scl cGvHD, qualifying the data obtained in this experiment. ATYR1923 at 0.4 mg/kg weekly beginning on Day 7 in murine scl cGvHD exerted therapeutic activity in both skin and lung as revealed by significantly decreased dermal thickness in the skin and histological fibrosis (Ashcroft score) in the lungs in comparison to untreated controls. The number of myofibroblasts and hydroxyproline (i.e., collagen) content was also significantly reduced in both organs. Observed effects with weekly dosing of ATYR1923 were similar to those observed with daily dosing of nintedanib. Late intervention with ATYR1923 was not significantly effective with this dosing paradigm. Conclusions: ATYR1923 is efficacious in a murine model of scl cGvHD when administered weekly at 0.4 mg/kg. ATYR1923 had robust activity when treatment was commenced early in the model and no significant activity when intervention commenced late. These observations are compatible with our hypothesis that the iMod domain’s primary effect is via modulation of immune response rather than fibrotic pathways. Based on the pre-clinical data, including in vitro, in vivo and toxicological experiments, clinical testing of ATYR1923 is ongoing. Sclerodermatous cGvHD Model Lung Skin 700cGy Balb/c recipient B10.D2 donor Bone marrow & splenocytes iMod domain Fc domain of an antibody nintedanib ATYR1923 nintedanib ATYR1923 0 56 Baseline Control Vehicle Control Early Intervention Late Intervention Syngeneic Control 21 7 Time (days) ATYR1923 administered once weekly at 0.4 mg/kg intravenously Nintedanib administered once daily at 60 mg/kg orally Skin and lung tissues collected ATYR1923 Mouse Bleomycin Rat Bleomycin Oral vehicle: 1x PBS, BID D8-D21 Intravenous (IV) vehicle: 50 mM L-His, 140 mM NaCl, QD D8-D21 Pirfenidone: 200 mg/kg PO BID D8-D21 Dexamethasone (Dex): 0.25 mg/kg D0-D21 ATYR1940: 3 mg/kg IV QD D8-D21 ATYR1923: 0.4 mg/kg IV QW D8, D15 ***p< 0.001 vs respective vehicle Oral vehicle: 1x PBS, BID D8-D21 Intravenous (IV) vehicle: 20 mM L-His, 150 mM NaCl, QD D8-D21 Nintedanib: 60 mg/kg PO D8-D21 ATYR1923: 1 or 3 mg/kg QW Early intervention D2, D9, D16; Late intervention D9, D16 *p< 0.05 vs IV vehicle Data were analyzed by Kruskal-Wallis non-parametric ANOVA followed by Dunn’s multiple comparison test to allogenic vehicle-treated controls terminated at 8 weeks. *p < 0.05, **p < 0.01, ***p<0.001, ****p<0.0001. Adapted from Manetti et al, 2010; Schroeder and DiPersio, 2011 Conclusions ATYR1923 is efficacious in a murine model of sclerodermatous chronic GvHD Weekly administration at only 0.4 mg/kg ATYR1923 has robust activity, comparable to nintedanib when treatment initiated at Day 7. ATYR1923 activity did not reach significance at 0.4 mg/kg weekly when treatment was initiated at Day 21. Data are compatible with our hypothesis that ATYR1923 modulates immune responses and inflammation following tissue injury. Based on the pre-clinical data, including in vitro, in vivo and toxicological experiments, a clinical trial with ATYR1923 for treatment of interstitial lung disease is planned to initiate later this year. Experimental Protocol Donor APC Donor T cells T and B cell Activation Antigens T and B cell Proliferation Autoantibodies Production Trafficking Tissue Injury Inflammation, Fibrosis Direct Cell Killing Host cell Donor B cell Skin, lung, liver, etc. Macrophage cytokines TGFβ Fibroblasts Collagen (fibrosis) Exhibit 99.2
