Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - UNITED THERAPEUTICS Corp | a2234069zex-23_1.htm |
| EX-31.1 - EX-31.1 - UNITED THERAPEUTICS Corp | a2234069zex-31_1.htm |
| EX-32.2 - EX-32.2 - UNITED THERAPEUTICS Corp | a2234069zex-32_2.htm |
| 10-K - 10-K - UNITED THERAPEUTICS Corp | a2234069z10-k.htm |
| EX-32.1 - EX-32.1 - UNITED THERAPEUTICS Corp | a2234069zex-32_1.htm |
| EX-31.2 - EX-31.2 - UNITED THERAPEUTICS Corp | a2234069zex-31_2.htm |
| EX-21 - EX-21 - UNITED THERAPEUTICS Corp | a2234069zex-21.htm |
| EX-10.52 - EX-10.52 - UNITED THERAPEUTICS Corp | a2234069zex-10_52.htm |
| EX-10.45 - EX-10.45 - UNITED THERAPEUTICS Corp | a2234069zex-10_45.htm |
Exhibit 10.51
Pursuant to 17 C.F.R §240.24b-2, confidential information (indicated as [***]) has been omitted and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission.
WHOLESALE PRODUCT PURCHASE AGREEMENT
THIS WHOLESALE PRODUCT PURCHASE AGREEMENT (the “Agreement”) is made this 1st day of January, 2018, (the “Effective Date”) by and between Priority Healthcare Distribution, Inc., doing business as CuraScript SD Specialty Distribution, a Florida corporation having offices at 255 Technology Park, Lake Mary, Florida 32746, (“Distributor”), and United Therapeutics Corporation (“UT”), a Delaware corporation having offices at 1040 Spring Street, Silver Spring, Maryland. Distributor and UT are each referred to in this Agreement as a “Party,” collectively, the “Parties.”
WHEREAS, UT manufactures Product; and
WHEREAS, Distributor wholesales certain products to its customers, which include physicians, physician group practices, and certain health care institutions and facilities located in the United States and Puerto Rico; and
WHEREAS, Distributor has represented that it possesses the necessary expertise, financial resources and organization to sell UT Product (as hereinafter defined) and desires to acquire from UT the right to sell, market, distribute and maintain UT Product in the Territory (as hereinafter defined); and
WHEREAS, the Parties desire to enter into this Agreement so that Distributor can sell, distribute and maintain UT Product in the Territory
NOW THEREFORE, in consideration of the mutual agreements and covenants contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereto, intending to be legally bound, hereby agree as follows:
ARTICLE I
PRODUCTS AND SERVICES
1.1 APPOINTMENT OF DISTRIBUTOR. This Agreement governs Distributor’s distribution of those UT products set forth in EXHIBIT A (“Product” or “Products”), which is attached hereto and incorporated by reference herein and which may be modified from time to time by the Parties upon written mutual consent. UT hereby appoints Distributor and Distributor hereby accepts such appointment, as a distributor of UT Product during the term of this Agreement in the Territory, subject to the terms and conditions of this Agreement. This appointment is non-exclusive, and UT reserves the right to appoint additional distributors in the Territory and to distribute UT Product in the Territory on its own behalf. UT will notify Distributor prior to adding additional distributors within the Territory.
1.2 PURCHASE ORDERS. Distributor shall submit written purchase orders to UT by electronic mail or in accordance with written instructions provided by UT. Except as otherwise agreed by UT, Purchase Orders shall be submitted one (1) time per month by the tenth (10th) day of the month. Each such order shall set forth: (a) the package reference (e.g. Remodulin/Tyvaso order quantities of ten (10); Orenitram order quantities of twelve (12)) for the UT Product ordered including item numbers, (b) UT Product Price per EXHIBIT A, (c) Quantity ordered for each product, (d) requested delivery dates, (e) specific shipping instructions, and (f) if applicable, any relevant export control information or documentation required of Distributor to enable UT to comply with Applicable Laws. Except as otherwise agreed by UT, Distributor shall submit such purchase orders at least five (5) business days prior to the requested delivery dates. Distributor is responsible for good inventory management processes and subsequent purchases should not deviate negatively by more than
[***]% from the previous purchase unless unexpected events occur and are communicated to UT in advance in writing. Distributor may, however, place a purchase order for UT Product that is more than [***]% of a previous purchase if needed. Distributor may only purchase UT Product from UT. Distributor may only sell UT Products to those customers listed in EXHIBIT C.
1.3 ACCEPTANCE OF ORDERS. Distributor purchase orders are subject to acceptance by UT in UT’s sole discretion. UT shall have no liability to Distributor or to the proposed Customer for orders that are not accepted. Each purchase order shall be deemed to be an offer by Distributor to purchase UT Product pursuant to the terms of this Agreement. Acceptance of an order by UT shall oblige the Parties to the terms and conditions set forth in this Agreement with respect to such order to the exclusion of any additional or contrary terms set forth in the Distributor purchase order. Any terms or conditions of such purchase order shall be null and void. Notwithstanding the foregoing, in the event of exigent circumstances, UT shall use its Commercially Reasonable Efforts to accept an emergency purchase order from Distributor two (2) business days prior to the requested delivery date.
1.4 REPORTS. Distributor will provide UT with data reports as specified on EXHIBIT E, which is attached hereto and incorporated by reference herein. The Parties intend that these reports will comply with all applicable laws, statutes, regulations, and rules (and reasonable interpretations thereof and guidance related thereto) (collectively, “Applicable Laws”). In the event of any inconsistency between the data file layouts set forth in EXHIBIT E and any Applicable Laws. Distributor shall be entitled to unilaterally modify the data reports without consent from, UT, if required by Applicable Law solely to the extent required to comply. If unilateral changes are made by the Distributor and if needed the Parties shall work in good faith to make additional modifications to the Report to reach a mutually agreed upon format. If UT requests additional report fields, changes to the report fields, or data configurations not specified on EXHIBIT E that require significant changes, Distributor shall notify UT of any additional fees pursuant to Section 2.8 below. If UT agrees to proceed, the Parties will execute an amendment to authorize such work and amend EXHIBIT E.
1.5 EVIDENCE OF PEDIGREE. In accordance with, and to the extent required by, Applicable Law, Distributor shall create and maintain all records, manifests, or other documentation, in electronic and/or written form, necessary to evidence the pedigree (i.e., a record of each distribution) of any Products purchased from UT and shipped, resold, or provided to another distributor or customer.
1.6 SERVICES. Distributor will fulfil their obligations as set forth in this Agreement and perform those services set forth in EXHIBIT B, which is attached hereto and incorporated by reference herein (the “Services”).
1.7 DELIVERY OF PRODUCT.
(a) Delivery Terms. Units of UT Product ordered by Distributor and accepted by UT shall be packed for shipment and storage in accordance with UT’s standard commercial shipping practices. UT shall use its Commercially Reasonable Efforts to deliver Units of UT Product into the possession of a common carrier for delivery within a reasonable period of time after acceptance of a purchase order by UT. Unless mutually agreed upon by Distributor and UT, no UT Product shall be shipped to Distributor on a Friday, Saturday or Sunday. Each order may only be shipped, and shall be addressed for shipment, to the Designated Shipment Locations specified in EXHIBIT G. Unless UT and Distributor otherwise agree in writing, all deliveries of UT Product shall be F.O.B., Distributor’s Designated Shipment Location. UT shall insure each shipment of UT Product with a reputable insurer for the full invoice price of such shipment.
