Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - Dova Pharmaceuticals Inc. | a2234415zex-23_1.htm |
| EX-5.1 - EX-5.1 - Dova Pharmaceuticals Inc. | a2234415zex-5_1.htm |
| EX-1.1 - EX-1.1 - Dova Pharmaceuticals Inc. | a2234415zex-1_1.htm |
Use these links to rapidly review the document
TABLE OF CONTENTS
As filed with the Securities and Exchange Commission on February 20, 2018
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Dova Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
2834 (Primary Standard Industrial Classification Code Number) |
81-3858961 (I.R.S. Employer Identification Number) |
240 Leigh Farm Road, Suite 245
Durham, North Carolina 27707
(919) 748-5975
(Address, including zip code, and telephone number, including area code, of registrant's principal executive offices)
Alex Sapir
President and Chief Executive Officer
Dova Pharmaceuticals, Inc.
240 Leigh Farm Road
Suite 245
Durham, NC 27707
(919) 748-5975
(Name, address, including zip code, and telephone number, including area code, of agent for service)
| Copies to: | ||
Divakar Gupta Darren DeStefano Mark Ballantyne Cooley LLP 1114 Avenue of the Americas New York, New York 10036 (212) 479-6000 |
Deanna Kirkpatrick Davis Polk & Wardwell LLP 450 Lexington Avenue New York, New York 10017 (212) 450-4000 |
|
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company," and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer ý (Do not check if a smaller reporting company) |
Smaller reporting company o Emerging growth company ý |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ý
CALCULATION OF REGISTRATION FEE
|
||||||||
| Title of each class of securities to be registered |
Amount to be registered(1) |
Proposed maximum offering price per share |
Proposed maximum aggregate offering price(2) |
Amount of registration fee |
||||
|---|---|---|---|---|---|---|---|---|
Common Stock, $0.001 par value per share |
2,875,000 | $31.15 | $89,556,250 | $11,149.76 | ||||
|
||||||||
(1) Includes shares the underwriters have the option to purchase.
(2) Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) under the Securities Act on the basis of the average of the high and low prices of the Registrant's common stock as reported on the NASDAQ Global Market on February 14, 2018.
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment that specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Subject to completion, dated February 20, 2018
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
2,500,000 Shares

Common Stock
We are offering 2,500,000 shares of our common stock. Our common stock is listed on The NASDAQ Global Market under the symbol "DOVA." The last reported sales price of our common stock on The NASDAQ Global Market on February 16, 2018 was $34.68 per share. The final public offering price will be determined through negotiation between us and the lead underwriters in the offering and the recent market price used throughout the prospectus may not be indicative of the final offering price.
We are an "emerging growth company" as defined under the federal securities laws and will be subject to reduced public company reporting requirements.
| | | | | | | | |
| |
Per share |
Total |
|||||
|---|---|---|---|---|---|---|---|
| | | | | | | | |
Public offering price |
$ | $ | |||||
Underwriting discounts and commissions(1) |
$ |
$ |
|||||
Proceeds to Dova Pharmaceuticals, Inc., before expenses |
$ |
$ |
|||||
| | | | | | | | |
(1) We have also agreed to reimburse the underwriters for certain FINRA-related expenses. See "Underwriting" in this prospectus for a description of compensation payable to the underwriters.
We have granted the underwriters an option for a period of 30 days to purchase up to 375,000 additional shares of common stock on the same terms and conditions set forth above.
Investing in our common stock involves a high degree of risk. See "Risk factors" beginning on page 13 of this prospectus, as well as in the documents incorporated or deemed to be incorporated by reference into this prospectus.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares in New York, New York against payment to investors on or about , 2018.
| J.P. Morgan | Jefferies | Evercore ISI |
Prospectus dated , 2018
Neither we nor the underwriters have authorized anyone to provide you with information other than that contained or incorporated by reference in this prospectus or any free writing prospectus prepared by or on behalf of us or to which we have referred you. We and the underwriters take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We and the underwriters are offering to sell, and seeking offers to buy, common stock only in jurisdictions where offers and sales are permitted. The information contained or incorporated by reference in this prospectus is accurate only as of the date on the front cover page of this prospectus or the date of the applicable document incorporated by reference, or other earlier date stated in this prospectus or in the applicable document incorporated by reference, regardless of the time of delivery of this prospectus or of any sale of our common stock.
To the extent there is a conflict between the information contained in this prospectus, on the one hand, and the information contained in any document incorporated by reference filed with the Securities and Exchange Commission before the date of this prospectus, on the other hand, you should rely on the information in this prospectus. If any statement in a document incorporated by reference is inconsistent with a statement in another document incorporated by reference having a later date, the statement in the document having the later date modifies or supersedes the earlier statement.
No action is being taken in any jurisdiction outside the United States to permit a public offering of our common stock or possession or distribution of this prospectus in that jurisdiction. Persons who come into possession of this prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus applicable to that jurisdiction.
This summary highlights information contained elsewhere in this prospectus and in the documents incorporated by reference. This summary does not contain all of the information you should consider before investing in our common stock. You should read this entire prospectus and the documents incorporated by reference in this prospectus carefully, especially the "Risk factors" section beginning on page 13 of this prospectus and in the documents incorporated by reference into this prospectus and our consolidated financial statements and the related notes incorporated by reference in this prospectus, before making an investment decision.
As used in this prospectus and in the documents incorporated by reference, unless the context otherwise requires, references to "we," "us," "our," "the company" and "Dova Pharmaceuticals" refer to Dova Pharmaceuticals, Inc. and our wholly-owned subsidiary, AkaRx, Inc.
Overview
We are a pharmaceutical company focused on acquiring, developing and commercializing drug candidates for diseases that are treated by specialist physicians, with an initial focus on addressing thrombocytopenia, a disorder characterized by a low blood platelet count. Our drug candidate, avatrombopag, which we acquired from Eisai, Inc., or Eisai, in March 2016, is an orally administered thrombopoietin receptor agonist, or TPO-RA, that we are developing for the treatment of thrombocytopenia. On September 21, 2017, we submitted a new drug application, or NDA, to the U.S. Food and Drug Administration, or FDA, for the treatment of thrombocytopenia in patients with chronic liver disease, or CLD, scheduled to undergo a procedure. The NDA submission was supported by two identically designed pivotal Phase 3 clinical trials, both of which met the primary and secondary endpoints with high statistical significance. The NDA was granted Priority Review by the FDA in November 2017 and the Prescription Drug User Fee Act goal date for an FDA decision is May 21, 2018. We intend to build a hepatology-focused sales organization in the United States and have initiated commercial activities to support the launch of avatrombopag.
We also plan to submit a Supplemental New Drug Application, or sNDA, in the second half of 2018 for the treatment of patients with immune thrombocytopenic purpura, or ITP, which is chronic thrombocytopenia that requires continuous treatment. To date, avatrombopag has been evaluated in one Phase 3 clinical trial and two Phase 2 clinical trials for the treatment of adults with chronic ITP. In the Phase 3 trial, the primary efficacy endpoint was achieved with high statistical significance (p<0.0001). Additionally, during the first quarter of 2018, we are initiating a Phase 3 trial in pre-surgery thrombocytopenia, or PST, and in the second quarter of 2018 we plan to initiate a Phase 3 clinical trial for the treatment of patients with chemotherapy-induced thrombocytopenia, or CIT.
We believe that avatrombopag's efficacy and safety profile in combination with its convenient oral dosing could provide advantages over other treatments for thrombocytopenia. To date, avatrombopag has been evaluated in more than 20 clinical trials involving more than 1,100 subjects and has been observed to be generally well tolerated. We believe that avatrombopag may have the potential to be used more broadly for patients with thrombocytopenia.
Thrombocytopenia and current treatments
Thrombocytopenia is characterized by a deficiency of platelets that impairs blood clot formation and increases bleeding risk. Thrombocytopenia is defined as having less than 150,000 platelets per microliter of circulating blood and is diagnosed with a routine blood test. Thrombocytopenia can result in significant
1
bleeding risk even in cases of minor injury and increases the risk of excessive, uncontrolled bleeding during or after a medical procedure. Physicians determine how to treat thrombocytopenia, either in the acute setting or chronically, based on a number of factors, including the patient's platelet count, etiology of the underlying cause of thrombocytopenia, duration of required platelet count elevation and the patient's overall health profile.
Acute setting
We have initially focused on developing avatrombopag for the treatment of thrombocytopenia in CLD patients scheduled to undergo a procedure. CLD involves the progressive destruction and regeneration of the liver over a period of more than six months. Patients with CLD have reduced platelet production when liver cell mass becomes severely damaged. In addition, these patients also have increased trapping of platelets in the spleen and thus even fewer platelets are present in circulating blood. For multiple reasons, these patients often develop thrombocytopenia, which often worsens with the severity of the liver disease. Approximately 1.1 million CLD patients in the United States are affected by thrombocytopenia.
Patients with CLD undergo numerous non-emergent medical procedures for diagnosis and treatment of their disease, including liver biopsies, fluid removal, liver transplantation and endoscopy. Multiple medical professional associations have guidelines that recommend that patients have at least 50,000 platelets per microliter of circulating blood prior to minimally to moderately invasive medical procedures. Approximately 70,000 CLD patients in the United States have a platelet count less than 50,000 platelets per microliter of circulating blood.
We estimate that approximately 60% of these 70,000 CLD patients are treated with platelet transfusions in order to raise platelet counts in advance of medical procedures. These patients generally undergo one to three medical procedures per year.
More broadly outside of this CLD patient population, approximately 125,000 platelet transfusions are administered annually for more invasive planned surgical procedures for thrombocytopenia patients irrespective of disease etiology. Additionally, approximately 125,000 platelet transfusions are administered for patients with chemotherapy-induced thrombocytopenia, or CIT. These are two follow-on indications we are pursuing.
Despite being the standard of care, platelet transfusions are associated with limitations that impact their use including variable efficacy, risk of transfusion reactions, antibody development in up to 50% of patients, short duration of effect of transfused platelets, limited supply and inconvenience of administration. There is no drug treatment approved by the FDA or the European Medicines Agency, or EMA, for thrombocytopenia in patients with CLD in the acute setting prior to a medical procedure.
Chronic setting
Chronic treatment of thrombocytopenia involves continuous treatment of the disorder. The substantial majority of patients who require chronic treatment suffer from immune thrombocytopenic purpura, or ITP. First-line therapy for ITP consists of corticosteroids or intravenous immunoglobulin, or IVIG. In addition to off-label rituximab and splenectomy, currently marketed TPO-RAs are used as a second-line treatment of ITP.
We estimate that chronic ITP affects approximately 60,000 adults in the United States, of which up to 27,000 may require continuous treatment beyond corticosteroids and IVIG. However, we believe these available treatments have limitations that impact their use, such as limited efficacy, risk to patient safety, patient non-compliance or inconvenience.
2
Because of the limitations of current therapies used for thrombocytopenia in the acute and chronic setting, we believe there remains a significant unmet need for a treatment that demonstrates reliable and durable effectiveness and a favorable safety profile, that can be conveniently administered and potentially reduce the burden on patients.
Our drug candidate
We believe our drug candidate, avatrombopag, has the potential to be a first-in-class drug treatment of thrombocytopenia in the acute setting and a best-in-class treatment of thrombocytopenia in the chronic setting. Avatrombopag is an orally administered, small molecule TPO-RA, which is intended to address the limitations of other existing treatments for thrombocytopenia.
Avatrombopag is designed to mimic the effects of thrombopoietin, or TPO, in vitro and in vivo. TPO is a hormone produced in the liver and kidneys that binds to its receptor, c-Mpl (myeloproliferative leukemia protein). Following TPO receptor binding, intracellular signaling leads to megakaryocyte growth and maturation, which results in increased platelet production. TPO-RAs, like TPO, stimulate the activation, proliferation and maturation of megakaryocytes, resulting in an increase in circulating platelets. Avatrombopag is a highly specific TPO-RA as it binds to the TPO receptor at a distinct site from native TPO, leaving the TPO receptor accessible to native TPO, enabling avatrombopag to have an additive effect on platelet production.
While TPO-RAs are a validated class of therapy for the chronic treatment of thrombocytopenia, they have not been approved for the acute treatment of thrombocytopenia due, in part, to the risk of side effects, including portal vein thrombosis, or PVT. In CLD patients, who often have excessive accumulation of scar tissue in the liver, portal blood flow may be significantly lower than normal putting the patient at an increased risk of developing PVT. Further, the use of some TPO-RAs may lead to an even greater risk of PVT in these patients as large increases in platelet counts can give rise to platelet accumulation and cause further blockage of the portal vein.
We believe avatrombopag's pharmacokinetic/pharmacodynamic, or PK/PD, profile and metabolic characteristics are the attributes that differentiate it from the currently marketed TPO-RAs and could make it a compelling treatment option for patients with thrombocytopenia in the acute setting. In clinical trials, avatrombopag has been observed to have a less variable PK/PD profile than other TPO-RAs. In addition, avatrombopag is not extensively metabolized—approximately 40% to 50% is metabolized and is mostly eliminated away from the biliary route. We believe these metabolic characteristics and this PK/PD profile further reduce the risk of adverse effects, including thromboembolic events such as PVTs, in patient populations that are liver compromised, such as those with CLD.
In the chronic setting, we believe that approved TPO-RAs suffer from limitations impacting their broader use such as the risk of severe hepatotoxicity, need for subcutaneous administration, and impact of food on drug absorption. We believe avatrombopag has the potential to provide an effective, predictable, convenient and safe alternative to currently available TPO-RAs.
