Attached files
| file | filename |
|---|---|
| 8-K - 8-K UPDATED INVESTOR DECK - Acer Therapeutics Inc. | acer-8k_20180629.htm |

Developing Therapeutics for the Treatment of Serious Rare Diseases with Significant Unmet Medical Needs July 2018 Nasdaq: ACER Exhibit 99.1

Forward-looking Statements This presentation contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this presentation regarding strategy, future operations, future financial position, future revenues, projected expenses, prospects, plans and objectives of management are forward-looking statements. Examples of such statements include, but are not limited to, statements relating to expectations regarding the capitalization and resources of the company; the potential for EDSIVO™ (celiprolol) and ACER-001 to safely and effectively target diseases; the commercial or market opportunity in any target indication; the adequacy of the company’s capital to support its future operations and its ability to successfully initiate and complete clinical trials and regulatory submissions; the ability to protect the company’s intellectual property rights; the nature, strategy and focus of the company; future economic conditions or performance; and the development, expected timeline and commercial potential of any product candidates of the company. Acer may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient resources of the company to meet its business objectives and operational requirements, the fact that the results of earlier studies and trials may not be predictive of future clinical trial results, the protection and market exclusivity provided by Acer’s intellectual property, risks related to the drug development and the regulatory approval process and the impact of competitive products and technological changes. Acer disclaims any intent or obligation to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. You should review additional disclosures we make in our filings with the Securities and Exchange Commission, including our Quarterly Reports on Form 10-Q and our Annual Report on Form 10-K. You may access these documents for no charge at http://www.sec.gov.

Acer is a pharmaceutical company that acquires, develops and commercializes therapies for serious rare and ultra-rare diseases with significant unmet medical needs. Headquartered: Newton, MA Headcount: 15 Founded: December 2013 Public: September 2017 Cash: $12.4M (3/31/18) Expected to have sufficient capital through the end of 2018 Acer Corporate Overview
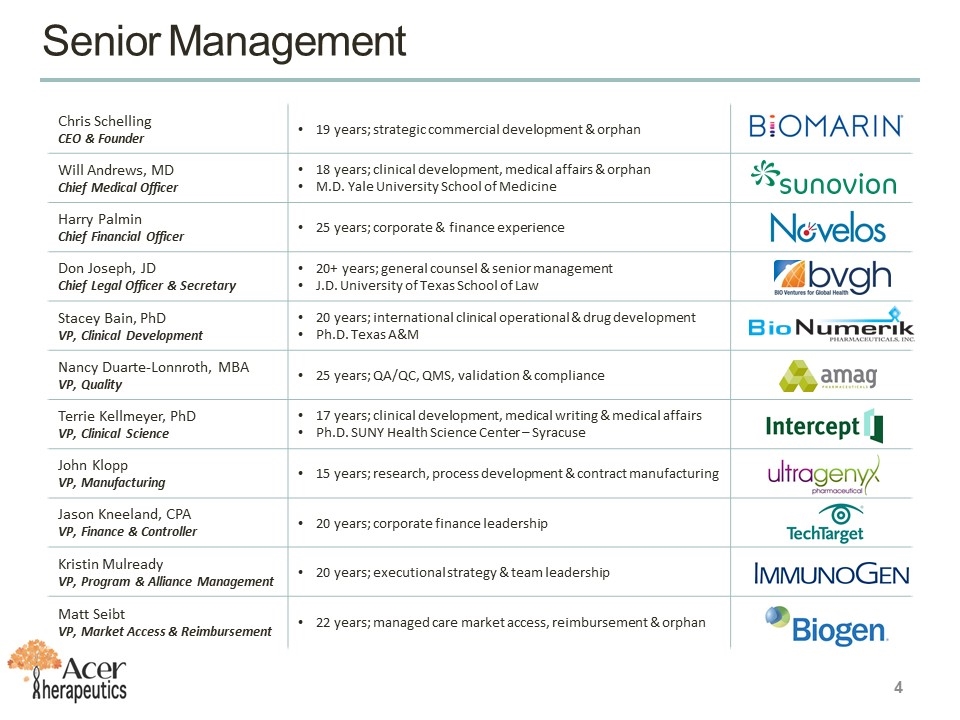
Senior Management Chris Schelling CEO & Founder 19 years; strategic commercial development & orphan Will Andrews, MD Chief Medical Officer 18 years; clinical development, medical affairs & orphan M.D. Yale University School of Medicine Harry Palmin Chief Financial Officer 25 years; corporate & finance experience Don Joseph, JD Chief Legal Officer & Secretary 20+ years; general counsel & senior management J.D. University of Texas School of Law Stacey Bain, PhD VP, Clinical Development 20 years; international clinical operational & drug development Ph.D. Texas A&M Nancy Duarte-Lonnroth, MBA VP, Quality 25 years; QA/QC, QMS, validation & compliance Terrie Kellmeyer, PhD VP, Clinical Science 17 years; clinical development, medical writing & medical affairs Ph.D. SUNY Health Science Center – Syracuse John Klopp VP, Manufacturing 15 years; research, process development & contract manufacturing Jason Kneeland, CPA VP, Finance & Controller 20 years; corporate finance leadership Kristin Mulready VP, Program & Alliance Management 20 years; executional strategy & team leadership Matt Seibt VP, Market Access & Reimbursement 22 years; managed care market access, reimbursement & orphan
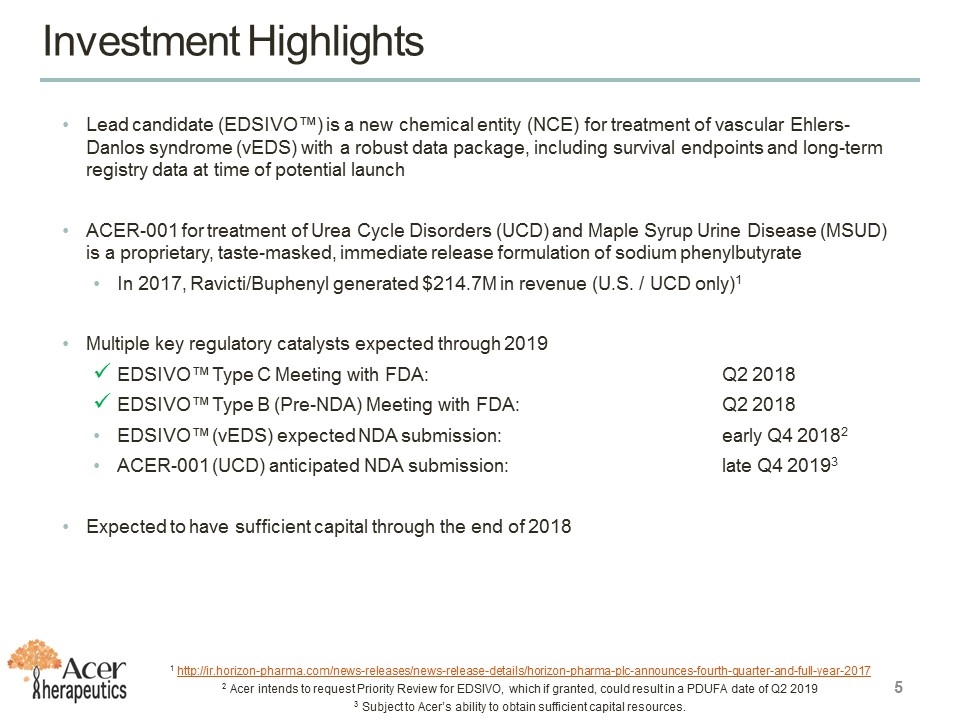
Investment Highlights Lead candidate (EDSIVO™) is a new chemical entity (NCE) for treatment of vascular Ehlers-Danlos syndrome (vEDS) with a robust data package, including survival endpoints and long-term registry data at time of potential launch ACER-001 for treatment of Urea Cycle Disorders (UCD) and Maple Syrup Urine Disease (MSUD) is a proprietary, taste-masked, immediate release formulation of sodium phenylbutyrate In 2017, Ravicti/Buphenyl generated $214.7M in revenue (U.S. / UCD only)1 Multiple key regulatory catalysts expected through 2019 EDSIVO™ Type C Meeting with FDA: Q2 2018 EDSIVO™ Type B (Pre-NDA) Meeting with FDA: Q2 2018 EDSIVO™ (vEDS) expected NDA submission: early Q4 20182 ACER-001 (UCD) anticipated NDA submission: late Q4 20193 Expected to have sufficient capital through the end of 2018 1 http://ir.horizon-pharma.com/news-releases/news-release-details/horizon-pharma-plc-announces-fourth-quarter-and-full-year-2017 2 Acer intends to request Priority Review for EDSIVO, which if granted, could result in a PDUFA date of Q2 2019 3 Subject to Acer’s ability to obtain sufficient capital resources.
