Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - Nuvectra Corp | ex_106475.htm |
| EX-31.2 - EXHIBIT 31.2 - Nuvectra Corp | ex_106474.htm |
| EX-31.1 - EXHIBIT 31.1 - Nuvectra Corp | ex_106473.htm |
| EX-23.1 - EXHIBIT 23.1 - Nuvectra Corp | ex_106472.htm |
| EX-21.1 - EXHIBIT 21.1 - Nuvectra Corp | ex_106471.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
☒ |
Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. For the fiscal year ended December 31, 2017. |
|
☐ |
Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. For the transition period from __________ to __________ |
Commission File No. 001-37525
Nuvectra Corporation
(Exact name of registrant as specified in its charter)
|
Delaware |
30-0513847 |
|
|
(Jurisdiction of incorporation) |
(I.R.S. Employer Identification No.) |
5830 Granite Parkway, Suite 1100,
Plano, Texas 75024
(Address of principal executive office)
(214) 474-3103
(Registrant's telephone number)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Name of each exchange on which registered |
|
Common Stock, par value $0.001 per share |
NASDAQ Global Market |
Securities registered pursuant to section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐ Accelerated filer ☐ Non-accelerated filer ☐ Smaller reporting company ☒Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Aggregate market value of voting and non-voting common equity of Nuvectra Corporation held by non-affiliates of the registrant as of June 30, 2017, based on the closing price of $13.28, as reported on the NASDAQ Global Market: approximately $134.2 million. Number of Common Stock shares outstanding on February 21, 2018: 14,109,967
DOCUMENTS INCORPORATED BY REFERENCE
Portions of Registrant’s Definitive Proxy Statement to be filed with the Securities and Exchange Commission in connection with its Annual Meeting of Stockholders to be held in 2018 are incorporated by reference into Part III of this Form 10-K.
|
|
||||
|
|
|
|
|
|
|
Item |
|
Description |
|
Page |
|
|
|
|
|
|
|
|
|
|
|
|
|
1. |
|
|
2 |
|
|
1A. |
|
|
17 |
|
|
1B. |
|
|
34 |
|
|
2. |
|
|
34 |
|
|
3. |
|
|
34 |
|
|
4. |
|
|
34 |
|
|
|
|
|
||
|
5. |
|
|
35 |
|
|
6. |
|
|
38 |
|
|
7. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
38 |
|
7A. |
|
|
48 |
|
|
8. |
|
|
49 |
|
|
9. |
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
|
77 |
|
9A. |
|
|
77 |
|
|
9B. |
|
|
77 |
|
|
|
|
|
||
|
10. |
|
|
78 |
|
|
11. |
|
|
78 |
|
|
12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
78 |
|
13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
78 |
|
14. |
|
|
78 |
|
|
|
|
|
||
|
15. |
|
|
79 |
|
|
|
|
|
82 |
|
Our History
Following its spin-off from Integer Holdings Corporation, formerly known as Greatbatch, Inc. (“Integer”), Nuvectra Corporation (“Nuvectra,” the “Company,” “we,” “us,” or “our”) became an independent, publicly-traded company as of March 14, 2016 and our common stock began trading on the NASDAQ Global Market (“NASDAQ”). Prior to the spin-off, we were a wholly-owned subsidiary of Integer. Nuvectra was initially formed as a limited liability company in Delaware on November 14, 2008 and was subsequently named QiG Group, LLC. In connection with the spin-off, QiG Group, LLC converted into a Delaware corporation and changed its name to Nuvectra Corporation. The shares of another Integer subsidiary, NeuroNexus Technologies, Inc., a Michigan corporation (“NeuroNexus”), were transferred to Nuvectra as part of the spin-off transaction and it became a wholly-owned subsidiary of Nuvectra.
Our Mission
Our mission is to help physicians improve the lives of people with chronic conditions through life-enhancing products and services.
Overview of Business
Nuvectra is a neuromodulation medical device company focused on the development and commercialization of our neurostimulation technology platform for the treatment of various disorders through stimulation of tissues associated with the nervous system. Our neurostimulation technology platform has the capability to provide treatment to patients in several established neurostimulation markets, including spinal cord stimulation (“SCS”), sacral neuromodulation (“SNM”), deep brain stimulation (“DBS”), and other emerging neurostimulation markets. Our Algovita® SCS system, or Algovita, is the first application of our neurostimulation technology platform and is indicated for the treatment of chronic pain of the trunk and/or limbs. Algovita received premarket approval from the FDA in November 2015, and we commercially launched Algovita in the United States (“U.S.”) during the first half of 2016. Outside of the United States, Algovita obtained CE mark approval in June 2014 and is indicated for the treatment of chronic intractable pain of the trunk or limbs. Algovita is reimbursable under existing SCS codes in the United States, the European Union (“EU”) and Australia, and has been commercially available to patients in Germany and several other European countries since November 2014.
We believe Algovita brings to market a user friendly, robust and flexible design with a broad set of product capabilities and advanced technology and is well positioned to compete in and help grow the existing SCS market, currently estimated at approximately $2.0 billion globally. In addition, we believe our neurostimulation technology platform is well positioned to compete in the SCS, SNM and DBS portions of the worldwide neurostimulation market, currently estimated at approximately $3.4 billion combined.
We have also developed our existing platform for use in the SNM market and have filed regulatory submissions with the FDA and CE mark authorities in January 2017 and December 2016, respectively, for Virtis™, the Company’s SNM system for the treatment of overactive bladder. In addition, in early 2016, we entered into a development agreement with Aleva Neurotherapeutics S.A. (“Aleva”), which was amended and restated on August 31, 2017, to develop our neurostimulation technology platform into a complete medical device for use in the DBS market for treatment of Parkinson’s disease and Essential Tremor. This platform is still under development.
Our NeuroNexus subsidiary is the neuroscience and clinical research portion of our business. NeuroNexus works closely with researchers to develop and refine new tools that aid and advance neuroscience research. NeuroNexus designs, manufactures and sells neural interface technologies including high quality, high density microelectrode arrays, custom designed probes, electrode instrumentation and accessories. In addition, the NeuroNexus team possesses years of neuroscience research experience to help facilitate successful research projects and provide insight to minimize known challenges.
Market Overview
The neurostimulation market is comprised of multiple individual markets each focused on the treatment of various indications through delivery of electrical stimulation to a targeted site of the body such as SCS, SNM and DBS. We estimate the combined SCS, SNM, and DBS market size at $3.4 billion in 2017, growing at an estimated 10% compound annual growth rate through 2020. We compete in the SCS market with our Algovita product. We intend to compete in the SNM market with our Virtis product, which is based on our neurostimulation technology platform and is currently in the regulatory review and approval process in the United States and Europe. In addition, in early 2016, we entered into a development agreement with Aleva, which was amended and restated on August 31, 2017. Through this agreement, we are leveraging our neurostimulation technology platform to develop a DBS system for Aleva to treat Parkinson’s disease and Essential Tremor. If we complete development of a DBS system to treat Parkinson’s disease and Essential Tremor for Aleva, we expect that Aleva will continue to commercialize the DBS system. If it does so and is successful, we would receive royalties on the sale of these DBS systems and components. There are additional and emerging neurostimulation markets that we may compete in with future products.
Spinal Cord Stimulation
SCS therapy has been used to treat chronic pain for over 40 years, and is indicated as a treatment option for chronic pain patients who have typically not achieved relief through pharmacological or conventional medical management. SCS therapy operates by delivering electrical signals to the spinal cord through thin wires called leads, which are placed near the spinal cord and are energized by a small battery-powered implantable pulse generator (“IPG”) implanted under the skin. Electrodes located at the end of the leads deliver electrical signals to the spinal cord. These electrical signals “override” the pain signals being sent to the brain resulting in relief for the patient.
Approximately 1.5 billion people worldwide and 100 million adults in the United States suffer from some form of chronic pain. Chronic pain can lead to reduced quality of life, increased incidence of depression and sleep deprivation. In the United States, chronic pain results in an estimated incremental cost of health care of approximately $300 billion per year.
According to market research and our internal estimates, in 2017, the size of the worldwide SCS market was estimated at approximately $2.0 billion, with approximately 75% of that market located in the United States. We believe the smaller market opportunity outside the United States is primarily the result of restrictions on procedure reimbursement. The worldwide size of the SCS market is projected to grow to an estimated $2.7 billion by 2020, an 11% compound annual growth rate, supported by additional penetration of the therapy in established markets, a growing base of physician implanters and increasing acceptance of SCS therapy as an effective and viable treatment option in emerging markets.
We believe SCS therapies have penetrated less than 10% of the potential United States market. We believe the following factors have limited market adoption of SCS therapies in the United States:
|
• |
Challenges in sustaining long-term pain therapy. SCS therapy historically has experienced challenges maintaining long-term effectiveness due to the limitations of existing systems and the nature of chronic pain. Historically, SCS systems have been prone to early therapy failures as a result of device malfunction and lead and extension breakage. SCS systems are also challenged by the dynamic nature of chronic pain, which can increase in intensity or spread to other areas in the body, and an inability to adjust the system to respond to changes in patient needs such as the need to deliver additional power to cover new pain locations. |
|
• |
Existing SCS devices are complicated and not user friendly. Most existing SCS systems on the market are a continuation of legacy designs. These systems, whether pre-operatively, intra-operatively or during long-term pain management, are generally difficult to use. Market research confirms that physicians and patients both want devices that are easier to use. Patients not only want effective pain relief and ease of use, but also want discreet and comfortable systems. |
|
• |
Lack of market awareness of successful SCS therapies. We believe the SCS market is under-penetrated and the patient population is under-served. We believe this results from a lack of awareness by patients and physicians of SCS therapies and their potential benefits, despite four decades of use. We believe referring physicians are generally unaware of recent advances in efficacy of SCS therapies and, in many cases, are unwilling to refer patients to physicians that specialize in chronic pain and the use of SCS therapies. |
Sacral Neuromodulation
SNM is a well-established treatment option for refractory symptoms of overactive bladder, including urinary frequency and/or urgency, with or without urge incontinence, and chronic fecal incontinence. Approved by the FDA in 1997 for initial indications of urinary frequency/urgency and urge incontinence, the American Urologic Association, or the AUA, includes the therapy in its treatment guidelines as a “third line” option to be considered after failure of first (behavioral) and second (drug) line options. According to the International Continence Society, there are approximately 400 million people worldwide who suffer from symptoms of urinary and fecal incontinence.
SNM involves sending mild electrical pulses to the sacral nerve, typically sacral spinal nerve S3, through a lead connected to an IPG, similar to the therapy provided by a pacemaker. The impulses modulate the reflexes between the pelvic floor, urethral sphincter, bladder and bowel. SNM helps the brain and nerves to communicate so that the bladder and related muscles can function properly. An advantage of SNM as compared to other potential therapies is that it is tested and evaluated by the patient and physician prior to long-term therapeutic use. This evaluation period gives patients and physicians an opportunity to determine whether adequate symptom relief is achievable, often in as few as three to seven days. Implantation of the SNM device is a minimally invasive procedure performed on an outpatient basis under sedation or general anesthesia, and lead placement is generally considered easier to perform than lead placement in SCS cases.
According to market research and our internal estimates, the worldwide SNM market in 2017 was estimated at $690 million and is expected to grow to $870 million by 2020, an 8% compound annual growth rate. SNM is the second most commonly performed neurostimulation therapy behind SCS with over 225,000 SNM devices implanted for overactive bladder since 1994. Currently, there is only one FDA-approved implantable SNM device available on the market in the United States.
Deep Brain Stimulation
DBS uses mild electrical pulses from leads connected to an IPG to stimulate specific targets in the brain. These pulses either inhibit or stimulate nerve signals, thereby offering relief for certain neurological conditions, which include movement and psychiatric disorders. Currently, the FDA has approved certain DBS devices for the treatment of Parkinson’s disease and Essential Tremor. An estimated one million people in the United States and between seven to ten million people worldwide suffer from Parkinson’s disease and over ten million people in the United States suffer from Essential Tremor. The FDA has also approved certain DBS devices for treatment of dystonia and obsessive-compulsive disorders under a humanitarian device exemption. DBS is also currently being investigated as a therapy for other neurological disorders, such as epilepsy, treatment-resistant major depression and Alzheimer’s disease.
According to market research and our internal estimates, the worldwide DBS market in 2017 was estimated at $650 million and is expected to grow to $850 million by 2020, a 9% compound annual growth rate. DBS is the third most commonly performed neurostimulation therapy behind SCS and SNM, with over 150,000 DBS devices implanted for Parkinson’s disease, Essential Tremor and dystonia since 1995. We expect that the DBS worldwide market will likely continue to experience high growth due to an increasingly aging population and an increase in neurodegenerative disorders.
We believe our multi-current neurostimulation technology platform may provide distinct advantages in providing DBS therapies where specific electrical field control and nerve selectivity can be very important. Our neurostimulation technology platform, in combination with new concepts in DBS lead design, may provide new benefits in DBS therapy delivery. In early 2016, we entered into a development agreement with Aleva, which was amended and restated on August 31, 2017. Through this agreement, we are leveraging our neurostimulation technology platform to develop a DBS system for Aleva to treat Parkinson’s disease and Essential Tremor. If we complete development of a DBS system to treat Parkinson’s disease and Essential Tremor for Aleva, we expect that Aleva will continue to commercialize the DBS system. If it does so and is successful, we would receive royalties on the sale of these DBS systems and components.
Additional and Emerging Indications
There are other established and emerging neurostimulation indications that may be a source of potential opportunity for Nuvectra and our neurostimulation technology platform. We believe we may be able to leverage our neurostimulation technology platform to capitalize on opportunities in indications such as Vagal Nerve Stimulation (“VNS”) and Peripheral Nerve Stimulation (“PNS”). VNS is approved for the treatment of epilepsy, depression and eating disorders. Research is ongoing for the use of VNS in the treatment of heart failure and rheumatoid arthritis. PNS is approved outside the United States for treatment of chronic pain. PNS is also an emerging approach to treat chronic headaches and post-amputation “phantom limb” pain. We are not yet commercially engaged in either of these indications.
Our Neurostimulation Technology Platform
Our neurostimulation technology platform was developed to provide the most innovative capabilities currently available on the market and to provide physicians and patients with improved solutions and tailored treatment options. Our platform is fundamental to the design of Algovita and provides the foundation for the development of future products. The key elements of our platform include:
|
• |
Innovative core technology. Our neurostimulation technology platform consists of core technology developed using our advanced engineering and design capabilities in IPGs, independent current sources, algorithmic programming, chipsets and leads. We own the core patents and patent applications that embody the intellectual property underlying our neurostimulation technology platform, and we also license certain other patents related to our leads and for potential SNM and DBS applications. |
|
• |
Durable, stretchable and flexible leads. Our leads feature coil-in-coil technology designed to allow our leads to stretch and to improve lead durability and flexibility, thereby reducing migration, breakage and kinking. In addition, the coil-in-coil design enhances steerability as compared to the straight wire lead designs used by many existing neurostimulation systems. |
|
• |
Advanced programmability. The algorithmic driven technologies in our platform are designed to allow physicians to program Algovita and other products incorporating our platform for rapid and sequential delivery of multiple stimulation programs. These products are capable of capturing feedback from patients, and thereby providing physicians and patients with the flexibility to select from a number of different stimulation programs and optimize treatment. |
|
• |
Multiple independent current sources. Our neurostimulation technology platform is capable of delivering multiple independent current sources that optimize current delivery and improve field control allowing for finer resolution and precision of therapy. |
|
• |
Unique safety features. Our neurostimulation technology platform was designed with unique safety features. The IPG has a deep discharge recovery battery (the patient can recharge even if the battery is exhausted), bi-directional recharge (the IPG can be recharged even if it flips over inside the patient’s body) and impedance checks to improve patient safety. The patient remote control indicates the battery status of the IPG, is paired to a single IPG, has quick “stim-off” functionality that permits immediate cessation of treatment and incorporates a patient feedback tool to encourage greater patient input thus improving safety. |
|
• |
Future offering capabilities. Our neurostimulation technology platform incorporates a proprietary chipset and hardware that is capable of being configured for use in next generation treatment offerings for Algovita and in other future neurostimulation systems. It is capable of delivering significantly higher frequencies than most other SCS systems presently available on the market, as well as pulse train stimulation and customized waveforms. |
Our Products – The Algovita System
Algovita delivers SCS therapy for the treatment of chronic pain. Algovita is based on our neurostimulation technology platform and contains what we believe are innovative capabilities as compared to other products currently available on the market. Algovita was developed to improve on existing SCS designs and utilizes new technologies to improve the patient’s and implanting physician’s experience, system robustness and overall treatment outcomes. Algovita was designed to permit physicians to implant the leads and the IPG efficiently and patients to operate the device easily. To this end, Algovita has straightforward controls and an interactive display that includes a stimulation diagram for quick visual confirmation of stimulation coverage.
Algovita obtained a CE mark and is currently available for sale in Germany and several other European countries. On November 30, 2015, Integer announced receipt of premarket approval for Algovita from the FDA. We launched Algovita commercially in the United States during the first half of 2016. In 2017, we filed regulatory submissions with FDA and CE mark authorities for full-body MRI Conditional approval for Algovita. We currently anticipate full-body MRI Conditional approval in the second quarter of 2018 for CE mark and in the fourth quarter of 2018 for FDA.
Algovita consists of the following components:
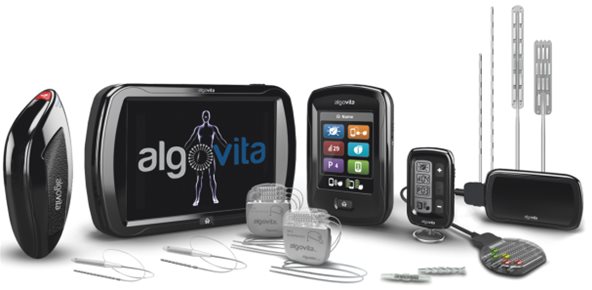
Implantable Pulse Generator: The IPG contains a rechargeable battery and electronics that deliver electrical pulses to the leads. The Algovita IPG has 26 output channels available in two different header configurations and can be connected to one, two or three leads. It is a programmable device and can deliver customized programs for each patient. The IPG is rechargeable and is surgically implanted under the skin, usually above the buttocks or in the abdomen.
Leads: The leads are thin, insulated wires that conduct electrical pulses to the spinal cord from the IPG. Algovita has both percutaneous and paddle leads that are inserted into the epidural space with a minimally invasive surgical procedure.
Patient Programmer: The patient programmer, called the Algovita Pocket Programmer, is a rechargeable, key fob-sized device that works like a remote control and allows patients to adjust their stimulation, change programs and monitor their stimulator battery charge levels.
Clinician Programmer: The clinician programmer contains proprietary software that allows customized programming of the IPG. It can non-invasively transmit a signal to the IPG, sending programming information and downloading diagnostic information. The Algovita programmer offers various 3D attributes, including virtual environment, pain mapping, stimulation mapping and stimulation overlap scores, which facilitate ease of use for clinicians.
Charger: The charger is a mobile device used to charge the IPG externally and to monitor the IPG battery charge levels. The patient can remain active while charging the IPG. Charging requirements depend on the patient’s power requirements.
Trial Stimulator: The trial stimulator contains electronics that deliver electrical pulses to the lead. It is a device that is worn externally during the evaluation period, which typically lasts several days.
Surgical Accessories: Algovita also contains accessories for implantation. These surgical accessories include components such as epidural needles, stylets, and lead anchors to assist the physician in the surgical procedure.
Our Competitive Strengths
We believe a number of competitive advantages distinguish us from our competitors:
|
• |
Differentiated neurostimulation technology platform. Our neurostimulation technology platform incorporates technological advances that we believe provide us with competitive advantages in the marketplace and provide meaningful benefits to both physicians and patients as compared to existing alternatives. The IPG component of our platform is capable of delivering a broad spectrum of outputs and pulse delivery ranges through its 26 independent current sources. The IPG also features a powerful chipset that enables new waveforms, stimulation outputs and embedded features that can be activated in the future. Our diverse lead portfolio provides additional capabilities for tailoring therapy to a wider spectrum of patients, and we believe our leads are easier to implant and steer than our competitors’ leads. |
|
• |
Broad range of Algovita capabilities. Algovita is based on our differentiated neurostimulation technology platform and features a broad range of technical capabilities, including 26 independent current sources, algorithmic programming, broad pulse delivery ranges and a powerful chip set for targeted SCS therapy delivery. We believe these capabilities provide Algovita with greater flexibility in tailoring therapy to a wider spectrum of SCS patients than the flexibility provided by the current generation of SCS systems that are presently available on the market. |
|
• |
Algovita’s robust design helps minimize therapy failures and enables greater control and precision in providing therapy. We believe Algovita’s robust design, including its leads and advanced programming features, help to minimize early SCS therapy failures and enable greater precision and control in targeting pain sites than the current generation of SCS systems that are presently available on the market. In addition, our advanced leads feature coil-in-coil technology, allowing for elasticity and greater flexibility than the leads of other SCS systems that are presently available on the market, which we believe results in a reduced likelihood of migration, breakage or kinking. Our 12-electrode lead provides the longest span of coverage available on the market and was designed to address loss of pain relief if the stimulation target changes. Additionally, our algorithmic driven clinician programming system allows for rapid localization of pain targets and use of many different stimulation programs. The stimulation field can also be further refined using direct patient inputs gathered through our patient feedback tool. |
|
• |
Algovita’s upgradeable technology enables next generation offerings. Algovita’s proprietary chip set and hardware is capable of being configured for use in next generation treatment offerings. This includes the ability to deliver significantly higher frequencies than most other SCS systems presently available on the market, as well as pulse train stimulation, including burst type stimulation, and customized waveforms. We believe these additional capabilities provide a strong base platform and system for potential new SCS and other treatment options that can be provided via a software or firmware upgrade. |
|
• |
Experienced management and engineering team with a track record of successful performance. Our team has a strong track record of successful performance and execution in the neurostimulation field. Collectively, our management team has over 100 years of combined experience in the neurostimulation and chronic pain industry. In addition, we have an experienced engineering team with significant expertise in designing and developing medical devices for the neurostimulation market. We believe physicians and customers value working with a team like ours comprised of highly skilled professionals who have in-depth knowledge of the industry, strong engineering and development capabilities and an understanding of the needs of both patients and physicians. |
Our Strategy
To achieve our objectives and capitalize on our competitive strengths, we are pursuing the following strategies:
|
• |
Expand our sales and marketing organization to drive adoption of Algovita. We will continue to build and scale our worldwide sales organization consisting of direct sales representatives and independent sales agents in the United States and a network of distributors and independent sales agents outside of the United States. Our direct sales representatives and independent sales agents in the United States target physician specialists involved with SCS treatment decisions located at strategic hospitals and outpatient surgery centers across the United States. Our marketing team offers education programs designed to create awareness and demand among other stakeholders involved in SCS treatment decisions, including third-party payers, hospital administrators and patients and their families. Internationally, specifically in the European Union, we will continue to expand our network of distributors and independent sales agents in target markets that we believe support SCS therapy and have strong reimbursement coverage. |
|
• |
Demonstrate the value of Algovita’s capabilities among surgeons, referring physicians and patients. Algovita was specifically designed to address the limitations of other currently available SCS technologies, which we believe has slowed adoption of SCS therapies. We are dedicating significant resources to demonstrate the value of Algovita’s broad capabilities, focusing on its ability to provide flexible treatment options for chronic pain patients. We are leveraging our growing sales force to promote awareness of Algovita by training and educating physicians, exhibiting at tradeshows and conducting focused advertising. |
|
• |
Invest in clinical and product development to drive product innovation. We are investing in clinical and product development to expand the capabilities of our neurostimulation technology platform. We expect this investment will result in further product innovations and expanded labeling and new indications for Algovita. These innovations are expected to include next generation IPG capabilities, additional lead offerings, MRI compatibility and advancements in algorithmic programming. We are also working to expand our product opportunities for our neurostimulation technology platform into other established neurostimulation markets, including SNM and DBS, and intend to invest in other emerging therapies in the future. We submitted a premarket approval application for Virtis to TÜV SÜD America and the FDA in December 2016 and January 2017, respectively. |
|
• |
Pursue strategic partnerships. We intend to pursue strategic partnerships to accelerate our expansion into other established neurostimulation markets. These strategic partnerships may partially or fully fund clinical and development costs for new products, expand our product distribution channels, improve our access to physicians and opinion leaders, supplement our product commercialization efforts, provide a partner that will perform or assist in performing clinical studies for new products, help us to add specialized clinical or regulatory expertise or provide access to or enable us to acquire complementary intellectual property. Our development agreement with Aleva is an example of this type of strategic partnership. |
|
• |
Leverage infrastructure and achieve operating efficiencies. We intend to leverage our existing infrastructure to achieve operating efficiencies as we grow sales volume. In addition, in connection with the spin-off, we entered into a long-term supply agreement with Integer and subsequently entered into a separate supply agreement with Minnetronix, Inc., a Minnesota corporation (“Minnetronix”), to benefit from their manufacturing capabilities. We continue to work with Integer and Minnetronix in an effort to decrease our manufacturing costs and increase product quality. |
Sales and Marketing
United States
Our commercialization in the United States has been led by a sales force comprised of sales management, national account coverage, direct sales representatives, sales agents and clinical specialists. As of February 21, 2018, we had 45 active territories in the United States. We expect to continue to scale and expand our sales force in the United States using direct sales representatives and independent sales agents. Our sales organization targets physician specialists involved in SCS treatment decisions, including neurosurgeons, interventional pain specialists and orthopedic spine surgeons, who are located at strategic hospitals and outpatient surgery centers across the United States. In addition, we have hired a sales leadership team to oversee our commercial activity including our President, a Vice President of U.S. Sales and a team of Regional Sales Directors. Complementing this group is our National Sales Director who facilitates administrative approvals in larger multi-site and regional hospital systems to help accelerate Algovita adoption and experienced SCS Clinical Specialists who support our sales representatives in trial and permanent implant procedures. Furthermore, our marketing team continues to increase awareness and grow demand for Algovita and SCS therapy in general by focusing on branding initiatives, physician and staff training on the use and benefits of Algovita and educating and providing ongoing support to physicians, patients, third-party payers and hospital administrators on the use of Algovita.
International
In Europe, we currently have three distributors through which we sell Algovita. As we continue to build our international sales organization, we expect that it will consist of a network of distributors and independent sales agents. We began our sales in Germany during 2014 and, to date, have expanded our sales efforts into Luxembourg, Switzerland, Austria, Sweden and the United Kingdom. We expect to expand our Algovita sales efforts into other European countries with health care systems that offer favorable reimbursement rates for SCS therapies, particularly rechargeable SCS systems, and where we believe we can successfully partner with independent sales agents or distributors that meet our qualifications.
Future Products
We expect sales and marketing of Virtis and other future neurostimulation medical device offerings that leverage our neurostimulation technology platform will be conducted through a network of distributors, independent sales agents, a direct sales force or through partnerships with third parties in specific neurostimulation fields of use. We intend to leverage our existing sales management team to manage sales people for Virtis and other future device offerings.
In connection with our development agreement with Aleva, we expect that, upon completion of a complete medical device for use in the DBS market for the treatment of Parkinson’s disease and Essential Tremor, Aleva will commercialize the DBS system using our licensed technology. If it does so and is successful, we would receive royalties on the sale of these DBS systems and components.
Customers
Algovita was designed to provide pain management solutions to patients who have evolving requirements and needs. We are still developing our customer base for Algovita, which includes distributors in Europe and hospitals, surgery centers and medical facilities in the U.S. served through a direct sales force and third-party distributors; therefore, the nature and extent of our selling relationships with each customer is different in terms of breadth of products purchased, purchased product volumes, length of contractual commitment, ordering patterns, inventory management, and selling prices.
Our NeuroNexus customers include institutions, scientists or universities throughout the world who perform research for the neuroscience and clinical markets.
Additionally, our customers include a company in the DBS market serviced through a strategic development agreement.
During fiscal year 2016, sales to Aleva were $3.1 million (or 25%) of the Company’s consolidated revenues. No other customers individually accounted for more than 10% of the Company’s consolidated revenues in 2016, and no customers individually accounted for more than 10% of the Company’s consolidated revenues in 2017.
Competition
The neuromodulation medical device industry is intensely competitive, subject to rapid change and highly sensitive to the introduction of new products and other market activities of industry participants. We currently compete in the SCS market for chronic pain. In the SCS market, the main competitors are Abbott Laboratories, formerly known as St. Jude Medical, Boston Scientific, Medtronic, and Nevro Corp. In addition, SCS therapy also competes against other potential therapies, including spinal surgeries, in particular spinal reoperation. All of the major medical device competitors in the SCS market have obtained United States and European Union regulatory approvals for their SCS systems, and some have recently launched new products or released additional clinical evidence supporting their product therapies, and others are expected to do so within the next few years. These major competitors are publicly-traded companies or divisions of publicly-traded companies, all of whom have significantly greater market share and resources than we have. In addition, these competitors also have more established operations, longer commercial histories and more extensive relationships with physicians than we have. Some of these competitors also have wider product offerings within neuromodulation and other medical device product categories. This may provide these competitors with greater negotiating power with customers and suppliers and with more opportunities to interact with the stakeholders involved in purchasing decisions.
We believe the primary competitive factors in the neurostimulation market are:
|
• |
Technological innovation, product enhancements and speed of innovation |
|
• |
Sales force experience and access |
|
• |
Ease of use |
|
• |
Product support and service |
|
• |
Clinical studies and research |
|
• |
Effective marketing and education |
|
• |
Pricing and reimbursement rates |
|
• |
Product reliability, safety and durability |
|
• |
Company brand recognition |
Research and Development
Our research and development team has significant experience in the design and development of medical devices, particularly in neurostimulation. The team includes specialists in software engineering, mechanical engineering, electrical engineering, graphical user interface design, clinical and regulatory expertise, as well as experts within our NeuroNexus subsidiary. NeuroNexus specializes in neural research, micro-neural interfaces and thin-film technology. NeuroNexus offers high-value neural interface technology and devices across a wide range of functions including neuromonitoring and recording, electrical and optical stimulation, and targeted drug delivery applications. By partnering with entrepreneurs and healthcare providers, we continually evaluate concepts for potential new therapies through early stage feasibility work that we expect will be completed by leveraging our NeuroNexus subsidiary.
