Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Evofem Biosciences, Inc. | d542933d8k.htm |

Presentation at RBC Capital Markets February 21, 2018 Global Healthcare Conference NASDAQ: EVFM Exhibit 99.1

Disclaimer This presentation contains forward looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 and other federal securities laws. In some cases, you can identify forward looking statements by terms such as “may,” ”will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “strategy,” “designed,” “suggest,” “currently,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward looking statements of this presentation are only predictions and are subject to a number of risks, uncertainties and assumptions, including, without limitation risks and uncertainties relating to: the outcome or success of Evofem’s clinical trials; Evofem’s ability to maintain and protect its intellectual property; the rate and degree of market acceptance of Amphora; Evofem’s ability to successfully commercialize Amphora and its ability to develop sales and marketing capabilities; Evofem’s ability to raise additional capital when needed and to rely on existing cash reserves to fund its current development plans and operations; Evofem’s ability to obtain the necessary regulatory approvals for its product candidates, including approvals from the united states food and drug administration for the use of Amphora® as a contraceptive, and the timing of such approvals; Evofem’s reliance on third party providers, such as third party manufacturers and clinical research organizations; the absence of any adverse events or side effects relating to the use of Amphora; and Evofem’s ability to retain members of its management and other key personnel. Many of these risks, and other risks and uncertainties, are discussed in further detail in the “Risk Factors-Risks Related to Evofem” section of a registration statement on Form S-4 initially filed with the Securities and Exchange Commission (the SEC) by Evofem Biosciences, Inc. (formerly known as Neothetics, Inc.) on November 15, 2017, a copy of which is available on the Evofem website (www.evofem.com).The forward looking statements in this presentation represent Evofem’s views only as of the date of this presentation, February 21, 2018, and Evofem undertakes no obligation to update or review any forward looking statement, whether