Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Armata Pharmaceuticals, Inc. | tv494260_ex99-1.htm |
| 8-K - FORM 8-K - Armata Pharmaceuticals, Inc. | tv494260_8k.htm |
Exhibit 99.2

Advancing bacteriophage therapeutics for patients with antibiotic - resistant infections Company Overview May 15, 2018 NYSE American: APHB

2 This presentation contains “forward - looking” statements that involve risks, uncertainties and assumptions. If the risks or uncer tainties materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by such for war d - looking statements. All statements other than statements of historical fact could be deemed forward - looking, including, but not limited to: the potential future of antibiotic resistance; the ability for bacteriophage therapies to disrupt and destroy biofilms an d r estore sensitivity to antibiotics; the planned development strategy, including the number of patients to be treated under expanded a cce ss; using data from expanded access cases to demonstrate the clinical utility of phage therapy; using data from expanded access c ase s to select indications and define treatment regimens for further development in 2018; presenting data to regulatory agencies and def ine studies required for registration in mid - 2018; the expected timing of additional clinical trials, including Phase II or registrational clinical trials; the drug product candidates to be supplied by AmpliPhi for clinical trials; bacteriophage technology being uniquely p osi tioned to address the global threat of antibiotic resistance; the protection of intellectual property; the activities to be performe d b y specific parties in connection with clinical trials or expanded access cases; the potential use of bacteriophages to treat bacterial i nfe ctions; research and development plans; the development of bacteriophage - based therapies; the ability to select combinations of phages t o formulate product candidates; the ability to manufacture product candidates; the safety and efficacy of product candidates; collaborations with third parties and the potential markets and market opportunities for product candidates; potential market gr owth; the expectation that existing cash resources will be sufficient to fund operations into the fourth quarter of 2018; and any s tat ements of assumptions underlying any of the items mentioned. These statements are based on estimates and information available to us at th e time of this presentation and are not guarantees of future performance. Actual results could differ materially from our curre nt expectations. You should not rely upon forward - looking statements as predictions of future events. Although we believe that the expectations reflected in the forward - looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward - looking statements will be achieved or occur. Moreover, we undertake no obligation to update publicly any forward - looking statements for any reason to conform these statements to actual results or to changes in our expectations except as required by law. We refer you to the documents that we file from time to time with the Securities and Exchange Commission (the “SEC”), includi ng our Annual Report on Form 10 - K, Quarterly Reports on Form 10 - Q and Current Reports on Form 8 - K. These documents, including the sections therein entitled “Risk Factors,” identify important factors that could cause the actual results to differ materially fr om those contained in forward - looking statements. Safe Harbor Statement

3 Novel therapies urgently needed to combat antimicrobial resistance AmpliPhi is leading development of bacteriophage therapeutics as novel, precisely targeted treatment modality for patients with serious, resistant bacterial infections • Pathogen - targeted mechanism of action that is differentiated from antibiotics • Kill bacteria by cell lysis, disrupt and destroy biofilm, restore sensitivity to antibiotics Two lead candidates target S. aureus ( AB - SA01) and P. aeruginosa (AB - PA01) • Pathogens on WHO Priority List • AB - SA01 successfully completed two Phase 1 studies • Received positive feedback from FDA in 2017 Announced January 2018: positive results from treatment of seven seriously ill patients, not responding to antibiotics, under Expanded Access Program (EAP) in 2017 • Serious or life - threatening infections: bacteremia, endocarditis, lung infections • Emergency IND (US FDA) or Special Access Scheme Category A (Australian TGA) • AB - SA01 and AB - PA01 well tolerated in all patients. 500+ doses administered IV or by inhalation • Treatment Success in 86% cases (physician’s assessment) • 28 - day all - cause mortality: 14%. (Mortality predicted by APACHE II scores: 46%) Plan for 2018: treat additional patients under EAP in 1H18, present data to FDA in mid - 2018 to define path to registration, and initiate Phase 2 or pivotal studies as early as 4Q18 AmpliPhi Biosciences Overview

4 “ The world is headed for a post - antibiotic era, in which common infections and minor injuries which have been treatable for decades can once again kill .” Dr. Keiji Fukuda, WHO’s Assistant Director - General for Health Security