The primary objective of our research and development program is to enhance Algovita for use in SCS and may include next generation IPGs, leads and accessories, expanded stimulation delivery methods and MRI compatibility. An additional objective of our research and development program includes enhancements to our neurostimulation technology platform for uses in indications outside of SCS, including Virtis, the second application of our platform and our first product submitted for approval for the SNM market.
In early 2016, we entered into a development agreement with Aleva to develop our neurostimulation technology platform into a complete medical device for use in the DBS market for treatment of Parkinson’s disease and Essential Tremor. In connection with our development agreement with Aleva, we expect that, upon completion of a complete medical device for use in the DBS market for the treatment of Parkinson’s disease and Essential Tremor, Aleva will commercialize the DBS system using our licensed technology. In addition to our development agreement with Aleva, as part of our research and development efforts, we also intend to pursue other strategic partnerships with third parties to, among other things, fund clinical and development costs, in part or in full, for new product offerings.
Net investments in research and development totaled $14.1 million and $14.5 million in fiscal year 2017 and 2016, respectively.
Intellectual Property
Protection of our intellectual property is important to our business. We rely on a combination of patent, trademark, trade secret, copyright and other intellectual property laws, non-disclosure agreements and other measures to protect our proprietary rights. As of February 21, 2018, we own 372 U.S. and foreign patents and 104 pending U.S. and foreign patent applications. Within our patent portfolio, we own the patents and patent applications that embody the core intellectual property underlying our neurostimulation technology platform.
We also own 8 U.S. trademark registrations, 14 pending U.S. trademark registrations, 23 foreign trademark registrations and 8 pending foreign trademark registrations.
The term of each of our individual patents depends on the legal term for patents in the countries in which they are granted. In most countries, including the United States, the patent term is generally 20 years from the earliest claimed filing date of a non-provisional patent application in the applicable country. The majority of our patents will expire between 2027 and 2035. Further, there has been substantial litigation regarding patent and other intellectual property rights in the medical device industry. In the future, we may need to engage in litigation to enforce patents issued or licensed to us, to protect our trade secrets or know-how, and/or to defend against claims of infringement of the rights of others or to determine the scope and validity of the proprietary rights of others.
We also license technology from various entities, including Integer, and as of February 21, 2018 we license 35 U.S. patents, 5 pending U.S. patents, 39 foreign patents and 9 foreign pending patent applications covering both SCS and the intra-spinal stimulation and SNM and DBS fields of use.
Under our license agreements with Integer entered into at the time of the spin-off, we license certain of our technology to Integer for use outside the field of use of neurostimulation, including outside the fields of SCS, SNM and DBS.
We also rely upon trade secrets, know-how and continuing technological innovation, and may rely upon other licensing opportunities in the future, to develop and maintain our competitive position. We seek to protect our proprietary rights through a variety of methods, including confidentiality agreements and proprietary information agreements with suppliers, employees, consultants and others who may have access to proprietary information, under which they are bound to assign to us inventions made during the term of their employment or service, as applicable.
Manufacturing and Supply
Integer Supply Agreement
At the time of the spin-off, we entered into a long-term supply agreement with Integer for the manufacture and supply of Algovita and most of its products, parts and components, including the IPG and the leads. For most products, parts and components of Algovita, other than our external peripheral devices, which are supplied by Minnetronix, Integer is our single or sole source supplier. Our supply agreement with Integer for Algovita has a term of five years and is also terminable upon mutual agreement by the parties, by either party upon material breach by the other, and by either party in the event the other party enters bankruptcy. Our supply agreement with Integer for Algovita also outlines the rights of each party with respect to quality assurance, inspection and compliance with applicable law and contains what we believe are customary indemnification provisions for commercial agreements. In addition, we also entered into a product component framework agreement providing Integer with the exclusive right to supply us with products, parts and components necessary for production of future SNM or DBS neurostimulation devices that we may seek to commercialize. Each of these agreements sets forth the process by which we order products, components or raw materials, as applicable, from our supplier (which process is either on a purchase order basis or based on quarterly or annual forecasts and in some cases require us to purchase minimum amounts) and the related fees for purchasing these items.
Minnetronix Supply Agreement
Effective December 9, 2016, we entered into a manufacturing and supply amendment with Minnetronix for the supply of our current platform of external peripheral devices used with our Algovita spinal cord stimulation system, including the clinician programmer, patient programmer, the patient charging paddle, the external pulse generator kit and the patient feedback tool. Minnetronix is our sole source supplier for these items, but we retain the right to manufacture these products ourselves if we establish our own facility in the future. This agreement is exclusive between Nuvectra and Minnetronix only for Nuvectra’s current platform of external peripheral products, allowing any next generation external devices to be manufactured by ourselves or a third party. This agreement will continue for so long as the supply relationship remains exclusive, unless terminated earlier by either party in the event of a material breach of the agreement by the other party (subject to customary cure periods). The exclusivity provision will survive the agreement’s termination in the event that Minnetronix terminates the agreement due to Nuvectra’s material breach. If Minnetronix discontinues the supply of the peripheral products, it must provide us with 18 months advance notice and provide us with a “last time buy” opportunity.
Other Suppliers
Within our supply chain we also have other suppliers, including some sole source suppliers, for some of our components, with whom we do not have agreements. ON Semiconductor, headquartered in Phoenix, Arizona, is one such sole source supplier, of the application-specific integrated circuit (“ASIC”) used in our IPGs and external pulse generators.
Manufacturing Requirements
Manufacturing facilities that produce medical devices or their component parts intended for distribution worldwide are subject to regulation and periodic unannounced inspection by the FDA and other domestic and international regulatory agencies. In the United States, companies are required to manufacture medical device products that are for sale in compliance with the FDA’s Quality System Regulations, which cover the methods used in, and the facilities used for, the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage and shipping of Algovita or other medical devices. In international markets, we are required to obtain and maintain various quality assurance and quality management certifications. Integer has obtained Quality Management System ISO13485 certification for manufacturing of SCS systems and accessories. We have obtained: Quality Management System ISO13485 and Full Quality Assurance Certification for the design and development of SCS systems and accessories and a Design Examination certificate for IPGs and accessories. We are required to demonstrate continuing compliance with applicable regulatory requirements to maintain these certifications and will continue to be periodically inspected by international regulatory authorities for certification purposes.
Product Liability and Insurance
The manufacture and sale of our products subject us to the risk of financial exposure for product liability claims. Our products are used in situations in which there is a risk of serious injury or death. We carry insurance policies, which we believe to be customary for similar companies in our industry. We cannot assure you that these policies will be sufficient to cover all or substantially all losses that we experience.
We endeavor to maintain executive and organization liability insurance in a form and with aggregate coverage limits that we believe are adequate for our business purposes, but our coverage limits may prove not to be adequate in some circumstances.
Third-Party Coverage and Reimbursement
For Algovita, the primary purchasers are hospitals and outpatient surgery centers in the United States. These purchasers typically bill various third-party payers, such as Medicare, Medicaid and private health insurance plans for the healthcare services associated with the SCS procedures. Government agencies and private payers then determine whether to provide coverage for specific procedures. We believe that SCS procedures using Algovita are adequately described by existing governmental and insurance reimbursement codes for the implantation of spinal cord stimulators and related leads performed in various sites of care. Medicare reimbursement rates for the same or similar procedures vary due to geographic location, nature of facility in which the procedure is performed, such as hospital outpatient department or outpatient surgery centers, and other factors. Although private payers’ coverage policies and reimbursement rates vary, Medicare is increasingly used as a model for how private payers and other governmental payers develop their coverage and reimbursement policies for healthcare items and services, including SCS procedures.
Outside the United States, reimbursement levels vary significantly by country, and by region within some countries. Reimbursement is obtained from a variety of sources, including government-sponsored and private health insurance plans, and combinations of both. In Germany, where Algovita has been commercially available to patients since November 2014, reimbursement for SCS by Germany’s established G-DRG system, which is a government-mandated pricing system pursuant to which German hospitals are paid for services provided to patents, is substantial enough that it makes economic sense to operate within Germany. Some countries require us to gather additional clinical data before granting broader coverage and reimbursement for Algovita. We will complete the requisite clinical studies and obtain coverage and reimbursement approval in those other countries where it also makes economic sense to do so.
SNM and DBS have established reimbursement pathways similar to those for SCS procedures. We will review and assess the reimbursement environment as part of our process of developing additional neurostimulation indications.
Regulation of our Business
Our products, including Algovita, and our operations generally, are subject to extensive and rigorous regulation by the FDA pursuant to its authority under the Federal Food, Drug, and Cosmetic Act, or Food and Drug Act, other federal and state authorities in the United States and comparable foreign regulatory authorities. To ensure that medical products distributed domestically and internationally are safe and effective for their intended use, the FDA and comparable foreign regulatory authorities have imposed regulations that govern, among other things, product design, development and testing, manufacturing, labeling and storage, premarket clearance, clinical investigations, advertising and promotion and product marketing, sales, distribution and recalls.
FDA Clearance and Approval of Medical Devices
The FDA regulates medical devices in the United States and the export of medical devices manufactured in the United States to help ensure that these medical devices are safe and effective for their intended uses. Any violation of these laws and regulations could have a material adverse effect on our business, financial condition and results of operations.
Under the Food and Drug Act, medical devices are classified as Class I, Class II or Class III depending on the degree of risk associated with the device and the extent of control needed to ensure its safety and effectiveness. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not “substantially equivalent” to a legally marked device are classified in Class III. The safety and effectiveness of Class III devices cannot be assured solely by general controls. Submission and FDA approval of a premarket approval application is required before marketing of a Class III device can begin. The premarket approval application process is considerably more demanding than the Class I and Class II 510(k) premarket notification process.
Algovita is a Class III device. On November 30, 2015, Integer announced the receipt of premarket approval for Algovita from the FDA and we launched Algovita commercially in the United States during the first half of 2016. In 2017, we filed a regulatory submission with FDA authorities for full-body MRI Conditional approval for Algovita. We currently anticipate FDA full-body MRI Conditional approval in the fourth quarter of 2018.
Similarly, Virtis is a Class III device. In January 2017, we filed a regulatory submission with FDA authorities for premarket approval of our SNM device. Although there can be no assurances, absent further regulatory requirements or delays, we currently anticipate FDA approval in the second half of 2018.
Continuing FDA Regulation
After a device is placed on the market, numerous regulatory requirements continue to apply. These include:
|
• |
compliance with the FDA’s Quality System Regulations, which requires medical device companies to follow design, testing, control, documentation and other quality assurance procedures during the manufacturing process; |
|
• |
labeling regulations; |
|
• |
the FDA’s general prohibition against promoting products for unapproved or “off-label” uses; |
|
• |
approval of product modifications that could significantly affect safety or effectiveness or that would constitute a major change in the intended use of Algovita or any other medical device using our neurostimulation technology platform; |
|
• |
medical device reporting regulations, which require that medical device companies comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur; |
|
• |
post-approval restrictions or conditions, including post-approval study commitments; |
|
• |
post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; |
|
• |
the FDA’s recall authority, whereby it can ask, or under certain conditions order, medical device companies to recall from the market a product that is in violation of governing laws and regulations; |
|
• |
regulations pertaining to voluntary recalls; and |
|
• |
notices of corrections or removals. |
Medical device companies are also required to register and list their devices with the FDA, based on which the FDA will conduct inspections to ensure continued compliance with applicable regulatory requirements.
The FDA has broad post-market and regulatory and enforcement powers. Failure to comply with the applicable U.S. medical device regulatory requirements could result in, among other things, warning letters, fines, injunctions, consent decrees and civil penalties, customer notifications or repair, replacement, refund, recall, administrative detention or seizure of Algovita systems, operating restrictions or partial suspension or total shutdown of production or criminal prosecution.
Other Healthcare Regulations
We are also subject to healthcare fraud and abuse regulation in the jurisdictions in which we will conduct business. These laws include, without limitation, applicable anti-kickback, false claims, healthcare reform, patient privacy and security laws, and physician payment transparency regulations.
Anti-Kickback Statute
The U.S. federal Anti-Kickback Statute is a criminal statute that prohibits persons from knowingly and willfully soliciting, offering, paying, or receiving “remuneration,” directly or indirectly to induce or reward the purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any health care items or services for which payment may be made under federal healthcare programs. The Anti-Kickback Statute prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. The Anti-Kickback Statute is broadly drafted and establishes penalties for parties on both sides of the prohibited transaction.
Federal False Claims Act
The U.S. Federal False Claims Act imposes civil liability on persons or entities who knowingly present or cause to be presented a false or fraudulent claim or knowingly use false statements to obtain payment from or approval by the federal government. Under the False Claims Act, a claim may be submitted directly to the federal government or to a recipient of federal funds, such as a federal contractor, where the funds are to be spent on the federal government’s behalf. In addition, private individuals have the ability to bring actions under the civil False Claims Act in the name of the government, known as qui tam actions, alleging false and fraudulent claims presented to or paid by the government or recipient of federal funds (or other violations of the statutes) and to share in any amounts paid by the entity to the government in fines or settlement. Medical device companies can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting an “off-label” use of a product.
Many states also have statutes or regulations similar to the U.S. federal Anti-Kickback and False Claims Act, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payer.
Healthcare Reform
In March 2010, the Affordable Care Act (“ACA”) was signed into law, which has substantially changed healthcare financing and delivery by both governmental and private insurers, and significantly impacts the medical device industry. The ACA impacted existing government healthcare programs and resulted in the development of new programs. The ACA’s provisions include a deductible 2.3% excise tax on any entity that manufactures or imports medical devices offered for sale in the United States, with certain limited exceptions. As part of the recently enacted Tax Cut and Jobs Act of 2017 (“TCJA”), this excise tax has been suspended through December 31, 2019. Absent future legislative action, this excise tax will be automatically reinstated beginning on January 1, 2020.
U.S. Privacy and Security Laws
We may be subject to data privacy and security laws and regulations of both the U.S. federal government and the individual states in which we operate. The Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and their respective implementing regulations impose obligations on entities covered thereby relating to the privacy, security and transmission of protected health information. These covered entities include health plans, health care clearing houses, and certain health care providers.
In addition, comparable state laws also govern the privacy and security of health information in certain circumstances. Many of these individual state laws differ from state to state in significant ways and may not have the same effect. In addition, certain of these state laws are more stringent than HIPAA and, in such circumstances, the more stringent state law must be followed.
Physician Payment Transparency Laws
In recent years, federal and state regulation of payments made to physicians and other healthcare providers and entities has increased. The ACA imposes new reporting requirements on some manufacturers, including some medical device manufacturers, for payments and other transfers of value provided to physicians or teaching hospitals. In addition, the ACA also requires reporting by physicians and their immediate family members of ownership or other investment interests in some medical device manufacturers.
Failure to submit the required information timely, accurately, or completely may result in civil monetary penalties of up to an aggregate of $150,000 per year and up to an additional aggregate of $1 million per year for “knowing failure to report.”
Some states also require medical device companies to comply with the industry’s voluntary compliance guidelines and/or the compliance guidelines promulgated by the U.S. federal government, which impose restrictions on device manufacturer marketing practices and/or require the tracking and reporting of gifts, compensation and other remuneration to healthcare providers and entities.
Regulations in the EU
Our international sales are subject to regulatory requirements in the countries in which Algovita is sold. The regulatory review process varies from country to country. Nuvectra is required to perform a post market approval clinical study. This study is in process.
In the European Economic Area, or EEA (which is comprised of the 28 Member States of the EU plus Norway, Liechtenstein and Iceland), we must comply with the requirements of the EU Active Implantable Medical Devices Directive 90/385/EEC (“AIMDD”), and appropriately affix the CE mark on Algovita to attest to such compliance. In achieving compliance, Algovita had to comply with the “Essential Requirements” described in Annex I of the AIMDD. In addition, to affix the CE mark on Algovita, we had to undergo a conformity assessment procedure, the requirements of which vary based upon the type of medical device and its classification. Except for low risk medical devices, a conformity assessment procedure also requires a third-party assessment by a Notified Body, which is an organization designated by a competent authority of an EEA country to conduct conformity assessments. The Notified Body audits and examines the technical file and the quality system for the manufacture, design and final inspection of the medical device. The Notified Body issues a CE Certificate of Conformity following successful completion of a conformity assessment procedure and confirmation of conformity with the Essential Requirements. Receipt of this CE Certificate entitles the medical device company to affix the CE mark to its medical device after preparing and signing an EC Declaration of Conformity. The assessment of the conformity for Algovita has been certified by our Notified Body, TÜV SÜD America.
In 2017, we filed a regulatory submission with CE mark authorities for full-body MRI Conditional approval for Algovita. We currently anticipate CE mark full-body MRI Conditional approval in the second quarter of 2018. In December 2016, we filed a regulatory submission with CE mark authorities for approval of our Virtis device. We currently anticipate CE mark approval for Virtis in the second quarter of 2018.
Algovita is subject to continued surveillance by its Notified Body, and we are required to report any serious adverse incidents related to Algovita to the appropriate authorities. We must also comply with additional requirements of individual countries in which Algovita is marketed and the requirements of certain EU Directives.
In September 2012, the European Commission published proposals for the revision of the EU regulatory framework for medical devices, which would replace the Medical Devices Directive 93/42/EEC (“MDD”) and the AIMDD with the new Medical Devices Regulation. The Medical Devices Regulation 2017/745 (“MDR”) was adopted April 5, 2017. The current AIMD remains in effect through the transition period ending May 26, 2020 per MDR Article 120, section 1. The new MDR will, among other things, impose additional reporting requirements on manufacturers of high risk medical devices, impose an obligation on medical device companies to appoint a “qualified person” responsible for regulatory compliance, and provide for stricter clinical evidence requirements.
EU Data Protection Directive
We are subject to laws and regulations in non-U.S. countries covering data privacy and the protection of health-related and other personal information. EU member states and other jurisdictions have adopted data protection laws and regulations, which impose significant compliance obligations. For example, the EU Data Protection Directive imposes strict obligations and restrictions on the ability to collect, analyze and transfer personal data, including health data from clinical trials and adverse event reporting. Failing to comply with these laws could lead to government enforcement actions and significant penalties against us.
Anti-Bribery Laws
The federal Foreign Corrupt Practices Act of 1997 and other similar anti-bribery laws in other jurisdictions, including the UK Bribery Act of 2010, generally prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more frequent and aggressive investigations and enforcement proceedings by both the Department of Justice and the Securities and Exchange Commission (“SEC”). Violations of United States or foreign laws or regulations could result in the imposition of substantial fines, interruptions of business, loss of supplier, vendor or other third-party relationships, termination of necessary licenses and permits, and other legal or equitable sanctions. Other internal or government investigations or legal or regulatory proceedings, including lawsuits brought by private litigants, may also follow as a consequence.
Employees
As of February 21, 2018, we had 195 employees of which 186 were full-time. We believe the success of our business will depend, in part, on our ability to continue to attract and retain qualified personnel. We are committed to developing our employees and providing them with opportunities to contribute to our growth and success. Our employees are not subject to a collective bargaining agreement, and we believe that we have good relations with our employees.
Corporate Information
Our principal executive offices are located at 5830 Granite Parkway, Suite 1100, Plano, Texas 75024 and our telephone number is (844) 727-7897. Our website address is www.nuvectramed.com. Information contained on, or connected to, our website is not part of this Annual Report on Form 10-K. We are traded on the Nasdaq Global Market under the symbol “NVTR.”
Emerging Growth Company Status
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act, or the JOBS Act, and are eligible to take advantage of certain exemptions from various reporting requirements applicable to public companies that are not emerging growth companies. These include, but are not limited to, (i) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, (ii) an exception from compliance with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, and (iii) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and the requirement to obtain stockholder approval of any golden parachute payments not previously approved.
In addition, Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
We would cease to be an emerging growth company upon the earliest of (1) December 31, 2021, which is the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement filed under the Securities Act; (2) the last day of the first fiscal year in which our total annual gross revenue exceeds $1.07 billion (as indexed for inflation); (3) the date on which we become a “large accelerated filer,” as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our common stock held by non-affiliates exceeds $700 million as of the last day of our most recently completed second fiscal quarter; and (4) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
Available Information
We make available on or through our website certain reports and amendments to those reports that we file with, or furnish to, the SEC in accordance with the Exchange Act. These include our Annual Reports on Form 10-K, our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act. We make this information available on or through our website free of charge as soon as reasonably practicable after we electronically file the information with, or furnish it to, the SEC. This information is also available by writing to us at the address on the cover of this Annual Report on Form 10-K to the attention of General Counsel. Copies of this information may be obtained at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains reports, proxy and information statements, and other information regarding our filings, at www.sec.gov. The information on, or that can be accessed through, our website is not incorporated by reference into this Annual Report on Form 10-K or any other filings we make with the SEC.
You should carefully consider the following risks, as well as the other information included in this Annual Report on Form 10-K, in evaluating us and our common stock. The occurrence of any of the risks described below could have a material adverse effect on our business, financial condition, results of operations, ability to raise capital and growth prospects. Additional risks and uncertainties may also impair our business, financial condition, results of operations, our ability to raise capital and growth prospects.
Risks Related to our Business
We are substantially dependent on the market acceptance in the United States for Algovita, and the failure of Algovita to gain market acceptance would negatively impact our business.
On November 30, 2015, Integer announced receipt of premarket approval for Algovita, and we launched Algovita commercially in the United States during the first half of 2016. If we are unable to achieve significant market acceptance in the United States, our results of operations will be adversely affected as the United States is expected to be the principal market for Algovita. We currently have Virtis and other complete medical devices incorporating our neurostimulation technology platform in development; however, if we are unsuccessful in commercializing Algovita, unable to market Algovita as a result of a quality problem, fail to maintain regulatory approval for Algovita, or experience unexpected or serious complications or other unforeseen negative effects related to Algovita, we would lose our expected main source of revenue, and our business will be adversely affected. Virtis and other extensions of our technology platform could be adversely affected as well.
If we fail to develop and retain an effective direct sales force in the United States, our business could suffer.
To commercialize Algovita in the United States, we must build and retain a substantial direct sales force. As we expand our commercial launch of Algovita in the United States and concurrently increase our marketing efforts, we will need to retain, grow and develop our direct sales representatives. There is significant competition for sales representatives experienced in medical device sales. Once hired, the training process is lengthy because it requires significant education for new sales representatives to achieve the level of clinical competency with Algovita expected by physicians. Upon completion of the training, sales representatives typically require lead-time in the field to grow their network of accounts and achieve the productivity levels we expect them to reach. If we are unable to attract, motivate, develop and retain a sufficient number of qualified sales representatives, and if our sales representatives do not achieve the productivity levels we expect them to reach, our revenue will not grow at the rate we expect and our financial performance will suffer. Also, to the extent we hire personnel from our competitors, we may have to wait until applicable non-competition provisions have expired before deploying that personnel in restricted territories or incur costs to relocate personnel outside of those territories, and we may be subject to future allegations that these new hires have been improperly solicited, or that they have divulged to us proprietary or other confidential information of their former employers. Any of these risks may adversely affect our business.
We must demonstrate to physicians the merits of Algovita compared to products marketed by our competitors.
Physicians play a significant role in determining the course of a patient’s treatment and, as a result, the type of product that will be used to treat a patient. As a result, our success depends, in large part, on effectively marketing Algovita and SCS therapy to physicians. To successfully commercialize Algovita, we must successfully demonstrate to physicians the merits of Algovita as compared to our competitors’ SCS systems. Acceptance of Algovita depends, in part, on educating physicians as to the distinctive characteristics, perceived benefits, safety, ease of use and cost-effectiveness of Algovita compared to our competitors’ SCS systems, and communicating to physicians the proper application of Algovita. If we are not successful in convincing physicians of the merits of Algovita or educating them on the use of Algovita, they may not use our products and we may be unable to increase our sales, sustain our growth or achieve profitability.
Additionally, an important part of our sales process also includes the education of physicians on the safe and effective use of Algovita. It is critical to the success of our commercialization efforts to educate physicians on the proper use of Algovita, and to provide them with adequate product support during clinical procedures. If physicians misuse or ineffectively use our products, it could result in unsatisfactory patient outcomes, patient injuries, negative publicity or lawsuits against us, any of which could have an adverse effect on our business. Finally, in the United States, for physicians to use Algovita, we expect that the hospital facilities where these physicians treat patients typically will require us to enter into purchasing contracts with them. If we do not receive access to hospital facilities via these contracting processes or otherwise, or if we are unable to secure contracts or tender successful bids, our sales and operating results may be adversely affected.
The seasonality of our business creates variance in our quarterly revenue, which makes it difficult to compare or forecast our financial results.
Our revenue fluctuates on a seasonal basis, which affects the comparability of our results between periods. We have experienced lower sales in the first quarter and third quarter of the year, which we believe is due to weather-related events, holidays, the buying patterns and implant volumes of our distributors, hospitals and clinics and reimbursement related issues such as patient deductibles. These seasonal variations are difficult to predict accurately, may vary amongst different markets and regions, and at times may be entirely unpredictable. This may create additional risk to our business as we rely upon forecasts of customer demand to build inventory in advance of anticipated sales. In addition, we believe our limited history commercializing our products has, in part, made our seasonal patterns more difficult to determine, making it more difficult to predict future seasonal patterns.
Our competitors are large, well-established companies with substantially greater resources than us and many have a long history of competing in the SCS market.
Our current and potential competitors are publicly-traded, or are divisions of publicly-traded, major medical device companies that have substantially greater financial, technical, sales and marketing resources than we do. For example, our major competitors, Medtronic, Inc., Boston Scientific Corporation, Abbott Laboratories, and Nevro Corp., each has an approved neuromodulation system in at least the United States, Europe, and Australia. Medtronic, Boston Scientific and Abbott Laboratories have each been established for several years while Nevro is a new entrant to the SCS market and is marketing its High Frequency 10 (HF10) SCS therapy for treatment of several chronic pain conditions. We expect that as we continue to sell Algovita commercially in the United States, our competitors will take aggressive action to protect their current market share and position. We expect to face significant competition in establishing our market share in the United States and may encounter currently unforeseen obstacles and competitive challenges.
In addition, we face a particular challenge in overcoming entrenched practices by some physicians who exclusively use the neurostimulation products produced by our larger, more established competitors. Physicians who have completed many successful procedures using neurostimulation products made by these competitors may be reluctant or unwilling to try a new product from a new entrant in the marketplace with which they are less familiar. If these physicians are unwilling to try or adopt Algovita, our business will be adversely affected.
Further, a number of our competitors are currently conducting, or we anticipate will be conducting, clinical trials to demonstrate the efficacy of their SCS systems, which may lead to regulatory approvals for use of their systems for additional indications or support for their marketing claims that their products are superior to Algovita. Competition may increase further as existing competitors enhance their product offerings to compete directly with Algovita or other companies enter the SCS market. If our competitors develop more effective or affordable products, achieve earlier patent protection or product commercialization, or produce clinical results that show greater efficacy than Algovita, our operations will likely be negatively affected. If we are forced to reduce our prices for Algovita due to increased competition, our revenues and operating results could be negatively affected.
We may never achieve full market acceptance in Europe.
Algovita received CE mark approval in June 2014, enabling us to commercialize it in Europe. We currently sell Algovita in Germany, Switzerland, Austria, Luxembourg, Sweden and the United Kingdom. We have limited experience engaging in commercial activities and limited established relationships with physicians and hospitals in those countries. We may be unable to gain broader market acceptance in these countries, which will adversely affect our sales and operating results.
We are dependent upon sole-source manufacturers and suppliers, including Integer and Minnetronix, making us vulnerable to supply shortages, manufacturing problems and price fluctuations, which could harm our business.
In connection with the spin-off, we entered into an exclusive supply agreement with Integer under which we purchase fully assembled Algovita systems and most products, parts and components necessary for the production of Algovita. We also entered into a product component framework agreement that provides Integer with the exclusive right to supply us with products, parts and components necessary for production of future SNM or DBS neurostimulation devices that we may seek to commercialize. Subject to conditions specified in these agreements, Integer is our exclusive and sole source manufacturer and supplier for most products, parts and components of Algovita, while Minnetronix is the sole-source supplier of our external peripheral devices.
Effective December 9, 2016, we entered into a manufacturing and supply amendment with Minnetronix for the supply of our current platform of external peripheral devices used with our Algovita spinal cord stimulation system, including the clinician programmer, patient programmer, the patient charging paddle, the external pulse generator kit and the patient feedback tool. Minnetronix is our sole-source supplier for these items (although we retain the right to manufacture the products ourselves).
As a result, we are vulnerable to supply shortages, failure to maintain adequate safety stock and manufacturing problems encountered by Integer or Minnetronix, including, for example, failure to follow specific protocols and procedures, failure to comply with applicable legal and regulatory requirements, equipment malfunction and environmental factors, failure to properly conduct its own business affairs, and infringement of third-party intellectual property rights, any of which could delay or impede its ability to meet our requirements. Integer or Minnetronix may also be unwilling to supply components for Algovita or our other products or suffer from disruptions in their own supply chains. Even if Integer or Minnetronix were in material default under the supply agreements, as a practical matter, it would require time and investment to qualify new suppliers and comply with regulatory requirements regarding manufacture and supply. In addition, we may not be able to take advantage of price fluctuations or competitive pricing that may become available from alternative supply sources. Our reliance on each of Integer and Minnetronix as our sole source suppliers also subjects us to other risks that could harm our business, including:
|
• |
we are not the only customer of either supplier, and they may therefore give other customers’ needs higher priority than ours; |
|
• |
in the event our supply agreement or relationship is terminated, we may have difficulty locating and qualifying alternative suppliers on a timely basis or at all; |
|
• |
in the event our supply agreement or relationship is terminated, switching suppliers would likely require product redesign and submission to FDA, or other foreign regulatory bodies, which would significantly impede or delay our commercial activities or even suspend them; |
|
• |
Integer or Minnetronix, which are dependent upon certain sole-source suppliers themselves, could suffer from shortages or delays in their supply chains, which could inhibit or delay their ability to fulfill our orders and meeting our requirements; and |
|
• |
Integer or Minnetronix could encounter other financial or business hardships unrelated to our demand, which could inhibit their ability to fulfill our orders and meet our requirements. |
Our ability to achieve profitability will depend, in part, on our ability to reduce the per unit cost of Algovita and improve our gross margins.
