Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - AIRXPANDERS INC | ex_105718.htm |
| EX-31.2 - EXHIBIT 31.2 - AIRXPANDERS INC | ex_105717.htm |
| EX-31.1 - EXHIBIT 31.1 - AIRXPANDERS INC | ex_105716.htm |
| EX-23.1 - EXHIBIT 23.1 - AIRXPANDERS INC | ex_105715.htm |
| EX-10.18 - EXHIBIT 10.18 - AIRXPANDERS INC | ex_105720.htm |
| EX-10.17 - EXHIBIT 10.17 - AIRXPANDERS INC | ex_105719.htm |
| EX-10.12 - EXHIBIT 10.12 - AIRXPANDERS INC | ex_106076.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2017
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 000-13470
AirXpanders, Inc.
(Exact name of registrant as specified in its charter)
|
Delaware |
20-2555438 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
1047 Elwell Court |
|
| Palo Alto, California | 94303 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: 650-390-9000
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the Act:
|
Title of each class |
Name of each exchange on which registered |
|
|
Class A Common Stock, par value $0.001 per share |
None |
Indicate by check mark if the Registrant is a well-known seasoned issuer as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or Section 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the Registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
Accelerated filer |
☒ |
|
Non-accelerated filer |
☐ (Do not check if a smaller reporting company) |
Smaller reporting company |
☐ |
| Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial reporting accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of the registrant’s Class A Common Stock, in the form of CHESS Depositary Interests, or CDIs, held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate), computed by reference to the price at which the CDIs were last sold on June 30, 2017, the last business day of the registrant’s most recently completed second fiscal quarter, as reported on the Australian Securities Exchange, was $142.9 million (A$186.1 million).
The number of shares of the Registrant’s common stock outstanding as of February 15, 2018 was 95,943,409.
DOCUMENTS INCORPORATED BY REFERENCE
The Registrant has incorporated by reference into Part III of this Annual Report on Form 10-K portions of its Proxy Statement for its 2018 Annual Meeting of Stockholders to be filed pursuant to Regulation 14A. The Proxy Statement will be filed within 120 days of Registrant’s fiscal year ended December 31, 2017.
TABLE OF CONTENTS
IMPLICATIONS OF BEING AN EMERGING GROWTH COMPANY
As a company with less than $1.0 billion in revenue during our most recently completed fiscal year, we qualify as an “emerging growth company” as defined in Section 2(a) of the Securities Act of 1933, as amended, or the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable, in general, to public companies that are not emerging growth companies. These provisions include:
|
• |
Reduced disclosure about our executive compensation arrangements; |
|
• |
No non-binding shareholder advisory votes on executive compensation or golden parachute arrangements; and |
|
• |
Exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting. |
We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.0 billion in annual revenues as of the end of a fiscal year, if we are deemed to be a large accelerated filer under the rules of the SEC, or if we issue more than $1.0 billion of non-convertible debt over a three-year-period. We intend to take advantage of the reduced disclosure obligations.
The JOBS Act permits an emerging growth company to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies until such standards would otherwise apply to a private company. We have elected to avail ourselves of this exemption to take advantage of the extended transition period for complying with new or revised accounting standards.
This Annual Report on Form 10-K for the year ended December 31, 2017, or "Form 10-K," contains forward-looking statements concerning our operating performance and events or developments that we expect or anticipate will occur in the future that are based on management’s beliefs, assumptions and expectations and on information currently available to management. Any statements contained in this Form 10-K that are not of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “could,” “believes,” “estimates,” “expects,” “intends,” or the negative of these words or other similar terms or expressions that involve risks and uncertainties which have not been based solely on historical facts but on our beliefs, assumptions and expectations about our future operating performance, events and results. The forward-looking statements are contained principally in the sections entitled “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” Forward-looking statements include, but are not limited to, statements about:
|
• |
our ability to continue as a going concern;
|
|
• |
the U.S. commercial market acceptance and U.S. sales of our product; |
|
• |
our ability or the ability of third-party contract manufacturer to build our product in sufficient quantities or at required quality standards to satisfy anticipated demand; |
|
• |
our ability to manufacture our product at a lower cost in order to generate positive gross margins; |
|
• |
our ability to obtain or maintain reimbursement for our current or new products; |
|
• |
our expectations with respect to the integrity or capabilities of our intellectual property positions. |
Management believes that these forward-looking statements are reasonable as and when made. These forward-looking statements are not guarantees of future performance or development and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. As a result, any or all of our forward-looking statements in this Form 10-K may turn out to be inaccurate. We have included important factors in the cautionary statements included in this Form 10-K, particularly in the section captioned “Item 1A. Risk Factors,” that could cause actual results or events to differ materially from the forward-looking statements that we make.
You are cautioned not to place undue reliance on the forward-looking statements because they speak only as of the date when made. Unless required by law, we do not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. We may not actually achieve the plans, projections or expectations disclosed in our forward-looking statements, and actual results, developments or events could differ materially from those disclosed in the forward-looking statements we make.
PART I
|
ITEM 1. |
BUSINESS. |
Overview
AirXpanders, Inc. ("AirXpanders", the "Company", or "we") is a U.S. based medical device company whose principal business is to design, manufacture, sell and distribute medical devices used in two-stage breast reconstruction procedures following mastectomy. Our AeroForm Tissue Expander System (AeroForm) is a needle-free, patient-controlled tissue expander used in patients undergoing two-stage breast reconstruction following mastectomy prior to the insertion of a breast implant. AeroForm was granted its first CE mark in Europe in October 2012, was approved by Australia’s Therapeutic Goods Administration, or TGA, in Australia in October 2013, commenced its initial marketing release of AeroForm in Australia in January 2015, was granted its U.S. Food and Drug Administration, or FDA, de novo marketing authorization in December 2016 (as a Class II medical device), and commenced its initial marketing release of AeroForm in the U.S. in January 2017. After we received FDA de novo clearance for AeroForm, we submitted a 510(k) application for a materials change related to enhanced film material. This 510(k) was cleared by the FDA in April 2017. To date, we have been primarily engaged in developing and launching our initial product technology, completing clinical trials, building the manufacturing infrastructure to support commercialization efforts, recruiting key personnel and raising capital.
AirXpanders was incorporated in Delaware in 2005 and is headquartered in Palo Alto, California. We have been publicly traded since 2015 (ASX: AXP). We have incurred net losses and cash flow deficits from operations since our inception. During the year ended December 31, 2017, we had net revenues of approximately $3.9 million and a net loss of $29.0 million. Our accumulated deficit was approximately $95.3 million at December 31, 2017. To date, our products have been approved for marketing and sales in Europe, Australia and the U.S.
Additional information about us is available on our website at http://www.airxpanders.com. The information that can be accessed through our website, however, is not part of this Annual Report. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and any amendments to those reports are available on the investor relations section of our website free of charge as soon as reasonably practicable after we electronically file or furnish such materials to the United States Securities and Exchange Commission, or SEC. In addition, the reports and materials that we file with the SEC are available at the SEC's website (http://www.sec.gov) and at the SEC's Public Reference Room at 100 F Street, NE, Washington DC 20549. Interested parties may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
Industry Background
Breast cancer is the second leading cause of death in women globally. Most women being treated for breast cancer undergo some type of surgery to address the primary breast tumor. The purpose of the surgery is to remove as much of the cancer as possible. Breast-conserving surgery (also known as lumpectomy or partial mastectomy) can be sufficient for early-stage breast cancer patients. However, for those patients who have a more advanced breast cancer or who have a genetic predisposition for developing breast cancer, treatment often requires mastectomy, which is the removal of one or both breasts.
In the U.S. alone, of the approximately 300,000 new diagnoses of female breast cancer every year, 36% of early-stage patients and 60% of late-stage patients receive a mastectomy. Mastectomies are also performed on a proportion of previously diagnosed patients who originally had a form of breast-conserving surgery, as well as on women undertaking mastectomy as a preventative measure prior to any cancer diagnosis. Approximately 60% of mastectomies in the U.S. involve the removal of both breasts. With increasing awareness, earlier detection and the emerging practice of pre-emptive breast removal surgery, death rates from breast cancer have been declining since the late 1980s, particularly in women younger than 50 years of age, while mastectomy rates have been increasing.
Although a traumatic and painful procedure, mastectomy has become increasingly accepted and adopted as a pre-emptive measure to prevent breast cancer. Women who have been diagnosed with cancer in one breast may elect to have the other breast removed as a precautionary measure. Similarly, preventative surgery to remove both breasts is an increasingly sought after option for women with a strong family history of breast cancer or who have been identified as having disease causing mutations in certain genes, such as BRCA1 or BRCA2, which predispose them to a significantly elevated risk of developing breast cancer. After a mastectomy, breast reconstruction is available to women who want to rebuild the shape and look of the breast.
Breast reconstruction restores breast symmetry after a mastectomy by creating a breast mound, similar in size, shape, and contour to the contralateral breast. The most common technique used in breast reconstruction is two-stage reconstruction which involves tissue expansion to create space for a breast implant. Tissue expansion involves expansion of the breast skin and muscle using a temporary tissue expander. Traditional tissue expanders on the market today are saline-based and require the patient to go to the surgeon’s office every few weeks to receive an injection of saline to fill the expander. Typically, it can take several weeks to several months to complete the process to reach the desired volume. This tissue expander is removed after a few months and microvascular flap reconstruction or the insertion of a breast implant is done at the time. Traditional saline-based tissue expanders have silicone outer shells and an external magnetic port to allow for saline fluid injections.
Instead of saline, AeroForm, a needle-free device, uses a controlled delivery of small amounts of carbon dioxide (CO2) gas to achieve the tissue expansion usually required prior to placement of a breast implant. It eliminates the need for the needle injections required for traditional saline-based tissue expanders, gives patients the ability to control the expansion process themselves and allows patients to achieve full expansion faster than is achieved using traditional saline-based tissue expanders. In a series of clinical trials, AeroForm has been shown to enable patients to proceed to the insertion of a breast implant faster than with a traditional saline-based tissue expander.
Our Business
Our business objective is to become a global market leader in the development and commercialization of medical devices for tissue expansion for breast reconstruction after mastectomy. Our principal strategies to achieve this objective are as follows:
|
• |
Convert the existing tissue expander market to AeroForm. |
|
• |
Expand the breast reconstruction market through general awareness. |
|
• |
Utilize traditional and social media to inform patients about AeroForm. |
|
• |
Leverage clinical results of our five clinical trials. |
|
• |
Seek strong margins through increased production volumes and manufacturing in a lower cost labor environment. |
|
• |
Obtain broad product distribution through a direct sales force in the U.S. |
Sole Product
Our sole product is the AeroForm Tissue Expander System, or AeroForm. AeroForm is equipped with a remote dosage controller that activates an internal CO2 gas reservoir in the temporary tissue expander implant. With the use of the remote control device, the patient is able to trigger the release of a small amount of gas to increase the volume of the implanted expander. This expansion process does not involve needles or injections, and enables the tissue expansion process to be undertaken in a short time frame and by the patient herself from wherever she chooses.
As the controlled release of the gas does not require the regular involvement of a surgeon or attendance at a clinic, the expansion of AeroForm can be achieved with a larger number of smaller expansions. The system is designed to allow a maximum number of three 10 milliliter (ml) expansions each day with a three-hour lockout between doses. By comparison, expansion with saline-based expanders typically involves administration of a 50ml to 100 ml volume of saline by a surgeon every one to three weeks. In addition to the greater convenience of AeroForm, expansion using a larger number of smaller expansion volumes results in a less painful experience for the patient, as she avoids expansion via delivery of a large amount of saline, which exerts pressure on the chest muscle and surrounding area. In addition, patients achieve required expansion in approximately half the time (21 versus 46 days on average).
AeroForm components
The following table describes each of the two components of AeroForm.
|
AeroForm Components |
Description |
|
|
Tissue Expander (expander) |
Implant that contains an outer silicone shell, inner gas barrier, reservoir of compressed CO2 gas and a receiving antenna. |
|
|
Dosage Controller (controller) |
Device that includes batteries, a transmitting antenna and circuitry to initiate and provide power to the antennae (in the expander). |
Sales & Marketing
We commenced commercial sales of AeroForm in Australia in 2015 and in the U.S. in 2017, and are focused on executing our commercial strategy to become the standard of care for tissue expanders in two-stage breast reconstruction post mastectomy. Following Australian reimbursement being granted in November 2014 (discussed below), we commenced an initial targeted launch of AeroForm in Australia in January 2015. Our initial focus has been on tissue expanders in the private health system, as they represent approximately 75% of tissue expanders purchased in Australia. We have an office in Australia that is responsible for developing and supporting our Australian sales and marketing activity.
Through 2016, our U.S. sales and marketing efforts centered on a range of preparatory activities to ensure that we are ready for a U.S. market release, including identifying and hiring the first tier of sales personnel; engaging in dialogue with targeted hospitals for initial adoption; and conducting early industry and patient awareness initiatives. In 2017, post FDA clearance, our sale efforts were initially concentrated on several key high volume academic and community hospitals that participated in our pivotal and continued access trials, as we broadened surgeon training and refined processes for seamless on-boarding with nursing, billing and inventory.
After we received FDA de novo clearance for AeroForm in December 2016, we submitted a 510(k) application for a materials change related to enhanced film material. This 510(k) was cleared by the FDA in April 2017. With this clearance, we launched a broader commercial release of AeroForm in the second half of 2017. To support the broader U.S. commercial release we hired a direct group of sales and regional managers, along with a group of independent representatives. The direct team is responsible for sales execution, team training and overall sales goal achievement and the indirect team will be compensated on a commission only basis. As a particular territory nears the targeted level of sales, we will consider inserting a direct representative to manage that particular maturing and developing territory.
Other key aspects of our sales and marketing strategy include close engagement and alignment with key opinion leaders in the fields of plastic surgery and breast reconstruction, and with key influencers and decision makers in patient advocacy groups.
Prior to 2017, all of our net revenues were generated from sales of AeroForm in Australia. In 2017, 80% of our net revenues were generated from customers in the U.S., with the remainder from customers in Australia.
See Note 3 of our consolidated financial statements in Item 8, "Financial Statements and Supplementary Data," for segment and geographical financial information, including revenues by geographic region, and our consolidated financial statements for net revenue information, which is incorporated by reference here.
Reimbursement for AeroForm
Australia
We secured Australian reimbursement for AeroForm in November 2014 by having both the AeroForm tissue expander and the dosage controller listed on the Prostheses List. The Prostheses List is the list of surgically implanted prostheses, human tissue items and other medical devices for which private health insurers must pay benefits for when they are provided to a patient with appropriate health insurance coverage as part of hospital treatment or hospital substitute treatment, and there is a Medicare benefit payable for the professional service. The Prostheses List covers all private health insurance plans in Australia.
U.S.
We believe we benefit from existing reimbursement codes in the U.S. that provide broad reimbursement coverage for breast reconstruction procedures (including tools and devices used in those procedures, such as tissue expanders). In addition, due to the Women’s Health and Cancer Rights Act of 1998 (U.S.) which federally mandates reimbursement of breast reconstruction procedures, private insurers that provide reimbursement for mastectomies (which we understand to be all major private insurers in the U.S.) are required to also provide reimbursement for procedures for breast reconstruction (inclusive of the use of tissue expanders such as AeroForm). The increasing U.S. policy focus on ensuring awareness of and access to breast reconstruction options for all breast cancer patients has ensured that public or private health systems broadly reimburse for tissue expansion, with the rates of reimbursement (reimbursement per procedure for surgeons and sites of service) increasing every year since 2010.
Rest of the World
Reimbursement is a lengthy country-by-country process. We will look to focus our reimbursement efforts to first obtain reimbursement in those countries where reimbursement is reasonably achievable in a timely manner and at rates that support profitable business operations.
Competition
We compete with large public pharmaceutical and medical device companies, such as Allergan, Inc. and Mentor Worldwide LLC, a division of Johnson & Johnson, which have been market leaders in the traditional saline-based tissue expander market for many years. Our competitors have greater brand recognition and longer histories in these markets. Furthermore, the resources and scale of these two dominant players in the tissue expander market provides them with advantages in terms of financing, research and development, manufacturing and marketing resources than are currently available to us. Additionally, these companies offer their customers access to a suite of products, including breast implants, which may allow them to offer favorable pricing on volume purchases or bundled purchases. In the United States, the benefits and advantages of AeroForm have allowed us to demand a premium to our competitors' pricing of traditional saline expanders; however, there is no guarantee we will be able to continue to realize this premium. In markets outside the United States, our ability to realize a premium will be influenced by a variety of factors, including local reimbursement environments.
Manufacturing
Our manufacturing operations consist of direct manufacturing capability in Palo Alto, CA, and San Jose, CA, as well as use of a third-party contract manufacturer in Costa Rica. Our facilities include a controlled environment assembly room and an engineering laboratory. Our primary manufacturing efforts are currently the final packaging of our product, and manufacture of key subassemblies, including the canister that contains the CO2 gas. Our third-party contract manufacturer performs final assembly of our product, excluding final packaging and sterilization. We have an ISO compliant Quality Management System that has been certified to the ISO 13485:2003 medical device standard. It is our strategy to outsource all assemblies that do not contain elements that we believe lead to a direct competitive advantage.
We currently outsource the manufacturing of certain AeroForm components and some subassemblies to qualified suppliers. During assembly, we send certain subassemblies to be sterilized by our approved sterilization suppliers. We package and label the final assembly before sending them for final sterilization by our approved sterilization partner. All of the critical suppliers and processes undergo monitoring and validation to control delivery and quality of the products. We produce key parts and components and make reasonable efforts to ensure that any externally purchased parts or raw materials are available from multiple suppliers, but this is not always possible. Certain components, subassemblies and services necessary for the manufacture of our systems are obtained either from a sole supplier or limited group of suppliers. We have established long-term supply agreements where possible. Although we seek to reduce our dependence on sole and limited source suppliers, partial or complete loss of these sources could disrupt production, delay scheduled deliveries to customers and have a material adverse effect on our revenues and results of operations.
Research and development
Our primary product is the AeroForm breast tissue expansion system, which includes an externally-controlled breast tissue expander that, when inflated, stretches breast tissue in order to accommodate a subsequently positioned permanent breast implant. We continue to invest in research and development to advance the features and functionality of the current technology and obtain yield and manufacturing improvements. We have developed and continue to develop technologies for remotely-controlled tissue expansion. Research and development expenses were $8.7 million in 2017, $7.2 million in 2016 and $4.8 million in 2015.
Clinical Trials
We have conducted successful clinical trials in Australia and the U.S., demonstrating the safety and efficacy of AeroForm. The first clinical trial of AeroForm, or PACE 1 trial, was conducted in 2009 and 2010 in Perth, Australia. The trial involved ten AeroForm devices implanted in seven patients. Following the success of the PACE 1 trial, we proceeded with the larger PACE 2 clinical trial in Perth, Australia, involving 33 patients and the implant of 61 AeroForm devices. This trial was completed in 2012. The successful data of both trials enabled us to successfully apply for and obtain CE Mark and Therapeutic Goods Administration (TGA) approval for the European and Australian markets, respectively.
After we obtained CE Mark and TGA approval for AeroForm, we conducted a further clinical trial in Australia, known as the ASPIRE trial. This trial allowed for the broader inclusion criteria than the earlier studies and allowed for and successfully demonstrated the use of concurrent radiotherapy without incident. The ASPIRE trial involved the recruitment of 21 patients who had 34 devices implanted. Five patients had AeroForm implanted and two patients received concurrent radiotherapy without incident.
In the U.S. we conducted a multicenter, prospective, randomized controlled pivotal trial under an investigational device exemption, or IDE. The XPAND trial, which commenced in 2012, enrolled 150 patients across 16 sites in the U.S. This trial was completed in 2016. Final results of the XPAND trial were published in October 2016. The results from the XPAND trial were submitted to the FDA in 2015 as part of our de novo submission, and served as the basis for the FDA’s clearance of the AeroForm system in the U.S. in 2016. AeroForm was cleared by the FDA for use for soft tissue expansion in breast reconstruction following mastectomy, for the treatment of underdeveloped breasts, and for the treatment of soft tissue deformities in the breast.
Intellectual Property
We protect key areas of invention for our products and technology through a combination of patents, copyright, trade secrets, trademark law and confidentiality agreements. We actively seek to file patent applications on our products and technology and as warranted by research and development activities. We also regularly monitor third party patents and applications of interest on an ongoing basis and analyze their relevance to us and our products.
We have sought patent protection of our technology in many of the countries and industries that are currently identified as target markets. As of December 31, 2017, our patent portfolio consisted of four issued and three pending U.S. patents, and 26 issued and 12 pending foreign patents. Our issued foreign patents include patents granted in Australia, Hong Kong, Japan and several of the major countries in the European Union. We will continue to seek intellectual property protection in as well as outside of the U.S.
Our patent portfolio includes 10 patents and 6 pending patent applications owned by Shalon Ventures and exclusively licensed by us, patents and a pending patent application co-owned by us and Shalon Ventures, as well as patent applications developed by and solely owned by us. We pay 3% royalties of net sales of the licensed inventions to Shalon Ventures with respect to the licensed patents and patent applications under an exclusive license entered into with Shalon Ventures on March 9, 2005, amended on March 9, 2009, January 9, 2012, and January 15, 2014, collectively referred to as the License Agreement. The License Agreement grants us certain exclusive rights in all human uses of self-expanding tissue expanders under certain patents and patent application owned or co-owned by Shalon Ventures. The License Agreement also gives us the first right to prosecute the licensed intellectual property. Our current U.S. issued patents expire between 2025 and 2035 and our current issued foreign patents expire between 2025 and 2030.
We protect our AIRXPANDERS, AIRXPANDER (in Australia) and AEROFORM trademarks by maintaining the registration of those marks for use in connection with surgical implants in Australia, the U.S., the European Union, Canada and Japan.
Our intellectual property is directed to technology incorporated into the AeroForm breast tissue expansion system, including its method of use. The term of a patent is limited, typically to 20 years from the date the application was filed or from the earliest non-provisional priority date in the applicable country.
Government Regulation of Medical Devices and of AeroForm
Governmental authorities in the United States, at the federal, state and local levels, and other countries extensively regulate, among other things, the research, development, testing, manufacture, labeling, promotion, advertising, distribution, marketing and export and import of products such as those we are commercializing and developing. Failure to obtain approval or clearance to market our products and products under development and to meet the ongoing requirements of these regulatory authorities could prevent us from continuing to market or develop our products and product candidates.
United States
Premarketing Regulation
In the United States, medical devices are regulated by the FDA. Unless an exemption applies, a new medical device will require either prior 510(k) clearance or approval of a premarket approval application, or PMA, before it can be marketed in the United States. The information that must be submitted to the FDA in order to obtain clearance or approval to market a new medical device varies depending on how the medical device is classified by the FDA. Medical devices are classified into one of three classes on the basis of the controls deemed by the FDA to be necessary to reasonably ensure their safety and effectiveness. Class I devices, which are those that have the lowest level or risk associated with them, are subject to general controls, including labeling, premarket notification and adherence to the quality system regulation, or QSR, which sets forth device-specific good manufacturing practices. Class II devices are subject to general controls and special controls, including performance standards. Class III devices, which have the highest level of risk associated with them, are subject to most of the previously identified requirements as well as to premarket approval. Most Class I devices and some Class II devices are exempt from the 510(k) requirement, although the manufacturers will still be subject to registration, listing, labeling and QSR requirements.