5 Bacteriophages Provide Hope Against Superbugs

6 Naturally - occurring viruses • Evolved to infect and kill only bacteria • Target specific bacterial strains Most abundant and diverse organisms on Earth • Humans co - exist with phages • An abundant variety of phage types exist, capable of infecting and killing most, if not all, bacterial strains Used broadly in Europe and U.S. to treat bacterial infections prior to development of antibiotics • Efficacy broadly, yet anecdotally, demonstrated • Early 20 th century challenges predated modern biotech era • Understanding phage MoA • Characterization and potency • Manufacturing and purification Bacteriophages: Novel Precisely Targeted Antibacterials

7 Today’s Advances in Bacteriophage Therapeutics Enabled by Modern Biotechnology • Biologics manufacturing • Purification (e.g., endotoxin removal) • Sequencing Advances in phage development Enabling technologies • Select and optimize proprietary phage combinations • Maximize efficacy and host coverage • Minimize resistance The Journal of the American Medical Association, October 2017 Growing recognition by medical community ID Week Conference, October 2018

8 Bacteriophages As Precisely Targeted Therapeutics: Preclinical Data 6h 8h 24h Untreated Phage Tx Ciprofloxacin (200 mg/kg SC) Biofilm thickness Targeted and selective 1 Directly kill bacteria by cell lysis 2 Destroy biofilm 3 Restore sensitivity to antibiotics 4
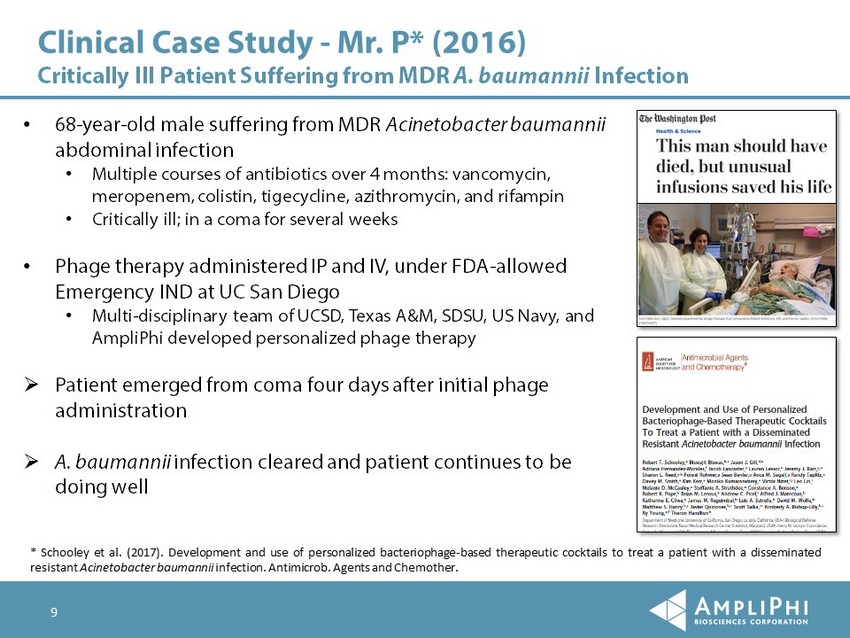
Clinical Case Study - Mr. P* (2016) Critically Ill Patient Suffering from MDR A. baumannii Infection • 68 - year - old male suffering from MDR Acinetobacter baumannii abdominal infection • Multiple courses of antibiotics over 4 months: vancomycin, meropenem, colistin , tigecycline, azithromycin, and rifampin • Critically ill; in a coma for several weeks • Phage therapy administered IP and IV, under FDA - allowed Emergency IND at UC San Diego • Multi - disciplinary team of UCSD, Texas A&M, SDSU, US Navy, and AmpliPhi developed personalized phage therapy » Patient emerged from coma four days after initial phage administration » A. baumannii infection cleared and patient continues to be doing well 9 * Schooley et al . ( 2017 ) . Development and use of personalized bacteriophage - based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection . Antimicrob . Agents and Chemother .

