Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - Alkermes plc. | alks-20171231ex32158c817.htm |
| EX-31.2 - EX-31.2 - Alkermes plc. | alks-20171231ex312d8393e.htm |
| EX-31.1 - EX-31.1 - Alkermes plc. | alks-20171231ex311324c03.htm |
| EX-23.1 - EX-23.1 - Alkermes plc. | alks-20171231ex231f3952d.htm |
| EX-21.1 - EX-21.1 - Alkermes plc. | alks-20171231ex211ad09af.htm |
| EX-10.17.1 - EX-10.17.1 - Alkermes plc. | alks-20171231ex10171f6a5.htm |
| 10-K - 10-K - Alkermes plc. | alks-20171231x10k.htm |
Exhibit 10.10
A complete version of this Exhibit has been filed separately with the Securities and Exchange Commission pursuant to an application requesting confidential treatment pursuant to Rule 24b-2 promulgated under the Securities Act of 1934, as amended. Certain confidential portions of this Exhibit were omitted and replaced with double asterisks ([**]).
Execution Version
LICENSE AND COLLABORATION AGREEMENT
BETWEEN
ALKERMES PHARMA IRELAND LIMITED
AND
BIOGEN SWISS MANUFACTURING GMBH
NOVEMBER 27, 2017
|
|
Page |
|
ARTICLE 1 DEFINITIONS |
1 |
|
1.1.Defined Terms |
1 |
|
12 |
|
|
1.3.Interpretation |
13 |
|
ARTICLE 2 MANAGEMENT OF THE COLLABORATION |
14 |
|
14 |
|
|
16 |
|
|
16 |
|
|
ARTICLE 3 DEVELOPMENT |
17 |
|
3.1.Development Team |
17 |
|
19 |
|
|
22 |
|
|
24 |
|
|
25 |
|
|
3.6.Compliance |
25 |
|
3.7.Safety Reporting |
25 |
|
26 |
|
|
26 |
|
|
ARTICLE 4 COMMERCIALIZATION |
27 |
|
27 |
|
|
27 |
|
|
27 |
|
|
4.4.Compliance |
27 |
|
ARTICLE 5 MANUFACTURE AND SUPPLY |
27 |
|
27 |
|
|
5.2.Supply Team |
29 |
|
ARTICLE 6 LICENSE GRANTS |
29 |
|
29 |
|
|
6.2.Patent Update |
30 |
-i-
Table of Contents
(continued)
|
|
Page |
|
|
30 |
|
6.4.Non-Suit |
30 |
|
6.5.Non-Interference |
31 |
|
6.6.Exclusivity |
31 |
|
ARTICLE 7 INTELLECTUAL PROPERTY RIGHTS |
32 |
|
32 |
|
|
33 |
|
|
7.3.Patent Committee |
33 |
|
7.4.Patent Filings |
33 |
|
35 |
|
|
36 |
|
|
7.7.Patent Marking |
37 |
|
37 |
|
|
38 |
|
|
ARTICLE 8 CONFIDENTIALITY; PUBLICITY |
38 |
|
8.1.Confidentiality |
38 |
|
39 |
|
|
40 |
|
|
8.4.Survival |
40 |
|
40 |
|
|
ARTICLE 9 PAYMENTS |
41 |
|
9.1.Up-Front Payment |
41 |
|
9.2.Option Payment |
41 |
|
41 |
|
|
9.4.Development Milestones for Products other than the Alkermes 8700 Product |
41 |
|
9.5.Royalties |
42 |
|
44 |
|
|
45 |
|
|
46 |
-ii-
Table of Contents
(continued)
|
|
Page |
|
|
46 |
|
46 |
|
|
9.11.Taxes |
46 |
|
ARTICLE 10 REPRESENTATIONS AND WARRANTIES; COVENANTS; DISCLAIMER |
47 |
|
10.1.Disclaimer |
47 |
|
48 |
|
|
48 |
|
|
51 |
|
|
51 |
|
|
ARTICLE 11 LIABILITY |
51 |
|
51 |
|
|
52 |
|
|
52 |
|
|
53 |
|
|
11.5.Cooperation |
53 |
|
11.6.Insurance |
53 |
|
ARTICLE 12 DISPUTE RESOLUTION |
54 |
|
12.1.Disputes |
54 |
|
12.2.Jurisdiction |
55 |
|
55 |
|
|
12.4.Equitable Relief |
55 |
|
ARTICLE 13 TERM AND TERMINATION |
56 |
|
13.1.Term |
56 |
|
56 |
|
|
56 |
|
|
56 |
|
|
56 |
|
|
13.6.Bankruptcy |
59 |
|
60 |
-iii-
Table of Contents
(continued)
|
|
Page |
|
ARTICLE 14 GENERAL PROVISIONS |
60 |
|
14.1.Notices |
60 |
|
14.2.Governing Law |
61 |
|
61 |
|
|
61 |
|
|
14.5.Waiver |
62 |
|
14.6.Severability |
62 |
|
62 |
|
|
14.8.Force Majeure |
62 |
|
14.9.Ambiguities |
62 |
|
14.10.Headings |
62 |
|
14.11.No Partnership |
62 |
|
63 |
|
|
63 |
|
|
14.14.Further Assurances |
63 |
-iv-
LICENSE AND COLLABORATION AGREEMENT
This License And Collaboration Agreement (the “Agreement”) is entered into effective as of November 27, 2017 (the “Effective Date”) by and between Alkermes Pharma Ireland Limited, a private limited company incorporated in Ireland (registered number 448848) whose registered address is Connaught House, 1 Burlington Road, Dublin 4, Ireland (“Alkermes”), and Biogen Swiss Manufacturing GmbH, a Swiss limited liability company with its principal office at Landis & Gyr Strasse 3, CHR-6300 Zug, Switzerland (“Biogen”).
Recitals:
Whereas, Alkermes is developing the Alkermes 8700 Product for the treatment of MS;
Whereas, Biogen has experience and expertise in the development and commercialization of pharmaceutical products;
Whereas, Alkermes will continue the development of the Alkermes 8700 Product in the U.S. for the treatment of MS through the Transfer Completion Date pursuant to the terms of this Agreement;
Whereas, Alkermes will manufacture and supply to Biogen Clinical Supplies of the Alkermes 8700 Product and potentially other Products pursuant to the terms of a Clinical Supply Agreement;
Whereas, on all of the terms and subject to the conditions set forth in this Agreement Biogen desires to appoint Alkermes as the toll manufacturer for Commercial Supplies of the Alkermes 8700 Product in the Territory at a site outside of the United States, and Alkermes is willing to accept such appointment, subject to the terms of a supply agreement to be negotiated by the Parties; and
Whereas, Alkermes wishes to grant to Biogen, and Biogen wishes to obtain, an exclusive license to Exploit the Products in the Field in the Territory, all on the terms and subject to the conditions set forth in this Agreement.
Now, Therefore, in consideration of the premises and the mutual covenants and agreements set forth herein, and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
1.1.Defined Terms. When used in this Agreement, each of the following terms shall have the meanings set forth in this Article 1:
1.1.1.“Affiliate” with respect to any Party, means any entity that, directly or indirectly, controls, is controlled by, or is under common control with such Party, but only for so long as such control continues. For these purposes, “control” will refer to: (i) the
1
possession, directly or indirectly, of the power to direct the management or policies of an entity, whether through ownership of voting securities, by contract or otherwise or (ii) the ownership, directly or indirectly, of at least fifty percent (50%) of the equity securities of the entity entitled to vote in the election of directors (or, in the case of an entity that is not a corporation, at least fifty percent (50%) of the equity securities of the entity entitled to vote in the election of the corresponding managing authority or entitled to direct the management and policies of such entity).
1.1.2.“Alkermes 8700 Product” means the oral Product in Development by Alkermes as of the Effective Date and containing as its active ingredient the compound having the structure set forth in Exhibit A.
1.1.3.“Alkermes 8700 Product 505(b)(2) NDA” means the NDA for the Alkermes 8700 Product in the Initial Indication to be submitted by Alkermes to the FDA in the U.S. pursuant to (a) the 505(b)(2) regulatory pathway, as set forth in the Initial Development Plan or (b) the 505(b)(1) regulatory pathway, solely in the event that Biogen grants to Alkermes the right of reference in accordance with Section 3.3.2.
1.1.4.“Alkermes Collaboration Know-How” means Collaboration Know-How invented solely by Alkermes’ or its Affiliates’ employees, agents or independent contractors in the performance of activities under the Initial Development Plan during the Term.
1.1.5.“Alkermes Collaboration Patents” means all Collaboration Patents that Cover Alkermes Collaboration Know-How.
1.1.6.“Allocable Overhead” means [**].
1.1.7.“Applicable Law” means all applicable laws, statutes, rules, regulations, ordinances and other pronouncements having the binding effect of law of any applicable Governmental Authority, including any rules, regulations, guidelines or other requirements of the Regulatory Authorities, that may be in effect from time to time in the Territory.
1.1.8.“Bankruptcy Code” means Title 11, U.S. Code, or analogous provisions of Applicable Law outside the U.S.
1.1.9.“Biogen Collaboration Know-How” means Collaboration Know-How invented solely by Biogen’s or its Affiliates’ or Sublicensees’ employees, agents or independent contractors in the performance of activities under the Initial Development Plan during the Term.
1.1.10.“Biogen Collaboration Patents” means all Collaboration Patents that Cover Biogen Collaboration Know-How.
1.1.11. “Business Day” means a day on which banking institutions in Cambridge, Massachusetts, Zug, Switzerland and Dublin, Ireland are open for business.
2
1.1.12.“Calendar Quarter” means each three (3)-month period of January through March, April through June, July through September and October through December.
1.1.13.“Calendar Year” means each annual twelve (12)-month period starting on January 1 and ending on December 31.
1.1.14.“Change of Control” means, with respect to a Party, (i) an acquisition, reorganization, merger or consolidation of such Party with a Third Party in which the holders of the voting securities of such Party outstanding immediately prior thereto cease to beneficially own at least fifty percent (50%) of the combined voting power of the surviving entity, directly or indirectly, immediately after such acquisition, reorganization, merger or consolidation, (ii) a transaction or series of related transactions in which a Third Party, together with its Affiliates (if applicable), becomes the beneficial owner of fifty percent (50%) or more of the combined voting power of the outstanding securities of such Party, or (iii) the sale or other transfer to a Third Party of all or substantially all of such Party’s assets.
1.1.15.“Clinical Supplies” means supplies of a Product and Placebo to be used for the conduct of pre-clinical studies, Post-Marketing Commitments or Clinical Trials of a Product in the Field in the Territory pursuant to this Agreement.
1.1.16.“Clinical Supply Agreement” means a supply agreement between the Parties for the Manufacture by Alkermes of Clinical Supplies.
1.1.17.“Clinical Trials” means human studies designed to measure the safety or efficacy of a Product.
1.1.18.“Code” means the Code on Interactions with Healthcare Professionals promulgated by the Pharmaceutical Research and Manufacturers of America (PhRMA) and the American Medical Association (AMA) Guidelines on Gifts to Physicians from Industry.
1.1.19.“Collaboration Know-How” means any Know-How invented during the Term by a Party’s or its Affiliates’, licensees’ or Sublicensees’ employees, agents or independent contractors, either alone or jointly with the other Party’s or its Affiliates’, licensees’ or Sublicensees’ employees, agents or independent contractors, in the performance of activities under the Initial Development Plan during the Term.
1.1.20.“Collaboration Patents” means any Patent Rights invented by a Party’s or its Affiliates’, licensees’ or Sublicensees’ employees, agents or independent contractors, either alone or jointly with the other Party’s or its Affiliates’, licensees’ or Sublicensees’ employees, agents or independent contractors, in the performance of activities under the Initial Development Plan during the Term that Cover any Collaboration Know-How.
1.1.21.“Collaboration Technology” means the Collaboration Know-How and the Collaboration Patents.
1.1.22.“Combination Product” means any single product in finished form containing as active ingredients both (i) a Product and (ii) one or more other pharmaceutically active compounds or substances that are not Products, whether co-formulated or co-packaged
3
(i.e., within a single box or sales unit); (such pharmaceutically active compounds or substances, the “Other Components”).
1.1.23.“Commercial Supplies” or “Commercial Supply” means supplies of a Product for commercial sale or as promotional samples, or for use in Post-Marketing Clinical Trials.
1.1.24.“Commercialization” means the performance of any and all activities directed to promoting, marketing, importing, exporting, distributing, selling or offering to sell a Product following or in expectation of receipt of Regulatory Approval (but excluding Development and Manufacture). When used as a verb, “Commercialize” means to engage in Commercialization.
1.1.25.“Commercially Reasonable Efforts” [**].
1.1.26.“Complete GI Tolerability Data Package” means, with respect to Part B of the GI Tolerability Clinical Trial and the Long-Term Safety Clinical Trial, in each case, full demographic and patient-level data for all patients enrolled in each such trial (including, for the Long-Term Safety Clinical Trial, a breakdown of those patients that were previously enrolled in the GI Tolerability Clinical Trial and those patients not previously enrolled in such trial) and a complete set of all data and results available for each such trial, including all data and results that would fall into the categories set forth on Schedule 1.1.26 for each of Part B of the GI Tolerability Clinical Trial and the Long-Term Safety Clinical Trial.
1.1.27.“Confidentiality Agreements” means (i) the Mutual Confidentiality Agreement dated September 9, 2015 by and between Alkermes and Biogen, Inc., as reinstated, amended and extended on September 15, 2017 and (ii) the Confidentiality Agreement dated April 8, 2013 between Alkermes, Inc. and Biogen, Inc. (formerly known as Biogen Idec Inc.).
1.1.28.“Controlled” means, with respect to Patent Rights or Know-How, that the applicable Party, in whole or in part, owns or has a license to such Patent Rights or Know-How (but without taking into account any rights granted by one Party to the other Party pursuant to this Agreement) and has the ability to grant a license or a sublicense, as applicable, or to otherwise disclose proprietary or trade secret information, to such other Party, without misappropriating the proprietary or trade secret information of a Third Party or violating the terms of any agreement or other arrangement with any Third Party existing and in effect at the time such Party would be required hereunder to grant the other Party such license or sublicensee; provided, however, that if a Party is acquired pursuant to a Change of Control, the acquired Party will not be deemed to Control any Know-How, Patent Rights or other intellectual property rights owned or controlled prior to such Change of Control by any entities that were not Affiliates of the acquired Party prior to such Change of Control and become Affiliates of the acquired Party in connection with such Change of Control.
1.1.29.“Cover” means, as to a particular subject matter at issue and a claim of a relevant Patent Right, that, in the absence of a license granted under, or ownership of, such Patent Right, the making, using, selling, offering for sale or importation of such subject
4
matter would infringe such Patent Right or, as to a pending claim included in such Patent Right, the making, using, selling, offering for sale or importation of such subject matter would infringe such pending claim if such claim were to issue in an issued patent without modification.
1.1.30.“Development” means the performance of any and all activities relating to preparation, submission, obtaining, supporting or maintaining Regulatory Approval of a Product in the Field in the Territory and to maintaining, supporting or expanding such Regulatory Approval, including pre-clinical studies, pharmacokinetic studies, toxicology studies, formulation, test method development, assay development and stability testing, manufacturing process development, manufacturing technical support, biomarker development, validation and scale-up (including bulk compound production), Manufacturing of Clinical Supplies and activities relating to developing the ability to Manufacture and to continue to Manufacture the Product, quality assurance and quality control for formulations of a Product, design and conduct of Clinical Trials or studies (including all Post-Marketing Commitments), report writing, statistical analysis and regulatory affairs including regulatory legal services. When used as a verb, “Develop” means to engage in Development.
1.1.31.“Development Costs” means all costs incurred (i.e., paid or accrued) by either Party or each such Party’s Affiliates or licensees or Sublicensees (as applicable), in each case, in accordance with GAAP applied on a consistent basis, to the extent attributable to Development of the Alkermes 8700 Product in the Initial Indication for the purpose of obtaining, supporting or maintaining Regulatory Approval of the Alkermes 8700 Product in the U.S. (including the performance of all Post-Marketing Commitments) or fulfilling such Party’s responsibilities under the Initial Development Plan in accordance therewith and with this Agreement. Such costs shall include:
(i)costs of research or Development, including costs of studies on the toxicological, pharmacokinetic, metabolic or clinical aspects of the Alkermes 8700 Product in the Initial Indication conducted internally or by individual investigators or consultants, necessary or desirable for the purpose of obtaining, supporting or maintaining Regulatory Approval of the Alkermes 8700 Product in the Initial Indication in the U.S. and for conducting Post-Marketing Commitments to support or maintain such Regulatory Approval, including the costs of personnel engaged in the foregoing activities at the applicable Development FTE Rate;
(ii)Fully Burdened Manufacturing Costs of Clinical Supplies;
(iii)costs of manufacturing process development, validations, scale-up, quality assurance and quality control for the Alkermes 8700 Product pursued by the Parties under the Initial Development Plan, to the extent not included in the Fully Burdened Manufacturing Costs of Clinical Supplies;
(iv)costs of preparing and reviewing data or information for the purpose of submitting the Alkermes 8700 Product 505(b)(2) NDA to the FDA;
(v)costs of communications and meetings with the FDA, and exchange of information and assistance related thereto, in each case, until the earlier of (a)
5
Regulatory Approval of the Alkermes 8700 Product 505(b)(2) NDA and completion of all associated Post-Marketing Commitments or (b) the date of an Alkermes Approval Failure; and
(vi)costs incurred in connection with receiving, investigating, recording, reviewing, communicating and exchanging adverse events and other reportable information, in each case, as provided in any safety data exchange agreement entered into between the Parties to the extent relating to the Development of the Alkermes 8700 Product in the Initial Indication in the U.S.
For clarity, any Development Cost incurred by a Party or its Affiliates or licensees or Sublicensees (as applicable) under this Agreement or any Supply Agreement shall be charged only once.
1.1.32.“Development FTE Rate” means initially an amount equal to [**] U.S. Dollars (US$[**]) per FTE per year; on January 1, 2019, and annually thereafter, such amount shall be adjusted to reflect any increase, since the prior adjustment (or the initial rate, as applicable), based on the most recent monthly index available as of the adjustment date set forth in the Bureau of Labor Statistics Consumer Price Index for Urban Wage Earners and Clerical Workers (CPI-W), Size Class A, all items less energy, which, for clarity, was 266.558 in June 2017.
1.1.33.“Distributor” means, with respect to a country, any Third Party that purchases its requirements for Products in such country from Biogen or its Affiliates or Sublicensees and is appointed as a distributor to distribute, market and resell such Product in such country, even if such Third Party is granted ancillary rights to develop, package or obtain Regulatory Approval of such Product in order to distribute, market or sell such Product in such country.
1.1.34.“DMF” means dimethyl fumarate.
1.1.35.“EMA” means the European Medicines Agency or any successor agency.
1.1.36.“Exploit” means to perform Medical Activities, Develop, Manufacture and Commercialize, including to make, have made, use, sell, offer for sale, import and export.
1.1.37.“FDA” means the U.S. Food and Drug Administration or any successor agency.
1.1.38.“Field” means the treatment, prevention or diagnosis of any human disease, disorder or condition.
1.1.39.“First Commercial Sale” means, with respect to a Product in a country in the Territory, the first sale for use or consumption by the general public of such Product by Biogen or an Affiliate or Sublicensee to a Third Party in such country after such Product has been granted Regulatory Approval by the appropriate Regulatory Authority(ies) in such country. Any transfer of a Product as part of an expanded access program, compassionate
6
sales or use program, an indigent program, as bona fide samples, as donations, for the performance of Clinical Trials or for similar bona fide business purposes in accordance with Applicable Law shall not constitute a “First Commercial Sale” hereunder.
1.1.40.“First FDA Approval” means the FDA’s approval of an NDA for the Alkermes 8700 Product in the Initial Indication in the U.S.
1.1.41.“FTE” means the equivalent of one (1) person who is employed by a Party or its Affiliates, or (solely with respect to technical personnel) hired as an independent contractor by a Party or its Affiliates in lieu of such Party’s own employees, who is qualified to perform the tasks assigned to such person. For FTEs performing Development activities pursuant to the Initial Development Plan, one (1) FTE shall perform a total of [**] hours of work per Calendar Year. Any FTE who devotes less or more than and [**] hours of work per Calendar Year to such work shall be treated as an FTE on a pro-rata basis calculated by dividing the actual number of hours spent on such work during such Calendar Year by and [**]. Such FTEs shall be charged at an hourly rate hereunder by the Parties.
1.1.42.“Fully Burdened Manufacturing Cost” means the costs incurred (i.e., paid or accrued) by a Party, its Affiliates or agents in the Manufacture (including shipping or storage costs) of a Product or Placebo, as applicable, which shall be the sum of direct labor, direct material and Allocable Overhead incurred in the Manufacture of such Product or Placebo as determined in accordance with GAAP as consistently applied by such Party or its Affiliates. Notwithstanding the foregoing, Fully Burdened Manufacturing Costs exclude (i) all payments (including upfront fees, milestones and royalties) to any Third Party to obtain rights (whether by acquisition, license or otherwise) to any intellectual property that is necessary or useful to Manufacture Clinical Supplies or Commercial Supplies in any country and (ii) any and all costs and expenses incurred in connection with the acquisition of any such rights.
1.1.43.“Fumaderm®” means Biogen’s oral fumaric acid ester pharmaceutical product.
1.1.44.“GAAP” means U.S. Generally Accepted Accounting Principles.
1.1.45.“GI” means gastrointestinal.
1.1.46.“GI Event” means any one or more of nausea, vomiting, diarrhea, abdominal pain, upper abdominal pain or lower abdominal pain, in any case, experienced by a patient in a Clinical Trial.
1.1.47.“GI Inferiority” will be deemed to exist if (i) the [**] of patients not previously enrolled in the GI Tolerability Clinical Trial or otherwise exposed to a fumarate that discontinue participation in the Long-Term Safety Clinical Trial due to a GI Event is greater than [**] or (ii) the percentage of patients who discontinue participation in the ALKS 8700 arm of Part B of the GI Tolerability Clinical Trial due to a GI Event is greater than the percentage of patients who discontinue participation in the Tecfidera® arm of Part B of the GI Tolerability Clinical Trial due to a GI Event, in all cases as set forth in the Complete GI Tolerability Data Package, provided that, if the rate of discontinuations due to a GI Event in Part A of the GI Tolerability Clinical Trial is lower in the Tecfidera® arm of such trial and the Parties jointly
7
agree in writing not to conduct Part B of the GI Tolerability Clinical Trial, then GI Inferiority will be deemed to exist. If the Parties jointly agree in writing not to conduct Part B of the GI Tolerability Clinical Trial for reasons other than because the rate of discontinuations due to a GI Event in Part A of the GI Tolerability Clinical Trial is lower in the Tecfidera® arm of such trial, then, unless the Parties jointly agree otherwise in writing, GI Inferiority will not be deemed to exist.
1.1.48.“GI Tolerability Clinical Trial” means the Clinical Trial sponsored by Alkermes, Inc., an Affiliate of Alkermes, with ClinicalTrials.gov identifier NCT03093324.
1.1.49.“Governmental Approvals” means all applications, notices, petitions, filings, ruling requests, and other documents and obtaining all consents, waivers, licenses, orders, registrations, approvals, permits, rulings, authorizations and clearances necessary or advisable to be obtained from any Governmental Authority in order to consummate the transactions contemplated by this Agreement.
1.1.50.“Governmental Authority” means any court, tribunal, agency, department, legislative body, commission or other instrumentality of any supra-national, national, state, county, city or other political subdivision in the world.
1.1.51.“HSR Act” means the Hart-Scott-Rodino Antitrust Improvements Act of 1976 and the rules and regulations thereunder, each as amended.
1.1.52.“IND” means an Investigational New Drug Application, as defined in the Federal Food, Drug, and Cosmetic Act, as amended or similar application or submission that is required to be filed with any Regulatory Authority before beginning Clinical Trials of a pharmaceutical product.
1.1.53.“Initial GI Tolerability Data Package” means (a) with respect to Part A of the GI Tolerability Clinical Trial, full demographic and patient-level data for all patients enrolled therein and those elements set forth on Schedule 1.1.26 and (b) with respect to the Long-Term Safety Clinical Trial, full demographic and patient-level data for all patients enrolled in such trial (including a breakdown of those patients that were previously enrolled in the GI Tolerability Clinical Trial and those patients not previously enrolled in such trial) and a complete set of all data and results available for such trial, including all data and results that would fall into the categories set forth on Schedule 1.1.26, provided that the information set forth in clause (b) will only be provided as of the date that the information set forth in the foregoing clause (a) of this definition is available.
1.1.54.“Initial Indication” means the treatment of MS.
1.1.55.“Know-How” means all non-public, proprietary data and results, technical information, know-how, inventions, discoveries, trade secrets, processes, procedures, techniques, new developments, compositions, products, compounds, material, methods, formulas, formulation, improvements, protocol, result of experimentation or testing, technology, ideas or other proprietary information and documentation thereof (including related papers, invention disclosures, blueprints, drawings, flowcharts, diagrams, diaries, notebooks, specifications,
8
methods of Manufacture, methods of service, data processing techniques, compilations of information, customer and supplier lists, pricing and cost information, and business and marketing plans and proposals), design or other know-how, whether or not patentable or copyrightable. Know-How shall not include any Patent Rights with respect thereto.