(b) Risk of Loss. Risk of loss and title to UT Product shall pass to Distributor upon delivery at its Designated Shipment Location. UT shall have no liability for any loss, theft, destruction or
damage to the Units of UT Product caused after they have been delivered to a Designated Shipment Location. Distributor shall, at its sole cost and expense, insure or self-insure the UT Products from the time of delivery at Distributor’s Designated Shipment Location until delivery of the Units of UT Product by Distributor to Customer has been completed.
(c) Inspection of Product. Distributor shall promptly inspect each shipment of UT Product. In the event of any shortage, damage, expiration, or discrepancy in a shipment of UT Product that is patently obvious, Distributor shall promptly report the same to UT and furnish such written evidence or other documentation as UT may reasonably request. Distributor shall be deemed to have accepted a shipment and UT shall not be liable for any such shortage, damage, expiration, or discrepancy in such shipment unless Distributor provides UT with such notice and substantiating evidence within five (5) days of receipt of the UT Product at Distributor’s Designated Shipment Location. Upon receipt of reasonable substantiating evidence of such shortage, damage or discrepancy, UT shall promptly provide additional UT product or substitute products to Distributor.
(d) Modification of Orders. No accepted purchase order shall be modified or canceled except upon the written agreement of both Parties.
(e) Change Order Charges. If Distributor requests modifications to an accepted order prior to the scheduled delivery date and prior to such time that delivery courier has accepted contents of order, then, in consideration for accepting such change order, UT may extend the scheduled delivery date.
(f) Product Changes. Subject to applicable regulatory approval, UT reserves the right, in its sole discretion and without incurring any liability to Distributor except as otherwise provided in this Agreement, to: (a) alter UT Product, (b) discontinue the manufacture of UT Product, or (c) commence the manufacture and sale of new products having features which compete with UT Product or make UT Product obsolete. UT also reserves the right, in its sole discretion and without incurring any liability to Distributor except as otherwise provided in this Agreement, immediately to alter the specifications or the manufacturing process for UT Product for reasons of health or safety. UT shall fill all accepted purchase orders from Distributor for altered or discontinued UT Product for which manufacturing and commercial deliveries have commenced prior to the effective date of such a change but otherwise shall have no obligation to do so unless the delivery date requested in the relevant purchase order is prior to the effective date of such a change.
1.8 PRODUCT RETURNS. UT will not accept the return of any UT Product, unless agreed in writing by UT, except if returned pursuant to a recall under Section 1.13 (b) below or products delivered under non-acceptable conditions as described in Section 1.7 (c). Notwithstanding anything herein to the contrary, all Product returns made in conjunction with this Agreement will be made on behalf of Distributor by Distributor’s designated third party product returns company (“Returns Agent”). UT will pay any reimbursement associated with Product returns directly to Distributor and not to the Returns Agent. All fees associated with the use of the Returns Agent’s services will be paid by Distributor and not UT.
1.9 DIVERSION. Distributor shall promptly notify UT upon learning of any activity that appears to illegally divert any Products. UT shall promptly notify Distributor if UT becomes aware of any diverted Product.
1.10 ADVERSE EVENT REPORTING (“AE”)/PRODUCT COMPLAINTS (“PC”). Distributor will not be responsible for United States Food and Drug Administration (“FDA”) reporting of adverse events/product complaints. Distributor shall notify UT at drugsafety@unither.com utilizing Distributor’s standard form within two (2) business days of any adverse event or product complaint from a third party being reported to Distributor that meets the definition as defined in Exhibit H of a serious adverse event or product complaint for purposes of regulatory reporting globally.
1.11 PRODUCT EDUCATION. Distributor will not promote UT’s Products, but Distributor will promote its own distribution services to its customers in accordance with Distributor’s standard business practices, which typically include, but are not limited to, informing its customers of pricing available for products distributed by Distributor. Distributor may, provide its customers with educational information concerning Product, additionally information may be provided by UT and reviewed and pre-approved by Distributor.
1.12 SALES OUTSIDE THE UNITED STATES. Distributor agrees not to distribute or sell Products outside of the United States, its territories or possessions (the “Territory”), unless otherwise agreed to between the Parties in a written amendment to this Agreement.
1.13 SUSPENSION OF DISTRIBUTION AND RECALL.
(a) Suspension of Distribution. If, for good reason and with written notification, UT requests that Distributor suspend distribution of any Product, Distributor shall use commercially reasonable efforts to suspend its distribution of such Product. If the suspension continues for more than six (6) weeks, UT will repurchase the Product held in inventory by Distributor at the Product Price, as defined in Section 2.1(a), paid for such Product by Distributor, and Distributor shall have the right to terminate this Agreement for material breach under Section 3.2(c)(vi) of this Agreement. All such repurchased Product shall be returned to UT at UT’s expense.
(b) Recalls. Any recalls of UT Product shall be conducted in compliance with FDA requirements and the UT standard operating procedure for recalls (“UT Recall SOP”) as provided to and accepted by Distributor. UT shall promptly notify Distributor of any recalls initiated by UT or required by the FDA. UT shall provide Distributor a third party (e.g., UPS or FedEx) billing number for shipping of recalled Products to UT (or UT’s designated agent) at UT’s expense. Distributor shall provide to UT the names and addresses of customers that may have received recalled Products. To the extent such recall does not result from a breach of any of Distributor’s representations and warranties under this Agreement or Distributor’s negligence or willful misconduct (in which even Distributor shall be responsible for all recall related expenses), UT shall be responsible for the mailing, shipping, and reasonable administrative expenses incurred by Distributor in connection with the recall, plus a reasonable service fee of one dollar ($1.00) per customer name and address, up to a maximum of one thousand dollars ($1,000) per recall. In addition, UT shall pay the cost of replacement Product for Distributor’s customers.
(c) Records of Recalls. Distributor shall maintain for two (2) years after termination or expiration of this Agreement such information as is reasonably required in the event of a Product recall after termination or expiration of this Agreement, and shall make such information available to UT, at UT’s expense, in the event of such a recall.
(d) Investigations. Distributor shall use its commercially reasonable efforts to cooperate with UT in investigating any Product failure that resulted in the need for a recall and any reasonable costs involved with such investigation will be paid by UT.
1.14 [***]
ARTICLE II
PRICES, FEES, AND PAYMENT
2.1 PRODUCT PRICING.
(a) Product Price. The price Distributor will pay UT for Products is set forth in EXHIBIT A (the “Product Price”).
(b) Resale Price. The Parties acknowledge that Distributor may offer the UT Product in the Territory at such prices or discounts as Distributor, in its sole discretion, may determine.
(c) Price Changes. At any time during the term of this Agreement, UT may increase or decrease its Prices for UT Product with advance written notice to Distributor of the effective date of the price change by sending such change notice to [***]. Any such price change shall not apply to purchase orders accepted prior to the effective date of the applicable price change. Distributor agrees to continue placing purchase orders at quantity volumes consistent with demand and inventory levels prior to the effective date of any such price change.
