Attached files
| file | filename |
|---|---|
| EX-31.2 - EX-31.2 - Aralez Pharmaceuticals Inc. | a18-12367_1ex31d2.htm |
| EX-31.1 - EX-31.1 - Aralez Pharmaceuticals Inc. | a18-12367_1ex31d1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K/A
(Amendment No. 1)
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2017
OR
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE TRANSITION PERIOD FROM TO
Commission File number 001-37691
ARALEZ PHARMACEUTICALS INC.
(Exact name of registrant as specified in its charter)
|
British Columbia, Canada |
|
98-1283375 |
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
7100 West Credit Avenue, Suite 101, Mississauga, Ontario, Canada L5N 0E4
(Address of principal executive offices)
(905) 876-1118
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Name of exchange on which registered |
|
Common Shares, without par value |
|
Nasdaq Global Market, Toronto Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act:
Common Shares, no par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act (Check one):
|
Large accelerated filer |
|
¨ |
|
Accelerated filer |
|
x |
|
Non-accelerated filer |
|
¨ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-accelerated filer |
|
¨ |
|
Emerging growth company |
|
¨ |
|
|
|
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No x
The aggregate market value of the common shares held by non-affiliates of the registrant (computed by reference to the closing sale price of $1.35 for the registrant’s common shares as reported on the Nasdaq Global Market on June 30, 2017) was approximately $83.1 million. As of the close of business on March 8, 2018, there were 67,010,887 common shares issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None
Explanatory Note
We are filing this Amendment No. 1 to Form 10-K (“Amendment No. 1”) to amend our Annual Report on Form 10-K for the fiscal year ended December 31, 2017, as filed with the Securities and Exchange Commission on March 14, 2018 (the “Original Form 10-K”), to include the information required by Part III of Form 10-K and to amend and restate the exhibit list in Item 15 of Part IV, solely to reflect the new certifications being filed by our principal executive officer and principal financial officer herewith. The Part III information was previously omitted from the Original Form 10-K in reliance on General Instruction G(3) to Form 10-K, which permits the information required by Items 10-14 of Part III of the Form 10-K to be incorporated by reference from our definitive proxy statement if such proxy statement is filed no later than 120 days after our fiscal year-end. The information required by Items 10-14 of Part III is no longer being incorporated by reference to the proxy statement relating to our 2018 Annual Meeting of Shareholders. The reference on the cover of the Original Form 10-K to the incorporation by reference to portions of our definitive proxy statement into Part III of the Original Form 10-K is hereby deleted. Except as otherwise expressly set forth in this Amendment No. 1, no portion of the Original Form 10-K is being amended or updated by this Amendment No. 1. Unless the context indicates otherwise, references to “Aralez” or the “Company” herein refer to Aralez Pharmaceuticals, Inc.
|
|
|
Page |
|
|
1 | |
|
|
|
|
|
1 | ||
|
6 | ||
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
33 | |
|
Certain Relationships and Related Transactions and Director Independence |
36 | |
|
37 | ||
|
|
|
|
|
|
| |
|
|
|
|
|
38 | ||
|
|
|
|
|
|
|
ITEM 10. Directors, Executive Officers and Corporate Governance
The Board in General
The board of directors (the “Board”) is currently comprised of seven directors, each of whose current term of office as a director expires at our 2018 annual meeting of shareholders. The Board is responsible for nominating directors for election to the Board and for filling vacancies on the Board that may occur between annual meetings of shareholders.
Biographical information for each of our directors is provided below. There are no familial relationships among any of the executive officers and directors of the Company.
|
Name |
|
Position with the Company |
|
Age |
|
Director Since |
|
Residence |
|
Adrian Adams |
|
Director and Chief Executive Officer |
|
67 |
|
2015 |
|
Pennsylvania, USA |
|
Neal F. Fowler |
|
Director |
|
56 |
|
2016 |
|
North Carolina, USA |
|
Rob Harris |
|
Director |
|
62 |
|
2016 |
|
Ontario, Canada |
|
Arthur S. Kirsch |
|
Director and Chairperson of the Board |
|
66 |
|
2016 |
|
New York, USA |
|
Kenneth B. Lee, Jr. |
|
Director |
|
70 |
|
2016 |
|
North Carolina, USA |
|
Seth A. Rudnick, M.D. |
|
Director |
|
69 |
|
2016 |
|
North Carolina, USA |
|
F. Martin Thrasher |
|
Director |
|
66 |
|
2016 |
|
Ontario, Canada |
Adrian Adams has been our Chief Executive Officer since February 5, 2016, and has been a director of the Company since December 11, 2015 and Chairperson of the Transaction Committee since November 3, 2016. From May 2015 through February 5, 2016, Mr. Adams was the Chief Executive Officer and a director of POZEN Inc. (“Pozen”), and served as a consultant to Pozen from April 2015 to May 2015. Previously, Mr. Adams served as Chief Executive Officer and President and as a director of Auxilium Pharmaceuticals Inc., a specialty biopharmaceutical company, from December 2011 until January 2015, when it was acquired by Endo International plc. Mr. Adams served as the Chairperson and Chief Executive Officer of and a director of Neurologix, Inc. (“Neurologix”), a company focused on the development of multiple innovative gene therapy development programs, from September 2011 to November 2011. Before Neurologix, Mr. Adams served as President, Chief Executive Officer and a director of Inspire Pharmaceuticals, Inc., a specialty pharmaceutical company, from February 2010 until May 2011, when it was acquired by Merck & Co., Inc. Previously, Mr. Adams served as President and Chief Executive Officer of Sepracor Inc., a specialty pharmaceutical company, from March 2007 and May 2007, respectively, until February 2010, when Sepracor was acquired by Dainippon Sumitomo Pharma Co., Ltd. Prior to his appointment as Chief Executive Officer of Sepracor, Mr. Adams served as its Chief Operating Officer. Prior to joining Sepracor, Mr. Adams served as the President and Chief Executive Officer of Kos Pharmaceuticals, Inc., a specialty pharmaceutical company, from 2002 until its acquisition by Abbott Laboratories in December 2006. Mr. Adams has also held general management and senior international and national marketing positions at SmithKline Beecham, Novartis and ICI (now part of AstraZeneca). Mr. Adams has served as Chairperson of the board of directors of AcelRx Pharmaceuticals, Inc. since February 2013 and served on the board of directors of Amylin Pharmaceuticals, Inc. from October 2007 to August 2012. Mr. Adams graduated from the Royal Institute of Chemistry at Salford University in the U.K.
Mr. Adams is a highly qualified pharmaceutical executive who brings to the Board over 30 years of experience in the industry. Mr. Adams has extensive national and international experience and has been instrumental in launching major global brands in addition to driving successful corporate development activities encapsulating financing, product and company acquisitions, in-licensing and company M&A activities.
Neal F. Fowler has been a director of the Company since February 5, 2016, and was previously a director of Pozen from 2010 through February 5, 2016. Mr. Fowler is Chief Executive Officer of Liquidia Technologies, Inc. (“Liquidia”), a biomedicines company, and has served in that capacity since 2008. Mr. Fowler is also a co-founder of Envisia Therapeutics Inc. (“Envisia”), an ophthalmology spin-out of Liquidia, and concurrently served as Chief Executive Officer of Envisia from its launch in 2013 through 2015. Mr. Fowler joined Liquidia in 2008 after seven years at Johnson & Johnson. While at Johnson & Johnson, he served as President of Centocor, Inc. (“Centocor”), a multi-billion dollar subsidiary focused on development and commercialization of industry leading biomedicines used in the treatment of chronic inflammatory diseases. Prior to Centocor, Mr. Fowler was President of Ortho-McNeil Neurologics Inc. and Vice President of the central nervous system franchise at Ortho-McNeil Pharmaceuticals. Mr. Fowler joined Johnson & Johnson after a 13-year career at Eli Lilly and Company where he held a variety of sales, marketing and business development roles with increasing responsibilities in both the pharmaceutical and medical device divisions. Mr. Fowler is a native of Raleigh, NC and received a Bachelor of Science degree in Pharmacy and Masters of Business Administration from the University of North Carolina at Chapel Hill (UNC-CH).
Mr. Fowler brings to the Board his extensive background in the pharmaceutical industry acquired through a variety of senior positions at several large pharmaceutical companies. He is currently chief executive officer at Liquidia, a position that has provided him with experience in running an emerging growth company.
Rob Harris has been a director of the Company since February 5, 2016. He previously served as President, Chief Executive Officer and a director of Tribute Pharmaceuticals Canada Inc. (now known as Aralez Pharmaceuticals Canada Inc.) (“Tribute”) from December 1, 2011 to February 2016. Mr. Harris founded Tribute Pharma, which later became Tribute Pharma Canada Inc. and Tribute Pharmaceuticals Canada Ltd. in November 2005. Tribute acquired both Tribute Pharma Canada Inc. and Tribute Pharmaceuticals Canada Ltd. on December 1, 2011. Mr. Harris was formerly the President and CEO of Legacy Pharmaceuticals Inc. from September 2004 to October 2005. As the VP of Business Development at Biovail Corporation from October 1997 to September 2004, Mr. Harris was involved in, led and successfully concluded numerous business development transactions, including the licensing of new chemical entities, the acquisition of mature products, the completion of co-promotion deals, distribution agreements, product development and reformulation transactions. Mr. Harris joined Biovail in 1997 as the GM of Biovail Pharmaceuticals Canada at a time when the company experienced rapid growth in the Canadian division. Before Biovail, Mr. Harris worked in various senior commercial management positions during his twenty-year tenure at Wyeth (Ayerst) from 1977 to 1997 and has been involved in numerous product launches during his career.
Mr. Harris brings to the Board over 35 years of pharmaceutical industry experience in both Canada and the United States in sales, marketing, business development and general management.
Arthur S. Kirsch has been a director of the Company, Chairperson of the Board, and Chairperson of the Audit Committee since February 5, 2016. Previously, he was a director of Pozen from 2004 through February 5, 2016. Mr. Kirsch has been Senior Advisor, GCA, LLC (formerly GCA Savvian, LLC), an investment bank, since June 2005. Mr. Kirsch was a Managing Director of Vector Securities, LLC, an investment and merchant banking firm, from 2001 to May 2005. He was a Managing Director and Head of Healthcare Research and Capital Markets of Prudential Vector Healthcare Group, a unit of Prudential Securities, Inc., a full-service brokerage firm, from 1999 to 2001. Mr. Kirsch was the Director, Equity Research of Vector Securities International, Inc., an investment banking firm, from 1995 to 1999. He served as a director of Immunomedics, Inc., a publicly-traded biopharmaceutical company, from August 2015 until October 2016. He currently serves as a director of Liquidia, a privately held biotechnology company, since December, 2016.
Mr. Kirsch has over 30 years of experience working in equity capital markets and has extensive knowledge of the healthcare and life sciences field. Mr. Kirsch, who has spent the majority of his career in investment banking with a focus on the healthcare industry, brings both financial and industry expertise to the Board.
Kenneth B. Lee, Jr. has been a director of the Company and Chairperson of the Compensation Committee since February 5, 2016. Previously, he was a director of Pozen from 2002 to February 5, 2016, and from 2002 was also Pozen’s lead Independent Director. Since June 2002 he has been an independent consultant and general partner of Hatteras Venture Partners (formerly Hatteras BioCapital, LLC and BioVista Capital, LLC), and the general partner of Hatteras BioCapital Fund, L.P., a venture capital fund focusing on life sciences companies, since 2003. Mr. Lee was President of A.M. Pappas & Associates, a venture capital firm, between January 2002 and June 2002. He was a Partner of Ernst & Young LLP from 1982 through 2000, and was the National Director of the Life Sciences Practice for the firm. He was a Partner of Ernst & Young Corporate Finance LLC from 2000 to 2001, where he served as the Managing Director of Ernst & Young’s Health Sciences Corporate Finance Group from 2000 to 2001. Mr. Lee has served on the board of directors of Biocryst Pharmaceuticals, Inc., a public company, since 2011, and is currently Chairperson of the audit committee and Chairperson of the finance committee. He has also served on the board of directors of Eyenovia, Inc., a public company, since March 2018, and is currently Chairperson of the audit committee. Mr. Lee is also a director of Clinipace Worldwide, a privately held company. Previously, he served on the boards of directors of CV Therapeutics, Inc., for which he served as lead independent director and Chairperson of the audit committee and a member of the compensation committee, Abgenix, Inc., for which he served on the audit committee and the compensation committee, OSI Pharmaceuticals, for which he served as a member of the audit committee, Inspire Pharmaceuticals Inc., for which he served as Chairperson of the board of directors, and Chairperson of the audit committee and a member of the compensation committee and finance committee, and Maxygen, Inc., for which he served as Chairperson of the audit committee and a member of the nominating/governance committee and the compensation committee. Mr. Lee served as a member of the executive committee of the board of directors of the North Carolina Biotechnology Industry Organization and as a member of the board of directors of Ibiliti, a nonprofit organization dedicated to building and expanding networks of resources for advanced medical technology companies. Mr. Lee is also a co-founder of the National Conference on Biotechnology Ventures.
Mr. Lee brings his extensive accounting and financial background to the Board, as well as expertise in the life sciences industry from his experience as a general partner of several venture capital funds specializing in life sciences. He has also served and is serving on the boards and audit committees of several public pharmaceutical companies similar in size to the Company, including serving as Chairperson of the board of directors of Biocryst Pharmaceuticals, Inc.
Seth A. Rudnick, M.D. has been a director of the Company and Chairperson of the Nominating/Corporate Governance Committee since February 5, 2016. Previously, he was a director of Pozen from 2011 through February 5, 2016. Dr. Rudnick was a venture partner and previously general partner at Canaan Partners, a venture capital firm from 1998 to December 2014. Formerly, Dr. Rudnick was the Chief Executive Officer and Chairperson of CytoTherapeutics Inc., a company developing stem cell-based therapies, from 1991 to 1998. He helped found and served as the Head of Research and Development for Ortho Biotech, a division of Johnson & Johnson focusing on cancer and chronic illnesses from 1986 to 1991. He currently serves on the boards of directors of the following privately held biotechnology companies: Liquidia Technologies, Inc., for which he serves as Chairperson, and G1 Therapeutics, for which he serves as Chairperson. Dr. Rudnick also served on the board of directors of Square 1 Financial, Inc., a public financial services company, from 2012 to October 2015. Currently he is a Clinical Adjunct Professor of Medicine at University of North Carolina, Chapel Hill.
Dr. Rudnick brings to the Board deep operational experience in the pharmaceutical and biotechnology industries acquired through a variety of senior research and development positions in several large and mid-size pharmaceutical companies and as Chief Executive Officer, and Chairperson of CytoTherapeutics, Inc., Chairperson of Liquidia Technologies, Inc., and Executive Chairperson of GI Therapeutics. Dr. Rudnick retired from Canaan Partners, a global venture capital firm with significant investments in the healthcare sector, where he served as general venture partner from 1998 to 2013, which provided him with significant experience in and insight into life sciences investments.
F. Martin Thrasher has been a director of the Company since February 5, 2016. Previously, he was a director of Tribute from 2009 to February 2016. Mr. Thrasher is a seasoned international executive. After graduating from the Richard Ivey School of Business in London, Ontario, Mr. Thrasher spent over 30 years working around the globe for companies such as General Foods from 1973 to 1977, McCormick & Co from 1977 to 1988, Campbell Soup Co. from 1988 to 2001 and ConAgra Foods Inc. from 2001 to 2004. He has served as President of FMT Consultants LLC since 2004. Mr. Thrasher has lived and worked in Canada, Australia, Belgium and the U.S. His responsibilities with Campbell Soup Co. included positions as President, International Grocery and President, North America Grocery. At ConAgra Foods Inc., he was President of the Retail Products Co, a $9 billion business with over 30,000 employees. Mr. Thrasher has been President of FMT Consulting, a boutique advisory and consulting firm since August 2004. In this capacity, he has served in a number of interim CEO and Executive Chairperson positions in Canada and the United States.
Mr. Thrasher brings to the Board extensive international business experience acquired from his time serving at several Fortune 500 companies. He has led large, complex organizations and overseen a variety of mergers and acquistions. Mr. Thrasher also has broad board experience having served on a number of private and public company boards.
Executive Officers
Our executive officers are as follows:
|
Name |
|
Age |
|
Position with the Company |
|
Adrian Adams |
|
67 |
|
Chief Executive Officer |
|
Andrew I. Koven |
|
60 |
|
President and Chief Business Officer |
|
Michael Kaseta |
|
42 |
|
Chief Financial Officer |
|
Jennifer L. Armstrong |
|
47 |
|
Executive Vice President, Human Resources and Administration |
|
James P. Tursi, M.D. |
|
53 |
|
Chief Medical Officer |
Our executive officers are appointed by, and serve at the discretion of, the Board. There are no familial relationships among any of our executive officers and directors of the Company.
Adrian Adams — See “The Board in General” for Mr. Adam’s biography.
Andrew I. Koven has been our President and Chief Business Officer since February 5, 2016. Previously, Mr. Koven was the President and Chief Business Officer of Pozen from June 2015 through February 5, 2016. Prior to joining Pozen, Mr. Koven served as Chief Administrative Officer and General Counsel of Auxilium Pharmaceuticals Inc., a specialty biopharmaceutical company, from February 2012 until January 2015, when it was acquired by Endo International plc. Mr. Koven served as President and Chief Administrative Officer and a member of the board of directors of Neurologix, Inc., a company focused on the development of multiple innovative gene therapy development programs, from September 2011 to December 2011. Before Neurologix, Inc., Mr. Koven served as Executive Vice President and Chief Administrative and Legal Officer of Inspire Pharmaceuticals, Inc., a specialty pharmaceutical company, from July 2010 until May 2011 when it was acquired by Merck & Co., Inc. Previously, Mr. Koven served as Executive Vice President, General Counsel and Corporate Secretary of Sepracor Inc., a specialty pharmaceutical company, from March 2007 until February 2010 when it was acquired by Dainippon Sumitomo Pharma Co., Ltd. Prior to joining Sepracor, Mr. Koven served as
Executive Vice President, General Counsel and Corporate Secretary of Kos Pharmaceuticals, Inc., a specialty pharmaceutical company, from August 2003 until its acquisition by Abbott Laboratories in December 2006. Mr. Koven began his career in the pharmaceutical industry first as an Assistant General Counsel and then as Associate General Counsel at Warner-Lambert Company from 1993 to 2000, followed by his role as Senior Vice President and General Counsel at Lavipharm Corporation from 2000 to 2003. From 1986 to 1992 he was a corporate associate at Cahill, Gordon & Reindel in New York. From 1992 to 1993 he served as Counsel, Corporate and Investment Division, at The Equitable Life Assurance Society of the U.S.
