Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | amag8-kxq417earningsrelease.htm |
| EX-99.1 - EXHIBIT 99.1 - AMAG PHARMACEUTICALS, INC. | ex991.htm |

AMAG Pharmaceuticals
2017 Financial Results
February 27, 2018

Forward-Looking Statements
2
This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of
1995 (PSLRA) and other federal securities laws. Any statements contained herein which do not describe historical facts,
including, among others, beliefs about the data for the new broad iron deficiency anemia Ferhamem label and the impact of
reports of hypophosphatemia; expectations regarding the market opportunity for Feraheme, including with the new broad
label; plans for the new Feraheme label’s launch strategy and growth drivers, including AMAG’s ability to expand access,
reinforce and enhance differentiation and expand patient types; beliefs about the importance patients place on the
attributes of the Makena auto-injector; expectations that the Makena auto-injector will be used in a majority of treated
patients assuming price parity; beliefs that the Makena auto-injector could become the new standard of care; attributes that
differentiate Intrarosa from other therapies; beliefs about the market opportunity for Intrarosa; plans for AMAG’s campaign
to educate and expand awareness of dyspareunia and treatment options; the key growth drivers for Intrarosa; beliefs that
AMAG has a strong liquidity profile; AMAG’s 2018 financial guidance, including total revenue, operating loss and adjusted
EBITDA; and AMAG’s 2018 key priorities are forward-looking statements which involve risks and uncertainties that could
cause actual results to differ materially from those discussed in such forward-looking statements.
Such risks and uncertainties include, among others, the possibility that AMAG’s expectations as to its 2018 plans will not be
realized, expectations for the recently approved Makena subcutaneous auto-injector and the Feraheme label expansion will
not be achievable, and that the entrance of generics to the Makena intramuscular formulation could happen more quickly
than anticipated in light of the loss of exclusivity in February 2018, and those other risks identified in AMAG’s filings with the
U.S. Securities and Exchange Commission (the “SEC”), including its Annual Report on Form 10-K for the year ended December
31, 2016 and subsequent filings with the SEC, including its upcoming Annual Report on Form 10-K for the year ended
December 31, 2017. Any such risks and uncertainties could materially and adversely affect AMAG’s results of operations, its
profitability and its cash flows, which would, in turn, have a significant and adverse impact on AMAG’s stock price. AMAG
cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made
AMAG disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in
events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that
actual results will differ from those set forth in the forward-looking statements.
AMAG Pharmaceuticals® and Feraheme® are registered trademarks of AMAG Pharmaceuticals, Inc. MuGard® is a registered
trademark of Abeona Therapeutics, Inc. Makena® is a registered trademark of AMAG Pharma USA, Inc. Cord Blood Registry®
and CBR® are registered trademarks of CbrSystems, Inc. Intrarosa® is a trademark of Endoceutics, Inc.

Today’s Agenda
3
2017 Highlights and Recent Events1
4 Key Priorities and Closing Remarks
3 2017 Financial Overview
5 Q&A
2 Product Portfolio Update

2017 Highlights and
Recent Events

Solid Execution
Intrarosa
Launched Intrarosa; drove broad awareness and access
Phase 3 hypoactive sexual desire disorder (HSDD) study initiated by partner, Endoceutics
Bremelanotide
Completed clinical work with partner, Palatin, for planned NDA submission in Q1-2018
Launched HCP condition awareness campaign
Feraheme
Received FDA approval for broad IDA label (February 2, 2018)
Grew volume prior to FDA approval of expanded label
Makena
Received FDA approval for subcutaneous (SC) auto-injector (February 14, 2018)
Achieved record sales of $387.2 million, an increase of 16% over 2016
Cord Blood Registry
Increased storage revenue by $7.4 million, or 9%, over 2016
Grew new first-time enrollments by 4% over 2016
Business Development
Expanded product portfolio with Intrarosa and bremelanotide; established new
women’s health commercial team
Financial
Achieved record revenue of $610M, with revenue growth of every key AMAG product over 2016
Reduced debt by nearly 20%, ending 2017 with total debt of $816M and cash of $329M
5

