Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Theravance Biopharma, Inc. | a18-13044_18k.htm |
| EX-99.1 - EX-99.1 - Theravance Biopharma, Inc. | a18-13044_1ex99d1.htm |
1Q 2018 Financial Results and Business Update May 8, 2018 Theravance Biopharma, Inc. (NASDAQ: TBPH)

Cautionary Statement Regarding Forward-Looking Statements Under the safe harbor provisions of Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, the company cautions investors that any forward-looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward-looking statements or projections. Examples of forward-looking statements in this presentation include statements relating to the company’s the Company’s strategies, plans and objectives, including financial and operating results, sales targets, the Company’s regulatory strategies, timing and results of clinical studies, the potential characteristics, benefits and mechanisms of action of the Company’s product and product candidates and the Company’s expectations for product candidates through development, potential regulatory approval and commercialization. The company’s forward-looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results to be materially different than those from those reflected in the forward-looking statements, such as risks related to delays or difficulties in commencing, enrolling or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective, risks that product candidates do not obtain approval from regulatory authorities, the feasibility of undertaking future clinical trials for our product candidates based on policies and feedback from regulatory authorities, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with or relying on third parties to discover, develop, conduct clinical studies, manufacture and commercialize products and risks associated with establishing and maintaining sales, marketing and distribution capabilities. Other risks affecting the company are described under the heading “Risk Factors” and elsewhere in the