Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Adicet Bio, Inc. | d549570dex991.htm |
| 8-K - FORM 8-K - Adicet Bio, Inc. | d549570d8k.htm |

July 25, 2018 RTB101 Phase 2b Topline Data Exhibit 99.2

This presentation may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding the safety, efficacy and regulatory and clinical progress of our product candidates, including RTB101 alone and in combination with everolimus. All such forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our clinical results and other future conditions. All statements other than statements of historical facts contained in this presentation, including statements regarding future results of operations and financial position, business strategy, current and prospective product candidates, planned clinical trials and preclinical activities, including the initiation, timing, progress and results of our preclinical and clinical studies and our research and development programs, product approvals, research and development costs, current and prospective collaborations, timing and likelihood of success, including our ability to advance RTB101 alone and in combination with everolimus into, and successfully complete, clinical studies, and the timing or likelihood of regulatory filings and approvals, expectations regarding market acceptance and size, plans for launch and commercialization, plans and objectives of management for future operations, and future results of anticipated product candidates, are forward-looking statements. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. These statements are also subject to a number of material risks and uncertainties that are discussed in the section entitled "Risk Factors" in resTORbio’s annual report on Form 10-K for the fiscal year ended December 31, 2017, as well as discussions of potential risks, uncertainties, and other important factors in resTORbio's subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. Neither we, nor our affiliates, advisors, or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified, and we make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. Forward-Looking Statements
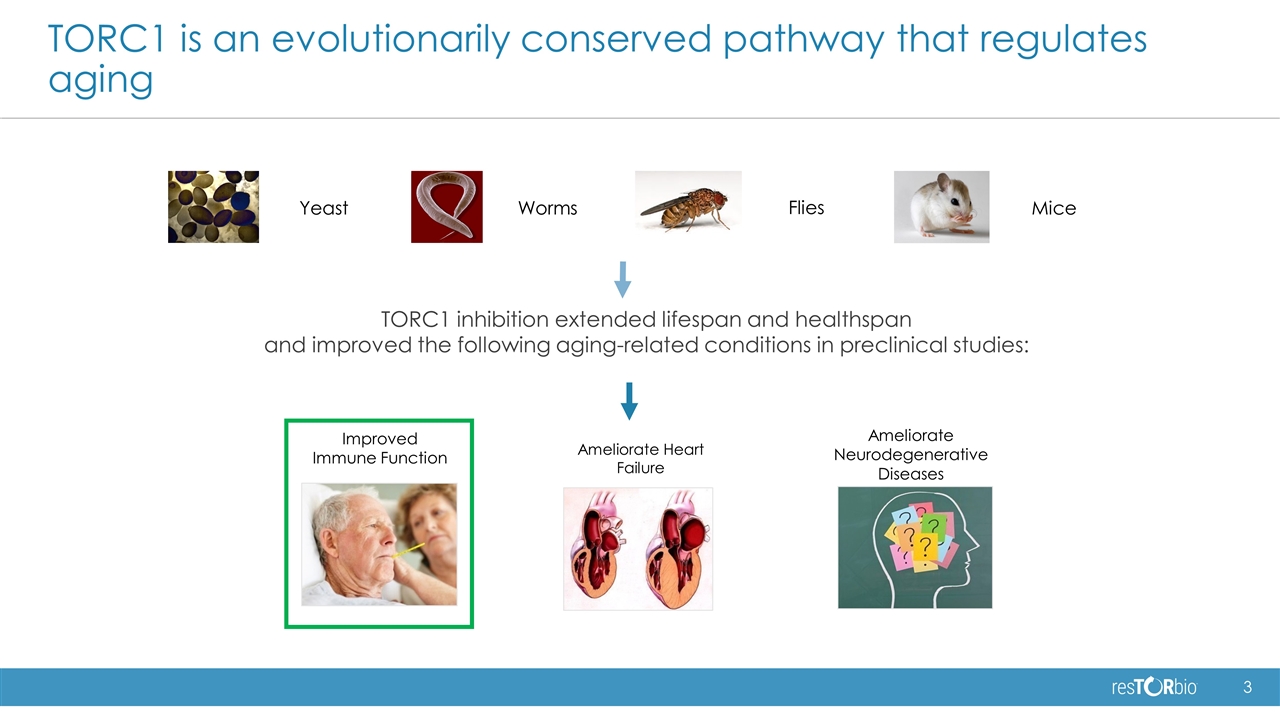
TORC1 is an evolutionarily conserved pathway that regulates aging Improved Immune Function Ameliorate Heart Failure Ameliorate Neurodegenerative Diseases TORC1 inhibition extended lifespan and healthspan and improved the following aging-related conditions in preclinical studies: Mice Flies Worms Yeast
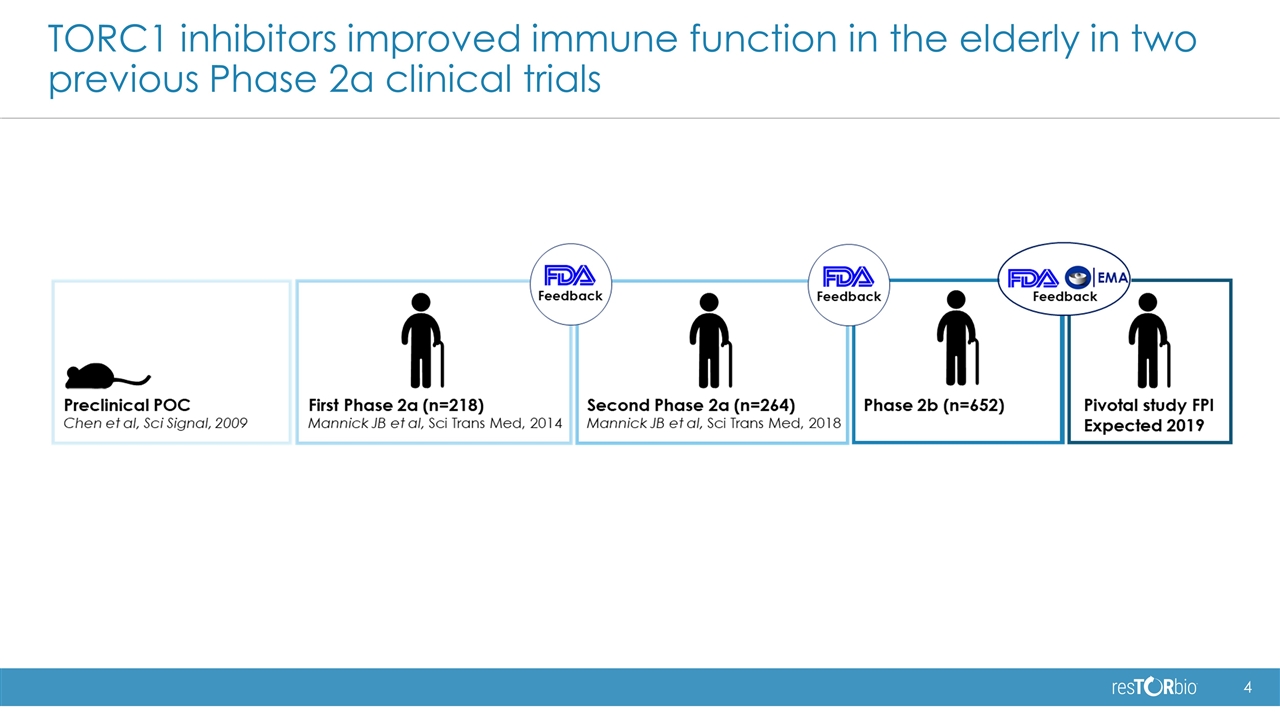
TORC1 inhibitors improved immune function in the elderly in two previous Phase 2a clinical trials
Positive topline results in Phase 2b trial of RTB101 Statistically significant and clinically meaningful 30.6% reduction in the percentage of patients with one or more laboratory-confirmed respiratory tract infections (RTIs), the primary endpoint of the trial, in the RTB101 10 mg once daily cohort (p=0.026) Greatest reductions were observed in pre-specified analyses of asthma patients 65 years and older (68.4% reduction, p=0.0002) and in patients 85 years and older (66.7% reduction, p=0.007) Successfully defined the dose and patient populations for pivotal trials: Dose: RTB101 10 mg once daily Patient population: 65 years or older with comorbidities, or 85 years and older RTB101 10 mg once daily was well-tolerated in the high-risk elderly patients enrolled in the study Plan to meet with regulatory authorities to discuss the design of our pivotal studies that we expect to initiate in 2019
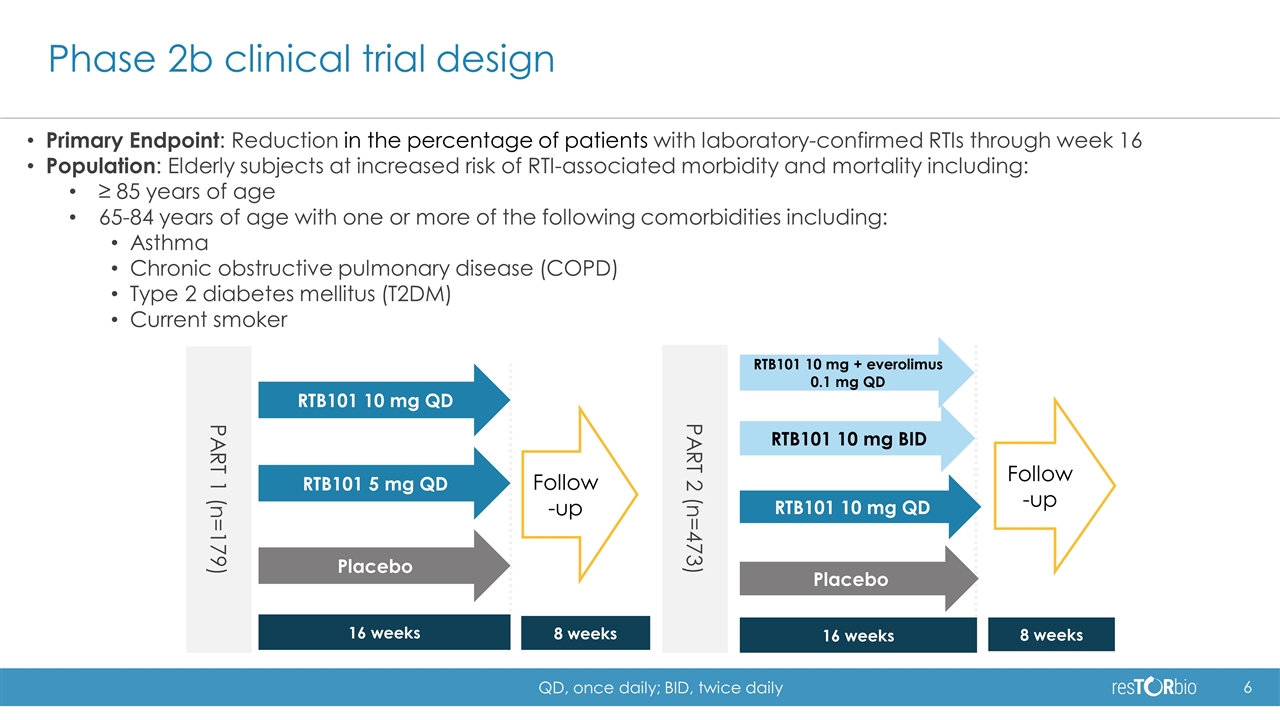
Phase 2b clinical trial design Primary Endpoint: Reduction in the percentage of patients with laboratory-confirmed RTIs through week 16 Population: Elderly subjects at increased risk of RTI-associated morbidity and mortality including: ≥ 85 years of age 65-84 years of age with one or more of the following comorbidities including: Asthma Chronic obstructive pulmonary disease (COPD) Type 2 diabetes mellitus (T2DM) Current smoker 16 weeks 8 weeks RTB101 5 mg QD RTB101 10 mg QD Placebo Follow-up PART 2 (n=473) RTB101 10 mg + everolimus 0.1 mg QD RTB101 10 mg QD Placebo 16 weeks 8 weeks Follow-up RTB101 10 mg BID PART 1 (n=179) QD, once daily; BID, twice daily
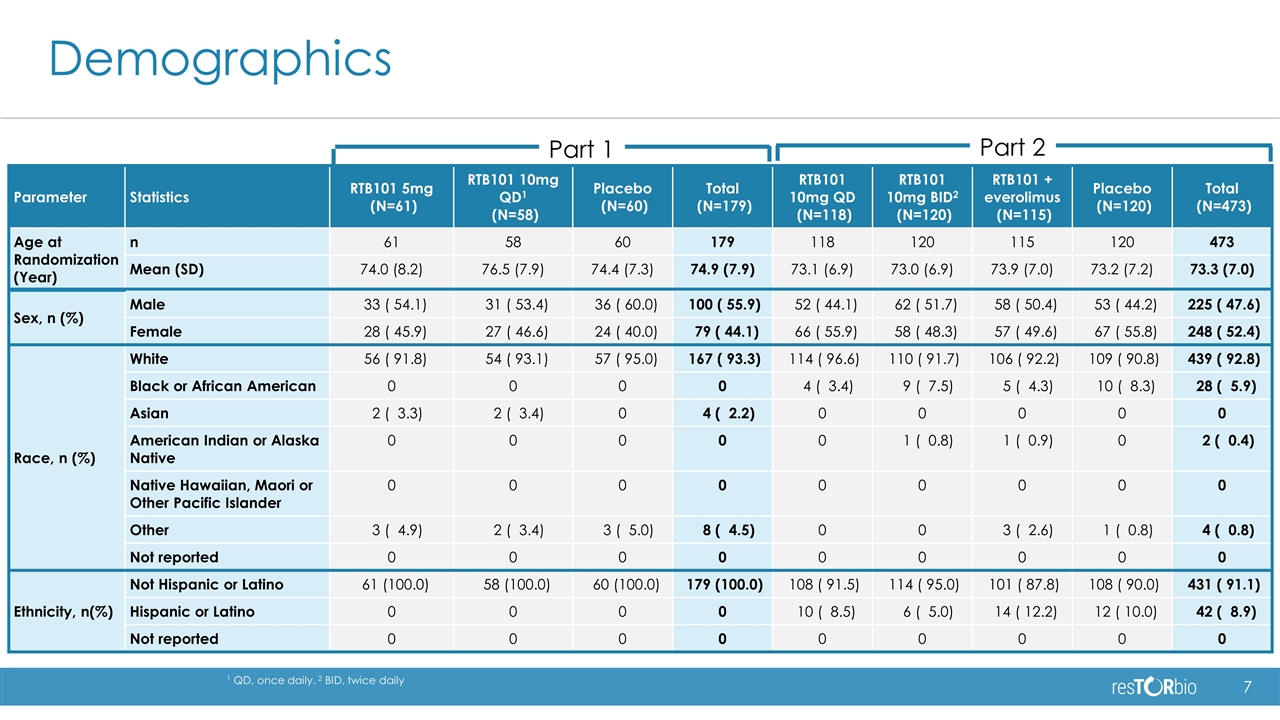
Demographics Part 1 Part 2 Parameter Statistics RTB101 5mg (N=61) RTB101 10mg QD1 (N=58) Placebo (N=60) Total (N=179) RTB101 10mg QD (N=118) RTB101 10mg BID2 (N=120) RTB101 + everolimus (N=115) Placebo (N=120) Total (N=473) Age at Randomization (Year) n 61 58 60 179 118 120 115 120 473 Mean (SD) 74.0 (8.2) 76.5 (7.9) 74.4 (7.3) 74.9 (7.9) 73.1 (6.9) 73.0 (6.9) 73.9 (7.0) 73.2 (7.2) 73.3 (7.0) Sex, n (%) Male 