Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Senseonics Holdings, Inc. | a18-14558_18k.htm |
Forward-Looking Statements In some cases, you can identify forward-looking statements by the words “expect,” “intend,” “target,” “plan,” “estimate,” “predict,” “opportunity,” and “continue,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward-looking statements include statements about future demand for our Eversense™ System and future products, if any, that we may develop; the growth of the CGM market; the factors that we believe drive demand for our Eversense™ System and our ability to sustain such demand; the size of the market and competitive landscape for our Eversense™ System; the initiation, timing, progress and results of our clinical trials; our plans for the Eversense™ System and our expectations about completion of its development and the timing of the submission for regulatory approval by the FDA, and the timing for receiving such approval; our enhancement of the Eversense™ System, including the extension of sensor life and improvements in accuracy; launch of the Eversense™ System in the United States and additional European countries; our plans for pursuing coverage and reimbursement for our Eversense™ System; our ability to protect and enforce our intellectual property rights; and anticipated trends and challenges in our business and the market in which we operate. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward- looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that these statements are based on a combination of facts and factors currently known by us and our expectations of the future, about which we cannot be certain. Factors that may cause actual results to differ materially from any future results expressed or implied by any forward-looking statements include the risk that we may not achieve greater market acceptance of our Eversense™ System; the risk that we may not be able to establish sales and marketing capabilities in order to meet demand for our Eversense™ System; risks associated with competition in the diagnostics industry; risks associated with the performance of our Eversense™ System; the risk that we may not receive regulatory clearance for our Eversense™ System in the United States; the risk that we may not be able to comply with extensive ongoing regulatory requirements; risks associated with reliance on a limited number of suppliers; the risk that we may not be able to successfully develop or introduce new or other applications of our technology at all or in a timely manner; the risk that we may not be able to maintain and expand coverage and reimbursement from health insurers and other third-party payers; risks associated with limited patent protection; the risk that we may not successfully manage our growth; and risks associated with reliance on outside financing to meet capital requirements. For further information regarding these risks, uncertainties and other factors you should read the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2017, our Quarterly Report on Form 10-Q for the quarter ended March 31, 2018 and our other filings we make with the Securities and Exchange Commission from time to time. Any forward-looking statements speak only as of the date of this presentation. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. This presentation contains certain forward-looking statements that involve risks and uncertainties that could cause actual results to be materially different from historical results or from any future results expressed or implied by such forward-looking statements. 2

confidently live life with ease develop and commercialize transformative glucose monitoring products. 3

