Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Cidara Therapeutics, Inc. | a8-k2018x03x19_02.htm |
| EX-99.1 - STRIVE PHASE 2 PART A TOPLINE DATA CONFERENCE CALL TRANSCRIPT - Cidara Therapeutics, Inc. | ex9912018-03x19_02.htm |

186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
STRIVE Part A Phase 2 topline data
March 2018
186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear
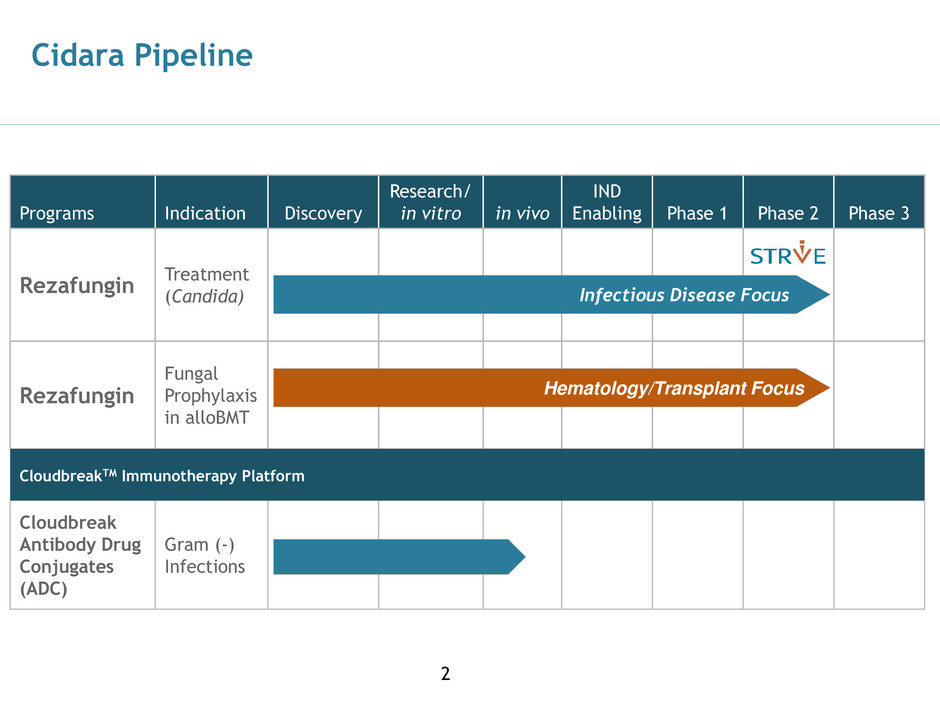
Cidara Pipeline
Programs
Indication
Discovery
Research/
in vitro
in vivo
IND
Enabling
Phase 1
Phase 2
Phase 3
Rezafungin
Treatment
(Candida)
Rezafungin
Fungal
Prophylaxis
in alloBMT
CloudbreakTM Immunotherapy Platform
Cloudbreak
Antibody Drug
Conjugates
(ADC)
Gram (-)
Infections
Infectious Disease Focus
Hematology/Transplant Focus
2

186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear
STRIVE Part A: Candidemia & Invasive Candidiasis
400/400/(400)mg n=30
400/200/(200)mg n=30
Week 1 2 3 4 5 6 7 8 9
Day
1 5 8 15 22 28
Dose Optional dose
Mycological
response
Mycological &
clinical response:
1 ENDPOINT
Mycological &
clinical response
(IC only)
45 35 42 49 56 59
Mycological &
clinical response
Caspofungin
70/50/(50)mg n=30
Week 1 2 3 4 5 6 7 8 9
Day
1 5 8 15 22 28 45 35 42 49 56 59
70mg Dose 50mg Dose
All cause mortality
Not powered for inferential statistics.
Rezafungin
Analysis Populations:
The Intent-to-treat (ITT) population: all randomized subjects
The Safety population: all subjects who received any amount of study drug
The Microbiological Intent-to-treat population (mITT): all subjects in safety population who had documented
Candida infection
3