33 ( 54.1) 31 ( 53.4) 36 ( 60.0) 100 ( 55.9) 52 ( 44.1) 62 ( 51.7) 58 ( 50.4) 53 ( 44.2) 225 ( 47.6) Female 28 ( 45.9) 27 ( 46.6) 24 ( 40.0) 79 ( 44.1) 66 ( 55.9) 58 ( 48.3) 57 ( 49.6) 67 ( 55.8) 248 ( 52.4) Race, n (%) White 56 ( 91.8) 54 ( 93.1) 57 ( 95.0) 167 ( 93.3) 114 ( 96.6) 110 ( 91.7) 106 ( 92.2) 109 ( 90.8) 439 ( 92.8) Black or African American 0 0 0 0 4 ( 3.4) 9 ( 7.5) 5 ( 4.3) 10 ( 8.3) 28 ( 5.9) Asian 2 ( 3.3) 2 ( 3.4) 0 4 ( 2.2) 0 0 0 0 0 American Indian or Alaska Native 0 0 0 0 0 1 ( 0.8) 1 ( 0.9) 0 2 ( 0.4) Native Hawaiian, Maori or Other Pacific Islander 0 0 0 0 0 0 0 0 0 Other 3 ( 4.9) 2 ( 3.4) 3 ( 5.0) 8 ( 4.5) 0 0 3 ( 2.6) 1 ( 0.8) 4 ( 0.8) Not reported 0 0 0 0 0 0 0 0 0 Ethnicity, n(%) Not Hispanic or Latino 61 (100.0) 58 (100.0) 60 (100.0) 179 (100.0) 108 ( 91.5) 114 ( 95.0) 101 ( 87.8) 108 ( 90.0) 431 ( 91.1) Hispanic or Latino 0 0 0 0 10 ( 8.5) 6 ( 5.0) 14 ( 12.2) 12 ( 10.0) 42 ( 8.9) Not reported 0 0 0 0 0 0 0 0 0 1 QD, once daily. 2 BID, twice daily
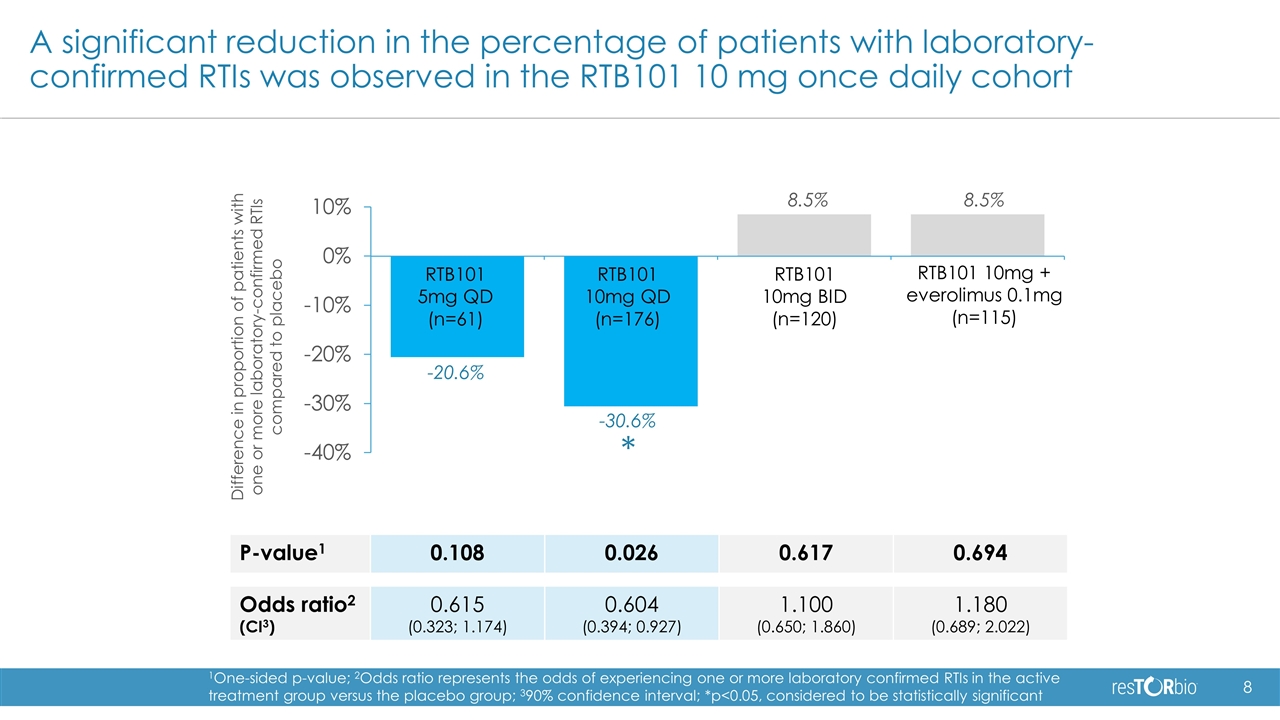
A significant reduction in the percentage of patients with laboratory-confirmed RTIs was observed in the RTB101 10 mg once daily cohort -20.6% 8.5% 8.5% -30.6% RTB101 5mg QD (n=61) RTB101 10mg QD (n=176) RTB101 10mg BID (n=120) RTB101 10mg + everolimus 0.1mg (n=115) P-value1 0.108 0.026 0.617 0.694 Odds ratio2 (CI3) 0.615 (0.323; 1.174) 0.604 (0.394; 0.927) 1.100 (0.650; 1.860) 1.180 (0.689; 2.022) 1One-sided p-value; 2Odds ratio represents the odds of experiencing one or more laboratory confirmed RTIs in the active treatment group versus the placebo group; 390% confidence interval; *p<0.05, considered to be statistically significant * Difference in proportion of patients with one or more laboratory-confirmed RTIs compared to placebo