Solid Execution
Intrarosa
Launched Intrarosa; drove broad awareness and access
Phase 3 hypoactive sexual desire disorder (HSDD) study initiated by partner, Endoceutics
Bremelanotide
Completed clinical work with partner, Palatin, for planned NDA submission in Q1-2018
Launched HCP condition awareness campaign
Feraheme
Received FDA approval for broad IDA label (February 2, 2018)
Grew volume prior to FDA approval of expanded label
Makena
Received FDA approval for subcutaneous (SC) auto-injector (February 14, 2018)
Achieved record sales of $387.2 million, an increase of 16% over 2016
Cord Blood Registry
Increased storage revenue by $7.4 million, or 9%, over 2016
Grew new first-time enrollments by 4% over 2016
Business Development
Expanded product portfolio with Intrarosa and bremelanotide; established new
women’s health commercial team
Financial
Achieved record revenue of $610M, with revenue growth of every key AMAG product over 2016
Reduced debt by nearly 20%, ending 2017 with total debt of $816M and cash of $329M
6

Strong 2017 Product Revenue Growth1
($M)
2016 2017
$531.8
$609.8
GAAP Product Revenue
14.7%
7
2016 2017
$548.8
$615.3
Non-GAAP Product Revenue2,3
12.1%
Makena revenue CBR revenueFeraheme/MuGard revenue Intrarosa revenue
1 Excludes “License fee, collaboration and other revenues” of $317,000 in 2016, and $124,000 in 2017.
2 Non-GAAP revenue includes purchase accounting adjustments related to CBR deferred revenue of $17M in 2016 and $5.5M in 2017.
3 See slide 34 for a reconciliation of GAAP to non-GAAP financials.

Treatment of iron
deficiency anemia (IDA)
in all eligible adult
patients
The only FDA-approved
therapy to reduce
recurrent preterm birth
in certain at-risk women
World’s largest umbilical
cord stem cell collection
and storage company
Candidate for the
treatment of severe
preeclampsia
An investigational
product for the
treatment of hypoactive
sexual desire disorder
(HSDD) in pre-
menopausal women
Management of oral
mucositis, a common
side effect of radiation or
chemotherapy
Maternal and Women’s HealthHematology /
Oncology Pregnancy
& Birth
Wellness
Post-Menopausal
Health
Velo
Option
Bremelanotide
FDA-approved locally
administered non-
estrogen1 product to
treat moderate to
severe dyspareunia
(pain during sex), a
symptom of vulvar and
vaginal atrophy (VVA),
due to menopause,
which does not carry a
boxed warning in its
label
81 Intrarosa is converted by enzymes in the body into androgens and estrogens, though the mechanism of action is not fully established.
AMAG’s Expanding Portfolio of Products

Product Portfolio Update
•Feraheme®
• Makena®
• Cord Blood Registry®
• Intrarosa®
9

