Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SELECTA BIOSCIENCES INC | selectabiosciences8k_june2.htm |

Jefferies Presentation Nasdaq: SELB June 2018

Safe Harbor / Disclaimer Any statements in this presentation about the future expectations, plans and prospects of Selecta Biosciences, Inc. (“the company”), including without limitation, the progress of the Phase 1/2 clinical program of SEL-212, the ability of SVP-Rapamycin to mitigate unwanted immunogenicity and unlock the full potential of biologic therapies, when the company will advance to Phase 3 for SEL-212 (if at all), the ability of SEL-212 to provide better and more sustained serum uric acid control, fewer flares, and less frequent dosing compared with recent data reported with the current FDA-approved uricase therapy, the ability of the company’s SVP platform, including SVP-Rapamycin, to enable new therapies or to improve the efficacy or safety of existing biologics by mitigating immune response, when the company will conduct an End-of-Phase 2 meeting for SEL-212 if at all, the potential of SEL-212 to treat severe gout patients and resolve their debilitating symptoms, whether the FDA approves the company’s plan to provide combination therapy of SEL-212 for the entire treatment period, whether the company will determine an appropriate dose regimen of SEL-212 for the Phase 3, whether SEL-212 has the potential to address the unmet needs of gout patients, whether patients receiving SEL- 212 will be able to complete full therapy cycles over 6 months, whether SEL-212 holds billion dollar potential, whether SEL-212 will continue to show low overall incidence of gout flares and continue to be generally well-tolerated, when the company will report further data from the Phase 2 trial, whether the data from patients receiving five monthly combination doses of SEL-212 will support the company’s plans for its Phase 3 trial, whether the SVP platform enables the biologic to be distributed broadly to desired sites of action, the potential treatment applications for products utilizing the SVP platform in areas such as enzyme therapy, gene therapy, oncology therapy, vaccines and treatments for allergies and autoimmune diseases, the potential of future collaborations or licenses based on the ability of SVP-Rapamycin, the potential of the SVP-Rapamycin platform, generally, statements regarding progress of the Phase 1 trial for SEL-403, whether mesothelioma patients would benefit from a combination therapy consisting of LMB-100 and SVP-Rapamycin, the company’s ability to locate and enroll a sufficient number of eligible patients to participate in the SEL-403 Phase 1 trial, whether preclinical data regarding SVP-Rapamycin and LMB-100 will be predictive of clinical trial results for SEL-403, whether the company files an IND for SEL-302 in 2019, the company's expectations about receiving additional payments from Spark Therapeutics, Inc. under the license agreement and/or the stock purchase agreement, the sufficiency of the company’s cash, cash equivalents, investments, and restricted cash into mid-2019 and other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “hypothesize,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including, but not limited to, the following: the uncertainties inherent in the initiation, completion and cost of clinical trials including their uncertain outcomes, the availability and timing of data from ongoing and future clinical trials and the results of such trials, whether preliminary results from a particular clinical trial will be predictive of the final results of that trial or whether results of early clinical trials will be indicative of the results of later clinical trials, the unproven approach of the company’s SVP technology, potential delays in enrollment of patients, undesirable side effects of the company’s product candidates, its reliance on third parties to manufacture its product candidates and to conduct its clinical trials, the company’s inability to maintain its existing or future collaborations or licenses, its inability to protect its proprietary technology and intellectual property, potential delays in regulatory approvals, the availability of funding sufficient for its foreseeable and unforeseeable operating expenses and capital expenditure requirements, substantial fluctuation in the price of its common stock, and other important factors discussed in the “Risk Factors” section of the company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission, or SEC, on May 9, 2018, and in other filings that the company makes with the SEC. In addition, any forward-looking statements included in this presentation represent the company’s views only as of the date of its publication and should not be relied upon as representing its views as of any subsequent date. The company specifically disclaims any obligation to update any forward-looking statements included in this presentation. 2

