Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Sio Gene Therapies Inc. | a18-16532_1ex99d1.htm |
| 8-K - 8-K - Sio Gene Therapies Inc. | a18-16532_18k.htm |
Building a leader in innovative neurological therapies Conference Call to Discuss Global Licensing Agreement for AXO-AAV-OPMD and Broader Platform Collaboration with Benitec Biopharma June 9, 2018

FORWARD LOOKING STATEMENTS Statements made in this presentation contain forward-looking statements, including statements regarding Axovant’s plans to advance the development of its AXO-AAV-OPMD program and expand its pipeline with additional gene therapy products, Axovant’s expectations about timing of the results for its Phase 1/2 clinical study for AXO-Lenti-PD in Parkinson’s disease and Phase 2 clinical study of REM Sleep Behavior Disorder in LBD, Axovant’s license arrangements with Benitec and Oxford BioMedica, and other elements of Axovant’s clinical development and regulatory strategy. Forward-looking statements can be identified by the words “believe,” “anticipate,” “continue,” “estimate,” “project,” “expect,” “plan,” “potential,” “intend,” “will,” “would,” “could,” “should” or the negative or plural of these words or other similar expressions that are predictions or indicate future events, trends or prospects. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially and reported results should not be considered as an indication of future performance. These risks and uncertainties include, but are not limited to: risks associated with the success, cost and timing of our product