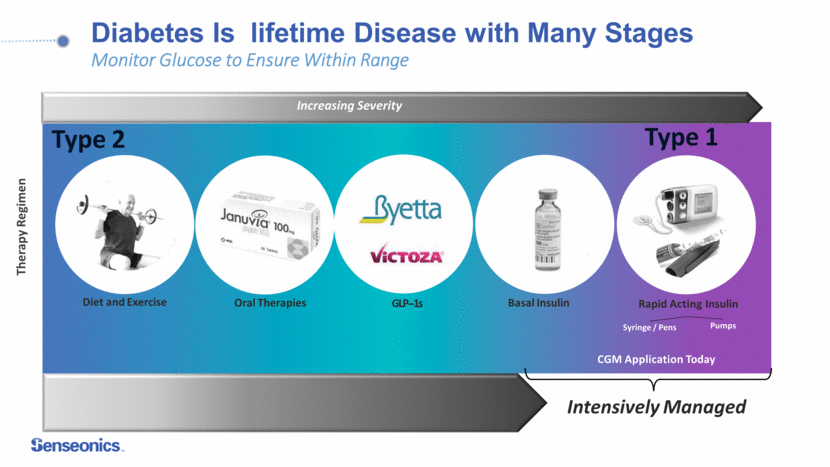
Diabetes Is lifetime Disease with Many Stages Monitor Glucose to Ensure Within Range Syringe / Pens Pumps Increasing Severity CGM Application Today Type 1 Therapy Regimen Oral Therapies Diet and Exercise GLP-1s Basal Insulin Rapid Acting Insulin Intensively Managed Type 2 4
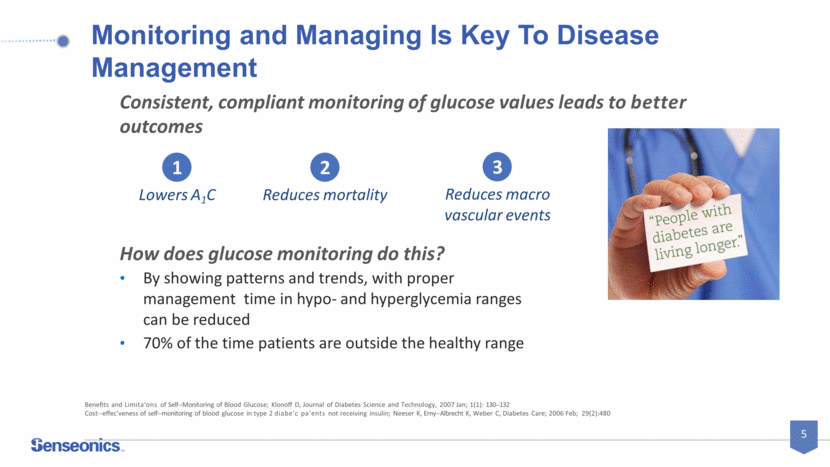
Monitoring and Managing Is Key To Disease Management How does glucose monitoring do this? By showing patterns and trends, with proper management time in hypo- and hyperglycemia ranges can be reduced 70% of the time patients are outside the healthy range Benefits and Limita'ons of Self-Monitoring of Blood Glucose; Klonoff D, Journal of Diabetes Science and Technology, 2007 Jan; 1(1): 130-132 Consistent, compliant monitoring of glucose values leads to better outcomes 1 Lowers A1C 2 Reduces mortality 3 Reduces macro vascular events Cost-effec'veness of self-monitoring of blood glucose in type 2 diabe'c pa'ents not receiving insulin; Neeser K, Erny-Albrecht K, Weber C, Diabetes Care; 2006 Feb; 29(2):480 5
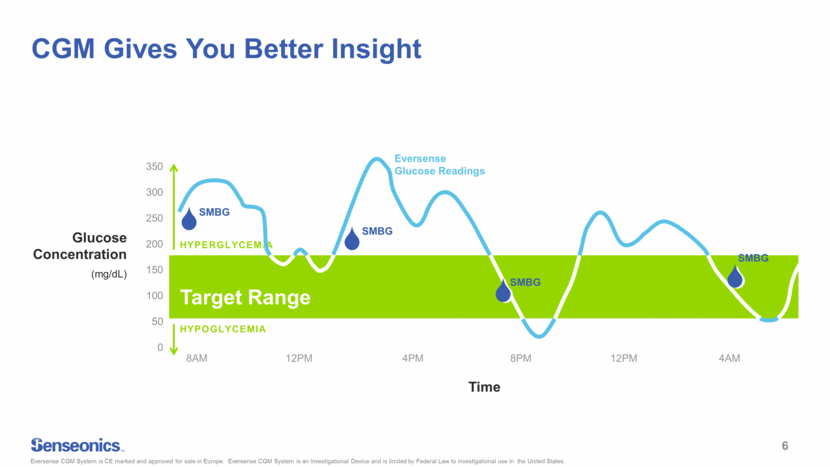
CGM Gives You Better Insight Glucose Concentration (mg/dL) Target Range HYPOGLYCEMIA HYPERGLYCEMIA Eversense Glucose Readings SMBG SMBG SMBG SMBG 8AM 12PM 4PM 8PM 12PM 4AM Eversense CGM System is CE marked and approved for sale in Europe. Eversense CGM System is an Investigational Device and is limited by Federal Law to investigational use in the United States. 6 0 50 100 150 200 250 300 350 14.00 22.00 0.00 2.00 4.00 6.00 8.00 10.00 Time
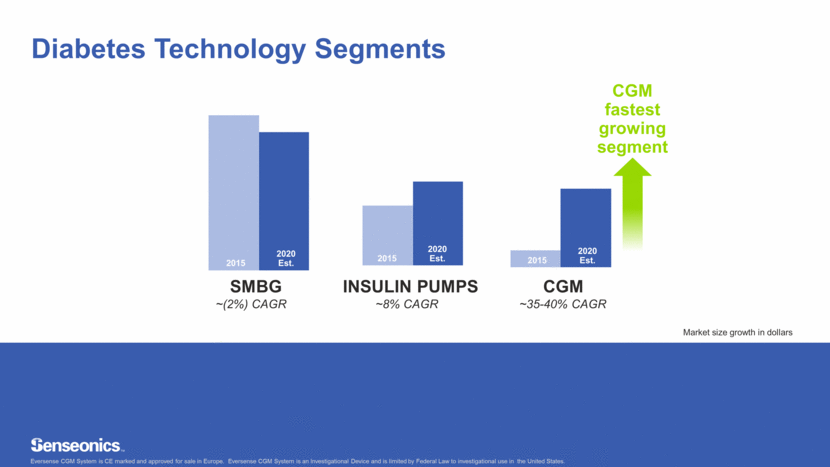
Diabetes Technology Segments 2015 2020 Est. 2015 2020 Est. 2015 2020 Est. CGM fastest growing segment Eversense CGM System is CE marked and approved for sale in Europe. Eversense CGM System is an Investigational Device and is limited by Federal Law to investigational use in the United States. ~35 – 40% CAGR ~8% CAGR ~(2%) CAGR SMBG INSULIN PUMPS CGM ~(2%) CAGR ~8% CAGR ~35-40% CAGR 2015 2020 Est. 2015 2020 Est. 2015 2020 Est. Market size growth in dollars
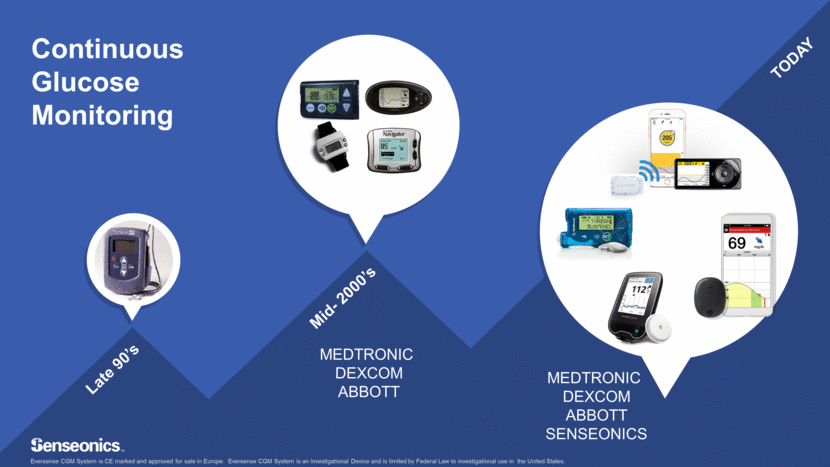
Mid- 2000’s Continuous Glucose Monitoring MEDTRONIC DEXCOM ABBOTT TODAY Late 90’s MEDTRONIC DEXCOM ABBOTT SENSEONICS Eversense CGM System is CE marked and approved for sale in Europe. Eversense CGM System is an Investigational Device and is limited by Federal Law to investigational use in the United States.
U.S. Market Potential – $18B 9 have an unmet need And CGM can contribute across the spectrum 6.9 M Insulin Taking Patients 23M Patients diagnosed with Diabetes 3.9M Insulin only (57%) 3M Insulin and oral (43%) 600K Pump $2.3B 250K CGM $750M Opportunity continues to expand National Diabetes Statistics Report, 2017 https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf Kelly Close Industry Roundup April 17, 2017 Eli Lilly Investor Report Q1 2017

