Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - Blueprint Medicines Corp | ex-99d3.htm |
| EX-99.1 - EX-99.1 - Blueprint Medicines Corp | ex-99d1.htm |
| 8-K - 8-K - Blueprint Medicines Corp | f8-k.htm |
Exhibit 99.2
|
|
1 Highly potent and selective RET inhibitor, BLU-667, achieves proof of concept in ARROW, a phase 1 study of advanced, RET-altered solid tumors Vivek Subbiah1, Matthew Taylor2, Jessica Lin3, Mimi Hu1, Sai-Hong Ignatius Ou4, Marcia S. Brose5, Elena Garralda6, Corinne Clifford7, Michael Palmer7, Meera Tugnait,7 Erica Evans7, Hongliang Shi7, Beni Wolf7, and Justin Gainor3 1Department of Investigational Cancer Therapeutics, The University of Texas MD Anderson Cancer Center, Houston, United States; 2The Knight Cancer Institute, Oregon Health & Science University, Portland, United States; 3Department of Medicine, Massachusetts General Hospital, Boston, United States, 4Chao Family Comprehensive Cancer Center, University of California Irvine Medical Center, United States; 5Abramson Cancer Center, University Of Pennsylvania, Philadelphia, United States; 6Vall d’Hebron Institute of Oncology, Vall d’Hebron University Hospital, Barcelona, Spain; 7Blueprint Medicines Corporation, Cambridge, United States; NCT03037385 ©2018 Blueprint Medicines Corporation |
|
|
2 Disclosures BLU-667 is an investigational agent discovered and currently in development by Blueprint Medicines Corporation (Blueprint Medicines) I have the following financial relationships to disclose: Grant/Research support from: Blueprint Medicines Corporation Novartis International AG Bayer AG GlaxoSmithKline plc NanoCarrier Co. Ltd Vegenics Pty Ltd Northwest Biotherapeutics Boston Biomedical Inc Berg Incyte Corporation Fujifilm Holdings Corporation PharmaMar D3 Pfizer Inc MultiVir Inc Amgen Inc AbbVie Inc Loxo Oncology F. Hoffmann-La Roche AG / Genentech Inc National Comprehensive Cancer Network National Cancer Institute-Cancer Therapy Evaluation Program |
