Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - INTERCEPT PHARMACEUTICALS, INC. | tv492548_ex32-1.htm |
| EX-31.2 - EXHIBIT 31.2 - INTERCEPT PHARMACEUTICALS, INC. | tv492548_ex31-2.htm |
| EX-31.1 - EXHIBIT 31.1 - INTERCEPT PHARMACEUTICALS, INC. | tv492548_ex31-1.htm |
| EX-10.6 - EXHIBIT 10.6 - INTERCEPT PHARMACEUTICALS, INC. | tv492548_ex10-6.htm |
| EX-10.5 - EXHIBIT 10.5 - INTERCEPT PHARMACEUTICALS, INC. | tv492548_ex10-5.htm |
| EX-10.2 - EXHIBIT 10.2 - INTERCEPT PHARMACEUTICALS, INC. | tv492548_ex10-2.htm |
| EX-10.1 - EXHIBIT 10.1 - INTERCEPT PHARMACEUTICALS, INC. | tv492548_ex10-1.htm |
| 10-Q - 10-Q - INTERCEPT PHARMACEUTICALS, INC. | tv492548_10q.htm |
Exhibit 10.3
CONFIDENTIAL TREATMENT REQUESTED UNDER 17 C.F.R. SECTIONS 200.80(b)(4) AND 240.24b-2. [*****] INDICATES OMITTED MATERIAL THAT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST FILED SEPARATELY WITH THE COMMISSION. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE COMMISSION.
AMENDMENT NO. 3
to
LICENSE AGREEMENT
THIS AMENDMENT NO. 3 (“Amendment”) to that certain License Agreement dated March 29, 2011 (as amended by the amendment No.1 as of June 8, 2011 and the amendment No.2 as of September 16, 2011, collectively, the “Agreement”) is made and entered into as of February 13, 2018, by and between Sumitomo Dainippon Pharma Co., Ltd. (“Sumitomo”), a company organized under the law of Japan, having a place of business at 6-8 Doshomachi 2-chome, Chuo-ku, Osaka 541-0045 Japan, and Intercept Pharmaceuticals, Inc. (“Intercept”), a company organized under the law of the State of Delaware, having a place of business at 10 Hudson Yards, 37th floors, New York, NY 10001 U.S.A.
RECITALS
WHEREAS, Sumitomo and Intercept have entered into the Agreement;
WHEREAS, Sumitomo provided Intercept with a notification letter (“Notice Letter”) for the partial termination of certain rights and obligations under the Agreement dated September 20, 2017;
WHEREAS, Sumitomo and Intercept agreed after Intercept’s receipt of the Notice Letter to extend the effective date of the termination of the rights and obligations in the Notice Letter until full execution of this Amendment;
WHEREAS, Sumitomo desires to obtain the Marketing Approval for and commercialize the Product in China based on the FDA approval and Intercept agrees to cooperate with Sumitomo;
WHEREAS, Sumitomo and Intercept now desire to amend and supplement certain terms and conditions of the Agreement as hereinafter specified;
WHEREAS, Sumitomo and Intercept desire that all other terms and conditions of the Agreement remain in full force and effect;
NOW, THEREFORE, for good and valuable consideration, the receipt and adequacy of which are hereby acknowledged, Sumitomo and Intercept agree as follows:
Capitalized terms in this Amendment shall have the same meaning as those in the Agreement, unless specifically defined otherwise in this Amendment. All Section references are in regard to the Agreement. References to the term “Agreement” in the Agreement shall be deemed to include this Amendment.
Except as expressly modified herein, the Agreement shall remain in full force and effect in accordance with its terms. To the extent that there are any inconsistencies between this Amendment and the Agreement, the terms of this Amendment shall supersede the Agreement.
| 1. | The parties hereby agree to revise Section 1 DEFINITIONS “Territory” of the Agreement to be amended and restated in its entirety as follows: |
“Territory” shall mean China (excluding Taiwan).
| 2. | The parties hereby agree to revise Section 2.2 (Registration) of the Agreement to be amended and restated in its entirety as follows: |
2.2 Registration. Upon DSP’s request, but only after Intercept’s receipt of the Upfront Fee set forth in Section 9.1, Intercept shall use Commercially Reasonable Efforts, at DSP’s sole expense, to register an exclusive license (and/or this Agreement) for DSP in the Territory with respect to the Intercept Technology and Intercept Patents, which registration shall be transferred or assigned to DSP by Intercept immediately upon issuance for no additional consideration.
