Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Axsome Therapeutics, Inc. | a18-7672_1ex99d1.htm |
| 8-K - 8-K - Axsome Therapeutics, Inc. | a18-7672_18k.htm |
Forward-Looking Statements & Safe Harbor Certain information contained in this presentation may include "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as "predicts," "believes," "potential," "continue," "estimates," "anticipates," "expects," "plans," "intends," "may," "could," "might," "will," "should" or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. In particular, the Company's statements regarding trends and potential future results are examples of such forward-looking statements. The forward looking statements include risks and uncertainties, including, but not limited to, the success, timing and cost of our ongoing clinical trials and anticipated clinical trials for our current product candidates, including statements regarding the timing of initiation and completion of the trials, interim analyses and receipt of interim results; the timing of and our ability to obtain and maintain U.S. Food and Drug Administration or other regulatory authority approval of, or other action with respect to, our product candidates; the Company's ability to obtain additional capital necessary to fund its operations; the Company's ability to generate revenues in the future; the Company's ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company's research and development programs; the enforceability of the Company's license agreements; the acceptance by the market of the Company's product candidates, if approved; and other factors, including general economic conditions and regulatory developments, not withi n the Company's control. These factors could cause actual results and developments to be materially different from those expressed in or implied by such statements. Forward-looking statements are not guarantees of future performance, and actual results may differ materially from those projected. The forward-looking statements are made only as of the date of this presentation and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstance. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, these projections, assumptions and estimates are necessarily subject to a high degree of uncertainty and risk. AXSOME THERAPEUTICS © AxsorreTherapeutics, Inc. 2

© Axsome Therapeutics, Inc. 3 Axsome is addressing growing markets, where current treatment options are limited or inadequate, by leveraging well-characterized compounds to create novel therapeutics to meet unmet medical needs and improve the lives of patients.
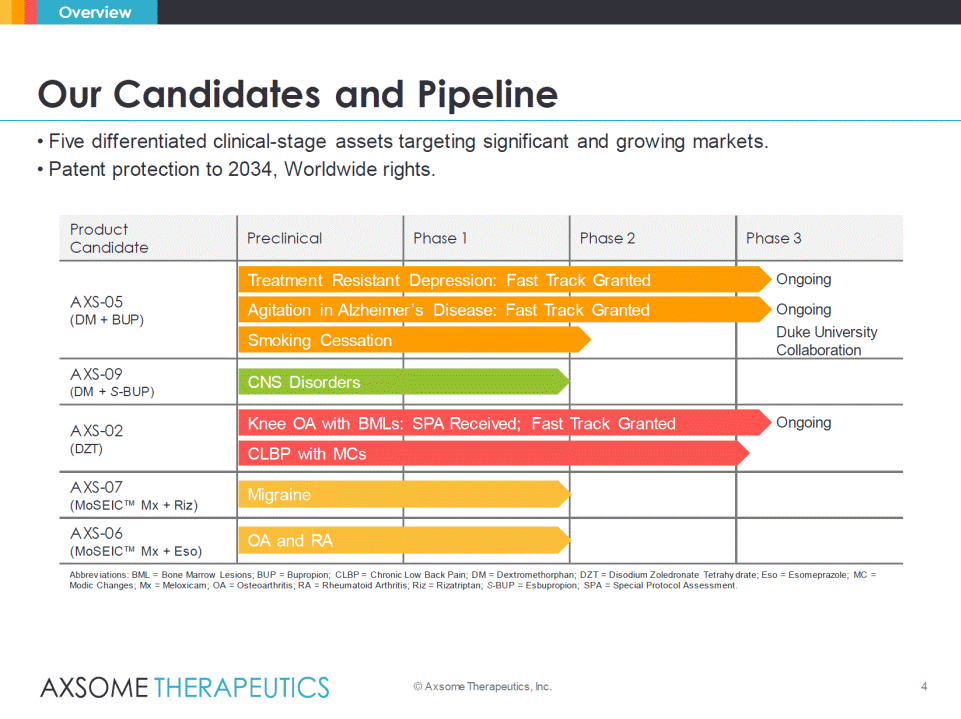
