Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GENOCEA BIOSCIENCES, INC. | gnca_682018.htm |

Pioneering Neoantigen Vaccines JUNE 2018

Disclaimer This presentation contains “forward-looking” statements that are within the meaning of federal securities laws and are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include information concerning our possible or assumed future results of operations, business strategies, clinical trials and pre-clinical studies, regulatory approval of our product candidates, liquidity position and capital needs, financing plans, industry environment, potential growth opportunities, potential market opportunities and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “expects,” “could,” “seeks,” “estimates,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions and the negatives of those terms. Forward-looking statements represent our management’s beliefs and assumptions only as of the date of this presentation. Our operations involve risks and uncertainties, many of which are outside our control, and any one of which, or combination of which, could materially affect our results of operations and whether the forward-looking statements ultimately prove to be correct. Factors that may materially affect our results of operations include, among other things, our ability to progress product candidates in preclinical and clinical trials, the ability of ATLAS™ to identify promising oncology vaccine and immunotherapy product candidates, the scope, rate and progress of our preclinical and clinical trials and other research and development activities, anticipated timing of new clinical trails, our estimates regarding the amount of funds we require to conduct our clinical trials for GEN-009, our plans to commercialize GEN-009, the timing of, and ability to, obtain and maintain necessary regulatory approvals for our product candidates, and those listed in our Annual Report on Form 10-K for the fiscal year ended December 31, 2017 and other filings with the Securities and Exchange Commission (“SEC”). Except as required by law, we assume no obligation to update these forward- looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. You may get copies of our Annual Report on Form 10-K, Quarterly Report on Form 10-Q and our other SEC filings for free by visiting EDGAR on the SEC website at http://www.sec.gov. 2

As Individual as the Cancer We Target Clinical Stage Company Selecting the Right Targets for the Right Patient UNIQUE POWERFUL APPROACH TECHNOLOGY Know, Don’t Guess ATLAS™ Platform POTENTIAL Identify new targets, The only neoantigen BEST-IN-CLASS specific to individuals, based discovery platform to be CANCER VACCINES on actual response validated in 750+ subjects A Decade of Expertise in Vaccinology Infectious Disease Oncology 3

Summary BROAD role of neoantigens in the IO revolution PROVEN ATLAS™ antigen identification technology differentiates Genocea IMPORTANT milestones expected over next 12 months Pioneering • First neoantigen vaccine, GEN-009, entering clinic this year Neoantigen • Pipeline expansion Cancer Vaccines • ATLAS™ partnership discussions ongoing FUNDED into Q4 2019 4
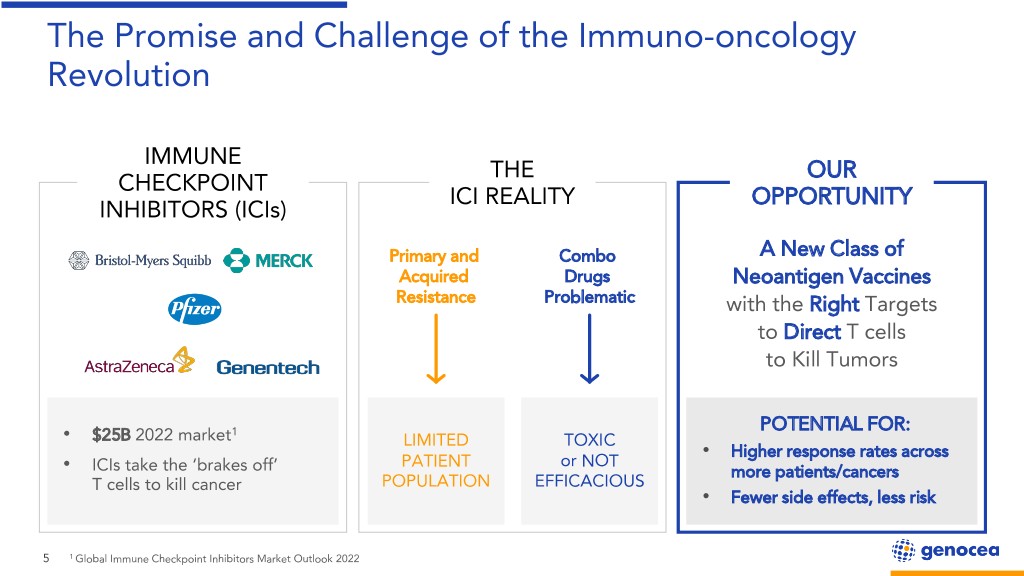
