Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - Cardiff Oncology, Inc. | d584926dex231.htm |
Table of Contents
As filed with the Securities and Exchange Commission on May 9, 2018
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER THE
SECURITIES ACT OF 1933
TROVAGENE, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 2836 | 27-2004382 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
11055 Flintkote Avenue
San Diego, CA 92121
(858) 952-7570
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
William J. Welch
Chief Executive Officer
Trovagene, Inc.
11055 Flintkote Avenue
San Diego, CA 92121
(858) 952-7570
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Jeffrey J. Fessler, Esq. Nazia J. Khan, Esq. Sheppard, Mullin, Richter & Hampton LLP 30 Rockefeller Plaza, 39th Floor New York, New York 10112 Tel: (212) 653-8700 Fax: (212) 653-8701 |
Gregory Sichenzia, Esq. Marcelle Balcombe, Esq. Sichenzia Ross Ference Kesner LLP 1185 Avenue of the Americas New York, New York 10036 Tel: (212) 930-9700 Fax: (212) 930-9725 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☐ (Do not check if a smaller reporting company) | Smaller reporting company | ☒ | |||
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
|
| ||||
| Title of Each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price(1)(2) |
Amount of Registration Fee | ||
| Class A Units consisting of (3): |
$15,000,000 | $1,868 | ||
| (i) Common Stock, par value $0.0001 per share |
||||
| (ii) Warrants to purchase Common Stock (4) |
||||
| Class B Units consisting of (3): |
$15,000,000 | $1,868 | ||
| (i) Series B Convertible Preferred Stock, par value $0.0001 per share |
||||
| (ii) Warrants to purchase Common Stock (4) |
||||
| (iii) Common Stock issuable upon conversion of the Series B Convertible Preferred Stock (4) |
||||
| Common Stock issuable upon exercise of warrants (3) |
$30,000,000 | $3,736 | ||
|
Total |
$60,000,000 | $7,472 | ||
|
| ||||
|
| ||||
| (1) | Estimated solely for the purpose of calculating the amount of the registration fee pursuant to Rule 457(o) of the Securities Act of 1933, as amended. Includes shares and warrants to be sold upon exercise of the underwriters’ option to purchase additional shares and/or warrants. See “Underwriting.” |
| (2) | Pursuant to Rule 416, the securities being registered hereunder include such indeterminate number of additional securities as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions. |
| (3) | The proposed maximum aggregate offering price of the Class A Units proposed to be sold in the offering will be reduced on a dollar-for-dollar basis based on the offering price of any Class B Units offered and sold in the offering, and as such the proposed maximum aggregate offering price of the Class A Units and Class B Units (including the common stock issuable upon exercise of the warrants included in the Class B Units), if any, is $15,000,000. |
| (4) | No fee pursuant to Rule 457(i) under the Securities Act of 1933, as amended. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement relating to these securities filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED MAY 9, 2018 |
[ ] Class A Units Consisting of Common Stock and Warrants and
[ ] Class B Units Consisting of Series B Convertible Preferred Stock and Warrants (and [ ] shares of common stock underlying shares of Series B Convertible Preferred Stock and [ ] shares of common stock underlying Warrants)

Trovagene, Inc.
We are offering [ ] Class A Units consisting of one share of our common stock and warrants to purchase one share of our common stock, at an exercise price equal to % of the public offering price of the Class A Units per share of common stock, which warrants will be exercisable upon issuance and will expire years from the date of issuance. The shares of common stock and warrants that are part of a Class A Unit are immediately separable and will be issued separately in this offering.
We are also offering to those purchasers, if any, whose purchase of Class A Units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding common stock immediately following the consummation of this offering, the opportunity, in lieu of purchasing Class A Units, to purchase Class B Units. Each Class B Unit will consist of one share of our newly designated Series B Convertible Preferred Stock (“Series B Preferred”) with a stated value of $1,000 and convertible into shares of our common stock at the public offering price of the Class A Units, together with the equivalent number of warrants as would have been issued to such purchaser of Class B Units if they had purchased Class A Units. For each Class B Unit we sell, the number of Class A Units we are offering will be decreased on a one-for-one basis. Because we will issue a common stock purchase warrant as part of each Class A Unit or Class B Unit, the number of warrants sold in this offering will not change as a result of a change in the mix of the Class A Units and Class B Units sold. The shares of Series B Preferred and warrants that are part of a Class B Unit are immediately separable and will be issued separately in this offering. We are also offering the shares of common stock issuable upon exercise of the warrants and conversion of the Series B Preferred.
The number of shares of our common stock outstanding after this offering will fluctuate depending on how many Class B Units are sold in this offering and whether and to what extent holders of Series B Preferred shares convert their shares to common stock.
Our common stock is listed on the Nasdaq Capital Market under the symbol “TROV.” On May 8, 2018, the last reported sale price of our common stock on the Nasdaq Capital Market was $0.28.
The final public offering price per Class A Unit will be determined through negotiation between us and the underwriter in this offering and will take into account the recent market price of our common stock, the general condition of the securities market at the time of this offering, the history of, and the prospects for, the industry in which we compete, and our past and present operations and our prospects for future revenues. The recent market price used throughout this prospectus may not be indicative of the public offering price per Class A Unit. The public offering price of the Class B Units will be $1,000 per unit.
Assuming an offering price of $[ ] per Class A Unit, the Series B Preferred included in the Class B Units will be convertible into an aggregate total of [ ] shares of common stock and the warrants included in the Class B Units will be exercisable for an aggregate total of [ ] shares of common stock.
There is no established trading market for the warrants or the Series B Preferred, and we do not expect an active trading market to develop. We do not intend to list the warrants or the Series B Preferred on any securities exchange or other trading market. Without an active trading market, the liquidity of the warrants and the Series B Preferred will be limited.
Investing in our securities involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” beginning on page 10 of this prospectus, and under similar headings in any amendments or supplements to this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| Per Class A Unit |
Per Class B Unit |
Total | ||||||||||
| Public offering price |
$ | $ | $ | |||||||||
| Underwriting discounts and commissions(1) |
$ | $ | $ | |||||||||
| Proceeds to us, before expenses |
$ | $ | $ | |||||||||
| (1) | Does not include a non-accountable expense allowance equal to [ ]% of the gross proceeds (excluding any proceeds from exercise of the over-allotment option) of this offering payable to ThinkEquity, a division of Fordham Financial Management, Inc. (“ThinkEquity”), the representative of the underwriters. See “Underwriting” for a description of compensation payable to the underwriters. |
We have granted a 45-day option to the underwriters to purchase a maximum of [ ] additional shares of common stock ([ ]% of the shares of common stock included in the Class A Units and Class B Units (on an as-converted basis with respect to any shares of Series B Preferred) sold in this offering) and/or warrants to purchase a maximum of [ ] shares of common stock ([ ]% of the warrants included as part of the Units sold in this offering), solely to cover over-allotments, if any.
The underwriters expect to deliver the securities to purchasers in the offering on or about , 2018.
ThinkEquity
A division of Fordham Financial Management, Inc.
, 2018
Table of Contents
|
|
We are a clinical-stage oncology therapeutics company. Our primary focus is to develop oncology therapeutics for the treatment of hematologic and solid tumor cancers for improved cancer care utilizing our technology in tumor genomics. Our lead drug candidate, PCM-075, a selective Polo-like Kinase 1 (PLK1), is initially being developed to treat Acute Myeloid Leukemia (AML) and metastatic Castration-Resistant Prostate Cancer (mCRPC). |
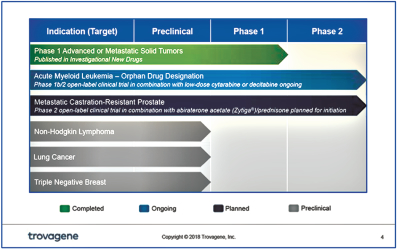
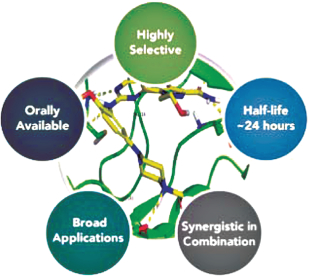
Table of Contents
| PAGE | ||||
| 1 | ||||
| 2 | ||||
| 10 | ||||
| 38 | ||||
| 39 | ||||
| 39 | ||||
| 41 | ||||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
42 | |||
| 58 | ||||
| 74 | ||||
| 81 | ||||
| Certain Relationships and Related Transactions and Director Independence |
86 | |||
| Security Ownership of Certain Beneficial Owners and Management |
88 | |||
| 90 | ||||
| 94 | ||||
| 102 | ||||
| 102 | ||||
| 102 | ||||
| F-1 | ||||
You should rely only on the information contained in this prospectus. We have not, and the underwriter has not, authorized anyone to provide you with any information other than that contained or incorporated by reference in this prospectus or in any applicable prospectus supplement or free writing prospectus prepared by or on behalf of us to which we have referred you. We are offering to sell, and seeking offers to buy, the securities covered hereby only in jurisdictions where offers and sales are permitted. You should not assume that the information contained in this prospectus or any prospectus supplement or free writing prospectus is accurate as of any date other than the date on the front cover of those documents, or that the information contained in any document incorporated by reference is accurate as of any date other than the date of the document incorporated by reference, regardless of the time of delivery of this prospectus or any sale of a security. Our business, financial condition, results of operations and prospects may have changed since those dates. We are not, and the underwriter is not, making an offer of these securities in any jurisdiction where the offer is not permitted.
For investors outside the United States: We have not, and the underwriter has not, taken any action that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities covered hereby the distribution of this prospectus outside the United States.
We further note that the representations, warranties and covenants made by us in any agreement that is incorporated by reference or filed as an exhibit to the registration statement of which this prospectus is a part were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
Information contained in, and that can be accessed through, our web site www.trovagene.com shall not be deemed to be part of this prospectus or incorporated herein by reference and should not be relied upon by any prospective investors for the purposes of determining whether to purchase the shares offered hereunder.
Table of Contents
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference herein contain, in addition to historical information, certain forward-looking statements. within the meaning of Section 27A of the Securities Act or 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended, that include information relating to future events, future financial performance, strategies, expectations, competitive environment, regulation and availability of resources. Such forward-looking statements include those that express plans, anticipation, intent, contingency, goals, targets or future development and/or otherwise are not statements of historical fact. These forward-looking statements are based on our current expectations and projections about future events and they are subject to risks and uncertainties known and unknown that could cause actual results and developments to differ materially from those expressed or implied in such statements.
In some cases, you can identify forward-looking statements by terminology, such as “expects,” “anticipates,” “intends,” “estimates,” “plans,” “believes,” “seeks,” “may,” “should”, “could” or the negative of such terms or other similar expressions. Accordingly, these statements involve estimates, assumptions and uncertainties that could cause actual results to differ materially from those expressed in them. Any forward-looking statements are qualified in their entirety by reference to the factors discussed throughout this prospectus or incorporated herein by reference.
You should read this prospectus and the documents we have incorporated by reference or filed as exhibits to the registration statement, of which this prospectus is part, completely and with the understanding that our actual future results may be materially different from what we expect. You should not assume that the information contained in this prospectus or any prospectus supplement or free writing prospectus is accurate as of any date other than the date on the front cover of those documents, or that the information contained in any document incorporated by reference is accurate as of any date other than the date of the document incorporated by reference, regardless of the time of delivery of this prospectus or any sale of a security.
Risks, uncertainties and other factors that may cause our actual results, performance or achievements to be different from those expressed or implied in our written or oral forward-looking statements may be found in this prospectus under the heading “Risk Factors” and in our Annual Report on Form 10-K for the year ended December 31, 2017 under the headings “Risk Factors” and “Business,” as updated in our Quarterly Report(s) on Form 10-Q.
Forward-looking statements speak only as of the date they are made. You should not put undue reliance on any forward-looking statements. We assume no obligation to update forward-looking statements to reflect actual results, changes in assumptions or changes in other factors affecting forward-looking information, except to the extent required by applicable securities laws. If we do update one or more forward-looking statements, no inference should be drawn that we will make additional updates with respect to those or other forward-looking statements.
New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. We qualify all of the information presented in this prospectus and incorporated herein by reference, and particularly our forward-looking statements, by these cautionary statements.
1
Table of Contents
The following summary highlights certain of the information contained elsewhere in or incorporated by reference into this prospectus. Because this is only a summary, however, it does not contain all the information you should consider before investing in our securities and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information included elsewhere in or incorporated by reference into this prospectus. Before you make an investment decision, you should read this entire prospectus carefully, including the risks of investing in our securities discussed under the section of this prospectus entitled “Risk Factors” and similar headings in the other documents that are incorporated by reference into this prospectus. You should also carefully read the information incorporated by reference into this prospectus, including our financial statements, and the exhibits to the registration statement of which this prospectus is a part.
Unless the context otherwise requires, references to “we,” “our,” “us,” “Trovagene” or the “Company” in this prospectus mean Trovagene, Inc. on a consolidated basis with its wholly-owned subsidiary, Trovagene, Srl, as applicable.
Overview
We are a clinical-stage oncology therapeutics company. Our primary focus is to develop oncology therapeutics for the treatment of hematologic and solid tumor cancers for improved cancer care utilizing our technology in tumor genomics.
On March 15, 2017, we announced that we licensed PCM-075, a PLK1 inhibitor, from Nerviano Medical Sciences S.r.l. (“Nerviano”) pursuant to a license agreement with Nerviano dated March 13, 2017. PCM-075 was developed to have high selectivity to PLK1 (at low nanomolar IC50 levels), to be administered orally, and to have a relatively short drug half-life of approximately 24 hours compared to other pan Polo-like inhibitors. A safety study of PCM-075 has been successfully completed in patients with advanced metastatic solid tumors and published in 2017 in Investigational New Drugs. We currently are enrolling a Phase 1b/2 open-label clinical trial of PCM-075 in combination with standard-of-care chemotherapy in patients with acute myeloid leukemia (“AML”). The Phase 1b/2 clinical trial is led by Hematologist Jorge Eduardo Cortes, M.D., Deputy Department Chair, Department of Leukemia, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center. In addition, we are working with Dr. David Einstein at the Genitourinary Oncology Program at Beth Israel Deaconess Medical Center and Harvard Medical School as the principal investigator on a Phase 2 open-label clinical trial of PCM-075 in combination with abiraterone acetate (Zytiga®) and prednisone in patients with metastatic Castration-Resistant Prostate Cancer (“mCRPC”).
Our intellectual property and proprietary technology enables us to analyze circulating tumor DNA (“ctDNA”) and clinically actionable markers to identify patients most likely to respond to specific cancer therapies. We plan to continue to vertically integrate our tumor genomics technology with the development of targeted cancer therapeutics.
We believe PCM-075 is the only PLK1 selective adenosine triphosphate (“ATP”) competitive inhibitor administered orally, with apparent antitumor activity in different preclinical models, currently in clinical trials. Polo-like kinase family consists of 5 members (PLK1-PLK5) and they are involved in multiple functions in cell division, including the regulation of centrosome maturation, checkpoint recovery, spindle assembly, cytokinesis, apoptosis and many others. PLK1 is essential for the maintenance of genomic stability during cell division (“mitosis”). The overexpression of PLK1 can lead to immature cell division followed by aneuploidy and cell death, a hallmark of cancer. PLK1 is over-expressed in a wide variety of hematologic and solid tumor malignancies including acute myeloid leukemia, prostate, lung, breast, ovarian and adrenocortical carcinoma. In addition, several studies have shown that over-expression of PLK1 is associate with poor prognosis.
2
Table of Contents
Studies have shown that inhibition of polo-like-kinases can lead to tumor cell death, including a Phase 2 study in AML where response rates with a different PLK inhibitor were up to 31% were observed when used in conjunction with a standard therapy for AML (low-dose cytarabine-LDAC) versus treatment with LDAC alone with a 13.3% response rate. We believe the more selective nature of PCM-075 to PLK1, its 24-hour half-life and oral bioavailability, as well as the reversibility of its on-target hematological toxicities may prove useful in addressing clinical therapeutic needs across a variety of cancers.
PCM-075 has been tested in vivo in different xenograft and transgenic models suggesting tumor growth inhibition or tumor regression when used in combination with other therapies. PCM-075 has been tested for antiproliferative activity on a panel of 148 tumor cell lines and appeared highly active with an IC50 (a measure concentration for 50% target inhibition) below 100 nM in 75 cell lines and IC50 values below 1 uM in 133 out of 148 cell lines. PCM-075 also appears active in cells expressing multi-drug resistant (“MDR”) transporter proteins and we believe PCM-075’s apparent ability to overcome the MDR transporter resistance mechanism in cancer cells could prove useful in broader drug combination applications.
In preclinical studies, synergy (interaction of discrete drugs such that the total effect is greater than the sum of the individual effects) has been demonstrated with PCM-075 when used in combination with more than ten different chemotherapeutics, including cisplatin, cytarabine, doxorubicin, gemcitabine and paclitaxel, as well as targeted therapies, such as abiraterone acetate (Zytiga®), histone deacetylase (“HDAC”) inhibitors, such as belinostat (Beleodaq®), Quizartinib (AC220), a development stage FLT3 inhibitor, and bortezomib (Velcade®). These therapeutics are used clinically for the treatment of many hematologic and solid tumor cancers, including AML, Non-Hodgkin Lymphoma (“NHL”), mCRPC, Adrenocortical Carcinoma (“ACC”), and Triple Negative Breast Cancer (“TNBC”).
On August 16, 2017, we announced results of preclinical research indicating potential synergy of PCM-075 with an investigational FLT3 Inhibitor, Quizartinib by Daiichi Sankyo, in FLT3 mutant xenograft mouse models. This synergy assessment study was conducted for us by a third-party contract research group. Approximately one third of AML patients harbor FLT3-mutated blood cancer cells. The U.S. Food and Drug Administration (“FDA”) recently approved Rydapt® (midostaurin) by Novartis for the treatment of newly diagnosed adult patients with AML that are FLT3 mutation-positive in combination with cytarabine and daunorubicin induction and cytarabine consolidation chemotherapy. There are three FLT3 inhibitors in ongoing phase 3 trials, including Quizartinib. We believe that a combination of PCM-075 with a FLT3 inhibitor for AML patients with a FLT3 mutation could extend treatment response and possibly slow or reduce resistance to FLT3 activity.
On August 21, 2017, we announced results of preclinical research indicating potential synergy of PCM-075 with a HDAC inhibitor in NHL cell lines. This synergy assessment study was conducted by Dr. Steven Grant, Associate Director for Translational Research and co-Leader, Developmental Therapeutics Program, Massey Cancer Center. Patients with relapsed or refractory NHL, such as cutaneous T cell lymphoma and peripheral T cell lymphoma, may be prescribed approved HDAC inhibitors and we believe this continues to be an area of unmet medical need. Dr. Grant’s data appeared to indicate that the combination of PCM-075 with Beleodaq® (belinostat), a HDAC inhibitor indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma, reduced cancer cells by up to 80% in two different forms of NHL (aggressive double-hit B-cell lymphoma and mantle cell lymphoma) cell lines.
On October 11, 2017, we entered into a Patent Option Agreement with Massachusetts Institute of Technology (“MIT”) for the exclusive rights to negotiate a royalty-bearing, limited-term exclusivity license to practice world-wide patent rights to US Patent 9,566,280, subject to the rights of MIT (research, testing, and educational purposes), Ortho McNeil Pharmaceuticals-Janssen Pharmaceuticals and its Affiliates (internal research and pre-clinical drug development purposes including some laboratory research) and the federal government (government-funded inventions claimed in any patent rights and to exercise march in rights). This
3
Table of Contents
patent is generally directed to combination therapies including an antiandrogen or androgen antagonist and polo-like kinase inhibitor for the treatment of cancer. The Patent Option Agreement expires one-year from the effective date and includes other requirements to maintain the option period.
On October 18, 2017, we announced results of preclinical research indicating potential synergy of PCM-075 with abiraterone acetate in C4-2 prostate cancer cells. This synergy assessment study was conducted by Dr. Michael Yaffe, David H. Koch Professor of Biology and Biological Engineering at MIT. The results appeared to indicate that the combination of PCM-075 with Zytiga® (abiraterone acetate) decreased cell viability in mCRPC tumor cells and the apparent synergy observed was greater than the expected effect of combining the two drugs. Zytiga is indicated for use in combination with prednisone for the treatment of patients with mCRPC who have received prior chemotherapy containing docetaxel. We believe there is an unmet medical need to improve on the resistance to hormone therapy and extend the benefit of response to Zytiga® for mCRPC patients.
On December 7, 2017, we announced results of preclinical research showing the sensitivity of triple negative breast cancer (“TNBC”) cell lines to PCM-075, data featured as a Poster Presentation at the 40th San Antonia Breast Cancer Symposium (SABCS). This synergy assessment study was conducted by Dr. Jesse Patterson and Dr. Michael Yaffe, at MIT. The results appeared to indicate that TNBC cell lines are 20-fold more sensitive to PCM-075 than estrogen receptor positive (ER+) breast cancer cell lines.
PCM-075 Phase 1 Safety Study in Solid Tumors
A Phase 1 safety study of PCM-075 was completed in patients with advanced metastatic solid tumor cancers with data published in July 2017 in the peer-reviewed journal Investigational New Drugs. Dr. Glen Weiss, Medical Oncologist at Goodyear, AZ and affiliated with Cancer Treatment Centers of America at Western Regional Medical Center, was the principal investigator and first author of the publication, entitled “Phase 1 Dose-Escalation Study of NMS-1286937, an Orally Available Polo-like Kinase 1 Inhibitor, in Patients with Advanced or Metastatic Solid Tumors.” This study evaluated first-cycle dose limiting toxicities and related maximum tolerated dose with data indicating a manageable safety profile for PCM-075 (formerly known as NMS-1286937) for the treatment of advanced or metastatic solid tumors, with transient adverse events that were likely related to the drug’s mechanism of action. The authors believe that data from preclinical work, coupled with the results of the Phase 1 trial, suggest that PCM-075 could become a new therapeutic option for the treatment of solid tumor and hematologic cancers.
In this trial, PCM-075 was administered orally, once daily for five consecutive days, every three weeks, to evaluate first-cycle dose-limiting toxicities and related maximum tolerated dose in adult subjects with advanced ormetastatic solid tumors. The study was also intended to evaluate PCM-075’s pharmacokinetic profile in plasma, its anti-tumor activity, and its ability to modulate intracellular targets in biopsied tissue. The study identified thrombocytopenia and neutropenia as the primary toxicities, which is consistent with the expected mechanism of action of PCM-075 and from results of preclinical studies. These hematologic toxicities were reversible, with recovery usually occurring within 3 weeks. No gastrointestinal disorders, mucositis, or alopecia was observed, confirming that bone marrow cells are the most sensitive to PCM-075 inhibition with the applied dosing schedule.
We are utilizing the existing Investigational New Drug (“IND”) application to develop PCM-075 in solid tumors as part of our clinical development expansion plans, with our initial focus in mCRPC.
PCM-075 Phase 2 Study in metastatic Castration-Resistant Prostate Cancer
On December 14, 2017, we announced the submission of our Phase 2 protocol of PCM-075 in combination with abiraterone acetate (Zytiga® - Johnson & Johnson) for the treatment of mCRPC, and our active solid tumor
4
Table of Contents
IND to the FDA. In this multi-center, open-label, Phase 2 trial, PCM-075 in combination with the standard dose of abiraterone acetate and prednisone, all administered orally, will be evaluated for safety and efficacy. The primary efficacy endpoint is the proportion of patients achieving disease control after 12 weeks of study treatment, as defined by lack of Prostate Specific Antigen (“PSA”) progression in patients who are showing signs of early progressive disease (rise in PSA but minimally symptomatic or asymptomatic) while currently receiving androgen deprivation therapy, abiraterone acetate and prednisone.
On January 24, 2018, we announced plans for our Phase 2 clinical trial evaluating the combination of PCM-075 and abiraterone acetate (Zytiga®) in patients with mCRPC. We plan to have 3 clinical sites for the Phase 2 study, with Beth Israel Deaconess Medical Center in Boston Massachusetts as the principal site. Dr. David Einstein at the Genitourinary Oncology Program at Beth Israel Deaconess Medical Center and Harvard Medical School is the principal investigator for the Phase 2 mCRPC trial.
PCM-075 Phase 1b/2 Study in Acute Myeloid Leukemia
In June, 2017, we announced the submission of our IND application and our Phase 1b/2 protocol of PCM-075 in combination with standard-of-care chemotherapy for the treatment of AML to the FDA. In July, 2017, we received notification from the FDA that our Phase 1b/2 clinical trial of PCM-075 in patients with AML “may proceed”. On October 9, 2017, we announced that the FDA granted Orphan Drug Designation to PCM-075 for the treatment of AML. We initiated our Phase 1b/2 AML trial in November, 2017.
The Phase 1b/2 is an open-label trial to evaluate the safety and anti-leukemic activity of PCM-075 in combination with standard-of-care chemotherapy in patients with AML. Phase 1b is a dose escalation trial to evaluate the safety, tolerability, dose and scheduling of PCM-075, and to determine a recommended clinical treatment dose for the Phase 2 continuation trial.
Pharmacokinetics of PCM-075 and correlative biomarker activity will be assessed prior to the initiation of Phase 2. The Phase 2 continuation trial is open-label with administration of the recommended PCM-075 clinical dose in combination with standard-of-care chemotherapy to further evaluate safety and assess preliminary efficacy. Doses of PCM-075 will be administered orally each day for five consecutive days in a 28-day cycle in both Phase 1b and Phase 2.
We announced in February 2018 that the first patient has completed the first cycle of dosing with PCM-075 in combination with low-dose cytarabine (“LDAC”) in our Phase 1b/2 multicenter trial of patients with AML. We currently have 8 sites activated and able to recruit, screen and enroll patients. We plan to have up to 10 clinical sites activated for the Phase 1b/2 trial. This trial is being led by Hematologist Jorge Cortes, M.D., Deputy Department Chair, Department of Leukemia, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center.
We announced in April 2018 the presentation of pharmacodynamics and biomarker data from the first patient to complete a treatment cycle of PCM-075 in combination with standard-of-care chemotherapy. We also announced that the combination regimen of PCM-075 plus low-dose cytarabine (“LDAC”) appeared to be well tolerated and that this patient went on to receive a second cycle of treatment. At this time, we have enrolled a total of three patients with the first two patients in the initial cohort at 12mg/m2 oral, daily dose of PCM-075 (Days 1-5 in a 28-day cycle) in combination with LDAC having successfully completed cycle 1 of treatment. The third patient is currently in cycle 1 of treatment. We also enrolled a total of three patients, with the first two patients in the initial cohort at 12 mg/m2 oral, daily dose of PCM-075 (Days 1-5 in a 28-day cycle) in combination with decitabine, having successfully completed cycle 1 of treatment. One patient in the decitabine arm was removed from the trial prior to the end of the 28-day cycle due to unrelated disease progression and will be replaced to complete the initial dosing cohort. The PCM-075 dose will be escalated in the Phase 1b segment of the ongoing trial until a maximum tolerated dose (MTD)/recommended Phase 2 dose (“RP2D”) is achieved.
5
Table of Contents
Company Information
We were incorporated in the State of Florida on April 26, 2002. On July 2, 2004, we acquired Xenomics, a California corporation, which was in business to develop and commercialize urine-based molecular diagnostics technology. In 2007, we changed our fiscal year end from January 31 to December 31 and in January 2010, we re-domesticated our state of incorporation from Florida to Delaware and our name was changed to Trovagene, Inc. We have trademarks for the name TROVAGENE, TROVAGENE PRECISION CANCER MONITORING and TROVAGENE TRANSRENAL MOLECULAR DIAGNOSTICS. Our principal executive offices are located at 11055 Flintkote Avenue, San Diego, CA 92121, and our telephone number is 858-952-7570. Our website address is www.trovagene.com. The information on our website is not part of this prospectus. We have included our website address as a factual reference and do not intend it to be an active link to our website.
6
Table of Contents
| Issuer |
Trovagene, Inc. |
| Class A Units offered |
[ ] Class A Units with each Class A Unit consisting of one share of our common stock and a warrant to purchase shares of our common stock at an exercise price equal to % of the public offering price of the Class A Units. The Class A Units will not be certificated and the shares of common stock and warrants that are part of such units will be immediately separable and will be issued separately in this offering. |
| Public offering price per Class A Unit |
$ per Class A Unit. |
| Class B Units offered |
[ ]Class B Units are also being offered to those purchasers, if any, whose purchase of Class A Units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding common stock immediately following the consummation of this offering. Each Class B Unit will consist of one share of our Series B Preferred, with a stated value of $1,000 and convertible into shares of our common stock, at the public offering price of the Class A Units, together with the equivalent number of warrants as would have been issued to such purchaser if they had purchased Class A Units. For each Class B Unit we sell, the number of Class A Units we are offering will be decreased on a one-for-one basis. Because we will issue a common stock purchase warrant as part of each Class A Unit or Class B Unit, the number of warrants sold in this offering will not change as a result of a change in the mix of the Class A Units and Class B Units sold. The Class B Units will not be certificated and the shares of Series B Preferred and warrants that are part of such units are immediately separable and will be issued separately in this offering |
| Public offering price per Class B Unit Warrants |
$1,000 per Class B Unit. |
| Each warrant included in the Units will have an exercise price equal to ___% of the public offering price of the Class A Units per share of common stock, will be exercisable upon issuance, and will expire ___ years from the date of issuance. |
| Over-allotment option |
We have granted a 45-day option to the underwriters to purchase a maximum of [ ] additional shares of common stock ([ ]% of the shares of common stock included in the Class A Units and Class B Units (on an as-converted basis with respect to any shares of Series B Preferred) sold in this offering) and/or warrants to purchase a maximum of [ ] shares of common stock ([ ]% of the warrants included as part of the Units sold in this offering), solely to cover over-allotments, if any. |
7
Table of Contents
| Common stock to be outstanding immediately after this offering |
[ ] shares, or [ ] shares if the underwriter exercises in full its option to purchase additional shares of common stock (on an as-converted to common stock basis with respect to any shares of Series B Preferred sold). |
| Series B Convertible Preferred Stock |
The Series B Preferred will be convertible into shares of our common stock at any time at the option of the holder, at a conversion price equal to the public offering price of the Class A Units. See “Description of Securities We Are Offering” for a discussion of the terms of the Series B Preferred. |
| Use of proceeds |
We intend to use the net proceeds from this offering for general corporate purposes, including working capital. See “Use of Proceeds” on page [ ]. |
| Risk factors |
This investment involves a high degree of risk. You should read the description of risks set forth under “Risk Factors” beginning on page [ ] of this prospectus for a discussion of factors to consider before deciding to purchase our securities. |
| Nasdaq Capital Market Trading Symbol of Common Stock |
“TROV” |
| There is no established public trading market for the warrants or Series B Preferred, and we do not expect an active trading market to develop. We do not intend to list the warrants or the Series B Preferred on any securities exchange or other trading market. Without an active trading market, the liquidity of the warrants and the Series B Preferred will be limited. |
| Lock-up |
We and our directors and executive officers have agreed with the underwriters not to offer for sale, issue, sell, contract to sell, pledge or otherwise dispose of any of our common stock or securities convertible into common stock for a period of 180 days commencing on the date of this prospectus in the case of our directors and executive officers and for a period of 90 days commencing on the date of this prospectus in case of us. See “Underwriting” beginning on page [ ]. |
| Registered Securities |
This prospectus also relates to the offering of the shares issuable upon conversion of the Series B Preferred and upon exercise of the warrants included in the Units. |
The number of shares of common stock shown above to be outstanding after this offering is based on 59,378,162 shares outstanding as of April 30, 2018, and excludes as of that date:
| • | 7,560,731 shares of our common stock issuable upon exercise of outstanding options at a weighted average price of $2.49 per share; |
| • | 371,025 shares of our common stock issuable upon vesting of restricted stock units; |
8
Table of Contents
| • | 17,873,853 shares of our common stock issuable upon exercise of outstanding warrants with a weighted-average exercise price of $1.11 per share; |
| • | 63,125 shares of our common stock issuable upon conversion of outstanding shares of Series A Convertible Preferred Stock; |
| • | 363,857 shares of our common stock that are reserved for equity awards that may be granted under our equity incentive plans; and |
| • | [ ] shares of our common stock underlying the warrants included in the Units. |
Unless otherwise indicated, all information in this prospectus assumes no exercise by the underwriters of their option to purchase additional shares of common stock and/or warrants to cover over-allotments, if any.
9
Table of Contents
Any investment in our securities involves a high degree of risk. Before deciding whether to purchase our securities, investors should carefully consider the risks described below together with the “Risk Factors” described in our Annual Report on Form 10-K for the year ended December 31, 2017 and any updates described in our Quarterly Reports on Form 10-Q, all of which are incorporated herein by reference, as may be amended, supplemented or superseded from time to time by other reports we file with the Securities Exchange Commission (“SEC”) as well as any risks and uncertainties described in any applicable prospectus supplement. Our business, financial condition, operating results and prospects are subject to the following material risks as well as those material risks incorporated by reference. Additional risks and uncertainties not presently foreseeable to us may also impair our business operations. Our business, financial condition or operating results could be materially adversely affected by any of these risks. In such case, the trading price of our common stock could decline, and our stockholders may lose all or part of their investment in our securities.
Risks Related to Our Business
We are a development stage company and may never earn a profit.
We are a development stage company and have incurred losses since our formation. As of December 31, 2017 and March 31, 2018, we have an accumulated total deficit of approximately $173.0 million and $177.7 million, respectively. For the fiscal years ended December 31, 2017 and 2016 and the three months ended March 31, 2018, we had a net loss attributable to common stockholders of approximately $24.9 million, $39.2 million and $4.8 million, respectively. To date, we have experienced negative cash flow from development of our product candidate PCM-075 and our cell-free molecular diagnostic technology. We have generated limited revenue from operations, and we expect to incur substantial net losses for the foreseeable future as we seek to further develop and commercialize PCM-075 and our cell-free molecular diagnostic technology. We cannot predict the extent of these future net losses, or when we may attain profitability, if at all. If we are unable to generate significant revenue from PCM-075 and our cell-free molecular diagnostic technology or attain profitability, we will not be able to sustain operations.
Because of the numerous risks and uncertainties associated with developing and commercializing PCM-075 and our cell-free molecular diagnostic technology and tests, we are unable to predict the extent of any future losses or when we will attain profitability, if ever. We may never become profitable and you may never receive a return on an investment in our securities. An investor in our securities must carefully consider the substantial challenges, risks and uncertainties inherent in the attempted development and commercialization of PCM-075 and tests in the medical diagnostic industry. We may never successfully commercialize PCM-075 and our cell-free molecular diagnostic technology or any future tests we may develop, and our business may not be successful.
We will need to raise substantial additional capital to develop and commercialize PCM-075 and our failure to obtain funding when needed may force us to delay, reduce or eliminate our product development programs or collaboration efforts.
As of March 31, 2018, our cash and cash equivalents balance was approximately $6.7 million and our working capital was approximately $4.0 million. Due to our recurring losses from operations and the expectation that we will continue to incur losses in the future, we will be required to raise additional capital to complete the development and commercialization of our current product candidates. We have historically relied upon private and public sales of our equity, as well as debt financings to fund our operations. In order to raise additional capital, we may seek to sell additional equity and/or debt securities or obtain a credit facility or other loan, which we may not be able to do on favorable terms, or at all. Our ability to obtain additional financing will be subject to a number of factors, including market conditions, our operating performance and investor sentiment. If we are unable to raise additional capital when required or on acceptable terms, we may have to significantly delay, scale
10
Table of Contents
back or discontinue the development and/or commercialization of one or more of our product candidates, restrict our operations or obtain funds by entering into agreements on unfavorable terms.
Our financial statements include an explanatory paragraph that expresses substantial doubt about our ability to continue as a going concern, indicating the possibility that we may not be able to operate in the future.
Primarily as a result of our losses incurred to date, our expected continued future losses, and limited cash balances, we have included an explanatory paragraph in our financial statements expressing substantial doubt about our ability to continue as a going concern. Our ability to continue as a going concern is contingent upon, among other factors, the sale of the shares of our common stock or obtaining alternate financing.
Our product candidate PCM-075 is in the early stages of development and its commercial viability remains subject to the successful outcome of PCM-075, current and future preclinical studies, clinical trials, regulatory approvals and the risks generally inherent in the development of a pharmaceutical product candidate. If we are unable to successfully advance or develop our product candidate, our business will be materially harmed.
In the near-term, failure to successfully advance the development of our product candidate may have a material adverse effect on us. To date, we have not successfully developed or commercially marketed, distributed or sold any product candidate. The success of our business depends primarily upon our ability to successfully advance the development of our product candidate through preclinical studies and clinical trials, have the product candidate approved for sale by the FDA or regulatory authorities in other countries, and ultimately have the product candidate successfully commercialized by us or a strategic partner. We cannot assure you that the results of our ongoing preclinical studies or clinical trials will support or justify the continued development of our product candidate, or that we will receive approval from the FDA, or similar regulatory authorities in other countries, to advance the development of our product candidate.
Our product candidate must satisfy rigorous regulatory standards of safety and efficacy before we can advance or complete its clinical development or it can be approved for sale. To satisfy these standards, we must engage in expensive and lengthy preclinical studies and clinical trials, develop acceptable manufacturing processes, and obtain regulatory approval of our product candidate. Despite these efforts, our product candidate may not:
| • | offer therapeutic or other medical benefits over existing drugs or other product candidates in development to treat the same patient population; |
| • | be proven to be safe and effective in current and future preclinical studies or clinical trials; |
| • | have the desired effects; |
| • | be free from undesirable or unexpected effects; |
| • | meet applicable regulatory standards; |
| • | be capable of being formulated and manufactured in commercially suitable quantities and at an acceptable cost; or |
| • | be successfully commercialized by us or by collaborators. |
Even if we demonstrate favorable results in preclinical studies and early-stage clinical trials, we cannot assure you that the results of late-stage clinical trials will be favorable enough to support the continued development of our product candidate. A number of companies in the pharmaceutical and biopharmaceutical industries have experienced significant delays, setbacks and failures in all stages of development, including late-stage clinical trials, even after achieving promising results in preclinical testing or early-stage clinical trials. Accordingly, results from completed preclinical studies and early-stage clinical trials of our product candidate
11
Table of Contents
may not be predictive of the results we may obtain in later-stage trials. Furthermore, even if the data collected from preclinical studies and clinical trials involving our product candidate demonstrate a favorable safety and efficacy profile, such results may not be sufficient to support the submission of a New Drug Application (“NDA”) to obtain regulatory approval from the FDA in the U.S., or other similar regulatory agencies in other jurisdictions, which is required to market and sell the product.
Our product candidate will require significant additional research and development efforts, the commitment of substantial financial resources, and regulatory approvals prior to advancing into further clinical development or being commercialized by us or collaborators. We cannot assure you that our product candidate will successfully progress through the drug development process or will result in commercially viable products. We do not expect our product candidate to be commercialized by us or collaborators for at least several years.
Our product candidate may exhibit undesirable side effects when used alone or in combination with other approved pharmaceutical products or investigational new drugs, which may delay or preclude further development or regulatory approval, or limit their use if approved.
Throughout the drug development process, we must continually demonstrate the safety and tolerability of our product candidate to obtain regulatory approval to further advance clinical development or to market it. Even if our product candidate demonstrates biologic activity and clinical efficacy, any unacceptable adverse side effects or toxicities, when administered alone or in the presence of other pharmaceutical products, which can arise at any stage of development, may outweigh potential benefits. In preclinical studies and clinical trials we have conducted to date, our product candidate’s safety profile is based on studies and trials that have involved a small number of subjects or patients over a limited period of time. We may observe adverse or significant adverse events or drug-drug interactions in future preclinical studies or clinical trial candidates, which could result in the delay or termination of development, prevent regulatory approval, or limit market acceptance if ultimately approved.
If the results of preclinical studies or clinical trials for our product candidate, including those that are subject to existing or future license or collaboration agreements, are unfavorable or delayed, we could be delayed or precluded from the further development or commercialization of our product candidate, which could materially harm our business.
In order to further advance the development of, and ultimately receive regulatory approval to sell, our product candidate, we must conduct extensive preclinical studies and clinical trials to demonstrate its safety and efficacy to the satisfaction of the FDA or similar regulatory authorities in other countries, as the case may be. Preclinical studies and clinical trials are expensive, complex, can take many years to complete, and have highly uncertain outcomes. Delays, setbacks, or failures can occur at any time, or in any phase of preclinical or clinical testing, and can result from concerns about safety or toxicity, a lack of demonstrated efficacy or superior efficacy over other similar products that have been approved for sale or are in more advanced stages of development, poor study or trial design, and issues related to the formulation or manufacturing process of the materials used to conduct the trials. The results of prior preclinical studies or clinical trials are not necessarily predictive of the results we may observe in later stage clinical trials. In many cases, product candidates in clinical development may fail to show desired safety and efficacy characteristics despite having favorably demonstrated such characteristics in preclinical studies or earlier stage clinical trials.
In addition, we may experience numerous unforeseen events during, or as a result of, preclinical studies and the clinical trial process, which could delay or impede our ability to advance the development of, receive regulatory approval for, or commercialize our product candidate, including, but not limited to:
| • | communications with the FDA, or similar regulatory authorities in different countries, regarding the scope or design of a trial or trials; |
| • | regulatory authorities (including an Institutional Review Board (“IRB”) or Ethical Committee (“EC”)) not authorizing us to commence or conduct a clinical trial at a prospective trial site; |
12
Table of Contents
| • | enrollment in our clinical trials being delayed, or proceeding at a slower pace than we expected, because we have difficulty recruiting patients or participants dropping out of our clinical trials at a higher rate than we anticipated; |
| • | our third party contractors, upon whom we rely for conducting preclinical studies, clinical trials and manufacturing of our trial materials, may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner; |
| • | having to suspend or ultimately terminate our clinical trials if participants are being exposed to unacceptable health or safety risks; |
| • | IRBs, ECs or regulators requiring that we hold, suspend or terminate our preclinical studies and clinical trials for various reasons, including non-compliance with regulatory requirements; and |
| • | the supply or quality of drug material necessary to conduct our preclinical studies or clinical trials being insufficient, inadequate or unavailable. |
Even if the data collected from preclinical studies or clinical trials involving our product candidates demonstrate a favorable safety and efficacy profile, such results may not be sufficient to support the submission of a NDA to obtain regulatory approval from the FDA in the U.S., or other similar foreign regulatory authorities in foreign jurisdictions, which is required to market and sell the product.
If third party vendors upon whom we intend to rely on to conduct our preclinical studies or clinical trials do not perform or fail to comply with strict regulations, these studies or trials of our product candidate may be delayed, terminated, or fail, or we could incur significant additional expenses, which could materially harm our business.
We have limited resources dedicated to designing, conducting and managing preclinical studies and clinical trials. We intend to rely on third parties, including clinical research organizations, consultants and principal investigators, to assist us in designing, managing, monitoring and conducting our preclinical studies and clinical trials. We intend to rely on these vendors and individuals to perform many facets of the drug development process, including certain preclinical studies, the recruitment of sites and patients for participation in our clinical trials, maintenance of good relations with the clinical sites, and ensuring that these sites are conducting our trials in compliance with the trial protocol, including safety monitoring and applicable regulations. If these third parties fail to perform satisfactorily, or do not adequately fulfill their obligations under the terms of our agreements with them, we may not be able to enter into alternative arrangements without undue delay or additional expenditures, and therefore the preclinical studies and clinical trials of our product candidate may be delayed or prove unsuccessful. Further, the FDA, or other similar foreign regulatory authorities, may inspect some of the clinical sites participating in our clinical trials in the U.S., or our third-party vendors’ sites, to determine if our clinical trials are being conducted according to Good Clinical Practices (“GCPs”). If we or the FDA determine that our third-party vendors are not in compliance with, or have not conducted our clinical trials according to, applicable regulations we may be forced to delay, repeat or terminate such clinical trials.
We have limited capacity for recruiting and managing clinical trials, which could impair our timing to initiate or complete clinical trials of our product candidates and materially harm our business.
We have limited capacity to recruit and manage the clinical trials necessary to obtain FDA approval or approval by other regulatory authorities. By contrast, larger pharmaceutical and bio-pharmaceutical companies often have substantial staff with extensive experience in conducting clinical trials with multiple product candidates across multiple indications. In addition, they may have greater financial resources to compete for the same clinical investigators and patients that we are attempting to recruit for our clinical trials. If potential competitors are successful in completing drug development for their product candidates and obtain approval from the FDA, they could limit the demand for PCM-075.
13
Table of Contents
As a result, we may be at a competitive disadvantage that could delay the initiation, recruitment, timing, completion of our clinical trials and obtaining regulatory approvals, if at all, for our product candidate.
We, and our collaborators, must comply with extensive government regulations in order to advance our product candidate through the development process and ultimately obtain and maintain marketing approval for our products in the U.S. and abroad.
The product candidate that we, or our collaborators, are developing require regulatory approval to advance through clinical development and to ultimately be marketed and sold, and are subject to extensive and rigorous domestic and foreign government regulation. In the U.S., the FDA regulates, among other things, the development, testing, manufacture, safety, efficacy, record-keeping, labeling, storage, approval, advertising, promotion, sale and distribution of pharmaceutical and biopharmaceutical products. Our product candidate is also subject to similar regulation by foreign governments to the extent we seek to develop or market it in those countries. We, or our collaborators, must provide the FDA and foreign regulatory authorities, if applicable, with preclinical and clinical data, as well as data supporting an acceptable manufacturing process, that appropriately demonstrate our product candidate’s safety and efficacy before it can be approved for the targeted indications. Our product candidate has not been approved for sale in the U.S. or any foreign market, and we cannot predict whether we or our collaborators will obtain regulatory approval for any product candidates we are developing or plan to develop. The regulatory review and approval process can take many years, is dependent upon the type, complexity, novelty of, and medical need for the product candidate, requires the expenditure of substantial resources, and involves post-marketing surveillance and vigilance and ongoing requirements for post-marketing studies or Phase 4 clinical trials. In addition, we or our collaborators may encounter delays in, or fail to gain, regulatory approval for our product candidate based upon additional governmental regulation resulting from future legislative, administrative action or changes in FDA’s or other similar foreign regulatory authorities’ policy or interpretation during the period of product development. Delays or failures in obtaining regulatory approval to advance our product candidate through clinical development, and ultimately commercialize them, may:
| • | adversely impact our ability to raise sufficient capital to fund the development of our product candidate; |
| • | adversely affect our ability to further develop or commercialize our product candidate; |
| • | diminish any competitive advantages that we or our collaborators may have or attain; and |
| • | adversely affect the receipt of potential milestone payments and royalties from the sale of our products or product revenues. |
Furthermore, any regulatory approvals, if granted, may later be withdrawn. If we or our collaborators fail to comply with applicable regulatory requirements at any time, or if post-approval safety concerns arise, we or our collaborators may be subject to restrictions or a number of actions, including:
| • | delays, suspension or termination of clinical trials related to our products; |
| • | refusal by regulatory authorities to review pending applications or supplements to approved applications; |
| • | product recalls or seizures; |
| • | suspension of manufacturing; |
| • | withdrawals of previously approved marketing applications; and |
| • | fines, civil penalties and criminal prosecutions. |
Additionally, at any time we or our collaborators may voluntarily suspend or terminate the preclinical or clinical development of a product candidate, or withdraw any approved product from the market if we believe
14
Table of Contents
that it may pose an unacceptable safety risk to patients, or if the product candidate or approved product no longer meets our business objectives. The ability to develop or market a pharmaceutical product outside of the U.S. is contingent upon receiving appropriate authorization from the respective foreign regulatory authorities. Foreign regulatory approval processes typically include many, if not all, of the risks and requirements associated with the FDA regulatory process for drug development and may include additional risks.
We have limited experience in the development of therapeutic product candidates and therefore may encounter difficulties developing our product candidate or managing our operations in the future.
We have limited experience in the discovery, development and manufacturing of therapeutic compounds. In order to successfully develop our product candidate, we must continuously supplement our research, clinical development, regulatory, medicinal chemistry, virology and manufacturing capabilities through the addition of key employees, consultants or third-party contractors to provide certain capabilities and skill sets that we do not possess.
