Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - TYME TECHNOLOGIES, INC. | d543275d8k.htm |
SM-88 First Human Study (FHS) and Compassionate Use Program Clinical Analysis June 2018 NASDAQ: TYME Exhibit 99.1

Safe Harbor Statement Certain statements in this presentation and associated oral statements are "forward-looking statements" under the Private Securities Litigation Reform Act. These forward-looking statements are based on our current expectations and beliefs and are subject to a number of risk factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Such risks and uncertainties include the risk that the Company does not complete its development work, the risks of FDA approval delay of (or failure to approve) our drug candidate as well as the risks inherent in commercializing a new product (including technology risks, market risks, financial risks and implementation risks, and other risks and uncertainties affecting the Company), as well as other risks that have been disclosed by us in our SEC filings. We disclaim and do not undertake any obligation to revise any forward-looking statements, including, without limitation, financial estimates, whether as a result of new information, future events, or otherwise. None of the first human study data, compassionate use data, Phase Ib/II data, or select case study data contained herein represent forward-looking statements, and such data is not necessarily representative of future patient outcomes for SM-88 and should not be relied upon as predictive information for therapeutic, regulatory approval or other purposes. This presentation does not constitute an offer to sell or the solicitation of an offer to buy any securities, nor will there be any sale of securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. June 2018
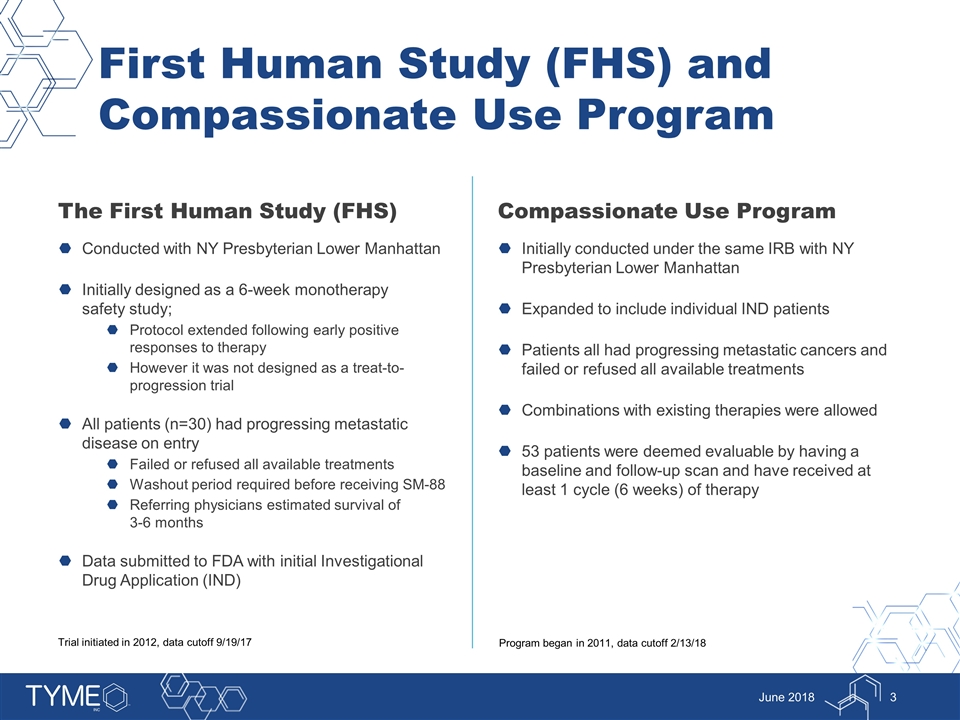