Currently, the gross profit generated from the sale of Algovita is not sufficient to cover our operating expenses. To achieve profitability, we need to, among other things, reduce the per unit cost for Algovita and improve our gross margins. This cannot be achieved without increasing the volume of systems and components that we purchase from Integer and Minnetronix. Any increase in manufacturing volumes is dependent upon a corresponding increase in sales. The occurrence of any factor that negatively impacts the sales of Algovita or reduces manufacturing efficiency may prevent us from achieving our desired reduction in per unit costs and increase in gross margins, which would negatively affect our operating results and may prevent us from attaining profitability.
Our international operations subject us to certain operating risks, which could adversely impact our results of operations and financial condition.
In 2014, following our receipt of CE mark for Algovita in June 2014, we began selling Algovita in Europe through a limited number of distributors. We began our sales in Germany and, to date, have expanded our sales efforts into Luxembourg, Switzerland, Austria, Sweden and the United Kingdom. Given that we do not have, or currently plan to use, any direct sales representatives in Europe, we are heavily dependent on the efforts of a limited number of distributors in Europe. The sale and shipment of Algovita and our other products across international borders exposes us and our distributors to risks inherent in operating in foreign jurisdictions. These risks include:
|
• |
difficulties in enforcing our intellectual property rights and in defending against third-party threats and intellectual property enforcement actions against us, our distributors, or our suppliers; |
|
• |
reduced or varied protection for intellectual property rights in some countries; |
|
• |
pricing pressure that we may experience internationally; |
|
• |
shortages in high-quality sales representatives and distributors; |
|
• |
third-party reimbursement policies that may require some of the patients who receive our products to directly absorb medical costs or that may necessitate the reduction of the selling prices of our products; |
|
• |
competitive disadvantage to competition with established business and customer relationships; |
|
• |
foreign currency exchange rate fluctuations; |
|
• |
imposition of additional U.S. and foreign governmental controls or regulations; |
|
• |
economic instability; |
|
• |
changes in duties and tariffs, license obligations and other non- tariff barriers to trade; |
|
• |
imposition of restrictions on the activities of foreign agents, representatives and distributors; |
|
• |
scrutiny of foreign tax authorities which could result in significant fines, penalties and additional taxes being imposed on us; |
|
• |
laws and business practices favoring local companies; |
|
• |
longer payment cycles; |
|
• |
difficulties in maintaining consistency with our internal guidelines; |
|
• |
difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; |
|
• |
imposition of costly and lengthy new export licensing requirements; and |
|
• |
imposition of new trade restrictions. |
In addition, our international operations subject us to laws regarding sanctioned countries, entities and persons, customs, import-export, laws regarding transactions in foreign countries, the U.S. Foreign Corrupt Practices Act of 1977 and local anti-bribery and other laws regarding interactions with healthcare professionals. Among other things, these laws restrict, and in some cases prohibit, United States companies from directly or indirectly selling goods, technology or services to people or entities in certain countries. In addition, these laws require that we exercise care in structuring our sales and marketing practices in foreign countries. Our failure to comply with these regulations and laws could subject us to penalties, fines, denial of export privileges, seizures of shipments, product recalls, restrictions on business activities or other criminal, civil or administrative actions.
If we experience any of these risks, our sales in non-U.S. jurisdictions may be harmed and our results of operations would suffer.
Issues with product quality could have a material adverse effect upon our business, subject us to regulatory actions, including product recalls and product liability litigation, and cause a loss of customer confidence in us or our products.
Our success depends upon the quality of our products. Quality management plays an essential role in meeting customer requirements, preventing defects and assuring the safety and efficacy of our products. Quality and safety issues may occur with respect to Algovita or any of our other products at any stage. A quality or safety issue, including a product recall, may result in adverse inspection reports, warning letters, product recalls or seizures, monetary sanctions, injunctions to halt manufacture and distribution of products, civil or criminal sanctions, costly product liability and other litigation, refusal of a government to grant approvals and licenses, restrictions on operations or withdrawal of existing approvals and licenses. An inability to address a quality or safety issue, including a product recall, in an effective and timely manner may also cause negative publicity, a diversion of management attention, a loss of customer confidence in us or our current or future products, which may result in the loss of sales and difficulty in successfully launching new products.
We may not be able to establish or strengthen our brand.
We believe that establishing and strengthening our brand is critical to achieving widespread acceptance for Algovita and our other products, particularly because of the highly competitive nature of the markets in which we operate. Promoting and positioning our brand depends largely on the success of our marketing efforts and the perception by physicians and our other customers of the quality and efficacy of Algovita and our other products.
Given the established nature of our competitors, it is likely that our future marketing efforts will require us to incur significant expenses. These brand promotion activities may not yield increased sales and, even if they do yield increased sales, any sales increases may not offset the expenses we incurred to promote our brand. If we fail to successfully promote and maintain our brand, or if we incur substantial expenses in an unsuccessful attempt to promote and maintain our brand, Algovita and our other products may not be accepted by physicians and our others customers, which would adversely affect our business, results of operations and financial condition.
Our business could suffer if we lose the services of key members of our senior management or fail to hire necessary personnel and sales representatives.
We are dependent upon the continued services of key members of our senior management. The loss of these individuals could disrupt our operations or our strategic plans. In addition, our future success will depend on, among other things, our ability to continue to hire or contract with, and retain, the necessary qualified scientific, technical and managerial personnel and sales representatives, for whom we compete with numerous other companies, academic institutions and organizations. The loss of members of our management team or our inability to attract or retain other qualified personnel could have a material adverse effect on our business, results of operations and financial condition.
If third-party payers do not provide adequate coverage and reimbursement for the use of Algovita and other neurostimulation devices we market for sale, we may be required to decrease our selling prices, which could have a negative effect on our financial performance.
Our success in marketing Algovita and any other neurostimulation devices we develop depends and will depend in large part on whether United States and international government health administrative authorities, including Medicare and Medicaid in the United States, private health insurers and other organizations adequately cover and reimburse customers for the cost of Algovita and those other devices. Third-party payers continually review their coverage and reimbursement policies and could, without notice, eliminate or reduce coverage or reimbursement for SCS, SNM or DBS therapy and/or Algovita and any other devices we develop.
Further, the trends toward managed care, healthcare cost containment and other changes in government and private sector initiatives are placing increased emphasis on the delivery of more cost-effective medical therapies. As the healthcare industry consolidates, competition to provide products and services to industry participants will continue to intensify, which will result in greater pricing pressures and the exclusion of certain suppliers from important market segments as group purchasing organizations, integrated delivery networks and large single accounts continue to use their market power to consolidate purchasing decisions. Access to adequate coverage and reimbursement for SCS, SNM or DBS therapy and, in particular, for Algovita by third-party payers is essential to the acceptance of Algovita.
In addition, reimbursement systems in international markets vary significantly by country and, in some cases, by region within some countries, and reimbursement approvals are often required to be obtained on a country-by-country basis. In many international markets, a product must be approved for reimbursement before it can be approved for sale in that country. Further, many international markets have government-managed healthcare systems that control reimbursement for new devices and procedures. If sufficient coverage and reimbursement is not available for Algovita and other SCS devices in our international markets, the demand for our products and our revenues will be adversely affected.
If we fail to properly manage our anticipated growth, our business could suffer.
We have a relatively short operating history. We intend to continue to grow and may experience periods of rapid growth and expansion, which could place a significant additional strain on our limited personnel, information technology systems and other resources. In particular, the hiring and retention of our direct sales representatives in the United States requires significant management, financial and other supporting resources. In order to manage our operations and growth, we will need to continue to improve our operational and management controls, reporting systems and control procedures, which we may be unable to do in a cost efficient manner or at all. If we are unable to manage our growth effectively, it may be difficult for us to execute our business strategy and our operating results and business could suffer.
If we are unable to successfully introduce new products or fail to keep pace with advances in technology, our business, financial condition and results of operations could be adversely affected.
Although we continue to develop or seek to develop additional products using our neurostimulation technology platform for commercial introduction, we may be substantially dependent on sales from Algovita for many years. Over the longer term, we will need to successfully introduce new products or advancements to Algovita in order to achieve our strategic business objectives. Product development requires substantial investment and there is inherent risk in the research and development process. A successful product development process depends on many factors, including our ability to properly anticipate and satisfy customer needs, adapt to new technologies, obtain regulatory approvals on a timely basis, demonstrate satisfactory clinical results, manufacture products in an economical and timely manner and differentiate our products from those of its competitors. If we cannot successfully introduce new products or adapt to changing technologies, our products may become obsolete and our revenue and profitability could suffer.
U.S. or European regulatory authorities may not approve the regulatory submission of Virtis, our SNM System.
We submitted Virtis to the FDA and to the CE mark authorities in Europe for the treatment of chronic urinary retention and the symptoms of overactive bladder in January 2017 and December 2016, respectively. The approval of these submissions may be delayed or rejected, or the authorities may require us to submit additional information for approval. In the event the submissions are not approved in either the U.S. or Europe, our market release of Virtis would be substantially delayed or may never occur, which could adversely impact our future revenues and our ability to effectively compete. If we are required to provide additional information to either authority, we may be required to increase spending for related research and development or clinical projects, which could adversely impact our profitability and require additional resources or personnel.
If clinical studies for future indications do not produce results necessary to support regulatory clearance or approval in the United States or elsewhere, we will be unable to commercialize our products for these indications.
We will likely need to conduct additional clinical studies and post marketing studies in the future to support approval for new indications and product claims. Clinical testing takes many years, is expensive and carries uncertain outcomes. The initiation and completion of any of these studies may be prevented, delayed, or halted for numerous reasons, including, but not limited to, the following:
|
• |
the FDA, institutional review boards or other regulatory authorities do not approve a clinical study protocol, force us to modify a previously approved protocol, or place a clinical study on hold; |
|
• |
patients do not enroll in, or enroll at a lower rate than we expect, or do not complete a clinical study; |
|
• |
patients or investigators do not comply with study protocols; |
|
• |
patients do not return for post-treatment follow-up at the expected rate; |
|
• |
patients experience serious or unexpected adverse side effects for a variety of reasons that may or may not be related to our products such as the advanced stage of two chronic diseases or conditions that may exist at the time of treatment, causing a clinical study to be put on hold; |
|
• |
sites participating in an ongoing clinical study withdraw, requiring us to engage new sites; |
|
• |
difficulties or delays associated with establishing additional clinical sites; |
|
• |
third-party clinical investigators decline to participate in our clinical studies, do not perform the clinical studies on the anticipated schedule, or perform in a manner inconsistent with the investigator agreement, clinical study protocol, good clinical practices or other regulations governing clinical trials; |
|
• |
third-party organizations do not perform data collection and analysis in a timely or accurate manner; |
|
• |
regulatory inspections of our clinical studies or manufacturing facilities require us to undertake corrective action or suspend or terminate our clinical studies; |
|
• |
changes in federal, state, or foreign governmental statutes, regulations or policies; |
|
• |
interim results are inconclusive or unfavorable as to immediate and long-term safety or efficacy; |
|
• |
the study design is inadequate to demonstrate safety and efficacy; or |
|
• |
the statistical endpoints are not met. |
Clinical failure can occur at any stage of the testing. Our clinical studies or post marketing studies may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical or non-clinical studies in addition to those we have planned. Our failure to adequately demonstrate the safety and effectiveness of any of our devices would prevent receipt of regulatory clearance or approval and, ultimately, the commercialization of that device or indication for use.
We could also encounter delays if the FDA concludes that our financial relationships with investigators results in a perceived or actual conflict of interest that may have affected the interpretation of a study, the integrity of the data generated at the applicable clinical trial site or the utility of the clinical trial itself. Principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and may receive cash and/or stock compensation in connection with such services. If these relationships and any related compensation to or ownership interest by the clinical investigator carrying out the study result in perceived or actual conflicts of interest, or if the FDA concludes that the financial relationship may have affected interpretation of the study, the integrity of the data generated at the applicable clinical trial site may be questioned and the utility of the clinical trial itself may be jeopardized, which could result in the delay or rejection of our premarket approval application by the FDA. Any such delay or rejection could prevent us from commercializing any of our products currently in development.
Our future success is highly dependent upon our use of our intellectual property rights, including trade secrets, which rights could be adversely impacted by many factors, each of which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
As a neuromodulation medical device company focused on the development and commercialization of our neurostimulation technology platform, we expect to be highly dependent upon our use of our intellectual property rights. These intellectual property rights could be adversely impacted by many factors, including:
|
• |
We may in the future become involved in lawsuits to defend ourselves against intellectual property disputes, which could be expensive and time consuming, and ultimately unsuccessful, and could result in the diversion of significant resources, and hinder our ability to commercialize our existing or future products; |
|
• |
Our patents and other intellectual property rights infringing or violating the proprietary rights of others, particularly given that our competitors have made substantial investments in patent portfolios and competing technologies and may have applied for or may in the future apply for and obtain, patents that may interfere with our ability to sell our products. For example, should a third party bring a claim against us, our customers, our suppliers or our distributors, whether merited or not, it could be costly to defend, require us to pay damages on behalf of our customers, suppliers, or distributors, interfere with our ability to make, use, sell, and/or export our products or require us to obtain a license (which we may not be able to obtain on commercially reasonable terms or at all); |
|
• |
Our intellectual property rights may not provide sufficient commercial protection for Algovita and any future complete medical device that incorporates our neurostimulation technology platform, and potentially enable third parties to use our technology or very similar technology and reduce our ability to compete in the market; |
|
• |
Third parties may seek to challenge our patents, and, as a result, these patents could be narrowed, invalidated or rendered unenforceable; |
|
• |
Our current and future patent applications may not result in the issuance of patents in the United States or foreign countries; |
|
• |
Patent reform legislation, including the Leahy-Smith America Invents Act, or any future patent reform legislation may affect the way patent applications are prosecuted, redefine prior art, affect patent litigation or switch the United States patent system from a “first-to-invent” system to a “first-to-file” system, any or all of which could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents; |
|
• |
If we may fail to maintain the patents and patent applications covering Algovita and our neurostimulation technology platform, whether through unintentional lapse or otherwise, a competitor could design, manufacture and market products that are the same or similar to our own; |
|
• |
We may become involved in interference or derivation proceedings or re-examination or opposition proceedings provoked by third parties or brought by the United States Patent and Trademark Office or any foreign patent authority to determine the priority of inventions or other matters of inventorship with respect to our patents or patent applications, which if the outcome were unfavorable could require us to cease using the related technology or to attempt to license rights to it from the prevailing party; |
|
• |
We rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position, which we endeavor to protect through non-disclosure and confidentiality agreements with parties who have access to these items; provided, however, despite our best efforts and contractual limitations, our trade secrets and other unpatented or unregistered proprietary information may get disclosed and thereafter are likely to lose trade secret protection; and |
|
• |
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our competitors or are in breach of non-competition or non-solicitation agreements with our competitors. |
If any of the foregoing occurs, we may have to withdraw existing products from the market or may be unable to commercialize one or more of our products, all of which could have a material adverse effect on our business, results of operations and financial condition.
We are subject to certain risks related to our license agreements with Integer, including the potential for Integer to develop competing or similar products.
We have licensed to Integer the right to use (i) specified non-core intellectual property underlying our neurostimulation technology platform for applications within the neurostimulation fields of use in the unrestricted license agreement and (ii) other specified intellectual property underlying our neurostimulation technology platform for applications outside of the neurostimulation fields of use in the restricted license agreement. In addition, NeuroNexus has licensed to Integer the right to use its intellectual property outside of the neurostimulation fields of use. Integer, through the use of this licensed intellectual property or through the use of other intellectual property that it separately owns or has developed, may seek to develop products, components or improvements to items that compete against or are similar to our own products and components. In addition, if Integer tries to develop a product that incorporates licensed intellectual property for applications within a prohibited field of use, we may seek to enforce the terms of the license agreements to prohibit these developments, which could subject us to costly litigation, distract management and negatively affect our supply agreements with Integer. In addition, pursuant to the terms of these license agreements, we will be required to indemnify Integer against third party infringement claims, which could result in our incurrence of significant expenses to defend any such matters or require us to make significant indemnification payments to Integer.
Our use of “open source” software in our products could subject us to possible litigation.
We use, and expect to continue to use, some open source software in our products. We may face claims from others claiming ownership of, or seeking to enforce the terms of, an open source license, including by demanding release of the open source software, derivative works or our proprietary source code that was developed using such software. These claims could also result in litigation, require us to purchase a costly license or require us to devote additional research and development resources to change our software, any of which would have an adverse effect on our business and operating results. Further, if the license terms for the open source code change, we may be forced to re-engineer our products, resulting in additional costs.
We may not be able to adequately protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents in all countries throughout the world would be prohibitively expensive. The requirements for patentability may differ in certain countries, particularly developing countries, and the breadth of patent claims allowed can be inconsistent. In addition, the laws of some foreign countries may not protect our intellectual property rights to the same extent as laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories in which we have patent protection that may not be sufficient to terminate infringing activities.
We do not have patent rights in certain foreign countries in which a market may exist. Moreover, in foreign jurisdictions where we do have patent rights, proceedings to enforce such rights could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, and our patent applications at risk of not issuing. Additionally, such proceedings could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Thus, we may not be able to stop a competitor from marketing and selling in foreign countries products that are the same as or similar to our products, and our competitive position in the international market would be harmed.
If our trademarks and trade names are not adequately protected, then we may not be able to build brand recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented, declared generic or determined to be infringing on other marks. We may not be able to protect our rights in these trademarks and trade names, which we need in order to build brand recognition in our markets of interest. In addition, third parties have registered trademarks similar to our trademarks in foreign jurisdictions and may in the future file for registration of such trademarks. If they succeed in registering or developing common law rights in such trademarks, and if we were not successful in challenging such third-party rights, we may not be able to use these trademarks to market our products in those countries. In any case, if we are unable to establish brand recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected.
We have a history of significant net operating losses. If we do not achieve and sustain profitability, our financial condition could suffer.
We have experienced significant net operating losses, and we expect to continue to incur net operating losses for the foreseeable future. We expect to continue to incur net operating losses as we continue to build our direct sales force in the United States, expand commercial sales of Algovita in the United States in 2018, continue to pursue regulatory approvals for Virtis, and assuming approval, begin to build a sales force for Virtis.
We intend to continue to increase our operating expenses substantially as we add sales representatives and independent sales agents in the United States and a network of distributors and independent sales agents outside of the United States to increase our geographic sales coverage and penetration, invest in research and development programs to accelerate new product launches, expand our marketing and training programs, conduct clinical studies, and increase our general and administrative functions as a result of operating as an independent publicly-traded company. We may not ever generate sufficient sales from our operations to achieve profitability, and even if we do achieve profitability, we may not be able to remain profitable for any substantial period of time. If our revenue grows more slowly than we anticipate, or if our operating expenses are higher than we expect, we may not be able to achieve profitability and our financial condition will suffer.
We will be required to obtain additional funds in the future, and these funds may not be available on acceptable terms or at all.
Our operations have consumed substantial amounts of cash since inception, and we anticipate our expenses will increase as we continue to build a direct sales force in the United States, develop the use of our neurostimulation technology platform for the treatment of other conditions, and continue to grow our business. Our existing resources, inclusive of the cash capital contribution of $75.0 million made by Integer immediately prior to the completion of the spin-off, borrowings under our credit facility, as amended (the availability of which is subject to compliance with specified conditions and covenants), and net proceeds from our follow-on common stock offering, may not allow us to conduct all of the activities that we believe would be beneficial for our future growth. As a result, we may need to seek additional funds in the future. If we are unable to raise additional funds on favorable terms, or at all, we may not be able to support our commercialization efforts for Algovita or increase our research and development activities, and the growth of our business may be negatively impacted.
Our cash requirements in the future may be significantly different from our current estimates and depend on many factors, including:
|
• |
the outcome, timing of, and costs involved in, seeking and obtaining supplementary or additional approvals from the FDA and other regulatory authorities; |
|
• |
the scope and timing of our investment in our United States commercial infrastructure and direct sales force; |
|
• |
the research and development activities we intend to undertake in order to expand the indications and product enhancements that we intend to pursue; |
|
• |
the costs of commercialization activities including product sales, marketing, manufacturing and distribution; |
|
• |
the degree and rate of market acceptance of Algovita; |
|
• |
changes or fluctuations in our inventory supply needs and forecasts of our supply needs; |
|
• |
the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
|
• |
our need to implement additional infrastructure and internal systems; |
|
• |
our ability to satisfy the conditions and covenants, including trailing six-month revenue milestones to be able to draw upon our $45 million of term loan financing under our credit facility; |
|
• |
our ability to hire additional personnel to support our operations as an independent publicly-traded company; and |
|
• |
the emergence of competing technologies or other adverse market developments. |
To finance these activities, we may seek additional funds through borrowings or rounds of financing, including private or public equity or debt offerings, and strategic partnerships. We may be unable to raise necessary funds on favorable terms, or at all.
If we borrow funds or issue debt securities, these securities will have payment rights superior to holders of our common stock and may contain covenants that will restrict our operations. We may have to obtain funds through arrangements with strategic partners that may require us to relinquish rights to our technologies, product candidates, or products that we otherwise may not wish to relinquish.
Our credit facility contains restrictions that limit our flexibility in operating our business.
In March 2016, we entered into a credit facility with Oxford Finance LLC and Silicon Valley Bank and borrowed $15.0 million under the facility. In February 2017, the credit facility was amended to extend the availability of the final two tranches of the facility. In September 2017 we drew down an additional $12.5 million under the facility. In February 2018, our credit facility was amended again to extend the amortization period and maturity date and to replace the $5 million revolving line commitment with a $5 million term loan commitment. In February 2018 we also drew down an additional $12.5 million under the facility. Our credit facility contains various covenants that limit our ability to engage in specified types of transactions. Subject to limited exceptions, these covenants limit our ability to, among other things:
|
• |
sell, lease, transfer, assign, or dispose of any part of our business or property; |
|
• |
create, incur, assume, or be liable for any indebtedness other than unsecured indebtedness to trade creditors incurred in the ordinary course of business and other permitted indebtedness as defined in our credit facility; |
|
• |
make restricted payments, including paying dividends on, repurchasing, or making distributions with respect to our capital stock; |
|
• |
make specified investments (including loans and advances); |
|
• |
merge or consolidate; and |
|
• |
enter into certain transactions with our affiliates. |
In addition, we are subject to a quarterly financial covenant requiring us to achieve specified minimum consolidated product revenues. The covenants in our credit facility may limit our ability to take certain actions and, in the event that we breach one or more covenants, our lenders may choose to declare an event of default and require that we immediately repay all amounts outstanding and foreclose on the collateral granted to it under the facility.
We may record future goodwill impairment charges or other asset impairment charges related to one or more of our reporting units, which could materially adversely impact our results of operations.
We assess our goodwill balances for impairment on the last day of each fiscal year, or more frequently if indicators are present or changes in circumstances suggest that impairment may exist. We assess goodwill for impairment at the reporting unit level and, in evaluating the potential for impairment of goodwill, we make assumptions regarding estimated revenue projections, growth rates, cash flows and discount rates. On December 31, 2017, we performed our annual qualitative analysis goodwill impairment test for all of our reporting units. In conjunction with our annual analysis, we determined that it was more likely than not that the fair value of each reporting unit exceeded its carrying value. Refer to Our Critical Accounting Estimates within our Management's Discussion and Analysis of Financial Condition and Results of Operations contained in Item 7 of this Annual Report on Form 10-K for more information regarding this testing.
On a quarterly basis, we monitor the key drivers of fair value to detect events or other changes that would warrant an interim impairment test of our goodwill and intangible assets. Relatively small declines in the future performance and cash flows of a reporting unit or asset group, changes in our reporting units or in the structure of our business as a result of future reorganizations, acquisitions or divestitures of assets or businesses, or small changes in other key assumptions, may result in the recognition of significant asset impairment charges, which could have a material adverse impact on our results of operations.
We are required to adopt a new revenue recognition standard in 2018, and we may have difficulties adopting this standard.
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Codification (“ASC”) Update No. 2014-09, Revenue from Contracts with Customers (“ASC 606”), which has been subsequently updated. The purpose of ASC 606 is to provide enhancements to the quality and consistency of how revenue is reported while also improving comparability in the financial statements of companies using accounting principles generally accepted in the United States of America (“GAAP”) and International Financial Reporting Standards. The core principle requires entities to recognize revenue in a manner that depicts the transfer of goods or services to customers in amounts that reflect the consideration an entity expects to be entitled to in exchange for those goods or services. We will adopt the provisions in ASC 606, as amended, effective January 1, 2018 under the modified retrospective method and will only apply this method to contracts that are not completed as of the date of adoption.
In order to comply with the requirements of ASC 606 on January 1, 2018, we are continuing to update and enhance our internal accounting systems and our internal controls over financial reporting. If we are not successful in updating our policies, procedures, information systems and internal controls over financial reporting, the revenue that we recognize and the related disclosures that we provide under ASC 606 may not be complete or accurate, which could harm our operating results or cause us to fail to meet our reporting obligations.
The application of the new standard will be based on all information available to us as of the date of adoption and up through subsequent interim reporting, including transition guidance published by the standard setters. However, we understand the interpretation of these new standards will continue to evolve as other public companies adopt ASC 606 and the standard setters issue new interpretive guidance related to these rules. As a result, changes in the interpretation of these rules could result in adjustments to our application of the new standard, which could have an adverse effect on our results of operations and financial condition.
We will need to maintain sufficient levels of inventory, which could consume a significant amount of our resources, reduce our cash flows and lead to inventory impairment charges.
We are subject to the risk of inventory obsolescence and expiration, which may lead to inventory impairment charges. In order to market and sell Algovita effectively, we often must maintain high levels of inventory. In particular, as we expand our commercial launch of Algovita in the United States, we intend to substantially increase our levels of inventory and our safety stock in order to meet our estimated demand and, as a result, incur significant expenditures associated with increases in our inventory and safety stock. The manufacturing process requires lengthy lead times, during which components of Algovita may become obsolete, and we may over- or under-estimate the amount needed of a given component, in which case we may expend extra resources or be constrained in the number of Algovita systems that we can produce. As compared to direct manufacturers, our dependence on Integer and Minnetronix as sole manufacturers of certain key parts and devices used in our products exposes us to greater lead times increasing our risk of inventory obsolescence. Furthermore, Algovita has a limited shelf life due to sterilization requirements, and part or all of a given product or component may expire and its value would become impaired and we would be required to record an impairment charge. If our estimates of required inventory are too high, we may be exposed to further inventory obsolesce risk. In the event we experience a supply chain imbalance, it could have a material adverse effect on our earnings and cash flows due to the resulting costs associated with the inventory impairment charges and costs required to replace such inventory.
If we increase our sales outside the Unites States, we may be subject to risks associated with currency fluctuations, and changes in foreign currency exchange rates could impact our results of operations.
If we increase our sales outside the Unites States, we may be subject to changes in the exchange rates between foreign currencies and the U.S. dollar, which could materially impact our reported results of operations and distort period-to-period comparisons. Fluctuations in foreign currency exchange rates also impact the reporting of our receivables and payables in non-U.S. currencies. As a result of foreign currency fluctuations, it could be more difficult to detect underlying trends in our business and results of operations. In addition, to the extent that fluctuations in currency exchange rates cause our results of operations to differ from our expectations or the expectations of our investors, the trading price of our common stock could be adversely affected.
In the future, we may engage in exchange rate-hedging activities in an effort to mitigate the impact of exchange rate fluctuations. If our hedging activities are not effective, changes in currency exchange rates may have a more significant impact on our results of operations.
We are subject to extensive governmental regulation, and our failure to comply with applicable requirements could cause our business to suffer.
Governmental authorities, principally the FDA and corresponding state and foreign regulatory agencies and authorities, regulate the medical device industry extensively and oversee virtually all aspects of a medical device’s development, testing, manufacturing, labeling, promotion, distribution and marketing, as well as modifications to existing products and the marketing of existing products for new indications.
Generally, unless an exemption applies, a medical device and modifications to a device or its indications must receive either premarket approval or premarket clearance from the FDA before it can be marketed in the United States. The approval process may involve lengthy and detailed laboratory and clinical testing procedures, sampling activities, extensive agency review processes, and other costly and time-consuming procedures. It may take several years to satisfy these requirements, depending on the complexity and novelty of the product or modification. We may not be successful in the future in receiving approvals and clearances in a timely manner or at all. Any delay in obtaining, or any failure to obtain, such approvals could negatively impact our marketing of any future products and reduce our product revenues.
The laws and regulations to which we are subject are complex and have tended to become more stringent over time. Legislative or regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales. See “Business – Regulation of our Business” for additional information regarding the regulatory schemes applicable to us and our business.
Our failure to comply with U.S. federal and state regulations or other foreign regulations applicable in the countries where we operate could lead to the issuance of warning letters or untitled letters, the imposition of injunctions, suspensions or loss of regulatory clearance or approvals, product recalls, termination of distribution, product seizures or civil penalties. In the most extreme cases, criminal sanctions or closure of manufacturing facilities are possible. If any of these risks materialize, our business would be adversely affected.
Algovita and other neurostimulation devices we develop may in the future be subject to notifications, recalls, or voluntary market withdrawals that could harm our reputation, business and financial results.