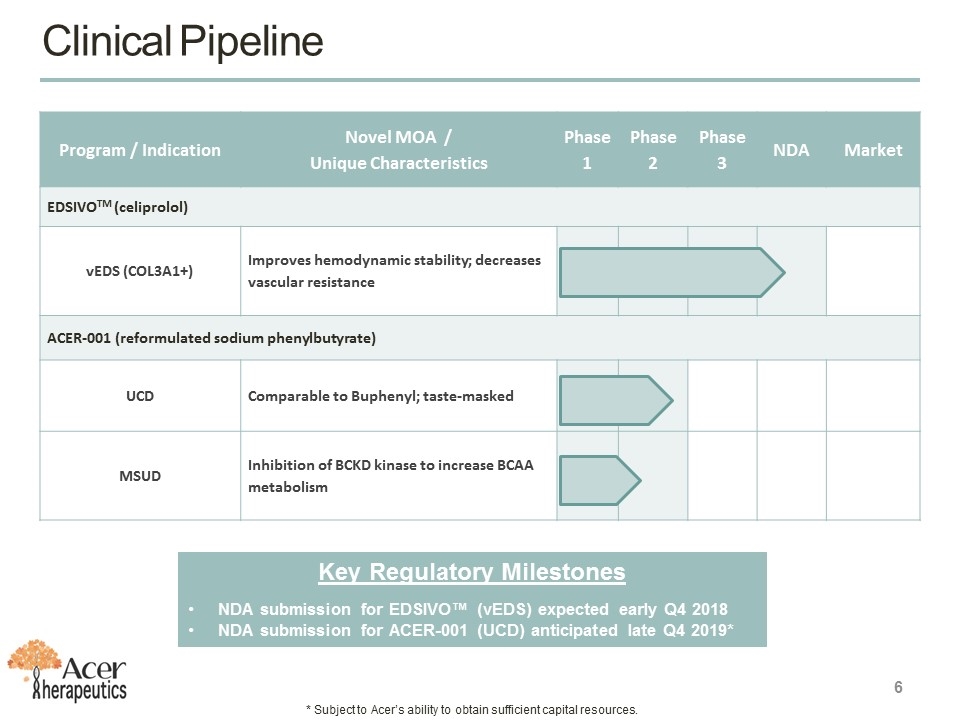
Program / Indication Novel MOA / Unique Characteristics Phase 1 Phase 2 Phase 3 NDA Market EDSIVOTM (celiprolol) vEDS (COL3A1+) Improves hemodynamic stability; decreases vascular resistance ACER-001 (reformulated sodium phenylbutyrate) UCD Comparable to Buphenyl; taste-masked MSUD Inhibition of BCKD kinase to increase BCAA metabolism Clinical Pipeline Key Regulatory Milestones NDA submission for EDSIVO™ (vEDS) expected early Q4 2018 NDA submission for ACER-001 (UCD) anticipated late Q4 2019* * Subject to Acer’s ability to obtain sufficient capital resources.
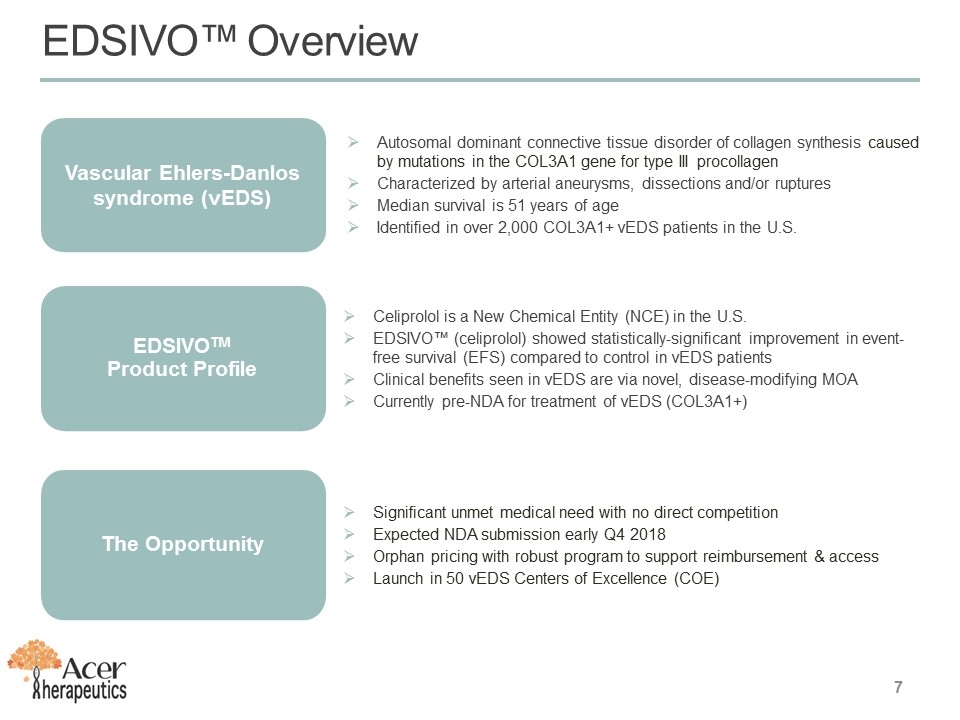
Autosomal dominant connective tissue disorder of collagen synthesis caused by mutations in the COL3A1 gene for type III procollagen Characterized by arterial aneurysms, dissections and/or ruptures Median survival is 51 years of age Identified in over 2,000 COL3A1+ vEDS patients in the U.S. Vascular Ehlers-Danlos syndrome (vEDS) Celiprolol is a New Chemical Entity (NCE) in the U.S. EDSIVO™ (celiprolol) showed statistically-significant improvement in event-free survival (EFS) compared to control in vEDS patients Clinical benefits seen in vEDS are via novel, disease-modifying MOA Currently pre-NDA for treatment of vEDS (COL3A1+) EDSIVOTM Product Profile Significant unmet medical need with no direct competition Expected NDA submission early Q4 2018 Orphan pricing with robust program to support reimbursement & access Launch in 50 vEDS Centers of Excellence (COE) The Opportunity EDSIVO™ Overview
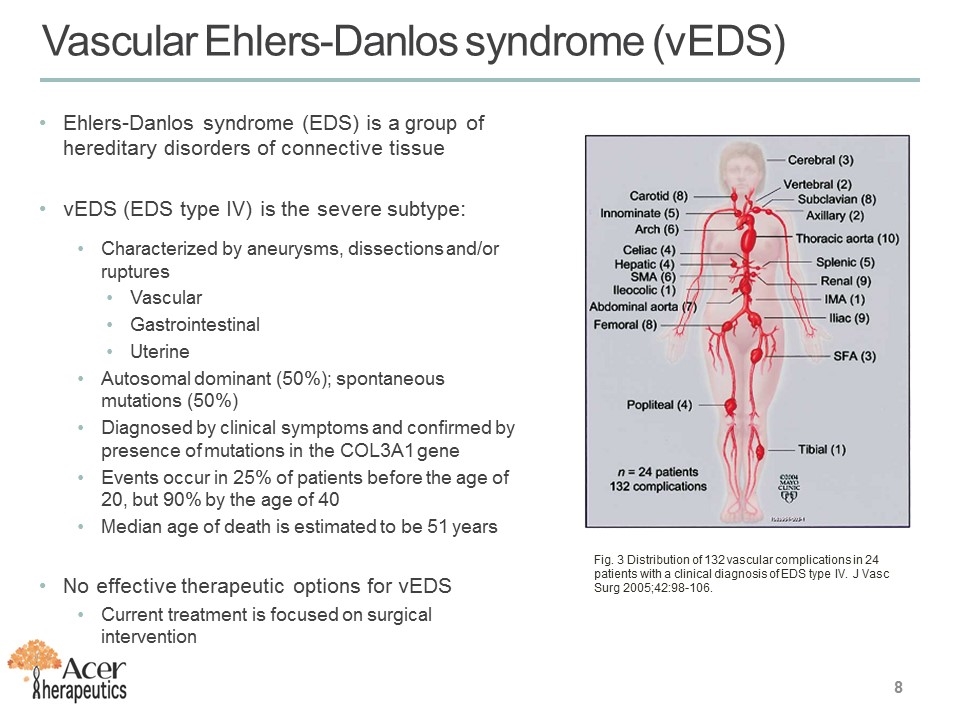
Ehlers-Danlos syndrome (EDS) is a group of hereditary disorders of connective tissue vEDS (EDS type IV) is the severe subtype: Characterized by aneurysms, dissections and/or ruptures Vascular Gastrointestinal Uterine Autosomal dominant (50%); spontaneous mutations (50%) Diagnosed by clinical symptoms and confirmed by presence of mutations in the COL3A1 gene Events occur in 25% of patients before the age of 20, but 90% by the age of 40 Median age of death is estimated to be 51 years No effective therapeutic options for vEDS Current treatment is focused on surgical intervention Vascular Ehlers-Danlos syndrome (vEDS) Fig. 3 Distribution of 132 vascular complications in 24 patients with a clinical diagnosis of EDS type IV. J Vasc Surg 2005;42:98-106.
