Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - NeuroBo Pharmaceuticals, Inc. | ex-99d2.htm |
| 8-K - 8-K - NeuroBo Pharmaceuticals, Inc. | f8-k.htm |
Exhibit 99.1
|
|
ADVANCING CARDIOVASCULAR AND NASH OPPORTUNITIES INDIGO-1 Trial Top-Line Results June 28th, 2018 |
|
|
Safe Harbor Statement This presentation includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Except for statements of historical fact, any information contained in this presentation may be a forward-looking statement that reflects the Company’s current views about future events and are subject to risks, uncertainties, assumptions and changes in circumstances that may cause events or the Company’s actual activities or results to differ significantly from those expressed in any forward-looking statement. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “could,” “would,” “should,” “plan,” “predict,” “potential,” “project,” “promising,” “expect,” “estimate,” “anticipate,” “intend,” “goal,” “strategy,” “believe,” and similar expressions and variations thereof. Forward-looking statements may include statements regarding the Company’s business strategy, market size, potential growth opportunities, capital requirements and use of proceeds, clinical development activities, the timing and results of clinical trials, regulatory submissions, potential regulatory approval and commercialization of the product candidate. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, the Company cannot guarantee future events, results, actions, levels of activity, performance or achievements. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under the heading “Risk Factors” in our filings with the SEC. These forward-looking statements speak only as of the date of this presentation and the Company undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market shares and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. 2 |
|
|
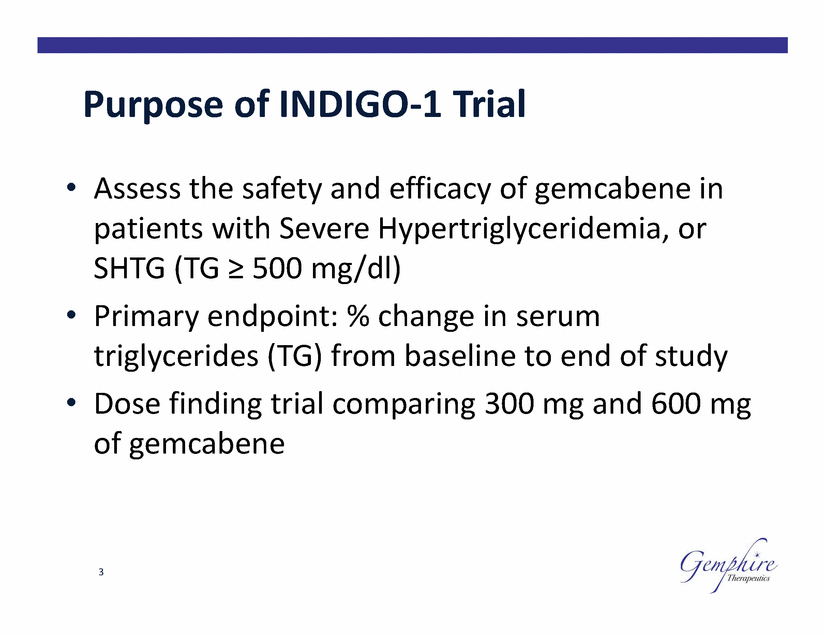
Purpose of INDIGO-1 Trial • Assess the safety and efficacy of gemcabene in patients with Severe Hypertriglyceridemia, or SHTG (TG ≥ 500 mg/dl) Primary endpoint: % change in serum • triglycerides (TG) Dose finding trial of gemcabene from baseline to end of study comparing 300 mg and 600 mg • 3 |
|
|
Study Design INDIGO-1: Phase 2b, Double-Blind, SHTG (TG ≥ 500 mg/dl) Baseline Placebo-Controlled Three Treatment Groups Placebo • • • Adults with TGs of 500-1500 mg/dL 91 pts, randomized into 3 groups Multiple baseline TGs ≥ 500 mg/dl Gemcabene 300 mg QD Gemcabene 600 mg QD 12 Weeks 4 Primary Endpoint: •% change in serum triglycerides (TG) from baseline to 12 weeks Secondary Endpoints: •% change in LDL-C, apoB, non-HDL-C, VLDL-C, and TC •% change in hsCRP and other inflammatory markers •% change in TGs with and without existing statin therapy •Safety and tolerability Locations: •Conducted at 39 sites across the U.S. (37) & Canada (2) |
|
|
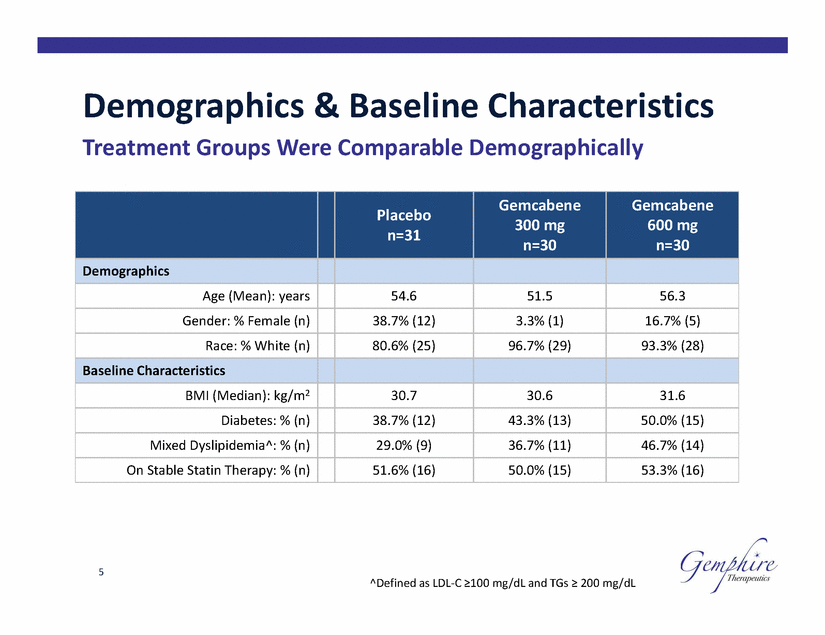
Demographics & Baseline Characteristics Treatment Groups Were Comparable Demographically 600 mg 5 ^Defined as LDL-C ≥100 mg/dL and TGs ≥ 200 mg/dL Placebo n=31 Gemcabene 300 mg n=30 Gemcabene n=30 Demographics Age (Mean): years 54.6 51.5 56.3 Gender: % Female (n) 38.7% (12) 3.3% (1) 16.7% (5) Race: % White (n) 80.6% (25) 96.7% (29) 93.3% (28) Baseline Characteristics BMI (Median): kg/m2 30.7 30.6 31.6 Diabetes: % (n) 38.7% (12) 43.3% (13) 50.0% (15) Mixed Dyslipidemia^: % (n) 29.0% (9) 36.7% (11) 46.7% (14) On Stable Statin Therapy: % (n) 51.6% (16) 50.0% (15) 53.3% (16) |
|
|
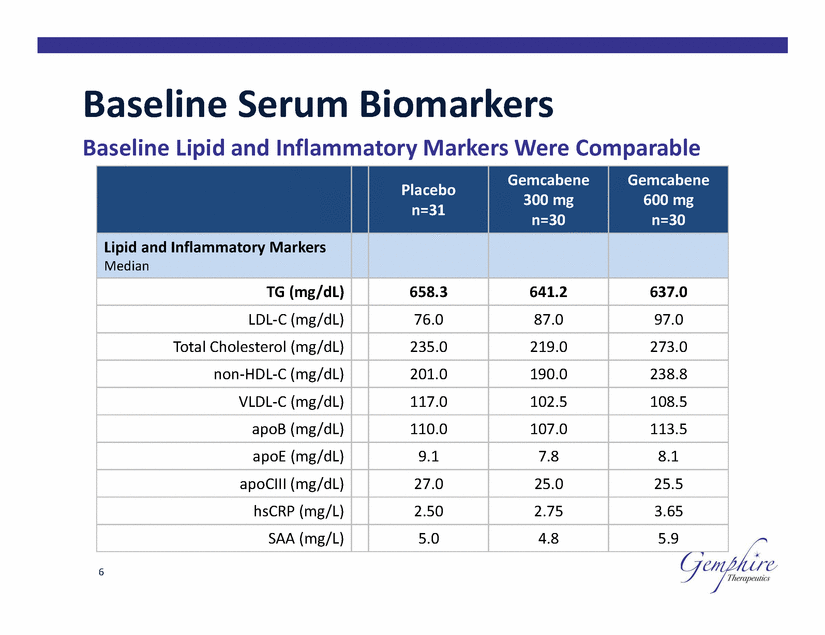
Baseline Serum Biomarkers Baseline Lipid and Inflammatory Markers Were Comparable 600 mg 6 Placebo n=31 Gemcabene 300 mg n=30 Gemcabene n=30 Lipid and Inflammatory Markers Median TG (mg/dL) 658.3 641.2 637.0 LDL-C (mg/dL) 76.0 87.0 97.0 Total Cholesterol (mg/dL) 235.0 219.0 273.0 non-HDL-C (mg/dL) 201.0 190.0 238.8 VLDL-C (mg/dL) 117.0 102.5 108.5 apoB (mg/dL) 110.0 107.0 113.5 apoE (mg/dL) 9.1 7.8 8.1 apoCIII (mg/dL) 27.0 25.0 25.5 hsCRP (mg/L) 2.50 2.75 3.65 SAA (mg/L) 5.0 4.8 5.9 |