Corporate Overview • Clinical-stage company applying proprietary Synthetic Vaccine Particle (SVP™) platform to mitigate unwanted immunogenicity and unlock the full potential of biologic therapies • Expect to begin Phase 3 in 2018 with SEL-212 (SVP-Rapamycin + pegsiticase) for chronic severe gout; current data suggest product profile may provide: 1) Better and more sustained serum uric acid control; 2) Fewer flares; and 3) Once monthly dosing; versus FDA approved uricase • Ongoing Phase 1 trial of SEL-403 (SVP-Rapamycin + LMB-100) for mesothelioma • Proprietary gene therapy candidates in preclinical development • License agreement in place with Spark Therapeutics, with additional potential for collaborations and licenses in a range of therapeutic areas 3

Immunogenicity is Well Recognized as a Serious Challenge for Biologic Therapies I M M U N O G E N I C I T Y ’ S I M P A C T COMPROMISED EFFICACY SAFETY RISK UNPREDICTABLE RESPONSE Anti-drug antibodies (ADAs) Hypersensitivity reactions can Changed PK/PD through neutralize therapeutic benefit impact patients drug-ADA interaction January 2018 Edition “With the explosion of biologic products on the market and in When the Immune System research pipelines, we’ve Thwarts Lifesaving Drugs become very concerned about the effectiveness and safety of these drugs.” – Amy Rosenberg, MD, Director, Division of Biotechnology Products Review and Research, FDA Patients often produce antibodies to the very treatments keeping them alive, sometimes to disastrous effect… 4

Mitigating Unwanted Immunogenicity via Selecta’s SVP-Rapamycin Technology Platform Spleen • By dosing the “free biologic” it distributes broadly to desired SVP-Rapamycin Biologic drug Dendritic cell sites of action • Some of the biologic co- localizes with dendritic cells Targeting that have taken up SVP- immune cells Rapamycin Sending precise instructions • The dendritic cells then induce Naïve T cell regulatory T cells that circulate Implementing Helper T cell throughout the body and the message suppress immune responses B cell against the biologic (i.e. ADAs) Inducing a tolerogenic Regulatory response to a biologic T cell drug (antigen) Preventing ADAs Potential to enable new therapies and improve efficacy/safety of existing biologics 5 Kishimoto et al., Improving the efficacy and safety of biologic drugs with tolerogenic nanoparticles, Nature Nanotechnology, Aug. 2016

SEL-212 for Chronic Severe Gout

SEL-212: Advancing a Potential New Treatment Option for Chronic Severe Gout Patients Ownership • In-licensed pegsiticase in 2014; combined with SVP-Rapamycin to form SEL-212 Rare and Serious Disease • ~160,000 adults with chronic severe gout treated by U.S. rheumatologists • Debilitating flares and joint-damaging arthritis caused by uric acid deposits; risk of renal and cardiovascular disease Immunogenicity Barrier • Uricases are highly effective in breaking down uric acid deposits, but are foreign to the human immune system, causing immunogenicity in nearly all patients that can negate efficacy and present safety risks Clear Clinical Path • Serum uric acid level reduction – a robust FDA/EMA primary endpoint for approval – can be seen rapidly upon dosing; easy to measure; maintenance strongly correlated with low/negative ADA titers • Adult patient population with rapid enrollment potential 7

Today’s Unmet Needs in Chronic Severe Gout • Monthly dosing (vs. biweekly for today’s FDA approved uricase therapy) • Ability to complete full therapy cycles (6 months) - Persistent reduction in Serum Uric Acid levels (SUA) - Elimination of tophi • Gout flare reduction • Avoidance of “off-label” and global immunosuppressive therapies We believe SEL-212 has the potential to address these unmet needs and holds billion-dollar potential 8