company’s Form 10-K filed with the Securities and Exchange Commission (SEC) on February 28, 2018, and other periodic reports filed with the SEC. In addition to the risks described above and in Theravance Biopharma's filings with the SEC, other unknown or unpredictable factors also could affect Theravance Biopharma's results.
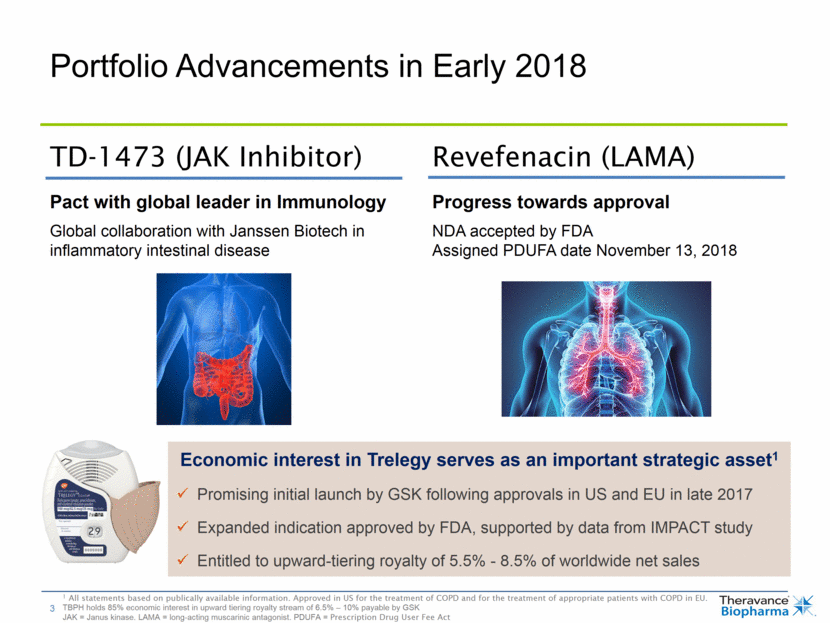
Portfolio Advancements in Early 2018 Economic interest in Trelegy serves as an important strategic asset1 Promising initial launch by GSK following approvals in US and EU in late 2017 Expanded indication approved by FDA, supported by data from IMPACT study Entitled to upward-tiering royalty of 5.5% - 8.5% of worldwide net sales Pact with global leader in Immunology Global collaboration with Janssen Biotech in inflammatory intestinal disease Progress towards approval NDA accepted by FDA Assigned PDUFA date November 13, 2018 TD-1473 (JAK Inhibitor) Revefenacin (LAMA) 1 All statements based on publically available information. Approved in US for the treatment of COPD and for the treatment of appropriate patients with COPD in EU. TBPH holds 85% economic interest in upward tiering royalty stream of 6.5% – 10% payable by GSK JAK = Janus kinase. LAMA = long-acting muscarinic antagonist. PDUFA = Prescription Drug User Fee Act
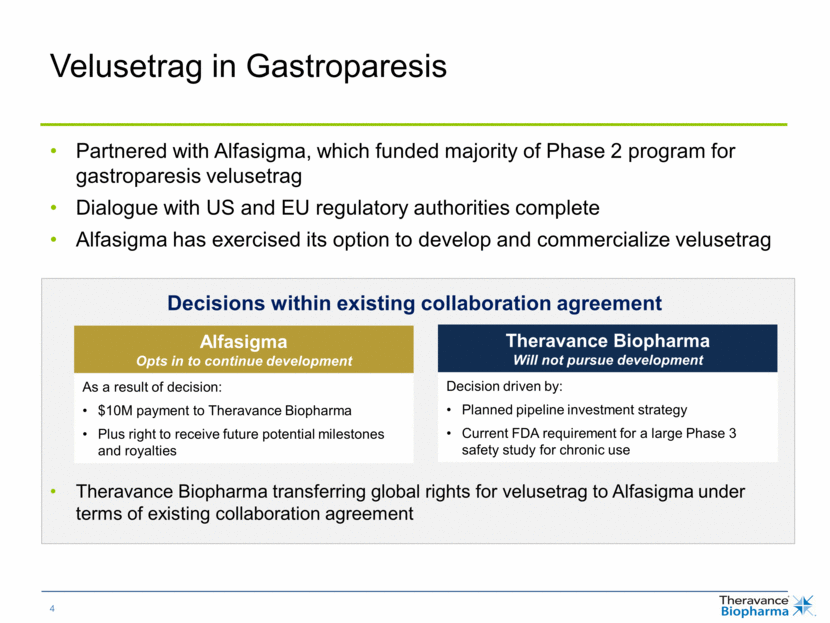
Velusetrag in Gastroparesis Partnered with Alfasigma, which funded majority of Phase 2 program for gastroparesis velusetrag Dialogue with US and EU regulatory authorities complete Alfasigma has exercised its option to develop and commercialize velusetrag Decisions within existing collaboration agreement Theravance Biopharma transferring global rights for velusetrag to Alfasigma under terms of existing collaboration agreement Alfasigma Opts in to continue development Theravance Biopharma Will not pursue development As a result of decision: $10M payment to Theravance Biopharma Plus future potential payments and royalties Decision driven by: Planned pipeline investment strategy Current FDA requirement for a large Phase 3 safety study