FERAHEME® is the only IV
iron demonstrating effective
treatment for IDA in adult
patients with 1g of iron
across 2 fifteen-minute
infusions 3-8 days apart
10
Feraheme Broad IDA Label1 Approved February 2, 2018
Strong Data in New Broad
IDA Feraheme Label…
I V I R O N D E F I C I E N C Y A N E M I A : F E R A H E M E
1 Feraheme is indicated for the treatment of iron deficiency anemia (IDA) in adult patients who have intolerance to oral iron or have had
unsatisfactory response to oral iron or who have chronic kidney disease (CKD).
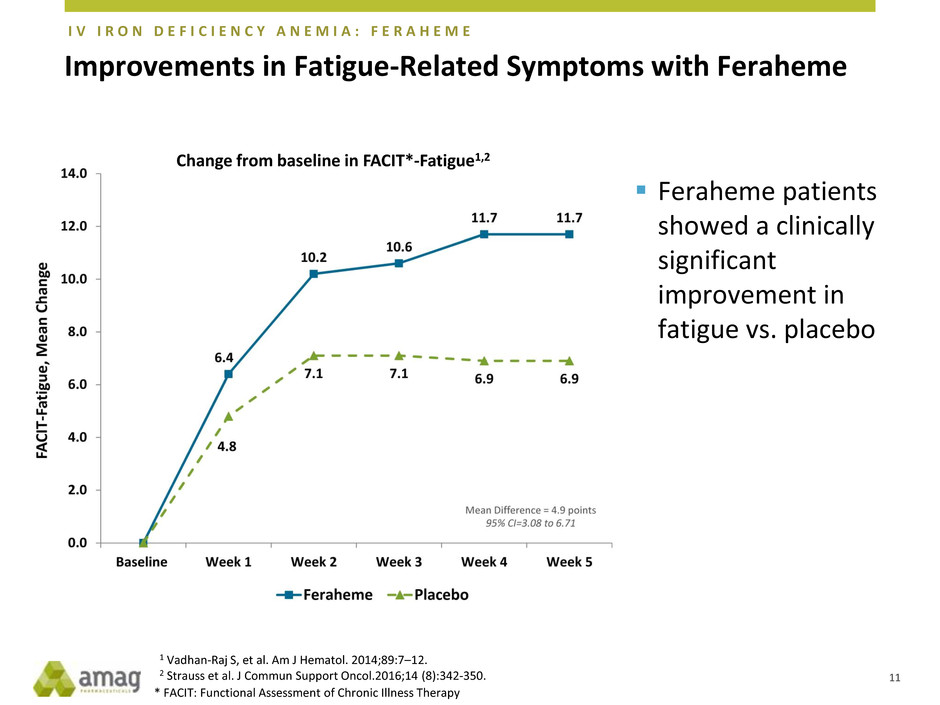
Improvements in Fatigue-Related Symptoms with Feraheme
11
I V I R O N D E F I C I E N C Y A N E M I A : F E R A H E M E
* FACIT: Functional Assessment of Chronic Illness Therapy
1 Vadhan-Raj S, et al. Am J Hematol. 2014;89:7–12.
2 Strauss et al. J Commun Support Oncol.2016;14 (8):342-350.
Change from baseline in FACIT*-Fatigue1,2
Feraheme patients
showed a clinically
significant
improvement in
fatigue vs. placebo

12
Lower Incidence of Severe Hypophosphatemia with Feraheme1
Hypophosphatemia can have important clinical implications2
– Acutely: muscle weakness and pain, fatigue
– Chronically: osteomalacia (impaired bone mineralization), bone pain, fractures, muscle weakness
I V I R O N D E F I C I E N C Y A N E M I A : F E R A H E M E
*** P<0.001, ANCOVA, Error bars represent 95% Confidence Interval
Shading represents lab normal reference range
Incidence of Serum Phosphate <0.6 mmol/L (2.0mg/dL) Mean Serum Phosphate (mg/dL)
1 Adkinson NF et al. Am J Hematol. 2018 Feb 8. doi: 10.1002/ajh.25060.
2 Blazevic A et al., The Netherlands Journal of Medicine, January 2014, Vol. 72, No. 1; Mani et al., Transplantation: October 15, 2010, Vol. 90, No. 7,
pp 804-805; Zoller H. et al, Curr Opin Nephrol Hypertens July 2017, Vol. 26, No. 4, pp.266-275.

