Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - SELECTA BIOSCIENCES INC | exhibit993panlarposterap.htm |
| EX-99.1 - EXHIBIT 99.1 - SELECTA BIOSCIENCES INC | exhibit991_pressrelease.htm |
| 8-K - 8-K - SELECTA BIOSCIENCES INC | selectabiosciences8k_prese.htm |

New SEL-212 Phase 2 Data
Presented at PANLAR
April 10, 2018

Safe Harbor / Disclaimer
2
Any statements in this presentation about the future expectations, plans and prospects of Selecta Biosciences, Inc. (“the company”), including without limitation,
statements regarding the progress of the Phase 1/2 clinical program of SEL-212, the potential of SEL-212 to treat severe gout patients and resolve their debilitating
symptoms, the ability of SVP-Rapamycin to induce immune tolerance against pegsiticase or otherwise mitigate immunogenicity, the ability of SEL-212 to improve
acute symptoms during a short induction cycle, whether the company participates in an End-of-Phase 2 meeting for SEL-212 in mid-2018 or at all, the ability of SEL-
212 to mitigate anti-drug antibodies, enable repeat dosing, achieve better and more sustained serum uric acid control and reduce gout flares, the ability of SEL-212 to
be re-administered if severe gout symptoms recur, whether results from patients receiving five monthly combination doses of SEL-212 will expand the three-month
SEL-212 clinical activity data across the entire five-month treatment period of the Phase 2 trial, when the company will report further data from the Phase 2 trial,
whether the FDA approves the company’s plan to provide combination therapy of SEL-212 for the entire treatment period, whether the data from patients receiving five
monthly combination doses of SEL-212 will support the company’s plans for its Phase 3 trial, whether the patient population for a Phase 3 for SEL-212 has a rapid
enrollment potential, when the company will advance to a Phase 3 for SEL-212 (if at all), whether SEL-212 has the potential to address the unmet needs of gout
patients, whether SEL-212 holds billion dollar potential, the ability of the company’s SVP platform, including SVP-Rapamycin, to mitigate immune response and induce
immune tolerance, the potential of the SVP-Rapamycin platform generally, and other statements containing the words “anticipate,” “believe,” “continue,” “could,”
“estimate,” “expect,” “hypothesize,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” and similar expressions, constitute forward-looking
statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking
statements as a result of various important factors, including, but not limited to, the following: the uncertainties inherent in the initiation, completion and cost of clinical
trials including their uncertain outcomes, the availability and timing of data from ongoing and future clinical trials and the results of such trials, whether preliminary
results from a particular clinical trial will be predictive of the final results of that trial or whether results of early clinical trials will be indicative of the results of later
clinical trials, the unproven approach of the company’s SVP technology, potential delays in enrollment of patients, undesirab le side effects of the company’s product
candidates, its reliance on third parties to manufacture its product candidates and to conduct its clinical trials, the company’s inability to maintain its existing or future
collaborations or licenses, its inability to protect its proprietary technology and intellectual property, potential delays in regulatory approvals, the availability of funding
sufficient for its foreseeable and unforeseeable operating expenses and capital expenditure requirements, substantial fluctuation in the price of its common stock, a
significant portion of the company’s total outstanding shares have recently become eligible to be sold into the market, and o ther important factors discussed in the
“Risk Factors” section of the company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission, or SEC, on March 15, 2018, and in other
filings that the company makes with the SEC. In addition, any forward-looking statements included in this presentation represent the company’s views only as of the
date of its publication and should not be relied upon as representing its views as of any subsequent date. The company specifically disclaims any obligation to update
any forward-looking statements included in this presentation.

