Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - Deciphera Pharmaceuticals, Inc. | d592942dex231.htm |
Table of Contents
As filed with the Securities and Exchange Commission on June 6, 2018.
Registration No. 333-225411
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
DECIPHERA PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 30-1003521 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
500 Totten Pond Road
Waltham, MA 02451
(781) 209-6400
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Michael D. Taylor, Ph.D.
President & Chief Executive Officer
Deciphera Pharmaceuticals, Inc.
500 Totten Pond Road
Waltham, MA 02451
(781) 209-6400
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Please send copies of all communications to:
| Richard A. Hoffman, Esq. Edwin M. O’Connor, Esq. Goodwin Procter LLP 100 Northern Avenue Boston, MA 02210 (617) 570-1000 |
Richard D. Truesdell, Jr., Esq. Marcel R. Fausten, Esq. Davis Polk & Wardwell LLP 450 Lexington Avenue New York, New York (212) 450-4000 |
Approximate date of commencement of the proposed sale to the public:
As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ (Do not check if a smaller reporting company) | Smaller reporting company | ☐ | |||
| Emerging growth company | ☒ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☒
CALCULATION OF REGISTRATION FEE
|
| ||||
| Title of Each Class of Securities to be Registered |
Proposed Maximum Aggregate Offering Price(1)(2) |
Amount of Registration Fee(3) | ||
| Common stock, $0.01 par value per share |
$165,211,875 | $20,569 | ||
|
| ||||
|
| ||||
| (1) | Includes additional shares of common stock that the underwriters have the option to purchase. |
| (2) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. |
| (3) | A registration fee of $13,912 was previously paid in connection with the Registration Statement. An additional $6,657 is paid herewith. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JUNE 6, 2018
PRELIMINARY PROSPECTUS
3,750,000 Shares

Common Stock
$ per share
This is an offering of 3,750,000 shares of common stock by Deciphera Pharmaceuticals, Inc.
Our common stock is listed on The Nasdaq Global Select Market under the symbol “DCPH.” The last reported sale price of our common stock on The Nasdaq Global Select Market on June 4, 2018 was $38.31 per share. The final public offering price will be determined through negotiation between us and the underwriters in the offering, and the recent market price used throughout this prospectus may not be indicative of the final public offering price.
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, have elected to comply with certain reduced public company reporting requirements and may elect to comply with reduced reporting requirements in future filings.
Investing in our common stock involves risks. See “Risk Factors” beginning on page 18 and in the documents incorporated by reference into this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Public Offering Price |
$ | $ | ||||||
| Underwriting Discounts and Commissions(1) |
$ | $ | ||||||
| Proceeds to Deciphera Pharmaceuticals, Inc. (before expenses) |
$ | $ | ||||||
| (1) | See “Underwriting” for a description of the compensation payable to the underwriters. |
To the extent that the underwriters sell more than 3,750,000 shares of our common stock, the underwriters have an option to purchase up to an additional 562,500 shares of common stock from us at the public offering price less the underwriting discounts and commissions.
The underwriters expect to deliver the shares against payment in New York, New York on , 2018.
| J.P. Morgan | Piper Jaffray |
Table of Contents
| 1 | ||||
| 18 | ||||
| 22 | ||||
| 24 | ||||
| 25 | ||||
| MARKET PRICE OF OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS |
26 | |||
| 27 | ||||
| 28 | ||||
| 29 | ||||
| 31 | ||||
| 34 | ||||
| MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS FOR NON-U.S. HOLDERS OF COMMON STOCK |
40 | |||
| 44 | ||||
| 46 | ||||
| 56 | ||||
| 56 | ||||
| 56 | ||||
| 57 | ||||
We are responsible for the information contained or incorporated by reference in this prospectus. We have not, and the underwriters have not, authorized anyone to provide you with any other information other than in this prospectus, and we take no responsibility for, and the underwriters have not taken responsibility for, any other information others may give you. We are not, and the underwriters are not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing or incorporated by reference in this prospectus is accurate only as of its date.
Through and including , 2018 (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
For investors outside the United States: Neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus outside of the United States.
This prospectus and the documents incorporated by reference contain references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
i
Table of Contents
This summary highlights information contained elsewhere and incorporated by reference in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before investing in our common stock, you should read the entire prospectus carefully, including the section entitled “Risk Factors” and the information in our filings with the U.S. Securities and Exchange Commission, or the SEC, incorporated by reference in this prospectus. Except where the context otherwise requires or where otherwise indicated, the terms “Deciphera,” “we,” “us,” “our,” “our company,” “the company,” and “our business” refer to Deciphera Pharmaceuticals, Inc. and its consolidated subsidiaries.
Deciphera Pharmaceuticals Overview
We are a clinical-stage biopharmaceutical company developing new drugs to improve the lives of cancer patients by addressing key mechanisms of drug resistance that limit the rate and durability of response of many cancer therapies. Our targeted, small molecule drug candidates, designed using our proprietary kinase switch control inhibitor platform, inhibit the activation of kinases, an important family of enzymes that, when mutated or over expressed, are known to be directly involved in the growth and spread of many cancers. We have built a diverse pipeline of wholly owned, orally administered drug candidates that includes three clinical-stage and two research-stage programs. We are studying our lead drug candidate DCC-2618 in an ongoing pivotal Phase 3 trial in fourth-line plus treatment of gastrointestinal stromal tumors, or GIST, where there are currently no approved therapies, and in an ongoing Phase 1 trial in patients with advanced malignancies. We presented interim results from this Phase 1 trial in September 2017 at the European Society for Medical Oncology 2017 Congress that demonstrate clinical proof-of-concept at well-tolerated doses in 57 heavily pre-treated GIST patients, of which 51 had KIT- or PDGFRα-driven GIST. We are currently enrolling expansion cohorts in this Phase 1 trial to study DCC-2618 in patients with different stages of GIST, as well as in patients with advanced systemic mastocytosis gliomas, including glioblastoma multiforme, and other solid tumors driven by KIT or PDGFRα. We expect to report initial data from some of these expansion cohorts in 2018. We expect to initiate enrollment in a second pivotal Phase 3 trial in second-line GIST comparing DCC-2618 to sunitinib in the second half of 2018. We are also developing two other clinical-stage, small molecule drug candidates, DCC-3014 and rebastinib, as immuno-oncology kinase, or immunokinase, switch control inhibitors targeting colony stimulating factor receptor 1, or CSF1R, and TIE2 kinase, respectively. Both drug candidates are in Phase 1 trials. We believe our proprietary kinase switch control inhibitor platform, supported by our experienced management team, enables us to develop advanced, differentiated kinase inhibitors that may provide significant benefits to cancer patients.
Corporate Update
DCC-2618
Ongoing Phase 1 Trial Update
We are studying DCC-2618 in an ongoing Phase 1 trial in patients with advanced malignancies. As of January 18, 2018, we had enrolled 169 total patients with 113 patients receiving 150 mg of DCC-2618 once daily, or QD. Sixty-eight patients were enrolled in the dose escalation portion and 101 patients were enrolled in the dose expansion portion of the Phase 1 trial. Of these 169 total patients, 142 were gastrointestinal stromal tumor, or GIST, patients, 100 of whom received 150 mg of DCC-2618 QD. We are enrolling patients with select advanced malignancies, including fourth- and fourth-line plus GIST, second- and third-line GIST, advanced systemic mastocytosis, or ASM, and gliomas, including glioblastoma multiforme, or GBM, in expansion cohorts of this Phase 1 trial.
1
Table of Contents
2018 American Society of Clinical Oncology Annual Meeting and Additional Interim Results
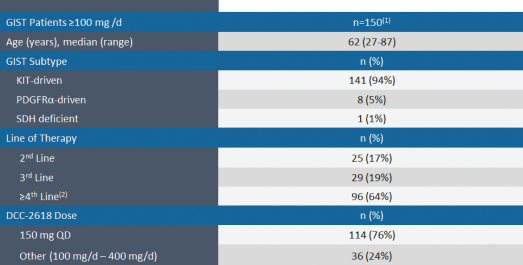
In June 2018, at the 2018 American Society of Clinical Oncology Annual Meeting, or ASCO 2018, we presented an update to the interim results from our ongoing Phase 1 trial of DCC-2618 in 150 GIST patients shown to harbor a broad range of KIT and PDGFRa mutations who received at least one dose at or above 100 mg of DCC-2618 daily, on or before February 26, 2018, with an efficacy cut-off date of April 18, 2018.
In the data presented at ASCO 2018 and as part of our additional interim update, we observed that 83% (115 of 138) of evaluable patients, defined as those patients with at least a baseline and one follow-up tumor assessment as of the efficacy cut-off date, had a best response of stable disease or partial response (defined as tumor size reduction of 30% or more), or PR, by Response Evaluation Criteria in Solid Tumors, or RECIST. In addition, we observed a disease control rate, or DCR, defined as the proportion of patients with either stable disease or a PR at a point in time, of 70% at three months in 145 evaluable patients, which excluded five patients that were on study at the efficacy cut-off date, but had not received a first tumor assessment. Disease control includes stable disease, PRs and complete responses, or CRs, measured by computerized tomography, or CT scan, or magnetic resonance imaging, or MRI scan, and assessed locally by RECIST. We also observed an objective response rate, or ORR, which is the proportion of patients with either CRs or PRs by RECIST of 15% in 145 patients. In 54 second- and third-line GIST patients, we also observed an ORR of 24%, a best response of 83% and a DCR of 80% at 3 months.
Interim results, including those presented at ASCO 2018, are summarized in the below table.
| Line of Therapy |
Total Patients(1) |
Active(1) | Best(2) Response (CR/PR/SD) |
DCR at 3 Months(2) |
ORR(2) | |||||||||||||||
| 2nd Line |
25 | 68 | % | 84 | % | 79 | % | 24 | % | |||||||||||
| 3rd Line |
29 | 76 | % | 83 | % | 82 | % | 24 | % | |||||||||||
|
³4th Line |
96 | 53 | % | 77 | %(3) | 64 | %(3) | 9 | %(3) | |||||||||||
| Total | 150 | 60 | % | 79 | %(3) | 70 | %(3) | 15 | %(3) | |||||||||||
| (1) | Reflects total number of GIST patients in the Phase 1 trial that received at least 100 mg of DCC-2618 daily and that began cycle one day one, or C1D1, on or before February 26, 2018. |
| (2) | Patients with C1D1 on or before February 2, 2018, or enrolled later with an available tumor assessment, based on the April 18, 2018 efficacy cut-off date. |
| (3) | Excludes five patients with C1D1 after February 2, 2018 and no assessment. |
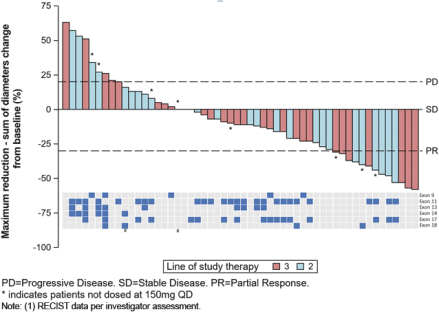
RECIST Responses in KIT- or PDGFRa-driven GIST Patients in Phase 1 Trial
At ASCO 2018, for the second- and third-line GIST patients in our Phase 1 trial of DCC-2618 who received both a baseline and post-treatment CT scan by the efficacy cut-off date (n = 54), we presented the greatest reduction or smallest increase in tumor size from baseline as measured by CT or MRI scan, or best response, for solid malignancies per RECIST as shown in the following figure.
2
Table of Contents
Best Response per RECIST(1)
In Second- and Third-Line KIT & PDGFRa GIST Patients Receiving
³ 100 mg DCC-2618 Daily (n = 54)
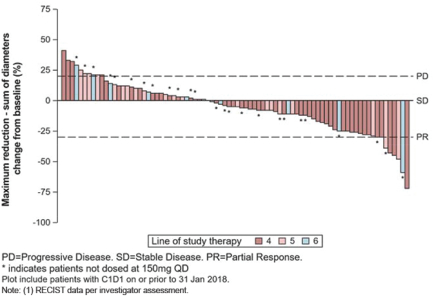
In addition to the data presented at ASCO 2018, for the fourth- and fourth-line plus GIST patients in our Phase 1 trial of DCC-2618 who received both a baseline and post-treatment CT scan by the efficacy cut-off date (n = 82), we also evaluated the best response for solid malignancies per RECIST as shown in the following figure.
Best Response per RECIST(1)
In Fourth- and Fourth-Line Plus KIT & PDGFRa GIST Patients Receiving
³ 100 mg DCC-2618 Daily (n = 82)
3
Table of Contents
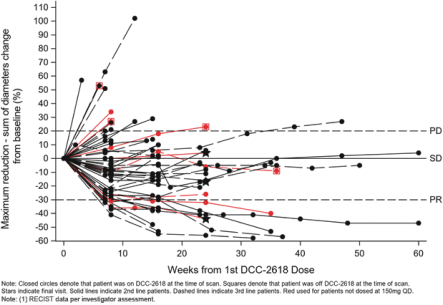
Disease Control Rate in KIT- and PDGFRa-driven GIST Patients
As presented at ASCO 2018, for the second- and third-line GIST patients in our Phase 1 trial of DCC-2618, we observed a DCR at three months of 79% and 82%, respectively. The chart below shows durability of response in 54 second- and third-line GIST patients receiving DCC-2618 at doses of at least 100 mg daily, where each cycle has a duration of 4 weeks.
Duration of Disease Control(1)
In Second- and Third-Line KIT & PDGFRa GIST Patients Receiving
³ 100 mg DCC-2618 Daily (n = 54)
4
Table of Contents
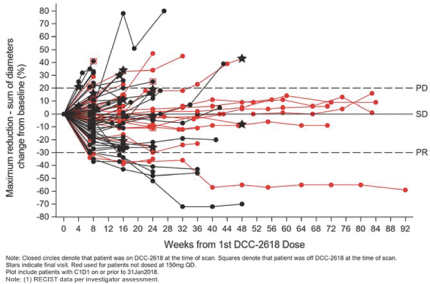
We also observed a DCR at three months of 64% in fourth- and fourth-line plus GIST patients in our Phase 1 trial of DCC-2618. In addition to the data presented at ASCO 2018, we evaluated the durability of response in 89 fourth- and fourth-line plus GIST patients receiving DCC-2618 at doses of at least 100 mg daily, where each cycle has a duration of 4 weeks as shown in the chart below.
Duration of Disease Control(1)
In Fourth- and Fourth-Line Plus KIT & PDGFRa GIST Patients Receiving
³ 100 mg DCC-2618 Daily (n = 89)
5
Table of Contents
Additional Interim Observations
In addition to the data presented at ASCO 2018, we also observed clinical activity in patients who were previously treated with the investigational drug avapritinib (BLU-285). Of the 150 patients in our ongoing Phase 1 trial of DCC-2618, there were 10 evaluable patients with KIT-driven GIST who previously received avapritinib and who were enrolled and being treated with DCC-2618 as of January 31, 2018. Six out of 10, or 60%, of these patients achieved stable disease as best response as of the efficacy cut-off date. One additional patient achieved stable disease following intra-patient dose escalation to 150 mg of DCC-2618 twice daily from 150 mg QD. As of the efficacy cut-off date, five out of 10, or 50%, of these patients remained on study and three out of 10, or 30%, of these patients had received DCC-2618 for at least six months. As of the efficacy cut-off date, of the three patients who had received DCC-2618 for at least six months, two achieved continued stable disease and remained on study. The third patient with progressive disease was dose escalated and reported as off-study as of the efficacy cut-off date. The chart below summarizes the treatment duration of the 10 evaluable patients who previously received avapritinib and the responses observed.