Cardiology in Review 2017;25: 247–253 EDSIVO™ Unique Mechanism of Action EDSIVO™ has a unique pharmacological profile: Selective β1 and ⍺2 adrenergic receptor antagonist β2 and β3 adrenergic receptor agonist Intrinsic sympathomimetic activity (ISA+) Lacks non-specific membrane effects Void of blood pressure lowering in normotensive people Most vEDS patients are normotensive, thus the protective effect of celiprolol is unlikely to be through blood pressure lowering (β1 antagonism) EDSIVO™ positive effects in vEDS patients are thought to be through: Providing more stable hemodynamic conditions that lead to a less fragile arterial wall Upregulation of collagen synthesis via TGF-β, thus strengthening the arterial wall and reducing its susceptibility to rupture EDSIVO™ is the only agent to show clinical benefit in patients with vEDS
Multicenter, prospective, randomized, open trial with blinded endpoint assessment Design: 9 sites: eight in France, one in Belgium Location: Inclusion criteria adapted Villefranche diagnostic criteria: 1 major criterion and 2 minor criteria, or 4 minor criteria needed for enrollment Eligibility: 53 patients with clinical vascular Ehlers-Danlos syndrome Randomly assigned to 5 years treatment with 1) celiprolol or 2) no treatment Enrollment: Ages 15 to 65 (mean 35), female/male ratio 2:1 Important phenotype characteristics equally balanced between celiprolol and control Demographics: Celiprolol administered twice daily Up-titrated every 6 mos. by 100 mg/day to max. 400 mg/day Dosing: Primary: arterial events (rupture/dissection, +/-fatal) Secondary: intestinal or uterine rupture Endpoints: Mean duration of follow-up 47 months; trial stopped early for treatment benefit Duration: French Ministry of Health; PI: Prof. Pierre Boutouyrie Funding / PI: ClinicalTrials.gov, number NCT00190411 Ong Lancet 2010; 376: 1476-84. EDSIVO™ Pivotal Clinical Trial
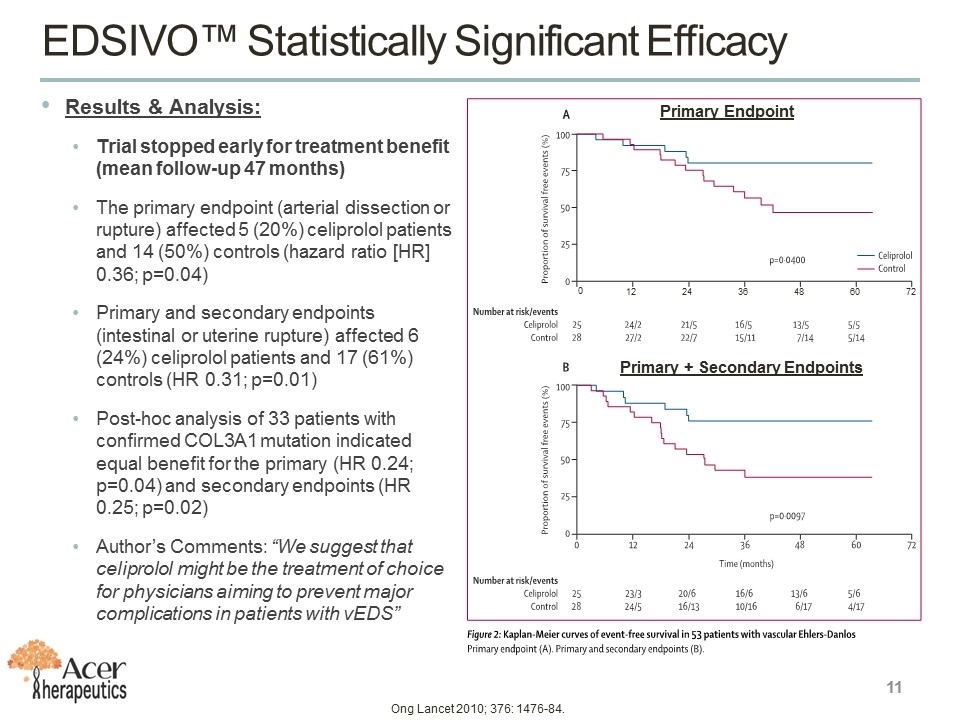
Results & Analysis: Trial stopped early for treatment benefit (mean follow-up 47 months) The primary endpoint (arterial dissection or rupture) affected 5 (20%) celiprolol patients and 14 (50%) controls (hazard ratio [HR] 0.36; p=0.04) Primary and secondary endpoints (intestinal or uterine rupture) affected 6 (24%) celiprolol patients and 17 (61%) controls (HR 0.31; p=0.01) Post-hoc analysis of 33 patients with confirmed