|
|
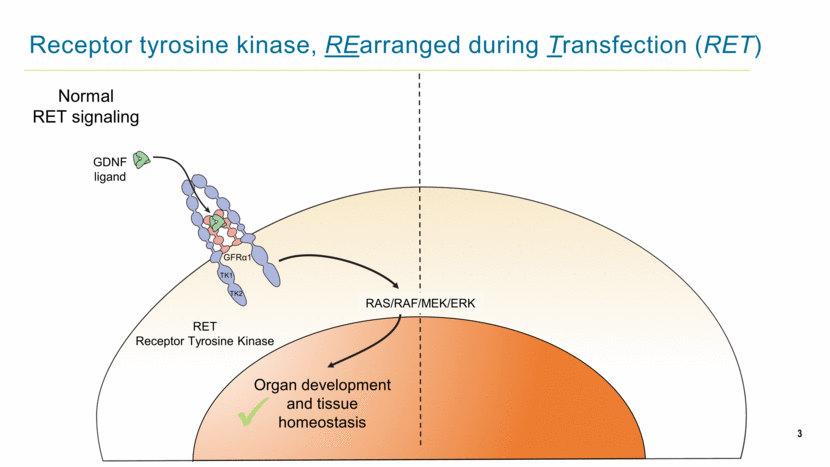
Receptor tyrosine kinase, REarranged during Transfection (RET) Organ development and tissue homeostasis RET Receptor Tyrosine Kinase GDNF ligand GFRα1 TK1 TK2 RAS/RAF/MEK/ERK Normal RET signaling 3 |
|
|
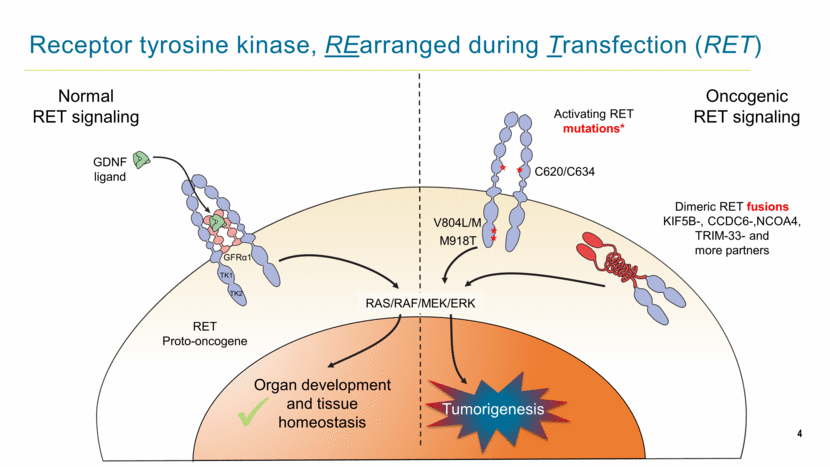
Receptor tyrosine kinase, REarranged during Transfection (RET) Activating RET mutations* C620/C634 Dimeric RET fusions KIF5B-, CCDC6-,NCOA4, TRIM-33- and more partners V804L/M M918T Organ development and tissue homeostasis RET Proto-oncogene GDNF ligand GFRα1 TK1 TK2 Oncogenic RET signaling Normal RET signaling RAS/RAF/MEK/ERK Tumorigenesis 4 |
|
|
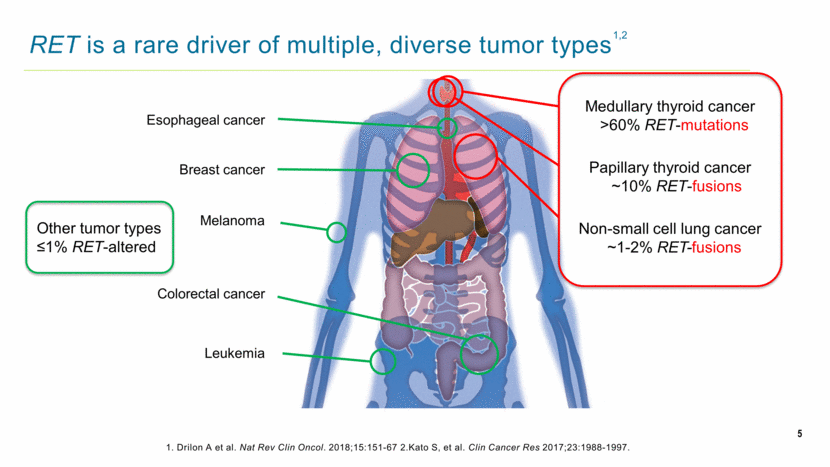
5 RET is a rare driver of multiple, diverse tumor types 1. Drilon A et al. Nat Rev Clin Oncol. 2018;15:151-67 2.Kato S, et al. Clin Cancer Res 2017;23:1988-1997. Colorectal cancer Non-small cell lung cancer ~1-2% RET-fusions Medullary thyroid cancer >60% RET-mutations Papillary thyroid cancer ~10% RET-fusions Esophageal cancer Breast cancer Melanoma Leukemia Other tumor types ≤1% RET-altered 1,2 |
|
|
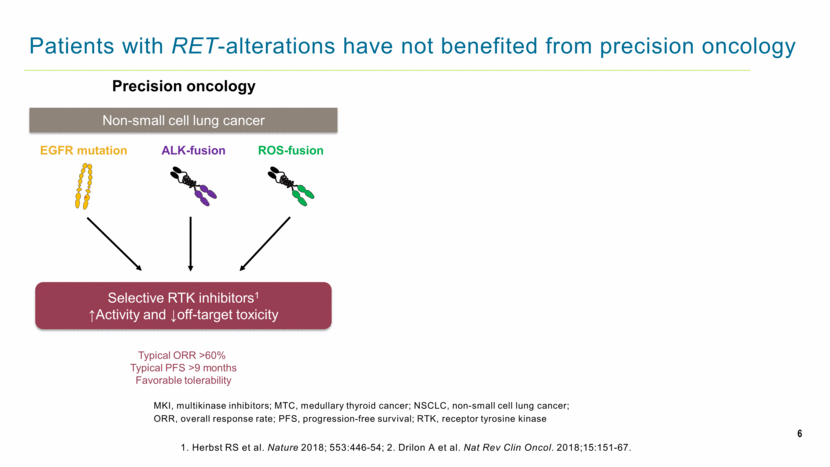
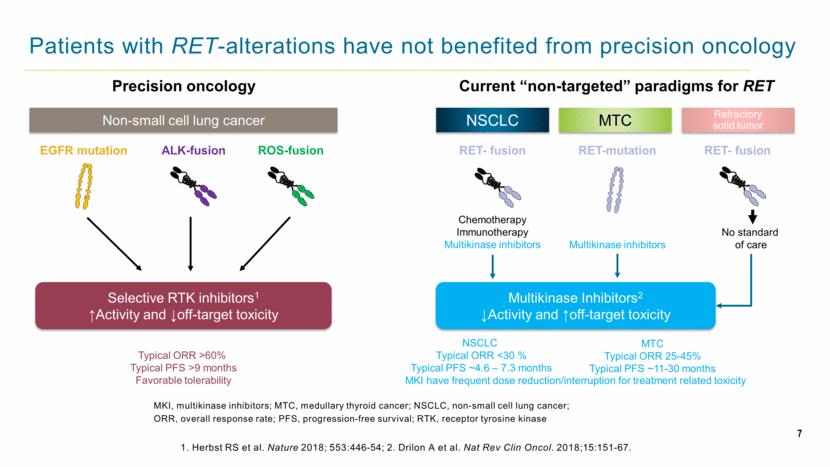
6 Patients with RET-alterations have not benefited from precision oncology MKI, multikinase inhibitors; MTC, medullary thyroid cancer; NSCLC, non-small cell lung cancer; ORR, overall response rate; PFS, progression-free survival; RTK, receptor tyrosine kinase Precision oncology Non-small cell lung cancer Typical ORR >60% Typical PFS >9 months Favorable tolerability EGFR mutation ALK-fusion ROS-fusion Selective RTK inhibitors1 ↑Activity and ↓off-target toxicity 1. Herbst RS et al. Nature 2018; 553:446-54; 2. Drilon A et al. Nat Rev Clin Oncol. 2018;15:151-67. |
|
|
