Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 PRESS RELEASE - bluebird bio, Inc. | blue-ex992_128.htm |
| 8-K - 8-K - bluebird bio, Inc. | blue-8k_20180601.htm |

ASCO Analyst & Investor Webcast June 1, 2018 NASDAQ: BLUE June 1, 2018 Exhibit 99.1

These slides and the accompanying oral presentation contain forward-looking statements and information relating to bluebird bio, its product candidate bb2121 and oncology research and development plans. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward-looking statements. For example, all statements we make regarding the initiation, timing, progress and results of our preclinical and clinical studies and our research and development programs, our ability to advance product candidates into, and successfully complete, clinical studies, and the timing or likelihood of regulatory filings and approvals are forward looking. All forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These statements are also subject to a number of material risks and uncertainties that are described in our most recent quarterly report on Form 10-Q, as well as our subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. Forward Looking Statements

Welcome Nick Leschly, chief bluebird

Making Hope A Reality
T H E G E N E T H E R A P Y P R O D U C T S C O M P A N Y LentiGlobin SCD Data-Driven Acceleration LentiGlobin TDT First Filing (2018) Lenti-D CALD First Filing (2019) Patient Impact ∞ bb2121 Multiple Myeloma First Filing (2019) Products on the Market 2+ Additional Programs in the Clinic 4+ Programs Nearing Commercialization 2+ Path to Patients Three Regulatory Filings Anticipated by End of 2019

Key Questions for Today What more have we learned about efficacy and durability of bb2121 in this heavily pretreated patient population? What are the new learnings? BCMA expression? Dose response? Is the safety profile still manageable? Is the path to earlier lines progressing?

CRB-401 Data Update David Davidson, M.D., chief medical officer
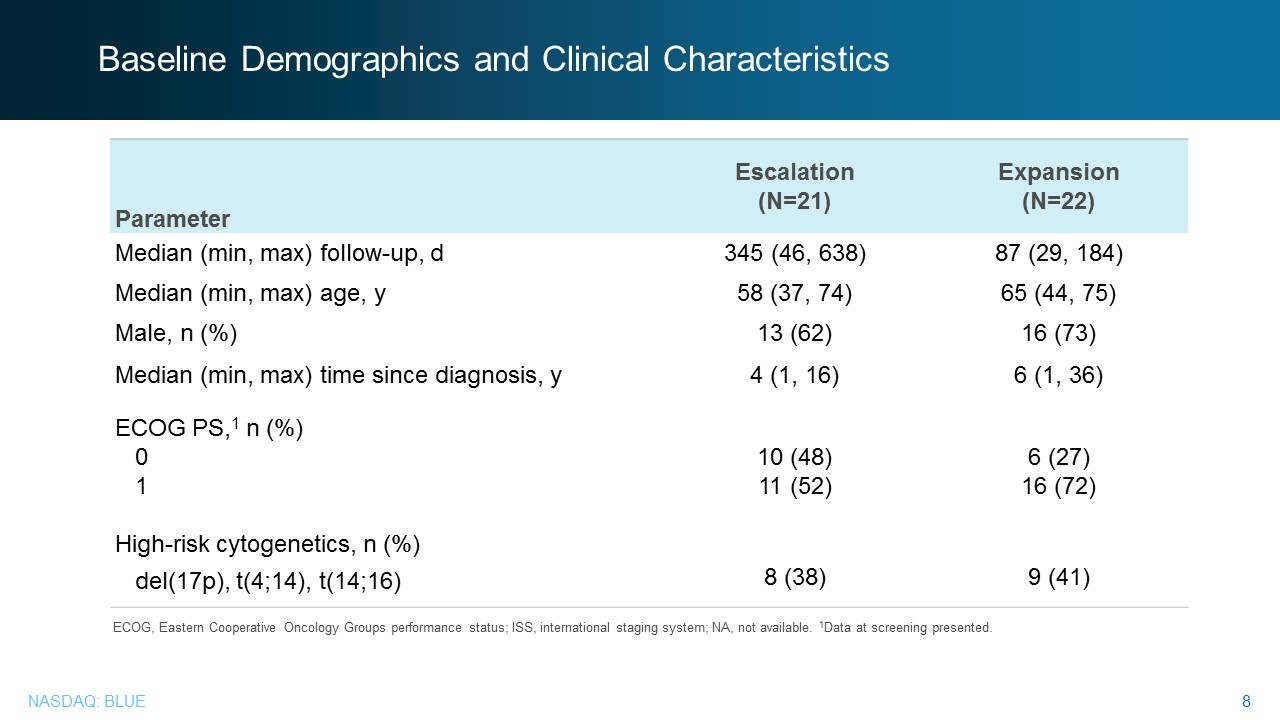
Baseline Demographics and Clinical Characteristics Parameter Escalation (N=21) Expansion (N=22) Median (min, max) follow-up, d 345 (46, 638) 87 (29, 184) Median (min, max) age, y 58 (37, 74) 65 (44, 75) Male, n (%) 13 (62) 16 (73) Median (min, max) time since diagnosis, y 4 (1, 16) 6 (1, 36) ECOG PS,1 n (%) 0 1 10 (48) 11 (52) 6 (27) 16 (72) High-risk cytogenetics, n (%) del(17p), t(4;14), t(14;16) 8 (38) 9 (41) ECOG, Eastern Cooperative Oncology Groups performance status; ISS, international staging system; NA, not available. 1Data at screening presented.