First Human Study (FHS) and Compassionate Use Program The First Human Study (FHS) Conducted with NY Presbyterian Lower Manhattan Initially designed as a 6-week monotherapy safety study; Protocol extended following early positive responses to therapy However it was not designed as a treat-to-progression trial All patients (n=30) had progressing metastatic disease on entry Failed or refused all available treatments Washout period required before receiving SM-88 Referring physicians estimated survival of 3-6 months Data submitted to FDA with initial Investigational Drug Application (IND) Compassionate Use Program Initially conducted under the same IRB with NY Presbyterian Lower Manhattan Expanded to include individual IND patients Patients all had progressing metastatic cancers and failed or refused all available treatments Combinations with existing therapies were allowed 53 patients were deemed evaluable by having a baseline and follow-up scan and have received at least 1 cycle (6 weeks) of therapy June 2018 Trial initiated in 2012, data cutoff 9/19/17 Program began in 2011, data cutoff 2/13/18
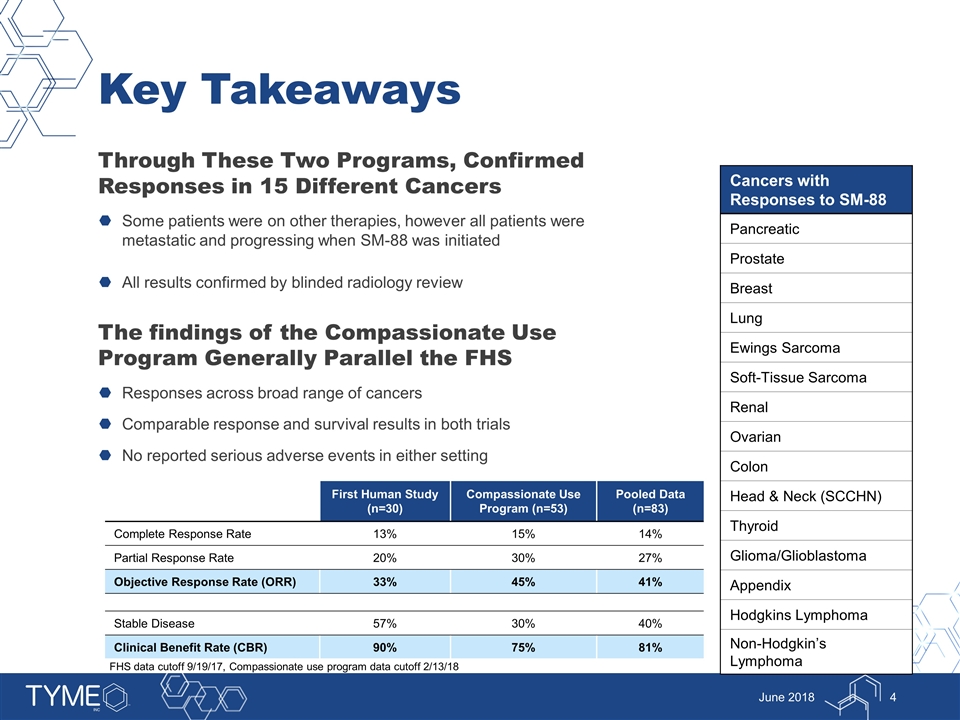
Key Takeaways Through These Two Programs, Confirmed Responses in 15 Different Cancers Some patients were on other therapies, however all patients were metastatic and progressing when SM-88 was initiated All results confirmed by blinded radiology review The findings of the Compassionate Use Program Generally Parallel the FHS Responses across broad range of cancers Comparable response and survival results in both trials No reported serious adverse events in either setting June 2018 Cancers with Responses to SM-88 Pancreatic Prostate Breast Lung Ewings Sarcoma Soft-Tissue Sarcoma Renal Ovarian Colon Head & Neck (SCCHN) Thyroid Glioma/Glioblastoma Appendix Hodgkins Lymphoma Non-Hodgkin’s Lymphoma First Human Study (n=30) Compassionate Use Program (n=53) Pooled Data (n=83) Complete Response Rate 13% 15% 14% Partial Response Rate 20% 30% 27% Objective Response Rate (ORR) 33% 45% 41% Stable Disease 57% 30% 40% Clinical Benefit Rate (CBR) 90% 75% 81% FHS data cutoff 9/19/17, Compassionate use program data cutoff 2/13/18
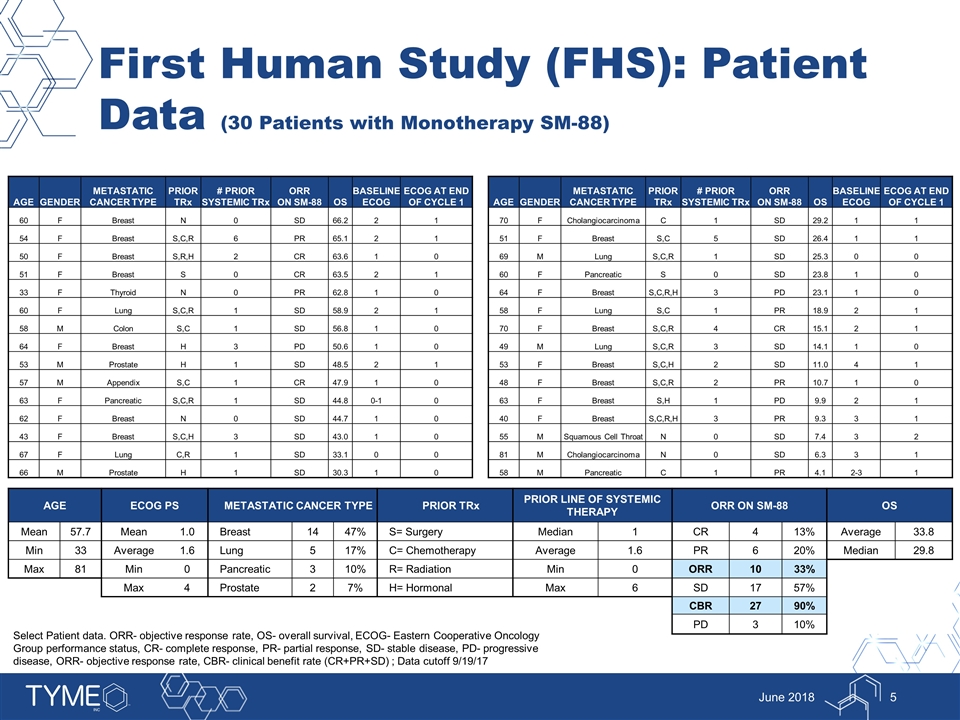