10 AmpliPhi’s Phage Library Targets Bacteria on WHO Priority Pathogens List Source: World Health Organization, 2017 Priority Pathogens List published in 2017 Priority 1: CRITICAL Acinetobacter baumannii , carbapenem - resistant Pseudomonas aeruginosa , carbapenem - resistant Enterbacteriaceae , carbapenem - resistant, 3 rd generation cephalosporin - resistant Enterococcus faecium, vancomycin - resistant Staphylococcus aureus, methicillin - resistant, vancomycin intermediate and resistant Helicobacter pylori, clarithromycin - resistant Campylobacter, fluoroquinolone - resistant Salmonella spp. , fluoroquinolone - resistant Neisseria gonorrhoeae , 3 rd generation cephalosporin - resistant, fluoroquinolone - resistant Priority 2: HIGH Priority 3: MEDIUM Streptococcus pneumoniae , penicillin - non - susceptible Haemophilus influenzae, ampicillin - resistant Shigella spp. , fluoroquinolone - resistant

AmpliPhi Development Pipeline Program Preclinical Phase 1/ Expanded Access Phase 2 Phase 3 AB - SA01 ( S. aureus) Intravenous: endocarditis, bacteremia, PJI Intrasinal : chronic rhinosinusitis Topical: various AB - PA01 ( P. aeruginosa) Intravenous: lung infections, cUTI , cIAI Inhaled: lung infections, CF Other ESKAPE pathogens 11 PJI, Prosthetic Joint Infection; cUTI , complicated Urinary Tract Infection; cIAI , complicated Intra - Abdominal Infection; CF, cystic fibrosis. ESKAPE pathogens are Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii , Pseudomonas aeruginosa, Enterobacter species.

12 In - House Facility Pioneering cGMP Manufacturing of Bacteriophage Therapeutics In - house CMC capabilities: • Process development • Bioanalytical • Fermentation • Purification • Aseptic fill • QC and QA Inspected by European regulatory authorities, most recently in 2018

13 Lead Product Candidates AB - SA01 and AB - PA01 AB - SA01 ( S. aureus ) • 3 lytic phages • 3 ¨ 10 9 PFU per dose • Coverage: ~96% of S. aureus strains, including MDR AB - PA01 ( P. aeruginosa ) • 4 lytic phages • 4 ¨ 10 9 PFU per dose • Coverage: ~80% of P. aeruginosa strains, including MDR PFU, plaque forming units AB - SA01 ( S. aureus ) AB - PA01 ( P. aeruginosa )

Positive Feedback from FDA in 2017 • FDA expressed support for phage therapy for patients with serious or life - threatening infections • Data from Expanded Access cases could inform approval pathway 14 “CBER acknowledged that phage therapy is an exciting approach to treatment of multidrug - resistant organisms and expressed a commitment to addressing the unique regulatory challenges that might arise during product development . ” “CBER stated that the clinical safety and effectiveness data collected during development, including from emergency case studies, could inform future discussions for clinical development and ultimately, the regulatory pathway to approval . ”

AmpliPhi Presented at FDA/NIH Bacteriophage Therapy Workshop in 2017 15 “…Though it was discovered… over a century ago, before the modern antibiotic era, bacteriophage may turn out to be important therapeutics in combatting antibiotic - resistant infections.” “… phage therapy appears to be non - toxic in humans and in animals, and phage have the benefit that their bacterial specificity allows sparing of the remainder of the beneficial microbiota.” “Though challenges clearly remain in the development of phage therapy for the prevention or treatment of infections in humans, their potential clinical utility seems quite promising in a time when other options seem much less so.” - Peter Marks, M.D., Ph.D. Director, FDA Center for Biologics Evaluation and Research

16 Expanded Access allows critically ill patients to receive experimental, unapproved therapies in attempt to save lives • Patients who are not responding to standard - of - care antibiotics • Emergency IND (US FDA) or Special Access Scheme (Australian TGA) AmpliPhi initiated Expanded Access Program for two lead therapeutic candidates AB - SA01 and AB - PA01 in May 2017 with objectives to: • Provide therapy for patients in dire need, demonstrate clinical utility of AB - SA01 and AB - PA01, and collect clinical and microbiological data • Refine treatment regimens and select indications for further development • Present data to FDA in mid - 2018 and define required registrational studies • Initiate Phase 2 or registrational studies for AB - SA01 and/or AB - PA01 as early as 4Q18 AmpliPhi’s Expanded Access Strategy Leverage Expanded Access Clinical Data to Pave Potential Path to Approval