COL3A1 mutation indicated equal benefit for the primary (HR 0.24; p=0.04) and secondary endpoints (HR 0.25; p=0.02) Author’s Comments: “We suggest that celiprolol might be the treatment of choice for physicians aiming to prevent major complications in patients with vEDS” Primary Endpoint Primary + Secondary Endpoints EDSIVO™ Statistically Significant Efficacy Ong Lancet 2010; 376: 1476-84. 0 12 24 36 48 60 72
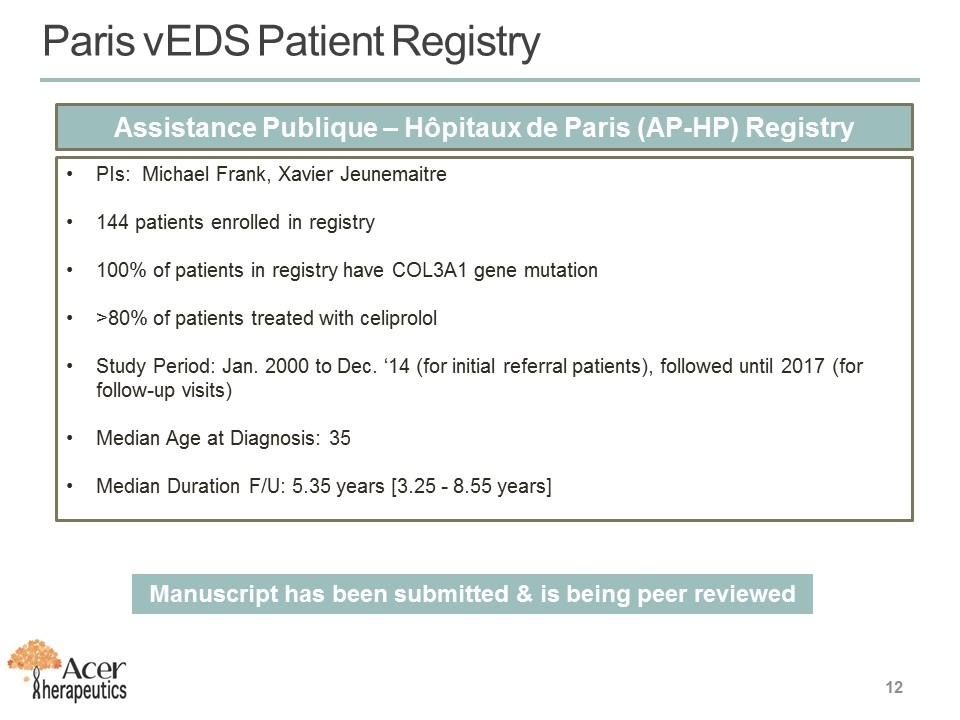
Paris vEDS Patient Registry Assistance Publique – Hôpitaux de Paris (AP-HP) Registry PIs: Michael Frank, Xavier Jeunemaitre 144 patients enrolled in registry 100% of patients in registry have COL3A1 gene mutation >80% of patients treated with celiprolol Study Period: Jan. 2000 to Dec. ‘14 (for initial referral patients), followed until 2017 (for follow-up visits) Median Age at Diagnosis: 35 Median Duration F/U: 5.35 years [3.25 - 8.55 years] Manuscript has been submitted & is being peer reviewed 12
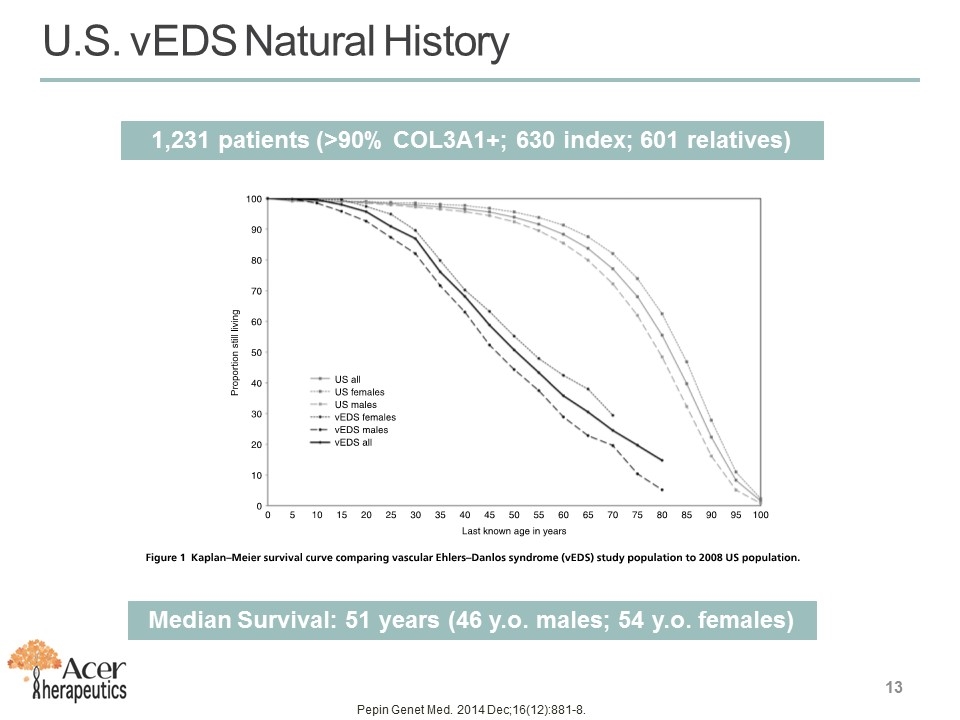
U.S. vEDS Natural History 13 Pepin Genet Med. 2014 Dec;16(12):881-8. Median Survival: 51 years (46 y.o. males; 54 y.o. females) 1,231 patients (>90% COL3A1+; 630 index; 601 relatives)
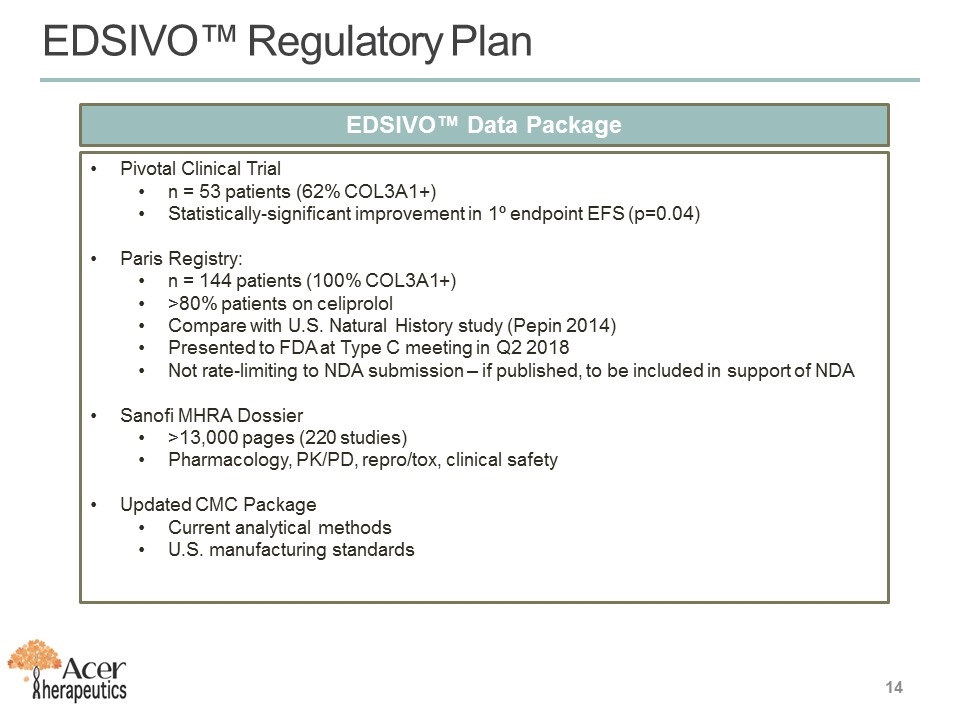
EDSIVO™ Data Package Pivotal Clinical Trial n = 53 patients (62% COL3A1+) Statistically-significant improvement in 1º endpoint EFS (p=0.04) Paris Registry: n = 144 patients (100% COL3A1+) >80% patients on celiprolol Compare with U.S. Natural History study (Pepin 2014) Presented to FDA at Type C meeting in Q2 2018 Not rate-limiting to NDA submission – if published, to be included in support of NDA Sanofi MHRA Dossier >13,000 pages (220 studies) Pharmacology, PK/PD, repro/tox, clinical safety Updated CMC Package Current analytical methods U.S. manufacturing standards EDSIVO™ Regulatory Plan
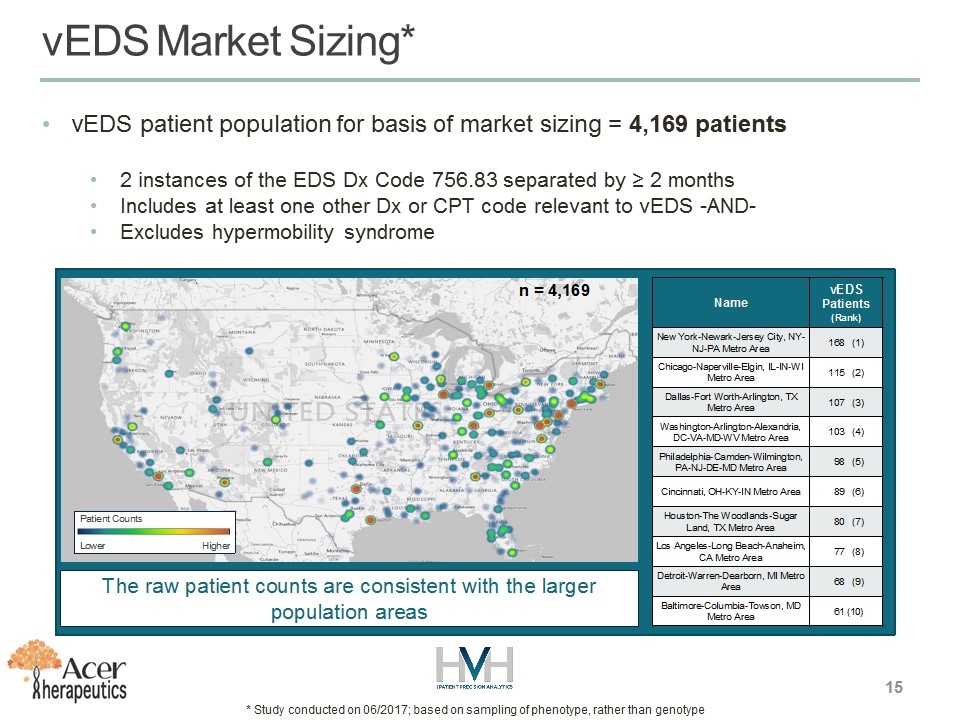
vEDS Market Sizing* vEDS patient population for basis of market sizing = 4,169 patients 2 instances of the EDS Dx Code 756.83 separated by ≥ 2 months Includes at least one other Dx or CPT code relevant to vEDS -AND- Excludes hypermobility syndrome * Study conducted on 06/2017; based on sampling of phenotype, rather than genotype
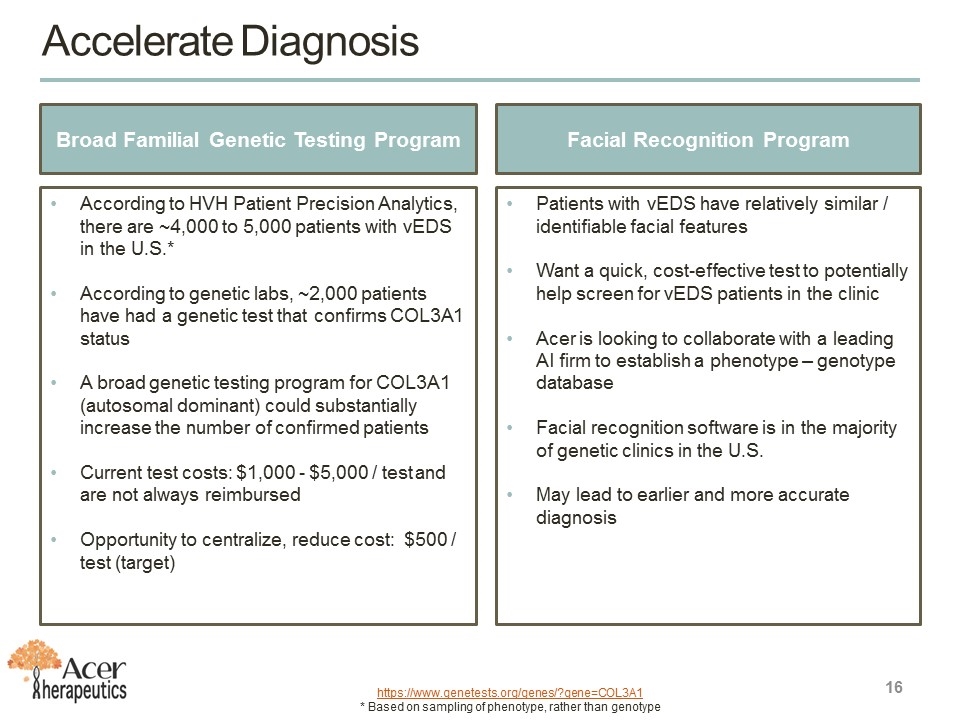