Current Stage of SEL- SEL-212 Clinical Development Plan 212 Development DETERMINATION OF DOSE REGIMEN TO TAKE INTO PHASE 3 PHASE 3 PROGRAM WITH DEFINED DOSE REGIMEN Phase 1 a Dose Finding Phase 2 Dose Ranging Pegsiticase Five monthly injections 6 Monthly Combination Injections of SEL-212 (N= 22) Matrix approach to find best doses of the two components: Phase 1 b Dose Finding • SVP-Rapamycin • Pegsiticase Primary Clinical Endpoint: SVP-Rapamycin +/- pegsiticase (Planned N ~ 140) Serum uric acid < 6 mg/dl (N= 63) measured at month 3 and 6

Phase 2 Trial Overview Enrollment Criteria • Patients with symptomatic gout and serum uric acid levels >6 mg/dL Primary/Secondary • Safety, tolerability and pharmacokinetics of multiple doses of SEL-212 and pegsiticase alone • Reduction of serum uric acid levels Endpoints • Reduction of ADA levels Design • Multiple ascending dose cohorts • Control cohorts: pegsiticase alone every 28 days for up to five doses Dosing • Cohorts 13,15,17: SEL-212 every 28 days for five doses • Every other cohort: SEL-212 every 28 days for three doses followed by two doses of pegsiticase alone Stopping Rules • Dosing stopped upon loss of sUA control at Days 21 after a dose As of April 2 • 117 patients dosed at 15 U.S. clinical sites 10

New Phase 2 Data at 12-weeks show 74% of Patients with Control of SUA <6 mg/dl Pegsiticase + SVP-Rapamycin SEL-212 1 0 0 Pegsiticase 74%; (14/19) 0.2 or 0.4 mg/kg SVP-Rapamycin 5 0 0.125 or 0.15 mg/kg 0 % Pts. SUA mg/dL SUA <6 Pts. % 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 Week 0.2 or 0.4 mg/kg Patients evaluable at 12 weeks who received Pegsiticase a full first dose and completed treatment cycle 1 1 0 0 Pegsiticase 0.2 or 0.4 mg/kg 5 0 5 0 17% 0 0 (1/6) % mg/dL SUA <6 Pts. % 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 Week 11 Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918

Patients Dosed With 0.125 mg/kg of SEL-110 + 0.4 mg/kg of SEL-037 Pegsiticase + SVP-Rapamycin Pegsiticase Alone Patient 1 0 1 0 5 8 4 6 1 0 4 1 0 3 106-0060 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 A 3 1 0 106-0061 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 F 3 1 0 111-0009 2 0 1 0 2 1 0 1 0 5 8 Anti 4 6 1 0 4 1 0 3 102-0019 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 - 4 1 0 3 Uricase Antibody Titer 107-0012 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 D 3 1 0 112-0002 2 0 1 0 2 1 0 1 0 5 8 0.125 mg/kg 1 0 4 6 4 E 1 0 3 112-0001 2 0 1 0 2 0.4 mg/kg 1 0 1 0 5 8 1 0 4 6 4 1 0 3 107-0014 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 111-0010 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 D 1 0 3 111-0011 2 0 1 0 2 5 Serum Uric Acid (mg/dL) Acid Uric Serum 1 0 1 0 8 4 6 1 0 4 G 1 0 3 117-0005 2 0 1 0 2 1 2 1 0 5 1 0 8 1 0 4 6 4 1 0 3 110-0031 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 H 1 0 3 107-0022 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 107-0024 2 0 1 0 2 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 Week A Stopping rules met D Withdrawn due to protocol deviation E Discontinuation due to infusion reaction F Withdrawal of consent G SAE; non-study drug related H Discontinuation due to TEAE 12 Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918