1.1.56.“Knowledge” means, with respect to Alkermes, the actual knowledge of [**] based on such individuals’ good faith understanding of the facts and information in their possession or control following reasonable inquiry and investigation of Alkermes personnel and [**], in each case, with relevant functional responsibilities with respect to such facts and information.
1.1.57.“Licensed Know-How” means (i) any Know-How Controlled by Alkermes or its Affiliates on the Effective Date or during the Term that (a) is or was used in the Development or Manufacture of Products, (b) is or was embodied in Products or (c) is necessary to Exploit the Products in the Field in the Territory, (ii) all Alkermes Collaboration Know-How and (iii) Alkermes’ interest in the Joint Collaboration Know-How.
1.1.58.“Licensed Patents” means (i) the Patent Rights set forth on Exhibit B, (ii) any Patent Rights Controlled by Alkermes or its Affiliates issuing from or claiming priority directly or indirectly to Patent Rights listed on Exhibit B, (iii) the Alkermes Collaboration Patents, (iv) Alkermes’ interest in the Joint Collaboration Patents and (v) any Patent Rights Controlled by Alkermes or its Affiliates on the Effective Date or during the Term that Cover Licensed Know-How or that are necessary to Exploit any compound or product Covered by any Patent Rights set forth in the foregoing clauses (i) through (iv), in each case, in the Field in the Territory. The Licensed Patents as of the Effective Date are listed on Exhibit B, provided that any Patent Right that is not listed on Exhibit B, but is otherwise described in this Section 1.1.58, shall still be considered a Licensed Patent hereunder.
1.1.59.“Long-Term Safety Clinical Trial” means the Clinical Trial sponsored by Alkermes, Inc., an Affiliate of Alkermes, with ClinicalTrials.gov identifier NCT02634307.
1.1.60.“Manufacturing” means the performance of any or all activities directed to producing, manufacturing, validating, scaling up, processing, filling, finishing, quality control, quality assurance, testing and release, shipping and warehousing of a Product or Placebo. When used as a verb, “Manufacture” means to engage in Manufacturing.
1.1.61.“Medical Activities” means any and all activities directed to the formulation and performance of (i) Post-Marketing Clinical Trials; (ii) market and key opinion leader plans for the development of the Products, including plans to support continuing medical education; (iii) publication plans for the Products; (iv) plans to ensure appropriate medical information responses with respect to the Products; (v) safety monitoring plans for the Products; (vi) plans and expected activities for field based medical affairs personnel for the Products; and (vii) other comparable medical affairs activities.
1.1.62.“MMF” means monomethyl fumarate.
1.1.63.“MS” means multiple sclerosis.
9
1.1.64.“NDA” means a New Drug Application filed with the FDA as described in 21 C.F.R. § 314 or any equivalent or corresponding application for Regulatory Approval (including pricing and reimbursement approval required by Applicable Law prior to sale of a pharmaceutical product) in any country or regulatory jurisdiction other than the U.S.
1.1.65. “Net Sales” means, with respect to any Product, the amount billed in arm’s-length transactions by a Party, an Affiliate of such Party, or any permitted Sublicensee [**].
1.1.66.“OIG Guidance” means the Office of Inspector General of the Department of Health and Human Services Compliance Program Guidance for Pharmaceutical Manufacturers.
1.1.67.“Party” means Biogen or Alkermes and, when used in the plural, will mean both Biogen and Alkermes.
1.1.68.“Patent Rights” means any and all of the following: (i) patent applications (including provisional patent applications) and patents (including the inventor’s certificates); (ii) any substitution, extension (including patent term extensions, patent term adjustments, supplementary protection certificates and pediatric exclusivity periods), registration, confirmation, reissue, continuation, divisional, continuation-in-part, reexamination, renewal, patent of addition or the like thereof or thereto; and (iii) all foreign counterparts of any of the foregoing, including PCT applications.
1.1.69.“Person” means any individual, firm, corporation, partnership, trust, business trust, joint venture, limited liability company, Governmental Authority, association or other entity.
1.1.70.“Placebo” means an inactive substitute for a Product.
1.1.71.“Post-Marketing Clinical Trial” means a clinical trial of a Product in human patients (including investigator-initiated trials) that is conducted for a purpose other than to obtain, support or maintain Regulatory Approval.
1.1.72.“Post-Marketing Commitments” means any and all items, tasks, activities, studies, trials or other commitments the completion of which is recommended or required by the FDA in connection with the initial grant of Regulatory Approval for the Alkermes 8700 Product in the Initial Indication in the U.S. or to support or maintain such Regulatory Approval.
1.1.73.“Product” means any product that is Covered by a Valid Claim of a Licensed Patent; provided, however, that, if any Product ceases to meet the preceding definition in a country due to the expiration of the last Valid Claim of a Licensed Patent Covering such product in such country, then such product shall continue to constitute a “Product” under this Agreement.
1.1.74.“Regulatory Approval” means all approvals necessary for the Commercialization of a pharmaceutical product for one or more indications in a country or
10
regulatory jurisdiction, which may include satisfaction of applicable regulatory and notification requirements and, where required by Applicable Law, separate pricing and reimbursement approvals prior to sale of a pharmaceutical product.
1.1.75.“Regulatory Authority” means any applicable supranational, national, regional, state or local regulatory agency, department, bureau, commission, council, or other government entity involved in regulating Development and granting Regulatory Approval for a pharmaceutical product in a regulatory jurisdiction within the Territory, including the FDA and the EMA.
1.1.76.“Serious Failure to Supply” shall mean that in a given Calendar Year, for reasons other than a Force Majeure Delay or a default of Biogen, Alkermes fails on at least [**] occasions to supply Biogen with those quantities of Product forecasted and ordered in accordance with the terms of the applicable Supply Agreement, and the cumulative shortfall for such Calendar Year attributable to such failure(s) is at least [**] of the aggregate amount so forecasted and ordered from Alkermes for delivery in such Calendar Year.
1.1.77.“Sublicense Agreement” means a written, definitive agreement for a sublicense between Biogen and a Sublicensee.
1.1.78.“Sublicensee” means any Third Party, other than a Distributor, to whom rights are granted under any of the rights licensed to Biogen by Alkermes under Section 6.1 with respect to any Product, including through any license, sublicense, co-development, co-discovery, co-promotion, distribution, joint venture, Development and Commercialization collaboration or similar transaction between Biogen (or an Affiliate of Biogen) and such Third Party.
1.1.79.“Supply Agreement” means the Clinical Supply Agreement or any supply agreement entered into between the Parties for the toll manufacture by Alkermes of Commercial Supplies.
1.1.80.“Tecfidera®” means Biogen’s oral DMF pharmaceutical product.
1.1.81.“Tecfidera® Competing Product” means any pharmaceutical product of any Third Party which contains the same active pharmaceutical ingredient as Tecfidera®, and is approved in reliance, in whole or in part, on a prior Regulatory Approval of Tecfidera®, provided that Tecfidera® Competing Product shall not include such pharmaceutical product of a Third Party where Biogen licenses or sublicenses rights to such Third Party to commercialize such pharmaceutical product (whether directly or by license, covenant not to sue, settlement agreement, release or any other arrangement or means).
1.1.82.“Tecfidera® Competition” means, with respect to a given Calendar Year in the U.S., that during such Calendar Year, the aggregate net revenue earned by Biogen and its Affiliates and their respective sublicensees and licensees from sales of Tecfidera® and the Alkermes 8700 Product, in each case in the U.S. in such Calendar Year, is less than [**] of the aggregate net revenue earned by Biogen and its Affiliates and their respective sublicensees and licensees from sales of Tecfidera® and the Alkermes 8700 Product, in each case, in the U.S. in
11
the last full Calendar Year prior to the Calendar Year in which a Tecfidera® Competing Product is first sold in the U.S.
1.1.83.“Territory” means all countries of the world.
1.1.84.“Third Party” means any Person other than the Parties or their respective Affiliates.
1.1.85.“U.S.” means the United States of America.
1.1.86.“U.S. Dollars” or “US$” means United States Dollars.
1.1.87.“Valid Claim” means a claim of an issued and unexpired Patent Right or pending claim of a patent application, which claim or pending claim has not been revoked or held unenforceable, unallowable, unpatentable or invalid by a decision of a court or other Governmental Authority of competent jurisdiction, which claim or pending claim is not appealable or has not been appealed within the time allowed for appeal, and which claim or pending claim has not been cancelled, withdrawn from consideration, abandoned, disclaimed, denied or admitted to be invalid or unenforceable through reissue, re-examination, inter partes review, post-grant review or disclaimer, opposition procedure, nullity suit, or otherwise; provided, however, that if the holding of such court or Governmental Authority is later reversed by a court or Governmental Authority with overriding authority, the claim shall be reinstated as a Valid Claim with respect to Net Sales made after the date of such reversal; and provided, further, that a claim of a patent application pending for more than [**] years ([**]) years from the earliest date from which such patent application claims priority shall not be considered to be a Valid Claim for purposes of this Agreement unless and until a patent with respect to such application issues with such claim, in which case such claim will be reinstated and be deemed to be a Valid Claim, but only as of the date of issuance of such patent.
1.2.Additional Definitions. The following terms have the meanings set forth in the corresponding Sections of this Agreement:
|
Terms |
Section |
|
Agreement |
Preamble |
|
Alkermes |
Preamble |
|
Alkermes Approval Failure |
3.3.1 |
|
Alkermes Indemnified Party |
11.2 |
|
Alkermes Manufacturing Know-How |
3.2.3(iv) |
|
ANDA |
7.5.2(ii) |
|
Auditing Party |
9.7.2 |
|
Biogen |
Preamble |
|
Biogen Indemnified Party |
11.3 |
|
Claim |
11.2 |
|
Complaining Party |
12.1.2 |
|
Confidential Information |
8.1 |
|
CPR Rules |
12.1.3 |
|
Dispute |
12.1.1 |
12
|
Terms |
Section |
|
Dispute Notice |
12.1.2 |
|
DT |
3.1.1 |
|
Effective Date |
Preamble |
|
Force Majeure Delay |
14.8 |
|
GI Tolerability Data Package Notice |
3.2.5(i) |
|
Hatch-Waxman Act |
7.5.1 |
|
Indemnitee |
11.4 |
|
Infringement |
7.5.1 |
|
Infringement Claim |
7.6.1 |
|
Initial Development Plan |
3.1.2 |
|
IP |
13.6.1 |
|
Joint Collaboration Know-How |
7.1.2(i) |
|
Joint Collaboration Patents |
7.1.2(i) |
|
Joint Collaboration Technology |
7.1.2(i) |
|
JSC |
2.1.1 |
|
Losses |
11.2 |
|
Minimum Annual Payment(s) |
9.5.1(ii) |
|
Minimum Annual Payment Term |
9.5.1(ii) |
|
Minimum Payment Commencement Date |
9.5.1(ii) |
|
Option Payment |
9.2 |
|
Other Component |
1.1.22 |
|
Patent Action |
6.3 |
|
Patent Committee |
7.3 |
|
Product Trademarks |
7.6.1 |
|
Prosecution |
7.4.1(i) |
|
Protection |
7.4.1(ii) |
|
Quality Agreement |
3.3.1 |
|
Recording Party |
9.7.1 |
|
Related Party |
1.1.65 |
|
Response |
12.1.2 |
|
Royalty Term |
9.5.3 |
|
Senior Management |
12.1.2 |
|
ST |
5.2 |
|
Sued Party |
7.6.1 |
|
Surviving Sublicensee |
13.5.4 |
|
Team Leader |
3.1.1 |
|
Term |
13.1 |
|
Transfer Completion Date |
3.3.1 |
|
Transition Plan |
3.2.3(iii) |
|
Up-Front Payment |
9.1 |
|
VAT |
9.11.4 |
1.3.Interpretation. Except where the context expressly requires otherwise in this Agreement, (a) the use of any gender herein shall be deemed to encompass references to either or
13
both genders, and the use of the singular shall be deemed to include the plural (and vice versa); (b) the words “include,” “includes,” and “including” shall be deemed to be followed by the phrase “without limitation” and shall not be interpreted to limit the provision to which it relates; (c) the word “will” shall be construed to have the same meaning and effect as the word “shall”; (d) any definition of or reference to any agreement, instrument or other document herein shall be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein); (e) any reference herein to any Person shall be construed to include the Person’s successors and assigns to the extent not prohibited by this Agreement; (f) the words “herein,” “hereof,” and “hereunder,” and words of similar import, shall be construed to refer to this Agreement in its entirety, as the context requires, and not to any particular provision hereof; (g) all references herein to Sections, Articles, Exhibits, or Schedules shall be construed to refer to Sections, Articles, Exhibits, or Schedules of this Agreement, and references to this Agreement include all Schedules and Exhibits hereto; (h) the word “notice” means notice in writing (whether or not specifically stated) and shall include notices, consents, approvals and other written communications contemplated under this Agreement; (i) provisions that require that a Party, the Parties or any committee hereunder “agree,” “consent,” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, approved minutes or otherwise (but excluding e-mail and instant messaging); (j) references to any specific law, rule or regulation, or article, Section or other division thereof, shall be deemed to include the then-current amendments thereto or any replacement or successor law, rule or regulation thereof; (k) the term “or” shall be interpreted in the inclusive sense commonly associated with the term “and/or”; (l) references to a particular statute or regulation include all rules and regulations thereunder and any predecessor or successor statute, rules or regulations, in each case, as amended or otherwise modified from time to time; and (m) whenever this Agreement refers to a number of days, such number shall refer to calendar days unless Business Days are specified.
Article 2
MANAGEMENT OF THE COLLABORATION
2.1.1.Establishment of the JSC. Within thirty (30) days after the Effective Date, the Parties shall establish the joint steering committee (the “JSC”), which will have overall responsibility for the collaboration between the Parties with respect to the Development of the Alkermes 8700 Product in the Initial Indication in the U.S. as contemplated by this Agreement. The JSC will comprise two (2) representatives of each Party, who will be appointed (and may be replaced at any time) by each Party upon notice to the other Party in accordance with this Agreement. Such representatives will include individuals of each Party with decision-making authority with respect to the matters within the authority of the JSC. To conduct the activities described in Section 2.1.2 below, the JSC will meet at least once each Calendar Quarter until disbandment of the JSC pursuant to Section 2.2, or more frequently if agreed by the JSC.
14
2.1.2.JSC Responsibilities. The JSC will perform the following functions:
(i)for the Alkermes 8700 Product in the Initial Indication in the U.S., review and, in its discretion, approve amendments to the Initial Development Plan, including the applicable annual budget or the regulatory strategy, in each case, as set forth therein;
(ii)review reports received from the ST (the supply team established pursuant to Section 5.2) and provide direction to the ST regarding the performance of its responsibilities under the Clinical Supply Agreement;
(iii)serve as the first forum for the settlement of disputes or disagreements between the Parties arising in the DT (the development team established pursuant to Section 3.1.1) or the ST; and
(iv)perform such other functions as appropriate to further the purposes of this Agreement as determined by mutual agreement of the Parties.
2.1.3.JSC Chairperson; Procedures. For a one (1)-year period commencing on the Effective Date, a Biogen representative to the JSC will serve as the chairperson of the JSC. For each subsequent one (1)-year period, JSC representatives of the Parties will alternate as the chairperson of the JSC. The chairperson will establish the timing and agenda for all JSC meetings and will send notice of such meetings, including the agenda therefor, to all JSC members; provided, however, that either Party may request that specific items be included in the agenda and may request that additional meetings be scheduled as needed. The location of each regularly scheduled JSC meeting will be agreed upon by the Parties. Meetings may also be held telephonically or by videoconference. A quorum of at least one (1) JSC representative appointed by each Party shall be present at or shall otherwise participate in each JSC meeting. If mutually agreed by the Parties on a case-by-case basis, the JSC may invite other non-members to participate in the discussions and meetings of the JSC, provided that the presence of such participants shall not be considered in determining whether there is a quorum at the JSC. The chairperson shall appoint one (1) person (who need not be a member of the JSC) to attend each meeting and record the minutes of such meeting in writing. Such minutes shall be circulated to the Parties promptly following each meeting for review, comment and approval. If no comments are received from a Party within thirty (30) days after receipt of the minutes by such Party, then such minutes shall be deemed to be approved by such Party.
2.1.4.JSC Decision Making. As a general principle, the JSC will operate by consensus, with the JSC representatives of each Party collectively having one (1) vote, respectively. In the event that the JSC members do not reach consensus with respect to a matter that is within the JSC’s decision-making authority within twenty (20) days after they have met and attempted to reach such consensus, such matter may be escalated to resolution by Senior Management by the written request of either Party. If Senior Management is unable to resolve such matter within ten (10) days of such written request, then:
15
(i)Alkermes shall have the final decision-making authority if such matter relates to (a) the contents of the Alkermes 8700 Product 505(b)(2) NDA or the conduct of any Clinical Trial of the Alkermes 8700 Product in the Initial Indication conducted by or on behalf of Alkermes pursuant to the Initial Development Plan, including the decision to remove Development activities (other than the GI Tolerability Clinical Trial) not required by the FDA for approval of the Alkermes 8700 Product 505(b)(2) NDA; (b) subject to Section 3.2.4(iv), the design and conduct of Part B of the GI Tolerability Clinical Trial (but, not whether to discontinue such trial); (c) the Manufacture of Clinical Supplies of the Alkermes 8700 Product, subject to the terms of the Clinical Supply Agreement; and (d) prior to the Transfer Completion Date, Regulatory Authority interactions, regulatory strategy (provided that Alkermes may not deviate from pursuit of obtaining Regulatory Approval of the Alkermes 8700 Product in the Initial Indication via the 505(b)(2) regulatory pathway unless the right of reference is granted to Alkermes in accordance with Section 3.3.2), communications, and activities related to the Alkermes 8700 Product in the Initial Indication and regulatory filings for, and Regulatory Approval of, the Alkermes 8700 Product in the Initial Indication in the U.S.;
(ii)with respect to any amendments to the Initial Development Plan to add one (1) or more Development activities, Alkermes shall have final decision-making authority with respect to the design and execution of such Development activities and Biogen shall have final decision-making authority with respect to any increase in the Initial Development Plan budget associated with such Development activities; and
(iii)Biogen shall have the final decision-making authority with respect to any other matter not set forth in Section 2.1.4(i) and Section 2.1.4(ii).
Notwithstanding anything to the contrary, to the extent any matters are required by Applicable Law or due to safety concerns with respect to a Product to be resolved within a shorter period of time than the periods set forth in this Section 2.1.4, the periods set forth in this Section 2.1.4 will be shortened as appropriate to permit the resolution of such matters within the required period.
2.2.Alkermes’ Participation in the JSC. The Parties agree that participation in the JSC is a right rather than an obligation of Alkermes, and Alkermes may elect at any time or from time to time not to participate in the JSC or any other committee, subcommittee, team or subteam contemplated hereunder. Accordingly, the Parties also agree that Alkermes’ decision not to participate in the JSC or any other committee, subcommittee, team or subteam contemplated hereunder will not constitute a breach of Alkermes’ material obligations hereunder. During any period that Alkermes has elected not to participate in the JSC or any other committee, subcommittee, team or subteam contemplated hereunder, each Party shall have the obligation to provide and the right to continue to receive the information it would otherwise be required to provide and entitled to receive under this Agreement and to participate directly with the other Party in discussions, reviews and approvals currently allocated to the JSC or such other committee, subcommittee, team or subteam pursuant to this Agreement.
2.3.Disbandment of the JSC. The JSC will automatically disband on the earlier of (a) the mutual written agreement of the Parties and (b) the Transfer Completion Date. Thereafter,
16
Biogen shall have the sole decision-making authority over all matters that were within the authority of the JSC prior to such disbandment.
3.1.1.Establishment of Development Team. Within thirty (30) days after the Effective Date, the Parties shall establish the development team (the “DT”) to coordinate and implement all activities for the Development of the Alkermes 8700 Product in the Initial Indication in the United States, within the annual budgets included in the Initial Development Plan. One (1) representative from each Party shall be designated as that Party’s “Team Leader” to act as the primary DT contact for that Party. Unless otherwise agreed by the Parties, the DT shall comprise an equal number of representatives of each Party as is reasonably necessary to accomplish the goals of the DT hereunder. Such representatives will include individuals with expertise and responsibilities in the areas of clinical development, process sciences, quality control, quality assurance, regulatory affairs and product development. Either Party may replace any or all of its DT representatives, including its Team Leader, at any time upon notice to the other Party in accordance with this Agreement.
3.1.2.Development Team Responsibilities. The DT will perform the following functions:
(i)for the Alkermes 8700 Product in the Initial Indication in the U.S., formulating amendments to the Initial Development Plan, including, in each case, the applicable annual budget or the regulatory strategy, in each case, set forth therein;
(ii)coordinating implementation of all Development activities for the Alkermes 8700 Product in the Initial Indication in the U.S. pursuant to the Initial Development Plan;
(iii)generating forecasts of supply requirements for the Alkermes 8700 Product and Placebo pursuant to the Initial Development Plan and delivering such forecasts to the ST;
(iv)exchanging information and facilitating cooperation and coordination between the Parties as they exercise their respective rights and meet their respective obligations under the Initial Development Plan;
(v)providing status updates to the JSC regarding Development activities for the Alkermes 8700 Product in the Initial Indication in the U.S. pursuant to the Initial Development Plan, including progress towards achieving key milestone events and Development Cost expenditures; and
(vi)performing such other functions as appropriate to further the purposes of this Agreement as determined by mutual agreement of the Parties.
17
In addition, the DT may designate subteams as appropriate to facilitate coordination and cooperation in key areas. The development plan for the Alkermes 8700 Product in the Initial Indication in the U.S. and the accompanying budget are attached hereto as Exhibit C (as amended from time to time in accordance with this Agreement, the “Initial Development Plan”). This Initial Development Plan shall be deemed to have been reviewed and approved by the JSC. The Initial Development Plan attached hereto as Exhibit C covers Development activities for the Alkermes 8700 Product in the Initial Indication in the U.S. through the filing of the Alkermes 8700 Product 505(b)(2) NDA (including the regulatory strategy for obtaining, supporting or maintaining Regulatory Approval of the Alkermes 8700 Product in the Initial Indication in the U.S.). The Initial Development Plan may be amended upon the written agreement of each Party to include additional Development activities for the Alkermes 8700 Product in the Initial Indication in the U.S. but, notwithstanding anything to the contrary set forth under this Agreement, will not include any Post-Marketing Commitments or Post-Marketing Clinical Trials for the Alkermes 8700 Product. The DT may formulate amendments to the Initial Development Plan at any time and submit such amendments to the JSC for review and approval in accordance with Section 2.1.2(i).
3.1.3.Development Team Procedures. For a one (1)-year period beginning on the Effective Date, the Team Leader of Alkermes shall serve as the chairperson of the DT. Thereafter, the Team Leader of Biogen shall serve as the chairperson of the DT. The chairperson shall establish the timing and agenda for all DT meetings and shall send notice of such meetings, including the agenda therefor, to all DT members; provided, however, that either Party may request that specific items be included in the agenda and may request that additional meetings be scheduled as needed. The DT will meet at least once each month or as agreed by the DT, until the disbandment of the DT pursuant to Section 3.1.5. The first DT meeting shall be held at Alkermes’ offices. Thereafter, the location of regularly scheduled DT meetings shall alternate between the offices of the Parties, unless otherwise agreed. Meetings may be held telephonically or by videoconference. A quorum of at least two (2) DT members appointed by each Party shall be present at or shall otherwise participate in each DT meeting. If mutually agreed by the Parties on a case-by-case basis, the DT may invite other non-members to participate in the discussions and meetings of the DT, provided that the presence of such participants shall not be considered in determining whether there is a quorum at the DT. The chairperson shall appoint one (1) person (who need not be a member of the DT) to attend each meeting and record the minutes of such meeting in writing. Such minutes shall be circulated to the Parties promptly following the meeting for review, comment and approval. If no comments are received from a Party within ten (10) Business Days after receipt of the minutes by such Party, such minutes shall be deemed to be approved by such Party.
3.1.4.Development Team Decision Making. As a general principle, the DT will operate by consensus, with the DT representatives of each Party collectively having one (1) vote, respectively. In the event that the DT members do not reach consensus with respect to a matter that is within the purview of the DT within twenty (20) days after they have met and attempted to reach such consensus, such matter shall be presented to the JSC for resolution.
3.1.5.Disbandment of the DT. The DT will automatically disband on the earlier of (i) the mutual written agreement of the Parties and (ii) the Transfer Completion
18
Date. Thereafter, Biogen shall have the sole decision-making authority over all matters that were within the authority of the DT prior to such disbandment.
3.2.Development Responsibilities and Rights.
3.2.1.Development Responsibilities of Alkermes. During the Term, Alkermes shall use commercially reasonable efforts to perform all activities allocated to Alkermes in the Initial Development Plan. During the Term, until the earlier of the Transfer Completion Date or an Alkermes Approval Failure, Alkermes shall be responsible for any and all interactions with the FDA in relation to the Initial Development Plan, activities conducted thereunder and the Alkermes 8700 Product 505(b)(2) NDA (other than in connection with any Post-Marketing Commitments, for which Biogen shall be responsible), including any required modifications to such Alkermes 8700 Product 505(b)(2) NDA. In addition, and without limiting the generality of the foregoing, during the Term, until the earlier of the Transfer Completion Date or an Alkermes Approval Failure, Alkermes shall use reasonable efforts to pursue and obtain Regulatory Approval of the Alkermes 8700 Product in the Initial Indication in the U.S. If Alkermes desires to perform any tasks, obligations or support that Alkermes is required to perform or provide hereunder through any of its Affiliates, contractors or agents, then Alkermes may engage such Affiliates, contractors or agents to perform such tasks, obligations or support, but Alkermes shall remain responsible for performance of its obligations hereunder.