Michael Kaseta has been our Chief Financial Officer (Principal Financial Officer, Principal Accounting Officer) since March 13, 2018. Mr. Kaseta previously served as our Head of Finance and Interim Chief Financial Officer beginning November 30, 2017 and Corporate Controller beginning September 26, 2016. Prior to joining the Company, Mr. Kaseta held various positions at Sanofi S.A. (“Sanofi”), including most recently Chief Financial Officer Sanofi North America, Global Services, from April 2015 through September 2016. Mr. Kaseta was previously the Vice President Sanofi NA Pharma Controlling from January 2013 through April 2015, Vice President, Sanofi Financial Shared Services from March 2007 through December 2013 and Director of Technical Accounting from 2005 to 2007.
Jennifer L. Armstrong has been our Executive Vice President, Human Resources and Administration since February 5, 2016. Ms. Armstrong was previously the Executive Vice President, Human Resources and Administration of Pozen from June 2015 through February 5, 2016. Prior to joining Pozen, she served as Senior Vice President of Human Resources at Auxilium Pharmaceuticals, Inc., a specialty biopharmaceutical company, from July 2009 to March 2015. Prior to that, she served at Senior Vice President of Human Resources and Corporate Communications at Genaera Corporation, a specialty biopharmaceutical company, from January 1998 to May 2009. Ms. Armstrong holds a Master’s degree in Arts Administration and a Bachelor’s degree in Corporate Communications, both from Drexel University.
James P. Tursi, M.D. has been our Chief Medical Officer since February 5, 2016. From October 2015 to February 5, 2016, Dr. Tursi was Chief Medical Officer of Pozen. Previously, Dr. Tursi served as Chief Medical Officer of Innocoll AG, a specialty pharmaceutical company, from March 2015 to September 2015, where he was responsible for managing all clinical research and development, medical affairs and safety activities. Prior to joining Innocoll, Dr. Tursi served as Chief Medical Officer at Auxilium Pharmaceuticals Inc., a specialty biopharmaceutical company, from August 2011 to March 2015, and as Vice President of Clinical Research & Development from March 2009 to August 2011. In these positions, Dr. Tursi was responsible for oversight of clinical and nonclinical development programs, clinical operations, medical affairs and global safety activities, and served as the clinical medical safety lead for all regulatory agency interactions with the FDA, Europe and Canada. Prior to Auxilium, he served as Director of Medical Affairs for GlaxoSmithKline Biologicals from January 2006 to March 2009 and directed all medical affairs responsibilities for the cervical cancer vaccine in North America. Dr. Tursi entered the pharmaceutical industry in 2004 as a Medical Director for Procter and Gamble Pharmaceuticals until 2006. He worked on several products and therapeutic areas, which included female sexual dysfunction, overactive bladder, and osteoporosis. His responsibilities included clinical development and medical affairs. Dr. Tursi was a board certified OB/GYN and practiced medicine and surgery for over 10 years. Dr. Tursi received his doctor of medicine degree from the Medical College of Pennsylvania and completed his residency training at the Johns Hopkins Hospital. Dr. Tursi has served as a member of the board of directors of Agile Therapeutics, a women’s health specialty pharmaceutical company, since October 2014, and is Chairperson of the compensation committee.
Audit Committee
The Company has a separately designated standing audit committee established in accordance with section 3(a)(58)(A) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and National Instrument 52-110 — Audit Committees (“NI 52-110”). The current members of the Audit Committee are Mr. Kirsch, who serves as Chairperson, Mr. Lee and Mr. Thrasher. The Board has determined that each of the members of the Audit Committee is independent as defined by the applicable Nasdaq listing standards, the Securities and Exchange Commission (“SEC”) rules applicable to audit committee members and within the meaning of NI 52-110. Each of the Audit Committee members is able to read and understand fundamental financial statements, including a company’s balance sheet, income statement, and cash flow statement, and has an understanding of the accounting principles used to prepare financial statements, as well as an understanding of the internal controls and procedures necessary for financial reporting. See “- The Board in General” above for the relevant education and experience of each current member of the Audit Committee. The Board has also determined that each member of the Audit Committee qualifies as an “audit committee financial expert” as defined in Item 407(d)(5) of Regulation S-K.
Code of Business Conduct and Ethics
We have adopted a Code of Conduct that applies to our employees, officers (including our principal executive officer, principal financial officer and other members of our finance and administration department) and our directors.
The objective of the Code of Conduct is to provide guidelines for maintaining integrity, honesty and ethical conduct, objectivity and impartiality of the Company. The Code of Conduct addresses, among other topics, conflicts of interest, protection and proper use of corporate assets and opportunities, confidentiality, fair dealing with the Company’s shareholders, customers, suppliers, competitors and employees, compliance with laws, rules and regulations and reporting of any illegal or unethical behavior.
As part of the Code of Conduct, any person subject to the Code of Conduct is required to avoid situations involving a conflict, or an appearance of a conflict, between their personal, family or business interests, and interests of those of the Company and must promptly disclose any such conflict, or an appearance of a conflict, to the Company. The Board has the ultimate responsibility for stewardship of the Code of Conduct. The Board has designated the Nominating/Corporate Governance Committee to oversee the administration of the Code of Conduct. The Nominating/Corporate Governance Committee reviews and approves all related party transactions that must be disclosed pursuant to applicable securities laws and regulations.
All persons subject to the Code of Conduct are required to provide, upon request, certification of compliance with the Code of Conduct, as well as compliance with all Company policies.
The foregoing description of the Code of Conduct is intended as summary only and does not purport to be complete and is subject to, and is qualified in its entirety by reference to, all of the provisions, of the Code of Conduct, a copy of which is available on our website at www.aralez.com and available on SEDAR at www.sedar.com. We will disclose on our website any future amendments to and/or waivers from the Conduct of Conduct that relate to our directors or executive officers.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act, and the rules issued thereunder, requires our directors and executive officers and beneficial owners of more than 10% of our equity securities to file reports of ownership and changes in beneficial ownership of our equity securities with the SEC. Copies of these reports are furnished to the Company. Based solely on our review of the copies of such reports furnished to us, and representations from the persons subject to Section 16(a) with respect to the Company, we believe that during 2017 all of our executive officers, directors and 10% shareholders complied with the Section 16(a) requirements, except that former director Jason Aryeh filed a late Form 4 on May 24, 2017 with respect to 51,041 shares that he sold on May 19, 2017.
ITEM 11. Executive Compensation
Compensation Committee Report
The Compensation Committee has reviewed and discussed with management the disclosures contained in the “Compensation Discussion and Analysis” below. Based on that review and discussion, the Compensation Committee has recommended to the Board that the Compensation Discussion and Analysis be included in this Annual Report on Form 10-K.
|
|
COMPENSATION COMMITTEE: |
|
|
|
|
|
Kenneth B. Lee, Jr. (Chairperson) |
|
|
Neal F. Fowler |
|
|
Seth A. Rudnick, M.D. |
Compensation Discussion and Analysis
This Compensation Discussion and Analysis explains our compensation program for the fiscal year ended December 31, 2017 (“Fiscal Year 2017”) as it pertains to our named executive officers. Our named executive officers for Fiscal Year 2017 consisted of the following:
· Adrian Adams, Chief Executive Officer (“CEO”);
· Andrew I. Koven, President and Chief Business Officer;
· Scott J. Charles, Chief Financial Officer until November 30, 2017;
· Michael Kaseta, Head of Finance and interim Chief Financial Officer beginning November 30, 2017, promoted to Chief Financial Officer on March 13, 2018;
· James P. Tursi, MD, Chief Medical Officer; and
· Mark A. Glickman, Chief Commercial Officer until March 30, 2018.
For purposes of this Compensation Discussion and Analysis, we refer to these persons as our “named executive officers.”
Executive Summary
2017 Compensation Actions
During 2017, we continued to refine our executive compensation program and to make adjustments to align the interests of our executive compensation program with the interests of our shareholders, including through the following actions:
· Reduction of 2016 Annual Bonuses. In March 2017, our Compensation Committee determined that the pre-determined Company performance metrics set by the Compensation Committee early in 2016 with respect to our 2016 Annual Incentive Bonus Plan had been achieved at the 196.76% level. However, due to our poor share price performance and certain business challenges, our Compensation Committee and our management determined that adjustments were appropriate in order to achieve a greater degree of alignment between executive pay and the interests of our shareholders. As such, our Compensation Committee agreed with management’s proposal that the 2016 annual incentive bonuses for our named executive officers would be paid out at the target level, rather than the much higher level of actual achievement under the pre-determined Company performance metrics for 2016.
· Removal of Eligibility for 2017 Annual Bonus. In addition to the reduction of the 2016 annual bonuses, Mr. Adams and Mr. Koven proposed in March 2017 that they remove themselves from eligibility for a cash bonus under our Annual Incentive Bonus Plan with respect to the 2017 performance period and, as such, received no bonuses for the 2017 performance period.
· Downward Adjustment of 2017 Annual Bonuses. In early 2018, our Compensation Committee reviewed our 2017 performance and determined that, while we achieved the Company performance metrics with respect to our 2017 Annual Incentive Bonus Plan at the 106.54% level, adjustments were appropriate in order to achieve a better alignment between our executives receiving bonuses and the interests of our shareholders. Instead, payments under our 2017 Annual Incentive Bonus Plan were made to those receiving bonuses at the 90% level for the Company performance metrics rather than the higher 106.54% level. This downward adjustment demonstrates our commitment to the alignment between executive pay and the interests of our shareholders. Individual performance metrics were paid out at the level determined with respect to each individual’s performance metrics to the extent they received any bonus.
· Updated Peer Group. Our Compensation Committee reviewed and updated our peer group during 2017 in order to ensure that our selected peers are comparable in market capitalization and revenue with respect to both our current and our projected market capitalization and revenue.
Compensation Best Practices
The executive compensation program developed by our Compensation Committee is designed to promote the short- and long-term objectives of Aralez, and to ensure that our executives are provided compensation based on their performance and achievement against these objectives. Our Compensation Committee also determined that our executive compensation program would include the following practices and policies, which help to align the interests of our executives and our shareholders and reduce any risks involved in our compensation program:
· Share Ownership Guidelines. Robust share ownership guidelines that apply to our CEO.
· Share Retention Policy. A share retention policy that applies to all of our named executive officers.
· Anti-Pledging Policy. Executives and directors are not permitted to pledge shares.
· Anti-Hedging Policy. Executives and directors may not enter into hedging transactions.
· Clawback Policy. Clawback policy that applies to incentive-based compensation awarded to executives if there is a restatement of financials.
· No Repricings. The Aralez 2016 Amended and Restated Long-Term Incentive Plan (the “2016 Plan”) prohibits repricings without shareholder approval.
· No Single Trigger Awards. Equity awards granted under the 2016 Plan will not automatically accelerate upon a Change of Control.
· No Section 280G Gross-Ups. Employment agreements no longer contain a Section 280G excise tax gross-up.
· Objective Performance Goals. Our annual incentive bonus awards are determined based on objective performance goals that align with the objectives of our business plan.
2017 Shareholder Say-on-Pay Vote
Aralez provides shareholders the opportunity to cast an annual, non-binding advisory vote on executive compensation (a “say-on-pay proposal”). At the Aralez annual meeting of shareholders held on May 3, 2017, approximately 60% of the votes cast on the say-on-pay proposal were voted in favor of the proposal.
In the beginning of 2017, our directors and management engaged in extensive dialogue with many of our shareholders regarding our executive compensation program. Our directors and management contacted shareholders representing an estimated 44% of our issued and outstanding Common Shares to discuss their concerns, if any, with our executive compensation program and the efforts that we have made to better align the compensation of our executives to the interests of our shareholders.
We understand that there is still some room to make improvements to our executive compensation program, but we are pleased that our shareholders understand the adjustments that we have made to date and the steps we have taken to align the interests of our named executive officers with those of our shareholders. We plan to continue the open dialogue with our shareholders on these and other issues.
Philosophy and Compensation Process
The following is a description of our compensation philosophy and process for determining executive compensation, which applied to the compensation decisions made during Fiscal Year 2017.
Objectives of Executive Compensation Program
Our compensation philosophy was developed by our Compensation Committee following the completion of the Tribute acquisition, and will continue to be refined to ensure alignment with our business and human capital objectives. Our executive compensation program is designed:
· to promote the achievement of our annual and long-term performance objectives as approved by the Compensation Committee and/or the Board;
· to ensure that our executive officers’ interests are aligned with maximizing shareholder value and the medium to long-term success of the Company; and
· to provide compensation packages that will attract, retain, and motivate superior executive personnel.
Our executive compensation program is designed to reward achievement of annual and long-term corporate goals, as well as individual goals that are supportive of our corporate and strategic objectives. We aim to provide higher levels of pay when executive and organizational performance exceeds performance standards. Likewise, individual and organizational performance that falls short of the pre-approved standards will result in payments and overall compensation that are at the lower end of competitive pay ranges. Our compensation programs are designed not only to reward past performance, but to provide incentives for continued high levels of executive performance, particularly through the multi-year vesting of time-based equity awards and the performance-based equity awards. All compensation decisions are guided by the overarching principle that the highest comparative levels of compensation should be paid to our highest performing executives when the Company is achieving high levels of performance.
Our Compensation Committee uses a mix of salary, variable cash and equity-based incentives in our executive compensation program in order to motivate our executive officers to work to fulfill our corporate goals and to build long-term value for our shareholders. Our Compensation Committee also believes that employees should be owners of the Company, and all of our executive officers are shareholders or hold unvested equity-based incentive awards.
Our Compensation Committee strives to mitigate excessive risk-taking that could harm our value or reward poor judgment by our executives. Several features of our executive compensation program reflect sound risk management practices that balance incentive opportunities with long-term value creation for our shareholders: (i) our annual cash incentives are based on the achievement of performance metrics that promote progress towards long-term Company goals rather than rewarding high-risk investments at the expense of long-term Company value; (ii) our Compensation Committee allocates compensation among base salary and short and long-term compensation target opportunities in such a way as to not encourage excessive risk-taking; and (iii) our Compensation Committee grants a mix of equity award instruments that include performance-based equity awards, full value awards, and appreciation awards, and have multi-year vesting periods, which mitigates risk and properly accounts for the time horizon of risk. Our Compensation Committee has determined that our policies, practices, and programs do not create risks that are likely to have a material adverse impact on the Company.
Role of Compensation Committee, Executive Officers, and Compensation Consultant
Our Compensation Committee is responsible for our executive compensation program. In accordance with its charter, our Compensation Committee’s responsibilities include reviewing and approving our overall compensation philosophy and the adequacy and market effectiveness of our compensation plans and programs; evaluating the performance of, and reviewing and approving total compensation for, its executive officers; and administering its equity-based and other incentive programs.
As required by its charter, our Compensation Committee is composed solely of independent directors under Nasdaq and SEC rules and “outside directors” as determined under Section 162(m) of the Internal Revenue Code, as amended (the “Code”) and the applicable Treasury Regulations.
Our Compensation Committee, when necessary, receives staff support from members of our executive management team, including Mr. Adams, Jennifer Armstrong, our Executive Vice President, Human Resources and Administration, and Eric Trachtenberg, our former General Counsel, Chief Compliance Officer and Corporate Secretary. Our management provides input to
our Compensation Committee regarding corporate goals and performance criteria for our annual incentive awards and long-term performance awards. In evaluating our executive officers other than the CEO, our Compensation Committee relies in part on the input and recommendations of our CEO. In evaluating our CEO’s compensation, our Compensation Committee considers, among other factors, an annual self-assessment submitted by our CEO, as well as a thorough review of corporate performance. Our CEO is not present during our Compensation Committee’s deliberations or determinations of his compensation.
In addition, our Compensation Committee has engaged Radford, an Aon Hewitt Company, and leading compensation consultant, to assist the Compensation Committee in the performance of its duties. During Fiscal Year 2017, Radford has assisted our Compensation Committee with the design and refinement of our executive compensation program, including an assessment of Board compensation and the establishment of our peer group (described below) and has provided data relating to the compensation practices of our peers. Other than services provided to our Compensation Committee, Radford has not performed any services for Aralez or any of its management during 2017.
Peer Group and Benchmarking
In November 2016, our Compensation Committee engaged Radford to perform an analysis of our peer group to determine its appropriateness and whether any peer companies should be added or removed. Our Compensation Committee considered many factors in selecting peer companies, including whether a potential peer has products on the market, whether a potential peer has executive positions of similar scope and responsibility, as well as whether investors might consider such company as a peer when considering investments in the Company. The peers range from 0.5x to 3.0x of our projected market capitalization and from 0.5x to 3.0x of our projected revenue as of November 2016. The peers have market capitalization ranging from approximately $223 million to $1.4 billion, and annual revenue ranging from approximately $60 million to $441 million.
The peer group, detailed below, was used for purposes of compensation benchmarking for 2017:
|
AMAG Pharmaceuticals |
|
Merrimack Pharmaceuticals |
|
|
Amarin |
|
Momenta Pharmaceuticals |
|
|
ANI Pharmaceuticals |
|
Retrophin |
|
|
Anika Therapeutics |
|
SciClone Pharmaceuticals |
|
|
Arena Pharmaceuticals |
|
Spectrum Pharmaceuticals |
|
|
Enanta Pharmaceuticals |
|
Sucampo Pharmaceuticals |
|
|
ImmunoGen |
|
Vanda Pharmaceuticals |
|
In determining our peer group, our Compensation Committee considered the peer group criteria used by institutional investor advisory groups (such as ISS and Glass Lewis) for making comparisons. Because the institutional investor advisory firms select peer companies from broad industry categories and do not focus on companies with products on the market and with similar business models, we have found that there is only limited overlap between the Aralez peer group and those used by the institutional investor advisory firms.