First Human Study (FHS): Patient Data (30 Patients with Monotherapy SM-88) June 2018 AGE ECOG PS METASTATIC CANCER TYPE PRIOR TRx PRIOR LINE OF SYSTEMIC THERAPY ORR ON SM-88 OS Mean 57.7 Mean 1.0 Breast 14 47% S= Surgery Median 1 CR 4 13% Average 33.8 Min 33 Average 1.6 Lung 5 17% C= Chemotherapy Average 1.6 PR 6 20% Median 29.8 Max 81 Min 0 Pancreatic 3 10% R= Radiation Min 0 ORR 10 33% Max 4 Prostate 2 7% H= Hormonal Max 6 SD 17 57% CBR 27 90% PD 3 10% Select Patient data. ORR- objective response rate, OS- overall survival, ECOG- Eastern Cooperative Oncology Group performance status, CR- complete response, PR- partial response, SD- stable disease, PD- progressive disease, ORR- objective response rate, CBR- clinical benefit rate (CR+PR+SD) ; Data cutoff 9/19/17 AGE GENDER METASTATIC CANCER TYPE PRIOR TRx # PRIOR SYSTEMIC TRx ORR ON SM-88 OS BASELINE ECOG ECOG AT END OF CYCLE 1 60 F Breast N 0 SD 66.2 2 1 54 F Breast S,C,R 6 PR 65.1 2 1 50 F Breast S,R,H 2 CR 63.6 1 0 51 F Breast S 0 CR 63.5 2 1 33 F Thyroid N 0 PR 62.8 1 0 60 F Lung S,C,R 1 SD 58.9 2 1 58 M Colon S,C 1 SD 56.8 1 0 64 F Breast H 3 PD 50.6 1 0 53 M Prostate H 1 SD 48.5 2 1 57 M Appendix S,C 1 CR 47.9 1 0 63 F Pancreatic S,C,R 1 SD 44.8 0-1 0 62 F Breast N 0 SD 44.7 1 0 43 F Breast S,C,H 3 SD 43.0 1 0 67 F Lung C,R 1 SD 33.1 0 0 66 M Prostate H 1 SD 30.3 1 0 AGE GENDER METASTATIC CANCER TYPE PRIOR TRx # PRIOR SYSTEMIC TRx ORR ON SM-88 OS BASELINE ECOG ECOG AT END OF CYCLE 1 70 F Cholangiocarcinoma C 1 SD 29.2 1 1 51 F Breast S,C 5 SD 26.4 1 1 69 M Lung S,C,R 1 SD 25.3 0 0 60 F Pancreatic S 0 SD 23.8 1 0 64 F Breast S,C,R,H 3 PD 23.1 1 0 58 F Lung S,C 1 PR 18.9 2 1 70 F Breast S,C,R 4 CR 15.1 2 1 49 M Lung S,C,R 3 SD 14.1 1 0 53 F Breast S,C,H 2 SD 11.0 4 1 48 F Breast S,C,R 2 PR 10.7 1 0 63 F Breast S,H 1 PD 9.9 2 1 40 F Breast S,C,R,H 3 PR 9.3 3 1 55 M Squamous Cell Throat N 0 SD 7.4 3 2 81 M Cholangiocarcinoma N 0 SD 6.3 3 1 58 M Pancreatic C 1 PR 4.1 2-3 1
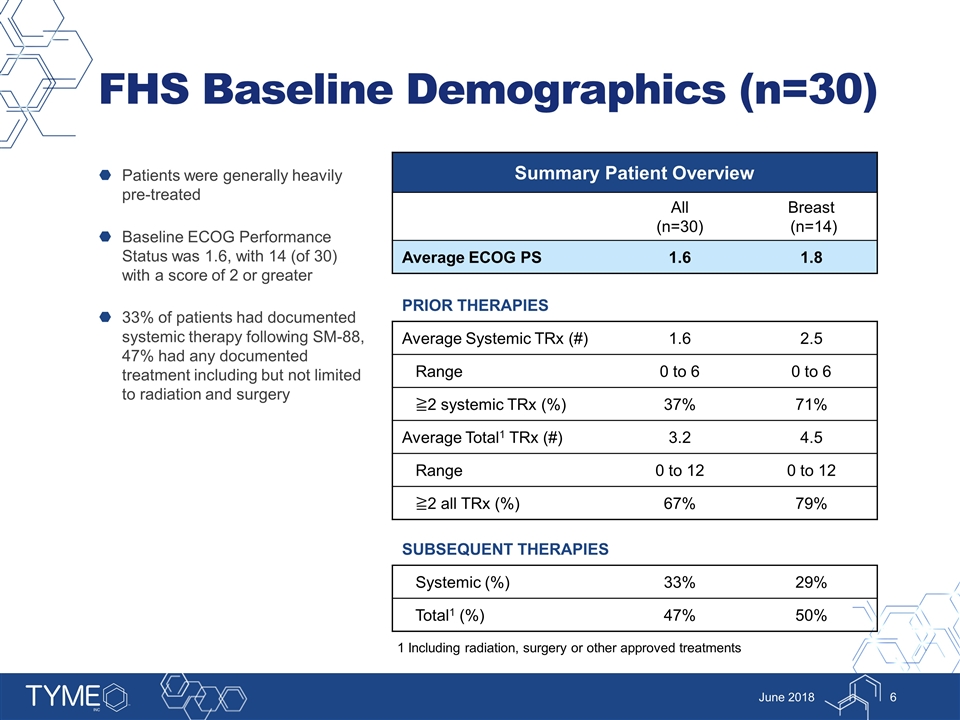