Clinical trials
In the first quarter of 2017, we completed two identically designed Phase 3 pivotal clinical trials, ADAPT 1 and ADAPT 2, in which all primary and secondary endpoints were met with high statistical significance. The primary endpoint for both studies was the percentage of CLD patients with thrombocytopenia scheduled to undergo a procedure, who did not require a platelet transfusion or any rescue procedure for bleeding at each of two doses of avatrombopag compared to placebo. In each trial, the percentage of subjects in each
3
of the two avatrombopag dosing cohorts requiring a platelet transfusion or a rescue procedure for bleeding was statistically significantly lower compared to placebo (across all cohorts, p-values ranging from p<0.0001 to p=0.0006). We also observed a larger percentage of avatrombopag-treated subjects who achieved the target platelet count of greater than or equal to 50,000 platelets per microliter of circulating blood on the procedure day and greater changes in platelet counts from baseline to procedure day, which were statistically significant improvements over placebo. We are initially developing avatrombopag for the acute treatment of thrombocytopenia in this population of patients with CLD scheduled to undergo a procedure.
In addition to ADAPT 1 and ADAPT 2, avatrombopag has also been evaluated in one Phase 3 trial in adults with chronic ITP, five Phase 2 trials in various thrombocytopenia patient populations, 15 Phase 1 trials and numerous preclinical studies, and has been observed to be generally well tolerated in over 1,100 patients. Based on the safety and efficacy profile observed in these trials and studies, we believe avatrombopag has the potential for use in the following acute settings: a broader population of thrombocytopenia patients regardless of disease etiology undergoing a broader set of medical procedures, including, for example, joint replacements, for which we are initiating a Phase 3 clinical trial in the first quarter of 2018, and patients who develop thrombocytopenia after receiving chemotherapy, for which we plan to initiate a Phase 3 clinical trial in the second quarter of 2018. In the chronic setting, we believe avatrombopag has the potential to treat adult patients with chronic ITP based on the results from a completed Phase 3 trial, and we plan to submit a sNDA to the FDA in the second half of 2018 for the treatment of patients with ITP.
Pipeline summary and addressable market
The following table summarizes our lead development programs. We hold the worldwide rights to avatrombopag for these indications.
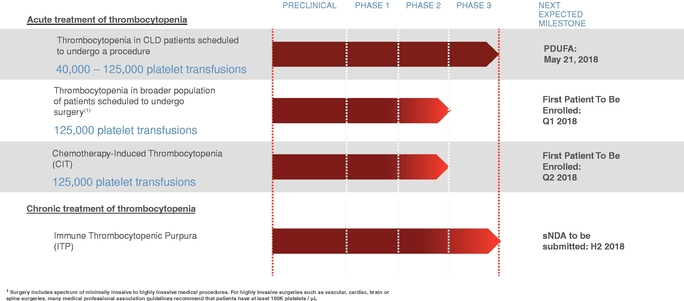
We believe that the total addressable market for avatrombopag is approximately $2.5 billion in the United States, with an approximately $800 million addressable market for our first indication, the acute treatment of thrombocytopenia in patients with CLD scheduled to undergo a procedure, and an approximately $1.7 billion addressable market for our follow-on indications depicted in the table. Furthermore, we believe that avatrombopag for the treatment of patients with chronic ITP alone has an approximately $600 million addressable market in the United States and an approximately $1.2 billion worldwide addressable market.
4
Management
Our management team has extensive experience ranging from identifying and acquiring drug candidates, drug development and global registrations through global commercial launches. Further, we are being supported by a leading group of biotech investors including PBM Capital Investments, LLC and Perceptive Advisors.
Our strategy
We are a pharmaceutical company focused on acquiring, developing and commercializing drug candidates for diseases that are treated by specialist physicians, with an initial focus on addressing thrombocytopenia. To achieve our goals, we are pursuing the following strategies:
- •
- Advance the development of our late-stage drug candidate, avatrombopag, for regulatory approval in the United States
and Europe. In the first quarter of 2017, we completed two identically designed pivotal Phase 3 clinical trials for avatrombopag in patients with CLD undergoing a
procedure. Based on these results, a NDA was submitted to the FDA for this initial indication on September 21, 2017. The NDA was granted Priority Review by the FDA in November 2017 and the
Prescription Drug User Fee Act goal date for an FDA decision is May 21, 2018. In addition, as our Phase 3 trials were also designed to be pivotal trials in Europe, we intend to submit a
Marketing Authorization Application, or MAA, to the European's Medicine's Agency, or EMA, in the first half of 2018.
- •
- Maximize the commercial potential of
avatrombopag. Our intent is to initially build a hepatology-focused sales organization in the United States. We have begun to execute this
strategy by hiring key executives with extensive U.S. commercial launch experience. In addition to the sales force, we intend to support the launch of avatrombopag with a dedicated team of
reimbursement support case managers as well as a network of specialty pharmacy service providers that will distribute the drug directly to the patient's home. In the future, we also may selectively
partner with leading companies that we believe can contribute additional resources and know-how for the development and commercialization of avatrombopag for additional indications and geographic
regions, further enhancing the value of our drug candidate.
- •
- Expand the breadth of indications for avatrombopag in other patient populations with
thrombocytopenia. We believe avatrombopag has the potential to be used more broadly for patients with thrombocytopenia in other acute settings
and chronically. During the first quarter of 2018, we are initiating a Phase 3 trial in PST, which will include patients with thrombocytopenia regardless of disease etiology undergoing
surgeries that require a platelet count of more than 100,000 platelets per microliter of circulating blood prior to surgery. Additionally, based on the results of one completed Phase 3 trial
and two Phase 2 trials evaluating the use of avatrombopag for the treatment of adults with chronic ITP and recent discussions with the FDA, we anticipate submitting a sNDA for avatrombopag in
the second half of 2018 for this indication. We also plan to initiate a Phase 3 clinical trial in the second quarter of 2018 for the treatment of patients with CIT.
- •
- Employ a value-driven approach to build a pipeline of drug
candidates. Using a similar approach to our identification and acquisition of avatrombopag, we intend to employ a value-driven strategy to
identify, acquire, develop and commercialize drug candidates for diseases that are treated by specialist physicians.
- •
- Maintain and strengthen our intellectual property portfolio. Our intellectual property strategy aims to protect and control the development and commercialization of our drug candidates. Our owned and
5
in-licensed patents for avatrombopag provide us with composition of matter and method of use exclusivity with respect to avatrombopag in the United States, including a composition of matter patent that expires in 2025, with possible patent term extension up to 2029. We also hold patents and applications in major world markets with respect to avatrombopag, which are projected to expire between 2023 and 2029, including available patent term adjustment and patent term extension. We will seek to broaden the scope of and increase the geographic reach of our patent protection throughout the world.
Risks associated with our business
Our ability to implement our business strategy is subject to numerous risks that you should be aware of before making an investment decision. See the "Risk Factors" section of this prospectus and in the documents incorporated by reference herein for a discussion of factors you should consider carefully before deciding to invest in our common stock. These risks include the following, among others:
- •
- We have a limited operating history and have never generated any product revenues. We expect to incur losses over the next several years and
may never achieve or maintain profitability.
- •
- We may require additional capital to fund our operations, and if we fail to obtain necessary financing, we may not be able to complete the
development and commercialization of our only current drug candidate, avatrombopag and any other potential drug candidates in the future.
- •
- We may be required to make significant payments in connection with our acquisition of avatrombopag from Eisai and our failure to make these
payments may adversely affect our ability to progress our development programs.
- •
- Our consolidated financial statements have been prepared assuming that we will continue as a going concern.
- •
- We are heavily dependent on the success of avatrombopag and if avatrombopag does not receive regulatory approval or is not successfully
commercialized, our business will be harmed.
- •
- If we are not able to obtain required regulatory approvals, we will not be able to commercialize avatrombopag, and our ability to generate
revenue will be materially impaired.
- •
- Even if we obtain FDA approval for avatrombopag in the United States, we may never obtain approval for or commercialize it in any other
jurisdiction, which would limit our ability to realize its full market potential.
- •
- Even if avatrombopag receives marketing approval, it may fail to achieve market acceptance by physicians, patients, third-party payors or
others in the medical community necessary for commercial success.
- •
- We do not have our own manufacturing capabilities and will rely on third parties to produce clinical and commercial supplies of avatrombopag
and any future drug candidate.
- •
- We rely on our license agreement with Astellas to provide rights to the core intellectual property relating to avatrombopag. Any termination or
loss of rights under that license agreement would have a material adverse effect on our development and commercialization of avatrombopag.
- •
- We currently have a limited number of employees, and we rely on Eisai and PBM Capital Group, LLC to provide various administrative, research and development and other services.
6
- •
- If we are unable to obtain and maintain patent protection for avatrombopag or any future drug candidate, or if the scope of the patent
protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, which could have a material adverse effect on our
ability to successfully commercialize our technology and drug candidates.
- •
- Concentration of ownership of our common stock among our existing executive officers, directors and principal stockholders may prevent new investors from influencing significant corporate decisions.
Implications of being an emerging growth company
We qualify as an "emerging growth company" as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of relief from certain reporting requirements and other burdens that are otherwise applicable generally to public companies. These provisions include:
- •
- an exception from compliance with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or
the Sarbanes-Oxley Act;
- •
- reduced disclosure about our executive compensation arrangements in our periodic reports, proxy statements and registration statements; and
- •
- exemptions from the requirements of holding non-binding advisory votes on executive compensation or golden parachute arrangements.
We may take advantage of these provisions until the earlier of December 31, 2022 or such time that we no longer qualify as an emerging growth company. We would cease to qualify as an emerging growth company if we have more than $1.07 billion in annual revenue, we are deemed to be a "large accelerated filer" under the rules of the U.S. Securities and Exchange Commission, or SEC, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, or we issue more than $1.0 billion of non-convertible debt over a three-year period. We may choose to take advantage of some but not all of these reduced reporting burdens. For example, we may take advantage of the exemption from auditor attestation on the effectiveness of our internal control over financial reporting. To the extent that we take advantage of these reduced reporting burdens, the information that we provide stockholders may be different than you might obtain from other public companies in which you hold equity interests.
In addition, under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Our corporate information
We were originally formed as a limited liability company under the laws of the state of Delaware in March 2016 under the name PBM AKX Holdings, LLC. In June 2016, we amended our certificate of formation to change our name to Dova Pharmaceuticals, LLC. In September 2016, we converted from a limited liability company to a corporation, Dova Pharmaceuticals, Inc. Our principal executive offices are located at 240 Leigh Farm Road, Suite 245, Durham, NC 27707, and our telephone number is (919) 748-5975. Our
7
website address is www.dova.com. The information contained in, or accessible through, our website is not incorporated by reference into this prospectus, and you should not consider any information contained in, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our common stock.
We own various U.S. federal trademark applications and unregistered trademarks, including our company name. All other trademarks or trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the symbols ® and ™, but such references should not be construed as any indication that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
8
| Common stock offered by us | 2,500,000 shares | |
Common stock to be outstanding immediately after this offering |
28,152,457 shares |
|
Option to purchase additional shares |
We have granted the underwriters an option for a period of 30 days from the date of this prospectus to purchase a maximum of 375,000 additional shares of common stock. |
|
Use of proceeds |
We estimate that the net proceeds to us from this offering, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, will be $80.9 million, or $93.1 million if the underwriters exercise their option to purchase additional shares in full, based on an assumed public offering price of $34.68 per share, the last reported sales price of our common stock on The NASDAQ Global Market on February 16, 2018. |
|
We anticipate that the majority of the net proceeds from this offering will be used to fund the commercial launch of avatrombopag, if approved, to fund the initiation of the Phase 3 clinical trial of avatrombopag for the treatment of a broader population of patients with thrombocytopenia undergoing surgery, to fund the initiation of the Phase 3 clinical trial of avatrombopag for the treatment of patients with CIT, to fund the sNDA submission for avatrombopag for the treatment of patients with ITP, and to repay a portion of our obligations under the Eisai note. The remainder may be used for other general corporate purposes, including general and administrative expenses and working capital. See "Use of proceeds" on page 17. |
||
Risk factors |
You should read the "Risk factors" section of this prospectus and in the documents incorporated by reference herein for a discussion of factors to consider carefully before deciding to invest in shares of our common stock. |
|
NASDAQ Global Market symbol |
DOVA |
The number of shares of our common stock that will be outstanding after this offering is based on 25,652,457 shares of common stock outstanding as of December 31, 2017 and excludes:
- •
- 2,128,641 shares of common stock issuable upon exercise of stock options awarded as of December 31, 2017 at a weighted average exercise
price of $7.90 per share;
- •
- 2,156,609 shares of our common stock reserved for future issuance under our equity incentive plans following this offering as of
December 31, 2017; and
- •
- 1,027,170 additional shares of our common stock reserved for future issuance under our equity incentive plans on January 1, 2018 as a result of an automatic annual increase in the share reserve.
9
Except as otherwise indicated herein, all information in this prospectus, including the number of shares that will be outstanding after this offering, assumes:
- •
- no exercise of outstanding options outstanding as of December 31, 2017; and
- •
- no exercise of the underwriters' option to purchase additional shares of common stock.
