Attached files
| file | filename |
|---|---|
| 8-K - 8-K - F-star Therapeutics, Inc. | sbph-8k_20180425.htm |
DRIVEN BY A NOVEL PHARMACEUTICAL PLATFORM FOCUSED ON SELECTIVE IMMUNOMODULATION Exhibit 99.1

Forward Looking Statements This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, among other things, statements, other than historical facts, regarding: the progress, scope, duration or results of clinical trials and preclinical studies of inarigivir soproxil (“inarigivir”), SB 11285 or any of our other product candidates or programs, such as the size, design, population, conduct, cost, objective or endpoints of any clinical trial, or the timing for initiation or completion of or availability of results from any clinical trial (including our Phase 2a clinical trial of inarigivir in patients with chronic Hepatitis B virus); the potential benefits that may be derived from any of our product candidates; our future operations, financial position, revenues, costs, expenses, uses of cash, capital requirements or our need for additional financing; or our strategies, goals, milestones, prospects, beliefs, intentions, plans, expectations, forecasts or objectives. Words such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “future,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “guidance,” and similar expressions sometimes identify forward-looking statements. Any forward-looking statement involves known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from those expressed or implied by such forward-looking statement, and, therefore, you are cautioned not to place undue reliance on any forward-looking statement. These factors include, but are not limited to: whether our cash resources will be sufficient to fund our continuing operations for the period anticipated; the components, timing, costs and results of our clinical trials, preclinical studies and other development activities involving our product candidates; whether certain top-line results from our clinical trials materially change as more information becomes available; whether results obtained in preclinical studies and clinical trials will be indicative of results obtained in future clinical trials; whether inarigivir, SB 11285 and any of our other product candidates will advance through the clinical trial process on a timely basis and receive approval from the United States Food and Drug Administration or equivalent foreign regulatory agencies; and whether, if inarigivir, SB 11285 or any of our other product candidates obtain regulatory approval, it will be successfully distributed and marketed. These and other risks and uncertainties that we face are described in our most recent Annual Report on Form 10-K, filed with the Securities and Exchange Commission (SEC) on February 20, 2018, and in other filings that we make with the SEC from time to time. All forward-looking statements speak only as of April 25, 2018 and should not be relied upon as representing our views as of any other date. We specifically disclaim any obligation to update any forward-looking statement, except as required by applicable law. All trademarks, service marks, trade names, logos and brand names identified in this presentation are the properties of their respective owners. This presentation also contains estimates and other statistical data generated by independent parties and by us relating to market size and statistics. These estimates involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. FORWARD LOOKING STATEMENT SPRING BANK PHARMACEUTICALS, INC. © 2018

