Attached files
| file | filename |
|---|---|
| 8-K - HUMANIGEN, INC | b651808k.htm |

Enhancing T-Cell Therapies in Oncology OTCQB: HGENwww.humanigen.com 1

Forward-Looking Statements This presentation contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements reflect management’s current knowledge, assumptions, judgment and expectations regarding future performance or events. Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct, and actual results could differ materially from the forward-looking statements.Words such as “will,” “expect,” “intend,” “plan,” “predict,” “potential,” “possible,” and similar expressions identify forward-looking statements, including, without limitation, statements related to the scope, progress, expansion, and costs of developing and commercializing the Company’s product candidates; opportunity to benefit from anticipated regulatory incentives for product candidates; and anticipated expenses related to development activities, clinical trials and the development and potential commercialization of product candidates.Forward-looking statements are subject to risks and uncertainties including, but not limited to, the Company’s lack of revenues, history of operating losses, limited cash reserves and ability to obtain additional capital to develop and commercialize its product candidates, including the additional capital which will be necessary to complete the clinical trials of the Company’s product candidates, and continue as a going concern; the Company’s ability to execute its strategy and business plan; the potential timing and outcomes of clinical studies of lenzilumab, ifabotuzumab, HGEN005 or any other product candidates and the uncertainties inherent in clinical testing; the ability of the Company to fund and timely source adequate supply of its development products from third-party manufacturers on which the Company depends; the potential, if any, for future development of any of its present or future products; the Company's ability to successfully progress, partner or complete further development of its programs; the ability of the Company to identify and develop additional products; the Company's ability to attain market exclusivity or to protect its intellectual property; competition; changes in the regulatory landscape that may prevent the Company from pursuing or realizing any of the expected benefits from the various regulatory incentives at the center of its strategy, or the imposition of regulations that affect the Company's products; and the various risks described in the "Risk Factors" and elsewhere in the Company's periodic and other filings with the Securities and Exchange Commission.You are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this presentation. The company has no obligation, and expressly disclaims any obligation to update, revise or correct any of the forward-looking statements, whether as a result of new information, future events or otherwise 2

Strategic Focus – Enhancing T-Cell Therapies in Oncology Short, Medium and Long-Term Growth Drivers: 3 Focus on CAR-T- induced AEs to prevent onset/severity of neurotoxicitySevere patient risk factor, also impacting clinical trial holds, commercial uptake and hospital resources Focus on optimizing CAR-T efficacyDemonstrate improved clinically significant cell expansion, leading to improved patient outcomes Establish both A/E and efficacy claims for lenzilumab across autologous and allogeneic CAR-T, other T-cell therapies Improve benefit/risk to enable CAR-T cell therapy to move to earlier lines of therapy, solid tumorsLeverage understanding of CNS/neurotox in T cells to advance development of ifabotuzumab in solid tumors with initial focus on GBMDevelop portfolio of optimized CARs utilizing ifabotuzumab and HGEN005 backbones