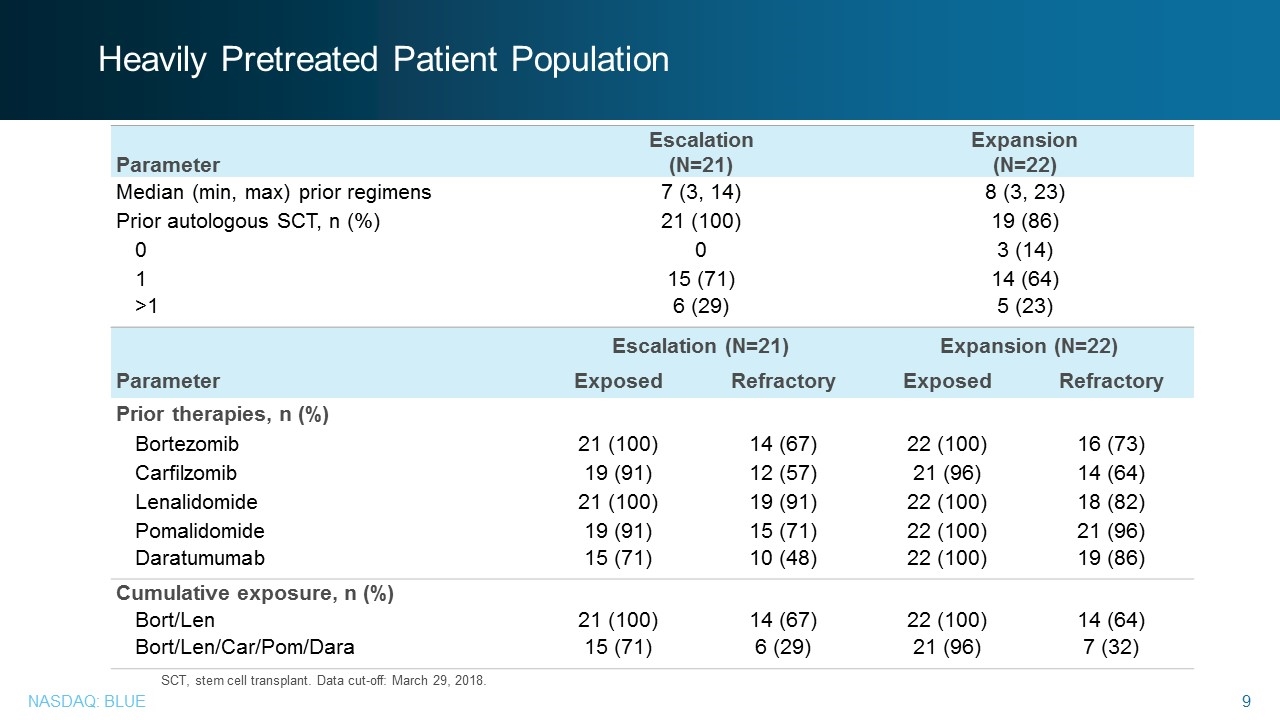
Parameter Escalation (N=21) Expansion (N=22) Exposed Refractory Exposed Refractory Prior therapies, n (%) Bortezomib 21 (100) 14 (67) 22 (100) 16 (73) Carfilzomib 19 (91) 12 (57) 21 (96) 14 (64) Lenalidomide 21 (100) 19 (91) 22 (100) 18 (82) Pomalidomide 19 (91) 15 (71) 22 (100) 21 (96) Daratumumab 15 (71) 10 (48) 22 (100) 19 (86) Cumulative exposure, n (%) Bort/Len 21 (100) 14 (67) 22 (100) 14 (64) Bort/Len/Car/Pom/Dara 15 (71) 6 (29) 21 (96) 7 (32) Parameter Escalation (N=21) Expansion (N=22) Median (min, max) prior regimens 7 (3, 14) 8 (3, 23) Prior autologous SCT, n (%) 21 (100) 19 (86) 0 0 3 (14) 1 15 (71) 14 (64) >1 6 (29) 5 (23) SCT, stem cell transplant. Data cut-off: March 29, 2018. Heavily Pretreated Patient Population
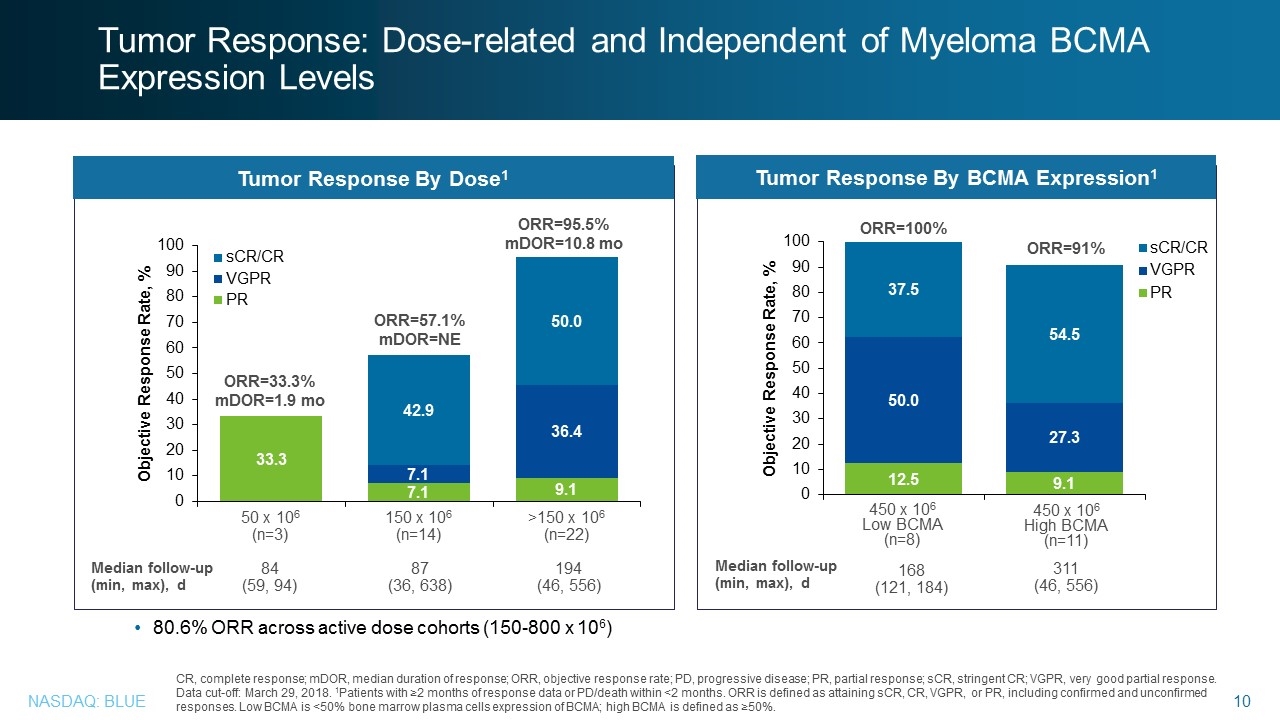
CR, complete response; mDOR, median duration of response; ORR, objective response rate; PD, progressive disease; PR, partial response; sCR, stringent CR; VGPR, very good partial response. Data cut-off: March 29, 2018. 