Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PIERIS PHARMACEUTICALS, INC. | form8-k.htm |

Deutsche Bank Health Care Conference Presentation May 2018 (Nasdaq: PIRS)

Forward Looking Statements This press release contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, references to novel technologies and methods and our business and product development plans, including the advancement of our proprietary and co- development programs into and through the clinic. Actual results could differ from those projected in any forward-looking statements due to numerous factors. Such factors include, among others, our ability to raise the additional funding we will need to continue to pursue our business and product development plans; the inherent uncertainties associated with developing new products or technologies and operating as a development stage company; our ability to develop, complete clinical trials for, obtain approvals for and commercialize any of our product candidates, including our ability to recruit and enroll patients in our studies; our ability to address the requests of the FDA; competition in the industry in which we operate and market conditions. These forward-looking statements are made as of the date of this press release, and we assume no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law. Investors should consult all of the information set forth herein and should also refer to the risk factor disclosure set forth in the reports and other documents we file with the SEC available at www.sec.gov, including without limitation the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2017 and the Company's Quarterly Reports on Form 10-Q. 2

Anticalin Proteins – A Novel Therapeutic Class Features Benefits Derived from lipocalins No observed (human epithelial proteins) immunogenicity to date Engineerable binding pocket Potent target engagement Unique bi/multispecific fusion Engineerable scaffold proteins Enhanced delivery, e.g., Small size (1/8th the size of a mAb) Inhaled therapeutics Our pipeline addresses clinically-validated targets in new ways by leveraging unique features of the Anticalin® protein drug class, effectively taking reduced target biology risk 3

Anticalin-based Drug Candidates can be Tailored to Multiple Formats Building Blocks Pure Anticalin Anticalin Multispecific Proteins Fc-Anticalin Proteins Antibody PRS-060 Fc Multispecific mAb-Anticalin PRS-343 Proteins Potent Multi-target Engagement • Novel MoA • Favorable Drug-like Properties 4

Financial Update (12/31/17) 2018 Anticipated Milestones (in millions) • PRS-343: Initial safety and PD data in 2H18 Cash & Cash Equivalents (proforma)* $172.4 Core Clinical • PRS-060: First-in-human data in 2H18 $0.0 Debt Non-Core • PRS-080: Phase IIa data in 2H18 (safety, PK, Clinical hemoglobin change post 5QW dosing) 2017 Opex $39.3 Next-Generation • Advance multiple programs in immuno-oncology CSO 45.0 Pipeline and respiratory *Includes $82.6 in cash and equivalents at year end plus $12.5 from AstraZeneca, $47.3 net from equity raise, $30 due from Seattle Genetics, and excludes YTD operating cash expenses Pipeline Highlights DISCOVERY PRECLINICAL PHASE I PHASE II PRS-080 PRS-343 PRS-060 Servier PRS-300’s AZ SeaGen Two IO INDs Planned in 2019 Advance additional respiratory programs under the AstraZeneca alliance in 2018 5

Immuno-oncology Franchise Proprietary Clinical • PRS-343: First-in-class bispecific to preferentially activate T cells in the tumor microenvironment (TME) • Committed to advancing several additional tumor-localized costimulatory bispecific fusion proteins Servier Alliance • 5-program deal (all bispecific fusion proteins) • Pieris retains full U.S. rights for 3 out of 5 programs • $31M upfront payment, $1.8B milestone potential • Up to low double-digit royalties on non-codev products Seattle Genetics Collaboration • 3-program partnership based on tumor-localized costimulatory bispecific fusion proteins • Pieris retains opt-in rights for 50/50 global profit split and US commercialization rights on one of the programs • $30 upfront payment, $1.2B milestone potential • Up to double-digit royalties on non-codev products 6

PRS-343: Why did we Design This? HER2-targeting antibody 4-1BB-targeting Anticalin proteins 4-1BB systemically agonizing antibody has shown mono-therapy efficacy yet significant toxicity in the clinic (narrow therapeutic window) PRS-343 preferentially agonizes 4-1BB in the TME by using its anti-HER2 component to drive drug clustering and, therefore, 4-1BB cross-linking 7

