Attached files
| file | filename |
|---|---|
| 8-K - VITRO DIAGNOSTICS, INC. - VITRO DIAGNOSTICS INC | vitro_8k.htm |
Vitro Bio-Pharma 1st Quarter 2018 Financial Results of Operations
Golden, Colorado-March 23, 2018-Vitro Diagnostics, Inc. (OTCQB: VODG), dba Vitro BioPharma, announced its 1st quarter ended January 31st 2018 financial results of operations.
Vitro Diagnostics Inc. (“Vitro”) is pleased to announce a record 1st quarter in Stem Cell Revenues. Vitro recorded 1st quarter revenues of $87,185 vs $57,041 an increase of 53% over the same comparative quarter last year. In addition, Stem Cell therapies accounted for 55% of the revenues up from 21% of the revenues in the prior comparative quarter last year. Current quarter stem cell revenues from our advanced stem cell therapies with our offshore partners was $48,530 for the 1st quarter ended January 31, 2018 vs $12,000 for the first quarter ended January 31, 2017.
The company’s gross profit margins improved to 68% up from 64% in the comparative prior year’s quarter. Gross margin improvement is in line with the strategic direction of the company to expand the market in its offshore Stem Cell therapies. The company’s clean-room lab expansion last year and increase in its batch Stem Cell manufacturing and production capacity to 2 Billion cells per harvest, using its patent-pending cell line, has increased efficiencies and lowered the cost per harvest.
Overall expenses increased in the quarter to $110,133 from $62,257 in the prior year’s comparative quarter. The increase in expenses reflects the addition of additional team resources as the Company expands its capability to service its strategic direction of offshore Stem Cell sales. The company has added internal operations staff in the stem cell lab; outside consultants supporting its efforts towards CLIA and ISO certification as well as the addition of internal accounting, finance and administrative support staff.
The company’s CEO and major shareholder continues to support the company with advances and deferred salary to provide working capital for the company to grow forward. During the quarter the company also added $75,000 of secured convertible notes receivable to support its expansion activities to be realized during the year.
The company is in discussions with its CEO and major shareholder regarding the conversion of his debts to equity under a re-capitalization plan that is in the interest of the debtholders and shareholders. Such discussions could result in the issuance of approximately 20M shares for the conversion of approximately $1M in debts to stock at $0.05 cents per share. This would increase the outstanding share capital to approximately 50M shares on a fully diluted basis. Such a re-capitalization has not yet been affected but it is being actively discussed amongst the company’s board and advisors.
During the quarter the company achieved and pursed the following company objectives;
Developed Stem Cell Therapy of TBI to Pre-market Status:
TBI (Traumatic Brain Injury) affects about 2 million Americans per year with therapy options limited to rehabilitation/palliative care. We developed our products & services to pre-market, beta testing levels. Our products include a nutraceutical formulation known to activate human stem cells together with diagnostic services providing biomarker profiling together with advanced brain imaging procedures. Our accomplishments included:
oFiling Trade Mark Registration with the USTPO for Brain Grow Technologies™
oSubmission of CLIA application. Vitro Biopharma’s clinical laboratory for biomarker profiling is now CLIA-registered and we anticipate certification soon.
oExpansion of partnership development to support TBI and other applications of our platform including: Stroke, Parkinson’s disease, Alzheimer’s Disease, etc.
oDeveloped products (including labeling & logo) and services to commercial status. The NutraVivo™ Brand of nutraceuticals is being launched. These are natural substances that activate stem cells to enhance recovery from injury such as TBI, etc while also enhancing overall cellular health.
Accelerated the Company’s Marketing Program
oUpdated its website and SEO
oEstablished initial social media presence
oDeveloped and expanded off-shore stem cell therapy partnerships.
Research and Development
oDeveloping stem cell products for use in cosmetic applications including skin rejuvenation and regeneration.
oDeveloping alternative forms of stem cells for enhanced distribution logistics and deployment methods
Dr. Jim Musick, CEO of Vitro Biopharma, said, “We are very pleased with the increased revenue growth during our first quarter 2018. We are continuing to develop our business model including growth of revenue from combined research product sales and revenues from off-shore markets for our clinical products and services.
This began about 5 years ago with sales of Clinical Grade MSC-Gro™ to support OA clinical trials in Australia. In 2017, we began sales of clinical grade stem cells to the DaVinci Centre in Grand Cayman Island that continued to grow in early 2018. We continue to expand off-shore partnerships for additional indications and revenue growth for the Company. In addition, we are beginning the initial marketing of our Stem Cell Therapy for Traumatic Brain Injury. Our products include a novel and patent-pending formulation of natural substances known to activate human stem cells together with diagnostic procedures to access molecular and brain structural injury and their response to therapy. Since we rely on unique combinations/formulations of natural dietary supplements, this therapeutic intervention is not subject to FDA regulation and our diagnostic procedures are conducted in compliance with CLIA. The TBI initiative will be initially launched in the US. In addition, we are considering the FDA’s fast track approval of stem cell therapies (RMAT) for transplants including novel deployment methods to treat TBI, Parkinson’s disease, Stroke and Alzheimer’s disease.”