Big Commercial, Partnering Universe in T-Cell Therapy 4

Experienced, Execution-Focused Leadership and Board; Extensive CAR-T and Immuno-Oncology Expertise Cameron Durrant, MD, MBACEOPharma and serial biotech execPharmacia/Pfizer, J+J in the US; Merck, GSK in EuropeBiotech Exec Chairman, Chairman, CEO, CFO Greg Jester, CPA CFO Decades of financial, operational and entrepreneurial experience in life sciencesVP, Finance, Tris PharmaCFO at private and public pharma companies, including Alvogen and InnoviveTarek Sahmoud, MD, PhDCMONovartis CAR-T clinical development consultant and former VP, Senior Clinical Program Head (oncology)Oncology dev expert: Celgene Global Head Clinical Dev (Corporate VP); BMS, Exec Dir, Global Medical AffairsRegistered Arimidex, Afinitor; responsibility for Kymriah, Yervoy, Abraxane, marizomib, dasatinib Bob Savage, MBA Board memberFormer Worldwide Chairman, J+J PharmaceuticalsFormer President, Worldwide Therapeutics and Inflammation, PharmaciaExtensive biotech board experience (12 boards over 20 years, including Depomed, The Medicines Company, Savient, Panacos, Noven, Epicept, Vela)Rainer Boehm, MD, MBABoard memberFormer interim CEO, Novartis PharmaFormer Chief Commercial and Chief Medical Affairs Officer, Novartis Pharma and EVP, Novartis OncologyBoard member, Cellectis (allogeneic CAR-T company)Tim Morris, CPABoard memberCFO IOVANCE Biotherapeutics, a leading immuno-oncology biotech ~$1.5Bn market capRaised almost $1Bn in equity and equity-linked securities in 22 years as a CFOExtensive deal experience: >70 transactions, combined value $2BnRon Barliant, JDBoard memberOf counsel with Goldberg KohnUS bankruptcy judge for N. Illinois for 14 years, extensive legal and bankruptcy experience Counseled companies, management and boards in distressed and complex situations 5

‘Achilles Heel’ of CAR-T: Neurotoxicity (NT), Cytokine Release Syndrome (CRS) Up to 87% with NT and 94% with CRS*One-third/one-half of patients have grade ≥3 NT, potential ICU admissionSeveral deaths *Package insert for KYMRIAH, YESCARTA www.cellculturedish.com 6

Myeloid and CAR-T Cells’ Increase in Brain Associated with NT 7 Cell Populations (in CSF) NE Grade 0-2 (n=15)Median cells/mL(Q1, Q3) NE Grade > 3 (N=10)Median cells/mL(Q1, Q3) NE Grade > 3/Grade 0-2Ratio CD45+ (leukocytes) 166 (18, 1504) 570 (170, 1700) 3.4 CD14+ (myeloid cells) 18 (11, 436) 306 (125, 1063) 17 CAR+ T cells CD3+ 35 (6, 324) 79 (47, 81) 2.3 CD4+ 26 (3, 213) 52 (34, 65) 2 CD8+ 17 (1, 85) 15 (10, 34) 0.9 CD4/CD8 T cell ratio 2 (1, 5) 3 (2, 5) 1.6 CAR+ CD4/CAR CD8 T cell ratio 9 (8, 53) 17 (11, 22) 2 Locke et al. ASH 2017, Abstract 1547 CSF = cerebrospinal fluid NE = neurologic event Q = quartile
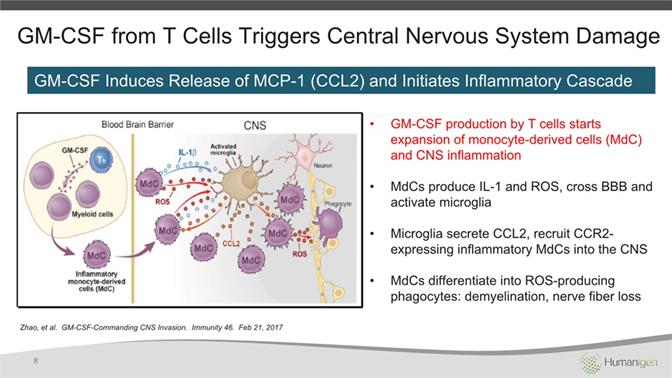
Zhao, et al. GM-CSF-Commanding CNS Invasion. Immunity 46. Feb 21, 2017 GM-CSF production by T cells starts expansion of monocyte-derived cells (MdC) and CNS inflammationMdCs produce IL-1 and ROS, cross BBB and activate microgliaMicroglia secrete CCL2, recruit CCR2-expressing inflammatory MdCs into the CNSMdCs differentiate into ROS-producing phagocytes: demyelination, nerve fiber loss 8 GM-CSF from T Cells Triggers Central Nervous System Damage GM-CSF Induces Release of MCP-1 (CCL2) and Initiates Inflammatory Cascade
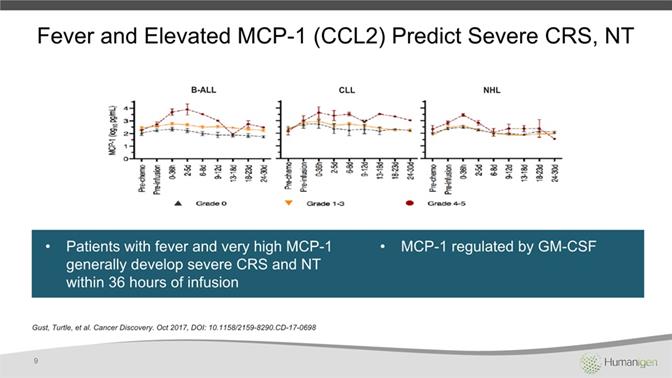
Patients with fever and very high MCP-1 generally develop severe CRS and NT within 36 hours of infusionMCP-1 regulated by GM-CSF Gust, Turtle, et al. Cancer Discovery. Oct 2017, DOI: 10.1158/2159-8290.CD-17-0698 9 Fever and Elevated MCP-1 (CCL2) Predict Severe CRS, NT B-ALL CLL NHL

