Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Natera, Inc. | a18-16102_18k.htm |
Safe Harbor This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding the market opportunity, products, commercial partners, user experience, clinical trials, financial performance, strategies, anticipated future performance and general business conditions of Natera, Inc. (“Natera”, the “Company”, “we” or “us”), are forward-looking statements. These forward-looking statements are subject to known and unknown risks and uncertainties that may cause actual results to differ materially, including: we may be unable to develop and successfully commercialize new products; our estimates of market opportunity and forecasts of market growth may prove to be inaccurate, and even if the market in which we compete achieves the forecasted growth, our business could fail to grow at similar rates; we may be unable to compete successfully with either existing or future prenatal testing or oncology diagnostic products or other test methods; our products may not perform as expected; the results of our clinical studies may not support the use of our tests, particularly in the average-risk pregnancy population or for microdeletions screening, or may not be able to be replicated in later studies required for regulatory approvals or clearances; if our sole CLIA-certified laboratory facility becomes inoperable, we will be unable to perform our tests and our business will be harmed; we rely on a limited number of suppliers or, in some cases, single suppliers, for some of our laboratory instruments and materials and may not be able to find replacements or immediately transition to alternative suppliers; if we are unable to successfully scale our operations, our business could suffer; the marketing, sale, and use of our products could result in substantial damages arising from product liability or professional liability claims that exceed our resources; we may be unable to expand third-party payer coverage and reimbursement for our tests, and we may be required to refund reimbursements already received; third-party payers may withdraw coverage or provide lower levels of reimbursement due to changing policies, billing complexities or other factors, such as the increased focus by third-party payers on requiring that prior