FHS Baseline Demographics (n=30) Patients were generally heavily pre-treated Baseline ECOG Performance Status was 1.6, with 14 (of 30) with a score of 2 or greater 33% of patients had documented systemic therapy following SM-88, 47% had any documented treatment including but not limited to radiation and surgery June 2018 Summary Patient Overview All (n=30) Breast (n=14) Average ECOG PS 1.6 1.8 PRIOR THERAPIES Average Systemic TRx (#) 1.6 2.5 Range 0 to 6 0 to 6 ≧2 systemic TRx (%) 37% 71% Average Total1 TRx (#) 3.2 4.5 Range 0 to 12 0 to 12 ≧2 all TRx (%) 67% 79% SUBSEQUENT THERAPIES Systemic (%) 33% 29% Total1 (%) 47% 50% 1 Including radiation, surgery or other approved treatments
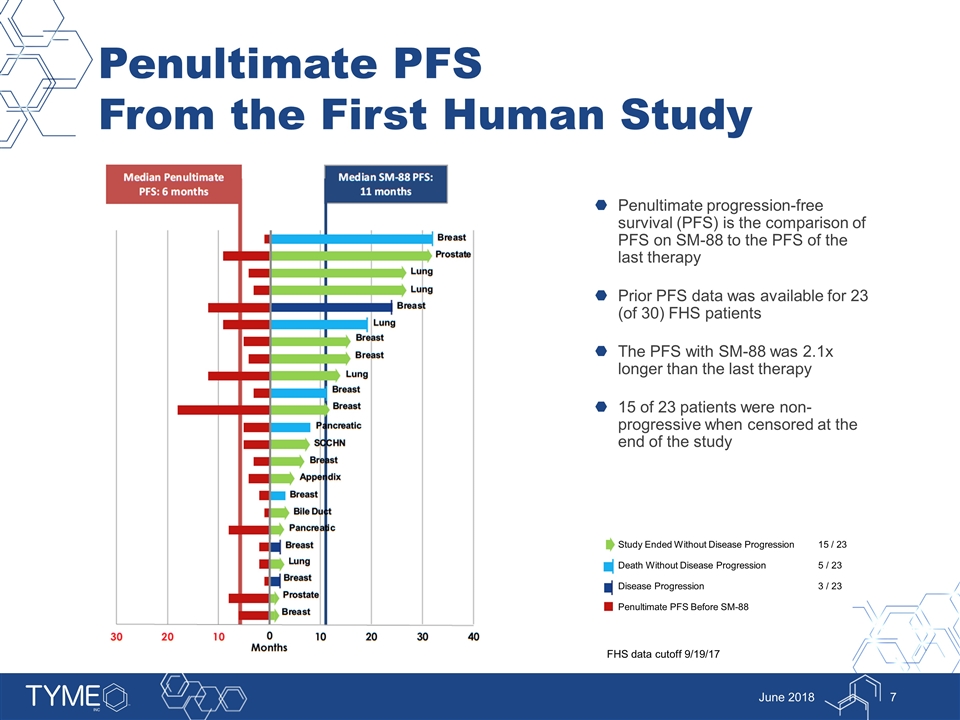
Study Ended Without Disease Progression 15 / 23 Death Without Disease Progression 5 / 23 Disease Progression 3 / 23 Penultimate PFS Before SM-88 Penultimate PFS From the First Human Study Penultimate progression-free survival (PFS) is the comparison of PFS on SM-88 to the PFS of the last therapy Prior PFS data was available for 23 (of 30) FHS patients The PFS with SM-88 was 2.1x longer than the last therapy 15 of 23 patients were non-progressive when censored at the end of the study June 2018 FHS data cutoff 9/19/17
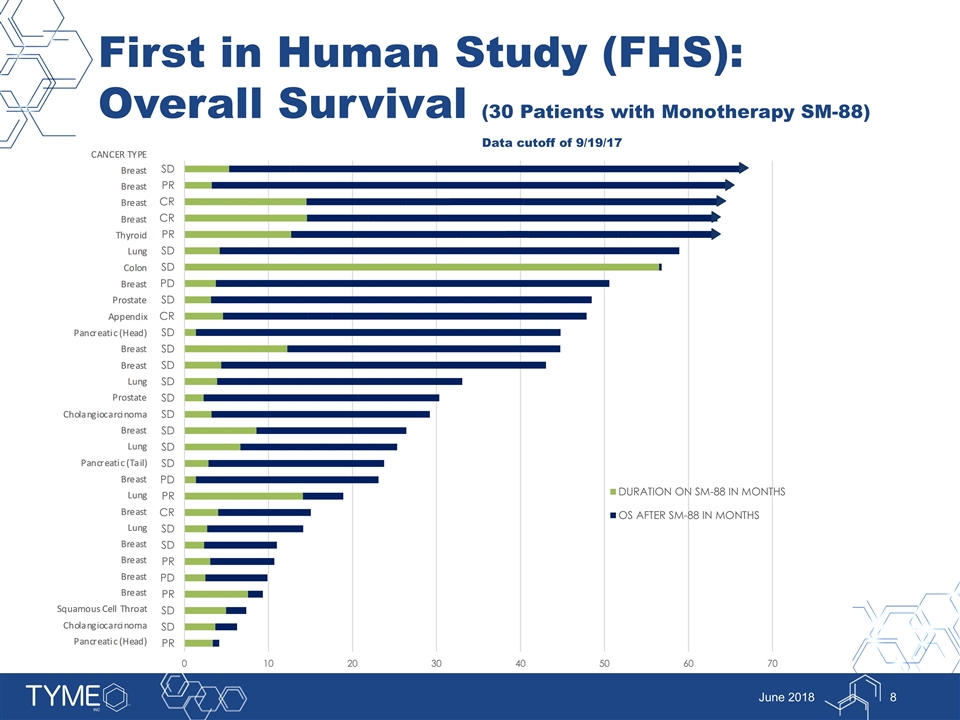
First in Human Study (FHS): Overall Survival (30 Patients with Monotherapy SM-88) Data cutoff of 9/19/17 June 2018
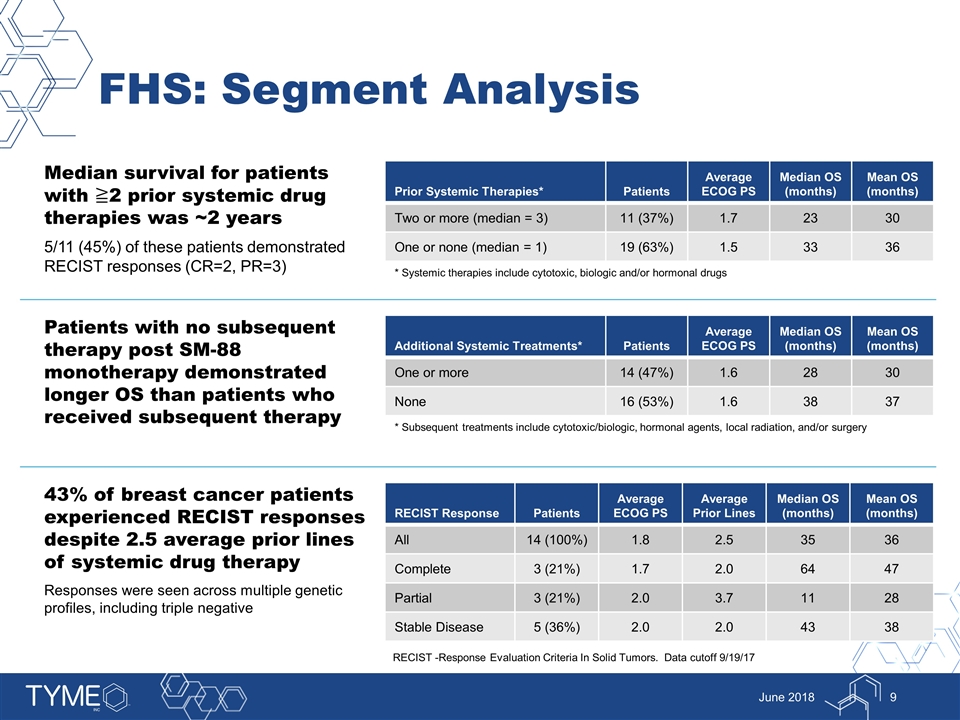
FHS: Segment Analysis June 2018 Median survival for patients with ≧2 prior systemic drug therapies was ~2 years 5/11 (45%) of these patients demonstrated RECIST responses (CR=2, PR=3) Prior Systemic Therapies* Patients Average ECOG PS Median OS (months) Mean OS (months) Two or more (median = 3) 11 (37%) 1.7 23 30 One or none (median = 1) 19 (63%) 1.5 33 36 * Systemic therapies include cytotoxic, biologic and/or hormonal drugs Patients with no subsequent therapy post SM-88 monotherapy demonstrated longer OS than patients who received subsequent therapy Additional Systemic Treatments* Patients Average ECOG PS Median OS (months) Mean