10
Summary consolidated financial data
The following tables set forth, for the periods and as of the dates indicated, our summary financial data. The consolidated statement of operations data for the period from March 24, 2016 (inception) through December 31, 2016 and for the year ended December 31, 2017 are derived from our audited consolidated financial statements incorporated by reference in this prospectus. The consolidated balance sheet as of December 31, 2017 is derived from our audited consolidated financial statements incorporated by reference in this prospectus. You should read this data together with our consolidated financial statements and related notes incorporated by reference in this prospectus and the information under the captions "Selected consolidated financial data" and "Management's discussion and analysis of financial condition and results of operations" included elsewhere in this prospectus or incorporated by reference herein. Our historical results are not necessarily indicative of our future results.
| |
|
|
|||||
|---|---|---|---|---|---|---|---|
| | | | | | | | |
| |
March 24, 2016 (inception) to December 31, 2016 |
Year ended December 31, 2017 |
|||||
| | | | | | | | |
|
(in thousands, except share and per share amounts) | ||||||
Statement of Operations Data: |
|||||||
Operating expenses: |
|||||||
Research and development |
$ | 20,842 | $ | 14,710 | |||
Research and development—licenses acquired |
5,000 | 1,000 | |||||
General and administrative |
1,201 | 13,499 | |||||
| | | | | | | | |
Total operating expenses |
27,043 | 29,209 | |||||
| | | | | | | | |
Loss from operations |
(27,043 | ) | (29,209 | ) | |||
| | | | | | | | |
Other income (expense): |
|||||||
Other income, net |
9 | 486 | |||||
Interest expense—related party |
(4 | ) | (1 | ) | |||
Interest expense |
(152 | ) | (1,231 | ) | |||
| | | | | | | | |
Total other expense |
(147 | ) | (746 | ) | |||
| | | | | | | | |
Net loss |
$ | (27,190 | ) | $ | (29,955 | ) | |
| | | | | | | | |
Net loss per share of common stock—basic and diluted(1) |
$ | (1.57 | ) | $ | (1.40 | ) | |
| | | | | | | | |
Weighted average common shares outstanding—basic and diluted(1) |
17,332,257 | 21,435,369 | |||||
| | | | | | | | |
(1) See Note 2 to our consolidated financial statements incorporated by reference in this prospectus for an explanation of the method used to calculate basic and diluted net loss per common share.
The following table presents our summary balance sheet data as of December 31, 2017:
- •
- on an actual basis; and
- •
- on an as adjusted basis to give effect to the sale of 2,500,000 shares of common stock in this offering at the assumed public offering price of $34.68 per share, the last reported sale price of our common
11
stock on The NASDAQ Global Market on February 16, 2018, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
| |
|
|
|||||
|---|---|---|---|---|---|---|---|
| | | | | | | | |
|
As of December 31, 2017 |
||||||
| |
Actual |
As adjusted |
|||||
| | | | | | | | |
|
(in thousands) | ||||||
Balance Sheet Data: |
|||||||
Cash and cash equivalents |
$ | 94,846 | $ | 175,744 | |||
Working capital |
61,120 | 142,018 | |||||
Total assets |
96,379 | 177,277 | |||||
Note payable, short-term |
30,212 | 30,212 | |||||
Total liabilities |
35,197 | 35,197 | |||||
Total stockholders' equity |
61,182 | 142,080 | |||||
| | | | | | | | |
The as adjusted information discussed above is illustrative only and will be revised based on the actual public offering price and other terms of our public offering determined at pricing. Each $1.00 increase (decrease) in the assumed public offering price of $34.68 per share would increase (decrease) the as adjusted amount of each of cash and cash equivalents, working capital, total assets and total stockholders' equity by $2.4 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of 100,000 shares in the number of shares offered by us at the assumed public offering price would increase (decrease) each of cash and cash equivalents, working capital, total assets and total stockholders' equity by $3.3 million.
12
Investing in our common stock involves a high degree of risk. Before you invest in our common stock, you should carefully consider the following risks, as well as general economic and business risks, including those set forth under the heading "Risk Factors" in our Annual Report on Form 10-K for the fiscal year ended December 31, 2017 incorporated by reference herein, and all of the other information contained in this prospectus and in the documents incorporated by reference herein. Any of the following risks, including those discussed in the documents incorporated by reference herein, could have a material adverse effect on our business, operating results and financial condition and cause the trading price of our common stock to decline, which would cause you to lose all or part of your investment. When determining whether to invest, you should also refer to the other information contained or incorporated by reference in this prospectus, including our financial statements and the related notes thereto. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us may also adversely affect our business.
Risks related to this offering and ownership of our common stock
If you purchase shares of our common stock in this offering, you will suffer immediate dilution of your investment.
The assumed public offering price of our common stock is substantially higher than the net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. Based on an assumed public offering price of $34.68 per share, you will experience immediate dilution of $29.63 per share, representing the difference between our as-adjusted net tangible book value per share after giving effect to this offering and the assumed public offering price.
In addition, as of December 31, 2017, we had outstanding stock options to purchase an aggregate of 2,128,641 shares of common stock at a weighted average exercise price of $7.90 per share. To the extent these outstanding options are exercised, there will be further dilution to investors in this offering.
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time, subject to the restrictions and limitations described below. If our stockholders sell, or the market perceives that our stockholders intend to sell, substantial amounts of our common stock in the public market, the market price of our common stock could decline significantly.
Upon the closing of this offering, based upon the number of shares outstanding as of December 31, 2017, we will have 28,152,457 outstanding shares of common stock. Of these shares, approximately 13.3 million shares are, including the 2,500,000 shares sold in this offering will be, freely tradable, subject, in the case of our affiliates, to the conditions of Rule 144 under the Securities Act. An additional 14.9 million shares are subject to a contractual lock-up with the underwriters for this offering for 90 days following this offering. J.P. Morgan Securities LLC and Jefferies LLC may release these stockholders from their lock-up agreements at any time and without notice, which would allow for earlier sales of shares in the public market subject to the conditions of Rule 144 under the Securities Act.
13
In addition, we have filed a registration statement on Form S-8 registering the issuance of approximately 6.0 million shares of common stock subject to options or other equity awards issued or reserved for future issuance under our equity incentive plans. Shares registered under this registration statement on Form S-8 are available for sale in the public market subject to vesting arrangements and exercise of options, the lock-up agreements described above and, in the case of our affiliates, the restrictions of Rule 144.
Additionally, the holders of an aggregate of approximately 21 million shares of our common stock, or their transferees, have rights, subject to some conditions, to require us to file one or more registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. If we were to register the resale of these shares, they could be freely sold in the public market without limitation. If these additional shares are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common stock could decline.
We will have broad discretion in the use of our existing cash and cash equivalents, including the proceeds from this offering, and may invest or spend our cash in ways with which you do not agree and in ways that may not increase the value of your investment.
We will have broad discretion over the use of our cash and cash equivalents, including the proceeds from this offering. You may not agree with our decisions, and our use of cash and cash equivalents may not yield any return on your investment. We expect to use the net proceeds to us from this offering, together with our existing cash and cash equivalents, to fund the commercialization of avatrombopag, if approved, to fund clinical trials of avatrombopag for additional indications beyond its initial indication, to repay a portion of our obligations under the Eisai note and for working capital and general corporate purposes. In addition, we may use a portion of the proceeds from this offering to pursue our strategy to in-license or acquire additional drug candidates. Our failure to apply the net proceeds from this offering effectively could compromise our ability to pursue our growth strategy and we might not be able to yield a significant return, if any, on our investment of these net proceeds. You will not have the opportunity to influence our decisions on how to use our net proceeds from this offering.
14
Special note regarding forward-looking statements
This prospectus and the documents incorporated by reference in this prospectus contain forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, that involve substantial risks and uncertainties. In some cases, you can identify forward-looking statements by terms such as "may," "will," "should," "expect," "plan," "anticipate," "could," "intend," "target," "project," "estimate," "believe," "estimate," "predict," "potential" or "continue" or the negative of these terms or other similar expressions intended to identify statements about the future. These statements speak only as of the date of this prospectus or as of any such date stated in the applicable document incorporated by reference in this prospectus and involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements include, without limitation, statements about the following:
- •
- the timing, progress and results of clinical trials of avatrombopag and any other drug candidates, including statements regarding the timing of
initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available and our research and development programs;
- •
- the timing of any submission of filings for regulatory approval of avatrombopag, and the timing of and our ability to obtain and maintain
regulatory approval of avatrombopag for any indication;
- •
- our expectations regarding the scope of any approved indication for avatrombopag;
- •
- our ability to expand the indications for which avatrombopag may be approved;
- •
- our expectations regarding the size of the patient populations for, market acceptance and opportunity for and clinical utility of avatrombopag
or any other drug candidates, if approved for commercial use;
- •
- our ability to rely on Eisai for transition services under the TSA, including with respect to the development of avatrombopag;
- •
- our manufacturing capabilities and strategy, including the scalability and commercial viability of our manufacturing methods and processes,
including our ability to maintain our supply agreement with Eisai;
- •
- our ability to successfully commercialize avatrombopag;
- •
- our estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain additional
financing;
- •
- our strategic plans and expectations for, and our ability to identify, develop and obtain regulatory approval for, new drug candidates;
- •
- the implementation of our strategic plan to identify and develop treatments for diseases treated by specialist physicians;
- •
- our ability to establish or maintain collaborations or strategic relationships;
- •
- our ability to identify, recruit and retain key personnel;
15
- •
- our ability to protect and enforce our intellectual property protection for avatrombopag, and the scope of such protection;
- •
- our expected use of proceeds from this offering;
- •
- our financial performance and expectation regarding future funding sources;
- •
- our competitive position and the development of and projections relating to our competitors or our industry;
- •
- the impact of laws and regulations; and
- •
- our expectations regarding the time during which we will be an emerging growth company under the JOBS Act.
Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. You should refer to the "Risk factors" section of this prospectus and in our Annual Report on Form 10-K for the year ended December 31, 2017, which is incorporated by reference in this prospectus, for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. As a result of these factors, we cannot assure you that the forward-looking statements in this prospectus and in the documents incorporated by reference in this prospectus will prove to be accurate. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. You should, however, review the factors and risks and other information we describe in the reports we will file from time to time with the SEC after the date of this prospectus.
You should read this prospectus, the documents that we incorporate by reference in this prospectus and those documents we have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
We obtained the industry, statistical and market data in this prospectus and the documents incorporated by reference in this prospectus from our own internal estimates and research as well as from industry and general publications and research, surveys and studies conducted by third parties. All of the market data used in this prospectus and the documents incorporated by reference in this prospectus involve a number of assumptions and limitations. While we believe that the information from these industry publications, surveys and studies is reliable, the industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of important factors, including those described in the section titled "Risk factors" in this prospectus and in the documents incorporated by reference herein. These and other factors could cause results to differ materially from those expressed in the estimates made by third parties and by us.
16
We estimate that the net proceeds from our issuance and sale of 2,500,000 shares of our common stock in this offering will be approximately $80.9 million (or $93.1 million if the underwriters exercise in full their option to purchase additional shares), based upon an assumed public offering price of $34.68 per share, which is the last reported sales price of our common stock on the NASDAQ Global Market on February 16, 2018, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
Each $1.00 increase (decrease) in the assumed public offering price of $34.68 per share would increase (decrease) the net proceeds to us from this offering by approximately $2.4 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Each increase (decrease) of 100,000 in the number of shares we are offering would increase (decrease) the net proceeds to us from this offering, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $3.3 million, assuming the assumed public offering price stays the same.
As of December 31, 2017, we had cash and cash equivalents of approximately $94.8 million. We anticipate that the majority of the net proceeds from this offering will be used to fund the commercial launch of avatrombopag, if approved, to fund the initiation of the Phase 3 clinical trial of avatrombopag for the treatment of a broader population of patients with thrombocytopenia undergoing surgery, to fund the initiation of the Phase 3 clinical trial of avatrombopag for the treatment of patients with CIT, to fund the sNDA submission for avatrombopag for the treatment of patients with ITP, and to repay a portion of our obligations under the Eisai note. The remainder may be used for other general corporate purposes, including general and administrative expenses and working capital. For a description of the terms of the Eisai note, see the section titled "Management's discussion and analysis of financial condition and results of operations—Eisai note and security agreement" in our Annual Report on Form 10-K for the year ended December 31, 2017, which is incorporated by reference herein.
Our expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot predict with complete certainty all of the particular uses for the net proceeds to be received upon the completion of this offering or the actual amounts that we will spend on the uses set forth above. We believe opportunities may exist from time to time to expand our current business through the acquisition or in-license of complementary drug candidates. While we have no current agreements for any specific acquisitions or in-licenses at this time, we may use a portion of the net proceeds for these purposes.
The amounts and timing of our actual expenditures will depend on numerous factors, including the progress of our clinical trials and other development and commercialization efforts for avatrombopag, as well as the amount of cash used in our operations. Based on our current operational plans and assumptions, we expect our cash and cash equivalents, together with the net proceeds from this offering, will be sufficient to enable us to commence the commercialization of avatrombopag, if approved. With respect to conducting clinical trials of avatrombopag for additional indications beyond its initial indication, we expect that we may require additional funds as these programs progress, the amounts of which will depend on the ultimate clinical development paths we pursue. However, we cannot estimate with certainty the amount of net proceeds to be used for the purposes described above. We may find it necessary or advisable to use the net proceeds for other purposes, and we will have broad discretion in the application of the net proceeds. Pending the uses described above, we plan to invest the net proceeds from this offering in short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
17
Market price of common stock
Our common stock has been quoted on the NASDAQ Global Market since June 29, 2017, under the symbol "DOVA." Prior to our IPO, there was no public market for our common stock.