authorization be obtained prior to conducting a test; if the FDA were to begin actively regulating our tests, we could incur substantial costs and delays associated with trying to obtain premarket clearance or approval and incur costs associated with complying with post-market controls; we could be subject to third party claims of intellectual property infringement, which could result in litigation or other proceedings and could limit our ability to commercialize our products or services; and any failure to obtain, maintain, and enforce our intellectual property rights could impair our ability to protect our proprietary technology and our brand. We discuss these and other risks and uncertainties in greater detail in the sections entitled “Risk Factors” and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in the filings we make with the SEC from time to time. Given these uncertainties, you should not place undue reliance on the forward-looking statements. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on its business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statement. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied. Except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations. We file reports, proxy statements, and other information with the SEC. Such reports, proxy statements, and other information concerning us can be read and copied at the SEC’s Public Reference Room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549 or on the Internet at http://www.sec.gov. Please call the SEC at 1-800-SEC-0330 for further information on the Public Reference Room. Our common stock is listed on the NASDAQ Global Select Market, and these reports, proxy statements and other information are also available for inspection at the offices of the NASDAQ Stock Market, Inc. located at 1735 K Street, NW, Washington, D.C. 20006. We will provide without charge upon written or oral request a copy of any or all of the documents that are incorporated by reference into this prospectus, other than exhibits which are specifically incorporated by reference into such documents. Requests should be directed to our Investor Relations department at Natera, Inc., 201 Industrial Road, Suite 410, San Carlos, California 94070. Our telephone number is (650) 249-9090. 2