Treatment Duration of Evaluable Patients Who Previously Received Avapritinib
(n = 10)
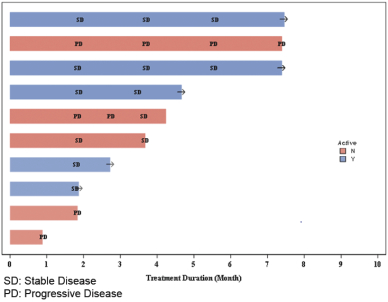
The evaluable patients in our ongoing Phase 1 clinical trial of DCC-2618 who were previously treated with avapritinib are limited in number. We are not able to draw any conclusions about why such patients were no longer enrolled in clinical trials for avapritinib. As a result, any clinical activity observed in these patients may not be representative of future results for these patients or indicative of results for other patients who were previously treated with avapritinib. References to such patients are not intended to be comparisons between avapritinib and DCC-2618.
6
Table of Contents
The chart below summarizes the demographic profile of the patients for whom data was presented at ASCO 2018 and the other additional interim data described above.
Demographic Profile of GIST Patients in Phase 1 Trial
AACR Annual Meeting 2018
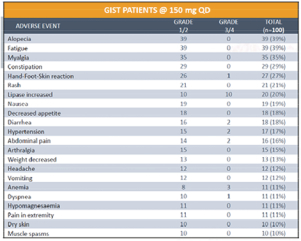
In April 2018, at the AACR Annual Meeting 2018, or AACR 2018, we reported updated safety on 100 patients with GIST who were treated at our recommended Phase 2 dose of 150 mg of DCC-2618 QD. The data showed that in the ongoing Phase 1 trial DCC-2618 continues to be generally well-tolerated at the dose of 150 mg QD as summarized in the table below.
Treatment Emergent Adverse Events
7
Table of Contents
At AACR 2018, we also reported that as of January 1, 2018, 137 GIST patients were enrolled in the Phase 1 trial of DCC-2618 at doses of at least 100 mg of DCC-2618 daily. As of March 19, 2018, 81 of the 137 patients remained on study, with 46, 21, 10 and seven of the patients on study for at least six, nine, 12 and 15 months, respectively.
Ongoing and Planned Phase 3 Trials for DCC-2618 in GIST
In January 2018, we initiated a pivotal Phase 3 trial, or INVICTUS, comparing treatment with DCC-2618 to placebo in 120 fourth-line plus GIST patients, 80 of whom will be treated with 150 mg of DCC-2618 QD and 40 of whom will receive placebo. We expect to initiate a second pivotal Phase 3 trial comparing treatment with DCC-2618 to sunitinib in up to 350 second-line GIST patients in 2018. We expect half of the patients enrolled in this trial will be treated with 150 mg of DCC-2618 QD and the other will be treated with 50 mg of sunitinib QD, with median PFS as the primary endpoint.
Preclinical Update
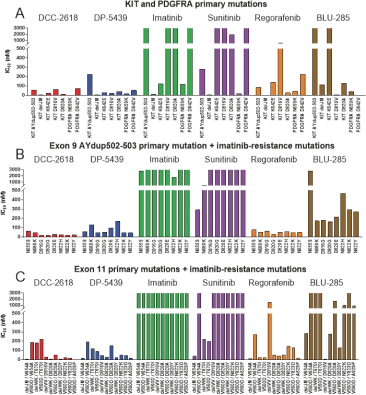
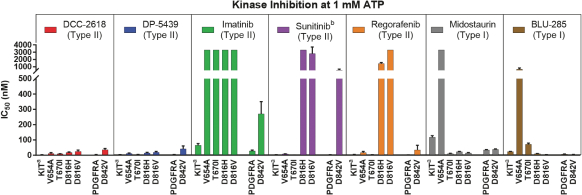
In April 2018, we presented preclinical data at AACR 2018 that describes the breadth of inhibition achieved with DCC-2618 and its active metabolite, DP-5439, across both primary and secondary KIT mutations and primary PDGFRa mutations compared to the in vitro profiles of the FDA-approved kinase inhibitors, imatinib, sunitinib, regorafenib, midostaurin and the investigational drug, avapritinib (BLU-285). Potency is measured by the concentration of DCC-2618 or DP-5439 required to inhibit kinase activity by 50%, or the inhibitory concentration 50%, or IC50. The lower the bar in the following graphs, the greater the potency. Compared to the approved and investigational compounds tested, DCC-2618 and its active metabolite, DP-5439, exhibited the broadest profile of inhibition across primary and secondary drug-resistant KIT mutations, and primary mutations in PDGFRa.
8
Table of Contents
In enzyme assays at relevant cellular levels of adenosine triphosphate, or ATP, DCC-2618 broadly inhibited primary and drug-resistant KIT mutations and primary PDGFRa mutations. DCC-2618 also broadly inhibited KIT and PDGFRa mutations in a panel of GIST, mastocytosis, leukemia, lung cancer, and transfected cell assays.
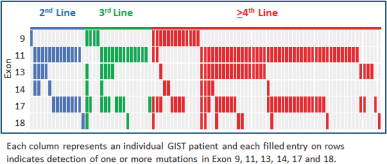
One of the exploratory objectives of our Phase 1 trial of DCC-2618 was to understand the KIT and PDGFRa mutation status at baseline identified in plasma ctDNA of GIST patients and their association with study drug response. At ASCO 2018, we presented data on the mutational status of 131 GIST patients, of which 95 had KIT-driven mutations in ctDNA, by exon, as summarized in the following figure.
KIT Mutations in ctDNA (n = 95)
In 131 GIST Patients by Line of Therapy
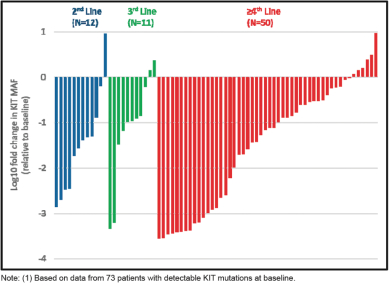
The following figure, which was also included in our ASCO 2018 presentation, shows the maximum change in ctDNA mutant allele frequency, or MAF, by exon in 73 patients with circulating KIT mutations observed at baseline and having data from at least one sample following treatment with DCC-2618. We observed in this group of KIT-positive GIST patients that treatment with DCC-2618 resulted in reductions of least 50% in the
9
Table of Contents
frequency of circulating ctDNA mutant KIT alleles in 78% (57 of 73) of patients, with 48% (35 of 73) of patients becoming KIT-negative on treatment.
Cumulative Reductions in Circulating MAF of KIT Exons 9, 11,
13, 14, 17 and 18 by Lines of Therapy (n = 73)(1)
(Note log scale: -1 = 10-fold reduction, -2 = 100-fold reduction)
Development of DCC-2618 in Gliomas, including GBM
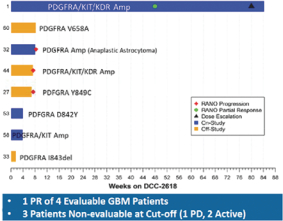
In November 2017, we presented data at the 22nd Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology, or SNO 2018, from eight patients diagnosed with malignant gliomas treated with DCC-2618. Of the five evaluable patients, four had GBM. Among these four GBM patients, we observed one PR, as defined by RANO, and three patients with progressive disease. In addition, of the three patients who were non-evaluable, two patients remained on study and one had progressive disease. DCC-2618 produced an encouraging partial response in a GBM patient with triple amplification of PDGFRa, KIT and KDR (4q12 amplicon). There was an observed tumor reduction from baseline of 94% on Cycle 23 Day 1 per RANO. However, other patients with similar amplifications or PDGFRa alterations did not derive similar benefit from treatment with DCC-2618. This patient population is very heterogeneous, and patients often exhibit multiple genetic alterations in addition to PDGFRa alterations. We believe that this single exceptional responder warrants
10
Table of Contents
further testing of DCC-2618 in patients with KIT- and PDGFRa-driven gliomas, and there is an open cohort in the ongoing expansion phase of the Phase 1 study.
Activity Observed in GBM
Rebastinib—TIE2 Inhibitor
Rebastinib is in clinical development for the treatment of multiple solid tumors and in combination with chemotherapy in an investigator-sponsored Phase 1b trial. The investigators presented preliminary clinical data at AACR 2018 from their ongoing Phase 1b trial of rebastinib. Based on data combining rebastinib with anti-PD1 antibodies or anti-tubulin chemotherapy in preclinical studies, we are evaluating opportunities for further development of these drug candidates in combination with other immuno-oncology therapies or chemotherapy. We expect to initiate a company-sponsored Phase 1b trial of rebastinib with chemotherapy in 2018.
Market Opportunity for our Drug Candidates
We are continuing to focus on our strategy to expand the market opportunity for DCC-2618 by pursuing development in second-line GIST, gliomas, including GBM, ASM and other solid tumors driven by KIT or PDGFRa. The chart below summarizes our estimates of the annual incidence of these diseases.
Estimated Market Opportunity
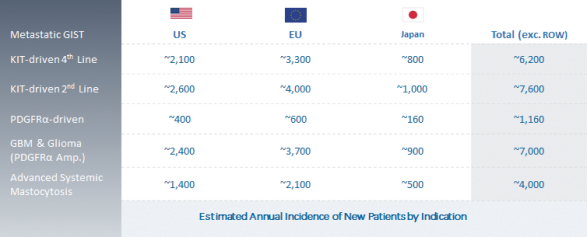
11
Table of Contents
Risks Associated with Our Business
Our ability to execute on our business strategy is subject to a number of risks, which are discussed more fully in the section of this prospectus entitled “Risk Factors.” You should carefully consider these risks before making an investment in our common stock. These risks include, among others, the following:
| • | We have incurred significant operating losses since our inception and have not generated any revenue from product sales. We expect to incur continued losses for the foreseeable future and may never achieve or maintain profitability. |
| • | We will require substantial additional funding. If we are unable to raise capital when needed, or on attractive terms, we could be forced to delay, reduce or eliminate our research or drug development programs or any future commercialization efforts. |
| • | We have a limited operating history, have not successfully completed late-stage clinical trials for any drug candidate, have not generated revenue from product sales or profits and do not expect to generate revenue or profits for the foreseeable future. We may never obtain approval for any of our drug candidates or achieve or sustain profitability. |
| • | All of our drug candidates target inhibition of the activation switch in kinases. If we are unable to commercialize our drug candidates or experience significant delays in doing so, our business will be materially harmed. |
| • | Clinical drug development involves a lengthy and expensive process. We may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of DCC-2618 and our other drug candidates. |
| • | If we experience delays or difficulties in the enrollment of patients in clinical trials, including in our planned second pivotal Phase 3 clinical trial for DCC-2618 in GIST, our receipt of necessary marketing approvals could be delayed or prevented. |
| • | The incidence and prevalence for target patient populations of our drug candidates have not been established with precision. If the market opportunities for our drug candidates are smaller than we estimate or if any approval that we obtain is based on a narrower definition of the patient population, our revenue potential and ability to achieve profitability will be adversely affected. |
| • | We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do. |
| • | We may enter into collaborations with third parties for the development and commercialization of our drug candidates. If those collaborations are not successful, we may not be able to capitalize on the market potential of these drug candidates. |
| • | Manufacturing pharmaceutical products is complex and subject to product loss for a variety of reasons. We contract with third parties for the manufacture of our drug candidates for preclinical testing and clinical trials and expect to continue to do so for commercialization. This reliance on third parties increases the risk that we will not have sufficient quantities of our drug candidates or products or such quantities at an acceptable cost or quality, which could delay, prevent or impair our development or commercialization efforts. |
| • | If we are unable to obtain and maintain sufficient patent protection for our drug candidates, or if the scope of the patent protection is not sufficiently broad, third parties, including our competitors, could develop and commercialize products similar or identical to ours, and our ability to commercialize our drug candidates successfully may be adversely affected. |
12
Table of Contents
Corporate Information
Deciphera Pharmaceuticals, LLC was formed and commenced operations in 2003. Deciphera Pharmaceuticals, Inc. was incorporated under the laws of Delaware on August 1, 2017 for the sole purpose of completing an initial public offering and related transactions in order to carry on the business of Deciphera Pharmaceuticals, LLC. We are the sole managing member of Deciphera Pharmaceuticals, LLC and conduct all our business through, operate and control all of the businesses and affairs of Deciphera Pharmaceuticals, LLC, our wholly owned subsidiary, directly or through blocker entities that are also wholly owned by us.
On October 2, 2017, we completed the initial public offering of our common stock, or IPO. On October 2, 2017, immediately prior to the completion of the IPO, we engaged in a series of transactions whereby Deciphera Pharmaceuticals, LLC became a wholly owned subsidiary of Deciphera Pharmaceuticals, Inc., a Delaware corporation. As part of the transactions, shareholders of Deciphera Pharmaceuticals, LLC exchanged their shares of Deciphera Pharmaceuticals, LLC for shares of Deciphera Pharmaceuticals, Inc. on a one-for-5.65 basis. We refer to these transactions as the Conversion.
Our principal executive offices are located at 500 Totten Pond Road, Waltham, MA 02451, and our telephone number is (781) 209-6400. Our corporate website address is www.deciphera.com. Information contained on or accessible through our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only.
Implications of Being an Emerging Growth Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and we may remain an emerging growth company for up to five years from December 31, 2017. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure and other requirements that are applicable to other public companies that are not emerging growth companies. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
13
Table of Contents
THE OFFERING
| Common stock we are offering |
3,750,000 shares |
| Common stock outstanding after giving effect to this offering |
36,344,128 shares |
| Underwriters’ option to purchase additional shares |
562,500 shares |
| Use of proceeds |
We estimate that our net proceeds from this offering will be approximately $134.3 million (or $154.6 million if the underwriters exercise their option to purchase additional shares in full), based upon an assumed public offering price of $38.31 per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on June 4, 2018, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| We intend to use the net proceeds from this offering, together with our existing cash and cash equivalents, as follows: |
| • | approximately $75 million to fund clinical trials for DCC-2618, including the dose escalation and expansion stages of our current Phase 1 clinical trial, a pivotal clinical trial in fourth-line GIST and additional clinical trials, including a pivotal clinical trial in second-line GIST, as well as clinical research outsourcing and manufacturing of clinical trial material, and pre-commercialization manufacturing process development and validation; |
| • | approximately $5 million to fund clinical trials for DCC-3014, including the dose escalation stage of our Phase 1 clinical trial, as well as clinical research outsourcing and manufacturing of clinical trial material; |
| • | approximately $9 million to fund clinical trials for rebastinib, as well as clinical research outsourcing and manufacturing of clinical trial material; |
| • | approximately $10 million to fund new and ongoing research activities for future drug candidates using our proprietary kinase switch control inhibitor platform; and |
| • | the remainder for working capital purposes, including general operating expenses. |
| See “Use of Proceeds” for additional information. |
| Risk factors |
See “Risk Factors” beginning on page 18 and the other information included in, or incorporated by reference into, this prospectus for a discussion of factors you should carefully consider before deciding to invest in our common stock. |
| Nasdaq Global Select Market symbol |
“DCPH” |
14
Table of Contents
The number of shares of our common stock to be outstanding after this offering is based on 32,594,128 shares of our common stock outstanding as of March 31, 2018, and excludes the following:
| • | 5,374,080 shares of common stock issuable upon the exercise of options outstanding as of March 31, 2018 under our 2015 Equity Incentive Plan and our 2017 Stock Option and Incentive Plan, at a weighted average exercise price of $8.55 per share; |
| • | 2,675,686 shares of our common stock available for future issuance under our 2017 Stock Option and Incentive Plan; and |
| • | 632,666 shares of our common stock reserved for issuance under our 2017 Employee Stock Purchase Plan. |
Except as otherwise indicated, all information in this prospectus assumes no exercise by the underwriters of their option to purchase a maximum of 562,500 additional shares of our common stock from us in this offering at the public offering price, less the underwriting discounts and commissions.