(d) Costs. All costs related to shipping, insuring, packing, handling and delivering UT Product to Distributor’s facility shall be at the sole expense of UT. All such costs incurred after the instant of delivery to the Designated Shipment Location shall be the responsibility of Distributor. Notwithstanding anything to the contrary in this Agreement, UT may, in its sole discretion, charge Distributor for any and all shipping, packing, handling or delivery charges associated with emergency purchase orders, or if Distributor places three (3) or more orders in a one (1) month period.
2.2 PAYMENT TERMS.
(a) Distributor shall make payments for UT Product within sixty (60) days of invoice date of an applicable invoice from UT payable by check and received by UT prior to the 60 day due date. Distributor shall be eligible for a two percent (2%) prompt pay discount if payment is received by UT within thirty (30) days of the date of invoice. All payments shall be made in United States Dollars.
2.3 TAX PAYMENTS. Each Party shall pay all taxes, duties, import deposits, assessments and other governmental charges, however designated, that are now or hereafter imposed upon such Party by any governmental authority or agency in connection with the performance of its obligations under this Agreement.
2.4 CHARGEBACKS. Subject to UT’s reimbursement of the Chargebacks (as described below) Distributor shall provide wholesale distribution to certain entities eligible for discounted government pricing (e.g., FSS, VA, PHS (340B)) (“Discounted Entity”) as described herein. The discounted government pricing is less than the price at which Distributor purchases UT Product (i.e., less than the Price set forth in Attachment A). Distributor shall create an account for each Discounted Entity purchasing UT Product from Distributor. As part of this process, Distributor shall use Commercially Reasonable Efforts to identify whether the proposed Discounted Entity is eligible for discounted government pricing through direct documentation from the proposed Discounted Entity or through review of data on the HRSA eligibility website or other database resource. UT agrees that all HRSA active entity codes are eligible for discounted government pricing. As an order for UT Product is received from the Discounted Entity, Distributor shall sell UT Product to the Discounted Entity at the discounted government price. At least five (5) business days prior to the effective date of any original contract, or update to any existing contract, UT will provide Distributor, via email, all original contract pricing and/or membership information, and any contract notifications or updates to ContractAdmin@CuraScript.com. The difference between the discounted government price and the Price as of the Discounted Entity’s invoice date for the UT Product is referred to as the “Chargeback.” The Chargeback shall be paid by UT to Distributor by check. When submitting a Chargeback request to UT, Distributor shall send Distributor’s chargeback template via systematic email to UTtrade@unither.com and shall include the information in an Excel format as set forth below. Chargeback request(s) shall be submitted to UT by the tenth (10th) day of each month for all activity in the previous calendar month. UT shall process Chargeback credits due to Distributor within thirty (30) days of receipt of the Chargeback submission. UT shall send Distributor any information or updates regarding Chargeback requests to ChargebackAdmin@CuraScript.com. UT will provide, at the time of payment, a reconciliation report for disputed Chargeback items.
Manual Chargeback Report
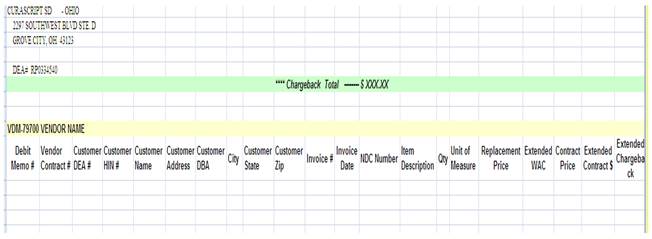
Distributor may resubmit disputed Chargebacks for reconsideration within (60) days from the date the reconciliation report is received. In the event that new information surfaces that causes corrections or adjustments to prior sales, Distributor may reopen and resubmit chargeback claims within eighteen (18) months of the original sale date or as otherwise may be required in a government contract. Distributor shall not set off Chargebacks owed by UT against any amounts owed by Distributor to UT. Upon termination of this Agreement, if there are any unapplied credits for a Chargeback, UT shall issue a check in the amount thereof to Distributor. Chargebacks paid hereunder constitute reimbursement to Distributor for debits incurred in administering UT discounts to Discounted Entities, and are not, and should not be construed as, remuneration intended to induce Distributor to purchase, order, lease, or recommend any UT product.
2.5 DATA REPORT MODIFICATIONS. UT shall pay Distributor a programming fee of $185 per hour within thirty (30) calendar days of receipt of Distributor’s invoice for such programming for any changes to the data specifications set forth on EXHIBIT E, including the addition of reporting fields, changes to the report fields, or data configurations not specified on EXHIBIT E. Late payments will accrue interest at the rate of eighteen percent (18%) per annum (or the maximum amount permissible under Applicable Law, if lower) for every invoice or statement past due. The late payment fee shall be calculated on the basis of a 365-day year for the actual number of days elapsed between the date upon which payment was due and the date upon which payment is made. UT agrees to pay reasonable attorney fees and expenses incurred by Distributor in enforcing its right of collection.
2.6 FEES FOR SERVICES.
(a) Services Fee. In consideration for Distributor’s performance of the Services, UT shall pay Distributor a fee (the “Services Fee”) in accordance with EXHIBIT B. Distributor shall invoice UT monthly, and UT shall pay such invoices within thirty (30) calendar days of receipt.
(b) Late Fee. Payment of the Services Fee other than as stated herein will result in a late payment fee equal to eighteen percent (18%) per annum (or the maximum amount permissible under Applicable Law, if lower) for every invoice or statement past due. The late payment fee shall be calculated on the basis of a 365-day year for the actual number of days elapsed between the date upon which payment was due and the date upon which payment is made. UT agrees to pay reasonable attorney fees and expenses incurred by Distributor in enforcing its right of collection.
ARTICLE III
TERM AND TERMINATION
3.1 TERM. Unless and until this Agreement is terminated as provided for herein, this Agreement shall have a term of Two (2) years, commencing on the Effective Date. Following the initial term, this Agreement shall be renewed automatically for additional one-year terms unless either Party shall have given the other written notice of non-renewal at least sixty (60) days prior to the expiration of the then current term.
3.2 TERMINATION. This Agreement is made in good faith based on the assumption that early termination shall not be required. Notwithstanding the foregoing, early termination shall be permissible as follows:
(a) By either Party with ninety (90) days’ written notice for any reason.
(b) Immediately by either Party if such Party provided written notice detailing a material breach of this Agreement and the breaching Party failed to cure the breach within thirty (30) days of the date of the notice.