The Promise and Challenge of the Immuno-oncology Revolution IMMUNE THE OUR CHECKPOINT ICI REALITY OPPORTUNITY INHIBITORS (ICIs) Primary and Combo A New Class of Acquired Drugs Neoantigen Vaccines Resistance Problematic with the Right Targets to Direct T cells to Kill Tumors 1 POTENTIAL FOR: • $25B 2022 market LIMITED TOXIC Higher response rates across PATIENT or NOT • • ICIs take the ‘brakes off’ more patients/cancers T cells to kill cancer POPULATION EFFICACIOUS • Fewer side effects, less risk 5 1 Global Immune Checkpoint Inhibitors Market Outlook 2022

Neoantigen Vaccines Have Come of Age Ott et al., Sahin et al., 2017 Today The time is right for Schumacher, developing Schreiber, 2015 2017 next-gen Possible to neoantigen Yadav et al., vaccinate against vaccines Gubin et al, 2014 2015 neoantigens Response to neoantigens drives 2014 checkpoint inhibitor Personalized tumor (ICI) efficacy mutations (neoantigens) are “foreign” to immune system 6
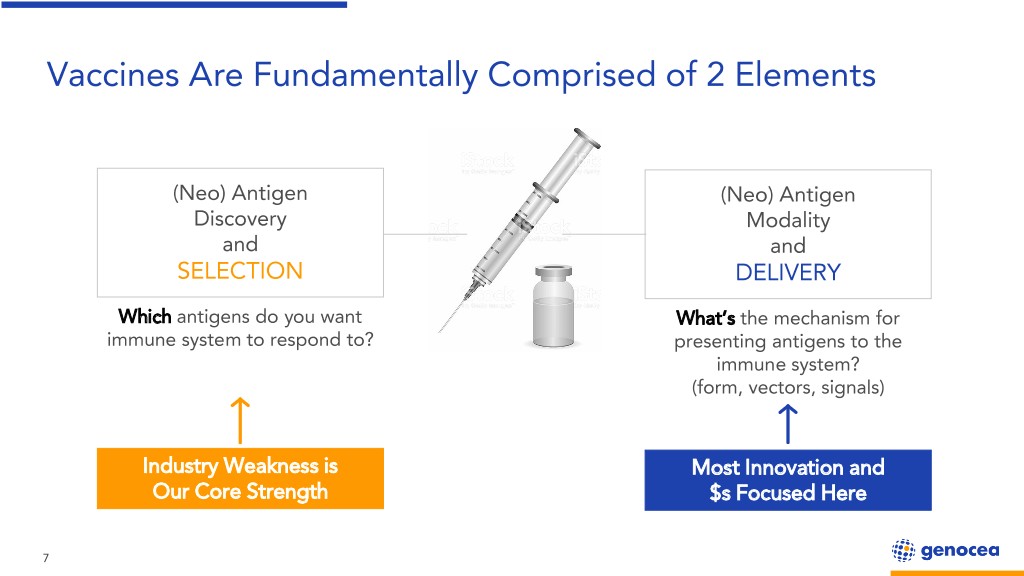
Vaccines Are Fundamentally Comprised of 2 Elements (Neo) Antigen (Neo) Antigen Discovery Modality and and SELECTION DELIVERY Which antigens do you want What’s the mechanism for immune system to respond to? presenting antigens to the immune system? (form, vectors, signals) Industry Weakness is Most Innovation and Our Core Strength $s Focused Here 7
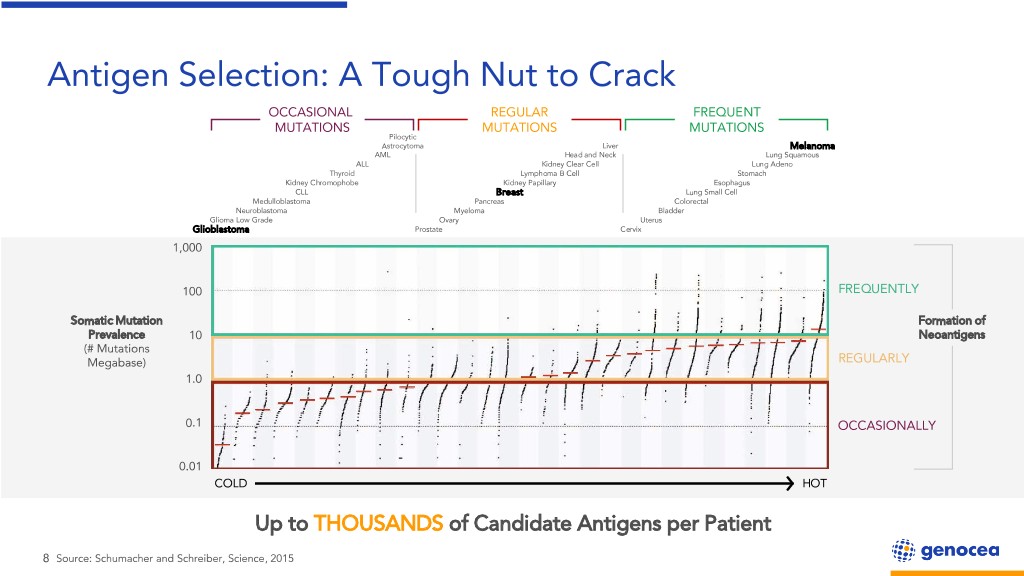
Antigen Selection: A Tough Nut to Crack OCCASIONAL REGULAR FREQUENT MUTATIONS MUTATIONS MUTATIONS Pilocytic Astrocytoma Liver Melanoma AML Head and Neck Lung Squamous ALL Kidney Clear Cell Lung Adeno Thyroid Lymphoma B Cell Stomach Kidney Chromophobe Kidney Papillary Esophagus CLL Breast Lung Small Cell Medulloblastoma Pancreas Colorectal Neuroblastoma Myeloma Bladder Glioma Low Grade Ovary Uterus Glioblastoma Prostate Cervix 1,000 100 FREQUENTLY Somatic Mutation Formation of Prevalence 10 Neoantigens (# Mutations Megabase) REGULARLY 1.0 0.1 OCCASIONALLY 0.01 COLD HOT Up to THOUSANDS of Candidate Antigens per Patient 8 Source: Schumacher and Schreiber, Science, 2015

