Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Dova Pharmaceuticals Inc. | a18-13054_1ex99d1.htm |
| 8-K - 8-K - Dova Pharmaceuticals Inc. | a18-13054_18k.htm |
Disclaimer Certain information contained in this presentation relates to or is based on studies, surveys and other data obtained from third-party sources and Dova’s own internal estimates and research. While Dova believes these sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. While Dova believes its internal research is reliable, such research has not been verified by any independent source. This presentation contains forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on Dova’s current beliefs, expectations and assumptions regarding the future of Dova’s business, future plans and strategies, including the timing of the commercial launch of DOPTELET. All statements other than statements of historical facts contained in this presentation, including statements regarding business strategy, degree of market acceptance of approved product, timing for commercial launch, timing and likelihood of success, and plans and objectives of management for future operations, are forward-looking statements. The words ”may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The forward-looking statements in this presentation represent Dova’s views as of the date of this presentation. Although Dova believes the expectations reflected in such forward-looking statements are reasonable, Dova can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Except as required by applicable law, Dova does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. New risk factors and uncertainties may emerge from time to time, and it is not possible to predict all risk factors and uncertainties. For further information regarding these risks, uncertainties and other factors, you should read the “Risk Factors” section of Dova’s Annual Report on Form 10-K for the year ended December 31, 2017, filed with the U.S. Securities and Exchange Commission (“SEC”) on February 16, 2018, Dova's Quarterly Report on Form 10-Q filed with the SEC on May 9, 2018 and Dova’s other Periodic Reports filed with the SEC. 2