Evidence Shows Strong GM-CSF Link to Neurotoxicity Elevated GM-CSF associated with grade > 3 NTIL-2 only other cytokine with this association Published clinical association in Zuma-1 trial Locke, Neelapu et al, AACR 2017 10

Sentman, et al. www.jimmunol.org/content/197/12/4674.long; Coxford, et al. Immunity 2015 (43)510-514; Ishii, et al. Blood 2016 128:3358; Teachey, et al. Cancer Discov. 2016 June ; 6(6): 664–679. doi:10.1158/2159-8290.CD-16-0040; Lee, et al. Blood 2016 124:2:188; David M. Barrett et al. Blood 2016;128:654 Scientific Rationale Points to GM-CSF Trigger to CAR-T A/Es GM-CSF from the CAR-T and other sources perpetuates inflammation and triggers A/Es through downstream cytokines GM-CSF k/o mice no sign of CRSIL-6 k/o mice still get CRS GM-CSF receptor k/o from CCR2+ myeloid cells abrogates cascade in neuro-inflammation models 11

Lenzilumab: First-in-Class, Anti-GM-CSF Monoclonal Antibody Lenzilumab Binds to and neutralizes circulating GM-CSF Safety and tolerability well established from one Phase I, two Phase II studies Compelling safety and tolerability profile in over 100 patientsEfficacy signals in Phase II eosinophilic asthma and RA studies Minimize incidence and severity of serious and potentially life-threatening side-effectsIncrease CAR-T expansion and persistence to improve efficacyDecrease ICU admissions and re-hospitalization rates Lenzilumab may improve utility of CAR-T to: 12 GM-CSF also target in many other autoimmune indications with significant unmet need

More robust proliferation after treatment with lenzilumab in vivo (~5x) Lenzilumab does not impede CAR-T cell function in vivo in the absence of PBMCsSurvival similar for CAR-T + control and CAR-T + lenzilumab In Vivo Preclinical Data: Positive Survival, Proliferation 13 Kenderian, S: Presentation at PEGS 5-1-18, Boston CD3+ (µg/mL)