| 3. | The parties hereby agree to revise Section 3.3 (JSC Meetings) of the Agreement to be amended and restated in its entirety as follows: |
3.3 JSC Meetings. The JSC shall hold meetings as necessity requires and within sixty (60) days upon either Party’s request. Meetings of the JSC shall be effective only if at least (1) representative of each Party is present or participating. The JSC may meet either (i) in person at either Party’s facilities, or (ii) by audio or video teleconference. Meetings of the JSC may be held with the consent of each Party. Each Party shall be responsible for all of its own expenses incurred in connection with participating in the JSC meetings or any of the other committee meetings.
| 4. | The parties hereby agree to revise Section 5.1 (Commercially Reasonable Efforts) of the Agreement to be amended and restated in its entirety as follows: |
5.1 Commercially Reasonable Efforts. DSP shall use Commercially Reasonable Efforts, at its own expense, with respect to all regulatory activities concerning the Development and Commercialization of the Products in the Field in the Territory. DSP shall have sole responsibility for all pricing and reimbursement approval proceedings relating to each Product in the Field in the Territory, and Intercept shall cooperate with DSP (including, but not limited to, filing, obtaining and holding the Import Drug License for the Product in the Territory), based on mutual good faith discussions and DSP shall bear the out-of-pocket expense incurred by Intercept relating to such cooperation solely, provided that, such expense shall be subject to prior written approval of DSP and shall not include the expenses for the Development outside the Territory. Upon reasonable prior notice and during normal business hours, Intercept shall, and shall cause its Affiliates and its Third Party sub-contractors to whom all or a part of the Development outside the Territory has been entrusted or contracted, to allow the inspection by a Regulatory Authority which is required as a condition of Regulatory Approval for the Product in the Field in the Territory. DSP shall use its Commercially Reasonable Efforts to provide any information concerning such inspection to Intercept in a timely manner. Intercept shall manage, but shall permit DSP or its designated representatives to be present at any inspection conducted by such Regulatory Authority. If any issue or concerns are raised concerning the Development of the Compound or the Product in connection with the inspection by such Regulatory Authority, Intercept shall immediately inform and discuss with DSP to solve the issue, including any recommendations made by the Regulatory Authority.
CONFIDENTIAL TREATMENT REQUESTED UNDER 17 C.F.R. SECTIONS 200.80(b)(4) AND 240.24b-2. [*****] INDICATES OMITTED MATERIAL THAT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST FILED SEPARATELY WITH THE COMMISSION. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE COMMISSION.
| 5. | The parties hereby agree to revise Section 6.2 (Commercial Supply), 6.2.1 and 6.2.2 of the Agreement to be amended and restated in its entirety as follows: |
6.2 Commercial Supply. Intercept shall supply DSP (or its Affiliates, sublicensees or sub-contractors) with all DSP’s requirements of the Commercial Supplies. Intercept shall be responsible for the Manufacture of the Commercial Supplies in compliance with the Specifications, GMP and all applicable Laws. The Parties shall discuss in good faith and cooperate with respect to the negotiation of a manufacturing and supply agreement (the “Commercial Supply Agreement”) governing the supply of the Commercial Supply by or on behalf of Intercept, to DSP (or its Affiliates, sublicensees or sub-contractors) for the Commercialization of the Product in the Field in the Territory prior to the initiation of the Phase III Clinical Trials in the Territory. The Commercial Supply Agreement shall contain, in addition to other customary terms, the following terms and conditions:
6.2.1 The transfer price for the [*****] of the Commercial Supply supplied to DSP by or on behalf of Intercept following receipt of Marketing Approval in the Territory shall be calculated at [*****] percent ([*****]%) of [*****] in effect on the date upon which each such order is sent to Intercept by DSP.