7 Patients with RET-alterations have not benefited from precision oncology MKI, multikinase inhibitors; MTC, medullary thyroid cancer; NSCLC, non-small cell lung cancer; ORR, overall response rate; PFS, progression-free survival; RTK, receptor tyrosine kinase Precision oncology Non-small cell lung cancer Typical ORR >60% Typical PFS >9 months Favorable tolerability EGFR mutation ALK-fusion ROS-fusion Selective RTK inhibitors1 ↑Activity and ↓off-target toxicity Current “non-targeted” paradigms for RET NSCLC MTC Refractory solid tumor RET-mutation RET- fusion RET- fusion Chemotherapy Immunotherapy Multikinase inhibitors No standard of care MTC Typical ORR 25-45% Typical PFS ~11-30 months NSCLC Typical ORR <30 % Typical PFS ~4.6 – 7.3 months Multikinase Inhibitors2 ↓Activity and ↑off-target toxicity Multikinase inhibitors MKI have frequent dose reduction/interruption for treatment related toxicity 1. Herbst RS et al. Nature 2018; 553:446-54; 2. Drilon A et al. Nat Rev Clin Oncol. 2018;15:151-67. |
|
|
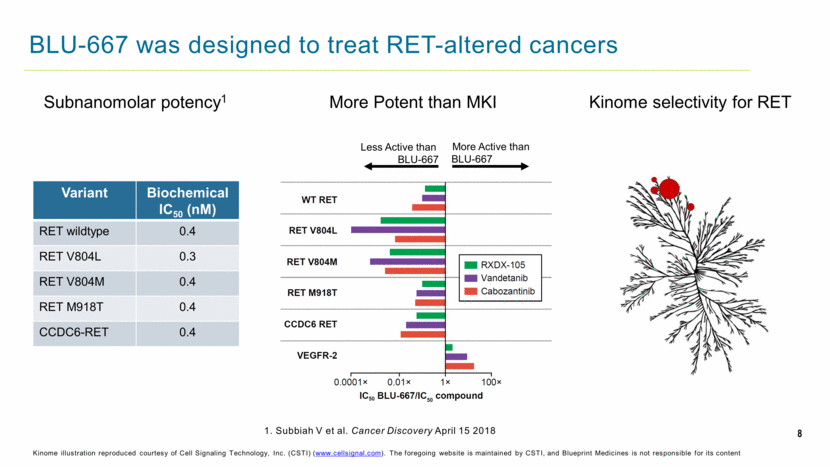
8 BLU-667 was designed to treat RET-altered cancers Kinome illustration reproduced courtesy of Cell Signaling Technology, Inc. (CSTI) (www.cellsignal.com). The foregoing website is maintained by CSTI, and Blueprint Medicines is not responsible for its content Kinome selectivity for RET Variant Biochemical IC50 (nM) RET wildtype 0.4 RET V804L 0.3 RET V804M 0.4 RET M918T 0.4 CCDC6-RET 0.4 Subnanomolar potency1 Less Active than BLU-667 More Active than BLU-667 More Potent than MKI 1. Subbiah V et al. Cancer Discovery April 15 2018 |
|
|
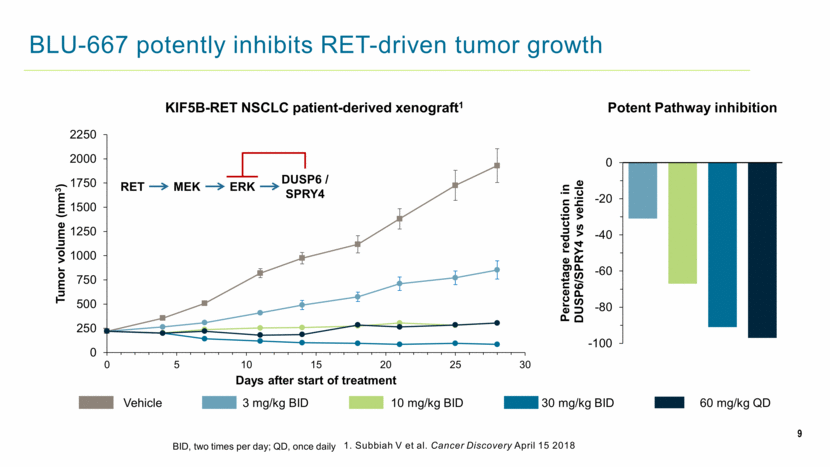
9 BLU-667 potently inhibits RET-driven tumor growth Potent Pathway inhibition KIF5B-RET NSCLC patient-derived xenograft1 Vehicle 3 mg/kg BID 10 mg/kg BID 30 mg/kg BID 60 mg/kg QD RET ERK DUSP6 / SPRY4 MEK BID, two times per day; QD, once daily 1. Subbiah V et al. Cancer Discovery April 15 2018 0 250 500 750 1000 1250 1500 1750 2000 2250 0 5 10 15 20 25 30 Tumor volume (mm 3 ) Days after start of treatment -100 -80 -60 -40 -20 0 Percentage reduction in DUSP6/SPRY4 vs vehicle |