|
|
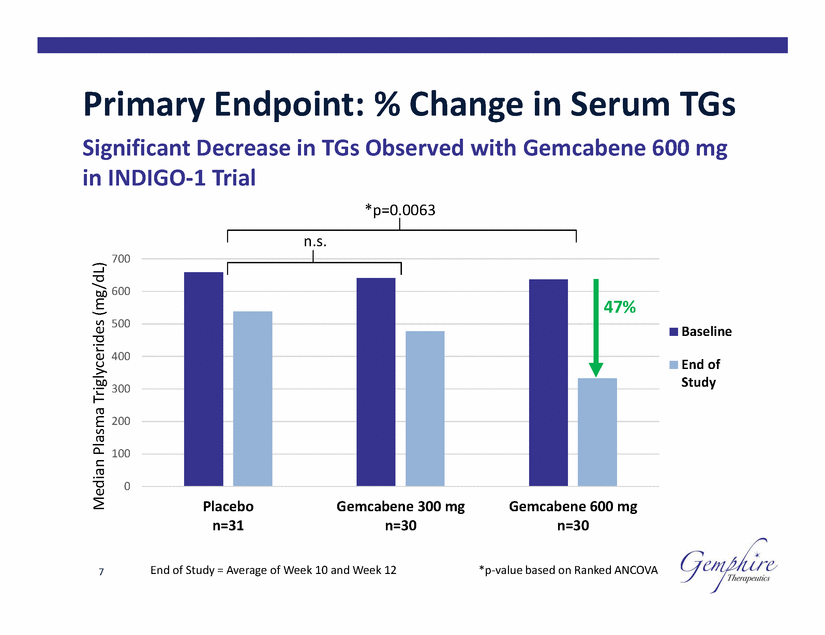
Primary Endpoint: % Change in Serum TGs Significant Decrease in TGs Observed with Gemcabene 600 mg in INDIGO-1 Trial *p=0.0063 n.s. 700 600 500 Baseline 400 End of Study 300 200 100 0 Placebo n=31 Gemcabene 300 mg n=30 Gemcabene 600 mg n=30 End of Study = Average of Week 10 and Week 12 *p-value based on Ranked ANCOVA 7 Median Plasma Triglycerides (mg/dL) 47% |
|
|
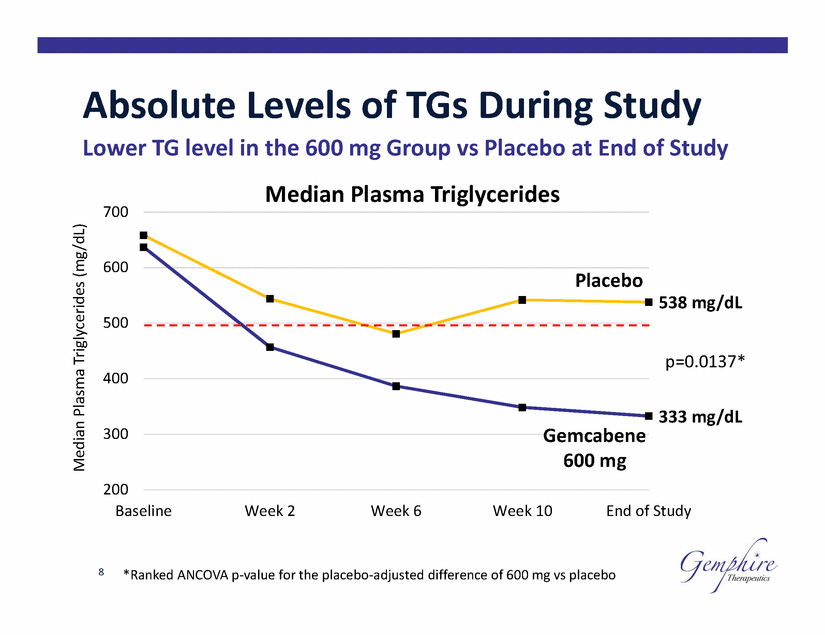
Absolute Levels of TGs During Study Lower TG level in the 600 mg Group vs Placebo at End of Study Median Plasma Triglycerides 700 600 Placebo 538 mg/dL 500 p=0.0137* 400 333 mg/dL 300 Gemcabene 600 mg 200 Baseline Week 2 Week 6 Week 10 End of Study 8 *Ranked ANCOVA p-value for the placebo-adjusted difference of 600 mg vs placebo Median Plasma Triglycerides (mg/dL) |
|
|
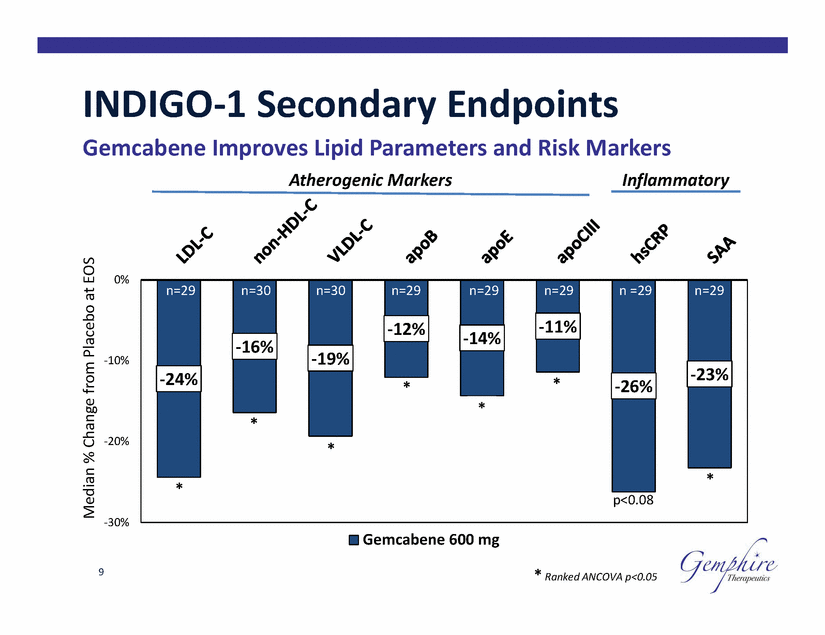
INDIGO-1 Secondary Endpoints Gemcabene Improves Lipid Parameters Atherogenic Markers and Risk Markers Inflammatory 0% -10% -20% * -30% Gemcabene 600 mg 9 * Ranked ANCOVA p<0.05 Median % Change from Placebo at EOS n=29 n=30 n=30 n=29 n=29 n=29 n =29 n=29 -11% -12% -14% -16% -19% -23% -24% *-26% * * * * p<0.08 |