186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear
Major objectives of STRIVE Part A
Select a dosing regimen for STRIVE Part B and Phase 3 based on:
• Clinical and mycological cure by time, severity and empiric treatment
• Tolerability, safety and adverse events
• Relative performance vs caspofungin in light of 20% NI margin in Phase 3
4
186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear 5
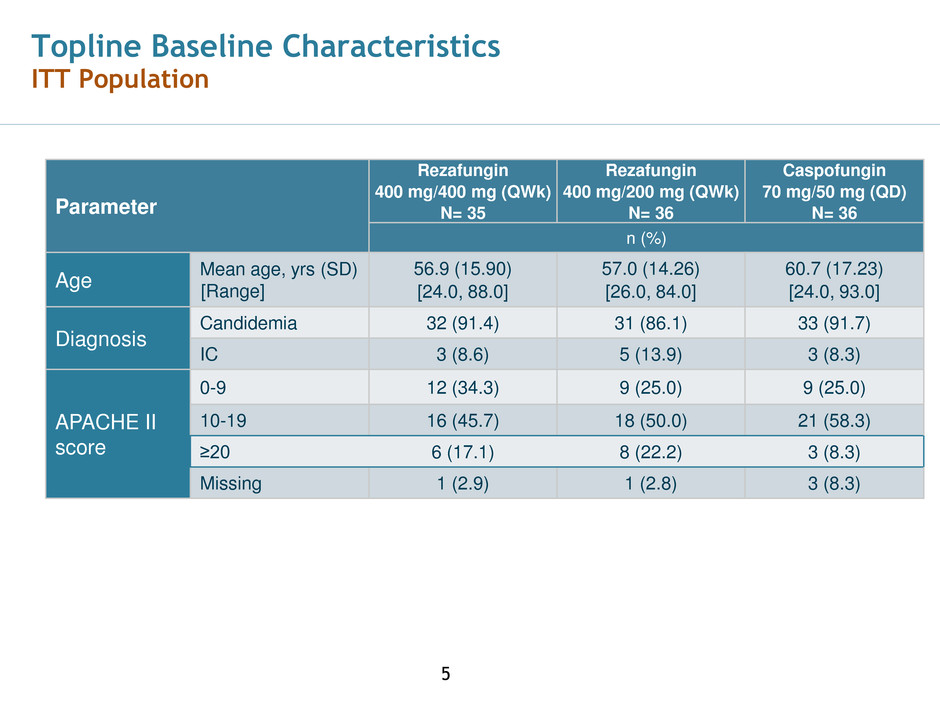
Topline Baseline Characteristics
ITT Population
Parameter
Rezafungin
400 mg/400 mg (QWk)
N= 35
Rezafungin
400 mg/200 mg (QWk)
N= 36
Caspofungin
70 mg/50 mg (QD)
N= 36
n (%)
Age
Mean age, yrs (SD)
[Range]
56.9 (15.90)
[24.0, 88.0]
57.0 (14.26)
[26.0, 84.0]
60.7 (17.23)
[24.0, 93.0]
Diagnosis
Candidemia 32 (91.4) 31 (86.1) 33 (91.7)
IC 3 (8.6) 5 (13.9) 3 (8.3)
APACHE II
score
0-9 12 (34.3) 9 (25.0) 9 (25.0)
10-19 16 (45.7) 18 (50.0) 21 (58.3)
≥20 6 (17.1) 8 (22.2) 3 (8.3)
Missing 1 (2.9) 1 (2.8) 3 (8.3)
5