Our Compensation Committee reviews our peer group annually to ensure that it remains appropriate based on our updated financial performance, market capitalization, and other criteria. In October 2017, our Compensation Committee approved a new peer group, for use in 2018, as detailed below:
|
AMAG Pharmaceuticals |
|
Retrophin |
|
Amarin |
|
SciClone Pharmaceuticals |
|
Amphastar Pharmaceuticals |
|
Spectrum Pharmaceuticals |
|
ANI Pharmaceuticals |
|
Sucampo Pharmaceuticals |
|
Depomed |
|
Teligent |
|
Eagle Pharmaceuticals |
|
Vanda Pharmaceuticals |
|
PDL BioPharma |
|
Vericel |
|
Progenics Pharmaceuticals |
|
VIVUS |
The updated peers range from 0.4x to 3.4x of our projected market capitalization and from 0.3x to 4.0x of our projected revenue as of October 2017. The new peers have market capitalization ranging from $100 million to $916 million, and annual revenue ranging from approximately $53 million to $593 million.
Elements of Compensation
Our executive compensation program, as designed by our Compensation Committee, incorporates four primary elements of compensation, described below:
· Base salary: Fixed compensation based on the role of the executive and the base salary for similar positions in our market for talent. Base salary provides compensation regardless of market performance or external factors and helps to retain our management team.
· Annual cash incentives: Variable compensation based on Company performance and individual performance. Annual cash incentives encourage our executives to work towards our corporate goals and provide our executives an opportunity to earn additional compensation for outstanding performance.
· Long-term incentive compensation: Variable compensation based on the value of our Common Shares. We grant both time-based awards and performance-based awards in order to retain our executives and to reward them for performance. Equity-based compensation aligns the interests of our executives with the interests of our shareholders.
· Other benefits: Our executives participate in our benefit plans on the same basis as other employees, and we do not provide material perquisites.
In addition, employment agreements with each of our named executive officers provide for potential payments upon certain terminations of employment and upon a change of control of our company, which are described in the narrative accompanying the Summary Compensation Table and Grants of Plan-Based Awards in 2017 Table and the section of this Annual Report on Form 10-K entitled “Potential Payments on Termination and Change of Control”. Our Compensation Committee believes that each of these compensation elements complements the others and that together they serve to achieve our compensation objectives.
Compensation awarded to our named executive officers is weighted towards performance-based compensation, with an emphasis on long-term equity incentive awards. We believe that putting a substantial proportion of our named executive officers’ compensation “at risk” motivates our executives to achieve our corporate objectives, and delivering compensation in the form of equity creates an ownership culture amongst our executives and ensures that our executives are focused on the interests of our shareholders.
The chart below illustrates the proportion of our CEO’s targeted compensation for 2017 that is allocated to each type of compensation.
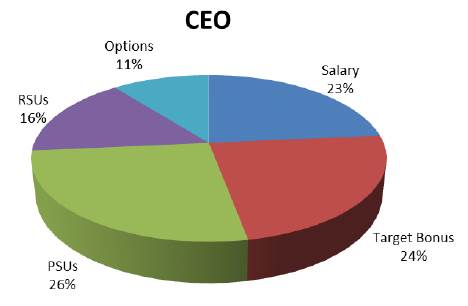
The target annual cash incentive bonus and performance share units are earned based on pre-established Company performance goals, meaning that approximately 50% of our CEO’s target compensation is only earned if the performance goals have been satisfied. Equity-based compensation makes up approximately 53% of our CEO’s target compensation, the value of which will increase or decrease with the price of our Common Shares, ensuring an alignment between our executives and our shareholders. Only 23% of our CEO’s target compensation is fixed, and the remaining 77% is variable, based on our performance and/or the price of our Common Shares. In addition, due to his removal of eligibility to receive an annual cash incentive bonus under our 2017 Annual Incentive Bonus
Plan, our CEO was not eligible to receive his target annual compensation for 2017 (which makes up 24% of his targeted compensation), and the value of restricted stock units (“RSUs”) and stock options granted to our CEO during 2017 was reduced by 30% from the targeted level.
Base Salary
The base salary of our CEO and other named executive officers is intended to provide a level of assured cash compensation that is commensurate with their senior professional status and career accomplishments. Accordingly, their base salaries are designed to be competitive with similar positions within the specialty biopharmaceutical industry. Our Compensation Committee relies on peer group analysis, surveys, and the advice of Radford to set base salaries for our named executive officers that are benchmarked to similar roles in the peer group.
Base salary adjustments include a combination of cost-of-living and merit increases, as appropriate, based on the executive’s performance of his or her key responsibilities and duties, and will be generally approved, communicated, and implemented in March of each year to allow for evaluation of the entire year, including our financial performance. Our Compensation Committee considers the CEO’s assessment of and recommendations with respect to each of the other executive officers. In addition, our Compensation Committee considers the market pay practices for the individual jobs.
Our Compensation Committee reviewed the base salaries of our named executive officers in March 2017 and approved a 3% merit-based increase for each of our named executive officers (other than Michael Kaseta). Michael Kaseta received a base salary of $300,000 until November 2017 when he was promoted to interim CFO, at which time his base salary was increased to $350,000. The 2017 base salary for each of our named executive officers is below:
|
Named Executive Officer |
|
2017 Base Salary |
| |
|
Adrian Adams |
|
$ |
721,000 |
|
|
Andrew I. Koven |
|
$ |
463,500 |
|
|
Scott J. Charles |
|
$ |
412,000 |
|
|
James P. Tursi, MD |
|
$ |
412,000 |
|
|
Mark A. Glickman |
|
$ |
397,000 |
|
|
Michael Kaseta |
|
$ |
350,000 |
|
Annual Cash Incentives
We award annual cash incentives to our named executive officers in order to reward them for the achievement of short-term goals relating to the financial performance of the Company as well as their execution of our operating plan and strategic initiatives. Annual cash incentives are paid pursuant to our Annual Incentive Bonus Plan, which is a sub-plan under our 2016 Plan, except that the annual cash incentive awarded to Mr. Kaseta for 2017 was paid pursuant to our bonus plan for non-executives. For 2018 and future years, Mr. Kaseta will be eligible to participate in our Annual Incentive Bonus Plan.
Target annual cash incentives are based on a percentage of each named executive officer’s base salary. The target annual cash incentive level for each named executive officer is specified in his employment agreement. Annual cash incentive targets were set based upon advice from the Compensation Committee’s independent consultants and through negotiations with our executives when they were hired. In 2017, Mr. Charles’ target annual cash incentive was increased from 45% to 50% based on our assessment of the annual cash incentive targets of other Chief Financial Officers in our industry.
|
Named Executive Officer |
|
2017 Annual Cash |
|
|
Adrian Adams |
|
100 |
% |
|
Andrew I. Koven |
|
75 |
% |
|
Scott J. Charles |
|
50 |
% |
|
James P. Tursi, MD |
|
45 |
% |
|
Mark A. Glickman |
|
45 |
% |
Under the Annual Incentive Bonus Plan, the annual cash incentive for our named executive officers is based 70% on the achievement of Company goals and 30% on the achievement of individual performance goals related to the named executive officer’s role with the Company (for the CEO, 75% based on Company goals and 25% based on individual performance goals).
In March 2017, Mr. Adams and Mr. Koven proposed that they remove themselves from eligibility for a cash bonus under the Annual Incentive Bonus Plan with respect to the 2017 performance period in order to align their compensation with the interests of
our shareholders to a very significant degree. As a result, neither Mr. Adams nor Mr. Koven received an annual cash incentive award with respect to 2017.
Company Performance Goals
For Fiscal Year 2017, our Compensation Committee set Company performance goals based on three separate performance metrics, with the weightings set forth below:
|
Company Operating Metric |
|
Weighting |
|
|
Net Revenues |
|
50 |
% |
|
Adjusted EBITDA |
|
25 |
% |
|
Adjusted Net Income |
|
25 |
% |
Adjusted EBITDA and Adjusted Net Income results are adjusted at the end of the performance period to take into account items of significant income or expense which are determined to be appropriate adjustments and to exclude the following one-time/discrete items:
· Items related to a change in accounting principle and tax law;
· Costs to evaluate, execute and integrate acquisitions, and related accounting implications, including acquired in-process research and development;
· Items related to the sale or disposition of a business, segment or product, including discontinued operations;
· Other items including changes in foreign currency exchange rates, asset impairment charges, income tax adjustments and losses on extinguishment or modification of debt;
· Charges related to share-based compensation including charges related to warrants;
· Depreciation and amortization;
· Restructuring costs; and
· Financing costs as well as related interest expense implications.
Performance goals are based on our corporate plan for Fiscal Year 2017, with performance at the budget level resulting in a payout at approximately the target level.
Each performance goal is assigned a threshold, target, stretch, and super-stretch level. Payout (as a percentage of target) for each level of achievement is set forth below:
|
|
|
Payout |
|
|
Below Threshold |
|
0 |
% |
|
Threshold |
|
75 |
% |
|
Target |
|
100 |
% |
|
Stretch |
|
150 |
% |
|
Super-Stretch |
|
250 |
% |
The threshold level of performance must be achieved on the Net Revenue goal and also either Adjusted EBITDA or Adjusted Net Income in order for payment on any performance goal to be paid out above the target level. The Net Revenue target for 2017 was $100.0 million, with a threshold performance target of $70.0 million and maximum payout at the performance target of $150.0 million. The Adjusted EBITDA target was $0.0 million, with a threshold performance target of ($25.0) million and maximum payout at the performance target of $10.0 million. The Adjusted Net Income target was ($35.0) million, with a threshold performance target of ($60.0) million and maximum payout at the performance target of ($10.0) million. Payout percentages are interpolated in a straight-line basis between band points. Our Compensation Committee set performance goals that are aligned with our corporate plan for Fiscal Year 2017 such that the stretch, and super-stretch payout levels would require extraordinary performance.
Individual Performance Goals
For 2017, 30% of each named executive officer’s potential bonus (25% of the CEO’s potential bonus), as set forth above, is based on the achievement of the executive’s individual performance goals for the year. The individual goals are set at the beginning of the year and are based on each executive’s role with the Company and to closely correlate to the Company’s strategic goals for 2017.
Mr. Koven’s individual goals primarily related to his role as President and Chief Business Officer, including management of Aralez Canada, Ireland, and certain business development functions including completion of transition of Zontivity and Toprol-XL® and its authorized generic (the “Toprol-XL Franchise”) to Aralez, and consideration of the sale, out-licensing, or discontinuation of Aralez Canada non-core products.
Dr. Tursi’s individual goals primarily related to his role as Chief Medical Officer, including support of the Zontivity launch and the Yosprala commercialization, submission of Yosprala in Europe, and business development.
Mr. Glickman’s individual goals primarily related to his role as Chief Commercial Officer, including achievement of net revenue targets for Zontivity and Yosprala, managed care initiatives, and executing plans to fully leverage the Toprol-XL Franchise in 2018.
2017 Performance
In the first quarter of 2018, our Compensation Committee reviewed 2017 performance and determined the level of achievement of the performance goals for the annual incentive bonuses and the individual performance of each of our named executive officers other than the CEO. The actual results with respect to 2017 Company performance are set forth below:
|
Company Operating Metric |
|
Actual |
|
Percentage of |
| |
|
Net Revenues |
|
$ |
105.9 |
|
109.83 |
% |
|
Adjusted EBITDA |
|
$ |
(4.5 |
) |
95.49 |
% |
|
Adjusted Net Income |
|
$ |
(32.8 |
) |
111.00 |
% |
|
Total Achievement (as percentage of target): |
|
|
|
106.54 |
% | |
With respect to individual performance, our Compensation Committee accepted Mr. Adams’ proposal that he achieved 25% of his individual objectives and Mr. Koven achieved 89% of his individual objectives, despite the fact that they had removed themselves from eligibility for a 2017 annual bonus. The Compensation Committee further determined that Dr. Tursi achieved 98% of his individual objectives and Mr. Glickman achieved 86% of his individual objectives.
Notwithstanding the higher achievement percentages under the 2017 pre-determined Company performance metrics (i.e., the 106.54% above), the Compensation Committee and our management have determined that the annual incentive bonuses earned under the 2017 formula should be adjusted downward to a flat 105%. However, in order to achieve a greater degree of alignment with our shareholders, our Compensation Committee and management further agreed that the annual cash incentive awards would be paid out only to our executives at the 90% level for the Company performance portion, rather than the approved higher level resulting from the use of the 105% Company performance factor. As a result of this determination, the annual cash incentive awards earned by Dr. Tursi and Mr. Glickman are less than they would have been entitled to receive under the pre-determined formula. In addition, Mr. Adams and Mr. Koven proposed in March 2017 to remove themselves from eligibility for a cash bonus under the Annual Incentive Bonus Plan with respect to the 2017 performance period. The reduction in annual cash incentive awards earned and the foregoing by Mr. Adams and Mr. Koven of their eligibility to receive cash bonuses for 2017 under the Annual Incentive Bonus Plan shows the commitment by the Compensation Committee and management to align our executive compensation program with the interests of our shareholders to a very significant degree. We note that annual incentive bonus is only one component of executive pay. The long-term equity incentive portion of executive pay is already fully aligned with the interests of our shareholders.
The amount each named executive would have been entitled to under the actual 2017 results (including, for Mr. Adams and Mr. Koven, if they had not removed themselves for eligibility to receive a cash bonus for 2017) and the actual amount paid to each named executive officer is set forth below:
|
Named Executive Officer |
|
2017 Annual Cash |
|
2017 Annual Cash |
| ||
|
Adrian Adams(1) |
|
$ |
612,850 |
|
$ |
0 |
|
|
Andrew I. Koven(1) |
|
$ |
348,320 |
|
$ |
0 |
|
|
Scott J. Charles(2) |
|
$ |
0 |
|
$ |
0 |
|
|
James P. Tursi, MD |
|
$ |
190,777 |
|
$ |
171,032 |
|
|
Mark A. Glickman |
|
$ |
177,400 |
|
$ |
158,508 |
|
(1) Mr. Adams and Mr. Koven removed themselves from eligibility to receive an annual bonus for 2017 and, as such, received no bonuses for 2017.
(2) Mr. Charles did not receive an annual bonus for 2017 due to his separation of employment on November 30, 2017.
Bonus Award for Mr. Kaseta
Mr. Kaseta was awarded an annual bonus for 2017 performance under the non-executive annual bonus plan in which he was eligible for a target bonus of 30% of his base salary, which was increased to 35% of his base salary for the portion of 2017 that he held the position of interim CFO, for a total target bonus of $92,708. Mr. Kaseta’s annual bonus was weighted 55% upon the achievement of corporate metrics and 45% upon his individual objectives.
The corporate metrics were identical to the corporate metrics discussed above for the other named executive officers. Mr. Kaseta’s individual goals primarily related to his position as the Company’s Corporate Controller, including financial process improvement, completion of integration of the Toprol-XL Franchise and Zontivity acquisitions, and support of the CFO in business development activities.
Mr. Kaseta received a 2017 annual bonus in the amount of $99,430 which represents achievement of 105% of the corporate metrics and 110% of his individual objectives.
Long-Term Incentive Compensation
Long-term equity-based incentive compensation is a key element of the Aralez executive compensation program. Equity-based incentive compensation ties a significant portion of compensation to Company performance by linking a significant portion of the executive’s total pay opportunity to the price of our Common Shares. The long-term equity-based compensation package granted to our executives is composed of time-based stock options and RSUs, and performance-based restricted stock units (“PSUs”), in the proportions set forth below:
|
Stock |
|
RSUs |
|
PSUs |
|
|
20 |
% |
30 |
% |
50 |
% |
Our Compensation Committee grants these types of awards together in order to balance the goals of retention and pay-for-performance.
|
Stock Options: |
|
Stock options vest over four years, with 25% vesting on the first anniversary of the date of grant and the remaining 75% vesting in equal installments on a monthly basis over the remaining three years, and have an exercise price equal to the fair market value of our Common Shares on the date of grant. Accordingly, the actual value an executive will realize is tied to future stock appreciation and is therefore aligned with corporate performance and shareholder returns. Stock options granted prior to May 2017 vest in equal installments on a monthly basis over four years. |
|
|
|
|
|
Restricted Stock Units: |
|
RSUs vest in equal annual installments over a three year vesting period. RSUs ensure that each of our executives is a true owner of the Company and help to retain our executives over the vesting period. |
|
Performance-Based Restricted Stock Units: |
|
PSUs vest at the end of a three-year performance period based on the achievement of pre-determined performance goals. Our Compensation Committee granted PSUs to our named executive officers in Fiscal Year 2016 with a three-year relative total shareholder return as the performance goal (measured against companies in the Nasdaq biotechnology index with annual revenue between $50 million and $500 million). Target performance (TSR in the 50th percentile) will pay out the target number of PSUs, while threshold performance (TSR in the 25th percentile) will pay out 50% of the PSUs, performance above target (TSR in the 75th percentile) will pay out 150%, and stretch performance (TSR in the 90th percentile) will pay out 200%. PSUs motivate our executives by providing them an opportunity to increase their compensation for extraordinary performance and align the interests of our executives with the interests of our shareholders. |
Our named executive officers (other than Mr. Kaseta) were granted PSUs on March 15, 2017 and stock options and RSUs on May 11, 2017, with the values set forth in the table below (at the target level). It is important to note that, although the value of the stock options and RSUs to be granted to these executives was established by the Committee on March 8, 2017, a decline in the Company’s stock price between March 8, 2017 and May 11, 2017 would have significantly increased the number of stock options and RSUs to which the executives would have been entitled had the awards been made as approved. In order to better align the compensation of these named executive officers with the interests of shareholders, the executives and the Committee agreed to reduce the number of stock options and RSUs to which the executives were entitled by 30%. Accordingly, the grant date fair value of the stock option and RSU awards actually made to the Company’s named executive officers as set forth in the table below are 30% less than those to which they were contractually entitled under their employment agreements and 30% less than the value originally approved by the Committee.
|
Named Executive Officer |
|
Stock Options(1) |
|
RSUs(1) |
|
PSUs(1) |
| |||
|
Adrian Adams |
|
$ |
228,005 |
|
$ |
340,673 |
|
$ |
811,125 |
|
|
Andrew I. Koven |
|
$ |
114,002 |
|
$ |
170,336 |
|
$ |
405,562 |
|
|
Scott J. Charles |
|
$ |
86,859 |
|
$ |
129,780 |
|
$ |
309,001 |
|
|
James P. Tursi, MD |
|
$ |
86,859 |
|
$ |
129,780 |
|
$ |
309,001 |
|
|
Mark A. Glickman |
|
$ |
83,697 |
|
$ |
125,054 |
|
$ |
297,750 |
|
(1) Grant date fair value of stock options was determined using a Black-Scholes model. The grant date fair value of RSUs and PSUs was determined using the “face value” of these awards, based on the closing price of our stock on Nasdaq on the date of grant (although, for accounting purposes and in the Summary Compensation Table, the PSUs will be subject to a “Monte Carlo” simulation).