• Five differentiated clinical-stage assets targeting significant and growing markets. • Patent protection to 2034, Worldwide rights. 4 Our Candidates and Pipeline © Axsome Therapeutics, Inc. Overview Product Candidate Preclinical Phase 1 Phase 2 Phase 3 AXS-05 (DM + BUP) AXS-09 (DM + S-BUP) AXS-02 (DZT) AXS-07 (MoSEIC™ Mx + Riz) AXS-06 (MoSEIC™ Mx + Eso) Abbreviations: BML = Bone Marrow Lesions; BUP = Bupropion; CLBP = Chronic Low Back Pain; DM = Dextromethorphan; DZT = Disodium Zoledronate Tetrahydrate; Eso= Esomeprazole; MC = ModicChanges; Mx = Meloxicam; OA = Osteoarthritis; RA = Rheumatoid Arthritis; Riz= Rizatriptan; S-BUP = Esbupropion; SPA = Special Protocol Assessment. CLBP with MCs Migraine Treatment Resistant Depression: Fast Track Granted Ongoing Knee OA with BMLs: SPA Received; Fast Track Granted Ongoing Agitation in Alzheimer’s Disease: Fast Track Granted Ongoing OA and RA Smoking Cessation Duke University Collaboration CNS Disorders
Novel therapy for CNS disorders: • Treatment ResistantDepression (TRD) • Agitation in Alzheimer’s Disease (AD) © Axsome Therapeutics, Inc. 5 Dextromethorphan (DM) + Bupropion (BUP) AXS-05
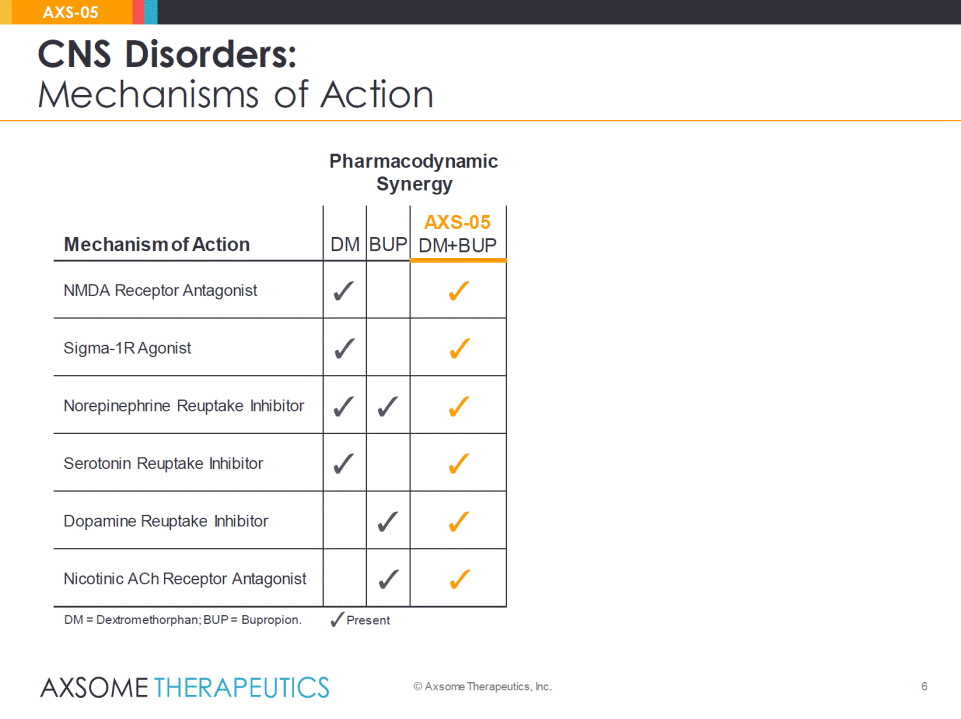
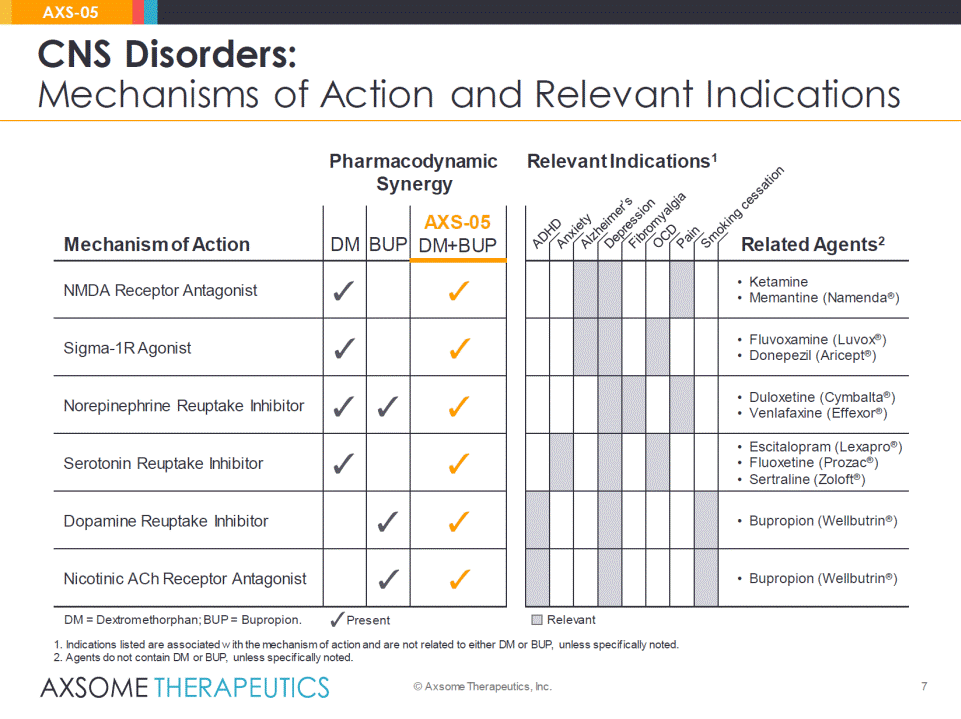
6 CNS Disorders: Mechanisms of Action © Axsome Therapeutics, Inc. AXS-05 Relevant Indications Pharmacodynamic Synergy Mechanism of Action DM BUP AXS-05DM+BUP Related Agents NMDA Receptor Antagonist •Ketamine •Memantine (Namenda®) Sigma-1R Agonist •Fluvoxamine (Luvox®) •Donepezil (Aricept®) Norepinephrine Reuptake Inhibitor •Duloxetine (Cymbalta®) •Venlafaxine (Effexor®) Serotonin Reuptake Inhibitor •Escitalopram (Lexapro®) •Fluoxetine (Prozac®) •Sertraline (Zoloft®) Dopamine Reuptake Inhibitor •Bupropion (Wellbutrin®) Nicotinic ACh Receptor Antagonist •Bupropion (Wellbutrin®) DM = Dextromethorphan; BUP = Bupropion. Relevant Present
7 CNS Disorders: Mechanisms of Action and Relevant Indications © Axsome Therapeutics, Inc. AXS-05 Relevant Indications1 Pharmacodynamic Synergy Mechanism of Action DM BUP AXS-05DM+BUP Related Agents2 NMDA Receptor Antagonist •Ketamine •Memantine (Namenda®) Sigma-1R Agonist •Fluvoxamine (Luvox®) •Donepezil (Aricept®) Norepinephrine Reuptake Inhibitor •Duloxetine (Cymbalta®) •Venlafaxine (Effexor®) Serotonin Reuptake Inhibitor •Escitalopram (Lexapro®) •Fluoxetine (Prozac®) •Sertraline (Zoloft®) Dopamine Reuptake Inhibitor •Bupropion (Wellbutrin®) Nicotinic ACh Receptor Antagonist •Bupropion (Wellbutrin®) DM = Dextromethorphan; BUP = Bupropion. Relevant Present 1. Indications listed are associated with the mechanism of action and are not related to either DM or BUP, unless specifically noted. 2.Agents do not contain DM or BUP, unless specifically noted.
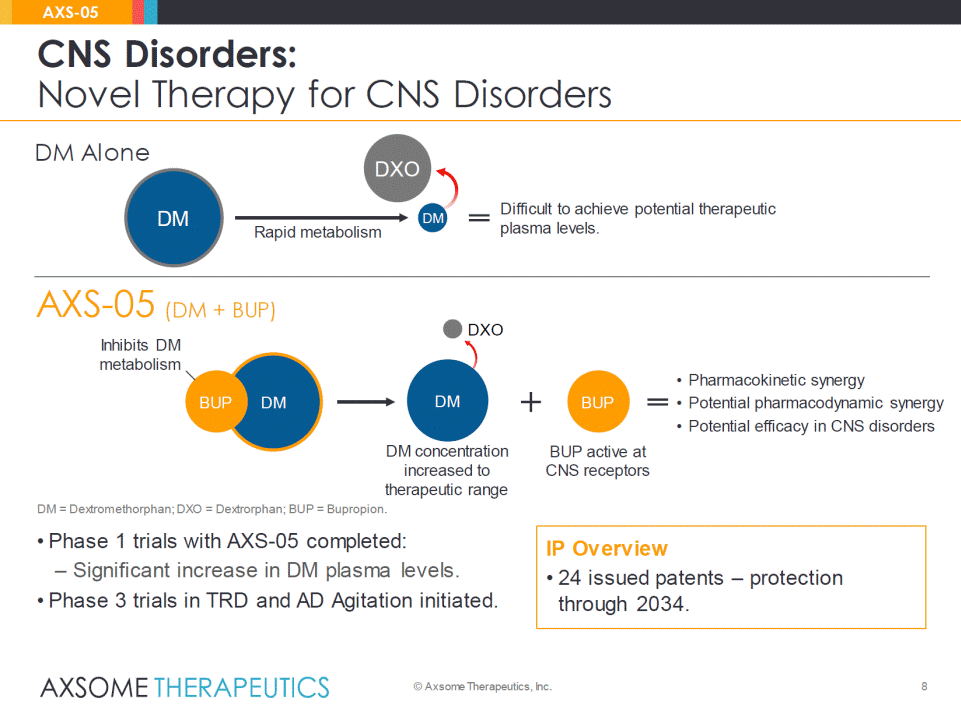
AXS-05 (DM + BUP) • Phase 1 trials with AXS-05 completed: – Significant increase in DM plasma levels. • Phase 3 trials in TRD and AD Agitation initiated. 8 CNS Disorders: Novel Therapy for CNS Disorders © Axsome Therapeutics, Inc. AXS-05 DM concentration increased to therapeutic range DM BUP DM BUP • Pharmacokinetic synergy •Potential pharmacodynamic synergy •Potential efficacy in CNS disorders Inhibits DM metabolism BUP active atCNS receptors Rapid metabolism DM DXO Difficult to achieve potential therapeutic plasma levels. DM DM = Dextromethorphan; DXO = Dextrorphan; BUP = Bupropion. IP Overview •24 issued patents –protection through 2034. DM Alone DXO