186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear 6
Topline Overall Response (Primary Outcome)
Day 14 – mITT Population
Response
Rezafungin
400 mg/400 mg (QWk)
N= 33
Rezafungin
400 mg/200 mg (QWk)
N= 31
Caspofungin
70 mg/50 mg (QD)
N= 28
n (%)
Success 19 (57.6) 22 (71.0) 18 (64.3)
Failure 7 (21.2) 6 (19.4) 8 (28.6)
Indeterminate 7 (21.2) 3 (9.7) 2 (7.1)
Excluding Indeterminate Response*
Success 19/26 (73.1) 22/28 (78.6) 18/26 (69.2)
Failure 7/26 (26.9) 6/28 (21.4) 8/26 (30.8)
*Indeterminate response indicates inability to assess outcome due to missing data point(s)
6

186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear 7
Topline Investigator Assessment of Clinical Response
Day 14 – mITT Population
Response
Rezafungin
400 mg/400 mg (QWk)
N= 33
Rezafungin
400 mg/200 mg (QWk)
N= 31
Caspofungin
70 mg/50 mg (QD)
N= 28
n (%)
Clinical Cure 25 (75.8) 24 (77.4) 20 (71.4)
Clinical Failure 7 (21.2) 4 (12.9) 8 (28.6)
Indeterminate 1 (3.0) 3 (9.7) 0
Excluding Indeterminate Response
Clinical Cure 25/32 (78.1) 24/28 (85.7) 20/28 (71.4)
Clinical Failure 7/32 (21.9) 4/28 (14.3) 8/28 (28.6)
Outcome most closely approximating primary outcome from prior IC clinical trials
7

186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear 8
Topline Overall Response
Day 5 – mITT Population
Response
Rezafungin
400 mg/400 mg (QWk)
N= 33
Rezafungin
400 mg/200 mg (QWk)
N= 31
Caspofungin
70 mg/50 mg (QD)
N= 28
n (%)
Success 19 (57.6) 21 (67.7) 15 (53.6)
Failure 10 (30.3) 8 (25.8) 12 (42.9)
Indeterminate 4 (12.1) 2 (6.5) 1 (3.6)
Excluding Indeterminate Response
Success 19/29 (65.5) 21/29 (72.4) 15/27 (55.6)
Failure 10/29 (34.5) 8/29 (27.6) 12/27 (44.4)
Comparing single dose of Rezafungin to five doses of Caspofungin
8

186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear 9
Topline Overall Success by Severity and Prior Therapy
Day 14 – mITT Population
Population
Rezafungin
400 mg/400 mg (QWk)
Rezafungin
400 mg/200 mg (QWk)
Caspofungin
70 mg/50 mg (QD)
n/N (%)
Overall Response 19/33 (57.6) 22/31 (71.0) 18/28 (64.3)
High APACHE II
score
≥15 6/10 (60) 8/10 (80) 7/12 (58.3)
Prior antifungal
therapy
No 8/11 (72.7) 10/10 (100) 7/9 (77.8)
Yes 11/22 (50) 12/21 (57.1) 11/19 (57.9)
9