Mr. Kaseta was not an executive officer at the beginning of 2017, and received grants of stock options and RSUs in 2017, but was not eligible to receive grants of PSUs. Mr. Kaseta received grants of stock options on March 15, 2017, May 11, 2017 and, in connection with his appointment as interim CFO, on November 30, 2017. Mr. Kaseta received a grant of RSUs on March 15, 2017. The vesting terms of Mr. Kaseta’s stock options and RSU grants are the same as set forth above with respect to the other named executive officers.
Procedures and Policies for Granting Equity-Based Awards
Our Compensation Committee approves the grant of all equity and equity-based awards to our CEO and other executive officers, as well as to the non-employee members of our Board. PSUs were granted to our named executive officers for Fiscal Year 2017 in March following the end of our year-end blackout period. Stock options and RSUs were granted to our named executive officers for Fiscal Year 2017 in May after our shareholders approved the increase of shares in the 2016 Plan. In all cases, stock options are granted at exercise prices equal to the closing price of our Common Shares as reported on Nasdaq on the date of grant.
New-hire grants for our executive officers are approved by our Compensation Committee prior to employment. As permitted under the 2016 Plan, our Compensation Committee has delegated to the CEO the authority to grant stock options and RSUs to new non-executive officer employees upon commencement of employment in accordance with a specified schedule of numbers of stock options or RSUs per grant, based on hiring position. However, at this time all stock options and RSUs granted to new non-executive officer employees are brought to and approved by the Compensation Committee.
Other Benefits
Benefits offered to our named executive officers serve as a safety net of protection against financial catastrophes that can result from illness, disability or death. Benefits offered to our named executive officers are substantially the same as those offered to all of our regular full-time employees. We maintain a 401(k) plan for our employees, including our named executive officers, to encourage our employees to save some portion of their cash compensation for their eventual retirement. Effective January 1, 2017, we match 50% of the first 6% of an employee’s eligible earnings in the 401(k) plan, with the 401(k) match capped at $12,000. We generally do not provide substantial perquisites to our executives. In addition, we offer Canadian tax preparation services for our named executive officers who are primarily located in the United States but are required to file tax returns in Canada. We also reimburse our named executive officers for the Canadian Pension Payment that they are required under Canadian law to pay, despite being ineligible to receive Canadian pension benefits, and we provide a tax equalization payment to reimburse these named executive officers for Canadian, U.S. and state taxes that apply to such payment.
Post-Employment Benefits
Providing reasonable severance benefits to our named executive officers in the context of termination by us without cause or by the executive for good reason (as defined in their employment agreements), either in connection with a change of control or otherwise, is an important part of maintaining a competitive executive compensation program and contributes to our ability to attract and retain high quality executives. In part, this reflects a recognition that it may be difficult for a senior executive to find a comparable position in a relatively short period of time following termination of employment. Providing reasonable protections to our named executive officers in the event of a change of control is helpful in aligning our executives’ interests with those of our shareholders in the event a potential change of control situation should occur.
Pozen entered into employment agreements with our named executive officers (other than Mr. Kaseta) when they were initially hired by Pozen. We entered into an employment agreement with Mr. Kaseta in March 2018, when he was appointed CFO. These agreements require that we provide severance and related benefits in the event of a termination of employment or a change of control. Our Compensation Committee received advice from its legal and compensation consultants as to practices and levels of such benefits among comparable companies. These provisions and benefits, as well as an estimate of the dollar value of these benefits that would be payable to our executive officers under specified assumed conditions are described in the section of this Annual Report on Form 10-K entitled “Potential Payments on Termination and Change of Control.”
We do not offer post-employment health or life insurance to our named executive officers other than the continuation of medical (and life insurance for Mr. Adams and Mr. Koven) benefits for a period following certain terminations (subject to payment of active employee rates) pursuant to their employment agreements.
Share Ownership Guidelines
Employee ownership is a core component of our operating culture, and we believe that share ownership encourages our executives to create value for our shareholders over the long term, and promotes retention and affiliation with the Company by allowing our employees to share in our long-term success while aligning employee and executive interests with those of our shareholders. To reflect this commitment to employee ownership, we have adopted share ownership guidelines, which require the CEO to hold shares with a value equal to six times base salary, as well as a share retention policy for all named executive officers, which requires such officers to retain at least 50% of the total equity credited from grants of equity awards (net of amounts required to pay taxes and exercise prices) while such individual remains a named executive officer. As of December 31, 2017, Mr. Adams owned vested Common Shares with a value greater than seven times his 2017 base salary. We expect that the newly hired members of our management team will comply with the share retention policy as their equity awards vest.
Anti-Hedging/Anti-Pledging Policy
Certain short-term or speculative transactions in our securities by directors or executive officers create the potential for heightened legal risk and/or appearance of improper or inappropriate conduct involving our securities. As a result, we do not allow any director or executive officer to hedge the economic risk of his or her ownership of Common Shares, which includes entering into any derivative transaction on Aralez securities (e.g., any short-sale, forward, option, collar). Further, we do not allow any director or executive officer to pledge Aralez securities at any time, which includes having Aralez securities in a margin account or using Aralez securities as collateral for a loan.
Clawback of Incentive Compensation
Our Board adopted an incentive-based compensation recovery policy that applies to all executives, including the named executive officers. The policy relates to the recoupment of incentive compensation awarded to these executives if there is a restatement of published financials.
CEO Pay Ratio
As a result of the recently adopted rules under the Dodd-Frank Act, the SEC now requires public companies to compare the compensation of their chief executive officer to the median compensation of their other employees, and disclose that information in proxy and information statements, registration statements and annual reports that must contain executive compensation information (“CEO Pay Ratio Disclosure”).
Our CEO’s annual total compensation for 2017, as reflected in the Summary Compensation Table, was $2,099,113. Our median employee’s annual total compensation for 2017 was $145,372. As a result, our 2017 CEO to median employee pay ratio is 14:1.
We identified the median employee by examining the 2017 W-2 compensation data from our payroll systems for employees, excluding our CEO, who were employed by us on December 31, 2017, the last day of our payroll year. We excluded employees located in Ireland, per the de minimis exemption which allows a company to exclude all non-US employees in certain jurisdictions if the aggregate number of employees in such jurisdictions account for 5% or less of the company’s total employees. We have 6 employees in Ireland out of 173 total employees, and thus Ireland employees account for 3.4% of Aralez’s total employee pool.
After identifying the median employee based on the 2017 W-2 compensation data, we calculated annual total compensation for such employee using the same methodology we use for our named executive officers as set forth in the 2017 Summary Compensation Table.
Tax and Accounting Implications
In setting elements of compensation, our Compensation Committee considers the impact of the following tax and accounting provisions:
· Section 162(m). In making compensation decisions, our Compensation Committee is mindful of the potential impact of Section 162(m) of the Code, which generally disallows a tax deduction to public companies for certain compensation over $1 million paid in any year to its chief executive officer and its three most highly compensated executive officers (other than its chief executive officer and chief financial officer). Qualifying performance-based compensation is not subject to this deduction limit if certain requirements are met. Our Compensation Committee generally seeks, where feasible, to structure the incentive compensation granted to our named executive officers in a manner that is intended to minimize or eliminate the impact of Section 162(m) of the Code. However, the Compensation Committee may at times elect to make awards that are subject to the Section 162(m) deduction limit, such as time-based RSUs or cash awards when it believes that such awards are appropriate to attract and retain top-quality executives or otherwise achieve its compensation objectives.
Beginning with the 2018 fiscal year, compensation in excess of $1 million that is paid to any named executive officer will no longer be exempt from the limitation on deductibility under the performance-based compensation exception except under certain limited circumstances where the arrangements were in effect on November 2, 2017. In addition, beginning in the 2018 fiscal year, the $1 million deduction limitation will apply to any individual who was subject to Section 162(m) of the Code during any preceding year beginning after December 31, 2016, regardless of whether the individual is a named executive officer for the current fiscal year.
· Section 409A. Section 409A of the Code, which governs the form and timing of payment of deferred compensation, generally changes the tax rules that affect most forms of deferred compensation that were not earned and vested prior to 2005. It also expands the types of compensation that are considered deferred compensation subject to these regulations. Section 409A imposes sanctions, including a 20% penalty and an interest penalty, on the recipient of deferred compensation that does not comply with Section 409A. Our Compensation Committee considers the potential implications of Section 409A of the Code in determining the form and timing of compensation awarded to our executives.
· Sections 280G and 4999. No employment agreement executed since January 1, 2009 provides for a gross-up payment to reimburse the executive for certain excise taxes imposed under Section 4999 of the Code as well as additional taxes resulting from such reimbursement, and there is no gross-up payment provision in the employment agreements with our named executive officers.
· Accounting Rules. Various rules under generally accepted accounting principles (GAAP) determine the manner in which grants for equity-based and other compensation are accounted for in our financial statements. Aralez records compensation expenses with respect to equity awards in accordance with FASB ASC Topic 718. Among the factors it has considered when making compensation decisions for our named executive officers, our Compensation Committee has taken into account the accounting treatment under FASB ASC Topic 718 of equity-based and alternative forms of compensation.
Summary Compensation Table (for fiscal years 2017, 2016 and 2015)
The following table summarizes the total compensation paid to or earned by, or with regard to stock awards and options, the grant date fair value of such awards granted during the fiscal years ended December 31, 2017, 2016 and 2015 to our named executive officers.
|
Name and Principal Position(1) |
|
Year |
|
Salary |
|
Bonus |
|
Stock |
|
Option |
|
Non-Equity |
|
All Other |
|
Total | |||||||
|
Adrian Adams |
|
2017 |
|
$ |
721,000 |
|
— |
|
$ |
1,138,108 |
|
$ |
228,005 |
|
$ |
0 |
|
$ |
12,000 |
(5) |
$ |
2,099,113 | |
|
Chief Executive Officer |
|
2016 |
|
$ |
700,000 |
|
— |
|
$ |
1,373,981 |
|
$ |
174,666 |
|
$ |
700,000 |
|
$ |
5,085,997 |
|
$ |
8,034,644 | |
|
|
|
2015 |
|
$ |
410,217 |
|
— |
|
$ |
14,858,944 |
|
— |
|
$ |
408,333 |
|
$ |
197,882 |
|
$ |
15,875,376 | ||
|
Andrew I. Koven |
|
2017 |
|
$ |
463,500 |
|
— |
|
$ |
569,053 |
|
$ |
114,002 |
|
$ |
0 |
|
$ |
12,000 |
(6) |
$ |
1,158,555 | |
|
President and Chief Business Officer |
|
2016 |
|
$ |
450,000 |
|
— |
|
$ |
686,557 |
|
$ |
87,277 |
|
$ |
337,500 |
|
$ |
4,229,174 |
|
$ |
5,790,508 | |
|
|
2015 |
|
$ |
264,383 |
|
— |
|
$ |
11,281,789 |
|
— |
|
196,875 |
|
$ |
221,919 |
|
$ |
11,964,966 | ||||
|
Scott J. Charles |
|
2017 |
|
$ |
375,667 |
|
— |
|
$ |
433,565 |
|
$ |
86,859 |
|
$ |
0 |
|
$ |
601,000 |
(7) |
$ |
1,497,091 | |
|
Former Chief Financial Officer |
|
2016 |
|
$ |
400,000 |
|
— |
|
$ |
1,014,194 |
|
$ |
66,540 |
|
$ |
180,000 |
|
$ |
545,688 |
|
$ |
2,206,422 | |
|
|
|
2015 |
|
$ |
175,572 |
|
$ |
400,000 |
|
$ |
355,471 |
|
— |
|
77,500 |
|
$ |
— |
|
$ |
1,008,543 | ||
|
Michael Kaseta |
|
2017 |
|
$ |
305,513 |
|
— |
|
$ |
5,925 |
|
$ |
51,181 |
|
$ |
99,430 |
|
$ |
9,000 |
(8) |
$ |
471,049 | |
|
Chief Financial Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
James P. Tursi, MD |
|
2017 |
|
$ |
412,000 |
|
— |
|
$ |
433,565 |
|
$ |
86,859 |
|
$ |
171,032 |
|
$ |
10,280 |
(9) |
$ |
1,113,736 | |
|
Chief Medical Officer |
|
2016 |
|
$ |
400,000 |
|
— |
|
$ |
1,014,194 |
|
$ |
66,540 |
|
$ |
180,000 |
|
$ |
544,631 |
|
$ |
2,205,365 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
Mark A. Glickman |
|
2017 |
|
$ |
397,000 |
|
— |
|
$ |
417,779 |
|
$ |
83,697 |
|
$ |
158,508 |
|
$ |
11,854 |
(10) |
$ |
1,068,838 | |
|
Former Chief Commercial Officer |
|
2016 |
|
$ |
385,000 |
|
— |
|
$ |
994,134 |
|
$ |
63,989 |
|
$ |
166,861 |
|
$ |
536,653 |
|
$ |
2,146,637 | |
|
|
|
2015 |
|
$ |
204,321 |
|
$ |
200,000 |
|
$ |
362,464 |
|
— |
|
$ |
90,956 |
|
$ |
9,660 |
|
$ |
867,401 | |
(1) Mr. Charles’ employment with the Company terminated on November 30, 2017. Mr. Kaseta was appointed interim CFO on November 30, 2017, and was promoted to CFO on March 13, 2018. Dr. Tursi joined Pozen on October 1, 2015, and was not a named executive officer with respect to 2015. Mr. Glickman resigned from the Company effective March 30, 2018.
(2) The amounts included in this column are the sign-on awards paid to Mr. Charles and Mr. Glickman at the time of hire.
(3) The amounts included in this column are the dollar amounts representing the full grant date fair value of each stock option, RSU or PSU award, as applicable, calculated in accordance with FASB ASC Topic 718 (excluding the effect of estimated forfeitures), and do not represent the actual value that may be recognized by the named executive officers upon option exercise or settlement of the RSU or PSU award. For stock options, amounts are computed by multiplying the fair value of the award (as determined under the Black-Scholes option pricing model) by the total number of options granted. For RSUs, fair value is computed by multiplying the total number of shares subject to the award by the closing market price of Common Shares on the date of grant. For PSUs, fair value is based on the Monte Carlo valuation model, and the amount disclosed in this column is based on the probable outcome of the performance conditions. The following amounts represent the maximum potential PSU value by individual for fiscal 2017 (based on the value of Common Shares on the date of grant): Mr. Adams: $1,622,251; Mr. Koven: $811,125; Mr. Charles: $617,999; Dr. Tursi: $617,999; and Mr. Glickman: $595,500. Mr. Kaseta did not receive PSUs in 2017. For information on the valuation assumptions used in calculating the grant date fair value of stock options, see Note 12 to Aralez’s audited financial statements included in the 2017 Annual Report.
(4) For 2017 and 2016, this amount represents the amount that was paid to our named executive officers (other than Mr. Kaseta) under our Annual Incentive Bonus Plan. For Mr. Kaseta, the amount represents the amount that he was paid under our bonus plan for non-executives. The results and final payouts with respect to 2017 are described above in the section titled “Compensation Discussion and Analysis — Elements of Compensation — Annual Cash Incentives.” The 2015 awards for Messrs. Adams, Koven, Charles and Glickman were guaranteed at the target level, pro-rated for the portion of Fiscal Year 2015 in which they were employed by Pozen.
(5) This amount includes $12,000 in employer matching contributions to Mr. Adams’ 401(k) plan account.
(6) This amount includes $12,000 in employer matching contributions to Mr. Koven’s 401(k) plan account.
(7) This amount includes $9,000 in employer matching contributions to Mr. Charles’ 401(k) plan account, and $592,000 attributable to severance accrued by Mr. Charles as of November 30, 2017, the date of his separation of employment.
(8) This amount includes $9,000 in employer matching contributions to Mr. Kaseta’s 401(k) plan account.
(9) This amount includes $10,280 in employer matching contributions to Dr. Tursi’s 401(k) plan account.
(10) This amount includes $11,854 in employer matching contributions to Mr. Glickman’s 401(k) plan account.