The following table sets forth the high and low sales prices of our common stock for the period indicated.
| |
|
|
|||||
|---|---|---|---|---|---|---|---|
| | | | | | | | |
| |
High |
Low |
|||||
| | | | | | | | |
2017 |
|||||||
Second quarter (beginning June 29, 2017) |
$ | 22.40 | $ | 18.18 | |||
Third quarter |
$ | 28.59 | $ | 16.98 | |||
Fourth quarter |
$ | 32.75 | $ | 22.00 | |||
2018 |
|||||||
First quarter (through February 16, 2018) |
$ | 35.00 | $ | 25.63 | |||
| | | | | | | | |
As of February 16, 2018, there were 25,679,266 shares of common stock outstanding held by 31 record stockholders. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities. The last reported sale price of our common stock on The NASDAQ Global Market on February 16, 2018 was $34.68 per share.
We have never declared or paid, and do not anticipate declaring or paying, in the foreseeable future, any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. Any future determination related to our dividend policy will be made at the discretion of our board of directors and will depend upon, among other factors, our results of operations, financial condition, capital requirements, contractual restrictions, business prospects and other factors our board of directors may deem relevant.
18
The following table sets forth our cash and cash equivalents and our capitalization as of December 31, 2017:
- •
- on an actual basis; and
- •
- on an as adjusted basis to give effect to our sale of 2,500,000 shares of common stock in this offering at an assumed public offering price of $34.68 per share, the last reported sale price of our common stock on The NASDAQ Global Market on February 16, 2018, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The following information is illustrative only of our cash and cash equivalents and capitalization following the completion of this offering and will change based on the actual public offering price and other terms of this offering determined at pricing. You should read this table together with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our financial statements and the related notes incorporated by reference in this prospectus.
| |
|
|
|||||
|---|---|---|---|---|---|---|---|
| | | | | | | | |
| |
As of December 31, 2017 |
||||||
| |
Actual |
As adjusted |
|||||
| | | | | | | | |
| |
(in thousands, except share and per share data) |
||||||
Cash and cash equivalents |
$ | 94,846 | $ | 175,744 | |||
| | | | | | | | |
Note payable, short-term |
30,212 | 30,212 | |||||
Stockholders' equity: |
|||||||
Common stock, $0.001 par value; 100,000,000 shares authorized and 25,652,457 shares issued and outstanding, actual; 100,000,000 shares authorized and 28,152,457 shares issued and outstanding, as adjusted |
26 | 28 | |||||
Additional paid-in capital |
118,301 | 199,197 | |||||
Accumulated deficit |
(57,145 | ) | (57,145 | ) | |||
| | | | | | | | |
Total stockholders' equity |
61,182 | 142,080 | |||||
| | | | | | | | |
Total capitalization |
$ | 91,394 | $ | 172,292 | |||
| | | | | | | | |
The number of shares of common stock outstanding in the table above assumes, with respect to the number of shares of common stock issued and outstanding after the offering, the issuance and sale in this offering of 2,500,000 shares of our common stock and does not include:
- •
- 2,128,641 shares of common stock issuable upon exercise of stock options awarded as of December 31, 2017 at a weighted average exercise
price of $7.90 per share; and
- •
- 2,156,609 shares of our common stock reserved for future issuance under our equity incentive plans following this offering as of
December 31, 2017; and
- •
- 1,027,170 additional shares of our common stock reserved for future issuance under our equity incentive plans on January 1, 2018 as a result of an automatic annual increase in the share reserve.
19
If you invest in our common stock in this offering, your interest will be diluted to the extent of the difference between the public offering price per share and the as adjusted net tangible book value per share of our common stock immediately after this offering. Net tangible book value per share is determined by dividing our total tangible assets less total liabilities by the number of outstanding shares of our common stock.
As of December 31, 2017, our net tangible book value was $61.2 million, or $2.39 per share of common stock.
Investors participating in this offering will incur immediate and substantial dilution. After giving effect to the issuance and sale of 2,500,000 shares of our common stock in this offering at an assumed public offering price of $34.68 per share, which is the last reported sales price of our common stock on The NASDAQ Global Market on February 16, 2018, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of December 31, 2017 would have been approximately $142.1 million, or approximately $5.05 per share of common stock. This represents an immediate increase in the net tangible book value of $2.66 per share to existing stockholders, and an immediate dilution in the net tangible book value of $29.63 per share to investors purchasing shares of our common stock in this offering. The following table illustrates this per share dilution:
| | | | | | | | |
Assumed public offering price per share |
$ | 34.68 | |||||
Actual net tangible book value per share as of December 31, 2017 |
$ | 2.39 | |||||
Increase in net tangible book value per share attributable to new investors participating in this offering |
2.66 | ||||||
| | | | | | | | |
As adjusted net tangible book value per share after this offering |
$ | 5.05 | |||||
| | | | | | | | |
Dilution per share to investors participating in this offering |
$ | 29.63 | |||||
| | | | | | | | |
The dilution information discussed above is illustrative only and will change based on the actual public offering price and other terms of this offering determined at pricing. Each $1.00 increase or decrease in the assumed public offering price of $34.68 per share would increase or decrease our as adjusted net tangible book value by $0.08 per share, and the dilution per share to investors participating in this offering by $0.92 per share, assuming that the number of shares offered by us, as set forth on the cover of this prospectus, remains the same. An increase of 100,000 shares offered by us at the assumed public offering price of $34.68 per share would increase our as adjusted net tangible book value by $0.10 per share and decrease the dilution to investors participating in this offering by $0.10 per share. A decrease of 100,000 shares offered by us at the assumed public offering price of $34.68 per share would decrease our as adjusted net tangible book value by $0.10 per share and increase the dilution to investors participating in this offering by $0.10 per share.
If the underwriters exercise their option in full to purchase 375,000 additional shares of common stock in this offering, the as adjusted net tangible book value per share after the offering would be $5.41 per share, the increase in the as adjusted net tangible book value per share to existing stockholders would be $3.02 per share and the dilution to investors purchasing common stock in this offering would be $29.27 per share.
20
The shares of our common stock reserved for future issuance under our equity benefit plans are subject to automatic annual increases in accordance with the terms of the plans. To the extent that options are exercised, new options or other equity awards are issued under our equity incentive plans, or we issue additional shares of common stock in the future, there will be further dilution to investors participating in this offering. In addition, we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
21
Selected consolidated financial data
The following tables set forth, for the periods and as of the dates indicated, our selected consolidated financial data. The balance sheet data as of December 31, 2016 and 2017 and the statement of operations data for the period from March 24, 2016 (inception) through December 31, 2016 and for the year ended December 31, 2017 are derived from our audited consolidated financial statements incorporated by reference in this prospectus. You should read this data together with our consolidated financial statements and related notes incorporated by reference in this prospectus and the information under the captions "Management's discussion and analysis of financial condition and results of operations" incorporated by reference herein. Our historical results are not necessarily indicative of our future results.
| |
|
|
|||||
|---|---|---|---|---|---|---|---|
| | | | | | | | |
| |
March 24, 2016 (Inception) to December 31, 2016 |
Year ended December 31, 2017 |
|||||
| | | | | | | | |
| |
(in thousands, except share and per share data) |
||||||
Statement of Operations Data: |
|||||||
Operating expenses: |
|||||||
Research and development |
$ | 20,842 | $ | 14,170 | |||
Research and development—licenses acquired |
5,000 | 1,000 | |||||
General and administrative |
1,201 | 13,499 | |||||
| | | | | | | | |
Total operating expenses |
27,043 | 29,209 | |||||
| | | | | | | | |
Loss from operations |
(27,043 | ) | (29,209 | ) | |||
| | | | | | | | |
Other income (expense): |
|||||||
Other income, net |
9 | 486 | |||||
Interest expense—related party |
(4 | ) | (1 | ) | |||
Interest expense |
(152 | ) | (1,231 | ) | |||
| | | | | | | | |
Total other expense |
(147 | ) | (746 | ) | |||
| | | | | | | | |
Net loss |
$ | (27,190 | ) | $ | (29,955 | ) | |
| | | | | | | | |
Net loss per share of common stock—basic and diluted(1) |
$ | (1.57 | ) | $ | (1.40 | ) | |
| | | | | | | | |
Weighted average common shares outstanding—basic and diluted(1) |
17,332,257 | 21,435,369 | |||||
| | | | | | | | |
(1) See Note 2 to our consolidated financial statements incorporated by reference in this prospectus for an explanation of the method used to calculate basic and diluted net loss per common share.
| |
|
|
|||||
|---|---|---|---|---|---|---|---|
| | | | | | | | |
| |
As of December 31, 2016 |
As of December 31, 2017 |
|||||
| | | | | | | | |
| |
(in thousands) |
||||||
Balance Sheet Data: |
|||||||
Cash and cash equivalents |
$ | 28,709 | $ | 94,846 | |||
Working capital |
20,435 | 61,120 | |||||
Total assets |
28,746 | 96,379 | |||||
Note payable, long-term and short-term |
13,640 | 30,212 | |||||
Total liabilities |
21,951 | 35,197 | |||||
Total stockholders' equity |
6,795 | 61,182 | |||||
| | | | | | | | |
22
Certain relationships and related party transactions
The following includes a summary of transactions since March 24, 2016 to which we have been a party, in which the amount involved in the transaction exceeded $120,000, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our voting securities or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest. Other than described below, there have not been, nor are there currently any proposed, transactions or series of similar transactions to which we have been or will be a party other than compensation arrangements, which include equity and other compensation, termination, change in control and other arrangements, which are described under "Executive compensation" in our Annual Report on Form 10-K for the year ended December 31, 2017, which is incorporated by reference herein.
Participation in initial public offering
In our initial public offering, certain of our directors, executive officers and 5% stockholders and their affiliates purchased an aggregate of 841,435 shares of our common stock. Each of those purchases was made through the underwriters at the initial public offering price. The following table sets forth the aggregate number of shares of our common stock that these 5% stockholders and their affiliates purchased in our initial public offering:
| | | | | |
| Purchaser |
Shares of common stock |
|||
|---|---|---|---|---|
| | | | | |
Perceptive Life Sciences Master Fund, Ltd. |
588,235 | |||
Entities affiliated with Paul Manning(1) |
150,000 | |||
Alex Sapir |
20,100 | |||
Steven M. Goldman |
42,000 | |||
Roger A. Jeffs |
31,400 | |||
Lee F. Allen |
3,500 | |||
Kevin Laliberte |
6,200 | |||
| | | | | |
(1) Consists of 100,000 shares purchased by Paul B. Manning together with his spouse as Joint Tenants with Right of Survivorship and 50,000 shares purchased by BKB Growth Investments, LLC, of which Mr. Manning is a co-manager.
Conversion from limited liability company to corporation
In September 2016, we converted from a Delaware limited liability company named Dova Pharmaceuticals, LLC (formerly known as PBM AKX Holdings, LLC), or the LLC, to Dova Pharmaceuticals, Inc., a Delaware corporation. We refer to this activity as the Conversion. The Conversion was effected pursuant to a plan of conversion whereby each unit of membership of the LLC was converted into 330 shares of our common stock. Additionally, we terminated the LLC's operating agreement in connection with the Conversion. As part of the Conversion, the members of the LLC became our stockholders in the same ownership proportions as immediately prior to the Conversion. Effective upon the Conversion, our stockholders entered into a stockholders agreement which contained provisions similar to those set forth in the LLC's operating agreement immediately prior to the Conversion.
Services agreements with PBM Capital Group, LLC
In April 2016, we entered into the Dova services agreement with PBM Capital Group, LLC, an affiliate of PBM Capital Investments, LLC, a beneficial owner of more than 5% of our common stock and an entity
23
controlled by Paul B. Manning, one of our directors, to engage PBM Capital Group, LLC for certain scientific and technical, accounting, operations and back office support services. We agreed to pay PBM Capital Group, LLC a flat fee of $25,000 per month for these services. The Dova services agreement had an initial term of 12 months and was extended on April 1, 2017 for an additional one-year term. Pursuant to the Dova services agreement, we paid $0.2 million and $0.3 million to PBM Capital Group, LLC during the period from March 24, 2016 through December 31, 2016 and for the year ended December 31, 2017, respectively.
In April 2016, our wholly-owned subsidiary, AkaRx, Inc., or AkaRx, entered into the AkaRx services agreement with PBM Capital Group, LLC to engage PBM Capital Group, LLC for certain scientific and technical, accounting, operations and back office support services. AkaRx agreed to pay PBM Capital Group, LLC a flat fee of $25,000 per month for these services. The AkaRx services agreement had an initial term of 12 months and was extended on April 1, 2017 for an additional one-year term. Pursuant to the AkaRx services agreement, we paid $0.2 million and $0.3 million to PBM Capital Group, LLC during the period from March 24, 2016 through December 31, 2016 and for the year ended December 31, 2017, respectively.
Guarantee by PBM Capital Investments, LLC
In March 2016, we entered into a transition services agreement with Eisai, or the TSA. In connection with the TSA, AkaRx issued Eisai note, which enables us to finance payments due to Eisai under the TSA. The principal amount of the Eisai note will be increased by the amount of unpaid service fees and out-of-pocket expenses due and owed to Eisai under the TSA. Principal and interest under the Eisai note can be prepaid at any time without penalty. Payments due pursuant to the Eisai note are currently guaranteed by PBM Capital Investments, LLC.