SEL-212 Phase 2 Overview and Summary of
New Data Presented at PANLAR
3
Phase 2 Trial Overview for new data presented at PANLAR
• Enrollment Criteria: Patients with symptomatic gout and serum uric acid (sUA) >6 mg/dl
• Primary/Secondary Endpoints:
• Safety, tolerability and pharmacokinetics of multiple doses of SEL-212 (SVP-Rapamycin + pegsiticase) and pegsiticase
alone
• Control of sUA levels
• Reduction in anti-drug antibody (ADA) levels
• Dosing (Cohort 10,11,12): SEL-212 every 28 days for three doses (months 0, 1 and 2) followed by two doses of pegsiticase
alone (months 3 and 4)
Summary of new data presented at PANLAR
• 3-month data show SEL-212 product profile provides:
• Mitigation of ADAs enabling repeat dosing and sustained serum uric acid control: ~74% of patients with sUA <6
mg/dl
• Low flare rate in the first month : 37% for new SEL-212 Cohorts; 26% for all SEL-212 Cohorts in the trial
• Less frequent dosing: Monthly compared to weekly/bi-weekly dosing for FDA-approved uricase
• 4-month data, of evaluable patients dosed at month 3 with pegsiticase alone, provide evidence for the ability of the
SVP platform to induce immune tolerance in a clinical setting with a highly immunogenic enzyme

SEL-212 Clinical Development Plan
Primary Clinical Endpoint:
Serum uric acid < 6 mg/dl
measured at month 3 and 6
DETERMINATION OF DOSE REGIMEN TO TAKE INTO PHASE 3 PHASE 3 PROGRAM WITH DEFINED DOSE REGIMEN
Phase 2 Dose Ranging
Five monthly injections
Matrix approach to find
best doses of the two
components:
• SVP-Rapamycin
• Pegsiticase
(Planned N ~ 140)
Phase 1 a
Dose Finding
Pegsiticase
(N= 22)
6 Monthly Combination Injections of SEL-212
Phase 1 b
Dose Finding
SVP-Rapamycin
+/- pegsiticase
(N= 63)
Current Stage of SEL-
212 Development
4

5
74%; (14/19)
0.2 or 0.4 mg/kg Pegsiticase
Patients evaluable at 12 weeks who received a full
first dose and completed treatment cycle 1
0.2 or 0.4 mg/kg
0.125 or 0.15 mg/kg
SEL-212
Pegsiticase
SVP-Rapamycin
0.2 or 0.4 mg/kg
Pegsiticase
17% (1/6)
Week
%
P
ts.
S
U
A
<6
mg
/d
L
Pegsiticase + SVP-Rapamycin
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2
0
5 0
1 0 0
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2
0
5 0
1 0 0
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2
0
5 0
1 0 0
%
P
ts.
S
U
A
<6
mg
/d
L
Week
Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918
New Phase 2 Data at 3 Months Show 74% of Patients
With Control of SUA <6 mg/dl

Patients Dosed With 0.125 mg/kg of SVP-Rapamycin + 0.4 mg/kg of
Pegsiticase
6
Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918
Patient
S
e
ru
m
U
ric
A
cid
(
m
g
/d
L
)
F
E
A
D
D
Week
G
106-0060
106-0061
111-0009
102-0019
107-0012
112-0002
112-0001
107-0014
111-0010
111-0011
117-0005
110-0031
107-0022
107-0024
H
A
n
ti-U
ric
a
s
e
A
n
ti
b
o
d
y
T
it
e
r
Pegsiticase Alone Pegsiticase + SVP-Rapamycin
0.125 mg/kg
0.4 mg/kg
Withdrawn due to protocol deviation Discontinuation due to infusion reaction Withdrawal of consent
Discontinuation due to TEAE SAE; non-study drug related
Stopping rules met A D E F
G H
0
2
4
6
8
1 0
1 0 2
1 0 3
1 0 4
1 0 5
0
2
4
6
8
1 0
1 0 2
3
4
5
0
2
4
6
8
1 0
1 0 2
3
4
5
0
2
4
6
8
1
1 0 2
3
4
5
0
2
4
6
8
1
1 0 2
3
4
5
0
2
4
6
8
1
1 0 2
3
4
5
8
1
0
2
4
6
1 0 2
3
4
5
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
2
4
6
8
1 0
1 0 2
1 0 3
1 0 4
1 0 5