Grants of Plan-Based Awards in 2017
The following table provides additional information about awards granted to our named executive officers in 2017.
|
|
|
Award |
|
|
|
Estimate Future Payouts Under Non- |
|
Estimated Future Payouts Under Equity |
|
All Other |
|
All Other |
|
Exercise |
|
Grant Date |
| ||||||||||
|
Name |
|
Type |
|
Grant |
|
Threshold |
|
Target |
|
Maximum |
|
Threshold |
|
Target |
|
Maximum |
|
Units |
|
Options |
|
Awards |
|
Awards |
| ||
|
Adrian Adams |
|
OPT |
|
5/11/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
252,316 |
|
$ |
1.56 |
|
$ |
228,005 |
|
|
|
RSU |
|
5/11/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
218,380 |
|
|
|
|
|
$ |
340,673 |
| ||
|
|
PSU |
|
3/15/2017 |
|
|
|
|
|
|
|
171,123 |
|
342,247 |
|
684,494 |
|
|
|
|
|
|
|
$ |
797,436 |
| ||
|
Andrew I. Koven |
|
OPT |
|
5/11/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
126,158 |
|
$ |
1.56 |
|
$ |
114,002 |
|
|
|
RSU |
|
5/11/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
109,190 |
|
|
|
|
|
$ |
170,336 |
| ||
|
|
PSU |
|
3/15/2017 |
|
|
|
|
|
|
|
85,562 |
|
171,123 |
|
342,247 |
|
|
|
|
|
|
|
$ |
398,717 |
| ||
|
Scott J. Charles |
|
AIC |
|
|
|
154,000 |
|
206,000 |
|
515,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
|
OPT |
|
5/11/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
96,121 |
|
$ |
1.56 |
|
$ |
86,859 |
| |
|
|
RSU |
|
5/11/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
83,192 |
|
|
|
|
|
$ |
129,780 |
| ||
|
|
PSU |
|
3/15/2017 |
|
|
|
|
|
|
|
65,190 |
|
130,380 |
|
260,759 |
|
|
|
|
|
|
|
$ |
303,785 |
| ||
|
Michael Kaseta |
|
AIC |
|
|
|
69,531 |
|
92,708 |
|
231,770 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
|
OPT |
|
3/15/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5,000 |
|
$ |
2.37 |
|
$ |
6,633 |
| |
|
|
RSU |
|
3/15/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
2,500 |
|
|
|
|
|
$ |
5,925 |
| ||
|
|
OPT |
|
5/11/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
25,000 |
|
$ |
1.56 |
|
$ |
22,591 |
| |
|
|
OPT |
|
11/30/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
25,000 |
|
$ |
1.51 |
|
$ |
21,957 |
| |
|
James P. Tursi, MD |
|
AIC |
|
|
|
139,050 |
|
185,400 |
|
463,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
|
OPT |
|
5/11/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
96,121 |
|
$ |
1.56 |
|
$ |
86,859 |
| |
|
|
RSU |
|
5/11/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
83,192 |
|
|
|
|
|
$ |
129,780 |
| ||
|
|
PSU |
|
3/15/2017 |
|
|
|
|
|
|
|
65,190 |
|
130,380 |
|
260,759 |
|
|
|
|
|
|
|
$ |
303,785 |
| ||
|
Mark A. Glickman |
|
AIC |
|
|
|
133,988 |
|
178,650 |
|
446,625 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
|
OPT |
|
5/11/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
92,621 |
|
$ |
1.56 |
|
$ |
83,697 |
| |
|
|
RSU |
|
5/11/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
80,163 |
|
|
|
|
|
$ |
125,054 |
| ||
|
|
PSU |
|
3/15/2017 |
|
|
|
|
|
|
|
62,816 |
|
125,633 |
|
251,266 |
|
|
|
|
|
|
|
$ |
292,725 |
| ||
(1) Award types are as follows: AIC is an annual incentive cash award, OPT is a stock option, RSU is a restricted stock unit, and PSU is a performance-based restricted stock unit.
(2) Each annual incentive cash award amount represents the individual’s current salary multiplied by their target bonus opportunity. The material terms of the annual cash incentive awards, including the actual results and individual payouts for 2017, are described above in the section titled “Compensation Discussion and Analysis — Elements of Compensation — Annual Cash Incentives.”
(3) PSU awards cliff vest at the end of the performance period (December 31, 2019) based on the actual percentile ranking of Aralez’s total shareholder return compared to peer performance. The material terms of the PSU awards are described above in the section titled “Compensation Discussion and Analysis — Elements of Compensation — Long-Term Incentive Compensation.”
(4) The RSU awards vest in equal annual installments over a three year vesting period.
(5) The stock option awards granted in May and November 2017 vest over a four year vesting period, with 25% vesting on the first anniversary of the date of grant and the remaining 75% vesting in equal installments on a monthly basis over the remaining three years. The stock options granted in March 2017 vest in equal installments on a monthly basis over a four-year period following the date of grant.
(6) The amounts included in this column are the dollar amounts representing the grant date fair value of each option, RSU, or PSU, as applicable, calculated in accordance with FASB ASC TOPIC 718 (excluding the effect of estimated forfeitures), and do not represent the actual value that may be recognized by the named executive officers upon option exercise or vesting of RSUs or PSUs. For PSUs, grant date fair value is based on the Monte Carlo valuation model, and the amount disclosed in this column is based on the probable outcome of the performance conditions.
Employment and Other Agreements
During Fiscal Year 2017, each of our named executive officers (other than Mr. Kaseta) was employed pursuant to employment agreements with us (Mr. Kaseta entered into an employment agreement effective March 13, 2018). Each employment agreement specifies, among other things, the named executive officer’s initial base salary, bonus and equity opportunity, entitlement to participate in the company’s benefits plans and post-termination benefits and obligations. The post-employment benefits are described in the section of this Annual Report on Form 10-K entitled “Potential Payments on Termination and Change of Control”.
Employment Agreement with Adrian Adams
Adrian Adams was appointed Chief Executive Officer on May 31, 2015. Under the terms of Mr. Adams’ employment agreement, which has an initial term of three years, he is entitled to (i) a base salary of $700,000, with annual increases, if any, to be made based on performance and in the sole discretion of the Board; (ii) an annual cash bonus, based on performance, payable in the discretion of the Compensation Committee, with a targeted amount of 100% of base salary; (iii) annual equity awards under the
Company’s equity compensation plan with a target value of not less than 225% of his base salary (50% of which will vest ratably over four years and 50% of which will vest based on the achievement of performance criteria); (iv) a one-time sign-on equity award in the form of 1,944,888 RSUs, which vest in equal annual installments on the first four anniversaries of the date of grant; and (v) reimbursement of up to $100,000 for reasonable legal fees associated with negotiating his employment agreement. He will also receive a tax equalization payment for any taxes imposed by Section 4985 of the Code. In addition, Mr. Adams’ employment agreement provides for benefits if his employment is terminated under certain circumstances which are described in the section of this Annual Report on Form 10-K entitled “Potential Payments on Termination and Change of Control”.
In March 2017, we amended Mr. Adams’ employment agreement to provide that he has agreed not to be eligible for a cash bonus under the Annual Incentive Bonus Plan with respect to the 2017 performance period, but that, for purposes of calculating any severance that he may become entitled to, his 2017 bonus will be deemed to have been paid at the target level.
Employment Agreement with Andrew I. Koven
Andrew I. Koven was appointed President and Chief Business Officer on May 31, 2015. Under the terms of Mr. Koven’s employment agreement, which has an initial term of three years, Mr. Koven will receive (i) an annual base salary of $450,000, with annual increases, if any, to be made based on performance and in the sole discretion of the Board; (ii) an annual cash bonus, based on performance, payable in the discretion of the Compensation Committee, with a targeted amount of 75% of base salary; (iii) annual equity awards under the Company’s equity compensation plan with a target value of not less than 175% of Mr. Koven’s base salary (50% of which will vest ratably over four years and 50% of which will vest based on the achievement of performance criteria); (iv) a one-time sign-on equity award in the form of 1,476,674 RSUs, which vest in equal annual installments on the first four anniversaries of the date of grant; (v) a tax equalization payment for any taxes imposed by Section 4985 of the Code; and (vi) reimbursement up to $100,000 for reasonable legal fees associated with negotiating his employment agreement. In addition, Mr. Koven’s employment agreement provides for benefits if his employment is terminated under certain circumstances which are described in the section of this Annual Report on Form 10-K entitled “Potential Payments on Termination and Change of Control”.
In March 2017, we amended Mr. Koven’s employment agreement to provide that he has agreed not to be eligible for a cash bonus under the Annual Incentive Bonus Plan with respect to the 2017 performance period, but that, for purposes of calculating any severance that he may become entitled to, his 2017 bonus will be deemed to have been paid at the target level.
Employment Agreement with Scott J. Charles
Scott J. Charles was appointed Senior Vice President, Finance on July 27, 2015, and was appointed Chief Financial Officer effective January 1, 2016. Under the terms of Mr. Charles’ employment agreement, which was effective as of July 27, 2015 and had an initial term of three years, Mr. Charles was eligible to receive (i) an annual base salary of $400,000, with annual increases, if any, to be made based on performance and in the sole discretion of the Compensation Committee; (ii) an annual cash bonus, based on performance, payable in the discretion of the Compensation Committee, with a targeted payout amount of 45% of Mr. Charles’ base salary; (iii) annual equity awards under the Company’s equity compensation plan with a target value of not less than 150% of Mr. Charles’ base salary; (iv) a one-time sign-on equity award in the form of 29,137 RSUs; (v) a signing bonus of $400,000; and (vi) a tax equalization payment for any taxes imposed by Section 4985 of the Code. In addition, Mr. Charles’ employment agreement provided for benefits if Mr. Charles’ employment had been terminated under certain circumstances. Mr. Charles’ employment terminated effective November 30, 2017, and the benefits he received upon his separation are described in the section of this Annual Report on Form 10-K entitled “Potential Payments on Termination and Change of Control”.
Employment Agreement with Michael Kaseta
Upon Mr. Charles’ separation, Michael Kaseta was appointed as the Company’s Head of Finance and Interim Chief Financial Officer, effective November 30, 2017. Mr. Kaseta was appointed Chief Financial Officer on March 13, 2018. In connection therewith, the Company entered into an employment agreement with Mr. Kaseta, which was not effective in 2017. Under the terms of Mr. Kaseta’s employment agreement, which has an initial term of three years, Mr. Kaseta will receive (i) an annual base salary of $375,000, with annual increases, if any, to be made based on performance and in the sole discretion of the Compensation Committee; (ii) an annual cash bonus, based on performance and peer company compensation practices, payable in the discretion of the Compensation Committee, with a targeted payout amount of 45% of Mr. Kaseta’s base salary; (iii) annual equity awards under the Company’s equity compensation plan with a target value of not less than 150% of Mr. Kaseta’s base salary; (iv) a promotion equity award valued at $281,000 in the form of 50% performance share units, 30% restricted stock units and 20% stock options, to be granted as soon as practicable; and (v) a tax equalization payment for any taxes imposed by Section 4985 of the Code. In addition, Mr. Kaseta’s employment agreement provides for benefits if Mr. Kaseta’s employment is terminated under certain circumstances which are described in the section of this Annual Report on Form 10-K entitled “Potential Payments on Termination and Change of Control”.
Employment Agreement with James P. Tursi
James P. Tursi, MD, was appointed Chief Medical Officer on October 1, 2015. Under the terms of Dr. Tursi’s employment agreement, which was effective as of October 1, 2015 and has an initial term of three years, Dr. Tursi will receive (i) an annual base salary of $400,000, with annual increases, if any, to be made based on performance and in the sole discretion of the Compensation Committee; (ii) an annual cash bonus, based on performance, payable in the discretion of the Compensation Committee, with a targeted payout amount of 45% of Dr. Tursi’s base salary; (iii) annual equity awards under the Company’s equity compensation plan with a target value of not less than 150% of Dr. Tursi base salary; (iv) a one-time sign-on equity award in the form of 29,137 RSUs; and (v) a tax equalization payment for any taxes imposed by Section 4985 of the Code. In addition, Dr. Tursi’s employment agreement provides for benefits if Dr. Tursi’s employment is terminated under certain circumstances which are described in the section of this Annual Report on Form 10-K entitled “Potential Payments on Termination and Change of Control”.
Employment Agreement with Mark A. Glickman
Mark A. Glickman was appointed Chief Commercial Officer on June 19, 2015 and resigned effective March 30, 2018. Under the terms of Mr. Glickman’s employment agreement, which was effective as of June 22, 2015 and had an initial term of three years, Mr. Glickman was eligible to receive (i) an annual base salary of $385,000, with annual increases, if any, to be made based on performance and in the sole discretion of the Compensation Committee; (ii) an annual cash bonus, based on performance, payable in the discretion of the Compensation Committee, with a targeted payout amount of 45% of Mr. Glickman’s base salary; (iii) annual equity awards under the Company’s equity compensation plan with a target value of not less than 150% of Mr. Glickman’s base salary; (iv) a one-time sign-on equity award in the form of 29,137 RSUs; (v) a signing bonus of $200,000; and (vi) a tax equalization payment for any taxes imposed by Section 4985 of the Code. In addition, Mr. Glickman’s employment agreement provided for benefits if Mr. Glickman’s employment had been terminated under certain circumstances which are described in the section of this Annual Report on Form 10-K entitled “Potential Payments on Termination and Change of Control”.
Outstanding Equity Awards at December 31, 2017
The following table summarizes the equity awards made to our named executive officers that had not been exercised and remained outstanding as of December 31, 2017.
|
|
|
Option Awards |
|
Stock Awards |
| |||||||||||||||||
|
Name |
|
Number of |
|
Number of |
|
Equity |
|
Option |
|
Option |
|
Number of |
|
Market |
|
Equity |
|
Equity |
| |||
|
Adrian Adams |
|
46,047 |
(3) |
59,204 |
|
— |
|
$ |
3.80 |
|
3/17/2026 |
|
1,273,720 |
(8) |
$ |
1,808,682 |
|
274,742 |
(9) |
$ |
390,134 |
|
|
|
|
0 |
(4) |
252,316 |
|
— |
|
$ |
1.56 |
|
5/11/2027 |
|
— |
|
— |
|
— |
|
|
| ||
|
Andrew I. Koven |
|
23,009 |
(3) |
29,583 |
|
— |
|
$ |
3.80 |
|
3/17/2026 |
|
888,949 |
(8) |
$ |
1,262,308 |
|
137,339 |
(9) |
$ |
195,021 |
|
|
|
|
0 |
(4) |
126,158 |
|
— |
|
$ |
1.56 |
|
5/11/2027 |
|
— |
|
— |
|
— |
|
— |
| ||
|
Scott J. Charles |
|
26,730 |
|
0 |
|
— |
|
$ |
3.80 |
|
3/17/2026 |
|
— |
|
— |
|
— |
|
— |
| ||
|
|
|
36,045 |
|
0 |
|
— |
|
$ |
1.56 |
|
5/11/2027 |
|
— |
|
— |
|
— |
|
— |
| ||
|
Michael Kaseta |
|
10,156 |
(5) |
27,344 |
|
|
|
$ |
4.90 |
|
11/11/2026 |
|
2,500 |
(10) |
$ |
3,550 |
|
— |
|
— |
| |
|
|
|
937 |
(6) |
4,063 |
|
— |
|
$ |
2.37 |
|
3/15/2027 |
|
— |
|
— |
|
— |
|
— |
| ||
|
|
|
0 |
(4) |
25,000 |
|
— |
|
$ |
1.56 |
|
5/11/2027 |
|
— |
|
— |
|
— |
|
— |
| ||
|
|
|
0 |
(7) |
25,000 |
|
— |
|
$ |
1.51 |
|
11/30/2027 |
|
— |
|
— |
|
— |
|
— |
| ||
|
James P. Tursi, MD |
|
17,542 |
(3) |
22,554 |
|
— |
|
$ |
3.80 |
|
3/17/2026 |
|
172,772 |
(8) |
$ |
245,336 |
|
104,664 |
(9) |
$ |
148,623 |
|
|
|
|
0 |
(4) |
96,121 |
|
— |
|
$ |
1.56 |
|
5/11/2027 |
|
— |
|
— |
|
|
|
|
| ||
|
Mark A. Glickman |
|
16,869 |
(3) |
21,690 |
|
— |
|
$ |
3.80 |
|
3/17/2026 |
|
168,533 |
(8) |
$ |
239,317 |
|
100,777 |
(9) |
$ |
143,103 |
|
|
|
|
0 |
(4) |
92,621 |
|
— |
|
$ |
1.56 |
|
5/11/2027 |
|
— |
|
— |
|
— |
|
— |
| ||
(1) The exercise price of each of the options included in this table is equal to the closing price of our Common Shares as reported by Nasdaq on the respective date of grant.
(2) Calculated by multiplying the closing market price of our Common Shares on December 29, 2017 ($1.42) by the unvested number of RSUs or unearned PSUs (at the threshold level of performance).
(3) The stock options were granted on March 17, 2016 and vest in equal installments on a monthly basis over a four-year vesting period.
(4) The stock options were granted on May 11, 2017 and vest as to 25% of the stock options on May 11, 2018. The remainder vest in equal installments on a monthly basis over the following three years.
(5) The stock options were granted on November 10, 2016 and vest in equal installments on a monthly basis over a four-year vesting period.
(6) The stock options were granted on March 15, 2017 and vest in equal installments on a monthly basis over a four-year vesting period.
(7) The stock options were granted on November 30, 2017 and vest as to 25% of the stock options on November 30, 2018. The remainder vest in equal installments on a monthly basis over the following three years.
(8) The vesting dates of the outstanding RSUs are as follows:
|
Named Executive Officer |
|
RSUs (#) |
|
Vesting Date |
|
|
Adrian Adams |
|
486,222 |
|
6/3/2018 |
|
|
|
|
486,222 |
|
6/3/2019 |
|
|
|
|
41,448 |
|
3/17/2018 |
|
|
|
|
41,448 |
|
3/17/2019 |
|
|
|
|
72,793 |
|
5/11/2018 |
|
|
|
|
72,793 |
|
5/11/2019 |
|
|
|
|
72,794 |
|
5/11/2020 |
|
|
Andrew I. Koven |
|
369,168 |
|
6/3/2018 |
|
|
|
|
369,169 |
|
6/3/2019 |
|
|
|
|
20,711 |
|
3/17/2018 |
|
|
|
|
20,711 |
|
3/17/2019 |
|
|
|
|
36,396 |
|
5/11/2018 |
|
|
|
|
36,397 |
|
5/11/2019 |
|
|
|
|
36,397 |
|
5/11/2020 |
|
|
Michael Kaseta |
|
833 |
|
3/15/2018 |
|
|
|
|
833 |
|
3/15/2019 |
|
|
|
|
834 |
|
3/15/2020 |
|
|
James P. Tursi, MD |
|
29,000 |
|
10/1/2018 |
|
|
|
|
29,001 |
|
10/1/2019 |
|
|
|
|
15,789 |
|
3/17/2018 |
|
|
|
|
15,790 |
|
3/17/2019 |
|
|
|
|
27,730 |
|
5/11/2018 |
|
|
|
|
27,731 |
|
5/11/2019 |
|
|
|
|
27,731 |
|
5/11/2020 |
|
|
Mark A. Glickman |
|
29,000 |
|
6/22/2018 |
|
|
|
|
29,001 |
|
6/22/2019 |
|
|
|
|
15,184 |
|
3/17/2018 |
|
|
|
|
15,185 |
|
3/17/2019 |
|
|
|
|
26,721 |
|
5/11/2018 |
|
|
|
|
26,721 |
|
5/11/2019 |
|
|
|
|
26,721 |
|
5/11/2020 |
|
(9) The PSUs vest as follows (based on the actual percentile ranking of Aralez’s total shareholder return over the performance period compared to peer performance): on December 31, 2018: 103,619 for Mr. Adams, 51,777 for Mr. Koven, 39,474 for Dr. Tursi, and 37,961 for Mr. Glickman; and on December 31, 2019: 171,123 for Mr. Adams, 85,562 for Mr. Koven, 65,190 for Dr. Tursi, and 62,816 for Mr. Glickman. The number of PSUs included in the table are based on achievement of the threshold level of performance.