A 510(k) premarket notification must demonstrate that the device in question is substantially equivalent to another legally marketed device, or predicate device, that did not require premarket approval. In evaluating the 510(k), the FDA will determine whether the device has the same intended use as the predicate device, and (a) has the same technological characteristics as the predicate device, or (b) has different technological characteristics, and (i) the data supporting the substantial equivalence contains information, including appropriate clinical or scientific data, if deemed necessary by the FDA, that demonstrates that the device is as safe and as effective as a legally marketed device, and (ii) does not raise different questions of safety and effectiveness than the predicate device. Most 510(k)s do not require clinical data for clearance, but the FDA may request such data. The FDA’s goal is to review and act on each 510(k) within 90 days of submission, but it may take longer based on requests for additional information. In addition, requests for additional data, including clinical data, will increase the time necessary to review the notice. If the FDA does not agree that the new device is substantially equivalent to the predicate device, the new device will be classified in Class III, and the manufacturer must submit a PMA. Since July 2012, however, with the enactment of the Food and Drug Administration Safety and Innovation Act, or FDASIA, a de novo pathway is directly available for certain low to moderate risk devices that do not qualify for the 510(k) pathway due to lack of a predicate device. Modifications to a 510(k)-cleared medical device may require the submission of another 510(k) or a PMA if the changes could significantly affect the safety or effectiveness or constitute a major change in the intended use of the device.
AeroForm, as a Class II medical device, was granted its FDA de novo marketing authorization in December 2016. AeroForm was cleared by the FDA for use for soft tissue expansion in breast reconstruction following mastectomy, for the treatment of underdeveloped breasts, and for the treatment of soft tissue deformities in the breast.
The PMA process is more complex, costly and time consuming than the 510(k) clearance procedure. A PMA must be supported by extensive data including, but not limited to, technical, preclinical, clinical, manufacturing, control and labeling information to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use. After a PMA is submitted, the FDA has 45 days to determine whether it is sufficiently complete to permit a substantive review. If the PMA is complete, the FDA will file the PMA. The FDA is subject to performance goal review times for PMAs and may issue a decision letter as a first action on a PMA within 180 days of filing, but if it has questions, it will likely issue a first major deficiency letter within 150 days of filing. It may also refer the PMA to an FDA advisory committee for additional review, and will conduct a preapproval inspection of the manufacturing facility to ensure compliance with the QSR, either of which could extend the 180-day response target. A PMA can take several years to complete and there is no assurance that any submitted PMA will ever be approved. Even when approved, the FDA may limit the indication for which the medical device may be marketed or to whom it may be sold. In addition, the FDA may request additional information or request the performance of additional clinical trials before it will reconsider the approval of the PMA or as a condition of approval, in which case the trials must be completed after the PMA is approved. Changes to the device, including changes to its manufacturing process, may require the approval of a supplemental PMA.
If a medical device is determined to present a “significant risk,” the manufacturer may not begin a clinical trial until it submits an investigational device exemption, or IDE, to the FDA and obtains approval of the IDE from the FDA. The IDE must be supported by appropriate data, such as animal and laboratory testing results and include a proposed clinical protocol. These clinical trials are also subject to the review, approval and oversight of an institutional review board, or IRB, at each institution at which the clinical trial will be performed. The clinical trials must be conducted in accordance with applicable regulations, including but not limited to the FDA’s IDE regulations and current good clinical practices. A clinical trial may be suspended by the FDA or the sponsor at any time for various reasons, including a belief that the risks to the study participants outweigh the benefits of participation in the trial. Even if a clinical trial is completed, the results may not demonstrate the safety and efficacy of a device, or may be equivocal or otherwise not be sufficient to obtain approval.
Post-Marketing Regulation
After a device is placed on the market, numerous regulatory requirements apply. These include:
|
• |
compliance with the QSR, which require manufacturers to follow stringent design, testing, control, documentation, record maintenance, including maintenance of complaint and related investigation files, and other quality assurance controls during the manufacturing process; |
|
• |
labeling regulations, which prohibit the promotion of products for uncleared or unapproved or “off-label” uses and impose other restrictions on labeling; |
|
• |
other post-marketing regulation of promotional activities, including standards and regulations for direct-to-consumer advertising, industry-sponsored scientific and educational activities, and promotional activities involving the internet and social media; and |
|
• |
medical device reporting obligations, which require that manufacturers investigate and report to the FDA adverse events, including deaths, or serious injuries that may have been or were caused by a medical device and malfunctions in the device that would likely cause or contribute to a death or serious injury if it were to recur. |
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
|
• |
warning letters; |
|
• |
fines, injunctions, and civil penalties; |
|
• |
recall or seizure of our products; |
|
• |
operating restrictions, partial suspension or total shutdown of production; |
|
• |
refusal to grant 510(k) clearance or PMA approvals of new products; |
|
• |
withdrawal of 510(k) clearance or PMA approvals; and |
|
• |
criminal prosecution. |
To ensure compliance with regulatory requirements, medical device manufacturers are subject to market surveillance and periodic, pre-scheduled and unannounced inspections by the FDA, and these inspections may include the manufacturing facilities of our third-party contract manufacturer and subcontractors.
International Regulation
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA approval, and the requirements may differ. For example, the primary regulatory authority with respect to medical devices in Europe is that of the European Union. The European Union consists of 28 countries and has a total population of over 500 million people. Norway, Iceland, Lichtenstein and Switzerland are not members of the European Union, but have transposed applicable European medical device laws into their national legislation. Thus, a device that is marketed in the European Union may also be recognized and accepted in those four non-member European countries as well.
The European Union has adopted numerous directives and standards regulating the design, manufacture, clinical trials, labeling and adverse event reporting for medical devices. Devices that comply with the requirements of relevant directives will be entitled to bear CE conformity marking, indicating that the device conforms to the essential requirements of the applicable directives and, accordingly, can be commercially distributed throughout the European Union. Actual implementation of these directives, however, may vary on a country-by-country basis. The CE Mark is a mandatory conformity mark on medical devices distributed and sold in the European Union and certifies that a medical device has met applicable requirements.
The method of assessing conformity varies, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a “Notified Body.” Notified Bodies are independent testing houses, laboratories, or product certifiers authorized by the E.U. member states to perform the required conformity assessment tasks, such as quality system audits and device compliance testing. An assessment by a Notified Body based within the European Union is required in order for a manufacturer to distribute the product commercially throughout the European Union. Medium and higher risk devices require the intervention of a Notified Body which will be responsible for auditing the manufacturer’s quality system. The Notified Body will also determine whether or not the product conforms to the requirements of the applicable directives. Devices that meet the applicable requirements of E.U. law and have undergone the appropriate conformity assessment routes will be granted CE “certification.”
The AeroForm has CE Mark designation for tissue expansion for patients undergoing tissue expansion. CE Marking requires demonstration of continued compliance with the directives, which apply to the continued safety and quality of the product. Our designated EU Notified Body regularly audits these parameters to ensure compliance with ISO 13485 certification.
The CE Mark is mandatory for medical devices sold not only within the countries of the European Union but more generally within all countries in Western Europe. As many of the European standards are converging with international standards, the CE Mark is often used on medical devices manufactured and sold outside of Europe (notably in Asia that exports many manufactured products to Europe). CE Marking gives companies easier access into not only the European market but also to Asian and Latin American markets, most of which recognize the CE Mark on medical devices as a mark of quality and adhering to international standards of consumer safety, health or environmental requirements.
In September 2012, the European Commission adopted a proposal for a regulation which, if adopted, will change the way most medical devices are regulated in the European Union, and may subject our products to additional requirements.
In a number of international markets, including Australia, regulatory approvals may be expedited once CE Mark approval has been received; although submissions are required in each country.
In Australia, the TGA is responsible for administering the Therapeutic Goods Act with AeroForm falling under the category of an active implantable medical device. In 2013, the TGA approved the AeroForm for inclusion on the Australian Register of Therapeutic Goods.
Health Care Regulatory Laws
In the United States, the research, manufacturing, distribution, marketing, sale and promotion of medical devices are subject to numerous regulations by various federal, state and local governmental authorities in addition to the FDA. Health care fraud and abuse laws and other regulatory laws and regulations that govern our business include the following:
|
• |
The federal anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce, or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any good, facility, item or service reimbursable under a federal health care program, such as Medicare or Medicaid. This statute has been interpreted broadly to apply to arrangements between pharmaceutical manufacturers and prescribers, purchasers, and formulary managers, among others. There are statutory exceptions and regulatory safe harbors available to protect certain common activities from prosecution or other regulatory sanctions that must be strictly followed. Failure to meet all of the requirements of a particular statutory exception or regulatory safe harbor does not make the conduct per se illegal under the anti-kickback statute, but subjects the arrangement to a case-by-case basis review of its facts and circumstances. The Affordable Care Act amended the federal anti-kickback statute such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation and codified case law that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act. |
|
• |
Federal false claims laws, including the civil False Claims Act, false statement laws and civil monetary penalty laws prohibit, among other things, any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. The False Claims Act contains qui tam provisions, which allow a private individual, or relator, to bring a civil action on behalf of the federal government alleging that the defendant submitted a false claim to the federal government and to share in any monetary recovery. Certain marketing practices, including off-label promotion, may violate federal false claims laws. |
|
• |
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created new federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any health care benefit program, including private third-party payers, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. Like the federal anti-kickback statute, the Affordable Care Act amended the intent standard for certain health care fraud provisions under HIPAA such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. |
|
• |
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their respective implementing regulations, imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information. |
|
• |
The federal Physician Payments Sunshine Act and its implementing regulations require that certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to annually report to the Centers for Medicare & Medicaid Services (CMS) information related to certain payments or other transfers of value made to physicians and teaching hospitals, and to report annually certain ownership and investment interests held by physicians and their immediate family members. |
|
• |
The U.S. Foreign Corrupt Practices Act, the U.K Anti-Bribery Act, and similar anti-bribery laws that generally prohibit companies and their intermediaries from making improper payments to officials for the purpose of obtaining or retaining business. |
|
• |
Analogous local, state and foreign laws, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving health care items or services reimbursed by non-governmental third-party payors, including private insurers; state laws that require medical device companies to comply with the device industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to health care providers and entities; state and foreign laws that require device manufacturers to report information related to payments and other transfers of value to health care professionals or entities; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable health care laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other health care laws.
Medical device and other health care companies have been prosecuted under these laws for a variety of promotional and marketing activities, such as providing free trips, free goods, sham consulting fees and grants and other monetary benefits to prescribers; and engaging in off-label promotion. If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to significant sanctions, including criminal fines, civil monetary penalties, administrative penalties, disgorgement, individual imprisonment, exclusion from participation in federal health care programs, integrity obligations, contractual damages, injunctions, recall or seizure of products, total or partial suspension of production, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Health Care Reform
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes to the health care system that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce health care costs. In March 2010, President Barack Obama signed into law the Affordable Care Act, or ACA, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of health care spending, enhance remedies against health care fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers and impose additional health policy reforms. Substantial new provisions affecting compliance also were enacted, which may affect our business practices with health care practitioners.
Some of the provisions of the ACA have yet to be implemented, and there have been judicial and Congressional challenges to certain aspects of the ACA. In addition, the current Trump administration and Congress may continue to seek legislative and regulatory changes, including repeal and replacement of certain provisions of the ACA. President Trump also signed an Executive Order in January 2017 directing federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, health care providers, health insurers, or manufacturers of pharmaceuticals or medical devices.
We expect that health care reform measures that have been and may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for our products. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payers. The implementation of cost containment measures or other health care reforms may affect our ability to generate revenue and profits or commercialize our product candidates.
Third-Party Coverage and Reimbursement
Because we typically receive payment directly from hospitals and health care facilities, we do not anticipate relying directly on payment for any of our products from third-party payers, such as Medicare, Medicaid, private insurers, and managed care companies. However, our business will be affected by policies administered by federal and state health care programs, such as Medicare and Medicaid, as well as private third-party payers, which often follow the policies of the state and federal health care programs. For example, our business will be indirectly impacted by the ability of a hospital or medical facility to obtain coverage and third-party reimbursement for procedures performed using our products. These third-party payers may deny reimbursement if they determine that a device used in a procedure was not medically necessary; was not used in accordance with cost-effective treatment methods, as determined by the third-party payer; or was used for an unapproved use. A national or local coverage decision denying Medicare coverage for one or more of our products could result in private insurers and other third party payers also denying coverage. Even if favorable coverage and reimbursement status is attained for our products, less favorable coverage policies and reimbursement rates may be implemented in the future. The cost containment measures that third-party payers and providers are instituting, both within the United States and abroad, could significantly reduce our potential revenues from the sale of our products and any product candidates. We cannot provide any assurances that we will be able to obtain and maintain third party coverage or adequate reimbursement for our products and product candidates in whole or in part.
For inpatient and outpatient procedures, including those that will involve use of our products, Medicare and many other third-party payers in the United States reimburse hospitals at a prospectively determined amount, generally based on one or more diagnosis related groups, or DRGs, associated with the patient’s condition for inpatient treatment and generally based on ambulatory payment classifications, or APCs, associated with the procedures performed during the patient’s stay, for outpatient treatment. Each DRG or APC is associated with a level of payment and may be adjusted from time to time, usually annually. Prospective payments are intended to cover most of the non-physician hospital costs incurred in connection with the applicable diagnosis and related procedures. However, the prospective payment amounts are typically set independent of a particular hospital’s actual costs associated with treating a particular patient and implanting a device. Therefore, the payment that a hospital would receive for a particular hospital visit would not typically take into account the cost of our products.
In international markets, health care payment systems vary by country and many countries have instituted price ceilings on specific product lines. There can be no assurance that our products will be considered cost-effective by third-party payers, that reimbursement will be available or, if available, that the third-party payers’ reimbursement policies will not adversely affect our ability to sell our products profitably.
Member countries of the European Union offer various combinations of centrally financed health care systems and private health insurance systems. The relative importance of government and private systems varies from country to country. Governments may influence the price of medical devices through their pricing and reimbursement rules and control of national health care systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may be marketed only once a reimbursement price has been agreed upon. Some of these countries may require, as condition of obtaining reimbursement or pricing approval, the completion of clinical trials that compare the cost-effectiveness of a particular product candidate to currently available therapies. Some E.U. member states allow companies to fix their own prices for devices, but monitor and control company profits. The choice of devices is subject to constraints imposed by the availability of funds within the purchasing institution. Medical devices are most commonly sold to hospitals or health care facilities at a price set by negotiation between the buyer and the seller. A contract to purchase products may result from an individual initiative or as a result of a competitive bidding process. In either case, the purchaser pays the supplier, and payment terms vary widely throughout the European Union. Failure to obtain favorable negotiated prices with hospitals or health care facilities could adversely affect sales of our products.
Material Contracts
We currently acquire certain materials and components required to manufacture our products from third parties and outsource certain aspects of our manufacturing process in addition to the warehousing and logistics arrangements that we have in Australia. Although alternative suppliers are available, we have identified the following agreements, which we consider to be material to our business as currently conducted. The key provisions of these material contracts are summarized below, however, the summary is not intended to be exhaustive.
Medplast — Contract Manufacturing Agreement
In January 2017, we entered into a Manufacturing and Supply Agreement with Medplast (formerly Vention Medical Costa Rica, S.A), pursuant to which Medplast agreed to manufacture and supply our AeroForm product. There are no specified minimum purchase quantities; however, we are required to provide, on a monthly basis, a twelve month demand forecast with the first three months considered binding and the remaining amount considered non-binding.
The agreement continues for four years unless terminated earlier. The term of the agreement automatically renews for additional one-year terms unless one party provides the other party with written notice of termination at least six months prior to the end of the applicable renewal period. The agreement may be terminated by either party for any reason upon 365 days’ written notice. In addition, the agreement may be terminated by mutual agreement of the parties, or by either party, with written notice, upon uncured material breach or insolvency of the other party.
Shalon Ventures — Royalty Agreement
Shalon Ventures and AirXpanders have entered into a License Agreement dated March 9, 2005 (as amended on March 9, 2009, January 9, 2012 and January 15, 2014) in relation to those inventions, collectively the Shalon Ventures License Agreement. Pursuant to the Shalon Ventures License Agreement, Shalon Ventures granted AirXpanders an exclusive license to develop, make, have made, use, offer for sale, sell, have sold, import and export products that, but for the license, would infringe one or more claims of the patents. The license covers all human uses of self-expanding tissue expanders anywhere in the world and includes the right to sublicense.
In consideration for the license, AirXpanders pays Shalon Ventures a running royalty of 3% of net sales of the licensed invention. AirXpanders indemnifies Shalon Ventures for any liability arising out of the commercialization of products using the license. Through the year ended December 31, 2017, the Company has incurred approximately $0.1 million in royalty fees.
The License Agreement may be terminated by either party, with ninety day written notice, upon uncured material breach, or in the case of insolvency, solely by AirXpanders.
Mr. Tadmor (Teddy) Shalon is the Chief Executive Officer and sole shareholder of Shalon Ventures. Additionally, Mr. Shalon and Mr. Barry Cheskin are each party to an agreement with Shalon Ventures, under which Shalon Ventures has agreed to pay Mr. Shalon 58%, and Mr. Cheskin 8%, of any royalties due to Shalon Ventures from AirXpanders under the Shalon Ventures License Agreement. Mr. Shalon and Mr. Cheskin are stockholders of AirXpanders. Mr. Cheskin is also a co-founder, director and chairman of the board of AirXpanders. Mr. Shalon served as a director of AirXpanders through May 22, 2017.
Financing
Since our incorporation on March 17, 2005 through August 31, 2017, we have been funded with a total of approximately $123.8 million through a series of equity, debt and convertible note financings.
We have raised approximately $78.4 million through public sales of our equity in our IPO, and a concurrent private placement under Regulation D of the Securities Act (Concurrent Placement), and through subsequent sales of our equity. This primarily consists of approximately $30.1 million raised in June 2015 in the IPO, Concurrent Placement and the issuance of convertible bridge notes, $14.2 million raised in June 2016 in an equity offering of our CDIs; and $32.6 million raised in February 2017 in an equity offering of our CDIs. Apart from the Concurrent Placement, these equity offerings were not made to U.S. persons (within the meaning of the Securities Act).
On June 22, 2015, we issued 29,629,654 shares of Common Stock in connection with an initial public offering (IPO) on the Australian Securities Exchange, or the ASX, a Concurrent Placement, and the conversion of convertible bridge notes payable and related accrued interest. We raised a total of approximately $30.1 million, net of issuance costs of approximately $2.9 million. Of this amount, $25.1 million were net cash proceeds directly from the IPO, and $5.0 million were cash proceeds from the Concurrent Placement. In connection with the IPO, all of our existing shares of preferred stock were converted into common stock.
In June 2016, we issued 8,771,930 shares of Common Stock in connection with an equity offering on the ASX. Our cash proceeds were approximately $14.2 million, net of issuance costs of approximately $0.7 million.
In February 2017, we issued 16,304,348 shares of Common Stock in connection with an equity offering on the ASX. We raised a total of $32.6 million, net of issuance costs of approximately $1.5 million.
In August 2017, we borrowed $15.0 million under a loan and security agreement with a financial institution which matures in August 2022.
Employees
As of December 31, 2017, we had 124 full-time employees. None of our employees are represented by a labor union or covered by a collective bargaining agreement. We consider our relations with our employees to be good.
Environmental Matters
Our operations are subject to various federal, state and local environmental protection regulations governing the use, storage, handling and disposal of hazardous materials, chemicals, and certain waste products. We believe that compliance with federal, state and local environmental protection regulations will not have a material adverse effect on our capital expenditures, earnings and competitive and financial position.
In the event that we fail to comply with such laws and regulations, we could be liable for damages, penalties and fines. We further discuss the impact of environmental regulation under “Risk Factors- We are subject to various environmental laws and regulations that could impose substantial costs upon us and may harm our business, operating results and financial condition.” in Item 1A.
Executive Officers of the Registrant
The names of our executive officers and their ages, titles and biographies as of December 31, 2017, are set forth below:
|
Name |
|
Age |
|
Position |
|
Scott Dodson |
|
53 |
|
President and Chief Executive Officer, Executive Director |
|
Scott Murcray |
|
46 |
|
Chief Financial Officer and Chief Operating Officer |
Scott Dodson has served as President and Chief Executive Officer, and director of AirXpanders since October 2010. Mr. Dodson has more than 25 years of executive management experience. Prior to AirXpanders, Mr. Dodson was President and CEO at Avantis Medical Systems from 2008 to 2010, a manufacturer of catheter-based endoscopic devices for the treatment of cancer and other abnormalities of the gastrointestinal tract. Mr. Dodson also served as President of Orthopedics at Orthofix International from 2006 to 2008, a manufacturer of reconstructive and regenerative orthopedic and spine solutions. Prior to that, Mr. Dodson held several senior management level positions with Boston Scientific for over 16 years. Mr. Dodson has a B.S. degree in Management from Indiana University in Bloomington, Indiana.
Scott Murcray joined AirXpanders as Chief Financial Officer and Chief Operating Officer in June 2016. Prior to AirXpanders, Mr. Murcray served at Nanometrics Incorporated, a semiconductor equipment and services company, from 2014 to 2016 as Vice President, Finance, and was responsible for accounting and finance. Prior to joining Nanometrics, from 2011 to 2014, Mr. Murcray served at ZOLL Medical Corporation, a medical device company, as a Vice President, Finance, and was responsible for accounting, finance, information technology, and human resources. From 1994 through 2011, Mr. Murcray held various accounting and finance leadership roles at VNUS Medical Technologies, Inc., Atrenta Inc., ePeople, Inc. and Arthur Andersen LLP. Mr. Murcray holds a B.S. degree in business administration from California Polytechnic State University, San Luis Obispo, and is a certified public accountant in the state of California.
|
ITEM 1A. |
RISK FACTORS. |
In addition to the other information contained in this Annual Report on Form 10-K, we have identified the following risks and uncertainties that may have a material adverse effect on our business, financial condition or results of operations. Investors should carefully consider the risks described below before making an investment decision. The risks described below are not the only ones we face. Additional risks not currently known to us or that we currently believe are immaterial may also impair our business operations. Our business could be harmed by any of these risks. The trading price of our CDIs could decline due to any of these risks and investors may lose all or part of their investment. This section should be read in conjunction with the Consolidated Financial Statements and Notes thereto, and Management’s Discussion and Analysis of Financial Condition and Results of Operations contained in this Annual Report on Form 10-K.
Risks Related to Our Business
We will need substantial additional funding and may be unable to raise capital when needed, which could force us to delay, reduce, or eliminate planned activities or may result in our inability to operate as a going concern.
As we have limited commercialization of our product, we are generating a small amount of revenue and are not cash flow positive or profitable. Our net revenue from sales of AeroForm was approximately $3.9 million for the year ended December 31, 2017 and, as of December 31, 2017, we had cash, cash equivalents and short-term investments of approximately $22.6 million. Our existing capital may be insufficient to meet our requirements. These requirements include, but not limited to, funding our continued commercial launch of AeroForm in the U.S., building our supporting manufacturing infrastructure and building inventory, continuing to build a dependable partnership with our current and other contract manufacturers, building our salesforce to support our commercialization efforts, and covering any losses. We will need to raise additional funds through financings or borrowings in 2018 in order to accomplish our planned objectives. Failure to raise additional funds could delay, reduce, or halt our commercialization and would impact our ability to continue as a going concern.
We have no committed sources of capital funding and there is no assurance that additional funding will be available to us in the future or be secured on acceptable terms. These factors raise substantial doubt about our ability to continue as a going concern within one year from the date the consolidated financial statements are issued. If adequate funding is not available, we may no longer be a going concern and may be forced to curtail operations, including our commercial activities and research and development programs, or cease operations altogether, file for bankruptcy, or undertake any combination of the foregoing. In such event, our stockholders may lose their entire investment in our company.
In addition, if we do not meet our payment obligations to third parties as they become due, we may be subject to litigation claims and our creditworthiness would be adversely affected. Even if we are successful in defending against these claims, litigation could result in substantial costs and would be a distraction to management, and may have other unfavorable results that could further adversely impact our financial condition.
There is substantial doubt about our ability to continue as a going concern.
Our independent registered accountants report on our December 31, 2017 consolidated financial statements contains an emphasis of a matter regarding substantial doubt about our ability to continue as a going concern. The consolidated financial statements have been prepared assuming we will continue as a going concern and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets, or the amounts and classification of liabilities that may result if we do not continue as a going concern. Based on our current operating plan, we do not have sufficient capital to continue our operations for a significant period of time beyond December 31, 2018. You should not rely on our consolidated balance sheet as an indication of the amount of proceeds that would be available to satisfy claims of creditors, and potentially be available for distribution to shareholders, in the event of liquidation.