17 Seven patients with serious or life - threatening infections, not responding to antibiotic therapy, treated with AB - SA01 or AB - PA01 in 2017 • Four patients with S. aureus infections treated with AB - SA01 • Three patients with P. aeruginosa infections treated with AB - PA01 • Emergency IND (US FDA) or Special Access Scheme Category A (Australian TGA) • Indications: bacteremia, endocarditis, prosthetic valve endocarditis, lung infection (cystic fibrosis), lung infection (post - transplant), and ventilator - associated pneumonia Bacteriophage treatment was well tolerated in all patients • 90 doses of AB - SA01 administered intravenously • 402 doses of AB - PA01 administered intravenously and 92 doses by nebulizer • No treatment related SAEs 86% Treatment Success (physician’s assessment) • Complete resolution or significant improvement of baseline signs and symptoms 28 - day all - cause mortality: 14% • Mortality predicted by APACHE II scores: 46% No bacterial resistance to AB - SA01/AB - PA01 detected during the course of bacteriophage treatment Expanded Access Interim Data Summary Data Announced January 2018

18 Expanded Access Interim Update: Safety and Tolerability ITT Population AB - SA01 (N=4) AB - PA01 (N=3) Total IV doses 90 402* Total inhaled doses - 92** Safety and tolerability Well tolerated in all patients No treatment - related SAEs Well tolerated in all patients No treatment - related SAEs * Includes 298 doses of AB - PA01 and 104 doses of AB - PA01 plus one additional bacteriophage ** Includes 56 doses of AB - PA01 and 36 doses of AB - PA01 plus one additional bacteriophage

19 Expanded Access Interim Update: Clinical Outcomes Clinical Outcome (Physician’s Assessment) (ITT Population) Value (N=7) Treatment Success 6 (86%) Improvement – Failure 1 ( 14%)* * The patient presented with septic shock, APACHE II score of 47 at baseline (predicted mortality risk 97%). Patient was not re sponding to best available antibiotic therapy, received 3 days of bacteriophage therapy, and died in surgery on Day 3. The patient’s dea th was deemed by treating physician as not related to treatment with bacteriophage. Physician’s Assessment: • Treatment Success: complete resolution or significant improvement of baseline signs and symptoms. • Improvement: clinically meaningful improvement of baseline signs and symptoms. • Failure: no resolution of baseline signs and symptoms, or death.

20 ISHLT 2018: Bacteriophage Treatment in a Lung Transplant Patient (AB - PA01)
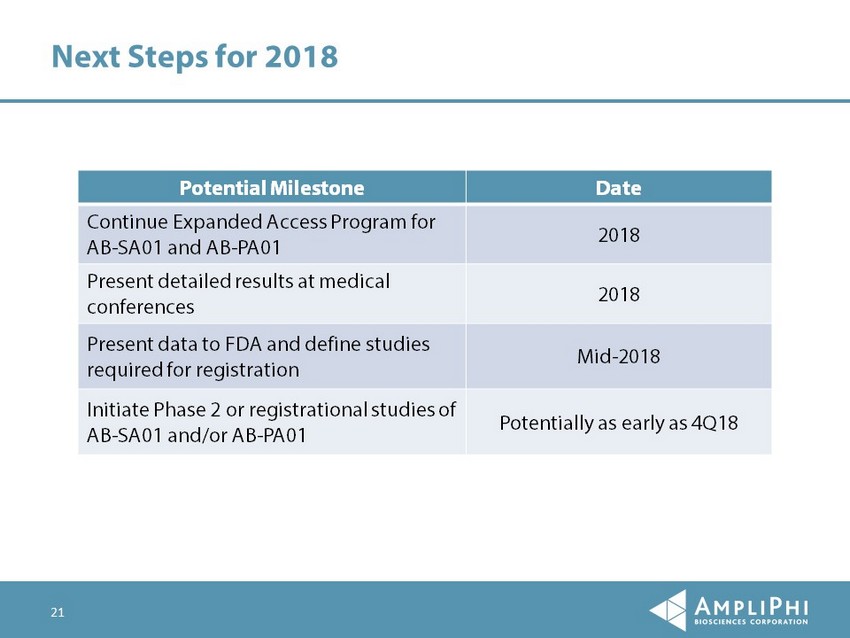
21 Next Steps for 2018 Potential Milestone Date Continue Expanded Access Program for AB - SA01 and AB - PA01 2018 Present detailed results at medical conferences 2018 Present data to FDA and define studies required for registration Mid - 2018 Initiate Phase 2 or registrational studies of AB - SA01 and/or AB - PA01 Potentially as early as 4Q18