RIG-I Agonists: HBV, HIV latency and other viral infections STING Agonists: immuno-oncology and autoimmune diseases ADDRESSING UNMET NEEDS USING OUR NOVEL SMNH PLATFORM 1 • SPRING BANK PHARMACEUTICALS, INC. © 2018 Clinical-stage biopharmaceutical company engaged in the discovery and development of a novel class of therapeutics using a proprietary small molecule nucleic acid hybrid (SMNH) technology A COMPANY WITH INNOVATIVE FOCUS
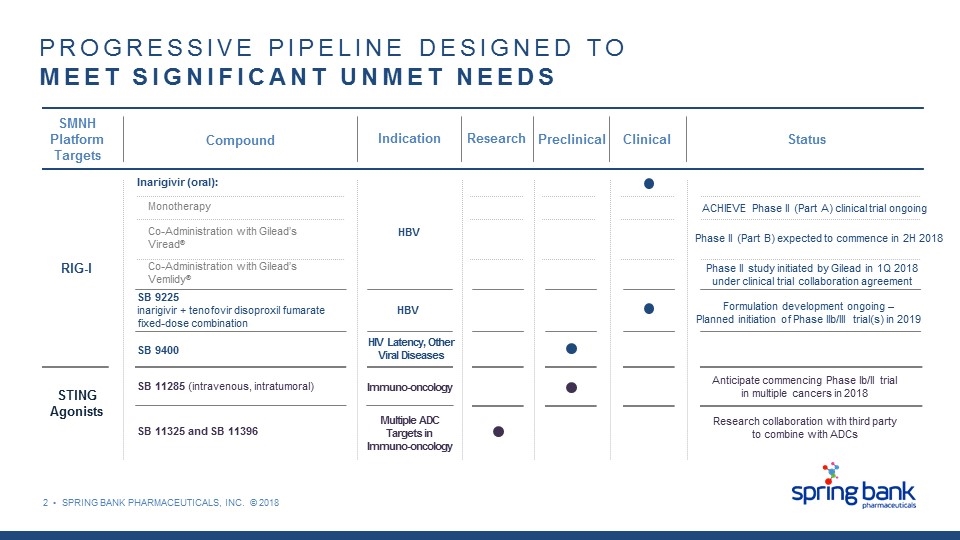
2 • SPRING BANK PHARMACEUTICALS, INC. © 2018 PROGRESSIVE PIPELINE DESIGNED TO MEET SIGNIFICANT UNMET NEEDS SMNH Platform Targets RIG-I STING Agonists Compound Inarigivir (oral): Monotherapy Co-Administration with Gilead’s Viread® Co-Administration with Gilead’s Vemlidy® SB 9225 inarigivir + tenofovir disoproxil fumarate fixed-dose combination SB 9400 Indication SB 11285 (intravenous, intratumoral) SB 11325 and SB 11396 HBV HIV Latency, Other Viral Diseases HBV Immuno-oncology Multiple ADC Targets in Immuno-oncology Research Preclinical Clinical Phase II (Part B) expected to commence in 2H 2018 ACHIEVE Phase II (Part A) clinical trial ongoing Phase II study initiated by Gilead in 1Q 2018 under clinical trial collaboration agreement Formulation development ongoing – Planned initiation of Phase IIb/III trial(s) in 2019 Anticipate commencing Phase Ib/II trial in multiple cancers in 2018 Research collaboration with third party to combine with ADCs Status
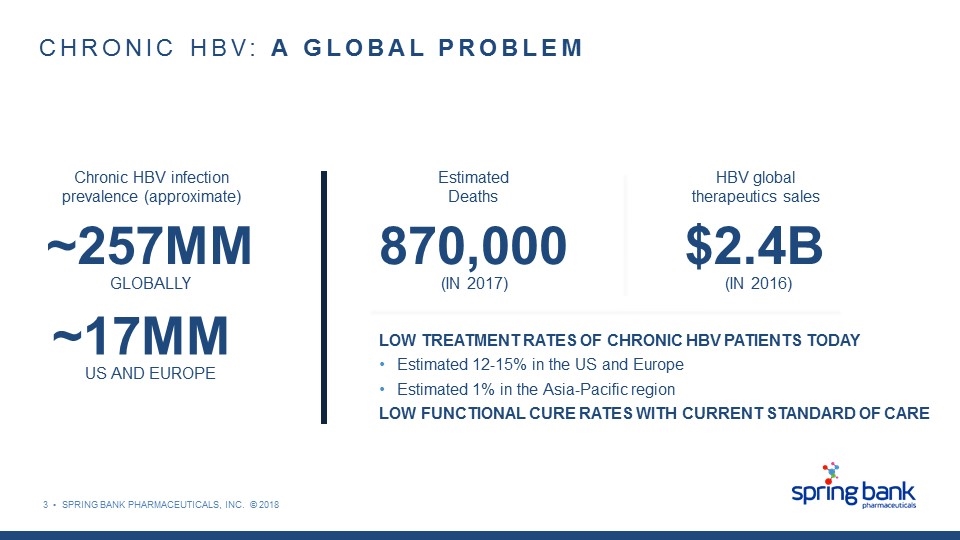
3 • SPRING BANK PHARMACEUTICALS, INC. © 2018 CHRONIC HBV: A GLOBAL PROBLEM Chronic HBV infection prevalence (approximate) ~257MM GLOBALLY ~17MM US AND EUROPE Estimated Deaths 870,000 (IN 2017) HBV global therapeutics sales $2.4B (IN 2016) LOW TREATMENT RATES OF CHRONIC HBV PATIENTS TODAY Estimated 12-15% in the US and Europe Estimated 1% in the Asia-Pacific region LOW FUNCTIONAL CURE RATES WITH CURRENT STANDARD OF CARE
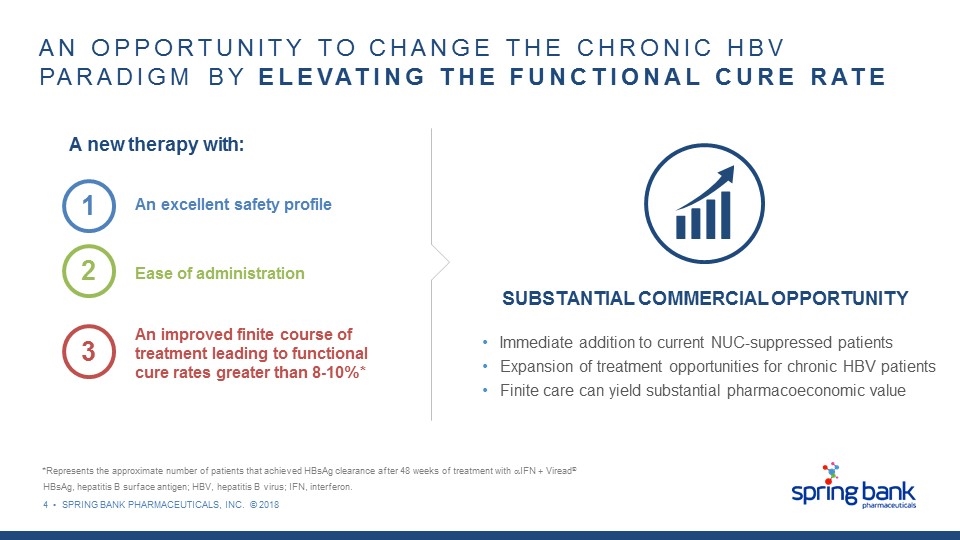
4 • SPRING BANK PHARMACEUTICALS, INC. © 2018 AN OPPORTUNITY TO CHANGE THE CHRONIC HBV PARADIGM BY ELEVATING THE FUNCTIONAL CURE RATE A new therapy with: 3 An improved finite course of treatment leading to functional cure rates greater than 8-10%* 2 Ease of administration 1 An excellent safety profile SUBSTANTIAL COMMERCIAL OPPORTUNITY Immediate addition to current NUC-suppressed patients Expansion of treatment opportunities for chronic HBV patients Finite care can yield substantial pharmacoeconomic value *Represents the approximate number of patients that achieved HBsAg clearance after 48 weeks of treatment with aIFN + Viread® HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IFN, interferon.