15
Table of Contents
SUMMARY CONSOLIDATED FINANCIAL DATA
You should read the following summary consolidated financial data together with the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included in our Annual Report on Form 10-K for the year ended December 31, 2017 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2018, each of which is incorporated by reference in this prospectus. We have derived the consolidated statement of operations data for the years ended December 31, 2017, 2016 and 2015 from our audited consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2017, which is incorporated by reference in this prospectus. We have derived the consolidated statement of operations data for three months ended March 31, 2018 and 2017 and the consolidated balance sheet data as of March 31, 2018 from our unaudited interim consolidated financial statements included in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2018, which is incorporated by reference in this prospectus. The unaudited interim financial statements have been prepared on the same basis as the audited financial statements and reflect, in the opinion of management, all adjustments of a normal, recurring nature that are necessary for a fair statement of the financial information included in those unaudited interim financial statements. Our historical results are not necessarily indicative of the results that may be expected in the future, and interim results are not necessarily indicative of results to be expected for the full year or any other period.
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||||||
| 2017 | 2016 | 2015 | 2018 | 2017 | ||||||||||||||||
| (in thousands, except share and per share data) | ||||||||||||||||||||
| Consolidated Statement of Operations Data: |
||||||||||||||||||||
| Revenue |
$ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating expenses: |
||||||||||||||||||||
| Research and development |
39,514 | 20,163 | 12,475 | 16,925 | 5,659 | |||||||||||||||
| General and administrative |
11,421 | 5,675 | 5,135 | 5,026 | 2,067 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
50,935 | 25,838 | 17,610 | 21,951 | 7,726 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(50,935 | ) | (25,838 | ) | (17,610 | ) | (21,951 | ) | (7,726 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest expense |
(95 | ) | (106 | ) | (2,209 | ) | (22 | ) | (25 | ) | ||||||||||
| Interest and other income, net |
746 | 4 | 3 | 543 | 42 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total other income (expense), net |
651 | (102 | ) | (2,206 | ) | 521 | 17 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (50,284 | ) | $ | (25,940 | ) | $ | (19,816 | ) | $ | (21,430 | ) | $ | (7,709 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss per share—basic and diluted |
$ | (2.99 | ) | $ | (2.23 | ) | $ | (4.67 | ) | $ | (0.66 | ) | $ | (0.66 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average common shares outstanding—basic and diluted(1) |
16,792,179 | 11,626,287 | 4,245,698 | 32,594,074 | 11,626,287 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | We did not have any common shares outstanding during the years ended December 31, 2016 and 2015 or for the period from January 1, 2017 through the closing of our IPO on October 2, 2017. To determine the weighted average shares outstanding for purposes of calculating net loss per share during those periods, we used the weighted average number of Series A convertible preferred shares outstanding because such shares represented the most subordinated share class outstanding during those periods. Share amounts for periods prior to the IPO have been retrospectively adjusted to give effect to the exchange of Series A convertible |
16
Table of Contents
| preferred shares into shares of common stock upon the Conversion (see Note 10 to our audited consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2017 and Note 2 to our unaudited consolidated financial statements included in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2018, each of which is incorporated by reference in this prospectus, for further details on the calculation of basic and diluted net loss per share). |
| As of March 31, 2018 | ||||||||
| Actual | As Adjusted(2) | |||||||
| (in thousands) | ||||||||
| Consolidated Balance Sheet Data: |
||||||||
| Cash and cash equivalents |
$ | 179,873 | $ | 314,216 | ||||
| Working capital(1) |
164,819 | 299,162 | ||||||
| Total assets |
182,141 | 316,484 | ||||||
| Notes payable to related party, including current portion |
1,435 | 1,435 | ||||||
| Total stockholders’ equity |
164,590 | 298,933 | ||||||
| (1) | We define working capital as current assets less current liabilities. |
| (2) | The as adjusted data reflects the sale by us of 3,750,000 shares of our common stock in this offering at an assumed public offering price of $38.31 per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on June 4, 2018, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
A $1.00 increase (decrease) in the assumed public offering price of $38.31 per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on June 4, 2018, would increase (decrease) the as adjusted amount of each of cash and cash equivalents, working capital, total assets and total stockholders’ equity by $3.5 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase (decrease) of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the as adjusted amount of each of cash and cash equivalents, working capital, total assets and total stockholders’ equity by $36.0 million, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. The as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
17
Table of Contents
Investing in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below together with all of the other information contained in this prospectus, or incorporated by reference, including our financial statements and the related notes and the risks and uncertainties discussed under “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2017 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2018, each of which is incorporated by reference herein in its entirety, before deciding to invest in our common stock. If any of these risks actually occur, our business, prospects, operating results and financial condition could suffer materially. In such event, the trading price of our common stock could decline and you might lose all or part of your investment.
Risks Related to this Offering
After this offering, our executive officers, directors and principal stockholders, if they choose to act together, will continue to have the ability to control or significantly influence all matters submitted to stockholders for approval.
Upon the closing of this offering, and disregarding any shares of common stock that they may purchase in the offering, our executive officers and directors, combined with our stockholders who owned more than 5% of our outstanding capital stock before this offering will, in the aggregate, beneficially own shares representing approximately 70.7% of our capital stock. As a result, if these stockholders were to choose to act together, they would be able to control all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, would control the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of ownership control may:
| • | delay, defer or prevent a change in control; |
| • | entrench our management and the board of directors; or |
| • | impede a merger, consolidation, takeover or other business combination involving us that other stockholders may desire. |
If you purchase shares of common stock in this offering, you will suffer immediate dilution of your investment.
The public offering price of our common stock will be substantially higher than the as adjusted net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that substantially exceeds our as adjusted net tangible book value per share after this offering. To the extent shares subsequently are issued under outstanding options, you will incur further dilution. Based on an assumed public offering price of $38.31 per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on June 4, 2018, you will experience immediate dilution of $30.08 per share, representing the difference between our as adjusted net tangible book value per share after giving effect to this offering and the public offering price. See the “Dilution” section for a more detailed description of the dilution to new investors in this offering.
An active trading market for our common stock may not be sustained.
Our shares of common stock began trading on The Nasdaq Global Select Market on September 28, 2017. Given the limited trading history of our common stock, there is a risk that an active trading market for our shares will not be sustained, which could put downward pressure on the market price of our common stock and thereby affect the ability of our stockholders to sell their shares.
18
Table of Contents
Future sales of common stock by stockholders may have an adverse effect on the then prevailing market price of our common stock.
In the event a public market for our common stock is sustained in the future, sales of our common stock may be made by holders of our public float or by holders of restricted securities in compliance with the provisions of Rule 144 of the Securities Act of 1933, as amended, or the Securities Act. In general, under Rule 144, a non-affiliated person who has satisfied a six-month holding period in a company registered under the Securities Exchange Act of 1934, as amended, or the Exchange Act, as amended, may, sell their restricted common stock without volume limitation, so long as the issuer is current with all reports under the Exchange Act in order for there to be adequate common public information. Affiliated persons may also sell their common shares held for at least six months, but affiliated persons will be required to meet certain other requirements, including manner of sale, notice requirements and volume limitations. Non-affiliated persons who hold their common shares for at least one year will be able to sell their common stock without the need for there to be current public information in the hands of the public. Future sales of shares of our public float or by restricted common stock made in compliance with Rule 144 may have an adverse effect on the then prevailing market price, if any, of our common stock.
The market prices for our common stock may be adversely impacted by future events.
Our common stock is currently quoted on The Nasdaq Global Select Market under the symbol “DCPH.” Market prices for our common stock will be influenced by a number of factors, including:
| • | the issuance of new equity securities pursuant to this offering or a future offering, including issuances of preferred stock; |
| • | changes in interest rates; |
| • | significant dilution caused by the anti-dilutive clauses in our financial agreements; |
| • | competitive developments, including announcements by competitors of new products or services or significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments; |
| • | variations in quarterly operating results; |
| • | change in financial estimates by securities analysts; |
| • | the depth and liquidity of the market for our common stock; |
| • | investor perceptions of our company and the pharmaceutical and biotech industries generally; and |
| • | general economic and other national conditions. |
The price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for purchasers of our common stock in this offering.
If you purchase shares in this offering, you may not be able to resell those shares at or above the public offering price. The trading price of the shares has fluctuated, and is likely to continue to fluctuate substantially. The trading price of our securities depends on a number of factors, including those described or incorporated by reference in this “Risk Factors” section, many of which are beyond our control and may not be related to our operating performance.
Since our common stock began trading on The Nasdaq Global Select Market on September 28, 2017, our stock has traded at prices as low as $15.15 per share and as high as $38.43 per share through June 4, 2018. The stock market in general and the market for smaller biopharmaceutical companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result
19
Table of Contents
of this volatility, you may not be able to sell your common stock at or above the public offering price. The market price for our common stock may be influenced by many factors, including:
| • | the degree of success of competitive products or technologies; |
| • | results of clinical trials and preclinical studies, of our drug candidates or those of our competitors; |
| • | regulatory or legal developments in the United States and other countries; |
| • | receipt of, or failure to obtain, regulatory approvals; |
| • | developments or disputes concerning patent applications, issued patents or other proprietary rights; |
| • | the recruitment or departure of key personnel; |
| • | the level of expenses related to any of our drug candidates or clinical development programs; |
| • | the results of our efforts to discover, develop, acquire or in-license additional technologies or drug candidates; |
| • | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
| • | variations in our financial results or those of companies that are perceived to be similar to us; |
| • | rumors or announcements regarding transactions involving our company or drug candidates; |
| • | changes in the structure of healthcare payment systems; |
| • | market conditions in the pharmaceutical and biotechnology sectors; |
| • | general economic, industry and market conditions; and |
| • | the other factors described or incorporated by reference in this “Risk Factors” section. |
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock to decline and delay the development of our drug candidates. Pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
A significant portion of our total outstanding shares are eligible to be sold into the market in the near future, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. After this offering, we will have 36,344,128 outstanding shares of common stock based on the number of shares outstanding as of March 31, 2018. Shares issued and sold in this offering may be resold in the public market immediately without restriction, unless purchased by our affiliates, officers, directors and certain stockholders subject to Rule 144 under the Securities Act or lock-up agreements. However, J.P. Morgan Securities LLC and Piper Jaffray & Co., as representatives of the underwriters, may, in their sole discretion, permit our officers, directors and stockholders who are subject to these lock-up agreements to sell shares prior to the expiration of the lock-up agreements. Any shares purchased by our existing stockholders through the underwriters in this offering would not be subject to these lock-up agreements. A significant portion of our shares outstanding prior to the completion of this offering will be subject to lock-up agreements as described in
20
Table of Contents
“Underwriting.” If, after the end of such lock-up agreements, these stockholders sell substantial amounts of our securities in the public market, or the market perceives that such sales may occur, the market price of our shares and our ability to raise capital through an issuance of equity securities in the future could be adversely affected. Moreover, holders of an aggregate of approximately 24.3 million shares of our common stock have rights, subject to specified conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders.
21
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference contain forward-looking statements, which reflect our current views with respect to, among other things, our operations and financial performance. All statements other than statements of historical facts contained in this prospectus, including statements regarding this offering and the use of proceeds from this offering, our strategy, future operations, future financial position, future revenue, projected costs, prospects, plan, objectives of management and expected market growth are forward-looking statements. You can identify these forward-looking statements by the use of words such as “outlook,” “believes,” “expects,” “potential,” “continues,” “may,” “will,” “should,” “seeks,” “approximately,” “predicts,” “intends,” “plans,” “estimates,” “anticipates” or the negative version of these words or other comparable words. Such forward-looking statements are subject to various risks and uncertainties. Accordingly, there are or will be important factors that could cause actual outcomes or results to differ materially from those indicated in these statements. We believe these factors include but are not limited to those described under “Risk Factors” and include, among other things:
| • | this offering and our anticipated use of proceeds from this offering; |
| • | the success, cost and timing of our product development activities and clinical trials, including the timing of our planned second pivotal Phase 3 trial for DCC-2618 in GIST; |
| • | our ability to obtain and maintain regulatory approval for DCC-2618 or any of our other current or future drug candidates, and any related restrictions, limitations, and/or warnings in the label of an approved drug candidate; |
| • | our expectations regarding the size of target patient populations for our drug candidates, if approved for commercial use, and any additional drug candidates we may develop; |
| • | our ability to obtain funding for our operations; |
| • | our ability to manufacture sufficient quantities of DCC-2618 to support our planned clinical trials and, if approved, commercialization; |
| • | the commercialization of our drug candidates, if approved; |
| • | our plans to research, develop and commercialize our drug candidates, including the timing of our second planned Phase 3 trial for DCC-2618 in GIST; |
| • | our ability to attract collaborators with development, regulatory and commercialization expertise; |
| • | our expectations regarding our ability to obtain, maintain, enforce and defend our intellectual property protection for our drug candidates; |
| • | future agreements with third parties in connection with the commercialization of DCC-2618, or any of our other current or future drug candidates; |
| • | the size and growth potential of the markets for our drug candidates, and our ability to serve those markets; |
| • | the rate and degree of market acceptance of our drug candidates, as well as the reimbursement coverage for our drug candidates; |
| • | regulatory and legal developments in the United States and foreign countries; |
| • | the performance of our third-party suppliers and manufacturers; |
| • | the success of competing therapies that are or may become available; |
| • | our ability to attract and retain key scientific or management personnel; |
| • | the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; |
22
Table of Contents
| • | our expectations regarding the period during which we qualify as an emerging growth company under the JOBS Act; and |
| • | our use of the proceeds from our IPO. |
These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in this prospectus and the documents incorporated by reference. We undertake no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise.
23
Table of Contents
MARKET, INDUSTRY AND OTHER DATA
This prospectus and the documents incorporated by reference include market, industry and other data and forecasts that we have derived from independent consultant reports, publicly available information, various industry publications, other published industry sources and our internal data and estimates. Independent consultant reports, industry publications and other published industry sources generally indicate that the information contained therein was obtained from sources believed to be reliable.
Our internal data and estimates are based upon information obtained from trade and business organizations and other contacts in the markets in which we operate and our management’s understanding of industry conditions. Although we believe that such information is reliable, we have not had this information verified by any independent sources.
24
Table of Contents
We estimate that the net proceeds to us from the sale of the shares of our common stock offered by us in this offering will be approximately $134.3 million, based on an assumed public offering price of $38.31 per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on June 4, 2018, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters’ option to purchase 562,500 additional shares in this offering is exercised in full, we estimate that our net proceeds from this offering will be approximately $154.6 million, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
A $1.00 increase (decrease) in the assumed public offering price of $38.31 per share, the last reported sale price of the our common stock on The Nasdaq Global Select Market on June 4, 2018, would increase (decrease) the net proceeds to us from this offering by approximately $3.5 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, an increase (decrease) of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us from this offering by approximately $36.0 million, assuming no change in the assumed public offering price and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
As of March 31, 2018, we had cash and cash equivalents of $179.9 million. We intend to use the net proceeds from this offering, together with our existing cash and cash equivalents, as follows:
| • | approximately $75 million to fund clinical trials for DCC-2618, including the dose escalation and expansion stages of our current Phase 1 clinical trial, a pivotal clinical trial in fourth-line GIST and additional clinical trials, including a pivotal clinical trial in second-line GIST, as well as clinical research outsourcing and manufacturing of clinical trial material, and pre-commercialization manufacturing process development and validation; |
| • | approximately $5 million to fund clinical trials for DCC-3014, including the dose escalation stage of our Phase 1 clinical trial, as well as clinical research outsourcing and manufacturing of clinical trial material; |
| • | approximately $9 million to fund clinical trials for rebastinib, as well as clinical research outsourcing and manufacturing of clinical trial material; |
| • | approximately $10 million to fund new and ongoing research activities for future drug candidates using our proprietary kinase switch control inhibitor platform; and |
| • | the remainder for working capital purposes, including general operating expenses. |
Our expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the closing of this offering or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual use of the net proceeds from this offering will vary depending on numerous factors, including the progress of our clinical trials and other development efforts for DCC-2618 and other factors described in “Risk Factors” beginning on page 18 or incorporated by reference herein, as well as the amount of cash we use in our operations. As a result, our management will have broad discretion in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the net proceeds from this offering. In addition, we might decide to postpone or not pursue clinical trials or preclinical activities if the net proceeds from this offering and the other sources of cash are less than expected.