Agenda Kidney transplant rejection New data from UCSF study demonstrates 92% sensitivity for acute rejection Targets greater than $2bn market (estimated) with established CMS pricing at ~$2,800 per test Breast cancer update Preliminary data very promising, to be submitted for peer review Two additional studies in pipeline Reinforces large pan-cancer market opportunity > $12bn 3
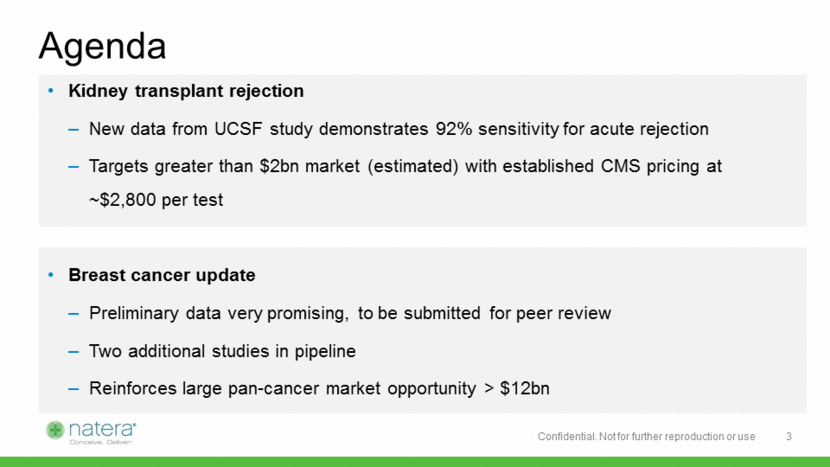
Natera’s Technology Designed to Analyze Cell-Free DNA 10+ years of experience with cfDNA, over 1 million tests performed Single molecule sensitivity in a tube of blood COGS below $200 per sample 4 Proprietary Molecular • >20,000 targets in one reaction, no custom equipment • Low DNA input and high yield: as low as single cell (6pg) Proprietary Bioinformatics • Proprietary algorithms using HapMap, TCGA, Cosmic • Cloud - based algorithms for signal processing on big data NIPT Assay IVF Assay Cancer Assay Prenatal Paternity Assay Forensics Assay Elastic Cloud Computing and Storage Platform Provisioning, Job Management, Billing Core Sequencing Security and Compliance Proton Other Platforms Copy Number Module Mutation Analysis Module Identify Module Illumina MLB Optim Cloud AA BB A B BBA AAB
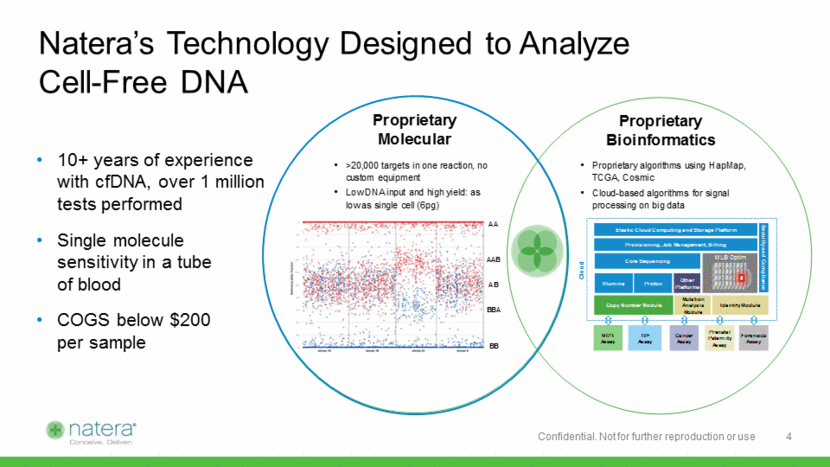
Applications Across Multiple Indications Panorama Horizon Vistara Spectrum Anora Prenatal Care Lung Bladder Colon Breast Additional indications Oncology Renal Additional indications Transplant 5
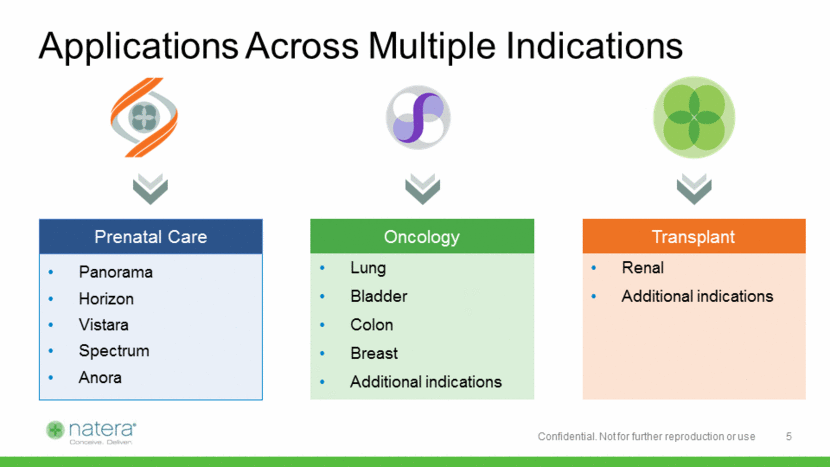
Unmet Need in Renal Transplant Monitoring 1 HTTPS://OPTN.transplant.hrsa.gov/ data/view-data/national data/# ~190,000 living kidney transplant recipients in the U.S. ~20,000 kidney transplants performed in U.S. per annum1 Approximately 15-20% suffer acute rejection in first year Patients must be monitored throughout their lifetime for graft rejection; patients often over-immunosuppressed Existing surveillance methods have significant limitations: Invasive/expensive (biopsy) or clinically inadequate (serum creatinine) Measurement of donor-derived cell free DNA emerging as a superior monitoring technology 6
UCSF Study Overview 292 samples from 187 patients were analyzed AR (52 samples) Non-AR (240 samples) 7 Acute Rejection, 52 Borderline Rejection , 82 Other Injury , 85 Stable , 73 Classified into 4 groups by biopsy: Acute Rejection Borderline Rejection Other Injury Stable