INARIGIVIR: A NOVEL, ORAL, SELECTIVE IMMUNOMODULATOR WITH A DUAL MECHANISM OF ACTION 5 • SPRING BANK PHARMACEUTICALS, INC. © 2018 Small molecule nucleic acid hybrid (SMNH) Hepatic-selective immunomodulator Favorable safety profile to date Orally bioavailable Physical properties allow for potential fixed-dose combination with DAAs Potent, selective RIG-I agonist
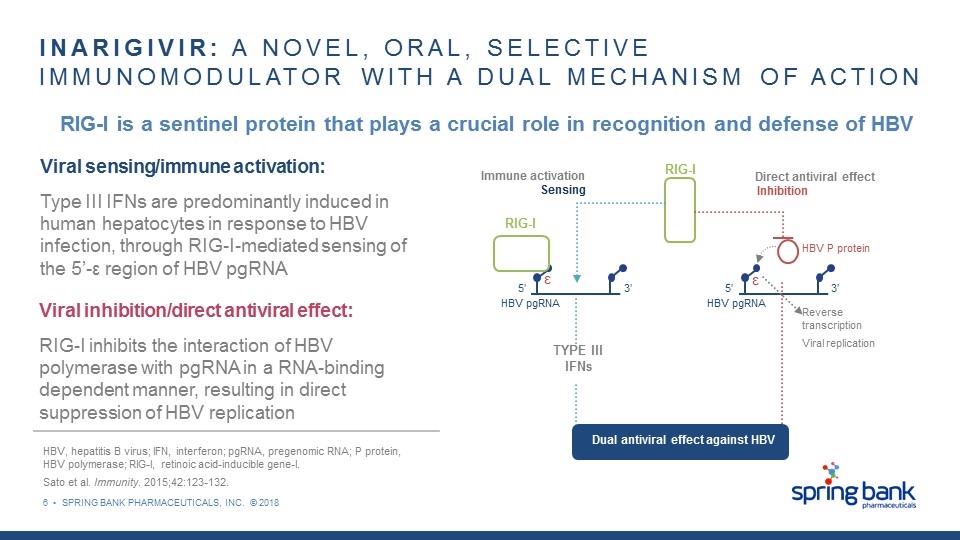
6 • SPRING BANK PHARMACEUTICALS, INC. © 2018 INARIGIVIR: A NOVEL, ORAL, SELECTIVE IMMUNOMODULATOR WITH A DUAL MECHANISM OF ACTION HBV, hepatitis B virus; IFN, interferon; pgRNA, pregenomic RNA; P protein, HBV polymerase; RIG-I, retinoic acid-inducible gene-I. Sato et al. Immunity. 2015;42:123-132. RIG-I is a sentinel protein that plays a crucial role in recognition and defense of HBV Viral sensing/immune activation: Type III IFNs are predominantly induced in human hepatocytes in response to HBV infection, through RIG-I-mediated sensing of the 5’-ε region of HBV pgRNA Viral inhibition/direct antiviral effect: RIG-I inhibits the interaction of HBV polymerase with pgRNA in a RNA-binding dependent manner, resulting in direct suppression of HBV replication RIG-I RIG-I TYPE III IFNs Immune activation Sensing Direct antiviral effect Inhibition HBV pgRNA 5’ 3’ HBV pgRNA 5’ 3’ Dual antiviral effect against HBV HBV P protein Reverse transcription Viral replication ε ε

7 • SPRING BANK PHARMACEUTICALS, INC. © 2018 INARIGIVIR’S DUAL MECHANISM OF ACTION: A UNIQUE, POTENTIAL BACKBONE AGENT FOR COMBINATION CURE STRATEGIES Evidence suggests host immune response is necessary for VIRAL CLEARANCE & FUNCTIONAL CURE “Combination of antiviral and immune modulatory therapies will likely be needed to achieve functional hepatitis B virus cure.” Lok A, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. J Hepatol. 2017;67:847-861.