Industry Recognizes Neoantigen Selection as Currently Inadequate … and yet the “Skeleton Key” Sole Focus: Tumor nEoantigen SeLection Alliance Neoantigen Selection (TESLA Consortium) BIG ACADEMIA BIOTECH PHARMA 9
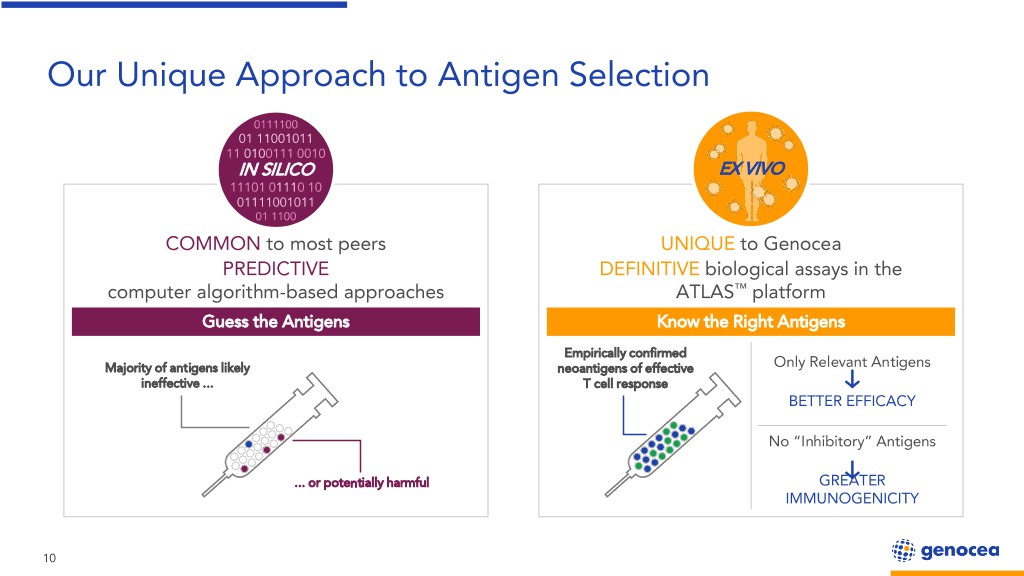
Our Unique Approach to Antigen Selection 0111100 01 11001011 11 0100111 0010 IN SILICO EX VIVO 11101 01110 10 01111001011 01 1100 COMMON to most peers UNIQUE to Genocea PREDICTIVE DEFINITIVE biological assays in the computer algorithm-based approaches ATLAS™ platform Guess the Antigens Know the Right Antigens Empirically confirmed Majority of antigens likely neoantigens of effective Only Relevant Antigens ineffective ... T cell response BETTER EFFICACY No “Inhibitory” Antigens ... or potentially harmful GREATER IMMUNOGENICITY 10
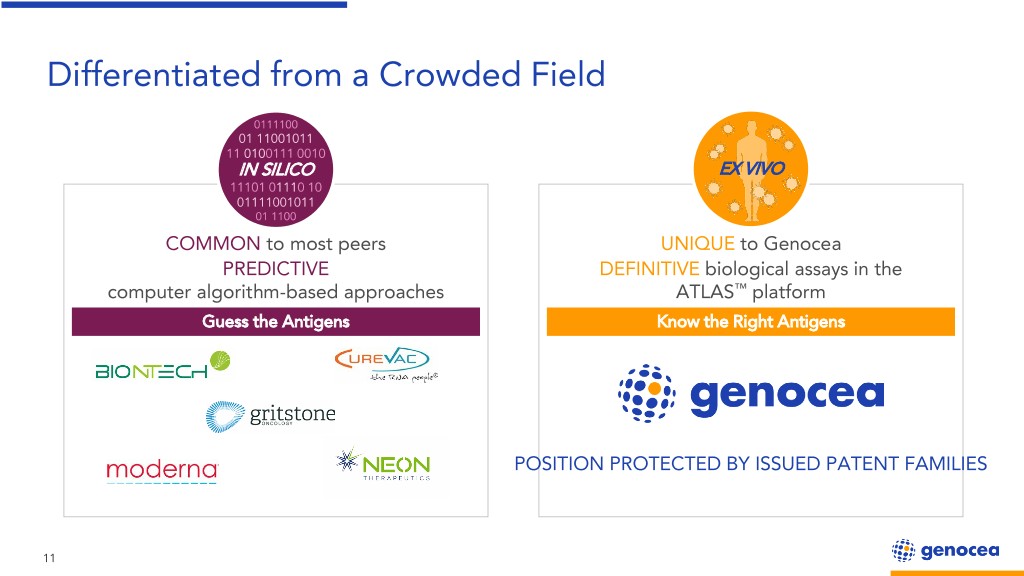
Differentiated from a Crowded Field 0111100 01 11001011 11 0100111 0010 IN SILICO EX VIVO 11101 01110 10 01111001011 01 1100 COMMON to most peers UNIQUE to Genocea PREDICTIVE DEFINITIVE biological assays in the computer algorithm-based approaches ATLAS™ platform Guess the Antigens Know the Right Antigens POSITION PROTECTED BY ISSUED PATENT FAMILIES 11
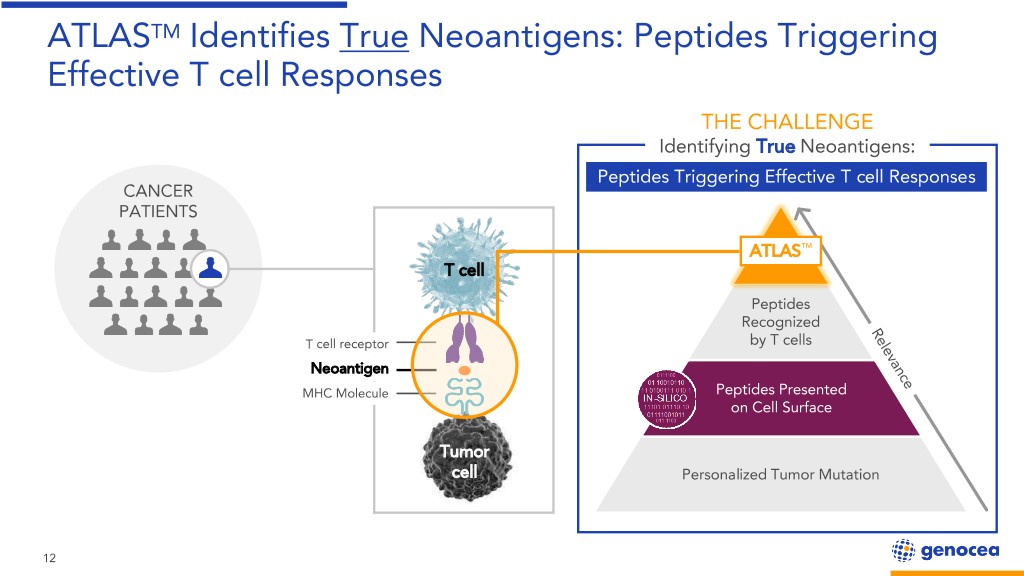
NeoantigensATLASTM Identifies are Powerful True Neoantigens: Because they Peptides Are “Foreign” Triggering to Effectivethe Immune T cell System Responses THE CHALLENGE Identifying True Neoantigens: Peptides Triggering Effective T cell Responses CANCER PATIENTS ATLAS™ T cell Peptides Recognized T cell receptor by T cells Neoantigen MHC Molecule Peptides Presented on Cell Surface Tumor cell Personalized Tumor Mutation 12
