Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Esperion Therapeutics, Inc. | a18-13085_18k.htm |
Study 1 Pivotal Phase (1002-040) 3 Long-Term Safety Study Supplemental Materials MAY 8, 2018

Safe Harbor Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward looking statements. For example, all statements we make regarding the regulatory approval pathway for the bempedoic acid / ezetimibe combination pill and bempedoic acid and the anticipated safety and efficacy profile of, clinical development plan for, the bempedoic acid / ezetimibe combination pill and bempedoic acid, including Esperion's timing, designs, plans and announcement of results regarding its global pivotal Phase 3 clinical development program for bempedoic acid and the bempedoic acid / ezetimibe combination pill, Esperion's timing and plans for submission of NDAs to the FDA and MAAs to the EMA and Esperion's expectations for the market for therapies to lower LDL-C, including the market adoption of bempedoic acid and the bempedoic acid / ezetimibe combination pill, if approved, are forward looking. All forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These statements are also subject to a number of material risks and uncertainties, including but not limited to, delays or failures in Esperion’s studies, that positive results from a clinical study of bempedoic acid may not be sufficient for FDA approval or necessarily be predictive of the results of future or ongoing clinical studies, that existing cash resources may be used more quickly than anticipated, and the risks detailed in Esperion's filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. Esperion disclaims any obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. 2 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Study 1 (040) Supplemental Materials Supplemental Materials Summary Comparison of Study 1 with pcsk9i Pivotal Phase 3 Long-Term Studies —Baseline Demographics —AE(s), SAE(s), Discontinuations due to AE(s), Fatal AEs Individual Patient Narratives for Fatal AE(s), none related to study treatment Adjudicated Cardiovascular Safety Events Tables Liver Function Test Tables Recent Precedents of the Efficacy of LDL-C Lowering Therapy Waning Over Time CVOT Powering Assumptions Bempedoic Acid Mechanism of Action (MOA) is inhibition of ATP-Citrate Lyase • • • • • • • • 3 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
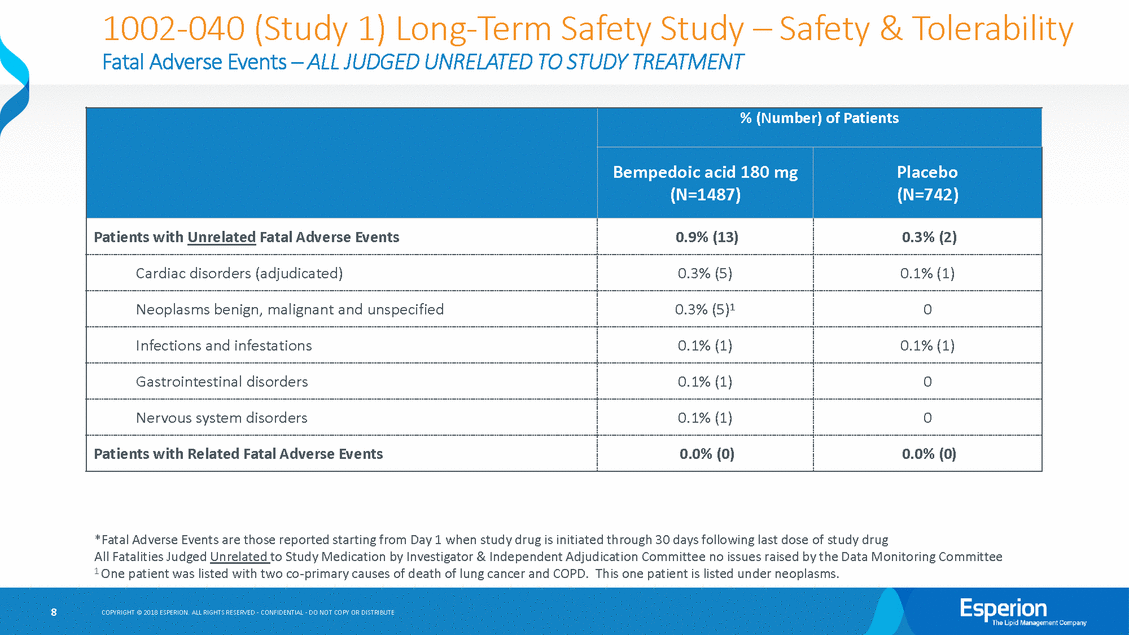
Study 1 (040) Supplemental Materials Summary By design, Study 1 enrolled the oldest and highest CV risk patient population of all recently completed long-term LDL-C lowering clinical studies with high levels of smoking, obesity and elevated blood pressure • As a result, safety event rates were in the range expected for this high-risk population • ALL fatal AE(s) were considered unrelated to study treatment, and pooled pcsk9i event rates (0.9%) were comparable to the 0.9% rate observed in Study 1 • 5 were due to cardiac events, 3 of which were heart attacks (MI) within the first 61 days after randomization in patients who had had coronary events prior to entering the study 5 were due to cancer; 4 lung cancer, by far the leading cause of cancer death in older people; with 80% of lung cancer deaths caused by smoking and for which symptoms typically don’t appear until the cancer is advanced. • • The fully-independent DMC has consistently reviewed both the Phase 3 and CVOT safety results and recommended continuation of the program Measures of adjudicated cardiovascular events ALL favored bempedoic acid over placebo Liver Function Tests (LFTs) elevations were as expected, very low overall, and consistent with statins and ezetimibe As expected, LDL-C lowering efficacy with bempedoic acid was less at 52 weeks than at 12 weeks, consistent with long-term studies of ALL approved LDL-C lowering therapies Bempedoic acid’s MOA has been conclusively demonstrated as inhibition of ATP-Citrate Lyase • • • • • 4 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