We have a history of net losses and we may never achieve or maintain profitability.
We are a U.S. based medical device company with a limited history of operations and have limited commercial experience with our product. Medical device product development is a speculative undertaking and involves a substantial degree of risk. To date, we have focused on developing our sole product, AeroForm, and currently have no other products in development. We have incurred net losses since our inception, including net losses of approximately $29.0 million in 2017, $19.4 million in 2016, and $11.2 million in 2015. As of December 31, 2017, our accumulated deficit was approximately $95.3 million. Although we have started to generate revenues from sales in Australia and the United States, we expect to continue to incur significant operating losses for the near future as we incur costs, including those associated with commercializing our products, building our supporting manufacturing infrastructure, building a dependable partnership with our current and other contract manufacturers, as well as the increased costs associated with being a public company in the U.S. with equity securities listed on the ASX.
Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Our failure to achieve sustained profitability would depress the value of our company and could impair our ability to raise capital, expand our business, diversify our research and development pipeline, market AeroForm or any other products we may identify and pursue, if approved, or continue our operations. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our stockholders’ equity and working capital. We cannot predict the extent of our future operating losses and accumulated deficit and we may never generate sufficient revenues to achieve or sustain profitability.
If we fail to comply with the covenants and other obligations under our security and loan agreement, the lender may be able to accelerate amounts owed under the facility and may foreclose upon the assets securing our obligations.
In August 2017, we entered into a loan and security agreement with Oxford Finance LLC, or Oxford, pursuant to which we borrowed $15 million from Oxford. Under the Oxford loan agreement, we are subject to a variety of affirmative and negative covenants. These covenants include required achievement of agreed financial milestones (minimum revenue levels), financial reporting, providing an unqualified auditor’s opinion together with our annual financial statements within 120 days of the end of our fiscal year (the unqualified audit opinion covenant), limitations on certain dispositions and licensing of assets, limitations on the incurrence of additional debt, and achievement of certain financial milestones. To secure our performance of our obligations under this loan and security agreement, we granted Oxford a security interest in all of our assets, with a negative pledge on our intellectual property. Our failure to comply with the terms of the loan and security agreement, including the unqualified audit opinion covenant, the occurrence of a material adverse change in our business, operations or condition (financial or otherwise) or prospects, the material impairment in our prospect of repayment, a material impairment in the perfection or priority of the Oxford’s lien on our assets or the value of Oxford’s collateral, failure to achieve agreed financial milestones, or the occurrence of certain other specified events could result in an event of default that, if not cured or waived, could result in the acceleration of all or a substantial portion of our loan, coupled with prepayment penalties, potential foreclosure on our assets, and other adverse results. If Oxford were to declare an event of default, it would have the option, among other things, of accelerating the debt under our loan and security agreement and foreclosing on the Company’s assets pledged as collateral for the term loan. Any declaration of an event of default would significantly harm our business and would likely cause the price of our common stock to decline.
Our business model will depend solely on the success of AeroForm for breast reconstruction procedures.
We expect to derive all of our revenue in the foreseeable future from sales of AeroForm for breast reconstruction procedures. We have no other commercial products or products in active development at this time. Acceptance of our product in the marketplace is uncertain, and our failure to achieve sufficient market acceptance will significantly limit our ability to generate revenue and be profitable. If we are unable to successfully launch and achieve meaningful market penetration with AeroForm, our commercial strategy will be unattainable and our business operations, financial results and growth prospects will be materially and adversely affected.
We are dependent on the acceptance, promotion and safe usage of AeroForm by surgeons and their patients.
Regulatory approval and clearance of AeroForm, including in Australia and the U.S., will not guarantee market adoption. In order to achieve commercial success, we are dependent on the acceptance and promotion of AeroForm by patients and surgeons. Reasons that patients and surgeons may be slow to adopt AeroForm include, but are not limited to:
|
• |
preference of the products of competitors due to familiarity with those products or for various other reasons; |
|
• |
limited clinical data illustrating the benefits of AeroForm to patients and surgeons; |
|
• |
concern over potential liability risks involved in using a new product; and |
|
• |
any delay in the qualification of AeroForm for reimbursement from relevant health care funding bodies in jurisdictions where approved reimbursement codes and reimbursement status for similar products does not already exist. |
While we already have early good relationships with a number of leading surgeons in Australia and the U.S., this in and of itself does not ensure the widespread support of AeroForm among surgeons. If a significant number of surgeons in our key markets do not adopt or recommend AeroForm, or continue to promote and use the products of competitors, this would adversely impact or delay our ability to generate revenue and achieve profitability.
We may be unable to compete successfully with current tissue expanders in the market for breast reconstruction.
The market for traditional tissue expander products in breast reconstruction procedures is well established and dominated by two large pharmaceutical and medical device companies, Allergan, Inc. and Mentor Worldwide LLC, a division of Johnson & Johnson, which have been market leaders for a number of years. Our AeroForm will compete against the traditional saline expanders which have been used for many years, are supported by clinical data, have a lower average selling price and have significantly greater brand recognition. Furthermore, the resources and scale of the two dominant players in the tissue expander market provides them with significant advantages in terms of financing, research and development, manufacturing and marketing resources and this may restrict out ability to secure market share for AeroForm. Additionally, these companies offer their customers access to a suite of products, including breast implants, which may allow them to offer favorable pricing on volume purchases or bundled purchases.
We have limited sales, marketing and distribution resources.
We currently have limited marketing resources and will need to commit significant resources to developing sales, distribution and marketing capabilities. We intend to utilize a hybrid sale distribution model in the U.S. but most other markets will likely entail the use of a distributor. We will need to ensure compliance with all legal and regulatory requirements for sales, marketing and distribution in each relevant market. There is a risk that we will be unable to develop sufficient sales, marketing and distribution capacity to effectively commercialize AeroForm.
We rely on key suppliers for product components.
Our contracts with key suppliers are generally standard in nature, in the form of purchase order arrangements that are common to medical device firms in the early stages of commercialization, with no minimum orders required. As we move further into our commercialization phase, we will increasingly rely on key suppliers for AeroForm components. A disruption at a key supplier could cause a substantial delay in the availability of AeroForm, leading to a potential loss of sales. Development of key manufacturing processes along with process validation testing, device verification testing, and regulatory approvals required for a manufacturing change could take up to six months to complete. However, we believe that alternative suppliers could ultimately be located, qualified and approved for all critical system components with the six month timeframe.
We intend to rely on a third party in Costa Rica to manufacture AeroForm.
We intend to have the main manufacturing of AeroForm managed by a contract manufacturer located in Costa Rica. There are inherent risks in relying on outsourced contract manufacturers particularly where the contract manufacturer is located outside of the U.S. These risks include risks of economic change, recession, labor strikes or disruptions, political turmoil, changes in tariffs or trade barriers, and lack of contract enforceability.
Should the manufacturer’s operations be disrupted for any reason or production halted, we may not be able to have enough AeroForm devices manufactured in a timely manner to satisfy product demand. While an alternative manufacturer could be appointed, it would take a significant amount of time to transfer the manufacturing process, which would include installation and validation of equipment, process and product qualifications and regulatory approvals. If such a disruption were to occur, it would adversely impact our ability to sell AeroForm and customers might instead purchase competing tissue expander products. There may also be an ongoing sales impact in the form of a reduction of goodwill as a result of our inability to supply hospitals and surgeons in a timely manner.
Third party payers, including government authorities and private health insurers, may not provide sufficient levels of reimbursement or any form of reimbursement for AeroForm.
Purchasers of tissue expanders for breast reconstruction procedures generally rely on third party payers, particularly government health administration authorities, including Medicare and Medicaid in the U.S., and private health insurers, to subsidize the cost of the products. We have to date secured reimbursement for AeroForm in Australia and expect that AeroForm will benefit from existing reimbursement codes for breast reconstruction procedures in the U.S. Although rates of reimbursement for breast reconstruction procedures in the U.S. have been increasing in recent years, reimbursement rates are lower than our cost to produce AeroForm, and no assurance can be given that reimbursement amounts will continue to increase or that the amounts will be sufficient to enable us to sell AeroForm on a profitable basis in the U.S. Moreover, we cannot predict what changes may be made in the future to third party coverage and reimbursement in Australia or the U.S. and what impact any such changes may have on our ability to sell AeroForm. The cost containment measures that third-party payers and providers are instituting, both within the United States and abroad, could significantly reduce our potential revenues from the sale of our products and any product candidates. We cannot provide any assurances that we will be able to obtain and maintain third party coverage or adequate reimbursement for our products and product candidates in whole or in part.
Reimbursement and healthcare payment systems in international markets vary significantly by country. Outside Australia and the U.S., we may not obtain international coverage and reimbursement approvals in a timely manner or at all.
In Australia, the report of the Competition Policy Review released on March 31, 2015 (commonly known as the Harper Report) stated that the regulation of prostheses should be further examined to see if pricing and supply can be made more competitive. However, it is not known whether any further review of prostheses regulation will occur and if it does occur, how resulting regulatory changes, if any, will affect the future reimbursement of AeroForm in Australia.
We may not be able to pass through the regulatory hurdles and gain the necessary approvals and clearances to sell AeroForm in certain other countries.
In the U.S., we received de novo clearance from the FDA, allowing us to commence sales to the U.S. market. We have received TGA and CE Mark approval for AeroForm, allowing us to commence sales to the Australian and European markets, respectively.
In other jurisdictions, AeroForm is still at various pre-commercialization phases. We cannot guarantee that we will receive all necessary regulatory approvals, nor can we accurately predict the product approval timelines, or other requirements that may be imposed by regulators (for example, further clinical trials or other requirements proving safety and effectiveness of AeroForm). Furthermore, there may be changes to regulatory standards, which could delay or prevent us from obtaining the necessary regulatory approvals. In addition, any future changes to AeroForm may require separate clearance or approval.
Any delays or barriers to our obtaining necessary regulatory clearances would limit the size of the market opportunity until such time, if any, that we will be able to obtain such clearances for AeroForm.
We are dependent on the protection and enforcement of our intellectual property rights.
The protection of the intellectual property we rely on is critical to our business and commercial success. If we are unable to protect or enforce the intellectual property rights embodied in AeroForm, there is a risk that other companies will incorporate the intellectual property into their technology, which could adversely affect our ability to compete in the market for tissue expanders.
As of December 31, 2017, our patent portfolio consisted of four issued and three pending U.S. patents, and 26 issued and 12 pending foreign patents. Our issued foreign patents were granted in Australia, Hong Kong, Japan and several of the major countries in the European Union.
In addition, some of the key patents related to AeroForm are co-owned by us and Shalon Ventures (includes U.S. patents) or licensed to us exclusively by Shalon Ventures (non-U.S. patents only). Although the license agreement between us and Shalon Ventures may only be terminated by a party in limited circumstances, if Shalon Ventures was to terminate the license agreement it could affect our ability to produce and sell AeroForm outside the U.S.
We may be subject to future third party intellectual property rights disputes.
We do not believe that our activities infringe any third party’s intellectual property rights. To date, no third party has asserted this to be the case. However, in the future we may be subjected to infringement claims or litigation arising out of patents and pending applications of our competitors, or additional proceedings initiated by third parties or intellectual property authorities to re-examine the patentability of licensed or owned patents. The defense and prosecution of intellectual property claims and litigation, and related legal and administrative proceedings are costly and time-consuming to pursue, and their outcome is uncertain. If we infringe the rights of third parties, we could be prevented from selling AeroForm or any future products and be forced to defend against litigation and to pay damages.
We have a limited operating history and may face difficulties encountered by companies early in their commercialization.
We have a limited operating history upon which to evaluate our business and forecast future net sales and operating results. In assessing our business prospects, you should consider the various risks and difficulties frequently encountered by companies early in their commercialization in competitive markets, particularly companies that develop and sell medical devices. These risks include our ability to:
|
• |
implement and execute our business strategy; |
|
• |
expand and improve the productivity of our sales force and marketing programs; |
|
• |
increase awareness of our brand and build loyalty among surgeons; |
|
• |
manage expanding operations; |
|
• |
respond effectively to competitive pressures and developments; and |
|
• |
successfully implement design changes to refine AeroForm over time and obtain any updates to regulatory approvals related to the changes. |
Ongoing regulation of our products may limit how we manufacture and market our product candidates, which could materially impair our ability to generate revenue.
Once regulatory approval has been granted, an approved product and its manufacturer are subject to ongoing review and regulation. Any approved or cleared product may only be promoted for its approved or cleared uses consistent with the products' labelling. In addition, product labeling, packaging, QSR requirements, adverse event reporting, advertising and promotion, scientific and educational activities, and promotional activities involving the internet and social media will be subject to extensive regulatory requirements. To ensure compliance with regulatory requirements, medical device manufacturers are subject to market surveillance and periodic, pre-scheduled and unannounced inspections by the FDA, and these inspections may include the manufacturing facilities of our subcontractors. Any modifications we make to our cleared devices may be subject to additional 510(k) or de novo clearance, and FDA may disagree with any determination we must first make on whether a new 510(k) clearance or de novo clearance is required under FDA guidance. Such disagreement may result in the need to recall products, send Dear Doctor letters and conduct corrective advertising.
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, including various sanctions such as warning letters; fines, injunctions, and civil penalties; recall or seizure of our products; operating restrictions, partial suspension or total shutdown of production; refusal to grant 510(k) clearance or PMA approvals of new products; withdrawal of 510(k) clearance or PMA approvals; and criminal prosecution. Further, the cost of compliance with post-approval regulations may have a negative effect on our operating results and financial condition.
If we market products in a manner that violates fraud and abuse and other health care laws, we may be subject to significant enforcement and sanctions.
In addition to FDA restrictions on marketing of medical device products, several other types of state, federal and foreign health care laws, including those commonly referred to as “fraud and abuse” laws, have been applied to restrict certain marketing practices in the medical device industry. These laws include, among others, the following:
|
• |
The federal anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce, or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any good, facility, item or service reimbursable under a federal health care program, such as Medicare or Medicaid. This statute has been interpreted broadly to apply to arrangements between pharmaceutical manufacturers and prescribers, purchasers, and formulary managers, among others. There are statutory exceptions and regulatory safe harbors available to protect certain common activities from prosecution or other regulatory sanctions that must be strictly followed. Failure to meet all of the requirements of a particular statutory exception or regulatory safe harbor does not make the conduct per se illegal under the anti-kickback statute, but subjects the arrangement to a case-by-case basis review of its facts and circumstances. The Affordable Care Act amended the federal anti-kickback statute such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation and codified case law that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act. |
|
• |
Federal false claims laws, including the civil False Claims Act, false statement laws and civil monetary penalty laws prohibit, among other things, any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. The False Claims Act contains qui tam provisions, which allow a private individual, or relator, to bring a civil action on behalf of the federal government alleging that the defendant submitted a false claim to the federal government and to share in any monetary recovery. Certain marketing practices, including off-label promotion, may violate federal false claims laws. |
|
• |
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created additional federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any health care benefit program, including private third-party payers, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. Like the federal anti-kickback statute, the Affordable Care Act amended the intent standard for certain health care fraud provisions under HIPAA such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. |
|
• |
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their respective implementing regulations, imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information on certain healthcare providers, health plans, healthcare clearinghouses and their respective business associates. . |
|
• |
The federal Physician Payments Sunshine Act and its implementing regulations require that certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to annually report to the Centers for Medicare & Medicaid Services (CMS) information related to certain payments or other transfers of value made to physicians and teaching hospitals, and to report annually certain ownership and investment interests held by physicians and their immediate family members. |
|
• |
The U.S. Foreign Corrupt Practices Act, the U.K Anti-Bribery Act, and similar anti-bribery laws that generally prohibit companies and their intermediaries from making improper payments to officials for the purpose of obtaining or retaining business. |
|
• |
Analogous local, state and foreign laws, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving health care items or services reimbursed by non-governmental third-party payors, including private insurers; state laws that require medical device companies to comply with the device industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to health care providers and entities; state and foreign laws that require device manufacturers to report information related to payments and other transfers of value to health care professionals or entities; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable health care laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other health care laws.
Medical device and other health care companies have been prosecuted under these laws for a variety of promotional and marketing activities, such as providing free trips, free goods, sham consulting fees and grants and other monetary benefits to prescribers; and engaging in off-label promotion. If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to significant sanctions, including criminal fines, civil monetary penalties, administrative penalties, disgorgement, individual imprisonment, exclusion from participation in federal health care programs, integrity obligations, contractual damages, injunctions, recall or seizure of products, total or partial suspension of production, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
We are exposed to the risk of product liability and product recalls.
We are exposed to the risk of product liability claims as a company that sells products to the public. This is a particularly sensitive issue for health care companies, and the medical device market has a history of product recalls and litigation. We may be exposed to the risk of product liability claims, which are inherent in the design, manufacturing, marketing and use of medical devices. Furthermore, we must comply with medical device reporting and vigilance requirements in each jurisdiction in which AeroForm and any future products are marketed.
Any product liability claim, with or without merit, may cause damage to our reputation and business. We have sought to minimize this risk by taking out product liability insurance, but this may not be sufficient if a large damages claim is awarded. If we are called as a defendant in a product liability suit, this could be a costly activity that may also divert management focus away from key strategic initiatives of the business, potentially adversely impacting financial performance and damaging our reputation.
Off-label use of AeroForm may harm its image or lead to substantial penalties.
We are only permitted to market AeroForm for the uses indicated on the labeling cleared by the relevant regulatory bodies in each market. We cannot prevent a surgeon or other third party from using or recommending the use of AeroForm for purposes outside of its approved intended use. This may lead to the increased likelihood of an adverse event, or inadequate treatment of a patient’s condition, which could harm our reputation in addition to potential claims for damages. If we were deemed to have marketed AeroForm for off-label use, we could be subject to civil or criminal sanctions, including fines, damages claims, injunctions or other penalties and our reputation within the industry may be damaged.
We must attract and retained skilled staff to pursue our business model.
Our long term growth and performance is dependent on attracting and retaining highly skilled staff. The medical device industry, and the San Francisco Bay area where we maintain our headquarters, has strong competition for highly skilled workers (including senior researchers, clinical staff, and management) due to the limited number of people with the appropriate skill set.
We currently employ, or engage as consultants, a number of key management and scientific personnel. There is a risk that we will be unable to attract and retain the necessary staff to pursue our business model. In particular, if Mr. Scott Dodson, our President and CEO, were to leave us, we would lose significant technical and business expertise, and we might not be able to find a suitable replacement in a timely manner. This would affect how efficiently we operate our business and our future financial performance could be impacted.
We have structured incentive programs for our key personnel, including an equity incentive plan. Despite these measures, there is no guarantee that we will be able to attract and retain suitable qualified personnel, which could negatively affect our ability to reach our goals.
Risks Related to Our Industry
We may be adversely affected by health care reform legislation in the U.S. and other countries.
In recent years, there have been numerous initiatives at the U.S. federal and state levels for comprehensive reforms affecting the payment for, the availability of and reimbursement for healthcare services. Recent legislation and many of the proposed bills include funding to assess the comparative effectiveness of medical devices. It is unclear what impact the comparative effectiveness analysis will have on our products or financial performance. If significant reforms are made to the healthcare system in the U.S., or in other jurisdictions, those reforms could adversely affect our financial condition and operating results.
In March 2010, President Obama signed into law comprehensive healthcare reform legislation known as the Affordable Care Act, or the ACA, as modified by the Health Care and Education Reconciliation Act of 2010 (U.S.). The ACA was a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of health care spending, enhance remedies against health care fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers and impose additional health policy reforms. Substantial new provisions affecting compliance also were enacted, which may affect our business practices with health care practitioners. Complying with the ACA could significantly increase our costs.
Some of the provisions of the ACA have yet to be implemented, and there have been judicial and Congressional challenges to certain aspects of the ACA, as well as recent efforts by the Trump administration to repeal or replace certain aspects of the ACA. Since January 2017, President Trump has signed two Executive Orders and other directives designed to delay the implementation of certain provisions of the ACA or otherwise circumvent some of the requirements for health insurance mandated by the ACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the ACA. While Congress has not passed comprehensive repeal legislation, two bills affecting the implementation of certain taxes under the ACA have been signed into law. The Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. Additionally, the continuing resolution on appropriations for fiscal year 2018, recently signed by President Trump, delays the implementation of certain PPACA-mandated fees, including the medical device excise tax on non-exempt medical devices. Further, the Bipartisan Budget Act of 2018, or the BBA, among other things, amends the ACA, effective January 1, 2019, to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole”. We continue to evaluate the effect that the ACA and its possible repeal and replacement has on our business.
We expect that health care reform measures that have been and may be adopted in the future may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for our products. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payers. The implementation of cost containment measures or other health care reforms may affect our ability to generate revenue and profits or commercialize our product candidates.
The manufacturing facilities of AeroForm must comply with stringent regulatory requirements.
The manufacturing facilities for AeroForm must meet stringent standards. As we intend to outsource our main manufacturing to a contract manufacturer located in Costa Rica, we will have limited direct control over the compliance of the facility which manufactures AeroForm. If the manufacturer does not comply with any relevant requirements, this may adversely affect our ability to sell AeroForm. The FDA routinely inspects all medical device companies, including contract manufacturers, for compliance with the Quality Systems Regulation, or QSR. Furthermore, to maintain the CE Mark, National Standards Authority of Ireland, our Notified Body, will regularly audit our suppliers and manufacturers. Failure to comply with the applicable regulatory requirements can result in, among other things, temporary manufacturing shutdowns, product recalls, product shortages, bans on imports and exports and a damaged brand name.
Our presence in the international marketplace exposes us to foreign operational risks.
We will seek to sell AeroForm in markets across Australia, the U.S., Europe, Canada, Latin America and Asia. As it is intended that the main manufacturing of AeroForm will be performed in Costa Rica, we will be exposed to risks of foreign regulations in Costa Rica and national trade laws, including import and export laws as well as customs regulations and laws. There are potentially high compliance costs associated with these laws and failure to comply with any applicable law or regulatory obligations could result in penalties and/or enforcement action (for example, stoppages or delays in clearing our products through customs).
Risks Related to our CDIs and Common Stock
Our principal stockholders could collectively exert control over us and may not make decisions that in the best interests of all stockholders.
Our principal stockholders beneficially own a substantial percentage of our voting stock. If these significant stockholders were to act together, they would be able to exert a significant degree of influence over our management and affairs and over matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. Accordingly, there is a risk that these stockholders, although unrelated to each other, may make collective decisions that do not accord with, or are not in the best interests of, other stockholders and CDI holders. For example, the principal stockholders could, through their concentration of ownership, delay or prevent a change of control, even if a change of control is in the best interests of our other stockholders and CDI holders.
Provisions of our Certificate of Incorporation, our Bylaws and Delaware law could make an acquisition of us more difficult and may prevent attempts by stockholders to replace or remove current members of the Board.
Certain provisions of Delaware law, our Certificate of Incorporation and Bylaws could discourage, delay or prevent a change of control or deter tender offers for our common stock that stockholders and CDI holders may consider favorable, including transactions in which CDI holders might otherwise receive a premium for their CDIs.
Our Certificate of Incorporation authorizes us to issue up to 10,000,000 shares of preferred stock in one or more different series with terms to be fixed by our board of directors. Stockholder or CDI holder approval is not necessary to issue preferred stock in this manner. Issuance of these shares of preferred stock could have the effect of making it more difficult and more expensive for a person or group to acquire control of us, and could effectively be used as an anti-takeover device.
Our Bylaws provide for an advance notice procedure for stockholders or CDI holders to nominate director candidates for election or to bring business before an annual meeting of stockholders, including proposed nominations of persons for election to our board of directors, and require that special meetings of stockholders be called only by our chairman of the board, chief executive officer, president or the board pursuant to a resolution adopted by a majority of the board.
The anti-takeover provisions of Delaware law and provisions in our organizational documents may prevent our stockholders from receiving the benefit from any premium to the market price of our common stock offered by a bidder in a takeover context. Even in the absence of a takeover attempt, the existence of these provisions may adversely affect the prevailing market price of our common stock if they are viewed as discouraging takeover attempts in the future.