OS (months) One or more 14 (47%) 1.6 28 30 None 16 (53%) 1.6 38 37 * Subsequent treatments include cytotoxic/biologic, hormonal agents, local radiation, and/or surgery 43% of breast cancer patients experienced RECIST responses despite 2.5 average prior lines of systemic drug therapy Responses were seen across multiple genetic profiles, including triple negative RECIST Response Patients Average ECOG PS Average Prior Lines Median OS (months) Mean OS (months) All 14 (100%) 1.8 2.5 35 36 Complete 3 (21%) 1.7 2.0 64 47 Partial 3 (21%) 2.0 3.7 11 28 Stable Disease 5 (36%) 2.0 2.0 43 38 RECIST -Response Evaluation Criteria In Solid Tumors. Data cutoff 9/19/17
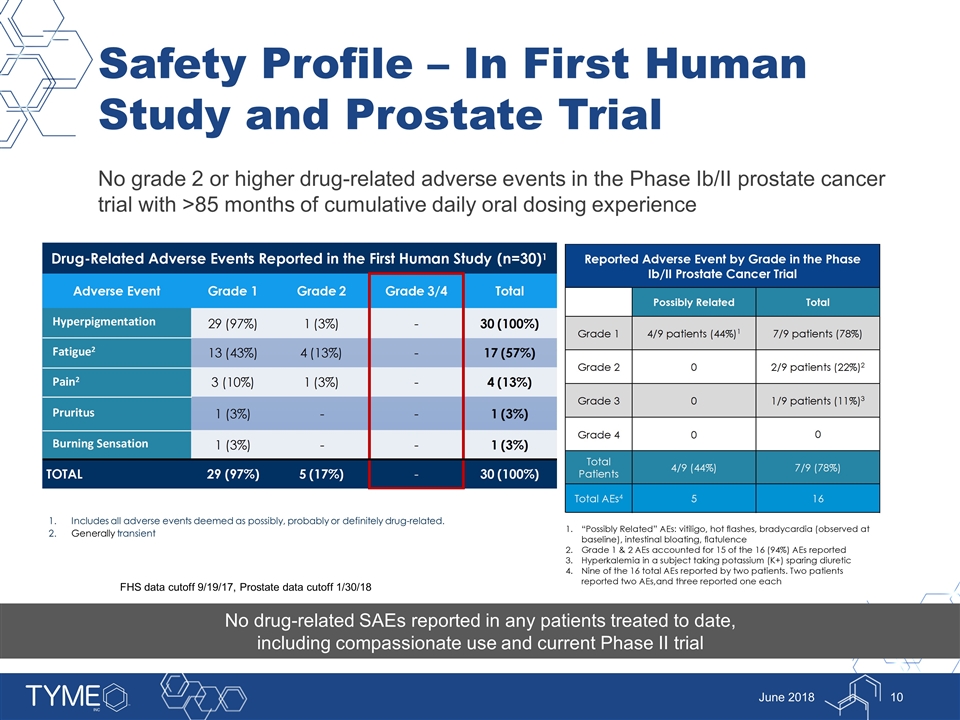
Safety Profile – In First Human Study and Prostate Trial No grade 2 or higher drug-related adverse events in the Phase Ib/II prostate cancer trial with >85 months of cumulative daily oral dosing experience June 2018 1.Includes all adverse events deemed as possibly, probably or definitely drug-related. 2.Generally transient No drug-related SAEs reported in any patients treated to date, including compassionate use and current Phase II trial FHS data cutoff 9/19/17, Prostate data cutoff 1/30/18
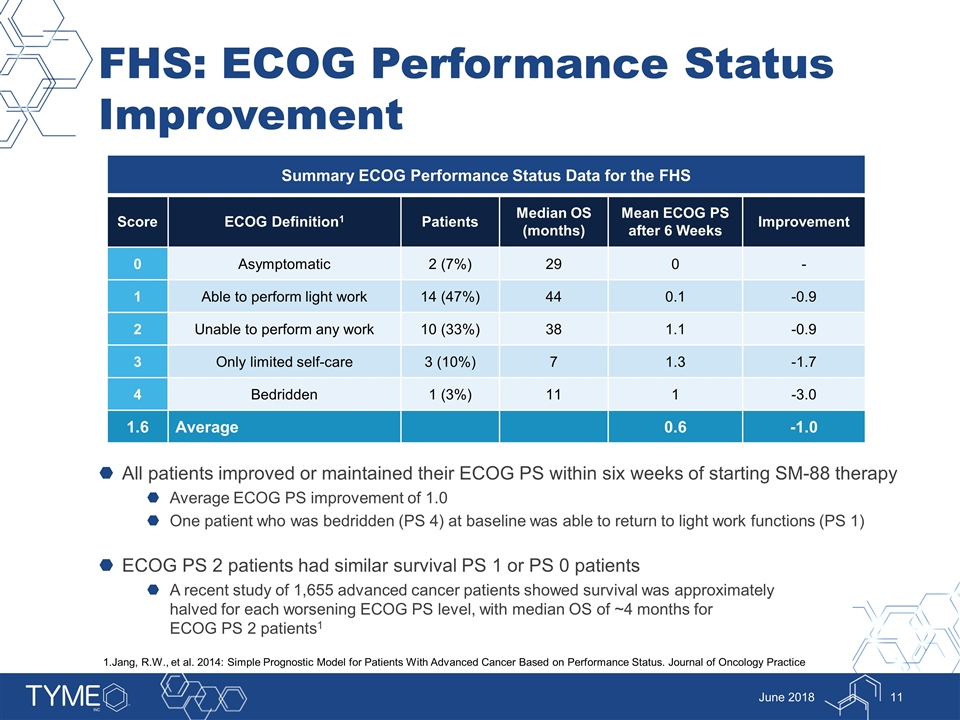
FHS: ECOG Performance Status Improvement All patients improved or maintained their ECOG PS within six weeks of starting SM-88 therapy Average ECOG PS improvement of 1.0 One patient who was bedridden (PS 4) at baseline was able to return to light work functions (PS 1) ECOG PS 2 patients had similar survival PS 1 or PS 0 patients A recent study of 1,655 advanced cancer patients showed survival was approximately halved for each worsening ECOG PS level, with median OS of ~4 months for ECOG PS 2 patients1 June 2018 Summary ECOG Performance Status Data for the FHS Score ECOG Definition1 Patients Median OS (months) Mean ECOG PS after 6 Weeks Improvement 0 Asymptomatic 2 (7%) 29 0 - 1 Able to perform light work 14 (47%) 44 0.1 -0.9 2 Unable to perform any work 10 (33%) 38 1.1 -0.9 3 Only limited self-care 3 (10%) 7 1.3 -1.7 4 Bedridden 1 (3%) 11 1 -3.0 1.6 Average 0.6 -1.0 1.Jang, R.W., et al. 2014: Simple Prognostic Model for Patients With Advanced Cancer Based on Performance Status. Journal of Oncology Practice