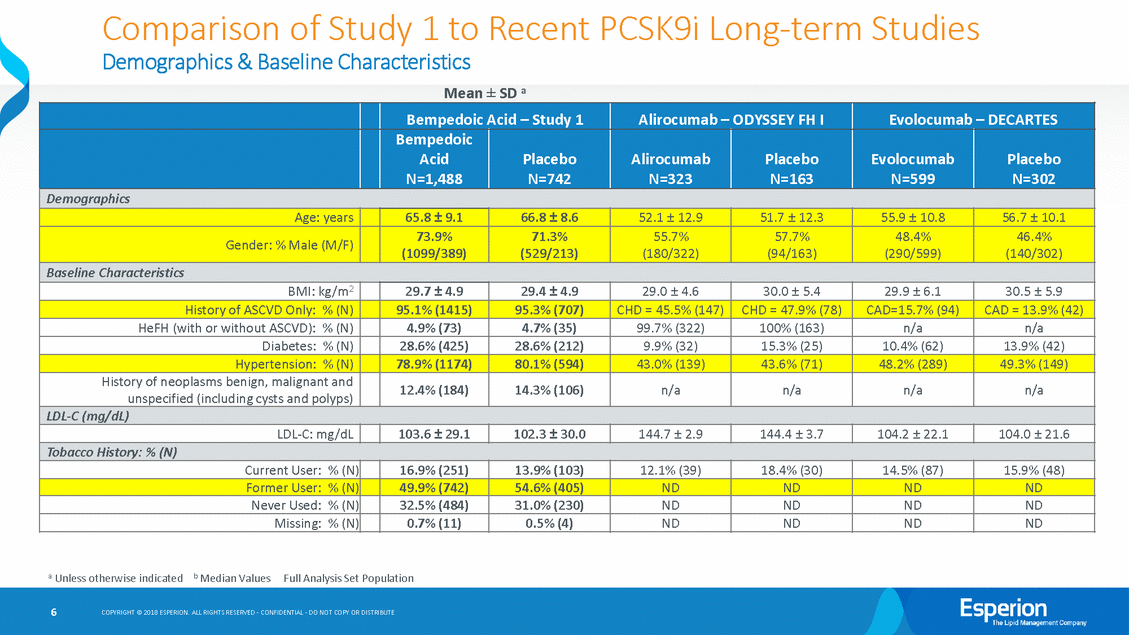
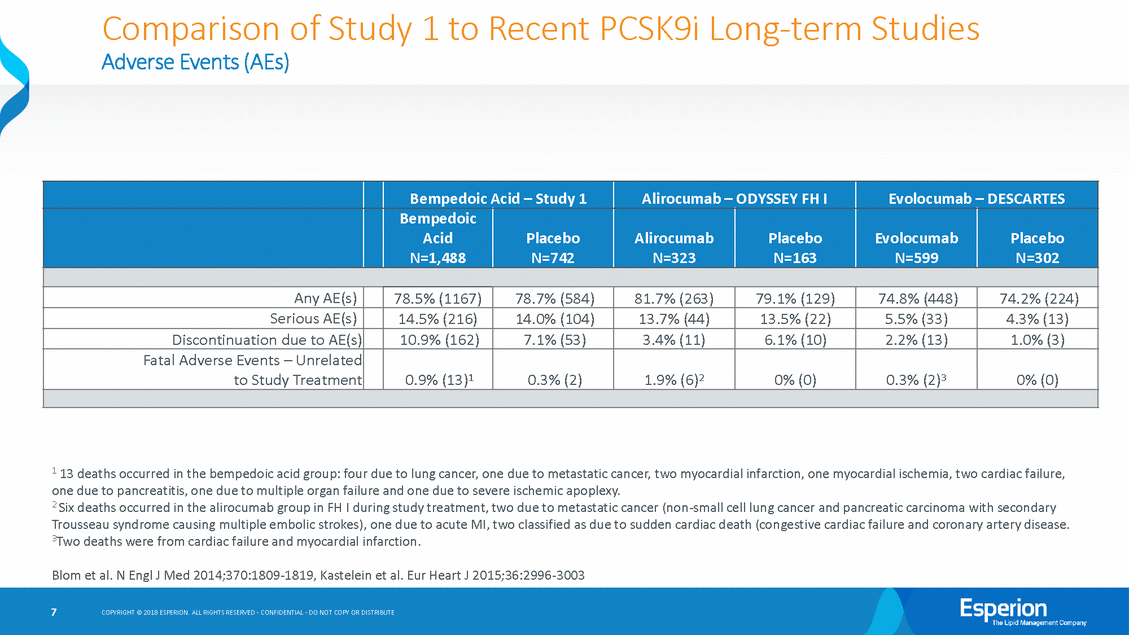
Comparison of Study 1 to Recent PCSK9i Long-term Studies Study 1 baseline demographics indicate patients are 10 – 15 years older than patients recent comparable long-term LDL-C lowering studies, at higher risk for cardiovascular disease due to obesity and other factors, and at higher risk for lung cancer as almost 70% are current or former smokers As shown on Slides 6 and 7: in • • —The overall rate of AE(s) is comparable with recent long-term studies of LDL-C lowering therapies —The rate of serious AE(s) is comparable with recent long-term studies of LDL-C lowering therapies —The rate of fatal AE(s) is low overall, but to be expected for the high-risk patient population enrolled in the study who have had at least one CV event and multiple other serious risk factors including smoking, obesity, high cholesterol, diabetes, hypertension, etc. —An imbalance in fatal AE(s) is a function of small numbers, and has also been observed in recent long-term studies of LDL-C lowering therapies We include individual patient narratives for each fatal AE —Neoplasms benign, malignant and unspecified —Cardiac Disorders —Other • 5 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Comparison of Study 1 to Recent PCSK9i Long-term Studies Demographics & Baseline Characteristics Mean ± SD a (140/302) a Unless otherwise indicated b Median ValuesFull Analysis Set Population 6 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE Bempedoic Acid – Study 1 Alirocumab – ODYSSEY FH I Evolocumab – DECARTES Bempedoic Acid N=1,488 Placebo N=742 Alirocumab N=323 Placebo N=163 Evolocumab N=599 Placebo N=302 Demographics Age: years 65.8 ± 9.1 66.8 ± 8.6 52.1 ± 12.9 51.7 ± 12.3 55.9 ± 10.8 56.7 ± 10.1 Gender: % Male (M/F) 73.9% (1099/389) 71.3% (529/213) 55.7% (180/322) 57.7% (94/163) 48.4% (290/599) 46.4% Baseline Characteristics BMI: kg/m2 29.7 ± 4.9 29.4 ± 4.9 29.0 ± 4.6 30.0 ± 5.4 29.9 ± 6.1 30.5 ± 5.9 History of ASCVD Only: % (N) 95.1% (1415) 95.3% (707) CHD = 45.5% (147) CHD = 47.9% (78) CAD=15.7% (94) CAD = 13.9% (42) HeFH (with or without ASCVD): % (N) 4.9% (73) 4.7% (35) 99.7% (322) 100% (163) n/a n/a Diabetes: % (N) 28.6% (425) 28.6% (212) 9.9% (32) 15.3% (25) 10.4% (62) 13.9% (42) Hypertension: % (N) 78.9% (1174) 80.1% (594) 43.0% (139) 43.6% (71) 48.2% (289) 49.3% (149) History of neoplasms benign, malignant and unspecified (including cysts and polyps) 12.4% (184) 14.3% (106) n/a n/a n/a n/a LDL-C (mg/dL) LDL-C: mg/dL 103.6 ± 29.1 102.3 ± 30.0 144.7 ± 2.9 144.4 ± 3.7 104.2 ± 22.1 104.0 ± 21.6 Tobacco History: % (N) Current User: % (N) 16.9% (251) 13.9% (103) 12.1% (39) 18.4% (30) 14.5% (87) 15.9% (48) Former User: % (N) 49.9% (742) 54.6% (405) ND ND ND ND Never Used: % (N) 32.5% (484) 31.0% (230) ND ND ND ND Missing: % (N) 0.7% (11) 0.5% (4) ND ND ND ND
Comparison of Adverse Events (AEs) Study 1 to Recent PCSK9i Long-term Studies 1 13 deaths occurred in the bempedoic acid group: four due to lung cancer, one due to metastatic cancer, two myocardial infarction, one myocardial ischemia, two cardiac failure, one due to pancreatitis, one due to multiple organ failure and one due to severe ischemic apoplexy. 2 Six deaths occurred in the alirocumab group in FH I during study treatment, two due to metastatic cancer (non-small cell lung cancer and pancreatic carcinoma with secondary Trousseau syndrome causing multiple embolic strokes), one due to acute MI, two classified as due to sudden cardiac death (congestive cardiac failure and coronary artery disease. 3Two deaths were from cardiac failure and myocardial infarction. Blom et al. N Engl J Med 2014;370:1809-1819, Kastelein et al. Eur Heart J 2015;36:2996-3003 7 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE Bempedoic Acid – Study 1 Alirocumab – ODYSSEY FH I Evolocumab – DESCARTES Bempedoic Acid N=1,488 Placebo N=742 Alirocumab N=323 Placebo N=163 Evolocumab N=599 Placebo N=302 Any AE(s) 78.5% (1167) 78.7% (584) 81.7% (263) 79.1% (129) 74.8% (448) 74.2% (224) Serious AE(s) 14.5% (216) 14.0% (104) 13.7% (44) 13.5% (22) 5.5% (33) 4.3% (13) Discontinuation due to AE(s) 10.9% (162) 7.1% (53) 3.4% (11) 6.1% (10) 2.2% (13) 1.0% (3) Fatal Adverse Events – Unrelated to Study Treatment 0.9% (13)1 0.3% (2) 1.9% (6)2 0% (0) 0.3% (2)3 0% (0)