3.2.2.Development Rights of Biogen.
(i)Following the Transfer Completion Date, Biogen shall have the unilateral right, itself or through its Affiliates, Sublicensees, subcontractors or Distributors, to conduct Development of the Alkermes 8700 Product in the Initial Indication in the U.S., and to perform Medical Activities, in each case, in its sole discretion (unless otherwise agreed by the Parties).
(ii)During the Term, Biogen shall have the unilateral right, itself or through its Affiliates, Sublicensees, subcontractors or Distributors, to conduct Development of, and perform Medical Activities with respect to, Products throughout the Territory, other than the Alkermes 8700 Product in the Initial Indication in the U.S. (which is covered in Section 3.2.2(i)), in each case, in its sole discretion.
(iii)Subject to Section 3.3.4, following receipt of Regulatory Approval for the Alkermes 8700 Product 505(b)(2) NDA, Biogen shall use Commercially Reasonable Efforts to conduct any Post-Marketing Commitments for the Alkermes 8700 Product in the Initial Indication in the U.S. required in connection with such Regulatory Approval.
3.2.3.Transfer of Data and Technology.
(i)As of the Effective Date. Promptly following the Effective Date, but in any event no later than sixty (60) days thereafter, Alkermes shall transfer to Biogen, at Biogen’s sole cost and expense [**] and thereafter at Alkermes’ cost, a true and complete copy of (a) all data and results generated from any Development activities conducted by or on behalf of Alkermes with respect to any Product prior to the Effective Date (as evidenced by all completed pre-clinical study reports and completed clinical study reports for Clinical Trials of
19
the Alkermes 8700 Product in the Initial Indication) and (b) all Trial Master Files (including any Trial Master File plans, tables of contents or indices and any evidence or certification of related quality checks) or equivalents thereof, for all completed or ongoing Clinical Trials of any Product conducted by or on behalf of Alkermes. If Biogen requests other tangible embodiments of the Licensed Know-How or additional data or results or any other documentation relating to such data or results in respect of Development activities conducted by or on behalf of Alkermes prior to the Effective Date, where the applicable pre-clinical study report or clinical study report for such activities has not yet been completed, then Alkermes shall attempt in good faith to provide such requested embodiments, data or results or other documentation related thereto to Biogen.
(ii)During the Term. Thereafter, on a quarterly basis until Alkermes’ completion of all of its obligations under the Initial Development Plan in accordance with Section 3.2.1, or more frequently as (a) new data and results with respect to the Products or (b) new or updated Trial Master Files, in each case ((a) and (b)), come into Alkermes’ possession or Control, and in any event sufficiently prior to the date of the First FDA Approval such that the transfer of the Alkermes 8700 Product 505(b)(2) NDA pursuant to Section 3.3.1 can occur immediately following the First FDA Approval, Alkermes shall transfer to Biogen, at Biogen’s sole cost and expense, a true and complete copy of any such new data and results (including all elements set forth on Schedule 1.1.26) generated from any Development activities conducted by or on behalf of Alkermes with respect to any Product for all ongoing Clinical Trials conducted by or on behalf of Alkermes (including the Long-Term Safety Clinical Trial and the GI Tolerability Clinical Trial), as evidenced by all completed pre-clinical study reports and completed clinical study reports for other Clinical Trials of the Alkermes 8700 Product in the Initial Indication, or new or updated Trial Master Files or new tangible embodiments of the Licensed Know-How. Without limiting the foregoing, Alkermes shall, prior to the Transfer Completion Date, transfer or have transferred to Biogen true and complete copies of (1) all data and results generated from any Development activities conducted by or on behalf of Alkermes with respect to the Products for all completed Clinical Trials conducted by or on behalf of Alkermes (including all pre-clinical studies and Clinical Trials of the Alkermes 8700 Product in the Initial Indication) and (2) all Trial Master Files (including any Trial Master File plans, tables of contents or indices and any evidence or certification of related quality checks) or equivalents thereof, for all completed Clinical Trials of the Alkermes 8700 Product conducted by or on behalf of Alkermes. Any transfer under this Section 3.2.3 shall be in such format as mutually agreed by the Parties, provided that upon Biogen’s request, Alkermes agrees to transfer such materials in electronic form. If Biogen requests other tangible embodiments of the Licensed Know-How or additional data or results or any other documentation relating to such data or results in respect of Development activities conducted by or on behalf of Alkermes under the Initial Development Plan, where the applicable pre-clinical study report or clinical study report for such activities has not yet been completed, then Alkermes shall attempt in good faith to provide such requested embodiments, data or results or other documentation related thereto to Biogen.
(iii)Transition Plan. Following the completion of Alkermes’ obligations under the Initial Development Plan in accordance with Section 3.2.1, the Parties shall cooperate with each other to ensure a smooth transition to Biogen or Biogen’s designee of ongoing Development activities related to the Alkermes 8700 Product in the Initial Indication,
20
including taking the actions specified in a transition plan that may be entered into between the Parties after the Effective Date by written agreement of the Parties, as such plan may be updated from time to time upon written agreement of the Parties (the “Transition Plan”). If there is any inconsistency between the Transition Plan and this Agreement, the terms of this Agreement shall prevail.
(iv)Manufacturing Technology Transfer. Upon Biogen’s written request, Alkermes will promptly make available to Biogen all Licensed Know-How (including all historical process or analytical information (i.e., all experimentally or literature-derived data used to Manufacture any Product)) to enable the Manufacture of all Products by or on behalf of Biogen (the “Alkermes Manufacturing Know-How”), by providing copies or samples of relevant documentation, materials and other embodiments of such Licensed Know-How, including data within reports, notebooks and electronic files. Alkermes will perform services reasonably requested by Biogen to facilitate the technology transfer described in this Section 3.2.3(iv) in a professional and workmanlike manner in accordance with Biogen’s reasonable instructions, and Biogen will reimburse Alkermes at the Development FTE Rate for all internal costs incurred in connection with the performance of such services performed and for all external expenses directly related thereto. Any materials provided by Alkermes in connection with the transfer of the Alkermes Manufacturing Know-How will remain the sole property of Alkermes.
3.2.4.Initial GI Tolerability Data Package Transfer.
(i)Initial GI Tolerability Data Package. Within a reasonable time period following the completion of the last visit for the last patient of Part A of the GI Tolerability Clinical Trial (including the two (2) week safety follow-up period for such last patient), Alkermes will provide written notice to Biogen (such notice, a “GI Tolerability Data Package Notice”) that includes:
(A)The Initial GI Tolerability Data Package; and
(B)Wire transfer instructions for payment of the Option Payment pursuant to Section 9.2 of this Agreement.
(ii)Incomplete Initial GI Tolerability Data Package. Following receipt of the Initial GI Tolerability Data Package Notice as set forth under Section 3.2.4(i), Biogen will have fifteen (15) days to notify Alkermes if the GI Tolerability Data Package included therein is missing any information related to Part A of the GI Tolerability Clinical Trial set forth in clause (a) of the definition of Initial GI Tolerability Data Package, which notice will describe the information that is missing from such Initial GI Tolerability Data Package. Alkermes will provide Biogen with such missing information identified in such notice, to the extent such information is Controlled by Alkermes, as soon as reasonably practicable.
(iii)Extension of the Initial GI Tolerability Data Package Period. If Alkermes provides any information to Biogen following the receipt of the GI Tolerability Data Package Notice pursuant to Section 3.2.4(i) and such information is, in Biogen’s reasonable discretion, material information that was not previously provided to Biogen, then upon written notice from Biogen to Alkermes, the [**]-day period within which Biogen would be obligated to
21
make the Option Payment in accordance with Section 9.2 will be extended such that there is at least [**] days between Biogen’s receipt of such information and the date on which Biogen would be obligated to make such Option Payment under Section 9.2.
(iv)Discussion Regarding Part B. Following receipt of the GI Tolerability Data Package Notice as set forth under Section 3.2.4(i), (a) the Parties will discuss in good faith whether to conduct Part B of the GI Tolerability Clinical Trial and (b) unless the Parties have agreed not to conduct such Part B of the GI Tolerability Clinical Trial, Alkermes will use commercially reasonable efforts to develop and provide to Biogen no later than thirty (30) days after the delivery of the GI Tolerability Data Package (1) a plan for Part B of such trial that is reasonably designed and powered to sufficiently differentiate between the Alkermes 8700 Product and Tecfidera® with respect to GI Events and (2) a proposed budget for the conduct of activities under such plan for Part B of the GI Tolerability Clinical Trial. Thereafter, at Biogen’s request, the Parties will discuss in good faith such plan and associated budget and if Biogen wishes to make any changes to the plan (including to increase the scope or power of such trial) in a manner that increases the associated budget therefor, then the Parties would discuss such proposed changes in good faith and attempt to reach agreement on such expanded scope or power and associated increase in budget. If the Parties mutually agree in writing with respect to the plan for the conduct of Part B of the GI Tolerability Clinical Trial and the associated budget for the Development activities to be conducted under such plan, then (A) the DT will amend the Initial Development Plan to include the conduct of Part B of the GI Tolerability Clinical Trial in accordance with the agreed-to plan and the agreed-to budget associated with such Development activities in accordance with Section 3.1.2 and (B) Alkermes will conduct Part B of the GI Tolerability Clinical Trial materially in accordance with such agreed-to plan, unless otherwise jointly agreed in writing by the Parties.
3.2.5.Complete GI Tolerability Data Package Transfer.
(i) Complete GI Tolerability Data Package. Within a reasonable time period following the completion of the last visit for the last patient of Part B of the GI Tolerability Clinical Trial (including any applicable safety follow-up period for such last patient), Alkermes will provide to Biogen the Complete GI Tolerability Data Package.
(ii) Incomplete Complete GI Tolerability Data Package. Following receipt of the Complete GI Tolerability Data Package, Biogen will have fifteen (15) days to notify Alkermes if the Complete GI Tolerability Data Package is missing any information, which notice will describe the information that is missing from such Complete GI Tolerability Data Package. Alkermes will provide Biogen with such missing information identified in such notice, to the extent such information is Controlled by Alkermes, as soon as reasonably practicable.
3.3.1.Alkermes 8700 Product in the Initial Indication in the U.S. Until the Transfer Completion Date, Alkermes shall be responsible for making all regulatory filings with the FDA in respect of the Alkermes 8700 Product in the Initial Indication, including the Alkermes 8700 Product 505(b)(2) NDA. Alkermes shall use commercially reasonable efforts to submit all such regulatory filings in accordance with the Initial Development Plan. All such
22
filings and Regulatory Approval (if granted) for the Alkermes 8700 Product 505(b)(2) NDA shall initially be held by and in the name of Alkermes until assignment thereof to Biogen pursuant to this Section 3.3.1. Alkermes will (A) send a letter to the FDA to transfer and assign to Biogen Alkermes’ entire right, title and interest in and to all regulatory filings related to the Alkermes 8700 Product in the Initial Indication, including the Alkermes 8700 Product 505(b)(2) NDA and (B) transfer to Biogen a complete copy of the approved Alkermes 8700 Product 505(b)(2) NDA and all regulatory filings, regulatory documentation and other supplements and records related to such NDA that are required to be kept under 21 C.F.R. § 314.81, in each case, within one (1) Business Day following:
(i)receipt of Regulatory Approval of the Alkermes 8700 Product 505(b)(2) NDA; or
(ii)Biogen’s request, if (a) Alkermes receives a complete response letter from the FDA in connection with the Alkermes 8700 Product 505(b)(2) NDA failing to grant Regulatory Approval for the Alkermes 8700 Product in the Initial Indication in the U.S. that suggests additional Development activities or other requirements, (b) after using reasonable efforts to discuss with the FDA, Alkermes is not able to narrow or eliminate such suggested additional Development activities or such other requirements and (c) even if Alkermes were to perform such additional Development activities or comply with such other requirements (including through the grant from Biogen of the right of reference in accordance with Section 3.3.2), there could be no reasonable expectation that the 8700 Product 505(b)(2) NDA could receive Regulatory Approval by December 31, 2021 (the occurrence of the events set forth in the foregoing clauses ((a) through (c)), an “Alkermes Approval Failure”);
The date on which Alkermes completes its obligations set forth in clauses (A) and (B) of this Section 3.3.1 shall be deemed the “Transfer Completion Date.”
3.3.2.Right of Reference. If, subsequent to filing the Alkermes 8700 Product 505(b)(2) NDA, (i) Alkermes receives feedback from the FDA, whether through a complete response letter or otherwise, that the FDA requires access to certain additional data to determine whether to grant approval for the Alkermes 8700 Product in the Initial Indication in the U.S. and (ii) the Parties, working in good faith, agree that such information is included in the U.S. Regulatory Approvals for Tecfidera® or related submissions filed with the FDA and should be included in the Alkermes 8700 Product 505(b)(2) NDA, then, until the earliest of the date of (a) approval of the Alkermes 8700 Product 505(b)(2) NDA, (b) an Alkermes Approval Failure or (c) any notice of termination of this Agreement, Biogen will, within two (2) Business Days of the occurrence of the events set forth in the foregoing clauses (i) and (ii), grant to Alkermes a fully paid-up and royalty-free right of reference to such U.S. Regulatory Approvals for Tecfidera® and related submissions filed with the FDA, for the sole purpose of obtaining Regulatory Approval of the Alkermes 8700 Product 505(b)(2) NDA. Upon such grant of a right of reference, Biogen will take such actions as may be reasonably requested by Alkermes to give effect to the intent and benefit of such right of reference as set forth in this Section 3.3.2.
3.3.3.Other Products. Biogen shall have the unilateral right, in its sole discretion and at its own cost and expense, to submit any regulatory filings, including INDs and NDAs for, and seek Regulatory Approval of, any Products in the Field in the Territory, other
23
than the Alkermes 8700 Product in the Initial Indication in the U.S., prior to the date of the Alkermes Approval Failure.
3.3.4.Alkermes Approval Failure. Notwithstanding anything else in this Agreement, in the event of an Alkermes Approval Failure, Biogen shall have no obligation to Exploit (including, for clarity, to seek Regulatory Approval for) the Alkermes 8700 Product. In addition, in the event of such an Alkermes Approval Failure, upon written agreement of the Parties, Alkermes may conduct additional Development of the Alkermes 8700 Product in the Initial Indication in the U.S. at its own cost and expense, including Clinical Trials (and, for clarity, the costs of such Development activities will not be treated as Development Costs for purposes of this Agreement unless otherwise agreed by the Parties).
3.4.Regulatory Meetings and Communications.
3.4.1.Responsibilities for the Alkermes 8700 Product in the Initial Indication in the U.S. Until the Transfer Completion Date, Alkermes shall be primarily responsible for conducting meetings and discussions with the FDA related to the Alkermes 8700 Product in the Initial Indication, subject to Section 3.4.3.
3.4.2.Responsibilities for Other Products. Biogen shall have the unilateral right, itself or through its Affiliates, Sublicensees, subcontractors or Distributors, to conduct meetings and discussions with Regulatory Authorities in the Territory related to Products in the Field, other than, prior to the Transfer Completion Date, discussions with the FDA related to the Alkermes 8700 Product in the Initial Indication, in its sole discretion.
3.4.3.Regulatory Authority Communications by Alkermes.
(i)Alkermes shall give Biogen reasonable advance notice of meetings and discussions, including meetings or discussions that take place via teleconference or videoconference, with the FDA related to the Alkermes 8700 Product in the Initial Indication, and Biogen shall have the right to send one (1) representative of its regulatory department with expertise in matters related to interactions with Regulatory Authorities to participate in person at all such meetings and discussions.
(ii)If Alkermes has written communications with the FDA (other than administrative correspondence) relating to the Alkermes 8700 Product in the Initial Indication, then Alkermes shall notify Biogen and, (a) as soon as practicable, but in no case later than forty-eight (48) hours following receipt of any such written communication from the FDA, provide a copy to Biogen of such communication(s) and (b) no later than twenty-four (24) hours in advance of sending any written communication to the FDA in response to such FDA communication, provide an advance copy to Biogen of any such written communication. Alkermes shall provide to Biogen substantive drafts of the NDA for the Alkermes 8700 Product in the Initial Indication prior to its submission, (a) following completion of material modules of such NDA or (b) at the request of Biogen. Alkermes shall consider in good faith any comments received from Biogen’s representative related to the draft NDA for the Alkermes 8700 Product in the Initial Indication, communications with the FDA as set forth in this Section 3.4.3(ii) and any Post-Marketing Commitments, in each case, as described in this Section 3.4. In the event that, at
24
any time prior to the Transfer Completion Date, Biogen receives any written communication from the FDA relating to the Alkermes 8700 Product in the Initial Indication, Biogen shall notify Alkermes and provide a copy to Alkermes of any such written communication promptly following such communication’s receipt. Prior to the Transfer Completion Date, Biogen shall not respond to any such communication but shall permit Alkermes to respond on Biogen’s behalf; provided, however, that Biogen shall have the right to respond to communications from the FDA to the extent reasonably required to comply with Applicable Laws.
(iii)In the event that, at any time during the Term, Alkermes receives any written communication from any Regulatory Authority relating to any Product, other than any such communication from the FDA relating to the Alkermes 8700 Product in the Initial Indication prior to the Transfer Completion Date, Alkermes shall notify Biogen and provide a copy to Biogen of any such written communication promptly following receipt of such communication. Alkermes shall not respond to any such communication and instead shall permit Biogen to respond on Alkermes’ behalf; provided, however, that during any period in which Alkermes is responsible for Manufacturing any Product, Alkermes shall have the right to respond to communications from the FDA or other Regulatory Authority to the extent solely related to the Manufacture of a Product by or on behalf of Alkermes or its Affiliate or reasonably required to comply with Applicable Laws.
3.5.Debarment Limitations. In the course of Development of the Products in the Territory by or on behalf of a Party, each Party shall not knowingly use any employee or consultant who is or has been debarred by the FDA or any other Regulatory Authority, or, to the best of such Party’s knowledge, who is or has been the subject of debarment proceedings by the FDA or any such Regulatory Authority. Such Party shall promptly notify the other Party of, and provide the other Party with a copy of, any correspondence or other reports that such Party receives from any Third Party with respect to any use of a debarred employee or consultant in connection with such Party’s performance of its obligations under this Agreement.
3.6.Compliance. Each Party shall conduct its Development activities under this Agreement in compliance with all Applicable Laws and the terms and conditions set forth in this Agreement.
3.7.Safety Reporting. Within sixty (60) days after the Effective Date, the Parties shall enter into a mutually acceptable safety data exchange agreement, setting forth guidelines and procedures for the receipt, investigation, recordation, review, communication, and exchange (as between the Parties) of adverse event reports, pregnancy reports, technical complaints and any other information concerning the safety of the Products, as well as safety governance and decision-making roles. Such guidelines and procedures shall be in accordance with, and enable the Parties and their Affiliates to fulfill, reporting obligations to the FDA or any other Regulatory Authority. Furthermore, such guidelines and procedures shall be consistent with relevant International Council on Harmonization (ICH) guidelines, except where said guidelines may conflict with reporting requirements of local Regulatory Authorities, in which case local reporting requirements shall prevail. The Parties’ costs incurred in connection with receiving, investigating, recording, reviewing, communicating and exchanging adverse events and other reportable information as provided in such safety data exchange agreement shall be included as an element of Development Costs (to the extent relating to the Development of a Product).
25
3.8.1.Development Costs Incurred by Alkermes. Biogen shall be responsible for fifty percent (50%) of all Development Costs in excess of the first Fifty-Six Million U.S. Dollars (US$56,000,000) incurred by Alkermes in the conduct of the activities under the Initial Development Plan during Calendar Year 2017. Starting with Calendar Year 2018 and thereafter until the completion of the activities allocated to Alkermes under the Initial Development Plan, Biogen shall be responsible for all Development Costs incurred by Alkermes in the conduct of its activities in the Initial Development Plan, to the extent such Development Costs do not exceed [**] percent ([**]%) of the aggregate costs and expenses set forth in the annual budget for the Initial Development Plan for each such Calendar Year. Subject to the last sentence of Section 3.9, (a) to the extent Alkermes incurs any Development Costs that exceed [**] percent ([**]%) of the aggregate costs and expenses set forth in the annual budget for the Initial Development Plan for such Calendar Year, each Party shall be responsible for [**] percent ([**]%) of such excess amounts, up to an amount equal to [**] percent ([**]%) of the aggregate costs and expenses set forth in the annual budget for the Initial Development Plan for such Calendar Year and (b) to the extent Alkermes incurs any Development Costs that exceed [**] percent ([**]%) of the aggregate costs and expenses set forth in the annual budget for the Initial Development Plan for such Calendar Year, Alkermes shall be responsible for such excess amounts; provided that, if the Alkermes 8700 Product 505(b)(2) NDA receives Regulatory Approval from the FDA prior to December 31, 2021, then Biogen will reimburse Alkermes for [**] percent ([**]%) of all such Development Costs in excess of [**] percent ([**]%) of the aggregate costs and expenses set forth in the annual budget for the Initial Development Plan for such Calendar Year to the extent that such Development Costs are incurred in the performance of activities that generate data and results that are submitted as part of such approved Alkermes 8700 Product 505(b)(2) NDA.
3.8.2.Development Costs Incurred by Biogen. Biogen shall be solely responsible for all Development Costs incurred by Biogen.
3.8.3.Development Costs Payments. Biogen will, within forty-five (45) days after the end of Calendar Year 2017, pay Alkermes the Development Costs due under Section 3.8.1 for such Calendar Year. Starting with Calendar Year 2018 and thereafter, for each Calendar Quarter for which Development Costs are payable by Biogen to Alkermes pursuant to Section 3.8.1, Biogen will, within forty-five (45) days after the receipt of an invoice from Alkermes for such Development Costs, pay Alkermes the amount due for such Calendar Quarter.
3.9.Initial Development Plan Budget. Responsibility for amendments to the Initial Development Plan budget shall rest with the DT, subject to review and approval by the JSC. Updates to such budget will be prepared on an annual basis as set forth in Section 3.1.2. In addition, if Alkermes identifies a potential overage of greater than [**] percent ([**]%) of the aggregate costs and expenses expected to be incurred in performing Development activities pursuant to the Initial Development Plan for a given Calendar Year, then Alkermes shall notify Biogen of such potential overage (through the DT). Following such notice, the DT shall discuss in good faith at its next meeting whether to make any amendment to the Initial Development Plan budget to account for such potential overage, which amendment, if applicable, shall be presented to the JSC for its review and approval. Notwithstanding anything to the contrary set
26
forth in this Agreement, Biogen shall not be responsible for bearing its share of any Development Costs that exceed [**] percent ([**]%) of the amounts set forth in the annual budget under the Initial Development Plan in accordance with Section 3.8.1 to the extent that such excess Development Costs are incurred as a result of (i) Alkermes’ failure to perform its Development obligations in accordance with the Initial Development Plan or this Agreement, or (ii) a delay in Development of the Alkermes 8700 Product due to the gross negligence or willful misconduct of Alkermes.
4.1.Commercialization Rights. During the Term, Biogen shall have the unilateral right, itself or through its Affiliates, Sublicensees, subcontractors or Distributors, to Commercialize the Products in the Field in the Territory in its sole discretion.
4.2.Commercialization Efforts. Subject to Section 3.3.4, following (i) receipt of Regulatory Approval for the Alkermes 8700 Product in the Initial Indication in the U.S., (ii) the Transfer Completion Date and (iii) the completion of any Post-Marketing Commitments that the FDA requires be completed prior to the commencement of Commercialization activities, Biogen shall use Commercially Reasonable Efforts to Commercialize the Alkermes 8700 Product in the Initial Indication in the U.S.
4.3.Commercialization Costs. Biogen shall be responsible for all costs of conducting Commercialization of Products.
4.4.Compliance. Biogen shall Commercialize the Products in the Field in the Territory in compliance with all Applicable Laws, the Code, the OIG Guidance and the terms and conditions set forth in this Agreement.
Article 5
MANUFACTURE AND SUPPLY
5.1.Supply and Quality Agreements.
5.1.1.Clinical Supplies. Within ninety (90) days after the Effective Date, the Parties will negotiate in good faith and execute a Clinical Supply Agreement pursuant to which Alkermes will Manufacture or have Manufactured, and Biogen and its Affiliates and Sublicensees will purchase exclusively from Alkermes, Clinical Supplies (provided that Biogen may qualify to Manufacture, or engage and qualify a Third Party to Manufacture, Clinical Supplies as a back-up manufacturer in the event of a Force Majeure Delay or a Serious Failure to Supply). The Clinical Supply Agreement will reflect the terms and conditions included in Exhibit D and such other commercially reasonable and customary terms and conditions, satisfactory in form and substance to the Parties and their legal advisors, as are necessary or appropriate for transactions of this type and that will allow Biogen to be supplied with Product in a manner appropriate to meet its obligations and exercise its rights under this Agreement, subject to both Parties’ compliance with the terms and conditions of such Clinical Supply Agreement.