Private placements of our securities
In March 2016, we issued PBM Capital Investments, LLC an aggregate of 50,000 units in exchange for its payment to Eisai of $5.0 million on our behalf in connection with our acquisition of worldwide rights to avatrombopag. In April 2016, we entered into a co-investment agreement, or the co-investment agreement, with PBM Capital Investments, LLC, and certain affiliates of PBM Capital Investments, LLC, which we refer to as the Co-Investors. Pursuant to the co-investment agreement, we issued and sold to the Co-Investors an aggregate of 2,522 units at a purchase price of $100.00 per unit for an aggregate purchase price of $252,200. Each unit was converted into 330 shares of our common stock in connection with the Conversion. Paul B. Manning, one of our directors, had sole voting and dispositive power over the shares held by PBM Capital Investments, LLC. Mr. Manning also had sole voting and shared dispositive power over the shares held by the Co-Investors.
Series A preferred stock financing
From September to November 2016, we sold an aggregate of 982,714 shares of our Series A preferred stock at a price of $29.51 per share for aggregate gross proceeds of $29.0 million. 338,868 shares were sold to Perceptive Life Sciences Master Fund, Ltd., a beneficial owner of more than 5% of our capital stock, for a purchase price of $10.0 million. In addition, 33,886 shares were sold to one of the Co-Investors for a purchase price of $1.0 million. Each share of Series A preferred stock converted into 3.3 shares of our common stock upon the closing of our IPO.
24
Investors' rights agreement
In connection with our Series A preferred stock financing, we entered into an investors' rights agreement, or the IRA. The IRA contains voting rights, information rights, board observer rights, pro rata participation rights and registration rights, among other things, with certain holders of our capital stock. In addition, the IRA entitles certain holders of our capital stock to designate a director to our board. Pursuant to the terms of the agreement, each of these rights terminated immediately prior to the closing of the IPO, except for the registration rights, as more fully described in "Description of capital stock—Registration rights."
Employment agreements
We have entered into employment-related agreements with our current executive officers, including our named executive officers. For more information regarding these agreements, see "Executive compensation—Employment agreements" and "Executive compensation—Payments upon termination or change in control" in our Annual Report on Form 10-K for the year ended December 31, 2017, which is incorporated by reference herein.
Indemnification agreements
We have entered into indemnification agreements with each of our directors and executive officers. These agreements, among other things, require us to indemnify each director (and in certain cases their related venture capital funds) and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys' fees, judgments, fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person's services as a director or executive officer.
Stock option grants to executive officers and directors
We have granted stock options to our named executive officers and directors, as more fully described in the section titled "Executive compensation" in our Annual Report on Form 10-K for the year ended December 31, 2017, which is incorporated by reference herein.
Policies and procedures for transactions with related persons
In connection with our IPO, we adopted a related person transaction policy that sets forth our procedures for the identification, review, consideration and approval or ratification of related person transactions. For purposes of our policy only, a "related person transaction" is a transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we and any related person are, were or will be participants in which the amount involved exceeds $120,000. Transactions involving compensation for services provided to us as an employee or director are not covered by this policy. A "related person" is any executive officer, director or beneficial owner of more than 5% of any class of our voting securities, including any of their immediate family members and any entity owned or controlled by such persons.
Under the policy, if a transaction has been identified as a related person transaction, including any transaction that was not a related person transaction when originally consummated or any transaction that was not initially identified as a related person transaction prior to consummation, our management must present information regarding the related person transaction to our audit committee, or, if audit committee approval would be inappropriate, to another independent body of our board of directors, for
25
review, consideration and approval or ratification. The presentation must include a description of, among other things, the material facts, the interests, direct and indirect, of the related persons, the benefits to us of the transaction and whether the transaction is on terms that are comparable to the terms available to or from, as the case may be, an unrelated third party or to or from employees generally. Under the policy, we will collect information that we deem reasonably necessary from each director, executive officer and, to the extent feasible, significant stockholder to enable us to identify any existing or potential related-person transactions and to effectuate the terms of the policy. In addition, under our Code of Conduct, our employees and directors will have an affirmative responsibility to disclose any transaction or relationship that reasonably could be expected to give rise to a conflict of interest. In considering related person transactions, our audit committee, or other independent body of our board of directors, will take into account the relevant available facts and circumstances including, but not limited to:
- •
- the risks, costs and benefits to us;
- •
- the impact on a director's independence in the event that the related person is a director, immediate family member of a director or an entity
with which a director is affiliated;
- •
- the availability of other sources for comparable services or products; and
- •
- the terms available to or from, as the case may be, unrelated third parties or to or from employees generally.
- •
- The policy requires that, in determining whether to approve, ratify or reject a related person transaction, our audit committee, or other independent body of our board of directors, must consider, in light of known circumstances, whether the transaction is in, or is not inconsistent with, our best interests and those of our stockholders, as our audit committee, or other independent body of our board of directors, determines in the good faith exercise of its discretion.
26
The following table sets forth information regarding beneficial ownership of our capital stock as of December 31, 2017 by:
- •
- each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common stock;
- •
- each of our directors;
- •
- each of our named executive officers; and
- •
- all of our current executive officers and directors as a group.
The percentage ownership information shown in the column titled "Before Offering" in the table is based upon 25,652,457 shares of common stock outstanding as of December 31, 2017. The percentage ownership information shown in the column titled "After Offering" in the table is based upon 28,152,457 shares, assuming the sale of 2,500,000 shares of our common stock by us in this offering.
We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of common stock issuable pursuant to the exercise of stock options or warrants that are exercisable on or before March 1, 2018, which is 60 days after December 31, 2017. These shares are deemed to be outstanding and beneficially owned by the person holding those options or warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. This table is based upon information supplied by officers, directors and principal stockholders and Schedules 13D and 13G, if any, filed with the SEC. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, we believe that each of the stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned.
27
Except as otherwise noted below, the address for persons listed in the table is c/o Dova Pharmaceuticals, Inc., 240 Leigh Farm Road, Suite 245, Durham, NC 27707.
| | | | | | | | | | | |
| |
|
Percentage of shares beneficially owned |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of shares beneficially owned |
|||||||||
| Name and address of beneficial owner |
Before offering |
After offering |
||||||||
| | | | | | | | | | | |
Greater than 5% stockholders |
||||||||||
Paul B. Manning and related entities(1) |
14,124,049 | 55.0% | 50.1% | |||||||
Perceptive Life Sciences Master Fund, Ltd.(2) |
1,809,015 | 7.1 | 6.4 | |||||||
FMR, LLC(3) |
1,888,583 | 7.4 | 6.7 | |||||||
Directors and named executive officers |
||||||||||
Alex Sapir(4) |
934,200 | 3.5 | 3.2 | |||||||
Doug Blankenship(5) |
228,525 | * | * | |||||||
Lee F. Allen, M.D., Ph.D.(6) |
254,966 | * | * | |||||||
Steven M. Goldman(7) |
130,169 | * | * | |||||||
Roger A. Jeffs(8) |
64,400 | * | * | |||||||
Paul B. Manning(1) |
14,124,049 | 55.0 | 50.1 | |||||||
Alfred J. Novak(9) |
33,000 | * | * | |||||||
Sean Stalfort(10) |
604,665 | 2.4 | 2.1 | |||||||
All executive officers and directors as a group (9 persons) |
16,520,424 |
60.5 |
55.5 |
|||||||
| | | | | | | | | | | |
* Represents beneficial ownership of less than 1%.
(1) Consists of (a) 12,390,823 shares of common stock held by Paul B. Manning and (b) 1,733,226 shares of common stock held by BKB Growth Investments, LLC, or BKB. Mr. Manning is a co-manager of BKB and has sole voting and investment power with respect to the shares held by BKB. The business address for Mr. Manning and BKB is 200 Garrett Street, Suite S, Charlottesville, VA 22902.
(2) This information has been obtained from a Schedule 13G/A filed on February 14, 2018 by entities and individuals associated with Perceptive Life Sciences Master Fund, Ltd. Shares beneficially owned prior to this offering consist of 1,809,015 shares of common stock directly held by Perceptive Life Sciences Master Fund, Ltd. Perceptive Advisors LLC serves as the investment manager to Perceptive Life Sciences Master Fund, Ltd. and may be deemed to beneficially own such shares. Joseph Edelman is the managing member of Perceptive Advisors LLC and may be deemed to beneficially own such shares. The principal business address of these persons and entities is 51 Astor Place, 10th Floor, New York, NY 10003.
(3) This information has been obtained from a Schedule 13G filed on February 13, 2018. The address of FMR, LLC is 245 Summer Street, Boston, Massachusetts 02210.
(4) Consists of 20,100 shares of common stock, 247,568 shares underlying options that are exercisable and vested within 60 days of December 31, 2017 and 666,532 shares that may be acquired pursuant to early exercise features of unvested options. Any shares issued upon the exercise of unvested options are subject to a repurchase right in favor of us if Mr. Sapir does not satisfy the option's vesting requirements. In any event, shares acquired upon an early exercise may not be disposed of until the vesting period has been satisfied.
(5) Consists of 228,525 shares that may be acquired pursuant to early exercise features of options that vest in accordance with their terms. Any shares issued upon the exercise of unvested options are subject to a repurchase right in favor of us if Mr. Blankenship does not satisfy the option's vesting requirements. In any event, shares acquired upon an early exercise may not be disposed of until the vesting period has been satisfied. On January 29, 2018, Mr. Blankenship resigned as our Chief Financial Officer, with his separation effective as of February 8, 2018.
(6) Consists of 3,500 shares of common stock and 251,466 shares that may be acquired pursuant to early exercise features of options that vest in accordance with their terms. Any shares issued upon the exercise of unvested options are subject to a repurchase right in favor of us if Dr. Allen does not satisfy the option's vesting requirements. In any event, shares acquired upon an early exercise may not be disposed of until the vesting period has been satisfied.
(7) Consists of (a) 92,169 shares of common stock held by Steven M. Goldman directly, (b) 5,000 shares held by the Steven M. Goldman Family LLC and (c) 33,000 shares that may be acquired pursuant to early exercise features of options that vest in accordance with their terms. Any shares issued upon the exercise of unvested options are subject to a repurchase right in favor of us if Mr. Goldman does not satisfy the option's vesting requirements. In any event, shares acquired upon an early exercise may not be disposed of until the vesting period has been satisfied. Mr. Goldman is the managing member of the Steven M. Goldman Family LLC and may be deemed to have voting and investment power with respect to the shares held by the Steven M. Goldman Family LLC.
(8) Consists of 31,400 shares of common stock and 33,000 shares that may be acquired pursuant to early exercise features of options that vest in accordance with their terms. Any shares issued upon the exercise of unvested options are subject to a repurchase right in favor of us if Dr. Jeffs does not satisfy the option's vesting requirements. In any event, shares acquired upon an early exercise may not be disposed of until the vesting period has been satisfied.
(9) Consists of 33,000 shares that may be acquired pursuant to early exercise features of options that vest in accordance with their terms. Any shares issued upon the exercise of unvested options are subject to a repurchase right in favor of us if Mr. Novak does not satisfy the option's vesting requirements. In any event, shares acquired upon an early exercise may not be disposed of until the vesting period has been satisfied.
(10) Consists of 604,665 shares of common stock.
28
The following description of our capital stock and provisions of our amended and restated certificate of incorporation and amended and restated bylaws are summaries. You should also refer to the amended and restated certificate of incorporation and the amended and restated bylaws, which are filed as exhibits to the registration statement of which this prospectus is part.
General
Our amended and restated certificate of incorporation authorizes us to issue up to 100,000,000 shares of common stock, $0.001 par value per share, and 10,000,000 shares of preferred stock, $0.001 par value per share, all of which shares of preferred stock will be undesignated. Our board of directors may establish the rights and preferences of the preferred stock from time to time.
Common stock
Voting rights
Each holder of our common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, including the election of directors. Under our amended and restated certificate of incorporation and amended and restated bylaws, our stockholders do not have cumulative voting rights. Because of this, the holders of a majority of the shares of common stock entitled to vote in any election of directors can elect all of the directors standing for election, if they should so choose. As of December 31, 2017, we had outstanding 25,652,457 shares of common stock, held of record by 31 stockholders. We believe the number of beneficial owners of our common stock at that date was substantially greater.
Dividends
Subject to preferences that may be applicable to any then-outstanding preferred stock, holders of common stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by the board of directors out of legally available funds.
Liquidation
In the event of our liquidation, dissolution or winding up, holders of common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any then-outstanding shares of preferred stock.
Rights and preferences
Holders of common stock have no preemptive, conversion or subscription rights and there are no redemption or sinking fund provisions applicable to the common stock. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate in the future.
Preferred stock
Our board of directors has the authority, without further action by our stockholders, to issue up to 10,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon, and to increase or
29
decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of our common stock. The purpose of authorizing our board of directors to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of us and may adversely affect the market price of our common stock and the voting and other rights of the holders of our common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock on the rights of holders of common stock until the board of directors determines the specific rights attached to that preferred stock.
There are no shares of preferred stock outstanding, and we have no present plans to issue any shares of preferred stock.