Patients Dosed With 0.15 mg/kg of SEL-110 + 0.2 mg/kg of SEL-037 Pegsiticase + SVP-Rapamycin Pegsiticase Alone Patient 1 0 1 0 5 8 4 6 1 0 4 E 3 106-0084 1 0 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 117-0003 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 106-0086 2 0 1 0 2 Anti 1 0 1 0 5 8 4 6 1 0 4 A 3 1 0 103-0022 2 0 1 0 2 1 0 1 0 5 - 8 Uricase Antibody Titer 4 6 1 0 4 1 0 3 111-0018 2 0 1 0 2 1 0 1 0 5 8 4 6 F 1 0 4 1 0 3 103-0025 2 0.15 mg/kg 0 1 0 2 1 0 1 0 5 8 4 6 D 1 0 0.2 mg/kg 4 1 0 3 111-0019 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 110-0034 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 104-2026 2 0 1 0 2 1 0 1 0 5 8 1 0 4 Serum Uric Acid (mg/dL) Acid Uric Serum 6 4 1 0 3 106-0092 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 106-0093 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 102-0024 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 104-2021 2 0 1 0 2 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 Week A Stopping rules met D Withdrawn due to protocol deviation E Discontinuation due to infusion reaction F Withdrawal of consent 13 Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918

Patients Dosed With 0.15 mg/kg of SEL-110 + 0.4 mg/kg of SEL-037 Pegsiticase + SVP-Rapamycin Pegsiticase Alone Patient 1 0 1 0 5 8 4 6 1 0 4 A 3 107-0017 1 0 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 A 3 1 0 106-0073 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 111-0012 2 0 1 0 2 Anti 1 0 1 0 5 8 4 6 1 0 4 1 0 3 111-0013 2 0 1 0 2 1 2 1 0 5 - 1 0 Uricase Antibody Titer 8 1 0 4 6 4 1 0 3 111-0015 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 G 3 4 1 0 110-0026 2 0.15 mg/kg 0 1 0 2 1 0 1 0 5 8 4 6 1 0 0.4 mg/kg 4 F 1 0 3 106-0077 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 110-0028 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 106-0079 2 0 1 0 2 1 0 1 0 5 8 1 0 4 Serum Uric Acid (mg/dL) Acid Uric Serum 6 4 1 0 3 107-0021 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 F 1 0 3 110-0030 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 103-0028 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 115-0002 2 0 1 0 2 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 Week A Stopping rules met F Withdrawal of consent G SAE; non-study drug related 14 Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918

SEL-212 Continues to Show Low Overall Incidence of Gout Flares In Total Phase 2 Patient Population Percent patients with gout flare by treatment month 6 0 50% 4 0 26% 19% 2 0 13% % of Patients w/Flare Patientsof % 4% 4% 0 month 1 1 2 3 4 5 Pegsiticase Alone SEL-212 15 Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918