PRS-343 Targets Local Biology 4-1BB (CD137) – Key Costimulatory Target T cell costimulation in TME • Marker for tumor-specific T cells in TME • Ameliorates T cell exhaustion • Critical for T cell expansion Tumor-specific • Induces anti-tumor cytolytic activity T cell • Drives central memory T cell differentiation for sustained response Clustering & T cell Activation HER2 – Strongly Validated Tumor Target • Restricted expression on normal tissue High HER2 • Multiple HER2+ tumors with high-unmet need Expression - Bladder, Gastric, Breast and several others - Mediates drug mobilization and immune receptor activation within the tumor bed 8

Multifunctional Immunomodulatory Effects of 4-1BB Therapy anti-4-1BB mAb A B anti-HER2 mAb T cell HER2 x 4-1BB bsAb NK 4-1BB FcγR NK TCR TUMOR DC HER2 TNFα Mφ IL-6 T cell Perforin Granzyme C D Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies Blood 131:49-57, 2018 9

4-1BB Costimulation Induces T Cell Mitochondrial Function and Biogenesis Enabling Cancer Immunotherapeutic Responses: Reversing the Exhausted Phenotype Untreated Immunotherapy Metabolic Support Immunotherapy and (ACT, PD-1) (4-1BB) Metabolic Support (ACT/PD-1+4-1BB) TRC 41BB Mitochondrial Biogenesis Mitochondrial Biogenesis Mitochondrial Biogenesis Mitochondrial Biogenesis Mitochondrial Function Mitochondrial Function Mitochondrial Function Mitochondrial Function T cell Activation T cell Activation T cell Activation T cell Activation Poor Immune Response Immunotherapy response Immunotherapy Improved immunotherapy dependent on metabolic response dependent on response tumor microenvironment T cell stimulation Menk et al J Exp Med 215: 1091-1100, 2018 10

PRS-343: T Cell Activation is HER2 Target-Dependent PRS-343-mediated T Cell activation PRS-343 PRS-343 + excess trastuzumab 11