development activities and clinical trials; the approval and commercialization of Axovant’s product candidates, including AXO-AAV-OPMD and AXO-Lenti-PD; the ability to obtain issued patents; identify and in-license or acquire third party patents; and increased regulatory requirements. These statements are also subject to the risk that clinical trial data are subject to differing interpretations, and regulatory agencies, medical and scientific experts and others may not share Axovant’s views of the clinical study data. In addition, promising interim results or other preliminary analyses do not in any way ensure that later or final results in a clinical trial or in related or similar clinical trials will replicate those interim results. The product candidates discussed are investigational and not approved and there can be no assurance that Axovant’s clinical programs including AXO-AAV-OPMD and AXO-Lenti-PD programs will be successful in demonstrating safety and/or efficacy, that Axovant will not encounter problems or delays in clinical development, or that any of Axovant’s product candidates will ever receive regulatory approval or be successfully commercialized. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Axovant’s business in general, see the “Risk Factors” section of Axovant’s annual report on Form 10-K filed with the Securities and Exchange Commission on June 11, 2018, and other filings that Axovant makes with the SEC from time to time. These forward-looking statements are based on information available to Axovant as of the date of this presentation and speak only as of the date of this presentation. Axovant disclaims any obligation to update these forward-looking statements, except as may be required by law.