186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear
Topline Day 30 All-Cause Mortality (1º Endpoint for Ph3)
mITT Population
Parameter
Rezafungin
400 mg/400 mg (QWk)
N= 33
Rezafungin
400 mg/200 mg (QWk)
N= 31
Caspofungin
70 mg/50 mg (QD)
N= 28
Deaths, n (%) 5 (15.2) 3 (9.7) 5 (17.9)
Deaths at Day 30 (%) 5 (15.2) 1 (3.2) 3 (10.7)
10
186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear
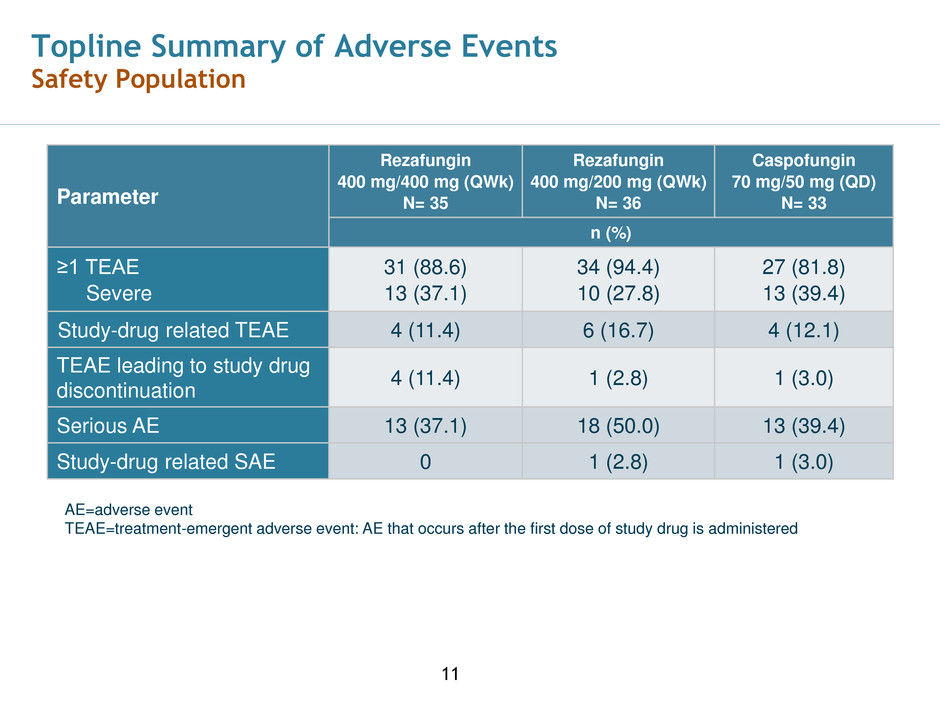
Topline Summary of Adverse Events
Safety Population
Parameter
Rezafungin
400 mg/400 mg (QWk)
N= 35
Rezafungin
400 mg/200 mg (QWk)
N= 36
Caspofungin
70 mg/50 mg (QD)
N= 33
n (%)
≥1 TEAE
Severe
31 (88.6)
13 (37.1)
34 (94.4)
10 (27.8)
27 (81.8)
13 (39.4)
Study-drug related TEAE 4 (11.4) 6 (16.7) 4 (12.1)
TEAE leading to study drug
discontinuation
4 (11.4) 1 (2.8) 1 (3.0)
Serious AE 13 (37.1) 18 (50.0) 13 (39.4)
Study-drug related SAE 0 1 (2.8) 1 (3.0)
AE=adverse event
TEAE=treatment-emergent adverse event: AE that occurs after the first dose of study drug is administered
11
186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear
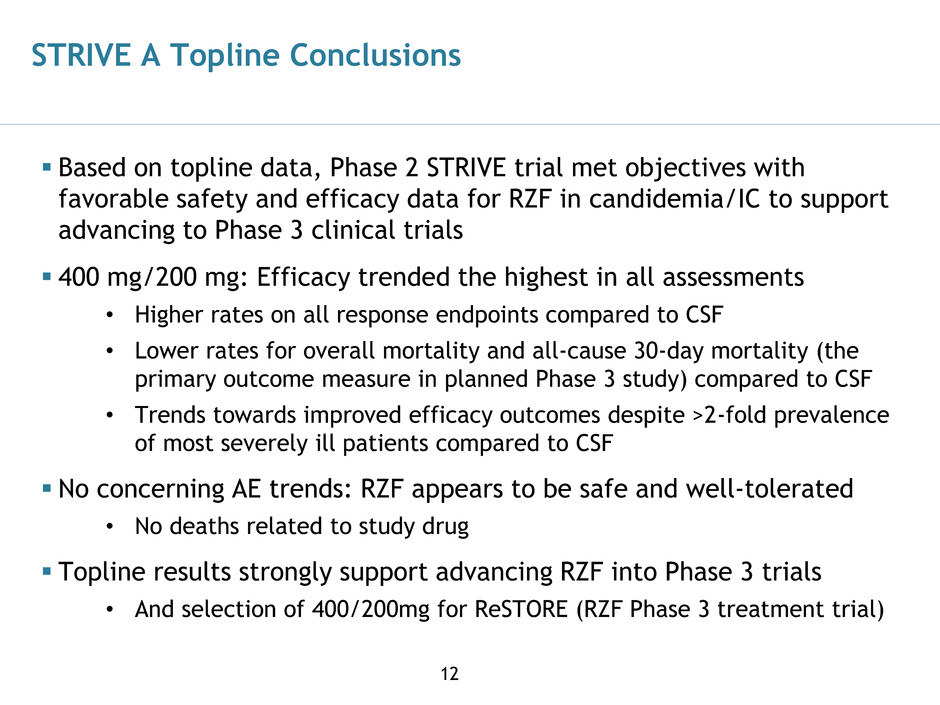
STRIVE A Topline Conclusions
Based on topline data, Phase 2 STRIVE trial met objectives with
favorable safety and efficacy data for RZF in candidemia/IC to support
advancing to Phase 3 clinical trials
400 mg/200 mg: Efficacy trended the highest in all assessments
• Higher rates on all response endpoints compared to CSF
• Lower rates for overall mortality and all-cause 30-day mortality (the
primary outcome measure in planned Phase 3 study) compared to CSF
• Trends towards improved efficacy outcomes despite >2-fold prevalence
of most severely ill patients compared to CSF
No concerning AE trends: RZF appears to be safe and well-tolerated
• No deaths related to study drug
Topline results strongly support advancing RZF into Phase 3 trials
• And selection of 400/200mg for ReSTORE (RZF Phase 3 treatment trial)
12