Accelerate Diagnosis https://www.genetests.org/genes/?gene=COL3A1 * Based on sampling of phenotype, rather than genotype Facial Recognition Program Patients with vEDS have relatively similar / identifiable facial features Want a quick, cost-effective test to potentially help screen for vEDS patients in the clinic Acer is looking to collaborate with a leading AI firm to establish a phenotype – genotype database Facial recognition software is in the majority of genetic clinics in the U.S. May lead to earlier and more accurate diagnosis Broad Familial Genetic Testing Program According to HVH Patient Precision Analytics, there are ~4,000 to 5,000 patients with vEDS in the U.S.* According to genetic labs, ~2,000 patients have had a genetic test that confirms COL3A1 status A broad genetic testing program for COL3A1 (autosomal dominant) could substantially increase the number of confirmed patients Current test costs: $1,000 - $5,000 / test and are not always reimbursed Opportunity to centralize, reduce cost: $500 / test (target)
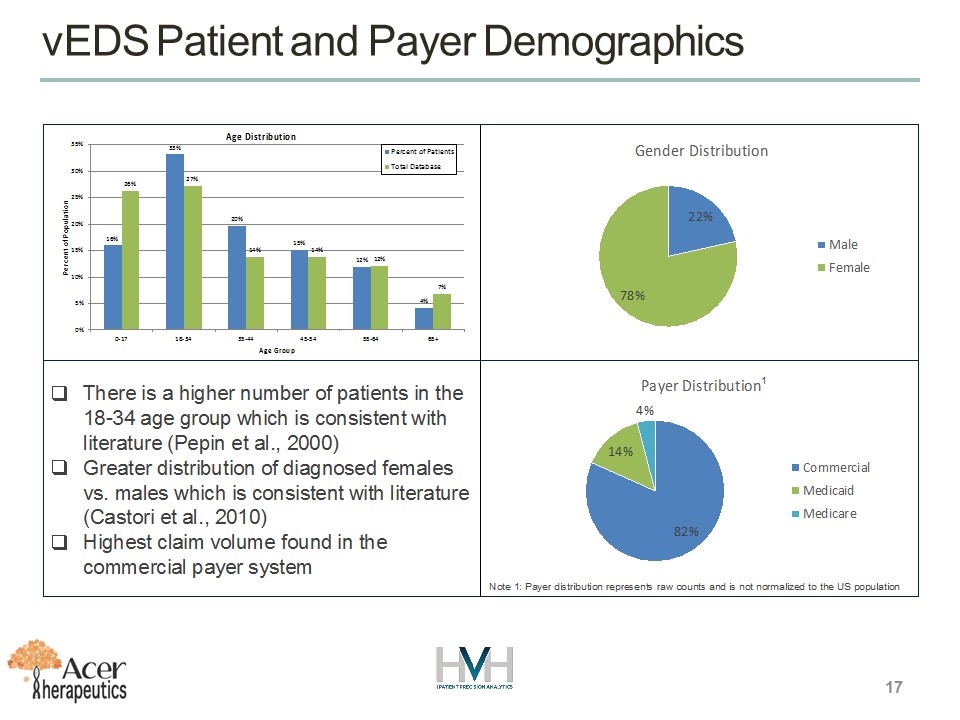
17 vEDS Patient and Payer Demographics
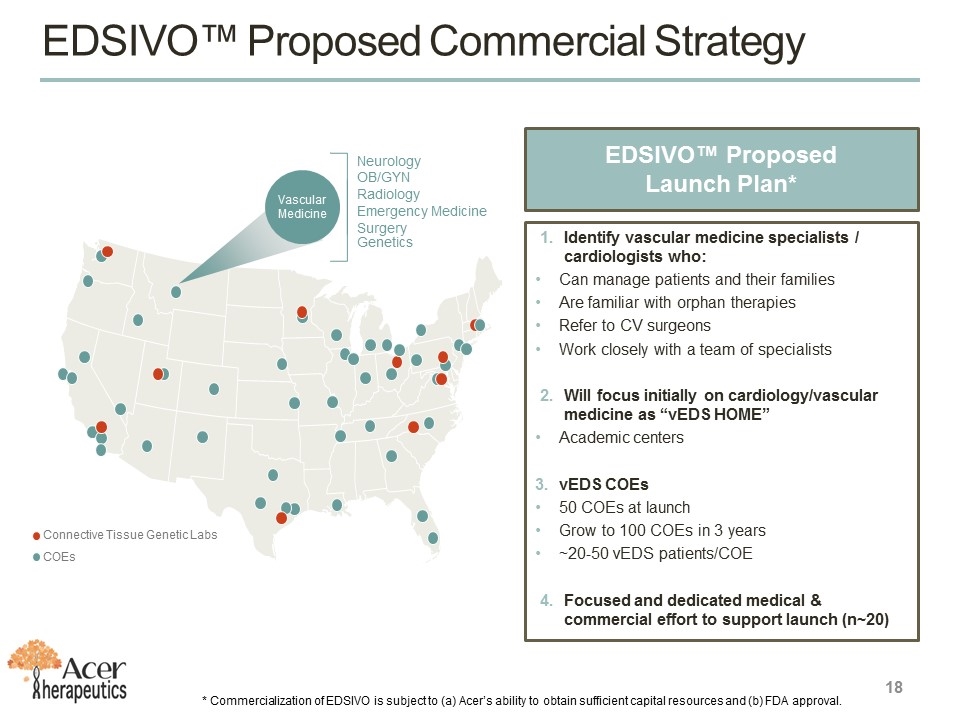
EDSIVO™ Proposed Commercial Strategy EDSIVO™ Proposed Launch Plan* Identify vascular medicine specialists / cardiologists who: Can manage patients and their families Are familiar with orphan therapies Refer to CV surgeons Work closely with a team of specialists Will focus initially on cardiology/vascular medicine as “vEDS HOME” Academic centers vEDS COEs 50 COEs at launch Grow to 100 COEs in 3 years ~20-50 vEDS patients/COE Focused and dedicated medical & commercial effort to support launch (n~20) 18 * Commercialization of EDSIVO is subject to (a) Acer’s ability to obtain sufficient capital resources and (b) FDA approval. Neurology OB/GYN Radiology Emergency Medicine Surgery Genetics Vascular Medicine Connective Tissue Genetic Labs COEs
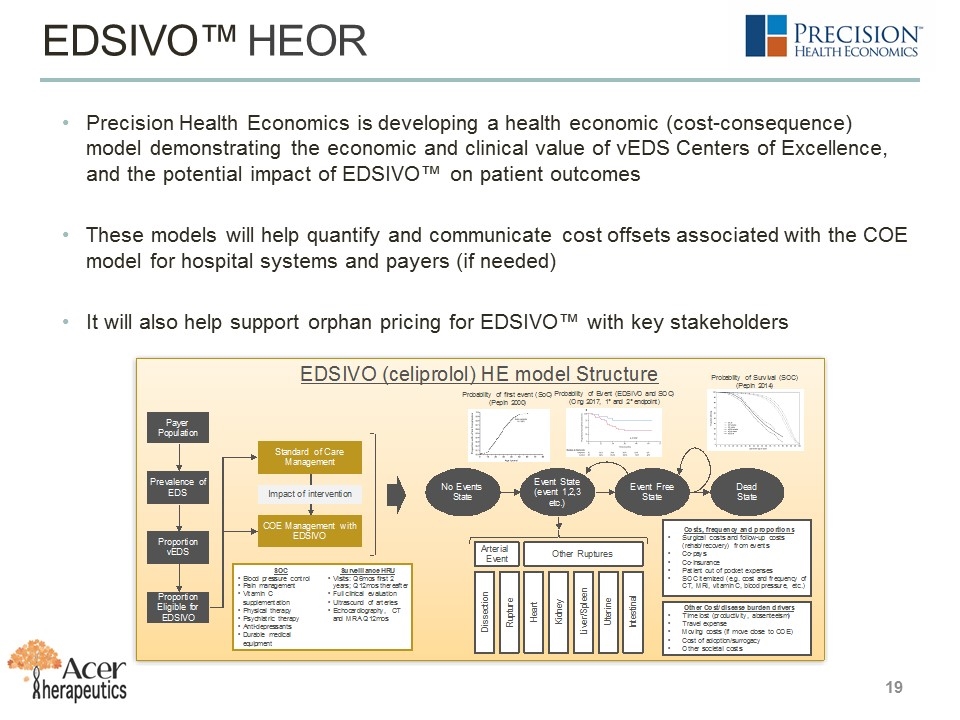
19 EDSIVO™ HEOR Precision Health Economics is developing a health economic (cost-consequence) model demonstrating the economic and clinical value of vEDS Centers of Excellence, and the potential impact of EDSIVO™ on patient outcomes These models will help quantify and communicate cost offsets associated with the COE model for hospital systems and payers (if needed) It will also help support orphan pricing for EDSIVO™ with key stakeholders