PRS-343 Shows Bifunctional Activity – Dose-dependent Tumor Growth Inhibition & CD8(+)TIL Expansion in HER2+ Ovarian Cancer Model • PRS-343 shows dose-dependent tumor growth inhibition in HER2-sensitive model • PRS-343 leads to strong and dose-dependent lymphocyte infiltration in tumors; monospecific anti-HER2 mAb (IgG4 backbone) inhibits tumor growth but lacks this immuno-stimulatory activity • Monospecific anti-4-1BB benchmark mAb shows insignificant response compared to isotype control and no significant tumor infiltration of lymphocytes Tumor growth (median) TIL phenotyping by IHC hCD3 hCD4 hCD8 Plot CD3 CD4 CD8 CD45 30 100µg 20 PRS-343 10 Frequency [%] 0 30 CD3 CD4 CD8 CD45 100µg 20 Isotype Ctrl 10 Frequency [%] 0 CD3 CD4 CD8 30 CD45 100µg 20 Incomplete Anti-4-1BB 10 group due to Frequency [%] 0 mortality SK-OV-3 tumor model 12
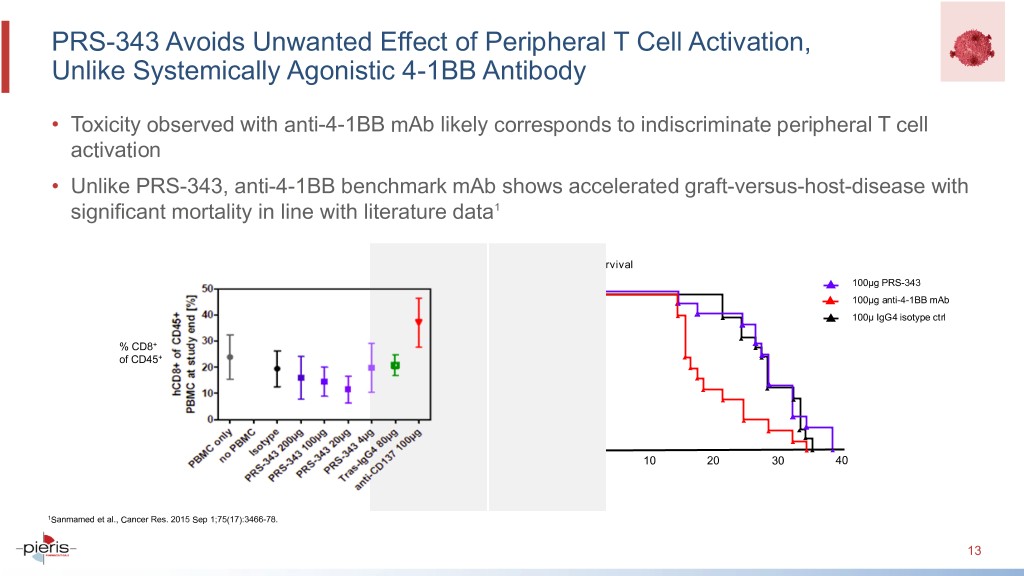
PRS-343 Avoids Unwanted Effect of Peripheral T Cell Activation, Unlike Systemically Agonistic 4-1BB Antibody • Toxicity observed with anti-4-1BB mAb likely corresponds to indiscriminate peripheral T cell activation • Unlike PRS-343, anti-4-1BB benchmark mAb shows accelerated graft-versus-host-disease with significant mortality in line with literature data1 Survival 100μg PRS-343 100 100μg anti-4-1BB mAb 100μ IgG4 isotype ctrl % CD8+ of CD45+ Survival 50 Percent 0 0 10 20 30 40 1Sanmamed et al., Cancer Res. 2015 Sep 1;75(17):3466-78. 13
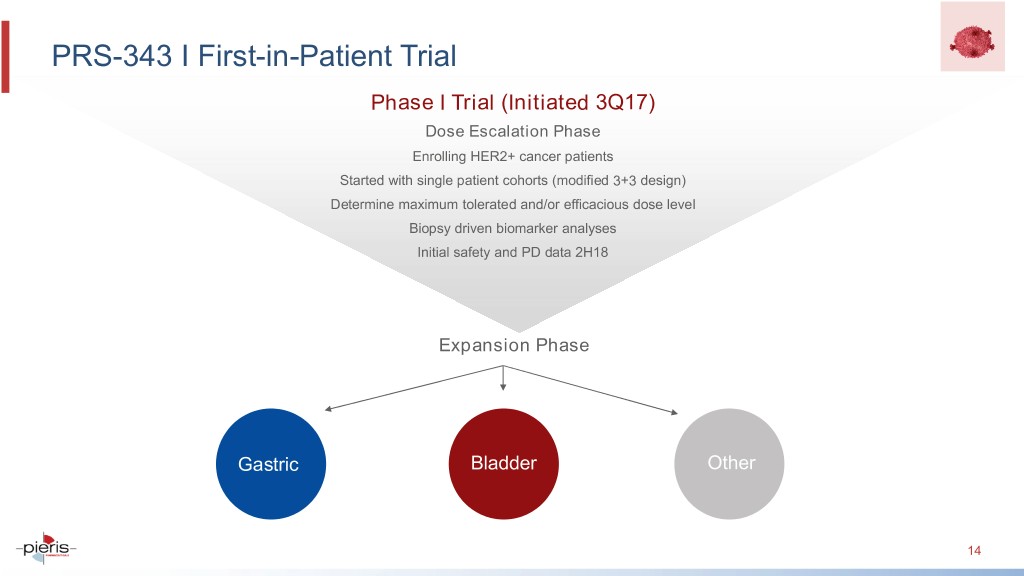
PRS-343 I First-in-Patient Trial Phase I Trial (Initiated 3Q17) Dose Escalation Phase Enrolling HER2+ cancer patients Started with single patient cohorts (modified 3+3 design) Determine maximum tolerated and/or efficacious dose level Biopsy driven biomarker analyses Initial safety and PD data 2H18 Expansion Phase Gastric Bladder Other 14