SEL-212 Safety For Total Phase 2 Patient Population •SEL-212 has been generally well tolerated at clinically active doses following >300 administrations •Fifteen SAEs reported in the ongoing Phase 2 trial: • Seven were reported not to be or unlikely to be related to study drug • Eight infusion reactions: • Four in cohorts receiving pegsiticase alone or pegsiticase in combination with the lowest dose of SVP-Rapamycin, as anticipated • Two due to protocol deviations related to dosing errors • Two during a repeat dose of SEL-212 in higher (0.1 – 0.15 mg/kg) dose cohorts • None occurred after treatment period 2 •All SAEs were successfully treated without further issues 16 Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918
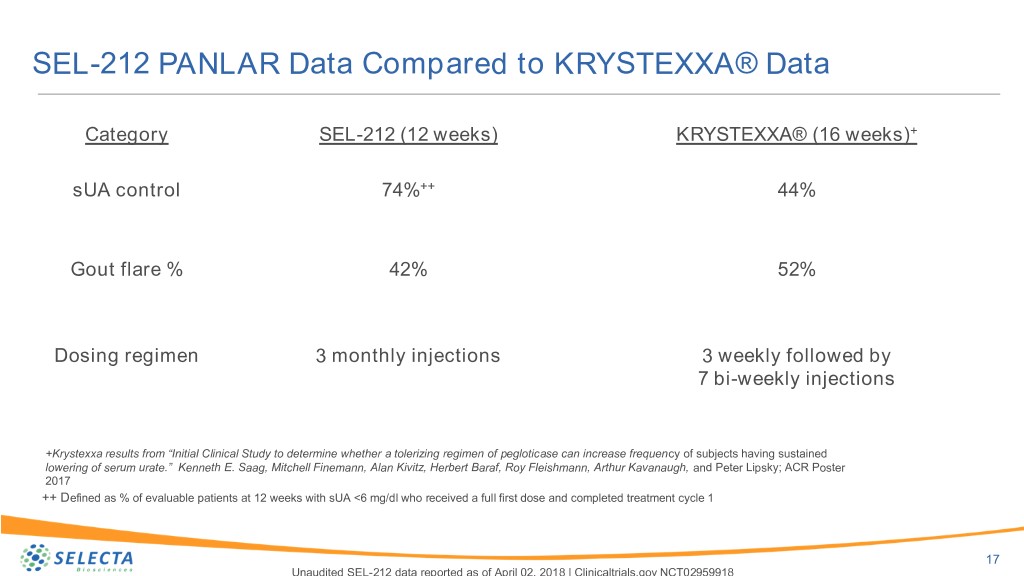
SEL-212 PANLAR Data Compared to KRYSTEXXA® Data Category SEL-212 (12 weeks) KRYSTEXXA® (16 weeks)+ sUA control 74%++ 44% Gout flare % 42% 52% Dosing regimen 3 monthly injections 3 weekly followed by 7 bi-weekly injections +Krystexxa results from “Initial Clinical Study to determine whether a tolerizing regimen of pegloticase can increase frequency of subjects having sustained lowering of serum urate.” Kenneth E. Saag, Mitchell Finemann, Alan Kivitz, Herbert Baraf, Roy Fleishmann, Arthur Kavanaugh, and Peter Lipsky; ACR Poster 2017 ++ Defined as % of evaluable patients at 12 weeks with sUA <6 mg/dl who received a full first dose and completed treatment cycle 1 17 Unaudited SEL-212 data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918

Next Step for SEL-212 in 2018 • Expansion of SEL-212 PHASE 3 PROGRAM PANLAR data set to be presented at EULAR (June 15th) • Data expected in 6 Monthly Combination Injections of SEL-212 third quarter from patients receiving five combination doses of SEL-212 • Patients now receiving 4th of 5 expected combination doses Primary Clinical Endpoint: • Phase 3 trial Serum uric acid < 6 mg/dl expected to begin in 2018 measured at month 3 and 6 18

SEL-403 for Mesothelioma

SEL-403: A Highly Potent Recombinant Pseudomonas Immunotoxin Targeting Mesothelin Ownership • In-licensed LMB-100 from NCI in April 2017; up to $9.25 million in milestones; low single-digit royalties • Combination with SVP-Rapamycin now known as SEL-403 Rare and Serious Disease • Mesothelin expressed in virtually all mesotheliomas (~3,000 annual U.S. diagnoses1) and pancreatic cancers (~50,000); high percentage of ovarian, lung, breast cancers • Certain solid tumors are particularly hard to treat and have remained evasive to immunotherapy approaches Immunogenicity Barrier • LMB-100 induces inhibitory antibodies upon first dose in almost all patients, limiting dosing to one or two administration cycles; insufficient to control tumor • Global immunosuppressants ineffective in preventing ADAs in a vast majority of patients • SVP allowed 3+ treatment cycles in pre-clinical models, restoring LMB-100 anti-tumor activity • Initial repeat dose data from ongoing SEL-212 Phase 2 encouraging for this application Clear Clinical Path • Both components of SEL-403 (SVP-Rapamycin and LMB-100) have been in the clinic in separate trials • FDA acceptance of IND for combination treatment announced in January; First patient dosed in March 2018 1. Beebe-Dimmer et al., Mesothelioma in the United States: a Surveillance, Epidemiology, and End Results (SEER) – Medicare investigation of treatment patterns and overall survival, Clin Epidemiol., Oct. 2016 20