The FDA and similar foreign governmental authorities, such as the Federal Institute for Drugs and Medical in Germany, have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture that could affect patient safety. Further, under the FDA and similar foreign medical device reporting regulations, we are required to submit information to the governmental agency when we receive a report or become aware that a device has or may have caused or contributed to a death or injury or has or may have a malfunction that could likely cause or contribute to death or injury if the malfunction were to recur, which may prompt action by the governmental authority. A government-mandated recall or voluntary recall by us or one of our distributors could occur as a result of component failures, manufacturing errors, design or labeling defects or other issues. Recalls, which include certain notifications and corrections as well as removals, of our products could divert managerial and financial resources and could have an adverse effect on our financial condition, harm our reputation with customers, and reduce our ability to achieve expected revenue.
In addition, the manufacturing of our products is subject to extensive post-market regulation by the FDA and foreign regulatory authorities, and any failure by us or our suppliers, including Integer and Minnetronix, to comply with regulatory requirements could result in recalls, facility closures, and other penalties. We and our suppliers are subject to the FDA’s Quality System Regulation and comparable foreign regulations that govern the methods used in, and the facilities and controls used for, the design, manufacture, quality assurance, labeling, packaging, sterilization, storage, shipping, and servicing of medical devices. These regulations are enforced through periodic inspections of manufacturing facilities. Any manufacturing issues at our or our suppliers’ facilities, including failure to comply with regulatory requirements, may result in warning or untitled letters, manufacturing restrictions, voluntary or mandatory recalls or corrections, fines, withdrawals of regulatory clearances or approvals, product seizures, injunctions, or the imposition of civil or criminal penalties, which would adversely affect our business results and prospects.
The misuse or off-label use of our products may harm our image in the marketplace, result in injuries that lead to product liability suits, which could be costly to our business, or result in costly investigations and sanctions from the FDA and other regulatory bodies if we are deemed to have engaged in off-label promotion.
Algovita has received premarket approval from the FDA and CE mark approval for use in the treatment of chronic pain of the trunk or limbs. We cannot, however, prevent a physician from using our product off-label, when in the physician’s independent professional medical judgment she deems it appropriate. There may be increased risk of injury to patients if physicians attempt to use our product off-label. Furthermore, the use of our product for indications other than those approved by the applicable regulatory body may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients. Physicians may also misuse our product or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability litigation. If our products are misused or used with improper technique, we may become subject to costly litigation, including product liability litigation, by our customers or their patients. In addition, if the FDA or other regulatory bodies determines that our promotional materials or training constitute promotion of an off-label use, it or they, as applicable, could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions that may result in fines, penalties, injunctions or other restrictions. Any of these events could significantly harm our business and results of operations.
We may be subject to federal, state and foreign healthcare laws and regulations, and a finding of failure to comply with such laws and regulations could have a material adverse effect on our business.
Although we do not provide healthcare services, submit claims for third-party reimbursement, or receive payments directly from Medicare, Medicaid or other third-party payers for our products, we are subject to healthcare fraud and abuse regulation and enforcement by federal, state and foreign governments, which could significantly impact our business.
In the United States, the laws that may affect our ability to operate include, but are not limited to:
|
• |
the U.S. federal Anti-Kickback Statute; |
|
• |
the U.S. federal False Claims Act and civil money penalties, including whistleblower and qui tam actions; |
|
• |
Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology and Clinical Health Act; |
|
• |
federal regulation of payments made to physicians and other healthcare providers (known as the physician “sunshine” requirements), which requirements have been recently expanded under the Patient Protection and ACA; |
|
• |
U.S. Foreign Corrupt Practices Act of 1977 and other anti-bribery laws; and |
|
• |
state and foreign law equivalents of each of the above federal laws. |
See “Business – Regulation of our Business” for a detailed description of each of these laws and their impact on our operations. The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Responding to investigations can be time-and resource-consuming and can divert management’s attention from the business. Additionally, as a result of these investigations, healthcare providers and entities may have to agree to additional onerous compliance and reporting requirements as part of a consent decree or corporate integrity agreement. Any such investigation or settlement could increase our costs or otherwise have an adverse effect on our business.
If our operations are found to be in violation of any of the laws or governmental regulations that apply to us now or in the future, we may be subject to penalties, including civil and criminal penalties, damages, fines, disgorgement, exclusion from governmental health care programs, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our financial results.
Healthcare legislative reform measures may have a material adverse effect on us.
In March 2010, the ACA was signed into law, which includes, among other things, a deductible 2.3% excise tax on any entity that manufactures or imports medical devices offered for sale in the United States, with limited exceptions. As a consequence of passage of the TCJA, this excise tax has been suspended through December 31, 2019, but if this suspension is not continued or made permanent thereafter, the excise tax will be reinstated starting on January 1, 2020 and would result in a significant increase in the tax burden on our industry. If any efforts we undertake to offset the excise tax in the future are unsuccessful, the increased tax burden could have an adverse effect on our results of operations and cash flows. Other elements of the ACA, including comparative effectiveness research, an independent payment advisory board and payment system reforms and shared savings pilots and other provisions, may significantly affect the payment for, and the availability of, healthcare services and result in fundamental changes to federal healthcare reimbursement programs, any of which may materially affect our business. The full impact of the ACA, its possible repeal or replacement and the impact of other laws and reform measures that may be proposed and adopted in the future, remains uncertain, but may continue the downward pressure on medical device pricing, especially under the Medicare program, and may also increase our regulatory burden and operating costs.
Additional state and federal healthcare reform measures may be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
Risks Related to our Common Stock
An active, liquid and orderly market for our common stock may not be sustained and the trading price of our common stock is volatile.
The trading price of our common stock is highly volatile and is subject to wide fluctuations in response to various factors, some of which are beyond our control. These factors include those discussed in this “Risk Factors” section of this Annual Report on Form 10-K, as well as:
|
• |
results from, or any delays in, clinical trial programs relating to our product candidates, including any additional planned clinical trials for Algovita or Virtis; |
|
• |
our reliance on each of Integer, our exclusive and sole manufacturer and supplier of parts and components for Algovita, and Minnetronix, our sole-source supplier of external peripheral devices; |
|
• |
any supply shortages or delays, manufacturing problems or price fluctuations related to Algovita or its components that could impact our inventory supply and inhibit our ability to meet demand as we expand our business; |
|
• |
announcements of new products by us or our competitors; |
|
• |
adverse actions taken by regulatory agencies with respect to our clinical trials, manufacturing supply chain or sales and marketing activities; |
|
• |
recalls of our products; |
|
• |
our operating results; |
|
• |
our cash-on-hand and overall liquidity; |
|
• |
dilution of our common stock resulting from the issuance of additional shares of common stock, preferred stock or securities convertible into additional shares of common stock; |
|
• |
changes or developments in laws or regulations applicable to Algovita and our other products; |
|
• |
failure to obtain regulatory approval for Virtis in the United States or Europe; |
|
• |
the success of our efforts to acquire or develop additional products; |
|
• |
any intellectual property infringement actions in which we may become involved; |
|
• |
announcements concerning our competitors or the medical device industry in general; |
|
• |
achievement of expected product sales and profitability; |
|
• |
manufacture, supply or distribution shortages; |
|
• |
FDA or foreign regulatory actions affecting us or our industry or other healthcare reform measures in the United States; |
|
• |
changes in financial estimates or recommendations by securities analysts; |
|
• |
trading volume of our common stock; |
|
• |
sales of our common stock by us, our executive officers and directors or our stockholders in the future; |
|
• |
general changes or uncertainty in the political, regulatory, safety or economic conditions in the United States or Europe, including as a result of the change in the United States presidential administration or the United Kingdom’s vote to leave the European Union; |
|
• |
general economic and market conditions and overall fluctuations in the United States equity markets; and |
|
• |
the loss of any of our key scientific personnel or executive officers. |
In addition, the stock markets in general, and the markets for equity securities of medical device companies in particular, have experienced volatility that may have been unrelated to the operating performance of the issuer. These broad market fluctuations may adversely affect the trading price or liquidity of our common stock. In the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the issuer. If any of our stockholders were to bring such a lawsuit against us, we could incur substantial costs defending the lawsuit and the attention of our management would be diverted from the operation of our business, which could seriously harm our financial position. Any adverse determination in litigation could also subject us to significant liabilities.
If securities or industry analysts issue inaccurate or unfavorable research regarding our stock, our stock price and trading volume could decline.
The trading market for our common stock is influenced by the research and reports that industry or securities analysts publish about us or our business. If analysts that choose to cover us downgrade our stock or issue inaccurate or unfavorable research regarding us, our business model or our stock performance, or if our operating results fail to meet the expectations of these analysts, our stock price could decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our trading volume to decline and, as a result, our stock price may become more volatile and could decline.
We are an “emerging growth company” and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, our common stock may be less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we have taken advantage of some of the exemptions from the reporting requirements that are afforded to emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may become more volatile. We may continue to take advantage of these exemptions until we are no longer an emerging growth company.
Your percentage of ownership in us may be diluted in the future.
As with any independent publicly-traded company, your percentage ownership in us may be diluted in the future because of equity issuances for acquisitions, capital market transactions or otherwise, including incentive equity awards that we have granted, and expect to continue to grant, to our directors, officers and employees. Our stockholders experienced dilution in connection with our follow-on common stock offering completed in February 2018. In addition, under our credit facility, we have issued, and expect to issue, warrants to the lenders to purchase a number of shares of our common stock with a notional value equal to 4.5% of the funded amount of such term loan tranches, with all warrants issued at the time of a tranche funding having an exercise price equal to the lower of the average closing price of our common stock for the ten previous days of trading or the closing price of our common stock on the day prior to such tranche funding. Each warrant is exercisable for ten years from the date of issuance. If we issue common stock, preferred stock or securities convertible into common stock, including the warrants issued to our lenders, our stockholders would experience dilution and, as a result, our stock price may decline.
Changes in tax law may affect the U.S. federal tax considerations of the purchase, ownership and disposition of our common stock.
On December 22, 2017, the TCJA was enacted into law resulting in significant changes to United States federal income taxation law, including changes to the U.S. federal income taxation of corporations, including us, and changes to the U.S. federal income taxation of stockholders in U.S. corporations, including investors in our common stock. The Treasury Department is expected to, but has not yet, issued regulations and other guidance regarding this new legislation. We are currently unable to predict what guidance will be issued with respect to the Tax Cuts and Jobs Act, whether any further changes to United States federal income taxation law will occur or the impact of any of the foregoing, including on the U.S. federal income tax considerations relating to the purchase, ownership and disposition of our common stock.
If we are unable to implement and maintain effective internal control over financial reporting, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our common stock could be adversely affected.
As an independent publicly-traded company, we are required to maintain internal control over financial reporting and to report any material weaknesses in our internal control. Section 404 of the Sarbanes-Oxley Act requires that we evaluate and determine the effectiveness of our internal control over financial reporting and provide a management report on internal control over financial reporting. The Sarbanes-Oxley Act also requires that our independent registered public accounting firm attest to our internal control over financial reporting, to the extent we are no longer an emerging growth company, as defined by the JOBS Act. We do not expect to have our independent registered public accounting firm attest to our internal control over financial reporting for so long as we are an emerging growth company. If we identify material weaknesses in our internal control over financial reporting, if we are unable to assert that our internal controls over financial reporting are effective, or, when required in the future, if our independent registered public accounting firm is unable to express an opinion as to the effectiveness of our internal control over financial reporting, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our common stock could be adversely affected, and we could become subject to investigations by the stock exchange on which our securities are then-listed, the SEC, or other regulatory authorities, which could require additional financial and management resources.
Provisions in our certificate of incorporation, by-laws and under Delaware law may discourage a takeover that stockholders may consider favorable and could lead to entrenchment of management.
Our certificate of incorporation and by-laws contain provisions that could significantly reduce the value of our shares to a potential acquirer or delay or prevent changes in control or changes in our management without the consent of our Board of Directors.
The provisions in our certificate of incorporation and by-laws include the following:
|
• |
a classified board of directors with three-year staggered terms, which may delay the ability of stockholders to change the membership of a majority of our board of directors; |
|
• |
no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
|
• |
the exclusive right of our Board of Directors to elect a director to fill a vacancy created by the expansion of our Board of Directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our Board of Directors; |
|
• |
the required approval of at least 66 2/3% of the voting power of all shares of capital stock then entitled to vote generally in the election of directors to remove a director for cause, and the prohibition on removal of directors without cause; |
|
• |
the ability of our Board of Directors to authorize the issuance of shares of preferred stock and to determine the price and other terms of those shares, including preferences and voting rights, without stockholder approval, which could be used to significantly dilute the ownership of a hostile acquirer; |
|
• |
the ability of our Board of Directors to alter our by-laws without obtaining stockholder approval; |
|
• |
the required approval of at least 66 2/3% of the voting power of all shares of capital stock then entitled to vote generally in the election of directors to amend, alter, change, repeal or adopt any provision of our by-laws and certain provisions of our certificate of incorporation; |
|
• |
a prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of our stockholders; |
|
• |
the requirement that a special meeting of stockholders may be called only by our Board of Directors, Chairman of our Board of Directors or our Chief Executive Officer, which may delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors for cause; and |
|
• |
advance notice procedures that stockholders must comply with in order to nominate candidates to our Board of Directors or to propose matters to be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of us. |
In addition, these provisions would apply even if we were to receive an offer that some stockholders may consider beneficial.
We are also subject to the anti-takeover provisions contained in Section 203 of the General Corporation Law of the State of Delaware, or the DGCL. Under Section 203, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other exceptions, the board of directors has approved the transaction.
Claims for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us and may reduce the amount of money available to us.
Our certificate of incorporation, by-laws and individual indemnity agreements with our officers and directors provide that we are required to indemnify our directors and officers, and, to the extent authorized from time to time by our Board of Directors, our other employees and agents, to the fullest extent permitted by Delaware law, subject to specified exceptions. Any claims for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us and may reduce the amount of money available to us.
Item 1B. Unresolved Staff Comments
None.
Our corporate headquarters is located in Plano, Texas, and we have research and development facilities located in Blaine, Minnesota, Broomfield, Colorado, and Ann Arbor, Michigan. Our NeuroNexus segment utilizes the Ann Arbor facility and our Nuvectra segment utilizes the remaining facilities. These facilities, with the exception of our Blaine facility, which is owned by Nuvectra, are leased and consist of approximately 71,522 square feet of office and laboratory space. The lease, as amended, for our Plano, Texas headquarters, which is with Integer, expires in March 2023. The leases, as amended, for our Broomfield, Colorado and Ann Arbor, Michigan facilities expire in September 2022 and June 2022, respectively. We believe the facilities we operate and our equipment are effectively utilized, well maintained, generally are in good condition, and will be able to accommodate our capacity needs to meet current levels of demand. We continuously review our anticipated requirements for facilities and, on the basis of that review, may from time to time acquire additional facilities, enhance existing facilities and/or dispose of existing facilities.
On September 19, 2017, Boston Scientific Corporation (“Boston Scientific”) filed a lawsuit in district court in Suffolk County, Massachusetts, against the Company and three former Boston Scientific employees recently hired by the Company, alleging tortious interference of contract on the part of the Company and breaches of contract related to non-solicitation and confidentiality agreements by Boston Scientific’s former employees. No demands for any monetary relief or specific performance have been made by Boston Scientific as of the date hereof. We intend to vigorously defend against the allegations.
Additionally, periodically we are a party to various legal actions, both threatened and filed, arising in the normal course of business. While we do not expect that the ultimate resolution of any such normal course actions will have a material effect on our results of operations, financial position, or cash flows, litigation is subject to inherent uncertainties. As such, there can be no assurance that any such normal course actions, which we currently believe to be immaterial, will not become material in the future.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Market for Registrant’s Common Equity, Related Shareholder Matters, and Issuer Purchases of Equity Securities
Market Information
Our common stock is traded on the Nasdaq Global Market (“Nasdaq”) under the symbol “NVTR.” The following table provides the market range for the closing price of our common stock since it began publically trading on March 14, 2016 based on reported sales prices on Nasdaq.
|
2017 |
High |
Low |
||||||
|
First Quarter |
$ | 8.02 | $ | 5.01 | ||||
|
Second Quarter |
13.89 | 6.36 | ||||||
|
Third Quarter |
13.26 | 10.29 | ||||||
|
Fourth Quarter |
14.52 | 7.72 | ||||||
|
2016 |
High |
Low |
||||||
|
First Quarter |
$ | 7.43 | $ | 4.30 | ||||
|
Second Quarter |
9.75 | 6.15 | ||||||
|
Third Quarter |
7.92 | 6.22 | ||||||
|
Fourth Quarter |
6.66 | 4.74 | ||||||
Holders
The closing price of our common stock on February 21, 2018 was $10.06. As of February 21, 2018, there were 111 holders of record of our common stock.
Dividend Policy
We did not pay a cash dividend in 2016 or 2017 and currently we do not intend to pay cash dividends on Nuvectra common stock. Our credit facility prohibits the payment of cash dividends without the prior written consent of our lenders, but allows the payment of stock dividends. The declaration and amount of any future cash or stock dividends, however, will be determined by our Board of Directors and will depend on our financial condition, earnings, corporate strategy and capital requirements, and any other factors that our Board of Directors believes are relevant.
Securities Authorized for Issuance under Equity Compensation Plans
Please see Item 12 "Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters" under Part III of this Annual Report for information on where to find information required by Item 201(d) of Regulation S-K.
Recent Sales of Unregistered Securities
Other than the issuance of warrants to Oxford Finance LLC and Silicon Valley Bank, as disclosed in Note 7 of the notes to the Consolidated Financial Statements contained in Item 8 of this Report, there were no unregistered sales of equity securities during the fiscal year ended December 31, 2017.
Use of Proceeds
Our Registration Statement on Form S-3 (File No. 333-220834) was declared effective by the SEC on October 24, 2017. On February 5, 2018, pursuant to an underwritten shelf takedown, with Piper Jaffray & Co. serving as representative of the several underwriters, we sold 3,248,750 shares of our common stock and received net proceeds of approximately $23.8 million, after deducting underwriting discounts and commissions and expenses. We intend to use these proceeds as set forth in our prospectus filed with the SEC pursuant to Rule 424(b)(5) on February 1, 2018.
Issuer Purchases of Equity Securities
There were no repurchases of shares of common stock made during the fourth quarter of the fiscal year ended December 31, 2017.
Transferability of Our Shares of Common Stock
Our shares of common stock that were distributed to Integer’s stockholders in the spin-off are freely transferable, unless the holder is considered an “affiliate” of ours under Rule 144 under the Securities Act. Persons who can be considered our affiliates generally include individuals or entities that directly, or indirectly through one or more intermediaries, control, are controlled by, or are under common control with, us, and may include some or all of our executive officers and directors. Our affiliates may sell our shares of common stock received in the spin-off only:
|
• |
under a registration statement that the SEC has declared effective under the Securities Act; or |
|
• |
under an exemption from registration under the Securities Act, such as the exemption afforded by Rule 144. |
We filed a registration statement on Form S-8 under the Securities Act to register shares of Nuvectra common stock authorized for issuance under our equity incentive plan. The shares covered by the S-8 registration statement are shares of our common stock underlying outstanding stock options, restricted stock units, stock appreciation rights, restricted stock and other equity awards issued under our equity incentive plan. This registration statement was effective immediately upon filing. Shares of our common stock issued pursuant to equity awards after the effective date of our registration statement on Form S-8, other than shares of our common stock issued to affiliates, generally are freely tradable without further registration under the Securities Act.
Stock Performance Graph
The graph below compares the twenty-two month total return to stockholders on our common stock with the return of the Standard & Poor’s (S&P) 500 Stock Index and the S&P Health Care Equipment Index. We relied upon information provided by another firm with respect to the stock performance graph. We did not attempt to validate the information supplied to us other than review it for reasonableness. The graph assumes $100 was invested in our common stock and in each of the named indices on March 14, 2016, and that all dividends were reinvested.
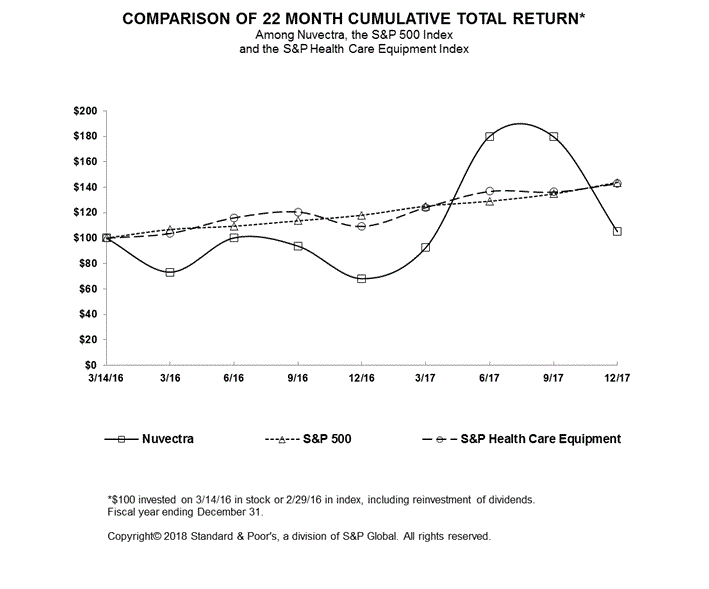
|
3/14/16 |
3/16 |
6/16 |
9/16 |
12/16 |
3/17 |
6/17 |
9/17 |
12/17 |
||||||||||||||||||||||||||||
|
Nuvectra |
$ | 100.00 | $ | 73.31 | $ | 100.27 | $ | 93.77 | $ | 68.16 | $ | 92.55 | $ | 179.95 | $ | 179.67 | $ | 105.15 | ||||||||||||||||||
|
S&P 500 |
100.00 | 106.78 | 109.41 | 113.62 | 117.97 | 125.12 | 128.98 | 134.76 | 143.72 | |||||||||||||||||||||||||||
|
S&P Health Care Equipment |
100.00 | 103.69 | 116.03 | 120.63 | 109.24 | 124.15 | 136.89 | 136.33 | 142.99 | |||||||||||||||||||||||||||
Note: The stock price performance shown on the graph and table above is not indicative of future price performance. This graph and table shall not be deemed "filed" for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, regardless of any general incorporation language in such filing.
Item 6. Selected Financial Data
Prior to fiscal year 2017, the Company utilized a fifty-two, fifty-three week fiscal year ending on the Friday nearest December 31. Fiscal year 2016 ended on December 30, 2016 and contained fifty-two weeks. Beginning in fiscal year 2017, the Company utilizes a calendar year end.
The selected consolidated financial data should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and the notes thereto included elsewhere in this report. The selected financial data in this section are not intended to replace our consolidated financial statements and the related notes. Our historical results are not necessarily indicative of our future results. Prior to March 14, 2016, our financial statements were prepared on a “combined” basis from the consolidated financial statements of Integer to represent our financial position and performance as if we had existed on a stand-alone basis in conformity with GAAP.
|
Year Ended |
||||||||
|
Statement of Operations Data: (in thousands) |
December 31, 2017 |
December 30, 2016 |
||||||
|
Sales |
$ | 31,836 | $ | 12,535 | ||||
|
Cost of sales |
15,886 | 6,430 | ||||||
|
Gross profit |
15,950 | 6,105 | ||||||
|
Operating expenses: |
||||||||
|
Selling, general and administrative expenses |
43,860 | 28,507 | ||||||
|
Research, development and engineering costs, net |
14,102 | 14,524 | ||||||
|
Other operating expenses, net |
— | 476 | ||||||
|
Total operating expenses |
57,962 | 43,507 | ||||||
|
Loss before provision for income taxes |
(44,575 | ) | (38,428 |
) |
||||
|
Provision for income taxes |
25 | — | ||||||
|
Net loss |
$ | (44,600 | ) | $ | (38,428 |
) |
||
|
Comprehensive loss |
$ | (44,599 | ) | $ | (38,430 |
) |
||
|
As of |
||||||||
|
Balance Sheet Data: (in thousands) |
December 31, 2017 |
December 30, 2016 |
||||||
|
Cash and cash equivalents |
$ | 28,165 | $ | 63,710 | ||||
|
Working capital |
28,978 | 56,523 | ||||||
|
Total assets |
91,103 | 119,302 | ||||||
|
Stockholders’ Equity |
48,173 | 88,578 | ||||||
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis should be read in conjunction with the consolidated financial statements and accompanying notes included in Item 8 of this Annual Report on Form 10-K.
The following discussion and analysis describes the factors that had a material effect on our financial position, results of operations and cash flows during the year ended December 31, 2017 as compared to the year ended December 30, 2016. You should read this discussion and analysis in conjunction with our consolidated financial statements and the notes to those consolidated financial statements in Item 8 of this Annual Report on Form 10-K. This Management’s Discussion and Analysis of Financial Condition and Results of Operations, or MD&A, contains forward-looking statements. Actual results could differ materially from those contained in any forward-looking statements. See “Risk Factors” for a discussion of the uncertainties, risks and assumptions associated with these statements.
Spin-Off From Integer
On March 14, 2016, Integer completed the spin-off of Nuvectra from the remainder of its business. The spin-off was accomplished by the distribution of all Nuvectra’s issued and outstanding shares of common stock to Integer’s stockholders on the basis of one share of Nuvectra common stock for every three shares of Integer common stock held on March 7, 2016, the record date of the distribution. Immediately prior to completion of the spin-off, QiG Group, LLC was converted from a limited liability company into Nuvectra Corporation, a Delaware corporation. At the time of the completion of the spin-off, Integer’s stockholders owned 100% of the outstanding common stock of both Integer and Nuvectra.
Our Business
Nuvectra is a neurostimulation medical device company focused on the development and commercialization of our neurostimulation technology platform for the treatment of various disorders through stimulation of tissues associated with the nervous system. Our neurostimulation technology platform has the capability to provide treatment to patients in several established neurostimulation markets, including SCS, SNM, DBS, and other emerging neurostimulation markets.
Our Algovita™ SCS system, or Algovita, is the first application of our neurostimulation technology platform and is indicated for the treatment of chronic pain of the trunk and limbs. Algovita received premarket approval from the FDA in November 2015, and we commercially launched Algovita in the United States during the first half of 2016. Outside of the United States, Algovita obtained CE mark approval in June 2014 and is indicated for the treatment of chronic intractable pain of the trunk or limbs. Algovita is reimbursable under existing SCS codes in the United States, the European Union (“EU”) and Australia, and has been commercially available to patients in Germany and several other European countries since November 2014.
We have also developed our existing platform for use in the SNM market and have filed regulatory submissions with the FDA and CE mark authorities in January 2017 and December 2016, respectively, for Virtis, the Company’s SNM system for the treatment of chronic urinary retention and the symptoms of overactive bladder. In addition, in early 2016, we entered into a development agreement with Aleva. This agreement provided that we would leverage our neurostimulation technology platform to develop a DBS system for Aleva to treat Parkinson’s disease. This platform is still under development. During the third quarter of 2017, Aleva received additional financing, and we entered into an Amended and Restated Development Agreement.
Our results also include the operations of our subsidiary NeuroNexus. NeuroNexus works closely with researchers to develop and refine new tools that aid and advance neuroscience research. NeuroNexus designs, manufactures and sells neural interface systems including high quality, high density microelectrode arrays, custom designed probes, electrode instrumentation and accessories. In addition, the NeuroNexus team possesses years of neuroscience research experience to help facilitate successful research projects and provide insight to minimize known challenges. Certain of NeuroNexus’ technologies could be incorporated into our neurostimulation technology platform in the future.
Our revenues include sales of Algovita, neural interface technology, components and systems to the neuroscience and clinical markets, and development and engineering service fees. We expect that our future revenues will come primarily from sales of neurostimulation medical device products, including Algovita, particularly as we continue our launch commercially in the United States, and from Virtis, the second application of our neurostimulation technology platform and our first product for the SNM market. From time to time, our future revenues may also include technology licensing fees, development and engineering service fees, and royalty fees.
Prior to the spin-off, our expenses included an allocation of general corporate overhead expenses from Integer relating to the following support functions provided for us by Integer: executive oversight, finance, legal, human resources, tax, information technology, product development, corporate procurement, and facilities. These expenses were charged to us on the basis of direct usage, when identifiable, with the remainder allocated primarily on a pro rata basis of estimated hours incurred, headcount, square footage, or other measures. We consider the expense allocation methodology and results to be reasonable for all periods presented. However, these allocations are not indicative of the actual expenses that would have been incurred if we were an independent publicly-traded company or of the costs we will incur in the future. Following the spin-off, Integer has continued to provide services related to certain of these functions for us on a transitional basis for a fee pursuant to our transition services agreement. At this time, we are unable to determine what our expenses would have been on a standalone basis if we had operated as an unaffiliated entity for each period in which a statement of operations is presented.
Our Customers
Algovita was designed to provide pain management solutions to patients who have evolving requirements and needs. We are still developing our customer base for Algovita, which includes distributors in Europe and hospitals, surgery centers and medical facilities in the U.S. served through a direct sales force and third-party distributors; therefore, the nature and extent of our selling relationships with each customer is different in terms of breadth of products purchased, purchased product volumes, length of contractual commitment, ordering patterns, inventory management, and selling prices. Our NeuroNexus customers include institutions, scientists or universities throughout the world who perform research for the neuroscience and clinical markets. Additionally, our customers include a company in the DBS market serviced through a strategic development agreement.
Strategic and Financial Overview
We are a neurostimulation medical device company formed in 2008 to design and develop a neurostimulation technology platform that could be utilized in multiple indications. Since our inception, the majority of our resources have been spent designing and developing Algovita. SCS was chosen as the first sector of the neurostimulation market to pursue, as we believe that it is a high growth and established market, there is an established regulatory and reimbursement pathway, and we believe that there are significant unmet needs in the SCS market. We currently have four significant competitors in the United States who may be better capitalized and who offer similar SCS devices that are already established and accepted in the market. While the competitive landscape for SCS remains challenging and we may face barriers to market acceptance of our product, we believe Algovita has certain differentiating features from other existing SCS systems that offer our patients and customers a broad set of capabilities and treatment options.
We have been leveraging our neurostimulation technology platform for other sectors of the neurostimulation market such as SNM and DBS, and are exploring other emerging indications. We submitted a premarket approval application for Virtis to TÜV SÜD America and the FDA in December 2016 and January 2017, respectively.