(10) The RSUs were granted on March 15, 2017 and vest in equal installments on the first, second and third anniversary of the date of grant.
Option Exercises and Stock Vested in Fiscal Year 2017
The following table provides information regarding our named executive officers’ exercise of stock options and vesting of restricted stock awards during Fiscal Year 2017.
|
|
|
Option Awards |
|
Stock Awards |
| |||||
|
Name |
|
Number of |
|
Value |
|
Number of |
|
Value |
| |
|
Adrian Adams |
|
— |
|
— |
|
527,668 |
|
$ |
744,791 |
|
|
Andrew I. Koven |
|
— |
|
— |
|
389,879 |
|
$ |
541,284 |
|
|
Scott J. Charles |
|
— |
|
— |
|
117,308 |
|
$ |
179,539 |
|
|
Michael Kaseta |
|
— |
|
— |
|
— |
|
— |
| |
|
James P. Tursi, MD |
|
— |
|
— |
|
44,789 |
|
$ |
101,935 |
|
|
Mark A. Glickman |
|
— |
|
— |
|
44,184 |
|
$ |
72,734 |
|
(1) Represents the value of RSUs that vested during 2017. Calculated by multiplying the number of Common Shares represented by the RSUs by the closing market price of our Common Shares on the vesting date.
Pension Benefits for Fiscal Year 2017
The table disclosing the value of accumulated benefits under and other information concerning defined benefit plans during the year is omitted because we do not have a defined benefit plan for our named executive officers or other employees. The only retirement plan available to our named executive officers in 2017 was our 401(k) plan which is available to all employees.
Nonqualified Deferred Compensation for Fiscal Year 2017
The table disclosing contributions to and aggregate earnings under or distributions from nonqualified defined contribution or other deferred compensation plans is omitted because we do not maintain any such nonqualified deferred compensation plans.
Potential Payments on Termination and Change of Control
Upon termination of employment or a change of control, our named executive officers are entitled to certain compensation and benefits under the terms of their employment agreements, as well as other plans and arrangements provided by us. The tables below list the potential compensation payable to our named executive officers under various hypothetical termination scenarios. The discussion and the amounts shown in the tables assume that the termination or change of control took place on December 31, 2017 (and thus include amounts earned through such time), and assume that the price per Common Share was the closing market price on December 29, 2017 ($1.42 per share). The amounts shown are estimates of the amounts that would have been paid out to the named executive officers for terminations on December 31, 2017. The amounts that the named executive officers would receive in an actual termination or change of control can only be determined at the time the event occurs.
Mr. Adams and Mr. Koven
Mr. Adams, our CEO, and Mr. Koven, our President and Chief Business Officer, entered into employment agreements with us which provide certain payments and benefits upon termination of employment under certain circumstances.
In the event the employment of Mr. Adams or Mr. Koven is terminated without cause, if either voluntarily terminates his employment for good reason, in the event of his death, or if either is terminated due to disability, Mr. Adams or Mr. Koven, as applicable, will receive: (i) accrued but unpaid base salary and vacation through the date of termination; (ii) a lump sum payment equal to 24 months of base salary; (iii) a lump sum payment equal to two times the greater of (x) the average annual bonus paid over the previous two years or (y) the annual bonus paid the year preceding the year in which his termination of employment occurs, provided that if Mr. Adams or Mr. Koven, as applicable, is not employed for a sufficient time to have received an annual cash bonus with respect to a full year, such calculation will assume that a target annual cash bonus was paid; (iv) continuation of medical and life insurance benefits for a period of 24 months following the date of termination (subject to his payment of active employee rates), or, if such benefits cannot be provided, a cash payment payable within 60 days in an amount equal to the fair market value of the benefits which were to be provided; and (v) acceleration of the vesting of all equity and equity-based awards that would otherwise vest in the next 24 month period.
In the event that, within 12 months of a change of control of Aralez, the employment of Mr. Adams or Mr. Koven is terminated without cause or if either voluntarily terminates his employment for good reason, Mr. Adams or Mr. Koven, as applicable, will receive: (i) accrued but unpaid base salary and vacation through the date of termination; (ii) a lump sum payment equal to 36 months of base salary; (iii) a lump sum payment equal to three times the greater of (x) the average annual bonus paid over the previous two years or (y) the annual bonus paid the year preceding the year in which his termination of employment occurs, provided that if Mr. Adams or Mr. Koven, as applicable, is not employed for a sufficient time to have received an annual cash bonus, such calculation will assume that a target annual cash bonus was paid; (iv) continuation of medical and life insurance benefits for a period of 36 months following the date of termination (subject to his payment of active employee rates), or, if such benefits cannot be provided, a cash payment payable within 60 days in an amount equal to the fair market value of the benefits which were to be provided; and (v) immediate and full vesting of all outstanding unvested equity awards. If Mr. Adams or Mr. Koven is terminated without cause or voluntarily terminates employment for good reason, in either case in anticipation of a change of control, and a change of control actually occurs during the six months thereafter, he will be entitled to receive the enhanced severance benefits set forth herein on the later of the 60th day following termination or the date of the change of control. In the event of a change of control, Mr. Adams and Mr. Koven will not be entitled to a tax gross-up with respect to excise taxes under Section 4999 of the Code. Instead, any payments to Mr. Adams or Mr. Koven that would be subject to the excise tax will be reduced to the level at which the excise tax will not be applied unless Mr. Adams or Mr. Koven would be in a better net after-tax position by receiving the full payments and paying the excise tax.
The payment of all severance benefits is contingent on Mr. Adams or Mr. Koven, as applicable, executing a general release of claims in favor of the Company and not revoking such release. Mr. Adams and Mr. Koven are subject to non-competition, non-solicitation and non-interference covenants for one year following termination of employment for any reason.
In March 2017, we amended Mr. Adams’ and Mr. Koven’s employment agreements to provide that they have agreed not to be eligible for a cash bonus under the Annual Incentive Bonus Plan with respect to the 2017 performance period, but that, for purposes of calculating any severance that they may become entitled to, their 2017 bonus will be deemed to have been paid at the target level.
Dr. Tursi and Mr. Glickman
Dr. Tursi, our Chief Medical Officer, and Mr. Glickman, our Chief Commercial Officer, entered into employment agreements with us that provide certain payments and benefits upon termination of employment under certain circumstances.
In the event the employment of Dr. Tursi or Mr. Glickman is terminated without cause or if either voluntarily terminates his employment for good reason, Dr. Tursi, or Mr. Glickman, as applicable, will receive (i) accrued but unpaid base salary and vacation through the date of termination; (ii) an amount, payable in 12 equal monthly installments, equal to the sum of (x) one times his base salary in effect immediately prior to the date of termination, and (y) one times the average annual cash bonus paid over the previous two years, provided that if Dr. Tursi, or Mr. Glickman, as applicable, is not employed for a sufficient time to have received an annual cash bonus with respect to a full year, such calculation will assume that a target annual cash bonus was paid; (iii) continuation of medical benefits for a period of 12 months following the date of termination (subject to his payment of active employee rates), or, if such benefits cannot be provided, a cash payment payable within 60 days in an amount equal to the fair market value of the benefits which were to be provided; and (iv) acceleration of the vesting of all equity and equity-based awards that would otherwise vest in the 12 month period following the date of termination.
In the event that, within 12 months of a change of control of Aralez, the employment of Dr. Tursi, or Mr. Glickman is terminated without cause or if he voluntarily terminates his employment for good reason, Dr. Tursi, or Mr. Glickman, as applicable, will receive (i) accrued but unpaid base salary and vacation through the date of termination; (ii) a lump sum cash amount, payable on the 60th day following the date of termination, equal to two times his base salary in effect immediately prior to date of termination; (iii) a lump sum cash amount, payable on the 60th day following date of termination, equal to two times the greater of (x) the average annual cash bonus received for each of the preceding two years and (y) the annual cash bonus received during the preceding year, provided that if Dr. Tursi, or Mr. Glickman, as applicable, is not employed for a sufficient time to have received an annual cash bonus with respect to a full year, such calculation will assume that a target annual cash bonus was paid; (iv) continuation of medical benefits for a period of 24 months following the date of termination (subject to his payment of active employee rates), or, if such benefits cannot be provided, a cash payment payable within 60 days in an amount equal to the fair market value of the benefits which were to be provided; and (v) immediate and full vesting of all outstanding equity or equity-based awards. In the event of a change of control, Dr. Tursi, and Mr. Glickman will not be entitled to a tax gross-up with respect to excise taxes under Section 4999 of the Code. Instead, any payments to Dr. Tursi, or Mr. Glickman that would be subject to the excise tax will be reduced to the level at which the excise tax will not be applied unless Dr. Tursi, or Mr. Glickman would be in a better net after-tax position by receiving the full payments and paying the excise tax.
The payment of all severance benefits is contingent on Dr. Tursi, or Mr. Glickman, as applicable, executing a general release of claims in favor of the Company and not revoking such release. Dr. Tursi, and Mr. Glickman are subject to non-competition, non-solicitation and non-interference covenants for one year following termination of employment for any reason.
Mr. Glickman resigned from his position with the Company on March 30, 2018, and did not receive any severance benefits in connection with his termination of employment.
Mr. Kaseta
Mr Kaseta was not subject to an employment agreement during 2017, and would not have been entitled to any benefits under an employment agreement if he had been terminated on December 31, 2017. Instead, Mr. Kaseta would have been entitled to benefits under the Aralez Pharmaceuticals Inc. U.S. Severance Plan (the “Severance Plan”). Under the Severance Plan, if Mr. Kaseta had been terminated by the Company for reasons other than job performance, misconduct, or a violation of Company policies or rules, or in the event he is deemed to have a constructive termination within 12 months following a change of control (as defined in the 2016 Plan), Mr. Kaseta would have been entitled to receive (i) an amount, payable in equal installments, equal to 11 months of his base salary (or, for a termination within 12 months following a change of control, 1.25 times that amount, payable in a lump sum 60 days after termination), (ii) his target bonus (or, for a termination within 12 months following a change of control, 1.25 times his target bonus), payable in equal installments, and (iii) if such termination is within 12 months of a change of control, immediate and full vesting of all outstanding equity or equity-based awards. The payment of all severance benefits under the Severance Plan is contingent on Mr. Kaseta executing a general release of claims in favor of the Company and not revoking such release.
We entered into an employment agreement with Mr. Kaseta effective March 13, 2018, in connection with his appointment as CFO. Pursuant to the employment agreement, in the event the employment of Mr. Kaseta is terminated without cause or if he voluntarily terminates his employment for good reason, Mr Kaseta will receive (i) accrued but unpaid base salary and vacation through the date of termination; (ii) an amount, payable in 12 equal monthly installments, equal to the sum of (x) one times his base salary in effect immediately prior to the date of termination, and (y) one times the average annual cash bonus paid over the previous two years, provided that if Mr. Kaseta is not employed for a sufficient time to have received an annual cash bonus with respect to a full year, such calculation will assume that a target annual cash bonus was paid; (iii) continuation of medical benefits for a period of 12 months following the date of termination (subject to his payment of active employee rates), or, if such benefits cannot be provided, a cash payment payable within 60 days in an amount equal to the fair market value of the benefits which were to be provided; and (iv) acceleration of the vesting of all equity and equity-based awards that would otherwise vest in the 12 month period following the date of termination.
In the event that, within 12 months of a change of control of Aralez, Mr. Kaseta’s employment is terminated without cause or if he voluntarily terminates his employment for good reason, Mr. Kaseta will receive (i) accrued but unpaid base salary and vacation through the date of termination; (ii) a lump sum cash amount, payable on the 60th day following the date of termination, equal to two times his base salary in effect immediately prior to date of termination; (iii) a lump sum cash amount, payable on the 60th day following date of termination, equal to two times the greater of (x) the average annual cash bonus received for each of the preceding two years and (y) the annual cash bonus received during the preceding year, provided that if Mr. Kaseta is not employed for a sufficient time to have received an annual cash bonus with respect to a full year, such calculation will assume that a target annual cash bonus was paid; (iv) continuation of medical benefits for a period of 24 months following the date of termination (subject to his payment of active employee rates), or, if such benefits cannot be provided, a cash payment payable within 60 days in an amount equal to the fair market value of the benefits which were to be provided; and (v) immediate and full vesting of all outstanding equity or equity-based awards. In the event of a change of control, Mr. Kaseta will not be entitled to a tax gross-up with respect to excise taxes under Section 4999 of the Code. Instead, any payments to Mr. Kaseta that would be subject to the excise tax will be reduced to the level at which the excise tax will not be applied unless Mr. Kaseta would be in a better net after-tax position by receiving the full payments and paying the excise tax.
The payment of all severance benefits is contingent on Mr. Kaseta executing a general release of claims in favor of the Company and not revoking such release. Mr. Kaseta is subject to non-competition, non-solicitation and non-interference covenants for one year following termination of employment for any reason.
Mr. Charles
Mr. Charles terminated employment with the Company on November 30, 2017. In connection with his separation of employment, he received the following benefits payable under his employment agreement: (a) the sum of (x) one (1) times his base salary as in effective immediately prior to his separation, and (y) one times his average annual bonus paid over the previous two (2) years, payable in twelve (12) substantially equal monthly installments commencing with the first regular payroll period following the expiration of any applicable revocation period with respect to the release, but within ninety (90) days after his date of separation; (b) reimbursement for monthly COBRA costs of continued coverage for twelve (12) months following his separation; (c) acceleration of the vesting of all equity and equity-based awards that would otherwise vest during the twelve (12) month period following his separation; and (d) any earned but unpaid salary, bonus, or other reimbursements. Prior to receiving these payments, Mr. Charles executed a release of all potential claims against the Company.
Applicable Definitions in Employment Agreements
Cause: In the employment agreements with our named executive officers, “cause” means:
· the executive is convicted of, or pleads guilty or nolo contendere to, a felony or a crime involving moral turpitude;
· in carrying out his duties, the executive engages in conduct that constitutes willful gross misconduct, or willful gross neglect and that, in either case, results in material economic or reputational harm to the company, which executive fails to cure after 30 days’ written notice; or
· the executive refuses to perform, or repeatedly fails to undertake good faith efforts to perform, the duties or responsibilities reasonably assigned to him, which has continued for 30 days following written notice of such non-performance.
Good Reason: In the employment agreements with our named executive officers, “good reason” means the occurrence, without the executive’s written consent, of any of the following:
· a change in authority, duties, responsibilities or reporting lines (including, for Mr. Koven, no longer reporting to Mr. Adams);
· a reduction in base salary;
· any relocation of principal office or principal place of employment to a location more than 50 miles from Philadelphia, Pennsylvania or such other corporate headquarters as is approved by the CEO;
· a material breach of the agreement by Aralez; or
· Aralez fails to extend the term of the employment agreement.
For Mr. Adams and Mr. Koven, “good reason” also occurs if Aralez ceases to have any class of securities registered under Section 12 of the Securities Exchange Act of 1934, as amended. For Mr. Adams, “good reason” also occurs if Aralez fails to appoint him to, or removes him from, the Board.
Change of control: In the employment agreements with our named executive officers, “change of control” means the first to occur of any of the following:
· a person or affiliated group acquires more than 50% of Aralez’s then outstanding voting securities;
· the shareholders of Aralez approve a plan of complete liquidation;
· the sale or disposition of all or substantially all of Aralez’s assets;
· a merger, consolidation or reorganization of Aralez with or involving another entity unless the holders of Aralez voting shares immediately prior to the merger have at least 50% of the combined voting power of the securities in the merged entity or its parent;
· a majority of the Board of Aralez are replaced during any 12-month period by directors whose appointment or election are not endorsed by a majority of the members of the Board before the date of appointment or election.
Accelerated Vesting of Options and Other Stock-Based Awards
2010 Plan
Under the change of control provisions of the 2010 Plan, unless the Compensation Committee determines otherwise, all outstanding options and stock appreciation rights, including those held by our named executive officers, will automatically accelerate and become fully exercisable, the restrictions and conditions on all outstanding stock awards will immediately lapse, and all stock units, dividend equivalents and other stock-based awards will become fully vested and will be paid at their target value or in such greater amounts as the Compensation Committee may determine. The Compensation Committee may also take certain other actions as provided in the 2010 Plan, including determining that outstanding options and stock appreciation rights that are not exercised will be assumed by, or replaced with comparable options or rights by, the surviving corporation (or a parent or subsidiary of the surviving corporation), and other outstanding grants that remain in effect after the change of control will be converted to similar grants of the surviving corporation or a parent or subsidiary of the surviving corporation). Dr. Tursi currently holds, and Mr. Glickman held (prior to his resignation) awards under the 2010 Plan. Following the completion of the Tribute acquisition, no new awards may be granted under the 2010 Plan.
For purposes of the 2010 Plan, a change of control is generally defined to include any of the following:
· a person, entity or affiliated group (with certain exceptions) acquires more than 50% of Aralez’s then outstanding voting securities;
· Aralez merges into another entity unless the holders of Aralez’s voting shares immediately prior to the merger have at least 50% of the combined voting power of the securities in the merged entity or its parent;
· Aralez sells or disposes of all or substantially all of its assets;
· Aralez is liquidated or dissolved; or
· a majority of the Aralez Board have been members of the Board for less than one year, unless the election or nomination for election of each new Director who was not a director at the beginning of such one year period was approved by a vote of at least two-thirds of the directors then still in office who were directors at the beginning of such period.