1Patients with ≥2 months of response data or PD/death within <2 months. ORR is defined as attaining sCR, CR, VGPR, or PR, including confirmed and unconfirmed responses. Low BCMA is <50% bone marrow plasma cells expression of BCMA; high BCMA is defined as ≥50%. Tumor Response By Dose1 Tumor Response By BCMA Expression1 ORR=33.3% mDOR=1.9 mo ORR=57.1% mDOR=NE 150 x 106 (n=14) >150 x 106 (n=22) 50 x 106 (n=3) ORR=95.5% mDOR=10.8 mo 450 x 106 High BCMA (n=11) Median follow-up (min, max), d 87 (36, 638) 84 (59, 94) 194 (46, 556) Median follow-up (min, max), d 450 x 106 Low BCMA (n=8) 311 (46, 556) ORR=100% ORR=91% 168 (121, 184) Tumor Response: Dose-related and Independent of Myeloma BCMA Expression Levels 80.6% ORR across active dose cohorts (150-800 x 106)
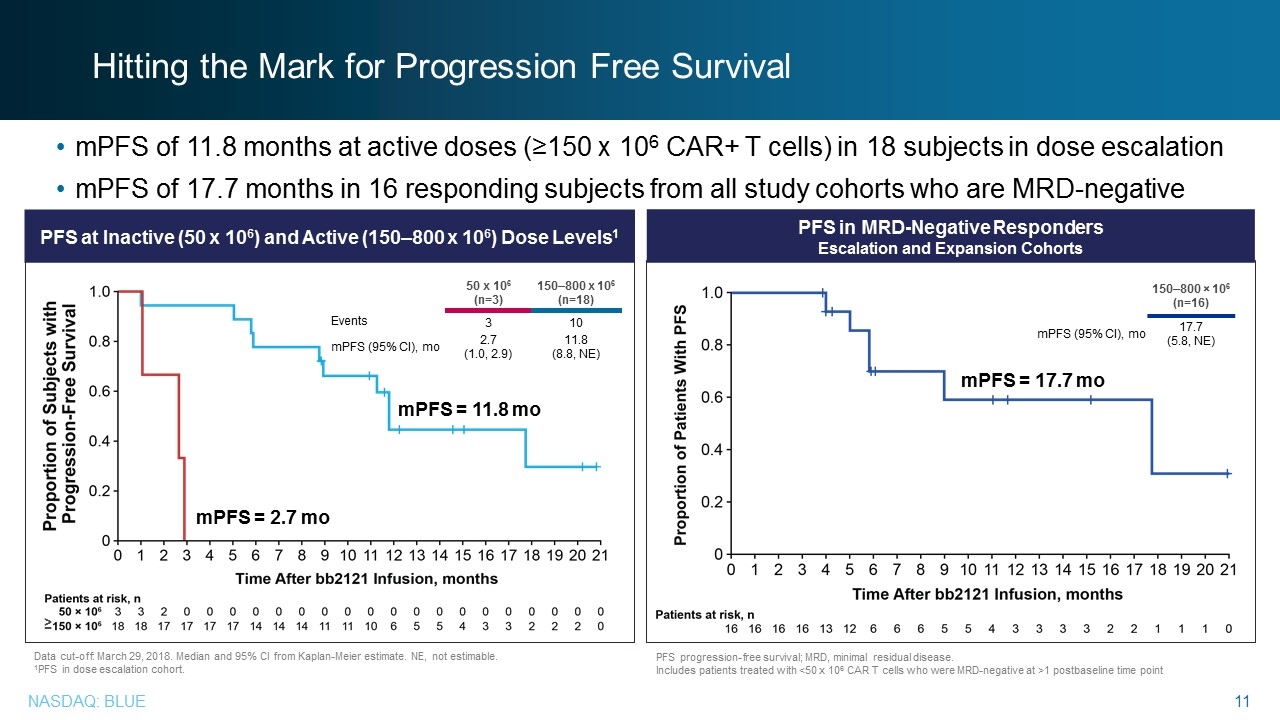
Data cut-off: March 29, 2018. Median and 95% CI from Kaplan-Meier estimate. NE, not estimable. 