In early 2016, we entered into a development agreement with Aleva, which was amended and restated on August 31, 2017. This agreement provided that we would leverage our neurostimulation technology platform to develop a DBS system for Aleva to treat Parkinson’s disease and Essential Tremor. Under its shareholders’ agreement with Aleva, certain privileges that Integer assigned to Nuvectra at the time of the Spin-off terminated because Integer did not fund a second tranche equity investment in Aleva as obligated under certain circumstances, including the right to receive a technology access success fee and an additional payment if Aleva were sold, sold its assets or were otherwise liquidated. However, Aleva has recently obtained additional financing from another source. If we complete development of a DBS system to treat Parkinson’s disease for Aleva, we expect that Aleva will continue to commercialize the DBS system. If it does so and is successful, we would receive royalties on the sale of these DBS systems and components.
We also intend to pursue other strategic partnerships to fund clinical and development costs of new products, expand our product distribution channels, supplement our product commercialization efforts, obtain assistance in designing and performing clinical studies and post market studies, add specialized clinical or regulatory expertise, or acquire or obtain access to complementary intellectual property.
We have a history of significant net losses, and we expect to continue to incur net losses for the foreseeable future. We expect that future revenue growth will come largely from sales of Algovita in the United States market.
Our Critical Accounting Estimates
The preparation of our consolidated financial statements in accordance with GAAP requires us to make estimates and assumptions that affect reported amounts and related disclosures. The methods, estimates, and judgments we use in applying our accounting policies have a significant impact on the results we report in our consolidated financial statements. We consider an accounting estimate to be critical if (1) it requires assumptions to be made that were uncertain at the time the estimate was made; and (2) changes in the estimate or different estimates that could have been selected could have a material impact on our consolidated results of operations, financial position or cash flows. Our most critical accounting estimates are described below. We also have other policies that we consider key accounting policies; however, these policies do not meet the definition of critical accounting estimates, because they do not generally require us to make estimates or judgments that are difficult or subjective.
Inventories
The value of inventories, comprised solely of finished goods, are stated at the lesser of market value or cost, determined using the first-in, first-out (“FIFO”) method. To value inventory, we must estimate excess or obsolete inventory, as well as inventory that is not of saleable quality. This valuation involves an inherent level of risk and uncertainty due to unpredictability of trends in the industry and customer demand for our products. In assessing the ultimate realization of inventories, we must make judgments as to future demand requirements and compare that with the current or committed inventory levels. Reserve requirements generally increase as demand decreases due to market conditions and technological and product life-cycle changes. Writedowns of excess and obsolete inventories were $417,000 and $156,000 in fiscal years 2017 and 2016, respectively.
Variations in methods or assumptions could have a material impact on our results. If our demand forecast for specific products is greater than actual demand and we fail to reduce our purchases accordingly, we could be required to record additional inventory write-downs, which would have a negative impact on our results of operations. A 5% write-down of our inventory would change 2017 net loss by approximately $0.2 million.
Tangible Long-lived Assets
Property, plant and equipment are carried at cost. The cost of property, plant and equipment is charged to depreciation expense over the estimated life of the operating assets primarily using straight-line rates. Tangible long-lived assets are subject to impairment assessment if certain indicators are present.
We assess the impairment of definite-lived long-lived assets or asset groups when events or changes in circumstances indicate that the carrying value may not be recoverable. Factors that are considered in deciding when to perform an impairment review include: a significant decrease in the market price of the asset or asset group; a significant change in the extent or manner in which a long-lived asset or asset group is being used or in its physical condition; a significant change in legal factors or in the business climate that could affect the value of a long-lived asset or asset group, including an action or assessment by a regulator; an accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction; a current-period operating or cash flow loss combined with a history of operating or cash flow losses or a projection or forecast that demonstrates continuing losses associated with the use of a long-lived asset or asset group; or a current expectation that, more likely than not, a long-lived asset or asset group will be sold or otherwise disposed of significantly before the end of its previously estimated useful life. The term more likely than not refers to a level of likelihood that is more than 50 percent.
Potential recoverability is measured by comparing the carrying amount of the asset or asset group to its related total future undiscounted cash flows. The projected cash flows for each asset or asset group considers multiple factors, including current revenue from existing customers, proceeds from the sale of the asset or asset group and expected profit margins giving consideration to historical and expected margins. If the carrying value is not recoverable, the asset or asset group is considered to be impaired. Impairment is measured by comparing the asset or asset group’s carrying amount to its fair value. When it is determined that useful lives of assets are shorter than originally estimated, and no impairment is present, the rate of depreciation is accelerated in order to fully depreciate the assets over their new shorter useful lives.
Estimation of the cash flows and useful lives of tangible assets that are long-lived requires significant management judgment. Events could occur that would materially affect our estimates and assumptions. Unforeseen changes in operations or technology could substantially alter the assumptions regarding the ability to realize the return of our investment in long-lived assets or the useful lives. Significant changes in these estimates and assumptions could change the amount of future depreciation expense or could create future impairments of these long-lived assets or asset groups.
Intangible Assets
Definite-lived intangible assets are amortized over their estimated useful lives and are assessed for impairment if certain indicators are present. Goodwill is non-amortizing as it is expected to generate cash flows indefinitely. Goodwill is assessed for impairment on an annual basis or more frequently if certain indicators are present. Goodwill is evaluated for impairment at the reporting unit level, which is defined as an operating segment or one level below an operating segment.
We base the fair value of identifiable tangible and intangible assets on detailed valuations that use information and assumptions provided by management. The fair values of intangible assets are determined using one of two valuation approaches: market or income. The selection of a particular method depends on the reliability of available data and the nature of the asset. The market approach values the asset based on available market pricing for comparable assets. The income approach values the asset based on the present value of risk-adjusted cash flows projected to be generated by that asset. The projected cash flows for each asset considers multiple factors from the perspective of a marketplace participant, including current revenue from existing customers, attrition trends, pricing, new product launches, cost synergies, and expected profit margins giving consideration to historical and expected margins.
We test our goodwill balances for impairment on the last day of each fiscal year, or more frequently if certain indicators are present or changes in circumstances suggest that impairment may exist. When evaluating goodwill for impairment, we compare the fair value of a reporting unit with its carrying amount. We recognize an impairment charge for the amount by which the carrying amount of a reporting unit, including goodwill, exceeds its fair value; however, the loss recognized would not exceed the total amount of goodwill allocated to the reporting unit. When evaluating goodwill for impairment, we first assess qualitative factors to determine whether it is necessary to perform the quantitative goodwill impairment test. If determined to be necessary, the quantitative impairment test is used to identify goodwill impairment and measure the amount for a goodwill impairment to be recognized, if any.
On December 31, 2017, we assessed qualitative factors and determined it was not necessary to perform the quantitative goodwill impairment test. We determined that it was more likely than not that the fair value of both our reporting units exceeded their carrying value.
On December 30, 2016, following our reassessment of our reporting units and reallocation of goodwill on a relative fair value basis, we performed a quantitative goodwill impairment test for both our reporting units. The implied fair value of goodwill for the reporting units was determined utilizing both the income approach, specifically the Discounted Cash Flow (“DCF”) method, and the market approach. The income approach calculates fair value by estimating the after-tax cash flows attributable to a reporting unit and then discounting these after-tax cash flows to a present value using a risk-adjusted discount rate. The market approach calculates fair value by analyzing market comparisons available. We believe that a combination of these approaches represented the most appropriate valuation technique for which sufficient data was available to determine the fair value of our reporting units.
In applying the income approach, we made assumptions about the amount and timing of future expected cash flows, terminal value growth rates and appropriate discount rates. The amount and timing of future cash flows within the DCF analysis is based on our most recent operational budgets, long range strategic plans and other estimates. The terminal value growth rate of approximately 4 percent was used to calculate the value of cash flows beyond the last projected period in our DCF analysis and reflects our best estimates for stable, perpetual growth of our reporting units. We used estimates of market-participant risk-adjusted weighted average cost of capital (“WACC”) of approximately 12 percent as a basis for determining the discount rates to apply to our reporting units’ future expected cash flows.
Upon completing the first step of the goodwill impairment test, we determined that the fair value of both reporting units exceeded their carrying value by 4.5% for the Nuvectra reporting unit and 34.1% for the NeuroNexus reporting unit.
The goodwill allocated to each of our reporting units may be subject to future impairment if their actual operating results deteriorate from expected results. Examples of a significant deterioration in operating conditions, which could impact the valuation and/or result in an impairment of goodwill are as follows: the loss of one or more significant customers, non-approval of new medical device systems, lack of market acceptance, discontinuation of significant development projects, technology obsolescence or failure of technology, product liability claims, among others.
Revenue Recognition
We recognize revenue when it is realized or realizable and earned. This occurs when persuasive evidence of an arrangement exists, delivery has occurred, the risk of loss is transferred, the price is fixed or determinable, the buyer is obligated to pay (i.e., not contingent on a future event), collectability is reasonably assured, the amount of future returns can reasonably be estimated, and no material remaining performance obligations are required of us. In cases where we utilize distributors or ship product directly to the end user, we generally recognize revenue upon shipment provided all revenue recognition criteria have been met. When sales representatives deliver products at the point of implantation at hospitals or medical facilities, we recognize revenue upon completion of the procedure and authorization. For the remaining sales, which are sent from the Company’s distribution center directly to hospitals and medical facilities, where product is ordered in advance of an implantation procedure and a valid purchase order has been received, the Company defers revenue until all post-delivery obligations are fulfilled.
Service revenue is recognized as the services are performed. Our development services are typically provided on a fixed-fee basis. The revenues for such longer duration projects are typically recognized using the proportional performance method. In using the proportional performance method, revenues are generally recorded based on the percentage of effort incurred to date on a contract relative to the estimated total expected contract effort. Estimating total contract effort and progress to completion on the arrangements, as well as whether a loss is expected to be incurred on the contract, requires significant judgment. We use historical experience, project plans and an assessment of the risks and uncertainties inherent in the arrangements to establish these estimates. Various uncertainties may or may not be within our control.
See Note 15 to our consolidated financial statements contained in this Annual Report on Form 10-K for a discussion of the impact of the new revenue recognition guidance provided by ASC 606 that we adopted using the modified retrospective method on January 1, 2018.
Stock-Based Compensation
We record compensation costs related to our stock-based awards, which currently include stock options and restricted stock units. We measure stock-based compensation cost at the grant date based on the fair value of the award. Compensation cost for service-based awards is recognized ratably over the applicable vesting period.
The Black-Scholes option-pricing model was used to estimate the fair value of stock options granted. We are required to make certain assumptions with respect to selected Black-Scholes model inputs, including expected volatility, expected life, expected dividend yield and the risk-free interest rate. Expected volatility is based on the historical volatilities for publicly traded stock of comparable companies. The expected life of stock options granted, which represents the period of time that the stock options are expected to be outstanding, is based on the simplified term methodology. The expected dividend yield is based on our history and expectation of dividend payouts. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for a period commensurate with the estimated expected life.
For service-based and nonmarket-based performance restricted stock unit awards, the fair market value of the award is determined based upon the closing value of the stock price on the grant date. The total expense recognized over the vesting period is only for those awards that ultimately vest, excluding market and nonmarket performance award considerations.
There is a high degree of subjectivity involved in selecting assumptions to be utilized to determine fair value. If factors change and result in different assumptions in future periods, the expense that we record for future grants may differ significantly from what we have recorded in the current period. A 5% change in our stock-based compensation expense would change 2017 net loss by approximately $0.1 million.
Provision for Income Taxes
Our consolidated financial statements have been prepared using the asset and liability approach in accounting for income taxes, which requires the recognition of deferred income taxes for the expected future tax consequences of net operating losses, credits, and temporary differences between the financial statement carrying amounts and the tax bases of assets and liabilities. A valuation allowance is provided on deferred tax assets if it is determined that it is more likely than not that the asset will not be realized.
In recording the provision for income taxes, management must estimate the future tax rates applicable to the reversal of temporary differences based upon the timing of expected reversal. Also, estimates are made as to whether taxable operating income in future periods will be sufficient to fully recognize any gross deferred tax assets. If recovery is not likely, we must increase our provision for income taxes by recording a valuation allowance against the deferred tax assets that we estimate will not ultimately be recoverable. Alternatively, we may make estimates about the potential usage of deferred tax assets that decrease our valuation allowances.
The calculation of our tax liabilities involves dealing with uncertainties in the application of complex tax regulations. Significant judgment is required in evaluating our tax positions and determining our provision for income taxes. During the ordinary course of business, there are many transactions and calculations for which the ultimate tax determination is uncertain. We establish reserves for uncertain tax positions when we believe that certain tax positions do not meet the more likely than not threshold. We adjust these reserves in light of changing facts and circumstances, such as the outcome of a tax audit or the lapse of statutes of limitations.
Changes could occur that would materially affect our estimates and assumptions regarding deferred taxes. Changes in current tax laws and tax rates could affect the valuation of deferred tax assets and liabilities, thereby changing the income tax provision. Utilization of our U.S. federal and state net operating losses may be subject to annual limitation due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such an annual limitation could result in the expiration of the net operating loss carryforwards before utilization.
Our Financial Results
Prior to fiscal year 2017, we utilized a fifty-two, fifty-three week fiscal year ending on the Friday nearest December 31. Fiscal year 2016 ended on December 30, 2016 and contained fifty-two weeks. Beginning in fiscal year 2017, we utilize a calendar year fiscal year. A summary of our financial results is as follows (in thousands, except per share data):
|
Year Ended |
||||||||||||||||
|
December 31, |
December 30, |
Change |
||||||||||||||
|
2017 |
2016 |
$ |
% |
|||||||||||||
|
Sales: |
||||||||||||||||
|
Algovita |
$ | 25,567 | $ | 4,162 | $ | 21,405 | 514 | % | ||||||||
|
Neural interface components and systems |
4,756 | 5,152 | (396 | ) | (8 | )% | ||||||||||
|
Development and engineering services |
1,513 | 3,221 | (1,708 | ) | (53 | )% | ||||||||||
|
Total sales |
31,836 | 12,535 | 19,301 | 154 | % | |||||||||||
|
Cost of sales |
15,886 | 6,430 | 9,456 | 147 | % | |||||||||||
|
Gross profit |
15,950 | 6,105 | 9,845 | 161 | % | |||||||||||
|
Gross profit as a % of sales |
50.1 |
% |
48.7 |
% |
||||||||||||
|
Selling, general and administrative expenses (SG&A) |
43,860 | 28,507 | 15,353 | 54 | % | |||||||||||
|
SG&A as a % of total operating expenses |
75.7 |
% |
65.5 |
% |
||||||||||||
|
Research, development and engineering costs, net (RD&E) |
14,102 | 14,524 | (422 |
) |
(3 | )% | ||||||||||
|
RD&E as a % of total operating expenses |
24.3 |
% |
33.4 |
% |
||||||||||||
|
Other operating expenses |
— | 476 | (476 | ) | (100 | )% | ||||||||||
|
Operating loss |
(42,012 |
) |
(37,402 |
) |
(4,610 |
) |
12 | % | ||||||||
|
Interest expense, net |
1,959 | 1,311 | 648 | 49 | % | |||||||||||
|
Other expense (income), net |
604 | (285 |
) |
889 | (312 | )% | ||||||||||
|
Provision for income taxes |
25 | — | 25 | 100 | % | |||||||||||
|
Effective tax rate |
0.0 |
% |
0.0 |
% |
||||||||||||
|
Net loss |
$ | (44,600 |
) |
$ | (38,428 |
) |
$ | (6,172 |
) |
16 | % | |||||
|
Diluted loss per share |
$ | (4.22 |
) |
$ | (3.74 |
) |
$ | (0.48 |
) |
13 | % | |||||
Sales
Algovita. The primary factor behind the 514% increase in sales from fiscal year 2016 to fiscal year 2017 was due to sales as a result of the commercial launch of Algovita in the United States during the first half of 2016. We expect to continue to develop our worldwide sales organization for Algovita consisting of direct sales representatives and independent sales agents in the United States and a network of distributors and independent sales agents outside of the United States to support future growth.
We expect our revenue to fluctuate from quarter to quarter due to a variety of factors, including seasonality, as we have historically experienced lower sales in the first quarter and third quarter of the year, which we believe is due to weather-related events, holidays, the buying patterns and implant volumes of our distributors, hospitals and clinics and reimbursement related issues such as patient deductibles. We anticipate that our total revenue will increase as we continue our commercialization in the United States.
Neural Interface Components and Systems. Neural interface components and systems are related to our NeuroNexus segment and consist of sales of neural interface technology, components, and systems to the neuroscience and clinical markets. The primary factor behind the 8% decrease in sales from fiscal year 2016 to fiscal year 2017 was due to decreased volume of component sales. Price fluctuations did not have a significant impact on sales for the periods presented.
Development and Engineering Service. We recognized $1.5 million and $3.2 million of development and engineering services revenue during fiscal year 2017 and 2016, respectively, from our development agreement with Aleva. See the section entitled “Strategic and Financial Overview” above for more information related to our development agreement with Aleva.
Cost of Sales
Cost of sales consists of the costs of components and materials, labor costs, amortization of technology intangibles, and plant and equipment depreciation and overhead. The primary driver behind the 147% increase in cost of sales from fiscal year 2016 to fiscal year 2017 was the increase in sales of our Algovita systems. We expect that our cost of sales will continue to increase as our sales of our Algovita products continue to grow.
From fiscal year 2016 to fiscal year 2017 our gross profit increased $9.8 million or 161%, and our gross profit as a percentage of sales, or Gross Margin, increased to 50.1% in fiscal year 2017 from 48.7% in fiscal year 2016. These increases were primarily due to an increase in the volume of Algovita sales in the United States.
Our gross margin has been and will continue to be affected by a variety of factors, including by our revenue mix as margins vary across each of our product lines, the costs to have our product manufactured for us, inventory-related charges and write-downs, the ratio of trial to permanent implants, and the average selling prices of our products. We expect our gross margin to be positively affected over time to the extent we are successful in increasing sales volume, reducing manufacturing costs and our revenue mix continues to shift towards Algovita and Virtis. However, our gross margin may continue to fluctuate from period to period.
Selling, General and Administrative Expenses
Selling, general and administrative (“SG&A”) expenses consist primarily of personnel costs, including salary and employee benefits for our sales and marketing personnel and for personnel that support our general operations, such as information technology, executive management, financial accounting, and human resources personnel. SG&A expenses increased $15.4 million, or 54%, from fiscal year 2016 to fiscal year 2017. This increase was primarily the result of increased personnel-related expenses as we have continued to build our Algovita worldwide sales organization and establish corporate support functions as an independent publicly-traded company.
Our SG&A expenses may fluctuate from period to period due to the seasonality of our revenue and the timing and extent of our SG&A expense. Going forward, we expect SG&A expenses to increase as we begin to build our Virtis worldwide sales organization consisting of direct sales representatives and independent sales agents in the United States and a network of distributors and independent sales agents outside of the United States. We expect that this will require recruiting appropriate and qualified direct sales representatives and independent sales agents, expanding our commercial infrastructure in the United States and training our direct sales representatives and independent sales agents. Thereafter, we expect that our sales representatives and independent sales agents will require lead time in the field to access and grow their network of accounts and produce sales results. We believe that successfully recruiting, training and retaining a sufficient number of productive sales representatives and independent sales agents is important in achieving our future growth objective.
After March 14, 2016, our SG&A expenses no longer include an allocation of corporate expenses from Integer but instead reflect fees paid to Integer to provide certain corporate support functions on a transitional basis under the transition services agreement. These corporate allocations included charges for executive oversight, finance, legal, human resources, tax, information technology, product development, corporate procurement, and facilities that totaled $0.2 million of SG&A expenses for fiscal year 2016. These expenses were charged to us on a pro rata basis based upon estimated hours incurred, headcount, square footage, or other measures. We consider the expense allocation methodology and results to be reasonable for all periods presented. However, these allocations are not indicative of the actual expenses that would have been incurred if we operated as an independent publicly-traded company or of the costs we will incur in the future. At this time, we are unable to determine what our expenses would have been on a standalone basis if we had operated as an unaffiliated entity for each period in which a statement of operations is presented.
Research, Development and Engineering Costs, Net
Research, development and engineering (“RD&E”) costs primarily include salary and employee benefits for our specialists in software engineering, mechanical engineering, electrical engineering, and graphical user interface design. Many of these specialists have considerable experience in neurostimulation-related products. Additionally, RD&E includes design verification testing expenses, which include salary and employee benefits for our engineers who test the design and materials used in our medical devices. RD&E costs also include salary, benefits and other personnel-related expenses for our regulatory, quality and clinical affairs employees.
RD&E costs for fiscal year 2017 were essentially flat compared to fiscal year 2016.
As we must continually strive to anticipate and meet our customers’ and patients’ evolving needs and preferences, we expect to invest in product development, product enhancements and improvements and future clinical studies to further develop and update our existing technologies and to expand the features offered in Algovita. We also intend to continue to pursue strategic partnerships to fund clinical and development costs, in part or in full, of new products, expand our product distribution channels, improve our access to physicians and opinion leaders, supplement our product commercialization efforts, obtain assistance in performing clinical studies, add specialized clinical or regulatory expertise, or acquire or obtain access to complementary intellectual property.
Other Operating Expenses
Other operating expenses were comprised of the following (in thousands):
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Performance restricted stock expense |
$ | — | $ | 469 | ||||
|
Other expenses |
— | 7 | ||||||
| $ | — | $ | 476 | |||||
For additional information, see Note 6 “Other Operating Expenses” of the notes to our Consolidated Financial Statements.
Interest Expense, Net
Interest expense, net for fiscal year 2017 and fiscal year 2016 was $2.0 million and $1.3 million, respectively. Interest expense, including amortization of deferred financing fees and discounts on debt, related to our credit facility was $2.3 million and $1.4 million for fiscal year 2017 and 2016, respectively. Interest income from investments was $0.3 million and $0.1 million for fiscal year 2017 and 2016, respectively. For additional information, see Note 7 “Debt” of the notes to our Consolidated Financial Statements.
Other Expense (Income), Net
Other expense (income), net for fiscal year 2017 and fiscal year 2016 related to the revaluation of our warrant liability due to the change in the estimated fair value.
Provision for Income Taxes
During fiscal years 2017 and 2016, we recorded a valuation allowance for the amount of the deferred tax asset that was generated from our net losses and federal research and development tax credit earned and Section 754 election to the extent they exceeded any deferred tax liability, as it was more likely than not that the deferred tax asset generated from those activities will not be realized. See Note 8 “Income Taxes” of the notes to our Consolidated Financial Statements for disclosures related to our income taxes.
Liquidity and Capital Resources
Background
We have incurred significant net losses and negative cash flows from operations since our inception and we expect to continue to incur additional net losses for the foreseeable future.
Immediately prior to the completion of the spin-off, Integer made a cash capital contribution of $75.0 million to us, which we have been using for the continued development and commercialization of Algovita, development of Virtis, and general corporate purposes. This cash capital contribution, together with our cash on hand, cash generated from sales, $23.8 million in net proceeds from our follow-on common stock offering of 3,248,750 shares in February 2018, and borrowings under our credit facility, as amended, $5 million of which is subject to achieving trailing six month revenue milestones and compliance with specified conditions and covenants, is in an amount that we estimate will, based on our current plans and expectations, meet our cash needs for at least the next twelve months. Based on our sequential quarterly revenue growth since the commercial release of our Algovita system into the United States market, and on our expectations of future and continued revenue growth, we believe that the Company should be able to achieve these trailing six month revenue milestones during the relevant periods, although there can be no assurances that we will be able to do so due to unforeseen obstacles that may negatively affect our business. We periodically evaluate our liquidity requirements, alternative uses of capital, capital needs and available resources. As a result of this process, we have in the past sought, and may in the future seek, to explore strategic alternatives to finance our business plan, including but not limited to, a public offering of our common stock, private equity or debt financings, or other sources, such as strategic partnerships. We have elected and may continue to elect to make near-term decisions, including engaging in various capital generating initiatives, to provide additional liquidity. Furthermore, on March 14, 2018 we will no longer be prohibited under the terms of the tax matters agreement with Integer from raising additional funds. However, if we are unable to raise additional funds when needed, we may be required to delay, reduce, or terminate some or all of our development plans. We are also focusing on increasing the sales of our products to generate cash flow to fund our operations. However, there can be no assurance that we will be successful in our plans described above or in attracting alternative debt or equity financing.
Currently, we expect our research and development expenditures for fiscal year 2018 to be between approximately $15 million and $20 million. These expenditures are primarily to continue our research and development program to enhance and improve Algovita and Virtis and to continue to develop our neurostimulation technology platform for uses in indications outside of SCS and SNM. Costs are primarily related to development engineering, regulatory, quality and clinical affairs. We expect to finance these expenditures using cash on-hand, net proceeds from our follow-on common stock offering, and from internally generated funds. We may increase, decrease or re-allocate these anticipated expenditures during any period based on industry conditions, the availability of capital, or other factors. We believe that nearly all of our anticipated research and development expenditures are discretionary.
Consolidated Cash Flows
Net cash used in operating activities was $48.2 million compared to a net loss of $44.6 million for fiscal year 2017. Net cash used in operating activities was $25.8 million compared to a net loss of $38.4 million for fiscal year 2016. The primary components driving the change in cash used in operating activities was the increase in our net loss from operations (adjusted to exclude non-cash charges) and changes in working capital accounts. The cash used in operating activities related to accounts payable and other current liabilities in fiscal year 2017 were related to the year-end inventory purchase from Integer in accordance with certain minimum order quantity requirements under our supply agreement and general timing of liability payments compared to fiscal year 2016. The provision for uncollectible accounts increased by $0.4 million in fiscal year 2017. There were no such charges during fiscal year 2016. Writedowns of excess and obsolete inventories increased by $0.3 million in fiscal year 2017. Depreciation and amortization decreased by $0.6 million in fiscal year 2017 compared to fiscal year 2016. In fiscal year 2016 we recorded accelerated depreciation of our former Enterprise Resource Planning software. No such accelerated depreciation was recorded in fiscal year 2017. Debt related amortization included in interest expense increased by $0.2 million in fiscal year 2017. The increase was related to amortization of our deferred financing fees and discount on debt. See Note 7 “Debt” of the notes to the Consolidated Financial Statements contained in Item 8 of this Annual Report on Form 10-K for additional information on debt related amortization.
Net cash used in investing activities was $1.2 million for fiscal year 2017 compared to $4.0 million for fiscal year 2016. Cash used in investing activities related to the purchases of property, plant, and equipment. As of December 31, 2017, we had no material commitments to purchase capital assets; however, planned capital expenditures for fiscal year 2018 are estimated at approximately $2.0 million.
Net cash provided by financing activities was $13.9 million for fiscal year 2017 compared to $93.3 million for fiscal year 2016. Cash provided by financing activities in fiscal year 2017 was composed of $12.5 million of borrowings under our Credit Facility and $1.4 million proceeds from the exercise of stock options and warrants partially offset by the payment of debt issuance costs. Cash provided by financing activities in fiscal year 2016 was primarily composed of the investment provided by Integer that was partially offset by the repurchase of non-controlling interests in Algostim and PelviStim, which was funded by a cash contribution from Integer, $15 million of borrowings under our Credit Facility partially offset by $1.5 million of debt issuance costs, and $249,000 of proceeds from the exercise of stock options.
Credit Facility
As of February 2018, the Credit Facility consists of (a) term loan facilities in an aggregate maximum principal amount of $45 million comprised of (i) a $27.5 million Term Loan A Commitment, which was funded in full in February 2018, (ii) a $12.5 million Term Loan B Commitment, which was funded in full at the closing of the agreement in February 2018 to repay the previously existing term loan, and (iii) a $5 million Term Loan C Commitment, which is available commencing on the date of achieving consolidated trailing six month product revenues of at least $25 million (the “Third Tranche Milestone”) and ending on the earlier of March 31, 2019 or sixty days following the Third Tranche Milestone.
Based on our sequential quarterly revenue growth since the commercial release of our Algovita system into the United States market, and on our expectations of future and continued revenue growth, we believe that the Company should be able to achieve the Third Tranche Milestone, although there can be no assurances that we will be able to do so due to unforeseen obstacles that may negatively affect our business. The term loan bears interest at the Wall Street Journal prime rate plus 4.15%, subject to an interest rate floor of 8.65%. At December 31, 2017 the interest rate on borrowings under the term loan was 8.40%. The Credit Facility provides for interest-only payments on outstanding term loan borrowings for 24 months after the first borrowing in February 2018 followed by 30 months of principal payments in equal amounts on outstanding term loan borrowings plus accrued interest payments.
On March 18, 2016, in connection with arranging the Credit Facility, we paid Piper Jaffray an arrangement fee of $1,125,000, which equaled 2.50% of the aggregate principal amount of the then-existing Credit Facility. On March 18, 2016, under the terms of the Credit Facility, we paid a commitment fee in an amount equal to 0.50% of the aggregate principal amount of the then-existing $40 million term loan and $5 million revolving line of credit. We also paid a fee of $25,000 plus the expenses of the lenders when we amended the Credit Facility in February 2017 and a second amendment final payment of approximately $0.8 million when we amended the Credit Facility in February 2018.
In addition, we will pay a final payment fee in an amount equal to 7.75% of the funded amount of the term loan, which final payment fee is due at the time of the final principal payment for the Credit Facility or upon early termination of the Credit Facility. If we satisfy the conditions and covenants to allow for drawing on the Term Loan C Commitment, but do not draw upon such commitment prior to the availability expiration date, we will pay a non-use fee of 5.00% of the unfunded amount of such commitment.
If available and we draw on the Term Loan C Commitment, we will issue warrants to the lenders to purchase a number of shares of our common stock with a notional value equal to 4.5% of the funded amount of such commitment, with all warrants issued at such time having an exercise price equal to the lower of the average closing price of our common stock for the ten previous days of trading or the closing price of our common stock on the day prior to such commitment funding. Each warrant is expected to be exercisable for ten years from the date of issuance.