Enhancing Focus on Strategic Priorities in 2018 Commitment to developing transformational medicines 1 Economic interest. FF/UMEC/VI= Fluticasone Furoate/Umeclidinium/Vilanterol. Innoviva formerly Theravance, Inc. NSRI = norepinephrine serotonin reuptake inhibitor; COPD = chronic obstructive pulmonary disease nOH = neurogenic orthostatic hypotension Revefenacin TD-1473 Research Opportunities to Create Transformational Medicines TD-9855 Trelegy Ellipta Managed by GSK and Innoviva1 Strategic Asset Nebulized LAMA in COPD (PDUFA date November 13, 2018) Intestinally-restricted JAK inhibitor for inflammatory intestinal diseases NSRI in symptomatic nOH, an orphan condition Inhaled JAK inhibitor for serious respiratory diseases (FF/UMEC/VI) Single inhaler triple therapy in COPD
TD-1473: Global Collaboration Agreement with Janssen Biotech Shared belief in TD-1473 as a localized medicine with potential to transform the treatment landscape in inflammatory intestinal disease Meaningful program enhancements for TD-1473 Apply Janssen expertise in IBD to optimize clinical strategy and execution Accelerate clinical development; plan to advance UC and Crohn’s in parallel Maximize worldwide commercial opportunity of TD-1473 Attractive deal economics reducing overall financial risk Phase 2b/3 study in ulcerative colitis and Phase 2 study in Crohn’s disease expected to initiate in 2H18
TD-9855: Exploratory Results Expected Mid-Year Intention to seek expedited development path Purpose: Phase 2a study to evaluate the effect of TD-9855 in improving symptoms of orthostatic intolerance Understanding totality of symptoms encompasses tests of function, orthostatic hypotension status, and other measures Dizziness a cardinal symptom Interest in patients who fail to accomplish 10-minute standing time at baseline Part A Part A: Single ascending dose portion Observed emerging signals of benefits to patients in Part A Part B: Single dose (response dose) or placebo Enrolling patients in open label design Up to 24 weeks (20 weeks dosing, 4 week wash out) Primary endpoint at 4 weeks Part C: Multiple dose portion to assess durability of response Part B Part C (Extension)