Furthermore, we have adopted an operating model that largely relies on the outsourcing of a number of responsibilities and key activities to third-party consultants, and contract research and manufacturing organizations in order to advance the development of our product candidate. Therefore, our success depends in part on our ability to retain highly qualified key management, personnel, and directors to develop, implement and execute our business strategy, operate the company and oversee the activities of our consultants and contractors, as well as academic and corporate advisors or consultants to assist us in this regard. We are currently highly dependent upon the efforts of our management team. In order to develop our product candidate, we need to retain or attract certain personnel, consultants or advisors with experience in drug development activities that include a number of disciplines, including research and development, clinical trials, medical matters, government regulation of pharmaceuticals, manufacturing, formulation and chemistry, business development, accounting, finance, regulatory affairs, human resources and information systems. We are highly dependent upon our senior management and scientific staff, particularly William Welch, our Chief Executive Officer. The loss of services of Mr. Welch or one or more of our other members of senior management could delay or prevent the successful completion of our planned clinical trials or the commercialization of our product candidate.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management, clinical and scientific personnel and on our ability to develop and maintain important relationships with leading academic institutions, clinicians and scientists. The competition for qualified personnel in the biotechnology and pharmaceuticals field is intense. We will need to hire additional personnel as we expand our clinical development and commercial activities. While we have not had difficulties recruiting qualified individuals, to date, we may not be able to attract and retain quality personnel on acceptable terms given the competition for such personnel among biotechnology, pharmaceutical and other companies. Although we have not experienced material difficulties in retaining key personnel in the past, we may not be able to continue to do so in the future on acceptable terms, if at all. If we lose any key managers or employees, or are unable to attract and retain qualified key personnel, directors, advisors or consultants, the development of our product candidate could be delayed or terminated and our business may be harmed.
Clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results.
Our product candidate may not prove to be safe and efficacious in clinical trials and may not meet all the applicable regulatory requirements needed to receive regulatory approval. In order to receive regulatory approval for the commercialization of our product candidate, we must conduct, at our own expense, extensive preclinical testing and clinical trials to demonstrate safety and efficacy of our product candidate for the intended indication of use. Clinical testing is expensive, can take many years to complete, if at all, and its outcome is uncertain. Failure can occur at any time during the clinical trial process.
15
Table of Contents
The results of preclinical studies and early clinical trials of new drugs do not necessarily predict the results of later-stage clinical trials. The design of our clinical trials is based on many assumptions about the expected effects of our product candidate, and if those assumptions are incorrect it may not produce statistically significant results. Preliminary results may not be confirmed on full analysis of the detailed results of an early clinical trial. Product candidates in later stages of clinical trials may fail to show safety and efficacy sufficient to support intended use claims despite having progressed through initial clinical testing. The data collected from clinical trials of our product candidates may not be sufficient to support the filing of an NDA or to obtain regulatory approval in the United States or elsewhere. Because of the uncertainties associated with drug development and regulatory approval, we cannot determine if or when we will have an approved product for commercialization or achieve sales or profits.
Delays in clinical testing could result in increased costs to us and delay our ability to generate revenue.
We may experience delays in clinical testing of our product candidate. We do not know whether planned clinical trials will begin on time, will need to be redesigned or will be completed on schedule, if at all. Clinical trials can be delayed for a variety of reasons, including delays in obtaining regulatory approval to commence a clinical trial, in securing clinical trial agreements with prospective sites with acceptable terms, in obtaining institutional review board approval to conduct a clinical trial at a prospective site, in recruiting patients to participate in a clinical trial or in obtaining sufficient supplies of clinical trial materials. Many factors affect patient enrollment, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the clinical trial, competing clinical trials and new drugs approved for the conditions we are investigating. Clinical investigators will need to decide whether to offer their patients enrollment in clinical trials of our product candidate versus treating these patients with commercially available drugs that have established safety and efficacy profiles. Any delays in completing our clinical trials will increase our costs, slow down our product development, timeliness and approval process and delay our ability to generate revenue.
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidate, our business will be substantially harmed.
The time required to obtain approval by the FDA and comparable foreign authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate and it is possible that our existing product candidates or any product candidate we may seek to develop in the future will ever obtain regulatory approval.
Our product candidate could fail to receive regulatory approval for many reasons, including the following:
| • | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
| • | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication; |
| • | the results of clinical trials may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval; |
| • | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| • | the data collected from clinical trials of our product candidates may not be sufficient to support the submission of an NDA or other submission or to obtain regulatory approval in the United States or elsewhere; |
16
Table of Contents
| • | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; |
| • | the FDA or comparable foreign regulatory authorities may fail to approve the companion diagnostics we contemplate developing with partners; and |
| • | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
This lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval to market our product candidate, which would significantly harm our business, results of operations and prospects.
In addition, even if we were to obtain approval, regulatory authorities may approve our product candidate for fewer or more limited indications than we request, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidate.
We have not previously submitted an NDA to the FDA, or similar drug approval filings to comparable foreign authorities, for our product candidate, and we cannot be certain that our product candidate will be successful in clinical trials or receive regulatory approval. Further, our product candidate may not receive regulatory approval even if it is successful in clinical trials. If we do not receive regulatory approvals for our product candidate, we may not be able to continue our operations. Even if we successfully obtain regulatory approvals to market one or more of our product candidates, our revenues will be dependent, in part, upon our collaborators’ ability to obtain regulatory approval of the companion diagnostics to be used with our product candidates, as well as the size of the markets in the territories for which we gain regulatory approval and have commercial rights. If the markets for patients that we are targeting for our product candidate are not as significant as we estimate, we may not generate significant revenues from sales of such products, if approved.
We plan to seek regulatory approval and to commercialize our product candidate, directly or with a collaborator, worldwide including the United States, the European Union and other additional foreign countries which we have not yet identified. While the scope of regulatory approval is similar in other countries, to obtain separate regulatory approval in many other countries we must comply with numerous and varying regulatory requirements of such countries regarding safety and efficacy and governing, among other things, clinical trials and commercial sales, pricing and distribution of our product candidates, and we cannot predict success in these jurisdictions.
We may be required to suspend or discontinue clinical trials due to unexpected side effects or other safety risks that could preclude approval of our product candidate.
Our clinical trials may be suspended at any time for a number of reasons. For example, we may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to the clinical trial patients. In addition, the FDA or other regulatory agencies may order the temporary or permanent discontinuation of our clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to the clinical trial patients.
Administering our product candidate to humans may produce undesirable side effects. These side effects could interrupt, delay or halt clinical trials of our product candidates and could result in the FDA or other regulatory authorities denying further development or approval of our product candidate for any or all targeted indications. Ultimately, our product candidate may prove to be unsafe for human use. Moreover, we could be subject to significant liability if any volunteer or patient suffers, or appears to suffer, adverse health effects as a result of participating in our clinical trials.
17
Table of Contents
If we fail to comply with healthcare regulations, we could face substantial enforcement actions, including civil and criminal penalties and our business, operations and financial condition could be adversely affected.
As a developer of pharmaceuticals, even though we do not intend to make referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payers, certain federal and state healthcare laws and regulations pertaining to fraud and abuse, false claims and patients’ privacy rights are and will be applicable to our business. We could be subject to healthcare fraud and abuse laws and patient privacy laws of both the federal government and the states in which we conduct our business. The laws include:
| • | the federal healthcare program anti-kickback law, which prohibits, among other things, persons from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual, for an item or service or the purchasing or ordering of a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs; |
| • | federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent, and which may apply to entities like us which provide coding and billing information to customers; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, which prohibits executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; |
| • | the Federal Food, Drug, and Cosmetic Act, which among other things, strictly regulates drug manufacturing and product marketing, prohibits manufacturers from marketing drug products for off-label use and regulates the distribution of drug samples; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payer, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by federal laws, thus complicating compliance efforts. |
If our operations are found to be in violation of any of the laws described above or any governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal and state privacy, security and fraud laws may prove costly.
If we are unable to satisfy regulatory requirements, we may not be able to commercialize our product candidate.
We need FDA approval prior to marketing our product candidate in the United States. If we fail to obtain FDA approval to market our product candidate, we will be unable to sell our product candidate in the United States and we will not generate any revenue.
The FDA’s review and approval process, including among other things, evaluation of preclinical studies and clinical trials of a product candidate as well as the manufacturing process and facility, is lengthy, expensive and uncertain. To receive approval, we must, among other things, demonstrate with substantial evidence from well-
18
Table of Contents
designed and well-controlled pre- clinical testing and clinical trials that the product candidate is both safe and effective for each indication for which approval is sought. Satisfaction of these requirements typically takes several years and the time needed to satisfy them may vary substantially, based on the type, complexity and novelty of the pharmaceutical product. We cannot predict if or when we will submit an NDA for approval for our product candidate currently under development. Any approvals we may obtain may not cover all of the clinical indications for which we are seeking approval or may contain significant limitations on the conditions of use.
The FDA has substantial discretion in the NDA review process and may either refuse to file our NDA for substantive review or may decide that our data is insufficient to support approval of our product candidate for the claimed intended uses. Following any regulatory approval of our product candidate, we will be subject to continuing regulatory obligations such as safety reporting, required and additional post marketing obligations, and regulatory oversight of promotion and marketing. Even if we receive regulatory approvals, the FDA may subsequently seek to withdraw approval of our NDA if we determine that new data or a reevaluation of existing data show the product is unsafe for use under the conditions of use upon the basis of which the NDA was approved, or based on new evidence of adverse effects or adverse clinical experience, or upon other new information. If the FDA does not file or approve our NDA or withdraws approval of our NDA, the FDA may require that we conduct additional clinical trials, preclinical or manufacturing studies and submit that data before it will reconsider our application. Depending on the extent of these or any other requested studies, approval of any applications that we submit may be delayed by several years, may require us to expend more resources than we have available, or may never be obtained at all.
We will also be subject to a wide variety of foreign regulations governing the development, manufacture and marketing of our products to the extent we seek regulatory approval to develop and market our product candidate in a foreign jurisdiction. As of the date hereof we have not identified any foreign jurisdictions which we intend to seek approval from. Whether or not FDA approval has been obtained, approval of a product by the comparable regulatory authorities of foreign countries must still be obtained prior to marketing the product in those countries. The approval process varies and the time needed to secure approval in any region such as the European Union or in a country with an independent review procedure may be longer or shorter than that required for FDA approval. We cannot assure you that clinical trials conducted in one country will be accepted by other countries or that an approval in one country or region will result in approval elsewhere.
If our product candidate is unable to compete effectively with marketed drugs targeting similar indications as our product candidate, our commercial opportunity will be reduced or eliminated.
We face competition generally from established pharmaceutical and biotechnology companies, as well as from academic institutions, government agencies and private and public research institutions. Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Small or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. Our commercial opportunity will be reduced or eliminated if our competitors develop and commercialize any drugs that are safer, more effective, have fewer side effects or are less expensive than our product candidate. These potential competitors compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient enrollment for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business.
If approved and commercialized, PCM-075 would compete with several currently approved prescription therapies for the treatment of AML. To our knowledge, other potential competitors are in earlier stages of development. If potential competitors are successful in completing drug development for their product candidates and obtain approval from the FDA, they could limit the demand for PCM-075.
19
Table of Contents
We expect that our ability to compete effectively will depend upon our ability to:
| • | successfully identify and develop key points of product differentiations from currently available therapies; |
| • | successfully and rapidly complete clinical trials and submit for and obtain all requisite regulatory approvals in a cost-effective manner; |
| • | maintain a proprietary position for our products and manufacturing processes and other related product technology; |
| • | attract and retain key personnel; |
| • | develop relationships with physicians prescribing these products; and |
| • | build an adequate sales and marketing infrastructure for our product candidates. |
Because we will be competing against significantly larger companies with established track records, we will have to demonstrate that, based on experience, clinical data, side-effect profiles and other factors, our products, if approved, are competitive with other products. If we are unable to compete effectively and differentiate our products from other marketed drugs, we may never generate meaningful revenue. If a competitor markets the same drug for the treatment of AML, before us, we may not receive orphan drug marketing exclusivity.
If the manufacturers upon whom we rely fail to produce our product candidate, in the volumes that we require on a timely basis, or fail to comply with stringent regulations applicable to pharmaceutical drug manufacturers, we may face delays in the development and commercialization of our product candidate.
We do not currently possess internal manufacturing capacity. We plan to utilize the services of contract manufacturers to manufacture our clinical supplies. Any curtailment in the availability of PCM-075, however, could result in production or other delays with consequent adverse effects on us. In addition, because regulatory authorities must generally approve raw material sources for pharmaceutical products, changes in raw material suppliers may result in production delays or higher raw material costs.
We continue to pursue active pharmaceutical ingredients (“API”) and drug product supply agreements with other manufacturers. We may be required to agree to minimum volume requirements, exclusivity arrangements or other restrictions with the contract manufacturers. We may not be able to enter into long-term agreements on commercially reasonable terms, or at all. If we change or add manufacturers, the FDA and comparable foreign regulators may require approval of the changes. Approval of these changes could require new testing by the manufacturer and compliance inspections to ensure the manufacturer is conforming to all applicable laws and regulations and good manufacturing practices (“GMP”). In addition, the new manufacturers would have to be educated in or independently develop the processes necessary for the production of our product candidate.
The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products may encounter difficulties in production, particularly in scaling up production. These problems include difficulties with production costs and yields, quality control, including stability of the product and quality assurance testing, shortages of qualified personnel, as well as compliance with federal, state and foreign regulations. In addition, any delay or interruption in the supply of clinical trial supplies could delay the completion of our clinical trials, increase the costs associated with conducting our clinical trials and, depending upon the period of delay, require us to commence new clinical trials at significant additional expense or to terminate a clinical trial.
We will be responsible for ensuring that each of our future contract manufacturers comply with the GMP requirements of the FDA and other regulatory authorities from which we seek to obtain product approval. These requirements include, among other things, quality control, quality assurance and the maintenance of records and
20
Table of Contents
documentation. The approval process for NDAs includes a review of the manufacturer’s compliance with GMP requirements. We will be responsible for regularly assessing a contract manufacturer’s compliance with GMP requirements through record reviews and periodic audits and for ensuring that the contract manufacturer takes responsibility and corrective action for any identified deviations. Manufacturers our product candidates may be unable to comply with these GMP requirements and with other FDA and foreign regulatory requirements, if any.
While we will oversee compliance by our contract manufacturers, ultimately we will not have control over our manufacturers’ compliance with these regulations and standards. A failure to comply with these requirements may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval. If the safety of our product candidate is compromised due to a manufacturers’ failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for or successfully commercialize our product candidates, and we may be held liable for any injuries sustained as a result. Any of these factors could cause a delay of clinical trials, regulatory submissions, approvals or commercialization of PCM-075 or other product candidates, entail higher costs or result in us being unable to effectively commercialize our product candidates. Furthermore, if our manufacturers fail to deliver the required commercial quantities on a timely basis and at commercially reasonable prices, we may be unable to meet demand for any approved products and would lose potential revenues.
We may not be able to manufacture our product candidate in commercial quantities, which would prevent us from commercializing our product candidate.
To date, our product candidate has been manufactured in small quantities for preclinical studies and clinical trials. If our product candidate is approved by the FDA or comparable regulatory authorities in other countries for commercial sale, we will need to manufacture such product candidate in larger quantities. We may not be able to increase successfully the manufacturing capacity for our product candidate in a timely or economic manner, or at all. Significant scale-up of manufacturing may require additional validation studies, which the FDA must review and approve. If we are unable to increase successfully the manufacturing capacity for a product candidate, the clinical trials as well as the regulatory approval or commercial launch of that product candidate may be delayed or there may be a shortage in supply. Our product candidate requires precise, high quality manufacturing. Our failure to achieve and maintain these high quality manufacturing standards in collaboration with our third-party manufacturers, including the incidence of manufacturing errors, could result in patient injury or death, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could harm our business, financial condition and results of operations.
Materials necessary to manufacture our product candidate may not be available on commercially reasonable terms, or at all, which may delay the development and commercialization of our product candidate.
We rely on Nerviano to purchase from third-party suppliers the materials necessary to produce bulk APIs, and product candidates for our clinical trials, and we will rely on such manufacturers to purchase such materials to produce the APIs and finished products for any commercial distribution of our products if we obtain marketing approval. Suppliers may not sell these materials to our manufacturers at the time they need them in order to meet our required delivery schedule or on commercially reasonable terms, if at all. We do not have any control over the process or timing of the acquisition of these materials by our manufacturers. Moreover, we currently do not have any agreements for the production of these materials. If our manufacturers are unable to obtain these materials for our clinical trials, testing of the affected product candidate would be delayed, which may significantly impact our ability to develop the product candidate. If we or our manufacturers are unable to purchase these materials after regulatory approval has been obtained for one of our products, the commercial launch of such product would be delayed or there would be a shortage in supply of such product, which would harm our ability to generate revenues from such product and achieve or sustain profitability.
21
Table of Contents
Our product candidate, if approved for sale, may not gain acceptance among physicians, patients and the medical community, thereby limiting our potential to generate revenues.
If our product candidate is approved for commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of any approved product by physicians, healthcare professionals and third-party payers and our profitability and growth will depend on a number of factors, including:
| • | demonstration of safety and efficacy; |
| • | changes in the practice guidelines and the standard of care for the targeted indication; |
| • | relative convenience and ease of administration; |
| • | the prevalence and severity of any adverse side effects; |
| • | budget impact of adoption of our product on relevant drug formularies and the availability, cost and potential advantages of alternative treatments, including less expensive generic drugs; |
| • | pricing, reimbursement and cost effectiveness, which may be subject to regulatory control; |
| • | effectiveness of our or any of our partners’ sales and marketing strategies; |
| • | the product labeling or product insert required by the FDA or regulatory authority in other countries; and |
| • | the availability of adequate third-party insurance coverage or reimbursement. |
If any product candidate that we develop does not provide a treatment regimen that is as beneficial as, or is perceived as being as beneficial as, the current standard of care or otherwise does not provide patient benefit, that product candidate, if approved for commercial sale by the FDA or other regulatory authorities, likely will not achieve market acceptance. Our ability to effectively promote and sell any approved products will also depend on pricing and cost-effectiveness, including our ability to produce a product at a competitive price and our ability to obtain sufficient third-party coverage or reimbursement. If any product candidate is approved but does not achieve an adequate level of acceptance by physicians, patients and third-party payers, our ability to generate revenues from that product would be substantially reduced. In addition, our efforts to educate the medical community and third-party payers on the benefits of our product candidates may require significant resources, may be constrained by FDA rules and policies on product promotion, and may never be successful.
Guidelines and recommendations published by various organizations can impact the use of our product.
Government agencies promulgate regulations and guidelines directly applicable to us and to our product. In addition, professional societies, practice management groups, private health and science foundations and organizations involved in various diseases from time to time may also publish guidelines or recommendations to the health care and patient communities. Recommendations of government agencies or these other groups or organizations may relate to such matters as usage, dosage, route of administration and use of concomitant therapies. Recommendations or guidelines suggesting the reduced use of our products or the use of competitive or alternative products that are followed by patients and health care providers could result in decreased use of our proposed product.
If third-party contract manufacturers upon whom we rely to formulate and manufacture our product candidate do not perform, fail to manufacture according to our specifications or fail to comply with strict regulations, our preclinical studies or clinical trials could be adversely affected and the development of our product candidate could be delayed or terminated or we could incur significant additional expenses.
We do not own or operate any manufacturing facilities. We intend to rely on third-party contractors, at least for the foreseeable future, to formulate and manufacture these preclinical and clinical materials. Our reliance on
22
Table of Contents
third- party contract manufacturers exposes us to a number of risks, any of which could delay or prevent the completion of our preclinical studies or clinical trials, or the regulatory approval or commercialization of our product candidate, result in higher costs, or deprive us of potential product revenues. Some of these risks include:
| • | our third-party contractors failing to develop an acceptable formulation to support later-stage clinical trials for, or the commercialization of, our product candidates; |
| • | our contract manufacturers failing to manufacture our product candidate according to their own standards, our specifications, GMPs, or otherwise manufacturing material that we or the FDA may deem to be unsuitable in our clinical trials; |
| • | our contract manufacturers being unable to increase the scale of, increase the capacity for, or reformulate the form of our product candidate. We may experience a shortage in supply, or the cost to manufacture our products may increase to the point where it adversely affects the cost of our product candidate. We cannot assure you that our contract manufacturers will be able to manufacture our products at a suitable scale, or we will be able to find alternative manufacturers acceptable to us that can do so; |
| • | our contract manufacturers placing a priority on the manufacture of their own products, or other customers’ products; |
| • | our contract manufacturers failing to perform as agreed or not remain in the contract manufacturing business; and |
| • | our contract manufacturers’ plants being closed as a result of regulatory sanctions or a natural disaster. |
Manufacturers of pharmaceutical products are subject to ongoing periodic inspections by the FDA, the U.S. Drug Enforcement Administration (“DEA”) and corresponding state and foreign agencies to ensure strict compliance with FDA-mandated current good marketing practices or GMPs, other government regulations and corresponding foreign standards. While we are obligated to audit their performance, we do not have control over our third-party contract manufacturers’ compliance with these regulations and standards. Failure by our third-party manufacturers, or us, to comply with applicable regulations could result in sanctions being imposed on us or the drug manufacturer from the production of other third-party products. These sanctions may include fines, injunctions, civil penalties, failure of the government to grant pre-market approval of drugs, delays, suspension or withdrawal of approvals, seizures or recalls of product, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect our business.
In the event that we need to change our third-party contract manufacturers, our preclinical studies, clinical trials or the commercialization of our product candidate could be delayed, adversely affected or terminated, or such a change may result in significantly higher costs.
Due to regulatory restrictions inherent in an IND or NDA, various steps in the manufacture of our product candidate may need to be sole-sourced. In accordance with GMPs, changing manufacturers may require the re-validation of manufacturing processes and procedures, and may require further preclinical studies or clinical trials to show comparability between the materials produced by different manufacturers. Changing our current or future contract manufacturers may be difficult for us and could be costly, which could result in our inability to manufacture our product candidate for an extended period of time and therefore a delay in the development of our product candidate. Further, in order to maintain our development time lines in the event of a change in our third-party contract manufacturer, we may incur significantly higher costs to manufacture our product candidate.
We do not currently have any internal drug discovery capabilities, and therefore we are dependent on in-licensing or acquiring development programs from third parties in order to obtain additional product candidates.
If in the future we decide to further expand our pipeline, we will be dependent on in-licensing or acquiring product candidates as we do not have significant internal discovery capabilities at this time. Accordingly, in order
23
Table of Contents
to generate and expand our development pipeline, we have relied, and will continue to rely, on obtaining discoveries, new technologies, intellectual property and product candidates from third-parties through sponsored research, in-licensing arrangements or acquisitions. We may face substantial competition from other biotechnology and pharmaceutical companies, many of which may have greater resources then we have, in obtaining these in-licensing, sponsored research or acquisition opportunities. Additional in-licensing or acquisition opportunities may not be available to us on terms we find acceptable, if at all. In-licensed compounds that appear promising in research or in preclinical studies may fail to progress into further preclinical studies or clinical trials.
If a product liability claim is successfully brought against us for uninsured liabilities, or such claim exceeds our insurance coverage, we could be forced to pay substantial damage awards that could materially harm our business.
The use of any of our existing or future product candidates in clinical trials and the sale of any approved pharmaceutical products may expose us to significant product liability claims. We currently do not have product liability insurance coverage for our proposed clinical trials but we intend to obtain such insurance. Such insurance coverage may not protect us against any or all of the product liability claims that may be brought against us in the future. We may not be able to acquire or maintain adequate product liability insurance coverage at a commercially reasonable cost or in sufficient amounts or scope to protect us against potential losses. In the event a product liability claim is brought against us, we may be required to pay legal and other expenses to defend the claim, as well as uncovered damage awards resulting from a claim brought successfully against us. In the event our product candidate is approved for sale by the FDA and commercialized, we may need to substantially increase the amount of our product liability coverage. Defending any product liability claim or claims could require us to expend significant financial and managerial resources, which could have an adverse effect on our business.
If we materially breach or default under the Nerviano agreement, Nerviano will have the right to terminate the agreement and we could lose critical license rights, which would materially harm our business.
Our business is substantially dependent upon certain intellectual property rights that we license from Nerviano. Therefore, our commercial success will depend to a large extent on our ability to maintain and comply with our obligations under the agreement. The agreement may be terminated by Nerviano in the event of an uncured breach by us. We expect that other technology in-licenses that we may enter into in the future will contain similar provisions and impose similar obligations on us. If we fail to comply with any such obligations such licensor will likely terminate their out-licenses to us, in which case we would not be able to market products covered by these licenses, including our PCM-075 asset. The loss of our license with Nerviano with respect to the PCM-075, and potentially other licenses that we enter into in the future, would have a material adverse effect on our business. In addition, our failure to comply with obligations under our material in-licenses may cause us to become subject to litigation or other potential disputes under any such license agreements.
In addition, the Nerviano agreement requires us to make certain payments, including license fees, milestone payments royalties, and other such terms typically required under licensing agreements and these types of technology in-licenses generally could make it difficult for us to find corporate partners and less profitable for us to develop product candidates utilizing these existing product candidates and technologies.
We may delay or terminate the development of a product candidate at any time if we believe the perceived market or commercial opportunity does not justify further investment, which could materially harm our business.
Even though the results of preclinical studies and clinical trials that have been conducted or we may conduct in the future may support further development of our product candidate, we may delay, suspend or terminate the
24
Table of Contents
future development of a product candidate at any time for strategic, business, financial or other reasons, including the determination or belief that the emerging profile of the product candidate is such that it may not receive FDA approval, gain meaningful market acceptance, generate a significant return to shareholders, or otherwise provide any competitive advantages in its intended indication or market.
We depend upon our officers and other key employees, and if we are not able to retain them or recruit additional qualified personnel, the commercialization of our product candidates and any future tests that we develop could be delayed or negatively impacted.
Our success is largely dependent upon the continued contributions of our officers, especially William J. Welch, our Chief Executive Officer, and other key employees. Our success also depends in part on our ability to attract and retain highly qualified scientific, commercial and administrative personnel. The specialized nature of our industry results in an inherent scarcity of experienced personnel in the field and, in order to pursue our test development and commercialization strategies, we will need to attract, hire and retain, or engage as consultants, additional personnel with specialized experience in a number of disciplines, including assay development, bioinformatics and statistics, laboratory and clinical operations, clinical affairs and studies, government regulation, sales and marketing, billing and reimbursement and information systems. Additionally, there is intense competition for personnel in the fields in which we operate. If we are unable to attract new employees and retain existing employees, the development and commercialization of our product candidates and any tests we may develop in the future could be delayed or negatively impacted.
We will need to increase the size of our organization, and we may experience difficulties in managing growth.
We are a small company with 17 full-time employees as of March 31, 2018. Future growth of our company will impose significant additional responsibilities on members of management, including the need to identify, attract, retain, motivate and integrate highly skilled personnel. We may increase the number of employees in the future depending on the progress of our development of our product candidates and our cell-free molecular diagnostic technology. Our future financial performance and our ability to commercialize our product candidates and cell-free molecular diagnostic tests and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to:
| • | manage our clinical studies effectively; |
| • | integrate additional management, administrative, manufacturing and regulatory personnel; |
| • | maintain sufficient administrative, accounting and management information systems and controls; and |
| • | hire and train additional qualified personnel. |
There is no guarantee that we will be able to accomplish these tasks, and our failure to accomplish any of them could materially adversely affect our business, prospects and financial condition.
All of our diagnostic technology and services are performed at a single laboratory, and in the event this facility is affected by a termination of the lease or a man-made or natural disaster, our operations could be severely impaired.
We are performing all of our diagnostic services in our laboratory located in San Diego, California. Despite precautions taken by us, any future natural or man-made disaster at this laboratory, such as a fire, flood, earthquake or terrorist act, could cause substantial delays in our operations, damage or destroy our equipment and urine samples or cause us to incur additional expenses.
In addition, we are leasing the facilities where our laboratory operates. We are currently in compliance with all of our lease obligations, but should the lease terminate for any reason, or if the laboratory is moved due to conditions outside of our control, it could cause substantial delay in our diagnostics operations, damage or
25
Table of Contents
destroy our equipment and biological samples or cause us to incur additional expenses. In the event of an extended shutdown of our laboratory, we may be unable to perform our services in a timely manner or at all and therefore would be unable to operate in a commercially competitive manner. This could materially adversely affect our operating results and financial condition.
Further, if we have to use a substitute laboratory while our facility is closed, we could only use another facility with established state licensure and accreditation under CLIA. We may not be able to find another CLIA-certified facility and comply with applicable procedures, or find any such laboratory that would be willing to perform the tests for us on commercially reasonable terms. Additionally, any new laboratory opened by us would be subject to certification under CLIA and licensure by various states, which would take a significant amount of time and expense and result in delays in our ability to continue our personalized medicine services operations.
Security threats to our information technology infrastructure and/or our physical buildings could expose us to liability and damage our reputation and business.
It is essential to our business strategy that our technology and network infrastructure and our physical buildings remain secure and are perceived by our customers and corporate partners to be secure. Despite security measures, however, any network infrastructure may be vulnerable to cyber-attacks by hackers and other security threats. We may face cyber-attacks that attempt to penetrate our network security, sabotage or otherwise disable our research, products and services, misappropriate our or our customers’ and partners’ proprietary information, which may include personally identifiable information, or cause interruptions of our internal systems and services. Despite security measures, we also cannot guarantee security of our physical buildings. Physical building penetration or any cyber-attacks could negatively affect our reputation, damage our network infrastructure and our ability to deploy our products and services, harm our relationship with customers and partners that are affected, and expose us to financial liability.
Additionally, there are a number of state, federal and international laws protecting the privacy and security of health information and personal data. For example, the Health Insurance Portability and Accountability Act (“HIPAA”) imposes limitations on the use and disclosure of an individual’s healthcare information by healthcare providers, healthcare clearinghouses, and health insurance plans, or, collectively, covered entities, and also grants individuals rights with respect to their health information. HIPAA also imposes compliance obligations and corresponding penalties for non-compliance on individuals and entities that provide services to healthcare providers and other covered entities. As part of the American Recovery and Reinvestment Act of 2009 (“ARRA”) the privacy and security provisions of HIPAA were amended. ARRA also made significant increases in the penalties for improper use or disclosure of an individual’s health information under HIPAA and extended enforcement authority to state attorneys general. As amended by ARRA and subsequently by the final omnibus rule adopted in 2013, HIPAA also imposes notification requirements on covered entities in the event that certain health information has been inappropriately accessed or disclosed: notification requirements to individuals, federal regulators, and in some cases, notification to local and national media. Notification is not required under HIPAA if the health information that is improperly used or disclosed is deemed secured in accordance with encryption or other standards developed by the U.S. Department of Health and Human Services. Most states have laws requiring notification of affected individuals and/or state regulators in the event of a breach of personal information, which is a broader class of information than the health information protected by HIPAA. Many state laws impose significant data security requirements, such as encryption or mandatory contractual terms, to ensure ongoing protection of personal information. Activities outside of the U.S. implicate local and national data protection standards, impose additional compliance requirements and generate additional risks of enforcement for non-compliance. We may be required to expend significant capital and other resources to ensure ongoing compliance with applicable privacy and data security laws, to protect against security breaches and hackers or to alleviate problems caused by such breaches.
26
Table of Contents
General economic or business conditions may have a negative impact on our business.
Continuing concerns over U.S. health care reform legislation and energy costs, geopolitical issues, the availability and cost of credit and government stimulus programs in the U.S. and other countries have contributed to increased volatility and diminished expectations for the global economy. If the economic climate deteriorates, our business, including our access to patient samples and the addressable market for tests that we may successfully develop, as well as the financial condition of our suppliers and our third-party payors, could be negatively impacted, which could materially adversely affect our business, prospects and financial condition.
We may become subject to federal and state tax assessments, penalties and interest with respect to past compensation paid to certain of our executives.
During our internal review process, contingencies were identified regarding various federal and state tax exposures with respect to past compensation paid to certain of our executives. We have not recorded any accrued liabilities related to the potential federal and state tax exposure. If we become subject to any material tax assessment, penalties and interest by federal and state tax authorities in the future, our results of operations, financial performance and cash flows could be materially adversely affected.
Complying with numerous regulations pertaining to our business is an expensive and time-consuming process, and any failure to comply could result in substantial penalties.
The establishment and operation of our laboratory is subject to regulation by numerous federal, state and local governmental authorities in the U.S. Our laboratory holds a CLIA certificate of compliance and is licensed by every state (other than the State of New York) and the District of Columbia, as required, which enables us to provide testing services to residents of almost every state. Failure to comply with state regulations or changes in state regulatory requirements could result in a substantial curtailment or even prohibition of the operations of our laboratory and could materially adversely affect our business. CLIA is a federal law that regulates clinical laboratories that perform testing on human specimens for the purpose of providing information for the diagnosis, prevention or treatment of disease. To renew CLIA certification, laboratories are subject to survey and inspection every two years. Moreover, CLIA inspectors may make unannounced inspections of these laboratories. If we were to lose our CLIA certification or our state licenses, whether as a result of a revocation, suspension or limitation of our license, we would no longer be able to continue our testing operations, which would materially adversely affect our business, prospects and financial condition. Potential sanctions for violations of these statutes and regulations also include significant fines, the suspension or loss of various licenses, certificates and authorizations, or product suspension or recalls.
We are subject to other regulation in the United States by both the federal government and the states in which we conduct our business, as well as in other jurisdictions outside of the United States, including:
| • | Medicare billing and payment regulations applicable to clinical laboratories; |
| • | the Federal Anti-kickback Law and state anti-kickback prohibitions; |
| • | the Federal physician self-referral prohibition, commonly known as the Stark Law, and the state equivalents; |
| • | the Federal Health Insurance Portability and Accountability Act of 1996; |
| • | the Medicare civil money penalty and exclusion requirements; |
| • | the Federal False Claims Act civil and criminal penalties and state equivalents; and |
| • | the Foreign Corrupt Practices Act, the United Kingdom Anti-bribery Act and the European Data Protection Directive, all of which apply to our international activities. |
We have adopted policies and procedures designed to comply with these laws. In the ordinary course of our business, we conduct internal reviews of our compliance with these laws. Our compliance is also subject to
27
Table of Contents
governmental review. The growth of our business and our expansion outside of the United States may increase the potential of violating these laws or our internal policies and procedures. The risk of our being found in violation of these or other laws and regulations is further increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of these laws and regulations, we may be subject to any applicable penalty associated with the violation, including civil and criminal penalties, damages and fines, we could be required to refund payments received by us, and we could be required to curtail or cease our operations. Any of the foregoing consequences could seriously harm our business and our financial results.
If we use biological and hazardous materials in a manner that causes injury, we could be liable for damages.
Our activities currently require the controlled use of potentially harmful biological materials and chemicals. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have. Additionally, we are subject to, on an ongoing basis, federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations may become significant and could materially adversely affect our business, prospects and financial condition. Moreover, in the event of an accident or if we otherwise fail to comply with applicable regulations, we could lose our permits or approvals or be held liable for damages or penalized with fines.
Health care reform measures could adversely affect our business.
In the United States and foreign jurisdictions, there have been, and continue to be, a number of legislative and regulatory changes and proposed changes to the healthcare system that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs. In 2010, the PPACA was enacted, which includes measures to significantly change the way health care is financed by both governmental and private insurers. Among the provisions of the PPACA of greatest importance to the pharmaceutical and biotechnology industry are the following:
| • | an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs; |
| • | implementation of the federal physician payment transparency requirements, sometimes referred to as the “Physician Payments Sunshine Act”; |
| • | a licensure framework for follow-on biologic products; |
| • | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; |
| • | establishment of a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending; |
| • | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program, to 23.1% and 13% of the average manufacturer price for most branded and generic drugs, respectively and capped the total rebate amount for innovator drugs at 100% of the Average Manufacturer Price, or AMP; |
28
Table of Contents
| • | a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for certain drugs and biologics, including our product candidates, that are inhaled, infused, instilled, implanted or injected; |
| • | extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; |
| • | expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for individuals with income at or below 133% of the federal poverty level, thereby potentially increasing manufacturers’ Medicaid rebate liability; |
| • | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; and |
| • | expansion of the entities eligible for discounts under the Public Health program. |
Some of the provisions of the PPACA have yet to be implemented, and there have been legal and political challenges to certain aspects of the PPACA. Since January 2017, President Trump has signed two executive orders and other directives designed to delay, circumvent, or loosen certain requirements mandated by the PPACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the PPACA. While Congress has not passed repeal legislation, the Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the PPACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. Congress may consider other legislation to repeal or replace elements of the PPACA.
Many of the details regarding the implementation of the PPACA are yet to be determined, and at this time, the full effect that the PPACA would have on our business remains unclear. In particular, there is uncertainty surrounding the applicability of the biosimilars provisions under the PPACA to our product candidates. The FDA has issued several guidance documents, but no implementing regulations, on biosimilars. A number of biosimilar applications have been approved over the past few years. It is not certain that we will receive 12 years of biologics marketing exclusivity for any of our products. The regulations that are ultimately promulgated and their implementation are likely to have considerable impact on the way we conduct our business and may require us to change current strategies. A biosimilar is a biological product that is highly similar to an approved drug notwithstanding minor differences in clinically inactive components, and for which there are no clinically meaningful differences between the biological product and the approved drug in terms of the safety, purity, and potency of the product.
Individual states have become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access, and marketing cost disclosure and transparency measures, and to encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce ultimate demand for our products or put pressure on our product pricing, which could negatively affect our business, results of operations, financial condition and prospects.
In addition, given recent federal and state government initiatives directed at lowering the total cost of healthcare, Congress and state legislatures will likely continue to focus on healthcare reform, the cost of
29
Table of Contents
prescription drugs and biologics and the reform of the Medicare and Medicaid programs. While we cannot predict the full outcome of any such legislation, it may result in decreased reimbursement for drugs and biologics, which may further exacerbate industry-wide pressure to reduce prescription drug prices. This could harm our ability to generate revenues. Increases in importation or re-importation of pharmaceutical products from foreign countries into the United States could put competitive pressure on our ability to profitably price our products, which, in turn, could adversely affect our business, results of operations, financial condition and prospects. We might elect not to seek approval for or market our products in foreign jurisdictions in order to minimize the risk of re-importation, which could also reduce the revenue we generate from our product sales. It is also possible that other legislative proposals having similar effects will be adopted.
Furthermore, regulatory authorities’ assessment of the data and results required to demonstrate safety and efficacy can change over time and can be affected by many factors, such as the emergence of new information, including on other products, changing policies and agency funding, staffing and leadership. We cannot be sure whether future changes to the regulatory environment will be favorable or unfavorable to our business prospects. For example, average review times at the FDA for marketing approval applications can be affected by a variety of factors, including budget and funding levels and statutory, regulatory and policy changes.
Risks Related to Our Intellectual Property
If we are unable to protect our intellectual property effectively, we may be unable to prevent third parties from using our technologies, which would impair our competitive advantage.
We rely on patent protection as well as a combination of trademark, copyright and trade secret protection, and other contractual restrictions, to protect our proprietary technologies, all of which provide limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. We may not be successful in defending challenges made in connection with our patents and patent applications. If we fail to protect our intellectual property, we will be unable to prevent third parties from using our technologies and they will be able to compete more effectively against us.
In addition to our patents, we rely on contractual restrictions to protect our proprietary technology. We require our employees and third parties to sign confidentiality agreements and our employees are also required to sign agreements assigning to us all intellectual property arising from their work for us. Nevertheless, we cannot guarantee that these measures will be effective in protecting our intellectual property rights. Any failure to protect our intellectual property rights could materially adversely affect our business, prospects and financial condition.
Our currently pending or future patent applications may not result in issued patents and any patents issued to us may be challenged, invalidated or held unenforceable. Furthermore, we cannot be certain that we were the first to make the invention claimed in our issued patents or pending patent applications in the U.S., or that we were the first to file for protection of the inventions claimed in our foreign issued patents or pending patent applications. In addition, there are numerous recent changes to the patent laws and proposed changes to the rules of the U.S. Patent and Trademark Office (“PTO”) which may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. For example, in September 2011, the U.S. enacted sweeping changes to the U.S. patent system under the Leahy-Smith America Invents Act, including changes that would transition the U.S. from a “first-to-invent” system to a “first-to-file” system and alter the processes for challenging issued patents. These changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. In addition, we may become subject to interference proceedings conducted in the patent and trademark offices of various countries to determine our entitlement to patents, and these proceedings may conclude that other patents or patent applications have priority over our patents or patent applications. It is also possible that a competitor may successfully challenge our patents through various proceedings and those challenges may result in the elimination or narrowing of our patents, and therefore reduce our patent protection. Accordingly, rights under
30
Table of Contents
any of our issued patents, patent applications or future patents may not provide us with commercially meaningful protection for our products or afford us a commercial advantage against our competitors or their competitive products or processes.
The patents issued to us may not be broad enough to provide any meaningful protection, one or more of our competitors may develop more effective technologies, designs or methods without infringing our intellectual property rights and one or more of our competitors may design around our proprietary technologies.
If we are not able to protect our proprietary technology, trade secrets and know-how, our competitors may use our inventions to develop competing products. We own certain patents relating to our cell-free molecular diagnostic technology. However, these patents may not protect us against our competitors, and patent litigation is very expensive. We may not have sufficient cash available to pursue any patent litigation to its conclusion because we currently do not generate revenues other than licensing, milestone and royalty income.
We cannot rely solely on our current patents to be successful. The standards that the PTO and foreign patent offices use to grant patents, and the standards that U.S. and foreign courts use to interpret patents, are not the same, are not always applied predictably or uniformly and can change, particularly as new technologies develop. As such, the degree of patent protection obtained in the U.S. may differ substantially from that obtained in various foreign countries. In some instances, patents have been issued in the U.S. while substantially less or no protection has been obtained in Europe or other countries.
We cannot be certain of the level of protection, if any, that will be provided by our patents if they are challenged in court, where our competitors may raise defenses such as invalidity, unenforceability or possession of a valid license. In addition, the type and extent of any patent claims that may be issued to us in the future are uncertain. Any patents that are issued may not contain claims that will permit us to stop competitors from using similar technology.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights and we may be unable to protect our rights to, or use, our cell-free molecular diagnostic technology.
Third parties may challenge the validity of our patents and other intellectual property rights, resulting in costly litigation or other time-consuming and expensive proceedings, which could deprive us of valuable rights. If we become involved in any intellectual property litigation, interference or other judicial or administrative proceedings, we will incur substantial expenses and the attention of our technical and management personnel will be diverted. An adverse determination may subject us to significant liabilities or require us to seek licenses that may not be available from third parties on commercially favorable terms, if at all. Further, if such claims are proven valid, through litigation or otherwise, we may be required to pay substantial monetary damages, which can be tripled if the infringement is deemed willful, or be required to discontinue or significantly delay development, marketing, selling and licensing of the affected products and intellectual property rights. In our European patent that covers using microRNAs to detect in vivo cell death, an anonymous third party has recently filed an opposition against the claims in the patent. Oppositions against the patentability of claims in a European patent are considered by a panel of examiners at the European Patent Office, and we are considering the full range of options available for defending against the opposition.
Our competitors may have filed, and may in the future file, patent applications covering technology similar to ours. Any such patent application may have priority over our patent applications and could further require us to obtain rights to issued patents covering such technologies. There may be third-party patents, patent applications and other intellectual property relevant to our potential products that may block or compete with our potential products or processes. If another party has filed a U.S. patent application on inventions similar to ours, we may have to participate in an interference proceeding declared by the PTO to determine priority of invention in the U.S. The costs of these proceedings could be substantial, and it is possible that such efforts would be
31
Table of Contents
unsuccessful, resulting in a loss of our U.S. patent position with respect to such inventions. In addition, we cannot assure you that we would prevail in any of these suits or that the damages or other remedies that we are ordered to pay, if any, would not be substantial. Claims of intellectual property infringement may require us to enter into royalty or license agreements with third parties that may not be available on acceptable terms, if at all. We may also be subject to injunctions against the further development and use of our technology, which could materially adversely affect our business, prospects and financial condition.
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could materially adversely affect our ability to raise the funds necessary to continue our operations.
Certain rights that we in-license from third-parties are not within our control, and we may be negatively impacted it we lose those rights.
We license some of the technology that is necessary for our products and services from third parties. In connection with such in-licenses, we may agree to pay the licensor royalties based on sales of our products, which become a cost of product revenues and impact the margins on our products and services. We may need to in-license other technologies in the future to commercialize on our products and services. We may also need to negotiate licenses after launching our products and services. Our business may suffer if any such licenses terminate, if the licensors fail to abide by the terms of the license, if the licensed patents or other rights are found to be invalid, or if we are unable to enter into necessary licenses on acceptable terms.
Risks Related to Ownership of Our Common Stock
If we discover material weaknesses and other deficiencies in our internal control and accounting procedures, our stock price could decline significantly and raising capital could be more difficult.
If we fail to comply with the rules under the Sarbanes-Oxley Act, related to disclosure controls and procedures, or if we discover additional material weaknesses and other deficiencies in our internal control and accounting procedures, our stock price could decline significantly and raising capital could be more difficult. Moreover, effective internal controls are necessary for us to produce reliable financial reports and are important in helping prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock could drop significantly. We previously identified a material weakness in our internal control over financial reporting as of December 31, 2012, which was remedied in the year ended December 31, 2013. We cannot be certain that additional material weaknesses or significant deficiencies in our internal controls will not be discovered in the future.
Our ability to use our net operating loss carry-forwards and certain other tax attributes is limited by Sections 382 and 383 of the Internal Revenue Code.
Net operating loss carryforwards allow companies to use past year net operating losses to offset against future years’ profits, if any, to reduce future tax liabilities. Sections 382 and 383 of the Internal Revenue Code of 1986, as amended (“Code”) limit a corporation’s ability to utilize its net operating loss carryforwards and certain other tax attributes (including research credits) to offset any future taxable income or tax if the corporation experiences a cumulative ownership change of more than 50% over any rolling three year period. State net operating loss carryforwards (and certain other tax attributes) may be similarly limited. An ownership change can therefore result in significantly greater tax liabilities than a corporation would incur in the absence of such a change and any increased liabilities could adversely affect the corporation’s business, results of operations, financial condition and cash flow.
32
Table of Contents
U.S. federal income tax reform could adversely affect us.
On December 22, 2017, President Trump signed into law the “Tax Cuts and Jobs Act” (“TCJA”) that significantly reforms the Code. The TCJA, among other things, includes changes to U.S. federal tax rates, imposes significant additional limitations on the deductibility of interest, allows for the expensing of capital expenditures, and puts into effect the migration from a “worldwide” system of taxation to a territorial system. We do not expect tax reform to have a material impact to our projection of minimal cash taxes or to our net operating losses. Our net deferred tax assets and liabilities will be revalued at the newly enacted U.S. corporate rate, and the impact will be recognized in our tax expense in the year of enactment with a corresponding adjustment to its valuation allowance for the period ended December 31, 2017. Further, any eligibility we may have or may someday have for tax credits associated with the qualified clinical testing expenses arising out of the development of orphan drugs will be reduced to 25% as a result of the TCJA; thus, our net future taxable income may be affected. We continue to examine the impact this tax reform legislation may have on our business. The impact of this tax reform on holders of our common stock is uncertain and could be adverse.
The rights of the holders of our common stock may be impaired by the potential issuance of preferred stock.
Our certificate of incorporation gives our board of directors the right to create one or more new series of preferred stock. As a result, the board of directors may, without stockholder approval, issue preferred stock with voting, dividend, conversion, liquidation or other rights that could adversely affect the voting power and equity interests of the holders of our common stock. Preferred stock, which could be issued with the right to more than one vote per share, could be used to discourage, delay or prevent a change of control of our Company, which could materially adversely affect the price of our common stock. We have designated 60,600 shares of preferred stock as Series A Convertible Preferred Stock and, subject to the terms of such series, we may create additional series of preferred stock in the future with voting, dividend, conversion, liquidation or other rights that could adversely affect the voting power and equity interests of the holders of our common stock.
Our common stock price may be volatile and could fluctuate widely in price, which could result in substantial losses for investors.
The market price of our common stock historically has been, and we expect will continue to be, subject to significant fluctuations over short periods of time. For example, during the year ended December 31, 2017, the closing price of our common stock ranged from a low of $0.25 to a high of $2.40. These fluctuations may be due to various factors, many of which are beyond our control, including:
| • | technological innovations or new products and services introduced by us or our competitors; |
| • | clinical trial results relating to our tests or those of our competitors; |
| • | announcements or press releases relating to the industry or to our own business or prospects; |
| • | coverage and reimbursement decisions by third party payors, such as Medicare and other managed care organizations; |
| • | regulation and oversight of our product candidates and services, including by the FDA, Centers for Medicare & Medicaid Services and comparable foreign agencies; |
| • | the establishment of partnerships with clinical reference laboratories; |
| • | healthcare legislation; |
| • | intellectual property disputes; |
| • | additions or departures of key personnel; |
| • | sales of our common stock; |
33
Table of Contents
| • | our ability to integrate operations, technology, products and services; |
| • | our ability to execute our business plan; |
| • | operating results below expectations; |
| • | loss of any strategic relationship; |
| • | industry developments; |
| • | economic and other external factors; and |
| • | period-to-period fluctuations in our financial results. |
In addition, market fluctuations, as well as general political and economic conditions, could materially adversely affect the market price of our securities. Because we are a development stage company with no revenue from operations to date, other than licensing, milestone and royalty income, you should consider any one of these factors to be material. Our stock price may fluctuate widely as a result of any of the foregoing.