|
|
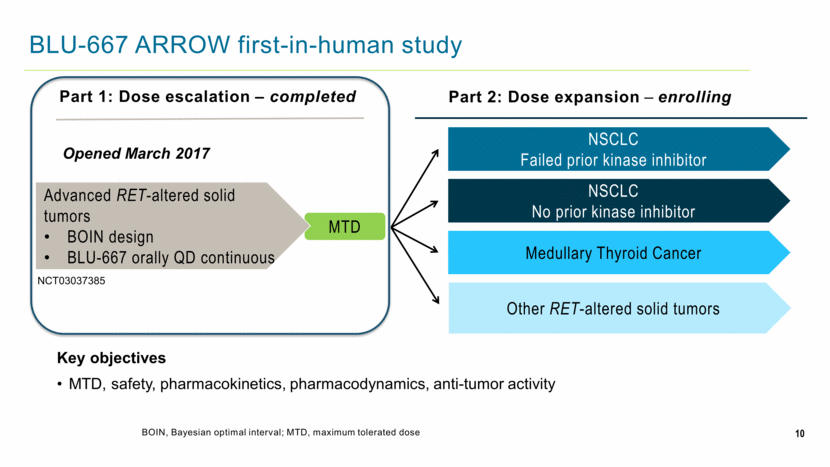
MTD Tablets will go to US first 10 BLU-667 ARROW first-in-human study BOIN, Bayesian optimal interval; MTD, maximum tolerated dose Part 1: Dose escalation – completed Part 2: Dose expansion – enrolling NSCLC No prior kinase inhibitor NSCLC Failed prior kinase inhibitor Medullary Thyroid Cancer Key objectives MTD, safety, pharmacokinetics, pharmacodynamics, anti-tumor activity Advanced RET-altered solid tumors BOIN design BLU-667 orally QD continuous Opened March 2017 Other RET-altered solid tumors NCT03037385 |
|
|
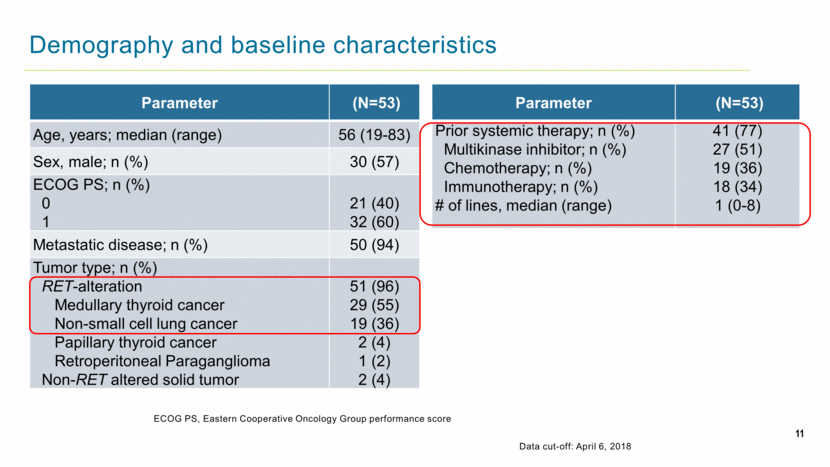
11 Demography and baseline characteristics ECOG PS, Eastern Cooperative Oncology Group performance score Data cut-off: April 6, 2018 Parameter (N=53) Age, years; median (range) 56 (19-83) Sex, male; n (%) 30 (57) ECOG PS; n (%) 0 1 21 (40) 32 (60) Metastatic disease; n (%) 50 (94) Tumor type; n (%) RET-alteration Medullary thyroid cancer Non-small cell lung cancer Papillary thyroid cancer Retroperitoneal Paraganglioma Non-RET altered solid tumor 51 (96) 29 (55) 19 (36) 2 (4) 1 (2) 2 (4) Parameter (N=53) Prior systemic therapy; n (%) Multikinase inhibitor; n (%) Chemotherapy; n (%) Immunotherapy; n (%) # of lines, median (range) 41 (77) 27 (51) 19 (36) 18 (34) 1 (0-8) |
|
|
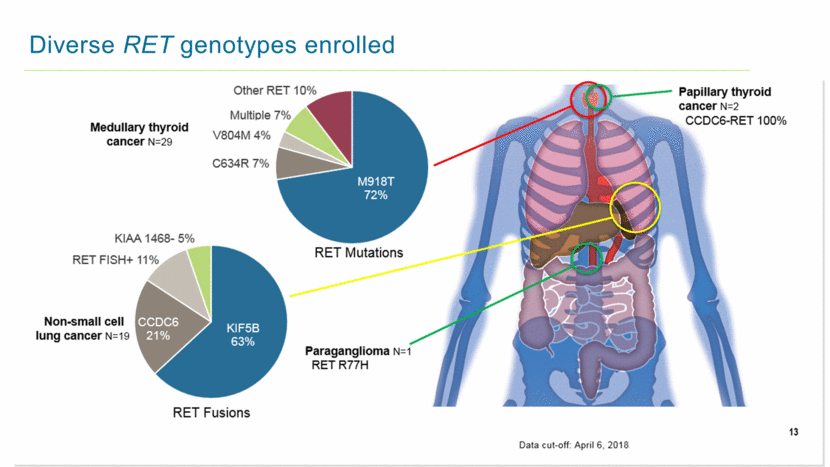
Diverse RET genotypes enrolled Medullar thyroid cancer N=29 Other RET 10% Multiple 7% V804M 4% C634R 7% M918T72% RET Mutations KIAA 1468-5% RET FISH+ 11% Non-small cell lung cancer N=19 RET Fusions CCDC6 21% KIF5B 63% Paraganglioma N=1 RET R77H Papillary thyroid cancer N=2 CCDC6-RET 100% Data cut-off April 6, 2018 13 |
|
|