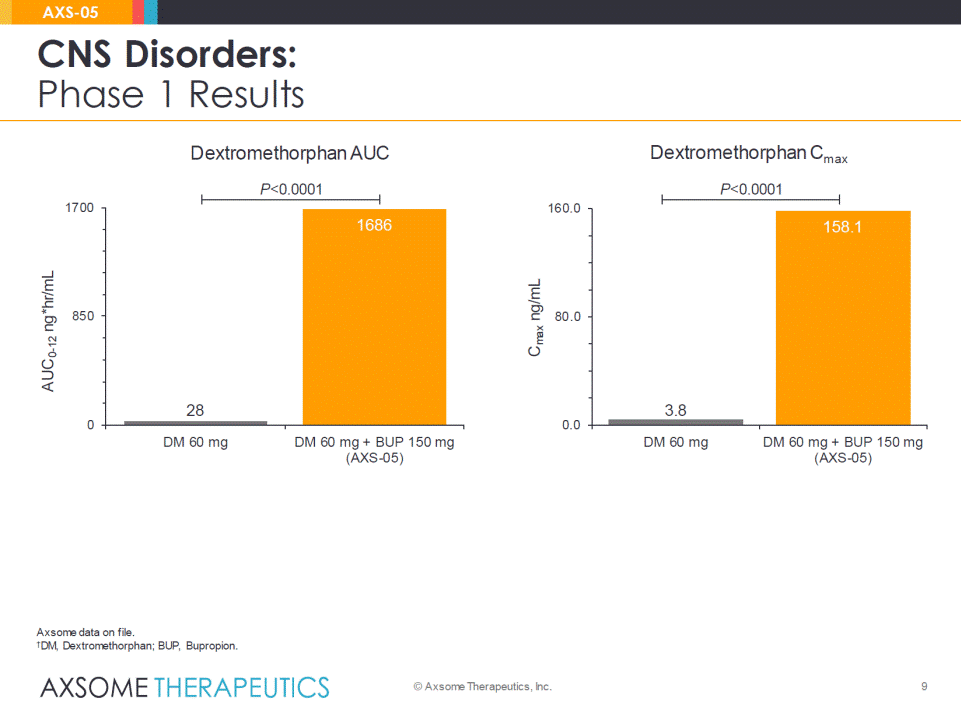
9 CNS Disorders: Phase 1 Results © Axsome Therapeutics, Inc. AXS-05 P<0.0001 P<0.0001 Dextromethorphan AUC Dextromethorphan Cmax Axsome data on file. †DM, Dextromethorphan; BUP, Bupropion. 28 1686 0 850 1700 DM 60 mg DM 60 mg + BUP 150 mg(AXS-05) AUC0-12ng*hr/mL 3.8 158.1 0.0 80.0 160.0 DM 60 mg DM 60 mg + BUP 150 mg(AXS-05) Cmaxng/mL
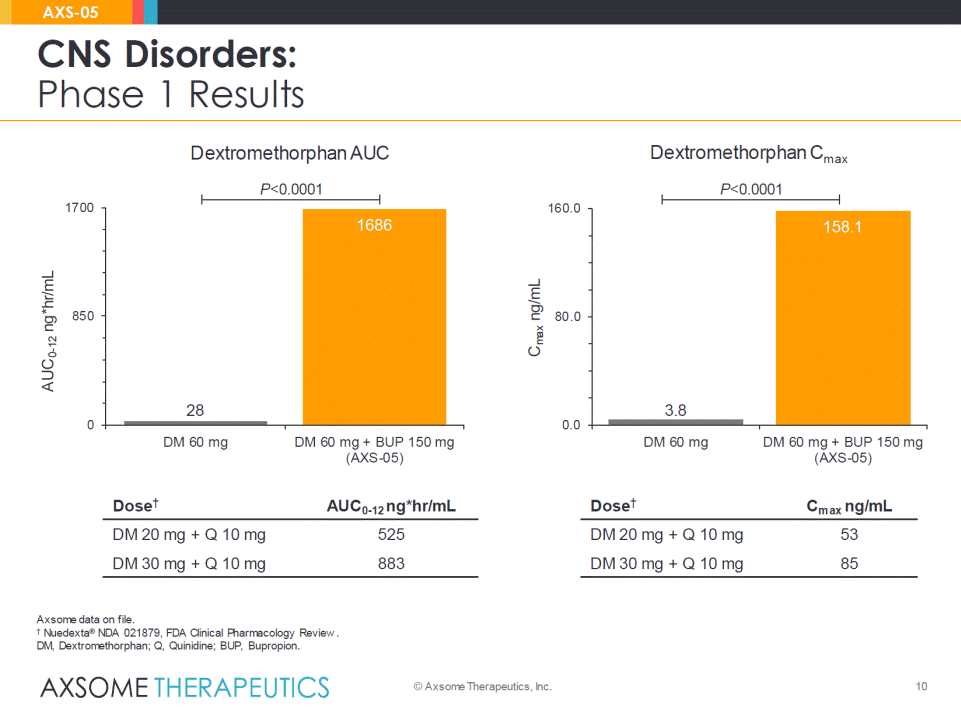
10 CNS Disorders: Phase 1 Results © Axsome Therapeutics, Inc. AXS-05 Dose† AUC0-12 ng*hr/mL DM 20 mg + Q 10 mg 525 DM 30mg + Q 10 mg 883 Dose† Cmaxng/mL DM 20 mg + Q 10 mg 53 DM 30mg + Q 10 mg 85 P<0.0001 P<0.0001 Dextromethorphan AUC Dextromethorphan Cmax Axsome data on file. †Nuedexta®NDA 021879, FDA Clinical Pharmacology Review. DM, Dextromethorphan; Q, Quinidine; BUP, Bupropion. 28 1686 0 850 1700 DM 60 mg DM 60 mg + BUP 150 mg(AXS-05) AUC0-12ng*hr/mL 3.8 158.1 0.0 80.0 160.0 DM 60 mg DM 60 mg + BUP 150 mg(AXS-05) Cmaxng/mL
• Major Depressive Disorder (MDD) is a leading cause of disease burden in the US.4 • 63% and 44% of MDD patients have inadequate response to initial therapy and second line therapy, respectively.2 • Only 1 approved drug for TRD = unmet medical need. • AXS-05 combines the MOA of 4 distinct anti-depressant drug classes into one novel oral therapeutic. • DM antidepressant effects demonstrated preclinically and clinically. • Phase 3 ongoing. 11 CNS Disorders: TRD Overview © Axsome Therapeutics, Inc. AXS-05 1. Marcus SC, Olfson M. Arch Gen Psychiatry 2010;67:1265-1273. 2. Rush AJ, et al. Am J Psychiatry 2006;163:1905-1917. 3. U.S. Census Bureau, Population April 1, 2010 to July 1, 2013. 4. Mathers CD, PLoS Med 2006; 3(11): e442. 3M patients in the U.S.1-3 Product Candidate Preclinical Phase 1 Phase 2 Phase 3 AXS-05 (DM + BUP) Treatment Resistant Depression: Fast Track Granted Initiated Abbreviations: DM = Dextromethorphan; BUP = Bupropion.
12 CNS Disorders: TRD Clinical Rationale AXS-05 • DM and metabolic inhibitor reduce depressive symptoms in TRD and in AD. Depressive Symptom Reduction in AD Agitation Patients Treated with DM and Metabolic Inhibitor2 P=0.002 1. MurroughJ, et al. J Affect Disord. 2017;218:277-283. 2.Cummings J, et al. JAMA. 2015;314:1242-1254. Symptom Reduction in TRD Patients Treated with DM and Metabolic Inhibitor1 -1.0 -0.5 0.0 0.5 Placebo DM+Q(30/10 mg) Change in Cornell Scale Score •Failed 2 to 10 prior treatments •45% of patients had 50% reduction in MADRS ** P<0.01 versus baseline Time (weeks) -20 -15 -10 -5 0 Wk 1 Wk 2 Wk 4 Wk 6 Wk 8 Wk 12 ** ** ** ** DM/Q Titration DM/Q 45/10 mg q 12 hrs Change in Montgomery-Asberg Depression Rating (MADRS) Scale © Axsome Therapeutics, Inc.