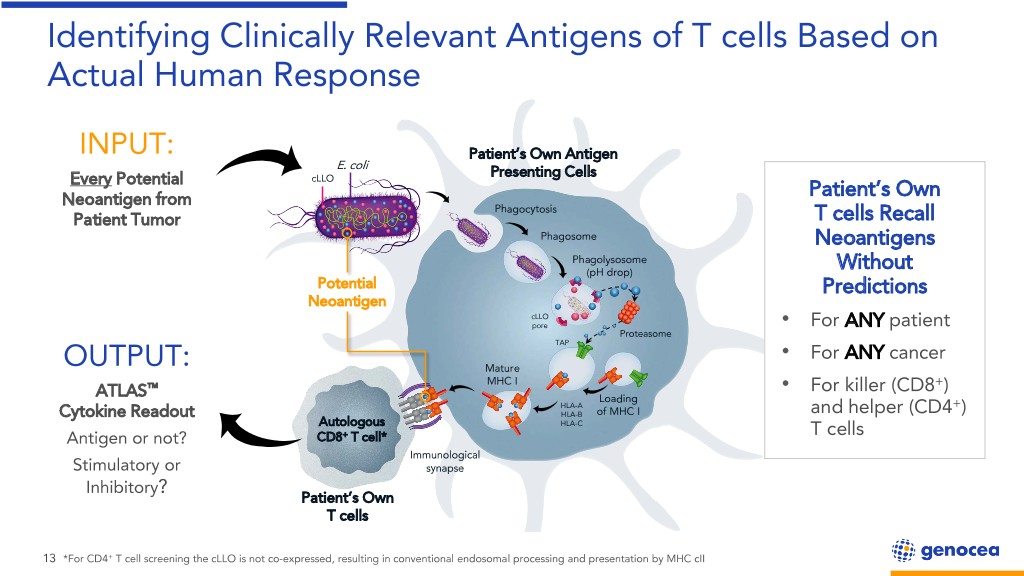
Identifying Clinically Relevant Antigens of T cells Based on Actual Human Response INPUT: Patient’s Own Antigen E. coli Presenting Cells Every Potential cLLO Neoantigen from Patient’s Own Phagocytosis Patient Tumor T cells Recall Phagosome Neoantigens Phagolysosome (pH drop) Without Potential Predictions Neoantigen cLLO pore • For ANY patient Proteasome TAP • For ANY cancer OUTPUT: Mature MHC I + ™ • For killer (CD8 ) ATLAS Loading HLA-A + Cytokine Readout HLA-B of MHC I and helper (CD4 ) Autologous HLA-C Antigen or not? CD8+ T cell* T cells Immunological Stimulatory or synapse Inhibitory? Patient’s Own T cells 13 *For CD4+ T cell screening the cLLO is not co-expressed, resulting in conventional endosomal processing and presentation by MHC cII
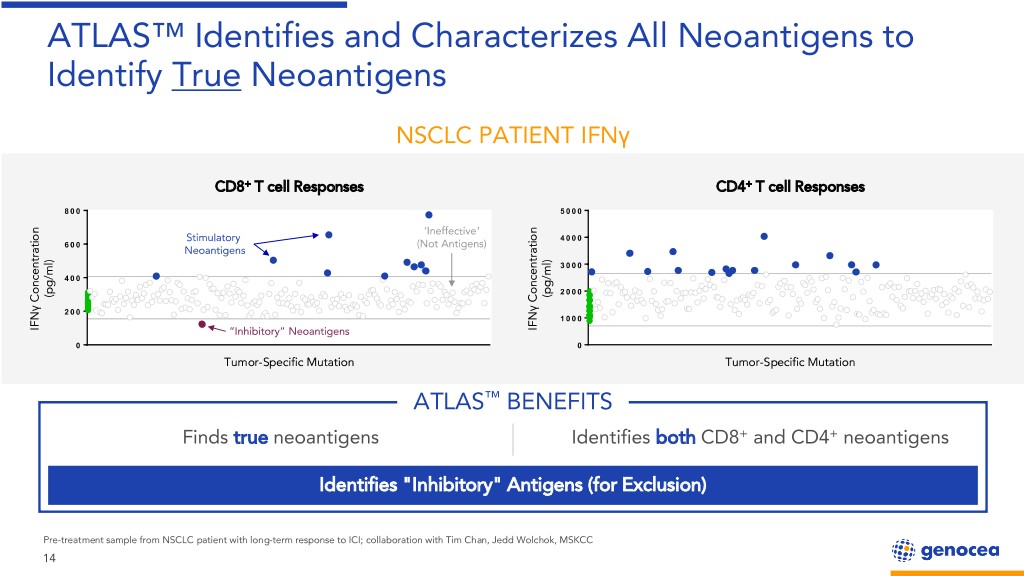
ATLAS™ Identifies and Characterizes All Neoantigens to Identify True Neoantigens NSCLC PATIENT IFNγ CD8+ T cell Responses CD4+ T cell Responses ‘Ineffective’ Stimulatory (Not Antigens) Neoantigens pg /ml) pg /ml) ( ( Concentration Concentration γ γ IFN “Inhibitory” Neoantigens IFN Tumor-Specific Mutation Tumor-Specific Mutation ATLAS™ BENEFITS Finds true neoantigens Identifies both CD8+ and CD4+ neoantigens Identifies "Inhibitory" Antigens (for Exclusion) Pre-treatment sample from NSCLC patient with long-term response to ICI; collaboration with Tim Chan, Jedd Wolchok, MSKCC 14
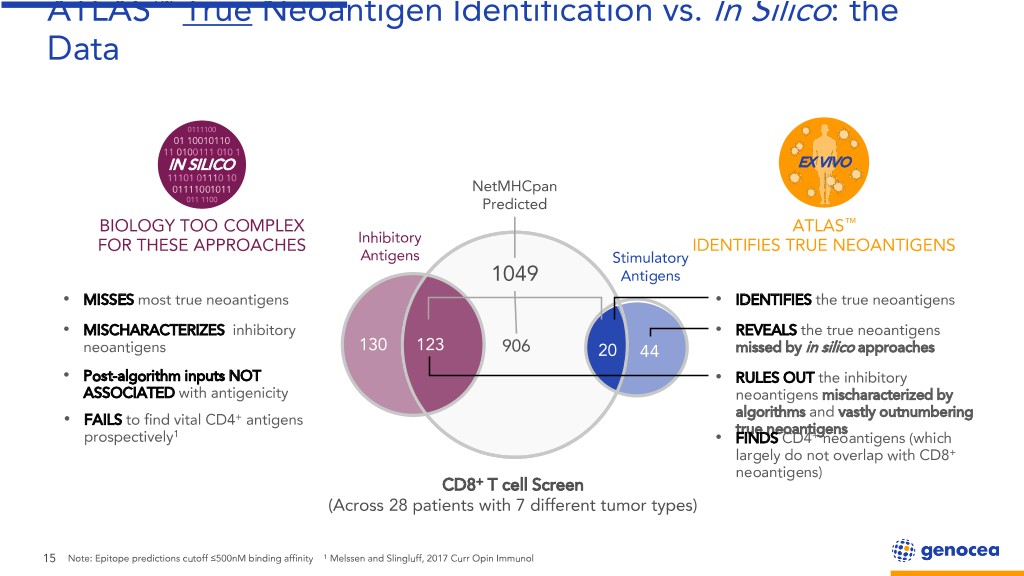