Immunotoxin LMB-100 LMB-100 Mesothelin is overexpressed on many solid tumors • LMB-100: Pseudomonas exotoxin A • Mesothelioma (>90%) linked to antibody Fab targeting mesothelin • Pancreatic cancer (>90%) • Technology was licensed to Roche Ira Pastan, M.D. • Ovarian cancer (70%) Head, Molecular but later returned to NCI Biology Section • Efficacy was limited by • Lung cancer (50%) National Cancer Institute immunogenicity after one or two • Breast cancer (34%) cycles in most patients • Currently in Phase 1 clinical trials 21

Clinical Activity of SS1P (LMB-100 Precursor) in Mesothelioma Patient 1 4 Treatment Cycles • Widely metastatic peritoneal mesothelioma • Survived 32 months Sum of target of target Sum(cm)lesions Months after treatment Before treatment 1.6 months 8 months 6 Treatment Cycles Patient 2 • Extensive pleural mesothelioma • Survival >6 years (still alive) Sum of target of target Sum(cm)lesions Months after treatment Day 12 3 months 15 months While patients receiving ≥4 cycles showed major anti-tumor response, immunogenicity limited treatment to 1 or 2 cycles for most patients despite use of immunosuppressive therapy 22 Science Translational Medicine. 2013 Oct 23;5(208).

Preclinical Data Supports the Benefits of SVP-Rapamycin + LMB-100 Combination Therapy SEL-403 Prevents formation of Restores LMB-100’s SVP alone does not anti-drug antibodies anti-tumor response accelerate tumor growth A n ti-L M B -1 0 0 A n tib o d y T u m o r G r o w t h T u m o r G r o w t h 2 5 0 0 1 5 0 0 4 0 0 S a l i n e L M B - 1 0 0 ) L M B - 1 0 0 + S V P l m ) ) 2 0 0 0 3 3 m / m m ( 3 0 0 ( g L M B -1 0 0 m G 1 0 0 0 ( e g L M B -1 0 0 + S V P z 1 5 0 0 I e i z 0 s S a l in e i 0 r s 1 2 0 0 S V P - R a p a m y c i n - o 1 0 0 0 r B o m M 5 0 0 u m L - i T u t 1 0 0 5 0 0 T n A 0 0 - 1 0 - 5 0 5 1 0 1 5 2 0 L M B -1 0 0 0 2 4 6 8 0 1 0 2 0 3 0 W e e k S V P D a y s s i n c e t u m o r i n o c u l a t i o n D a y s s i n c e t u m o r i n o c u l a t i o n 23 Proc Natl Acad Sci U S A. 2018 Jan 23;115(4):E733-E742

SEL-403 In Clinical Phase 1 at NCI • Enrolled the first patient of a dose-escalating Phase 1 trial in March 2018 under a CRADA at NCI (NCT03436732) • Enrolling up to 18 patients with malignant pleural or peritoneal mesothelioma who have undergone at least one regimen of chemotherapy • Patients to receive four treatment cycles of the combination product candidate • Primary objective: Evaluate the safety and tolerability of the combination therapeutic candidate in the study population • Additional measurements: Objective Response Rates and ADA titers 24 CRADA #3157 with NCI