186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear
STRIVE Part B provides an early read on treatment Phase 3
Seamless Phase 2 to Phase 3 Strategy
Phase 2
STRIVE ( Part A )
Phase 2
STRIVE ( Part B )
Phase 3
ReSTORE
Dosing Regimen
Global , multicenter , randomized , double blind ,
active comparator ( caspofungin ) , / invasive candidiasis
Low Dose
Selected Dose
:
1 1
20
%
3
High Dose Selected Dose
from Part A
)
2 :
1
Enrollment
momentum
Original
Protocol
Amended
Protocol
Phase 3
Protocol
Rollover Rollover
Increasing statistical
robustness
Not powered for inferential statistics 20 NI Margin %
The patient populations from STRIVE and ReSTORE will bolster the
global safety database needed for regulatory filings.
13

186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear
…two P3 studies, one in each population, with distinct commercial opportunities
Topline
Phase 2
STRIVE A
Data
Phase 3
Treatment
Trial Start
Phase 3
Prophylaxis
Trial Start
Phase 3
Prophylaxis
Interim data
STRIVE B
Treatment
Data
Phase 3
Prophylaxis
Data
2018 2019 2020
Phase 3
Treatment
Data
STRIVE Program Enables Phase 3 Treatment and Prophylaxis
14

186, 89, 21
43, 124, 154
28, 83, 102
102, 102, 102
170, 170, 170
Keep this area clear
Keep this area clear
Rezafungin Overall Phase 3 Development Plan
Phase 3 Treatment Trial
Indication
Phase 3
Size
Duration of therapy,
Endpoints and
Comparators
Timing
for initiation
and data
Treatment of
Candidemia & Invasive
Candidiasis in patients with
limited treatment options
~ 150 patients
2-4 week treatment
Day 30 all-cause mortality
Caspofungin
Mid-2018
Mid-2020
Phase 3 Prophylaxis Trial
Prophylaxis of Aspergillus,
Candida & PCP in patients
undergoing allogeneic bone
marrow transplant
~450 patients
w/ adaptive design
90 day prophylaxis
90 day fungal free survival
Fluconazole, Posaconazole,
Bactrim
Mid-2018
Mid-2020
Based on meetings with FDA in 4Q17
15