ATLAS™ True Neoantigen Identification vs. In Silico: the Data 0111100 01 10010110 11 0100111 010 1 ININ -SILICOSILICO EX VIVO 11101 01110 10 01111001011 NetMHCpan 011 1100 Predicted BIOLOGY TOO COMPLEX ATLAS™ FOR THESE APPROACHES Inhibitory IDENTIFIES TRUE NEOANTIGENS Antigens Stimulatory 1049 Antigens • MISSES most true neoantigens • IDENTIFIES the true neoantigens • MISCHARACTERIZES inhibitory • REVEALS the true neoantigens neoantigens 130 123 906 20 44 missed by in silico approaches • Post-algorithm inputs NOT • RULES OUT the inhibitory ASSOCIATED with antigenicity neoantigens mischaracterized by algorithms and vastly outnumbering FAILS to find vital CD4+ antigens • true neoantigens prospectively1 • FINDS CD4+ neoantigens (which largely do not overlap with CD8+ neoantigens) CD8+ T cell Screen (Across 28 patients with 7 different tumor types) 15 Note: Epitope predictions cutoff ≤500nM binding affinity 1 Melssen and Slingluff, 2017 Curr Opin Immunol
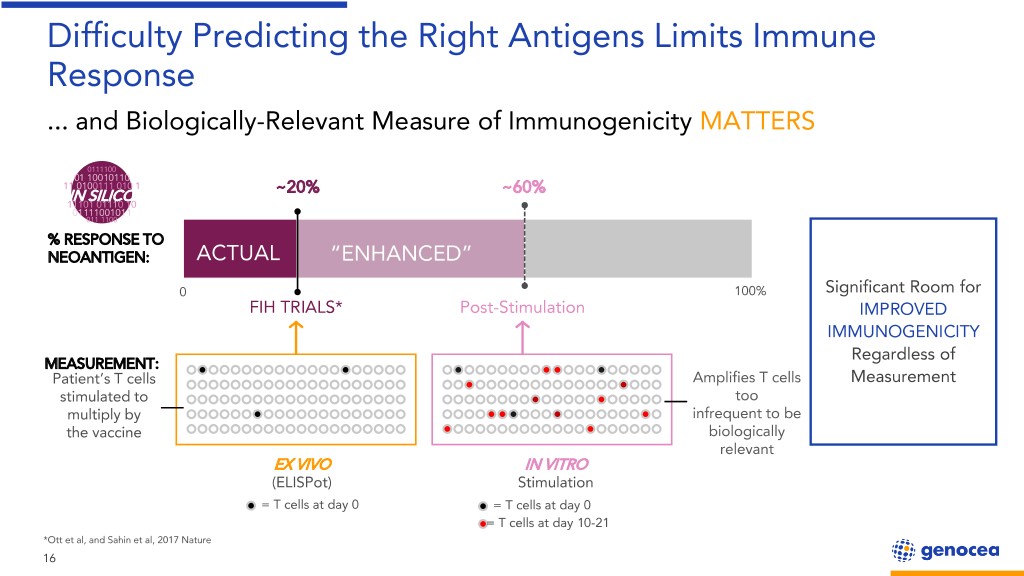
Difficulty Predicting the Right Antigens Limits Immune Response ... and Biologically-Relevant Measure of Immunogenicity MATTERS 0111100 01 10010110 11 0100111 010 1 ~20% ~60% IN SILICO 11101 01110 10 01111001011 011 1100 % RESPONSE TO NEOANTIGEN: ACTUAL “ENHANCED” 0 100% Significant Room for FIH TRIALS* Post-Stimulation IMPROVED IMMUNOGENICITY Regardless of MEASUREMENT: Patient’s T cells Amplifies T cells Measurement stimulated to too multiply by infrequent to be the vaccine biologically relevant EX VIVO IN VITRO (ELISPot) Stimulation = T cells at day 0 = T cells at day 0 = T cells at day 10-21 *Ott et al, and Sahin et al, 2017 Nature 16
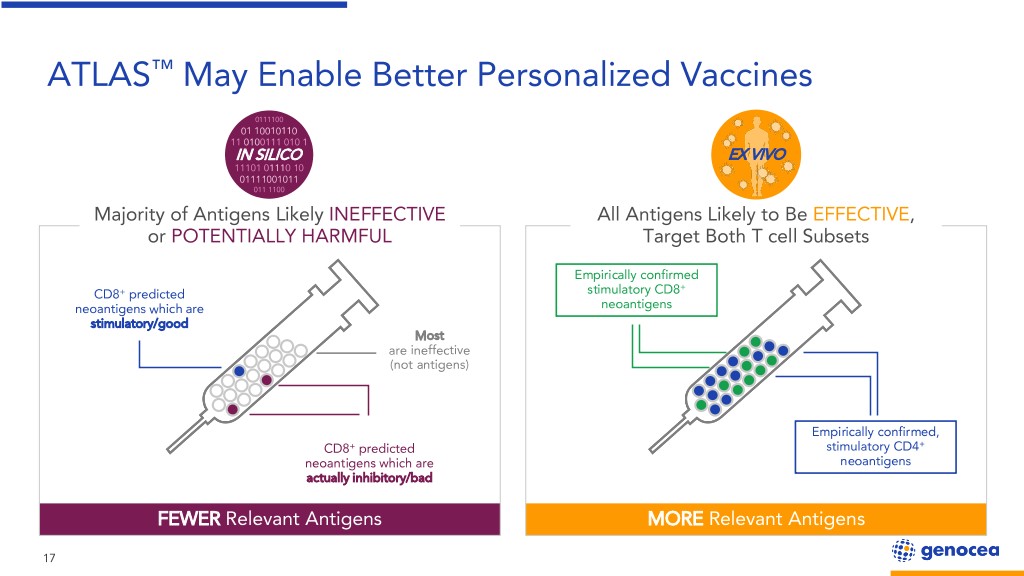