Inducement Grants
Mr. Adams and Mr. Koven each hold RSUs that were made as “inducement grants” under the Nasdaq rules, and were not granted under the 2010 Plan. The RSUs subject to the inducement grants will become fully vested and will be paid upon a change of control. For purposes of the inducement grants, “change of control” has the same meaning as the term has in the 2010 Plan.
2016 Plan
Our named executive officers each hold stock options, RSUs, and performance-based RSUs under the 2016 Plan. Under the change of control provisions of the 2016 Plan, unless the awards are assumed by, or replaced with comparable options or rights by, the surviving corporation (or a parent or subsidiary of the surviving corporation), all outstanding options and stock appreciation rights, including those held by our named executive officers, will automatically accelerate and become fully exercisable, all outstanding restricted stock awards and restricted stock units which do not vest based on the achievement of performance goals will become fully vested, and all outstanding restricted stock awards and restricted stock units which vest based on the achievement of performance goals will become vested at the target level of performance (unless the award agreements for such awards provide for a higher level of vesting). The award agreements for the performance-based RSUs held by our named executive officers provide that, unless the awards are assumed or replaced with identical awards by the surviving company, the awards will vest at the greater of the target level of performance or the actual level of performance assuming that the change of control date was the last day of the performance period. The Compensation Committee may also specify in award agreements that awards will vest upon certain terminations following a change of control.
For purposes of the 2016 Plan, a change of control is generally defined to include any of the following:
· a person, entity or affiliated group (with certain exceptions) acquires more than 50% of the total voting power or the total fair market value of the capital stock of Aralez;
· a person, entity or affiliated group acquires assets from Aralez with a total fair market value equal to or more than 50% of the total gross fair market value of the assets of Aralez immediately prior to such acquisition; or
· a majority of the Aralez Board has been replaced during a 12-month period by directors whose appointment or election is not endorsed by a majority of the members of the Aralez Board immediately prior to such appointment or election.
Performance-Based RSUs Under the 2016 Plan
The PSUs granted under the 2016 Plan provide that, unless otherwise provided in another agreement with the Company, the PSUs will be forfeited upon termination of employment of the participant prior to the last day of the performance period. However, in the event an agreement with the Company purports to accelerate the vesting of the PSUs upon a termination of employment, the accelerated PSUs will be paid on the date such PSUs would have been paid had the participant’s employment continued through the end of the performance period, with the number of PSUs that are earned based on the achievement of the performance goals through the end of the performance period and pro-rated based on the number of full months during the performance period that the participant was employed by the Company.
Upon a change of control of the Company, unless the PSUs are continued or assumed by the surviving or successor entity, the participant will be deemed to have earned and vest in the number of PSUs equal to the greater of (i) the target number of PSUs and (ii) the actual number of PSUs that would have been vested and earned if the last day of the performance period had been the date of the change of control. If the PSUs are continued or assumed by the surviving or successor entity following a change of control, and the participant is terminated without Cause or resigns for Good Reason (each as defined in the participant’s employment agreement) following the change of control but prior to the last day of the performance period, the participant will be deemed to have earned and vest in the number of PSUs determined as set forth above if the PSUs had not been continued or assumed.
Estimated Payments Upon Termination or Change of Control
The following table illustrates the value of the payments and benefits our named executive officers (other than Mr. Charles) would be entitled to receive upon a termination of employment or upon a change on control of Aralez, in either case as of December 31, 2017.
|
Executive Benefits and Payments Upon Termination |
|
Termination |
|
Termination |
|
Death or |
|
Change of |
|
Change of |
| |||||
|
Adrian Adams |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Cash Severance—Salary |
|
$ |
— |
|
$ |
1,442,000 |
|
$ |
1,442,000 |
|
$ |
2,163,000 |
|
$ |
— |
|
|
Cash Severance—Bonus(1) |
|
$ |
— |
|
$ |
1,400,000 |
|
$ |
1,400,000 |
|
$ |
2,100,000 |
|
$ |
— |
|
|
Stock Options—Accelerated(2) |
|
$ |
— |
|
$ |
0 |
|
$ |
0 |
|
$ |
0 |
|
$ |
— |
|
|
RSUs and PSUs—Accelerated(2) |
|
$ |
— |
|
$ |
1,705,315 |
|
$ |
1,705,315 |
|
$ |
2,588,950 |
|
$ |
1,380,870 |
|
|
Health Care Continuation(3) |
|
$ |
— |
|
$ |
54,705 |
|
$ |
54,705 |
|
$ |
82,058 |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Andrew I. Koven |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Cash Severance—Salary |
|
$ |
— |
|
$ |
927,000 |
|
$ |
927,000 |
|
$ |
1,390,500 |
|
$ |
— |
|
|
Cash Severance—Bonus(1) |
|
$ |
— |
|
$ |
675,000 |
|
$ |
675,000 |
|
$ |
1,012,500 |
|
$ |
— |
|
|
Stock Options—Accelerated(2) |
|
$ |
— |
|
$ |
0 |
|
$ |
0 |
|
$ |
0 |
|
$ |
— |
|
|
RSUs and PSUs—Accelerated(2) |
|
$ |
— |
|
$ |
1,210,624 |
|
$ |
1,210,624 |
|
$ |
1,652,348 |
|
$ |
1,048,439 |
|
|
Health Care Continuation(3) |
|
$ |
— |
|
$ |
54,705 |
|
$ |
54,705 |
|
$ |
82,058 |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Michael Kaseta |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Cash Severance—Salary |
|
$ |
— |
|
$ |
320,833 |
|
$ |
— |
|
$ |
401,041 |
|
$ |
— |
|
|
Cash Severance—Bonus(1) |
|
$ |
— |
|
$ |
122,500 |
|
$ |
— |
|
$ |
153,125 |
|
$ |
— |
|
|
Stock Options—Accelerated(2) |
|
$ |
— |
|
$ |
0 |
|
$ |
— |
|
$ |
0 |
|
$ |
— |
|
|
RSUs and PSUs—Accelerated(2) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
3,550 |
|
$ |
— |
|
|
Health Care Continuation(3) |
|
$ |
— |
|
$ |
27,353 |
|
$ |
— |
|
$ |
54,705 |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
James P. Tursi, MD |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Cash Severance—Salary |
|
$ |
— |
|
$ |
412,000 |
|
$ |
— |
|
$ |
824,000 |
|
$ |
— |
|
|
Cash Severance—Bonus(1) |
|
$ |
— |
|
$ |
180,000 |
|
$ |
— |
|
$ |
360,000 |
|
$ |
— |
|
|
Stock Options—Accelerated(2) |
|
$ |
— |
|
$ |
0 |
|
$ |
— |
|
$ |
0 |
|
$ |
— |
|
|
RSUs and PSUs—Accelerated(2) |
|
$ |
— |
|
$ |
102,977 |
|
$ |
— |
|
$ |
542,581 |
|
$ |
82,361 |
|
|
Health Care Continuation(3) |
|
$ |
— |
|
$ |
27,352 |
|
$ |
— |
|
$ |
54,705 |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
Mark A. Glickman |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Cash Severance—Salary |
|
$ |
— |
|
$ |
397,000 |
|
$ |
— |
|
$ |
794,000 |
|
$ |
— |
|
|
Cash Severance—Bonus(1) |
|
$ |
— |
|
$ |
170,056 |
|
$ |
— |
|
$ |
333,722 |
|
$ |
— |
|
|
Stock Options—Accelerated(2) |
|
$ |
— |
|
$ |
0 |
|
$ |
— |
|
$ |
0 |
|
$ |
— |
|
|
RSUs and PSUs—Accelerated(2) |
|
$ |
— |
|
$ |
100,685 |
|
$ |
— |
|
$ |
525,524 |
|
$ |
82,361 |
|
|
Health Care Continuation(3) |
|
$ |
— |
|
$ |
27,352 |
|
$ |
— |
|
$ |
54,705 |
|
$ |
— |
|
(1) Pursuant to the terms of the employment agreements, the amount used as a basis for the cash severance—bonus calculation is based on the 2016 annual bonus paid for Mr. Adams and Mr.Koven and, for Dr. Tursi and Mr. Glickman, the average of the 2015 bonus (at the target level since the executive was not employed for the full year) and 2016 annual bonus paid to the executive for a termination that is not following a change of control, and the 2016 annual bonus paid to the executive for a termination within 12 months following a change of control. For Mr. Kaseta, the cash severance—bonus calculation is based on the target bonus.
(2) Calculated by multiplying the closing market price of Common Shares on December 29, 2017 ($1.42) by the accelerated number of RSUs or PSUs. For stock options, the aggregate value is based on the spread between the closing market price of Common Shares on December 29, 2017 ($1.42) and the exercise price of the options.
(3) Health care continuation is based on Aralez’s rates for coverage during the 2018 plan year, assuming that each executive elected to participate in COBRA at the same level as the executive currently participates.
The following table sets forth the value of the payments and benefits paid to Mr. Charles upon his separation of employment on November 30, 2017.
|
Scott Charles |
|
|
| |
|
Cash Severance—Salary |
|
$ |
412,000 |
|
|
Cash Severance—Bonus(1) |
|
$ |
180,000 |
|
|
Stock Options—Accelerated(2) |
|
$ |
0 |
|
|
RSUs and PSUs—Accelerated(2) |
|
$ |
107,994 |
|
|
Health Care Continuation(3) |
|
$ |
27,353 |
|
(1) Pursuant to the terms of the employment agreements, the amount used as a basis for the cash severance—bonus calculation is the target bonus.
(2) Calculated by multiplying the closing market price of Common Shares on November 30, 2017 ($1.51) by the accelerated number of RSUs or PSUs. For stock options, the aggregate value is based on the spread between the closing market price of Common Shares on November 30, 2017 ($1.51) and the exercise price of the options.
(3) Health care continuation is based on Aralez’s rates for coverage during the 2018 plan year.
Director Compensation
Discussed in the following paragraphs and tables is the compensation paid to the non-employee directors of Aralez. Each non-employee director receives a cash retainer in the amount of $40,000, paid in equal quarterly installments, and an annual equity award of 15,000 stock options and 7,500 RSUs, each with a one-year vesting term. Directors may elect to defer the receipt of the shares subject to the RSUs until such time as the director ceases to provide services to the Company.
In addition, the non-employee directors are eligible to receive the following cash retainer fees for service on the Board:
· an additional annual retainer for the Chairperson of the Board in the amount of $30,000;
· an annual retainer for Board committee Chairpersons, as follows: $17,500 for service as Chairperson of the Transaction Committee; $12,000 for service as Chairperson of the Nominating/Corporate Governance Committee; $17,500 for service as Chairperson of the Compensation Committee; and $25,000 for service as Chairperson of the Audit Committee; and
· an annual retainer for Board committee members (other than committee Chairpersons), as follows: $10,000 for service on the Transaction Committee; $8,000 for service on the Nominating/Corporate Governance Committee; $10,000 for service on the Compensation Committee; and $12,500 for service on the Audit Committee.
Each new director will receive a sign-on grant of 30,000 stock options and 15,000 RSUs, which will vest in three equal annual installments.
Reduction of 2017 Cash Retainer Fees
In May 2017, the Board agreed to modify the director compensation program with respect to 2017 in light of the Company’s circumstances and share price performance. The Board modified the 2017 director compensation program to provide the annual cash retainer payable to each non-employee member of the Board by 50%. The director compensation program was reinstated effective January 1, 2018 without the reduction.
Director Compensation for Fiscal Year 2017
The following table further summarizes the compensation paid by Aralez to the non-employee directors during Fiscal Year 2017. Except as noted below, all of the Aralez directors are paid at the same rate. The differences among directors in the table below are a function of additional compensation for chairing a committee and/or serving on one or more committees.
|
Name |
|
Fees |
|
Stock |
|
Option Awards |
|
Total |
| ||||
|
Jason M. Aryeh |
|
$ |
16,302 |
|
$ |
11,700 |
|
$ |
12,211 |
|
$ |
40,213 |
|
|
Neal F. Fowler |
|
$ |
28,709 |
|
$ |
11,700 |
|
$ |
12,211 |
|
$ |
52,620 |
|
|
Rob Harris |
|
$ |
23,675 |
|
$ |
11,700 |
|
$ |
12,211 |
|
$ |
47,586 |
|
|
Arthur S. Kirsch |
|
$ |
50,073 |
|
$ |
11,700 |
|
$ |
12,211 |
|
$ |
73,984 |
|
|
Kenneth B. Lee, Jr. |
|
$ |
33,969 |
|
$ |
11,700 |
|
$ |
12,211 |
|
$ |
57,880 |
|
|
Seth A. Rudnick, M.D. |
|
$ |
30,602 |
|
$ |
11,700 |
|
$ |
12,211 |
|
$ |
54,513 |
|
|
F. Martin Thrasher |
|
$ |
28,429 |
|
$ |
11,700 |
|
$ |
12,211 |
|
$ |
52,340 |
|
(1) The amounts included in this column are the dollar amounts representing the full grant date fair value of each restricted stock unit award or option award, as applicable, calculated in accordance with FASB ASC Topic 718. For information on the valuation assumptions used in calculating this amount, see Note 12 to Aralez’s audited financial statements included in the 2017 Annual Report.
The following table lists the number of outstanding RSUs and the number of outstanding options held by each of the directors as of December 31, 2017, each of which was granted at an exercise price equal to the closing price of Common Shares as reported by Nasdaq on the respective date of grant.
|
Name |
|
Restricted Stock |
|
Options |
|
|
Jason M. Aryeh |
|
7,500 |
|
30,000 |
|
|
Neal F. Fowler |
|
15,000 |
|
30,000 |
|
|
Rob Harris |
|
7,500 |
|
220,831 |
|
|
Arthur S. Kirsch |
|
7,500 |
|
42,214 |
|
|
Kenneth B. Lee, Jr. |
|
15,000 |
|
30,000 |
|
|
Seth A. Rudnick, M.D. |
|
15,000 |
|
30,000 |
|
|
F. Martin Thrasher |
|
15,000 |
|
43,095 |
|
Compensation Committee Interlocks and Insider Participation
During Fiscal Year 2017, the members of the Compensation Committee were Messrs. Lee, Aryeh (who resigned from the Board effective June 7, 2017) and Fowler and Dr. Rudnick. None of these individuals ever served as an officer or employee of the Company. None of the executive officers of the Company served on the board of directors or compensation committee of a company that had an executive officer that served on the Board or the Compensation Committee.
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Security Ownership of Certain Beneficial Owners and Management
The following tables sets forth information known to us concerning the beneficial ownership of our outstanding Common Shares as of April 16, 2018 (unless otherwise noted) for:
· each person known by us to beneficially own 5% or more of our outstanding Common Shares;
· each of our directors;
· each of our named executive officers; and
· all of our directors and current executive officers as a group.
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. In computing the number of Common Shares beneficially owned by a person and the percentage ownership of that person, Common Shares that could be issued upon the exercise of outstanding options held by that person that are currently exercisable at April 16, 2018, or that will become exercisable within 60 days of April 16, 2018, are considered outstanding. These Common Shares, however, are not considered outstanding when computing the percentage ownership of each other person.
Except as indicated in the footnotes to this table and pursuant to state community property laws, each shareholder named in the table has sole voting and investment power for the Common Shares shown as beneficially owned by them. Percentage of ownership is based on 67,194,277 Common Shares issued and outstanding on April 16, 2018.
|
Name and Address of Beneficial Owner |
|
Number of Common |
|
Percentage |
|
|
Entities affiliated with Deerfield Management Company, L.P. |
|
10,701,187 |
(1) |
9.9 |
% |
|
Par Investment Partners, L.P. |
|
5,942,309 |
(2) |
8.8 |
% |
(1) Based on information disclosed in a Schedule 13G/A filed with the SEC on February 14, 2018 by Deerfield Mgmt, L.P., Deerfield Management Company, L.P., Deerfield Mgmt III, L.P., Deerfield Private Design Fund III, L.P., Deerfield International Master Fund, L.P., Deerfield Partners, L.P. and James E. Flynn (the “Deerfield Reporting Persons”). As reported in the Schedule 13G/A filed by the Deerfield Reporting Persons, as of December 31, 2017: (i) Deerfield Mgmt, L.P. had shared voting power and shared dispositive power with respect to 5,372,885 Common Shares, comprised of an aggregate of 844,583 Common Shares and 4,528,302 Common Shares underlying convertible notes held by Deerfield Partners, L.P. and Deerfield International Master Fund, L.P., of which Deerfield Mgmt, L.P. is the General Partner; (ii) Deerfield Management Company, L.P. had shared voting power and shared dispositive power with respect to 10,701,187 Common Shares, comprised of an aggregate of 1,644,583 Common Shares and 9,056,604 Common Shares underlying convertible notes held by Deerfield Private Design Fund III, L.P., Deerfield Partners, L.P. and Deerfield International Master Fund, L.P., of which Deerfield Management Company, L.P. is the investment advisor; (iii) Deerfield Mgmt III, L.P. had shared voting power and shared dispositive power with respect to 5,328,302 Common Shares, comprised of an aggregate of 800,000 Common Shares and 4,528,302 Common Shares underlying convertible notes held by Deerfield Private Design Fund III, L.P., of which Deerfield Mgmt III, L.P. is the general partner; (iv) Deerfield Private Design Fund III, L.P. had shared voting power and shared dispositive power with respect to 5,328,302 Common Shares, comprised of an aggregate of 800,000 Common Shares and 4,528,302 Common Shares underlying convertible notes; (v) Deerfield International Master Fund, L.P. had shared voting power and shared dispositive power with respect to 3,008,815 Common Shares, comprised of an aggregate of 472,966 Common Shares and 2,535,849 Common Shares underlying convertible notes; (vi) Deerfield Partners, L.P. had shared voting power and shared dispositive power with respect to 2,364,070 Common Shares, comprised of an aggregate of 371,617 Common Shares and 1,992,453 Common Shares underlying convertible notes; and (vii) James E. Flynn had shared voting power and shared dispositive power with respect to 10,701,187 Common Shares, comprised of an aggregate of 1,644,583 Common Shares and 9,056,604 Common Shares underlying convertible notes held by Deerfield Private Design Fund III, L.P., Deerfield Partners, L.P. and Deerfield International Master Fund, L.P. The provisions of the convertible notes beneficially owned by the Deerfield Reporting Persons restrict the conversion of such securities to the extent that, upon such conversion, the number of Common Shares then beneficially owned by the holder and any other person or entities with which such holder would constitute a Section 13(d) “group” would exceed 9.985% of the total number of Common Shares of the Company then outstanding (the “Ownership Cap”). Accordingly, notwithstanding the number of Common Shares reported, the Deerfield Reporting Persons disclaim beneficial ownership of the Common Shares issuable upon the conversion of such convertible notes to the extent that
upon such conversion the number of Common Shares beneficially owned by the Deerfield Reporting Persons, in the aggregate, would exceed the Ownership Cap. The address of the Deerfield Reporting Persons is 780 Third Avenue, 37th Floor, New York, NY 10017.