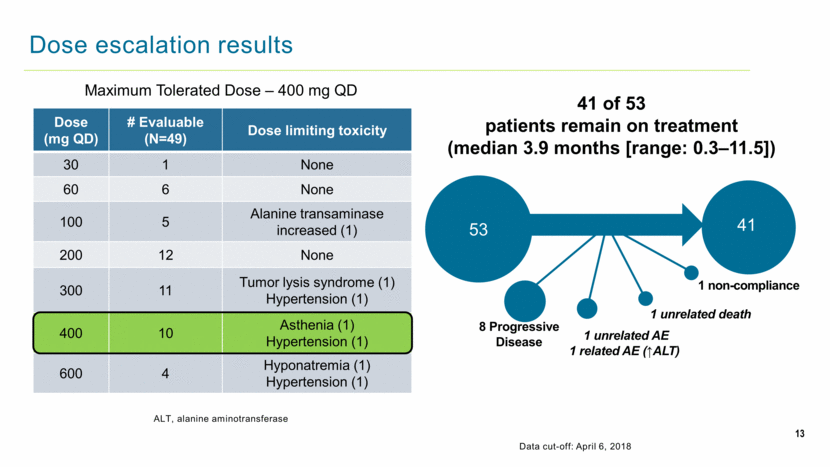
13 Dose escalation results ALT, alanine aminotransferase Data cut-off: April 6, 2018 Dose (mg QD) # Evaluable (N=49) Dose limiting toxicity 30 1 None 60 6 None 100 5 Alanine transaminase increased (1) 200 12 None 300 11 Tumor lysis syndrome (1) Hypertension (1) 400 10 Asthenia (1) Hypertension (1) 600 4 Hyponatremia (1) Hypertension (1) 1 unrelated death 8 Progressive Disease 1 non-compliance 1 unrelated AE 1 related AE (↑ALT) 53 41 Maximum Tolerated Dose – 400 mg QD 41 of 53 patients remain on treatment (median 3.9 months [range: 0.3–11.5]) |
|
|
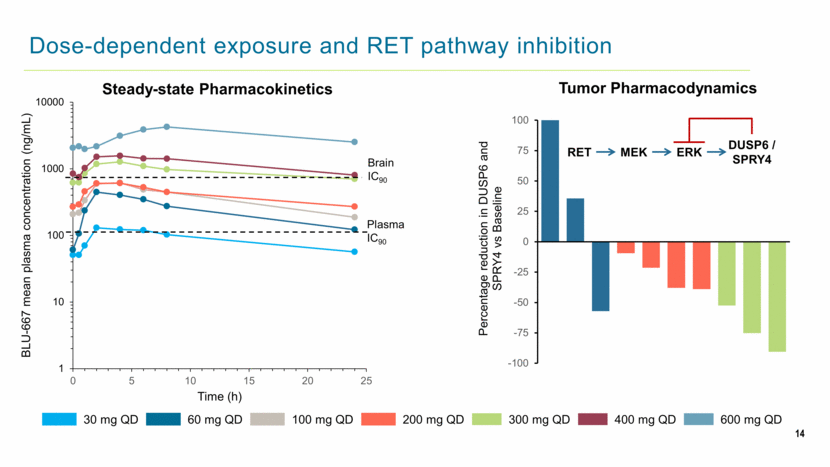
14 Dose-dependent exposure and RET pathway inhibition Tumor Pharmacodynamics Steady-state Pharmacokinetics Plasma IC90 Brain IC90 30 mg QD 60 mg QD 100 mg QD 200 mg QD 300 mg QD 400 mg QD 600 mg QD RET ERK DUSP6 / SPRY4 MEK 1 10 100 1000 10000 0 5 10 15 20 25 BLU - 667 mean plasma concentration (ng/mL) Time (h) |
|
|
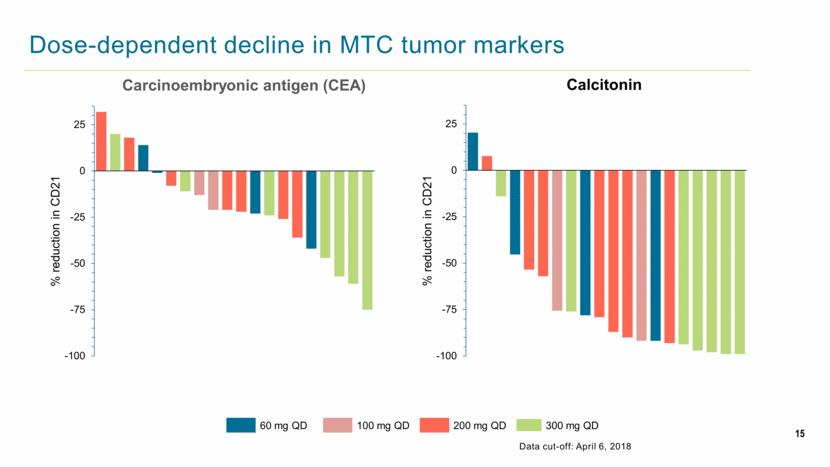
15 Dose-dependent decline in MTC tumor markers 60 mg QD 100 mg QD 200 mg QD 300 mg QD Data cut-off: April 6, 2018 -100 -75 -50 -25 0 25 200 300 200 60 60 200 300 100 100 200 200 60 300 200 200 60 300 300 300 300 % reduction in CD21 Carcinoembryonic antigen (CEA) -100 -75 -50 -25 0 25 60 200 300 60 200 200 100 300 60 200 200 200 100 60 200 300 300 300 300 300 % reduction in CD21 Calcitonin |
|
|
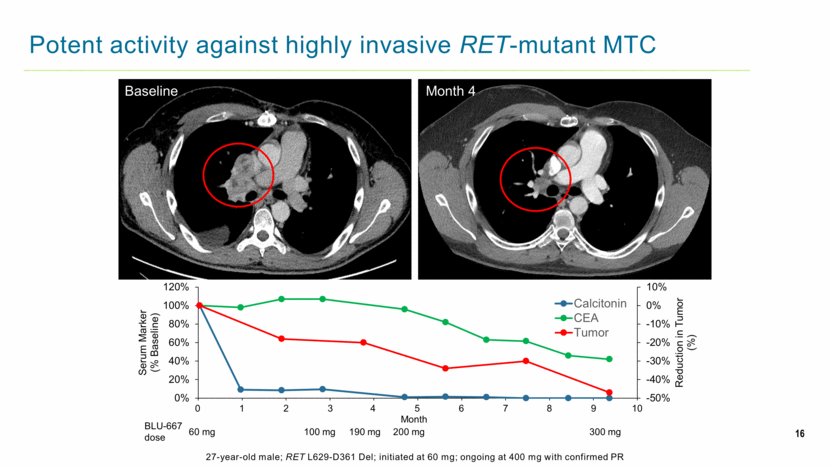
16 Potent activity against highly invasive RET-mutant MTC 27-year-old male; RET L629-D361 Del; initiated at 60 mg; ongoing at 400 mg with confirmed PR Baseline Month 4 60 mg 100 mg 190 mg 200 mg 300 mg BLU-667 dose 0 1 2 3 4 5 6 7 8 9 10 Month -50% -40% -30% -20% -10% 0% 10% 0% 20% 40% 60% 80% 100% 120% 0 30 60 90 120 150 180 210 240 270 300 Reduction in Tumor (%) Serum Marker (% Baseline) Calcitonin CEA Tumor |
|
|