22 Significant Market Opportunity 500,000+ U.S. Annual Incidence of Infections Targeted by AB - SA01 & AB - PA01 Indication S. aureus P. aeruginosa Bacteremia 150,000 50,000 Cardiac implant infections (LVAD and pacemaker) 1,000 200 CF lung infections 10,000 20,000 Complicated intra - abdominal infections ( cIAI ) * 140,000 Complicated urinary tract infections ( cUTI ) * 130,000 Endocarditis (native and prosthetic valve) 20,000 2,000 Hospital/ventilator - associated pneumonia (HAP/VAP) 100,000 50,000 Prosthetic joint infections (PJI) 10,000 - ~300,000 ~400,000 Source: AmpliPhi market research. (*) not an initial target population, generally well served by antibiotics.

23 Bacteriophages as Targeted Therapy: Infectious Diseases, Microbiome, Oncology, and Beyond Selectively remove bacterial populations Targeted therapy Precisely modulate microbiome Preserve commensal bacterial species
24 Experienced Management Team Management Paul Grint, M.D. Chief Executive Officer Igor Bilinsky, Ph.D. Chief Operating Officer Steve Martin Chief Financial Officer Alex Gaidamaka, Ph.D., D.V.M. VP CMC Sandra Morales, Ph.D. VP Research Carrie Langlais Furr, Ph.D. VP Regulatory & Project Management Board of Directors Jeremy Curnock Cook, Chair Louis Drapeau Wendy Johnson Mike Perry, D.V.M., Ph.D . Vijay Samant Paul Grint, M.D.

25 Advisors Robert T. Schooley, MD Professor of Medicine, Division of Infectious Diseases UCSD Editor - in - Chief, Clinical Infectious Diseases Timothy K. Lu, MD, PhD Associate Professor of Biological Engineering and Electrical Engineering and Computer Science MIT

26 • Completed two common stock only fund - raises under the existing S - 3 shelf in January and March of 2018. Raised approximately $7M of gross proceeds • Cash resources expected to be sufficient to fund operations into the fourth quarter of 2018 • 16.5M common shares outstanding and 25.9M fully diluted as of May 15, 2018 * • Received $2.0M in cash in September 2017 from the Australian Government as a tax rebate based on R&D activities performed in Australia in 2016 • Publicly - traded NYSE American exchange – APHB Funding and Capitalization *Share amounts outstanding include common stock only. Fully diluted includes outstanding warrants and stock options. See the mos t recent Quarterly Report on Form 10 - Q as filed with the SEC

27 Novel therapies urgently needed to combat antimicrobial resistance AmpliPhi is leading development of bacteriophage therapeutics as novel, precisely targeted treatment modality for patients with serious, resistant bacterial infections • Pathogen - targeted mechanism of action that is differentiated from antibiotics Two lead candidates target S. aureus ( AB - SA01) and P. aeruginosa (AB - PA01) • AB - SA01 successfully completed two Phase 1 studies • Received positive feedback from FDA in 2017 Announced January 2018: positive results from treatment of seven seriously ill patients, not responding to antibiotics, under Expanded Access Program (EAP) in 2017 • AB - SA01 and AB - PA01 well tolerated in all patients. 500+ doses administered IV or by inhalation • Treatment Success in 86% cases (physician’s assessment) • 28 - day all - cause mortality: 14%. (Mortality predicted by APACHE II scores: 46%) Plan to treat additional patients under EAP in 1H18, present data to FDA in mid - 2018 to define path to registration, and initiate Phase 2 or pivotal studies as early as 4Q18 • Seek most rapid path to approval AmpliPhi Biosciences Overview