1002-040 (Study 1) Long-Term Safety Study Fatal Adverse Events – ALL JUDGED UNRELATED TO STUDY TREATMENT – Safety & Tolerability *Fatal Adverse Events are those reported starting from Day 1 when study drug is initiated through 30 days following last dose of study drug All Fatalities Judged Unrelated to Study Medication by Investigator & Independent Adjudication Committee no issues raised by the Data Monitoring Committee 1 One patient was listed with two co-primary causes of death of lung cancer and COPD. This one patient is listed under neoplasms. 8 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE % (Number) of Patients Bempedoic acid 180 mg (N=1487) Placebo (N=742) Patients with Unrelated Fatal Adverse Events 0.9% (13) 0.3% (2) Cardiac disorders (adjudicated) 0.3% (5) 0.1% (1) Neoplasms benign, malignant and unspecified 0.3% (5)1 0 Infections and infestations 0.1% (1) 0.1% (1) Gastrointestinal disorders 0.1% (1) 0 Nervous system disorders 0.1% (1) 0 Patients with Related Fatal Adverse Events 0.0% (0) 0.0% (0)
Individual Patient Narratives 9 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE

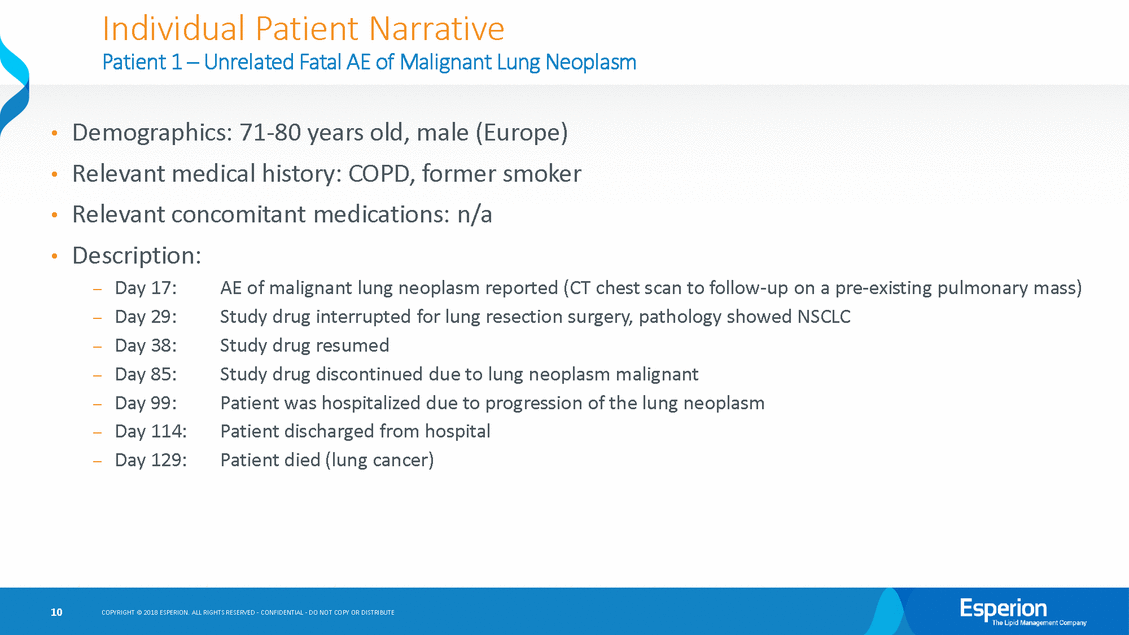
Individual Patient Narrative Patient 1 – Unrelated Fatal AE of Malignant Lung Neoplasm Demographics: 71-80 years old, male (Europe) Relevant medical history: COPD, former smoker Relevant concomitant medications: n/a Description: • • • • Day 17: Day 29: Day 38: Day 85: Day 99: Day 114: Day 129: AE of malignant lung neoplasm reported (CT chest scan to follow-up on a pre-existing pulmonary mass) Study drug interrupted for lung resection surgery, pathology showed NSCLC Study drug resumed Study drug discontinued due to lung neoplasm malignant Patient was hospitalized due to progression of the lung neoplasm Patient discharged from hospital Patient died (lung cancer) — — — — — — — 10 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative Patient 2 – Unrelated Fatal AE of Lung Adenocarcinoma Demographics: 71-80 years old, male (Europe) Relevant medical history: puncture of a large pleural effusion malignancy, nicotine abuse Relevant concomitant medications: n/a Description: • • on right with no evidence of • • Day 3: Day 27: Day 43: Day 61: Study drug discontinued due to muscle cramps (Note: study drug never re-started) Patient hospitalized for pleural effusion (fluid buildup in lung) Patient underwent partial pleurectomy, with drains inserted Patient diagnosed with severe lung adenocarcinoma as result of lung biopsy revealed right pleural carcinoma with pronounced tumor load Patient discharged from hospital AE reported Patient died (lung adenocarcinoma) — — — — Day 61: Day 61: Day 87: — — — 11 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative Patient 3 – Unrelated Fatal AE of Malignant Lung Squamous Cell Carcinoma Demographics: 61-70 years old, female (Europe) Relevant medical history: former smoker Relevant concomitant medications: n/a Description: • • • • Day 87: Day 87: Day 141: Day 148: Day 156: Day 204: Patient diagnosed with lung squamous cell carcinoma metastatic AE reported Patient hospitalized, underwent radiation therapy Study drug discontinued due to lung carcinoma. Patient rehospitalized, underwent radiation therapy. Patient later entered home hospice care Patient died (lung adenocarcinoma) — — — — — — 12 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative Patient 4 – Unrelated Fatal AE of Malignant Lung Neoplasm and COPD