Large Market Opportunity with New Broad Label
Feraheme
12%
Injectafer
29%
Other IV irons
59%
13
I V I R O N D E F I C I E N C Y A N E M I A : F E R A H E M E
Q4-2017 U.S. Non-dialysis IV Iron Market1
1 AMAG estimates current market using IQVIA data and internal analytics.
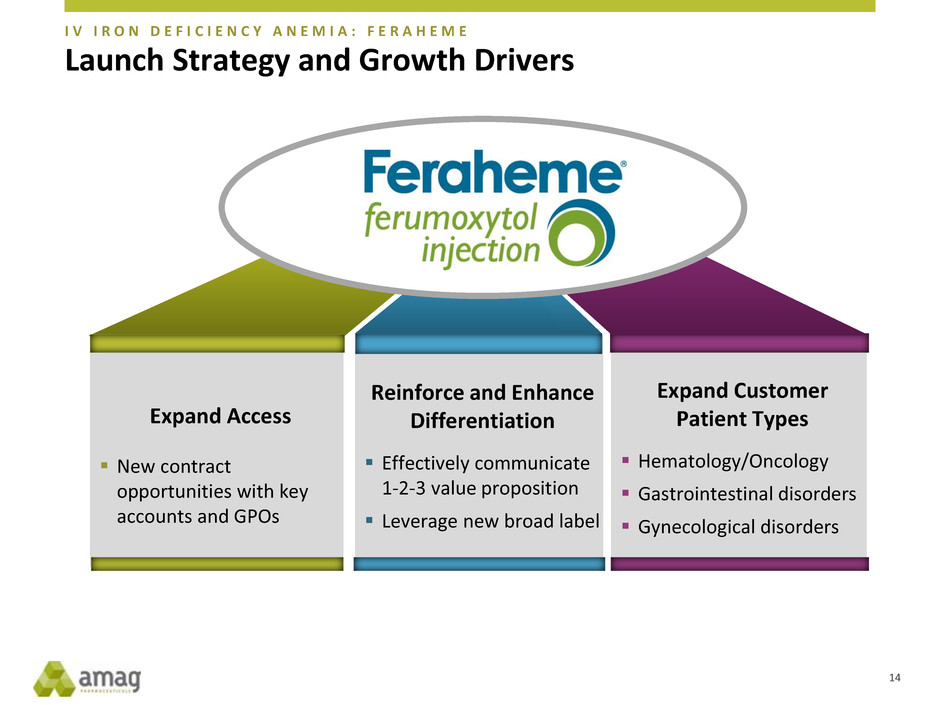
Launch Strategy and Growth Drivers
14
Expand Access
New contract
opportunities with key
accounts and GPOs
Expand Customer
Patient Types
Hematology/Oncology
Gastrointestinal disorders
Gynecological disorders
Reinforce and Enhance
Differentiation
Effectively communicate
1-2-3 value proposition
Leverage new broad label
I V I R O N D E F I C I E N C Y A N E M I A : F E R A H E M E

Product Portfolio Update
• Feraheme®
•Makena®
• Cord Blood Registry®
• Intrarosa®
15

Efficient
Discreet
Administration friendly
16
Makena SC Auto-injector Approved February 14, 2018
M A T E R N A L H E A L T H : M A K E N A
Subcutaneous injection Intramuscular injection

17
SC Attributes Important to Patients
1%
2%
10%
25%
28%
34%
0% 5% 10% 15% 20% 25% 30% 35% 40%
Site of injection
Needle thickness
Route of administration
Needle visibility
Needle length
Time for administration
Relative Importance
Statistically significant impact on overall preference
M A T E R N A L H E A L T H : M A K E N A
Top Drivers in Discreet Choice Analysis1
1 AMAG sponsored “Discrete Choice” patient survey conducted by Trinity Healthcare; n=183.

18
Makena SC Auto-injector Expected to be Used in
Majority of Treated Patients Assuming Price Parity1
M A T E R N A L H E A L T H : M A K E N A
6%
2%
2%
8%
83%
Future Prescribing3
(MDs, n=20)
Current Prescribing2
(HCPs, n=30)
8%
18%
67%
N/A
N/A Makena SC4
Makena IM
Generic HPC
single-dose vial4
Compounded
IM / HPC
Vaginal
Progesterone
Makena SC4
Makena IM
Generic HPC
single-dose vial4
Compounded
IM / HPC
Vaginal
Progesterone
Current and Future Use of Treatments to Reduce Risk of Preterm Birth
1 AMAG sponsored qualitative research conducted by Thinkgen.
2 Re-percentaged to exclude those patients who did not receive treatment. Respondents were asked “Of the at-risk patients you personally
managed in the past 12 months, what number received the following treatments? Your best estimate is fine.”
3 Respondents were asked to allocate their next 10 patients across treatments, adding the instruction after fielding began to assume that
Makena subcutaneous and a generic single-dose vial were available. Respondents were told not to consider product cost and coverage
when allocating future patients.
4 Included in the “Future Prescribing” allocation only.