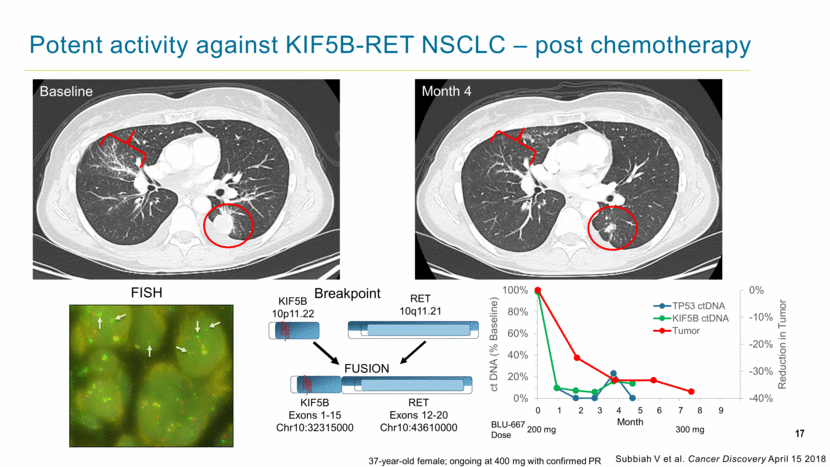
Potent activity against KIF5B-RET NSCLC – post chemotherapy 17 FISH Breakpoint FUSION KIF5B 10p11.22 RET 10q11.21 KIF5B Exons 1-15 Chr10:32315000 RET Exons 12-20 Chr10:43610000 Baseline Month 4 37-year-old female; ongoing at 400 mg with confirmed PR 300 mg BLU-667 Dose 200 mg 0 1 2 3 4 5 6 7 8 9 Month Subbiah V et al. Cancer Discovery April 15 2018 -40% -30% -20% -10% 0% 0% 20% 40% 60% 80% 100% 0 30 60 90 120 150 180 210 240 270 300 Reduction in Tumor ct DNA (% Baseline) TP53 ctDNA KIF5B ctDNA Tumor |
|
|
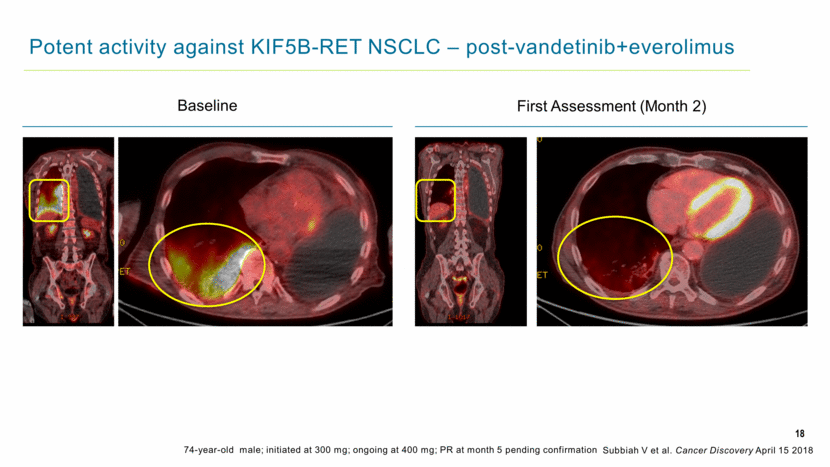
18 Potent activity against KIF5B-RET NSCLC – post-vandetinib+everolimus 74-year-old male; initiated at 300 mg; ongoing at 400 mg; PR at month 5 pending confirmation Baseline First Assessment (Month 2) Subbiah V et al. Cancer Discovery April 15 2018 |
|
|
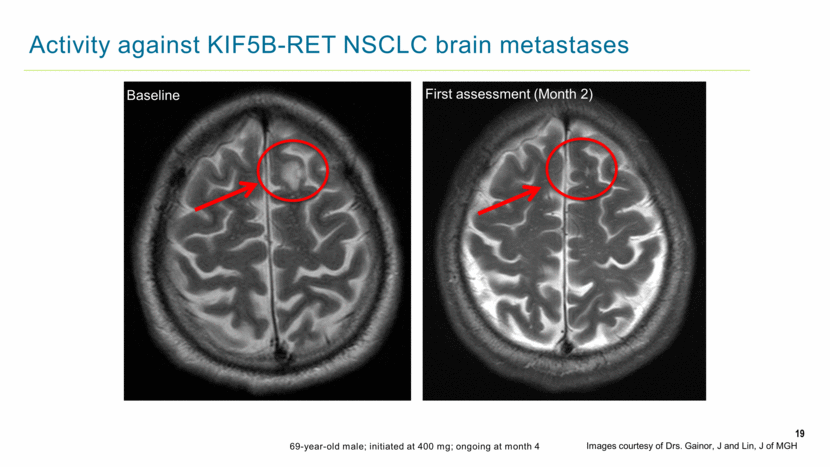
19 Activity against KIF5B-RET NSCLC brain metastases 69-year-old male; initiated at 400 mg; ongoing at month 4 Baseline First assessment (Month 2) Images courtesy of Drs. Gainor, J and Lin, J of MGH |
|
|
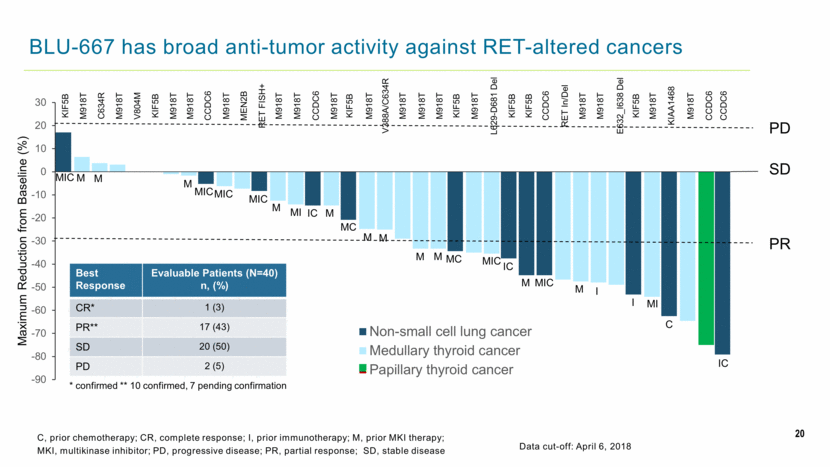
20 BLU-667 has broad anti-tumor activity against RET-altered cancers Data cut-off: April 6, 2018 C, prior chemotherapy; CR, complete response; I, prior immunotherapy; M, prior MKI therapy; MKI, multikinase inhibitor; PD, progressive disease; PR, partial response; SD, stable disease PD SD PR Best Response Evaluable Patients (N=40) n, (%) CR* 1 (3) PR** 17 (43) SD 20 (50) PD 2 (5) KIF5B MIC M M MIC M MIC MIC MI M M IC MC M M M M M IC MIC I M I MI IC C KIF5B M918T M918T C634R V804M KIF5B M918T M918T CCDC6 M918T MEN2B RET FISH+ M918T M918T CCDC6 M918T M918T V388A/C634R M918T M918T L629-D681 Del KIF5B CCDC6 RET In/Del M918T E632_I638 Del KIF5B M918T M918T CCDC6 CCDC6 KIAA1468 M918T M918T KIF5B KIF5B M918T MIC MC * confirmed ** 10 confirmed, 7 pending confirmation -90 -80 -70 -60 -50 -40 -30 -20 -10 0 10 20 30 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 Maximum Reduction from Baseline (%) Non-small cell lung cancer Medullary thyroid cancer Papillary thyroid cancer |