The Credit Facility includes affirmative and negative covenants, including an affirmative covenant regarding minimum revenue requirements, prohibitions on the payment of cash dividends on our capital stock, and restrictions on mergers, sales of assets, investments, incurrence of liens, incurrence of indebtedness and transactions with affiliates. The Credit Facility includes a prepayment fee for the prepayment of the outstanding term loan balance prior to the maturity date in an amount equal to 3.00% of the prepaid term loan balance for a prepayment made during the first year after closing, 2.00% of the prepaid term loan balance for a prepayment made during the second year after closing and 1.00% of the prepaid term loan balance for a prepayment made thereafter. Our obligations under the Credit Facility are secured by substantially all of our assets, except for our intellectual property, which is subject to a negative pledge covenant.
Common Stock Offering
Our Registration Statement on Form S-3 (File No. 333-220834) was declared effective by the SEC on October 24, 2017. On February 5, 2018, pursuant to an underwritten shelf takedown, with Piper Jaffray & Co. serving as representative of the several underwriters, we sold 3,248,750 shares of our common stock and received net proceeds of approximately $23.8 million, after deducting underwriting discounts and commissions and expenses. We intend to use these proceeds as set forth in our prospectus filed with the SEC pursuant to Rule 424(b)(5) on February 1, 2018.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements within the meaning of Item 303(a)(4) of Regulation S-K.
Recently Issued Accounting Standards
In the normal course of business, we evaluate all new accounting pronouncements issued by the FASB to determine the potential impact they may have on our Consolidated Financial Statements. See Note 15 “Recently Issued Accounting Standards” of the notes to the Consolidated Financial Statements contained in Item 8 of this Annual Report on Form 10-K for additional information about these recently issued accounting standards and their potential impact on our financial condition or results of operations.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Risk – The interest rate on any outstanding balance under the term loan of our Credit Facility is based upon the Wall Street Journal prime rate plus 4.15%, subject to an interest rate floor, thus subjecting us to interest rate risk. At December 31, 2017 our interest rate on the $27.5 million outstanding balance was 8.40%. A hypothetical 50 basis point increase in the interest rate would have increased annual interest expense on this credit facility by approximately $137,500.
Item 8. Financial Statements and Supplementary Data
Index to Consolidated Financial Statements
|
|
Page |
|
|
Consolidated Financial Statements as of and for the Years Ended December 31, 2017 and December 30, 2016: |
|
|
|
|
|
|
|
50 |
||
|
|
||
|
51 |
||
|
Consolidated Statements of Operations and Comprehensive Loss |
52 |
|
|
53 |
||
|
54 |
||
|
55 |
||
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of Nuvectra Corporation
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Nuvectra Corporation and subsidiaries (the "Company") as of December 31, 2017 and December 30, 2016, the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2017, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and December 30, 2016, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2017, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Deloitte & Touche LLP
Dallas, Texas
March 6, 2018
We have served as the Company's auditor since 2015.
NUVECTRA CORPORATION
(in thousands, except share and per share data)
|
As of |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
ASSETS |
||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ | 28,165 | $ | 63,710 | ||||
|
Trade accounts receivable, net of allowance for doubtful accounts of $417 in fiscal 2017 and $10 in fiscal 2016 |
10,875 | 3,177 | ||||||
|
Inventories |
4,978 | 5,233 | ||||||
|
Prepaid expenses and other current assets |
1,011 | 443 | ||||||
|
Total current assets |
45,029 | 72,563 | ||||||
|
Property, plant and equipment, net |
6,219 | 6,317 | ||||||
|
Intangible assets, net |
1,428 | 1,714 | ||||||
|
Goodwill |
38,182 | 38,182 | ||||||
|
Other long-term assets |
245 | 526 | ||||||
|
Total assets |
$ | 91,103 | $ | 119,302 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 2,043 | $ | 9,928 | ||||
|
Accrued liabilities |
8,827 | 3,355 | ||||||
|
Accrued compensation |
4,392 | 2,757 | ||||||
|
Short-term debt |
789 | — | ||||||
|
Total current liabilities |
16,051 | 16,040 | ||||||
|
Other long-term liabilities |
993 | 940 | ||||||
|
Long-term debt, net |
25,886 | 13,744 | ||||||
|
Total liabilities |
$ | 42,930 | $ | 30,724 | ||||
|
Commitments and contingencies (Note 9) |
||||||||
|
Stockholders’ equity: |
||||||||
|
Common stock, $0.001 par value, 100,000,000 shares authorized; 10,849,385 and 10,319,627 shares issued and outstanding in fiscal 2017 and fiscal 2016, respectively |
11 | 10 | ||||||
|
Additional paid-in capital |
125,999 | 121,806 | ||||||
|
Accumulated other comprehensive loss |
(1 |
) |
(2 |
) |
||||
|
Accumulated deficit |
(77,836 |
) |
(33,236 |
) |
||||
|
Total stockholders’ equity |
48,173 | 88,578 | ||||||
|
Total liabilities and stockholders’ equity |
$ | 91,103 | $ | 119,302 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
NUVECTRA CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
AND COMPREHENSIVE LOSS
(in thousands, except per share data)
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Sales: |
||||||||
|
Product |
$ | 30,323 | $ | 9,314 | ||||
|
Service |
1,513 | 3,221 | ||||||
|
Total sales |
31,836 | 12,535 | ||||||
|
Cost of sales: |
||||||||
|
Product |
14,989 | 4,806 | ||||||
|
Service |
897 | 1,624 | ||||||
|
Total cost of sales |
15,886 | 6,430 | ||||||
|
Gross profit |
15,950 | 6,105 | ||||||
|
Operating expenses: |
||||||||
|
Selling, general and administrative expenses |
43,860 | 28,507 | ||||||
|
Research, development and engineering costs, net |
14,102 | 14,524 | ||||||
|
Other operating expenses |
— | 476 | ||||||
|
Total operating expenses |
57,962 | 43,507 | ||||||
|
Operating loss |
(42,012 |
) |
(37,402 |
) |
||||
|
Interest expense, net |
1,959 | 1,311 | ||||||
|
Other expense (income), net |
604 | (285 |
) |
|||||
|
Loss before provision for income taxes |
(44,575 |
) |
(38,428 |
) |
||||
|
Provision for income taxes |
25 | — | ||||||
|
Net loss |
$ | (44,600 |
) |
$ | (38,428 |
) |
||
|
Other comprehensive loss: |
||||||||
|
Unrealized holding gain (loss) on investments arising during period |
1 |
|
(2 |
) |
||||
|
Other comprehensive loss |
1 |
|
(2 |
) |
||||
|
Comprehensive loss |
$ | (44,599 |
) |
$ | (38,430 |
) |
||
|
Basic and diluted net loss per share |
$ | (4.22 |
) |
$ | (3.74 |
) |
||
|
Basic and diluted weighted average shares outstanding |
10,576 | 10,277 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
NUVECTRA CORPORATION
CONSOLIDATED CASH FLOW STATEMENTS
(in thousands)
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Cash flows from operating activities: |
||||||||
|
Net loss |
$ | (44,600 |
) |
$ | (38,428 |
) |
||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Provision for uncollectible accounts |
407 | — | ||||||
|
Writedowns of excess and obsolete inventories |
417 | 156 | ||||||
|
Depreciation and amortization |
1,615 | 2,243 | ||||||
|
Debt related amortization included in interest expense |
759 | 510 | ||||||
|
Stock-based compensation |
2,315 | 2,370 | ||||||
|
Loss on disposal of property, plant and equipment |
— | 226 | ||||||
|
Changes in operating assets and liabilities: |
||||||||
|
Trade accounts receivable |
(8,105 |
) |
(2,760 |
) |
||||
|
Inventories |
(162 |
) |
(5,365 |
) |
||||
|
Prepaid expenses and other current assets |
(568 |
) |
(322 |
) |
||||
|
Accounts payable and other current liabilities |
(2,412 |
) |
12,610 | |||||
|
Accrued compensation |
1,635 | 2,035 | ||||||
|
Other long-term liabilities |
502 | 940 | ||||||
|
Net cash used in operating activities |
(48,197 |
) |
(25,785 |
) |
||||
|
Cash flows from investing activities: |
||||||||
|
Acquisition of property, plant and equipment |
(1,231 |
) |
(4,031 |
) |
||||
|
Net cash used in investing activities |
(1,231 |
) |
(4,031 |
) |
||||
|
Cash flows from financing activities: |
||||||||
|
Borrowings under credit facility |
12,500 | 15,000 | ||||||
|
Purchase of non-controlling interests |
— | (6,818 |
) |
|||||
|
Net funding and capital contribution provided by Integer |
— | 86,421 | ||||||
|
Proceeds from the exercise of stock options and warrants |
1,430 | 249 | ||||||
|
Payment of debt issuance costs |
(47 |
) |
(1,528 |
) |
||||
|
Net cash provided by financing activities |
13,883 | 93,324 | ||||||
|
Net (decrease) increase in cash and cash equivalents |
(35,545 |
) |
63,508 | |||||
|
Cash and cash equivalents, beginning of period |
63,710 | 202 | ||||||
|
Cash and cash equivalents, end of period |
$ | 28,165 | $ | 63,710 | ||||
|
Supplemental Disclosure of Cash Flow Information: |
||||||||
|
Interest paid |
$ | 1,427 | $ | 822 | ||||
|
Income taxes paid |
13 | — | ||||||
|
Acquisition of property, plant and equipment accrued not paid |
— | 129 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
NUVECTRA CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands)
|
Additional |
Accumulated Other |
Integer’s |
Total |
|||||||||||||||||||||||||
|
Common Stock |
Paid-In |
Accumulated |
Comprehensive |
Net |
Stockholders’ |
|||||||||||||||||||||||
|
Shares |
Amount |
Capital |
Deficit |
Loss |
Investment |
Equity |
||||||||||||||||||||||
|
At January 1, 2016 |
— | $ | — | $ | — | $ | (125,094 |
) |
$ | — | $ | 162,934 | $ | 37,840 | ||||||||||||||
|
Issuance of common stock |
10,258 | 10 | 119,624 | 125,094 | — | (244,728 |
) |
— | ||||||||||||||||||||
|
Issuance of common stock warrants |
— | — | 238 | — | — | — | 238 | |||||||||||||||||||||
|
Option exercises |
51 | — | 249 | — | — | — | 249 | |||||||||||||||||||||
|
Restricted stock issued, net of stock forfeited |
11 | — | — | — | — | — | — | |||||||||||||||||||||
|
Stock-based compensation |
— | — | 1,695 | — | — | 675 | 2,370 | |||||||||||||||||||||
|
Capital contribution from Integer |
— | — | — | — | — | 75,000 | 75,000 | |||||||||||||||||||||
|
Net funding provided by Integer |
— | — | — | — | — | 11,311 | 11,311 | |||||||||||||||||||||
|
Unrealized holding period loss |
— | — | — | — | (2 |
) |
— | (2 |
) |
|||||||||||||||||||
|
Net loss |
— | — | — | (33,236 |
) |
— | (5,192 |
) |
(38,428 |
) |
||||||||||||||||||
|
At December 30, 2016 |
10,320 | $ | 10 | $ | 121,806 | $ | (33,236 |
) |
$ | (2 |
) |
$ | — | $ | 88,578 | |||||||||||||
|
Issuance of common stock warrants |
— | — | 449 | — | — | — | 449 | |||||||||||||||||||||
|
Option and warrant exercises |
293 | 1 | 1,429 | — | — | — | 1,430 | |||||||||||||||||||||
|
Restricted stock issued, net of stock forfeited |
236 | — | — | — | — | — | — | |||||||||||||||||||||
|
Stock-based compensation |
— | — | 2,315 | — | — | — | 2,315 | |||||||||||||||||||||
|
Unrealized holding period gain |
— | — | — | — | 1 | — | 1 | |||||||||||||||||||||
|
Net loss |
— | — | — | (44,600 |
) |
— | — | (44,600 |
) |
|||||||||||||||||||
|
At December 31, 2017 |
10,849 | $ | 11 | $ | 125,999 | $ | (77,836 |
) |
$ | (1 |
) |
$ | — | $ | 48,173 | |||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
NUVECTRA CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
1. |
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Nature of Operations – Nuvectra Corporation, together with its wholly-owned subsidiaries (i) Algostim, LLC (“Algostim”), (ii) PelviStim LLC (“PelviStim”), and (iii) NeuroNexus Technologies, Inc. (“NeuroNexus”) (collectively “Nuvectra” or the “Company”), is a neurostimulation company committed to helping physicians improve the lives of people with chronic conditions. The Algovita® Spinal Cord Stimulation (“SCS”) System (“Algovita”) is the Company’s first commercial offering and is Conformité Européene (“CE”) marked and United States Food & Drug Administration (“FDA”) approved for the treatment of chronic pain of the trunk and/or limbs. Nuvectra’s innovative technology platform also has capabilities under development to support other neurological indications such as sacral neuromodulation (“SNM”) for the treatment of overactive bladder and deep brain stimulation (“DBS”) for the treatment of Parkinson’s disease and Essential Tremor. In addition, the Company’s NeuroNexus subsidiary designs, manufactures and markets neural-interface technologies for the neuroscience clinical research market.
Basis of Presentation – The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and with the instructions to Form 10-K and Regulation S-X.
On March 14, 2016 Integer Holdings Corporation, formerly known as Greatbatch, Inc. (“Integer”), completed the spin-off of the Company, at which time the Company became a separate public company (the “Spin-off”). Prior to the Spin-off, the Company was a limited liability company named QiG Group, LLC (“QiG”). Prior to March 14, 2016, the financial statements of QiG were prepared on a “combined” basis from the consolidated financial statements of Integer to represent the financial position and performance of QiG as if it had existed on a stand-alone basis in conformity with GAAP. Those combined financial statements included the assets and liabilities that had historically been held at Integer but which were specifically identifiable or attributable to the Company or were transferred to the Company in connection with the spin-off. All intercompany transactions and accounts within the Company have been eliminated. All transactions between the Company and Integer were considered to be effectively settled in the combined financial statements at the time the transaction was recorded. The total net effect of the settlement of these intercompany transactions were reflected in the 2016 combined cash flow statements as a financing activity and in the combined balance sheets as Integer’s Net Investment.
Prior to March 14, 2016, those combined financial statements included an allocation of expenses related to certain Integer corporate functions, including executive oversight, finance, legal, human resources, tax, information technology, product development, corporate procurement, and facilities. These expenses were charged to the Company on the basis of direct usage, when identifiable, with the remainder allocated primarily on a pro rata basis of estimated hours incurred, headcount, square footage, or other measures. The Company’s management considers the expense allocation methodology and results to be reasonable for all periods presented. However, these allocations are not indicative of the actual expenses that would have been incurred if the Company had been an independent publicly-traded company or of the costs the Company will incur in the future. Following the spin-off, Integer has continued to provide many of these services on a transitional basis for a fee. See Note 14 “Related Party Transactions” for additional information. Additionally, Integer maintains a number of employee benefit and stock-based compensation programs at a corporate level. Nuvectra’s employees historically participated in those programs, and as such, the Company was charged a portion of the expenses associated with these programs. Any benefit plan liabilities that are the Company’s direct obligation, such as certain performance-based bonus plans, are reflected in the Consolidated Balance Sheets, as well as within the Company’s operating expenses. See Note 5 “Employee Benefit Plans” for further description of these plans.
Liquidity and Capital Resources – The Company has incurred significant net losses and negative cash flows from operations since inception and expects to incur additional net losses for the foreseeable future. Immediately prior to completion of the Spin-off, Integer made a cash capital contribution to Nuvectra of $75 million. This cash capital contribution, together with the Company’s cash on hand, cash generated from sales, $23.8 million in net proceeds from our follow-on common stock offering of 3,248,750 shares in February 2018, and borrowings under its credit facility, $5 million of which is subject to achieving a trailing six month revenue milestone and compliance with specified conditions and covenants, is an amount that the Company estimates will, based on its current plans and expectations, meet its cash needs for at least the next twelve months. Based on the Company’s sequential quarterly revenue growth since the commercial release of its Algovita system into the United States market, and on its expectations of future and continued revenue growth, the Company believes that it should be able to achieve the Third Tranche Milestone, although there can be no assurances that the Company will be able to do so due to unforeseen obstacles that may negatively affect its business. The Company periodically evaluates its liquidity requirements, alternative uses of capital, capital needs and available resources. As a result of this process, the Company has in the past sought, and may in the future seek, to explore strategic alternatives to finance its business plan, including but not limited to, a public offering of its common stock, private equity or debt financings, or other sources, such as strategic partnerships. The Company has elected and may continue to elect to make near-term decisions, including engaging in various capital generating initiatives, to provide additional liquidity. If the Company is unable to raise additional funds when needed, it may be required to delay, reduce, or terminate some or all of its development plans. The Company is also focusing on increasing the sales of its products to generate cash flow to fund its operations. However, there can be no assurance that the Company will be successful in its plans described above or in attracting alternative debt or equity financing.
Fiscal Year End – Beginning in fiscal year 2017, the Company utilizes a calendar year end. Prior to fiscal year 2017, the Company utilized a fifty-two, fifty-three week fiscal year ending on the Friday nearest December 31. Fiscal year 2016 ended on December 30, 2016 and contained fifty-two weeks.
Use of Estimates – The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of sales and expenses during the reporting period. Actual results could differ materially from those estimates. Significant items subject to such estimates and assumptions include inventories, tangible and intangible asset valuations, revenue, stock-based compensation, and income tax accounts.
Fair Value Measurements – Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (i.e. the “exit price”) in an orderly transaction between market participants at the measurement date. Accounting Standards Codification (“ASC”) establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. The hierarchy is broken down into three levels based on the reliability of inputs as follows:
Level 1 – Valuation is based on quoted prices in active markets for identical assets or liabilities that the Company has the ability to access. Level 1 valuations do not entail a significant degree of judgment.
Level 2 – Valuation is determined from quoted prices for similar assets or liabilities in active markets, quoted prices for identical instruments in markets that are not active or by model-based techniques in which all significant inputs are observable in the market.
Level 3 – Valuation is based on unobservable inputs that are significant to the overall fair value measurement. The degree of judgment in determining fair value is greatest for Level 3 valuations.
The availability of observable inputs can vary and is affected by a wide variety of factors, including, the type of asset/liability, whether the asset/liability is established in the marketplace, and other characteristics particular to the valuation. To the extent that a valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, for disclosure purposes the level in the fair value hierarchy within which the fair value measurement in its entirety falls is determined based on the lowest level input that is significant to the fair value measurement in its entirety.
Fair value is a market-based measure considered from the perspective of a market participant rather than an entity-specific measure. Therefore, even when market assumptions are not readily available, assumptions are required to reflect those that market participants would use in pricing the asset or liability at the measurement date. The carrying amounts of cash and cash equivalents, trade accounts receivable, accounts payable and other current liabilities and accrued bonuses approximate fair value because of the short-term nature of these items. Note 11 “Fair Value Measurements” contains additional information on assets and liabilities recorded at fair value in the Consolidated Financial Statements.
Cash and Cash Equivalents – Cash and Cash Equivalents consist of cash and highly liquid, short-term investments with maturities at the time of purchase of three-months or less.
Concentration of Credit Risk – Financial instruments that potentially subject the Company to concentration of credit risk consist principally of trade accounts receivable owed to the Company by its customers. The Company performs on-going credit evaluations of its customers. During fiscal year 2016, sales to Aleva Neurotherapeutics S.A. (“Aleva”) were $3.1 million (or 25%) of the Company’s consolidated revenues. No other customers individually accounted for more than 10% of the Company’s consolidated revenues in 2016, and no customers individually accounted for more than 10% of the Company’s consolidated revenues in 2017. Included in accounts receivable at December 30, 2016 was $0.3 million due from Aleva. No other customers individually accounted for more than 10% of the Company’s accounts receivable at December 30, 2016, and no customers individually accounted for more than 10% of the Company’s accounts receivable at December 31, 2017. Additionally, the Company maintains cash deposits with major banks, which from time to time may exceed insured limits. The Company performs on-going credit evaluations of its banks. See Note 13 “Business Segments, Geographic and Concentration Risk Information” for additional information.
Allowance for Doubtful Accounts – The Company provides credit, in the normal course of business, to its customers in the form of trade accounts receivable. Credit is extended based on evaluation of a customer’s financial condition and collateral is not required. The Company maintains an allowance for those customer receivables that it does not expect to collect. The Company accrues its estimated losses from uncollectable accounts receivable to the allowance based upon recent historical experience, the length of time the receivable has been outstanding and other specific information as it becomes available. Provisions to the allowance for doubtful accounts are charged to current operating expenses. Actual losses are charged against this allowance when incurred. The allowance for doubtful accounts was $0.4 million and $0.01 million at the end of fiscal years 2017 and 2016, respectively.
Inventories – The value of inventories, comprised solely of finished goods, are stated at the lesser of net realizable value or cost, determined using the first-in, first-out (“FIFO”) method. To value inventory, management must estimate excess or obsolete inventory, as well as inventory that is not of saleable quality. This valuation involves an inherent level of risk and uncertainty due to unpredictability of trends in the industry and customer demand for the Company’s products. In assessing the ultimate realization of inventories, management must make judgments as to future demand requirements and compare that with the current or committed inventory levels. Reserve requirements generally increase as demand decreases due to market conditions and technological and product life-cycle changes. Writedowns of excess and obsolete inventories were $0.4 million and $0.2 million in fiscal years 2017 and 2016, respectively. Future events and variations in valuation methods or assumptions may cause significant fluctuations in this estimate and could have a material impact on the Company’s results of operations.
Property, Plant and Equipment, Net (“PP&E”) – PP&E is carried at cost less accumulated depreciation. Depreciation is computed by the straight-line method over the estimated useful lives of the assets, as follows: buildings and building improvements 7-40 years; machinery and equipment 3-8 years; office equipment 3-10 years; and leasehold improvements over the remaining lives of the improvements or the lease term, if less. The cost of repairs and maintenance are expensed as incurred; renewals and betterments are capitalized. Upon retirement or sale of an asset, its cost and related accumulated depreciation or amortization is removed from the accounts and any gain or loss is recorded in operating income or expense.
The Company is a party to various operating lease agreements for buildings, machinery, and equipment. Lease expense includes the effect of escalation clauses, which are accounted for ratably over the lease term. Note 2 “Property, Plant and Equipment, Net” contains additional information on the Company’s PP&E.
Amortizing Intangible Assets, Net – Amortizing Intangible Assets, Net consists primarily of purchased technology and patents, and customer lists. The Company amortizes its definite-lived intangible assets over their estimated useful lives utilizing an accelerated method of amortization, which approximates the projected cash flows used to fair value those intangible assets at the time of acquisition. The amortization period for the Company’s amortizing intangible assets are as follows: purchased technology and patents 6 years and customer lists 7 years. See Note 3 “Intangible Assets, Net” for additional information on the Company’s amortizing intangible assets.
Impairment of Long-Lived Assets – The Company assesses the impairment of definite-lived long-lived assets or asset groups when events or changes in circumstances indicate that the carrying value may not be recoverable. Factors that are considered in deciding when to perform an impairment review include: a significant decrease in the market price of the asset or asset group; a significant change in the extent or manner in which a long-lived asset or asset group is being used or in its physical condition; a significant change in legal factors or in the business climate that could affect the value of a long-lived asset or asset group, including an action or assessment by a regulator; an accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction; a current-period operating or cash flow loss combined with a history of operating or cash flow losses or a projection or forecast that demonstrates continuing losses associated with the use of a long-lived asset or asset group; or a current expectation that, more likely than not, a long-lived asset or asset group will be sold or otherwise disposed of significantly before the end of its previously estimated useful life. The term more likely than not refers to a level of likelihood that is more than 50 percent.
Potential recoverability is measured by comparing the carrying amount of the asset or asset group to its related total future undiscounted cash flows. The projected cash flows for each asset or asset group considers multiple factors, including current revenue from existing customers, proceeds from the sale of the asset or asset group and expected profit margins giving consideration to historical and expected margins. If the carrying value is not recoverable, the asset or asset group is considered to be impaired. Impairment is measured by comparing the asset or asset group’s carrying amount to its fair value. When it is determined that useful lives of assets are shorter than originally estimated, and no impairment is present, the rate of depreciation is accelerated in order to fully depreciate the assets over their new shorter useful lives.
Goodwill Valuation – The Company assesses its goodwill balances for impairment on the last day of each fiscal year, or more frequently if certain indicators are present or changes in circumstances, as described above, suggest that impairment may exist. The Company first assesses qualitative factors to determine whether it is necessary to perform the quantitative goodwill impairment test. If determined to be necessary, the quantitative impairment test is used to identify goodwill impairment and measure the amount for a goodwill impairment to be recognized, if any. When evaluating goodwill for impairment, the Company compares the fair value of a reporting unit with its carrying amount. The Company recognizes an impairment charge for the amount by which the carrying amount of a reporting unit, including goodwill, exceeds its fair value; however, the loss recognized would not exceed the total amount of goodwill allocated to the reporting unit. As of December 31, 2017 and December 30, 2016 the Company determined that it was more likely than not that the fair value of both reporting units exceeded their carrying value.
The following represents our goodwill balance by reportable segment. Changes to goodwill during the years ended December 31, 2017 and December 30, 2016 were as follows (in thousands):
|
Nuvectra |
NeuroNexus |
Total |
||||||||||
|
Balance – January 1, 2016 |
||||||||||||
|
Goodwill, gross |
$ | 33,491 | $ | 4,691 | $ | 38,182 | ||||||
|
Accumulated impairment losses |
— | — | — | |||||||||
|
Goodwill, net |
33,491 | 4,691 | 38,182 | |||||||||
|
Goodwill impairment charge |
— | — | — | |||||||||
|
Balance – December 30, 2016 |
||||||||||||
|
Goodwill, gross |
33,491 | 4,691 | 38,182 | |||||||||
|
Accumulated impairment losses |
— | — | — | |||||||||
|
Goodwill, net |
33,491 | 4,691 | 38,182 | |||||||||
|
Goodwill impairment charge |
— | — | — | |||||||||
|
Balance – December 31, 2017 |
||||||||||||
|
Goodwill, gross |
33,491 | 4,691 | 38,182 | |||||||||
|
Accumulated impairment losses |
— | — | — | |||||||||
|
Goodwill, net |
$ | 33,491 | $ | 4,691 | $ | 38,182 | ||||||
Warranty Reserve – The Company offers a warranty on certain of its products and has established a warranty reserve, as a component of other current liabilities, for any potential claims. The Company estimates its warranty reserve based upon an analysis of all identified or expected claims and an estimate of the cost to resolve those claims. Factors that affect the Company’s warranty liability include the number of units sold, historical and anticipated rates of warranty claims, and differences between actual and expected warranty costs per claim. The Company periodically assesses the adequacy of its warranty liabilities and adjusts the amounts as necessary.
Revenue Recognition – The Company recognizes revenue when it is realized or realizable and earned. This occurs when persuasive evidence of an arrangement exists, delivery has occurred, the risk of loss is transferred, the price is fixed or determinable, the buyer is obligated to pay (i.e., not contingent on a future event), collectability is reasonably assured, the amount of future returns can reasonably be estimated, and no material remaining performance obligations are required of the Company. In cases where the Company utilizes distributors or ships product directly to the end user, it generally recognizes revenue upon shipment provided all revenue recognition criteria have been met. When sales representatives deliver products at the point of implantation at hospitals or medical facilities, the Company recognizes revenue upon completion of the procedure and authorization. For the remaining sales, which are sent from the Company’s distribution center directly to hospitals and medical facilities, where product is ordered in advance of an implantation procedure and a valid purchase order has been received, the Company defers revenue until all post-delivery obligations are fulfilled. Deferred revenue primarily consists of amounts billed or collected upon delivery of products with material remaining post-delivery performance obligations. Deferred revenue is recorded in accrued liabilities in the Consolidated Balance Sheets. Related cost of sales have also been deferred and are recorded in prepaid expenses and other current assets in the Consolidated Balance Sheets.
Service revenue is recognized as the services are performed. The Company’s development services are typically provided on a fixed-fee basis. The revenues for such longer duration projects are typically recognized using the proportional performance method. In using the proportional performance method, revenues are generally recorded based on the percentage of effort incurred to date on a contract relative to the estimated total expected contract effort. Estimating total contract effort and progress to completion on the arrangements, as well as whether a loss is expected to be incurred on the contract, requires significant judgment. Management uses historical experience, project plans and an assessment of the risks and uncertainties inherent in the arrangements to establish these estimates. Various uncertainties may or may not be within the Company’s control. Deferred revenue also consists of amounts contractually billable or collected for service revenue not yet performed.
Research, Development and Engineering Costs, Net (“RD&E”) – RD&E costs are expensed as incurred. The primary costs are salary and employee benefits for our specialists in software engineering, mechanical engineering, electrical engineering, and graphical user interface design, design verification testing expenses, which include salary and employee benefits for our engineers who test the design and materials used in our medical devices, salary, benefits and other personnel-related expenses for our regulatory, quality and clinical affairs employees, material costs used in development projects and subcontracting costs. Any reimbursements received from government grants are recorded as a reduction of the research, development and engineering costs incurred.
Stock-Based Compensation – Certain of the Company’s employees participated in the stock-based compensation programs of Integer and prior to the Spin-off received awards of time-based stock options and time- and performance-based restricted stock units, which typically vest over a three-year period and are settled in shares of Integer common stock. The stock-based payment compensation expense includes the compensation expense directly attributable to Nuvectra employees from these Integer equity incentives. In addition, certain incentive awards that were originally granted under an Integer equity incentive award plan adjusted into an incentive award of Nuvectra common stock at the time of the Spin-off. Additionally, subsequent to the spin-off, the Company’s employees participate in the stock-based compensation programs of Nuvectra. The compensation costs related to stock-based awards granted to employees is based upon their estimated fair value on the grant date. Compensation cost for service-based awards is recognized ratably over the applicable vesting period. Compensation cost for Integer nonmarket-based performance awards was reassessed each period and recognized based upon the probability that the performance targets would be achieved. Compensation cost for Integer market-based performance awards was expensed ratably over the applicable vesting period and was recognized each period whether the performance metrics were achieved or not.