Patients Dosed With 0.15 mg/kg of SVP-Rapamycin + 0.2 mg/kg of
Pegsiticase
7
Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918
Patient
S
e
ru
m
U
ric
A
cid
(
m
g
/d
L
)
Week
A
n
ti-U
ric
a
s
e
A
n
ti
b
o
d
y
T
it
e
r
0.15 mg/kg
0.2 mg/kg
Withdrawn due to protocol deviation Discontinuation due to infusion reaction Withdrawal of consent Stopping rules met A D E F
106-0084
117-0003
106-0086
103-0022
111-0018
103-0025
111-0019
110-0034
104-2026
106-0092
106-0093
102-0024
104-2021
E
A
F
D
Pegsiticase Alone Pegsiticase + SVP-Rapamycin
0
2
4
6
8
1 0
1 0 2
1 0 3
1 0 4
1 0 5
2
3
4
5
0
2
4
6
8
1 0
1 0
2
3
4
5
0
2
4
6
8
1
1 0 2
3
4
5
2
3
4
5
0
2
4
6
8
1
1 0
2
3
4
5
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
2
4
6
8
1 0
1 0 2
1 0 3
1 0 4
1 0 5

Patients Dosed With 0.15 mg/kg of SVP-Rapamycin + 0.4 mg/kg of
Pegsiticase
8
Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918
S
e
ru
m
U
ric
A
cid
(
m
g
/d
L
)
Week
A
n
ti-U
ric
a
s
e
A
n
ti
b
o
d
y
T
it
e
r
0.15 mg/kg
0.4 mg/kg
Stopping rules met A Withdrawal of consent F SAE; non-study drug related G
107-0017
106-0073
111-0012
111-0013
111-0015
110-0026
106-0077
110-0028
106-0079
107-0021
110-0030
103-0028
115-0002
Patient
A
G
A
F
F
Pegsiticase Alone Pegsiticase + SVP-Rapamycin
0
2
4
6
8
1 0
1 0 2
1 0 3
1 0 4
1 0 5
2
3
4
5
0
2
4
6
8
1 0
1 0
8
1 0
2
2
3
4
5
0
2
4
6
1 0 2
3
4
5
8
1
2
3
4
5
0
2
4
6
1 0
2
3
4
5
8
1
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0
0
2
4
6
8
1 0
1 0 2
1 0 3
1 0 4
1 0 5

1 1 2 3
0
2 0
4 0
6 0
9
Percent patients with gout flare by treatment month
%
o
f
P
a
ti
e
n
ts
w
/Fl
a
re
SEL-212*
50%
month
Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918
%
o
f
P
a
ti
e
n
ts
w
/Fl
a
re
Pegsiticase
Alone
37%
26%
5%
Pegsiticase 0.2 or 0.4 mg/kg with SVP-Rapamycin 0.125 or 0.15 mg/kg; Patients evaluable at 12
weeks who received a full first dose and completed treatment cycle 1
*
New PANLAR Data Continue to Show Low Overall
Incidence of Gout Flares
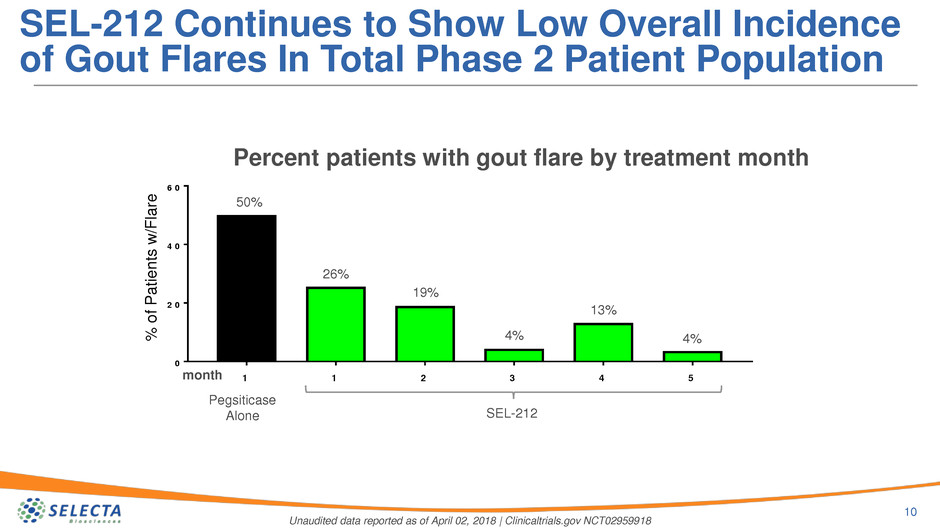
SEL-212 Continues to Show Low Overall Incidence
of Gout Flares In Total Phase 2 Patient Population
10
Percent patients with gout flare by treatment month
1 1 2 3 4 5
0
2 0
4 0
6 0
%
o
f
P
a
ti
e
n
ts
w
/Fl
a
re
Pegsiticase
Alone SEL-212
50%
26%
19%
4%
13%
4%
month
Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918