ATLAS™ May Enable Better Personalized Vaccines 0111100 01 10010110 11 0100111 010 1 IN SILICO EX VIVO 11101 01110 10 01111001011 011 1100 Majority of Antigens Likely INEFFECTIVE All Antigens Likely to Be EFFECTIVE, or POTENTIALLY HARMFUL Target Both T cell Subsets Empirically confirmed + CD8+ predicted stimulatory CD8 neoantigens which are neoantigens stimulatory/good Most are ineffective (not antigens) Empirically confirmed, CD8+ predicted stimulatory CD4+ neoantigens which are neoantigens actually inhibitory/bad FEWER Relevant Antigens MORE Relevant Antigens 17
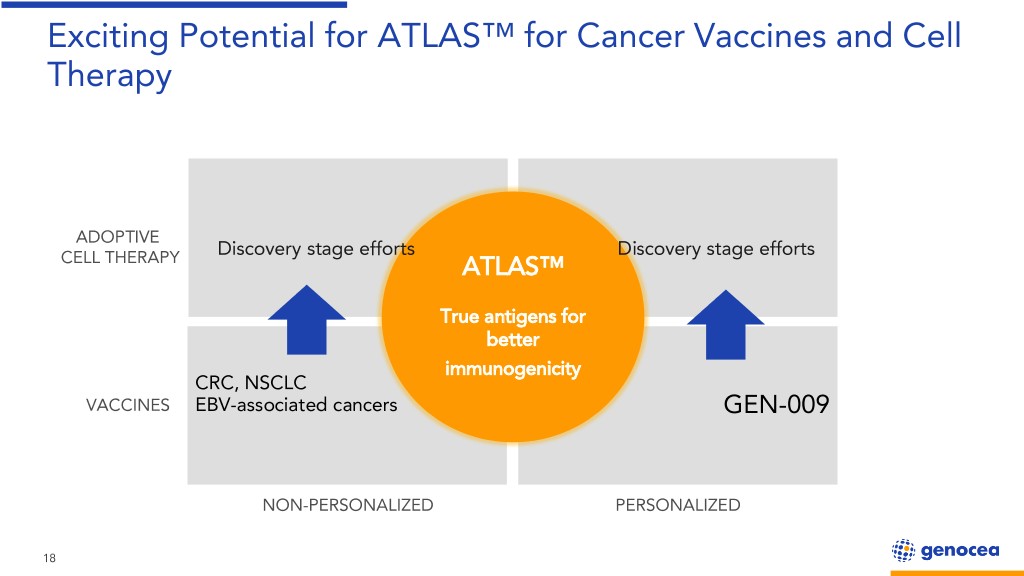
Exciting Potential for ATLAS™ for Cancer Vaccines and Cell Therapy ADOPTIVE Discovery stage efforts Discovery stage efforts CELL THERAPY ATLAS™ True antigens for better immunogenicity CRC, NSCLC VACCINES EBV-associated cancers GEN-009 NON-PERSONALIZED PERSONALIZED 18
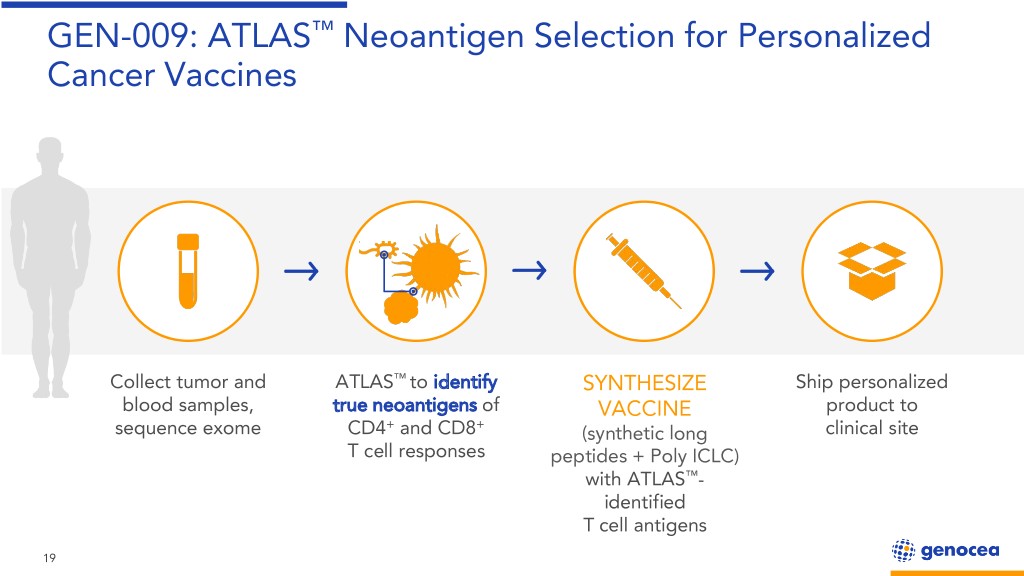
GEN-009: ATLAS™ Neoantigen Selection for Personalized Cancer Vaccines Collect tumor and ATLAS™ to identify SYNTHESIZE Ship personalized blood samples, true neoantigens of VACCINE product to sequence exome CD4+ and CD8+ (synthetic long clinical site T cell responses peptides + Poly ICLC) with ATLAS™- identified T cell antigens 19
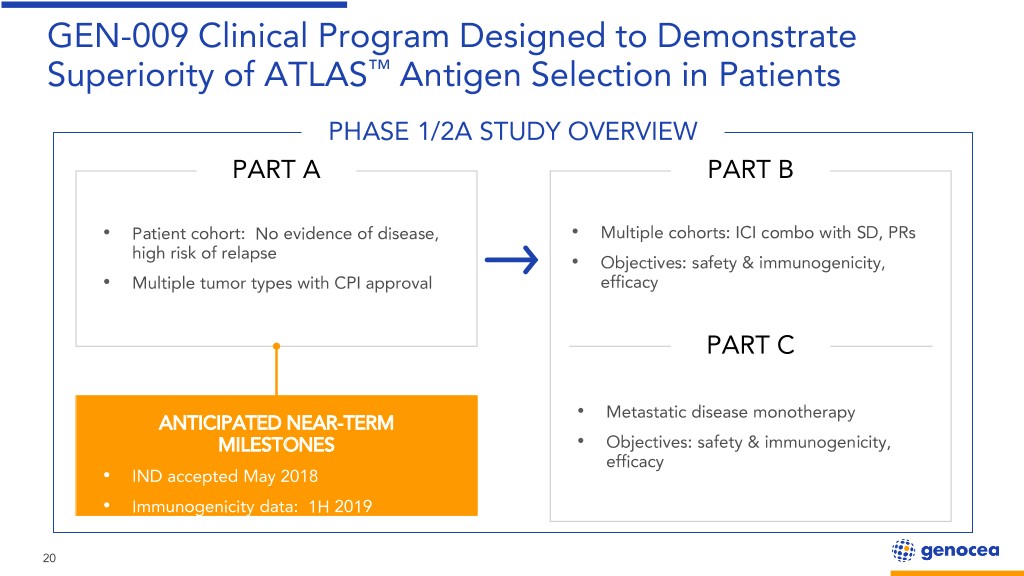
GEN-009 Clinical Program Designed to Demonstrate Superiority of ATLAS™ Antigen Selection in Patients PHASE 1/2A STUDY OVERVIEW PART A PART B • Patient cohort: No evidence of disease, • Multiple cohorts: ICI combo with SD, PRs high risk of relapse • Objectives: safety & immunogenicity, • Multiple tumor types with CPI approval efficacy PART C • Metastatic disease monotherapy ANTICIPATED NEAR-TERM MILESTONES • Objectives: safety & immunogenicity, efficacy • IND accepted May 2018 • Immunogenicity data: 1H 2019 20