19
Ensure SC Auto-injector Becomes the New Standard of Care
M A T E R N A L H E A L T H : M A K E N A
Expand use through
alternative treatment sites
(i.e. retail pharmacies)
Rapid and easy dispensing
of SC auto-injector
Key Imperatives Key Tactics
Manage access (price,
patient out-of-pocket,
reimbursement)
Easy access to SC auto-
injector with strong payer
value proposition
Effectively communicate the
benefits of SC auto-injector
~100 Makena sales reps,
Makena Care Connection
and distribution partners

Product Portfolio Update
• Feraheme®
• Makena®
•Cord Blood Registry®
• Intrarosa®
20

Returned to Growth
21
14,767
15,379
Q4-2016 Q4-2017
W O M E N ’ S H E A L T H : C O R D B L O O D R E G I S T R Y
Growth in First New EnrollmentsStrong commercial execution
driving new enrollments
2/3 of revenue driven by growing,
recurring annual storage fees

Product Portfolio Update
• Feraheme®
• Makena®
• Cord Blood Registry®
•Intrarosa®
22

Non-estrogen
treatment2
Unique safety profile
(no boxed warning)
Differentiated
MOA
23
Intrarosa is Differientiated1
W O M E N ’ S H E A L T H : I N T R A R O S A
1 Market research sponsored by AMAG and conducted by Roscow Market Research.
2 Intrarosa is converted by enzymes in the body into androgens and estrogens, though the mechanism of action is not fully established.

24
Dyspareunia: 90% of Affected Women Not on Rx Therapy
W O M E N ’ S H E A L T H : I N T R A R O S A
20M women in U.S. suffer from dyspareunia, a symptom of VVA1
+18 Million2
U.S. women with dyspareunia
not on prescription therapy
~1.7 Million2
U.S. women with dyspareunia
treated with prescription therapy
1 Intrarosa is a steroid indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause.
2 AMAG estimate based on Wysocki et al. Management of Vaginal Atrophy: Implications from the REVIVE Survey. Clinical Medicine Insights: Reproductive
Health 2014:8 23–30; Kingsberg et al. Vulvar and Vaginal Atrophy in Postmenopausal Women: Findings from the REVIVE Survey. J Sex Med 2013;101790-
1799; and F. Palma et al: Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study.
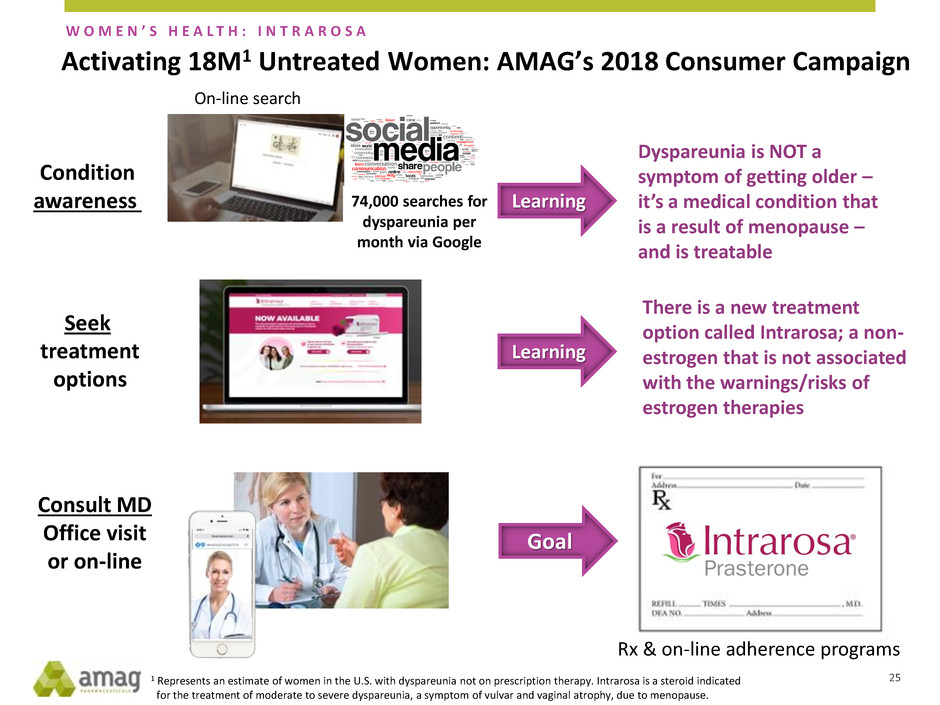
Activating 18M1 Untreated Women: AMAG’s 2018 Consumer Campaign
W O M E N ’ S H E A L T H : I N T R A R O S A
25
Seek
treatment
options
Consult MD
Office visit
or on-line
Condition
awareness
On-line search
74,000 searches for
dyspareunia per
month via Google
Dyspareunia is NOT a
symptom of getting older –
it’s a medical condition that
is a result of menopause –
and is treatable
Learning
There is a new treatment
option called Intrarosa; a non-
estrogen that is not associated
with the warnings/risks of
estrogen therapies
Learning
Goal
Rx & on-line adherence programs
1 Represents an estimate of women in the U.S. with dyspareunia not on prescription therapy. Intrarosa is a steroid indicated
for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause.