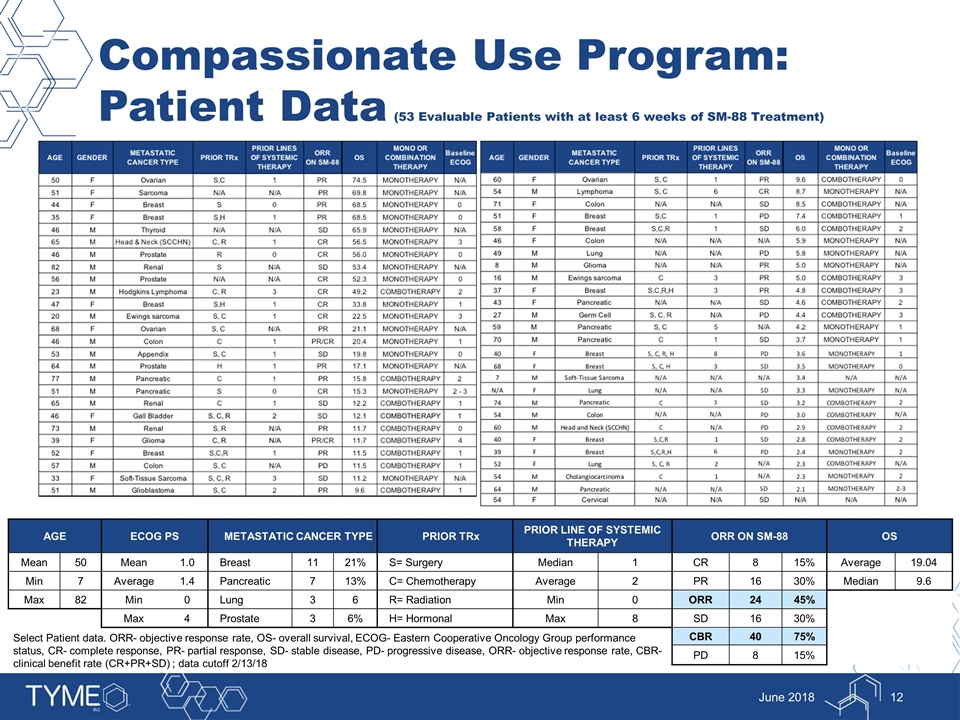
Compassionate Use Program: Patient Data (53 Evaluable Patients with at least 6 weeks of SM-88 Treatment) June 2018 2 AGE ECOG PS METASTATIC CANCER TYPE PRIOR TRx PRIOR LINE OF SYSTEMIC THERAPY ORR ON SM-88 OS Mean 50 Mean 1.0 Breast 11 21% S= Surgery Median 1 CR 8 15% Average 19.04 Min 7 Average 1.4 Pancreatic 7 13% C= Chemotherapy Average 2 PR 16 30% Median 9.6 Max 82 Min 0 Lung 3 6 R= Radiation Min 0 ORR 24 45% Max 4 Prostate 3 6% H= Hormonal Max 8 SD 16 30% CBR 40 75% PD 8 15% Select Patient data. ORR- objective response rate, OS- overall survival, ECOG- Eastern Cooperative Oncology Group performance status, CR- complete response, PR- partial response, SD- stable disease, PD- progressive disease, ORR- objective response rate, CBR- clinical benefit rate (CR+PR+SD) ; data cutoff 2/13/18
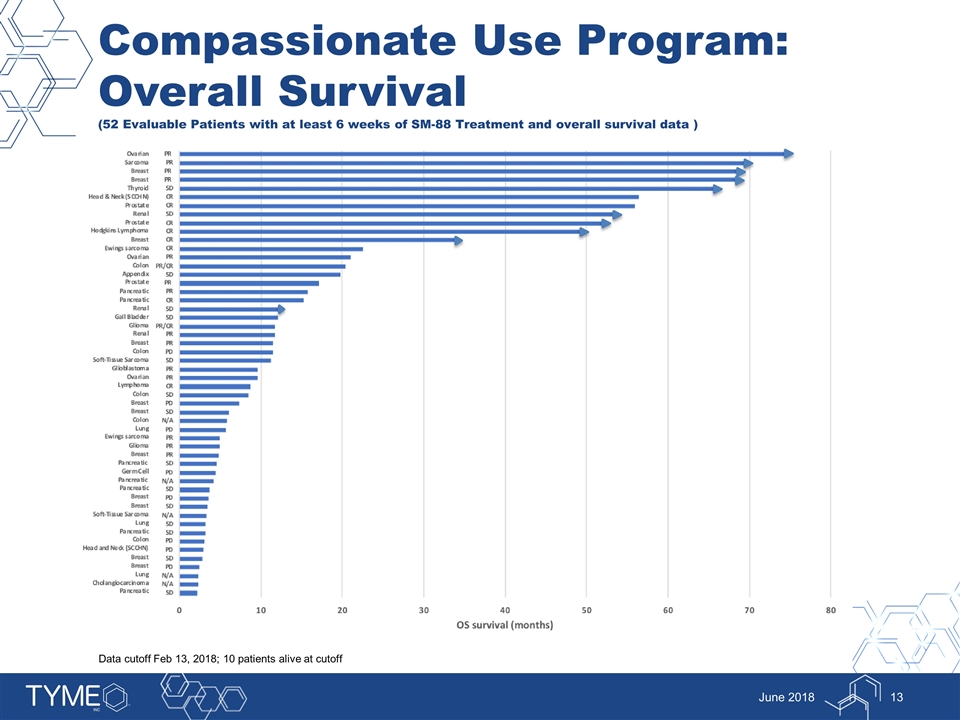
Compassionate Use Program: Overall Survival (52 Evaluable Patients with at least 6 weeks of SM-88 Treatment and overall survival data ) June 2018 Data cutoff Feb 13, 2018; 10 patients alive at cutoff
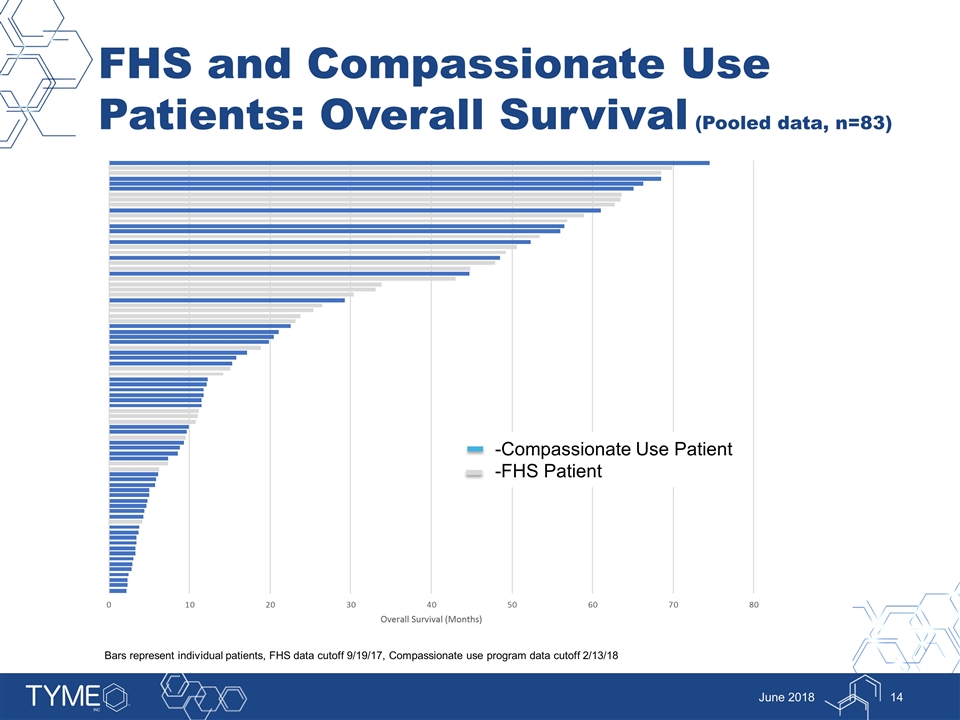
FHS and Compassionate Use Patients: Overall Survival (Pooled data, n=83) June 2018 -Compassionate Use Patient -FHS Patient Bars represent individual patients, FHS data cutoff 9/19/17, Compassionate use program data cutoff 2/13/18
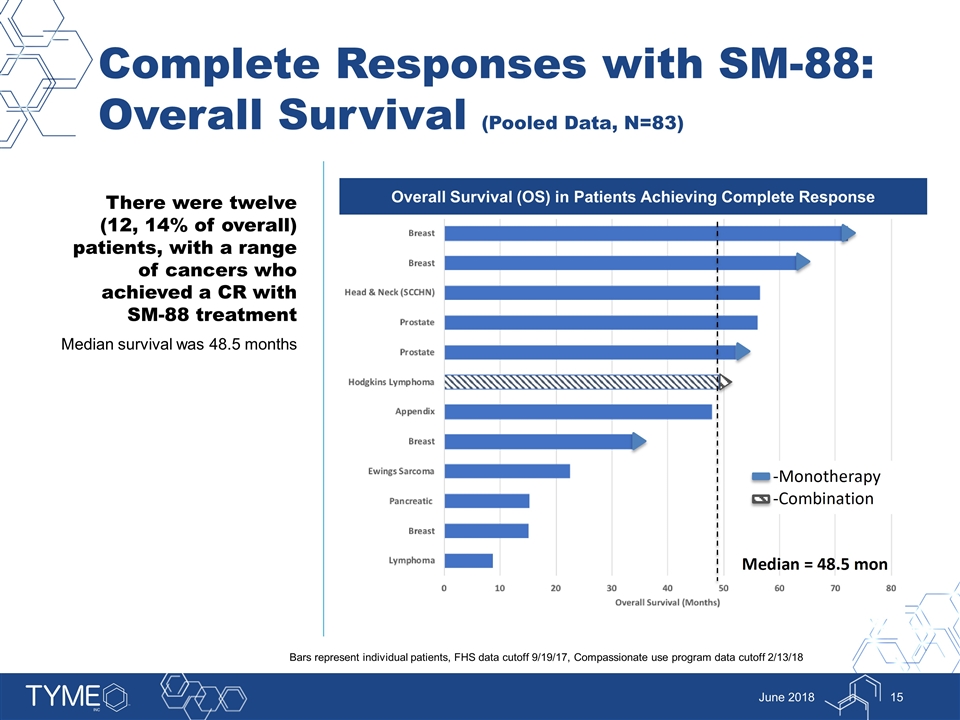
Complete Responses with SM-88: Overall Survival (Pooled Data, N=83) June 2018 Overall Survival (OS) in Patients Achieving Complete Response There were twelve (12, 14% of overall) patients, with a range of cancers who achieved a CR with SM-88 treatment Median survival was 48.5 months Bars represent individual patients, FHS data cutoff 9/19/17, Compassionate use program data cutoff 2/13/18
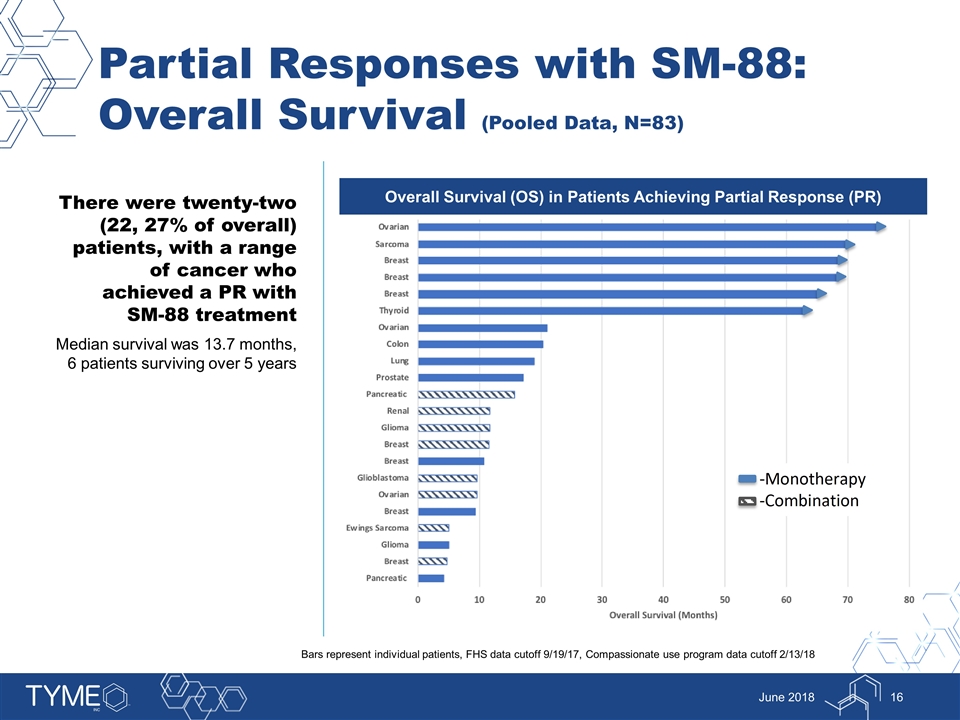