Pending application of the net proceeds, we intend to invest the net proceeds from this offering in short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
25
Table of Contents
MARKET PRICE OF OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS
Our common stock trades under the symbol “DCPH” on The Nasdaq Global Select Market and has been publicly traded since September 28, 2017. Prior to this time, there was no public market for our common stock. The following table sets forth the high and low sales price of our common stock as reported on The Nasdaq Global Select Market for the periods indicated:
| High | Low | |||||||
| Year Ended December 31, 2017 |
||||||||
| Third Quarter (from September 28, 2017) |
$ | 20.53 | $ | 16.11 | ||||
| Fourth Quarter |
$ | 24.50 | $ | 15.15 | ||||
| High | Low | |||||||
| Year Ending December 31, 2018 |
||||||||
| First Quarter |
$ | 29.98 | $ | 19.74 | ||||
| Second Quarter (through June 4, 2018) |
$ | 38.43 | $ | 19.19 | ||||
On June 4, 2018, the last reported sale price of our common stock on The Nasdaq Global Select Market was $38.31 per share.
As of June 4, 2018, there were approximately 16 holders of record of shares of our common stock. This number does not include stockholders for whom shares are held in “nominee” or “street” name.
26
Table of Contents
We currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any dividends on our common stock in the foreseeable future. Any future determination to declare dividends will be made at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant.
27
Table of Contents
The following table sets forth our cash and cash equivalents and our capitalization as of March 31, 2018:
| • | on an actual basis; and |
| • | on an as adjusted basis to give effect to the sale by us of 3,750,000 shares of our common stock in this offering at an assumed public offering price of $38.31 per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on June 4, 2018, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
The as adjusted information below is illustrative only, and our capitalization following the completion of this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this table together with the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included in our Annual Report on Form 10-K for the year ended December 31, 2017 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2018, each of which is incorporated by reference in this prospectus.
| As of March 31, 2018 | ||||||||
| Actual | As Adjusted | |||||||
| (in thousands, except share data) |
||||||||
| Cash and cash equivalents |
$ | 179,873 | $ | 314,216 | ||||
|
|
|
|
|
|||||
| Notes payable to related party, including current portion |
$ | 1,435 | $ | 1,435 | ||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.01 par value; 5,000,000 shares authorized, actual and as adjusted; no shares issued or outstanding, actual and as adjusted |
— | — | ||||||
| Common stock, $0.01 par value; 125,000,000 shares authorized, actual and as adjusted; 32,594,128 shares issued and outstanding, actual; 36,344,128 shares issued and outstanding, as adjusted |
326 | 363 | ||||||
| Additional paid-in capital |
381,563 | 515,869 | ||||||
| Accumulated deficit |
(217,299 | ) | (217,299 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
164,590 | 298,933 | ||||||
|
|
|
|
|
|||||
| Total capitalization |
$ | 166,025 | $ | 300,368 | ||||
|
|
|
|
|
|||||
A $1.00 increase (decrease) in the assumed public offering price of $38.31 per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on June 4, 2018, would increase (decrease) the as adjusted amount of each of cash and cash equivalents, total stockholders’ equity and total capitalization by $3.5 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase (decrease) of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the as adjusted amount of each of cash and cash equivalents, total stockholders’ equity and total capitalization by $36.0 million, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The table above does not include:
| • | 5,374,080 shares of common stock issuable upon the exercise of options outstanding as of March 31, 2018 under our 2015 Equity Incentive Plan and our 2017 Stock Option and Incentive Plan, at a weighted average exercise price of $8.55 per share; |
| • | 2,675,686 shares of our common stock available for future issuance under our 2017 Stock Option and Incentive Plan; and |
| • | 632,666 shares of our common stock reserved for issuance under our 2017 Employee Stock Purchase Plan. |
28
Table of Contents
If you invest in our common stock in this offering, your ownership interest will be diluted immediately to the extent of the difference between the public offering price per share of our common stock and the as adjusted net tangible book value per share of our common stock immediately after this offering.
As of March 31, 2018, our historical net tangible book value was $164.6 million, or $5.05 per share of common stock. Our historical net tangible book value per share is equal to our total tangible assets, less total liabilities, divided by the number of outstanding shares of our common stock. After giving effect to the sale by us of 3,750,000 shares of our common stock in this offering at an assumed public offering price of $38.31 per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on June 4, 2018, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of March 31, 2018 would have been approximately $298.9 million, or approximately $8.23 per share of common stock. This represents an immediate increase in as adjusted net tangible book value of $3.18 per share to our existing stockholders and immediate dilution of $30.08 per share to new investors purchasing common stock in this offering. Dilution per share to new investors is determined by subtracting the as adjusted net tangible book value per share after this offering from the assumed public offering price per share paid by new investors. The following table illustrates this dilution on a per share basis:
| Assumed public offering price per share |
$ | 38.31 | ||||||
| Historical net tangible book value per share as of March 31, 2018 |
$ | 5.05 | ||||||
| Increase in as adjusted net tangible book value per share attributable to new investors purchasing common stock in this offering |
3.18 | |||||||
|
|
|
|||||||
| As adjusted net tangible book value per share after this offering |
8.23 | |||||||
|
|
|
|||||||
| Dilution per share to new investors purchasing common stock in this offering |
$ | 30.08 | ||||||
|
|
|
A $1.00 increase (decrease) in the assumed public offering price of $38.31 per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on June 4, 2018, would increase (decrease) our as adjusted net tangible book value per share after this offering by $0.10 and dilution per share to new investors purchasing common stock in this offering by $0.90, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase our as adjusted net tangible book value per share after this offering by $0.74 and decrease the dilution per share to new investors purchasing common stock in this offering by $0.74, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. A decrease of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would decrease our as adjusted net tangible book value per share after this offering by $0.79 and increase the dilution per share to new investors purchasing common stock in this offering by $0.79, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters fully exercise their option to purchase additional shares of common stock in this offering, our as adjusted net tangible book value per share after this offering would be $8.65 and the dilution in as adjusted net tangible book value per share to new investors purchasing common stock in this offering would be $29.66, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The following table summarizes, as of March 31, 2018, on the as adjusted basis described above, the total number of shares of common stock purchased from us, the total consideration paid or to be paid, and the average price per share paid or to be paid by existing stockholders and by new investors in this offering at an assumed
29
Table of Contents
public offering price of $38.31 per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on June 4, 2018, before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. As the table shows, new investors purchasing common stock in this offering will pay an average price per share substantially higher than our existing stockholders paid.
| Shares Purchased | Total Consideration | Average Price Per Share |
||||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||||
| Existing stockholders |
32,594,128 | 89.7 | % | $ | 385,817,684 | 72.9 | % | $ | 11.84 | |||||||||||
| New investors |
3,750,000 | 10.3 | 143,662,500 | 27.1 | $ | 38.31 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
36,344,128 | 100.0 | % | $ | 529,480,184 | 100.0 | % | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
A $1.00 increase (decrease) in the assumed public offering price of $38.31 per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on June 4, 2018, would increase (decrease) the total consideration paid by new investors by $3.8 million and, in the case of an increase, would increase the percentage of total consideration paid by new investors by 0.5 percentage points and, in the case of a decrease, would decrease the percentage of total consideration paid by new investors by 0.5 percentage points, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. An increase (decrease) of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the total consideration paid by new investors by $38.3 million and, in the case of an increase, would increase the percentage of total consideration paid by new investors by 4.9 percentage points and, in the case of a decrease, would decrease the percentage of total consideration paid by new investors by 5.7 percentage points, assuming no change in the assumed public offering price per share.
The table above assumes no exercise of the underwriters’ option to purchase additional shares in this offering. If the underwriters’ option to purchase additional shares is fully exercised, the number of shares of our common stock held by existing stockholders would be reduced to 88.3% of the total number of shares of our common stock outstanding after this offering, and the number of shares of common stock held by new investors purchasing common stock in this offering would be increased to 11.7% of the total number of shares of our common stock outstanding after this offering.
The table above is based on 32,594,128 shares of our common stock outstanding as of March 31, 2018, and excludes the following:
| • | 5,374,080 shares of common stock issuable upon the exercise of options outstanding as of March 31, 2018 under our 2015 Equity Incentive Plan and our 2017 Stock Option and Incentive Plan, at a weighted average exercise price of $8.55 per share; |
| • | 2,675,686 shares of our common stock available for future issuance under our 2017 Stock Option and Incentive Plan; and |
| • | 632,666 shares of our common stock reserved for issuance under our 2017 Employee Stock Purchase Plan. |
If additional shares of common stock are issued in connection with the exercise of outstanding options, new options or other securities are issued under our equity incentive plans, or if we issue additional shares of common stock in the future, there will be further dilution to investors purchasing common stock in this offering.
30
Table of Contents
The following table sets forth information with respect to the beneficial ownership of our common stock, as of May 18, 2018 by:
| • | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common stock; |
| • | each of our directors; |
| • | each of our named executive officers; and |
| • | all of our executive officers and directors as a group. |
The column entitled “Percentage of Shares Beneficially Owned—Before Offering” is based on a total of 32,594,128 shares of our common stock outstanding as of May 18, 2018. The column entitled “Percentage of Shares Beneficially Owned—After Offering” is based on shares of our common stock to be outstanding after this offering, including the shares of our common stock that we are selling in this offering, but not including any additional shares issuable pursuant to the underwriters’ option to purchase additional shares. The number of shares beneficially owned by each stockholder is determined under rules issued by the SEC and includes voting or investment power with respect to securities. Under these rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power. In computing the number of shares beneficially owned by an individual or entity and the percentage ownership of that person, shares of common stock subject to options, warrants, or other rights held by such person that are currently exercisable or will become exercisable within 60 days after May 18, 2018 are considered outstanding, although these shares are not considered outstanding for purposes of computing the percentage ownership of any other person. Unless otherwise indicated, the address of all listed stockholders is 500 Totten Pond Rd, Waltham, Massachusetts 02451. Each of the stockholders listed has sole voting and investment power with respect to the shares beneficially owned by the stockholder unless noted otherwise, subject to community property laws where applicable.
| Shares Beneficially Owned |
Percentage of Shares Beneficially Owned |
|||||||||||
| Name of Beneficial Owner |
Before Offering |
After Offering |
||||||||||
| 5% Stockholders |
||||||||||||
| Brightstar Associates LLC(1) |
16,572,370 | 50.84 | % | 45.60 | % | |||||||
| Entities affiliated with New Leaf Venture Partners(2) |
4,104,140 | 12.59 | % | 11.29 | % | |||||||
| Entities affiliated with Viking Global Investors LP(3) |
2,394,625 | 7.35 | % | 6.59 | % | |||||||
| Entities affiliated with SV Health Investors(4) |
1,716,303 | 5.27 | % | 4.27 | % | |||||||
| Named Executive Officers and Directors |
||||||||||||
| Michael D. Taylor, Ph.D.(5) |
842,174 | 2.52 | % | 2.26 | % | |||||||
| Christopher J. Morl(6) |
126,179 | * | * | |||||||||
| Daniel L. Flynn, Ph.D.(7) |
937,640 | 2.81 | % | 2.52 | % | |||||||
| Patricia L. Allen(8) |
17,362 | * | * | |||||||||
| Edward J. Benz, Jr., M.D.(9) |
17,362 | * | * | |||||||||
| James A. Bristol, Ph.D.(10) |
203,262 | * | * | |||||||||
| Steven L. Hoerter(11) |
1,334 | * | * | |||||||||
| John R. Martin(12) |
44,417 | * | * | |||||||||
| Liam Ratcliffe, M.D., Ph.D.(2) |
4,140,974 | 12.69 | % | 11.38 | % | |||||||
| Michael Ross, Ph.D.(13) |
33,584 | * | * | |||||||||
| Dennis L. Walsh(14) |
44,417 | * | * | |||||||||
| All executive officers and directors as a group (12 persons)(15) |
6,902,083 | 19.58 | % | 17.70 | % | |||||||
| * | Less than 1%. |
31
Table of Contents
| (1) | Based on the Schedule 13G filed with the SEC by Brightstar Associates LLC, or Brightstar, on January 26, 2018. Consists of an aggregate of 16,572,370 shares of common stock directly held by Brightstar. Brightstar is managed by a three-person managing board consisting of Mark K. Fallon, Gary L. Muller and Timothy Fritzel, and all action relating to the voting or disposition of these shares requires approval of a majority of the board. Such individuals expressly disclaim any such beneficial ownership. The address of Brightstar is 1020 Central Street, Suite 300, Kansas City, Missouri 64105. |
| (2) | Based on the Schedule 13D filed with the SEC by New Leaf Ventures III, L.P., or NLV III, New Leaf Biopharma Opportunities I, L.P., or Biopharma I, New Leaf Venture Associates III, L.P., or NLV Associates III, BPO Associates I, L.P., or NLBA I, New Leaf Venture Management III, L.L.C., or NLV Management III, Liam Ratcliffe, Jeani Delagardelle, Ronald M. Hunt and Vijay K. Lathi on October 11, 2017. Consists of an aggregate of 4,104,140 shares of common stock, of which (i) 1,957,832 shares are directly owned by NLV III, and (ii) 2,146,308 shares are directly owned by Biopharma I, except that (a) NLBA I, the sole general partner of Biopharma I, may be deemed to have sole power to vote or dispose of such shares owned by Biopharma I, (b) NLV Associates III, the sole general partner of NLV III, may be deemed to have sole power to vote or dispose of such shares owned by NLV III, (c) NLV Management III, as the sole general partner of NLBA I and ultimate general partner of Biopharma I, may be deemed to have sole power to vote or dispose of all of the shares directly owned by Biopharma I, and as the sole general partner of NLV Associates III and ultimate general partner of NLV III, may be deemed to have sole power to vote or dispose of all of the shares directly owned by NLV III, and (d) Liam Ratcliffe, a member of our board of directors, Jeani Delagardelle, Ronald M. Hunt and Vijay K. Lathi, the sole members of NLV Management III, may be deemed to have shared power to vote or dispose of all of such shares. The address of the principal business office of NLV III, NLV Associates III, Biopharma I, NLBA I, NLV Management III, Liam Ratcliffe and Ronald M. Hunt is c/o New Leaf Ventures, 7 Times Square, Suite 3502, New York, New York 10036. The address of the principal business office of Vijay K. Lathi and Jeani Delagardelle is c/o New Leaf Venture Partners, 1200 Part Place, Suite 300, San Mateo, California 94043. Additionally, Dr. Ratcliffe beneficially owns 36,834 shares of common stock underlying options that are exercisable as of May 18, 2018 or will become exercisable within 60 days after such date. |
| (3) | Based on the Schedules 13G and 13G/A filed with the SEC by Viking Global Investors LP, or VGI, Viking Global Opportunities GP LLC, or Opportunities GP, Viking Global Opportunities Portfolio GP LLC, or Opportunities Portfolio GP, Viking Global Opportunities Liquid Portfolio Sub-Mater LP, or Opportunities Liquid Fund, Viking Global Opportunities Illiquid Investments Sub-Master LP, or Opportunities Illiquid Fund, Viking Global Opportunities Intermediate LP, or Opportunities Intermediate, DRAGSA 14 LLC, or DRAGSA, O. Andreas Halvorsen, David C. Ott and Rose S. Shabet on October 11, 2017 and February 14, 2018, respectively. Consists of an aggregate of 2,394,625 shares of common stock, of which (i) 903,083 shares are directly owned by Opportunities Liquid Fund, and (ii) 1,491,542 shares are directly owned by Opportunities Iliquid Fund, except that (a) Opportunities Portfolio GP, the general partner of Opportunities Liquid Fund and Opportunities Illiquid Fund, may be deemed to have shared power to vote or dispose of all shares directly owned by Opportunities Liquid Fund and Opportunities Illiquid Fund, (b) Opportunities GP, the sole member of Opportunities Portfolio GP, may be deemed to have shared power to vote or dispose of all shares controlled by Opportunities Portfolio GP, which consists of the shares directly owned by Opportunities Liquid Fund and Opportunities Illiquid Fund, (c) VGI, an affiliate of Opportunities Portfolio GP, which provides managerial services to Opportunities Liquid Fund and Opportunities Illiquid Fund, may be deemed to have shared power to vote or dispose of all shares directly owned by Opportunities Liquid Fun and Opportunities Illiquid Fund, and (d) O. Andreas Halvorsen, David C. Ott and Rose S. Shabet, as Executive Committee Members of Viking Global Partners LLC, the general partner of VGI and Opportunities GP, may be deemed to have shared power to vote or dispose of shares beneficially owned by VGI and Opportunities GP, which includes all shares directly owned by Opportunities Liquid Fund and Opportunities Illiquid Fund. The address of the principal business address for each of the entities and individuals listed above is c/o Viking Global Investors LP, 55 Railroad Avenue, Greenwich, Connecticut 06830. |
32
Table of Contents
| (4) | Based on the Schedule 13D filed with the SEC by SV Life Sciences Fund VI, L.P., or SVLS VI LP, and SV Life Sciences Fund VI Strategic Partners, L.P., or Strategic Partners, SV Life Sciences Fund VI (GP), L.P., or SVLS VI GP, and SVLSF VI, LLC on October 6, 2017. Consists of an aggregate of 1,716,303 shares of common stock, of which (i) 1,659,487 shares are directly owned by SVLS VI LP, and (ii) 56,816 shares are directly owned by Strategic Partners, except that (a) SVLSF VI GP, the general partner of SVLS VI LP and Strategic Partners, may be deemed to have sole power to vote or dispose of such shares owned directly by SVLS VI LP and Strategic Partners and (b) SVLSF VI, LLC, the general partner of SVLS VI GP, may be deemed to have sole power to vote and dispose of such shares owned directly by SVLS VI LP and Strategic Partners. Each of SVLS VI GP and SVLSF VI, LLC disclaims beneficial ownership of the shares held by SVLS VI LP and Strategic Partners except to the extent of any pecuniary interest therein. The address of the principal business office for each of the entities and individuals listed above is c/o SV Health Investors, One Boston Place, Suite 3900, 201 Washington Street, Boston, Massachusetts 02108. |
| (5) | Consists of 842,174 shares of common stock issuable pursuant to stock options exercisable within 60 days of May 18, 2018. |
| (6) | Consists of 126,179 shares of common stock issuable pursuant to stock options exercisable within 60 days of May 18, 2018. |
| (7) | Consists of 790,591 shares of common stock issuable pursuant to stock options exercisable within 60 days of May 18, 2018 and 147,049 shares of common stock held by Biochenomix, LLC, of which Dr. Flynn is a Managing Director and the beneficial owner of such shares. |
| (8) | Consists of 17,362 shares of common stock issuable pursuant to stock options exercisable within 60 days of May 18, 2018. |
| (9) | Consists of 17,362 shares of common stock issuable pursuant to stock options exercisable within 60 days of May 18, 2018. |
| (10) | Consists of 203,262 shares of common stock issuable pursuant to stock options exercisable within 60 days of May 18, 2018. |
| (11) | Consists of 1,334 shares of common stock issuable pursuant to stock options exercisable within 60 days of May 18, 2018. |
| (12) | Consists of 44,417 shares of common stock issuable pursuant to stock options exercisable within 60 days of May 18, 2018. |
| (13) | Consists of 33,584 shares of common stock issuable pursuant to stock options exercisable within 60 days of May 18, 2018. |
| (14) | Consists of 44,417 shares of common stock issuable pursuant to stock options exercisable within 60 days of May 18, 2018. |
| (15) | See notes 2, 5, 6, 7, 8, 9, 10, 11, 12, 13 and 14 above. Also includes shares of common stock issuable pursuant to stock options exercisable within 60 days of May 18, 2018 held by Oliver Rosen, M.D. and Thomas P. Kelly, who are executive officers but not named executive officers. |
33
Table of Contents
The following description of our capital stock and provisions of our amended and restated certificate of incorporation and amended and restated bylaws are summaries of material terms and provisions and are qualified by reference to our amended and restated certificate of incorporation and amended and restated bylaws, copies of which have been filed with the SEC and are incorporated by reference as exhibits to the registration statement of which this prospectus is a part.