6.2.2 The [*****] of the Commercial Supply supplied to DSP by or on behalf of Intercept following receipt of Marketing Approval in the Territory shall be based on [*****] percent ([*****]%) of [*****]. The [*****] is less than or equal to $[*****] (the “[*****]”). In the event that the [*****] exceeds [*****], Intercept shall use Commercially Reasonable Efforts to reduce the [*****]. Should that not be possible, the Parties shall discuss in good faith an increased [*****] for the Product.
| 6. | The parties hereby agree to revise Section 9.2.1 and 9.2.3 (Royalty Tiers) of the Agreement to be amended and restated in its entirety as follows: |
9.2.1 Within thirty (30) calendar days following the occurrence of each of the events set forth below for the Product, DSP shall pay to Intercept each of the non-refundable, non-creditable milestone payments set forth below:
| Milestone Event | Milestone Payment |
| Development Milestones | |
| [*****] | |
| [*****] | US$[*****] |
CONFIDENTIAL TREATMENT REQUESTED UNDER 17 C.F.R. SECTIONS 200.80(b)(4) AND 240.24b-2. [*****] INDICATES OMITTED MATERIAL THAT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST FILED SEPARATELY WITH THE COMMISSION. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE COMMISSION.
| [*****] | US$[*****] |
| [*****] | US$[*****] |
| [*****] | US$[*****] |
| [*****] | US$[*****] |
| [*****] | US$[*****] |
| [*****] | |
| [*****] | US$[*****]* |
In the event that the [*****] occurs [*****], then US$[*****] milestone payment, instead of US$[*****] milestone payment of as set forth in the chart above, shall be due.
For the avoidance of doubt, [*****] shall be deemed to have occurred when [*****].
9.2.3 Royalty Tiers. DSP shall pay to Intercept a royalty of [*****] percent ([*****]%) based on total annual Net Sales of all Products in the Field in the Territory for each fiscal year (i.e. ending on March 31 of each calendar year) in which the Net Sales of all Products in the Territory for such year is less than US$[*****] (the “First Tier Royalty Rate”). DSP shall pay to Intercept a royalty of [*****] percent ([*****]%) based on total annual Net Sales of all Products in the Field in the Territory for each fiscal year in which the Net Sales of all Products in the Territory for such year is US$[*****] or more but less than US$[*****] (the “Second Tier Royalty Rate”). DSP shall pay to Intercept a royalty of [*****] percent ([*****]%) based on total annual Net Sales of all Products in the Field in the Territory for each fiscal year in which the Net Sales of all Products in the Territory for such year exceeds US$[*****] (the “Third Tier Royalty Rate”). Notwithstanding the foregoing, the transfer price for the [*****] of the Commercial Supplies to DSP by Intercept following receipt of Marketing Approval in the Territory shall be calculated in accordance with Section 6.2.1 and shall be deemed to [*****], and accordingly [*****]; however in no event will the transfer price be less than [*****] percent ([*****]%) plus the applicable First, Second or Third Tier Royalty Rate.
The parties hereby acknowledge and agree that (i) milestone payment of US$[*****] for [*****] (US$[*****] and [*****] US$[*****]) and (ii) milestone payment of US$[*****] for [*****] have already been fully paid by Sumitomo and fully received by Intercept.
| 7. | The parties hereby agree to delete Section 9.3.2 (Reduced Royalty Rates in Japan) of the Agreement in its entirety. |
| 8. | The parties hereby agree to revise Section 9.4 (Necessary Third Party Technology Payments) of the Agreement to be amended and restated in its entirety as follows: |
9.4 Necessary Third Party Technology Payments. DSP shall be entitled to deduct [*****] percent ([*****]%) of all royalties it is required to pay to a Third Party for Necessary Third Party IP under any agreement to license or acquire intellectual property used in the Development or Commercialization of the Product in the Field in the Territory up to a maximum of [*****] percent ([*****]%) for purposes of Section 9.2.3, or [*****] percent ([*****]%) for purposes of Section 9.3.1 of the applicable royalty rate.
CONFIDENTIAL TREATMENT REQUESTED UNDER 17 C.F.R. SECTIONS 200.80(b)(4) AND 240.24b-2. [*****] INDICATES OMITTED MATERIAL THAT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST FILED SEPARATELY WITH THE COMMISSION. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE COMMISSION.