as a result of new information, future developments or otherwise, except as required by law. This presentation is for informational purposes only and is not an offer to sell or a solicitation of an offer to buy any security. Any such offering would only be made pursuant to definitive documents relating specifically to the offering of such securities. This presentation includes certain information obtained from trade and statistical services, third party publications and other sources. Evofem has not independently verified such information and there can be no assurance of its accuracy.

Evofem Biosciences is developing innovative products to fill unmet needs of women in reproductive and sexual health Our Mission
Nasdaq-listed 18 Jan 2018 “EVFM”, $20M investment from Invesco Asset Mgmt. Next-generation women’s health company Amphora® poised to compete in $5.7B Rx contraceptive market1 On demand, woman-controlled, hormone-free vaginal gel Top-line results from confirmatory Phase 3 trial expected Q1 2019 Additional opportunities for Amphora: prevention of gonorrhea and chlamydia Next in pipeline: vaginal gel to reduce bacterial vaginosis recurrence Experienced management team with strong track record in women’s health About Evofem Biosciences 1 IMS MIDAS Data - December 2014.

Seasoned Management Team with Established Track Record of Clinical and Commercial Execution Officer / Title Selected Achievements Saundra Pelletier Chief Executive Officer & President Extensive global experience as a pharmaceutical executive, entrepreneur and advocate for women’s health Founding CEO of WCG, an internationally recognized non-profit organization 30 years experience in executive management in women’s health Jay File Chief Financial Officer Over $560M in equity and debt offerings in the biotech and healthcare industries Commercial launch of four diagnostic tests in the women’s health market during a two-year period 25 years experience in accounting and finance in public and private biotechnology companies Kelly Culwell, M.D. Chief Medical Officer Medical advisor to the International Planned Parenthood Federation Medical Officer with the WHO and board-certified gynecologist and obstetrician 20 years experience medical oversight in women’s health Russ Barrans Chief Commercial Officer Managed the launch of over half a dozen brands worldwide including Mirena®, and Plan B One-Step® OTC Oversight of global and domestic product launches in women’s health 25 years experience marketing world wide product sales and distribution