Partial Responses with SM-88: Overall Survival (Pooled Data, N=83) June 2018 There were twenty-two (22, 27% of overall) patients, with a range of cancer who achieved a PR with SM-88 treatment Median survival was 13.7 months, 6 patients surviving over 5 years Overall Survival (OS) in Patients Achieving Partial Response (PR) Bars represent individual patients, FHS data cutoff 9/19/17, Compassionate use program data cutoff 2/13/18
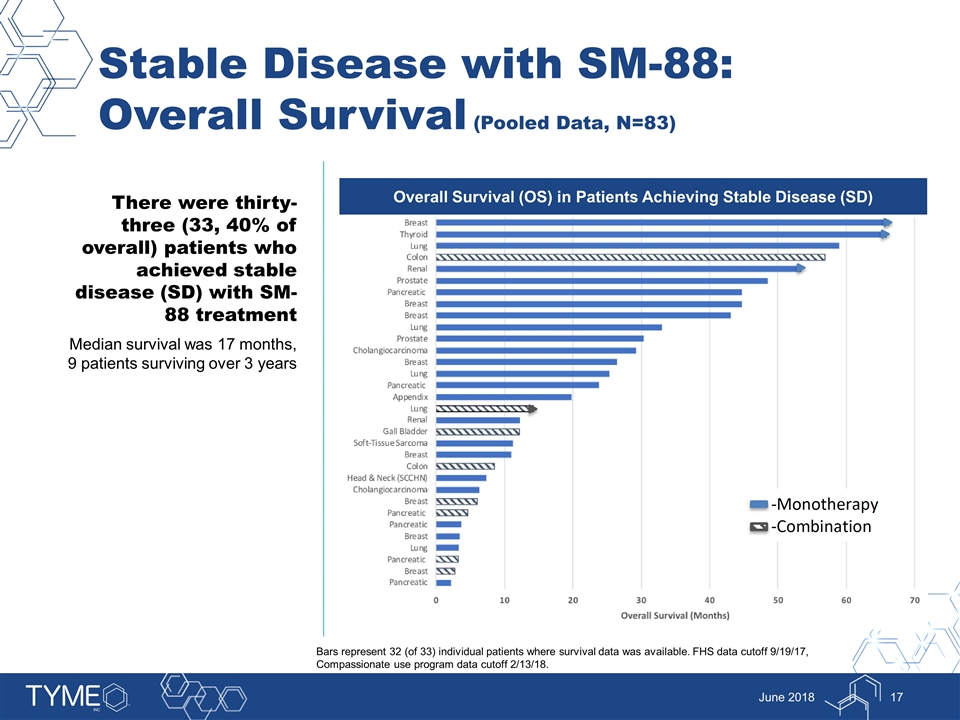
Stable Disease with SM-88: Overall Survival (Pooled Data, N=83) June 2018 There were thirty-three (33, 40% of overall) patients who achieved stable disease (SD) with SM-88 treatment Median survival was 17 months, 9 patients surviving over 3 years Overall Survival (OS) in Patients Achieving Stable Disease (SD) Bars represent 32 (of 33) individual patients where survival data was available. FHS data cutoff 9/19/17, Compassionate use program data cutoff 2/13/18.
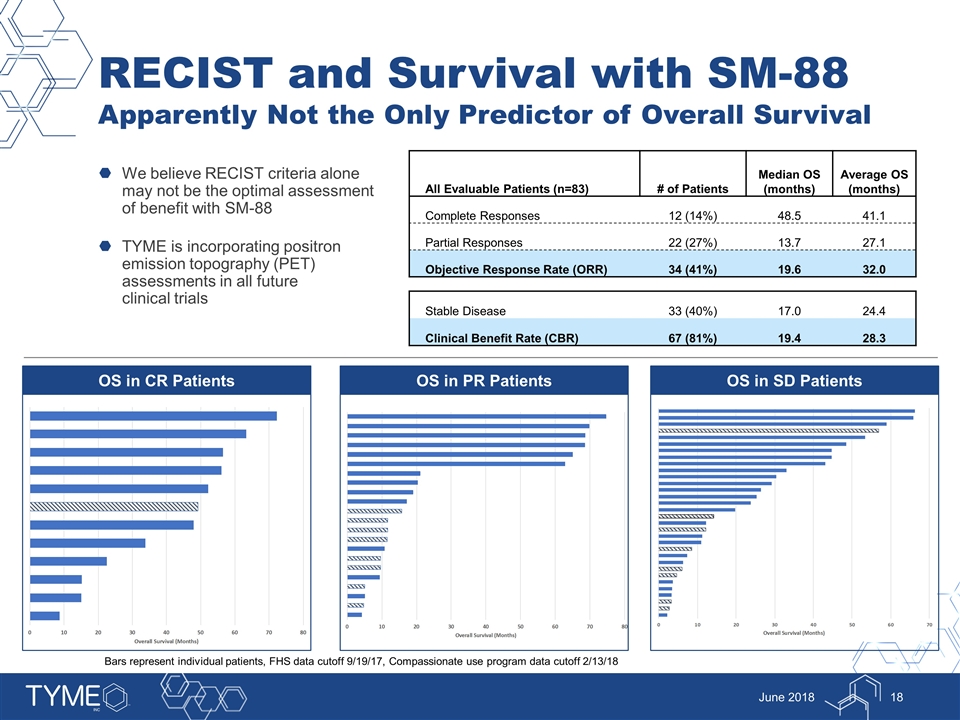
RECIST and Survival with SM-88 Apparently Not the Only Predictor of Overall Survival We believe RECIST criteria alone may not be the optimal assessment of benefit with SM-88 TYME is incorporating positron emission topography (PET) assessments in all future clinical trials June 2018 All Evaluable Patients (n=83) # of Patients Median OS (months) Average OS (months) Complete Responses 12 (14%) 48.5 41.1 Partial Responses 22 (27%) 13.7 27.1 Objective Response Rate (ORR) 34 (41%) 19.6 32.0 Stable Disease 33 (40%) 17.0 24.4 Clinical Benefit Rate (CBR) 67 (81%) 19.4 28.3 OS in PR Patients OS in SD Patients OS in CR Patients Bars represent individual patients, FHS data cutoff 9/19/17, Compassionate use program data cutoff 2/13/18
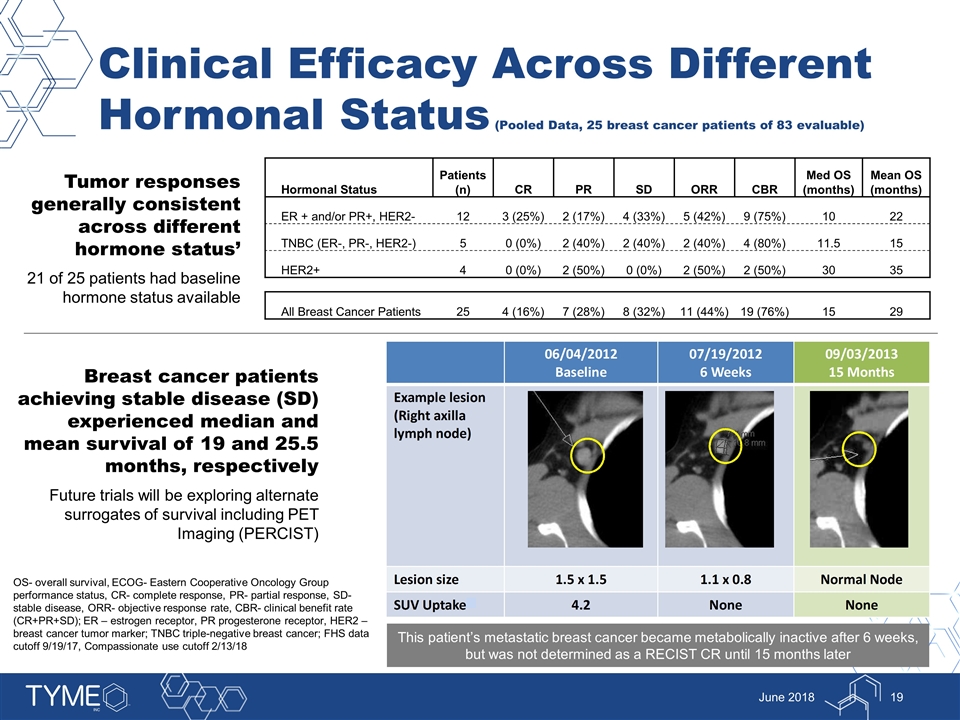