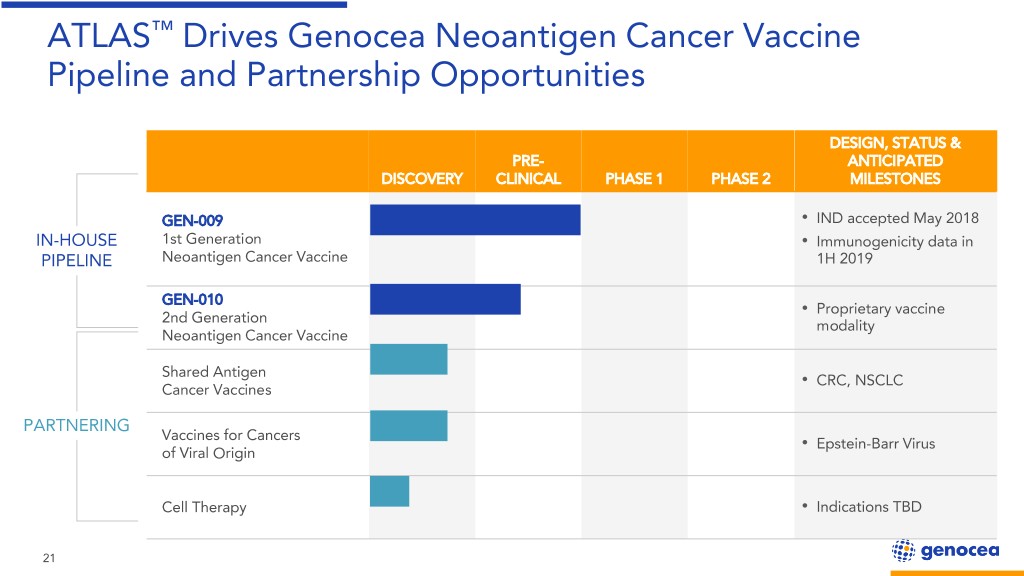
ATLAS™ Drives Genocea Neoantigen Cancer Vaccine Pipeline and Partnership Opportunities DESIGN, STATUS & PRE- ANTICIPATED DISCOVERY CLINICAL PHASE 1 PHASE 2 MILESTONES GEN-009 • IND accepted May 2018 IN-HOUSE 1st Generation • Immunogenicity data in PIPELINE Neoantigen Cancer Vaccine 1H 2019 GEN-010 Proprietary vaccine 2nd Generation • modality Neoantigen Cancer Vaccine Shared Antigen CRC, NSCLC Cancer Vaccines • PARTNERING Vaccines for Cancers Epstein-Barr Virus of Viral Origin • Cell Therapy • Indications TBD 21

Deep Expertise in Vaccinology and Immuno-Oncology MANAGEMENT TEAM SCIENTIFIC ADVISORY BOARD Chip Clark President President & CEO Elizabeth Jaffe, MD Deputy Director Chair Pamela Carroll, PhD Chuck Drake, MD, PhD SVP Immuno-oncology Director of Genitourinary Oncology Kwok Wong, MD Chief of Hematology and Medical Oncology Jessica Baker Flechtner, PhD Chief Scientific Officer George Siber, MD, PhD Vaccines Former CSO SCIENTIFIC FOUNDERS Eric Hoffman, PhD Chief Business Officer Darren Higgins, PhD Narinder Singh SVP Pharmaceutical Science & Manufacturing David Sinclair, PhD 22

Summary BROAD role of neoantigens in the IO revolution PROVEN ATLAS™ antigen identification technology differentiates Genocea IMPORTANT milestones expected over next 12 months Pioneering • First neoantigen vaccine, GEN-009, entering clinic this year Neoantigen • Pipeline expansion Cancer Vaccines • ATLAS™ partnership discussions ongoing FUNDED into Q4 2019 23

Genocea Biosciences, Inc. NASDAQ: GNCA Cambridge Discovery Park 100 Acorn Park Drive, 5th floor Cambridge, MA 02140 USA Phone: +1 617.876.8191 www.genocea.com