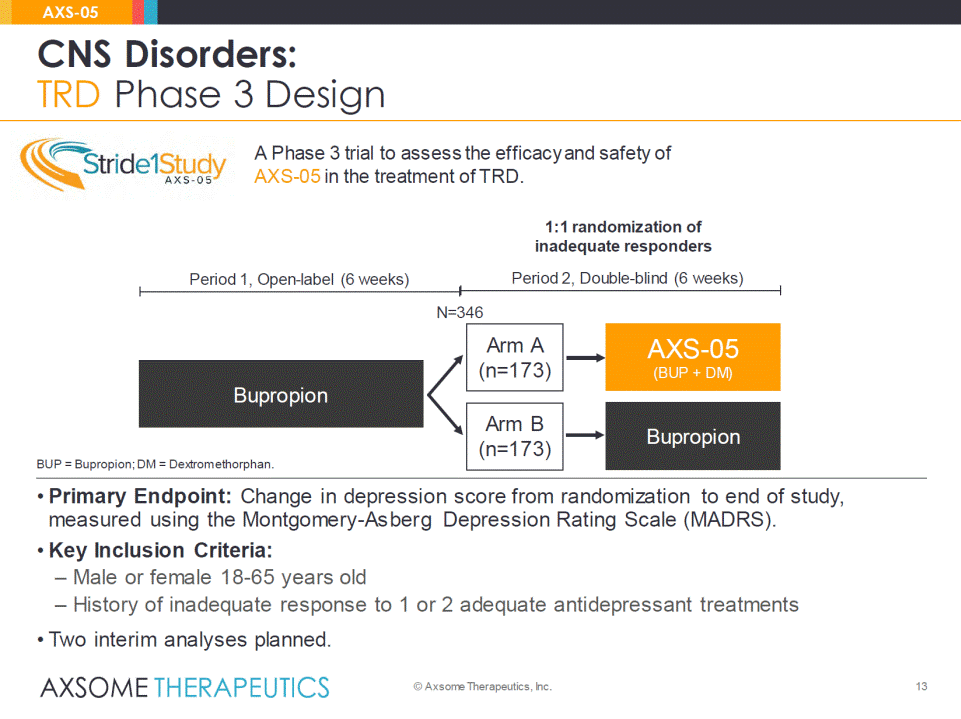
• Primary Endpoint: Change in depression score from randomization to end of study, measured using the Montgomery-Asberg Depression Rating Scale (MADRS). • Key Inclusion Criteria: – Male or female 18-65 years old – History of inadequate response to 1 or 2 adequate antidepressant treatments • Two interim analyses planned. 13 CNS Disorders: TRD Phase 3 Design AXS-05 © Axsome Therapeutics, Inc. BUP = Bupropion; DM = Dextromethorphan. Bupropion AXS-05 (BUP + DM) Period 1, Open-label (6 weeks) Period 2, Double-blind (6 weeks) 1:1 randomization of inadequate responders Bupropion N=346 A Phase 3 trial to assess the efficacy and safety ofAXS-05in the treatment of TRD. Arm A(n=173) Arm B(n=173)
• Agitation and aggression seen in approximately 45% of AD patients during 5-year period.3 • Characterized by emotional distress, aggressive behaviors, disruptive irritability, disinhibition, and caregiver burden.4 • Associated with4,5: – Accelerated cognitive decline – Earlier nursing home placement – Increased mortality • No approved medication = unmet medical need. • Proof of concept: DM plus metabolic inhibitor reduced agitation in AD patients. • Phase 2/3 ongoing. 14 CNS Disorders: Agitation in AD Overview © Axsome Therapeutics, Inc. AXS-05 1. Ryu, SH, et al. Am J Geriatr Psychiatry. 2005;13:976-983. 2. Hebert, LE, et al. Neurology. 2013;80:1778-1783. 3. Steinberg M, et al. Int J Geriatr Psychiatry. 2008;2:170-177. 4. Antonsdottir IM, et al. Expert Opin Pharmacother. 2015;11:1649-1656. 5. Rabins PV et al. Alzheimers Dement. 2013; 9:204-207. 2M patients in the U.S.1,2 Product Candidate Preclinical Phase 1 Phase 2 Phase 3 AXS-05 (DM + BUP) Agitation in Alzheimer’s Disease: Fast Track Granted Initiated Abbreviations: DM = Dextromethorphan; BUP = Bupropion.

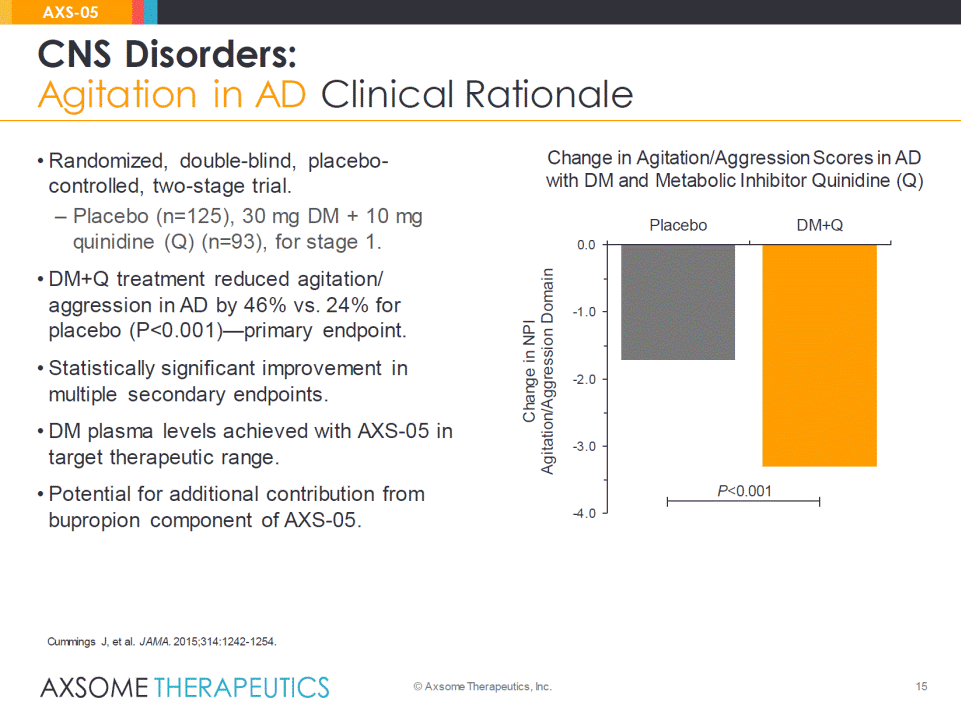
15 CNS Disorders: Agitation in AD Clinical Rationale © Axsome Therapeutics, Inc. AXS-05 Change in Agitation/Aggression Scores in AD with DM and Metabolic Inhibitor Quinidine (Q) P<0.001 Cummings J, et al. JAMA. 2015;314:1242-1254. -4.0 -3.0 -2.0 -1.0 0.0 Placebo DM+Q Change in NPI Agitation/Aggression Domain • Randomized, double-blind, placebo-controlled, two-stage trial. –Placebo (n=125), 30 mg DM + 10 mg quinidine (Q) (n=93), for stage 1. •DM+Q treatment reduced agitation/ aggression in AD by 46% vs. 24% for placebo (P<0.001)—primary endpoint. •Statistically significant improvement in multiple secondary endpoints. •DM plasma levels achieved with AXS-05 in target therapeutic range. •Potential for additional contribution from bupropion component of AXS-05.