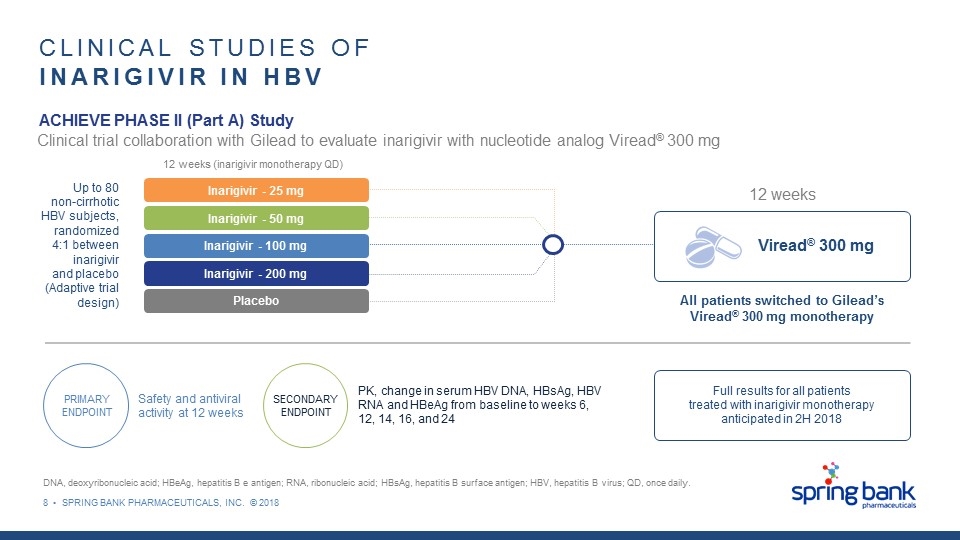
8 • SPRING BANK PHARMACEUTICALS, INC. © 2018 CLINICAL STUDIES OF INARIGIVIR IN HBV DNA, deoxyribonucleic acid; HBeAg, hepatitis B e antigen; RNA, ribonucleic acid; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; QD, once daily. PRIMARY ENDPOINT SECONDARY ENDPOINT Safety and antiviral activity at 12 weeks PK, change in serum HBV DNA, HBsAg, HBV RNA and HBeAg from baseline to weeks 6, 12, 14, 16, and 24 Full results for all patients treated with inarigivir monotherapy anticipated in 2H 2018 Up to 80 non-cirrhotic HBV subjects, randomized 4:1 between inarigivir and placebo (Adaptive trial design) Inarigivir - 200 mg Placebo Inarigivir - 100 mg Inarigivir - 50 mg Inarigivir - 25 mg Viread® 300 mg All patients switched to Gilead’s Viread® 300 mg monotherapy 12 weeks (inarigivir monotherapy QD) 12 weeks Clinical trial collaboration with Gilead to evaluate inarigivir with nucleotide analog Viread® 300 mg ACHIEVE PHASE II (Part A) Study
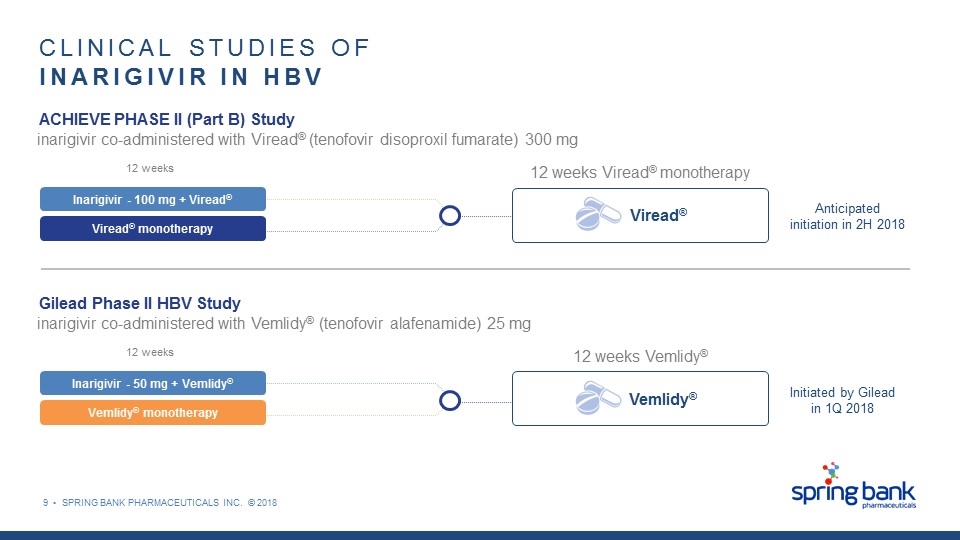
9 • SPRING BANK PHARMACEUTICALS INC. © 2018 CLINICAL STUDIES OF INARIGIVIR IN HBV inarigivir co-administered with Viread® (tenofovir disoproxil fumarate) 300 mg ACHIEVE PHASE II (Part B) Study Viread® monotherapy Inarigivir - 100 mg + Viread® Viread® 12 weeks 12 weeks Viread® monotherapy Anticipated initiation in 2H 2018 inarigivir co-administered with Vemlidy® (tenofovir alafenamide) 25 mg Gilead Phase II HBV Study Vemlidy® monotherapy Inarigivir - 50 mg + Vemlidy® Vemlidy® 12 weeks 12 weeks Vemlidy® Initiated by Gilead in 1Q 2018
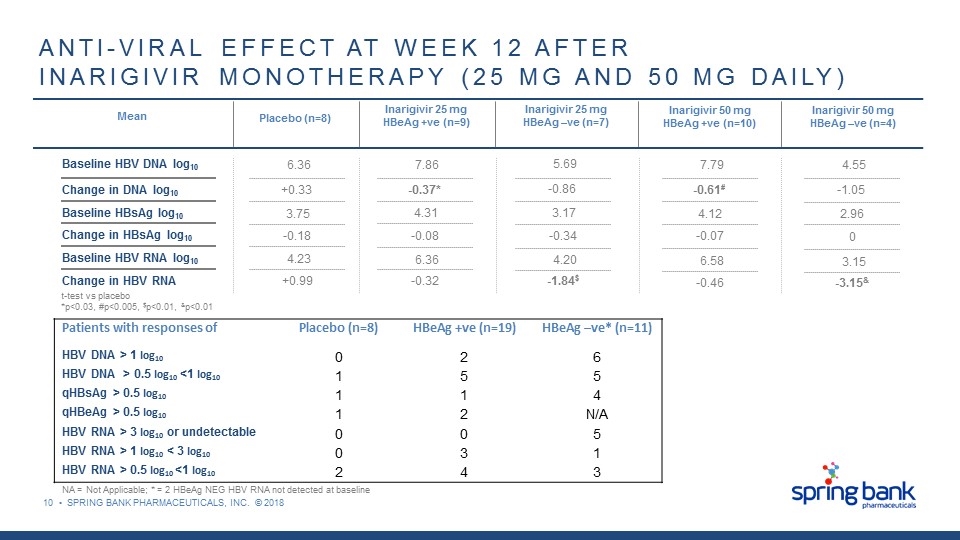