Proprietary & Licensed Gene Therapy Programs

Selecta’s Proprietary Gene Therapy Programs Ownership • Two proprietary gene therapies utilizing Anc80 and AAV + SVP-Rapamycin (SEL-302 & SEL-313) Rare and Serious Disease • Two rare inborn error of metabolism: Methylmalonic Acidemia (MMA) and Ornithine Transcarbamylase (OTC) Deficiency • Onset in early infancy; significantly reduces life expectancy Immunogenicity Barrier • Infants require treatment prior to metabolic crisis to avoid CNS effects; retreatment likely needed as patients grow • Repeat systemic gene therapy dosing currently not possible due to neutralizing antibodies to viral capsid • Cellular immune responses to the liver are an additional potential barrier Clear Clinical Path • Lead gene therapy program is SEL-302 for MMA • Clinical endpoints include: Methylmalonyl-CoA mutase and MMA levels • Expect to file IND in 2019 26

Benefits of ADA Mitigation in Gene Therapy Inhibiting Liver Allowing for Repeat Dosing Inflammation from First Dose And Dose Titration SVP or Empty NP SVP or Empty NP SVP or Empty NP + AAV8 + AAV8-Luc AAV8-Factor IX Day 0 Day 0 21 54 CD8 T cell Liver Infiltrates Anti-AAV8 Antibody Titer r ) 5 t e 2 5 0 0 0 t i C 4 T 2 0 0 0 0 8 ( V 3 1 5 0 0 0 A A A N 2 1 0 0 0 0 - i R t 5 0 0 0 1 n m A 8 0 0 D 0 2 0 4 0 6 0 C -1 D a y s S V P E m p ty N P Serum Factor IX Expression Serum ALT Enzyme Levels ) 200000 L m 2 0 ** /ml) SVP-Rapamycin / 160000 U ng m Empty NP 1 5 ( 120000 y t i 80000 v 1 0 i t c 40000 a 5 T Human FIX ( FIX Human 0 L A 0 S V P E m p ty N P 34 41 54 Days 27 Preclinical data generated in mice in collaboration with Dr. Federico Mingozzi, Genethon

Demonstration of the Role of Regulatory T Cells Effect can be Transferred to a Recipient T Reg Depletion Negates Effect AAV8-Luc +/- SVP AAV8-Luc +/- SVP PRIME CHALLENGE DONOR Transfer splenocytes Day 0 14 AAV8-hFIX Day 0 19 20 21 32 RECIPIENT Anti-CD25 Treg Depletion Day 0 1 28 Anti-AAV8 IgG levels in recipient mice (Day +14) Anti-AAV8 IgG levels (Day 32) 5 0 ) *** ) 2 0 L L * * * ** m / 4 0 m g / n g ( 1 5 ( 3 0 G G g I g I 8 1 0 2 0 V 8 A V A - A i 1 0 t A 5 n - i a t n 0 a 0 S V P -R a p a m y c in + + - - E m p ty N P S V P ** P < 0.01, *** P < 0.001 T re g d e p le tio n - + - + 28 Preclinical data generated in mice in collaboration with Dr. Federico Mingozzi, Genethon
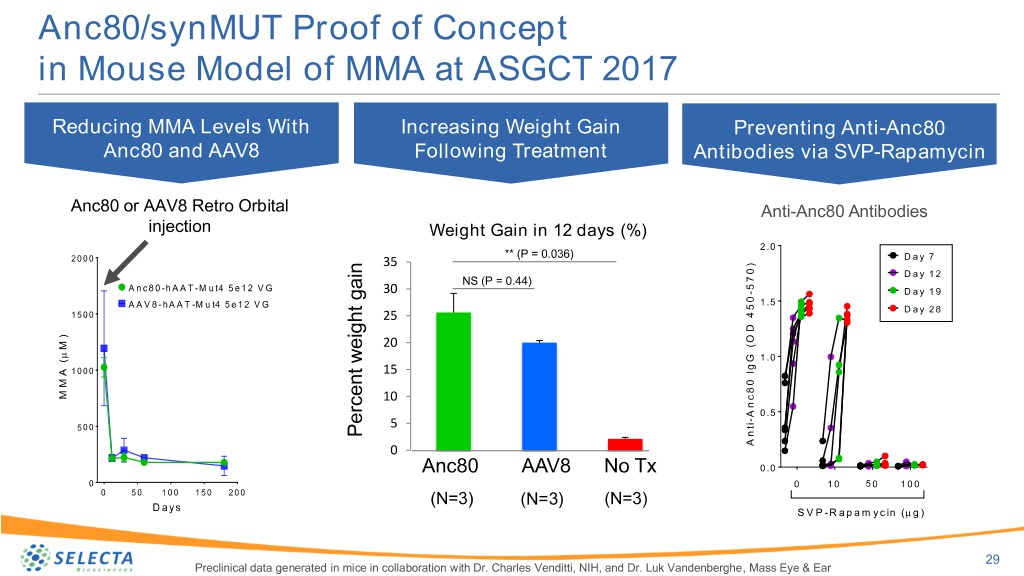
Anc80/synMUT Proof of Concept in Mouse Model of MMA at ASGCT 2017 Reducing MMA Levels With Increasing Weight Gain Preventing Anti-Anc80 Anc80 and AAV8 Following Treatment Antibodies via SVP-Rapamycin Anc80 or AAV8 Retro Orbital Anti-Anc80 Antibodies injection Weight Gain in 12 days (%) 2 .0 2 0 0 0 ** (P = 0.036) D a y 7 35 ) 0 D a y 1 2 NS (P = 0.44) 7 A n c 8 0 -h A A T -M u t4 5 e 1 2 V G 5 30 - D a y 1 9 A A V 8 -h A A T -M u t4 5 e 1 2 V G 0 1 .5 5 D a y 2 8 1 5 0 0 4 25 D ) O ( M 20 1 .0 G ( g 1 0 0 0 15 I A 0 M 8 M 10 c n A 0 .5 - i 5 0 0 5 t n Percent weight gain weight Percent 0 A Anc80 AAV8 No Tx 0 .0 0 0 1 0 5 0 1 0 0 0 5 0 1 0 0 1 5 0 2 0 0 (N=3) (N=3) (N=3) D a y s S V P -R a p a m y c in ( g ) 29 Preclinical data generated in mice in collaboration with Dr. Charles Venditti, NIH, and Dr. Luk Vandenberghe, Mass Eye & Ear