27
Notwithstanding anything to the contrary set forth in this Agreement or the Clinical Supply Agreement, the Fully Burdened Manufacturing Costs of Clinical Supplies will be included in the Development Costs under this Agreement and will not be charged to Biogen under the Clinical Supply Agreement. Within one hundred and twenty (120) days after the Effective Date, the Parties will negotiate in good faith and execute a technical and quality agreement, which will be appended to the Clinical Supply Agreement and which will specify certain quality assurance and quality control requirements relating to the Manufacture of such Clinical Supplies. Notwithstanding anything to the contrary set forth in this Section 5.1.1, if (i) Alkermes foregoes its exclusive right to Manufacture or have Manufactured Clinical Supplies or (ii) there is a Serious Failure to Supply, then, in any case ((i) – (ii)), (a) Biogen and its Affiliates and Sublicensees shall have no further obligation to purchase Clinical Supplies from Alkermes, (b) Biogen shall have the exclusive right to Manufacture or to have Manufactured Clinical Supplies and (c) Alkermes shall promptly conduct a transfer of Manufacturing technology to Biogen or its designee to enable Biogen or such designee to Manufacture Clinical Supplies. In addition, in the event of a Force Majeure Delay (and for the duration thereof), until such time as Alkermes is able to resume sufficient Manufacturing to meet Biogen’s demand for Clinical Supplies, Biogen may Manufacture itself or have Manufactured by its back-up manufacturer, all Clinical Supplies for which Alkermes is unable to meet Biogen’s demand.
5.1.2.Commercial Supplies. Pursuant to this Agreement, Biogen has the right to Manufacture or have Manufactured Commercial Supplies. Biogen has considered in good faith, and hereby appoints, Alkermes as the toll manufacturer for such Commercial Supplies for Commercialization in the Territory at a site outside of the United States, and Biogen and its Affiliates and Sublicensees will purchase Commercial Supplies exclusively from Alkermes (provided that Biogen may qualify to Manufacture, or engage and qualify a Third Party to Manufacture, Commercial Supplies as a back-up manufacturer so long as such Third Party Manufacturer does not Manufacture more than [**] percent ([**]%) of Commercial Supplies in the aggregate in any Calendar Year, except in the event of a Force Majeure Delay or a Serious Failure to Supply). This appointment is subject to the Parties negotiating in good faith and entering into a commercial supply agreement pursuant to which Biogen would engage Alkermes on a toll manufacturing basis to Manufacture Commercial Supplies and Biogen would purchase such Commercial Supplies in accordance with the terms and conditions included in Exhibit E and such other commercially reasonable and customary terms and conditions, satisfactory in form and substance to the Parties and their legal advisors, as are necessary or appropriate for transactions of this type and that will allow Biogen to be supplied with Product in a manner appropriate to meet its obligations and exercise its rights under this Agreement, subject to both Parties’ compliance with the terms and conditions of such commercial supply agreement. In connection with such commercial supply agreement, the Parties will also negotiate in good faith and execute a technical and quality agreement, which will be appended to the commercial supply agreement and which will specify certain quality assurance and quality control requirements relating to the Manufacture of such Commercial Supplies. If, following the use of good faith efforts, the Parties are unable to reach agreement on the terms of a commercial supply agreement within a period of six (6) months from the commencement of negotiations, then Biogen shall have the right (in its sole discretion) to Manufacture or have Manufactured Commercial Supplies. Notwithstanding anything to the contrary set forth in this Section 5.1.2, if (i) Alkermes foregoes its exclusive right to Manufacture or have Manufactured Commercial Supplies, (ii) Alkermes undergoes a Change of Control in which the acquirer is a competitor of
28
Biogen set forth on Schedule 5.1 or a Third Party toll manufacturer that Manufactures a competing fumarate product or (iii) there is a Serious Failure to Supply, then, in any case ((i) – (iii)), (a) Biogen and its Affiliates and Sublicensees shall have no further obligation to exclusively purchase Commercial Supplies from Alkermes, (b) Biogen shall have the exclusive right to Manufacture or to have Manufactured Commercial Supplies and (c) Alkermes shall promptly conduct a transfer of Manufacturing technology to Biogen or its designee to enable Biogen or such designee to Manufacture Commercial Supplies. In addition, in the event of a Force Majeure Delay (and for the duration thereof), until such time as Alkermes is able to resume sufficient Manufacturing to meet Biogen’s demand for Commercial Supplies, Biogen may Manufacture itself or have Manufactured by its back-up manufacturer, all Commercial Supplies for which Alkermes is unable to meet Biogen’s demand.
5.2.Supply Team. The Parties will establish a supply team (the “ST”) pursuant to the provisions of the Clinical Supply Agreement. The ST will report to the JSC.
6.1.Patent and Know-How License Grant.
6.1.1.Grant to Biogen. Subject to the terms and conditions of this Agreement, Alkermes hereby grants to Biogen an exclusive (even as to Alkermes and its Affiliates, except as expressly set forth in Section 6.1.4), royalty-bearing, worldwide license, with the right to sublicense through multiple tiers pursuant to Section 6.1.2, under the Licensed Patents and the Licensed Know-How to Exploit the Products in the Field in the Territory.
6.1.2.Sublicenses. Biogen will have the right to grant sublicenses through multiple tiers to Affiliates and Third Parties of the rights granted to Biogen under this Agreement in accordance with the terms and conditions of this Section 6.1.2. The grant of any such sublicense will not relieve Biogen of its obligations under this Agreement (including its financial obligations). In addition, such sublicense will be consistent in all respects with all applicable terms and conditions of this Agreement. With respect to any sublicense with a Sublicensee other than an Affiliate, Biogen shall provide Alkermes with a copy of such sublicense, and any modification or termination thereof, promptly after execution of such sublicense, modification or termination (and in any event within thirty (30) days after such sublicense has been fully executed or modified or termination of such sublicense has occurred); provided that any such copy may be redacted to remove any confidential, proprietary or competitive information of Biogen or its Sublicensee, and any other information not necessary for Alkermes to assess Biogen’s compliance with the terms of this Section 6.1.2.
6.1.3.No Implied Licenses; Negative Covenant. Except as expressly granted herein, neither Party grants to the other Party any right or license under any intellectual property rights Controlled by such first Party.
6.1.4.Alkermes Reservation of Rights. The license granted in Section 6.1.1 is subject to a reserved non-exclusive, non-transferable, and, except as necessary or
29
useful for Alkermes to Manufacture Clinical Supplies and Commercial Supplies in accordance with this Agreement and the applicable Supply Agreements, non-sublicenseable right of Alkermes under the Licensed Patents and Licensed Know-How solely to exercise its rights and perform its obligations under this Agreement and any Supply Agreement.
6.2.Patent Update. At least once annually, Alkermes will provide to Biogen an update to the list of Licensed Patents set forth on Exhibit B, and Exhibit B shall automatically be modified to include such updates. Notwithstanding the foregoing, and for the avoidance of doubt, any Patent Right that satisfies the definition of a “Licensed Patent” under Section 1.1.58 shall automatically constitute a Licensed Patent under this Agreement, notwithstanding any failure or delay in including such Patent Right on the list of Licensed Patents set forth on Exhibit B.
6.3.Termination of License to Contested Patent Rights. If Biogen or any of its Affiliates or Sublicensees initiates or provides financial support (other than equity funding) or information to a Third Party for purposes of initiating any action or proceeding in any forum of competent jurisdiction in the Territory (including a court, a patent office or an arbitral tribunal, and whether in the form of petitions for declaratory relief, claims, counterclaims, defenses, interferences, petitions for reexamination, inter partes review, post-grant review, or otherwise, but excluding any action that may be necessary or reasonably required in response to a subpoena or court or administrative law request or order) that any Patent Right (or any claim thereof) within the Licensed Patents is unpatentable, invalid, unenforceable, or not infringed (any such action or proceeding, a “Patent Action”) and a final, non-appealable order is made in any such forum that such Patent Right or claim thereof is patentable, valid, enforceable or infringed, as applicable, then Alkermes, as the licensor of the Licensed Patents under this Agreement, may at its discretion, (a) invoice Biogen for all expenses incurred by Alkermes in such Patent Action, including reasonable attorneys’ fees, experts’ fees and other costs of investigation or defense, court costs and other litigation expenses, and Biogen shall pay all undisputed amounts set forth in such invoice within sixty (60) days after receipt of such invoice, (b) terminate its license to Biogen pursuant to this Article 6 to such Patent Right, whereupon such Patent Right will no longer be deemed to be within the Licensed Patents or (c) take both actions outlined in clauses (a) and (b) of this sentence. Notwithstanding the foregoing, (1) if any Affiliate of Biogen that becomes an Affiliate of Biogen through a Change of Control of Biogen is engaged in a Patent Action at the time of such Change of Control, the provisions of this Section 6.3 shall not be deemed to apply as a result of such Patent Action by such Affiliate of Biogen, (2) Biogen shall have the right to defend itself against any action or proceeding in any forum of competent jurisdiction in the Territory brought by Alkermes or any of its Affiliates or Sublicensees alleging infringement of any Patent Right and (3) in the case of a Patent Action by a Sublicensee, Alkermes shall not have the right to take the actions described in the preceding sentence unless Biogen fails to either terminate the applicable sublicense or cause the Sublicensee to cease pursuing such Patent Action within sixty (60) days of the date that Biogen becomes aware of such Patent Action.
6.4.Non-Suit. During the Term until the Transfer Completion Date, Biogen, on behalf of itself and of any Affiliates, Sublicensees, successors and assigns, agrees on a worldwide basis not to file or maintain any lawsuit, cause of action or other legal action against Alkermes or its Affiliates under any Patent Rights Controlled by Biogen that Cover the Alkermes 8700 Product solely for purposes of Exploiting the Alkermes 8700 Product in accordance with
30
this Agreement. Biogen shall cause all of its Affiliates, Sublicensees, successors and assigns to be bound by the obligations of this Section 6.4.
6.5.Non-Interference. During the Term until the Transfer Completion Date, Biogen and its Affiliates, Sublicensees, successors and assigns shall not, and shall not cause or provide support to any Third Party to, initiate or otherwise undertake any of the following actions against the Alkermes 8700 Product: interfering with efforts to obtain and maintain Regulatory Approval of the Alkermes 8700 Product in the U.S., or advocating for non-approval or limited Regulatory Approval of the Alkermes 8700 Product in the U.S., including the filing of a lawsuit against the FDA or the filing or submission of any Citizen Petitions, correspondence or other written submissions with the FDA. Biogen shall cause all of its Affiliates, Sublicensees, successors and assigns to be bound by these obligations.
6.6.Exclusivity. During the Term, neither Party nor any of such Party’s Affiliates, or Sublicensees (who are granted research and Development rights under this Agreement), shall (i) directly or indirectly research, develop or commercialize any pro-drug or salt of MMF or DMF or (ii) acquire any right, title or interest from a Third Party in any pro-drug or salt of MMF or DMF, except, in each case (i) or (ii), with respect to the conduct of such activities by Biogen or its Affiliates with respect to Tecfidera® and Fumaderm® (in each case, using the same active pharmaceutical ingredient(s) included in Tecfidera® or Fumaderm®, as applicable, as of the Effective Date, in any form, formulation, combination, administration, dosage or delivery mechanism (but not including any pro-drug or salt of DMF or MMF (other than DMF))) or any Product. Notwithstanding anything to the contrary in this Agreement, upon a finding of GI Inferiority of the Alkermes 8700 Product, (a) if Biogen has Commercialized the Alkermes 8700 Product, then the exclusivity obligations of Alkermes and its Affiliates set forth in this Section 6.6 shall remain in effect, and the exclusivity obligations of Biogen and its Affiliates and Sublicensees (who are granted research and Development rights under this Agreement) set forth in this Section 6.6 shall automatically terminate or (b) if Biogen has not Commercialized the Alkermes 8700 Product in the U.S. within six (6) months after the receipt of Regulatory Approval for the Alkermes 8700 Product in the Initial Indication in the U.S. and the completion of any Post-Marketing Commitments that the FDA requires be completed prior to the commencement of Commercialization activities, then the exclusivity obligations of Alkermes and Biogen and their respective Affiliates, and with respect to Biogen, Sublicensees (who are granted research and Development rights under this Agreement), set forth in this Section 6.6 shall automatically terminate. In the event that a Party or its Affiliate, or Sublicensee (who is granted research and development rights under this Agreement), obtains, whether through acquisition, merger or other similar transaction, control of any entity that is engaging in any activities that would cause such Party to breach this Section 6.6, then such Party shall not be deemed in breach of this Section 6.6 if (1) such Party or its Affiliate or, with respect to Biogen, Sublicensee, or the applicable entity divests such rights (through sale, exclusive license or other transfer) within six (6) months from the date such rights are obtained and (2) all activities by such Party or its Affiliate or Sublicensee with respect to the applicable competitive compound or product during such six (6)-month period are conducted independently of the activities conducted by such Party or its Affiliates or Sublicensees pursuant to this Agreement with customary firewall separations, and no intellectual property of such Party or its Affiliates or the other Party or its Affiliates relating to any Product is used in the conduct of such activities. Notwithstanding anything to the contrary in this Section 6.6, if a Party or its Affiliate or
31
Sublicensee undergoes a Change of Control (in the case of a Sublicensee, applying that term as if such Sublicensee were a Party) in which the acquirer or its Affiliate (in each case, that was not an Affiliate of such Party prior to such Change of Control) is engaging in any activities that would cause such Party to breach this Section 6.6, such Party shall not be deemed to be in breach of this Section 6.6 as a result of such Change of Control, provided that, during the period in which such Party is subject to exclusivity obligations under this Section 6.6, all activities by such Party or its Affiliate or Sublicensee with respect to the applicable competitive compound or product are conducted independently of the activities conducted pursuant to this Agreement with customary firewall separations, and no intellectual property of such Party or its Affiliates or the other Party or its Affiliates relating to any Product is used in the conduct of such activities.
Article 7
INTELLECTUAL PROPERTY RIGHTS
7.1.Ownership of Intellectual Property.
7.1.1.Product Trademarks. Biogen may, in its sole discretion, select any trademarks, trade dress, designs, logos or slogans to be used in connection with the Exploitation of the Products in the Field in the Territory (collectively, the “Product Trademarks”) and will own all such Product Trademarks. Neither Alkermes nor its Affiliates shall use or seek to register, anywhere in the world, any trademarks that are confusingly similar to any Product Trademark.
7.1.2.Collaboration Technology.
(i)Ownership. Alkermes shall own all rights, title and interests in and to any and all Alkermes Collaboration Know-How and Alkermes Collaboration Patents. Biogen shall own all rights, title and interests in and to any and all Biogen Collaboration Know-How and Biogen Collaboration Patents. The Parties shall jointly own any and all Collaboration Know-How invented jointly by a Party’s or its Affiliates’ employees, agents or independent contractors, on the one hand, and the other Party’s or its Affiliates’ employees, agents or independent contractors, on the other hand, in the performance of activities under the Initial Development Plan during the Term (“Joint Collaboration Know-How”), and any and all Collaboration Patents Covering such Collaboration Know-How (“Joint Collaboration Patents” and, collectively with the Joint Collaboration Know-How, the “Joint Collaboration Technology”). Subject to Section 6.6 and the licenses granted under this Agreement, each Party shall be free to exploit, either itself or through the grant of licenses to Third Parties (which Third Party licenses may be further sublicensable), its rights in and to the Joint Collaboration Technology, throughout the world without restriction, without the need to obtain further consent from the other Party, and without any duty to account or payment of any compensation to the other Party; provided, however, that if either Party disclaims in writing its ownership interest in any Joint Collaboration Technology, then such Joint Collaboration Technology shall become solely owned by the other Party and such disclaiming Party will and hereby does assign to the other Party its rights, title and interests in and to such disclaimed Collaboration Technology. Inventorship of any Collaboration Technology shall be determined in accordance with United States patent laws.
32
(ii)Assignment of Inventions. To the extent either Party or any of its Affiliates obtains any rights, title or interests in or to any Collaboration Technology of the other Party, such first Party, on behalf of itself and its Affiliates, hereby assigns to the other Party all such rights, title and interests to the extent necessary to effectuate the allocation of ownership set forth in Section 7.1.2(i). Each employee, agent or independent contractor (including all subcontractors) of a Party or its respective Affiliates performing work under this Agreement shall, prior to commencing such work, be bound by invention assignment obligations, including: (a) promptly reporting any invention, discovery, process or other intellectual property right; (b) presently assigning to the applicable Party or Affiliate all of his or her right, title and interest in and to any invention, discovery, process or other intellectual property; (c) cooperating in the preparation, filing, prosecution, maintenance and enforcement of any patent or patent application; and (d) performing all acts and signing, executing, acknowledging and delivering any and all documents required for effecting the obligations and purposes of this Agreement. It is understood and agreed that such invention assignment agreement need not reference or be specific to this Agreement.
7.2.Disclosure of Inventions. During the Term, each Party will promptly (but no later than sixty (60) days following such Party’s receipt of an invention disclosure) provide to the other Party any invention disclosure submitted to such Party that discloses any Collaboration Technology.
7.3.Patent Committee. Each Party will appoint one (1) representative with patent and intellectual property expertise no later than forty-five (45) days after the Effective Date. Such representatives (the “Patent Committee”) will meet (in person, by telephone or videoconference) upon request by either Party during the Term to coordinate, discuss, and review strategies with respect to Prosecuting and enforcing the Licensed Patents and the Joint Collaboration Patents.
(i)By Alkermes. Alkermes will have the first right, at its own cost and expense and using patent counsel of its choosing, to prepare, file, prosecute and maintain the Licensed Patents and the Joint Collaboration Patents, including any appeal proceeding made at the applicable patent office following such patent office’s failure to issue any such patent (collectively, “Prosecution” and when used as a verb, “Prosecute” means to engage in Prosecution). Alkermes will provide Biogen with copies of all material documents and correspondence relating to the Prosecution of the Licensed Patents and the Joint Collaboration Patents (a) promptly after receipt, with respect to communications from applicable patent authorities and (b) a reasonable time in advance of filing, for documents to be filed by Alkermes, in each case (a) and (b), to allow Biogen time to review such materials and comment thereon. Alkermes will reasonably consider Biogen’s reasonable comments on the documents filed, but Alkermes will not be obligated to implement such comments. Biogen will provide Alkermes all reasonable assistance in the Prosecution of such Licensed Patents and Joint Collaboration Patents, including by making its employees, agents and consultants reasonably available to Alkermes (or
33
Alkermes’ authorized attorneys, agents or representatives), to the extent reasonably necessary to enable Alkermes to undertake Prosecution as contemplated by this Agreement.
(ii)By Biogen. If Alkermes elects to halt Prosecution of any patents or patent applications within the Licensed Patents or the Joint Collaboration Patents in any country in the Territory, then Alkermes will notify Biogen in a timely manner to permit the preservation of any rights in such country with respect to such Licensed Patents or Joint Collaboration Patents. Following written notification from Biogen to Alkermes that Biogen wishes to assume Prosecution of such Licensed Patents or Joint Collaboration Patents in such country, Alkermes will permit Biogen to so assume Prosecution of such Licensed Patents or Joint Collaboration Patents (as applicable), at its own cost and expense and using patent counsel of its choosing. In addition, and notwithstanding anything to the contrary set forth in this Agreement, Biogen will have the first right, at its sole cost and expense and using patent counsel of its choosing, to direct and control any patent interferences, reexaminations, inter partes reviews, reissuances, revocations, oppositions and appeals from any such proceedings of the Licensed Patents and the Joint Collaboration Patents (collectively, “Protection”). Alkermes will provide Biogen reasonable assistance in the Prosecution and Protection of such Licensed Patents or Joint Collaboration Patents, including by making its employees, agents and consultants reasonably available to Biogen (or Biogen’s authorized attorneys, agents or representatives), to the extent reasonably necessary to enable Biogen to undertake Prosecution and Protection as contemplated by this Agreement. If Biogen elects to halt Protection of any patents or patent applications within the Licensed Patents or the Joint Collaboration Patents in any country in the Territory, then Biogen will notify Alkermes in a timely manner to permit the preservation of any rights in such country with respect to such Licensed Patents or Joint Collaboration Patents. Following written notification from Alkermes to Biogen that Alkermes wishes to assume Protection of such Licensed Patents or Joint Collaboration Patents in such country, Biogen will permit Alkermes to so assume Protection of such Licensed Patents or Joint Collaboration Patents (as applicable) at its own cost and expense and using patent counsel of its choosing. Biogen will provide reasonable assistance in the Protection of such Licensed Patents or Joint Collaboration Patents, including by making its employees, agents and consultants reasonably available to Alkermes (or Alkermes’ authorized attorneys, agents or representatives), to the extent reasonably necessary to enable Alkermes to undertake Protection as contemplated by this Agreement. Notwithstanding the foregoing, Biogen shall forfeit its right to control Protection of any Licensed Patents and Joint Collaboration Patents that are or become subject to a Patent Action under Section 6.3.
(iii)Other Patent Rights. Except as expressly provided in this Section 7.4.1, each Party shall have the sole right, in its sole discretion, to conduct Prosecution of any Patent Rights owned by such Party.
7.4.2.Common Interest. All information exchanged between the Parties or between the Parties’ outside patent counsel regarding Prosecution of the Licensed Patents, and the Joint Collaboration Patents shall be deemed Confidential Information of the prosecuting Party subject to Article 8. In addition, the Parties acknowledge and agree that, with regard to Prosecution of the Licensed Patents and the Joint Collaboration Patents, the interests of the Parties as licensor and licensee are aligned and are legal in nature. The Parties agree and acknowledge that they have not waived, and nothing in this Agreement constitutes a waiver of,
34
any legal privilege concerning the Licensed Patents or the Joint Collaboration Patents, including privilege under the common interest doctrine and similar or related doctrines.
7.4.3.Patent Term Extensions. The Parties will use reasonable efforts and cooperate with one another to obtain all available supplementary protection certificates, patent term restorations and other patent extensions with respect to the Products, and to make any filings with respect thereto. Alkermes will cooperate with Biogen with respect to any such filings, including by executing such authorizations and other documents and taking such other actions as may be reasonably requested by Biogen to obtain such extensions.
7.5.1.Notification of Infringement. If either Party learns of any actual or threatened infringement by a Third Party of a Licensed Patent or Collaboration Patent in the Territory or, other than Prosecution-related matters, any attack by a Third Party on the validity or enforceability of a Licensed Patent or Collaboration Patent in the Territory, including any certification received by such Party under the U.S. Drug Price Competition and Patent Term Restoration Act of 1984 (Public Law 98-417, as amended, the “Hatch-Waxman Act”), with respect to a Licensed Patent or Collaboration Patent and a Product in the Field (each, an “Infringement”), such Party will promptly, and in any event within five (5) days, notify the other Party and will provide the other Party with available evidence of such events.
7.5.2.Enforcement of Licensed Patents and Joint Collaboration Patents.
(i)Biogen shall have the first right, but not the obligation, at its own cost and expense and using counsel of its choosing, to institute any action, suit or proceeding against any Infringement of a Licensed Patent or Joint Collaboration Patent. Biogen shall have the right to cause Alkermes to join Biogen as a party plaintiff to any such action, suit or proceeding, at Biogen’s sole expense. Biogen will keep Alkermes reasonably informed regarding such action, suit or proceeding and will reasonably consider Alkermes’ input regarding such action, suit or proceeding.
(ii)If, after sixty (60) days following the date of notice given pursuant to Section 7.5.1 (or at least twenty (20) days before the expiration of any time limit set forth under 21 U.S.C. §355), Biogen has not provided written notification to Alkermes that Biogen will institute an action, suit or proceeding against the applicable Infringement or provided Alkermes with information and arguments demonstrating that there is insufficient basis for the allegation of such Infringement, then Alkermes shall have the right, but not the obligation, at its own cost and expense and using counsel of its choosing, to institute any action, suit or proceeding against such Infringement, provided that, if Biogen provides notice to Alkermes that Biogen has determined, for reasons related to the benefit of the Alkermes 8700 Product in the aggregate not to institute an action, suit or proceeding against such Infringement, Alkermes shall not have the right to institute an action, suit or proceeding against such Infringement. Notwithstanding the foregoing provisions of this Section 7.5.2(ii), if Biogen forms a good faith belief that instituting an action, suit or proceeding against such Infringement would not benefit the Alkermes 8700 Product in the aggregate, then Biogen shall consult with Alkermes in
35
reasonable detail regarding Biogen’s reasonable good faith rationale for such determination, and if after such consultation with Alkermes, Biogen maintains such belief in good faith, then Alkermes shall agree to a reasonable request from Biogen not to institute any such action, suit or proceeding against such Infringement; provided that, if the Parties disagree as to whether any such request from Biogen not to institute any such action, suit or proceeding against such Infringement is reasonable, then Alkermes will not institute any such action, suit or proceeding against such Infringement unless and until (a) in the case of an abbreviated new drug application (“ANDA”) under the Hatch-Waxman Act, Senior Management has attempted in good faith to discuss and resolve such disagreement at least five (5) days before the expiration of any time limit set forth under 21 U.S.C. §355 (and each Party agrees that it will use its best efforts to make its respective Senior Management available for such discussion within such timeframes) and (b) other than in the case of an ANDA, such request by Biogen is determined not to be reasonable in accordance with the Senior Management escalation and dispute resolution procedure set forth in Section 12.1.2 and, if such dispute remains unresolved after such procedure, then in accordance with the procedure set forth in Section 12.1.3, which escalation and dispute resolution procedures shall be conducted in an expedited manner so as to allow Alkermes to proceed with an action, suit or proceeding against such Infringement if such request by Biogen is determined not to be reasonable. In connection with any action, suit or proceeding pursuant to this Section 7.5.2, the Parties will cooperate with and assist each other in all reasonable respects.