10 • SPRING BANK PHARMACEUTICALS, INC. © 2018 t-test vs placebo *p<0.03, #p<0.005, $p<0.01, &p<0.01 ANTI-VIRAL EFFECT AT WEEK 12 AFTER INARIGIVIR MONOTHERAPY (25 MG AND 50 MG DAILY) Mean Placebo (n=8) Inarigivir 25 mg HBeAg +ve (n=9) Inarigivir 25 mg HBeAg –ve (n=7) Inarigivir 50 mg HBeAg +ve (n=10) Inarigivir 50 mg HBeAg –ve (n=4) Baseline HBV DNA log10 Change in DNA log10 Baseline HBsAg log10 Change in HBsAg log10 Baseline HBV RNA log10 Change in HBV RNA 6.36 +0.33 3.75 -0.18 4.23 +0.99 7.86 -0.37* 4.31 -0.08 6.36 -0.32 5.69 -0.86 3.17 -0.34 4.20 -1.84$ 7.79 -0.61# 4.12 -0.07 6.58 -0.46 4.55 -1.05 2.96 0 3.15 -3.15& Patients with responses of Placebo (n=8) HBeAg +ve (n=19) HBeAg –ve* (n=11) HBV DNA > 1 log10 0 2 6 HBV DNA > 0.5 log10 <1 log10 1 5 5 qHBsAg > 0.5 log10 1 1 4 qHBeAg > 0.5 log10 1 2 N/A HBV RNA > 3 log10 or undetectable 0 0 5 HBV RNA > 1 log10 < 3 log10 0 3 1 HBV RNA > 0.5 log10 <1 log10 2 4 3 NA = Not Applicable; * = 2 HBeAg NEG HBV RNA not detected at baseline
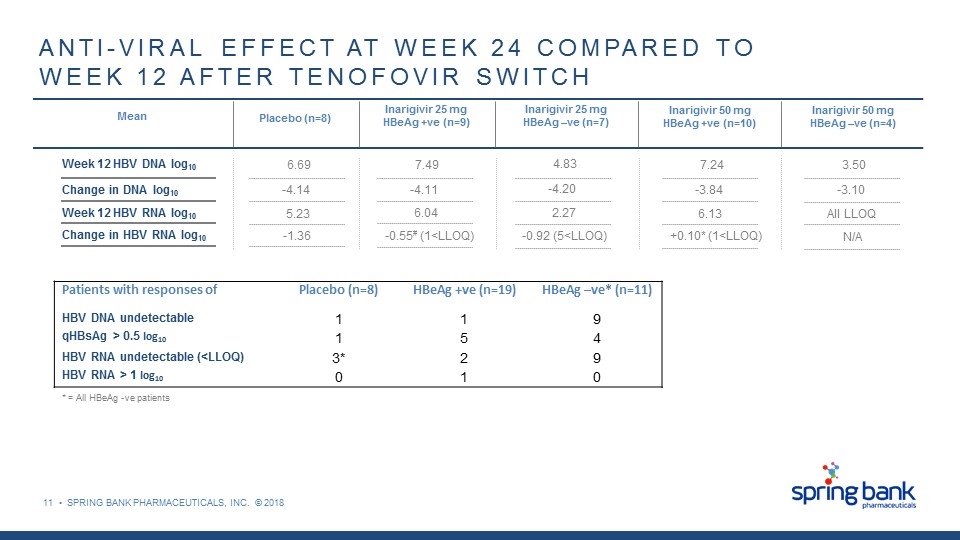
11 • SPRING BANK PHARMACEUTICALS, INC. © 2018 ANTI-VIRAL EFFECT AT WEEK 24 COMPARED TO WEEK 12 AFTER TENOFOVIR SWITCH Mean Placebo (n=8) Inarigivir 25 mg HBeAg +ve (n=9) Inarigivir 25 mg HBeAg –ve (n=7) Inarigivir 50 mg HBeAg +ve (n=10) Inarigivir 50 mg HBeAg –ve (n=4) Week 12 HBV DNA log10 Change in DNA log10 Week 12 HBV RNA log10 Change in HBV RNA log10 6.69 -4.14 5.23 -1.36 7.49 -4.11 6.04 -0.55# (1<LLOQ) 4.83 -4.20 2.27 -0.92 (5<LLOQ) 7.24 -3.84 6.13 +0.10* (1<LLOQ) 3.50 -3.10 All LLOQ N/A Patients with responses of Placebo (n=8) HBeAg +ve (n=19) HBeAg –ve* (n=11) HBV DNA undetectable 1 1 9 qHBsAg > 0.5 log10 1 5 4 HBV RNA undetectable (<LLOQ) 3* 2 9 HBV RNA > 1 log10 0 1 0 * = All HBeAg -ve patients
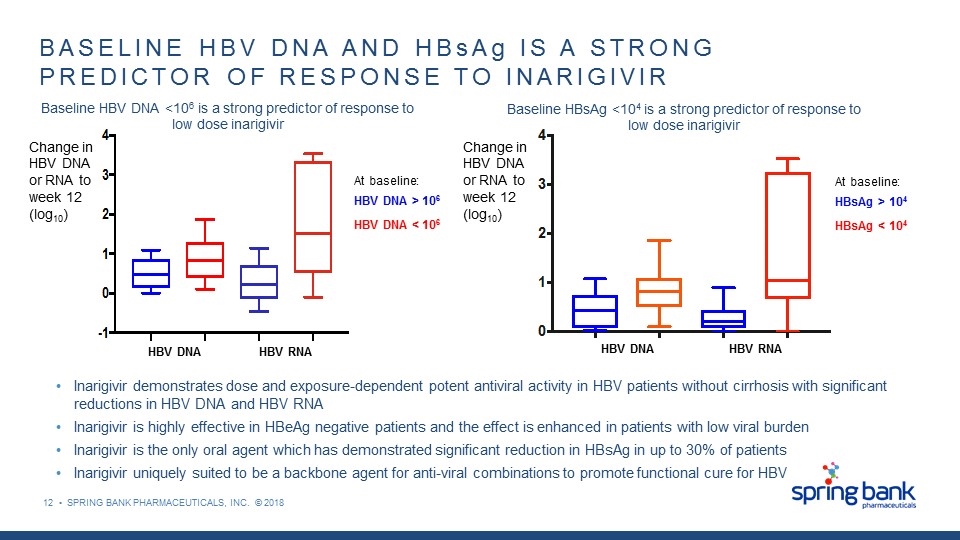