Spark Therapeutics License Agreement • December 2016 agreement provides Spark Therapeutics with exclusive worldwide rights to Selecta's SVP technology for up to five gene therapy targets • Among the largest gene therapy and SMID-cap to SMID-cap biotech deals announced to date • Initial focus on combination of SVP with Spark’s Hemophilia A gene therapy • Received $30 million of initial cash payments and investments in Selecta equity • Subject to the terms of the license agreement, Spark also agreed to pay to Selecta: - Up to $430 million in milestone payments for each target - Mid-single to low-double-digit royalties on worldwide annual net sales of any resulting commercialized gene therapy 30

Pipeline Indication Preclinical Phase 1 Phase 2 Phase 3 Proprietary ADA Mitigation Programs Chronic Severe Gout SEL-212 Mesothelioma & Pancreatic Cancer SEL-403 Methylmalonic Acidemia (MMA) SEL-302 Ornithine Transcarbamylase Deficiency (OTC) SEL-313 ADA Mitigation Program License Hemophilia A 31

Financial Overview For the Quarter Ended March 31, 2018 December 31, 2017 (In thousands, except share and per share data) Grant & Collaboration Revenue $ - $17 Research & Development Expenses 11,139 13,623 General & Administrative Expenses 4,674 5,671 Net Loss Attributable to Common Stockholders $(15,866) $(19,544) Net Loss Per Basic & Diluted Share $(0.71) $(0.89) Wtd. Avg. Common Shares Outstanding – Basic & Diluted 22,345,523 20,425,050 As of March 31, 2018 December 31, 2017 (In thousands) Cash, Cash Equivalents, Marketable Securities, Restricted Cash $83,472 $96,967 Cash runway into mid-2019 32