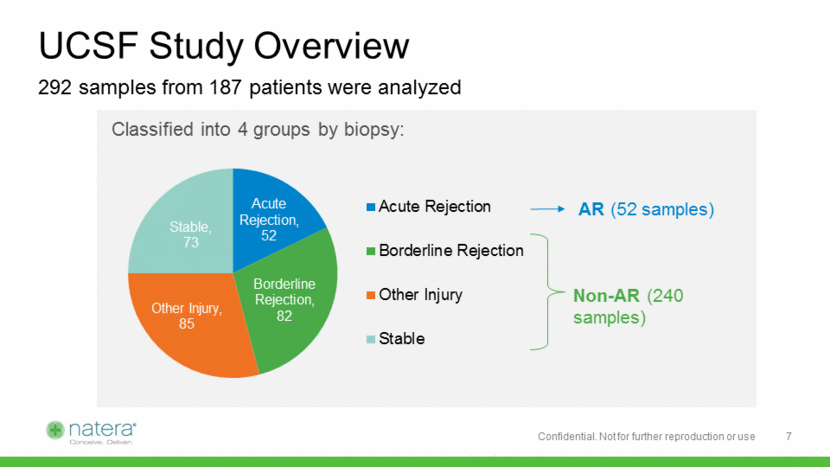
Levels of Donor DNA Significantly Higher in Patients Suffering Acute Rejection Boxes indicate inter-quartile range, horizonal lines represent medians.*Non-AR includes Stable, Borderline Rejection, and Other Injury Positive Negative dd-cfDNA (%) 1% threshold Acute Rejection (n=52) Non-AR (n=240) Significance level p<0.0001 2.8% 0.5% *** Sensitivity: 92.3% Specificity: 72.9% Area Under Curve (AUC): 0.90 2.8% 0.5% 8 > 95% of positive results had clinically meaningful findings
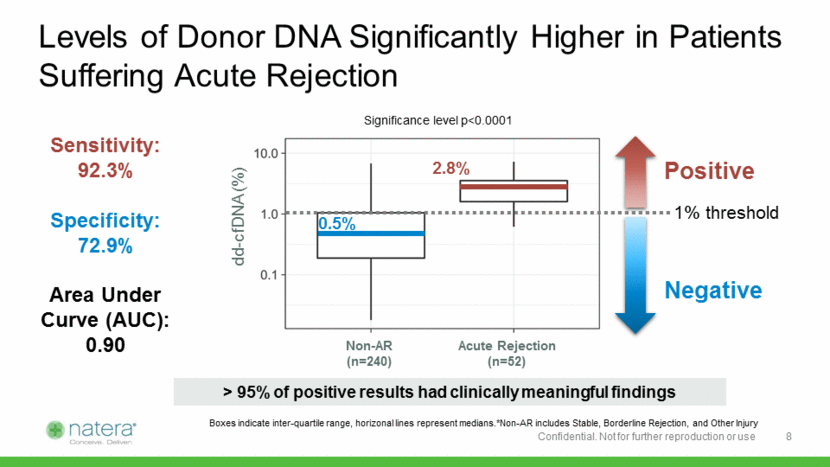
Specificity Among Stable Patients is Even Higher Positive Negative dd-cfDNA (%) 1% threshold 2.8% 0.2% Acute Rejection (n=52) Stable Only (n=73) Sensitivity: 92.3% Specificity: 93.2% Area Under Curve (AUC): 0.95 Significance level p<0.0001 Boxes indicate inter-quartile range, horizonal lines represent medians 9
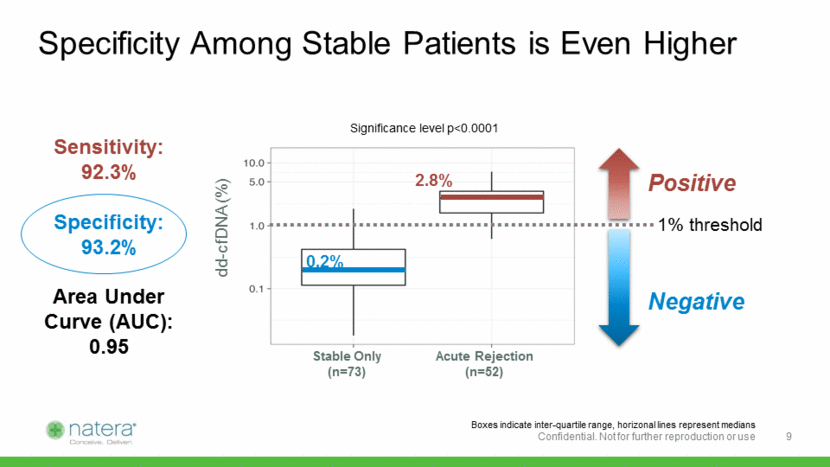
Natera Assay Outperforms Competition Natera study1 (n=292 samples) Bloom et al.2 (n=107 samples) Performance Metrics Sensitivity 92% (n=52) 59% (n=27) Specificity 73% (n=240) 85% (n=80) AUC 0.90 0.74 Assuming 25% Prevalence (higher risk population) NPV 97% 86% PPV 53% 57% Assuming 15% Prevalence (average risk population) NPV 98% 92% PPV 38% 41% 1 Based on SNP-based dd-cfDNA analyses described here (292 plasma specimens from 187 unique kidney transplant recipients). 2 From Bloom RD, et al. Cell-Free DNA and Active Rejection in Kidney Allografts. J Am Soc Nephrol. 2017;28(7):2221-2232. 10
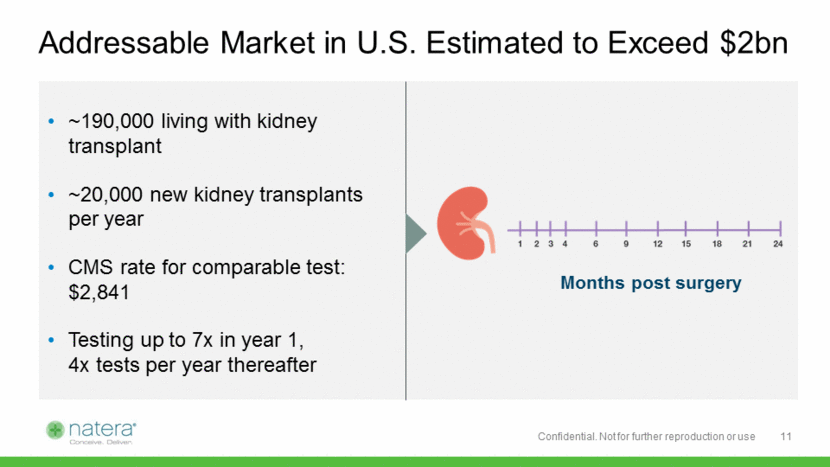
Addressable Market in U.S. Estimated to Exceed $2bn ~190,000 living with kidney transplant ~20,000 new kidney transplants per year CMS rate for comparable test: $2,841 Testing up to 7x in year 1, 4x tests per year thereafter Months post surgery 11
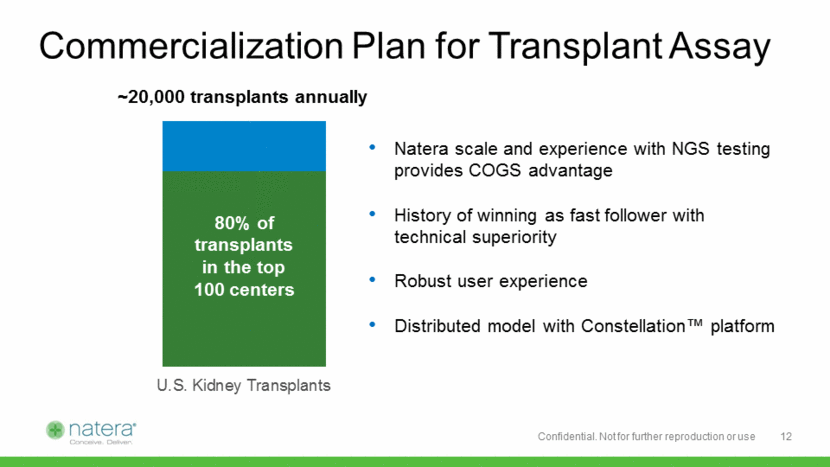
Commercialization Plan for Transplant Assay Natera scale and experience with NGS testing provides COGS advantage History of winning as fast follower with technical superiority Robust user experience Distributed model with Constellation™ platform 12 80% of transplants in the top 100 centers U.S. Kidney Transplants ~20,000 transplants annually