Being a public company is expensive and administratively burdensome.
We are subject to the periodic reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Although we have been listed on the ASX since June 2015 and have been required to file financial information and make certain other filings with the ASX, our status as a U.S. reporting company under the Exchange Act will cause us to incur additional legal, accounting and other expenses that we have not previously incurred. We expect these rules and regulations to increase our legal and financial compliance costs and to make some activities more time-consuming and costly. We also expect these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors (and the Audit and Risk Committee in particular) or as executive officers. We cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
The costs and management time involved in complying with Delaware laws, Australian laws and U.S. reporting requirements are likely to be significant.
As a Delaware company with an ASX listing and a registration as a foreign company in Australia, we will need to ensure continuous compliance with Delaware law and relevant Australian laws and regulations, including the listing rules and certain provisions of the Corporations Act. To the extent of any inconsistency between Delaware law and Australian law and regulations, we may need to make changes to our business operations, structure or policies to resolve such inconsistency. If we are required to make such changes, this is likely to result in interruptions to our operations, additional demands on key employees and extra costs.
|
ITEM 1B. |
UNRESOLVED STAFF COMMENTS. |
None.
|
ITEM 2. |
PROPERTIES. |
We lease approximately 15,000 square feet for office, research and development and manufacturing operations in Palo Alto, California under a non-cancelable operating lease. These premises have also been certified to the ISO 13485:2003 medical device standard. The term of the sublease expires on September 30, 2019. In April 2017, we signed a sublease for an additional approximately 24,000 square foot facility is San Jose, CA. The sublease expires in August 2019. In the event that the leases were terminated early and provided there is sufficient notice, we believe we could find suitable alternative premises with no interruption in operations. If the leases are terminated without notice or the premises were severely damaged there could be an impact on our operations and potentially an interruption in manufacturing, while we relocated and arranged for certification of the new premises. We believe that these premises are suitable and adequate for our needs now and for the foreseeable future.
|
ITEM 3. |
LEGAL PROCEEDINGS. |
From time to time, we may be involved in litigation relating to claims arising out of our operations. We are not currently involved in any material legal proceedings, and our management believes there are currently no claims or actions pending against us, the ultimate disposition of which could have a material adverse effect on our operations, financial condition, or cash flows. We may, however, be involved in material legal proceedings in the future. Such matters are subject to uncertainty and there can be no assurance that such legal proceedings will not have a material adverse effect on our business, results of operations, financial position or cash flows.
|
ITEM 4. |
MINE SAFETY DISCLOSURES. |
None.
PART II
|
ITEM 5. |
MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES. |
Market Information
Our CDIs, each representing one-third of one share of our Class A Common Stock, have been listed on the Australian Securities Exchange under the trading symbol “AXP” since June 22, 2015. Prior to such time there was no public market for our securities. There is no principal market in the U.S. for our CDIs or shares of our common stock. Our high and low sales prices on the ASX for the respective periods are shown below, both in Australian dollars per CDI and in U.S. dollars per share of common Stock. All currency conversions are based on the prevailing Australian dollar to U.S. dollar exchange rate applicable on the relevant date as reported by the Reserve Bank of Australia.
|
High per share |
Low per share |
|||||||||||||||
|
High per |
Low per |
of common |
of common |
|||||||||||||
|
CDI |
CDI |
stock |
stock |
|||||||||||||
|
Period |
(A$) |
(A$) |
(US$) |
(US$) |
||||||||||||
|
Fiscal Year 2017: |
||||||||||||||||
| First Quarter | 1.27 | 0.73 | 2.78 | 1.68 | ||||||||||||
| Second Quarter | 0.93 | 0.65 | 2.14 | 1.49 | ||||||||||||
| Third Quarter | 0.83 | 0.60 | 1.98 | 1.42 | ||||||||||||
|
Fourth Quarter |
0.83 | 0.65 | 1.96 | 1.48 | ||||||||||||
|
Fiscal Year 2016: |
||||||||||||||||
|
First Quarter |
1.34 | 1.02 | 2.83 | 2.17 | ||||||||||||
|
Second Quarter |
1.15 | 0.78 | 2.66 | 1.74 | ||||||||||||
|
Third Quarter |
1.49 | 0.90 | 3.40 | 2.03 | ||||||||||||
|
Fourth Quarter |
1.45 | 0.99 | 3.34 | 2.22 | ||||||||||||
Holders
As of February 15, 2018, we had 95,943,409 shares of our Common Stock issued and outstanding with approximately 1,996 holders of record. The holders included CHESS Depositary Nominees Pty Limited, which held 88,993,750 shares of our Common Stock in the form of CDIs on behalf of the CDI holders; there were approximately 1,967 registered owners of our CDIs on February 15, 2018. In some instances, because brokers and the institutions on behalf of stockholders hold many of our CDIs, we are unable to estimate the total number of holders represented by these record holders.
There were no shares of Class B common stock issued or outstanding as of December 31, 2017.
Dividends
During 2017, 2016 and 2015 we did not declare or pay any dividends on our common stock and do not currently anticipate declaring or paying dividends on our common stock in the foreseeable future. We currently intend to retain all of our future earnings, if any, to finance the operation and expansion of our business. Any future determination relating to our dividend policy will be made at the discretion of the Board and will depend on a number of factors, including future earnings, capital requirements, financial conditions, future prospects, contractual restrictions and covenants and other factors that the Board may deem relevant.
The loan and security agreement with Oxford prohibits us from paying any dividends without prior written consent.
Recent Sales of Unregistered Securities
None.
Issuer Purchases of Equity Securities
None.
Corporate Performance Graph
The following performance graph and related information shall not be deemed to be “soliciting material” or to be “filed” with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933, as amended, or the Exchange Act, except to the extent that we specifically incorporate it by reference into such filing.
The following performance graph compares the performance of our Common Stock from June 22, 2015 (the first day of trading of our CDIs) through December 31, 2017, after giving effect to the three-for-one CDI-to-Common Stock exchange ratio and after converting to U.S. dollars using the closing exchange rate applicable on the relevant date, to the performance of the S&P/ASX 300 from June 30, 2015 through December 31, 2017. The comparison assumes $100 was invested in our Common Stock after the market closed on June 22, 2015 and $100 was invested after the market closed on June 30, 2015 in each of the presented indices. Pursuant to applicable SEC rules, all values assume reinvestment of the full amount of all dividends, however no dividends have been declared on our Common Stock to date. The stockholder return shown on the performance graph below is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future stockholder returns.
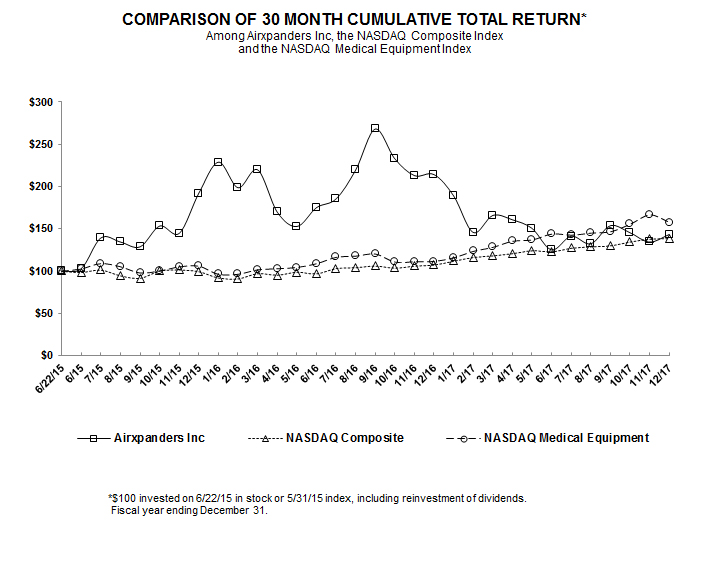
|
ITEM 6. |
SELECTED FINANCIAL DATA. |
SELECTED CONSOLIDATED FINANCIAL DATA
The following table sets forth our selected consolidated financial data for the periods, and as of the dates, indicated. You should read the following selected consolidated financial data in conjunction with our audited consolidated financial statements and the related notes thereto included elsewhere in this registration statement and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this registration statement.
| Years Ended December 31, | |||||||||||||||
|
2017 |
2016 |
2015 |
2014 |
||||||||||||
|
(in thousands, except share and per share data) |
|||||||||||||||
|
Consolidated Statements of Operations and Comprehensive Loss Data: |
|||||||||||||||
|
Revenue |
$ | 3,906 | $ | 570 | $ | 293 | $ | — | |||||||
|
Cost of goods sold |
9,190 | 4,543 | 1,906 | — | |||||||||||
|
Gross loss |
(5,284 | ) | (3,973 | ) | (1,613 | ) | — | ||||||||
|
Operating expenses: |
|||||||||||||||
|
Research and development |
8,726 | 7,164 | 4,827 | 4,143 | |||||||||||
|
Selling, general and administrative |
14,393 | 7,986 | 4,640 | 2,229 | |||||||||||
|
Total operating expenses |
23,119 | 15,150 | 9,467 | 6,372 | |||||||||||
|
Operating loss |
(28,403 | ) | (19,123 | ) | (11,080 | ) | (6,372 | ) | |||||||
|
Other expense (income): |
|||||||||||||||
|
Interest expense |
796 | 249 | 422 | 561 | |||||||||||
|
Other expense (income) |
(217 | ) | 50 | (341 | ) | 45 | |||||||||
|
Total other expense (income), net |
579 | 299 | 81 | 606 | |||||||||||
|
Loss before income taxes |
(28,982 | ) | (19,422 | ) | (11,161 | ) | (6,978 | ) | |||||||
|
Provision for income taxes |
1 | 1 | — | — | |||||||||||
|
Net loss |
$ | (28,983 | ) | $ | (19,423 | ) | $ | (11,161 | ) | $ | (6,978 | ) | |||
|
Basic and diluted net loss per Class A common share |
$ | (0.31 | ) | $ | (0.26 | ) | $ | (0.32 | ) | $ | (7.74 | ) | |||
|
Weighted-average number of shares used in computing basic and diluted net loss per Class A common share |
93,544,173 | 74,793,530 | 35,377,588 | 901,666 | |||||||||||
| December 31, | |||||||||||||||
|
2017 |
2016 |
2015 |
2014 |
||||||||||||
|
(in thousands) |
|||||||||||||||
|
Consolidated Balance Sheet Data: |
|||||||||||||||
|
Cash, cash equivalents and short-term investments |
$ | 22,590 | $ | 11,477 | $ | 19,113 | $ | 1,651 | |||||||
|
Working capital |
$ | 26,897 | $ | 10,206 | $ | 17,143 | $ | 279 | |||||||
|
Total assets |
$ | 37,325 | $ | 15,529 | $ | 20,898 | $ | 2,138 | |||||||
|
Long-term debt, including current portion |
$ | 14,624 | $ | 1,195 | $ | 2,584 | $ | 3,564 | |||||||
|
Total liabilities |
$ | 20,489 | $ | 3,360 | $ | 3,852 | $ | 4,325 | |||||||
|
Total stockholders' equity (deficit) |
$ | 16,836 | $ | 12,169 | $ | 17,045 | $ | (2,187 | ) | ||||||
|
ITEM 7. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
The following discussion and analysis of our financial condition and results of operations should be read together with our consolidated financial statements and related notes appearing elsewhere in this Annual Report on Form 10-K. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. You should review “Risk Factors” in Item 1A and elsewhere in this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements described in the following discussion and analysis. Please see "FORWARD-LOOKING STATEMENTS" at the beginning of this Form 10-K for additional information you should consider regarding forward-looking statements.
Overview
AirXpanders is a U.S. based medical device company whose principal business is to design, manufacture, sell and distribute medical devices used in two-stage breast reconstruction procedures following mastectomy. Our AeroForm Tissue Expander System (AeroForm) is a needle-free, patient-controlled tissue expander used in patients undergoing two-stage breast reconstruction following mastectomy prior to the insertion of a breast implant. AeroForm was granted its first CE mark in Europe in October 2012, was approved by Australia’s Therapeutic Goods Administration in October 2013, commenced its initial marketing release of AeroForm in Australia in January 2015, and was granted its U.S. Food and Drug Administration (FDA) de novo marketing authorization in December 2016 (as a Class II medical device). After we received FDA de novo clearance for AeroForm, we submitted a 510(k) application for a materials change related to enhanced film material. This 510(k) was cleared by the FDA in April 2017. To date, we have been primarily engaged in developing and launching our initial product technology, completing clinical trials, building the manufacturing infrastructure to support commercialization efforts, recruiting key personnel and raising capital.
In the U.S. alone, of the approximately 300,000 new diagnoses of female breast cancer every year, 36% of early-stage patients and 60% of late-stage patients receive a mastectomy. Mastectomies are also performed on a proportion of previously diagnosed patients who originally had a form of breast-conserving surgery, as well as on women undertaking mastectomy as a preventative measure prior to any cancer diagnosis. Approximately 60% of mastectomies in the U.S. involve the removal of both breasts. After a mastectomy, breast reconstruction is available to women who want to rebuild the shape and look of the breast.
Breast reconstruction restores breast symmetry after a mastectomy by creating a breast mound, similar in size, shape, and contour to the contralateral breast. The most common technique used in breast reconstruction is two-stage reconstruction which involves tissue expansion to create space for a breast implant. Tissue expansion involves expansion of the breast skin and muscle using a temporary tissue expander. AeroForm is a needle-free device, uses a controlled delivery of small amounts of carbon dioxide (CO2) gas to achieve the tissue expansion usually required prior to placement of a breast implant. It eliminates the need for the needle injections required for traditional saline-based tissue expanders, gives patients the ability to control the expansion process themselves, and allows patients to achieve full expansion faster than is achieved using traditional saline tissue expanders. In a series of clinical trials, AeroForm has been shown to enable patients to proceed to the insertion of a breast implant faster than with a traditional saline-based tissue expander.
We commenced commercial sales of AeroForm in 2015 and are focused on executing our strategy to become the standard of care for tissue expanders in breast reconstruction post mastectomy. Through 2016, our commercial activities were limited to Australia, where a number of our early clinical trials were performed. Commencing in 2017, the majority of our commercial effort has been focused on the U.S. Once sales are established in the U.S., we may extend our presence into Europe, where we have already obtained approval to market and sell AeroForm, and Asia, where we will need to obtain regulatory approvals.
We secured Australian reimbursement for AeroForm in November 2014. In December 2016, the FDA granted de novo marketing authorization for AeroForm as a Class II medical device, and we commenced commercial operations in January 2017. In the U.S. we believe we will benefit from existing reimbursement codes that provide broad reimbursement coverage for breast reconstruction procedures (including tools and devices used in those procedures, such as tissue expanders). In addition, due to the U.S. Women’s Health and Cancer Rights Act of 1998, which federally mandates reimbursement of breast reconstruction procedures, private insurers that provide reimbursement for mastectomies (which we understand to be all major private insurers in the U.S.) are required to also provide reimbursement for procedures for breast reconstruction (inclusive of the use of tissue expanders such as AeroForm). Although rates of reimbursement for breast reconstruction procedures in the U.S. have been increasing in recent years, reimbursement rates are lower than our cost to produce AeroForm, and no assurance can be given that reimbursement amounts will continue to increase or that the amounts will be sufficient to enable us to sell AeroForm on a profitable basis in the U.S. Moreover, we cannot predict what changes may be made in the future to third party coverage and reimbursement in Australia or the U.S. and what impact any such changes may have on our ability to sell AeroForm.
AirXpanders was incorporated in Delaware in 2005 and is headquartered in Palo Alto, California. We have incurred net losses and cash flow deficits from operations since our inception. During the year ended December 31, 2017, we had net revenues of approximately $3.9 million and a net loss of $29.0 million. Our accumulated deficit was approximately $95.3 million at December 31, 2017. To date, our products have been approved for marketing and sales in Europe, Australia and the United States. We commenced the sale of our product in Australia in 2015, and in the U.S. in the first quarter of 2017.
On June 22, 2015, we issued 29,629,654 shares of Common Stock in connection with an initial public offering (IPO) on the Australian Securities Exchange (ASX), a concurrent private placement under Regulation D of the Securities Act (or Concurrent Placement) and the conversion of convertible bridge notes payable and related accrued interest. We raised a total of approximately $30.1 million, net of issuance costs of approximately $2.9 million. Of this amount, $25.1 million were net cash proceeds directly from the IPO, and $5.0 million were cash proceeds from the Concurrent Placement and private placement of convertible bridge notes payable. In connection with the IPO, all of our existing shares of preferred stock were converted into common stock.
In June 2016, we issued 8,771,930 shares of Common Stock in connection with an equity offering on the ASX. Our cash proceeds were approximately $14.2 million, net of issuance costs of approximately $0.7 million.
In February 2017, we issued 16,304,348 shares of Common Stock in connection with an equity offering on the ASX. We raised a total of $32.6 million, net of issuance costs of approximately $1.5 million.
In August 2017, we borrowed $15.0 million under a loan and security agreement with a financial institution which matures in August 2022.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements prepared in conformity with U.S. GAAP. The preparation of these financial statements requires us to make certain estimates and assumptions that may affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reported periods. These estimates and assumptions, including those related to valuation of our common stock prior to the IPO, valuation of stock options and valuation of our inventory, have been our most significant to date. We base our estimates on our historical experience and various other assumptions that are believed to be reasonable under the circumstances. Actual results could differ materially from our estimates under different assumptions or conditions.
We believe that our application of the following accounting policies, each of which require significant judgments and estimates on the part of management, are the most critical to aid in fully understanding and evaluating our financial condition and results of operations. Our significant accounting policies are more fully described in Note 3, “Summary of Significant Accounting Policies,” to our consolidated financial statements appearing elsewhere in this registration statement.
Revenue Recognition
We generate all of our revenue from sales of AeroForm. We recognize revenue from sales of our products in accordance with the Revenue Recognition Topic ASC 605. We recognize revenue from product sales when the following four criteria are met: delivery has occurred, there is persuasive evidence of an arrangement, the fee is fixed or determinable, and collectability of the related receivable is reasonably assured. We recognize revenue when title to the product and risk of loss transfer to customers, provided there are no remaining performance obligations required or any written matters requiring customer acceptance, which generally occurs upon either shipment or implantion of our device. In the U.S., we offer a thirty day return policy and recognize revenue net of sales returns and allowances. Appropriate reserves are established for anticipated sales returns based on historical experience, recent gross sales and any notification of pending returns. Actual sales returns in any future period are inherently uncertain and thus may differ from the estimates. If actual sales returns differ significantly from the estimates, an adjustment to revenue in the current or subsequent period would be recorded. We have established an allowance for sales returns of $0.2 million as of December 31, 2017 recorded net against accounts receivable in the accompanying consolidated balance sheet. No amount was recorded as of December 31, 2016 as all sales were recorded in Australia.
Inventory
We state inventory at the lower of cost or market value, with cost determined by the first-in, first-out method. When needed, we provide reserves for excess or obsolete inventory. Inventory cost is written down to market value when cost exceeds market value, which we estimate using current levels of inventory, as well as historical sales data and forecasts of sales, as well as current reimbursement rates. Additionally, we record a provision for excess, expired, and obsolete inventory based primarily on estimates of forecasted revenues. A significant change in the timing or level of demand for products as compared to forecasted amounts may result in recording additional provisions for excess, expired, and obsolete inventory in the future. When capitalizing inventory, we consider factors such as status of regulatory approval, alternative use of inventory, and anticipated commercial use of the product.
Stock-Based Compensation
Stock-based compensation expense for employee awards is measured at the grant date, based on the fair value of the awards ultimately expected to vest and is recognized as an expense, on a straight-line basis, over the requisite service period, which is generally the vesting period. Forfeitures are required to be estimated at the time of grant and revised if necessary in subsequent periods if actual forfeitures or vesting differ from those estimates. Such revisions could have a material effect on our operating results.
We use the Black-Scholes option-pricing model (Black-Scholes model) to determine the estimated fair value of our stock options utilizing various inputs with respect to expected term, expected stock price volatility, expected dividend yield and risk-free interest rates. We use the simplified method to estimate expected term which is a weighted average of the vesting periods and the total term of the award. We estimate expected volatility using comparable public companies’ volatility for similar terms as we do not have a long enough operating period as a public company to estimate our own volatility. As we develop our own volatility history over time, we will incorporate that history into our expected volatility estimates. The Black-Scholes model calls for a single expected dividend yield as an input. We have never paid a dividend and do not have any current plans to do so. We base our risk-free interest rate on the U.S. Treasury zero coupon issues in effect at the time of the grant that correspond to the expected term of the option.
We recognize the fair value of awards granted to nonemployees as stock-based compensation expense over the period in which the related services are received.
Going Concern
As we have limited commercialization of our product, we are generating a small amount of revenue and are not cash flow positive or profitable. Our net revenue from sales of AeroForm was approximately $3.9 million for the year ended December 31, 2017, and, as of December 31, 2017, we had cash and cash equivalents of approximately $22.6 million. We believe our current cash balances will be sufficient to meet our anticipated cash requirements to fund our commercial efforts in the U.S. in 2018, and build our supporting manufacturing infrastructure and salesforce to support our commercialization efforts through at least December 31, 2018.
Emerging Growth Company Status
The Jumpstart our Business Startups Act of 2012 (the JOBS Act) permits an “emerging growth company” such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We have elected to avail ourselves of this exemption to take advantage of the extended transition period for complying with new or revised accounting standards.
Results of Operations
The following table sets forth significant components of our results of operations for the periods presented.
|
Years Ended December 31, |
||||||||||||
|
2017 |
2016 |
2015 |
||||||||||
|
(in thousands) |
||||||||||||
|
Revenue |
$ | 3,906 | $ | 570 | $ | 293 | ||||||
|
Cost of goods sold |
9,190 | 4,543 | 1,906 | |||||||||
|
Gross loss |
(5,284 | ) | (3,973 | ) | (1,613 | ) | ||||||
|
Operating expenses: |
||||||||||||
|
Research and development |
8,726 | 7,164 | 4,827 | |||||||||
|
Selling, general and administrative |
14,393 | 7,986 | 4,640 | |||||||||
|
Total operating expenses |
23,119 | 15,150 | 9,467 | |||||||||
|
Operating loss |
(28,403 | ) | (19,123 | ) | (11,080 | ) | ||||||
|
Other expense (income): |
||||||||||||
|
Interest expense |
796 | 249 | 422 | |||||||||
|
Other expense (income) |
(217 | ) | 50 | (341 | ) | |||||||
|
Total other expense (income), net |
579 | 299 | 81 | |||||||||
|
Operating loss before income tax provision |
(28,982 | ) | (19,422 | ) | (11,161 | ) | ||||||
|
Provision for income taxes |
1 | 1 | — | |||||||||
|
Net loss |
$ | (28,983 | ) | $ | (19,423 | ) | $ | (11,161 | ) | |||
2017 Compared to 2016 Compared to 2015
Net Revenues and Cost of Goods Sold
|
Years Ended December 31, |
||||||||||||||||||||
|
2017 |
2016 |
2015 |
Year-over-Year % Change |
|||||||||||||||||
|
(in thousands) |
||||||||||||||||||||
|
Net revenues |
$ | 3,906 | $ | 570 | $ | 293 | 585 | % | 94 | % | ||||||||||
|
Cost of goods sold |
9,190 | 4,543 | 1,906 | 102 | % | 138 | % | |||||||||||||
|
Gross loss |
$ | (5,284 | ) | $ | (3,973 | ) | $ | (1,613 | ) | 32 | % | 146 | % | |||||||
The Company commenced commercial operations in the United States in 2017. Prior to that, all net revenues were generated from sales of AeroForm in Australia.
Net Revenues. The increase in net revenues of $3.3 million in 2017 relative to 2016 was the result of the commencement of commercial operations in the United States in the first quarter of 2017, resulting in net revenues of $3.1 million in 2017, as well as an increase in net revenues generated from sales in Australia of $0.2 million due to continued growth in unit sales. The increase in net revenues of $0.3 million in 2016 relative to 2015 was a result of continued market share growth as we began to penetrate and gain adoption at a number of hospitals in Australia.