Because certain of our stockholders control a significant number of shares of our common stock, they may have effective control over actions requiring stockholder approval.
As of March 31, 2018, our directors, executive officers and principal stockholders, and their respective affiliates, beneficially owned approximately 8.3% of our outstanding shares of common stock. As a result, these stockholders, acting together, would have the ability to control the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. In addition, these stockholders, acting together, would have the ability to control the management and affairs of our Company. Accordingly, this concentration of ownership may harm the market price of our common stock by:
| • | delaying, deferring or preventing a change in control of our Company; |
| • | impeding a merger, consolidation, takeover or other business combination involving us; or |
| • | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
We have not paid dividends on our common stock in the past and do not expect to pay dividends on our common stock for the foreseeable future. Any return on investment may be limited to the value of our common stock.
We have never paid any cash dividends on our common stock. We expect that we will devote any income we receive from operations to our future operations and growth. We do not expect to pay cash dividends on our common stock in the near future. Payment of dividends would depend upon our profitability at the time, cash available for those dividends, and other factors that our board of directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on an investor’s investment will only occur if our stock price appreciates. In addition, the terms of the Series A Convertible Preferred Stock prohibit us from paying dividends to the holders of our common stock so long as any dividends due on the Series A Convertible Preferred Stock remain unpaid. Investors in our common stock should not rely on an investment in our Company if they require dividend income.
Delaware law and our corporate charter and bylaws contain anti-takeover provisions that could delay or discourage takeover attempts that stockholders may consider favorable.
Provisions in our certificate of incorporation and bylaws may have the effect of delaying or preventing a change of control of our company or changes in our management. For example, our board of directors has the
34
Table of Contents
authority to issue up to 20,000,000 shares of preferred stock in one or more series and to fix the powers, preferences and rights of each series without stockholder approval. The ability to issue preferred stock could discourage unsolicited acquisition proposals or make it more difficult for a third party to gain control of our Company, or otherwise could materially adversely affect the market price of our common stock.
Furthermore, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the General Corporation Law of the State of Delaware. This provision may prohibit or restrict large stockholders, in particular those owning 15% or more of our outstanding voting stock, from merging or combining with us, which could discourage potential takeover attempts, reduce the price that investors may be willing to pay for shares of our common stock in the future and result in our market price being lower than it would without these provisions.
A sale of a substantial number of shares of our common stock may cause the price of our common stock to decline and may impair our ability to raise capital in the future.
Our common stock is traded on the Nasdaq Capital Market and could be considered “thinly-traded,” meaning that the number of investors interested in purchasing our common stock at or near bid prices at any given time may be relatively small or non-existent. Finance transactions resulting in a large amount of newly issued shares that become readily tradable, or other events that cause current stockholders to sell shares, could place downward pressure on the trading price of our common stock. In addition, the lack of a robust resale market may require a stockholder who desires to sell a large number of shares of common stock to sell the shares in increments over time to mitigate any adverse impact of the sales on the market price of our stock.
If our stockholders sell, or the market perceives that our stockholders may sell for various reasons, including the ending of restriction on resale, substantial amounts of our common stock in the public market, including shares issued upon the exercise of outstanding options or warrants, the market price of our common stock could fall. Sales of a substantial number of shares of our common stock may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
We may be subject to stockholder litigation, thereby diverting our resources, which could materially adversely affect our profitability and results of operations.
The market for our common stock is characterized by significant price volatility, and we expect that our share price will continue to be at least as volatile for the indefinite future. In the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price for its securities. In addition, stockholders may bring actions against companies relating to past transactions or other matters. Any such actions could give rise to substantial damages and thereby materially adversely affect our consolidated financial position, liquidity or results of operations. Even if an action is not resolved against us, the uncertainty and expense associated with stockholder actions could materially adversely affect our business, prospects and financial condition. Litigation can be costly, time-consuming and disruptive to business operations. The defense of lawsuits could also result in diversion of our management’s time and attention away from business operations, which could harm our business.
Risks Related to this Offering
Our management will have broad discretion as to the use of the net proceeds from this offering.
We cannot specify with certainty the particular uses of the net proceeds we will receive from this offering, and these uses may vary from our current plans. Our management will have broad discretion in the application of the net proceeds, including for any of the purposes described in “Use of Proceeds.” Accordingly, you will have to rely upon the judgment of our management with respect to the use of the proceeds. Our
35
Table of Contents
management may spend a portion or all of the net proceeds from this offering in ways that holders of our securities may not desire or that may not yield a significant return or any return at all. The failure by our management to apply these funds effectively could harm our business. Pending their use, we may also invest the net proceeds from this offering in a manner that does not produce income or that loses value.
If we fail to comply with the continued minimum closing bid requirements of the Nasdaq Capital Market LLC (“Nasdaq”) or other requirements for continued listing, our common stock may be delisted and the price of our common stock and our ability to access the capital markets could be negatively impacted.
On September 5, 2017, we received a written notice (the “Notice”) from the Nasdaq Stock Market LLC (“Nasdaq”) that we were not in compliance with Nasdaq Listing Rule 5550(a)(2), as the minimum bid price of our common stock has been below $1.00 per share for 30 consecutive business days. The Notice had no immediate effect on the listing of our common stock, and our common stock continues to trade on the Nasdaq Capital Market. In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we initially had a period of 180 calendar days, or until March 5, 2018, to regain compliance with the minimum bid price requirement. On March 6, 2018, we were notified by Nasdaq that we are eligible for an additional 180 calendar day period until September 4, 2018 to regain compliance with the minimum $1.00 bid price per share requirement. To regain compliance, the closing bid price of our common stock must meet or exceed $1.00 per share for at least 10 consecutive business days during this 180 calendar day period. If we do not regain compliance within the allotted compliance period(s), including any extensions that may be granted by Nasdaq or fail to comply with or other requirements for continued listing, our common stock may be delisted and the price of our common stock and our ability to access the capital markets could be negatively impacted. A delisting of our common stock from the Nasdaq Capital Market could materially reduce the liquidity of our common stock and result in a corresponding material reduction in the price of our common stock. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, employees and fewer business development opportunities.
The warrants are speculative in nature.
The warrants do not confer any rights of common stock ownership on its holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at a fixed price for a limited period of time. Specifically, for a period of ___ years commencing upon the date of issuance, holders of the warrants may exercise their right to acquire the common stock and pay an exercise price equal to ___% of the offering price per Class A Unit. Moreover, the market value of the warrants is uncertain and the warrants will not be listed or quoted for trading on any market or exchange. There can be no assurance that the market price of the common stock will ever equal or exceed the exercise price of the warrants, and consequently, whether it will ever be profitable for holders of the warrants to exercise the warrants.
A large number of shares issued in this offering may be sold in the market following this offering, which may depress the market price of our common stock.
A large number of shares issued in this offering may be sold in the market following this offering, which may depress the market price of our common stock. Sales of a substantial number of shares of our common stock in the public market following this offering could cause the market price of our common stock to decline. If there are more shares of common stock offered for sale than buyers are willing to purchase, then the market price of our common stock may decline to a market price at which buyers are willing to purchase the offered shares of common stock and sellers remain willing to sell the shares. All of the shares of common stock issued in the offering will be freely tradable without restriction or further registration under the Securities Act.
There is no public market for the warrants or the Series B Preferred.
There is no established public trading market for the warrants or the Series B Preferred offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the warrants or
36
Table of Contents
the Series B Preferred on any national securities exchange or other nationally recognized trading system, including the Nasdaq Capital Market. Without an active market, the liquidity of the warrants and the Series B Preferred will be limited.
You will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future.
You will incur immediate and substantial dilution as a result of this offering. After giving effect to the sale by us of shares offered in this offering at an assumed public offering price of $[ ] per share, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us, investors in this offering can expect an immediate dilution of approximately $[ ] per share. See “Dilution” below for a more detailed discussion of the dilution you will incur if you purchase our common stock in the offering.
Holders of Series B Preferred will have limited voting rights.
Holders of Series B Preferred will vote with the common stock on an as-converted to common stock basis, provided, however, that in no event will a holder of shares of Series B Preferred Stock be entitled to vote a number of shares in excess of such holder’s beneficial ownership limitation. See “Description of Securities We Are Offering.”
37
Table of Contents
We estimate that the net proceeds of this offering will be approximately $[ ] million, from the sale of our securities in this offering (or $ if the underwriter exercises in full its over-allotment option) after deducting the underwriter fees and estimated offering expenses payable by us. The public offering price per unit was negotiated between us and the underwriter based on market conditions at the time of pricing, and represents a discount to the current market price of our common stock. This amount excludes the proceeds, if any, from the exercise of warrants in this offering. If all of the warrants sold in this offering were to be exercised in cash at an assumed exercise price of $[ ] per share, we would receive additional net proceeds of approximately $[ ] million (excluding any proceeds from exercise of warrants issued pursuant to the over-allotment option). We cannot predict when or if these warrants will be exercised. It is possible that these warrants may expire and may never be exercised.
We intend to use the net proceeds received from this offering to fund our research and development activities and for working capital and general corporate purposes.
We have not yet determined the amount of net proceeds to be used specifically for any of the foregoing purposes. Accordingly, we will retain broad discretion over the use of these proceeds. Pending any use as described above, we intend to invest the net proceeds in high-quality, short-term, interest-bearing securities.
38
Table of Contents
We have never declared or paid cash dividends on our common stock. We currently intend to retain our future earnings, if any, for use in our business and therefore do not anticipate paying cash dividends in the foreseeable future. Payment of future dividends, if any, will be at the discretion of our board of directors after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs and plans for expansion. Pursuant to the terms of the Series A Convertible Preferred Stock, dividends cannot be paid to the holders of our common stock so long as any dividends due on the Series A Convertible Preferred Stock remain unpaid.
If you purchase shares in this offering, your interest will be diluted to the extent of the difference between the public offering price per Class A Unit and the net tangible book value per share of our common stock after this offering. Our net tangible book value as of March 31, 2018 was $4,442,185, or $0.08 per share of common stock (based upon 58,832,953 outstanding shares of common stock). “Net tangible book value” is total assets minus the sum of liabilities and intangible assets. “Net tangible book value per share” is net tangible book value divided by the total number of shares of common stock outstanding.
After giving effect to the sale by us in this offering of [ ] Class A Units at an assumed public offering price of $[ ] per Class A Unit (the closing price of our common stock as quoted on the Nasdaq Capital Market on [ ], 2018) and [ ] Class B Units at a public offering price of $1,000 per Class B Unit, and assuming all Series B Preferred Shares included in the Class B units were converted to common stock, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses that we will pay, our net tangible book value as of March 31, 2018 would have been approximately $[ ], or $[ ] per share of common stock. This amount represents an immediate increase in net tangible book value of $[ ] per share to existing stockholders and an immediate dilution of $[ ] per share to purchasers in this offering.
The following table illustrates the dilution:
| Assumed public offering price per Class A Unit |
$ | |||||||
| Net tangible book value per share as of March 31, 2018 |
$ | 0.08 | ||||||
| Increase in net tangible book value per share attributable to this offering |
$ | |||||||
|
|
|
|||||||
| Pro forma net tangible book value per share after this offering |
$ | |||||||
|
|
|
|||||||
| Dilution per share to new investors |
$ | |||||||
|
|
|
The above table is based on 58,832,953 shares of common stock outstanding as of March 31, 2018, assumes no exercise by the underwriter of its over-allotment option and excludes as of that date:
| • | 7,588,306 shares of our common stock issuable upon exercise of outstanding options at a weighted average price of $2.49 per share; |
| • | 369,600 shares of our common stock issuable upon vesting of restricted stock units; |
| • | 18,418,853 shares of our common stock issuable upon exercise of outstanding warrants with a weighted-average exercise price of $1.11 per share; |
| • | 63,125 shares of our common stock issuable upon conversion of outstanding shares of Series A Convertible Preferred Stock; |
| • | 338,957 shares of our common stock that are reserved for equity awards that may be granted under our equity incentive plans; and |
| • | [ ] shares of our common stock issuable upon exercise of the warrants offered hereby. |
39
Table of Contents
If the underwriters exercise in full their over-allotment option, our net tangible book value per share after giving effect to this offering would be approximately $[ ], or $[ ] per share, which amount represents an immediate increase in net tangible book value of $[ ] per share to existing stockholders and a dilution to new investors of $[ ] per share.
If we issue any additional shares in connection with outstanding options or warrants, there will be additional dilution.
40
Table of Contents
The following table sets forth our cash and cash equivalents and our capitalization as of March 31, 2018 on:
| • | an actual basis; and |
| • | on a pro forma basis to give effect to the sale by us in this offering of [ ] Class A Units, at the assumed public offering price of $[ ] per Class A Unit and [ ] Class B Units, at the public offering price of $1,000 per Class B Unit, assuming conversion of all Series B Preferred Shares included in the Class B Units, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
| As of March 31, 2018 | ||||||||
| Actual | Pro Forma | |||||||
| Cash, cash equivalents and restricted cash |
$ | 6,657,158 | $ | |||||
|
|
|
|
|
|||||
| Stockholders’ equity: |
||||||||
| Preferred Stock, par value $0.001; 20,000,000 shares authorized; 60,600 shares of Series A Convertible Preferred Stock issued and outstanding |
60 | |||||||
| Common Stock, par value $0.0001; 150,000,000 shares authorized; 58,832,953 shares issued and outstanding, actual; [ ] shares issued and outstanding pro forma |
5,883 | |||||||
| Additional paid-in capital |
182,401,648 | |||||||
| Accumulated deficit |
(177,728,501 | ) | ||||||
|
|
|
|||||||
| Total stockholders’ equity |
4,679,090 | |||||||
|
|
|
|||||||
| Total capitalization |
4,679,090 | |||||||
|
|
|
|||||||
The above table is based on 58,832,953 shares of common stock outstanding as of March 31, 2018, assumes no exercise by the underwriter of its over-allotment option and excludes as of that date:
| • | 7,588,306 shares of our common stock issuable upon exercise of outstanding options at a weighted average price of $2.49 per share; |
| • | 369,600 shares of our common stock issuable upon vesting of restricted stock units; |
| • | 18,418,853 shares of our common stock issuable upon exercise of outstanding warrants with a weighted-average exercise price of $1.11 per share; |
| • | 63,125 shares of our common stock issuable upon conversion of outstanding shares of Series A Convertible Preferred Stock; |
| • | 338,957 shares of our common stock that are reserved for equity awards that may be granted under our equity incentive plans; and |
| • | [ ] shares of our common stock issuable upon exercise of the warrants offered hereby. |
41
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
We are a clinical-stage oncology therapeutics company. Our primary focus is to develop oncology therapeutics for the treatment of hematologic and solid tumor cancers for improved cancer care utilizing our technology in tumor genomics.
On March 15, 2017, we announced that we licensed PCM-075, a PLK1 inhibitor, from Nerviano, pursuant to a license agreement with Nerviano dated March 13, 2017. PCM-075 was developed to have high selectivity to PLK1 (at low nanomolar IC50 levels), to be administered orally, and to have a relatively short drug half-life of approximately 24 hours compared to other pan PLK inhibitors. A safety study of PCM-075 has been successfully completed in patients with advanced metastatic solid tumors and published in 2017 in Investigational New Drugs. We currently are enrolling a Phase 1b/2 open-label clinical trial of PCM-075 in combination with standard-of-care chemotherapy in patients with AML. The Phase 1b/2 clinical trial is led by Hematologist Jorge Eduardo Cortes, M.D., Deputy Department Chair, Department of Leukemia, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center. In addition, we are working with Dr. David Einstein at the Genitourinary Oncology Program at Beth Israel Deaconess Medical Center and Harvard Medical School as the principal investigator on a Phase 2 open-label clinical trial of PCM-075 in combination with abiraterone acetate (Zytiga®) and prednisone in patients with mCRPC with plans to enroll patients later this year.
Our intellectual property and proprietary technology enables us to analyze ctDNA and clinically actionable biomarkers to identify patients most likely to respond to specific cancer therapies. We plan to continue to vertically integrate our tumor genomics technology with the development of targeted cancer therapeutics.
We believe PCM-075 is the only PLK1 selective ATP competitive inhibitor, administered orally, with apparent antitumor activity in different preclinical models, currently in clinical trials. Polo-like kinase family consists of 5 members (PLK1-PLK5) and they are involved in multiple functions in cell division, including the regulation of centrosome maturation, checkpoint recovery, spindle assembly, cytokinesis, apoptosis and many others. PLK1 is essential for the maintenance of genomic stability during cell division (“mitosis”). The overexpression of PLK1 can lead to immature cell division followed by aneuploidy and cell death, a hallmark of cancer. PLK1 is over-expressed in a wide variety of hematologic and solid tumor malignancies, including acute myeloid leukemia, prostate, lung, breast, ovarian and adrenocortical carcinoma. In addition, several studies have shown that over-expression of PLK1 is associated with poor prognosis.
Studies have shown that inhibition of polo-like-kinases can lead to tumor cell death, including a Phase 2 study in AML where response rates with a different PLK inhibitor were up to 31% were observed when used in conjunction with a standard therapy for AML (low-dose cytarabine-LDAC) versus treatment with LDAC alone with a 13.3% response rate. We believe the more selective nature of PCM-075 to PLK1, its 24-hour half-life and oral bioavailability, as well as the reversibility of its on-target hematological toxicities may prove useful in addressing clinical therapeutic needs across a variety of cancers.
PCM-075 has been tested in vivo in different xenograft and transgenic models suggesting tumor growth inhibition or tumor regression when used in combination with other therapies. PCM-075 has been tested for antiproliferative activity on a panel of 148 tumor cell lines and appeared highly active with an IC50 (a measure concentration for 50% target inhibition) below 100 nM in 75 cell lines and IC50 values below 1 uM in 133 out of 148 cell lines. PCM-075 also appears active in cells expressing multi-drug resistant (“MDR”) transporter proteins and we believe PCM-075’s apparent ability to overcome the MDR transporter resistance mechanism in cancer cells could prove useful in broader drug combination applications.
In preclinical studies, synergy (interaction of discrete drugs such that the total effect is greater than the sum of the individual effects) has been demonstrated with PCM-075 when used in combination with more than ten
42
Table of Contents
different chemotherapeutics, including cisplatin, cytarabine, doxorubicin, gemcitabine and paclitaxel, as well as targeted therapies, such as abiraterone acetate (Zytiga®), HDAC inhibitors, such as belinostat (Beleodaq®), Quizartinib (AC220), a development stage FLT3 inhibitor, and bortezomib (Velcade®). These therapeutics are used clinically for the treatment of many hematologic and solid tumor cancers, including AML, NHL, mCRPC, ACC, and TNBC.
On August 16, 2017, we announced results of preclinical research indicating potential synergy of PCM-075 with an investigational FLT3 Inhibitor, Quizartinib by Daiichi Sankyo, in FLT3 mutant xenograft mouse models. This synergy assessment study was conducted for us by a third-party contract research group. Approximately one third of AML patients harbor FLT3-mutated blood cancer cells. The FDA recently approved Rydapt® (midostaurin) by Novartis for the treatment of newly diagnosed adult patients with AML that are FLT3 mutation-positive in combination with cytarabine and daunorubicin induction and cytarabine consolidation chemotherapy. There are three FLT3 inhibitors in ongoing phase 3 trials, including Quizartinib. We believe that a combination of PCM-075 with a FLT3 inhibitor for AML patients with a FLT3 mutation could extend treatment response and possibly slow or reduce resistance to FLT3 activity.
On August 21, 2017, we announced results of preclinical research indicating potential synergy of PCM-075 with a HDAC inhibitor in NHL cell lines. This synergy assessment study was conducted by Dr. Steven Grant, Associate Director for Translational Research and co-Leader, Developmental Therapeutics Program, Massey Cancer Center. Patients with relapsed or refractory NHL, such as cutaneous T-cell lymphoma and peripheral T cell lymphoma, may be prescribed approved HDAC inhibitors and we believe this continues to be an area of unmet medical need. Dr. Grant’s data appeared to indicate that the combination of PCM-075 with Beleodaq® (belinostat), a HDAC inhibitor indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma, reduced cancer cells by up to 80% in two different forms of NHL (aggressive double-hit B-cell lymphoma and mantle-cell lymphoma) cell lines.
On October 11, 2017, we entered into a Patent Option Agreement with Massachusetts Institute of Technology (“MIT”) for the exclusive rights to negotiate a royalty-bearing, limited-term exclusivity license to practice world-wide patent rights to US Patent 9,566,280, subject to the rights of MIT (research, testing, and educational purposes), Ortho McNeil Pharmaceuticals-Janssen Pharmaceuticals and its Affiliates (internal research and pre-clinical drug development purposes including some laboratory research) and the federal government (government-funded inventions claimed in any patent rights and to exercise march in rights). This patent is generally directed to combination therapies including an antiandrogen or androgen antagonist and polo-like kinase inhibitor for the treatment of cancer. The Patent Option Agreement expires one-year from the effective date and includes other requirements to maintain the option period.
On October 18, 2017, we announced results of preclinical research indicating potential synergy of PCM-075 with abiraterone acetate in C4-2 prostate cancer cells. This synergy assessment study was conducted by Dr. Michael Yaffe, David H. Koch Professor of Biology and Biological Engineering at MIT. The results appeared to indicate that the combination of PCM-075 with Zytiga® (abiraterone acetate) decreased cell viability in mCRPC tumor cells and the apparent synergy observed was greater than the expected effect of combining the two drugs. Zytiga® is indicated for use in combination with prednisone for the treatment of patients with mCRPC who have received prior chemotherapy containing docetaxel. We believe there is an unmet medical need to improve on the resistance to hormone therapy and extend the benefit of response to abiraterone acetate for mCRPC patients.
On December 7, 2017, we announced results of preclinical research showing the sensitivity of TNBC cell lines to PCM-075, data featured as a Poster Presentation at the 40th San Antonio Breast Cancer Symposium. This synergy assessment study was conducted by Dr. Jesse Patterson and Dr. Michael Yaffe, at MIT. The results appeared to indicate that TNBC cell lines are 20-fold more sensitive to PCM-075 than estrogen receptor positive (ER+) breast cancer cell lines.
43
Table of Contents
Our accumulated deficit through March 31, 2018 is $177,728,501. To date, we have generated minimal revenues and expect to incur additional losses to perform further research and development activities and expand commercial operations.
During 2018, we have advanced our business with the following activities:
| • | Announced plans for a Phase 2 clinical trial evaluating the combination of PCM-075 and abiraterone acetate (Zytiga®- Johnson & Johnson) in patients with mCRPC. This study is designed to have 3 clinical sites, with Dr. David Einstein at the Genitourinary Oncology Program at Beth Israel Deaconess Medical Center and Harvard Medical School as the principal investigator. |
| • | Presented data showing synergy of PCM-075 in combination with Zytiga® in a mCRPC model at the 2018 Genitourinary Cancers Symposium (ASCO GU). |
| • | Activated six additional clinical trial sites, for a total of eight sites actively screening and enrolling patients, for our Phase1b/2 multicenter trial of PCM-075 in patients with AML. |
| • | Announced that the first patient successfully completed the cycle 1 of treatment in our Phase1b/2 multicenter trial of PCM-075 in combination with LDAC in patients with AML. The patient tolerated the combination well and correlative analyses of blood samples, taken at specified time points, also indicated activity on circulating leukemic cells. |
| • | Announced that two additional patients in the initial dose escalation cohort are on treatment and receiving a 12 mg/m2 oral, daily dose of PCM-075 (Days 1-5 in a 28-day cycle) in combination with LDAC, completing enrollment of the three patients in this cohort. Additionally, patient enrollment is also complete in the first Phase 1b dose-escalation cohort of three patients to receive a 12 mg/m2 oral, daily dose of PCM-075 (Days 1-5 in a 28-day cycle) in combination with decitabine. Subsequent to this announcement, one patient in the decitabine arm was removed from the trial prior to the end of the 28-day cycle due to unrelated disease progression and will be replaced to complete this dosing cohort. |
| • | Presented data showing that PCM-075 exhibits synergistic activity when combined with FLT3 inhibitors in a human xenograft AML model, at the American Association for Cancer Research (“AACR”) Annual Meeting in Chicago, IL. |
| • | Presented the methodology developed to track dynamic changes in blood leukemic cells, genomic alterations and PLK1 inhibition in patients treated with PCM-075 in combination with LDAC in its Phase 1b/2 clinical trial in AML, at the AACR Annual Meeting in Chicago, IL. |
Our drug development efforts are in their early stages, and we cannot estimate the costs or the time that our development efforts will take to complete, or the timing and amount of revenues we may generate from the sale of our drugs. The risk of completion of any program is high because of the many uncertainties involved in developing new drug candidates to market, including the long duration of clinical testing, the specific performance of proposed products under stringent clinical trial protocols, extended regulatory approval and review cycles, our ability to raise additional capital, the nature and timing of research and development expenses, and competing technologies being developed by organizations with significantly greater resources.
Critical Accounting Policies
Financial Reporting Release No. 60 requires all companies to include a discussion of critical accounting policies or methods used in the preparation of financial statements. Our accounting policies are described in Item 8. Financial Statements—Note 2 Basis of Presentation and Summary of Significant Accounting Policies in this prospectus. The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates. We believe that the following discussion represents our critical accounting policies.
44
Table of Contents
Revenue Recognition
Historically, our revenues have been generated from royalty, license and milestones related to agreements we have with other healthcare companies, medical laboratories and biotechnology partners. We also have revenues from our diagnostic services and clinical research services.
We recognize revenues when persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed or determinable, and collection is reasonably assured.
Royalty and License Revenues
We license and sublicense our patent rights to healthcare companies, medical laboratories and biotechnology partners. These agreements may involve multiple elements such as license fees, royalties and milestone payments. Revenue is recognized when the criteria described above have been met as well as the following:
| • | Up-front nonrefundable license fees pursuant to agreements under which we have no continuing performance obligations are recognized as revenues on the effective date of the agreement and when collection is reasonably assured. |
| • | Minimum royalties are recognized as earned, and royalties are earned based on the licensee’s use. We are unable to predict licensee’s sales and thus revenue is recognized upon receipt of notification from licensee and payment when collection is assured. Notification is generally one quarter in arrears. |
Diagnostic Service Revenue
Diagnostic service revenue, which consists of fees for clinical laboratory tests may come from several sources, including commercial third-party payors, such as insurance companies and health maintenance organizations, government payors, such as Medicare and Medicaid in the U.S., patient self-pay and, in some cases, from hospitals or referring laboratories who, in turn, bill third-party payors for testing.
Diagnostic service revenue will be recognized when the criteria described above has been met as well as upon cash collection until we can reliably estimate the amount that will be ultimately collected for our LDTs, at which time we will recognize revenues on an accrual basis.
Clinical Research Services Revenue
Revenue from clinical research services consists primarily of revenue from the sale of urine and blood collection supplies under agreements with our clinical research and business development partners. Revenue is recognized when supplies are delivered.
Cost of Revenue
Cost of revenue represents the cost of materials, personnel costs and costs associated with processing specimens including pathological review, quality control analyses, and delivery charges necessary to render an individualized test result. Costs associated with performing tests are recorded as the tests are processed. However, the revenue on diagnostic services is recognized on a cash collection basis resulting in costs incurred before the collection of related revenue.
Derivative Financial Instruments—Warrants
Our derivative financial instruments—warrants liabilities are related to warrants issued in connection with financing transactions and are therefore not designated as hedging instruments. All derivatives are recorded on our consolidated balance sheet at fair value in accordance with current accounting guidelines for such complex financial instruments.
45
Table of Contents
We have issued common stock warrants in connection with the execution of certain equity financings. Such warrants are classified as derivative liabilities under the provisions of the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 815, Derivatives and Hedging (“ASC 815”) or ASC 480 Distinguishing Liabilities from Equity (“ASC 480”) and are recorded at their fair market value as of each reporting period. Such warrants do not meet the exemption that a contract should not be considered a derivative instrument if it is (1) indexed to its own stock and (2) classified in stockholders’ equity. The warrants within the scope of ASC 480 contain a feature that could require the transfer of cash in the event a change of control occurs without an authorization of our Board of Directors, and therefore classified as a liability. Changes in fair value of derivative liabilities are recorded in the consolidated statement of operations under the caption “Gain (loss) from change in fair value of derivative financial instruments—warrants.”
The fair value of warrants is determined using the Black-Scholes option-pricing model using assumptions regarding volatility of our common stock price, remaining life of the warrant, and risk-free interest rates at each period end. Therefore we use model-derived valuations where inputs are observable in active markets to determine the fair value and accordingly classify such warrants in Level 3 per ASC Topic 820, Fair Value Measurements and Disclosures (“ASC 820”). At December 31, 2017 and 2016, the fair value of such warrants was $649,387 and $834,940, respectively, and was recorded as a liability under the caption “Derivative financial instruments—warrants” on the consolidated balance sheet.
Research and Development
Research and development expense, which includes expenditures in connection with an in-house research and development laboratory, salaries and staff costs, application and filing for regulatory approval of proposed products, regulatory and scientific consulting fees and clinical samples, as well as clinical collaborators and insurance, are accounted for in accordance with FASB ASC Topic 730-10-55-2, Research and Development. Also, as prescribed by this guidance, patent filing and maintenance expenses are considered legal in nature and therefore classified as general and administrative expense. We are providing the following summary of our research and development expense to supplement the more detailed discussions under “Results of Operations” below. Costs are not allocated to projects as the majority of the costs relate to employees and facilities costs and we do not track employees’ hours by project or allocate facilities costs on a project basis.
| For the years ended December 31, |
||||||||
| 2017 | 2016 | |||||||
| Salaries and staff costs |
$ | 2,568,263 | $ | 7,698,632 | ||||
| Outside services, consultants and lab supplies |
2,125,374 | 5,573,362 | ||||||
| Facilities |
1,064,561 | 1,434,101 | ||||||
| Other |
2,124,452 | 300,547 | ||||||
|
|
|
|
|
|||||
| Total research and development |
$ | 7,882,650 | $ | 15,006,642 | ||||
|
|
|
|
|
|||||
While certain of our research and development costs may have future benefits, our policy of expensing all research and development expenditures is predicated on the fact that we have no history of successful commercialization of molecular diagnostic products to base any estimate of the number of future periods that would be benefited.
FASB ASC Topic 730, Research and Development requires that non-refundable advance payments for goods or services that will be used or rendered for future research and development activities be deferred and capitalized. As the related goods are delivered or the services are performed, or when the goods or services are no longer expected to be provided, the deferred amounts are recognized as an expense.
46
Table of Contents
License Fees
We expense amounts paid to acquire licenses associated with products under development when the ultimate recoverability of the amounts paid is uncertain and the technology has no alternative future use when acquired. Acquisitions of technology licenses are charged to expense or capitalized based upon management’s assessment regarding the ultimate recoverability of the amounts paid and the potential for alternative future use. We have determined that technological feasibility for its product candidates is reached when the requisite regulatory approvals are obtained to make the product available for sale.
Restructuring
Restructuring costs are included in loss from operations in the consolidated statements of operations. We have accounted for these costs in accordance with ASC Topic 420, Exit or Disposal Cost Obligations. One-time termination benefits are recorded at the time they are communicated to the affected employees.
Stock-based Compensation
We rely heavily on incentive compensation in the form of stock options, restricted stock units (“RSU”) and restricted stock awards (“RSA”) to recruit, retain and motivate directors, executive officers, employees and consultants. Incentive compensation in the form of stock options, RSU, RSA and warrants is designed to provide long-term incentives, develop and maintain an ownership stake and conserve cash. Stock-based compensation expense related to stock options for employees and directors is recognized in the consolidated statement of operations based on estimated amounts, including the grant date fair value and the expected service period. We estimate the grant date fair value using a Black-Scholes model. Stock-based compensation recorded in our consolidated statement of operations is based on awards expected to ultimately vest and has been reduced for estimated forfeitures. We recognize the value of the awards on a straight-line basis over the awards’ requisite service periods. The requisite service period is generally the time over which our stock-based awards vest. Compensation expense for RSU and RSA is measured at the grant date and recognized ratably over the vesting period in the consolidated statement of operations. The fair value of RSU and RSA is determined based on the closing market price of our common stock on the grant date.
We account for equity instruments granted to non-employees in accordance with FASB ASC Topic 505-50 “Equity-Based Payment to Non-Employees”, where the value of the stock-based compensation is based upon the measurement date as determined at either: (1) the date at which a performance commitment is reached, or (2) the date at which the necessary performance to earn the equity instruments is complete. Accordingly, the fair value of these options is being “marked to market” quarterly until the measurement date is determined.
Fair Value of Financial Instruments
Financial instruments consist of cash and cash equivalents, short- term investments, accounts receivable, accounts payable, debt and derivative liabilities. We have adopted ASC 820 for financial assets and liabilities that are required to be measured at fair value and non-financial assets and liabilities that are not required to be measured at fair value on a recurring basis. These financial instruments are stated at their respective historical carrying amounts, which approximate fair value due to their short term nature as they reflect current market interest rates. Debt is stated at its respective historical carrying amounts, which approximate fair value as balances reflect current market interest rates.
47
Table of Contents
ASC 820 provides that the measurement of fair value requires the use of techniques based on observable and unobservable inputs. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect our market assumptions. The inputs create the following fair value hierarchy:
| • | Level 1 — Quoted prices for identical instruments in active markets. |
| • | Level 2 — Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations where inputs are observable or where significant value drivers are observable. |
| • | Level 3 — Instruments where significant value drivers are unobservable to third parties. |
Off-Balance Sheet Arrangements
As of March 31, 2018, we did not have any off-balance sheet arrangements as described by Item 303(a)(4) of Regulation S-K.
Recent Accounting Pronouncements
See Item 8. Financial Statements—Note 2 Basis of Presentation and Summary of Significant Accounting Policies in prospectus for a discussion of recent accounting pronouncements.
Results of Operations
Three Months Ended March 31, 2018 and 2017
Revenues
Our total revenues were $100,136 and $95,038 for the three months ended March 31, 2018 and 2017, respectively. The components of our revenues were as follows:
| Three Months Ended March 31, | ||||||||||||
| 2018 | 2017 | Increase (Decrease) | ||||||||||
| Royalties |
$ | 49,055 | $ | 65,826 | $ | (16,771 | ) | |||||
| Diagnostic services |
40,002 | 28,862 | 11,140 | |||||||||
| Clinical research |
11,079 | 350 | 10,729 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
$ | 100,136 | $ | 95,038 | $ | 5,098 | ||||||
|
|
|
|
|
|
|
|||||||
The decrease in royalty income is mainly a result of adoption of ASC 606. Based on the new revenue standards, we recorded approximately $78,000 to accumulated deficit rather than recognize it to revenue in the first quarter of 2018. See Note 3 to the condensed consolidated financial statements for detailed information. Revenue from diagnostic services is recognized when payment is received for the test results. Payments received was higher in 2018 as compared to the same period in the prior year. Revenue from clinical research consists of revenue from the sale of urine and blood collection supplies and tests performed under agreements with our clinical research and business development partners. Revenue is recognized when control of supplies and/or test results are transferred to customers (upon delivery). There were more sales for the three months ended March 31, 2018 as compared to the same period of 2017.
We expect our royalties to fluctuate as the royalties are sales-based or usage-based royalties on our IP license. Revenue recognition of the royalty depends on the timing and overall sales activities of the licensees. In addition, we expect a decrease in our diagnostic service revenue and clinical research revenue as we focus on develop oncology therapeutics.
48
Table of Contents
Cost of Revenues
Our total cost of revenues was $366,344 for the three months ended March 31, 2018, compared to $616,426 in the same period of 2017. Cost of revenues mainly relates to the costs of our diagnostic service revenues. The costs are recognized at the completion of testing. Decrease in cost of revenues for the three months ended March 31, 2018 compared to the same period of last year is mainly due to the lower volume of tests processed.
Research and Development Expenses
Research and development expenses consisted of the following:
| Three Months Ended March 31, | ||||||||||||
| 2018 | 2017 | Increase (Decrease) | ||||||||||
| Salaries and staff costs |
$ | 402,068 | $ | 875,377 | $ | (473,309 | ) | |||||
| Stock-based compensation |
395,709 | 372,200 | 23,509 | |||||||||
| Outside services, consultants and lab supplies |
849,988 | 634,794 | 215,194 | |||||||||
| Facilities |
191,391 | 367,901 | (176,510 | ) | ||||||||
| Travel and scientific conferences |
39,218 | 16,040 | 23,178 | |||||||||
| Fees, license and other |
5,464 | 2,013,518 | (2,008,054 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total research and development |
$ | 1,883,838 | $ | 4,279,830 | $ | (2,395,992 | ) | |||||
|
|
|
|
|
|
|
|||||||
Research and development expenses decreased by $2,395,992 to $1,883,838 for the three months ended March 31, 2018 from $4,279,830 for the same period in 2017. Our costs have decreased due primarily to the decreases in fees, license and other and salaries and staff costs. The decrease in fees, license and other was due to the $2.0 million license fee payment in March 2017 to Nerviano for development and commercialization rights to PCM-075. Our average internal research and development personnel decreased from nineteen to seven, resulting in a decrease of expenses in salaries and staff costs. In addition, as a result of the shifting of our business focus, we entered in new clinical studies related to oncology therapeutics which drove the increase in outside services costs. We expect a reduction of research and development costs that relate to CLIA services; however, other costs may increase as we complete the development of PCM-075.
Selling, General and Administrative Expenses
Selling, general and administrative expenses consisted of the following:
| Three Months Ended March 31, | ||||||||||||
| 2018 | 2017 | Increase (Decrease) | ||||||||||
| Salaries and staff costs |
$ | 690,170 | $ | 1,421,593 | $ | (731,423 | ) | |||||
| Board of Directors’ fees |
128,328 | 113,619 | 14,709 | |||||||||
| Stock-based compensation |
970,791 | 601,309 | 369,482 | |||||||||
| Outside services and consultants |
191,062 | 343,620 | (152,558 | ) | ||||||||
| Legal and accounting fees |
163,020 | 460,682 | (297,662 | ) | ||||||||
| Facilities and insurance |
255,053 | 269,338 | (14,285 | ) | ||||||||
| Travel and conferences |
56,457 | 283,933 | (227,476 | ) | ||||||||
| Fees, license and other |
50,096 | 110,530 | (60,434 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total general and administrative |
$ | 2,504,977 | $ | 3,604,624 | $ | (1,099,647 | ) | |||||
|
|
|
|
|
|
|
|||||||
Selling, general and administrative expenses decreased by $1,099,647 to $2,504,977 for the three months ended March 31, 2018, from $3,604,624 for the same period in 2017. The overall decrease in selling, general and administrative expenses was primarily due to the reduction in force. During the three months ended March 31, 2018 we decreased the number of our selling, marketing, and administrative personnel, bringing our average
49
Table of Contents
headcount to nine from seventeen in the same period of the prior year. The decrease of selling, general and administrative expenses was offset by an increase in stock-based compensation. Stock-based compensation, a non-cash expense, will fluctuate based on the timing and amount of options granted, forfeitures and the fair value of the options at the time of grant or remeasurement. Our selling, general and administrative costs may increase in future periods in order to support fundraising activities and general business activities as we continue to develop and introduce new product offerings.
Restructuring
On March 15, 2017, we announced a strategic restructuring plan in connection with the expansion of precision medicine therapeutics to our business. The restructuring plan included a reduction in force and was completed in the last quarter of 2017. Restructuring charges of approximately $1.7 million were incurred and had been included as a component of operating loss for the three months ended March 31, 2017.
Net Interest Expense
Net interest expense was $2,465 and $429,397 for the three months ended March 31, 2018 and 2017, respectively. The decrease of net interest expense is primarily due to a decrease in interest expense, resulting from pay-off of our $15.0 million term loan. We expect net interest expense to decrease as a result of repayment of our equipment line of credit.
Change in Fair Value of Derivative Financial Instruments — Warrants
We have issued warrants that are accounted for as derivative liabilities. As of March 31, 2018, the derivative financial instruments—warrants liabilities were revalued to $779,076, resulting in an increase in value of $129,689 from December 31, 2017, based primarily upon the increase in our stock price as well as the changes in the expected term, volatility, and risk free interest rates for the expected term. The increase in value was recorded as a loss from the change in fair value of derivative financial instruments—warrants in the condensed consolidated statement of operations.
Net Loss
Net loss and per share amounts were as follows:
| Three Months Ended March 31, | ||||||||||||
| 2018 | 2017 | Increase (Decrease) | ||||||||||
| Net loss attributable to common shareholders |
$ | (4,792,237 | ) | $ | (10,005,597 | ) | $ | (5,213,360 | ) | |||
| Net loss per common share — basic |
$ | (0.09 | ) | $ | (0.32 | ) | $ | (0.23 | ) | |||
| Net loss per common share — diluted |
$ | (0.09 | ) | $ | (0.32 | ) | $ | (0.23 | ) | |||
| Weighted average shares outstanding — basic |
55,364,438 | 30,961,014 | 24,403,424 | |||||||||
| Weighted average shares outstanding — diluted |
55,364,438 | 30,961,014 | 24,403,424 | |||||||||
The $5,213,360 decrease in net loss attributable to common shareholders and the $0.23 decrease in basic net loss per share was primarily the result of a decrease in operating expenses of $5,465,525 for the three months ended March 31, 2018 compared to the same period in the prior year. Basic net loss per share in 2018 was also impacted by the increase in basic weighted average shares outstanding resulting from the issuance of approximately 6.0 million shares of common stock upon the exercise of warrants as well as vesting of RSU.
50
Table of Contents
Years Ended December 31, 2017 and 2016
Revenues
Our total revenues were $505,404 and $381,072 for the years ended December 31, 2017 and 2016, respectively. Total revenues consisted of the following:
| For the years ended December 31, | ||||||||||||
| 2017 | 2016 | (Decrease)/Increase | ||||||||||
| Royalty income |
$ | 285,444 | $ | 258,062 | $ | 27,382 | ||||||
| Diagnostic service revenue |
196,111 | 86,137 | 109,974 | |||||||||
| Clinical research services |
23,849 | 36,873 | (13,024 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
$ | 505,404 | $ | 381,072 | $ | 124,332 | ||||||
|
|
|
|
|
|
|
|||||||
The $27,382 increase in royalty income in the year ended December 31, 2017 is primarily a result of higher royalty payments earned in excess of minimum royalty payments in the current year compared to the year ended December 31, 2016. According to our revenue recognition policy, we do not record royalty revenues in excess of minimum royalty amounts until we have received payment of such royalties.
Diagnostic service revenue is recognized when payment is received for the test results as long as all the other revenue criteria are completed. The number of tests payments received were higher in the year ended December 31, 2017 as compared to the prior year.
Revenue from clinical research services consists primarily of revenue from the sale of urine and blood collection supplies and sample processing under agreements with our clinical research and business development partners. Revenue is recognized when supplies are delivered. We sold and delivered fewer supplies during the year ended December 31, 2017 as compared to the year ended December 31, 2016.
We expect our royalty income to fluctuate as the royalties are based on the minimum royalty payments as well as the timing of when payments are received for royalties in excess of minimum royalties. Our diagnostic service revenue will be impacted by our focus on precision cancer therapeutics. In addition, we expect revenue from clinical research services to fluctuate based on timing of delivery of supplies under agreements.
Cost of Revenue
Our total cost of revenue was $1,811,424 in the year ended December 31, 2017, as compared to $1,730,512 in the year ended December 31, 2016. Cost of revenue mainly relates to the costs of our diagnostic service revenues and these costs are recognized at the completion of testing. Due to revenue being recognized when cash is received, costs incurred in one period may relate to revenue recognized in a later period. Gross margins are negative related to the timing of cash received as compared to the services performed as well as inefficiencies in realizing capacity-related issues. The increase in cost of revenues in the year ended December 31, 2017 compared to the prior period is mainly due to the higher percentage allocation of cost to cost of revenue versus to research and development expense related to clinical studies and to sales and marketing expense related to our clinical experience program.
51
Table of Contents
Research and Development Expenses
Research and development expenses consisted of the following:
| For the years ended December 31, | ||||||||||||
| 2017 | 2016 | Increase/(Decrease) | ||||||||||
| Salaries and staff costs |
$ | 1,541,766 | $ | 5,277,936 | $ | (3,736,170 | ) | |||||
| Stock-based compensation |
1,026,497 | 2,420,696 | (1,394,199 | ) | ||||||||
| Outside services, consultants and lab supplies |
2,125,374 | 5,573,362 | (3,447,988 | ) | ||||||||
| Facilities |
1,064,561 | 1,434,101 | (369,540 | ) | ||||||||
| Travel and scientific conferences |
80,714 | 213,419 | (132,705 | ) | ||||||||
| Fees, license and other |
2,043,738 | 87,128 | 1,956,610 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total research and development expenses |
$ | 7,882,650 | $ | 15,006,642 | $ | (7,123,992 | ) | |||||
|
|
|
|
|
|
|
|||||||
Research and development expenses decreased by $7,123,992 to $7,882,650 for the year ended December 31, 2017 from $15,006,642 for the year ended December 31, 2016. Our costs have decreased primarily due to the average number of our internal research and development personnel decreasing from thirty-one to ten as a result of our strategic restructuring activities. In addition, research and development expenses incurred related to clinical studies, samples processed and validated in connection with the clinical collaborations, as well as lab supplies, decreased for the year ended December 31, 2017 as compared to the prior year as a result of the shifting of our business focus. The total decrease of research and development expenses was offset by the increase in fees, license and other. The increase in fees, license and other was primarily due to the $2.0 million license fee payment in March 2017 to Nerviano for development and commercialization rights to PCM-075. We expect a reduction of research and development costs that relate to CLIA services as a result of our focus on precision cancer therapeutics; however, other costs may increase as we continue the development of PCM-075.
Selling and Marketing Expenses
Selling and marketing expenses consisted of the following:
| For the years ended December 31, | ||||||||||||
| 2017 | 2016 | Increase/(Decrease) | ||||||||||
| Salaries and staff costs |
$ | 1,004,887 | $ | 5,336,941 | $ | (4,332,054 | ) | |||||
| Stock-based compensation |
676,635 | 2,111,366 | (1,434,731 | ) | ||||||||
| Outside services and consultants |
250,550 | 1,260,354 | (1,009,804 | ) | ||||||||
| Facilities and insurance |
273,099 | 496,881 | (223,782 | ) | ||||||||
| Trade shows, conferences and marketing |
398,425 | 1,312,749 | (914,324 | ) | ||||||||
| Travel |
74,662 | 889,265 | (814,603 | ) | ||||||||
| Other |
57,152 | 115,588 | (58,436 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total selling and marketing expenses |
$ | 2,735,410 | $ | 11,523,144 | $ | (8,787,734 | ) | |||||
|
|
|
|
|
|
|
|||||||
Selling and marketing expenses decreased by $8,787,734 to $2,735,410 for the year ended December 31, 2017, from $11,523,144 for the year ended December 31, 2016. The overall decrease in selling and marketing expenses was primarily due to our strategic restructuring activities. As part of our restructuring, we reduced the number of our field sales, customer support and marketing personnel, thereby bringing down our average headcount to four from nineteen in the prior year. We expect decreases in personnel and related costs due to the reduction in force.
52
Table of Contents
General and Administrative Expenses
General and administrative expenses consisted of the following:
| For the years ended December 31, | ||||||||||||
| 2017 | 2016 | Increase/(Decrease) | ||||||||||
| Personnel and outside services costs |
$ | 3,445,296 | $ | 4,058,213 | $ | (612,917 | ) | |||||
| Stock-based compensation |
2,350,962 | 2,910,156 | (559,194 | ) | ||||||||
| Board of Directors’ fees |
474,676 | 456,498 | 18,178 | |||||||||
| Legal and accounting fees |
3,885,613 | 2,916,508 | 969,105 | |||||||||
| Facilities and insurance |
963,285 | 641,715 | 321,570 | |||||||||
| Travel |
96,134 | 184,217 | (88,083 | ) | ||||||||
| Fees, licenses, taxes and other |
281,500 | 308,640 | (27,140 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total general and administrative expenses |
$ | 11,497,466 | $ | 11,475,947 | $ | 21,519 | ||||||
|
|
|
|
|
|
|
|||||||
General and administrative expenses increased by $21,519 to $11,497,466 for the year ended December 31, 2017 from $11,475,947 for the year ended December 31, 2016. This increase was primarily due to an increase in legal and accounting fees, offset by a decrease in personnel and outside services costs and stock-based compensation. During the year ended December 31, 2017, we have decreased our average internal headcount to eight from ten in the prior year. We also decreased the utilization of outside services to support our information technology, human resources, and investor relations activities, resulting in the decrease in personnel and outside services costs. Stock-based compensation, a non-cash expense, will fluctuate based on the timing and amount of options granted, forfeitures and the fair value of the options at the time of grant or remeasurement. The increase in legal and accounting fees primarily resulted from the $2.1 million litigation settlement with the former CEO and CFO offset by decreases in general legal matters and patent related legal fees. Our general and administrative costs may increase in future periods in order to support fundraising activities and general business activities as we continue to develop and introduce new product offerings.