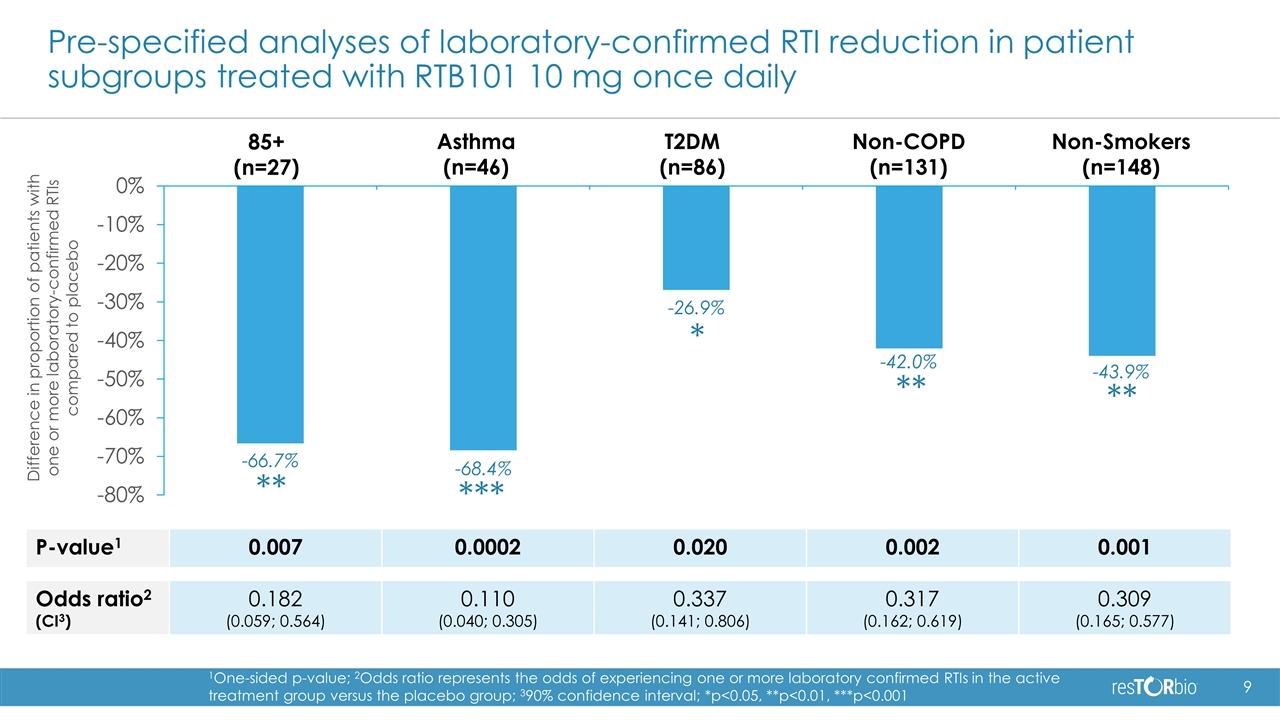
Pre-specified analyses of laboratory-confirmed RTI reduction in patient subgroups treated with RTB101 10 mg once daily 85+ (n=27) Asthma (n=46) T2DM (n=86) Non-COPD (n=131) P-value1 0.007 0.0002 0.020 0.002 0.001 Odds ratio2 (CI3) 0.182 (0.059; 0.564) 0.110 (0.040; 0.305) 0.337 (0.141; 0.806) 0.317 (0.162; 0.619) 0.309 (0.165; 0.577) Non-Smokers (n=148) 1One-sided p-value; 2Odds ratio represents the odds of experiencing one or more laboratory confirmed RTIs in the active treatment group versus the placebo group; 390% confidence interval; *p<0.05, **p<0.01, ***p<0.001 ** *** ** * ** -66.7% -68.4% -26.9% -43.9% -42.0% Difference in proportion of patients with one or more laboratory-confirmed RTIs compared to placebo
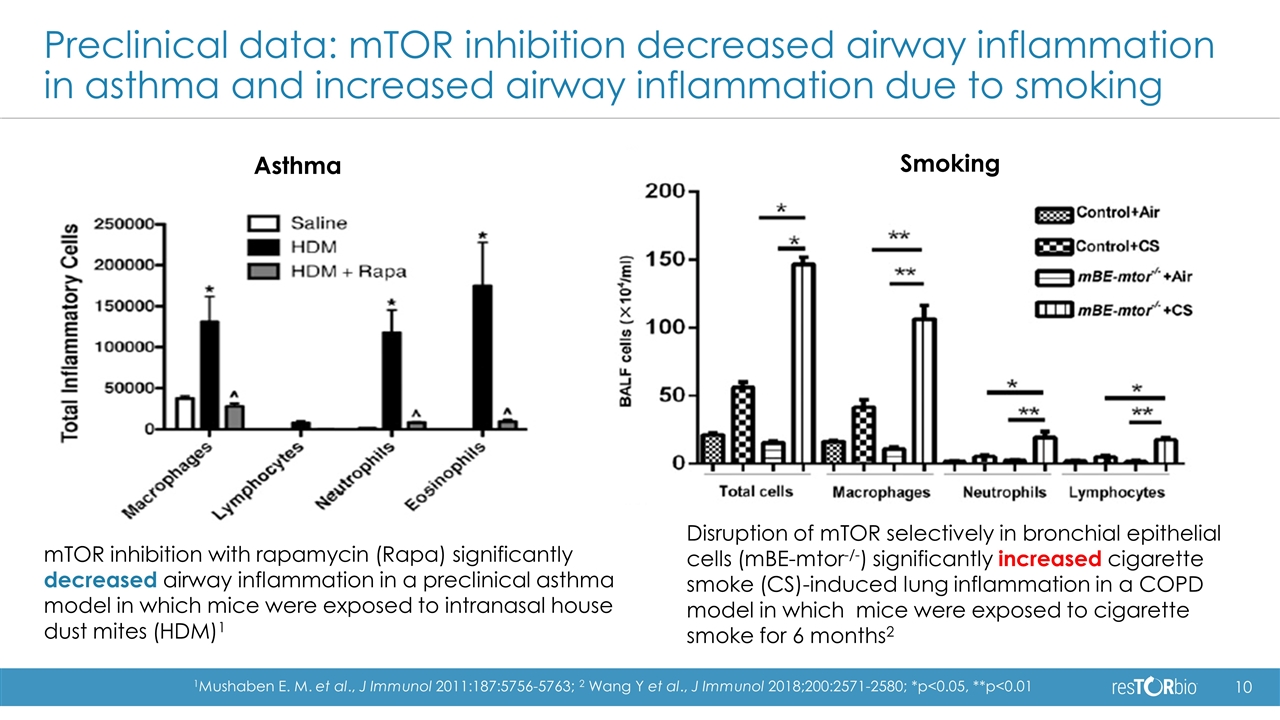
Preclinical data: mTOR inhibition decreased airway inflammation in asthma and increased airway inflammation due to smoking Asthma Smoking mTOR inhibition with rapamycin (Rapa) significantly decreased airway inflammation in a preclinical asthma model in which mice were exposed to intranasal house dust mites (HDM)1 Disruption of mTOR selectively in bronchial epithelial cells (mBE-mtor-/-) significantly increased cigarette smoke (CS)-induced lung inflammation in a COPD model in which mice were exposed to cigarette smoke for 6 months2 1Mushaben E. M. et al., J Immunol 2011:187:5756-5763; 2 Wang Y et al., J Immunol 2018;200:2571-2580; *p<0.05, **p<0.01