Future revenue growth consistent with or greater than the growth experienced in 2017 is dependent on continued increased utilization of AeroForm by existing customers and the acquisition of new customers in the U.S.
Cost of Goods Sold. The increase in cost of goods sold of approximately $4.6 million in 2017 compared to 2016 was primarily related to an increase in net revenues. In addition, we recorded an inventory write-down and other inventory reserves of $3.0 million in 2017 relative to approximately $1.5 million in 2016, primarily related to lower of cost or market, or LCM, adjustments, as the cost to manufacture our products continue to exceed the market value of our products. The increase in cost of goods sold of approximately $2.6 million in 2016 compared to 2015 was primarily related to the ongoing scale up of our manufacturing capability in 2016, primarily related to increased personnel expenses. In addition, we recorded an increase of $1.5 million in our inventory provision in 2016 compared to 2015, primarily related to LCM adjustments.
We expect to generate gross profit in 2018 based on an anticipated increase in sales volume which should allow us to absorb our fixed overhead costs over larger manufacturing volumes, as well as improved per unit manufacturing assembly times, which is expected to result in a decreased direct labor cost per unit. If we do not realize an increase in sales sufficient to obtain these expected efficiencies, or if we are unable to realize improved per unit manufacturing assembly times, we will be unable to realize gross profits in 2018.
Total Operating Expenses
|
Years Ended December 31, |
||||||||||||||||||||
|
2017 |
2016 |
2015 |
Year-over-Year % Change |
|||||||||||||||||
|
(in thousands) |
||||||||||||||||||||
|
Research and development |
$ | 8,726 | $ | 7,164 | $ | 4,827 | 22 | % | 48 | % | ||||||||||
|
Selling, general and administrative |
14,393 | 7,986 | 4,640 | 80 | % | 72 | % | |||||||||||||
|
Total operating expenses |
$ | 23,119 | $ | 15,150 | $ | 9,467 | 52 | % | 60 | % | ||||||||||
Research and Development Expense. Research and development expenses increased by $1.6 million in 2017 compared to 2016, primarily due to an increase in personnel expenses of $0.8 million driven by the increase in the number of employees and the increase in the allocation of manufacturing resources dedicated to pre-commercial manufacturing-related development projects, and an increase in nonrecurring engineering expenses of $0.7 million associated with the expansion of manufacturing capability at our third-party contract manufacturer and other engineering projects. Research and development expenses increased by $2.3 million in 2016 compared to 2015, primarily due to an increase in personnel expenses of $1.5 million driven by the increase in the number of employees, an increase of $0.4 million for clinical expenses associated with conducting our clinical trials, and an increase in expenses attributable to expanding our office space.
Selling, General and Administrative Expense. Selling, general and administrative expense increased by $6.4 million in 2017 compared to 2016 principally due an increase in personnel expenses of $3.8 million as we hired our initial sales force in the United States and other key personnel to develop infrastructure to support commercialization efforts in the United States. Marketing program expenses, primarily related to public relations, advertising and product evaluations and demonstration units, increased by $0.9 million to support the commercial launch in the United States. Professional service fees also increased by $0.5 million primarily due to the additional compliance expenses as a result of becoming a United States reporting company in 2017. Selling, general and administrative expense increased by $3.3 million in 2016 compared to 2015 principally due an increase in personnel expenses of $2.5 million as we hired key personnel to develop infrastructure to support anticipated commercialization efforts in the U.S. Professional service fees also increased by $0.8 million primarily due to the additional compliance expenses as a result of a listing on the ASX in June 2015.
Overall operating expenses in 2018 are expected to decrease as compared to 2017, based on expected increased expenses for U.S. sales personnel and consultants, offset by anticipated decreases in market programs, engineering programs, clinical trials expenses and personnel expenses in other areas.
Total Other Expense (Income), Net
|
Years Ended December 31, |
Year-over-Year % Change |
|||||||||||||||||||
|
2017 |
2016 |
2015 |
||||||||||||||||||
|
(in thousands) |
||||||||||||||||||||
|
Interest expense |
$ | 796 | $ | 249 | $ | 422 | 220 | % | (41 | %) | ||||||||||
|
Other expense (income), net |
(217 | ) | 50 | (341 | ) | (534 | %) | 115 | % | |||||||||||
|
Total other expense (income), net |
$ | 579 | $ | 299 | $ | 81 | 94 | % | 269 | % | ||||||||||
Interest Expense. The increase in interest expense of $0.5 million in 2017 as compared to 2016 resulted from higher outstanding debt balances as the result of our August 2017 borrowing of $15.0 million. The decrease in interest expense of $0.2 million in 2016 as compared to 2015 resulted from the conversion of outstanding convertible notes into equity upon our listing on ASX in June 2015 and lower overall outstanding debt balances.
Other expense (income), net. The change in other expense (income), net, of $0.3 million in 2017 as compared to 2016 is primarily attributed to interest income of $0.2 million in 2017 due to an increase in cash balances invested in interest bearing investments. The change in other expense (income), net of $0.4 million is primarily attributed to a loss of under $0.1 million in 2016 and a gain of $0.3 million in 2015 due to fluctuations in the foreign exchange rate.
Liquidity and Capital Resources
We have incurred losses since our inception in 2005 including net losses after taxes of $29.0 million, $19.4 million, and $11.2 million in 2017, 2016 and 2015, respectively, and as of December 31, 2017, we had an accumulated deficit of approximately $95.3 million. We have financed our operations from a combination of sales of equity securities and issuances of convertible term notes.
Our independent registered accountants report on our December 31, 2017 consolidated financial statements contains an emphasis of a matter regarding substantial doubt about our ability to continue as a going concern. The consolidated financial statements have been prepared assuming we will continue as a going concern and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets, or the amounts and classification of liabilities that may result if we do not continue as a going concern.
In January 2014, the Company borrowed $3,500,000 under a loan and security agreement with a financial institution which matured in July 2017. The loan was paid in full at maturity in July 2017. No amounts are available to borrow under the agreement.
In 2015, we raised net proceeds of $30.1 million through the issuance of 29,629,654 shares of Common Stock in connection with an IPO on the ASX, a Concurrent Placement and the issuance of convertible bridge notes, which were converted to common shares at the IPO. In connection with the IPO, all of our existing shares of preferred stock were converted into common stock. In June 2016, we generated $14.2 million, net of issuance costs in an equity offering. In February 2017, we generated net proceeds of $32.6 million.
In August 2017, we borrowed $15,000,000 under a loan and security agreement with Oxford which matures in August 2022. Interest is paid monthly on the principal amount at a variable rate equal to the greater of (a) the thirty day LIBOR rate, or (b) 0.99%, plus 7.26% per annum. The loan is secured by substantially all of our assets, excluding intellectual property, which intellectual property is subject to a negative pledge in favor of Oxford. Under the terms of the agreement, interest-only payments are due monthly through September 2019, with principal payments commencing in October 2019, due in 35 equal monthly installments. If we are in compliance with certain financial milestones, the interest-only payments can be extended by twelve months through September 2020, in which case the principal payments would commence in October 2020, due in 23 equal monthly installments. A final fee of $1,200,000 is due at maturity (or acceleration or prepayment). Subject to a prepayment fee equal to between 0.5% to 2.0% of the principal amount of the prepaid amount, we can prepay the entire loan amount by providing a written five-day notice prior to such prepayment and paying all outstanding principal, interest, final payment fees and prepayment fees plus any default fees and all other sums that shall have become due and payable.
Under the loan and security agreement we are required to maintain a certain minimum level of revenues on a trailing six-month basis, subject to quarterly measurement through 2018, and monthly thereafter, in addition to complying with certain other covenants. The loan and security agreement also includes events of default, the occurrence and continuation of any of which provides the financial institution with the right to exercise remedies against us and the collateral securing the loans, including cash. These events of default include, among other things, the failure to pay amounts due under the credit facilities, insolvency, the occurrence of a material adverse event, which includes a material adverse change in our business, operations or properties (financial or otherwise) or a material impairment of the prospect of repayment of any portion of the obligations. A violation of any of these covenants or the occurrence of a material adverse change could result in a default under the loan and security, which would result in termination of all commitments and loans under the agreement and all amounts owing under the agreement to become immediately due and payable. As of September 30, 2017, we were in violation of one of these covenants. On November 9, 2017, we entered into the waiver and first amendment to the loan and security agreement with Oxford, pursuant to which we received a waiver of the event default for the September 30, 2017 noncompliance with a financial covenant, and modified certain future covenants. We are in compliance with our loan covenants as of December 31, 2017.
Based on our current operating plan, we do not have sufficient capital to continue our operations for a significant period of time beyond December 31, 2018. We plan to raise additional capital in the future to support our operations. There is no assurance, however, that we will be successful in raising the needed capital on acceptable terms or at all, or that the proceeds will be received in a timely manner to fully support our operations.
The following table sets forth the major sources and uses of cash for each of the periods set forth below:
|
Years Ended December 31, |
||||||||||||
|
2017 |
2016 |
2015 |
||||||||||
|
(in thousands) |
||||||||||||
|
Net cash used in operating activities |
$ | (32,455 | ) | $ | (19,154 | ) | $ | (10,835 | ) | |||
|
Net cash used in investing activities |
(21,015 | ) | (1,151 | ) | (840 | ) | ||||||
|
Net cash provided by financing activities |
46,155 | 12,669 | 29,317 | |||||||||
|
Net (decrease) increase in cash and cash equivalents |
$ | (7,315 | ) | $ | (7,636 | ) | $ | 17,462 | ||||
Cash Flows from Operating Activities
In 2017, cash used in operating activities of $32.5 million resulted primarily from a net loss of $28.9 million, reduced by noncash adjustments, primarily inventory reserve adjustments of $3.0 million, depreciation and amortization of $0.7 million and stock based compensation of $0.7 million. Additional operating cash requirements consisted of $9.4 million for inventory purchases and builds to support a ramp up in commercial activity in connection with the U.S. launch, offset by $1.7 million due to timing of payments for 2017 expenditures and $1.1 million due to the timing of customer payments.
In 2016, cash used in operating activities of $19.2 million resulted primarily from a net loss of $19.4 million, reduced by noncash adjustments, primarily inventory reserve adjustments of $1.5 million and stock based compensation of $0.4 million. Additional operating cash requirements consisted of $2.4 million for inventory purchases to support a ramp up in commercial activity in advance of a U.S. launch, offset by $0.5 million due to timing of payments for 2016 expenditures.
In 2015, cash used in operating activities of $10.8 million resulted primarily from a net loss of approximately $11.2 million, reduced by noncash adjustments, primarily inventory LCM adjustments of $0.5 million and stock based compensation of $0.1 million. Additional operating cash requirements consisted of $0.9 million for inventory purchases to support a ramp up in commercial activity in Australia, offset by $0.5 million due to timing of payments for 2015 expenditures.
We anticipate cash outflows from operating activities to decrease in 2018 as a result of increasing sales, offset by decreases in overall operating expenses and a decrease in the rate of increase in inventory. If we do not experience increasing revenues, a decrease in the rate of build of inventory or a decrease in overall operating expenses, cash outflows from operating activities may equal or exceed the amount experienced in 2017.
Cash Flows from Investing Activities
In 2017, cash used in investing activities consisted of $18.4 million for the purchase of short-term investments, net of maturities, and $2.6 million for capital expenditures to support scaling up of manufacturing capacity.
Cash used in 2016 and 2015 is primarily attributable to capital expenditures to support scaling up of manufacturing capability.
Absent any further sources of capital, we anticipate cash from investing activities in 2018 to consist primarily of maturities of short-term investments. Overall investments in additional capital equipment are expected to decrease in 2018 as compared to 2017.
Cash Flows from Financing Activities
In 2017, cash provided by financing activities of $46.2 million resulted primarily from approximately $32.6 million in net proceeds from our February 2017 equity offering in which we issued approximately 16.3 million shares of Common Stock, and $14.7 million in net proceeds from our August 2017 debt issuance. This was slightly offset by $1.3 million in principal payments we made on our outstanding note due July 2017.
In 2016, cash provided by financing activities of $12.7 million resulted primarily from approximately $14.2 million in net proceeds from our June 2016 equity offering in which we issued approximately 8.8 million shares of Common Stock. This was slightly offset by $1.5 million in principal payments we made on our outstanding note due July 2017.
In 2015, cash provided by financing activities of $29.1 million resulted from approximately $30.1 million in net proceeds from our IPO in June 2015, the Concurrent Placement and the issuance of convertible notes payable. Together, such net proceeds more than offset $1.0 million in principal payments we made on our outstanding note due July 2017.
Funding Requirements
Based on our current operating plan, we do not have sufficient capital to continue our operations for a significant period of time beyond December 31, 2018. We plan to raise additional capital in the future to support our operations. However, we may not be able to obtain sufficient additional funding on acceptable terms or at all.
Our forecast of the period of time through which our financial resources will be adequate to support our operations and further expand the commercialization of our product, and our expectations about raising additional funds, are forward-looking statements and involve risks and uncertainties, and actual results could vary materially and negatively as a result of a number of factors, including the factors discussed in the “Risk Factors” section of this registration statement. We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect.
Due to the numerous risks and uncertainties associated with the development and commercialization of AeroForm, we are unable to estimate precisely the amounts of capital and operating expenditures necessary to complete the development of, and to obtain full commercial scale launch in the U.S. Our funding requirements will depend on many factors, including, but not limited to, the following:
|
• |
the rate of progress and cost of our commercialization activities; |
|
• |
the expenses we incur in marketing and selling AeroForm; |
|
• |
the revenue generated by sales of AeroForm; |
|
• |
the success of our investment in our manufacturing and supply chain infrastructure; |
|
• |
the time and costs involved in obtaining regulatory approvals for AeroForm in new markets; |
|
• |
the success of our research and development efforts; |
|
• |
the emergence of competing or complementary developments; and |
|
• |
the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights. |
We will continue to manage our capital structure and to consider all financing opportunities, whenever they may occur, that could strengthen our long-term liquidity profile. Any such capital transactions may or may not be similar to transactions in which we have engaged in the past. There can be no assurance that any such financing opportunities will also be available on acceptable terms, if at all.
Off–Balance Sheet Arrangements
We do not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, that would have been established for the purpose of facilitating off-balance sheet arrangements (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) or other contractually narrow or limited purposes. As such, we are not exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in those types of relationships. We enter into guarantees in the ordinary course of business related to the guarantee of our own performance and the performance of our branch office in Australia.
Contractual Obligations and Commitments
Our commitments for operating leases below relate to our leases and subleases of office, laboratory and manufacturing space in Palo Alto, California, San Jose, California and Sydney, Australia, net of sublease income from subleases for our office space in Palo Alto, CA.
The following table summarizes our outstanding contractual obligations as of December 31, 2017:
|
|
||||||||||||||||||||
|
Total |
Less Than 1 Year |
1-3 Years | 3-5 Years |
More Than 5 Years |
||||||||||||||||
|
(in thousands) |
||||||||||||||||||||
|
Operating lease obligations, net of sublease income |
$ | 1,534 | $ | 835 | $ | 699 | $ | — | $ | — | ||||||||||
| Inventory purchase commitments | 2,038 | 2,038 | — | — | — | |||||||||||||||
|
Debt repayments (interest and principal) |
20,289 | 1,291 | 14,713 | 4,285 | — | |||||||||||||||
| $ | 23,861 | $ | 4,164 | $ | 15,412 | $ | 4,285 | $ | — | |||||||||||
Recent Accounting Pronouncements
For additional discussions on recent accounting pronouncements please refer to Note 3, “Summary of Significant Accounting Policies”, to our consolidated financial statements included in this Annual Report on Form 10-K.
|
ITEM 7A. |
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK. |
We design, manufacture, sell and distribute the AeroForm Tissue Expander System in Australia. We commenced initial marketing launch in the U.S. in January 2017. Our earnings and cash flows are exposed to market risk from changes in currency exchange rates and interest rates.
Interest Rate Sensitivity
Our cash and cash equivalents of $22.6 million at December 31, 2017 consisted of cash, money market funds and investments in U.S. Treasury Securities, all of which will be used or be available for working capital purposes. We do not enter into investments for trading or speculative purposes. When excess cash is available for investment, the goals of our investment policy are preservation of capital, fulfillment of liquidity needs and fiduciary control of cash and investments. We also seek to maximize income from our investments without assuming significant risk. Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of interest rates in the United States and Australia. Because of the short-term nature of our cash, cash equivalents and short-term investments, we do not believe that we have any material exposure to changes in their fair values as a result of changes in interest rates. The continuation of historically low interest rates in the United States in addition to our expected lower overall balances in short-term investments will limit our earnings on investments held in U.S. dollars.
Our debt bears interest at a variable rate, so interest expense and cash flows could be adversely affected by changes in interest rates. Interest rates for our debt can fluctuate based on changes in market interest rates. As of December 31, 2017, the outstanding principal balance of short-term and long-term debt was $15.0 million.
Foreign Currency Risk
We conduct business in foreign currencies in Australia. Our reporting currency is the U.S. dollar (U.S.$). For U.S. reporting purposes of our non-U.S. operations, we translate all monetary assets and liabilities at the period-end exchange rate, all nonmonetary assets and liabilities at historical rates and revenue and expenses at the average exchange rates in effect during the periods. The net effect of these translation adjustments is shown in the accompanying consolidated financial statements within other expense (income) as a component of net loss.
Through 2016, we generated all of our revenue and receivables in the Australian dollar (A$). Fluctuations in the exchange rate of the U.S. dollar against the Australian dollar, may result in foreign currency exchange gains and losses that may significantly impact our financial results. In 2017, 2016 and 2015, foreign currency translation and remeasurement gains or losses included in other expense (income), net, in the consolidated statements of operations and comprehensive loss was a de minimus gain, a loss of under $0.1 million and a gain of $0.3 million, respectively.
All of the proceeds from our 2017, 2016 and 2015 offerings were denominated in Australian dollars and as of December 31, 2017 we held approximately U.S.$0.2 million denominated as Australian dollars. Accordingly, we have had and will continue to have exposure to foreign currency exchange rate fluctuations. A change of 10% or more in foreign currency exchange rates of the Australian dollar could have a material impact on our financial position and results of operations if our revenue continues to be denominated in or if we retain a substantial portion of our cash and cash equivalents in Australian dollars.
|
ITEM 8. |
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
AirXpanders, Inc.
Index to Consolidated Financial Statements
|
|
Page |
|
Report of Independent Registered Public Accounting Firm |
32 |
|
Consolidated Financial Statements |
|
|
Consolidated Balance Sheets |
33 |
|
Consolidated Statements of Operations and Comprehensive Loss |
34 |
|
Consolidated Statements of Stockholders’ Equity (Deficit) |
35 |
|
Consolidated Statements of Cash Flows |
36 |
|
Notes to Consolidated Financial Statements |
37 |
| Supplementary Data | |
| Selected Quarterly Financial Data Results (Unaudited) | 51 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
AirXpanders, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of AirXpanders, Inc. (collectively, the “Company”) as of December 31, 2017 and 2016, the related consolidated statements of operations and comprehensive loss, stockholders' equity (deficit) and cash flows for the years ended December 31, 2017, 2016 and 2015, and the related notes to the consolidated financial statements (collectively, the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for the years ended December 31, 2017, 2016 and 2015, in conformity with accounting principles generally accepted in the United States of America.
Emphasis of Matter Regarding Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has incurred net losses and cash flow deficits from operations since its inception and has an accumulated deficit of $95.3 million at December 31, 2017. This raises substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters also are described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ SingerLewak LLP
We have served as the Company's auditor since 2013.
San Jose, California
February 27, 2018
AirXpanders, Inc.
Consolidated Balance Sheets
(In thousands, except share and per share amounts)
|
December 31, |
||||||||
|
2017 |
2016 |
|||||||
|
ASSETS |
||||||||
|
Current assets |
||||||||
|
Cash and cash equivalents |
$ | 4,162 | $ | 11,477 | ||||
| Short-term investments | 18,428 | — | ||||||
|
Accounts receivable, net |
1,022 | 118 | ||||||
|
Inventory |
8,132 | 1,413 | ||||||
|
Prepaid expenses and other current assets |
1,018 | 558 | ||||||
|
Total current assets |
32,762 | 13,566 | ||||||
|
Property and equipment |
4,435 | 1,879 | ||||||
|
Other assets |
129 | 84 | ||||||
|
Total assets |
$ | 37,326 | $ | 15,529 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY |
||||||||
|
Current liabilities |
||||||||
|
Current portion of long-term debt, net of discount |
$ | — | $ | 1,195 | ||||
|
Accounts payable |
3,345 | 1,249 | ||||||
|
Accrued expenses |
2,521 | 916 | ||||||
|
Total current liabilities |
5,866 | 3,360 | ||||||
|
Long-term debt, less current portion, net of discount |
14,624 | — | ||||||
|
Total liabilities |
20,490 | 3,360 | ||||||
|
Commitments and Contingencies (Note 8) |
||||||||
|
Stockholders' equity |
||||||||
|
Preferred stock, $0.001 par value; 10,000,000 authorized; no shares issued and outstanding at December 31, 2017 and 2016 |
— | — | ||||||
|
Class A common stock, $0.001 par value; 200,000,000 authorized; 95,943,409 and 79,241,708 issued and outstanding at December 31, 2017 and 2016, respectively |
96 | 79 | ||||||
|
Class B common stock, $0.001 par value; 100,000,000 authorized; no shares issued and outstanding at December 31, 2017 and 2016 |
— | — | ||||||
|
Additional paid-in capital |
112,045 | 78,418 | ||||||
| Accumulated other comprehensive income | 6 | — | ||||||
|
Accumulated deficit |
(95,311 | ) | (66,328 | ) | ||||
|
Total stockholders' equity |
16,836 | 12,169 | ||||||
|
Total liabilities and stockholders' equity |
$ | 37,326 | $ | 15,529 | ||||
See accompanying notes to financial statements.
AirXpanders, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
|
Years Ended December 31, |
||||||||||||
|
2017 |
2016 |
2015 |
||||||||||
|
Revenue |
$ | 3,906 | $ | 570 | $ | 293 | ||||||
|
Cost of goods sold |
9,190 | 4,543 | 1,906 | |||||||||
|
Gross loss |
(5,284 | ) | (3,973 | ) | (1,613 | ) | ||||||
|
Operating expenses: |
||||||||||||
|
Research and development |
8,726 | 7,164 | 4,827 | |||||||||
|
Selling, general and administrative |
14,393 | 7,986 | 4,640 | |||||||||
|
Total operating expenses |
23,119 | 15,150 | 9,467 | |||||||||
|
Operating loss |
(28,403 | ) | (19,123 | ) | (11,080 | ) | ||||||
|
Other expense (income): |
||||||||||||
|
Interest expense |
796 | 249 | 422 | |||||||||
|
Other expense (income), net |
(217 | ) | 50 | (341 | ) | |||||||
|
Total other expense (income), net |
579 | 299 | 81 | |||||||||
|
Operating loss before income tax provision |
(28,982 | ) | (19,422 | ) | (11,161 | ) | ||||||
|
Provision for income taxes |
1 | 1 | — | |||||||||
|
Net loss |
(28,983 | ) | (19,423 | ) | (11,161 | ) | ||||||
|
Net loss per Class A common share: basic and diluted |
$ | (0.31 | ) | $ | (0.26 | ) | $ | (0.32 | ) | |||
|
Weighted average number of Class A common shares used in computing net loss per Class A common share: basic and diluted |
93,544,173 | 74,793,530 | 35,377,588 | |||||||||
| Comprehensive loss: | ||||||||||||
| Net Loss | $ | (28,983 | ) | $ | (19,423 | ) | $ | (11,161 | ) | |||
| Unrealized gain on investments | 6 | — | — | |||||||||
| Total comprehensive loss | $ | (28,977 | ) | $ | (19,423 | ) | $ | (11,161 | ) | |||
See accompanying notes to consolidated financial statements.
AirXpanders, Inc.