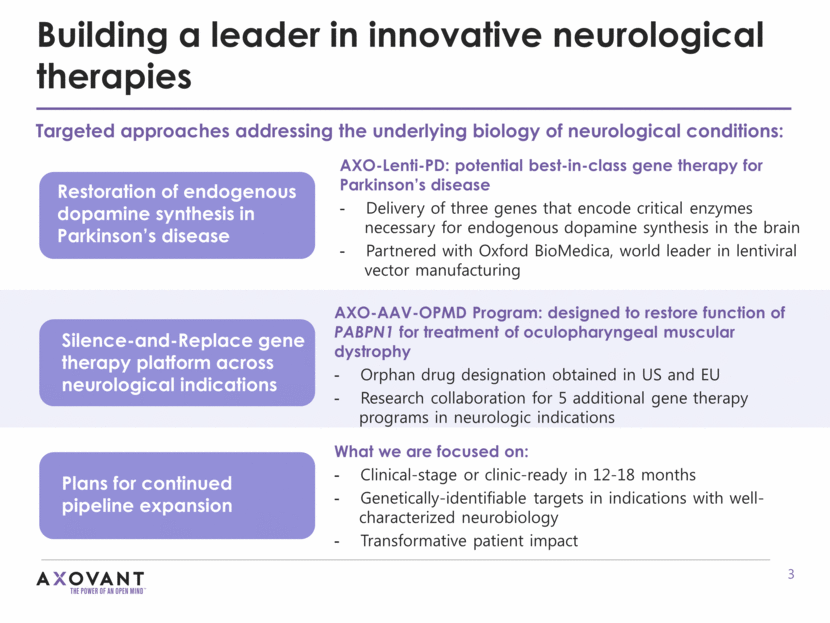
Building a leader in innovative neurological therapies Targeted approaches addressing the underlying biology of neurological conditions: Silence-and-Replace gene therapy platform across neurological indications Restoration of endogenous dopamine synthesis in Parkinson’s disease AXO-Lenti-PD: potential best-in-class gene therapy for Parkinson’s disease Delivery of three genes that encode critical enzymes necessary for endogenous dopamine synthesis in the brain Partnered with Oxford BioMedica, world leader in lentiviral vector manufacturing Plans for continued pipeline expansion AXO-AAV-OPMD Program: designed to restore function of PABPN1 for treatment of oculopharyngeal muscular dystrophy Orphan drug designation obtained in US and EU Research collaboration for 5 additional gene therapy programs in neurologic indications What we are focused on: Clinical-stage or clinic-ready in 12-18 months Genetically-identifiable targets in indications with well-characterized neurobiology Transformative patient impact
Global license for AXO-AAV-OPMD Program and broader platform collaboration Axovant granted worldwide rights to AXO-AAV-OPMD program and 5 investigational gene therapy products in neurological conditions Plan to initiate a placebo-controlled clinical study of AXO-AAV-OPMD gene therapy in oculopharyngeal muscular dystrophy (OPMD) patients in 2019 First additional gene therapy product will focus on C9orf72 gene associated with amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) Unique Silence-and-Replace approach is designed to correct the underlying genetic defect causing OPMD Delivers a combination of DNA-directed RNA interference (Silence) along with a functional copy of gene (Replace) to produce a long-term restoration of normal gene function One-time administration using single adeno-associated virus (AAV) vector Axovant to pay $10M upfront; additional payments tied to development, regulatory, and commercial sales milestones on all programs Benitec to receive 30% of net profits on worldwide sales of AXO-AAV-OPMD Program and tiered royalties on any additional products from research collaboration

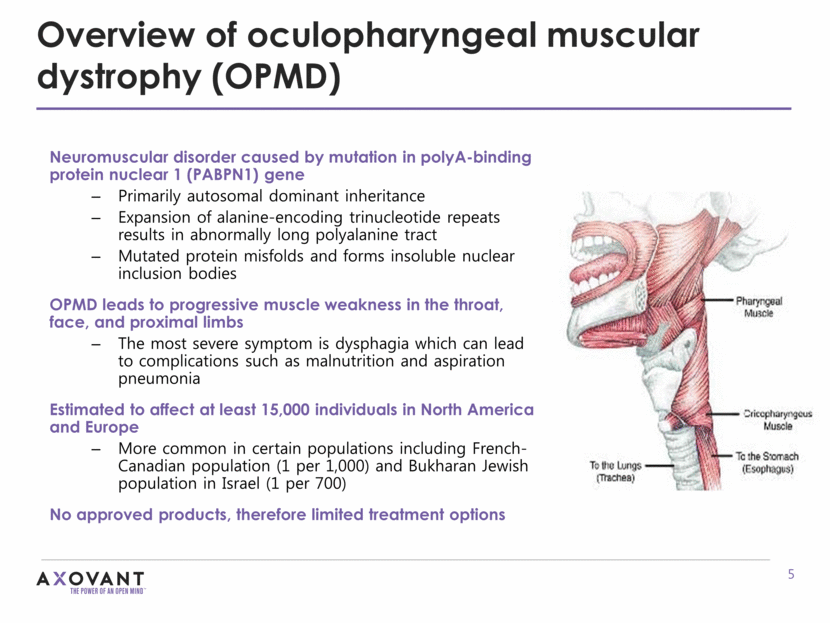
Overview of oculopharyngeal muscular dystrophy (OPMD) Neuromuscular disorder caused by mutation in polyA-binding protein nuclear 1 (PABPN1) gene Primarily autosomal dominant inheritance Expansion of alanine-encoding trinucleotide repeats results in abnormally long polyalanine tract Mutated protein misfolds and forms insoluble nuclear inclusion bodies OPMD leads to progressive muscle weakness in the throat, face, and proximal limbs The most severe symptom is dysphagia which can lead to complications such as malnutrition and aspiration pneumonia Estimated to affect at least 15,000 individuals in North America and Europe More common in certain populations including French-Canadian population (1 per 1,000) and Bukharan Jewish population in Israel (1 per 700) No approved products, therefore limited treatment options