Patent-Protected Technology 13 Natera approach does not use transplant-specific markers and does not require advance determination of donor or recipient genotypes Natera technology already protected by two granted European patents, one issued U.S. patent, and at least four pending EU and US patent applications Patents corresponding to the first-generation cfDNA-based approaches do not apply to us
Oncology Update Signatera™(RUO) in Breast Cancer

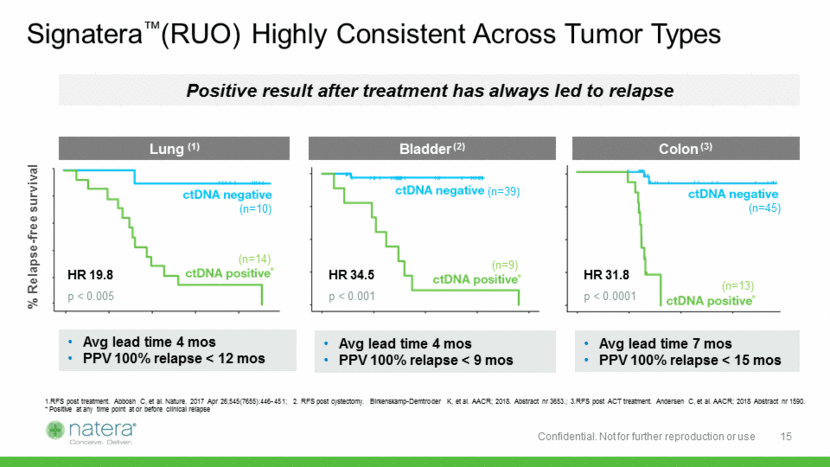
% Relapse-free survival Signatera™(RUO) Highly Consistent Across Tumor Types (n=10) (n=39) (n=14) (n=9) (n=45) (n=13) HR 19.8 p < 0.005 HR 34.5 p < 0.001 HR 31.8 p < 0.0001 1.RFS post treatment. Abbosh C, et al. Nature. 2017 Apr 26;545(7655):446-451; 2. RFS post cystectomy. Birkenskamp-Demtroder K, et al. AACR; 2018. Abstract nr 3653.; 3.RFS post ACT treatment. Andersen C, et al. AACR; 2018 Abstract nr 1590. * Positive at any time point at or before clinical relapse Lung (1) Bladder (2) Colon (3) Avg lead time 4 mos PPV 100% relapse < 12 mos Avg lead time 4 mos PPV 100% relapse < 9 mos Avg lead time 7 mos PPV 100% relapse < 15 mos * * * Positive result after treatment has always led to relapse 15
1 2 3 ISPY-2: Neoadjuvant Leicester/ICL: Adjuvant/RM Jules Bordet: Neoadjuvant/RM PI: Laura Esserman, MD, PhD 182 patients 327 plasma time points Objective: Evaluate Signatera as marker of molecular response to neoadjuvant therapy, in comparison with radiological and pathological response criteria PIs: Jacqui Shaw, MD, PhD Charles Coombes, MD, PhD 49 patients 218 plasma time points Objective: Evaluate Signatera as marker of molecular relapse, after completion surgery and adjuvant chemotherapy, in comparison to radiology PI: Michail Ignatiadis, MD,PhD 80 patients 300 plasma time points Objectives: Evaluate Signatera as marker of molecular response to neoadjuvant therapy, in comparison with radiological and pathological response criteria Evaluate Signatera as marker of molecular relapse, after completion of neoadjuvant therapy and definitive surgery, in comparison to radiology 16 3 Prospective Breast Cancer Studies for Different Indications
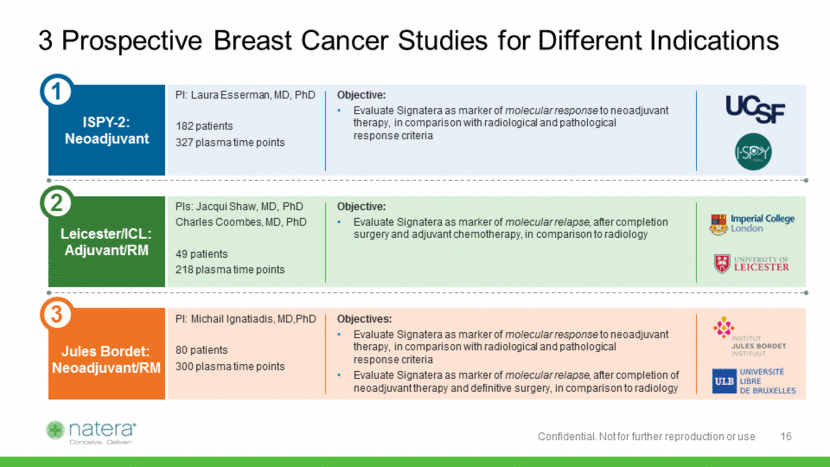
Study design: Blood collection every 6 months, in a cohort of 49 breast cancer patients starting at least 3 years after conclusion of treatment Key output metrics: relapse detection rate, lead time vs. imaging Adjuvant 36 42 48 54 60 Months Longitudinal Plasma Samples Scan Scan Tumor Scan Study Design in Post-Treatment Setting Collaboration with University of Leicester and Imperial College of London Subtype Patients ER/PR+/HER2- 34 HER2+ 8 TNBC 7 Total 49 17