Multiple Synergies Between 4-1BB and Other Therapeutic Modalities Have Been Demonstrated with in vivo Cancer Models Landscape of potentially synergistic interactions based on the combination of 4-1BB-based and other anticancer therapeutics. Arrows represent described combinations. Other agonistic Cancer monoclonal antibodies vaccines Chemotherapy Radiotherapy Virotherapy 4-1BB-based Immunotherapy ADCC-inducing Checkpoint Therapy with Cytokine gene Adoptive T cell monoclonal antibodies inhibitors gene-transferred T cells therapy therapy with TILs Cytoplasmic domain of 4-1BB as a Sorting of the fittest T cells component in CARTs Costimulation of T cell in culture Coadministration of mAb with TCR-transferred cells Coadministration of mAb with TILs 15

Novel Inhaled Biologics Platform: Targeting Lung Diseases Locally • PRS-060 (Part of AstraZeneca alliance) - First-in-class inhaled IL-4Ra antagonist for asthma - Phase1a SAD initiated in 4Q17 Alliance Highlights - Pieris retains opt-in for co-development/co- commercialization rights in the US 5 committed novel inhaled • Proprietary inhaled discovery programs ongoing Anticalin protein programs Including lead asthma program PRS-060 (IL-4Ra) Retained co-development and co-commercialization (US) options on PRS-060 and up to 2 additional programs $57.5M upfront & Phase I MS in 2017; up to ~$2.1B in milestones, plus double-digit royalties Access to complementary formulation and device know-how for inhaled delivery 16
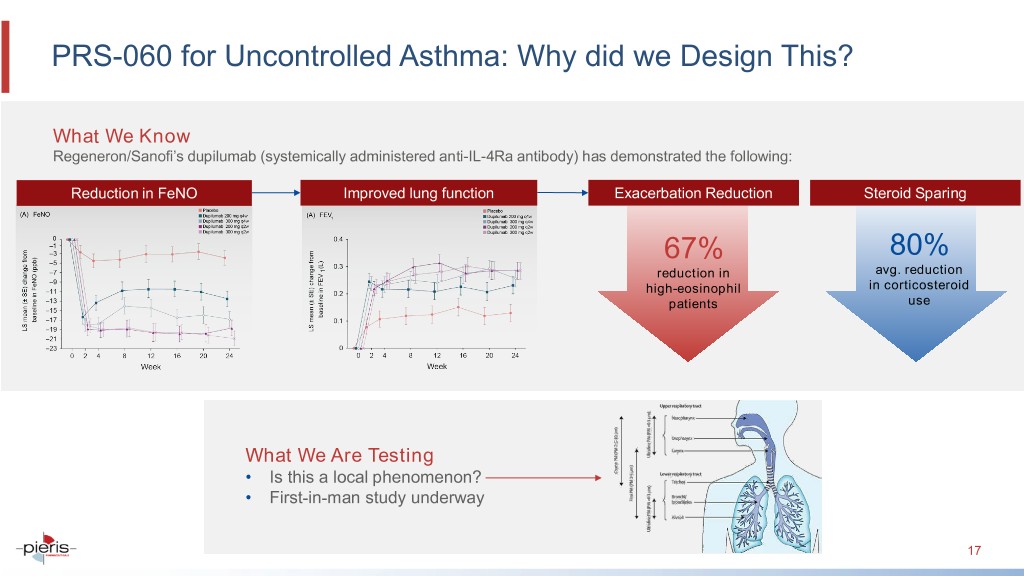
PRS-060 for Uncontrolled Asthma: Why did we Design This? What We Know Regeneron/Sanofi’s dupilumab (systemically administered anti-IL-4Ra antibody) has demonstrated the following: Reduction in FeNO Improved lung function Exacerbation Reduction Steroid Sparing 67% 80% reduction in avg. reduction high-eosinophil in corticosteroid patients use What We Are Testing • Is this a local phenomenon? • First-in-man study underway 17
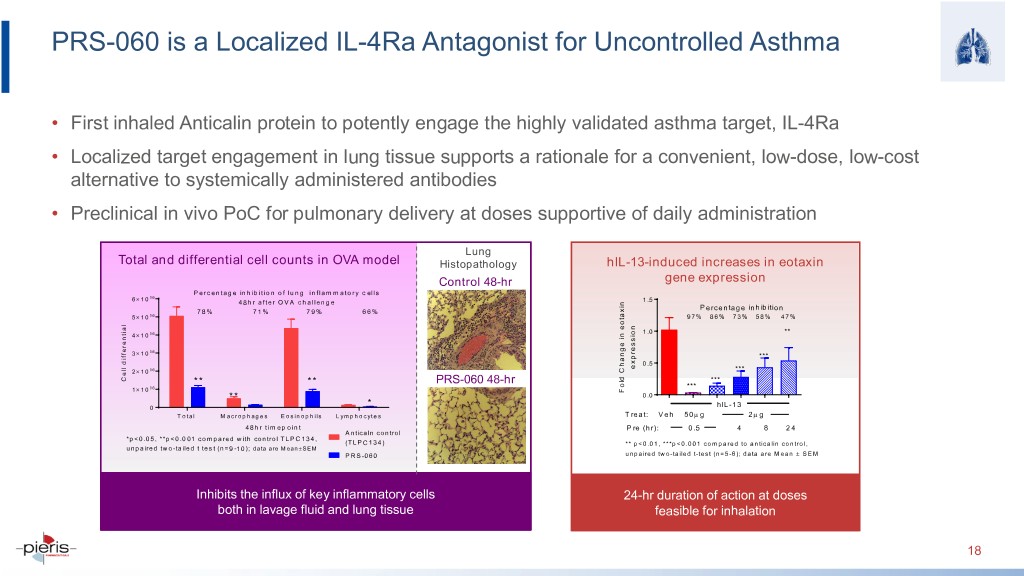