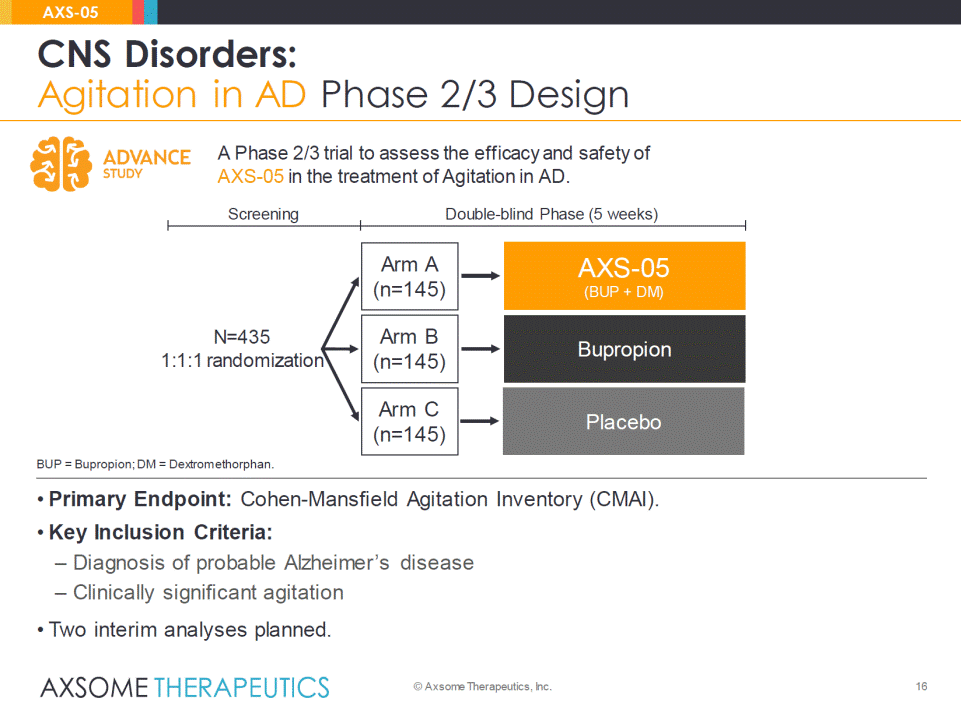
• Primary Endpoint: Cohen-Mansfield Agitation Inventory (CMAI). • Key Inclusion Criteria: – Diagnosis of probable Alzheimer’s disease – Clinically significant agitation • Two interim analyses planned. 16 CNS Disorders: Agitation in AD Phase 2/3 Design AXS-05 © Axsome Therapeutics, Inc. BUP = Bupropion; DM = Dextromethorphan. A Phase 2/3 trial to assess the efficacy and safety of AXS-05 in the treatment of Agitation in AD. N=435 1:1:1 randomization Placebo AXS-05 (BUP + DM) Arm A(n=145) Arm C(n=145) Double-blind Phase (5 weeks) Screening Bupropion Arm B(n=145)
• Smoking is single largest cause of preventable death in the U.S.1 • 70% of smokers want to quit and only 3-5% who attempt to quit without assistance are successful for 6-12 months.2 • DM component of AXS-05 significantly reduced nicotine self-administration in nicotine-dependent rats. • Bupropion component of AXS-05 has been found to be effective for smoking cessation in clinical trials. • Axsomeentered into a research collaboration with Duke University to evaluate AXS-05 in a Phase 2 clinical trial in smokers attempting to quit. • Phase 2 controlled trial initiation anticipated in 1Q 2018. 17 CNS Disorders: Smoking Cessation Overview © Axsome Therapeutics, Inc. AXS-05 1. U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. 2014 . 2. Hughes JR, et al. Addiction. 2004;99(1):29-38 . 40M patients in the U.S.1 Product Candidate Preclinical Phase 1 Phase 2 Phase 3 AXS-05 (DM + BUP) Smoking Cessation Duke University Collaboration Abbreviations: DM = Dextromethorphan; BUP = Bupropion.
Novel therapy for chronic pain: • Knee Osteoarthritis (OA) with Bone Marrow Lesions (BMLs) • Chronic Low Back Pain (CLBP) with ModicChanges (MCs) © Axsome Therapeutics, Inc. 18 Disodium Zoledronate Tetrahydrate AXS-02

IP Overview • 75 issued patents* –protection through 2034. • Drug delivery, pharmacokinetic, composition of matter, and method of use claims. 19 Chronic Pain: Differentiated Therapy © Axsome Therapeutics, Inc. AXS-02 AXS-02 Disodium Zoledronate Tetrahydrate Oral Dose Long- acting Targeted Therapy Non- opioid NovelMechanism *Claims cover AXS-02 and related substances and disease indications.
Decreased bone resorption. Farnesyl pyrophosphate synthase (FPPS).1 ASIC1a expression. ASIC activation.2 TRPV1 activation.2,3 TNFproduction.4 IL-6 production.5 IL-8 production.6 Angiogenesis.7 c-FOS expression.2 20 Chronic Pain: Therapy via Multiple Mechanisms of Action © Axsome Therapeutics, Inc. AXS-02 AXS-02 Disodium Zoledronate Tetrahydrate Inhibits bone-resorbing osteoclasts Downregulates acid-sensing* ion channels Reduces pro-inflammatory cytokine production Anti-angiogenic * Acid is a well known cause of pain. 1. Green JR, Rogers MJ. Drug Dev Res. 2002;55:210-24. 2.Nagae M, et al. Bone.2006;39:1107-15. 3.Abe Y, et al. J Bone Miner Metab.2015;33:125–134. 4.Wolf AM, et al. Haematologica. 2006;91:1165-71. 5.Derenne S, et al. Bone Miner Res. 1999;14:2048-56. 6.Stathopoulos GT, et al. Am J Respir Crit Care Med. 2008;178:50-9. 7.Misso G, et al. Cancer Biol Ther.2012;13:1491-500.