Restructuring
On March 15, 2017, we announced a strategic restructuring plan in connection with the focus on precision medicine therapeutics to our business. The restructuring plan includes a reduction in force and was completed in the last quarter of 2017. Restructuring charges of approximately $2.2 million were incurred and have been included as a component of operating loss for the year ended December 31, 2017. Of the total restructuring charges, approximately $1.1 million was related to termination of employees and an approximately $0.5 million charge related to impaired license fees.
Interest Income and Interest Expense
Interest expense was $1,033,939 and $1,674,341 for the years ended December 31, 2017 and 2016, respectively. The decrease in the year ended December 31, 2017 is due to a decrease in interest expense resulting from pay-off of our $15.0 million term loan. Interest income was $147,883 and $298,829 for the years ended December 31, 2017 and 2016, respectively. The decrease in interest income of approximately $151,000 is a result of liquidation of our short-term investments. We expect interest expense to fluctuate due to the potential changes in the variable interest rate of our equipment line of credit.
Change in Fair Value of Derivative Financial Instruments—Warrants
We have issued warrants to purchase shares of our common stock that are accounted for as derivative liabilities. As of December 31, 2017, the derivative financial instruments—warrants liabilities related to securities issued were revalued to $649,387, resulting in a decrease in fair value of $3,401,072 from December 31, 2016 based primarily upon the change in our stock price from $2.10 at December 31, 2016 to $0.31 at December 31, 2017, and the changes in the expected term, volatility and risk-free interest rates for the expected
53
Table of Contents
term, offset by an issuance of derivative financial instruments of $3,215,519. The decrease in value was recorded as non-operating gain for the year ended December 31, 2017.
Net Loss
Net loss and per share amounts were as follows:
| For the years ended December 31, | ||||||||||||
| 2017 | 2016 | Increase/(Decrease) | ||||||||||
| Net loss attributable to common stockholders |
$ | (24,930,984 | ) | $ | (39,227,959 | ) | $ | (14,296,975 | ) | |||
| Net loss per common share — basic |
$ | (0.72 | ) | $ | (1.30 | ) | $ | (0.58 | ) | |||
| Net loss per common share — diluted |
$ | (0.72 | ) | $ | (1.37 | ) | $ | (0.65 | ) | |||
| Weighted-average shares outstanding — basic |
34,680,362 | 30,174,838 | 4,505,524 | |||||||||
| Weighted-average shares outstanding — diluted |
34,680,362 | 30,281,263 | 4,399,099 | |||||||||
The decrease in net loss attributable to common stockholders of $14,296,975 to $24,930,984 for the year ended December 31, 2017 from $39,227,959 for the year ended December 31, 2016 resulted primarily from a decrease in operating expenses as compared to the prior year. Basic and diluted net loss per share for the year ended December 31, 2017 were impacted by the increase in both basic and diluted weighted-average shares outstanding resulting from the sale and issuance of approximately 21.0 million shares of common stock through a public offering, direct registered offering and controlled equity offering through our agreement with Cantor Fitzgerald & Co., and issuance of approximately 1.1 million shares of common stock in connection with the vesting of restricted stock units as well as restricted stock awards.
Liquidity and Capital Resources
As of March 31, 2018, we had $6,657,158 in cash and cash equivalents. Net cash used in operating activities for the three months ended March 31, 2018 was $2,856,147, compared to $8,758,208 for the three months ended March 31, 2017. Our use of cash was a result of the net loss of $4,786,177 for the three months ended March 31, 2018, adjusted for non-cash items related to stock-based compensation of $1,406,131, depreciation and amortization of $252,480, deferred rent of $79,586, and the loss from the change in fair value of derivative financial instruments—warrants of $129,689. The changes in our operating assets and liabilities consisted of higher accounts payable and accrued expenses, lower prepaid expenses, as well as decreased accounts receivable and unbilled receivable. At our current and anticipated level of operating loss, we expect to continue to incur an operating cash outflow for the next several years.
Net cash used in investing activities was $5,100 during the three months ended March 31, 2018, compared to $5,183,944 provided by investing activities for the same period in 2017. Investing activities during the three months ended March 31, 2018 consisted of net purchases for capital equipment of $5,100, while investing activities during the three months ended March 31, 2017 consisted primarily of net maturities of short-term investments of $5,195,396.
Net cash provided by financing activities was $1,292,641 during the three months ended March 31, 2018, compared to $156,526 used in financing activities for the same period in 2017. Financing activities during the three months ended March 31, 2018 related primarily to the proceeds from exercise of warrants of $1,449,167.
As of December 31, 2017, we had $8,225,764 in cash and cash equivalents. Net cash used in operating activities for the year ended December 31, 2017 was $23,281,067, compared to $31,039,855 for the year ended December 31, 2016. Our use of cash was primarily a result of the net loss of $24,906,744 for the year ended December 31, 2017, adjusted for items mainly related to stock-based compensation of $4,012,585, depreciation and amortization of $1,247,576, loss on extinguishment of debt of $1,655,825, and gain from the
54
Table of Contents
change in fair value of derivatives of $3,401,072. The changes in our operating assets and liabilities consisted primarily of lower accounts payable and accrued expenses, a decrease in accounts receivable and increased prepaid expenses. At our current and anticipated levels of operating losses, we expect to continue to incur an operating cash outflow for the next several years. As of December 31, 2017 and 2016, we had working capital of $5,522,917 and $31,152,936, respectively. The decrease in working capital is primarily due to the decrease in cash and cash equivalents and short-term investments. In June 2017, the lenders took the total of $16,668,583 out of our bank accounts to satisfy all of our outstanding obligations under the Loan and Security Agreement dated as of June 30, 2014, which caused a significant decrease of our cash position as compared to 2016.
Net cash provided by investing activities was $23,962,225 during the year ended December 31, 2017, compared to $24,833,649 used in investing activities for the year ended December 31, 2016. Investing activities during the year ended December 31, 2017 consisted primarily of net sales and maturities of short-term investments offset by purchases for capital equipment.
Net cash used in financing activities was $6,378,057 during the year ended December 31, 2017, compared to $2,301,376 provided by financing activities during the year ended December 31, 2016. Financing activities during the year ended December 31, 2017 related to the pay-off of long-term debt resulting in debt extinguishment of $16,613,067 and $626,104 repayment of equipment line of credit, offset by $10,861,114 from the sales of common stock and warrants, net of expenses. Financing activities during the year ended December 31, 2016 consisted of net proceeds from the sale of our common stock of $2,285,415, $366,966 from proceeds related to the exercise of options, and $740,076 from net borrowings on equipment lines of credit, offset by $1,091,018 of net repayment on long-term debt.
As of March 31, 2018, and December 31, 2017, we had working capital of $3,985,883 and $5,522,917, respectively.
Based on our current business plan and assumptions, we expect to continue to incur significant losses and require significant additional capital to further advance our clinical trial programs and support our other operations. Considering our current cash resources, we believe our existing resources (not including any proceeds from this offering) will be sufficient to fund our planned operations through July 2018. In addition, we have based our cash sufficiency estimates on our current business plan and assumptions that may prove to be wrong. We could utilize our available capital resources sooner than we currently expect, and we could need additional funding to sustain our operations even sooner than currently anticipated. These circumstances raise substantial doubt about our ability to continue as a going concern.
Our working capital requirements will depend upon numerous factors including but not limited to the nature, cost and timing of our research and development programs. To date, our sources of cash have been primarily limited to the sale of equity securities. We cannot be certain that additional funding will be available on acceptable terms, or at all. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience significant dilution. If we are unable to raise additional capital when required or on acceptable terms, we may have to significantly delay, scale back or discontinue the development and/or commercialization of one or more product candidates, all of which may have a material adverse impact on our operations. We may also be required to (i) seek collaborators for product candidates at an earlier stage than otherwise would be desirable and on terms that are less favorable than might otherwise be available; or (ii) relinquish or otherwise dispose of rights to technologies, product candidates or products that we would otherwise seek to develop or commercialize ourselves on unfavorable terms. We are evaluating all options to raise additional capital, increase revenue, as well as reduce costs, in an effort to strengthen our liquidity position, which may include the following: (1) Raising capital through public and private equity offerings; (2) Introducing operation and business development initiatives to bring in new revenue streams; (3) Reducing operating costs by identifying internal synergies; (4) Engaging in strategic partnerships. We continually assess any spending plans, including a review of our discretionary spending in connection with certain strategic contracts, to effectively and efficiently address our liquidity needs.
55
Table of Contents
Nasdaq Notice
On September 5, 2017, we received a written notice from the Nasdaq Stock Market LLC (“Nasdaq”) that we were not in compliance with Nasdaq Listing Rule 5550(a)(2) for continued listing on the Nasdaq Capital Market, as the minimum bid price of our common stock had been below $1.00 per share for 30 consecutive business days. The Notice had no immediate effect on the listing of our common stock, and our common stock continue to trade on the Nasdaq Capital Market. In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we have a period of 180 calendar days, or until March 5, 2018, to regain compliance with the minimum bid price requirement.
On March 6, 2018, the Nasdaq Capital Market informed us that we are eligible for an additional 180 calendar day period until September 4, 2018 to regain compliance with the minimum $1.00 bid price per share requirement. To regain compliance, the closing bid price of our common stock must meet or exceed $1.00 per share for at least ten consecutive business days during this 180 calendar day period.
Controlled Equity Offering and Public Offerings
On May 27, 2016 we filed a Form S-3 Registration Statement to offer and sell in one or more offerings, any combination of common stock, preferred stock, debt securities, warrants, or units having an aggregate initial offering price not exceeding $250,000,000. The preferred stock, debt securities, warrants, and units may be convertible or exercisable or exchangeable for common stock or preferred stock or other securities. This Registration Statement was declared effective on June 13, 2016. We received gross proceeds of $2.4 million from the sale of 421,810 shares of our common stock at a weighted-average price of $5.61 under a Controlled Equity Offering Sales Agreement with Cantor Fitzgerald & Co. as sales agent (the “Agent”) since the date of effectiveness of the Form S-3 on June 13, 2016.
On March 15, 2017, we filed a supplement to our Form S-3 registration statement to offer and sell additional shares of our common stock having an aggregate offering price up to $20,698,357 through the Agent. We received gross proceeds of approximately $110,000 in 2017 through the Controlled Equity Offering Agreement with the Agent.
On July 19, 2017, we closed a registered direct offering of 6,191,500 shares of our common stock. In a concurrent private placement, we also issued warrants to purchase up to 4,643,626 shares of its common stock. The warrants are exercisable six months following the date of issuance, will expire on the fifth anniversary of the initial exercise date and have an exercise price of $1.41 per share. The combined purchase price for one registered share of common stock and one unregistered warrant to purchase 0.75 of an unregistered share of common stock was $1.15. The net proceeds to us were approximately $6.5 million.
On December 19, 2017 we closed a public offering of 14,683,333 shares of our common stock and warrants to purchase up to an aggregate of 15,000,000 shares of common stock. Each share of common stock was sold together with a warrant to purchase one share of common stock at a combined effective price to the public of $0.30 per share and accompanying warrant. The warrants are exercisable immediately at an exercise price of $0.30 per share and will expire five years from the date of issuance. The net proceeds to us was approximately $4.1 million.
56
Table of Contents
Contractual Obligations and Commitments
The following table is a summary of contractual obligations that existed as of December 31, 2017, and is based on information appearing in the notes to Consolidated Financial Statements included elsewhere in prospectus.
| Payments Due by period | ||||||||||||||||||||
| Total | Less than 1 Year |
1-3 Years | 3-5 Years | More than 5 Years |
||||||||||||||||
| Operating leases |
$ | 3,679,552 | $ | 881,815 | $ | 1,838,336 | $ | 959,401 | $ | — | ||||||||||
| Debt obligation (1) |
1,461,327 | 1,461,327 | — | — | — | |||||||||||||||
| Service agreement (2) |
1,414,117 | 222,717 | 1,191,400 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total obligations |
$ | 6,554,996 | $ | 2,565,859 | $ | 3,029,736 | $ | 959,401 | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Debt is in default. Represents principal, interest under default rate and final fee payment. |
| (2) | Represents amounts that will become due upon future delivery of supplies and services from various parties under service contracts as of December 31, 2017. |
57
Table of Contents
We are a clinical-stage oncology therapeutics company. Our primary focus is to develop oncology therapeutics for the treatment of hematologic and solid tumor cancers for improved cancer care utilizing our technology in tumor genomics.
On March 15, 2017, we announced that we licensed PCM-075, a PLK1 inhibitor, from Nerviano, pursuant to a license agreement with Nerviano dated March 13, 2017. PCM-075 was developed to have high selectivity to PLK1 (at low nanomolar IC50 levels), to be administered orally, and to have a relatively short drug half-life of approximately 24 hours compared to other pan PLK inhibitors. A safety study of PCM-075 has been successfully completed in patients with advanced metastatic solid tumors and published in 2017 in Investigational New Drugs. We currently are enrolling a Phase 1b/2 open-label clinical trial of PCM-075 in combination with standard-of-care chemotherapy in patients with AML. The Phase 1b/2 clinical trial is led by Hematologist Jorge Eduardo Cortes, M.D., Deputy Department Chair, Department of Leukemia, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center. In addition, we are working with Dr. David Einstein at the Genitourinary Oncology Program at Beth Israel Deaconess Medical Center and Harvard Medical School as the principal investigator on a Phase 2 open-label clinical trial of PCM-075 in combination with abiraterone acetate (Zytiga®) and prednisone in patients with mCRPC with plans to enroll patients later this year.
Our intellectual property and proprietary technology enables us to analyze ctDNA and clinically actionable biomarkers to identify patients most likely to respond to specific cancer therapies. We plan to continue to vertically integrate our tumor genomics technology with the development of targeted cancer therapeutics.
We believe PCM-075 is the only PLK1 selective ATP competitive inhibitor, administered orally, with apparent antitumor activity in different preclinical models, currently in clinical trials. Polo-like kinase family consists of 5 members (PLK1-PLK5) and they are involved in multiple functions in cell division, including the regulation of centrosome maturation, checkpoint recovery, spindle assembly, cytokinesis, apoptosis and many others. PLK1 is essential for the maintenance of genomic stability during cell division (“mitosis”). The overexpression of PLK1 can lead to immature cell division followed by aneuploidy and cell death, a hallmark of cancer. PLK1 is over-expressed in a wide variety of hematologic and solid tumor malignancies, including acute myeloid leukemia, prostate, lung, breast, ovarian and adrenocortical carcinoma. In addition, several studies have shown that over-expression of PLK1 is associated with poor prognosis.
Studies have shown that inhibition of polo-like-kinases can lead to tumor cell death, including a Phase 2 study in AML where response rates with a different PLK inhibitor were up to 31% were observed when used in conjunction with a standard therapy for AML (low-dose cytarabine-LDAC) versus treatment with LDAC alone with a 13.3% response rate. We believe the more selective nature of PCM-075 to PLK1, its 24-hour half-life and oral bioavailability, as well as the reversibility of its on-target hematological toxicities may prove useful in addressing clinical therapeutic needs across a variety of cancers.
PCM-075 has been tested in vivo in different xenograft and transgenic models suggesting tumor growth inhibition or tumor regression when used in combination with other therapies. PCM-075 has been tested for antiproliferative activity on a panel of 148 tumor cell lines and appeared highly active with an IC50 (a measure concentration for 50% target inhibition) below 100 nM in 75 cell lines and IC50 values below 1 uM in 133 out of 148 cell lines. PCM-075 also appears active in cells expressing multi-drug resistant (“MDR”) transporter proteins and we believe PCM-075’s apparent ability to overcome the MDR transporter resistance mechanism in cancer cells could prove useful in broader drug combination applications.
In preclinical studies, synergy (interaction of discrete drugs such that the total effect is greater than the sum of the individual effects) has been demonstrated with PCM-075 when used in combination with more than ten different chemotherapeutics, including cisplatin, cytarabine, doxorubicin, gemcitabine and paclitaxel, as well as targeted therapies, such as abiraterone acetate (Zytiga®), HDAC inhibitors, such as belinostat (Beleodaq®),
58
Table of Contents
Quizartinib (AC220), a development stage FLT3 inhibitor, and bortezomib (Velcade®). These therapeutics are used clinically for the treatment of many hematologic and solid tumor cancers, including AML, NHL, mCRPC, ACC, and TNBC.
On August 16, 2017, we announced results of preclinical research indicating potential synergy of PCM-075 with an investigational FLT3 Inhibitor, Quizartinib by Daiichi Sankyo, in FLT3 mutant xenograft mouse models. This synergy assessment study was conducted for us by a third-party contract research group. Approximately one third of AML patients harbor FLT3-mutated blood cancer cells. The FDA recently approved Rydapt® (midostaurin) by Novartis for the treatment of newly diagnosed adult patients with AML that are FLT3 mutation-positive in combination with cytarabine and daunorubicin induction and cytarabine consolidation chemotherapy. There are three FLT3 inhibitors in ongoing phase 3 trials, including Quizartinib. We believe that a combination of PCM-075 with a FLT3 inhibitor for AML patients with a FLT3 mutation could extend treatment response and possibly slow or reduce resistance to FLT3 activity.
On August 21, 2017, we announced results of preclinical research indicating potential synergy of PCM-075 with a HDAC inhibitor in NHL cell lines. This synergy assessment study was conducted by Dr. Steven Grant, Associate Director for Translational Research and co-Leader, Developmental Therapeutics Program, Massey Cancer Center. Patients with relapsed or refractory NHL, such as cutaneous T cell lymphoma and peripheral T cell lymphoma, may be prescribed approved HDAC inhibitors and we believe this continues to be an area of unmet medical need. Dr. Grant’s data appeared to indicate that the combination of PCM-075 with Beleodaq® (belinostat), an HDAC inhibitor indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma, reduced cancer cells by up to 80% in two different forms of NHL (aggressive double-hit B-cell lymphoma and mantle cell lymphoma) cell lines.
On October 11, 2017, we entered into a Patent Option Agreement with Massachusetts Institute of Technology (“MIT”) for the exclusive rights to negotiate a royalty-bearing, limited-term exclusivity license to practice world-wide patent rights to US Patent 9,566,280, subject to the rights of MIT (research, testing, and educational purposes), Ortho McNeil Pharmaceuticals-Janssen Pharmaceuticals and its Affiliates (internal research and pre-clinical drug development purposes including some laboratory research) and the federal government (government-funded inventions claimed in any patent rights and to exercise march in rights). This patent is generally directed to combination therapies including an antiandrogen or androgen antagonist and polo-like kinase inhibitor for the treatment of cancer. The Patent Option Agreement expires one-year from the effective date and includes other requirements to maintain the option period.
On October 18, 2017, we announced results of preclinical research indicating potential synergy of PCM-075 with abiraterone acetate in C4-2 prostate cancer cells. This synergy assessment study was conducted by Dr. Michael Yaffe, David H. Koch Professor of Biology and Biological Engineering at MIT. The results appeared to indicate that the combination of PCM-075 with Zytiga® (abiraterone acetate) decreased cell viability in mCRPC tumor cells and the apparent synergy observed was greater than the expected effect of combining the two drugs. Zytiga® is indicated for use in combination with prednisone for the treatment of patients with mCRPC who have received prior chemotherapy containing docetaxel. We believe there is an unmet medical need to improve on the resistance to hormone therapy and extend the benefit of response to abiraterone for mCRPC patients.
On December 7, 2017, we announced results of preclinical research showing the sensitivity of TNBC cell lines to PCM-075, data featured as a Poster Presentation at the 40th San Antonio Breast Cancer Symposium. This synergy assessment study was conducted by Dr. Jesse Patterson and Dr. Michael Yaffe, at MIT. The results appeared to indicate that TNBC cell lines are 20-fold more sensitive to PCM-075 than estrogen receptor positive (ER+) breast cancer cell lines.
59
Table of Contents
PCM-075 Phase 1 Safety Study in Solid Tumors
A Phase 1 safety study of PCM-075 was completed in patients with advanced metastatic solid tumor cancers with data published in July 2017, in the peer-reviewed journal Investigational New Drugs. Dr. Glen Weiss, Medical Oncologist at Goodyear, AZ and affiliated with Cancer Treatment Centers of America at Western Regional Medical Center, was the principal investigator and first author of the publication, entitled “Phase 1 Dose-Escalation Study of NMS-1286937, an Orally Available Polo-like Kinase 1 Inhibitor, in Patients with Advanced or Metastatic Solid Tumors.” This study evaluated first-cycle dose limiting toxicities and related maximum tolerated dose with data indicating a manageable safety profile for PCM-075 (formerly known as NMS-1286937) for the treatment of advanced or metastatic solid tumors, with transient adverse events that were likely related to the drug’s mechanism of action. The authors believe that data from preclinical work, coupled with the results of the Phase 1 trial, suggest that PCM-075 could become a new therapeutic option for the treatment of solid tumor and hematologic cancers.
In this trial, PCM-075 was administered orally, once daily for five consecutive days, every three weeks, to evaluate first cycle dose-limiting toxicities and related maximum tolerated dose in adult subjects with advanced/metastatic solid tumors. The study was also intended to evaluate PCM-075’s pharmacokinetic profile in plasma, its anti-tumor activity, and its ability to modulate intracellular targets in biopsied tissue. The study identified thrombocytopenia and neutropenia as the primary toxicities, which is consistent with the expected mechanism of action of PCM-075 and results from preclinical studies. These hematologic toxicities were reversible, with recovery usually occurring within 3 weeks. No GI disorders, mucositis, or alopecia was observed, confirming that bone marrow cells are the most sensitive to PCM-075 inhibition with the applied dosing schedule.
We are utilizing the existing IND application to develop PCM-075 in solid tumors as part of our clinical development expansion plans, with our initial focus in mCRPC.
PCM-075 Phase 2 Study in metastatic Castration-Resistant Prostate Cancer
On December 14, 2017, we announced the submission of our Phase 2 protocol of PCM-075 in combination with abiraterone acetate (Zytiga® - Johnson & Johnson) for the treatment of mCRPC, and our active solid tumor IND to the FDA. In this multi-center, open-label, Phase 2 trial, PCM-075 in combination with the standard dose of abiraterone and prednisone, all administered orally, will be evaluated for safety and efficacy. The primary efficacy endpoint is the proportion of patients achieving disease control after 12 weeks of study treatment, as defined by lack of Prostate Specific Antigen (“PSA”) progression in patients who are showing signs of early progressive disease (rise in PSA but minimally symptomatic or asymptomatic) while currently receiving androgen deprivation therapy, abiraterone and prednisone.
On January 24, 2018, we announced plans for our Phase 2 clinical trial evaluating the combination of PCM-075 and abiraterone acetate (Zytiga®) in patients with mCRPC. We plan to have 3 clinical sites for the Phase 2 study, with Beth Israel Deaconess Medical Center in Boston Massachusetts as the principal site. Dr. David Einstein at the Genitourinary Oncology Program at Beth Israel Deaconess Medical Center and Harvard Medical School is the principal investigator for the Phase 2 mCRPC trial.
PCM-075 Phase 1b/2 Study in Acute Myeloid Leukemia
In June, 2017, we announced the submission of our IND application and our Phase 1b/2 protocol of PCM-075 in combination with standard-of-care chemotherapy for the treatment of AML to the FDA. In July, 2017, we received notification from the FDA that our Phase 1b/2 clinical trial of PCM-075 in patients with AML “may proceed”. On October 9, 2017, we announced that the FDA granted Orphan Drug Designation to PCM-075 for the treatment of AML. We initiated our Phase 1b/2 AML trial in November, 2017.
The Phase 1b/2 is an open-label trial to evaluate the safety and anti-leukemic activity of PCM-075 in combination with standard-of-care chemotherapy in patients with AML. Phase 1b is a dose escalation trial to
60
Table of Contents
evaluate the safety, tolerability, dose and scheduling of PCM-075, and to determine a recommended clinical treatment dose for the Phase 2 continuation trial.
Pharmacokinetics of PCM-075 and correlative biomarker activity will be assessed prior to the initiation of Phase 2. The Phase 2 continuation trial is open-label with administration of the recommended PCM-075 clinical dose in combination with standard-of-care chemotherapy to further evaluate safety and assess preliminary efficacy. Doses of PCM-075 will be administered orally each day for five consecutive days in a 28-day cycle in both Phase 1b and Phase 2.
We announced in February 2018 that the first patient has completed the first cycle of dosing with PCM-075 in combination with low-dose cytarabine in our Phase 1b/2 multicenter trial of patients with AML. We currently have eight sits activated and able to recruit, screen and enroll patients. We plan to have up to 10 clinical sites activated for the Phase 1b/2 trial. This trial is being led by Hematologist Jorge Cortes, M.D., Deputy Department Chair, Department of Leukemia, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center.
We announced in April 2018 the presentation of pharmacodynamics and biomarker data from the first patient to complete a treatment cycle of PCM-075 in combination with standard-of-care chemotherapy. We also announced that the combination regimen of PCM-075 plus LDAC appeared to be well tolerated and that this patient went on to receive a second cycle of treatment. At this time, we have enrolled a total of three patients with the first two patients in the initial cohort at 12mg/m2 oral, daily dose of PCM-075 (Days 1-5 in a 28-day cycle) in combination with LDAC having successfully completed cycle 1 of treatment. The third patient is currently in cycle 1 of treatment. We also enrolled a total of three patients, with the first two patients in the initial cohort at 12 mg/m2 oral, daily dose of PCM-075 (Days 1-5 in a 28-day cycle) in combination with decitabine, having successfully completed cycle 1 of treatment. One patient in the decitabine arm was removed from the trial prior to the end of the 28-day cycle due to disease progression and will be replaced to complete the initial dosing cohort. The PCM-075 dose will be escalated in the Phase 1b segment of the ongoing trial until a maximum tolerated dose (MTD)/recommended Phase 2 dose (“RP2D”) is achieved.
Optimizing Drug Development with Correlative Biomarker Analysis using Circulating Tumor DNA
We have significant experience and expertise with biomarkers and technology in cancer, including AML. We are one of the patent holders of NPM1 for diagnosis and monitoring of patients. NPM1-mutated AML is a genetic marker in leukemia and accounts for approximately one-third of all AML patients. We plan to use our PCM technology to profile other dominant AML markers, such as FLT3, DNMT3A, NRAS, and KIT, as well as to measure PLK1 enzymatic activity to potentially identify patients most likely to respond to PCM-075 and to measure patient therapy response.
Technological advancements in the molecular characterization of cancers have enabled researchers to identify an increasing number of key molecular drivers of cancer progression. These discoveries have led to multiple novel anticancer therapeutics, and clinical benefit in selected patient populations. As a precision medicine biotechnology company developing targeted therapies to treat hematologic and solid tumor cancers, our objective is to optimize drug development by using our proprietary precision medicine technology as part of our approach to genomic profiling of tumors.
Our CLIA-certified/CAP-accredited laboratory in San Diego, California, enables us to use our technology platform to optimize drug development and patient care. In the clinical development of our lead drug candidate, PCM-075, correlative biomarker analysis will be used to help inform decisions in the evaluation of dose-response and optimal regimen for desired pharmacologic effect and safety. Additionally, some biomarkers can be used as a surrogate endpoint for efficacy and/or toxicity, as well as predicting patients’ response by identifying certain patient populations that are more likely to respond to the drug therapy.
61
Table of Contents
Targeting cell-free nucleic acid markers allows for the development of genetic tests that use noninvasive and easy-to-obtain urine samples, as well as blood samples, rather than other more traditional and more invasive, expensive and/or often unreliable methods, such as radiographic imaging and tissue biopsy. Using our proprietary technology, we developed NextCollect, a first of its kind high-volume urine specimen collection and DNA preservation kit. Formulated DNA preservative solution is integrated into the NextCollect reservoir cap, and dispensed when secured the NextCollect cup. When added to the urine specimen, NextCollect preserves DNA for up to 2 weeks at room temperature NextCollect™ is designed to collect a higher volume of urine specimen, containing more DNA available for testing methods. NextCollect™ urine extracted DNA can be used for a range of applications across oncology, urology, virology and infectious disease. NextCollect™ is manufactured for Research Use Only and we began making it available in December 2017, for purchase by academic institutions, cancer centers and research laboratories for their clinical research purposes.
Operating Segment and Geographic Information
We operate in one business segment, using one measurement of profitability to manage our business. We do not assess the performance of our geographic regions on measures of revenue or comprehensive income or expense. In addition, all of our principal operations, assets and decision-making functions are located in the U.S. We do not produce reports for, or measure the performance of, our geographic regions on any asset-based metrics. Therefore, geographic information is not presented for revenues or long-lived assets.
The Market
PCM-075
We are a clinical-stage biotechnology company with our primary focus on the development of our lead drug candidate, PCM-075, a PLK1 inhibitor that may treat multiple hematologic and solid tumor cancers.
There have been several drug candidates in this class of targeted oncology therapeutics to enter clinical trials; however, PCM-075 is the lead candidate and is differentiated from other ATP competitive inhibitors in that:
| • | its inhibition of PLK1 is highly-selective and the half maximal inhibitory concentration (IC50) for PLK2 and PLK3 is over 5,000-fold of that for PLK1; |
| • | it has a relatively short half-life of approximately 24 hours; and |
| • | it is available in an oral gelcap formulation. |
The unacceptable toxicity of prior PLK inhibitors, such as volasertib from Boehringer Ingelheim, may be due to non-selective inhibition of PLK2 and PLK3 and a much longer half-life (approximately 135 hours) that could result in drug accumulation, which ultimately may have led to unsatisfactory clinical outcomes.
We believe the efficacy of PLK1 inhibition in AML has already been shown in the proof-of-concept trial of volasertib. Therefore, PCM-075’s highly-selective activity, oral dosing and short half-life could enable favorable efficacy and safety with potential survival benefits in AML patients with relapsed/refractory disease or newly-diagnosed disease and ineligible for intensive induction therapy.
We recently initiated a Phase 1b/2 clinical trial of PCM-075 in combination with standard-of-care chemotherapy in AML patients to evaluate the safety/tolerability, determine the maximum tolerated dose (“MTD”), and assess preliminary efficacy. This study is on file at ClinicalTrials.gov with the Identifier NCT03303339. We also announced a Phase 2 open-label clinical trial in adult patients with mCRPC in combination with abiraterone acetate (Zytiga®) and prednisone. The mCRPC Phase 2 trial is on file at ClinicalTrials.gov with the Identifier NCT03414034. As such, we have two active IND applications in place with the FDA, one with the hematologic division and one with the solid tumor division. This enables us to quickly activate to conduct clinical trials of our lead drug candidate, PCM-075, in both hematologic and solid tumor cancers.
62
Table of Contents
Drug Development and Monitoring of Therapeutic Outcomes
Cell-free DNA diagnostic technology has significant potential as a simple, quick, noninvasive way of monitoring clinical responses to drugs in clinical development and evaluating patient-specific responses to already approved and marketed therapies. Specific target applications include, but are not limited to, optimizing drug development to identify patients most likely to respond to targeted therapeutics.
One of the largest costs associated with development of a new therapy is the phases and size of human clinical studies required to identify the cohort of responders, and the resulting statistical power required. By measuring specific genetic markers, it may be possible to pre-identify, and subsequently screen, for the most likely responders to the therapy, and to limit patient recruitment to this subset. This strategy could significantly reduce the cost to develop a drug and improve development time lines. We believe that there is significant research potential for our molecular diagnostic technology to be incorporated into these clinical trial protocols, and ultimately into post-approval patient identification protocol.
Infectious Diseases — HPV
The rationale for screening for HPV is that high-risk subtypes cause virtually all cases of cervical cancer. We have developed a urine-based HPV test capable of screening for known high-risk HPV types that are associated with the development of cervical cancer. Cervical cancer is the third most commonly diagnosed cancer, and the fourth leading cause of cancer deaths in females, worldwide. Deaths due to cervical cancer are a significant global problem, especially in developing countries where screening practices are inadequate.
Other areas beyond HPV detection and monitoring include those infectious diseases caused by viruses, bacteria, fungi, and parasites. Cell-free nucleic acid assays that detect molecular targets in organisms can provide a quick, accurate, simple, and cost effective method for screening and monitoring disease. Specific areas of interest include testing for molecular targets from organisms that cause Lyme disease, John Cunningham Virus, valley fever, and various fungal infections. These organisms all tend to be difficult to identify with current technology, making differential diagnosis especially challenging, thus delaying the start of potentially curative anti-infective treatment.
Our investment in the research and development of new nucleic acid preservatives or methods, which improve the stability of urine as a cell-free nucleic acid specimen led to the development of a new urine collection and DNA preservation cup (“NextCollect™”). It is our expectation that we will continue to provide the NextCollect™ as a stand-alone kit for research use to academic researchers and institutions that they can purchase and utilize in their own laboratories.
Our Business Strategy
We are a precision medicine biotechnology company developing oncology therapeutics for improved cancer care, optimizing drug development by leveraging our proprietary PCM technology in tumor genomics. Our broad intellectual property and proprietary technology enables us to measure ctDNA in urine and blood to identify and quantify clinically actionable markers for predicting response to cancer therapies. We offer our PCM technology at our CLIA-certified/CAP-accredited laboratory and plan to continue to vertically integrate our PCM technology with the development of precision cancer therapeutics.
We believe we have an opportunity to utilize precision diagnostics to improve treatment outcomes for cancer patients using our proprietary technology to detect clinically actionable mutations and monitor patient response to therapy. The licensing of global development and commercialization rights to PCM-075 allows us to execute our strategy to vertically integrate our PCM technology with precision cancer therapeutics, by developing drugs where our deep understanding of tumor genomics may allow for effective targeting of appropriate cancer patients.
63
Table of Contents
Research and Development
We have historically made substantial investments in research and development. Our research and development efforts prioritize discovering, developing and testing our clinical and preclinical candidates and platform technologies. Our research and development team is composed of researchers and scientists (PhD’s), laboratory associate scientists, and experts in drug development and tumor genomics.
Research and development expenses for the years ended December 31, 2017 and 2016 were approximately $7.9 million and $15.0 million, respectively.
Intellectual Property
We consider the protection of our proprietary technologies and products, as well as our ability to maintain patent protection intended to cover the composition of matter of our product candidates, their methods of use, and other related technology and inventions, to be a critical element in the success of our business. As of March 31, 2018, our wholly-owned and licensed intellectual property included over 78 issued patents and 44 pending patent applications in the U.S. and abroad. The pending applications include multiple international applications filed under the Patent Cooperation Treaty (“PCT applications”) that may be used as the basis for multiple additional patent applications.
We plan to protect our intellectual property position by, among other things, licensing or filing our own U.S. and foreign patent applications related to our proprietary technology, and any inventions or improvements that are important to the development and implementation of our business. We also may seek patent protection, if available, with respect to biomarkers and diagnostic methods that may be used to determine optimal patient populations for use of our product candidates.
Our license agreement related to PCM-075 grants us exclusive, worldwide licenses under a portfolio of patents covering three broad areas: (1) Directed to PCM-075, related compounds and processes for making compounds; pharmaceutical compositions and methods of treating diseases characterized by dysregulated protein kinase activity; (2) Directed to salts and pharmaceutical compositions of PCM-075; methods of treating mammals in need of PLK inhibition; and (3) Directed to synergistic combinations of PCM-075 and one or more of a broad range of antineoplastic agents, and pharmaceutical compositions of those combinations. Members of this patent group expire between 2026 and 2029.
On October 11, 2017, we entered into a Patent Option Agreement with Massachusetts Institute of Technology (“MIT”) for the exclusive rights to negotiate a royalty-bearing, limited-term exclusivity license to practice world-wide patent rights to US Patent 9,566,280, subject to the rights of MIT (research, testing, and educational purposes), Ortho McNeil Pharmaceuticals-Janssen Pharmaceuticals and its Affiliates (internal research and pre-clinical drug development purposes including some laboratory research) and the federal government (government-funded inventions claimed in any patent rights and to exercise march in rights). This patent is generally directed to combination therapies including an antiandrogen or androgen antagonist and polo-like kinase inhibitor for the treatment of cancer. The Patent Option Agreement expires one-year from the effective date and includes other requirements to maintain the option period.
Another group of patents and patent applications are directed to various methods relating to detecting nucleic acid sequences in urine and nucleic acid modifications and alterations in urine; detecting and monitoring cancer through urine-based testing, nucleic acid screening, and monitoring in cases of transplantation and infectious diseases, detecting specific gene mutations and indicators of disease (including NPM1 mutations). Applications are also pending to protect proprietary methods of collecting, extracting, detecting and enriching small concentrations of short nucleic acid sequences, and detecting and monitoring mutations in diseases, such as cancer, over time. Members of this patent group expire between 2018 and 2034.
64
Table of Contents
Wherever possible, we seek to protect our inventions by filing U.S. patents as well as foreign counterpart applications in select other countries. Because patent applications in the U.S. are maintained in secrecy for at least eighteen months after the applications are filed, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that we were the first to make the inventions covered by each of our issued or pending patent applications, or that we were the first to file for protection of inventions set forth in such patent applications. Our planned or potential products may be covered by third-party patents or other intellectual property rights, in which case continued development and marketing of our products would require a license. Required licenses may not be available to us on commercially acceptable terms, if at all. If we do not obtain these licenses, we could encounter delays in product introductions while we attempt to design around the patents, or we could find that the development, manufacture or sale of products requiring such licenses are not possible.
In addition to patent protection, we also rely on know-how, trade secrets and the careful monitoring of proprietary information, all of which can be difficult to protect. We seek to protect some of our proprietary technology and processes by entering into confidentiality agreements with our employees, consultants, and contractors. These agreements may be breached, we may not have adequate remedies for any breach and our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our employees or our consultants or contractors use intellectual property owned by others in their work for us, disputes may also arise as to the rights in related or resulting know-how and inventions.
Manufacturing and Distribution
We have a supplier agreement with NerPharMa, S.r.l., a pharmaceutical manufacturing company and a subsidiary of Nerviano, to manufacture drug product for PCM-075. The agreement covers the clinical and commercial supply of PCM-075, and includes both Active Pharmaceutical Ingredients (“API”) and Good Manufacturing Process (“GMP”) production of capsules.
In 2018, we will continue offering laboratory testing services of LDTs from our CLIA-certified/CAP-accredited laboratory. Our primary customers for these LDT’s are pharmaceutical companies and third party laboratories. In addition, we plan to offer our NextCollect™ urine collection and DNA preservation cup for research use by academic institutions, cancer centers and research laboratories.
Government Regulation
We operate in a highly regulated industry that is subject to significant federal, state, local and foreign regulation. Our present and future business has been, and will continue to be, subject to a variety of laws including, the Federal Food, Drug, and Cosmetic Act, or FDC Act, and the Public Health Service Act, among others.
The FDC Act and other federal and state statutes and regulations govern the testing, manufacture, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion of our products. As a result of these laws and regulations, product development and product approval processes are very expensive and time-consuming.
FDA Approval Process
In the United States, pharmaceutical products, including biologics, are subject to extensive regulation by the FDA. The FDC Act and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications, or NDAs, or biologic license applications, or BLAs, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties, and criminal prosecution.
65
Table of Contents
Pharmaceutical product development in the United States typically involves preclinical laboratory and animal tests, the submission to the FDA of an IND, which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug or biologic for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Preclinical tests include laboratory evaluation as well as animal trials to assess the characteristics and potential pharmacology and toxicity of the product. The conduct of the preclinical tests must comply with federal regulations and requirements including good laboratory practices. The results of preclinical testing are submitted to the FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has not objected to the IND within this 30-day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the investigational drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted in compliance with federal regulations and good clinical practices, or GCP, as well as under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary or permanent discontinuation of a clinical trial at any time or impose other sanctions if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The clinical trial protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions.
Clinical trials to support NDAs or BLAs, which are applications for marketing approval, are typically conducted in three sequential Phases, but the Phases may overlap. In Phase 1, the initial introduction of the investigational drug candidate into healthy human subjects or patients, the investigational drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses and, if possible, early evidence on effectiveness. Phase 2 usually involves trials in a limited patient population, to determine the effectiveness of the investigational drug for a particular indication or indications, dosage tolerance and optimum dosage, and identify common adverse effects and safety risks. In the case of product candidates for severe or life-threatening diseases such as pneumonia, the initial human testing is often conducted in patients rather than in healthy volunteers.
If an investigational drug demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 clinical trials are undertaken to obtain additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit-risk relationship of the investigational drug and to provide adequate information for its labeling.
After completion of the required clinical testing, an NDA or, in the case of a biologic, a BLA, is prepared and submitted to the FDA. FDA approval of the marketing application is required before marketing of the product may begin in the United States. The marketing application must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture, and controls.
66
Table of Contents
The FDA has 60 days from its receipt of an NDA or BLA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of marketing applications. Most such applications for non-priority drug products are reviewed within ten months. The review process may be extended by the FDA for three additional months to consider new information submitted during the review or clarification regarding information already provided in the submission. The FDA may also refer applications for novel drug products or drug products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving a marketing application, the FDA will typically inspect one or more clinical sites to assure compliance with GCP.
Additionally, the FDA will inspect the facility or the facilities at which the drug product is manufactured. The FDA will not approve the NDA or, in the case of a biologic, the BLA unless compliance with cGMPs is satisfactory and the marketing application contains data that provide substantial evidence that the product is safe and effective in the indication studied. Manufacturers of biologics also must comply with FDA’s general biological product standards.
After the FDA evaluates the NDA or BLA and the manufacturing facilities, it issues an approval letter or a complete response letter. A complete response letter outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed in a resubmission of the marketing application, the FDA will re-initiate review. If the FDA is satisfied that the deficiencies have been addressed, the agency will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included. It is not unusual for the FDA to issue a complete response letter because it believes that the drug product is not safe enough or effective enough or because it does not believe that the data submitted are reliable or conclusive.
An approval letter authorizes commercial marketing of the drug product with specific prescribing information for specific indications. As a condition of approval of the marketing application, the FDA may require substantial post-approval testing and surveillance to monitor the drug product’s safety or efficacy and may impose other conditions, including labeling restrictions, which can materially affect the product’s potential market and profitability. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Other Regulatory Requirements
Once a NDA or BLA is approved, a product will be subject to certain post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of therapeutic products, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet.
Biologics may be marketed only for the approved indications and in accordance with the provisions of the approved labeling. Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new BLA or BLA supplement, before the change can be implemented. A BLA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing BLA supplements as it does in reviewing BLAs. We cannot be certain that the FDA or any other regulatory agency will grant approval for our product candidates for any other indications or any other product candidate for any indication on a timely basis, if at all.
67
Table of Contents
Adverse event reporting and submission of periodic reports is required following FDA approval of a BLA. The FDA also may require post-marketing testing, known as Phase 4 testing, risk evaluation and mitigation strategies, and surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control as well as product manufacturing, packaging, and labeling procedures must continue to conform to cGMPs after approval. Manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA during which the agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered. U.S. Foreign Corrupt Practices Act.
The U.S. Foreign Corrupt Practices Act, to which we are subject, prohibits corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity.
Federal and State Fraud and Abuse Laws
Healthcare providers, physicians and third-party payors play a primary role in the recommendation and prescription of drug and biologic product candidates which obtain marketing approval. In addition to FDA restrictions on marketing of pharmaceutical products, pharmaceutical manufacturers are exposed, directly, or indirectly, through customers, to broadly applicable fraud and abuse and other healthcare laws and regulations that may affect the business or financial arrangements and relationships through which a pharmaceutical manufacturer can market, sell and distribute drug and biologic products. These laws include, but are not limited to:
The federal Anti-Kickback Statute which prohibits, any person or entity from, among other things, knowingly and willfully offering, paying, soliciting, or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in-kind, to induce or reward either the referring of an individual for, or the purchasing, leasing, ordering, or arranging for the purchase, lease, or order of any healthcare item or service reimbursable, in whole or in part, under Medicare, Medicaid, or any other federally financed healthcare program. The term “remuneration” has been broadly interpreted to include anything of value. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other hand. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases, or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor.
The federal false claims and civil monetary penalty laws, including the Federal False Claims Act, which imposes significant penalties and can be enforced by private citizens through civil qui tam actions, prohibits any person or entity from, among other things, knowingly presenting, or causing to be presented, a false, fictitious or fraudulent claim for payment to the federal government, or knowingly making, using or causing to be made, a false statement or record material to a false or fraudulent claim to avoid, decrease or conceal an obligation to pay money to the federal government. In addition, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes “any request or demand” for money or property presented to the U.S. government. In addition, manufacturers can be held liable under the False Claims Act even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. Criminal prosecution is also possible for
68
Table of Contents
making or presenting a false, fictitious or fraudulent claim to the federal government. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the company’s marketing of the product for unapproved, and thus non-reimbursable, uses.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which, among other things, imposes criminal liability for executing or attempting to execute a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and creates federal criminal laws that prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statements or representations, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry in connection with the delivery of, or payment for, benefits, items or services.
HIPAA, as amended by the Health Information Technology and Clinical Health Act of 2009, or HITECH, and its implementing regulations, which impose certain requirements relating to the privacy, security, transmission and breach reporting of individually identifiable health information upon entities subject to the law, such as health plans, healthcare clearinghouses and healthcare providers and their respective business associates that perform services for them that involve individually identifiable health information. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in U.S. federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions.
The federal physician payment transparency requirements, sometimes referred to as the “Physician Payments Sunshine Act,” and its implementing regulations, which require certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the United States Department of Health and Human Services, or HHS, information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members.
State and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, that may impose similar or more prohibitive restrictions, and may apply to items or services reimbursed by non-governmental third-party payors, including private insurers.
State and foreign laws that require pharmaceutical companies to implement compliance programs, comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or to track and report gifts, compensation and other remuneration provided to physicians and other healthcare providers, and other federal, state and foreign laws that govern the privacy and security of health information or personally identifiable information in certain circumstances, including state health information privacy and data breach notification laws which govern the collection, use, disclosure, and protection of health-related and other personal information, many of which differ from each other in significant ways and often are not pre-empted by HIPAA, thus requiring additional compliance efforts.
Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some business activities can be subject to challenge under one or more of such laws. The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry.
69
Table of Contents
Ensuring that business arrangements with third parties comply with applicable healthcare laws and regulations is costly and time consuming. If business operations are found to be in violation of any of the laws described above or any other applicable governmental regulations a pharmaceutical manufacturer may be subject to penalties, including civil, criminal and administrative penalties, damages, fines, disgorgement, individual imprisonment, exclusion from governmental funded healthcare programs, such as Medicare and Medicaid, contractual damages, reputational harm, diminished profits and future earnings, additional reporting obligations and oversight if subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, and curtailment or restructuring of operations, any of which could adversely affect a pharmaceutical manufacturer’s ability to operate its business and the results of its operations.
Healthcare Reform in the United States
In the United States, there have been, and continue to be, a number of legislative and regulatory changes and proposed changes to the healthcare system that could affect the future results of pharmaceutical manufactures’ operations. In particular, there have been and continue to be a number of initiatives at the federal and state levels that seek to reduce healthcare costs. Most recently, the Patient Protection and Affordable Care Act, or PPACA, was enacted in March 2010, which includes measures to significantly change the way healthcare is financed by both governmental and private insurers. Among the provisions of the PPACA of greatest importance to the pharmaceutical and biotechnology industry are the following:
| • | an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs; |
| • | implementation of the federal physician payment transparency requirements, sometimes referred to as the “Physician Payments Sunshine Act”; |
| • | a licensure framework for follow-on biologic products; |
| • | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; |
| • | establishment of a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending; |
| • | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program, to 23.1% and 13% of the average manufacturer price for most branded and generic drugs, respectively and capped the total rebate amount for innovator drugs at 100% of the Average Manufacturer Price, or AMP; |
| • | a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for certain drugs and biologics, including our product candidates, that are inhaled, infused, instilled, implanted or injected; |
| • | extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; |
| • | expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for individuals with income at or below 133% of the federal poverty level, thereby potentially increasing manufacturers’ Medicaid rebate liability; |
| • | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries |
70
Table of Contents
| during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; and |
| • | expansion of the entities eligible for discounts under the Public Health program. |
Some of the provisions of the PPACA have yet to be implemented, and there have been legal and political challenges to certain aspects of the PPACA. Since January 2017, President Trump has signed two executive orders and other directives designed to delay, circumvent, or loosen certain requirements mandated by the PPACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the PPACA. While Congress has not passed repeal legislation, the Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the PPACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. Congress may consider other legislation to repeal or replace elements of the PPACA.
Many of the details regarding the implementation of the PPACA are yet to be determined, and at this time, the full effect that the PPACA would have on a pharmaceutical manufacturer remains unclear. In particular, there is uncertainty surrounding the applicability of the biosimilars provisions under the PPACA. The FDA has issued several guidance documents, but no implementing regulations, on biosimilars. A number of biosimilar applications have been approved over the past few years. The regulations that are ultimately promulgated and their implementation are likely to have considerable impact on the way pharmaceutical manufacturers conduct their business and may require changes to current strategies. A biosimilar is a biological product that is highly similar to an approved drug notwithstanding minor differences in clinically inactive components, and for which there are no clinically meaningful differences between the biological product and the approved drug in terms of the safety, purity, and potency of the product.
