Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Ra Pharmaceuticals, Inc. | a18-15004_1ex99d1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): June 7, 2018
RA PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
001- 37926 |
|
26-2908274 |
|
(State or other jurisdiction of |
|
(Commission |
|
(I.R.S. Employer |
|
87 Cambridge Park Drive |
|
02140 |
|
(Address of principal executive offices) |
|
(Zip Code) |
(617) 401-4060
(Registrant’s telephone number, include area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. x
Item 7.01. Regulation FD Disclosure.
Ra Pharmaceuticals, Inc. (the “Company”) from time to time presents and/or distributes to the investment community at various industry and other conferences slide presentations to provide updates and summaries of its business. A copy of its current corporate slide presentation (the “Presentation”) is attached to this Current Report on Form 8-K as Exhibit 99.1. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
The information in Item 7.01 of this Form 8-K, including Exhibit 99.1 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 8.01. Other Events.
On June 7, 2018, in connection with the presentation and/or distribution of the Presentation, the Company presented the following reticulocyte count data for the eculizumab switch cohort from the Company’s Phase 2 clinical trial of RA101495 SC in paroxysmal nocturnal hemoglobinuria, or PNH:
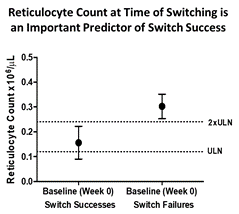
As depicted in the figure, a post-hoc analysis of the eculizumab switch cohort data demonstrated that an elevated absolute reticulocyte count (> 2 times the upper limit of normal) at the time of switching from eculizumab to RA101495 SC was an important predictor of breakthrough hemolysis during washout. These data are consistent with the observation that transfusion dependency on eculizumab was associated with switch failure.
Recent data from the PNH National Service in the United Kingdom has identified reticulocyte count as the single best indicator of extravascular hemolysis in PNH patients on eculizumab. These data demonstrated that reticulocyte count appears to be a better indicator of extravascular hemolysis than lactate dehydrogenase, correlating more strongly with raised bilirubin, increased C3-loading of PNH red blood cells and an increased transfusion requirement. We therefore believe that assessing reticulocyte count will allow us to better identify those patients who are more likely to be able to successfully switch from eculizumab to RA101495 SC.
Forward-Looking Statements Disclaimer
This Current Report on Form 8-K (the “Current Report”) contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this Current Report that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding our ability to identify those patients who are more likely to be able to successfully switch from eculizumab to RA101495 SC by assessing reticulocyte count. These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the risk that assessing reticulocyte count will not allow us to better identify those patients who are more likely to be able to successfully switch from eculizumab to RA101495 SC. These and other important factors discussed under the caption “Risk Factors” in our Annual Report on Form 10-K filed with the Securities and Exchange Commission, or SEC, on March 14, 2018 and our other reports filed with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this Current Report. Any such forward-looking statements represent management’s estimates as of the date of this Current Report. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this Current Report.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
|
Exhibit |
|
Description |
|
|
|
|
|
99.1 |
|
Corporate slide presentation of Ra Pharmaceuticals, Inc., dated June 2018 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
RA PHARMACEUTICALS, INC. | |
|
|
|
|
|
|
|
|
|
Date: June 7, 2018 |
By: |
/s/ David C. Lubner |
|
|
|
David C. Lubner |
|
|
|
Executive Vice President and Chief Financial Officer |