Demographics: 81-90 years old, male (Europe) Relevant medical history: end-stage COPD, abdominal aortic aneurysm, Relevant concomitant medications: n/a Description: • • • • smoker Day 120: Day 142: Day 142 Day 252: Day 268: Day 270: Day 303: Patient underwent CT for AAA, evidence for cancer noted (approximate date) Diagnosis of lung cancer confirmed by PET scan AE reported Study drug discontinued Patient hospitalized due to increased breathlessness Patient transferred to palliative care Patient died (lung cancer and COPD) — — — — — — — 13 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative Patient 5 – Unrelated Fatal AE of Metastases to Liver Demographics: 71-80 years old, male (Europe) Relevant medical history: Bowen’s disease (squamous Relevant concomitant medications: n/a Description: • • • • cell carcinoma in situ), smoker Day 235: Day 235: Day 240: Day 240: Day 242: Day 252: Patient presented with decreased appetite, weight loss, rash Study drug discontinued Abdominal ultrasound shows liver metastases with no primary source identified AE reported Patient hospitalized with jaundice and abdominal pain; CT scan showed diffuse malignancy within liver Patient died (metastases to liver) — — — — — — 14 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative Patient 6 – Unrelated Fatal AE of Heart Failure Demographics: 71-80 years old, male (Europe) Relevant medical history: acute MI, stage III chronic kidney disease, heart failure, coronary revascularization, aortic aneurysm, type 2 diabetes, hypertension, CHD • • Relevant concomitant medications: aspirin, metoprolol, trapidil (anti-platelet), (diuretic), sacubitril/valsartan (Entresto), ezetimibe, atorvastatin Description: torsemide • • Day 164: Day 169: Day 183: Day 192: Day 228: Day 228: Day 228: Patient hospitalized for edema secondary to cardiac decompensation Event reported resolved Patient hospitalized for GI discomfort Event considered resolved Patient hospitalized with severe heart failure Study drug discontinued AE reported — — — — — — — — — Day 228-232: Patient had tracheobronchitis, was intubated, X-ray confirmed congested upper left lobe focal abscess Day 233: Patient died 15 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative Patient 7 – Unrelated Fatal AE of Heart Failure Demographics: 61-70 years old, female (Europe) Relevant medical history (including co-morbidities): diabetes • • hypertension, ischemic heart disease, type 2 Relevant concomitant medications: aspirin, lercanidipine, nebivolol, Description: aspirin, rosuvastatin • • Day 114: Day 114: Day 114: Patient felt weak at dinner, lost consciousness, could not be revived AE reported Patient died — — — 16 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative Patient 8 – Unrelated Fatal AE of Myocardial Infarction Demographics: 61-70 years old, female (Europe) Relevant medical history: acute MI, type 2 diabetes Relevant concomitant medications: lisinopril, amlodipine, simvastatin Description: • • • bisoprolol, gliclazide, metformin, • Day 11: Day 11: Day 17: Day 18: Day 60: Day 61: Day 61: Day 61: Patient hospitalized; laparoscopy revealed internal hernia. Keyhole surgery performed Study drug interrupted Event considered resolved; patient discharged from hospital Study drug resumed Last day of study drug Patient woke with shoulder pain and emergency services called. Patient went into cardiac arrest Patient died AE reported — — — — — — — — 17 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative Patient 9 – Unrelated Fatal AE of Myocardial Ischemia, Hypertensive Heart Disease Demographics: 61-70 years old, male (Europe) Relevant medical history: ischemic heart disease, triple CABG, double CABG, atrial fibrillation, ventricular heart failure, cardiomyopathy, angina, PAD requiring angioplasty • • left Relevant concomitant medications: aspirin, bisoprolol, furosemide, ramipril, warfarin, • eplerenone, Description: simvastatin • Day 48: Day 48: Patient found dead at home AE reported — — 18 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative Patient 10 – Unrelated Fatal AE of Myocardial Infarction Demographics: 61-70 years old, male (Europe) Relevant medical history: acute MI, heart failure, coronary smoking, hypertension • • revascularization, type 2 diabetes, Relevant concomitant medications: aspirin, insulin, metformin, liraglutide, empagliflozin, • bisoprolol, nitrates, ivabradine, nicorandil, Description: nitroglycerine, simvastatin • Day 28: Day 28: Patient suffered MI and died AE reported — — — Date of last known dose of study drug unknown 19 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative Patient 11 – Unrelated Fatal AE of Acute Pancreatitis, Acute Cholecystitis, Pancreatic Pseudocyst, and Pancreatic Adenocarcinoma Demographics: 61-70 years old, male (U.S.) Relevant medical history: upper abdominal pain Relevant concomitant medications: aspirin, ondansetron, pantoprazole, oxycodone/acetaminophen, lisinopril, pravastatin Description: • • • • Day 30: Day 47: Day 228: Day 230: Day 232: Day 238: Day 239: Study drug discontinued – increase in pancreatic enzymes day 3, day 15 AE of pancreatic pseudocyst reported Patient hospitalized for abdominal pain; diagnosed with hyponatremia, cholecytitis, pancreatic mass Patient diagnosed with pancreatic adenocarcinoma Patient had seizure, suffered cerebral infarct Patient discharged from hospital to hospice care Patient died — — — — — — — 20 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative Patient 12 – Unrelated Fatal AE of Ischemic Cerebral Infarction/Severe Ischemic Apoplex Demographics: 51-60 years old, male (Europe) Relevant medical history: coronary artery disease, hypertension, coronary revascularization, acute MI Relevant concomitant medications: aspirin, candesartan, bisoprolol, atorvastatin Description: • • • • Day 190: Patient hospitalized with severe ischemic stroke; lysis unsuccessful; thrombectomy performed; developed cerebral bleeding AE reported Patient died — Day 190: Day 192: — — 21 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative Patient 13 – Unrelated Fatal AE of Multiple Organ Failure Demographics: 71-80 years old, male (Europe) Relevant medical history: COPD, angina, coronary artery disease, hypertension, ischemic stroke Relevant concomitant medications: atenolol, clopidogrel, candesartan, bendroflumethiazide, atorvastatin Description: • • • • Day 219: Day 232: Day 328: Day 331: Day 331: Day 332: Patient admitted to hospital for epigastric and chest pain, confirmed cholecystitis Event reported resolved, patient discharged from hospital Patient hospitalized, underwent cholecystectomy, bile duct exploration, stenting Patient developed sepsis and bile leak Study drug discontinued Patient developed multiple organ failure and died — — — — — — 22 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative (Placebo) Patient 1– Unrelated Fatal AE of Sepsis/Multiple Organ Failure Demographics: 61-70 years old, male (U.S.) Relevant medical history: hernia repair, sleep apnea Relevant concomitant medications: n/a Description: • • • • Day 170: Day 170: Day 171: Day 171: Day 174: Patient hospitalized with multiple GI complaints Study drug (placebo) discontinued due to colon neoplasm Patient underwent unsuccessful colonoscopy due to narrowing of colon Patient developed peritonitis due to a ruptured colon Patient developed septic shock with multiple organ failure and died — — — — — 23 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Individual Patient Narrative (Placebo) Patient 2 – Unrelated Fatal AE of Myocardial Infarction (adjudicated) Demographics: 61-70 years old, male (Europe) Relevant medical history: type 2 diabetes, diabetic neuropathy, peripheral artery bypass • • peripheral artery disease, Relevant concomitant medications: Description: Lisinopril, aspirin, insulin, liraglutide, atorvastatin • • Day 160: Patient died at home — 24 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Adjudicated Cardiovascular Event Tables 25 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Study 1 Adjudicated Cardiovascular Events The following slide shows adjudicated cardiovascular events for Study —All adjudicated CV events —5-component MACE events —4-component MACE events 1 • These early results show a trend favoring bempedoic acid over placebo in every analysis of CV events in Study 1 which ranged from a 19% to 8% CV event reduction.This is • particularly notable since event (K-M) curves in recently completed outcomes studies for the — — — pcsk9i’s didn’t separate For all CV events For 5-component MACE For 4-component MACE until after the first year. 26 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
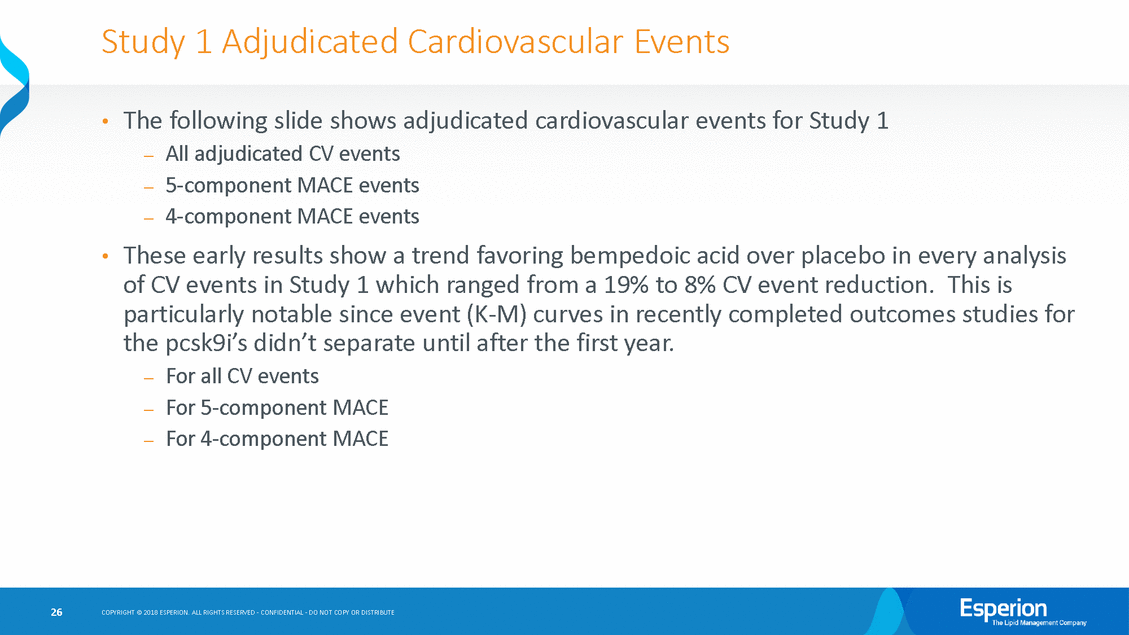
1002-040 (Study 1) – Adverse Events of Special Interest Rates Clinical Endpoint Adjudicated Events – Treatment Emergent AE (TEAE) Analysis 1Includes Coronary Revascularization, CV Death, Non-Fatal MI, non-Fatal Stroke, Hospitalization For Unstable Angina 2Includes Coronary Revascularization, CV Death, Non-Fatal MI, non-Fatal Stroke 27 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE Event % (Number) of Patients Bempedoic Acid (N= 1487) Placebo (N=742) All Adjudicated CV Events 4.6% (68) 5.7% (42) Five-Component MACE Adjudicated Events1 3.8% (57) 4.6% (34) Four-Component MACE Adjudicated Events2 3.6% (54) 3.9% (29)

Rates of LFT Elevations for Oral LDL-C Lowering Therapies 28 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Rates of LFT Elevations for Oral LDL-C Lowering Therapies LFT elevations have been seen with EVERY previously approved and successful once-daily LDL-C lowering therapy —Statins – 0.2% - 2.3% —Ezetimibe – 0.5% oral, • LFT elevations with approved oral LDL-C lowering never been associated with serious liver injury —No increases in bilirubin —No cases of Hy’s Law therapies and bempedoic acid have • As expected, bempedoic acid has shown a confirmed rate of LFT elevations that is on the • low-end of reported rates of LFT elevations for approved oral LDL-C lowering and almost exactly the same as ezetimibe —For Study 1 – 0.40% —Completed Integrated Data – 0.49% —Total Ongoing Phase 3 Program – 0.54% (all blinded data in both arms) therapies 29 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
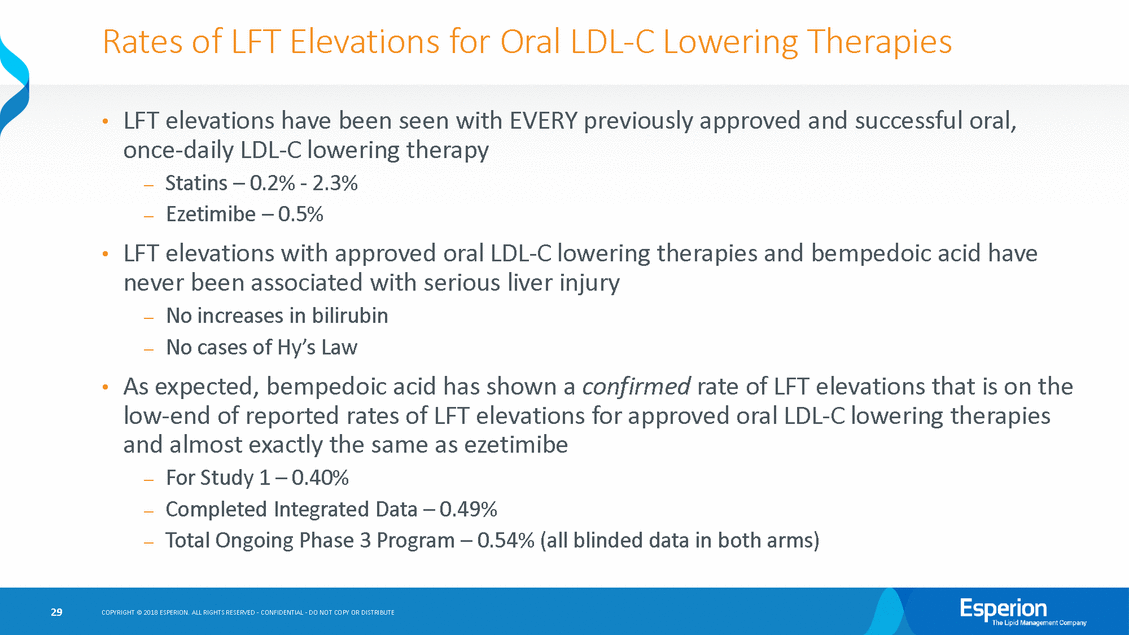
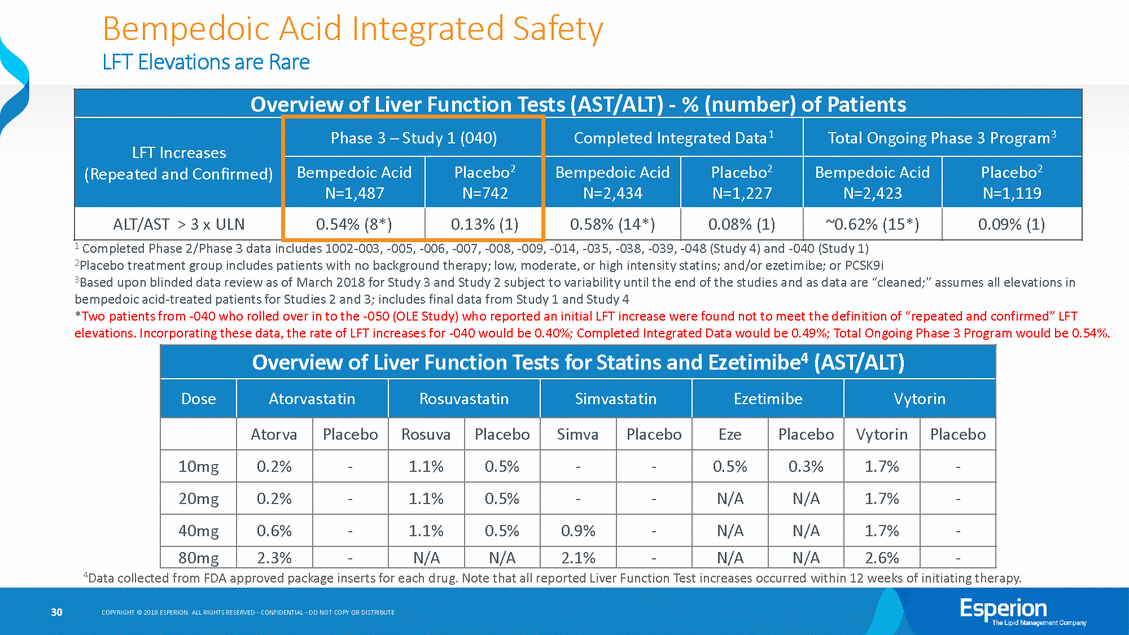
Bempedoic Acid LFT Elevations are Rare Integrated Safety 1 Completed Phase 2/Phase 3 data includes 1002-003, -005, -006, -007, -008, -009, -014, -035, -038, -039, -048 (Study 4) and -040 (Study 1) 2Placebo treatment group includes patients with no background therapy; low, moderate, or high intensity statins; and/or ezetimibe; or PCSK9i 3Based upon blinded data review as of March 2018 for Study 3 and Study 2 subject to variability until the end of the studies and as data are “cleaned;” assumes all elevations in bempedoic acid-treated patients for Studies 2 and 3; includes final data from Study 1 and Study 4 *Two patients from -040 who rolled over in to the -050 (OLE Study) who reported an initial LFT increase were found not to meet the definition of “repeated and confirmed” LFT elevations. Incorporating these data, the rate of LFT increases for -040 would be 0.40%; Completed Integrated Data would be 0.49%; Total Ongoing Phase 3 Program would be 0.54%. 4Data collected from FDA approved package inserts for each drug. Note that all reported Liver Function Test increases occurred within 12 weeks of initiating therapy. 30 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE Overview of Liver Function Tests for Statins and Ezetimibe4 (AST/ALT) Dose Atorvastatin Rosuvastatin Simvastatin Ezetimibe Vytorin Atorva Placebo Rosuva Placebo Simva Placebo Eze Placebo Vytorin Placebo 10mg 0.2% - 1.1% 0.5% - - 0.5% 0.3% 1.7% - 20mg 0.2% - 1.1% 0.5% - - N/A N/A 1.7% - 40mg 0.6% - 1.1% 0.5% 0.9% - N/A N/A 1.7% - 80mg 2.3% - N/A N/A 2.1% - N/A N/A 2.6% - Overview of Liver Function Tests (AST/ALT) - % (number) of Patients LFT Increases (Repeated and Confirmed) Phase 3 – Study 1 (040) Completed Integrated Data1 Total Ongoing Phase 3 Program3 Bempedoic Acid N=1,487 Placebo2 N=742 Bempedoic Acid N=2,434 Placebo2 N=1,227 Bempedoic Acid N=2,423 Placebo2 N=1,119 ALT/AST > 3 x ULN 0.54% (8*) 0.13% (1) 0.58% (14*) 0.08% (1) ~0.62% (15*) 0.09% (1)