Options
As of December 31, 2017, under our 2017 Plan and our IPO Plan, options to purchase an aggregate of 2,128,641 shares of common stock were outstanding. For additional information regarding the terms of these plans, see "Executive Compensation—Equity Incentive Plans" in our Annual Report on Form 10-K for the year ended December 31, 2017, which is incorporated by reference herein.
Registration rights
The registration rights provisions of the IRA provide those holders with demand and piggyback registration rights with respect to their shares of our common stock held by them, which we refer to herein as registrable shares. After registration pursuant to these rights, such shares of common stock will become freely tradable without restriction under the Securities Act. Approximately 21 million shares of common stock are entitled to these registration rights. The IRA restricts us from granting additional registration rights to any other party without the consent of a majority of the holders of registrable securities unless such additional registration rights are no more favorable than those in the IRA.
Demand registration rights
The holders of at least a majority of the registrable shares, voting as a single class, who are party to the IRA have the right to demand that we file a Form S-1 registration statement for the registration of their shares of common stock. These registration rights are subject to specified conditions and limitations, including the right of a managing underwriter to limit the number of shares included in any such registration under specified circumstances. Upon such a request, we are required to effect the registration as expeditiously as possible. We are not obligated to file a registration statement pursuant to this provision on more than one occasion (unless such registration statement was not declared effective by the SEC).
Piggyback registration rights
If we propose to register any of our common stock under the Securities Act of 1933, as amended, or the Securities Act, either for our own account or for the account of other stockholders, other than pursuant to certain specified registrations (including relating to company stock option plans), the holders of registrable shares will each be entitled to notice of the registration and will be entitled to include their registrable
30
shares in the related registration statement. These piggyback registration rights are subject to specified conditions and limitations, including the right of a managing underwriter to limit the number of shares included in any such registration under specified circumstances. All piggyback registration rights have been waived in connection with this offering.
Registration on Form S-3
At any time after we become eligible to file a registration statement on Form S-3, the holders of at least a majority of our shares of common stock have the right to demand that we file a registration statement on Form S-3, and holders of such shares will be entitled, upon their written request, to have such shares registered by us on a Form S-3 registration statement at our expense, provided that such requested registration has an anticipated aggregate offering size to the public of at least $5.0 million, net of offering expenses, and subject to other specified conditions and limitations. We are not obligated to file a registration statement pursuant to this provision on more than one occasion in any 12-month period (unless such registration statement was not declared effective by the SEC).
In the event that any registration in which the holders of registrable shares participate pursuant to our IRA is an underwritten public offering, we agree to enter into an underwriting agreement containing customary terms for such offering.
Expenses of registration
We are required to pay all expenses, including fees and expenses of one counsel to represent the selling stockholders (up to $75,000 total), relating to any demand, piggyback or Form S-3 registration, other than underwriting discounts and commissions, stock transfer taxes and any additional fees of counsel for the selling stockholders, subject to specified conditions and limitations. We are not required to pay registration expenses if a demand registration request is withdrawn at the request of a majority of holders of registrable securities to be registered, unless holders of a majority of the registrable securities agree to forfeit their right to one demand registration.
The IRA contains customary cross-indemnification provisions, pursuant to which we are obligated to indemnify the selling stockholders in the event of material misstatements or omissions in the applicable registration statement attributable to us, and the selling stockholders are obligated to indemnify us for material misstatements or omissions in the registration statement attributable to them, subject to certain limitations.
Termination of registration rights
The registration rights granted under the IRA will terminate upon the earlier of the fifth anniversary of the completion of our IPO and a liquidation event for the Company.
Anti-takeover provisions
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
- •
- before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder;
31
- •
- upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at
least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding, but not the outstanding voting stock
owned by the interested stockholder, those shares owned (i) by persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have the
right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
- •
- on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 662/3% of the outstanding voting stock that is not owned by the interested stockholder.
In general, Section 203 defines a "business combination" to include the following:
- •
- any merger or consolidation involving the corporation and the interested stockholder;
- •
- any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder;
- •
- subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to
the interested stockholder;
- •
- any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the
corporation beneficially owned by the interested stockholder; or
- •
- the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits by or through the corporation.
In general, Section 203 defines an "interested stockholder" as an entity or person who, together with the person's affiliates and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Certificate of incorporation and bylaws
Our amended and restated certificate of incorporation, or our restated certificate, provides for our board of directors to be divided into three classes with staggered three-year terms. Only one class of directors is elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Because our stockholders do not have cumulative voting rights, stockholders holding a majority of the shares of common stock outstanding are able to elect all of our directors. Our restated certificate and our amended and restated bylaws, or our restated bylaws, also provide that directors may be removed by the stockholders only for cause upon the vote of 662/3% or more of our outstanding common stock. Furthermore, the authorized number of directors may be changed only by resolution of the board of directors, and vacancies and newly created directorships on the board of directors may, except as otherwise required by law or determined by the board, only be filled by a majority vote of the directors then serving on the board, even though less than a quorum.
Our restated certificate and restated bylaws also provide that all stockholder actions must be effected at a duly called meeting of stockholders and eliminated the right of stockholders to act by written consent without a meeting. Our restated bylaws also provide that only our chairman of the board, chief executive
32
officer or the board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors may call a special meeting of stockholders.
Our restated bylaws also provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide timely advance notice in writing, and specify requirements as to the form and content of a stockholder's notice.
Our restated certificate and restated bylaws provide that the stockholders cannot amend many of the provisions described above except by a vote of 662/3% or more of our outstanding common stock.
The combination of these provisions make it more difficult for our existing stockholders to replace our board of directors as well as for another party to obtain control of us by replacing our board of directors. Since our board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management. In addition, the authorization of undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change our control.
These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to reduce our vulnerability to hostile takeovers and to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and may have the effect of delaying changes in our control or management. As a consequence, these provisions may also inhibit fluctuations in the market price of our stock that could result from actual or rumored takeover attempts. We believe that the benefits of these provisions, including increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure our company, outweigh the disadvantages of discouraging takeover proposals, because negotiation of takeover proposals could result in an improvement of their terms.
Choice of forum
Our restated certificate provides that the Court of Chancery of the State of Delaware will be the exclusive forum for:
- •
- any derivative action or proceeding brought on our behalf;
- •
- any action asserting a breach of fiduciary duty;
- •
- any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, our restated certificate, or our amended and
restated bylaws; or
- •
- any action asserting a claim against us that is governed by the internal affairs doctrine.
The enforceability of similar choice of forum provisions in other companies' certificates of incorporation has been challenged in legal proceedings, and it is possible that, in connection with any action, a court could find the choice of forum provisions contained in our restated certificate to be inapplicable or unenforceable in such action.
33
Transfer agent and registrar
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company. The transfer agent's address is 6201 15th Avenue, Brooklyn, NY 11219.
NASDAQ Global Market listing
Our common stock is listed on The NASDAQ Global Market under the trading symbol "DOVA."
34
Shares eligible for future sale
Based on the number of shares outstanding as of December 31, 2017, upon completion of this offering, 28,152,457 shares of common stock will be outstanding. All of the these shares will be freely tradable without restrictions or further registration under the Securities Act, except for any shares held by our "affiliates," as that term is defined under Rule 144 under the Securities Act. Restricted securities may be sold in the public market only if registered or if their resale qualifies for exemption from registration described below under Rule 144 promulgated under the Securities Act or another available exemption. Of these shares, approximately 14.9 million shares will be eligible for sale in the public market upon expiration of lock-up agreements 90 days after the date of this prospectus, subject in certain circumstances to the volume, manner of sale and other limitations under Rule 144.
Rule 144
In general, non-affiliate persons who have beneficially owned restricted shares of our common stock for at least six months, and any affiliate of the company who owns either restricted or unrestricted shares of our common stock, are entitled to sell their securities without registration with the SEC under an exemption from registration provided by Rule 144 under the Securities Act.
Non-affiliates
Any person who is not deemed to have been one of our affiliates at the time of, or at any time during the three months preceding, a sale may sell an unlimited number of restricted securities under Rule 144 if:
- •
- the restricted securities have been held for at least six months, including the holding period of any prior owner other than one of our
affiliates (subject to certain exceptions); and
- •
- we are current in our Exchange Act reporting at the time of sale.
Any person who is not deemed to have been an affiliate of ours at the time of, or at any time during the three months preceding, a sale and has held the restricted securities for at least one year, including the holding period of any prior owner other than one of our affiliates, will be entitled to sell an unlimited number of restricted securities without regard to whether we are current in our Exchange Act reporting. Non-affiliate resales are not subject to the manner of sale, volume limitation or notice filing provisions of Rule 144.
Affiliates
Persons seeking to sell restricted securities who are our affiliates at the time of, or any time during the three months preceding, a sale, would be subject to the restrictions described above. They are also subject to additional restrictions, by which such person would be required to comply with the manner of sale and notice provisions of Rule 144 and would be entitled to sell within any three-month period only that number of securities that does not exceed the greater of either of the following:
- •
- 1% of the number of shares of our common stock then outstanding, which will equal approximately 280,000 shares immediately after the completion
of this offering based on the number of shares outstanding as of December 31, 2017; or
- •
- the average weekly trading volume of our common stock on The NASDAQ Global Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale.
35
Additionally, persons who are our affiliates at the time of, or any time during the three months preceding, a sale may sell unrestricted securities under the requirements of Rule 144 described above, without regard to the six month holding period of Rule 144, which does not apply to sales of unrestricted securities.
Form S-8 registration statements
We have filed a registration statement on Form S-8 under the Securities Act to register all shares of common stock subject to outstanding stock options and common stock issued or issuable under our 2017 Plan and IPO Plan, permitting the resale of such shares by non-affiliates in the public market without restriction under the Securities Act and the sale by affiliates in the public market, subject to compliance with the resale provisions of Rule 144 and expiration or release from the terms of the lock-up agreements described above.
Lock-up agreements
In connection with this offering, we, and all of our executive officers, directors and their affiliated entities owning in the aggregate approximately 14.9 million shares of our common stock, have entered into lock-up agreements or otherwise agreed that we and they will not, subject to limited exceptions, (i) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, or (ii) enter into any swap or other arrangement that transfers all or a portion of the economic consequences associated with the ownership of any shares of common stock or any such other securities (regardless of whether any of these transactions are to be settled by the delivery of shares of common stock or such other securities, in cash or otherwise), in each case without the prior written consent of J.P. Morgan Securities LLC and Jefferies LLC for a period of 90 days after the date of this prospectus.
Registration rights
Upon the closing of this offering, the holders of approximately 21 million shares of our common stock will be entitled to specified rights with respect to the registration of their registrable shares under the Securities Act, subject to certain limitations and the expiration, waiver or termination of the lock-up agreements. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act immediately upon effectiveness of the registration. See "Description of capital stock—Registration rights" for additional information.
36
Material U.S. federal income tax consequences to non-U.S. holders
The following is a discussion of the material U.S. federal income tax considerations applicable to non-U.S. holders (as defined below) with respect to their ownership and disposition of shares of our common stock issued pursuant to this offering, but does not purport to be a complete analysis of all potential tax effects. All prospective non-U.S. holders of our common stock should consult their own tax advisors with respect to the U.S. federal income tax consequences of the purchase, ownership and disposition of our common stock, as well as any consequences arising under the U.S. estate tax or under the laws of any other taxing jurisdiction, including any state, local and non-U.S. tax consequences and any U.S. federal non-income tax consequences. In general, a non-U.S. holder means a beneficial owner of our common stock (other than a partnership or an entity or arrangement treated as a partnership for U.S. federal income tax purposes) that is not, for U.S. federal income tax purposes:
- •
- an individual who is a citizen or resident of the United States;
- •
- a corporation, or an entity treated as a corporation for U.S. federal income tax purposes, created or organized in the United States or under
the laws of the United States or of any state thereof or the District of Columbia;
- •
- an estate, the income of which is subject to U.S. federal income tax regardless of its source; or
- •
- a trust if (1) a U.S. court can exercise primary supervision over the trust's administration and one or more U.S. persons have the authority to control all of the trust's substantial decisions or (2) the trust has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a U.S. person.
This discussion is based on current provisions of the U.S. Internal Revenue Code of 1986, as amended, which we refer to as the Code, existing U.S. Treasury Regulations promulgated thereunder, published administrative rulings and judicial decisions, all as in effect as of the date of this prospectus supplement. These laws are subject to change and to differing interpretation, possibly with retroactive effect. Any change or differing interpretation could alter the tax consequences to non-U.S. holders described in this prospectus supplement.
This discussion is limited to non-U.S. holders that hold shares of our common stock as a capital asset within the meaning of Section 1221 of the Code (generally, for investment). This discussion does not address all aspects of U.S. federal income taxation that may be relevant to a particular non-U.S. holder in light of that non-U.S. holder's individual circumstances, nor does it address any aspects of U.S. estate or gift tax, or any state, local or non-U.S. taxes. This discussion also does not consider any specific facts or circumstances that may apply to a non-U.S. holder and does not address the special tax rules applicable to particular non-U.S. holders, such as holders that own, or are deemed to own, more than 5% of our capital stock (except to the extent specifically set forth below), corporations that accumulate earnings to avoid U.S. federal income tax, tax-exempt organizations, banks, financial institutions, insurance companies, brokers, dealers or traders in securities, commodities or currencies, tax-qualified retirement plans, holders subject to the alternative minimum tax or Medicare contribution tax, holders holding our common stock as part of a hedge, straddle or other risk reduction strategy, conversion transaction or other integrated investment, holders deemed to sell our common stock under the constructive sale provisions of the Code, controlled foreign corporations, passive foreign investment companies and U.S. expatriates and certain former citizens or long-term residents of the United States.