PRS-060 is a Localized IL-4Ra Antagonist for Uncontrolled Asthma • First inhaled Anticalin protein to potently engage the highly validated asthma target, IL-4Ra • Localized target engagement in lung tissue supports a rationale for a convenient, low-dose, low-cost alternative to systemically administered antibodies • Preclinical in vivo PoC for pulmonary delivery at doses supportive of daily administration Lung Total and differential cell counts in OVA model Histopathology hIL-13-induced increases in eotaxin Control 48-hr gene expression P ercentage inhibition of lung inflam m atory cells 6 × 10 06 48hr after OVA challenge 1 .5 Percentage inhibition 78% 71% 79% 66% 5 × 10 06 97% 86% 73% 58% 47% 1 .0 ** 4 × 10 06 × 06 3 10 *** 0 .5 expression 2 × 10 06 *** Cell differential ** ** PRS-060 48-hr *** 06 *** 1 × 10 eotaxin Change in Fold ** 0 .0 * 0 hIL-13 T otal M acrophages Eosinophils Lymphocytes Treat: Veh 50µ g 2µ g 48hr timepoint Pre (hr): 0.5 4 8 24 Anticaln control *p<0.05, **p<0.001 compared with control TLPC134, (TLPC134) ** p<0.01, ***p<0.001 compared to anticalin control, unpaired two-tailed t test (n=9-10); data are Mean± SEM PRS-060 unpaired two-tailed t-test (n=5-6); data are Mean ± SEM Inhibits the influx of key inflammatory cells 24-hr duration of action at doses both in lavage fluid and lung tissue feasible for inhalation 18

Pieris Investment Opportunity • An industry-validated class of novel therapeutics - Anticalin proteins - $120+M in upfront payments and milestones since January 2017 Immuno-oncology • Potentially transformative, wholly owned IO program - Clinical-stage, tumor-targeted 4-1BB bispecific • High-value, inhaled targeted respiratory program - Clinical-stage inhaled IL-4Ra antagonist - partnered with AstraZeneca – retained co-dev/US comm rights • All three anchor partnerships include US-focused commercialization rights Respiratory • Robust IND engine that has yielded several clinical-stage candidates with excellent drug-like properties ANCHOR PARTNERSHIPS 19