|
|
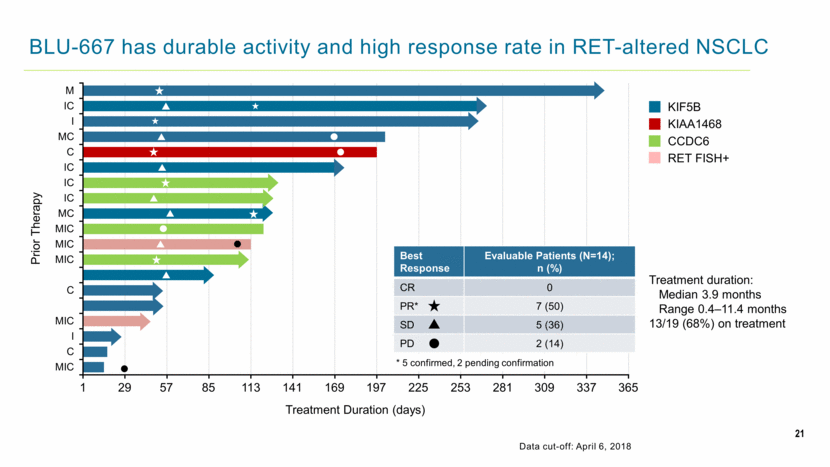
21 BLU-667 has durable activity and high response rate in RET-altered NSCLC Treatment Duration (days) KIF5B KIAA1468 CCDC6 RET FISH+ Prior Therapy 1 29 57 85 113 141 169 197 225 253 281 309 337 365 Treatment duration: Median 3.9 months Range 0.4–11.4 months 13/19 (68%) on treatment Best Response Evaluable Patients (N=14); n (%) CR 0 PR* 7 (50) SD 5 (36) PD 2 (14) * 5 confirmed, 2 pending confirmation Data cut-off: April 6, 2018 MIC C I MIC C MIC MIC MIC MC IC IC IC C MC I IC M |
|
|
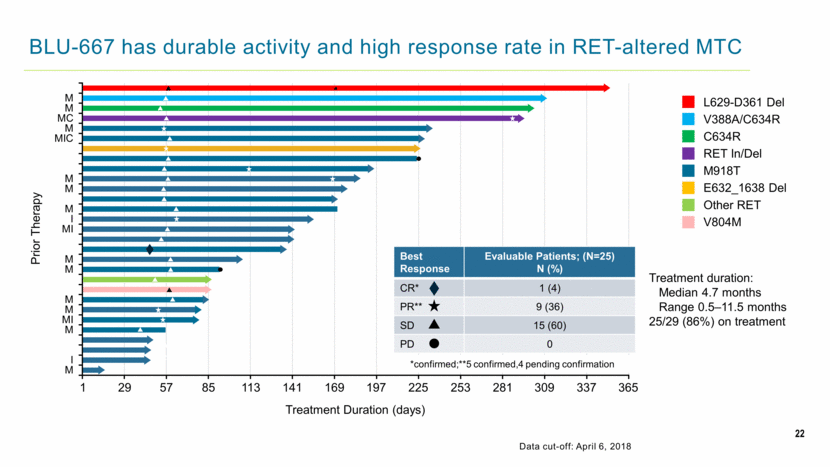
22 BLU-667 has durable activity and high response rate in RET-altered MTC L629-D361 Del V388A/C634R C634R RET In/Del M918T E632_1638 Del Other RET V804M Prior Therapy Treatment duration: Median 4.7 months Range 0.5–11.5 months 25/29 (86%) on treatment Treatment Duration (days) 1 29 57 85 113 141 169 197 225 253 281 309 337 365 Best Response Evaluable Patients; (N=25) N (%) CR* 1 (4) PR** 9 (36) SD 15 (60) PD 0 *confirmed;**5 confirmed,4 pending confirmation Data cut-off: April 6, 2018 |
|
|
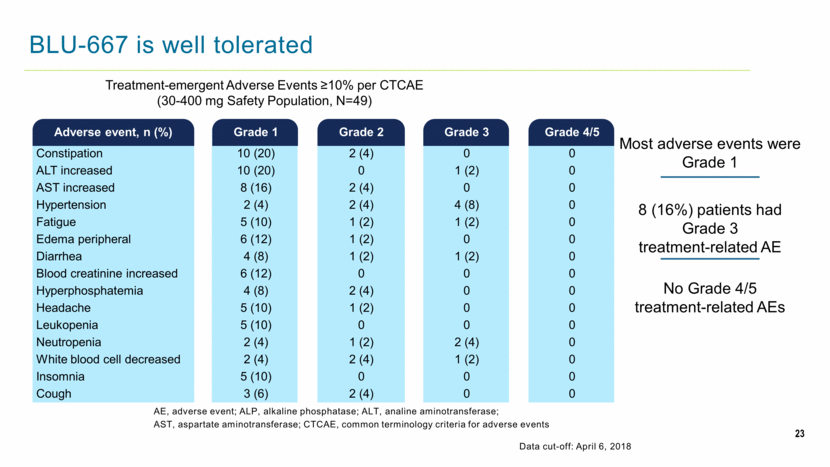
23 BLU-667 is well tolerated AE, adverse event; ALP, alkaline phosphatase; ALT, analine aminotransferase; AST, aspartate aminotransferase; CTCAE, common terminology criteria for adverse events Data cut-off: April 6, 2018 Treatment-emergent Adverse Events ≥10% per CTCAE (30-400 mg Safety Population, N=49) Most adverse events were Grade 1 No Grade 4/5 treatment-related AEs 8 (16%) patients had Grade 3 treatment-related AE Adverse event, n (%) Grade 1 Grade 2 Grade 3 Grade 4/5 Constipation 10 (20) 2 (4) 0 0 ALT increased 