1PFS in dose escalation cohort. mPFS of 11.8 months at active doses (≥150 x 106 CAR+ T cells) in 18 subjects in dose escalation mPFS of 17.7 months in 16 responding subjects from all study cohorts who are MRD-negative PFS at Inactive (50 x 106) and Active (150–800 x 106) Dose Levels1 PFS in MRD-Negative Responders Escalation and Expansion Cohorts 50 x 106 (n=3) 150–800 x 106 (n=18) Events 3 10 mPFS (95% CI), mo 2.7 (1.0, 2.9) 11.8 (8.8, NE) ≥ mPFS = 11.8 mo mPFS = 2.7 mo Hitting the Mark for Progression Free Survival PFS progression-free survival; MRD, minimal residual disease. Includes patients treated with <50 x 106 CAR T cells who were MRD-negative at >1 postbaseline time point 150–800 × 106 (n=16) mPFS (95% CI), mo 17.7 (5.8, NE) mPFS = 17.7 mo
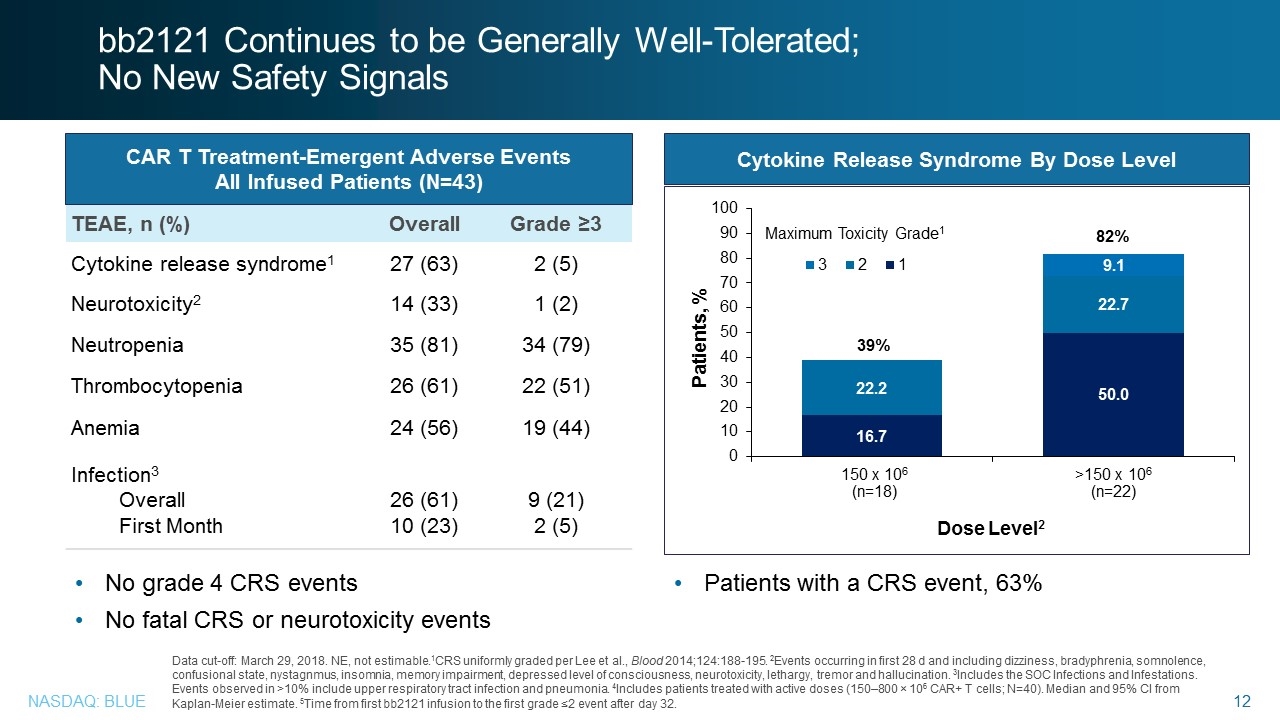
TEAE, n (%) Overall Grade ≥3 Cytokine release syndrome1 27 (63) 2 (5) Neurotoxicity2 14 (33) 1 (2) Neutropenia 35 (81) 34 (79) Thrombocytopenia 26 (61) 22 (51) Anemia 24 (56) 19 (44) Infection3 Overall First Month 26 (61) 10 (23) 9 (21) 2 (5) CAR T Treatment-Emergent Adverse Events All Infused Patients (N=43) Data cut-off: March 29, 2018. NE, not estimable.1CRS uniformly graded per Lee et al., Blood 2014;124:188-195. 