Consolidated Statements of Stockholders’ Equity (Deficit)
(In thousands, except share and per share amounts)
|
Convertible Preferred Stock |
Common Stock |
|||||||||||||||||||||||||||||||
|
Issued and Outstanding Shares |
Amount |
Issued and Outstanding Shares |
Amount |
Additional Paid-In Capital | Accumulated Other Comprehensive Income | Accumulated Deficit | Total Stockholders' Equity (Deficit) | |||||||||||||||||||||||||
|
Balance, December 31, 2014 |
130,509,868 | $ | 130 | 901,665 | $ | 1 | $ | 33,425 | $ | — | $ | (35,744 | ) | $ | (2,188 | ) | ||||||||||||||||
|
Issuance of common stock for cash (net of issuance costs of $2,873) |
— | — | 25,217,180 | 25 | 25,055 | — | — | 25,080 | ||||||||||||||||||||||||
|
Exercise of warrants to purchase common stock |
— | — | 9,780,489 | 10 | 39 | — | — | 49 | ||||||||||||||||||||||||
|
Exercise of stock options |
— | — | 2,965 | — | 1 | — | — | 1 | ||||||||||||||||||||||||
|
Conversion of convertible bridge notes payable and accrued interest of $70 into common stock |
— | — | 4,412,474 | 4 | 5,026 | — | — | 5,030 | ||||||||||||||||||||||||
|
Conversion of convertible preferred stock into common stock |
(130,509,868 | ) | (130 | ) | 30,112,422 | 30 | 100 | — | — | — | ||||||||||||||||||||||
|
Conversion of warrants to purchase preferred stock into warrants to purchase common stock |
— | — | — | — | 123 | — | — | 123 | ||||||||||||||||||||||||
|
Stock-based compensation |
— | — | — | — | 111 | — | — | 111 | ||||||||||||||||||||||||
|
Net loss |
— | — | — | — | — | — | (11,161 | ) | (11,161 | ) | ||||||||||||||||||||||
|
Balance, December 31, 2015 |
— | — | 70,427,195 | 70 | 63,880 | — | (46,905 | ) | 17,045 | |||||||||||||||||||||||
|
Issuance of common stock for cash (net of issuance costs of $731) |
— | — | 8,771,930 | 9 | 14,150 | — | — | 14,159 | ||||||||||||||||||||||||
|
Exercise of stock options |
— | — | 42,583 | — | 11 | — | — | 11 | ||||||||||||||||||||||||
|
Stock-based compensation |
— | — | — | — | 377 | — | — | 377 | ||||||||||||||||||||||||
|
Net loss |
— | — | — | — | — | — | (19,423 | ) | (19,423 | ) | ||||||||||||||||||||||
|
Balance, December 31, 2016 |
— | — | 79,241,708 | 79 | 78,418 | — | (66,328 | ) | 12,169 | |||||||||||||||||||||||
| Issuance of Class A Common Stock for cash (net of issuance costs of $1,462) | — | — | 16,304,348 | 16 | 32,633 | — | 32,649 | |||||||||||||||||||||||||
| Exercise of stock options and warrants, 425,124 shares net of 27,771 shares traded for exercise price | — | — | 397,353 | 1 | 60 | — | — | 61 | ||||||||||||||||||||||||
| Issuance of warrants to purchase Class A Common Stock in connection with issuance of debt | — | — | — | — | 227 | — | — | 227 | ||||||||||||||||||||||||
|
Stock-based compensation |
— | — | — | — | 707 | — | — | 707 | ||||||||||||||||||||||||
| Unrealized gain on investments | — | — | — | — | — | 6 | — | 6 | ||||||||||||||||||||||||
|
Net loss |
— | — | — | — | — | — | (28,983 | ) | (28,983 | ) | ||||||||||||||||||||||
|
Balance, December 31, 2017 |
— | $ | — | 95,943,409 | $ | 96 | $ | 112,045 | $ | 6 | $ | (95,311 | ) | $ | 16,836 | |||||||||||||||||
See accompanying notes to consolidated financial statements.
AirXpanders, Inc.
Consolidated Statements of Cash Flows
(In thousands)
|
For the Years Ended December 31, |
||||||||||||
|
2017 |
2016 |
2015 |
||||||||||
|
Cash flows from operating activities |
||||||||||||
|
Net loss |
$ | (28,983 | ) | $ | (19,423 | ) | $ | (11,161 | ) | |||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
|
Depreciation and amortization |
667 | 237 | 86 | |||||||||
|
Amortization of debt discount and deferred issuance cost |
211 | 111 | 37 | |||||||||
|
Loss of disposal of assets |
— | 14 | — | |||||||||
|
Interest on convertible bridge notes payable converted to common stock |
— | — | 70 | |||||||||
|
Changes in fair value of warrant liabilities |
— | — | (42) | |||||||||
|
Inventory write-down |
2,990 | 1,537 | 527 | |||||||||
|
Stock-based compensation |
707 | 377 | 111 | |||||||||
|
Sales returns reserve |
179 | |||||||||||
|
Changes in operating assets and liabilities: |
||||||||||||
|
Accounts receivable |
(1,083 | ) | (41 | ) | (77 | ) | ||||||
|
Inventory |
(9,383 | ) | (2,423 | ) | (883 | ) | ||||||
|
Prepaid expenses and other assets |
(499 | ) | (390 | ) | (175 | ) | ||||||
|
Accounts payable |
1,460 | 325 | 473 | |||||||||
|
Accrued expenses |
1,279 | 522 | 199 | |||||||||
|
Net cash used in operating activities |
(32,455 | ) | (19,154 | ) | (10,835 | ) | ||||||
|
Cash flows from investing activities |
||||||||||||
|
Purchase of property and equipment |
(2,587 | ) | (1,151 | ) | (840 | ) | ||||||
| Maturities of short-term investments | 13,564 | — | — | |||||||||
| Purchases of short-term investments | (31,992 | ) | — | — | ||||||||
|
Net cash used in investing activities |
(21,015 | ) | (1,151 | ) | (840 | ) | ||||||
|
Cash flows from financing activities |
||||||||||||
|
Proceeds from debt issuance, net of issuance costs |
14,717 | — | — | |||||||||
|
Proceeds from convertible bridge notes payable |
— | — | 4,960 | |||||||||
|
Principal payments on notes payable |
(1,272 | ) | (1,500 | ) | (953 | ) | ||||||
|
Proceeds from issuance of common stock, net of issuance costs |
32,649 | 14,159 | 25,080 | |||||||||
|
Proceeds from exercise of stock options |
61 | 10 | 1 | |||||||||
|
Proceeds from exercise of warrants for common stock |
— | — | 49 | |||||||||
|
Net cash provided by financing activities |
46,155 | 12,669 | 29,137 | |||||||||
|
Net (decrease) increase in cash and cash equivalents |
(7,315 | ) | (7,636) | 17,462 | ||||||||
|
Cash and cash equivalents -- beginning of period |
11,477 | 19,113 | 1,651 | |||||||||
|
Cash and cash equivalents -- end of period |
$ | 4,162 | $ | 11,477 | $ | 19,113 | ||||||
|
Supplemental disclosure: |
||||||||||||
|
Cash paid for interest |
$ | 445 | $ | 138 | $ | 240 | ||||||
|
Cash paid for taxes |
$ | 1 | $ | 1 | $ | 1 | ||||||
|
Supplemental schedule of noncash investing and financing activities: |
||||||||||||
| Issuance of warrant for 277,778 shares of Class A Common Stock in connection with debt | $ | 277 | $ | — | $ | — | ||||||
|
Conversion of preferred stock to common stock in connection with initial public offering (IPO) |
$ | — | $ | — | $ | 34,633 | ||||||
|
Conversion of convertible bridge notes payable and accrued interest to common stock in connection with IPO |
$ | — | $ | — | $ | 5,030 | ||||||
|
Conversion of warrant liabilities to equity |
$ | — | $ | — | $ | 123 | ||||||
See accompanying notes to consolidated financial statements.
AirXpanders, Inc.
Notes to Consolidated Financial Statements
NOTE 1 – DESCRIPTION OF BUSINESS
AirXpanders, Inc. and its Australian branch (“AirXpanders” or the “Company”) is a Delaware corporation formed on March 17, 2005, and is headquartered in Palo Alto, California. The Company's principal business is to design, manufacture, sell and distribute medical devices used in two-stage breast reconstruction procedures following mastectomy. AirXpanders' AeroForm Tissue Expander System (AeroForm) is a needle-free, patient-controlled tissue expander used in patients undergoing two-stage breast reconstruction following mastectomy prior to the insertion of a breast implant. AeroForm was granted its first CE mark in Europe in October 2012, was approved by Australia’s Therapeutic Goods Administration in October 2013, commenced its initial marketing release of AeroForm in Australia in January 2015, and was granted its U.S. Food and Drug Administration, or FDA, de novo marketing authorization in December 2016 (as a Class II medical device). To date, the Company has been primarily engaged in developing and launching our initial product technology, completing clinical trials, building the manufacturing infrastructure to support commercialization efforts, recruiting key personnel and raising capital.
NOTE 2 – LIQUIDITY AND GOING CONCERN
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business for the foreseeable future. The Company has incurred net losses and cash flow deficits from operations since its inception and has an accumulated deficit of $95.3 million at December 31, 2017. To date, the Company’s products have been approved for marketing and sales in Europe, Australia and the United States, and the Company started selling its product in Australia in 2015, and in the United States in 2017. Management expects operating losses and cash flow deficits to continue for the foreseeable future. The Company’s ability to achieve profitability is dependent primarily on its ability to gain market share in the U.S, build and maintain manufacturing capacity to support commercial launch in the U.S. and obtain a more profitable per unit manufacturing cost for its products. These conditions raise substantial doubt about the Company’s ability to continue as a going concern for at least a year after the issuance date of the accompanying consolidated financial statements.The Company plans to address these conditions by raising capital through equity or debt financings, or a combination of both, from outside current and new investors and institutions. There is no assurance, however, that the Company will be successful in raising the needed capital and, if funding is available, that it will be available on terms acceptable to the Company. The accompanying consolidated financial statements do not include any adjustments that may be needed if the Company were unable to continue as a going concern.
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (U.S. GAAP). The consolidated financial statements include the accounts of AirXpanders, Inc. and its Australian branch office. Intercompany transactions and balances have been eliminated in consolidation. Certain amounts presented in prior periods have been reclassified to the current year presentation. Such changes had no effect on the previously reported net loss or accumulated deficit.
Foreign Currency
The Company transacts business in Australia. The functional currency of its Australian branch is the U.S. dollar. Monetary assets and liabilities are translated at the year-end exchange rate and non-monetary assets and liabilities are translated at historical rates and items in the statement of operations are translated at average rates with gains and losses from remeasurement being recorded in other expense (income), net in the accompanying consolidated statements of operations and comprehensive loss. Foreign currency translation and remeasurement gains or losses included in other expense (income), net in the accompanying consolidated statements of operations and comprehensive loss was a de minimus gain during the year ended December 31, 2017, a de minimus loss during the year ended December 31, 2016 and a gain of $0.3 million during the year ended December 31, 2015.
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of expenses during the reporting period. Actual results could differ materially from those estimates. The Company’s most significant estimates relate to the valuation of its common stock prior to the IPO, valuation of stock options, estimate of sales returns and valuation of its inventory at the lower of cost or market.
Certain Significant Risks and Uncertainties
The Company operates in a dynamic, highly competitive industry and believes that changes in any of the following areas could have a material adverse effect on the Company’s future financial position, results of operations, or cash flows: ability to obtain future financing; advances and trends in new technologies and industry standards; regulatory approval and market acceptance of the Company’s products; development of sales channels; certain supplier relationships; litigation or claims against the Company based on intellectual property, patent, product, regulatory, or other factors including the Company’s ability to attract and retain employees necessary to support its growth.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to credit risk consist primarily of cash and cash equivalents. The Company maintains all of its U.S. cash balances at one financial institution, which at times may exceed the Federal Deposit Insurance Corporation (FDIC) limits of $250,000 for interest-bearing accounts. At December 31, 2017 and 2016, the Company had unrestricted cash balances of approximately $3.7 million and $10.8 million, respectively, that were in excess of the FDIC limits. The Company currently maintains its Australian cash balances at two financial institutions, which, at times, may exceed the Australian government guaranteed limit of USD $0.2 million (AU$ 250,000). At December 31, 2017, the Company had no cash balances that were in excess of the Australian guaranteed limit. At December 31, 2016, the Company had a cash balance of approximately $0.8 million that was in excess of the Australian guaranteed limit. At December 31, 2017 and 2016, the Company maintained cash and investment balances of $22.4 million and $10.5 million, respectively, with one U.S. financial institution.
Cash, Cash Equivalents and Short-term Investments
The Company considers all highly liquid investments with an original maturity of three months or less, when purchased, to be cash equivalents. Short term investments are classified as “available-for-sale” and are reported at fair value with unrealized gains and losses reported in stockholders' equity as a component of other comprehensive loss. Gross realized gains and losses on sales and maturities of securities are recorded in other expense (income), net, in our statement of operations. As of December 31, 2017, the Company's investments consisted of U.S. Treasury Securities, and amortized cost approximated fair value. The cost of securities sold is based on the specific identification method. The Company classifies its investments as current based on the nature of the investment and their availability for use in current operations. The Company reviews its investment portfolio quarterly to determine if any securities may be other-than-temporarily impaired due to increased credit risk, changes in industry or sector of a certain instrument or ratings downgrades.
Inventory
Inventory is valued at the lower of cost or market value, with cost determined by the first-in, first-out method. When needed, the Company provides reserves for excess or obsolete inventory.
Property and Equipment
Property and equipment are stated at cost, net of accumulated depreciation and amortization. Depreciation and amortization is computed using the straight-line method over the following estimated useful lives of the assets:
|
Machinery and equipment |
5 years |
|
Computer equipment |
3 years |
|
Furniture and fixtures |
5 years |
|
Software licenses |
1 - 3 years |
|
Office equipment |
3 years |
Leasehold improvements and property and equipment under capital leases are amortized over the shorter of the estimated useful lives of the assets or the lease terms. Construction in process assets are stated at cost and will be depreciated over their estimated useful lives once placed in service.
Expenditures for repairs and maintenance are charged to expense as incurred. Upon disposition of an asset, the cost and related accumulated depreciation are removed from the accounts and the resulting gain or loss is reflected in the consolidated statement of operations.
Impairment of Long-Lived Assets
The Company’s long-lived assets and other assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. Recoverability of an asset to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the asset. If such asset is considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the asset exceeds its fair value. Through December 31, 2017, the Company had not experienced impairment losses on its long-lived assets.
Fair Value of Financial Instruments
The Company follows Financial Accounting Standards Board (“FASB”) Accounting Standards Codification Topic No. 820, Fair Value Measurement (“ASC 820”), which clarifies fair value as an exit price, establishes a hierarchal disclosure framework for measuring fair value, and requires extended disclosures about fair value measurements. The provisions of ASC 820 apply to all financial assets and liabilities measured at fair value.
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
As defined in ASC 820, fair value represents the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As a result, fair value is a market-based approach that should be determined based on assumptions that market participants would use in pricing an asset or a liability. As a basis for considering these assumptions, ASC 820 defines a three-tier value hierarchy that prioritizes the inputs used in the valuation methodologies in measuring fair value.
|
Level 1 – |
Quoted prices in active markets for identical assets or liabilities. |
|
|
Level 2 – |
Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
|
|
Level 3 – |
Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
The following table sets forth by level, within the fair value hierarchy, the Company’s assets measured at fair value on a recurring basis in the balance sheet as of the following dates (in thousands):
|
December 31, 2017 |
||||||||||||||||
|
Fair Value Measurements |
||||||||||||||||
|
Using Input Types |
||||||||||||||||
|
Level 1 |
Level 2 |
Level 3 |
Total |
|||||||||||||
| Cash and cash equivalents | $ | 4,162 | $ | 4,162 | ||||||||||||
|
Short-term investments |
18,428 | - | - | 18,428 | ||||||||||||
|
Total assets at fair value |
$ | 22,590 | $ | — | $ | — | $ | 22,590 | ||||||||
|
December 31, 2016 |
||||||||||||||||
|
Fair Value Measurements |
||||||||||||||||
|
Using Input Types |
||||||||||||||||
|
Level 1 |
Level 2 |
Level 3 |
Total |
|||||||||||||
|
Cash and cash equivalents |
$ | 11,477 | - | - | $ | 11,477 | ||||||||||
|
Total assets at fair value |
$ | 11,477 | $ | — | $ | — | $ | 11,477 | ||||||||
Long-term debt is valued at carrying value which is considered to be representative of its fair value based on current market rates available to the Company for comparable borrowing facilities (Level 2 measurement).
Revenue Recognition
The Company recognizes revenue from sales of its products in accordance with the Revenue Recognition Topic ASC 605. The Company recognizes revenue from product sales when the following four criteria are met: delivery has occurred, there is persuasive evidence of an arrangement, the fee is fixed or determinable, and collectability of the related receivable is reasonably assured. The Company recognizes revenue when title to the product and risk of loss transfer to customers, provided there are no remaining performance obligations required of the Company or any written matters requiring customer acceptance. Revenue recognition generally occurs upon either shipment or implantion of our device. In the United States, the Company offers a thirty day return policy and recognizes revenue net of sales returns and allowances. Appropriate reserves are established for anticipated sales returns based on historical experience, recent gross sales and any notification of pending returns. Actual sales returns in any future period are inherently uncertain and thus may differ from the estimates. If actual sales returns differ significantly from the estimates, an adjustment to revenue in the current or subsequent period would be recorded.
The Company has established an allowance for sales returns of $0.2 million as of December 31, 2017 recorded net against accounts receivable in the balance sheet. No amount was recorded as of December 31, 2016 as all sales were recorded in Australia.
Shipping and Handling Costs
Shipping and handling costs are included as a component of cost of goods sold.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are stated at cost, net of allowance for doubtful accounts and reserves for sales returns. Credit is extended to customers based on an evaluation of their financial condition and other factors. The Company does not charge interest on past due balances. The Company generally does not require collateral or other security to support accounts receivable. The Company performs ongoing credit evaluations of its customers and maintains an allowance for doubtful accounts.
The Company estimates its allowance for doubtful accounts by evaluating specific accounts where information indicates that customers may have an inability to meet their financial obligations and receivable amounts are outstanding for an extended period beyond the invoice terms. In these cases, the Company uses assumptions and judgment, based on the best available facts and circumstances, to either record a specific allowance against these customer balances or to write the balances off. The accounts receivable aging is reviewed on a regular basis and write-offs are recorded on a case-by-case basis net of any amounts that may be collected. Allowance charges are recorded as operating expenses. Based on the Company’s customer analysis, it did not have an allowance for doubtful accounts at December 31, 2017 and 2016.
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
Revenue and Receivables Concentration
No customer accounts for more than 10% of net revenues for year ended December 31, 2017, or of the accounts receivable balance as of December 31, 2017. Two customers each contributed 16% of the Company’s revenue for the year ended December 31, 2016 and two customers accounted for 33% of the accounts receivable balance at December 31, 2016. One customer contributed 24% of the Company’s revenue for the year ended December 31, 2015. U.S. product sales are to hospitals and accounted for 80% of total net revenues for the year ended December 31, 2017, with the remainder to hospitals in Australia. All product sales in 2016 and 2015 were to hospitals in Australia.
Reverse Stock Split
In May 2015 the Company’s stockholders approved a 5-for-1 reverse stock split of all outstanding common stock and all securities exercisable into common stock. All amounts for common stock, preferred stock conversion ratios, stock options and warrants in the consolidated financial statements have been retroactively adjusted to reflect the effect of the reverse stock split.
Stock-Based Compensation
Stock-based compensation is measured at the grant date based on the fair value of the award. The fair value of the award that is ultimately expected to vest is recognized as expense on a straight-line basis over the requisite service period, which is generally the vesting period. The expense recognized for the portion of the award that is expected to vest has been reduced by an estimated forfeiture rate. The forfeiture rate is determined at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
The Company uses the Black-Scholes option-pricing model (the “Black-Scholes model”) as the method for determining the estimated fair value of stock options.
Expected Term
The Company’s expected term represents the period that the Company’s stock-based awards are expected to be outstanding and is determined using the simplified method, which essentially equates to a weighted average of the vesting periods and total term of the award.
Expected Volatility
Expected volatility is estimated using comparable public company’s volatility for similar terms as the Company does not have a long enough operating period as a public company to estimate its own volatility. Over time as the Company develops its own volatility history it will begin to incorporate that history into its expected volatility estimates. Prior to the Company’s listing on the ASX it used the same methodology.
Expected Dividend
The Black-Scholes model calls for a single expected dividend yield as an input. The Company has never paid dividends and has no current plans to pay dividends on its common stock.
Risk-Free Interest Rate
The risk-free interest rate used in the Black-Scholes model is based on the U.S. Treasury zero coupon issues in effect at the time of grant for periods corresponding with the expected term of the option.
The Company recognizes the fair value of stock options granted to nonemployees as stock-based compensation expense over the period in which the related services are received.
Research and Development
Costs incurred in research and development activities (including clinical trials) are expensed as incurred. Research and development costs include, but are not limited to, payroll and personnel expenses, laboratory supplies, consulting costs, travel, parts and materials, equipment expenses, and equipment depreciation.
Income Taxes
The Company accounts for income taxes using the asset and liability method. Under this method, deferred income tax assets and liabilities are recorded based on the estimated future tax effects of differences between the financial statement and income tax basis of assets and liabilities. In addition, deferred tax assets are recorded for the future benefit of utilizing net operating loss and tax credit carryovers. Deferred tax assets and liabilities are measured using the enacted tax rates applied to taxable income. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is provided against the Company’s deferred income tax assets when it is more likely than not that the asset will not be realized.
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
Significant judgment is required in determining any valuation allowance recorded against deferred tax assets. In assessing the need for a valuation allowance, the Company considers all available evidence, including past operating results, estimates of future taxable income and the feasibility of tax planning strategies. In the event that the Company changes its determination as to the amount of deferred tax assets that are more likely than not to be realized, the Company will adjust its valuation allowance with a corresponding impact to the provision for income taxes in the period in which such determination is made.
The Company follows authoritative guidance regarding uncertain tax positions. This guidance requires that realization of an uncertain income tax position must be more likely than not (i.e. greater than 50% likelihood of receiving a benefit) before it can be recognized in the financial statements. The guidance further prescribes the benefit to be realized assumes a review by tax authorities having all relevant information and applying current conventions. The interpretation also clarifies the financial statement classification of tax related penalties and interest and sets forth disclosures regarding unrecognized tax benefits. The Company recognizes potential accrued interest and penalties related to unrecognized tax benefits as income tax expense.
Segments
The Company has determined the chief executive officer is the chief operating decision maker. The Company’s chief executive officer reviews financial information presented for purposes of assessing performance and making decisions on how to allocate resources. The Company has determined that it operates in a single reporting segment.
Basic and Diluted Net Loss Per Share
Basic net loss per share is computed by dividing the net loss by the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share is computed by giving effect to all potential shares of common stock, resulting from the conversion or exercise of stock options, stock warrants, convertible debt and convertible preferred stock to the extent dilutive. For the periods presented, all such common stock equivalents have been excluded from diluted net loss per share as the effect to net loss per share would be anti-dilutive.
Following is a table summarizing the potentially dilutive common shares that were excluded from diluted weighted-average common shares outstanding for the periods presented below (in thousands):
|
December 31, |
||||||||||||
|
2017 |
2016 |
2015 |
||||||||||
|
Shares of Class A common stock issuable upon conversion of warrants and convertible preferred stock |
615 | 337 | 387 | |||||||||
|
Potential Class A common stock excluded from diluted net loss per share |
6,521 | 5,356 | 4,171 | |||||||||
|
Basic and diluted net loss per Class A common share |
7,136 | 5,693 | 4,558 | |||||||||
Recent Accounting Pronouncements
In May 2017, the FASB issued Accounting Standards Update 2017-09, “Compensation—Stock Compensation (Topic 718): Scope of Modification Accounting,” that provides guidance about which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting. The new standard is effective for the Company in the first quarter of fiscal 2018. Early adoption is permitted. The new guidance must be applied on a prospective basis. The Company does not anticipate that the adoption of this standard will have a significant impact on our consolidated financial statements or the related disclosures.