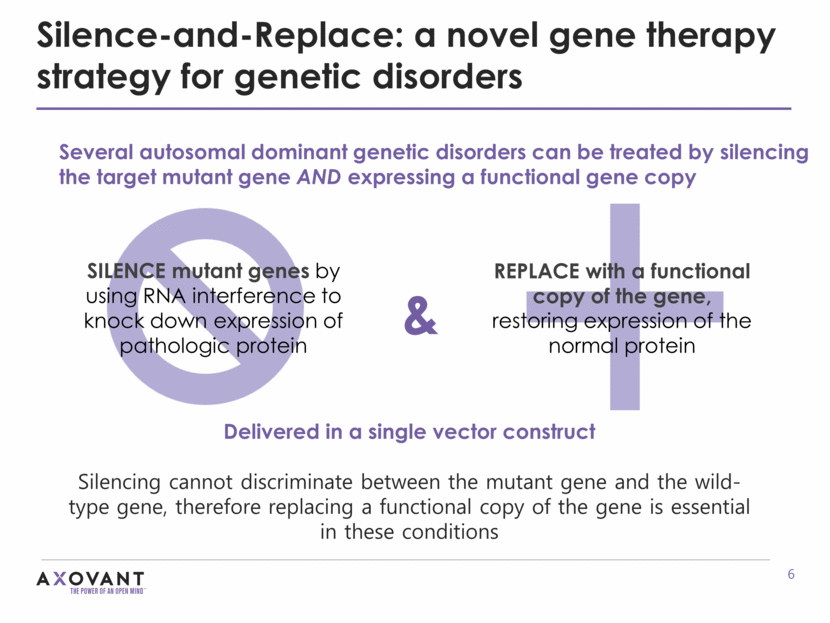
Silence-and-Replace: a novel gene therapy strategy for genetic disorders Delivered in a single vector construct Silencing cannot discriminate between the mutant gene and the wild-type gene, therefore replacing a functional copy of the gene is essential in these conditions SILENCE mutant genes by using RNA interference to knock down expression of pathologic protein REPLACE with a functional copy of the gene, restoring expression of the normal protein & Several autosomal dominant genetic disorders can be treated by silencing the target mutant gene AND expressing a functional gene copy
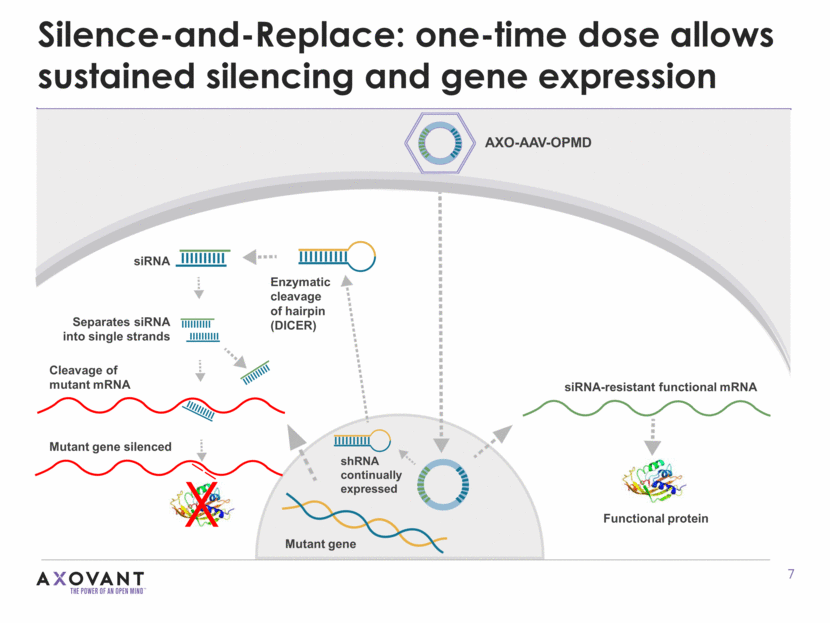
Silence-and-Replace: one-time dose allows sustained silencing and gene expression Enzymatic cleavage of hairpin (DICER) siRNA Separates siRNA into single strands Cleavage of mutant mRNA Mutant gene silenced Protein Mutant gene X shRNA continually expressed siRNA-resistant functional mRNA AXO-AAV-OPMD Functional protein
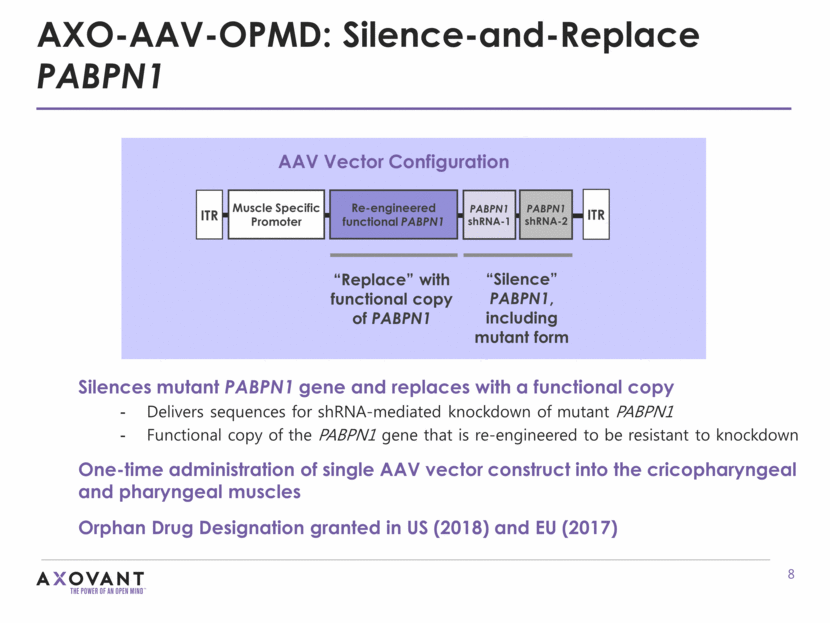
AXO-AAV-OPMD: Silence-and-Replace PABPN1 GOALS OF THERAPY: Less troublesome dyskinesia Less OFF time More ON time Lower requirement for exogenous L-dopa Silences mutant PABPN1 gene and replaces with a functional copy Delivers sequences for shRNA-mediated knockdown of mutant PABPN1 Functional copy of the PABPN1 gene that is re-engineered to be resistant to knockdown One-time administration of single AAV vector construct into the cricopharyngeal