12 • SPRING BANK PHARMACEUTICALS, INC. © 2018 BASELINE HBV DNA AND HBsAg IS A STRONG PREDICTOR OF RESPONSE TO INARIGIVIR At baseline: HBV DNA > 106 HBV DNA < 106 Change in HBV DNA or RNA to week 12 (log10) Baseline HBV DNA <106 is a strong predictor of response to low dose inarigivir Change in HBV DNA or RNA to week 12 (log10) Baseline HBsAg <104 is a strong predictor of response to low dose inarigivir HBV DNA HBV RNA At baseline: HBsAg > 104 HBsAg < 104 HBV DNA HBV RNA Inarigivir demonstrates dose and exposure-dependent potent antiviral activity in HBV patients without cirrhosis with significant reductions in HBV DNA and HBV RNA Inarigivir is highly effective in HBeAg negative patients and the effect is enhanced in patients with low viral burden Inarigivir is the only oral agent which has demonstrated significant reduction in HBsAg in up to 30% of patients Inarigivir uniquely suited to be a backbone agent for anti-viral combinations to promote functional cure for HBV
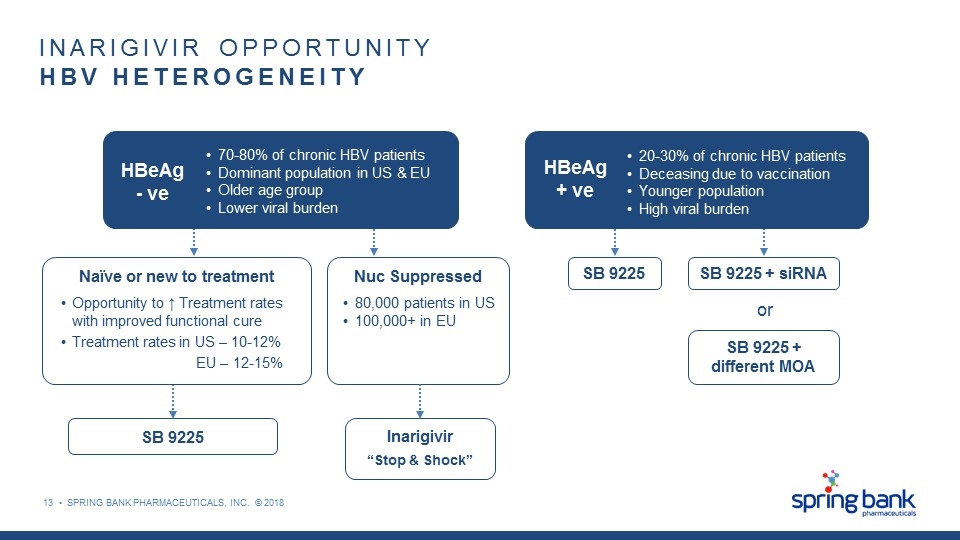
HBeAg - ve 70-80% of chronic HBV patients Dominant population in US & EU Older age group Lower viral burden SB 9225 SB 9225 + siRNA SB 9225 + different MOA or Inarigivir “Stop & Shock” SB 9225 INARIGIVIR OPPORTUNITY HBV HETEROGENEITY Naïve or new to treatment Nuc Suppressed 80,000 patients in US 100,000+ in EU Opportunity to ↑ Treatment rates with improved functional cure Treatment rates in US – 10-12% EU – 12-15% HBeAg + ve 20-30% of chronic HBV patients Deceasing due to vaccination Younger population High viral burden 13 • SPRING BANK PHARMACEUTICALS, INC. © 2018
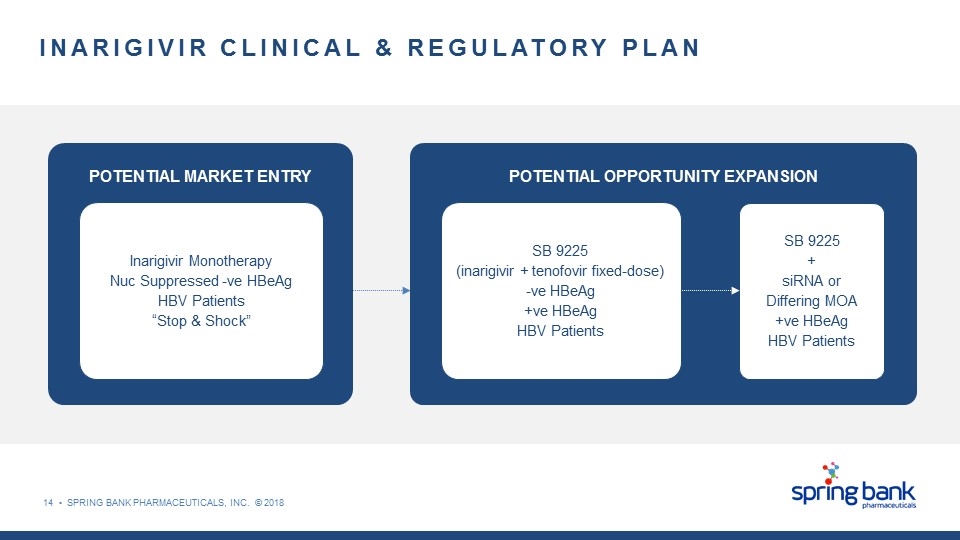
INARIGIVIR CLINICAL & REGULATORY PLAN Inarigivir Monotherapy Nuc Suppressed -ve HBeAg HBV Patients “Stop & Shock” POTENTIAL MARKET ENTRY POTENTIAL OPPORTUNITY EXPANSION SB 9225 + siRNA or Differing MOA +ve HBeAg HBV Patients SB 9225 (inarigivir + tenofovir fixed-dose) -ve HBeAg +ve HBeAg HBV Patients 14 • SPRING BANK PHARMACEUTICALS, INC. © 2018
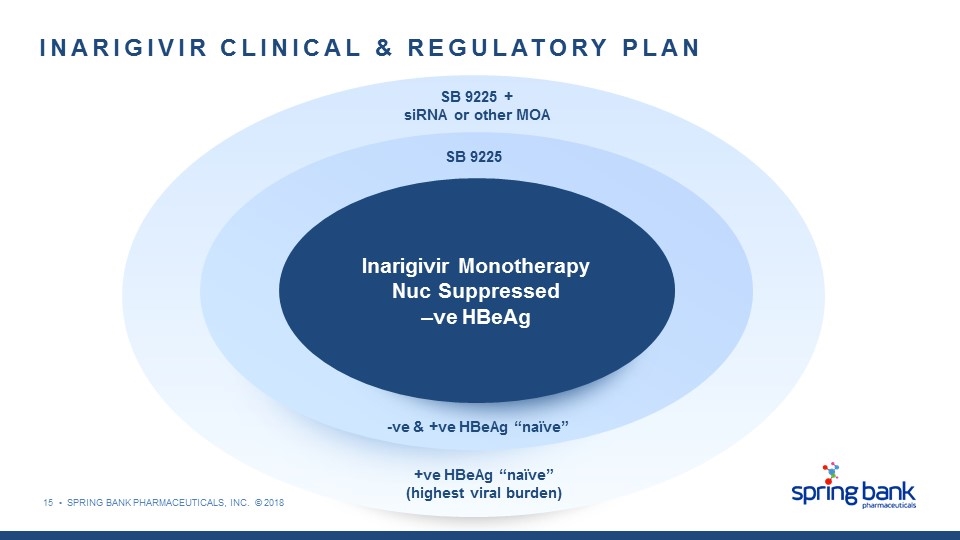