37
In addition, this discussion does not address the tax treatment of partnerships (or entities or arrangements that are treated as partnerships for U.S. federal income tax purposes) or persons that hold their common stock through such partnerships or such entities or arrangements. If a partnership, including any entity or arrangement treated as a partnership for U.S. federal income tax purposes, holds shares of our common stock, the U.S. federal income tax treatment of a partner in such partnership will generally depend upon the status of the partner, the activities of the partnership and certain determinations made at the partner level. Such partners and partnerships should consult their own tax advisors regarding the tax consequences of the purchase, ownership and disposition of our common stock.
There can be no assurance that the Internal Revenue Service, which we refer to as the IRS, will not challenge one or more of the tax consequences described herein, and we have not obtained, nor do we intend to obtain, a ruling with respect to the U.S. federal income tax consequences with respect to the matters discussed below.
Distributions on our common stock
Distributions, if any, on our common stock generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the non-U.S. holder's investment, up to such holder's adjusted tax basis in the common stock. Any remaining excess will be treated as capital gain from the sale or exchange of such common stock, subject to the tax treatment described below in "—Gain on sale, exchange or other disposition of our common stock."
Subject to the discussion below regarding backup withholding and foreign accounts, dividends paid to a non-U.S. holder will generally be subject to withholding of U.S. federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. A non-U.S. holder that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. A non-U.S. holder of our common stock who claims the benefit of an applicable income tax treaty generally will be required to provide a properly executed IRS Form W-8BEN or W-8BEN-E (or successor form) and satisfy relevant certification and other requirements. Non-U.S. holders are urged to consult their tax advisors regarding their entitlement to benefits under a relevant income tax treaty.
Dividends that are treated as effectively connected with a trade or business conducted by a non-U.S. holder within the United States and, if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed base maintained by the non-U.S. holder within the United States are generally exempt from the 30% withholding tax if the non-U.S. holder satisfies applicable certification and disclosure requirements. To claim the exemption, the non-U.S. holder must furnish to us or the applicable withholding agent a valid IRS Form W-8ECI (or applicable successor form), certifying that the dividends are effectively connected with the non-U.S. holder's conduct of a trade or business within the United States. However, such U.S. effectively connected income, net of specified deductions and credits, is taxed at the same U.S. federal income tax rates applicable to U.S. persons (as defined in the Code). Any U.S. effectively connected income received by a non-U.S. holder that is a corporation may also, under certain circumstances, be subject to an additional "branch profits tax" at a 30% rate or such lower rate as may be specified by an applicable income tax treaty.
38
Gain on sale, exchange or other disposition of our common stock
Subject to the discussions below regarding backup withholding and foreign accounts, in general, a non-U.S. holder will not be subject to any U.S. federal income tax on any gain realized upon such holder's sale, exchange or other disposition of shares of our common stock unless:
- •
- the gain is effectively connected with a U.S. trade or business of the non-U.S. holder and, if an applicable income tax treaty so provides, is
attributable to a permanent establishment or a fixed base maintained in the United States by such non-U.S. holder, in which case the non-U.S. holder generally will be taxed at the U.S. federal income
tax rates applicable to U.S. persons (as defined in the Code) and, if the non-U.S. holder is a foreign corporation, the branch profits tax described above in "Distributions on our common stock" may
also apply;
- •
- the non-U.S. holder is a nonresident alien individual who is present in the United States for 183 days or more in the taxable year of
the disposition and certain other conditions are met, in which case the non-U.S. holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty) on the
net gain derived from the disposition, which may be offset by U.S. source capital losses of the non-U.S. holder, if any (even though the individual is not considered a resident of the United States),
provided the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses; or
- •
- our common stock constitutes a U.S. real property interest because we are, or have been, at any time during the five-year period preceding such disposition (or the non-U.S. holder's holding period, if shorter) a "U.S. real property holding corporation." Even if we are or become a U.S. real property holding corporation, provided that our common stock is regularly traded on an established securities market, our common stock will be treated as a U.S. real property interest only with respect to a non-U.S. holder that holds more than 5% of our outstanding common stock, directly or indirectly, actually or constructively, during the shorter of the five-year period ending on the date of the disposition or the period that the non-U.S. holder held our common stock. In such case, such non-U.S. holder generally will be taxed on its net gain derived from the disposition at the graduated U.S. federal income tax rates applicable to U.S. persons (as defined in the Code). Generally, a corporation is a U.S. real property holding corporation only if the fair market value of its U.S. real property interests equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance, we do not believe that we are, or have been, a U.S. real property holding corporation, or that we are likely to become one in the future. We expect that our common stock will be regularly traded on an established securities market, but no assurance can be provided that our common stock will be regularly traded.
Backup withholding and information reporting
We must report annually to the IRS and to each non-U.S. holder the gross amount of the dividends on our common stock paid to such holder and the tax withheld, if any, with respect to such dividends. Non-U.S. holders will have to comply with specific certification procedures to establish that the holder is not a U.S. person (as defined in the Code) in order to avoid backup withholding at the applicable rate with respect to dividends on our common stock. U.S. backup withholding generally will not apply to a non-U.S. holder who provides a properly executed IRS Form W-8BEN or W-8BEN-E or otherwise establishes an exemption.
Information reporting and backup withholding will generally apply to the proceeds of a disposition of our common stock by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or foreign,
39
unless the holder certifies its status as a non-U.S. holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker. Non-U.S. holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Copies of information returns may be made available to the tax authorities of the country in which the non-U.S. holder resides or is incorporated under the provisions of a specific treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a non-U.S. holder may be allowed as a credit against the non-U.S. holder's U.S. federal income tax liability, if any, and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Foreign accounts
The Code generally imposes a U.S. federal withholding tax of 30% on dividends and the gross proceeds of a disposition of our common stock paid to a "foreign financial institution" (as specifically defined for this purpose), unless such institution enters into an agreement with the U.S. government to, among other things, withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which may include certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners). Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing these withholding and reporting requirements may be subject to different rules. This U.S. federal withholding tax of 30% also applies to dividends and the gross proceeds of a disposition of our common stock paid to a non-financial foreign entity, unless such entity provides the withholding agent with either a certification that it does not have any substantial direct or indirect U.S. owners or information regarding substantial direct and indirect U.S. owners of the entity. The withholding tax described above will not apply if the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from the rules. The withholding provisions described above currently apply to dividends on our common stock and will apply with respect to gross proceeds of a sale or other disposition of our common stock on or after January 1, 2019. Under certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. Non-U.S. holders are encouraged to consult with their own tax advisors regarding the possible implications of the legislation on their investment in our common stock.
EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY RECENTLY ENACTED CHANGE IN APPLICABLE LAW, AS WELL AS TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL, NON-U.S. OR U.S. FEDERAL NON-INCOME TAX LAWS.
40
We are offering the shares of common stock described in this prospectus through a number of underwriters. J.P. Morgan Securities LLC, Jefferies LLC and Evercore Group L.L.C. are acting as book-running managers of the offering and as representatives of the underwriters. We have entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of shares of common stock listed next to its name in the following table:
| |
|
|||
|---|---|---|---|---|
| | | | | |
| Name |
Number of shares |
|||
| | | | | |
J.P. Morgan Securities LLC |
||||
Jefferies LLC |
||||
Evercore Group L.L.C. |
||||
| | | | | |
Total |
||||
| | | | | |
The underwriters are committed to purchase all the common shares offered by us if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may also be increased or the offering may be terminated.
The underwriters propose to offer the common shares directly to the public at the offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per share. Any such dealers may resell shares to certain other brokers or dealers at a discount of up to $ per share from the initial public offering price. After the initial offering of the shares to the public, the offering price and other selling terms may be changed by the underwriters. Sales of shares made outside of the United States may be made by affiliates of the underwriters.
The underwriters have an option to buy up to 375,000 additional shares of common stock from us to cover sales of shares by the underwriters which exceed the number of shares specified in the table above. The underwriters have 30 days from the date of this prospectus to exercise this option to purchase additional shares. If any shares are purchased with this option to purchase additional shares, the underwriters will purchase shares in approximately the same proportion as shown in the table above. If any additional shares of common stock are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered.
The underwriting fee is equal to the public offering price per share of common stock less the amount paid by the underwriters to us per share of common stock. The underwriting fee is $ per share. The following table shows the per share and total underwriting discounts and commissions to be paid to the
41
underwriters assuming both no exercise and full exercise of the underwriters' option to purchase additional shares.
| |
|
|
|||||
|---|---|---|---|---|---|---|---|
| | | | | | | | |
| |
Without option to purchase additional shares exercise |
With full option to purchase additional shares exercise |
|||||
| | | | | | | | |
Per Share |
$ | $ | |||||
Total |
$ | $ | |||||
| | | | | | | | |
We estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions, will be approximately $600,000. We have agreed to reimburse the underwriters for expenses relating to the clearance of this offering with the Financial Industry Regulatory Authority up to $20,000.
A prospectus in electronic format may be made available on the web sites maintained by one or more underwriters, or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make Internet distributions on the same basis as other allocations.
We have agreed that we will not (i) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, or (ii) enter into any swap or other arrangement that transfers all or a portion of the economic consequences associated with the ownership of any shares of common stock or any such other securities (regardless of whether any of these transactions are to be settled by the delivery of shares of common stock or such other securities, in cash or otherwise), in each case without the prior written consent of J.P. Morgan Securities LLC and Jefferies LLC for a period of 90 days after the date of this prospectus.
We have agreed that, subject to certain conditions, the foregoing restrictions shall not apply to:
(i) the shares of our common stock to be sold in this offering;
(ii) any shares of our common stock issued pursuant to equity compensation plans described in this prospectus, provided that no filing under the Exchange Act or other public announcement shall be made, subject to certain exceptions;
(iii) any options and awards granted under an equity compensation plan described in this prospectus, provided that the recipient executes a lock-up agreement for the remainder of the 90-day period referred to above;
(iv) the filing of a registration statement on Form S-8 relating to an equity compensation plan described in this prospectus; or
42
(v) up to 5% of our outstanding securities by us in connection with certain commerical or strategic transactions, including acquisitions, provided that the recipient executes a lock-up agreement for the remainder of the 90-day period referred to above.
Our executive officers, directors and their affiliated entities owning in the aggregate approximately 14.9 million shares of our common stock have entered into lock-up agreements prior to the commencement of this offering pursuant to which each of these persons or entities for a period of 90 days after the date of this prospectus, may not, without the prior written consent of J.P. Morgan Securities LLC and Jefferies LLC, (1) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock (including, without limitation, common stock or such other securities which may be deemed to be beneficially owned by such directors, executive officers and stockholders in accordance with the rules and regulations of the SEC and securities which may be issued upon exercise of a stock option or warrant) or (2) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the common stock or such other securities, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of common stock or such other securities, in cash or otherwise, or (3) make any demand for or exercise any right with respect to the registration of any shares of our common stock or any security convertible into or exercisable or exchangeable for our common stock.
Each such director, executive officer and stockholder has agreed that, subject to certain conditions, the foregoing restrictions shall not apply to any shares of our common stock purchased in this offering or in the public market following this offering, or any transfers of shares of our common stock:
(i) as a bona fide gift or gifts or by will, testamentary document or intestate succession;
(ii) to any trust for the direct or indirect benefit of such directors, executive officers and stockholders or their immediate family;
(iii) to partners, members, stockholders or trust beneficiaries of such directors, executive officers and stockholders;
(iv) in the event such stockholder is a corporation, partnership, limited liability company, trust or other business entity, to any direct or indirect affiliate of such stockholder or any investment fund or other entity controlled or managed by such stockholder or any investment fund or other entity that controls such stockholder;
(v) by operation of law;
(vi) to the Company pursuant to agreements under which the Company has the option to repurchase such shares or a right of first refusal with respect to transfers of such shares upon termination of service of the locked-up party; or
(vii) pursuant to a bona fide third-party tender offer, merger, consolidation or other similar transaction made to all holders of the Company's securities involving a change of control of the Company, provided, that in the event that such tender offer, merger, consolidation or other such transaction is not completed, such securities held by such directors, executive officers and stockholders will remain subject to the lock-up provisions.
43
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act.
Our common stock is listed on The NASDAQ Global Market under the trading symbol "DOVA."
In connection with this offering, the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling shares of common stock in the open market for the purpose of preventing or retarding a decline in the market price of the common stock while this offering is in progress. These stabilizing transactions may include making short sales of the common stock, which involves the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in this offering, and purchasing shares of common stock on the open market to cover positions created by short sales. Short sales may be "covered" shorts, which are short positions in an amount not greater than the underwriters' option to purchase additional shares referred to above, or may be "naked" shorts, which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their option to purchase additional shares, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market compared to the price at which the underwriters may purchase shares through the option to purchase additional shares. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market that could adversely affect investors who purchase in this offering. To the extent that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of the common stock, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase common stock in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
These activities may have the effect of raising or maintaining the market price of the common stock or preventing or retarding a decline in the market price of the common stock, and, as a result, the price of the common stock may be higher than the price that otherwise might exist in the open market. If the underwriters commence these activities, they may discontinue them at any time. The underwriters may carry out these transactions on The NASDAQ Global Market, in the over-the-counter market or otherwise.