| 9. | The parties hereby agree that Sumitomo hereby waives any and all rights and option described in Section 8 COUNTRY OPTION of the Agreement. |
| 10. | The parties hereby agree that (i) any and all rights and obligations relating to DSP Improvements specified in Exhibit A attached in this Amendment shall be assigned or transferred from Sumitomo to Intercept, (ii) Intercept shall bear the expenses for assignment or transferring of such DSP Improvements and (iii) Sumitomo shall have no obligation relating to such DSP Improvements that has not accrued prior to the effective date of this Amendment. For clarification, such DSP Improvements shall be deemed to Intercept Improvements. |
| 11. | The parties hereby agree that, effective on and after the effective date of this Amendment, Section 12.5.2 (ii) (Prosecution and Maintenance outside the Territory) of the Agreement shall apply to the prosecution and maintenance of Joint Improvement for Japan and Korea which is specified in Exhibit B attached in this Amendment and Section 15.4.2 (i) (Prosecution and Maintenance in the Territory) thereof shall not apply to its prosecution and maintenance. |
| 12. | The parties hereby agree that (i) any and all rights and obligations relating to the trademarks specified in Exhibit C attached in this Amendment shall be assigned or transferred from Sumitomo to Intercept, (ii) Intercept shall bear the expenses for assignment or transferring of such trademarks and (iii) Sumitomo shall have no obligation relating to such trademarks that has not accrued prior to the effective date of this Amendment. |
| 13. | The parties hereby agree to replace Section 11.2 in its entirety as follows: |
11.2 Publication. If either party plans to publish or present the results of any studies or other data regarding the Compound or the Product in the Field and conducted in and outside the Territory, the Party shall submit the draft of the publication, translated into English, to the other no later than two (2) weeks prior to the planned submission for publication for review, unless such disclosure requires immediate publication due to disclosure requirements of the U.S. Securities and Exchange Commission, the NASDAQ stock exchange or any other stock exchange on which securities issued by a Party are traded and Intercept has advised DSP of the deadline for disclosure in a sufficiently timely manner. As soon as a Party is aware of a deadline for submitting an abstract for an upcoming scientific meeting, it shall notify the other Party in writing and the Parties shall use Commercially Reasonable Efforts to exchange comments on the proposed abstract in a timely manner to facilitate the publication / presentation of the proposed abstract. Otherwise, any publication shall need the other Party’s prior written consent, which shall not be unreasonably withheld. Any comment, reasonable request for modification or reasonable rejection must be made within as quickly as practically possible from the receipt of the draft. Failure to quickly make such comments shall be conclusively deemed to constitute approval of such publication or presentation. For the avoidance of doubt, this Section 11.2 shall apply to publications made by either Party both in the Territory and outside the Territory.
CONFIDENTIAL TREATMENT REQUESTED UNDER 17 C.F.R. SECTIONS 200.80(b)(4) AND 240.24b-2. [*****] INDICATES OMITTED MATERIAL THAT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST FILED SEPARATELY WITH THE COMMISSION. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE COMMISSION.
| 14. | The parties hereby agree to replace Section 15.1 in its entirety as follows: |
15.1 Term; Expiration. The term of this Agreement (the “Term”) shall commence on the Effective Date and expire on a country-by-country basis on the later to occur of (i) the tenth (10th) anniversary of the First Commercial Sale of the Product for the first or second indication in such country (whichever is later) or (ii) the expiration date of the Exclusive Period in such country. Notwithstanding the foregoing, in the event that [*****] in China has not [*****] (as defined in Section 9.2.1) by December 31, 2020, DSP may choose, after a good faith discussion with Intercept, either that DSP pays to Intercept US$[*****] as a milestone payment or that the Agreement is terminated as a whole, but not both.
| 15. | The parties hereby agree that, notwithstanding the provision of Section 15.4.5 (Surviving Provisions) of the Agreement, the obligation of Sumitomo described in Section 15.4.2 (a), (b) and (c) for Japan and Korea shall survive four (4) years after the date of this Amendment. |
CONFIDENTIAL TREATMENT REQUESTED UNDER 17 C.F.R. SECTIONS 200.80(b)(4) AND 240.24b-2. [*****] INDICATES OMITTED MATERIAL THAT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST FILED SEPARATELY WITH THE COMMISSION. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE COMMISSION.
IN WITNESS WHEREOF, this Amendment has been executed by the parties hereto to be made effective as of February 13, 2018, through their duly authorized officers on the date(s) set forth below.