The first & only fully implantable CGM sensor for confident long-term glucose monitoring Highest Accuracy Longest Sensor Life Best Convenience 10 Eversense Advantage

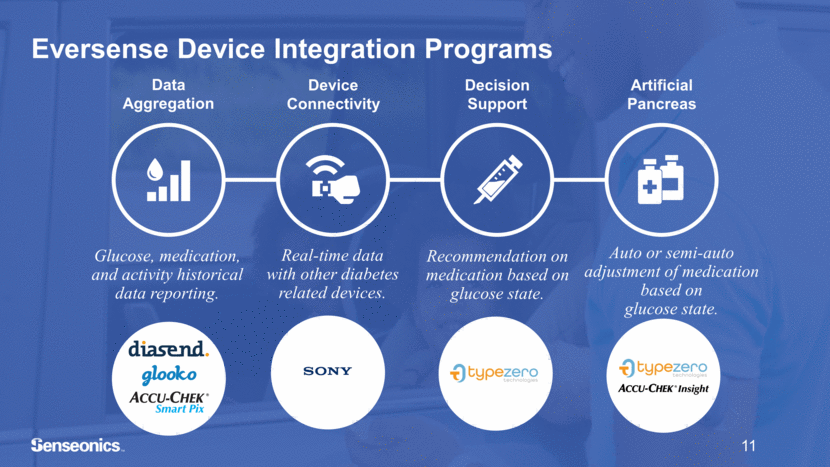
Data Aggregation Device Connectivity Artificial Pancreas Glucose, medication, and activity historical data reporting. Real-time data with other diabetes related devices. Recommendation on medication based on glucose state. Auto or semi-auto adjustment of medication based on glucose state. 11 Eversense Device Integration Programs Decision Support
The Eversense future 2020 and beyond 12 On-Demand: Type 2 FGM Long-term wear with no transmitter Type 1 : Gemini FGM +CGM Flash when desired, CGM for peace of mind 365 Day Sensor Life 1x/week calibration 365 Day Sensor Life 1x/week calibration

An Implantable CGM What does it mean for patients? Less Hassle Fewer Decisions More Flexibility, Freedom 13 A new Standard for CGM Enabling enhanced adherence and actionable information