In summary, Vitro Biopharma is advancing as a key player in regenerative medicine with 10-years’ experience in the development and commercialization of stem cell products for research, recognized by a Best in Practice Technology Innovation Leadership award for Stem Cell Tools and Technology and a growing track record of successful translation to therapy. We plan to leverage our proprietary technology platform to the establishment of international Stem Cell Centers of Excellence and regulatory approvals in the US.
Sincerely yours,
James R. Musick, PhD.
President, CEO & Chairman of the Board
www.vitrobiopharma.com
Forward-Looking Statements
Statements herein regarding financial performance have not yet been reported to the SEC nor reviewed by the Company’s auditors. Certain statements contained herein and subsequent statements made by and on behalf of the Company, whether oral or written may contain “forward-looking statements”. Such forward looking statements are identified by words such as “intends,” “anticipates,” “believes,” “expects” and “hopes” and include, without limitation, statements regarding the Company’s plan of business operations, product research and development activities, potential contractual arrangements, receipt of working capital, anticipated revenues and related expenditures. Factors that could cause actual results to differ materially include, among others, acceptability of the Company’s products in the market place, general economic conditions, receipt of additional working capital, the overall state of the biotechnology industry and other factors set forth in the Company’s filings with the Securities and Exchange Commission. Most of these factors are outside the control of the Company. Investors are cautioned not to put undue reliance on forward-looking statements. Except as otherwise required by applicable securities statutes or regulations, the Company disclaims any intent or obligation to update publicly these forward-looking statements, whether as a result of new information, future events or otherwise.
CONTACT:
Dr. James Musick
Chief Executive Officer
Vitro BioPharma
(303) 999-2130 Ext. 3
E-mail: jim@vitrobiopharma.com
Source: Vitro Diagnostics, Inc.
www.vitrobiopharma.com
Vitro Diagnostics Income Statement;
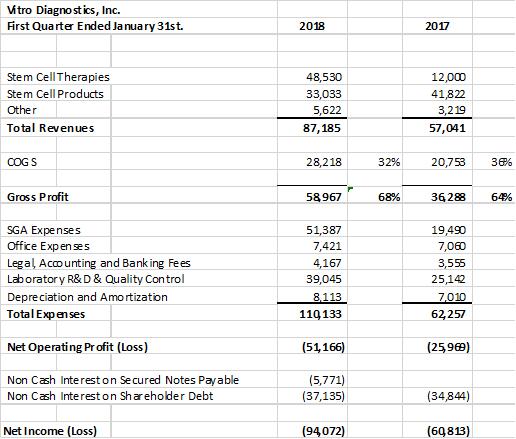
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant
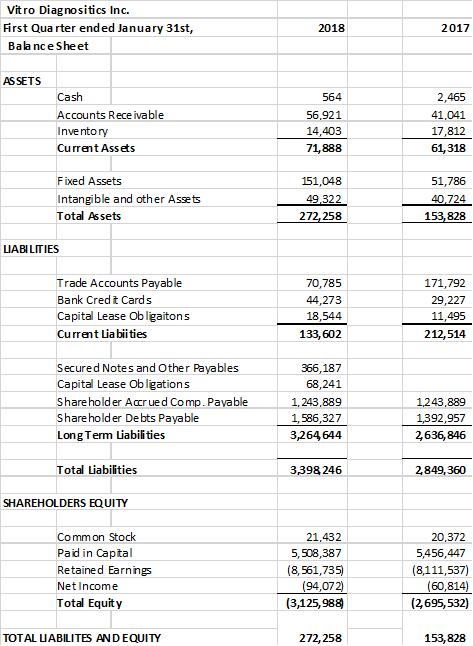
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant
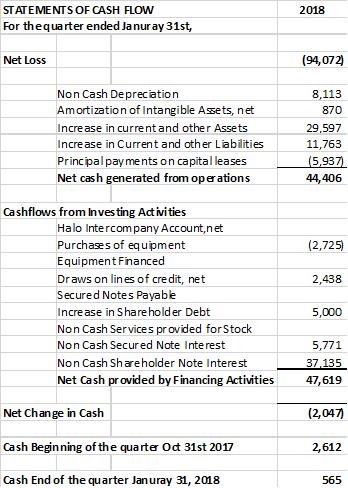
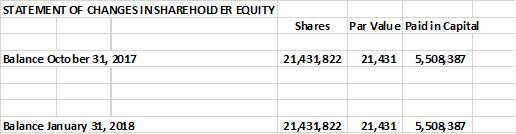
The company provides its financial information for investor purposes only, the results published are not audited or necessarily SEC or GAAP compliant.