7.5.3.Recoveries. In the event that either Party exercises the rights conferred in Section 7.5.2 and recovers any damages or other sums in such action, suit or proceeding or in settlement thereof, such damages or other sums recovered shall first be applied to all reasonable out-of-pocket costs and expenses incurred by the Parties in connection therewith, including attorneys’ fees. If such recovery is insufficient to cover all such costs and expenses of both Parties, it shall be shared pro rata in proportion to the total of such costs and expenses incurred by each Party. If, after such reimbursement, any funds shall remain from such damages or other sums recovered, and (i) if Biogen institutes the action, suit or proceeding pursuant to Section 7.5.2(i) that results in such damages or other sums recovered, then Biogen shall pay Alkermes a payment calculated by multiplying the applicable royalty rate for the Alkermes 8700 Product (that Biogen would have paid to Alkermes if such damages or other sums recovered were treated as Net Sales hereunder) by the amount of such damages or other sums recovered and Biogen shall retain the remainder or (ii) if Alkermes institutes the action, suit or proceeding pursuant to Section 7.5.2(ii) that results in such damages or other sums recovered, then Alkermes shall pay Biogen a payment calculated by multiplying the applicable royalty rate for the Alkermes 8700 Product (that Biogen would have paid to Alkermes if such damages or other sums recovered were treated as Net Sales hereunder) by the amount of such damages or other sums recovered and Alkermes shall retain the remainder.
7.5.4.Other Patent Rights. Except as expressly provided in Section 7.5.2, each Party shall have the sole right, in its sole discretion, to institute any action, suit or proceeding against any actual or threatened infringement by a Third Party of any Patent Right owned by such Party.
36
7.6.1.Notice. In the event that a Third Party at any time provides written notice of a claim to, or brings an action, suit or proceeding against, either Party, or any of their respective Affiliates or sublicensees (each Person so sued being referred to herein as a “Sued Party”), claiming infringement of such Third Party’s Patent Rights or unauthorized use or misappropriation of its Know-How based upon an assertion or claim arising out of the Exploitation of a Product in the Field in the Territory (“Infringement Claim”), such Party shall promptly notify the other Party of the Infringement Claim or the commencement of such action, suit or proceeding, enclosing a copy of the Infringement Claim and all papers served.
7.6.2.Right to Defend. If the Sued Party with respect to any Infringement Claim is entitled to indemnification under Article 11 with respect to such Infringement Claim, then the terms and conditions of Article 11 and not this Section 7.6.2 shall apply to such Infringement Claim. In all other cases, Biogen shall have the right, but not the obligation, at its own cost and expense and using counsel of its choosing, to defend against any Infringement Claim brought against Biogen or its Affiliates or Sublicensees and Alkermes shall have the right, but not the obligation, at its own cost and expense and using counsel of its choosing, to defend against any Infringement Claim brought against Alkermes or its Affiliates or sublicensees. The Sued Party shall keep the other Party reasonably informed of all material developments in connection with any such suit and shall not, without the other Party’s prior written consent, enter into any settlement or consent decree that requires any payment by or admits or imparts any other liability to the other Party. The other Party shall make available to the Sued Party its advice and counsel regarding any Infringement Claim and shall offer reasonable assistance in connection with any Infringement Claim to the Sued Party, at the Sued Party’s cost and expense.
7.7.Patent Marking. Biogen agrees to mark, and to require any of its Affiliates or Sublicensees to mark, any Products (or their containers or labels) made, sold, or otherwise distributed by it or them with any notice of Patent Rights required under Applicable Law to enable such Patent Rights to be enforced to their full extent in any country where Products are made, used, sold, or offered for sale.
7.8.Orange Book Listings. With respect to patent listings in the FDA Orange Book for issued patents for the Alkermes 8700 Product in the Initial Indication, the Parties shall determine by mutual agreement which patents to list in the FDA Orange Book (a) prior to the submission of the Alkermes 8700 Product 505(b)(2) NDA, or any other NDA for the Alkermes 8700 Product submitted to the FDA and (b) within twenty (20) days after the receipt of Regulatory Approval for the Alkermes 8700 Product in the Initial Indication. If the Parties are unable to reach agreement on any such listings within twenty (20) days before submission of the Alkermes 8700 Product 505(b)(2) NDA, or any other NDA for the Alkermes 8700 Product submitted to the FDA or within twenty (20) days after receipt of Regulatory Approval for the Alkermes 8700 Product in the Initial Indication in the U.S. prior to the Transfer Completion Date, then Alkermes will have the right to make the determination with respect to which patents to list in the FDA Orange Book. After the Transfer Completion Date, Biogen shall have the sole right, in its sole discretion, to determine which patents to list in the FDA Orange Book (or its foreign equivalent in any country in the Territory) for the Alkermes 8700 Product. With respect to patent listings in the FDA Orange Book (or its foreign equivalent in any country in the Territory) for issued patents for any other Product or for the Alkermes 8700 Product in any other indication
37
than the Initial Indication in the Field, in each case, Biogen shall have the sole right, in its sole discretion, to determine which patents to list.
7.9.1.Notification of Infringement. If Alkermes learns that a Third Party is infringing any Product Trademark in the Territory, Alkermes will promptly notify Biogen.
7.9.2.Infringement Action. Biogen will have the sole right, at its own cost and expense and in its sole discretion, to take any action with respect to any infringement of a Product Trademark in the Territory, with counsel of its own choice. Any recovery from any settlement or judgment from such action will be retained by Biogen.
Article 8
CONFIDENTIALITY; PUBLICITY
8.1.Confidentiality. Except to the extent authorized by this Agreement or otherwise agreed upon in writing, the Parties agree that the receiving Party will keep confidential and will not publish or otherwise disclose or use for any purpose, any proprietary and confidential information and materials furnished to it by the disclosing Party pursuant to this Agreement or the Confidentiality Agreements (collectively, “Confidential Information”), except to the extent that it can be established by the receiving Party that such Confidential Information:
8.1.1.was already known to the receiving Party or its Affiliates, as demonstrated by competent written records, other than under an obligation of confidentiality, at the time of disclosure by the disclosing Party;
8.1.2.was generally available to the public or otherwise part of the public domain at the time of its disclosure by the disclosing Party;
8.1.3.became generally available to the public or otherwise part of the public domain after its disclosure by the disclosing Party and other than through any act or omission of the receiving Party or its Affiliates in breach of this Agreement;
8.1.4.was disclosed to the receiving Party or its Affiliates, other than under an obligation of confidentiality, by a Third Party who had no obligation to the disclosing Party not to disclose such information to others; or
8.1.5.was subsequently developed by the receiving Party or its Affiliates without use of or reference to the Confidential Information of the disclosing Party as demonstrated by competent written records.
Licensed Know-How and unpublished Licensed Patents (to the extent that such Licensed Know-How or unpublished Licensed Patents are not subject to any of the foregoing exceptions set forth under this Section 8.1) will be considered Confidential Information of Alkermes, provided that Biogen may use or disclose such Licensed Know-How and Licensed Patents in
38
accordance with Article 8. Notwithstanding anything to the contrary set forth in this Agreement, during the Term Alkermes will use at least the same degree of care to protect the secrecy of such Licensed Know-How and unpublished Licensed Patents that it uses to prevent the disclosure of its own other confidential information of similar importance and in any event a reasonable duty of care.
8.2.Authorized Use and Disclosure. Each Party will maintain the Confidential Information of the other Party in confidence and may use the Confidential Information of the other Party only in performance of its obligations under this Agreement and any Supply Agreement. Each Party may disclose such Confidential Information to its employees, Affiliates, sublicensees, agents, consultants or other Third Parties who need to know such Confidential Information in connection with the performance of such Party’s obligations under this Agreement or any Supply Agreement and who are bound by obligations of confidentiality and non-use at least substantially equivalent to the obligations of this Article 8. Each Party will be liable for any unauthorized use or disclosure of Confidential Information by its employees, Affiliates, sublicensees, agents, consultants or other Third Parties to which it has disclosed or transferred such Confidential Information.
Without limiting the generality of the foregoing paragraph, a Party may disclose Confidential Information of the other Party to the extent that such disclosure is reasonably necessary in connection with:
8.2.1.filing or prosecuting patent or trademark applications relating to the Products;
8.2.2.prosecuting or defending litigation relating to the Products;
8.2.3.Exploiting the Products;
8.2.4.seeking Regulatory Approval of a Product, including Regulatory Approval of a Manufacturing facility for a Product;
8.2.5.seeking reimbursement or pricing approvals for a Product from Governmental Authorities;
8.2.6.complying with Applicable Laws, including securities laws and the rules of any securities exchange or market on which a Party’s or its Affiliates’ securities are listed or traded; or
8.2.7.complying with subpoenas or requests for information from Governmental Authorities.
In making any disclosures set forth in Section 8.2.1 through Section 8.2.7 above, the disclosing Party will, except where impracticable for necessary disclosures (as in the event of medical emergency), give such advance notice to the other Party of such disclosure requirement as is reasonable under the circumstances and, except to the extent inappropriate (as in the case of patent applications), use its reasonable efforts to cooperate with the other Party in order to secure confidential treatment of such Confidential Information required to be disclosed, except to the
39
extent that the disclosing Party receives advice from its legal counsel or independent registered public accounting firm that such information is required to be disclosed under Applicable Laws, including securities laws and the rules of any securities exchange or market on which a Party’s or its Affiliates’ securities are listed or traded.
8.3.Disclosure to Investors. The Parties acknowledge that each Party may, from time to time, engage or have engaged in fundraising or other business activities. The Parties may disclose a copy of this Agreement, under terms of confidentiality no less strict than those contained in this Agreement, to their respective actual or bona fide potential investors or business partners (and to their respective bankers, lawyers, accountants and agents); provided that any such copy may be redacted to remove any confidential, proprietary or competitive information of the disclosing Party and any other information not necessary in connection with their evaluation of such potential or actual investment.
8.4.Survival. This Article 8 will survive the termination or expiration of this Agreement for a period of [**] years.
8.5.Publications or Presentations.
8.5.1.General. Biogen shall have the sole right, in its sole discretion, to present at symposia, national or regional professional meetings and to publish in journals regarding the Products and any activities under this Agreement, provided that any such presentation or publication shall not include any Confidential Information of Alkermes. Alkermes shall have no right to present at symposia, national or regional professional meetings or to publish in journals regarding the Products or any activities under this Agreement.
8.5.2.Publicity. A joint press release approved by each Party announcing this Agreement is attached hereto as Exhibit F. Biogen retains the right to make publications about its activities under the Agreement (i) prior to the Transfer Completion Date and with respect to the Alkermes 8700 Product, with the consent of Alkermes, which consent shall not be unreasonably withheld or delayed, and (ii) (a) with respect to any Product other than the Alkermes 8700 Product and, (b) following the Transfer Completion Date, with respect to the Alkermes 8700 Product, in each case (a) and (b), without the consent of Alkermes. Neither Party shall be required to seek the permission of the other Party to repeat any information regarding the terms of this Agreement or the arrangements hereunder to the extent the same has already been publicly disclosed by such Party or by the other Party; provided that such information remains true, correct and consistent with the most recent information related thereto that has been publically disclosed. Routine references to this Agreement and the arrangements hereunder in the context of disclosures or publications regarding a Party’s business in general will be allowed in the usual course of a Party’s business, including the use of other Party’s name. Each Party may use the other Party’s corporate logo(s) or Product Trademarks only with the prior written consent of the other Party.
40
9.1.Up-Front Payment. Within ten (10) Business Days after the Effective Date, as an upfront, one-time, nonrefundable and non-creditable fee in consideration of the grant of the licenses set forth in Section 6.1, Biogen will pay to Alkermes the amount of Twenty Eight Million U.S. Dollars (US$28,000,000) (the “Up-Front Payment”).
9.2.Option Payment. Within [**] days after Biogen’s receipt from Alkermes of the Initial GI Tolerability Data Package in accordance with Section 3.2.4(i), subject to any extension pursuant to Section 3.2.4(iii), Biogen will make a one-time, nonrefundable and non-creditable payment to Alkermes in the amount of Fifty Million U.S. Dollars (US$50,000,000) (the “Option Payment”), unless, following the Parties’ discussions as set forth in Section 3.2.4(iv), the Parties jointly agree in writing not to conduct Part B of the GI Tolerability Clinical Trial because the rate of discontinuations due to a GI Event in Part A of the GI Tolerability Clinical Trial is lower in the Tecfidera® arm of such trial (in which case GI Inferiority will be deemed to exist and Biogen will not be obligated to make the Option Payment). If Biogen does not make the Option Payment in accordance with this Section 9.2 for any reason other than the Parties jointly agreeing in writing not to conduct Part B of the GI Tolerability Clinical Trial because the rate of discontinuations due to a GI Event in Part A of the GI Tolerability Clinical Trial is lower in the Tecfidera® arm of such trial, and Biogen fails to make such Option Payment within five (5) Business Days following written notice from Alkermes of such failure, then Biogen will be deemed to have given notice of termination for convenience in accordance with Section 13.3.
9.3.NDA Approval Payment. If the Alkermes 8700 Product 505(b)(2) NDA approval is obtained on or prior to December 31, 2021, then within [**] days after the Transfer Completion Date, Biogen will make a one-time, nonrefundable and non-creditable payment to Alkermes in the amount of One Hundred Fifty Million U.S. Dollars (US$150,000,000).
9.4.Development Milestones for Products other than the Alkermes 8700 Product. As further consideration of the grant of the licenses set forth in Section 6.1 and the performance of Alkermes’ other obligations hereunder, Biogen shall pay to Alkermes the amounts set forth below no later than [**] days after the earliest date on which the corresponding milestone event has first been achieved with respect to the first two Products other than the Alkermes 8700 Product:
|
Development Milestone Event |
Amount |
|
The first administration to the first patient in a Clinical Trial of such Product. |
[**] |
|
The first administration of such Product to the first patient in a phase 3 Clinical Trial. |
[**] |
|
Receipt of Regulatory Approval of an NDA from the FDA in the U.S. for such Product. |
[**] |
41
The milestone payments set forth in this Section 9.4 will be paid on a Product-by-Product basis on the first occurrence of each such applicable milestone for each of the first two Products (other than the Alkermes 8700 Product).
9.5.1.Royalty Payments for Alkermes 8700 Product.
(i)Royalty Percentages. As further consideration of the grant of the licenses set forth in Section 6.1 and the performance of Alkermes’ other obligations hereunder, Biogen will pay to Alkermes royalty payments on Net Sales of the Alkermes 8700 Product in the Territory on a country-by-country basis during the applicable Royalty Term at the rate of [**] percent ([**]%) of Net Sales. Notwithstanding the foregoing, in the event of a determination of GI Inferiority, if Biogen has made the Option Payment to Alkermes, then the royalty rate during each Royalty Term for the Alkermes 8700 Product in each country in the Territory shall be [**] percent ([**]%) of Net Sales until such time as the aggregate royalty payments paid to Alkermes across all countries equal Fifty Million U.S. Dollars ($50,000,000), after which time such royalty rate shall return to its prior level, before the determination of GI Inferiority that resulted in such royalty rate of [**] percent ([**]%) (but subject in any event to Section 9.5.5, Section 9.5.6 and Section 9.5.7).
(ii)Minimum Annual Payments. As further consideration for the grant of the licenses set forth in Section 6.1 and the performance of Alkermes’ other obligations hereunder, Biogen will pay Alkermes a minimum aggregate royalty payment during each twelve (12)-month period (each, a “Minimum Annual Payment” and together, the “Minimum Annual Payments”) in the amounts set forth in the table below, commencing on the first day of the first month following the date of the First FDA Approval (such first day of such first month, the “Minimum Payment Commencement Date”) and continuing for a period of five (5) years, unless earlier terminated as set forth in Section 9.5.1(iii) (the “Minimum Annual Payment Term”).
42
For clarity, for each twelve (12)-month period during the Minimum Annual Payment Term in which the aggregate amount payable to Alkermes as royalties under Section 9.5.1(i) is less than the applicable Minimum Annual Payment, Biogen shall pay Alkermes an amount equal to the applicable Minimum Annual Payment for such twelve (12)-month period, minus the aggregate amount already paid to Alkermes as royalties under Section 9.5.1(i) for such twelve (12)-month period.
|
Twelve (12)-Month Period |
Minimum Annual Payment |
|
First twelve (12)-month period following the Minimum Payment Commencement Date |
[**] |
|
Second twelve (12)-month period following the Minimum Payment Commencement Date |
[**] |
|
Third twelve (12)-month period following the Minimum Payment Commencement Date |
[**] |
|
Fourth twelve (12)-month period following the Minimum Payment Commencement Date |
[**] |
|
Fifth twelve (12)-month period following the Minimum Payment Commencement Date |
[**] |
(iii)Termination of Minimum Annual Payments. Notwithstanding anything to the contrary in Section 9.5.1(ii), the Minimum Annual Payment Term shall immediately terminate, and no further Minimum Annual Payments shall be payable under this Agreement, if any of the following conditions is met:
(A)(i) [**] and (ii) [**];
Notwithstanding anything to the contrary, if any of the foregoing conditions in clauses (A) through (C) exist prior to the Minimum Payment Commencement Date, then the Minimum Annual Payment Term shall be deemed not to commence and no Minimum Annual Payments shall be payable under this Agreement.
9.5.2.Royalty Payments for Products other than the Alkermes 8700 Product. As further consideration of the grant of the licenses set forth in Section 6.1 and the performance of Alkermes’ other obligations hereunder, Biogen will pay to Alkermes tiered royalty payments on annual aggregate worldwide Net Sales of Products, other than the Alkermes 8700 Product, on a Product-by-Product basis during the applicable Royalty Term as follows: (i) for Net Sales less than or equal to [**] U.S. Dollars (US$[**]), [**] percent ([**]%) of such Net Sales and (ii) for Net Sales greater than [**] U.S. Dollars (US$[**]), [**] percent ([**]%) of such Net Sales.
9.5.3.Duration of Royalty Payments. Biogen will pay royalties to Alkermes, as set forth in Section 9.5.1(i) and Section 9.5.2, on a country-by-country and
43
Product-by-Product basis, during the period commencing on the First Commercial Sale of a Product in a country and ending on the later of (i) the expiration of all Valid Claims of the Joint Collaboration Patents and Licensed Patents that Cover the use or sale of such Product in such country and (ii) [**] years after the First Commercial Sale of such Product in such country (any such period, a “Royalty Term”). Following expiration of the Royalty Term for any Product in a country, no further royalties shall be payable in respect of sales of such Product in such country and, thereafter, the license granted to Biogen under Section 6.1.1 with respect to such Product in such country shall be non-exclusive, fully paid-up, perpetual, irrevocable and royalty-free.
9.5.4.Cumulative Royalties. The obligation to pay royalties under this Agreement shall be imposed only once with respect to a single unit of a Product regardless of how many Valid Claims of the Licensed Patents Cover the use or sale of such Product in the applicable country.
9.5.5.No Valid Claim. On a country-by-country and Product-by-Product basis, in any country in which a Product is Commercialized and there are no remaining Valid Claims of the Licensed Patents that Cover the use or sale of such Product in such country, the royalties payable to Alkermes on Net Sales of such Product pursuant to (i) Section 9.5.1(i) will be reduced to [**] percent ([**]%) of such Net Sales for the Alkermes 8700 Product and (ii) Section 9.5.2 will be reduced to [**] percent ([**]%) of the applicable royalty rate for any Product (other than the Alkermes 8700 Product).
9.5.6.Third Party Payment Obligations. If Biogen or its Affiliates or Sublicensees are required to make any payments (including upfront fees, milestones or royalties) to a Third Party to obtain rights to any intellectual property that is necessary to Exploit a Product in the Field in any country, then Biogen may deduct up to [**] percent ([**]%) of such Third Party payments as and when incurred from any royalty payment or Minimum Annual Payment due to Alkermes under Section 9.5.1 or Section 9.5.2 with respect to such Product and such country; provided that Biogen may not deduct from any payments due to Alkermes hereunder any such Third Party payments due under any arrangement entered into prior to the Effective Date.
9.5.7.Royalty Floor. Notwithstanding anything to the contrary herein, in no event shall the royalty payments or Minimum Annual Payments due to Alkermes under Section 9.5.1 or Section 9.5.2 for any Product in a given Calendar Quarter be reduced as a result of the application of the reductions and offsets described in this Section 9.5 below [**] percent ([**]%) of the amounts otherwise payable to Alkermes under Section 9.5.1 or Section 9.5.2 with respect to such Product. Any reductions or offsets that are not used to reduce payments under Section 9.5.1 or Section 9.5.2 in a given Calendar Quarter as a result of the foregoing limitations may be carried over to reduce payments due under Section 9.5.1 and Section 9.5.2 in subsequent Calendar Quarters.
9.6.Reporting and Paying Net Sales. For each Calendar Quarter for which royalties are payable by Biogen to Alkermes pursuant to Section 9.5.1(i) or Section 9.5.2, Biogen will (i) deliver to Alkermes, within five (5) days after the end of each such Calendar Quarter, a non-binding estimated report prepared in good faith, (ii) deliver to Alkermes, within forty-five (45) days after the end of each such Calendar Quarter a true and accurate report, in each case,
44
providing in reasonable detail (A) an accounting of all Net Sales made on a country-by-country and Product-by-Product basis in the Territory during such Calendar Quarter, including the amount of gross sales of Products and the aggregate allowable deductions therefrom, (B) the number of units of Products sold, (C) the currency conversion rates used, (D) the U.S. Dollar-equivalent of such Net Sales during such Calendar Quarter and (E) a calculation of the amount of royalty payment due on such Net Sales, and (iii) within forty-five (45) days after the end of each such Calendar Quarter, pay Alkermes the royalties due under Section 9.5.1(i) and Section 9.5.2 with respect to such Calendar Quarter as provided for in the report delivered under (ii) above. In addition, within forty-five (45) days of the end of the first Calendar Quarter following each twelve (12)-month period during the Minimum Annual Payment Term, Biogen shall pay Alkermes any amount due under Section 9.5.1(ii) for such twelve (12)-month period of the Minimum Annual Payment Term. Each report delivered hereunder shall be considered Confidential Information of Biogen, subject to the terms and conditions of Article 8 hereof. Any payments due hereunder for less than a full Calendar Quarter will be prorated.
9.7.Records and Reporting; Audits.
9.7.1.Records and Reporting. Each Party shall keep, and shall cause its Affiliates and Sublicensees to keep, such accurate and complete records of Net Sales and its Development Costs as are necessary to determine the amounts due to Alkermes under this Agreement, including time records. Records of Net Sales and Development Costs shall be retained by each Party or any of its Affiliates and Sublicensees (in such capacity, the “Recording Party”) for three (3) years following the end of the Calendar Year to which they pertain.
9.7.2.Audits. During normal business hours and with reasonable advance notice to the Recording Party, such records shall be made available for inspection, review and audit, at the request and expense of the other Party (the “Auditing Party”), by an independent certified public accountant, appointed by such Auditing Party and reasonably acceptable to the Recording Party, for the sole purpose of verifying the accuracy of the Recording Party’s accounting reports and payments made or to be made pursuant to this Agreement or any Supply Agreement; provided, however, that such audits may not be performed by the Auditing Party more than once per Calendar Year, that such audits may only cover records pertaining to any period commencing not more than two (2) Calendar Years prior to the date of such audit, and that such Auditing Party shall not be permitted to audit the same period of time more than once. Such accountants, prior to any review hereunder, shall have entered into an appropriate confidentiality agreement with the Recording Party on mutually acceptable terms and shall have been instructed not to reveal to the Auditing Party the details of their review, except for (i) such information as is required to be disclosed under this Agreement and (ii) such information presented in a summary fashion as is necessary to report the accountants’ conclusions to the Auditing Party. The report prepared by such accountants shall be sent or otherwise provided to the Recording Party by such accountants at the same time it is sent or otherwise provided to the Auditing Party. All costs and expenses incurred in connection with performing any such audit shall be paid by the Auditing Party unless the audit uncovers a net underpayment of amounts owed or overreporting of expenses by a Recording Party of five percent (5%) of total amounts owed or expenses reported by such Recording Party for any Calendar Year period covered by the audit, in which case the Recording Party will bear the full cost of such audit. If either Party is found to have been underpaid any amounts payable to such
45
Party hereunder or to have overpaid to the other Party any amounts payable hereunder, such first Party will be entitled to recover any undisputed discrepancy, plus interest calculated in accordance with Section 9.9, within forty-five (45) days after receipt of such audit report. If either Party disagrees with any discrepancy identified during the course of any audit conducted pursuant to this Section 9.7.2, then either Party may submit the issue for resolution in accordance with Article 12.
9.8.Manner of Payments. All sums due to Alkermes or Biogen under this Agreement shall be payable in U.S. Dollars by bank wire transfer in immediately available funds to such bank account(s) as Alkermes and Biogen, respectively, shall designate from time to time. Each Party shall endeavor to notify the other Party as to the date and amount of any such wire transfer to the other Party at least two (2) Business Days prior to such transfer, but in no event later than the Business Day of such transfer.
9.9.Interest on Late Payments. Without limitation on other available rights or remedies, any payments or portions thereof due hereunder that are not paid at the latest five (5) days following the date such payments are due under this Agreement will bear interest at the lower of (i) the Prime Rate as determined by Bank of America in effect on the due date, or (ii) the maximum rate permitted by Applicable Law, calculated on the number of days such payment is delinquent.
