Attached files
| file | filename |
|---|---|
| 8-K - 8-K INVESTOR PRESENTATION - ANTARES PHARMA, INC. | atrs-8k_20180509.htm |

NASDAQ: ATRS Deutsche Bank Securities 43rd Annual Health Care Conference May 9, 2018 Robert F. Apple President & Chief Executive Officer Exhibit 99.1

Safe Harbor Statement This presentation contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: the Company’s ability to resolve the deficiencies identified by the FDA in the Complete Response Letter for XYOSTED™, FDA approval of the Company’s NDA for XYOSTED™ and future market acceptance and revenue for XYOSTED™; future market acceptance and revenue from AMAG Pharmaceutical’s Makena subcutaneous auto injector product; successful completion of the transaction with Ferring International Center, S.A. and satisfaction of the various conditions in the Ferring asset purchase agreement and payment of the full purchase price; Teva’s ability to successfully commercialize VIBEX® Sumatriptan Injection USP and the amount of revenue from the same; continued growth of prescriptions and sales of OTREXUP®; the timing and results of the Company’s or its partners’ research projects or clinical trials of product candidates in development; actions by the FDA or other regulatory agencies with respect to the products or product candidates of the Company or of its partners including Teva’s ANDA’s for epinephrine, teriparatide and exenatide; continued growth in product, development, licensing and royalty revenue; the Company’s ability to obtain financial and other resources for its research, development, clinical, and commercial activities and other statements regarding matters that are not historical facts, and involve predictions. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially different from any future results, performance, achievements or prospects expressed in or implied by such forward-looking statements. In some cases you can identify forward-looking statements by terminology such as ''may'', ''will'', ''should'', ''would'', ''expect'', ''intend'', ''plan'', ''anticipate'', ''believe'', ''estimate'', ''predict'', ''potential'', ''seem'', ''seek'', ''future'', ''continue'', or ''appear'' or the negative of these terms or similar expressions, although not all forward-looking statements contain these identifying words. Additional information concerning these and other factors that may cause actual results to differ materially from those anticipated in the forward-looking statements is contained in the "Risk Factors" section of the Company's Annual Report on Form 10-K for the year ended December 31, 2017, and in the Company's other periodic reports and filings with the Securities and Exchange Commission. The Company cautions investors not to place undue reliance on the forward-looking statements contained in this press release. The Company cautions investors not to place undue reliance on the forward-looking statements contained in this presentation. All forward-looking statements are based on information currently available to the Company on the date hereof, and the Company undertakes no obligation to revise or update these forward-looking statements to reflect events or circumstances after the date of this presentation, except as required by law. ©2018 Copyright Antares Pharma, Inc. All Rights Reserved.

Antares Pharma A Growing Specialty Pharmaceutical Company with 2017 revenue of $54.5 million An Innovative Leader in Self-Administered Injection Technology Novel Drug Delivery Technology Can Provide Multiple Product Opportunities and Life Cycle Management Solutions Strong Balance Sheet - $28.1 million in cash and short term investments at March 31, 2018

Antares Pharma Two combination products approved and on the U.S. market (OTREXUP®, Sumatriptan) AMAG’s Makena® subcutaneous auto injector launched in the U.S. in March 2018 utilizing our QuickShot® device One NDA for a Drug Device Combination Product submitted by Antares to the FDA (XYOSTED™) Three ANDA Drug Device Combination Products submitted by Teva to U.S. FDA with first to file status (Epinephrine pen, Exenatide, Teriparatide)
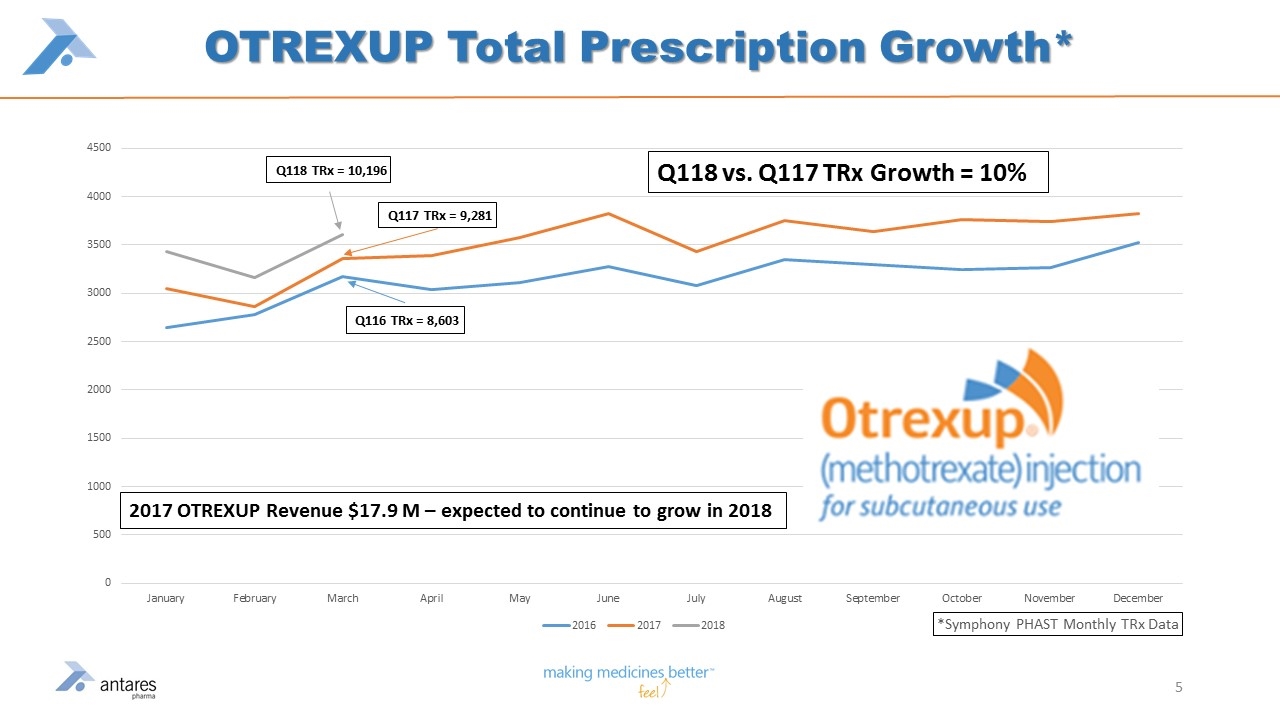
OTREXUP Total Prescription Growth* 2017 OTREXUP Revenue $17.9 M – expected to continue to grow in 2018
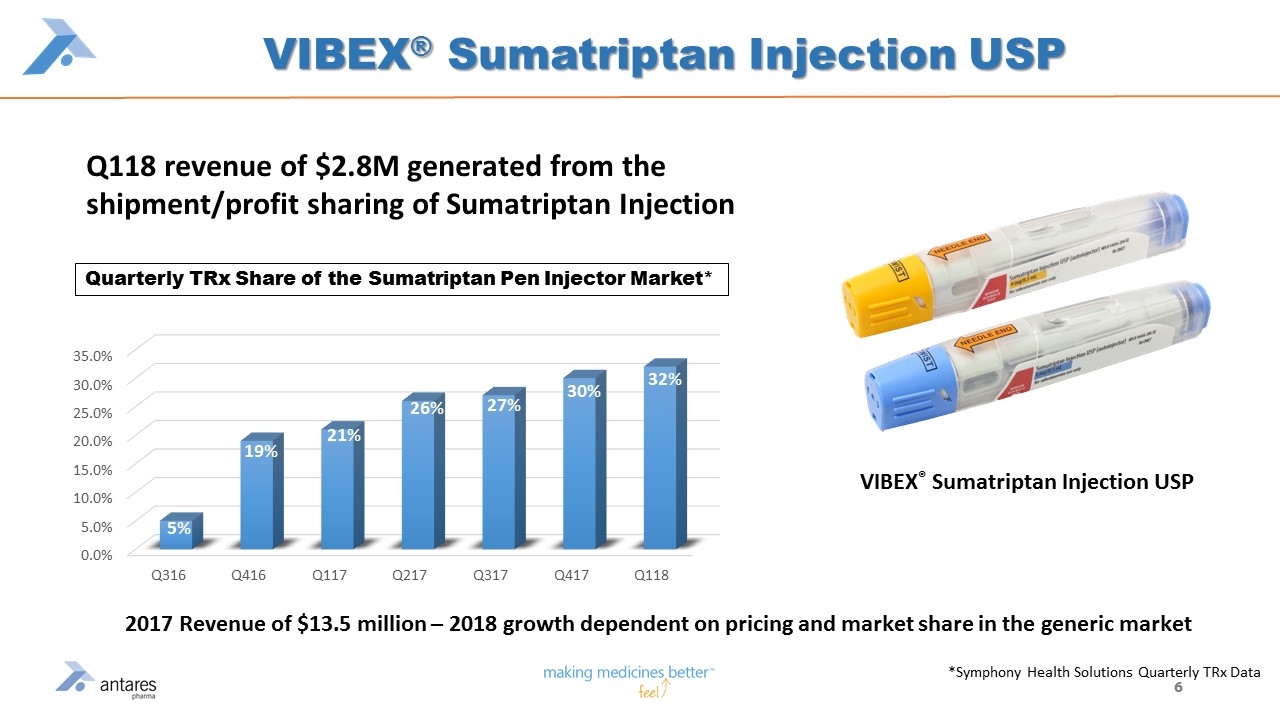
VIBEX® Sumatriptan Injection USP Q118 revenue of $2.8M generated from the shipment/profit sharing of Sumatriptan Injection VIBEX® Sumatriptan Injection USP *Symphony Health Solutions Quarterly TRx Data 30% 32% Quarterly TRx Share of the Sumatriptan Pen Injector Market* 2017 Revenue of $13.5 million – 2018 growth dependent on pricing and market share in the generic market
Makena® Subcutaneous Auto Injector– FDA Approved AMAG/Makena® collaboration began in 2014 Alliance terms: Cost plus product transfer price (fully packaged QuickShot® device), plus royalty on net sales and sales performance milestones QuickShot® device used to develop a once-weekly subcutaneous injection of Makena® Potentially better patient