2Events occurring in first 28 d and including dizziness, bradyphrenia, somnolence, confusional state, nystagnmus, insomnia, memory impairment, depressed level of consciousness, neurotoxicity, lethargy, tremor and hallucination. 3Includes the SOC Infections and Infestations. Events observed in >10% include upper respiratory tract infection and pneumonia. 4Includes patients treated with active doses (150–800 × 106 CAR+ T cells; N=40). Median and 95% CI from Kaplan-Meier estimate. 5Time from first bb2121 infusion to the first grade ≤2 event after day 32. No grade 4 CRS events No fatal CRS or neurotoxicity events Cytokine Release Syndrome By Dose Level Dose Level2 39% 82% >150 x 106 (n=22) 150 x 106 (n=18) Maximum Toxicity Grade1 Patients with a CRS event, 63% bb2121 Continues to be Generally Well-Tolerated; No New Safety Signals
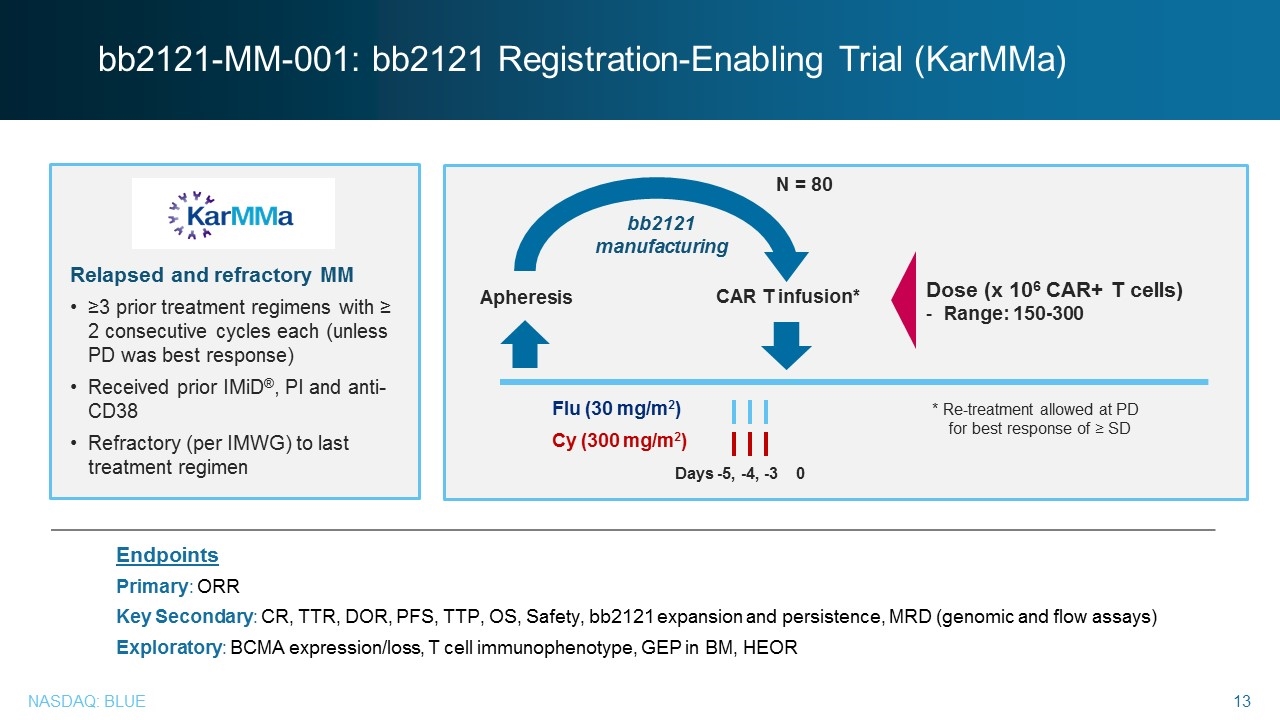