26
Key Intrarosa Growth Drivers
Launch
Priority
1
Launch
Priority
2
Launch
Priority
3
Create affordable access for all patients
Increase market awareness
Increase HCP prescribing
Launch
Priority
4
Launch
Priority
5
Make Intrarosa the 1st choice treatment among HCPs
Educate potential patients about condition
and Intrarosa as therapy
New in 2018
Intrarosa
Growth
Strategy
W O M E N ’ S H E A L T H : I N T R A R O S A

Financial Overview
27

$151.61 $158.3
1
$14.6
($7.1)
$531.81
$609.81
$78.9
($293.3)
Q4-2016 Q4-2017
4.4%
FY 2016 FY 2017
14.7%
3-months Ended December 31 12-months Ended December 31
1 Excludes “License fee, collaboration and other revenues” of $4,000 in Q4-2016, $70,000 in Q4-2017, $317,000 in 2016, and $124,000 in 2017.
Strong Financial Results While Investing in the Future
28
GAAP Product Revenue and Operating Income/Loss ($M)
Makena revenue CBR revenueFeraheme/MuGard revenue
GAAP operating income (loss)Intrarosa revenue

$153.02 $159.62
$77.4 $65.7
Strong Financial Results While Investing in the Future
$548.82
$230.1
$615.32
$265.7
FY 2016 FY 2017
12.1%
Q4-2016 Q4-2017
4.3%
1 See slide 34 for a reconciliation of GAAP to non-GAAP financials.
2 Excludes “License fee, collaboration and other revenues” of $4,000 in Q4-2016, $70,000 in Q4-2017, $317,000 in 2016, and $124,000 in 2017.
3 Includes purchase accounting adjustments related to CBR deferred revenue of $1.4M in Q4-2016 and Q4-2017, $17.0M in 2016, and $5.5M in 2017.
29
3-months Ended December 31 12-months Ended December 31
Non-GAAP Product Revenue and Adjusted EBITDA ($M)1
Makena revenue CBR revenue3Feraheme/MuGard revenue
Non-GAAP adjusted EBITDAIntrarosa revenue