Pieris Pharmaceuticals, Inc. Corporate HQ: 255 State Street, 9th Floor, Boston, MA 02109, USA R&D Hub: Freising, Germany (Munich) info@pieris.com www.pieris.com

Appendix

Bispecific Geometry Impacts Immune Synapse, Efficacy A Varied Immune Synapse... ... Does Not Materially Impact Target Engagement... ...But Impacts Efficacy The Natural Immune Synapse TNFRSL ~15nm (e.g. 4-1BB Ligand) 13.4 nm C-terminal Heavy chain fusion TNFRS (e.g. 4-1BB) ~8nm C-terminal Light chain fusion Efficacy Experimental Design IFN-γ IL-2 ~5nm Activation 4-1BB/HER-2 T Cell bispecific N-terminal Heavy chain fusion Signal 1 Signal 2 4-1BB HER-2 ~5nm α-CD3 α-CD3 Antibody antibody Culture Dish HER-2+ N-terminal Light chain fusion Tumor Cell Stand-alone building Bispecific-based 22 block affinity building block affinity

PRS-080 Shows Consistent Effects in Healthy Volunteers & CKD5 Patients – Ongoing Ph IIa Study will Evaluate Hemoglobin • In both healthy volunteers and CKD5 Ph I SAD in Healthy Volunteers* Ph Ib SAD in CKD5 patients** patients, PRS-080 Mean Iron Concentrations† Mean Iron Concentrations 60 60 o Was safe and well-tolerated 50 50 Showed a dose-proportional increase of PK o 40 40 parameters (data not shown) 30 30 o Demonstrated dose-dependent PD effects on Serum Iron [µM] serum iron and TSAT 20 Serum Iron [µM] 20 10 10 o Led to an immediate dose-dependent 0 0 decrease in circulating free hepcidin (data 0 48 96 144 192 240 0 48 96 144 not shown) Mean TSAT (%)† Mean TSAT (%) • A Phase IIa trial is underway in Germany 100 100 and Czech Republic 80 80 • Planning 5 QW infusions in ESRD FID 60 60 anemia patients TSAT (%) TSAT 40 (%) TSAT 40 • Two dose cohorts: 4 mg/kg and 8 mg/kg body weight (4 drug; 2 placebo per cohort) 20 20 • Safety, tolerability hemoglobin (Hb) and 0 0 0 48 96 144 192 240 0 48 96 144 reticulocyte concentration of Hb as endpoints Time after start of infusion [hours] Time after start of infusion [hours] • Presented at 57th ASH Conference December 2015 ** Presented at 54th ERA-EDTA Conference June 2017 • If data are positive, Pieris will seek to out- † Subjects achieving iron response > 34.5 µM N=24 (6 patients per dose cohort, 6 patients on placebo) license beyond Japan (avg. 3 out of 6 subjects / dose cohort) 23

Management and Board Executive Management Team Stephen Yoder, J.D. Louis Matis, M.D. Allan Reine, M.D. President & CEO SVP, Chief Development Officer SVP, Chief Financial Officer CGI Pharmaceuticals Board of Directors Stephen Yoder Michael Richman Jean-Pierre Bizzari, M.D. Christopher Kiritsy Ann Barbier, M.D., Ph.D. President & CEO CEO, NextCure Director CEO, Arisaph Pharmaceuticals CMO, Translate Bio Amplimune, Chiron, Celgene, Servier, Rhone-Poulenc, Kos Pharmaceuticals MedImmune, Macrogenics Sanofi-Aventis Steven Prelack Julian Adams, Ph.D. James Geraghty SVP & COO, VetCor President & CEO, Gamida Cell Director Aerpio, Galectin Therapeutics, Clal BioTech Industries,Ltd., Infinity, Third Rock Ventures, Sanofi, Genzyme, BioVex Group Millennium Pharm., LeukoSite Inc. Bain and Company 24