In addition, in connection with this offering, the underwriters may engage in passive market making transactions in our common stock on The NASDAQ Global Market prior to the pricing and completion of this offering. Passive market making consists of displaying bids on The NASDAQ Global Market no higher than the bid prices of independent market makers and making purchases at prices no higher than these independent bids and effected in response to order flow. Net purchases by a passive market maker on each day are generally limited to a specified percentage of the passive market maker's average daily trading volume in the common stock during a specified period and must be discontinued when such limit is reached. Passive market making may cause the price of our common stock to be higher than the price that otherwise would exist in the open market in the absence of these transactions. If passive market making is commenced, it may be discontinued at any time.
44
Selling restrictions
General
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Notice to prospective investors in Canada
The shares may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the shares must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser's province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser's province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Notice to prospective investors in the European Economic Area
In relation to each Member State of the European Economic Area (each, a "Relevant Member State"), no offer of shares may be made to the public in that Relevant Member State other than:
A. to any legal entity which is a qualified investor as defined in the Prospectus Directive;
B. to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representatives; or
C. in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of shares shall require the Company or the representatives to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
45
Each person in a Relevant Member State who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed to and with each of the representatives and the Company that it is a "qualified investor" within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive. In the case of any shares being offered to a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares to the public other than their offer or resale in a Relevant Member State to qualified investors as so defined or in circumstances in which the prior consent of the representatives has been obtained to each such proposed offer or resale.
For the purposes of this provision, the expression an "offer of shares to the public" in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in the Relevant Member State by any measure implementing the Prospectus Directive in the Relevant Member State and the expression "Prospectus Directive" means Directive 2003/71/EC (as amended by Directive 2010/73/EU), and includes any relevant implementing measure in the Relevant Member State.
Notice to prospective investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are "qualified investors" (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19 (5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the "Order") and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as "relevant persons") or otherwise in circumstances which have not resulted and will not result in an offer to the public of the shares in the United Kingdom within the meaning of the Financial Services and Markets Act 2000.
Any person in the United Kingdom that is not a relevant person should not act or rely on the information included in this document or use it as basis for taking any action. In the United Kingdom, any investment or investment activity that this document relates to may be made or taken exclusively by relevant persons.
Notice to prospective investors in Hong Kong
The shares have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to "professional investors" as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do
not result in the document being a "prospectus" as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the shares has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons
46
outside Hong Kong or only to "professional investors" as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
Notice to prospective investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the "SFA"), (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
(a) a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
(b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor,
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries' rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the shares pursuant to an offer made under Section 275 of the SFA except:
(a) to an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA;
(b) where no consideration is or will be given for the transfer;
(c) where the transfer is by operation of law;
(d) as specified in Section 276(7) of the SFA; or
(e) as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore
Notice to prospective investors in Japan
The shares have not been and will not be registered pursuant to Article 4, Paragraph 1 of the Financial Instruments and Exchange Act. Accordingly, none of the shares nor any interest therein may be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term, as used herein, means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to or for the benefit of a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Act and any other applicable laws, regulations and ministerial guidelines of Japan in effect at the relevant time.
47
Other relationships
Certain of the underwriters and their affiliates have provided in the past to us and our affiliates and may provide from time to time in the future certain commercial banking, financial advisory, investment banking and other services for us and such affiliates in the ordinary course of their business, for which they have received and may continue to receive customary fees and commissions. In addition, from time to time, certain of the underwriters and their affiliates may effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future.
48
The validity of the shares of common stock offered hereby will be passed upon for us by Cooley LLP, New York, New York. As of the date of this prospectus, a partner of Cooley LLP beneficially owns an aggregate of 13,668 shares of our common stock. Certain legal matters will be passed upon for the underwriters by Davis Polk & Wardwell LLP.
The consolidated financial statements of Dova Pharmaceuticals, Inc. as of December 31, 2017 and 2016 and for the year ended December 31, 2017 and for the period from March 24, 2016 (inception) to December 31, 2016, have been incorporated by reference herein and in the registration statement in reliance upon the report of KPMG LLP, independent registered public accounting firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing.
The audit report covering the December 31, 2017 consolidated financial statements contains an explanatory paragraph that states we have suffered recurring losses from operations that raises substantial doubt about our ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of that uncertainty.
Where you can find additional information
We have filed with the SEC a registration statement on Form S-1 under the Securities Act, with respect to the shares of common stock being offered by this prospectus. This prospectus, which constitutes part of the registration statement, does not contain all of the information in the registration statement and its exhibits. For further information with respect to our company and the common stock offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration statement, over the internet at the SEC's website at www.sec.gov. You may also read and copy any document we file with the SEC at its public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
We are subject to the information reporting requirements of the Exchange Act, and we will file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information will be available for inspection and copying at the public reference room and website of the SEC referred to above. We also maintain a website at www.dova.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of, and is not incorporated into, this prospectus.
49
Incorporation of certain information by reference
The SEC allows us to "incorporate by reference" information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC (File No. 001-38135):
- •
- our Annual Report on Form 10-K for the year ended December 31, 2017, filed with the SEC on February 16, 2018; and
- •
- our Current Report on Form 8-K filed with the SEC on January 31, 2018.
Notwithstanding the statements in the preceding paragraphs, no document, report or exhibit (or portion of any of the foregoing) or any other information that we have "furnished" to the SEC pursuant to the Exchange Act shall be incorporated by reference into this prospectus.
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to Dova Pharmaceuticals, Inc., Attn: Investor Relations, 240 Leigh Farm Road, Suite 245, Durham, NC 27707.
You also may access these filings on our website at www.dova.com. We do not incorporate the information on our website into this prospectus or any supplement to this prospectus and you should not consider any information on, or that can be accessed through, our website as part of this prospectus or any supplement to this prospectus (other than those filings with the SEC that we specifically incorporate by reference into this prospectus or any supplement to this prospectus).
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement.
50
2,500,000 Shares

Dova Pharmaceuticals, Inc.
Common Stock
Prospectus
| J.P. Morgan | Jefferies | Evercore ISI |
, 2018
PART II
Information not required in prospectus
Item 13. Other expenses of issuance and distribution.
The following table indicates the expenses to be incurred in connection with the offering described in this registration statement, other than underwriting discounts and commissions, all of which will be paid by us. All amounts are estimated except the SEC registration fee, the Financial Industry Regulatory Authority, Inc., or FINRA, filing fee.
| | | | | |
| |
Amount to be Paid |
|||
|---|---|---|---|---|
| | | | | |
SEC registration fee |
$ | 11,150 | ||
FINRA filing fee |
13,933 | |||
Printing and engraving expenses |
75,000 | |||
Legal fees and expenses |
300,000 | |||
Accounting fees and expenses |
140,000 | |||
Transfer agent and registrar fees and expenses |
10,000 | |||
Miscellaneous fees and expenses |
49,917 | |||
| | | | | |
Total |
$ | 600,000 | ||
| | | | | |
Item 14. Indemnification of directors and officers.
We are incorporated under the laws of the State of Delaware. Section 102 of the Delaware General Corporation Law permits a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit.
Section 145 of the Delaware General Corporation Law provides that a corporation has the power to indemnify a director, officer, employee or agent of the corporation and certain other persons serving at the request of the corporation in related capacities against expenses (including attorneys' fees), judgments, fines and amounts paid in settlements actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he is or is threatened to be made a party by reason of such position, if such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
As permitted by the Delaware General Corporation Law, our amended and restated certificate of incorporation and bylaws provide that: (i) we are required to indemnify our directors to the fullest extent permitted by the Delaware General Corporation Law; (ii) we may, in our discretion, indemnify our officers, employees and agents as set forth in the Delaware General Corporation Law; (iii) we are required, upon satisfaction of certain conditions, to advance all expenses incurred by our directors in connection with
certain legal proceedings; (iv) the rights conferred in the bylaws are not exclusive; and (v) we are authorized to enter into indemnification agreements with our directors, officers, employees and agents.
We have entered into agreements with each of our directors and executive officers that require us to indemnify them against expenses, judgments, fines, settlements and other amounts that any such person becomes legally obligated to pay (including with respect to a derivative action) in connection with any proceeding, whether actual or threatened, to which such person may be made a party by reason of the fact that such person is or was a director or officer of us or any of our affiliates, provided such person acted in good faith and in a manner such person reasonably believed to be in, or not opposed to, our best interests. The indemnification agreements also set forth certain procedures that will apply in the event of a claim for indemnification thereunder. We intend to enter into similar indemnification agreements with our executive officers prior to the completion of this offering. At present, no litigation or proceeding is pending that involves any of our directors or officers regarding which indemnification is sought, nor are we aware of any threatened litigation that may result in claims for indemnification.
We maintain a directors' and officers' liability insurance policy. The policy insures directors and officers against unindemnified losses arising from certain wrongful acts in their capacities as directors and officers and reimburses us for those losses for which we have lawfully indemnified the directors and officers. The policy contains various exclusions.
In addition, the underwriting agreement filed as Exhibit 1.1 to this Registration Statement provides for indemnification by the underwriters of us and our officers and directors for certain liabilities arising under the Securities Act, or otherwise.
Our investor rights agreement with certain investors also provides for cross-indemnification in connection with the registration of our common stock on behalf of such investors.
Item 15. Recent sales of unregistered securities.
Issuances of capital stock, promissory notes and warrants
The following list sets forth information regarding all unregistered securities sold by us since March 24, 2016 through the date of the prospectus that forms a part of this registration statement.
1) In March 2016, we issued PBM Capital Investments, LLC an aggregate of 50,000 units in exchange for its payment to Eisai of $5.0 million on our behalf in connection with our acquisition of worldwide rights to avatrombopag. In April 2016, we issued and sold to the Co-Investors an aggregate of 2,522 units at a purchase price of $100.00 per unit for aggregate consideration of $252,200. Each unit was converted into 330 shares of our common stock in connection with the Conversion.
2) From September to November 2016, we issued an aggregate of 982,714 shares of our Series A preferred stock to 12 investors at a purchase price of $29.51 per share for aggregate consideration of $29.0 million.
The offers, sales and issuances of the securities described in the paragraphs above were exempt from registration under Section 4(a)(2) of the Securities Act and Regulation D promulgated under the Securities Act. The recipients represented to us that they acquired the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the securities issued in these transactions. The recipients also represented to us that they were accredited investors as defined in Rule 501 promulgated under the Securities Act.
In addition, upon the completion of our IPO in June 2017, all outstanding shares of our convertible preferred stock, including the shares described in paragraph 2 above, automatically converted into an
aggregate of 3,242,950 shares of common stock. The issuance of such shares was exempt from registration under Section 3(a)(9) of the Securities Act.
Stock option grants
From March 24, 2016 through June 28, 2017, we granted options under the 2017 Plan to purchase an aggregate of 1,725,741 shares of our common stock to employees at a weighted average exercise price of $3.96 per share. Of these, no options have been cancelled without being exercised and 26,809 shares were issued upon the exercise of stock options.
The offers, sales and issuances of the securities described in the foregoing paragraph were exempt from registration under Rule 701 promulgated under the Securities Act in that the transactions were under compensatory benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of such securities were our employees, directors or consultants and received the securities under our 2017 Plan. Appropriate legends were affixed to the securities issued in these transactions.
Item 16. Exhibits and financial statement schedules.
^ Pursuant to Item 601(b)(2) of Regulation S-K, the Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the Stock Purchase Agreement to the Securities and Exchange Commission upon request.
+ Indicates management contract or compensatory plan.
# Confidential treatment has been granted with respect to portions of this exhibit (indicated by asterisks) and those portions have been separately filed with the Securities and Exchange Commission.
Item 17. Undertakings.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling
person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Durham, State of North Carolina, on this 20th day of February, 2018.
| DOVA PHARMACEUTICALS, INC. | ||||
By: |
/s/ ALEX SAPIR Alex Sapir President and Chief Executive Officer |
|||
KNOW ALL BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Alex Sapir and Mark W. Hahn, and each of them, his true and lawful agent, proxy and attorney-in-fact, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to (i) act on, sign and file with the Securities and Exchange Commission any and all amendments (including post-effective amendments) to this registration statement together with all schedules and exhibits thereto and any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, together with all schedules and exhibits thereto, (ii) act on, sign and file such certificates, instruments, agreements and other documents as may be necessary or appropriate in connection therewith, (iii) act on and file any supplement to any prospectus included in this registration statement or any such amendment or any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and (iv) take any and all actions which may be necessary or appropriate to be done, as fully for all intents and purposes as he might or could do in person, hereby approving, ratifying and confirming all that such agent, proxy and attorney-in-fact or any of his substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ ALEX SAPIR Alex Sapir |
President, Chief Executive Officer and Director (Principal Executive Officer) | February 20, 2018 | ||
/s/ MARK W. HAHN Mark W. Hahn |
Chief Financial Officer (Principal Financial and Accounting Officer) |
February 20, 2018 |
||
/s/ STEVEN M. GOLDMAN Steven M. Goldman |
Director |
February 20, 2018 |
||
/s/ ROGER A. JEFFS Roger A. Jeffs |
Director |
February 20, 2018 |
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ PAUL B. MANNING Paul B. Manning |
Director | February 20, 2018 | ||
/s/ ALFRED J. NOVAK Alfred J. Novak |
Director |
February 20, 2018 |
||
/s/ SEAN STALFORT Sean Stalfort |
Director |
February 20, 2018 |