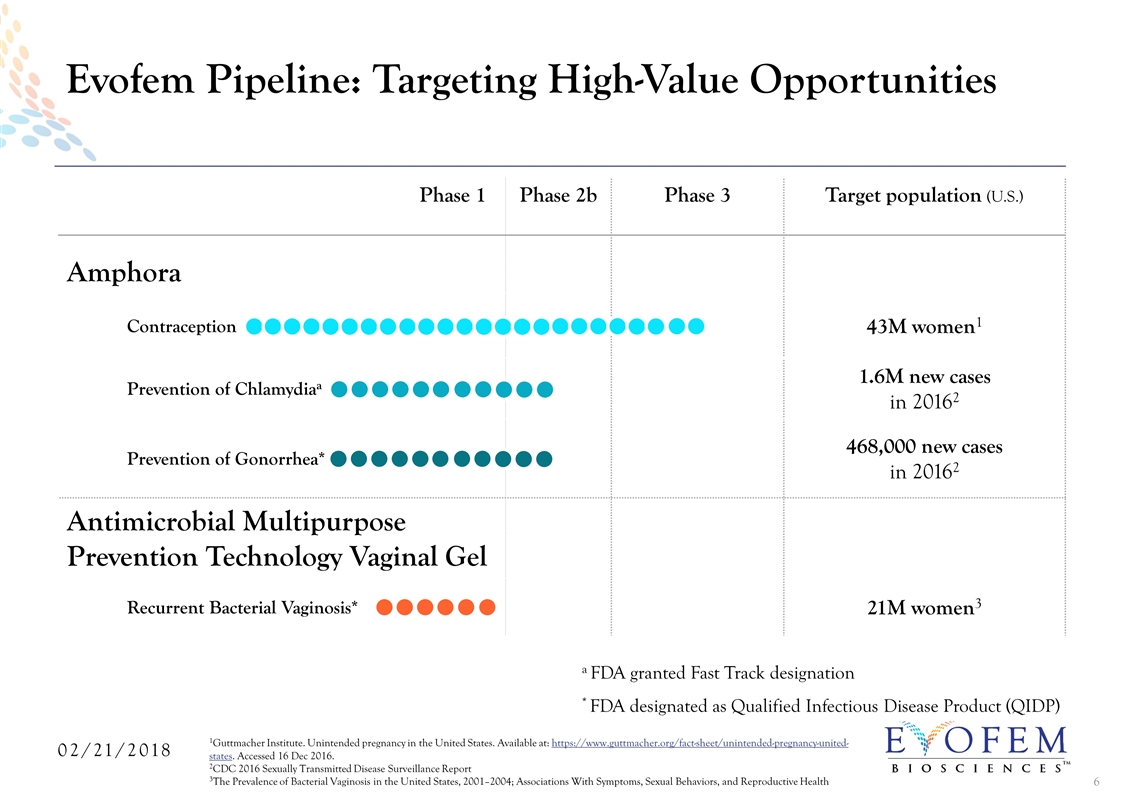
Phase 1 Phase 2b Phase 3 Target population (U.S.) Amphora Contraception 43M women1 Prevention of Chlamydiaa 1.6M new cases in 20162 Prevention of Gonorrhea* 468,000 new cases in 20162 Antimicrobial Multipurpose Prevention Technology Vaginal Gel Recurrent Bacterial Vaginosis* 21M women3 Evofem Pipeline: Targeting High-Value Opportunities 1Guttmacher Institute. Unintended pregnancy in the United States. Available at: https://www.guttmacher.org/fact-sheet/unintended-pregnancy-united-states. Accessed 16 Dec 2016. 2CDC 2016 Sexually Transmitted Disease Surveillance Report 3The Prevalence of Bacterial Vaginosis in the United States, 2001–2004; Associations With Symptoms, Sexual Behaviors, and Reproductive Health a FDA granted Fast Track designation * FDA designated as Qualified Infectious Disease Product (QIDP)
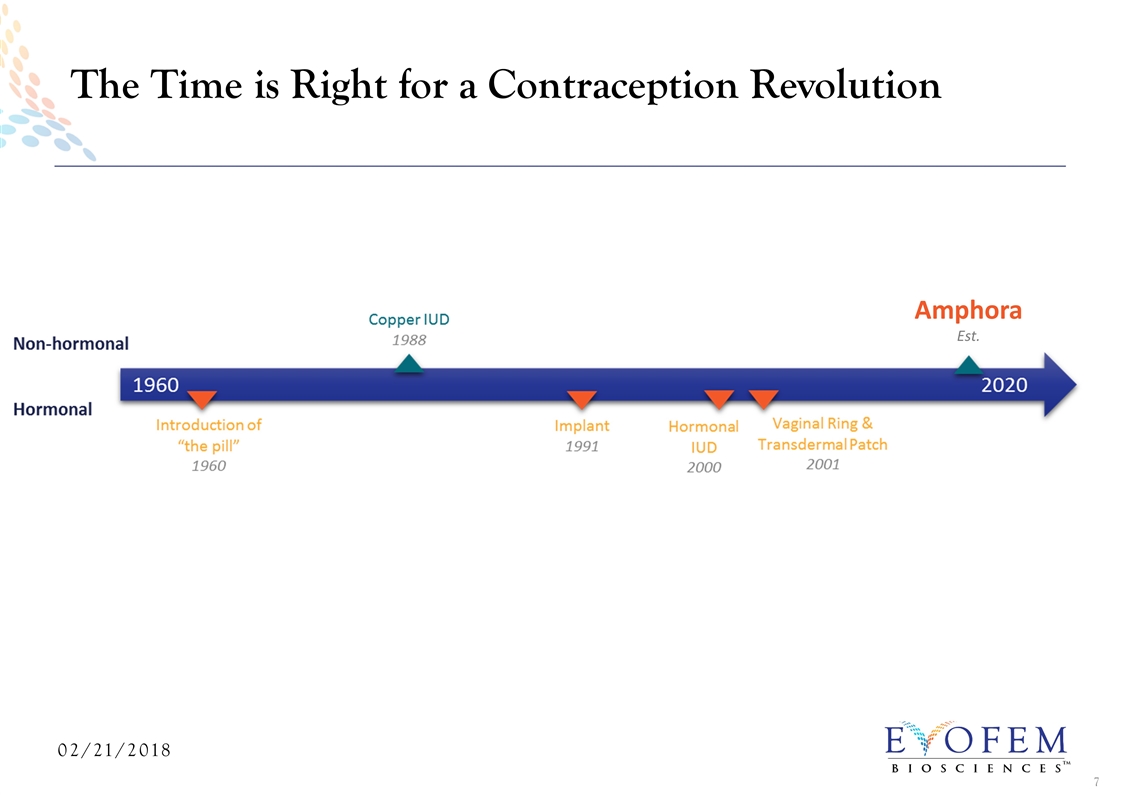
The Time is Right for a Contraception Revolution Amphora Est.

Women manage their fertility for decades Contraception is switched 3-4 times throughout their lifetime due to side effects driven by hormones Loss of libido, headaches, moodiness, nausea Nontraditional forms of contraception have led to U.S. market growth of $700M over the last 5 years1 43M women are at risk for pregnancy2 71% of women have concerns about hormone exposure3 58% are not currently satisfied with their contraceptive choice3 30% want to avoid hormones completely 28% indicated that Amphora was an effective addition to their current method Will Women Use Amphora? 1 IMS MIDAS Data - December 2014. 2 Guttmacher Institute. Unintended pregnancy in the United States. Available at: https://www.guttmacher.org/fact-sheet/unintended-pregnancy-united-states. Accessed 16 Dec 2016. 3 US Primary Market Research with Consumers - January 2016 (n=287).
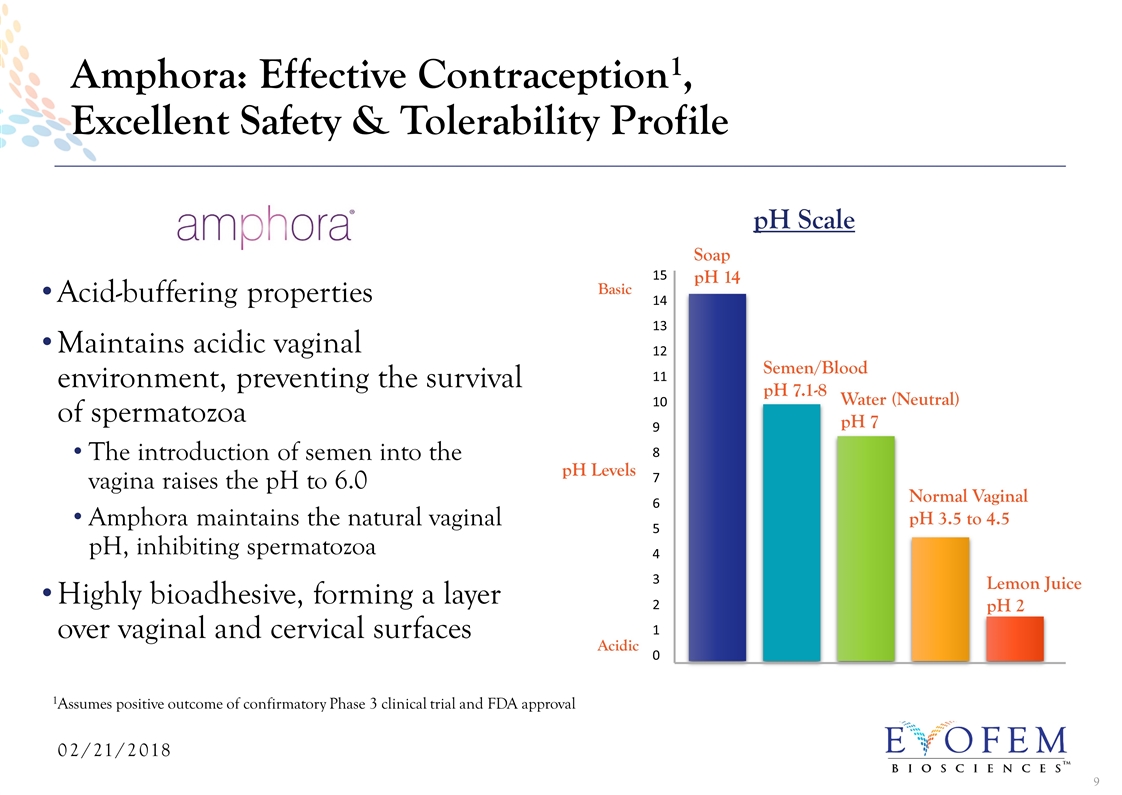
Acid-buffering properties Maintains acidic vaginal environment, preventing the survival of spermatozoa The introduction of semen into the vagina raises the pH to 6.0 Amphora maintains the natural vaginal pH, inhibiting spermatozoa Highly bioadhesive, forming a layer over vaginal and cervical surfaces Amphora: Effective Contraception1, Excellent Safety & Tolerability Profile Normal Vaginal pH 3.5 to 4.5 Water (Neutral) pH 7 Semen/Blood pH 7.1-8 Lemon Juice pH 2 pH Scale 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 Acidic Basic pH Levels Soap pH 14 1Assumes positive outcome of confirmatory Phase 3 clinical trial and FDA approval