Upon consummation of this offering, and assuming the same capitalization as of March 31, 2018, our authorized capital stock will consist of 125,000,000 shares of common stock, par value $0.01 per share, and 5,000,000 shares of preferred stock, par value $0.01 per share, all of which preferred stock will be undesignated, and there will be 36,344,128 shares of our common stock outstanding and no shares of preferred stock outstanding. Unless our board of directors determines otherwise, we will issue all shares of our capital stock in uncertificated form. As of June 4, 2018, we had approximately 16 record holders of our capital stock. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Common Stock
The holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of the stockholders. The holders of our common stock do not have any cumulative voting rights. Holders of our common stock are entitled to receive ratably any dividends declared by our board of directors out of funds legally available for that purpose, subject to any preferential dividend rights of any outstanding preferred stock. Our common stock has no preemptive rights, conversion rights or other subscription rights or redemption or sinking fund provisions.
In the event of our liquidation, dissolution or winding up, holders of our common stock will be entitled to share ratably in all assets remaining after payment of all debts and other liabilities and any liquidation preference of any outstanding preferred stock. The shares to be issued by us in this offering will be, when issued and paid for, validly issued, fully paid and non-assessable.
Preferred Stock
Our board of directors has the authority, without further action by our stockholders, to issue up to 5,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting, or the designation of, such series, any or all of which may be greater than the rights of common stock. The issuance of our preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon our liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change in control of our company or other corporate action. We have no shares of preferred stock outstanding, and we have no present plan to issue any shares of preferred stock.
Options
As of March 31, 2018, we had options outstanding to purchase 5,374,080 shares of our common stock that were issued to employees and directors as compensation for services. These options are subject to our equity incentive plans and vesting terms approved by our board of directors and set forth in written option award agreements.
34
Table of Contents
Authorized but Unissued Capital Stock
The Delaware General Corporation Law does not require stockholder approval for any issuance of authorized shares. However, the listing requirements of Nasdaq, which would apply so long as our common stock remains listed on Nasdaq, require stockholder approval of certain issuances equal to or exceeding 20% of the then outstanding voting power or then outstanding number of shares of common stock. These additional shares may be used for a variety of corporate purposes, including future public offerings, to raise additional capital or to facilitate acquisitions.
One of the effects of the existence of unissued and unreserved common stock or preferred stock may be to enable our board of directors to issue shares to persons friendly to current management, which issuance could render more difficult or discourage an attempt to obtain control of our company by means of a merger, tender offer, proxy contest or otherwise, and thereby protect the continuity of our management and possibly deprive our stockholders of opportunities to sell their shares of common stock at prices higher than prevailing market prices.
Anti-Takeover Effects of Provisions of Delaware Law and Our Amended and Restated Certificate of Incorporation and Bylaws
Our amended and restated certificate of incorporation and amended and restated bylaws include a number of provisions that may have the effect of delaying, deferring or preventing another party from acquiring control of us and encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our board of directors rather than pursue non-negotiated takeover attempts. These provisions include the items described below.
Board Composition and Filling Vacancies
Our amended and restated certificate of incorporation provides for the division of our board of directors into three classes serving staggered three-year terms, with one class being elected each year. Our amended and restated certificate of incorporation also provides that directors may be removed only for cause and then only by the affirmative vote of the holders of 75% or more of the shares then entitled to vote at an election of directors. Furthermore, any vacancy on our board of directors, however occurring, including a vacancy resulting from an increase in the size of our board, may only be filled by the affirmative vote of a majority of our directors then in office even if less than a quorum. The classification of directors, together with the limitations on removal of directors and treatment of vacancies, has the effect of making it more difficult for stockholders to change the composition of our board of directors.
No Written Consent of Stockholders
Our amended and restated certificate of incorporation provides that all stockholder actions are required to be taken by a vote of the stockholders at an annual or special meeting, and that stockholders may not take any action by written consent in lieu of a meeting. This limit may lengthen the amount of time required to take stockholder actions and would prevent the amendment of our bylaws or removal of directors by our stockholders without holding a meeting of stockholders.
Meetings of Stockholders
Our amended and restated certificate of incorporation and amended and restated bylaws provide that only a majority of the members of our board of directors then in office may call special meetings of stockholders and only those matters set forth in the notice of the special meeting may be considered or acted upon at a special meeting of stockholders. Our amended and restated bylaws limit the business that may be conducted at an annual meeting of stockholders to those matters properly brought before the meeting.
35
Table of Contents
Advance Notice Requirements
Our amended and restated bylaws establish advance notice procedures with regard to stockholder proposals relating to the nomination of candidates for election as directors or new business to be brought before meetings of our stockholders. These procedures provide that notice of stockholder proposals must be timely given in writing to our corporate secretary prior to the meeting at which the action is to be taken. Generally, to be timely, notice must be received at our principal executive offices not less than 90 days nor more than 120 days prior to the first anniversary date of the annual meeting for the preceding year. Our amended and restated bylaws specify the requirements as to form and content of all stockholders’ notices. These requirements may preclude stockholders from bringing matters before the stockholders at an annual or special meeting.
Amendment to Certificate of Incorporation and Bylaws
Any amendment of our amended and restated certificate of incorporation must first be approved by a majority of our board of directors, and if required by law or our amended and restated certificate of incorporation, must thereafter be approved by a majority of the outstanding shares entitled to vote on the amendment and a majority of the outstanding shares of each class entitled to vote thereon as a class, except that the amendment of the provisions relating to stockholder action, board composition, limitation of liability and the amendment of our bylaws and certificate of incorporation must be approved by not less than 75% of the outstanding shares entitled to vote on the amendment, and not less than 75% of the outstanding shares of each class entitled to vote thereon as a class. Our amended and restated bylaws may be amended by the affirmative vote of a majority of the directors then in office, subject to any limitations set forth in the bylaws; and may also be amended by the affirmative vote of at least 75% of the outstanding shares entitled to vote on the amendment, or, if our board of directors recommends that the stockholders approve the amendment, by the affirmative vote of the majority of the outstanding shares entitled to vote on the amendment, in each case voting together as a single class.
Undesignated Preferred Stock
Our amended and restated certificate of incorporation provide for 5,000,000 authorized shares of preferred stock. The existence of authorized but unissued shares of preferred stock may enable our board of directors to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise. For example, if in the due exercise of its fiduciary obligations, our board of directors were to determine that a takeover proposal is not in the best interests of our stockholders, our board of directors could cause shares of preferred stock to be issued without stockholder approval in one or more private offerings or other transactions that might dilute the voting or other rights of the proposed acquirer or insurgent stockholder or stockholder group. In this regard, our amended and restated certificate of incorporation grants our board of directors broad power to establish the rights and preferences of authorized and unissued shares of preferred stock. The issuance of shares of preferred stock could decrease the amount of earnings and assets available for distribution to holders of shares of common stock. The issuance may also adversely affect the rights and powers, including voting rights, of these holders and may have the effect of delaying, deterring or preventing a change in control of us.
Exclusive Jurisdiction for Certain Actions
Our amended and restated bylaws provide that, unless we consent in writing to an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers and employees to us or our stockholders, (iii) any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or our bylaws, or (iv) any action asserting a claim that is governed by the internal affairs doctrine, in each case subject to the Court of Chancery having personal jurisdiction over the indispensable parties named as defendants therein. Although we believe this provision benefits us by providing increased consistency in the application of Delaware law in the
36
Table of Contents
types of lawsuits to which it applies, the provision may have the effect of discouraging lawsuits against our directors and officers. The enforceability of similar exclusive forum provisions in other corporation’s bylaws has been challenged in legal proceedings, and it is possible that a court could rule that this provision in our amended and restated bylaws is inapplicable or unenforceable.
Section 203 of the Delaware General Corporation Law
We are subject to the provisions of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. Under Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions:
| • | before the stockholder became interested, our board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| • | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee stock plans, in some instances, but not the outstanding voting stock owned by the interested stockholder; or |
| • | at or after the time the stockholder became interested, the business combination was approved by our board of directors and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, lease, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| • | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| • | subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; and |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Registration Rights
The holders of approximately 24.3 million shares of our common stock, or their transferees, are entitled to the registration rights set forth below with respect to registration of the resale of such shares under the Securities Act pursuant to the registration rights agreement, by and among us and certain of our stockholders.
37
Table of Contents
Demand Registration Rights
Upon the written request of at least 40% of the holders of the registrable securities then outstanding, or a lesser percentage in certain cases, that we file a registration statement under the Securities Act covering the registration of registrable securities owned by such holder(s) having an anticipated aggregate offering price, net of selling expenses, of at least $25.0 million, we will be obligated to notify all holders of registrable securities of such request. As soon as practicable thereafter, and in any event within 60 days after the date such request is given, we will be required to register the sale on a registration statement on Form S-1 of all registrable securities that holders may request to be registered, subject to specified exceptions, conditions and limitations. We may postpone the filing of a registration statement for up to 90 days once in any 12-month period if in the good faith judgment of our board of directors such registration would be detrimental to us, and we are not required to effect the filing of a registration statement during the period starting with the date that is 60 days prior to our good faith estimate of the date of filing of a registration statement initiated by us and ending on a date 180 days, in the case of our initial public offering, or 90 days, in all other cases, after the effective date of a registration statement initiated by us. We are required to effect only three registrations pursuant to this provision. The underwriters of any underwritten offering will have the right to limit the number of shares having registration rights to be included in the registration statement.
“Piggyback” Registration Rights
If we register any securities for public sale, holders of registration rights will have the right to include their shares in the registration statement. The underwriters of any underwritten offering will have the right to limit the number of registrable securities to be included in the registration statement, but such number may not be below 20% of the total number of shares included in such registration statement.
Form S-3 Registration Rights
If we are eligible to file a registration statement on Form S-3, holders of at least 10% of our registrable securities then outstanding, or a lesser percentage in certain cases, have the right to request that we file a registration statement on Form S-3, so long as the aggregate price to the public of the securities to be sold under the registration statement on Form S-3 is at least $5.0 million. As soon as practicable thereafter, and in any event within 45 days after the date such request is given, we will be required to register the sale on a registration statement on Form S-3 of all registrable securities that holders may request to be registered, subject to specified exceptions, conditions and limitations. We may postpone the filing of a registration statement for up to 90 days once in any 12-month period if in the good faith judgment of our board of directors such registration would be detrimental to us, and we are not required to effect the filing of a registration statement during the period starting with the date that is 30 days prior to our good faith estimate of the date of filing of a registration statement initiated by us and ending on a date 90 days after the effective date of a registration statement initiated by us. We are required to effect only two registrations in any 12-month period. The underwriters of any underwritten offering will have the right to limit the number of shares having registration rights to be included in the registration statement.
Expenses of Registration
Pursuant to the registration rights agreement, we are generally required to bear all registration expenses, including the fees and expenses of one counsel representing the selling holders, incurred in connection with the demand, piggyback and Form S-3 registrations described above. We are not required to bear selling expenses, which include all underwriting discounts and commissions, selling commissions, stock transfer taxes applicable to the sale of registrable securities, and fees and disbursements of any additional counsel for any selling holder. We are not required to pay registration expenses if the registration request is withdrawn at the request of the holders of a majority of the registrable securities unless (i) the holders of a majority of the registrable securities agree to forfeit their right to one registration, or (ii) the withdrawal is due to the discovery of a material adverse change in our business.