If approved, EDSIVO™ will be the only FDA-approved therapy to treat vEDS patients 2,000 to 5,000 vEDS patients in the U.S. Orphan pricing well supported by initial payer research, with additional validation from HEOR models Provide a robust patient assistance program (PAP) to help offset costs (so there will be little/no incentive for vEDS patients in the U.S. to attempt to obtain celiprolol elsewhere) Granted U.S. Orphan Drug Designation for vEDS (January 2015) If approved, would grant 7 years market exclusivity in vEDS Potential +0.5 years pediatric exclusivity Use patents filed may provide additional exclusivity 50-100 Centers of Excellence Focused, dedicated field support (n~20-25 people) EDSIVO™ Market Opportunity

A group of metabolic genetic diseases that lead to toxic build-up of NH4+ Currently treated with Ravicti, Buphenyl, Ammonul, and a highly-restricted diet >2,000 patients with UCD in the U.S.; ~600 patients treated with sodium / glycerol phenylbutyrate Urea Cycle Disorders (UCD) A metabolic genetic disease that leads to toxic build-up of leucine and other branched-chain amino acids Currently managed with a highly-restricted diet; poor compliance Well-defined patient population with ~800 eligible patients in the U.S. Maple Syrup Urine Disease (MSUD) Anticipate NDA submission for UCD late Q4 2019* Issued U.S. / EU patents covering methods of use in MSUD Orphan drug designation in MSUD Advantageous orphan pricing with robust program to support reimbursement and patient access The Opportunity ACER-001 Overview A taste-masked, immediate release formulation of sodium phenylbutyrate First office action (USPTO) on formulation patent PK/BE study to show equivalence to sodium phenylbutyrate ACER-001 Product Profile * Subject to Acer’s ability to obtain sufficient capital resources.
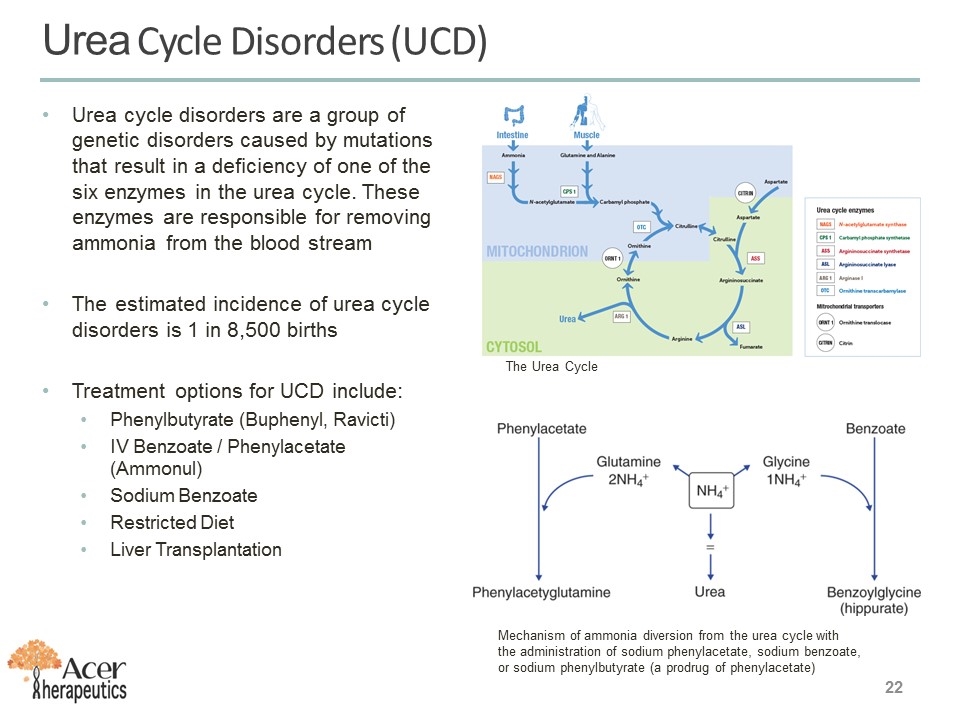
Urea cycle disorders are a group of genetic disorders caused by mutations that result in a deficiency of one of the six enzymes in the urea cycle. These enzymes are responsible for removing ammonia from the blood stream The estimated incidence of urea cycle disorders is 1 in 8,500 births Treatment options for UCD include: Phenylbutyrate (Buphenyl, Ravicti) IV Benzoate / Phenylacetate (Ammonul) Sodium Benzoate Restricted Diet Liver Transplantation The Urea Cycle Mechanism of ammonia diversion from the urea cycle with the administration of sodium phenylacetate, sodium benzoate, or sodium phenylbutyrate (a prodrug of phenylacetate) Urea Cycle Disorders (UCD)