In November 2016, the FASB issued ASU 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash, which requires that a statement of cash flows explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. The new guidance is effective for annual reporting periods beginning after December 15, 2017, and interim periods within that reporting period. Early adoption is permitted including adoption in an interim period. The Company does not anticipate that the adoption of this standard will have a significant impact on our consolidated financial statements or the related disclosures.
In August 2016, the FASB issued ASU 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments, which addresses certain issues where diversity in practice was identified and may change how an entity classifies certain cash receipts and cash payments on its statement of cash flows. The new guidance is effective for annual reporting periods beginning after December 15, 2017, and interim periods within that reporting period. Early adoption is permitted. The Company does not anticipate that the adoption of this standard will have a significant impact on our consolidated financial statements or the related disclosures.
In March 2016, the FASB issued ASU 2016-09, Improvements to Employee Share-Based Payment Accounting, which amends ASC Topic 718, Compensation—Stock Compensation. The new guidance simplifies several aspects of the accounting for employee share-based payment transactions for both public and nonpublic entities, including the accounting for income taxes, forfeitures, and statutory tax withholding requirements, as well as classification in the statement of cash flows. As the Company has elected to avail itself of the exemption permitting an emerging growth company to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies until such standards would otherwise apply to a private company, the new standard will be effective for the Company for the annual reporting period beginning after December 15, 2017, and interim periods within that reporting period. The Company does not anticipate that the adoption of this standard will have a significant impact on our consolidated financial statements or the related disclosures.
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
In February 2016, the FASB issued a new standard, Leases, ASC 842. Lessees will need to recognize all lease arrangements with terms longer than twelve months on their balance sheet as a right-of-use asset and a corresponding lease liability. For income statement purposes, the FASB retained a dual model, requiring leases to be classified as either operating or finance. Lessor accounting is similar to the current model. Existing sale-leaseback guidance, including guidance for real estate, is replaced with a new model applicable to both lessees and lessors. As the Company has elected to avail itself of the exemption permitting an emerging growth company to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies until such standards would otherwise apply to a private company, the new standard will be effective for the Company in the fiscal year beginning after December 15, 2019. The Company expects the valuation of our right-of-use assets and lease liabilities, previously described as operating leases, to approximate the present value of our forecasted future lease commitments. We are currently evaluating process and system changes required in order to comply with the measurement and disclosure requirements.
In May 2014, the FASB issued ASU No. 2014-09, “Revenue from Contracts with Customers (Topic 606)”. The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should 1) identify the contract(s) with a customer, 2) identify the performance obligations in the contract, 3) determine the transaction price, 4) allocate the transaction price to the performance obligations in the contract, and 5) recognize revenue when (or as) the entity satisfies a performance obligation. In July 2015, the FASB deferred for one year the effective date of the new revenue standard, but early adoption will be permitted. As the Company has elected to avail itself of the exemption permitting an emerging growth company to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies until such standards would otherwise apply to a private company, the new standard will be effective for the Company on January 1, 2019. The Company does not anticipate that the adoption of this standard will have a significant impact on our consolidated financial statements or the related disclosures.
NOTE 4 – INVENTORY
Inventory consisted of the following as of December 31 (in thousands):
|
2017 |
2016 |
|||||||
|
Raw materials |
$ | 1,777 | $ | 760 | ||||
|
Work in progess |
4,863 | 439 | ||||||
|
Finished goods |
1,492 | 214 | ||||||
|
Inventory |
$ | 8,132 | $ | 1,413 | ||||
The Company had written down its inventory to market value by $3.0 million, $1.5 million, $0.5 million for the years ended December 31, 2017, 2016 and 2015, respectively.
NOTE 5 – PROPERTY AND EQUIPMENT
Property and equipment consisted of the following as of December 31 (in thousands):
|
2017 |
2016 |
|||||||
|
Machinery and equipment |
$ | 3,356 | $ | 1,084 | ||||
|
Computer equipment |
284 | 161 | ||||||
|
Furniture and fixtures |
386 | 84 | ||||||
|
Leasehold improvements |
810 | 170 | ||||||
|
Software licenses |
284 | 189 | ||||||
|
Office equipment |
23 | 11 | ||||||
|
Construction in progress |
651 | 872 | ||||||
|
Property and equipment, gross |
5,794 | 2,571 | ||||||
|
Accumulated depreciation |
(1,359 | ) | (692 | ) | ||||
|
Property and equipment, net |
$ | 4,435 | $ | 1,879 | ||||
Depreciation and amortization expense amounted to $0.7 million, $0.2 million and $0.1 million for the years ended December 31, 2017, 2016 and 2015, respectively.
NOTE 6 – ACCRUED EXPENSES
Accrued expenses consisted of the following as of December 31 (in thousands):
|
2017 |
2016 |
|||||||
|
Accrued compensation and benefits |
$ | 967 | $ | 425 | ||||
|
Accrued rent payable |
204 | 69 | ||||||
|
Accrued clinical trials services |
75 | 177 | ||||||
|
Accrued inventory supplies |
391 | 93 | ||||||
|
Accrued other |
884 | 152 | ||||||
|
Total accrued expenses |
$ | 2,521 | $ | 916 | ||||
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
NOTE 7 – DEBT FINANCING
Convertible Bridge Notes Payable
In February and June 2015, the Company raised through a private placement of convertible bridge notes payable a total of $5.0 million in net cash proceeds. The convertible bridge notes had a stated interest rate of 7% per annum. All the outstanding convertible bridge notes payable and accrued unpaid interest of $0.1 million were converted into 4,412,474 shares of common stock as part of Company’s IPO in June 2015.
January 2014 Debt
In January 2014, the Company borrowed $3.5 million under a loan and security agreement with a financial institution which matured in July 2017. Interest was paid monthly on the principal amount at 7.34% per annum. The loan was secured by substantially all of the Company’s assets, excluding intellectual property. Under the terms of the agreement and amendment, interest-only payments were made monthly through April 2015, with principal payments commencing in May 2015, due in 27 equal monthly installments. A fee of $271,250 was due at maturity, which was accrued over the term of the loan.
In connection with the loan agreement and security agreement, the Company granted a warrant to the financial institution for the purchase of 52,500 shares of Series E convertible preferred stock (“Series E”) at $1.00 per share. As a result of the Company’s IPO in June 2015 and conversion of all outstanding preferred stock into common stock, the warrants were converted into warrants for 52,500 shares of common stock at an exercise price of $1.00 per share. The fair value of the warrant of $32,000 on the date of issuance was recorded as a debt discount.
The Company recorded $35,000, $35,000 and $37,000 to interest expense related to amortization of the debt discount and issuance costs for the years ended December 31, 2017, 2016 and 2015, respectively.
The Company recorded $0.1 million, $0.2 million and $0.3 million of interest expense on the loans for the years ended December 31, 2017, 2016 and 2015, respectively.
The loan was paid in full at maturity in July 2017. No amounts are available to borrow under the agreement.
August 2017 Debt
In August 2017, the Company borrowed $15.0 million under a loan and security agreement with a financial institution which matures in August 2022. Interest is paid monthly on the principal amount at a variable rate equal to the greater of (a) the thirty day LIBOR rate, or (b) 0.99%, plus 7.26% per annum. The loan is secured by substantially all of our assets, excluding intellectual property, which intellectual property is subject to a negative pledge in favor of the financial institution. Under the terms of the agreement, interest-only payments are due monthly through September 2019, with principal payments commencing in October 2019, due in 35 equal monthly installments. If we are in compliance with certain financial milestones, the interest-only payments can be extended by twelve months through September 2020, in which case the principal payments would commence in October 2020, due in 23 equal monthly installments. A final fee of $1.2 million is due at maturity (or acceleration or prepayment). Subject to a prepayment fee equal to between 0.5% to 2.0% of the principal amount of the prepaid amount, we can prepay the entire loan amount by providing a written five-day notice prior to such prepayment and paying all outstanding principal, interest, final payment fees and prepayment fees plus any default fees and all other sums that shall have become due and payable.
Under the loan and security agreement the Company is required to maintain a certain minimum level of revenues on a trailing six-month basis, subject to quarterly measurement through 2018, and monthly thereafter, in addition to complying with certain other covenants. The loan and security agreement also includes events of default, the occurrence and continuation of any of which provides the financial institution with the right to exercise remedies against us and the collateral securing the loans, including cash. These events of default include, among other things, the failure to pay amounts due under the credit facilities, insolvency, the occurrence of a material adverse event, which includes a material adverse change in our business, operations or properties (financial or otherwise) or a material impairment of the prospect of repayment of any portion of the obligations. A violation of any of these covenants or the occurrence of a material adverse change could result in a default under the loan and security, which would result in termination of all commitments and loans under the agreement and all amounts owing under the agreement to become immediately due and payable. As of September 30, 2017, the Company was in violation of one of these covenants. On November 9, 2017, the Company and the financial institution entered into the waiver and first amendment to the loan and security agreement, pursuant to which it received a waiver of the event of default for the September 30, 2017 noncompliance with a financial covenant, and modified certain financial covenants. We are in compliance with our loan covenants as of December 31, 2017.
In connection with the loan and security agreement, the Company issued warrants to the financial institution for the purchase of 277,778 shares of its Common Stock with an exercise price of $1.62 per share. The fair value of the warrants of $0.2 million on the date of issuance was recorded as additional debt discount.
The Company recorded $0.2 million to interest expense related to amortization of the debt discount, issuance costs and the final fee for the year ended December 31, 2017. As of December 31, 2017, the unamortized discount and issuance cost is $0.5 million.
The Company recorded $0.7 million of interest expense on the loans for the year ended December 31, 2017. At December 31, 2017, $15.0 million was outstanding under this loan and security agreement.
As of December 31, 2017, the future principal payments, including the final fee, due under the loan and security agreement are (in thousands):
|
Year ending December 31, |
||||
|
2018 |
$ | |||
|
2019 |
1,714 | |||
| 2020 | 5,143 | |||
| 2021 | 5,143 | |||
|
2022 |
4,200 | |||
|
Total |
$ | 16,200 |
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
NOTE 8 – COMMITMENTS AND CONTINGENCIES
The Company leases approximately 15,000 square feet for office, research and development and manufacturing operations in Palo Alto, California under a non-cancelable operating lease. The term of the lease expires on September 30, 2019. In April 2017, The Company signed a non-cancelable operating sublease for an additional approximately 24,000 square foot facility in San Jose, California. The sublease expires in August 2019. The Company maintains a sales office in Sydney, Australia under a non-cancelable lease that expires in April 2020. The terms of the leases provide for rental payments on a graduated scale. The Company recognizes rent expense on a straight-line basis over the lease term. Rent expense for the years ended December 31, 2017, 2016 and 2015 was $0.5 million, $0.4 million and $0.2 million, respectively.
As of December 31, 2017, the future rental commitments due under the Company's operating leases are (in thousands):
|
Year ending December 31, |
||||
|
2018 |
$ | 835 | ||
|
2019 |
681 | |||
|
2020 |
18 | |||
|
Total |
$ | 1,534 |
The Company maintains an inventory purchase agreements with its third-party contract manufacturer in Costa Rica. The Company’s liability under this purchase commitment is generally restricted to a forecasted three month period. The Company estimates its open inventory purchase commitment as of December 31, 2017 was approximately $2.0 million. The Company recorded a $0.3 million inventory write-down to market value related to this commitment, which is included in accrued expenses in the accompanying consolidated balance sheet.
Indemnifications
The Company has agreed to indemnify its officers and directors for certain events or occurrences arising as a result of the officers or directors serving in such capacity. The Company has a directors and officers’ liability insurance policy that limits its exposure and enables the Company to recover a portion of any future amounts paid resulting from the indemnification of its officers and directors. In addition, the Company enters into indemnification agreements with other parties in the ordinary course of business. The Company has not incurred material costs to defend lawsuits or settle claims related to these indemnification agreements. The Company’s management believes the estimated fair value of these indemnification agreements is minimal and has not recorded a liability for these agreements as of December 31, 2017 and 2016.
Contingencies
From time to time we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not presently a party to any legal proceedings that, if determined adversely to us, would individually or taken together have a material adverse effect on our business, operating results, financial condition or cash flows.
Royalties
The Company uses AeroForm technology in the products it is developing. AeroForm embodies inventions that have been patented in certain key jurisdictions. Certain of those patents are held by Shalon Ventures (either alone or jointly with AirXpanders). Shalon Ventures and AirXpanders have entered into a License Agreement dated March 9, 2005 (as amended on March 9, 2009, January 9, 2012 and January 15, 2014) in relation to those inventions (Shalon Ventures License Agreement). Pursuant to the Shalon Ventures License Agreement, Shalon Ventures granted AirXpanders an exclusive license to develop, make, have made, use, offer for sale, sell, have sold, import and export products that, but for the license, would infringe one or more claims of the patents. The license covers all human uses of self-expanding tissue expanders anywhere in the world and includes the right to sublicense.
In consideration for the license, AirXpanders pays Shalon Ventures a running royalty of 3% of net sales of the licensed invention. If the amount of royalties paid in a calendar year is less than $10,000, then AirXpanders shall also pay Shalon Ventures’ out of pocket costs for prosecuting and maintaining the relevant patents. Each party indemnifies the other for any liability arising out of its material breach of the license, or its gross negligence, intentional misconduct and illegal actions. AirXpanders also indemnifies Shalon Ventures for any liability arising out of the commercialization of products using the license. Through the years ended December 31, 2017, 2016, and 2015, the Company has incurred $0.1 million, $17,000 and $9,000, respectively, in royalty fees, which is included in cost of goods sold in the accompanying consolidated financial statements. Mr. Teddy Shalon and Mr. Barry Cheskin are stockholders of the Company. Mr. Cheskin is also the co-founder and chairman of the board of the Company. Mr. Shalon is the Chief Executive Officer and sole shareholder of Shalon Ventures. Mr. Shalon and Mr. Cheskin are each party to an agreement with Shalon Ventures, under which Shalon Ventures has agreed to pay Mr. Shalon 58%, and Mr. Cheskin 8%, of any royalties due to Shalon Ventures from AirXpanders under the Shalon Ventures License Agreement.
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
NOTE 9 – COMMON STOCK
In May 2015, the Company’s stockholders approved a 5-for-1 reverse stock split of all outstanding common stock and all securities exercisable into common stock.
The Company’s Certificate of Incorporation, as amended, authorize the Company to issue 300,000,000 shares of $0.001 par value common stock consisting of 200,000,000 shares of common stock Class A and 100,000,000 shares of common stock Class B. Class A Common Stockholders are entitled to dividends when and if declared by the Board of Directors, Class B common stockholders are not entitled to any dividends. The holder of each share of Class A Common Stock is entitled to one vote and holders of Class B common stock are not entitled to vote. At December 31, 2017 and 2016, no dividends had been declared for common stock. At December 31, 2017, 95,943,409 and no shares of common stock Class A and Class B, respectively, were issued and outstanding.
As of December 31, 2017 and 2016, common stock that the Company had reserved for issuance was as follows (in thousands):
|
2017 |
2016 |
|||||||
|
Warrants for common and convertible preferred shares |
615 | 337 | ||||||
|
Stock option plans |
8,147 | 6,957 | ||||||
|
Total |
8,762 | 7,294 | ||||||
In June 2015, the Company issued 29,629,654 shares of common stock in connection with an IPO on the ASX and conversion of convertible bridge notes payable and related accrued interest. The Company raised a total of $30.1 million, net of issuance costs of $2.9 million. Of this amount $28.0 million (AU$36.5 million) were cash proceeds directly from the IPO; $5.0 million were net cash proceeds from the private placement of convertible bridge notes payable.
In June 2016, the Company issued 8,771,930 shares of common stock in connection with an equity offering on the ASX. The Company cash proceeds were $14.2 million, net of issuance costs of $0.7 million.
In February 2017, the Company issued 16,304,348 shares of common stock in connection with an equity offering on the ASX. The Company raised a total of $32.6 million, net of issuance costs of $1.5 million.
NOTE 10 – CONVERTIBLE PREFERRED STOCK
The Company issued Series A, B, B-1, C, D and E convertible preferred stock during the period from 2005 through 2014, raising a total of $30.4 million in cash, net of issuance costs of $0.6 million.
As part of Company’s IPO in June 2015, all outstanding convertible preferred stock was converted into common stock. Series A, B, B-1 and E convertible preferred stock were converted to common stock at a 1:1 ratio, Series C convertible preferred stock was converted to common at a 1.25:1 ratio and Series D convertible preferred stock was converted to common at a 1.35:1 ratio. After conversion of all preferred stock to common stock, the Company cancelled all authorized series of preferred stock.
The Company’s Certificate of Incorporation, as amended, authorize the Company to issue 10,000,000 authorized shares of preferred stock, with rights and privileges for preferred stock to be determined by Company’s Board of Directors before issuing preferred shares. At December 31, 2017, there are no outstanding shares of preferred stock.
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
NOTE 11 – WARRANTS
The Company accounts for warrants in accordance with ASC 480, “Distinguishing Liabilities from Equity” (“ASC 480”). Under ASC 480, warrants containing certain features, such as put rights and anti-dilution protection, are required to be accounted for as liabilities and recorded at fair value, with changes in fair value being recorded in the consolidated statement of operations. The Company’s preferred stock warrants prior to conversion to common stock warrants had contained such features, requiring liability accounting.
As part of Company’s IPO in June 2015, all outstanding preferred stock warrants were converted into warrants for common stock. As a result of this conversion, the warrants liabilities for $0.1 million were reclassified to additional paid in capital. The Company is required to reserve authorized but unissued shares of its common stock in an amount equal to the number of warrant shares purchasable under the arrangements described below.
The warrant liabilities were revalued at the end of each reporting period and through June 2015 the date of conversion to common stock warrants with the changes in fair value recorded in other income (expense) in the statements of operations.
The changes in fair value of these warrants recorded as other income (expense) for the year ended December 31, 2015 totaled $42,000.
The fair value of the warrant liabilities was estimated using Black-Scholes model using the following assumptions:
|
2015 |
||||||
|
Stock price |
$1.16 | |||||
|
Expected terms (years) |
0.62 | — | 8.67 | |||
|
Volatility |
33.06 | — | 43.08% | |||
|
Risk-free rate |
0.24 | — | 1.99% | |||
|
Dividend yield |
—% | |||||
In connection with the loan and security agreement, the Company issued warrants to the financial institution for the purchase of 277,778 shares of its Common Stock with an exercise price of $1.62 per share. The fair value of the warrants of $0.2 million on the date of issuance was recorded as additional debt discount. The Company assessed these warrants and determined they were instruments that qualified for equity classification.
The fair value of the warrant issued in connection with the loan and security agreement was estimated using Black-Scholes model using the following assumptions:
|
2017 Warrant |
|||
|
Stock price |
$1.62 | ||
|
Expected terms (years) |
10 | ||
|
Volatility |
35% | ||
|
Risk-free rate |
2.3% | ||
|
Dividend yield |
—% |
The Company’s outstanding warrants consisted of the following as of December 31 (in thousands):
|
2017 |
2016 | |||||||
|
Warrants; exercisable in common stock, exercise price $0.05 per share, expiring in June 2018 |
119 | 119 | ||||||
|
Warrants; exercisable in common stock, exercise price $0.05 per share, expiring in October 2018 |
5 | 5 | ||||||
|
Warrants; exercisable in common stock, exercise price $1.25 per share, expiring in February 2021 |
120 | 120 | ||||||
|
Warrants; exercisable in common stock, exercise price $1.00 per share, expiring in January 2023 |
40 | 40 | ||||||
|
Warrants; exercisable in common stock, exercise price $1.00 per share, expiring in January 2024 |
53 | 53 | ||||||
|
Warrants; exercisable in common stock, exercise price $1.62 per share, expiring in August 2027 |
278 | — | ||||||
|
Total |
615 | 337 | ||||||
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
NOTE 12 – STOCK-BASED COMPENSATION
In March 2005, the Company adopted the 2005 Equity Incentive Plan (the “2005 Plan”). In May 2015 the Company adopted the 2015 Equity Incentive Plan (the “2015 Plan”) collectively, (the “Plans”). The Plans provide for the granting of stock options to employees and consultants of the Company. Options granted under the Plan may be either incentive stock options or nonqualified stock options. Incentive stock options (ISO) may be granted only to Company employees (including officers and directors who are also employees). Nonqualified stock options (NSO) may be granted to Company employees and consultants.
In May 2013, the 2005 Plan was amended to increase the number of shares reserved for issuance under the Plan to 6,170,159 shares of common stock.
During the year ended December 31, 2015, the 2005 Plan expired and no future options can be granted under the 2005 Plan.
The Company has reserved 1,500,000 shares under the 2015 Plan in addition a total of 4,099,835 shares reserved under the 2005 Plan that will be added to 2015 plan if and when the underlying options are cancelled. Pursuant to the 2015 Plan’s “evergreen” provision, on the first day of each calendar year beginning in 2016, the number of shares reserved and available for issuance will be increased by an amount equal to 2.0% of the total number of shares of capital stock outstanding on December 31st of the preceding calendar year, subject to a cap equal to 10% of the fully diluted number of shares of capital stock of the Company as of the same date.
Options under the Plans may be granted for periods of up to 10 years and at prices no less than 100% of the estimated fair value of the shares on the date of grant. In the case of an Incentive Stock Option granted to a holder who, at the time the Option is granted, owns stock representing more than 10% of the voting power of all classes of stock of the Company, the term of the Option shall be up to 5 years from the date of grant and at no less than 110% of the estimated fair value of the shares on the date of grant. Options granted generally vest 1/4 on the 12-month anniversary of the vesting commencement date and 1/48 on each monthly anniversary thereafter.
At December 31, 2017 and 2016, 6,387,060 and 5,216,327 options were vested and expected to vest with a weighted-average exercise price of $1.18 and $0.38 and weighted average remaining contractual life of 6.8 and 6.6 years, respectively. The weighted average grant date fair value per share of options granted during the years ended December 31, 2017, 2016 and 2015 was $0.67, $0.86, and $0.34, respectively. The fair value of shares vested during the years ended December 31, 2017, 2016 and 2015 was $0.6 million, $0.2 million and $0.1 million, respectively. The intrinsic value of the options exercised during the years ended December 31, 2017, 2016 and 2015 was $0.8 million, $0.1 million and $5,000, respectively.
In connection with the grant of stock options to employees and non-employees, the Company recorded stock compensation expense as follows (in thousands):
|
Years Ended December 31, |
||||||||||||
|
2017 |
2016 |
2015 |
||||||||||
|
Cost of goods sold |
$ | 101 | $ | 44 | $ | — | ||||||
|
Research and development |
51 | 32 | — | |||||||||
|
Selling, general and administrative |
555 | 301 | 111 | |||||||||
|
Total |
$ | 707 | $ | 377 | $ | 111 | ||||||
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
As of December 31, 2017, unrecognized compensation expense related to employees and to non-employees totaled $1.7 million and $1.0 million, respectively, and will be recognized over approximately 2.6 years and 3.1 years, respectively.