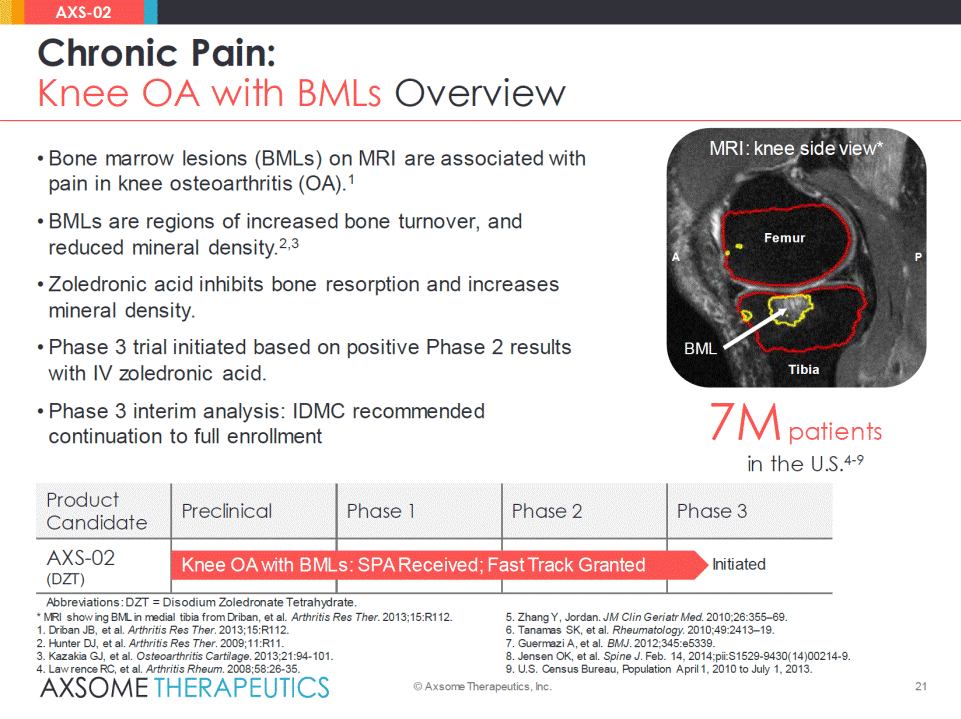
Product Candidate Preclinical Phase 1 Phase 2 Phase 3 AXS-02 (DZT) Knee OA with BMLs: SPA Received; Fast Track Granted 7M patients in the U.S.4-9 BML MRI: knee side view* Femur Tibia A P • Bone marrow lesions (BMLs) on MRI are associated with pain in knee osteoarthritis (OA).1 • BMLs are regions of increased bone turnover, and reduced mineral density.2,3 • Zoledronic acid inhibits bone resorption and increases mineral density. • Phase 3 trial initiated based on positive Phase 2 results with IV zoledronicacid. • Phase 3 interim analysis: IDMC recommended continuation to full enrollment 21 Chronic Pain: Knee OA with BMLs Overview © Axsome Therapeutics, Inc. AXS-02 * MRI show ing BML in medial tibia from Driban, et al. Arthritis Res Ther. 2013;15:R112. 1. Driban JB, et al. Arthritis Res Ther. 2013;15:R112. 2. Hunter DJ, et al. Arthritis Res Ther. 2009;11:R11. 3. Kazakia GJ, et al. Osteoarthritis Cartilage. 2013;21:94-101. 4. Law rence RC, et al. Arthritis Rheum. 2008;58:26-35. 5. Zhang Y, Jordan. JM Clin Geriatr Med. 2010;26:355–69. 6. Tanamas SK, et al. Rheumatology. 2010;49:2413–19. 7. Guermazi A, et al. BMJ. 2012;345:e5339. 8. Jensen OK, et al. Spine J. Feb. 14, 2014;pii:S1529-9430(14)00214-9. 9. U.S. Census Bureau, Population April 1, 2010 to July 1, 2013. Initiated Abbreviations: DZT = Disodium Zoledronate Tetrahydrate.
• Primary Endpoint: Change in pain intensity from baseline to week 24, measured using the 0-10 Numerical Rating Scale (NRS). • Key Inclusion Criteria: – Male at least 50 years of age or postmenopausal female, with knee OA and BMLs – Moderate or worse knee pain • Dosage: Once per week for six weeks; no drug for remainder of double-blind phase. 22 Chronic Pain: Knee OA with BMLs Phase 3 Design © Axsome Therapeutics, Inc. AXS-02 N=346 1:1 randomization Placebo AXS-02 (disodium zoledronate tetrahydrate) Arm A(n=173) Arm B(n=173) Double-blind Phase (24 weeks) Screening, Baseline A Phase 3 trial to assess the efficacy and safety ofAXS-02in the treatment of pain of knee OA associated with BMLs. Special Protocol Assessment (SPA) received Clinical Knee OASymptom Treatment 1 Study
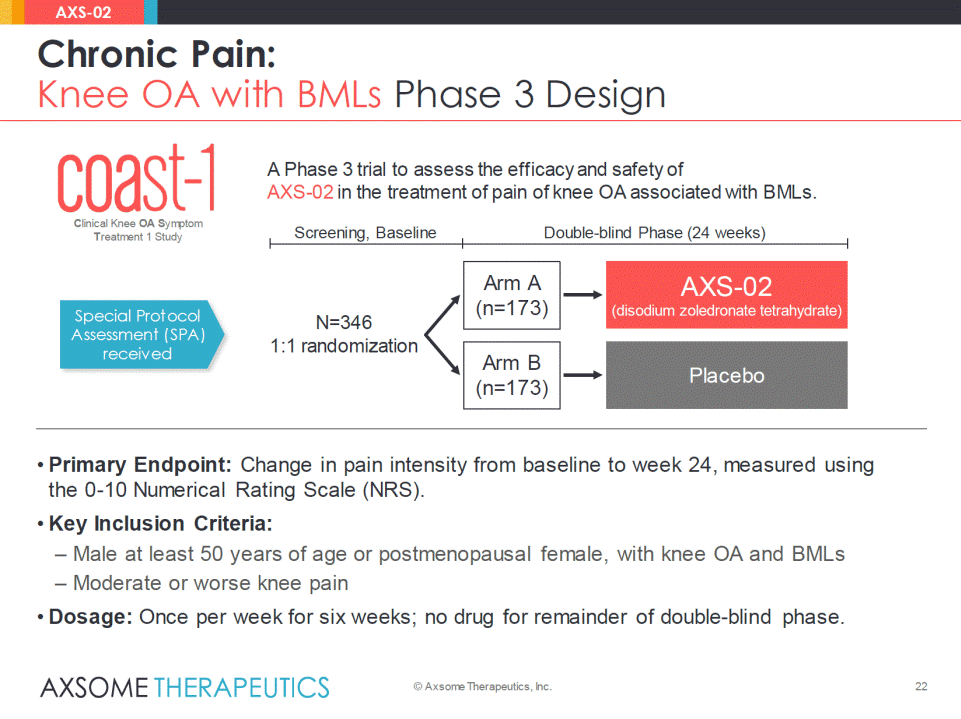
Product Candidate Preclinical Phase 1 Phase 2 Phase 3 AXS-02 (DZT) CLBP with MCs 1.6M patients in the U.S.4-7 • Modic changes (MCs) type 1 (M1) on MRI are associated withchronic low back pain (CLBP).1 • Increased bone turnover on bone scan is seen in M1 lesions.2 • Increased pro-inflammatory cytokines, and vascular densityseen in M1 lesions.3 • Zoledronic acid reduces bone turnover, suppresses the production of inflammatory mediators, and is anti-angiogenic. • Phase 2 results: Zoledronic acid reduced pain in patientswith CLBP. • FDA clearance received for IND for Phase 3 trial –initiation planned following readouts from CREATE-1 and STRIDE-1. • Issued U.S. patents: protection into 2034 –uses of oral zoledronic acid for low back pain. 23 Chronic Pain: CLBP with MCs Overview © Axsome Therapeutics, Inc. AXS-02 * MRI show ing modic type 1 lesions from Luoma K, et al. European Congress of Radiology (ECR). 2014;Poster B-0458. 1. Zhang Y, et al. Eur Spine J. 2008;17:1289-1299. 2. Järvinen J, et al. Spine: ISSLS Society Meeting Abstracts. Oct. 2011;Volume Suppl, Abstract GP127. 3. Rahme R, Moussa R. Am J Neuroradiol. 2008;29:838–42. 4. Law rence RC, et al. Arthritis Rheum. 2008;58:26-35. 5. Zhang Y, Jordan. JM Clin Geriatr Med. 2010;26:355–69. 6. Jensen OK, et al. Spine J. Feb. 14, 2014;pii:S1529-9430(14)00214-9. 7. U.S. Census Bureau, Population April 1, 2010 to July 1, 2013. A P Disc M1 M1 MRI: lumbar side view* Abbreviations: DZT = Disodium Zoledronate Tetrahydrate.