Relapsed and refractory MM ≥3 prior treatment regimens with ≥ 2 consecutive cycles each (unless PD was best response) Received prior IMiD®, PI and anti-CD38 Refractory (per IMWG) to last treatment regimen bb2121-MM-001: bb2121 Registration-Enabling Trial (KarMMa) Endpoints Primary: ORR Key Secondary: CR, TTR, DOR, PFS, TTP, OS, Safety, bb2121 expansion and persistence, MRD (genomic and flow assays) Exploratory: BCMA expression/loss, T cell immunophenotype, GEP in BM, HEOR Apheresis Flu (30 mg/m2) Cy (300 mg/m2) CAR T infusion* bb2121 manufacturing Dose (x 106 CAR+ T cells) Range: 150-300 Days -5, -4, -3 0 * Re-treatment allowed at PD for best response of ≥ SD N = 80
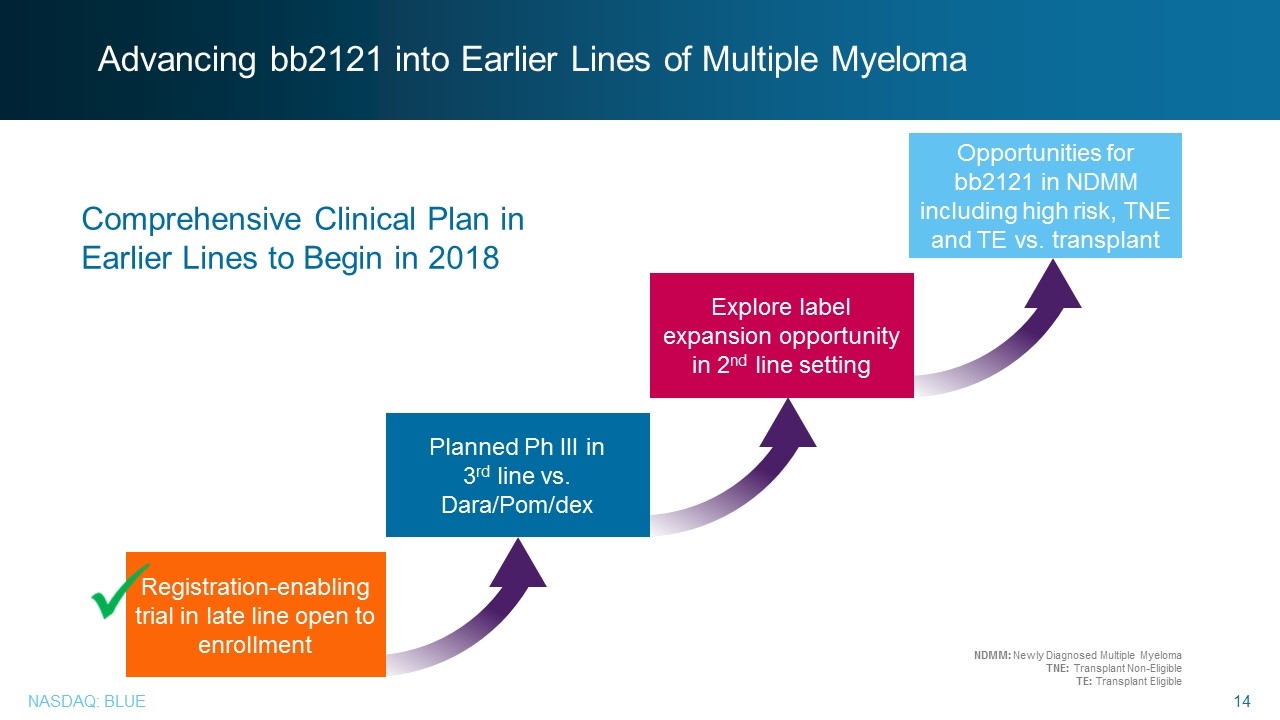
Advancing bb2121 into Earlier Lines of Multiple Myeloma Registration-enabling trial in late line open to enrollment Planned Ph III in 3rd line vs. Dara/Pom/dex Explore label expansion opportunity in 2nd line setting Opportunities for bb2121 in NDMM including high risk, TNE and TE