9.10.Currency of Payments/Exchange Rates. All payments to be made under this Agreement will be made in U.S. Dollars. The royalty due on Net Sales and the price for any applicable Product sold in the Territory (other than in the U.S.) will be calculated on the basis of the local currency sales figures translated into U.S. Dollars according to Biogen’s standard currency translation methodology. As of Effective Date, Biogen converts revenue in local currency using the average FX rates for the month downloaded from the Bloomberg end of day rates on the second last business day of the month. The methodology employed by Biogen will be that methodology used by Biogen from time to time in the translation of its foreign currency operating results for external reporting and will be consistent with GAAP, and in any event will not be impacted by Biogen’s hedging program.
9.11.1.Withholding. Biogen will make all payments to Alkermes under this Agreement without deduction or withholding except to the extent that any such deduction or withholding is required by Applicable Law. It is the intention of the Parties that all payments will be made by Biogen hereunder from its offices in Zug, Switzerland. No such payments will be made by Biogen hereunder from any other jurisdiction, unless Biogen and Alkermes agree in advance that such payments would not adversely affect the withholding tax position of such payments. In the event of Biogen’s breach of this Section 9.11.1 resulting in an adverse effect on Alkermes’ withholding tax position of such payments, Biogen shall make true-up payments to Alkermes as compensation for such adverse effect.
9.11.2.Payment of Taxes. Any tax required to be withheld by Applicable Law on amounts payable under this Agreement will promptly be paid by Biogen on behalf of Alkermes or Alkermes on behalf of Biogen, as applicable, to the appropriate Governmental
46
Authority, and the Party making such payment will furnish the other Party with proof of payment of such tax within one (1) calendar month of such payment. The Party making tax payments hereunder will give ten (10) days’ advance notice of its intention to begin withholding any such tax in advance of such withholding.
9.11.3.Cooperation and Documentation. Biogen and Alkermes will cooperate (i) in all respects necessary to take advantage of any double taxation agreements or similar agreements as may, from time to time, be available in order for the payments under this Agreement to be made without any deduction or withholding and (ii) with respect to producing all documentation required by any Governmental Authority including an IRS Form W-8BEN-E with respect to taxes or as reasonably requested by Biogen or Alkermes, as applicable, to secure a reduction in the rate of applicable withholding taxes or to secure a credit or refund for withheld taxes. Alkermes shall prepare and deliver to Biogen a complete, accurate IRS Form W-8BEN-E for the Up-Front Payment within three (3) days after the Effective Date.
9.11.4.Value Added Tax. All payments to Alkermes under this Agreement are exclusive of any applicable value added tax (“VAT”), for which, if applicable, Biogen will be responsible; provided that Alkermes will issue an appropriate VAT invoice to Biogen.
9.11.5.Pharmaceutical Excise Taxes. Biogen may invoice Alkermes each Calendar Quarter for its pro rata share of any pharmaceutical excise taxes (based on the applicable Product royalty rate(s) in effect at the time of such Product sales to which the tax applies) imposed by the United States Patient Protection and Affordable Care Act of 2010 due with respect to sales of the Products in the U.S. at the time Biogen accrues such taxes in accordance with GAAP. Biogen will provide a summary of the amount invoiced and a roll-forward of the accrual balance. Alkermes will, within forty-five (45) days after the receipt of an invoice from Biogen for such pharmaceutical excise taxes, pay Biogen the undisputed amounts set forth in such invoice. Alkermes will notify Biogen of any disputed amounts within ten (10) days of receipt of any such invoice and will work with Biogen to resolve such issues on a timely basis. If such issue cannot be resolved by the Parties, then such matter will be resolved in accordance with the dispute resolution process set forth in Article 12. If the amount of such pharmaceutical excise taxes owed by Biogen differs from the amount accrued by Biogen in respect of such taxes for the applicable period, then Biogen will reflect such differences in Alkermes’ next accrual payment.
Article 10
REPRESENTATIONS AND WARRANTIES; COVENANTS; DISCLAIMER
10.1.Disclaimer. EXCEPT AS EXPRESSLY PROVIDED IN THIS AGREEMENT, EACH PARTY DISCLAIMS ALL REPRESENTATIONS AND WARRANTIES, EXPRESS OR IMPLIED, INCLUDING WARRANTIES OF COMMERCIAL UTILITY, MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, VALIDITY OR SCOPE OF PATENT RIGHTS OR NON-INFRINGEMENT OF THIRD PARTY INTELLECTUAL PROPERTY RIGHTS. Each Party acknowledges and agrees that nothing in this Agreement will be construed as representing any estimate or projection of (a) the successful Development or
47
Commercialization of any Product under this Agreement, (b) the number of Products that will or may be successfully Developed or Commercialized under this Agreement, (c) anticipated sales or the actual value of any Products that may be successfully Developed or Commercialized under this Agreement or (d) the damages, if any, that may be payable if this Agreement is terminated for any reason. Without limiting the foregoing, Biogen makes no representation, warranty or covenant, either express or implied, that (i) it will successfully Develop, Manufacture, Commercialize or continue to Commercialize any Product in any country, (ii) if Commercialized, that any Product will achieve any particular sales level, whether in any individual country or cumulatively throughout the Territory or (iii) other than is expressly required under Section 4.2, that it will devote, or cause to be devoted, any level of diligence or resources to Developing or Commercializing any Product in any country, or in the Territory in general.
10.2.Mutual Representations, Warranties and Covenants. Each Party represents, warrants and covenants to the other as of the Effective Date as follows:
10.2.1.this Agreement has been duly executed and delivered by such Party and constitutes the valid and binding obligation of such Party, enforceable against such Party in accordance with its terms, except as enforceability may be limited by bankruptcy, fraudulent conveyance, insolvency, reorganization, moratorium and other Applicable Laws relating to or affecting creditors’ rights generally and by general equitable principles;
10.2.2.such Party has the full right, power and authority to execute, deliver and perform this Agreement;
10.2.3.the execution, delivery and performance of this Agreement have been duly authorized by all necessary action on the part of such Party and its officers and directors;
10.2.4.the execution, delivery and performance of this Agreement do not breach, violate, contravene or constitute a default under any contracts, arrangements or commitments to which such Party is a party or by which it is bound nor do the execution, delivery and performance of this Agreement by such Party violate any order or Applicable Law of any court or Governmental Authority having authority over it; and
10.2.5.such Party will not enter into any contract, arrangement or commitment in the future that conflicts with or violates any term or provision of this Agreement.
10.3.Alkermes Representations and Warranties. Alkermes further represents and warrants to Biogen as of the Effective Date as follows:
10.3.1.Exhibit B contains a complete and correct list of all Patent Rights Controlled by Alkermes or its Affiliates as of the Effective Date that (i) are necessary for the Exploitation of the Alkermes 8700 Product in the Field in the Territory or (ii) Cover any Know-How Controlled by Alkermes or its Affiliates on the Effective Date that (a) is or was used in the Development or Manufacture of Products or (b) is or was embodied in Products;
48
10.3.2.Alkermes has and will have the full right, power and authority to grant, and is not required to obtain the consent of any Third Party to grant, the rights and licenses granted to Biogen under Article 6;
10.3.3.Except as set forth in Schedule 10.3.3, Alkermes owns the entire right, title and interest in and to the Licensed Patents and the Licensed Know-How, free of any encumbrance, lien, charge, license grant, option grant or other burden, and any such encumbrance, lien, charge, license grant, option grant or other burden set forth in Schedule 10.3.3 will not negatively affect Alkermes’ right to grant the license to Biogen pursuant to Article 6;
10.3.4.the polymorph used by Alkermes in the Alkermes 8700 Product was disclosed in WO2014/152494, which published September 25, 2014 and is owned or otherwise Controlled by Alkermes, as compound (14) of Example 1, and which is further characterized in Example 7 and Figure 7 therein;
10.3.5.the data provided by Alkermes to Biogen that characterizes the polymorph used by Alkermes in the Alkermes 8700 Product, including the x-ray powder diffraction data, is true and complete and correct in all material respects;
10.3.6.Alkermes has complied in all material respects with all Applicable Laws in connection with the Prosecution of the Licensed Patents, including, with respect to any issued patents and pending patent applications, any disclosure requirements of the United States Patent and Trademark Office or any other Governmental Authority and has timely paid all filing and renewal fees payable with respect thereto;
10.3.7.Alkermes has obtained, or caused its Affiliates, as applicable, to obtain, assignments from the inventors of all inventorship rights to the Licensed Patents, and all such assignments are valid and enforceable, and the inventorship of the Licensed Patents is properly identified on each patent or patent application;
10.3.8.to Alkermes’ Knowledge, no Third Party is infringing any Licensed Patent;
10.3.9.to Alkermes’ Knowledge, the Exploitation of the Alkermes 8700 Product in the Field in the Territory does not infringe any issued Patent Right of any Third Party and Alkermes has disclosed to Biogen, to Alkermes’ Knowledge, any pending Patent Rights that, if issued, may be infringed by the Exploitation of the Alkermes 8700 Product in the Field in the Territory;
10.3.10.Alkermes has not received notice of any claims, and there are no judgments or settlements against or owed by Alkermes or, to Alkermes’ Knowledge, any pending or threatened claims or litigation, in each case, claiming that a Patent Right owned by such Third Party would be infringed by Exploitation of the Products in the Field in the Territory;
10.3.11.to Alkermes’ Knowledge, Alkermes has the right to use, and to permit Biogen, Biogen’s Affiliates and Biogen’s Sublicensees to use, the Licensed Know-How for all permitted purposes under this Agreement;
49
10.3.12.to Alkermes’ Knowledge, the Manufacture of the Alkermes 8700 Product as conducted or as currently planned to be conducted (including for Commercial Supplies), in each case, as of the Effective Date does not and will not infringe any Patent Right of any Third Party or misappropriate any Know-How of any Third Party (for clarity, this provision applies to Patent Rights and Know-How of Third Parties that Cover the process of manufacturing the Alkermes 8700 Product);
10.3.13.Alkermes and its Affiliates have taken commercially reasonable measures consistent with industry practices to protect the secrecy, confidentiality and value of all Licensed Know-How that constitutes trade secrets under Applicable Law (including requiring all employees, consultants and independent contractors to execute binding and enforceable agreements requiring all such employees, consultants and independent contractors to maintain the confidentiality of such Licensed Know-How) and, to Alkermes’ Knowledge, such Licensed Know-How has not been used, disclosed to or discovered by any Third Party except pursuant to such confidentiality agreements and there has not been a breach by any party to such confidentiality agreements;
10.3.14.the Licensed Patents are, to Alkermes’ Knowledge, valid and enforceable, and no Third Party has made any claim made against Alkermes or its Affiliates asserting the invalidity, unenforceability or non-infringement of any Licensed Patents (including, by way of example, through the institution or written threat of institution of interference, nullity, opposition, inter partes or post-grant review or similar invalidity proceedings before the United States Patent and Trademark Office or any analogous foreign Governmental Authority);
10.3.15.Except as set forth in Schedule 10.3.3, the Licensed Patents and Licensed Know-How are not subject to any funding agreement with any Governmental Authority or any other Third Party, and are not subject to the requirements of the Bayh-Dole Act or any similar provision of any Applicable Law;
10.3.16.neither Alkermes nor any of its Affiliates (i) are subject to any obligation to or with any Third Party that causes Alkermes or its Affiliates not to Control (or otherwise not have rights to) any Patent Right or Know-How that would, but for such obligation, be included in the Licensed Patents or the Licensed Know-How or (ii) hold for use or otherwise have rights to, but do not Control, any Patent Rights or Know-How that would otherwise be included in the Licensed Patents or Licensed Know-How if such Patent Rights or Know-How were Controlled by Alkermes or an Affiliate;
10.3.17.there is no action, claim, demand, suit, proceeding, arbitration, grievance, citation, summons, subpoena, inquiry or investigation of any nature, civil, criminal, regulatory or otherwise, in law or in equity, pending or, to Alkermes’ Knowledge, threatened, with any judicial or arbitrative body against Alkermes or any of its Affiliates in connection with the Licensed Patents, the Licensed Know-How or the Products;
10.3.18.the Development and Manufacture of the Alkermes 8700 Product have been conducted in all material respects in accordance with Applicable Law;
50
10.3.19.in the Development and Manufacture of the Alkermes 8700 Product, Alkermes is not, as of the Effective Date, using any employee who is debarred by the FDA or any other Regulatory Authority, or who is the subject of debarment proceedings by the FDA or any such Regulatory Authority;
10.3.20.to Alkermes’ Knowledge, in the Development and Manufacture of the Alkermes 8700 Product, Alkermes has not previously used any employee and has not previously used, and is not currently using, any consultant, in each case, who is or has been debarred by the FDA or any other Regulatory Authority, or, to Alkermes’ Knowledge, who is or has been the subject of debarment proceedings by the FDA or any such Regulatory Authority; and
10.3.21.Alkermes has not knowingly withheld from Biogen information relating to the Licensed Patents, the Licensed Know-How and the Alkermes 8700 Product, in each case, that Alkermes reasonably believes would be material to Biogen’s decision to enter into this Agreement and undertake the commitments and obligations set forth herein.
10.4.Biogen Representations and Warranties. Biogen further represents and warrants to Alkermes that, as of the Effective Date, Biogen has undertaken an assessment of the reportability of the transactions contemplated by this Agreement under the HSR Act, and following such assessment, Biogen has concluded that pursuant to 16 CFR 801.10, a filing under the HSR Act is not required as of the Effective Date.
10.5.Responsibility for Government Approvals. Biogen, as the acquirer of the licenses granted in Article 6, shall determine, control and direct strategy regarding requesting Governmental Approvals in connection with the transactions contemplated by this Agreement, including with respect to determining the necessity of requesting Governmental Approvals for the transactions contemplated by this Agreement by any Governmental Authority, and if applicable, all matters relating to any review by any Governmental Authority, or any litigation by, or negotiations with, any Governmental Authority related thereto, and will take the lead in all meetings, discussions, and communications, if any, with any Governmental Authority relating to requesting or obtaining all Governmental Approvals for the transactions contemplated by this Agreement (provided that this Section 10.5 shall not apply to any Regulatory Approvals that may be required to Exploit the Alkermes 8700 Product prior to the Transfer Completion Date).
11.1.Limitation of Liability. EXCEPT FOR (i) LIABILITY FOR EITHER PARTY’S BREACH OF ARTICLE 8, (ii) THE PARTIES’ INDEMNIFICATION OBLIGATIONS PURSUANT TO SECTIONS 11.2 AND 11.3 OR (iii) ANY LIABILITY ARISING FROM A PARTY’S FRAUD OR WILLFUL MISCONDUCT, NEITHER PARTY WILL BE LIABLE TO THE OTHER PARTY OR ANY OF SUCH OTHER PARTY’S REPRESENTATIVES OR STOCKHOLDERS FOR ANY INDIRECT, INCIDENTAL, SPECIAL, PUNITIVE, EXEMPLARY OR CONSEQUENTIAL DAMAGES OR LOST PROFITS OR LOST REVENUES ARISING OUT OF OR RESULTING FROM THIS
51
AGREEMENT, REGARDLESS OF WHETHER IT HAS BEEN INFORMED OF THE POSSIBILITY OR LIKELIHOOD OF SUCH DAMAGES OR THE TYPE OF CLAIM, CONTRACT OR TORT (INCLUDING NEGLIGENCE).
11.2.Biogen Indemnification. Biogen will indemnify, defend and hold harmless Alkermes and its Affiliates and their respective directors, officers, employees and agents (each an “Alkermes Indemnified Party”) from and against all costs, losses, liabilities, expenses (including reasonable attorneys’ fees, experts’ fees and other costs of investigation or defense at any stage of the proceedings) and damages (collectively, “Losses”) to the extent relating to a claim, action or demand (“Claim”) by a Third Party or Governmental Authority arising out of or resulting from:
11.2.1.any breach of this Agreement by Biogen;
11.2.2.the violation of any Applicable Law by or on behalf of Biogen, its Affiliates or its Sublicensees;
11.2.3.Development or Commercialization of any Product in the Field in the Territory by or on behalf of Biogen, its Affiliates or its Sublicensees, including use of the Products by Third Parties;
11.2.4.a trademark infringement action pursuant to Section 7.9.2; or
11.2.5.the gross negligence or willful misconduct of any Biogen Indemnified Party;
except to the extent such Claim is subject to an indemnification, defense or hold harmless obligation of Alkermes set forth in Section 11.3 or in any Supply Agreement.
11.3.Alkermes Indemnification. Alkermes will indemnify, defend and hold harmless Biogen and its Affiliates and their respective directors, officers, employees and agents (each a “Biogen Indemnified Party”) from and against all Losses relating to a Claim by a Third Party or Governmental Authority to the extent arising out of or resulting from:
11.3.1.any breach of this Agreement by Alkermes;
11.3.2.the violation of any Applicable Law by or on behalf of Alkermes or its Affiliates;
11.3.3.Development of any Product in the Field in the Territory by or on behalf of Alkermes or its Affiliates;
11.3.4.Development or Commercialization of the Alkermes 8700 Product by or on behalf of Alkermes or its Affiliates after the effective date of termination of this Agreement with respect to the Alkermes 8700 Product or in its entirety; or
11.3.5.the gross negligence or willful misconduct of any Alkermes Indemnified Party;
52
except to the extent such Claim is subject to an indemnification, defense or hold harmless obligation of Biogen set forth in Section 11.2 or in any Supply Agreement.
11.4.Indemnification Procedures. In the event of any Claim by a Third Party or Governmental Authority against any Alkermes Indemnified Party or Biogen Indemnified Party (individually, an “Indemnitee”), the indemnified Party shall promptly notify the other Party in writing of the Claim and the indemnifying Party shall manage and control, at its sole expense, the defense of the Claim and any settlement thereof. The Indemnitee shall cooperate with the indemnifying Party and may, at its option and expense, be represented in any such action or proceeding. The indemnifying Party shall not be liable for any settlements, litigation costs or expenses incurred by any Indemnitee without the indemnifying Party’s prior written authorization. Notwithstanding the foregoing, if the indemnifying Party believes that any of the exceptions to its obligation of indemnification of the Indemnitees set forth in Sections 11.2 or 11.3, as applicable, may apply, the indemnifying Party shall promptly notify the Indemnitees, which may be represented in any such action or proceeding by separate counsel at their expense; provided, however, that the indemnifying Party shall be responsible for payment of such expenses if the Indemnitees are ultimately determined to be entitled to indemnification from the indemnifying Party. Notwithstanding any other provision of this Article 11 to the contrary, no Indemnitee under this Agreement shall be required to waive a conflict of interest under any applicable rules of professional ethics or responsibility if such waiver would be required for a single law firm to defend both the indemnifying Party and one or more Indemnitees. In such case, the indemnifying Party shall provide a defense of the affected Indemnitees through a separate law firm reasonably acceptable to the affected Indemnitees at the indemnifying Party’s expense. Except with the approval of an Indemnitee, which approval will not be unreasonably withheld or delayed, the indemnifying Party will not consent to entry of any judgment or enter into any settlement that would admit any wrongdoing by, or result in injunctive or other relief being imposed against, an Indemnitee.
11.5.Cooperation. The indemnified Party and each Indemnitee will cooperate in the defense or prosecution of any action or proceeding with respect to which it is being indemnified and will furnish such records, information and testimony, provide such witnesses and attend such conferences, discovery proceedings, hearings, trials and appeals as may be reasonably requested by the indemnifying Party in connection with such action or proceeding. Such cooperation will include access during normal business hours afforded to the indemnifying Party to, and reasonable retention by the indemnified Party and the Indemnitee of, records and information that are reasonably relevant to such action or proceeding, and making Indemnitees and other employees and agents available on a mutually convenient basis to provide additional information and explanation of any material provided hereunder, and the indemnifying Party will reimburse the indemnified Party for all its reasonable out-of-pocket expenses incurred in connection with such cooperation.
11.6.Insurance. As of the Effective Date each Party shall procure and maintain, at its sole cost and expense, commercial general liability insurance and products liability coverage in amounts not less than Ten Million U.S. Dollars (US$10,000,000) per incident and Twenty Million U.S. Dollars (US$20,000,000) annual aggregate. In the event of an indemnification claim pursuant to Sections 11.2 or 11.3 above, such insurance will be primary to any insurance owned, secured or put in place by the Indemnitee. All such policies will be written by insurance
53
companies with an A.M. Best’s rating (or its equivalent) of A-VII or higher. In the event that any of these policies are written on a claims-made basis, then such policies shall be maintained during the Term and until the later of (i) three (3) years after expiration of Term or (ii) sixty (60) days following expiration of all applicable statutes of limitation for any potential Claims that may be indemnified Losses pursuant to Sections 11.2 or 11.3, as applicable. Upon written request, each Party will provide the other Party with a certificate of insurance attesting to such coverage. The minimum amounts of insurance coverage required under this Section 11.6 shall not be construed to create a limit of either Party’s liability with respect to its indemnification obligation under Sections 11.2 or 11.3 above, as applicable. Notwithstanding the foregoing, Biogen and Alkermes may self-insure to the extent that such Party self-insures for its other products.
12.1.1.Objective. The Parties recognize that disputes, controversies or claims arising out of or relating to this Agreement or any Supply Agreement, or the interpretation, breach, termination or invalidity hereof or thereof (each a “Dispute”), may from time to time occur during the Term. It is the objective of the Parties to establish procedures to facilitate the resolution of Disputes occurring with respect to this Agreement or any Supply Agreement, in an expedient manner by mutual cooperation and without resorting to litigation. To accomplish this objective, the Parties agree to follow the procedures set forth in this Article 12 if and when a Dispute occurs with respect to this Agreement or any Supply Agreement. Notwithstanding the foregoing or anything to the contrary in this Agreement, with respect to any matter under this Agreement, (a) if such matter is within the scope of the JSC’s authority, the dispute resolution provisions of Section 2.1.4 and not this Article 12 shall apply with respect to such matter and (b) if this Agreement expressly provides that such matter is subject to a Party’s sole discretion or to a Party’s sole or final decision-making authority, such matter shall not be subject to dispute resolution under this Article 12, but may be finally determined by such Party in accordance with the terms of this Agreement.
12.1.2.Escalation. With respect to any Dispute under this Agreement, other than any Dispute relating to the scope, validity or enforceability of a Licensed Patent or a Collaboration Patent (which may only be determined in accordance with Section 12.3 hereof), either Party (the “Complaining Party”) may present such Dispute for resolution by senior management of each of Alkermes and Biogen (in the case of Alkermes, its Chief Executive Officer or a designee, and in the case of Biogen, its Chief Executive Officer or a designee) (collectively, “Senior Management”) by providing a dispute notice (the “Dispute Notice”) to Senior Management and the other Party. The Dispute Notice will concisely set forth the Dispute, the Parties’ respective positions, and the specific relief requested. Within ten (10) days after receipt of a Dispute Notice, the Party receiving the Dispute Notice will provide a concise written response (the “Response”) to such Dispute Notice to Senior Management and the Complaining Party. Senior Management will attempt to resolve such Dispute within ten (10) days after receipt by Senior Management of the Response. In the event that Senior Management cannot resolve a
54
Dispute within the ten (10)-day period, unless otherwise agreed by the Parties, such Dispute may be referred by either Party to arbitration in accordance with Section 12.1.3 upon written notice to the other Party.
12.1.3.Arbitration. The Parties agree that any Dispute referred for arbitration by a Party pursuant to Section 12.1 will be resolved through binding arbitration in accordance with the CPR International Institute for Conflict Prevention and Resolution Rules for Non-Administered Arbitration, as amended from time to time (the “CPR Rules”). If either Party receives a Dispute Notice, then any associated time to cure will be stayed pending the resolution of the issue pursuant to this Section 12.1.3. Any Dispute in which either Party seeks in excess of Twenty Million U.S. Dollars (US$20,000,000) in damages will be resolved by an arbitral tribunal consisting of three (3) arbitrators, one (1) of whom will be designated by each Party in accordance with the CPR Rules, and a third arbitrator who will chair the tribunal and who will be selected as provided in the CPR Rules. Any other Dispute, aside from those seeking equitable relief, will be submitted to a sole arbitrator, appointed pursuant to the CPR Rules. Any suit seeking equitable relief shall be heard by a court of competent jurisdiction pursuant to Section 12.2. The arbitrator(s) will render a written opinion setting forth findings of fact and conclusions of law with the reasons therefor stated. Arbitration pursuant to this Section 12.1.3 will be governed by the Federal Arbitration Act, 9 U.S.C. §§ 1-16, and judgment upon the award rendered by the arbitrators may be entered by any court having jurisdiction thereof. The arbitration proceedings for all Disputes will be conducted in New York, New York in English and will be confidential in nature. Each Party will continue to perform its obligations under the Agreement pending final resolution of any Dispute unless to do so would be impossible or impracticable under the circumstances. The Parties agree that they will share equally the cost of the arbitration filing and hearing fees, and the cost of the arbitrators. Each Party must bear its own attorneys’ fees and associated costs and expenses.
12.2.Jurisdiction. The Parties agree to the exclusive jurisdiction of the federal courts located in the State of New York for the purposes of enforcing awards entered pursuant to this Article 12 and for enforcing the agreements reflected in this Article 12.
12.3.Determination of Disputes Relating to Patents. Notwithstanding anything to the contrary herein, any Dispute relating to the determination of scope, validity or enforceability of a Licensed Patent or Collaboration Patent will be submitted exclusively to the national court or other tribunal having jurisdiction over the disputed patent.