and pharyngeal muscles Orphan Drug Designation granted in US (2018) and EU (2017) AAV Vector Configuration Re-engineered functional PABPN1 PABPN1 shRNA-1 PABPN1 shRNA-2 Muscle Specific Promoter ITR ITR “Replace” with functional copy of PABPN1 “Silence” PABPN1, including mutant form
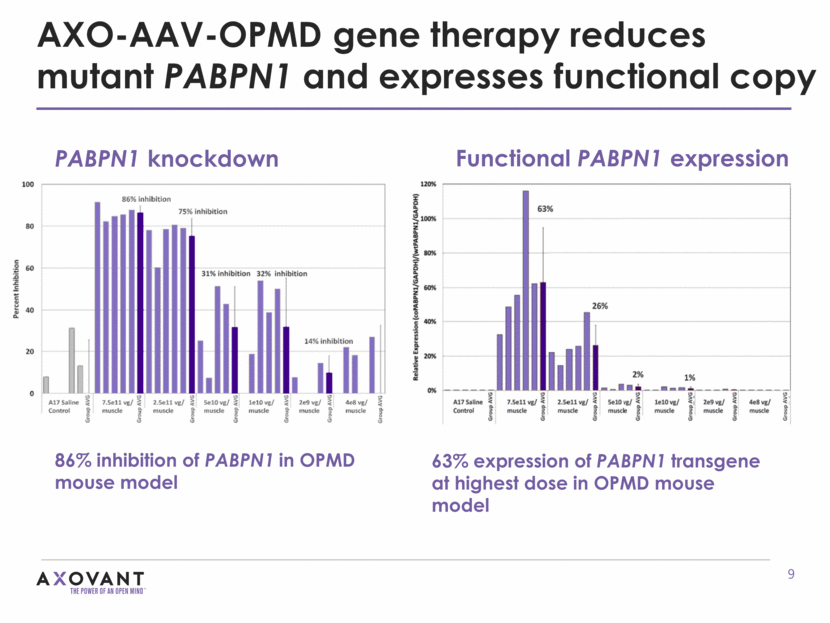
86% inhibition of PABPN1 in OPMD mouse model AXO-AAV-OPMD gene therapy reduces mutant PABPN1 and expresses functional copy PABPN1 knockdown 63% expression of PABPN1 transgene at highest dose in OPMD mouse model Functional PABPN1 expression
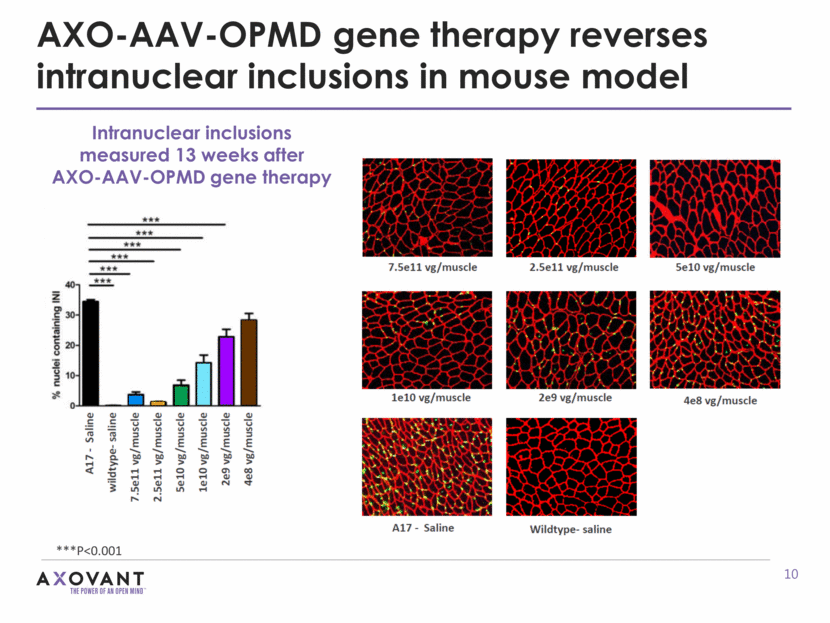
AXO-AAV-OPMD gene therapy reverses intranuclear inclusions in mouse model Intranuclear inclusions measured 13 weeks after AXO-AAV-OPMD gene therapy ***P<0.001
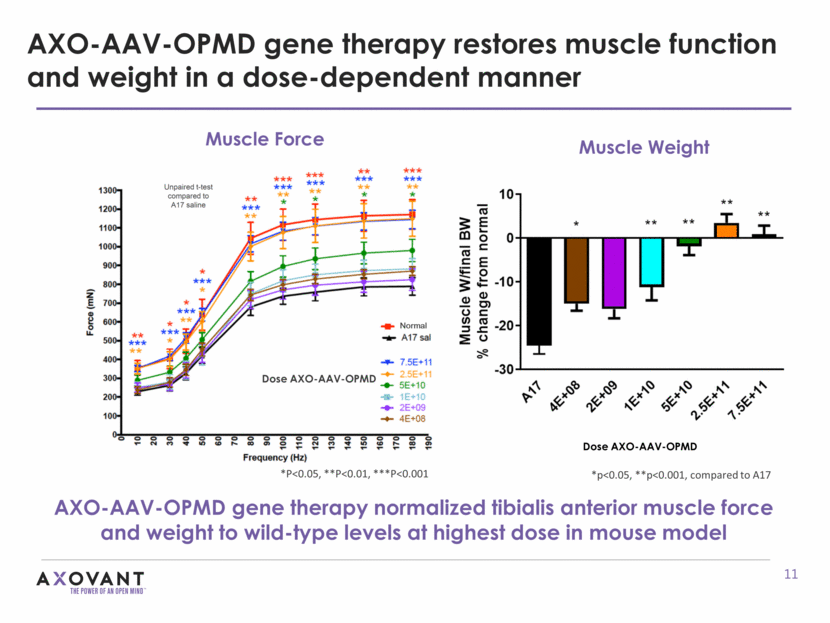
AXO-AAV-OPMD gene therapy restores muscle function and weight in a dose-dependent manner AXO-AAV-OPMD gene therapy normalized tibialis anterior muscle force and weight to wild-type levels at highest dose in mouse model Normal Dose AXO-AAV-OPMD Muscle Force *P<0.05, **P<0.01, ***P<0.001 Dose AXO-AAV-OPMD Muscle Weight *p<0.05, **p<0.001, compared to A17
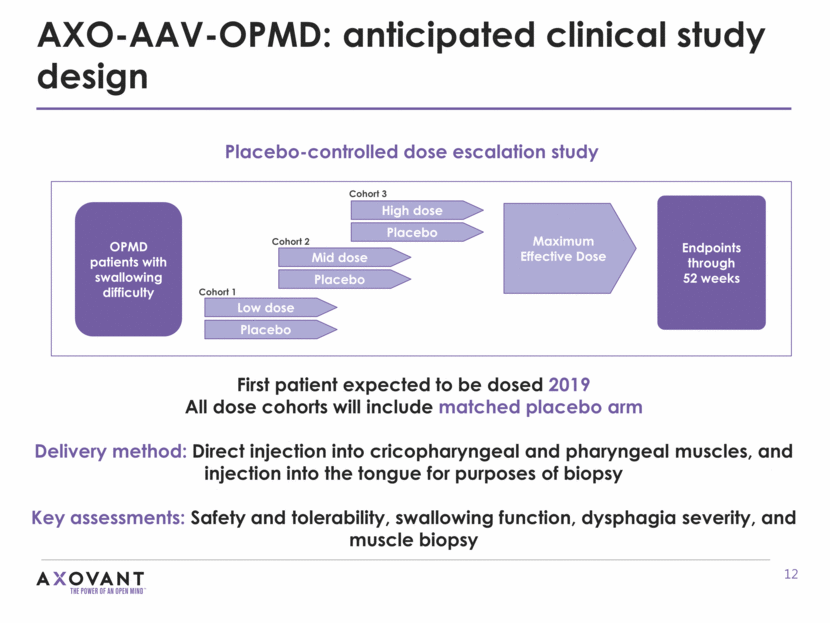
AXO-AAV-OPMD: anticipated clinical study design First patient expected to be dosed 2019 All dose cohorts will include matched placebo arm Delivery method: Direct injection into cricopharyngeal and pharyngeal muscles, and injection into the tongue for purposes of biopsy Key assessments: Safety and tolerability, swallowing function, dysphagia severity, and muscle biopsy Placebo-controlled dose escalation study OPMD patients with swallowing difficulty Low dose Placebo Mid dose Placebo High dose Maximum Effective Dose Placebo Cohort 1 Cohort 2 Cohort 3 Endpoints through 52 weeks