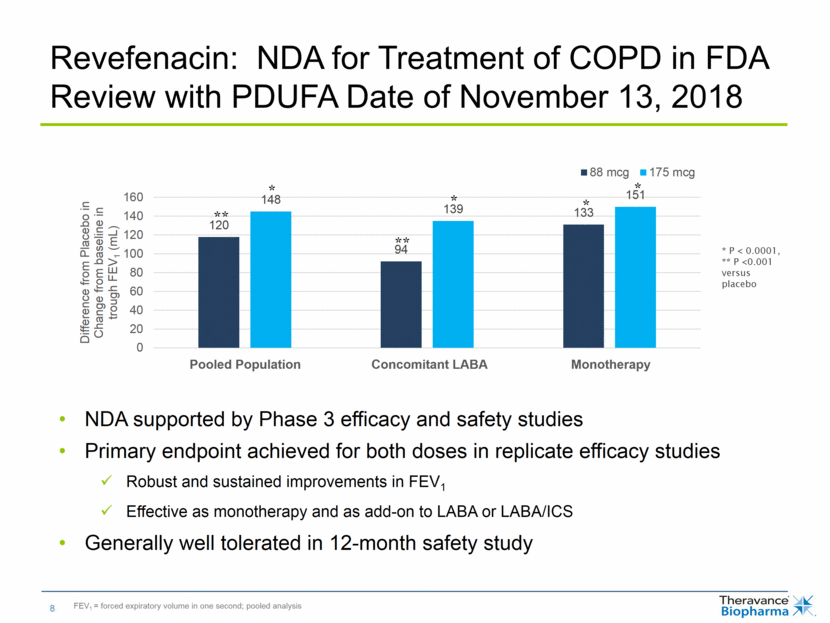
NDA supported by Phase 3 efficacy and safety studies Primary endpoint achieved for both doses in replicate efficacy studies Robust and sustained improvements in FEV1 Effective as monotherapy and as add-on to LABA or LABA/ICS Generally well tolerated in 12-month safety study Revefenacin: NDA for Treatment of COPD in FDA Review with PDUFA Date of November 13, 2018 FEV1 = forced expiratory volume in one second; pooled analysis * P < 0.0001, ** P <0.001 versus placebo * ** * * * ** Monotherapy Concomitant LABA Pooled Population
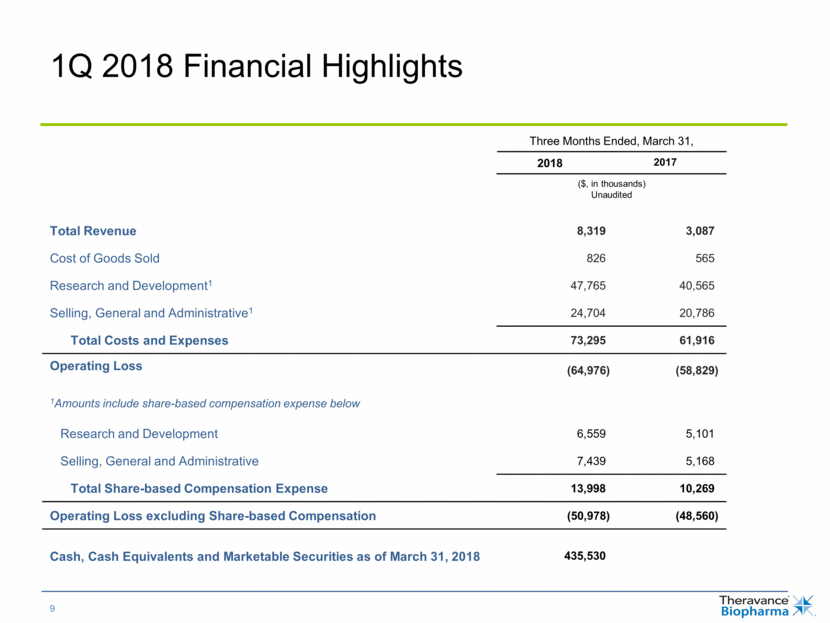
1Q 2018 Financial Highlights Three Months Ended, March 31, 2018 2017 ($, in thousands) Unaudited Total Revenue 8,319 3,087 Cost of Goods Sold 826 565 Research and Development1 47,765 40,565 Selling, General and Administrative1 24,704 20,786 Total Costs and Expenses 73,295 61,916 Operating Loss (64,976) (58,829) 1Amounts include share-based compensation expense below Research and Development 6,559 5,101 Selling, General and Administrative 7,439 5,168 Total Share-based Compensation Expense 13,998 10,269 Operating Loss excluding Share-based Compensation (50,978) (48,560) Cash, Cash Equivalents and Marketable Securities as of March 31, 2018 435,530
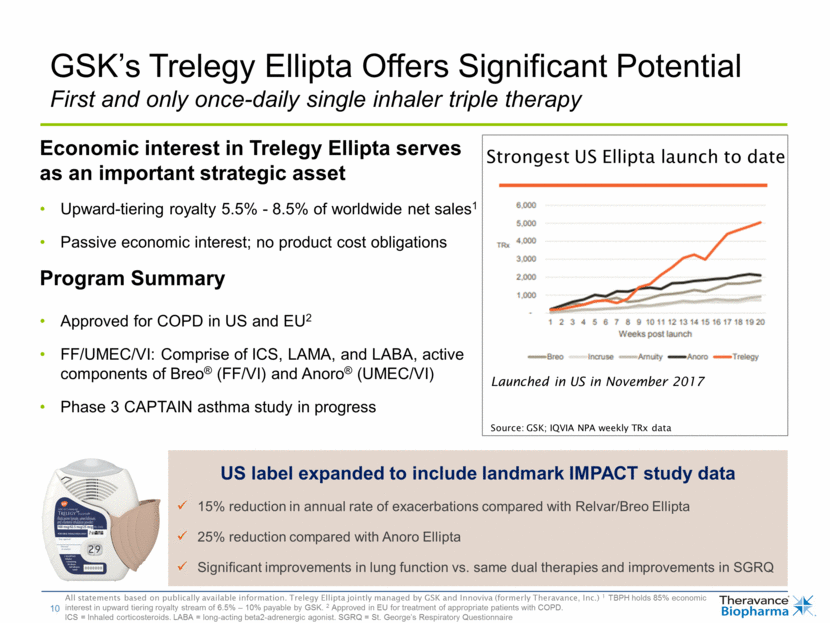
Economic interest in Trelegy Ellipta serves as an important strategic asset Upward-tiering royalty 5.5% - 8.5% of worldwide net sales1 Passive economic interest; no product cost obligations Program Summary Approved for COPD in US and EU2 FF/UMEC/VI: Comprise of ICS, LAMA, and LABA, active components of Breo® (FF/VI) and Anoro® (UMEC/VI) Phase 3 CAPTAIN asthma study in progress GSK’s Trelegy Ellipta Offers Significant Potential First and only once-daily single inhaler triple therapy