12.4.Equitable Relief. The Parties agree that irreparable harm may occur in the event any of the provisions of Article 6, Article 7, Article 8, in each case, are not performed in accordance with the terms of this Agreement or are otherwise breached and that money damages may not be a sufficient remedy for such a breach of this Agreement. Therefore, in addition to, and not in limitation of, any other remedy available to either Party, a Party will be entitled to seek, at its sole expense, injunctive relief or other equitable relief in the event of any such breach or threatened breach of this Agreement by the other Party from a court of competent jurisdiction, and such an action may be filed and maintained notwithstanding any ongoing arbitration proceeding. Such remedies, and all other remedies provided for in this Agreement, shall be cumulative and not exclusive and will be in addition to any other remedies a Party may have under Applicable Law or in equity or otherwise.
55
Article 13
TERM AND TERMINATION
13.1.Term. This Agreement will commence as of the Effective Date and, unless sooner terminated as provided in this Article 13, will continue in effect until the expiration of the last Royalty Term as set forth in Section 9.5.3 (such period, the “Term”).
13.2.Right to Terminate for Government Prohibition. Either Party will have the right to terminate this Agreement effective immediately upon written notice to the other Party, following the issuance of any order, decree or judgment by any Governmental Authority that makes illegal, enjoins or prohibits the transactions effected by this Agreement.
13.3.Biogen’s Right to Terminate for Convenience. Biogen may terminate this Agreement, on a Product-by-Product basis or in its entirety, for any reason or for no reason, upon fifteen (15) days’ prior written notice to Alkermes; provided that, from and after the date of the First FDA Approval, Biogen may only terminate this Agreement, with respect to the Alkermes 8700 Product or in its entirety, upon one hundred and eighty (180) days’ prior written notice to Alkermes. Notwithstanding anything to the contrary set forth in this Agreement, from and after any notice of termination provided by Biogen to Alkermes under this Section 13.3 that terminates the Agreement with respect to the Alkermes 8700 Product or in its entirety, Biogen will not be obligated to make any further Minimum Annual Payments.
13.4.Right to Terminate Upon Bankruptcy. Either Party may, in addition to any other remedies available to it under Applicable Law or in equity, terminate this Agreement, effective immediately upon written notice to the other Party in the event (i) the other Party has made an assignment for the benefit of its creditors; (ii) there has been appointed an administrator, trustee or receiver for the other Party or for all or a substantial part of its property; or (iii) any case or proceeding has been commenced or other action taken by or against the other Party in bankruptcy or seeking reorganization, liquidation, dissolution, winding-up, arrangement, composition or readjustment of its debts or any other relief under any bankruptcy, insolvency, reorganization or other similar act or Applicable Law of any jurisdiction now or hereafter in effect, and any such event has continued for sixty (60) days undismissed.
13.5.1.Termination of License Grants. Upon any termination (but not expiration) of this Agreement in its entirety, except for the licenses and rights granted under Sections 13.5.2 and 13.5.3, all other licenses and rights granted by a Party to the other Party hereunder will terminate. Upon any termination of this Agreement with respect to a Product pursuant to Section 13.3, except for the licenses and rights granted under Sections 13.5.2 and 13.5.3, all other licenses and rights granted by a Party to the other Party hereunder with respect to such Product(s) (as applicable) shall terminate.
13.5.2.Alkermes 8700 Product Rights. In the event of a termination (but not expiration) of this Agreement with respect to the Alkermes 8700 Product or in its entirety, so
56
long as at such time (1) GI Inferiority does not exist or GI Inferiority exists and Biogen has Commercialized the Alkermes 8700 Product, and (2) Alkermes is not then in breach of its obligations under this Agreement (unless such breach is curable and has been cured no later than sixty (60) days after written notice from Biogen to Alkermes requesting cure of such breach), then at Alkermes’ written request, Biogen shall:
(i)within a reasonable time period, transfer to Alkermes all ongoing Clinical Trials being conducted by the Parties for the Alkermes 8700 Product as of the effective date of such termination of this Agreement, if permitted by Applicable Law and the applicable Regulatory Authorities (or any data monitoring review board or internal safety review board), and provide cooperation reasonably requested by Alkermes in connection with such transfer;
(ii)if such termination occurs after the Transfer Completion Date, then no later than one (1) Business Day following the effective date of such termination, (a) send a letter to the FDA to transfer and assign to Alkermes all Regulatory Approvals and regulatory filings related to the Alkermes 8700 Product, including the Alkermes 8700 Product 505(b)(2) NDA and (b) transfer to Alkermes a complete copy of the Alkermes 8700 Product 505(b)(2) NDA and any other application for Regulatory Approval of the Alkermes 8700 Product and all regulatory filings, regulatory documentation and other supplements and records related to such NDA and applications that are required to be kept under 21 C.F.R. § 314.81, in each case, in Biogen’s possession and Control as of the effective date of such termination of this Agreement;
(iii)no later than one (1) Business Day following the effective date of such termination, transfer to Alkermes a true and complete copy of (a) all data and results generated from any Development activities conducted by or on behalf of Biogen with respect to the Alkermes 8700 Product prior to the effective date of such termination of this Agreement, (b) all Trial Master Files (including any Trial Master File plans, tables of contents or indices and any evidence or certification of related quality checks) or equivalents thereof, for all completed or ongoing Clinical Trials of the Alkermes 8700 Product conducted by or on behalf of Biogen and (c) all other tangible embodiments of the Collaboration Know-How, in each case, in Biogen’s possession and Control as of the effective date of such termination of this Agreement;
(iv)as of the effective date of such termination, grant to Alkermes an exclusive (even as to Biogen and its Affiliates), fully paid-up, perpetual, irrevocable and royalty-free, worldwide license, with the right to grant sublicenses through multiple tiers, under Biogen Collaboration Know-How and Biogen Collaboration Patents to Exploit the Alkermes 8700 Product in the Initial Indication in the Territory; and
(v)as of the effective date of such termination, grant to Alkermes a non-exclusive, fully paid-up, perpetual, irrevocable and royalty-free, worldwide license, with the right to grant sublicenses through multiple tiers, under any Know-How and Patent Rights that (a) are Controlled by Biogen or its Affiliates as of the effective date of such termination, (b) are invented after the Effective Date and prior to the effective date of such termination in the course of performance of activities by Biogen or its Affiliates or Sublicensees under this Agreement and (c) relate to (with respect to Know-How) or Cover (with respect to Patent Rights) the Alkermes 8700 Product in the form such product exists as of the effective date of such termination to
57
Exploit the Alkermes 8700 Product in the form such product exists as of the effective date of such termination in the Initial Indication in the Territory.
13.5.3.License to Biogen Know-How and Biogen Patents. In the event of a termination (but not expiration) of this Agreement with respect to the Alkermes 8700 Product or in its entirety, so long as at such time (i) GI Inferiority does not exist or GI Inferiority exists and Biogen has Commercialized the applicable Product and (ii) Alkermes is not then in breach of its obligations under this Agreement (unless such breach is curable and has been cured no later than sixty (60) days after written notice from Biogen to Alkermes requesting cure of such breach), then at Alkermes’ written request, the Parties will negotiate in good faith the terms and conditions of the grant of a license to Alkermes under Know-How and Patent Rights Controlled by Biogen prior to the Effective Date that Cover the Alkermes 8700 Product and that are not licensed to Alkermes pursuant to Sections 13.5.2(iv) or Section 13.5.2(v).
13.5.4.Costs of Product Reversion to Alkermes. Alkermes shall be responsible for all costs and expenses incurred [**] in connection with the activities set forth in Section 13.5.2, and thereafter Biogen shall be responsible for all such costs and expenses incurred.
13.5.5.Surviving Sublicensee. Following the effective date of any termination of this Agreement, at the request of any Sublicensee with a market capitalization (or other independent valuation) of at least Ten Billion U.S. Dollars (US$10,000,000,000), and who is not then in breach of its Sublicense Agreement and is otherwise in good standing, subject to Alkermes’ receipt of a copy from such Sublicensee of the Sublicense Agreement with such Sublicensee (together with all modifications or amendments); provided that any such copy may be redacted to remove any confidential, proprietary or competitive information of Biogen, Alkermes will enter into, without any assistance by Biogen, a direct license agreement with such Sublicensee under the Licensed Know-How and Licensed Patents that are sublicensed to such Sublicensee on substantially the same terms, i.e., provides Sublicensee and Alkermes (as a substitute thereunder for Biogen) the same rights and obligations, as set forth in such Sublicense Agreement between Biogen and such Sublicensee effective as of the date of termination of the Sublicense Agreement granted to Sublicensee by Biogen; provided, however, that (a) such direct license agreement would not impose on Alkermes any obligations over and above its obligations under this Agreement and would not impose on any such Sublicensee any obligations over and above its obligations under the applicable Sublicense Agreement, and (b) as consideration for such direct license, the direct license agreement would require Sublicensee to pay Alkermes the same amount as Alkermes would have received from Biogen (had this Agreement survived) as a result of the Sublicensee’s performance under such Sublicense Agreement. During the pendency of any negotiation of a direct license agreement between Alkermes and the applicable Sublicensee in accordance with this Section 13.5.5, so as to ensure no disruption in the rights granted to such Sublicensee, such Sublicensee is hereby licensed to continue to exercise its rights and will continue to perform its obligations, in each case, as set forth under such Sublicense Agreement and the applicable terms under such Sublicense Agreement will apply mutatis mutandis to Alkermes rather than Biogen, except that Alkermes will not have any obligations over and above its obligations under this Agreement. As provided in this Section 13.5.5, in the event of any termination of this Agreement, Alkermes will not have the right to terminate or otherwise restrict any rights granted to a Sublicensee that is not also in breach of this Agreement
58
or the applicable Sublicense Agreement. For any Sublicensee with a market capitalization (or other independent valuation) of less than Ten Billion U.S. Dollars (US$10,000,000,000) and who is not then in breach of its Sublicense Agreement and is otherwise in good standing, Alkermes agrees to discuss in good faith with such Sublicensee a direct license agreement with such Sublicensee under the Licensed Know-How and Licensed Patents that are sublicensed to such Sublicensee.
13.6.1.All rights and licenses granted under or pursuant to this Agreement are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the Bankruptcy Code or analogous provisions of Applicable Law outside the U.S., licenses of right to “intellectual property” as defined under Section 101 of the Bankruptcy Code or analogous provisions of Applicable Law outside the U.S. (hereinafter “IP”). The Parties agree that the licensee of any such rights under this Agreement shall retain and may fully exercise all of its rights and elections under the Bankruptcy Code or any other provisions of Applicable Law outside the U.S. that provide similar protection for IP. The license-granting Party shall, during the Term, create and maintain current copies of all IP licensed to the other Party under this Agreement. Upon the bankruptcy of a Party, the other Party shall further be entitled to a complete duplicate of (or complete access to, as appropriate) any such IP, and such IP, if not already in such other Party’s possession, shall be promptly delivered to such other Party. Each Party acknowledges and agrees that “embodiments” of such IP within the meaning of Section 365(n) include, without limitation, laboratory notebooks, product samples and inventory, research studies and data, all Regulatory Approvals and rights of reference therein, and all embodiments of any Licensed Know-How. If (a) a case under the Bankruptcy Code is commenced by or against a license-granting Party, (b) this Agreement is rejected as provided in the Bankruptcy Code, and (c) the other Party elects to retain its rights hereunder as provided in Section 365(n) of the Bankruptcy Code, the license-granting Party (in any capacity, including debtor-in-possession) and its successors and assigns (including a trustee) shall:
(i)provide to the other Party all such IP (including all embodiments thereof) in such license-granting Party’s possession on terms agreed by the Parties, promptly upon the other Party’s written request; and
(ii)not interfere with the other Party’s rights under this Agreement, or any agreement supplemental hereto, to such IP (including such embodiments), including any right to obtain such IP (or such embodiments) from another entity, to the extent provided in Section 365(n) of the Bankruptcy Code.
13.6.2.All rights, powers and remedies provided herein are in addition to and not in substitution for any and all other rights, powers and remedies now or hereafter existing at law or in equity (including the Bankruptcy Code) in the event of the commencement of a case under the Bankruptcy Code with respect to either Party. The Parties agree that they intend the following rights to extend to the maximum extent permitted by law, and to be enforceable under Bankruptcy Code Section 365(n) upon any rejection of this Agreement: (a) the right of access to any IP (including all embodiments thereof) of the license-granting Party or any Third Party with whom the license-granting Party contracts to perform any of its obligations
59
under this Agreement; and (b) the right to contract directly with any such Third Party to complete the contracted work.
13.7.Survival of Certain Provisions. Termination of this Agreement for any reason or expiration of this Agreement will not release either Party from any obligation arising prior to the date of expiration or termination. The rights and obligations under Section 3.8.1 and Section 3.8.3 (in each case solely with respect to amounts accruing prior to the effective date of termination or expiration of this Agreement and record-keeping and audits with respect to such amounts), Section 6.1.3, Section 7.1, Section 10.1, Section 13.5 and this Section 13.7 and Article 1 (solely to the extent necessary to give effect to the other surviving provisions), Article 8, Article 9 (solely with respect to amounts accruing prior to the effective date of termination or expiration of this Agreement, and record-keeping and audits with respect to such amounts), Article 11, Article 12 and Article 14, in each case, only in the event and to the extent applicable, and subject to the terms and conditions stated therein, will survive any expiration or termination of this Agreement. Any right to terminate this Agreement, and any rights a Party has under Section 13.5, as applicable, shall be cumulative and not exclusive and will be in addition to any other rights or remedies that the Party giving notice of termination may have under Applicable Law or in equity or otherwise.
14.1.Notices. All notices, reports, requests or demands required or permitted under this Agreement will be sent by hand or overnight courier, properly addressed to the respective Parties as follows:
If to Alkermes:
Alkermes Pharma Ireland Limited
Connaught House
1 Burlington Road
Dublin 4, Ireland
Attention: President
With a copy to:
Alkermes Public Limited Company
Connaught House
1 Burlington Road
Dublin 4, Ireland
Attention: Chief Legal Officer
If to Biogen:
Biogen MA Inc.
225 Binney Street
Cambridge, MA 02142
Attention: Executive Vice President, Head of R&D
60
With a copy to:
Biogen MA Inc.
225 Binney Street
Cambridge, MA 02142
Attention: Chief Legal Officer
and
Ropes & Gray LLP
Prudential Tower, 800 Boylston Street
Boston, MA 02199-3600
Attention: Susan Galli, Esq.
or to such address or addresses as the Parties hereto may designate for such purposes during the Term. Notices will be deemed to have been sufficiently given or made: (i) if by hand, when delivered, and (ii) if by overnight courier, upon receipt by the applicable Party.
14.2.Governing Law. This Agreement and all questions regarding the existence, validity, interpretation, breach or performance of this Agreement will be governed by and construed in accordance with the laws of the State of New York (other than its choice of law principles).
14.3.Entire Agreement; Amendment. This Agreement, together with the Exhibits hereto, represent the entire agreement between the Parties regarding the subject matter hereof, and supersedes all prior or contemporaneous written or oral promises or representations relating such subject matter not incorporated herein (including the Confidentiality Agreements). The Parties are not relying, and have not relied, on any representations or warranties whatsoever regarding the subject matter of this Agreement, express or implied, except for the representations and warranties set forth in this Agreement. No amendment or modification of the terms and conditions of this Agreement will be binding on either Party unless reduced to writing referencing this Agreement and signed by a duly authorized officer of each Party.
14.4.Binding Effect and Assignment. This Agreement will be binding upon and inure to the benefit of the Parties hereto and their respective successors and permitted assigns. This Agreement will not be assignable by either Party without the other Party’s prior written consent; provided, however, that either Party may assign its rights or obligations under this Agreement (in whole or in part), without the other Party’s written consent but with notice to the other Party, to an Affiliate. If any Affiliate to which a Party has assigned its rights or obligations under this Agreement thereafter ceases to be an Affiliate of such Party, then such assignment will be deemed to require the consent of the other Party pursuant to this Section 14.4. To the extent that the assigning Party survives as a legal entity, the assigning Party shall remain responsible for the performance by its assignee of this Agreement or any obligations hereunder so assigned to such assignee. Either Party may also assign this Agreement (in whole or in part) without the other Party’s written consent, but with notice to the other Party, to any successor pursuant to a Change of Control, and Biogen may assign this Agreement (in whole or in part), without Alkermes’ written consent but with notice to Alkermes in connection with the sale or other
61
transfer to a Third Party of all or substantially all of Biogen’s assets to which this Agreement relates (i.e., Tecfidera®, Fumaderm® and any Product).
14.5.Waiver. No provision of this Agreement shall be waived by any act, omission or knowledge of a Party or its agents or employees except by an instrument in writing expressly waiving such provision and signed by a duly authorized officer of the waiving Party. A waiver by either Party of any of the terms and conditions of this Agreement in any instance will not be deemed or construed to be a waiver of such term or condition for the future, or of any other term or condition hereof. All rights, remedies, undertakings, obligations and agreements contained in this Agreement will be cumulative and none of them will be in limitation of any other remedy, right, undertaking, obligation or agreement of either Party.
14.6.Severability. If any part of this Agreement will be found to be invalid, illegal or unenforceable under Applicable Law in any jurisdiction, such part will be ineffective only to the extent of such invalidity, illegality or unenforceability in such jurisdiction, without in any way affecting the remaining parts of this Agreement in that jurisdiction or the validity, legality or enforceability of the Agreement as a whole in any other jurisdiction. In addition, the part that is ineffective will be reformed in a mutually agreeable manner so as to as nearly approximate the intent of the Parties as possible.
14.7.Counterparts and Signatures. This Agreement may be executed in two or more counterparts, each of which will be deemed an original for all purposes, but all of which together will constitute one and the same instrument. Signatures provided by facsimile transmission or in Adobe™ Portable Document Format (PDF) sent by electronic mail shall be deemed to be original signatures.
14.8.Force Majeure. Neither Party will be held liable or responsible to the other Party or be deemed to have breached or defaulted under this Agreement for failure or delay in performing its obligations hereunder (except for payment of money) to the extent, and as long as, such failure or delay is caused by or results from causes beyond the reasonable control of the affected Party (a “Force Majeure Delay”), including fire, floods, embargoes, war, civil commotions, terrorism, strikes, lockouts or other labor disturbances, acts of God, acts of a Governmental Authority or judicial orders or decrees. In the event of a Force Majeure Delay, the affected Party will give prompt notice thereof to the other Party (to the extent possible), will use commercially reasonable efforts to mitigate the adverse consequences thereof and will resume performance hereunder with dispatch whenever the consequences of the Force Majeure Delay have been mitigated.
14.9.Ambiguities. Ambiguities, if any, in this Agreement will not be construed against any Party, irrespective of which Party may be deemed to have authored the ambiguous provision.
14.10.Headings. Headings are for the convenience of reference only and will not control the construction or interpretation of any of the provisions of this Agreement.
14.11.No Partnership. Nothing in this Agreement is intended or will be deemed to constitute a partnership, agency, or joint venture relationship between the Parties.
62
Notwithstanding any of the provisions of this Agreement, neither Party will at any time enter into, incur, or hold itself out to Third Parties as having authority to enter into or incur, on behalf of the other Party, any commitment, expense, or liability whatsoever.
14.12.No Third Party Beneficiaries. No Person other than Alkermes, Biogen and their respective successors and permitted assigns shall be deemed an intended beneficiary hereunder or have any right to enforce any provision of this Agreement.
14.13.Performance by an Affiliate. Each of Biogen and Alkermes acknowledges that obligations under this Agreement may be performed by Affiliates of Biogen and Alkermes. Each of Biogen and Alkermes will remain responsible for any obligations of such Party under this Agreement undertaken by one or more of its Affiliates.
14.14.Further Assurances. Each Party agrees to do and perform all such further acts and things and shall execute and deliver such other agreements, certificates, instruments and documents necessary or that the other Party may deem advisable in order to carry out the intent and accomplish the purposes of this Agreement and to evidence, perfect or otherwise confirm its rights hereunder.
[Signature page follows]
63
In Witness Whereof, each of the Parties has caused this Agreement to be executed and delivered by its duly authorized representatives to be effective as of the Effective Date.
|
ALKERMES PHARMA IRELAND LIMITED |
|
BIOGEN SWISS MANUFACTURING GMBH |
||
|
By: |
/s/ Shane Cooke |
|
By: |
/s/ Fred Lawson |
|
Name: |
Shane Cooke |
|
Name: |
F. Lawson |
|
Title: |
Director |
|
Title: |
Director |
|
Date: |
November 27, 2017 |
|
Date: |
26/11/2017 |
Signature Page to License and Collaboration Agreement
Alkermes 8700 Chemical Structure
The chemical structure of the active ingredient in the Alkermes 8700 Product:
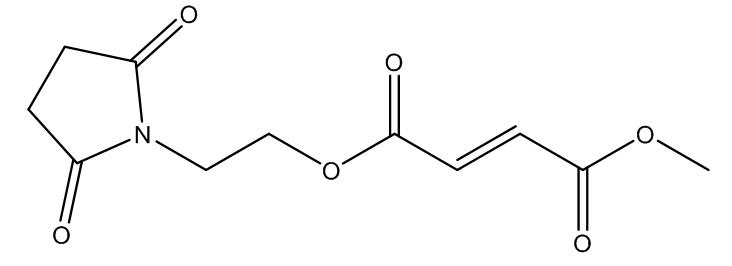
A-1
B-1
B-2
B-3
B-4
C-1
ALKS 8700 Product High‐Level Development Plan
* The activities that are allocated to Alkermes, unless otherwise agreed in writing by the Parties, are (1) all activities prior to the
Transfer Completion Date and (2) transition of the P3 Long‐Term Safety Study (‐A301) to Biogen following the Transfer
Completion Date.
[**]
C-2
Detailed ALKS 8700 Product Plan – Multiple Sclerosis (505b2)
[**]
* The activities that are allocated to Alkermes, unless otherwise agreed in writing by the Parties, are (1) all activities prior to the Transfer Completion Date and (2) transition of the P3 Long‐Term Safety Study (‐ A301) to Biogen following the Transfer Completion Date.