Multicenter, randomized, open-label trial Primary endpoint: Contraceptive efficacy compared to Conceptrol (nonoxynol-9) 3,400 women at 62 sites (49 U.S., 13 Russia)1 No safety concerns Phase 3 Comparative Contraceptive Trial: AMP001 Source: Company materials, ClinicalTrials.gov. 18-35 year old adult women, sexually active, at risk for pregnancy and desiring contraception.
April 28, 2016: Complete Response Letter (CRL) Issues outlined: Russian subjects considered not generalizable to the U.S. population Certain cycles excluded from analysis Cycle 0 Cycles <21 days or >42 days Results and Safety Data of AMP001 Phase 3 Clinical Trial 6 Months Amphora 6 Months Conceptrol Difference Perfect Use Ϯ 4.1% 4.2% -0.1% (95% Confidence Interval)* (2.7% - 5.4%) (2.8% - 5.6%) (-2.1%, 1.8%) Typical Use^ 10.5% 10% 0.5% (95% Confidence Interval)* (8.6% - 12.3%) (8.1% - 11.9%) (-2.2%, 3.2%) Amphora Conceptrol All Subjects System organ class (N=1458) (N=1477) (N=2935) Preferred term n(%) n(%) n(%) Total number (%) of subjects with at least one serious adverse event 11 (0.8) 19 (1.3) 30 (1.0) Total number (%) of subjects with at least one adverse event 833 (57.1) 857 (58.0) 1690 (57.6) Ϯ Perfect use defined as trial subjects who used the product correctly at every episode of coitus within a given cycle ^Typical use defined as trial subjects who had at least one episode of coitus without using the product correctly during the study and without any backup or emergency contraception *If upper bound of the CI for the difference at 6 months is ≤ 5.5%, then non-inferiority is concluded
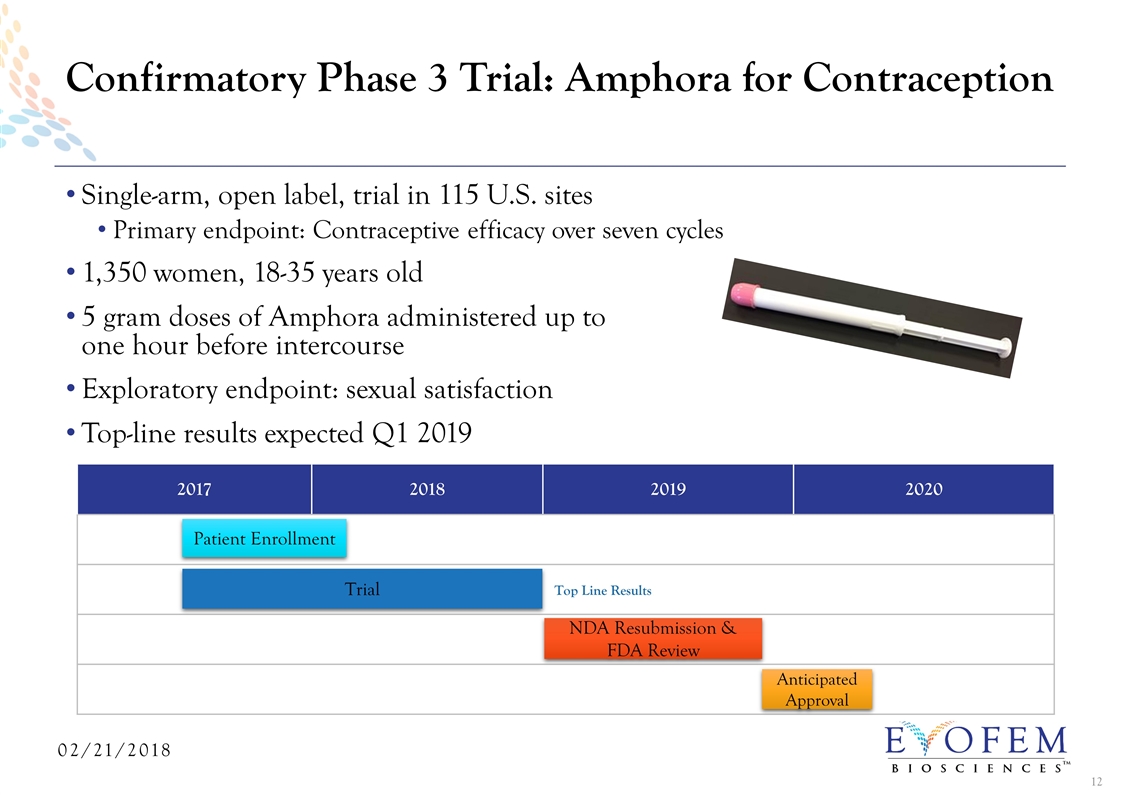
Single-arm, open label, trial in 115 U.S. sites Primary endpoint: Contraceptive efficacy over seven cycles 1,350 women, 18-35 years old 5 gram doses of Amphora administered up to one hour before intercourse Exploratory endpoint: sexual satisfaction Top-line results expected Q1 2019 Confirmatory Phase 3 Trial: Amphora for Contraception 2017 2018 2019 2020 Patient Enrollment Trial NDA Resubmission & FDA Review Anticipated Approval Top Line Results