Novel therapies: • AXS-07 –Migraine • AXS-06 –OA and RA MoSEIC™ Meloxicam © AxsomeTherapeutics, Inc. 24
Migraine, OA and RA: MoSEIC™ Meloxicam Overview MoSEIC™ • MoSEICmeloxicam is a potent, oral, rapidly-absorbed, once-daily, non-opioid, COX-2 preferential, pain therapeutic. •Standard meloxicam has an extended Tmax(4-6 hours) which delays its onset of action.1,2 •Axsome’sMoSEIC(Molecular Solubility Enhanced Inclusion Complex) technology substantially increases the rate of absorption of meloxicam while maintaining its approximately 20-hour half-life. •Phase 1 results: 9 times faster Tmax, higher Cmaxand similar half-life, compared to Mobic®. •Potential utility for migraine, and the signs and symptoms of OA and RA. •AXS-07 is a fixed-dose combination of MoSEICmeloxicam and rizatriptan. •AXS-06 is a fixed-dose combination of MoSEICmeloxicam and esomeprazole (to reduce risk of NSAID-associated ulcers). 1. Mobic® (meloxicam) FDA Package Insert. 2. Euller-Ziegler et al., InflammRes 50, Supplement 1 (2001) S5–S9. IP Overview •1 issued patent –protection through 2036. •Pharmacokinetic patents •14 pending U.S. and international applications. © AxsomeTherapeutics, Inc. 25
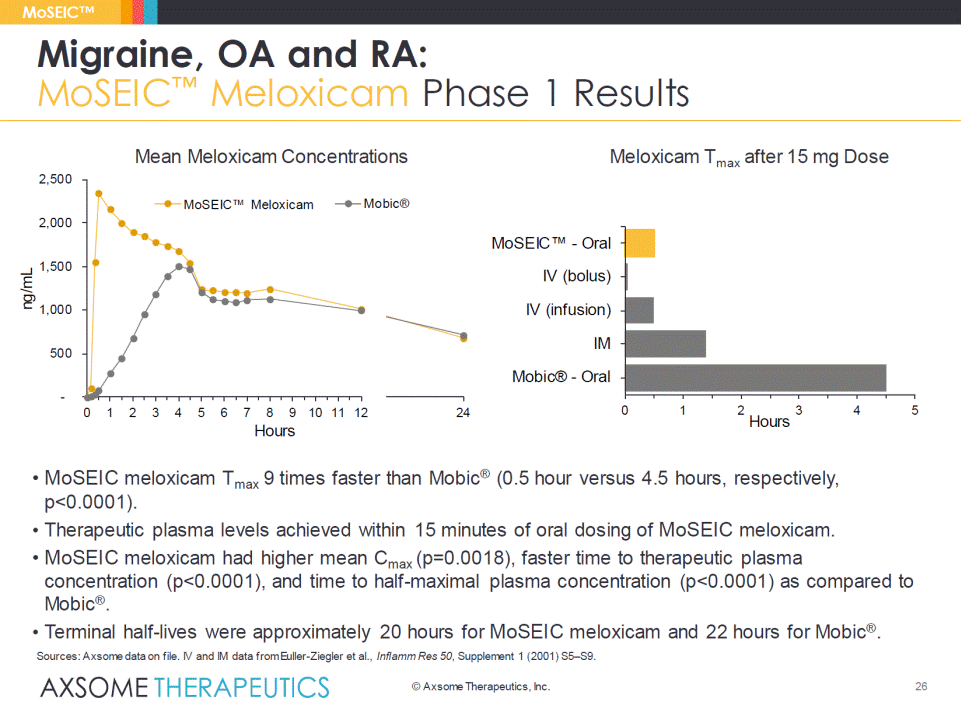
• MoSEICmeloxicam Tmax9 times faster than Mobic®(0.5 hour versus 4.5 hours, respectively, p<0.0001). • Therapeutic plasma levels achieved within 15 minutes of oral dosing of MoSEICmeloxicam. • MoSEICmeloxicam had higher mean Cmax(p=0.0018), faster time to therapeutic plasma concentration (p<0.0001), and time to half-maximal plasma concentration (p<0.0001) as compared to Mobic®. • Terminal half-lives were approximately 20 hours for MoSEICmeloxicam and 22 hours for Mobic®. Migraine, OA and RA: MoSEIC™ Meloxicam Phase 1 Results MoSEIC™ - 500 1,000 1,500 2,000 2,500 0 1 2 3 4 5 6 7 8 9 10 11 12 ng/mL Hours MoSEIC™ Meloxicam Mobic® 14 24 Sources: Axsomedata on file. IV and IM data from Euller-Ziegler et al., InflammRes 50, Supplement 1 (2001) S5–S9. 0 1 2 3 4 5 Mobic® - Oral IM IV (infusion) IV (bolus) MoSEIC™ -Oral Hours Mean Meloxicam Concentrations Meloxicam Tmaxafter 15 mg Dose © AxsomeTherapeutics, Inc. 26
• Meloxicam is a new molecule for migraine—not currently approved or used for this indication due to prolonged Tmax • MoSEICdelivery enables its use in abortive treatment of migraine – Rapid Tmaxof MoSEICmeloxicam is ideal for migraine treatment – Extended half-life of MoSEICmeloxicam should lead to lower symptom recurrence • AXS-07 combines unique PK of MoSEICmeloxicam with proven efficacy of rizatriptan • FDA Pre-IND written guidance received • Phase 3 initiation anticipated in 2018 AXS-07: MoSEIC™ Meloxicam + Rizatriptan for Migraine MoSEIC™ 37M patients in the U.S.1 Product Candidate Preclinical Phase 1 Phase 2 Phase 3 AXS-07 (MoSEIC™ Mx + Riz) Abbreviations: Mx= Meloxicam; Riz= Rizatriptan. Migraine 1. PleisJR, et al., Summary health statistics for U.S. adults: National Health Interview Survey, 2009. National Center for Health Statistics. Vital Health Stat 10(249). 2010. © AxsomeTherapeutics, Inc. 27
• Rapid absorption and onset of action – Based on rapid absorption of MoSEICmeloxicam and expected additive effect of AXS-07 components • Strong and consistent pain relief – Potential for superior efficacy as compared to current treatments based on expected additive effect of AXS-07 components • Sustained pain relief – Based on extended MoSEICmeloxicam half-life and expected additive effect of AXS-07 components • Pharmacoeconomicbenefits – Potentially superior efficacy expected to result in reduced use of medication and medical services, reduced absenteeism and loss of productivity AXS-07: Differentiated Clinical Profile for Migraine MoSEIC™ © AxsomeTherapeutics, Inc. 28