SB 9225 + siRNA or other MOA +ve HBeAg “naïve” (highest viral burden) SB 9225 -ve & +ve HBeAg “naïve” INARIGIVIR CLINICAL & REGULATORY PLAN Inarigivir Monotherapy Nuc Suppressed –ve HBeAg 15 • SPRING BANK PHARMACEUTICALS, INC. © 2018

16 • SPRING BANK PHARMACEUTICALS, INC. © 2018 SB 11285 A NOVEL SYNTHETIC STING AGONIST LEAD COMPOUND A NOVEL SYNTHETIC
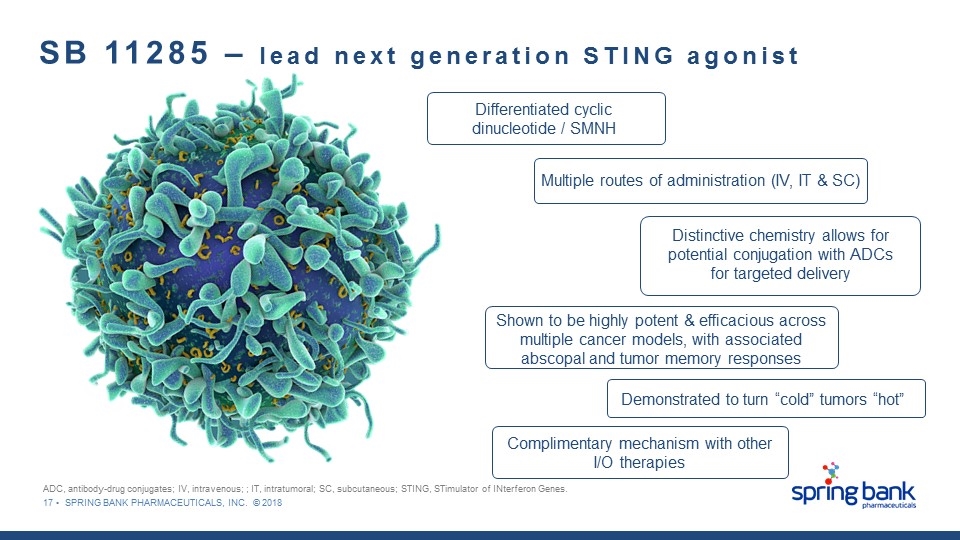
17 • SPRING BANK PHARMACEUTICALS, INC. © 2018 SB 11285 – lead next generation STING agonist Shown to be highly potent & efficacious across multiple cancer models, with associated abscopal and tumor memory responses Multiple routes of administration (IV, IT & SC) Differentiated cyclic dinucleotide / SMNH Distinctive chemistry allows for potential conjugation with ADCs for targeted delivery Demonstrated to turn “cold” tumors “hot” ADC, antibody-drug conjugates; IV, intravenous; ; IT, intratumoral; SC, subcutaneous; STING, STimulator of INterferon Genes. Complimentary mechanism with other I/O therapies
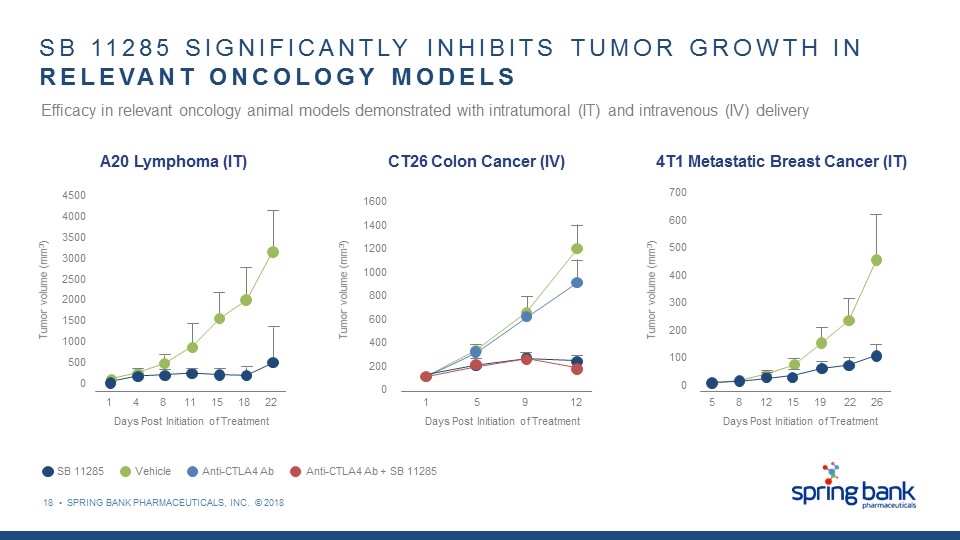
18 • SPRING BANK PHARMACEUTICALS, INC. © 2018 SB 11285 SIGNIFICANTLY INHIBITS TUMOR GROWTH IN RELEVANT ONCOLOGY MODELS 4500 4000 3500 3000 2500 2000 1500 1000 500 0 1 4 8 11 15 18 22 Tumor volume (mm3) Days Post Initiation of Treatment A20 Lymphoma (IT) 1600 1400 1200 1000 800 600 400 200 0 1 5 9 12 Days Post Initiation of Treatment Tumor volume (mm3) CT26 Colon Cancer (IV) Efficacy in relevant oncology animal models demonstrated with intratumoral (IT) and intravenous (IV) delivery 700 600 500 400 300 200 100 0 Tumor volume (mm3) 5 8 12 15 19 22 26 Days Post Initiation of Treatment 4T1 Metastatic Breast Cancer (IT) Vehicle SB 11285 Anti-CTLA4 Ab Anti-CTLA4 Ab + SB 11285
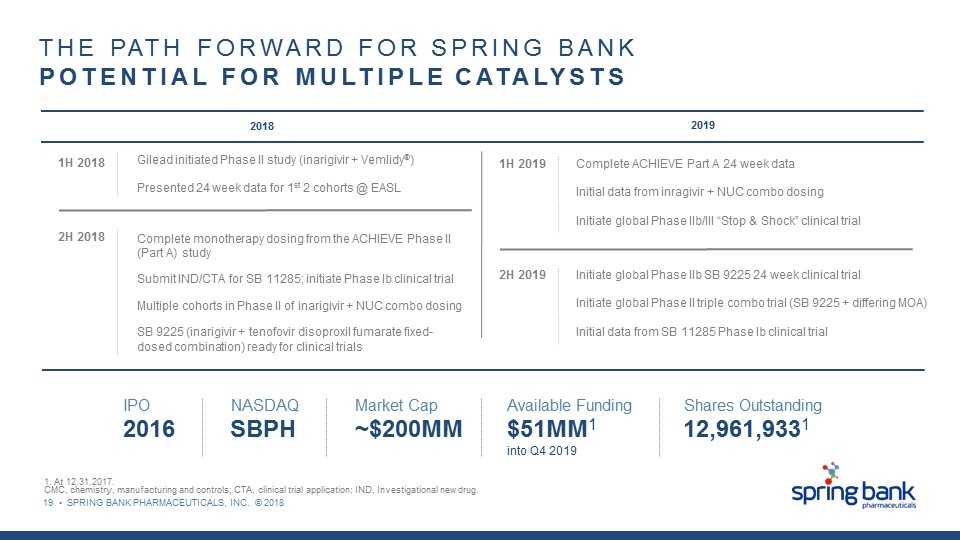