Clinical Efficacy Across Different Hormonal Status (Pooled Data, 25 breast cancer patients of 83 evaluable) June 2018 Tumor responses generally consistent across different hormone status’ 21 of 25 patients had baseline hormone status available Hormonal Status Patients (n) CR PR SD ORR CBR Med OS (months) Mean OS (months) ER + and/or PR+, HER2- 12 3 (25%) 2 (17%) 4 (33%) 5 (42%) 9 (75%) 10 22 TNBC (ER-, PR-, HER2-) 5 0 (0%) 2 (40%) 2 (40%) 2 (40%) 4 (80%) 11.5 15 HER2+ 4 0 (0%) 2 (50%) 0 (0%) 2 (50%) 2 (50%) 30 35 All Breast Cancer Patients 25 4 (16%) 7 (28%) 8 (32%) 11 (44%) 19 (76%) 15 29 Breast cancer patients achieving stable disease (SD) experienced median and mean survival of 19 and 25.5 months, respectively Future trials will be exploring alternate surrogates of survival including PET Imaging (PERCIST) This patient’s metastatic breast cancer became metabolically inactive after 6 weeks, but was not determined as a RECIST CR until 15 months later OS- overall survival, ECOG- Eastern Cooperative Oncology Group performance status, CR- complete response, PR- partial response, SD- stable disease, ORR- objective response rate, CBR- clinical benefit rate (CR+PR+SD); ER – estrogen receptor, PR progesterone receptor, HER2 – breast cancer tumor marker; TNBC triple-negative breast cancer; FHS data cutoff 9/19/17, Compassionate use cutoff 2/13/18
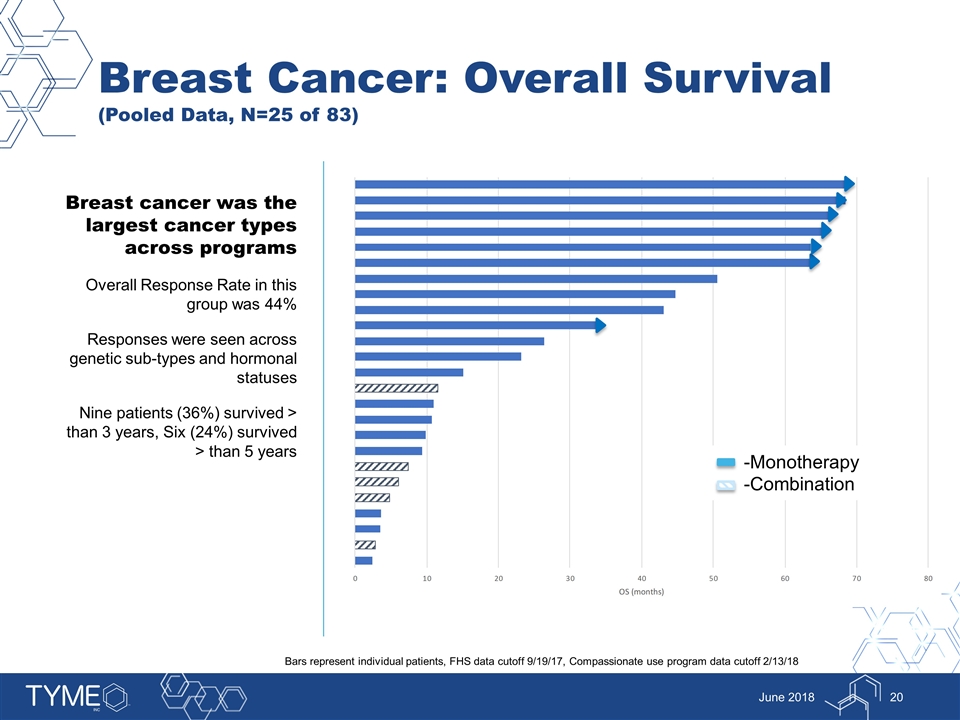
Breast Cancer: Overall Survival (Pooled Data, N=25 of 83) June 2018 Breast cancer was the largest cancer types across programs Overall Response Rate in this group was 44% Responses were seen across genetic sub-types and hormonal statuses Nine patients (36%) survived > than 3 years, Six (24%) survived > than 5 years -Monotherapy -Combination Bars represent individual patients, FHS data cutoff 9/19/17, Compassionate use program data cutoff 2/13/18
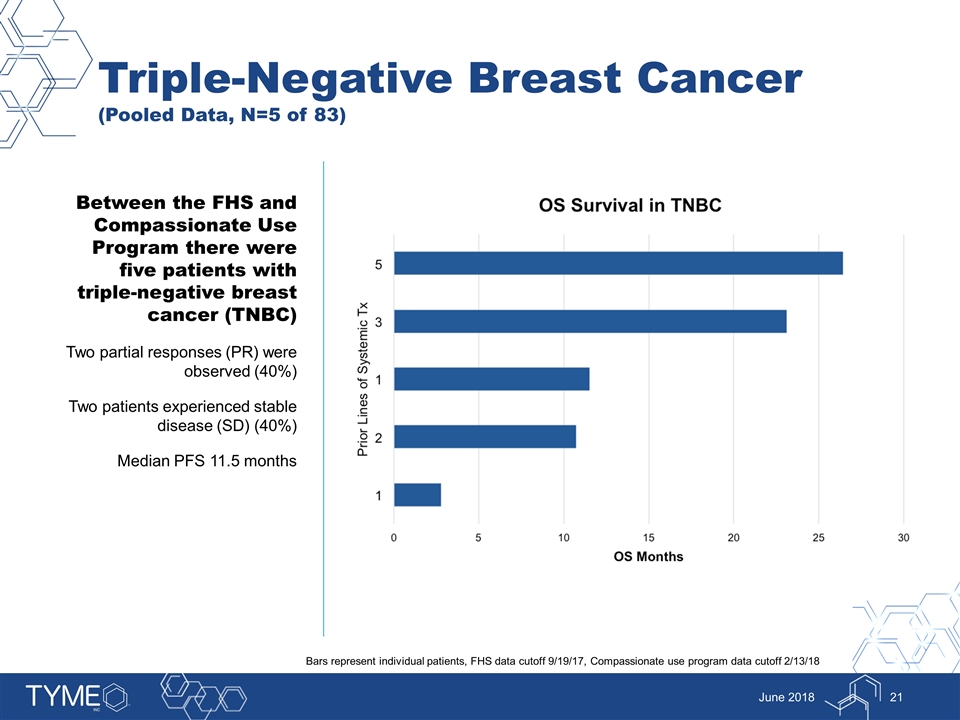
Triple-Negative Breast Cancer (Pooled Data, N=5 of 83) June 2018 Between the FHS and Compassionate Use Program there were five patients with triple-negative breast cancer (TNBC) Two partial responses (PR) were observed (40%) Two patients experienced stable disease (SD) (40%) Median PFS 11.5 months Bars represent individual patients, FHS data cutoff 9/19/17, Compassionate use program data cutoff 2/13/18

June 2018