AXS-06: MoSEIC™ Meloxicam + Esomeprazole for OA and RA MoSEIC™ • AXS-06 is a fixed-dose combination of MoSEIC™ meloxicam and esomeprazole •Being developed to treat OA and RA, and to reduce the risk of NSAID-associated upper GI ulcers •Potentially best-in-class NSAID profile: –Oral administration with IV-like onset of action –Long half-life for sustained effect and once-daily dosing –Improved GI safety from esomeprazole component •Positive Phase 1 results: therapeutic meloxicam concentrations within 15 mins, gastroprotectiveesomeprazole concentrations •FDA Pre-IND written guidance received •AXS-06 is Phase 3-ready 120M NSAID TRx per year in the U.S. Product Candidate Preclinical Phase 1 Phase 2 Phase 3 AXS-06 (MoSEIC™ Mx + Eso) Abbreviations: Eso= Esomeprazole; Mx= Meloxicam; OA = Osteoarthritis; RA = Rheumatoid Arthritis. Phase 3 ready OA and RA © AxsomeTherapeutics, Inc. 29
30 Corporate © Axsome Therapeutics, Inc. Barriers to Entry *Claims cover AXS-02 and related substances and disease indications. MoSEIC™ Meloxicam (AXS-07, AXS-06) AXS-05 AXS-02 23 Issued U.S. Patents*1 Issued O-U.S. Patent Claims extending to 2034 >40 pending Proprietary Manufacturing Drug Product Formulation 1 issued U.S. PatentClaims extending to 2036>10 pending Proprietary Manufacturing Drug Product Formulation Proprietary Manufacturing API Synthesis 66 Issued U.S. Patents* 9 Issued O-U.S. Patents Claims extending to 2034 >60 pending

Management Herriot Tabuteau, MDFounder & CEO John Golubieski, MBA CFO Cedric O’Gorman, MD, MBASVP, Clinical Development & Medical Affairs Mark Jacobson, MASVP, Operations Robert Niecestro, PhDVP, Clinical & Regulatory 31 Our Team © Axsome Therapeutics, Inc. Corporate Board of Directors Roger Jeffs, PhD Former President, Co-CEO, Director United Therapeutics Corp. Prior positions at Amgen and Burroughs Wellcome Myrtle Potter Former President, COO Genentech Prior positions at Bristol-Myers Squibb and Merck Mark Saad Former CFO Bird Rock Bio, Inc. Former COO of the Global Healthcare Group at UBS Mark Coleman, MD Medical Director National Spine and Pain Centers Diplomat of the American Board of Anesthesiology Herriot Tabuteau, MD Chairman

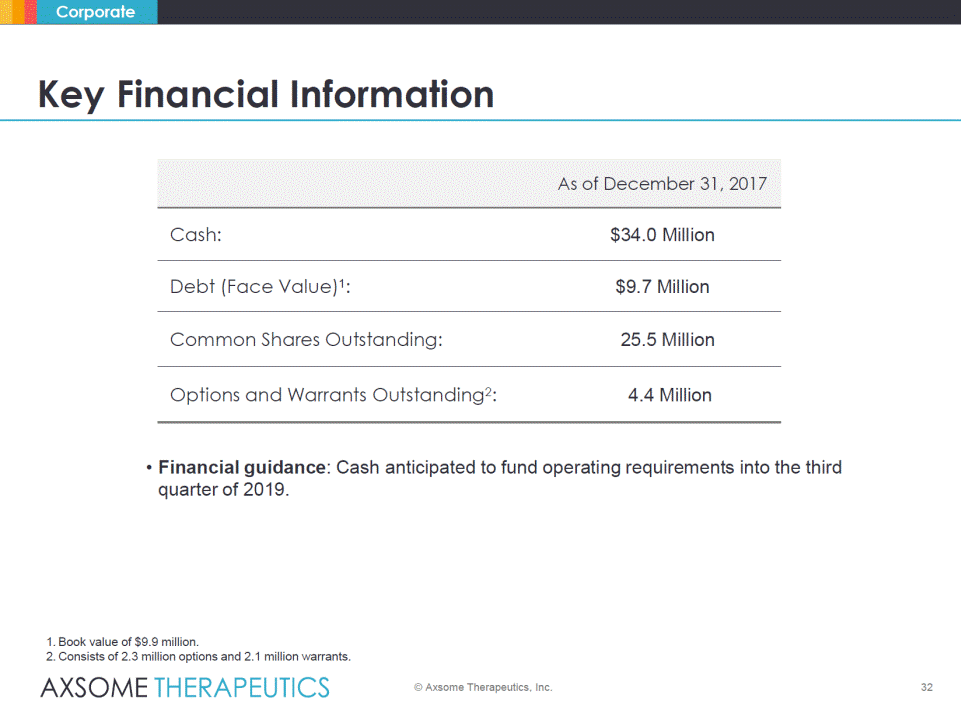
Key Financial Information As of December 31, 2017 Cash: $34.0 Million Debt (Face Value) 1: $9.7 Million Common Shares Outstanding: 25.5 Million Options and Warrants Outstanding2: 4.4 Million • Financial guidance: Cash anticipated to fund operating requirements into the third quarter of 2019.1. Book value of $9.9million. 2.Consists of 2.3 million options and 2.1 million warrants © Axsome Therapeutics, Inc. 32
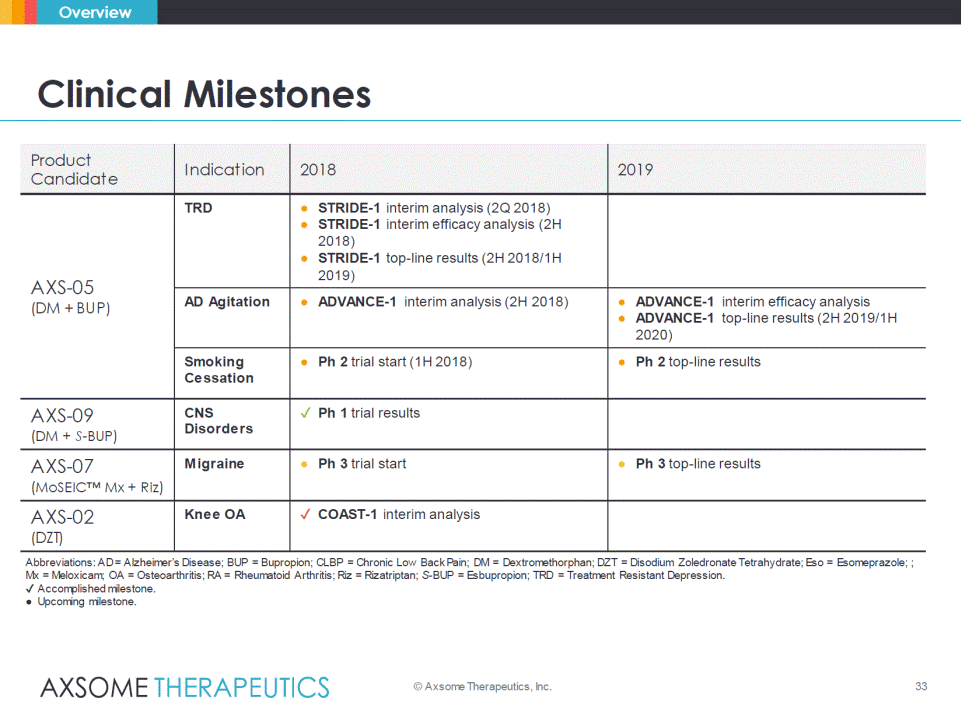
33 Clinical Milestones © Axsome Therapeutics, Inc. Overview Product Candidate Indication 2018 2019 AXS-05 (DM + BUP) TRD • STRIDE-1 interim analysis (2Q 2018) • STRIDE-1 interim efficacy analysis (2H 2018) • STRIDE-1 top-line results (2H 2018/1H 2019) AD Agitation • ADVANCE-1 interim analysis (2H 2018) • ADVANCE-1 interim efficacy analysis • ADVANCE-1 top-line results (2H 2019/1H 2020) Smoking Cessation • Ph 2 trial start (1H 2018) • Ph 2 top-line results AXS-09 (DM + S-BUP) CNS Disorders Ph 1 trial results AXS-07 (MoSEIC™ Mx + Riz) Migraine • Ph 3 trial start • Ph 3 top-line results AXS-02 (DZT) Knee OA COAST-1 interim analysis Abbreviations: AD = Alzheimer’s Disease; BUP = Bupropion; CLBP = Chronic Low Back Pain; DM = Dextromethorphan; DZT = Disodium Zoledronate Tetrahydrate; Eso = Esomeprazole; ; Mx = Meloxicam; OA = Osteoarthritis; RA = Rheumatoid Arthritis; Riz = Rizatriptan; S-BUP = Esbupropion; TRD = Treatment Resistant Depression. Accomplished milestone. • Upcoming milestone.
For more information, please contact Mark Jacobson SVP, Operations 212-332-3243 mjacobson@Axsome.com axsome.com