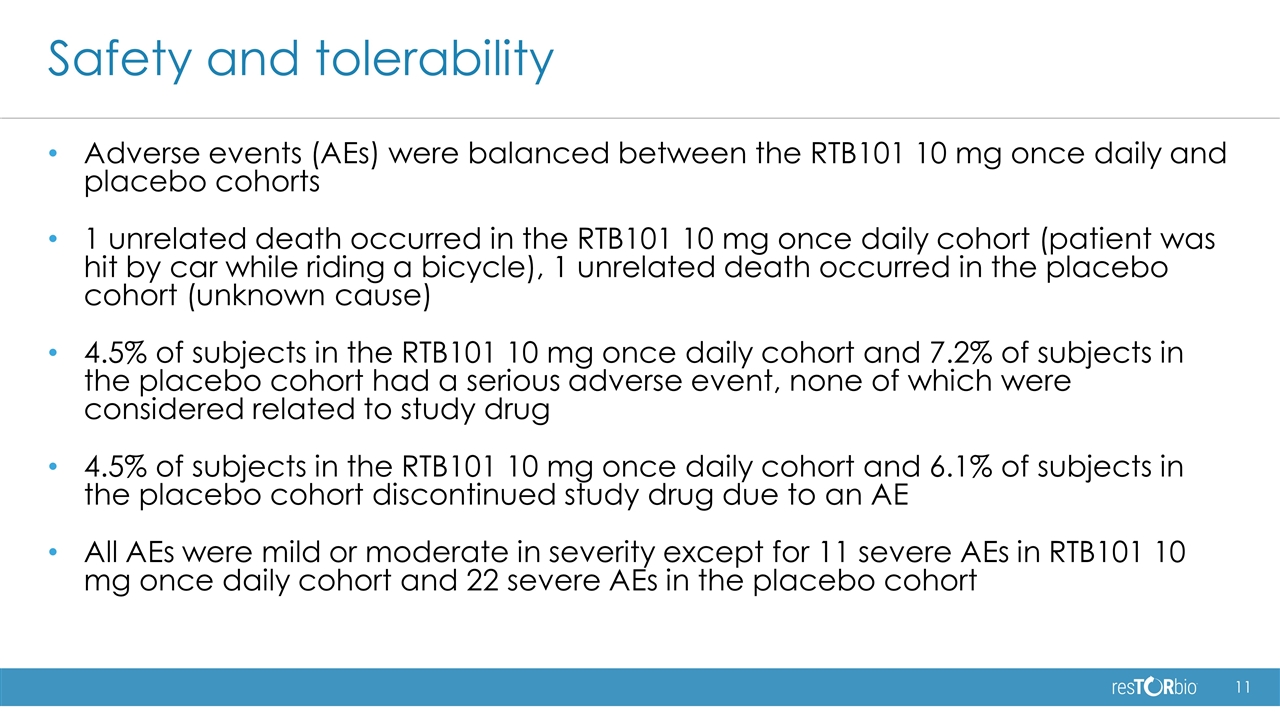
Safety and tolerability Adverse events (AEs) were balanced between the RTB101 10 mg once daily and placebo cohorts 1 unrelated death occurred in the RTB101 10 mg once daily cohort (patient was hit by car while riding a bicycle), 1 unrelated death occurred in the placebo cohort (unknown cause) 4.5% of subjects in the RTB101 10 mg once daily cohort and 7.2% of subjects in the placebo cohort had a serious adverse event, none of which were considered related to study drug 4.5% of subjects in the RTB101 10 mg once daily cohort and 6.1% of subjects in the placebo cohort discontinued study drug due to an AE All AEs were mild or moderate in severity except for 11 severe AEs in RTB101 10 mg once daily cohort and 22 severe AEs in the placebo cohort
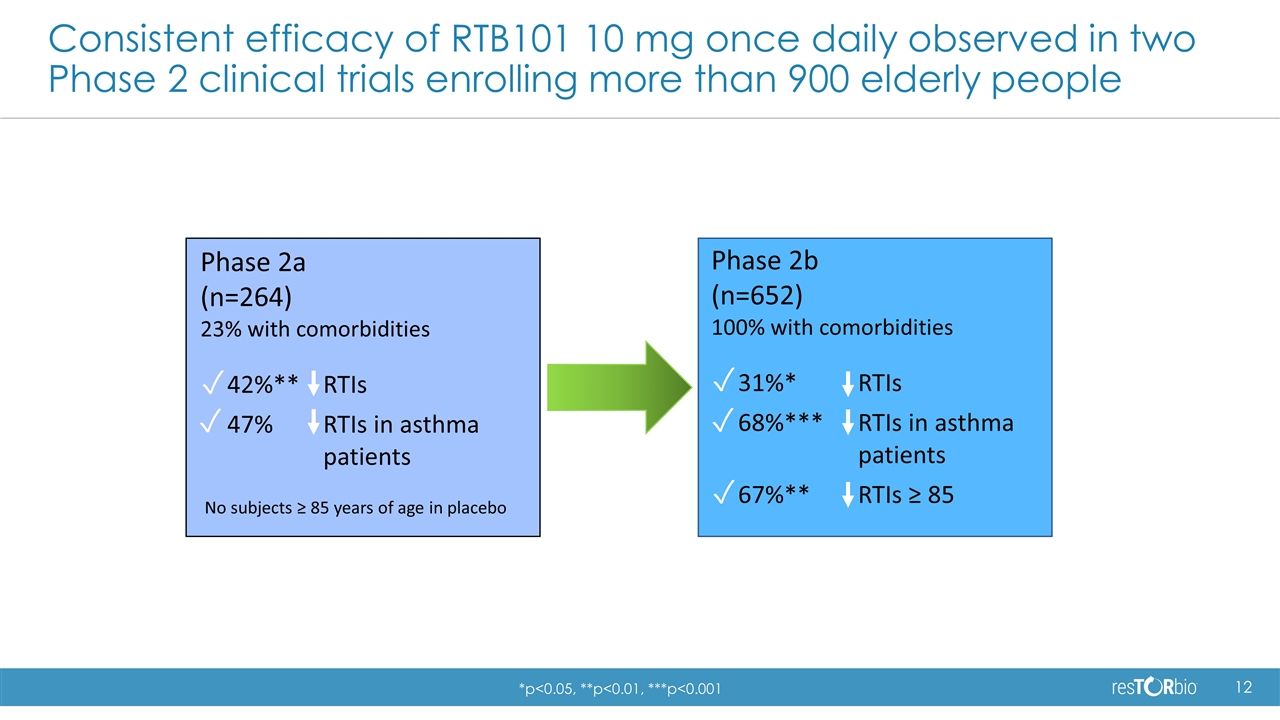
Consistent efficacy of RTB101 10 mg once daily observed in two Phase 2 clinical trials enrolling more than 900 elderly people Phase 2a (n=264) 23% with comorbidities 42%**RTIs 47%RTIs in asthma patients No subjects ≥ 85 years of age in placebo ✓ ✓ Phase 2b (n=652) 100% with comorbidities 31%* RTIs 68%*** RTIs in asthma patients 67%** RTIs ≥ 85 ✓ ✓ ✓ *p<0.05, **p<0.01, ***p<0.001
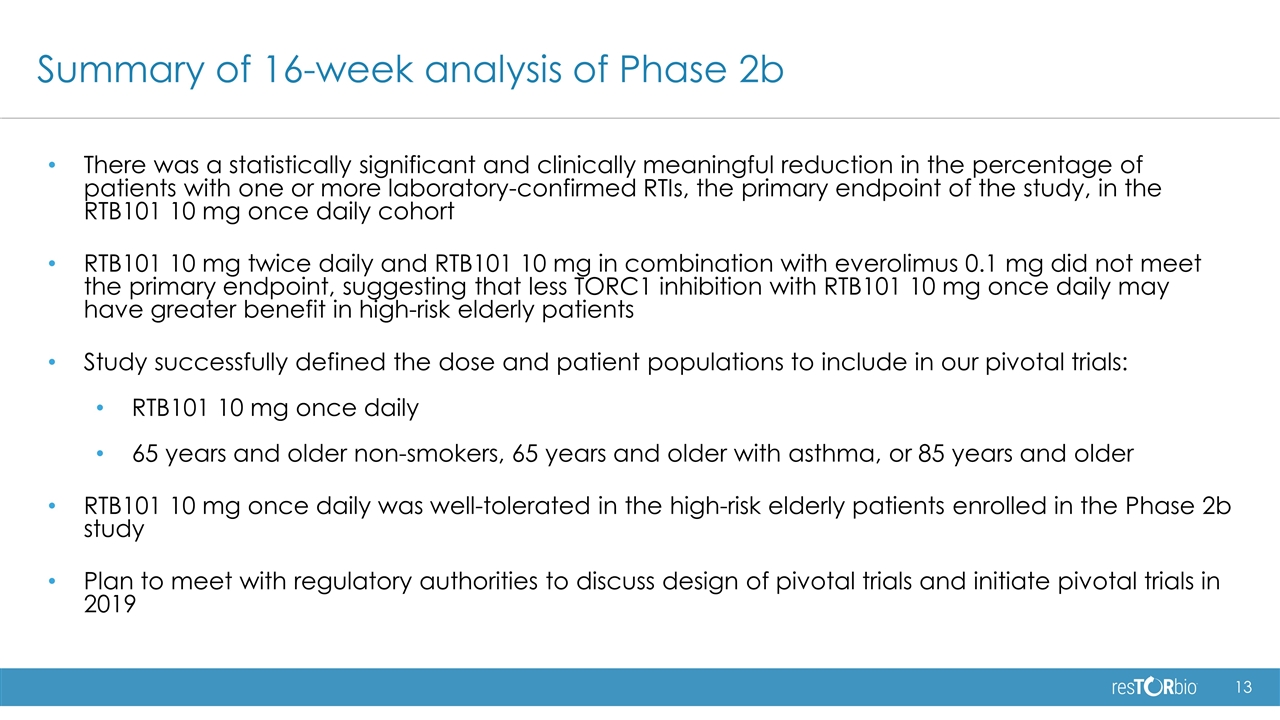