Strong Liquidity Profile
($M) 12/31/17 12/31/16
Cash, cash equivalents and investments $329 $ 579
Principal debt outstanding
Convertible senior notes (2.5%) due 2019 $ 21 $ 200
Convertible senior notes (3.25%) due 2022 320 --
Term loan facility (4.75%) due 2021 (repaid in May 2017) -- 328
Senior notes (7.875%) due 2023 475 500
Total debt outstanding $816 $1,028
Balance sheet improved to align with evolving business strategy:
‒ De-levered by ~20%
‒ Extended maturities and repaid term loan
‒ Forward net leverage ratio ~4.2x1
30
1 Net leverage ratio derived from netting cash balance as of 12/31/17 from debt balance as of 12/31/17, divided by
mid-point of EBITDA guidance for 2018.
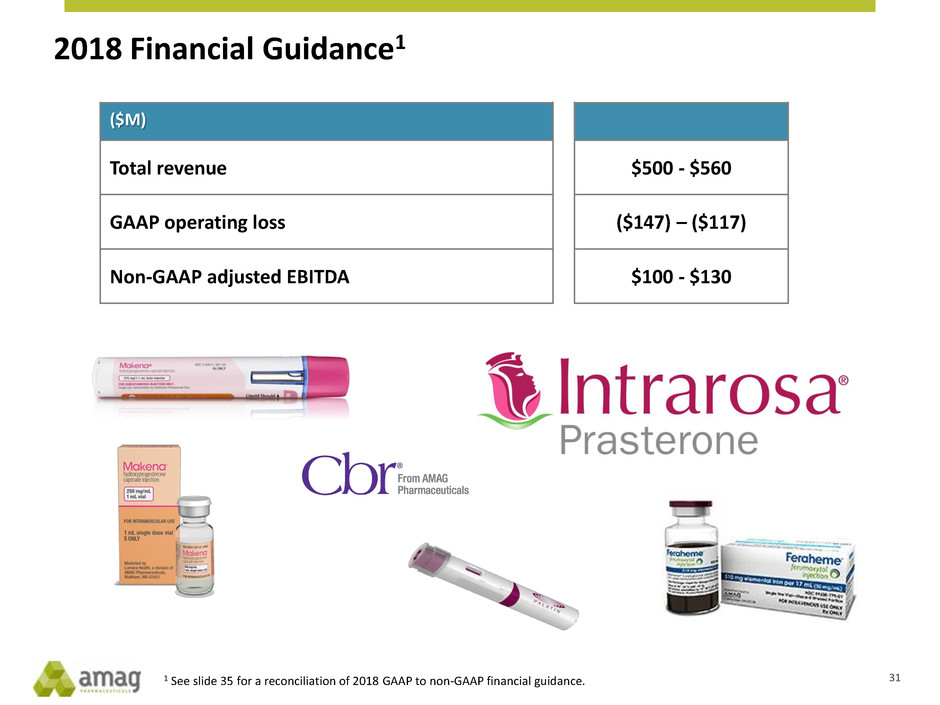
2018 Financial Guidance1
($M)
Total revenue $500 - $560
GAAP operating loss ($147) – ($117)
Non-GAAP adjusted EBITDA $100 - $130
1 See slide 35 for a reconciliation of 2018 GAAP to non-GAAP financial guidance. 31
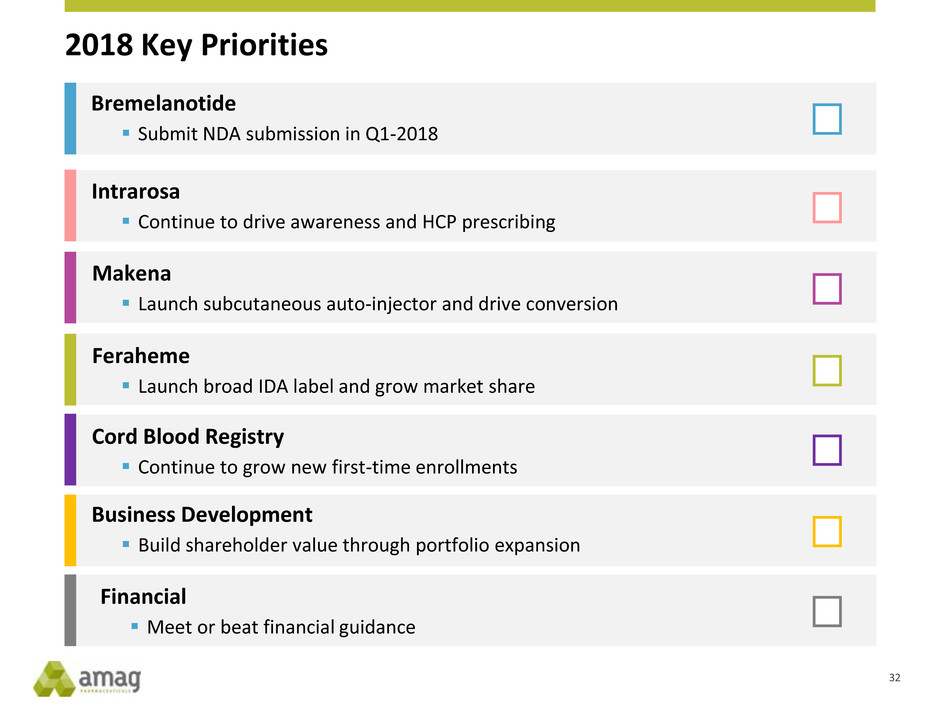
2018 Key Priorities
Intrarosa
Continue to drive awareness and HCP prescribing
Feraheme
Launch broad IDA label and grow market share
Makena
Launch subcutaneous auto-injector and drive conversion
Cord Blood Registry
Continue to grow new first-time enrollments
Business Development
Build shareholder value through portfolio expansion
Financial
Meet or beat financial guidance
Bremelanotide
Submit NDA submission in Q1-2018
32