|
|
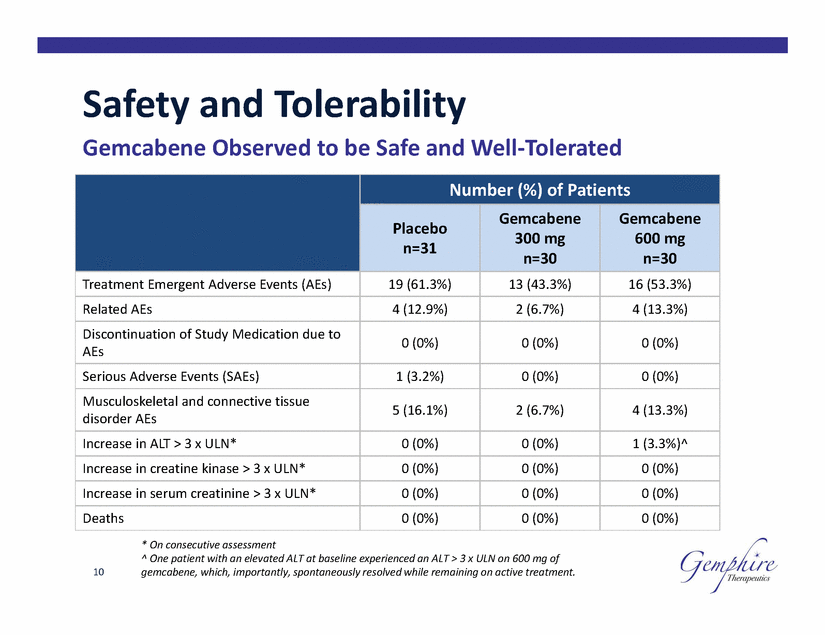
Safety and Tolerability Gemcabene Observed to be Safe and Well-Tolerated 600 mg * On consecutive assessment ^ One patient with an elevated ALT at baseline experienced an ALT > 3 x ULN on 600 mg of gemcabene, which, importantly, spontaneously resolved while remaining on active treatment. 10 Number (%) of Patients Placebo n=31 Gemcabene 300 mg n=30 Gemcabene n=30 Treatment Emergent Adverse Events (AEs) 19 (61.3%) 13 (43.3%) 16 (53.3%) Related AEs 4 (12.9%) 2 (6.7%) 4 (13.3%) Discontinuation of Study Medication due to AEs 0 (0%) 0 (0%) 0 (0%) Serious Adverse Events (SAEs) 1 (3.2%) 0 (0%) 0 (0%) Musculoskeletal and connective tissue disorder AEs 5 (16.1%) 2 (6.7%) 4 (13.3%) Increase in ALT > 3 x ULN* 0 (0%) 0 (0%) 1 (3.3%)^ Increase in creatine kinase > 3 x ULN* 0 (0%) 0 (0%) 0 (0%) Increase in serum creatinine > 3 x ULN* 0 (0%) 0 (0%) 0 (0%) Deaths 0 (0%) 0 (0%) 0 (0%) |
|
|
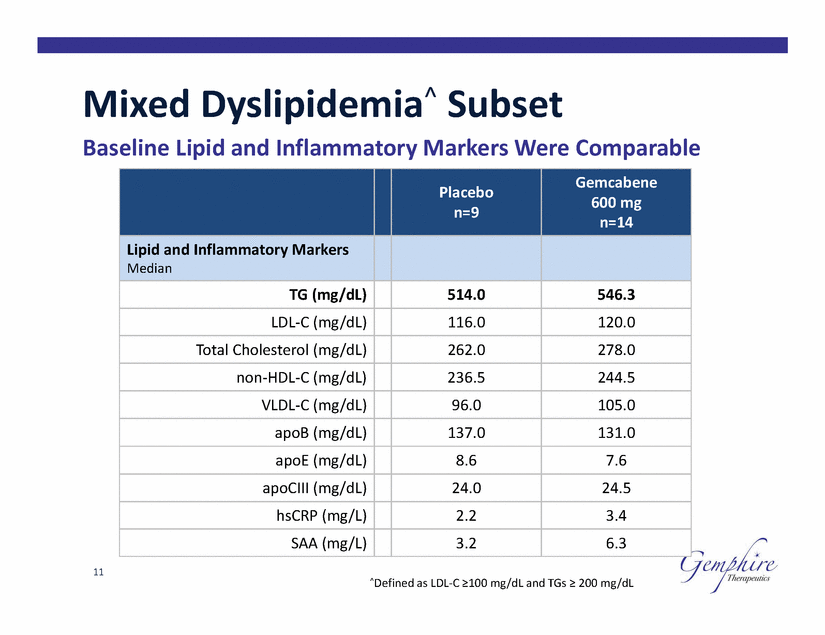
Mixed Dyslipidemia^ Subset Baseline Lipid and Inflammatory Markers Were Comparable 600 mg 11 ^Defined as LDL-C ≥100 mg/dL and TGs ≥ 200 mg/dL Placebo n=9 Gemcabene n=14 Lipid and Inflammatory Markers Median TG (mg/dL) 514.0 546.3 LDL-C (mg/dL) 116.0 120.0 Total Cholesterol (mg/dL) 262.0 278.0 non-HDL-C (mg/dL) 236.5 244.5 VLDL-C (mg/dL) 96.0 105.0 apoB (mg/dL) 137.0 131.0 apoE (mg/dL) 8.6 7.6 apoCIII (mg/dL) 24.0 24.5 hsCRP (mg/L) 2.2 3.4 SAA (mg/L) 3.2 6.3 |