Individual states have become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access, and marketing cost disclosure and transparency measures, and to encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm a pharmaceutical manufacturer’s business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce ultimate demand for certain products or put pressure product pricing, which could negatively affect a pharmaceutical manufacturer’s business, results of operations, financial condition and prospects.
In addition, given recent federal and state government initiatives directed at lowering the total cost of healthcare, Congress and state legislatures will likely continue to focus on healthcare reform, the cost of prescription drugs and biologics and the reform of the Medicare and Medicaid programs. While no one cannot predict the full outcome of any such legislation, it may result in decreased reimbursement for drugs and biologics, which may further exacerbate industry-wide pressure to reduce prescription drug prices. This could harm a pharmaceutical manufacturer’s ability to generate revenue. Increases in importation or re-importation of pharmaceutical products from foreign countries into the United States could put competitive pressure on a pharmaceutical manufacturer’s ability to profitably price products, which, in turn, could adversely affect business, results of operations, financial condition and prospects. A pharmaceutical manufacturer might elect not to seek approval for or market products in foreign jurisdictions in order to minimize the risk of re-importation, which could also reduce the revenue generated from product sales. It is also possible that other legislative proposals having similar effects will be adopted.
Furthermore, regulatory authorities’ assessment of the data and results required to demonstrate safety and efficacy can change over time and can be affected by many factors, such as the emergence of new information,
71
Table of Contents
including on other products, changing policies and agency funding, staffing and leadership. No one can be sure whether future changes to the regulatory environment will be favorable or unfavorable to business prospects. For example, average review times at the FDA for marketing approval applications can be affected by a variety of factors, including budget and funding levels and statutory, regulatory and policy changes.
Regulation in the European Union
Biologics are also subject to extensive regulation outside of the United States. In the European Union, for example, there is a centralized approval procedure that authorizes marketing of a product in all countries of the European Union, which includes most major countries in Europe. If this procedure is not used, approval in one country of the European Union can be used to obtain approval in another country of the European Union under two simplified application processes, the mutual recognition procedure or the decentralized procedure, both of which rely on the principle of mutual recognition. After receiving regulatory approval through any of the European registration procedures, pricing and reimbursement approvals are also required in most countries.
Other Regulations
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances and biological materials. We may incur significant costs to comply with such laws and regulations now or in the future.
Some drugs benefit from additional government incentives. Orphan drugs receive special consideration from the FDA in order to encourage pharmaceutical companies to develop treatments for rare diseases. Incentives for the development of orphan drugs include quicker approval time and potential financial assistance, including waiver of Prescription Drug User Fee Act (“PDUFA”). Companies are often permitted to charge substantial prices for orphan drugs, making them more profitable than they would be without government intervention. As a result, the development of orphan drugs continues to grow at a faster rate than the development of traditional pharmaceuticals. The FDA granted Orphan Drug Designation (“ODD”) to PCM-075 in the treatment of AML in October, 2017.
Competition
PCM-075 is not the first PLK inhibitor that has entered clinical development; however, it currently is the only oral PLK inhibitor in active clinical development and delivers highly-selective PLK1 inhibition, which suggests that it could demonstrate survival benefits in elderly AML patients without the adverse events that have prohibited the advancement of other PLK1 inhibitors. PCM-075 has completed a Phase 1 trial in advanced metastatic solid tumor cancers and a Phase 1b/2 trial in AML was initiated in November 2017. Additionally, a Phase 2 trial in mCRPC is filed with FDA and we are working towards the activation up to three sites including Beth Israel Deaconess Medical Center.
The most prominent PLK inhibitor tested in late-stage clinical development, thus far, is volasertib, developed by Boehringer Ingelheim. In a randomized Phase 2 trial of volasertib plus low-dose cytarabine (“LDAC”) in 87 AML patients not eligible for induction therapy, patients received LDAC 20mg twice-daily subcutaneously on days 1-10 or LDAC plus volasertib 350 mg IV on days 1 + 15 every four weeks. The response rate (complete remission and complete remission with incomplete blood count recovery) was higher for LDAC + volasertib vs LDAC (31.0% vs 13.3%; p=0.052). Median event-free survival was significantly prolonged by LDAC + volasertib compared with LDAC (5.6 vs 2.3 months). The encouraging results led to the Phase 3 POLO-AML-2 study in early 2013, which enrolled 666 elderly patients (65 years or older) with newly diagnosed AML, who were not eligible for intensive induction therapy. However; in June, 2016, Boehringer Ingelheim reported that LDAC + volasertib did not meet the primary endpoint of objective response; although better than LDAC, alone, the difference was not statistically significant. The data also showed an unfavorable overall
72
Table of Contents
survival trend for the experimental arm, with the safety profile of the LDAC + volasertib dosing regimen considered as the main reason for the trend. The fact that volasertib demonstrated survival benefits in the Phase 2 trial provided proof-of-concept for PLK inhibition as a mechanism of action for an AML therapy; however, its unacceptable safety profile may have resulted from the fact that volasertib’s inhibition of PLK1 is not highly selective and it also inhibits PLK2 and PLK3. By contrast, PCM-075 is able to deliver much more selective inhibition of PLK1 than volasertib. PCM-075 also has a half-life of 24 hours vs volasertib’s 135 hours.
GSK461364, developed by GSK, appears to have less sensitivity to PLK2 and PLK3 than volasertib, although it is not as specific to PLK1 as PCM-075. GSK461364 was investigated in a Phase 1 study in patients with advanced solid tumor cancers. The best response was prolonged stable disease of more than 16 weeks that occurred in 15% of patients. However, GSK461364 had off target adverse events including grade 4 pulmonary emboli. Venous thrombotic emboli (VTE) and myelosuppression were the most common grade 3-4 drug-related events; and VTE occurred in 20% of patients, which demanded co-administration of anticoagulants. There are no further clinical updates for GSK461364 after the Phase 1 study.
Other PLK inhibitors that have been evaluated include rogosertib - Oncova, a non-targeted broad-spectrum multi-kinase inhibitor (RAF, PI3K, PLK), evaluated for pancreatic cancer and Myelodysplastic Syndrome (“MDS”), which failed a Phase 3 trial in MDS. Currently, Oncova is testing an IV formualtion of rogosertib in high-risk MDS patients. CY140 - Cyclacel, a PLK1, 2, 3 inhibitor, is currently in preclinical studies for the treatment of esophageal cancer.
Employees
As of March 31, 2018, we had a total of 17 employees, all of whom were full-time. None of our employees are covered by a collective bargaining agreement, and we consider our relations with our employees to be good.
Properties
We currently lease approximately 26,100 square feet of laboratory and office space for our headquarters in San Diego, California under a lease agreement, as amended from time to time, that expires in December 2021. We believe that our facilities are adequate for our needs for the immediate future and that, should it be needed, suitable additional space will be available to accommodate expansion of our operations on commercially reasonable terms.
Legal Proceedings
From time to time, we may become involved in various lawsuits and legal proceedings that arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in matters may arise from time to time that may harm our business. As of the date of this prospectus, we are not party to any material legal proceedings.
73
Table of Contents
The following table sets forth the names and ages of all of our directors and executive officers as of March 31, 2018. Our Board of Directors is currently comprised of eight members, who are elected annually to serve for one year or until their successor is duly elected and qualified, or until their earlier resignation or removal. Executive officers serve at the discretion of the Board of Directors and are appointed by the Board of Directors.
| Name |
Age | Position | ||||
| Thomas H. Adams, Ph.D. |
75 | Chairman of the Board | ||||
| William (Bill) Welch |
56 | Chief Executive Officer and Director | ||||
| Mark Erlander, Ph.D. |
58 | Chief Scientific Officer | ||||
| John Brancaccio |
69 | Director | ||||
| Gary S. Jacob, Ph.D. |
70 | Director | ||||
| Dr. Paul Billings |
65 | Director | ||||
| Dr. Stanley Tennant |
66 | Director | ||||
| Dr. Rodney S. Markin |
61 | Director | ||||
| Dr. Athena Countouriotis |
46 | Director | ||||
Executive Biographies
The principal occupations for the past five years (and, in some instances, for prior years) of each of our directors and executive officers are as follows:
Thomas H. Adams. Thomas H. Adams, Ph.D., has been our Chairman of the Board since April 2009. Dr. Adams served as our interim Chief Executive Officer from March 28, 2016 until April 25, 2016. Dr. Adams has served as the Chairman of Clearbridge BioPhotonics, Inc., an imaging solutions company, since April 2013. From June 2005 through 2011, Dr. Adams served as a director of IRIS International, Inc., a diagnostics company, and has served as Chief Technology Officer of IRIS since April 2006. Dr. Adams was the Head of Iris Molecular Diagnostics from 2006 until November 2012 and has served as the President of Iris Personalized Medicine since 2011. In November 2012, IRIS was acquired by Danaher Corporation. Dr. Adams served as Chairman and Chief Executive Officer of Leucadia Technologies, a privately held medical-device company, from 1998 to April 2006, when Leucadia was acquired by IRIS. In 1989, Dr. Adams founded Genta, Inc., a publicly held biotechnology company in the field of antisense technology, and served as its Chief Executive Officer until 1997. Dr. Adams founded Gen-Probe, Inc. in 1984 and served as its Chief Executive Officer and Chairman until its acquisition by Chugai Biopharmaceuticals, Inc. in 1989. Dr. Adams has served as a director of Synergy Pharmaceuticals Inc., a biotechnology company, since July 2009 and has served as a director of Gensignia Life Sciences, Inc., a molecular diagnostics company, since October 2014. Dr. Adams has served as a director of ContraVir Pharmaceuticals, Inc., an antiviral biotechnology company, since September 2016. Dr. Adams holds a Ph.D. in Biochemistry from the University of California, at Riverside. The Board believes that Dr. Adams’ executive leadership, particularly in the diagnostic field, and the extensive healthcare expertise he has developed qualifies Dr. Adams to serve as a director of our company.
William (Bill) Welch. William Welch has served as our Chief Executive Officer since April 2016 and as a director of our company since May 2016. Mr. Welch was President and Chief Executive Officer of Sequenom, Inc. from June 2014 to September 2015 where he introduced the first non-invasive prenatal test (NIPT) utilizing maternal blood sample to identify fetal chromosomal abnormalities. Mr. Welch began his career at Sequenom as Senior Vice President, Diagnostics in January 2011 and became President and Chief Operating Officer in June 2014. Prior to joining Sequenom, Mr. Welch was a consultant to molecular diagnostic companies in the personalized medicine sector. From August 2005 to September 2009, Mr. Welch was senior vice president and chief commercial officer at Monogram Biosciences, a leader in HIV and oncology diagnostic testing services. Prior to his time at Monogram, Mr. Welch was vice president of sales and marketing at La Jolla Pharmaceuticals, an immunology based biotechnology company and vice president of global marketing with Dade Behring
74
Table of Contents
MicroScan. Mr. Welch entered the healthcare field with Abbott Laboratories where he held progressive management positions, including General Manager. Mr. Welch earned a B.S. with honors in chemical engineering from the University of California at Berkeley and received his M.B.A. from Harvard University.
Mark Erlander, Ph.D. Mark Erlander, Ph.D., has been our Chief Scientific Officer since March 2013. Dr. Erlander has more than 18 years of experience directing and leading research and development for gene discovery, with a strong focus on molecular diagnostics. Prior to joining Trovagene, Dr. Erlander was Chief Scientific Officer at bioTheranostics (a bioMerieux company) a molecular diagnostic testing company that is focused on clinical applications in oncology, from September 2008 to February 2013. From March 2013 to March 2014, Dr. Erlander served as Chief Scientific Officer of Gensignia Life Sciences, Inc., a molecular diagnostics company. Previously, Dr. Erlander was a group leader and subsequently a research fellow at the R.W. Johnson Pharmaceutical Research Institute (Johnson & Johnson). He was also an assistant member and postdoctoral fellow at The Scripps Research Institute in the Department of Molecular Biology. Dr. Erlander holds a BS degree in Biochemistry from the University of California, Davis; an MS degree in Biochemistry from Iowa State University; and a Ph.D. in Neuroscience from the University of California, Los Angeles. Dr. Erlander is an accomplished researcher with 32 issued U.S. patents and 38 U.S. patent applications, and is a lead or contributing author on more than 70 scientific papers and review articles.
John Brancaccio. John Brancaccio, a retired CPA, has served as a director of our company since December 2005. From April 2004 until his retirement in May 2017, Mr. Brancaccio was the Chief Financial Officer of Accelerated Technologies, Inc., an incubator for medical device companies. Mr. Brancaccio served as a director of Callisto Pharmaceuticals, Inc. from April 2004 until its merger with Synergy Pharmaceuticals, Inc. in January 2013 and has been a director of Tamir Biotechnology, Inc. (formerly Alfacell Corporation) since April 2004, as well as a director of Synergy Pharmaceuticals Inc. since July 2008 and ContraVir Pharmaceuticals, Inc. since December 2013 and Rasna Therapeutics, Inc. since August 2016. The Board believes that Mr. Brancaccio’s experience as a chief financial officer provides him with valuable financial and accounting expertise that qualifies him to serve as a director of our company.
Gary S. Jacob. Gary S. Jacob, Ph.D., has served as a director of our company since February 2009. Since July 2008, Dr. Jacob has been President, Chief Executive Officer and a Director of Synergy Pharmaceuticals Inc., and he has served as its Chairman since September 2013. Dr. Jacob has been Chairman of ContraVir Pharmaceuticals, Inc. since May 2013. Dr. Jacob also served as a director of Callisto Pharmaceuticals, Inc. from October 2004 until its merger with Synergy Pharmaceuticals, Inc. in January 2013. Prior to 1999, Dr. Jacob served as a Monsanto Science Fellow, specializing in the field of glycobiology, and from 1997 to 1998, he was Director of Functional Genomics, Corporate Science & Technology, at Monsanto Company. Dr. Jacob earned a B.S. in Chemistry from the University of Missouri, and holds a Ph.D. in Biochemistry from the University of Wisconsin-Madison. The Board believes that Dr. Jacob’s broad management expertise in the pharmaceutical and biotechnology industries provides relevant experience in a number of strategic and operational areas and qualifies him to serve as a director of our company.
Dr. Paul Billings. Paul Billings, M.D., Ph.D., was appointed to the Board in October 2013 and has been a member of our Scientific Advisory Board since November 2012. Since April 2018, Dr. Billings has served as Chief Medical Officer and Vice President, Medical Affairs for Natera, Inc. Dr. Billings is a board certified internist and clinical geneticist, and served as Executive-in-Residence at the California Innovation Center of Johnson and Johnson, Inc. from 2014 to 2015. He has also served as the Medical Director of the IMPACT program at Thermo Fisher Scientific, Inc. (TFS) from 2013 to 2015. He recently finished serving as the first and only Chief Medical Officer at Life Technologies Corporation (from 2011 to 2014) and the Genetic Sciences Division of TFS. Dr. Billings has extensive healthcare experience in many aspects of genomics and molecular medicine. He has also served on the Scientific Advisory Board of the U.S. Food and Drug Administration from 2011 to 2014, the Genomic Medicine Advisory Committee at the Department of Veterans Affairs from 2010 to 2014, and the National Academy of Sciences Institute of Medicine’s Roundtable on Genomics from 2011 to 2015. In addition to Trovagene, Dr. Billings has served as a director of Rennova Health, Inc. (formerly CollabRx, Inc.) from November 2015 to June 2017 and served as a director of Ancestry.com Inc. from February 2012 to
75
Table of Contents
May 2013. He serves as an advisor or director for many companies, including Omicia, Inc., BioScale Inc., Applied Immunology, Inc., Aueon, Inc. and PAX Neuroscience Inc. Dr. Billings holds an M.D. from Harvard Medical School and a Ph.D. in immunology, also from Harvard University. The Board believes that Dr. Billings’ medical and managerial experience in the diagnostic field qualifies him to serve as a director of our company.
Dr. Stanley Tennant. Stanley Tennant, M.D., has served as a director of our company since December 2010. From July 1983 to June 2012, Dr. Tennant was a cardiologist in Greensboro, North Carolina. Since January 1992, Dr. Tennant has served as the president of Five Star Management, a real estate company. Dr. Tennant has served as a director of Oak Ridge Financial Services, Inc. since July 2011. He graduated from Wake Forest University School of Medicine in 1978 and completed postgraduate training in Internal Medicine and Cardiology at Vanderbilt University in 1983. The Board believes that Dr. Tennant’s practical experience in the healthcare field qualifies him to serve as a director of our company.
Dr. Rodney S. Markin. Rodney S. Markin, M.D., Ph.D., has been a director of our company since February 2014. Dr. Markin has served as Chief Operating Officer of University of Nebraska since August 2017. Dr. Markin has served as Chief Technology Officer and Associate Vice Chancellor for Business Development at the University of Nebraska Medical Center from 2011 to July 2017; as a Professor of Pathology and Microbiology since 1985; as David T. Purtilo Distinguished Professor Pathology and Microbiology since 2005; as Courtesy Professor of Surgery since 1990 and as Courtesy Professor of Psychiatry since 2013. Dr. Markin is also a director on the Board of Children’s Hospital and Medical Center Foundation, on the Board of Trustees for Keck Graduate Institute, on the Board of the Make-A-Wish Foundation and on the Board of PerceptiMed since July 2015. Dr. Markin served on the Board of Directors of Transgenomic, Inc. from March 2007 to December 2014. The Board believes that Dr. Markin’s valuable executive experience in the healthcare business qualifies him to serve as a director of our company.
Dr. Athena Countouriotis. Dr. Athena Countouriotis has been a director of our company since September 2017. Dr. Countouriotis brings significant experience leading clinical development programs, from preclinical through clinical stages, and approval. Over the course of her career, she has been involved in multiple clinical programs, with a focus within oncology, both hematologic and solid tumor indications, that have supported regulatory approvals in the U.S. and Europe. Since June 2017, Dr. Countouriotis has been Senior Vice President, Chief Medical Officer at Adverum Biotechnologies. From January 2015 to May 2017, she served as Senior Vice President and Chief Medical Officer at Halozyme Therapeutics. From February 2012 to January 2015, Dr. Countouriotis was Chief Medical Officer at Ambit Biosciences through the initial development of quizartinib, a small molecule FLT3 inhibitor for the treatment of Acute Myeloid Leukemia, and ultimate acquisition of the company by Daiichi Sankyo. Dr. Countouriotis also worked at both Pfizer and Bristol-Meyers Squibb in various roles leading clinical development of oncology focused therapeutics. She holds a M.D. from Tufts University School of Medicine, completed her pediatric residency at the University of California, Los Angeles, and did additional training at Fred Hutchinson Cancer Research Center in the pediatric hematology-oncology program. The Board believes that Dr. Countouriotis’s medical and clinical research expertise in oncology provides relevant experience to the Board and management and qualifies her to serve as a director of our company.
Family Relationships and Other Arrangements
There are no family relationships among our directors and executive officers. There are no arrangements or understandings between or among our executive officers and directors pursuant to which any director or executive officer was or is to be selected as a director or executive officer.
76
Table of Contents
Involvement in Certain Legal Proceedings
To our knowledge, during the last ten years, none of our directors, executive officers (including those of our subsidiaries), promoters or control persons have:
| • | had a bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| • | been convicted in a criminal proceeding or been subject to a pending criminal proceeding, excluding traffic violations and other minor offenses; |
| • | been subject to any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; |
| • | been found by a court of competent jurisdiction (in a civil action), the Securities and Exchange Commission, or SEC, or the Commodities Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended or vacated; and |
| • | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization, any registered entity, or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Board Leadership Structure and Role in Risk Oversight
Since April 2009, we have separated the roles of Chairman of the Board (“Chairman”) and Chief Executive Officer (“CEO”). Although the separation of roles has been appropriate for us during this time period, in the view of the Board, the advisability of the separation of these roles depends upon the specific circumstances and dynamics of our leadership.
As Chairman, Dr. Adams serves as the primary liaison between the CEO and the independent directors and provides strategic input and counseling to the CEO. With input from other members of the Board, committee chairs and management, he presides over meetings of the Board. Dr. Adams has developed an extensive knowledge of our company, its challenges and opportunities and has a productive working relationship with our senior management team.
The Board, as a unified body and through committee participation, organizes the execution of its monitoring and oversight roles and does not expect the Chairman to organize those functions. Our primary rationale for separating the positions of Chairman and CEO is the recognition of the time commitments and activities required to function effectively as the Chairman and as the CEO of a company with a relatively flat management structure. The separation of roles has also permitted the Board to recruit senior executives into the CEO position with skills and experience that meet the Board’s planning for the position, some of which such individuals may not have extensive public company board experience.
The Board has three standing committees-Audit, Compensation and Corporate Governance/Nominating. The membership of each of the committees of the Board is comprised of independent directors, with each of the committees having a separate chairman, each of whom is an independent director. Our non-management members of the Board meet in executive session at each regular Board meeting.
Management is responsible for the day-to-day management of the risks we face, while the Board, as a whole and through its committees, has responsibility for the oversight of risk management. In its risk oversight role, the Board is responsible for satisfying itself that the risk management processes designed and implemented by management are adequate and functioning as designed.
77
Table of Contents
The Board believes that establishing the right “tone at the top” and that full and open communication between executive management and the Board are essential for effective risk management and oversight. Our CEO communicates frequently with members of the Board to discuss strategy and challenges facing our company. Senior management usually attends our regular quarterly Board meetings and is available to address any questions or concerns raised by the Board on risk management-related and any other matters. Each quarter, the Board receives presentations from senior management on matters involving our key areas of operations.
Audit Committee
We have a separately-designated standing Audit Committee established in accordance with Section 3(a)(58)(A) of the Securities Exchange Act of 1934, as amended, or the Exchange Act. The Audit Committee’s responsibilities include, among other things: (i) selecting and retaining an independent registered public accounting firm to act as our independent auditors, setting the compensation for our independent auditors, overseeing the work done by our independent auditors and terminating our independent auditors, if necessary, (ii) periodically evaluating the qualifications, performance and independence of our independent auditors, (iii) pre-approving all auditing and permitted non-audit services to be provided by our independent auditors, (iv) reviewing with management and our independent auditors our annual audited financial statements and our quarterly reports prior to filing such reports with the Securities and Exchange Commission, or the SEC, including the results of our independent auditors’ review of our quarterly financial statements, and (v) reviewing with management and our independent auditors significant financial reporting issues and judgments made in connection with the preparation of our financial statements. The Audit Committee also prepares the Audit Committee report that is required to be included in our annual proxy statement pursuant to the rules of the SEC.
The Audit Committee currently consists of John P. Brancaccio, chairman of the Audit Committee, Dr. Rodney Markin and Dr. Stanley Tennant. Under the applicable rules and regulations of NASDAQ, each member of a company’s audit committee must be considered independent in accordance with NASDAQ Listing Rule 5605(c)(2)(A)(i) and (ii) and Rule 10A-3(b)(1) under the Exchange Act. The Board has determined that each of Mr. Brancaccio, Dr. Markin and Dr. Tennant is “independent” as that term is defined under applicable NASDAQ and SEC rules. Mr. Brancaccio is our audit committee financial expert. The Board has adopted a written charter setting forth the authority and responsibilities of the Audit Committee, which is available on our website at http://trovagene.investorroom.com/ under “Corporate Governance”.
Compensation Committee
The purpose of the Compensation Committee is to discharge the Board’s responsibilities relating to compensation of our directors and executive officers. The Compensation Committee has responsibility for, among other things, (i) recommending to the Board for approval the overall compensation philosophy for our company and periodically reviewing the overall compensation philosophy for all employees to ensure it is appropriate and does not incentivize unnecessary and excessive risk taking, (ii) reviewing annually and making recommendations to the Board for approval, as necessary or appropriate, with respect to our compensation plans, (iii) based on an annual review, determining and approving, or at the discretion of the Compensation Committee, recommending to the Board for determination and approval, the compensation and other terms of employment of each of our officers, (iv) reviewing and making recommendations to the Board with respect to the compensation of directors, (v) overseeing our regulatory compliance with respect to compensation matters, (vi) reviewing and discussing with management, prior to the filing of our annual proxy statement or annual report on Form 10-K, our disclosure relating to executive compensation, including our Compensation Discussion and Analysis and executive and director compensation tables as required by SEC rules, and (vii) preparing an annual report regarding executive compensation for inclusion in our annual proxy statement or our annual report on Form 10-K. The Compensation Committee has the power to form one or more subcommittees, each of which may take such actions as may be delegated by the Compensation Committee.
The charter of the Compensation Committee grants the Compensation Committee authority to select, retain, compensate, oversee and terminate any compensation consultant to be used to assist in the evaluation of director,
78
Table of Contents
chief executive officer, officer and our other compensation and benefit plans and to approve the compensation consultant’s fees and other retention terms. The Compensation Committee is directly responsible for the appointment, compensation and oversight of the work of any internal or external legal, accounting or other advisors and consultants retained by the Compensation Committee. The Compensation Committee may also select or retain advice and assistance from an internal or external legal, accounting or other advisor as the Compensation Committee determines to be necessary or advisable in connection with the discharge of its duties and responsibilities and will have the direct responsibility to appoint, compensate and oversee any such advisor. During the past year, the Compensation Committee engaged Barney & Barney, LLC (“Barney & Barney”) as a compensation consultant.
The Compensation Committee currently consists of Dr. Rodney Markin, chairman of the Compensation Committee, Dr. Stanley Tennant and Dr. Athena Countouriotis. The Board has determined that all of the members are “independent” under NASDAQ Listing Rule 5602(a)(2). The Board has adopted a written charter setting forth the authority and responsibilities of the Compensation Committee, which is available on our website at http://trovagene.investorroom.com/ under “Corporate Governance”.
Corporate Governance/Nominating Committee
The Corporate Governance/Nominating Committee has responsibility for assisting the Board in, among other things, (i) effecting Board organization, membership and function, including identifying qualified board nominees, (ii) effecting the organization, membership and function of the committees of the Board, including the composition of the committees of the Board and recommending qualified candidates for the committees of the Board, (iii) evaluating and providing successor planning for the chief executive officer and our other executive officers, (iv) identifying and evaluating candidates for director in accordance with certain general and specific criteria, (v) developing and recommending to the Board Corporate Governance Guidelines and any changes thereto, setting forth the corporate governance principles applicable to us, and overseeing compliance with the our Corporate Governance Guidelines, and (vi) reviewing potential conflicts of interest involving directors and determining whether such directors may vote on issues as to which there may be a conflict. The Corporate Governance/Nominating Committee is responsible for identifying and evaluating candidates for director. Potential nominees are identified by the Board based on the criteria, skills and qualifications that are deemed appropriate by the Corporate Governance/Nominating Committee. The Corporate Governance/Nominating Committee believes that candidates for director should have certain minimum qualifications, including high character and integrity, an inquiring mind and vision, willingness to ask hard questions, ability to work well with others, freedom from conflicts of interest, willingness to devote sufficient time to the Company’s affairs, diligence in fulfilling his or her responsibilities and the capacity and desire to represent the best interests of the Company and our stockholders as a whole and not primarily a special interest group or constituency. While our nominating criteria does not prescribe specific diversity standards, the Corporate Governance/Nominating Committee and its independent members seek to identify nominees that have a variety of perspectives, professional experience, education, difference in viewpoints and skills, and personal qualities that will result in a well-rounded Board.
The Corporate Governance/Nominating Committee currently consists of Dr. Rodney Markin, chairman of the Corporate Governance/Nominating Committee, Mr. John Brancaccio and Dr. Gary S. Jacob. The Board has determined that all of the members are “independent” under NASDAQ Listing Rule 5605(a)(2). The Board has adopted a written charter setting forth the authority and responsibilities of the Corporate Governance/Nominating Committee, which is available on our website at http://trovagene.investorroom.com/ under “Corporate Governance”.
Code of Business Conduct and Ethics
We have adopted a formal Code of Business Conduct and Ethics applicable to all Board members, officers and employees. Our Code of Business Conduct and Ethics can be found on our website (www.trovagene.com). A
79
Table of Contents
copy of our Code of Business Conduct and Ethics may be obtained without charge upon written request to Secretary, Trovagene, Inc., 11055 Flintkote Avenue, San Diego, California 92121. If we make any substantive amendments to our Code of Business Conduct and Ethics or grant any waiver from a provision of the Code of Business Conduct and Ethics to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on our website (www.trovagene.com) and/or in our public filings with the SEC.
Corporate Governance Guidelines
The Board has adopted Corporate Governance Guidelines, which are designed to help us achieve our goals, govern us with high standards of integrity and increase stockholder value. These Corporate Governance Guidelines provide a framework for the conduct of the Board’s business.
The Corporate Governance Guidelines also set forth the practices our Board will follow with respect to Board composition and selection, Board meetings and Board committees and Chief Executive Officer performance evaluation and compensation. Our Corporate Governance Guidelines can be found on our website (www.trovagene.com).
Hedging and Pledging Policies
As part of our Insider Trading Policy, all of our officers, all of our directors, certain of our employees and consultants and family members or others sharing a household with any of the foregoing are prohibited from engaging in short sales of our securities, any hedging or monetization transactions involving our securities and in transactions involving puts, calls or other derivative securities based on our securities. Our Insider Trading Policy further prohibits such persons from purchasing our securities on margin, borrowing against any account in which our securities are held or pledging our securities as collateral for a loan unless pre-cleared by our Insider Trading Compliance Officer. As of March 31, 2018, none of our directors or executive officers had pledged any shares of our common stock.
80
Table of Contents
Summary Compensation Table
The following table provides certain summary information concerning compensation awarded to, earned by or paid to our Principal Executive Officer and Principal Financial Officer and our other highest paid executive officer whose total annual salary and bonus exceeded $100,000 (collectively, the “named executive officers”) for fiscal year 2017.
| Name and Principal Position |
Year | Salary ($) | Non-Equity Incentive Plan Compensation ($)(1) |
Option Awards ($) (2) |
Stock Awards ($) (3) |
Total ($) | ||||||||||||||||||
| William Welch, CEO |
2017 | 475,000 | 811,388 | (4)(5) | — | 1,123,314 | 2,409,702 | |||||||||||||||||
| 2016 | 319,712 | 115,781 | 3,204,294 | — | 3,639,787 | |||||||||||||||||||
| Dr. Mark Erlander, CSO |
2017 | 374,400 | 125,229 | (2)(6) | 225,866 | 296,252 | 1,021,747 | |||||||||||||||||
| 2016 | 374,400 | 234,000 | 458,166 | 199,500 | 1,266,066 | |||||||||||||||||||
| (1) | The amounts in this column relate to amounts earned by the Named Executive Officers in 2017 and 2016, as applicable, pursuant to our variable pay program. |
| (2) | Amounts shown in this column do not reflect dollar amounts actually received by our named executive officers. Instead, these amounts represent the aggregate grant date fair value of stock option awards determined in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 718. The valuation assumptions used in determining 2017 and 2016 amounts are described in Note 5 to our financial statements included in this prospectus. Our named executive officers will only realize compensation to the extent the trading price of our common stock is greater than the exercise price of such stock options on the date the options are exercised. |
| (3) | This reflects the grant date fair value of awards granted during fiscal 2017. |
| (4) | Amounts shown in this column do not reflect dollar amounts actually received by our named executive officer. Instead, these amounts represent (1) a total of $652,511 income taxes we paid for our named executive officer related to the restricted stock awards granted and vested during the fiscal year ended December 31, 2017; and (2) the aggregate grant date fair value of stock option awards determined in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 718. The valuation assumptions used in determining 2017 and 2016 amounts are described in Note 5 to our financial statements included in our Annual Reports on Form 10-K for the fiscal years ended December 31, 2017 and 2016. Our named executive officer will only realize compensation to the extent the trading price of our common stock is greater than the exercise price of such stock options on the date the options are exercised. |
| (5) | Received stock options to purchase 662,500 shares of common stock in lieu of cash bonus. |
| (6) | Received stock options to purchase 522,189 shares of common stock in lieu of cash bonus. |
81
Table of Contents
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information for the named executive officers regarding the number of shares subject to both exercisable and unexercisable stock options, as well as the exercise prices and expiration dates thereof, as of December 31, 2017. Except for the options set forth in the table below, no other equity awards were held by any our named executive officers as of December 31, 2017.
| Option Awards(1) | Stock Awards | |||||||||||||||||||||||
| Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
Number of shares or units of stock that have not vested (#) |
Market value of shares or units of stock that have not vested ($) |
||||||||||||||||||
| William Welch |
312,500 | 437,500 | 4.73 | 4/25/2026 | 200,000 | 61,500 | ||||||||||||||||||
| — | — | — | — | 130,000 | 39,975 | |||||||||||||||||||
| Mark Erlander |
5,000 | — | 2.84 | 9/13/2022 | 75,000 | 23,063 | ||||||||||||||||||
| 10,000 | — | 4.87 | 12/10/2022 | 90,000 | 27,675 | |||||||||||||||||||
| 200,000 | — | 7.04 | 1/28/2023 | |||||||||||||||||||||
| 100,000 | — | 5.53 | 12/11/2023 | |||||||||||||||||||||
| 150,000 | 50,000 | 3.29 | 7/16/2024 | |||||||||||||||||||||
| 45,000 | 15,000 | 4.39 | 12/11/2024 | |||||||||||||||||||||
| 71,875 | 78,125 | 5.18 | 1/4/2026 | |||||||||||||||||||||
| 96,250 | 288,750 | 0.85 | 8/22/2027 | |||||||||||||||||||||
| (1) | For each executive officer, the shares listed in this table are subject to a single stock option award carrying the varying exercise prices as set forth herein. The option awards remain exercisable until they expire ten years from the date of grant, subject to earlier expiration following termination of employment. |
Director Compensation
Under our non-employee director compensation policy, a new non-employee director receives an initial grant of options to purchase a number of shares of common stock equal to 0.1% of our shares of common stock issued and outstanding as of the date of grant (subject to adjustment for recapitalizations, stock split, stock dividends and the like). In addition, each non-employee director receives the following annual compensation for his or her service: (i) an annual retainer fee of $50,000, payable quarterly, and an equity grant of options to purchase a number of shares of common stock equal to 0.1% of shares of our common stock issued and outstanding as of the date of grant (subject to adjustment for recapitalizations, stock split, stock dividends and the like), all of which vest on the one year anniversary of the date of grant, (ii) an additional annual retainer fee of $30,000, payable quarterly, if such non-employee director serves as the Chairman of the Board of Directors, (iii) an additional annual retainer fee of $16,000, $10,000 and $8,000 payable quarterly, if such non-employee director serves as the chair of the Audit Committee, Compensation Committee or Nominating/Corporate Governance Committee, respectively, and (iv) an additional annual retainer fee of $8,000, $6,000 and $4,000 to such non-employee director if he or she serves as a non-chair member of the Audit Committee, Compensation Committee and Nominating/Corporate Governance Committee, respectively, per committee. We also reimburse all of our directors for out-of-pocket expenses incurred in connection with the rendering of services as a director.
82
Table of Contents
The following table sets forth summary information concerning the total compensation paid to our non-employee directors in 2017 for services to our company as director.
| Name |
Fees Earned or Paid in Cash ($) |
Option Awards ($)(1) |
Stock Awards ($) (2) |
Total ($) | ||||||||||||
| Thomas H. Adams(3) |
90,000 | 18,768 | 52,220 | 160,988 | ||||||||||||
| John P. Brancaccio(4) |
70,000 | 18,768 | 60,135 | 148,903 | ||||||||||||
| Gary S. Jacob(5) |
54,000 | 18,768 | 53,206 | 125,974 | ||||||||||||
| Stanley Tennant(6) |
64,000 | 18,768 | 51,560 | 134,328 | ||||||||||||
| Paul Billings(7) |
64,000 | 18,768 | 47,488 | 130,256 | ||||||||||||
| Rodney Markin(8) |
72,000 | 18,768 | 46,351 | 137,119 | ||||||||||||
| (1) | Amounts shown in this column do not reflect dollar amounts actually received by our non-employee directors. Instead, these amounts represent the aggregate grant date fair value of stock option awards determined in accordance with FASB ASC Topic 718. The valuation assumptions used in determining 2017 amounts are described in Note 5 to our financial statements included in this prospectus. Our non-employee directors will only realize compensation to the extent the trading price of our common stock is greater than the exercise price of such stock options on the date the options are exercised. |
| (2) | This reflects the grant date fair value of awards granted during fiscal 2017. |
| (3) | As of December 31, 2017, 412,995 stock options were outstanding, of which 374,849 were exercisable. 16,667 stock awards were unvested as of December 31, 2017. |
| (4) | As of December 31, 2017, 170,517 stock options were outstanding, of which 132,411 were exercisable. 16,667 stock awards were unvested as of December 31, 2017. |
| (5) | As of December 31, 2017, 185,445 stock options were outstanding, of which 147,339 were exercisable. 16,667 stock awards were unvested as of December 31, 2017. |
| (6) | As of December 31, 2017, 131,264 stock options were outstanding, of which 93,158 were exercisable. 16,667 stock awards were unvested as of December 31, 2017. |
| (7) | As of December 31, 2017, 121,336 stock options were outstanding, of which 83,230 were exercisable. 16,667 stock awards were unvested as of December 31, 2017. |
| (8) | As of December 31, 2017, 106,336 stock options were outstanding, of which 68,230 were exercisable. 16,667 stock awards were unvested as of December 31, 2017. |
Employment Agreements
William Welch Employment Agreement
On May 6, 2016, we entered into an employment agreement with Mr. Welch (the “Welch Employment Agreement”). The term of the Welch Employment Agreement commenced on May 6, 2016 and will continue until May 6, 2020, following which time the Welch Employment Agreement will be automatically renewed for successive one year periods at the end of each term, unless either party delivers written notice to the other party of their intent to not renew the agreement. Pursuant to the Welch Employment Agreement, Mr. Welch’s current base compensation is $475,000 per year. Mr. Welch is eligible to receive a cash bonus of up to 50% of his base salary per year based on meeting certain performance objectives and bonus criteria. In addition, upon entering into the Welch Employment Agreement, Mr. Welch was granted 750,000 stock options, which have an exercise price of $4.73 per share, 187,500 of which vest on April 25, 2017 and 15,625 vest monthly subsequent thereto.
If Mr. Welch’s employment is terminated by us for cause or as a result of Mr. Welch’s death or permanent disability, or if Mr. Welch terminates his employment agreement voluntarily, Mr. Welch will be entitled to receive a lump sum equal to (i) any portion of unpaid base compensation then due for periods prior to termination, (ii) any bonus earned but not yet paid through the date of his termination, and (iii) all business expenses reasonably and necessarily incurred by Mr. Welch prior to the date of termination. If Mr. Welch’s employment is terminated by us without cause or by Mr. Welch for good reason, Mr. Welch will be entitled to receive the amounts due upon termination of his employment by us for cause or as a result of his death or
83
Table of Contents
permanent disability, or upon termination by Mr. Welch of his employment voluntarily, in addition to (provided that Mr. Welch executes a written release with respect to certain matters) a severance payment equal to his base compensation for 24 months from the date of termination and the bonus and any benefits that Mr. Welch would be eligible for during such 24-month period. In addition, if Mr. Welch’s employment is terminated: (a) by us without cause within 12 months prior to a change of control (as defined in the Welch Employment Agreement) that was pending during such 12 month period, (b) by Mr. Welch for good reason within 12 months after a change of control, or (c) by us without cause at any time upon or within 12 months after a change of control, Mr. Welch will be entitled to receive the amounts due upon termination of his employment by us for cause or as a result of his death or permanent disability, or upon termination by Mr. Welch of his employment voluntarily, in addition to the severance payments due if Mr. Welch’s employment is terminated by us without cause or by Mr. Welch for good reason, and all of Mr. Welch’s unvested stock options and other equity awards would immediately vest and become fully exercisable (x) in the event a change of control transaction is pending, for a period of six months following the date of termination, and (y) in the event a change of control transaction is not then pending, for the period of time set forth in the applicable agreement evidencing the award.
William Welch Stock Award Agreement
On August 15, 2017, we entered into a stock award agreement (the “Agreement”) with Mr. Welch, pursuant to which an initial grant of 745,392 shares of common stock was issued to Mr. Welch under our 2014 Equity Incentive Plan, all of which shares vested upon grant. In addition, we agreed to make additional grants of common stock (the “Additional Grants”) to the Mr. Welch over two year time period. All grants will be vested upon date of grant and are subject to a one year lock-up from each date of grant. It is intended that, in the aggregate, the shares issued to Mr. Welch under the Agreement will constitute 5% of the issued and outstanding shares of common stock as of the last grant date scheduled for October 15, 2019 (the “Award Shares”). The Additional Grants shall be adjusted as is necessary to maintain such percentage.
Pursuant to the Agreement, we have agreed to pay to or on behalf of Mr. Welch the amount necessary to satisfy the full amount of Mr. Welch’s federal, state and local taxes as a result of the grant of the Award Shares. Upon the date the Executive ceases to be our employee for any reason, we may elect to repurchase all or any portion of the vested Award Shares at a price equal to the fair market value of the Award Shares. In addition, upon the consummation of our sale, a termination by us without Cause (as defined in the employment agreement dated May 6, 2016 between us and Mr. Welch (the “Employment Agreement”)) or Mr. Welch’s resignation for Good Reason as defined in the Employment Agreement, Mr. Welch shall be entitled to receive either (i) cash in an amount equal to the difference between the fair market value of the Award Shares then held by Mr. Welch and the fair market value of the Award Shares Mr. Welch would have received if he held 5% of the issued and outstanding shares of our common stock or (ii) such additional grants as is necessary to increase Mr. Welch’s total Award Shares to equal 5% of the shares of common stock issued and outstanding as of such date.
Employment Agreement with Dr. Mark Erlander
On February 18, 2016, we entered into an employment agreement with Dr. Erlander (the “Erlander Employment Agreement”). The term of the Erlander Employment Agreement commenced on February 18, 2016 and will continue until January 1, 2020, following which time the Erlander Employment Agreement will be automatically renewed for successive one year periods at the end of each term, unless either party delivers written notice to the other party of their intent to not renew the agreement. Pursuant to the Erlander Employment Agreement, Mr. Erlander’s current base compensation is $374,400 per year. Mr. Erlander is eligible to receive a cash bonus of up to 50% of his base salary per year based on meeting certain performance objectives and bonus criteria.
If Mr. Erlander’s employment is terminated by us for cause or as a result of Mr. Erlander’s death or permanent disability, or if Mr. Erlander terminates his employment agreement voluntarily, Mr. Erlander will be entitled to receive a lump sum equal to (i) any portion of unpaid base compensation then due for periods prior to
84
Table of Contents
termination, (ii) any bonus earned but not yet paid through the date of his termination, and (iii) all business expenses reasonably and necessarily incurred by Mr. Erlander prior to the date of termination. If Mr. Erlander’s employment is terminated by us without cause or by Mr. Erlander for good reason, Mr. Erlander will be entitled to receive the amounts due upon termination of his employment by us for cause or as a result of his death or permanent disability, or upon termination by Mr. Erlander of his employment voluntarily, in addition to (provided that Mr. Erlander executes a written release with respect to certain matters) a severance payment equal to his base compensation for 12 months from the date of termination and the bonus and any benefits that Mr. Erlander would be eligible for during such 12-month period. In addition, if Mr. Erlander’s employment is terminated: (a) by us without cause within 12 months prior to a change of control (as defined in the Erlander Employment Agreement) that was pending during such 12 month period, (b) by Mr. Erlander for good reason within 12 months after a change of control, or (c) by us without cause at any time upon or within 12 months after a change of control, Mr. Erlander will be entitled to receive the amounts due upon termination of his employment by us for cause or as a result of his death or permanent disability, or upon termination by Mr. Erlander of his employment voluntarily, in addition to the severance payments due if Mr. Erlander’s employment is terminated by us without cause or by Mr. Erlander for good reason, and all of Mr. Erlander’s unvested stock options and other equity awards would immediately vest and become fully exercisable (x) in the event a change of control transaction is pending, for a period of six months following the date of termination, and (y) in the event a change of control transaction is not then pending, for the period of time set forth in the applicable agreement evidencing the award.
Potential Payments Upon Termination Or Change In Control
Other than the provisions of the executive severance benefits to which our named executive officers would be entitled to at December 31, 2017 as set forth above, we have no liabilities under termination or change in control conditions. We do not have a formal policy to determine executive severance benefits. Each executive severance arrangement is negotiated on an individual basis.
The tables below estimate the current value of amounts payable to our named executive officers in the event that a termination of employment occurred on December 31, 2017. The closing price of our common stock, as reported on the Nasdaq Capital Market, was $0.31on December 29, 2017. The following tables exclude certain benefits, such as accrued vacation, that are available to all employees generally. The actual amount of payments and benefits that would be provided can only be determined at the time of a change in control and/or the named executive officer’s qualifying separation from our Company.
William Welch
| Termination | ||||||||
| By Trovagene Without Cause Outside a Change In Control |
By Trovagene Without Cause or by Mr. Welch for Good Reason in Connection with a Change In Control(1) |
|||||||
| Value of Equity Securities Accelerated |
$ | — | $ | 129,641 | ||||
| Cash Payments |
998,195 | 1,585,393 | ||||||
|
|
|
|
|
|||||
| Total Cash Benefits and Payments |
$ | 998,195 | $ | 1,715,034 | ||||
|
|
|
|
|
|||||
| (1) | Relates to the termination of the Welch Employment Agreement: (a) by us without cause within 12 months prior to a change of control that was pending during such 12 month period, (b) by Mr. Welch for good reason within 12 months after a change of control, or (c) by us without cause at any time upon or within 12 months after a change of control. |
85
Table of Contents
Mark Erlander, Ph.D.
| Termination | ||||||||
| By Trovagene Without Cause Outside a Change In Control |
By Trovagene Without Cause or by Mr. Erlander for Good Reason in Connection with a Change In Control(1) |
|||||||
| Value of Equity Securities Accelerated |
$ | — | $ | 110,772 | ||||
| Cash Payments |
386,813 | 386,813 | ||||||
|
|
|
|
|
|||||
| Total Cash Benefits and Payments |
$ | 386,813 | $ | 497,585 | ||||
|
|
|
|
|
|||||
| (1) | Relates to the termination of the Erlander Employment Agreement: (a) by us without cause within 12 months prior to a change of control that was pending during such 12 month period, (b) by Dr. Erlander for good reason within 12 months after a change of control, or (c) by us without cause at any time upon or within 12 months after a change of control. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The following is a description of transactions or series of transactions since January 1, 2016, or any currently proposed transaction, to which we were or are to be a participant and in which the amount involved in the transaction or series of transactions exceeds $120,000, and in which any of our directors, executive officers or persons who we know hold more than five percent of any class of our capital stock, including their immediate family members, had or will have a direct or indirect material interest, other than compensation arrangements with our directors and executive officers.
In March 2016, we engaged Rutan & Tucker, LLP, a law firm to represent us with respect to various lawsuits. One of the partners from Rutan & Tucker, LLP, is the son of our Chairman of the Board. The fees for legal services are based on the hourly rates of the individuals performing the legal services. During the years ended December 31, 2017 and 2016, we incurred and recorded approximately $650,000 and approximately $537,000, respectively, of legal expenses for services performed by Rutan & Tucker, LLP.
We have entered into indemnification agreements with our directors and executive officers under which we agreed to indemnify those individuals under the circumstances and to the extent provided for in the agreements, for expenses, damages, judgments, fines, settlements and any other amounts they may be required to pay in actions, suits or proceedings which they are or may be made a party or threatened to be made a party by reason of their position as a director, officer or other agent of ours, and otherwise to the fullest extent permitted under Delaware law and our By-Laws. We also have an insurance policy covering our directors and executive officers with respect to certain liabilities, including liabilities arising under the Securities Act of 1933, as amended, or otherwise.
Our board has adopted a written related party transaction policy to set forth the policies and procedures for the review, approval and ratification of related party transactions. This policy covers any financial transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships (including any indebtedness or guarantee of indebtedness) in which we are or are to be a participant, the amount involved will or may be expected to exceed $50,000 since the beginning of our last completed fiscal year, and a related party has or will have a direct or indirect material interest. A related party is any individual who is, or who has been since the beginning of our last fiscal year, an executive officer, director or nominee for election as a director, or any person known to be the record or beneficial owner of more than 5% of any class of our voting securities, any immediate family member of any of the foregoing persons or any entity which is owned or controlled by any of the foregoing persons, or any entity in which one of the foregoing persons has a substantial ownership interest in or control over such entity. Transactions involving the employment or compensation of our executive officers or compensation to our directors, transactions with another company at which a related party’s only relationship is as a director and/or beneficial owner of less than 10% of such company’s equity interests, transactions in which
86
Table of Contents
all of our stockholders receive proportional benefits, certain regulated transactions and certain banking-related services are not considered related-person transactions under this policy. Under our Audit Committee Charter and our related party transaction policy, our Audit Committee is responsible for reviewing and approving in advance any related party transaction. In connection with its review of a related party transaction, the Audit Committee will take into account, among other factors it deems appropriate, whether the related party transaction is on terms no less favorable than terms generally available to an unaffiliated third-party under the same or similar circumstances and the extent of the related party’s interest in the related party transaction.