AMAG Pharmaceuticals
Q&A
February 27, 2018

Appendix

Reconciliation of GAAP to Non-GAAP 2017 Financial Results
35
($M)
GAAP operating income (loss)
Purchase accounting adjustments related to CBR
deferred revenue
Depreciation and intangible asset amortization
Non-cash inventory step-up adjustments
Stock-based compensation
Adjustments to contingent consideration
Impairment charges of intangible assets
Acquisition related costs
Acquired IPR&D
Restructuring costs
Non-GAAP adjusted EBITDA
Q4-2017 Q4-2016 2017 2016
($7.1) $14.6 ($293.3) $78.9
1.4 1.4 5.5 17.0
65.6 29.1 153.3 94.2
0.9 1.0 2.3 5.7
5.4 5.7 23.5 22.5
(0.5) 20.6 (47.7) 25.7
-- 3.7 319.2 19.7
-- 1.3 1.5 1.3
-- -- 65.8 --
-- -- -- 0.7
$65.7 $77.4 $230.1 $265.7

Reconciliation of GAAP to Non-GAAP 2018 Financial Guidance
36
($M) 2018 Financial Guidance
Operating loss ($147) – ($117)
Depreciation & intangible asset amortization 200
Stock-based compensation 23
Non-cash inventory step up and adjustments to contingent consideration 4
Acquired IPR&D 20
Non-GAAP adjusted EBITDA $100 - $130

Share Count Reconciliation
37
(M)
Weighted average basic shares outstanding
Employee equity incentive awards
GAAP diluted shares outstanding
Employee equity incentive awards
Non-GAAP diluted shares outstanding
4Q-2017 4Q-2016
34.8 34.3
0.1 --1
34.9 34.3
-- 0.8
34.9 35.1
2017 2016
34.9 34.3
--1 --1
34.9 34.3
0.32 0.5
35.2 34.8
1 Employee equity incentive awards would be anti-dilutive in this period.
2 Reflects the non-GAAP dilutive impact of employee equity incentive awards.
22

AMAG Pharmaceuticals
2017 Financial Results
February 27, 2018