In Long-Term Studies, LDL-C Lowering Wanes Over Time 31 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE

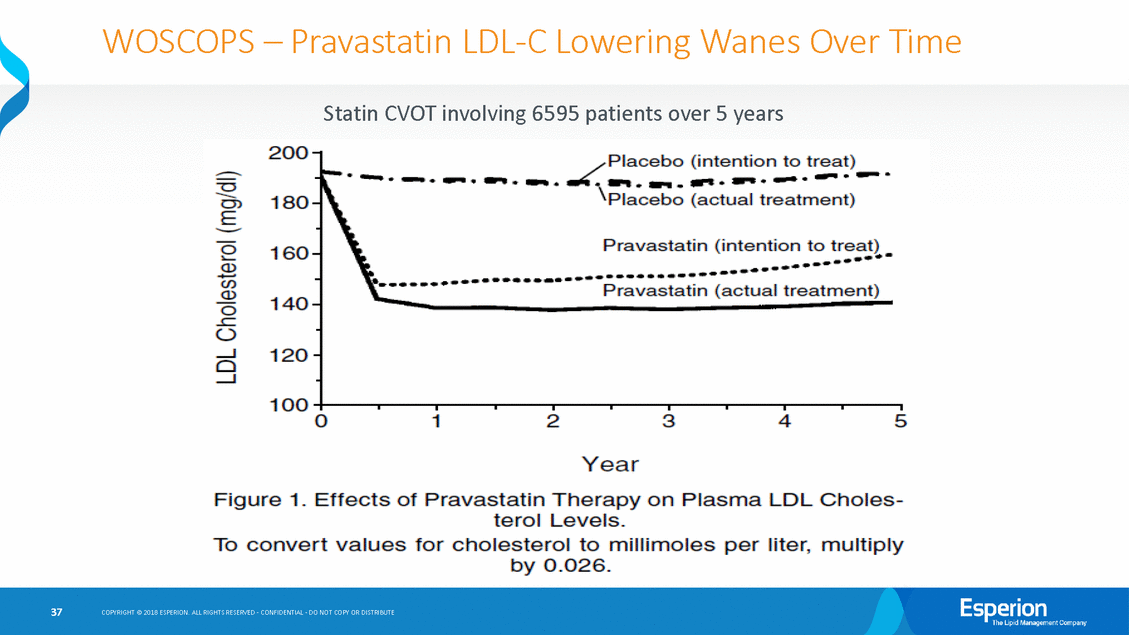
In Long-Term Studies, LDL-C Lowering Wanes Over Time As shown in the next several slides, the efficacy of every approved LDL-C lowering • therapy has waned over time (e.g., —Ezetimibe —Vytorin (simvastatin/ezetimibe) —Alirocumab —Evolocumab —Pravastatin —Atorvastatin during long-term studies): The LDL-C lowering of bempedoic 16% at 52-weeks (on-treatment) acid also waned over time from 20% at 12-weeks to — 32 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
SEAS Study-Ezetimibe LDL-C Lowering Wanes Over Time Placebo H 125-z --"I .... --::.;: --:iii:--- - - - ..I. 0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 COPYRIGHT@ 2018 ESPERION ALL RIGHTS RESERVEDC· ONFIDENTIAL0· 0 NOT COPY OR DISTRIBUTE 33 ASerurn LDL Chol esterol 150-:.:--a-_ _ :a: _ _ - _ -_ _ ..... _ _ -:r: TI 100-P<O.OOl 75-T 50-v-----Si mvastat in p l us ezetim i be 25-0--. .------.--.----.--. --.----. ---.------.--.------. Year
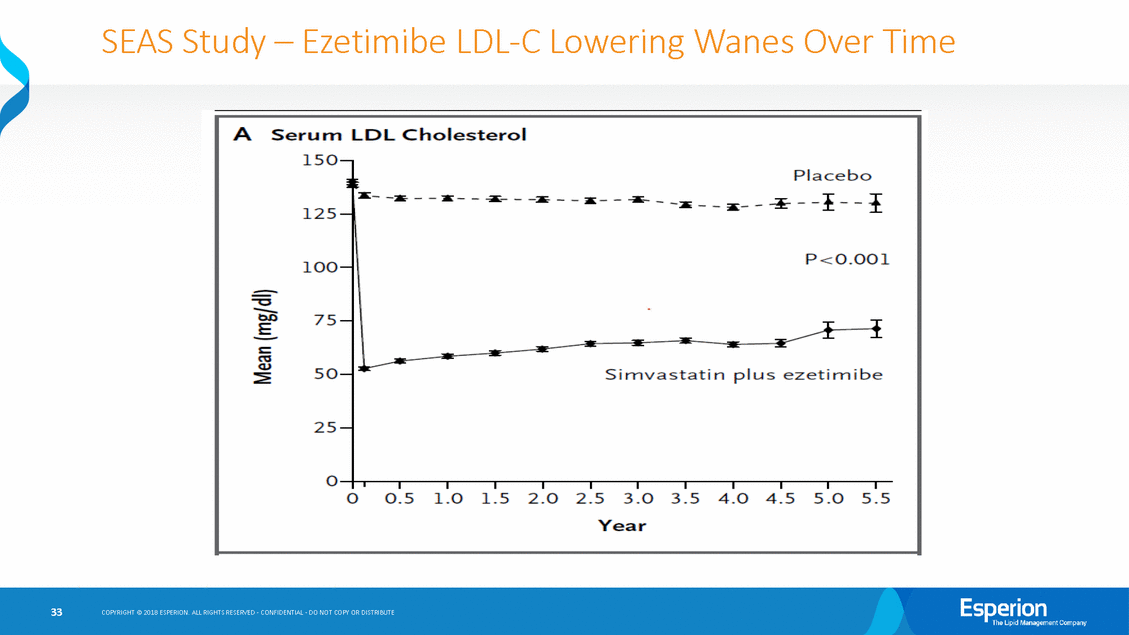
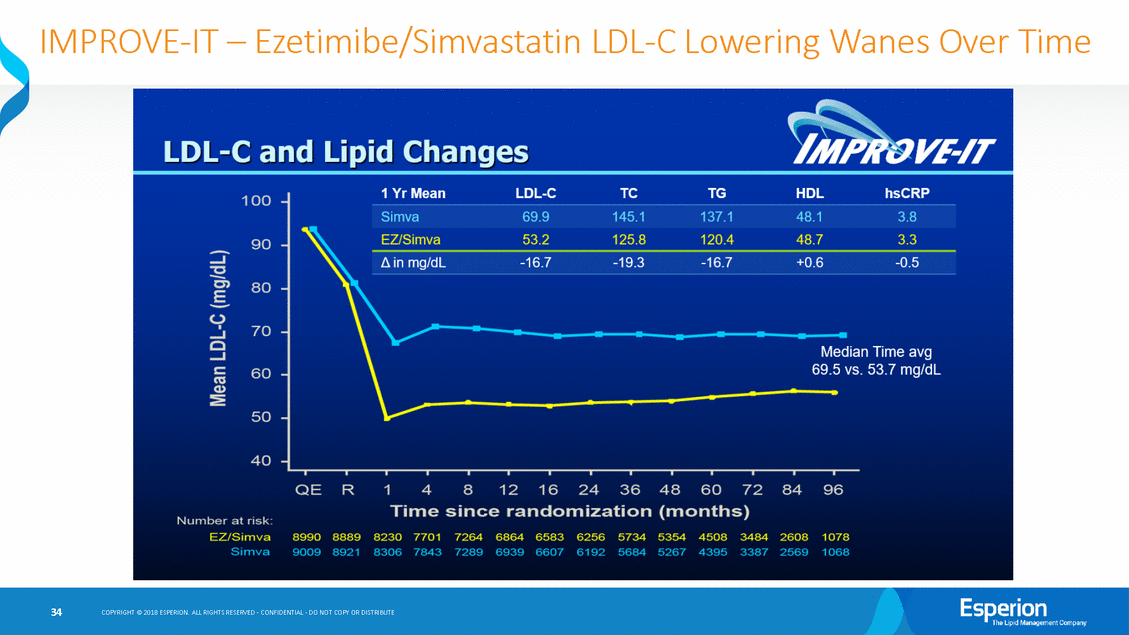
IMPROVE-IT-Ezetimibe/Simvastatin LDL-C Lowering Wanes Over Time COPYRIGHT@ 2018 ESPERION ALL RIGHTS RESERVED-CONFIDENTIAL-00 NOT COPY OR DISTRIBUTE
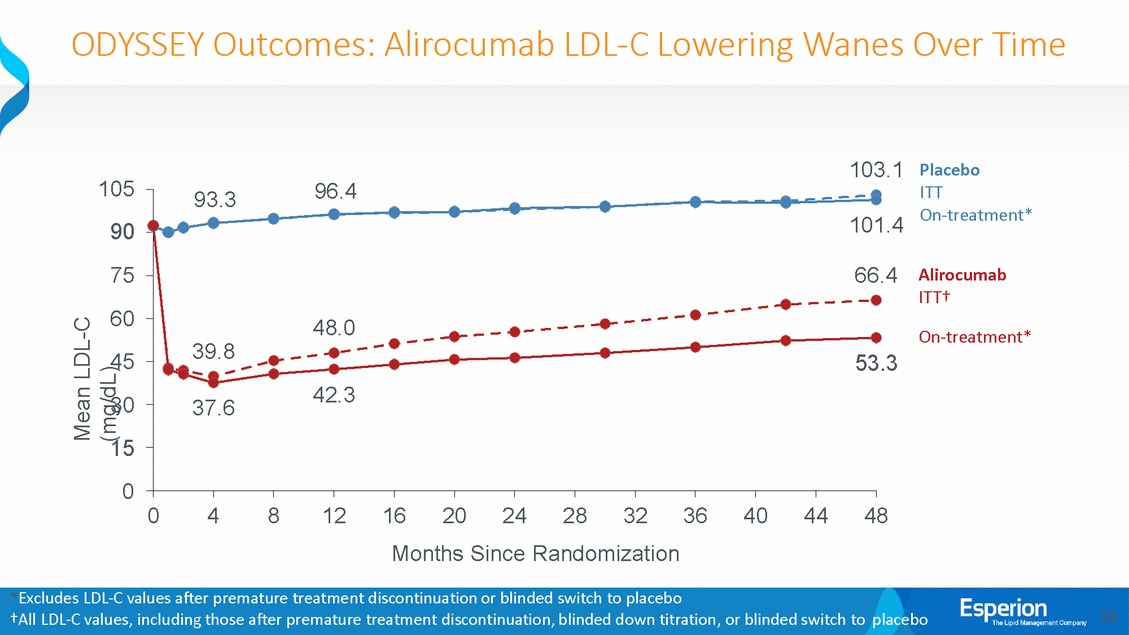
ODYSSEY Outcomes: Alirocumab LDL-C Lowering Wanes Over Time 103.1 Placebo ITT On-treatment* 105 90 75 60 45 30 15 0 101.4 66.4 Alirocumab ITT† On-treatment* 53.3 0 4 8 12 16 20 24 28 32 36 40 44 48 Months Since Randomization *Excludes LDL-C values after premature treatment discontinuation or blinded switch to placebo †All LDL-C values, including those after premature treatment discontinuation, blinded down titration, or blinded switch to placebo 28 Mean LDL-C (mg/dL) 93.396.4 48.0 39.8 37.642.3
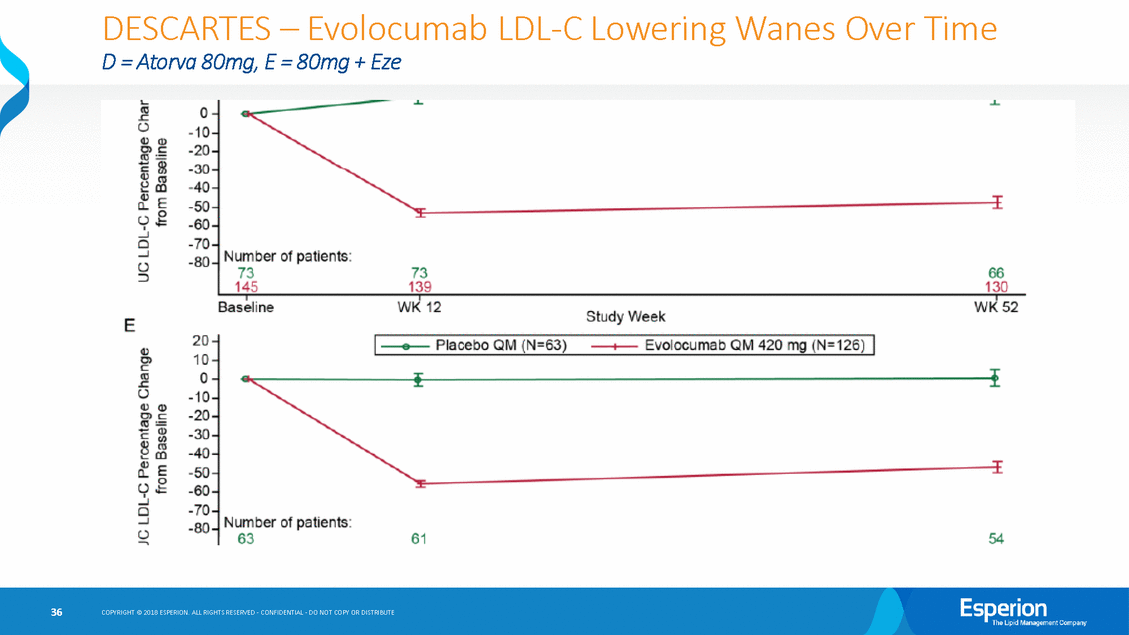
DESCARTES-Evolocumab D =Atorva BOmg, E =BOmg + Eze LDL-C Lowering Wanes Over Time _._ L 0 -10 -20 -30 -40 -50 -60 -70 -80 0 4) (I) O..>,=C: m (j cu m 0".. E u,g I _j c _j Number of patients: 73 145 CJ ::l 73 139 66 130 Baseline WK 12 WK52 Study Week E 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 e 0) c .! 0 4l.l (J) 0) c co= cs0<» aJ t.=. CO cf E 0 0 "-' -. ...J Q ...J 0--. Number of patients: 63 54 61 COPYRIGHT@ 2018 ESPERION ALL RIGHTS RESERVED-CONFIDENTIAL-00 NOT COPY OR DISTRIBUTE 36 •P llaoebo QM (N=63) ----lt--Evolocumab QIM 420 mg (N=126) J
WOSCOPS – Pravastatin LDL-C Lowering Wanes Over Time Statin CVOT involving 6595 patients over 5 years 37 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
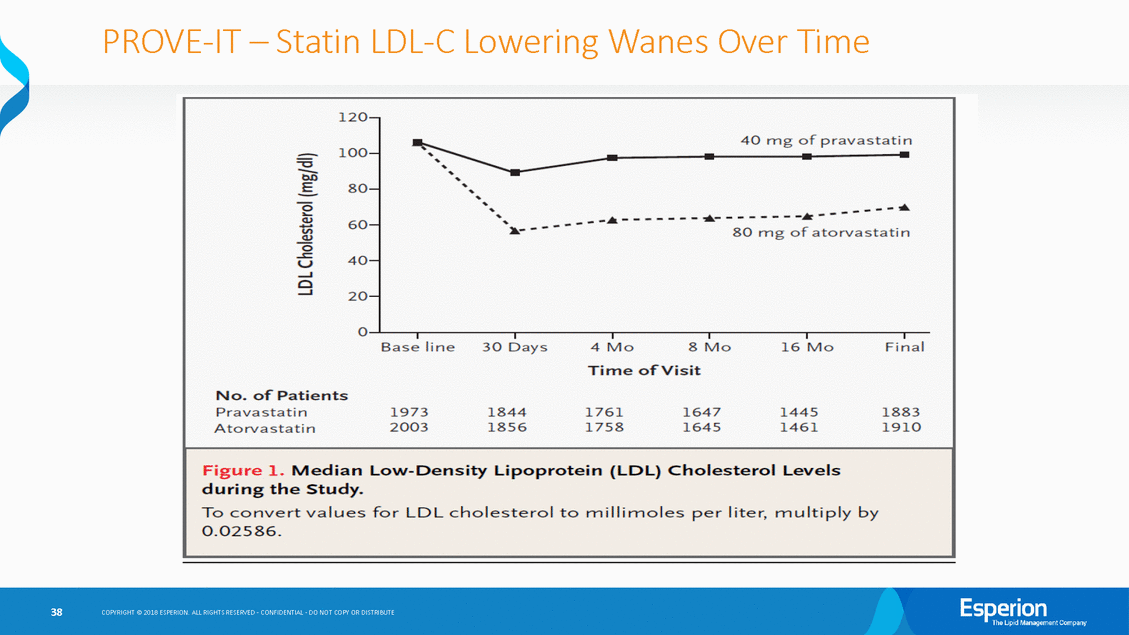
PROVE-IT-Statin LDL-C Lowering Wanes Over Time ,, '' b:O _.., - - - - - - - ·--- -· - - - -·--E 80-------· ' 0..... v; 0 u . .... ----' ' lll.---60-80 mg of atorvastatin 40-20-Base line 30 Day s 4 Mo 8 Mo 16 Mo Final COPYRIGHT@ 2018 ESPERION ALL RIGHTS RESERVEDC· ONFIDENTIAL0· 0 NOT COPY OR DISTRIBUTE 38 120-=-a100-'-·----------- ----------•-----4-0 n_n_gf_-•p r_a_v_a_s ta ti ' Q) Q) -C 0 . 0 ----.-----------r-.----------.r-----------.------------.-----------.--Tirne of"Visit No. of" Patients Pravastatin 19731844 17611647 1445 1883 A torvasta tin 2003185617581645 14611910 Figure 1. Median Low-Density Lipoprotei n (LDL) Cholesterol Levels during the Study. To convert v alues for LDL cholesterol to rnillirnoles per liter, multiply by 0.02586.