vs. transplant Comprehensive Clinical Plan in Earlier Lines to Begin in 2018 NDMM: Newly Diagnosed Multiple Myeloma TNE: Transplant Non-Eligible TE: Transplant Eligible

Closing Nick Leschly, chief bluebird
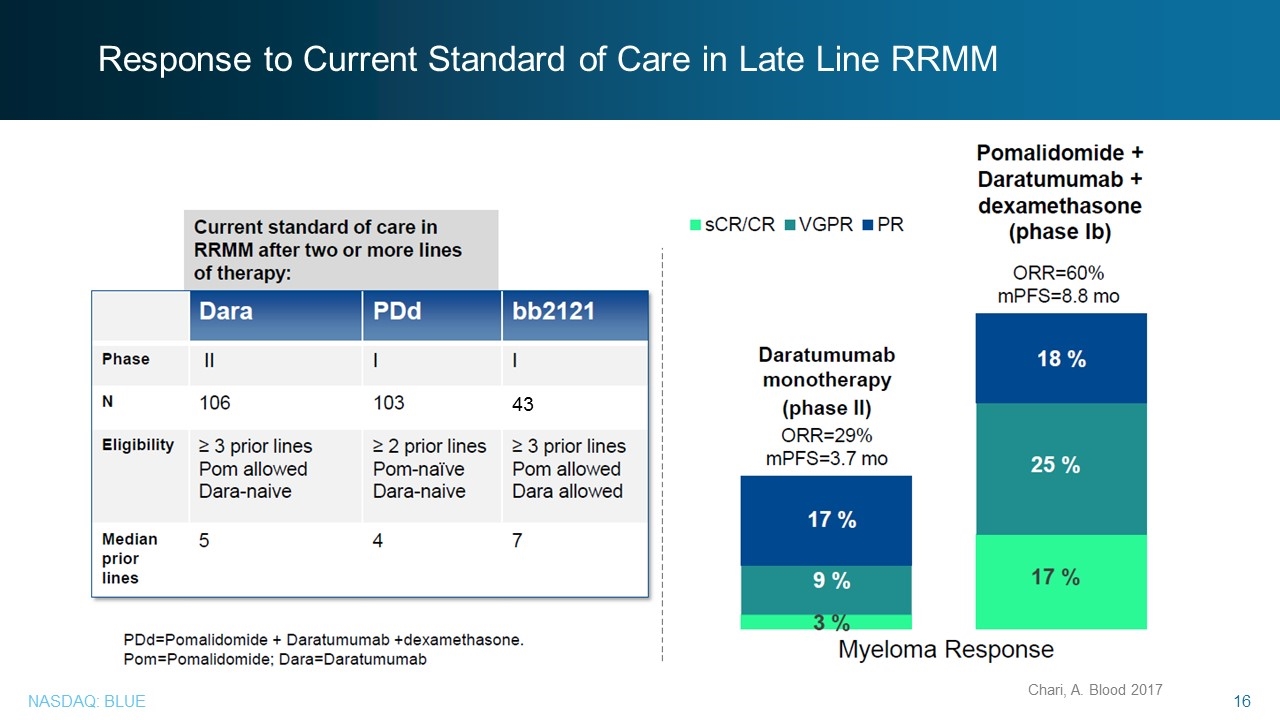
Response to Current Standard of Care in Late Line RRMM Chari, A. Blood 2017 43
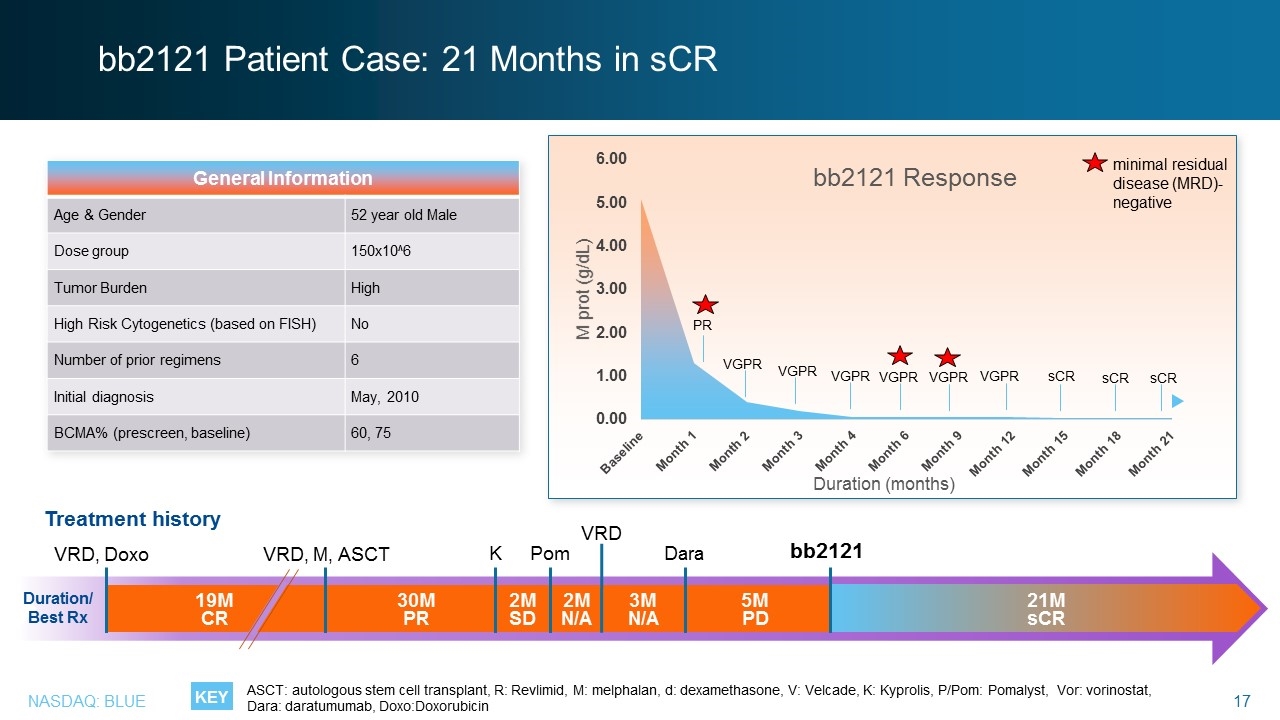
bb2121 Patient Case: 21 Months in sCR General Information Age & Gender 52 year old Male Dose group 150x10^6 Tumor Burden High High Risk Cytogenetics (based on FISH) No Number of prior regimens 6 Initial diagnosis May, 2010 BCMA% (prescreen, baseline) 60, 75 VRD, Doxo VRD, M, ASCT K Pom bb2121 Duration/ Best Rx 30M PR 2M SD 3M N/A 5M PD 2M N/A 19M CR VRD Dara Treatment history 21M sCR KEY minimal residual disease (MRD)-negative PR VGPR VGPR VGPR VGPR sCR VGPR VGPR sCR sCR ASCT: autologous stem cell transplant, R: Revlimid, M: melphalan, d: dexamethasone, V: Velcade, K: Kyprolis, P/Pom: Pomalyst, Vor: vorinostat, Dara: daratumumab, Doxo:Doxorubicin