10 (20) 0 1 (2) 0 AST increased 8 (16) 2 (4) 0 0 Hypertension 2 (4) 2 (4) 4 (8) 0 Fatigue 5 (10) 1 (2) 1 (2) 0 Edema peripheral 6 (12) 1 (2) 0 0 Diarrhea 4 (8) 1 (2) 1 (2) 0 Blood creatinine increased 6 (12) 0 0 0 Hyperphosphatemia 4 (8) 2 (4) 0 0 Headache 5 (10) 1 (2) 0 0 Leukopenia 5 (10) 0 0 0 Neutropenia 2 (4) 1 (2) 2 (4) 0 White blood cell decreased 2 (4) 2 (4) 1 (2) 0 Insomnia 5 (10) 0 0 0 Cough 3 (6) 2 (4) 0 0 |
|
|
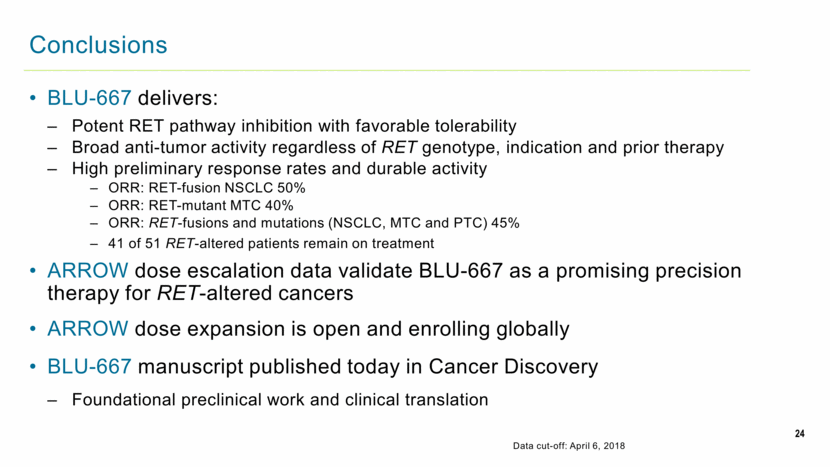
BLU-667 delivers: Potent RET pathway inhibition with favorable tolerability Broad anti-tumor activity regardless of RET genotype, indication and prior therapy High preliminary response rates and durable activity ORR: RET-fusion NSCLC 50% ORR: RET-mutant MTC 40% ORR: RET-fusions and mutations (NSCLC, MTC and PTC) 45% 41 of 51 RET-altered patients remain on treatment ARROW dose escalation data validate BLU-667 as a promising precision therapy for RET-altered cancers ARROW dose expansion is open and enrolling globally BLU-667 manuscript published today in Cancer Discovery Foundational preclinical work and clinical translation 24 Conclusions Data cut-off: April 6, 2018 |
|
|
We thank the participating patients, their families, all study co-investigators, and research co-ordinators at the following institutions: Department of Investigational Cancer Therapeutics, The University of Texas MD Anderson Cancer Center, Houston, United States The Knight Cancer Institute Oregon Health & Science University Portland, United States Department of Medicine, Massachusetts General Hospital Cancer Center, Boston, United States Chao Family Comprehensive Cancer Center University of California Irvine Medical Center, United States Abramson Cancer Center, University Of Pennsylvania, United States Vall d’Hebron Institute of Oncology Vall d’Hebron University Hospital, Barcelona, Spain 25 Acknowledgements |