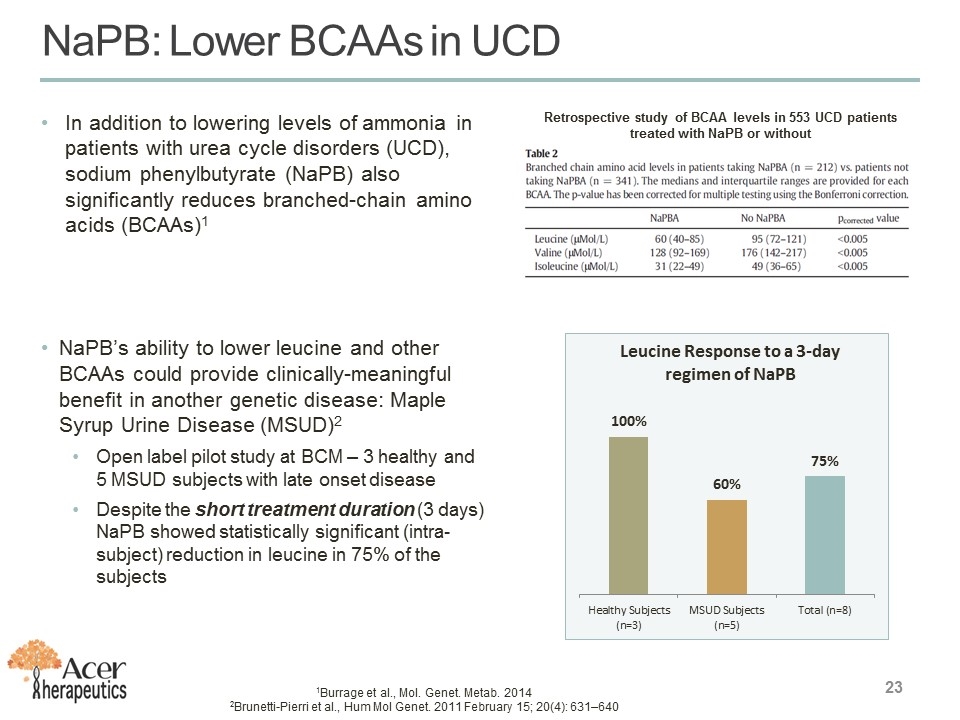
In addition to lowering levels of ammonia in patients with urea cycle disorders (UCD), sodium phenylbutyrate (NaPB) also significantly reduces branched-chain amino acids (BCAAs)1 NaPB’s ability to lower leucine and other BCAAs could provide clinically-meaningful benefit in another genetic disease: Maple Syrup Urine Disease (MSUD)2 Open label pilot study at BCM – 3 healthy and 5 MSUD subjects with late onset disease Despite the short treatment duration (3 days) NaPB showed statistically significant (intra-subject) reduction in leucine in 75% of the subjects 1Burrage et al., Mol. Genet. Metab. 2014 2Brunetti-Pierri et al., Hum Mol Genet. 2011 February 15; 20(4): 631–640 Retrospective study of BCAA levels in 553 UCD patients treated with NaPB or without NaPB: Lower BCAAs in UCD
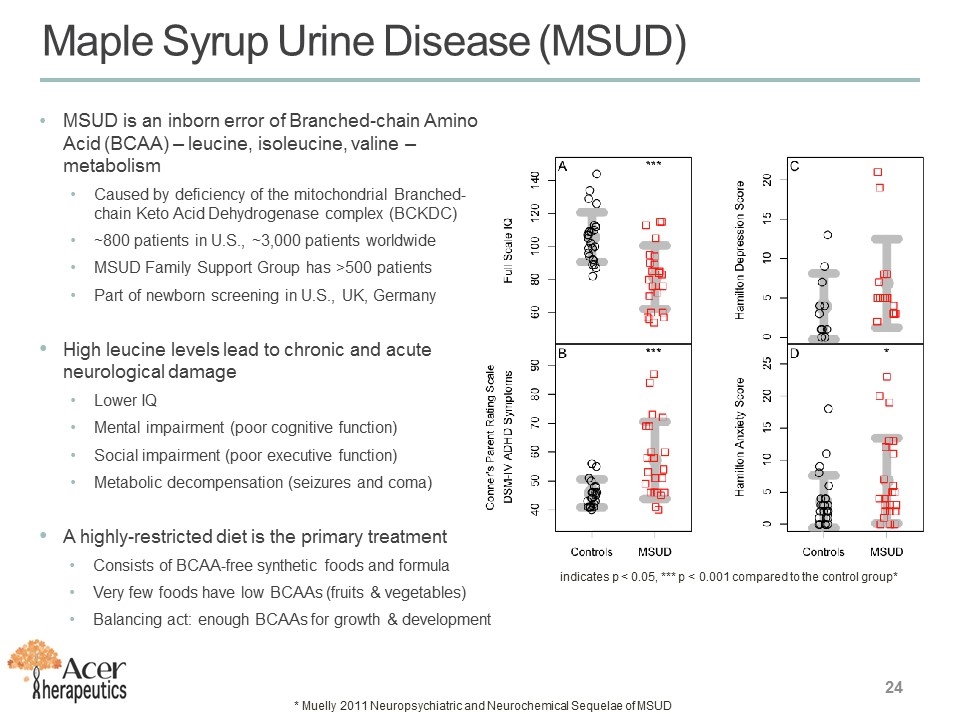
MSUD is an inborn error of Branched-chain Amino Acid (BCAA) – leucine, isoleucine, valine – metabolism Caused by deficiency of the mitochondrial Branched-chain Keto Acid Dehydrogenase complex (BCKDC) ~800 patients in U.S., ~3,000 patients worldwide MSUD Family Support Group has >500 patients Part of newborn screening in U.S., UK, Germany High leucine levels lead to chronic and acute neurological damage Lower IQ Mental impairment (poor cognitive function) Social impairment (poor executive function) Metabolic decompensation (seizures and coma) A highly-restricted diet is the primary treatment Consists of BCAA-free synthetic foods and formula Very few foods have low BCAAs (fruits & vegetables) Balancing act: enough BCAAs for growth & development Maple Syrup Urine Disease (MSUD) indicates p < 0.05, *** p < 0.001 compared to the control group* * Muelly 2011 Neuropsychiatric and Neurochemical Sequelae of MSUD
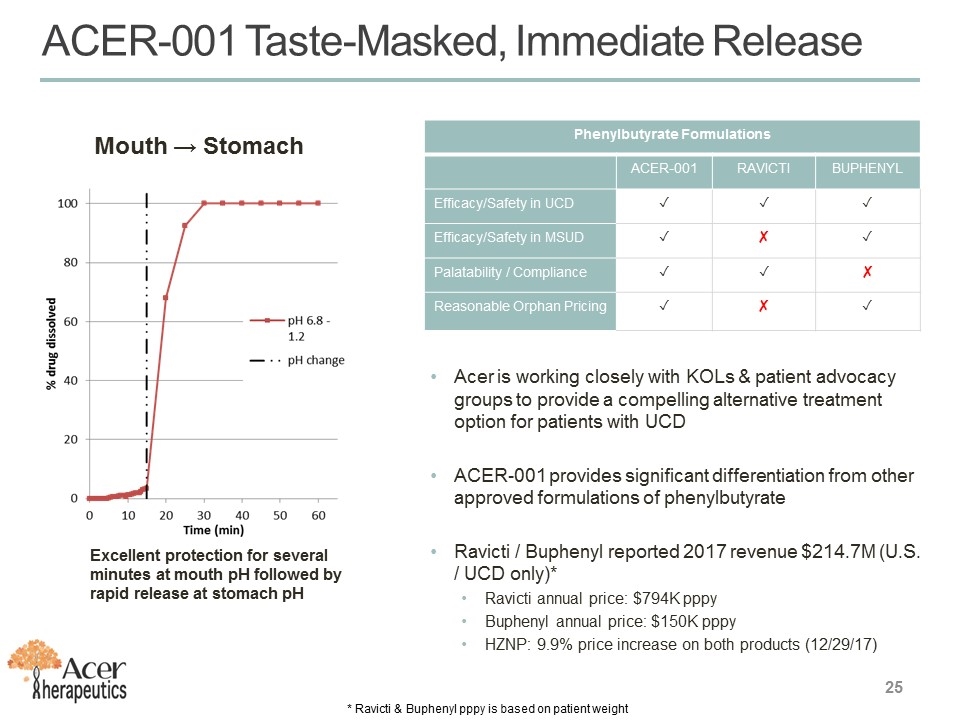
ACER-001 Taste-Masked, Immediate Release Mouth → Stomach Excellent protection for several minutes at mouth pH followed by rapid release at stomach pH Phenylbutyrate Formulations ACER-001 RAVICTI BUPHENYL Efficacy/Safety in UCD ✓ ✓ ✓ Efficacy/Safety in MSUD ✓ ✘ ✓ Palatability / Compliance ✓ ✓ ✘ Reasonable Orphan Pricing ✓ ✘ ✓ Acer is working closely with KOLs & patient advocacy groups to provide a compelling alternative treatment option for patients with UCD ACER-001 provides significant differentiation from other approved formulations of phenylbutyrate Ravicti / Buphenyl reported 2017 revenue $214.7M (U.S. / UCD only)* Ravicti annual price: $794K pppy Buphenyl annual price: $150K pppy HZNP: 9.9% price increase on both products (12/29/17) * Ravicti & Buphenyl pppy is based on patient weight