Activity under the Plan is set forth below:
|
Weighted |
Weighted |
|||||||||||||||
|
Average |
Average |
|||||||||||||||
|
Options |
Number of |
Exercise |
Remaining |
|||||||||||||
|
Available |
Options |
Price |
Contractual |
|||||||||||||
|
for Grant |
Outstanding |
per Share |
Life in Years |
|||||||||||||
|
Balance — December 31, 2014 |
1,516,823 | 3,845,709 | $ | 0.30 | 7.33 | |||||||||||
|
Additional shares reserved (net of released) |
231,052 | — | $ | - | ||||||||||||
|
Options granted |
(357,127 | ) | 357,127 | $ | 0.90 | |||||||||||
|
Options exercised |
— | (2,965 | ) | $ | 0.18 | |||||||||||
|
Options forfeited/cancelled |
28,579 | (28,579 | ) | $ | 0.95 | |||||||||||
|
Balance — December 31, 2015 |
1,419,327 | 4,171,292 | $ | 0.33 | 6.57 | |||||||||||
|
Additional shares reserved (net of released) |
1,408,544 | — | $ | - | ||||||||||||
|
Options granted |
(1,531,169 | ) | 1,531,169 | $ | 2.44 | |||||||||||
|
Options exercised |
— | (42,583 | ) | $ | 0.25 | |||||||||||
|
Options forfeited/cancelled |
304,176 | (304,176 | ) | $ | 0.60 | |||||||||||
|
Balance — December 31, 2016 |
1,600,878 | 5,355,702 | $ | 0.92 | 6.60 | |||||||||||
| Additional shares reserved (net of released) | 1,584,834 | |||||||||||||||
|
Options granted |
(1,877,517 | ) | 1,877,517 | $ | 1.88 | |||||||||||
|
Options exercised |
— | (421,837 | ) | $ | 0.28 | |||||||||||
| Shares traded for option exercises | 27,771 | — | ||||||||||||||
|
Options forfeited/cancelled |
290,236 | (290,236 | ) | $ | 1.13 | |||||||||||
|
Balance — December 31, 2017 |
1,601,202 | 6,521,146 | $ | 1.19 | 6.90 | |||||||||||
The fair value of options granted to employees and non-employees was estimated at the date of grant using the following assumptions for the years ended December 31:
|
2017
|
2016
|
2015
|
||||||||||||||||
|
Expected terms (years) |
5.27 | — | 10.0 | 5.54 | — | 6.08 | 5.83 | — | 6.58 | |||||||||
|
Volatility |
31.9 | — | 41.0% | 33.73 | — | 34.87% | 34.91 | — | 43.52% | |||||||||
|
Risk-free rate |
1.83 | — | 2.4% | 1.18 | — | 1.86% | 1.44 | — | 1.85% | |||||||||
|
Dividend yield |
—% | —% | —% | |||||||||||||||
For non-employees, the Company revalues the stock option at each reporting date and recognizes the calculated fair value as stock-based compensation expense over the period in which the related services are received.
NOTE 13 – INCOME TAXES
The net loss for the following years consist of the following (in thousands):
|
Years Ended December 31, |
||||||||||||
|
2017 |
2016 |
2015 |
||||||||||
|
Domestic |
$ | (26,124 | ) | $ | (17,381 | ) | $ | (10,068 | ) | |||
|
Foreign - branch |
(2,859 | ) | (2,041 | ) | (1,093 | ) | ||||||
|
Total |
$ | (28,983 | ) | $ | (19,422 | ) | $ | (11,161 | ) | |||
The Company had an effective tax rate of zero percent in each of the three years ended December 31, 2017, 2016, and 2015, respectively. The provision for income taxes in the statement of operations is comprised of minimum state taxes.
Reconciliations of the provision for income taxes at the statutory rate to the Company’s provision for income tax are as follows (in thousands):
|
Years Ended December 31, |
||||||||||||
|
2017 |
2016 |
2015 |
||||||||||
|
U.S. Federal (tax benefit) provision at statutory rate |
$ | (9,854 | ) | $ | (6,604 | ) | $ | (3,795 | ) | |||
|
State (tax benefit) income taxes, net of federal benefit |
(1,139 | ) | — | (83 | ) | |||||||
|
Stock-based compensation |
140 | 53 | 27 | |||||||||
|
Change in valuation allowance |
210 | 6,813 | 4,064 | |||||||||
|
Research and development credits |
(252 | ) | (277 | ) | (224 | ) | ||||||
| Tax Rate Differential Impact, Tax Cuts and Jobs Act | 10,851 | — | — | |||||||||
| Minimum state taxes | 1 | 1 | — | |||||||||
|
Other permanent differences |
44 | 15 | 11 | |||||||||
|
Total |
$ | 1 | $ | 1 | $ | — | ||||||
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
The significant components of the net deferred tax asset are as follows (in thousands):
|
December 31, |
||||||||
|
2017 |
2016 |
|||||||
|
Gross deferred income tax assets |
||||||||
|
Net operating loss carryforwards |
$ | 23,534 | $ | 23,641 | ||||
|
Research and development credit carryforwards |
1,400 | 1,053 | ||||||
|
Property and equipment (depreciation) |
(382 | ) | (46 | ) | ||||
|
Others |
1,428 | 1,411 | ||||||
|
Total deferred tax assets |
25,980 | 26,059 | ||||||
|
Valuation allowance |
(25,980 | ) | (26,059 | ) | ||||
|
Total |
$ | — | $ | — | ||||
On December 22, 2017, the Tax Cuts and Jobs Act (“Act”) was signed into law. Among other changes is a permanent reduction in the federal corporate income tax rate from 35% to 21% effective January 1, 2018. As a result of the reduction in the corporate income tax rate, the Company revalued its net deferred tax asset at December 31, 2017. This results in a reduction in the value of the Company’s net deferred tax asset of approximately $10.9 million, which was fully offset by the change in valuation allowance.
On December 22, 2017, the SEC staff issued Staff Accounting Bulletin No. 118 (SAB 118) to the accounting for certain income tax effects of the Act. The Company has computed reasonable estimates related to the income tax effects of the Act. The Company has considered tax law changes effective 2018. The Company is reporting the estimates as a provisional amount in its financial statements for which the accounting under ASC 740 is completed. Primarily due to the lack state issued guidance, the Company will finalize the calculation in 2018. The Company does not expect tax reform legislation to have a significant impact on the results of operations.
A valuation allowance has been recorded for the entire amount of the Company’s deferred tax assets as a result of uncertainties regarding the realization of the deferred tax assets. The change in the valuation allowance totaled $0.3 million, $7.4 million and $4.0 million for the years ended December 31, 2017, 2016, and 2015, respectively, principally due to increases in the valuation allowance associated with increased net operating losses. The 2017 change is primarily due to the change in the valuation allowance caused by tax effects of the current year net operating loss (“NOL”), $10.7 million, which is offset by the $10.9 million tax charge related to remeasurement of the deferred tax assets.
As of December 31, 2017, the Company had NOL carryforwards for federal, state and Australian income tax reporting purposes of approximately $91.9 million, $56.4[JS1] million, and $6.5 million, respectively. As of December 31, 2017, the Company also had Federal and California research and development tax credit carryforwards of approximately $0.8 million and $0.7 million, respectively. The Federal NOL and tax credit carryforwards will expire at various dates beginning in 2025 through 2037. The California NOL carryforwards will expire at various dates beginning in 2028 through 2037. The California research and development tax credit carryforwards are carried forward indefinitely. Subject to continuity of ownership requirements, Australian NOL are carried forward indefinitely.
Pursuant to Internal Revenue Code Sections 382 and 383, annual use of the Company’s NOL and tax credit carryforwards may be limited in the event a cumulative change in ownership of more than 50% occurs within a three-year period. The Company has not completed a study to assess whether an ownership change has occurred or whether there have been multiple ownership changes since the Company’s formation, primarily due to the complexity and cost associated with such a study; and the possibility that there may be additional ownership changes in the future. If the Company has experienced an ownership change, the utilization of the NOL or tax credit carryforwards to offset future taxable income and taxes, respectively, would be subject to an annual limitation. Any limitation may effectively eliminate of all or a portion of the NOL or tax credit carryforwards before utilization.
The Company maintains a full valuation allowance for its deferred tax assets due to its historical losses and uncertainties surrounding its ability to generate future taxable income to realize these assets. Due to the existence of the valuation allowance, future changes in any unrecognized tax benefits and recognizable deferred tax benefits after the completion of an ownership change analysis is not expected to impact its effective tax rate.
The following table displays by contributing factor the changes in the valuation allowance for deferred tax assets for the years ended (in thousands):
|
December 31, |
||||||||||||
|
2017 |
2016 |
2015 |
||||||||||
|
Balance at the beginning of the period |
$ | 26,059 | $ | 18,708 | $ | 14,675 | ||||||
|
Net operating loss generated |
10,398 | 6,079 | 3,593 | |||||||||
|
Research and development tax credit increase (decrease) |
252 | 252 | 211 | |||||||||
|
Depreciation and amortization increase (decrease) |
(574 | ) | 30 | (44 | ) | |||||||
| Tax Rate Differential Impact, Tax Cuts and Jobs Act | (10,851 | ) | — | — | ||||||||
|
Reserves and accruals increase |
696 | 990 | 273 | |||||||||
|
Balance at the end of the period |
$ | 25,980 | $ | 26,059 | $ | 18,708 | ||||||
AirXpanders, Inc.
Notes to Consolidated Financial Statements (Cont.)
The following table reflects changes in the unrecognized tax benefits since January 1, 2016 (in thousands):
|
December 31, |
||||||||
|
2017 |
2016 |
|||||||
|
Gross amount of unrecognized tax benefits as of the beginning of the period |
$ | 488 | $ | 356 | ||||
|
Increase related to current year tax provision |
116 | 132 | ||||||
|
Gross amount of unrecognized tax benefits as of the end of the period |
$ | 604 | $ | 488 | ||||
The Company’s major tax jurisdictions are the United States, California, and Australia. All the Company’s tax years from 2005 through 2017 will remain open for examination by the federal, state and foreign tax authorities for three and four years, respectively, from the date of utilization of any net operating loss or tax credits. The Company is not currently subject to income tax examinations by any authority.
NOTE 14 – RETIREMENT PLAN
The Company has a salary deferral plan under Section 401(k) of the Internal Revenue Code. The plan allows eligible employees to defer a portion of their compensation ranging from 1% to the maximum allowable dollar limit which is set by law. Such deferrals accumulate on a tax deferred basis until the employee withdraws the funds. The Company, at its option, may match a portion of the employees’ contribution. During the year ended December 31, 2017, the Company made matching contributions of $0.1 million. During the years ended December 31, 2016 and 2015, the Company made no matching contributions.
NOTE 15 – RELATED PARTIES
Mrs. Lynae Dodson, the wife of Mr. Scott Dodson, chief executive officer and director, owns and operates a marketing consulting firm, Bridge Marketing. Bridge Marketing manages trade shows, social media and provides other market services for the Company. During the years ended December 31, 2017, 2016, and 2015, Bridge Marketing received payments of $0.2 million in each year, respectively, for marketing support services rendered to the Company. These amounts are including in selling, general and administrative costs in the accompanying consolidated statements of operations.
See Note 8 of these Notes to the Consolidated Financial Statements for discussion regarding royalty payments to Shalon Ventures under a patent license agreement. Mr. Teddy Shalon is the Chief Executive Officer and sole shareholder of Shalon Ventures. Mr. Shalon and Mr. Barry Cheskin are each party to an agreement with Shalon Ventures, under which Shalon Ventures has agreed to pay Mr. Shalon 58%, and Mr. Cheskin 8%, of any royalties due to Shalon Ventures from AirXpanders. Mr. Shalon and Mr. Cheskin are stockholders of the Company. Mr. Cheskin is also the co-founder and chairman of the board of the Company. Mr. Shalon was a director of the Company through May 2017.
SUPPLEMENTAL FINANCIAL INFORMATION
Selected Quarterly Financial Results (Unaudited)
The following table sets forth selected consolidated quarterly results of operations for the years ended December 31, 2017 and 2016 (in thousands, except share and per share amounts):
|
|
|
Quarters Ended |
|
|||||||||||||
|
|
|
December 31, 2017 |
|
|
September 30, 2017 |
|
|
June 30, 2017 |
|
|
March 31, 2017 |
|
||||
|
Net revenues |
|
$ |
1,789 |
|
$ |
1,159 |
|
|
$ |
701 |
|
|
$ |
257 |
|
|
|
Gross loss |
|
$ |
(1,037 |
) |
|
$ |
(479 |
) |
|
$ |
(1,995 |
) |
|
$ |
(1,773 |
) |
|
Operating loss |
|
$ |
(7,231 |
) |
|
$ |
(5,802 |
) |
|
$ |
(8,038 |
) |
|
$ |
(7,332 |
) |
|
Net loss |
|
$ |
(7,664 |
) |
|
$ |
(5,999 |
) |
|
$ |
(8,022 |
) |
|
$ |
(7,298 |
) |
|
Net loss per common share - basic and diluted |
|
$ |
(0.08 |
) |
|
$ |
(0.06 |
) |
|
$ |
(0.08 |
) |
|
$ |
(0.08 |
) |
|
Shares used in per common share computations - basic and diluted |
|
|
95,906,958 |
|
|
|
95,896,120 |
|
|
|
95,890,248 |
|
|
|
87,431,858 |
|
|
|
|
Quarters Ended |
|
|||||||||||||
|
|
|
December 31, 2016 |
|
|
September 30, 2016 |
|
|
June 30, 2016 |
|
|
March 31, 2016 |
|
||||
|
Net revenues |
|
$ |
195 |
|
$ |
159 |
|
|
$ |
122 |
|
|
$ |
94 |
|
|
|
Gross loss |
|
$ |
(1,159 |
) |
|
$ |
(1,121 |
) |
|
$ |
(756 |
) |
|
$ |
(937 |
) |
|
Operating loss |
|
$ |
(5,208 |
) |
|
$ |
(5,198 |
) |
|
$ |
(4,776 |
) |
|
$ |
(3,941 |
) |
|
Net loss |
|
$ |
(5,379 |
) |
|
$ |
(5,229 |
) |
|
$ |
(4,916 |
) |
|
$ |
(3,899 |
) |
|
Net loss per common share - basic and diluted |
|
$ |
(0.07 |
) |
|
$ |
(0.07 |
) |
|
$ |
(0.07 |
) |
|
$ |
(0.06 |
) |
|
Shares used in per common share computations - basic and diluted |
|
|
79,210,877 |
|
|
|
79,201,098 |
|
|
|
71,969,512 |
|
|
|
70,427,195 |
|
|
ITEM 9. |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
None.
|
ITEM 9A. |
CONTROLS AND PROCEDURES. |
Evaluation of Disclosure Controls and Procedures
As required by Rule 13a-15(b) of the Securities Exchange Act of 1934, or the Exchange Act, our management, including our principal executive officer and our principal financial officer, conducted an evaluation as of the end of the period covered by this Annual Report on Form 10-K of the effectiveness of the design and operation of our disclosure controls and procedures. Based on that evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures are effective at the reasonable assurance level in ensuring that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports we file under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Management's Report on Internal Controls Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act as the process designed by, or under the supervision of, our chief executive officer and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that:
(1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of assets;
(2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with the authorizations of management and directors; and
(3) provide reasonable assurance regarding the prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on our financial statements.
Under the supervision and with the participation of our management, including our Chief Executive Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework provided in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2017.
Changes in Internal Control
As required by Rule 13a-15(d) of the Exchange Act, our management, including our principal executive officer and our principal financial officer, conducted an evaluation of the internal control over financial reporting to determine whether any changes occurred during the quarter ended December 31, 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. Based on that evaluation, our principal executive officer and principal financial officer concluded no such changes during the quarter ended December 31, 2017 materially affected, or were reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Effectiveness of Controls and Procedures
Our management, including our principal executive officer and principal financial officer, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. Thus, misstatements due to error or fraud may occur and not be detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of controls.
|
ITEM 9B. |
OTHER INFORMATION. |
None.
PART III
|
ITEM 10. |
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE. |
The information required by this Item is incorporated by reference to our Proxy Statement for our 2018 Annual Meeting of Stockholders (the “Proxy Statement”) to be filed with the SEC not later than 120 days after the end of our fiscal year ended December 31, 2017, specifically:
|
|
• |
Information regarding our directors and any persons nominated to become a director, as well as with respect to some other required board matters, is set forth under Proposal 1 entitled “Election of Directors” and under “Corporate Governance.” |
|
|
• |
Information regarding our audit committee and our designated “audit committee financial expert” is set forth under the caption “Corporate Governance.” |
|
|
• |
Information on our code of business conduct and ethics for directors, officers and employees is set forth under the caption “Code of Ethics” under “Corporate Governance.” |
|
|
• |
Information regarding Section 16(a) beneficial ownership reporting compliance is set forth under the caption “Section 16(a) Beneficial Ownership Reporting Compliance.” |
|
|
• |
Information regarding procedures by which stockholders may recommend nominees to our board of directors is set forth under the caption “Nominating and Governance Committee” under “Corporate Governance.” |
Information regarding our executive officers is set forth at the end of Item 1, Part 1 of this Annual Report on Form 10-K under the caption “Executive Officers of the Registrant."
|
ITEM 11. |
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE. |
Information regarding compensation of our named executive officers is set forth under the caption “Executive Compensation” in the Proxy Statement, which information is incorporated herein by reference.
Information regarding compensation of our directors is set forth under the caption "Compensation of Directors" in the Proxy Statement, which information is incorporated herein by reference.
Information regarding compensation committee interlocks is set forth under the caption "Compensation Committee Interlocks and Insider Participation" in the Proxy Statement, which information is incorporated herein by reference.
The Compensation Committee Report is set forth under the caption "Compensation Committee Report" in the Proxy Statement, which report is incorporated herein by reference.
|
ITEM 12. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
Information regarding security ownership of certain beneficial owners, directors and executive officers is set forth under the caption "Security Ownership of Certain Beneficial Owners and Management" in the Proxy Statement, which information is incorporated herein by reference.
Equity Compensation Plan Information
The following table provides certain aggregate information with respect to all of the Company’s equity compensation plans in effect as of December 31, 2017.
|
|
Options, Warrants | |||||||||||
|
Number of |
Exercise |
and Rights |
||||||||||
|
Options |
Price |
Available |
||||||||||
|
Plan Category |
Outstanding(2) |
per Share |
For Grant(1) |
|||||||||
|
Equity compensation plans approved by security holders |
7,135,738 | $ | 1.19 | 1,626,202 | ||||||||
|
Equity compensation plans not approved by security holders |
— | — | — | |||||||||
|
Total |
7,135,738 | $ | 1.19 | 1,626,202 | ||||||||
|
(1) |
Consists of 1,626,202 shares of our common stock available for issuance under our 2015 Equity Incentive Plan and no shares of our common stock available for future issuance under our 2005 Equity Incentive Plan which was superseded by our 2015 Equity Incentive Plan. |
|
(2) |
Includes options to purchase 6,521,146 shares of our common stock issued under our 2005 and 2015 Equity Incentive Plan and warrants to purchase 614,592 shares of our common stock. |
|
ITEM 13. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE. |
Information regarding certain relationships and related transactions is set forth under the caption "Related Person Transaction Policy" under the caption "Corporate Governance" in the Proxy Statement, which information is incorporated herein by reference.
Information regarding director independence is set forth under the caption “Board of Directors Meetings and Committees” under “Corporate Governance” in the Proxy Statement, which information is incorporated herein by reference.
|
ITEM 14. |
PRINCIPAL ACCOUNTING FEES AND SERVICES. |
Information regarding principal auditor fees and services is set forth under the proposal entitled “Ratification of Appointment of Independent Registered Public Accounting Firm” in the Proxy Statement, which information is incorporated herein by reference.
PART IV
|
ITEM 15. |
EXHIBITS, FINANCIAL STATEMENT SCHEDULES. |
|
(a) |
The following documents are filed as part of this report on Form 10-K: |
|
|
(1) |
Consolidated Financial Statements. |
See Index to Consolidated Financial Statements in Item 8 on page 31 of this Annual Report on Form 10-K.
|
|
(2) |
Consolidated Financial Statement Schedule. |
All schedules have been omitted because they are not applicable or are not required or the information required to be set forth therein is included in the Consolidated Financial Statements or notes thereto.
|
|
(3) |
Exhibits. |
See Exhibit Index below of this Annual Report on Form 10-K, which is incorporated by reference here.
| Incorporation by Reference | |||||||||||
|
Exhibit No. |
Exhibit Description | Form | File Number | Exhibit/Appendix Reference | Filing Date | Filed Herewith | |||||
|
3.1 |
10-12G | 000-55781 | 3.1 | May 1, 2017 | |||||||
|
3.2 |
10-12G | 000-55781 | 3.2 | May 1, 2017 | |||||||
|
4.1 |
Reference is made to Exhibits 3.1 and 3.2 |
10-12G | 000-55781 | 4.1 | May 1, 2017 | ||||||
|
4.3 |
10-12G | 000-55781 | 4.3 | May 1, 2017 | |||||||
|
4.4 |
Warrant to Purchase Stock, dated January 31, 2013, between AirXpanders, Inc. and Oxford Finance LLC |
10-12G | 000-55781 | 4.4 | May 1, 2017 | ||||||
|
4.5 |
10-12G | 000-55781 | 4.5 | May 1, 2017 | |||||||
|
10.1* |
10-12G | 000-55781 | 10.1 | May 1, 2017 | |||||||
|
10.2* |
10-12G | 000-55781 | 10.2 | May 1, 2017 | |||||||
|
10.3* |
2015 Equity Incentive Plan and Australian Sub-Plan, as amended |
10-12G | 000-55781 | 10.3 | May 1, 2017 | ||||||
|
10.4* |
10-12G | 000-55781 | 10.4 | May 1, 2017 | |||||||
|
10.5 |
License Agreement, dated March 9, 2005, between AirXpanders, Inc. and Shalon Ventures, Inc. |
10-12G | 000-55781 | 10.7 | May 1, 2017 | ||||||
|
10.6 |
10-12G | 000-55781 | 10.8 | May 1, 2017 | |||||||
|
10.7 |
10-12G | 000-55781 | 10.9 | May 1, 2017 | |||||||
|
10.8 |
10-12G | 000-55781 | 10.10 | May 1, 2017 | |||||||
|
10.9 |
Standard Industrial Lease, dated July 14, 2010, between AirXpanders, Inc. and McCandless Limited |
10-12G | 000-55781 | 10.11 | May 1, 2017 | ||||||
|
10.10 |
First Amendment to Lease, dated May 1, 2013, between AirXpanders, Inc. and McCandless Limited |
10-12G | 000-55781 | 10.12 | May 1, 2017 | ||||||
|
10.11 |
Second Amendment to Lease, dated July 1, 2015, between AirXpanders, Inc. and McCandless Limited |
10-12G | 000-55781 | 10.13 | May 1, 2017 | ||||||
|
10.12 |
X | ||||||||||
EXHIBIT INDEX
(continued)
|
* |
Indicates management contract or compensatory plan. |
|
** |
Confidential treatment has been granted for certain portions of this exhibit. Omitted information has been filed separately with the Securities and Exchange Commission. |
|
ITEM 16. |
FORM 10-K SUMMARY. |
None.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
AirXpanders, Inc. (Registrant) |
||||
|
|
By: |
/s/ Scott Murcray |
|||
|
|
|
Name: |
Scott Murcray |
||
|
Date: February 27, 2018 |
|
Title: |
Chief Financial Officer and Chief Operating Officer |
||
| (Duly Authorized Officer, Principal Financial Officer and Principal Accounting Officer) | |||||
POWER OF ATTORNEY KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Scott Dodson and Scott Murcray, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution for him, and in his name in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, and any of them or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/S/ Scott Dodson |
|
President and Chief Executive Officer and Director |
|
February 27, 2018 |
|
Scott Dodson |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
/S/ Scott Murcray |
|
Chief Financial Officer and Chief Operating Officer |
|
February 27, 2018 |
|
Scott Murcray |
|
(Principal Financial Officer and Principal Accounting Officer) |
|
|
|
|
|
|
|
|
|
/S/ Barry Cheskin |
|
Chairman of the Board of Directors |
|
February 27, 2018 |
|
Barry Cheskin |
|
|
|
|
|
|
|
|
|
|
|
/S/ Gregory Lichtwardt |
|
Director |
|
February 27, 2018 |
| Gregory Lichtwardt |
|
|
|
|
|
|
|
|
|
|
|
/S/ Dennis Condon |
|
Director |
|
February 27, 2018 |
|
Dennis Condon |
|
|
|
|
|
|
|
|
|
|
|
/S/ Elizabeth Hammack |
|
Director |
|
February 27, 2018 |
|
Elizabeth Hammack |
|
|
|
|
|
|
|
|
|
|
|
/S/ Zita Peach |
|
Director |
|
February 27, 2018 |
|
Zita Peach |
|
|
|
|