(2) Based on information disclosed in a Schedule 13G/A filed with the SEC on February 14, 2018 by PAR Investment Partners, L.P., PAR Group, L.P. and PAR Capital Management, Inc. According to the Schedule 13G/A, as of December 31, 2017, PAR Group, L.P. was the sole general partner of PAR Investment Partners, L.P. PAR Capital Management, Inc. is the sole general partner of PAR Group, L.P. The address of PAR Capital Management, Inc. is 200 Clarendon Street, F1 48, Boston, MA 02116.
|
Name of Beneficial Owner(1) |
|
Number of Common |
|
Percentage |
|
|
Adrian Adams |
|
3,239,698 |
(2) |
4.8 |
% |
|
Neal F. Fowler |
|
88,869 |
(3) |
* |
|
|
Rob Harris |
|
1,422,612 |
(2) |
2.1 |
% |
|
Michael Kaseta |
|
24,008 |
(4) |
* |
|
|
Arthur S. Kirsch |
|
318,823 |
(5) |
* |
|
|
Andrew I. Koven |
|
953,936 |
(7) |
1.4 |
% |
|
Kenneth B. Lee, Jr. |
|
117,952 |
(8) |
* |
|
|
Seth A. Rudnick, M.D. |
|
131,891 |
(9) |
* |
|
|
F. Martin Thrasher |
|
320,272 |
(10) |
* |
|
|
James P. Tursi, M.D. |
|
169,897 |
(11) |
* |
|
|
All current directors, director nominees and executive officers as a group (11 persons) |
|
6,889,279 |
(12) |
10.3 |
% |
* Less than 1%
(1) Unless otherwise set forth herein, the address of the named beneficial owners is c/o Aralez Pharmaceuticals Inc., 7100 West Credit Avenue, Suite 101, Mississauga, Ontario, Canada, L5N 0E4.
(2) This number includes 125,345 Common Shares that are issuable to Mr. Adams pursuant to stock options that are currently exercisable or will become exercisable within 60 days of April 16, 2018 and 559,014 Common Shares that are issuable pursuant to RSUs that will vest within 60 days of April 16, 2018.
(3) This number includes 30,000 Common Shares that are issuable to Mr. Fowler pursuant to stock options that are currently exercisable or will become exercisable within 60 days of April 16, 2018.
(4) This number includes 600,188 Common Shares held by Mr. Harris’ spouse. This number also includes 220,830 Common Shares that are issuable to Mr. Harris pursuant to stock options that are currently exercisable or will become exercisable within 60 days of April 16, 2018 and 7,500 Common Shares that are issuable pursuant to RSUs that will vest within 60 days of April 16, 2018.
(5) This number includes 23,175 Common Shares that are issuable to Mr. Kaseta pursuant to stock options that are currently exercisable or will become exercisable within 60 days of April 16, 2018.
(6) This number includes 5,000 Common Shares that are held by the Laura A. Kirsch Trust, dated 4/12/2000, for which Mr. Kirsch acts as a trustee, and includes 104,000 Common Shares that are held in an IRA for Mr. Kirsch. This number also includes 42,214 Common Shares that are issuable to Mr. Kirsch pursuant to stock options that are currently exercisable or will become exercisable within 60 days of April 16, 2018 and 7,500 Common Shares that are issuable pursuant to RSUs that will vest within 60 days of April 16, 2018.
(7) This number includes 62,654 Common Shares that are issuable to Mr. Koven pursuant to stock options that are currently exercisable or will become exercisable within 60 days of April 16, 2018 and 405,564 Common Shares that are issuable pursuant to RSUs that will vest within 60 days of April 16, 2018.
(8) This number includes 30,000 Common Shares that are issuable to Mr. Lee pursuant to stock options that are currently exercisable or will become exercisable within 60 days of April 16, 2018.
(9) This number includes 30,000 Common Shares held in the Seth A. Rudnick 2014 GST Trust U/A Dated 3/01/2014 (the “Trust”) for the benefit of Mr. Rudnick’s heirs. Mr. Rudnick’s spouse is the trustee of the Trust. This number also includes 30,000 Common Shares that are issuable to Mr. Rudnick pursuant to stock options that are currently exercisable or will become exercisable within 60 days of April 16, 2018.
(10) This number includes 272,812 Common Shares that are held by 2089636 Ontario Ltd., for whom Mr. Thrasher is the sole owner and President. This number also includes 43,095 Common Shares that are issuable to Mr. Thrasher pursuant to stock options that are currently exercisable or will become exercisable within 60 days of April 16, 2018.
(11) This number includes 47,750 Common Shares that are issuable to Dr. Tursi pursuant to stock options that are currently exercisable or will become exercisable within 60 days of April 16, 2018 and 27,730 Common Shares that are issuable pursuant to RSUs that will vest within 60 days of April 16, 2018.
(12) This number includes 683,652 Common Shares issuable pursuant to stock options that are currently exercisable or will become exercisable within 60 days of April 16, 2018 and 1,052,339 Common Shares that are issuable pursuant to RSUs that will vest within 60 days of April 16, 2018.
Equity Compensation Plan Information
The following table sets forth information as of December 31, 2017 regarding our compensation plans, including individual compensation agreements, under which equity securities were authorized for issuance:
|
Plan Category |
|
Number of |
|
Weighted |
|
Number of |
| |
|
|
|
(a) |
|
(b) |
|
(c) |
| |
|
Equity compensation plans approved by security holders |
|
6,092,781 |
(1) |
$ |
3.28 |
(2) |
3,552,770 |
|
|
Equity compensation plans not approved by security holders |
|
1,710,781 |
(3) |
— |
|
— |
| |
|
Total |
|
7,803,562 |
|
$ |
3.28 |
|
3,552,770 |
|
(1) Represents 707,834 Common Shares associated with outstanding options and RSUs that were granted under the Pozen equity compensation plans approved by security holders. The awards outstanding under these plans as of February 5, 2016 were assumed by Aralez upon the closing of the Tribute acquisition, and no further awards will be granted under the Pozen equity compensation plans. This number also includes 5,384,947 Common Shares associated with outstanding options, RSUs and performance share units (at target) that were granted under the 2016 Plan.
(2) Represents the weighted average exercise price of the outstanding options reported in column (a). Does not take into account the outstanding RSUs reported in column (a).
(3) Represents Common Shares underlying inducement RSU grants to certain executive officers that were issued outside of security holder approved equity plans.
ITEM 13. Certain Relationships and Related Transactions, and Director Independence
Certain Relationships and Related Party Transactions
The Board has adopted written policies and procedures for the review, approval or ratification of transactions involving the Company and any executive officer, director, director nominee, 5% shareholder and certain of their immediate family members (each of whom we refer to as a “related person”). The policies and procedures cover any transaction involving $120,000 or more with a related person (a “related person transaction”) in which the related person has a material interest and which does not fall under an explicitly stated exception set forth in the applicable disclosure rules of the SEC.
Any proposed related person transaction must be reported to the Chairperson of the Nominating/Corporate Governance Committee. The policy calls for the transaction to be reviewed and, if deemed appropriate, approved by the Nominating/Corporate Governance Committee. The transaction should be approved in advance whenever practicable. If not practicable, the Nominating/Corporate Governance Committee will review, and may, if deemed appropriate, ratify the related person transaction. The policy also permits the Chairperson of the Nominating/Corporate Governance Committee to approve related person transactions that arise between committee meetings, subject to ratification by the Nominating/Corporate Governance Committee at its next meeting. Any related person transaction that is ongoing in nature will be reviewed annually.
A related person transaction will be considered approved or ratified if it is authorized by the Nominating/Corporate Governance Committee or Chairperson of the Nominating/Corporate Governance Committee after full disclosure of the related person’s interest in the transaction. The transaction may be approved or ratified only if the Nominating/Corporate Governance Committee determines that the transaction is not inconsistent with the Company’s best interests. In considering related person transactions, the Nominating/Corporate Governance Committee will consider any information considered material to investors and the following factors:
· the related person’s interest in the transaction;
· the approximate dollar value of the transaction;
· whether the transaction was undertaken in the ordinary course of our business;
· whether the terms of the transaction are no less favorable to us than terms that we could have reached with an unrelated third party; and
· the purpose and potential benefit to us of the transaction.
The policy provides that transactions involving the compensation of our executive officers will be reviewed and approved by the Compensation Committee or the Board, in accordance with the Compensation Committee’s charter.
Except as described below, there have been no related party transactions since January 1, 2017.
We paid $236,810 in legal fees to the attorneys representing Deerfield Private Design Fund III, L.P., Deerfield International Master Fund, L.P. and Deerfield Partners, L.P. (the “Deerfield Entities”) since January 1, 2017 in connection with matters related to the Second Amended and Restated Facility Agreement, dated December 7, 2015, by and among the Company, Pozen, Tribute and the Deerfield Entities.
Director Independence
The Board has determined that five of the seven incumbent directors (or 71% of the Board) have no material relationship with the Company, either directly or indirectly, and are “independent” within the meaning of the applicable Nasdaq listing standards and National Instrument 58-101 — Disclosure of Corporate Governance Practices (“NI 58-101”) (such directors, the “Independent Directors”). Specifically, the Board has identified each of the members of the Board, with the exception of Mr. Adams, who serves as our Chief Executive Officer, and Mr. Harris, who previously served as President and Chief Executive Officer of Tribute, as independent for the purposes of the applicable Nasdaq listing standards and NI 58-101.
ITEM 14. Principal Accountant Fees and Services
Fees and Services of E&Y
The following table sets forth the aggregate fees billed to the Company for professional services rendered to it by Ernst & Young LLP for Fiscal Year 2017 and for the fiscal year ended December 31, 2016 (“Fiscal Year 2016”):
|
|
|
2017 |
|
2016 |
| ||
|
Audit Fees(1) |
|
$ |
1,019,754 |
|
$ |
1,514,000 |
|
|
Audit-Related Fees |
|
— |
|
— |
| ||
|
Tax Fees(2) |
|
— |
|
15,000 |
| ||
|
All Other Fees(3) |
|
1,985 |
|
1,985 |
| ||
|
Total |
|
$ |
1,021,739 |
|
$ |
1,530,985 |
|
(1) Audit Fees in Fiscal Year 2017 consisted of fees associated with the annual audit and the reviews of the Company’s quarterly reports on Form 10-Q. Audit Fees in Fiscal Year 2016 consisted of fees associated with the annual audit and the reviews of the Company’s quarterly reports on Form 10-Q.
(2) Tax Fees in Fiscal Year 2016 consisted of fees associated with New Jersey tax incentives consultations.
(3) All Other Fees consists of fees for any other products or services provided by Ernst & Young, LLP not described above. The All Other Fees for 2017 and 2016 are related to licensed accounting research software.
Pre-Approval Policies and Procedures.
Our Audit Committee has adopted policies and procedures relating to the pre-approval of all audit and non-audit services to be provided by our independent auditors. Under these policies and procedures, the Audit Committee approves in advance the provision of services and fees for such services that are specifically identified in the independent auditor’s annual engagement letter for the audits and reviews, in management’s annual budget relating to services to be provided by the independent auditors and any amendments to the annual budget reflecting additional services to be provided by or higher fees of the independent auditors. All other services to be provided by the independent auditors are pre-approved by the Audit Committee as they arise. The Chairperson of the Audit Committee has been delegated authority to pre-approve services in accordance with these policies and procedures. The Chairperson is to report any such approval of services to the Audit Committee at its next meeting. The Audit Committee considers, among other things, whether the provision of such audit or non-audit services is consistent with applicable regulations regarding maintaining auditor independence, whether the provision of such services would impair the independent auditors’ independence and whether the independent auditors are best positioned to provide the most effective and efficient service.
ITEM 15. Exhibits and Financial Statement Schedules
|
Exhibit |
|
Index Description of Exhibit |
|
|
|
|
|
2.1 |
|
|
|
|
|
|
|
2.2 |
|
|
|
|
|
|
|
2.3 |
|
|
|
|
|
|
|
2.4 |
|
|
|
|
|
|
|
2.5 |
|
|
|
|
|
|
|
2.6 |
|
|
|
|
|
|
|
3.1 |
|
|
|
|
|
|
|
3.2 |
|
|
|
|
|
|
|
10.1 |
|
|
|
|
|
|
|
10.2 |
|
|
|
|
|
|
|
10.3 |
|
|
|
|
|
|
|
10.4 |
|
|
|
|
|
|
|
10.5 |
|
|
|
|
|
|
|
10.6 |
|
|
Exhibit |
|
Index Description of Exhibit |
|
|
|
|
|
10.7 |
|
|
|
|
|
|
|
10.8 |
|
|
|
|
|
|
|
10.9 |
|
|
|
|
|
|
|
10.10 |
|
|
|
|
|
|
|
10.11 |
|
|
|
|
|
|
|
10.12 |
|
|
|
|
|
|
|
10.13 |
|
|
|
|
|
|
|
10.14 |
|
|
|
|
|
|
|
10.15 |
|
|
|
|
|
|
|
10.16 |
|
|
|
|
|
|
|
10.17 |
|
|
|
|
|
|
|
10.18 |
|
|
|
|
|
|
|
10.19 |
|
|
|
|
|
|
|
10.20 |
|
|
|
|
|
|
|
10.21 |
|
|
|
|
|
|
|
10.22 |
|
|
|
|
|
|
|
10.23 |
|
|
|
|
|
|
|
10.24 |
|
|
Exhibit |
|
Index Description of Exhibit |
|
|
|
|
|
10.25 |
|
|
|
|
|
|
|
10.26 |
|
|
|
|
|
|
|
10.27 |
|
|
|
|
|
|
|
10.28 |
|
|
|
|
|
|
|
10.29 |
|
|
|
|
|
|
|
10.30 |
|
|
|
|
|
|
|
10.31 |
|
|
|
|
|
|
|
10.32 |
|
|
|
|
|
|
|
10.33 |
|
|
|
|
|
|
|
10.34 |
|
|
|
|
|
|
|
10.35 |
|
|
|
|
|
|
|
10.36 |
|
|
|
|
|
|
|
10.37 |
|
|
|
|
|
|
|
10.38 |
|
|
|
|
|
|
|
21.1 |
|
|
|
|
|
|
|
23.1 |
|
|
Exhibit |
|
Index Description of Exhibit |
|
|
|
|
|
31.1 |
|
|
|
|
|
|
|
31.2 |
|
|
|
|
|
|
|
32.1 |
|
|
|
|
|
|
|
32.2 |
|
|
|
|
|
|
|
101 |
|
The following materials from Aralez Pharmaceuticals Inc.’s Annual Report on Form 10-K for the year ended December 31, 2017, formatted in Extensible Business Reporting Language (XBRL): (i) Consolidated Balance Sheets at December 31, 2017 and 2016, (ii) Consolidated Statements of Operations for the years ended December 31, 2017, 2016 and 2015, (iii) Consolidated Statements of Comprehensive (Loss) Income for the years ended December 31, 2017, 2016 and 2015 (iv) Consolidated Statements of Shareholders’ Equity at December 31, 2017, 2016 and 2015, (v) Consolidated Statements of Cash Flows for the years ended December 31, 2017, 2016 and 2015, and (vi) Notes to Consolidated Financial Statements (incorporated by reference to Exhibit 101 to Aralez Pharmaceutical Inc.’s Annual Report on Form 10-K filed March 14, 2018). |
+ Compensation Related Contract.
† Confidential treatment requested. Confidential materials omitted and filed separately with Securities and Exchange Commission.
* Form may also be utilized in connection with A&R LTIP and any such form as amended to refer to A&R LTIP is incorporated herein, as applicable.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
Registrant: | |
|
|
| |
|
|
Aralez Pharmaceuticals Inc. | |
|
|
| |
|
Date: April 30, 2018 |
By: |
/s/ Adrian Adams |
|
|
|
Adrian Adams |
|
|
|
Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Adrian Adams |
|
Chief Executive Officer (Principal Executive Officer), Director |
|
April 30, 2018 |
|
Adrian Adams |
|
|
|
|
|
|
|
|
|
|
|
/s/ Michael J. Kaseta |
|
Chief Financial Officer (Principal Financial Officer, Principal Accounting Officer) |
|
April 30, 2018 |
|
Michael J. Kaseta |
|
|
|
|
|
|
|
|
|
|
|
/s/ Arthur S. Kirsch |
|
Director |
|
April 30, 2018 |
|
Arthur S. Kirsch |
|
|
|
|
|
|
|
|
|
|
|
/s/ Neal F. Fowler |
|
Director |
|
April 30, 2018 |
|
Neal F. Fowler |
|
|
|
|
|
|
|
|
|
|
|
/s/ Seth A. Rudnick, M.D. |
|
Director |
|
April 30, 2018 |
|
Seth A. Rudnick, M.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ Kenneth B. Lee, Jr. |
|
Director |
|
April 30, 2018 |
|
Kenneth B. Lee, Jr. |
|
|
|
|
|
|
|
|
|
|
|
/s/ Rob Harris |
|
Director |
|
April 30, 2018 |
|
Rob Harris |
|
|
|
|
|
|
|
|
|
|
|
/s/ F. Martin Thrasher |
|
Director |
|
April 30, 2018 |
|
F. Martin Thrasher |
|
|
|
|