ACCEPTED AND AGREED TO:
|
Sumitomo Dainippon Pharma Co., Ltd. |
Intercept Pharmaceuticals, Inc. |
|
By: /s/Masayo Tada_______________ (signature) |
By: /s/ Mark Pruzanski__________ (signature) |
|
Print Name: Masayo Tada__________ |
Print Name: Mark Pruzanski_____ |
|
Title: C.E.O._____________________ |
Title: C.E.O.__________________ |
|
Date: 2/13/2018 |
Date: 2/9/2018 |
CONFIDENTIAL TREATMENT REQUESTED UNDER 17 C.F.R. SECTIONS 200.80(b)(4) AND 240.24b-2. [*****] INDICATES OMITTED MATERIAL THAT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST FILED SEPARATELY WITH THE COMMISSION. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE COMMISSION.
Exhibit A
The DSP Improvements assigned to Intercept
| Country | Title | Serial No. | Filing Date | Status |
| PCT and Taiwan | Film–Coated Tablet Having High Chemical Stability of Active Ingredient | PCT/JP2017/013214 and TW106110706 | March 30, 2017 | Pending |
| PCT and Taiwan | Oral Preparation Having Exceptional Elutability | PCT/JP2017/013221 and TW106110707 | March 30, 2017 | Pending |
| PCT and Taiwan | MEDICINE OBTAINED BY COMBINING FXR AGONIST AND ARB | PCT/JP2017/012448 and TW106110180 | March 27, 2017 | Pending |
CONFIDENTIAL TREATMENT REQUESTED UNDER 17 C.F.R. SECTIONS 200.80(b)(4) AND 240.24b-2. [*****] INDICATES OMITTED MATERIAL THAT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST FILED SEPARATELY WITH THE COMMISSION. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE COMMISSION.
Exhibit B
The Joint Improvements
| Country | Title | Serial No. | Filing Date | Status |
| See below caption*1 | Compositions of Obeticholic Acid and Methods of Use | PCT/US2016/029369; US15/139,138 and their worldwide counterparts *1 | April 26, 2016 | Pending |
*1) Total 44 of the countries or areas where the patent applications have been filed are as follows:
United Arab Emirates; Argentina (12-month, non-PCT country); Australia; Bosnia & Herzegovina; Brazil; Belize; Canada; Chile; China (current Sumitomo Territory); Colombia; Costa Rica; Algeria; Eurasia; Ecuador; Egypt; Europe; Guatemala; Honduras; Indonesia (former Option Country); Israel; India; Japan (former Sumitomo Territory); South Korea (former Sumitomo Territory); Morocco; Montenegro; Mexico; Malaysia (former Option Country); Nicaragua; New Zealand; Oman; Panama; Peru; Philippines (former Option Country); Qatar; Saudi Arabia; Singapore(former Option Country); El Salvador; Taiwan (12-month, non-PCT country) (former Option Country); Thailand (former Option Country); Tunisia; Ukraine; United States; Vietnam (former Option Country); South Africa.
CONFIDENTIAL TREATMENT REQUESTED UNDER 17 C.F.R. SECTIONS 200.80(b)(4) AND 240.24b-2. [*****] INDICATES OMITTED MATERIAL THAT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST FILED SEPARATELY WITH THE COMMISSION. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE COMMISSION.
Exhibit C
| Country | Trademark |
Registration date |
Registration number |
International class | |
| Japan | OCALIVA | January 29, 2016 | 5823112 | 5 | |
 |
January 29, 2016 | 5823113 | 5 | Katakana for ”OCALIVA” | |
 |
January 29, 2016 | 5823114 | 5 | Katakana for ”OCALIVA” | |
| QIJUVA | January 29, 2016 | 5823115 | 5 | ||
 |
January 29, 2016 | 5823116 | 5 | Katakana for ”QIJUVA” | |
 |
January 29, 2016 | 5823117 | 5 | Katakana for ”QIJUVA” | |
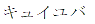 |
January 29, 2016 | 5823118 | 5 | Katakana for ”QIJUVA” | |
 |
January 29, 2016 | 5823119 | 5 | Katakana for ”QIJUVA” | |
 |
January 29, 2016 | 5823129 | 5 | ||
| Korea | OCALIVA | August 23, 2016 | 40-1198094 | 5 | |
| QIJUVA | August 23, 2016 | 40-1198095 | 5 | ||
 |
August 23, 2016 | 40-1198096 | 5 |
CONFIDENTIAL TREATMENT REQUESTED UNDER 17 C.F.R. SECTIONS 200.80(b)(4) AND 240.24b-2. [*****] INDICATES OMITTED MATERIAL THAT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST FILED SEPARATELY WITH THE COMMISSION. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE COMMISSION.