CVOT Powering Assumptions 39 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE

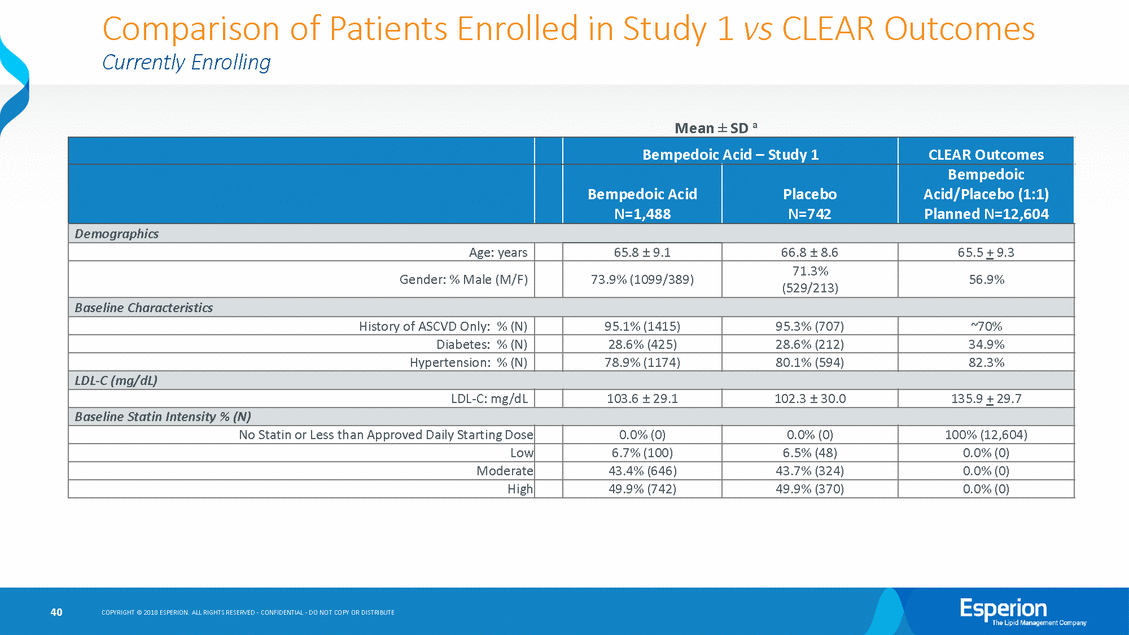
Comparison Currently Enrolling of Patients Enrolled in Study 1 vs CLEAR Outcomes Mean ± SD a 40 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE Bempedoic Acid – Study 1 CLEAR Outcomes Bempedoic Acid N=1,488 Placebo N=742 Bempedoic Acid/Placebo (1:1) Planned N=12,604 Demographics Age: years 65.8 ± 9.1 66.8 ± 8.6 65.5 + 9.3 Gender: % Male (M/F) 73.9% (1099/389) 71.3% (529/213) 56.9% Baseline Characteristics History of ASCVD Only: % (N) 95.1% (1415) 95.3% (707) ~70% Diabetes: % (N) 28.6% (425) 28.6% (212) 34.9% Hypertension: % (N) 78.9% (1174) 80.1% (594) 82.3% LDL-C (mg/dL) LDL-C: mg/dL 103.6 ± 29.1 102.3 ± 30.0 135.9 + 29.7 Baseline Statin Intensity % (N) No Statin or Less than Approved Daily Starting Dose 0.0% (0) 0.0% (0) 100% (12,604) Low 6.7% (100) 6.5% (48) 0.0% (0) Moderate 43.4% (646) 43.7% (324) 0.0% (0) High 49.9% (742) 49.9% (370) 0.0% (0)
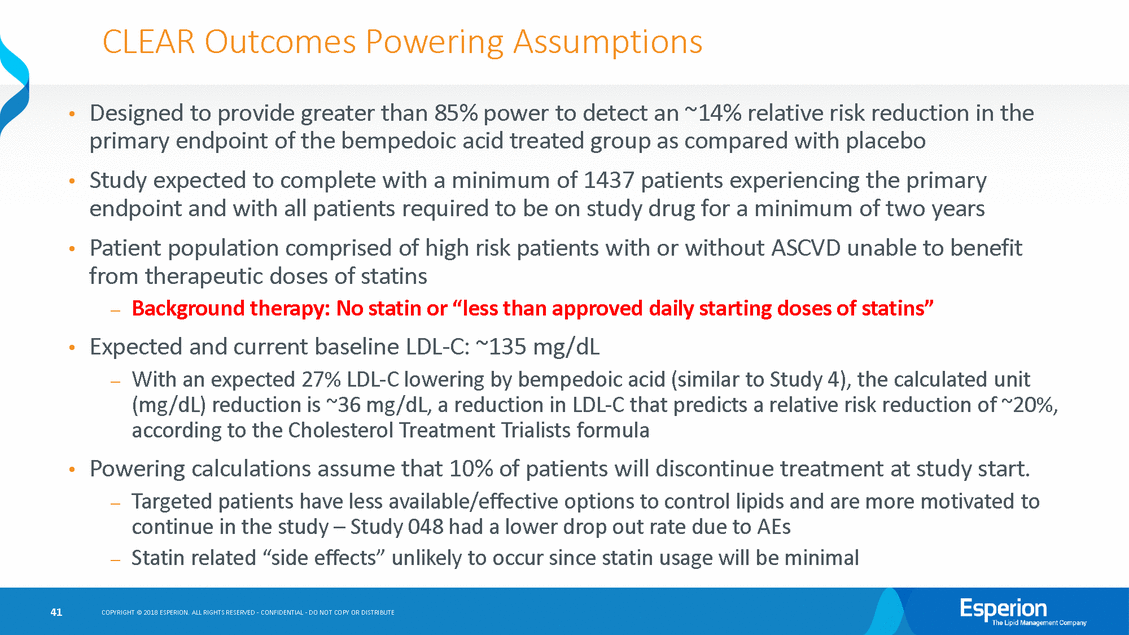
CLEAR Outcomes Powering Assumptions Designed to provide greater than 85% power to detect an ~14% relative risk reduction in the primary endpoint of the bempedoic acid treated group as compared with placebo Study expected to complete with a minimum of 1437 patients experiencing the primary endpoint and with all patients required to be on study drug for a minimum of two years Patient population comprised of high risk patients with or without ASCVD unable to benefit from therapeutic doses of statins —Background therapy: No statin or “less than approved daily starting doses of statins” Expected and current baseline LDL-C: ~135 mg/dL —With an expected 27% LDL-C lowering by bempedoic acid (similar to Study 4), the calculated unit (mg/dL) reduction is ~36 mg/dL, a reduction in LDL-C that predicts a relative risk reduction of ~20%, according to the Cholesterol Treatment Trialists formula Powering calculations assume that 10% of patients will discontinue treatment at study start. —Targeted patients have less available/effective options to control lipids and are more motivated to continue in the study – Study 048 had a lower drop out rate due to AEs —Statin related “side effects” unlikely to occur since statin usage will be minimal • • • • • 41 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
Mechanism of Action 42 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE

Bempedoic Acid Mechanism-ATP-citrate lyase inhibition _ nature COMMUNICAnGNS CLE OPeN by 31 May 2016 1 Aa:.epted 6 Oct 2016 1 Published 28 Nov 2016 er -specific ATP-citrate lyase inhibition bempedoic acid decreases LDL-C and enuates atherosclerosis ndra D. Lalwani1·• wi despread use o f stat1ns to reduce low-density 1\po,protein cholesterol (LDL..C) and ACL) and AMP-activated protein lcinase CAMPK) activity in rodents> However, its unknown. Here we show that ETC-1002 is a prodthat requires activation by very s to LDL receptor upregulatiol\ decreased LDL·C and attenuation of atherosclerosis, pendently of AMPK. Furthermore, we demonstrate that the absence o f AC'SVL1in COPYRIGHT@ 2018 ESPERION ALL RIGHTS RESERVEDC· ONFIDENTIAL0· 0 NOT COPY OR DISTRIBUTE 43 _ L ARTI Received Liv att Stephen Carolyn & Nare Despite as.so<lJa te lowering requirecL hyperchol lyase ( medhanis are long-chai ACL lead inde skeleta1 myotOlti c L. Pinkosky1.2, Roger S. Newton1, Emily A. Da, Rebecca J. Ford 2, Sar ka Lhotak3, Richard C. Austin3, M. Birch 1, Brennan K Smit. Sergey Filippov 1, Pieter H.E. Groot\ Gregory R. Steinberg-1.4·" d athe rasderobc cardiovascula r risk,. many patients do not adhieve suffiotent LDL..C due to rn.sclel ated side eff ects. indicat1ng novel treatment strategies are Bempedoic acid (E:lL-1002) is a small molecule intended to lower LDL..C 1n es terolemc patients.and has been previously shown to modul ate !both ATP-ortrate m for LDL C l owenang. e fficacy in models o f atherosclerosi s and relevanoe in humans n acyi.CoA synthetase·1(ACSVLl) to modulate both targets, and Lhat inhibition of muscle provides a mechani stic basis for ETC-1002 to potentially avoid the ity associated with s tatin thera,py. 001:tO.'IOJa/........,..'0457

ETC-1002/Bempedoic Acid Mechanism Summary LDL-C Lowering via Tissue Specific ACL Inhibition • ETC-1002 is a prodrug and inhibits cholesterol synthesis by inhibition of ACL – an enzyme upstream of HMG-CoA reductase ACL inhibition by ETC-1002-CoA • - - - ETC-1002-CoA directly inhibits recombinant human ACL while parent ETC-1002 does not ETC-1002-CoA inhibits cholesterol synthesis in primary human liver cells ETC-1002-CoA increases LDL receptor activity in human liver cells • ETC-1002 (prodrug) conversion to ETC-1002-CoA (active form) - - - Catalyzed by ACSVL1, an enzyme highly expressed in liver and not expressed skeletal muscle ETC-1002-CoA is not detected in rodent skeletal muscle in vitro or in vivo ETC-1002-CoA is not detected in primary human skeletal muscle cells in vitro or human skeletal muscle microsomes Unlike statins, treatment with ETC-1002/bempedoic acid does not result in cholesterol synthesis inhibition nor promote skeletal muscle toxicity in vitro - Adapted from Pinkosky et al. Nature Communications. 2016 Nov 28; DOI: 10.1038/ncomms13457 44 COPYRIGHT © 2018 ESPERION. ALL RIGHTS RESERVED - CONFIDENTIAL - DO NOT COPY OR DISTRIBUTE
www.esperion.com