Director Independence
Our The Board has determined that a majority of the Board consists of members who are currently “independent” as that term is defined under Nasdaq Listing Rule 5605(a)(2). The Board considers Drs. Jacob, Billings, Tennant, Countouriotis, and Markin and Mr. Brancaccio to be “independent.”
87
Table of Contents
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding beneficial ownership of shares of our common stock as of March 31, 2018 by (i) each person known to beneficially own more than 5% of our outstanding common stock, (ii) each of our directors, (iii) each of our named executive officers, and (iv) all of our directors and executive officers as a group. Except as otherwise indicated, the persons named in the table below have sole voting and investment power with respect to all shares beneficially owned, subject to community property laws, where applicable.
| Name of Beneficial Owner (1) |
Shares of Common Stock Beneficially Owned |
Percentage(2) | ||||||
| Executive officers and directors: |
||||||||
| Thomas Adams |
772,494 | (3) | 1.3 | % | ||||
| William Welch |
1,895,770 | (4) | 3.2 | % | ||||
| Paul Billings |
106,395 | (5) | * | |||||
| John Brancaccio |
189,441 | (6) | * | |||||
| Gary Jacob |
306,793 | (7) | * | |||||
| Stanley Tennant |
394,219 | (8) | * | |||||
| Rodney S. Markin |
90,840 | (9) | * | |||||
| Athena Countouriotis |
— | — | ||||||
| Mark Erlander |
1,416,556 | (10) | 2.4 | % | ||||
| All Officers and Directors as a Group (9 persons) |
5,172,508 | (11) | 8.3 | % | ||||
| * | less than 1% |
| (1) | The address of each person is c/o Trovagene, Inc., 11055 Flintkote Avenue, Suite A, San Diego, CA 92121 unless otherwise indicated herein. |
| (2) | The calculation in this column is based upon 58,832,953 shares of common stock outstanding on March 31, 2018. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to the subject securities. Shares of common stock that are currently exercisable or exercisable within 60 days of March 31, 2018 are deemed to be beneficially owned by the person holding such securities for the purpose of computing the percentage beneficial ownership of such person, but are not treated as outstanding for the purpose of computing the percentage beneficial ownership of any other person. |
| (3) | Includes (i) 374,849 shares of common stock issuable upon exercise of stock options that are exercisable within 60 days after March 31, 2018, and (ii) 45,686 shares of common stock issuable upon exercise of warrants that are exercisable within 60 days after March 31, 2018. |
| (4) | Includes 1,053,125 shares of common stock issuable upon exercise of stock options that are exercisable within 60 days after March 31, 2018. |
| (5) | Includes 83,230 shares of common stock issuable upon exercise of stock options that are exercisable within 60 days after March 31, 2018. |
| (6) | Includes (i) 132,441 shares of common stock issuable upon exercise of stock options that are exercisable within 60 days after March 31, 2018, and (ii) 13,833 shares of common stock issuable upon exercise of warrants that are exercisable within 60 days after March 31, 2018. |
| (7) | Includes (i) 147,339 shares of common stock issuable upon exercise of stock options that are exercisable within 60 days after March 31, 2018, and (ii) 10,500 shares of common stock issuable upon exercise of warrants that are exercisable within 60 days after March 31, 2018. |
| (8) | Includes (i) 93,158 shares of common stock issuable upon exercise of stock options that are exercisable within 60 days after March 31, 2018, and (ii) 75,000 shares of common stock issuable upon exercise of warrants that are exercisable within 60 days after March 31, 2018. |
| (9) | Includes 68,230 shares of common stock issuable upon exercise of stock options that are exercisable within 60 days after March 31, 2018. |
88
Table of Contents
| (10) | Includes 1,312,189 shares of common stock issuable upon exercise of stock options that are exercisable within 60 days after March 31, 2018. |
| (11) | Includes (i) 3,264,561 shares of common stock issuable upon exercise of stock options that are exercisable within 60 days after March 31, 2018 and (ii) 145,019 shares of common stock issuable upon exercise of warrant to purchase shares of common stock. |
89
Table of Contents
DESCRIPTION OF SECURITIES WE ARE OFFERING
We are offering [ ] Class A Units consisting of one share of our common stock and one warrant to purchase shares of our common stock, at an exercise price equal to % of the public offering price of the Class A Units per share of common stock, which warrants will be exercisable upon issuance and will expire years from date of issuance. The shares of common stock and warrants that are part of a Class A Unit are immediately separable and will be issued separately in this offering.
We are also offering to those purchasers, if any, whose purchase of Class A Units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding common stock immediately following the consummation of this offering, the opportunity, in lieu of purchasing Class A Units, to purchase Class B Units. Each Class B Unit will consist of one share of our newly designated Series B Preferred with a stated value of $1,000 and convertible into shares of our common stock at the public offering price of the Class A Units, together with the equivalent number of warrants as would have been issued to such purchaser of Class B Units if they had purchased Class A Units. For each Class B Unit we sell, the number of Class A Units we are offering will be decreased on a one-for-one basis. The shares of Series B Preferred and warrants that are part of a Class B Unit are immediately separable and will be issued separately in this offering. We are also offering the shares of common stock issuable upon exercise of the warrants and conversion of the Series B Preferred.
General
We are authorized to issue up to 150,000,000 shares of common stock, $0.0001 par value per share, and 20,000,000 shares of preferred stock, $0.001 par value per share.
As of April 30, 2018, a total of 59,378,162 shares of our common stock were issued and outstanding and 60,600 shares of our Series A Convertible Preferred Stock were issued and outstanding.
Common Stock
The holders of our common stock are entitled to one vote per share. Our certificate of incorporation does not provide for cumulative voting. The holders of our common stock are entitled to receive ratably such dividends, if any, as may be declared by our board of directors out of legally available funds; however, the current policy of our board of directors is to retain earnings, if any, for operations and growth. Upon liquidation, dissolution or winding-up, the holders of our common stock are entitled to share ratably in all assets that are legally available for distribution. Except for certain stockholders who have the right to participate, until January 19, 2019, in any issuance by us of common stock in a subsequent financing up to 35% of the subsequent financing, the holders of our common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of any series of preferred stock, which may be designated solely by action of our board of directors and issued in the future.
Preferred Stock
The following is a summary of the material terms of our Series A Convertible Preferred Stock and the Series B Preferred. This summary is not complete. The following summary is qualified in its entirety by reference to the Certificate of Designation of the Series A Convertible Preferred Stock, and the form of Certificate of Designation of Series B Preferred Stock, each of which has been filed as an exhibit to the registration statement of which this prospectus is a part.
90
Table of Contents
Series A Convertible Preferred Stock
The material terms of the Series A Convertible Preferred Stock consist of:
1) Dividends. Holders of our Series A Convertible Preferred Stock are entitled to receive cumulative dividends at the rate per share of 4% per annum, payable quarterly on March 31, June 30, September 30 and December 31, beginning with September 30, 2005. Dividends are payable, at our sole election, in cash or shares of common stock. As of December 31, 2017 and 2016, we had $316,775 and $292,535, respectively in accrued cumulative unpaid preferred stock dividends, included in accrued liabilities in our consolidated balance sheets, and $24,240 and $24,240 of accrued dividends was recorded during the years ended December 31, 2017 and 2016, respectively.
2) Voting Rights. Shares of the Series A Convertible Preferred Stock have no voting rights. However, so long as any shares of Series A Convertible Preferred Stock are outstanding, we may not, without the affirmative vote of the holders of the shares of Series A Convertible Preferred Stock then outstanding, (a) adversely change the powers, preferences or rights given to the Series A Convertible Preferred Stock, (b) authorize or create any class of stock senior or equal to the Series A Convertible Preferred Stock, (c) amend our certificate of incorporation or other charter documents, so as to affect adversely any rights of the holders of Series A Convertible Preferred Stock or (d) increase the authorized number of shares of Series A Convertible Preferred Stock.
3) Liquidation. Upon any liquidation, dissolution or winding-up of our company, the holders of the Series A Convertible Preferred Stock are entitled to receive an amount equal to the Stated Value per share, which is currently $10 per share plus any accrued and unpaid dividends.
4) Conversion Rights. Each share of Series A Convertible Preferred Stock is convertible at the option of the holder into that number of shares of common stock determined by dividing the Stated Value, currently $10 per share, by the conversion price, originally $2.15 per share.
5) Subsequent Equity Sales. The conversion price is subject to adjustment for dilutive issuances for a period of 12 months beginning upon registration of the common stock underlying the Series A Convertible Preferred Stock. The relevant registration statement became effective on March 17, 2006 and during the following twelve month period the conversion price was adjusted to $9.60 per share.
6) Automatic Conversion. If the price of our common stock equals $25.80 per share for 20 consecutive trading days, and an average of 8,333 shares of common stock per day are traded during the 20 trading days, we will have the right to deliver a notice to the holders of the Series A Convertible Preferred Stock, requesting the holders to convert any portion of the shares of Series A Convertible Preferred Stock into shares of common stock at the applicable conversion price.
Series B Preferred Stock
General. Our board of directors has designated up to [ ] shares of the 20,000,000 authorized shares of preferred stock as Series B Convertible Preferred Stock. When issued, the shares of Series B Preferred will be validly issued, fully paid and non-assessable. Each share of Series B Preferred will have a stated value of $1,000 per share.
Rank. The Series B Preferred will rank on parity to our common stock.
Conversion. Each share of Series B Preferred will be convertible into shares of our common stock at any time at the option of the holder at a conversion price equal to the public offering price of the Class A Units in this offering (subject to adjustment as provided in the certificate of designation). Holders of Series B Preferred will
91
Table of Contents
be prohibited from converting Series B Preferred into shares of our common stock if, as a result of such conversion, the holder, together with its affiliates, would beneficially own more than 4.99% (or upon the election by a holder prior to the issuance of any shares of Series B Preferred, 9.99%) of the total number of shares of our common stock then issued and outstanding.
Liquidation Preference. In the event of our liquidation, dissolution or winding-up, holders of Series B Preferred will be entitled to receive the same amount that a holder of our common stock would receive if the Series B Preferred were fully converted into shares of our common stock at the conversion price (disregarding for such purposes any conversion limitations) which amounts shall be paid pari passu with all holders of common stock.
Voting Rights. Shares of Series B Preferred will vote on an as-converted to common stock basis; provided, however, in no event will a holder of shares of Series B Preferred be entitled to vote a number of shares in excess of such holder’s beneficial ownership limitation. In addition, the affirmative vote of the holders of a majority of the then outstanding shares of Series B Preferred will be required to, (a) alter or change adversely the powers, preferences or rights given to the Series B Preferred, (b) amend our certificate of incorporation or other charter documents in any manner that materially adversely affects any rights of the holders, (c) increase the number of authorized shares of Series B Preferred, or (d) enter into any agreement with respect to any of the foregoing.
Dividends. Shares of Series B Preferred will not be entitled to receive any dividends, unless and until specifically declared by our board of directors. The holders of the Series B Preferred will participate, on an as-if-converted-to-common stock basis, in any dividends to the holders of common stock.
Redemption. We will be not obligated to redeem or repurchase any shares of Series B Preferred. Shares of Series B Preferred will not otherwise be entitled to any redemption rights or mandatory sinking fund or analogous fund provisions.
Exchange Listing. We do not plan on making an application to list the Series B Preferred on any national securities exchange or other nationally recognized trading system.
Warrants
As of April 30, 2018, we had outstanding warrants to purchase an aggregate of 17,873,853 shares of our common stock.
Warrants to be issued in this offering
The following is a summary of the material terms of the warrants. This summary is not complete and is qualified in its entirety by reference to the warrants, the form of which has been filed as an exhibit to the registration statement of which this prospectus is a part.
Form. The warrants will be issued as individual warrant agreements to the investors in the offering. You should review a copy of the form of warrant, which is filed as an exhibit to the registration statement of which this prospectus forms a part, for a complete description of the terms and conditions applicable to the warrants.
Exercisability. The warrants will be exercisable at any time after their original issuance, expected to be , 2018, and at any time up to the date that is years after their original issuance. The warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and, at any time a registration statement registering the issuance of the shares of common stock underlying the warrants under the Securities Act is effective and available for the issuance of such shares, by payment in full in immediately available funds for the number of shares of common stock purchased upon such exercise. If a registration statement registering the issuance of the shares of common stock underlying the warrants under the
92
Table of Contents
Securities Act is not effective or available, the holder may, in its sole discretion, elect to exercise the warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of common stock determined according to the formula set forth in the warrant. No fractional shares of common stock will be issued in connection with the exercise of a warrant. In lieu of fractional shares, we will, at our sole discretion, either pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up such fractional amount to the next whole share
Exercise Limitation. A holder will not have the right to exercise any portion of the warrant if the holder (together with its affiliates) would beneficially own in excess of 4.99% (or, upon election by a holder prior to the issuance of any warrants, 9.99%) of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants.
Exercise Price. The exercise price per share of common stock purchasable upon exercise of the warrants will be equal to % of the public offering price per Class A Unit. The warrants may also be exercised via cashless exercise, whereby the holder will receive upon exercise of the warrant (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the warrant. The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Transferability. Subject to applicable laws, the warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. We do not plan on making an application to list the warrants on any national securities exchange or other nationally recognized trading system.
Fundamental Transactions. In the event of a fundamental transaction, as described in the warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the warrants will be entitled to receive upon exercise of the warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the warrants immediately prior to such fundamental transaction.
Rights as a Stockholder. Except as otherwise provided in the warrants or by virtue of such holder’s ownership of shares of our common stock, the holder of a warrant will not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the warrant.
93
Table of Contents
ThinkEquity, a division of Fordham Financial Management, Inc. (“ThinkEquity”), is acting as representative of the underwriters of the offering. We have entered into an underwriting agreement dated , 2018 with the representative. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to each underwriter named below, and each underwriter named below has severally agreed to purchase, at the public offering price less the underwriting discounts set forth on the cover page of this prospectus, the number of Units listed next to its name in the following table:
| Name |
Number of Class A Units |
Number of Class B Units | ||
| ThinkEquity, a division of Fordham Financial Management, Inc. | ||||
| Total: |
The underwriters are committed to purchase all the Units offered by us other than those covered by the over-allotment option to purchase additional shares and/or warrants described below, if they purchase any Units. The obligations of the underwriters may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’ obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement, such as receipt by the underwriters of officers’ certificates and legal opinions.
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters may be required to make in respect thereof.
The underwriters are offering the Units, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel and other conditions specified in the underwriting agreement. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
We have granted the underwriters an over-allotment option. This option, which is exercisable for up to 45 days after the date of this prospectus, permits the underwriters to purchase a maximum of additional shares (15% of the shares of common stock included in the Class A Units and Class B Units sold in this offering, on an as-converted to common stock basis with respect to any Series B Preferred sold) and/or warrants to purchase shares of common stock (15% of the warrants included as part of the Units sold in this offering) from us to cover over-allotments, if any. If the underwriters exercise all or part of this option, they will purchase shares covered by the option at the public offering price that appears on the cover page of this prospectus, less the underwriting discount. If this option is exercised in full, the total price to the public will be $ and the total net proceeds, before expenses, to us will be $ .
Discounts, Commissions and Reimbursement
The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Class A Unit |
Per Class B Unit |
Total Without Over- Allotment Option |
Total With Full Over- Allotment Option |
|||||||||||||
| Public offering price |
$ | $ | $ | $ | ||||||||||||
| Underwriting discount (7%) |
$ | $ | $ | $ | ||||||||||||
| Non-accountable expense allowance (1%)(1) |
$ | $ | $ | $ | ||||||||||||
| Proceeds, before expenses, to us |
$ | $ | $ | $ | ||||||||||||
94
Table of Contents
| (1) | We have agreed to pay a non-accountable expense allowance to the representative equal to 1.0% of the gross proceeds received in this offering (excluding any proceeds received from exercise of the underwriters’ over-allotment option). |
The underwriters propose to offer the securities to the public at the public offering price set forth on the cover of this prospectus. In addition, the underwriters may offer some of the shares to other securities dealers at such price less a concession of $ per share. If all of the securities offered by us are not sold at the public offering price, the representative may change the offering price and other selling terms by means of a supplement to this prospectus.
We have also agreed to reimburse the representative for the full amount of its actual accountable expenses incurred in connection with the offering, up to a maximum of $100,000 (including, without limitation, for the representative’s external counsel, “road show” expenses, mailing, printing, and reproduction expenses, and due diligence expenses, including background checks of our officers and directors.
We estimate that the total expenses of the offering payable by us, excluding the total underwriting discount and non-accountable expense allowance, will be approximately $ .
Discretionary Accounts
The underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
Lock-Up Agreements
Pursuant to “lock-up” agreements, we and our executive officers and directors, have agreed, subject to limited exceptions, without the prior written consent of the Representative not to directly or indirectly, offer to sell, sell, pledge or otherwise transfer or dispose of any of shares of (or enter into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person at any time in the future of) our common stock, enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of shares of our common stock, make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any shares of common stock or securities convertible into or exercisable or exchangeable for common stock or any of our other securities or publicly disclose the intention to do any of the foregoing, subject to customary exceptions, for a period of 180 days from the date of this prospectus, in the case of our directors and officers, and for a period of 90 days from the date of this prospectus, in the case of us.
Right of First Refusal
Until nine months after the closing date of the offering, the representative will have an irrevocable right of first refusal to act as sole investment banker, sole book-runner, and/or sole placement agent at the representative’s sole and discretion, for each and every future public and private equity and debt offering, including all equity linked financings during such nine month period, of the Company on terms and conditions customary to the representative.
Electronic Offer, Sale and Distribution of Securities
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters or selling group members. The representative may agree to allocate a number of securities to underwriters and selling group members for sale to its online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us, and should not be relied upon by investors.
95
Table of Contents
Listing
Our common stock is listed on the Nasdaq Capital Market under the symbol “TROV.”
Stabilization
In connection with this offering, the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate-covering transactions, penalty bids and purchases to cover positions created by short sales.
Stabilizing transactions permit bids to purchase shares so long as the stabilizing bids do not exceed a specified maximum, and are engaged in for the purpose of preventing or retarding a decline in the market price of the shares while the offering is in progress.
Over-allotment transactions involve sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares that they may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. The underwriters may close out any short position by exercising their over-allotment option and/or purchasing shares in the open market.
Syndicate covering transactions involve purchases of shares in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared with the price at which they may purchase shares through exercise of the over-allotment option. If the underwriters sell more shares than could be covered by exercise of the over-allotment option and, therefore, have a naked short position, the position can be closed out only by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that after pricing there could be downward pressure on the price of the shares in the open market that could adversely affect investors who purchase in the offering.
Penalty bids permit the representative to reclaim a selling concession from a syndicate member when the shares originally sold by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions.
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our shares of common stock or preventing or retarding a decline in the market price of our shares of common stock. As a result, the price of our common stock in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Passive market making
In connection with this offering, underwriters and selling group members may engage in passive market making transactions in our common stock on the Nasdaq Capital Market in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales of the shares and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, then that bid must then be lowered when specified purchase limits are exceeded.
96
Table of Contents
Other Relationships
Certain of the underwriters and their affiliates may in the future provide various investment banking, commercial banking and other financial services for us and our affiliates for which they may in the future receive customary fees..
Offer restrictions outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer to the offeree under this prospectus.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
European Economic Area—Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
| • | to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
97
Table of Contents
| • | to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statements); |
| • | to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to obtaining the prior consent of the Company or any underwriter for any such offer; or |
| • | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Directive. |
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D. 744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority (the ISA), or ISA, nor have such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing the prospectus; nor has it
98
Table of Contents
authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Societ—$$—Aga e la Borsa, “CONSOB” pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
| • | to Italian qualified investors, as defined in Article 100 of Decree no.58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and |
| • | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
| • | Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be: |
| • | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and |
| • | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or
99
Table of Contents
indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissăo do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA).
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by the Company.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of
100
Table of Contents
section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to the Company.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws. Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor. Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
101
Table of Contents
The validity of the securities being offered by this prospectus will be passed upon for us by Sheppard Mullin Richter & Hampton LLP, New York, New York. Certain legal matters in connection with this offering have been passed upon for the underwriter by Sichenzia Ross Ference Kesner LLP, New York, New York.
The consolidated financial statements as of December 31, 2017 and 2016 and for each of the two years in the period ended December 31, 2017 included in this prospectus have been so included in reliance on the report of BDO USA, LLP, an independent registered public accounting firm (the report on the consolidated financial statements contains an explanatory paragraph regarding the Company’s ability to continue as a going concern), appearing elsewhere herein, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus, which constitutes a part of the registration statement on Form S-1 that we have filed with the SEC under the Securities Act, does not contain all of the information in the registration statement and its exhibits. For further information with respect to us and the securities offered by this prospectus, you should refer to the registration statement and the exhibits filed as part of that document. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, and file annual, quarterly and current reports, proxy statements and other information with the SEC. You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at http://www.sec.gov. We also maintain a website at www.trovagene.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus.
You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street, N.E., Room 1580, Washington, DC 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities. You may also request a copy of these filings, at no cost, by writing or telephoning us at: 11055 Flintkote Avenue, San Diego, California, 92121, (858) 952-7570.
102
Table of Contents
Index to Consolidated Financial Statements
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Trovagene, Inc.
San Diego, California
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Trovagene, Inc. and Subsidiary (the “Company”) as of December 31, 2017 and 2016 and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2017, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2017 and 2016, and the results of their operations and their cash flows for each of the two years in the period ended December 31, 2017, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ BDO USA, LLP
We have served as the Company’s auditor since 2007.
San Diego, California
February 26, 2018
F-2
Table of Contents
Trovagene, Inc. and Subsidiary
Consolidated Balance Sheets
| December 31, 2017 |
December 31, 2016 |
|||||||
| Assets | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 8,225,764 | $ | 13,915,094 | ||||
| Short-term investments |
— | 23,978,022 | ||||||
| Accounts receivable |
77,095 | 100,460 | ||||||
| Prepaid expenses and other current assets |
1,165,828 | 956,616 | ||||||
|
|
|
|
|
|||||
| Total current assets |
9,468,687 | 38,950,192 | ||||||
| Property and equipment, net |
2,426,312 | 3,826,915 | ||||||
| Other assets |
389,942 | 1,173,304 | ||||||
|
|
|
|
|
|||||
| Total Assets |
$ | 12,284,941 | $ | 43,950,411 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 825,244 | $ | 1,130,536 | ||||
| Accrued liabilities |
1,454,587 | 4,021,365 | ||||||
| Deferred rent |
334,424 | 285,246 | ||||||
| Current portion of long-term debt (in default) |
1,331,515 | 2,360,109 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
3,945,770 | 7,797,256 | ||||||
| Long-term debt, less current portion |
— | 14,176,359 | ||||||
| Derivative financial instruments—warrants |
649,387 | 834,940 | ||||||
| Deferred rent, net of current portion |
1,183,677 | 1,373,717 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
5,778,834 | 24,182,272 | ||||||
| Commitments and contingencies (Note 10) |
||||||||
| Stockholders’ equity |
||||||||
| Preferred stock, $0.001 par value, 20,000,000 shares authorized, 60,600 shares outstanding at each of December 31, 2017 and 2016, designated as Series A Convertible Preferred Stock with liquidation preference of $606,000 at each of December 31, 2017 and 2016 |
60 | 60 | ||||||
| Common stock, $0.0001 par value, 150,000,000 shares authorized at December 31, 2017 and 2016; 52,791,584 and 30,696,791 issued and outstanding at December 31, 2017 and 2016, respectively |
5,279 | 3,070 | ||||||
| Additional paid-in capital |
179,546,954 | 167,890,984 | ||||||
| Accumulated other comprehensive loss |
— | (10,773 | ) | |||||
| Accumulated deficit |
(173,046,186 | ) | (148,115,202 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
6,506,107 | 19,768,139 | ||||||
|
|
|
|
|
|||||
| Total Liabilities and Stockholders’ Equity |
$ | 12,284,941 | $ | 43,950,411 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Table of Contents
Trovagene, Inc. and Subsidiary
Consolidated Statements of Operations
| Year Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Revenues: |
||||||||
| Royalties |
$ | 285,444 | $ | 258,062 | ||||
| Diagnostic services |
196,111 | 86,137 | ||||||
| Clinical research services |
23,849 | 36,873 | ||||||
|
|
|
|
|
|||||
| Total revenues |
505,404 | 381,072 | ||||||
|
|
|
|
|
|||||
| Costs and expenses: |
||||||||
| Cost of revenue |
1,811,424 | 1,730,512 | ||||||
| Research and development |
7,882,650 | 15,006,642 | ||||||
| Selling and marketing |
2,735,410 | 11,523,144 | ||||||
| General and administrative |
11,497,466 | 11,475,947 | ||||||
| Restructuring charges |
2,174,251 | 790,438 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
26,101,201 | 40,526,683 | ||||||
|
|
|
|
|
|||||
| Loss from operations |
(25,595,797 | ) | (40,145,611 | ) | ||||
|
|
|
|
|
|||||
| Interest income |
147,883 | 298,829 | ||||||
| Interest expense |
(1,033,939 | ) | (1,674,341 | ) | ||||
| Other loss, net |
(170,138 | ) | (144,733 | ) | ||||
| Loss on extinguishment of debt |
(1,655,825 | ) | — | |||||
| Gain from changes in fair value of derivative financial instruments—warrants |
3,401,072 | 2,462,137 | ||||||
|
|
|
|
|
|||||
| Net loss |
(24,906,744 | ) | (39,203,719 | ) | ||||
| Preferred stock dividend |
(24,240 | ) | (24,240 | ) | ||||
|
|
|
|
|
|||||
| Net loss attributable to common stockholders |
$ | (24,930,984 | ) | $ | (39,227,959 | ) | ||
|
|
|
|
|
|||||
| Net loss per common share — basic |
$ | (0.72 | ) | $ | (1.30 | ) | ||
|
|
|
|
|
|||||
| Net loss per common share — diluted |
$ | (0.72 | ) | $ | (1.37 | ) | ||
|
|
|
|
|
|||||
| Weighted-average shares outstanding — basic |
34,680,362 | 30,174,838 | ||||||
|
|
|
|
|
|||||
| Weighted-average shares outstanding — diluted |
34,680,362 | 30,281,263 | ||||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
Trovagene, Inc. and Subsidiary
Consolidated Statements of Comprehensive Loss
| Year Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Net loss |
$ | (24,906,744 | ) | $ | (39,203,719 | ) | ||
| Other comprehensive loss: |
||||||||
| Foreign currency translation loss or reversal of previous loss |
1,708 | (1,708 | ) | |||||
| Unrealized gain or reversal of previous loss on securities available-for-sale |
9,065 | (9,065 | ) | |||||
|
|
|
|
|
|||||
| Total other comprehensive loss |
10,773 | (10,773 | ) | |||||
|
|
|
|
|
|||||
| Total comprehensive loss |
(24,895,971 | ) | (39,214,492 | ) | ||||
|
|
|
|
|
|||||
| Preferred stock dividend |
(24,240 | ) | (24,240 | ) | ||||
|
|
|
|
|
|||||
| Comprehensive loss attributable to common stockholders |
$ | (24,920,211 | ) | $ | (39,238,732 | ) | ||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
Trovagene, Inc. and Subsidiary
Consolidated Statements of Stockholders’ Equity
| Preferred Stock Shares |
Preferred Stock Amount |
Common Stock Shares |
Common Stock Amount |
Additional Paid-In Capital |
Accumulated other comprehensive loss |
Accumulated Deficit |
Total Stockholders’ Equity |
|||||||||||||||||||||||||
| Balance, January 1, 2016 |
60,600 | $ | 60 | 29,737,601 | $ | 2,974 | $ | 157,585,498 | $ | — | $ | (108,887,243 | ) | $ | 48,701,289 | |||||||||||||||||
| Sale of common stock, net of expenses |
— | — | 421,810 | 42 | 2,285,373 | — | — | 2,285,415 | ||||||||||||||||||||||||
| Stock based compensation |
— | — | — | — | 7,504,316 | — | — | 7,504,316 | ||||||||||||||||||||||||
| Issuance of warrant in connection with debt agreement |
— | — | — | — | 148,885 | — | — | 148,885 | ||||||||||||||||||||||||
| Issuance of common stock upon net exercise of stock options |
— | — | 341,333 | 34 | (34 | ) | — | — | — | |||||||||||||||||||||||
| Issuance of common stock upon exercise of stock options |
— | — | 98,396 | 10 | 366,956 | — | — | 366,966 | ||||||||||||||||||||||||
| Issuance of common stock upon net exercise of warrant |
— | — | 2,651 | — | — | — | — | — | ||||||||||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units |
— | — | 95,000 | 10 | (10 | ) | — | — | — | |||||||||||||||||||||||
| Unrealized loss from foreign currency translation |
— | — | — | — | — | (1,708 | ) | — | (1,708 | ) | ||||||||||||||||||||||
| Unrealized loss on securities available-for-sale |
— | — | — | — | — | (9,065 | ) | — | (9,065 | ) | ||||||||||||||||||||||
| Preferred stock dividend |
— | — | — | — | — | — | (24,240 | ) | (24,240 | ) | ||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (39,203,719 | ) | (39,203,719 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance, December 31, 2016 |
60,600 | 60 | 30,696,791 | 3,070 | 167,890,984 | (10,773 | ) | (148,115,202 | ) | 19,768,139 | ||||||||||||||||||||||
| Sale of common stock, net of expenses |
— | — | 20,976,914 | 2,097 | 10,859,016 | — | — | 10,861,113 | ||||||||||||||||||||||||
| Stock-based compensation |
— | — | — | — | 4,012,585 | — | — | 4,012,585 | ||||||||||||||||||||||||
| Derivative liability-fair value of warrants issued |
— | — | — | — | (3,215,519 | ) | — | — | (3,215,519 | ) | ||||||||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units |
— | — | 372,487 | 37 | (37 | ) | — | — | — | |||||||||||||||||||||||
| Issuance of common stock upon vesting of restricted stock awards |
— | — | 745,392 | 75 | (75 | ) | — | — | — | |||||||||||||||||||||||
| Reversal of previous loss from foreign currency translation |
— | — | — | — | — | 1,708 | — | 1,708 | ||||||||||||||||||||||||
| Reversal of previous loss on securities available-for-sale |
— | — | — | — | — | 9,065 | — | 9,065 | ||||||||||||||||||||||||
| Preferred stock dividend |
— | — | — | — | — | — | (24,240 | ) | (24,240 | ) | ||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (24,906,744 | ) | (24,906,744 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance, December 31, 2017 |
60,600 | $ | 60 | 52,791,584 | $ | 5,279 | $ | 179,546,954 | $ | — | $ | (173,046,186 | ) | $ | 6,506,107 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
Trovagene, Inc. and Subsidiary
Consolidated Statements of Cash Flows
| Year ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Operating activities |
||||||||
| Net loss |
$ | (24,906,744 | ) | $ | (39,203,719 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Loss on disposal of assets |
455,051 | 577,314 | ||||||
| Impairment loss |
589,700 | — | ||||||
| Depreciation and amortization |
1,247,576 | 1,069,547 | ||||||
| Stock-based compensation expense |
4,012,585 | 7,504,316 | ||||||
| Loss on extinguishment of debt |
1,655,825 | — | ||||||
| Accretion of final fee premium |
293,614 | 390,548 | ||||||
| Amortization of discount on debt |
113,780 | 173,803 | ||||||
| Net realized loss on short-term investments |
6,400 | — | ||||||
| Amortization of premiums on short-term investments |
9,230 | 107,261 | ||||||
| Deferred rent |
(140,863 | ) | (201,037 | ) | ||||
| Interest income accrued on short-term investments |
(90,330 | ) | (84,182 | ) | ||||
| Change in fair value of derivative financial instruments—warrants |
(3,401,072 | ) | (2,462,137 | ) | ||||
| Changes in operating assets and liabilities: |
||||||||
| Increase in other assets |
— | (789,739 | ) | |||||
| Decrease (increase) in accounts receivable |
23,365 | (1,724 | ) | |||||
| Increase in prepaid expenses and other current assets |
(208,185 | ) | (277,327 | ) | ||||
| (Decrease) increase in accounts payable and accrued expenses |
(2,940,999 | ) | 2,157,221 | |||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(23,281,067 | ) | (31,039,855 | ) | ||||
| Investing activities |
||||||||
| Capital expenditures |
(101,101 | ) | (823,483 | ) | ||||
| Proceeds from disposals of capital equipment |
1,540 | — | ||||||
| Maturities of short-term investments |
16,431,837 | 13,750,000 | ||||||
| Purchases of short-term investments |
(8,804,604 | ) | (37,760,166 | ) | ||||
| Sales of short-term investments |
16,434,553 | — | ||||||
|
|
|
|
|
|||||
| Net cash provided by (used in) investing activities |
23,962,225 | (24,833,649 | ) | |||||
| Financing activities |
||||||||
| Proceeds from sale of common stock and warrants |
11,727,153 | 2,364,801 | ||||||
| Payments of stock issuance costs |
(866,039 | ) | (79,386 | ) | ||||
| Proceeds from exercise of options |
— | 366,966 | ||||||
| Borrowings under equipment line of credit |
— | 792,251 | ||||||
| Repayments under equipment line of credit |
(626,104 | ) | (52,175 | ) | ||||
| Proceeds from borrowings under long-term debt, net of costs |
— | 7,805,085 | ||||||
| Payment upon debt extinguishment |
(1,613,067 | ) | — | |||||
| Repayments of long-term debt |
(15,000,000 | ) | (8,896,166 | ) | ||||
|
|
|
|
|
|||||
| Net cash (used in) provided by financing activities |
(6,378,057 | ) | 2,301,376 | |||||
| Effect of exchange rate changes on cash and cash equivalents |
7,569 | (5,825 | ) | |||||
|
|
|
|
|
|||||
| Net change in cash and cash equivalents |
(5,689,330 | ) | (53,577,953 | ) | ||||
| Cash and cash equivalents—Beginning of period |
13,915,094 | 67,493,047 | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents—End of period |
$ | 8,225,764 | $ | 13,915,094 | ||||
|
|
|
|
|
|||||
| Supplementary disclosure of cash flow activity: |
||||||||
| Cash paid for taxes |
$ | 800 | $ | 4,560 | ||||
| Cash paid for interest |
$ | 668,465 | $ | 1,103,677 | ||||
| Supplemental disclosure of non-cash investing and financing activities: |
||||||||
| Warrants issued in connection with long-term debt |
$ | — | $ | 148,885 | ||||
| Preferred stock dividends accrued |
$ | 24,240 | $ | 24,240 | ||||
| Leasehold improvements paid for by lessor |
$ | — | $ | 1,860,000 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Table of Contents
Trovagene, Inc. and Subsidiary
Notes to Consolidated Financial Statements
1. Business Overview and Going Concerns
Business Organization and Overview
Trovagene, Inc. (“Trovagene” or the “Company”) headquartered in San Diego, California, is a clinical-stage, precision medicine oncology therapeutics company. The Company’s primary focus is to develop oncology therapeutics for improved cancer care and to optimize drug development by leveraging its proprietary Precision Cancer Monitoring® (“PCM”) technology in tumor genomics.
Trovagene’s lead drug candidate, PCM-075, is a Polo-like Kinase 1 (“PLK1”) selective adenosine triphosphate (“ATP”) competitive inhibitor. PCM-075 has shown preclinical antitumor activity as a single agent and synergy in combination with more than ten different chemotherapeutics and targeted therapies, such as Zytiga® (abiraterone acetate), Beleodaq® (belinostat), Quizartinib (AC220), a development stage FLT3 inhibitor, and Velcade® (bortezomib) in Acute Myeloid Leukemia (“AML”), metastatic Castration-Resistant Prostate Cancer (“mCRPC”) and other hematologic and solid tumor cancers.
PCM-075 was developed to have high selectivity to PLK1, to be administered orally, and to have a relatively short drug half-life of approximately 24 hours compared to other PLK inhibitors. PCM-075 has completed a safety study in patients with advanced metastatic solid tumors, has a phase 1b/2 clinical trial in patients with AML underway, and a Phase 2 clinical trial in mCRPC planned.
Going Concern Uncertainty
Trovagene’s consolidated financial statements as of December 31, 2017 have been prepared under the assumption that Trovagene will continue as a going concern, which assumes that the Company will realize its assets and satisfy its liabilities in the normal course of business. The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from the outcome of the uncertainty concerning the Company’s ability to continue as a going concern.
The Company has incurred net losses since its inception and has negative operating cash flows. Considering the Company’s current cash resources, including the net proceeds received from the offerings of its equity securities in July and December 2017, management believes the Company’s existing resources will be sufficient to fund the Company’s planned operations through June 2018. The Company also received a default letter from Silicon Valley Bank (“SVB”) regarding the Loan and Security Agreement entered in November 2015 which stated that events of default had occurred and SVB will decide in its sole discretion whether or not to exercise rights and remedies. Based on its current business plan and assumptions, the Company expects to continue to incur significant losses and require significant additional capital to further advance its clinical trial programs and support its other operations. The Company has based its cash sufficiency estimates on its current business plan and its assumptions that may prove to be wrong. The Company could utilize its available capital resources sooner than it currently expects, and it could need additional funding to sustain its operations even sooner than currently anticipated. These circumstances raise substantial doubt about the Company’s ability to continue as a going concern. For the foreseeable future, the Company’s ability to continue its operations is dependent upon its ability to obtain additional capital.
The Company cannot be certain that additional funding will be available on acceptable terms, or at all. To the extent that the Company can raise additional funds by issuing equity securities, the Company’s stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants that impact the Company’s ability to conduct its business.
F-8
Table of Contents
If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly delay, scale back or discontinue the development and/or commercialization of one or more of its product candidates, all of which would have a material adverse impact on the Company’s operations. The Company may also be required to:
| • | Seek collaborators for product candidates at an earlier stage than otherwise would be desirable and on terms that are less favorable than might otherwise be available; and |
| • | Relinquish licenses or otherwise dispose of rights to technologies, product candidates or products that the Company would otherwise seek to develop or commercialize themselves, on unfavorable terms. |
The Company is evaluating the following options to both raise additional capital as well as reduce costs, in an effort to strengthen its liquidity position:
| • | Raising capital through public and private equity offerings; |
| • | Adding capital through short-term and long-term borrowings; |
| • | Introducing operation and business development initiatives to bring in new revenue streams; |
| • | Reducing operating costs by identifying internal synergies; |
| • | Engaging in strategic partnerships; and |
| • | Taking actions to reduce or delay capital expenditures. |
As of February 20, 2018, the Company has received approximately $452,000 upon exercise of 1,814,167 warrants in connection with the December 2017 public offering. The Company continually assesses its spending plans to effectively and efficiently address its liquidity needs.
2. Basis of Presentation and Summary of Significant Accounting Policies
The accompanying consolidated financial statements of Trovagene, which include its wholly owned subsidiary, Trovagene S.r.l., have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). All intercompany balances and transactions have been eliminated.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash and cash equivalents consist of operating and money market accounts as of December 31, 2017 and operating, money market accounts and commercial paper as of December 31, 2016 on deposit. Cash equivalents are considered by the Company to be highly liquid investments purchased with original maturities of three months or less from the date of purchase.
Short-Term Investments
Short-term investments consist of corporate debt securities, U.S. treasury securities, and commercial paper. The Company classifies its short-term investments as available-for-sale, as the sale of such securities may be required prior to maturity to execute management strategies. Investments classified as available-for-sale are carried at fair value, with the unrealized gains and losses reported as a component of consolidated accumulated
F-9
Table of Contents
other comprehensive income (loss) in stockholders’ equity until realized. Realized gains and losses from the sale of available-for-sale securities, if any, are determined on a specific identification basis. A decline in the market value of any available-for-sale security below cost that is determined to be other than temporary will result in an impairment charge to earnings and a new cost basis for the security is established. No such impairment charges were recorded for any period presented. Premiums and discounts are amortized or accreted over the life of the related security as an adjustment to yield using the straight-line method and included in interest income. Interest income is recognized when earned. Realized gains and losses on investments in securities were included in other income (loss) within the consolidated statements of operations. As of December 31, 2017, all of the short-term investments have been sold to satisfy the Company’s outstanding obligations under the Loan and Security Agreement dated as of June 30, 2014 upon demanding repayment by the lenders. As a result, the Company recognized net realized loss of approximately $6,400 for the year ended December 31, 2017.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash and cash equivalents and short-term investments. The Company maintains deposit accounts at financial institutions that are in excess of federally insured limits. The Company has not experienced any losses in such accounts and believes it is not exposed to significant risk on its cash due to the financial position of the depository institution in which those deposits are held. We limit our exposure to credit loss by generally placing our cash and short-term investments in high credit quality financial institutions and investment in fixed income instruments denominated and payable in U.S. dollars. Additionally, we have established guidelines regarding diversification of our investments and their maturities, which are designed to maintain principal and maximize liquidity.
Revenues
Revenue is recognized when persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed or determinable, and collection is reasonably assured.
Royalty and License Revenues
The Company licenses and sublicenses its patent rights to healthcare companies, medical laboratories and biotechnology partners. These agreements may involve multiple elements such as license fees, royalties and milestone payments. Revenue is recognized when the criteria described above have been met as well as the following:
| • | Up-front nonrefundable license fees pursuant to agreements under which the Company has no continuing performance obligations are recognized as revenues on the effective date of the agreement and when collection is reasonably assured. |
| • | Minimum royalties are recognized as earned, and royalties are earned based on the licensee’s use. The Company is unable to predict licensee’s sales and thus revenue is recognized upon receipt of notification from licensee and payment when collection is assured. Notification is generally one quarter in arrears. |
Diagnostic Service Revenues
Revenue for clinical laboratory tests may come from several sources, including commercial third-party payors, such as insurance companies and health maintenance organizations, government payors, such as Medicare and Medicaid in the United States, patient self-pay and, in some cases, from hospitals or referring laboratories who, in turn, might bill third-party payors for testing. The Company is recognizing diagnostic service revenue on the cash collection basis until such time as it is able to properly estimate collections on third party reimbursements.
F-10
Table of Contents
Clinical Research Services Revenue
Revenue from clinical research services consists primarily of revenue from the sale of urine and blood collection supplies under agreements with our clinical research and business development partners. Revenue is recognized when supplies are delivered.
Allowance for Doubtful Accounts
The Company reviews the collectability of accounts receivable based on an assessment of historic experience, current economic conditions, and other collection indicators. At December 31, 2017 and 2016 the Company had not recorded an allowance for doubtful accounts. When accounts are determined to be uncollectible, they are written off against the reserve balance and the reserve is reassessed. When payments are received on reserved accounts, they are applied to the individual’s account and the reserve is reassessed.
Derivative Financial Instruments—Warrants
The Company has issued common stock warrants in connection with the execution of certain equity financings. Such warrants are classified as derivative liabilities under the provisions of Financial Accounting Standards Board (“FASB”) ASC 815 Derivatives and Hedging (“ASC 815”) or ASC 480 Distinguishing Liabilities from Equity (“ASC 480”) are recorded at their fair market value as of each reporting period. Such warrants do not meet the exemption that a contract should not be considered a derivative instrument if it is (1) indexed to its own stock and (2) classified in stockholders’ equity. The warrants within the scope of ASC 480 contain a feature that could require the transfer of cash in the event a change of control occurs without an authorization of our Board of Directors, and therefore classified as a liability. Changes in fair value of derivative liabilities are recorded in the consolidated statement of operations under the caption “Change in fair value of derivative instruments.”
The fair value of warrants is determined using the Black-Scholes option-pricing model using assumptions regarding the volatility of Trovagene’s common stock price, the remaining life of the warrants, and the risk-free interest rates at each period end. The Company thus uses model-derived valuations where inputs are observable in active markets to determine the fair value and accordingly classifies such warrants in Level 3 per FASB ASC Topic 820, Fair Value Measurements (“ASC 820”). At December 31, 2017 and 2016, the fair value of these warrants was $649,387 and $834,940, respectively, and was recorded as a liability under the caption “derivative financial instruments—warrants” on the consolidated balance sheets.
Stock-Based Compensation
FASB ASC Topic 718 “Compensation—Stock Compensation” (“ASC 718”) requires companies to measure the cost of employee services received in exchange for the award of equity instruments based on the estimated fair value of the award at the date of grant. The expense is recognized ratably over the period during which an employee is required to provide services in exchange for the award. ASC 718 did not change the way Trovagene accounts for non-employee stock-based compensation. Trovagene continues to account for shares of common stock, stock options and warrants issued to non-employees based on the fair value of the stock, stock option or warrant, if that value is more reliably measurable than the fair value of the consideration or services received. The Company accounts for stock options issued and vesting to non-employees in accordance with FASB ASC Topic 505-50 “Equity-Based Payment to Non-Employees”, and, accordingly, the value of the stock compensation to non-employees is based upon the measurement date as determined at either (1) the date at which a performance commitment is reached, or (2) the date at which the necessary performance to earn the equity instruments is complete. Therefore, the fair value of these options is being “marked to market” quarterly until the measurement date is determined.
F-11
Table of Contents
Fair Value of Financial Instruments
Financial instruments consist of cash and cash equivalents, short-term investments, accounts receivable, accounts payable, debt and derivative liabilities. The Company has adopted ASC 820 for financial assets and liabilities that are required to be measured at fair value and non-financial assets and liabilities that are not required to be measured at fair value on a recurring basis. These financial instruments are stated at their respective historical carrying amounts, which approximate fair value due to their short term nature as they reflect current market interest rates. Debt is stated at its respective historical carrying amounts, which approximate fair value as they reflect current market interest rates.
In accordance with FASB ASC Subtopic 820-10, the Company measures certain assets and liabilities at fair value on a recurring basis using the three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The three tiers include:
| • | Level 1 — Quoted prices for identical instruments in active markets. |
| • | Level 2 — Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations where inputs are observable or where significant value drivers are observable. |
| • | Level 3 — Instruments where significant value drivers are unobservable to third parties. |
Long-Lived Assets
Long-lived assets consist of property and equipment and finite-lived intangible assets. The Company records property and equipment at cost, and records other intangible assets based on their fair values at the date of acquisition. Depreciation on property and equipment is calculated using the straight-line method over the estimate useful life of five years for laboratory equipment and three to five years for furniture and office equipment. Amortization of leasehold improvements is computed based on the shorter of the life of the asset or the term of the lease. Amortization of intangible assets is calculated using the straight line method over the estimate useful life of the assets, based on when the Company expect to receive cash inflows generated by the intangible assets.
Impairment losses on long-lived assets used in operations are recorded when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets carrying amount. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the estimated fair value of the assets. During the year ended December 31, 2017, the Company recorded $104,700 of impairment loss on long-lived intangible assets. No impairment losses were recorded on long-lived assets to be held and used during the year ended December 31, 2016.
Restructuring
Restructuring costs are included in loss from operations in the consolidated statements of operations. The Company has accounted for these costs in accordance with ASC Topic 420, Exit or Disposal Cost Obligations. One-time termination benefits are recorded at the time they are communicated to the affected employees. In March 2017, the Company announced a restructuring plan which was completed as of December 31, 2017. See Note 12 to the consolidated financial statements for further information.
Income Taxes
Income taxes are determined using the asset and liability approach of accounting for income taxes. Under this approach, deferred taxes represent the future tax consequences expected to occur when the reported amounts of assets and liabilities are recovered or paid. Deferred taxes result from differences between the financial
F-12
Table of Contents
statement and tax bases of Trovagene’s assets and liabilities and are adjusted for changes in tax rates and tax laws when changes are enacted. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized. The assessment of whether or not a valuation allowance is required often requires significant judgment.
Contingencies
In the normal course of business, Trovagene is subject to loss contingencies, such as legal proceedings and claims arising out of its business, that cover a wide range of matters, including, among others, government investigations, stockholder lawsuits, product and environmental liability, and tax matters. In accordance with FASB ASC Topic 450, Accounting for Contingencies, Trovagene records such loss contingencies when it is probable that a liability has been incurred and the amount of loss can be reasonably estimated. Trovagene, in accordance with this guidance, does not recognize gain contingencies until realized.
Cost of Revenue
Cost of revenue represents the cost of materials, personnel costs, costs associated with processing specimens including pathological review, quality control analyses, and delivery charges necessary to render an individualized test result. Costs associated with performing tests are recorded as the tests are processed. However, the revenue on diagnostic services is recognized on a cash collection basis resulting in costs incurred before the collection of related revenue.
Research and Development
Research and development expenses, which include expenditures in connection with an in-house research and development laboratory, salaries and staff costs, purchased in-process research and development and regulatory and scientific consulting fees, as well as contract research and insurance, are accounted for in accordance with FASB ASC Topic 730-10-55-2, Research and Development. Also, as prescribed by this guidance, patent filing and maintenance expenses are considered legal in nature and therefore classified as general and administrative expense, if any.