19 • SPRING BANK PHARMACEUTICALS, INC. © 2018 2018 2019 THE PATH FORWARD FOR SPRING BANK POTENTIAL FOR MULTIPLE CATALYSTS 1. At 12.31.2017. CMC, chemistry, manufacturing and controls; CTA, clinical trial application; IND, Investigational new drug. 1H 2018 Gilead initiated Phase II study (inarigivir + Vemlidy®) Presented 24 week data for 1st 2 cohorts @ EASL 2H 2018 Complete monotherapy dosing from the ACHIEVE Phase II (Part A) study Submit IND/CTA for SB 11285; initiate Phase Ib clinical trial Multiple cohorts in Phase II of inarigivir + NUC combo dosing SB 9225 (inarigivir + tenofovir disoproxil fumarate fixed-dosed combination) ready for clinical trials IPO 2016 NASDAQ SBPH Market Cap ~$200MM Available Funding $51MM1 into Q4 2019 Shares Outstanding 12,961,9331 1H 2019 2H 2019 Complete ACHIEVE Part A 24 week data Initial data from inragivir + NUC combo dosing Initiate global Phase IIb/III “Stop & Shock” clinical trial Initiate global Phase IIb SB 9225 24 week clinical trial Initiate global Phase II triple combo trial (SB 9225 + differing MOA) Initial data from SB 11285 Phase Ib clinical trial
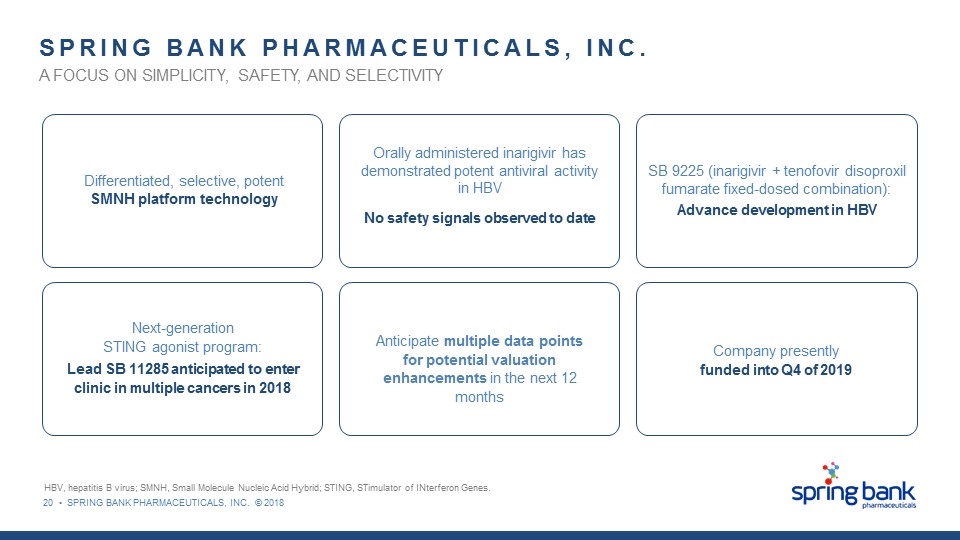
20 • SPRING BANK PHARMACEUTICALS, INC. © 2018 SPRING BANK PHARMACEUTICALS, INC. A FOCUS ON SIMPLICITY, SAFETY, AND SELECTIVITY SB 9225 (inarigivir + tenofovir disoproxil fumarate fixed-dosed combination): Advance development in HBV Next-generation STING agonist program: Lead SB 11285 anticipated to enter clinic in multiple cancers in 2018 Anticipate multiple data points for potential valuation enhancements in the next 12 months Company presently funded into Q4 of 2019 HBV, hepatitis B virus; SMNH, Small Molecule Nucleic Acid Hybrid; STING, STimulator of INterferon Genes. Differentiated, selective, potent SMNH platform technology Orally administered inarigivir has demonstrated potent antiviral activity in HBV No safety signals observed to date