Lenzilumab CAR-T Development Plan Has Top KOL Support 14 Top KOLs understand GM-CSF as target KOLs proactively offered to study neurotoxicity prevention Lenzilumab pivotal study as prophylactic therapy prior to CAR-T infusion to prevent NT with YESCARTA, expected start 2H 2018 at MD Anderson (with lead investigator for Gilead/Kite’s YESCARTA) with KYMRIAH, expected start 2H 2018 at Mayo Clinic (with director of T cell program)
HGEN Scientific Advisory Board Input From Top KOLs at Leading Centers 15 Massachusetts General Hospital, HarvardMoffittOlivia Newton-John Cancer Research InstituteMemorial Sloan Kettering Cancer CenterCHOPU PennDana Farber Cancer Institute HarvardMayo ClinicSeattle Children’sUniversity of FloridaMD AndersonNIHCHOPEmory – Winstar Cancer Institute

Framing Lenzilumab’s Extensive Value Potential GM-CSF neutralization reduces MDSC IDO and PD-L1 expression Lenzilumab as ‘booster’ of CAR-T: in vivo cell expansion data One-third to a half of patients require ICU stay Prophylactic lenzilumab to prevent onset of CAR-T potentially life-threatening side-effects CAR-T toxicity creates challenges for sales growth; managing cancer treatment side effects a high-value category 16

Ifabotuzumab: First-in-Class, EphA3 Monoclonal Antibody Targets Eph receptor A3 Phase I bio-imaging study in glioblastoma multiforme (GBM), promising initial resultsCAR being designed Developed an Antibody Drug Conjugate EphA3 Receptor Ifabotuzumab INTRACELLULAR Inhibition of cell positioning, survival and tumor growth Not expressed in normal tissue - overexpressed in certain blood cancers, solid tumors and tumor vasculature 17 Ifabotuzumab

Monoclonal Antibody Pipeline Preclinical Phase I Phase II Phase III BLA Lenzilumab(anti-GM-CSF mAb) Prophylaxis CAR-T-induced serious A/Es, potential improved efficacy (CAR-T companion/booster) CMML (monotherapy in salvage patients - fully enrolled) Ifabotuzumab(anti-EphA3 mAb) GBM (monotherapy in salvage patients) Antibody drug conjugate(currently in animal testing for various cancers) CAR-T construct potential for various cancers HGEN005(anti-EMR1 mAb) Eosinophilic asthma Eosinophilic esophagitis Eosinophilic granulomatosis with polyangiitis CAR-T construct potential for eosinophilic leukemia PhaseI PhaseI Potential pivot to JMML on interim CMML data PotentialPivotal 18
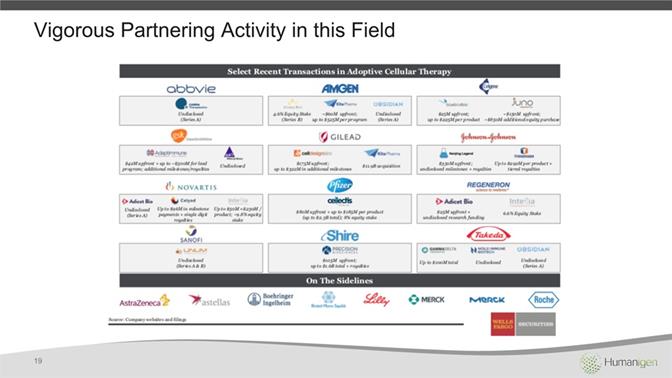
Vigorous Partnering Activity in this Field 19

Summary Top KOLs back scientific rationale for lenzilumab starting pivotal trials Lenzilumab has potential to make CAR-T safer, more effective and a more routine out-patient procedure Not enough ICU beds for numbers of patients necessary to hit analyst forecasts; hospital reimbursement additional gating factor impeding stronger uptake of CAR-TOffer significant strategic value, sales acceleration for CAR-T treatment, plus generate standalone revenue stream More shots on goal: Lenzilumab phase I trial in CMML (fully recruited) Ifabotuzumab phase I in GBM underway with positive early signs, plus ADC developmentHGEN005 preclinical asset for multiple serious eosinophilic diseasesCAR-T platform utilizing ifabotuzumab and HGEN005Relatively modest capital requirements, near term read out and significant potential upside 20