While certain of the Company’s research and development costs may have future benefits, the Company’s policy of expensing all research and development expenditures is predicated on the fact that Trovagene has no history of successful commercialization of molecular diagnostic products to base any estimate of the number of future periods that would be benefited.
FASB ASC Topic 730, Research and Development requires that non-refundable advance payments for goods or services that will be used or rendered for future research and development activities be deferred and capitalized. As the related goods are delivered or the services are performed, or when the goods or services are no longer expected to be provided, the deferred amounts are recognized as an expense.
F-13
Table of Contents
Net Loss Per Share
Basic and diluted net loss per share is presented in conformity with FASB ASC Topic 260, Earnings per Share, for all periods presented. In accordance with this guidance, basic and diluted net loss per common share is determined by dividing net loss applicable to common stockholders by the weighted-average common shares outstanding during the period. Preferred dividends are included in income available to common stockholders in the computation of basic and diluted earnings per share. Shares used in calculating diluted net loss per common share exclude as anti-dilutive the following share equivalents:
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Options to purchase Common Stock |
4,490,475 | 5,528,628 | ||||||
| Warrants to purchase Common stock |
23,555,520 | 4,538,606 | ||||||
| Restricted Stock Units |
1,274,302 | 272,000 | ||||||
| Series A Convertible Preferred Stock |
63,125 | 63,125 | ||||||
|
|
|
|
|
|||||
| 29,383,422 | 10,402,359 | |||||||
|
|
|
|
|
|||||
The following table summarizes the Company’s diluted net loss per share:
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Numerator: |
||||||||
| Net loss attributable to common stockholders |
$ | (24,930,984 | ) | $ | (39,227,959 | ) | ||
| Adjustment for gain from change in fair value of derivative financial instruments—warrants |
— | (2,321,053 | ) | |||||
|
|
|
|
|
|||||
| Net loss used for diluted loss per share |
$ | (24,930,984 | ) | $ | (41,549,012 | ) | ||
|
|
|
|
|
|||||
| Denominator: |
||||||||
| Weighted-average shares used to compute basic net loss per share |
34,680,362 | 30,174,838 | ||||||
| Adjustments to reflect assumed exercise of warrants |
— | 106,425 | ||||||
|
|
|
|
|
|||||
| Weighted-average shares used to compute diluted net loss per share |
34,680,362 | 30,281,263 | ||||||
|
|
|
|
|
|||||
| Net loss per share attributable to common stockholders: |
||||||||
| Basic |
$ | (0.72 | ) | $ | (1.30 | ) | ||
|
|
|
|
|
|||||
| Diluted |
$ | (0.72 | ) | $ | (1.37 | ) | ||
|
|
|
|
|
|||||
Change in Accounting Principle
In March 2016, the FASB issued ASU 2016-09, Improvements to Employee Share-Based Payment Accounting (“ASU 2016-09”), which aims to simplify the accounting for share-based payment transactions, including accounting for income taxes, classification on the statement of cash flows, accounting for forfeitures, and classification of awards as either liabilities or equity. In addition, under the ASU 2016-09, excess income tax benefits from share-based compensation arrangements are classified as cash flow from operations, rather than cash flow from financing activities. The Company adopted ASU 2016-09 as of January 1, 2017 and has elected to continue estimating forfeitures based on historical experience. The adoption of ASU 2016-09 had no impact on the Company’s financial statements.
Recent Accounting Pronouncements
In August 2016, the FASB issued Accounting Standards Update (“ASU”) 2016-15, Classification of Certain Cash Receipts and Cash Payments (“ASU 2016-15”), which includes amendments that clarify how certain cash
F-14
Table of Contents
receipts and cash payments are presented in the statement of cash flows. ASU 2016-15 also provides guidance clarifying when an entity should separate cash receipts and cash payments and classify them into more than one class of cash flows. The new amendments and guidance are effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. Early adoption is permitted provided that all amendments are adopted in the same period. The Company is currently evaluating the impact of adoption of ASU 2016-15 on its consolidated statements of cash flows.
In February 2016, the FASB issued ASU 2016-02, Leases. The new standard establishes a right-of-use (“ROU”) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for most leases. The new standard is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. A modified retrospective transition approach is required for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. The new standard will impact the Company’s accounting for its office leases and the Company is currently evaluating the impact of the new standard on its consolidated financial statements.
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers (“ASU 2014-09”). The new standard is based on the principle that revenue should be recognized to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. Since its initial release, the FASB has issued several amendments to the standard, which include clarification of accounting guidance related to identification of performance obligations, intellectual property licenses, and principle versus agent considerations. ASU 2014-09 and all subsequent amendments (collectively, “ASC 606”) became effective for the Company on January 1, 2018 and was adopted the standard using the modified retrospective method. The cumulative effect of applying the new standard is immaterial and we will recognize the amount in retained earnings on the date of initial application. Under ASC 606, the Company will accrue for royalties as earned and no longer record them on a lag. The Company has reviewed its revenue streams to identify potential differences in accounting under the new revenue recognition standard. The Company’s timing and measurement of revenue recognition will not be materially affected by the adoption and implementation of ASC 606.
Currently, the Company does not have any significant contracts with customers given its stage of development. The Company has derived its revenues primarily from a limited number of royalty, license and diagnostic service agreements. The consideration the Company is eligible to receive under these agreements includes upfront license payments, milestone payments and royalties. Each of these agreements has unique terms that have been evaluated separately under the new standards. The new standards differ from the current accounting standard in many respects, such as in the accounting for variable consideration, including milestone payments. For example, the Company currently recognizes milestone revenue using the milestone method specified in ASC 605-28, which generally results in recognition of milestone revenue in the period that the milestone event is achieved. However, under the new standards, it is possible to start to recognize milestone revenue before the milestone is achieved if management determines with a high degree of certainty that amounts recorded as revenues will not have to be reversed when the uncertainty associated with the variable consideration is subsequently resolved. The Company has assessed the potential impact that the new standards may have with respect to its diagnostic service revenue and has determined to recognize its diagnostic service revenue on a cash collection basis as it does currently. The Company has completed its full assessment of the impact the new standards will have on its financial statements before the year-end 2017. The assessment concludes the Company will not have a significant change in the timing and measurement of its revenue upon adoption of the new standards. The Company will adopt the new standards effective January 1, 2018 using the modified retrospective transition method. The Company’s current assessment identifies a highly immaterial adjustment to beginning retained earnings for the cumulative effect of the change.
F-15
Table of Contents
3. Supplementary Balance Sheet Information
Short-term Investments
As of December 31, 2017, all short-term investments have been sold to satisfy the Company’s outstanding obligations under the Loan and Security Agreement dated as of June 30, 2014 upon demanding repayment by the lenders. The following table sets forth the composition of short-term investments as of December 31, 2016.
| Unrealized | ||||||||||||||||||
| Maturity in Years |
Cost | Gains | Losses | Fair Value | ||||||||||||||
| Corporate debt securities |
Less than 1 year | $ | 14,165,915 | $ | 44 | $ | (5,273 | ) | $ | 14,160,686 | ||||||||
| Commercial paper |
Less than 1 year | 1,195,444 | — | — | 1,195,444 | |||||||||||||
| U.S. treasury securities |
Less than 1 year | 8,625,728 | 330 | (4,166 | ) | 8,621,892 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| Total investment |
$ | 23,987,087 | $ | 374 | $ | (9,439 | ) | $ | 23,978,022 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
Property and Equipment
Fixed assets consist of laboratory, testing and computer equipment and fixtures stated at cost. Depreciation and amortization expense for property and equipment for the years ended December 31, 2017 and 2016 was $1,053,913 and $969,833, respectively. Property and equipment consisted of the following:
| As of December 31, | ||||||||
| 2017 | 2016 | |||||||
| Furniture and office equipment |
$ | 1,076,709 | $ | 1,144,741 | ||||
| Leasehold improvements |
1,994,514 | 1,994,514 | ||||||
| Laboratory equipment |
1,426,581 | 2,449,645 | ||||||
|
|
|
|
|
|||||
| 4,497,804 | 5,588,900 | |||||||
| Less—accumulated depreciation and amortization |
(2,071,492 | ) | (1,761,985 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 2,426,312 | $ | 3,826,915 | ||||
|
|
|
|
|
|||||
Accrued Liabilities
Accrued liabilities consisted of the following:
| As of December 31, | ||||||||
| 2017 | 2016 | |||||||
| Accrued compensation |
$ | 618,128 | $ | 2,203,876 | ||||
| Accrued research agreements |
135,139 | 736,199 | ||||||
| Accrued professional fees |
— | 421,314 | ||||||
| Other accrued liabilities |
701,320 | 659,976 | ||||||
|
|
|
|
|
|||||
| Total accrued liabilities |
$ | 1,454,587 | $ | 4,021,365 | ||||
|
|
|
|
|
|||||
4. Stockholders’ Equity
Common Stock
During the year ended December 31, 2016, the Company issued a total of 959,190 shares of common stock. The Company received gross proceeds of approximately $2.4 million from the sale of 421,810 shares of its common stock at a weighted-average price of $5.61 under the agreement with the Agent. In addition, 98,396 shares were issued upon exercise of options for a weighted-average price of $3.73, 341,333 shares were issued upon net exercise of 1,236,875 options at a weighted average exercise price of $3.81, 2,651 shares were issued upon net exercise of 8,333 warrants at a weighted-average exercise price of $3.00, and 95,000 shares were issued upon vesting of restricted stock units.
F-16
Table of Contents
During the year ended December 31, 2017, the Company issued a total of 22,094,793 shares of common stock. The Company received gross proceeds of approximately $11.6 million from the sale of 20,874,833 shares of its common stock and 19,960,293 share of warrants and pre-funded warrants through public offering, registered direct offering and private placement in July and December 2017. The Company received gross proceeds of approximately $0.1 million from the sale of 102,081 shares of its common stock at a weighted-average price of $1.08 under the agreement with the Agent. In addition, 372,487 shares were issued upon vesting of restricted stock units (“RSU”), and 745,392 shares were issued upon vesting of restricted stock awards (“RSA”).
Warrants
A summary of warrant activity and changes in warrants outstanding, including both liability and equity classifications, is presented below:
| Number of Warrants (1) |
Weighted- Average Exercise Price Per Share (1) |
Weighted-Average | ||||||||
| Balance outstanding, December 31, 2015 |
5,533,242 | $ | 3.86 | 2.5 | ||||||
| Granted |
30,992 | $ | 4.84 | |||||||
| Exercised |
(8,333 | ) | $ | 3.00 | ||||||
| Expired |
(50,000 | ) | $ | 8.00 | ||||||
|
|
|
|||||||||
| Balance outstanding, December 31, 2016 |
5,505,901 | $ | 3.83 | 1.6 | ||||||
| Granted |
19,643,626 | $ | 0.56 | |||||||
| Expired |
(1,910,674 | ) | $ | 5.32 | ||||||
|
|
|
|||||||||
| Balance outstanding, December 31, 2017 |
23,238,853 | $ | 0.95 | 4.4 | ||||||
|
|
|
|||||||||
| (1) | Excluded the pre-funded warrants to purchase 316,667 shares of common stock at a nominal exercise price of $0.01 per share. The pre-warrants expire when exercised in full. |
The Company issued warrants to purchase 30,992 shares of common stock at an exercise price of $4.84 per share during the year ended December 31, 2016. The warrants were issued in connection with the fifth amendment to the $15.0 million debt agreement. The estimated fair value of the warrants was determined on the date of grant using the Black-Scholes option valuation model using the following assumptions: a risk-free interest rate of 1.59%, dividend yield of 0%, expected volatility of 130.66% and expected term of ten years. The resulting fair value of $148,885 was recorded as a debt discount and was amortized to interest expense over the new term of the loan using the effective interest method. In June 2017, Company received a Notice of Event of Default from the lenders which stated that Events of Default had occurred and all of the obligation under the Agreement were immediately due and payable. Upon termination of the Agreement, unamortized debt discount was recorded as loss on debt extinguishment.
In connection with a direct registered offering occurred in July 2017, the Company issued warrants to purchase 4,643,626 shares of common stock at an exercise price of $1.41 per share which expire on the five years anniversary of the original issuance date. In December 2017, the Company issued warrants to purchase 15,000,000 shares of common stock at an exercise price of $0.30 per share in a public offering which expire on the five years anniversary of the original issuance date. The Company also issued pre-funded warrants to purchase 316,667 shares of common stock which expire when exercised in full. $0.29 of the pre-funded warrant exercise price was paid upfront on the closing date of the public offering and the remaining exercise price is $0.01.
F-17
Table of Contents
Series A Convertible Preferred Stock
The material terms of the Series A Convertible Preferred Stock consist of:
1) Dividends. Holders of the Company’s Series A Convertible Preferred Stock are entitled to receive cumulative dividends at the rate per share of 4% per annum, payable quarterly on March 31, June 30, September 30 and December 31, beginning with September 30, 2005. Dividends are payable, at the Company’s sole election, in cash or shares of common stock. As of December 31, 2017 and 2016, the Company had $316,775 and $292,535, respectively in accrued cumulative unpaid preferred stock dividends, included in accrued liabilities in the Company’s consolidated balance sheets, and $24,240 and $24,240 of accrued dividends was recorded during the years ended December 31, 2017 and 2016, respectively.
2) Voting Rights. Shares of the Series A Convertible Preferred Stock have no voting rights. However, so long as any shares of Series A Convertible Preferred Stock are outstanding, the Company may not, without the affirmative vote of the holders of the shares of Series A Convertible Preferred Stock then outstanding, (a) adversely change the powers, preferences or rights given to the Series A Convertible Preferred Stock, (b) authorize or create any class of stock senior or equal to the Series A Convertible Preferred Stock, (c) amend its certificate of incorporation or other charter documents, so as to affect adversely any rights of the holders of Series A Convertible Preferred Stock or (d) increase the authorized number of shares of Series A Convertible Preferred Stock.
3) Liquidation. Upon any liquidation, dissolution or winding-up of the Company, the holders of the Series A Convertible Preferred Stock are entitled to receive an amount equal to the Stated Value per share, which is currently $10 per share plus any accrued and unpaid dividends.
4) Conversion Rights. Each share of Series A Convertible Preferred Stock is convertible at the option of the holder into that number of shares of common stock determined by dividing the Stated Value, currently $10 per share, by the conversion price, originally $2.15 per share.
5) Subsequent Equity Sales. The conversion price is subject to adjustment for dilutive issuances for a period of 12 months beginning upon registration of the common stock underlying the Series A Convertible Preferred Stock. The relevant registration statement became effective on March 17, 2006 and during the following twelve month period the conversion price was adjusted to $9.60 per share.
6) Automatic Conversion. If the price of the Company’s common stock equals $25.80 per share for 20 consecutive trading days, and an average of 8,333 shares of common stock per day are traded during the 20 trading days, the Company will have the right to deliver a notice to the holders of the Series A Convertible Preferred Stock, requesting the holders to convert any portion of the shares of Series A Convertible Preferred Stock into shares of common stock at the applicable conversion price. As of the date of these financial statements, such conditions have not been met.
As of each of December 31, 2017 and 2016, there were 60,600 shares of Series A Convertible Preferred Stock outstanding.
5. Stock-Based Compensation
The Trovagene, Inc. 2014 Equity Incentive Plan (the “2014 EIP’), authorizing up to 2,500,000 shares of common stock for issuance under the 2014 EIP, was approved by the Board in June 2014 and approved by the stockholders of the Company at the September 17, 2014 Annual Meeting of Stockholders. An additional 2,500,000 shares of common stock was authorized for issuance by the Board in March 2015 and was approved by the stockholders at the June 10, 2015 Annual Meeting of Stockholders. Stockholder approval was obtained on May 17, 2016 to increase the number of authorized shares in the 2014 EIP from 5,000,000 to 7,500,000. The adoption of an amendment to the Company’s 2014 EIP to increase the number of shares of common stock reserved for issuance to 9,500,000 was approved by the stockholders at the June 13, 2017 Annual Meeting of Stockholders.
F-18
Table of Contents
As of December 31, 2017, there were 3,436,788 shares available for issuance under the 2014 EIP.
Stock-based compensation has been recognized in operating results as follows:
| Years ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| In cost of revenue |
$ | 83,713 | $ | 122,301 | ||||
| In research and development expenses |
1,026,497 | 2,420,696 | ||||||
| In selling and marketing expense |
676,635 | 2,111,366 | ||||||
| In general and administrative expenses |
2,350,962 | 2,910,156 | ||||||
| Benefit from restructuring |
(125,222 | ) | (60,203 | ) | ||||
|
|
|
|
|
|||||
| Total stock-based compensation |
$ | 4,012,585 | $ | 7,504,316 | ||||
|
|
|
|
|
|||||
Stock Options
The estimated fair value of stock option awards was determined on the date of grant using the Black-Scholes option valuation model with the following assumptions during the years indicated below:
| Years ended December 31, | ||||
| 2017 | 2016 | |||
| Risk-free interest rate |
1.82% - 2.03% | 0.93% - 1.89% | ||
| Dividend yield |
0% | 0% | ||
| Expected volatility (range) |
86% - 117% | 80% - 134% | ||
| Expected volatility (weighted-average) |
87% | 103% | ||
| Expected term (in years) |
5.3 years | 5.5 years | ||
Risk-free interest rate — Based on the daily yield curve rates for U.S. Treasury obligations with maturities that correspond to the expected term of the Company’s stock options.
Dividend yield — Trovagene has not paid any dividends on common stock since its inception and does not anticipate paying dividends on its common stock in the foreseeable future.
Expected volatility — Based on the historical volatility of Trovagene’s common stock.
Expected term — The expected option term represents the period that stock-based awards are expected to be outstanding based on the simplified method provided in Staff Accounting Bulletin (“SAB”) No. 107, Share-Based Payment (“SAB No. 107”), which averages an award’s weighted-average vesting period and expected term for “plain vanilla” share options. Under SAB No. 107, options are considered to be “plain vanilla” if they have the following basic characteristics: (1) are granted “at-the-money”; (2) exercisability is conditioned upon service through the vesting date; (3) termination of service prior to vesting results in forfeiture; (4) limited exercise period following termination of service; and (5) are non-transferable and non-hedgeable.
Forfeitures — FASB ASC Topic 718 (“ASC 718”) required forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. FASB ASU 2016-09 allows the Company to make an entity-wide accounting policy election to either estimate the number of awards that are expected to vest or account for forfeitures when they occur. The Company elected to estimate forfeitures based on its historical experience.
The weighted-average fair value per share of all options granted during the years ended December 31, 2017 and 2016, estimated as of the grant date using the Black-Scholes option valuation model, was $0.56 and $3.60 per share, respectively.
F-19
Table of Contents
The unrecognized compensation cost related to non-vested stock options outstanding at December 31, 2017 and 2016 was $2,915,970 and $8,211,896, respectively. The weighted-average remaining amortization period at December 31, 2017 and 2016 for non-vested stock options was 2.0 years and 2.8 years, respectively.
The total intrinsic value of stock options exercised was $0 and $1,932,799 during the years ended December 31, 2017 and 2016, respectively. The total fair value of shares vested during the years ended December 31, 2017 and 2016 was $3,992,127 and $6,261,655, respectively.
A summary of stock option activity and of changes in stock options outstanding is presented below:
| Number of Options |
Weighted- Average Exercise Price Per Share |
Intrinsic Value |
Weighted- Average Remaining Contractual Life |
|||||||||||||
| Balance outstanding, December 31, 2015 |
6,948,630 | $ | 5.45 | $ | 5,903,466 | 7.8 years | ||||||||||
| Granted |
3,246,250 | $ | 5.02 | |||||||||||||
| Exercised |
(1,335,271 | ) | $ | 3.81 | ||||||||||||
| Forfeited |
(3,330,981 | ) | $ | 5.63 | ||||||||||||
|
|
|
|||||||||||||||
| Balance outstanding, December 31, 2016 |
5,528,628 | $ | 5.49 | $ | — | 7.7 years | ||||||||||
| Granted |
1,059,242 | $ | 0.82 | |||||||||||||
| Forfeited |
(2,079,938 | ) | $ | 6.24 | ||||||||||||
| Expired |
(17,457 | ) | $ | 4.74 | ||||||||||||
|
|
|
|||||||||||||||
| Balance outstanding, December 31, 2017 |
4,490,475 | $ | 4.04 | $ | — | 7.1 years | ||||||||||
|
|
|
|||||||||||||||
| Vested and exercisable, December 31, 2017 |
2,745,350 | $ | 4.66 | $ | — | 6.0 years | ||||||||||
|
|
|
|||||||||||||||
Upon adoption of ASU 2016-09, the cash flows resulting from tax deductions in excess of the cumulative compensation cost recognized for options exercised (excess tax benefits) are classified within operating activities in the statement of cash flows. Due to Trovagene’s accumulated deficit position, no tax benefits have been recognized in the cash flow statement.
Restricted Stock Units
Under guidance provided by ASC Topic 718 “Compensation—Stock Compensation” for share-based payments, stock-based compensation cost for RSU is measured at the grant date based on the closing market price of the Company’s common stock at the grant date and recognized ratably over the service period through the vesting date. All RSU were granted with no purchase price. Vesting of the RSU is generally subject to service conditions.
F-20
Table of Contents
A summary of the RSU activity is presented below:
| Number of Shares |
Weighted Average Grant Date Fair Value Per Share |
Intrinsic Value |
||||||||||
| Non-vested RSU outstanding, December 31, 2015 |
— | $ | — | $ | — | |||||||
| Granted |
402,000 | $ | 4.06 | |||||||||
| Vested |
(95,000 | ) | $ | 4.27 | ||||||||
| Forfeited |
(35,000 | ) | $ | 3.99 | ||||||||
|
|
|
|||||||||||
| Non-vested RSU outstanding, December 31, 2016 |
272,000 | $ | 3.99 | $ | 571,200 | |||||||
| Granted |
2,249,242 | $ | 1.59 | |||||||||
| Vested |
(372,487 | ) | $ | 3.47 | ||||||||
| Forfeited |
(874,453 | ) | $ | 1.75 | ||||||||
|
|
|
|||||||||||
| Non-vested RSU outstanding, December 31, 2017 |
1,274,302 | $ | 1.43 | $ | 391,848 | |||||||
|
|
|
|||||||||||
At December 31, 2017 and 2016, total unrecognized compensation costs related to non-vested RSU were $689,365 and $4,430, which are expected to be recognized over 2.9 years and one day, respectively. The total intrinsic values of RSU vested was $647,885 and $293,781 during the year ended December 31, 2017 and 2016, respectively. The total fair values of RSU vested during the year ended December 31, 2017 and 2016 were $1,291,878 and $405,550, respectively.
Restricted Stock Awards
During the year ended December 31, 2017, a total of 745,392 shares of RSA were granted, all of which were vested immediately. The total fair value of vested RSA during the year ended December 31, 2017 was $596,314. The weighted-average grant date fair value of the RSA was $0.80 per share during the year ended December 31, 2017. There were no such awards granted during the year ended December 31, 2016.
6. Derivative Financial Instruments — Warrants
Based upon the Company’s analysis of the criteria contained in ASC Topic 815-40, Contracts in Entity’s Own Equity (“ASC 815-40”) or ASC Topic 480-10, Distinguishing Liabilities from Equity (“ASC 480-10”), Trovagene determined that certain warrants issued in connection with the execution of certain equity financings must be recorded as derivative liabilities. In accordance with ASC 815-40 and ASC 480-10, the warrants are also being re-measured at each balance sheet date based on estimated fair value, and any resultant change in fair value is being recorded in the Company’s consolidated statements of operations. The Company estimates the fair value of these warrants using the Black-Scholes option pricing model.
The range of assumptions used to determine the fair value of the warrants valued using the Black-Scholes option pricing model during the periods indicated was:
| 2017 | 2016 | |||
| Estimated fair value of Trovagene common stock |
$0.31 - $1.26 | $2.10 - $4.65 | ||
| Expected warrant term |
1.0 - 5.5 years | 2.0 - 2.8 years | ||
| Risk-free interest rate |
1.27% - 2.21% | 0.71% - 1.20% | ||
| Expected volatility |
86% - 116% | 82% - 94% | ||
| Dividend yield |
— % | — % |
Expected volatility is based on the historical volatility of Trovagene’s common stock. The warrants have a transferability provision and based on guidance provided in SAB No. 107 for instruments issued with such a provision, Trovagene used the full contractual term as the expected term of the warrants. The risk-free interest rate is based on the U.S. Treasury security rates consistent with the expected remaining term of the warrants at each balance sheet date.
F-21
Table of Contents
The following table sets forth the components of changes in the Company’s derivative financial instruments—warrants liability balance, valued using the Black-Scholes option pricing method, for the periods indicated.
| Date |
Description |
Number of Warrants |
Derivative Instrument Liability |
|||||||
| December 31, 2015 |
Balance of derivative financial instruments—warrants liability | 967,295 | $ | 3,297,077 | ||||||
|
|
Change in fair value of derivative financial instruments—warrants during the year recognized as a gain in the statement of operations | — | (2,462,137 | ) | ||||||
|
|
|
|
|
|||||||
| December 31, 2016 |
Balance of derivative financial instruments—warrants liability | 967,295 | 834,940 | |||||||
|
|
Issuance of Derivative Financial Instruments | 4,643,626 | 3,215,519 | |||||||
|
|
Change in fair value of derivative financial instruments—warrants during the year recognized as a gain in the statement of operations | — | (3,401,072 | ) | ||||||
|
|
|
|
|
|||||||
| December 31, 2017 |
Balance of derivative financial instruments—warrants liability | 5,610,921 | $ | 649,387 | ||||||
|
|
|
|
|
|||||||
The remaining contractual term of these warrants outstanding at December 31, 2017 and 2016 was approximately 4.4 and 2.0 years, respectively.
At December 31, 2017 and 2016, the total fair value of the above warrants accounted for as derivative financial instruments—warrants, valued using the Black-Scholes option pricing model, was $649,387 and $834,940, respectively, and is classified as derivative financial instruments—warrants liability on the balance sheet.
F-22
Table of Contents
7. Fair Value Measurements
The following table presents the Company’s assets and liabilities that are measured and recognized at fair value on a recurring basis classified under the appropriate level of the fair value hierarchy as of December 31, 2017 and 2016:
| Fair Value Measurements at December 31, 2017 |
||||||||||||||||
| Quoted Prices in Active Markets for Identical Assets and Liabilities (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||||||
| Assets: |
| |||||||||||||||
| Money market fund (1) |
$ | 4,522,631 | $ | — | $ | — | $ | 4,522,631 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Assets |
$ | 4,522,631 | $ | — | $ | — | $ | 4,522,631 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities: |
| |||||||||||||||
| Derivative financial instruments—warrants |
$ | — | $ | — | $ | 649,387 | $ | 649,387 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Liabilities |
$ | — | $ | — | $ | 649,387 | $ | 649,387 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Fair Value Measurements at December 31, 2016 |
||||||||||||||||
| Quoted Prices in Active Markets for Identical Assets and Liabilities (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||||||
| Assets: |
||||||||||||||||
| Money market fund (1) |
$ | 12,095,620 | $ | — | $ | — | $ | 12,095,620 | ||||||||
| Corporate debt securities (2) |
— | 14,160,686 | — | 14,160,686 | ||||||||||||
| Commercial paper (3) |
— | 2,393,948 | — | 2,393,948 | ||||||||||||
| U.S. treasury securities (2) |
— | 8,621,892 | — | 8,621,892 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Assets |
$ | 12,095,620 | $ | 25,176,526 | $ | — | $ | 37,272,146 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities: |
||||||||||||||||
| Derivative financial instruments—warrants |
$ | — | $ | — | $ | 834,940 | $ | 834,940 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Liabilities |
$ | — | $ | — | $ | 834,940 | $ | 834,940 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Included as a component of cash and cash equivalents on the accompanying consolidated balance sheet. |
| (2) | Included in short-term investments on the accompanying consolidated balance sheet. |
| (3) | $1,198,504 of commercial paper was included as a component of cash and cash equivalents, and the rest of amount was included in short-term investments on the accompanying consolidated balance sheet. |
F-23
Table of Contents
The following table sets forth a summary of changes in the fair value of the Company’s Level 3 liabilities for the years ended December 31, 2017 and 2016:
| Description |
Balance at December 31, 2016 |
Issuance of Derivative Financial Instruments |
Unrealized (gains) or losses |
Balance at December 31, 2017 |
||||||||||||
| Derivative financial instruments—Warrants |
$ | 834,940 | $ | 3,215,519 | $ | (3,401,072 | ) | $ | 649,387 | |||||||
| Description |
Balance at December 31, 2015 |
Unrealized (gains) or losses |
Balance at December 31, 2016 |
|||||||||
| Derivative financial instruments—Warrants |
$ | 3,297,077 | $ | (2,462,137 | ) | $ | 834,940 | |||||
The unrealized gains or losses on the derivative financial instruments—warrants are recorded as a change in fair value of derivative financial instruments—warrants in the Company’s consolidated statement of operations. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. At each reporting period, the Company reviews the assets and liabilities that are subject to ASC Topic 815-40. At each reporting period, all assets and liabilities for which the fair value measurement is based on significant unobservable inputs or instruments that trade infrequently and therefore have little or no price transparency are classified as Level 3.
8. Debt
Equipment Line of Credit
In November 2015, the Company entered into a Loan and Security Agreement (“Equipment Line of Credit”) with Silicon Valley Bank that provided for cash borrowings for equipment (“Equipment Advances”) of up to $2.0 million, secured by the equipment financed. Under the terms of the agreement, interest is equal to 1.25% above the Prime Rate. At December 31, 2017, the interest rate was 5.75%. Interest only payments are due on borrowings through November 30, 2016, with both interest and principal payments commencing in December 2016. Any equipment advances after November 30, 2016 are subject to principal and interest payments immediately over a 36-month period following the advance. All unpaid principal and interest on each Equipment Advance will be due on November 1, 2019. The Company has an obligation to make a final payment equal to 7% of total amounts borrowed at the loan maturity date.
On June 20, 2017, the Company received a Notice of Event of Default (“Default Letter”) from SVB which stated that Events of Default had occurred and SVB will decide in its sole discretion whether or not to exercise rights and remedies. Pursuant to the Default Letter, the Company has classified the entire balance of $1,331,515 as a current liability as of December 31, 2017 and also started recording accrued interest at a default rate. The Company recorded $232,765 in interest expense related to the Equipment Line of Credit during the year ended December 31, 2017. The Company is currently working with lender for resolution.
Loan and Security Agreement
In June 2014, the Company entered into a $15,000,000 loan and security agreement (“Agreement”) with two banks pursuant to which the lenders provided the Company with a term loan, which was funded at closing. In connection with the loan, each of the lenders received a warrant to purchase up to an aggregate of 85,470 shares of the Company’s common stock at an exercise price of $3.51 per share, which such warrants are exercisable for ten years from the date of issuance. On July 20, 2016, the Company signed the 5th Amendment to Loan and Security Agreement (“Amendment”) to refinance its existing term loan. Under the Amendment, the interest rate was adjusted to 3.75% plus the Wall Street Journal Prime Rate (subject to a floor of 7.25%). The Company is required to make interest only payments on the outstanding amount of the loan on a monthly basis through September 1, 2017, after which equal monthly payments of principal and interest are due
F-24
Table of Contents
until the loan maturity date of February 1, 2020. In addition, the lenders received a warrant to purchase an aggregate 30,992 shares of the Company’s common stock at an exercise price of $4.84 per share exercisable for ten years from the date of issuance. As of December 31, 2017, warrants to purchase 73,727 shares of common stock remains outstanding, of which 42,735 of these warrants were in connection with the original Agreement.
On June 1, 2017, the Company received a Notice of Event of Default from the lenders which stated that Events of Default had occurred and all of the obligation under the Agreement were immediately due and payable. On June 6, 2017, the lenders took the total pay-off amount of $16,668,583 for the principal, interest, final payment, and other amounts out of the Company’s bank accounts which satisfied all of the Company’s outstanding obligations under the Agreement. Accordingly, the Agreement was terminated in June 2017. Upon termination of the Agreement, the prepayment fee of $450,000, unamortized debt discount of $400,562 and unamortized final fee of $738,196 were recorded as loss on debt extinguishment. The Company recorded total interest expense of $801,173 related to the Agreement during the year ended December 31, 2017.
9. Income Taxes
At December 31, 2017, Trovagene had federal net operating loss carryforwards (NOLs) of approximately $130.5 million, which, if not used, expire beginning in 2020. Trovagene also has California NOLs of approximately $72.1 million that will begin to expire in 2029. Trovagene also has research and development tax credits available for federal and California purposes of approximately $2.0 million and $1.1 million, respectively. The federal research and development tax credits will begin to expire on January 31, 2025. The California research and development tax credits are not set to expire. The utilization of these NOLs and research and development tax credits is subject to limitations based on past and future changes in ownership of Trovagene pursuant to Section 382 (“Section 382”) of the Internal Revenue Code of 1986, as amended (the “Code”). The Company has determined that ownership changes have occurred for purposes of Section 382 and, therefore, the ability of the Company to utilize its NOLs is limited.
The provision for income taxes based on losses from continuing operations consists of the following at December 31 (in thousands):
| Years ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Current: |
||||||||
| State |
$ | 1 | $ | — | ||||
|
|
|
|
|
|||||
| Total current provision |
1 | — | ||||||
| Deferred: |
||||||||
| Federal |
9,781 | (14,035 | ) | |||||
| State |
3,171 | (2,443 | ) | |||||
| Foreign |
— | (114 | ) | |||||
|
|
|
|
|
|||||
| Total deferred expense (benefit) |
12,952 | (16,592 | ) | |||||
| Valuation allowance |
(12,953 | ) | 16,592 | |||||
|
|
|
|
|
|||||
| Total income tax provision |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
F-25
Table of Contents
Significant components of the Company’s taxes and the rates as of December 31 are shown below (in thousands, except percentages):
| Years ended December 31, | ||||||||||||||||
| 2017 | 2016 | |||||||||||||||
| Tax computed at the federal statutory rate |
$ | (8,591 | ) | 34 | % | $ | (13,206 | ) | 34 | % | ||||||
| State tax, net of federal tax benefit |
(697 | ) | 3 | % | (2,286 | ) | 6 | % | ||||||||
| Foreign tax |
— | — | % | (114 | ) | — | % | |||||||||
| Permanent Items |
(706 | ) | 3 | % | (114 | ) | — | % | ||||||||
| Tax credits |
(431 | ) | 2 | % | (1,276 | ) | 3 | % | ||||||||
| Valuation allowance increase |
(11,029 | ) | 43 | % | 16,996 | (43 | )% | |||||||||
| Tax rate change |
21,454 | (85 | )% | — | — | % | ||||||||||
|
|
|
|
|
|||||||||||||
| Provision for income taxes |
$ | — | — | % | $ | — | — | % | ||||||||
|
|
|
|
|
|||||||||||||
The Tax Cuts and Jobs Act of 2017 (“TCJA”) was signed into law on December 22, 2017. The TCJA significantly revises the U.S. corporate income tax by, among other things, lowering the statutory corporate tax rate from 35% to 21%, eliminating certain deductions, imposing a mandatory one-time tax on accumulated earnings of foreign subsidiaries, introducing new tax regimes, and changing how foreign earnings are subject to U.S. tax. The TCJA also enhanced and extended through 2026 the option to claim accelerated depreciation deductions on qualified property. We have not completed our determination of the accounting implications of the TCJA on our tax accruals. However, we have reasonably estimated the effects of the TCJA and recorded in our financial statements as of December 31, 2017 the provisional amounts for the revaluation of our net deferred tax assets and liabilities resulting from the permanent reduction in the U.S. statutory corporate tax rate to 21% from 35%. The provision estimate results in $19.5 million of tax expense offset by an adjustment to the valuation allowance. As we complete our analysis of the TCJA, collect and prepare necessary data, and interpret any additional guidance issued by the U.S. Treasury Department, the IRS, and other standard-setting bodies, we may make adjustments to the provisional amounts. Those adjustments may materially impact our provision for income taxes in the period in which the adjustments are made.
Significant components of the Company’s deferred tax assets and liabilities from federal and state income taxes as of December 31 are shown below (in thousands):
| Years ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Deferred tax assets: |
||||||||
| Tax loss carryforwards |
$ | 29,713 | $ | 41,502 | ||||
| Research and development credits and other tax credits |
3,084 | 2,817 | ||||||
| Stock-based compensation |
3,565 | 4,658 | ||||||
| Other |
945 | 1,283 | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
37,307 | 50,260 | ||||||
| Valuation allowance |
(37,307 | ) | (50,260 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax asset |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
Trovagene records a valuation allowance against deferred tax assets to the extent that it is more likely than not that some portion, or all of, the deferred tax assets will not be realized. Due to the substantial doubt related to Trovagene’s ability to utilize its deferred tax assets, the Company recorded a valuation allowance against the deferred tax.
FASB ASC Topic 740-10-30-7, Accounting for Income Taxes had no effect on Trovagene’s financial position, cash flows or results of operations upon adoption, as Trovagene does not have any unrecognized tax
F-26
Table of Contents
benefits. Trovagene’s practice is to recognize interest and/or penalties related to income tax matters in income tax expense, and none have been incurred to date.
10. Commitments and Contingencies
License and Service Agreements
In March 2017, the Company entered into a license agreement with Nerviano which granted the Company development and commercialization rights to NMS-1286937, which Trovagene refers to as PCM-075. PCM-075 is an oral, investigative drug and a highly-selective adenosine triphosphate competitive inhibitor of the serine/threonine PLK 1. The Company plans to develop PCM-075 initially in patients with AML. Upon execution of the agreement, the Company paid $2.0 million in license fees which were expensed to research and development costs during the year ended December 31, 2017. The Company is committed to pay $1.0 million for future services provided by Nerviano, such as the costs to manufacture drug product, no later than June 30, 2019. Terms of the agreement also provide for the Company to pay royalties based on certain development and sales milestones.
The Company is a party to various agreements under which it licenses technology on an exclusive basis in the field of human diagnostics. License fees are generally calculated as a percentage of product revenues, with rates that vary by agreement. To date, payments have not been material.
Litigation
Trovagene does not believe that the Company has legal liabilities that are probable or reasonably possible that require either accrual or disclosure, except for the following: On March 28, 2016 the Company filed a complaint in the Superior Court of the State of California for the County of San Diego against the Company’s former CEO and CFO, for, among other things, breach of fiduciary duty for failing to present a lucrative corporate opportunity to the Company concerning promising new therapeutics in the field of precision medicine and instead taking that opportunity for their own personal benefit (the “Complaint”). The Complaint asks that these two former executives be required to turn over their interests in these new therapeutics to the Company. The former CEO and CFO filed a cross complaint in the Superior Court of the State of California for the County of San Diego against the Company on May 23, 2016 for, among other things, breach of contract (the “Cross Complaint”, and together with the Complaint, collectively, the “Litigation”). On July 28, 2017, the parties settled the Litigation. The settlement involved mutual releases by all parties involved. The net cost to Trovagene in connection with the settlement is approximately $2.1 million. Of that amount, $975,000 was the net amount paid directly to the former CEO and CFO. From time to time, the Company may become involved in various lawsuits and legal proceedings that arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in matters may arise from time to time that may harm the Company’s business. As of the date of this report, management believes that there are no claims against the Company, which it believes will result in a material adverse effect on the Company’s business or financial condition.
Employment Agreements
The Company has longer-term contractual commitments with various employees. Certain employment agreements provide for severance payments.
Lease Agreements
The Company currently leases approximately 26,100 square feet facilities in San Diego under an operating lease that expires on December 31, 2021 at a monthly rental rate of approximately $68,000. The Company leased certain lab and office space in Torino, Italy, of approximately 2,300 square feet at a monthly rental rate of approximately $3,100. The lease was terminated at the end of September 2017. Rent expense for the years ended December 31, 2017 and 2016 was approximately $663,000 and $602,000, respectively.
F-27
Table of Contents
The Company is also a party to various non-cancelable operating lease agreements for office equipment.
Total annual commitments under non-cancelable lease agreements for each of the years ended December 31 are as follows:
| Operating Leases |
Sublease Income |
Net Operating Leases |
||||||||||
| 2018 |
$ | 881,815 | $ | (216,504 | ) | $ | 665,311 | |||||
| 2019 |
906,879 | (183,124 | ) | 723,755 | ||||||||
| 2020 |
931,457 | — | 931,457 | |||||||||
| 2021 |
959,401 | — | 959,401 | |||||||||
| 2022 |
— | — | — | |||||||||
| Thereafter |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 3,679,552 | $ | (399,628 | ) | $ | 3,279,924 | |||||
|
|
|
|
|
|
|
|||||||
Public Offering and Controlled Equity Offering
On March 15, 2017, the Company filed a Form 424B5 to amend and supplement the information in the Company’s registration statement and prospectus, dated June 13, 2016, to offer and sell additional shares of the Company’s common stock having an aggregate offering price up to $20,698,357. The Company entered into an agreement with Cantor Fitzgerald & Co. (“Agent”) on January 25, 2013 to issue and sell up to $30,000,000 of shares of common stock through the Agent. As payment for its services, the Agent is entitled to a 3% commission on gross proceeds. Gross proceeds of $110,000 have been raised in 2017.
Database Usage
In March 2016 the Company entered into an agreement with an outside vendor to develop an online database for test requisition and test results. Under the agreement, the Company is obligated to pay a fixed development fee, and a usage fee each time an external user completes and submits a test order form to the database. To date, the Company has paid the fixed development fee. Costs incurred in connection with the usage fees were immaterial.
11. Employee Benefit Plan
The Company has a retirement savings plan under Section 401(k) of the Code covering its employees. The plan allows employees to defer, up to the maximum allowed, a percentage of their income on a pre-tax basis through contributions to the plans, plus any employee age 50 and over can participate in the caught-up dollars as allowed by Internal Revenue Service codes. The Company also has a Roth investment plan that is taken after taxes. The Company does not currently make matching contributions.
12. Restructuring Charges
On March 15, 2017, in connection with the addition of precision medicine therapeutics to its business, the Company announced a restructuring plan (the “Restructuring”) which included a reduction in force. As part of this restructuring, the Company elected to dissolve its wholly owned subsidiary, Trovagene Srl, in 2017, resulting in a reversal of foreign currency translation losses. The financial results of the dissolution is represented in the restructuring cost in the December 31, 2017 financial statements.
This restructuring has been completed as of December 31, 2017. The Company incurred approximately $2.2 million in restructuring charges, which has been included as a component of operating loss for the year ended December 31, 2017. Restructuring charges include approximately $1.1 million of costs related to termination of
F-28
Table of Contents
employees, which is net of a $125,000 stock-based compensation expense reversal for certain terminated employees. The remaining restructuring charges of approximately $485,000 were related to impairment of capitalized license fees. Of the total restructuring expenses recorded, approximately $262,000 remains to be paid as of December 31, 2017 and is included in accrued liabilities on the Company’s consolidated balance sheet.
13. Related Party Transactions
In March 2016, the Company engaged Rutan & Tucker, LLP, a law firm to represent Trovagene, Inc. with respect to various lawsuits. One of the partners from Rutan & Tucker, LLP, is the son of the Company’s Chairman of the Board. The fees for legal services are based on the hourly rates of the individuals performing the legal services. During the year ended December 31, 2017 and 2016, the Company incurred and recorded approximately $650,000 and $537,000 of legal expenses, net of insurance reimbursements, for services performed by Rutan & Tucker, LLP, respectively.
14. Selected Quarterly Financial Data (Unaudited)
The following is a summary of the quarterly results of operations of the Company for years ended December 31, 2017 and 2016:
| Quarter Ended(1) | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| (dollars in thousands, except per share data) | ||||||||||||||||
| 2017 |
||||||||||||||||
| Revenues |
$ | 95 | $ | 102 | $ | 123 | $ | 185 | ||||||||
| Operating expenses |
$ | 10,221 | $ | 5,990 | $ | 5,921 | $ | 3,969 | ||||||||
| Net loss attributable to common stockholders |
$ | (10,005 | ) | $ | (8,052 | ) | $ | (4,298 | ) | $ | (2,576 | ) | ||||
| Net loss per common share - basic |
$ | 0.32 | $ | (0.26 | ) | $ | (0.12 | ) | $ | (0.06 | ) | |||||
| Net loss per common share - diluted |
$ | (0.32 | ) | $ | (0.26 | ) | $ | (0.12 | ) | $ | (0.06 | ) | ||||
| Shares used in the calculation of net loss attributable to common stockholders - basic |
30,961,014 | 30,991,740 | 36,465,672 | 40,182,071 | ||||||||||||
| Shares used in the calculation of net loss attributable to common stockholders - diluted |
30,961,014 | 30,991,740 | 36,465,672 | 40,182,071 | ||||||||||||
| 2016 |
||||||||||||||||
| Revenues |
$ | 120 | $ | 104 | $ | 89 | $ | 68 | ||||||||
| Operating expenses |
$ | 10,579 | $ | 10,084 | $ | 10,013 | $ | 9,850 | ||||||||
| Net loss attributable to common stockholders |
$ | (10,269 | ) | $ | (10,208 | ) | $ | (10,197 | ) | $ | (8,554 | ) | ||||
| Net loss per common share - basic |
$ | (0.35 | ) | $ | (0.34 | ) | $ | (0.34 | ) | $ | (0.28 | ) | ||||
| Net loss per common share - diluted |
$ | (0.36 | ) | $ | (0.34 | ) | $ | (0.34 | ) | $ | (0.34 | ) | ||||
| Shares used in the calculation of net loss attributable to common stockholders - basic |
29,755,184 | 29,958,037 | 30,339,774 | 30,639,440 | ||||||||||||
| Shares used in the calculation of net loss attributable to common stockholders - diluted |
30,108,377 | 29,958,037 | 30,339,774 | 30,711,946 |
| (1) | Basic and diluted net loss per common share are computed independently for each of the periods presented. Accordingly, the sum of the quarterly net loss per common share amount may not agree to the total for the year. |
F-29
Table of Contents
CONDENSED CONSOLIDATED BALANCE SHEETS
(Unaudited)
| March 31, 2018 |
December 31, 2017 |
|||||||
| Assets | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 6,657,158 | $ | 8,225,764 | ||||
| Accounts receivable and unbilled receivable |
114,343 | 77,095 | ||||||
| Prepaid expenses and other current assets |
1,068,144 | 1,165,828 | ||||||
|
|
|
|
|
|||||
| Total current assets |
7,839,645 | 9,468,687 | ||||||
| Property and equipment, net |
2,223,597 | 2,426,312 | ||||||
| Other assets |
345,277 | 389,942 | ||||||
|
|
|
|
|
|||||
| Total Assets |
$ | 10,408,519 | $ | 12,284,941 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 651,671 | $ | 825,244 | ||||
| Accrued expenses |
1,685,178 | 1,454,587 | ||||||
| Deferred rent |
341,924 | 334,424 | ||||||
| Current portion of long-term debt |
1,174,989 | 1,331,515 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
3,853,762 | 3,945,770 | ||||||
| Derivative financial instruments—warrants |
779,076 | 649,387 | ||||||
| Deferred rent, net of current portion |
1,096,591 | 1,183,677 | ||||||
|
|
|
|
|
|||||
| Total Liabilities |
5,729,429 | 5,778,834 | ||||||
| Commitments and contingencies (Note 9) |
||||||||
| Stockholders’ equity |
||||||||
| Preferred stock, $0.001 par value, 20,000,000 shares authorized; 60,600 shares outstanding at March 31, 2018 and December 31, 2017; designated as Series A Convertible Preferred Stock with liquidation preference of $606,000 at March 31, 2018 and December 31, 2017 |
60 | 60 | ||||||
| Common stock, $0.0001 par value, 150,000,000 shares authorized; 58,832,953 and 52,791,584 shares issued and outstanding at March 31, 2018 and December 31, 2017, respectively |
5,883 | 5,279 | ||||||
| Additional paid-in capital |
182,401,648 | 179,546,954 | ||||||
| Accumulated deficit |
(177,728,501 | ) | (173,046,186 | ) | ||||
|
|
|
|
|
|||||
| Total Stockholders’ Equity |
4,679,090 | 6,506,107 | ||||||
|
|
|
|
|
|||||
| Total Liabilities and Stockholders’ Equity |
$ | 10,408,519 | $ | 12,284,941 | ||||
|
|
|
|
|
|||||
See accompanying notes to the unaudited condensed consolidated financial statements.