HER2+ Therapeutic Decision Making in the MRD Setting 18 Unmet need: Neratinib recently approved for treatment in HER2+ patients after a full year of trastuzumab. Only 2% survival benefit with severe side effects. Patients: > 40,000 new diagnoses per year with HER2+ disease1 Cost of neratinib treatment: $10,400 per month Use of neratinib could be considered in patients with early stage HER2-positive breast cancer and clinical features that indicate a higher likelihood of relapse.2 Dr. Alexandra Zimmer, M.D. Women’s Malignancies Branch of NCI Center for Cancer Research “ “ 1 American Cancer Society. Breast Cancer Facts & Figures 2017-2018. 2https://www.cancer.gov/news-events/cancer-currents-blog/2017/neratinib-breast-cancer-fda
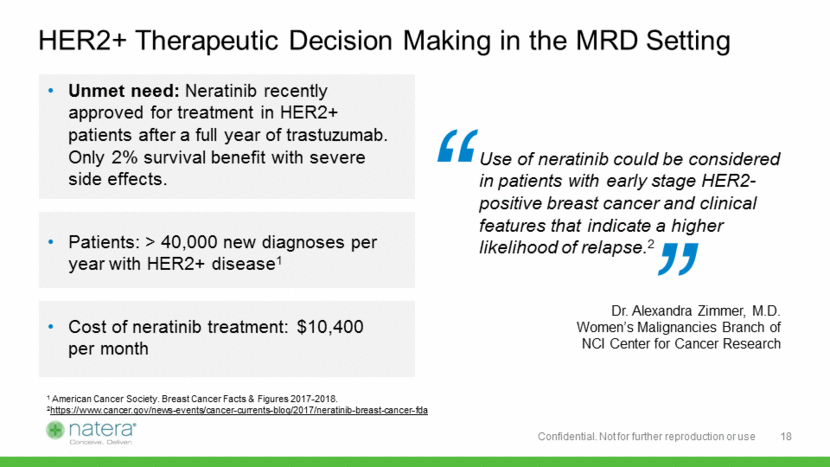
Establishing the Recurrence Monitoring Indication 19 Recurrence Monitoring Protocols Oncologist / Radiologist Setting Primary Care / OBGYN Setting DCIS + 30%5 of non-TNBC Stage 1 0 – 5 years post-dx New cases: 96,000/yr1,2,5 Prevalence: > 400,0002,3 Disease-free patients 5 – 15 years post dx New cases: 230,000/yr1,2 Prevalence: >1.5M2,3 Stage 2+ and 70%5 of Stage 1 0 – 5 years post-dx New cases: 232,000/yr1,2,5 Prevalence: >800,0002,3 ACS Breast Cancer Facts & Figures 2017-2018 JAMA. 2015;313(2):165-173. doi:10.1001/jama.2014.17322 SEER database Prevalence Estimates: link Del Turco. JAMA 1994;271:1593-1597 Internal estimates Confidential. Not for further reproduction or use Over 70% of relapses are distant, not local2,4
Commercialization Strategy 20 Confidential. Not for further reproduction or use Pharma: RUO & CLIA, existing team with 18 signed deals Oncologists, other providers – targeted directly and/or with channel partners Low hanging fruit within current guidelines: HER2+, CRC II, Lung Ib Other indications: leverage clinical studies funded by pharma and others to develop utility evidence for regulatory and CMS approval LCD and private payor coverage for near-term indications NCD via Joint FDA/CMS pathway Cash pay upfront & INTL – inbound interest for recurrence monitoring Channels Indications Reimbursement
Powered By NATERA
[LOGO]