Summary of 16-week analysis of Phase 2b There was a statistically significant and clinically meaningful reduction in the percentage of patients with one or more laboratory-confirmed RTIs, the primary endpoint of the study, in the RTB101 10 mg once daily cohort RTB101 10 mg twice daily and RTB101 10 mg in combination with everolimus 0.1 mg did not meet the primary endpoint, suggesting that less TORC1 inhibition with RTB101 10 mg once daily may have greater benefit in high-risk elderly patients Study successfully defined the dose and patient populations to include in our pivotal trials: RTB101 10 mg once daily 65 years and older non-smokers, 65 years and older with asthma, or 85 years and older RTB101 10 mg once daily was well-tolerated in the high-risk elderly patients enrolled in the Phase 2b study Plan to meet with regulatory authorities to discuss design of pivotal trials and initiate pivotal trials in 2019

Medical Need & Market Opportunity
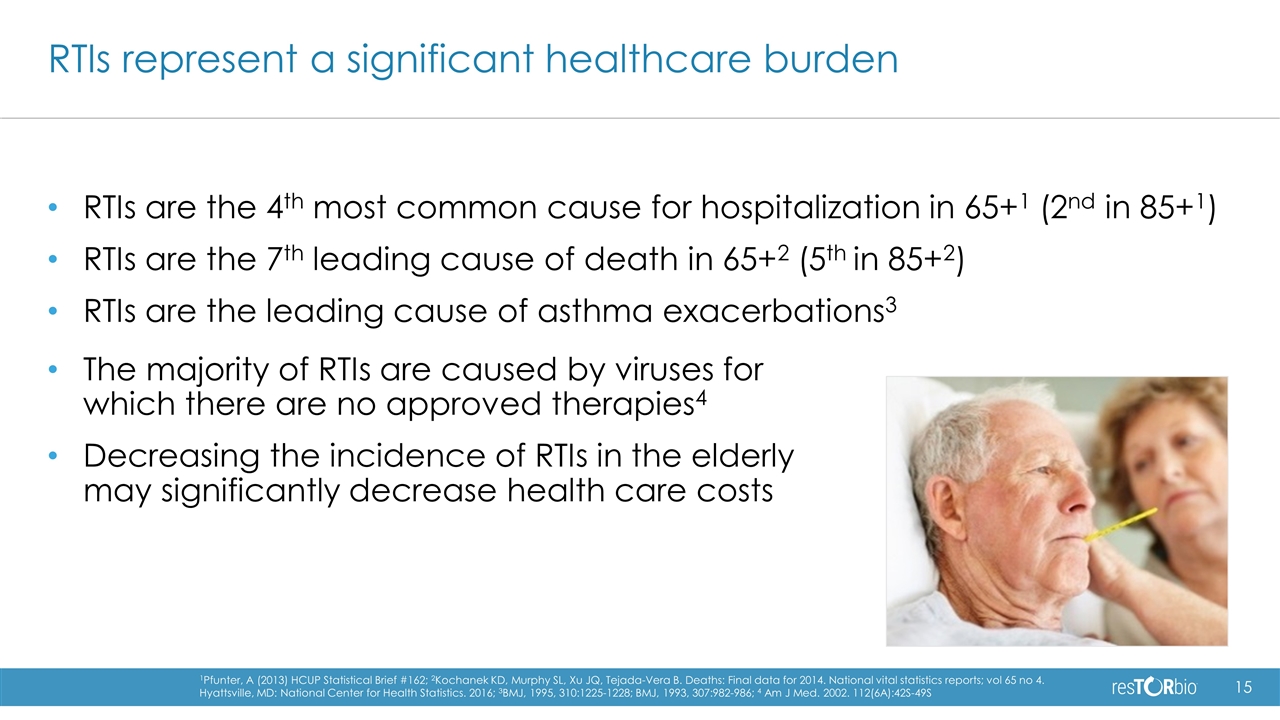
RTIs are the 4th most common cause for hospitalization in 65+1 (2nd in 85+1) RTIs are the 7th leading cause of death in 65+2 (5th in 85+2) RTIs are the leading cause of asthma exacerbations3 RTIs represent a significant healthcare burden 1Pfunter, A (2013) HCUP Statistical Brief #162; 2Kochanek KD, Murphy SL, Xu JQ, Tejada-Vera B. Deaths: Final data for 2014. National vital statistics reports; vol 65 no 4. Hyattsville, MD: National Center for Health Statistics. 2016; 3BMJ, 1995, 310:1225-1228; BMJ, 1993, 307:982-986; 4 Am J Med. 2002. 112(6A):42S-49S The majority of RTIs are caused by viruses for which there are no approved therapies4 Decreasing the incidence of RTIs in the elderly may significantly decrease health care costs
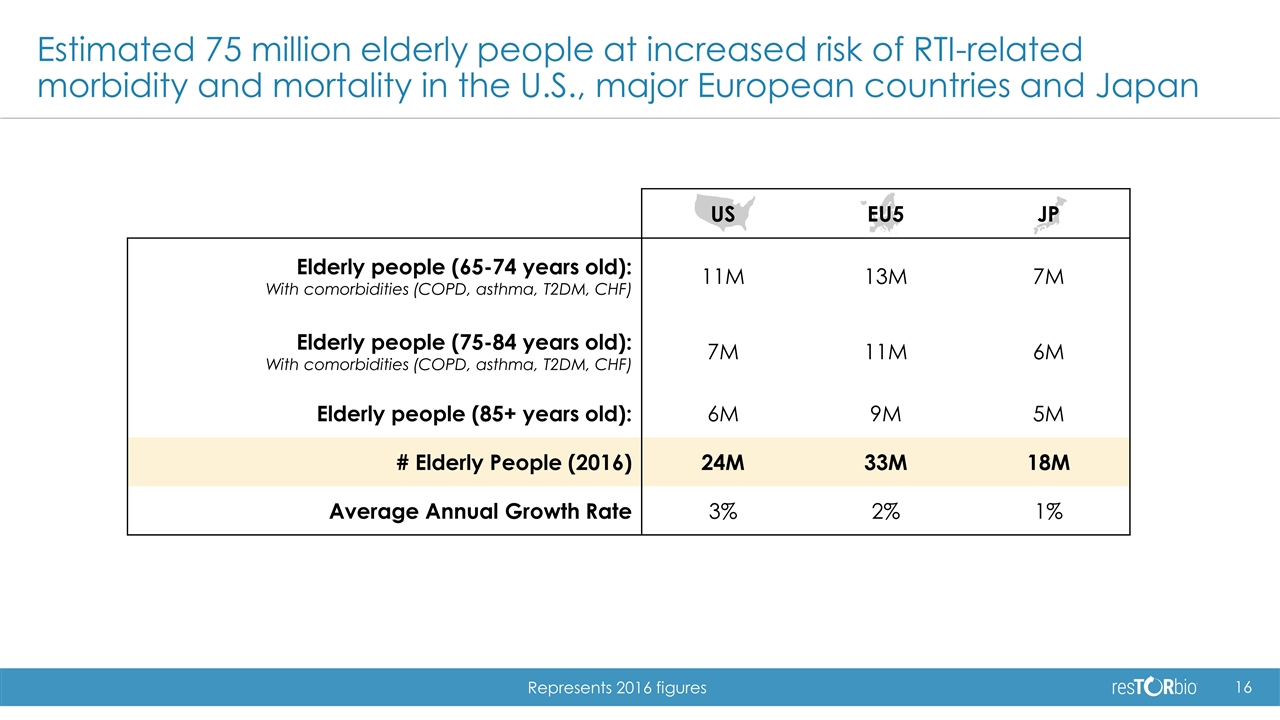