Phase 2b/3 trial underway Double-blinded placebo-controlled efficacy trial 850 women, 18-35 years old, with chlamydia or gonorrhea infection in past 4 months 20 U.S. sites Four-month interventional period + one-month follow-up Primary objective: Prevention of chlamydia trachomatis in women Secondary objective: Prevention of neisseria gonorrhea in women Favorable regulatory position FDA granted Fast Track designation for prevention of urogenital chlamydia in women FDA designated as a QIDP for prevention of gonorrhea in women Eligible for FDA’s Fast Track Adds five years market exclusivity Amphora for Prevention of STIs
BV ~40% recurrence rate1 Phase 1 dose-finding study completed pH levels maintained to seven days Trial design in development Differentiated formulation under assessment Anticipated to enroll ~230 women at up to 36 U.S sites Anticipated First Patient In: Q4 2018 FDA designated as a Qualified Infectious Disease Product Eligible for FDA’s Fast Track Adds five years market exclusivity Phase 2b/3 Clinical Trial: MPT for Bacterial Vaginosis 1Koumans EH et al., The prevalence of bacterial vaginosis in the United States, 2001-2004; associations with symptoms, sexual behavior, and reproductive health. Sex. Transm Dis 34, 864-869 (2007).
Nasdaq: EVFM Shares outstanding 17.8M 52-week range (02/15/2018) $1.80 - $15.78 Recent share price (02/15/2018)$ 7.06 Market Cap (02/15/2018)$125.4M Key Stock Data
Multiple Near-term Catalysts Anticipated Initiated Phase 2b/3 Amphora STI trial (first patient in) Jan 2018 Completed enrollment in Phase 3 Amphora contraceptive trial Feb 2018 Initiate Phase 2b/3 MPT vaginal gel BV trial (first patient in) Q4 2018 Top-line results from Phase 3 Amphora contraceptive trial Q1 2019 Resubmit Amphora NDA for contraception Q2 2019 Anticipated FDA action on Amphora NDA for contraception Q4 2019
Leveraging MPT vaginal gel technology platform to develop and commercialize novel, first-in-class products for women Preparing lead candidate Amphora for 2020 entry into $5.7B U.S. Rx contraceptive market* Addresses significant gaps in the market -- on demand, woman-controlled, hormone-free contraception Label expansion opportunities for Amphora in STI prevention Led by experienced management team with history of success in women’s health Why Evofem? Why Now? *Assumes positive outcome of confirmatory Phase 3 clinical trial and FDA approval