(c) Immediately with written notice, by either Party, except that only Distributor may terminate this Agreement with respect to subsection (vi), in the event that:
(i) the other Party shall file any petition under any bankruptcy, reorganization, insolvency or moratorium laws, or any other law or laws for the relief of or in relation to the relief of debtors;
(ii) there shall be filed against the other Party any involuntary petition under any bankruptcy statute or a receiver or trustee shall be appointed to take possession of all or a substantial
part of the assets of the Party that has not been dismissed or terminated within sixty (60) days of the date of such filing or appointment;
(iii) the other Party shall make a general assignment for the benefit of creditors or shall become unable, or admit in writing its inability, to meet its obligations as they mature;
(iv) the other Party shall institute any proceedings for liquidation or the winding up of its business other than for purposes of reorganization, consolidation, or merger;
(v) the other Party’s financial condition shall become such as to endanger completion of its performance in accordance with the terms and conditions of this Agreement;
(vi) UT suspends distribution of Products for more than six (6) weeks pursuant to Section 1.13(a); or
(vii) the other Party is unable to perform its duties for a period of thirty (30) days pursuant to Section 7.10.
(d) Immediately upon notification by either Party if the terms of this Agreement are determined by either Party in good faith to be inconsistent with any Applicable Law, or upon a change in law pursuant to Section 7.11.
(e) [***]
ARTICLE IV
CONFIDENTIALITY AND DATA
4.1 CONFIDENTIALITY. Each Party shall take all reasonable actions and do all things reasonably necessary to ensure that any information contained in this Agreement, as well as any information that is disclosed by one Party to the other under this Agreement (in any case, “Confidential Information”) shall not be disclosed or used for purposes outside this Agreement. The foregoing prohibition shall not apply to disclosures: (a) to the disclosing Party’s attorney or accountant; (b) made pursuant to a request from a legal or regulatory authority; (c) by the disclosing Party to its Affiliate, as defined below (provided such Affiliate is subject to the confidentiality restrictions herein), and for the purpose of this section “Affiliate” shall mean an entity in which the disclosing Party maintains an ownership position or an entity under common ownership or control with the disclosing Party; or (d) that are required pursuant to a court order or by law. The foregoing prohibition shall not apply to information that: (i) a Party can show it knew prior to disclosure without obligation of confidentiality; (ii) is or becomes public knowledge through no fault of said Party; or (iii) is lawfully disclosed by a third party under no obligation of confidentiality. This Section 4.1 shall survive any termination of this Agreement for a period of five (5) years thereafter. Each Party shall either return to the other Party, or destroy, all Confidential Information received hereunder upon the expiration or termination of this Agreement, except that each Party may retain one (1) copy of such Confidential Information in order to satisfy any future legal obligations it may have. Notwithstanding anything to the contrary contained herein, if reasonably necessary, Distributor shall be permitted to disclose to potential and existing customers of Distributor (and any potential purchaser of Distributor) the general terms of this Agreement.
4.2 Notwithstanding anything to the contrary in this Agreement, Distributor shall not sell its purchasing data, which may include data relating to the Product, to third parties (e.g., IMS or NDC).
ARTICLE V
REPRESENTATIONS AND WARRANTIES
5.1 STATUTORY AND REGULATORY COMPLIANCE. Distributor and UT shall comply with all Applicable Laws governing their activities related to this Agreement, including without limitation, laws related to
fraud and abuse, false claims, provision of samples, and prohibition on kickbacks. Without limiting the generality of the foregoing, the Parties further agree as follows:
(a) Discounts/Rebates. Although Distributor does not submit claims or requests for payment to Medicare or Medicaid, the Parties have structured any discounts and rebates under this Agreement in a manner consistent with the applicable characteristics of the statutory discount exception (42 U.S.C. § 1320a-7b(b)(3)(A)) and the discount safe harbor (42 C.F.R. § 1001.952(h)). The terms pursuant to which any discount or rebate will be paid are fixed and are set forth in this Agreement and the attached Exhibits. This Agreement is not dependent on, and does not operate in conjunction with, either explicitly or implicitly, any other arrangement or agreement between UT and Distributor. UT represents and warrants that: (i) it will refrain from doing anything that would impede Distributor from meeting any reporting obligations Distributor may have under Applicable Law; (ii) it will comply with all reporting requirements for pharmaceutical manufacturers under all Federal health care programs and, in particular, will include any and all discounts and rebates paid hereunder in its calculations of “average manufacturer price” or “best price” under the Medicaid drug rebate program, and in the calculations of “average sales price” under Medicare, to the extent applicable; (iii) it will properly report the existence of the discounts/rebates on the invoices or statements submitted by UT to Distributor; and (iv) no discount/rebate paid pursuant to this Agreement is intended in any way as a discount related to a drug formulary or drug formulary activities and no discount/rebate has been negotiated or discussed between the Parties in connection with any such drug formulary or formulary activities. To the extent required under Applicable Law, Distributor will report the discounts/rebates to appropriate Federal health care programs, and will, upon the request of a governmental agency, including the Secretary of Health and Human Services or a state healthcare agency, disclose information regarding the discounts/rebates to the requesting agency.
(b) Services Fee. UT represents and warrants that: (i) it has engaged Distributor to perform bona fide, legitimate, reasonable, and necessary Services; (ii) the Services are not intended to serve, either directly or indirectly, as a means of marketing the Product; (iii) the Services do not involve the counseling or promotion of any off-label use of the Products or a business arrangement or other activity that violates any Applicable Laws; (iv) the Service Fees (as set forth at EXHIBIT B) do not constitute a discount or other form of compensation that must be included in “best price,” “average manufacturer price,” or “average sales price” reporting; (v) the Service Fees are not intended in any way as remuneration for referrals or for other business generated; (vi) the Service Fees represent fair market value for the services based on arms-length negotiations; and (vii) the Service Fees paid pursuant to this Agreement are not intended in any way as payments related to a drug formulary or drug formulary activities and have not been negotiated or discussed between the Parties in connection with any such drug formulary or formulary activities.
(c) Compliance With Drug Distribution Laws. By executing this Agreement, UT hereby designates Distributor, and Distributor accepts such designation, as an authorized distributor of record for the Products for purposes of the Parties’ compliance with the Prescription Drug Marketing Act of 1987, as amended by the Prescription Drug Amendments of 1992 and the Drug Quality and Security Act of 2013, including the Drug Supply Chain Security Act (DQSA), and as may be further amended from time to time, and any and all other applicable laws and regulations requiring the same or similar designation as an authorized pharmacy of record or authorized distributor of record. UT and Distributor represent and warrant that, to their knowledge, they are authorized trading partners under the DQSA and a party that experiences a change in its authorized status will notify the other party in writing promptly. UT agrees to provide any documentation of Distributor’s authorized status and documentation of and statements relating
to pharmaceutical transactions as may be reasonably requested by Distributor as necessary for compliance with Applicable Laws.
(d) Regulatory Approvals. UT represents and warrants that it has received all applicable regulatory and statutory approvals in order for it to lawfully distribute the Product(s) to Distributor hereunder.
(e) FDA Compliance. UT hereby represents that, at the time of commercial sale of the Product, UT will have received clearance from FDA to market the Product in the United States. In the event FDA or any other governmental entity withdraws its marketing clearance for the Product, UT shall promptly notify Distributor. Furthermore, UT represents and warrants that, at the time of shipment or delivery from UT, the Products (i) shall not be adulterated, misbranded, or otherwise prohibited within the meaning of the Federal Food, Drug and Cosmetic Act, 21 U.S.C.A. 301 et seq., as amended, and in effect at the time of shipment or delivery (“FFDCA”) or within the meaning of any Applicable Law in which the definition of adulteration or misbranding are substantially the same as those contained in the FFDCA; and (ii) shall not be merchandise that may not be introduced or delivered for introduction into interstate commerce under the provisions of Sections 301, 404 or 505 of the FFDCA (21 U.S.C.A. 331, 344 and 355).