Topics for Discussion • • • • • DOPTELET Approved Packaging and Labelling Addressing a Market with Unmet Needs Launch Plans / Launch Metrics Additional Important Milestones in 2018 Q&A 3

Rx Only HOC71J0020 1S NDC1Utt0l0 10 R.IOnly Doptelet. (avatrombopag)tablets 20mg Doptelet. (avatrombopag)tablets 20mg ·=···•·•.D •: •:lova 4 •••.• PHARIIA AC E UTICALS

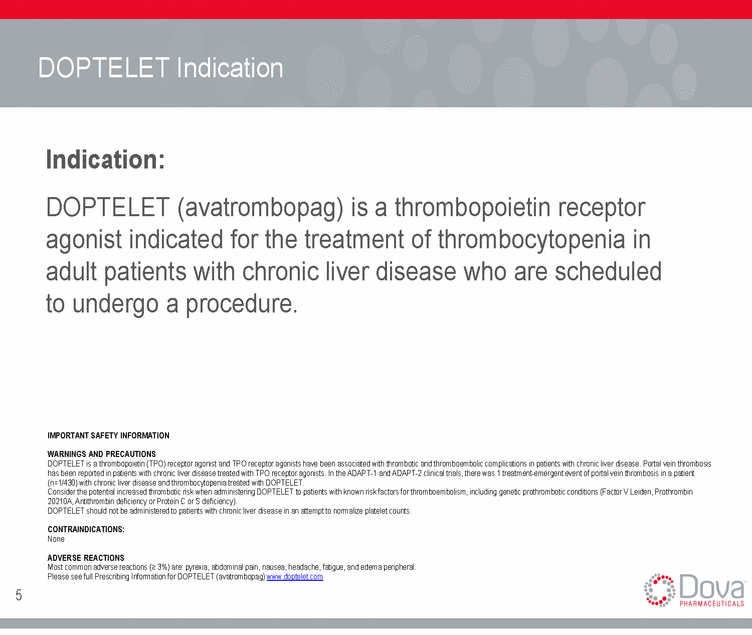
DOPTELET Indication Indication: DOPTELET (avatrombopag) is a thrombopoietin receptor agonist indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. IMPORTANT SAFETY INFORMATION WARNINGS AND PRECAUTIONS DOPTELET is a thrombopoietin (TPO) receptor agonist and TPO receptor agonists have been associated with thrombotic and thromboembolic complications in patients with chronic liver disease. Portal vein thrombosis has been reported in patients with chronic liver disease treated with TPO receptor agonists. In the ADAPT-1 and ADAPT-2 clinical trials, there was 1 treatment-emergent event of portal vein thrombosis in a patient (n=1/430) with chronic liver disease and thrombocytopenia treated with DOPTELET. Consider the potential increased thrombotic risk when administering DOPTELET to patients with known risk factors for thromboembolism, including genetic prothrombotic conditions (Factor V Leiden, Prothrombin 20210A, Antithrombin deficiency or Protein C or S deficiency). DOPTELET should not be administered to patients with chronic liver disease in an attempt to normalize platelet counts. CONTRAINDICATIONS: None ADVERSE REACTIONS Most common adverse reactions (> 3%) are: pyrexia, abdominal pain, nausea, headache, fatigue, and edema peripheral. Please see full Prescribing Information for DOPTELET (avatrombopag) www.doptelet.com 5
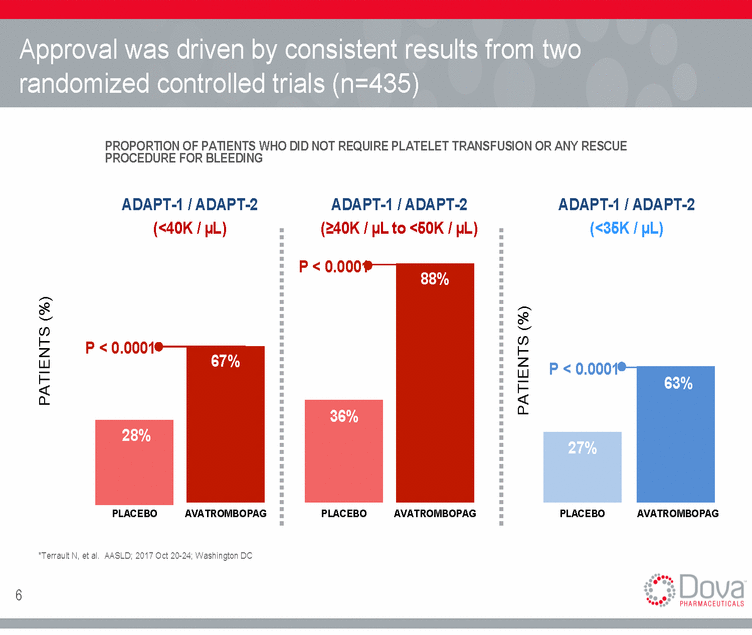