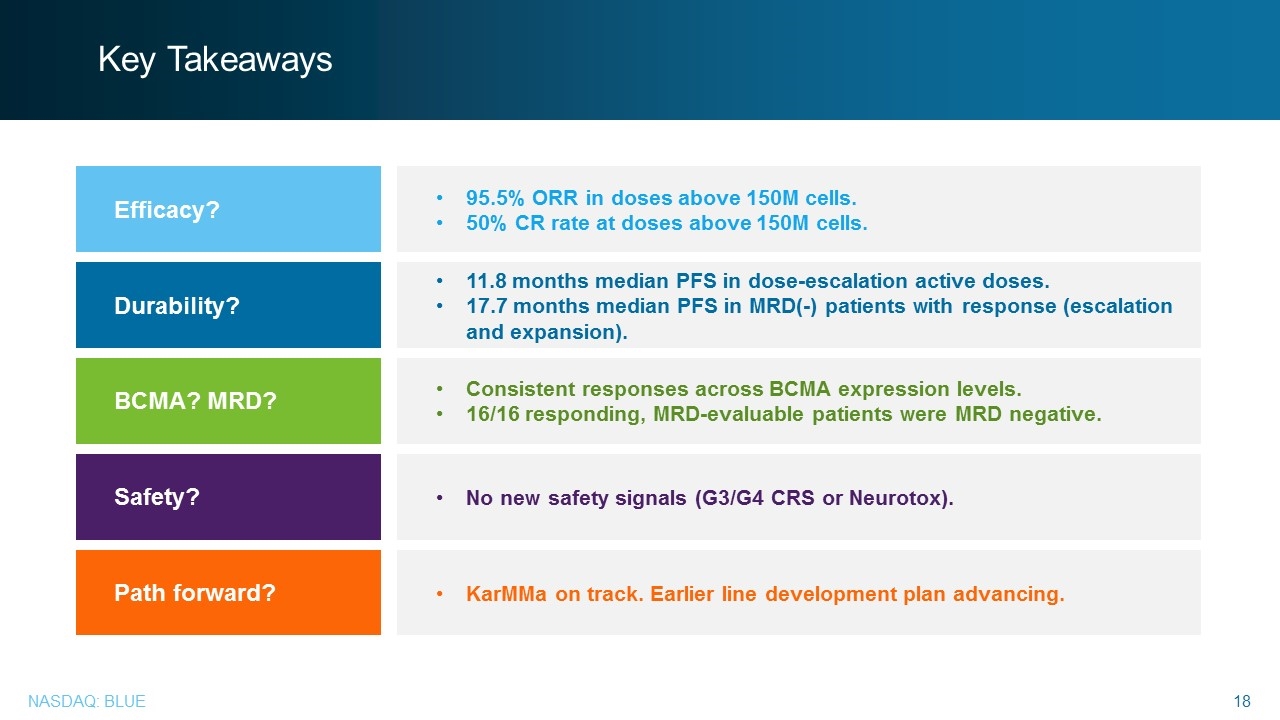
Key Takeaways Efficacy? Durability? BCMA? MRD? Safety? Path forward? 95.5% ORR in doses above 150M cells. 50% CR rate at doses above 150M cells. 11.8 months median PFS in dose-escalation active doses. 17.7 months median PFS in MRD(-) patients with response (escalation and expansion). Consistent responses across BCMA expression levels. 16/16 responding, MRD-evaluable patients were MRD negative. No new safety signals (G3/G4 CRS or Neurotox). KarMMa on track. Earlier line development plan advancing.
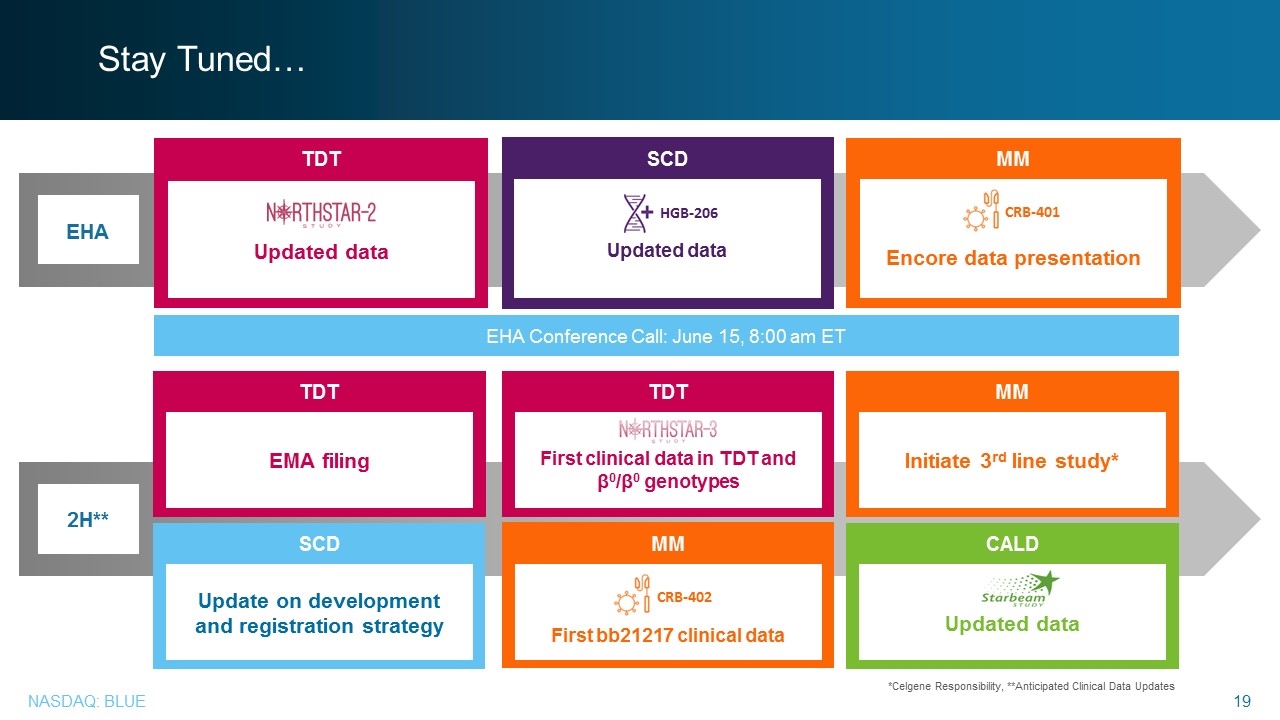
Stay Tuned… *Celgene Responsibility, **Anticipated Clinical Data Updates EHA MM Encore data presentation TDT Updated data SCD Updated data EHA Conference Call: June 15, 8:00 am ET 2H** TDT First clinical data in TDT and β0/β0 genotypes TDT EMA filing MM First bb21217 clinical data CALD Updated data SCD Update on development and registration strategy MM Initiate 3rd line study* CRB-401 CRB-402 HGB-206

Q&A