F-30
Table of Contents
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
| Three Months Ended March 31, | ||||||||
| 2018 | 2017 | |||||||
| Revenues: |
||||||||
| Royalties |
$ | 49,055 | $ | 65,826 | ||||
| Diagnostic services |
40,002 | 28,862 | ||||||
| Clinical research |
11,079 | 350 | ||||||
|
|
|
|
|
|||||
| Total revenues |
100,136 | 95,038 | ||||||
| Costs and expenses: |
||||||||
| Cost of revenues |
366,344 | 616,426 | ||||||
| Research and development |
1,883,838 | 4,279,830 | ||||||
| Selling, general and administrative |
2,504,977 | 3,604,624 | ||||||
| Restructuring charges |
— | 1,719,804 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
4,755,159 | 10,220,684 | ||||||
|
|
|
|
|
|||||
| Loss from operations |
(4,655,023 | ) | (10,125,646 | ) | ||||
|
|
|
|
|
|||||
| Net interest expense |
(2,465 | ) | (429,397 | ) | ||||
| (Loss) gain from change in fair value of derivative financial instruments—warrants |
(129,689 | ) | 555,506 | |||||
| Other income |
1,000 | — | ||||||
|
|
|
|
|
|||||
| Net loss |
(4,786,177 | ) | (9,999,537 | ) | ||||
| Preferred stock dividend |
(6,060 | ) | (6,060 | ) | ||||
|
|
|
|
|
|||||
| Net loss attributable to common stockholders |
$ | (4,792,237 | ) | $ | (10,005,597 | ) | ||
|
|
|
|
|
|||||
| Net loss per common share — basic |
$ | (0.09 | ) | $ | (0.32 | ) | ||
|
|
|
|
|
|||||
| Net loss per common share — diluted |
$ | (0.09 | ) | $ | (0.32 | ) | ||
|
|
|
|
|
|||||
| Weighted-average shares outstanding — basic |
55,364,438 | 30,961,014 | ||||||
|
|
|
|
|
|||||
| Weighted-average shares outstanding — diluted |
55,364,438 | 30,961,014 | ||||||
|
|
|
|
|
|||||
See accompanying notes to the unaudited condensed consolidated financial statements.
F-31
Table of Contents
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(Unaudited)
| Three Months Ended March 31, | ||||||||
| 2018 | 2017 | |||||||
| Net loss |
$ | (4,786,177 | ) | $ | (9,999,537 | ) | ||
| Other comprehensive loss: |
||||||||
| Foreign currency translation loss |
— | (2,399 | ) | |||||
| Unrealized gain or reversal of previous losses on securities available-for-sale |
— | (454 | ) | |||||
|
|
|
|
|
|||||
| Total other comprehensive loss |
— | (2,853 | ) | |||||
|
|
|
|
|
|||||
| Total comprehensive loss |
(4,786,177 | ) | (10,002,390 | ) | ||||
| Preferred stock dividend |
(6,060 | ) | (6,060 | ) | ||||
|
|
|
|
|
|||||
| Comprehensive loss attributable to common stockholders |
$ | (4,792,237 | ) | $ | (10,008,450 | ) | ||
|
|
|
|
|
|||||
See accompanying notes to the unaudited condensed consolidated financial statements.
F-32
Table of Contents
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(Unaudited)
| Preferred Stock Shares |
Preferred Stock Amount |
Common Stock Shares |
Common Stock Amount |
Additional Paid-In Capital |
Accumulated Deficit |
Total Stockholders’ Equity |
||||||||||||||||||||||
| Balance, January 1, 2018 |
60,600 | $ | 60 | 52,791,584 | $ | 5,279 | $ | 179,546,954 | $ | (173,046,186 | ) | $ | 6,506,107 | |||||||||||||||
| Stock-based compensation |
— | — | — | — | 1,406,131 | — | 1,406,131 | |||||||||||||||||||||
| Issuance of common stock upon exercise of warrants |
— | — | 5,136,667 | 514 | 1,448,653 | — | 1,449,167 | |||||||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units |
— | — | 904,702 | 90 | (90 | ) | — | — | ||||||||||||||||||||
| Preferred stock dividend |
— | — | — | — | — | (6,060 | ) | (6,060 | ) | |||||||||||||||||||
| Cumulative adjustment upon adoption of ASC 606 |
— | — | — | — | — | 109,922 | 109,922 | |||||||||||||||||||||
| Net loss |
— | — | — | — | — | (4,786,177 | ) | (4,786,177 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance, March 31, 2018 |
60,600 | $ | 60 | 58,832,953 | $ | 5,883 | $ | 182,401,648 | $ | (177,728,501 | ) | $ | 4,679,090 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
See accompanying notes to the unaudited condensed consolidated financial statements.
F-33
Table of Contents
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
| Three Months Ended March 31, |
||||||||
| 2018 | 2017 | |||||||
| Operating activities |
||||||||
| Net loss |
$ | (4,786,177 | ) | $ | (9,999,537 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Impairment loss |
— | 485,000 | ||||||
| Depreciation and amortization |
252,480 | 330,968 | ||||||
| Stock based compensation expense |
1,406,131 | 920,799 | ||||||
| Accretion of final fee premium |
— | 125,012 | ||||||
| Amortization of discount on debt |
— | 68,223 | ||||||
| Amortization of premiums on short-term investments |
— | 10,877 | ||||||
| Deferred rent |
(79,586 | ) | (66,119 | ) | ||||
| Interest income accrued on short-term investments |
— | 151,583 | ||||||
| Change in fair value of derivative financial instruments—warrants |
129,689 | (555,506 | ) | |||||
| Changes in operating assets and liabilities: |
||||||||
| Decrease in accounts receivable and unbilled receivable |
72,674 | 20,112 | ||||||
| Decrease in prepaid expenses and other current assets |
97,684 | 110,957 | ||||||
| Increase (decrease) in accounts payable and accrued expenses |
50,958 | (360,577 | ) | |||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(2,856,147 | ) | (8,758,208 | ) | ||||
|
|
|
|
|
|||||
| Investing activities: |
||||||||
| Capital expenditures, net |
(5,100 | ) | (11,452 | ) | ||||
| Maturities of short-term investments |
— | 14,000,000 | ||||||
| Purchases of short-term investments |
— | (8,804,604 | ) | |||||
|
|
|
|
|
|||||
| Net cash (used in) provided by investing activities |
(5,100 | ) | 5,183,944 | |||||
|
|
|
|
|
|||||
| Financing activities: |
||||||||
| Proceeds from exercise of warrants |
1,449,167 | — | ||||||
| Repayments of equipment line of credit |
(156,526 | ) | (156,526 | ) | ||||
|
|
|
|
|
|||||
| Net cash provided by (used in) financing activities |
1,292,641 | (156,526 | ) | |||||
| Effect of exchange rate changes on cash and cash equivalents |
— | (844 | ) | |||||
|
|
|
|
|
|||||
| Net change in cash and equivalents |
(1,568,606 | ) | (3,731,634 | ) | ||||
| Cash and cash equivalents—Beginning of period |
8,225,764 | 13,915,094 | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents—End of period |
$ | 6,657,158 | $ | 10,183,460 | ||||
|
|
|
|
|
|||||
| Supplementary disclosure of cash flow activity: |
||||||||
| Cash paid for interest |
$ | 16,417 | $ | 300,040 | ||||
| Supplemental disclosure of non-cash investing and financing activities: |
||||||||
| Preferred stock dividends accrued |
$ | 6,060 | $ | 6,060 | ||||
See accompanying notes to the unaudited condensed consolidated financial statements.
F-34
Table of Contents
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1. Organization and Basis of Presentation
Business Organization and Overview
Trovagene, Inc. (“Trovagene” or the “Company”) headquartered in San Diego, California, is a clinical-stage, oncology therapeutics company. The Company’s primary focus is to develop oncology therapeutics for the treatment of hematologic and solid tumor cancers for improved cancer care, utilizing its proprietary technology in tumor genomics.
Trovagene’s lead drug candidate, PCM-075, is a Polo-like Kinase 1 (“PLK1”) highly-selective adenosine triphosphate (“ATP”) competitive inhibitor. PCM-075 has shown preclinical antitumor activity as a single agent and synergy in combination with more than ten different chemotherapeutics, including cisplatin, cytarabine, doxorubicin, gemcitabine and paclitaxel, as well as targeted therapies, such as abiraterone acetate (Zytiga®), histone deacetylase (“HDAC”) inhibitors, such as belinostat (Beleodaq®), Quizartinib (AC220), a development stage FLT3 inhibitor, and bortezomib (Velcade®). These therapeutics are used clinically for the treatment of many hematologic and solid tumor cancers, including Acute Myeloid Leukemia (“AML”), Non-Hodgkin Lymphoma (“NHL”), metastatic Castration-Resistant Prostate Cancer (“mCRPC”), Adrenocortical Carcinoma (“ACC”), and Triple Negative Breast Cancer (“TNBC”).
PCM-075 was developed to have high selectivity to PLK1 (at low nanomolar IC50 levels), to be administered orally, and to have a relatively short drug half-life of approximately 24 hours compared to other pan PLK inhibitors. A safety study of PCM-075 has been successfully completed in patients with advanced metastatic solid tumors and published in 2017 in Investigational New Drugs. The Company has two active Investigational New Drug (“INDs”) applications in place with the U.S. Food and Drug Administration (“FDA”) for PCM-075, allowing the Company to pursue clinical development in hematologic malignancies and solid tumor cancers. Trovagene is currently enrolling a Phase 1b/2 open-label clinical trial of PCM-075 in combination with standard-of-care chemotherapy in patients with AML. The Phase 1b/2 clinical trial is led by Hematologist Jorge Eduardo Cortes, M.D., Deputy Department Chair, Department of Leukemia, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center. In addition, the Company is working with Dr. David Einstein at the Genitourinary Oncology Program at Beth Israel Deaconess Medical Center and Harvard Medical School as the principal investigator on a Phase 2 open-label clinical trial of PCM-075 in combination with abiraterone acetate (Zytiga®) and prednisone in patients with mCRPC with plans to enroll patients later this year.
Trovagene’s intellectual property and proprietary technology enables the Company to analyze circulating tumor DNA (“ctDNA”) and clinically actionable markers to identify patients most likely to respond to specific cancer therapies. The Company plans to continue to vertically integrate its tumor genomics technology with the development of targeted cancer therapeutics.
Basis of Presentation
The accompanying unaudited interim condensed consolidated financial statements of Trovagene, which include all accounts of its wholly owned subsidiary, Trovagene, Srl (dissolved in October 2017), have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). All intercompany balances and transactions have been eliminated.
The accompanying unaudited interim condensed consolidated financial statements have been prepared in accordance with GAAP and the rules and regulations of the Securities and Exchange Commission (“SEC”) related to a quarterly report on Form 10-Q. Certain information and note disclosures normally included in annual financial statements prepared in accordance with GAAP have been condensed or omitted pursuant to those
F-35
Table of Contents
rules and regulations. The unaudited interim condensed consolidated financial statements reflect all adjustments consisting of normal recurring adjustments which, in the opinion of management, are necessary for a fair statement of the Company’s financial position and the results of its operations and cash flows for the periods presented. The unaudited condensed balance sheet at December 31, 2017 has been derived from the audited financial statements at that date but does not include all of the information and disclosures required by GAAP for annual financial statements. The operating results presented in these unaudited interim condensed consolidated financial statements are not necessarily indicative of the results that may be expected for any future periods. These unaudited interim condensed consolidated financial statements should be read in conjunction with the audited consolidated financial statements and the notes thereto for the year ended December 31, 2017 included in the Company’s annual report on Form 10-K filed with the SEC on February 26, 2018.
Liquidity
Trovagene’s condensed consolidated financial statements as of March 31, 2018 have been prepared under the assumption that Trovagene will continue as a going concern, which assumes that the Company will realize its assets and satisfy its liabilities in the normal course of business. The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from the outcome of the uncertainty concerning the Company’s ability to continue as a going concern.
The Company has incurred net losses since its inception and has negative operating cash flows. Considering the Company’s current cash resources, management believes the Company’s existing resources will be sufficient to fund the Company’s planned operations through July 2018. On April 6, 2018, the Company paid off the outstanding Loan and Security Agreement (“Equipment Line of Credit”) entered in November 2015 to Silicon Valley Bank (“SVB”). Based on its current business plan and assumptions, the Company expects to continue to incur significant losses and require significant additional capital to further advance its clinical trial programs and support its other operations. The Company has based its cash sufficiency estimates on its current business plan and its assumptions that may prove to be wrong. The Company could utilize its available capital resources sooner than it currently expects, and it could need additional funding to sustain its operations even sooner than currently anticipated. These circumstances raise substantial doubt about the Company’s ability to continue as a going concern. For the foreseeable future, the Company’s ability to continue its operations is dependent upon its ability to obtain additional capital.
The Company cannot be certain that additional funding will be available on acceptable terms, or at all. To the extent that the Company can raise additional funds by issuing equity securities, the Company’s stockholders may experience significant dilution.
If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly delay, scale back or discontinue the development and/or commercialization of one or more of its product candidates, all of which would have a material adverse impact on the Company’s operations. The Company may also be required to:
| • | Seek collaborators for product candidates at an earlier stage than otherwise would be desirable and on terms that are less favorable than might otherwise be available; and |
| • | Relinquish licenses or otherwise dispose of rights to technologies, product candidates or products that the Company would otherwise seek to develop or commercialize themselves, on unfavorable terms. |
The Company is evaluating all options to raise additional capital as well as reduce costs, in an effort to strengthen its liquidity position, which may include the following:
| • | Raising capital through public and private equity offerings; |
| • | Introducing operation and business development initiatives to bring in new revenue streams; |
F-36
Table of Contents
| • | Reducing operating costs by identifying internal synergies; and |
| • | Engaging in strategic partnerships. |
As of April 30, 2018, the Company has received approximately $1.6 million upon exercise of 5,681,667 warrants in connection with the December 2017 public offering. The Company continually assesses its spending plans to effectively and efficiently address its liquidity needs.
NASDAQ Notice
On September 5, 2017, the Company received a written notice from the NASDAQ Stock Market LLC (“NASDAQ”) that it was not in compliance with NASDAQ Listing Rule 5550(a)(2) for continued listing on the NASDAQ Capital Market, as the minimum bid price of the Company’s common stock had been below $1.00 per share for 30 consecutive business days. In accordance with NASDAQ Listing Rule 5810(c)(3)(A), the Company has a period of 180 calendar days, or until March 5, 2018, to regain compliance with the minimum bid price requirement.
On March 6, 2018, the NASDAQ Capital Market informed the Company that it is eligible for an additional 180 calendar day period until September 4, 2018 to regain compliance with the minimum $1.00 bid price per share requirement. To regain compliance, the closing bid price of the Company’s common stock must meet or exceed $1.00 per share for at least ten consecutive business days during this 180 calendar day period.
2. Summary of Significant Accounting Policies
During the three months ended March 31, 2018, there have been no changes to the Company’s significant accounting policies as described in its Annual Report on Form 10-K for the fiscal year ended December 31, 2017, except as described below.
Revenue Recognition
The Company recognizes revenue when control of its products and services is transferred to its customers in an amount that reflects the consideration it expects to receive from its customers in exchange for those products and services. This process involves identifying the contract with a customer, determining the performance obligations in the contract, determining the contract price, allocating the contract price to the distinct performance obligations in the contract, and recognizing revenue when the performance obligations have been satisfied. A performance obligation is considered distinct from other obligations in a contract when it provides a benefit to the customer either on its own or together with other resources that are readily available to the customer and is separately identified in the contract. The Company considers a performance obligation satisfied once it has transferred control of a good or service to the customer, meaning the customer has the ability to use and obtain the benefit of the good or service. The Company recognizes revenue for satisfied performance obligations only when it determines there are no uncertainties regarding payment terms or transfer of control. For sales-based royalties, the Company recognizes revenue at the later of (i) when the related sales occur, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied).
Royalty and License Revenues
The Company licenses and sublicenses its patent rights to healthcare companies, medical laboratories and biotechnology partners. These agreements may involve multiple elements such as license fees, royalties and milestone payments. Revenue is recognized when the criteria described above have been met as well as the following:
| • | Up-front nonrefundable license fees pursuant to agreements under which the Company has no continuing performance obligations are recognized as revenues on the effective date of the agreement and when collection is reasonably assured. |
F-37
Table of Contents
| • | Minimum royalties are recognized as earned, and royalties are earned based on the licensee’s use. The Company estimates and records licensee’s sales based on historical usage rate and collectability. |
Diagnostic Service Revenues
Revenue for clinical laboratory tests may come from several sources, including commercial third-party payors, such as insurance companies and health maintenance organizations, government payors, such as Medicare and Medicaid in the United States, patient self-pay and, in some cases, from hospitals or referring laboratories who, in turn, might bill third-party payors for testing. This revenue stream does not meet the criteria for contracts with a customer under ASC 606 because it is not probable that the Company will collect substantially all the consideration to which it will be entitled in exchange for the goods and services transferred, nor can it reliably determine the expected transaction price. Therefore, the Company is recognizing diagnostic service revenue on the cash collection basis until such time as it is able to properly estimate collections on third party reimbursements.
Clinical Research Revenue
Revenue from clinical research consists of revenue from the sale of urine and blood collection supplies and tests performed under agreements with our clinical research and business development partners. Revenue is recognized when supplies and/or test results are delivered, which is when control of the product is deemed to be transferred.
Refer to Note 3 to the condensed consolidated financial statements for further information.
Net Loss Per Share
Basic and diluted net loss per share is presented in conformity with ASC Topic 260, Earnings per Share, for all periods presented. In accordance with this guidance, basic net loss per common share was determined by dividing net loss applicable to common stockholders by the weighted-average common shares outstanding during the period. Preferred dividends are included in income available to common stockholders in the computation of basic and diluted earnings per share. Diluted net loss per share is computed by dividing the net loss by the weighted average number of common shares and common share equivalents outstanding for the period. Common share equivalents are only included when their effect is dilutive.
The following table sets forth the computation of basic and diluted earnings per share:
| Three Months Ended March 31, |
||||||||
| 2018 | 2017 | |||||||
| Numerator: Net loss attributable to common shareholders |
$ | (4,792,237 | ) | $ | (10,005,597 | ) | ||
| Adjustment for gain from change in fair value of derivative financial instruments—warrants |
— | — | ||||||
|
|
|
|
|
|||||
| Net loss used for diluted loss per share |
$ | (4,792,237 | ) | $ | (10,005,597 | ) | ||
|
|
|
|
|
|||||
| Denominator for basic and diluted net loss per share: |
||||||||
| Weighted-average shares used to compute basic loss per share |
55,364,438 | 30,961,014 | ||||||
| Adjustments to reflect assumed exercise of warrants |
— | — | ||||||
|
|
|
|
|
|||||
| Weighted-average shares used to compute diluted net loss per share |
55,364,438 | 30,961,014 | ||||||
|
|
|
|
|
|||||
| Net loss per share attributable to common stockholders: |
||||||||
| Basic |
$ | (0.09 | ) | $ | (0.32 | ) | ||
|
|
|
|
|
|||||
| Diluted |
$ | (0.09 | ) | $ | (0.32 | ) | ||
|
|
|
|
|
|||||
F-38
Table of Contents
The following table sets forth the outstanding potentially dilutive securities that have been excluded in the calculation of diluted net loss per share because their effect was anti-dilutive:
| March 31, | ||||||||
| 2018 | 2017 | |||||||
| Options to purchase Common Stock |
7,588,306 | 4,687,031 | ||||||
| Warrants to purchase Common Stock |
18,418,853 | 5,505,901 | ||||||
| Restricted Stock Units |
369,600 | 976,991 | ||||||
| Series A Convertible Preferred Stock |
63,125 | 63,125 | ||||||
|
|
|
|
|
|||||
| 26,439,884 | 11,233,583 | |||||||
|
|
|
|
|
|||||
Change in Accounting Principle
In August 2016, the FASB issued Accounting Standards Update (“ASU”) 2016-15, Classification of Certain Cash Receipts and Cash Payments (“ASU 2016-15”), which includes amendments that clarify how certain cash receipts and cash payments are presented in the statement of cash flows. ASU 2016-15 also provides guidance clarifying when an entity should separate cash receipts and cash payments and classify them into more than one class of cash flows. The Company adopted ASU 2016-15 as of January 1, 2018. The adoption of ASU 2016-15 had no material impact on its consolidated statements of cash flows.
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers (“ASU 2014-09”). The new standard is based on the principle that revenue should be recognized to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. Since its initial release, the FASB has issued several amendments to the standard, which include clarification of accounting guidance related to identification of performance obligations, intellectual property licenses, and principle versus agent considerations. ASU 2014-09 and all subsequent amendments (collectively, “ASC 606”) became effective for the Company on January 1, 2018. The Company adopted ASC 606 on January 1, 2018 using the modified retrospective method for all contracts not completed as of the date of adoption. Refer to Note 3 to the condensed consolidated financial statements for further details.
Recent Accounting Pronouncements
In February 2016, the FASB issued ASU 2016-02, Leases. The new standard establishes a right-of-use (“ROU”) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for most leases. The new standard is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. A modified retrospective transition approach is required for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. The new standard will impact the Company’s accounting for its office leases and the Company is currently evaluating the impact of the new standard on its consolidated financial statements.
3. Revenue
Financial Statement Impact of Adopting ASC 606
The Company adopted ASC 606 using the modified retrospective method. This resulted in a cumulative adjustment to decrease the Company’s accumulated deficit by $109,922 to reflect the acceleration of revenue recognition related to its sales-based royalties for agreements with customers that were not completed as of January 1, 2018. As a result of applying the modified retrospective method to adopt the new revenue guidance, the Company recorded $109,922 to unbilled receivables under the condensed consolidated balance sheet as of January 1, 2018.
F-39
Table of Contents
Impact of New Revenue Guidance on Financial Statement Line Items
The following summarizes the significant changes to the Company’s condensed consolidated balance sheet and condensed consolidated statement of operations for the three months ended March 31, 2018 as a result of the adoption of ASC 606 on January 1, 2018 compared to what would have been recognized under ASC 605:
| • | Total reported assets and equity were $30,667 greater than what would have been reported under ASC 605 as of March 31, 2018. This was due to the acceleration of future minimum customer sales-based royalty revenues under ASC 606 through the potential contract cancellation period. |
| • | $77,589 reduction of recorded revenues related to prior periods. Previously under ASC 605, there was a lag of at least one quarter before the Company was notified of customers’ sales-based royalties, and thus royalty revenues in excess of the minimum guaranteed amounts were recognized in arrears. This would have resulted in recording additional royalty revenue in the first quarter of 2018 related to eligible 2017 customer sales. For customers that only report royalty-eligible sales annually, this typically resulted in the recognition of a full year’s worth of royalties in excess of the minimum in the first quarter of the following year. However, ASC 606 requires recognition in the period earned even if amounts are unknown (subject to the constraint that a significant future reversal of this estimated revenue is not probable). Because the modified retrospective approach was applied upon adoption on January 1, 2018, this cumulative difference (amount in arrears) was adjusted to the Company’s accumulated deficit rather than recording this revenue in the first quarter of 2018. |
| • | Partially offsetting the reduction above is the $18,326 acceleration of first quarter 2018 sales-based royalty revenue in excess of minimum guaranteed amounts to the extent the amounts are known or can be estimated, and a significant reversal is not probable. |
The net impact of accounting for revenue under the new guidance increased net loss and net loss per share by $59,263 and $0.001 per basic and diluted share, respectively for the three months ended March 31, 2018.
The adoption of ASC 606 had no impact on the Company’s cash flows from operations. The aforementioned impacts resulted in offsetting shifts in cash flows between net loss and changes in working capital balances.
Revenue Recognitions
The Company has historically derived its revenues from the following sources: (i) royalties from sublicense and patent transfer agreements, (ii) up-front fees from sublicense and patent transfer agreements, (iii) milestone payments from sublicense and patent transfer agreements, (iv) diagnostic services revenue and (v) clinical research revenue. These revenue streams are discussed in greater detail below.
Royalty Revenue
Royalties have comprised the majority of the Company’s revenues to date. Its licensing and patent transfer agreements provide for ongoing royalties, generally calculated as a percentage of net revenues related to the licensed or transferred intellectual property (“IP”). In addition, many of its agreements specify a minimum annual royalty amount beginning in the year of the customer’s first related sale. Because minimum royalty amounts are contractually guaranteed, they are considered fixed consideration and allocated to the performance obligations at the stated amounts in the agreements. Sales-based royalties in excess of the minimum amount are considered variable consideration as the amounts are not known until the related customer sales occur, and are therefore excluded from the transaction price. Royalty amounts are reported by customers on a quarterly or annual basis, depending on the agreement, and are typically collected by the Company in the following quarter.
Under ASC 606, fixed consideration is recognized as revenue when all performance obligations have been satisfied. For existing licensing and patent transfer agreements, the sole performance obligation was the issuance of the sublicense or the transfer of the patent which occurred at the agreements’ inception. However, as these
F-40
Table of Contents
agreements are generally cancellable by either party with 60-90 days’ notice, a fixed contractual minimum cannot be determined at the outset of the agreement. Thus, at a given point the Company may only recognize minimum royalty revenue to be received 60-90 days in the future, as there are no guarantees beyond the minimum cancellation period. This is a slight acceleration compared to previous guidance, which did not permit future minimum royalties to be recognized in an earlier period. The cumulative adjustment to accumulated deficit upon adoption at January 1, 2018 related to this acceleration in revenue recognition was not material, at approximately $32,000.
Sales-based royalties in excess of annual minimums are considered variable consideration. Sales-based or usage-based royalty based on an intellectual property license prohibits recognition of the royalty until sales or other activities occur. Historically, there has been a short lag before the Company was notified of a customer’s previous period sales, and thus sales greater than minimum royalties were recognized in arrears as these amounts became known. Under ASC 606 the Company is now required to record an estimate of sales in excess of minimums even if the exact amount is unknown. Given the Company’s relatively low revenues overall and the unpredictable nature of these sales-based royalties, such acceleration under ASC 606 has not been material. A cumulative adjustment of approximately $78,000 was recognized upon adoption as a result of this acceleration. Amounts that have been recognized as revenue but not yet billed to customers are presented as unbilled receivables on the Company’s balance sheet.
Up-Front Licensing and Patent Transfer Fees
Each of the Company’s licensing agreements contains a non-refundable up-front licensing fee for use by the customer of the related IP. The Company’s IP license grants and patent transfers are considered to be functional IP as each has immediate standalone value and distinct performance obligations and as such, revenue is recognized upon transfer of control to the customer. This is considered fixed consideration under ASC 606 and is allocated entirely to the IP grant at the amount stated in the agreement. This is consistent with the previous guidance and as such, the adoption of ASC 606 had no effect on this revenue stream as all performance obligations under existing agreements had already been satisfied, fees had been collected from customers, and the related revenues had already been recognized prior to adoption.
Milestone Payments from Sublicense and Patent Transfer Agreements
A few of the Company’s agreements with customers contain payments related to the achievement of specific milestones. However, as no milestones have been reached under these agreements in several years and the Company does not expect to achieve the remaining milestones under existing agreements, these potential amounts are excluded from the transaction price, and the adoption of ASC 606 had no effect on this revenue stream. The Company will, however, continue to update its assessment in future reporting periods regarding the likelihood of achieving outstanding milestones.
Diagnostic Service Revenue
This revenue stream is related to the performance of clinical laboratory tests and has come primarily from insurance companies and government payors, such as Medicare and Medicaid in the United States. Some revenue also comes from international private payors. Diagnostic services revenue to date has been recognized on a cash collection basis due to (i) the highly complex insurance and governmental regulations and practices that vary based on state, third party payor, etc., (ii) the Company’s relatively short commercial history with uncollected billings, (iii) the Company’s fairly high percentage of services that are billed and not collected, and (iv) significant lag times between when a sample is processed and when payment is received. While distinct performance obligations and stand-alone selling prices can be identified, we do not believe these agreements meet the criteria for contracts with a customer under ASC 606 because it is not probable that the entity will collect substantially all the consideration to which it will be entitled in exchange for the goods and services transferred, nor can it reliably determine the expected transaction price. Therefore, the Company has continued to
F-41
Table of Contents
recognize this revenue on a cash basis as it did under the previous guidance. Thus, the adoption of ASC 606 did not affect this revenue stream.
Clinical Research Revenue
This revenue stream consists primarily of sales of urine and blood collection supplies and testing services under agreements with distributors and with pharmaceutical companies. These agreements meet the criteria for contracts with a customer, have fixed prices and quantities for goods (supplies) and services (tests), and each good or service represents a distinct performance obligation and has a stand-alone selling price that is independent of other purchases by the customer. Performance obligations are satisfied when goods or services are provided to the customer under ASC 606. Because testing services are very short in duration (less than two weeks) and have relatively low prices and low volumes, related costs are expensed immediately rather than recorded as contract assets, as the results would not differ significantly. Standard payment terms apply to these agreements, and thus there is no financing component nor prepayments that would result in a contract liability. Customers are invoiced and revenue is recognized simultaneously upon shipment or delivery of test results at the stated amounts per the contract, which is consistent with previous guidance. Thus, the adoption of ASC 606 did not affect reported amounts for this revenue stream.
Transaction Price Allocated to Future Performance Obligations
Licensing and patent transfer agreements may contain three possible revenue sources: up-front licensing fees, sales-based royalties and potential milestone revenue. However, all of the Company’s existing agreements of this type contained only a single performance obligation to provide the functional IP to the customer at the outset of the agreement. While the Company continues to receive related sales-based royalties, the related performance obligations were satisfied in previous years and thus the Company has no future performance obligations under these agreements.
4. Fair Value Measurements
The following table presents the Company’s assets and liabilities that are measured and recognized at fair value on a recurring basis classified under the appropriate level of the fair value hierarchy as of March 31, 2018 and December 31, 2017:
| Fair Value Measurements at March 31, 2018 |
||||||||||||||||
| Quoted Prices in Active Markets for Identical Assets and Liabilities (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||||||
| Assets: |
||||||||||||||||
| Money market fund (1) |
$ | 6,840,505 | $ | — | $ | — | $ | 6,840,505 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Assets |
$ | 6,840,505 | $ | — | $ | — | $ | 6,840,505 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities: |
||||||||||||||||
| Derivative financial instruments—warrants |
$ | — | $ | — | $ | 779,076 | $ | 779,076 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Liabilities |
$ | — | $ | — | $ | 779,076 | $ | 779,076 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-42
Table of Contents
| Fair Value Measurements at December 31, 2017 |
||||||||||||||||
| Quoted Prices in Active Markets for Identical Assets and Liabilities (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||||||
| Assets: |
||||||||||||||||
| Money market fund (1) |
$ | 8,309,964 | $ | — | $ | — | $ | 8,309,964 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Assets |
$ | 8,309,964 | $ | — | $ | — | $ | 8,309,964 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities: |
||||||||||||||||
| Derivative financial instruments—warrants |
$ | — | $ | — | $ | 649,387 | $ | 649,387 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Liabilities |
$ | — | $ | — | $ | 649,387 | $ | 649,387 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Included as a component of cash and cash equivalents on the accompanying condensed consolidated balance sheets. |
The following table sets forth a summary of changes in the fair value of the Company’s Level 3 liabilities for the three months ended March 31, 2018:
| Description |
Balance at December 31, 2017 |
Realized (gains) or losses |
Balance at March 31, 2018 |
|||||||||
| Derivative financial instruments—warrants |
$ | 649,387 | $ | 129,689 | $ | 779,076 | ||||||
|
|
|
|
|
|
|
|||||||
The change in the fair value of the “derivative financial instruments—warrants” is recorded as a gain or loss in the Company’s consolidated statement of operations. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. At each reporting period, the Company reviews the assets and liabilities that are subject to ASC Topic 815-40 and ASC Topic 480-10. At each reporting period, all assets and liabilities for which the fair value measurement is based on significant unobservable inputs or instruments that trade infrequently and therefore have little or no price transparency are classified as Level 3.
5. Property and Equipment
Property and equipment consist of the following:
| As of March 31, 2018 |
As of December 31, 2017 |
|||||||
| Furniture and office equipment |
$ | 1,076,709 | $ | 1,076,709 | ||||
| Leasehold improvements |
1,994,514 | 1,994,514 | ||||||
| Laboratory equipment |
1,431,681 | 1,426,581 | ||||||
|
|
|
|
|
|||||
| 4,502,904 | 4,497,804 | |||||||
| Less—accumulated depreciation and amortization |
(2,279,307 | ) | (2,071,492 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 2,223,597 | $ | 2,426,312 | ||||
|
|
|
|
|
|||||
6. Equipment Line of Credit
In November 2015, the Company entered into a Loan and Security Agreement (“Equipment Line of Credit”) with SVB that provided for cash borrowings for equipment (“Equipment Advances”) of up to $2.0 million, secured by the equipment financed. Under the terms of the agreement, interest is equal to 1.25% above the Prime Rate. At March 31, 2018, the interest rate was 6.00%. Interest only payments are due on borrowings through November 30, 2016, with both interest and principal payments commencing in December 2016. All unpaid principal and interest on each Equipment Advance will be due on November 1, 2019. The Company has an
F-43
Table of Contents
obligation to make a final payment equal to 7% of total amounts borrowed at the loan maturity date. The Company is also subject to certain affirmative and negative covenants under the Equipment Line of Credit.
On June 20, 2017, the Company received a Notice of Event of Default (“Default Letter”) from SVB which stated that Events of Default had occurred and SVB will decide in its sole discretion whether or not to exercise rights and remedies. The Company does not agree that the loan is in Default, but pursuant to the Default Letter from SVB, the Company classified the entire balance of $1,174,989 as a current liability as of March 31, 2018 and also recorded accrued interest at a default rate. The Company recorded $24,236 in interest expense related to the Equipment Line of Credit during the three months ended March 31, 2018.
The Company paid off the Equipment Line of Credit on April 6, 2018. Refer to Note 10 to the condensed consolidated financial statements for further information.
7. Derivative Financial Instruments — Warrants
Based upon the Company’s analysis of the criteria contained in ASC Topic 815-40, Contracts in Entity’s Own Equity (“ASC 815-40”) or ASC Topic 480-10, Distinguishing Liabilities from Equity (“ASC 480-10”), Trovagene determined that certain warrants issued in connection with the execution of certain equity financings must be recorded as derivative liabilities. In accordance with ASC 815-40 and ASC 480-10, the warrants are also being re-measured at each balance sheet date based on estimated fair value, and any resultant change in fair value is being recorded in the Company’s condensed consolidated statements of operations. The Company estimates the fair value of these warrants using the Black-Scholes option pricing model.
The range of assumptions used to determine the fair value of the warrants valued using the Black-Scholes option pricing model during the periods indicated was:
| Three Months Ended March 31, | ||||||||
| 2018 | 2017 | |||||||
| Estimated fair value of Trovagene common stock |
0.31-0.35 | 1.15-2.10 | ||||||
| Expected warrant term |
0.8-5.1 years | 1.8-2.0 years | ||||||
| Risk-free interest rate |
1.76-2.54 | % | 1.20-1.27 | % | ||||
| Expected volatility |
91-116 | % | 94-98 | % | ||||
| Dividend yield |
0 | % | 0 | % | ||||
Expected volatility is based on historical volatility of Trovagene’s common stock. The warrants have a transferability provision and based on guidance provided in Staff Accounting Bulletin (“SAB”) No. 107, Share-Based Payment (“SAB No. 107”), for instruments issued with such a provision, Trovagene used the remaining contractual term as the expected term of the warrants. The risk free rate is based on the U.S. Treasury security rates consistent with the expected remaining term of the warrants at each balance sheet date.
The following table sets forth the components of changes in the Company’s derivative financial instruments—warrants liability balance, valued using the Black-Scholes option pricing method, for the periods indicated.
| Date |
Description |
Number of Warrants |
Derivative Instrument Liability |
|||||||
| December 31, 2017 |
Balance of derivative financial instruments—warrants liability | 5,610,921 | $ | 649,387 | ||||||
| Change in fair value of derivative financial instruments—warrants during the period recognized as a loss in the condensed consolidated statements of operations | — | 129,689 | ||||||||
|
|
|
|
|
|||||||
| March 31, 2018 |
Balance of derivative financial instruments—warrants liability | 5,610,921 | $ | 779,076 | ||||||
|
|
|
|
|
|||||||
F-44
Table of Contents
8. Stockholders’ Equity
Common Stock
During the three months ended March 31, 2018, the Company issued a total of 6,041,369 shares of Common Stock. 5,136,667 shares were issued upon exercise of warrants for a weighted-average price of $0.28. In addition, 904,702 shares were issued upon vesting of restricted stock units (“RSU”).
Stock Options
Stock-based compensation expense related to Trovagene equity awards have been recognized in operating results as follow:
| Three Months Ended March 31, |
||||||||
| 2018 | 2017 | |||||||
| Included in research and development expense |
$ | 395,709 | $ | 372,200 | ||||
| Included in cost of revenue |
39,631 | 26,156 | ||||||
| Included in selling, general and administrative expense |
970,791 | 601,309 | ||||||
| Benefit from restructuring |
— | (78,866 | ) | |||||
|
|
|
|
|
|||||
| Total stock-based compensation expense |
$ | 1,406,131 | $ | 920,799 | ||||
|
|
|
|
|
|||||
The unrecognized compensation cost related to non-vested stock options outstanding at March 31, 2018 and 2017, net of expected forfeitures, was $2,662,066 and $5,677,247, respectively, which is expected to be recognized over a weighted-average remaining vesting period of 1.8 and 2.6 years, respectively. The weighted-average remaining contractual term of outstanding options as of March 31, 2018 was approximately 8.1 years. The total fair value of stock options vested during the three months ended March 31, 2018 and 2017 was $971,488 and $1,526,211, respectively.
The estimated fair value of stock option awards was determined on the date of grant using the Black-Scholes option valuation model with the following weighted-average assumptions during the following periods indicated:
| Three Months Ended March 31, |
||||||||
| 2018 | 2017 (1) | |||||||
| Risk-free interest rate |
2.43 | % | — | % | ||||
| Dividend yield |
0 | % | 0 | % | ||||
| Expected volatility |
90.28 | % | — | % | ||||
| Expected term |
5.2 years | 0 | ||||||
| (1) | No options granted during the three months ended March 31, 2017. |
A summary of stock option activity and changes in stock options outstanding is presented below:
| Total Options | Weighted-Average Exercise Price Per Share |
Intrinsic Value |
||||||||||
| Balance outstanding, December 31, 2017 |
4,490,475 | $ | 4.04 | $ | — | |||||||
| Granted |
3,132,831 | $ | 0.30 | |||||||||
| Canceled / Forfeited |
(35,000 | ) | $ | 5.82 | ||||||||
|
|
|
|||||||||||
| Balance outstanding, March 31, 2018 |
7,588,306 | $ | 2.49 | $ | 154,135 | |||||||
|
|
|
|||||||||||
| Exercisable at March 31, 2018 |
5,156,223 | $ | 2.71 | $ | 109,892 | |||||||
|
|
|
|||||||||||
F-45
Table of Contents
On June 13, 2017, the number of authorized shares in the Trovagene 2014 Equity Incentive Plan (“2014 EIP”) was increased from 7,500,000 to 9,500,000. As of March 31, 2018 there were 338,957 shares available for issuance under the 2014 EIP.
Restricted Stock Units
There were no RSU granted during the three months ended March 31, 2018. The weighted-average grant date fair value of the RSU $2.05 per share during the three months ended March 31, 2017.
A summary of the RSU activity is presented below:
| Number of Shares |
Weighted-Average Grant Date Fair Value Per Share |
Intrinsic Value |
||||||||||
| Non-vested RSU outstanding, December 31, 2017 |
1,274,302 | $ | 1.43 | $ | 391,848 | |||||||
| Vested |
(904,702 | ) | $ | 1.18 | $ | 266,461 | ||||||
|
|
|
|||||||||||
| Non-vested RSU outstanding, March 31, 2018 |
369,600 | $ | 2.05 | $ | 129,064 | |||||||
|
|
|
|||||||||||
At March 31, 2018 and 2017, total unrecognized compensation costs related to non-vested RSU were $602,134 and $1,603,214, which are expected to be recognized over a weighted-average period of 2.8 and 3.3 years, respectively. The total fair values of vested RSU during the three months ended March 31, 2018 and 2017 were $1,070,914 and $1,091,580, respectively.
Warrants
A summary of warrant activity and changes in warrants outstanding, including both liability and equity classifications is presented below:
| Total Warrants (1) | Weighted-Average Exercise Price Per Share |
Weighted-Average Remaining Contractual Term (1) |
||||||||||
| Balance outstanding, December 31, 2017 |
23,238,853 | $ | 0.95 | 4.4 | ||||||||
| Exercised |
(4,820,000 | ) | $ | 0.30 | ||||||||
|
|
|
|||||||||||
| Balance outstanding, March 31, 2018 |
18,418,853 | $ | 1.11 | 4.0 | ||||||||
|
|
|
|||||||||||
| (1) | Excluded the pre-funded warrants to purchase 316,667 shares of common stock at a nominal exercise price of $0.01 per share. The pre-warrants were exercised in full during the three months ended March 31, 2018. |
9. Commitments and Contingencies
Employment Agreements
The Company has longer-term contractual commitments with various employees. Certain employment agreements provide for severance payments.
Lease Agreements
The Company leases approximately 26,100 square feet of office and laboratory space at a monthly rental rate of approximately $68,000. The lease will expire on December 31, 2021. The Company currently subleases certain office space and records the rental receipt under the subleases as a reduction of its rent expense.
F-46
Table of Contents
License and Service Agreements
In March 2017, the Company entered into a license agreement with Nerviano Medical Sciences S.r.l. (“Nerviano”) which granted the Company development and commercialization rights to NMS-1286937, which Trovagene refers to as PCM-075. PCM-075 is an oral, investigative drug and a highly-selective adenosine triphosphate competitive inhibitor of the serine/threonine PLK 1. The Company plans to develop PCM-075 in patients with hematologic malignancies and solid tumor cancers. Upon execution of the agreement, the Company paid $2.0 million in license fees which were expensed to research and development costs during the year ended December 31, 2017. Under the agreement, the Company is committed to pay $1.0 million for services provided by Nerviano, such as the costs to manufacture drug product, no later than June 30, 2019. As of March 31, 2018, approximately $200,000 has been paid for services provided. Terms of the agreement also provide for the Company to pay royalties based on certain development and sales milestones.
The Company is a party to various agreements under which it licenses technology on an exclusive basis in the field of human diagnostics. License fees are generally calculated as a percentage of product revenues, with rates that vary by agreement. To date, payments have not been material.
Litigation
Trovagene does not believe that the Company has legal liabilities that are probable or reasonably possible that require either accrual or disclosure. From time to time, the Company may become involved in various lawsuits and legal proceedings that arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in matters may arise from time to time that may harm the Company’s business. As of the date of this report, management believes that there are no claims against the Company, which it believes will result in a material adverse effect on the Company’s business or financial condition.
10. Subsequent Event
On April 6, 2018, the Company paid approximately $1,100,000 to SVB. This payment repaid the outstanding Equipment Line of Credit loan in full.
F-47
Table of Contents
[ ] Class A Units Consisting of Common Stock and Warrants and
[ ] Class B Units Consisting of Series B Convertible Preferred Stock and Warrants (and [ ] shares of common stock underlying shares of Series B Convertible Preferred Stock and [ ] shares of common stock underlying Warrants)

PROSPECTUS
ThinkEquity
A division of Fordham Financial Management, Inc.
, 2018
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. Other Expenses of Issuance and Distribution.
The following table sets forth the costs and expenses, payable by the Company in connection with the registration and sale of the Class A Units and Class B Units being registered other than estimated fees and commissions in connection with our public offering. All amounts are estimates except the SEC registration fee and the Financial Industry Regulatory Authority, Inc. (“FINRA”) filing fee.
| Amount | ||||
| SEC registration fee |
$ | 7,472 | ||
| FINRA filing fee |
9,500 | |||
| Accounting fees and expenses |
* | |||
| Legal fees and expenses |
* | |||
| Transfer agent fees and expenses |
* | |||
| Printing and mailing expenses |
* | |||
| Miscellaneous fees and expenses |
* | |||
|
|
|
|||
| Total expenses |
$ | * | ||
|
|
|
|||
| * | To be filed by amendment |
ITEM 14. Indemnification of Directors and Officers.
The Company’s amended and restated certificate of incorporation eliminates the personal liability of directors to the fullest extent permitted by the Delaware General Corporation Law and, together with the Company’s bylaws, provides that the Company shall indemnify and hold harmless, to the fullest extent permitted by applicable law as it may be amended or supplemented, any person who was or is made or is threatened to be made a party or is otherwise involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that such person, or a person for whom such person is the legal representative, is or was a director or officer of the Company or, while a director or officer of the Company, is or was serving at the request of the Company as a director, officer, employee or agent of another corporation or of a partnership, joint venture, trust, enterprise or nonprofit entity, including service with respect to employee benefit plans, against all liability and loss suffered and expenses (including attorneys’ fees) reasonably incurred by such person.
We have also obtained a liability insurance policy that insures our directors and officers, within the limits and subject to the limitations of the policy, against certain expenses in connection with the defense of actions, suits or proceedings, and certain liabilities that might be imposed as a result of such actions, suits or proceedings, to which they are parties by reason of being or having been directors or officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that, in the opinion of the SEC, this indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
ITEM 15. Recent Sales of Unregistered Securities.
The Company has sold the securities described below within the past three years which were not registered under the Securities Act. All of the sales listed below were made pursuant to an exemption from registration afforded by Section 4(a)(2) of the Securities Act and Regulation D thereunder.
On July 20, 2016, the Company issued to each of Oxford Finance LLC and Silicon Valley Bank a warrant to purchase an aggregate 15,496 shares of Company common stock at an exercise price of $4.84 per share exercisable for ten years from the date of issuance.
II-1
Table of Contents
On July 13, 2017, the Company entered into a securities purchase agreement, whereby the Company issued and sold to certain purchasers warrants to purchase up to 4,643,625 shares of common stock with an exercise price of $1.41 per share.
ITEM 16. Exhibits and Financial Statement Schedules.
(a) The exhibits listed under the caption “Exhibit Index” following the signature page are filed herewith or incorporated by reference herein.
(b) Financial Statement Schedules
No financial statement schedules are provided because the information required to be set forth therein is not applicable or is shown in the consolidated financial statements or notes thereto.
ITEM 17. Undertakings.
(a) The undersigned Registrant hereby undertakes:
(1) to file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement:
(i) to include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) to reflect in the prospectus any facts or events arising after the effective date of the Registration Statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the Registration Statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective Registration Statement; and
(iii) to include any material information with respect to the plan of distribution not previously disclosed in the Registration Statement or any material change to such information in the Registration Statement;
provided, however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the Registrant pursuant to Section 13 or Section 15(d) of the Exchange Act that are incorporated by reference in the Registration Statement.
(2) that, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant to this Registration Statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned Registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
II-2
Table of Contents
(i) any preliminary prospectus or prospectus of the undersigned Registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) any free writing prospectus relating to the offering prepared by or on behalf of the undersigned Registrant or used or referred to by the undersigned Registrant;
(iii) the portion of any other free writing prospectus relating to the offering containing material information about the undersigned Registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) The undersigned Registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the Registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Exchange Act) that is incorporated by reference in the Registration Statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(d) The undersigned Registrant hereby undertakes that:
(1) for purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective.
(2) for the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-3
Table of Contents
II-4
Table of Contents
II-5
Table of Contents
| * | to be filed by amendment. |
| ** | Filed herewith. |
| *** | The U.S. Securities and Exchange Commission (SEC) has granted confidential treatment with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC. |
| + | Indicates a management contract or compensatory plan or arrangement. |
II-6
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in San Diego, California, on the 9th day of May 2018.
| TROVAGENE, INC. | ||
| By: | /s/ William Welch | |
| William Welch | ||
| Chief Executive Officer | ||
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints William Welch, his/her true and lawful attorney-in-fact and agent with full power of substitution and re-substitution, for him/her and in his/her name, place and stead, in any and all capacities to sign any or all amendments (including, without limitation, post-effective amendments) to this Registration Statement, any related Registration Statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and any or all pre- or post-effective amendments thereto, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully for all intents and purposes as he or she might or could do in person, hereby ratifying and confirming that said attorney-in-fact and agent, or any substitute or substitutes for him, may lawfully do or cause to be done by virtue hereof. Pursuant to the requirements of the Securities Act of 1933, the following persons in the capacities and on the dates indicated have signed this Registration Statement below.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated
| Signature |
Title |
Date | ||
| /s/ William Welch William Welch |
Chief Executive Officer and Director (Principal Executive Officer and Principal Financial and Accounting Officer) | May 9, 2018 | ||
| /s/ Thomas H. Adams Thomas H. Adams |
Chairman of the Board |
May 9, 2018 | ||
| /s/ John P. Brancaccio John P. Brancaccio |
Director |
May 9, 2018 | ||
| /s/ Gary S. Jacob Gary S. Jacob |
Director |
May 9, 2018 | ||
|
Paul Billings |
Director |
May , 2018 | ||
| /s/ Stanley Tennant Stanley Tennant |
Director |
May 9, 2018 | ||
| /s/ Rodney S. Markin Rodney S. Markin |
Director |
May 9, 2018 | ||
| /s/ Athena Countouriotis Athena Countouriotis |
Director |
May 9, 2018 | ||
II-7