compliance and easier administration Currently administered IM with a large-gauge needle from a single dose vial, QuickShot® product administered sub-Q through a fine-gauge nonvisible needle First FDA approval of QuickShot® auto injector Makena® sNDA approved by FDA February 14, 2018 AMAG launched Makena® SC AI March 2018 AMAG recently disclosed that 47% of new patient enrollments in the Makena Cares Connection Program were for the auto injector according to the most recent weekly enrollment data – off to a fast start
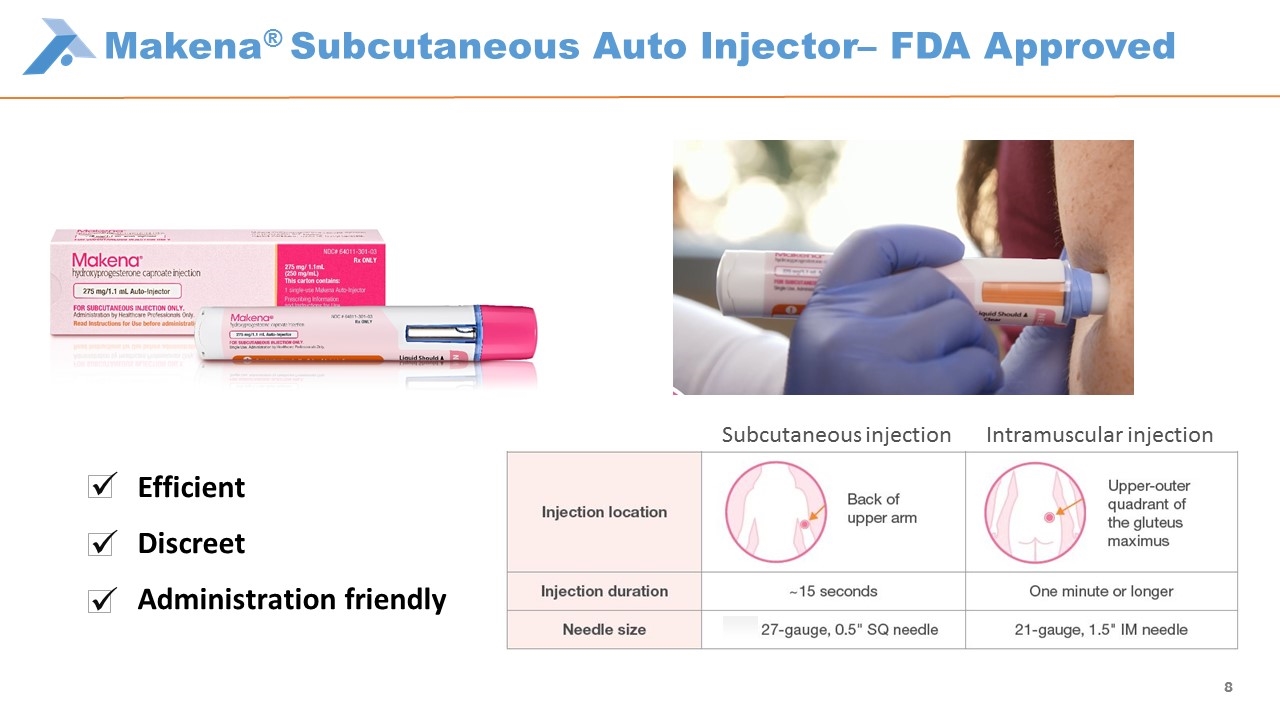
Makena® Subcutaneous Auto Injector– FDA Approved Subcutaneous injection Intramuscular injection Efficient Discreet Administration friendly ü ü ü
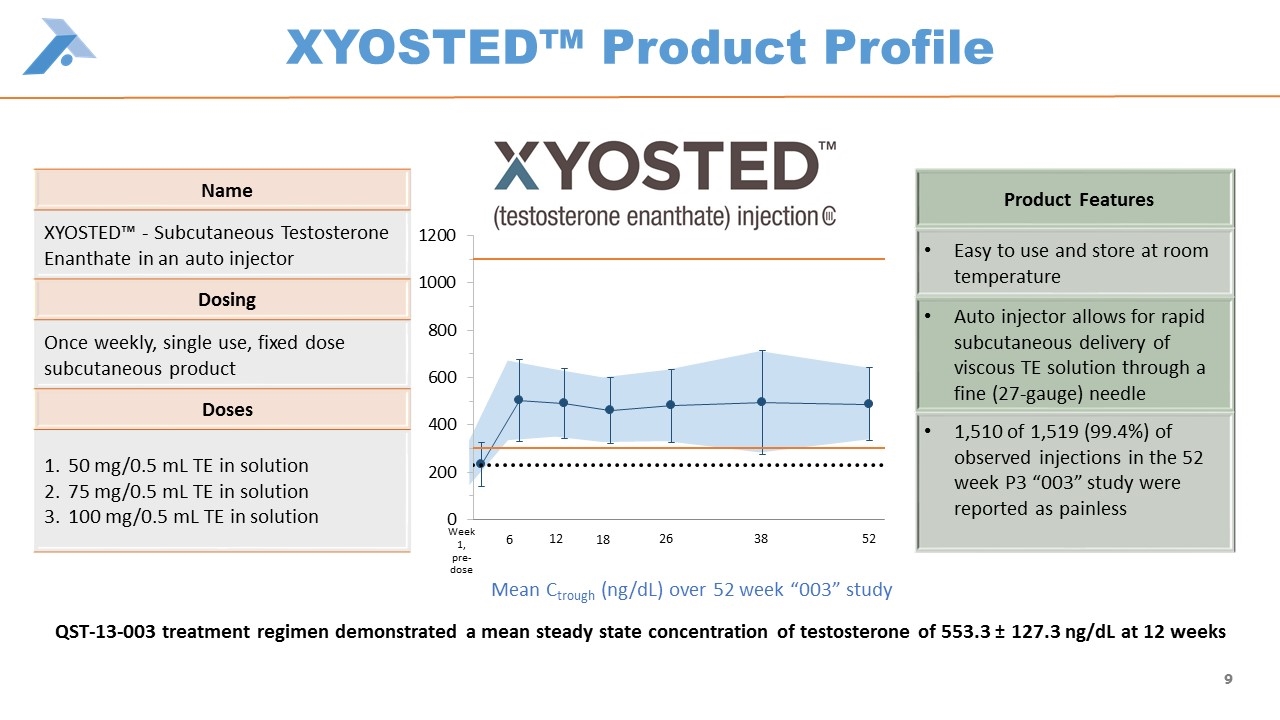
Name XYOSTED™ - Subcutaneous Testosterone Enanthate in an auto injector Dosing Once weekly, single use, fixed dose subcutaneous product Doses 50 mg/0.5 mL TE in solution 75 mg/0.5 mL TE in solution 100 mg/0.5 mL TE in solution Product Features Easy to use and store at room temperature Auto injector allows for rapid subcutaneous delivery of viscous TE solution through a fine (27-gauge) needle 1,510 of 1,519 (99.4%) of observed injections in the 52 week P3 “003” study were reported as painless XYOSTED™ Product Profile Mean Ctrough (ng/dL) over 52 week “003” study 6 12 18 26 38 52 Week 1, pre-dose QST-13-003 treatment regimen demonstrated a mean steady state concentration of testosterone of 553.3 ± 127.3 ng/dL at 12 weeks
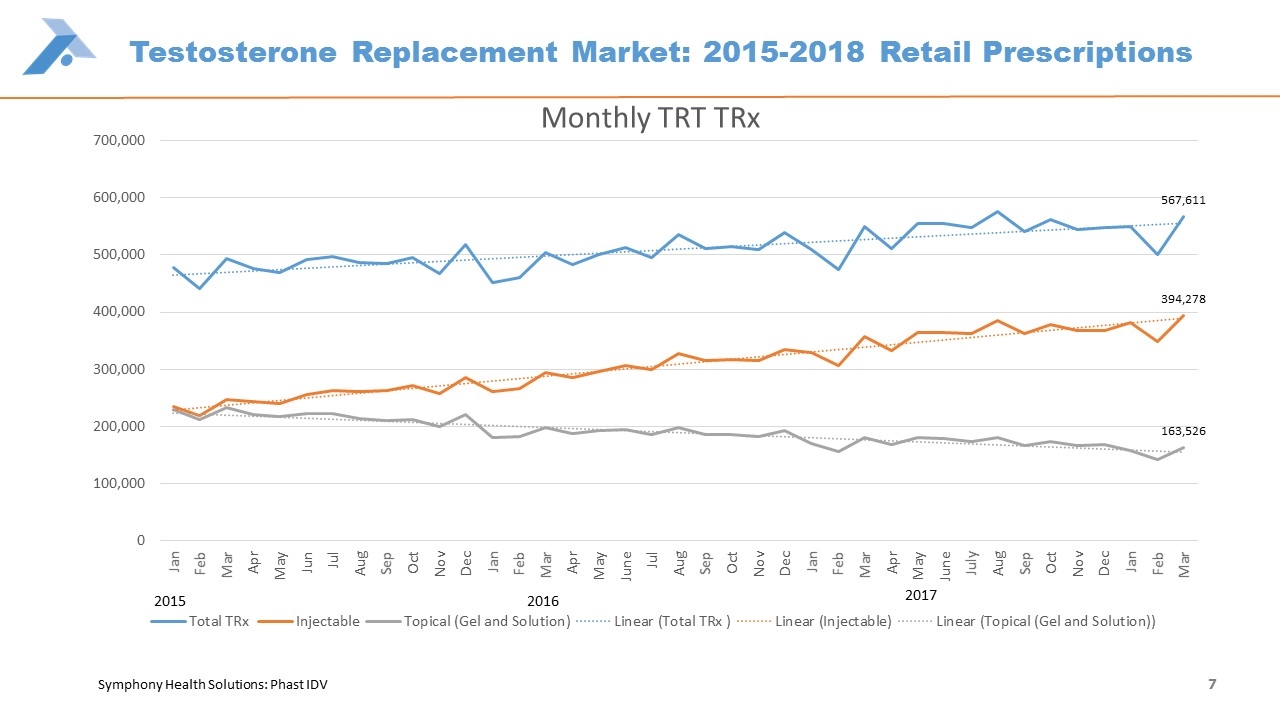
Testosterone Replacement Market: 2015-2018 Retail Prescriptions Symphony Health Solutions: Phast IDV 2016 2015 7
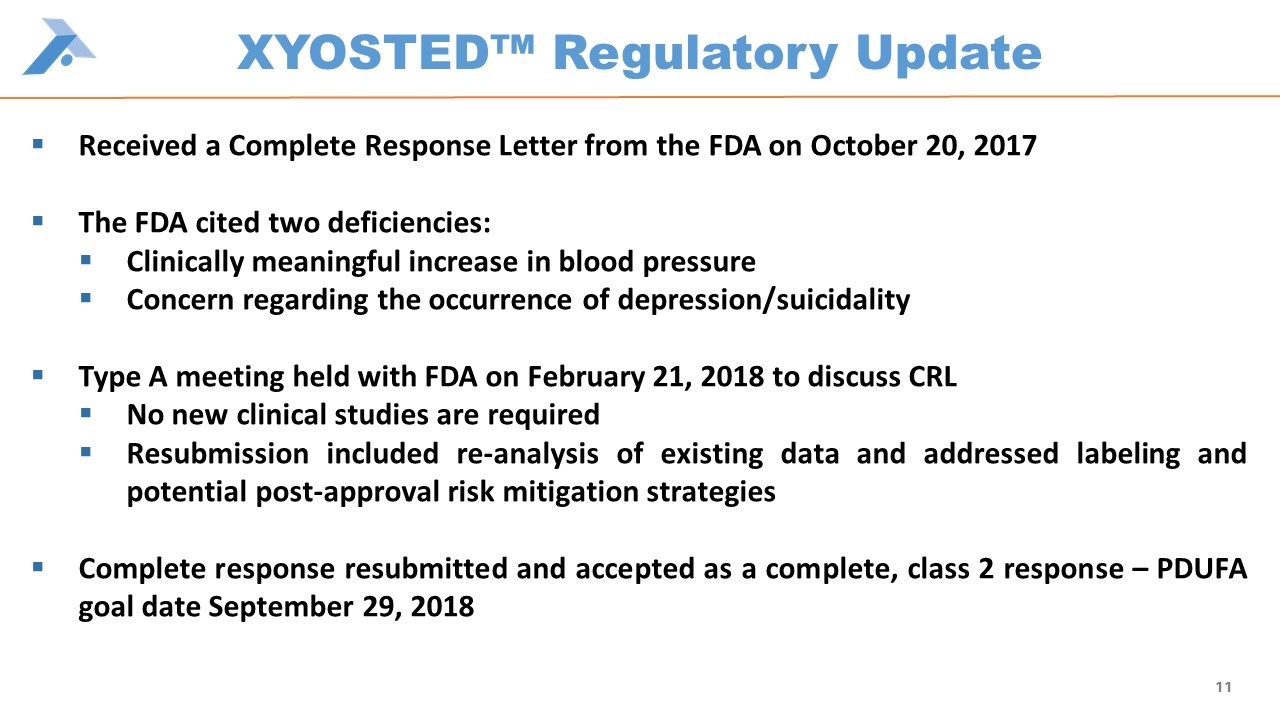
XYOSTED™ Regulatory Update Received a Complete Response Letter from the FDA on October 20, 2017 The FDA cited two deficiencies: Clinically meaningful increase in blood pressure Concern regarding the occurrence of depression/suicidality Type A meeting held with FDA on February 21, 2018 to discuss CRL No new clinical studies are required Resubmission included re-analysis of existing data and addressed labeling and potential post-approval risk mitigation strategies Complete response resubmitted and accepted as a complete, class 2 response – PDUFA goal date September 29, 2018

Teva Alliance Development Update Epinephrine Teva filed against EpiPen® (epinephrine) - ANDA still under active review at FDA - ~$22 M pre-launch devices shipped to date Exenatide Teriparatide Teva filed against Byetta® (exenatide) and they are working