Approval was driven by consistent results from two randomized controlled trials (n=435) PROPORTION OF PATIENTS WHO DID NOT REQUIRE PLATELET TRANSFUSION OR ANY RESCUE PROCEDURE FOR BLEEDING ADAPT-1 / ADAPT-2 (<40K / µL) ADAPT-1 / ADAPT-2 (>40K / µL to <50K / µL) ADAPT-1 / ADAPT-2 (<35K / µL) P < 0.0001 P < 0.0001 P < 0.0001 PLACEBO AVATROMBOPAG PLACEBO AVATROMBOPAG PLACEBO AVATROMBOPAG *Terrault N, et al. AASLD; 2017 Oct 20-24; Washington DC 6 PATIENTS (%) PATIENTS (%) 27% 28% 36% 63% 67% 88%
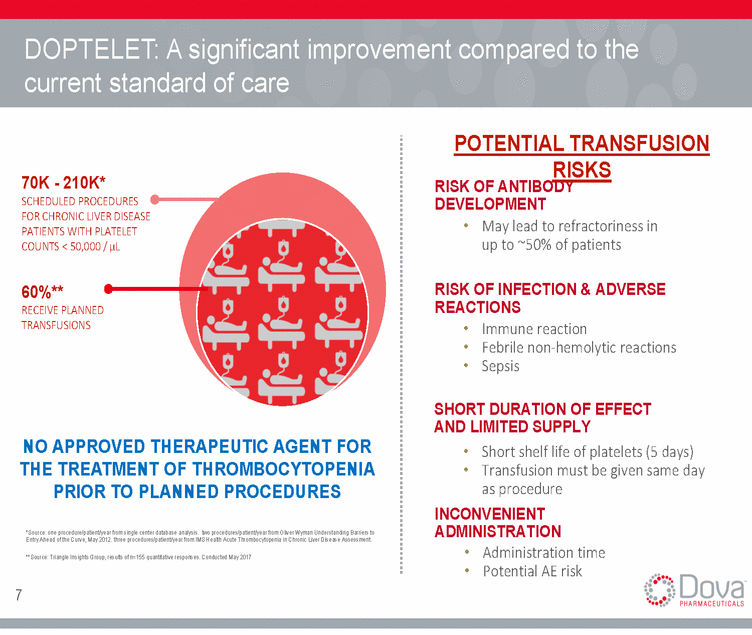
DOPTELET: A significant improvement compared to the current standard of care POTENTIAL TRANSFUSION RISKS RISK OF ANTIBODY DEVELOPMENT • May lead to refractoriness in up to ~50% of patients 70K - 210K* SCHEDULED PROCEDURES FOR CHRONIC LIVER DISEASE PATIENTS WITH PLATELET COUNTS < 50,000 / µL RISK OF INFECTION & ADVERSE REACTIONS • Immune reaction • Febrile non-hemolytic reactions • Sepsis 60%** RECEIVE PLANNED TRANSFUSIONS SHORT DURATION OF EFFECT AND LIMITED SUPPLY • Short shelf life of platelets (5 days) • Transfusion must be given same day as procedure INCONVENIENT ADMINISTRATION • Administration time • Potential AE risk NO APPROVED THERAPEUTIC AGENT FOR THE TREATMENT OF THROMBOCYTOPENIA PRIOR TO PLANNED PROCEDURES *Source: one procedure/patient/year from single center database analysis. two procedures/patient/year from Oliver Wyman Understanding Barriers to Entry Ahead of the Curve, May 2012. three procedures/patient/year from IMS Health Acute Thrombocytopenia in Chronic Liver Disease Assessment. **Source: Triangle Insights Group, results of n=155 quantitative responses. Conducted May 2017 7
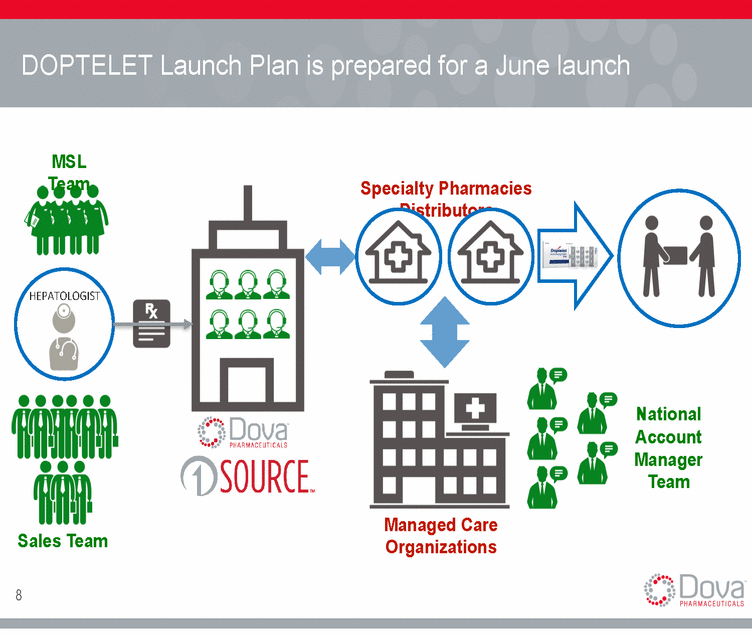
DOPTELET Launch Plan is prepared for a June launch MSL Team Specialty Pharmacies Distributors HEPATOLOGIST National Account Manager Team Managed Care Organizations Sales Team 8