SEL-212 Safety For the Total Phase 2 Patient
Population
11
•SEL-212 has been generally well tolerated at clinically active
doses following >300 administrations
•Fifteen SAEs reported in the ongoing Phase 2 trial:
• Seven were reported not to be or unlikely to be related to study drug
• Eight infusion reactions:
• Four in cohorts receiving pegsiticase alone or pegsiticase in combination
with the lowest dose of SVP-Rapamycin, as anticipated
• Two due to protocol deviations related to dosing errors
• Two during a repeat dose of SEL-212 in higher (0.1 – 0.15 mg/kg) dose
cohorts
• None occurred after treatment period 2
•All SAEs were successfully treated without further issues
Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918
12
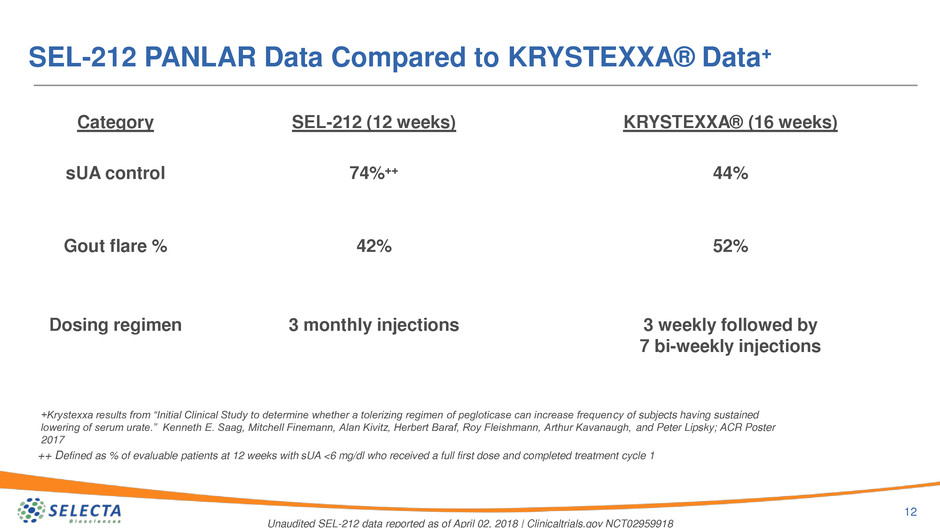
SEL-212 PANLAR Data Compared to KRYSTEXXA® Data+
SEL-212 (12 weeks) KRYSTEXXA® (16 weeks)
74%++ 44%
42% 52%
3 monthly injections 3 weekly followed by
7 bi-weekly injections
+Krystexxa results from “Initial Clinical Study to determine whether a tolerizing regimen of pegloticase can increase frequency of subjects having sustained
lowering of serum urate.” Kenneth E. Saag, Mitchell Finemann, Alan Kivitz, Herbert Baraf, Roy Fleishmann, Arthur Kavanaugh, and Peter Lipsky; ACR Poster
2017
Unaudited SEL-212 data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918
Category
sUA control
Gout flare %
Dosing regimen
++ Defined as % of evaluable patients at 12 weeks with sUA <6 mg/dl who received a full first dose and completed treatment cycle 1