through the regulatory approval process using the ANDA pathway. ATRS believes Teva has first to file status and 180 days of marketing exclusivity, launch pending approval Teva filed against Forteo® (teriparatide) and they are working through the regulatory approval process using the ANDA pathway. Antares believes Teva has first to file status and 180 days of marketing exclusivity. US patent litigation has been settled - Lilly does not expect competitive products to enter the market earlier than 2H19. Approved in Europe in 17 countries which addresses the majority of value in Europe – awaiting IP clearance prior to launch

ZOMAJET™ Sale to Ferring Pharmaceuticals Needle-free asset sale to Ferring executed October 10, 2017 for up to $14.5 million Milestone Payments: $2.0M paid upon signing in October 2017 $2.75M received Q118 $4.75M at Closing (2H 2018) $5M at Completion (2H 2018) Continue to earn royalties and product sales until completion of transaction
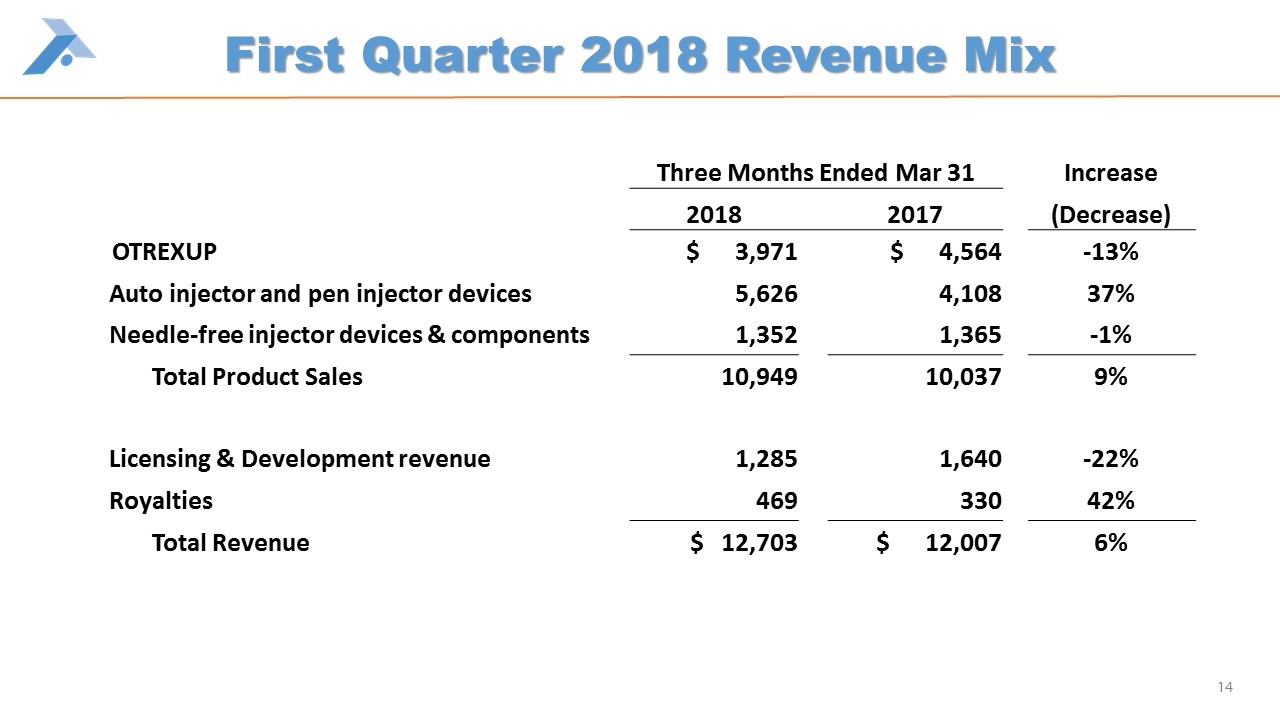
First Quarter 2018 Revenue Mix Three Months Ended Mar 31 Increase 2018 2017 (Decrease) OTREXUP $ 3,971 $ 4,564 -13% Auto injector and pen injector devices 5,626 4,108 37% Needle-free injector devices & components 1,352 1,365 -1% Total Product Sales 10,949 10,037 9% Licensing & Development revenue 1,285 1,640 -22% Royalties 469 330 42% Total Revenue $ 12,703 $ 12,007 6%