38
Table of Contents
Termination of Registration Rights
The demand, piggyback and Form S-3 registration rights discussed above will terminate as to a given holder of registrable securities upon the earlier of (i) three years following the closing of our IPO, except with respect to shares held by certain principal investors whose registration rights shall not terminate until any such principal investor first holds less than one percent of our outstanding capital stock, (ii) the closing of a change of control or (iii) when all shares held by the holders can be sold under SEC Rule 144 within a 90-day period.
Market Listing
Our common stock is listed on The Nasdaq Global Select Market under the symbol “DCPH.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A.
39
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS FOR NON-U.S. HOLDERS OF COMMON STOCK
The following discussion is a summary of the material U.S. federal income tax considerations applicable to non-U.S. holders (as defined below) with respect to their ownership and disposition of shares of our common stock issued pursuant to this offering. For purposes of this discussion, a non-U.S. holder means a beneficial owner of our common stock that is for U.S. federal income tax purposes:
| • | a non-resident alien individual; |
| • | a foreign corporation or any other foreign organization taxable as a corporation for U.S. federal income tax purposes; or |
| • | a foreign estate or trust, the income of which is not subject to U.S. federal income tax on a net income basis. |
This discussion does not address the tax treatment of partnerships or other entities that are pass-through entities for U.S. federal income tax purposes or persons that hold their common stock through partnerships or other pass-through entities. A partner in a partnership or other pass-through entity that will hold our common stock should consult his, her or its own tax advisor regarding the tax consequences of acquiring, holding and disposing of our common stock through a partnership or other pass-through entity, as applicable.
This discussion is based on current provisions of the U.S. Internal Revenue Code of 1986, as amended, which we refer to as the Code, existing and proposed U.S. Treasury Regulations promulgated thereunder, current administrative rulings and judicial decisions, all as in effect as of the date of this prospectus and, all of which are subject to change or to differing interpretation, possibly with retroactive effect. Any such change or differing interpretation could alter the tax consequences to non-U.S. holders described in this prospectus. There can be no assurance that the Internal Revenue Service, which we refer to as the IRS, will not challenge one or more of the tax consequences described herein and we have not obtained, nor do we intend to obtain, a ruling from the IRS with respect to the U.S. federal income tax consequences to a non-U.S. holder of the ownership, or disposition, of our common stock. We assume in this discussion that a non-U.S. holder holds shares of our common stock as a capital asset, generally property held for investment.
This discussion does not address all aspects of U.S. federal income that may be relevant to a particular non-U.S. holder in light of that non-U.S. holder’s individual circumstances nor does it address any aspects of any U.S. federal tax other than the income tax, U.S. state, local or non-U.S. taxes, the alternative minimum tax, any tax considerations resulting from a non-U.S. holder having a functional currency other than the U.S. dollar, or the Medicare tax on net investment income. This discussion also does not consider any specific facts or circumstances that may apply to a non-U.S. holder and does not address the special tax rules applicable to particular non-U.S. holders, such as:
| • | insurance companies; |
| • | tax exempt or governmental organizations; |
| • | financial institutions; |
| • | brokers or dealers in securities; |
| • | regulated investment companies; |
| • | pension plans; |
| • | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | “qualified foreign pension funds,” or entities wholly owned by a “qualified foreign pension fund”; |
40
Table of Contents
| • | persons deemed to sell our common stock under the constructive sale provisions of the Code; |
| • | persons that hold our common stock as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment; |
| • | persons who hold or receive our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; and |
| • | certain U.S. expatriates. |
This discussion is for general information only and is not tax advice. Accordingly, all prospective non-U.S. holders of our common stock should consult their own tax advisors with respect to the U.S. federal, state, local and non-U.S. tax consequences of the purchase, ownership and disposition of our common stock.
Distributions on Our Common Stock
Distributions, if any, on our common stock generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the non-U.S. holder’s investment, up to such holder’s tax basis in the common stock. Any remaining excess will be treated as capital gain, subject to the tax treatment described below in “Gain on Sale or Other Taxable Disposition of Our Common Stock.” Any such distributions will also be subject to the discussion below under the section titled “Withholding and Information Reporting Requirements—FATCA.”
Subject to the discussion in the following two paragraphs in this section, dividends paid to a non-U.S. holder generally will be subject to withholding of U.S. federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence.
Dividends that are treated as effectively connected with a trade or business conducted by a non-U.S. holder within the United States and, if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed base maintained by the non-U.S. holder within the United States, are generally exempt from the 30% withholding tax if the non-U.S. holder satisfies applicable certification and disclosure requirements. However, such U.S. effectively connected income, net of specified deductions and credits, is taxed at the same graduated U.S. federal income tax rates applicable to United States persons (as defined in the Code). Any U.S. effectively connected income received by a non-U.S. holder that is a corporation may also, under certain circumstances, be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence.
A non-U.S. holder of our common stock who claims the benefit of an applicable income tax treaty between the United States and such holder’s country of residence generally will be required to provide a properly executed IRS Form W-8BEN or W-8BEN-E (or successor form) and satisfy applicable certification and other requirements. Non-U.S. holders are urged to consult their tax advisors regarding their entitlement to benefits under a relevant income tax treaty. A non-U.S. holder that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty may obtain a refund or credit of any excess amounts withheld by timely filing a U.S. tax return with the IRS.
Gain on Sale or Other Taxable Disposition of Our Common Stock
Subject to the discussion below under “Backup Withholding and Information Reporting” and “Withholding and Information Reporting Requirements—FATCA,” a non-U.S. holder generally will not be subject to any U.S. federal income or withholding tax on any gain realized upon such holder’s sale or other taxable disposition of shares of our common stock unless:
| • | the gain is effectively connected with the non-U.S. holder’s conduct of a U.S. trade or business and, if an applicable income tax treaty so provides, is attributable to a permanent establishment or a fixed-base |
41
Table of Contents
| maintained by such non-U.S. holder in the United States, in which case the non-U.S. holder generally will be taxed on a net income basis at the graduated U.S. federal income tax rates applicable to United States persons (as defined in the Code) and, if the non-U.S. holder is a foreign corporation, the branch profits tax described above in “Distributions on Our Common Stock” also may apply; |
| • | the non-U.S. holder is a nonresident alien individual who is present in the United States for 183 days or more in the taxable year of the disposition and certain other conditions are met, in which case the non-U.S. holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence) on the net gain derived from the disposition, which may be offset by certain U.S. source capital losses of the non-U.S. holder, if any (even though the individual is not considered a resident of the United States), provided that the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses; or |
| • | we are, or have been, at any time during the five-year period preceding such sale of other taxable disposition (or the non-U.S. holder’s holding period, if shorter) a “U.S. real property holding corporation,” unless our common stock is regularly traded on an established securities market and the non-U.S. holder holds no more than 5% of our outstanding common stock, directly or indirectly, actually or constructively, during the shorter of the 5-year period ending on the date of the disposition or the period that the non-U.S. holder held our common stock. If we are determined to be a U.S. real property holding corporation and the foregoing exception does not apply, then a purchaser may be required to withhold 15% of the proceeds payable to a non-U.S. holder from a sale of our common stock and the non-U.S. holder generally will be taxed on its net gain derived from the disposition at the graduated U.S. federal income tax rates applicable to United States persons (as defined in the Code). Generally, a corporation is a U.S. real property holding corporation only if the fair market value of its U.S. real property interests equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance, we do not believe that we are, or have been, a U.S. real property holding corporation, or that we are likely to become one in the future. No assurance can be provided that our common stock will be regularly traded on an established securities market for purposes of the rules described above. |
Backup Withholding and Information Reporting
We must report annually to the IRS and to each non-U.S. holder the gross amount of the distributions on our common stock paid to such holder and the tax withheld, if any, with respect to such distributions. Non-U.S. holders may have to comply with specific certification procedures to establish that the holder is not a United States person (as defined in the Code) in order to avoid backup withholding at the applicable rate, currently 24%, with respect to dividends on our common stock. Dividends paid to non-U.S. holders subject to withholding of U.S. federal income tax, as described above in “Distributions on Our Common Stock,” generally will be exempt from U.S. backup withholding.
Information reporting and backup withholding will generally apply to the proceeds of a disposition of our common stock by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a non-U.S. holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker. Non-U.S. holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them. Copies of information returns may be made available to the tax authorities of the country in which the non-U.S. holder resides or is incorporated under the provisions of a specific treaty or agreement. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a non-U.S. holder
42
Table of Contents
can be refunded or credited against the non-U.S. holder’s U.S. federal income tax liability, if any, provided that an appropriate claim is filed with the IRS in a timely manner.
Withholding and Information Reporting Requirements—FATCA
The Foreign Account Tax Compliance Act, or FATCA, generally imposes a U.S. federal withholding tax at a rate of 30% on payments of dividends on, or gross proceeds from the sale or other disposition of, our common stock paid to a foreign entity unless (i) if the foreign entity is a “foreign financial institution,” such foreign entity undertakes certain due diligence, reporting, withholding, and certification obligations, (ii) if the foreign entity is not a “foreign financial institution,” such foreign entity identifies certain of its U.S. investors, if any, or (iii) the foreign entity is otherwise exempt under FATCA. Under applicable U.S. Treasury regulations, withholding under FATCA currently applies to payments of dividends on our common stock, but will only apply to payments of gross proceeds from a sale or other disposition of our common stock made after December 31, 2018. Under certain circumstances, a non-U.S. holder may be eligible for refunds or credits of this withholding tax. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Non-U.S. holders should consult their tax advisors regarding the possible implications of this legislation on their investment in our common stock and the entities through which they hold our common stock, including, without limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of the 30% withholding tax under FATCA.
43
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
We cannot predict the effect, if any, that future sales of shares of common stock, or the availability for future sale of shares of common stock, will have on the market price of shares of our common stock prevailing from time to time. The sale of substantial amounts of shares of our common stock in the public market, or the perception that such sales could occur, could harm the prevailing market price of shares of our common stock.
Based on the number of shares outstanding as of March 31, 2018, upon completion of this offering, we will have a total of 36,344,128 shares of our common stock outstanding (or 36,906,628 shares of common stock if the underwriters exercise in full their option to purchase additional shares of common stock). Of the outstanding shares, all of the shares sold in this offering will be freely tradable, except that any shares, including shares sold to an entity affiliated with an existing shareholder that may purchase shares in this offering, held by our affiliates, as that term is defined in Rule 144 under the Securities Act, may only be sold in compliance with the limitations described below.
Rule 144
In general, a person who has beneficially owned restricted stock for at least six months would be entitled to sell their securities provided that (i) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days preceding, a sale and (ii) we are subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Persons who have beneficially owned restricted shares for at least six months but who are our affiliates at the time of, or any time during the 90 days preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of either of the following:
| • | 1% of the number of shares then outstanding, which will equal approximately 363,441 shares immediately after this offering assuming no exercise of the underwriters’ option to purchase additional shares, based on the number of shares outstanding as of March 31, 2018; or |
| • | the average weekly trading volume of our common stock on The Nasdaq Global Select Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Such sales both by affiliates and by non-affiliates must also comply with the manner of sale, current public information and notice provisions of Rule 144.
Rule 701
Rule 701 under the Securities Act, as in effect on the date of this prospectus, permits resales of shares in reliance upon Rule 144 but without compliance with certain restrictions of Rule 144, including the holding period requirement. Most of our employees, executive officers or directors who purchased shares under a written compensatory plan or contract may be entitled to rely on the resale provisions of Rule 701. However, certain Rule 701 shares are subject to lock-up agreements as described below and under “Underwriting” included elsewhere in this prospectus and will become eligible for sale upon the expiration of the restrictions set forth in those agreements.
Lock-Up Agreements
In connection with this offering, we, along with our directors, executive officers and certain stockholders have agreed with the underwriters that for a period of 90 days (the restricted period), after the date of this prospectus, subject to specified exceptions, we or they will not offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, or enter into any swap or other
44
Table of Contents
arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock. Upon expiration of the “restricted” period, certain of our stockholders will have the right to require us to register their shares under the Securities Act. See “—Registration Rights” below and “Description of Capital Stock—Registration Rights.”
Certain of our employees, including our executive officers and/or directors, may enter into written trading plans that are intended to comply with Rule 10b5-1 under the Exchange Act. Sales under these trading plans would not be permitted until the expiration of the lock-up agreements relating to the offering described above.
Registration Rights
We are party to a registration rights agreement pursuant to which the holders of approximately 24.3 million shares of our common stock have rights with respect to the registration of their shares under the Securities Act, subject to the lock-up agreements described under “—Lock-Up Agreements” above. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates. Any sales of shares by these stockholders could have a material adverse effect on the trading price of our common stock. See “Description of Capital Stock—Registration Rights.”
Equity Incentive Plans
We have filed registration statements on Form S-8 under the Securities Act to register shares of our common stock subject to awards outstanding or to be granted under our 2015 Equity Incentive Plan, our 2017 Stock Option and Incentive Plan and our 2017 Employee Stock Purchase Plan. Shares covered by these registration statements are eligible for sale in the public markets, subject to the lock-up agreements described above and Rule 144 limitations applicable to affiliates. For a more complete discussion of our equity incentive plans, see our Definitive Proxy Statement for our 2018 annual meeting of stockholders, as filed with the SEC on April 3, 2018.
45
Table of Contents
We are offering the shares of common stock described in this prospectus through a number of underwriters. J.P. Morgan Securities LLC and Piper Jaffray & Co. are acting as joint book-running managers of the offering and as representatives of the underwriters. We have entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of shares of common stock listed next to its name in the following table:
| Name |
Number of Shares |
|||
| J.P. Morgan Securities LLC |
||||
| Piper Jaffray & Co. |
||||
|
|
|
|||
| Total |
3,750,000 | |||
|
|
|
|||
The underwriters are committed to purchase all the common shares offered by us if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may also be increased or the offering may be terminated.
The underwriters propose to offer the common shares directly to the public at the public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per share. Any such dealers may resell shares to certain other brokers or dealers at a discount of up to $ per share from the public offering price. After the initial offering of the shares to the public, the offering price and other selling terms may be changed by the underwriters.
The underwriters have an option to buy up to 562,500 additional shares of common stock from us to cover sales of shares by the underwriters which exceed the number of shares specified in the table above. The underwriters have 30 days from the date of this prospectus to exercise this option. If any shares are purchased with this option, the underwriters will purchase shares in approximately the same proportion as shown in the table above. If any additional shares of common stock are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered.
The underwriting fee is equal to the public offering price per share of common stock less the amount paid by the underwriters to us per share of common stock. The underwriting fee is $ per share. The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Without exercise of option to purchase additional shares |
With full exercise of option to purchase additional shares |
|||||||
| Per Share |
$ | $ | ||||||
| Total |
$ | $ | ||||||
We estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions, will be approximately $0.7 million. We have agreed to reimburse the underwriters for expenses relating to clearance of this offering with the Financial Industry Regulatory Authority up to $30,000.
A prospectus in electronic format may be made available on the web sites maintained by one or more underwriters, or selling group members, if any, participating in the offering. The underwriters may agree to
46
Table of Contents
allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make Internet distributions on the same basis as other allocations.
We have agreed that we will not (i) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, or (ii) enter into any swap or other arrangement that transfers all or a portion of the economic consequences associated with the ownership of any shares of common stock or any such other securities (regardless of whether any of these transactions are to be settled by the delivery of shares of common stock or such other securities, in cash or otherwise), in each case without the prior written consent of J.P. Morgan Securities LLC and Piper Jaffray & Co. for a period of 90 days after the date of this prospectus, other than the shares of our common stock to be sold hereunder, any shares of our common stock issued upon the exercise of options granted under our existing management incentive plans and other limited exceptions.