|
|
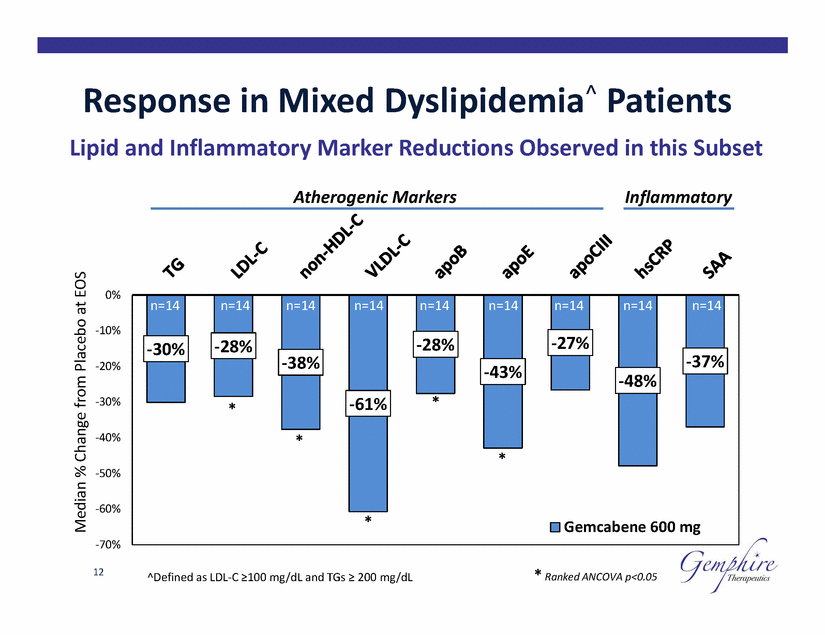
Response in Mixed Dyslipidemia^ Patients Lipid and Inflammatory Marker Reductions Observed in this Subset Atherogenic Markers Inflammatory 0% -10% -20% -30% -40% -50% -60% -70% 12 * Ranked ANCOVA p<0.05 ^Defined as LDL-C ≥100 mg/dL and TGs ≥ 200 mg/dL Median % Change from Placebo at EOS n=14 n=14 n=14 n=14 n=14 n=14 n=14 n=14 n=14 -27% -30% -28% -28% -38% -37% -43% -48% -61% * * * *Gemcabene 600 mg |
|
|
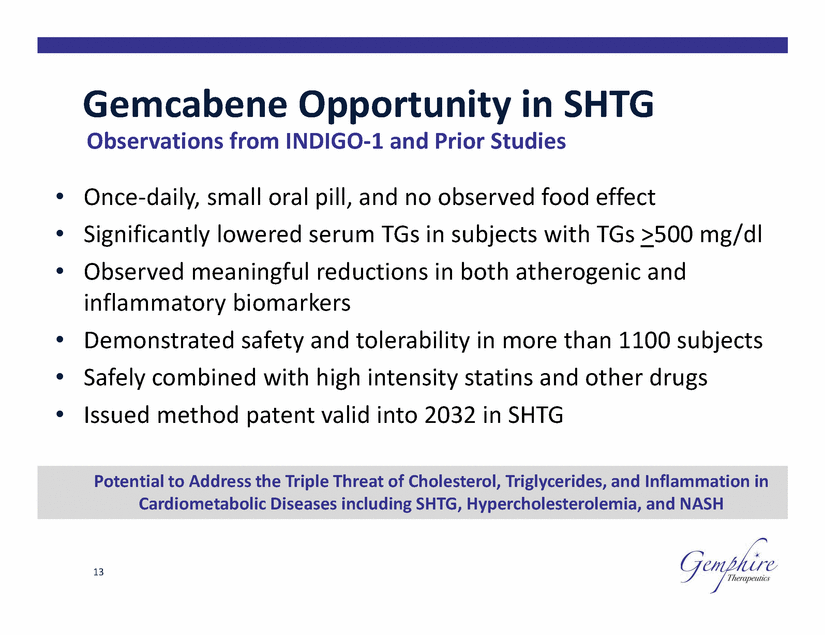
Gemcabene Opportunity in SHTG Observations from INDIGO-1 and Prior Studies • • • Once-daily, small oral pill, and no observed food effect Significantly lowered serum TGs in subjects with TGs >500 Observed meaningful reductions in both atherogenic and inflammatory biomarkers mg/dl • • • Demonstrated safety and tolerability in more than 1100 subjects Safely combined with high intensity statins and Issued method patent valid into 2032 in SHTG other drugs 13 Potential to Address the Triple Threat of Cholesterol, Triglycerides, and Inflammation in Cardiometabolic Diseases including SHTG, Hypercholesterolemia, and NASH |