5.2 PRODUCT MATERIALS. UT represents and warrants that any materials relating to Products that it provides to Distributor: (a) are limited to communications that are intended to describe the Product or provide important Product-related information; (b) if required under Applicable Law, have received all appropriate regulatory approvals prior to use (e.g., FDA approval); and (c) do not involve the counseling or promotion of any off-label use.
5.3 PRODUCT WARRANTY. UT hereby authorizes Distributor to rely upon, and to pass on the UT standard warranty set forth in EXHIBIT D to Distributor’s Customers in the Territory, which may be revised by UT upon written notice to Distributor.
5.4 EXCLUDED CLAIMS. UT shall not have any additional warranty obligations to Distributor or Customers under Section 5.3 above or otherwise to the extent that Distributor has made any warranties, oral or written, beyond those expressly set forth in the standard UT warranty, set forth in EXHIBIT D hereto. Distributor shall not offer its customers any warranties different from or in addition to those given by UT hereunder.
5.5 FEDERAL PROGRAMS. UT represents, warrants, and certifies that neither it nor any of its principals were or are debarred, suspended, proposed for debarment, otherwise determined to be ineligible to participate in Federal health care programs (as that term is defined in 42 U.S.C. 1320a-7b(f)), convicted of a criminal offense related to the provision of health care items or services, or currently the subject of any Office of Inspector General investigation (collectively, an “Adverse Enforcement Action”). UT shall notify Distributor immediately if UT or any of its principals becomes the subject of an Adverse Enforcement Action.
ARTICLE VI
INDEMNIFICATION, LIMITATION OF LIABILITY, AND INSURANCE
6.1 MUTUAL INDEMNIFICATION.
(a) Distributor Indemnification. Distributor will indemnify and hold UT and its affiliates, officers, directors, agents and employees harmless from and against any loss, cost, damage, expense, or other liability, including, without limitation, reasonable costs and attorney fees (collectively, “Damages”) incurred in connection with any and all actual or threatened third party claims, suits, investigations, enforcement actions, or any other judicial or quasi-judicial proceeding (“Claims”)
arising out of (i) Distributor’s negligent acts or omissions or willful misconduct, or (ii) Distributor’s breach of this Agreement. Distributor shall have no obligation to indemnify UT in connection with any Claims caused by or based upon the negligence or intentional misconduct of UT or UT’s breach of this Agreement.
(b) UT Indemnification. UT will indemnify and hold Distributor and its affiliates, officers, directors, agents and employees harmless from and against any Damages incurred in connection with any and all Claims arising out of (i) UT’s manufacturing of the Products or any harm caused to a third party resulting from the use of the Product; (ii) any recall, quarantine, warning, or withdrawal of any Products; (iii) UT’s negligent acts or omissions or willful misconduct; (iv) UT’s breach of this Agreement; or (v) the use by any third party of any Product. UT shall have no obligation to indemnify Distributor in connection with any Claims caused by or based upon the negligence or intentional misconduct of Distributor or Distributor’s breach of this Agreement.
(c) Notification. As a condition of indemnification, the Party seeking indemnification shall notify, to the extent possible under Applicable Law, the indemnifying Party in writing promptly upon learning of any Claim for which indemnification may be sought hereunder. The indemnifying Party shall have a right to participate in the defense of such Claim, and the Parties will cooperate in good faith in such defense. No Party shall have an obligation to indemnify the other Party as described herein with respect to any Claim settled without the mutual written consent of both Parties, which consent shall not be unreasonably withheld.
6.2 LIMITATION OF LIABILITY. In no event shall either Party be liable to the other under this Agreement for any special, incidental, indirect, exemplary, or consequential damages, whether based on breach of contract, warranty, tort (including negligence), lost profits or savings, punitive damages, injury to reputation, loss of customers or business, product liability, or otherwise, regardless of whether such Party has been advised of the possibility of such damage. The Parties acknowledge and agree that the foregoing limitations of liability are a condition and material consideration for their entry into this Agreement.
6.3 INSURANCE. Distributor and UT shall maintain such policies of general liability, professional liability, and other insurance of the types and in amounts customarily carried by their respective businesses. Notwithstanding the foregoing, UT shall, at a minimum, maintain throughout the term of this Agreement commercial products liability coverage, either through commercial insurance or a self-insured retention pool, in an amount no less than ten million dollars ($10,000,000). Each Party shall provide the other with reasonable proof of insurance upon written request.
ARTICLE VII
GENERAL TERMS
7.1 NON-EXCLUSIVITY. Nothing herein shall be construed to limit Distributor from entering into other agreements with other manufacturers or wholesalers that allow Distributor to distribute or wholesale products that compete with UT’s Products.
7.2 NOTICE. Any notice, demand, request, consent, or approval required or permitted hereunder shall be in writing and shall be delivered: (a) personally; (b) by certified mail, return receipt requested, postage prepaid; (c) by facsimile transmission; or (d) by overnight courier by a nationally recognized courier service, to the address indicated below or to such other address as may be designated in writing by each Party from time to time.
If to UT:
United Therapeutics Corporation
1040 Spring Street
Silver Spring, Maryland 20910
Attention: Chief Financial Officer
Telefax: 301-508-9291
With a copy to:
United Therapeutics Corporation
1735 Connecticut Ave. NW, 2nd Floor
Washington, DC 20009
Attention: General Counsel
Telefax: 202-483-4005
If to Distributor:
Express Scripts, Inc.
c/o Priority Healthcare Distribution, Inc.
One Express Way
St. Louis, MO 63121
Attn: Legal Department
With a copy to:
Priority Healthcare Distribution, Inc.
255 Technology Park Drive
Lake Mary, FL 32746
Attn: General Manager
All such communications shall be deemed to have been received by the intended recipient: (i) on the day actually received if delivered personally; (ii) five (5) business days following deposit in the United States Mail if sent by certified mail; (iii) upon confirmation of receipt of a facsimile transmission if sent by facsimile; or (iv) on the next business day if sent by overnight courier.
7.3 SEVERABILITY. In the event any portion of this Agreement not material to the remaining portions hereof shall be held illegal, void, or ineffective, the remaining portions hereof shall remain in full force and effect. Subject to the consent of both Parties, such consent not to be unreasonably withheld, if any of the terms or provisions of this Agreement are in conflict with any Applicable Laws, then such terms or provisions shall be deemed inoperative to the extent that they may conflict with such Applicable Laws and shall be deemed to be modified to conform to such Applicable Laws.