Our directors and executive officers, and certain of our stockholders holding an aggregate of 22,392,813 shares of our common stock as of May 18, 2018 have entered into lock-up agreements with the underwriters prior to the commencement of this offering pursuant to which each of these persons or entities, with limited exceptions, for a period of 90 days after the date of this prospectus, may not, without the prior written consent of J.P. Morgan Securities LLC and Piper Jaffray & Co., (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock (including without limitation, common stock or such other securities which may be deemed to be beneficially owned by such directors, executive officers, managers and members in accordance with the rules and regulations of the SEC and securities which may be issued upon exercise of a stock option or warrant), or publicly disclose the intention to make any offer, sale, pledge or disposition, (2) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of our common stock or such other securities, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of our common stock or such other securities, in cash or otherwise or (3) make any demand for or exercise any right with respect to the registration of any shares of our common stock or any security convertible into or exercisable or exchangeable for our common stock, in each case subject to certain exceptions, including:
(i) transfers of shares of our common stock as a bona fide gift; provided that such donee agrees to be bound by the lock-up provisions;
(ii) in the event such stockholder is a corporation, partnership, limited liability company, trust or other business entity, (a) transfers to another corporation, partnership, limited liability company, trust or other affiliate or (b) distributions without consideration to its stockholders, partners, members or other equity holders; provided that in each case, such transferee agrees to be bound by the lock-up provisions;
(iii) sales or other transfers of any such director, officer or stockholder’s shares of common stock acquired in this offering or transactions relating to any such director, officer or stockholder’s shares of common stock acquired in open market transactions after the effective date of the registration statement for this offering;
(iv) transfers of shares of our common stock to an immediate family member of such director, officer or stockholder or any trust or other legal entity for the direct or indirect benefit of such director, officer or stockholder or the immediate family member of such director, officer or stockholder, or if the stockholder is a trust, to any beneficiary of such stockholder; provided that any such transferee agrees to be bound by the lock-up provisions, and provided further that any such transfer shall not involve a disposition for value;
(v) transfers of shares of our common stock by will or intestate succession upon the death of a director, officer or stockholder;
47
Table of Contents
(vi) transfers of shares of our common stock by operation of law or by order of a court of competent jurisdiction pursuant to a qualified domestic order or in connection with a divorce settlement;
(vii) the surrender or forfeiture of such director, officer or stockholder’s shares of our common stock to satisfy (x) tax withholding obligations upon exercise or vesting or (y) the exercise price upon a cashless net exercise, in each case, of share options, equity awards, warrants or other right to acquire shares of our common stock pursuant to equity compensation plans described or incorporated by reference in this prospectus;
(viii) the exercise of any option, warrant or other rights to acquire shares of our common stock or other securities, the settlement of any share-settled share appreciation rights, restricted shares or restricted share units or the conversion of any convertible security into shares of our common stock; provided that any such shares or other securities issued continue to be subject to the lock-up provisions;
(ix) pursuant to a bona fide third-party tender offer, merger, consolidation or other similar transaction in each case made to all holders of our common stock, involving a change of control; provided that in the event that such transaction is not completed, such director, officer or stockholder’s shares shall remain subject to the lock-up provisions; and
(x) transfers of such director, officer or stockholder arising as a result of the termination of employment and pursuant to employment agreements under which we have the option to repurchase such director, officer or stockholder’s shares or a right of first refusal with respect to the transfer of such director, officer or stockholder’s shares.
The lock-up agreements will also not apply to the establishment of a trading plan by such director, officer or stockholder pursuant to Rule 10b5-1 under the Exchange Act for the transfer of shares of common stock; provided that such plan does not provide for the transfer of common stock during the 90-day period referred to above.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act.
Our common stock is listed on The Nasdaq Global Select Market under the symbol “DCPH.”
In connection with this offering, the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling shares of common stock in the open market for the purpose of preventing or retarding a decline in the market price of the common stock while this offering is in progress. These stabilizing transactions may include making short sales of the common stock, which involves the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in this offering, and purchasing shares of common stock on the open market to cover positions created by short sales. Short sales may be “covered” shorts, which are short positions in an amount not greater than the underwriters’ option to purchase additional shares referred to above, or may be “naked” shorts, which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their option to purchase additional shares, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market compared to the price at which the underwriters may purchase shares through the option to purchase additional shares. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market that could adversely affect investors who purchase in this offering. To the extent that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of the common stock, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase common stock in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
48
Table of Contents
These activities may have the effect of raising or maintaining the market price of the common stock or preventing or retarding a decline in the market price of the common stock, and, as a result, the price of the common stock may be higher than the price that otherwise might exist in the open market. If the underwriters commence these activities, they may discontinue them at any time. The underwriters may carry out these transactions on The Nasdaq Global Select Market, in the over-the-counter market or otherwise.
Other Relationships
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities.
Certain of the underwriters and their respective affiliates have, from time to time, performed, and may in the future perform, various financial advisory and investment banking services for us, for which they may receive customary fees and expenses.
In addition, in the ordinary course of business, the underwriters and their respective affiliates may make or hold a broad array of investments including serving as counterparties to certain derivative and hedging arrangements and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of the issuer. The underwriters and their respective affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Selling Restrictions
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Notice to Prospective Investors in the European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”), with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State, no offer of shares may be made to the public in that Relevant Member State other than:
| A. | to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| B. | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), subject to obtaining the prior consent of the underwriters; or |
| C. | in any other circumstances falling within Article 3(2) of the Prospectus Directive, |
provided that no such offer of shares shall require the Company or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the
49
Table of Contents
Prospectus Directive and each person who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed to and with each of the underwriters and the Company that it is a “qualified investor” within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive.
In the case of any shares being offered to a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares to the public other than their offer or resale in a Relevant Member State to qualified investors as so defined or in circumstances in which the prior consent of the representatives has been obtained to each such proposed offer or resale.
For the purposes of this provision, the expression an “offer of shares to the public” in relation to any shares in any Relevant Member State means the communication in any form and by means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase shares, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive” means Directive 2003/71/EC (as amended, including by Directive 2010/73/EU), and includes any relevant implementing measure in the Relevant Member State.
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the “Order”) and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”) or otherwise in circumstances which have not resulted and will not result in an offer to the public of the shares in the United Kingdom within the meaning of the Financial Services and Markets Act 2000.
Any person in the United Kingdom that is not a relevant person should not act or rely on the information included in this document or use it as basis for taking any action. In the United Kingdom, any investment or investment activity that this document relates to may be made or taken exclusively by relevant persons.
Notice to Prospective Investors in Canada
The shares may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the shares must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
50
Table of Contents
Notice to Prospective Investors in Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document does not constitute a prospectus within the meaning of, and has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company, the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (FINMA), and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (“CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
Notice to Prospective Investors in the Dubai International Financial Centre (“DIFC”)
This document relates to an Exempt Offer in accordance with the Markets Rules 2012 of the Dubai Financial Services Authority (“DFSA”). This document is intended for distribution only to persons of a type specified in the Markets Rules 2012 of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus supplement nor taken steps to verify the information set forth herein and has no responsibility for this document. The securities to which this document relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the securities offered should conduct their own due diligence on the securities. If you do not understand the contents of this document you should consult an authorized financial advisor.
In relation to its use in the DIFC, this document is strictly private and confidential and is being distributed to a limited number of investors and must not be provided to any person other than the original recipient, and may not be reproduced or used for any other purpose. The interests in the securities may not be offered or sold directly or indirectly to the public in the DIFC.
Notice to Prospective Investors in the United Arab Emirates
The shares have not been, and are not being, publicly offered, sold, promoted or advertised in the United Arab Emirates (including the Dubai International Financial Centre) other than in compliance with the laws of the United Arab Emirates (and the Dubai International Financial Centre) governing the issue, offering and sale of securities. Further, this prospectus does not constitute a public offer of securities in the United Arab Emirates (including the Dubai International Financial Centre) and is not intended to be a public offer. This prospectus has not been approved by or filed with the Central Bank of the United Arab Emirates, the Securities and Commodities Authority or the Dubai Financial Services Authority.
Notice to Prospective Investors in Australia
This prospectus:
| • | does not constitute a product disclosure document or a prospectus under Chapter 6D.2 of the Corporations Act 2001 (Cth) (the “Corporations Act”); |
| • | has not been, and will not be, lodged with the Australian Securities and Investments Commission (“ASIC”), as a disclosure document for the purposes of the Corporations Act and does not purport to include the information required of a disclosure document under Chapter 6D.2 of the Corporations Act; |
51
Table of Contents
| • | does not constitute or involve a recommendation to acquire, an offer or invitation for issue or sale, an offer or invitation to arrange the issue or sale, or an issue or sale, of interests to a “retail client” (as defined in section 761G of the Corporations Act and applicable regulations) in Australia; and |
| • | may only be provided in Australia to select investors who are able to demonstrate that they fall within one or more of the categories of investors, or Exempt Investors, available under section 708 of the Corporations Act. |
The shares may not be directly or indirectly offered for subscription or purchased or sold, and no invitations to subscribe for or buy the shares may be issued, and no draft or definitive offering memorandum, advertisement or other offering material relating to any shares may be distributed in Australia, except where disclosure to investors is not required under Chapter 6D of the Corporations Act or is otherwise in compliance with all applicable Australian laws and regulations. By submitting an application for the shares, you represent and warrant to us that you are an Exempt Investor.
As any offer of shares under this document will be made without disclosure in Australia under Chapter 6D.2 of the Corporations Act, the offer of those securities for resale in Australia within 12 months may, under section 707 of the Corporations Act, require disclosure to investors under Chapter 6D.2 if none of the exemptions in section 708 applies to that resale. By applying for the shares you undertake to us that you will not, for a period of 12 months from the date of issue of the shares, offer, transfer, assign or otherwise alienate those securities to investors in Australia except in circumstances where disclosure to investors is not required under Chapter 6D.2 of the Corporations Act or where a compliant disclosure document is prepared and lodged with ASIC.
Notice to Prospective Investors in Japan
The shares have not been and will not be registered pursuant to Article 4, Paragraph 1 of the Financial Instruments and Exchange Act. Accordingly, none of the shares nor any interest therein may be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any “resident” of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to or for the benefit of a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Act and any other applicable laws, regulations and ministerial guidelines of Japan in effect at the relevant time.
Notice to Prospective Investors in Hong Kong
The shares have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the shares has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or
52
Table of Contents
invitation for subscription or purchase, of shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”), (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275 of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| (a) | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| (b) | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, |
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the shares pursuant to an offer made under Section 275 of the SFA except:
| (a) | to an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA; |
| (b) | where no consideration is or will be given for the transfer; |
| (c) | where the transfer is by operation of law; |
| (d) | as specified in Section 276(7) of the SFA; or |
| (e) | as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore. |
Notice to Prospective Investors in Bermuda
Shares may be offered or sold in Bermuda only in compliance with the provisions of the Investment Business Act of 2003 of Bermuda which regulates the sale of securities in Bermuda. Additionally, non-Bermudian persons (including companies) may not carry on or engage in any trade or business in Bermuda unless such persons are permitted to do so under applicable Bermuda legislation.
Notice to Prospective Investors in Saudi Arabia
This document may not be distributed in the Kingdom of Saudi Arabia except to such persons as are permitted under the Offers of Securities Regulations as issued by the board of the Saudi Arabian Capital Market Authority (“CMA”) pursuant to resolution number 2-11-2004 dated 4 October 2004 as amended by resolution number 1-28-2008, as amended (the “CMA Regulations”). The CMA does not make any representation as to the accuracy or completeness of this document and expressly disclaims any liability whatsoever for any loss arising from, or incurred in reliance upon, any part of this document. Prospective purchasers of the securities offered hereby should conduct their own due diligence on the accuracy of the information relating to the securities. If you do not understand the contents of this document, you should consult an authorised financial adviser.
Notice to Prospective Investors in the British Virgin Islands
The shares are not being, and may not be offered to the public or to any person in the British Virgin Islands for purchase or subscription by or on behalf of the Company. The shares may be offered to companies
53
Table of Contents
incorporated under the BVI Business Companies Act, 2004 (British Virgin Islands),”BVI Companies”), but only where the offer will be made to, and received by, the relevant BVI Company entirely outside of the British Virgin Islands.
Notice to Prospective Investors in China
This prospectus does not constitute a public offer of shares, whether by sale or subscription, in the People’s Republic of China (the “PRC”). The shares are not being offered or sold directly or indirectly in the PRC to or for the benefit of, legal or natural persons of the PRC.
Further, no legal or natural persons of the PRC may directly or indirectly purchase any of the shares or any beneficial interest therein without obtaining all prior PRC’s governmental approvals that are required, whether statutorily or otherwise. Persons who come into possession of this document are required by the issuer and its representatives to observe these restrictions.
Notice to Prospective Investors in Korea
The shares have not been and will not be registered under the Financial Investments Services and Capital Markets Act of Korea and the decrees and regulations thereunder (the “FSCMA”), and the shares have been and will be offered in Korea as a private placement under the FSCMA. None of the shares may be offered, sold or delivered directly or indirectly, or offered or sold to any person for re-offering or resale, directly or indirectly, in Korea or to any resident of Korea except pursuant to the applicable laws and regulations of Korea, including the FSCMA and the Foreign Exchange Transaction Law of Korea and the decrees and regulations thereunder (the “FETL”). The shares have not been listed on any of securities exchanges in the world including, without limitation, the Korea Exchange in Korea. Furthermore, the purchaser of the shares shall comply with all applicable regulatory requirements (including but not limited to requirements under the FETL) in connection with the purchase of the shares. By the purchase of the shares, the relevant holder thereof will be deemed to represent and warrant that if it is in Korea or is a resident of Korea, it purchased the shares pursuant to the applicable laws and regulations of Korea.
Notice to Prospective Investors in Malaysia
No prospectus or other offering material or document in connection with the offer and sale of the shares has been or will be registered with the Securities Commission of Malaysia (“Commission”) for the Commission’s approval pursuant to the Capital Markets and Services Act 2007. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Malaysia other than (i) a closed end fund approved by the Commission; (ii) a holder of a Capital Markets Services Licence; (iii) a person who acquires the shares, as principal, if the offer is on terms that the shares may only be acquired at a consideration of not less than RM250,000 (or its equivalent in foreign currencies) for each transaction; (iv) an individual whose total net personal assets or total net joint assets with his or her spouse exceeds RM3 million (or its equivalent in foreign currencies), excluding the value of the primary residence of the individual; (v) an individual who has a gross annual income exceeding RM300,000 (or its equivalent in foreign currencies) per annum in the preceding 12 months; (vi) an individual who, jointly with his or her spouse, has a gross annual income of RM400,000 (or its equivalent in foreign currencies), per annum in the preceding 12 months; (vii) a corporation with total net assets exceeding RM10 million (or its equivalent in a foreign currencies) based on the last audited accounts; (viii) a partnership with total net assets exceeding RM10 million (or its equivalent in foreign currencies); (ix) a bank licensee or insurance licensee as defined in the Labuan Financial Services and Securities Act 2010; (x) an Islamic bank licensee or takaful licensee as defined in the Labuan Financial Services and Securities Act 2010; and (xi) any other person as may be specified by the Commission; provided that, in the each of the preceding categories (i) to (xi), the distribution of the shares is made by a holder of a Capital Markets Services Licence who
54
Table of Contents
carries on the business of dealing in securities. The distribution in Malaysia of this prospectus is subject to Malaysian laws. This prospectus does not constitute and may not be used for the purpose of public offering or an issue, offer for subscription or purchase, invitation to subscribe for or purchase any securities requiring the registration of a prospectus with the Commission under the Capital Markets and Services Act 2007.
Notice to Prospective Investors in Taiwan
The shares have not been and will not be registered with the Financial Supervisory Commission of Taiwan pursuant to relevant securities laws and regulations and may not be sold, issued or offered within Taiwan through a public offering or in circumstances which constitutes an offer within the meaning of the Securities and Exchange Act of Taiwan that requires a registration or approval of the Financial Supervisory Commission of Taiwan. No person or entity in Taiwan has been authorized to offer, sell, give advice regarding or otherwise intermediate the offering and sale of the shares in Taiwan.