C-3
ALKS 8700 Development Plan Budget – Multiple Sclerosis
|
Spend in $M |
FY'17 |
FY'17 |
FY'17 |
FY'17 |
FY'17 |
FY'18 |
FY'18 |
FY'18 |
FY'18 |
FY'18 |
FY'19 |
FY'19 |
FY'19 |
FY'19 |
FY'19 |
|
Total |
Q1 |
Q2 |
Q3 |
Q4 |
Total |
Q1 |
Q2 |
Q3 |
Q4 |
Total |
Q1 |
Q2 |
Q3 |
Q4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Base ALKS 8700 Development (FY16 - FY'21) |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
Clinical |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Phase 1 PK and Clinical Pharmacology Studies |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
ALKS 8700 FIH Study (-001) |
[**] |
|
|
|
|
[**] |
|
|
|
|
[**] |
|
|
|
|
|
ALKS 8700 MAD / FE Study (-A102) |
[**] |
|
|
|
|
[**] |
|
|
|
|
[**] |
|
|
|
|
|
ALKS 8700 Relative BA Study Fasted Condition (-A103) |
[**] |
|
|
|
|
[**] |
|
|
|
|
[**] |
|
|
|
|
|
ALKS 8700 Relative BA Study Fed (HF) Condition (-A104) |
[**] |
|
|
|
|
[**] |
|
|
|
|
[**] |
|
|
|
|
|
ALKS 8700 Mass Balance Study (-A105) |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
|
|
|
[**] |
|
|
|
|
|
ALKS 8700 Alcohol Dose Dumping Study (-A106) |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
|
|
|
[**] |
|
|
|
|
|
ALKS 8700 DDI Study (-A107) |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
|
|
|
[**] |
|
|
|
|
|
ALKS 8700 Renal Study (-A108) |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
|
|
|
[**] |
|
|
|
|
|
ALKS 8700 Additional Food Effect Study (-A109) |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
|
|
|
|
|
|
|
|
|
ALKS 8700 Potential tQT Study |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
|
[**] |
|
|
|
|
|
Phase 3 Long-term Safety Study (-A301) |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
Phase 3 Tolerability Study (-A302) |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
|
|
|
Clinical CRO Credits |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
Translational Medicine |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
[**] |
|
|
|
|
|
Non-Clinical |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2-Yr Rat Study |
[**] |
[**] |
|
[**] |
[**] |
[**] |
[**] |
|
|
|
[**] |
|
|
|
|
|
Mouse Carci Studies |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
|
|
|
[**] |
|
|
|
|
|
Additional Non-Clinical Studies/Activities |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
[**] |
|
|
|
|
|
CMC |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
API & Drug Product Development, Manufacturing, and Supply |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
Medical Affairs |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PRO Development & Additional Medical Affairs Activities |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
NDA Submission Fee & supporting preparation spend |
[**] |
|
|
|
|
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
|
|
|
|
Other – All |
[**] |
|
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
Pediatric Plan Activities |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
Non-clinical Studies |
[**] |
|
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
|
|
|
|
Adult PK Sprinkling Study |
[**] |
|
|
|
|
[**] |
|
|
|
|
[**] |
|
|
|
|
|
Pediatric PK Study |
[**] |
|
|
|
|
[**] |
|
|
|
|
[**] |
|
|
|
|
|
Pediatric Extension |
[**] |
|
|
|
|
[**] |
|
|
|
|
[**] |
|
|
|
|
|
ALKS 8700 External Spend Projection |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
[**] |
|
|
|||||||||||||||
|
ALKS 8700 Internal Spend Projection |
[**] |
|
|
|
|
[**] |
[**] |
[**] |
[**] |
|
|||||
|
|
|||||||||||||||
|
Total R&D |
[**] |
|
|
|
|
[**] |
|
|
|
|
[**] |
|
|
|
|
C-4
Phase 3 Long‐Term Safety Study (‐A301)
|
Name of Sponsor/ Company: Alkermes, Inc. |
|
|
Name of Investigational Product: ALKS 8700 |
|
|
Name of Active Ingredient: ALKS 8700 |
|
|
Title of study: A Phase 3 Open Label Study to Evaluate the Long-term Safety and Tolerability of ALKS 8700 in Adults with Relapsing Remitting Multiple Sclerosis |
|
|
Investigators: This study will be conducted at approximately 125 sites in North America and Europe. |
|
|
Study Period: Estimated date of first subject’s consent: Q4 2015 Estimated date of last subject’s last visit: Q4 2020 |
Phase of Development: 3 |
|
Objectives: • Evaluate the long-term safety and tolerability of ALKS 8700 for up to 96 weeks of treatment in adult subjects with relapsing remitting multiple sclerosis (RRMS) • Evaluate treatment effect over time in adult subjects with RRMS treated with ALKS 8700 |
|
|
Methodology: [**] |
|
C-5
|
Study Design Schematic [**]
|
|
Number of Subjects Planned: [**] |
|
Main Criteria for Inclusion: [**]
|
|
Investigational Product, Dosage, Duration and Mode of Administration: [**]
|
|
Reference Therapy, Dosage, Duration and Mode of Administration: [**]
|
|
Duration of Study: [**]
|
C-6
|
Criteria for Evaluation: Safety and Tolerability: [**]
Efficacy: [**]
Pharmacokinetics: [**]
|
|
Statistical Methods: [**]
Study Populations: [**]
|
C-7
|
Safety: [**]
Efficacy: [**]
Pharmacokinetics: [**]
Sample Size Considerations: [**]
|
C-8
Phase 3 Tolerability Study (‐A302)
|
Name of Sponsor/ Company: Alkermes, Inc. |
|
|
Name of Investigational Product: ALKS 8700 |
|
|
Name of Active Ingredient: ALKS 8700 |
|
|
Title of study: A Phase 3 Study in Subjects with Relapsing Remitting Multiple Sclerosis to Evaluate the Tolerability of ALKS 8700 and Dimethyl Fumarate |
|
|
Investigators: This study will be a multicenter study. |
|
|
Study Period: Estimated date of first subject’s consent: Q1 2017 Estimated date of last subject’s last visit: [**] |
Phase of Development: 3 |
|
Objectives: • Evaluate the utility of two GI symptom scales (IGISIS and GGISIS) and endpoints derived from the scales in assessing GI tolerability in adult subjects with relapsing remitting multiple sclerosis (RRMS) (after administration of ALKS 8700 or DMF in Part A • Compare the GI tolerability of ALKS 8700 and DMF in adult subjects with RRMS using two GI symptom scales (IGISIS and GGISIS) in Part B with endpoints informed from Part A • Evaluate the safety and tolerability of ALKS 8700 in adult subjects with RRMS in Parts A and B |
|
|
Methodology (Part A and Part B): [**]
|
|
C-9
|
Number of Subjects Planned (Part A and Part B): [**]
|
|
Main Criteria for Inclusion (Part A and Part B): [**]
|
|
Investigational Product, Dosage, Duration and Mode of Administration (Part A and Part B): [**]
|
|
Reference Therapy, Dosage, Duration and Mode of Administration (Part A and Part B): [**]
|
C-10
|
Duration of Study (Part A and Part B): [**]
|
|
Criteria for Evaluation (Part A and Part B):
Endpoints:
Primary Endpoint: [**]
Secondary Endpoints: [**]
Safety and Tolerability (Part A and Part B): [**]
|
|
Exploratory Efficacy (Part A and Part B): [**]
|
|
Pharmacokinetics (Part A): [**]
|
C-11
|
Statistical Methods (Part A and Part B): Sample Size Considerations (Part A and Part B): [**]
Study Populations (Part A and Part B): [**]
Safety Analyses (Part A and Part B): [**]
GI Tolerability Analyses (Part A and Part B): [**]
Exploratory Efficacy Analyses (Part A and Part B): [**]
Pharmacokinetics Analyses (Part A): [**] |
C-12
|
[**]
|
C-13
ALKS 8700 Renal Study (‐A108)
|
Name of Sponsor/ Company: Alkermes, Inc. |
|
|
Name of Investigational Product: ALKS 8700 |
|
|
Name of Active Ingredient: ALKS 8700 |
|
|
Title of study: A Phase 1 Study of the Pharmacokinetics, Safety and Tolerability of ALKS 8700 in Subjects with Renal Impairment |
|
|
Investigators: This study will be conducted at multiple centers study in the United States. |
|
|
Study Period: Estimated date of first subject’s consent: Q4 2016 Estimated date of last subject’s last visit: Q2 2017 |
Phase of Development: 1 |
|
Objectives: The objective of this study is to compare the pharmacokinetics (PK), safety and tolerability of ALKS 8700 in subjects with mild, moderate and severe renal impairment versus healthy control subjects following single dose administration. |
|
|
Methodology: [**]
|
|
C-14
|
Study Design Schematic [**]
|
|
Number of Subjects Planned: [**]
|
|
Main Criteria for Inclusion: [**]
|
|
Investigational Product, Dosage, Duration and Mode of Administration: [**]
|
|
Reference Therapy, Dosage, Duration and Mode of administration: [**] |
C-15
|
Duration of Study: [**]
|
|
Criteria for Evaluation: Pharmacokinetics: [**]
Safety: [**]
|
|
Statistical Methods: [**]
Analyses Populations: [**]
Pharmacokinetic Analysis: [**]
|
C-16
|
Safety Analysis: [**]
Sample Size Considerations: [**]
|
C-17
ALKS 8700 tQT Study (‐A110)
|
Name of Sponsor/ Company: Alkermes, Inc. |
|
|
Name of Investigational Product: ALKS 8700 |
|
|
Name of Active Ingredient: Monomethyl fumarate |
|
|
Title of study: A Phase 1 Study to Evaluate the Effect of Multiple Doses of ALKS 8700 on QTc Interval in Healthy Volunteers |
|
|
Investigator(s): Single center study in the US |
|
|
Study Period (years): Estimated date of first patient visit: Q4 2017 Estimated date of last patient completed: Q1 2018 |
Phase of Development: 1 |
|
Objectives: Primary: To evaluate the effects of multiple doses of therapeutic and supratherapeutic oral dose strengths of ALKS 8700 on the heart rate-corrected QT interval using Fridericia’s formula (QTcF) Secondary: • To evaluate the effect of ALKS 8700 on other electrocardiogram (ECG) parameters: heart rate, PR and QRS intervals, and T-wave morphology and U-wave presence • To demonstrate sensitivity of the study to detect a small QT effect using moxifloxacin as a positive control • To evaluate the safety and tolerability of ALKS 8700 • To evaluate the pharmacokinetic/pharmacodynamic relationship between the effect of ALKS 8700 on ECG and plasma concentrations of the metabolites monomethyl fumarate (MMF) and RDC-6567 |
|
|
Methodology: [**]
|
|
C-18
|
Number of Subjects Planned: [**]
|
|
Main Criteria for Inclusion: [**]
|
|
Investigational Product, Dosage, Duration, and Mode of Administration: [**]
|
|
Reference Therapy, Dosage, Duration, and Mode of Administration: [**]
|
|
Duration of Study: [**]
|
|
Criteria for Evaluation: Primary Endpoint: [**] Secondary Endpoints: [**]
|
C-19
|
Pharmacokinetics/Pharmacodynamics: [**]
Safety: [**]
|
C-20
|
Statistical Methods: [**]
Analyses Populations: [**]
Baseline: [**]
Safety Analyses: [**]
Concentration-Response Analysis: [**]
|
C-21
|
Criteria for Negative QT Assessment: [**]
Investigation of Hysteresis: [**]
Assessment of Appropriateness of Model: [**]
|
C-22
|
Assay Sensitivity: [**]
By-Time Point Analysis: [**]
Categorical Analyses: [**]
Safety: [**]
Sample Size Considerations: [**]
|
C-23
|
[**]
|
C-24
ALKS 8700 Juvenile Animal Toxicity Study
|
[**]
|
C-25
Clinical Supply Agreement Terms
Clinical Supply Agreement and Quality Agreement
-The Parties will negotiate and execute a Clinical Supply Agreement and an associated Technical and Quality Agreement (“Clinical Supply Quality Agreement”) in accordance with Section 5.1.1 of the Agreement.
Program of Manufacturing, Forecasts and Delivery of Products
-A detailed program of Manufacture with a rolling Calendar Quarter forecast and order and delivery mechanisms will be outlined in the Clinical Supply Agreement.
-Biogen to supply Tecfidera® [**].
-Alkermes shall have no liability as a result of any failure or delay in Manufacturing Clinical Supplies.
Quality Assurance
-Alkermes will Manufacture the Products in accordance with Applicable Law and cGMPs, the specifications for the Products and the terms and conditions of the Clinical Supply Agreement and the Clinical Supply Quality Agreement.
-Alkermes will perform and document those tests and checks required to assure the quality of the Product that are established by the Clinical Supply Quality Agreement.
-The Clinical Supply Agreement will contain terms establishing standards for the release, acceptance and rejection of the Products and will establish a minimum shelf life for the Products.
-In accordance with the terms of the Clinical Supply Agreement and the Clinical Supply Quality Agreement, Biogen will be entitled to inspect or have inspected those portions of the facilities that are used by Alkermes in the Manufacture of the Products as well as the relevant Manufacturing records.
-Validation batches will be treated as Clinical Supplies until such time as they are utilized in the Commercialization of Product, at which time Biogen will pay Alkermes the difference between the clinical and commercial supply prices.
Supply Price for Clinical Supplies of Products
D-1
Commercial Supply Agreement Terms
Clinical Supply Agreement and Quality Agreement
-The Parties will negotiate and execute a Commercial Supply Agreement and an associated Technical and Quality Agreement (“Commercial Supply Quality Agreement”) in accordance with Section 5.1 of the Agreement.
Program of Manufacturing, Forecasts and Delivery of Products
-A detailed program of Manufacture with a rolling Calendar Quarter forecast and order and delivery mechanisms will be outlined in the Commercial Supply Agreement.
-Product in the form of “finished goods” to be delivered Ex Works (Incoterms 2010) Athlone, Ireland.
-Biogen to provide (A) 3-year rolling forecasts and (B) 4-quarter rolling forecasts, the proximate two quarters of which shall be binding and the subsequent two quarters of which may be increased or decreased by 20% prior to becoming binding.
Quality Assurance
-Alkermes will Manufacture the Products in accordance with Applicable Law and cGMPs, the specifications for the Products and the terms and conditions of the Commercial Supply Agreement and the Commercial Supply Quality Agreement.
-Alkermes will perform and document those tests and checks required to assure the quality of the Products that are established by the Commercial Supply Quality Agreement.
-The Commercial Supply Agreement will contain terms establishing standards for the release, acceptance and rejection of the Products and will establish a minimum shelf life for the Products.
-In accordance with the terms of the Commercial Supply Agreement and the Commercial Supply Quality Agreement, Biogen will be entitled to inspect or have inspected those portions of the facilities that are used by Alkermes in the Manufacture of the Products as well as the relevant Manufacturing records.
Supply Price for Commercial Supplies of Products
[**]
Products in Process upon Termination
-For any Product that Alkermes has commenced the Manufacture of, but for which Manufacture is incomplete on the Commercial Supply Agreement termination date, Biogen shall either request that Alkermes (i) complete the Manufacture of such incomplete Product, in which case Biogen shall pay Alkermes [**] or (ii) cease the
E-1
Manufacture of such incomplete Product, in which case Biogen shall pay Alkermes the costs incurred by Alkermes for such incomplete Product, as calculated in accordance with GAAP.
Indemnification and Liability for Manufacture of Commercial Supplies of Products
-Customary toll manufacturer indemnification provisions.
-Limitation of liability equal to one year of amounts paid to Alkermes under the Commercial Supply Agreement.
-In the event of a Serious Failure to Supply, Alkermes shall transfer to Biogen or its designee, at Biogen’s cost and expense, the Manufacturing technology to enable Biogen or its designee to Manufacture Commercial Supplies.
E-2
F-1
|
|
Biogen Contacts: |
|
|
For Investors: Matt Calistri +1 781 464 2442 |
|
|
For Media: Matt Fearer +1 781 464 3260 |
|
|
|
|
|
|
|
|
Alkermes Contacts: |
|
|
For Investors: Sandy Coombs +1 781 609 6377 |
|
|
Eva Stroynowski +1 781 609 6823 |
|
|
For Media: Jennifer Snyder +1 781 609 6166 |
BIOGEN AND ALKERMES ANNOUNCE LICENSE AND COLLABORATION AGREEMENT TO DEVELOP AND COMMERCIALIZE ALKS 8700 FOR THE TREATMENT OF MULTIPLE SCLEROSIS
–– Novel, Oral, Fumarate Therapy Intended to Provide a Differentiated Gastrointestinal Tolerability Profile ––
–– Biogen Brings Multiple Sclerosis Expertise to Commercialization of ALKS 8700 ––
–– New Drug Application Anticipated for Submission in 2018 ––
Cambridge, Mass and Dublin, Ireland., Nov. 27, 2017 — Biogen (Nasdaq: BIIB) and Alkermes plc (Nasdaq: ALKS) today announced that they have entered into a global license and collaboration agreement to develop and commercialize ALKS 8700, a novel, oral, monomethyl fumarate (MMF) small drug molecule in Phase 3 development for the treatment of relapsing forms of multiple sclerosis (MS).
“This partnership is further evidence of Biogen’s ongoing commitment to multiple sclerosis and builds upon our deep experience in neuroscience and particularly in MS,” stated Michel Vounatsos, Chief Executive Officer at Biogen. “We aim to provide patients with a new oral therapy which may bring differentiated benefits.”
“This collaboration has the potential to provide important benefits to patients with multiple sclerosis and immediately increases the value of ALKS 8700 to Alkermes,” said Richard Pops, Chief Executive Officer at Alkermes. “Biogen has a broad product portfolio and a highly experienced commercial team. In Biogen’s hands, we believe that patients will have broader and more rapid access to this important medicine. Meanwhile, we will focus our growing commercial capabilities on our expanding portfolio of medicines in psychiatry, including addiction, schizophrenia and depression.”
Under the terms of the agreement, Biogen will receive an exclusive, worldwide license to commercialize ALKS 8700 and will pay Alkermes a mid-teens royalty on worldwide net sales of ALKS 8700.
This collaboration aligns the interests of Alkermes and Biogen in the successful development and commercialization of ALKS 8700 as an important potential treatment option for patients
F – Press Release
suffering from MS. Biogen will reimburse Alkermes for fifty percent (50%) of the 2017 ALKS 8700 development costs, with Alkermes receiving an upfront payment of $28 million representing Biogen’s share of development expenses already incurred in 2017. Beginning Jan. 1, 2018, Biogen will be responsible for all development expenses related to ALKS 8700. Alkermes may also receive milestone payments for ALKS 8700 with a maximum aggregate value of $200 million upon certain clinical and regulatory achievements. Biogen anticipates the initial milestone payment of $50 million will be recorded as an expense in 2017.
Alkermes will maintain responsibility for regulatory interactions with the U.S. Food and Drug Administration (FDA) through the potential approval of the New Drug Application (NDA) for ALKS 8700 for the treatment of MS. Biogen shall be responsible for all commercialization activities for ALKS 8700.
ALKS 8700 is currently in Phase 3 development for MS. Alkermes plans to seek approval of ALKS 8700 under the 505(b)(2) regulatory pathway referencing Biogen’s TECFIDERA® (dimethyl fumarate). The registration package for ALKS 8700 will include pharmacokinetic bridging studies that establish bioequivalence to TECFIDERA and data from a two-year safety study known as EVOLVE-MS-1. Initial safety data from EVOLVE-MS-1 were recently presented at MSParis2017, the 7th Joint Meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) in October. Safety data from the first month of the EVOLVE-MS-1 study (N=580) showed that treatment with ALKS 8700 was associated with low rates of gastrointestinal (GI) adverse events (AEs) leading to discontinuation and no occurrence of serious GI AEs. The most common AEs during the first month of treatment with ALKS 8700 were flushing, pruritus and diarrhea.
Also, currently underway is a head-to-head study (EVOLVE-MS-2) evaluating the GI tolerability of ALKS 8700 compared to TECFIDERA. Initial data from EVOLVE-MS-2 are expected in the first half of 2018.
About the EVOLVE-MS Clinical Development Program
The key components of the EVOLVE-MS (Endeavoring to Advance Treatment for Patients Living with Multiple Sclerosis) clinical development program of ALKS 8700 include a two-year safety study and pharmacokinetic bridging studies comparing ALKS 8700 and TECFIDERA. In addition, the program includes an elective head-to-head study comparing the GI tolerability of ALKS 8700 and TECFIDERA.
About ALKS 8700
ALKS 8700 is an oral, novel and proprietary monomethyl fumarate (MMF) prodrug candidate in development for the treatment of relapsing forms of multiple sclerosis (MS). ALKS 8700 is designed to rapidly and efficiently convert to MMF in the body and to offer differentiated features as compared to the currently marketed dimethyl fumarate, TECFIDERA®.
About Multiple Sclerosis
Multiple sclerosis (MS) is an unpredictable, often disabling disease of the central nervous system (CNS), which interrupts the flow of information within the brain, and between the brain and
F – Press Release
body.1 MS symptoms can vary over time and from person to person. Symptoms may include extreme fatigue, impaired vision, problems with balance and walking, numbness or pain and other sensory changes, bladder and bowel symptoms, tremors, problems with memory and concentration and mood changes, among others.1Approximately 400,000 individuals in the U.S. and 2.5 million people worldwide have MS, and most are diagnosed between the ages of 15 and 50.2
About Alkermes plc
Alkermes plc is a fully integrated, global biopharmaceutical company developing innovative medicines for the treatment of central nervous system (CNS) diseases. The company has a diversified commercial product portfolio and a substantial clinical pipeline of product candidates for chronic diseases that include schizophrenia, depression, addiction and multiple sclerosis. Headquartered in Dublin, Ireland, Alkermes plc has an R&D center in Waltham, Massachusetts; a research and manufacturing facility in Athlone, Ireland; and a manufacturing facility in Wilmington, Ohio. For more information, please visit Alkermes’ website at www.alkermes.com.
About Biogen
At Biogen, our mission is clear: we are pioneers in neuroscience. Biogen discovers, develops and delivers worldwide innovative therapies for people living with serious neurological and neurodegenerative diseases. Founded in 1978 as one of the world’s first global biotechnology companies by Charles Weissman, Heinz Schaller, Kenneth Murray and Nobel Prize winners Walter Gilbert and Phillip Sharp, today Biogen has the leading portfolio of medicines to treat multiple sclerosis; has introduced the first and only approved treatment for spinal muscular atrophy; and is focused on advancing neuroscience research programs in Alzheimer’s disease and dementia, neuroimmunology, movement disorders, neuromuscular disorders, pain, ophthalmology, neuropsychiatry, and acute neurology. Biogen also manufactures and commercializes biosimilars of advanced biologics.
We routinely post information that may be important to investors on our website at www.biogen.com. To learn more, please visit www.biogen.com and follow us on social media Twitter, LinkedIn, Facebook, YouTube.
Biogen Safe Harbor
This press release contains forward-looking statements, made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including statements relating to the potential benefits and results, including financial and operating results, that may be achieved through Biogen’s license agreement with Alkermes, risks and uncertainties associated with drug development and commercialization, the potential benefits, safety, efficacy and clinical effects of ALKS 8700, the timing and status of regulatory filings, and the potential of Biogen’s commercial business and pipeline programs, including ALKS 8700. These forward-looking statements may be accompanied by such words as “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “intend,” “may,” “plan,” “potential,” “project,” “target,” “will,” and other words and terms of similar meaning. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Results in early stage clinical trials may not be indicative of full results or results from later stage or larger scale clinical trials and do not ensure
F – Press Release
regulatory approval. You should not place undue reliance on these statements or the scientific data presented.
These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements, including, without limitation: uncertainty as to whether the anticipated benefits and potential of Biogen’s license agreement with Alkermes can be achieved; risks that Biogen and/or Alkermes may not fully enroll the clinical trials for ALKS 8700 or will take longer than expected; risks of unexpected costs or delays; uncertainty of success in the development and potential commercialization of ALKS 8700, which may be impacted by, among other things, unexpected concerns that may arise from additional data or analysis, the occurrence of adverse safety events, failure to obtain regulatory approvals in certain jurisdictions, failure to protect and enforce Biogen’s data, intellectual property, and other proprietary rights and uncertainties relating to intellectual property claims and challenges; third party collaboration risks; and uncertainty of Biogen’s success in developing, licensing, or acquiring other product candidates or additional indications for existing products. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from Biogen’s expectations in any forward-looking statement. Investors should consider this cautionary statement, as well as the risk factors identified in Biogen’s most recent annual or quarterly report and in other reports Biogen has filed with the U.S. Securities and Exchange Commission. These statements are based on Biogen’s current beliefs and expectations and speak only as of the date of this press release. Biogen does not undertake any obligation to publicly update any forward-looking statements, whether as a result of new information, future developments or otherwise.
Alkermes Note Regarding Forward-Looking Statements
Certain statements set forth in this press release constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: the continued expansion of Alkermes’ portfolio of medicines in psychiatry, the continued clinical development and the potential therapeutic and commercial value of ALKS 8700 for the treatment of relapsing forms of MS, the number of patients enrolled in the ALKS 8700 Phase 3 studies, the timing of expected initial data from EVOLVE-MS-2, the regulatory strategy for filing of an NDA for ALKS 8700 and the adequacy of the EVOLVE-MS development program for ALKS 8700 to serve as the basis for an NDA, the timing of the submission of an NDA to the FDA for ALKS 8700 and the potential financial, commercial and therapeutic benefits that may be achieved through collaboration with Biogen under the license and collaboration agreement between Alkermes and Biogen. Alkermes cautions that forward-looking statements are inherently uncertain. Although Alkermes believes that such statements are based on reasonable assumptions within the bounds of its knowledge of its business and operations, the forward-looking statements are neither promises nor guarantees and they are necessarily subject to a high degree of uncertainty and risk. Actual performance and results may differ materially from those expressed or implied in the forward-looking statements due to various risks and uncertainties. These risks and uncertainties include, among others: whether the results from the head-to-head study to evaluate the GI tolerability of ALKS 8700 compared to TECFIDERA will show that ALKS 8700 has more favorable GI tolerability; whether preclinical and early clinical results for ALKS 8700 will be predictive of future clinical study results or real-world results; whether clinical trials for ALKS 8700 will be completed on time or at all; changes in the cost, scope and duration of the ALKS 8700 clinical trials; whether
F – Press Release
ALKS 8700 could be shown ineffective or unsafe during clinical studies, and whether, in such instances, Alkermes may not be permitted by regulatory authorities to undertake new or additional clinical studies of ALKS 8700; whether regulatory submissions for ALKS 8700 will be submitted on time or at all; whether adverse decisions by regulatory authorities will occur; whether the pharmacokinetic, Phase 3 and other studies conducted for ALKS 8700 will meet the FDA’s requirements for approval; whether the potential financial, commercial and therapeutic benefits of collaboration with Biogen under the license and collaboration agreement between Alkermes and Biogen will be achieved; and those risks described in the Alkermes Annual Report on Form 10-K for the fiscal year ended December 31, 2016, and Quarterly Reports on Form 10-Q for the quarters ended March 31, 2017 and September 30, 2017 and in subsequent filings made by Alkermes with the U.S. Securities and Exchange Commission (SEC), which are available on the SEC’s website at www.sec.gov. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the company disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release.
TECFIDERA® is a registered trademark of Biogen Inc.
1 National Multiple Sclerosis Society. Multiple Sclerosis: Just the Facts. Accessed from http://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Brochure-Just-the-Facts.pdf on Nov. 27, 2017.
2 Multiple Sclerosis Association of America. MS Overview. Accessed from http://mymsaa.org/ms-information/overview/who-gets-ms/ on Nov. 27, 2017.
###
F – Press Release
Schedule 1.1.26
Schedule 5.1
Amended and Restated Credit Agreement, dated as of September 16, 2011, as amended and restated on September 25, 2012, as further amended by that certain Amendment No. 2 to Amended and Restated Credit Agreement dated as of February 14, 2013, and as amended by that certain Amendment No. 3 and Waiver to Amended and Restated Credit Agreement dated as of May 22, 2013 and by that certain Amendment No. 4 to Amended and Restated Credit Agreement dated as of October 12, 2016, among ALKERMES, INC., a corporation organized under the laws of the Commonwealth of Pennsylvania, ALKERMES PLC, a company incorporated under the laws of the Republic of Ireland (registered number 498284) (“Holdings”), ALKERMES PHARMA IRELAND LIMITED, a private limited company organized under the laws of the Republic of Ireland (registered number 448848) and a wholly owned indirect subsidiary of Holdings (the “Intermediate Holdco”), ALKERMES US HOLDINGS, INC., a Delaware corporation and a wholly owned subsidiary of Intermediate Holdco, certain other subsidiaries of Holdings, the several banks and other financial institutions or entities from time to time parties to the Credit Agreement as lenders, MORGAN STANLEY SENIOR FUNDING, INC., as administrative agent, MORGAN STANLEY SENIOR FUNDING, INC., Citigroup Global Markets, Inc. and JPMorgan Chase Bank, N.A. as co-syndication agents, and MORGAN STANLEY SENIOR FUNDING, INC., as collateral agent.
Schedule 10.3.3