The Black-Scholes option-pricing model was used to estimate the fair value of stock options granted. For service-based and nonmarket-based performance restricted stock unit awards, the fair market value of the award is determined based upon the closing value of the stock price on the grant date. Historically, for market-based performance restricted stock unit awards, the fair market value of the award was determined utilizing a Monte Carlo simulation model, which projected the value of Integer’s stock under numerous scenarios and determined the value of the award based upon the present value of those projected outcomes.
The total expense recognized over the vesting period will only be for those awards that ultimately vest, excluding market and nonmarket performance award considerations. Note 5 “Employee Benefit Plans” contains additional information on stock-based compensation.
Insurance – The Company historically participated in Integer’s various insurance programs, to insure for property and casualty risks, product liability, employee health care, workers’ compensation and other casualty losses. Many of the potential losses were covered by Integer under conventional insurance programs with third-party carriers with high deductible limits. In other areas, Integer was self-insured with stop-loss coverage. The Company was charged a portion of the expenses associated with these programs. As of March 14, 2016 the Company is covered by its own insurance policies and, since that time, no insurance fee has been allocated from Integer. See Note 14 “Related Party Transactions” for additional information.
Interest Expense, Net – Interest expense, including amortization of deferred financing fees and discounts on debt, related to the Company’s Credit Facility was $2.3 million and $1.4 million for fiscal year 2017 and 2016, respectively. Interest income from investments was $0.3 million and $0.1 million for fiscal year 2017 and 2016, respectively.
Comprehensive Loss – The Company’s comprehensive loss as reported in the Consolidated Statements of Operations and Comprehensive Loss is comprised of the Company’s net loss and unrealized holding period gains and / or losses related to investments.
Income Taxes – The Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined on the basis of the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date. The Company recognizes deferred tax assets to the extent that it believes these assets are more likely than not to be realized. In making such a determination, the Company considers all available positive and negative evidence. If the Company determines that it would be able to realize its deferred tax assets in the future in excess of their net recorded amount, the Company would make an adjustment to the deferred tax asset valuation allowance, which would reduce the provision for income taxes.
The Company records uncertain tax positions in accordance with ASC 740 on the basis of a two-step process whereby (1) the Company determines whether it is more likely than not that the tax positions will be sustained on the basis of the technical merits of the position and (2) for those tax positions that meet the more-likely-than-not recognition threshold, the Company recognizes the largest amount of tax benefit that is more than 50 percent likely to be realized upon ultimate settlement with the related tax authority.
The Company recognizes interest and penalties related to unrecognized tax benefits within the income tax expense line in the accompanying Consolidated Statement of Operations.
Subsequent Events – The Company considers events or transactions that occur after the balance sheet date but prior to the issuance of the financial statements to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure.
On February 5, 2018, the Company announced the closing of its underwritten follow-on public offering of 3,248,750 shares of its common stock at a price to the public of $8.00 per share, which included the exercise in full by the underwriters of their option to purchase 423,750 additional shares of common stock on the same terms and conditions. Gross proceeds to Nuvectra were approximately $26.0 million.
See Note 7 “Debt” for additional information on the Company’s credit facility as amended in February 2018.
|
2. |
PROPERTY, PLANT AND EQUIPMENT, NET |
PP&E, net is comprised of the following (in thousands):
|
At |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Machinery and equipment |
$ | 2,329 | $ | 1,675 | ||||
|
Buildings and building improvements |
2,855 | 2,854 | ||||||
|
Information technology hardware and software |
4,431 | 3,191 | ||||||
|
Furniture and fixtures |
374 | 314 | ||||||
|
Land and land improvements |
390 | 390 | ||||||
|
Construction work in process |
342 | 1,066 | ||||||
|
Total, gross |
10,721 | 9,490 | ||||||
|
Accumulated depreciation |
(4,502 |
) |
(3,173 |
) |
||||
|
Total, net |
$ | 6,219 | $ | 6,317 | ||||
Depreciation and rent expense for PP&E were as follows (in thousands):
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Depreciation expense |
$ | 1,329 | $ | 1,974 | ||||
|
Rent expense |
520 | 519 | ||||||
Minimum future estimated annual operating lease payments as of December 31, 2017 were expected to be (in thousands):
|
2018 |
$ | 445 | ||
|
2019 |
401 | |||
|
2020 |
408 | |||
|
2021 |
415 | |||
|
2022 |
282 | |||
|
Thereafter |
— | |||
|
Total estimated operating lease payments |
$ | 1,951 |
|
3. |
INTANGIBLE ASSETS, NET |
Intangible assets, net are comprised of the following (in thousands):
|
Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
||||||||||
|
At December 30, 2016 |
||||||||||||
|
Technology and patents |
$ | 1,058 | $ | (498 |
) |
$ | 560 | |||||
|
Customer lists |
1,869 | (715 |
) |
1,154 | ||||||||
|
Total intangible assets |
$ | 2,927 | $ | (1,213 |
) |
$ | 1,714 | |||||
|
At December 31, 2017 |
||||||||||||
|
Technology and patents |
$ | 1,058 | $ | (624 |
) |
$ | 434 | |||||
|
Customer lists |
1,869 | (875 |
) |
994 | ||||||||
|
Total intangible assets |
$ | 2,927 | $ | (1,499 |
) |
$ | 1,428 | |||||
Aggregate intangible asset amortization expense is classified as follows (in thousands):
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Cost of sales |
$ | 126 | $ | 110 | ||||
|
Selling, general and administrative expenses |
160 | 159 | ||||||
|
Total intangible asset amortization expense |
$ | 286 | $ | 269 | ||||
Estimated future intangible asset amortization expense based on the current carrying value is as follows for the years ending December 31 (in thousands):
|
Estimated Amortization Expense |
||||
|
2018 |
$ | 298 | ||
|
2019 |
293 | |||
|
2020 |
209 | |||
|
2021 |
194 | |||
|
2022 |
108 | |||
|
Thereafter |
326 | |||
|
Total estimated amortization expense |
$ | 1,428 | ||
|
4. |
Accrued Liabilities |
Accrued liabilities consisted of the following (in thousands):
|
At |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Inventory purchases |
$ | 5,825 | $ | 547 | ||||
|
Regulatory, clinical and quality |
358 | 640 | ||||||
|
Legal |
358 | 98 | ||||||
|
Deferred revenue |
335 | — | ||||||
|
Sales and marketing |
321 | 108 | ||||||
|
Operations engagement fee |
200 | 600 | ||||||
|
Interest |
199 | 99 | ||||||
|
Warranty reserve |
195 | 98 | ||||||
|
Research and development |
164 | 165 | ||||||
|
Sales and use tax |
145 | 56 | ||||||
|
Insurance |
127 | — | ||||||
|
Information technology system implementations |
114 | 327 | ||||||
|
Travel and entertainment |
— | 285 | ||||||
|
Accrued other |
486 | 332 | ||||||
|
Total accrued liabilities |
$ | 8,827 | $ | 3,355 | ||||
|
5. |
EMPLOYEE BENEFIT PLANS |
Nuvectra Corporation 2016 Equity Incentive Plan – The Nuvectra Corporation 2016 Equity Incentive Plan (the “2016 Equity Plan”) was initially adopted by the Board of Managers of QiG Group, LLC and was subsequently ratified and approved by Nuvectra’s Board of Directors effective as of March 14, 2016. The 2016 Equity Plan provides that the Compensation and Organization Committee of the Board of Directors (the “Compensation Committee”) may award eligible participants, as it may determine from time to time, the following types of awards: stock options, stock appreciation rights, restricted stock, restricted stock units and stock bonuses. Subject to the adjustment clauses in the 2016 Equity Plan, the total number of shares of Nuvectra common stock reserved for issuance under the 2016 Equity Plan is 1,541,195.
During fiscal year 2017, the Compensation Committee granted equity awards aggregating 548,057 shares of common stock under the 2016 Equity Plan in the form of both restricted stock units and non-qualified stock options to its directors and certain officers and key employees. Compensation cost related to the 2016 Equity Plan for the fiscal year 2017 was approximately $2.3 million.
During fiscal year 2016, the Compensation Committee granted equity awards aggregating 805,222 shares of common stock under the 2016 Equity Plan in the form of both restricted stock units and non-qualified stock options to its directors and certain officers and key employees. Compensation cost related to the 2016 Equity Plan for fiscal year 2016 was approximately $1.3 million.
Stock-Based Compensation – Certain of the Company’s employees participated in the stock-based compensation programs of Integer and prior to the Spin-off received awards of time-based stock options and time- and performance-based restricted stock units, which typically vest over a three-year period and are settled in shares of Integer common stock. The stock-based payment compensation expense includes the compensation expense directly attributable to Nuvectra employees from these Integer equity incentives. In addition, certain incentive awards that were originally granted under an Integer equity incentive award plan adjusted into an incentive award of Nuvectra common stock at the time of the Spin-off. Compensation cost related to these Integer equity incentives was approximately $0.2 million and $1.1 million for fiscal years 2017 and 2016, respectively. As of December 31, 2017, there was approximately $3.3 million of total unrecognized compensation expense that is expected to be recognized over a weighted-average period of 1.6 years.
The components and classification of stock-based compensation expense were as follows (in thousands):
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Stock options |
$ | 864 | $ | 638 | ||||
|
Restricted stock and restricted stock units |
1,451 | 1,732 | ||||||
|
Total stock-based compensation expense |
$ | 2,315 | $ | 2,370 | ||||
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Selling, general and administrative expense |
$ | 1,992 | $ | 1,565 | ||||
|
Research, development and engineering costs, net |
323 | 336 | ||||||
|
Other operating expenses |
— | 469 | ||||||
|
Total stock-based compensation expense |
$ | 2,315 | $ | 2,370 | ||||
The fair value of each stock option grant is estimated on the date of grant using the Black-Scholes option-pricing model with weighted-average assumptions based on the grant date. The weighted average fair value and assumptions used to value options granted under the 2016 Equity Plan were as follows:
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Weighted average fair value |
$ | 3.64 | $ | 4.52 | ||||
|
Risk-free interest rate |
2.06 |
% |
1.72 |
% |
||||
|
Expected volatility |
55 |
% |
55 |
% |
||||
|
Holding period (in years) |
6 | 10 | ||||||
|
Expected dividend yield |
— |
% |
— |
% |
||||
The following table summarizes the stock option activity during fiscal year 2017:
|
Number of Time-Vested Stock Options |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
|||||||||||||
|
Outstanding at December 30, 2016 |
873,992 | $ | 6.49 | |||||||||||||
|
Granted |
338,839 | 6.84 | ||||||||||||||
|
Exercised |
(261,748 |
) |
5.80 | |||||||||||||
|
Forfeited or expired |
(80,263 |
) |
7.18 | |||||||||||||
|
Outstanding at December 31, 2017 |
870,820 | $ | 6.78 | 7.59 | $ | 1,203,654 | ||||||||||
|
Exercisable at December 31, 2017 |
423,547 | $ | 6.53 | 6.18 | $ | 736,883 | ||||||||||
The Company received proceeds totaling $1.4 million upon the exercise of 261,748 stock options and 32,425 warrants during fiscal year 2017. The Company received proceeds totaling $0.2 million upon the exercise of 50,794 stock options during fiscal year 2016.
The following table summarizes the restricted stock and restricted stock unit activity during fiscal year 2017:
|
Time-Vested Activity |
Weighted Average Fair Value |
|||||||
|
Nonvested at December 30, 2016 |
551,224 | $ | 6.90 | |||||
|
Granted |
209,218 | 6.76 | ||||||
|
Vested |
(229,667 |
) |
8.76 | |||||
|
Forfeited |
(101,770 |
) |
6.52 | |||||
|
Nonvested at December 31, 2017 |
429,005 | $ | 6.88 | |||||
Nuvectra Bonus Plan – The terms of the Nuvectra Corporation Bonus Plan (the “Bonus Plan”) provide for both annual discretionary defined contribution cash bonuses and performance-based bonuses based upon Nuvectra’s company-wide performance measures and, for certain employees, individual performance measures that are set by Nuvectra’s executive management and, in some instances, members of the Board of Directors. Compensation cost related to the Bonus Plan for fiscal year 2017 and 2016 was approximately $1.9 million and $1.0 million, respectively.
Defined Contribution Plans – The Company sponsors a defined contribution 401(k) plan for its employees. The plan provides for the deferral of employee compensation under Section 401(k) of the Internal Revenue Code of 1986, as amended (“Section 401(k)”), and a discretionary match. In fiscal year 2017 and 2016 this match was 25% per dollar of participant deferral, up to 6% of the total compensation for each participant. Direct costs related to this defined contribution plan were $0.3 million and $0.2 million in fiscal year 2017 and 2016, respectively.
Integer sponsors a defined contribution 401(k) plan for its employees, in which the Company’s employees historically participated. The plan provides for the deferral of employee compensation under Section 401(k) and a discretionary match. Until the spin-off in the first quarter of fiscal year 2016 this match was 35% per dollar of participant deferral, up to 6% of the total compensation for each participant. The 401(k) compensation expense for the first quarter of fiscal year 2016 recognized in these Consolidated Financial Statements includes all of the compensation expenses directly attributable to Nuvectra employees. Direct costs related to this defined contribution plan allocated to the Company were $0.2 million in fiscal year 2016.
|
6. |
OTHER OPERATING EXPENSES |
Performance Restricted Stock Expense – As a result of the Spin-off, certain 2015 performance stock units for Nuvectra employees were effectively cancelled with no new issuance. Therefore, the remaining unvested expense totaling $469,000 as of March 14, 2016 was accelerated and expensed immediately.
Asset Disposals – During fiscal year 2016, the Company recorded charges in connection with various asset disposals. There were no such charges in fiscal year 2017.
|
7. |
DEBT |
Long-term debt is comprised of the following (in thousands):
|
At |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Term loan |
$ | 29,631 | $ | 16,163 | ||||
|
Deferred financing fees |
(962 |
) |
(1,262 |
) |
||||
|
Discount on debt |
(1,994 |
) |
(1,157 |
) |
||||
|
Total debt |
26,675 | 13,744 | ||||||
|
Less current portion of long-term debt |
789 | — | ||||||
|
Total long-term debt |
$ | 25,886 | $ | 13,744 | ||||
Credit Facility – The Company has a credit facility, as amended in February 2018 (the “Credit Facility”), that consists of (a) term loan facilities in an aggregate maximum principal amount of $45,000,000 comprised of (i) a $27,500,000 Term Loan A Commitment, which was funded in full at the closing of the agreement in February 2018 to repay the previously existing term loan, (ii) a $12,500,000 Term Loan B Commitment, which was funded in full in February 2018, and (iii) a $5,000,000 Term Loan C Commitment, which is available commencing on the date of achieving consolidated trailing six-month product revenues of at least $25 million (the “Third Tranche Milestone”) and ending on the earlier of March 31, 2019 or sixty days following the Third Tranche Milestone (collectively, the “Term Loans). Based on the Company’s sequential quarterly revenue growth since the commercial release of its Algovita system into the United States market, and on its expectations of future and continued revenue growth, the Company believes that it should be able to achieve the Third Tranche Milestone, although there can be no assurances that the Company will be able to do so due to unforeseen obstacles that may negatively affect its business.
The Term Loans bear interest at a floating rate equal to the prime rate plus 4.15%, with a floor of 8.65%. At December 31, 2017 the interest rate on borrowings under the term loan was 8.40%. The Company pays monthly accrued interest only on the Term Loans through March 2020 and thereafter the Company will pay monthly accrued interest on the Term Loans plus equal payments of principal for 30 months. At the maturity of the Term Loans, on September 1, 2022, all principal on the Term Loans then outstanding, plus an additional 7.75% of the funded loan amounts (the “Final Payment”), will be due and payable. This Final Payment has been treated as an in substance discount and is being amortized using the straight-line method over the life of the loan.
On March 18, 2016 the Company paid an arrangement fee of $1,125,000 to Piper Jaffray Companies (“Piper Jaffray”), which equaled 2.50% of the then-existing $40 million term loan and $5 million revolving line of credit, a commitment fee of $200,000 for all of the Term Loan A, B, and C Commitments, and one-half of a $25,000 commitment fee for the Revolving Loans, the remaining one-half of which was due and paid on the one year anniversary of the initial closing. In connection with the amendment dated February 14, 2017, the Company also paid an amendment fee of $25,000 plus expenses of the lenders. In connection with the amendment in February 2018, the Company also paid a second amendment final payment of approximately $0.8 million. The Term Loan C Commitment is subject to a non-use fee of 5% of the amount of such commitment if the commitment becomes available, but the Company declines to borrow the Term Loan thereunder. If any Term Loans are voluntarily prepaid prior to their scheduled maturity, the Company must pay, in addition to the Final Payment, a prepayment fee equal to 3% of the prepaid principal if paid in the first year after the initial closing, 2% of the prepaid principal if paid in the second year after the initial closing, and 1% of the prepaid principal if paid thereafter.
The Loans are secured by a first priority lien on substantially all of the assets of the Company, including, without limitation, all cash, deposit accounts, accounts receivable, equipment, inventory, contract rights, and the Company’s real property located in Blaine, Minnesota, but excluding all intellectual property of the Company (other than accounts receivable and proceeds of intellectual property). The Company’s intellectual property is subject to a negative pledge. The Company must maintain its primary operating and investment accounts with Silicon Valley Bank, which accounts are subject to customary control agreements.
The Credit Facility contains customary representations and warranties, reporting and other covenants for credit facilities of this kind including prohibitions on the payment of cash dividends on the Company’s capital stock and restrictions on mergers, sales of assets, investments, incurrence of liens, incurrence of indebtedness and transactions with affiliates. The Company is subject to a quarterly financial covenant requiring the Company to achieve specified minimum consolidated product revenues. As of December 31, 2017, the Company was in compliance with the financial covenant. The events of default in the Credit Facility are customary for credit facilities of this kind, and include failure to pay interest or principal, breaches of affirmative and negative covenants, a material adverse change occurring, and cross defaults to other material agreements of the Company.
On February 16, 2018, the credit facility was amended, and the new Term A loan commitment ($27.5 million) and Term B loan commitment ($12.5 million) were funded in full, with the Term A loan proceeds used to pay off the loans then outstanding under the credit facility. As a condition to the lenders’ funding the loans under the initial credit facility, concurrently with the funding under the initial term loan A commitment on March 18, 2016, (i) the Company issued to Oxford Finance LLC a warrant to purchase 56,533 shares of Nuvectra common stock at an exercise price of $5.97 per share, which warrant is exercisable until March 18, 2026, and (ii) the Company issued to Silicon Valley Bank a warrant to purchase 56,533 shares of Nuvectra common stock at an exercise price of $5.97 per share, which warrant is exercisable until March 18, 2026. The fair value of the warrants on the date of grant totaled approximately $0.2 million and was recorded as a discount on long-term debt and as additional paid-in capital in the Consolidated Balance Sheet, as the warrants met the criteria under the relevant accounting standard for treatment as an equity instrument. The debt discount is being amortized over the term of the Term Loan A Commitment.
As a condition to the lenders’ funding the loans under the Credit Facility, concurrently with the funding under the initial term loan B commitment on September 28, 2017, (i) the Company issued to Oxford Finance LLC a warrant to purchase 22,844 shares of Nuvectra common stock at an exercise price of $12.31 per share, which warrant is exercisable until September 28, 2027, and (ii) the Company issued to Silicon Valley Bank a warrant to purchase 22,844 shares of Nuvectra common stock at an exercise price of $12.31 per share, which warrant is exercisable until September 28, 2027. The fair value of the warrants on the date of grant totaled approximately $0.4 million and was recorded as additional paid-in capital in the Consolidated Balance Sheet, as the warrants met the criteria under the relevant accounting standard for treatment as an equity instrument. The debt discount is being amortized over the term of the Term Loan B Commitment.
As a condition to the lenders’ funding the loans under the Credit Facility, concurrently with the funding under the Term Loan B Commitment in February 2018, (i) the Company issued to Oxford Finance LLC a warrant to purchase 30,245 shares of Nuvectra common stock at an exercise price of $9.30 per share, which warrant is exercisable until February 18, 2028, and (ii) the Company issued to Silicon Valley Bank a warrant to purchase 30,245 shares of Nuvectra common stock at an exercise price of $9.30 per share, which warrant is exercisable until February 18, 2028. The fair value of the warrants on the date of grant was recorded as additional paid-in capital in the Consolidated Balance Sheet in the first quarter of 2018, as the warrants met the criteria under the relevant accounting standard for treatment as an equity instrument. The debt discount is being amortized over the term of the Term Loan B Commitment.
Upon the funding of the term loan C commitment, Oxford Finance LLC and Silicon Valley Bank were each entitled to additional warrants for the purchase of Nuvectra common stock. The number of shares under each warrant were to be equal to the amount of the term loan made by each lender multiplied by 4.50% and divided by the then current trading price of Nuvectra common shares. The exercise price of the warrants were to be equal to the trading price of Nuvectra common shares on the date of funding. The fair value of these future warrants as of December 31, 2017 was approximately $0.4 million and was recorded in other long-term liabilities in the Consolidated Balance Sheet. These warrants were classified as a derivative liability because the Company did not meet the criteria under the relevant accounting standard for treatment as equity instruments. As a result, the derivative liability warrants will be re-measured to its fair value at the end of each reporting period until it meets the requirements for equity treatment or is cancelled. See Note 11 “Fair Value Measurements” for additional information.
Upon the funding of the Term Loan C Commitment, Oxford Finance LLC and Silicon Valley Bank will each be entitled to additional warrants for the purchase of Nuvectra common stock. The number of shares under each warrant will be equal to the amount of the Term Loan made by each lender multiplied by 4.50% and divided by the then current trading price of Nuvectra common shares. The exercise price of the warrants will be equal to the trading price of Nuvectra common shares on the date of funding.
Deferred Financing Fees – The change in deferred financing fees is as follows (in thousands):
|
At January 1, 2016 |
$ | — | ||
|
Additions during the period |
1,528 | |||
|
Amortization during the period |
(266 |
) |
||
|
At December 30, 2016 |
$ | 1,262 |
|
At December 30, 2016 |
$ | 1,262 | ||
|
Additions during the period |
47 | |||
|
Amortization during the period |
(347 |
) |
||
|
At December 31, 2017 |
$ | 962 |
In accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) 2015-03, “Interest-Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs,” the Company has presented debt issuance costs as a direct deduction from Long-Term Debt in the Consolidated Balance Sheets.
|
8. |
INCOME TAXES |
The provision for income taxes associated with the Company was comprised of the following (in thousands):
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Current tax expense |
$ | 25 | $ | — | ||||
|
Deferred tax benefit |
(2,743 | ) | (13,997 |
) |
||||
|
Change in valuation allowance |
2,743 |
|
13,997 | |||||
|
Total provision for income taxes |
$ | 25 | $ | — | ||||
The provision for income taxes differs from the United States statutory rate as a result of the following (in thousands):
|
At |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Tax benefit at U.S. statutory rate |
$ | (15,155 |
) |
$ | (13,117 |
) |
||
|
State taxes, net of federal benefit |
(822 |
) |
(1,146 |
) |
||||
|
Impact of tax reform rate change |
11,696 | — | ||||||
|
Other |
1,562 | 266 | ||||||
|
Valuation allowance – tax reform rate change |
(11,696 |
) |
— | |||||
|
Valuation allowance – other changes |
14,440 | 13,997 | ||||||
|
Total provision for income taxes |
$ | 25 | $ | — | ||||
Deferred tax assets (liabilities) consist of the following (in thousands):
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Net operating loss carryforwards |
$ | 16,834 | $ | 10,998 | ||||
|
Research and development tax credits |
420 | — | ||||||
|
Property, plant and equipment |
127 | 637 | ||||||
|
Intangible assets |
3,281 | 5,603 | ||||||
|
Accruals |
503 | 415 | ||||||
|
Other |
861 | 1,629 | ||||||
|
Gross deferred tax assets |
22,026 | 19,282 | ||||||
|
Less valuation allowance |
(22,026 |
) |
(19,282 |
) |
||||
|
Net deferred tax assets |
— | — | ||||||
|
Net deferred tax asset (liability) |
$ | — | $ | — | ||||
Deferred income tax assets or liabilities reflect temporary differences between amounts of assets and liabilities, including net operating loss (“NOL”) carryforwards, for financial and tax reporting. A valuation allowance is provided on deferred tax assets if it is determined that it is more likely than not that the asset will not be realized.
On December 22, 2017, the President of the United States signed into law the Tax Cuts and Jobs Act (“The Act”). This legislation makes significant changes in U.S. tax law including a reduction in the corporate tax rates, changes to net operating loss carryforwards and carrybacks, and a repeal of the corporate alternative minimum tax. The legislation reduced the U.S. corporate tax rate from the current maximum rate of 35% to 21% for tax years beginning after December 31, 2017. As a result of the enacted law, the Company was required to revalue deferred tax assets and liabilities and the valuation allowance existing as of December 31 2017 from the 34% federal rate for Nuvectra in effect through the end of 2017, to the new 21% statutory federal rate. As a result of the change in law, the Company recorded a decrease in its deferred tax assets of $11.7 million and a corresponding decrease in its valuation allowance.
The Company has approximately $72.2 million in federal and $29.0 million in state net operating losses that could be used to offset taxable income in future periods and reduce its income taxes payable in those future periods. In addition, the Company has approximately $560 thousand of federal research and development tax credit carryforwards. Many of these net operating loss carryforwards and the federal research and development tax credits will expire if they are not used within certain periods.
The Company considers all available positive and negative evidence, including the Company’s current and past performance, the market environment in which the Company operates, the utilization of past tax credits, length of carry back and carry forward periods, existing contracts or sales backlog that will result in future profits, as well as other factors, to determine whether, based on the weight of that evidence, a valuation allowance is needed for some portion or all of a net deferred income tax asset. Judgment is used in considering the relative impact of negative and positive evidence. In arriving at these judgments, the weight given to the potential effect of negative and positive evidence is commensurate with the extent to which such evidence can be objectively verified. In evaluating the objective evidence and the need for a valuation allowance, the Company considered the past three years of cumulative operating losses.
Based on an assessment of the available positive and negative evidence, including the historical operating losses, the Company has concluded that it is more likely than not that the net deferred tax assets will not be realized. As such, the Company has provided a full valuation allowance on the net deferred income tax assets as of December 31, 2017 and December 30, 2016. Until an appropriate level of profitability is sustained, the Company expects to continue to record a full valuation allowance on future tax benefits.
At December 31, 2017, the gross amount of unrecognized tax benefits was 140 thousand, this amount would not impact our effective tax rate, if recognized due to the full valuation allowance on our deferred tax assets. Under ASC 740-10-45-10A, the unrecognized tax benefit is presented in the financial statements as a reduction to a deferred tax asset and no liability is recorded. We classify interest and penalties as a component of income tax expense. As of December 31, 2017 and December 30, 2016, the Company has not accrued any interest and penalties.
A reconciliation of the beginning and ending amounts of unrecognized tax benefits is as follows (in thousands):
|
December 31, 2017 |
||||
|
Balance at beginning of fiscal year |
$ | — | ||
|
Gross amount of increases for current year tax positions |
140 | |||
|
Balance at ending of fiscal year |
$ | 140 | ||
The Company files annual income tax returns in the United States and various state and local jurisdictions. There are currently no examinations in process. Generally, the Company is no longer subject to examination for years before 2014.
Pursuant to the terms of the tax matters agreement with Integer, for a period of two years following the date of the spin-off, the Company will be prohibited from (i) causing or permitting to occur any transaction or series of transactions, subject to certain exceptions provided under the U.S. federal income tax rules, in connection with which one or more persons would (directly or indirectly) acquire an interest in its capital stock that, when combined with any other acquisition of an interest in its capital stock that occurs after the spin-off, comprises 30% or more of the value or the total combined voting power of all interests that are treated as outstanding equity of Nuvectra for U.S. federal income tax purposes immediately after such transaction or, in the case of a series of related transactions, immediately after any transaction in such series; (ii) transferring, selling or otherwise disposing of 35% or more of its gross assets if such transfer, sale or other disposition would violate the IRS’ rules and regulations; (iii) liquidating its business or (iv) ceasing to maintain its active business. If the Company takes any of these actions and such actions result in tax-related costs for Integer, then the Company would generally be required to indemnify Integer for such costs. On March 14, 2018 we will no longer be prohibited under the terms of the tax matters agreement with Integer from raising additional funds.
|
9. |
COMMITMENTS AND CONTINGENCIES |
Litigation – Periodically the Company is a party to various legal actions, both threatened and filed, arising in the normal course of business. While the Company does not expect that the ultimate resolution of any pending actions will have a material effect on its results of operations, financial position, or cash flows, litigation is subject to inherent uncertainties. As such, there can be no assurance that any pending or threatened legal action, which the Company currently believes to be immaterial, does not become material in the future.
On September 19, 2017, Boston Scientific Corporation filed a lawsuit in district court in Suffolk County, Massachusetts, against the Company and three former Boston Scientific employees recently hired by the Company, alleging tortious interference of contract on the part of the Company and breaches of contract related to non-solicitation and confidentiality by Boston Scientific’s former employees. No demands for any monetary relief or specific performance have been made by Boston Scientific at this time. We intend to vigorously defend against the allegations.
Purchase Commitments – Contractual obligations for purchase of goods or services are defined as agreements that are enforceable and legally binding on the Company and that specify all significant terms, including: fixed or minimum quantities to be purchased; fixed, minimum, or variable price provisions; and the approximate timing of the transaction. The Company’s purchase orders are normally based on its current manufacturing or other operational needs. Inventory to be purchased by Nuvectra in fiscal year 2018 under its supply agreements with Integer and Minnetronix, Inc. is subject to certain minimum order quantity requirements. As of December 31, 2017, the Company had no material commitments to purchase capital assets; however, planned capital expenditures for 2018 are estimated at approximately $2.0 million and will primarily be financed by existing cash and cash equivalents, cash generated from sales, or the Credit Facility. The Company also enters into contracts for outsourced services; however, the contracts generally contain clauses allowing for cancellation without significant penalty.