Notice to Prospective Investors in South Africa
Due to restrictions under the securities laws of South Africa, the shares are not offered, and the offer shall not be transferred, sold, renounced or delivered, in South Africa or to a person with an address in South Africa, unless one or other of the following exemptions applies:
| i | the offer, transfer, sale, renunciation or delivery is to: |
| (a) | persons whose ordinary business is to deal in securities, as principal or agent; |
| (b) | the South African Public Investment Corporation; |
| (c) | persons or entities regulated by the Reserve Bank of South Africa; |
| (d) | authorized financial service providers under South African law; |
| (e) | financial institutions recognized as such under South African law; |
| (f) | a wholly owned subsidiary of any person or entity contemplated in (c), (d) or (e), acting as agent in the capacity of an authorized portfolio manager for a pension fund or collective investment scheme (in each case duly registered as such under South African law); or |
| (g) | any combination of the person in (a) to (f); or |
| ii | the total contemplated acquisition cost of the securities, for any single addressee acting as principal is equal to or greater than ZAR1,000,000. |
No “offer to the public” (as such term is defined in the South African Companies Act, No. 71 of 2008 (as amended or re-enacted) (the “South African Companies Act”)) in South Africa is being made in connection with the issue of the shares. Accordingly, this document does not, nor is it intended to, constitute a “registered prospectus” (as that term is defined in the South African Companies Act) prepared and registered under the South African Companies Act and has not been approved by, and/or filed with, the South African Companies and Intellectual Property Commission or any other regulatory authority in South Africa. Any issue or offering of the shares in South Africa constitutes an offer of the shares in South Africa for subscription or sale in South Africa only to persons who fall within the exemption from “offers to the public” set out in section 96(1)(a) of the South African Companies Act. Accordingly, this document must not be acted on or relied on by persons in South Africa who do not fall within section 96(1)(a) of the South African Companies Act (such persons being referred to as “SA Relevant Persons”). Any investment or investment activity to which this document relates is available in South Africa only to SA Relevant Persons and will be engaged in South Africa only with SA relevant persons.
55
Table of Contents
The validity of the shares of common stock offered hereby will be passed upon for us by Goodwin Procter LLP, Boston, Massachusetts. Certain legal matters in connection with this offering will be passed upon for the underwriters by Davis Polk & Wardwell LLP, New York, New York.
The financial statements incorporated in this prospectus by reference to the Annual Report on Form 10-K for the year ended December 31, 2017 have been so incorporated in reliance on the report (which contains an explanatory paragraph relating to the Company’s requirement for additional financing to fund future operations) of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 (including exhibits, schedules, and amendments) under the Securities Act with respect to the shares of common stock offered by this prospectus. This prospectus does not contain all the information set forth in the registration statement and the exhibits and schedules filed and incorporated by reference as part of this registration statement. For further information about us and the shares of common stock to be sold in this offering, we refer you to the registration statement and exhibits and schedules filed as part of the registration statement. Statements contained in this prospectus or incorporated by reference relating to the contents of any contract, agreement or other document are not necessarily complete and are qualified in all respects by the complete text of the applicable contract, agreement or other document, a copy of which has been filed or incorporated by reference as an exhibit to the registration statement. Whenever this prospectus refers to any contract, agreement, or other document, we refer you to the exhibits that are a part of the registration statement for a copy of the contract, agreement, or document.
You may read and copy all or any portion of the registration statement or any other information we file at the SEC’s public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You can request copies of these documents, upon payment of a duplicating fee, by writing to the SEC. Please call the SEC at 1-800-SEC-0330 for further information about the operation of the public reference rooms. Our SEC filings, including the registration statement, are also available to you on the SEC’s website (http://www.sec.gov).
We are subject to the information and periodic reporting requirements of the Exchange Act. Under the Exchange Act, we will file annual, quarterly and current reports, as well as proxy statements and other information with the SEC. These periodic reports, proxy statements, and other information will be available for inspection and copying at the SEC’s Public Reference Room and the website of the SEC referred to above.
56
Table of Contents
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information from other documents that we file, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below (other than Current Reports on Form 8-K furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed or furnished on such form that are related to such items) that we have filed with the SEC (SEC File No. 001-38219):
| • | our Annual Report on Form 10-K for the year ended December 31, 2017, filed with the SEC on March 28, 2018; |
| • | the information specifically incorporated by reference into our Annual Report on Form 10-K for the year ended December 31, 2017 from our Definitive Proxy Statement on Schedule 14A, filed with the SEC on April 3, 2018; |
| • | our Quarterly Report on Form 10-Q for the quarter ended March 31, 2018, filed with the SEC on May 8, 2018; |
| • | our Current Reports on Form 8-K filed with the SEC on May 21, 2018, May 31, 2018 and June 4, 2018; and |
| • | the description of our common stock contained in our registration statement on Form 8-A filed with the SEC on September 27, 2017, including any amendments or reports filed for the purposes of updating this description. |
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to Deciphera Pharmaceuticals, Inc., 500 Totten Pond Road, 6th Floor, Waltham, Massachusetts 02451, Attention: Chief Financial Officer.
You also may access these filings on our website at www.deciphera.com. We do not incorporate the information contained on or accessible through our website into this prospectus or any supplement to this prospectus and you should not consider any information on, or that can be accessed through, our website as part of this prospectus or any supplement to this prospectus (other than those filings with the SEC that we specifically incorporate by reference into this prospectus or any supplement to this prospectus).
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement.
57
Table of Contents
3,750,000 Shares
Common Stock

PRELIMINARY PROSPECTUS
| J.P. Morgan | Piper Jaffray |
, 2018
Through and including , 2018 (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the fees and expenses in connection with the issuance and distribution of the securities being registered (excluding the underwriting discounts and commissions). Except for the SEC registration fee and the FINRA filing fee, all amounts are estimates.
| Amount paid or to be paid |
||||
| SEC registration fee |
$ | 20,569 | ||
| FINRA filing fee |
25,282 | |||
| Legal fees and expenses |
330,000 | |||
| Accountants’ fees and expenses |
205,000 | |||
| Printing expenses |
75,000 | |||
| Transfer agent and registrar fees |
5,000 | |||
| Miscellaneous |
39,149 | |||
|
|
|
|||
| Total |
$ | 700,000 | ||
|
|
|
|||
Item 14. Indemnification of Directors and Officers.
Section 145 of the Delaware General Corporation Law authorizes a corporation’s board of directors to grant, and authorizes a court to award, indemnity to officers, directors, and other corporate agents.
Our amended and restated certificate of incorporation contains provisions that limit the liability of our directors for monetary damages to the fullest extent permitted by Delaware law. Consequently, our directors will not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duties as directors, except liability for the following:
| • | any breach of their duty of loyalty to our company or our stockholders; |
| • | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
| • | any transaction from which they derived an improper personal benefit. |
Any amendment to, or repeal of, these provisions will not eliminate or reduce the effect of these provisions in respect of any act, omission or claim that occurred or arose prior to that amendment or repeal. If the Delaware General Corporation Law is amended to provide for further limitations on the personal liability of directors of corporations, then the personal liability of our directors will be further limited to the greatest extent permitted by the Delaware General Corporation Law.
In addition, pursuant to our amended and restated bylaws, we will indemnify, to the fullest extent permitted by law, any person who is or was a party or is threatened to be made a party to any action, suit or proceeding by reason of the fact that he is or was one of our directors or officers or is or was serving at our request as a director or officer of another corporation, partnership, joint venture, trust, or other enterprise. Our amended and restated bylaws provide that we may indemnify to the fullest extent permitted by law any person who is or was a party or is threatened to be made a party to any action, suit, or proceeding by reason of the fact that he is or was one of
II-1
Table of Contents
our employees or agents or is or was serving at our request as an employee or agent of another corporation, partnership, joint venture, trust, or other enterprise. Our amended and restated bylaws also provide that we must advance expenses incurred by or on behalf of a director or officer in advance of the final disposition of any action or proceeding, subject to very limited exceptions.
We have also entered into indemnification agreements with each of our directors and executive officers that are broader than the specific indemnification provisions contained in the Delaware General Corporation Law. These indemnification agreements require us, among other things, to indemnify our directors and executive officers against liabilities that may arise by reason of their status or service. These indemnification agreements also require us to advance all expenses incurred by the directors and executive officers in investigating or defending any such action, suit, or proceeding. We believe that these agreements are necessary to attract and retain qualified individuals to serve as directors and executive officers.
The limitation of liability and indemnification provisions that are included in our amended and restated certificate of incorporation, amended and restated bylaws, and indemnification agreements that we entered into with our directors and executive officers may discourage stockholders from bringing a lawsuit against our directors and executive officers for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against our directors and executive officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder’s investment may be harmed to the extent that we pay the costs of settlement and damage awards against directors and executive officers as required by these indemnification provisions. At present, we are not aware of any pending litigation or proceeding involving any person who is or was one of our directors, officers, employees or other agents or is or was serving at our request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, for which indemnification is sought, and we are not aware of any threatened litigation that may result in claims for indemnification.
We have obtained insurance policies under which, subject to the limitations of the policies, coverage is provided to our directors and executive officers against loss arising from claims made by reason of breach of fiduciary duty or other wrongful acts as a director or executive officer, including claims relating to public securities matters, and to us with respect to payments that may be made by us to these directors and executive officers pursuant to our indemnification obligations or otherwise as a matter of law.
The underwriting agreement filed as Exhibit 1.1 to this registration statement provides for indemnification by the underwriters of us and our officers and directors for certain liabilities arising under the Securities Act and otherwise.
Item 15. Recent Sales of Unregistered Securities.
Set forth below is information regarding securities we have issued within the past three years that were not registered under the Securities Act:
(1) Issuances of Capital Stock
On September 14, 2015, we issued and sold to two accredited investors an aggregate of 1,855,250 series A preferred shares, in exchange for the cancellation of an aggregate of $95.6 million of outstanding indebtedness under convertible promissory notes we had previously issued.
On September 14, 2015, we issued and sold to two accredited investors an aggregate of 73,811 series B-1 preferred shares, in exchange for cancellation of an aggregate of $3.7 million of outstanding indebtedness under convertible promissory notes we had previously issued.
On September 14, 2015, we issued and sold to four accredited investors an aggregate of 426,764 series B-1 preferred shares at a price per share of $50.50 for aggregate cash consideration of $21.6 million.
II-2
Table of Contents
On December 30, 2015, we issued and sold to an accredited investor an aggregate of 198,020 series B-1 preferred shares at a price per share of $50.50 for aggregate cash consideration of $10.0 million.
On July 11, 2016, we issued and sold to five accredited investors an aggregate of 876,366 series B-2 preferred shares at a price per share of $63.13 for aggregate cash consideration of $55.3 million.
On May 26, 2017, we issued and sold to seven accredited investors an aggregate of 690,333 series C preferred shares at a price per share of $75.76 for aggregate cash consideration of $52.3 million.
On October 2, 2017, we issued to direct and indirect equity holders of Deciphera Pharmaceuticals, LLC an aggregate of 24,425,190 shares of our common stock in exchange for, directly and indirectly, all of the outstanding preferred shares of Deciphera Pharmaceuticals, LLC, or the Exchange. The Exchange was part of a series of transactions pursuant to which Deciphera Pharmaceuticals, LLC became our wholly owned subsidiary.
(2) Stock Option Grants
Since January 1, 2015, we have granted to our employees, directors, consultants and other service providers an aggregate of 12,405 options to purchase units of our membership interests under the 2012 Unit Option Plan, which is no longer in effect and all such grants have since been cancelled.
From December 18, 2015, when the 2015 Equity Incentive Plan was adopted, through September 27, 2017, the date of effectiveness of the registration statement on Form S-1 for our IPO, we granted to our employees, directors, consultants and other service providers options to purchase an aggregate of 3,398,780 shares of our common stock under the 2015 Equity Incentive Plan.
(3) Stock Appreciation Rights Grants
Since December 18, 2015, when the 2015 Equity Incentive Plan was adopted, we have granted to our employees, directors, consultants and other service providers an aggregate of 839,810 share appreciation rights under the 2015 Equity Incentive Plan.
No underwriters were involved in the foregoing issuances of securities. The offers, sales and issuances of the securities described above were deemed to be exempt from registration under the Securities Act in reliance upon Rule 701 or Section 4(a)(2) of the Securities Act. The offers, sales and issuances of the securities that were deemed to be exempt in reliance on Rule 701 were transactions under compensatory benefit plans and contracts relating to compensation as provided under Rule 701. The offers, sales and issuances of the securities that were deemed to be exempt in reliance upon Section 4(a)(2) were each transactions not involving any public offering, and all recipients of these securities were accredited investors within the meaning of Rule 501 of Regulation D of the Securities Act who were acquiring the applicable securities for investment and not distribution and had represented that they could bear the risks of the investment. Each of the recipients of securities in these transactions had adequate access, through employment, business or other relationships, to information about us. On October 6, 2017, we filed a registration statement on Form S-8 under the Securities Act to register all of the shares of our common stock subject to outstanding options and all shares of our common stock otherwise issuable pursuant to our equity compensation plans.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits
See the Exhibit Index on the page immediately following the signature page for a list of exhibits filed as part of this registration statement on Form S-1, which Exhibit Index is incorporated herein by reference.
II-3
Table of Contents
(b) Financial Statement Schedules
All schedules have been omitted because they are not required or because the required information is given in the financial statements or notes to those statements.
Item 17. Undertakings.
The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (2) | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-4
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this Amendment No. 1 to the registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Waltham, Commonwealth of Massachusetts on June 6, 2018.
| DECIPHERA PHARMACEUTICALS, INC. | ||
| /s/ Michael D. Taylor | ||
| By: |
Michael D. Taylor, Ph.D. President and Chief Executive Officer | |
Pursuant to the requirements of the Securities Act of 1933, this Amendment No. 1 to the registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Name |
Title |
Date | ||
| /s/ Michael D. Taylor |
||||
| Michael D. Taylor, Ph.D. | President, Chief Executive Officer and Director (Principal Executive Officer) |
June 6, 2018 | ||
| /s/ Thomas P. Kelly |
||||
| Thomas P. Kelly | Chief Financial Officer (Principal Financial and Accounting Officer) |
June 6, 2018 | ||
| * |
||||
| Patricia L. Allen | Director |
June 6, 2018 | ||
| * |
||||
| Edward J. Benz, Jr., M.D. | Director |
June 6, 2018 | ||
| * |
||||
| James A. Bristol, Ph.D. | Director |
June 6, 2018 | ||
| * |
||||
| Steven L. Hoerter | Director |
June 6, 2018 | ||
II-5
Table of Contents
| Name |
Title |
Date | ||
| * |
||||
| John R. Martin | Director |
June 6, 2018 | ||
| * |
||||
| Liam Ratcliffe, M.D., Ph.D. | Director |
June 6, 2018 | ||
| * |
||||
| Michael Ross, Ph.D. | Director |
June 6, 2018 | ||
| * |
||||
| Dennis L. Walsh | Director |
June 6, 2018 | ||
| *By: /s/ Michael D. Taylor | Attorney-in-fact | June 6, 2018 | ||
| Michael D. Taylor |
||||
II-6
Table of Contents
EXHIBIT INDEX
II-7
Table of Contents
| * | Previously filed. |
| # | Indicates management contract or compensation plan. |
| (1) | Schedules and exhibits have been omitted from this filing pursuant to Item 601(b)(2) of Regulation S-K. The Registrant agrees to furnish supplementally a copy of any omitted schedule or exhibit to the Securities and Exchange Commission upon its request; provided, however, that the Registrant may request confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, for any schedule or exhibit so furnished. |
II-8