US EU5 JP Elderly people (65-74 years old): With comorbidities (COPD, asthma, T2DM, CHF) 11M 13M 7M Elderly people (75-84 years old): With comorbidities (COPD, asthma, T2DM, CHF) 7M 11M 6M Elderly people (85+ years old): 6M 9M 5M # Elderly People (2016) 24M 33M 18M Average Annual Growth Rate 3% 2% 1% Estimated 75 million elderly people at increased risk of RTI-related morbidity and mortality in the U.S., major European countries and Japan Represents 2016 figures
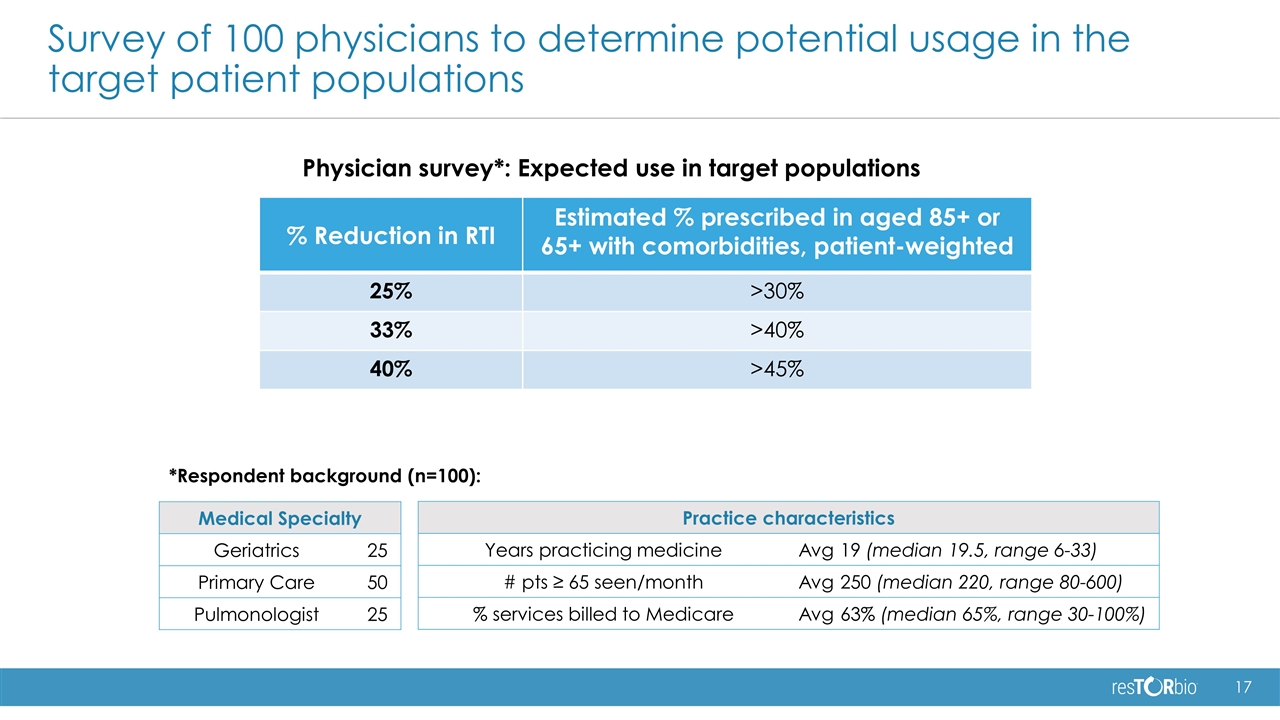
% Reduction in RTI Estimated % prescribed in aged 85+ or 65+ with comorbidities, patient-weighted 25% >30% 33% >40% 40% >45% Survey of 100 physicians to determine potential usage in the target patient populations Medical Specialty Geriatrics 25 Primary Care 50 Pulmonologist 25 Practice characteristics Years practicing medicine Avg 19 (median 19.5, range 6-33) # pts ≥ 65 seen/month Avg 250 (median 220, range 80-600) % services billed to Medicare Avg 63% (median 65%, range 30-100%) *Respondent background (n=100): Physician survey*: Expected use in target populations
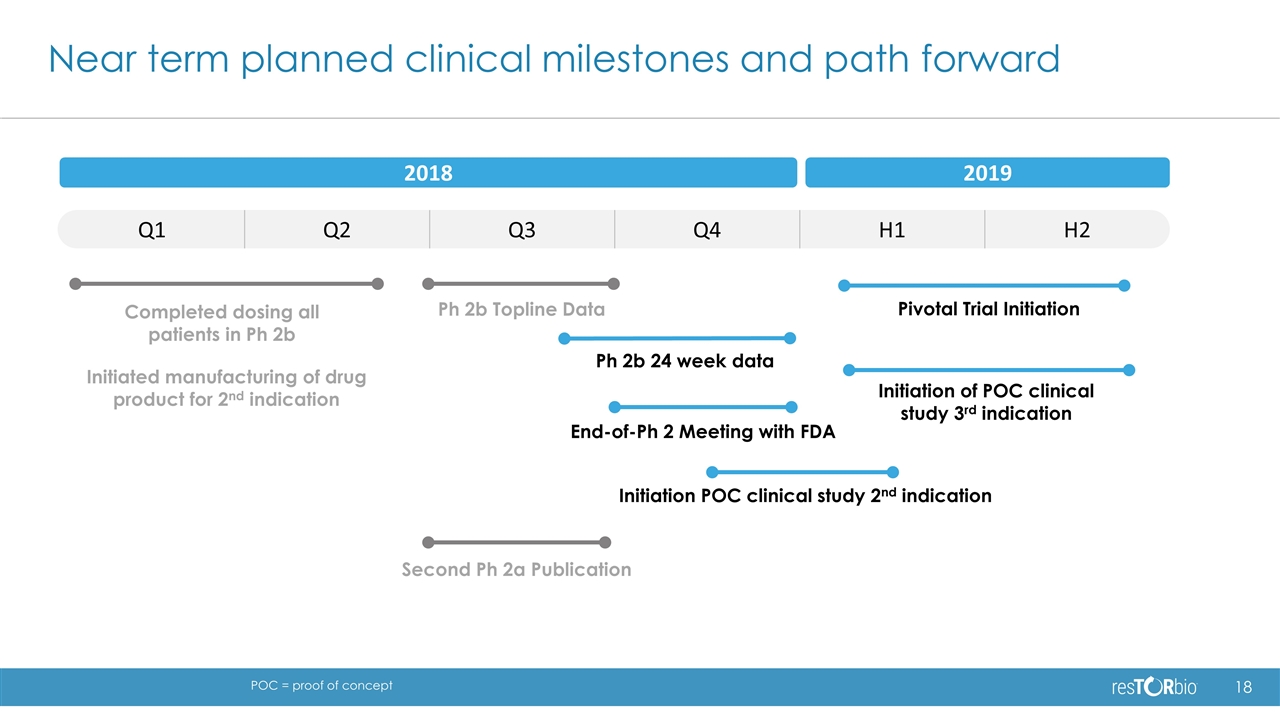
Q1 Q2 Q3 Q4 H1 H2 Near term planned clinical milestones and path forward Ph 2b Topline Data Initiation POC clinical study 2nd indication Initiation of POC clinical study 3rd indication Pivotal Trial Initiation 2018 2019 Second Ph 2a Publication Ph 2b 24 week data Completed dosing all patients in Ph 2b Initiated manufacturing of drug product for 2nd indication End-of-Ph 2 Meeting with FDA POC = proof of concept

July 2018 RTB101 Phase 2b Data Review