Operating Leases – The Company is a party to various operating lease agreements. See Note 2 “Property, Plant and Equipment, Net” for information on the Company’s future lease obligations, which primarily relates to building leases.
|
10. |
EARNINGS PER SHARE (“EPS”) |
Basic net loss per share is calculated by dividing net loss by the weighted average number of common shares outstanding during the period. Diluted net loss per share is equal to basic net loss per share as the Company had no potentially dilutive securities outstanding for any of the periods presented.
The following table illustrates the calculation of Basic and Diluted EPS (in thousands, except per share amounts):
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Basic net loss per share: |
||||||||
|
Net loss |
$ | (44,600 |
) |
$ | (38,428 |
) |
||
|
Weighted average common shares outstanding |
10,576 | 10,277 | ||||||
|
Basic net loss per share |
$ | (4.22 |
) |
$ | (3.74 |
) |
||
|
Diluted net loss per share: |
||||||||
|
Net loss |
$ | (44,600 |
) |
$ | (38,428 |
) |
||
|
Weighted average common shares outstanding |
10,576 | 10,277 | ||||||
|
Dilutive stock options, restricted stock and restricted stock units |
— | — | ||||||
|
Weighted average common shares outstanding – assuming dilution |
10,576 | 10,277 | ||||||
|
Diluted net loss per share |
$ | (4.22 |
) |
$ | (3.74 |
) |
||
|
Outstanding securities and warrants that were not included in the diluted calculation because their effect would be anti-dilutive |
1,402 | 1,538 | ||||||
|
11. |
FAIR VALUE MEASUREMENTS |
The carrying amounts of cash and cash equivalents, accounts receivable, accounts payable, and accrued expenses approximate fair value because of the short-term nature of these items. As of December 31, 2017, the fair value of the Company’s variable rate long-term debt approximates its carrying value and is categorized in Level 2 of the fair value hierarchy.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
Fair value measurement standards apply to certain financial assets and liabilities that are measured at fair value on a recurring basis (each reporting period).
The Company has categorized its warrants measured at fair value on a recurring basis in Level 3 of the fair value hierarchy. The fair value of the warrants classified as liability awards was determined by utilizing a Monte Carlo simulation model, which projects the value of Nuvectra stock versus its peer group under numerous scenarios and determines the value of the award based upon the present value of these projected outcomes. The estimated fair value of the warrants as of the date of issuance was approximately $0.2 million and was recorded as a long-term asset and a liability in the Consolidated Balance Sheet. The estimated fair value of the warrant liability will be revalued on a periodic basis and any resulting increases or decreases in the estimated fair value will be recorded as an adjustment to earnings. During fiscal year 2017, the Company recorded an expense of approximately $0.6 million in fair value adjustments, which is included in Other Expense on the Consolidated Statement of Operations. The estimated fair value of the warrants as of December 31, 2017 was approximately $0.4 million.
The Company’s investments in marketable securities primarily consist of investments in debt securities, which are classified as available-for-sale and presented as current assets within Cash and Cash Equivalents on the consolidated balance sheet because of their original maturities of three months or less. Unrealized gains or losses for the periods presented are included in other comprehensive loss.
The fair values of marketable securities were estimated using the market approach using prices and other relevant information generated by market transactions involving identical or comparable assets. The Company uses quoted market prices in active markets or quoted market prices in markets that are not active to measure fair value. When developing fair value estimates, the Company maximizes the use of observable inputs and minimizes the use of unobservable inputs. As of December 31, 2017, the fair market value of marketable securities was approximately $21.3 million, all of which had original maturities of three months or less.
Marketable securities, measured at fair value, by level within the fair value hierarchy were as follows (in thousands):
|
December 31, 2017 |
|||||||||||||
|
Fair Value Hierarchy |
Cost |
Unrealized Loss |
Fair Value |
||||||||||
|
Cash |
Level 1 |
$ | 7,336 | $ | — | $ | 7,336 | ||||||
|
Government |
Level 1 |
1,499 | — | 1,499 | |||||||||
|
Financial |
Level 2 |
3,799 | (1 |
) |
3,798 | ||||||||
|
Industrial |
Level 2 |
8,649 | — | 8,649 | |||||||||
|
Total |
$ | 21,283 | $ | (1 |
) |
$ | 21,282 | ||||||
|
December 30, 2016 |
|||||||||||||
|
Fair Value Hierarchy |
Cost |
Unrealized Loss |
Fair Value |
||||||||||
|
Cash |
Level 1 |
$ | 33,821 | $ | — | $ | 33,821 | ||||||
|
Government |
Level 1 |
2,005 | — | 2,005 | |||||||||
|
Financial |
Level 2 |
8,064 | (1 |
) |
8,063 | ||||||||
|
Industrial |
Level 2 |
11,125 | (1 |
) |
11,124 | ||||||||
|
Total |
$ | 55,015 | $ | (2 |
) |
$ | 55,013 | ||||||
Assets and Liabilities Measured at Fair Value on a Nonrecurring Basis
Fair value standards also apply to certain assets and liabilities that are measured at fair value on a nonrecurring basis. A summary of the valuation methodologies for assets and liabilities measured on a nonrecurring basis is as follows:
Long-lived Assets – The Company reviews the carrying amount of its long-lived assets to be held and used, other than goodwill, for potential impairment whenever certain indicators are present as described in Note 1 “Summary of Significant Accounting Policies.”
Goodwill – Goodwill recorded is not amortized but is periodically tested for impairment. The Company assesses goodwill for impairment on the last day of each fiscal year, or more frequently if certain events occur as described in Note 1 “Summary of Significant Accounting Policies.” During fiscal years 2017 and 2016, no impairment charges were recorded related to the Company’s Goodwill.
Warrants – In order to determine the fair value of the warrants classified as equity awards, the Company used a Monte Carlo simulation model. The risk-free interest rate represents the 10-Year U.S. Treasury rate as of March 18, 2016. The expected volatility assumption is based on historical volatilities for publicly traded stock of comparable companies.
The following table summarizes the assumptions as of December 31, 2017 used for estimating the fair value of the warrants classified as liability awards:
|
Risk-free interest rate |
2.35 |
% |
||
|
Expected volatility |
70.00 |
% |
||
|
Contractual term (in years) |
10 | |||
|
Dividend yield |
— |
% |
|
12. |
STOCKHOLDERS’ EQUITY |
The Company is authorized to issue 100 million shares of common stock, $0.001 par value per share. Holders of common stock are entitled to one vote per share. Holders of common stock are entitled to receive dividends, if and when declared by the Board of Directors, and to share ratably in the Company’s assets legally available for distribution to its stockholders in the event of liquidation. Holders of common stock have no preemptive, subscription, redemption, or conversion rights. The holders of common stock do not have cumulative voting rights. The holders of a majority of the shares of common stock can elect all of the directors and can control our management and affairs. On March 14, 2016, in connection with the spin-off, the Company issued 10,258,278 shares of common stock to Integer shareholders.
|
13. |
BUSINESS SEGMENTS, GEOGRAPHIC AND CONCENTRATION RISK INFORMATION |
The Company has two reportable segments and two reporting units consisting of: Nuvectra and NeuroNexus.
Nuvectra is a neurostimulation company committed to helping physicians improve the lives of people with chronic conditions. Algovita is the Company’s first commercial offering and is approved for the treatment of chronic pain of the trunk and/or limbs. Nuvectra’s innovative technology platform also has capabilities under development to support other neurological indications such as SNM for the treatment of overactive bladder and DBS for the treatment of Parkinson’s disease and Essential Tremor. Revenue includes development and engineering service fees and sales from the limited release of Algovita in the United States and Europe. Future revenues of Nuvectra are expected to come primarily from sales of Algovita, particularly after expansion of its launch commercially in the United States, and Virtis, the second application of the Company’s neurostimulation technology platform and its first product for the SNM market.
NeuroNexus designs, manufactures and markets neural-interface technologies for the neuroscience clinical research market. Revenues includes sales of neural interface technology, components and systems to the neuroscience and clinical markets.
An analysis and reconciliation of the Company’s product lines, business segments and geographic information to the respective information in the Consolidated Financial Statements follows. Sales by geographic area are presented by allocating sales from external customers based on where the products are shipped or services are rendered (in thousands):
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Product line sales: |
||||||||
|
Algovita |
$ | 25,567 | $ | 4,162 | ||||
|
Neural interface components and systems |
4,756 | 5,152 | ||||||
|
Development and engineering service |
1,513 | 3,221 | ||||||
|
Total sales |
$ | 31,836 | $ | 12,535 | ||||
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Business segment sales: |
||||||||
|
Nuvectra |
$ | 27,080 | $ | 7,383 | ||||
|
NeuroNexus |
4,756 | 5,152 | ||||||
|
Total sales |
$ | 31,836 | $ | 12,535 | ||||
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Segment (loss) income from operations: |
||||||||
|
Nuvectra |
$ | (42,231 |
) |
$ | (37,702 |
) |
||
|
NeuroNexus |
219 | 300 | ||||||
|
Total segment loss from operations |
(42,012 |
) |
(37,402 |
) |
||||
|
Unallocated operating expenses |
— | — | ||||||
|
Operating loss |
(42,012 |
) |
(37,402 |
) |
||||
|
Unallocated other expense, net |
(2,563 |
) |
(1,026 |
) |
||||
|
Loss before provision for income taxes |
$ | (44,575 |
) |
$ | (38,428 |
) |
||
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Depreciation and amortization: |
||||||||
|
Nuvectra |
$ | 1,249 | $ | 1,908 | ||||
|
NeuroNexus |
366 | 335 | ||||||
|
Total depreciation and amortization included in segment income from operations |
1,615 | 2,243 | ||||||
|
Unallocated depreciation and amortization |
— | — | ||||||
|
Total depreciation and amortization |
$ | 1,615 | $ | 2,243 | ||||
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Expenditures for tangible long-lived assets: |
||||||||
|
Nuvectra |
$ | 1,140 | $ | 3,950 | ||||
|
NeuroNexus |
91 | 81 | ||||||
|
Total reportable segments |
1,231 | 4,031 | ||||||
|
Unallocated tangible long-lived assets |
— | — | ||||||
|
Total expenditures |
$ | 1,231 | $ | 4,031 | ||||
|
At |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Identifiable assets: |
||||||||
|
Nuvectra |
$ | 83,696 | $ | 111,503 | ||||
|
NeuroNexus |
7,407 | 7,799 | ||||||
|
Total reportable segments |
91,103 | 119,302 | ||||||
|
Unallocated assets |
— | — | ||||||
|
Total assets |
$ | 91,103 | $ | 119,302 | ||||
|
Year Ended |
||||||||
|
December 31, 2017 |
December 30, 2016 |
|||||||
|
Sales by geographic area: |
||||||||
|
United States |
$ | 26,976 | $ | 5,800 | ||||
|
Non-Domestic locations: |
||||||||
|
Switzerland |
1,576 | 3,461 | ||||||
|
Germany |
1,234 | 1,567 | ||||||
|
Rest of world |
2,050 | 1,707 | ||||||
|
Total sales |
$ | 31,836 | $ | 12,535 | ||||
All of the Company’s long-lived tangible assets are located in the United States.
|
14. |
RELATED PARTY TRANSACTIONS |
Corporate Overhead Allocations from Integer – As discussed in Note 1 “Summary of Significant Accounting Policies,” prior to March 14, 2016, the Company operated as part of Integer and as a result shared many overhead functions and services performed by various Integer corporate departments. Costs of these departments were allocated across the Integer entities, including the Company, that benefited from those services during the periods prior to March 14, 2016. The indirect costs allocated included executive oversight, finance, legal, human resources, tax, information technology, product development, corporate procurement, and facilities. These expenses have been charged to the Company on a pro rata basis based upon estimated hours incurred, headcount, square footage, or other measures. The Company considers the expense allocation methodology and results to be reasonable. However, these allocations are not indicative of the actual expenses that would have been incurred if the Company was an independent publicly-traded company or of the costs the Company will incur in the future. At this time, the Company is unable to determine what its expenses would have been on a standalone basis if the Company had operated as an unaffiliated entity for the periods prior to March 14, 2016. Corporate overhead allocations from Integer totaled $0.2 million for fiscal year 2016 and were classified as selling, general and administrative expenses. There were no such allocations in fiscal year 2017.
On March 14, 2016 the Company entered into, or amended, various agreements with Integer to effect the Spin-off and to provide a framework for the Company’s relationship with Integer going forward after the Spin-off including a supply agreement, license agreements, a separation and distribution agreement, a tax matters agreement, a transition services agreement and an employee matters agreement, which provided for the allocation between Nuvectra and Integer of assets, employees, liabilities and obligations (including PP&E, employee benefits, and tax-related assets and liabilities) attributable to the Company’s business for the period prior to, at, and after the Spin-off. Immediately prior to the completion of the Spin-off, Integer made a cash capital contribution to Nuvectra of $75.0 million.
Employee Benefit Plans – Certain of the Company’s employees historically participated in various Integer defined contribution and stock-based compensation plans. Compensation expense allocated to Nuvectra for these plans from Integer was based upon the costs directly attributable to Nuvectra employees. See Note 5 “Employee Benefit Plans” for additional information.
Supply Agreement – The Company has a supply agreement with Integer pursuant to which Integer manufactures Algovita and certain of its components. Total charges incurred under this supply agreement are included in cost of sales.
Purchase of Non-controlling Interests – During the fourth quarter of 2015, the Company purchased the outstanding non-controlling interests of Algostim and PelviStim for $16.7 million, of which $9.9 million was paid in 2015 and $6.8 million was accrued at January 1, 2016 and paid by Integer in January 2016 prior to the spin-off. Included in the purchased amount was $6.9 million paid to Drees Holding LLC, which is a limited liability company of which Scott F. Drees, CEO of Nuvectra, is the principal owner and the sole managing director and approximately $0.8 million paid to Norbert Kaula, Executive Vice President of Research and Development of Nuvectra. Mr. Drees and Mr. Kaula each received their interests in Algostim and PelviStim in connection with entering into a long-term consulting agreement with Nuvectra and prior to being appointed to their current positions. The consulting agreements were terminated in connection with Mr. Drees and Mr. Kaula agreeing to serve in their current roles for Nuvectra. The buyout of the non-controlling interests was funded by a cash contribution from Integer.
|
15. |
RECENTLY ISSUED ACCOUNTING STANDARDS |
In the normal course of business, management evaluates all new accounting pronouncements issued by the FASB to determine the potential impact they may have on the Company’s Consolidated Financial Statements. Based upon this review, except as noted below, management does not expect any of the recently issued accounting pronouncements, which have not already been adopted, to have a material impact on the Company’s Consolidated Financial Statements.
Recently Adopted
In January 2017, the FASB issued Accounting Standard Update (“ASU”) 2017-04, “Intangibles—Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment.” This guidance simplifies the subsequent measurement of goodwill and eliminates the two-step goodwill impairment test. Under the new guidance, an annual or interim goodwill impairment test is performed by comparing the fair value of a reporting unit with its carrying amount, and an impairment charge is recognized for the amount by which the carrying amount exceeds the reporting unit’s fair value; however, the loss recognized should not exceed the total amount of goodwill allocated to that reporting unit. Additionally, an entity should consider income tax effects from any tax deductible goodwill on the carrying amount of the reporting unit when measuring the goodwill impairment loss, if applicable. An entity still has the option to perform the qualitative assessment for a reporting unit to determine if the quantitative impairment test is necessary. The Company adopted ASU 2017-04 and has applied the guidance during its fiscal year 2017 annual impairment testing.
In March 2016, the FASB issued ASU 2016-09, “Improvements to Employee Share-Based Payment Accounting,” which amends ASC Topic 718, Compensation – Stock Compensation. ASU 2016-09 simplifies several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. The Company has adopted the guidance in this ASU in the first quarter of fiscal year 2017 on a prospective basis as permitted by the new standard. There has been no impact on the income taxes line item of the Company’s Consolidated Statements of Operations and Comprehensive Loss related to excess tax benefits upon vesting or settlement, no impact to its cash flow presentation and no impact to the computation of diluted earnings per share. Under the new guidance, the company has elected to account for forfeitures as they occur. Due to the Spin-off and related change to the Company’s equity incentive plans, this election did not result in an adjustment to the Company’s stock-based compensation expense. The adoption of ASU 2016-09 did not have a material impact on the Company’s Consolidated Financial Statements.
In July 2015, the FASB issued ASU 2015-11, “Inventory (Topic 330): Simplifying the Measurement of Inventory.” This update requires inventory within the scope of the standard to be measured at the lower of cost and net realizable value. Net realizable value is defined as the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The amendments in this update do not apply to inventory that is measured using last-in, first-out or the retail inventory method. The amendments apply to all other inventory, which includes inventory that is measured using FIFO or average cost. The guidance in this ASU became effective for the Company in the first quarter of fiscal year 2017. The adoption of ASU 2015-11 did not have a material impact on the Company’s Consolidated Financial Statements.
Not Yet Adopted
In May 2017, the FASB issued ASU 2017-09, “Compensation-Stock Compensation (Topic 718): Scope of Modification Accounting.” ASU 2017-09 clarifies when changes to the terms or conditions of a share-based payment award must be accounted for as modifications. Under ASU 2017-09, an entity will not apply modification accounting to a share-based payment award if the award’s fair value, vesting conditions and classification as an equity or liability instrument are the same immediately before and after the change. ASU 2017-09 will be applied prospectively to awards modified on or after the adoption date. The guidance is effective for annual periods, and interim periods within those annual periods, beginning after December 15, 2017. ASU 2017-09 is effective for the Company beginning in fiscal 2018. The Company does not expect its pending adoption of ASU 2017-09 to have a material impact on its Consolidated Financial Statements.
In January 2017, the FASB issued ASU 2017-01, “Business Combinations (Topic 805): Clarifying the Definition of a Business.” ASU 2017-01 provides a screen to determine when an integrated set of assets and activities (collectively referred to as a “set”) does not constitute a business. The screen requires that when substantially all of the fair value of the gross assets acquired (or disposed of) is concentrated in a single identifiable asset or a group of similar identifiable assets, the set is not a business. This screen reduces the number of transactions that need to be further evaluated. If the screen is not met, the amendments in ASU 2017-01 (i) require that to be considered a business, a set must include, at a minimum, an input and a substantive process that together significantly contribute to the ability to create output and (ii) remove the evaluation of whether a market participant could replace missing elements. This update is effective for annual and interim periods beginning after December 15, 2017, which will require the Company to adopt these provision in the first quarter of fiscal 2018 using a prospective approach. The Company does not expect its pending adoption of ASU 2017-01 to have a material impact on its Consolidated Financial Statements.
In August 2016, the FASB issued ASU 2016-15, “Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments.” This update provides clarification regarding how certain cash receipts and cash payments are presented and classified in the statement of cash flows. ASU 2016-15 addresses eight specific cash flow issues with the objective of reducing the existing diversity in practice. This update is effective for annual and interim periods beginning after December 15, 2017, which will require the Company to adopt these provisions in the first quarter of fiscal 2018 using a retrospective approach. The Company does not expect its pending adoption of ASU 2016-15 to have a material impact on its Consolidated Financial Statements.
In February 2016, the FASB issued ASU 2016-02, “Leases” in order to increase transparency and comparability among organizations by recognizing lease assets and lease liabilities on the balance sheet for those leases classified as operating leases under previous GAAP. ASU 2016-02 requires that a lessee should recognize a liability to make lease payments (the lease liability) and a right-of-use asset representing its right to use the underlying asset for the lease term on the balance sheet. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018 (including interim periods within those periods), using a modified retrospective approach, and early adoption is permitted. The Company is currently in the process of evaluating the impact of adoption of the ASU on its Consolidated Financial Statements.
In January 2016, the FASB issued ASU 2016-01, “Recognition and Measurement of Financial Assets and Financial Liabilities,” which updates certain aspects of recognition, measurement, presentation and disclosure of financial instruments. The standard is effective for interim and annual periods beginning after December 15, 2017, which will require the Company to adopt these requirements in the first quarter of fiscal year 2018. The Company is currently in the process of evaluating the impact of adoption of the ASU on its Consolidated Financial Statements.
In May 2014, the FASB issued ASC Update No. 2014-09, Revenue from Contracts with Customers (“ASC 606”), which has been subsequently updated. The purpose of ASC 606 is to provide enhancements to the quality and consistency of how revenue is reported while also improving comparability in the financial statements of companies using GAAP and International Financial Reporting Standards (IFRS). ASC 606 outlines a single comprehensive model for companies to use in accounting for revenue arising from contracts with customers and supersedes most current revenue recognition guidance, including industry-specific guidance. The core principle requires entities to recognize revenue in a manner that depicts the transfer of goods or services to customers in amounts that reflect the consideration an entity expects to be entitled to in exchange for those goods or services.
ASC 606, as amended, is effective for annual periods beginning after December 15, 2017. Companies have the option of using either a full retrospective or a modified retrospective approach to adopt the guidance. On January 1, 2018 the Company adopted ASC 606 under the modified retrospective method and will only apply this method to contracts that are not completed as of the date of adoption. The modified retrospective method results in a cumulative effect of initially applying the standard as an adjustment to the opening balance of retained earnings at the date of initial application for the impact to changes in the timing and amount of revenue recognition as well as the timing of capitalization and amortization of certain contract costs.
The Company has completed an implementation plan to assess the impact of the new guidance on its operations, financial results and related disclosures. To date, we have completed our assessment of the revenue streams and areas of the balance sheet and financial statement components impacted, including assessing the impact of the new guidance on our results of operations and internal controls.
The Company’s revenue streams identified include (i) Algovita US product sales, (ii) Algovita foreign product sales, (iii) NeuroNexus product sales, including those with custom design services and (iv) development and engineering service revenue from the agreement with Aleva Neurotherapeutics, S.A. (“Aleva”). Based on the Company’s analysis of open contracts as of the adoption date, there will be no material impact on the timing or amount of revenue recognized for the Algovita and NeuroNexus product sales revenue streams as its contracts include only point-in-time performance obligations which are fully satisfied within the same reporting period, consistent with current revenue recognition. Going forward, to the extent that a contract includes multiple performance obligations which are not fully satisfied and one or more were not priced at its standalone selling price (“SSP”), the Company will be required to perform an allocation of the transaction price which may result in a difference in the amount of revenue recognized in any period. Additionally, for the development and engineering service revenue stream there will not be a material change in the timing and amount of revenue recognized as the services and distribution license are priced at their respective SSPs and the consideration, the sales-based royalties, for the distribution license for intellectual property are constrained until the customer’s subsequent sale.
Upon adopting ASC 606, the Company will be required to provide additional disclosures in the notes to the consolidated financial statements, specifically related to disaggregated revenue, contract balances and performance obligations. Additionally, as part of the Company’s implementation of ASC 606 the Company has implemented new internal controls in order to address risks associated with applying the five-step model, specifically related to judgments made in connection to performance obligations, estimated standalone selling prices and implied or explicit options that represent material rights. Additionally, the Company has established monitoring controls to identify new sales arrangements and changes in its business environment that could potentially impact our current accounting assessment.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)). Disclosure controls and procedures are designed to ensure that material information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and to ensure that such material information is accumulated and communicated to our management, including our CEO and CFO, as appropriate to allow timely decisions regarding required disclosure. Based on this evaluation, our CEO and CFO concluded that as of the end of the period covered by this Annual Report on Form 10-K, our disclosure controls and procedures were effective.
Management’s Annual Report on Internal Control Over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) under the Securities Exchange Act of 1934 as a process designed by, or under the supervision of, the Company’s principal executive and principal financial officers and effected by the Company’s board of directors, management and other associates, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
|
(1) |
Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
|
(2) |
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and |
|
(3) |
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2017. In making this assessment, the Company’s management used the criteria set forth by COSO in “Internal Control – Integrated Framework (2013).” Based on the results of its evaluation, the Company’s management has concluded that the internal control over financial reporting was effective as of December 31, 2017.
Because we are a smaller reporting company, in accordance with Section 404(c) of the Sarbanes-Oxley Act of 2002, this annual report does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting.
Changes in Internal Control over Financial Reporting
We maintain a system of internal controls that are designed to provide reasonable assurance that our books and records accurately reflect, in all material respects, the transactions of the Company and that we meet and achieve our control objectives. From time to time, we may experience changes to our internal controls due to, for example, employee turnover, re-balancing of workloads, extended absences, and promotions of employees.
There have been no changes in our internal controls over financial reporting during the quarter ended December 31, 2017 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Not applicable
Item 10. Directors, Executive Officers, and Corporate Governance
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 2018 Annual Meeting of Stockholders, which we intend to file with the Securities and Exchange Commission within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
Item 11. Executive Compensation
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 2018 Annual Meeting of Stockholders, which we intend to file with the Securities and Exchange Commission within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 2018 Annual Meeting of Stockholders, which we intend to file with the Securities and Exchange Commission within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 2018 Annual Meeting of Stockholders, which we intend to file with the Securities and Exchange Commission within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
Item 14. Principal Accounting Fees and Services
The information required by this Item is incorporated herein by reference to the information that will be contained in our proxy statement related to the 2018 Annual Meeting of Stockholders, which we intend to file with the Securities and Exchange Commission within 120 days of the end of our fiscal year pursuant to General Instruction G(3) of Form 10-K.
Item 15. Exhibits and Financial Statement Schedules
(a) The following documents are filed as part of this report:
(1) Financial Statements:
See Part II, Item 8. “Financial Statements and Supplementary Data.”
(2) Financial Statement Schedules:
All financial statement schedules have been omitted because they are not applicable, not required or the information required is shown in the financial statements or the notes thereto.
(3) Exhibits.
The exhibits filed as part of this Annual Report on Form 10-K are set forth on the following Exhibit Index:
|
Exhibit No. |
Description |
|||
|
|
|
|||
|
3.1 |
||||
|
|
|
|||
|
3.2 |
||||
|
|
|
|||
|
4.1 |
||||
|
|
|
|||
|
4.2 |
||||
|
|
|
|||
|
4.3 |
||||
|
10.1 |
||||
|
10.2 |
||||
|
10.3 |
||||
|
10.4 |
||||
|
10.5 |
||||
|
10.6 |
||||
|
10.7 |
||||
|
10.8 |
||||
|
10.9 |
||||
|
10.10 |
||||
|
10.11 |
||||
|
10.12 |
||||
|
10.13 |
||||
|
10.14 |
||||
|
10.15 |
||||
|
10.16 |
||||
|
10.17 |
||||
|
10.18 |
||||
|
|
|
|||
|
10.19 |
||||
|
10.20 |
||||
|
10.21 |
||||
|
10.22 |
||||
|
10.23 |
||||
|
10.24 |
||||
|
10.28 |
|
|
10.29 |
|
|
10.30 |
|
21.1 |
|
|
23.1 |
|
|
31.1 |
Certification of Chief Executive Officer pursuant to Rule 13a-14(a) of the Securities Exchange Act* |
|
|
|
|
31.2 |
Certification of Chief Financial Officer pursuant to Rule 13a-14(a) of the Securities Exchange Act* |
|
|
|
|
32.1 |
|
|
|
|
|
101.INS |
XBRL Instance Document* |
|
|
|
|
101.SCH |
XBRL Extension Schema Document* |
|
|
|
|
101.CAL |
XBRL Extension Calculation Linkbase Document* |
|
|
|
|
101.LAB |
XBRL Extension Label Linkbase Document* |
|
|
|
|
101.PRE |
XBRL Extension Presentation Linkbase Document* |
|
|
|
|
101.DEF |
XBRL Extension Definition Linkbase Document* |
* Filed herewith.
** Furnished herewith.
† Management contract or compensatory plan or arrangement.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
NUVECTRA CORPORATION |
|
|
|
|
|
|
|
|
Date: March 6, 2018 |
/s/ Scott F. Drees |
|
|
|
|
Scott F. Drees |
|
|
|
|
Chief Executive Officer (Principal Executive Officer) |
|
|
|
|
|
|
|
|
Date: March 6, 2018 |
/s/ Walter Z. Berger |
|
|
|
|
Walter Z. Berger |
|
|
|
|
Chief Operating Officer and Chief Financial Officer |
|
|
|
|
(Principal Financial and Accounting Officer) |
|
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Dated: March 6, 2018 |
|
By: |
|
/s/ Dr. Joseph A. Miller, Jr. |
|
|
|
|
|
|
|
|
|
|
|
Dr. Joseph A. Miller, Jr. |
|
|
|
|
|
Chairman of the Board |
|
|
|
|
|
|
|
Dated: March 6, 2018 |
|
By: |
|
/s/ Anthony P. Bhil III |
|
|
|
|
|
|
|
|
|
|
|
Anthony P. Bhil III |
|
|
|
|
|
Director |
|
|
|
|
|
|
|
Dated: March 6, 2018 |
|
By: |
|
/s/ Scott F. Drees |
|
|
|
|
|
|
|
|
|
|
|
Scott F. Drees |
|
|
|
|
|
Director |
|
|
|
|
|
|
|
Dated: March 6, 2018 |
|
By: |
|
/s/ Kenneth G. Hawari |
|
|
|
|
|
|
|
|
|
|
|
Kenneth G. Hawari |
|
|
|
|
|
Director |
|
|
|
|
|
|
|
Dated: March 6, 2018 |
|
By: |
|
/s/ David D. Johnson |
|
|
|
|
|
|
|
|
|
|
|
David D. Johnson |
|
|
|
|
|
Director |
|
|
|
|
|
|
|
Dated: March 6, 2018 |
|
By: |
|
/s/ Dr. Fred B. Parks, PhD |
|
|
|
|
|
|
|
|
|
|
|
Dr. Fred B. Parks, PhD |
|
|
|
|
|
Director |
|
|
|
|
|
|
|
Dated: March 6, 2018 |
|
By: |
|
/s/ Jon T. Tremmel |
|
|
|
|
|
|
|
|
|
|
|
Jon T. Tremmel |
|
|
|
|
|
Director |
|
|
|
|
|
|
|
Dated: March 6, 2018 |
|
By: |
|
/s/ Thomas E. Zelibor |
|
|
|
|
|
|
|
|
|
|
|
Thomas E. Zelibor |
|
|
|
|
|
Director |
82