AXO-AAV-OPMD: scalable, high-quality manufacturing process already in place AXO-AAV-OPMD will be produced with scalable, high-yield baculovirus based methodologies and purification processes to control COGS GMP-grade clinical material produced at a top-three, US-based gene therapy contract manufacturing organization Product-specific process developed to produce high upstream productivity and industry-standard purification yield Uses a modified AAV capsid for the generation of highly active viral vector particles Currently manufacturing at 50L scale, with plans for clinical product to be generated at 250L scale
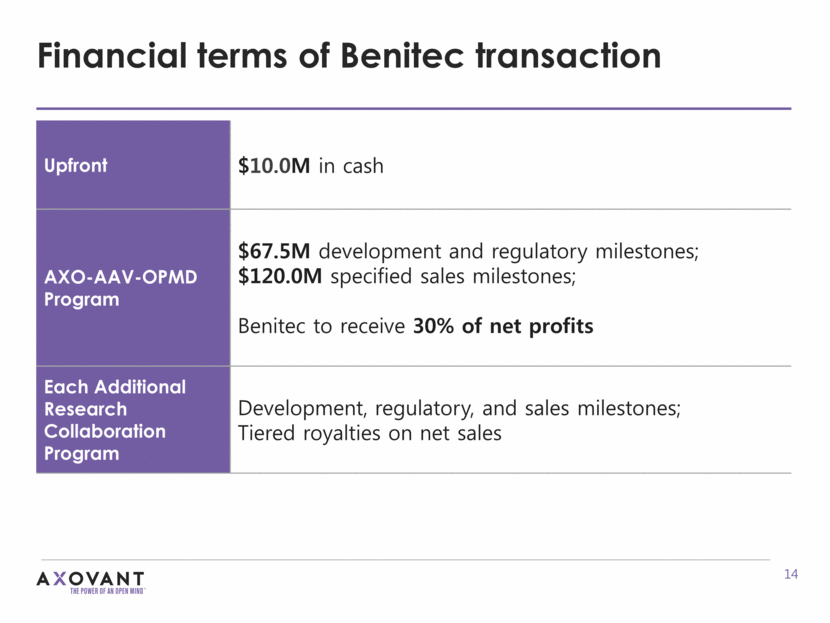
Financial terms of Benitec transaction Upfront $10.0M in cash AXO-AAV-OPMD Program $67.5M development and regulatory milestones; $120.0M specified sales milestones; Benitec to receive 30% of net profits Each Additional Research Collaboration Program Development, regulatory, and sales milestones; Tiered royalties on net sales
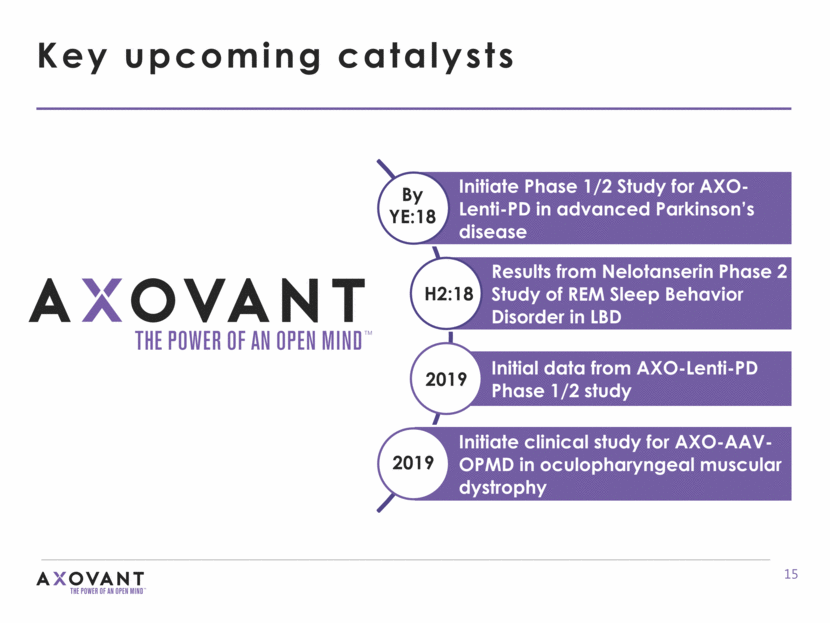
By YE:18 H2:18 2019 Key upcoming catalysts 2019 Initiate Phase 1/2 Study for AXO-Lenti-PD in advanced Parkinson’s disease Results from Nelotanserin Phase 2 Study of REM Sleep Behavior Disorder in LBD Initial data from AXO-Lenti-PD Phase 1/2 study Initiate clinical study for AXO-AAV-OPMD in oculopharyngeal muscular dystrophy