All statements based on publically available information. Trelegy Ellipta jointly managed by GSK and Innoviva (formerly Theravance, Inc.) 1 TBPH holds 85% economic interest in upward tiering royalty stream of 6.5% – 10% payable by GSK. 2 Approved in EU for treatment of appropriate patients with COPD. ICS = Inhaled corticosteroids. LABA = long-acting beta2-adrenergic agonist. SGRQ = St. George’s Respiratory Questionnaire US label expanded to include landmark IMPACT study data 15% reduction in annual rate of exacerbations compared with Relvar/Breo Ellipta 25% reduction compared with Anoro Ellipta Significant improvements in lung function vs. same dual therapies and improvements in SGRQ Strongest US Ellipta launch to date So Launched in US in November 2017 Source: GSK; IQVIA NPA weekly TRx data
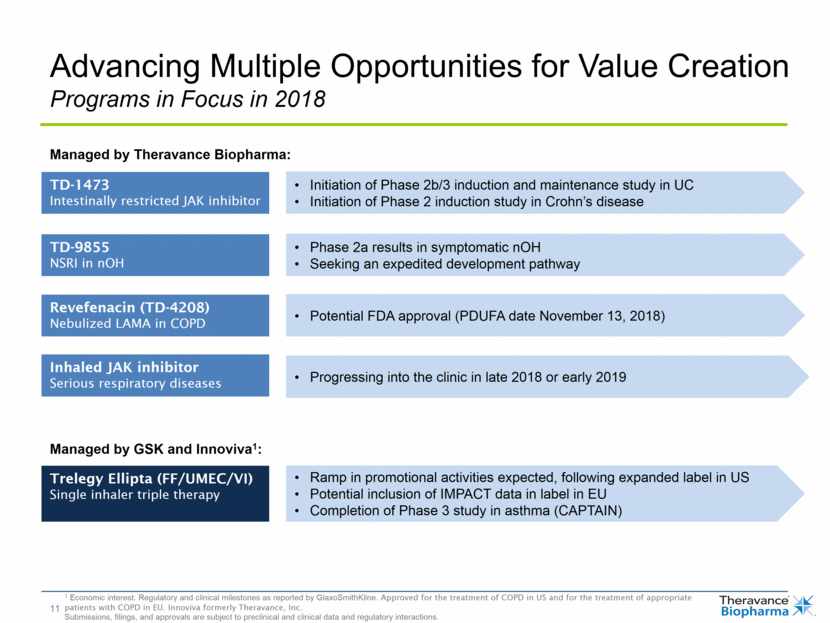
Advancing Multiple Opportunities for Value Creation Programs in Focus in 2018 TD-1473 Intestinally restricted JAK inhibitor TD-9855 NSRI in nOH Revefenacin (TD-4208) Nebulized LAMA in COPD Inhaled JAK inhibitor Serious respiratory diseases Trelegy Ellipta (FF/UMEC/VI) Single inhaler triple therapy Initiation of Phase 2b/3 induction and maintenance study in UC Initiation of Phase 2 induction study in Crohn’s disease Phase 2a results in symptomatic nOH Seeking an expedited development pathway Potential FDA approval (PDUFA date November 13, 2018) Progressing into the clinic in late 2018 or early 2019 Ramp in promotional activities expected, following expanded label in US Potential inclusion of IMPACT data in label in EU Completion of Phase 3 study in asthma (CAPTAIN) Managed by GSK and Innoviva1: Managed by Theravance Biopharma: 1 Economic interest. Regulatory and clinical milestones as reported by GlaxoSmithKline. Approved for the treatment of COPD in US and for the treatment of appropriate patients with COPD in EU. Innoviva formerly Theravance, Inc. Submissions, filings, and approvals are subject to preclinical and clinical data and regulatory interactions.