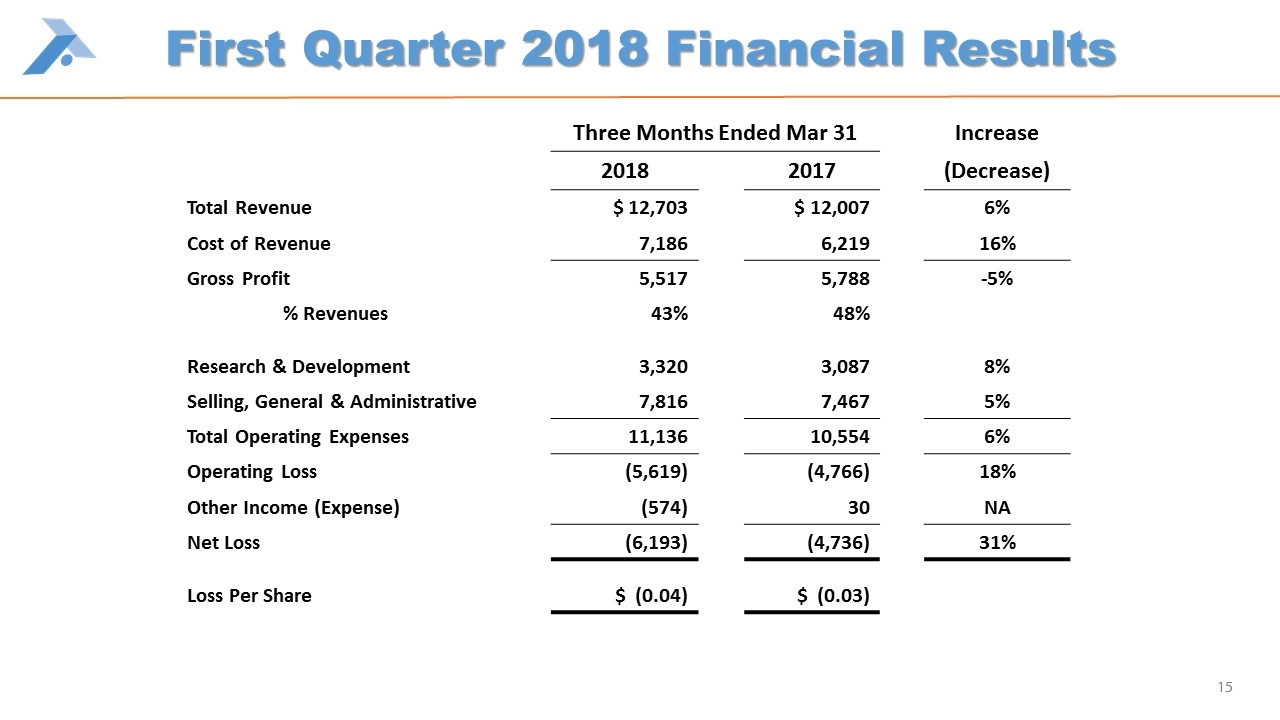
First Quarter 2018 Financial Results Three Months Ended Mar 31 Increase 2018 2017 (Decrease) Total Revenue $ 12,703 $ 12,007 6% Cost of Revenue 7,186 6,219 16% Gross Profit 5,517 5,788 -5% % Revenues 43% 48% Research & Development 3,320 3,087 8% Selling, General & Administrative 7,816 7,467 5% Total Operating Expenses 11,136 10,554 6% Operating Loss (5,619) (4,766) 18% Other Income (Expense) (574) 30 NA Net Loss (6,193) (4,736) 31% Loss Per Share $ (0.04) $ (0.03)
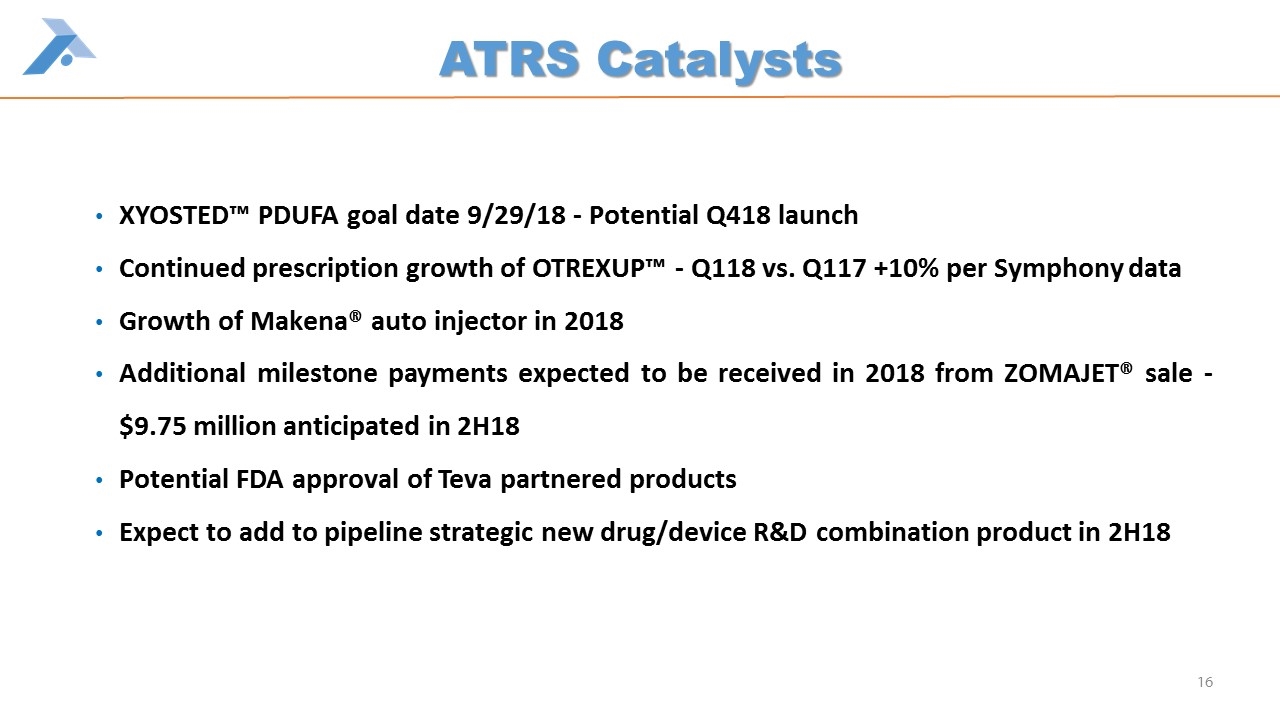
ATRS Catalysts XYOSTED™ PDUFA goal date 9/29/18 - Potential Q418 launch Continued prescription growth of OTREXUP™ - Q118 vs. Q117 +10% per Symphony data Growth of Makena® auto injector in 2018 Additional milestone payments expected to be received in 2018 from ZOMAJET® sale - $9.75 million anticipated in 2H18 Potential FDA approval of Teva partnered products Expect to add to pipeline strategic new drug/device R&D combination product in 2H18 16

NASDAQ: ATRS Deutsche Bank Securities 43rd Annual Health Care Conference May 9, 2018 Robert F. Apple President & Chief Executive Officer