Experienced and Dedicated Sales Force • • All sales territories have been filled Over 300 years experience in hepatology / liver disease Previous hepatology experience includes Salix, BMS, Gilead, Intercept Sales Force Training currently underway in preparation for a June launch • • 9

Dova 1Source: Streamlining access for both patients and healthcare providers MANAGES THE LOGISTICS COORDINATES FINANCIAL SUPPORT DELIVERS DOPTELET ON TIME FOR PROCEDURE / SUPPORTS ADHERENCE 10

··-T·a--i-CI-IC·C-IIT-a-.l·\'W-I-M·P-TB-.nT-&afll ·==c=:."U:tl.:........ ··-·--. --;':::-.:.:=·'-".'-..-...-...-....----.....-..---·-·-·-···.·..-··-· ... A - - --.•.. .X... ..... .J ac,p:.,.,, D oPtelet. -·-a., ·.__-.._--_._--_-_-------...._, ... ·--·------------- ---::---------·-----·-----·-·-·----·-·-·---··--· ...... - s:-·.:=::•:-..::.==::-.;:=.:':.-.... - Dif 11 "••·•" PHARIIA AC E UTICALS ova ..,.. _-_-_·.... .-...._-·· :: ------ --WELCOME ·..··-"" · "

DOPTELET: Strong Conference Presence in 2018 • Digestive Disease Week (DDW) June 2-5, Washington, DC American College of Gastroenterology (ACG) October 5-10, Philadelphia, PA American Association for the Study of Liver Diseases (AASLD) November 9-13, San Francisco, CA American Society of Hematology (ASH) December 1-4, San Diego, CA • • • 12

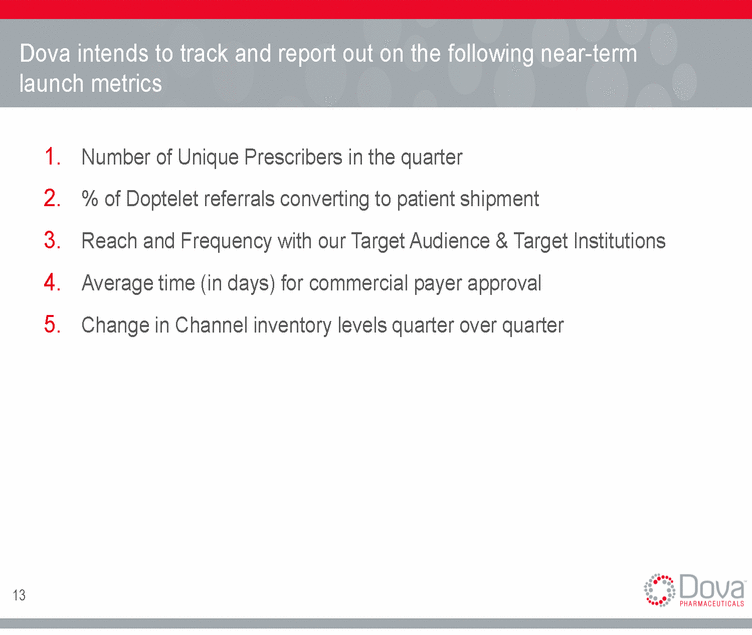
Dova intends to track and report out on the following near-term launch metrics 1. 2. 3. 4. 5. Number of Unique Prescribers in the quarter % of Doptelet referrals converting to patient shipment Reach and Frequency with our Target Audience & Target Institutions Average time (in days) for commercial payer approval Change in Channel inventory levels quarter over quarter 13
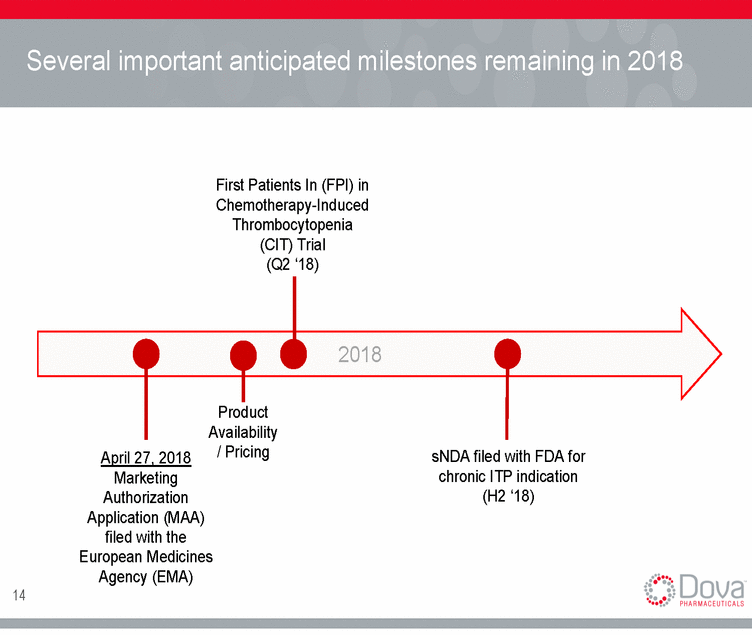
Several important anticipated milestones remaining in 2018 First Patients In (FPI) in Chemotherapy-Induced Thrombocytopenia (CIT) Trial (Q2 ‘18) 2018 Product Availability / Pricing sNDA filed with FDA for chronic ITP indication (H2 ‘18) April 27, 2018 Marketing Authorization Application (MAA) filed with the European Medicines Agency (EMA) 14