Phase 3 Initiation Expected in 2018
Primary Clinical Endpoint:
Serum uric acid < 6 mg/dl
measured at month 3 and 6
6 Monthly Combination Injections of SEL-212
SEL-212 PHASE 3 PROGRAM+
• Data expected in
third quarter from
patients receiving
five combination
doses of SEL-212
• Phase 3 trial
expected to begin
in 2018
13
+Will include placebo controlled trials; potentially positive controlled trials (e.g., Head to Head with KRYSTEXXA®)

Preclinical Studies Demonstrate Induction of Antigen-
Specific Immune Tolerance by SVP-Rapamycin
Preclinical mechanism of action studies
• Antigen-specific1, 2, 5
• Induction of tolerogenic DCs in vivo2
• Induction of antigen-specific Tregs in vivo1, 2, 4
• Biodistribution of SVP-Rapamycin to antigen-
presenting cells in the spleen1, 2, 3
• Free rapamycin does not induce immune tolerance2
• Reversal of tolerance by depletion of Tregs5
• Transfer of tolerance from SVP-Rapa-treated mice
to naïve mice3, 4, 5
14
SVP-Rapamycin
Pegsiticase
Regulatory T cell
Naïve T cell
B cell
Helper
T cell
Dendritic cell (DC)
Induction of
tolerogenic DC
Immune suppression mediated
by regulatory T cells
Naïve T cell
1. Maldonado et al., PNAS, 2015, 112(2):E156-65
2. Kishimoto et al, Nature Nanotech, 2016, 11(10):890-899
3. Mazor et al., PNAS, 2018,115(4):E733-E742
4. LaMothe et al. Frontiers Immunol, 2018, 9:281
5. Amine et al., Mol Therapy, 2016, 24, Suppl 1, S34,

15
0
5 , 0 0 0
1 0 , 0 0 0
S p l e n o c y t e s R e c e i v e d
A
n
t
i-
L
M
B
-
1
0
0
t
it
e
r
Cell number - 107 107 107 107
LMB-100 - - + - +
SVP-Rapa - - - + +
PBS - +
Transfer of Tolerance from Treated Mice to Naïve Mice
CONFIDENTIAL
4 5
TREATED
DONOR
+/-LMB-100
+/- SVP
1 3 Week
Week
NAÏVE
RECIPIENT LMB-100
Transfer splenocytes
LMB-100
+/-LMB-100
+/- SVP
Mazor et al., PNAS 2018
T cells from mice treated with SVP-Rapamycin and LMB-100 are able to transfer
tolerance to naïve mice that have never been exposed to SVP-Rapamycin
Anti-LMB-100 Antibody Titer

16
Unaudited data reported as of April 02, 2018 | Clinicaltrials.gov NCT02959918
Current Phase 2 Data Provides Evidence for Immune
Tolerance Induction
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2
0
5 0
1 0 0
0.2 or 0.4 mg/kg
0.125 or 0.15 mg/kg
SEL-212
Pegsiticase
SVP-Rapamycin
Patients evaluable at 16 weeks who received a
full first dose and completed treatment cycle 1
Week
%
P
ts.
S
U
A
<6
mg
/d
L
Pegsiticase + SVP-Rapamycin
Weeks after dose of
pegsiticase alone
0.2 or 0.4 mg/kg Pegsiticase
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6
0
5 0
1 0 0
0.2 or 0.4 mg/kg Pegsiticase
0.2 or 0.4 mg/kg
Pegsiticase
Treatment naïve patients after
1 dose of pegsiticase alone
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2
0
5 0
1 0 0
%
P
ts.
S
U
A
<6
mg
/d
L
SEL-212-treated patients with
control of sUA at 12 weeks after
1 dose of pegsiticase alone
91%
17%
91% of patients who make it to week 12
sustain control of sUA through 16 weeks

We thank all of the patients that participated in our clinical
trials. We are very grateful to the clinical trial site
investigators and their staff.