Cannibalize existing sodium phenylbutyrate market share in UCD Taste-masked, immediate-release formulation Competitively priced Life cycle expansion opportunity in MSUD Barriers to entry: Filed formulation patent application (January 2016) Issued patent (US/EP) “Methods of modulation of branched chain acids and uses thereof” 505(b)(2) Exclusivity: 3 years in U.S. for UCD Obtained U.S. Orphan Drug Designation for MSUD (August 2014) Pediatric Exclusivity: 6 months Provide a robust PAP to help offset costs ACER-001 Market Opportunity
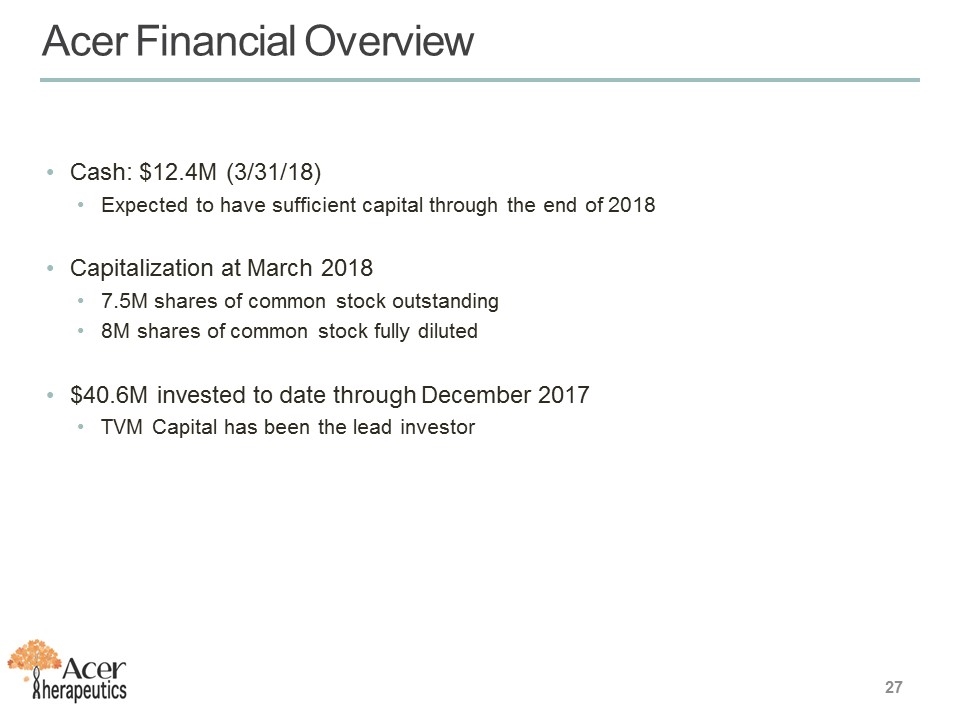
Acer Financial Overview Cash: $12.4M (3/31/18) Expected to have sufficient capital through the end of 2018 Capitalization at March 2018 7.5M shares of common stock outstanding 8M shares of common stock fully diluted $40.6M invested to date through December 2017 TVM Capital has been the lead investor
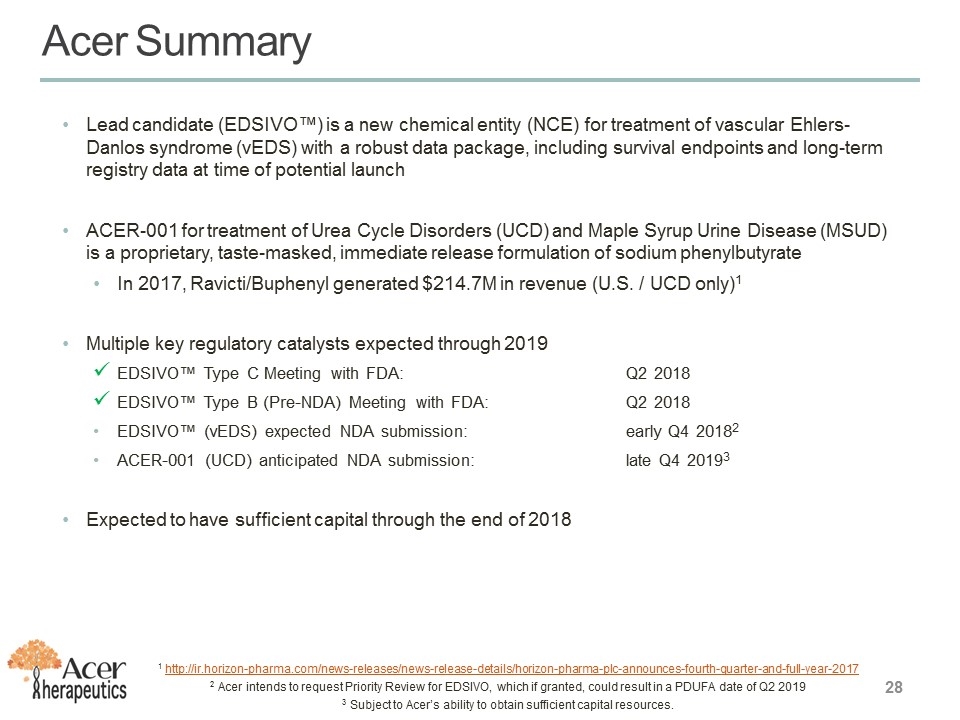
Acer Summary Lead candidate (EDSIVO™) is a new chemical entity (NCE) for treatment of vascular Ehlers-Danlos syndrome (vEDS) with a robust data package, including survival endpoints and long-term registry data at time of potential launch ACER-001 for treatment of Urea Cycle Disorders (UCD) and Maple Syrup Urine Disease (MSUD) is a proprietary, taste-masked, immediate release formulation of sodium phenylbutyrate In 2017, Ravicti/Buphenyl generated $214.7M in revenue (U.S. / UCD only)1 Multiple key regulatory catalysts expected through 2019 EDSIVO™ Type C Meeting with FDA: Q2 2018 EDSIVO™ Type B (Pre-NDA) Meeting with FDA: Q2 2018 EDSIVO™ (vEDS) expected NDA submission: early Q4 20182 ACER-001 (UCD) anticipated NDA submission: late Q4 20193 Expected to have sufficient capital through the end of 2018 1 http://ir.horizon-pharma.com/news-releases/news-release-details/horizon-pharma-plc-announces-fourth-quarter-and-full-year-2017 2 Acer intends to request Priority Review for EDSIVO, which if granted, could result in a PDUFA date of Q2 2019 3 Subject to Acer’s ability to obtain sufficient capital resources.