7.4 AUDIT. No more than once during any twelve (12) month period with 30 business days written notice accompanied by a detailed scope during the term of this Agreement and for one hundred eighty (180) days thereafter, either Party shall permit a certified public accountant, engaged by the auditing Party and reasonably acceptable to the other Party (“Auditor”) to audit the other’s records relating to the twelve (12) month period preceding the date when the audit is conducted. Such audit shall be limited to tracking of rebates, data reports, and chargeback reports. This audit may include Distributor’s facilities and quality systems as they relate to the Services covered by Exhibit B, with the exception of information and operations regarded by the Distributor as Proprietary information. If either Party elects to conduct an audit, the other Party agrees to make available upon thirty (30) days’ advance written notice, during normal business hours, such documents and personnel in a manner as not to unduly interfere with the audited Party’s operations. If any audit reveals (a) an error in the calculation, reporting, or payment of any rebates; or (b) that an overcharge or undercharge incurred, in the case of an error by either Party, such Party shall provide a written response or explanation, correct any error, and remit any monies
due within fifteen (15) days after receiving notice of the error or overcharge. Any Auditor hired by either Party must both enter into a confidentiality agreement executed by both Parties and be retained on an hourly or fixed rate basis, and not a contingency basis. Each Party shall pay their respective expenses associated with the audit. If an independent third party is used to conduct the audit, such third party shall execute a confidentiality agreement with the Distributor prior to any such audit. Audits during December and January are excluded unless the request is related to an inspection and timing stipulated by a government regulator that impacts the services defined in this agreement. Notwithstanding the foregoing, “for cause” audits may be performed with less than thirty (30) business days’ notice, but with as much notice as reasonably practicable taking into account the level of urgency associated with a “for cause” audit. DISTRIBUTOR will issue responses in writing to UT within an agreed timeline to any “for cause” audit observations. These timelines may be accelerated for critical audit observations, relating to the distribution of UT Product.
7.5 ENTIRE AGREEMENT. With regard to the issues addressed herein, this Agreement and the Exhibits attached hereto contain the entire agreement and understanding of the Parties, and supersede any and all prior agreements and understandings regarding the same subject matter.
7.6 AMENDMENT. No amendment, modification, revision, representation, warranty, promise or waiver of or to this Agreement shall be effective unless the same shall be in writing and signed by both Parties. Notwithstanding the foregoing, EXHIBIT A (Product Pricing) may be modified with a written notification from UT to Distributor. Upon the effective date of the change, the Exhibit(s) will be deemed amended to reflect such change.
7.7 COUNTERPARTS. This Agreement may be executed in any number of counterparts, all of which together shall constitute one and the same instrument.
7.8 ASSIGNMENT. Neither Party may assign this Agreement without the written consent of the other; provided, however, that Distributor may assign this Agreement to any entity that, directly or indirectly, wholly owns or controls Distributor or any affiliate that is, directly or indirectly, wholly owned or controlled by any entity that, directly or indirectly, wholly owns or controls Distributor.
7.9 DELEGATION OF RESPONSIBILITIES. Distributor may engage a third party to conduct certain administrative functions on its behalf and may subcontract portions of certain limited functions and responsibilities of this Agreement, including, but not limited to, data compilation and reporting services, financial accounting and processing services, or any other function relating to any of Distributor’s obligations set forth herein. UT agrees to cooperate with Distributor’s reasonable requests relating to Distributor’s engagement of any such third party. Such third party must perform in a manner conforming to this Agreement and will be bound by confidentiality restrictions no less restrictive than are set forth in Section 4.1 of this Agreement. Distributor shall retain full responsibility and liability for the performance of any subcontracted service.
7.10 FORCE MAJEURE. Notwithstanding anything to the contrary herein, neither Party shall be liable in any manner for any delay to perform its obligations under this Agreement where the cause of such delay is beyond a Party’s reasonable control, including, without limitation, any delay or failure due to strikes, labor disputes, riots, earthquakes, storms, hurricanes, floods or other extreme weather conditions, fires, explosions, acts of God, embargoes, war or other outbreak of hostilities, government acts or regulations, or the failure or inability of carriers, suppliers, delivery services, or telecommunications providers to provide services necessary to enable a Party to perform its obligations hereunder. In any such circumstance, the Party unable to perform its obligations shall notify the other Party of such circumstance, and said other Party shall have the right to terminate this Agreement immediately pursuant to Section 3.2(c)(vii) if the Party continues to be unable to perform its obligations hereunder for a period of thirty (30) days.
7.11 CHANGE IN LAW. If, subsequent to the Effective Date (a) there is a change to any existing Applicable Laws; (b) any Applicable Law is promulgated, enacted, enforced, or otherwise applied; or (c) any decree, order, judgment, or permanent injunction is entered or enforced by any court of competent jurisdiction or any other government agency relating to the terms of this Agreement, which in the good faith opinion of UT or Distributor adversely and materially affects or will adversely and materially affect its business by reason of the terms of this Agreement, the affected Party shall notify the other Party in writing and the Parties will promptly negotiate alternative terms that would not adversely and materially affect the affected Party’s business, and that would, subject to Applicable Law, provide reasonably equivalent benefits to both Parties as the modified or deleted terms. If the Parties do not so reach a mutually satisfactory agreement within thirty (30) days after notice from the affected Party, the relevant adverse terms may be terminated or, if the relevant adverse terms are material to the overall Agreement, the Agreement may be terminated pursuant to Section 3.2(d).
7.12 WAIVER. No waiver of any term of this Agreement shall be valid unless waived in writing and signed by the Party against whom the waiver is sought. The failure of either Party to require performance by the other Party of any provision of this Agreement shall not affect, in any way, the right to require such performance at any time thereafter.
7.13 INDEPENDENT CONTRACTORS. Nothing in this Agreement is intended to create any relationship between Distributor and UT other than as independent contractors and neither Party, nor any of their employees, staff, agents, officers, or directors shall be construed to be the agent, fiduciary, employee, or representative of the other.
7.14 CHOICE OF LAW. This Agreement and performance of the obligations hereunder, shall be governed by, and construed in accordance with, the laws of the State of Delaware, without regard to the conflicts of laws provision therein.
7.15 SURVIVAL. The confidentiality and indemnification obligations described in this Agreement shall survive the termination of this Agreement. These ongoing obligations shall be binding upon both Parties regardless of the reason for the termination of this Agreement.
7.16 THIRD PARTY BENEFICIARIES. This Agreement is not a third party beneficiary contract, and, therefore, there are no third party beneficiaries to this Agreement.
[SIGNATURE PAGE FOLLOWS]
IN WITNESS WHEREOF, the undersigned, duly authorized, has executed this Agreement, effective as of the Effective Date.
|
PRIORITY HEALTHCARE DISTRIBUTION, INC. |
|
UNITED THERAPEUTICS CORPORATION | ||
|
|
|
| ||
|
|
|
| ||
|
By: |
/s/ Earl English |
|
By: |
/s/ Kevin T. Gray |
|
|
|
| ||
|
|
|
| ||
|
Name: |
Earl English |
|
Name: |
Kevin T. Gray |
|
|
|
| ||
|
Title: |
President |
|
Title: |
SVP, Strategic Operations |
|
|
|
| ||
|
Date: |
10.6.17 |
|
Date: |
12/20/2017 |
EXHIBIT A
PRODUCT PRICING
UT shall notify the Distributor in writing of any change (and the amount of the change) in the Price of any respective UT Product in accordance with Section 7.2.
UT shall provide Distributor with a current list of UT Product prices to Discounted Entities, including FSS prices, Federal Ceiling Prices, and prices to section 340B entities, and shall promptly notify Distributor of any and all changes in such prices as well as the effective dates of such changes.
|
UT PRODUCT NAME |
|
NDC |
|
STRENGTH |
|
PRICE |
|
Orenitram |
|
66302-300-01 |
|
0.125 mg |
|
$487.50 |
|
Orenitram |
|
66302-300-01 |
|
0.25 mg |
|
$975.00 |
|
Orenitram |
|
66302-300-01 |
|
1.0 mg |
|
$3,900.00 |
|
Orenitram |
|
66302-300-01 |
|
2.5 mg |
|
$9,750.00 |
|
Orenitram |
|
66302-350-01 |
|
5.0 MG |
|
$19,500.00 |
|
Orenitram |
|
66302-300-10 |
|
0.125 mg |
|
$48.75 |
|
Orenitram |
|
66302-300-10 |
|
0.25 mg |
|
$97.50 |
|
Orenitram |
|
66302-300-10 |
|
1.0 mg |
|
$390.00 |
|
Orenitram |
|
66302-300-10 |
|
2.5 mg |
|
$975.00 |
|
Orenitram |
|
66302-350-10 |
|
5.0 mg |
|
$1,950.00 |
|
Remodulin 1mg |
|
66302-0101-01 |
|
1mg/20ml |
|
$1,179.00 |
|
Remodulin 2.5mg |
|
66302-0102-01 |
|
2.5mg/20ml |
|
$2,947.50 |
|
Remodulin 5 mg |
|
66302-0105-01 |
|
5mg/20ml |
|
$5,895.00 |
|
Remodulin 10 mg |
|
66302-0110-01 |
|
10mg/20ml |
|
$11,790.00 |
|
Remodulin Diluent |
|
66302-150-50 |
|
50 mL vial, carton of 1 |
|
No-Charge |
|
Tyvaso Patient Starter Kit (PSK) |
|
66302-206-01 |
|
|
|
$16,750.00 |
|
Tyvaso Patient Resupply Kit (RSK) |
|
66302-206-02 |
|
|
|
$15,015.00 |
|
Tyvaso Supplemental Refill 4 ct |
|
66302-206-03 |
|
|
|
$2,145.00 |
|
Tyvaso Institutional Starter Kit (ISK) |
|
66302-206-04 |
|
|
|
$3,880.00 |
NDC 66302-206-01 Tyvaso Starter Kit includes:
· 28 ampules of Tyvaso
· Sets of Autoclavable Parts
· Tyvaso Inhalation Devices
· 2 AC Power Adapters
· 1 Rechargeable Battery Pack
· 1 Car Power Cord
· 1 Leather Carrying Case
· 32 Medicine Cups
· 64 Filter Membranes
· 1 Nose Clip
· 1 Measuring Cup
· 1 Safety Box
· 2 Sets of Safety Plugs
NDC 66303-206-02 Tyvaso Re-Supply Kit includes:
· 28 ampoules of Tyvaso
· 1 Set of Autoclavable Parts
· 32 Medicine Cups
· 64 Filter Membranes
NOC 66302-206-03 Tyvaso Supplemental Refill includes:
· 4 ampoules of Tyvaso
NDC 66302-206-04 Tyvaso Institutional Starter Kit (ISK) includes:
· 4 ampules of Tyvaso
· 2 Sets of Autoclavable Parts
· 2 Tyvaso Inhalation Devices
· 2 AC Power Adapters
· 1 Rechargeable Battery Pack
· 1 Car Power Cord
· 1 Leather Carrying Case
· 32 Medicine Cups
· 64 Filter Membranes
· 1 Nose Clip
· 1 Measuring Cup
· 1 Safety Box
· 2 Sets of Safety Plugs
EXHIBIT B
I. DISTRIBUTION SERVICES
|
SERVICE |
|
DESCRIPTION |
|
Development, Implementation and Management of internal requirements |
|
· Hiring and ongoing participation in training of staff related to UT Product. Distributor will maintain, throughout the Territory, adequate order fulfillment staff who are adequately trained on PAH and UT Product.
· Data and System set up to support timely and appropriate delivery of all required reports and data. |
|
Account Management |
|
· Call Center staffed to meet nationwide business hours of customer base
· Online Order functionality, including inquiry features (not available at Memphis location)
· Active management of Customer Relationships including but not limited to: responding to Product Inquiries; triage to clinical support for physicians and patients as appropriate; triage to sales and/or reimbursement support as provided by UT; designated account managers by disease state, etc. |
|
Rush/Special Order |
|
Orders that are received and processed outside normal parameters, such as expedited shipping, special instructions, etc. or at UT’s requests. This service should include Saturday delivery service as well as early AM delivery options as requested. |
|
Product Storage |
|
Controlled temperature Product storage. |
|
Order Processing |
|
Order is defined as a shipment to a unique address that leaves the distribution center, regardless of the number of cartons or packages that constitute that shipment and/or the number of inbound requests for said Order. Line is defined as each SKU or product line picked on the order. |
|
Receiving |
|
Receiving product into the warehouse, including review and monitoring of any temp tale devices used in shipments to assure proper specifications were maintained for inbound receipts |
|
Packing Supplies |
|
Any packing materials that Distributor must provide for to ship Products. |
|
SERVICE |
|
DESCRIPTION |
|
Credit/Rebill Transactions |
|
Any UT requested/caused credit or rebill transactions keyed in the system. |
|
RGA Initiation |
|
RGA: Returned Goods Authorization. |
|
Return Processing |
|
Receipt of physical return at the distribution center; includes itemizing contents of the return if at request of or as the result of an issue caused by UT. |
|
Returns Storage |
|
Returns Storage, including providing controlled room temperature pallet storage in Distributor morgue until product is returnable to UT along with tracking of return quantities and reasons. |
|
Chargeback Processing |
|
Process chargebacks, if applicable, to manufacturer or designee. |
|
Daily and Monthly Reports |
|
See Exhibit E and F for reporting details. |
|
Inventory Management |
|
In accordance with Section 1.2, establish mechanism to ensure appropriate inventory to meet the needs of the Customers |
II. [***]
III. SERVICE FEES
For sales to specialty pharmacies, Distributor will provide the Services described in Section I of this Exhibit B, and UT agrees to pay a Service Fee of [***] basis points from the WAC price for each Product purchased net returns for the Products defined below. Distributor will invoice UT monthly for this Service Fee.
· Remodulin 1MG VL
· Remodulin 2.5MG VL
· Remodulin 5MG VL
· Remodulin 10 MG VL
· Tyvaso 2.9ML ampules (4pack)
· Tyvaso starter kit
· Tyvaso refill kit
· Orenitram 5.0 MG tablet
· Orenitram 2.5MG tablet
· Orenitram 0.25MG tablet
· Orenitram 0.125MG tablet
· Orenitram 1MG tablet
The Service Fee is compensation to Distributor for all services described in exhibits and herein, except as otherwise noted below:
|
Custom Reports |
|
UT to pay Distributor $[***]/hour |
|
Fee for reports created that are not part of the standard reports provided by Distributor. Hourly report creation fees assessed for initial report creation but not thereafter for running the same report. |
|
Custom Development Services |
|
UT to pay Distributor $[***]/hour |
|
Fee for customized processes developed at UT’s request. Hourly fees will be assessed and approved by UT before development work is to begin. |
|
[***] |
|
[***] |
|
[***] |
EXHIBIT C
CUSTOMER LISTING
· Hospitals
· FSS (including VA and 340B)
· Specialty Pharmacies limited to the following:
· Accredo Health Group
EXHIBIT D
UT WARRANTY
UT warrants that all of its UT Product shall as of the date such UT Product leaves UT’ s facility:
(i) be free from defects in design, material and workmanship, (ii) be in compliance with all applicable law and regulation, including without limitation all regulatory requirements of the FDA, including those related to the adulteration or misbranding of UT Product within the meaning of Section 501 and 502 of the Food Drug and Cosmetics Act, (iii) not be articles which may not be introduced into interstate commerce pursuant to the requirements of Sections 505, 514, 515, 516 or 520 thereof, and (iv) be manufactured in accordance with current FDA Good Manufacturing Practice as required by 21 C.F.R. 210 and 820.
EXHIBIT E
DATA SPECIFICATIONS
Shipment
|
Field Name |
|
Data Type |
|
Required |
|
Description |
|
Ship Date |
|
Date |
|
Y |
|
MMDDYYYY |
|
Quantity Shipped |
|
Numeric |
|
Y |
|
Including negative quantities |
|
Customer # |
|
Numeric |
|
Y |
|
CSD Ship To Account # |
|
Ship to Name |
|
Varchar |
|
Y |
|
|
|
Ship to Address #1 |
|
Varchar |
|
Y |
|
|
|
Ship to Address #2 |
|
Varchar |
|
N |
|
Field labeled “Additional Heading” |
|
Ship to City |
|
Varchar |
|
Y |
|
|
|
Ship to State |
|
Varchar |
|
Y |
|
|
|
Ship to Zip |
|
Numeric |
|
Y |
|
5 digits only |
|
Ship to Phone |
|
Varchar |
|
N |
|
|
|
Bill to Name |
|
Varchar |
|
Y |
|
|
|
Bill to Address #1 |
|
Varchar |
|
Y |
|
|
|
Bill to Address #2 |
|
Varchar |
|
N |
|
Field labeled “Additional Heading” |
|
Bill to City |
|
Varchar |
|
Y |
|
|
|
Bill to State |
|
Varchar |
|
Y |
|
|
|
Bill to Zip |
|
Numeric |
|
Y |
|
5 digits only |
|
NDC Number |
|
Varchar |
|
Y |
|
|
|
HIN # |
|
Varchar |
|
N |
|
|
|
DEA# |
|
Varchar |
|
N |
|
|
|
NPI # |
|
Varchar |
|
N |
|
|
|
Warehouse |
|
Varchar |
|
Y |
|
Warehouse location shipped from |
|
Lot# |
|
Varchar |
|
Y |
|
|
|
Lot Expiration Date |
|
Varchar |
|
Y |
|
|
|
Invoice # |
|
Numeric |
|
Y |
|
Document # |
|
Order # |
|
Numeric |
|
Y |
|
|
|
Format |
|
CSV |
|
Frequency |
|
Daily & Monthly |
|
Distribution |
|
FTP or email |
|
Products to Report |
|
TBD |
|
File Name |
|
CURASCRIPT_SD_SHIPMENT_DRUGXXXX_MMYYYY.CSV (monthly) or |
|
Other |
|
Zero byte file if no records exist |
EXHIBIT E (CONT.)
DATA SPECIFICATIONS
|
Field Name |
|
Data Type |
|
Required |
|
Description |
|
NDC # |
|
Varchar |
|
Y |
|
|
|
Drug Name |
|
Varchar |
|
Y |
|
|
|
Reporting Period Start Date |
|
Date |
|
Y |
|
MMDDYYYY |
|
Reporting Period End Date |
|
Date |
|
Y |
|
MMDDYYYY |
|
Reporting Date |
|
Date |
|
Y |
|
MMDDYYYY |
|
Warehouse Name |
|
Varchar |
|
Y |
|
|
|
Quantity On Hand |
|
Numeric |
|
Y |
|
If none then “0” |
|
Quantity Received |
|
Numeric |
|
Y |
|
If none then “0” |
|
Quantity On Order |
|
Numeric |
|
Y |
|
If none then “0” |
|
Quantity Adjustment |
|
Numeric |
|
Y |
|
If none then “0”; include negative quantity |
|
Quantity Shipped |
|
Numeric |
|
Y |
|
If none then “0” |
|
Quantity Transferred |
|
Numeric |
|
Y |
|
If none then “0” |
|
Quantity Returned |
|
Numeric |
|
Y |
|
If none then “0” |
|
Format |
|
CSV |
|
Frequency |
|
Daily & Monthly |
|
Distribution |
|
FTP or email |
|
Products to Report |
|
TBD |
|
File Name |
|
CURASCRIPT_SD_INVENTORY_DRUGXXXX_MMYYYY.CSV (monthly) or |
|
Other |
|
Zero byte file if no records exist |
EXHIBIT G
DISTRIBUTOR LOCATIONS
Priority Healthcare Distribution Inc.
2297 Southwest Blvd., Suite D
Grove City, OH 43123
614-539-8074 (Phone)
614-539-2798 (Fax)
DEA # RP0334540
HIN #K771N1N00
Priority Healthcare Distribution Inc.
2040 W. Rio Salado Parkway, Suite 101A
Tempe, AZ 85281
480-403-3689 (Phone)
480-403-3672 (Fax)
HIN# J343K1E00
Priority Healthcare Distribution
1680 Century Center Parkway Ste 8
Memphis, TN 38134
901-385-3600 (Phone)
866-628-8942 (Fax)
DEA # RA0401416
Other locations must be mutually agreed to in writing by the Parties.
Exhibit H
Adverse Events and Product Complaints Definitions
|
Adverse Drug Experience |
|
Includes and adverse experience, LOE pregnancy or medication error, each as defined below |
|
Adverse Event (“AE”)/Adverse Experience |
|
Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have to have a causal relationship with this treatment. |
|
Lack of Effect repot (“LOE”) |
|
A report of a situation where there is apparent failure of the company product or medicinal technology to bring about the intended beneficial effect on individuals in a defined population with a given medical problem, under ideal condition of use. |
|
Medication Error |
|
Any preventable event that can cause or lead to inappropriate medication use or patient harm while the medication is in the control of a healthcare professional, patient or consumer. |
|
Special Situations |
|
Special Situations are not AEs but are similar to “events of special interests” that require similar reporting to regulatory authorities worldwide & they are: use of product during pregnancy/breastfeeding, product overdose (accidental or intentional), product misuse/abuse (accidental or intentional), Off Label product use (product used for indication not approved in USPI), accidental or occupational exposure. |
|
Product Complaint (“PC”) |
|
Information concerning any incident that causes the drug or its label to be mistaken for, or applied to another article, and bacteriological contamination, or any significant changes in distributed drug product (chemical, physical, deterioration or |
|
|
|
other change), and or failure of a batch of distributed drug product to meet specifications established for it in the application. |
